Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
1
PERCUTANEOUS URINARY CATHETER
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority from United States Patent Application
No.
16/206,207, filed November 30, 2018, which is a continuation-in-part of United
States Patent
Application No. 15/879,770 filed January 25, 2018, which is a continuation-in-
part of United
States Patent Application No. 15/687,064 filed August 25, 2017, which is a
continuation-in-
part of United States Patent Application No. 15/411,884 filed January 20,
2017, which is a
continuation-in-part of United States Patent Application No. 15/214,955 filed
July 20, 2016,
which claims the benefit of United States Provisional Application No.
62/300,025 filed
February 25, 2016, United States Provisional Application No. 62/278,721, filed
January 14,
2016, United States Provisional Application No. 62/260,966 filed November 30,
2015, and
United States Provisional Application No. 62/194,585, filed July 20, 2015,
each of which is
incorporated by reference herein in their entireties.
[0002] Also, United States Patent Application No. 15/879,770 filed January 25,
2018 is a
continuation-in-part of United States Patent Application No. 15/687,083 filed
August 25,
2017, which is a continuation-in-part of United States Patent Application No.
15/411,884
filed January 20, 2017, which is a continuation-in-part of United States
Patent Application
No. 15/214,955 filed July 20, 2016, which claims the benefit of United States
Provisional
Application No. 62/300,025 filed February 25, 2016, United States Provisional
Application
No. 62/278,721, filed January 14, 2016, United States Provisional Application
No.
62/260,966 filed November 30, 2015, and United States Provisional Application
No.
62/194,585, filed July 20, 2015, each of which is incorporated by reference
herein in its
entirety.
[0003] Also, United States Patent Application No. 15/879,770 filed January 25,
2018 is a
continuation-in-part of United States Patent Application No. 15/745,823 filed
January 18,
2018, which is the U.S. national phase of PCT/U52016/043101, filed July 20,
2016, which
claims the benefit of United States Provisional Application No. 62/300,025
filed February 25,
2016, United States Provisional Application No. 62/278,721, filed January 14,
2016, United
States Provisional Application No. 62/260,966 filed November 30, 2015, and
United States
Provisional Application No. 62/194,585, filed July 20, 2015, each of which is
incorporated by
reference herein in its entirety.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
2
[0004] Also, United States Patent Application No. 15/879,770 filed January 25,
2018
claims the benefit of United States Provisional Application No. 62/489,789
filed April 25,
2017 and United States Provisional Application No. 62/489,831 filed April 25,
2017.
BACKGROUND
Technical Field
[0005] The present disclosure relates to devices and methods for treating
impaired renal
function across a variety of disease states and, in particular, to devices and
methods for
collection of urine and inducement of negative and/or positive pressure in
portions of a
patient's urinary tract through a percutaneously implanted catheter.
Background
[0006] The renal or urinary system includes a pair of kidneys, each kidney
being connected
by a ureter to the bladder, and a urethra for draining fluid or urine produced
by the kidneys
from the bladder. The kidneys perform several vital functions for the human
body including,
for example, filtering the blood to eliminate waste in the form of urine. The
kidneys also
regulate electrolytes (e.g., sodium, potassium and calcium) and metabolites,
blood volume,
blood pressure, blood pH, fluid volume, production of red blood cells, and
bone metabolism.
Adequate understanding of the anatomy and physiology of the kidneys is useful
for
understanding the impact that altered hemodynamics other fluid overload
conditions have on
their function.
[0007] In normal anatomy, the two kidneys are located retroperitoneally in the
abdominal
cavity. The kidneys are bean-shaped encapsulated organs. Urine is formed by
nephrons, the
functional unit of the kidney, and then flows through a system of converging
tubules called
collecting ducts. The collecting ducts join together to form minor calyces,
then major
calyces, which ultimately join near the concave portion of the kidney (renal
pelvis). A major
function of the renal pelvis is to direct urine flow to the ureter. Urine
flows from the renal
pelvis into the ureter, a tube-like structure that carries the urine from the
kidneys into the
bladder. The outer layer of the kidney is called the cortex, and is a rigid
fibrous
encapsulation. The interior of the kidney is called the medulla. The medulla
structures are
arranged in pyramids.
[0008] Each kidney is made up of approximately one million nephrons. Each
nephron
includes the glomerulus, Bowman's capsule, and tubules. The tubules include
the proximal
convoluted tubule, the loop of Henle, the distal convoluted tubule, and the
collecting duct.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
3
The nephrons contained in the cortex layer of the kidney are distinct from the
anatomy of
those contained in the medulla. The principal difference is the length of the
loop of Henle.
Medullary nephrons contain a longer loop of Henle, which, under normal
circumstances,
allows greater regulation of water and sodium reabsorption than in the cortex
nephrons.
[0009] The glomerulus is the beginning of the nephron, and is responsible for
the initial
filtration of blood. Afferent arterioles pass blood into the glomerular
capillaries, where
hydrostatic pressure pushes water and solutes into Bowman's capsule. Net
filtration pressure
is expressed as the hydrostatic pressure in the afferent arteriole minus the
hydrostatic pressure
in Bowman's space minus the osmotic pressure in the efferent arteriole.
Net Filtration Pressure = Hydrostatic Pressure (Afferent
Arteriole) - Hydrostatic Pressure (Bowman's Space) - Osmotic
Pressure (Efferent Arteriole) (Equation 1)
[0010] The magnitude of this net filtration pressure defined by Equation 1
determines how
much ultra-filtrate is formed in Bowman's space and delivered to the tubules.
The remaining
blood exits the glomerulus via the efferent arteriole. Normal glomerular
filtration, or delivery
of ultra-filtrate into the tubules, is about 90 ml/min/1.73m2.
[0011] The glomerulus has a three-layer filtration structure, which includes
the vascular
endothelium, a glomerular basement membrane, and podocytes. Normally, large
proteins
such as albumin and red blood cells, are not filtered into Bowman's space.
However,
elevated glomerular pressures and mesangial expansion create surface area
changes on the
basement membrane and larger fenestrations between the podocytes allowing
larger proteins
to pass into Bowman's space.
[0012] Ultra-filtrate collected in Bowman's space is delivered first to the
proximal
convoluted tubule. Re-absorption and secretion of water and solutes in the
tubules is
performed by a mix of active transport channels and passive pressure
gradients. The
proximal convoluted tubules normally reabsorb a majority of the sodium
chloride and water,
and nearly all glucose and amino acids that were filtered by the glomerulus.
The loop of
Henle has two components that are designed to concentrate wastes in the urine.
The
descending limb is highly water permeable and reabsorbs most of the remaining
water. The
ascending limb reabsorbs 25% of the remaining sodium chloride, creating a
concentrated
urine, for example, in terms of urea and creatinine. The distal convoluted
tubule normally
reabsorbs a small proportion of sodium chloride, and the osmotic gradient
creates conditions
for the water to follow.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
4
[0013] Under normal conditions, there is a net filtration of approximately 14
mmHg. The
impact of venous congestion can be a significant decrease in net filtration,
down to
approximately 4 mmHg. See Jessup M., The cardio renal syndrome: Do we need a
change of
strategy or a change of tactics?, JACC 53(7):597-600, 2009 (hereinafter
"Jessup"). The
second filtration stage occurs at the proximal tubules. Most of the secretion
and absorption
from urine occurs in tubules in the medullary nephrons. Active transport of
sodium from the
tubule into the interstitial space initiates this process. However, the
hydrostatic forces
dominate the net exchange of solutes and water. Under normal circumstances, it
is believed
that 75% of the sodium is reabsorbed back into lymphatic or venous
circulation. However,
because the kidney is encapsulated, it is sensitive to changes in hydrostatic
pressures from
both venous and lymphatic congestion. During venous congestion the retention
of sodium
and water can exceed 85%, further perpetuating the renal congestion. See
Verbrugge et al.,
The kidney in congestive heart failure: Are natriuresis, sodium, and diruetucs
really the
good, the bad and the ugly? European Journal of Heart Failure 2014:16,133-42
(hereinafter
"Verbrugge").
[0014] Venous congestion can lead to a prerenal form of acute kidney injury
(AM). Prerenal AM is due to a loss of perfusion (or loss of blood flow)
through the
kidney. Many clinicians focus on the lack of flow into the kidney due to
shock. However,
there is also evidence that a lack of blood flow out of the organ due to
venous congestion can
be a clinically important sustaining injury. See Damman K, Importance of
venous congestion
for worsening renal function in advanced decompensated heart failure, JACC
17:589-96,
2009 (hereinafter "Damman").
[0015] Prerenal AKI occurs across a wide variety of diagnoses requiring
critical care
admissions. The most prominent admissions are for sepsis and Acute
Decompensated Heart
Failure (ADHF). Additional admissions include cardiovascular surgery, general
surgery,
cirrhosis, trauma, burns, and pancreatitis. While there is wide clinical
variability in the
presentation of these disease states, a common denominator is an elevated
central venous
pressure. In the case of ADHF, the elevated central venous pressure caused by
heart failure
leads to pulmonary edema, and, subsequently, dyspnea in turn precipitating the
admission. In
the case of sepsis, the elevated central venous pressure is largely a result
of aggressive fluid
resuscitation. Whether the primary insult was low perfusion due to hypovolemia
or sodium
and fluid retention, the sustaining injury is the venous congestion resulting
in inadequate
perfusion.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
[0016] Hypertension is another widely recognized state that creates
perturbations within
the active and passive transport systems of the kidney(s). Hypertension
directly impacts
afferent arteriole pressure and results in a proportional increase in net
filtration pressure
within the glomerulus. The increased filtration fraction also elevates the
peritubular capillary
pressure, which stimulates sodium and water re-absorption. See Verbrugge.
[0017] Because the kidney is an encapsulated organ, it is sensitive to
pressure changes in
the medullary pyramids. The elevated renal venous pressure creates congestion
that leads to
a rise in the interstitial pressures. The elevated interstitial pressures
exert forces upon both
the glomerulus and tubules. See Verbrugge. In the glomerulus, the elevated
interstitial
pressures directly oppose filtration. The increased pressures increase the
interstitial fluid,
thereby increasing the hydrostatic pressures in the interstitial fluid and
peritubular capillaries
in the medulla of the kidney. In both instances, hypoxia can ensue leading to
cellular injury
and further loss of perfusion. The net result is a further exacerbation of the
sodium and water
re-absorption creating a negative feedback. See Verbrugge, 133-42. Fluid
overload,
particularly in the abdominal cavity is associated with many diseases and
conditions,
including elevated intra-abdominal pressure, abdominal compartment syndrome,
and acute
renal failure. Fluid overload can be addressed through renal replacement
therapy. See Peters,
C.D., Short and Long-Term Effects of the Angiotensin II Receptor Blocker
Irbesartanon
Intradialytic Central Hemodynamics: A Randomized Double-Blind Placebo-
Controlled One-
Year Intervention Trial (the SAFIR Study), PLoS ONE (2015) 10(6): e0126882.
doi:10.1371/journal.pone.0126882 (hereinafter "Peters"). However, such a
clinical strategy
provides no improvement in renal function for patients with the cardiorenal
syndrome. See
Bart B, Ultrafiltration in decompensated heart failure with cardiorenal
syndrome, NEJM
2012;367:2296-2304 (hereinafter "Bart"). In view of such problematic effects
of fluid
retention, systems and methods for improving removal of fluid such as urine
from the patient
and, specifically for increasing quantity and quality of fluid output from the
kidneys, are
needed.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
6
SUMMARY
[0018] The present disclosure improves upon previous systems by providing a
specialized
catheter for percutaneous insertion and for deployment within a renal pelvis
and/or kidney of
a patient.
[0019] According to some examples, a catheter configured to be deployed in a
urinary tract
of a patient includes a proximal portion configured to pass through a
percutaneous opening;
and a distal portion including a retention portion configured to be deployed
in a kidney, renal
pelvis, and/or bladder of the patient. The retention portion includes one or
more protected
drainage holes, ports or perforations and is configured, when deployed, to
establish an outer
periphery or protective surface area that inhibits mucosal tissue from
occluding the one or
more protected drainage holes, ports, or perforations upon application of
negative pressure
through the catheter.
[0020] According to some other examples, a system for inducing negative
pressure in a
portion of a urinary tract of a patient includes a catheter configured to be
deployed within a
portion of the urinary tract of the patient. The catheter includes a proximal
portion
configured to pass through a percutaneous opening and a distal portion
including a retention
portion configured to be deployed in a kidney, renal pelvis, and/or bladder of
the patient. The
retention portion includes one or more protected drainage holes, ports or
perforations and is
configured, when deployed, to establish an outer periphery or protective
surface area that
inhibits mucosal tissue from occluding the one or more protected drainage
holes, ports, or
perforations upon application of negative pressure through the catheter. The
system also
includes a pump external to the patient's body for application of the negative
pressure at the
proximal portion of the catheter. The pump induces negative pressure to a
portion of the
urinary tract, which causes fluid from the urinary tract to be drawn into the
catheter at least
partially through the one or more protected drainage holes, ports, or
perforations.
[0021] According to some other examples, a method for removing fluid from a
urinary
tract of a patient includes inserting a urinary catheter through a
percutaneous opening and
into a kidney, renal pelvis, and/or bladder of the patient and deploying a
retention portion of
the catheter within the kidney, renal pelvis, and/or bladder of the patient to
maintain patency
of fluid flow from the kidney of the patient through at least a portion of the
catheter. The
catheter includes a proximal portion configured to pass through the
percutaneous opening and
a distal portion including the retention portion configured to be deployed in
the kidney, renal
pelvis, and/or bladder of the patient. The retention portion includes one or
more protected
drainage holes, ports or perforations and is configured, when deployed, to
establish an outer
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
7
periphery or protective surface area that inhibits mucosal tissue from
occluding the one or
more protected drainage holes, ports, or perforations upon application of
negative pressure
through the catheter.
[0022] Non-limiting examples, aspects or embodiments of the present invention
will now
be described in the following numbered clauses:
[0023] Clause 1: A catheter configured to be deployed in a urinary tract of a
patient,
comprising: a proximal portion configured to pass through a percutaneous
opening; and a
distal portion comprising a retention portion configured to be deployed in a
kidney, renal
pelvis, and/or bladder of the patient, wherein the retention portion comprises
one or more
protected drainage holes, ports or perforations and is configured, when
deployed, to establish
an outer periphery or protective surface area that inhibits mucosal tissue
from occluding the
one or more protected drainage holes, ports, or perforations upon application
of negative
pressure through the catheter.
[0024] Clause 2: The catheter of clause 1, wherein the catheter is configured
to transition
between a contracted configuration, in which the catheter can pass through the
percutaneous
opening, and a deployed configuration, in which the retention portion is
configured to retain
at least the distal portion of the catheter within the kidneys, renal pelvis,
and/or bladder of the
patient.
[0025] Clause 3: The catheter of clause 1 or clause 2, wherein, when deployed,
a
maximum outer diameter of the retention portion is greater than a diameter of
a drainage
lumen of the catheter.
[0026] Clause 4: The catheter of any of clauses 1-3, wherein the retention
portion
comprises an expandable retention portion which, when deployed, defines a
three-
dimensional shape sized and positioned to maintain patency of fluid flow
between a kidney
and a proximal end of the catheter, such that at least a portion of the fluid
flow flows through
the expandable retention portion.
[0027] Clause 5: The catheter of clause 4, wherein an area of two-dimensional
slices of
the three-dimensional shape defined by the deployed expandable retention
portion in a plane
transverse to a central axis of the expandable retention portion decreases
towards a distal end
of the expandable retention portion.
[0028] Clause 6: The catheter of clause 4 or clause 5, wherein a maximum cross-
sectional
area of the three-dimensional shape defined by the deployed expandable
retention portion in a
plane transverse to a central axis of the expandable retention portion is less
than or equal to
about 500 mm2.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
8
[0029] Clause 7: The catheter of any of clauses 1-6, wherein the retention
portion
comprises a proximal end sized to be positioned in a kidney and a distal end
sized to be
positioned in the renal pelvis.
[0030] Clause 8: The catheter of any of clauses 1-7, wherein the retention
portion
comprises a coiled retention portion comprising at least a first coil having a
first diameter and
at least a second coil having a second diameter, the first diameter being
greater than the
second diameter.
[0031] Clause 9: The catheter of clause 8, wherein the first coil is closer to
the proximal
portion of the catheter than is the second coil.
[0032] Clause 10: The catheter of any of clauses 1-8, wherein the retention
portion
comprises a coiled retention portion comprising a plurality of coils, and
wherein a distal-most
coil of the plurality of coils has a smaller diameter than other coils of the
plurality of coils.
[0033] Clause 11: The catheter of clause 10, wherein the coiled retention
portion
comprises a straight portion extending through the retention portion, and
wherein the
plurality of coils are wrapped around the straight portion.
[0034] Clause 12: The catheter of any of clauses 1-11, wherein the retention
portion is
coextensive with other portions of the catheter.
[0035] Clause 13: The catheter of any of clauses 1-12, wherein an axial length
of the
retention portion from a proximal end to a distal end thereof is from about 5
mm to about 100
mm.
[0036] Clause 14: The ureteral catheter of any of clauses 1-13, wherein the
one or more
protected drainage holes, ports, or perforations have a diameter ranging from
0.0005 mm to
about 2.0 mm.
[0037] Clause 15: The catheter of any of clauses 1-14, wherein the catheter
comprises an
elongated tube extending from a proximal end of the proximal portion to a
distal end of the
distal portion.
[0038] Clause 16: The catheter of clause 15, wherein the elongated tube is
from about 30
cm to about 60 cm in length.
[0039] Clause 17: The catheter of clause 15 or clause 16, wherein the
elongated tube has
an outer diameter of from about 1.0 mm to about 10.0 mm, and/or an inner
diameter of about
0.5 mm to about 9.5 mm.
[0040] Clause 18: The catheter of any of clauses 1-17, wherein a proximal end
of the
proximal portion of the catheter is configured to be connected to a pump for
applying the
negative pressure through the catheter.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
9
[0041] Clause 19: The catheter of any of clauses 1-18, wherein the proximal
portion is
essentially free of or free of perforations and/or drainage ports.
[0042] Clause 20: A system for inducing negative pressure in a portion of a
urinary tract
of a patient, the system comprising: a catheter configured to be deployed
within a portion of
the urinary tract of the patient, comprising: a proximal portion configured to
pass through a
percutaneous opening; and a distal portion comprising a retention portion
configured to be
deployed in a kidney, renal pelvis, and/or bladder of the patient, wherein the
retention portion
comprises one or more protected drainage holes, ports or perforations and is
configured,
when deployed, to establish an outer periphery or protective surface area that
inhibits
mucosal tissue from occluding the one or more protected drainage holes, ports,
or
perforations upon application of negative pressure through the catheter; and a
pump external
to the patient's body for application of the negative pressure at the proximal
portion of the
catheter, wherein the pump induces negative pressure to a portion of the
urinary tract, which
causes fluid from the urinary tract to be drawn into the catheter at least
partially through the
one or more protected drainage holes, ports, or perforations.
[0043] Clause 21: The system of clause 20 further comprising a controller
electrically
connected to the pump configured to actuate the pump to control the
application of the
negative pressure to the proximal end of the catheter.
[0044] Clause 22: The system of clause 21, further comprising one or more
physiological
sensors associated with the patient, the physiological sensors being
configured to provide
information representative of at least one physical parameter to the
controller, and wherein
the controller is configured to actuate or cease operation of the pump based
on the at least one
physical parameter.
[0045] Clause 23: The system of any of clauses 20-22, wherein the negative
pressure is
provided within a range of about 2 mmHg to about 50 mmHg.
[0046] Clause 24: The system of any of clauses 20-23, wherein the pump
provides a
sensitivity of about 10 mmHg or less.
[0047] Clause 25: A method for removing fluid from a urinary tract of a
patient, the
method comprising: inserting a urinary catheter through a percutaneous opening
and into a
kidney, renal pelvis, and/or bladder of the patient; and deploying a retention
portion of the
catheter within the kidney, renal pelvis, and/or bladder of the patient to
maintain patency of
fluid flow from the kidney of the patient through at least a portion of the
catheter, wherein the
catheter comprises a proximal portion configured to pass through the
percutaneous opening;
and a distal portion comprising the retention portion configured to be
deployed in the kidney,
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
renal pelvis, and/or bladder of the patient, and wherein the retention portion
comprises one or
more protected drainage holes, ports or perforations and is configured, when
deployed, to
establish an outer periphery or protective surface area that inhibits mucosal
tissue from
occluding the one or more protected drainage holes, ports, or perforations
upon application of
negative pressure through the catheter.
[0048] Clause 26: The method of clause 25, wherein inserting the urinary
catheter through
the percutaneous opening comprises: inserting a needle of the ureteral
catheter into a portion
of the patient's body to create the percutaneous opening; inserting the needle
into a kidney of
the patient and advancing the needle through the kidney to the renal pelvis of
the patient; and
inserting an elongated tube of the ureteral catheter over the needle, such
that a distal end of
the elongated tube advances from the kidney into the renal pelvis.
[0049] Clause 27: The method of clause 26, wherein inserting the urinary
catheter
comprises inserting the needle of the urinary catheter into the abdominal
region of the patient.
[0050] Clause 28: The method of any of clauses 25-27, further comprising
attaching a
proximal end of the urinary catheter directly or indirectly to a fluid pump
and applying a
negative pressure to a proximal end of the proximal portion of the urinary
catheter by
actuating the pump, thereby inducing the negative pressure in the kidney,
renal pelvis, and/or
bladder of the patient.
BRIEF DESCRIPTION OF THE DRAWINGS
[0051] These and other features and characteristics of the present disclosure,
as well as the
methods of operation and functions of the related elements of structures and
the combination
of parts and economies of manufacture, will become more apparent upon
consideration of the
following description and the appended clauses with reference to the
accompanying
drawings, all of which form a part of this specification, wherein like
reference numerals
designate corresponding parts in the various figures. It is to be expressly
understood,
however, that the drawings are for the purpose of illustration and description
only and are not
intended as a definition of the limit of the invention.
