Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03068201 2019-12-20
MAGNESIUM ALLOY SHEET AND MANUFACTURING METHOD THEREFOR
[DESCRIPTION]
[Technical Field]
An embodiment of the present invention relates to a magnesium alloy
sheet and a manufacturing method therefor.
[Background Art]
At present, the limitation of carbon dioxide emission and the importance
of renewable energy are becoming a hot issue in the international community.
Accordingly, lightweight alloys, which are a type of structural materials, are
recognized as very attractive research fields.
In particular, magnesium is the lightest metal with a density of 1.74 g/cm3
and has various advantages such as vibration absorbing ability and
electromagnetic wave shielding ability as compared with other structural
materials such as aluminum and steel. Therefore, research of related industry
has been actively carried out to utilize magnesium.
An alloy containing magnesium has been currently applied not only in the
field of electronic device but also in the field of vehicle, but it has
fundamental
problems in corrosion resistance, flame resistance, and formability, and thus
there are limitations in expanding the application range thereof.
In particular, with regard to formability, magnesium has a hexagonal
1
CA 03068201 2019-12-20
closed packed (HCP) structure, such that a slip system is not enough at room
temperature, which makes it difficult to perform a processing process thereof.
That is, a large amount of heat is required in a processing process of
magnesium, which leads to an increase in the cost of the processing process.
Meanwhile, among the magnesium alloys, an AZ-based alloy contains
aluminum (Al) and zinc (Zn), and corresponds to a commercialized magnesium
alloy, because it is inexpensive while securing physical properties of a
somewhat
appropriate strength and ductile.
However, the physical properties mentioned above mean only an
appropriate level among the magnesium alloys. The strength of the AZ-based
alloy is lower than that of aluminum (Al) which is a competitive material.
Therefore, it is necessary to improve the physical properties such as a
low formability and strength of the AZ-based magnesium alloy, but there is a
lack
of research on this.
[Disclosure]
[Technical Problem]
The present invention has been made in an effort to provide a
magnesium alloy sheet and a manufacturing method therefor.
Specifically, the present invention is to improve formability of a
magnesium sheet by suppressing center segregation consisting of Al-Ca
secondary phase particles. Accordingly, the present invention is to provide a
magnesium alloy sheet in which Al-Ca secondary phases are dispersed without
2
CA 03068201 2019-12-20
being segregated in the center of the magnesium alloy sheet.
In addition, the present invention is to improve a strength of a
magnesium alloy sheet by controlling a twinned crystal structure through skin
pass rolling while maintaining the formability of the magnesium alloy sheet.
Specifically, a strength of a magnesium alloy sheet may be increased while
maintaining formability of the magnesium alloy sheet by minimizing a
development change in texture of (0001) through skin pass rolling.
[Technical Solution]
An exemplary embodiment of the present invention provides a
magnesium alloy sheet containing, relative to 100 wt% of the entire magnesium
alloy sheet, 2.7 to 5.0 wt% of Al, 0.75 to 1.0 wt% of Zn, 0.1 to 1.0 wt% of
Ca, 1.0
wt% or less of Mn (excluding 0 wt%), and the balance of Mg and other
inevitable
impurities, wherein a volume fraction of bottom crystal grains, relative to
100
vol% of overall crystal grains of the magnesium alloy sheet, is 30% or less,
and
.. the bottom crystal grains are crystal grains in a <0001>//C-axis direction.
The magnesium alloy sheet may include Al-Ca secondary phase
particles, and a difference in area fraction of the Al-Ca secondary phase
particles between a quarter portion (1/4) of a surface of the magnesium alloy
sheet and a center portion (1/2) of the surface of the magnesium alloy sheet
may
be 10% or less.
Specifically, a ratio of a length of center segregation to a total length of
the magnesium alloy sheet in a rolling direction may be less than 5%.
A ratio of a thickness of the center segregation to a total thickness of the
3
CA 03068201 2019-12-20
magnesium alloy sheet in a thickness direction may be less than 2.5%.
Therefore, in the magnesium alloy sheet, the Al-Ca secondary phase particles
may be uniformly distributed without being segregated in the center portion of
the magnesium alloy sheet.
The Al-Ca secondary phase particle may contain, relative to 100 wt% of
the entire Al-Ca secondary phase particle, 20.0 to 25.0 wt% of Al, 5.0 to 10.0
wt% of Ca, 0.1 to 0.5 wt% of Mn, 0.5 to 1.0 wt% of Zn, and the balance of Mg
and other inevitable impurities.
An average particle size of the Al-Ca secondary phase particles may be
0.01 to 4 pm.
2 to 15 Al-Ca secondary phase particles may be included per area of 100
pm2 of the magnesium alloy sheet.
A limiting dome height (LDH) of the magnesium alloy sheet may be 7 mm
or more.
A maximum texture intensity of a (0001) surface of the magnesium alloy
sheet may be 1 to 4.
A yield strength of the magnesium alloy sheet may be 150 to 190 MPa.
Another exemplary embodiment of the present invention provides a
magnesium alloy sheet containing: relative to 100 wt% of the entire magnesium
alloy sheet, 2.7 to 5.0 wt% of Al, 0.75 to 1.0 wt% of Zn, 0.1 to 1.0 wt% of
Ca, 1.0
wt% or less of Mn (excluding 0 wt%), and the balance of Mg and other
inevitable
impurities, wherein a volume fraction of a twinned crystal structure, relative
to
100 vol% of the entire area of the magnesium alloy sheet, is 35% or less.
4
CA 03068201 2019-12-20
Specifically, the volume fraction of the twinned crystal structure, relative
to 100 vol /0 of the entire area of the magnesium alloy sheet, may be 5 to
35%.
The magnesium alloy sheet in which a volume fraction of bottom crystal
grains, relative to 100 vol% of overall crystal grains of the magnesium alloy
sheet,
is 30% or less, and the bottom crystal grains are crystal grains in a
<0001>//C-axis direction may be provided.
A limiting dome height of the magnesium alloy sheet may be 7 mm or
more.
A maximum texture intensity of a (0001) surface of the magnesium alloy
sheet may be 1 to 4.
A yield strength of the magnesium alloy sheet may be 200 to 300 MPa.
