Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 2018/125930
PCT/US2017/068571
INTRAOCULAR DRUG DELIVERY DEVICE AND ASSOCIATED METHODS
FIELD OF THE INVENTION
The present invention relates to systems, methods, and devices for the
sustained and
targeted (local) delivery of a pharmaceutical active agent into a subject's
eye. Accordingly,
the present invention involves the fields of polymer chemistry, material
science, polymer
science, drug delivery, formulation science, pharmaceutical sciences, and
medicine,
particularly ophthalmology.
BACKGROUND
Age-related macular degeneration (AMD) and glaucoma are two of the leading
causes
of blindness in the United States and across the world. Present glaucoma
therapies generally
require polypharmacy, where subjects are often prescribed several topical
agents that must be
applied to the eye with varying frequencies, in some cases up to 3 or 4 times
a day. These
dosing regimens are often difficult for subjects to consistently follow, and
many individuals
progress to needing surgical treatments such as intraocular shunts or
trabeculectomies, which
have significant attendant complications.
Subjects having macular degeneration are often required to have monthly
intravitreal
injections. Such injections are painful and may lead to retinal detachment,
endophthalmitis,
and other complications. Furthermore, these injections are generally performed
only by
retinal surgeons, a small fraction of the ophthalmic community, producing a
bottleneck in eye
care delivery and increased expense.
Postoperative surgery inflammation is associated with raise intraocular
pressure
(TOP), and increase the likelihood of cystoid macular edema (CME), synechial
formation,
posterior capsule opacification (PCO), and secondary glaucoma. Patient
compliance is of
concern in the management of postoperative inflammation because multiple eye
drops must
be taken multiple times per day at regular intervals over the course of weeks.
Poor
compliance compromises the efficacy of topical drugs, which are further
limited by corneal
absorption and have highly variable intraocular concentrations during the
therapeutic course.
Uveitis specifically refers to inflammation of the middle layer of the eye,
termed the "uvea"
but in common usage may refer to any inflammatory process involving the
interior of the eye.
Uveitis is estimated to be responsible for approximately 10% of the blindness
in the United
States.
CA 3050771 2019-07-24
WO 2018/125930
PCT/US2017/068571
Postoperative cataract surgery inflammation can be well controlled by
improving
patient compliance. Available literature and experience shows penetration of
the drug after
topical administration is poor and higher systemic concentration means
frequent systemic
adverse events. All of these factors highlight the need for sustained
intraocular delivery for
pharmaceutical active agents to effectively control inflammation.
SUMMARY
A method of delivering an active agent to a posterior segment of an eye of a
subject
can include placing a biodegradable active agent matrix within a lens capsule
of the subject to
deliver the active agent to a posterior segment of the eye. The biodegradable
active agent
matrix comprises an active agent present in an amount to deliver a
therapeutically effective
dose of the active agent to the posterior segment of the eye from the lens
capsule.
There has thus been outlined, rather broadly, the more important features of
the
invention so that the detailed description thereof that follows may be better
understood, and
so that the present contribution to the art may be better appreciated. Other
features of the
present invention will become clearer from the following detailed description
of the
invention, taken with the accompanying drawings and claims, or may be learned
by the
practice of the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a photograph showing a bioerodible dexamethasone implant (BDI), in
accordance with some examples of the present disclosure.
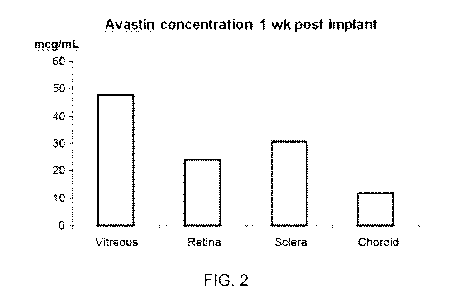
FIG. 2 is a bar graph showing the amount of an active agent present in various
eye
tissues following implantation of an intraocular device in accordance with a
further aspect of
the present invention.
FIG. 3 is a graph of in-vitro release kinetics of an example BDI implant. Data
are
presented as mean SD (n=3).
FIG. 4 time vs concentration profile of an example BDI implant with 120 to 160
[tg of
dexamethasone (DXM) in aqueous and vitreous humor of New Zealand White (NZW)
rabbits.
FIG. 5 time vs concentration profile of an example BDI implant with 120 to 160
[tg of
DXM in iris/ciliary body and retina/choroid of NZW rabbits.
FIG. 6 is a graph of time vs. concentration profile of an example BDI implant
and
topical drops in aqueous humor of New Zealand white rabbits.
- 2 -
CA 3050771 2019-07-24
WO 2018/125930
PCT/US2017/068571
FIG. 7 is a graph of time vs. concentration profile of an example BDI implant
and
topical drops in vitreous humor of New Zealand white rabbits.
FIG. 8 is a graph of time vs. concentration profile of an example BDI implant
and
topical drops in retina/choroid of New Zealand white rabbits.
FIG. 9 is a graph of time vs. concentration profile of an example BDI implant
and
topical drops in iris/ciliary body of New Zealand white rabbits.
FIG. 10 is a graph illustrating the effect of croscarmellose concentration on
drug
release for some example BDI implants.
FIG. 11 is a graph of in-vitro release kinetics of an example BDI implant.
Data are
presented as mean SD (n=3).
FIG. 12 is a graph of in-vitro release kinetics of another example BDI
implant. Data
are presented as mean SD (n=3).
FIG. 13 is a graph of time vs. concentration profile of an example BDI implant
and
topical drops in aqueous humor of New Zealand white rabbits.
FIG. 14 is a graph of time vs. concentration profile of an example BDI implant
and
topical drops in vitreous humor of New Zealand white rabbits.
FIG. 15 is a graph of time vs. concentration profile of an example BDI implant
and
topical drops in retina/choroid of New Zealand white rabbits.
FIG. 16 is a graph of time vs. concentration profile of an example BDI implant
and
topical drops in iris/ciliary body of New Zealand white rabbits.
FIG. 17 is a graph of retinal thickness vs. time profile of an example BDI
implant and
topical drops as compared to normal control.
FIG. 18 is a graph of time vs. concentration profile of an example BDI implant
and
topical drops in aqueous humor of New Zealand white rabbits.
FIG. 19 is a graph of time vs. concentration profile of an example BDI implant
and
topical drops in vitreous humor of New Zealand white rabbits.