[0052] Further features and other examples and advantages will become apparent
from the
following detailed description made with reference to the drawings in which:
[0053] FIG. lA is a schematic drawing of an indwelling portion of a system
comprising a
ureteral stent and a bladder catheter deployed in a urinary tract of a
patient, according to an
example of the present invention;
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
11
[0054] FIG. 1B is a schematic drawing of an indwelling portion of a system
comprising a
ureteral catheter and a bladder catheter deployed in a urinary tract of a
patient, according to
an example of the present invention;
[0055] FIG. 1C is a schematic drawing of an indwelling portion of a system
comprising a
ureteral catheter and a bladder catheter deployed in a urinary tract of a
patient, according to
an example of the present invention;
[0056] FIG. 1D is a perspective view of a retention portion of a bladder
catheter, according
to an example of the present invention;
[0057] FIG. 1E is a cross sectional view of the retention portion of FIG. 1D,
taken along
line 1E-1E of FIG. 1D, according to an example of the present invention;
[0058] FIG. 1F is a schematic drawing of an indwelling portion of a system
comprising a
ureteral catheter and a bladder catheter deployed in a urinary tract of a
patient, according to
an example of the present invention;
[0059] FIG. 1G is a perspective view of a retention portion of a bladder
catheter, according
to an example of the present invention;
[0060] FIG. 1H is a side elevational view of the retention portion of FIG. 1G,
according to
an example of the present invention;
[0061] FIG. II is a top plan view of the retention portion of FIG. 1G,
according to an
example of the present invention;
[0062] FIG. 1J is a perspective view of a retention portion of a bladder
catheter, according
to an example of the present invention;
[0063] FIG. 1K is a perspective view of a retention portion of a bladder
catheter, according
to an example of the present invention;
[0064] FIG. 1L is a side elevational view of a retention portion of a bladder
catheter prior
to deployment, according to an example of the present invention;
[0065] FIG. 1M is a side elevational view of the retention portion of FIG. 1L
after
deployment, according to an example of the present invention;
[0066] FIG. 1N is a perspective view of a retention portion of a bladder
catheter, according
to an example of the present invention;
[0067] FIG. 10 is a cross-sectional view of a portion of the retention portion
of FIG. 1N,
according to an example of the present invention;
[0068] FIG. 1P is a schematic drawing of an indwelling portion of a system
comprising a
ureteral catheter and a bladder catheter deployed in a urinary tract of a
patient, according to
an example of the present invention;
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
12
[0069] FIG. 1Q is a perspective view of a retention portion of a bladder
catheter, according
to an example of the present invention;
[0070] FIG. 1R is a cross-sectional view of a portion of the retention portion
of FIG. 1Q,
according to an example of the present invention;
[0071] FIG. 1S is a perspective view of a retention portion of a bladder
catheter, according
to an example of the present invention;
[0072] FIG. 1T is a cross-sectional view of a portion of the retention portion
of FIG. is,
according to an example of the present invention;
[0073] FIG. 1U is a schematic drawing of an indwelling portion of a system
comprising a
ureteral catheter and a bladder catheter deployed in a urinary tract of a
patient, according to
an example of the present invention;
[0074] FIG. 1V is a perspective view of a retention portion of a bladder
catheter, according
to an example of the present invention;
[0075] FIG. 1W is a cross sectional view of the retention portion of FIG. 1V,
taken along
line 1W-1W of FIG. 1V, according to an example of the present invention;
[0076] FIG. 2A is a schematic drawing of an indwelling portion of a system
comprising a
ureteral catheter deployed in a urinary tract of a patient, according to an
example of the
present invention;
[0077] FIG. 2B is a schematic drawing of an indwelling portion of a system
comprising a
ureteral catheter deployed in a urinary tract of a patient, according to an
example of the
present invention;
[0078] FIG. 3 is a dimetric view of an example of a prior art transformable
ureteral stent
according to FIG. 1 of PCT Patent Application Publication WO 2017/019974,
wherein the
image on the left represents the uncompressed state of the stent and the image
on the right
represents the compressed state of the stent;
[0079] FIG. 4 is a perspective view of an example of a prior art ureteral
stent according to
FIG. 4 of US Patent Application Publication No. 2002/0183853 Al;
[0080] FIG. 5 is a perspective view of an example of a prior art ureteral
stent according to
FIG. 5 of US Patent Application Publication No. 2002/0183853 Al;
[0081] FIG. 6 is a perspective view of an example of a prior art ureteral
stent according to
FIG. 7 of US Patent Application Publication No. 2002/0183853 Al;
[0082] FIG. 7A is a schematic drawing of another example of an indwelling
portion of a
system comprising a ureteral catheter and a bladder catheter deployed in a
urinary tract of a
patient, according to an example of the present invention;
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
13
[0083] FIG. 7B is a schematic drawing of a system for inducing negative
pressure to the
urinary tract of a patient according to an example of the present invention;
[0084] FIG. 7C is a an enlarged schematic drawing of a portion of a ureteral
catheter
according to the present invention positioned in the renal pelvis region of
the kidney showing
in phantom general changes believed to occur in the renal pelvis tissue in
response to
application of negative pressure through the ureteral catheter;
[0085] FIG. 8A is a perspective view of an exemplary catheter according to an
example of
the present invention;
[0086] FIG. 8B is a front view of the catheter of FIG. 8A;
[0087] FIG. 9A is a schematic drawing of an example of a retention portion for
a catheter
according to an example of the present invention;
[0088] FIG. 9B is a schematic drawing of another example of a retention
portion for a
catheter according to an example of the present invention;
[0089] FIG. 9C is a schematic drawing of another example of a retention
portion for a
catheter according to an example of the present invention;
[0090] FIG. 9D is a schematic drawing of another example of a retention
portion for a
catheter according to an example of the present invention;
[0091] FIG. 9E is a schematic drawing of another example of a retention
portion for a
catheter according to an example of the present invention;
[0092] FIG. 10 is a front view of another example of a catheter according to
an example of
the present invention;
[0093] FIG. 10A is a perspective view of the retention portion of the catheter
of FIG. 10
enclosed by circle 10A according to an example of the present invention;
[0094] FIG. 10B is a front view of the retention portion of FIG. 10A according
to an
example of the present invention;
[0095] FIG. 10C is a rear view of the retention portion of FIG. 10A according
to an
example of the present invention;
[0096] FIG. 10D is a top view of the retention portion of FIG. 10A according
to an
example of the present invention;
[0097] FIG. 10E is a cross sectional view of the retention portion of FIG. 10A
taken along
line 10E-10E according to an example of the present invention;
[0098] FIG. 1OF is s a cross sectional view of the retention portion of FIG.
10A taken
along line 10E-10E according to an example of the present invention positioned
in the renal
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
14
pelvis region of the kidney showing in general changes believed to occur in
the renal pelvis
tissue in response to application of negative pressure through the ureteral
catheter;
[0099] FIG. 10G is s a cross sectional view of the retention portion of FIG.
10A taken
along line 10E-10E according to an example of the present invention positioned
in the
bladder showing in general changes believed to occur in the bladder tissue in
response to
application of negative pressure through the bladder catheter;
[00100] FIG. 11 is a schematic drawing of a retention portion of a catheter in
a constrained
or linear position according to an example of the present invention;
[00101] FIG. 12 is a schematic drawing of another example of a retention
portion of a
catheter in a constrained or linear position according to an example of the
present invention;
[00102] FIG. 13 is a schematic drawing of another example of a retention
portion of a
ureteral catheter in a constrained or linear position according to an example
of the present
invention;
[00103] FIG. 14 is a schematic drawing of another example of a retention
portion of a
catheter in a constrained or linear position according to an example of the
present invention;
[00104] FIG. 15A is a graph showing a percentage of fluid flow through
openings of an
exemplary catheter as a function of position according to an example of the
present invention;
[00105] FIG. 15B is a graph showing a percentage of fluid flow through
openings of
another exemplary catheter as a function of position according to an example
of the present
invention;
[00106] FIG. 15C is a graph showing a percentage of fluid flow through
openings of
another exemplary catheter as a function of position according to an example
of the present
invention;
[00107] FIG. 16 is a schematic drawing of a retention portion of a catheter
showing
stations for calculating fluid flow coefficients for a mass transfer balance
evaluation
according to an example of the present invention;
[00108] FIG. 17 is a schematic drawing of an indwelling portion of a system
comprising a
ureteral catheter and a bladder catheter deployed in a urinary tract of a
patient, according to
another example of the present invention;
[00109] FIG. 18A is side elevational view of a retention portion of a catheter
according to
an example of the present invention;
[00110] FIG. 18B is cross-sectional view of the retention portion of the
catheter of FIG.
18A taken along lines B-B of FIG. 18A;
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
[00111] FIG. 18C is a top plan view of the retention portion of the catheter
of FIG. 18A
taken along lines C-C of Fig. 18A;
[00112] FIG. 18D is cross sectional view of a retention portion of a ureteral
catheter
according to an example of the present invention positioned in the renal
pelvis region of the
kidney showing in general changes believed to occur in the renal pelvis tissue
in response to
application of negative pressure through the ureteral catheter;
[00113] FIG. 18E is cross sectional view of a retention portion of a bladder
catheter
according to an example of the present invention positioned in the bladder
showing in general
changes believed to occur in the bladder tissue in response to application of
negative pressure
through the bladder catheter;
[00114] FIG. 19 is a side elevational view of a retention portion of another
catheter
according to an example of the present invention;
[00115] FIG. 20 is a side elevational view of a retention portion of another
catheter
according to an example of the present invention;
[00116] FIG. 21 is a side elevational view of a retention portion of another
catheter
according to an example of the present invention;
[00117] FIG. 22A is a perspective view of a retention portion of another
ureteral catheter
according to an example of the present invention;
[00118] FIG. 22B is a top plan view of the retention portion of the catheter
of FIG. 22A
taken along lines 22B-22B of FIG. 22A;
[00119] FIG. 23A is a perspective view of a retention portion of another
catheter according
to an example of the present invention;
[00120] FIG. 23B is a top plan view of the retention portion of the catheter
of FIG. 23A
taken along lines 23B-23B of FIG. 23A;
[00121] FIG. 24A is a perspective view of a retention portion of another
catheter according
to an example of the present invention;
[00122] FIG. 24B is a cross sectional view of a retention portion of a
ureteral catheter
according to an example of the present invention positioned in the renal
pelvis region of the
kidney showing in general changes believed to occur in the renal pelvis tissue
in response to
application of negative pressure through the ureteral catheter;
[00123] FIG. 24C is a cross sectional view of a retention portion of a bladder
catheter
according to an example of the present invention positioned in the bladder
showing in general
changes believed to occur in the bladder tissue in response to application of
negative pressure
through the bladder catheter;
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
16
[00124] FIG. 25 is a side elevational view of a retention portion of another
catheter
according to an example of the present invention;
[00125] FIG. 26 is a side elevational view of a retention portion of another
catheter
according to an example of the present invention;
[00126] FIG. 27 is a cross-sectional side view of a retention portion of
another catheter
according to an example of the present invention;
[00127] FIG. 28A is a perspective view of a retention portion of another
catheter according
to an example of the present invention;
[00128] FIG. 28B is a top plan view of the retention portion of the catheter
of FIG. 28A;
[00129] FIG. 29A is a perspective view of a retention portion of another
catheter according
to an example of the present invention;
[00130] FIG. 29B is a top plan view of the retention portion of the catheter
of FIG. 29A;
[00131] FIG. 29C is a cross sectional view of a retention portion of a
ureteral catheter
according to an example of the present invention positioned in the renal
pelvis region of the
kidney showing in general changes believed to occur in the renal pelvis tissue
in response to
application of negative pressure through the ureteral catheter;
[00132] FIG. 30 is a perspective view of a retention portion of another
catheter according
to an example of the present invention;
[00133] FIG. 31 is a top plan view of the retention portion of the catheter of
FIG. 30;
[00134] FIG. 32A is a perspective view of a retention portion of another
catheter according
to an example of the present invention;
[00135] FIG. 32B is a top plan view of the retention portion of the catheter
of FIG. 32A;
[00136] FIG. 33 is a cross-sectional side elevational view of a retention
portion of another
catheter according to an example of the present invention;
[00137] FIG. 34 is a cross-sectional side elevational view of a retention
portion of another
catheter according to an example of the present invention;
[00138] FIG. 35A is a perspective view of a retention portion of another
catheter according
to an example of the present invention;
[00139] FIG. 35B is a cross-sectional side elevational view of the retention
portion of the
catheter of FIG. 35A taken along lines B-B of FIG. 35A;
[00140] FIG. 36 is a side elevational view showing a cut away cross-sectional
view of the
sheath surrounding a catheter according to an example of the present invention
in a
contracted configuration for insertion into a patient's ureter;
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
17
[00141] FIG.37A is a schematic drawing of another example of a retention
portion for a
catheter according to an example of the present invention;
[00142] FIG. 37B is a schematic drawing of a cross-sectional view of a portion
of the
retention portion of FIG. 37A, taken along lines B-B of FIG. 37A;
[00143] FIG. 38A is a schematic drawing of another example of a retention
portion for a
catheter according to an example of the present invention;
[00144] FIG. 38B is a schematic drawing of a portion of a cross-sectional view
of the
retention portion of FIG. 5A, taken along lines B-B of FIG. 38A;
[00145] FIG. 39A is a schematic drawing of another example of a retention
portion for a
catheter according to an example of the present invention;
[00146] FIG. 39B is a schematic drawing of a cross section of another example
of a
retention portion for a ureteral catheter according to an example of the
present invention
positioned in the renal pelvis region of the kidney showing in general changes
believed to
occur in the renal pelvis tissue in response to application of negative
pressure through the
ureteral catheter;
[00147] FIG. 39C is a schematic drawing of a cross section of another example
of a
retention portion for a bladder catheter according to an example of the
present invention
positioned in the bladder showing in general changes believed to occur in the
bladder tissue
in response to application of negative pressure through the bladder catheter;
[00148] FIG. 40A is a schematic drawing of a cross section of another example
of a
retention portion for a catheter according to an example of the present
invention;
[00149] FIG. 40B is a schematic drawing of a cross section of another example
of a
retention portion for a ureteral catheter according to an example of the
present invention
positioned in the renal pelvis region of the kidney showing in general changes
believed to
occur in the renal pelvis tissue in response to application of negative
pressure through the
ureteral catheter;
[00150] FIG. 40C is a schematic drawing of a cross section of another example
of a
retention portion for a bladder catheter according to an example of the
present invention
positioned in the bladder showing in general changes believed to occur in the
bladder tissue
in response to application of negative pressure through the bladder catheter;
[00151] FIG. 41A is a schematic drawing of another example of a retention
portion for a
catheter according to an example of the present invention;
[00152] FIG. 41B is a schematic drawing of a cross section of another example
of a
retention portion for a ureteral catheter according to an example of the
present invention
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
18
positioned in the renal pelvis region of the kidney showing in general changes
believed to
occur in the renal pelvis tissue in response to application of negative
pressure through the
ureteral catheter;
[00153] FIG. 41C is a schematic drawing of a cross section of another example
of a
retention portion for a bladder catheter according to an example of the
present invention
positioned in the bladder showing in general changes believed to occur in the
bladder tissue
in response to application of negative pressure through the bladder catheter;
[00154] FIG. 42A is a flow chart illustrating a process for insertion and
deployment of a
system according to an example of the present invention;
[00155] FIG. 42B is a flow chart illustrating a process for applying negative
pressure using
a system according to an example of the present invention;
[00156] FIG. 43 is a schematic drawing of a nephron and surrounding
vasculature showing
a position of the capillary bed and convoluted tubules;
[00157] FIG. 44 is a schematic drawing of a system for inducing negative
pressure to the
urinary tract of a patient according to an example of the present invention;
[00158] FIG. 45A is a plan view of a pump for use with the system of FIG. 44
according to
an example of the present invention;
[00159] FIG. 45B is a side elevation view of the pump of FIG. 45A;
[00160] FIG. 46 is a schematic drawing of an experimental set-up for
evaluating negative
pressure therapy in a swine model according to the present invention;
[00161] FIG. 47 is a graph of creatinine clearance rates for tests conducted
using the
experimental set-up shown in FIG. 21;
[00162] FIG. 48A is a low magnification photomicrograph of kidney tissue from
a
congested kidney treated with negative pressure therapy;
[00163] FIG. 48B is a high magnification photomicrograph of the kidney tissue
shown in
FIG. 48A;
[00164] FIG. 48C is a low magnification photomicrograph of kidney tissue from
a
congested and untreated (e.g., control) kidney;
[00165] FIG. 48D is a high magnification photomicrograph of the kidney tissue
shown in
FIG. 23C
[00166] FIG. 49 is a flow chart illustrating a process for reducing creatinine
and/or protein
levels of a patient according to an example of the present invention;
[00167] FIG. 50 is a flow chart illustrating a process for treating a patient
undergoing fluid
resuscitation according to an example of the present invention;
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
19
[00168] FIG. 51 is a graph of serum albumin relative to baseline for tests
conduct on swine
using the experimental method described herein;
[00169] FIG. 52A is a perspective view of a catheter configured to be inserted
to the renal
pelvis through a percutaneous access site;
[00170] FIG. 52B is a side view of the catheter of FIG. 52A;
[00171] FIG. 53 is a cross-sectional view of the catheter of FIG. 52A;
[00172] FIG. 54 is a schematic drawing showing a ureteral catheter inserted
through a
percutaneous access site and deployed in a renal pelvis of a patient;
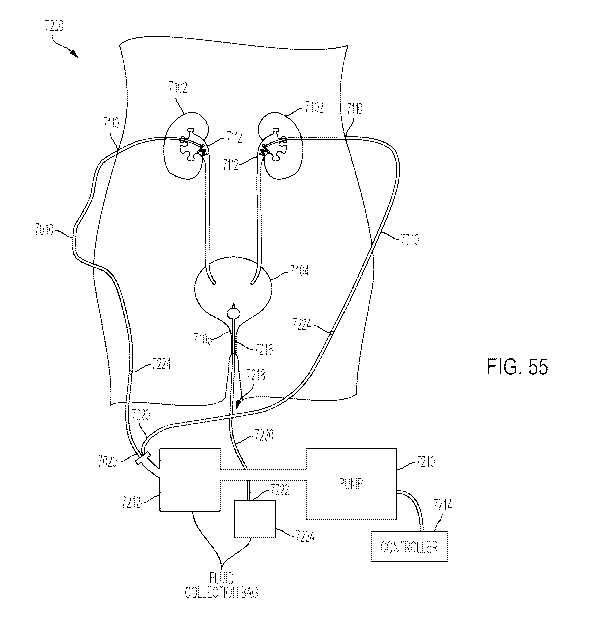
[00173] FIG. 55 is a schematic drawing of a urinary tract of a patient showing
a system for
collecting fluid including the ureteral catheter of FIG. 54;
[00174] FIG. 56 is a flow chart of a method for deploying a ureteral catheter
in the renal
pelvis through a percutaneous access site;
[00175] FIGS. 57A-57E are schematic drawings showing steps for inserting a
ureteral
catheter to a renal pelvis of a patient;
[00176] FIG. 58A is a perspective view of another example of a catheter
configured to be
inserted to the renal pelvis through a percutaneous access site, according to
an aspect of the
disclosure; and
[00177] FIG. 58B is a cross-sectional view of the catheter of FIG. 58A.
DETAILED DESCRIPTION OF THE INVENTION
[00178] As used herein, the singular form of "a", "an", and "the" include
plural referents
unless the context clearly states otherwise.
[00179] As used herein, the terms "right", "left", "top", and derivatives
thereof shall relate
to the invention as it is oriented in the drawing figures. The term "proximal"
refers to the
portion of the catheter device that is manipulated or contacted by a user
and/or to a portion of
an indwelling catheter nearest to the urinary tract access site. The term
"distal" refers to the
opposite end of the catheter device that is configured to be inserted into a
patient and/or to the
portion of the device that is inserted farthest into the patient's urinary
tract. However, it is to
be understood that the invention can assume various alternative orientations
and, accordingly,
such terms are not to be considered as limiting. Also, it is to be understood
that the
invention can assume various alternative variations and stage sequences,
except where
expressly specified to the contrary. It is also to be understood that the
specific devices and
processes illustrated in the attached drawings, and described in the following
specification,
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
are examples. Hence, specific dimensions and other physical characteristics
related to the
embodiments disclosed herein are not to be considered as limiting.
[00180] For the purposes of this specification, unless otherwise indicated,
all numbers
expressing quantities of ingredients, reaction conditions, dimensions,
physical characteristics,
and so forth used in the specification and claims are to be understood as
being modified in all
instances by the term "about." Unless indicated to the contrary, the numerical
parameters set
forth in the following specification and attached claims are approximations
that can vary
depending upon the desired properties sought to be obtained by the present
invention.
[00181] Notwithstanding that the numerical ranges and parameters setting forth
the broad
scope of the invention are approximations, the numerical values set forth in
the specific
examples are reported as precisely as possible. Any numerical value, however,
inherently
contains certain errors necessarily resulting from the standard deviation
found in their
respective testing measurements.
[00182] Also, it should be understood that any numerical range recited herein
is intended
to include all sub-ranges subsumed therein. For example, a range of "1 to 10"
is intended to
include any and all sub-ranges between and including the recited minimum value
of 1 and the
recited maximum value of 10, that is, all subranges beginning with a minimum
value equal to
or greater than 1 and ending with a maximum value equal to or less than 10,
and all
subranges in between, e.g., 1 to 6.3, or 5.5 to 10, or 2.7 to 6.1.
[00183] As used herein, the terms "communication" and "communicate" refer to
the
receipt or transfer of one or more signals, messages, commands, or other type
of data. For
one unit or component to be in communication with another unit or component
means that
the one unit or component is able to directly or indirectly receive data from
and/or transmit
data to the other unit or component. This can refer to a direct or indirect
connection that can
be wired and/or wireless in nature. Additionally, two units or components can
be in
communication with each other even though the data transmitted can be
modified, processed,
routed, and the like, between the first and second unit or component. For
example, a first unit
can be in communication with a second unit even though the first unit
passively receives data,
and does not actively transmit data to the second unit. As another example, a
first unit can be
in communication with a second unit if an intermediary unit processes data
from one unit and
transmits processed data to the second unit. It will be appreciated that
numerous other
arrangements are possible.
[00184] As used herein, "maintain patency of fluid flow between a kidney and a
bladder of
the patient" means establishing, increasing or maintaining flow of fluid, such
as urine, from
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
21
the kidneys through the ureter(s), ureteral stent(s) and/or ureteral
catheter(s) to the bladder
and outside of the body. In some examples, the fluid flow is facilitated or
maintained by
providing a protective surface area 1001 in the upper urinary tract and/or
bladder to prevent
the uroendothelium from contracting or collapsing into the fluid column or
stream. As used
herein, "fluid" means urine and any other fluid from the urinary tract.
[00185] As used herein, "negative pressure" means that the pressure applied to
the
proximal end of the bladder catheter or the proximal end of the ureteral
catheter, respectively,
is below the existing pressure at the proximal end of the bladder catheter or
the proximal end
of the ureteral catheter, respectively, prior to application of the negative
pressure, e.g., there
is a pressure differential between the proximal end of the bladder catheter or
the proximal end
of the ureteral catheter, respectively, and the existing pressure at the
proximal end of the
bladder catheter or the proximal end of the ureteral catheter, respectively,
prior to application
of the negative pressure. This pressure differential causes fluid from the
kidney to be drawn
into the ureteral catheter or bladder catheter, respectively, or through both
the ureteral
catheter and the bladder catheter, and then outside of the patient's body. For
example,
negative pressure applied to the proximal end of the bladder catheter or the
proximal end of
the ureteral catheter can be less than atmospheric pressure (less than about
760 mm Hg or
about 1 atm), or less than the pressure measured at the proximal end of the
bladder catheter or
the proximal end of the ureteral catheter prior to the application of negative
pressure, such
that fluid is drawn from the kidney and/or bladder. In some examples, the
negative pressure
applied to the proximal end of the bladder catheter or the proximal end of the
ureteral catheter
can range from about 0.1 mmHg to about 150 mm Hg, or about 0.1 mm Hg to about
50 mm
Hg, or about 0.1 mm Hg to about 10 mm Hg, or about 5 mm Hg to about 20 mm Hg,
or about
45 mm Hg (gauge pressure at the pump 710 or at a gauge at the negative
pressure source). In
some examples, the negative pressure source comprises a pump external to the
patient's body
for application of negative pressure through both the bladder catheter and the
ureteral
catheter, which in turn causes fluid from the kidney to be drawn into the
ureteral catheter,
through both the ureteral catheter and the bladder catheter, and then outside
of the patient's
body. In some examples, the negative pressure source comprises a vacuum source
external to
the patient's body for application and regulation of negative pressure through
both the
bladder catheter and the ureteral catheter, which in turn causes fluid from
the kidney to be
drawn into the ureteral catheter, through both the ureteral catheter and the
bladder catheter,
and then outside of the patient's body. In some examples, the vacuum source is
selected from
the group consisting of a wall suction source, vacuum bottle, and manual
vacuum source, or
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
22
the vacuum source is provided by a pressure differential. In some examples,
the negative
pressure received from the negative pressure source can be controlled
manually,
automatically, or combinations thereof. In some examples, a controller is used
to regulate
negative pressure from the negative pressure source. Non-limiting examples of
negative and
positive pressure sources are discussed in detail below.
[00186] As used herein, "positive pressure" means that the pressure applied to
the
proximal end of the bladder catheter or the proximal end of the ureteral
catheter, respectively,
is above the existing pressure at the proximal end of the bladder catheter or
the proximal end
of the ureteral catheter, respectively, prior to application of the negative
pressure, and causes
fluid present in the ureteral catheter or bladder catheter, respectively, or
through both the
ureteral catheter and the bladder catheter, to flow back towards the bladder
or kidney. In
some examples, the positive pressure applied to the proximal end of the
bladder catheter or
the proximal end of the ureteral catheter can range from about 0.1 mmHg to
about 150 mm
Hg, or about 0.1 mm Hg to about 50 mm Hg, or about 0.1 mm Hg to about 10 mm
Hg, or
about 5 mm Hg to about 20 mm Hg, or about 45 mm Hg (gauge pressure at the pump
710 or
at a gauge at the positive pressure source). The positive pressure source can
be provided by a
pump or wall pressure source, or pressurized bottle, for example, and can be
controlled
manually, automatically, or combinations thereof. In some examples, a
controller is used to
regulate positive pressure from the positive pressure source.
[00187] Fluid retention and venous congestion are central problems in the
progression to
advanced renal disease. Excess sodium ingestion coupled with relative
decreases in excretion
leads to isotonic volume expansion and secondary compartment involvement. In
some
examples, the present invention is generally directed to devices and methods
for facilitating
drainage of urine or waste from the bladder, ureter, and/or kidney(s) of a
patient. In some
examples, the present invention is generally directed to systems and methods
for inducing a
negative pressure in at least a portion of the bladder, ureter, and/or
kidney(s), e.g., urinary
system, of a patient. While not intending to be bound by any theory, it is
believed that
applying a negative pressure to at least a portion of the bladder, ureter,
and/or kidney(s), e.g.,
urinary system, can offset the medullary nephron tubule re-absorption of
sodium and water in
some situations. Offsetting re-absorption of sodium and water can increase
urine production,
decrease total body sodium, and improve erythrocyte production. Since the
intra-medullary
pressures are driven by sodium and, therefore, volume overload, the targeted
removal of
excess sodium enables maintenance of volume loss. Removal of volume restores
medullary
hemostasis. Normal urine production is 1.48-1.96 L/day (or 1-1.4 ml/min).
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
23
[00188] Fluid retention and venous congestion are also central problems in the
progression
of prerenal Acute Kidney Injury (AKI). Specifically, AKI can be related to
loss of perfusion
or blood flow through the kidney(s). Accordingly, in some examples, the
present invention
facilitates improved renal hemodynamics and increases urine output for the
purpose of
relieving or reducing venous congestion. Further, it is anticipated that
treatment and/or
inhibition of AM positively impacts and/or reduces the occurrence of other
conditions, for
example, reduction or inhibition of worsening renal function in patients with
NYHA Class III
and/or Class IV heart failure. Classification of different levels of heart
failure are described
in The Criteria Committee of the New York Heart Association, (1994),
Nomenclature and
Criteria for Diagnosis of Diseases of the Heart and Great Vessels, (9th ed.),
Boston: Little,
Brown & Co. pp. 253-256, the disclosure of which is incorporated by reference
herein in its
entirety. Reduction or inhibition of episodes of AKI and/or chronically
decreased perfusion
may also be a treatment for Stage 4 and/or Stage 5 chronic kidney disease.