[Advantageous Effect]
In the magnesium alloy sheet according to an embodiment of the present
invention, the center segregations consisting of Al-Ca secondary phase
particles
are dispersed, such that the formability the magnesium sheet may be improved.
Accordingly, according to an embodiment of the present invention, it is
possible
to provide the magnesium alloy sheet in which the Al-Ca secondary phases are
dispersed without being segregated in the center of the magnesium alloy sheet.
Specifically, it is possible to provide the magnesium alloy sheet in which a
difference in area fraction of the Al-Ca secondary phase particles between a
quarter portion (1/4) of a surface of the magnesium alloy sheet and a center
portion (1/2) of the surface of the magnesium alloy sheet is 10% or less.
According to an embodiment of the present invention, it is possible to
5
CA 03068201 2019-12-20
obtain, through skin pass rolling, the magnesium alloy sheet in which an area
fraction of a twinned crystal structure, relative to 100% of the entire area
of the
magnesium alloy sheet, is 35% or less. Specifically, the strength of the
magnesium alloy sheet may be improved by controlling the twinned crystal
structure while maintaining the formability of the magnesium alloy sheet by
minimizing the development of a texture of (0001) through a skin pass process.
[Description of the Drawings]
FIG. 1 is a flowchart schematically illustrating a manufacturing method
for a magnesium alloy sheet according to an embodiment of the present
invention.
FIG. 2 is a photograph obtained by observing a magnesium alloy sheet
produced in Example 1a with optical microscopy.
FIG. 3 is a photograph obtained by observing a magnesium alloy sheet
produced in Comparative Example la with optical microscopy.
FIG. 4 is a photograph obtained by observing the magnesium alloy sheet
produced in Example la with secondary electron microscopy.
FIG. 5 shows a result of measuring a limiting dome height of the
magnesium alloy sheet produced in Example 1a.
FIG. 6 shows a maximum texture intensity of a (0001) surface of
Example 1a.
FIG. 7 shows a maximum texture intensity of a (0001) surface of
Comparative Example la.
FIG. 8 shows a result of electron backscatter diffraction (EBSD) analysis
6
CA 03068201 2019-12-20
of the magnesium alloy sheet produced in Example 1a.
FIG. 9 is a graph illustrating fractions of crystal orientations of Example
= la.
FIG. 10 is a result of EBSD analysis of a magnesium alloy sheet
according to a reduction ratio of skin pass.
FIG. 11 shows a maximum texture intensity of each of (0001) surfaces of
Example 2 and Comparative Example 2, depending on a skin pass condition.
[Mode for Invention]
The advantages and features of the present invention, and methods of
accomplishing these will become obvious with reference to the embodiments to
be described below in detail along with the accompanying drawings. However,
the present invention is not limited to embodiments to be disclosed below, but
various forms different from each other may be implemented. The
embodiments are merely provided to make the present invention complete and
to completely notify those skilled in the art to which the present invention
pertains, of the scope of the present invention, and the present invention is
only
defined by the scope of the claims. The same reference numerals throughout
the specification denote the same elements.
Accordingly, in some embodiments, well-known techniques are not
described in detail in order to avoid obscuring the present invention. Unless
otherwise defined, all terms (including technical and scientific terms) used
in the
specification have the same meaning as commonly understood by those skilled
in the art to which the present invention pertains. Throughout the present
7
CA 03068201 2019-12-20
specification, unless explicitly described to the contrary, "comprising" any
components will be understood to imply the inclusion of other elements rather
than the exclusion of any other elements. In
addition, unless explicitly
described to the contrary, a singular form includes a plural form.
A magnesium alloy sheet according to an embodiment of the present
invention may contain, relative to 100 wt% of the entire magnesium alloy
sheet,
2.7 to 5.0 wt% of Al, 0.75 to 1.0 wt% of Zn, 0.1 to 1.0 wt% of Ca, 1.0 wt% or
less
of Mn (excluding 0 wt%), and the balance of Mg and other inevitable
impurities.
Hereinafter, the reason for limiting the components and compositions will
be described.
First, aluminum (Al) improves the mechanical properties of the
magnesium alloy sheet and castability of a molten metal. When Al is added in
an amount of more than 5.0 wt%, the castability may rapidly deteriorate. On
the other hand, when Al is added in an amount of less than 2.7 wt%, the
mechanical properties of the magnesium alloy sheet may deteriorate.
Therefore, a content of Al may be adjusted within the above-mentioned range.
Zinc (Zn) improves the mechanical properties of the magnesium alloy
sheet. When Zn is added in an amount of more than 1.0 wt%, a large number
of surface defects or center segregations is generated, and the castability
may
thus rapidly deteriorate. On the other hand, when Zn is added in an amount of
less than 0.75 wt%, the mechanical properties of the magnesium alloy sheet
may deteriorate. Therefore, a content of Zn may be adjusted within the
above-mentioned range.
8
CA 03068201 2019-12-20
Calcium (Ca) imparts flame resistance to the magnesium alloy sheet.
When Ca is added in an amount of more than 1.0 wt%, the castability may
rapidly deteriorate due to reduction of fluidity of the molten metal, and the
formability of the magnesium alloy sheet may deteriorate due to an increase of
the center segregation consisting of an Al-Ca-based intermetallic compound.
When Ca is added in an amount of less than 0.1 wt%, the flame resistance may
not be sufficiently imparted. Therefore, a content of Ca may be adjusted
within
the above-mentioned range. More specifically, Ca may be contained in an
amount of 0.5 to 0.8 wt%.
Manganese (Mn) improves the mechanical properties of the magnesium
alloy sheet. When Mn is added in an amount of more than 1.0 wt%, a heat
dissipation property may deteriorate and uniform distribution control may be
difficult.
Therefore, a content of Mn may be adjusted within the
above-mentioned range.
The volume fraction of bottom crystal grains, relative to 100 vol% of
overall crystal grains of the magnesium alloy sheet may be 30% or less.
In an embodiment of the present invention, a bottom crystal grain refers
to a crystal grain with a bottom orientation. Specifically, magnesium has a
hexagonal closed pack (HCP) crystal structure, here, a crystal grain when a
C-axis of the crystal structure is parallel to a thickness direction of the
sheet
refers to a crystal grain with a bottom crystal orientation (that is, bottom
crystal
grain). Accordingly, in the present specification, the bottom crystal grain
may
be expressed as a "<0001>//C-axis".
