Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03095159 2020-09-24
WO 2019/191198 PCMJS2019/024237
Ti TICK ENED CATALYZED DYE SYSTEM
HELD OF THE INVENTION
The present invention relates to catalyzed hair dyeing systems and methods
which may
reduce the time of exposure of hair to oxidative dye treatments, thereby
reducing overall hair
damage from the dyeing process.
BACKGROUND OF THE INVENTION
Coloring of hair has become an increasingly popular practice. People desire
hair
coloration for reasons spanning from style choices to achieving more youthful
appearances. As
people age, the production of melanin slows, resulting in hair greying.
Melanin can be purposely
altered by chemical treatments to give lighter shades. The lightening is
achieved by oxidizing
the melanin pigments, usually with an oxidizing agent in alkaline solution,
also called bleaches.
Examples of oxidizing agents that can be used are hydrogen peroxide,
potassium, sodium or
ammonium salts of perborate or percarbonate, persulfate and percarbamide.
Bleaches are also used during oxidative dyeing treatments. Oxidative (or
"permanent")
dye compositions comprise "precursor dyes" which are small molecules capable
of diffusing
into the hair. These molecules mainly belong to three classes of aromatic
compounds: diamines,
aminophenols and phenols. They are sufficiently small to diffuse in the hair
shaft where, once
activated by an oxidizing agent such as hydrogen peroxide, they further react
with other
precursors to form larger colored complexes. Oxidative hair dye compositions
commonly
contain, in addition to the dye precursors and a source of peroxide, a variety
of additional
cosmetic and peroxide stabilizing agents.
Oxidizing agents can activate oxidative dye precursors across a range of pH.
However,
it is known that enhanced dye oxidation can be achieved via the use of a hair-
swelling agent
(HSA) that can adjust the pH of the oxidizing solution. Such HSA's further
enhance the
oxidizing and dyeing process by swelling the hair fibers to aid both the
diffusion of the peroxide
and dyeing agents into the hair and enabling faster, more thorough dye
oxidization and hair
dyeing. Preferred hair-swelling agents for adjusting the pH of peroxide hair
oxidizing
compositions are aqueous alkaline solutions containing ammonia (ammonium
hydroxide) or
monoethanolamine (MEA).
1
CA 03095159 2020-09-24
WO 2019/191198 PCT1US2019/024237
Low levels of chelants are routinely used as stabilizers or preservatives in
various
oxidizing compositions. For example, EDT A (ethyl enedi am i netetraaceti c
acid) is commonly
used as a stabilizer in hydrogen peroxide solution, which would otherwise
decompose too
rapidly and could not be stored for a long time. Ethylene diaminedissucinnic
acid (EDDS) is
also known as a good stabilizing agent component to increase the stability of
laundry bleaching
products. Amounts as low as 0.1% by weight of the oxidizing composition are
usually used to
stabilize the oxidizing agent contained in the oxidizing compositions.
Oxidative treatments of hair such as bleaching (decoloration) and oxidative
dyeing give
good results and are very commonly used. They are however not without
drawbacks. The
.. oxidizing agents used for bleaching and oxidative dyeing damage hair, to
some extent. The
mechanism by which damage is caused to the hair fibers is not perfectly
understood. However,
it is known that some of the disulphide bonds linking the keratin chains break
in the presence of
oxidizing compositions. Repeated oxidative treatments leave weak, brittle
hairs, which have
little shine and luster. An enormous effort has been made to address this
problem, and various
solutions have been proposed.
Today, most dyeing or bleaching compositions are sold with a conditioner,
which is
applied on hair after the bleaching or dyeing composition has been rinsed off.
Examples of
conditioning agents are silicones, cationic surfactants and cationic polymers.
However efficient,
conditioners cannot prevent successive chemical treatments causing premature
hair breakage.
.. In fact, conditioners do not bring the hair back to its initial condition
but merely conceal the
damage under a protective layer of the conditioning agent, which only results
in an improved
feel of the hair.
U.S. Patent No. 7,686,849 to Forbes et al. addresses the use of
organotitanates as
catalysts for oxidative hair color. The patent acknowledges that these
organotitanate catalyst
are prone to hydrolysis, offering hydrolysis as the reasoning and mechanism
for organotitanates
acting as bonding agents. The high pH instability of organotitanates is known
in the literature,
academic as well as vendor, and it is fully expected that these catalysts will
hydrolyze under
high pH formulation conditions to make titanium dioxide.
There is an ongoing need to provide hair color with a stabilized approach for
minimizing
.. or reducing hair damage in an oxidative hair dye system.
2
CA 03095159 2020-09-24
WO 2019/191198 PCT1US2019/024237
SUMMARY OF THE INVENTION
The present invention relates to a hair dye system. The hair dye system
includes at least
one dye precursor, an oxidizing agent, and a catalyst. The catalyst is a
homogeneous catalyst or
.. a heterogeneous catalyst and the dye system has a viscosity of less than
about 2,500 mPa-s,
when measured at a shear rate of about 25s' and at about 25 C.
The present invention further relates to methods for making and using the dye
system.