Chronic kidney
disease progression is described in National Kidney Foundation, K/DOQI
Clinical Practice
Guidelines for Chronic Kidney Disease: Evaluation, Classification and
Stratification. Am. J.
Kidney Dis. 39:S1-S266, 2002 (Suppl. 1), the disclosure of which is
incorporated by
reference herein in its entirety.
[00189] Also, the ureteral catheters, ureteral stents and/or bladder catheters
disclosed
herein can be useful for preventing, delaying the onset of, and/or treating
end-stage renal
disease ("ESRD"). The average dialysis patient consumes about $90,000 per year
in
healthcare utilization for a total cost to the US government of $33.9 Billion.
Today, ESRD
patients comprise only 2.9% of Medicare's total beneficiaries, yet they
account over 13% of
total spending. While the incidence and costs per patient have stabilized in
recent years, the
volume of active patients continues to rise.
[00190] The five stages of advanced chronic kidney disease ("CKD") are based
upon
glomerular filtration rate (GFR). Stage 1 (GFR >90) patients have normal
filtration, while
stage 5 (GFR <15) have kidney failure. Like many chronic diseases, the
diagnosis capture
improves with increasing symptom and disease severity.
[00191] The CKD 3b/4 subgroup is a smaller subgroup that reflects important
changes in
disease progression, healthcare system engagement and transition to ESRD.
Presentation to
the emergency department rises with severity of CKD. Among the US Veteran's
Administration population, nearly 86% of the incident dialysis patients had a
hospital
admission within the five years preceding the admission. Of those, 63% were
hospitalized at
initiation of dialysis. This suggests a tremendous opportunity to intervene
prior to dialysis.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
24
[00192] Despite being further down the arterial tree than other organs, the
kidneys receive
a disproportionate amount of cardiac output at rest. The glomerular membrane
represents a
path of least resistance of filtrate into the tubules. In healthy states, the
nephron has multiple,
intricate, redundant means of auto-regulating within normal ranges of arterial
pressure.
[00193] Venous congestion has been implicated in reduced renal function and is
associated
with the systemic hypervolemia found in later stages of CKD. Since the kidney
is covered
with a semi-rigid capsule, small changes in venous pressure translate into
direct changes in
the intratubule pressures. This shift in intratubule pressure has been shown
to upregulate
reabsorption of sodium and water, perpetuating the vicious cycle.
[00194] Regardless of the initial insult and early progression, more advanced
CKD is
associated with decreased filtration (by definition) and greater azotemia.
Regardless of
whether the remaining nephrons are hyperabsorbing water or they are just
unable to filtrate
sufficiently, this nephron loss is associated with fluid retention and a
progressive decline in
renal function.
[00195] The kidney is sensitive to subtle shifts in volume. As pressure in
either the tubule
or capillary bed rises, the pressure in the other follows. As the capillary
bed pressure rises,
the production of filtrate and elimination of urine can decline dramatically.
While not
intending to be bound by any theory, it is believe that mild and regulated
negative pressure
delivered to the renal pelvis decreases the pressure among each of the
functioning nephrons.
In healthy anatomy, the renal pelvis is connected via a network of calyces and
collecting
ducts to approximately one million individual nephrons. Each of these nephrons
are
essentially fluid columns connecting Bowman's space to the renal pelvis.
Pressure
transmitted to the renal pelvis translates throughout. It is believed that, as
negative pressure
is applied to the renal pelvis, the glomerular capillary pressure forces more
filtrate across the
glomerular membrane, leading to increased urine output.
[00196] It is important to note that the tissues of the urinary tract are
lined with urothelium,
a type of transitional epithelium. The tissues lining the inside of the
urinary tract are also
referred to as uroendothelial or urothelial tissues, such as mucosal tissue
1003 of the ureter
and/or kidney and bladder tissue 1004. Urothelium has a very high elasticity,
enabling a
remarkable range of collapsibility and distensibility. The urothelium lining
the ureter lumen
is surrounded first by the lamina propria, a thin layer of loose connective
tissue, which
together comprise the urothelial mucosa. This mucosa is then surrounded by a
layer of
longitudinal muscle fibers. These longitudinal muscle fibers surrounding the
urothelial
mucosa and the elasticity of the urothelial mucosa itself allow the ureter to
relax into a
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
collapsed stellate cross-section and then expand to full distention during
diuresis. Histology
of any normal ureteral cross-section reveals this star-shaped lumen in humans
and other
mammals generally used in translational medical research. Wolf et al.,
"Comparative
Ureteral Microanatomy", JEU 10: 527-31 (1996).
[00197] The process of transporting urine from the kidney to the bladder is
driven by
contractions through the renal pelvis and peristalsis distally through the
rest of the
ureter. The renal pelvis is the widening of the proximal ureter into a funnel-
shape where the
ureter enters the kidney. The renal pelvis has actually been shown to be a
continuation of the
ureter, comprised of the same tissue but with one additional muscle layer that
allows it to
contract. Dixon and Gosling, "The Musculature of the Human Renal Calyces,
Pelvis and
Upper Ureter", J. Anat. 135: 129-37 (1982). These contractions push urine
through the renal
pelvis funnel to allow peristaltic waves to propagate the fluid through the
ureter to the
bladder.
[00198] Imaging studies have shown that the ureter of the dog can readily
increase to up to
17x its resting cross-sectional area to accommodate large volumes of urine
during diuresis.
Woodburne and Lapides, "The Ureteral Lumen During Peristalsis", AJA 133: 255-8
(1972).
Among swine, considered to be the closest animal model for the human upper
urinary tract,
the renal pelvis and most proximal ureter are actually shown to be the most
compliant of all
ureteral sections. Gregersen, et al., "Regional Differences Exist in Elastic
Wall Properties in
the Ureter", SJUN 30: 343-8 (1996). Wolf's comparative analysis of various
research
animals' ureteral microanatomy to that of humans revealed comparable thickness
of lamina
propria layer relative to whole ureter diameter in dogs (29.5% in humans and
34% in dogs)
and comparable percentage of smooth muscle relative to total muscular cross
sectional area in
pigs (54% in humans and 45% in pigs). While there are certainly limitations to
the
comparisons between species, dogs and pigs have historically been strong foci
in studying
and understanding human ureter anatomy and physiology, and these reference
values support
this high level of translatability.
[00199] There is much more data available on structure and mechanics of pig
and dog
ureters and renal pelves than on human ureters. This is due partly to the
invasiveness
required for such detailed analyses as well as the inherent limitations of
various imaging
modalities (MRI, CT, ultrasound, etc.) to attempt to accurately identify size
and composition
of such small, flexible, and dynamic structures clinically. Nevertheless, this
ability for the
renal pelvis to distend or completely collapse in humans is a hurdle for
nephrologists and
urologists seeking to improve urine flow.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
26
[00200] While not intending to be bound by any theory, the present inventors
theorized
that the application of negative pressure might help to facilitate fluid flow
from the kidney,
and that a very particular tool, designed to deploy a protective surface area
in order to open or
maintain the opening of the interior of the renal pelvis while inhibiting the
surrounding
tissues from contracting or collapsing into the fluid column under negative
pressure, is
needed to facilitate the application of negative pressure within the renal
pelvis. The catheter
designs of the present invention disclosed herein provide a protective surface
area to inhibit
surrounding urothelial tissues from contracting or collapsing into the fluid
column under
negative pressure. It is believed that the catheter designs of the present
invention disclosed
herein can successfully maintain the stellate longitudinal folding of the
ureteral wall away
from the central axis and protected holes of the catheter drainage lumen, and
can inhibit
natural sliding of the catheter down the stellate cross-sectional area of the
ureteral lumen
and/or downward migration by peristaltic waves.
[00201] Also, catheter designs of the present invention disclosed herein can
avoid an
unprotected open hole at the distal end of the drainage lumen which fails to
protect
surrounding tissues during suction. While it is convenient to think of the
ureter as a straight
tube, the true ureter and renal pelvis can enter the kidney at a variety of
angles. Lippincott
Williams & Wilkins, Annals of Surgery, 58, Figs 3 & 9 (1913). Therefore, it
would be
difficult to control the orientation of an unprotected open hole at the distal
end of the drainage
lumen when deploying such a catheter in the renal pelvis. This single hole may
present a
localized suction point that has no means of either reliable or consistent
distancing from
tissue walls, thereby permitting tissue to occlude the unprotected open hole
and risking
damage to the tissue. Also, catheter designs of the present invention
disclosed herein can
avoid placement of a balloon having an unprotected open hole at the distal end
of the
drainage lumen close to the kidney which may result in suction against and/or
occlusion of
the calyces. Placement of a balloon having an unprotected open hole at the
distal end of the
drainage lumen at the very base of the uretero ¨ renal pelvis junction may
result in suction
against and occlusion by renal pelvis tissue. Also, a rounded balloon may
present a risk of
ureteral avulsion or other damage from incidental pulling forces on the
balloon.
[00202] Delivering negative pressure into the kidney area of a patient has a
number of
anatomical challenges for at least three reasons. First, the urinary system is
composed of
highly pliable tissues that are easily deformed. Medical textbooks often
depict the bladder as
a thick muscular structure that can remain in a fixed shape regardless of the
volume of urine
contained within the bladder. However, in reality, the bladder is a soft
deformable structure.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
27
The bladder shrinks to conform to the volume of urine contained in the
bladder. An empty
bladder more closely resembles a deflated latex balloon than a ball. In
addition, the mucosal
lining on the interior of the bladder is soft and susceptible to irritation
and damage. It is
desirable to avoid drawing the urinary system tissue into the orifices of the
catheter to
maintain adequate fluid flow therethrough and avoid injury to the surrounding
tissue.
[00203] Second, the ureters are small tube-like structures that can expand and
contract to
transport urine from the renal pelvis to the bladder. This transport occurs in
two ways:
peristaltic activity and by a pressure gradient in an open system. In the
peristaltic activity, a
urine portion is pushed ahead of a contractile wave, which almost completely
obliterates the
lumen. The wave pattern initiates in the renal pelvis area, propagates along
the ureter, and
terminates in the bladder. Such a complete occlusion interrupts the fluid flow
and can
prevent negative pressure delivered in the bladder from reaching the renal
pelvis without
assistance. The second type of transport, by pressure gradient through a wide-
open ureter,
may be present during large urine flow. During such periods of high urine
production, the
pressure head in the renal pelvis would not need to be caused by contraction
of the smooth
muscles of the upper urinary tract, but rather is generated by the forward
flow of urine, and
therefore reflects arterial blood pressure. Kiil F., "Urinary Flow and
Ureteral Peristalsis" in:
Lutzeyer W., Melchior H. (Eds.) Urodynamics. Springer, Berlin, Heidelberg (pp.
57-70)
(1973).
[00204] Third, the renal pelvis is at least as pliable as the bladder. The
thin wall of the
renal pelvis can expand to accommodate multiple times the normal volume, for
example as
occurs in patients having hydronephrosis.
[00205] More recently, the use of negative pressure in the renal pelvis to
remove blood
clots from the renal pelvis by the use of suction has been cautioned against
because of the
inevitable collapse of the renal pelvis, and as such discourages the use of
negative pressure in
the renal pelvis region. Webb, Percutaneous Renal Surgery: A Practical
Clinical Handbook.
p 92. Springer (2016).
[00206] While not intending to be bound by any theory, the tissues of the
renal pelvis and
bladder are flexible enough to be drawn inwardly during delivery of negative
pressure to
conform to the shape and volume of the tool being used to deliver negative
pressure.
Analogous to the vacuum sealing of a husked ear of corn, the urothelial tissue
will collapse
around and conform to the source of negative pressure. To prevent the tissue
from occluding
the lumen and impeding the flow of urine, the present inventors theorized that
a protective
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
28
surface area sufficient to maintain the fluid column when mild negative
pressure is applied
would prevent or inhibit occlusion.
[00207] The present inventors determined that there are specific features that
enable a
catheter tool to be deployed successfully in and deliver negative pressure
through the
urological region that have not been previously described. These require a
deep
understanding of the anatomy and physiology of the treatment zone and adjacent
tissues. The
catheter must comprise a protective surface area within the renal pelvis by
supporting the
urothelium and inhibiting the urothelial tissue from occluding openings in the
catheter during
application of negative pressure through the catheter lumen. For example,
establishing a
three dimensional shape or void volume, that is free or essentially free from
urothelial tissue,
ensures the patency of the fluid column or flow from each of the million
nephrons into the
drainage lumen of the catheter.
[00208] Since the renal pelvis is comprised of longitudinally oriented smooth
muscle cells,
the protective surface area would ideally incorporate a multi-planar approach
to establishing
the protected surface area. Anatomy is often described in three planes,
sagittal (vertical front
to back that divides the body into right and left parts), coronal (vertical
side to side dividing
the body into dorsal and ventral parts) and transverse (horizontal or axial
that divides the
body into superior and inferior parts, and is perpendicular to the sagittal
and coronal planes).
The smooth muscle cells in the renal pelvis are oriented vertically. It is
desirable for the
catheter to also maintain a radial surface area across the many transverse
planes between the
kidney and the ureter. This enables a catheter to account for both
longitudinal and horizontal
portions of the renal pelvis in the establishment of a protective surface area
1001. In
addition, given the flexibility of the tissues, the protection of these
tissues from the openings
or orifices that lead to the lumen of the catheter tool is desirable. The
catheters discussed
herein can be useful for delivering negative pressure, positive pressure, or
can be used at
ambient pressure, or any combination thereof.
[00209] In some examples, a deployable/retractable expansion mechanism is
utilized that,
when deployed, creates and/or maintains a patent fluid column or flow between
the kidney
and the catheter drainage lumen. This deployable/retractable mechanism, when
deployed,
creates the protective surface area 1001 within the renal pelvis by supporting
the urothelium
and inhibiting the urothelial tissue from occluding openings in the catheter
during application
of negative pressure through the catheter lumen. In some examples, the
retention portion is
configured to be extended into a deployed position in which a diameter of the
retention
portion is greater than a diameter of the drainage lumen portion.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
29
[00210] With reference to FIGS. 1A-1C, 1F, 1P, 1U, 2A, 2B, 7A, 7B, 17, and 44,
the
urinary tract, indicated generally at 1, comprises a patient's right kidney 2
and left kidney 4.
As discussed above, the kidneys 2, 4 are responsible for blood filtration and
clearance of
waste compounds from the body through urine. Urine produced by the right
kidney 2 and the
left kidney 4 is drained into a patient's bladder 10 through tubules, namely a
right ureter 6
and a left ureter 8. For example, urine may be conducted through the ureters
6, 8 by
peristalsis of the ureter walls, as well as by gravity. The ureters 6, 8 enter
the bladder 10
through a ureter orifice or opening 16. The bladder 10 is a flexible and
substantially hollow
structure adapted to collect urine until the urine is excreted from the body.
The bladder 10 is
transitionable from an empty position (signified by reference line E) to a
full position
(signified by reference line F). When the bladder is in the empty position E,
the bladder
superior wall 70 can be positioned adjacent to and/or conform to the outer
periphery 72, 1002
or protective surface area 1001 of the distal end 136 of the bladder catheter
56, 116, shown
for example in FIGS. 1A and 1B as mesh 57, in FIGS. 1C, 1U and 7A as coil
1210, in FIG.
1F as a basket shaped structure or support cap 212 of a bladder superior wall
support 210, in
FIG. 1P as an annular balloon 310, and in FIG. 17 as funnel 116. Normally,
when the
bladder 10 reaches a substantially full state, urine is permitted to drain
from the bladder 10 to
a urethra 12 through a urethral sphincter or opening 18 located at a lower
portion of the
bladder 10. Contraction of the bladder 10 can be responsive to stresses and
pressure exerted
on a trigone region 14 of the bladder 10, which is the triangular region
extending between the
ureteral openings 16 and the urethral opening 18. The trigone region 14 is
sensitive to stress
and pressure, such that as the bladder 10 begins to fill, pressure on the
trigone region 14
increases. When a threshold pressure on the trigone region 14 is exceeded, the
bladder 10
begins to contract to expel collected urine through the urethra 12.
[00211] Similarly, as shown in FIGS. 1A, 1B, 1C, 1F, 1P, 1U, 2A and 2B, for
example, the
outer periphery 72, 1002 or protective surface area 1001 of the ureteral
catheters 112, 114 of
the present invention can support tissue 1003 of the ureter and/or kidney to
maintain patency
of fluid flow between the kidney and the bladder of the patient.
[00212] In some examples, methods and systems 50, 100, as shown for example in
FIGS. 1A, 1B, 1C, 1F, 1P, 1U, 2A, 2B, 7, 17, and 44, are provided for removing
fluid (such
as urine) from a patient, the method comprising: deploying a ureteral stent
52, 54 (shown in
FIG. 1A) or ureteral catheter 112, 114 (shown in FIGS. 1B, 1C, 1F, 1P, 1U, 2A,
2B, 7, 17,
and 44) into a ureter 6, 8 of a patient to maintain patency of fluid flow
between a kidney 2, 4
and a bladder 10 of the patient; and/or deploying a bladder catheter 56, 116
into the bladder
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
10 of the patient, wherein the bladder catheter 56, 116 comprises a distal end
136 configured
to be positioned in a patient's bladder 10, a drainage lumen portion 140
having a proximal
end 117, and a sidewall 119 extending therebetween; and applying negative
pressure to the
proximal end 117 of the bladder catheter 56, 116 and/or ureteral catheter(s)
112, 114 to
induce negative pressure in a portion of the urinary tract of the patient to
remove fluid from
the patient. In some examples, the method further comprises deploying a second
ureteral
stent or second ureteral catheter into a second ureter or kidney of the
patient to maintain
patency of fluid flow between a second kidney and the bladder of the patient,
as shown in
FIGS. 1A, 1B, 1C, 1F, 1P, 1U, 2A, 2B, 7, 17, and 44. Specific characteristics
of exemplary
ureteral stents or ureteral catheters of the present invention are described
in detail herein.
[00213] In some non-limiting examples, the ureteral or bladder catheter 56,
112, 114, 116,
312, 412, 512, 812, 1212, 5000, 5001 comprises (a) a proximal portion 117,
128, 1228, 5006,
5007, 5017 and (b) a distal portion 118, 318, 1218, 5004, 5005, the distal
portion comprising
a retention portion 130, 330, 410, 500, 1230, 1330, 2230, 3230, 4230, 5012,
5013 that
comprises one or more protected drainage holes, ports or perforations 133,
533, 1233 and is
configured to establish an outer periphery 1002 or protective surface area
1001 that inhibits
urothelial tissue, such as mucosal tissue 1003 of the ureter and/or kidney and
bladder tissue
1004, from occluding the one or more protected drainage holes, ports or
perforations 133,
533, 1233 upon application of negative pressure through the catheter.
Exemplary ureteral catheters:
[00214] As shown in FIGS. 2A, 7, 17, and 44, examples of systems 100 including
ureteral
catheters 112, 114 configured to be positioned within the urinary tract of a
patient are
illustrated. For example, distal ends 120, 121, 1220, 5019, 5021 of the
ureteral catheters 112,
114 can be configured to be deployed in at least one of the patient's ureters
2, 4; renal pelvis
20, 21 area of the kidneys 6, 8; or the kidneys 6, 8.
[00215] In some examples, suitable ureteral catheters are disclosed in US
Patent
No. 9,744,331, US Patent Application Publication No. US 2017/0021128 Al, US
Patent
Application No. 15/687,064, and US Patent Application No. 15/687,083, each of
which is
incorporated by reference herein.
[00216] In some examples, the system 100 can comprise two separate ureteral
catheters,
such as a first catheter 112 disposed in or adjacent to the renal pelvis 20 of
the right kidney 2
and a second catheter 114 disposed in or adjacent to the renal pelvis 21 of
the left kidney 4.
The catheters 112, 114 can be separate for their entire lengths, or can be
held in proximity to
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
31
one another by a clip, ring, clamp, or other type of connection mechanism
(e.g., connector) to
facilitate placement or removal of the catheters 112, 114. As shown in FIGS.
2A, 7, 17, 27,
and 44, the proximal end 113, 115 of each catheter 112, 114 is positioned
within the bladder
10, or at the proximal end of the ureter near the bladder 10, such that the
fluid or urine drains
into the bladder. In some examples, the proximal end 113, 115 of each catheter
112, 114 can
be in fluid communication with the distal portion or end 136 of a bladder
catheter 56, 116. In
some examples, catheters 112, 114 can merge or be connected together within
the bladder to
form a single drainage lumen that drains into the bladder 10.
[00217] As shown in FIG. 2A, in some examples, the proximal end 113, 115 of
one or both
of the catheters 112, 114 can be positioned within the urethra 12 and
optionally connected to
additional drainage tubing to drain fluid to the outside of the body of the
patient. As shown
in FIG. 2B, in some examples, the proximal end 113, 115 of one or both of the
catheters 112,
114 can be positioned to extend from the urethra 12 outside of the body of the
patient.
[00218] In other examples, the catheters 112, 114 can be inserted through or
enclosed
within another catheter, tube, or sheath along portions or segments thereof to
facilitate
insertion and retraction of the catheters 112, 114 from the patient's body.
For example, a
bladder catheter 116 can be inserted over and/or along the same guidewire as
the ureteral
catheters 112, 114, or within the same tubing used to insert the ureteral
catheters 112, 114.
[00219] With reference to FIGS. 1B, 1C, 1F, 1P, 1U, 2A, 2B, 7, 8A, and 8B, an
exemplary
ureteral catheter 112, 1212, 5000 can comprise at least one elongated body or
tube 122, 1222,
5009 the interior of which defines or comprises one or more drainage
channel(s) or lumen(s),
such as drainage lumen 124, 1224, 5002. The tube 122, 1222, 5009 size can
range from
about 1 Fr to about 9 Fr (French catheter scale). In some examples, the tube
122, 1222, 5009
can have an external diameter ranging from about 0.33 to about 3 mm, and an
internal
diameter ranging from about 0.165 to about 2.39 mm. In one example, the tube
122 is 6 Fr
and has an outer diameter of 2.0 0.1 mm. The length of the tube 122, 1222,
5009 can range
from about 30 cm to about 120 cm depending on the age (e.g., pediatric or
adult) and gender
of the patient.
[00220] The tube 122, 1222, 5009 can be formed from a flexible and/or
deformable
material to facilitate advancing and/or positioning the tube 122, 1222, 5009
in the bladder 10
and ureters 6, 8 (shown in FIGS. 2 and 7). The catheter material should be
flexible and soft
enough to avoid or reduce irritation of the renal pelvis and ureter, but
should be rigid enough
that the tube 122, 1222, 5009 does not collapse when the renal pelvis or other
portions of the
urinary tract exert pressure on the exterior of the tube 122, 1222, 5009, or
when the renal
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
32
pelvis and/or ureter are drawn against the tube 122, 1222, 5009 during
inducement of
negative pressure. For example, the tube 122, 1222, 5009 or drainage lumen can
be formed,
at least in part, from one or more materials including copper, silver, gold,
nickel-titanium
alloy, stainless steel, titanium, and/or polymer such as biocompatible
polymer(s),
polyurethane, polyvinyl chloride, polytetrafluoroethylene (PTFE), latex,
silicon coated latex,
silicon, silicone, polyglycolide or poly(glycolic acid) (PGA), Polylactide
(PLA), Poly(lactide-
co-glycolide), Polyhydroxyalkanoates, Polycaprolactone and/or Poly(propylene
fumarate).
In one example, the tube 122, 1222, 5009 is formed from a thermoplastic
polyurethane. The
tube 122, 1222, 5009 can also include or be impregnated with one or more of
copper, silver,
gold, nickel-titanium alloy, stainless steel, and titanium. In some examples,
the tube 122,
1222, 5009 is impregnated with or formed from a material viewable by
fluoroscopic imaging.
For example, the biocompatible polymer which forms the tube 122, 1222, 5009
can be
impregnated with barium sulfate or a similar radiopaque material. As such, the
structure and
position of the tube 122, 1222, 5009 is visible to fluoroscopy.
[00221] At least a portion or all of the interior or exterior of the catheter
112, 1212, 5000,
for example tube 122, 1222, 5009 can be coated with a hydrophilic coating to
facilitate
insertion and/or removal, and/or to enhance comfort. In some examples, the
coating is a
hydrophobic and/or lubricious coating. For example, suitable coatings can
comprise
ComfortCoat@ hydrophilic coating which is available from Koninklijke DSM N.V.
or
hydrophilic coatings comprising polyelectrolyte(s) such as are disclosed in
United States
Patent No. 8,512,795, which is incorporated herein by reference.
