Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
USING TARGETED RADIOTHERAPY (TRT) TO DRIVE ANTI-TUMOR
IMMUNE RESPONSE TO IMMUNOTHERAPIES
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit of U.S. Application No.
15/809,427, filed
November 10, 2017, which is incorporated by reference herein in its entirety.
STATEMENT REGARDING FEDERALLY SPONSORED
RESEARCH OR DEVELOPMENT
[0002] This invention was made with government support under 0D024576 and
CA197078 awarded by the National Institutes of Health. The government has
certain
rights in the invention.
FIELD OF THE DISCLOSURE
[0003] This disclosure relates generally to methods of treating cancer. In
particular,
the disclosure is directed to methods of treating a cancer comprising one or
more
malignant solid tumors in a subject by (1) systemically administering to the
subject an
immunomodulatory dose of a targeted radiotherapy (TRT) agent, such as a
radioactive
metal chelate compound, a radiohalogenated compound, radiolabeled antibody, or
a
radiosiotope that is differentially taken up by and retained within solid
tumor tissue; and
(2) systemically administering to the subject one or more immunostimulatory
agents,
such as one or more immune checkpoint inhibitors.
1
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
BACKGROUND
[0004] Current cancer treatment typically involves systemic chemotherapy
whereby
non-targeted small molecule or antibody directed cytotoxic agents
preferentially enter, or
bind to (in the case of antibody directed agents) and kill cancer cells by a
variety of
mechanisms. External beam radiation therapy (xRT), which is often combined
with
chemotherapy, kills cancer cells by inducing nuclear DNA double strand breaks
resulting
in cell-cycle death. Unlike systemic chemotherapy, xRT depends on the ability
to
accurately determine the anatomic location of the tumor. Surgical resection of
tumors
also depends on the ability to see the tumor and on complete removal, since
residual
tumor cells will quickly reestablish the tumor following surgery. Surgery and
xRT are
generally limited to the local treatment of malignant tumors and thus are
limited in
treating disseminated or metastatic disease, which is why chemotherapy is
often used in
conjunction with these treatment modalities. Although systemic chemotherapy is
capable
of reaching many distant metastatic sites, with the possible exception of
brain metastases,
for all too many patients, responses are typically short-lived (months to
several years) and
ultimately result in tumor recurrence.
[0005] Because the body's natural immune system is also capable of
destroying
cancer cells following their recognition, immunologic approaches are rapidly
becoming
more prevalent in cancer treatment paradigms. However, some cancer cells, and
to a
greater extent cancer stem cells, manage to initially avoid immune-
surveillance and
actually acquire the ability to evolve and ultimately survive by remaining
relatively
immune invisible [Gaipi et al, Immunotherapy 6:597-610, 2014].
[0006] One specific immunologic approach that is being increasingly
investigated is
"in situ vaccination," a strategy that seeks to enhance tumor immunogenicity,
generate
tumor infiltrating lymphocytes (TEL) and drive a systemic anti-tumor immune
response
directed against "unvaccinated," disseminated tumors. In in situ vaccination;
a malignant
solid tumor is injected with (or treated with) one or more agents that
facilitate the release
of tumor antigens while simultaneously providing pro-inflammatory signals to
reverse the
immune-tolerizing microenvironment of the tumor [Pierce et al, Human Vaccines
&
Immunotherapoeutics 11(8):1901-1909, 2015; Marabelle eta!, Clin. Cancer Res.
20(7):1747-56, 2014; Morris eta!, Cancer Res; 76(13); 3929-41, 20161.
2
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[0007] A second and quite different approach is systemically-administered
i in in unotherapy. . In systemically-administered immunotherapy, an
iMMUTIOStiMUlatory
agent, such as an immune checkpoint inhibitor, is administered to circulate
through the
entire body (e.g., intravenously), rather than being locally injected into the
tumor. Such
agents can be used to treat tumors in which an anti-tumor immune response is
present,
but has been "exhausted" or rendered ineffective. In the case of checkpoint
inhibitors, the
tumor cells express "checkpoint ligands" or other checkpoint molecules that
interact with
"checkpoint receptors" on the existing anti-tumor immune cells, triggering the
inactivation of these cells, By blocking this interaction, systemically-
administered
checkpoint inhibitors turn on the exhausted, pre-existing immune response in
cancer
patients, facilitating a more effective attack on the cancer cells by the
patient's own
immune system.
[0008] Although recent data from clinical trials and pre-clinical models
illustrate the
potential of these approaches, there is a great need in the art for
systemically-
administered immunotherapy methods exhibiting improved systemic efficacy.
[0009] Radiation hormesis is a decades-old hypothesis that low doses of
ionizing RT
can be beneficial by stimulating the activation of natural protective repair
mechanisms
that are not activated in the absence of ionizing RT [Cameron and Moulder,
Med. Phys.
25:1407, 1998]. The reserve repair mechanisms are hypothesized to be
sufficiently
effective when stimulated as to not only cancel the detrimental effects of
ionizing RT but
also inhibit disease not related to RT exposure. Perhaps related, the abscopal
effect is a
phenomenon reported in the 1950's, whereby, xRT treatment of one tumor
actually
causes shrinkage of another tumor outside the RT treatment area. Although
rare, this
phenomenon is thought to be dependent on activation of the immune system.
Together,
hormesis and the abscopal effect support the potential interaction and
stimulation of the
immune system by low dosage (immune stimulatory but non-cytotoxic) RT, which
may
then be combined with other immunologic approaches, such as systemically-
administered
immunotherapy.
[0010] We have previously published that the combination of local xRT + in
situ
vaccination and/or systemic checkpoint inhibitor immunotherapy are potently
synergistic
in treating large established tumors in mice, when there is a single tumor
present [Morris
3
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
et al, Cancer Res; 76(13); 3929-41, 2016]. However, we have surprisingly
discovered
that the combination of in situ vaccination and xRT does not result in
inhibited tumor
growth in the presence of a second, non-radiated tumor. Apparently, the non-
radiated
tumor exhibits a dampening effect (which we have designated as "concomitant
immune
tolerance") on the immunomodulatory effect of the xRT and in situ vaccine on
the
radiated tumor.
[0011] This concomitant immune tolerance can be overcome, enabling efficacy
of in
situ vaccination, when xRT is given to all areas of tumor. However, xRT cannot
be
effectively used in combination with in situ vaccination methods in the
presence of
multiple tumors, particularly if the tumors are not few in number, or if the
location of one
or more of the tumors is not precisely known, or if it is not feasible to
deliver xRT to all
sites of tumor. Furthermore, administering xRT to all tumor sites in patients
with
metastatic disease would likely result in systemic immune suppression,
defeating the
central purpose of systemically-administered immunotherapy.
[0012] Accordingly, in combination with systemically-administered
immunotherapy,
there is a need for improved methods of delivering an immunomodulatory dose of
RT to
all tumors within a subject, regardless of their number and anatomic location.
BRIEF SUMMARY
[0013] We have previously shown that certain alkylphosphocholine analogs
are
preferentially taken up and retained by malignant solid tumor cells. In U.S.
Patent
Publication No. 2014/0030187, which is incorporated by reference herein in its
entirety,
Weichert et al. disclose using analogs of the base compound 18-(p-
iodophenyl)octadecyl
phosphocholine (NM404; see Figure 1) for detecting and locating, as well as
for treating,
a variety of malignant solid tumors. If the iodo moiety is an imaging-
optimized
radionuclide, such as iodine-124 ([124I]_NM404), the analog can be used in
positron
emission tomography¨computed tomography (PET/CT) or single-photon emission
computed tomography (SPECT) imaging of solid tumors. Alternatively, if the
iodo
moiety is a radionuclide optimized for delivering therapeutic doses of RT to
the solid
tumors cells in which the analog is taken up, such as iodine-125 or iodine-131
([1251]-
NM404 or [131I]-NM404), the analog can be used to treat the solid tumors.
4
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[0014] Such analogs not only target a wide variety of solid tumor types in
vivo, but
also undergo prolonged selective retention in tumor cells, thus affording high
potential as
RT agents. Moreover, tumor uptake is limited to malignant cancer and not
premalignant
or benign lesions.
[0015] However, there are metal isotopes that have better properties for
optimized
imaging and/or RT than the radioactive iodine isotopes used in the previously
disclosed
alkylphosphocholine analogs. For example, as an imaging isotope, 1-124 suffers
from
poor positron output (only about 24% of the emissions are positrons), and it
suffers
further from a confounding gamma emission (600 KeV), which actually interferes
with
normal 511 KeV PET detection. Certain positron emitting metals have better
imaging
characteristics. As another example, as an RT isotope, 1-131 produces other
non-
therapeutic emissions at other energies, which add undesired radiation
dosimetry to
neighboring normal tissue, including bone marrow. The beta particle range of 1-
131 is
also quite long, which contributes to off target toxicity. Several metallic
radiotherapy
isotopes offer a cleaner emission profile and shorter pathlength and thus less
potential
toxicity.
[0016] We have developed improved alkylphosphocholine analogs that include
a
chelated radioactive metal isotope instead of a radioactive iodine isotope
(see, e.g., U.S.
Patent Publication No. 2018/0022768, which is incorporated by reference herein
in its
entirety). The analogs include the same backbone as the previously disclosed
radioiodinated compounds, so they are still selectively taken up and retained
in tumor
cells. However, the chelated radioactive metal isotope provides improved
emissions for
imaging and/or radiotherapy applications. Such agents are well suited for
delivering a
sub-cytotoxic but immunomodulatory dose of ionizing RT to all malignant tumors
present within a subject, regardless of whether their number and locations are
known.
[0017] Accordingly, in a first aspect, the disclosure encompasses a method
of treating
a cancer comprising one or more malignant solid tumors in a subject. The
method
includes the steps of systemically administering to the subject (a) an
immunomodulatory
dose of a targeted radiotherapy (TRT) agent that is differentially taken up by
and retained
within the malignant solid tumor tissue; and (b) one or more immunostimulatory
agents.
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[0018] In some embodiments, the one or more immunostimulatory agents are
immune checkpoint inhibitors capable of targeting one or more checkpoint
molecules.
[0019] Non-limiting examples of the one or more immune checkpoint
inhibitors
include agents that are capable of targeting one or more of the following
checkpoint
molecules: A2AR (adenosine A2a receptor), BTLA (B and T lymphocyte
attenuator),
CTLA4 (cytotoxic T lymphocyte-associated protein 4), KIR (killer cell
immunoglobulin-
like receptor), LAG3 (Lymphocyte Activation Gene 3), PD-1 (programmed death
receptor 1), PD-Li (programmed death ligand 1), CD40 (cluster of
differentiation 40),
CD27 (cluster of differentiation 27), CD28 (cluster of differentiation 28),
CD137 (cluster
of differentiation 137), 0X40 (CD134; cluster of differentiation 134), OX4OL
(0X40
ligand; cluster of differentiation 252), GITR (glucocorticoid-induced tumor
necrosis
factor receptor-related protein), GITRL (glucocorticoid-induced tumor necrosis
factor
receptor-related protein ligand), ICOS (inducible T-cell costimulatory), ICOSL
(inducible
T-cell costimulatory ligand), B7H3 (CD276; cluster of differentiation 276),
B7H4
(VTCN1; V-set domain-containing T-cell activation inhibitor 1), IDO
(Indoleamine 2,3-
dioxygenase), TIM-3 (T-cell Immunoglobulin domain and Mucin domain 3), Gal-9
(galectin-9), or VISTA (V-domain Ig suppressor of T cell activation).
[0020] In some embodiments, the one or more immune checkpoint inhibitors
include
one or more anti-immune checkpoint molecule antibodies. In some such
embodiments,
the one or more anti-immune checkpoint molecule antibodies include at least
one
monoclonal antibody.
[0021] In some embodiments, the one or more immune checkpoint inhibitors
include
one or more small molecules capable of inhibiting or blocking one or more
immune
checkpoint molecules. Non-limiting examples of such small molecule checkpoint
inhibitors include CA-170 and CA-327, which both target PD-Li.
[0022] In some embodiments, the one or more anti-immune checkpoint molecule
antibodies include an anti-CTLA4 antibody, an anti-PD-1 antibody, an anti-PD-
Li
antibody, an anti-LAG3 antibody, an anti-KIR antibody, an anti-A2AR antibody,
and
anti-BTLA antibody, an anti-CD40 antibody, an anti-CD27 antibody, an anti-CD28
antibody, an anti-CD137 antibody, an anti-0X40 antibody, an anti-OX4OL
antibody, an
anti-GITR antibody, an anti-GITRL antibody, an anti-ICOS antibody, an anti-
ICOSL
6
CA 03082056 2020-05-06
WO 2019/094657 PCT/US2018/059927
antibody, an anti-B7H3 antibody, an anti-B7H4 antibody, an anti-IDO antibody,
an anti-
TIM-3 antibody, an anti-Gal-9 antibody, or an anti-VISTA antibody.
[0023] In some embodiments, the TRT agent is metaiodobenzylguanidine
(MIBG),
where the iodine atom in the MIBG is a radioactive iodine isotope.
[0024] In some embodiments, the TRT agent is a radiolabeled tumor-targeting
antibody.
[0025] In some embodiments, the TRT agent is radioactive isotope of radium,
such as
Ra-223.
[0026] In some embodiments, the TRT agent is a radioactive phospholipid
ether
metal chelate having the formula:
RI ft( \--hs .. (C H2) n (OCH2CHYCH2)m01 1:,) C HC H2 -R2 lib
L \
0 -
or a salt thereof Ri includes a chelating agent that is chelated to a metal
atom, wherein
the metal atom is an alpha, beta or Auger emitting metal isotope with a half-
life of greater
than 6 hours and less than 30 days; a is 0 or 1; n is an integer from 12 to
30; m is 0 or 1;
Y is ¨H, ¨OH, -COOH, -COOX, -0C0X, or ¨OX, wherein X is an alkyl or an aryl;
R2 is
-N-112Z, -N+HZ2, or -N-13, wherein each Z is independently an alkyl or an
aroalkyl; and b is 1 or 2. Non-limiting examples of metal isotopes that could
be used
include Sc-47, Lu-177, Y-90, Ho-166, Re-186, Re-188, Cu-67, Au-199, Rh-105, Ra-
223,
Ac-225, Pb-212, or Th-227.
[0027] In some embodiments, the chelating agent is 1,4,7,10-
tetraazacyclododecane-
1,4,7-triacetic acid (DO3A) or one of its derivatives; 1,4,7-triazacyclononane-
1,4-diacetic
acid (NODA) or one of its derivatives; 1,4,7-triazacyclononane-1,4,7-triacetic
acid
(NOTA) or one of its derivatives; 1,4,7,10-tetraazacyclododecane-1,4,7,10-
tetraacetic
acid (DOTA) or one of its derivatives; 1,4,7-triazacyclononane,1-glutaric acid-
4,7-
diacetic acid (NODAGA) or one of its derivatives; 1,4,7,10-
tetraazacyclodecane,1-
glutaric acid-4,7,10-triacetic acid (DOTAGA) or one of its derivatives;
1,4,8,11-
tetraazacyclotetradecane-1,4,8,11-tetraacetic acid (TETA) or one of its
derivatives;
7
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
1,4,8,11-tetraazabicyclo[6.6.2]hexadecane-4,11-diacetic acid (CB-TE2A) or one
of its
derivatives; diethylene triamine pentaacetic acid (DTPA), its diester, or one
of its
derivatives; 2-cyclohexyl diethylene triamine pentaacetic acid (CHX-A"-DTPA)
or one
of its derivatives; deforoxamine (DFO) or one of its derivatives; 1,24[6-
carboxypyridin-
2-yl]methylamino]ethane (H2dedpa) or one of its derivatives; and DADA or one
of its
derivatives, wherein DADA comprises the structure:
0
I ______________________ 'µ
0 NH
I.
HS SH =
[0028] In some embodiments, a is 1 (aliphatic aryl-alkyl chain). In other
embodiments, a is 0 (aliphatic alkyl chain).
[0029] In some embodiments, m is 1 (acylphospholipid series). In some such
embodiments, n is an integer between 12 and 20. In some embodiments, Y is
¨0C0X,
-COOX or ¨OX.
[0030] In some embodiments, X is ¨CH2CH3 or ¨CH3.
[0031] In some embodiments, m is 0 (alkylphospholipid series).
[0032] In some embodiments, b is 1.
[0033] In some embodiments, n is 18.
[0034] In some embodiments, R2 is -N-73. In some such embodiments, each Z
is
independently ¨CH2CH3 or ¨CH3. In some such embodiments, each Z is ¨CH3.
8
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[0035] In some embodiments, the chelating agent chelated to the metal atom
is:
0
\I\
o
'OH
1 \õ...,,rf,---/) 0
OH
0
HO
NThi
0
1
HO
,OH OH
N ________________________________ \
HO' HO
9
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
OH
0
-,---\
µ'
N ,
HO1s-
, .- N
li i
0
I
(Y01-1 ,
0,,, ADH OH
r 4
(/\
f----""
I '0
µN N HN -
,
,
1
,-----'-'4.,
N
N I
..,.." t 0
,,, ...-,
7 i=
\ ______________________________ ,
, ......,-
k
,
V µ
.,,,-'ikR.>,
H 0 'O HO
OH
e-., ...k...,
0,-\\
'
\ N,,,
H 0,-7-- N HN1
i
ii 0 N' / <\\ '-,,,,,,,s \ // b
I ,
0\\OH
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
O\N .0H OH
,...,,,,..-
t
µ
\/ \/
0
/
---N N _______________________________ \
i
\----N N-7 __ 1
/
(\ _______________________________ t '`
,
µ
k
/'=. O ,
HO '.0- HO
OH
\
/I i 1
Li
c------N N------\
/\ ,...._.\\
s.---N N-1
( \ ______________________________ i
\
HO ND
/ 1
________________________________________ /
i \/ \
r _________________________ N N N\
HO1C
i \ /
it
/ \ \ CO2H
H02C 00-)11 C
.02H
,
11
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
/ ............................... \
=
/ .. N N
HO,7C. CO2H
HO2C CO1H CO2H
0
/ __________________________________ /
t-o N Nil Ni OH
HOnC-1( \--0O21-1
H0,-)C'"
,0
0¨<\ IN-41
N +115 \
HO )=0
HO¨N,
HN
\= 0
0 / __ s,
N¨<µ
HO 0
12
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
/
r .............. NH
/_\
\-,OH HO -K
0 Q , or
0
\--NH
(
?
NH HN
HS SH
13
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
100361 In some
embodiments, the radioactive phospholipid ether metal chelate is one
of the following compounds, wherein the selected compound is chelated to the
metal
atom:
0
(OH
Nr (CH2)1800CH2CH2NMe3
j0 0
OH
HO
0
0
(OH
0
N¨(CH2)1801=1)0CH2CH2NMe3
Nr 0
OTh
j 0
OH
HO
0
HO
0
1\1- 7
NTh(kil = (CH2)180112)0CH2CH2NMe3
oj\)
HO
HO
N
CNN¨(CH2)180POCH2CH2NMe3
HO
14
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
00H OH
r\ 1---0
rN N
LN N 0
(CH2)180POCH2CH2NMe3
e
/0
HOO HO ,
00H OH
( /--\r--o
rN I\1
L 0 H 0
N N--"\----(CH2)18010CH2CH2NMe3
e
/0
HOO HO ,
OH
0
r\C
HO N H ---r @
0 Q/N 11 0
0 (CH2)180POCH2CH2NMe3
6
e
OH ,
OH
0
r\N-,
0
c/N--\.---(CH2)180POCH2CH2NMe3
0 6
e
o)\OH ,
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
00H OH
rN I\1 HN . (CH2)180P110CH2CH2NMe3
LN N0 6e
HOO HO 0 ,
00H OH
(JO
N HN¨(0H2)18010CH2CH2NMe3
L o
0
e
0
HOO HO ,
OH
0
r\N 0
HN II (CH2)180POCH2CH2NMe3
HO-1 D ,
o/ QN ________ /-
0 o
e
0)\OH ,
OH
0
r\N 0
ii O
HN¨(CH2)180POCH2CH2NMe3
HO-{N
D
N 6
0 c/ /¨
___________________ o e
0.
OH ,
00H OH
\ / _________________ (CH2)18012'0CH2CH2C)NMe3
\ _______ N N 6
0
HO 0 HO ,
16
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
00H OH
(
C"¨)7
0
(CH2)180POCH2CH2NMe3
N N
0
HOO HO
OH
N N 0
C __________
\--(CH2)18011,0CH2CH2C)NMe3
N N 0
HOO
OH
N N
CNND7
0
(CH2)18010CH2CH2NMe3
0
HOO
0
(CH2)180POCH2CH2NMe3
0
/N N N¨\
HO2C ( CO2H
HO2C CO2H CO2H
9
c (CH2)18 010CH2CH2NMe3
/ 0
/N N N¨\
HO2C ( CO2H
HO2C CO2H CO2H
17
CA 03082056 2020-05-06
WO 2019/094657 PCT/US2018/059927
0 0
Q (0H2)180,00H20H2Nme3
0
/¨N N N¨\ 0
HO2C ) ( ( CO2H
HO2C co2H CO2H
,
i:i
1 e
a __ i (CH)18 oiocH2cH2Nme3
o
/¨N N N¨\ e
HO2C ) ( ( co2H
HO2C co2H co2H
,
o o
e 9 , \ /__\ /__\ / o o
Me3NCH2CH20c)0(CH2)18-0 N N N 0¨(CH2)180112)0CH2CH2NMe3
HO2C¨" )
Cb HO2C \¨CO2H O
e ,
o
04 HN
N-(-
HO 5 0
HO-Ns, NH
\
e o
II HN H-
Me3NCH2CH20-1?-0(CH2)18
N(
HO o ,
o
04 HN
N-(-'5HO 0
HO-Ns, µ
e o HN¨\ H-NH
Me3NCH2CH20+0(CH2)18¨ \ \
0 0 \ ____
N(
e
HO 0 ,
18
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
0
(CH2)180POCH2CH2NMe3
6
(NH HN
N ( N
¨OH H04-
0 0
0
H
c (CH)18 010CH2CH2NMe3
0
iNH HN
OH
HO
0 0
0
)\-NH = (CH2)18010CH2CH2NMe3
/ ( 0
NH HN
rO
HS SH ,or
0 0
,-NH-(CH2)18010CH2CH2NMe3
/ (
NH HN
rO
HS SH
[0037] In some
embodiments, in the phospholipid ether metal chelate structure, a is 1,
b is 1, m is 0, n is 18, and R2 is ¨N+(CH3)3. In some such embodiments, the
phospholipid
ether metal chelate is N1V1600 chelated to the metal atom, such as (but not
limited to) 90Y-
NM600.