9
CA 03068201 2019-12-20
More specifically, in a case where a fraction of the bottom crystal grains
is in the above-mentioned range, a magnesium alloy sheet having an excellent
formability may be obtained.
Specifically, the volume fraction of bottom crystal grains in the
<0001>//C-axis direction, relative to 100 vol% of overall crystal grains of
the
magnesium alloy sheet may be 30% or less. More specifically, the volume
fraction of bottom crystal grains in the <0001>//C-axis direction, relative to
100
vol% of overall crystal grains of the magnesium alloy sheet may be 25% or
less.
Still more specifically, the volume fraction of bottom crystal grains in the
<00014/C-axis direction, relative to 100 vol% of overall crystal grains of the
magnesium alloy sheet may be 20% or less. A lower limit of the volume fraction
of bottom crystal grains in the <0001>//C-axis may be more than 0%. This
means that in a case where the volume fraction of crystal grains in the
<0001>//C-axis direction is in any range (more than 0%), the magnesium alloy
sheet may be included in the present invention.
In the magnesium alloy sheet, the fraction of crystal grains in the
<0001>//C-axis direction may be decreased due to an increase of the
orientation
distribution of crystal grains.
In a case where the fraction of crystal grains in the <0001>//C-axis
direction satisfies the above-mentioned range, the texture intensity of the
magnesium alloy sheet is decreased, such that a magnesium alloy sheet having
an excellent formability may be obtained.
The magnesium alloy sheet according to an embodiment of the present
CA 03068201 2019-12-20
invention may include Al-Ca secondary phase particles.
Specifically, the magnesium alloy sheet according to an embodiment of
the present invention may include the Al-Ca secondary phase particles, but may
hardly include the center segregation. More specifically, the magnesium alloy
sheet according to an embodiment of the present invention may have a form in
which the Al-Ca secondary phase particles are uniformly dispersed. The center
segregation refers to that Al-Ca secondary phase particles are segregated in
the
center portion of the magnesium alloy sheet in the thickness direction (ND),
and
as described above, as the center segregation increases, the formability of
the
magnesium alloy sheet may deteriorate.
Accordingly, in the magnesium alloy sheet according to an embodiment
of the present invention, a difference in area fraction of the Al-Ca secondary
phase particles between a quarter portion (1/4) of a surface of the magnesium
alloy sheet and the center portion (1/2) of the surface of the magnesium alloy
sheet may be 10% or less. Therefore, the Al-Ca secondary phase particles are
entirely and uniformly dispersed without segregation in the center portion of
the
magnesium alloy sheet, and the formability of the magnesium alloy sheet may
thus be improved. Here, the area fraction refers to a fraction with respect to
an
area of the Al-Ca secondary phase particles per same area of the quarter
portion
and the center portion.
More specifically, a ratio of a length of the center segregation to the total
length of the magnesium alloy sheet in the rolling direction (RD) may be less
than 5%. In addition, a ratio of a thickness of the center segregation to the
total
11
CA 03068201 2019-12-20
thickness of the magnesium alloy sheet in the thickness direction (ND) may be
less than 2.5%.
The above description means that the center segregation is hardly
generated, and the above range is a range in which both the length and
thickness of the center segregation are decreased as compared to center
segregation which is generally generated when Al and Ca are added.
Therefore, the formability of the magnesium alloy sheet according to an
embodiment of the present invention may be improved.
The total length of the magnesium sheet may be based on a magnesium
sheet with a constant length unit. Specifically, the length unit may be 1,000
to
3,000 pm.
Specifically, the Al-Ca secondary phase particle may contain, relative to
100 wt% of the entire Al-Ca secondary phase particle, 20.0 to 25.0 wt% of Al,
5.0
to 10.0 wt /0 of Ca, 0.1 to 0.5 wt% of Mn, 0.5 to 1.0 wt% of Zn, and the
balance of
Mg and other inevitable impurities.
In general, in a case where Al and Ca are added to magnesium to from
an alloy, center segregation consisting of Al-Ca secondary phase particles is
generated, which causes the significant deterioration of the formability of
the
magnesium alloy sheet. On the other hand, the magnesium alloy sheet
according to an embodiment of the present invention may improve the
formability of the magnesium sheet by suppressing the generation of the center
segregation consisting of Al-Ca secondary phase particles. Specifically, the
magnesium alloy sheet in which Al-Ca secondary phase particles are dispersed
12
CA 03068201 2019-12-20
may be provided.
An average particle size of the Al-Ca secondary phase particles may be
0.01 to 4 pm. As the average particle size of the Al-Ca secondary phase
particles is large, as described above, the formability of the magnesium alloy
.. sheet may deteriorate due to the generation of the center segregation.
Within
the above-mentioned range of the particle size, the improved formability may
be
exhibited.
2 to 15 Al-Ca secondary phase particles may be included per area of 100
pm2 of the magnesium alloy sheet. The number of Al-Ca secondary phase
particles is in the above-mentioned range, the formability of the magnesium
alloy
sheet may be improved.
In an embodiment of the present invention, in order to control the Al-Ca
secondary phase particles, composition ranges of Al, Zn, Mn, and Ca,
temperature and time conditions during homogenization heat treatment,
temperature and rolling ratio during warm-rolling, and the like may be
precisely
controlled.
The magnesium alloy sheet according to an embodiment of the present
invention includes crystal grains, and an average particle size of the crystal
grains may be 5 to 30 pm. Within the above particle size range of the crystal
grains, the formability of the magnesium alloy sheet may be improved.
In addition, a limiting dome height of the magnesium alloy sheet
according to an embodiment of the present invention may be 7 mm or more.
More preferably, the limiting dome height of the magnesium alloy sheet may be
7
13
CA 03068201 2019-12-20
to 10 mm.
In general, a limiting dome height is utilized as an index for evaluating
formability (in particular, pressability) of a material, and as the limiting
dome
height is increased, the formability of the material is improved.
A limiting dome height within the above limited range is a significantly
higher limiting dome height than that of a magnesium alloy sheet generally
known, which caused by an increase in orientation distribution of the crystal
grain in the magnesium alloy sheet.
