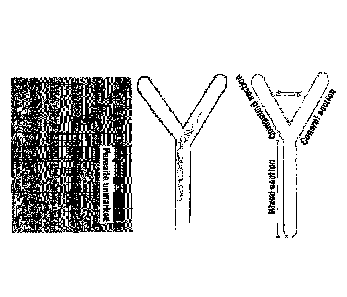Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
1
COMPOSITIONS FOR PREVENTING OR TREATING FISH PARASITIC INFECTIONS,
INCLUDING EXTRACTS OF SOPHOR4 FLAVESCENS ALTON OR FRACTIONS THEREOF
[Technical Field]
[1] The present invention relates to a composition for preventing or treating
a parasitic infectious disease of fish, comprising an extract of Sophora
flavescens Alton as an active ingredient, more specifically, a pharmaceutical
composition for preventing or treating a parasitic infectious disease of
fish, comprising the extract of Sophora flavescens Alton, a fraction thereof
or a compound isolated therefrom as an active ingredient, a feed additive
composition for preventing or improving, and a method for preventing or
treating a parasitic infectious disease of a fish, comprising the step of
putting the composition directly into a fish or in a farm.
[Background Art]
[2] Worldwide, fish aquaculture is an alternative to the depletion of fishery
resources, focusing on fish species that are favorable to food and have
excellent merchantability, and among them, salmon contains many unsaturated
fatty acids such as DHA and EPA which are beneficial to our body, and is
excellent in taste and is the most preferred fish species in the world. The
world's largest salmon aquaculture country is Norway, followed by Britain,
Chile, Canada and Australia. The common point of salmon cultivation stations
is that they are located in the waters where cold seawater flows. The global
salmon market is estimated at 4.3 million tons and 20 trillion won a year,
including about 60 trillion won if it includes processing market.
Date Recue/Date Received 2022-03-23
2
[3] In Korea, Salmonidae, Cherry salmon is mainly cultivated, and Cherry
salmon is a salmonidae living in rivers and seas of Korea, China, Japan and
Russia, and the size is 60 cm smaller than 1 meter of Norwegian salmon. We
call it Shima salmon which is Japanese name. That is, Salmonidae trout
inhabits in Korea, but they do not use the name salmon but cherry salmon
(trout). Trout fishing and festivals in winter are famous. In Korea, the
development of freshwater-based inland water trout farming has developed,
and in 2015 it was the 50th anniversary of trout farming. Meanwhile, salmon
can be classified into Pacific salmon and Atlantic salmon. In Korea, silver
salmon belonging to Pacific salmon is being tried. Atlantic salmon, on the
other hand, are cultivated in large scale in the North Sea fjord such as
Norway, and in the Pacific coast in Chile and Canada. Korea is still in trial
study.
[4] Norway's most known form of salmon, Marine Harvest, succeeded in salmon
farming in the 1960s, and small businesses with local fishermen have grown
into global companies today. Currently, the largest salmon farming company
in the world, hatches 100 million salmon eggs per year and earns about 3
trillion won in salmon farming.
[5] However, the biggest problem of the current aquaculture industry is
mortality rate, which is more than 20%, and the major causes are the incidence
of intractable diseases and infections (viral, bacterial, parasitic) due to
the rise in water temperature, and reduction of the effects of medicines
treatment. In particular, "sea lice" is essential for farms with high
breeding density, and salmon with sea lice has many side effects due to
physical and chemical damage. 'Sea lice is an external parasite that is
Date Recue/Date Received 2022-03-23
3
largely parasitic to Atlantic salmon farmed in Norway as well as Scotland
and Canada. Once a sea lice occurs, it can spread explosively. Specifically,
sea lice for fish are external parasites that live on the mucus, epidermal
tissue or blood of the host fish. The protein components present in the sea
lice are attached to the in vitro tissues of the fish and become parasitic
life as a host. When sea lice are parasitized on fish, they amplify
inflammation, pathogens, etc., and they have a serious problem that the
mortality rate increases and the fish population decreases. In Norway and
Scotland, there are cases where salmon are killed by 30 to 40% because fish
are concentrated and cultivated intensively within the cage farm.
[6] In the past, farmers tried to solve this problem by putting insecticides
on feed for salmon farming, but in recent years, sea lice resistant to this
chemical has appeared. Currently, Marine Harvest is trying to reduce the
number of sea lice by introducing a feeder fish which naturally eats the sea
lice into the fish farm. Experts predict that sea lice extermination will be
possible with convergence technologies, including old management means such
as pesticides and new strategies such as genetic resistance culture, but the
performance is not yet successful, and to date, the use of insecticides
containing emamectin benzoate has been the most effective means.