FIG. 20 is a graph of time vs. concentration profile of an example BDI implant
and
topical drops in retina/choroid of New Zealand white rabbits.
FIG. 21 is a graph of time vs. concentration profile of an example BDI implant
and
topical drops in iris/ciliary body of New Zealand white rabbits.
FIG. 22 is a graph of retinal thickness vs. time profile of an example BDI
implant and
topical drops as compared to normal control. These drawings merely depict
exemplary
embodiments of the present invention and they are, therefore, not to be
considered limiting of
its scope. It will be readily appreciated that the components of the present
invention, as
- 3 -
CA 3050771 2019-07-24
WO 2018/125930
PCT/US2017/068571
generally described and illustrated in the figures herein, could be arranged,
sized, and
designed in a wide variety of different configurations.
DETAILED DESCRIPTION OF THE INVENTION
The following detailed description of exemplary embodiments of the invention
makes
reference to the accompanying drawings, which form a part hereof and in which
are shown,
by way of illustration, exemplary embodiments in which the invention may be
practiced.
While these exemplary embodiments are described in sufficient detail to enable
those skilled
in the art to practice the invention, it should be understood that other
embodiments may be
realized and that various changes to the invention may be made without
departing from the
spirit and scope of the present invention. Thus, the following more detailed
description of the
embodiments of the present invention is not intended to limit the scope of the
invention, as
claimed, but is presented for purposes of illustration only and not limitation
to describe the
features and characteristics of the present invention, to set forth the best
mode of operation of
the invention, and to sufficiently enable one skilled in the art to practice
the invention.
Accordingly, the scope of the present invention is to be defined solely by the
appended
claims.
It must be noted that, as used in this specification and the appended claims,
the
singular forms "a," "an," and "the" include plural referents unless the
context clearly dictates
otherwise. Thus, for example, reference to -a drug" includes reference to one
or more of
such drugs, "an excipient" includes reference to one or more of such
excipients, and "filling"
refers to one or more of such steps.
Definitions
In describing and claiming the present invention, the following terminology
will be
used in accordance with the definitions set forth below.
As used herein, "active agent," "bioactive agent," "pharmaceutically active
agent,"
and "drug," may be used interchangeably to refer to an agent or substance that
has
measurable specified or selected physiologic activity when administered to a
subject in a
significant or effective amount. These terms of art are well-known in the
pharmaceutical and
medicinal arts.
As used herein, "formulation" and "composition" may be used interchangeably
herein, and refer to a combination of two or more elements, or substances. In
some
embodiments a composition can include an active agent, an excipient, or a
carrier to enhance
delivery, depot formation, etc.
- 4 -
CA 3050771 2019-07-24
WO 2018/125930
PCT/US2017/068571
As used herein, "effective amount" refers to an amount of an ingredient which,
when
included in a composition, is sufficient to achieve an intended compositional
or physiological
effect. Thus, a "therapeutically effective amount" refers to a substantially
non-toxic, but
sufficient amount of an active agent, to achieve therapeutic results in
treating or preventing a
condition for which the active agent is known to be effective. It is
understood that various
biological factors may affect the ability of a substance to perform its
intended task.
Therefore, an "effective amount" or a "therapeutically effective amount" may
be dependent
in some instances on such biological factors. Further, while the achievement
of therapeutic
effects may be measured by a physician or other qualified medical personnel
using
evaluations known in the art, it is recognized that individual variation and
response to
treatments may make the achievement of therapeutic effects a subjective
decision. However,
the determination of an effective amount is well within the ordinary skill in
the art of
pharmaceutical and nutritional sciences as well as medicine.
As used herein, "subject" refers to a mammal that may benefit from the
administration
of a composition or method as recited herein. Examples of subjects include
humans, and can
also include other animals such as horses, pigs, cattle, dogs, cats, rabbits,
aquatic marnmals,
etc.
As used herein, the term "intraocular lens" refers to a lens that is utilized
to replace a
lens in the eye of a subject. Such intraocular lenses can be synthetic or
biological in nature.
Furthermore, in some aspects the term -intraocular lens" can also refer to the
original natural
lens that is associated with the eye.
As used herein, the term "ciliary sulcus" refers to the space between the
posterior root
of the iris and the ciliary body of the eye.
As used herein, the term "substantially" refers to the complete or nearly
complete
extent or degree of an action, characteristic, property, state, structure,
item, or result. For
example, an object that is "substantially" enclosed would mean that the object
is either
completely enclosed or nearly completely enclosed. The exact allowable degree
of deviation
from absolute completeness may in some cases depend on the specific context.
However,
generally speaking the nearness of completion will be so as to have the same
overall result as
if absolute and total completion were obtained. The use of "substantially- is
equally
applicable when used in a negative connotation to refer to the complete or
near complete lack
of an action, characteristic, property, state, structure, item, or result. For
example, a
composition that is "substantially free of' particles would either completely
lack particles, or
so nearly completely lack particles that the relevant effect would be the same
as if it
- 5 -
CA 3050771 2019-07-24
WO 2018/125930
PCT/US2017/068571
completely lacked particles. In other words, a composition that is
"substantially free of" an
ingredient or element may still actually contain such item as long as there is
no measurable
effect thereof.
As used herein, the term "about" is used to provide flexibility to a numerical
range
endpoint by providing that a given value may be "a little above" or "a little
below" the
endpoint.
As used herein, a plurality of items, structural elements, compositional
elements,
and/or materials may be presented in a common list for convenience. However,
these lists
should be construed as though each member of the list is individually
identified as a separate
and unique member. Thus, no individual member of such list should be construed
as a de
facto equivalent of any other member of the same list solely based on their
presentation in a
common group without indications to the contrary.
As used herein, the term "at least one of' is intended to be synonymous with
"one or
more of" For example, "at least one of A, B and C" explicitly includes only A,
only B, only
C, and combinations of each.
Concentrations, amounts, and other numerical data may be expressed or
presented
herein in a range format. It is to be understood that such a range format is
used merely for
convenience and brevity and thus should be interpreted flexibly to include not
only the
numerical values explicitly recited as the limits of the range, but also to
include all the
individual numerical values or sub-ranges encompassed within that range as if
each
numerical value and sub-range is explicitly recited. As an illustration, a
numerical range of
"about 1 to about 5" should be interpreted to include not only the explicitly
recited values of
about 1 to about 5, but also include individual values and sub-ranges within
the indicated
range. Thus, included in this numerical range are individual values such as 2,
3, and 4 and
sub-ranges such as from 1-3, from 2-4, and from 3-5, etc. This same principle
applies to
ranges reciting only one numerical value. Furthermore, such an interpretation
should apply
regardless of the breadth of the range or the characteristics being described.