Therefore, the maximum texture intensity of a (0001) surface of the
magnesium alloy sheet may be 1 to 4. In a case where the limiting dome height
is out of the above-mentioned range, the formability of the magnesium alloy
sheet may deteriorate.
In addition, a yield strength of the magnesium alloy sheet according to an
embodiment of the present invention may be in a range of 150 to 190 MPa.
In the magnesium alloy sheet according to an embodiment of the present
invention, through skin pass in a production step described below, an area
fraction of a twinned crystal structure may be 35% or less relative to 100% of
the
entire area of the magnesium alloy sheet. More specifically, the area fraction
of
the twinned crystal structure may be 5 to 35%. Still more specifically, the
area
fraction of the twinned crystal structure may be 5 to 33%. By controlling the
fraction of the twinned crystal structure to the above range, the yield
strength of
the magnesium alloy sheet according to an embodiment of the present invention
may be 200 to 300 MPa. This range is considered as an excellent range in the
14
CA 03068201 2019-12-20
magnesium sheet according to an embodiment of the present invention.
In addition, a thickness of the magnesium alloy sheet according to an
embodiment of the present invention may be 0.4 to 3 mm. The magnesium
sheet according to an embodiment of the present invention may be selected
depending on properties required in the above thickness range. However, the
present invention is not limited to this thickness range.
FIG. 1 is a flowchart schematically illustrating a manufacturing method
for a magnesium alloy sheet according to an embodiment of the present
invention. The flowchart of the manufacturing method for a magnesium alloy
sheet of FIG. 1 is merely to illustrate the present invention, and the present
invention is not limited thereto. Therefore, the manufacturing method for a
magnesium alloy sheet may be variously modified.
A manufacturing method for a magnesium alloy sheet according to an
embodiment of the present invention includes: a step (S10) of preparing a cast
material by casting a molten metal, the molten metal containing, relative to
100
wt% of the entire molten metal, 2.7 to 5.0 wt% of Al, 0.75 to 1.0 wt% of Zn,
0.1 to
1.0 wt% of Ca, 1.0 wt% or less of Mn (excluding 0 wt%), and the balance of Mg
and other inevitable impurities; a step (S20) of subjecting the cast material
to
homogenization heat treatment; and a step (S30) of subjecting the cast
material
.. subjected to the homogenization heat treatment to warm-rolling.
In addition, the manufacturing method for a magnesium alloy sheet may
further include other steps, as necessary.
First, the step (S10) of preparing a cast material by casting a molten
CA 03068201 2019-12-20
metal may be performed, the molten metal containing, relative to 100 wt% of
the
entire molten metal, 2.7 to 5.0 wt% of Al, 0.75 to 1.0 wt% of Zn, 0.1 to 1.0
wt% of
Ca, 1.0 wt% or less of Mn (excluding 0 wt%), and the balance of Mg and other
inevitable impurities.
The reason for limiting the numeral values of the respective components
is the same as that mentioned above, and the repeated description thereof will
thus be omitted.
At this time, as the method (S10) of preparing the cast material, a die
casting method, a strip casting method, a billet casting method, a centrifugal
casting method, a tilting casting method, a sand casting method, a direct
chill
casting method, or combination thereof may be used.
More specifically, a strip casting method may be used. However, the
present invention is not limited thereto.
More specifically, in the step (S10) of preparing the cast material, a
rolling force may be 0.2 ton/mm2 or more. Still more specifically, the rolling
force may be 1 ton/mm2 or more. Still more specifically, the rolling force may
be 1.5 ton/mm2. The cast material is coagulated and a rolling force is
simultaneously applied thereto, and at this time, the formability of the
magnesium alloy sheet may be improved by adjusting the rolling force to the
above range.
Next, the step (S20) of subjecting the cast material to homogenization
heat treatment may be carried out.
At this time, the heat treatment may be performed at a temperature of
16
CA 03068201 2019-12-20
350 C to 500 C for 1 to 28 hours. More specifically, the homogenization heat
treatment may be performed for 18 to 28 hours.
In a temperature range of lower than 350 C, the homogenization heat
treatment is not properly performed, and beta phases such as Mg17A112 may not
be solid-dissolved in the matrix.
In a temperature range of higher than 500 C, the beta phases
condensed in the cast material may melt, resulting in an occurrence of a fire
or
formation of holes in the magnesium sheet. Therefore, the homogenization
heat treatment may be performed within the above-mentioned temperature
range.
Next, the step (S30) of subjecting the cast material subjected to the
homogenization heat treatment to warm-rolling may be carried out.
At this time, a temperature condition of the warm-rolling may be 150 C to
350 C. In a temperature range of lower than 150 C, a large amount of edge
cracks may be generated. In a temperature range of higher than of 500 C, the
magnesium alloy sheet may not be appropriate for mass production. Therefore,
the warm-rolling may be performed in the above-mentioned temperature range.
The step of subjecting the cast material subjected to the homogenization
heat treatment to warm-rolling may be carried out a plurality of times, and
the
warm-rolling may be performed at a reduction ratio of 10 to 30% per time. The
reduction ratio of the warm-rolling refers to a "value(%)" relative to 100% of
the
thickness (length(%) of the cast material. By performing warm-rolling a
plurality
of times, finally, the rolling may be performed until the cast material has a
thin
17
CA 03068201 2019-12-20
thickness of about 0.4 mm.
At least one time of a step of performing intermediate annealing in the
middle of a plurality of times of warm-rolling may be further included. By
further
including the step of performing intermediate annealing in the middle of a
plurality of times of warm-rolling, the formability of the magnesium alloy
sheet
may be further improved. Specifically, the step of performing intermediate
annealing may be carried out at 300 to 500 C for 1 to 10 hours. More
specifically, the intermediate annealing step may be carried out at 450 to 500
C.
Within the above-mentioned range, the formability of the magnesium alloy sheet
may be further improved.
After the warm-rolling step, the method may further include a step of
performing subsequent heat treatment. By including the step of performing
subsequent heat treatment, the formability of the magnesium alloy sheet may be
further improved. The step of performing subsequent heat treatment may be
carried out at 300 to 500 C for 1 to 15 hours. Specifically, the step of
performing subsequent heat treatment may be carried out for 1 to 10 hours.
