Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03088882 2020-07-02
WO 2019/139777
PCT/US2018/067051
METHODS OF FORMING SPHERICAL METALLIC PARTICLES
BACKGROUND
[0001] Embodiments of the present disclosure generally relate to methods
of
forming spherical titanium-based metallic particles. More particularly,
embodiments
of the present disclosure relate to methods of forming spherical titanium
alloy
particles using microwave plasma.
[0002] An important aspect of preparing some forms of industrial powders
is
the spheroidization process, which transforms irregularly shaped or angular
powders
produced by conventional crushing methods, into spherical low-porosity
particles.
Spherical powders are homogenous in shape, dense, less porous, and exhibit
better
flowability. Such powders may exhibit superior properties in applications such
as
injection molding, thermal spray coatings, or additive manufacturing.
[0003] Titanium and titanium-alloy particles are particularly useful in
additive
manufacturing of industrial grade components. Additive manufacturing of
titanium
components may require high-quality, low-cost spherical titanium or titanium
alloy
powder as a feedstock for good flowability. Conventional methods for
processing of
titanium alloys to produce spherical powders typically involve multiple steps,
such as,
producing titanium ingots from sponges and utilizing melting and atomization
processes on the titanium ingots to produce spherical powder. The formation of
titanium powder can be facilitated by one of several approaches, such as, the
Kroll
process, the Hunter process, or the Armstrong process. However, most of these
commercial processes are typically carried out as large-scale processes and
are batch
segregated, which increases the complexity and associated cost. Furthermore,
the
intermediate metallurgical processes for conversion to alloys may add to the
cost of
the resulting spherical titanium alloy powder.
[0004] Other methods for forming spherical titanium particles employ
thermal
arc plasma or radio-frequency generated plasma for spheroidization of titanium-
based
feedstock material. However, these two methods may present limitations
inherent to
the thermal non-uniformity of radio-frequency and thermal arc plasmas. Some
other
spheroidization methods employ inductively coupled plasma (ICP), where angular
powder obtained from a Hydride-Dehydride (HDH) process is entrained within a
gas
1
CA 03088882 2020-07-02
WO 2019/139777
PCT/US2018/067051
and injected though a hot plasma environment to melt the powder particles.
However,
this method also suffers from non-uniformity of the plasma, which leads to
incomplete spheroidization of the feedstock. Further, the HDH process involves
several time-consuming complex steps, which may again add to the cost of the
resulting spherical powder.
BRIEF DESCRIPTION
[0005] In one aspect, the present disclosure relates to a method of
forming
spherical metallic particles including titanium. The method includes
contacting a
feedstock material including a metal halide with a reductant in the presence
of a
microwave plasma discharge to form the spherical metallic particles. In
another
aspect, the present disclosure relates to a plurality of spherical metallic
particles
including titanium, formed by contacting a feedstock material including a
titanium
halide with a reductant in the presence of a microwave plasma discharge.
[0006] In yet another aspect, the present disclosure relates to a method
of
forming spherical titanium-based particles. The method includes contacting a
feedstock material including a titanium halide with a hydrogen gas in the
presence of
a microwave plasma discharge to reduce the titanium halide and form the
spherical
titanium-based particles.
DRAWINGS
[0007] These and other features, aspects, and advantages of the present
disclosure will become better understood when the following detailed
description is
read with reference to the accompanying drawings, wherein:
[0008] FIG. 1 illustrates a schematic of an apparatus for forming
spherical
metallic particles, in accordance with some embodiments of the present
disclosure.
DETAILED DESCRIPTION
[0009] In the following specification and the claims, which follow,
reference
will be made to a number of terms, which shall be defined to have the
following
meanings. The singular forms "a", "an" and "the" include plural referents
unless the
context clearly dictates otherwise. As used herein, the term "or" is not meant
to be
exclusive and refers to at least one of the referenced components being
present and
2
CA 03088882 2020-07-02
WO 2019/139777
PCT/US2018/067051
includes instances in which a combination of the referenced components may be
present, unless the context clearly dictates otherwise.