[7] (Patent Document 1) KR1020010109335 A
DETAILED DESCRIPTION OF THE INVENTION
[Summary]
[8] The present invention provides a composition for preventing or treating
an infectious disease caused by a fish parasite including sea lice, using a
Sophora flavescens Alton, which is a food material harmless to human body
Date Recue/Date Received 2022-03-23
4
and environmentally friendly and without causing tolerance, toxicity and
environmental pollution.
[9] It is another object of the present invention to control, insect and
eliminate fish parasites by using a feed additive composition containing an
extract of Sophora flavescens Alton as an active ingredient.
[Technical Solution]
[10] The present invention provides a pharmaceutical composition for
preventing or treating a fish parasitic infectious disease comprising an
extract of Sophora flavescens Alton or a fraction thereof as an active
ingredient. The extract of Sophora flavescens Alton is a leaf, stem, root,
flower, fruit or whole of Sophora flavescens Alton, and extracts include
matrine, oxymatrine, anagyrine, sophoranol, cytisine, methylcytisine,
kurarinone, xanthohumol, Noranhydroicaritin, isoanhydroicaritin,
glucoluteolin, kaempferol-3-sophoroside, Heilsteine, and Heilsteine-4-
rhamnosyl glucoside.
[11] The extract of Sophora flavescens Alton according to the present
invention can be obtained by ultrasonic extraction, hot water extraction or
solvent extraction, but is not limited thereto.
[12] In the present invention, the fish parasite is selected from the group
consisting of sea lice, Cymothoa Exigua, Ichthyophthirius multifiliis,
Bivagina tai, Anisakiasis, Distoma, Tapeworm, Scutica, Benedenia or Anisakis
Simplex, and preferably in the genus Lepeophtheirus or in the caligus, in
the caligidae, and more preferably, it is sea lice, Lepeophtheirus salmonis
or caligus rogercresseyi.
Date Recue/Date Received 2022-03-23
5
[13] In the present invention, fish can be collectively referred to as any
fish to which the parasitic infectious disease according to the present
invention can develop, and it is preferable to use salmon, trout, catfish,
perch, tuna, halibut, arctic char, sturgeon, flounder, tonguefish, carp,
tilapia, striped bass, eel, sea bream, yellow tail, amber jack, grouper,
Korean rockfish, red sea bream, rock bream, flat fish, mackerel, sea bass,
tuna blue-fin, black sea bream, yellow tail and goldstriped amberjack. It is
also preferably, but not exclusively, aquaculture. It is preferably
Salmonidae.
[14] The present invention also provides a feed additive composition for
preventing or improving a fish parasitic infection comprising an extract of
Sophora flavescens Alton or a fraction thereof as an active ingredient.
[15] The present invention also provides a composition for the control or
insecticides of a fish parasite comprising Sophora flavescens Afton extract
or a fraction thereof as an active ingredient.
[16] As another example, the present invention provides a method for
preventing or treating parasitic infestation in a fish by injecting a
composition comprising an extract of Sophora flavescens Alton or a fraction
thereof as an active ingredient into fish.
[Effects of the Invention]
The composition comprising the extract of Sophora flavescens Alton of the
present invention or the fraction thereof as an active ingredient may be
used as a preventive or therapeutic agent for fish parasites because it
induces sea lice to be avoided or the fish to inhibit or eliminate parasitic
access at the time of ingestion.
Date Recue/Date Received 2022-03-23
6
BRIEF DESCRIPTION OF THE DRAWINGS
[18] Fig.1 shows a Y-tube chamber for sea lice avoidance experiments.
[19] Fig. 2 shows the results of the sea lice avoidance effect of the extract
of Sophora flavescens Alton.
[20] Figures 3(a) and 3(h) show the death, blunting, and survival rate of
sea lice in a concentration-dependent manner.
DETAILED DESCRIPTION
Sophora flavescens Alton is a perennial plant belonging to the rosemary bean
family of dicotyledonous plants and a wild flower native to the mountain
areas of the country and Flowers bloom in raceme. The scientific name of
Gosam is Sophorae flavescens Aiton, and the name Gosam uses the word 'Go'
because the taste is bitter and use the letter 'Sam because the efficacy is
similar to ginseng. It' s roots are mainly used as medicines, and it has the
effect of elimination of stroke, the cooling the heat of the body, the
efficacy of taking urine out of it and extracting heat through it and the
efficacy of cooling the heat and drying the moisture, and therefore, it is
used for leukopenia treatment and anti-radioactivity, blood sugar lowering,
antitumor, antibacterial, immunosuppression and so on.