Any steps recited in any method or process claims may be executed in any order
and
are not limited to the order presented in the claims unless otherwise stated.
Means-plus-
function or step-plus-function limitations will only be employed where for a
specific claim
limitation all of the following conditions are present in that limitation: a)
"means for" or "step
for" is expressly recited; and b) a corresponding function is expressly
recited. The structure,
material or acts that support the means-plus function are expressly recited in
the description
herein. Accordingly, the scope of the invention should be determined solely by
the appended
- 6 -
CA 3050771 2019-07-24
WO 2018/125930
PCT/US2017/068571
claims and their legal equivalents, rather than by the descriptions and
examples given herein.
Intraocular Drug Delivery Device
An intraocular drug delivery device can provide improved ophthalmic drug
delivery
by alleviating the need for multiple injections or complex eyedrop regimens by
providing an
intra-capsular reservoir which is implantable and biodegrades such that
subsequent surgery is
often unnecessary. Further, the device can deliver a variety or combination of
different
medicines.
A novel intraocular drug delivery device, system, and associated methods for
providing sustained release of ocular active agents for extended periods of
time are disclosed
and described. One problem with many eye diseases such as Age-related Macular
Degeneration (AMD) is the constant need for a subject to receive painful
ocular injections,
which have significant risks of retinal detachment, vitreous hemorrhage, and
endophthalmitis.
The intraocular drug delivery device allows for sustained release of an active
agent over time,
thus eliminating the need for frequent ocular injections.
It should be noted that neovascularization is a key pathobiological process in
a variety
of eye diseases, such as AMD, proliferative diabetic retinopathy, vascular
occlusive disease,
and radiation retinopathy. Additionally, the incidence of glaucoma is
increasing worldwide.
Many other disorders, including severe uveitis and geographic atrophy in AMD,
can be
treated using such an intraocular drug delivery device. Thus, an implantable,
generally
sutureless drug delivery device for placement in the anterior segment of the
eye has great
potential to improve the quality of life for subjects.
The drug delivery device can continuously deliver dexamethasone or other anti-
inflammatory or therapeutic agents with near zero order kinetics for two weeks
or more.
Treatment of uveitis needs long term (6-8 weeks) sustained delivery of anti-
inflammatory
agents. The biggest disadvantage with topical drops is that negligible
concentrations of drugs
will reach the posterior segment of the eye and especially the retina/choroid.
The designed
and disclosed drug delivery device can deliver dexamethasone and/or other
therapeutic agents
continuously with near zero order kinetics both to the anterior and posterior
segments of the
eye, thus effectively controlling the inflammation.
Therefore, the opportunity exists to improve management of AMD, postoperative
surgery inflammation and uveitis patients undergoing cataract surgery by
sustained release of
pharmaceutical active agent(s).Accordingly, the present invention provides
systems, devices,
and associated methods for the delivery of active agents into the eye of a
subject. In some
- 7 -
CA 3050771 2019-07-24
WO 2018/125930
PCT/US2017/068571
examples, the systems, devices, and associated methods can be positioned
within the anterior
segment of the eye (e.g. within the lens capsule) of a subject to deliver an
active agent to the
posterior segment of an eye of the subject. Non-limiting examples of ocular
regions found
within the posterior segment of the eye can include at least one of the
vitreous humor, the
choroid, and the retina. In addition to delivering the active agent to the
posterior segment of
the eye, the active agent can also be delivered to the anterior segment of the
eye. The anterior
segment of the eye can include at least one of the aqueous humor, the iris,
and the lens
capsule. In one aspect, the intraocular device can be sutureless. A sutureless
device can be
defined as a device or structure that can be inserted and retained within a
lens capsule without
the need for a suture to hold the device in place.
In further detail, in some aspects, the device can be implantable within the
lens
capsule during cataract surgery, essentially "piggybacking" on the cataract
extraction, and
thus eliminating the need for additional surgical procedures. One benefit to
"piggybacking"
on the cataract extraction is the ability to deliver steroids, antibiotics,
and/or various non-
steroidal agents directly to the eye after surgery, thus helping to minimize
complications such
as cystoid macular edema. In other aspects, the device can be implanted in a
surgery that is
separate from a cataract procedure, e.g., subsequent to a previous cataract
extraction with
reopening of the lens capsule. For example, the device can be implanted post-
cataract surgery
for treatment of macular degeneration, retinal vein or artery occlusion,
diabetic retinopathy,
macular edema (e.g. from diabetes, uveitis, intraocular surgery, etc.),
retinal degenerations
where a neuroprotectant delivery is indicated, or the like.
In one embodiment, the device can be provided in the form of an implant
containing
an active agent within a biodegradable or bioerodible polymer matrix. The
biodegradable
active agent matrix can include an active agent in an amount to deliver a
therapeutically
effective amount or therapeutically effective dose of the active agent to the
posterior segment
of the eye from the lens capsule. A therapeutically effective amount or
therapeutically
effective dose can vary depending on the particular therapeutic agent being
employed in the
biodegradable active agent matrix. Further, the therapeutically effective
amount or
therapeutically effective dose can vary depending on the severity of the
condition being
treated. Nonetheless, the active agent can be present in an amount to
facilitate delivery of the
active agent from the anterior segment of the eye (e.g. from the lens capsule)
to the posterior
segment of the eye.
The therapeutically effective amount or therapeutically effective dose can
typically
range from about 50 micrograms (mcg) to about 10 milligrams (mg), depending on
the active
- 8 -
CA 3050771 2019-07-24
WO 2018/125930
PCT/US2017/068571
agent being employed and the severity of the condition. In some specific
examples, the
therapeutically effective amount or therapeutically effective dose can range
from about 50
mcg to about 600 mcg. In yet other examples, the therapeutically effective
amount or
therapeutically effective dose can range from about 100 mcg to about 400 mcg,
from about
100 mcg to about 300 mcg, or from about 200 mcg to about 400 mcg. In yet
further detail,
the active agent can typically be present in the implant at a concentration of
from about 5
wt% to about 25 wt%, or from about 5 wt% to about 15 wt%, or from about 10 wt%
to about
20 wt%. Further still, depending on the dosage requirements, one, two or more
implants can
be implanted per eye to achieve a therapeutically effective dose.