Within the above-mentioned range, the formability of the magnesium alloy sheet
may be further improved.
A manufacturing method for a magnesium alloy sheet according to
another embodiment of the present invention may include: a step of preparing a
cast material by casting a molten metal, the molten metal containing, relative
to
100 wt% of the entire molten metal, 2.7 to 5.0 wt% of Al, 0.75 to 1.0 wt% of
Zn,
0.1 to 1.0 wt% of Ca, 1.0 wt% or less of Mn (excluding 0 wt%), and the balance
18
CA 03068201 2019-12-20
of Mg and other inevitable impurities; a step of subjecting the cast material
to
homogenization heat treatment; a step of preparing a rolled material by
subjecting the cast material subjected to the homogenization heat treatment to
warm-rolling; a step of subjecting the rolled material to subsequent heat
treatment; and a step of producing a magnesium alloy sheet by subjecting the
rolled material subjected to the subsequent heat treatment to skin pass.
First, the step of preparing a cast material by casting a molten metal may
be performed, the molten metal containing, relative to 100 wt% of the entire
molten metal, 2.7 to 5.0 wt% of Al, 0.75 to 1.0 wt% of Zn, 0.1 to 1.0 wt% of
Ca,
1.0 wt% or less of Mn (excluding 0 wt%), and the balance of Mg and other
inevitable impurities.
In the above step, the molten metal may a commercially available AZ31
alloy, AL5083 alloy, or a combination thereof. However, the present invention
is not limited thereto.
More specifically, the molten metal may be prepared in a temperature
range of 650 to 750 C. Thereafter, a cast material may be produced by casting
the molten metal. At this time, a thickness of the cast material may be 3 to 7
mm.
At this time, as the method of preparing the cast material, a die casting
method, a strip casting method, a billet casting method, a centrifugal casting
method, a tilting casting method, a sand casting method, a direct chill
casting
method, or combination thereof may be used. More specifically, a strip casting
method may be used. However, the present invention is not limited thereto.
19
CA 03068201 2019-12-20
More specifically, in the step of preparing the cast material, a rolling force
may be 0.2 ton/mm2 or more. Still more specifically, the rolling force may be
1
ton/mm2 or more. Still more specifically, the rolling force may be 1.5
ton/mm2.
Next, the step of subjecting the cast material to homogenization heat
treatment may be carried out.
More specifically, the step of subjecting the cast material to
homogenization heat treatment may include: a primary heat treatment step in a
temperature range of 300 C to 400 C; and a secondary heat treatment step in a
temperature range of 400 C to 500 C. The temperature ranges of the primary
heat treatment step and the secondary heat treatment step may be different
from
each other.
Still more specifically, the primary heat treatment step in a temperature
range of 300 C to 400 C may be carried out for 5 hours to 20 hours. In
addition,
the secondary heat treatment step in a temperature range of 400 C to 500 C
may be carried out for 5 hours to 20 hours.
By carrying out the primary heat treatment step in the above temperature
range, a Mg-Al-Zn ternary system Pi-phase generated in the casting step may
be removed. In a case where the ternary system Pi-phase is present, the
subsequent process may be adversely affected. In addition, by carrying out the
secondary heat treatment step in the above temperature range, a stress in a
slab
may be released. Further, the formation of recrystallization of the cast
structure
may be more actively induced.
Next, the step of preparing a rolled material by subjecting the cast
CA 03068201 2019-12-20
material subjected to the homogenization heat treatment to warm-rolling may be
carried out.
The cast material subjected to the heat treatment may be rolled to a
thickness range of 0.4 to 3 mm through 1 to 15 times of rolling. In addition,
the
.. rolling may be performed at 150 to 350 C.
More specifically, in a case where the rolling temperature is lower than
150 C, a crack on the surface when rolling may be induced, and in a case where
the rolling temperature is higher than 350 C, it may not be suitable for
actual
production facilities. Therefore, the rolling may be performed at 150 C to
350 C.
Next, a step of subjecting the rolled material to intermediate annealing
may be carried out. In the rolling step, when the cast material is rolled a
plurality of times, heat treatment may be performed in a temperature range of
390 C to 550 C for 1 hour to 15 hours in an interval between the pass and the
pass.
For example, the intermediate annealing is performed one time after
performing the rolling two times, and the rolled material may thus be rolled
to the
final target thickness. As another example, the intermediate annealing is
performed one time after performing the rolling three times, and the rolled
material may thus be rolled to the final target thickness. More specifically,
in a
case where the rolled cast material is annealed in the above temperature
range,
the stress generated by the rolling may be released. Therefore, the rolling
may
be performed several times to obtain a desired thickness of the cast material.
21
CA 03068201 2019-12-20
Next, the step of subjecting the rolled material to subsequent heat
treatment may be carried out.
The step of subjecting the cast material to subsequent heat treatment
may be carried out at 300 to 500 C for 1 to 15 hours. Specifically, the step
of
.. subjecting the cast material to subsequent heat treatment may be carried
out for
1 to 10 hours. Within the above-mentioned range, the formability of the
magnesium alloy sheet may be further improved.
Finally, the step of producing a magnesium alloy sheet by subjecting the
rolled material subjected to the subsequent heat treatment to skin pass may be
carried out.
More specifically, the skin pass is also referred to as skin pass rolling or
temper rolling, which means that a deformation pattern generated in a cold
rolled
sheet after heat treatment is removed, and cold rolling is performed with a
light
pressure to improve the strength.
Therefore, in an embodiment of the present invention, the skin pass may
be performed one time in a temperature range of 250 C to 350 C.
The magnesium alloy sheet produced by performing the skin pass may
be rolled at a reduction ratio of 2 to 15% with respect to the thickness of
the
rolled material. More specifically, the reduction ratio may be related to the
skin
pass temperature.
As a specific example, when the skin pass temperature is 250 C, the
reduction ratio of the skin pass may be 5 to 15%. At this time, a yield
strength
may be in a range of 200 to 260 MPa. Further, at this time, a limiting dome
22
CA 03068201 2019-12-20
height may be in a range of 7.3 to 8.1.
As a specific example, when the skin pass temperature is 300 C, the
reduction ratio of the skin pass may be 5 to 15%. More preferably, the
reduction ratio of the skin pass may be 7 to 12%. At this time, a yield
strength
may be in a range of 200 to 250 MPa. Further, at this time, a limiting dome
height may be in a range of 7.3 to 8.1.
