Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03078929 2020-04-09
WO 2018/071378
PCT/US2017/055872
CLOSED LOOP ENHANCED OIL RECOVERY
CROSS-REFERENCE TO RELATED APPLICATION
This application claims the benefit of U.S. Provisional Application No.
62/406,102, entitled "CLOSED LOOP ENHANCED OIL RECOVERY," filed October 10,
2016, the disclosure of which is incorporated herein by reference in its
entirety.
BACKGROUND
Hydrocarbons, particularly heavy hydrocarbons, obtained from subterranean
formations
are often used as energy resources, as feedstocks, and as consumer products.
Concerns over
depletion of available hydrocarbon resources and concerns over declining
overall quality of
produced hydrocarbons have led to development of processes for more efficient
recovery,
processing and/or use of available hydrocarbon resources. In situ processes
may be used to
remove hydrocarbon materials from subterranean formations. The chemical and/or
physical
properties of the hydrocarbon material in subterranean formations may need to
be changed to
allow the hydrocarbon material to be more easily removed from the subterranean
formation.
Examples of chemical and physical changes may include in situ reactions that
produce
removable fluids, composition changes, solubility changes, density changes,
phase changes,
and/or viscosity changes of the hydrocarbon material in the formation. In some
cases, heaters
may be placed in wellbores to heat a formation during an in situ process. U.S.
Pat. No.
7,575,052 discloses in more detail heat sources, which may be used to heat a
subterranean
formation. In-situ thermal conduction, in the past, has been slow, and thus
considered
impractical for heavy oil recovery.
In oil shale work, attempts have been made to visualize alternatives to the
use of
expensive electric heating. Transferring heat directly from a heater to the
formation would save
almost half the energy of electric heating. In some production processes, high
temperature
circulating fluids in pipes have been used to efficiently transmit heat by
convection. In
refineries, molten salts are pumped in pipes to transfer intense heat.
1
CA 03078929 2020-04-09
WO 2018/071378
PCT/US2017/055872
There is a need in the art for cost effective methods for enhanced oil
recovery using
thermal conduction. Provided herein are systems and methods addressing these
and other needs
in the art.
SUMMARY
In general terms, this disclosure is directed to heating an underground
reservoir,
particularly an underground reservoir comprised of heavy oils. This
disclosure's main purpose
is to reduce the viscosity of the heavy oil to a point at which the fluids
become mobile and flow
at an economic rate. The process of this disclosure involves heat conduction,
which has, in the
past been considered less efficient than convective heat transfer, as
discussed in the
Background. Operational expenditures may, however, be minimized in this
disclosure. If steam,
for example, is used in the closed loop, there is no water lost, and there is
little concern about
multi-phase flow. The circulating fluid can, however, be any fluid that allows
for the greatest
heat transfer to the reservoir fluids. Various aspects are described in this
disclosure.
In one possible configuration and by non-limiting example, a fully enclosed
wellbore is
formed through an underground reservoir that contains an oil shale
formation/heavy oil. In
some embodiments, the heavy hydrocarbon can have an API gravity of less than
20 (e.g., an
API gravity of less than 10 ). In some embodiments, the heavy hydrocarbon can
have a density
of at least 1,000 kg/m3. In some embodiments, the heavy hydrocarbon can have a
viscosity of
at least 100 centipoise at 15 C. In some embodiments, the portion of the
reservoir can have a
permeability of 10 millidarcy or more (e.g., 100 millidarcy or more).
A fluid is passed through the wellbore to heat the shale/heavy oil. The
temperature of
the heating fluid can be greater than the temperature of the heavy hydrocarbon
in the heavy
hydrocarbon reservoir. In some cases, the temperature of the heating fluid is
an initial
temperature of the heating fluid before the heating fluid passes through the
portion of the heavy
hydrocarbon reservoir. In some embodiments, the heating fluid can have a
temperature of from
700 C to 920 C (e.g., from 770 C to 870 C, or from 800 C to 850 C). The
wellbore formed in
the underground reservoir can include first and second ends. The first end and
the second ends
of the wellbore may be coupled to a first wellhead and a second wellhead,
respectively.
As discussed herein, the heating fluid can be steam. In some examples, the
heating
fluid can be a hydrocarbon supplied from a hydrocarbon well after extraction
of the
hydrocarbon from a hydrocarbon reservoir. After the well is filled with the
fluid, the volume of
2
CA 03078929 2020-04-09
WO 2018/071378
PCT/US2017/055872
the fluid entering the first end of the wellbore may be substantially equal to
a volume of the
fluid exiting the second end of the wellbore. After the fluid has passed
through the wellbore,
the fluid may be free of heavy hydrocarbon from the heavy hydrocarbon
reservoir.