[0010] Approximating language, as used herein throughout the
specification
and claims, may be applied to modify any quantitative representation that
could
permissibly vary without resulting in a change in the basic function to which
it is
related. Accordingly, a value solidified by a term or terms, such as "about",
and
"substantially" is not to be limited to the precise value specified. In some
instances,
the approximating language may correspond to the precision of an instrument
for
measuring the value. Similarly, "free" may be used in combination with a term,
and
may include an insubstantial number, or trace amounts, while still being
considered
free of the solidified term. Here and throughout the specification and claims,
range
limitations may be combined and/or interchanged, such ranges are identified
and
include all the sub-ranges contained therein unless context or language
indicates
otherwise.
[0011] As mentioned earlier, conventional methods for producing spherical
titanium-based particles may involve expensive feedstock material such as
metallic
sponges. Further, these processes may involve multiple intermediate
metallurgical
steps and batch processing of the feedstock material, which in turn may affect
the cost
and consistency of the final product. Embodiments of the present disclosure
described herein address the noted shortcomings in the art.
[0012] A method of forming spherical metallic particles including
titanium is
presented. The method includes contacting a feedstock material including a
metal
halide with a reductant in the presence of a microwave plasma discharge.
[0013] The term "metallic particles" as used herein refers to a plurality
of
particles including an elemental metal, a metal alloy, or a combination
thereof.
Therefore, the term metallic particles as used herein includes elemental
titanium, a
titanium-based metal alloy, or a combination thereof. The term "elemental
metal" as
used herein means that an amount of a base metal in the metallic particles is
greater
than 97 weight percent. In certain embodiments, an amount of the base metal in
the
metallic particles is greater than 99 weight percent. Therefore, the term
"elemental
titanium" as used herein means than an amount of titanium in the metallic
particles is
greater than 97 weight percent. In certain embodiments, the spherical metallic
3
CA 03088882 2020-07-02
WO 2019/139777
PCT/US2018/067051
particles include a metal alloy including titanium. The metal alloy may
further
include aluminum, vanadium, or a combination thereof In certain embodiments,
the
spherical metallic particles include titanium alloy particles, such as,
Ti6A14V. In
some such instances, the amount of aluminum in the titanium alloy may be in a
range
of from about 4 weight percent to about 7 weight percent, and the amount of
vanadium in the titanium alloy may be in a range from about 3 weight percent
to
about 5 weight percent.
[0014] The term "spherical metallic particles" as used herein refers to a
plurality of particles having an average aspect ratio that is less than 1.1.
In some
embodiments, the spherical metallic particles may have an average aspect ratio
that is
less than 1.05. The spherical metallic particles may have an average diameter
in a
range of from about 1 micron to about 500 microns. In some embodiments, the
spherical metallic particles may have an average diameter in a range of from
about 10
microns to about 150 microns.
[0015] As noted herein, the method includes contacting the feedstock
material
with the microwave plasma discharge. The feedstock material includes a metal
halide
such as titanium halide. In embodiments, wherein the spherical metallic
particles
include elemental titanium, the feedstock material includes at least one
titanium
halide. A non-limiting example of a suitable titanium halide includes titanium
chloride.
[0016] In some embodiments, wherein the spherical metallic particles
include
a metal alloy, the feedstock material includes a metal halide mixture. Non-
limiting
examples of suitable halides in the metal halide mixture may include titanium
chloride
and one or both of vanadium chloride and aluminum chloride. In certain
embodiments, the feedstock material may be in the form of a liquid.
[0017] The microwave plasma discharge may be generated using a suitable
microwave plasma torch. The method may include introducing the feedstock
material
into the microwave¨plasma torch using any suitable means, for example, a
fluidized
bed feeder. Within the microwave plasma torch, the feedstock materials are
exposed
to a plasma discharge causing the materials to melt. Because of the uniformity
of the
microwave plasma discharge, the feedstock material may be exposed to a
substantially uniform temperature profile, and rapidly heated and melted. In
one
4
CA 03088882 2020-07-02
WO 2019/139777
PCT/US2018/067051
example, the feedstock material may be exposed to a uniform temperature
profile in a
range from about 4,000 K to about 8,000 K within the plasma. During the same
time
(i.e., time that the feedstock material is exposed to the plasma discharge), a
reductant
may be introduced into the microwave plasma torch such that the reductant also
contacts the feedstock material. Therefore, the metal halide in the feedstock
material
undergoes a reduction reaction, thereby forming a metal or a metal alloy
(depending
on the feedstock material composition). A non-limiting example of a suitable
reductant includes hydrogen. In certain embodiments, the reductant includes
hydrogen gas.