BRIEF DESCRIPTION OF THE DRAWINGS
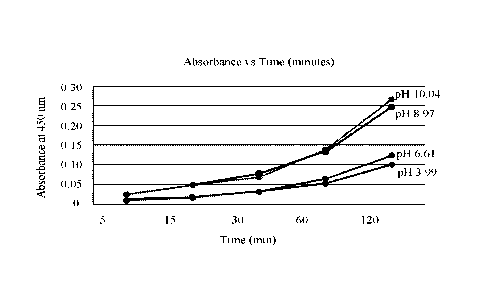
FIG. 1 shows the rates of reaction of the dye system herein at varying pH
levels.
FIG. 2 is a comparative graph, illustrating changes to viscosity vs. shear
rate of
exemplary compositions herein.
FIGS. 3A-3C show the formula of Example 1, when formulated according to Method
I
herein, under increasing magnification (4x, 20x, and 10x, respectively), 24
hours after
formulation. FIGS. 4A-4C show the formula of Example 2, when formulated
according to
Method I herein, under increasing magnification (4x, 20x, and 10x,
respectively), 24 hours after
formulation. FIGS. 5A-5C show the formula of Example 3, when formulated
according to
Method II herein, under increasing magnification (4x, 20x, and 10x,
respectively), 24 hours after
formulation.
FIGS. 6A-6C show the formula of Example 4, when formulated according to Method
ill herein, under increasing magnification (4x, 20x, and 10x, respectively),
24 hours after
formulation.
FIG. 7A is a photo of a sample composition according to Example 3, formulated
according to Method II herein.
FIG. 7B is a photo of a sample composition according to Example 2, forinulated
according to Method I herein.
FIG. 8A is a photo of a sample composition according to Example 4, formulated
according to Method III herein.
FIG. 8B is a photo of a sample composition according to Example 1.
3
CA 03095159 2020-09-24
WO 2019/191198 PCT1US2019/024237
DETAILED DESCRIPTION OF THE INVENTION
Dye Precursor Compounds
The following conventional primary intermediate and coupler substances may be
used
as the oxidation dye pre-cursor compounds.
As primary intermediate substances the following can be used: standard primary
aromatic amines with an additional free or substituted hydroxy or amino group
substituent in
the ortho- or para-position, indole derivative compounds or substituted
heterocyclic compounds,
especially from the classes of pyrimidines and pyrazoles, such as 1,4-
diaminobenzene (p-
phenylendiamine), 1,4-diamino-2-methylbenzene (p-toluene-diamine), 1,4-diamino-
2,6-
dimethylbenzene, 1,4-diamino-2,5-dimethylbenzene, 1,4-diamino-2,3-
dimethylbenzene, 1,4-
di am i no-2-chl orobenzene, 4-di (2-hydroxyethyl)am i no aniline, N,N-bi s-(2-
hydroxyethyl )-p-
phenylenediamine, 4-(2-methoxyethypamino aniline, 1,4-diamino-2-(2-hydroxy-
ethyl)-
benzene, 1,3-bis-N-(2-hydroxy-ethyl)-N-(4-amino-phenyl )-am ino-2-
propanol, 2',2-1,2-
ethanediyl-bis(oxy-2,1-ethanediyloxy)-bis-1,4-diaminobenzene, 4-amino-phenol,
4-amino-3-
methylphenol, 4-methylaminophenol, 4-amino-2-(aminomethyl)phenol, 4-amino-2-(2-
hydroxyethypamino-methylphenol; 4-amino-2-(methoxymethyl)-phenol, 5-amino-
salicylic
acid, 2,4,5,6-tetra aminopyri mi di ne, 2,5,6-tri-amino-4-hydroxy-pyri mi di
ne, 4,5-di amino-142-
hydroxyethyl)-1H-pyrazol e, 4,5-di ami no-1-(1-methy I ethyl)-1H-pyrazol e,
4,5-diami no-1-(4-
methyl phenyl)methy1-1H-pyrazole, 4,5-diamino-1-(4-chloro-phenyl)methy1-1H-
pyrazole, 4,5-
diamino-1-methylpyrazol e, 2,5-dimethylpyridine, 2-amino-6-methylphenol or 2-
amino-5-
methyl-phenol, alone or in combination with each other.