The biodegradable active agent matrix can be configured to bioerode to provide
controlled release of the therapeutically effective amount over a period of
days, weeks, or
months. In some examples, the therapeutically effective amount can be released
over a
period ranging from about 1 week to about 10 weeks. In other examples, the
therapeutically
effective amount can be released over a period ranging from about 1 week to
about 3 weeks,
from about 2 weeks to about 6 weeks, or from about 5 weeks to about 8 weeks.
In cases such
as retinal vein/artery occlusion, diabetic retinopathy, macular edema or
retinal degenerations,
the period can often range from 2 months to 12 months, and in some cases from
2.5 months
to 5 months. For example, bioerodible lipid polymers and/or bioerodible
polycaprolactone
can be used as an extended release matrix material.
It is noted that in addition to the amount of the active agent present in the
biodegradable active agent matrix, other additional factors can affect the
delivery of the
active agent to the posterior segment of the eye. For example, the
intracapsular positioning
of the device within the lens capsule can affect delivery of the active agent
to the posterior
segment of the eye. In some examples, positioning of the implant at a location
inferior and
peripheral to the intraocular lens (TOL) or within the inferior peripheral
capsule can provide
suitable delivery of the active agent to the posterior segment of the eye.
Typically, the
implant can also be located at a lower portion within the lens capsule. For
example, the
implant can be oriented within the inferior periphery, annular periphery
encircling the
intraocular lens for at least 180 degrees, or the like as long as a line of
sight is not obstructed.
Further still, the molecular weight and molecular size of the active agent can
affect
delivery of the active agent to the posterior segment of the eye. Thus, in
some examples, the
active agent can have a molecular weight of 250,000 daltons (Da) or less. In
yet other
examples, the active agent can have a molecular weight of 170,000 Da or less.
In yet
additional examples, the active agent can have a molecular weight of 500 Da or
less.
- 9 -
CA 3050771 2019-07-24
WO 2018/125930
PCT/US2017/068571
Numerous active agents are known for the treatment or prophylaxis of various
eye
conditions, such as AMD (neovascular form or atrophic form), glaucoma,
diabetic
retinopathy, Retinopathy of Prematurity, uveitis, corneal transplant
rejection, capsular
fibrosis, posterior capsule opacification, retinal vein occlusions,
infections, and the like. Any
suitable active agent for incorporation into a biodegradable active agent
matrix can be used,
such as steroids, NSAIDs, antibiotics, anti-VEGF agents, PDGF-B inhibitors
(Fovistat),
integrin antagonists, complement antagonists, the like, or combinations
thereof Non-limiting
examples of suitable active agents can include dexamethasone, prednisolone,
bevacizumab
(Avastin0), ranibizumab (Lucentisk), sunitinib, pegaptanib (Macugenk),
moxifloxacin,
gatifloxicin, besifloxacin, timolol, latanoprost, brimonidine, nepafenac,
bromfenac,
diclofenac, ketorolac, triamcinolone, difluprednate, fluocinolide,
aflibercept, the like, or
combinations thereof Treatment regimens can additionally include various
photodynamic
therapies, and the like. In one specific example, the active agent can include
dexamethasone.
The bioerodible polymer matrix can include one or several excipients, which
can
depend on the duration of active agent delivery. Non-limiting examples of
active agent
matrix materials can include polymeric and non-polymeric materials. Specific
non-limiting
examples of suitable matrix materials include biodegradable polymers such as
PLGA
(different ratios of lactic to glycolide content and end groups such as acid
or ester
termination), PVA, PEG, PLA, PGA, hydroxypropylcellulose, sodium
carboxymethylcellulose, croscarmellose sodium, polycaprolactone, hyaluronic
acid, albumin,
sodium chloride block copolymers thereof, and the like. Specific copolymers
such as
polylactic-polyglycolic acid block copolymers (PLGA), polyglycolic acid-
polyvinyl alcohol
block copolymers (PGA/PVA), hydroxypropylmethylcellulose (HPMC),
polycaprolactone-
polyethylene glycol block copolymers, croscarmellose, and the like can be
particularly
effective. In one aspect, the active agent matrix can be a PLGA having about
45-80% PLA
and 55-20% PGA such as about 65% PLA and 35% PGA, and in one case about 50%
PLA
and 500/0 PGA. In another alternative embodiment, the weight ratios of PLGA,
dexamethasone, and Croscarmellose sodium can be 60-90/5-25/5-25 or 50-75/10-
40/10-40
ratios. In another aspect, the biodegradable active agent matrix comprises a
low melt fatty
acid such as, but not limited to, lauric acid, myrisitic acid, palmitic acid,
stearic acid,
arachidic acid, capric acid, oleic acid, palmitoleic acid, and mixtures
thereof In one aspect,
the biodegradable active agent matrix can comprise a pharmaceutically
acceptable
disintegrant. In one example, the distintegrant can be a superdistintegrant.
Non-limiting
examples of suitable disintigrants include crosslinked celluloses (e.g.
croscarmellose, Ac-Di-
- 10 -
CA 3050771 2019-07-24
WO 2018/125930
PCT/US2017/068571
Sol, NYMCE ZSX, PRIMELLOSE, SOLUTAB, VIVASOL), microcrystalline cellulose,
alginates, crosslinked PVP (e.g. CROSSPOVIDONE, KOLLIDON, POLYPLASDONE),
crosslinked starch, soy polysaccharides, calcium silicate, salts thereof, and
the like.
Typically, the delivery device herein can be targeted for a relatively short
delivery
duration, such as less than eight weeks. In some examples, the active agent
has a delivery
duration of from about two weeks to about six weeks. Delivery duration can be
a function of
the type of polymer used in the matrix, copolymer ratios, and other factors.
Although other
biodegradable polymers can be suitable such as those listed previously,
particularly suitable
polymers can include at least one of poly(lactic-co-glycolide), hydroxypropyl
methyl
cellulose, hydroxyl methyl cellulose, polyglycolide-polyvinyl alcohol,
croscarmellose,
polycaprolactone, eudragit L100, eudragit RS100, poly(ethylene glycol) 4000,
poly(ethylene
glycol) 8000 and poly(ethylene glycol) 20,000. In one example, the
biodegradable active
agent matrix can comprise poly(lactic-co-glycolide) having a copolymer ratio
from 10/90 to
90/10 and in another case from 52/48 to 90/10. One particular aspect, the
copolymer ratio can
be about 50/50. In another specific example, the copolymer ratio can be 52-
78/48-22 and in
another specific example from 60-90/40-10. Although degradation rates can be
dependent on
such proportions, additional alternative approaches can also be useful such as
device
coatings, particle encapsulation, and the like.