19
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[0038] In some embodiments, the TRT agent is a radiohalogenated
phospholipid
ether having the formula:
0
11-
,se a OCH2CHYCH2
) 0 (
P ) - R2 ,
C FIS. H2
0
or a salt thereof Ri comprises a radioactive halogen isotope; a is 0 or 1; n
is an integer
from 12 to 30; m is 0 or 1; Y is selected from the group consisting of ¨H,
¨OH, -COOH, -
COOX, ¨OX, and ¨0C0X, wherein X is an alkyl or an arylalkyl; and R2 is
selected from
the group consisting of -NH3, -1\11-12Z, -N+HZ2, and -N-13, wherein each Z is
independently an alkyl or an aryl.
[0039] In some embodiments, the radioactive halogen isotope is 1231, 1241,
1251, 1311,
211At,
76Br, or 77Br.
[0040] In some embodiments, a is 1 and m is 0.
[0041] In some embodiments, n is 18.
[0042] In some embodiments, R2 is -N+(CH3)3. In some such embodiments, a is
1, m
is 0, and n is 18. In some such embodiments, the radioactive halogen isotope
is 1231, 1241,
125 or 1, 1311 =
(the radiohalogenated phospholipid ether is [123I].4M404, [1241].4M404,
[125I]-N1404, [131I]-NM404, [211 A
NM404, [76B1]-NM404, or [77B1]-NM404).
[0043] In some embodiments, the TRT agent, the immunostimulatory agent, or
both,
are administered intravenously.
[0044] In some embodiments, the subject is a human.
[0045] Non-limiting examples of the cancers presenting as malignant solid
tumors
that can be treated using the method include melanoma, neuroblastoma, lung
cancer,
adrenal cancer, colon cancer, colorectal cancer, ovarian cancer, prostate
cancer, liver
cancer, subcutaneous cancer, squamous cell cancer of the skin or head or neck,
intestinal
cancer, retinoblastoma, cervical cancer, glioma, breast cancer, pancreatic
cancer, soft
tissue sarcoma, Ewings sarcoma, rhabdomyosarcoma, osteosarcoma, Wilms' tumor,
and
pediatric brain tumors.
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[0046] In some embodiments, the cancer is treated without administering to
the
subject an antibody to a tumor antigen that is not a checkpoint molecule.
[0047] In some embodiments, an anti-GD2 antibody is not adminstered to the
subject.
[0048] In a second aspect, the disclosure encompasses the use of a TRT
agent and
one or more immunostimulatory agents for treating a cancer comprising one or
more
malignant solid tumors in a subject, as further described above.
[0049] In a third aspect, the disclosure encompasses the use of a TRT agent
and/or
one or more immunostimulatory agents for the manufacture of a medicament
treating a
cancer comprising one or more malignant solid tumors in a subject, as further
described
above.
[0050] Other objects, features and advantages of the present invention will
become
apparent after review of the specification, claims and drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
[0051] The patent or application file contains at least one drawing
executed in color.
Copies of this patent or patent application publication with color drawing(s)
will be
provided by the Office upon request and payment of the necessary fee.
[0052] Fig. 1 shows the chemical structure of the base compound 18- (p-
iodophenyl)
octadecyl phosphocholine (NM404).
[0053] Fig. 2A is a graph showing that xRT + IT-IC elicits in situ tumor
vaccination.
More specifically, Fig. 2A shows tumor growth curves that show synergy between
xRT
and IT-hu14.18-IL2. 71% (22/31) of mice treated with xRT + IT-IC are rendered
disease-
free.
[0054] Fig. 2B is another graph showing that xRT + IT-IC elicits in situ
tumor
vaccination. More specifically, Fig. 2B shows Kaplan-Meier survival curves
that show
synergy between xRT and IT-hu14.18-IL2. 71% (22/31) of mice treated with xRT +
IT-
IC are rendered disease-free.
[0055] Fig. 2C is another graph showing that xRT + IT-IC elicits in situ
tumor
vaccination. More specifically, Fig. 2C shows that 90% of the treated mice
reject
subsequent engraftment with B78 melanoma.
[0056] Fig. 3 is a graph demonstrating concomitant immune tolerance.
Primary tumor
response is shown. A distant un-treated tumor suppresses response to xRT + IT-
IC in a 2-
21
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
tumor B78 melanoma model, and this suppression can be overcome be radiating
the
second tumor.
[0057] Fig. 4 is a graph showing that concomitant immune tolerance is due
to Tregs.
Primary tumor response is shown. A distant un-treated tumor suppresses
response to xRT
+ IT-IC in a 2-tumor B78 melanoma model and this suppression can be overcome
by
depleting Tregs (using transgenic DEREG mice that express diphtheria toxin
receptors on
their Tregs, and thus depleting Tregs by administering diphtheria toxin).
[0058] Fig. 5 is an image showing selective uptake of '24I-N\/1404 by B78
melanoma.
A mouse bearing a ¨200mm3 B78 tumor received IV '24I-N\4404 and had serial
PET/CT
scans done. This image at 71h shows selective uptake by the tumor with some
residual
background uptake by the heart and liver.
[0059] Fig. 6 is a graph demonstrating that in situ vaccination can be
elicited in the
presence of residual levels of molecular targeted radiation therapy (TRT).
Treatment with
combined xRT + IT-IC is equally effective in the presence or absence of 3
1..tfi 1311-
NM404. This approximates the residual activity of TRT that will be present
when we
deliver xRT (d0) followed by IT-IC (d6-10), as described in Example 4.
[0060] Fig. 7 shows a time course MRI image of a tumor-bearing mouse
following
injection of Gd-NM600 showing enhancement of the tumor (T) by 24 hours.
[0061] Fig. 8A is a graph showing tumor-specific inhibition of primary
tumor
response to the combination of local RT+IT-IC by a distant untreated tumor in
murine
melanoma and pancreatic tumor models. C57BL/6 mice bearing a syngeneic,
disialoganglioside-expressing (GD2+), primary flank tumor +/- a secondary
tumor on the
contralateral flank were treated to the primary tumor only, as indicated, with
xRT on day
"1" and intra-tumor (IT) injection of 50 mcg of the anti-GD2 immunocytokine
(IC),
hu14.18-1L2 (a fusion of anti-GD2 mAb and IL2), on day 6-10. Mean primary
tumor
volumes are displayed in Fig. 8A. More specifically, Fig. 8A shows that in
mice bearing
a primary B78 melanoma tumor, the presence of an untreated secondary B78 tumor
antagonized primary tumor response to RT+IT-IC. We describe this effect as
"concomitant immune tolerance" ¨ an antagonistic effect of a non-treated
distant tumor
on the local response of a treated tumor to xRT + IT-IC.
22
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[0062] Fig. 8B is another graph showing tumor-specific inhibition of
primary tumor
response to the combination of local RT+IT-IC by a distant untreated tumor in
murine
melanoma and pancreatic tumor models. C57BL/6 mice bearing a syngeneic,
disialoganglioside-expressing (GD2+), primary flank tumor +/- a secondary
tumor on the
contralateral flank were treated to the primary tumor only, as indicated, with
xRT on day
"1" and intra-tumor (IT) injection of 50 mcg of the anti-GD2 immunocytokine
(IC),
hu14.18-IL2 (a fusion of anti-GD2 mAb and IL2), on day 6-10. More
specifically, Fig.
8B shows Kaplan-Meier survival curves for mice plus replicate experiments.
Nearly all
mice were euthanized due to primary tumor progression.
[0063] Fig. 8C is another graph showing tumor-specific inhibition of
primary tumor
response to the combination of local RT+IT-IC by a distant untreated tumor in
murine
melanoma and pancreatic tumor models. C57BL/6 mice bearing a syngeneic,
disialoganglioside-expressing (GD2+), primary flank tumor +/- a secondary
tumor on the
contralateral flank were treated to the primary tumor only, as indicated, with
xRT on day
"1" and intra-tumor (IT) injection of 50 mcg of the anti-GD2 immunocytokine
(IC),
hu14.18-IL2 (a fusion of anti-GD2 mAb and IL2), on day 6-10. More
specifically, Fig.
8C shows that in mice bearing a primary Panc02-GD2+ pancreatic tumor, with or
without
a secondary Panc02-GD2¨ tumor on the opposite flank, the presence of an
untreated
Panc02 secondary tumor suppressed the response of a primary Panc02-GD2+ tumor
to
RT+IT-IC.
[0064] Fig. 8D is another graph showing tumor-specific inhibition of
primary tumor
response to the combination of local RT+IT-IC by a distant untreated tumor in
murine
melanoma and pancreatic tumor models. C57BL/6 mice bearing a syngeneic,
disialoganglioside-expressing (GD2+), primary flank tumor +/- a secondary
tumor on the
contralateral flank were treated to the primary tumor only, as indicated, with
xRT on day
"1" and intra-tumor (IT) injection of 50 mcg of the anti-GD2 immunocytokine
(IC),
hu14.18-IL2 (a fusion of anti-GD2 mAb and IL2), on day 6-10. More
specifically, Fig.
8D shows that in mice bearing a primary B78 melanoma tumor, a secondary B78
tumor
suppressed primary tumor response to RT+IT-IC but a secondary Panc02-GD2+
pancreatic tumor did not exert this effect.
23
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[0065] Fig. 8E is another graph showing tumor-specific inhibition of
primary tumor
response to the combination of local RT+IT-IC by a distant untreated tumor in
murine
melanoma and pancreatic tumor models. C57BL/6 mice bearing a syngeneic,
disialoganglioside-expressing (GD2+), primary flank tumor +/- a secondary
tumor on the
contralateral flank were treated to the primary tumor only, as indicated, with
xRT on day
"1" and intra-tumor (IT) injection of 50 mcg of the anti-GD2 immunocytokine
(IC),
hu14.18-1L2 (a fusion of anti-GD2 mAb and IL2), on day 6-10. More
specifically, Fig.
8E shows that in mice bearing a primary Panc02-GD2+ tumor a secondary Panc02-
GD2¨
tumor suppressed primary tumor response to combined xRT and IT-hu14.18-1L2,
while a
B78 secondary tumor did not. n=number of mice per group. NS=non-significant,
***p<0.001.
[0066] Fig. 9A includes immunohistochemistry images (left and center) and
graphs
(right) showing that concomitant immune tolerance is circumvented by specific
depletion
of regulator T cells (Tregs). More specifically, Fig. 9A shows
immunohistochemistry for
the Treg marker, FoxP3 (representative 400x images are shown) for tumors
evaluated on
day 6 after xRT in mice with one (Fig. 9A, leftmost panels Al and A2) or two
(Fig. 9A.,.
center panels A3 and A4) tumors. Mice received no xRT, or xRT only to the
primary
tumor. The primary tumor is shown in Fig. 9A, panels Al-A3 and the secondary
is shown
in Fig. 9A,. panel A4. Small arrows point out some of the FoxP3+ cells (brown
nuclei =
FoxP3+, blue = hematoxylin counterstain). The graphs on the right display
blinded
quantification of FoxP3+ cells per 200x field, corresponding to the conditions
shown in
Fig. 9A, panels Al, A2, A3 and A4, respectively.
[0067] Fig. 9B is another graph showing that concomitant immune tolerance
is
circumvented by specific depletion of regulator T cells (Tregs). More
specifically, Fig.
9B shows that DEREG mice express diphtheria toxin receptor under control of
the Treg-
specific FoxP3 promoter, enabling specific depletion of Tregs upon IP
injection of
diphtheria toxin. DEREG mice bearing primary and secondary B78 melanoma tumors
were treated with xRT+IT-IC to the primary tumor and IP injection of either
diphtheria
toxin or PBS (the first of replicate experiments are shown). Concomitant
immune
tolerance is eliminated following depletion of Tregs in these mice, resulting
in improved
(Fig. 9B) primary tumor response. n=number of mice per group. **p<0.01,
***p<0.001.
24
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[0068] Fig. 9C is another graph showing that concomitant immune tolerance
is
circumvented by specific depletion of regulator T cells (Tregs). More
specifically, Fig.
9C shows that DEREG mice express diphtheria toxin receptor under control of
the Treg-
specific FoxP3 promoter, enabling specific depletion of Tregs upon IP
injection of
diphtheria toxin. DEREG mice bearing primary and secondary B78 melanoma tumors
were treated with xRT+IT-IC to the primary tumor and IP injection of either
diphtheria
toxin or PBS (the first of replicate experiments are shown). Concomitant
immune
tolerance is eliminated following depletion of Tregs in these mice, resulting
in improved
(Fig. 9C) secondary tumor response. n=number of mice per group. **p<0.01,
***p<0.001.
[0069] Fig. 10A and is a graph showing that concomitant immune tolerance is
overcome by delivering xRT to both tumor sites. In mice bearing primary and
secondary
B78 tumors, the secondary tumor suppresses primary tumor response to primary
tumor
treatment with xRT + IT-IC. This is overcome by delivering 12 Gy xRT to both
the
primary and secondary tumors and IT-IC to the primary tumor, resulting in
improved
(Fig. 10A) primary tumor response (the first of replicate experiments is
shown) from
replicate experiments. n=number of mice per group. **p<0.01, ***p<0.001.
[0070] Fig. 10B is another graph showing that concomitant immune tolerance
is
overcome by delivering xRT to both tumor sites. In mice bearing primary and
secondary
B78 tumors, the secondary tumor suppresses primary tumor response to primary
tumor
treatment with xRT + IT-IC. This is overcome by delivering 12 Gy xRT to both
the
primary and secondary tumors and IT-IC to the primary tumor, resulting in
improved
(Fig. 10B) aggregate animal survival from replicate experiments. n=number of
mice per
group. **p<0.01, ***p<0.001.
[0071] Fig. 11A is a graph showing that low dose xRT alone does not elicit
in situ
vaccination but does overcome concomitant immune tolerance when delivered to
distant
tumor sites together with 12 Gy + IT-IC treatment of an in situ vaccine site.
More
specifically, Fig. 11A shows that in mice bearing a primary B78 tumor only, 12
Gy + IT-
IC elicits in situ vaccination (as shown previously) and results in complete
tumor
regression in most mice (4/6 in this experiment) and a memory immune response
(Morris,
Cancer Res, 2016). On the other hand no animals exhibit complete tumor
regression
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
following either IT-IC alone or low dose (2 Gy) xRT + IT-IC (0/6 in both
groups)
p<0.05.
[0072] Fig. 11B is another graph showing that low dose xRT alone does not
elicit in
situ vaccination but does overcome concomitant immune tolerance when delivered
to
distant tumor sites together with 12 Gy + IT-IC treatment of an in situ
vaccine site. More
specifically, Fig. 11B shows that in mice bearing a primary and secondary B78
melanoma tumor, low dose xRT (2 Gy or 5 Gy) delivered to the secondary tumor
is
comparable to 12 Gy in its capacity to overcome concomitant immune tolerance
at the
primary tumor.
[0073] Fig. 11C is another graph showing that low dose xRT alone does not
elicit in
situ vaccination but does overcome concomitant immune tolerance when delivered
to
distant tumor sites together with 12 Gy + IT-IC treatment of an in situ
vaccine site. More
specifically, Fig. 11C shows that in these same animals, it is apparent that
overcoming
concomitant immune tolerance by delivery of low dose xRT to the secondary
tumor
rescues a systemic response to IT-IC immunotherapy. In this context, when xRT
is
delivered to all tumor sites then IT-IC injection of the primary tumor
triggers a systemic
anti-tumor effect that renders secondary tumor response to 2 Gy or 5 Gy
greater than the
response to 12 Gy xRT in absence of primary tumor IT-IC injection.
[0074] Fig. 12A is a PET image showing that low dose TRT with 131I-NM404
effectively depletes tumor infiltrating FoxP3+ Tregs without systemic
leukopenia or
depletion of tumor infiltrating CD8+ effector T cells. In most clinical
scenarios, it is not
feasible to deliver external beam, even low dose, to all tumor sites without
eliciting
marked bone marrow depletion and leukopenia that would result in
immunosuppression.
Here we tested whether TRT could be administered systemically to specifically
deplete
tumor infiltrating suppressive immune cells (Tregs), without triggering
systemic immune
cell depletion and leukopenia. More specifically, Fig. 12A shows dosimetry
studies in
this B78 melanoma tumor model using positron-emitting 1241-N1404 confirm tumor-
selective uptake of NM404. C57BL/6 mice bearing B78 tumors were treated with
60 tCi
131I-NM404. This activity approximates the amount of 131I-NM404 necessary to
deliver
2 Gy TRT to a B78 tumor. Peripheral blood and tumor samples were collected in
26
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
untreated control mice (designated "C") and at 8 day intervals (Ti = d8, T2 =
d16, T3 =
d24, T4 = d32) thereafter.
[0075] Fig. 12B is a bar graph showing that low dose TRT with 131I-NM404
effectively depletes tumor infiltrating FoxP3+ Tregs without systemic
leukopenia or
depletion of tumor infiltrating CD8+ effector T cells. In most clinical
scenarios, it is not
feasible to deliver external beam, even low dose, to all tumor sites without
eliciting
marked bone marrow depletion and leukopenia that would result in
immunosuppression.
Here we tested whether TRT could be administered systemically to specifically
deplete
tumor infiltrating suppressive immune cells (Tregs), without triggering
systemic immune
cell depletion and leukopenia. More specifically, Fig. 12B shows that this
dose of TRT
did not result in any significant systemic leukopenia,
[0076] Fig. 12C is another bar graph showing that low dose TRT with 131I-
NM404
effectively depletes tumor infiltrating FoxP3+ Tregs without systemic
leukopenia or
depletion of tumor infiltrating CD8+ effector T cells. In most clinical
scenarios, it is not
feasible to deliver external beam, even low dose, to all tumor sites without
eliciting
marked bone marrow depletion and leukopenia that would result in
immunosuppression.
Here we tested whether TRT could be administered systemically to specifically
deplete
tumor infiltrating suppressive immune cells (Tregs), without triggering
systemic immune
cell depletion and leukopenia. More specifically, Fig. 12C shows that this
dose of TRT
did not significantly affect the level of tumor infiltrating CD8+ effector T
cells (ANOVA
p=0.25).
[0077] Fig. 12D is another bar graph showing that low dose TRT with 131I-
NM404
effectively depletes tumor infiltrating FoxP3+ Tregs without systemic
leukopenia or
depletion of tumor infiltrating CD8+ effector T cells. In most clinical
scenarios, it is not
feasible to deliver external beam, even low dose, to all tumor sites without
eliciting
marked bone marrow depletion and leukopenia that would result in
immunosuppression.
Here we tested whether TRT could be administered systemically to specifically
deplete
tumor infiltrating suppressive immune cells (Tregs), without triggering
systemic immune
cell depletion and leukopenia. More specifically, Fig. 12D shows that tumor
infiltrating
FoxP3+ Tregs were significantly depleted by this dose of TRT (ANOVA p=0.03; *
p<0.05).
27
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[0078] Fig. 13A is a graph showing that low dose TRT with 131I-NM404
effectively
overcomes concomitant immune tolerance and rescues the systemic anti-tumor
effect of
in situ vaccination. Given the capacity of low dose 131I-NM404 TRT to deplete
tumor-
infiltrating Tregs without rendering a mouse leukopenic, we tested whether low
dose 131J
NM404 might effectively overcome concomitant immune tolerance. C57BL/6 mice
bearing two B78 tumors were treated with 60-mcCi 131I-NM404 on day 1 (NM404),
as
indicated. After one half-life (day 8), animals received 12 Gy xRT or no xRT
to the
primary tumor (in situ vaccine site). Control mice receiving no 131I.4M404
were treated
to the secondary tumor as indicated (0, 2, or 12 Gy). Mice received daily IT
injections of
IC to the primary tumor (in situ vaccine site), as indicated, on days 13-17.
More
specifically, Fig. 13A shows that primary tumor response is shown and
demonstrates that
administration of low dose TRT effectively overcomes concomitant immune
tolerance
and rescues the systemic anti-tumor effect of in situ vaccination.
[0079] Fig. 13B is another graph showing that low dose TRT with 131I-NM404
effectively overcomes concomitant immune tolerance and rescues the systemic
anti-
tumor effect of in situ vaccination. Given the capacity of low dose 131I-NM404
TRT to
deplete tumor-infiltrating Tregs without rendering a mouse leukopenic, we
tested whether
low dose 131I-N1404 might effectively overcome concomitant immune tolerance.
C57BL/6 mice bearing two B78 tumors were treated with 60-mcCi 131I-NM404 on
day 1
(NM404), as indicated. After one half-life (day 8), animals received 12 Gy xRT
or no
xRT to the primary tumor (in situ vaccine site). Control mice receiving no
131I-NM404
were treated to the secondary tumor as indicated (0, 2, or 12 Gy). Mice
received daily IT
injections of IC to the primary tumor (in situ vaccine site), as indicated, on
days 13-17.
More specifically, Fig. 13B shows that secondary tumor response is shown and
demonstrates that administration of low dose TRT effectively overcomes
concomitant
immune tolerance and rescues the systemic anti-tumor effect of in situ
vaccination.
