Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
METHODS AND COMPOSITIONS FOR THE TREATMENT OF
EPIDERMOLYSIS BULLOSA
RELATED APPLICATIONS
[001] This application claims priority to USSN 15/712,294, filed on September
22, 2017,
the contents of which are herein incorporated by reference.
FIELD OF THE INVENTION
[002] The present invention relates to the fields of medicine, cell biology,
molecular biology
and genetics. In particular, the present invention relates to compositions and
methods for
treating epidermolysis bullosa.
BACKGROUND
[003] Epidermolysis bullosa (EB) is a group of genodermatoses that cause
blisters in the
skin and mucosa' membranes, with an incidence of 20 per million newborns in
the United
States. It is a result of a defect in anchoring between the epidermis and
dermis, resulting in
skin fragility. Its severity ranges from mild to lethal.
[004] Dystrophic epidermolysis bullosa (DEB) is an inherited variant affecting
the skin and
other organs. Children born with this disease are referred to as "butterfly
children" as their
skin is described to be as delicate and fragile as a butterfly's wings. The
skin of DEB
patients is highly susceptible to severe blistering. Open wounds on the skin
heal slowly or
not at all, often scarring extensively, and are particularly susceptible to
infection. Many
individuals bathe in a bleach and water mixture to fight off these infections.
The chronic
inflammation leads to errors in the DNA of the affected skin cells, which in
turn causes
squamous cell carcinoma (SCC). The majority of DEB patients die before the age
of 30,
either of SCC or complications related to DEB.
[005] DEB is caused by mutations within the human COL7A1 gene encoding the
protein
type VII collagen (collagen VII). DEB-causing mutations can be either
autosomal dominant
(DDEB) or autosomal recessive (RDEB). COL7A1 is located on the short arm of
human
chromosome 3, in the chromosomal region denoted 3p21.31. The gene is
approximately
1
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
31,000 base pairs in size and is remarkable for the extreme fragmentation of
its coding
sequence into 118 exons. COL7A1 is transcribed into an mRNA of 9,287 base
pairs. In the
skin, the type Vii collagen protein is synthesized by keratinocytes and dermal
fibroblasts.
[006] Collagen VII is a 300 kDa protein that dimerizes to form a semicircular
looping
structure: the anchoring fibril. Anchoring fibrils are thought to form a
structural link between
the epidermal basement membrane and the fibrillar collagens in the upper
dermis. Collagen
VII is also associated with the epithelium of the esophageal lining, and DEB
patients may
suffer from chronic scarring, webbing, and obstruction of the esophagus.
Affected
individuals are often severely malnourished due to trauma to the oral and
esophageal mucosa
and require feeding tubes for nutrition. They also suffer from iron-deficiency
anemia of
uncertain origin, which leads to chronic fatigue.
[007] There remains a need to provide methods and compositions to treat EB.
SUMMARY
[008] The present invention provides methods to isolate microvesicles (MVs),
e.g.,
extracellular vesicles (MVs) from biological fluids without damaging the
structural and/or
functional integrity of the microvesicles. The present invention also provides
methods to
isolate ectosomes, microparticles, microvesicles, nanovesicles, shedding
vesicles, apoptotic
bodies, or membrane particles from biological fluids without damaging their
structural and/or
functional integrity. The present invention further provides MVs (e.g., EVs)
and methods of
using MVs(e.g., EVs) for the treatment of EB (e.g., RDEB and/or DDEB).
[009] In one aspect, a method of treating epidermolysis bullosa in a subject
in need thereof
comprising administering a pharmaceutical composition comprising isolated
microvesicles
purified by precipitation from a biological fluid to the subject, and
alleviating or reducing one
or more symptoms of epidermolysis bullosa in the subject is provided.
[010] In certain exemplary embodiments, the epidermolysis bullosa is
dystrophic
epidermolysis bullosa, e.g., recessive dystrophic epidermolysis bullosa.
[011] In certain exemplary embodiments, the isolated microvesicles are
extracellular
vesicles that are optionally precipitated from a biological fluid using a
precipitating agent
2
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
selected from the group consisting of calcium ions, magnesium ions, sodium
ions,
ammonium ions, iron ions, ammonium sulfate, alginate, and polyethylene glycol.
[012] In certain exemplary embodiments, the biological fluid is selected from
the group
consisting of peripheral blood, serum, plasma, ascites, urine, cerebrospinal
fluid (CSF),
sputum, saliva, bone marrow, synovial fluid, aqueous humor, amniotic fluid,
cerumen, breast
milk, broncheo alveolar lavage fluid, semen, prostatic fluid, Cowper's fluid,
female ejaculate,
sweat, fecal matter, hair, tears, cyst fluid, pleural and peritoneal fluid,
pericardial fluid,
lymph, chyme, chyle, bile, interstitial fluid, menses, pus, sebum, vomit,
vaginal secretions,
mucosal secretion, stool water, pancreatic juice, lavage fluid, fluid derived
from a cell, fluid
derived from a tissue sample, and cell culture fluid. In certain exemplary
embodiments, the
biological fluid is from mammalian (e.g., human) cells. In certain exemplary
embodiments,
the mammalian cells are mesenchymal cells.
[013] In certain exemplary embodiments, the precipitating agent is
polyethylene glycol, that
optionally has a molecular weight of about 6,000 Da, about 8,000 Da, about
10,000 Da or
about 20,000 Da.
[014] In certain exemplary embodiments, the one or more symptoms of
epidermolysis
bullosa are selected from the group consisting of any combination of thickened
calluses,
epidermal blistering (e.g., of the hands, the feet, the elbows and/or the
knees), blistering of
oral mucosa, thickened fingernails and/or toenails, sepsis, malnutrition,
dehydration,
electrolyte imbalance, obstructive airway complications, defective collagen
VII expression,
anemia, esophageal strictures, growth retardation, webbing or fusion of
fingers and/or toes,
malformation of teeth, microstomia and corneal abrasions.
[015] In certain exemplary embodiments, treatment comprises increasing
collagen VII
expression in the subject.
[016] In another aspect, a method of treating epidermolysis bullosa in a
subject in need
thereof comprising administering a pharmaceutical composition comprising
isolated
extracellular vesicles to the subject and alleviating or reducing one or more
symptoms of
epidermolysis bullosa in the subject is provided.
[017] In certain exemplary embodiments, the epidermolysis bullosa is
dystrophic
epidermolysis bullosa, e.g., recessive dystrophic epidermolysis bullosa.
3
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
[018] In certain exemplary embodiments, the isolated microvesicles are
extracellular
vesicles that are optionally precipitated from a biological fluid using a
precipitating agent
selected from the group consisting of calcium ions, magnesium ions, sodium
ions,
ammonium ions, iron ions, ammonium sulfate, alginate, and polyethylene glycol.
[019] In certain exemplary embodiments, the biological fluid is selected from
the group
consisting of peripheral blood, serum, plasma, ascites, urine, cerebrospinal
fluid (CSF),
sputum, saliva, bone marrow, synovial fluid, aqueous humor, amniotic fluid,
cerumen, breast
milk, broncheo alveolar lavage fluid, semen, prostatic fluid, Cowper's fluid,
female ejaculate,
sweat, fecal matter, hair, tears, cyst fluid, pleural and peritoneal fluid,
pericardial fluid,
lymph, chyme, chyle, bile, interstitial fluid, menses, pus, sebum, vomit,
vaginal secretions,
mucosal secretion, stool water, pancreatic juice, lavage fluid, fluid derived
from a cell, fluid
derived from a tissue sample, and cell culture fluid. In certain exemplary
embodiments, the
biological fluid is from mammalian (e.g., human) cells. In certain exemplary
embodiments,
the mammalian cells are mesenchymal cells.
[020] in certain exemplary embodiments, the precipitating agent is
polyethylene glycol, that
optionally has a molecular weight of about 6,000 Da, about 8,000 Da, about
10,000 Da or
about 20,000 Da.
[021] In certain exemplary embodiments, the one or more symptoms of
epidermolysis
bullosa are selected from the group consisting of any combination of thickened
calluses,
epidermal blistering (e.g., of the hands, the feet, the elbows and/or the
knees), blistering of
oral mucosa, thickened fingernails and/or toenails, sepsis, malnutrition,
dehydration,
electrolyte imbalance, obstructive airway complications, defective collagen
VII expression,
anemia, esophageal strictures, growth retardation, webbing or fusion of
fingers and/or toes,
malformation of teeth, microstomia and corneal abrasions.
[022] in certain exemplary embodiments, treatment comprises increasing
collagen VII
expression in the subject.
[023] In another aspect, a method of increasing collagen Vii levels in a cell,
comprising
contacting the cell with an isolated extracellular vesicle from a mammalian
fluid, wherein the
cell expresses an epidermolysis bullosa genotype, is provided.
4
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
[024] In certain exemplary embodiments, the mammalian fluid is selected from
the group
consisting of peripheral blood, serum, plasma, ascites, urine, CSF, sputum,
saliva, bone
marrow, synovial fluid, aqueous humor, amniotic fluid, cerumen, breast milk,
broncheo
alveolar lavage fluid, semen, prostatic fluid, Cowper's fluid, female
ejaculate, sweat, fecal
matter, hair, tears, cyst fluid, pleural and peritoneal fluid, pericardial
fluid, lymph, chyme,
chyle, bile, interstitial fluid, menses, pus, sebum, vomit, vaginal
secretions, mucosal
secretion, stool water, pancreatic juice, lavage fluid, fluid derived from a
cell, fluid derived
from a tissue sample, and cell culture fluid. In certain exemplary
embodiments, the
mammalian fluid is a conditioned medium. In certain exemplary embodiments, the
conditioned medium is derived from mesenchymal stem cells.
[025] in certain exemplary embodiments, the cell comprises a mutation in the
COL7A1
gene.
[026] In certain exemplary embodiments, the epidermolysis bullosa genotype is
recessive
dystrophic epidermolysis bullosa.
[027] In certain exemplary embodiments, one or both of proliferation of the
cell is
stimulated, and resistance of the cell to trypsin digestion is enhanced.
[028] In certain exemplary embodiments, the isolated extracellular vesicle
delivers collagen
Vii protein and/or COL7A1 mRNA to the cell.
[029] In another aspect, a method of delivering one or more bioactive agents
to a cell,
comprising contacting the cell with an isolated extracellular vesicle from a
mammalian fluid
is provided.
[030] In certain exemplary embodiments, the mammalian fluid is selected from
the group
consisting of peripheral blood, serum, plasma, ascites, urine, CSF, sputum,
saliva, bone
marrow, synovial fluid, aqueous humor, amniotic fluid, cerumen, breast milk,
broncheo
alveolar lavage fluid, semen, prostatic fluid, Cowper's fluid, female
ejaculate, sweat, fecal
matter, hair, tears, cyst fluid, pleural and peritoneal fluid, pericardial
fluid, lymph, chyme,
chyle, bile, interstitial fluid, menses, pus, sebum, vomit, vaginal
secretions, mucosal
secretion, stool water, pancreatic juice, lavage fluid, fluid derived from a
cell, fluid derived
from a tissue sample, and cell culture fluid. In certain exemplary
embodiments, the
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
mammalian fluid is a conditioned medium. In certain exemplary embodiments, the
conditioned medium is derived from mesenchymal stem cells.
[031] In certain exemplary embodiments, the cell comprises a mutation in the
COL7A1
gene.
[032] In certain exemplary embodiments, the one or more bioactive agents are
selected from
the group consisting of collagen VII protein, collagen VII mRNA, a STAT3
signalling
activator (e.g., an interferon, epidermal growth factor, interleukin-5,
interleukin-6, a MAP
kinase, and/or a c-src non-receptor tyrosine kinase), and a canonical Wnt
activator.
[033] In certain exemplary embodiments, STAT3 is phosphorylated.
[034] In certain exemplary embodiments, the one or more bioactive agents are
one or more
pharmaceutical compounds.
[035] In certain exemplary embodiments, the cell has a recessive dystrophic
epidermolysis
bullosa genotype.
[036] in certain exemplary embodiments, one or both of proliferation of the
cell is
stimulated, and resistance of the cell to trypsin digestion is enhanced.
[037] In another aspect, a method for isolating and/or purifying microvesicles
from cell
culture supernatants or biological fluids utilizing precipitation agent that
precipitates the
microvesicle from the cell culture supernatant or biological fluid by
displacing the water of
solvation is provided.
[038] In another aspect, an isolated preparation of microvesicles is provided.
In one
embodiment, the isolated preparation of microvesicles is subsequently
purified. In one
embodiment, the isolated preparation of microvesicles is subsequently purified
to yield a
preparation of ectosomes. In one embodiment, the isolated preparation of
microvesicles is
subsequently purified to yield a preparation of microparticles. In one
embodiment, the
isolated preparation of microvesicles is subsequently purified to yield a
preparation of
nanovesicles. In one embodiment, the isolated preparation of microvesicles is
subsequently
purified to yield a preparation of shedding vesicles. In one embodiment, the
isolated
preparation of microvesicles is subsequently purified to yield a preparation
of membrane
6
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
particles. In one embodiment, the isolated preparation of microvesicles is
subsequently
purified to yield a preparation of apoptotic bodies.
[039] In another aspect, an isolated preparation of microvesicles that
promotes or enhances
angiogenesis is provided. In one embodiment, the isolated preparation of
microvesicles
promotes or enhances angiogenesis in a patient.
[040] In another aspect, an isolated preparation of microvesicles that
promotes or enhances
neuronal regeneration is provided. In one embodiment, the isolated preparation
of
microvesicles promotes or enhances neuronal regeneration in a patient.
[041] In another aspect, an isolated preparation of microvesicles that
promotes or enhances
cellular proliferation is provided. In one embodiment, the isolated
preparation of
microvesicles promotes or enhances cellular proliferation in a patient.
[042] In another aspect, an isolated preparation of microvesicles that
promotes or enhances
cellular migration is provided. In one embodiment, the isolated preparation of
microvesicles
promotes or enhances cellular migration in a patient. In one embodiment, the
present
invention provides an isolated preparation of microvesicles that promotes or
enhances wound
healing. In one embodiment, the wound is a full-thickness burn. In one
embodiment, the
wound is a second-degree burn.
[043] In another aspect, an isolated preparation of microvesicles that reduces
scar formation
in a patient is provided.
[044] In another aspect, an isolated preparation of microvesicles that reduces
wrinkle
formation in the skin of a patient is provided.
[045] In another aspect, an isolated preparation of microvesicles that is used
to diagnose the
presence and/or progression of a disease in a patient is provided. In one
embodiment, the
disease is metastatic melanoma. In an alternative embodiment the disease in an
infiammatory/autoimmune disorder such as rheumatoid arthritis. In one
embodiment, the
disease is graft versus host disease.
[046] In another aspect, an isolated preparation of microvesicles that can
promote functional
regeneration and organization of complex tissue structures is provided. In one
embodiment,
an isolated preparation of microvesicles that can regenerate hematopoietic
tissue in a patient
7
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
with aplastic anemia is provided. In one embodiment, an isolated preparation
of
microvesicles that can regenerate at least one tissue in a patient with
diseased, damaged or
missing skin selected from the group consisting of: epithelial tissue, stromal
tissue, nerve
tissue, vascular tissue and adnexal structures, is provided. In one
embodiment, the present
invention provides an isolated preparation of microvesicles that can
regenerate tissue and/or
cells from all three germ layers.
[047] In another aspect, an isolated preparation of microvesicles that is used
to modulate the
immune system of a patient is provided.
[048] in another aspect, an isolated preparation of microvesicles that
enhances the survival
of tissue or cells that is transplanted into a patient is provided. In one
embodiment, the
patient is treated with the isolated preparation of microvesicles prior to
receiving the
transplanted tissue or cells. In an alternate embodiment, the patient is
treated with the isolated
preparation of microvesicles after receiving the transplanted tissue or cells.
In an alternate
embodiment, the tissue or cells is treated with the isolated preparation of
microvesicles. In
one embodiment, the tissue or cells is treated with the isolated preparation
of microvesicles
prior to transplantation.
[049] In another aspect, an isolated preparation of microvesicles containing
at least one
molecule selected from the group consisting of RNA, DNA, and protein from a
host cell is
provided. In one embodiment, the host cell is engineered to express at least
one molecule
selected from the group consisting of RNA, DNA, and protein. In one
embodiment, the
isolated preparation of microvesicles containing at least one molecule
selected from the group
consisting of RNA, DNA, and protein from a host cell is used as a therapeutic
agent.
BRIEF DESCRIPTION OF THE DRAWINGS
[050] The accompanying drawings, which are incorporated herein and form part
of the
specification, illustrate various embodiments of the present invention and,
together with the
description, further serve to explain the principles of the invention and to
enable a person of
ordinary skill in the art to make and use the invention. In the drawings, like
reference
numbers indicate identical or functionally similar elements. A more complete
appreciation of
the invention and many of the attendant advantages thereof will be readily
obtained as the
8
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
same becomes better understood by reference to the following detailed
description when
considered in connection with the accompanying drawings, wherein:
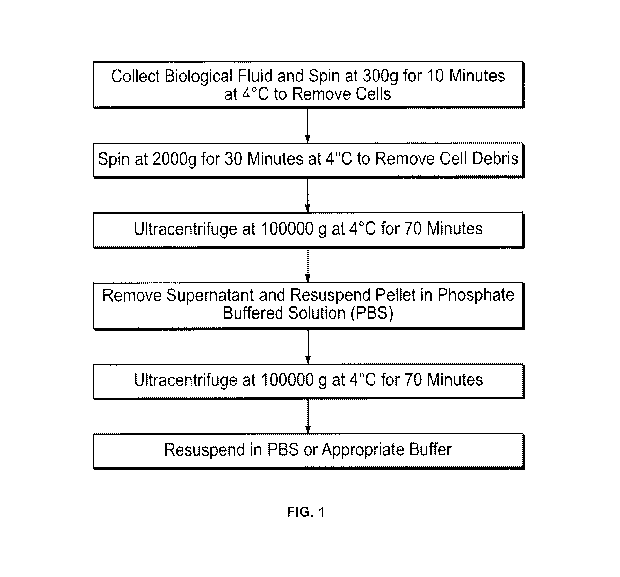
[051] Fig. 1 shows a schematic outline of a protocol used to isolate
microvesicles by
ultracentrifugation.
[052] Fig. 2 shows one embodiment of a microvesicle isolation method of the
present
invention.
[053] Fig. 3 shows an alternate embodiment of a microvesicle isolation method
of the
present invention.
[054] Fig. 4 shows one embodiment of an apparatus of the present invention
that facilitates
the clarification of the biological fluid and the collection of the
precipitated microvesicles by
filtration.
11155:1 Figs. SA ¨ Fig. SD show electron micrographs of microvesicles derived
from medium
conditioned using human bone marrow-derived mesenchymal stem cells isolated by
the
ultracentrifuge method described in Example 1 (Fig. 5A & Fig. 5B) and isolated
according to
the methods of the present invention (Fig. 5C & Fig. 5D) at the magnifications
shown in the
panels.
11156:1 Figs. 6A ¨ Fig. 6D show electron micrographs of microvesicles derived
from medium
conditioned using porcine bone marrow-derived mesenchymal stem. cells isolated
by the
ultracentrifuge method described in Example 1 (Fig. 6A & Fig. 6B) and isolated
according to
the methods of the present invention (Fig. 6C & Fig. 6D) at the magnifications
shown in the
panels.
11157:1 Figs. 7A ¨ Fig. 7D show electron micrographs of microvesicles derived
from medium
conditioned using murine bone marrow-derived mesenchymal stem cells isolated
by the
ultracentrifuge method described in Example 1 (Fig. 7A & Fig. 7B) and isolated
according to
the methods of the present invention (Fig. 7C & Fig. 7D) at the magnifications
shown in the
panels.
[058] Figs. 8A ¨ Fig. 8C show electron micrographs of microvesicles isolated
from human
plasma according to the methods of the present invention. Fig. 8A through Fig.
8C show the
microvesicles under increasing magnification, as shown by the scale bars in
the panels.
9
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
[059] Figs. 9A ¨ Fig. 9C show electron micrographs of microvesicles isolated
from porcine
plasma according to the methods of the present invention. Fig. 9A through Fig.
9C show the
microvesicles under increasing magnification, as shown by the scale bars in
the panels.
[060] Figs. 10A ¨ Fig. 10C show electron micrographs of microvesicles isolated
from
human urine according to the methods of the present invention. Fig. 10A
through Fig. 10C
show the microvesicles under increasing magnification, as shown by the scale
bars in the
panels.
[061] Fig. 11 shows a Western blot, reporting the expression of HSP70, CD63,
STAT 3 and
phosphorylated STAT3 in lysates of human bone marrow-derived mesenchymal stem
cells,
microvesicles isolated from medium conditioned using human bone marrow-derived
stem
cells, prepared by ultracentrifugation (hMSC MV Ultracentrifuge), or the
methods of the
present invention, as described in Example 3 (hMSC PEG Precipitation).
Microvesicles
derived from human plasma and human urine, prepared by the methods of the
present
invention, as described in Example 3 were also analyzed. (Human plasma PEG
Precipitation)
and (human urine PEG Precipitation) respectively.
[062] Figs. 12A ¨ Fig. 12C show the effect of microvesicles isolated from
medium
conditioned using human bone marrow-derived mesenchymal stem cells on the
proliferation
of normal human dermal fibroblasts (Fig. 12A), dermal fibroblasts obtained
from a diabetic
foot ulcer (Fig. 12B), and dermal fibroblasts obtained from a pressure foot
ulcer (Fig. 12C).
The effect of microvesicles isolated by ultracentrifugation (MV U/C) and
microvesicles
isolated by the methods of the present invention (MV PEG) were compared.
Fibroblasts
treated with PBS or microvesicle depleted culture medium were included as a
control.
Proliferation was determined using an MIT assay.
[063] Figs. 13A ¨ Fig. 13G show the effect of microvesicles isolated from
medium
conditioned using human bone marrow-derived mesenchymal stem cells on the
migration of
human dermal fibroblasts, as determined by the ability of the fibroblasts to
migrate into a
region that had been scratched off. The panel labeled "pretreatment" shows a
representative
area of a cell culture plate where the cells were removed, prior to the
addition of the test
treatments. The effect of fibroblast migration was tested using microvesicles
isolated
according to the methods of the present invention (PEG precipitation) and
microvesicles
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
isolated by ultracentrifugation (Ultracentrifuge) at the concentrations shown.