[23] In the present invention, Sophorae flavescens Aiton is used as raw, or
it is possible to use a Sophorae flavescens Aiton which has undergone more
than one process through a drying method such as natural drying, oven drying,
hot air drying, freeze drying, or a method such as juicing, grinding, cutting
and crushing.
Date Recue/Date Received 2022-03-23
7
[24] The term "extract" in the present invention means an extract obtained
by extracting the Sophorae flavescens Aiton, a diluent or concentrate of the
extract, a dried product obtained by drying the extract, an adjusted product
or a purified product of the extract, or a mixture thereof, and extracts of
all formulations which can be formed using an extract.
[25] In the extract of Sophora flavescens Alton of the present invention,
the method for extracting Sophora flavescens Alton is not particularly
limited and may be carried out according to a method commonly used in the
art. Nonlimiting examples of the extraction method include hydrothermal
extraction method, ultrasonic extraction method, filtration method, and
ref lux extraction method. These methods may be carried out singly or in
combination of two or more methods.
[26] In the present invention, the kind of the extraction solvent used for
extracting the Sophora flavescens Alton is not particularly limited, and any
solvent known in the art can be used. Non-limiting examples of the extraction
solvent include water; Cl to C4 lower alcohols such as methanol, ethanol,
propyl alcohol and butyl alcohol; Polyhydric alcohols such as glycerin,
butylene glycol and propylene glycol; and hydrocarbon solvents such as methyl
acetate, ethyl acetate, acetone, benzene, hexane, diethyl ether, and
dichloromethane; or mixtures thereof, and preferably, water, lower alcohol,
1,3-buty1eneg1yco1, and ethyl acetate may be used alone or in combination of
two or more. In the present invention, the extract may be extracted with a
solvent selected from the group consisting of water, an alcohol having 1 to
4 carbon atoms and a mixed solvent thereof, and the alcohol may be methanol,
Date Recue/Date Received 2022-03-23
8
ethanol, propyl alcohol or butanol, but is not limited to.
[27] As a method for obtaining the extract of Sophora flavescens Alton of
the present invention, it is more preferable to dry the Sophora flavescens
Aiton at a shade and a room temperature for 5 to 7 days after harvest, and
to pulverize, and polar solvent of (Cl) to (C4) lower alcohols such as water,
methanol, ethanol, and butanol of about 1 to 20 times, preferably about 1 to
times, of the dry weight, or a mixed solvent thereof having a mixing ratio
of about 1: 0.1 to 1:10 is used as an elution solvent, and the extraction
temperature is 20 C to 100 C, preferably room temperature, and the
extraction period can be extracted using extraction methods such as hot water
extraction, cold extraction, reflux cooling extraction, or ultrasonic
extraction for about 12 hours to 6 days, preferably 5 days.
[28] The term "fraction" in the present invention means the product
obtained by performing fractionation to separate a specific component or a
specific component group from a mixture containing various components.
[29] The fractionation method for obtaining the fraction in the present
invention is not particularly limited and may be carried out according to a
method commonly used in the art. As a non-limiting example of the above
mentioned fractionation method, there can be mentioned a method in which the
extract obtained by extracting Sophora flavescens Afton is treated with a
predetermined solvent to obtain a fraction from the extract.
[30] In the present invention, the compounds contained in the extract of
Sophora flavescens Alton include matrine, oxymatrine, anagyrine, sophoranol,
Date Recue/Date Received 2022-03-23
9
cytisine, methylcytisine, kaurinone, xanthohumol, Noranhydroicaritin,
isoanhydroicaritin, glucoluteolin, kaempferol-3-sophoroside, Heilsteine,
Heilsteine-4-rhamnosyl glucoside, and the like. And such compounds may be
obtained from the extract or fraction by conventional separation and
purification processes.
[32] In the present invention, fish can he collectively referred to as any
fish to which the parasitic infectious disease according to the present
invention can develop, and it is preferable to use salmon, trout, catfish,
perch, tuna, halibut, arctic char, sturgeon, flounder, tonguefish, carp,
tilapia, striped bass, eel, sea bream, yellow tail, amber jack, grouper,
Korean rockfish, red sea bream, rock bream, flat fish, mackerel, sea bass,
tuna blue-fin, black sea bream, yellow tail and goldstriped amberjack. It is
also preferably, but not exclusively, aquaculture. In one embodiment of the
present invention, an experiment was conducted using salmon as an example.