[00222] In some examples, as shown in FIG. 8B, for example, the tube 122 can
comprise:
a distal portion 118 (e.g., a portion of the tube 122 configured to be
positioned in the ureter 6,
8 and renal pelvis 20, 21); a middle portion 126 (e.g., a portion of the tube
122 configured to
extend from the distal portion 118 through the ureteral openings 16 into the
patient's bladder
and urethra 12); and a proximal portion 128 (e.g., a portion of the tube 122
extending into
the bladder 10, or urethra 12, or extending from the urethra 12 outside of the
body of the
patient). In one example, the combined length of the proximal portion 128 and
the middle
portion 126 of the tube 122 is about 54 2 cm. In some examples, the tube 122
terminates in
the bladder 10. In that case, fluid drains from the proximal end of the
ureteral catheter 112,
114 and is directed from the body through the additional indwelling bladder
catheter. In
other examples, the tube 122 terminates in the urethra 12, e.g., a bladder
catheter is not
required. In other examples, the tube extends from the urethra 12 outside of
the body of the
patient, e.g., a bladder catheter is not required.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
33
Exemplary ureteral retention portions:
[00223] Any of the retention portions disclosed herein can be formed from the
same
material as the drainage lumen discussed above, and can be unitary with or
connected to the
drainage lumen, or the retention portion can be formed from a different
material, such as
those that are discussed above for the drainage lumen, and connected thereto.
For example,
the retention portion can be formed from any of the aforementioned materials,
for example a
polymer such as polyurethane, flexible polyvinyl chloride,
polytetrafluoroethylene (PTFE),
latex, silicone, silicon, polyglycolide or poly(glycolic acid) (PGA),
Polylactide (PLA),
Poly(lactide-co-glycolide), Polyhydroxyalkanoates. Polycaprolactone and/or
Poly(propylene
fumarate).
[00224] Generally, and as shown for example in FIGS. 2A-C, 8A, and 8B, the
distal
portion 118 of the ureteral catheter 112 comprises a retention portion 130 for
maintaining the
distal end 120 of the catheter 112 at a desired fluid collection position
proximate to or within
the renal pelvis 20, 21 of the kidney 2, 4. In some examples, the retention
portion 130 is
configured to be flexible and bendable to permit positioning of the retention
portion 130 in
the ureter and/or renal pelvis. The retention portion 130 is desirably
sufficiently bendable to
absorb forces exerted on the catheter 112 and to prevent such forces from
being translated to
the ureters. For example, if the retention portion 130 is pulled in the
proximal direction P
(shown in FIG. 9A) toward the patient's bladder, the retention portion 130 can
be sufficiently
flexible to begin to unwind or be straightened so that it can be drawn through
the ureter.
Similarly, when the retention portion 130 can be reinserted into the renal
pelvis or other
suitable region within the ureter, it can be biased to return to its deployed
configuration.
[00225] In some examples, the retention portion 130 is integral with the tube
122. In that
case, the retention portion 130 can be formed by imparting a bend or curl to
the catheter
body 122 that is sized and shaped to retain the catheter at a desired fluid
collection location.
Suitable bends or coils can include a pigtail coil, corkscrew coil, and/or
helical coil, such as
are shown in FIGS. 1, 2A, 7A, and 8A-10G. For example, the retention portion
130 can
comprise one or more radially and longitudinally extending helical coils
configured to contact
and passively retain the catheter 112 within the ureter 6, 8 proximate to or
within the renal
pelvis 20, 21, as shown for example in FIGS. 2A, 7A, and 8A-10G. In other
examples, the
retention portion 130 is formed from a radially flared or tapered portion of
the catheter body
122. For example, the retention portion 130 can further comprise a fluid
collecting portion,
as shown in FIGS. 17-41C, such as a tapered or funnel-shaped inner surface
186. In other
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
34
examples, the retention portion 130 can comprise a separate element connected
to and
extending from the catheter body or tube 122.
[00226] In some examples, the retention portion 130 can further comprise one
or more
perforated sections, such as drainage holes, perforations or ports 132, 1232
(shown, for
example, in FIGS. 9A-9E, 10A, 10E, 11-14, 27, 32A, 32B, 33, 34, and 39-41A-C).
A
drainage port 132 can be located, for example, at the open distal end 120, 121
of the tube 122,
as shown in FIG. 10D. In other examples, perforated sections and/or drainage
ports 132,
1232 are disposed along the sidewall 109 of the distal portion 118 of the
catheter tube 122, as
shown in FIGS. 9A-9E, 10A, 10E, 11-14, 27, 32A, 32B, 33, 34, and 41A-C, or
within the
material of the retention portion, such as the sponge material of FIGS. 39 and
40. The
drainage ports or holes 132, 1232 can be used for assisting in fluid
collection by which fluid
can flow into the drainage lumen for removal from the patient's body. In other
examples, the
retention portion 130 is solely a retention structure and fluid collection
and/or imparting
negative pressure is provided by structures at other locations on the catheter
tube 122.
[00227] In some examples, such as are shown in FIGS. 9B-E, 10D-G, 18B, 18C-E,
20,
22A-35, 37B, 38A, 39B, 40A-41C, at least a portion of, most, or all of the
drainage holes,
ports or perforations 132, 1232 are positioned in the ureteral catheter 112,
114 or bladder
catheter 116 in protected surface areas or inner surface areas 1000, such that
tissue 1004,
1003 from the bladder or kidney does not directly contact or partially or
fully occlude the
protected drainage holes, ports or perforations 133. For example, as shown in
FIGS. 2A-2C,
7A, 7B, 10F, 17, 18D, 24B, 29C, 39B, 40B, and 41B, when negative pressure is
induced in
the ureter and/or renal pelvis, a portion of the mucosal tissue 1003 of the
ureter and/or kidney
may be drawn against the outer periphery 72, 1002 or protective surface areas
1001 or outer
regions of the retention portion 130 and may partially or fully occlude some
drainage holes,
ports or perforations 134 positioned on the outer periphery 72, 1002 or
protective surface
areas 1001 of the retention portion 130. Similarly, as shown in FIGS. 2A-2C,
7A, 7B, 10G,
17, 18E, 24C, 39C, 40C, and 41C, when negative pressure is induced in the
bladder, a portion
of the bladder tissue 1004, such as the transitional epithelial tissue lining,
lamina propria
connective tissue, muscularis propria and/or fatty connective tissue, may be
drawn against the
outer periphery 72, 1002 or protective surface areas 1001 or outer regions of
the retention
portion 130 and may partially or fully occlude some drainage holes, ports or
perforations 134
positioned on the outer periphery 1002 or protective surface areas 1001 or
outer regions of
the retention portion 130.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
[00228] At least a portion of protected drainage ports 133 located on the
protected surface
areas or inner surface areas 1000 of the retention portion 130 would not be
partially or fully
occluded when such tissues 1003, 1004 contact the outer periphery 72, 1002 or
protective
surface areas 1001 or outer regions of the retention portion 130. Further,
risk of injury to the
tissues 1003, 1004 from pinching or contact with the drainage ports 133 can be
reduced or
ameliorated. The configuration of the outer periphery 72, 1002 or protective
surface areas
1001 or outer regions of the retention portion 130 depends upon the overall
configuration of
the retention portion 130. Generally, the outer periphery 72, 1002 or
protective surface areas
1001 or outer regions of the retention portion 130 contacts and supports the
bladder 1004 or
kidney tissue 1003, and thereby inhibits occlusion or blockage of the
protected drainage
holes, ports or perforations 133.
[00229] For example, as shown in FIG. 10E-G, there is shown an exemplary
retention
portion 1230 comprising a plurality of helical coils 1280, 1282, 1284. The
outer periphery
1002 or protective surface areas 1001 or outer regions of the helical coils
1280, 1282, 1284
contact and support the bladder tissue 1004 or kidney tissue 1003 to inhibit
occlusion or
blockage of protected drainage holes, ports or perforations 1233 positioned in
protected
surface areas or inner surface areas 1000 of the helical coils 1280, 1282,
1284. The outer
periphery 1002 or protective surface areas 1001 or outer regions of the
helical coils 1280,
1282, 1284 provides protection for the protected drainage holes, ports or
perforations 1233.
In FIG. 10F, the kidney tissue 1003 is shown surrounding and contacting at
least a portion of
the outer periphery 1002 or protective surface areas 1001 or outer regions of
the helical coils
1280, 1282, 1284, which inhibits contact of the kidney tissue 1003 with the
protected surface
areas or inner surface areas 1000 of the helical coils 1280, 1282, 1284, and
thereby inhibits
partial or full blockage of the protected drainage holes, ports or
perforations 1233 by the
kidney tissue 1003. In FIG. 10G, the bladder tissue 1004 is shown surrounding
and
contacting at least a portion of the outer periphery 1002 or protective
surface areas 1001 or
outer regions of the helical coils 1280, 1282, 1284, which inhibits contact of
the bladder
tissue 1004 with the protected surface areas or inner surface
areas 1000 of the helical coils 1280, 1282, 1284, and thereby inhibits partial
or full blockage
of the protected drainage holes, ports or perforations 1233 by the bladder
tissue 1004.
[00230] Similarly, other examples of configurations of bladder and/or ureteral
retention
portions shown in FIGS. 1, 2A, 7A, 17, 18A, 18B, 18C, 19, 20, 21, 22A, 22B,
23A, 23B, 24,
25, 26, 27, 28A, 28B, 29A, 29B, 30, 31, 32A, 32B, 33, 34, 35A, 35B, 36, 37A,
37B, 38A,
38B, 39, 40, and 41 provide an outer periphery 1002 or protective surface
areas 1001 or outer
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
36
regions which can contact and support the bladder tissue 1004 or kidney tissue
1003 to inhibit
occlusion or blockage of protected drainage holes, ports or perforations 133,
1233 positioned
in protected surface areas or inner surface areas 1000 of the retention
portions. Each of these
examples will be discussed further below.
[00231] Referring now to FIGS. 8A, 8B, and 9A-9E, exemplary retention portions
130 for
ureteral catheters or bladder catheters comprising a plurality of helical
coils, such as one or
more full coils 184 and one or more half or partial coils 183, are
illustrated. The retention
portion 130 is capable of moving between a contracted position and the
deployed position
with the plurality of helical coils. For example, a substantially straight
guidewire can be
inserted through the retention portion 130 to maintain the retention portion
130 in a
substantially straight contracted position. When the guidewire is removed, the
retention
portion 130 can transition to its coiled configuration. In some examples, the
coils 183, 184
extend radially and longitudinally from the distal portion 118 of the tube
122. With specific
reference to FIGS. 8A and 8B, in an exemplary embodiment, the retention
portion 130
comprises two full coils 184 and one half coil 183. For example, as shown in
FIGS. 8A and
8B, the outer diameter of the full coils 184, shown by line D1, can be about
18 2 mm, the
half coil 183 diameter D2 can be about 14 mm 2 mm, and the coiled retention
portion 130
can have a height H of about 16 2 mm.
[00232] The retention portion 130 can further comprise the one or more
drainage holes
132, 1232 (shown in FIGS. 9A-9E, 10A and 10E, for example) configured to draw
fluid into
an interior of the catheter tube 122. In some examples, the retention portion
130 can
comprise two, three, four, five, six, seven, eight or more drainage holes 132,
1232, plus an
additional hole 110 at the distal tip or end 120 of the retention portion. In
some examples, the
diameter of each of the drainage holes 132, 1232 (shown in FIGS. 9A-9E, 10A
and 10E, for
example) can range from about 0.7 mm to 0.9 mm and, preferably, is about 0.83
0.01 mm.
In some examples, the diameter of the additional hole 110 at the distal tip or
end of the
retention portion 130 (shown in FIGS. 9A-9E, 10A and 10E, for example) can
range from
about 0.165 mm to about 2.39 mm, or about 0.7 to about 0.97 mm. The distance
between
adjacent drainage holes 132, specifically the linear distance between the
closest outer edges
of adjacent drainage holes 132, 1232 when the coils are straightened, can be
about 15 mm
2.5 mm, or about 22.5 2.5 mm or more.
[00233] As shown in FIGS. 9A-9E, in another exemplary embodiment, the distal
portion
118 of the drainage lumen 124 proximal to the retention portion 130 defines a
straight or
curvilinear central axis L. In some examples, at least a half or first coil
183 and a full or
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
37
second coil 184 of the retention portion 130 extend about an axis A of the
retention portion
130. The first coil 183 initiates or begins at a point where the tube 122 is
bent at an angle a
ranging from about 15 degrees to about 75 degrees from the central axis L, as
indicated by
angle a, and preferably about 45 degrees. As shown in FIGS. 9A and 9B, prior
to insertion in
the body, the axis A can be coextensive with the longitudinal central axis L.
In other
examples, as shown in FIGS. 9C-9E, prior to insertion in the body, the axis A
extends from
and is curved or angled, for example at angle 13, relative to the central
longitudinal axis L.
[00234] In some examples, multiple coils 184 can have the same or different
inner and/or
outer diameter D and height H2 between adjacent coils 184. In that case, the
outer diameter
D1 of each of the coils 184 may range from about 10 mm to about 30 mm. The
height H2
between each of the adjacent coils 184 may range from about 3 mm to about 10
mm.
[00235] In other examples, the retention portion 130 is configured to be
inserted in the
tapered portion of the renal pelvis. For example, the outer diameter D1 of the
coils 184 can
increase toward the distal end 120 of the tube 122, resulting in a helical
structure having a
tapered or partially tapered configuration. For example, the distal or maximum
outer
diameter D of the tapered helical portion ranges from about 10 mm to about 30
mm, which
corresponds to the dimensions of the renal pelvis, and the outer diameter D1
of each adjacent
coil can decrease closer to the proximal end 128 of the retention portion 130.
The overall
height H of the retention portion 130 can range from about 10 mm to about 30
mm.
[00236] In some examples, the outer diameter D1 of each coil 184 and/or height
H2
between each of the coils 184 can vary in a regular or irregular fashion. For
example, the
outer diameter D1 of coils or height H2 between adjacent coils can increase or
decrease by a
regular amount (e.g., about 10% to about 25% between adjacent coils 184). For
example, for
a retention portion 130 having three coils (as shown, for example, in FIGS. 9A
and 9B) an
outer diameter D2 of a proximal-most coil or first coil 183 can be about 6 mm
to 18 mm, an
outer diameter D3 of a middle coil or second coil 185 can be about 8 mm to
about 24 mm,
and an outer diameter D13 of a distal-most or third coil 187 can be between
about 10 mm and
about 30 mm.
[00237] The retention portion 130 can further comprise the drainage
perforations, holes or
ports 132 disposed on or through the sidewall 109 of the catheter tube 122 on,
or adjacent to,
the retention portion 130 to permit urine waste to flow from the outside of
the catheter tube
122 to the inside drainage lumen 124 of the catheter tube 122. The position
and size of the
drainage ports 132 can vary depending upon the desired flow rate and
configuration of the
retention portion 130. The diameter D1 1 of each of the drainage ports 132 can
range
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
38
independently from about 0.005 mm to about 1.0 mm. The spacing D12 between the
closest
edge of each of the drainage ports 132 can range independently from about 1.5
mm to about 5
mm. The drainage ports 132 can be spaced in any arrangement, for example,
random, linear
or offset. In some examples, the drainage ports 132 can be non-circular, and
can have a
surface area of about 0.00002 to 0.79 mm2.
[00238] In some examples, as shown in FIG. 9A, the drainage ports 132 are
located around
the entire outer periphery 72, 1002 or protective surface area 1001 of the
sidewall 109 of the
catheter tube 122 to increase an amount of fluid that can be drawn into the
drainage lumen
124 (shown in FIGS. 2, 9A, and 9B). In other examples, as shown in FIGS. 9B-9E
and 10-
10E, the drainage holes, ports or perforations 132 can be disposed essentially
only or only on
the protected surface areas or inner surface areas 1000 or radially inwardly
facing side 1286
of the coils 184 to prevent occlusion or blockage of the drainage ports 132,
1232 and the
outwardly facing side 1288 of the coils may be essentially free of drainage
ports 132, 1232 or
free of drainage ports 132, 1232. The outer periphery 72, 189, 1002 or
protective surface
area 1001 or outer regions 192 of the helical coils 183, 184, 1280, 1282, 1284
can contact and
support the bladder tissue 1004 or kidney tissue 1003 to inhibit occlusion or
blockage of
protected drainage holes, ports or perforations 133, 1233 positioned in
protected surface areas
or inner surface areas 1000 of the helical coils 183, 184, 1280, 1282, 1284.
For example,
when negative pressure is induced in the ureter and/or renal pelvis, mucosal
tissue of the
ureter and/or kidney may be drawn against the retention portion 130 and may
occlude some
drainage ports 134 on the outer periphery 72, 189, 1002 of the retention
portion 130.
Drainage ports 133, 1233 located on the radially inward side 1286 or protected
surface areas
or inner surface areas 1000 of the retention structure would not be
appreciably occluded when
such tissues 1003, 1004 contact the outer periphery 72, 189, 1002 or
protective surface area
1001 or outer regions of the retention portion 130. Further, risk of injury to
the tissues from
pinching or contact with the drainage ports 132, 133, 1233, or protected
drainage holes, ports
or perforations 133, 1233 can be reduced or ameliorated.
[00239] With reference to FIGS. 9C and 9D, other examples of ureteral
catheters 112
having a retention portion 130 comprising a plurality of coils 184 are
illustrated. As shown
in FIG. 9C, the retention portion 130 comprises three coils 184 extending
about the axis A.
The axis A is a curved arc extending from the central longitudinal axis L of
the portion of the
drainage lumen 181 proximal to the retention portion 130. The curvature
imparted to the
retention portion 130 can be selected to correspond to the curvature of the
renal pelvis, which
comprises a cornucopia-shaped cavity.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
39
[00240] As shown in FIG. 9D, in another exemplary embodiment, the retention
portion
130 can comprise two coils 184 extending about an angled axis A. The angled
axis A
extends at an angle from a central longitudinal axis L, and is angled, as
shown by angle 13,
relative to an axis generally perpendicular to the central axis L of the
portion of the drainage
lumen. The angle 13 can range from about 15 to about 75 degrees (e.g., about
105 to about
165 degrees relative to the central longitudinal axis L of the drainage lumen
portion of the
catheter 112).
[00241] FIG. 9E shows another example of a ureteral catheter 112. The
retention portion
comprises three helical coils 184 extending about an axis A. The axis A is
angled, as shown
by angle 13, relative to the horizontal. As in the previously-described
examples, the angle
can range from about 15 to about 75 degrees (e.g., about 105 to about 165
degrees relative to
the central longitudinal axis L of the drainage lumen portion of the catheter
112).
[00242] In some examples shown in FIGS. 10-10E, the retention portion 1230 is
integral
with the tube 1222. In other examples, the retention portion 1230 can comprise
a separate
tubular member connected to and extending from the tube or drainage lumen
1224.
[00243] In some examples, the retention portion comprises a plurality of
radially extending
coils 184. The coils 184 are configured in the shape of a funnel, and thereby
form a funnel
support. Some examples of coil funnel supports are shown in FIGS. 2A-C, 7A,
7B, 8A, and
8A-10E.
[00244] In some examples, the at least one sidewall 119 of the funnel support
comprises at
least a first coil 183 having a first diameter and a second coil 184 having a
second diameter,
the first diameter being less than the second diameter, wherein the maximum
distance
between a portion of a sidewall of the first coil and a portion of an adjacent
sidewall of the
second coil ranges from about 0 mm to about 10 mm. In some examples, the first
diameter of
the first coil 183 ranges from about 1 mm to about 10 mm and the second
diameter of the
second coil 184 ranges from about 5 mm to about 25 mm. In some examples, the
diameter of
the coils increases toward a distal end of the drainage lumen, resulting in a
helical structure
having a tapered or partially tapered configuration. In some embodiments, the
second coil
184 is closer to an end of the distal portion 118 of the drainage lumen 124
than is the first coil
183. In some examples, the second coil 184 is closer to an end of the proximal
portion 128 of
the drainage lumen 124 than is the first coil 183.
[00245] In some examples, the at least one sidewall 119 of the funnel support
comprises an
inwardly facing side 1286 and an outwardly facing side 1288, the inwardly
facing side 1286
comprising at least one opening 133, 1233 for permitting fluid flow into the
drainage lumen,
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
the outwardly facing side 1288 being essentially free of or free of openings,
as discussed
below. In some examples, the at least one opening 133, 1233 has an area
ranging from about
0.002 mm2 to about 100 mm2.
[00246] In some examples, the first coil 1280 comprises a sidewall 119
comprising a
radially inwardly facing side 1286 and a radially outwardly facing side 1288,
the radially
inwardly facing side 1286 of the first coil 1280 comprising at least one
opening 1233 for
permitting fluid flow into the drainage lumen.
[00247] In some examples, the first coil 1280 comprises a sidewall 119
comprising a
radially inwardly facing side 1286 and a radially outwardly facing side 1288,
the radially
inwardly facing side 1286 of the first coil 1280 comprising at least two
openings 1233 for
permitting fluid flow into the drainage lumen 1224.
[00248] In some examples, the first coil 1280 comprises a sidewall 119
comprising a
radially inwardly facing side 1286 and a radially outwardly facing side 1288,
the radially
outwardly facing side 1288 of the first coil 1280 being essentially free of or
free of one or
more openings 1232.
[00249] In some examples, the first coil 1280 comprises a sidewall 119
comprising a
radially inwardly facing side 1286 and a radially outwardly facing side 1288,
the radially
inwardly facing side 1286 of the first coil 1280 comprising at least one
opening 1233 for
permitting fluid flow into the drainage lumen 1224 and the radially outwardly
facing side
1288 being essentially free of or free of one or more openings 1232.
[00250] Referring now to FIGS. 10-10E, in some examples, the distal portion
1218
comprises an open distal end 1220 for drawing fluid into the drainage lumen
1224. The distal
portion 1218 of the ureteral catheter 1212 further comprises a retention
portion 1230 for
maintaining the distal portion 1218 of the drainage lumen or tube 1222 in the
ureter and/or
kidney. In some examples, the retention portion 1230 comprises a plurality of
radially
extending coils 1280, 1282, 1284. The retention portion 1230 can be flexible
and bendable to
permit positioning of the retention portion 1230 in the ureter, renal pelvis,
and/or kidney. For
example, the retention portion 1230 is desirably sufficiently bendable to
absorb forces
exerted on the catheter 1212 and to prevent such forces from being translated
to the ureters.
Further, if the retention portion 1230 is pulled in the proximal direction P
(shown in FIGS.
9A-9E) toward the patient's bladder 10, the retention portion 1230 can be
sufficiently flexible
to begin to unwind or be straightened so that it can be drawn through the
ureter 6, 8. In some
examples, the retention portion 1230 is integral with the tube 1222. In other
examples, the
retention portion 1230 can comprise a separate tubular member connected to and
extending
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
41
from the tube or drainage lumen 1224. In some examples, the catheter 1212
comprises a
radiopaque band 1234 (shown in FIG. 29) positioned on the tube 1222 at a
proximal end of
the retention portion 1230. The radiopaque band 1234 is visible by
fluoroscopic imaging
during deployment of the catheter 1212. In particular, a user can monitor
advancement of the
band 1234 through the urinary tract by fluoroscopy to determine when the
retention portion
1230 is in the renal pelvis and ready for deployment.
[00251] In some examples, the retention portion 1230 comprises perforations,
drainage
ports, or openings 1232 in a sidewall of the tube 1222. As described herein, a
position and
size of the openings 1232 can vary depending upon a desired volumetric flow
rate for each
opening and size constraints of the retention portion 1230. In some examples,
a diameter
Dll of each of the openings 1232 can range independently from about 0.05 mm to
about 2.5
mm and have an area of about 0.002 mm2 to about 5 mm2. Openings 1232 can be
positioned
extending along on a sidewall 119 of the tube 1222 in any direction desired,
such as
longitudinal and/or axial. In some examples, spacing between the closest
adjacent edge of
each of the openings 1232 can range from about 1.5 mm to about 15 mm. Fluid
passes
through one or more of the perforations, drainage ports, or openings 1232 and
into the
drainage lumen 1234. Desirably, the openings 1232 are positioned so that they
are not
occluded by tissues 1003 of the ureters 6, 8 or kidney when negative pressure
is applied to the
drainage lumen 1224. For example, as described herein, openings 1233 can be
positioned on
interior portions or protected surfaces areas 1000 of coils or other
structures of the retention
portion 1230 to avoid occlusion of the openings 1232, 1233. In some examples,
the middle
portion 1226 and proximal portion 1228 of the tube 1222 can be essentially
free of or free
from perforations, ports, openings or openings to avoid occlusion of openings
along those
portions of the tube 1222. In some examples, a portion 1226, 1228 which is
essentially free
from perforations or openings includes substantially fewer openings 1232 than
other portions
such as distal portion 1218 of the tube 1222. For example, a total area of
openings 1232 of
the distal portion 1218 may be greater than or substantially greater than a
total area of
openings of the middle portion 1226 and/or the proximal portion 1228 of the
tube 1222.