[0080] Figure 14 shows the chemical structure of an exemplary
alkylphosphocholine
metal chelate (64Cu-NM600). Other metals may be used in place of mCu.
[0081] Figure 15 is a PET/CT image of two single tumor B78 mice from a scan
taken
48 hours post-injection with 86Y-NM600.
28
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[0082] Figure 16 is a PET/CT image of two two-tumor B78 mice from a scan
taken
48 hours post-injection with 86Y-NM600.
[0083] Figure 17 includes PET/CT images for a U87MG mouse from scans taken
3
hours (left panel), 24 hours (center panel) and 48 hours (right panel) post-
injection with
64Cu-NM600. The images show tissue activity calculated as a percent of
injected dose/g
tissue (%ID/g, scale shown on far right).
[0084] Figure 18 includes PET/CT images for a 4T1 mouse from scans taken 3
hours
(left panel), 24 hours (center panel) and 48 hours (right panel) post-
injection with 64Cu-
NM600. The images show tissue activity calculated as a percent of injected
dose/g tissue
(%ID/g, scale shown on far right).
[0085] Figure 19 includes PET/CT images for an HCT-116 mouse from scans
taken 3
hours (left panel), 24 hours (center panel) and 48 hours (right panel) post-
injection with
64Cu-NM600. The images show tissue activity calculated as a percent of
injected dose/g
tissue (%ID/g, scale shown on far right).
[0086] Figure 20 includes PET/CT images for an A549 mouse from scans taken
3
hours (left panel), 24 hours (center panel) and 48 hours (right panel) post-
injection with
64Cu-NM600. The images show tissue activity calculated as a percent of
injected dose/g
tissue (%ID/g, scale shown on far right).
[0087] Figure 21 includes PET/CT images for a PC-3 mouse from scans taken 3
hours (left panel), 24 hours (center panel) and 48 hours (right panel) post-
injection with
64Cu-NM600. The images show tissue activity calculated as a percent of
injected dose/g
tissue (%ID/g, scale shown on far right).
[0088] Figure 22 includes PET/CT images for an HT-29 mouse from scans taken
3
hours (left panel), 24 hours (center panel) and 48 hours (right panel) post-
injection with
64Cu-NM600. The images show tissue activity calculated as a percent of
injected dose/g
tissue (%ID/g, scale shown on far right).
[0089] Figure 23 includes PET/CT images for a MiaPaca mouse from scans
taken 3
hours (left panel), 24 hours (center panel) and 48 hours (right panel) post-
injection with
64Cu-NM600. The images show tissue activity calculated as a percent of
injected dose/g
tissue (%ID/g, scale shown on far right).
29
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[0090] Figure 24 includes PET/CT images for a 4T1 mouse from scans taken 3
hours
(left panel), 24 hours (center panel) and 48 hours (right panel) post-
injection with 86Y-
NM600. The images show tissue activity calculated as a percent of injected
dose/g tissue
(%ID/g, scale shown on far right).
[0091] Figure 25 includes PET/CT images for a 4T1 mouse from scans taken 3
hours
(left panel), 24 hours (center panel) and 48 hours (right panel) post-
injection with 89Zr-
NM600. The images show tissue activity calculated as a percent of injected
dose/g tissue
(%ID/g, scale shown on far right).
[0092] Figure 26 includes PET/CT images for an HT-29 mouse from scans taken
4
hours (left panel) and 1 day (right panel) post-injection with 52Mn-NM600. The
images
show tissue activity calculated as a percent of injected dose/g tissue (%ID/g,
scale shown
on far right).
[0093] Figure 27 includes PET/CT images for a PC-3 mouse from scans taken 4
hours (left panel) and 1 day (right panel) post-injection with 52Mn-NM600. The
images
show tissue activity calculated as a percent of injected dose/g tissue (%ID/g,
scale shown
to the right of each image).
[0094] Figure 28 includes PET/CT images for an HT-29 mouse from scans taken
2
days (left panel), 3 days (second panel from the left), 5 days (second panel
form the right)
and 7 days (right panel) post-injection with 52Mn-NM600. The images show
tissue
activity calculated as a percent of injected dose/g tissue (%ID/g, scale shown
to the right
of the images).
[0095] Figure 29 includes PET/CT images for a PC-3 mouse from scans taken 2
days
(left panel), 3 days (second panel from the left), 5 days (second panel form
the right) and
7 days (right panel) post-injection with 52Mn-NM600. The images show tissue
activity
calculated as a percent of injected dose/g tissue (%ID/g, scale shown to the
right of the
images).
[0096] Figure 30 is a graph showing PET quantitative region of interest
data (chelate
uptake as a function of time) for 4T1 tumor tissue in 4T1 mice injected with
86Y-NM600,
64Cu-NM600 and 89Zr-NM-600.
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[0097] Figure 31 is a graph showing PET quantitative region of interest
data
(chelate uptake as a function of time) for heart tissue in 4T1 mice injected
with 86Y-
NM600, 64Cu-NM600 and 89Zr-NM-600.
[0098] Figure
32 is a graph showing PET quantitative region of interest data (chelate
uptake as a function of time) for liver tissue in 4T1 mice injected with 86Y-
NM600, 64Cu-
NM600 and 89Zr-NM-600.
[0099] Figure
33 is a graph showing PET quantitative region of interest data (chelate
uptake as a function of time) for whole body in 4T1 mice injected with 86Y-
NM600,
64Cu-NM600 and 89Zr-NM-600.
[00100] Figure
32 is a bar graph illustrating ex vivo chelate biodistribution in healthy
and tumor tissues in 4T1 mice 48 hours (86Y-NM600, 64Cu-NM600, 89Zr-NM-600 and
177Lu-NM600) and 96 hours (177Lu-NM600) post-injection of the metal chelates.
[00101] Figure 35 shows the chemical structure of an exemplary
alkylphosphocholine
metal chelate (177Lu-NM600). Other metals may be used in place of 177Lu.
[00102] Figure 36 is an audioradiographic image of three B78 mice taken 48
hours
after injection with 90Y-NM600. Xenografted B78 tumors are seen as large dark
spots at
the lower right of each mouse image.
[00103] Figure 37 is an audioradiographic image of three B78 mice taken 96
hours
after injection with 90Y-NM600. Xenografted B78 tumors are seen as large dark
spots at
the lower right of each mouse image.
[00104] Figure 38 is an audioradiographic image of a B78 mouse taken on day 5
after
injection with 177Lu-NM600. Xenografted B78 tumors are seen as two dark spots
at the
bottom of the mouse image.
[00105] Figure 39 is an audioradiographic image of a B78 mouse taken on day 13
after
injection with 177Lu-NM600. Xenografted B78 tumors are seen as two dark spots
at the
bottom of the mouse image.
[00106] Figure 39 is an audioradiographic image of a B78 mouse taken on day 13
after
injection with 177Lu-NM600. Xenografted B78 tumors are seen as two dark spots
at the
bottom of the mouse image.
31
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[00107] Figure 40 is an audioradiographic image of a MiaPaca mouse taken 10
days
after injection with 177Lu-NM600. The location of the xenografted MiaPaca
tumor is
indicated by the arrow and dashed circle.
[00108] Figure 41 is an audioradiographic image of three 4T1 mice taken 48
hours
after injection with 177Lu-NM600. The locations of the xenografted 4T1 tumors
are
indicated by the arrows and dashed circles.
[00109] Figure 42 is an audioradiographic image of three 4T1 mice taken 96
hours
after injection with 177Lu-NM600. The locations of the xenografted 4T1 tumors
are
indicated by the dashed circles.
[00110] Figure 43 is an audioradiographic image of three 4T1 mice taken 4
hours after
injection with 90Y-NM600. The locations of the xenografted 4T1 tumors are
indicated by
the arrows and dashed circles.
[00111] Figure 44 is an audioradiographic image of three 4T1 mice taken 48
hours
after injection with 90Y-NM600. The xenografted 4T1 tumors are seen as large
dark
spots on the lower right of each mouse image.
[00112] Figure 45 is an audioradiographic image of three 4T1 mice taken 96
hours
after injection with 90Y-NM600. The xenografted 4T1 tumors are seen as large
dark
spots on the lower right of each mouse image.
[00113] Figure 46 is a graph illustrating the radiotherapeutic effect of 90Y-
NM600 at
two different doses (150 Ci and 300 Ci) in a B78 xenograft mouse model,
versus a
control (excipient only). Data is presented as measured tumor volume in mm3 as
a
function of time in days after injection.
[00114] Figure 47 is a graph illustrating the radiotherapeutic effect of a
single 500 Ci
dose of 177Lu-NM600 in a B78 xenograft mouse model, versus a control
(excipient only).
Data is presented as measured tumor volume in mm3 as a function of time in
days after
injection.
[00115] Figure 48 is a graph illustrating the radiotherapeutic effect of a
single 400 Ci
dose of 177Lu-NM600 in a MiaPaca xenograft mouse model, versus a control
(excipient
only). Data is presented as measured tumor volume in mm3 as a function of time
in days
after injection.
32
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[00116] Figure 49 is a graph illustrating the radiotherapeutic effect of a
single 500 Ci
dose of 177Lu-NM600 in a 4T1 xenograft mouse model, versus a control
(excipient only).
Data is presented as measured tumor volume in mm3 as a function of time in
days after
injection. * P < 0.05; ** P < 0.01; *** P < 0.001.
[00117] Figure 50 is a graph illustrating the radiotherapeutic effect of two
serial doses
of 177Lu-NM600 (500 Ci and 250 Ci) in a 4T1 xenograft mouse model, versus a
control (excipient only). Data is presented as measured tumor volume in mm3 as
a
function of time in days after injection.
[00118] Figure 51 is a graph illustrating the radiotherapeutic effect of 177Lu-
NM600 at
two different doses (500 Ci and 250 Ci) in a 4T1 xenograft mouse model,
versus a
control (excipient only). Data is presented as measured tumor volume in mm3 as
a
function of time in days after injection.
[00119] Figure 52 is a graph illustrating the impact of tumor mass on the
comparative
therapeutic efficacy of 90Y-NM600 and 1-31-I-NM404 in conventional TRT.
[00120] Figure
53 is a bar graph comparing average albumin binding energies of three
different metal chelate analogs of NM404, along with an amine analog. For
comparison,
the binding energy of I-NM404 is shown as a dotted line.
[00121] Figure 54 is a graph illustrating tumor volume (mm3) as a function of
time
(days) in B78 melanoma flank tumor mice treated with anti-CTLA4 immune
checkpoint
inhibitor (CTLA4) and/or varying doses (25 Ci, 50 Ci, or 100 Ci) of the
targeted
radiotherapy (TRT) agent Y90-NM600. Control mice were administered vehicle
without
anti-CTLA4 or the TRT agent (PBS). After Day 18, combination treatment with 50
or
100 Ci of Y90-NM600 with anti-CTLA4 had significantly (p < 0.05 by ANOVA)
reduced tumor growth compared to PBS, Y90-NM600 alone, or anti-CTLA4 alone.
The
25 1..1Ci Y90-NM600 combination treatment group with anti-CTLA-4 had an
intermediate
growth delay response that showed a trend towards dose response.
[00122] Figure 55 is a graph showing aggregate animal survival for mice
administered
a combination of TRT (50 Ci Y90-NM600) and checkpoint blockade (anti-CTLA4),
compared to mice administered TRT alone, checkpoint blockade alone (anti-
CTLA4), or
PBS vehicle.
33
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[00123] Figure 56 is a graph showing aggregate animal survival for mice
administered
three different combinations of TRT (25 Ci, 50 Ci, and 100 Ci Y90-NM600)
with
checkpoint blockade (anti-CTLA4).
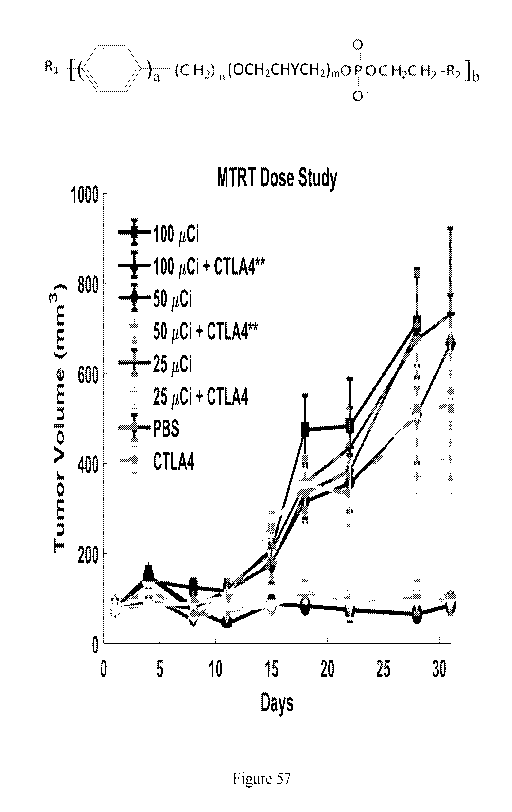
[00124] Figure 57 is a graph illustrating tumor volume (mm3) as a function of
time
(days) in B78 melanoma flank tumor mice treated with anti-CTLA4 immune
checkpoint
inhibitor (CTLA4) and/or varying doses (25 Ci, 50 Ci, or 100 Ci) of the
molecularly
targeted radiotherapy (MTRT) agent 90Y-NM600. Control mice were administered
vehicle without anti-CTLA4 or the MTRT agent (PBS). Combination treatment with
50
or 100 Ci of 90Y-NM600 with anti-CTLA4 had significantly (p < 0.05 by ANOVA)
reduced tumor growth compared to PBS, 90Y-NM600 alone, or anti-CTLA4 alone.
[00125] Figure 58 is a graph showing aggregate animal survival for B78
melanoma
flank tumor mice treated with anti-CTLA4 immune checkpoint inhibitor (CTLA4)
and/or
varying doses (25 Ci, 50 Ci, or 100 Ci) of the molecularly targeted
radiotherapy
(MTRT) agent 90Y-NM600. Mice administered a combination of MTRT (50 Ci 90Y-
NM600 or 100 Ci 90Y-NM600) and checkpoint blockade (anti-CTLA4) exhibited
significantly increased survival as compared to other groups.
[00126] Figure 59 is a graph illustrating tumor volume (mm3) as a function of
time
(days) in NXS2 neuroblastoma tumor mice treated with anti-CTLA4 immune
checkpoint
inhibitor (CTLA4) and/or 50 Ci of the molecularly targeted radiotherapy
(MTRT) agent
90Y-NM600. Control mice were administered vehicle without anti-CTLA4 or the
TRT
agent (PBS). Combination treatment of 90Y-NM600 MTRT with anti-CTLA4 had
significantly reduced tumor growth compared to PBS, 90Y-NM600 alone, or anti-
CTLA4
alone.
[00127] Figure 60 is a graph illustrating tumor volume (mm3) as a function of
time
(days) in 4T1 breast cancer tumor mice treated with anti-CTLA4 immune
checkpoint
inhibitor (CTLA4) and/or 50 Ci of the molecularly targeted radiotherapy
(MTRT) agent
90Y-NM600. Control mice were administered vehicle without anti-CTLA4 or the
TRT
agent (PBS). Combination treatment of 90Y-NM600 MTRT with anti-CTLA4 had
significantly reduced tumor growth compared to PBS, 90Y-NM600 alone, or anti-
CTLA4
alone.
34
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[00128] Figure 61 is a graph illustrating tumor volume (mm3) as a function of
time
(days) for the irradiated primary B78 tumor in B78 melanoma flank tumor mice
having
both primary and secondary (distant) tumors. Mice were treated with various
combinations
of EBRT of the primary tumor only (12 Gy, secondary tumor was shielded), anti-
CTLA4
immune checkpoint inhibitor (CTLA4) and/or 50 Ci of the molecularly targeted
radiotherapy (MTRT) agent 90Y-NM600. Combination treatment of 12 Gy EBRT, 90Y-
NM600 MTRT, and anti-CTLA4 caused significantly reduced primary tumor growth
compared to other groups.
[00129] Figure 62 is a graph illustrating tumor volume (mm3) as a function of
time
(days) for the shielded secondary (distant) B78 tumor in B78 melanoma flank
tumor mice
having both primary and secondary tumors. Mice were treated with various
combinations
of EBRT of the primary tumor only (12 Gy, secondary tumor was shielded), anti-
CTLA4
immune checkpoint inhibitor (CTLA4) and/or 50 Ci of the molecularly targeted
radiotherapy (MTRT) agent 90Y-NM600. Combination treatment of EBRT to the
primary
tumor, 90Y-NM600 MTRT, and anti-CTLA4 caused significantly reduced secondary
tumor
growth compared to other groups.
DETAILED DESCRIPTION
I. IN GENERAL
[00130] It is understood that this disclosure is not limited to the
particular
methodology, protocols, materials, and reagents described, as these may vary.
The
terminology used herein is for the purpose of describing particular
embodiments only,
and is not intended to limit the scope of the present invention, which will be
limited only
by any later-filed nonprovisional applications.
[00131] As used herein and in the appended claims, the singular forms "a",
"an", and
"the" include plural reference unless the context clearly dictates otherwise.
As well, the
terms "a" (or "an"), "one or more" and "at least one" can be used
interchangeably herein.
The terms "comprising" and variations thereof do not have a limiting meaning
where
these terms appear in the description and claims. Accordingly, the terms
"comprising",
"including", and "having" can be used interchangeably.
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[00132] Unless defined otherwise, all technical and scientific terms used
herein have
the same meanings as commonly understood by one of ordinary skill in the art
to which
this invention belongs. Although any methods and materials similar or
equivalent to
those described herein can be used in the practice or testing of the present
invention, the
preferred methods and materials are now described. All publications and
patents
specifically mentioned herein are incorporated by reference for all purposes
including
describing and disclosing the chemicals, instruments, statistical analysis and
methodologies which are reported in the publications which might be used in
connection
with the invention. All references cited in this specification are to be taken
as indicative
of the level of skill in the art.
[00133] The terminology as set forth herein is for description of the
embodiments only
and should not be construed as limiting of the invention as a whole. Unless
otherwise
specified, "a," "an," "the," and "at least one" are used interchangeably and
mean one or
more than one.
[00134] The disclosure is inclusive of the compounds described herein
(including
intermediates) in any of their pharmaceutically acceptable forms, including
isomers (e.g.,
diastereomers and enantiomers), tautomers, salts, solvates, polymorphs,
prodrugs, and the
like. In particular, if a compound is optically active, the invention
specifically includes
each of the compound's enantiomers as well as racemic mixtures of the
enantiomers. It
should be understood that the term "compound" includes any or all of such
forms,
whether explicitly stated or not (although at times, "salts" are explicitly
stated).
[00135] "Pharmaceutically acceptable" as used herein means that the compound
or
composition or carrier is suitable for administration to a subject to achieve
the treatments
described herein, without unduly deleterious side effects in light of the
necessity of the
treatment.
[00136] The term "effective amount," as used herein, refers to the amount of
the
compounds or dosages that will elicit the biological or medical response of a
subject,
tissue or cell that is being sought by the researcher, veterinarian, medical
doctor or other
clinician.
[00137] As used herein, "pharmaceutically-acceptable carrier" includes any and
all dry
powder, solvents, dispersion media, coatings, antibacterial and antifungal
agents, isotonic
36
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
agents, absorption delaying agents, and the like. Pharmaceutically-acceptable
carriers are
materials, useful for the purpose of administering the compounds in the method
of the
present invention, which are preferably non-toxic, and may be solid, liquid,
or gaseous
materials, which are otherwise inert and pharmaceutically acceptable, and are
compatible
with the compounds of the present invention. Examples of such carriers
include, without
limitation, various lactose, mannitol, oils such as corn oil, buffers such as
PBS, saline,
polyethylene glycol, glycerin, polypropylene glycol, dimethylsulfoxide, an
amide such as
dimethylacetamide, a protein such as albumin, and a detergent such as Tween
80, mono-
and oligopolysaccharides such as glucose, lactose, cyclodextrins and starch.
[00138] The term "administering" or "administration," as used herein,
refers to
providing the compound or pharmaceutical composition of the invention to a
subject
suffering from or at risk of the diseases or conditions to be treated or
prevented.
[00139] A route of administration in pharmacology is the path by which a drug
is
taken into the body. Routes of administration may be generally classified by
the location
at which the substance is applied. Common examples may include oral and
intravenous
administration. Routes can also be classified based on where the target of
action is.
Action may be topical (local), enteral (system-wide effect, but delivered
through the
gastrointestinal tract), or parenteral (systemic action, but delivered by
routes other than
the GI tract), via lung by inhalation.
[00140] In an enteral administration, the desired effect is systemic (non-
local),
substance is given via the digestive tract. In a parenteral administration,
the desired
effect is systemic, and substance is given by routes other than the digestive
tract.
[00141] Enteral administration may be administration that involves any part of
the
gastrointestinal tract and has systemic effects. The examples may include
those by mouth
(orally), many drugs as tablets, capsules, or drops, those by gastric feeding
tube, duodenal
feeding tube, or gastrostomy, many drugs and enteral nutrition, and those
rectally, various
drugs in suppository.
[00142] Examples of parenteral administrations may include intravenous (into a
vein),
e.g. many drugs, total parenteral nutrition intra-arterial (into an artery),
e.g., vasodilator
drugs in the treatment of vasospasm and thrombolytic drugs for treatment of
embolism,
intraosseous infusion (into the bone marrow), intra-muscular, intracerebral
(into the
37
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
brain parenchyma), intracerebroventricular (into cerebral ventricular system),
intrathecal
(an injection into the spinal canal), and subcutaneous (under the skin). Among
them,
intraosseous infusion is, in effect, an indirect intravenous access because
the bone
marrow drains directly into the venous system. Intraosseous infusion may be
occasionally used for drugs and fluids in emergency medicine and pediatrics
when
intravenous access is difficult.