Suitable coupler substances include, for example, substituted m-
diaminobenzenes, m-
aminophenol, resorcinol derivative compounds, indole derivative compounds,
naphthols or
substituted heterocyclic compounds can be used, especially from the classes of
pyrimidines and
pyridines, such as N,N-dimethy1-3-ureidoaniline, 2,6-diamino-pyridine, 2-amino-
4- (2-
hydroxy ethyl)am i no an i sole, 2,4-di am i n o-1-fl uoro-5-methy l -benzene,
2,4-di am i n o-1-m eth oxy-
5-methylbenzene, 2,4-di amino-1 -ethoxy-5-m ethylbenzene, 2,4-diami no-1-(2-
hydroxyethoxy)-
5-methylbenzene, 2,4-di -(2-hydroxy ethyl)ami no-1,5-di rnethoxy -benzene, 2,3-
diamino-6-
methoxy py ri di ne, 3-amino-6-methoxy-2-(methylami no)pyri di ne, 2,6-di
amino-3,5-di methoxy -
pyridine, 3,5-di ami no-2,6-di methoxy py ri di ne,
1,3-di ami nobenzene, 2,4-di amino-142-
hydroxyethoxy)-benzene, 3-di-(2-hydroxyethyp-amino aniline, 4-amino-l-ethoxy-2-
di- (2-
hydroxyethyl)amino-benzene, 5-methyl-2-(1-methylethyl)phenol, 3-(2-hydroxy-
ethyl)amino
aniline. 3-(2-aminoethyl)amino aniline, 1,3-di-(2,4-diaminophenoxy)propane,
2,4-dimethoxy-
4
CA 03095159 2020-09-24
WO 2019/191198 PCT1US2019/024237
1,3-di ami no-benzene, 2,6-bi s-(2-hydroxy ethy I )am inotoluene, 3-di methyl -
am i nophenol , 5-
amino-2-methyl-phenol, 5-amino-4-fluoro-2-methylphenol, 5-ami no-4-m eth oxy-2-
methyl -
phenol, 5-amino-4-ethoxy-2-methyl-phenol, 3-amino-
2,4-dichl orophenol, 3-
diethylaminophenol, 3-amino-2-chloro-6-methyl-
phenol, 3-aminophenol, 3-
(amidomethyl)aminophenol, 5-(2-hydroxy-ethyl)amino-2-methyl-phenol, 3- (2-
Hydroxyethyl)ami no-phenol, 5-amino-2-ethy I phenol , 5-
(3-hydroxy propyl)ami no-2-
methylphenol, 3-(2,3-dihydroxypropyl)amino-2-methyl-phenol, 3-(2-hydroxy-
ethyl)amino-2-
methylphenol, 5-(2-hydroxyethyl)amino-1,3-benzodioxole, 1,3-dihydroxybenzene,
4-chloro-
1,3-dihydroxybenzene, 1,3-dihydroxy-2-methyl-benzene, 3,4-
methylendioxybenzene, 3,4-
methylendioxyani line, 1-hydroxy-6-bromo-3,4-methylendioxybenzene, 5-amino-4-
chl oro-2-
methylphenol, 3,4-diaminobenzoic acid, 6-hydroxy-
2H-1,4-benzoxazine, 2,7-
dihydroxynaphthalene, 1-naphthol, 1,7-dihydroxynaphthalene, 1,5-
dihydroxynaphthalene, 2,6-
dihydroxy-4-methylpyridine, 2,6-dihydroxypyridine, 2-
methyl- 1-naphthol acetate,
phenyl m ethyl pyrazol one, 2,6-di hy droxy-3,4-
di m et hylpyridine, 4-hy droxyi ndol e, 5,6-
di hydroxyindole, 5-hydroxyindole, 6-hydroxyi ndol e, 7-hydroxyi ndole, 2,3-i
ndol i di one, 2 -
amino-3-hydroxypyrimidine or 4,5,6-dihydroxy-indoline, alone or in combination
with each
other.
The composition may contain one or more of the previously named primary
intermediate
and coupler substances. These dye compounds, in so far as they are bases can
also be used in
the form of their physiologically compatible acid addition salts, for example
as the
hydrochlorides and/or sulfates, or, in so far as they have aromatic OH groups,
in the form of
salts with bases, for example as alkali phenolates.
The oxidation dye pre-cursor compounds are, based on the ready-to-use dye
mixture,
contained in the oxidation dye composition according to the invention in a
total amount of from
0.001 to 20 percent by weight, preferably in a total amount of from 0.01 to 5
percent by weight
of the dye system. The primary intermediate and coupler substances are
preferably used in
equimolar amounts. It is however not disadvantageous when one of these classes
of substances
is present in excess with respect to the other, or vice versa. The primary
intermediate and coupler
substance can be present, for example, in a ratio of from about 1:0.5 to about
1:2.
5
CA 03095159 2020-09-24
WO 2019/191198 PCT1US2019/024237
Oxidizing Agent
The present invention includes an oxidizing agent. Exemplary oxidizing agents
include,
for example, percarbonates, persulfates, organic peracids and organic
hydroperoxides. In certain
circumstances, molecular oxygen (including air) may also be used. According to
the present
invention, a preferred oxidizing agent is hydrogen peroxide. In one
embodiment, the oxidizing
agent may be present at a level of from about 0.75 to about 6 percent by
weight of the dye
system.
Dye Catalyst
The present invention includes at least one metal-containing compound for use
as a
catalyst. The metal-containing compound preferably comprises at least one
inorganic metal
compound. Preferred inorganic metal compounds comprise compounds of d-block
transition
metals such as scandium, titanium, vanadium, chromium, molybdenum, iron,
manganese,
cobalt, nickel, copper, zirconium and zinc including, but not limited to, the
acetates,
acetylacetonates, aluminates, bicarbonates, borates, bromates, carbonates,
chlorites, cyanides,
diethylcitrates, halides, hexafluoroacetylacetonates, hexafluorophosphates,
hexafluorosilicates,
dihydrogen phosphates, hydrogen carbonates, hydrogen sulfates, hydrogen
sulfides, hydrogen
sulfites, hydroxides, hypochlorites, iodates, nitrates, nitrites, oxalates,
oxides,
perfluorophthalocyanines, peroxides, phosphates, phthalocyanines,
pyrophosphates, silicates,
sulfamates, sulfates, sulfides, sulfites, tartrates, tetrafluoroborates,
thiocyanates, thiolates,
thiosulfates, tosylates and triflates of these metals.
