Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03054748 2019-08-27
Method for Producing Lithium Hydroxide from Lithium-Containing Ore
The invention relates to a method for producing lithium hydroxide, in
particular highly
pure lithium hydroxide, for use in batteries and/or accumulators, from lithium-
containing ore and/or mineral and/or from lithium-containing earths.
For some years, a worldwide increase in demand for the light metal lithium has
already been observed. Lithium, for example in the form of lithium hydroxide
and
lithium carbonate, is mainly used for battery applications, in particular for
rechargeable batteries and/or accumulators, so-called lithium-ion batteries,
due to its
electrochemical properties. These batteries are used in particular in portable
electrical devices, such as mobile phones, laptops or the like. Also, in the
automotive
industry, the lithium-ion battery in electric and hybrid vehicles is becoming
increasingly important as an alternative or add-on to the internal combustion
engine.
In the future, therefore, a further increase in the demand for lithium
hydroxide and
highly pure lithium carbonate can be expected.
The extraction of lithium currently takes place predominantly from brines or
sols,
which are obtained, for example, from salt lakes, by means of an absorption,
evaporation, precipitation and/or ion exchange method. However, these sources
will
not be sufficient for the future demand for lithium. In the article by MESHRAM
et al.,
Extraction of lithium from primary and secondary sources by pre-treatment,
leaching
and separation, in: Hydrometallurgy 150 (October 2014) 192-208, various
natural
sources of lithium and methods for its extraction are disclosed. Accordingly,
the
lithium extraction from ores and minerals, such as pegmatite, spodumene and
petalite or clays, such as hectorite, is more expensive than the extraction
from brines
or sols, but can also be achieved by various methods, such as the sulfate
process or
alkaline digesting. Usually, the extraction of lithium hydroxide from lithium-
containing
ores is performed by addition of sulfate salts or sulfuric acid and through
the
production of lithium carbonate as an intermediate product. The lithium-
containing
ores are first roasted or calcined, resulting in the leachable lithium mineral
13-
spodumene. The 8-spodumene is then leached with sulfuric acid to obtain an
aqueous lithium sulfate solution. By adding lime milk and sodium carbonate,
1
=
CA 03054748 2019-08-27
magnesium, iron and calcium are then gradually removed from the solution. By
adding more sodium carbonate to the solution, up to 98% of the lithium
contained in
the solution can be precipitated as lithium carbonate. In a further step, the
resulting
lithium carbonate is converted into lithium hydroxide.
Recent developments are aimed at the direct production of lithium hydroxide by
the
chlor-alkali process without the prior production of lithium carbonate. For
example,
US 2011/0044882 discloses a process for the preparation of lithium hydroxide
from a
lithium chloride solution. A solution containing lithium, which can be
obtained from
sols or ores, is first concentrated and then subjected to various purification
steps,
such as a pH adjustment for the precipitation of divalent or trivalent ions or
an ion
exchange to reduce the total concentration of calcium and magnesium.
The concentrated and purified lithium chloride solution is subjected to
electrolysis,
wherein a semipermeable membrane is permeable to lithium ions, so that a
lithium
hydroxide solution with chlorine and hydrogen as by-products is obtained.
Chlorine
gas is obtained at the anode of the electrolyzer and lithium hydroxide and
hydrogen
at the cathode. The total amount of calcium and magnesium in the high purity
lithium
hydroxide solution is less than 150 ppb (parts per billion).
In order to produce the lithium chloride solution, AU 2013 20 18 33 B2 and the
parallel application US 2015/152523 propose extracting the lithium contained
in the
ore by leaching f3-spodumene with hydrochloric acid. In a subsequent
purification
step, the resulting solution is purified and concentrated to be subsequently
supplied
to the electrolysis. The lithium extraction rate for the resulting lithium
chloride solution
according to this production route is less than 84%, according to the article
by
NOGUEI RA et al., Comparison of Processes for Lithium Recovery from Lepidolite
by
H2SO4 Digestion or HCI Leaching, Proc. Inter. Con. Min. Mater. and Metal. Eng.