[00252] In some examples, the openings 1232 are sized and spaced to improve
fluid flow
through the retention portion 1230. In particular, the present inventors have
discovered that
when a negative pressure is applied to the drainage lumen 1224 of the catheter
1212 a
majority of fluid is drawn into the drainage lumen 1224 through proximal-most
perforations
or openings 1232. In order to improve flow dynamics so that fluid is also
received through
more distal openings and/or through the open distal end 1220 of the tube 1222,
larger size or
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
42
a greater number of openings 1232 can be provided towards the distal end 1220
of the
retention portion 1230. For example, a total area of openings 1232 on a length
of tube 1222
near a proximal end 1228 of the retention portion 1230 may be less than a
total area of
openings 1232 of a similar sized length of the tube 1222 located near the open
distal end
1220 of the tube 1222. In particular, it may be desirable to produce a flow
distribution
through the drainage lumen 1224 in which less than 90%, preferably less than
70%, and,
more preferably, less than 55% of fluid flow is drawn into the drainage lumen
1224 through a
single opening 1232 or a small number of openings 1232 positioned near the
proximal end
1228 of the retention portion 1230.
[00253] In many examples, the openings 1232 are generally a circular shape,
although
triangular, elliptical, square-shaped, diamond shaped, and any other opening
shapes may also
be used. Further, as will be appreciated by one of ordinary skill in the art,
a shape of the
openings 1232 may change as the tube 1222 transitions between an uncoiled or
elongated
position and a coiled or deployed position. It is noted that while the shape
of the openings
1232 may change (e.g., the orifices may be circular in one position and
slightly elongated in
the other position), the area of the openings 1232 is substantially similar in
the elongated or
uncoiled position compared to the deployed or coiled position.
[00254] In some examples, the drainage lumen 1224 defined by tube 1222
comprises: a
distal portion 1218 (e.g., a portion of the tube 1222 configured to be
positioned in the ureter
6, 8 and renal pelvis 20, 21 (shown for example in FIGS. 7A and 10)); a middle
portion 1226
(e.g., a portion of the tube 1222 configured to extend from the distal portion
through ureteral
openings 16 into the patient's bladder 10 and urethra 12 (shown in FIGS. 7A
and 10)); and a
proximal portion 1228 (e.g., a portion of the tube 1222 extending from the
urethra 12 to an
external fluid collection container and/or pump 2000). In one example, the
combined length
of the proximal portion 1228 and the middle portion 1226 of the tube 1222 is
about 54 2
cm. In some examples, the middle portion 1226 and proximal portion 1228 of the
tube 1222
includes distance markings 1236 (shown in FIG. 10) on a sidewall of the tube
1222 which
can be used, during deployment of the catheter 1212, to determine how far the
tube 1222 is
inserted into the urinary tract of the patient.
[00255] As shown in FIGS. 7A and 10-14, an exemplary ureteral catheter 1212
comprises
at least one elongated body or tube 1222, the interior of which defines or
comprises one or
more drainage channel(s) or lumen(s), such as drainage lumen 1224. The tube
1222 size can
range from about 1 Fr to about 9 Fr (French catheter scale). In some examples,
the tube 1222
can have an external diameter ranging from about 0.33 to about 3.0 mm, and an
internal
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
43
diameter ranging from about 0.165 to about 2.39 mm. In one example, the tube
1222 is 6 Fr
and has an outer or external diameter of 2.0 0.1 mm. The overall length of
the tube 1222
can range from about 30 cm to about 120 cm depending on the age (e.g.,
pediatric or adult)
and gender of the patient.
[00256] The tube 1222 can be formed from a flexible and/or deformable material
to
facilitate advancing and/or positioning the tube 1222 in the bladder 10 and
ureters 6, 8
(shown in FIG. 7), such as any of the materials discussed above. For example,
the tube 1222
can be formed from one or more materials such as biocompatible polymers,
polyvinyl
chloride, polytetrafluoroethylene (PTFE) such as Teflon , silicon coated
latex, or silicon. In
one example, the tube 1222 is formed from a thermoplastic polyurethane.
Helical coil retention portion
[00257] Referring now to FIGS. 10A-10E, an exemplary retention portion 1230
comprises
helical coils 1280, 1282, 1284. In some examples, the retention portion 1230
comprises a
first or half coil 1280 and two full coils, such as a second coil 1282 and a
third coil 1284. As
shown in FIGS. 10A-10D, in some examples, the first coil 1280 comprises a half
coil
extending from 0 degrees to 180 degrees around a curvilinear central axis A of
the retention
portion 1230. In some examples, as shown the curvilinear central axis A is
substantially
straight and co-extensive with a curvilinear central axis of the tube 1222. In
other examples,
the curvilinear central axis A of the retention portion 1230 can be curved
giving the retention
portion 1230, for example, a cornucopia shape. The first coil 1280 can have a
diameter D1 of
about 1 mm to 20 mm and preferably about 8 mm to 10 mm. The second coil 1282
can be a
full coil extending from 180 degrees to 540 degrees along the retention
portion 1230 having a
diameter D2 of about 5 mm to 50 mm, preferably about 10 mm to 20 mm, and more
preferably about 14 mm 2 mm. The third coil 1284 can be a full coil
extending between
540 degrees and 900 degrees and having a diameter D3 of between 5 mm and 60
mm,
preferably about 10 mm to 30 mm, and more preferably about 18 mm 2 mm. In
other
examples, multiple coils 1282, 1284 can have the same inner and/or outer
diameter. For
example, an outer diameter of the full coils 1282, 1284, can each be about 18
2 mm.
[00258] In some examples, an overall height H of the retention portion 1230
ranges from
about 10 mm to about 30 mm and, preferably about 18 2 mm. A height H2 of a
gap
between adjacent coils 1284, namely between the sidewall 1219 of the tube 1222
of the first
coil 1280 and the adjacent sidewall 1221 of the tube 122 of the second coil
1282 is less than
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
44
3.0 mm, preferably between about 0.25 mm and 2.5 mm, and more preferably
between about
0.5 mm and 2.0 mm.
[00259] The retention portion 1230 can further comprise a distal-most curved
portion
1290. For example, the distal most portion 1290 of the retention portion 1230,
which
includes the open distal end 1220 of the tube 1222, can be bent inwardly
relative to a
curvature of the third coil 1284. For example, a curvilinear central axis X1
(shown in FIG.
10D) of the distal-most portion 1290 can extend from the distal end 1220 of
the tube 1222
towards the curvilinear central axis A of the retention portion 1230.
[00260] The retention portion 1230 is capable of moving between a contracted
position, in
which the retention portion 1230 is straight for insertion into the patient's
urinary tract, and
the deployed position, in which the retention portion 1230 comprises the
helical coils 1280,
1282, 1284. Generally, the tube 1222 is naturally biased toward the coiled
configuration.
For example, an uncoiled or substantially straight guidewire can be inserted
through the
retention portion 1230 to maintain the retention portion 1230 in its straight
contracted
position, as shown for example in FIGS. 11-14. When the guidewire is removed,
the
retention portion 1230 naturally transitions to its coiled position.
[00261] In some examples, the openings 1232, 1233 are disposed essentially
only or only
on a radially inwardly facing side 1286 or protected surface area or inner
surface area 1000 of
the coils 1280, 1282, 1284 to prevent occlusion or blockage of the openings
1232, 1233. A
radially outwardly facing side 1288 of the coils 1280, 1282, 1284 may be
essentially free of
the openings 1232. In similar examples, a total area of openings 1232, 1233 on
the inwardly
facing side 1286 of the retention portion 1230 can be substantially greater
than a total area of
openings 1232 on the radially outwardly facing side 1288 of the retention
portion 1230.
Accordingly, when negative pressure is induced in the ureter and/or renal
pelvis, mucosal
tissue of the ureter and/or kidney may be drawn against the retention portion
1230 and may
occlude some openings 1232 on the outer periphery 1002 or protective surface
area 1001 of
the retention portion 1230. However, openings 1232 located on the radially
inward side 1286
or protected surface area or inner surface area 1000 of the retention portion
1230 are not
appreciably occluded when such tissues contacts the outer periphery1002 or
protective
surface area 1001 of the retention portion 1230. Therefore, risk of injury to
the tissues from
pinching or contact with the drainage openings 1232 can be reduced or
eliminated.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
Hole or opening distribution examples
[00262] In some examples, the first coil 1280 can be free or essentially free
from
openings 1232. For example, a total area of openings 1232 on the first coil
1280 can be less
than or substantially less than a total area of openings 1232 of the full
coils 1282, 1284.
Examples of various arrangements of openings or openings 1232, which could be
used for a
coiled retention portion (such as coiled retention portion 1230 shown in FIGS.
10A-10E), are
illustrated in FIGS 11-14. As shown in FIGS. 11-14, a retention portion 1330
is depicted in
its uncoiled or straight position, as occurs when a guidewire is inserted
through the drainage
lumen.
[00263] An exemplary retention portion 1330 is illustrated in FIG. 11. In
order to more
clearly describe positioning of openings of the retention portion 1330, the
retention portion
1330 is referred to herein as being divided into a plurality of sections or
perforated sections,
such as a proximal-most or first section 1310, a second section 1312, a third
section 1314, a
fourth section 1316, a fifth section 1318, and a distal-most or sixth section
1320. One of
ordinary skill in the art would understand that fewer or additional sections
can be included, if
desired. As used herein, "section" refers to a discrete length of the tube
1322 within the
retention portion 1330. In some examples, sections are equal in length. In
other examples,
some sections can have the same length, and other sections can have a
different length. In
other examples, each section has a different length. For example, each of
sections 1310,
1312, 1314, 1316, 1318 and 1320 can have a length L1-L6, respectively, ranging
from about
5 mm to about 35 mm, and preferably from about 5 mm to 15 mm.
[00264] In some examples, each section 1310, 1312, 1314, 1316, 1318 and 1320
comprises
one or more openings 1332. In some examples, each section each comprises a
single opening
1332. In other examples, the first section 1310 includes a single opening 1332
and other
sections comprise multiple openings 1332. In other examples, different
sections comprise
one or more openings 1332, each of the opening(s) having a different shape or
different total
area.
[00265] In some examples, such as the retention portion 1230 shown in FIGS.
10A-10E,
the first or half coil 1280, which extends from 0 to about 180 degrees of the
retention portion
1230 can be free from or essentially free from openings. The second coil 1282
can include
the first section 1310 extending between about 180 and 360 degrees. The second
coil 1282
can also include the second and third sections 1312, 1314 positioned between
about 360
degrees and 540 degrees of the retention portion 1230. The third coil 1284 can
include the
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
46
fourth and fifth sections 1316, 1318 positioned between about 540 degrees and
900 degrees
of the retention portion 1230.
[00266] In some examples, the openings 1332 can be sized such that a total
area of
openings of the first section 1310 is less than a total area of openings of
the adjacent second
section 1312. In a similar manner, if the retention portion 1330 further
comprises a third
section 1314, then openings of a third section 1314 can have a total area that
is greater than
the total area of the openings of the first section 1310 or the second section
1312. Openings
of the forth 1316, fifth 1318, and sixth 1320 sections may also have a
gradually increasing
total area and/or number of openings to improve fluid flow through the tube
1222.
[00267] As shown in FIG. 11, the retention portion 1230 of the tube includes
five sections
1310, 1312, 1314, 1316, 1318, each of which includes a single opening 1332,
1334, 1336,
1338, 1340. The retention portion 1330 also includes a sixth section 1320
which includes the
open distal end 1220 of the tube 1222. In this example, the opening 1332 of
the first section
1310 has the smallest total area. For example, a total area of the opening
1332 of the first
section can range from about 0.002 mm2 and about 2.5 mm2, or about 0.01 mm2
and 1.0 mm2,
or about 0.1 mm2 and 0.5 mm2. In one example, the opening 1332 is about 55 mm
from the
distal end 1220 of the catheter, has a diameter of 0.48 mm, and an area of
0.18 mm2. In this
example, a total area of openings 1334 of the second section 1312 is greater
than the total
area of openings 1232 of the first section 1310 and can range in size from
about 0.01 mm2 to
about 1.0 mm2. The third 1336, fourth 1338, and fifth 1350 openings can also
range in size
from about 0.01 mm2 to about 1.0 mm2. In one example, the second opening 1334
is about
45 mm from the distal end of the catheter 1220, has a diameter of about 0.58
mm, and an area
of about 0.27 mm2. The third opening 1336 can be about 35 mm from the distal
end of the
catheter 1220 and have a diameter of about 0.66 mm. The fourth opening 1338
can be about
25 mm from the distal end 1220 and have a diameter of about 0.76 mm. The fifth
opening
1340 can be about 15 mm from the distal end 1220 of the catheter and have a
diameter of
about 0.889 mm. In some examples, the open distal end 1220 of the tube 1222
has the largest
opening having an area ranging from about 0.5 mm2 to about 5.0 mm2 or more. In
one
example, the open distal end 1220 has a diameter of about 0.97 mm and an area
of about 0.74
mm2.
[00268] As described herein, openings 1332 1334, 1336, 1338, 1340 can be
positioned
and sized so that a volumetric flow rate of fluid passing through the first
opening 1332 more
closely corresponds to a volumetric flow rate of openings of more distal
sections, when
negative pressure is applied to the drainage lumen 1224 of the catheter 1212,
for example
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
47
from the proximal portion 1228 of the drainage lumen 1224. As described above,
if each
opening were the same area, then, when negative pressure is applied to the
drainage lumen
1224, the volumetric flow rate of fluid passing through the proximal-most of
first opening
1332 would be substantially greater than a volumetric flow rate of fluid
passing through
openings 1334 closer to the distal end 1220 of the retention portion 1330.
While not
intending to be bound by any theory, it is believed that when negative
pressure is applied, the
pressure differential between the interior of the drainage lumen 1224 and
external to the
drainage lumen 1224 is greater in the region of the proximal-most opening and
decreases at
each opening moving towards the distal end of the tube. For example, sizes and
positions of
the openings 1332 1334, 1336, 1338, 1340 can be selected so that a volumetric
flow rate for
fluid which flows into openings 1334 of the second section 1312 is at least
about 30% of a
volumetric flow rate of fluid which flows into the opening(s) 1332 of the
first section 1310.
In other examples, a volumetric flow rate for fluid flowing into the proximal-
most or first
section 1310 is less than about 60% of a total volumetric flow rate for fluid
flowing through
the proximal portion of the drainage lumen 1224. In other examples, a
volumetric flow rate
for fluid flowing into openings 1332, 1334 of the two proximal-most sections
(e.g., the first
section 1310 and the second section 1312) can be less than about 90% of a
volumetric flow
rate of fluid flowing through the proximal portion of the drainage lumen 1224
when a
negative pressure, for example a negative pressure of about -45 mmHg, is
applied to the
proximal end of the drainage lumen.
[00269] As will be appreciated by one of ordinary skill in the art, volumetric
flow rate and
distribution for a catheter or tube comprising a plurality of openings or
perforations can be
directly measured or calculated in a variety of different ways. As used
herein, "volumetric
flow rate" means actual measurement of the volumetric flow rate downstream and
adjacent to
each opening or using a method for "Calculated Volumetric Flow Rate" described
below.
[00270] For example, actual measurement of the dispersed fluid volume over
time can be
used to determine the volumetric flow rate through each opening 1332, 1334,
1336, 1338,
1340. In one exemplary experimental arrangement, a multi-chamber vessel
comprising
individual chambers sized to receive sections 1310, 1312, 1314, 1316, 1318,
1320 of the
retention portion 1330 could be sealed around and enclose the retention
portion 1330. Each
opening 1332, 1334, 1336, 1338, 1340 could be sealed in one of the chambers.
An amount of
fluid volume drawn from the respective chamber into the tube 3222 through each
opening
1332, 1334, 1336, 1338, 1340 could be measured to determine an amount of fluid
volume
drawn into each opening over time when a negative pressure is applied. The
cumulative
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
48
amount of fluid volume collected in the tube 3222 by a negative pressure pump
system would
be equivalent to the sum of fluid drawn into each opening 1332, 1334, 1336,
1338, 1340.
[00271] Alternatively, volumetric fluid flow rate through different openings
1332, 1334,
1336, 1338, 1340 can be calculated mathematically using equations for modeling
fluid flow
through a tubular body. For example, volumetric flow rate of fluid passing
through openings
1332 1334, 1336, 1338, 1340 and into the drainage lumen 1224 can be calculated
based on a
mass transfer shell balance evaluation, as described in detail below in
connection with the
Mathematical Examples and FIGS. 15A-15C. Steps for deriving mass balance
equations and
for calculating a flow distribution between or volumetric flow rates for the
openings 1332
1334, 1336, 1338, 1340 are also described in detail below in connection with
FIGS. 15A-
15C.
[00272] Another exemplary retention portion 2230 with openings 2332, 2334,
2336, 2338,
2340 is illustrated in FIG. 12. As shown in FIG. 12, the retention portion
2230 comprises
numerous smaller perforations or openings 2332, 2334, 2336, 2338, 2340. Each
of the
openings 2332, 2334, 2336, 2338, 2340 can have a substantially identical cross-
sectional area
or one or more openings 2332, 2334, 2336, 2338, 2340 can have different cross-
sectional
areas. As shown in FIG. 12, the retention portion 2330 comprises six sections
2310, 2312,
2314, 2316, 2318, 2320, such as are described above, wherein each section
comprises a
plurality of the openings 2332, 2334, 2336, 2338, 2340. In the example shown
in FIG. 12, a
number of openings 2332, 2334, 2336, 2338, 2340 per section increases towards
the distal
end 2220 of the tube 2222, such that a total area of openings 1332 in each
section increases
compared to a proximally adjacent section.
[00273] As shown in FIG. 12, openings 2332 of the first section 2310 are
arranged along a
first virtual line V1, which is substantially parallel to a central axis X1 of
the retention
portion 2230. Openings 2334, 2336, 2338, 2340 of the second 2312, third 2314,
fourth 2316,
and fifth 2318 sections, respectively, are positioned on the sidewall of the
tube 2222 in a
gradually increasing number of rows, such that openings 2334, 2336, 2338, 2340
of these
sections also line up around a circumference of the tube 2222. For example,
some of the
openings 2334 of the second section 2312 are positioned such that a second
virtual line V2
extending around a circumference of the sidewall of the tube 2222 contacts at
least a portion
of multiple openings 2334. For example, the second section 2312 can comprise
two or more
rows of perforations or openings 2334, in which each opening 2334 has an equal
or different
cross-sectional area. Further, in some examples, at least one of the rows of
the second
section 2312 can be aligned along a third virtual line V3, which is parallel
with the central
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
49
axis X1 of the tube 2222, but is not co-extensive with the first virtual line
Vi. In a similar
manner, the third section 2314 can comprise five rows of perforations or
openings 2336, in
which each opening 2336 has an equal or different cross-sectional area; the
fourth section
2316 can comprise seven rows of perforations or openings 2338; and the fifth
section 2318
can comprise nine rows of perforations or openings 2340. As in previous
examples, the sixth
section 2320 comprises a single opening, namely the open distal end 2220 of
the tube 2222.
In the example of FIG. 12, each of the openings has the same area, although
the area of one
or more openings can be different if desired.
[00274] Another exemplary retention portion 3230 with openings 3332, 3334,
3336,
3338, 3340 is illustrated in FIG. 13. The retention portion 3230 of FIG. 13
includes a
plurality of similarly sized perforations or openings 3332, 3334, 3336, 3338,
3340. As in
previous examples, the retention portion 3230 can be divided into six sections
3310, 3312,
3314, 3316, 3318, 3320, each of which comprises at least one opening. The
proximal-most
or first section 3310 includes one opening 3332. The second section 3312
includes two
openings 3334 aligned along the virtual line V2 extending around a
circumference of the
sidewall of the tube 3222. The third section 3314 comprises a grouping of
three openings
3336, positioned at vertices of a virtual triangle. The fourth section 3316
comprises a
grouping of four openings 3338 positioned at corners of a virtual square. The
fifth section
3318 comprises ten openings 3340 positioned to form a diamond shape on the
sidewall of the
tube 3222. As in previous examples, the sixth section 3320 comprises a single
opening,
namely the open distal end 3220 of the tube 3222. The area of each opening can
range from
about 0.001 mm2 and about 2.5 mm2. In the example of FIG. 13, each of the
openings has the
same area, although the area of one or more openings can be different if
desired.
[00275] Another exemplary retention portion 4230 with openings 4332, 4334,
4336, 4338,
4340 is illustrated in FIG. 14. The openings 4332 4334, 4336, 4338, 4340 of
the retention
portion 4330 have different shapes and sizes. For example, the first section
4310 includes a
single circular opening 4332. The second section 4312 has a circular opening
4334 with a
larger cross sectional area than the opening 4332 of the first section 4310.
The third section
4314 comprises three triangular shaped openings 4336. The fourth section 4316
comprises a
large circular opening 4338. The fifth section 4318 comprises a diamond shaped
opening
4340. As in previous examples, the sixth section 4320 comprises the open
distal end 4220 of
the tube 4222. FIG. 14 illustrates one example of an arrangement of different
shapes of
openings in each section. It is understood that the shape of each opening in
each section can
be independently selected, for example the first section 4310 can have one or
more diamond-
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
shaped openings or other shapes. The area of each opening can be the same or
different and
can range from about 0.001 mm2 and about 2.5 mm2.
Examples
Calculation of volumetric flow rate and percentage of flow distribution
[00276] Having described various arrangements of openings for retention
portions of the
ureteral catheter 1212, a method for determining the Calculated Percentage of
How
Distribution and Calculated Volumetric Flow Rate through the catheter will now
be described
in detail. A schematic drawing of an exemplary catheter with sidewall openings
showing a
position of portions of the tube or drainage lumen used in the following
calculations is shown
in FIG. 16. Calculated Percentage of Flow Distribution refers to a percentage
of total fluid
flowing through proximal portions of the drainage lumen which entered the
drainage lumen
through different openings or sections of the retention portion. Calculated
Volumetric Flow
rate refers to fluid flow per unit time through different portions of the
drainage lumen or
openings of the retention portion. For example, a volumetric flow rate for a
proximal portion
of the drainage lumen describes a rate of flow for a total amount of fluid
passing through the
catheter. A volumetric flow rate for an opening refers to a volume of fluid
which passes
through the opening and into the drainage lumen per unit time. In Tables 3-5
below flow is
described as a percentage of total fluid flow or of a total volumetric flow
rate for a proximal
portion of the drainage lumen. For example, an opening having a flow
distribution of 100%
means that all fluid entering the drainage lumen passed through the opening.
An opening
having a distribution of 0% would indicate that none of the fluid in the
drainage lumen
entered the drainage lumen through that opening.
[00277] These volumetric flow rate calculations were used to determine and
model fluid
flow through the retention portion 1230 of the ureter catheter 1212 shown in
FIGS. 7A and
10-10E. Further, these calculations show that adjusting the area of openings
and linear
distribution of openings along the retention portion effects a distribution of
fluid flow through
different openings. For example, reducing the area of the proximal-most
opening decreases
the proportion of fluid drawn into the catheter through the proximal most
opening and
increases the proportion of fluid drawn into more distal openings of the
retention portion.
[00278] For the following calculations, a tube length of 86 cm having an inner
diameter of
0.97 mm and an end hole inner diameter of 0.97 mm was used. Density of urine
was 1.03
g/mL and had a coefficient of friction of 8.02 x 10-3 Pa. S (8.02 x 10-3
kg/s.m) at 37 C.
CA 03118062 2021-04-28
WO 2020/110046 PCT/IB2019/060255
51
The urine volumetric flow rate passing through the catheter was 2.7 ml per
minute (QT0tai) as
determined by experimental measurement.
[00279] Calculated Volumetric Flow Rate is determined by a volumetric mass
balance
equation in which a sum total of volumetric flow through all perforations or
openings 1232 of
the five sections of the retention portion (referred to herein as volumetric
flow Q2 to Q6) and
through the open distal end 1220 (referred to herein as volumetric flow Qi)
equals the total
volumetric flow (C) Total) exiting the proximal end of the tube 1222 at a
distance of 10 cm to 60
,
cm away from the last proximal opening, as shown in Equation 2.
QTota1=Q1+ Q2+ Q3+ Q4+ Q5+ Q6 (Equation 2)
[00280] A Modified Loss Coefficient (K') for each of the sections is based on
three types
of loss coefficients within the catheter model, namely: an Inlet Loss
Coefficient taking into
account a pressure loss resulting at a pipe inlet (e.g., the openings and open
distal end of the
tube 1222); a Friction Loss Coefficient which takes into account pressure loss
resulting from
friction between the fluid and pipe wall; and a Flow Junction Loss Coefficient
taking into
account pressure loss resulting from the interaction of two flows coming
together.
[00281] The Inlet Loss Coefficient is dependent on a shape of the orifice or
opening. For
example, a tapered or nozzle shaped orifice would increase flow rate into the
drainage
lumen 1224. In a similar manner, a sharp-edged orifice would have different
flow properties
than an orifice with less defined edges. For purposes of the following
calculations, it is
assumed that the openings 1232 are side orifice openings and the open distal
end 1220 of the
tube 1222 is a sharp-edged opening. The cross sectional area of each opening
is considered
constant through the tube sidewall.