[33] The parasite of the present invention collectively refers to an
invertebrate living in parasitic life outside and in the body of another
organism (fish, crustacean, animal, plant, etc.). In detail, there are the
sea lice, Cymothoa Exigua, Ichthyophthirius multifiliis, Bivagina tai,
Anisakiasis, Distoma, Tapeworm, Scutica, Benedenia, Anisakis Simplex, and
the like, but are not limited to. It also includes at least one of internal
parasites (protozoa, annular animals, linear animals, etc.) and external
parasites (flat animals, arthropods, etc.).
[34] In one embodiment of the present invention, it has been confirmed that
the extract of Sophora flavescens Aiton has an effect of eliminating sea
Date Recue/Date Received 2022-03-23
10
lice (FIGS. 2 and 3), and therefore, it has been found that the composition
comprising the extract of Sophora flavescens Afton of the present invention
is suitable for use in the prevention, improvement or treatment of parasitic
infection of fish.
[35] The "prevention" in the present invention means any action to inhibit
or delay the onset of parasitic infectious disease of fish by the composition,
and "treatment" means any action whereby the symptoms of a parasitic
infectious disease of a fish are improved or beneficially altered by the
composition.
[36] The "pharmaceutical composition" in the present invention means a
preparation for the purpose of prevention or treatment of disease, and may
be formulated into various forms according to conventional methods. For
example, it can be formulated into oral formulations such as powders,
granules, tablets, capsules, suspensions, emulsions and syrups, and can be
formulated in the form of external preparations, suppositories, and
sterilized injection solutions. However, it is most preferable that the
composition of the present invention is provided in the form of a bathing
agent for the purpose of treating diseases of fishes.
[37] In addition, each of the formulations may be further formulated with
pharmaceutically acceptable carriers such as buffers, preserving agents,
wettability agents, solubilizers, isotonic agents, stabilizers, bases,
excipients, lubricants and the like known in the art.
Date Recue/Date Received 2022-03-23
11
[38] Meanwhile, the pharmaceutical composition of the present invention is
administered in a pharmaceutically effective amount. The " pharmaceutically
effective amount" of the present invention means an amount that is
sufficient to treat a disease at a reasonable benefit / risk ratio applicable
to veterinary treatment and does not cause side effects, and "effective
dose levels" may be determined by factors including the health of a fish,
severity, activity of the drug, sensitivity to the drug, method of
administration, time of administration, route of administration and rate of
release, duration of treatment, composition or factors including c0
administered drugs and other factors well known in the veterinary arts.
[40] In another aspect, the present invention provides a feed additive
composition for preventing or improving a parasitic infestation disease of
fish, comprising an extract of Sophora flavescens Alton, a fraction thereof,
or a compound isolated therefrom as an active ingredient.
[42] For the purpose of the present invention, the extract of Sophora
flavescens Alton, fractions thereof, or compounds isolated therefrom can be
added to feed compositions as a feed additive, since they can be used for
the purpose of preventing or improving a parasitic infectious disease of
fish.
[43] In the present invention, the feed additives are collectively referred
to as substances added to feed in small quantities for nutritive or specific
purposes, therefore, in the present invention, the feed additives are
substances added for the purpose of preventing or improving parasitic
infectious diseases of fish. The extract of Sophora flavescens Alton of the
Date Recue/Date Received 2022-03-23
12
present invention or the compound isolated therefrom may be included in the
feed composition as the feed additive, and the feed may be used to
collectively mean all the foods supplied to the fish.
[44] The feed composition of the present invention may further contain a
binder, an emulsifying agent, a preservative, etc. to be added for the purpose
of preventing deterioration of quality, and may further contain an amino
acid, a vitamin, an enzyme, a prophylactic agent, a flavoring agent, a non-
protein nitrogenous substance, a silicate, a buffer, a colorant, an
extractant, an oligosaccharide and the like for the purpose of enhancing the
utility, and in addition, feed mixtures and the like may be further included,
but the present invention is not limited thereto.
[45] The compound feed commonly used in the feed composition for fish includes
at least one protein source selected from fish meal, casein, meat meal, and
shrimp powder, or at least one carbohydrate source selected from sweet potato
starch, potato starch, wheat starch, or at least one lipid source selected
from rapeseed oil, soybean oil, corn oil, pollack liver oil, squid liver oil,
but the present invention is not limited thereto, and even when used in
combination with the compounded feed, the effect of preventing or improving
the parasitic infectious disease described in the present invention occurs
in the same manner.
[46] In another aspect, the present invention provides a method for
preventing or treating parasitic infestation in a fish, comprising the step
of administering a composition comprising the extract of Sophora flavescens
Aiton, a fraction thereof or a compound isolated therefrom as an active
Date Recue/Date Received 2022-03-23
13
ingredient directly to a fish or to a fish farm.