Particularly preferred compounds in this context include VBr3, VCI2, VCI3,
VC14, V203,
V204, V205, VO(SO4), V0C13, VOF3, V(C5H702)3, VO(C5H702)2, VO(OR)3,
Mo2(000CH3)4,
Mo(C0)6, MoC13, MoC15, M002C12, MoF6, Mo02, Mo03, MoS2, Mo0C14, MoSO4,
Mn(OCOCR3)2, Mn(OCOCH3)2.xH20, Mn(C5H702)2, MnBr2, MnBrIxH20, MnCO3,
MnCO3.xH20, Mn2(C0)1o, MnC12, MnC12.xH20, M11F2, MnF3, Mn(HCO2)2.xH20, MnI2,
Mn(NO3)2, Mn(NO3)2AH20, Mn304, IvIn203, Mn02, Mn(C32H16N8), MnSO4, MnSO4.x1-
120,
MnS, Fe(OCOCH3)2, Fe(OCOCH3)3, FeBr2, FeBr3, FeCl2, FeC12.xH20, FeCl3,
FeC13.xH20,
Fe(0Et)3, FeSO4.NH3CH2CH2NH3SO4.4H20, Fe4[Fe(CN)6]3, FeF2, FeF3, FeF3.xH20,
FeI2,
Fe(CH3CHOHC00)2.xH20, Fe(NO3)3.xH20, Fe(C204).xH20, FeO, Fe203, Fe304,
FePO4.xH20, Fe(C,32H161\18), FeSO4.xH20, FeS, Fe(BF)4.xH20, Fe(SCN)2,
Co(OCOC,H3)2,
Co(OCOCH3)2.xH20, Co(C5H702)2, Co(C5H702)2.x11.20, Al2Co04, CoBr2, CoBr2.xH20,
CoCO3, CoCO3.x1120. CO2(C0)8, CoCl2, COC12.x1E120, CoF2, Co[C1-13(CFI2)3CH(C21-
15)C042,
Co(OH)2, Cob, Co(NO3)2, Co(NO3)2.xH20, Co(0204), Co(C204).xH20, C0504,
CoSO4.xH20,
6
CA 03095159 2020-09-24
WO 2019/191198 PCT1US2019/024237
Co(BF4)2, Co(BF4)2.XH20, CO(SCN)2, Ni(OCOCI-13)2, Ni(OCOCH3).x1120,
Ni(C5H702)2,
NiBr2, NiBr2.xH20, NiCO3, Ni(CO3).xNi(OH)2, NiC12, NiC12.xH20, Ni0CoO,
Ni[CH3(CH2)3CH(C2H5)CO2]2, Ni F2, Ni(OH)2, N112, Ni(NO3)2, Ni(NO3)2.xH20,
Ni(C204),
Ni(C204).xH20, Ni02, Ni02.xH20, Ni(C32H16N8), Ni(SO3NH2)2, Ni(SO3N1-12)2AH20,
NiSO4,
NiSO4.xH20, Ni3 S2, NiZnFe4 04, CUOCOCH3, CU(OCOCH3)2, CU(OCOCH3)2.X.H20,
Cu(C511702)2, CuBr, CuBr2, CuCO3, CuCO3.Cu(OH)2, CuCl, CuC12, CuC12.xH20,
Cu[CH3(CH2)3CH(C2H5)CO2]2, CuF2, CuF2.xH20, Cu(HCO2)2, Cu(HCO2)2.xH20,
Cu(OH)2,
Cu2(OH)PO4, CuI, CuFe204, Cu(NO3)2, Cu(NO3)2.xH20, Cu2O, CuO, Cu(C32H161\18),
Cu2P207.xH20, CuSO4, CuSO4.xII20, CuS, Cu[02CCH(OH)CH(OH)CO21.xH20, Cu(BF4)2,
Cu(BF4).xH20, Cu(SCN), Zn(OCOCH3)2, Zn(OCOCH3)2.xH20, Zn(C5H702)2,
Zn(C5H702)2AH20, ZnBr2, ZnBr2.xH20, ZnC12, ZnF2, Zn(C32F16N8), Zn(C5HF602)2,
Zn(C5HF602)2.xH20, ZnSiF6.xH20, Zn12, ZnFe204, Zn(NO3)2, Zn(NO3)2.xH20,
Zn(C204),
Zn(C204).xH20, ZnO, ZnO.xH20, Zn02, Zn3(PO4)2, Zn(C321116N8), ZnSO4,
ZnSO4.xH20, ZnS,
Zn(BF4)2, Zn(BF4)2.xH20, Zr(OCOCH3)4, Zr(OCOCH3)x(OH)4-x, Zr(C5H702)4,
Zr(C261144016), ZrCO3(0H2)2.Zr02, ZrCi4, ZrF4, ZrF4.xH2O, Zr(HPO4)2, Zr(OH)4,
Zr14,
ZrO(NO3)2, ZrO(NO3)2.xH20, Zr(SO4)2, Zr(SO4)2.x1120, ZrOC12 and ZrOC12.xIT20.
These
compounds may, for example, be applied in combination with readily available
amino phenolic
compounds, such as p- or m-aminophenol, and oxidizing agents such as hydrogen
peroxide.