[00143] The following abbreviations are used in this disclosure: ADCC,
Antibody
dependent cell-mediated cytotoxicity; anti-CTL4, an antibody that targets
cytotoxic T
lymphocyte-associated antigen 4 (CTLA4), which is found on cytotoxic T
lymphocytes
(CTLs); B16, A melanoma syngeneic to C57B1/6 mice; B78, A variant of B16 that
expresses GD2, due to transfection with GD2 synthase; D, day; Hu14.18-IL2, The
primary immunocytokine (reacts against GD2) used in the studies disclosed in
the
examples; IC, Immunocytokine (a fusion protein of a tumor-reactive mAb linked
to IL2);
ICI, immune checkpoint inhibitor; IL2, Interleukin 2; IT, Intratumoral; IV,
Intravenous;
mAb, Monoclonal antibody; MAHA, Mouse anti-human antibody; NM404, used to
designate the phospholipid ether shown in Figure 1, which is selectively taken
up by most
tumors and used for TRT in the studies disclosed in the examples; NM600, used
to
designate the phospholipid ether shown in Figure 14, which can be chelated
with any
metal, and which is also selectively taken up by most tumors and used for TRT
in the
studies disclosed in the examples; NXS2, A neuroblastoma syngeneic to AJ mice;
Panc02-GD2, A pancreatic cancer syngeneic to C57B1/6 mice, expressing GD2, due
to
transfection with GD2 synthase; PLE, Phospholipid ether; RT, Radiation
therapy; TRT,
Targeted radiotherapy; W, week; 9464D-GD2, A neuroblastoma syngeneic to
C57B1/6
mice, expressing GD2, due to transfection with GD2 synthase.
THE INVENTION
[00144] This disclosure is directed to methods of treating any cancer that
presents as
one or more malignant solid tumors. The disclosed methods combine two
treatment steps,
with an unexpected synergy resulting in a much improved effect against the
malignant
solid tumors. Specifically, an immunomodulatory dose of a radioactive
phospholipid
metal chelate compound or radiohalogenated phospholipid compound that is
38
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
differentially taken up by and retained within malignant solid tumor tissue is
administered to the patient, and further immunomodulation is performed by
systemically
administering (e.g., by IV injection) a composition that includes one or more
agents
capable of stimulating specific immune cells, either with or without
additional xRT to at
least one of the malignant solid tumors being treated with immune-stimulating
agents.
[00145] The immunomodulatory dose of the radioactive phospholipid metal
chelate or
radiohalogenated compound likely reduces Treg levels (and other immune-
suppressive
elements) and prevents the immune system dampening (concomitant immune
tolerance)
that occurs when xRT is used against a tumor and one or more additional tumors
are not
radiated, although an understanding of the mechanism is not necessary to
practice the
invention and the invention is not limited to any particular mechanism of
action.
A. Systemically-administered immunotherapy: immune checkpoint
inhibitors as exemplary immunostimulatory agents.
[00146] In direct contrast to methods of immunostimulation by administering an
immunomodulatory agent directly into a tumor (such as intratumoral
immunization by in
situ vaccination, as illustrated in some of the examples below), systemically-
administered
immunotherapy is performed by administering an immunostimulatory agent
systemically.
The immunostimulatory agent circulates through the whole body of the subject,
stimulating the body's natural immune response.
[00147] Immune checkpoint inhibitors are non-limiting examples of such
immunostimulatory agents. Activated T cells express multiple immune co-
inhibitory
receptors, such as lymphocyte-activation gene 3 (LAG-3), programmed cell death
protein
1 (PD-1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA4). These and
other
immune checkpoint molecules have been shown to modulate T cell responses to
tumor
antigens in the tumor microenvironment through unique and non-redundant
pathways.
[00148] More specifically, cancer growth is partly mediated by immune
suppression
induced by cancers. Tumors can activate suppressive immune checkpoint pathways
in
order to diminish the general immune response to the tumor. Accordingly,
blockade of
key immune checkpoint pathways can induce anti-tumor immunity, facilitated by
the
patient's own immune system.
39
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[00149] CTLA4 was the first immune checkpoint molecule to be clinically
targeted, by
administering CTLA4-targeting (anti-CLA4) mAbs. To date, the most promising
immune checkpoint inhibitor strategies for the treatment of cancers involve
administering
mAbs targeting CTLA-4 and/or PD-1/PD-Ll. Other immune checkpoint inhibitor
strategies are currently in development, and the disclosed combination method
is not
limited to targeting any specific immune checkpoint pathway.
[00150] A series of reviews covering checkpoint inhibitors and cancer
immunotherapy
was recently published in volume 276 of Immunological Reviews. These reviews,
including the introductory overview, Sharpe, A.H., "Introduction to checkpoint
inhibitors
and cancer immunotherapy," Immunol Rev. 276 (4 March 2017): 5-8, are
incorporated by
reference herein in their entirety.
B. Immunomodulatory dose of a radioactive phospholipid metal chelate
compound
[00151] The radioactive phospholipid metal chelate compound used should
selectively
target a wide range of solid tumor cell types, such that the RT emitted by the
metal
isotope chelated to the metal chelate compound is directed to malignant solid
tumor
tissue without substantially exposing other tissue types to the emitted RT.
The radioactive
metal isotope included in the radioactive phospholipid metal chelate compound
may be
any radioactive metal isotope known to emit ionizing RT in a form that would
result in
immunostimulation of the cells that take up the compound. Non-limiting
examples of
radioactive metal isotopes that could be used include Lu-177, Y-90, Ho-166, Re-
186, Re-
188, Cu-67, Au-199, Rh-105, Ra-223, Ac-225, Pb-212, or Th-227.
[00152] The immunomodulatory RT dose (as opposed to injected dose) of the
radioactive phospholipid metal chelate compound is much less than the dose
that would
be used for conventional RT against malignant solid tumors. Specifically, the
dose
should be sufficient to stimulate a response in immune cells within the tumor
microenvironment (likely by reducing immune-suppressing Treg levels and other
immunosuppressive cells or molecules), while not ablating the desired immune
cells that
are responsible for the immunostimulatory effect.
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[00153] The proper immunomodulatory dose can be calculated from imaging data
obtained after administering a "detection-facilitating" dose of a radioactive
metal chelate
compound. The detection-facilitating dose may be quite different than the
immunomodulatory dose, and the radioactive metal isotope that is chelated into
the
radioactive metal chelate compound may be different (although the rest of the
compound
structure should be the same). The radioactive metal isotope used in the
detection step
and dosimetry calculations may be any radioactive metal isotope known to emit
RT in a
form that is readily detectable by conventional imaging means. Non-limiting
examples
of "conventional imaging means" include gamma ray detection, PET scanning, and
SPECT scanning. Non-limiting examples of radioactive metal isotopes that could
be used
include Ga-66, Cu-64, Y-86, Co-55, Zr-89, Sr-83, Mn-52, As-72, Sc-44, Ga-67,
In-111,
or Tc-99m.
C. Metal chelates of PLE analogs
[00154] The disclosed structures utilize an alkylphosphocholine (APC) carrier
backbone. Once synthesized, the agents should harbor formulation properties
that render
them suitable for injection while retaining tumor selectivity as was
demonstrated
previously with the related radiohalogenated compounds. The disclosed
structures
include a chelating moiety to which the radioactive metal isotope will chelate
to produce
the final imaging or therapeutic agent.
D. Methods of Synthesizing Exemplary M-PLE Analogs
[00155] Proposed synthesis of compound 1 is shown below. The first step of the
synthesis is similar to described in Org Synth, 2008, 85, 10-14. The synthesis
is started
from cyclen which is converted into DO3A tris-Bn ester. This intermediate is
then
conjugated with NM404 in the presence of the base and Pd catalyst. Finally,
benzyl
protecting groups are removed by the catalytic hydrogenation.
41
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
0
?\--013n
(-N> BrCH2CO2Bn BnO{C-N
N 0
n 0
__µ _2,18_ 2_ 2 _
_ _3
HN + I . (CH 1 P CH CH NiVie
NH HN AcONa, DMAc j.- cNi O
cidj e
0¨
cyclen Bn0
0
rOBn \L
(--N 0 H2, Pd/C
Pd catalyst N N 0/¨c 11 (CH2)180POCH2CH2C)NMe3
¨0,- 01
base Ni e
OBn )
Bn0¨ 0
0
?\--OH
(.--N-
N 11 0 0
(CH2)180POCH2CH2NMe3
1
0/-1CN j 0
e
OH )
HO¨ 1
0
[00156] Synthesis of compound 2 is shown below. It begins with DO3A tris-Bn
ester
which is alkylated with 3-(bromo-prop-1-yny1)-trimethylsilane. After
alkylation, the
trimethylsilyl group is removed and the intermediate acetylene is coupled with
NM404
by the Sonogashira reaction. The benzyl groups are removed and the triple bond
is
hydrogenated simultaneously in the last step of the synthesis.
42
CA 03082056 2020-05-06
WO 2019/094657 PCT/US2018/059927
0
0
?
?-0L'OBn Bn
0
0 ,KI-N K2CO3
Bn0 - (--N->
BrCH2-CEC-SiMe3 Bn0-1 N
\-N HN ,.- N Me0H
c NJ i-Pr2NEt c N1-1\
SiMe3
Bn0
Bn0-
0
0
DO3A tris-Bn ester
0
?"\--0Bn
0 paci2(PPh3)2
0
Bn0.-_ (--N->
+ II e
\ (cH N N
(I) I . x _ 2)180POCH2CH2NMe3 .
cNi-\
e Et3N, Me0H
H
Bn0-
0
0
?L'OBn
? 0 H2, Pd/C
)-kk-1-12)180POCH2CH2NMe3
Orc NJ (5
e
OBn )
Bn0-
0
0
(OH
N 0
II e
N \ / __ (CH2)180POCH2CH2NMe3
ocN--..) (!)
e
OH ) 2
HO-
0
43
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[00157] Compounds 5 and 6 can be synthesized from same precursors, DTPA
dianhydride and 18-p-(3-hydroxyethyl-phenyl)-octadecyl phosphocholine as shown
in the
schemes below.
0\\ rCO2H HO o e
7 __ \ / __ \ =
(CH2)180AOCH2CH2NMe3 (1 eq)
6
O N N N 0 +
\ ___________________ µ e
O 0
DTPA dianhydride
0
HO2C ¨\ /¨\ /¨\ /
_õ.. N N N 0 0 e
\¨co2H . (CH2)180AOCH2CH2NMe3
HO2C 6
e
0, rco, io
/ \ 0
O NNN/ 'o + HO . (CH2)180POCH2CH22Me3 (2
eq) ¨'-
O / \ µo O
e
DTPA dianhydride
o o
ii 0 N N N 0 0
n e
Me3NCH2CH2OPO(CH2)18 . HO2C- ) \¨CO2H
. (CH2)180POCH2CH2NMe3
6e HO2C O
e
6
44
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[00158] NOTA-NM404 conjugates can be synthesized in an analogous manner. One
example of NOTA-NM404 conjugate 7:
1-7 CO2H
HO2C?N
0
(CH2)18010CH2CH2NMe3
0
LCO2H
7
E. Dosage Forms and Administration Methods
[00159] For the synergistic targeted RT, any route of administration may be
suitable.
In one embodiment, the disclosed alkylphosphocholine analogs may be
administered to
the subject via intravenous injection. In another embodiment, the disclosed
alkylphosphocholine analogs may be administered to the subject via any other
suitable
systemic deliveries, such as parenteral, intranasal, sublingual, rectal, or
transdermal
administrations.
[00160] In another embodiment, the disclosed alkylphosphocholine analogs may
be
administered to the subject via nasal systems or mouth through, e.g.,
inhalation.
[00161] In another embodiment, the disclosed alkylphosphocholine analogs may
be
administered to the subject via intraperitoneal injection or IP injection.
[00162] In certain embodiments, the disclosed alkylphosphocholine analogs may
be
provided as pharmaceutically acceptable salts. Other salts may, however, be
useful in the
preparation of the alkylphosphocholine analogs or of their pharmaceutically
acceptable
salts. Suitable pharmaceutically acceptable salts include, without limitation,
acid
addition salts which may, for example, be formed by mixing a solution of the
alkylphosphocholine analog with a solution of a pharmaceutically acceptable
acid such as
hydrochloric acid, sulfuric acid, methanesulfonic acid, fumaric acid, maleic
acid, succinic
acid, acetic acid, benzoic acid, oxalic acid, citric acid, tartaric acid,
carbonic acid or
phosphoric acid.
[00163] Where the disclosed alkylphosphocholine analogs have at least one
asymmetric center, they may accordingly exist as enantiomers. Where the
disclosed
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
alkylphosphocholine analogs possess two or more asymmetric centers, they may
additionally exist as diastereoisomers. It is to be understood that all such
isomers and
mixtures thereof in any proportion are encompassed within the scope of the
present
disclosure.
[00164] The disclosure also includes methods of using pharmaceutical
compositions
comprising one or more of the disclosed alkylphosphocholine analogs in
association with
a pharmaceutically acceptable carrier. Preferably these compositions are in
unit dosage
forms such as tablets, pills, capsules, powders, granules, sterile parenteral
solutions or
suspensions, metered aerosol or liquid sprays, drops, ampoules, auto-injector
devices or
suppositories; for parenteral, intranasal, sublingual or rectal
administration, or for
administration by inhalation or insufflation.
[00165] For preparing solid compositions such as tablets, the principal active
ingredient is mixed with a pharmaceutically acceptable carrier, e.g.
conventional
tableting ingredients such as corn starch, lactose, sucrose, sorbitol, talc,
stearic acid,
magnesium stearate, dicalcium phosphate or gums, and other pharmaceutical
diluents,
e.g. water, to form a solid preformulation composition containing a
homogeneous
mixture for a compound of the present invention, or a pharmaceutically
acceptable salt
thereof. When referring to these preformulation compositions as homogeneous,
it is
meant that the active ingredient is dispersed evenly throughout the
composition so that
the composition may be easily subdivided into equally effective unit dosage
forms such
as tablets, pills and capsules. This solid pre-formulation composition is then
subdivided
into unit dosage forms of the type described above containing from 0.1 to
about 500 mg
of the active ingredient of the present invention. Typical unit dosage forms
contain from
1 to 100 mg, for example, 1, 2, 5, 10, 25, 50 or 100 mg, of the active
ingredient. The
tablets or pills of the novel composition can be coated or otherwise
compounded to
provide a dosage affording the advantage of prolonged action. For example, the
tablet or
pill can comprise an inner dosage and an outer dosage component, the latter
being in the
form of an envelope over the former. The two components can be separated by an
enteric
layer which, serves to resist disintegration in the stomach and permits the
inner
component to pass intact into the duodenum or to be delayed in release. A
variety of
materials can be used for such enteric layers or coatings, such materials
including a
46
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
number of polymeric acids and mixtures of polymeric acids with such materials
as
shellac, cetyl alcohol and cellulose acetate.
[00166] The liquid forms in which the alkylphosphocholine analogs may be
incorporated for administration orally or by injection include aqueous
solutions, suitably
flavored syrups, aqueous or oil suspensions, and flavored emulsions with
edible oils such
as cottonseed oil, sesame oil, coconut oil or peanut oil, as well as elixirs
and similar
pharmaceutical vehicles. Suitable dispersing or suspending agents for aqueous
suspensions include synthetic and natural gums such as tragacanth, acacia,
alginate,
dextran, sodium caboxymethylcellulose, methylcellulose, polyvinylpyrrolidone
or
gelatin.
[00167] The disclosed alkylphosphocholine analogs are particularly useful when
formulated in the form of a pharmaceutical injectable dosage, including in
combination
with an injectable carrier system. As used herein, injectable and infusion
dosage forms
(i.e., parenteral dosage forms) include, but are not limited to, liposomal
injectables or a
lipid bilayer vesicle having phospholipids that encapsulate an active drug
substance.
Injection includes a sterile preparation intended for parenteral use.
[00168] Five distinct classes of injections exist as defined by the USP:
emulsions,
lipids, powders, solutions and suspensions. Emulsion injection includes an
emulsion
comprising a sterile, pyrogen-free preparation intended to be administered
parenterally.
Lipid complex and powder for solution injection are sterile preparations
intended for
reconstitution to form a solution for parenteral use. Powder for suspension
injection is a
sterile preparation intended for reconstitution to form a suspension for
parenteral use.
Powder lyophilized for liposomal suspension injection is a sterile freeze
dried preparation
intended for reconstitution for parenteral use that is formulated in a manner
allowing
incorporation of liposomes, such as a lipid bilayer vesicle having
phospholipids used to
encapsulate an active drug substance within a lipid bilayer or in an aqueous
space,
whereby the formulation may be formed upon reconstitution. Powder lyophilized
for
solution injection is a dosage form intended for the solution prepared by
lyophilization
("freeze drying"), whereby the process involves removing water from products
in a
frozen state at extremely low pressures, and whereby subsequent addition of
liquid
creates a solution that conforms in all respects to the requirements for
injections. Powder
47
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
lyophilized for suspension injection is a liquid preparation intended for
parenteral use that
contains solids suspended in a suitable fluid medium, and it conforms in all
respects to
the requirements for Sterile Suspensions, whereby the medicinal agents
intended for the
suspension are prepared by lyophilization. Solution injection involves a
liquid
preparation containing one or more drug substances dissolved in a suitable
solvent or
mixture of mutually miscible solvents that is suitable for injection.
[00169]
Solution concentrate injection involves a sterile preparation for parenteral
use
that, upon addition of suitable solvents, yields a solution conforming in all
respects to the
requirements for injections. Suspension injection involves a liquid
preparation (suitable
for injection) containing solid particles dispersed throughout a liquid phase,
whereby the
particles are insoluble, and whereby an oil phase is dispersed throughout an
aqueous
phase or vice-versa. Suspension liposomal injection is a liquid preparation
(suitable for
injection) having an oil phase dispersed throughout an aqueous phase in such a
manner
that liposomes (a lipid bilayer vesicle usually containing phospholipids used
to
encapsulate an active drug substance either within a lipid bilayer or in an
aqueous space)
are formed. Suspension sonicated injection is a liquid preparation (suitable
for injection)
containing solid particles dispersed throughout a liquid phase, whereby the
particles are
insoluble. In addition, the product may be sonicated as a gas is bubbled
through the
suspension resulting in the formation of microspheres by the solid particles.
[00170] The parenteral carrier system includes one or more pharmaceutically
suitable
excipients, such as solvents and co-solvents, solubilizing agents, wetting
agents,
suspending agents, thickening agents, emulsifying agents, chelating agents,
buffers, pH
adjusters, antioxidants, reducing agents, antimicrobial preservatives, bulking
agents,
protectants, tonicity adjusters, and special additives.
[00171] The following examples are offered for illustrative purposes only, and
are not
intended to limit the scope of the present invention in any way. Indeed,
various
modifications of the invention in addition to those shown and described herein
will
become apparent to those skilled in the art from the foregoing description and
the
following examples and fall within the scope of the appended claims.
48
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
III. EXAMPLES
Introduction to the Examples
[00172] These examples demonstrate the potential of bringing together two very
distinct cutting-edge disciplines in cancer treatment research, capitalizing
on an
unexpected and very potent synergy. These disciplines are: 1) systemically
administered
TRT; and 2) locally directed, antibody-mediated cancer immunotherapy or
systemically
administered cancer immunotherapy. The data presented herein suggest that
powerful
synergy results from combining these approaches. Together, these two
strategies can be
used to destroy visible macroscopic tumor in a way that enables the destroyed
cancer
cells to function as a potent immunostimulator that creates tumor-specific T
cell
immunity able to eradicate persistent residual metastatic disease, for any
type of solid
tumor in any location.
[00173] Our ongoing preclinical work has shown that combination of tumor-
specific
mAb with IL2 (to activate innate immune cells) results in augmented antibody-
dependent
cell-mediated cytotoxicity (ADCC) [1,2]; a process that has already been
translated into
clinical benefit for children with neuroblastoma [3]. Recent preclinical data
demonstrate
more potent antitumor efficacy when the mAb-IL2 fusion protein is injected
intratumorally (IT) [4,5]. Remarkably, large tumors that do not respond to
these mAb/IL2
injections and continue growing if treated only with local xRT, can be
completely
eradicated if the xRT is combined with the mAb/IL2 treatment. Most mice are
cured and
develop T cell memory that rejects re-challenge with similar tumor cells [6];
demonstrating that the combined xRT + mAb/IL2 is acting as a potent "in situ"
anti-
cancer vaccine.
[00174] A key limitation is that if there is another macroscopic tumor present
in these
animals when they receive xRT+ mAb/IL2 treatment to the primary (first) tumor,
the
second tumor will continue to grow and, to our surprise, suppress the immune
response,
preventing any shrinkage of the 1st treated tumor. This "concomitant immune
tolerance"
results, in part, from suppressive regulatory T cells (Tregs) in the 2nd
tumor. Delivering
RT alone to both tumors has minimal anti-tumor effect, but does deplete these
Tregs.
Thus, when first tumors are treated with xRT + mAb/IL2, the addition of RT to
the
second tumor circumvents this immune tolerance, enabling eradication of both
tumors
49
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[7]. These observations indicate a limitation of in situ tumor vaccination in
the metastatic
setting, but also suggest a robust capacity of RT to overcome this limitation.
[00175] xRT cannot typically be delivered to all metastatic sites without
prohibitive
normal tissue toxicity and immune suppression. Yet not delivering xRT to all
sites of
macroscopic disease may leave inhibitory immune lineages intact, which are
capable of
suppressing the immunologic response to our local xRT + mAb/IL2 immunotherapy.
What is needed, therefore, is a means to deliver RT to all tumor sites in a
cancer patient
in a targeted manner.