[0018] After the reduction of the metal halides in the feedstock
material,
within the microwave plasma discharge, the reduced and melted metals may be
inherently spheroidized, at least in part, due to liquid surface tension. As
the
microwave generated plasma exhibits a substantially uniform temperature
profile,
more than 90% spheroidization of particles may be achieved. Therefore, by
exposing
the feedstock material to the microwave plasma discharge in the presence of
the
reductant, both dehalogenation and spheroidization are achieved. Thus,
separate or
distinct processing steps may not be needed to achieve dehalogenation and
spheroidization.
[0019] Various parameters of the microwave plasma discharge, as well as
feedstock material parameters, may be adjusted in order to achieve the desired
results.
These parameters may include one or more of microwave power, feedstock
material
size, feedstock material insertion rate, gas flow rates, plasma temperature,
and cooling
rates.
[0020] After the spheroidization step in the microwave plasma discharge,
the
plurality of spherical metallic particles may exit the microwave plasma
discharge,
resulting in cooling and further solidification of the particles. In some
embodiments,
the spherical metallic particles exiting from the microwave plasma discharge
may be
further subjected to one or more additional cooling steps to facilitate
solidification and
collection. The cooled and solidified spherical metallic particles may be
subsequently
collected using appropriate collection mechanisms, e.g., collection bins.
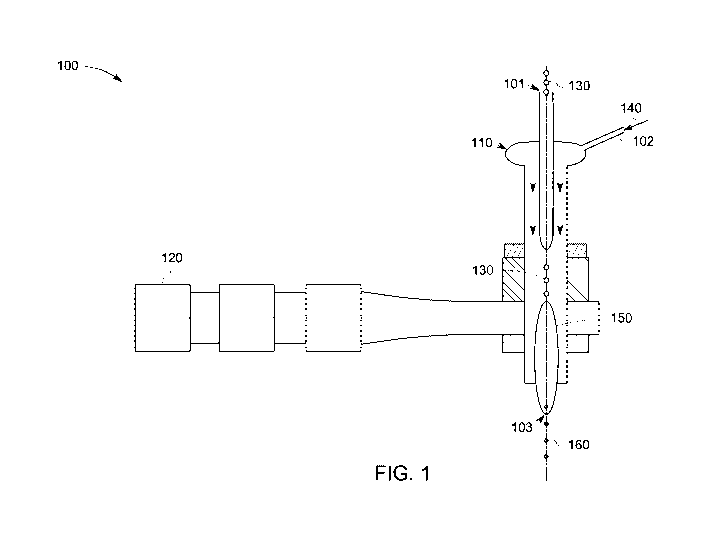
[0021] Fig. 1 illustrates a schematic of an apparatus 100 for forming
spherical
metallic particles, in accordance with some embodiments of the present
disclosure.
CA 03088882 2020-07-02
WO 2019/139777
PCT/US2018/067051
The apparatus includes a plasma torch 110 having a first inlet 101 and a
second inlet
102. The plasma torch 110 is configured to generate and sustain a microwave
plasma
discharge 150 upon ignition from a suitable microwave radiation source 120. A
feedstock material 130 is fed into the plasma torch 110 via the first inlet
101 and a
reductant 140 is fed into the plasma torch 110 via the second inlet 102. As
further
illustrated in Fig. 1, the feedstock material 130 is introduced into the
microwave
plasma discharge 150 in the presence of the reductant 140. The feedstock
material
130 melts within the microwave plasma discharge 150, and is simultaneously
reduced
within the microwave plasma discharge because of the reductant 140. The
reduced
and melted metals are inherently spheroidized, at least in part, due to liquid
surface
tension. Spherical metallic particles 160 are discharged from the plasma torch
110 via
an outlet 103. The location and configuration of the first inlet 101, the
second inlet
102, and the outlet 103 are depicted in Fig. 1 for illustration purposes only,
and any
other suitable locations and configurations are also envisaged within the
scope of the
present disclosure. As noted earlier, the discharged spherical metallic
particles 160
may be subjected to one or more cooling steps and subsequently collected (not
shown
in Figure).