In the present invention, a limit dome height (LDH) is an index for
evaluating formability of the sheet, in particular, pressability, and the
formability
may be measured by measuring a deformed height of a specimen obtained by
applying a deformation to the specimen. A high value of the limiting dome
height means that the formability of the sheet is excellent.
More specifically, the skin pass is performed under the conditions of the
above temperature and pressure, the development of the texture of (0001) is
suppressed, the formability may be secured. That is, in a case where the skin
pass is performed under the above conditions, a change of the texture
intensity
may be minimized and the strength may thus be increased.
Hereinafter, the present invention will be described in detail with
reference to examples. However, the following examples are only to illustrate
the present invention, and the contents of the present invention are not
limited by
the following examples.
Example 1
A molten metal containing, relative to 100 wt% of the entire molten metal,
Al and Ca in amounts as shown in Table 1, 0.8 wt% of Zn, 0.5 wt% of Mn, and
23
CA 03068201 2019-12-20
the balance of Mg and inevitable impurities was prepared.
The molten metal was passed between two cooling rolls to prepare a
magnesium cast material. At this time, a rolling force of the cooling roll is
as
shown in Table 1.
Next, the magnesium cast material was subjected to homogenization
heat treatment at 400 C while varying time as shown in Table 1.
The magnesium cast material subjected to the homogenization heat
treatment was subjected to warm-rolling at a temperature of 250 C at a
reduction ratio of 15%. Next, the magnesium cast material subjected to the
warm-rolling was subjected to intermediate annealing at a temperature as shown
in Table 1, and then subjected to warm-rolling again at a temperature of 250 C
at a reduction ratio of 15%, thereby producing a magnesium alloy sheet.
Comparative Example 1
A molten metal containing, relative to 100 wt% of the entire molten metal,
Al and Ca in amounts as shown in Table 1, 0.8 wt% of Zn, and the balance of Mg
and inevitable impurities was prepared.
A magnesium alloy sheet was produced in the same manner as that of
Example 1, except for the conditions as shown in Table 1.
[Table 1]
Al Ca Casting Homogenizatio Rolling Intermediate
conten conten roll n temperatur annealing
t t Rolling Annealing time e temperature(
(wt%) (wt%) force (hr) ( C) C)
24
CA 03068201 2019-12-20
(ton/mm2
Example la 3 0.6 1.2 24 250 450
Example lb 4 0.6 1.2 24 250 450
Example 1 c 5 0.6 1 24 250 450
Example Id 3 0.6 1.2 24 250 300
Example le 3 0.6 1.2 24 250 400
Example lf 3 0.6 1.2 24 250 500
Example lg 3 0.7 0.2 24 250 500
Example lh 3 0.7 1.2 24 250 450
Example Ii 3 0.6 1 1 250 400
Comparativ
e Example 3 0.6 0.8 24 250
la
Cornparativ
e Example 3 0.7 1.2 24 400 250
lb
Comparativ
e Example 3 0.7 1 48 250 400
1 c
Comparativ 3 0.7 0.8 24 100 400
CA 03068201 2019-12-20
e Example
Id
In order to compare and evaluate physical properties of the magnesium
alloy sheets produced in examples and comparative examples, the following
experimental examples were performed.
Experimental Example 1: Observation of Microstructure of Magnesium
Alloy Sheet
Microstructures of the magnesium alloy sheets produced in examples
and comparative examples were observed with a scanning electron microscope
(SEM).
The observed results are illustrated in FIGS. 2 to 4 of the present
invention.
FIG. 2 is a photograph obtained by observing a magnesium alloy sheet
produced in Example la with a scanning electron microscope (SEM). FIG. 3 is
a photograph obtained by observing a magnesium alloy sheet produced in
Comparative Example 1 a with a scanning electron microscope (SEM).
Specifically, in each of FIGS. 2 and 3, a horizontal direction is a rolling
direction (RD) of the magnesium alloy sheet and a vertical direction is a
thickness direction (ND) of the magnesium alloy sheet.
As illustrated in FIG. 2, it can be appreciated that center segregation of
the magnesium alloy sheet was hardly generated in Example la. Specifically, it
can be appreciated that a ratio of a length of the center segregation to the
total
26
CA 03068201 2019-12-20
length of about 2000 pm in the rolling direction in Example la was less than
5%.
On the other hand, as illustrated in FIG. 3, it can be appreciated that, a
large amount of center segregation of the magnesium alloy sheet was generated
in Comparative Example 1 a. Specifically, it can be appreciated that a ratio
of a
length of the center segregation to the total length of about 2000 pm in the
rolling
direction in Comparative Example la was 5% or more. Further, it was
confirmed that, a thickness of the center segregation to the total thickness
of
about 1200 pm in the thickness direction in Comparative Example la was about
30 pm. From this fact, it can be appreciated that a ratio of the thickness of
the
center segregation to the total thickness of the magnesium alloy sheet in the
thickness direction was 2.5%. Therefore, it could be confirmed that a large
amount of center segregation was generated in Comparative Example la.
As described above, since the center segregation causes deterioration of
formability of the magnesium alloy sheet, as the center segregation is not
generated, a magnesium alloy sheet having an excellent formability may be
obtained.
FIG. 4 is a photograph obtained by observing the magnesium alloy sheet
produced in Example la with secondary electron microscopy.
The white dots in FIG. 4 are Al-Ca secondary phase particles. More
specifically, as a result of analyzing compositions of the white dots in FIG.
4, it
was analyzed that the white dots contain 24.61 wt% of Al, 8.75 wt% of Ca, 0.36
wt% of Mn, 0.66 wt% of Zn, and the balance of Mg and other inevitable
impurities.
27
CA 03068201 2019-12-20
From this fact, it was confirmed that the magnesium alloy sheet produced
in Example la includes the Al-Ca secondary phase particles. Specifically, it
can be appreciated that 50 Al-Ca secondary phase particles were distributed
per
area of 1600 pm2 of the magnesium alloy sheet in FIG. 4.