The temperature of the heating fluid exiting the reservoir may be in a range
from 350 C
to 580 C (e.g., from 400 C to 530 C, or from 450 C to 500 C). At least a
portion of the
hydrocarbon material in the hydrocarbon reservoir, may be heated (using the
heating fluid) to a
temperature ranging from 100 C to 240 C (e.g., from 150 C to 230 C, or from
200 C to
220 C). In some embodiments, some or all of the hydrocarbons may be
transformed into
lighter hydrocarbons, water, and gas during heating. In some embodiments,
temperature within
at least a portion of the hydrocarbon reservoir can be increased to a
pyrolyzation temperature
(e.g., to a temperature of between 250 C and 400 C).
Methods of producing a heavy hydrocarbon from a hydrocarbon reservoir are also
disclosed herein. The method can include heating a portion of the hydrocarbon
reservoir by
passing a heating fluid through a fully enclosed well in the hydrocarbon
reservoir; and
extracting the heavy hydrocarbon from the hydrocarbon reservoir. In some
embodiments,
extracting the heavy hydrocarbon from the hydrocarbon reservoir can include
perforating the
fully enclosed well to extract the heavy hydrocarbon after heating the portion
of the
hydrocarbon reservoir. In some embodiments, the hydrocarbon reservoir can
include one or
more additional wells for extraction of the heavy hydrocarbon after heating
the portion of the
hydrocarbon reservoir.
The method of producing the heavy hydrocarbon can further include contacting
the
heavy hydrocarbon with a material to displace the heavy hydrocarbon from the
hydrocarbon
reservoir. The material can be selected from water, an alcohol, a solvent, a
surfactant, a
hydrocarbon, an alkali agent, a polymer, carbon disulfide, carbon dioxide, or
a combination
thereof.
DESCRIPTION OF DRAWINGS
Figure 1 shows a sequence of a pump 10, condenser 20, steam generator 30, and
compressor 40. The compressed steam 50 is piped through a closed loop 55
through a heavy
oil reservoir 60, thereby heating up surrounding material in the reservoir 60
to a viscosity
suitable for removing as product 70 through pipe 75.
3
CA 03078929 2020-04-09
WO 2018/071378
PCT/US2017/055872
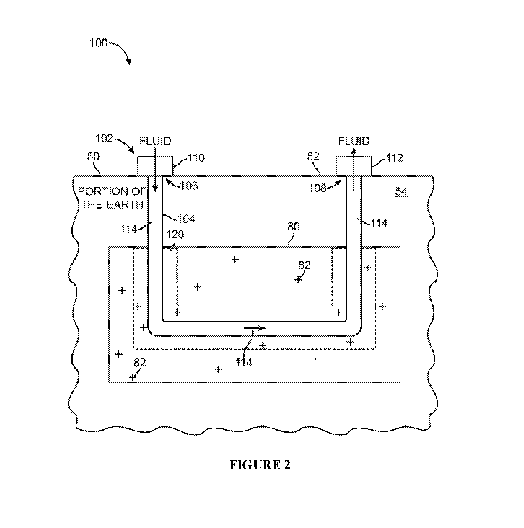
Figure 2 is a schematic block diagram illustrating a portion of the earth
including an oil
shale reservoir, and further illustrating an example heating system for
heating the oil shale
reservoir.
DETAILED DESCRIPTION
Provided herein are systems and methods for treating a subsurface formation.
In some
embodiments, the disclosure provides an in situ conversion system for
producing hydrocarbons
from a subsurface formation, including: a plurality of wellbores in the
formation; piping
positioned in at least one of the wellbores and a fluid circulation system
coupled to the piping,
wherein the fluid circulation system is configured to circulate a heat
transfer fluid through at
least a portion of the piping to form at least one heated portion of the
formation. There may
also be an electrical power supply, configured to provide electrical current
to at least a portion
of the piping located below an overburden in the formation to resistively heat
at least a portion
of the piping, and wherein heat transfers from the piping to the formation.
The following definitions generally relates to systems and methods for
treating
hydrocarbons in the formations. Such formations may be treated to yield
hydrocarbon products,
hydrogen, and other products.