Homogeneous delivery devices can be formed, for example, by mixing a polymer
material with a loading amount of active agent to form a matrix dispersion.
The loading
amount can be chosen to correspond to the desired dosage during diffusion.
Loading amount
can take into account diffusion characteristics of the polymer and active
agent, residual active
agent, delivery time, and the like. The matrix dispersion can then be formed
into the device
shape using any suitable technique. For example, the matrix dispersion can be
cast, sprayed
and dried, extruded, stamped, or the like. Such configurations will most often
be formed
using a biodegradable matrix, although non-biodegradable materials can also be
used. In one
alternative formulation, the device can be formed in situ from a suspension of
the active agent
within a biodegradable polymer matrix precursor. Upon delivery into the target
site, the
biodegradable polymer matrix precursor can form (via precipitation and/or
polymerization)
the biodegradable active agent matrix in situ.
With the above homogeneous delivery device, particular efficacy can be
provided for
treatment of uveitis and post-operative cataract surgery inflammation. For
example,
dexamethasone can be dispersed within a biodegradable active agent matrix.
Although
dexamethasone dosage amounts can vary, generally from about 100 mcg to about
400 mcg
- 11 -
CA 3050771 2019-07-24
WO 2018/125930
PCT/US2017/068571
can be effective for these indications. More specifically, some patients may
be categorized as
low risk while others can be categorized as high risk due to various factors
such as age,
secondary complications, pre-existing conditions, etc. Most often, a low risk
patient can
benefit from a low dosage of about 100 mcg to about 150 mcg. In contrast, a
high risk
individual can be administered a high dosage of about 250 mcg to about 350
mcg. Some
biodegradable implants can specifically designed and tested for the treatment
of postoperative
surgery inflammation and can deliver pharmaceutical active agent up to or
about 2 weeks or
more. Yet other biodegradable implants can be designed and tested for the
treatment of
postoperative surgery inflammation and uveitis and can deliver active agent up
to or about 6-
8 weeks or more. Further, depending on the severity of the inflammation, one,
two, more
implants can be implanted per eye during surgery.
The active agent delivery devices can optionally include additional active
agents or
other desired therapeutically beneficial substances. In one aspect, for
example, the device
can include at least one secondary active agent. Where a plurality of active
agents is included
in the active agent delivery device, the active agent matrix can be homogenous
or non-
homogenous. In some examples, the active agent delivery device can include a
plurality of
active agents and can be homogenous. In some examples, the active agent
delivery device
can include a plurality of active agents and can be non-homogenous. For
example, one active
agent can be coated on the surface of the implant. In yet other examples, the
implant can be
formulated to have pre-designated regions or layers including different active
agents. In yet
other examples, the implant can be formulated to have pre-designated regions
or layers
having the same active agent, but at different concentrations. For example, in
some cases, an
outer region or layer of the implant can have a higher concentration of the
active agent to
deliver a higher initial dose or burst of the active agent followed by a
prolonged lower dose
over a period of days or weeks. Further, in some examples, different regions
of the implant
can be adapted to biodegrade at different rates. In yet other examples, agents
can optionally
be coated on the implant to reduce the incidence of capsular fibrosis. Non-
limiting examples
of such agents include anti-cell proliferative agents, anti-TGF-beta agents,
a5b1 integrin
antagonists, rapamycin, and the like.
The ocular active agent delivery device can be configured to fit within a lens
capsule
or ciliary sulcus of an eye. The delivery device can be shaped in any geometry
which allows
for insertion into the lens capsule or ciliary sulcus. For example, the
implant can be in the
shape of round, square shape, crescent, or donut shape. However, other
suitable shapes can
include, but are not limited to, discs, pellets, rods, and the like. Although
dimensions can
- 12 -
CA 3050771 2019-07-24
WO 2018/125930
PCT/US2017/068571
vary, typical dimensions can range from about 0.5 mm to about 4 mm width and
about 0.2
mm to about 1 mm thickness. Although the total mass of the delivery device can
vary, most
often the total mass can be from 0.2 mg to 4 mg, or from about 1.5 mg to about
2.5 mg. For
example, about 2 mg total mass can provide effective active agent volume,
while also
balancing overall size to fit within the target tissue areas.
In some specific examples, the implant can be shaped as a disc or pellet.
Where this
is the case, the implant can typically have a diameter ranging from about 0.4
millimeters
(mm) to about 3 mm, from about 0.5 mm to about 1.5 mm, or from about 0.7 mm to
about 1.3
mm. Further, in some examples, the disc- or pellet-shaped implant can
typically have a
thickness ranging from about 0.2 mm to about 2 mm, from about 0.8 mm to about
1.5 mm, or
from about 0.3 mm to about 1.0 mm. One example of a pellet or disc is
illustrated in FIG. 1,
which has a diameter of about 2 to 2.5 mm and a thickness of about 1.0-1.5 mm.
In one
specific example, the implant can have rounded edges, hemispherical, or semi-
circular
shapes.
In yet other specific examples, the implant can be shaped as a rod. Where this
is the
case, the implant can typically have a diameter ranging from about 0.05 mm to
about 2 mm,
from about 0.1 mm to about 1.0 mm, or from about 0.2 mm to about 0.8 mm.
Further, in
some examples, the rod-shaped implant can typically have a length ranging from
about 0.5
mm to about 5 mm, from about 1.0 mm to about 3.0 mm, or from about 1.5 mm to
about 2.5
mm.
Yet another aspect of the present invention provides a method of delivering an
active
agent into an eye of a subject. It is noted that when discussing various
examples and
embodiments of the implantable devices, systems, and methods described herein,
each of
these respective discussions can also apply to each of the other aspects of
the present
invention. Thus, for example, when discussing the implantable device per se,
this discussion
is also relevant to the methods discussed herein, and vice versa.
With this in mind, in some specific examples, the method can include placing a
biodegradable active agent matrix, as described herein, within a lens capsule
of a subject to
deliver the active agent to a posterior segment of the eye. The biodegradable
active agent
matrix can include an active agent in an amount to deliver a therapeutically
effective dose of
the active agent to the posterior segment of the eye from the lens capsule.