Alternative metal compounds for use as catalysts comprise salts of the alkali
metals of
Group 1, such as potassium, or the alkaline earth metals of Group 2, for
example magnesium.
Specific examples of suitable salts include acetates, acetylacetonates,
aluminates, bicarbonates,
borates, bromates, carbonates, chlorites, cyanides, diethylcitrates, halides,
hexafluoroacetylacetonates, hexafluorophosphates, hexafluorosilicates,
dihydrogen phosphates,
hydrogen carbonates, hydrogen sulfates, hydrogen sulfides, hydrogen sulfites,
hydroxides,
hypochlorites, iodates, nitrates, nitrites, oxalates, oxides,
perfluorophthalocyanines, peroxides,
phosphates, phthalocyanines, pyrophosphates, silicates, sulfamates, sulfates,
sulfides, sulfites,
tartrates, tetrafluoroborates, thiocyanates, thiolates, thiosulfates,
tosylates and triflates, such as
KAI(SO4)2 K2CO3, K3PO4, KNO3, KCl, MgSO4, Mg3(PO4)2, MgCO3, Mg(NO3)2 and
MgCl2.
In certain embodiments of the invention wherein the at least one metal-
containing
compound comprises at least one inorganic metal compound, the catalyst may
comprise at least
one mineral or clay. Preferred examples of the minerals or clays include
anatase, brookite,
eudi al yte, ilmenite, perovskite, rutile, sabaite, zircon, zi rconolite,
zircohylite or zirkelite.
7
CA 03095159 2020-09-24
WO 2019/191198 PCT1US2019/024237
When applying the dye systems to hair fibers, it is preferred that the at
least one metal-
containing compound for use as a catalyst comprises at least one metal complex
comprising at
least one organic ligand. It is also preferred that the at least one dye
precursor comprises an
aromatic amino compound, a phenolic compound or an amino phenolic compound
such as p- or
m-aminophenol, and that the oxidizing agent is hydrogen peroxide.
In the context of the present invention, particularly suitable catalysts which
comprise at
least one metal complex comprising at least one organic ligand are metal
chelates, most
particularly zirconium complexes comprising at least one organic ligand.
Typical ligands
include optionally substituted alkyl ligands. A particularly preferred example
of such a catalyst
is aluminum zirconium glycinate (AZG) chelate complex.
AZG is particularly preferred relative to titanium-based metal compounds. Both
zirconium and titanium are known to undergo hydrolytic polymerization (known
as olation) to
form large molecular weight oligomers. In the case of aluminum zirconium
chlorohydrates and
glycinates this reaction does not substantially affect catalytic reactivity
nor does it
fundamentally alter the structure of the material. In contrast organotitanates
are structurally
modified in this environment to form titanium oxide which is catalytically
inactive under these
conditions. Accordingly, in one embodiment, the dye catalyst is free of
titanium. And in a
further embodiment, the compositions of the present invention are free of
titanium.
The dye catalyst may be present at a level of from about 0.0001 to 20% by
weight of the
.. dye system.
Alkalizer
The present invention may also include an alkalizer. Suitable alkalizers
include, for
example, alkanolamines such as aminomethylpropanol (AMP) and monoethanolamine
(MEA).
A particularly preferred alkalizer is ammonia.
Alkalizers are known in the art of dying hair for the purpose of raising the
cuticle of the
hair to facilitate delivery of dye compounds below the cuticle. While efforts
have been
undertaken to avoid ammonia, primarily, due to its smell and perception of
hair damage, it has
been found that the combination of an alkalizer with the catalyst, herein,
increases the rate of
reaction and may therefore reduce hair damage.
It has also been found that the present invention functions advantageously at
a pH of
about 9 or greater. Therefore, the alkalizer herein may be used as a pH
adjuster, to facilitate a
pH at or above about 9. Preferably, the pH of the present invention is from
about 9 to about 10.
8
Preferably, the alkalizer may be present at a level of from about 0.1 to 25
percent by
weight of the dye system.
FIG. I shows the rates of reaction of the dye system herein at varying pH
levels, The
self-coupling reaction of m-aminophenol (3.0 mmol) mediated by hydrogen
peroxide (5 eq) in
water (20 mL) was monitored by UV-vis spectroscopy at various pH values
(adjusted with RC'
or NRIGH) in the presence of 5 mol % aluminum zirconium pentachlorohydrex
(Rezal 67,
SummitReheis). The rates of absorption are directly proportional to the pH of
the dye system,
including the catalyst herein. For example, at pH 8.97 (about pH 91), the rate
of absorption is
more than double the rate of absorption at pH levels below 6.61. Therefore,
the catalyst herein
.. operates synergistically with the pH level to determine the rate of
absorption. Accordingly, at
a pH level at about 9 or above, the dyeing process occurs faster than at pH
levels below for
example, 6.61,
Thickening Agents
Thickening agents, including thickener or gelling agents, 'include substances
which can
increase or control the viscosity of a composition. Thickeners include those
that can increase
the viscosity of a composition without substantially modifying the efficacy of
the active
ingredients within the composition. Thickeners can also increase the stability
of compositions.