"Hydrocarbons" are generally defined as molecules formed primarily by carbon
and
hydrogen atoms. Hydrocarbons may also include other elements such as, but not
limited to,
halogens, metallic elements, nitrogen, oxygen, and/or sulfur. Hydrocarbons may
be, but are not
limited to, kerogen, bitumen, pyrobitumen, oils, natural mineral waxes, and
asphaltites.
Hydrocarbons may be located in or adjacent to mineral matrices in the earth.
Matrices may
include, but are not limited to, sedimentary rock, sands, silicilytes,
carbonates, diatomites, and
other porous media. "Hydrocarbon fluids" are fluids that include hydrocarbons.
Hydrocarbon
fluids may include, entrain, or be entrained in non-hydrocarbon fluids such as
hydrogen,
nitrogen, carbon monoxide, carbon dioxide, hydrogen sulfide, water, and
ammonia.
A "formation" can include one or more hydrocarbon containing layers, one or
more
non-hydrocarbon layers, an overburden, and/or an underburden. The "overburden"
and/or the
underburden" include one or more different types of impermeable materials. For
example,
overburden and/or underburden may include rock, shale, mudstone, or wet/tight
carbonate. In
some embodiments of in situ conversion processes, the overburden and/or the
underburden may
include a hydrocarbon containing layer or hydrocarbon containing layers that
are relatively
4
CA 03078929 2020-04-09
WO 2018/071378
PCT/US2017/055872
impermeable and are not subjected to temperatures during in situ conversion
processing that
result in significant characteristic changes of the hydrocarbon containing
layers of the
overburden and/or the underburden. For example, the underburden may contain
shale or
mudstone, but the underburden is not allowed to heat to pyrolysis temperatures
during the in
situ conversion process. In some cases, the overburden and/or the underburden
may be
somewhat permeable.
"Formation fluids" refer to fluids present in a formation and may include
pyrolyzation
fluid, synthesis gas, mobilized hydrocarbon, and water (steam). Formation
fluids may include
hydrocarbon fluids as well as non-hydrocarbon fluids. The term "mobilized
fluid" refers to
fluids in a hydrocarbon containing formation that are able to flow as a result
of thermal
treatment of the formation. "Produced fluids" refer to fluids removed from the
formation.
"Heavy hydrocarbons" are viscous hydrocarbon fluids. Heavy hydrocarbons may
include highly viscous hydrocarbon fluids such as heavy oil, tar, and/or
asphalt. Heavy
hydrocarbons may include carbon and hydrogen, as well as smaller
concentrations of sulfur,
oxygen, and nitrogen. Additional elements may also be present in heavy
hydrocarbons in trace
amounts. Heavy hydrocarbons may be classified by API gravity. Heavy
hydrocarbons generally
have an API gravity below about 20 . Heavy oil, for example, generally has an
API gravity of
about 10-20 , whereas tar generally has an API gravity below about 10 . The
viscosity of heavy
hydrocarbons is generally greater than about 100 centipoise at 15 C. Heavy
hydrocarbons may
include aromatics or other complex ring hydrocarbons.
Heavy hydrocarbons may be found in a relatively permeable formation. The
relatively
permeable formation may include heavy hydrocarbons entrained in, for example,
sand or
carbonate. "Relatively permeable" is defined, with respect to formations or
portions thereof, as
an average permeability of 10 millidarcy or more (for example, 10 or 100
millidarcy).
In some cases, a portion or all of a hydrocarbon portion of a relatively
permeable
formation may be predominantly heavy hydrocarbons and/or tar with no
supporting mineral
grain framework and only floating (or no) mineral matter (for example, asphalt
lakes).
Certain types of formations that include heavy hydrocarbons may also be, but
are not
limited to, natural mineral waxes, or natural asphaltites. "Natural mineral
waxes" typically
occur in substantially tubular veins that may be several meters wide, several
kilometers long,
and hundreds of meters deep. "Natural asphaltites" include solid hydrocarbons
of an aromatic
composition and typically occur in large veins. In situ recovery of
hydrocarbons from
5
CA 03078929 2020-04-09
WO 2018/071378
PCT/US2017/055872
formations such as natural mineral waxes and natural asphaltites may include
melting to form
liquid hydrocarbons and/or solution mining of hydrocarbons from the
formations.
"Thermally conductive fluid" includes fluid that has higher thermal
conductivity than
air at standard temperature and pressure (STP) (0 C and 101.325 kPa).