In some examples, the method can include performing a cataract removal surgery
on
the eye of the subject, further including removing an existing lens from the
eye of the subject,
inserting an intraocular lens into the eye of the subject, and placing the
biodegradable active
- 13 -
CA 3050771 2019-07-24
WO 2018/125930
PCT/US2017/068571
agent matrix within the lens capsule. In some examples, the biodegradable
active agent
matrix or implant can be associated with the intraocular lens. Where this is
the case, the
delivery device may be attached or detached from an intraocular lens. The
delivery device
can be associated by actual contact or sufficient proximity while allowing
effective diffusion
of active agent to target areas of the eye. A biodegradable system can have
substantial value
in routine cataract surgery to provide short-term/time-limited delivery of
postoperative
medicines while minimizing or eliminating the need for eyedrop usage by the
patient. The
lens that is removed can be the original natural lens of the eye, or it can be
a lens that was
previously inserted into the eye as a result of a prior procedure.
Numerous methods of placing the device into the eye are contemplated. For
example,
in one aspect, the implant can be associated with the intraocular lens prior
to inserting the
intraocular lens into the eye. In such cases it can be necessary to configure
the implant to
comply with any requirements of the surgical procedure. For example, cataract
surgeries are
often performed through a small incision. One standard size incision is about
2.75 mm;
although this device can be compatible with smaller or larger incision sizes
as well. As such,
the intraocular lens assembly can be shaped to allow insertion through this
small opening.
Thus the active agent delivery device can also be configured to be inserted
with the
intraocular lens assembly, e.g. by shape and/or choice of resilient and
flexible material for the
implant. Additionally, the active agent delivery device can also be physically
coupled or
decoupled to the intraocular lens assembly prior to insertion of the assembly
into the eye. In
another aspect, the implant can be positioned within the lens capsule, and
optionally
associated with the intraocular lens assembly, following insertion of the lens
into the eye.
The capsular bag can be readily reopened for a patient having prior cataract
surgery. Thus,
the insertion of the delivery device can be performed immediately prior to
insertion of an
intraocular lens or later in time as a separate procedure.
Examples:
Example 1:
A standard clear-corneal phacoemulsification with intraocular lens (Acrysof
SA6OAT;
Alcon) implantation was performed on 35 rabbits. At the time of each surgery,
an intraocular
device containing an active agent was inserted into a lens capsule of each
rabbit. The rabbits
were divided into 4 groups, depending on the active agent in the intraocular
device. Devices
were loaded with 5-15 mg of either Avastin, Timolol, Brimonidine, or
Latanoprost. Each
group was evaluated to determine the intraocular device and lens stability,
capsular fibrosis,
- 14 -
CA 3050771 2019-07-24
WO 2018/125930
PCT/US2017/068571
and healing of cataract wounds and anterior segment. A subgroup of eyes was
evaluated
weekly for 4 weeks for inflammation and harvested at 1 month for
histopathologic evaluation
of capsular and CDR integrity.
Example 2:
The surgery and setup as described in Example 1 was repeated, with the
exception
that aqueous and vitreous taps were performed biweekly and assayed for drug
concentrations
with HPLC and/or ELISA. In each drug group, half of the eyes were harvested at
one month
and the other half at two months. This was accomplished as follows:
immediately after
sacrificing the rabbit and enucleating the eye, the eye was frozen in liquid
nitrogen to prevent
perturbation and redistribution of drug in eye tissues. The eye was then
dissected into 3 parts
(aqueous humor, vitreous and retina/choroid layer) to evaluate anatomic
toxicity and tissue
drug concentration. The intraocular device was retrieved and assessed for
remaining drug
amounts. The distribution profile of the intraocular device was compared with
the
conventional intravitreal injection of 2.5 mg/0.1 cc Avastin for direct
comparison of the
different delivery methods.
At 2 and 4 months, eyes from the remaining subgroups of rabbits were
enucleated,
fixed by 10% formalin, embedded in paraffin, step sectioned, stained by
hematoxyline and
eosin (H & E), and examined for histological changes.
Example 3:
Three intraocular devices were implanted into eyes of New Zealand white
rabbits
under general anesthesia after lens extraction (phacoemulsification
technique). Two of the
devices were loaded with Avastin and one was loaded with the contrast agent
Galbumin as a
control. Proper intraocular device position was verified by MM as well as
clinical
examination.
The rabbits were sacrificed and the eyes are removed and assayed after 1 week
post
implantation. Avastin was detected by ELISA in the retina and vitreous at
concentrations of
24-48 mcg/mL, and was not present in the control rabbit eye. FIG. 5 shows the
amount of
Avastin assayed per ocular region at 1 week post implantation.
Example 4:
To confirm that placement of implant in the capsular bag and delivers drugs
both to
the front and back of the eye for short and long term, microparticles were
prepared using
PLGA [poly(d,l-lactide-co-glycolide), MW. 7000-17000, acid terminated],
hydroxypropyl
methyl cellulose (HPMC) and dexamethasone. Dexamethasone loaded PLGA
microspheres
were prepared using standard oil-in-water (o/w) emulsion-solvent extraction
method. An
- 15 -
CA 3050771 2019-07-24
WO 2018/125930
PCT/US2017/068571
amount of 160 mg PLGA was dissolved in 4 mL methylene chloride and 1 mL
acetonitrile.
An amount of 40 mg dexamethasone and 10 mg of HPMC was dispersed in the PLGA
solution by vortexing for 5 min. This organic phase was then emulsified in 20
mL of a 2%
(w/v) PVA (MW 90 kDa) solution and homogenized. The resultant emulsion was
poured into
200 n-iL of a 2.0% (w/v) PVA (MW 90 kDa) solution and stirred in an ice bath
for 6 min. The
contents were stirred for 8 hr at room temperature to evaporate the
dichloromethane and
acetonitrile to form a turbid microparticulate suspension. The microparticles
were separated
by centrifugation, washed twice, resuspended in deionized water, and freeze-
dried to obtain
lyophilized particles. The prepared microparticles were characterized and
pelleted using
bench top pellet press with 2 mm die set to form an implant.