[47] In another aspect, the present invention provides a method for
preventing or treating parasitic infestation in fish, comprising the step of
administering to a fish directly or in a fish farm a pharmaceutical
composition or feed additive composition comprising an extract of Sophora
flavescons Afton as an active ingredient.
[48] The method for preventing or treating a parasitic infectious disease of
fish may include any step directly or indirectly delivered to a fish by
including the step of administering directly to the fish or to a farm.
[49] Specifically, for example, the purpose of the present invention can be
achieved by directly injecting or feeding to a fish, and can also be
accomplished through means such as bathing or eating. Preferably, the
effective concentration of the composition may be coated on the feed and fed
to a fish having lesions due to parasitic infectious disease, but the present
invention is not limited thereto. At this time, the dose of the composition
may vary depending on the weight of the fish to be treated, the health
condition, the administration time, and the severity of the disease.
[51] Hereinafter, effects of the present invention will be described with
reference to examples.
[53] [Example 11: Preparation of extract of Sophora flavescens Alton
[54] The roots were thoroughly washed with water, naturally dried for 5 to
7 days, and cut. Three times the weight of methanol was added to 50 kg of
Date Recue/Date Received 2022-03-23
14
the excised Sophora flavescens Alton, and the mixture was extracted at room
temperature for 5 days and then filtered. It was concentrated under reduced
pressure, lyophilized and powdered.
[56] [Example 21: Sea lice ( avoi dance) repellent effect of Sophora
flavescens
Alton
[57] Using the Y-Tuhe choice camber, the movement of the sea lice and the
time spent in each section were measured to confirm the avoidance effect of
the sea lice repellent.
[58] The control section is flushed with clean sea water and the compound
section is flushed with the substance to be tested. Mixed section is a section
where control and compound sections are mixed and drained.
[59] The sea lice are positioned at the point where the mixed section starts
and the movement is observed, and the analysis of the test results confirmed
the effect of sea lice eradication by analyzing how long the sea lice moved
and stayed in the opposite direction of flow of the test material, that is,
the control section and the mixed section. (see Fig. 1)
[61] According to the above experimental method, the movement of the sea
lice was observed by flowing the seawater in the control section and flowing
the substance of the present invention in the compound section, as shown in
FIG. 2.
[62] In Fig. 2, Con.1 was treated with seawater as a control, 0H4_A was
treated with 0.5 mg / ml of Sophora flavescens Alton and 0H4_13 was treated
Date Recue/Date Received 2022-03-23
15
with 0.25 mg / ml of Sophora flavescens Alton respectively and therefore it
shows the effect of avoiding sea lice. In Con.1, the sea lice did not move
to other sections because the seawater flows in all sections of the Y-tube
chamber, and the time spent of sea lice in 0H4_A treated with 0.5 mg / ml
Sophora flavescens Alton was about 68% in the control section, about 20% in
the mixed section, and about 12% in the compound section, and the time spent
of sea lice in 0H4_B treated with 0.25 mg / ml of Sophora flavescens Afton
was about 29% in the control section, about 24% in the mixed section, and
about 47% in the compound section. This means that it has sea lice avoidance
effect depending on the concentration of Sophora flavescens Aiton.
[64] [Example 3]: The survival rate of sea lice about Sophora flavescens
Aiton.
[65] In order to confirm the effectiveness of Sophora flavescens Alton that
the sea lice had a repelling effect, experiments were carried out using a
24-well plate. Sea water, sea lice and active substances were added to the
24-well plate at various concentrations, and then incubated in an incubator,
and all solutions used in the experiments were kept at 10 C during the
experiment. After incubation for 24 hours, the effect of sea lice was
confirmed by observing the condition of sea lice.
[67] As a result of the experiment, as shown in Fig. 3 (a), about 95% of the
sea lice survive in the control without any treatment, but as the treatment
concentration of extract of Sophora flavescens Alton increased, the number
of alive species decreased and the number of dead species increased. In
addition, when the concentration of extract of Sophora flavescens Alton was
Date Recue/Date Received 2022-03-23
16
0.31 mg / ml, 50% (LD50) of the sea lice population was dead, and at 0.12 mg
/ ml, the sea lice population 50% (EC50) showed moribund state or swimming
inhibition and showed excellent effect on sea lice.
[69] In conclusion, the extract of Sophora flavescens Alton of the present
invention or its fractions thereof have anti-sea lice activity, and thus can
he used for the prevention or treatment of parasitic infectious diseases of
fish, for the parasite elimination, and for the control thereof.
[INDUSTRIAL APPLICABILITY]
The present invention is recognized as being industrially applicable in the
fields of pharmaceutical biotechnology and agriculture and fisheries.
Date Recue/Date Received 2022-03-23