The term "wellbore" refers to a hole in a formation made by drilling or
insertion of a
conduit into the formation. A wellbore may have a substantially circular cross
section, or
another cross-sectional shape. As used herein, the terms "well" and "opening,"
when referring
to an opening in the formation may be used interchangeably with the term
"wellbore."
A "u-shaped wellbore" refers to a wellbore that extends from a first opening
in the
formation, through at least a portion of the formation, and out through a
second opening in the
formation. In this context, the wellbore may be only roughly in the shape of a
"v" or "u", with
the understanding that the "legs" of the "u" do not need to be parallel to
each other, or
perpendicular to the "bottom" of the "u" for the wellbore to be considered "u-
shaped."
"Triad" refers to a group of three items (for example, heaters, wellbores, or
other
objects) coupled together. "Orifices" refer to openings, such as openings in
conduits, having a
wide variety of sizes and cross-sectional shapes including, but not limited
to, circles, ovals,
squares.
"Upgrade" refers to increasing the quality of hydrocarbons. For example,
upgrading
heavy hydrocarbons may result in an increase in the API gravity of the heavy
hydrocarbons.
"Thermal fracture" refers to fractures created in a formation caused by
expansion or
contraction of a formation and/or fluids in the formation, which is in turn
caused by increasing/
decreasing the temperature of the formation and/or fluids in the formation,
and/or by
increasing/decreasing a pressure of fluids in the formation due to heating.
The term "polymer" refers to a molecule having a structure that essentially
includes the
multiple repetitions of units derived, actually or conceptually, from
molecules of low relative
molecular mass. In some embodiments, the polymer is an oligomer.
Methods
Methods of heating a hydrocarbon containing formation, particularly a
hydrocarbon
reservoir comprised of heavy oil are provided herein. As the formation is
heated, the viscosity
of the hydrocarbon may be reduced to a point at which the fluids in the
reservoir become
mobile and flow at an economic rate.
6
CA 03078929 2020-04-09
WO 2018/071378
PCT/US2017/055872
The methods disclosed herein can include forming a fully enclosed circulation
system in
at least a portion of a hydrocarbon reservoir. The fully enclosed circulation
system provides
passage of the heating fluid through the hydrocarbon reservoir without being
injected directly
into the formation. A possible benefit of using the fully enclosed wellbore is
that there is little
concern about multi-phase flow.
The circulation system used in the hydrocarbon reservoir can be defined by a
wellbore
or a conduit. A typical wellbore includes perforations that enable fluid to
flow between the
wellbore and an underground reservoir. However, the fully enclosed wellbore
disclosed herein
is substantially non-perforated to prevent communication of the heating fluid
into the
hydrocarbon reservoir, and of the heavy hydrocarbon, or other constituents of
the portion of the
reservoir into the heating fluid. In some embodiments, the fully enclosed
wellbore can
comprise a conduit. For example, the wellbore can include a drill pipe, a
wired drill pipe, a
tube, or a casing. In some examples, the wellbore can include a tubular member
defining an
internal flow channel that provides thermal communication with the internal
flow channel and
the hydrocarbon reservoir. Optionally, the conduit can be equipped with
electrical cables and
heaters that can be used to heat the hydrocarbon reservoir.
The fully enclosed wellbore can have any suitable shape for efficient heat
transfer to the
heavy oil in the reservoir. In some embodiments, the wellbore can be a U-
shaped wellbore. In
some embodiments, the wellbore can be a network of connected wellbores.
The circulation system in the hydrocarbon reservoir can be a closed loop
circulation
system. The term "closed loop" as used herein refers to a circulation system
that re-circulates
the same fluid and have no need of a well or a place to discharge the fluid.
The closed loop
circulation system can circulate fluids continuously between the active and
passive spaces of
the circulation system without being removed (e.g., by or to an external
pumping mechanism).
The closed loop circulation system provides that after the well is filled with
a heating fluid, the
volume of the fluid entering the first end of the well is substantially equal
to a volume of fluid
exiting the second end of the well. Operational expenditures may be minimized
using the
closed-loop system. For example, if steam, for example, is used in the closed
loop, there is no
water lost, and there is little concern about multi-phase flow.
In some embodiments, the wellbore can be coupled to wellheads, which can then
form
the closed loop circulation system. For example, the wellbore can include a
first and a second
end. The first end and the second end of the wellbore are located above the
hydrocarbon
7
CA 03078929 2020-04-09
WO 2018/071378
PCT/US2017/055872
reservoir. The first end of the wellbore can be coupled to a first wellhead
and the second end of
the wellbore can be coupled to a second wellhead. However, the number of
wellheads coupled
to a wellbore (or the number of wellbore coupled to a wellhead) are not
limited. In a further
embodiment, the hydrocarbon reservoir can include multiple fully enclosed
wellbores.