[00176] We have developed TRT vehicles capable of targeting systemically
administered RT to both primary and metastatic cancers. One such TRT agent,
131I-
NM404, an intravenously (IV) administered phospholipid ether (PLE) analog, has
shown
nearly universal tumor targeting properties in over 60 in vivo cancer and
cancer stem cell
models. This agent is currently being evaluated clinically in multiple imaging
and therapy
trials [8,9]. A systemic injection of 131I-N1V1404 localizes in all tumors
regardless of
anatomic location and internally provide sufficient RT to ablate intratumoral
immunosuppressive pathways that can prevent development of an effective, tumor-
eradicating, immune response. The unique attributes of this approach are the
near
universal tumor targeting capability of NM404, as well as the ability to
deliver
immunomodulatory sub-lethal doses of RT to all tumor sites, something that is
not
typically feasible with xRT. What is new about this is that our TRT Agents may
immuno-
modulate all tumors regardless of anatomic location, overcoming concomitant
tolerance,
which will result in a long-term in situ tumor vaccination effect following
local xRT
followed by injection of a tumor specific mAb + IL2. As an increasing number
of tumor
specific mAbs are becoming approved for clinical use, this combination
strategy provides
an expanded approach for any tumor type that can be targeted by a tumor-
reactive mAb.
Furthermore, the approach can be readily generalized to all in situ tumor
vaccination
strategies.
[00177] Recently, we have discovered that the iodine in 131I-NM404 can be
substituted
with chelators capable of carrying a wide variety of metallic imaging (MRI and
PET) and
TRT radiotherapy moieties. In these examples, we describe how to assess the
ability of
131I-N1V1404 (and thus, the related metal chelate analogs) to initiate the
systemic
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
immunomodulatory response necessary to enable combined xRT + immunotherapy
treatment to induce a potent radioimmune-facilitated response against
cancerous solid
timuors. A similar approach can be used for combined PLE analog-delivered TRT
with
other immunotherapy methods used against cancerous solid tumors. For example,
we
have illustrated below that the combination method can use an immunodulation
step that
is quite different from local in situ tumor vaccination: the systemic
administration of an
immunostumulatory agent such as an immune checkpoint inhibitor.
[00178] In sum, we disclose herein therapeutic and research processes that
combine
two different methods from seemingly disconnected cancer therapy disciplines
into a
single unified treatment. The data presented in these examples indicate that
the two
methods can be synergistically combined to effectively eliminate malignant
solid tumors
and to prevent tumor recurrence.
[00179] In Example 1, we present background data from our B78 GD2+ model in
support of the method.
[00180] In Example 2, we provide guidance for determining the dose of xRT
needed
for optimal in situ vaccine effect to a primary tumor, and the lowest dose of
xRT to a
distant tumor needed to prevent concomitant immune tolerance.
[00181] In Example 3, we provide guidance for determining the 131I-NM404
dosing
that approximates the required dosing of xRT to metastases, as determined in
Example 2,
and subsequently evaluating the effects of that 131I4\flM404 dose on in vivo
immune
function. Such guidance can be similarly applied when using the disclosed
radioactive
phospholipid metal chelate compounds as the TRT agent.
[00182] In Example 4, we provide guidance for using data from Examples 2
and 3 to
design/test/develop a regimen of 131I-NM404 + local xRT + IT-mAb/IL2 in mice
bearing
two or more tumors in order to destroy the locally treated tumors and induce T-
cell
mediated eradication of all distant tumors. Critical issues of TRT and xRT
dose and time
are optimized for antitumor efficacy. Again, such guidance can be similarly
applied when
using the disclosed radioactive phospholipid metal chelate compounds as the
TRT agent.
[00183] In Example 5, we provide an exemplary synthesis that also finds use to
the
synthesis of analogous compounds chelating radioactive metal isotopes.
51
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[00184] In Example 6, we demonstrate that an analog having a chelating agent
and
chelated metal substituted for the iodine moiety of NM404 (Gd-NM600) is taken
up by
(and can be imaged in) solid tumor tissue, thus providing proof of concept for
using the
disclosed metal chelates as a TRT agent.
[00185] In Examples 7, 8, 9 and 10, we provide information and specific data
from
experimental studies performed in accordance with the guidance of Examples 1-
4.
[00186] In Examples 11 and 12, we demonstrate that additional analogs having a
chelating agents and chelated metals substituted for the iodine moiety of
NM404 are
taken up by, and can be imaged in, and can be used therapeutically for TRT in
a range of
solid tumor in vivo models, thus providing additional proof of concept for
using the
disclosed metal chelates as TRT agents in the disclosed methods.
[00187] In Example 13, we discuss how dosimetry in combination with known
radiosensitivities can be used by the skilled artisan to optimize treatment
dosages for any
solid tumors.
[00188] In Example 14, we discuss differences and advantages in using
alkylphosphocholine metal chelates in the disclosed methods, rather than the
iodinated
compounds exemplified in Examples 1-4 and 7-10.
[00189] In Examples 15 and 16, we demonstrate that TRT in combination with
systemically-administered immunotherapy, rather than in situ vaccination, is
also
effective is treating solid tumors. The immunostimulatory agent that is
systemically
administered may be an immune checkpoint blocker or inhibitor (in this case,
anti-
CTLA4).
Example 1: Background Supporting Data
[00190] The Sondel lab has shown that tumor-specific mAb + IL2 activates
innate
immune cells to mediate ADCC in mice [2], with clinical benefit for children
with
neuroblastoma [3]. In mice, IV administration of the hu14.18-IL2 is more
potent than IV
administration of anti-GD2 mAb + IL2 [2, 10]. This can provide dramatic
antitumor
effects against very small recently established GD2+ tumors or very small
microscopic
metastases, potentially accounting for the clinical use of this approach in
patients in
remission but at great risk for relapse [3]. More potent antitumor efficacy
against
52
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
measurable, macroscopic tumors [i.e. ¨ 50 mm3 GD2+ tumors] can be achieved
when the
IC is injected intratumorally (IT-IC) rather than IV [4,5].
[00191] We are now focusing on ways to provide benefit in the setting of much
larger,
macroscopic tumors. Mice bearing a moderately large (200 mm3) B78 melanoma
tumor,
established five weeks earlier, show no response to IV-IC, and are slowed in
their growth
by IT-IC, but the tumors continue to grow. These same 200 mm3 tumors also grow
after
12 Gy of xRT. In contrast, when the IT-IC and xRT are combined, 73% of the
animals
become tumor-free and appear cured of their disease (Figs. 2A and 2B). These
mice then
show T-cell mediated rejection of rechallenge with the same tumor (Fig. 2C).
Thus IT-IC
+ xRT synergize, inducing the tumor to become an "in situ tumor vaccine" [6].
[00192] In order to simulate clinical metastases, we inoculate mice with B78
in one
flank on d-1, and the other flank at week 2. At week 5, the first tumor is 200
mm3, and
the second is 50 mm3. We anticipated that xRT + IT-IC would destroy the first
tumor and
that the resultant T cell response would then destroy the second. However,
adding IT-IC
to the xRT had virtually no effect on either the 50 mm3 tumor or the 200 mm3
tumor (Fig.
3). This demonstrated a key limitation to the therapy we delivered; namely, if
there is
another tumor present when these mice receive xRT + IT-IC to the first tumor,
the second
tumor will cause a systemic tumor-specific concomitant immune tolerance
effect,
preventing any shrinkage of either tumor. Importantly, we have found that
local xRT (12
Gy) to the first and second tumor simultaneously, abrogates this tolerance
effect,
allowing IT-IC to the first tumor to induce an immune response that eradicates
both
tumors in most mice (Fig. 4) [7]. Recent data, using a Treg depleting mAb (not
shown) or
transgenic mice that allow selective Treg depletion (Fig. 4) [7], demonstrate
that this
immune tolerance is mediated, in part, by regulatory T cells (Tregs); RT to
the first and
second tumors partially deplete these Tregs, potentially explaining how
irradiating both
tumors circumvents the tolerance effect [7].
[00193] While local xRT to both the first and second tumors circumvents
tolerance,
clinical metastatic disease is often in several locations. All macroscopic
metastatic
disease must receive RT to block immune tolerance and enable xRT + IT-IC to
effectively eradicate all tumor sites. However, delivery of 12 Gy xRT to all
sites of
53
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
disease may be akin to "total body RT" with major dose-dependent (potentially
lethal)
toxicity and profound systemic immune suppression.
[00194] Previously, the Weichert lab has pioneered the development of TRT, in
order
to deliver RT to all systemic tumor sites, while minimizing "off-target" RT to
normal
tissue (especially marrow and immune tissue).
[00195] Based on the finding that tumor cells contain an overabundance of
phospholipid ethers (PLE) [11], we synthesized over thirty radioiodinated PLE
analogs in
hopes of identifying analogs that would selectively target tumors [12]. One of
these,
NM404, not only displayed near universal tumor uptake in all but three of over
70 in vivo
models examined regardless of anatomic location, including brain metastases
and cancer
stem cells, but also underwent prolonged selective retention once it entered
tumor cells
[8]. These diapeutic PLE analogs are unique in that they avoid premalignant
and
inflammatory lesions. Surface membrane lipid rafts, which are overexpressed on
cancer
cells relative to normal cells, serve as portals of entry for PLE's, including
NM404, into
cancer and cancer stem cells [8]. Radioiodinated NM404 (1-124 and I-131),
which has
now been evaluated in five phase 1 and 2 PET imaging trials and three phase 1
TRT
radiotherapy trials, respectively, affords similar tumor uptake and retention
properties in
over a dozen human cancer types [8]. Excellent tumor uptake in the cancer
models
relevant to these examples (the B78 GD2+ murine melanoma) have been confirmed
with
124I-NM404 PET imaging (Fig. 5).
Example 2: Determining Dosages of xRT
[00196] Our data suggest these four hypotheses: (1) the dose of xRT we have
used to
treat a single tumor causes modest direct in vivo tumor death and increases
susceptibility
to immune mediated death (via both ADCC and T cells); (2) the strong T-cell
response
provided by the addition of IT-IC, but not IT mAb, suggests that mAb binding
to radiated
tumor cells, in the presence of IL2, facilitates antigen presentation and
augmented
induction of adaptive immunity; (3) the presence of a second tumor prevents
the xRT +
IT-IC to the first tumor from causing virtually any anti-tumor effect, due to
tolerance
caused largely by the systemic actions of immunosuppressive cells present in
the second
tumor [such as Tregs and possibly myeloid derived suppressor cells (MDSC)];
this
54
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
tolerance can be circumvented by depletion of Tregs (Fig. 4) or irradiating
the second
tumor (Fig. 3); (4) the dose of RT needed at the second tumor to circumvent
tolerance
might be much lower than the xRT dose needed for the first tumor to become an
"in situ
vaccine" [14].
[00197] Optimizing xRT dose for the primary ("in situ vaccine") tumor site.
[00198] Our in vivo studies of xRT + IT-IC have focused on one dose of 12 Gy
to the
first tumor. This is based on our data showing that in vitro RT induces a dose-
dependent
functional upregulation of Fas on B78 tumor cells (nearing peak at >12 Gy),
coupled to
our in vivo data demonstrating our in situ vaccine effect of xRT + IT-IC
requires mice
with functional Fas-L (6). We conducted in vivo pilot studies prior to
selecting the 12 Gy
dose, which showed higher dose (16 Gy) or increased fractionation flank RT had
toxicity
(dermatitis, ulceration, and late limb edema) and no improvement in tumor
response.
While we chose a 12 Gy single fraction of xRT for our in vivo studies, as we
move
towards clinical translation, it will be beneficial to better understand the
mechanism of
the local xRT effect and its dose requirements, in order to safely and
effectively induce
the in situ vaccine effect.
[00199] Our mouse data (Figs. 2A, 2B and 2C) show that we can induce a potent
vaccine effect with 12 Gy xRT + IT-IC, even though 12 Gy of xRT alone causes
no
shrinkage of the tumor; it merely slows the progressive growth. It is
contemplated that we
might see just as potent an in situ vaccine effect using lower doses of RT. To
test this, we
will evaluate a range of xRT doses (from 4 ¨ 16 Gy) as a single fraction in
mice bearing a
¨ 200 mm3 B78 tumor, followed by our standard IT-IC regimen (50 mcg/d on days
6-10).
We will determine which xRT doses give optimal tumor eradication and T-cell
memory,
when combined with IT-IC. If doses lower than 12 Gy are less toxic and show
comparable efficacy, such lower doses would be better targets for our xRT dose
to the "in
situ vaccine" site in Examples 3 and 4. Similar approaches may be used to
optimize
dosing for particular targets or subjects.
[00200] Optimizing xRT dose at a distant tumor to prevent tolerance from
blocking "in situ vaccination."
[00201] Treating both the first and second tumors with 12 Gy (Fig. 3) enables
IT-IC to
the first tumor to induce a potent response that eradicates both tumors. Our
goal is to be
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
able to accomplish this same in situ vaccine effect by providing xRT + IT-IC
to a single
tumor while using the minimal RT dose necessary at metastatic sites to
circumvent
tolerance. We recognize that xRT itself, especially if widespread, can be
myelo/immunosuppressive. This is why we are pursuing TRT in Examples 3 and 4.
Even
though it is targeted, TRT does have some systemic delivery of RT. In order to
minimize
the systemic immune suppression from TRT, we wish to give as low of a dose of
TRT as
is needed to effectively inhibit the tumor-induced immune tolerance, while not
causing
systemic RT-induced global immune suppression. Therefore, it is best to select
the lowest
dose of xRT needed to be delivered to the distant tumor in order to enable a
higher xRT
dose to the first tumor to function as an in situ vaccine when combined with
IT-IC to the
first tumor.
[00202] As an exemplary optimization experiment, mice bearing a 200 mm3 first
B78
tumor and a ¨50 mm3 second B78 tumor will receive 12 Gy of xRT to the first
tumor on
day-0 (-5 weeks after implantation of the first B78 tumor). This will be
followed by our
standard regimen of IT-IC on days 6-10. Separate groups of mice will receive
varying
doses of xRT to the second tumor. Based on data from the lab of B. Johnson
demonstrating that a total body xRT of 3 Gy can prevent an immunosuppressive
effect in
a myeloma model (15), we will evaluate doses of 0, 1, 5 and 8 Gy (in addition
to the 12
Gy dose we know is effective). We will see if doses substantially less than 12
Gy to the
second tumor can be as effective as the full 12 Gy dose at eliminating the
immune
tolerance.
[00203] Once we have selected the critical dose of xRT where we lose the
beneficial
effect, we will perform subsequent analyses to better optimize the critical
dose. For
example, if 5 Gy is as effective as 12 Gy, but 1 Gy is not much better than 0
Gy, we
would then compare 2, 3, and 4 Gy to identify the critical lowest effective RT
dose
needed to eliminate tolerance and obtain efficacy in this two tumor model,
receiving 12
Gy + IT-IC to the first tumor.
[00204] Repeat studies are then be done to confirm if this lowest effective
dose to the
second tumor still enables an effective in situ vaccine when the dose to the
first is the
lowest effective dose in the 1-tumor model (tested in Example 2, above) rather
than the
12 Gy dose. In summary, the studies of Example 2 optimize what the lowest xRT
doses
56
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
are for the first and second tumors, without losing the efficacy we have
demonstrated
with 12 Gy to both.
[00205] Initiating studies of required xRT dose to first and second tumors in
mice
bearing tumors other than B78.
[00206] To allow our mouse studies to suggest more clinical generalizability,
we will
initiate analyses of RT + IT-IC in additional models of GD2+ tumors. We have
published
on IT-IC with hu14.18-1L2 IC in AJ mice bearing the GD2+ NXS2 neuroblastoma
[5].
We are also evaluating IT-IC with this same IC in C57BL/6 mice bearing the
GD2+
9464D-GD2 neuroblastoma, and the Panc02-GD2 pancreatic cancer that express GD2
via
our insertion of the gene for GD2 synthase. As for Example 2, for each model
we will
determine the lowest effective xRT dose needed to the primary and the
secondary tumors
to retain the in situ vaccine effect.
Example 3:
Determining Dosage of '31I-NM404 and Evaluating Effects on Immune Function
Dosimetry with TRT and immunesuppression from TRT in C57BL/6 mice.
[00207] 131I-NM404 has shown selective uptake in vitro in >95% of tumor lines
(human and mouse), with poor uptake by non-malignant cells, and with similar
tumor
specificity seen in vivo. This includes selective uptake in vivo with the B78
tumor (Fig.
5). In our preliminary dosimetry study, we gave 124I-NM404 to C57BL/6 mice and
characterized the time course of TRT exposure by serial PET/CT imaging (as in
Fig. 5).
Monte Carlo dosimetry calculations [16-18] based on this study indicated that
¨ 6011Ci
of 131I-NM404 would be needed to deliver ¨ 3 Gy to an established B78 tumor
over a
four week period of decay. After those four weeks, the remaining TRT dose to
the B78
tumor would be less than 0.25 Gy. We will replicate the data we obtained in
our 2-tumor
model using xRT (Fig. 3), but use the lowest possible dose of targeted 131I-
NM404 TRT
to enable effective elimination of tumor-induced tolerance at all sites of
distant disease.
However, unlike xRT, which delivers all dose in minutes and is then done, TRT
deposits
dose over time, depending upon both the biological and physical half-life of
the targeted
isotope (8 day t1/2 for 1314 We want an initial TRT effect at the distant
tumor sites to
eradicate immune tolerance; however we want the immunosuppressive TRT effect
to
57
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
then be minimal when we give the IT-IC to induce ADCC and the in situ vaccine
anti-
tumor effects. This is essential to allow full tumor destruction at all sites.
[00208] Using the dosimetry calculations from our preliminary data, we
estimated that
a dose of 3 pfi of 131I-NM404, should deliver an equivalent of ¨0.2 Gy to the
tumor site,
a dose that we hypothesized should not be immunosuppressive and should not
prevent
lymphocyte-mediated tumor destruction. As noted above, this is the dose we
estimated
would remain yet to be delivered 28 days after an initial '3'I-NM404 dose of
60 pfi. We
thus evaluated groups of mice bearing a single 200 mm3 B78 tumor. On day 0,
all mice
got 12 Gy xRT to their tumor, and on days 6-10, all got 50 mcg/d of IT-IC. One
group
also got 3 pfi of 131I-NM404 on d-0 (-0.2 Gy). Fig. 6 shows that the group
receiving the
131I-NM404 had the same degree of tumor eradication as the group without 131I-
NM404,
demonstrating that this low dose of "residual" TRT in the tumor does not block
immune
mediated destruction by the RT + IT-IC in situ vaccine. We thus hypothesize
that if we
use an initial dose of 60 pfi of 131I-NM404 TRT on day-22, it would
effectively block
the tolerogenic effect of distant tumors, yet enable xRT on day 0 and IT-IC on
days 6-10
(28d after the TRT) to the first tumor to function as an in situ vaccine,
inducing an
adaptive response that then eradicates all tumors.
[00209] The experiments outlined in this example optimize the dose
relationships
tested in Fig. 6. In our 1-tumor B78 model, we will test a range of doses of
131I-NM404
TRT to select the best TRT dose that results in enough unwanted systemic
immune
suppression to interfere with the desired in situ vaccine effect (and thereby
slow or
prevent eradication of the first tumor). This is important to Example 4, as it
allows us to
make sure the residual radioactivity of the TRT has decayed to less than this
value at the
time we initiate IT-IC to the first tumor in mice with distant disease. We
will also
evaluate the kinetics of the TRT response after varying TRT doses to select an
optimal
time period for how long we should wait after the "tolerance-preventing TRT
dose" is
given to animals with multiple tumors to allow the RT + IT-IC treatment of the
first
tumor to still induce the in situ vaccine effect and eradicate the primary as
well as distant
tumors.
[00210] Related studies will also look at what dose of TRT, given as single
agent
treatment, are most beneficial to cause slowing, versus shrinkage, versus
eradication of a
58
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
single B78 tumor. The dose of TRT that is most beneficial to eliminate the
tumor-induced
immune tolerance will be substantially less than the TRT dose needed to
actually induce
complete tumor destruction (from the TRT alone).
[00211] Finally, once the effects of various optimized doses of TRT are
determined in
the 1-tumor model, we will evaluate the subtle immune-suppressive effects of
TRT, by
evaluating sera from these subject for their immune response to the human IgG
component of the IC. We have shown that immunocompetent mice generate a
readily
quantified level of Mouse Anti-Human Antibody (MAHA) following treatment with
these humanized ICs (19). We will use this as a means of determining at what
dose we
are seeing the TRT cause a detectible dose-dependent decrease in the strength
of the
murine immune response, to gauge the overall immunosuppressive effects from
the
systemic doses of RT these mice will receive from this TRT. The low TRT dose
that we
will need to block the tumor-induced immune tolerance will cause minimal
systemic
immune suppression.
Example 4: Developing an optimal Regimen of 131I-NM404 + local xRT + IT-
mA13/1L2 in Mice Bearing Two or More Tumors
[00212] Testing the efficacy of TRT + RT + IT-IC in the 2-tumor B78 model.
[00213] The dose and timing information learned from the studies outlined in
Examples 2 and 3 will provide the information we need to optimize TRT dosing
and
timing required for efficacy in our 2-tumor model. C57BL/6 mice will be
inoculated with
B78 in the left (L) and right (R) flanks simultaneously. Each tumor should be
¨ 50 mm3
after two weeks and ¨ 200 mm3 after five weeks. If we assume that our
dosimetry
calculations in Example 3 suggest that we need to deliver 6011Ci of TRT to
approximate
3 Gy RT to the second tumor (to block the immune tolerance), our external beam
xRT
studies predict that this dose should have minimal slowing effect on tumor
growth. We
would plan to treat different groups of mice with 30, 60 or 90 tCi at the 2 w
time point
(when the tumors are ¨ 50 mm3). Three weeks later the tumors should be ¨ 200
mm3; at
that time we will give xRT (dose determined as outlined in Example 2) followed
six days
later (¨ 28 d after the TRT) by five daily injections of IT-IC to the tumor in
the L flank,
to induce the in situ vaccine effect. Control mice would get no TRT, and only
the xRT
59
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
and IT-IC to the L flank, anticipating no in situ vaccine due to tolerance
from the distant
tumor. A separate group would get local xRT to both tumors and IT-IC to the L
flank,
anticipating eradication of both tumors via the in situ vaccine effect.