[0022] A method of forming spherical titanium-based particles is also
presented. The method includes contacting a feedstock material including a
titanium
halide with a hydrogen gas in the presence of a microwave plasma discharge, to
reduce the titanium halide and form the spherical titanium-based particles.
[0023] In one example, the method includes contacting liquid mixtures of
titanium tetrachloride and other metal chlorides (such as aluminum and
vanadium
chlorides) to form a liquid halide mixture. The liquid halide mixture is used
as a
feedstock in a microwave-based plasma system containing a reducing atmosphere,
for
example, hydrogen gas. In the reducing atmosphere plasma environment, the
metal
halides are directly reduced to metal, and subsequently converted to spherical
titanium
alloy powder.
[0024] A plurality of spherical metallic particles including titanium,
formed
by the method described herein, is also presented. The plurality of spherical
metallic
particles includes an elemental metal, a metal alloy, or a combination
thereof. In
some embodiments, the spherical metallic particles include elemental titanium,
a
6
CA 03088882 2020-07-02
WO 2019/139777
PCT/US2018/067051
titanium alloy, or a combination thereof In certain embodiments, the plurality
of
spherical metallic particles includes a titanium alloy. The titanium alloy may
further
include aluminum, vanadium, or a combination thereof.
[0025] The
spherical titanium-based metallic particles and methods of
producing such particles, in accordance with embodiments of the present
disclosure,
may provide a number of advantages. For example, the methods as described
herein
may allow for a continuous process that simultaneously reduces and
spheroidizes the
feedstock materials. That is, the separate and distinct steps required in
conventional
processes (e.g., HDH process) can be replaced with a single processing step
using a
microwave plasma discharge. Reduction in the number of intermediate steps may
reduce the cost of the resulting spherical metallic particles. Further, use of
simple
metal halide mixtures as feedstock materials, instead of the more expensive
traditional
sponge-based feedstock materials may further significantly reduce the cost of
the
resulting spherical metallic particles.
[0026]
Reduction in the number of processing steps also reduces the
possibility for contamination by oxygen and other contaminants. Additionally,
the
continuous spheroidization process disclosed herein may improve the
consistency of
the end products by reducing or eliminating variations associated with typical
batch-
based dehydrogenation processes.
[0027] The
methods as described herein can achieve additional improvements
in consistency due to the homogeneity and control of the energy source (i.e.,
microwave plasma process).
Specifically, if the plasma conditions are well
controlled, particle agglomeration can be reduced, if not eliminated, thus
leading to a
better particle size distribution, which could result in high-quality, low-
cost, high
flowability titanium-based powder. As mentioned earlier, high-quality, low-
cost, high
flowability titanium-based powder may be particularly desirable for additive
manufacturing of titanium-based components.
[0028] The
appended claims are intended to claim the invention as broadly as
it has been conceived and the examples herein presented are illustrative of
selected
embodiments from a manifold of all possible embodiments. Accordingly, it is
the
Applicants' intention that the appended claims are not to be limited by the
choice of
examples utilized to illustrate features of the present disclosure. As used in
the
7
CA 03088882 2020-07-02
WO 2019/139777
PCT/US2018/067051
claims, the word "comprises" and its grammatical variants logically also
subtend and
include phrases of varying and differing extent such as for example, but not
limited
thereto, "consisting essentially of' and "consisting of." Where necessary,
ranges have
been supplied; those ranges are inclusive of all sub-ranges there between. It
is to be
expected that variations in these ranges will suggest themselves to a
practitioner
having ordinary skill in the art and where not already dedicated to the
public, those
variations should where possible be construed to be covered by the appended
claims.
It is also anticipated that advances in science and technology will make
equivalents
and substitutions possible that are not now contemplated by reason of the
imprecision
of language and these variations should also be construed where possible to be
covered by the appended claims.
8