The method further include passing a heating fluid through the wellbore to
heat the heavy oil.
The fluid circulation system can absorb heat or reject heat into the
surrounding reservoir. The
heating fluid can be any fluid that allows for the greatest heat transfer to
the reservoir fluids.
For example, the heating fluid can be a liquid, a gas, an emulsion, a slurry,
a stream of solid
particles that have flow characteristics, or a mixed phase fluid. In some
embodiments, the
heating fluid can include steam. Steam is chemically stable at the required
temperatures and
pressures and has a relatively high molecular weight that results in a high
volumetric heat
capacity. The steam used in the wellbore can be derived from seawater, or
fresh water from an
aquifer, river or lake. In some embodiments, the steam can be derived from
hard brine water or
soft brine water.
In some embodiments, the heating fluid can include a hydrocarbon supplied from
a
hydrocarbon well after extraction of the hydrocarbon from a hydrocarbon
reservoir. Other
suitable heating fluids can include carbon dioxide (CO2), air, helium and/or
nitrogen.
The heating fluid can be delivered to the wellbore by pumping the heating
fluid into the
wellhead and through the first end of the wellbore. The heating fluid
continues through the
wellbore and through a lateral segment of the wellbore before completing the
cycle through the
second end of the wellbore. Any suitable mechanism for pumping the heating
fluid can be
utilized that allows the fluid to go into the wellbore such as those typically
used in hydraulic
fracturing.
The temperature of the heating fluid can be greater than the temperature of
the heavy
hydrocarbon in the heavy hydrocarbon reservoir. In some embodiments, the
heating fluid has a
temperature of from 700 C to 920 C, from 770 C to 870 C, or from 800 C to 850
C. The
temperature of the heating fluid exiting the reservoir may be in a range from
350 C to 580 C,
from 400 C to 530 C, or from 450 C to 500 C. A heat supply, such as a heater,
may be placed
inside or outside the wellbores to heat the heating fluid and as such the
hydrocarbon reservoir.
The heat supply can be in the form of a piping that is positioned in the U-
shaped wellbore to
form a U-shaped heater. U.S. Patent No. 7,575,052 describes downhole heaters
that can be used
in the methods described herein, the entirety of which is incorporated by
reference.
8
CA 03078929 2020-04-09
WO 2018/071378
PCT/US2017/055872
At least a portion of the hydrocarbon material in the hydrocarbon reservoir,
may be
heated (using the heating fluid) to a temperature ranging from 100 C to 240 C,
from 150 C to
230 C, or from 200 C to 220 C. During heating, hydrocarbons may be transformed
into lighter
hydrocarbons, water, and gas. In some embodiments, temperature within at least
a portion of
the hydrocarbon reservoir can be increased to a pyrolyzation temperature (for
example,
between 250 C and 400 C).
FIG. 2 is schematic block diagram illustrating a portion of the earth 50
including a
reservoir comprising heavy hydrocarbon 80, and further illustrating an example
heating system
100 for heating the heavy hydrocarbon reservoir 80. The portion of the earth
50 includes a
surface 52 and a subsurface portion 54. The portion of the earth 50 may
include land, or land
and sea, for example. 80 is located in the subsurface portion 54, and includes
heavy
hydrocarbon 82. Typically the heavy hydrocarbon reservoir 80 is naturally
occurring, and is
located some distance below the surface 52.
In this example, the heating system 100 includes a well 102 having a wellbore
104. The
wellbore 104 can be formed by drilling through the portion of the earth 50,
for example. The
wellbore 104 typically includes a first end 106 and a second end 108, and
defines a passageway
between the first end 106 and the second end 108. In some embodiments, the
well 102 also
includes well heads. For example, the first end 106 of the wellbore 104 is
coupled to a well
head 110, and the second end 108 of the wellbore 104 is coupled to the well
head 112.
To heat the heavy hydrocarbon 82 within the heavy hydrocarbon reservoir 80, a
fluid
114, e.g. steam, is passed through the well 100. As one example, fluid 114 is
pumped into the
well head 110 and through the first end 106 of the wellbore 104. The fluid 114
continues
through the wellbore 104 and into the heavy hydrocarbon reservoir 80. The
fluid 114 has a
temperature that is greater than a temperature of the heavy hydrocarbon
reservoir. Therefore,
heat from the fluid 114 is transferred into the heavy hydrocarbon reservoir,
forming a heated
portion 120 adjacent the wellbore 104. The fluid 114 proceeds along the
wellbore 104 to the
second end 108 where the fluid 114 exits the wellbore through the well head
112.