Another group get
TRT + IT-IC, but without local xRT, anticipating an incomplete vaccine effect.
[00214] Follow-up experiments further evaluate varying doses of TRT and
variations
in the timing between the TRT and the local xRT + IT-IC to the primary tumor
(L flank).
The readouts will be: (A) eradication of the primary tumor; (B) eradication of
the
secondary tumor; and (C) systemic immune suppression, via ELISA analyses of
the
MAHA response. Our goal is to identify optimal TRT dose and timing with a
particular
subject and disease model, to add to the local xRT + IT-IC regimen that can
eradicate
both tumors in most subject, while minimizing systemic immunosuppression (as
measured by MAHA response).
[00215] Optimizing TRT + xRT + IT-IC in mice bearing more than two B78
tumors.
[00216] This section of Example 4 is most analogous to the relevant clinical
setting;
namely, patients with an injectable tumor that could be used as an in situ
vaccine site, but
with multiple distant metastases that could each be causing tumor-induced
immune
tolerance. These studies will replicate the conditions found to be most
effective in the
first part of Example 4 (above). The important difference is that these
subject will each
have four separate tumors, in L and R flanks, and L and R para-scapular
regions. The
TRT is given at the dose and timing found most effective in the studies
outlined in the
first section of Example 4, with xRT + IT-IC subsequently given only to the L-
flank
lesion. The goal here is to select TRT dose and timing issues to enable most
effective in
situ vaccine, because the TRT would effectively eliminate the tumor-induced
immune
tolerance caused by the three sites not getting xRT. The measure of efficacy
is
elimination of all four tumors in most subjects. Modifications in TRT dose and
timing are
tested in order to generate an optimized regimen that is most effective. Such
a regimen
finds use in the clinic for patients with multiple distant metastases, that
could not all be
irradiated via external beam, but could be irradiated via TRT, when combined
with local
xRT + IT-IC to the "in situ vaccine" site.
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
Example 5: Synthesis of metal chelated NM600
[00217] In this Example, we show the synthetic scheme used to synthesize one
exemplary phospholipid chelate, Gd-NM600. Analogs incorporating various
radioactive
isotopes could be synthesized in a similar manner, where the radioactive
isotope in
questions is substituted for Gd.
61
3152.303.P180116W001
[00218] Scheme for synthesizing Gd-1\11\4600 (the disclosed
radioactive metal isotopes could be substituted for Gd):
0
t,..)
o
,-,
Bn0 OBn Bn0 OBn
CB:z
0 0
/--\ /--\ /--µ
CNN HN...i Br)(OBn BrOtBu
cA
________________________________ Jo- 0 cN N...i 0
___________________________________________________________________ v.-
0 cN N-1 0 un
--.1
NH HN-j Na0Ac, DMAC
0 N HN") K2CO3, MeCN
0 N N"--1 0
\/ 75% / \/ = HBr 93%
Bn0
Bn0 OtBu
cyclen
Bn0 OBn
HCI 0 CN N...i 0
0
ii 0
COMU, Et3N
+ H2N * (CH2)180POCH2CH2NMe3
1
,,
dioxane 0 N N"--1 0
CHCI3, 80% L.
\__/ \_4 00
.
,,
cA Bn0 OH
.
u,
n.)
.
,,
.
N,
0 0
.
,
.
?L'OBn (OH
u,
cn
c-,,- 0
9 e H2, Pd/C
-)p.. (--N- 0
N NkA.N # Nk.)1.
9 e
(CH2)180c)OCH2CH2NMe3 Et0H (j¨/N 1
11 # (CH2)180c)OCH2CH2NMe3
OcNi H 80% \õN--/
OBn ) 00 OH )
e
Bn0¨ HO-
0 0
0
IV
0-1
n
,-i
Gda3 r.H
Py-H20 0--ANI. * ( 9 e
k.,..2)180 c)OCH2CH2NMe3
00
C 1..,P
t=.)
-1,..
=
0 0
oe
CB
82%
un
04
n.)
0
--.1
101273789.DOCX/ 162
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
Example 6: In vivo Imaging Proof of Concept
[00219] In this example, we demonstrate the successful in vivo MRI imaging of
a
tumor, using Gd-NM600 as the MRI contrast agent. The data demonstrates that
the
backbone phospholipid and chelating agent are taken up and retained by solid
tumors,
demonstrating that such chelates incorporating various radioactive metals, as
disclosed herein, would exhibit similar properties
[00220] For proof-of-concept in vivo imaging of tumor uptake of the Gd-NM404
agent, nude athymic mouse with a flank A549 tumor (non small cell lung cancer)
xenograft was scanned. The Gd-NM600 agent (2.7 mg) was delivered via tail vein
injection. Mice were anesthetized and scanning performed prior to contrast
administration and at 1, 4, 24, 48, and 72 hours following contrast delivery.
Imaging
was performed on a 4.7T Varian preclinical MRI scanner with a volume
quadrature
coil. Ti-weighted images were acquired at all imaging time points using a fast
spin
echo scan with the following pulse sequence parameters: repetition time (TR) =
206
ms, echo spacing = 9 ms, echo train length = 2, effective echo time (TE) = 9
ms, 10
averages, with a 40x40 mm2 field of view, 192x192 matrix, 10 slices of
thickness lmm
each.
[00221] As seen in Figure 7, MRI imaging of the tumor was significantly
enhanced
by 24 hours post-injection.
[00222] These results demonstrate that the differential uptake and retention
of
alkylphosphocholine analogs is maintained for the metal chelated analogs
disclosed
herein. Thus, the disclosed metal chelates can readily be applied to clinical
therapeutic and imaging applications.
Example 7: Experiments determining the dose of xRT needed for optimal in situ
vaccine effect to a primary tumor, and the lowest dose of xRT to a distant
tumor
needed to prevent concomitant immune tolerance
[00223] As a follow-up to Examples 1-4, dose titration experiments, evaluating
a
variety of xRT doses, to mice with 1 or 2 tumors have been performed. The
first goal
has been to test the dose of xRT needed in mice with one tumor to facilitate
synergy
and an "in situ vaccine" with IT-IC, tumor-reactive mAb linked to IL2. Initial
experiments have confirmed our prior observation that 12 Gy RT alone does not
eradicate or even regress the growth of established B78 melanoma tumors (0%
complete regression), whereas 12 Gy + IT-IC results in complete regression of
most
63
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
B78 tumors (66%) in mice bearing a single tumor. On the other hand, 2 Gy + IT-
IC
slows tumor progression compared to IT-IC alone (mean tumor size day 32 = 472
mm3 vs 1214 mm3, respectively) but did not render any mice disease free (0%
complete regression).
[00224] In our "2-tumor model", we have previously shown that treatment of one
"primary" tumor with xRT + IT-IC is not effective in treating either the
treated
primary tumor or the untreated "secondary" tumor. In fact, in this 2-tumor
model we
have observed that the presence of the second tumor eliminates the efficacy of
IT-IC
injection following xRT. We have designated this phenomenon as "concomitant
immune tolerance" (CIT), and demonstrated that this results, at least in part,
from T
regulatory cells (Tregs) in the distant (non-irradiated) secondary tumor,
which
circulate systemically and repopulate the xRT-treated /IT-IC injected primary
tumor.
These Tregs that return to the primary tumor appear to interfere with the
desired "in
situ vaccine" effect.
[00225] We have now confirmed our prior observation that CIT can be overcome
by delivering 12 Gy xRT to both the primary and the secondary tumor.
Importantly,
given that Tregs are quite sensitive to RT, we hypothesized that a lower dose
of RT
could be delivered to the secondary tumor in order to overcome CIT and rescue
response to in situ vaccination at the primary tumor (primary tumor treated
with 12
Gy + IT-IC). We have now tested this and observed that xRT doses of 2 Gy or 5
Gy
to the secondary tumor are comparable to 12 Gy in their capacity to blunt CIT
and
rescue response to primary tumor treatment with 12 Gy + IT-IC. These important
experiments have been repeated in duplicate, and suggest (as hypothesized)
that the
dose of xRT that must be given to distant tumors to prevent CIT is much less
than the
dose needed at the IT-IC injected primary tumor site for the purpose of
generating an
in situ vaccine effect.
[00226] This supports our overarching hypothesis in this disclosure, and
suggests
that in animals bearing multiple tumors we will be able to deliver a
relatively low
dose of RT to all sites of disease using the targeted radiotherapeutic (TRT)
NM600,
and thereby overcome CIT when this is combined with local xRT and IT-IC
injection
of a single tumor site (the in situ vaccine site).
64
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
Example 8: Experiments determining the 1311-NM404 dosing that approximates
the required dosing of xRT to metastases, as determined above, and then
evaluating the effects of that 1311-NM404 dose on in vivo immune function
[00227] Based on the preliminary data described above in Examples 1-4, studies
have been done to move these concepts into in vivo testing using TRT.
Dosimetry
studies have been performed on mice bearing 1 or 2 B78 tumors (the tumor model
that we have used to demonstrate best our in situ vaccine approach and the
hurdle of
CIT). This was done in order to estimate the amount of 131I-NM404 that would
be
needed to approximate ¨ 2 Gy of xRT.
[00228] In order to then determine if a ¨2 Gy equivalent dose of 131I-NM404
would have the desired effects against intratumor lymphoid cells (particularly
Tregs),
2 separate approaches have been pursued. First, we administered this dose of
1311-
NM404 to mice bearing a radiosensitive lymphoma tumor, which exhibits
comparable
NM404 uptake to B78 tumors. Following this we have documented potent lymphoid
tumor shrinkage/dose-dependent inhibition under conditions that did not cause
either
substantial shrinkage/slowing of the B78 tumor or any evident depletion of
circulating
lymphoid cells (as gauged by peripheral complete blood counts). These data are
consistent with the fact that lymphoid cells are much more sensitive to low-
dose RT
than are typical solid tumor cells, and suggest that selective uptake of TRT
in tumor
may enable intratumor lymphoid cell depletion without systemic lymphopenia.
These
studies also suggest that such a lymphoid tumor could serve as an in vivo
biological
"dosimeter" for identifying and monitoring the effect of TRT on intratumor
lymphoid
cells.
[00229] A second approach involved treating mice with B78 tumors with these
same doses of 131I-NM404. These animals were then sacrificed at half-life (8d)
intervals, and after sufficient delay for radioactive decay, the tumors were
stained for
the presence of effector T cells and Tregs by immunohistochemistry
Intriguingly, the
animals receiving 131I-NM404 in this initial experiment showed no systemic
lymphopenia at any time point (by peripheral complete blood count) but did
show a
decrease in intratumor FoxP3+ Tregs at 2 half-lifes following TRT
administration. At
this 2-half-life time point, we also observed a decrease in intratumor
effector CD8+ T
cells. Importantly, however at subsequent 3 and 4 half-life time points we
observed an
increase in intratumor CD8+ effector T cells but a further decline in the
levels of
intratumor Tregs, both compared to untreated baseline and211d half-life
levels. This
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
observation again supports our hypothesis that it will be feasible to use TRT
to
overcome Treg-mediated CIT in order to rescue an in situ vaccine effect in
animals
bearing multiple tumors.
[00230] Finally, to characterizing the immunological effects of TRT on the
immune cells within tumors, we have treated B78 bearing mice with 131I-NM404
and
collected tumor tissue at pretreatment and at half-life (8d) intervals
thereafter. These
tissues were then analyzed by RT-PCR for gene expression of a panel of immune
signatures. The results indicate that TRT treatment alone causes striking
changes in
expression of tumor cell markers of immunsusceptibility and in genes normally
expressed only by immune cells, with the latter showing a clear time course of
decreased expression followed by rebound over-expression.
Example 9: Experiments using data from Examples 5 and 6 to develop a regimen
of "11-NM404 + local xRT + IT-mAb/IL2 in mice bearing two or more tumors
and induce T-cell mediated eradication of all distant tumors
[00231] This Example illustrates treating animals bearing tumors in at least 2
locations. Our strategy involves using xRT and local IT-IC at the in situ
vaccine site,
in combination with TRT systemically to inhibit CIT, in order to obtain
enhanced
anti-tumor immune activity at all tumor sites. Critical issues of TRT and xRT
dose
and timing will be optimized for antitumor efficacy.
[00232] Using the data summarized in Examples 7 and 8, a study was done in
mice
bearing 2 separate B78 tumors. Mice received the estimated required systemic
1311-
NM404 dose followed by xRT and local immunotherapy to the in situ vaccine
site.
With appropriate controls, this dose of 131I-NM404 did appear to attenuate
CIT, as
desired in mice with 2 tumors. In addition, in mice with one tumor, this TRT
dose did
not appear to interfere with the local in situ vaccine effect (as hypothesized
and
desired). Further testing, and modification of some of the experimental
variables, is
underway in order to try to maximize the desired effect of blocking CIT
without
suppressing the in situ vaccine effect. More details regarding these
experiments are
disclosed in Example 10 below.
66
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
Example 10: Data from mice bearing two or more tumors
[00233] Tumor-specific inhibition of primary tumor response to the
combination of local xRT+IT-IC by a distant untreated tumor in murine
melanoma and pancreatic tumor models.
[00234] C57BL/6 mice bearing a syngeneic, GD2+ primary flank tumor +/- a
secondary tumor on the contralateral flank were treated to the primary tumor
only, as
indicated, with xRT on day "1" and IT injection of 50 mcg of the anti-GD2 IC,
hu14.18-
IL2 on day 6-10.
[00235] In mice bearing a primary B78 melanoma tumor, the presence of an
untreated secondary B78 tumor antagonized primary tumor response to xRT+IT-IC
(Figure 8A). We describe this effect as "concomitant immune tolerance" ¨ an
antagonistic effect of a non-treated distant tumor on the local response of a
treated
tumor to xRT + IT-IC. Kaplan-Meier survival curves were obtained for these
mice plus
replicate experiments (Figure 8B). Nearly all mice were euthanized due to
primary
tumor progression.
[00236] In mice bearing a primary Panc02-GD2+ pancreatic tumor, with or
without
a secondary Panc02-GD2¨ tumor on the opposite flank, the presence of an
untreated
Panc02 secondary tumor suppressed the response of a primary Panc02-GD2+ tumor
to
xRT+IT-IC (Figure 8C). In mice bearing a primary B78 melanoma tumor, a
secondary
B78 tumor suppressed primary tumor response to xRT+IT-IC but a secondary
Panc02-
GD2+ pancreatic tumor did not exert this effect (Figure 8D). In mice bearing a
primary
Panc02-GD2+ tumor a secondary Panc02-GD2¨ tumor suppressed primary tumor
response to combined xRT and IT-hu14.18-IL2, while a B78 secondary tumor did
not
(Figure 8E).
[00237] Concomitant immune tolerance is circumvented by specific depletion
of regulator T cells (Tregs).
[00238] Immunohistochemistry images were obtained for the Treg marker, FoxP3
for tumors evaluated on day 6 after xRT in mice with one or two tumors (Figure
9A).
Mice received no xRT, or xRT only to the primary tumor. DEREG mice express
diphtheria toxin receptor under control of the Treg-specific FoxP3 promoter,
enabling
specific depletion of Tregs upon IP injection of diphtheria toxin (Figures 9B
and 9C).
DEREG mice bearing primary and secondary B78 melanoma tumors were treated with
xRT+IT-IC to the primary tumor and IP injection of either diphtheria toxin or
PBS.
Concomitant immune tolerance is eliminated following depletion of Tregs in
these
67
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
mice, resulting in improved primary (Figure 9B) and secondary (Figure 9C)
tumor
response.
[00239] Concomitant immune tolerance is overcome by delivering xRT to both
tumor sites.
[00240] In mice bearing primary and secondary B78 tumors, the secondary tumor
suppresses primary tumor response to primary tumor treatment with xRT + IT-IC.
This is overcome by delivering 12 Gy xRT to both the primary and secondary
tumors
and IT-IC to the primary tumor, resulting in improved primary tumor response
(Figure 10A) and aggregate animal survival (Figure 10B) from replicate
experiments.
[00241] Low dose xRT alone does not elicit in situ vaccination but does
overcome concomitant immune tolerance when delivered to distant tumor sites
together with 12 Gy + IT-IC treatment of an in situ vaccine site.
[00242] In mice bearing a primary B78 tumor only, 12 Gy + IT-IC elicits in
situ
vaccination (as shown previously) and results in complete tumor regression in
most
mice (Figure 11A) and a memory immune response (Morris, Cancer Res, 2016). On
the other hand no animals exhibit complete tumor regression following either
IT-IC
alone or low dose (2 Gy) xRT + IT-IC (0/6 in both groups) p<0.05.
[00243] In mice bearing a primary and secondary B78 melanoma tumor, low dose
xRT (2 Gy or 5 Gy) delivered to the secondary tumor is comparable to 12 Gy in
its
capacity to overcome concomitant immune tolerance at the primary tumor (Figure
11B). In these same animals, it is apparent that overcoming concomitant immune
tolerance by delivery of low dose xRT to the secondary tumor rescues a
systemic
response to IT-IC immunotherapy (Figure 11C). In this context, when RT is
delivered
to all tumor sites then IT-IC injection of the primary tumor triggers a
systemic anti-
tumor effect that renders secondary tumor response to 2 Gy or 5 Gy greater
than the
response to 12 Gy RT in absence of primary tumor IT-IC injection.
[00244] Low dose TRT with "11-NM404 effectively depletes tumor infiltrating
FoxP3+ Tregs without systemic leukopenia or depletion of tumor infiltrating
CD8+ effector T cells.
[00245] In most clinical scenarios, it is not feasible to deliver external
beam, even
low dose, to all tumor sites without eliciting marked bone marrow depletion
and
leukopenia that would result in immunosuppression. Here we tested whether TRT
could be administered systemically to specifically deplete tumor infiltrating
suppressive immune cells (Tregs), without triggering systemic immune cell
depletion
68
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
and leukopenia. Dosimetry studies in this B78 melanoma tumor model were
performed using positron-emitting '241-NM404 confirm tumor-selective uptake of
NM404 (Figure 12A). C57BL/6 mice bearing B78 tumors were treated with 60
1.1.Ci
131I-NM404. This activity approximates the amount of 131I-NM404 necessary to
deliver ¨ 2 Gy TRT to a B78 tumor. Peripheral blood and tumor samples were
collected in untreated control mice (C) and at 8 day intervals (Ti = d8, T2 =
dl 6, T3
= d24, T4 = d32) thereafter. This dose of TRT did not result in any
significant
systemic leukopenia (Figure 12B) and did not significantly affect the level of
tumor
infiltrating CD8+ effector T cells (Figure 12C). However, tumor infiltrating
FoxP3+
Tregs were significantly depleted by this dose of TRT (Figure 12D).
[00246] Low dose TRT with 1311-NM404 effectively overcomes concomitant
immune tolerance and rescues the systemic anti-tumor effect of in situ
vaccination.
[00247] Given the capacity of low dose 131I-NM404 TRT to deplete tumor-
infiltrating Tregs without rendering a mouse leukopenic, we tested whether low
dose
131I-NM404 might effectively overcome concomitant immune tolerance. C57BL/6
mice bearing two B78 tumors were treated with 60- Ci 131I-NM404 on day 1
(NM404), as indicated. After one half-life (day 8), animals received 12 Gy xRT
or no
xRT to the primary tumor (in situ vaccine site). Control mice receiving no
1311-
NM404 were treated to the secondary tumor as indicated (0, 2, or 12 Gy). Mice
received daily IT injections of IC to the primary tumor (in situ vaccine
site), as
indicated, on days 13-17. Primary tumor (Figure 13A) and secondary tumor
(Figure
13B) response demonstrates that administration of low dose TRT effectively
overcomes concomitant immune tolerance and rescues the systemic anti-tumor
effect
of in situ vaccination.
69
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
References Cited in the Examples 1-4 and 7-10:
[1] Hank JA, Robinson RR, Surfus J, Mueller BM, Reisfeld RA, Cheung N--K and
Sondel PM. Augmentation of antibody dependent cell mediated cytotoxicity
following in vivo therapy with recombinant Interleukin-2. Cancer Res. 50:5234-
9.
1990.
[2] Neal ZC, Yang JC, Rakhmilevich AL, Buhtoiarov I, Lum HE, Imboden M, Hank
JA, Lode HN, Reisfeld RA, Gillies SD, Sondel PM. Enhanced activity of hu14.18-
1L2
IC against the murine NXS2 neuroblastoma when combined with IL2 therapy. Clin
Cancer Res. 2004 Jul 15;10(14):4839-47.
[3] Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman S, Chen H, Smith M,
Anderson B, Villablanca J, Matthay KK, Shimada H, Grupp SA, Seeger R, Reynolds
CP, Buxton A, Reisfeld RA, Gillies SD, Cohn SL, Mans JM, Sondel PM. Anti-GD2
antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N
Engl J.
Med. 2010 Sep 30;363(14):1324-34.
[4] Johnson EE, Yamane BH, Lum HD, Buhtoiarov IN, Rakhmilevich AL, Mahvi
DM, Gillies SD, Sondel, PM. Radiofrequency Ablation Combined with KS-IL2 IC
(EMD 273066) Results in an Enhanced Anti-tumor Effect Against Murine Colon
Adenocarcinoma. Clin Cancer Res. 2009 Aug 1;15(15):4875-84.
[5] Yang RK, Kalogriopoulos NA, Rakhmilevich AL, Ranheim EA, Seo S, Kim KM,
Alderson KL, Gan J, Reisfeld RA, Gillies SD, Hank JA, Sondel PM. Intratumoral
hu14.18-1L2 (IC) Induces Local and Systemic Antitumor Effects that Involve
Both
Activated T- and NK cells as well as Enhanced IC Retention. J Immunol. 2012
Sep
1;189(5):2656-64.