Non-lin)iting examples of thickening agents that can be used in the context of
the present
invention include carboxylic acid polymers, crosslinked polyacrylate polymers,
polyacrylamide
.. polymers, polysaccharides, and gums. Examples of carboxylic acid polymers
include
crosslinked compounds containing one or more monomers derived from acrylic
acid, substituted
acrylic acids, and salts and esters of these acrylic acids and the substituted
acrylic acids, wherein
the crosslinking agent contains two or more carbon-carbon double bonds and is
derived from a
polyhydric alcohol (see U.S. Pat. Nos. 5,087,445; 4,509,949; 2,798,053; CTFA
International
Cosmetic Ingredient Dictionary, Fourth edition, 1991, pp. 12 and 80). Examples
of
commercially available carboxylic acid polymers include carbomers, which are
homopolymers
of acrylic acid crosslinked with ally! ethers of sucrose or pentaerytritol
(e.g., CarbopolTM 900
series from B. F. GoodrichTm).
Non-limiting examples of crosslinked polya,crylate polymers include cationic
and
nonionic polymers. Examples are described in U.S. Pat. Nos. 5,100,660;
4,849,484; 4,835,206;
4,628,078; 4,599,379.
Non-limiting examples of polya.crylami de polymers (including, nonionic
polyacrylamide
polymers including substituted branched or unbranched polymers) include
polyacrylamide,
9
Date Recue/Date Received 2022-03-16
CA 03095159 2020-09-24
WO 2019/191198 PCT1US2019/024237
i soparaffi n and laureth-7, multi -block copolymers of ac ry I am i des and
substituted acry I ami des
with acrylic acids and substituted acrylic acids.
Non-limiting examples of polysaccharides include cellulose, carboxymethyl
hydroxyethylcellulose, cellulose acetate propionate carboxylate,
hydroxyethylcellulose,
hydroxyethyl ethylcellulose, hydroxypropylcellulose, hydroxypropyl
methylcellulose, methyl
hydroxyethylcellulose, microcrystalline cellulose, sodium cellulose sulfate,
and mixtures
thereof Another example is an alkyl substituted cellulose where the hydroxy
groups of the
cellulose polymer are hydroxyalkylated (preferably hydroxyethylated or
hydroxypropylated) to
form a hydroxyalkylated cellulose, which is then further modified with a C io-
Cm straight chain
or branched chain alkyl group through an ether linkage. Other useful
polysaccharides include
scleroglucans comprising a linear chain of (1-3) linked glucose units with a(1-
6) linked glucose
every three unit
Non-limiting examples of gums that can be used with the present invention
include
acacia, agar, algin, alginic acid, ammonium alginate, amylopectin, calcium
alginate, calcium
carrageenan, carnitine, carrageenan, chitosan, dextrin, gelatin, gellan gum,
guar gum, guar
hydroxypropyltri moni urn chloride, hectorite, hyaluroinic acid, hydrated
silica, hydroxypropyl
chitosan, hydroxypropyl guar, karaya gum, kelp, locust bean gum, natto gum,
potassium
alginate, potassium carrageenan, propylene glycol alginate, sclerotium gum,
sodium
carboxymethyl dextran, sodium carrageenan, tragacanth gum, xanthan gum, and
mixtures
thereof.
Further non-limiting examples of thickening agents include carbomer, cetyl
alcohol, ammonium
acryloydimethyltaurate/VP copolymer, aluminum starch actenylsuccinate,
cocamidopropyl betaine,
PPG-2 hydroxyethyl coco/isostearamide, tin oxide, hexadecane copolymer,
calcium aluminum
borosilicate, alumina, calcium sodium borosilicate, aluminum calcium sodium
silicate, synthetic
fluorphlogopite, dipropylene glycol, polyethylene glycol. quaternium-90
bentonite. kaolin, and disodium
EDTA.
NON-LIMITING EXAMPLES
The following Examples illustrate specific embodiments of the compositions of
the
present invention, but are not intended to be limiting thereof. Other
modifications can be
undertaken by the skilled artisan without departing from the spirit and scope
of this invention.
The compositions illustrated in the following Examples are prepared according
to the
methods described hereinafter. All exemplified amounts are listed as weight
percents and
exclude minor materials such as diluents, preservatives, color solutions,
imagery ingredients,
botanicals, and so forth, unless otherwise specified.
The following are representative of dye system of the present invention:
1 2 3 4 5 6 7 8
Phase A POI'
1 1 iii0;:::,'.!:;.411070.11111111511M1101113
Water q.s. q.s. q.s. q.s. q.s. q.s.
q.s.