Fibroblasts
treated with PBS or microvesicle depleted culture medium were included as a
control.
[064] Figs. 14A ¨ Fig. 14G show the effect of microvesicles isolated from
medium
conditioned using human bone marrow-derived mesenchymal stem cells on the
migration of
human dermal fibroblasts obtained from a diabetic foot ulcer, as determined by
the ability of
the fibroblasts to migrate into a region that had been scratched off. The
panel labeled
"pretreatment" shows a representative area of a cell culture plate where the
cells were
removed, prior to the addition of the test treatments. The effect of
fibroblast migration was
tested using microvesicles isolated according to the methods of the present
invention (PEG
precipitation) and microvesicles isolated by ultracentrifugation
(Ultracentrifuge) at the
concentrations shown. Fibroblasts treated with PBS or microvesicle depleted
culture medium
were included as a control.
[065] Figs. 15A ¨ Fig. I5D show the uptake of the microvesicles of the present
invention
into human dermal fibroblasts. Cell nuclei, resolved using Hoechst 33342 dye
are shown in
the panels labeled "Hoechst33342." Cells, resolved using vybrant dye are shown
in the panel
labeled "Vybrant-Dio." Microvesicles, resolved using PKH dye are shown in the
panel
labeled "PKH labeled MV". A panel where images obtained from all three dyes
are overlaid
is seen in the panel labeled "Composite."
[066] Figs. 16A ¨ Fig. 16D show the uptake of the microvesicles of the present
invention
into human dermal fibroblasts. Cell nuclei, resolved using Hoechst 33342 dye
are shown in
the panels labeled "Hoechst33342." Cells, resolved using vybrant dye are shown
in the panel
labeled "Vybrant- Dio." Microvesicles, resolved using PKH dye are shown in the
panel
labeled "PKH labeled MV". A panel where images obtained from all three dyes
are overlaid
is seen in the panel labeled "Composite."
[067] Fig. 17 shows a Western blot of lysates of human dermal fibroblasts
treated with:
microvesicles isolated according to the methods of the present invention from
plasma
obtained from a patient suffering from rheumatoid arthritis (Human Plasma MV
PEG
Precipitation); microvesicles isolated according to the methods of the present
invention from
medium conditioned with bone marrow-derived mesenchymal stem cells (Human hMSC
MV
PEG Precipitation); microvesicles isolated via ultracentrifugation from medium
conditioned
11
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
with bone marrow-derived mesenchymal stem cells (Human hMSC MV
ultracentrifugation);
PBS control; and a depleted medium control (hMSC conditioned medium depleted
of MV).
[068] Fig. 18 shows the presence of the region containing exon 15 of BRAF
containing the
T1799A mutation, in: SK-MEL28 cells, from RNA amplified using primer 1 (lane
3); SK-
MEL28 cells, from RNA amplified using primer 2 (lane 4); microvesicles
isolated according
to the methods of the present invention from medium conditioned with SK-MEL28
cells,
from RNA amplified using primer 1 (lane 5); microvesicles isolated according
to the methods
of the present invention from medium conditioned with SK-MEL28 cells, from RNA
amplified using primer 2 (lane 6); SK-MEL28 cells, from DNA amplified using
primer 1
(lane 7); SK-MEL28 cells, from DNA amplified using primer 2 (lane 8);
microvesicles
isolated according to the methods of the present invention from medium
conditioned with
SK-MEL28 cells, from DNA amplified using primer 1 (lane 9); and microvesicles
isolated
according to the methods of the present invention from medium conditioned with
SK-MEL28
cells, from DNA amplified using primer 2 (lane 10).
[069] Fig. 19 shows the presence of V600E BRAF in a lysate of SK-MEL28 cells
and a
lysate of microvesicles isolated according to the methods of the present
invention from
medium conditioned with SK-MEL28 cells.
[070] Figs. 20A ¨ Fig. 20D show the uptake of the microvesicles isolated
according to the
methods of the present invention from culture medium conditioned using bone
marrow-
derived stern cells obtained from a green fluorescent protein (GFP) expressing
mouse into
human dermal fibroblasts. Cell nuclei, resolved using Hoechst 33342 dye are
shown in the
panels labeled "Hoechst33342." Cells, resolved using vybrant dye are shown in
the panel
labeled "Vybrant- Dio." GFP-labeled microvesicles are shown in the panel
labeled "GFP."
A panel where images obtained from all three dyes are overlaid is seen in the
panel labeled
"Composite."
[071] Figs. 2IA ¨ Fig. 21D show the uptake of the microvesicles isolated
according to the
methods of the present invention from culture medium conditioned using bone
marrow-
derived stem cells obtained from a GFP expressing mouse into human dermal
fibroblasts.
Cell nuclei, resolved using Hoechst 33342 dye are shown in the panels labeled
"Hoechst33342." Cells, resolved using vybrant dye are shown in the panel
labeled "Vybrant-
12
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
Dio." GFP-labeled microvesicles are shown in the panel labeled "GFP." A panel
where
images obtained from all three dyes are overlaid is seen in the panel labeled
"Composite."
[072] Figs. 22A ¨ Fig. 22D show histological sections of full-thickness wounds
from: Fig.
22A - untreated animals; Fig. 22B - microvesicles isolated from medium
conditioned using
autologous bone marrow-derived mesenchymal stem cells according to the methods
of the
present invention; Fig. 22C - saline; and Fig 22D - microvesicles isolated
from autologous
bone marrow-derived mesenchymal stem cells by ultracentrifugation, 5 days post
wound.
[073] Figs. 23A- Fig. 23D show pictures of second degree burns on animals
treated with:
Fig. 23A - microvesicles isolated from medium conditioned using autologous
bone marrow-
derived mesenchymal stem cells by ultracentrifugation; Fig. 23B -
microvesicles isolated
from medium conditioned using autologous bone marrow-derived mesenchymal stem
cells
according to the methods of the present invention; and Fig. 23C - untreated
animals, 7 days
post wound. Fig 23D - shows a full thickness wound in an animal treated with
microvesicles
isolated from medium conditioned using autologous bone marrow-derived
mesenchymal
stem cells by ultracentrifugation 7 days post wound. Arrows indicate abscess
formation in a
full thickness wound treated with microvesicles isolated by
ultracentrifugation at Day 7
(40X). This was not observed in full thickness wounds treated with
microvesicles prepared
according to the methods of the present invention.
[074] Fig. 24 shows a histological slide of a second degree wound, 28 days
post wound,
from an animal treated with microvesicles isolated from medium conditioned
using
autologous bone marrow-derived mesenchymal stem cells according to the methods
of the
present invention.
[075] Fig. 25 shows a histological slide of a second-degree wound, 28 days
post wound,
from an animal treated with saline.
[076] Fig. 26 shows a histological slide of a full-thickness wound, 28 days
post wound,
from an animal treated with microvesicles isolated from medium conditioned
using
autologous bone marrow-derived mesenchymal stem cells according to the methods
of the
present invention.
[077] Figs. 27A ¨ Fig. 27C show a histological slide of a full-thickness
wound, 28 days
post wound, from an animal treated with microvesicles isolated from medium
conditioned
13
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
using autologous bone marrow-derived mesenchymal stem cells according to the
methods of
the present invention. Fig. 27A shows new nerve growth (arrows) and
angiogenesis (circles).
Fig. 27B shows new nerve growth (arrows). Fig. 27C shows new blood vessel
growth
(arrows).
[078] Fig. 28 shows a histological slide of a full-thickness wound, 7 days
post wound in an
animal treated with microvesicles derived from medium conditioned using
autologous bone
marrow-derived mesenchymal stem cells.
[079] Figs. 29A ¨ Fig. 29B show the presence or absence of chimerism in
irradiated
animals following administration of GFP-labeled bone marrow.
[080] Figs. 30A ¨ Fig. 30C show the effects of MSC treatment on hair growth
following
gamma irradiation (Fig. 30A and Fig. 30B), and the absence of chimerism in
irradiated
animals following administration of GFP-labeled bone marrow (Fig. 30C).
[081] Fig. 31A ¨ Fig. 31F show the effect of bone marrow-derived microvesicles
obtained
using the method of the present invention on blood vessel formation, using an
in vitro assay
of angiogenesis. The upper three panels are representative images taken using
an
epifluorescent microscope of cultures of HUVEC cells treated with bone marrow-
derived
microvesicles obtained using the method of the present invention ("Bone Marrow
Aspirate
MV"). The lower three panels are representative images taken using an
epifluorescent
microscope of cultures of HUVEC cells treated with vehicle control ("Vehicle
Control").
[082] Figs. 32A ¨ Fig. 32C show the effect of bone marrow-derived
microvesicles obtained
using the method of the present invention on cell growth or proliferation,
using an in vitro
assay of cell growth. Fig. 32A shows representative images taken using an
epifluorescent
microscope of cultures of normal adult fibroblasts treated with bone marrow-
derived
microvesicles obtained using the method of the present invention ("Bone Marrow
MV") or
PBS ("PBS"). three days post treatment. Fig. 32B shows the average cell number
in cultures
of normal adult fibroblasts treated with bone marrow-derived microvesicles
obtained using
the method of the present invention ("Bone Marrow MV") or PBS ("PBS"), three
days post
treatment. Fig. 32C graphically depicts cell numbers.
[083] Figs. 33A ¨ Fig. 33B show the results of chronic wound treatment with
bone marrow
stem cells (including BM-MSCs). Fig. 33A - Prior to treatment and before wound
14
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
debridement. A necrotic Achilles tendon is visible. Fig. 33B - Healed post-
administration
(i.e., topical administration) of bone marrow cells.
[084] Figs. 34A ¨ Fig. 34 C show dermal rebuilding in wounds treated with bone
marrow
stem cells. (A) Fig. 34A - pre-treatment biopsy of a fibrotic, scarred wound.
Post-treatment
biopsies with the generation of numerous reticulin fibers (Fig. 34B) and
elastic fibers (Fig.
34C) are shown.
[085] Figs. 35A ¨ Fig. 35C show a deep second degree burn injury. The patient
was given
two administrations of BM-MSCs 11 days apart. Fig. 35A - Deep second degree
burn injury
day 0 (prior to treatment). The circled area represents the deepest portion of
the burn injury.
Fig. 35B - Hair follicle accentuation 11 days after the first administration
(i.e., topical
administration) of BM-MSCs. The accentuated follicles are noted in the circled
area of A.
Fig. 35C - Hair growth in in the circled area of Fig. 35A, 34 days after the
second
administration of BM-MSCs.
[086] Figs. 36A ¨ Fig. 36C show the healing of a burn patient treated with two
topical
administrations of MSCs given ten days apart. Fig. 36A - Prior to treatment.
Fig. 36B - 10
days post-treatment (i.e., topical administration) with first dose of MSCs.
Fig. 36CA -7 days
post-treatment with second dose of BM-MSCs (i.e., 17 days after Fig. 36A).
[087] Figs. 37A ¨ Fig. 37B show no evidence of scarring in burn patient
assessed one year
post-treatment with BM-MSCs. Upper panel: left ventral forearm (bottom panel
shows area
outlined in yellow). The patient's skin showed evidence of normal elasticity
with no
evidence of scarring in the original burned areas.
[088] Figs. 38A ¨ Fig. 38B show full thickness wounds (day 5) created on
Yorkshire pigs.
Fig. 38A - Untreated control. Fig. 38B - Wound treated with BM-MSC EVs
according to
certain embodiments of the invention. There was significantly greater closure
of the wound
after treatment with BM-MSC EVs. Arrows indicate areas of increased dermal
remodeling
according to certain embodiments of the invention.
[089] Figs. 39A ¨ Fig. 39C show full thickness wounds (day 28) created on
Yorkshire pigs
treated with BM-MSC EVs according to certain exemplary embodiments. Fig. 39A -
Arrows
highlight nerve growth and stars illustrate vascular growth. Fig. 39B - Higher
magnification
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
illustrating vascular growth (arrows). Fig. 39C - Higher magnification
illustrating nerve
growth (arrows).
[090] Figs. 40A ¨ Fig. 40B show second degree burn wounds in pigs 5 days post-
treatment
with intralesional injection of porcine BM-MSC EVs according to certain
exemplary
embodiments. Left: EVs prepared by ultracentrifugation methods known in the
art were used
to treat a burn wound. The wound was raised and grossly inflamed with sterile
pustule
formation (indicative of an induced inflammatory response and not infection)
and reduced
healing. Right: EVs prepared using exemplary methods described herein were
used to treat a
burn wound. The wound has accelerated healing with reduced inflammation
compared to
traditional EVs prepared by ultracentrifugation.
[091] Figs. 41A ¨ Fig. 41B graphically depict enrichment of COL7A1 mRNA in BM-
MSC
EVs (middle bars in each panel). EV treatment increased COL7A1 expression in
RDEB
fibroblasts. Left panel shows COL7A1 expression detected with primer pair 1;
right panel
shows COL7A1 expression detected with primer pair 2. Gene expression was
normalized by
beta-actin expression, a common EV housekeeping gene.
[092] Fig. 42 graphically depicts a chemoselective ligation assay (utilizing
"click iT"
reaction chemistry) that revealed production of new collagen VII from RDEB
fibroblasts
following co-treatment with BM-MSC EVs (10 g/mL) and the L-methionine analog
L-
homopropargylglycine (HPG) (a modified amino acid) which incorporates into
newly
synthesized proteins.
[093] Figs. 43A ¨ Fig. 43B graphically depict that BM-MSC EVs significantly
promote
both RDEB proliferation (Fig. 43A) and resistance to trypsin digestion (Fig.
43B), both
standard in vitro assays to assess gain-of-function support the pro-wound
healing potential of
RDEB dermal fibroblasts.
[094] Figs. 44A ¨ Fig. 44C show the validation of an in vitro cell line
derived from an
infant diagnosed as having RDEB (Hallopeau-Siemens type). The RDEB fibroblasts
expressed significantly less COL7A1 compared to fibroblasts derived from non-
affected
subjects (NHF). Fig. 44A - Primer pairs 1 and 2 designed near 3' end of cDNA
corresponding to 5' end of mRNA. Fig. 44B - COL7A1 gene expression in normal
human
16
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
fibroblasts (NHFs) and in RDEB fibroblasts. Fig. 44C - RDEB cells secreted low
levels of
collagen VII protein relative to normal (control) human fibroblasts.
[095] Figs. 45A ¨ Fig. 45B show vesicle exchange between BM-MSCs and RDEB
fibroblasts. RDEBFs (stained with lipid dye Dil (red)) and BM-MSCs (stained
with lipid dye
DiO (green)) were co-cultured, and, within one day, began to uptake
extracellular vesicles
(yellow). Scale bar, 10 gm.
[096] Figs. 46A ¨ Fig. 46D show that collagen VII protein co-isolated with BM-
MSC
extracellular vesicles (EVs). Fig. 46A - Transmission electron micrograph of
an extracellular
vesicle isolated from BM-MSC serum-free conditioned media (CM). Fig. 46B -
NanoSight
image of BM-MSC EVs, diluted 1:500. Fig. 46C - Histogram of size vs
concentration
(diluted 1:500). Inset shows EVs contain CD63 exosome marker. Fig. 46D -
Collagen VII
protein in BM-MSC CM and associated with purified BM-MSC EVs.
[097] Figs. 47A¨ Fig. 47B show enrichment of COL7A1 mRNA in BM-MSC EVs (middle
bars in each panel). EV treatment increased COL7A1 expression in RDEB
fibroblasts. Left
panel shows COL7A1 expression detected with primer pair 1; right panel shows
COL7A1
expression detected with primer pair 2. Gene expression was normalized by beta-
actin
expression, a common EV housekeeping gene.
[098] Figs. 48A ¨ Fig. 48C show that RDEB fibroblasts treated with BM-MSC EVs
contained more collagen VII protein in media 3 days after washing. Fig. 48A -
Treatment
schematic. Fig. 48B - Western blot of collagen VII in RDEB media. Fig. 48C -
Densitometry quantification of Fig. 48B (above baseline collagen VII
detection).
[099] Figs. 49A ¨ Fig. 49C depict a chemoselective ligation assay (utilizing
"click iT"
reaction chemistry) (Fig. 49A and Fig, 49B) that revealed production of new
collagen VII
from RDEB fibroblasts following co-treatment with BM-MSC EVs (10 g/mL) and L-
methionine analog L-homopropargylglycine (HPG) (a modified amino acid) which
incorporates into newly synthesized proteins (Fig. 49C).
[0100] Figs. SOA ¨ Fig. SOB show that BM-MSC EVs increased in vitro surrogate
assays
related to wound healing (proliferation and trypsin-resistance) of RDEB
fibroblasts. Fig. 50A
- Proliferation (MTT) assay. Fig. 50B - Trypsin resistance assay.
17
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
[0101] Figs. 51A ¨ Fig. 51E show BM-MSCs that were delivered in saline to burn
patients
in a clinical trial. BM-MSCs secreted large numbers of EVs (CD63 positive) in
saline within
hours (shown, 4 hours). Upper left panel, NanoSight of saline buffer
background; upper right
panel, NanoSight EVs in saline (diluted 1:500); lower panels: histogram of
1:500 dilution of
saline delivered in burn clinical trial, bar graph quantification. Western
blot inset shows
CD63 (exosome marker) secreted by BM-MSCs within 4 hours.
[0102] Fig. 52 depicts a model according to certain exemplary embodiments of
the invention
in which the secretome of BM-MSCs contains EV-associated and non-EV-associated
proteins
that deliver multiple pro-wound healing functions to RDEB fibroblasts,
including collagen
VII protein, collagen VII mRNA, STAT3-signaling activators, and canonical Wnt
activators.
DETAILED DESCRIPTION
[0103] For clarity of disclosure, and not by way of limitation, the detailed
description of the
invention is divided into the following subsections that describe or
illustrate certain features,
embodiments or applications of the present invention.
Methods to Isolate the Microvesicles of the Present invention
[0104] As used herein, the term "microvesicles" refers to vesicles comprising
lipid bilayers,
formed from the plasma membrane of cells, and are heterogeneous in size,
ranging from
about 2 nm to about 5000 nm. The cell from which a microvesicle is formed is
herein
referred to as "the host cell." Microvesicles are a heterogeneous population
of vesicles and
include, but are not limited to, extracellular vesicles (EVs), ectosomes,
microparticles,
microvesicles, nanovesicles, shedding vesicles, membrane particles and the
like.
[0105] Microvesicles exhibit membrane proteins from their host cell on their
membrane
surface, and may also contain molecules within the microvesicle from the host
cell, such as,
for example, mRNA, miRNA, tRNA, RNA, DNA, lipids, proteins or infectious
particles.
These molecules may result from, or be, recombinant molecules introduced into
the host cell.
Microvesicles play a critical role in intercellular communication, and can act
locally and
distally within the body, inducing changes in cells by fusing with a target
cell, introducing the
molecules transported on and/or in the microvesicle to the target cell. For
example,
microvesicles have been implicated in anti-tumor reversal, cancer, tumor
immune
suppression, metastasis, tumor-stroma interactions, angiogenesis and tissue
regeneration.
18
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
Microvesicles may also be used to diagnose disease, as they have been shown to
carry bio-
markers of several diseases, including, for example, cardiac disease, HIV and
leukemia.
[0106] In one embodiment, microvesicles are isolated from a biological fluid
containing
microvesicles in a method comprising the steps of:
a) obtaining a biological fluid containing microvesicles,
b) clarifying the biological fluid to remove cellular debris,
c) precipitating the microvesicles by adding a precipitating agent to the
clarified
biological fluid,
d) collecting the precipitated microvesicles and washing the material to
remove the
precipitating agent, and
e) suspending the washed microvesicles in a solution for storage or subsequent
use.
[0107] In one embodiment, the biological fluid is clarified by centrifugation.
In an alternate
embodiment, the biological fluid is clarified by filtration.
[0108] In one embodiment, the precipitated microvesicles are collected by
centrifugation. In
an alternate embodiment, the precipitated microvesicles are collected by
filtration.
[0109] in one embodiment, microvesicles are isolated from a biological fluid
containing
microvesicles in a method comprising the steps of:
a) obtaining a biological fluid containing microvesicles,
b) clarifying the biological fluid to remove cellular debris,
c) precipitating the microvesicles by adding a precipitating agent to the
clarified
biological fluid,
d) collecting the precipitated microvesicles and washing the material to
remove the
precipitating agent,
e) suspending the washed microvesicles in a solution, and
19
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
f) processing the microvesicles to analyze the nucleic acid, carbohydrate,
lipid, small
molecules and/or protein content.
[0110] In one embodiment, the biological fluid is clarified by centrifugation.
In an alternate
embodiment, the biological fluid is clarified by filtration.
[0111] In one embodiment, the precipitated microvesicles are collected by
centrifugation. In
an alternate embodiment, the precipitated microvesicles are collected by
filtration.
[0112] In one embodiment, the present invention provides reagents and kits to
isolate
microvesicles from biological fluids according to the methods of the present
invention.
[0113] The biological fluid may be peripheral blood, sera, plasma, ascites,
urine,
cerebrospinal fluid (CSF), sputum, saliva, bone marrow, synovial fluid,
aqueous humor,
amniotic fluid, cerumen, breast milk, broncheo alveolar lavage fluid, semen
(including
prostatic fluid), Cowper's fluid or pre-ejaculatory fluid, female ejaculate,
sweat, fecal matter,
hair, tears, cyst fluid, pleural and peritoneal fluid, pericardial fluid,
lymph, chyme, chyle, bile,
interstitial fluid, menses, pus, sebum, vomit, vaginal secretions, mucosal
secretion, stool
water, pancreatic juice, lavage fluids from sinus cavities, bronchopulmonary
aspirates or
other lavage fluids.
[0114] The biological fluid may also be derived from the blastocyl cavity,
umbilical cord
blood, or maternal circulation, which may be of fetal or maternal origin. The
biological fluid
may also be derived from a tissue sample or biopsy.