[6] Morris ZS, Emily I. Guy El, Francis DM, Gressett MM, Carmichael LL, Yang
RK, Armstrong EA, Huang S, Navid F, Gillies SD, Korman A, Hank JA,
Rakhmilevich AL, Harari PM, Sondel PM. Combining Local Radiation and tumor-
specific antibody or IC to elicit in situ tumor vaccination. Cancer Research,
e-pub
ahead of print, 2016.
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[7] Morris ZS, G.E., Francis DM, Gressett MM, Armstrong EA, Huan S, Gillies
SD,
Korman AJ, Hank JA, Rakhmilevich AL, Harari PM, and Sondel PM., IC augments
local and abscopal response to radiation and CTLA-4 checkpoint inhibition in a
murine melanoma model. Am. Soc.Therapeutic Radiation Oncology. Abstract
accepted Oct. 2015 (and selected as the meeting's winning abstract in the
basic---
translational science category).
[8] Weichert JP, Clark PA, Kandela IK, Vaccaro AM, Clarke W, Longino MA,
Pinchuk
AN, Farhoud M, Swanson KI, Floberg JM, Grudzinski J, Titz B, Traynor AM, Chen
HE, Hall LT, Pazoles CJ, Pickhardt PJ, Kuo JS. Alkylphosphocholine Analogs for
Broad Spectrum Cancer Imaging and Therapy. Science Translational Medicine 6,
240ra75, 1-10. 2014.
[9] Morris ZS, JP Weichert, J Sakera, EA Armstrong, A Besemer, B Bednarz, R
Kimple, PM Harari. Therapeutic combination of radiolabeled NM404 with external
beam radiation in head and neck cancer model systems. Radiotherapy and
Oncology. J.
Radiation Oncology, DOT: 10.1016. 2015.
[10] Lode HN, Xiang R, Dreier T, Varki NM, Gillies SD, Reisfeld RA. Natural
killer
cell¨mediated eradication of neuroblastoma metastases to bone marrow by
targeted
interleukin-2 therapy. Blood 91(5), 1706-1715. 1998.
[11] Snyder F, Wood R. Alkyl and alk-1 -enyl ethers of glycerol in lipids from
normal
and neoplastic human tissues. Cancer Res 29, 251-257. 1969.
[12] Pinchuk AN, Rampy MA, Longino MA, Skinner RW, Gross MD, Weichert JP,
Counsell RE, Synthesis and structure-activity relationship effects on the
tumor avidity
of radioiodinated phospholipid ether analogues. J Med Chem 49, 2155- 2165.
2006.
[13] Swanson KI, Clark PA, Pinchuk AN, Longino MA, Farhoud M, Weichert JP, Kuo
JS. Initial Studies on Novel Cancer-Selective Alkylphosphocholine Analogs
CLR1501
71
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
and CLR1502 for Fluorescence-guided Neurosurgery. Neurosurgery. 76(2): 115-
123.
2015.
[14] Filatenkov A, Baker J, Mueller AM, Kenkel J, Ahn GO, Dutt S, Zhang N,
Kohrt
H, Jensen K, Dejbakhsh-Jones S, Shizuru JA, Negrin RN, Engleman EG, Strober S.
Ablative Tumor Radiation Can Change the Tumor Immune Cell Microenvironment to
Induce Durable Complete Remissions. Clin Cancer Res. 21:3727-39. 2015.
[15] Jing W, Gershan JA, Weber J, Tlomak D, McOlash L, Sabatos-Peyton C,
Johnson
BD. Combined immune checkpoint protein blockade and low dose whole body
irradiation as immunotherapy for myeloma. J Immunother Cancer. 3:2.
2015.
[16] Bednarz B., Besemer A., Yang Y. A Monte Carlo-Based Small Animal
Dosimetry
Platform for Pre-Clinical Trials: Proof of Concept. Med. Phys. 39, 3899. 2012.
[17] Besemer et al. Towards Personalized Dosimetry Using Diapeutic
Radiopharmaceuticals. Med. Phys. 40, 382. 2013.
[18] Besemer A. and Bednarz B. Validation of a patient-specific Monte Carlo
targeted
radionuclide therapy dosimetry platform. Med. Phys. 41, 303. 2014.
[19] Imboden M, Murphy KR, Rakhmilevich AL, Neal ZC, Xiang R, Reisfeld RA,
Gillies SD and Sondel PM. The level of MHC Class I expression on murine
adenocarcinoma can change the antitumor effector mechanism of immunocytokine
therapy. Cancer Res. 61:1500-7. 2001.
72
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
Example 11:
In vivo uptake of multiple NM600 metal chelates in mice xenografted with eight
different solid tumor types, demonstrated by PET imaging
[00248] In this example, we demonstrate the differential uptake of NM600
chelated
with four different metals in a range of solid tumors in vivo, as demonstrated
by
PET/CT imaging of such tumors. These data provide additional support for the
use of
metal chelated alkylphosphocholine analogs as TRT agents for eliminating tumor-
induced immune tolerance, as disclosed herein. The structure of NM600 is shown
in
Figure 14, as an example species chelated with 64Cu (64Cu-NM600); however, any
metal can be readily chelated to NM600.
[00249] Specifically, mice were each xenografted with one of eight
different solid
tumor cell lines (B78 (melanoma), U87MG (glioblastoma), 4T1 (breast
carcinoma),
HCT-116 (colorectal carcinoma), A549 (lung carcinoma), PC-3 (prostate
carcinoma),
HT-29 (colorectal adenocarcinoma), or MiaPaca (pancreatic carcinoma). For each
of
the xenografted mice, cell suspension containing tumor cells was inoculated
into
subcutaneous tissue of one or both flanks of the mouse. Once xenograft tumors
reached a minimum size, each mouse was injected with between 150-300 nCi of
64 89 86 52
NM600 radiolabeled with Cu, Zr, Y, or Mn via lateral tail vein injection.
After
an uptake period, PET imaging was performed in an Inveon micro PET/CT. Right
before each scan, mice were anesthetized with isoflurane (2%) and placed in a
prone
position in the scanner. Longitudinal 40-80 million coincidence event static
PET
scans were acquired at 3, 12, 24, and 48 hours post-injection of the
radiotracer and the
images were reconstructed using an OSEM3D/MAP reconstruction algorithm.
[00250] Figure 15 shows the resulting images 48 hours post-injection-for
single-
tumor B78 mice injected with 86Y-NM600; Figure 16 shows the resulting images
48
hours post-injection-for two-tumor B78 mice injected with 86Y-NM600; Figure 17
shows the resulting images 3, 24 and 48 hours post-injection for a U87MG mouse
injected with 64Cu-NM600; Figure 18 shows the resulting images 3, 24 and 48
hours
post-injection for a 4T1 mouse injected with 64Cu-NM600; Figure 19 shows the
resulting images 3, 24 and 48 hours post-injection for an HCT-116 mouse
injected
with 64Cu-NM600; Figure 20 shows the resulting images 3, 24 and 48 hours post-
injection for an A549 mouse injected with 64Cu-NM600; Figure 21 shows the
resulting images 3, 24 and 48 hours post-injection for a PC-3 mouse injected
with
73
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
64Cu-NM600; Figure 22 shows the resulting images 3, 24 and 48 hours post-
injection
for a HT-29 mouse injected with 64Cu-NM600; Figure 23 shows the resulting
images
3, 24 and 48 hours post-injection for a MiaPaca mouse injected with 64Cu-
NM600;
Figure 24 shows the resulting images 3, 24 and 48 hours post-injection for a
4T1
mouse injected with 86Y-NM600; Figure 25 shows the resulting images 3, 24 and
48
hours post-injection for a 4T1 mouse injected with 89Zr-NM600.
[00251] For HT-29 and PC3 mice injected with 52Mn-NM600, PET images were
obtained at 4 hours, and one day post-injection (Figure 26 for HT-29; Figure
27 for
PC3), as well as on days 2, 3, 5 and 7 post-injection (Figure 28 for HT-29;
Figure 29
for PC-3).
[00252] As seen in Figures 15-29, the scanned mice produced PET/CT three-
dimensional volume renderings showing cumulative absorbed dose distribution
concentrated in the xenografted tumor. The results confirm the differential
uptake of
metal chelated NM600 into the xenografted solid tumor tissue, and demonstrate
the
potential use of NM600 analogs incorporating radioactive metal isotopes in the
disclosed treatment methods.
[00253] Quantitative region-of-interest analysis of the images was performed
by
manually contouring the tumor and other organs of interest. Quantitative data
was
expressed as percent injected doe per gram of tissue (%ID/g). Exemplary data
show
that 4T1 tumor tissue increased its uptake over time and effectively retained
all three
tested NM600 chelates (86Y-NM600, 64Cu-NM600 and 89Zr-NM600, see Figure 30),
while healthy heart (Figure 31), liver (Figure 32) and whole body tissue
(Figure 33)
all exhibited significantly decreased uptake/retention over time.
[00254] Ex vivo biodistribution analysis was performed after the last
longitudinal
PET scan. Mice were euthanized and tissues harvested, wet-weighed, and counted
in
an automatic gamma counter (Wizard 2480, Perkin Elmer). Exemplary
biodistribution data show significant uptake and retention in tumor tissue
(4T1) for
different NM-600 chelates (86Y-NM600, 64Cu-NM600, 89Zr-NM600 and 177Lu-
NM600, see Figure 34),
[00255] Together, these results demonstrate that the disclosed metal chelates
can
readily be used for the TRT step of the disclosed treatment methods.
74
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
Example 12:
Demonstrating anti-tumor activity and tumor autoradiography with two
different NM600 metal chelates against multiple solid tumor types in
xenografted
mice
[00256] In this example, using three different solid tumor models, we show
that
alkylphosphocholine metal chelate analogs can be effectively used to
facilitate
conventional TRT. These results further demonstrate the potential for using
the metal
chelates in the TRT step of the presently disclosed treatment methods.
[00257] B78, MiaPaca and 4T1 subcutaneous flank xenografts were induced in
mice,
as described previously. Subsequently, the mice were administered therapeutic
doses
(250-500 CO of 90Y-NM600, 177Lu-NM600, or a control solution via lateral tail
vein
injection.
[00258] Planar 2D phosphor images of the biodistribution of the agent were
taken
using a Cyclone Phosphorimager (Perkin Elmer). Mice were anesthetized and
place in
direct contact with the phosphor plate in a supine position, where they
remained for a
period of 15-30 min; plates were then read in the phosphorimager. Various
images were
recorded between 4 and 96 h post-injection of the radioactive dose. The
resulting
autoradiography images demonstrate rapid and selective uptake and long term
retention
of the chelates in all of the solid tumor tissues types tested (see Figures
40, 41, 42, 43,
44 and 45).
[00259] Tumor response was assessed by comparing tumor growth of the treated
vs.
control mice. Tumor volume was determined by measuring tumor's length and
width
with calipers and calculating the volume using the formula for the volume of
the
ellipsoid. Mice weight was also recorded. Humane endpoints were defined as:
tumor
volume >2500 m3 or significant weight lost below 13 g.
[00260] As seen in Figures 46, 47, 48, 49, 50 and 51, the results demonstrate
that
the two tested NM600 chelates had a statistically significant in vivo
therapeutic effect
when compared with the control, resulting in decreased mean tumor volumes for
double doses of 177Lu-NM600 in 4T1 xenografts (see Fig. 50), and reducing
growth to
near zero or slowing the growth rate of MiaPaca, 4T1 or B78 xenografts given a
single dose of 177Lu-NM600 (see Figures 47, 48, and 49) or B78 or 4T1
xenografts
given a single dose of 90Y-NM600 (see Figures 46 and 51).
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[00261] These results further demonstrate the efficacy of using the disclosed
alkylphosphocholine metal chelates to deliver TRT to effectively treat solid
tumors of
various types.
Example 13:
Coupling radiation dosimetry and radiosensitivity index to predict TRT
response
in a wide range of solid tumor types
[00262] In this example, we discuss factors for determining chelate dosages
appropriate for the TRT step of the disclosed methods in a range of solid
tumor types.
[00263] Estimation of tumor absorbed doses
[00264] Whether the amount of 177Lu/90Y-NM600 that is administered is immuno-
stimulatory or cytotoxic depends on the tumor absorbed dose. The diapeutic
property
of NM600, that 64Cu/86Y-NM600 can be used as an imaging surrogate for
therapeutic
metals 177Lu/90Y-NM600, respectively, was leveraged to estimate tumor
dosimetry.
Ultimately, 64Cu/86Y-NM600 PET/CT was used to quantitatively measure in vivo
biodistribution and estimate radiation dosimetry which can help identify dose
limiting
organs and potential tumor efficacy of 177Lu/90Y-NM600 TRT.
[00265] The general concept is as follows: (1) the concentration of 64Cu/86Y-
NM600 within the tumor is quantified over time using longitudinal PET/CT
imaging,
(2) the concentration of 64Cu/86Y-NM600 is decay corrected to account for the
difference in decay rates between the 64Cu/86Y-NM600 and 177Lu/90Y-NM600, (3)
the
concentration of 177Lu/90Y-NM600 within the tumor is time-integrated to
compute the
cumulative activity, or total number of decays, (4) the deposition of the
radionuclide
decays is modeled within the tumor and quantified.
[00266] Steps (1) through (3) can be performed with any medical image
processing
software package whereas step (4) requires sophisticated radiation dosimetry
software. OLINDA/EXM (Stabin, Sparks and Crowe 2005) is a dosimetry estimation
software with 510(k) approval that uses the formalism developed by the Medical
Internal Radiation Dose (MIRD) committee of the Society for Nuclear Medicine
(Bolch et al., 2009). The MIRD approach estimates the mean absorbed dose
received
by a tissue or organ due to the radiation emitted from within the organ itself
or from
another source organ. The simplest form of the MIRD equation,
D (t s) = A sS(t s),
76
CA 03082056 2020-05-06
WO 2019/094657 PCT/US2018/059927
gives the absorbed dose, D [mGy], to a target region t from the radionuclide
activity
within a source region s. The radionuclide activity of s is expressed as a
cumulated
activity As which is the total number of radionuclide decays given in units of
MBq-s.
The S-factor, s) [mGy/ MBq-s], is the fraction of the energy released by
one
radionuclide decay within the source region s which is deposited within the
target
region t normalized by the mass of the target region t, mt. The S-factor is a
tabulated
value calculated using Monte Carlo in a set of standard phantoms and organs.
Typically, we are concerned with the dose per unit of injected activity, D
[mGy/MBql. The equation is written in terms of the residence time, rh,[MBq-
s/MBqinil,
As
Th =
Ainj'
which is the ratio of the cumulative activity and the injection activity, Ain
[MBql, as
D(t s) (As
s) = _____________________________ = ¨)= S = Th. S.
Ain] Ain]
[00267] In the case of calculating tumor dosimetry, OLINDA/EXM models the
tumor as an isolated unit density sphere whose volume was estimated from the
tumor
region of interest (ROT) created as part of step (1). The concentration of
NM600
(%ID/g) within the tumor was determined at each time point and decay
corrected.
Cumulative activity was then calculated by integrating the concentrations over
all
time using trapezoidal piecewise integration.
[00268] Radiation dosimetry results for many cell lines are shown in Table 1.
This
information can be used to estimate the absorbed dose for radiotherapy studies
aimed
to either eradicate tumors or stimulate the immune system.
77
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
Table 1: Dosimetry estimates for both 177Lu-NM600 and 90Y-NM600 (Gy/MBqini)
using either 64Cu-NM600 or 86Y-NM600 PET imaging as a surrogate
PC3 A549 HT-29 MiaPaca U87MG 4T1 B78
Lu-177 0.39 0.30 0.49 0.24 0.58 1.50 0.92
Y-90 0.69 0.53 0.84 0.45 1.01 4.68 2.86
[00269] Radiosensitivity Index to Predict Dose-response
[00270] Intrinsic
radiosensitivity is a crucial factor underlying radiotherapy
response; and, knowing it a priori for a cancer type could help predict how it
may
respond to radiation from TRT. However, since there is no method for its
routine
assessment in tumors, radiosensitivity is measured as the surviving fraction
(between
0 and 1) following irradiation with 2 Gy (SF2) by clonogenic assay. The
relative
radiosensitivity of cancer cell phenotypes ranges from those that have very
low
radiosensitivities (pancreas, colorectal, glioma and breast) to those with
high
radiosensitivities (lymphomas). Cancers can be categorized or ranked by their
radiosensitivity indices (Table 2).
[00271] If we can demonstrate good tumor uptake and growth inhibition with APC
metal chelates in a highly radiosensitive tumor like lymphoma and in a highly
radiation resistant tumor like glioma, breast, pancreatic or colorectal, then
it can be
implied that these agents would be effective against any tumor with an SF2
value
between that of lymphoma and glioma (0.3-0.82) if they are able to target the
tumor in
vivo. It would also be expected then that the radiation dose needed to
eradicate glioma
tumor cells would be higher than that needed to treat the more radiosensitive
lymphoma cells.
[00272] We currently have in vivo imaging to confirm tumor selectivity and
therapy response (tumor growth inhibition) data in all the tumor cell lines
listed in
Table 2. In some cases, it may be necessary to give multiple doses of the APC
chelates to elicit sufficient cancer cell kill. By using quantitative imaging
coupled
with radiation dosimetry calculation, we can estimate the tumor absorbed dose
necessary to either kill the cancer cells (higher doses) or stimulate the
immune
system, as disclosed herein (lower doses).
[00273] Coupling dosimetry estimates for a variety of cancer cell lines (Table
1)
with their respective radiosensitivity indices (Table 2) supports the
establishment of a
dose response landscape for NM600. By knowing the tumor targeting
characteristics
78
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
and efficacy of NM600 within a series of cell lines, it is possible to
estimate the
absorbed tumor dose and potential efficacy of cell lines with similar
radiosensitivity
indices. Furthermore, treatment doses can be linearly scaled according to
Table 1,
depending on the desired outcome of tumor eradication or immuno-stimulation
(as
disclosed herein).
Table 2: Relative Radiosensitivity of Cancer Cells
Imaging uptake Refs.
and or growth
Tumor Type Cell Line SF2 value
inhibition with
APC chelates
Breast MDA-MB- 0.82 Yes 8
231
Pancreatic Mia-Paca 0.80 Yes 6, 7
Colorectal HCT-29 0.75 Yes 7
Melanoma B-78 0.65 Yes 3, 4, 7
Glioma (brain) U-87 0.63 Yes 1, 2, 7
Lung A-549 0.61 Yes 5, 7
(NSCLC)
Prostate P0-3 0.55 Yes 4
Lymphoma EL-4 0.30 Yes 3, 7
SF2=surviving fraction following exposure to 2 Gy of in vitro radiation
exposure
* Several cell lines
iTaghiari. Alphonse, et al. In vivo radiation sensitivity of glioblastoma
multiforme."
International Journal of Radiation Oncology* Biology* Physics 32.1(1995): 99-
104.
2Rainsay-, J., R. Ward, and N. M. Bieehen. "Radiosensitivity testing of human
malignant
gliomas." International Journal of Radiation Oncology* Biology* Physics 24.4
(1992): 675-
680.
3Fertil, B., and F. P. Malaise. "Intrinsic radiosensitivity of human cell
lines is correlated with
radioresponsiveness of human tumors: analysis of 101 published survival
curves."
International Journal ofRadiation Oncology* Biology* Physics 11.9 (1985): 1699-
1707.
79
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
4Wo11in, Michael, et al. "Radio sensitivity of human prostate cancer and
malignant melanoma
cell lines." Radiotherapy and Oncology 15.3 (1989): 285-293.
5Kodyni, Elisabeth, et al. The small-molecule CDK inhibitor, SNS-032, enhances
cellular
radiosensitivity in quiescent and hypoxic non-small cell lung cancer cells."
Lung Cancer 66.1
(2009): 37-47.
qinicel, Steffen, Claus &Aka, and Kirsten Lauber. "On the analysis of
clonogenic survival
data: Statistical alternatives to the linear-quadratic model." Radiation
Oncology 11.1(2016):
11.
'EP Malaise, Patrick J. Deschavanne, and Bernard Fertil. "Intrinsic
radiosensitivity of human
cells." Advances in radiation biology 15 (2016): 37-70.
8Siles, E., et al. "Relationship between p53 status and radiosensitivity in
human tumour cell
lines." British journal of cancer 73.5 (1996): 581-588.
[00274] References cited in Example 13:
[00275] Bolch, W. E., K. F. Eckerman, G. Sgouros, and S. R. Thomas. 2009.
"MIRD Pamphlet No. 21: A Generalized Schema for Radiopharmaceutical
Dosimetry¨Standardization of Nomenclature." Journal of Nuclear Medicine 50
(3):
477-84. doi:10.2967/jnumed.108.056036.
[00276] Stabin, M G, R B Sparks, and E Crowe. 2005. "OL1NDA/EXM: The
Second-Generation Personal Computer Software for Internal Dose Assessment in
Nuclear Medicine." J Nucl Med 46 (6): 1023-27.
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
Example 14:
Advantages of and differences when using alkylphosphocholine metal chelates in
place of radioiodinated compounds, such as those exemplified in Examples 1-4
and 7-10
[00277] In this example, we discuss the advantages of using APC metal chelates
instead of radioiodinated compounds (the compounds exemplified in Examples 1-4
and 7-10). We also discuss factors to be considered by the skilled artisan
when
optimizing dosages of metal chelates to be used in the TRT step of the
disclosed
methods.
[00278] Chelates permit the use of a wide variety of stable or radioactive
metal ions
for imaging and therapy. They can be conjugated with a wide variety of alpha,
beta,
Auger, gamma and positron emitters whereas iodine is limited to one positron
(1-124),
one beta (I-131), one gamma (1-123) and 1 Auger (1-125) isotope.