(2014). Further, in the article by YAN et al., Extraction of lithium from
lepidolite using
chlorination roasting ¨ water leaching process, Trans. Nonferrous Met. Soc.
China 22
(2012), 1753, an alternative method for producing a lithium chloride solution
is
known. According to the described method, lepidolite is initially crushed and
mixed
with a mixture of sodium chloride and calcium chloride for chlorination. The
resulting
lithium chloride solution contains 92% of the lithium portion of the ore.
2
Furthermore, in BARBOSA LUCIA I et al.: "Extraction of lithium from [beta]-
spodumene using chlorination roasting with calcium chloride," THERMOCHIMICA
ACTA, vol. 605, (2015), a possible process route for the extraction of lithium
from
lithium-containing ore is known. In particular, the naturally occurring alpha-
crystalline form of spodumene is calcined, in order to transfer the spodumene
into
its beta-crystalline form. In particular, the starting material is roasted
with calcium
chloride in a fixed bed reactor, wherein subsequently the roasted material is
leached out with water.
A disadvantage of the known prior art consists in the high cost and the
sometimes low yield of lithium or lithium hydroxide from the lithium-
containing
ores and/or minerals and/or earths. In particular, in the precipitation of
lithium
carbonate from a lithium sulfate solution, high amounts of sodium carbonate
are
consumed. Sodium carbonate, however, is subject to a highly fluctuating price
curve in the market, whereby a process for the production of lithium hydroxide
via
lithium carbonate as an intermediate product is subject to an increased cost
risk.
In AU 2013 20 18 33 B2, the production of the intermediate lithium carbonate
is
circumvented by recovering lithium hydroxide from the lithium chloride
solution by
means of the chlor-alkali process. The lithium chloride solution is obtained
by
leaching 8-spodumene with hydrochloric acid. Also in this process route, the
yield
of lithium through leaching with hydrochloric acid is comparatively low.
Higher
lithium extraction rates could be achieved through longer process times and an
increase in process temperature, but this results in lower process economic
efficiency.
The object of the invention is therefore to provide in a method for producing
lithium hydroxide from lithium-containing ore and/or mineral and/or lithium-
containing earths, a solution which makes it possible to increase the
extraction
rate of highly pure lithium hydroxide by applying a chlor-alkali process.
This object is achieved by embodiments of the present invention. In the method
according to the invention for producing lithium hydroxide from lithium-
containing
ore and/or mineral and/or lithium-containing earths, in particular for
producing
highly pure lithium
3
CA 3054748 2021-03-09
=
CA 03054748 2019-08-27
hydroxide for use in batteries and/or accumulators, in a calcining and
leaching step, a
lithium chloride solution is produced, the lithium-containing ores and/or
minerals
and/or earths first being roasted by using one or more metal chlorides and/or
a
mixture of metal chlorides, and then leached out, in particular by using
water. In a
subsequent purification step a highly pure lithium chloride solution is
produced, in
particular by removing cations, such as sodium and/or potassium, and/or
calcium,
and/or magnesium, and/or iron, from the lithium chloride solution. In a
subsequent, in
particular final, electrolysis step, lithium hydroxide, in particular highly
pure lithium
hydroxide, is produced, the highly pure lithium chloride solution being
subjected to a
membrane electrolysis process, which produces chlorine gas and hydrogen as
byproducts.
According to the invention, therefore, a direct production of lithium
hydroxide from an
ore and/or mineral and/or earth is proposed, which can take place without the
production of lithium carbonate as an intermediate product, with greatly
reduced use
of chemicals, in particular with greatly reduced use or even without the use
of sodium
carbonate and/or without the use of acids, in particular hydrochloric acid or
sulfuric
acid, in contrast to the known prior art. For this purpose, the lithium-
containing ore
and/or mineral and/or the lithium-containing earth is initially roasted in a
calcining and
leaching step by using metal chlorides, preferably by using a mixture of metal
chlorides. The roasting with metal chlorides instead of hydrochloric acid or
other
chlorides improves the extraction rate and/or yield of lithium contained in
the
produced lithium chloride solution based on the lithium content of the ore
and/or the
mineral and/or the earth. For leaching the lithium chloride water is
preferably used,
also in view of the subsequent electrolysis step.