These implants were sterilized, implanted in the capsular bag of rabbit's
eyes. Two
dose groups were used (300 and 600 pg), two rabbits were sacrificed from each
of low and
high dose group at 1, 2, 4, 6 weeks and various tissue samples (aqueous humor,
vitreous
humor, IOL, iris/ciliary body and retina/choroid) were collected and samples
were analyzed
by a validated LC/MS/MS method. Microspheres were in the range of 6 + 2 p,M as
confirmed
by Zetasizer nano and SEM photomicrographs. Drug loading in the microparticles
was >99%
and the final yield was 60% (i.e. encapsulation efficiency). Drug loading was
determined as
percent drug loading = (weight of drug loaded/weight of microspheres) x100.
Dose related
pharmacokinetics with near zero order kinetics was observed in rabbits up to 6
weeks.
Further, dexamethasone flow was bidirectional from the endocapsular space into
both the
anterior and posterior chambers. There were also no cells or formation of
fibrin in the anterior
and posterior chambers of the eye. Histological examinations revealed all the
tissues
examined were normal and showed no signs of inflammation.
All the study animals were acquainted to study room conditions once they are
out of
quarantine and randomized. All the positive control group and implantation
groups
underwent phacoemulsification and insertion of an intraocular lens (TOL) in
both the eyes.
Group III and IV received one and two implants per eye respectively.
Group I: Normal control group; n=6
Group II: Phacoemulsification and inserting IOL; DXM drops (up to 4 weeks with
tapering) and antibiotic drops (up to 2 days); positive control group; n=6
Group-III: Phacoemulsification and inserting IOL; BDI implant low dose (one
implant per eye) and antibiotic drops up to 2 days (bid.) after surgery; n=8
Group-IV: Phacoemulsification and inserting IOL; BDI implant high dose (two
implants per eye) and antibiotic drops up to 2 days (b.i.d.) after surgery,
n=8
- 16 -
CA 3050771 2019-07-24
WO 2018/125930
PCT/US2017/068571
Results of in vitro release kinetics are presented in FIG. 10. All the batches
exhibited
biphasic release pattern with initial burst release on day-1 and thereafter
slow and sustained
release. The burst effect was slightly higher with implants containing HPMC.
A total of 16 animals (32 eyes) received the implant. Dexamethasone
concentrations
are presented in FIG. 11 through FIG. 14. The implants degraded slowly over 4
weeks and by
week 6 were completely disappeared. Therapeutic concentrations of DXM was
found up to
week 6 with minimal systemic exposure (<40 ng/mL with high dose), whereas,
with
dexamethasone drops systemic exposure was higher (>150 ng/mL during week 1).
Mean PK
parameters for BDI-2 implant and positive control group in aqueous humor,
vitreous humor,
retina/choroid, and iris/ciliary body are shown in Table 1 and 2.
Table 1: Pharmacokinetics in aqueous humor and vitreous humor
Low dose: 300 lig High dose: 600 lig Dexamethasone Drops
Parameter Aqueous Vitreous Aqueous Vitreous Aqueous Vitreous
humor humor humor humor humor humor
1570 1379
Cmax (ng/mL) 650+ 109 892 + 151 62 + 24 3 + 0
113 233
Tmax (day) 19+8 28+0 7 + 0 28+0 14+0 16 + 11
AUCo-t 15231 18317 28202 32933 1023
61 5
(day*ng/mL) 361 2435 3369 4027 320
Clast (ng/mL) 8 + 3 2 + 1 52 + 18 85 + 23 6 + 2 2 + 1
Table 2: Pharmacokinetics in retina/choroid and iris/ciliary body
Low dose: 300 lig High dose: 600 lig Dexamethasone Drops
Parameter Retina/ Retina/ Retina/
Iris/CB Iris/CB Iris/CB
Choroid Choroid Choroid
Cmax ( 1\4) 21 + 4 35 + 5 117 + 40 209 + 24 3 + 1 3 + 2
Tmax (day) 14 0 7 0 23 8 14 0 14 0 9 4
AUCo-t 2226 +
455 61 759 132 3913 685 48 16 42 27
(day*04) 1105
Clast (04) 1.3 0.6 1.5 0.5 12 8 13 10 0.2 0.1
0.5 0.3
Intraocular pressure was normal in all the groups. Further, there were no
signs of
- 17 -
CA 3050771 2019-07-24
WO 2018/125930
PCT/US2017/068571
anterior or posterior chamber inflammation as assessed with Slit lamp
biomicroscopy and
confirmed by histological examination. There was a trend in increase in
retinal thickness in
animals treated with dexamethasone drops whereas, implants maintained retinal
thickness.
The PLGA polymer degrades in to lactic and glycolic acid through hydrolysis,
then
further degrades in to carbon dioxide and water before eliminating from the
body. Implants
did not migrate to the center to obstruct the visual field.
BDI-1 implant was manufactured by following partial solvent casting method
with
subsequent evaporation and removing the residual solvent by drying the product
under high
vacuum for 3 days. Various implants were prepared using PLGA [poly(d,l-lactide-
co-
glycolide), MW. 7000-17000, acid terminated], hydroxypropyl methyl cellulose
(HPMC),
croscarmellose sodium (cross linked sodium carboxymethylcellulose),
hydroxypropyl
cellulose and dexamethasone in several different compositions.
The dried particles were directly pelleted using bench top pellet press with a
2 mm die
set to form an implant.
The selected BDI-1 implants (from in-vitro release studies, FIG. 7) were
sterilized,
implanted in the capsular bag of rabbit's eyes. Two implants with different
composition and
dose were tested in-vivo in NZW rabbits to establish pharmacokinetics. Two
rabbits were
sacrificed at 2, 6, 10, 15 days and various tissue samples (aqueous humor,
vitreous humor,
IOL, iris/ciliary body and retina/choroid) were collected and samples were
analyzed by a
validated LC/MS/MS method. Pharmacokinetics with near zero order kinetics was
observed
in rabbits up to 15 days. Further, dexamethasone flow was bidirectional from
the
endocapsular space into both the anterior and posterior chambers. There were
also no cells or
formation of fibrin in the anterior and posterior chambers of the eye.
Histological
examinations revealed all the tissues examined were normal and showed no signs
of
inflammation.
Results of in vitro release kinetics are presented in FIG. 7. All the batches
exhibited
smooth release pattern with initial burst release on day-1 and thereafter slow
and sustained
release. The burst effect was slightly higher with implants containing HPMC.
A total of 8 animals (16 eyes) received the implant containing 120 lig of DXM.