As illustrated in FIG. 4, it can be appreciated that the center segregation
of the Al-Ca secondary phase particles was not generated in Example 1 a, and
the Al-Ca secondary phase particles were dispersed. From this fact, as shown
in Table 2, it can be appreciated that a limiting dome height of the magnesium
alloy sheet produced in Example la of the present invention is 9.4 mm, whereas
a limiting dome height of the magnesium alloy sheet produced in Comparative
Example 1a is 2.5 mm, which shows that the formability of the magnesium alloy
sheet produced in Comparative Example 1 a is inferior to that of the magnesium
alloy sheet produced in Example 1 a.
Experimental Example 2: Measurement of Limiting Dome Height of
Magnesium Alloy Sheet
In the present invention, a limit dome height (LDH) is an index for
evaluating formability of the sheet, in particular, pressability, and the
formability
may be measured by measuring a deformed height of a specimen obtained by
applying a deformation to the specimen.
The limiting dome height was measured by inserting each of the
magnesium alloy sheets of examples and comparative examples between an
upper die and a lower die, and fixing an outer periphery of each specimen with
a
force of 5 kN. Here, a known press oil was used as a lubricant. Then, a
28
CA 03068201 2019-12-20
spherical punch having a diameter of 20 mm was used to deform the specimen
at a rate of 5 to 10 mm/min, the punch was inserted until each specimen was
fractured, and then a deformed height of each specimen at the time of
fracturing
was measured. That is, the deformed height of the specimen was measured.
The results are illustrated in FIG. 5 of the present invention.
FIG. 5 shows a result of measuring a limiting dome height of the
magnesium alloy sheet produced in Example la.
As illustrated in FIG. 5, it can be appreciated that the magnesium alloy
sheet produced in Example la has an excellent formability.
The results can also be confirmed in Tables 2 and 3.
Experimental Example 3: Analysis of Crystal Orientation of Crystal Grain
The crystal orientation of crystal grain of each of the magnesium alloy
sheets produced in examples and comparative examples were confirmed with
an XRD analyzer, and the results are illustrated in FIGS. 6 to 11.
Specifically, a =
texture of the crystal grains obtained by using an XRD pole figure method is
illustrated.
More specifically, the pole figure is represented by stereographic
projection of an orientation of an arbitrarily fixed crystal coordinate system
onto a
coordinate system of the specimen. Still more specifically, poles of the
crystal
grains with various orientations with respect to a {0001} surface are
represented
on a standard coordinate system, and a density contour of the poles is drawn
according to a pole density distribution, thereby representing the pole
figure. At
this time, the poles are fixed in a specific lattice direction by Bragg's
angle, and a
29
CA 03068201 2019-12-20
plurality of poles may be represented for a single crystal.
Accordingly, it can be construed that as a density distribution value of the
contour represented by the pole figure method is small, crystal grains with
various orientations are distributed, and as the density distribution value is
large,
a large amount of crystal grains in a <0001>//C-axis direction is distributed.
The results may be compared through FIGS. 6 and 7 of the present
invention.
FIG. 6 shows a maximum texture intensity of a (0001) surface of
Example 1a. FIG. 7 shows a maximum texture intensity of a (0001) surface of
Comparative Example la.
Specifically, the maximum texture intensity of each of the (0001)
surfaces of FIGS. 6 and 7 is the result obtained by analyzing the crystal
orientation of the magnesium alloy sheet with the XRD analyzer as described
=
above.
As illustrated in FIG. 6, it could be confirmed that the maximum density
distribution value (texture intensity) of the (0001) surface in examples was
2.73,
which is low, whereas the maximum density distribution value in comparative
examples was 12.1, which is high as compared to that of each example.
That is, since a value of the maximum texture intensity is small, and the
contour is widely spread in examples, it can be derived that the crystal
grains
with various orientations are distributed.
On the other hand, since a value of the maximum texture intensity is
large, and the contour is concentrated in comparative examples, it can be
CA 03068201 2019-12-20
appreciated that a large amount of crystal grains in the <0001>//C-axis
direction
is included.
From the above results, it can be appreciated that the magnesium alloy
sheets of examples have a more excellent formability.
This may be appreciated through FIGS. 8 and 9 of the present invention.
FIG. 8 shows a result of electron backscatter diffraction (EBSD) analysis
of the magnesium alloy sheet produced in Example 1a.
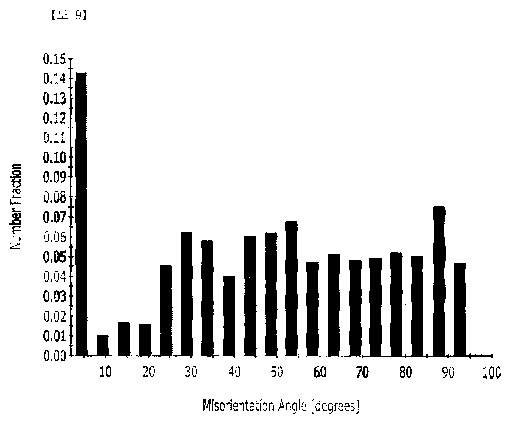
FIG. 9 is a graph illustrating fractions of crystal orientations of Example
1a.
First, as illustrated in FIG. 8, the crystal orientation of crystal grain may
also be measured by EBSD. More specifically, the crystal orientation of
crystal
grain may be measured by EBSD by injecting electrons into a specimen through
e-electron beam and using inelastic scattering diffraction at the back of the
specimen.
In addition, as illustrated in FIG. 9, crystal grains having a misorientation
angle of 200 or less between grains may be bottom crystal grains. Therefore,
it
was confirmed that the volume fraction of the crystal grains in the
<0001>//C-axis direction relative to 100% of the volume fraction of the entire
crystal grains was about 18.5%.
In addition, as illustrated in FIG. 8, it can be appreciated that the crystal
grains with various orientations were distributed in various colors, and the
crystal
grains (red) corresponding to the crystal grains in the <0001>//C-axis
direction
could be confirmed with naked eyes from the EBSD results.