[00282] The Friction Loss Coefficient approximates pressure loss resulting
from friction
between the fluid and the adjacent inner wall of the tube 1222. Friction loss
is defined
according to the following equations:
Re = ¨19" (Equation 3.1)
C f
f = (Equation 3.2)
Re
L
K1 - 2 = K2 - 3 = K3 - 3 = K4 - 3 = K5 - 3 = = (Equation
3.3)
[00283] The How Junction Loss Coefficients are derived from loss coefficients
for
combining flow at a branch angle of 90 degrees. Values for the loss
coefficients were
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
52
obtained from Charts 13.10 and 13.11 of Miller DS, Internal Flow Systems,
1990,
incorporated by reference herein. The charts use the ratio of the inlet
orifice area (referred to
as Al in the charts) to the pipe cross-sectional area (referred to as A3 in
the charts) and the
ratio of the inlet orifice volumetric flow rate (Q1 in the charts) to the
resulting combined pipe
volumetric flow rate (Q3 in the charts). For example, for an area ratio of 0.6
between an area
of the opening and an area of the drainage lumen, the following Flow Junction
Loss
Coefficients (K13 and 1(23) would be used.
Flow
Ratio
0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0
(Qt/Q3)
K13
-0.58 -0.04 0.11 0.45 0.75 1.13 1.48 1.81 2.16
2.56
K23
0.15 0.27 0.39 0.48 0.56 0.63 0.69 0.72 0.74
0.76
[00284] To calculate the Total Manifold Loss Coefficient (K), it is necessary
to separate
the model into so-called "reference stations" and progressively work through
and balance the
pressure and flow distributions of the two paths (e.g., flow through the
opening and flow
through the drainage lumen of the tube) to reach each station starting from
the distal tip to the
most proximal "Station". A graphical representation of the different stations
used for this
calculation is shown in FIG. 16. For example, a most-distal "Station" A is the
distal open end
1220 of the tube 122. A second Station A' is the distal most opening on the
sidewall of the
tube 122 (e.g., the opening(s) of the fifth section 1318 in FIGS. 11-14). The
next station B is
for flow through the drainage lumen 1224 just proximal to the A' opening.
[00285] To calculate loss between Station A (the distal opening) and Station B
for fluid
entering through the open distal end of the tube 1222 (Path 1), the modified
loss coefficient
(K') is equal to:
K' = Inlet Loss + Friction Loss + Flow Junction Loss (Equation 4.1)
2
Apipe
ICE? = K1-1 X (- X Q1) + K1_2 X Q12 + K1_3 X (Q1 + Q2)2 (Equation
Ai
4.2)
[00286] In a similar manner, a second path to Station B is through the
opening(s) 1334 of
the fifth section 1318 (shown in FIGS. 11-14) of the retention portion 1330. A
modified loss
calculation for Path 2 is calculated as follows:
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
53
K' = Inlet Loss + Flow Junction Loss (Equation 5.1)
2
Apipe
K13 = K2_1 X (-A2 X Q2) + K2_2 X (Q1 + Q2)2 (Equation 5.2)
[00287] The modified loss coefficients of both Path 1 and Path 2 must equate
to ensure the
volumetric flow rates (Qi and Q2) reflect the balanced distribution within the
manifold at
Station B. The volumetric flow rates are adjusted until equal modified loss
coefficients for
both paths is achieved. The volumetric flow rates can be adjusted because they
represent a
fractional portion of a total volumetric flow rate (Q'Totai), which is assumed
to be unity for the
purpose of this step-by-step solution. Upon equating the two modified loss
coefficients, one
can then proceed to equating the two paths to reach station C (the fourth
section 1316 in
FIGS. 11-14).
[00288] Loss coefficients between Station B (flow through drainage lumen in
the fifth
section 1318) and Station C (flow through lumen in the fourth section 1316)
are calculated in
a similar manner as shown by Equations 5.1 and 5.2). For example, for Path 1
(Station B to
Station C), the modified loss coefficient (K') for the opening(s) of the
fourth section 1316 is
defined as:
K' = Loss to Station B + Friction Loss + Flow Junction Loss (Equation
6.1)
= K13 + K2_3 X (Q1 + Q2)2 + K2_4 X (Q1 + Q2 + Q3)2 (Equation 6.2)
[00289] For Path 2 (Station B to C), the modified loss coefficient (K') based
on the flow
area of the opening(s) of the fourth section 1316 are defined as:
K' = Inlet Loss + Flow Junction Loss (Equation 7.1)
2
Apipe
= K3_1 X (-A3 X Q3) + K3_2 X (Q1 + Q2 + Q3)2 (Equation 7.2)
[00290] As with the previous stations, the modified loss coefficients of both
Path 1 and
Path 2 must equate to ensure the volumetric flow rates (Q1, Q2, and Q3)
reflect the balanced
distribution within the manifold up to Station C. Upon equating the two
modified loss
coefficients, one can then proceed to equating the two paths to reach Station
D, Station E and
Station F. The step-by-step solution process proceeds through each station as
demonstrated
until calculating the modified loss coefficient for the final station, Station
F in this case. The
Total Loss Coefficient (K) for the manifold can then be calculated using an
actual QTotal
(volumetric flow rate through a proximal portion of the drainage lumen)
determined through
experimental measurement.
K = -4' (Equation 8)
(2Total
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
54
[00291] The fractional volumetric flow rates calculated through the step-by-
step exercise
can then be multiplied by the actual total volumetric flow rate (QT.tai) to
determine the flow
through each opening 1232 (shown in FIGS. 10-10E) and open distal end 1220.
Examples
[00292] Examples are provided below and shown in Tables 3-5 and FIGS. 15A-15C
for
the calculated volumetric flow rates.
Example /
[00293] Example 1 illustrates a distribution of fluid flow for a retention
member tube with
different sized openings, which corresponds to the embodiment of the retention
member 1330
shown in FIG. 11. As shown in Table 3, the proximal most opening (Q6) had a
diameter of
0.48 mm, the distal-most opening (Q5) on the sidewall of the tube had a
diameter of 0.88
mm, and the open distal end (Q6) of the tube had a diameter of 0.97 mm. Each
of the
openings was circular.
[00294] The Percentage of Flow Distribution and Calculated Volumetric Flow
Rate were
determined as follows.
Path to Station B through distal end of tube (Path 1)
8.4 = Cf / Re (Cf = 64 for circular cross-section)
KINLET 0.16 (Contraction coefficient. for sharp edged orifice entering
pipe)
KORIFICE 2.8 (Contraction coefficient. for sharp edged orifice w/ no
outlet pipe)
KFRICTION = f*(L/D) (Dependent on the length between orifices)
Part 1-1 = Inlet loss coef x (AT/Ai X Q1)2
Part 1-2 = Catheter friction loss x Q'12
Part 1-3 = Through flow junction loss to station 2 x (Q'i +
A2/AT= 0.82
Q'2/(Q'i +
Q'2) = 0.83
K13 = 0.61 (From Miller, see table above)
Part]-] = 0.0000
Part 1-2 = 0.0376
Part 1-3 = 0.0065
K' = 0.0442
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
Path to Station B through sidewall opening (Path 2)
Part 2-1 = Orifice loss coef x (AT/A2 x
Part 2-2 = Branch flow junction loss to station 2 x (Qi +
A2/AT= 0.82
Q2/(Q'1 + Q2) = 0.83
K22 = 1.3 (From Chart 13.10 of Miller)
Part 2-1 = 0.0306
Part 2-2 = 0.0138
K' = 0.0444
Path to Station C from Station B (Path 1 + Path 2)
Part 2-3 = Catheter friction loss x (Qi +
Part 2-4 = Through flow junction loss to station 3 x (Qi + Q'2 +
A3/AT = 0.61
Q'3/(Q'1 + Q2 + Q3) = 0.76
K24 = 0.71 (From Chart 13.11 of Miller)
Loss coefficient to
Station 2 = 0.044
Part 2-3 = 0.921
Part 2-4 = 0.130
K' = 1.095
Path to Station C through sidewall opening (Path 3)
Part 3-1 = Orifice loss coef x (AT/A3 x
Part 3-2 = Branch flow junction loss to station 3 x (Q'i + Q'2 +
A3/AT = 0.61
Q'3/(Q'1 + Q'2 + Q'3) = 0.76
K32 = 1.7 (From Chart 13.10 of Miller)
Part 3-1 = 0.785
Part 3-2 = 0.311
K' = 1.096
Path to Station D from Station C (Path 1 + Path 2 + Path 3)
Part 3-3 = Catheter friction loss x (Qi + +
Part 3-4 = Through flow junction loss to station 4 x (Qi + Q'2 + Q3 Q4)2
AVAT = 0.46
Q'4/(Q'1 + Q'2 + Q3 Q'4) = 0.70
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
56
K34 = 0.77 (From Chart 13.11 of Miller)
Loss coefficient to Station 3 = 1.10
Part 3-3 = 15.90
Part 3-4 = 1.62
K' = 18.62
Path to Station D through sidewall opening (Path 4)
Part 4-1 = Orifice loss coef x (AT/A4. x Q4)2
Part 4-2 = Branch flow junction loss to station 4 x + Q'2+ Q'3 +
AVAT = 0.46
Q'4/(Q'1 + Q'2 + Q3 + (24)
= 0.70
K4 2 = 2.4 (From Chart 13.10 of Miller)
Part 4-1 = 13.59
Part 4-2 = 5.04
K' = 18.62
Path to Station E from Station D (Path 1 + Path 2 + Path 3 + Path 4)
Part 4-3 = Catheter friction loss x (gi + Q'2 + Q'3 Q'4)2
Part 4-4 = Through flow junction loss to station 5 x (gi + Q'2+ Q'3 +
AS/AT= 0.36
Q5/(Q'l Q'2 Q3 Q4 Q's) = 0.65
K34= 0.78 (From Chart 13.11 of Miller)
Loss coefficient to Station 4 = 18.6
Part 4-3 = 182.3
Part 4-4= 13.3
K' = 214.2
CA 03118062 2021-04-28
WO 2020/110046 PCT/IB2019/060255
57
Path to Station E through sidewall opening (Path 5)
Part 5-1 = Orifice loss coef x (AT/As x
Part 5-2 = Branch flow junction loss to station 5 x (Q'i + Q'2 + Q'3 +
As/AT = 0.36
Q's/(Q'i Q'2 Q'3 Q'a + Q's) = 0.65
K42 = 3.3 (From Chart 13.10 of Miller)
Part 5-1 = 157.8
Part 5-2 = 56.4
K' = 214.2
Path to Station F from Station E (through paths 1-5)
Part 5-3 = Catheter friction loss x (Q'i + Q'2 + Q'3 Q'a +
Part 5-4 = Through flow junction loss to station 6 x (Q'i + Q'2 + Q'3 Q'a +
Q's +
A6/AT = 0.24
Q'6/(Q'1 + Q'2 + Q'3 Q'a + Q'S = 0.56
K34 = 0.77 (From Chart 13.11 of Miller)
Loss coefficient to Station 5 = 214.2
Part 5-3 = 1482.9
Part 5-4 = 68.3
K' = 1765.4
Path to Station F through sidewall opening (path 6)
Part 6-1 = Orifice loss coef x (AT/A6 x
Part 6-2 = Branch flow junction loss to station 6 x (Q'i + Q'2 + Q'a +
Q's +
A6/AT 0.24
Q'6/(Q'1 + Q'2 + Q'3 Q'a + Q's + Q'6)
0.56
K42 = 5.2 (From Chart 13.10 of Miller)
Part 6-1 = 1304.3
Part 6-2 = 461.2
K' = 1765.5
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
58
[00295] In order to calculate flow distribution for each "Station" or opening,
the calculated
K' values were multiplied by actual total volumetric flow rate (0
-,Total) to determine the flow
through each perforation and distal end hole. Alternatively, calculated
results could be
presented as a percentage of total flow or a flow distribution as shown in
Table 3. As shown
in Table 3 and in FIG. 15C, the Percentage of Flow Distribution (% Flow
Distribution)
through the proximal most opening (Q6) was 56.1%. Flow through the two
proximal-most
openings (Q6 and Q5) was 84.6%.
Table 3
Cumulative
% Flow Diameter Length
Position Length
Distribution (mm) (mm)
(mm)
Q6'
56.1%
(proximal) 0.48 0 0
Qs' 28.5% 0.58 10 10
Q4' 10.8% 0.66 10 20
Q3, 3.5% 0.76 10 30
Q2' 0.9% 0.88 10 40
Qi' (distal) 0.2% 0.97 15 55
QTOTAL 100%
[00296] As demonstrated in Example 1, the increasing diameters of perforations
going
from the proximal to distal regions of the retention portion of the tube
results in more evenly
distributed flow across the entire retention portion.
Example 2
[00297] In Example 2, each opening has the same diameter and area. As shown in
Table 4
and FIG. 15A, in that case, flow distribution through the proximal-most
opening is 86.2% of
total flow through the tube. Flow distribution through the second opening is
11.9%.
Therefore, in this example, it was calculated that 98.1% of fluid passing
through the drainage
lumen entered the lumen through the two proximal-most openings. Compared to
Example 1,
Example 2 has increased flow through the proximal end of the tube. Therefore,
Example 1
provides a wider flow distribution in which a greater percentage of fluid
enters the drainage
lumen through openings other than the proximal-most opening. As such, fluid
can be more
efficiently collected through multiple openings reducing fluid backup and
improving
distribution of negative pressure through the renal pelvis and/or kidneys.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
59
Table 4:
Cumulative
% Flow Diameter Length
Position . . . Length
Distribution (mm) (mm)
(mm)
Q6'
86.2% 0.88 0 0
(proximal)
Qs,
11.9% 0.88 22 22
Q4' 1.6% 0.88 22 44
Q3, 0.2% 0.88 22 66
Q2' 0.03% 0.88 22 88
Qi' (distal) 0.01% 0.97 22 110
QTOTAL 100%
Example 3
[00298] Example 2 also illustrates flow distribution for openings having the
same
diameter. However, as shown in Table 5, the openings are closer together (10
mm vs. 22
mm). As shown in Table 5 and FIG. 15B, 80.9% of fluid passing through the
drainage lumen
entered the drainage lumen through the proximal most opening (Q6). 96.3% of
fluid in the
drainage lumen entered the drainage lumen through the two proximal-most
openings (Q5 and
Q6).
Table 5
Cumulative
% Flow Diameter Length
Position Length
Distribution (mm) (mm)
(mm)
Q6'
0.88
(proximal) 80.9% 0 0
Qs,
15.4% 0.88 10 10
Q4' 2.9% 0.88 10 20
Q3,
0.6% 0.88 10 30
Q2' 0.1% 0.88 10 40
Qi' (distal) 0.02% 0.97 15 55
QTOTAL 100%
[00299] Referring generally now to FIGS. 17-41C, and more specifically to FIG.
17, there
are shown two exemplary ureteral catheters 5000, 5001 positioned within the
urinary tract of
a patient, and a bladder catheter 116. The ureteral catheter 5000, 5001
comprises: a drainage
lumen 5002, 5003 for draining fluid such as urine from at least one of a
patient's kidney 2, 4,
renal pelvis 20, 21 or in the ureter 6, 8 adjacent to the renal pelvis 20, 21.
The drainage
lumen 5002, 5003 comprises a distal portion 5004, 5005 configured to be
positioned in a
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
patient's kidney 2, 4, renal pelvis 20, 21 and/or in the ureter 6, 8 adjacent
to the renal
pelvis 20, 21 and a proximal portion 5006, 5007 through which fluid 5008 is
drained to the
bladder 10 or outside of the body of the patient, as shown in FIGS. 2B and 2C.
[00300] In some examples, the distal portion 5004, 5005 comprises an open
distal end
5010, 5011 for drawing fluid into the drainage lumen 5002, 5003. The distal
portion 5004,
5005 of the ureteral catheter 5000, 5001 further comprises a retention portion
5012, 5013 for
maintaining the distal portion 5004, 5005 of the drainage lumen or tube 5002,
5003 in the
ureter and/or kidney. The retention portion 5012, 5013 can be flexible and/or
bendable to
permit positioning of the retention portion 5012, 5013 in the ureter, renal
pelvis, and/or
kidney. For example, the retention portion 5012, 5013 is desirably
sufficiently bendable to
absorb forces exerted on the catheter 5000, 5001 and to prevent such forces
from being
translated to the ureters. Further, if the retention portion 5012, 5013 is
pulled in the proximal
direction P (shown in FIG. 17) toward the patient's bladder 10, the retention
portion 5012,
5013 can be sufficiently flexible to begin to unwind, straightened or
collapsed so that it can
be drawn through the ureter 6, 8.
[00301] In some examples, the retention portion comprises a funnel support.
Non-limiting
examples of different shapes of funnel supports are shown in FIGS. 7A, 7B, 17,
and 18A-
41C, which are discussed in detail below. Generally, the funnel support
comprises at least
one sidewall. The at least one sidewall of the funnel support comprises a
first diameter and a
second diameter, the first diameter being less than the second diameter. The
second diameter
of the funnel support is closer to an end of the distal portion of the
drainage lumen than the
first diameter.
[00302] The proximal portion of the drainage lumen or drainage tube is
essentially free of
or free of openings. While not intending to be bound by any theory, it is
believed that when
negative pressure is applied at the proximal end of the proximal portion of
the drainage
lumen, that openings in the proximal portion of the drainage lumen or drainage
tube may be
undesirable as such openings may diminish the negative pressure at the distal
portion of the
ureteral catheter and thereby diminish the draw or flow of fluid or urine from
the kidney and
renal pelvis of the kidney. It is desirable that the flow of fluid from the
ureter and/or kidney
is not prevented by occlusion of the ureter and/or kidney by the catheter.
Also, while not
intending to be bound by any theory, it is believed that when negative
pressure is applied at
the proximal end of the proximal portion of the drainage lumen, ureter tissue
may be drawn
against or into openings along the proximal portion of the drainage lumen,
which may irritate
the tissues.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
61
[00303] Some examples of ureteral catheters comprising a retention portion
comprising a
funnel support according to the present invention are shown in FIGS. 7A, 7B,
17, and 18A-
41C. In FIGS. 7A-10E, the funnel support is formed by a coil of tubing. In
FIGS. 17-41C,
other examples of the funnel support are shown. Each of these funnel supports
according to
the present invention will be discussed in detail below.
[00304] Referring now to FIGS. 18A-D, in some examples, there is shown a
distal
portion 5004 of the ureteral catheter, indicated generally as 5000. The distal
portion 5004
comprises a retention portion 5012 comprising a funnel-shaped support 5014.
The funnel-
shaped support 5014 comprises at least one sidewall 5016. As shown in FIGS.
18C and 18D,
the outer periphery 1002 or protective surface area 1001 comprises the outer
surface or outer
wall 5022 of the funnel-shaped support 5014. The one or more drainage holes,
ports or
perforations, or interior opening 5030, are disposed on the protected surface
areas or inner
surface areas 1000 of the funnel-shaped support 5014. As shown in FIGS. 18C
and 18D,
there is a single drainage hole 5030 at the base portion 5024 of the funnel-
shaped support,
although multiple holes can be present.
[00305] The at least one sidewall 5016 of the funnel support 5014 comprises a
first (outer)
diameter D4 and a second (outer) diameter D5, the first outer diameter D4
being less than the
second outer diameter D5. The second outer diameter D5 of the funnel support
5014 is closer
to the distal end 5010 of the distal portion 5004 of the drainage lumen 5002
than is the first
outer diameter D4. In some examples the first outer diameter D4 can range from
about 0.33
mm to 4 mm (about 1 Fr to about 12 Fr (French catheter scale)), or about 2.0
0.1 mm. In
some examples, the second outer diameter D5 is greater than first outer
diameter D4 and can
range from about 1 mm to about 60 mm, or about 10 mm to 30 mm, or about 18 mm
2 mm.
[00306] In some examples, the at least one sidewall 5016 of the funnel support
5014 can
further comprise a third diameter D7 (shown in FIG. 18B), the third diameter
D7 being less
than the second outer diameter D5. The third diameter D7 of the funnel support
5014 is
closer to the distal end 5010 of the distal portion 5004 of the drainage lumen
5002 than is the
second diameter D5. The third diameter D7 is discussed in greater detail below
regarding the
lip. In some examples, the third diameter D7 can range from about 0.99 mm to
about 59 mm,
or about 5 mm to about 25 mm.
[00307] The at least one sidewall 5016 of the funnel support 5014 comprises a
first (inner)
diameter D6. The first inner diameter D6 is closer to the proximal end 5017 of
the funnel
support 5014 than is the third diameter D7. The first inner diameter D6 is
less than the third
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
62
diameter D7. In some examples the first inner diameter D6 can range from about
0.05 mm to
3.9 mm, or about 1.25 0.75 mm.
[00308] In some examples, an overall height H5 of the sidewall 5016 along a
central axis
5018 of the retention portion 5012 can range from about 1 mm to about 25 mm.
In some
examples, the height H5 of the sidewall can vary at different portions of the
sidewall, for
example if the sidewall has an undulating edge or rounded edges such as is
shown in FIG. 24.
In some examples, the undulation can range from about 0.01 mm to about 5 mm or
more, if
desired.
[00309] In some examples, as shown in FIGS. 7A-10E, and 17-41C, the funnel
support
5014 can have a generally conical shape. In some examples, the angle 5020
between the
outer wall 5022 near the proximal end 5017 of the funnel support 5014 and the
drainage
lumen 5002 adjacent to the base portion 5024 of the funnel support 5014 can
range from
about 100 degrees to about 180 degrees, or about 100 degrees to about 160
degrees, or about
120 degrees to about 130 degrees. The angle 5020 may vary at different
positions about the
circumference of the funnel support 5014, such as is shown in Fig. 22A, in
which the angle
5020 ranges from about 140 degrees to about 180 degrees.
[00310] In some examples, the edge or lip 5026 of the distal end 5010 of the
at least one
sidewall 5016 can be rounded, square, or any shape desired. The shape defined
by the edge
5026 can be, for example, circular (as shown in FIGS. 18C and 23B), elliptical
(as shown in
FIG. 22B), lobes (as shown in FIGS. 28B, 29B and 31), square, rectangular, or
any shape
desired.
[00311] Referring now to FIGS. 28A-31, there is shown a funnel support 5300
wherein the
at least one sidewall 5302 comprises a plurality of lobe-shaped longitudinal
folds 5304 along
the length L7 of the sidewall 5302. The outer periphery 1002 or protective
surface area 1001
comprises the outer surface or outer wall 5032 of the funnel-shaped support
5300. The one
or more drainage holes, ports or perforations, or interior opening, are
disposed on the
protected surface areas or inner surface areas 1000 of the funnel-shaped
support 5300. As
shown in FIG. 28B, there is a single drainage hole at the base portion of the
funnel-shaped
support, although multiple holes can be present.
[00312] The number of folds 5304 can range from 2 to about 20, or about 6, as
shown. In
this example, the folds 5304 can be formed from one or more flexible
materials, such as
silicone, polymer, solid material, fabric, or a permeable mesh to provide the
desired lobe
shape. The folds 5304 can have a generally rounded shape as shown in cross-
sectional view
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
63
51B. The depth D100 of each fold 5304 at the distal end 5306 of the funnel
support 5300 can
be the same or vary, and can range from about 0.5 mm to about 5 mm.
[00313] Referring now to FIGS. 29A and 29B, one or more folds 5304 can
comprise at
least one longitudinal support member 5308. The longitudinal support member(s)
5308 can
span the entire length L7 or a portion of the length L7 of the funnel support
5300. The
longitudinal support members 5308 can be formed from a flexible yet partially
rigid material,
such as a temperature sensitive shape memory material, for example nitinol.
The thickness of
the longitudinal support members 5308 can range from about 0.01 mm to about 1
mm, as
desired. In some examples, the nitinol frame can be covered with a suitable
waterproof
material such as silicon to form a tapered portion or funnel. In that case,
fluid is permitted to
flow down the inner surface 5310 of the funnel support 5300 and into the
drainage lumen
5312. In other examples, the folds 5304 are formed from various rigid or
partially rigid
sheets or materials bended or molded to form a funnel-shaped retention
portion.
[00314] Referring now to FIGS. 30 and 31, the distal end or edge 5400 of the
folds 5402
can comprise at least one edge support member 5404. The edge support member(s)
5404 can
span the entire circumference 5406 or one or more portions of the
circumference 5406 of the
distal edge 5400 of the funnel support 5408. The edge support member(s) 5404
can be
formed from a flexible yet partially rigid material, such as a temperature
sensitive shape
memory material, for example nitinol. The thickness of the edge support
member(s) 5404
can range from about 0.01 mm to about 1 mm, as desired.
[00315] In some examples, such as are shown in FIGS. 18A-C, the distal end
5010 of the
drainage lumen 5002 (or funnel support 5014) can have an inwardly facing lip
5026 oriented
towards the center of the funnel support 5014, for example of about 0.01 mm to
about 1 mm,
to inhibit irritating the kidney tissue. Thus, the funnel support 5014 can
comprise a third
diameter D7 less than the second diameter D5, the third diameter D7 being
closer to an end
5010 of the distal portion 5004 of the drainage lumen 5002 than the second
diameter D5. The
outer surface 5028 of the lip 5026 can be rounded, a square edge, or any shape
desired. The
lip 5026 may assist in providing additional support to the renal pelvis and
internal kidney
tissues.
[00316] Referring now to FIGS. 24A-C, in some examples, the edge 5200 of the
distal
end 5202 of the at least one sidewall 5204 can be shaped. For example, the
edge 5200 can
comprise a plurality of generally rounded edges 5206 or scallops, for example
about 4 to
about 20 or more rounded edges. The rounded edges 5206 can provide more
surface area
than a straight edge to help support the tissue of the renal pelvis or kidney
and inhibit
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
64
occlusion. The edge 5200 can have any shape desired, but preferably is
essentially free of or
free of sharp edges to avoid injuring tissue.