In a purification step, a highly pure lithium chloride solution is obtained
from the
produced, still contaminated lithium chloride solution. This means, in
particular, that
the proportion of lithium in the solution is increased compared to other ions.
In
particular, cations, such as sodium and/or potassium and/or calcium and/or
magnesium and/or iron, are removed from the lithium chloride solution. This is
likewise advantageous in view of the electrolysis step to be carried out
subsequently,
in which unwanted cations are deposited on the cathode in addition to the
lithium to
4
CA 03054748 2019-08-27
be extracted.
The invention thus combines the advantages of two methods known in the art for
the
production of lithium hydroxide, by adopting individual steps of a production
method
using the extraction of lithium carbonate as an intermediate product in a
chlor-alkali
production route and/or by replacing and/or adapting individual steps of the
chlor-
alkali production route to the same.
In an advantageous embodiment, the method according to the invention is
characterized in that the one or more metal chlorides or the mixture of metal
chlorides used in the calcining and leaching step comprises/comprise at least
sodium
chloride and/or potassium chloride and/or lithium chloride and/or magnesium
chloride
and/or calcium chloride. Preferably, a mixture of sodium chloride and calcium
chloride is used for roasting, because the melting temperature of the mixture
is well
below the melting temperature of the other metal chlorides. Due to the
increased
fluidity of the melt obtained at a lower temperature, the chlorides more
readily diffuse
to the surface of the lithium-containing ore and/or mineral and/or the lithium-
containing earth, thereby improving lithium extraction. In comparison to the
sulfate
process, in which the lithium-containing ore and/or mineral and/or the lithium-
containing earth, in particular lepidolite, is roasted and the resulting P-
spodumene is
digested by means of sulfuric acid, roasting with salts, in particular with
metal
chlorides, provides an increased lithium yield and improved roasting
properties. The
excess salts contained in the lithium chloride solution are usually removed
with soda
ash, i.e. with sodium carbonate. The leaching of the solution is
advantageously
carried out with water, so that lithium hydroxide and HCI can then be obtained
from
the prepared lithium chloride solution by means of the chlor-alkali process.
Advantageously, before the electrolysis step, cations contained in the lithium
chloride
solution and affecting the electrolysis, in particular iron and/or calcium
and/or
magnesium, should be reduced to very low concentrations. In an advantageous
embodiment, the invention therefore provides that the lithium chloride
solution is
purified in the purification step by adjusting the pH of the lithium chloride
solution, in
particular to a pH higher than 8, wherein the pH is preferably increased by
adding a
=
CA 03054748 2019-08-27
lye containing in particular hydroxides and/or carbonates and/or an alkaline
solution.
By increasing the pH, in particular to a pH greater than 8, undesirable ions,
such as
aluminum, iron, magnesium and manganese, can be precipitated as corresponding
hydroxides from the lithium chloride solution and then removed. Another
possibility is
provided, for example, by the oxidation of iron contained in the lithium
chloride
solution, wherein chemical substances, which are suitable for the oxidation of
iron,
are added to the lithium chloride solution. Expediently, calcium can be
removed from
the lithium chloride solution in the purification step by adding alkali
carbonate, in
particular lithium carbonate and/or sodium carbonate. The invention therefore
provides in a further embodiment that the lithium chloride solution is
purified in the
purification step by adding alkali carbonate, in particular lithium carbonate
and/or
sodium carbonate, wherein, in particular, calcium is removed from the lithium
chloride
solution. The resulting calcium carbonate can be separated from the lithium
chloride
solution, whereas the added lithium is extracted in the electrolysis step.