In order to heat a larger portion of the hydrocarbon reservoir, the heating
system can
include two or more adjacent wells. A heating fluid is supplied through the
wells, which heats
the heavy hydrocarbon adjacent to the wells generating a larger heated
portion.
Once heating has been completed, the produced hydrocarbon can be extracted.
The
hydrocarbon reservoir can include multiple wellbores such that at least one of
the wellbore can
9
CA 03078929 2020-04-09
WO 2018/071378
PCT/US2017/055872
be used for heating while another of the wellbore can be used for production
(extraction) of the
produced hydrocarbon. The multiple wellbores can be operated simultaneously,
for example, to
heat the heavy hydrocarbon through a first well at the same time that the
heavy hydrocarbon is
being produced from the reservoir through a second well. Alternately, after
the hydrocarbon
reservoir is heated, the fully enclosed wellbore can be perforated and
converted into a portion
of a production system. In this example, the wellbore is perforated to permit
extraction of the
heavy hydrocarbon.
A fluid may be introduced into the hydrocarbon reservoir before and/or after
the heat
treatment of the reservoir to enhance recovery of the hydrocarbon material.
"Enhanced
recovery" or "enhanced oil recovery" refers to techniques for increasing the
amount of
hydrocarbon that may be extracted from a formation (e.g., an oil reservoir).
Examples of
enhanced oil recovery operations include, for example, miscible gas injection
(which includes,
for example, carbon dioxide flooding), chemical injection (sometimes referred
to as chemical
enhanced oil recovery (CEOR), and which includes, for example, polymer
flooding, alkaline
flooding, surfactant flooding, conformance control operations, as well as
combinations thereof
such as alkaline-polymer flooding or alkaline-surfactant-polymer flooding),
microbial injection,
and thermal recovery (which includes, for example, cyclic steam, steam
flooding, and fire
flooding). In some embodiments, the enhanced oil recovery operation can
include a polymer
(P) flooding operation, an alkaline-polymer (AP) flooding operation, a
surfactant-polymer (SP)
flooding operation, an alkaline-surfactant-polymer (ASP) flooding operation, a
conformance
control operation, or any combination thereof. The terms "operation" and
"application" may be
used interchangeability herein, as in enhanced oil recovery operations or
enhanced oil recovery
applications. The mixture formed may have a reduced viscosity compared to the
initial
viscosity of the fluids in the formation. The mixture may flow and/or be
mobilized towards
production wells in the formation.
In some embodiments, the method can include contacting the hydrocarbon
material in
the hydrocarbon reservoir with a fluid such as water, an alcohol, a solvent, a
surfactant, a
hydrocarbon, alkaline water solutions (for example, sodium carbonate
solutions), caustic,
polymers, carbon disulfide, carbon dioxide, or a combination thereof.
In some embodiments, the method can include contacting the hydrocarbon
material with
a surfactant. A surfactant, as used herein, is a compound within the aqueous
composition that
functions as a surface active agent when the aqueous composition is in contact
with a crude oil
CA 03078929 2020-04-09
WO 2018/071378
PCT/US2017/055872
(e.g., an unrefined petroleum). The surfactant can act to lower the
interfacial tension and/or
surface tension of the unrefined petroleum. The surfactant can comprise an
anionic surfactant, a
non-ionic surfactant, a zwitterionic surfactant, a cationic surfactant, or a
combination thereof.
The surfactant can be any appropriate surfactant useful in the field of
enhanced oil recovery.
For example, in some embodiments, the surfactant can comprise an internal
olefin sulfonate
(I0S), an alpha olefin sulfonate (AOS), an alkyl aryl sulfonate (ARS), an
alkane sulfonate, a
petroleum sulfonate, an alkyl diphenyl oxide (di)sulfonate, an alcohol
sulfate, an alkoxy sulfate,
an alkoxy sulfonate, an alcohol phosphate, an alkoxy phosphate, a
sulfosuccinate ester, an
alcohol ethoxylate, an alkyl phenol ethoxylate, a quaternary ammonium salt, a
betaine or
sultaine. The surfactant as provided herein, can also be a soap.
Suitable surfactants are disclosed, for example, in U.S. Patent Nos.