DXM
concentrations are presented in FIG. 8 FIG. 9. The implants eroded slowly over
10 days and
reaching trough concentrations of DXM by day 15. The implants are degraded by
80% of its
mass by day 15 and expected to fully degrade by day 20. Therapeutic
concentrations of DXM
was found up to day 15 with minimal systemic exposure (<23 ng/mL), whereas,
with
dexamethasone drops systemic exposure was higher (>150 ng/mL during week 1, in-
house
- 18 -
CA 3050771 2019-07-24
WO 2018/125930 PCT/US2017/068571
data).
Example 5:
A number of biodegradable implants were prepared with PLGA, croscarmellose
sodium, and dexamethasone in accordance with Table 3 below.
Table 3 ¨ Effect of CrosCarmellose on Drug Release Rate
Formulation PLGA Croscarmellose Dexamethasone
(wt%) (wt%) (wt%)
Series 1 85 0 15
Series 2 84 1 15
Series 3 83.5 1.5 15
Series 4 83 2 15
Series 5 82.5 2.5 15
Series 6 82 3 15
Series 7 80 5 15
Series 8 77.5 7.5 15
Drug release profiles for each of the listed formulations were obtained using
an in-
vitro drug release model. Individual drug release profiles are presented in
FIG. 10. As
illustrated in FIG. 10, increasing amounts of croscarmellose can increase the
drug release rate
from the biodegradable implant as compared to a biodegradable implant prepared
with only
PLGA.
Example 6:
Two biodegradable dexamethasone implants (BDI) were prepared using different
formulations including PLGA, croscarmellose, and dexamethasone. The first BDI
was
configured to deliver 200 mcg dexamethasone over a period of two weeks. The
second BDI
was configured to deliver 300 mcg dexamethasone over a period of six weeks.
The drug
release profiles were evaluating using an in-vitro drug release model. The
release profile for
the first and second BDIs are illustrated in FIGs. 11 and 12, respectively.
Further, these implants were sterilized and implanted in the capsular bag of
rabbit's
eyes. The implants degraded slowly over a number of weeks until they
completely
- 19 -
CA 3050771 2019-07-24
WO 2018/125930
PCT/US2017/068571
disappeared. Mean PK parameters for the first BDI implant and positive control
group in
aqueous humor, vitreous humor, retina/choroid, and iris/ciliary body are shown
in Tables 4
and 5.
Table 4: Pharmacokinetics in aqueous humor and vitreous humor for first BDI
BDI: 200 lig Dexamethasone Drops
Parameter Aqueous Vitreous Aqueous Vitreous
humor humor humor humor
Cmax (ng/mL) 259 20 296 32 92 12 15 4
Tmax (day) 6 0 10 0 2 + 0 3 2
AUCo-t 2365 2769
517 43 104 42
(day*ng/mL) 182 276
Clast (ng/mL) 52 20 34 14 23 6 1 1
Table 5: Pharmacokinetics in retina/choroid and iris/ciliary body for first
BDI
BDI: 200 lig Dexamethasone Drops
Parameter Retina/ Retina/
Iris/CB Iris/CB
Choroid Choroid
Cmax (tam) 133 12 38 + 6 3.3 0.8 2.2 0.5
Tmax (day) 9 2 6 4 6 0 3 2
AUCo-t
1264 66 312 46 23 4 19 8
(day* M)
Clast ( M) 26 10 16 6 0.9 0.2 0.6 0.2
Dexamethasone concentration profiles for each of the ocular regions are
presented in
FIGs. 13 through FIG. 16. Further, the retinal thicknesses for each of the
test subjects were
measured over time and compared to retinal thicknesses for test subjects
treated with a topical
formulation and control subjects with normal retinal thickness. These results
are depicted in
FIG. 17. As illustrated in FIG. 17, a therapeutically effective dose can
reduce retinal
thickening associated with an ocular condition as compared to retinal
thickening without
treatment or as compared to treatment with a topical formulation.
Mean PK parameters for the second BDI implant and positive control group in
aqueous humor, vitreous humor, retina/choroid, and iris/ciliary body are shown
in Tables 6
- 20 -
CA 3050771 2019-07-24
WO 2018/125930
PCT/US2017/068571
and 7.
Table 6: Pharmacokinetics in aqueous humor and vitreous humor for second BDI
BDI: 300 lig Dexamethasone Drops
Parameter Aqueous Vitreous Aqueous Vitreous
humor humor humor humor
Cmax (ng/mL) 314 55 86 10 72 19 2.2 0.3
Tmax (day) 28 0 21 12 7 0 9 4
AUCo4 8377 2329 1533
68 5
(day*ng/mL) 1055 219 325
Clast (ng/mL) 232 29 35 8 17 8 2 1
Table 7: Pharmacokinetics in retina/choroid and iris/ciliary body for second
BDI
BDI: 300 lig Dexamethasone Drops
Parameter Retina/ Retina/
Iris/CB Iris/CB
Choroid Choroid
0.13 0.06
Cmax (04) 6.4 0.7 1.1 0.2
0.06 0.01
Tmax (day) 7 0 14 0 14 12 23 8
AUCo-t
99 19 28 6 2.3 0.7 1.2 0.2
(day*MVI)
0.11 0.06
Clast (ttM) 1.7 0.2 0.8 0.2
0.06 0.01
Dexamethasone concentration profiles for each of the ocular regions are
presented in
FIGs. 18 through FIG. 21. Further, the retinal thicknesses for each of the
test subjects were
measured over time and compared to retinal thicknesses for test subjects
treated with a topical
formulation and control subjects with normal retinal thickness. These results
are depicted in
FIG. 22. As illustrated in FIG. 22, a therapeutically effective dose can
reduce retinal
thickening associated with an ocular condition as compared to retinal
thickening without
treatment or as compared to treatment with a topical formulation.
It should be understood that the above-described arrangements are only
illustrative of
application of the principles of the present invention Numerous modifications
and
- 21 -
CA 3050771 2019-07-24
WO 2018/125930
PCT/US2017/068571
alternative arrangements may be devised by those skilled in the art without
departing from
the spirit and scope of the present invention. Thus, while the present
invention has been
described above with particularity and detail in connection with what is
presently deemed to
be the most practical and preferred embodiments of the invention, it will be
apparent to those
of ordinary skill in the art that numerous modifications, including, but not
limited to,
variations in size, materials, shape, form, function and manner of operation,
assembly and use
may be made without departing from the principles and concepts set forth
herein
- 22 -
CA 3050771 2019-07-24