[0115] The biological fluid may be derived from plant cells of cultures of
plant cells. The
biological fluid may be derived from yeast cells or cultures of yeast cells.
[0116] In one embodiment, the biological fluid is cell culture medium. In one
embodiment,
the cell culture medium is conditioned using tissues and/or cells prior to the
isolation of
microvesicles according to the methods of the present invention.
[0117] The term "conditioned" or "conditioned medium" refers to medium,
wherein a
population of cells or tissue, or combination thereof is grown, and the
population of cells or
tissue, or combination thereof contributes factors to the medium. In one such
use, the
population of cells or tissue, or combination thereof is removed from the
medium, while the
factors the cells produce remain. In one embodiment, the factors produced are
microvesicles.
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
Medium may be conditioned via any suitable method selected by one of ordinary
skill in the
art. For example, medium may be cultured according to the methods described in
EP1780267A2.
[0118] in one embodiment, microvesicles are isolated from cells or tissue that
have been pre-
treated prior to the isolation of the microvesicles. Pretreatment may include,
for example,
culture in a specific medium, a medium that contains at least one additive,
growth factor,
medium devoid of serum, or a combination thereof. Alternatively, pretreatment
may
comprise contacting cells or tissues with additives (e.g. interleukin, VEGF,
inducers of
transcription factors, transcription factors, hormones, neurotransmitters,
pharmaceutical
compounds, microRNA), transforming agents (e.g. liposome, viruses, transfected
agents,
etc.). Alternatively, pretreatment may comprise exposing cells or tissue to
altered physical
conditions (e.g. hypoxia, cold shock, heat shock and the like).
[0119] In one embodiment, microvesicles are isolated from medium conditioned
using cells
or tissue that have been pre-treated prior to the isolation of the
microvesicles. Pretreatment
may include, for example, culture in a specific medium, a medium that contains
at least one
additive, growth factor, medium devoid of serum, or a combination thereof.
Alternatively,
pretreatment may comprise contacting cells or tissues with additives (e.g.
interleukin, VEGF,
inducers of transcription factors, transcription factors, hormones,
neurotransmitters,
pharmaceutical compounds, microRNA), transforming agents (e.g. Liposome,
viruses,
transfected agents, etc.). Alternatively, pretreatment may comprise exposing
cells or tissue to
altered physical conditions (e.g. hypoxia, cold shock, heat shock and the
like).
[0120] In one embodiment, the biological fluid is an extract from a plant. In
an alternate
embodiment, the biological fluid is a cell culture medium from a culture of
plant cells. In an
alternate embodiment, the biological fluid is yeast extract. In an alternate
embodiment, the
biological fluid is a cell culture medium from a culture of yeast cells.
[0121] While the methods of the present invention may be carried out at any
temperature,
one of ordinary skill in the art can readily appreciate that certain
biological fluids may
degrade, and such degradation is reduced if the sample is maintained at a
temperature below
the temperature at which the biological fluid degrades. In one embodiment, the
method of
the present invention is carried out at 4 C. In an alternate embodiment, at
least one step of
the method of the present invention is carried out at 4 C. In certain
embodiments, the
21
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
biological fluid may be diluted prior to being subjected to the methods of the
present
invention. Dilution may be required for viscous biological fluids, to reduce
the viscosity of
the sample, if the viscosity of the sample is too great to obtain an
acceptable yield of
microvesicles. The dilution may be a 1:2 dilution. Alternatively, the dilution
may be a 1:3
dilution. Alternatively, the dilution may be a 1:4 dilution. Alternatively,
the dilution may be
a 1:5 dilution. Alternatively, the dilution may be a 1:6 dilution.
Alternatively, the dilution
may be a 1:7 dilution. Alternatively, the dilution may be a 1:8 dilution.
Alternatively, the
dilution may be a 1:9 dilution. Alternatively, the dilution may be a 1: 10
dilution.
Alternatively, the dilution may be a 1:20 dilution. Alternatively, the
dilution may be a 1:30
dilution. Alternatively, the dilution may be a 1:40 dilution. Alternatively,
the dilution may
be a 1:50 dilution. Alternatively, the dilution may be a 1:60 dilution.
Alternatively, the
dilution may be a 1:70 dilution. Alternatively, the dilution may be a 1:80
dilution.
Alternatively, the dilution may be a 1:90 dilution. Alternatively, the
dilution may be a 1: 100
dilution.
[0122] The biological fluid may be diluted with any diluent, provided the
diluent does not
affect the functional and/or structural integrity of the microvesicles. One of
ordinary skill in
the art may readily select a suitable diluent. Diluents may be, for example,
phosphate
buffered saline, cell culture medium, and the like.
[0123] In one embodiment, the biological fluid is clarified by the application
of a centrifugal
force to remove cellular debris. The centrifugal force applied to the
biological fluid is
sufficient to remove any cells, lysed cells, tissue debris from the biological
fluid, but the
centrifugal force applied is insufficient in magnitude, duration, or both, to
remove the
microvesicles. The biological fluid may require dilution to facilitate the
clarification.
[0124] The duration and magnitude of the centrifugal force used to clarify the
biological fluid
may vary according to a number of factors readily appreciated by one of
ordinary skill in the
art, including, for example, the biological fluid, the pH of the biological
fluid, the desired
purity of the isolated microvesicles, the desired size of the isolated
microvesicles, the desired
molecular weight of the microvesicles, and the like. In one embodiment, a
centrifugal force
of 2000 x g is applied to the biological fluid for 30 minutes.
[0125] The clarified biological fluid is contacted with a precipitation agent
to precipitate the
microvesicles. In one embodiment, the precipitation agent may be any agent
that surrounds
22
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
the microvesicles and displaces the water of solvation. Such precipitation
agents may be
selected from the group consisting of polyethylene glycol, dextran, and
polysaccharides.
[0126] In an alternate embodiment, the precipitation agent may cause
aggregation of the
microvesicles.
[0127] In an alternate embodiment, the precipitation agent is selected from
the group
consisting of calcium ions, magnesium ions, sodium ions, ammonium ions, iron
ions, organic
solvents such as ammonium sulfate, and flocculating agents, such as alginate.
[0128] The clarified biological fluid is contacted with the precipitation
agent for a period of
time sufficient to precipitate the microvesicles. The period of time
sufficient to precipitate
the microvesicles may vary according to a number of factors readily
appreciated by one of
ordinary skill in the art, including, for example, the biological fluid, the
pH of the biological
fluid, the desired purity of the isolated microvesicles, the desired size of
the isolated
microvesicles, the desired molecular weight of the microvesicles, and the
like. In one
embodiment, the period of time sufficient to precipitate the microvesicles is
6 hours.
[0129] In one embodiment, the clarified biological fluid is contacted with the
precipitation
agent for a period of time sufficient to precipitate the microvesicles at 4
C.
[0130] The concentration of the precipitation agent used to precipitate the
microvesicles from
a biological fluid may vary according to a number of factors readily
appreciated by one of
ordinary skill in the art, including, for example, the biological fluid, the
pH of the biological
fluid, the desired purity of the isolated microvesicles, the desired size of
the isolated
microvesicles, the desired molecular weight of the microvesicles, and the
like.
[0131] In one embodiment, the precipitation agent is polyethylene glycol. The
molecular
weight of polyethylene glycol used in the methods of the present invention may
be from
about 200 Da to about 10,000 Da. In one embodiment, the molecular weight of
polyethylene
glycol used in the methods of the present invention may be greater than 10,000
Da. In certain
embodiments, the molecular weight of polyethylene glycol used in the methods
of the present
invention is 10,000 Da or 20,000 Da. The choice of molecular weight may be
influenced by
a variety of factors including, for example, the viscosity of the biological
fluid, the desired
punity of the microvesicles, the desired size of the microvesicles, the
biological fluid used,
and the like. In one embodiment, the molecular weight of polyethylene glycol
used in the
23
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
methods of the present invention may be from about 200 Da to about 8,000 Da,
or is
approximately any of 200 Da, 300 Da, 400 Da, 600 Da, 1000 Da, 1450 Da, 1500
Da, 2000
Da, 3000 Da, 3350 Da, 4000 Da, 6000 Da, 8000 Da, 10000 Da, 20000 Da or 35000
Da or any
ranges or molecular weights in between.
[0132] In one embodiment, the molecular weight of polyethylene glycol used in
the methods
of the present invention is about 6000 Da.
[0133] In one embodiment, the average molecular weight of polyethylene glycol
used in the
methods of the present invention is about 8000 Da.
[0134] In one embodiment, the average molecular weight of polyethylene glycol
used in the
methods of the present invention is about 10000 Da.
[0135] In one embodiment, the average molecular weight of polyethylene glycol
used in the
methods of the present invention is about 20000 Da.
[0136] The concentration of polyethylene glycol used in the methods of the
present invention
may be from about 0.5% w/v to about 100% w/v. The concentration of
polyethylene glycol
used in the methods of the present invention may be influenced by a variety of
factors
including, for example, the viscosity of the biological fluid, the desired
purity of the
microvesicles, the desired size of the microvesicles, the biological fluid
used, and the like.
[0137] In certain embodiments, the polyethylene glycol is used in the
concentration of the
present invention at a concentration between about 5% and 25% w/v. In certain
embodiments, the concentration is about 5%, 6%, 7%, 8%, 9%, 10%, 11%, 12%,
13%, 14%,
or 15%, or a range between any two of these values.
[0138] In one embodiment, the concentration of polyethylene glycol used in the
methods of
the present invention is about 8.5% w/v.
[0139] in one embodiment, the concentration of polyethylene glycol used in the
methods of
the present invention is about 6% w/v.
[0140] In one embodiment, polyethylene glycol having an average molecular
weight of 6000
Da is used, at a concentration of 8.5% w/v. In one embodiment, the
polyethylene glycol is
diluted in 0.4M sodium chloride.
24
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
[0141] In one embodiment, the concentration of the polyethylene glycol used in
the methods
of the present invention is inversely proportional to the average molecular
weight of the
polyethylene glycol. For example, in one embodiment, polyethylene glycol
having an
average molecular weight of 4000 Da is used, at a concentration of 20% w/v. In
an alternate
embodiment, polyethylene glycol having an average molecular weight of 8000 Da
is used, at
a concentration of 10% w/v. In an alternate embodiment, polyethylene glycol
having an
average molecular weight of 20000 Da is used, at a concentration of 4% w/v.
[0142] In one embodiment, the precipitated microvesicles are collected by the
application of
centrifugal force. The centrifugal force is sufficient and applied for a
duration sufficient to
cause the microvesicles to form a pellet, but insufficient to damage the
microvesicles.
[0143] The duration and magnitude of the centrifugal force used to precipitate
the
microvesicles from a biological fluid may vary according to a number of
factors readily
appreciated by one of ordinary skill in the art, including, for example, the
biological fluid, the
pH of the biological fluid, the desired purity of the isolated microvesicles,
the desired size of
the isolated microvesicles, the desired molecular weight of the microvesicles,
and the like. In
one embodiment, the precipitated microvesicles are collected by the
application of a
centrifugal force of 10000 x g for 60 minutes.
[0144] The precipitated microvesicles may be washed with any liquid, provided
the liquid
does not affect the functional and/or structural integrity of the
microvesicles. One of ordinary
skill in the art may readily select a suitable liquid. Liquids may be, for
example, phosphate
buffered saline, cell culture medium, and the like.
[0145] In one embodiment, the washing step removes the precipitating agent. In
one
embodiment, the microvesicles are washed via centrifugal filtration, using a
filtration device
with a 100 kDa molecular weight cut oft
[0146] The isolated microvesicles may be suspended with any liquid, provided
the liquid
does not affect the functional and/or structural integrity of the
microvesicles. One of ordinary
skill in the art may readily select a suitable liquid. Liquids may be, for
example, phosphate
buffered saline, cell culture medium, and the like.
[0147] In one embodiment, the isolated microvesicles may be further processed.
The further
processing may be the isolation of a microvesicle of a specific size.
Alternatively, the further
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
processing may be the isolation of microvesicles of a particular size range.
Alternatively, the
further processing may be the isolation of a microvesicle of a particular
molecular weight.
Alternatively, the further processing may be the isolation of microvesicles of
a particular
molecular weight range. Alternatively, the further processing may be the
isolation of a
microvesicle exhibiting or containing a specific molecule.
[0148] In one embodiment, the microvesicles of the present invention are
further processed
to isolate a preparation of microvesicles having a size of about 2 nm to about
1000 nm as
determined by electron microscopy. In an alternate embodiment, the
microvesicles of the
present invention are further processed to isolate a preparation of
microvesicles having a size
of about 2 nm to about 500 nm as determined by electron microscopy. In an
alternate
embodiment, the microvesicles of the present invention are further processed
to isolate a
preparation of microvesicles having a size of about 2 nm to about 400 nm as
determined by
electron microscopy. In an alternate embodiment, the microvesicles of the
present invention
are further processed to isolate a preparation of microvesicles having a size
of about 2 nm to
about 300 nm as determined by electron microscopy. In an alternate embodiment,
the
microvesicles of the present invention are further processed to isolate a
preparation of
microvesicles having a size of about 2 nm to about 200 nm as determined by
electron
microscopy. In an alternate embodiment, the microvesicles of the present
invention are
further processed to isolate a preparation of microvesicles having a size of
about 2 nm to
about 100 nm as determined by electron microscopy. In an alternate embodiment,
the
microvesicles of the present invention are further processed to isolate a
preparation of
microvesicles having a size of about 2 nm to about 50 mu as determined by
electron
microscopy. In an alternate embodiment, the microvesicles of the present
invention are
further processed to isolate a preparation of microvesicles having a size of
about 2 nm to
about 20 nm as determined by electron microscopy. In an alternate embodiment,
the
microvesicles of the present invention are further processed to isolate a
preparation of
microvesicles having a size of about 2 run to about 10 nm as determined by
electron
microscopy.
[0149] in one embodiment, the subsequent purification is performed using a
method selecting
from the group consisting of immunoaffinity, HPLC, tangential flow filtration,
phase
separation/partitioning, and microfluidics.
26
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
[0150] In one embodiment, the isolated microvesicles are further processed to
analyze the
molecules exhibited on, or contained within the microvesicles. The molecules
analyzed are
selected from the group consisting of nucleic acid, carbohydrate, lipid, small
molecules, ions,
metabolites, protein, and combinations thereof.
[0151] Biological fluid comprising cell culture medium conditioned using
cultured cells: In
one embodiment, microvesicles are obtained from medium conditioned using
cultured cells.
Any cultured cell, or population of cells may be used in the methods of the
present invention.
The cells may be stem cells, primary cells, cell lines, tissue or organ
explants, or any
combination thereof. The cells may be allogeneic, autologous, or xenogeneic in
origin.
[0152] In one embodiment, the cells are cells derived from bone-marrow
aspirate. In one
embodiment, the cells derived from bone marrow aspirate are bone marrow-
derived
mesenchymal stem cells. In one embodiment, the cells derived from bone marrow
aspirate
are mononuclear cells. In one embodiment, the cells derived from bone marrow
aspirate are
a mixture of mononuclear cells and bone marrow-derived mesenchymal stem cells.
[0153] In one embodiment, bone marrow-derived mesenchymal stem cells are
isolated from
bone marrow aspirate by culturing bone marrow aspirate in plastic tissue
culture flasks for a
period of time of up to about 4 days, followed by a wash to remove the non-
adherent cells.
[0154] in one embodiment, mononuclear cells are isolated from bone marrow
aspirate by
low- density centrifugation using a ficoll gradient, and collecting the
mononuclear cells at the
interface.
[0155] In one embodiment, prior to isolation of microvesicles according to the
methods of
the present invention, the cells are cultured, grown or maintained at an
appropriate
temperature and gas mixture (typically, 37 C, 5% CO2 for mammalian cells) in
a cell
incubator. Culture conditions vary widely for each cell type, and are readily
determined by
one of ordinary skill in the art.
[0156] In one embodiment, one, or more than one culture condition is varied.
In one
embodiment, this variation results in a different phenotype.
[0157] In one embodiment, where the cells require serum in their culture
medium, to begin
the microvesicle isolation procedure, the cell culture medium is supplemented
with
27
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
microvesicle- free serum and then added to the cells to be conditioned. The
microvesicles are
collected from the conditioned cell culture medium. Serum may be depleted by
any suitable
method, such as, for example, ultracentrifugation, filtration, precipitation,
and the like. The
choice of medium, serum concentration, and culture conditions are influenced
by a variety of
factors readily appreciated by one of ordinary skill in the art, including,
for example, the cell
type being cultured, the desired purity of the microvesicles, the desired
phenotype of the
cultured cell, and the like. In one embodiment, the cell culture medium that
is conditioned
for the microvesicle isolation procedure is the same type of cell culture
medium that the cells
were grown in, prior to the microvesicle isolation procedure.
[0158] In one embodiment, to begin the microvesicle isolation procedure, the
cell culture
medium is removed, and serum-free medium is added to the cells to be
conditioned. The
microvesicles are then collected from the conditioned serum free medium. The
choice of
medium, and culture conditions are influenced by a variety of factors readily
appreciated by
one of ordinary skill in the art, including, for example, the cell type being
cultured, the
desired purity of the microvesicles, the desired phenotype of the cultured
cell, and the like.
In one embodiment, the serum-free medium is supplemented with at least one
additional
factor that promotes or enhances the survival of the cells in the serum free
medium. Such
factor may, for example, provide trophic support to the cells, inhibit, or
prevent amtosis of
the cells.
[0159] The cells are cultured in the culture medium for a period of time
sufficient to allow
the cells to secrete microvesicles into the culture medium. The period of time
sufficient to
allow the cells to secrete microvesicles into the culture medium is influenced
by a variety of
factors readily appreciated by one of ordinary skill in the art, including,
for example, the cell
type being cultured, the desired purity of the microvesicles, the desired
phenotype of the
cultured cell, desired yield of microvesicles, and the like.
[0160] The microvesicles are then removed from the culture medium by the
methods of the
present invention.
[0161] In one embodiment, prior to the microvesicle isolation procedure, the
cells are treated
with at least one agent selected from the group consisting of an anti-
inflammatory compound,
an anti-apoptotic compound, an inhibitor of fibrosis, a compound that is
capable of enhancing
angiogenesis, an immunosuppressive compound, a compound that promotes survival
of the
28
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
cells, a chemotherapeutic, a compound capable of enhancing cellular migration,
a neurogenic
compound, and a growth factor. In one embodiment, while the cells are being
cultured in the
medium from which the microvesicles are collected, the cells are treated with
at least one
agent selected from the group consisting of an anti-inflammatory compound, an
anti-
apoptotic compound, an inhibitor of fibrosis, a compound that is capable of
enhancing
angiogenesis, an inununosuppressive compound, a compound that promotes
survival of the
cells, and a growth factor.
[0162] In one embodiment, the anti-inflammatory compound may be selected from
the
compounds disclosed in U. S. Patent. No. 6,509,369.
[0163] In one embodiment, the anti-apoptotic compound may be selected from the
compounds disclosed in U. S. Patent. No. 6,793,945.
[0164] In one embodiment, the inhibitor of fibrosis may be selected from the
compounds
disclosed in U. S. Patent. No. 6,331,298.
[0165] In one embodiment, the compound that is capable of enhancing
angiogenesis may be
selected from the compounds disclosed in U. S. Patent Application 2004/0220393
or U. S.
Patent Application 2004/0209901.
[0166] In one embodiment, the immunosuppressive compound may be selected from
the
compounds disclosed in U. S. Patent Application 2004/0171623.
[0167] In one embodiment, the compound that promotes survival of the cells may
be selected
from the compounds disclosed in U. S. Patent Application 2010/0104542.
[0168] In one embodiment, the growth factor may be at least one molecule
selected from the
group consisting of members of the TGF-I3 family, including TGF-I31, 2, and 3,
bone
morphogenic proteins (BMP-2, -3,4, -5, -6, -7, -11, -12, and -13), fibroblast
growth factors-1
and -2, platelet-derived growth factor-AA, -AB, and -BB, platelet rich plasma,
insulin growth
factor (IGF-I, II) growth differentiation factor (GDF-5, -6, -8, -10, -15),
vascular endothelial
cell- derived growth factor (VEGF), pleiotrophin, endothelin, among others.
Other
pharmaceutical compounds can include, for example, nicotinamide, hypoxia
inducible factor
1 -alpha, glucagon like peptide-1 (GLP-1), GLP-1 and GLP-2 mimetibody, and II,
Exendin-4,
nodal, noggin, NGF, retinoic acid, parathyroid hormone, tenascin-C,
tropoelastin, thrombin-
29
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
derived peptides, cathelicidins, defensins, laminin, biological peptides
containing cell- and
heparin- binding domains of adhesive extracellular matrix proteins such as
fibronectin and
vitronectin, and MAPK inhibitors, such as, for example, compounds disclosed in
U. S. Patent
Application 2004/ 0209901 and U. S. Patent Application 2004/0132729.
[0169] In one embodiment, microvesicles are isolated from a biological fluid
comprising cell
culture medium conditioned using a culture of bone marrow-derived mesenchymal
stem cells
comprising the steps of:
a) obtaining a population of bone marrow-derived mesenchymal stem cells and
seeding flasks at a 1:4 dilution of cells,
b) culturing the cells in medium until the cells are 80 to 90% confluent,
c) removing and clarifying the medium to remove cellular debris,
d) precipitating the microvesicles by adding a precipitating agent to the
clarified
culture medium,
e) collecting the precipitated microvesicles and washing the material to
remove the
precipitating agent, and
f) suspending the washed microvesicles in a solution for storage or subsequent
use.
[0170] in one embodiment, microvesicles are isolated from a biological fluid
comprising cell
culture medium conditioned using a culture of bone marrow-derived mononuclear
cells
comprising the steps of:
a) obtaining a population of bone marrow-derived mononuclear cells and seeding
flasks at a 1:4 dilution of cells,
b) culturing the cells in medium until the cells are 80 to 90% confluent,
c) removing and clarifying the medium to remove cellular debris,
d) precipitating the microvesicles by adding a precipitating agent to the
clarified
culture medium,
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
e) collecting the precipitated microvesicles and washing the material to
remove the
precipitating agent, and
I) suspending the washed microvesicles in a solution for storage or subsequent
use.