Furthermore, the prepared lithium chloride solution may also be subject to ion
exchange, in particular cation exchange, to further reduce the cations
contained in
the lithium chloride solution. The invention therefore also provides that the
lithium
chloride solution in the purification step is subject to an ion exchange, in
particular a
cation exchange, for further reduction of the cations contained in the lithium
chloride
solution.
Likewise useful is an optional purification of the lithium chloride solution
in the
purification step by fractional crystallization, wherein lithium and/or sodium
and/or
potassium are separated from each other and the sodium and/or potassium
precipitate in the form of sodium chloride or potassium chloride, as provided
by a
further development of the invention.
The lithium chloride solution can also be further purified by solvent
extraction. In this
case, the lithium to be extracted is separated from other alkali metal salts,
in
particular sodium chloride. The invention is therefore further characterized
in that the
lithium chloride solution is purified in the purification step by solvent
extraction,
wherein lithium is separated from other alkali salts, in particular sodium
chloride.
6
=
CA 03054748 2019-08-27
According to the invention, sodium chloride obtained during the purification
step, in
particular by fractional crystallization or solvent extraction, is used for
roasting the
lithium-containing ores and/or minerals and/or earths and is fed to the
calcining and
leaching step. In the invention, it is therefore also provided that the sodium
chloride
obtained in the purification step is used in the calcining and leaching step
for roasting
the lithium-containing ores and/or minerals and/or the lithium-containing
earths.
In this way, the need for sodium chloride for the method according to the
invention
can be further reduced.
Further possible solutions known from the prior art for the purification of
the lithium
chloride solution can alternatively or optionally be applied in the previously
described
purification solutions during the purification step of the production method
according
to the invention.
To increase the efficiency of the method according to the invention it is
finally
provided, in a further development of the invention, that the chlorine gas
generated in
the electrolysis step is recombined with the hydrogen also generated in the
electrolysis step, in particular by means of an HCI producer, in order to form
hydrochloric acid. The hydrochloric acid produced thereby can be removed as a
by-
product of the lithium hydroxide production process.
The invention is explained in more detail below by way of an example with
reference
to a drawing. In particular
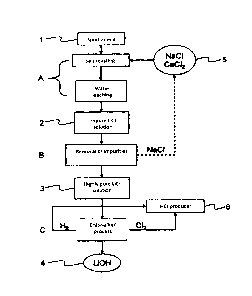
The Figure shows a flow diagram of an exemplary method according to the
invention
for the production of lithium hydroxide from lithium-containing ore and/or
mineral
and/or a lithium-containing earth.
The Figure shows a flow diagram of an exemplary method according to the
invention
for the production of lithium hydroxide from lithium-containing ore and/or
mineral
and/or a lithium-containing earth (1). According to the exemplary embodiment,
the
7
CA 03054748 2019-08-27
lithium-containing mineral or earth (1) spodumene (LiAl[Si206]) serves as a
starting
material for the production of lithium hydroxide (4), which is extracted as
the end
product of the production method according to the invention for further use
for battery
applications, in particular for rechargeable lithium-ion batteries. In
particular, by
means of the production method according to the invention, it is possible to
obtain
highly pure lithium hydroxide, whose total content of disturbing foreign
cations, such
as calcium and magnesium, is less than 150 ppb (parts per billion). Spodumene
is
found in lithium-containing ores (1), in particular in lepidolite. The
preparation of the
lithium-containing ore and/or mineral (1) for further processing according to
the
invention can be carried out in the usual way by crushing and grinding the
rocks. The
following is the overall reaction of the inventive embodiment for the
production of
lithium hydroxide (4) from the lithium-containing mineral (1) spodumene:
2 LiAlSi206 + 2 H20 + CaCl2 -> 2 LiOH + CaA12514012
In a calcining and leaching step (A), the lithium-containing mineral (1)
spodumene is
initially roasted at a temperature of 880 C for 30 minutes with the addition
of a
mixture of metal chlorides (5). In the exemplary embodiment, since the melting
point
of a mixture with lepidolite, depending on the respective mixing proportions,
is below
the melting point of mixtures with other metal chlorides, the mixture is
composed of
sodium and calcium chloride, and thus an extraction of the lithium is favored.