Catalyse - 2.24 2.24 2.24 2.24 2.24 2.24
2.24
Jaguarm S - - - - - , - - 1.00
Butylene glycol - - - - - 5.00 - 5.00
Decyl glucoside 5.00 5.00 5.00 5.00 ' 5.00 -
5.00
Glycerine 2.00 2.00 2.00 2.00 2.00 5.00
2.00 2.00
Lauramidopropyl betaine - - - - - 2.00 - -
Erhythorbic acid 0.2 0.2 0.2 0.2 0.2 0.2 0.2
0.50
Tetrasodium EDTA 0.40 0.40 0.40 0.40 0.40 4.2 0.40
1.00
Sodium sulfite 0.20 0.20 0.20 0.20 0.20 0.20
0.20 0.50
Arginine - - - - - 2.00 - -
p-aminophenol 0.20 0.20 0.20 0.20 - - - 0.50
4-amino-2-hydroxytoluene 0.22 0.22 0.22 0.22 1.50 1.50 -
-
2,4-diaminophenoxy ethanol - - - - 1.46 .1.46 0.10
-
LI-amino-in-cresol - - - - - - 0.30 -
. -
p-aminophenol - - - - - - 0.50
rn-aminophenol - - - - - - 0.60 0.25
=
tetraaminopyrimidine - - . - - - 0.10 -
sulfate
Resorcinol - - - - - - 0.50 -
Phase B
1111111113181001110.1Mearini
Peg-40 hydrogenated - - - - - 3.00 - -
castor oil
Cocamide MEA 5.00 5.00 5.00 5.00 10.00 - -
-
Glyceryl Stearate/PEG-100 - - - - - 1.50 - -
Stearate2 ,
Glyceryl Stearate ' 4.00 4.00 4.00 4.00 ' 4.00 - -
-
Cetearyl alcohol 2.50 2.50 2.50 ' 2.50 2.50 4.00
- -
Steareth-21 2.50 2.50 2.50 2.50 3.00 - -
ii
Date Recue/Date Received 2022-03-16
Candellila wax 2.00 2.00 2.00 2.00 2.00
Oleic acid 1.00 1.00 1.00 1.00 1.00 9.00
Sorbi tan ol eate 3.00
Sunflower oil 2.00
Phase C
Ammonium hydroxide 6.9 0.9 6.9 6.9 4.5 6.4
Amin om ethyl propanol 8.7 8.5
'Aluminum Zirconium Glycinate, available from SummitReheis
SP ARLACEL 165-MBAL-PW-(AP), available from CrodaTm
Methods for Forming the Dye System
The components of the dye system may be combined according to a variety of
conditions. Exemplary methods for forming the dye system are described
hereinafter.
For the purpose of comparison, Example 1, provided above, illustrates a dye
composition
which is absent of the catalyst of the present invention. This formula may be
prepared according
to the following method.
Method for formulating a dye system without a catalyst
Example 1 is formed according to the following method. Phase A is prepared by
adding
the water, decyl glucoside, and glycerine to a beaker with moderate stirring
using a propeller.
Once homogeneous, the erythorbic acid, tetrasodium EDTA, and sodium sulfite
are added. The
.. mixture is then heated to 80 C. At approximately 70 C the p-aminophenol
and 4-amino-2-
hydroxytoluene are added with an increase in stirring rate to aid dissolution.
Phase B is prepared by weighing the components into a single beaker and
heating to 80
C on a hot plate.
Once both phases have reached 80 C they are combined with one another and the
heat
.. is removed, allowing this system to cool.
Once the mixture has cooled to 35 C the ammonium hydroxide of Phase C is
combined
with the mixture of Phases A and B, with vigorous mixing, until a thickened
homogeneous
emulsion is obtained. The final emulsion is then transferred to suitable
containers for later use.
Methods for forming a dye system including a catalyst
Examples 2-8, herein above, are illustrative of dye systems which include a
catalyst
according to the present invention. Various methods for formulating such dye
systems are
provided hereinafter.
12
Date Recue/Date Received 2022-03-16
CA 03095159 2020-09-24
WO 2019/191198 PCT1US2019/024237
Method I
Example 2 is prepared according to the following method. Phase A is prepared
by
adding the water, decyl glucoside, and glycerine to a beaker with moderate
stirring using a
propeller. Once homogeneous, the erythorbic acid, tetrasodium EDTA, and sodium
sulfite are
added. The mixture is then heated to 80 C. At approximately 70 C the p-
aminophenol and 4-
amino-2-hydroxytoluene are added with an increase in stirring rate to aid
dissolution.
Phase B is prepared by weighing the components into a single beaker and
heating to 80
C on a hot plate.
Once both phases have reached 80 C they are combined and the heat is removed,
allowing this system to cool.
In this example, once the mixture has cooled to 40 C the AZG catalyst is
added to the
mixture. After the mixture has further cooled to 35 C the ammonium hydroxide
of Phase C is
combined with the mixture of Phases A and B, with vigorous mixing, until a
thickened
homogeneous emulsion is obtained. The final emulsion is then transferred to
suitable containers
for later use.
Method 11
Example 3 is prepared according to the following method. Phase A is prepared
by
adding the water, decyl glucoside, and glycerine to a beaker with moderate
stirring using a
propeller. Once homogeneous, the erythorbic acid, tetrasodium EDTA, and sodium
sulfite are
added. The mixture is then heated to 80 C. In this example, at approximately
70 C the AZG
catalyst is added to the aqueous phase. This is accompanied by a change in
haziness of the liquid.
Once homogeneous the p-aminophenol and 4-amino-2-hydroxytoluene are added with
an
increase in stirring rate to aid dissolution.
Phase B is prepared by weighing the components into a single beaker and
heating to 80
C on a hot plate.
Once both phases have reached 80 C they are combined and the heat is removed,
allowing this system to cool.