[00317] In some examples, such as are shown in FIGS. 18A-C and 22A-23B, the
funnel
support 5014 comprises a base portion 5024 adjacent to the distal portion 5004
of the
drainage lumen 5002. The base portion 5024 comprises at least one interior
opening 5030
aligned with an interior lumen 5032 of the drainage lumen 5002 of the proximal
portion 5006
of the drainage lumen 5002 for permitting fluid flow into the interior lumen
5032 of the
proximal portion 5006 of the drainage lumen 5002. In some examples, the cross-
section of
the opening 5030 is circular, although the shape may vary, such as ellipsoid,
triangular,
square, etc.
[00318] In some examples, such as is shown in FIGS. 22A-23B, a central axis
5018 of the
funnel support 5014 is offset with respect to a central axis 5034 of the
proximal portion 5006
of the drainage lumen 5002. The offset distance X from the central axis 5018
of the funnel
support 5014 with respect to the central axis 5034 of the proximal portion
5006 can range
from about 0.1 mm to about 5 mm.
[00319] The at least one interior opening 5030 of the base portion 5024 has a
diameter D8
(shown, for example, in FIGS. 18C and 23B) ranging from about 0.05 mm to about
4 mm. In
some examples, the diameter D8 of the interior opening 5030 of the base
portion 5024 is
about equal to the first inner diameter D6 of the adjacent proximal portion
5006 of the
drainage lumen.
[00320] In some examples, the ratio of the height H5 of the at least one
sidewall 5016
funnel support 5014 to the second outer diameter D5 of the at least one
sidewall 5016 of the
funnel support 5014 ranges from about 1:25 to about 5:1.
[00321] In some examples, the at least one interior opening 5030 of the base
portion 5024
has a diameter D8 ranging from about 0.05 mm to about 4 mm, the height H5 of
the at least
one sidewall 5016 of the funnel support 5014 ranges from about 1 mm to about
25 mm, and
the second outer diameter D5 of the funnel support 5014 ranges from about 5 mm
to about 25
mm.
[00322] In some embodiments, the thickness Ti (shown in FIG. 18B, for example)
of the
at least one sidewall 5016 of the funnel support 5014 can range from about
0.01 mm to about
1.9 mm, or about 0.5 mm to about 1 mm. The thickness Ti can be generally
uniform
throughout the at least one sidewall 5016, or it may vary as desired. For
example, the
thickness Ti of the at least one sidewall 5016 can be less or greater near the
distal end 5010
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
of the distal portion 5004 of the drainage lumen 5002 than at the base portion
5024 of the
funnel support 5014.
[00323] Referring now to FIGS. 18A-21, along the length of the at least one
sidewall 5016,
the sidewall 5016 can be straight (as shown in FIGS. 18A and 20), convex (as
shown in FIG.
19), concave (as shown in FIG. 21), or any combination thereof. As shown in
FIGS. 19 and
21, the curvature of the sidewall 5016 can be approximated from the radius of
curvature R
from the point Q such that a circle centered at Q meets the curve and has the
same slope and
curvature as the curve. In some examples, the radius of curvature ranges from
about 2 mm to
about 12 mm. In some examples, the funnel support 5014 has a generally
hemispherical
shape, as shown in FIG. 19.
[00324] In some examples, the at least one sidewall 5016 of the funnel support
5014 is
formed from a balloon 5100, for example as shown in FIGS. 35A, 35 B, 38A and
38B. The
balloon 5100 can have any shape that provides a funnel support to inhibit
occlusion of the
ureter, renal pelvis, and/or the rest of the kidney. As shown in FIGS. 35A and
35B, the
balloon 5100 has the shape of a funnel. The balloon can be inflated after
insertion or deflated
before removal by adding or removing gas or air through the gas port(s) 5102.
The gas
port(s) 5102 can simply be contiguous with the interior 5104 of the balloon
5100, e.g., the
balloon 5100 can be adjacent to the interior 5106 or encase the exterior 5108
of an adjacent
portion of the proximal portion 5006 of the drainage lumen 5002. The diameter
D9 of the
sidewall 5110 of the balloon 5100 can range from about 1 mm to about 3 mm, and
can vary
along its length such that the sidewall has a uniform diameter, tapers toward
the distal end
5112 or tapers toward the proximal end 5114 of the funnel support 5116. The
outer diameter
D10 of the distal end 5112 of the funnel support 5116 can range from about 5
mm to about 25
mm.
[00325] In some examples, the at least one sidewall 5016 of the funnel support
5014 is
continuous along the height H5 of the at least one sidewall 5016, for example
as shown in
FIGS. 18A, 19, 20, and 21. In some examples, the at least one sidewall 5016 of
the funnel
support 5014 comprises a solid wall, for example the sidewall 5016 is not
permeable through
the sidewall after 24 hours of contact with a fluid such as urine on one side.
[00326] In some examples, the at least one sidewall of the funnel support is
discontinuous
along the height or the body of the at least one sidewall. As used herein,
"discontinuous"
means that the at least one sidewall comprises at least one opening for
permitting the flow of
fluid or urine therethrough into the drainage lumen, for example by gravity or
negative
pressure. In some examples, the opening can be a conventional opening through
the sidewall,
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
66
or openings within a mesh material, or openings within a permeable fabric. The
cross-
sectional shape of the opening can be circular or non-circular, such as
rectangular, square,
triangular, polygonal, ellipsoid, as desired. In some examples, an "opening"
is a gap between
adjacent coils in a retention portion of a catheter comprising a coiled tube
or conduit.
[00327] As used herein, "opening" or "hole" means a continuous void space or
channel
through the sidewall from the outside to the inside of the sidewall, or vice
versa. In some
examples, each of the at least one opening(s) can have an area which can be
the same or
different and can range from about 0.002 mm2 to about 100 mm2, or about 0.002
mm2 to
about 10 mm2. As used herein, the "area" or "surface area" or "cross-sectional
area" of an
opening means the smallest or minimum planar area defined by a perimeter of
the opening.
For example, if the opening is circular and has a diameter of about 0.36 mm
(area of 0.1
mm2) at the outside of the sidewall, but a diameter of only 0.05 mm (area of
0.002 mm2) at
some point within the sidewall or on the opposite side of the sidewall, then
the "area" would
be 0.002 mm2 since that is the minimum or smallest planar area for flow
through the opening
in the sidewall. If the opening is square or rectangular, the "area" would be
the length times
the width of the planar area. For any other shapes, the "area" can be
determined by
conventional mathematical calculations well known to those skilled in the art.
For example,
the "area" of an irregular shaped opening is found by fitting shapes to fill
the planar area of
the opening, calculating the area of each shape and adding together the area
of each shape.
[00328] In some examples, at least a portion of the sidewall comprises at
least one (one or
more) openings. Generally, the central axis of the opening(s) can be generally
perpendicular
to the planar outer surface of the sidewall, or the opening(s) can be angled
with respect to the
planar outer surface of the sidewalls. The dimensions of the bore of the
opening may be
uniform throughout its depth, or the width may vary along the depth, either
increasing,
decreasing, or alternating in width through the opening from the exterior
surface of the
sidewall to the interior surface of the sidewall.
[00329] Referring now to FIGS. 9A-9E, 10A, 10E, 11-14, 27, 32A, 32B, 33 and
34, in
some examples at least a portion of the sidewall comprises at least one (one
or more)
openings. The opening(s) can be positioned anywhere along the sidewall. For
example, the
openings can be uniformly positioned throughout the sidewall, or positioned in
specified
regions of the sidewall, such as closer to the distal end of the sidewall or
closer to the
proximal end of the sidewall, or in vertical or horizontal or random groupings
along the
length or circumference of the sidewall. While not intending to be bound by
any theory, it is
believed that, when negative pressure is applied at the proximal end of the
proximal portion
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
67
of the drainage lumen, openings in the proximal portion of the funnel support
that are directly
adjacent to the ureter, renal pelvis and/or other kidney tissue may be
undesirable as such
openings may diminish the negative pressure at the distal portion of the
ureteral catheter and
thereby diminish the draw or flow of fluid or urine from the kidney and renal
pelvis of the
kidney, as well as perhaps irritate the tissue.
[00330] The number of openings can vary from 1 to 1000 or more, as desired.
For
example, in FIG. 27, six openings (three on each side) are shown. As discussed
above, in
some examples, each of the at least one opening(s) can have an area which can
be the same or
different and can range from about 0.002 mm2 to about 50 mm2, or about 0.002
mm2 to about
mm2.
[00331] In some examples, as shown in FIG. 27, the openings 5500 can be
positioned
closer the distal end 5502 of the sidewall 5504. In some examples, the
opening(s) are
positioned in the distal half 5506 of the sidewall towards the distal end
5502. In some
examples, the openings 5500 are evenly distributed around the circumference of
the distal
half 5506 or even closer to the distal end 5502 of the sidewall 5504.
[00332] In contrast, in FIG. 32B, the openings 5600 are positioned near the
proximal end
5602 of an inner sidewall 5604, and do not directly contact the tissue since
there is an outer
sidewall 5606 between the opening 5600 and the tissue. Alternatively or
additionally, one or
more opening(s) 5600 can be positioned near the distal end of the inner
sidewall, as desired.
The inner sidewall 5604 and outer sidewall 5606 can be connected by one or
more supports
5608 or ridges connecting the outside 5610 of the inner sidewall 5604 to the
inside 5612 of
the outer sidewall 5606.
[00333] In some non-limiting examples, such as are shown in FIGS. 9A-9E, 10A,
10D-
10G, 18B, 18D, 18E, 20, 22A, 22B, 23A, 23B, 24A-24C, 25, 26, 27, 28A, 28B, 29A-
29C, 30,
31, 32A, 32B, 33, 34, 35A, 35B, 37B, 38A, 39B, 39C, 40A-40C, and 41A-41C, a
protected
surface area(s) or inner surface area(s) 1000 can be established by a variety
of different
shapes or materials. Non-limiting examples of protected surface areas or inner
surface areas
1000 can comprise, for example, the interior portions 152, 5028, 5118, 5310,
5410, 5510,
5616, 5710, 5814, 6004 of a funnel 150, 5014, 5116, 5300, 5408, 5508, 5614,
5702, 5802,
6000, the interior portions 164, 166, 168, 170, 338, 1281, 1283, 1285 of a
coil 183, 184, 185,
187, 334, 1280, 1282, 1284, the interior portions 5902, 6003 of a porous
material 5900, 6002,
the interior portions 162, 5710, 5814 of a mesh 57, 5704, 5804, or the
interior portions 536 of
a cage 530 with protected drainage holes 533.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
68
[00334] In some non-limiting examples, one or more protected drainage holes,
ports or
perforations 133, 1233 are disposed on the protected surface area 1000. Upon
application of
negative pressure therapy through the catheter, the urothelial or mucosal
tissue 1003, 1004
conforms or collapses onto the outer periphery 189, 1002 or protective surface
area 1001 of
the retention portion 130, 330, 410, 500, 1230, 1330, 2230, 3230, 4230, 5012,
5013 of the
catheter and is thereby prevented or inhibited from occluding one or more of
the protected
drainage holes, ports or perforations 133, 1233 disposed on the protected
surface area or inner
surface area 1000, and thereby a patent fluid column or flow is established,
maintained, or
enhanced between the renal pelvis and calyces and the drainage lumen 124, 324,
424, 524,
1224, 5002, 5003, 5312, 5708, 5808.
[00335] In some examples, the retention portion 130, 330, 410, 500, 1230,
1330, 2230,
3230, 4230, 5012, 5013 comprises one or more helical coils having outwardly
facing sides
1288 and inwardly facing sides 1286, and wherein the outer periphery 1002 or
protective
surface area 1001 comprises the outwardly facing sides 1288 of the one or more
helical coils,
and the one or more protected drainage holes, ports or perforations 133, 1233
are disposed on
the inwardly facing sides 1286 (protected surface area or inner surface area
1000) of the one
or more helical coils.
[00336] For example, a funnel shape, as shown in FIG. 25, can create a
sidewall 5700 that
conforms to the natural anatomical shape of the renal pelvis preventing the
urothelium from
constricting the fluid column. The interior 5710 of the funnel support 5702
provides a
protected surface area 1000 having openings 5706 therethrough which provide a
passageway
through which a fluid column can flow from the calyces into the drainage lumen
5708.
Similarly, the mesh form of FIG. 26 can also create a protected surface area
1000, such as
interior 5814 of the mesh 5804, between the calyces and the drainage lumen
5808 of the
catheter. The mesh 5704, 5804 comprises a plurality of openings 5706, 5806
therethrough
for permitting fluid flow into the drainage lumen 5708, 5808. In some
examples, the
maximum area of an opening can be less than about 100 mm2, or less than about
1 mm2, or
about 0.002 mm2 to about 1 mm2, or about 0.002 mm2 to about 0.05 mm2. The mesh
5704,
5804 can be formed from any suitable metallic or polymeric material such as
are discussed
above.
[00337] In some examples, the funnel support further comprises a cover portion
over the
distal end of the funnel support. This cover portion can be formed as an
integral part of the
funnel support or connected to the distal end of the funnel support. For
example, as shown in
FIG. 26, the funnel support 5802 comprises a cover portion 5810 across the
distal end 5812
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
69
of the funnel support 5802 and projecting from the distal end 5812 of the
funnel support
5802. The cover portion 5810 can have any shape desired, such as flat, convex,
concave,
undulating, and combinations thereof. The cover portion 5810 can be formed
from mesh or
any polymeric solid material as discussed above. The cover portion 5810 can
provide an
outer periphery 1002 or protective surface area 1001 to assist in supporting
the pliant tissue in
the kidney region to facilitate urine production.
[00338] In some examples, the funnel support comprises a porous material, for
example as
shown in FIGS. 39A-40C. FIGS. 39A-40C and suitable porous materials are
discussed in
detail below. Briefly, in FIGS. 39 and 40, the porous material itself is the
funnel support. In
FIG. 39, the funnel support is a wedge of porous material. In FIG. 40, the
porous material is
in the shape of a funnel. In some examples, such as FIG. 33, the porous
material 5900 is
positioned within the interior 5902 of the sidewall 5904. In some examples,
such as FIG. 34,
the funnel support 6000 comprises a porous liner 6002 positioned adjacent to
the interior
6004 of the sidewall 6006. The thickness T2 of the porous liner 6002 can range
from about
0.5 mm to about 12.5 mm, for example. The area of the openings within the
porous material
can be about 0.002 mm2 to about 100 mm2, or less.
[00339] Referring now to FIGS. 37A and 37B, for example, a retention portion
130 of a
ureteral catheter 112 comprises a catheter tube 122 having a widened and/or
tapered distal
end portion which, in some examples, is configured to be positioned in the
patient's renal
pelvis and/or kidney. For example, the retention portion 130 can be a funnel-
shaped structure
comprising an outer surface 185 configured to be positioned against the ureter
and/or kidney
wall and comprising an inner surface 186 configured to direct fluid toward a
drainage lumen
124 of the catheter 112. The retention portion can be configured into a funnel-
shaped support
having an outer surface 185 and an inner surface 186, and wherein the outer
periphery 189 or
protective surface area 1001 comprises the outer surface 185 of the funnel-
shaped support,
and the one or more drainage holes, ports or perforations 133, 1233 are
disposed on the inner
surface 186 at the base of the funnel-shaped support. In another example shown
in FIGS.
32A and 32B, the retention portion can be configured into a funnel-shaped
support 5614
having an outer surface and an inner surface 5616, and wherein the outer
periphery 1002 or
protective surface area 1001 comprises the outer surface of the outer sidewall
5606. The
protected surface area 1000 can comprise the inner sidewall 5604 of the inner
funnel and the
one or more drainage holes, ports or perforations 5600 can be disposed on the
inner sidewall
5604 of the funnel-shaped support.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
[00340] Referring to FIGS. 37A and 37B, the retention portion 130 can comprise
a
proximal end 188 adjacent to the distal end of the drainage lumen 124 and
having a first
diameter D1 and a distal end 190 having a second diameter D2 that is greater
than the first
diameter D1 when the retention portion 130 is in its deployed position. In
some examples,
the retention portion 130 is transitionable from a collapsed or compressed
position to the
deployed position. For example, the retention portion 130 can be biased
radially outward
such that when the retention portion 130 is advanced to its fluid collecting
position, the
retention portion 130 (e.g., the funnel portion) expands radially outward to
the deployed state.
[00341] The retention portion 130 of the ureteral catheter 112 can be made
from a variety
of suitable materials that are capable of transitioning from the collapsed
state to the deployed
state. In one example, the retention portion 130 comprises a framework of
tines or elongated
members formed from a temperature sensitive shape memory material, such as
nitinol. In
some examples, the nitinol frame can be covered with a suitable waterproof
material such as
silicon to form a tapered portion or funnel. In that case, fluid is permitted
to flow down the
inner surface 186 of the retention portion 130 and into the drainage lumen
124. In other
examples, the retention portion 130 is formed from various rigid or partially
rigid sheets or
materials bended or molded to form a funnel-shaped retention portion as
illustrated in FIGS.
37A and 37B.
[00342] In some examples, the retention portion of the ureteral catheter 112
can include
one or more mechanical stimulation devices 191 for providing stimulation to
nerves and
muscle fibers in adjacent tissues of the ureter(s) and renal pelvis. For
example, the
mechanical stimulation devices 191 can include linear or annular actuators
embedded in or
mounted adjacent to portions of the sidewall of the catheter tube 122 and
configured to emit
low levels of vibration. In some examples, mechanical stimulation can be
provided to
portions of the ureters and/or renal pelvis to supplement or modify
therapeutic effects
obtained by application of negative pressure. While not intending to be bound
by theory, it is
believed that such stimulation affects adjacent tissues by, for example,
stimulating nerves
and/or actuating peristaltic muscles associated with the ureter(s) and/or
renal pelvis.
Stimulation of nerves and activation of muscles may produce changes in
pressure gradients or
pressure levels in surrounding tissues and organs which may contribute to or,
in some cases,
enhance therapeutic benefits of negative pressure therapy.
[00343] With reference to FIGS. 38A and 38B, according to another example, a
retention
portion 330 of a ureteral catheter 312 comprises a catheter tube 322 having a
distal portion
318 formed in a helical structure 332 and an inflatable element or balloon 350
positioned
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
71
proximal to the helical structure 332 to provide an additional degree of
retention in the renal
pelvis and/or fluid collection location. A balloon 350 can be inflated to
pressure sufficient to
retain the balloon in the renal pelvis or ureter, but low enough to avoid
distending or
damaging these structures. Suitable inflation pressures are known to those
skilled in the art
and are readily discernible by trial and error. As in previously-described
examples, the
helical structure 332 can be imparted by bending the catheter tube 322 to form
one or more
coils 334. The coils 334 can have a constant or variable diameter and height
as described
above. The catheter tube 322 further comprises a plurality of drainage ports
336 disposed on
the sidewall of the catheter tube 322 to allow urine to be drawn into the
drainage lumen 324
of the catheter tube 322 and to be directed from the body through the drainage
lumen 324, for
example on the inwardly facing and/or outwardly facing sides of the coil 334.
[00344] As shown in FIG. 38B, the inflatable element or balloon 350 can
comprise an
annular balloon-like structure having, for example, a generally heart-shaped
cross section and
comprising a surface or cover 352 defining a cavity 353. The cavity 353 is in
fluid
communication with an inflation lumen 354 extending parallel to the drainage
lumen 324
defined by the catheter tube 322. The balloon 350 can be configured to be
inserted in the
tapered portion of the renal pelvis and inflated such that an outer surface
356 thereof contacts
and rests against an inner surface of the ureter and/or renal pelvis. The
inflatable element or
balloon 350 can comprise a tapered inner surface 358 extending longitudinally
and radially
inward towards the catheter tube 322. The inner surface 358 can be configured
to direct urine
toward the catheter tube 322 to be drawn into the drainage lumen 324. The
inner surface 358
can also be positioned to prevent fluid from pooling in the ureter, such as
around the
periphery of the inflatable element or balloon 350. The inflatable retention
portion or balloon
350 is desirably sized to fit within the renal pelvis and can have a diameter
ranging from
about 10 mm to about 30 mm.
[00345] With reference to FIGS. 39A-40C, in some examples, an assembly 400
including a
ureteral catheter 412 comprising a retention portion 410 is illustrated. The
retention portion
410 is formed from a porous and/or sponge-like material that is attached to a
distal end 421 of
a catheter tube 422. The porous material can be configured to channel and/or
absorb urine
and direct the urine toward a drainage lumen 424 of the catheter tube 422. The
retention
portion 410 can be configured into a funnel-shaped support having an outer
surface and an
inner surface, and wherein the outer periphery 1002 or protective surface area
1001
comprises the outer surface of the funnel-shaped support, and the one or more
drainage holes,
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
72
ports or perforations in the porous material can be disposed within the porous
material or on
the inner surface 426 of the funnel-shaped support.
[00346] As shown in FIG. 40, the retention portion 410 can be a porous wedge
shaped-
structure configured for insertion and retention in the patient's renal
pelvis. The porous
material comprises a plurality of holes and/or channels. Fluid can be drawn
through the
channels and holes, for example, by gravity or upon inducement of negative
pressure through
the catheter 412. For example, fluid can enter the wedge-shaped retention
portion 410
through the holes and/or channels and is drawn toward a distal opening 420 of
the drainage
lumen 424, for example, by capillary action, peristalsis, or as a result of
the inducement of
negative pressure in the holes and/or channels. In other examples, as shown in
FIG. 40, the
retention portion 410 comprises a hollow, funnel structure formed from the
porous sponge-
like material. As shown by arrow A, fluid is directed down an inner surface
426 of the funnel
structure into the drainage lumen 424 defined by the catheter tube 422. Also,
fluid can enter
the funnel structure of the retention portion 410 through holes and channels
in the porous
sponge-like material of a sidewall 428. For example, suitable porous materials
can include
open-celled polyurethane foams, such as polyurethane ether. Suitable porous
materials can
also include laminates of woven or non-woven layers comprising, for example,
polyurethane,
silicone, polyvinyl alcohol, cotton, or polyester, with or without
antimicrobial additives such
as silver, and with or without additives for modifying material properties
such as hydrogels,
hydrocolloids, acrylic, or silicone.
[00347] With reference to FIG. 41, according to another example, a retention
portion 500
of a ureteral catheter 512 comprises an expandable cage 530. The expandable
cage 530
comprises one or more longitudinally and radially extending hollow tubes 522.
For example,
the tubes 522 can be formed from an elastic, shape memory material such as
nitinol. The
cage 530 is configured to transition from a contracted state, for insertion
through the patient's
urinary tract, to a deployed state for positioning in the patient's ureters
and/or kidney. The
hollow tubes 522 comprise a plurality of drainage ports 534 which can be
positioned on the
tubes, for example, on radially inward facing sides thereof. The ports 534 are
configured to
permit fluid to flow or be drawn through the ports 534 and into the respective
tubes 522. The
fluid drains through the hollow tubes 522 into a drainage lumen 524 defined by
a catheter
body 526 of the ureteral catheter 512. For example, fluid can flow along the
path indicated
by the arrows 532 in FIG. 41. In some examples, when negative pressure is
induced in the
renal pelvis, kidneys, and/or ureters, portions of the ureter wall and/or
renal pelvis may be
drawn against the outward facing surfaces of the hollow tubes 522. The
drainage ports 534
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
73
are positioned and configured so as not to be appreciably occluded by ureteral
structures upon
application of negative pressure to the ureters and/or kidney.
[00348] In some examples, the ureteral catheter comprising a funnel support
can be
deployed into a patient's urinary tract, and more specifically in the renal
pelvis region/kidney
using a conduit through the urethra and into the bladder. The funnel support
6100 is in a
collapsed state (shown in FIG. 36) and sheathed in a ureteral sheath 6102. To
deploy the
ureteral catheter, the medical professional would insert a cystoscope into the
urethra to
provide a channel for tools to enter the bladder. The ureteral orifice would
be visualized and
guide wire would be inserted through the cystoscope and ureter until the tip
of the guide wire
reaches the renal pelvis. The cystoscope likely would be removed, and a
"pusher tube"
would be fed over the guide wire up to the renal pelvis. The guidewire would
be removed
while the "pusher tube" stays in place to act as deployment sheath. The
ureteral catheter
would be inserted through the pusher tube / sheath and the catheter tip would
be actuated
once it extends beyond the end of the pusher tube / sheath. The funnel support
would expand
radially to assume the deployed position.
Exemplary ureteral stents:
[00349] Referring now to FIG. 1A, in some examples, the ureteral stent 52, 54
comprises
an elongated body comprising a proximal end 62, a distal end 58, a
longitudinal axis, and at
least one drainage channel that extends along the longitudinal axis from the
proximal end to
the distal end to maintain patency of fluid flow between a kidney and a
bladder of the patient.
In some examples, the ureteral stent further comprises a pigtail coil or
loop(s) on at least one
of the proximal end or the distal end. In some examples, the body of the
ureteral stent further
comprises at least one perforation on a sidewall thereof. In other examples,
the body of the
ureteral stent is essentially free of or free of perforation(s) on a sidewall
thereof.