[00279] Metal Isotopes are diapeutically more efficacious than 1-131 and 1-
124.
[00280] Lu-177 has fewer high energy gammas which make it more favorable for
SPECT imaging and dosimetry. However, its beta energy is slightly less than 1-
131,
making it more ideal for treating smaller tumors.
[00281] 1-131 and Lu-177 are comparable in therapeutic efficacy "horse power",
but
there is significantly less contribution to the overall dose from gamma-
emissions for
Lu-177. In the case of Y-90, there is negligible contribution to the radiation
dose from
gamma-emissions.
[00282] Relative to 1-131, Y-90 is more efficacious for killing cancer
cells by
conventional TRT than 1-131, as seen in Figure 52 and discussed further below.
[00283] The Committee on Medical Internal Radiation Dose (MIRD) develops
standard methods, models, assumptions, and mathematical schema for assessing
internal radiation doses from administered radiopharmaceuticals. The MIRD
approach,
which simplifies the problem of assessing radiation dose for many different
radionuclides, has been implemented in the widely used 510(k) approved
software,
OLINDA/EXMl. Along with its many standard anthropomorphic phantoms,
OLINDA/EXM has a Spheres Model which can be used to approximate tumor doses.
The Spheres Model assumes homogeneous distribution of a radiopharmaceutical
within
unit-density spheres of a range of tumor masses (0.01 ¨ 6,000 g).
[00284] Using this standard model, we compared Y-90 to 1-131 in terms of
radiation
dose normalized by administered radioactivity. The results of this comparison,
for
81
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
tumor masses between 1 to 100 g, are displayed in Figure 52. Note that the Y-
90-to-I-
131 ratio reaches 4 for a 4 g tumor, and remains between 4.0 and 4.2 up to a
100 g
tumor, strongly suggesting that on a mCi per mCi basis that Y-90 is between
3.6 and
4.1 times as cytotoxic as 1-131 in tumors up to lOg in size, and about 4.1
times more
effective in tumors greater than 10 grams in size.
[00285] Different Pharmacokinetic Properties
[00286] Unlike iodinated analogs, APC chelates are too large to fit into known
albumin binding pockets in the plasma and therefore exhibit different in vivo
pharmacokinetic and biodistribution profiles (see Figure 53). Lower binding
energies
lead to larger fractions of free molecule in the plasma which affords more
rapid tumor
uptake. Some APC chelates are cleared via the renal system, whereas iodinated
analogs
are eliminated through the hepatobiliary system. APC chelates also accumulate
in
tumors and clear from the blood much quicker than iodinated analog. Faster
blood
clearance is directly associated with lower bone marrow and off-target
toxicity of
therapeutic radiopharmaceuticals.
[00287] These differences in PK and biodistribution profiles lead to differing
dose
limiting organ toxicity and ultimate utility. Moving from hematological
toxicity to renal
or liver for dose limiting toxicity would increase the utility of radiometal
chelates for
TRT.
[00288] Moreover, the pharmacokinetic profile of the APC chelates can easily
be
manipulated by minor changes in the structure of the chelate (e.g. chelate
charge). The
choice of chelators is vast. Faster clearance from normal tissues improves
imaging
contrast and therapeutic windows, resulting in higher maximum tolerable doses.
[00289] APC chelates possess different physico-chemical characteristics than
iodinated analogs. They are much more water-soluble, and therefore do not need
surfactants to render them suitable for intravenous injection. APC chelates
are based on
ionic binding of the metal to the chelate, whereas iodinated compounds form
covalent
bonds with their carrier molecules. In vivo de-iodination is quite common in
alkyl
iodides whereas chelates tend to be extremely stable in vivo.
[00290] Once de-iodination occurs, free iodide rapidly accumulates in the
thyroid
with a very long subsequent excretion half-life, whereas free radiometals are
in general
excreted from the body or detoxified much more quickly.
[00291] In vivo biodistribution of APC chelates can be quite different
depending on
the metal ion so the metal and chelate also both contribute to the tumor
targeting
82
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
characteristics of the APC. Not all chelates target tumors. Tumor targeting
depends on
the cumulative properties of the APC carrier, the type of chelate (linear
chelates
undergo rapid renal elimination whereas macrocyclic chelates undergo
hepatobiliary
excretion), and the metal ion. Even slight changes in chelate structure result
in
significant variations on the in vivo properties. Simple changes in isotope
can result in
changes in tumor targeting larger than 50%.
[00292] Radioactive APC-metal chelates are easily radiolabeled in nearly
quantitative (>98%) yields under facile conditions, whereas radioiodination
yields of
iodinated analogs are much lower (typically about 50% for 1-131 and 60% for 1-
124).
Moreover, high specific activities can be achieved with chelates. Synthesis
can be done
using a radiolabeling kit in any nuclear pharmacy without the requirement of
sophisticated ventilation equipment or training. Radioiodination must be done
in a fume
hood fitted with effluent monitoring equipment due to the volatility of
radioactive
iodine during the labeling reaction.
[00293] Imaging agents don't necessarily make good therapy agents and vice
versa.
[00294] It cannot be assumed that because there is good tumor uptake with an
imaging agent that it implies that therapy is obvious. In addition to having
good tumor
uptake, a therapy agent needs to have prolonged tumor retention relative to
normal
tissues and must be cleared from the blood quickly in order to lower bone
marrow
exposure and associated toxicity. Iodinated analogs have prolonged blood
residence
resulting in dose limiting bone marrow toxicity. In contrast, our APC chelates
exhibit
much faster blood clearance kinetics most likely, as stated above, due to
lower albumin
binding in the plasma.
[00295] Finally, due to the short path length and physical nature of metallic
beta-
and alpha-emitters relative to Iodine-131, there are no exposure concerns for
health care
workers or family members following injection. Patients undergoing 1-131
therapy
often have to be held for some time (up to a week) in a lead shielded room
prior to being
released from the hospital. Patients injected with radioactive alpha and beta-
emitting
APC chelates will not be required to remain hospitalized
83
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
Example 15:
TRT delivered by Y90-NM600 in combination with administering an anti-CLA4
immune checkpoint inhibitor synergistically inhibits cancer in an in vivo
melanoma model
[00296] In this example, we demonstrate the efficacy of the disclosed
combination
method, where the in vivo immunization is performed by systemically
administering
an immune checkpoint inhibitor (an anti-CTLA4 antibody), and the TRT is
performed
by systemically administering the 90Y-NM600 chelate used in previous examples.
[00297] B78 melanoma subcutaneous flank xenografts were implanted in male
C57BL/6 mice, as described previously. Subsequently, the mice were randomized
to
be to be treated with varying doses (25 uCi, 50 uCi, or 100 uCi) of9 Y-NM600
(Day
1), both with and without anti-CTLA4 antibody (an immune-checkpoint inhibitor)
(200 ug on Days 4, 7, and 11) (n=6 for each experimental group). Both agents
were
administered by via lateral tail vein injection (i.e., intravenously). Control
groups of
PBS treatment alone and anti-CTLA4 alone were also included. Tumors were
measured with calipers twice a week, and animal survival was monitored for 60
days.
[00298] As shown in Figure 54, the three combination therapies (anti-CTLA4 +
90Y-NM600 at three different dosages) showed substantial tumor growth
inhibition, as
compared to any of the single therapies (anti-CTLA4 or 90Y-NM600 alone at
three
different dosages) or the PBS control. After Day 18, combination treatment
with 50
or 100 uCi of 90Y-NM600 with anti-CTLA4 had significantly (p <0.05 by ANOVA)
reduced tumor growth compared to PBS, 90Y-NM600 alone, or anti-CTLA4 alone.
The 25 uCi 90Y-NM600 combination treatment group with anti-CTLA-4 had an
intermediate growth delay response that showed a trend towards dose response.
[00299] As seen in Figure 55, mice treated with 50 uCi of9 Y-NM600 combined
with anti-CTLA4 exhibited significantly greater aggregate survival than mice
treated
with TRT alone or PBS vehicle (p < 0.05). The log rank was p = 0.06 for the
combination treatmnent, as compared to anti-CTLA4 alone.
[00300] As seen in Figure 56, all three combination treatments significantly
improved survival. Significantly, there were 6/12 (50%) complete responders in
the
combination TRT + CTLA4 arms at therapeutic 50 and 100 Ci doses of9 Y-NM600,
as compared to 0/24 complete responders in the non-combination control arms
(PBS,
TRT alone at 50 iCi, TRT alone at 100 Ci, and anti-CTLA4 alone).
84
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[00301] These results illustrate the therapeutic potential of combining the
use of a
molecular targeted radiotherapeutic agent with any agent that causes immune
checkpoint inhibition (ICI). The results show that a combination of
molecularly
targeted TRT and an ICI affords a synergistic effect, relative to treatment
with each
agent alone. In addition to demonstrating significant tumor regression, the
combined
method also has the potential to generate immunologic memory and ultimately
afford
a potent in situ cancer vaccine effect that prevents tumor recurrence.
Example 16:
Utilization of Molecular Targeted Radiotherapy to Enhance the Efficacy of
Systemic Checkpoint Inhibition in Metastatic Cancer Models
[00302] In this follow-up to Example 15, we provide greatly expanded
supporting
data demonstrating the efficacy of the disclosed method combining systemically
administering an immune checkpoint inhibitor and TRT performed by systemically
administering the 90Y-NM600 chelate used in previous examples. Efficacy is
demonstrated in mouse melanoma, neuroblastoma and breast cancer models, as
well
as in multiple tumor melanoma models having disseminated "cold" tumors.
[00303] Clinical studies demonstrate that a subset of patients treated with
immune
checkpoint inhibitors (ICIs) experience durable and complete response (CR) at
all
disease sites. However, ICIs are not typically effective in patients with
immunologically "cold" tumors characterized by low levels of T cell infiltrate
and/or
few mutation-created neo-antigens. In this example, we demonstrate using the
disclosed combination method to stimulate an immune response in such tumors
and to
enhance response in "hot" tumors. More specifically, we enhanced the efficacy
of
systemic ICI by combining it with systemic molecular targeted radiotherapy
(MTRT),
which can deliver immunostimulatory low dose radiation to all sites of disease
without causing resultant systemic lymphodepletion that would be
counterproductive
in generating an anti-tumor immunotherapy response.
[00304] Methods:
[00305] For tumor uptake studies of MTRT, flank tumors (n = 3 for each of B78
melanoma and Panc02) were established by injection of 1-2x106 cells in 100 pL
PBS
into C57BL/6 mice on an approved IACUC protocol. Both B78 and Panc02 tumors
are poor to moderately immunogenic, slow growing, radio-resistant tumor lines
and
this profile makes them useful for studies of MTRT where slow growth permits
time
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
for MTRT decay and radio-resistance plus poor immunogenicity enables testing
for
cooperative improvements in efficacy with combined MTRT + ICI.
[00306] After tumors were well established, approximately 5 weeks after
injection,
animals were treated with a dose of IV 86Y-NM600 and serial PET/CT images were
collected at 1,2, and 3 days after MTRT injection. PET uptake values were
compared
to areas of background activity including the heart and liver. A paired t-test
was
performed to test for significant differences in 86Y-NM600 uptake between
background organs and tumor sites.
[00307] To demonstrate the ability of9 Y-NM600 and/or ICI to decrease
immunosuppressive Treg cell populations within B78 Melanoma flank tumors, we
generated flank tumor models (n = 4 for each group) of B78 melanoma. MTRT (50
pCi), anti-CTLA4 (200 pg Days 4,7,10), MTRT and CTLA4, and PBS placebo
control were our treatment groups. The effect of treatment on tumor immune
cell
populations was examined at day 1, 7, and 14 after radiation or saline placebo
delivery by harvesting tumor tissue and freezing one portion for histology and
saving
another portion for quantitative PCR. The rest of the tumor sample was prepped
for
mRNA and RT-PCR analysis. Quantitative RT-PCR was used to evaluate changes in
tumor cell expression of immune susceptibility markers (e.g. Fas, MHC-I, and
PD-
L1).
[00308] For efficacy studies, 2 bilateral flank tumors models of B78 melanoma
were generated in C57BL/6 mice. Once tumors grew to 80-120 mm3, they were
randomized into the following treatment groups: anti-CTLA-4 alone at 200 pg IP
on
days 4, 7, 10, 90Y-NM600 IV (50 pCi) on day 1 and anti-CTLA4, 12 Gy whole body
radiation (EBRT) and anti-CTLA4, 12 Gy EBRT + 50 pCi 90Y-NM600, 12 Gy
EBRT + 50 pCi 90Y-NM600 and anti-CTLA4. Tumor measurements were made
twice a week for 30 days, and survival was tracked to 60 days with a
euthanasia
endpoint of 15 mm diameter for tumor burden.
[00309] Mice with complete response to therapy were re-challenged with 2x106
B78 or 1x106 Panc02 cells to the opposite flank 90 days after MTRT and then
again at
120 days with Panc02 (only for B78) and B16 melanoma to test for tumor
specific
immune memory response.
[00310] Results:
[00311] The selective uptake of our MTRT agent, 90Y-NM600, was confirmed in
both B78 and Panc02 tumor models. For B78 melanoma, tumor uptake of 90Y-
86
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
NM600 demonstrated that after initial injection the majority of the agent was
in the
blood pool as expected, however by 48 hours after injection the majority of
the agent
was retained in the tumor or organs of elimination (liver, kidney). Gamma
counts of
tissue sections taken at Day 48 confirms PET imaging uptake values with higher
radioactivity counts in tumor tissue that increase over time with lower values
in
marrow spaces that decrease over time. Monte Carlo dosimetry performed as a
collaborative effort shows that approximately 2-3 Gy are delivered over the
life of the
MTRT agent when our experimental dose of 50 p.Ci is delivered. PET uptake and
tissue biodistribution studies in Panc02 pancreatic cancer also demonstrated
increased
uptake and retention of 90Y-NM600 in tumor tissue compared to marrow tissue at
72
hours.
[00312] To study the effect of treatment on tumor immune cell populations,
tumor
tissue samples were collected at various time points after radiation. At Day
14 after
MTRT treatment (50 pCi 90Y-NM600), we found that combination MTRT and anti-
CTLA4 significantly increases effector/suppressive immune t-cell ratios as
determined by the CD4/FoxP3 and CD8/FoxP3 infiltrates in tumor tissue.
Quantitative PCR (qPCR) studies of gene expression also showed increased
inflammatory gene expression including genes that are part of the stimulator
of
interferon gene pathway (STING). Levels of Mxl, IFNa, IFNO, and PDL1, which
are
all downstream of STING activation, were upregulated compared to PBS control.
[00313] We next established a single B78 R flank tumor in mice and once they
reached approximately 80 mm3, randomized them into 25, 50, and 100 uCi MTRT
dose treatment groups given on Day 1 with and without anti CTLA4 given on days
4,7,and 10, as well as PBS and anti CTLA4 alone as controls. We found that
combination MTRT at 50 and 100 uCi dose levels and anti-CTLA4 demonstrated
significantly improved tumor growth delay (Figure 57) and survival (Figure 58)
compared to other groups. At 25 uCi of MTRT there was an intermediate
response.
Additionally, the only mice that had a complete response to therapy were in
the
combination therapy groups with 66%, 33, and 16% of the animals in the 50 uCi,
100,
and 25 uCi MTRT dose groups. All mice that had a complete response at Day 60
after MTRT injection were challenged with B78 cells on the contralateral flank
and
there was a 100% rejection rate compared to naïve controls, demonstrating that
our
treatment was able to generate an immune memory response.
87
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
[00314] This study has since been replicated showing similar trends and
survival
across both studies showed a significantly improved overall survival in mice
treated
with combination MTRT (50, 100 uCi) and anti-CTLA4 compared to other groups by
log rank test.
[00315] We next extended this study to similar mouse models of neuroblastoma
(NXS2) and breast cancer (4T1). As seen in Figure 59 (NXS2) and in Figure 60
(4T1), the CTLA4 MTRT combination again was the only group demonstrating
significantly reduced tumor growth (in fact, tumor volume reduction).
[00316] Next, we extended the study to demonstrate improved response rates in
mice with multiple bulky tumors. We designed a study MTRT treatment in a two-
tumor mouse model, the goal being to treat mice with multiple bulky tumors,
which
would correspond to a patient with bulky metastatic disease at multiple sites.
For this
experiment, we examined if MTRT can improve response rates over the current
clinical paradigm of delivering EBRT to one site in combination with immune
checkpoint blockade.
[00317] Two-tumor models of Panc02 and B78 melanoma were established. First in
B78 melanoma, traditional immunosensitizing EBRT (12 Gy) to a primary site of
disease (secondary site shielded) was combined with anti-CTLA4 and compared to
radiation alone, MTRT and anti-CTLA4, or combination treatment with EBRT to a
primary site with MTRT to all sites and anti-CTLA4. Tumor growth curves show
that
triple combination treatment results in improved tumor regression of both
primary
(Figure 61) and secondary (Figure 62) tumors compared to other groups.
Additionally,
survival was significantly improved with triple combination treatment (p <
0.01)
compared to dual combination treatment groups. Triple combination treatment
resulted
in a 40% complete response rate (16% CR in MTRT + anti-CTLA4, 0% other groups)
with all responding animals having tumor specific immune memory to B78 or
related
B16 melanoma.
[00318] Finally, we simulated advanced multi-site "cold" cancers (i.e.,
multi-site
tumors that do not provoke a strong immune system response, and thus are
largely
resistant to checkpoint inhibition) using a mouse model having two distant
macrospcopic tumors and disseminated microscopic metastases.
[00319] To form the large primary tumor, mice were injected in one flank with
2 x
106 B78 melanoma tumor cells. To form the small secondary tumor, twelve days
later, mice were injected in the opposite flank with 5 x 105 B78 melanoma
tumor
88
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
cells. Seventeen days after this (Day 1), to create the disseminated
metastases, mice
were injected intravenously with 2 x 105 B16 melanoma cells.
[00320] Mice were the exposed to various single or combination treatments: PBS
control injection; MTRT, 50 Ci IV on Day 1; ICI, Anti-CTLA4/PD1 on Days 4, 7
and 10; In Situ Vaccine (IS), 12 Gy local RT on Day 1 + intratumoral injection
of
anti-GD2 mAb and IL2 on Days 6-10. Tested single and combination treatments
were PBS, MTRT, ICI, IS, MTRT + ICI, MTRT + IS, ICI + IS, and MTRT + IS +
ICI. Beginning on Day 60, mice were monitored for tumor growth and animal
survival, and tumor-free mice were rechallenged with B78 on Day 90.
[00321] On Day 90, less than 20% of the ICI mice survived, while about half of
the
MTRT + IS and the ICI + IS mice survived (the MTRT + IS had a somewhat higher
survival rate). Surprisingly, the MTRT + IS mice were 100 % alive (the
survival rate
was zero for all the other groups). Notably, 83% of these mice were found to
be
tumor-free, exhibiting complete remission (CR) with immune cell memory (i.e.,
were
cured), while the remainder retained an uncontrolled secondary tumor.
[00322] We also confirmed uptake and dose delivery in a variety of other
cancers,
including neuroblastoma (NXS2, 9464D), rhabdomyosarcoma (M3-9-M), high grade
glioma, lewis lung carcinoma, and head and neck cancer (MOC-2). In addition to
tumor
uptake and dosimetry, toxicity analysis was conducted, and no radiation-
induced
marrow toxicity (as measured by seum white cells or lymphocytes) was observed
at our
therapeutic radiation dose of 50 Ci (2-3 Gy tumor dose). We have also
irradiated mice
with both external beam and varying doses of 90Y-NM600 and collected histology
stained with IHC as well as tissue for mRNA analysis by PCR. Data from these
studies
show upregulation of the interferon signaling pathway with 50 Ci of 90Y-NM600
as
well as increased PDL1 expression. In addition, we have found that tumor
infiltrating
regulatory T-cells are reduced wuth molecularly targeted radiotherapy.
[00323] In sum, our findings from this study suggest that low dose NM600 MTRT
can enhance abscopal response in tumors when combined with checkpoint
blockade.
Notably, the NM600 MTRT radiotherapeutic delivery agent demonstrates the
ability
to improve response in "cold" tumors that normally do not respond to immune
checkpoint blockade alone. In addition, a relatively low MTRT dose, 50 Ci
(2.5 Gy
tumor dose) is sufficient to achieve immunostimulatory effects to enhance ICI
efficacy without systemic lymphodepletion. MTRT can be added to single site
EBRT
89
CA 03082056 2020-05-06
WO 2019/094657
PCT/US2018/059927
and checkpoint blockade to achieve greater tumor response and cure rates at
both
local and distant tumor sites. Our results show that MTRT has great potential
to
improve therapeutic efficacy of immunotherapy treatments in patients.
Conclusion to the Examples
[00324] These examples illustrate an anti-cancer strategy based on the
synergistic
and widely applicable combination of targeted systemic delivery of
radiotherapy with
systemic delivery of an immunostimulatory agent, such as an immune checkpoint
inhibitor. As the disclosed metal chelated and radiohalogenated
alkylphosphocholine
analogs can target cancers of virtually any histology, the systemic
administration of
immune checkpoint-targeting mAbs or small molecules (immune checkpoint
inhibitors) finds use for virtually any cancer type (tumor reactive mAbs are
approved
or in clinical testing for nearly all cancer histological types). Accordingly,
the clinical
translation of the two different combined strategies have wide application for
virtually
all high risk cancers.
[00325] Other embodiments and uses of the invention will be apparent to those
skilled in the art from consideration from the specification and practice of
the
invention disclosed herein. All references cited herein for any reason,
including all
journal citations and U.S./foreign patents and patent applications, are
specifically and
entirely incorporated herein by reference. It is understood that the invention
is not
confined to the specific reagents, formulations, reaction conditions, etc.,
herein
illustrated and described, but embraces such modified forms thereof as come
within
the scope of the following claims.