3,811,504,
3,811,505, 3,811,507, 3,890,239, 4,463,806, 6,022,843, 6,225,267, and
7,629,299; International
Patent Application Publication Nos. WO/2008/079855, WO/2012/027757 and WO
2011/094442; as well as U.S. Patent Application Publication Nos. 2005/0199395,
2006/0185845, 2006/018486, 2009/0270281, 2011/0046024, 2011/0100402,
2011/0190175,
2007/0191633, 2010/004843. 2011/0201531, 2011/0190174, 2011/0071057,
2011/0059873,
2011/0059872, 2011/0048721, 2010/0319920, 2010/0292110, and 2013/0281327, all
of which
are incorporated herein by reference in their entirety. Additional suitable
surfactants are
surfactants known to be used in enhanced oil recovery methods, including those
discussed in D.
B. Levitt, A. C. Jackson, L. Britton and G. A. Pope, "Identification and
Evaluation of High-
Performance EOR Surfactants," SPE IX89, conference contribution for the SPE
Symposium on
Improved Oil Recovery Annual Meeting, Tulsa, Okla., Apr. 24-26, 2006. In some
embodiments, the total surfactant concentration can be from about 0.05% w/w to
about 10%
w/w.
In some embodiments, the method can include contacting the hydrocarbon
material with
a viscosity enhancing water-soluble polymer. The water-soluble polymer may be
a biopolymer
such as xanthan gum or scleroglucan, a synthetic polymer such as
polyacryamide, hydrolyzed
polyarcrylamide or co-polymers of acrylamide and acrylic acid, 2-acrylamido 2-
methyl
propane sulfonate or N-vinyl pyrrolidone, a synthetic polymer such as
polyethylene oxide, or
any other high molecular weight polymer soluble in water or brine. In some
embodiments, the
polymer is polyacrylamide (PAM), partially hydrolyzed polyacrylamides (HPAM),
and
copolymers of 2-acrylamido-2-methylpropane sulfonic acid or sodium salt or
mixtures thereof,
11
CA 03078929 2020-04-09
WO 2018/071378
PCT/US2017/055872
and polyacrylamide (PAM) commonly referred to as AMPS copolymer and mixtures
of the
copolymers thereof. In one embodiment, the viscosity enhancing water-soluble
polymer is
polyacrylamide or a co-polymer of polyacrylamide. Examples of commercially
available
polymers for use herein include Florpaam 3330S and Florpaam 3360S. The
viscosity enhancing
water-soluble polymer can be used in the range of about 500 to about 5000 ppm
concentration,
such as from about 1000 to 2000 ppm (e.g., in order to match or exceed the
reservoir oil
viscosity under the reservoir conditions of temperature and pressure).
In some embodiments, the method can include contacting the hydrocarbon
material with
an alkali agent. An alkali agent as provided herein can be a basic, ionic salt
of an alkali metal
(e.g., lithium, sodium, potassium) or alkaline earth metal element (e.g.,
magnesium, calcium,
barium, radium). Examples of suitable alkali agents include, for example,
NaOH, KOH, Li0H,
Na2CO3, NaHCO3, Na-metaborate, Na silicate, Na orthosilicate, Na acetate or
NH4OH. The
alkali agent can be present in the hydrocarbon reservoir at a concentration
from 0.1% w/w to
10% w/w (e.g., from 0.1% w/w to 5% w/w). In some embodiments, the alkali agent
can be
present in an effective amount to afford a pH of from 8 to 12 (e.g., 10 to
12).
In some embodiments, the method can include contacting the hydrocarbon
material with
a co-solvent. The co-solvent can be an alcohol, alcohol ethoxylate, glycol
ether, glycols, or
glycerol. In embodiments, the hydrocarbon reservoir includes a plurality of
different additional
co-solvents. Suitable co-solvents include alcohols (e.g., C1-C6 alcohols and
C1-C4 alcohols),
alkyl alkoxy alcohols (e.g., Ci-C6 alkoxy alcohols and C1-C4 alkoxy alcohols),
and phenyl
alkoxy alcohols. The alkyl alkoxy alcohols or phenyl alkoxy alcohols provided
herein have a
hydrophobic portion (alkyl or aryl chain), a hydrophilic portion (e.g., an
alcohol) and optionally
an alkoxy (ethoxylate or propoxylate) portion. Thus, the co-solvent can be an
alcohol, alkoxy
alcohol, glycol ether, glycol or glycerol. Suitable co-solvents are known in
the art, and include,
for example, co-solvents described in U.S. Patent Application Publication No.