[0171] In one embodiment, the bone marrow-derived mesenchymal stem cells are
cultured in
medium comprising a-MEM supplemented with 20% fetal bovine serum and 1%
penicillin/streptomycin/glutamine at 37 C in 95% humidified air and 5% CO2.
[0172] In one embodiment, the bone marrow-derived mononuclear cells are
cultured in
medium comprising a-MEM supplemented with 20% fetal bovine serum and 1%
penicillin/streptomycin/glutamine at 37 C in 95% humidified air and 5% CO,.
[0173] In one embodiment, the medium is clarified by centrifugation.
[0174] In one embodiment, the precipitating agent is polyethylene glycol
having an average
molecular weight of 6000. In one embodiment, the polyethylene glycol is used
at a
concentration of about 8.5 w/v %. In one embodiment, the polyethylene glycol
is diluted in a
sodium chloride solution having a final concentration of 0.4 M.
[0175] In one embodiment, the precipitated microvesicles are collected by
centrifugation.
[0176] In one embodiment, the isolated microvesicles are washed via
centrifugal filtration,
using a membrane with a 100 kDa molecular weight cut-off, using phosphate
buffered saline.
[0177] Biological fluid comprising plasma: In one embodiment, microvesicles
are obtained
from plasma. The plasma may be obtained from a healthy individual, or,
alternatively, from
an individual with a particular disease phenotype.
[0178] In one embodiment, microvesicles are isolated from a biological fluid
comprising
plasma comprising the steps of:
a) obtaining plasma and diluting the plasma with cell culture medium,
b) precipitating the microvesicles by adding a precipitating agent to the
diluted
plasma,
31
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
c) collecting the precipitated microvesicles and washing the material to
remove the
precipitating agent, and
d) suspending the washed microvesicles in a solution for storage or subsequent
use.
[0179] In one embodiment, the plasma is diluted 1: 10 with culture medium. In
one
embodiment, the culture medium is a-MEM.
[0180] In one embodiment, the precipitating agent is polyethylene glycol
having an average
molecular weight of 6000. In one embodiment, the polyethylene glycol is used
at a
concentration of about 8.5 w/v %. In one embodiment, the polyethylene glycol
is diluted in a
sodium chloride solution having a final concentration of 0.4 M.
[0181] In one embodiment, the precipitated microvesicles are collected by
centrifugation.
[0182] In one embodiment, the isolated microvesicles are washed via
centrifugal filtration,
using a membrane with a 100 kDa molecular weight cut-off, using phosphate
buffered saline.
[0183] Biological fluid comprising bone marrow aspirate: In one embodiment,
microvesicles
are obtained from bone marrow aspirate. In one embodiment, microvesicles are
obtained
from the cellular fraction of the bone marrow aspirate. In one embodiment,
microvesicles are
obtained from the acellular fraction of the bone marrow aspirate.
[0184] in one embodiment, microvesicles are obtained from cells cultured from
bone marrow
aspirate. In one embodiment, the cells cultured from bone marrow aspirate are
used to
condition cell culture medium, from which the microvesicles are isolated.
[0185] In one embodiment, microvesicles are isolated from a biological fluid
comprising
bone marrow aspirate comprising the steps of:
a) obtaining bone marrow aspirate and separating the bone marrow aspirate into
an
acellular portion and a cellular portion,
b) diluting the acellular portion,
c) clarifying the diluted acellular portion to remove cellular debris,
32
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
d) precipitating the microvesicles in the acellular portion by adding a
precipitating
agent to the diluted acellular portion,
e) collecting the precipitated microvesicles and washing the material to
remove the
precipitating agent, and
f) suspending the washed microvesicles in a solution for storage or subsequent
use.
[0186] in one embodiment, the acellular portion is diluted 1: 10 with culture
medium.
[0187] in one embodiment, the culture medium is a-MEM.
[0188] In one embodiment, the diluted acellular portion is claiified by
centrifugation.
[0189] In one embodiment, the precipitating agent is polyethylene glycol
having an average
molecular weight of 6000. In one embodiment, the polyethylene glycol is used
at a
concentration of about 8.5 w/v %. In one embodiment, the polyethylene glycol
is diluted in a
sodium chloride solution having a final concentration of 0.4 M.
[0190] In one embodiment, the precipitated microvesicles are collected by
centrifugation.
[0191] In one embodiment, the isolated microvesicles are washed via
centrifugal filtration,
using a membrane with a 100 kDa molecular weight cut-off, using phosphate
buffered saline.
[0192] In one embodiment the cellular portion is further processed to isolate
and collect cells.
In one embodiment, the cellular portion is further processed to isolate and
collect bone
marrow- derived mesenchymal stem cells. In one embodiment, the cellular
portion is further
processed to isolate and collect bone marrow-derived mononuclear cells. In one
embodiment, the cellular portion is used to condition medium, from which
microvesicles may
later be derived.
[0193] In one embodiment, microvesicles are isolated from the cellular
portion. The cellular
portion may be incubated for a period of time prior to the isolation of the
microvesicles.
Alternatively, the microvesicles may be isolated from the cellular portion
immediately after
the cellular portion is collected.
[0194] In one embodiment, the cellular portion is also treated with at least
one agent selected
from the group consisting of an anti-inflammatory compound, an anti-apoptotic
compound,
33
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
an inhibitor of fibrosis, a compound that is capable of enhancing
angiogenesis, an
immunosuppressive compound, a compound that promotes survival of the cells, a
chemotherapeutic, a compound capable of enhancing cellular migration, a
neurogenic
compound, and a growth factor.
[0195] In one embodiment, the anti-inflammatory compound may be selected from
the
compounds disclosed in U. S. Patent. No. 6,509,369.
[0196] In one embodiment, the anti-apoptotic compound may be selected from the
compounds disclosed in U. S. Patent. No. 6,793,945.
[0197] In one embodiment, the inhibitor of fibrosis may be selected from the
compounds
disclosed in U. S. Patent. No. 6,331,298.
[0198] In one embodiment, the compound that is capable of enhancing
angiogenesis may be
selected from the compounds disclosed in U. S. Patent Application 2004/0220393
or U. S.
Patent Application 2004/0209901.
[0199] In one embodiment, the immunosuppressive compound may be selected from
the
compounds disclosed in U. S. Patent Application 2004/0171623.
[0200] In one embodiment, the compound that promotes survival of the cells may
be selected
from the compounds disclosed in U. S. Patent Application 2010/0104542.
[0201] In one embodiment, the growth factor may be at least one molecule
selected from the
group consisting of members of the TGF-13 family, including TGF-31, 2, and 3,
bone
morphogenic proteins (BMP-2, -3,-4, -5, -6, -7, -11, -12, and -13), fibroblast
growth factors-1
and -2, platelet-derived growth factor-AA, -AB, and -BB, platelet rich plasma,
insulin growth
factor (IGF-I, II) growth differentiation factor (GDF-5, -6, -8, -10, -15),
vascular endothelial
cell- derived growth factor (VEGF), pleiotrophin, endothelin, among others.
Other
pharmaceutical compounds can include, for example, nicotinamide, hypoxia
inducible factor
1-alpha, glucagon like peptide-1 (GLP-1), GLP-1 and GLP-2 mimetibody, and II,
Exendin-4,
nodal, noggin, NGF, retinoic acid, parathyroid hormone, tenascin-C,
tropoelastin, thrombin-
derived peptides, cathelicidins, defensins, laminin, biological peptides
containing cell- and
heparin-binding domains of adhesive extracellular matrix proteins such as
fibronectin and
vitronectin, and MAPK inhibitors, such as, for example, compounds disclosed in
U. S. Patent
34
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
Application 2004/0209901 and U. S. Patent Application 2004/0132729. In one
embodiment,
the cellular portion is cultured under hypoxic conditions. In one embodiment,
the cellular
portion is heat- shocked.
[0202] Biological fluid comprising urine: In one embodiment, microvesicles are
obtained
from urine. The urine may be obtained from a healthy individual, or,
alternatively, from an
individual with a particular disease phenotype.
[0203] In one embodiment, microvesicles are isolated from a biological fluid
comprising
urine comprising the steps of:
a) obtaining a urine sample,
b) clarifying the urine to remove cellular debris,
c) precipitating the microvesicles by adding a precipitating agent to the
clarified urine,
d) collecting the precipitated microvesicles and washing the material to
remove the
precipitating agent, and
e) suspending the washed microvesicles in a solution for storage or subsequent
use.
[0204] In one embodiment, the urine is clarified by centrifugation.
[0205] in one embodiment, the precipitating agent is polyethylene glycol
having an average
molecular weight of 6000. In one embodiment, the polyethylene glycol is used
at a
concentration of about 8.5 w/v %. In one embodiment, the polyethylene glycol
is diluted in a
sodium chloride solution having a final concentration of 0.4 M.
[0206] In one embodiment, the precipitated microvesicles are collected by
centrifugation.
[0207] In one embodiment, the isolated microvesicles are washed via
centrifugal filtration,
using a membrane with a 100 kDa molecular weight cut-off, using phosphate
buffered saline.
[0208] In an alternate embodiment of the present invention, the biological
fluids are clarified
by filtration. In an alternate embodiment, the precipitated microvesicles are
collected by
filtration. In an alternate embodiment, the biological fluids are clarified
and the precipitated
microvesicles are collected by filtration. In certain embodiments, filtration
of either the
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
biological fluid, and/or the precipitated microvesicles required the
application of an external
force. The external force may be gravity, either normal gravity or centrifugal
force.
Alternatively, the external force may be suction.
[0209] in one embodiment, the present embodiment provides an apparatus to
facilitate the
clarification of the biological fluid by filtration. In one embodiment, the
present invention
provides an apparatus to facilitate collection of the precipitated
microvesicles by filtration. In
one embodiment, the present invention provides an apparatus that facilitates
the clarification
of the biological fluid and the collection of the precipitated microvesicles
by filtration. In one
embodiment, the apparatus also washes the microvesicles.
[0210] In one embodiment, the apparatus is the apparatus shown in Figure 4. In
this
embodiment, the biological fluid is added to the inner chamber. The inner
chamber has a first
filter with a pore size that enables the microvesicles to pass, while
retaining any particle with
a size greater than a mierovesicle in the inner chamber. In one embodiment,
the pore size of
the filter of the inner chamber is 1 gm. In this embodiment, when the
biological fluid passed
from the inner chamber through the filter, particles greater than 1 pm are
retained in the inner
chamber, and all other particles collect in the region between the bottom of
the inner chamber
and a second filter.
[0211] The second filter has a pore size that does not allow microvesicles to
pass. In one
embodiment, the pore size of the second filter of the inner chamber is 0.01
pm. In this
embodiment, when the biological fluid passed through the second filter, the
microvesicles are
retained in the region between the bottom of the inner chamber and the second
filter, and all
remaining particles and fluid collect in the bottom of the apparatus.
[0212] One of ordinary skill in the art can readily appreciate that the
apparatus can have more
than two filters, of varying pore sizes to select for microvesicles of desired
sizes, for example.
[0213] In one embodiment, a precipitating agent is added to the biological
fluid in the inner
chamber. In one embodiment, a precipitating agent is added to the filtrate
after it has passed
through the first filter. The filter membranes utilized by the apparatus of
the present
invention may be made from any suitable material, provided the filter membrane
does not
react with the biological fluid, or bind with components within the biological
fluid. For
example, the filter membranes may be made from a low bind material, such as,
for example,
36
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
polyethersulfone, ny10n6, polytetrafluoroethylene, polypropylene, zeta
modified glass
microfiber, cellulose nitrate, cellulose acetate, polyvinylidene fluoride,
regenerated cellulose.
The Microvesicles of the Present Invention
[0214] In one embodiment, the microvesicles of the present invention have a
size of about 2
nm to about 5000 nm as determined by electron microscopy. In an alternate
embodiment, the
microvesicles of the present invention have a size of about 2 urn to about
1000 tun as
determined by electron microscopy. In an alternate embodiment, the
microvesicles of the
present invention have a size of about 2 urn to about 500 nm as determined by
electron
microscopy. In an alternate embodiment, the microvesicles of the present
invention have a
size of about 2 nm to about 400 nm as determined by electron microscopy. In an
alternate
embodiment, the microvesicles of the present invention have a size of about 2
nm to about
300 nm as determined by electron microscopy. In an alternate embodiment, the
microvesicles of the present invention have a size of about 2 urn to about 200
nm as
determined by electron microscopy. In an alternate embodiment, the
microvesicles of the
present invention have a size of about 2 nm to about 100 nm as determined by
electron
microscopy. In an alternate embodiment, the microvesicles of the present
invention have a
size of about 2 nm to about 50 nm as determined by electron microscopy. In an
alternate
embodiment, the microvesicles of the present invention have a size of about 2
nm to about 20
rim as determined by electron microscopy. In an alternate embodiment, the
microvesicles of
the present invention have a size of about 2 urn to about 10 nm as determined
by electron
microscopy.
[0215] In one embodiment, the microvesicles of the present invention have a
molecular
weight of at least 100 kDa.
[0216] Microvesicles isolated according to the methods of the present
invention may be used
for therapies. Alternatively, microvesicles isolated according to the methods
of the present
invention may be used for diagnostic tests. Alternatively, the microvesicles
of the present
invention may be used to alter or engineer cells or tissues. In the case where
the
microvesicles of the present invention are used to alter or engineer cells or
tissues, the
microvesicles may be loaded, labeled with RNA, DNA, lipids, carbohydrates,
protein, drugs,
small molecules, metabolites, or combinations thereof, that will alter or
engineer a cell or
tissue. Alternatively, the microvesicles may be isolated from cells or tissues
that express
37
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
and/or contain the RNA, DNA, lipids, carbohydrates, protein, drugs, small
molecules,
metabolites, or combinations thereof.
Use of the Microvesicles of the Present Invention in Diagnostic Tests
[0217] The microvesicles of the present invention can be used in a diagnostic
test that detects
biomarkers that identify particular phenotypes such as, for example, a
condition or disease, or
the stage or progression of a disease. Biomarkers or markers from cell-of-
origin specific
microvesicles can be used to determine treatment regimens for diseases,
conditions, disease
stages, and stages of a condition, and can also be used to determine treatment
efficacy.
Markers from cell-of-origin specific microvesicles can also be used to
identify conditions of
diseases of unknown origin.
[0218] As used herein, the term "biomarker" refers to an indicator of a
biological state. It is
a characteristic that is objectively measured and evaluated as an indicator of
normal
biological processes, pathogenic processes, or pharmacologic responses to a
therapeutic
intervention. One or more biomarkers of microvesicle can be assessed for
characterizing a
phenotype. The biomarker can be a metabolite, a nucleic acid, peptide,
protein, lipid,
antigen, carbohydrate or proteoglycan, such as DNA or RNA. The RNA can be
mRNA,
miRNA, snoRNA, snRNA, rRNAs, tRNAs, siRNA, hnRNA, or shRNA.
[0219] A phenotype in a subject can be characterized by obtaining a biological
sample from
the subject and analyzing one or more microvesicles from the sample. For
example,
characterizing a phenotype for a subject or individual may include detecting a
disease or
condition (including pre- symptomatic early stage detecting), determining the
prognosis,
diagnosis, or theranosis of a disease or condition, or determining the stage
or progression of a
disease or condition. Characterizing a phenotype can also include identifying
appropriate
treatments or treatment efficacy for specific diseases, conditions, disease
stages and condition
stages, predictions and likelihood analysis of disease progression,
particularly disease
recurrence, metastatic spread or disease relapse. A phenotype can also be a
clinically distinct
type or subtype of a condition or disease, such as a cancer or tumor.
Phenotype
determination can also be a determination of a physiological condition, or an
assessment of
organ distress or organ rejection, such as post-transplantation. The products
and processes
described herein allow assessment of a subject on an individual basis, which
can provide
benefits of more efficient and economical decisions in treatment.
38
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
[0220] The phenotype can be any phenotype listed in U.S. Patent 7,897,356. The
phenotype
can be a tumor, neoplasm, or cancer. A cancer detected or assessed by products
or processes
described herein includes, but is not limited to, breast cancer, ovarian
cancer, lung cancer,
colon cancer, hyperplastic polyp, adenoma, colorectal cancer, high grade
dysplasia, low grade
dysplasia, prostatic hyperplasia, prostate cancer, melanoma, pancreatic
cancer, brain cancer
(such as a glioblastoma), hematological malignancy, hepatocellular carcinoma,
cervical
cancer, endometrial cancer, head and neck cancer, esophageal cancer,
gastrointestinal stromal
tumor (GIST), renal cell carcinoma (RCC) or gastric cancer. The colorectal
cancer can be
CRC Dukes B or Dukes C-D. The hematological malignancy can be B-Cell Chronic
Lymphocytic Leukemia, B-Cell Lymphoma-DLBCL, B-Cell Lymphoma-DLBCL-germinal
center-like, B-Cell Lymphoma-DLBCL-activated B-cell-like, and Burkitt's
lymphoma. The
phenotype may also be a premalignant condition, such as Barrett's Esophagus.
[0221] The phenotype can also be an inflammatory disease, immune disease, or
autoimmune
disease. For example, the disease may be inflammatory bowel disease (IBD),
Crohn's
disease (CD), ulcerative colitis (UC), pelvic inflammation, vasculitis,
psoriasis, diabetes,
autoimmune hepatitis, Multiple Sclerosis, Myasthenia Gravis, Type I diabetes,
Rheumatoid
Arthritis, Psoriasis, Systemic Lupus Erythematosis (SLE), Hashimoto's
Thyroiditis, Grave's
disease, Ankylosing Spondylitis Sjorgen's Disease, CREST syndrome,
Scleroderma,
Rheumatic Disease, organ rejection, graft versus host disease, Primary
Sclerosing
Cholangitis, or sepsis. In certain exemplary embodiments, the disease is EB,
e.g., RDEB
and/or DDEB, junctional EB, EB simplex and/or acquired forms of EB.
[0222] The phenotype can also be a cardiovascular disease, such as
atherosclerosis,
congestive heart failure, vulnerable plaque, stroke, or ischemia. The
cardiovascular disease
or condition can be high blood pressure, stenosis, vessel occlusion or a
thrombotic event.
[0223] The phenotype can also be a neurological disease, such as Multiple
Sclerosis (MS),
Parkinson's Disease (PD), Alzheimer's Disease (AD), schizophrenia, bipolar
disorder,
depression, autism, Prion Disease, Pick's disease, dementia, Huntington
disease (HD),
Down's syndrome, cerebrovascular disease, Rasmussen's encephalitis, viral
meningitis,
neuropsychiatric systemic lupus erythematosus (NPSLE), amyotrophic lateral
sclerosis,
Creutzfeldt-Jacob disease, Gerstmann-Straussler-Scheinker disease,
transmissible spongiform
encephalopathy, ischemic reperfusion damage (e.g. stroke), brain trauma,
microbial infection,
39
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
or chronic fatigue syndrome. The phenotype may also be a condition such as
fibromyalgia,
chronic neuropathic pain, or peripheral neuropathic pain.
[0224] The phenotype may also be an infectious disease, such as a bacterial,
viral or yeast
infection. For example, the disease or condition may be Whipple's Disease,
Prion Disease,
cirrhosis, methicillin-resistant staphylococcus aureus, HIV, hepatitis,
syphilis, meningitis,
malaria, tuberculosis, or influenza. Viral proteins, such as HIV or HCV-like
particles can be
assessed in an exosome, to characterize a viral condition.
[0225] The phenotype can also be a perinatal or pregnancy related condition
(e.g.,
preeclampsia or preterm birth), metabolic disease or condition, such as a
metabolic disease or
condition associated with iron metabolism. The metabolic disease or condition
can also be
diabetes, inflammation, or a perinatal condition.
[0226] The phenotype may be detected via any suitable assay method, such as,
for example,
western blots, ELISA, PCR, and the like. The assay methods may be combined to
perform
multiplexed analysis of more than one phenotype. Examples of assay methods
that may be
applied to the microvesicles of the present invention are disclosed in PCT
Applications
W02009092386A3 and W02012108842A1.
[0227] In the case where the biomarker is RNA, the RNA may be isolated from
the
microvesicles of the present invention by the methods disclosed in U.S. Patent
8,021,847.
[0228] In one embodiment, the microvesicles of the present invention are
utilized in a
diagnostic test for the diseases disclosed in U.S. Patent 7,897,356.
[0229] In one embodiment, the microvesicles of the present invention are
utilized in a
diagnostic test for cancer according to the methods disclosed in U.S. Patent
8,211,653.
[0230] In one embodiment, the microvesicles of the present invention are
utilized in a
diagnostic test for cancer according to the methods disclosed in U.S. Patent
8,216,784.
[0231] In one embodiment, the microvesicles of the present invention are
utilized in a
diagnostic test for prostate cancer according to the methods disclosed in U.S.
Patent
8,278,059. In one embodiment, the microvesicles of the present invention are
utilized in a
diagnostic test for the prognosis for cancer survival according to the methods
disclosed in
U.S. Patent 8,343,725.
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
[0232] In one embodiment, the microvesicles of the present invention are
utilized in a
diagnostic test for the prognosis for cancer survival according to the methods
disclosed in
U.S. Patent 8,349,568.
[0233] in one embodiment, the microvesicles of the present invention are
utilized in a
diagnostic test for acute lymphomic leukemia according to the methods
disclosed in U.S.
Patent 8,349,560.
[0234] In one embodiment, the microvesicles of the present invention are
utilized in a
diagnostic test for acute lymphomic leukemia according to the methods
disclosed in U.S.
Patent 8,349,561.
[0235] In one embodiment, the microvesicles of the present invention are
utilized in a
diagnostic test for hepatitis C virus. In one embodiment, hepatitis C viral
RNA is extracted
from the microvesicles of the present invention according to the methods
described in U.S.
Patent 7,807,438 to test for the presence of hepatitis C virus in a patient.
[0236] In one embodiment, the microvesicles of the present invention are
utilized in a
diagnostic test for determining the response of a patient to cancer therapy
according to the
methods disclosed in U.S. Patent 8,349,574.