Once the mixture has cooled to 35 C the ammonium hydroxide of Phase C is
combined
with the mixture of Phases A and B, with vigorous mixing, until a thickened
homogeneous
emulsion is obtained. The final emulsion is then transferred to suitable
containers for later use.
Method III
Example 4 is prepared according to the following method. Phase A is prepared
by
adding the water, decyl glucoside, and glycerine to a beaker with moderate
stirring using a
13
CA 03095159 2020-09-24
WO 2019/191198 PCT1US2019/024237
propeller. Once homogeneous, the erythorbic acid, tetrasodium EDTA, and sodium
sulfite are
added The mixture is then heated to 80 C. At approximately 70 C the p-
aminophenol and 4-
amino-2-hydroxytoluene are added with an increase in stirring rate to aid
dissolution.
Phase B is prepared by weighing the components into a single beaker and
heating to 80
C on a hot plate.
Once both phases have reached 80 C they are combined and the heat is removed,
allowing this system to cool.
Once the mixture has cooled to 35 C the ammonium hydroxide of Phase C is
combined
with the mixture of Phases A and B, with vigorous mixing, until a thickened
homogeneous
emulsion is obtained. Finally, the AZG catalyst is added slowly to the
thickened emulsion. Once
addition is complete and homogeneous the final emulsion is then transferred to
suitable
containers for later use.
Examples 2-4 are identical to one another regarding their overall ingredient
list, but are
formulated according to the discrete methods discussed hereinabove. Examples 5-
8 are
provided for further illustrative purposes, and may be prepared according to
any of the methods
described herein. Each of examples 2-4 differ from Example 1 in that Example 1
does not
include a catalyst, and therefore is also formulated according to a different
method. FIG. 2
illustrates the impact of addition of the catalyst to the base formula on its
viscosity. According
to formulation Methods I and 11, the addition of the catalyst increases the
overall viscosity of
the dye system. These methods result in enhanced spreadability and even
distribution of the
catalyst system herein. However, the technique described in Method III fails
to achieve the
viscosity benefits of Methods I and II.
Therefore, it has been found that addition of an alkalizer, such as NH3,
before the catalyst
is introduced, results in uneven distribution of the catalyst.
FIGS. 3A-3C show the formula of Example 1, under increasing magnification (4x,
20x,
and 10x, respectively), 24 hours after formulation. The orientation of the dye
structure is
believed to correlate to the viscosity profile of Example 1 illustrated in
FIG. 3. As the dye
system thickens, the structure becomes increasingly dense.
FIGS. 4A-4C show the formula of Example 2, when formulated according to Method
I
herein, under increasing magnification (4x, 20x, and 10x, respectively), 24
hours after
formulation. As is illustrated in these depictions, the dye system is more
dense, with an even
distribution of the catalyst.
14
FIGS. 5A-5C show the formula of Example 3, herein, under increasing
magnification
(4x, 20x, and 10x, respectively), 24 hours after formulation. As is
illustrated in these depictions,
the dye system formulated according to Method II results in a dense
consistency.
FIGS. 6A-6C show the formula of EXample 4, under increasing magnification (4x,
20x,
and 1.0x, respectively), 24 hours after formulation. As is illustrated in
these depictions, the the
system formulated according to Method 111. results in a structure that
generally conforms to the
dye system depicted in:FIGS. 3A-3C. Therefore, no thickening benefit results
from application
of Method HI.
FIGs. 7A, 7B, and 8B show the dye system having a homogeneous consistency.
FIC.is
7A and 7B particularly show a thickened system, containing a homogeneous
distribution of the
catalyst herein.
In contrast, FIG. 8A shoWs a heterogeneous system, with interspersed clusters
of the
catalyst herein. This system results in an uneven distribution of the
catalyst, creating catalytic
"hot zones" with the system, which is unsuitable for controlled application of
the catalyst system
to hair. This further demonstrates the drawbacks of alkalizing the system
before addition of the
catalyst, as described by Method UI herein.
Furthermore, in view of the enhanced rate of reaction achieved via the
formulas herein,
known- sensitizers such as parapheriyienediamine (PPD) and paratoluenediamine
(PTD) are not
essential to the dye systems herein, Therefore ; in one embodiment, the dye
system herein is free
from PPD or PTD-
The dimensions and values disclosed herein are not to be understood as being
strictly
limited to the exact numerical values recited. Instead, unless otherwise
specified, each such
dimension is intended to mean both the recited value and a functionally
equivalent range
surrounding that value. For example, a dimension disclosed as "40 mm" is
intended to mean
"about 40 mm."
The citation of any document is not an admission that it is prior art with
respect to any
invention disclosed or claimed herein or that it alone, or in any combination
with any other
reference or references, teaches, suggests or discloses any such invention.
Further, to the
extent that any meaning or definition of a term in this document conflicts
with any meaning or
definition of the same term in a document incorporated by reference, the
meaning or
definition assigned to that term in this document shall govern.
Date Recue/Date Received 2022-03-16
CA 03095159 2020-09-24
WO 2019/191198 PCT1US2019/024237
While particular embodiments of the present invention have been illustrated
and
described, it would be obvious to those skilled in the art that various other
changes and
modifications can be made without departing from the spirit and scope of the
invention It is
therefore intended to cover in the appended claims all such changes and
modifications that are
within the scope of this invention.
16