31
CA 03068201 2019-12-20
[Table 2]
Size of crystal Sheet Yield Limiting dome
grain thickness strength height
(1-1m) (mm) (MPa) (LDH, mm)
Example la 19 0.7 164 9.4
Example lb 7 0.6 161 8.2
,
Example 1 c 6 1 166 8.1
Example id 13 1 155 7.5
Example le 21 1 157 8
Example If 25 1 154 9.9
Example 1 g 16 0.7 151 9
Example lh 15 3 155 9.1
Example li 17 1 164 9
Comparative
0.7 188 2.5
Example la
Comparative
11 0.6 155 5
Example lb
Comparative
40 1.5 145 5.1
Example 1 c
Comparative
8 1 166 4.9
Example ld
32
CA 03068201 2019-12-20
As a result, it was confirmed that in Comparative Examples of la to id
which did not satisfy the conditions of homogenization annealing time, rolling
temperature, and intermediate annealing temperature, the formability was
inferior to that of each example. In addition, it can be appreciated that a
yield
strength of each of Comparative Examples of la to id was inferior to that of
each example. In Comparative Example lc, an average size of the crystal
grains was about 40 pm, that is, the formability was relatively excellent as
compared to that of the other comparative examples, but a level of the
formability was less than that of each example.
Example 2
A molten metal containing, relative to 100 wt% of the entire molten metal,
3.0 wt% of Al, 0.1 wt% of Zn, 1.0 wt% of Ca, 0.3 wt% of Mn, and the balance of
Mg and inevitable impurities was prepared.
The molten metal was casted to prepare a magnesium cast material.
The magnesium cast material was subjected to a primary
homogenization heat treatment at 350 C for 10 hours. The magnesium cast
material subjected to the primary homogenization heat treatment was subjected
to a secondary homogenization heat treatment at 450 C for 10 hours.
A rolled material was prepared by casting the cast material subjected to
homogenization heat treatment.
Thereafter, the rolled material was subjected to subsequent heat
treatment at 400 C for 10 hours.
Finally, a magnesium sheet was produced by subjecting the rolled
33
CA 03068201 2019-12-20
material subjected to the subsequent heat treatment to skin pass, and the skin
pass temperature and reduction ratio are as shown in Table 2.
Comparative Example 2
A magnesium alloy sheet was produced in the same manner as that of
Example 2, except for the conditions of skin pass temperature and reduction
ratio.
In order to compare and evaluate physical properties of the magnesium
alloy sheets produced in examples and comparative examples, the following
experimental examples were performed
In addition, an experimental example for measurement of the limiting
dome height and analysis of the crystal orientation was carried out, and the
experimental method is the same as described above.
Experimental Example 4: Comparison of Physical Properties Depending
On Reduction Ratio of Skin Pass and Temperature
[Table 3]
Maximum
Limitin
Yield
Skin pass tensile Skin pass g dome
strengt ' stren g th Elongatio
temperatur Reduction ratio height
n rate ( /0)
e ( C) (%) 7.ct..(MPa (LDH,
(MPa)
mm)
)
Example 2a 5 202 257 22 8.1
250
Example 2b 9 211 254 22 8.0
34
CA 03068201 2019-12-20
Example 2c 15 252 272 16 7.3
Comparativ
e Example 22 272 289 8.6 7.0
2a
Comparativ X
e Example DELETEDTEXT 140
233 23 8-9
2b
Example 2d 7 203 253 22 8
300
Example 2e 12 247 267 18 7.3
Comparativ
e Example 17 247 272 10 7.3
2c
As shown in Table 3, as a result of subjecting the magnesium alloys
having the same compositions and components as each other, a yield strength
was improved without a large change of the formability. More specifically, the
formability may be measured by comparing numerical values of an elongation
rate and a limiting dome height.
In addition, the formability may be secured by minimizing the change of
the texture, and the change of the texture depending on the reduction ratio of
the
skin pass may be confirmed through FIG. 10.
FIG. 10 is a result of EBSD analysis of a magnesium alloy sheet
depending on a reduction ratio of skin pass.
CA 03068201 2019-12-20
As illustrated in FIG. 10, it can be confirmed that even in a case where
the skin pass after rolling was further performed, the crystal grains with
various
orientations were distributed. In addition, in a case where the rolling was
performed in a state in which the reduction ratio of the skin pass was
increased,
the orientation change of the texture was minimized, and the strength of the
magnesium alloy sheet was improved, due to the development of the twinned
crystal (black) structure and potential.
Specifically, it was confirmed that in a case where the reduction ratio of
the skin pass is 2 to 6%, the area fraction of the twinned crystal structure,
relative to 100% of the entire area of the magnesium alloy, was 15%. It was
confirmed that in a case where the reduction ratio of the skin pass is 6 to
15%,
the area fraction of the twinned crystal structure, relative to 100% of the
entire
area of the magnesium alloy, was 30%.
As described above, due to the twinned crystal structure and potential,
the strength of the magnesium alloy sheet may be maintained and the
formability
of the magnesium alloy sheet may also be improved.
Therefore, in a case where the rolling is performed by exceeding the
reduction ratio of 15% (Comparative Example 2a), the texture of the (0001)
surface is developed again, which causes deterioration of the formability of
the
magnesium alloy sheet.
FIG. 11 shows a maximum texture intensity of each of (0001) surfaces of
Example 2 and Comparative Example 2, depending on a skin pass condition.
As illustrated in FIG. 11, even in a case where the skin pass was
36
CA 03068201 2019-12-20
performed, the change of the texture of examples was not large. However, it
could be appreciated that in a case where the reduction ratio of the skin pass
was excessive as in Comparative Example 2a, the intensity of the texture was
largely changed. Therefore, as illustrated in Table 3, it was confirmed that a
phenomenon in which an increase effect of the yield strength was excellent,
but
the elongation rate significantly deteriorated.
In addition, it was also confirmed that an increase effect of the yield
strength depending on the reduction ratio of the skin pass was significantly
exhibited as compared to an increase effect of the yield strength depending on
the change in skin pass temperature.
Although the embodiments of the present invention has been described
with reference to the accompanying drawings, those skilled in the art will
appreciate that various modifications and alterations may be made without
departing from the spirit or essential feature of the present invention.
Therefore, it should be understood that the aforementioned
embodiments are illustrative in terms of all aspects and are not limited. The
scope of the present invention is defined by the appended claims rather than
the
detailed description, and all changes or modifications derived from the
meaning
and scope of the appended claims and their equivalents should be interpreted
as
falling within the scope of the present invention.
37