[0237] In one embodiment, the microvesicles of the present invention are
utilized in a
diagnostic test for diagnosing malignant tumors according to the methods
disclosed in U.S.
Patent Application US20120058492A1.
[0238] In one embodiment, the microvesicles of the present invention are
utilized in a
diagnostic test for diagnosing cancer or adverse pregnancy outcome according
to the methods
disclosed in U.S. Patent Application U520120238467A1.
[0239] In one embodiment, the microvesicles of the present invention are
utilized in a
diagnostic test for HIV in urine according to the methods disclosed in U.S.
Patent Application
US20120214151A1. In one embodiment, the microvesicles of the present invention
are
utilized in a diagnostic test for cardiovascular events according to the
methods disclosed in
U.S. Patent Application US20120309041A1.
41
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
[0240] In one embodiment, the microvesicles of the present invention are
utilized in a
diagnostic test for cardiovascular events according to the methods disclosed
in PCT
Application W020121 10099A1.
[0241] in one embodiment, the microvesicles of the present invention are
utilized in a
diagnostic test for cardiovascular events according to the methods disclosed
in PCT
Application W02012126531A1.
[0242] In one embodiment, the microvesicles of the present invention are
utilized in a
diagnostic test for cardiovascular events according to the methods disclosed
in PCT
Application W02013110253A3.
[0243] In one embodiment, the microvesicles of the present invention are
utilized in a
diagnostic test for melanoma according to the methods disclosed in PCT
Application
W02012135844A2.
[0244] In one embodiment, the microvesicles of the present invention are
utilized in a
diagnostic test for metastatic melanoma by testing microvesicles isolated
according to the
methods of the present invention for the presence of the biomarker BRAF. The
presence of
BRAF may be determined via western blot, or, alternatively, by PCR. In one
embodiment,
the metastatic melanoma test is capable of detecting wild type and malignant
BRAF. In one
embodiment, the metastatic melanoma test is capable of detecting splice
variants of the
malignant BRAF.
[0245] In one embodiment, the microvesicles that are utilized in the
diagnostic test for
metastatic melanoma are isolated using a method comprising the steps outlined
in Figure 3.
[0246] In one embodiment, microvesicles are obtained from a patient wishing to
be
diagnosed for the presence of metastatic melanoma. In one embodiment, the
microvesicles
are obtained from the patient's plasma.
[0247] In one embodiment, the presence of metastatic melanoma is determined
via PCR,
using one of the two primer sets below:
Sequence 1:
Forward: AGACCTCACAGTAAAAATAGGTGA (SEQ ID NO: 1)
Reverse: CTGATGGGACCCACTCCATC (SEQ ID NO: 2)
Amplicon length: 70
42
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
Sequence 2:
Forward: GAAGACCTCACAGTAAAAATAGGTG (SEQ ID NO: 3)
Reverse: CTGATGGGACCCACTCCATC (SEQ ID NO: 4)
Amplicon length: 82
f02481 In another embodiment, the presence of metastatic melanoma is
determined via
western blot, using the mouse anti-BRAF V600E antibody (NewEast Biosciences,
Malvern,
PA).
Use of the Microvesieles of the Present Invention in Therapies
[0249] The microvesicles of the present invention can be used as a therapy to
treat a disease.
[0250] In one embodiment, the microvesicles of the present invention are used
as vaccines
according to the methods described in U.S. Patent Application US20030198642A1.
[0251] In one embodiment, the microvesicles of the present invention are used
to modulate or
suppress a patient's immune response according to the methods described in
U.S. Patent
Application US20060116321 Al.
[0252] In one embodiment, the microvesicles of the present invention are used
to modulate or
suppress a patient's immune response according to the methods described in PCT
Patent
Application W006007529A3.
[02531 In one embodiment, the microvesicles of the present invention are used
to modulate or
suppress a patient's immune response according to the methods described in PCT
Patent
Application W02007103572A3.
[0254] In one embodiment, the microvesicles of the present invention are used
to modulate or
suppress a patient's immune response according to the methods described in
U.S. Patent
8,288,172.
[0255] In one embodiment, the microvesicles of the present invention are used
as a therapy
for cancer according to the methods described in PCT Patent Application
W02011000551A1. In one embodiment, the microvesicles of the present invention
are used
as a therapy for cancer or an inflammatory disease according to the methods
described in
U.S. Patent Application US20120315324A1.
43
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
[0256] In one embodiment, the microvesicles of the present invention are used
as a therapy
for vascular injury according to the methods described in U.S. Patent
8,343,485.
[0257] In one embodiment, the microvesicles of the present invention are used
to deliver
molecules to cells. The delivery of molecules may be useful in treating or
preventing a
disease. In one embodiment, the delivery is according to the methods described
in PCT
Application W004014954A1. In an alternate embodiment, the delivery is
according to the
methods described in PCT Application W02007126386A1. In an alternate
embodiment, the
delivery is according to the methods described in PCT Application
W02009115561A1. In an
alternate embodiment, the delivery is according to the methods described in
PCT Application
W02010119256A1.
[0258] In one embodiment, the microvesicles of the present invention are used
to promote or
enhance wound healing. In one embodiment, the wound is a full-thickness burn.
In one
embodiment, the wound is a second-degree burn.
[0259] In one embodiment, the microvesicles of the present invention are used
to promote or
enhance angiogenesis in a patient.
[0260] In one embodiment, the microvesicles of the present invention are used
to promote or
enhance neuronal regeneration in a patient.
[0261] In one embodiment, the microvesicles of the present invention are used
to reduce scar
formation in a patient.
[0262] In one embodiment, the microvesicles of the present invention are used
to reduce
wrinkle formation in the skin of a patient.
[0263] In one embodiment, the microvesicles of the present invention are used
to orchestrate
complex tissue regeneration in a patient.
[0264] in one embodiment, the present invention provides an isolated
preparation of
microvesicles that can promote functional regeneration and organization of
complex tissue
structures. In one embodiment the present invention provides an isolated
preparation of
microvesicles that can regenerate hematopoietic tissue in a patient with
aplastic anemia. In
one embodiment the present invention provides an isolated preparation of
microvesicles that
can regenerate at least one tissue in a patient with diseased, damages or
missing skin selected
44
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
from the group consisting of: epithelial tissue, stromal tissue, nerve tissue,
vascular tissue and
adnexal structures. In one embodiment, the present invention provides an
isolated
preparation of microvesicles that can regenerate tissue and/or cells from all
three germ layers.
[0265] in one embodiment, the present invention provides an isolated
preparation of
microvesicles that is used to modulate the immune system of a patient.
[0266] In one embodiment, the present invention provides an isolated
preparation of
microvesicles that is used to alleviate one or more symptoms of EB (e.g., RDEB
and/or
DDEB, junctional EB, EB simplex and/or acquired forms of EB) in a patient.
[0267] In another embodiment, the present invention provides an isolated
preparation of
microvesicles that is used to increase collagen VII expression in a patient
having EB (e.g.,
RDEB and/or DDEB, junctional EB, EB simplex and/or acquired forms of EB).
[0268] In one embodiment, the present invention provides an isolated
preparation of
microvesicles that enhances the survival of tissue or cells that is
transplanted into a patient.
In one embodiment, the patient is treated with the isolated preparation of
microvesicles prior
to receiving the transplanted tissue or cells. In an alternate embodiment, the
patient is treated
with the isolated preparation of microvesicles after receiving the
transplanted tissue or cells.
In an alternate embodiment, the tissue or cells is treated with the isolated
preparation of
microvesicles. In one embodiment, the tissue or cells is treated with the
isolated preparation
of microvesicles prior to transplantation.
[0269] In one embodiment, the present invention provides an isolated
preparation of
microvesicles containing at least one molecule selected from the group
consisting of RNA,
DNA, lipid, carbohydrate, metabolite, protein, and combination thereof from a
host cell. In
one embodiment, the host cell is engineered to express at least one molecule
selected from
the group consisting of RNA, DNA, lipid, carbohydrate, metabolite, protein,
and combination
thereof. In one embodiment, the isolated preparation of microvesicles
containing at least one
molecule selected from the group consisting of RNA, DNA, lipid, carbohydrate,
metabolite,
protein, and combination thereof from a host cell is used as a therapeutic
agent.
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
Use of the Microvesicles of the Present Invention in Therapies
[0270] For therapeutic use, MVs are preferably combined with a
pharmaceutically acceptable
carrier. As used herein, "pharmaceutically acceptable carrier" means buffers,
carriers, and
excipients suitable for use in contact with the tissues of human beings and
animals without
excessive toxicity, irritation, allergic response, or other problem or
complication,
commensurate with a reasonable benefit/risk ratio. The carrier(s) should be
"acceptable" in
the sense of being compatible with the other ingredients of the formulations
and not
deleterious to the recipient. Pharmaceutically acceptable carriers include
buffers, solvents,
dispersion media, coatings, isotonic and absorption delaying agents, and the
like, that are
compatible with pharmaceutical administration. The use of such media and
agents for
pharmaceutically active substances is known in the art.
[0271] Accordingly, EV compositions of the present invention can comprise at
least one of
any suitable excipients, such as, but not limited to, diluent, binder,
stabilizer, buffers, salts,
lipophilic solvents, preservative, adjuvant or the like. Pharmaceutically
acceptable excipients
are preferred. Non-limiting examples of, and methods of preparing such sterile
solutions are
well known in the art, such as, but not limited to, those described in
Gennaro, Ed.,
Remington's Pharmaceutical Sciences, 18th Edition, Mack Publishing Co.
(Easton, Pa.)
1990. Pharmaceutically acceptable carriers can be routinely selected that are
suitable for the
mode of administration, solubility and/or stability of EV composition as well
known in the art
or as described herein.
[0272] Pharmaceutical excipients and additives useful in the present
composition include but
are not limited to proteins, peptides, amino acids, lipids, and carbohydrates
(e.g., sugars,
including monosaccharides, di-, tri-, tetra-, and oligosaccharides;
derivatized sugars such as
alditols, aldonic acids, esterified sugars and the like; and polysaccharides
or sugar polymers),
which can be present singly or in combination, comprising alone or in
combination 1-99.99%
by weight or volume. Exemplary protein excipients include serum albumin such
as human
serum albumin (HSA), recombinant human albumin (rHA), gelatin, casein, and the
like.
Representative amino acid/antibody molecule components, which can also
function in a
buffering capacity, include alanine, glycine, arginine, betaine, histidine,
glutamic acid,
aspartic acid, cysteine, lysine, leucine, isoleucine, valine, methionine,
phenylalanine,
aspartame, and the like.
[0273] Carbohydrate excipients suitable for use in the invention include, for
example,
monosaccharides such as fructose, maltose, galactose, glucose, D-mannose,
sorbose, and the
46
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
like; disaccharides, such as lactose, sucrose, trehalose, cellobiose, and the
like;
polysaccharides, such as raffinose, melezitose, maltodextrins, dextrans,
starches, and the like;
and alditols, such as mannitol, xylitol, maltitol, lactitol, xylitol sorbitol
(glucitol), myoinositol
and the like. Preferred carbohydrate excipients for use in the present
invention are mannitol,
trehalose, and raffmose.
[0274] EV compositions can also include a buffer or a pH adjusting agent;
typically, the
buffer is a salt prepared from an organic acid or base. Representative buffers
include organic
acid salts such as salts of citric acid, acetic acid, ascorbic acid, gluconic
acid, carbonic acid,
tartaric acid, succinic acid, or phthalic acid; Tris, tromethamine
hydrochloride, or phosphate
buffers.
[0275] Additionally, EV compositions of the invention can include polymeric
excipients/additives such as polyvinylpyrrolidones, ficolls (a polymeric
sugar), dextrates
(e.g., cyclodextrins, such as 2-hydroxypropy1-13-cyclodextrin), polyethylene
glycols, flavoring
agents, antimicrobial agents, sweeteners, antioxidants, antistatic agents,
surfactants (e.g.,
polysorbates such as "TWEEN 20" and "TWEEN 80"), lipids (e.g., phospholipids,
fatty
acids), steroids (e.g., cholesterol), and chelating agents (e.g., EDTA).
[0276] These and additional known pharmaceutical excipients and/or additives
suitable for
use in the antibody molecule compositions according to the invention are known
in the art,
e.g., as listed in "Remington: The Science & Practice of Pharmacy," 19th ed.,
Williams &
Williams, (1995), and in the "Physician's Desk Reference," 52nd ed., Medical
Economics,
Montvale, N.J. (1998). Preferred carrier or excipient materials are
carbohydrates (e.g.,
saccharides and alditols) and buffers (e.g., citrate) or polymeric agents.
[0277] The present invention provides for stable compositions, comprising MVs
in a
pharmaceutically acceptable formulation. Preserved formulations contain at
least one known
preservative or optionally selected from the group consisting of at least one
phenol, m-cresol,
p-cresol, o-cresol, chlorocresol, benzyl alcohol, phenylmercuric nitrite,
phenoxyethanol,
formaldehyde, chlorobutanol, magnesium chloride (e.g., hexahydrate),
alkylparaben (methyl,
ethyl, propyl, butyl and the like), benzalkonium chloride, benzethonium
chloride, sodium
dehydroacetate and thimerosal, or mixtures thereof in an aqueous diluent. Any
suitable
concentration or mixture can be used as known in the art, such as 0.001-5%, or
any range or
value therein, such as, but not limited to 0.001, 0.003, 0.005, 0.009, 0.01,
0.02, 0.03, 0.05,
0.09Ø1. 0.2, 0.3,0.4, 0.5, 0.6, 0.7, 0.8,0.9, 1.0, 1.1, 1.2, 1.3, 1.4, 1.5,
1.6, 1.7, 1.8, 1.9, 2.0,
2.1, 2.2, 2.3, 2.4, 2.5, 2.6, 2.7, 2.8, 2.9, 3.0, 3.1, 3.2, 3.3, 3.4, 3.5,
3.6, 3.7, 3.8, 3.9, 4.0, 4.3,
47
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
4.5, 4.6, 4.7, 4.8, 4.9, or any range or value therein. Non-limiting examples
include, no
preservative, 0.1-2% m-cresol (e.g., 0.2, 0.3, 0.4, 0.5, 0.9, or 1.0%), 0.1-3%
benzyl alcohol
(e.g., 0.5, 0.9, 1.1., 1.5, 1.9, 2.0, or 2.5%), 0.001-0.5% thimerosal (e.g.,
0.005 or 0.01%),
0.001-2.0% phenol (e.g., 0.05, 0.25, 0.28, 0.5, 0.9, or 1.0%), 0.0005-1.0%
alkylparaben(s)
(e.g., 0.00075, 0.0009, 0.001, 0.002, 0.005, 0.0075, 0.009, 0.01, 0.02, 0.05,
0.075, 0.09, 0.1,
0.2, 0.3, 0.5,0.75, 0.9, or 1.0%), and the like.
[0278] Pharmaceutical compositions containing MVs as disclosed herein can be
presented in
a dosage unit form and can be prepared by any suitable method. A
pharmaceutical
composition should be formulated to be compatible with its intended route of
administration.
Examples of routes of administration are intravenous (TV), intradermal,
inhalation,
transdermal, topical, transmucosal, and rectal administration. A preferred
route of
administration for MVs is topical administration. Useful formulations can be
prepared by
methods known in the pharmaceutical art. For example, see Remington's
Pharmaceutical
Sciences (1990) supra. Formulation components suitable for parenteral
administration
include a sterile diluent such as water for injection, saline solution, fixed
oils, polyethylene
glycols, glycerine, propylene glycol or other synthetic solvents;
antibacterial agents such as
benzyl alcohol or methyl parabens; antioxidants such as ascorbic acid or
sodium bisulfite;
chelating agents such as EDTA; buffers such as acetates, citrates or
phosphates; and agents
for the adjustment of tonicity such as sodium chloride or dextrose.
[0279] The carrier should be stable under the conditions of manufacture and
storage, and
should be preserved against microorganisms. The carrier can be a solvent or
dispersion
medium containing, for example, water, ethanol, polyol (for example, glycerol,
propylene
glycol, and liquid polyethylene glycol), and suitable mixtures thereof.
[0280] Pharmaceutical formulations are preferably sterile. Sterilization can
be accomplished
by any suitable method, e.g., filtration through sterile filtration membranes.
Where the
composition is lyophilized, filter sterilization can be conducted prior to or
following
lyophilization and reconstitution.
[0281] The compositions of this invention may be in a variety of forms. These
include, for
example, liquid, semi-solid and solid dosage forms, such as liquid solutions
(e.g., injectable
and infusible solutions), dispersions or suspensions, and Liposomes. The
preferred form
depends on the intended mode of administration and therapeutic application.
Typical
preferred compositions are in the form of injectable or infusible solutions.
The preferred
mode of administration is parenteral (e.g., intravenous, subcutaneous,
intraocular,
48
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
intraperitoneal, intramuscular). In a preferred embodiment, the preparation is
administered
by intravenous infusion or injection. In another preferred embodiment, the
preparation is
administered by intramuscular or subcutaneous injection.
[0282] The phrases "parenteral administration" and "administered parenterally"
as used
herein means modes of administration other than enteral and topical
administration, usually
by injection, and includes, without limitation, intravenous, intramuscular,
subcutaneous,
intraarterial, intrathecal, intracapsular, intraorbital, intravitreous,
intracardiac, intradermal,
intraperitoneal, transtracheal, inhaled, subcutaneous, subcuticular,
intraarticular, subcapsular,
subarachnoid, intraspinal, epidural and intrasternal injection and infusion.
[0283] The present invention provides a kit, comprising packaging material and
at least one
vial comprising a solution of MVs with the prescribed buffers and/or
preservatives,
optionally in an aqueous diluent. The aqueous diluent optionally further
comprises a
pharmaceutically acceptable preservative. Preservatives include those selected
from the
group consisting of phenol, m-cresol, p-cresol, o-cresol, chlorocresol, benzyl
alcohol,
alkylparaben (methyl, ethyl, propyl, butyl and the like), benzalkonium
chloride,
benzethonium chloride, sodium dehydroacetate and thimerosal, or mixtures
thereof The
concentration of preservative used in the formulation is a concentration
sufficient to yield an
anti-microbial effect. Such concentrations are dependent on the preservative
selected and are
readily determined by the skilled artisan.
[0284] Other excipients, e.g. isotonicity agents, buffers, antioxidants,
preservative enhancers,
can be optionally and preferably added to the diluent. An isotonicity agent,
such as glycerin,
is commonly used at known concentrations. A physiologically tolerated buffer
can be added
to provide improved pH control. The formulations can cover a wide range of
pHs, such as
from about pH 4.0 to about pH 10.0, from about pH 5.0 to about pH 9.0, or
about pH 6.0 to
about pH 8Ø
[0285] Other additives, such as a pharmaceutically acceptable solubilizers
like TWEEN 20
(polyoxyethylene (20) sorbitan monolaurate), TWEEN 40 (polyoxyethylene (20)
sorbitan
monopalmitate), TWEEN 80 (polyoxyethylene (20) sorbitan monooleate), Pluronic
F68
(polyoxyethylene polyoxypropylene block copolymers), and PEG (polyethylene
glycol) or
non-ionic surfactants such as polysorbate 20 or 80 or poloxamer 184 or 188,
Pluronic
polyls, other block co-polymers, and chelators such as EDTA and EGTA can
optionally be
added to the formulations or compositions to reduce aggregation. These
additives are
particularly useful if a pump or plastic container is used to administer the
formulation. The
49
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
presence of pharmaceutically acceptable surfactant mitigates the propensity
for the protein to
aggregate.
[0286] Various delivery systems can be used to administer MVs to a subject. In
certain
exemplary embodiments, administration of MVs is topical, optionally with the
addition of a
dressing, bandage, medical tape, pad, gauze or the like. Suitable dressings to
aid in topical
delivery are well-known in the art and are commercially available. In other
embodiments,
MVs are administered by pulmonary delivery, e.g., by intranasal
administration, or by oral
inhalative administration. Pulmonary delivery may be achieved via a syringe or
an inhaler
device (e.g., a nebulizer, a pressurized metered-dose inhaler, a multi-dose
liquid inhaler, a
thermal vaporization aerosol device, a dry powder inhaler or the like).
Suitable methods for
pulmonary delivery are well-known in the art and are commercially available.
[0287] Any of the formulations described above can be stored in a liquid or
frozen form and
can be optionally subjected to a preservation process.
[0288] In certain exemplary embodiments of the invention, EVs described herein
are used to
deliver one or more bioactive agents to a target cell. The term "bioactive
agent" is intended
to include, but is not limited to, proteins (e.g., non-membrane-bound
proteins), peptides (e.g.,
non-membrane-bound peptides), transcription factors, nucleic acids and the
like, that are
expressed in a cell and/or in a cellular fluid and are added during the
purification and/or
preparation of EVs described herein, and/or pharmaceutical compounds, proteins
(e.g., non-
membrane-bound proteins), peptides (e.g., non-membrane-bound peptides),
transcription
factors, nucleic acids and the like, that EVs described herein are exposed to
during one or
more purification and/or preparation steps described herein. In certain
embodiments, a
bioactive agent is a collagen VII protein, a collagen VII mRNA, a STAT3
signalling activator
(e.g., an interferon, epidermal growth factor, interleukin-5, interleukin-6, a
MAP kinase, a c-
src non-receptor tyrosine kinase or another molecule that phosphorylates
and/or otherwise
activates STAT3) and/or a canonical Wnt activator (see, e.g., McBride et al.
(2017)
Transgenic expression of a canonical Wnt inhibitor, kallistatin, is associated
with decreased
circulating CD19+ B lymphocytes in the peripheral blood. International Journal
of
Hematology, 1-10. DO!: 10.1007/s12185-017-2205-5, incorporated herein by
reference in its
entirety). In other embodiments, a bioactive agent is one or more
pharmaceutical compounds
known in the art.
[0289] It will be readily apparent to those skilled in the art that other
suitable modifications
and adaptations of the methods described herein may be made using suitable
equivalents
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
without departing from the scope of the embodiments disclosed herein. Having
now
described certain embodiments in detail, the same will be more clearly
understood by
reference to the following examples, which are included for purposes of
illustration only and
are not intended to be limiting. All patents, patent applications and
references described
herein are incorporated by reference in their entireties for all purposes.