2013/0281327
which is hereby incorporated herein in its entirety. The co-solvent can be
used in combinatino
with a surfactant.
In some embodiments, the method can include contacting the hydrocarbon
material with
a gas. For instance, the gas may be combined with the hydrocarbon composition
to reduce its
mobility by decreasing the liquid flow in the pores of the solid material
(e.g., rock). In some
embodiments, the gas may be supercritical carbon dioxide, nitrogen, natural
gas or mixtures of
these and other gases.
12
CA 03078929 2020-04-09
WO 2018/071378
PCT/US2017/055872
The compositions and methods of the appended claims are not limited in scope
by the
specific compositions and methods described herein, which are intended as
illustrations of a
few aspects of the claims. Any compositions and methods that are functionally
equivalent are
intended to fall within the scope of the claims. Various modifications of the
compositions and
methods in addition to those shown and described herein are intended to fall
within the scope of
the appended claims. Further, while only certain representative compositions
and method steps
disclosed herein are specifically described, other combinations of the
compositions and method
steps also are intended to fall within the scope of the appended claims, even
if not specifically
recited. Thus, a combination of steps, elements, components, or constituents
may be explicitly
mentioned herein or less, however, other combinations of steps, elements,
components, and
constituents are included, even though not explicitly stated.
The term "comprising" and variations thereof as used herein is used
synonymously with
the term "including" and variations thereof and are open, non-limiting terms.
Although the
terms "comprising" and "including" have been used herein to describe various
embodiments,
the terms "consisting essentially of' and "consisting of' can be used in place
of "comprising"
and "including" to provide for more specific embodiments of the disclosure and
are also
disclosed. Other than where noted, all numbers expressing geometries,
dimensions, and so forth
used in the specification and claims are to be understood at the very least,
and not as an attempt
to limit the application of the doctrine of equivalents to the scope of the
claims, to be construed
in light of the number of significant digits and ordinary rounding approaches.
It is understood that when combinations, subsets, groups, etc. of elements are
disclosed
(e.g., combinations of components in a composition, or combinations of steps
in a method), that
while specific reference of each of the various individual and collective
combinations and
permutations of these elements may not be explicitly disclosed, each is
specifically
contemplated and described herein. By way of example, if a composition is
described herein as
including a component of type A, a component of type B, a component of type C,
or
combination thereof, it is understood that this phrase describes all of the
various individual and
collective combinations and permutations of these components. For example, in
some
embodiments, the composition described by this phrase could include only a
component of type
A. In some embodiments, the composition described by this phrase could include
only a
component of type B. In some embodiments, the composition described by this
phrase could
include only a component of type C. In some embodiments, the composition
described by this
13
CA 03078929 2020-04-09
WO 2018/071378
PCT/US2017/055872
phrase could include a component of type A and a component of type B. In some
embodiments, the composition described by this phrase could include a
component of type A
and a component of type C. In some embodiments, the composition described by
this phrase
could include a component of type B and a component of type C. In some
embodiments, the
composition described by this phrase could include a component of type A, a
component of
type B, and a component of type C. In some embodiments, the composition
described by this
phrase could include two or more components of type A (e.g., Al and A2). In
some
embodiments, the composition described by this phrase could include two or
more components
of type B (e.g., B1 and B2). In some embodiments, the composition described by
this phrase
could include two or more components of type C (e.g., Cl and C2). In some
embodiments, the
composition described by this phrase could include two or more of a first
component (e.g.., two
or more components of type A (Al and A2)), optionally one or more of a second
component
(e.g., optionally one or more components of type B), and optionally one or
more of a second
component (e.g., optionally one or more components of type C). In some
embodiments, the
composition described by this phrase could include two or more of a first
component (e.g.., two
or more components of type B (B1 and B2)), optionally one or more of a second
component
(e.g., optionally one or more components of type A), and optionally one or
more of a second
component (e.g., optionally one or more components of type C). In some
embodiments, the
composition described by this phrase could include two or more of a first
component (e.g.., two
or more components of type C (Cl and C2)), optionally one or more of a second
component
(e.g., optionally one or more components of type A), and optionally one or
more of a second
component (e.g., optionally one or more components of type B).
Unless defined otherwise, all technical and scientific terms used herein have
the same
meanings as commonly understood by one of skill in the art to which the
disclosure belongs.
Publications cited herein and the materials for which they are cited are
specifically incorporated
by reference.
14