The
leaching is then carried out with water at a temperature of 90 C. Compared
with
leaching with acid, for example hydrochloric acid or sulfuric acid, the use of
water is
safer, less expensive and advantageous for a subsequent chlor-alkali process.
The yield, i.e. the extracted amount of lithium relative to the total amount
of lithium
contained in the starting product is at least 92% in the exemplary embodiment.
The
proportion of excess salts in the still contaminated lithium chloride solution
(2) is
about 31%. The excretion of the excess salts can be carried out by addition of
alkali
carbonate, wherein expediently sodium carbonate or alternatively lithium
carbonate is
added. A precipitation reaction for calcium chloride by addition of sodium
carbonate
is as follows:
8
-
CA 03054748 2019-08-27
CaCl2 + Na2003-> CaCO3 + 2 NaCI
In a purification step (B), the lithium chloride solution (2) is further
purified in order to
obtain a highly pure lithium chloride solution (3). A highly pure lithium
chloride
solution (3) is characterized in particular by a very low proportion of
disturbing foreign
cations, such as sodium, potassium, magnesium, calcium and iron. In
particular, the
total amount of magnesium and calcium is less than 150 ppb (parts per billion)
based
on the total amount of ions. As described above, the removal of the foreign
cations
and other purification can be carried out by adjusting the pH to pH > 8 of the
lithium
chloride solution (2) by adding chemicals for oxidizing iron, by fractional
crystallization separation, solvent extraction and/or by ion exchange. If a
separation
between lithium and sodium or lithium and other alkali metal salts is carried
out by
means of fractional crystallization and/or solvent extraction, separated
sodium
chloride and/or if appropriate calcium chloride can be used for roasting the
lithium-
containing mineral (1) spodumene in the calcining and leaching step (A). In
this way,
waste products obtained in the purification step (B) can be supplied to the
calcining
and leaching step (A) in order to reduce the requirement for substances
required for
the production process according to the invention, in particular sodium
chloride
and/or calcium chloride.
The highly pure lithium chloride solution (3) obtained by the purification
step (B) is
subjected to an electrolysis step (C) to obtain lithium hydroxide (4). By
means of a
membrane electrolyzing device having a semipermeable membrane, a chlor-alkali
process is carried out in the electrolysis step (C). An anode and a cathode of
the
electrolyzer are separated by the semipermeable membrane. By applying a
voltage,
the ions contained in the highly pure lithium chloride solution (3) are
separated from
each other, wherein lithium hydroxide (4) is obtained as the main product and
hydrogen as a by-product of the electrolysis at the cathode and chlorine gas
is
extracted as a by-product at the anode. Since disturbing foreign cations have
already
been removed from the lithium chloride solution (2) in the purification step
(B) to
obtain a highly pure lithium chloride solution (3), the lithium hydroxide (4)
accumulating at the cathode can also be extracted in a highly pure state, i.e.
almost
free of interfering cations.
9
CA 03054748 2019-08-27
The extracted by-products, hydrogen and chlorine gas, can be recombined by an
HCI
producer (6) with hydrochloric acid. By producing a readily available by-
product, such
as hydrochloric acid, the cost-effectiveness of the process according to the
invention
can be further increased.
The extracted, in particular highly pure lithium hydroxide is suitable for use
in battery
applications, in particular for use in rechargeable lithium-ion batteries or
for further
processing, for example to obtain lithium carbonate, in particular highly pure
lithium
carbonate.
List of reference numerals:
1 lithium-containing ores and/or minerals and/or earths
2 lithium chloride solution
3 highly pure lithium chloride solution
4 lithium hydroxide
metal chlorides
6 HCI producer
A calcining and leaching step
B purification step
C electrolysis step