EXAMPLES
Example 1: Isolation of Mierovesieles from Cell culture Medium by
Ultracentrifugation
[0290] This example illustrates the typical method by which microvesicles are
isolated from
cell culture medium, or any biological fluid. An outline of the method to
isolate
microvesicles from cell culture medium is shown in Figure 1. In summary, the
cells are
cultured in medium supplemented with microvesicle-free serum (the serum may be
depleted
of microvesicles by ultracentrifugation, filtration, precipitation, etc.).
After culturing the cells
for a period of time, the medium is removed and transferred to conical tubes
and centrifuged
at 400 x g for 10 minutes at 4 C to pellet the cells. Next, the supernatant
is transferred to
new conical tubes and centrifuged at 2000 x g for 30 minutes at 4 C to
further remove cells
and cell debris. This may be followed by another centrifugation step (e.g.
10000 x g for 30
minutes to further deplete cellular debris and/or remove larger
microvesicles). The resultant
supernatant is transferred to ultracentrifuge tubes, weighed to ensure equal
weight and
ultracentrifuged at 70000+ x g for 70 minutes at 4 C to pellet the
microvesicles.
R12911 This supernatant is subsequently discarded and the pellet is
resuspended in ice cold
PBS. The solution is ultracentrifuged at 70000+ x g for 70 minutes at 4 C to
pellet the
microvesicles. The microvesicle enriched pellet is resuspended in a small
volume
(approximately 50-100 IA) of an appropriate buffer (e.g. PBS).
Example 2: Isolation of Microvesicles from Cell culture Medium by the Methods
of the
Present Invention
[0292] This example illustrates how microvesicles are isolated from cell
culture medium by
the methods of the present invention. An outline of the method to isolate
microvesicles from
medium that has cultured cells is shown in Figures 2 and 3. In summary, the
cells are
cultured in medium supplemented with microvesicle-free serum (the serum may be
depleted
51
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
of microvesicles by ultracentrifugation, filtration, precipitation, etc.).
After culturing the cells
for a period of time, the medium is removed and transferred to conical tubes
and centrifuged
at 400 x g for 10 minutes at 4 C to pellet the cells. Next, the supernatant
is transferred to
new conical tubes and centrifuged at 2000 x g for 30 minutes at 4 C to
further remove cells
and cell debris. This may be followed by another centrifugation step (e.g.
10000 x g for 30
minutes to further deplete cellular debris and remove larger particles).
[0293] Microvesicles are then precipitated at 4 C using 8.5% w/v PEG 6000 and
0.4 M
NaCI. This mixture is spun at 10000 x g at 4 C for 30 minutes. The
supernatant is removed
and the pellet is resuspended in an appropriate buffer (e.g. PBS). It may be
used for
immediate downstream reactions or further purified. Further purification
procedures can
include the use of centrifugal filters (e.g. MWCO of 100 klla),
immunoaffinity, HPLC,
tangential flow filtration, phase separation/partitioning, microfluidics, etc.
Example 3: Isolation of Microvesicles from Culture Medium Conditioned Using
Bone Marrow Derived Stem Cells by the Methods of the Present Invention
[0294] Normal donor human bone marrow was acquired from AllCells LLC
(Emeryville,
CA, http://www.alIcells.com). MSCs were isolated by a standard plastic
adherence method.
Bone marrow mononuclear cells were isolated by low-density centrifugation
using Fico11-
Paque Premium (density: 1.077 g/m1) according to the manufacturer's protocol
(GE
Healthcare Life Sciences, Pittsburgh, PA). The mononuclear cells were
collected at the
interface, washed three times in phosphate-buffered saline (PBS) supplemented
with 2% FBS
(Atlanta Biologics, Atlanta, GA) , and resuspended in MSC medium consisting of
alpha-
minimum essential medium (a-MEM) (Mediatech Inc., Manassas, VA) and 20% FBS,
1%
Penicillin/Streptomycin (Lonza, Allendale, NJ) and 1% glutamine (Lonza).
[0295] Initial cultures of either MSCs or mononuclear cells were seeded
between 2-3 x 105
cells/cm2 in tissue culture-treated dishes (BD Biosciences, San Jose, CA) and
placed in a cell
incubator at 37 C in 95% humidified air and 5% CO2. After 48-72 hours, the non-
adherent
cells were removed, the culture flasks were rinsed once with PBS, and fresh
medium was
added to the flask. The cells were grown until 80% confluence was reached and
then
passaged by Trypsin-EDTA (Life technologies, Carlsbad, CA). Cells were split
at a 1:4 ratio
into 5-layer multi-flasks (BD Biosciences). Alternatively, cryopreserved MSC
were thawed
at 37 C and immediately cultured in a-MEM supplemented with 20% microvesicle-
free fetal
52
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
bovine serum and 1% penicillin/streptomycin/glutamine at 37 C in 95%
humidified air and
5% CO2. They were expanded similar to above.
[0296] The cells were grown in the multi-flasks until 80-90% confluence was
reached. The
flasks were rinsed twice with PBS and a -MEM supplemented with 1%
Penicillin/Streptomycin/Glutamine was added. After 24 hours, the conditioned
medium
transferred to 50mL conical centrifuge tubes (Thermo Fisher Scientific Inc.,
Weston, FL) and
immediately centrifuged at 400 x g for 10 minutes at 4 C to pellet any non-
adherent cells.
The supernatant was transferred to new 50mL conical centrifuge tubes and
centrifuged at
2000 x g for 30 minutes at 4 C to further remove cells and cell debris. The
supernatants
were collected and placed into 250 ml sterile, polypropylene disposable
containers (Corning,
Corning, NY). To the supernatant, RNase and protease free polyethylene glycol
average
molecular weight 6000 (Sigma Aldrich, Saint Louis, MO) at 8.5 w/v % and sodium
chloride
(final concentration 0.4 M) were added. The solution was placed in a cold room
at 4 C
overnight with rocking. The solution was transferred to 50 rriL conical
centrifuge tubes and
centrifuged at 10000 x g at 4 C for 30 minutes. The supernatant was decanted
and the
microvesicle enriched pellet resuspended in phosphate-buffered saline (PBS).
The
microvesicle enriched solution was transferred to Amicon ultra-15 centrifugal
filter units
(nominal molecular weight limit 100 kDa) (Millipore, Billerica, MA) and
centrifuged at 5000
x g for 30 minutes. The filter units were washed with phosphate-buffered
saline and
centrifuged again at 5000 x g for 30 minutes. The concentrated sample was
recovered
(approximately 200 pi) from the bottom of the filter device. Protein
concentration was
determined by the micro BSA Protein assay kit (Pierce, Rockford, IL) and the
enriched
microvesicle solution was stored at -70 degrees or processed for downstream
use (e.g.
protein, RNA, and DNA extraction).
Example 4: Isolation of Microvesicles from Plasma by the Methods of the
Present
Invention
f02971 Approximately 6-8 ml of blood (human and pig) was collected via
venipuncture and
placed into BD Vacutainer plastic EDTA lavender tubes (BD Biosciences, San
lose, CA).
The venipuncture tubes were centrifuged at 400 x g for 30 minutes at room
temperature.
Plasma was removed (approximately 3-4 nil) and placed into new 50 ml conical
centrifuge
53
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
tubes (Thermo Fisher Scientific Inc., Weston, FL). Sterile alpha-minimum
essential medium
(a-MEM) (Mediatech Inc., Manassas, VA) was added in a 1: 10 (Plasma to medium)
ratio.
[0298] To the solution, RNase and protease free polyethylene glycol average
molecular
weight 6000 (Sigma Aldrich, Saint Louis, MO) at 8.5 w/v % and sodium chloride
(fmal
concentration 0.4 M) were added. The solution was placed in a cold room at 4
C overnight
with rocking. The solution was centrifuged at 10000 x g at 4 C for 30
minutes. The
supernatant was decanted and the microvesicle enriched pellet resuspended in
phosphate-
buffered saline (PBS). The microvesicle enriched solution was transferred to
Amicon ultra-
15 centrifugal filter units (nominal molecular weight limit 100 kDa)
(Millipore, Billerica,
MA) and centrifuged at 5000 x g for 30 minutes. The filter units were washed
with
phosphate-buffered saline and centrifuged again at 5000 x g for 30 minutes.
The
concentrated sample was recovered (approximately 200-400 p.1) from the bottom
of the filter
device. Protein concentration was determined by the micro BSA Protein assay
kit (Pierce,
Rockford. IL) and the enriched microvesicle solution was stored at -70 degrees
or processed
for downstream use (e.g. protein, RNA, and DNA extraction).
Example 5: Isolation of Micro vesicles from Bone Marrow Aspirate by the
Methods of
the Present Invention
[0299] Pig bone marrow was isolated from the iliac crest. The skin area was
carefully
cleaned with povidine iodine 7.5% and isopropanol 70%. An 11-gauge 3 mm trocar
(Ranafac, Avon, MA) was inserted into the iliac crest. An aspiration syringe
with loaded
with 5000 - 1000 units of heparin to prevent clotting of the marrow sample.
Approximately
20-25 ml of marrow was aspirated and the solution transferred to 50 ml conical
centrifuge
tubes. Alternatively, normal donor human bone marrow (approximately 50 ml) was
acquired
from AllCells LLC (Emeryville, CA, URL: alIcells.com).
[0300] The 50 ml conical tubes were centrifuged at 400 x g for 30 minutes at
room
temperature. The supernatant (the acellular portion) was collected
(approximately 10-12 ml
per 50 ml) and placed into new 50 ml conical centrifuge tubes (Thermo Fisher
Scientific Inc.,
Weston, FL). Sterile alpha-minimum essential medium (cc-MEM) (Mediatech Inc..
Manassas, VA) was added in a 1: 10 (bone marrow supernatant to medium) ratio.
The
solution was transferred to new 50 ml conical tubes and centrifuged at 2000 x
g for 30
minutes at 4 C. The supernatant was transferred to new 50 ml conical tubes
and to this
54
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
solution, RNase and protease free polyethylene glycol average molecular weight
6000 (Sigma
Aldrich, Saint Louis, MO) at 8.5 w/v % and sodium chloride (final
concentration 0.4 M) were
added.
[0301] The solution was placed in a cold room at 4 C overnight with rocking.
The solution
was centrifuged at 10000 x g at 4 C for 30 minutes. The supernatant was
decanted and the
microvesicle enriched pellet resuspended in phosphate-buffered saline (PBS).
The
microvesicle enriched solution was transferred to Amicon ultra-15 centrifugal
filter units
(nominal molecular weight limit 100 kDa) (Millipore, Billerica, MA) and
centrifuged at 5000
x g for 30 minutes. The filter units were washed with phosphate-buffered
saline and
centrifuged again at 5000 x g for 30 minutes. The concentrated sample was
recovered
(approximately 200-400 1) from the bottom of the filter device. Protein
concentration was
determined by the micro BSA Protein assay kit (Pierce, Rockford, IL) and the
enriched
microvesicle solution was stored at -70 degrees or processed for downstream
use (e.g.
protein, RNA, and DNA extraction).
[0302] The cellular portion was collected and processed for mesenchymal stem
isolation or
for bone marrow complete isolation.
Example 6: Isolation of Microvesicles from Urine by the Methods of the Present
Invention
[0303] Approximately 500 ml of clean catch human urine was isolated and placed
into 50 ml
conical tubes (Thermo Fisher Scientific Inc., Weston, FL).
[0304] The 50 ml conical tubes were centrifuged at 400 x g for 30 minutes at 4
C. The
supernatant was removed and placed into new 50 ml conical centrifuge tubes
(Thermo Fisher
Scientific Inc., Weston, FL). The solution was transferred to new 50 ml
conical tubes and
centrifuged at 2000 x g for 30 minutes at 4 C. The supernatant was transferred
to new 50 ml
conical tubes and to this solution, RNase and protease free polyethylene
glycol average
molecular weight 6000 (Sigma Aldrich, Saint Louis, MO) at 8.5 w/v % and sodium
chloride
(final concentration 0.4 M) were added.
[0305] The solution was placed in a cold room at 4 C overnight with rocking.
The solution
was centrifuged at 10000 x g at 4 C for 30 minutes. The supernatant was
decanted and the
microvesicle enriched pellet resuspended in phosphate-buffered saline (PBS).
The
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
microvesicle enriched solution was transferred to Amicon ultra-15 centrifugal
filter units
(nominal molecular weight limit 100 kDa) (Millipore, Billerica, MA) and
centrifuged at 5000
x g for 30 minutes. The filter units were washed with phosphate-buffered
saline and
centrifuged again at 5000 x g for 30 minutes. The concentrated sample was
recovered
(approximately 200-400 gl) from the bottom of the filter device. Protein
concentration was
determined by the micro BSA Protein assay kit (Pierce, Rockford, IL) and the
enriched
microvesicle solution was stored at -70 degrees or processed for downstream
use (e.g.
protein, RNA, and DNA extraction).
Example 7: Isolation of Microvesicles from Medium from a Long-Term Culture of
Bone
Marrow Cells by the Methods of the Present Invention
[0306] Bone marrow was obtained from an aspirate (see Example 1) and red blood
cells were
lysed using 0.8% ammonium chloride solution containing 0.1 mM EDTA (Stem Cell
Technologies, Vancouver, BC). The nucleated cells were pelleted under a fetal
bovine serum
(Atlanta Biologics, Atlanta, GA) cushion at 400 X g for 5 minutes. Nucleated
cells were
washed in McCoy's 5a media (Mediatech Inc., Manassas, VA) by pelleting at 400
x g for 5
min. The cells were resuspended in culture media at a density of 1 x 106
cells/ml and plated
in 25,75 or 225 cm2 flasks (Corning, Corning, NY).
[0307] Culture media consisted of McCoy's 5a media, 1% sodium bicarbonate
(Life
technologies, Carlsbad, CA), 0-4% MEM non-essential amino acids (Life
technologies), 0-
8% MEM essential amino acids (Life technologies), 1% L-glutamine (Lonza,
Allendale, NJ),
0.1 uM Hydrocortisone (Life technologies), 1% penicillin/streptomycin (Lonza),
12-5% fetal
calf serum (Atlanta Biologics) and 12-5% horse serum (Stem Cell Technology).
The cultures
were incubated at 33 C and 5% CO2. Feeding was performed weekly by adding half
of the
original volume of media without removing any media during the first nine
weeks of culture.
If the cultures were grown beyond nine weeks, the volume of culture media was
reduced to
the original volume and half the original volume of fresh media was added each
week
[0308] After approximately nine weeks of culture, the original medium was
removed and
stored. The cells were washed twice with phosphate buffered saline (PBS) and
incubated for
24 hours in media consisting of McCoy's 5a media, 1% sodium bicarbonate, 0-4%
MEM
nonessential amino acids, 0-8% MEM essential amino acids (Life technologies),
1% L-
glutamine (Lonza, Allendale, NJ), and 1% penicillin/streptomycin (Lonza).
56
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
[0309] After 24 hours, the supernatant was transferred to 50mL conical
centrifuge tubes
(Thermo Fisher Scientific Inc., Weston, FL) and immediately centrifuged at 400
x g for 10
minutes at 4 C to pellet any non-adherent cells. The original medium that was
stored was
added back to the cells. The supernatant were transferred to new 50 mL conical
centrifuge
tubes and centrifuged at 2000 x g for 30 minutes at 4 C to further remove
cells and cell
debris.
[0310] The supernatant was collected and placed into 250 ml sterile,
polypropylene
disposable containers (Corning, Corning, NY). To the supernatant, RNase and
protease free
polyethylene glycol average molecular weight 6000 (Sigma Aldrich, Saint Louis,
MO) at 8.5
w/v % and sodium chloride (final concentration 0.4 M) was added. The solution
was placed
in a cold room at 4 C overnight with rocking. The solution was transferred to
50 mL conical
centrifuge tubes and centrifuged at 10000 x g at 4 C for 30 minutes. The
supernatant was
decanted and the microvesicle enriched pellet resuspended in phosphate-
buffered saline
(PBS). The microvesicle enriched solution was transferred to Amicon ultra-15
centrifugal
filter units (nominal molecular weight limit 100 kDa) (Millipore, Billerica,
MA) and
centrifuged at 5000 x g for 30 minutes. The filter units were washed with
phosphate-buffered
saline and centrifuged again at 5000 x g for 30 minutes. The concentrated
sample was
recovered (approximately 200 i.d) from the bottom of the filter device.
Protein concentration
was determined by the micro BSA Protein assay kit (Pierce, Rockford, IL) and
the enriched
microvesicle solution stored at -70 degrees or processed for downstream use
(e.g. protein,
RNA, and DNA extraction).
Example 8: Analysis of the Microvesides of the Present Invention
[0311] Samples of microvesicles were analyzed by electron microscopy. For
transmission
electron microscopy (TEM), each specimen of microvesicles was loaded on
formvar-coated.
150 mesh copper grids (Electron Microscopy Sciences, Fort Washington, PA) for
20 minutes.
The grids were drained and floated on drops of 2% glutaraldehyde for 5
minutes, then washed
in double distilled water (DDOH), followed by staining on drops of 4% aqueous
uranyl
acetate and multiple washes in DDOH. The grids were examined at 80kV in a
Philips CM10
electron microscope.
[0312] Figure 5 shows electron micrographs of microvesicles derived from human
bone
marrow- derived mesenchymal stem cells isolated by the ultracentrifuge method
described in
57
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
Example 1 (panels A&B) and according to the methods of the present invention
as described
in Example 3 (panels C&D). Figure 6 shows electron micrographs of
microvesicles derived
from porcine bone marrow-derived mesenchymal stem cells isolated by the
ultracentrifuge
method described in Examples 1 (panels A&B) and according to the methods of
the present
invention as described in Example 3 (panels C&D). Figure 7 shows electron
micrographs of
microvesicles derived from murine bone marrow-derived mesenchymal stem cells
isolated by
the ultracentrifuge method described in Examples 1 (panels A&B) and according
to the
methods of the present invention as described in Example 3 (panels C&D).
[0313] Figures 5 to 7 illustrate the differences between microvesicles
isolated by the methods
of the present invention compared to ultracentrifuge isolation. The
microvesicles isolated
according to the methods of the present invention have borders that are
smoother,
unconnated and appear more "intact."
[0314] Figure 8 shows electron micrographs of microvesicles isolated from
human plasma
according to the methods of the present invention. The heterogeneity of the
shapes and sizes
achieved with PEG isolation suggests that all types of microvesicles were
isolated. Similar
heterogeneity was observed in microvesicles from porcine plasma (Figure 9) and
human
urine (Figure 10) that were isolated according to the methods of the present
invention.
[0315] To analyze protein expression in samples of microvesicles, cells and
microvesicles
were lysed in RIPA buffer (Cell signaling technology, Danvers, MA) and protein
concentration estimated by the microBSA assay kit (Pierce, Rockford, IL).
Approximately
20 micrograms of lysate were loaded in each lane and the membranes were probed
overnight
(1:1000) by either Rabbit anti-63 antibody (SBI Biosciences, Mountain View,
CA), Rabbit
anti-hsp70 (SB1 Biosciences), rabbit STAT3 (Cell signaling technology), and/or
rabbit
phospho-STAT3 (Cell signaling technology).
[0316] The presence of the exosomal markers (HSP 70 and CD63) confirmed that
the
methods of the present invention were capable of isolating exosomes. Further,
the exosomes
also contained the transcription factor STAT3 and the activated phosphorylated
form
phospho-STAT3. See Figure 11.
58
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
Example 9: The Effect of the Microvesicles of the Present Invention on
Fibroblast
Proliferation and Migration
[0317] To study the ability the microvesicles of the present invention to
promote or enhance
wound healing, the ability of the microvesicles to stimulate the proliferation
of dermal
fibmblasts was tested. Normal human adult dermal fibroblasts were obtained
from Life
Technology (Carlsbad, CA). Chronic wound patient fibroblasts (pressure foot
ulcer and
diabetic foot ulcer) were collected under an IRB approved protocol (IND# BB
IND 13201)
from wounds of 2 years duration without evidence of healing despite standard
of care and
advanced wound care treatments. Normal and Chronic wound fibroblasts were
plated at 5 x
103 cells per well on 24 well tissue culture plates (BD Biosciences, San Jose,
CA). MTT cell
proliferation assays were performed at day 0 and day 3. Microvesicles were
added on day 0.
Both PEG isolated and ultracentrifuge isolated microvesicles were
approximately equivalent
in increasing growth of both normal and chronic wound fibroblasts after 3
days. Phosphate
buffered saline (PBS) and conditioned MSC medium depleted of microvesicles
showed little
growth. See Figure 12.
[0318] in co-culture experiments, normal adult fibroblasts and fibroblast from
a diabetic foot
ulcer were seeded in twenty four well plates. Each well was seeded to achieve
100%
confluency (approximately 1 x 105 cells per well). To prevent the influence of
cell
proliferation, 2 hours prior to scratch, the medium was substituted with a
fresh serum-free
culture medium containing mitomycin at 10 g/ml. The confluent monolayer was
then
scored with a 1 ml sterile pipette tip to leave a scratch of 0.4-0.5 mm in
width. Culture
medium was then immediately removed (along with any dislodged cells). The
removed
medium was replaced with fresh culture medium (10% FBS) containing either
microvesicles
(PEG or ultracentrifuge derived). PBS, or MSC conditioned medium depleted of
microvesicles. The scratched area was monitored by collecting digitized images
immediately
after the scratch and 3 days after treatment. Digitized images were captured
with an inverted
IX81 Olympus microscope (Olympus America, Center Valley, PA, URL:
olympusamerica.com) and ORCA-AG Hamamatsu digital camera (Hamamatsu Photonics
K.K., Hamamatsu City, Shizuoka Pref., Japan, URL: hamamatsu.com). Three days
after
treatment, microvesicles isolated according to the methods of the present
invention showed
the greatest in migration (essentially closing the wound), followed by
microvesicles derived
59
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
from ultracentrifuge. The controls (PBS) and MSC conditioned medium depleted
of
microvesicles (Depleted) showed little migration. See Figure 13.
[0319] Figure 14 shows the effects of microvesicles on cell migration
fibroblasts derived
from a diabetic foot ulcer. Similar to the results in Figure 13, microvesicles
isolated
according to the methods of the present invention evoked the greatest
migration, followed by
microvesicles isolated using the ultracentrifuge method described in Example
1. The controls
(PBS) and MSC conditioned medium depleted of microvesicles (Depleted) showed
little
migration.
Example 10: Uptake of the Microvesicles of the Present Invention into Cells
[0320] Human MSC microvesicles isolated from conditioned medium according to
the
methods of the present invention were labeled with the phospholipid cell
linker dye PKH-26
(red) per manufacturer's instruction (Sigma- Aldrich, St. Louis, MO). Normal
skin
fibroblasts were labeled with Vybrant-Dio (Life technology) per manufacturer
instructions.
Normal skin fibroblasts were plated on fibronectin (Sigma-Aldrich) coated 4-
well Nunc*
Lab-Tek* 11 Chamber Slides (Thermo Fisher Scientific Inc., Weston, FL) (5 x 10
cells per
well). Cells were stained with the nuclear dye Hoechst 33342 (Life technology)
per
manufacturer's instructions. Dio labeled fibroblasts were treated with PKH-26
labeled
microvesicles for 24 hours. Images were captured with an inverted 1X81 Olympus
microscope and ORCA-AG Hamamatsu digital camera. Normal dermal fibroblasts
(stained
with the green lipid membrane dye Dio) demonstrated uptake of PKH-26 labeled
human
MSC MV isolated by PEG precipitation in a pen-nuclear location. See Figures 15
and 16. In
Figure 16, the microvesicles are seen in a pen-nuclear location.
Example 11: Use of the Microvesicles of the Present Invention as a Diagnostic
for
Rheumatoid Arthritis
[0321] Normal dermal fibroblasts were plated at a density of 1 x 105
cells/well in a 6-well
tissue culture plate (BD Biosciences). Fibroblasts were serum starved
overnight and treated
with PBS (control), 10 micrograms of either microvesicles isolated according
to the methods
of the present invention from plasma obtained from a patient suffering from
rheumatoid
arthritis (Human Plasma MV PEG Precipitation); microvesicles isolated
according to the
methods of the present invention from medium conditioned with bone marrow-
derived
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
mesenchymal stem cells (Human hMSC MV PEG Precipitation); microvesicles
isolated
according via ultracentrifugation from medium conditioned with bone marrow-
derived
mesenchymal stem cells (Human hMSC MV ultracentrifugation); PBS control; and a
depleted medium control (hMSC conditioned medium depleted of MV). The amount
of
STAT3 phosphorylation observed in the fibroblasts was greater in the
microvesicles isolated
according to the methods of the present invention. See Figure 17.
Example 12: Use of the Microvesicles of the Present Invention as a Diagnostic
for
Metastatic Melanoma
[0322] BRAF is a human gene that makes a protein called B-Raf. More than 30
mutations of
the BRAF gene associated with human cancers have been identified. We have
designed per
primers to amplify the mutated form of BRAF that is linked to metastatic
melanoma. The
mutation is a T1799A mutation in exon 15 in BRAF. This leads to valine (V)
being
substituted for by glutamate (E) at codon 600 (now referred to as V600E). The
presence of
this mutation is required for treatment by the BRAF inhibitor Vemurafenib. The
SK-Me128
cell line, obtained from ATCC (Washington DC, Maryland) is known to have the
T1799A
mutation in exon 15 in BRAF. Microvesicles, isolated according to the methods
of the
present invention were obtained from medium conditioned by a 3 day incubation
in EMEM
(ATCC) + 10% serum (Atlanta Biologics, Atlanta, Georgia).
[0323] The isolated microvesicles were processed for DNA and RNA isolation
using
Qiagen's (Hilden, Germany) AUPrep DNA/RNA kit. Approximately 50 ng of RNA from
SK-MEL28 cells and microvesicles were reverse transcribed using iScriptTM
Reverse
Transcription Supermix (BioRad, Hercules, CA). A 2 ml aliquot was used for PCR
utilizing
Platinum PCR SuperMix (Life technology) per manufacturer's instructions. In
addition, 80
ng of DNA from SK-MEL28 cells and microvesicles was used for PCR utilizing
Platinum
PCR SuperMix per manufacturer's instructions. PCR products were run on a 3%
agarose gel
and visualized by Bio-Rad's gel-doc system. The results are shown in Figure
18.
The primers used were:
Sequence 1:
Forward: AGACCTCACAGTAAAAATAGGTGA (SEQ ID NO: 1)
Reverse: CTGATGGGACCCACTCCATC (SEQ ID NO: 2)
Amplicon length: 70
61
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
Sequence 2:
Forward: GAAGACCTCACAGTAAAAATAGGTG (SEQ ID NO: 3)
Reverse: CTGATGGGACCCACTCCATC (SEQ ID NO: 4)
Amplicon length: 82
[0324] In addition, samples of the microvesicles were lysed in RIPA buffer and
protein
concentration estimated by the microBSA assay kit. Approximately 50 microgram
were
loaded in each lane and the membranes were probed overnight (1:1000) by mouse
anti-BRAF
V600E antibody (NewEast Biosciences, Malvern, PA). Secondary antibody, goat
anti-mouse
(Pierce) was applied at 1: 10000 dilution for 1 hour. The Western blot shows
BRAF V600E
detection in SICMEL28 cell and MV lysate.
Example 13: Isolation of Microvesicles from Medium Conditioned Using a Culture
of
GFP- Labeled Bone Marrow-Derived Mesenchymal Stem Cells by the Methods of the
Present Invention
[0325] Homozygous transgenic mice expressing the enhanced Green Fluorescent
Protein
(GFP) under the direction of the human ubiquitin C promoter (C57BL/6-Tg(UBC-
GFP)30Scha/J) were obtained from Jackson Laboratories (Bar Harbor, Maine).
These mice
are known to express GFP in all tissues.
[0326] GFP-Mice (approximately 3-4 weeks of age) were euthanized by CO2
asphyxiation.
The limbs were cut above the hip and below the ankle joint. The hind limbs
were harvested
and skin, muscle, and all connective tissue was removed. The bones were then
placed in a
dish of ice cold sterile IX PBS and washed several times in PBS. The ends of
each bone were
snipped off with scissors. A 10 cc syringe with warmed medium (a-MEM
supplemented
with 20% fetal bovine serum and 1% penicillin/streptomycin/glutamine) was
forced through
the bone shaft to extract all bone marrow into a 150 mm plate. This was
repeated several
times to ensure all the marrow was removed. The cell mixture was pipetted
several times to
dissociate cells and the cell suspension was passed through a cell strainer
(70 p.m size) (BD
Biosciences, San Jose, CA) to remove large cell clumps or bone particles.
[0327] Initial cultures were seeded between 2-3 x 105 cells/cm2 in tissue
culture-treated
dishes (BD Biosciences, San Jose, CA) and placed in a cell incubator at 37 C
in 95%
humidified air and 5% CO2. After 72-96 hours, the non-adherent cells were
removed, the
culture flasks were rinsed once with PBS, and fresh medium was added to the
flask. The
62
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
cells were grown until 80% confluence was reached and then passaged by Trypsin-
EDTA
(Life technologies, Carlsbad, CA). Cells were split at a 1:4 ratio.
[0328] Alternatively, cryopreserved GFP Mouse-MSC's were thawed at 37 C and
immediately cultured in a-MEM supplemented with 20% fetal bovine serum and 1%
penicillin/streptomycin/glutamine at 37 C in 95% humidified air and 5% CO2.
They were
expanded similar to above.
[0329] The cells were grown in the flasks until 100% confluence was reached
(approximately
1 week). The supernatant were transferred to 50 mL conical centrifuge tubes
(Thermo Fisher
Scientific Inc., Weston, FL) and immediately centrifuged at 400 x g for 10
minutes at 4 C to
pellet any non-adherent cells. The supernatant was transferred to new 50 mL
conical
centrifuge tubes and centrifuged at 2000 x g for 30 minutes at 4 C to further
remove cells
and cell debris. The supernatants were collected and placed into 250 ml
sterile,
polypropylene disposable containers (Corning, Corning, NY). To the
supernatant, RNase and
protease free polyethylene glycol average molecular weight 6000 (Sigma
Aldrich, Saint
Louis, MO) at 8.5 w/v % and sodium chloride (final concentration 0.4 M) were
added. The
solution was placed in a cold room at 4 C overnight with rocking. The
solution was
transferred to 50 mL conical centrifuge tubes and centrifuged at 10000 x g at
4 C for 30
minutes. The supernatant was decanted and the microvesicle enriched pellet
resuspended in
phosphate-buffered saline (PBS). The microvesicle enriched solution was
transferred to
Amicon ultra-15 centrifugal filter units (nominal molecular weight limit 100
kDa) (Millipore,
Billerica, MA) and centrifuged at 5000 x g for 30 minutes. The filter units
were washed with
phosphate-buffered saline and centrifuged again at 5000 x g for 30 minutes.
The concentrated
sample was recovered (approximately 200-400 1) from the bottom of the filter
device.
Protein concentration was determined by the micro BSA Protein assay kit
(Pierce, Rockford,
IL) and the enriched microvesicle solution was stored at -70 degrees or
processed for
downstream use (e.g. protein, RNA, and DNA extraction).
[0330] To determine cellular uptake of the microvesicles, normal human skin
fibroblasts
were labeled with Vybrant-Dio (Life technology) per manufacturer instructions.
Normal skin
fibroblasts were plated on fibronectin (Sigma-Aldrich) coated 4-well Nunc* Lab-
Tek* 11
Chamber Slide (Thermo Fisher Scientific Inc.) (5 x 10 cells per well). Cells
were stained
with the nuclear dye Hoechst 33342 (Life technology) per manufacturer's
instructions. Dil
63
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
labeled fibroblasts were treated with microvesicles isolated from GFP
expressing mouse
MSC for 24 hours. Images were captured with an inverted IX81 Olympus
microscope and
ORCA-AG Hamamatsu digital camera. See Figures 20 and 21. Importantly, these
images
show that the microvesicles containing GFP were taken up by the cells.
Example 14: Use of the Microvesicles of the Present Invention as a Therapy to
Promote
or Enhance Wound Healing
[0331] Full thickness wounds were created on the backs of pigs using a 10 mm
punch biopsy
instrument. Microvesicles were isolated from culture medium conditioned using
autologous
bone marrow-derived mesenchymal stem cells, either according to the methods
described in
Example 1 (the "conventional ultracentrifugation method"), or by the methods
described in
Example 3. 30 micrograms of microvesicles were administered to the wounds by
local
injection at the time of wounding and at Days 1 and 2. Controls were treated
with saline or
allowed to heal air exposed. After 5 days, the animals were euthanized, and
the wounds
examined.
[0332] Figure 22 shows the histology of the wounds 5 days post-wounding. At 5
days,
wounds treated with microvesicles isolated according to the methods of the
present invention
(i.e., according to the methods described in Example 3) appeared smaller than
saline controls,
air exposed controls and wounds treated with microvesicles prepared by
ultracentrifugation.
The wounds treated with microvesicles prepared by ultracentrifugation showed
an enhanced
inflammatory response, compared to those treated with microvesicles prepared
according to
the methods of the present invention and both controls.
[0333] In another study, second degree burn wounds were created on the backs
of pigs using
a brass rod heated to 100 C. Microvesicles were isolated from culture medium
conditioned
using autologous bone marrow-derived mesenchymal stem cells, either according
to the
methods described in Example 1 (the "conventional ultracentrifugation
method"), or by the
methods described in Example 3. 30 micrograms of microvesicles were
administered to the
wounds by local injection at the time of wounding and at days 1 and 2.
Controls were treated
with saline or allowed to heal air exposed.
[0334] Over the course of the experiment (up to 28 days post-burn injury)
wounds treated
with microvesicles prepared by ultracentrifugation were significantly more
inflamed than
64
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
those treated with microvesicles prepared according to the methods of the
present invention
(i.e., according to the methods described in Example 3). See Figure 23.
Similarly, wounds
treated with microvesicles prepared by ultracentrifugation were significantly
more inflamed
than saline controls and air exposed controls. Burn wounds treated with
microvesicles
prepared according to the methods of the present invention did not appear
significantly more
inflamed than controls.
[0335] Figure 23 illustrates the difference in inflammation at Day 7 post-
wounding between
wounds treated with microvesicles prepared by ultracentrifugation,
microvesicles prepared
according to the methods of the present invention and an air exposed control.
Microscopically, abscess formation was seen in both full thickness and burn
wounds treated
with microvesicles prepared by ultracentrifugation. Without intending to be
bound by
scientific theory, the inflammation noted with microvesicles prepared by
ultracentrifugation
was thought to be due to damaged microvesicles, which can easily stimulate an
inflammatory
cascade. The microvesicles of the present invention may also confer additional
benefits by
including additional particles.
[0336] Figure 24 shows a second degree porcine burn wound treated with
microvesicles
isolated by the methods of the present invention 28 days after burn injury.
There is a
significant remodeling of collagen, with the appearance of ground substance.
These findings
are indicative of dermal remodeling with collagen type ill formation. There is
also denim!
epidermal induction resulting in a thickened epidermis that appears well
anchored to the
dermis. These findings are not observed in scar formation and are more
consistent with
dermal regeneration. An epidermis forming over a scar is easily subject to re-
injury due to
the inability to anchor well to a scarred dermis.
[0337] Figure 25 shows a second degree porcine burn wound treated with saline
28 days after
burn injury. There is minimal dermal regeneration with a flattened epidermis.
The lack of
significant rete ridge formation is highly suggestive of an inadequately
anchored epidermis.
These findings are much more indicative of scar formation with the risk of
continued injury.
[0338] Figure 26 shows a full thickness porcine wound treated with
microvesicles isolated
according to the methods of the present invention 28 days after injury. There
is ingrowth of a
nerve (illustrated by the arrows) into the remodeling derails, likely
stimulated by the
application of the microvesicles. The nerve grown is accompanied by an
angiogenic response
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
(circled areas). The nerve appears to be a developed structure and is not due
to simple axon
sprouting. This is a unique finding and has never been reported and was also
not observed in
control wounds or wounds treated with microvesicles prepared by
ultracentrifugation. These
observations are highly indicative of complex tissue regeneration with the
ability to generate
mature elements from all germ layers including epidermis, stoma, vasculature
and nervous
tissue. These methods then appear to be widely applicable to the treatment of
numerous
conditions including traumatic, inflammatory, neoplastic and degenerative
disorders of
ectodermal, endodemial and mesodemial derived tissues.
[0339] Figure 27 shows a full thickness porcine wound treated with
microvesicles isolated by
the methods of the present invention 28 days after injury. This Figure
illustrates the
observations described in Figure 26 at greater magnification. In A) the nerve
growth appears
to be following a path related to the angiogenic response. This finding is
interesting as nerve
growth is well known to follow angiogenesis in embryologic development. Again,
these
findings are indicative of tissue regeneration. B) shows the nerve at higher
power. C) better
illustrates the angiogenesis adjacent to the nerve growth.
[0340] Bone formation was seen in all treatment groups (control and
microvesicle treated) in
the porcine full thickness wound model. See Figure 28. Animals received a
total of 1.44 mg
microvesicles (half prepared according to the methods of the present invention
and half by
ultracentrifugation). There then appeared to be a systemic effect stimulating
the formation of
bone in all wounds. Bone formation tended to occur more in more inflammatory
wounds
suggesting a synergistic effect of local inflammatory mediators and the
systemic effect of
microvesicles.
Example 15: Use of the Microvesides of the Present Invention as a Therapy to
Repopulate Bone Marrow and Regenerate Complex Structures
[0341] C57/CJ6 (GFP) mice were lethally irradiated with two cycles of 400 cGy
gamma
irradiation to ablate their host bone marrow progenitors. After irradiation,
mice were treated
in an approximately 2cm area with an ablative fractional Erbium: YAG laser.
After laser
treatment, a plastic chamber was adhered to the skin, and bone marrow derived
cells obtained
from a syngeneic GFP<+>transgenic mouse were added to the chamber. The GFP+
bone
marrow cells included, freshly harvested total bone marrow cells, lineage
negative selected
66
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
bone marrow cells, mesenchymal stem cells and bone marrow complete cultured
cells (as
described in this application). In only a few animals was chimerism able to be
achieved;
detected by circulating GFP+ cells 4 to 6 weeks after administration of cells.
(See Figure 29.)
Surprisingly, many animals survived without evidence of donor bone marrow
engraftment.
Overall (in all groups of cells given) 30% of animals receiving cells
survived. Among the
different groups, survival rates were highest for animals receiving lineage
negative selected
cells (45%) and fresh bone marrow cells (30%). Control irradiated animals
receiving no cells
had a 100% mortality rate. Cytokines have failed to similarly rescue similarly
lethally
irradiated animals and no functional donor bone marrow engraftment could be
demonstrated
in these surviving animals. Microvesicles secreted by the delivered cells are
likely
responsible for the recovery of the host bone marrow leading to survival of
these animals.
We have demonstrated that fresh bone marrow (which includes lineage negative
cells) and
mesenchymal stem cells produce ample amounts of microvesicles that could
accomplish this
effect. In another study, C57/CJ6 (OFF) mice were lethally irradiated with two
cycles of 400
cGy gamma irradiation to inhibit their hair growth and partially ablate their
bone marrow.
After irradiation, the backs of the mice were shaved and the mice were then in
an
approximately 2cm area with an ablative fractional Erbium: YAG laser. After
laser
treatment, a plastic chamber was adhered to the skin and bone marrow derived
cells obtained
from a syngeneic GFP+ transgenic mouse were added to the chamber. The GFP+
bone
marrow cells included, freshly harvested bone marrow cells, lineage negative
selected bone
marrow cells, mesenchyrnal stem cells and bone marrow complete cultured cells
(as
described in this application). In no animals was chimerism able to be
achieved; detected by
circulating GFP+ cells 4 to 6 weeks after administration of cells. See Figure
30. Animals
receiving laser treatment alone had no to very minimal short stubby hair
growth. Figure 30
(A). In animals given bone marrow cells, there was significant, long lasting
hair growth.
Figure 30 (A & B). These findings were most dramatic in mice treated with GFP+
lineage
negative selected cells and total fresh GFP+ bone marrow cells. Hair growth
could be
detected in 2 weeks and continued to grow for several months. Skin biopsies
were taken in
the area of new hair growth but no GFP+ cells were detected. Functional
engraftment of bone
marrow cells could also not be detected in any animal by FACS analysis. Figure
30 (C). As
with the example in Figure 29, cytokines have not been demonstrated to have
this effect in
restoring hair growth. Microvesicles secreted by the delivered cells are
likely responsible for
the stimulation of hair growth.
67
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
Example 16: Use of the Microvesicles of the Present Invention to Promote or
Stimulate
Angiogenesis and to Promote or Stimulate Fibroblast Proliferation
[0342] Isolation of Bone Marrow Aspirate Microvesicles: Approximately 25 ml of
fresh
whole bone marrow was obtained from AllCells, Inc. (Alameda, CA). The bone
marrow was
carefully placed into new 50 ml conical centrifuge tubes and centrifuged at
400 x g for 30
minutes at room temperature. The supernatant was carefully removed
(approximately 15 ml)
and placed into new 50 ml conical centrifuge tubes (Thermo Fisher Scientific
Inc., Weston,
FL) and centrifuged at 2000 x g for 30 minutes at 4 C. The supernatant was
again carefully
removed and placed into new 50 ml conical centrifuge tubes to which sterile
alpha-minimum
essential medium (a-MEM) (Mediatech Inc., Manassas, VA) was added in a 1:10
(Bone
marrow supernatant to medium) ratio. To the solution, RNase and protease free
polyethylene
glycol average molecular weight 6000 (Sigma Aldrich, Saint Louis, MO) at 8.5
w/v % and
sodium chloride (final concentration 0.4 M) were added. The solution was
placed in a cold
room at 4 C overnight with rocking. The solution was centrifuged at 10000 x g
at 4 C for
30 minutes. The supernatant was decanted and the microvesicle enriched pellet
resuspended
in phosphate-buffered saline (PBS). The microvesicle enriched solution was
transferred to
Amicon ultra-15 centrifugal filter units (nominal molecular weight limit 100
kDa) (Millipore,
Billerica, MA) and centrifuged at 5000 x g for 30 minutes. The filter units
were washed with
phosphate-buffered saline and centrifuged again at 5000 x g for 30 minutes.
The
concentrated sample was recovered (approximately 200-400 pl) from the bottom
of the filter
device.
[0343] Angiogenesis assay: Angiogenesis was measured using an endothelial tube
formation
assay (Invitrogen Life Technologies, Grand Island, NY). Cryopreserved primary
Human
Umbilical Vein Endothelial cells (HUVEC) (Invitrogen Life Technologies) were
grown in a
75-cm tissue-culture flask for 6 days in Medium 200PRF supplemented with 2%
low serum
growth supplement (Invitrogen Life Technologies). Cells were then plated at a
density of 3 x
10<4>in a 24 well tissue culture plate containing medium without supplement.
HUVEC Cells
were subsequently treated with bone marrow microvesicles (approximately 100
g). PBS
was used as the vehicle control. Treated cells were incubated for 6 hours at
37 C and 5%
CO2. Calcein AM fluorescent dye at a concentration of 2 pg/m1 was used for
visualization of
tube formation. Fluorescent images were captured with an inverted IX81 Olympus
68
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
microscope (Olympus America, Center Valley, PA). Bone marrow MV showed
significant
tube formation capacity as compared to the vehicle (PBS) control (see Figure
31).
[0344] Growth assay: Normal adult fibroblasts were plated onto 24-well plates
(10000
cells/well) in growth media (5% FBS, 1% glutamine, 1% Penicillin/Streptomycin)
for the
assays. After overnight incubation, three wells were randomly selected and the
cells were
stained with NucBlue Live ReadyProbes Reagent (Invitrogen Life technologies)
(Day 0).
Fluorescent images were captured using the EVOS FL Auto Cell Imaging System
(Invitrogen
Life technologies). Fibroblasts were re-fed with fresh medium containing bone
marrow-
derived microvesicles (approximately 100 jig) or PBS (vehicle control) and
after three days
(Day 3), cells were stained and imaged. Bone marrow-derived microvesicle
treated
fibroblasts increased approximately three fold in number (compared to Day 0)
and at a
significant greater rate than the vehicle control (Figure 32, panel A and
Figure 32, panel B).
Example 17: F,V-Mediated Delivery of Bioactive Materials to Target Cells
[0345] According to certain exemplary embodiments of the invention, EVs
described herein
are useful for delivering one or more bioactive agent (e.g., collagen VII
proteins or peptides,
collagen VII mRNA, STAT3-signalling activators, canonical Wnt activators and
the like) to a
target cell. This example demonstrates delivery of EVs to RDEB fibroblast
cells that were
deficient in COL7A1 expression compared to wild-type fibroblast cells. The EVs
stimulated
collagen VII expression in the RDEB fibroblasts. The EVs also stimulated the
expression of
markers related to wound healing in the RDEB fibroblasts.
[0346] Figure 44 shows the validation of an in vitro cell line derived from an
infant
diagnosed as having RDEB (Hallopeau-Siemens type). Vesicle exchange was
observed
between BM-MSCs and RDEB fibroblasts (Figure 45). Collagen VII was co-isolated
with
BM-MSC EVs (Figure 46), and COL7A1 mRNA was enriched in MB-MSC EVs (Figure
47).
[0347] RDEB fibroblasts were treated with BM-MSC EVs on day 1, were washed on
day
three, and showed an increase in collagen VII expression on day six (Figure
48). A
chemoselective ligation assay (utilizing "click iT" reaction chemistry)
revealed the
production of new collagen VII from RDEB fibroblasts following co-treatment
with BM-
MSC EVs (Figure 49). BM-MSC EVs were shown to increase in vitro surrogate
assays
69
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
related to wound healing (e.g., proliferation and trypsin-resistance) of RDEB
fibroblasts
(Figure 50).
[0348] BM-MSCs that were delivered in saline to burn patients in a clinical
trial were shown
to secrete large numbers of EVs (CD63 positive) in saline within hours (shown,
4 hours)
(Figure 51).
[0349] A model of BM-MSC-mediated wound healing is set forth in Figure 52.
Example 18: Extracellular Vesicles Derived From Bone Marrow Mesenchymal Stem
Cells in the Treatment of Recessive Dystrophic Epidermolysis Bullosa
[0350] Local and intravenous injections of allogeneic bone marrow-derived
mesenchymal
stem cells (BM-MSCs) have been shown to promote wound healing in Recessive
Dystrophic
Epidermolysis Bullosa (RDEB). We have described that extracellular vesicles
(EVs) derived
from BM-MSCs (BM-MSC EVs) are largely responsible for the healing effects
attributed to
BM-MSCs. We have also discovered that EVs can transfer collagen VII (Col VII)
to RDEB
cells. We propose the first clinical trial where BM-MSC EVs from healthy
allogeneic donors
will be applied topically to wounds in RDEB patients, maximizing safety and
comfort for the
patient while enhancing wound healing. Treating with EVs has many advantages
over
cellular therapy including much lower risks of genetic instability and
malignant
transformation. We will secure FDA Investigational New Drug (IND) approval and
manufacture clinical grade BM-MSC EVs for an open-label dose-escalation Phase
I study of
topically applied allogeneic BM-MSC-derived EVs in 30 RDEB patients.
Specific Aims
Aim I - Gain IND approval from FDA for a Phase I dose escalation trial of
topically applied
BM-MSCEVs in RDEB patients and prepare optimal clinical grade BM-MSC-EVs
[0351] We will apply specific criteria for donor BM-MSC EV screening,
selection and
functional characterization for use in our Phase I clinical trial. This
includes defining details
of EV manufacturing and product characteristics for the IND as they relate
specifically to
RDEB. We will employ analyses found to be important in our stem cell based
clinical trials
and assess donors for BM-MSC EVs based on functional performance on recipient
RDEB
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
cells. We will select optimal BM-MSC donors and manufacture BM-MSC EVs to be
used
for treatment of RDEB patients in our trial.
Aim 2--- Conduct an open-label, dose-escalation clinical trial of topical,
alloacneic BM-MSC-
derived EVs in the treatment of wounds in 30 RDEB patients
[0352] Dosing will be based on the PI's successful clinical trial of topically
applied BM-
MSCs to burn patients. There will be 3, serially escalating, dosing groups
with 10 patients to
complete each group. The dosing schedule will be first dost at treatment day 0
with three
additional doses given monthly (total of four treatments over three months).
Primary
outcomes will assess safety and tolerability of topically applied BM-MSC EV;
secondary
outcomes will assess wound healing, pain, itch, and cosmesis (including
pigment, scar
assessment and evidence of tissue regeneration). Integrium Contract Research
Organization
will assist in the clinical trial.
Research Strategy
Aim 1 ¨ Gain IND approval from FDA for use of topical BM-MSC-derived
extracellular
vesicles (EVs) for Phase I clinical trial to treat wounds of RDEB patients.
[0353] Direct application of bone marrow-derived stem and progenitor cells to
burns and
recalcitrant chronic non-healing wounds lead to wound closure and dermal
rebuilding.
Chronic wound patients (of greater than one year in duration) were treated
with bone marrow
stem and progenitor cells. MSC's represented approximately 30% of the cells
given to
patients. Evidence of healing was observed in all treated patients, with many
achieving full
closure of their wounds. Some subjects have remained healed for more than 7
years
(eventually lost to follow-up). Among the clinical findings noted were dermal
rebuilding and
the lack of scarring in healing wounds noted both clinically and
histologically. Clinically,
there is elevation of the wound bed with little to no atrophy/depression on
closure of healed
wounds (Figure 33). Histologic evidence supports dermal rebuilding in treated
wounds.
[0354] Microscopic findings included increased collagen formation and ground
substance
deposition. Among the most surprising observations were the restoration of
structures such
as reti.cul.in and elastic fibers (Figure 34). These fibers are
characteristically lost in the
healing of even uncomplicated acute and chronic wounds. Overall, these
findings support the
ability to induce healing in a non-healing wound, restore tissue volume
deficits, stimulate
71
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
tissue regeneration and greatly reduce scarring using topically applied bone
marrow derived
stem cells, with no adverse events.
[0355] Allogeneic BM-MSCs applied topically to burn wounds showed evidence of
rapid
epithelialization, reduced scarring, restoration of pigment and regeneration
of hair follicles.
No related severe adverse events nor evidence of rejection were observed.
Follicular
regeneration (Figure 35) consistent with tissue regeneration was noted in
after topical
application of BM-MSCs to burn wounds. Regeneration of hair follicles was,
however, not
observed in non-treated areas of the burn injury. Dramatic re-pigmentation
(Figure 36)
indicative of tissue regeneration has also been observed.
[0356] The rapid re-pigmentation noted in patients is unheard of by any other
means of
treatment as these wounds typically undergo a prolonged (often permanent)
period of post
burn leukoderma. Restoration of elasticity in burned skin was also an
extraordinary finding
(Figure 37). What is especially remarkable about these results are that they
occur after only
short term topical application of BM-MSCs. Especially within this time frame
is not likely
that cells will survive long and/or engraft by this means of administration.
This strongly
indicates that the delivered cells are able to rapidly communicate complex
messages leading
to a robust regenerative and healing effect. Naked cytokines, nucleic acids
and transcription
factors would not survive long in the burn wound environment nor is it
feasible that single
factors would be capable of generating such a complex response.
[0357] Without intending to be bound by scientific theory, it was hypothesized
that
membrane bound EVs are capable of generating such a clinical response. In
examining just
the saline vehicle in which the cells are delivered to patients (after removal
of cells) we have
found more than 1.6 X 1011 EV particles / mL present, confirming that we are
delivering
substantial numbers of EVs to patients. The EVs within the samples
administered to patients
are intact and possess characteristic EV markers. Recently, we have published
that EVs
stimulate normal and chronic wound fibroblast proliferation and migration, and
enhances
angiogenesis via activation of STAT3-mediated target genes (Shabbir A, Cox A,
Rodriguez-
Menocal L, Salgado M, Van Badiavas E. Mesenchymal Stem Cell Exosomes Induce
Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and
Enhance
Angiogenesis In Vitro. Stem Cells Dev 2015;24:1635-47). Our preclinical
studies also
strongly support BM-MSC EV stimulation of wound healing and tissue
regeneration (Figures
38 and 39). Evidence of tissue regeneration in our preclinical studies, such
as nerve growth,
have not been realized by other means. In particular, our preclinical studies
have
72
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
demonstrated that other methods of EV isolation damage vesicles which lead to
the
generation of an undesirable inflammatory response. Our novel method does not
damage
EVs and has been shown to induce rapid healing without the generation of an
inflammatory
response (Figure 40). Preliminary data indicated that these vesicles, aside
from delivering
pro-healing factors, could transport Col VII protein and functional COL7A1
mRNA to RDEB
fibroblasts (Figures 41 and 42).
[0358] In an assay to capture Col VII protein in combination with a
chemoselective ligation
reaction (Click iT , ThermoFisher) we found RDEB fibroblasts were actually
induced by
BM-MSC EVs (at a dose of 10 lig/mL) to make new Col VII protein (Figure 42).
BM-MSC
EVs significantly promote both RDEB proliferation and resistance to trypsin
digestion
(Figure 43). These are standard assays used to assess gain-of-function and the
pro-wound
healing potential of RDEB dermal fibroblasts. These data provide evidence that
BM-MSC
EVs have benefits for RDEB in addition to their potential to improve wound
healing. To
gain IND approval for the clinical trial, we will establish manufacturing
within contracted
GMP manufacturing facilities that the PI has successfully worked with in
previous trials.
Aside from meeting general manufacturing requirements, we will address issues
relating
specifically to the treatment of RDEB. We will establish criteria for donor
screening and
selecting to optimize potential pro-regenerative activities of EVs. BM-MSC EV
product
characteristics will be defined. These parameters include protein
concentrations, EV size
distributions (e.g., using NanoSight NS300), surface marker characterization,
removal of
reagents used during manufacturing, and stability testing of the product.
Using mass
spectrometry and RNA sequencing, we will define protein and RNA cargo contents
of several
BM-MSC EV donors, correlating cargo with functional assay performance. BM-MSC
EV
functional activities will be defined on recipient RDEB cells, including in
vitro studies to
establish potency for wound healing and reversal of phenotype, including RDEB
fibroblast
proliferation and trypsin resistance assays. Additionally, endothelial
angiogenesis assays will
be examined in vitro.
Aim 2 - Conduct an open-label, dose-escalation, clinical trial of topical,
allogeneic BM-
MSC-derived EVs in the treatment of wounds in 30 RDEB patients
Approach;
[0359] The clinical trial will be an open-label, pilot study with three
escalating treatment
dose groups (10 patients per dose level). Investigators will identify target
lesions between 5
73
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
and 50 cm2 for treatment. EVs in saline will be applied underneath a thin
silicone sheet
dressing as a primary layer with an overlying secondary standard-of-care wound
dressings.
Control wounds will be treated with saline underneath a silicone sheet.
Treatment frequency
will at baseline, 4 weeks, 8 weeks, and 12 weeks. Dose levels will be derived
from levels
administered in the PI' s burn trial. Digital images will be taken of
treatment and control
wounds. Treatment and control wounds will be measured using the Silhouette
device.
[0360] Outcomes will be assessed monthly for 12 weeks. Given our previous
experience we
expect to see a greater than 50% stimulation in healing. Clinically and from
the standpoint of
laboratory variation, we have assumed a pooled standard deviation of 20. Here
a statistical
power (not to be confused with probability) of 0.8 is recommended for clinical
studies
examining differences in this range (Breau RH, Carnat TA, Gaboury I.
Inadequate statistical
power of negative clinical trials in urological literature. The Journal of
urology
2006;176:263-6; Ichihara K, Boyd JC. An appraisal of statistical procedures
used in
derivation of reference intervals. Clinical chemistry and laboratory medicine
: CCLM /
FESCC 2010;48:1537-51). In fact, evaluating differences of greater than 50%
could require
a statistical power of less than 0.8. The probability that a chance difference
will be called
significant is denoted by a (type I error) and typically should have a
threshold of 0.05, below
which a p value is deemed significant. The chance of missing a real difference
(type II error)
is designated by 0. With these values, which we think are realistic, we have
calculated the
following sample size (Table 1) that will be required. The a refers to the
probability that a
chance difference will be called significant. As is customary, the threshold
is 0.05 (95%
confidence level), below which a p value is deemed significant. The values in
Table I are for
two-tailed tests. The sample size chosen of 10 patients per group will be more
than adequate
even when increasing the statistical power well beyond the recommended 0.8 to
0.95. While
this is more stringent than we might need, it does ensure that we have proper
statistical power
with the number of subjects proposed.
Number of Patients
Power 11 a = 0,10 = 0,05 a= 0.02 a = 0,01
0.80 0.2 3 4 $ 6
0.90 0.1 4 5 6 7
0.95 0.05 5 6 7 8
74
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
Eligibility Criteria:
[0361] Key inclusion criteria includes: Male or female patients, 12 years or
older at time of
screening, and given consent with guardian, if under 18 years of age; Have
confirmed RDEB
diagnosis, as defined by clinical presentation and histologic confirmation;
Have at least 1
active wound between 5 and 50 cm2 on arms and/or legs; Females of childbearing
potential
must have a negative urine or serum pregnancy test at screening and be using
an acceptable
form of birth control (oral/ implant/ injectable/transdermal contraceptives,
intrauterine
device, or other forms of birth control). Key exclusion criteria will include:
Clinical evidence
of infection; Concurrent immunosuppressive therapy of any kind for any reason.
Primary Outcome Measures:
[0362] The following primary outcome measures will be assessed.
1) Screening for and documenting all adverse events, particularly those
suspected to be
related to treatment.
2) Characterization and analysis of all reported related adverse events.
3) Evaluating participants that have discontinued due to voluntary withdrawal
or intolerance
of the treatment.
[0363] Of note, in this application we are proposing the use of EVs derived
from allogeneic
BM-MSCs in multiple doses to improve wound healing and possibly introduce Col
VII in
RDEB wounds. The EU and US clinical registries list well over 1,000 clinical
trials
worldwide using BM-MSCs and therefore the paracrine materials they produce
(including
EVs) with approximately half of all studies employing allogeneic sources. To
date, no
serious related adverse events have been reported. In our trial using
allogeneic BM-MSCs in
burn patients we have not detected any evidence of a related adverse event nor
immune
response to the delivered material which we know contains ample amounts of BM-
MSC EVs.
Analysis of an immune response in our study includes a sensitive ELISA assay
cleared by the
FDA which will detect minute subclinical evidence of an immune response in
mixed
lymphocyte reactions. Despite giving multiple doses in our trial, we have not
detected any
immune response in these assays. This is however not unexpected as BM-MSCs are
known
to have immune modulatory properties (Bartholomew A, Polchert D, Szilagyi E,
Douglas
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
GW, Kenyon N. Mesenchymal stem cells in the induction of transplantation
tolerance.
Transplantation 2009;87:S55-7; Siegel G, Schafer R, Dazzi F. The
immunosuppressive
properties of mesenchymal stem cells. Transplantation 2009;87:S45-9; Sundin M,
Barrett AJ,
Ringden 0, et al. HSCT recipients have specific tolerance to MSC but not to
the MSC donor.
J Immunother 2009;32:755-64) which have been shown to be mediated by the EVs
they
produce (Bruno S. Deregibus MC, Camussi G. The secretome of mesenchymal
stromal cells:
Role of extracellular vesicles in immunomodulation. Immunology letters
2015;168:154-8;
Chen W, Huang Y, Han J, et al. Immunomodulatory effects of mesenchymal stromal
cells-
derived exosome. Immunologic research 2016;64:831-40; Li X, Liu L, Yang J, et
al.
Exosome Derived From Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-
181c Attenuating Burn-induced Excessive Inflammation. EBioMedicine 2016;8:72-
82). We
believe that carefully moving forward with an EV based therapy for RDEB is
justified given
that numerous trials, including our own, have been delivering allogeneic
mesenchymal stem
cell based EVs for years without evidence of immune response or rejection. We
will of
course monitor patients very closely for any evidence of immune response or
rejection using
measures well established in our trials. As we have recently found that BM-MSC
EVs can
deliver Col VII and induce RDEB cells to make Col VII, a question has been
raised as to the
potential for patients to develop antibodies to Col VII. This has been a
concern in many
clinical trials performed on RDEB patients but none have demonstrated an
adverse response
even in trials where Col VII antibodies were detected (Petrof, infra). In pre-
clinical trials
directly administering Col VII protein, even when circulating antibodies to
Col VII were
detected, worsening of disease, increased blistering and binding of these
antibodies to skin
was not observed (Riazifar, infra; Palazzi X, Marchal T, Chabanne L, Spadafora
A, Magnol
JP, Meneguzzi G. Inherited dystrophic epidermolysis bullosa in inbred dogs: A
spontaneous
animal model for somatic gene therapy. J Invest Dermatol 2000;115:135-7; South
AP, Uitto
J. Type VII Collagen Replacement Therapy in Recessive Dystrophic Epidermolysis
Bullosa-
How Much, How Often? J Invest Dermatol 2016;136:1079-81; Woodley DT, Cogan J,
Wang
X, et al. De novo anti-type VII collagen antibodies in patients with recessive
dystrophic
epidermolysis bullosa. J Invest Dermatol 2014;134:1138-40). BM-MSC trials in
RDEB
patients also failed to demonstrate worsening of disease, increased blistering
or evidence of
induction of autoimmunity, even when the presence of Col VII could be
established and/or
anti-collagen antibodies could be detected (El-Darouti M. Fawzy M, Amin I, et
al. Treatment
of dystrophic epidermolysis bullosa with bone marrow non-hematopoietic stem
cells: a
76
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
randomized controlled trial. Dermatol Ther 2016;29:96-100; Riazifar M, Pone
EJ, Lotvall J,
Zhao W. Stem Cell Extracellular Vesicles: Extended Messages of Regeneration.
Annu Rev
Pharmacol Toxicol 2017;57:125-54; Petrof G, Lwin SM, Martinez-Queipo M, et at.
Potential
of Systemic Allogeneic Mesenchymal Stromal Cell Therapy for Children with
Recessive
Dystrophic Epidermolysis Bullosa. J Invest Dermatol 2015;135:2319-21). Both
chemical
(Woodley, infra) and bone marrow stem transplantation (Wagner JE, Ishida-
Yamamoto A,
McGrath JA, et al. Bone marrow transplantation for recessive dystrophic
epidermolysis
bullosa. The New England journal of medicine 2010;363:629-39) induced
expression of Col
VII in RDEB patients could not establish any concern about induced anti-Col
VII antibodies
in treated patients. This is not surprising as more than half of RDEB patients
normally
express the predominant antigenic portions of Col VII responsible for antibody
production
(Woodley DT, Cogan J. Hou Y, et al. Gentamicin induces functional type VII
collagen in
recessive dystrophic epidermolysis bullosa patients. J Clin Invest 2017; Jones
DA, Hunt SW,
3rd, Prisayanh PS, Briggaman RA, Gammon WR. Immunodominant autoepitopes of
type VII
collagen are short, paired peptide sequences within the fibronectin type III
homology region
of the noncollagenous (NCI) domain. J Invest Dermatol 1995;104:231-5; Lapiere
JC,
Woodley DT, Parente MG, et al. Epitope mapping of type VII collagen.
Identification of
discrete peptide sequences recognized by sera from patients with acquired
epidermolysis
bullosa. J Clin Invest 1993;92:1831-9; Pfendner E, Uitto J, Fine JD.
Epidermolysis bullosa
carrier frequencies in the US population. J Invest Dermatol 2001;116:483-4).
[0364] The findings that many RDEB patients undergo focal reverse mosaicism
(with areas
durable skin containing of intact Col VII) also provides strong evidence that
introduction of
Col VII into RDEB patients is not likely induce a pathogenic response. Even
so, if the
production of Col VII antibodies were to produce a clinically relevant effect,
it would more
resemble a form of epidermolysis bullosa acquisita; a much more manageable
disease than
RDEB. It also needs to pointed out that we are proposing the use EV's and not
stem cells.
Stem cells have the ability to engraft and are not retrievable. EV's are not
viable, cannot
persist and do not replicate. Therefore, if we detect any evidence or
suspicion of an adverse
outcome (including rising anti Col VII antibody titers), the treatment would
be stopped and is
reversible.
77
CA 03076610 2020-03-20
WO 2019/060719
PCT/US2018/052213
Secondary Outcome Measures:
[0365] The following secondary outcome measures will be assessed:
1) Blister/erosion reduction based on change in body surface area index
(BSAI).
2) Target wound size reduction or closure. Wound size reduction is an
assessment to
determine possible efficacy of the EV treatment. Target wounds will be
measured using
Silhouette (Aranz Medical), an FDA-approved medical device wound imaging, 3D
measurement and documentation system using noninvasive laser technology
providing
accurate, precise and repeatable wound assessments.
3) Physician Assessment of Individual Signs ¨ this scale evaluates blistering
and erosions,
oozing/crusting/weeping, pruritis, erythema on unblistered surrounding skin
and pain. Body
areas will include head/neck, upper limbs, trunk and lower limbs.
4) Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI).
5) VAS Pain Score questionnaire and pain medication use.
6) ItchyQuant scale score (validated scale to assess itch) (Haydek CO, Love E,
Mollanazar
NK, et al. Validation and Banding of the ItchyQuant: A Self-Report Itch
Severity Scale. J.
Invest. Dermatol. 2017;137:57-61.).
7) Children's Dermatology Life Quality Index (CDLQI). Initial trial site is
the University of
Miami (with world renowned Pediatric Dermatologist Dr. Lawrence Schachner).
Secondary
sites will be determined.
[0366] Although the various aspects of the invention have been illustrated
above by reference
to examples and preferred embodiments, it will be appreciated that the scope
of the invention
is defined not by the foregoing description but by the following claims
properly construed
under principles of patent law.
78