[00350] Some examples of ureteral stents 52, 54 that can be useful in the
present systems
and methods include CONTOURTm ureteral stents, CONTOUR VLTM ureteral stents,
POLARISTM Loop ureteral stents, POLARISTM Ultra ureteral stents, PERCUFLEXTM
ureteral stents, PERCUFLEXTM Plus ureteral stents, STRETCHTm VL Flexima
ureteral
stents, each of which are commercially available from Boston Scientific
Corporation of
Natick, Massachusetts. See "Ureteral Stent Portfolio", a publication of Boston
Scientific
Corp., (July 2010), hereby incorporated by reference herein. The CONTOURTm and
CONTOUR VLTM ureteral stents are constructed with soft PercuflexTM Material
that becomes
soft at body temperature and is designed for a 365-day indwelling time.
Variable length coils
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
74
on distal and proximal ends allow for one stent to fit various ureteral
lengths. The fixed
length stent can be 6F ¨ 8F with lengths ranging from 20cm ¨ 30cm, and the
variable length
stent can be 4.8F ¨ 7F with lengths of 22 ¨ 30cm. Other examples of suitable
ureteral stents
include INLAY ureteral stents, INLAY OPTIMA ureteral stents, BARDEX double
pigtail ureteral stents, and FLUORO4TM silicone ureteral stent, each of which
are
commercially available from C.R. Bard, Inc. of Murray Hill, NJ. See "Ureteral
Stents",
http://www.bardmedical.com/products/kidney- stone-management/ureteral-stents/
(January
21, 2018), hereby incorporated by reference herein.
[00351] The stents 52, 54 can be deployed in one or both of the patient's
kidneys or kidney
area (renal pelvis or ureters adjacent to the renal pelvis), as desired.
Typically, these stents
are deployed by inserting a stent having a nitinol wire therethrough through
the urethra and
bladder up to the kidney, then withdrawing the nitinol wire from the stent,
which permits the
stent to assume a deployed configuration. Many of the above stents have a
planar loop 58, 60
on the distal end (to be deployed in the kidney), and some also have a planar
loop 62, 64 on
the proximal end of the stent which is deployed in the bladder. When the
nitinol wire is
removed, the stent assumes the pre-stressed planar loop shape at the distal
and/or proximal
ends. To remove the stent, a nitinol wire is inserted to straighten the stent
and the stent is
withdrawn from the ureter and urethra.
[00352] Other examples of suitable ureteral stents 52, 54 are disclosed in PCT
Patent
Application Publication WO 2017/019974, which is incorporated by reference
herein. In
some examples, as shown, for example, in FIGS. 1-7 of WO 2017/019974 and in
FIG. 3
herein (same as FIG. 1 of WO 2017/019974), the ureteral stent 100 can
comprise: an
elongated body 101 comprising a proximal end 102, a distal end 104, a
longitudinal axis 106,
an outer surface 108, and an inner surface 110, wherein the inner surface 110
defines a
transformable bore 111 that extends along the longitudinal axis 106 from the
proximal end
102 to the distal end 104; and at least two fins 112 projecting radially away
from the outer
surface 108 of the body 101; wherein the transformable bore 111 comprises: (a)
a default
orientation 113A (shown on the left in FIG. 59) comprising an open bore 114
defining a
longitudinally open channel 116; and (b) a second orientation 113B (shown on
the right in
FIG. 59) comprising an at least essentially closed bore 118 or closed bore
defining a
longitudinally essentially closed drainage channel 120 along the longitudinal
axis 106 of the
elongated body 101, wherein the transformable bore 111 is moveable from the
default
orientation 113A to the second orientation 113B upon radial compression forces
122 being
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
applied to at least a portion of the outer surface 108 of the
body 101.
[00353] In some examples, as shown in FIG. 3, the drainage channel 120 of the
ureteral
stent 100 has a diameter D which is reduced upon the transformable bore 111
moving from
the default orientation 113A to the second orientation 113B, wherein the
diameter is
reducible up to the point above where urine flow through the transformable
bore 111 would
be reduced. In some examples, the diameter D is reduced by up to about 40%
upon the
transformable bore 111 moving from the default orientation 113A to the second
orientation
113B. In some examples, the diameter D in the default orientation 113A can
range from
about 0.75 to about 5.5 mm, or about 1.3 mm or about 1.4 mm. In some examples,
the
diameter D in the second orientation 113B can range from about 0.4 to about 4
mm, or about
0.9 mm.
[00354] In some examples, one or more fins 112 comprise a flexible material
that is soft to
medium soft based on the Shore hardness scale. In some examples, the body 101
comprises a
flexible material that is medium hard to hard based on the Shore hardness
scale. In some
examples, one or more fins have a durometer between about 15 A to about 40 A.
In some
examples, the body 101 has a durometer between about 80A to about 90 A. In
some
examples, one or more fins 112 and the body 101 comprise a flexible material
that is medium
soft to medium hard based on the Shore hardness scale, for example having a
durometer
between about 40 A to about 70 A.
[00355] In some examples, one or more fins 112 and the body 101 comprise a
flexible
material that is medium hard to hard based on the Shore hardness scale, for
example having a
durometer between about 85 A to about 90 A.
[00356] In some examples, the default orientation 113A and the second
orientation 113B
support fluid or urine flow around the outer surface 108 of the stent 100 in
addition to
through the transformable bore 111.
[00357] In some examples, one or more fins 112 extend longitudinally from the
proximal
end 102 to the distal end 104. In some examples, the stent has two, three or
four fins.
[00358] In some examples, the outer surface 108 of the body has an outer
diameter in the
default orientation 113A ranging from about 0.8 mm to about 6 mm, or about 3
mm. In some
examples, the outer surface 108 of the body has an outer diameter in the
second orientation
113B ranging from about 0.5 mm to about 4.5 mm, or about 1 mm. In some
examples, one or
more fins have a width or tip ranging from about 0.25 mm to about 1.5 mm, or
about 1 mm,
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
76
projecting from the outer surface 108 of the body in a direction generally
perpendicular to the
longitudinal axis.
[00359] In some examples, the radial compression forces are provided by at
least one of
normal ureter physiology, abnormal ureter physiology, or application of any
external force.
In some examples, the ureteral stent 100 purposefully adapts to a dynamic
ureteral
environment, the ureteral stent 100 comprising: an elongated body 101
comprising a
proximal end 102, a distal end 104, a longitudinal axis 106, an outer surface
108, and an inner
surface 110, wherein the inner surface 110 defines a transformable bore 111
that extends
along the longitudinal axis 106 from the proximal end 102 to the distal end
104; wherein the
transformable bore 111 comprises: (a) a default orientation 113A comprising an
open bore
114 defining a longitudinally open channel 116; and (b) a second orientation
113B
comprising an at least essentially closed bore 118 defining a longitudinally
essentially closed
channel 120, wherein the transformable bore is moveable from the default
orientation 113A
to the second orientation 113B upon radial compression forces 122 being
applied to at least a
portion of the outer surface 108 of the body 101, wherein the inner surface
110 of the body
101 has a diameter D which is reduced upon the transformable bore 111 moving
from the
default orientation 113A to the second orientation 113B, wherein the diameter
is reducible up
to the point above where fluid flow through the transformable bore 111 would
be reduced. In
some examples, the diameter D is reduced by up to about 40% upon the
transformable bore
111 moving from the default orientation 113A to the second orientation 113B.
[00360] Other examples of suitable ureteral stents are disclosed in US Patent
Application
Publication US 2002/0183853 Al, which is incorporated by reference herein. In
some
examples, as shown, for example, in FIGS. 4, 5 and 7 of US 2002/0183853 Al and
in FIGS.
4-6 herein (same as FIGS. 1 of 4, 5 and 7 of US 2002/0183853 Al), the ureteral
stent
comprises an elongated, body 10 comprising a proximal end 12, a distal end 14
(not shown) ,
a longitudinal axis 15, and at least one drainage channel (for example, 26,
28, 30 in FIG. 4;
32, 34, 36 and 38 in FIG. 5; and 48 in FIG. 6) that extends along the
longitudinal axis 15
from the proximal end 12 to the distal end 14 to maintain patency of fluid
flow between a
kidney and a bladder of the patient. In some examples, the at least one
drainage channel is
partially open along at least a longitudinal portion thereof. In some
examples, the at least one
drainage channel is closed along at least a longitudinal portion thereof. In
some examples,
the at least one drainage channel is closed along the longitudinal length
thereof. In some
examples, the ureteral stent is radially compressible. In some examples, the
ureteral stent is
radially compressible to narrow the at least one drainage channel. In some
examples, the
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
77
elongated body 10 comprises at least one external fin 40 along the
longitudinal axis 15 of the
elongated body 10. In some examples, the elongated body comprises one to four
drainage
channels. The diameter of the drainage channel can be the same as described
above.
Systems for inducing negative pressure
[00361] In some examples, a system for inducing negative pressure in a portion
of a
urinary tract of a patient or for removing fluid from the urinary tract of a
patient is provided,
comprising: a ureteral stent or ureteral catheter for maintaining patency of
fluid flow between
at least one of a kidney and a bladder of the patient; a bladder catheter
comprising a drainage
lumen for draining fluid from the bladder of the patient; and a pump in fluid
communication
with a distal end of the drainage lumen, the pump comprising a controller
configured to
actuate the pump to apply negative pressure to the proximal end of the
catheter to induce
negative pressure in a portion of the urinary tract of the patient to remove
fluid from the
urinary tract of the patient.
[00362] In some examples, a system for inducing negative pressure in a portion
of a
urinary tract of a patient is provided, the system comprising: (a) a ureteral
catheter
comprising a distal portion for insertion within the patient's kidney and a
proximal portion;
(b) a bladder catheter comprising a distal portion for insertion within the
patient's bladder and
a proximal portion for application of negative pressure, the proximal portion
extending
outside of the patient's body; and (c) a pump external to the patient's body
for application of
negative pressure through both the bladder catheter and the ureteral catheter,
which in turn
causes fluid from the kidney to be drawn into the ureteral catheter, through
both the ureteral
catheter and the bladder catheter, and then outside the patient's body.
[00363] In some examples, a system for inducing negative pressure in a portion
of a
urinary tract of a patient is provided, the system comprising: (a) at least
one ureteral catheter,
the at least one ureteral catheter comprising a distal portion for insertion
within the patient's
kidney and a proximal portion; (b) a bladder catheter comprising a distal
portion for insertion
within the patient's bladder and a proximal portion for receiving negative
pressure from a
negative pressure source, wherein at least one of the at least one ureteral
catheter(s) or the
bladder catheter comprises (a) a proximal portion; and (b) a distal portion,
the distal portion
comprising a retention portion that comprises one or more protected drainage
holes, ports or
perforations and is configured to establish an outer periphery or protective
surface area that
inhibits mucosal tissue from occluding the one or more protected drainage
holes, ports or
perforations upon application of negative pressure through the catheter; and
(c) a negative
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
78
pressure source for application of negative pressure through both the bladder
catheter and the
ureteral catheter(s), which in turn causes fluid from the kidney to be drawn
into the ureteral
catheter(s), through both the ureteral catheter(s) and the bladder catheter,
and then outside of
the patient's body.
[00364] In some examples, a system for inducing negative pressure in a portion
of a
urinary tract of a patient, the system comprising: (a) at least one ureteral
catheter, the at least
one ureteral catheter comprising a distal portion for insertion within the
patient's kidney and
a proximal portion; (b) a bladder catheter comprising a distal portion for
insertion within the
patient's bladder and a proximal portion for receiving a pressure
differential, wherein the
pressure differential causes fluid from the kidney to be drawn into the
ureteral catheter(s),
through both the ureteral catheter(s) and the bladder catheter, and then
outside of the patient's
body, the pressure differential being applied to increase, decrease and/or
maintain fluid flow
therethrough, wherein at least one of the at least one ureteral catheter(s) or
the bladder
catheter comprises (a) a proximal portion; and (b) a distal portion, the
distal portion
comprising a retention portion that comprises one or more protected drainage
holes, ports or
perforations and is configured to establish an outer periphery or protective
surface area that
inhibits mucosal tissue from occluding the one or more protected drainage
holes, ports or
perforations upon application of differential pressure through the catheter.
[00365] With reference to FIGS. 1A, 1B, 1C, 1F, 1P, 1U, 2A, 2B, 7A and 7B, an
exemplary system 1100 for inducing negative pressure in a urinary tract of a
patient for
increasing renal perfusion is illustrated. The system 1100 comprises one or
two ureteral
catheters 1212 (or alternatively ureteral stents shown in FIG. 1A) connected
to a fluid pump
2000 for generating the negative pressure. More specifically, the patient's
urinary tract
comprises the patient's right kidney 2 and left kidney 4. The kidneys 2, 4 are
responsible for
blood filtration and clearance of waste compounds from the body through urine.
Urine or
fluid produced by the right kidney 2 and the left kidney 4 is drained into a
patient's bladder
through tubules, namely a right ureter 6 and a left ureter 8, which are
connected to the
kidneys at the renal pelvis 20, 21. Urine may be conducted through the ureters
6, 8 by
peristalsis of the ureter walls, as well as by gravity. The ureters 6, 8 enter
the bladder 10
through a ureter orifice or opening 16. The bladder 10 is a flexible and
substantially hollow
structure adapted to collect urine until the urine is excreted from the body.
The bladder 10 is
transitionable from an empty position (signified by reference line E) to a
full position
(signified by reference line F). Normally, when the bladder 10 reaches a
substantially full
state, fluid or urine is permitted to drain from the bladder 10 to a urethra
12 through a urethral
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
79
sphincter or opening 18 located at a lower portion of the bladder 10.
Contraction of the
bladder 10 can be responsive to stresses and pressure exerted on a trigone
region 14 of the
bladder 10, which is the triangular region extending between the ureteral
openings 16 and the
urethral opening 18. The trigone region 14 is sensitive to stress and
pressure, such that as the
bladder 10 begins to fill, pressure on the trigone region 14 increases. When a
threshold
pressure on the trigone region 14 is exceeded, the bladder 10 begins to
contract to expel
collected urine through the urethra 12.
[00366] As shown in FIGS. 1, 2A, 7A and 7B, distal portions of the ureteral
catheter(s) are
deployed in the renal pelvis 20, 21 near the kidneys 2, 4. Proximal portions
of the one or
more of the catheter(s) 1212 empty into the bladder, into the urethra or
outside of the body.
In some examples, the proximal portion 1216 of the ureteral catheter 1212 is
in fluid
communication with the distal portion or end 136 of the bladder catheter 56,
116. A proximal
portion 1216 of the bladder catheter 56, 116 is connected to a source of
negative pressure,
such as a fluid pump 2000. The shape and size of the connector can be selected
based on the
type of pump 2000 being used. In some examples, the connector can be
manufactured with a
distinctive configuration so that it can only be connected to a particular
pump type, which is
deemed to be safe for inducing negative pressure in a patient's bladder,
ureter, or kidneys. In
other examples, as described herein, the connector can be a more generic
configuration
adapted for attachment to a variety of different types of fluid pumps. System
1100 is but one
example of a negative pressure system for inducing negative pressure that can
be used with
the bladder catheters disclosed herein.
[00367] Referring now to FIGS. 1A, 1B, 1C, 1F, 1P, 1U, 2A, 2B 7A, 7B, 17, in
some
examples the system 50, 100 comprises a bladder catheter 116. The distal ends
120, 121 of
the ureteral catheters 112, 114 can drain directly into the bladder, and the
fluid can drain
through the bladder catheter 116, and optionally along the sides of the
bladder catheter tube.
Exemplary bladder catheters
[00368] Any of the ureteral catheters disclosed herein can be used as bladder
catheters
useful in the present methods and systems. In some examples, the bladder
catheter 116
comprises a retention portion 123 or deployable seal and/or anchor 136 for
anchoring,
retaining, and/or providing passive fixation for indwelling portions of the
urine collection
assembly 100 and, in some examples, to prevent premature and/or untended
removal of
assembly components during use. The retention portion 123 or anchor 136 is
configured to
be located adjacent to the lower wall of the patient's bladder 10 (shown in
FIGS. 1A, 1B, 1C,
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
1F, 1P, 1U, 2A, 2B, 7A, 7B, 17) to prevent patient motion and/or forces
applied to indwelling
catheters 112, 114, 116 from translating to the ureters. The bladder catheter
116 comprises
an interior of which defines a drainage lumen 140 configured to conduct urine
from the
bladder 10 to an external urine collection container 712 (shown in FIG. 44).
In some
examples, the bladder catheter 116 tube size can range from about 8 Fr to
about 24 Fr. In
some examples, the bladder catheter 116 can have an external tube diameter
ranging from
about 2.7 to about 8 mm. In some examples, the bladder catheter 116 can have
an internal
diameter ranging from about 2.16 to about 10 mm. The bladder catheter 116 can
be available
in different lengths to accommodate anatomical differences for gender and/or
patient size.
For example, the average female urethra length is only a few inches, so the
length of a tube
138 can be rather short. The average urethra length for males is longer due to
the penis and
can be variable. It is possible that woman can use bladder catheters 116 with
longer length
tubes 138 provided that the excess tubing does not increase difficulty in
manipulating and/or
preventing contamination of sterile portions of the catheter 116. In some
examples, a sterile
and indwelling portion of the bladder catheter 116 can range from about 1 inch
to 3 inches
(for women) to about 20 inches for men. The total length of the bladder
catheter 116
including sterile and non-sterile portions can be from one to several feet.
[00369] In some examples, such as are shown in FIGS. 1A, 1B, 1C, 1F, 1P, 1U,
2A, 2B,
7A and 7B, the distal portion 136 of the bladder catheter 56, 116 comprises a
retention
portion 123 that includes one or more drainage holes, ports or perforations
142 and is
configured to establish an outer periphery 1002 or protective surface area
1001 that inhibits
mucosal tissue from occluding the one or more drainage holes, ports or
perforations 142 upon
the application of negative pressure by the pump 710, 2000.
[00370] In some examples in which the retention portion 123 comprises a tube
138, the
tube 138 can comprise one or more drainage holes, ports or perforations 142
configured to be
positioned in the bladder 10 for drawing urine into the drainage lumen 140.
For example,
fluid or urine that flows into the patient's bladder 10 from the ureteral
catheters 112, 114 is
expelled from the bladder 10 through the ports 142 and drainage lumen 140. The
drainage
lumen 140 may be pressurized to a negative pressure to assist in fluid
collection.
[00371] In some examples, such as are shown in FIGS. 1A, 1B, 1C, 1F, 1P, 1U,
2A, 2B,
7A and 7B, the one or more drainage holes, ports or perforations 142, 172 of
the bladder
catheter 56, 116, like the ureteral catheters discussed above, are disposed on
a protected
surface area or inner surface area 1000 of the retention portion 123, and
wherein, upon
application of negative pressure, the mucosal tissue 1003, 1004 conforms or
collapses onto
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
81
the outer periphery 1002 or protective surface area 1001 of the retention
portion 173 of the
bladder catheter 56, 116 and is thereby prevented or inhibited from occluding
the one or more
of the protected drainage holes, ports or perforations 172 of the bladder
catheter 56, 116
[00372] With specific reference to FIGS. 1A, 1B, 1C, 1F, 1P, 1U, 2A, 2B, 7A
and 7B, the
retention portion 123 or deployable seal and/or anchor 136 is disposed at or
adjacent to a
distal end 148 of the bladder catheter 116. The retention portion 123 or
deployable anchor
136 is configured to transition between a contracted state for insertion into
the bladder 10
through the urethra 12 and urethral opening 18 and a deployed state. The
retention portion
123 or deployable anchor 136 is configured to be deployed in and seated
adjacent to a lower
portion of the bladder 10 and/or against the urethral opening 18. For example,
the retention
portion 123 or deployable anchor 136 can be positioned adjacent to the
urethral opening 18 to
enhance suction of a negative pressure applied to the bladder 10 or to
partially, substantially,
or entirely seal the bladder 10 to ensure that urine in the bladder 10 is
directed through the
drainage lumen 140 and to prevent leakage to the urethra 12. For a bladder
catheter 116
including an 8 Fr to 24 Fr elongated tube 138, the retention portion 123 or
deployable anchor
136 can have a diameter of about 10 mm to about 100 mm) in the deployed state.
Exemplary bladder anchor structures
[00373] Any of the ureteral catheters disclosed herein can be used as bladder
catheters
useful in the present methods and systems. For example, the bladder catheter
can comprise a
mesh as a bladder anchor, such as is shown in FIGS. 1A, 1B and 7B. In another
example, the
bladder catheter 116 can comprise a coil 36, 38, 40, 183, 184, 185, 334, 1210
as a bladder
anchor, such as is shown in FIGS. 1C-1W and 7A. In another example, the
bladder catheter
116 can comprise a mesh funnel 57 as a bladder anchor, such as is shown in
FIG. 7B. In
another example, the bladder catheter 116 can comprise a funnel 150 as a
bladder anchor,
such as is shown in FIG. 17. Regardless of the embodiment selected, the
retention portion
123 creates an outer periphery 1002 or protective surface area 1001 to prevent
the tissues
1003, 1004 from contracting or collapsing into the fluid column under negative
pressure.
[00374] In some examples, the retention portion 123 comprises a coiled
retention portion
similar to the retention portions of the ureteral catheters described in
connection with FIGS.
2A and 7A-14. In some examples such as are shown in FIGS. 1C-1E, 1U-1W, the
coiled
retention portion 123 can comprise a plurality of helical coils 36, 38, 40 or
438, 436, 432
arranged such that an outer periphery 1002 or outer region of the helical
coils 36, 38, 40 or
438, 436, 432 contacts and supports bladder tissue 1004 to inhibit occlusion
or blockage of
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
82
protected drainage holes, ports or perforations 172 positioned in protected
surface areas or
inner surface areas of the helical coils 36, 38, 40 or 438, 436, 432.
[00375] The coiled retention portion 123 can comprise at least the first coil
36, 438 having
an outer diameter D1 (see FIG. 1E), at least a second coil 38, 436 having an
outer diameter
D2, and at least a third coil 40, 432 having an outer diameter D3. The
diameter D3 of the
distal-most or third coil 40, 432 can be smaller than a diameter of either the
first coil 36, 438
or the second coil 38, 436. Accordingly, a diameter of the coils 36, 38, 40 or
438, 436, 432,
and/or a step distance or height between adjacent coils 36, 38, 40 or 438,
436, 432 can vary in
a regular or irregular manner. In some examples, the plurality of coils 36,
38, 40 or 438, 436,
432 can form a tapered or reverse pyramid shape in which D1>D2>D3. In some
examples,
the coiled retention portion 123 can comprise a plurality of similarly sized
coils or, for
example, can include a plurality of proximal similarly sized coils and a
distal-most coil
having a smaller diameter than other coils of the plurality of coils. The
diameter of the coils
36, 38, 40 or 438, 436, 432 and distance or height between adjacent coils is
selected so that
the retention portion 123 remains in the bladder for a desired period of time,
such as hours,
days or up to about 6 months. The coiled retention portion 123 can be large
enough so that it
remains in the bladder 10 and does not pass into the urethra until the
catheter is ready to be
removed from the bladder 10. For example, the outer diameter D1 of the
proximal most or
first coil 36 438 can range from about 2 mm to 80 mm. The outer diameter D2 of
the second
coil 38, 436 can range from about 2 mm to 60 mm. The distal-most or third coil
40, 432 can
have an outer diameter D3 ranging from about 1 mm to 45 mm. The diameter of
the coil tube
can range from about 0.33 mm to 9.24 mm (about 1 Fr to about 28 Fr (French
catheter scale).
[00376] The configurations, sizes and positions of the holes, ports or
perforations 142, 172
can be any of the configurations, sizes and positions discussed above for the
ureteral or other
catheters. In some examples, holes, ports or perforations 142 are present on
the outer
periphery 1002 or protective surface area 1001 and protected holes, ports or
perforations 172
are present on the protected surface areas or inner surface areas 1000. In
some examples, the
outer periphery 1002 or protective surface area 1001 is essentially free of or
free of holes,
ports or perforations 142, and the protected holes, ports or perforations 172
are present on the
protected surface areas or inner surface areas 1000.
[00377] The retention portion 416 shown in FIGS. 1U-1W is a coiled retention
portion
comprising a plurality of coils wrapped around a substantially linear or
straight portion 430 of
the elongated tube 418. In some examples, the coiled retention portion 416
comprises a
straight portion 430 and a distal-most coil 432 formed from a bend 434 of from
about 90
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
83
degrees to 180 degrees in the elongated tube 418. The retention portion 416
further
comprises one or more additional coils, such as a second or middle coil 436
and a third or
proximal most coil 438, which are wrapped around the straight portion 430. The
elongated
tube 418 can further comprise a distal end 440 after the proximal most coil
438. The distal
end 440 can be closed or can be open to receive urine or fluid from the
bladder 10.
[00378] An area of two-dimensional slices 34 (shown in FIG. 1E) of the three-
dimensional
shape 32 defined by the deployed expandable retention portion 123 in a plane
transverse to a
central axis A of the expandable retention portion 16 can decrease towards the
distal end 22
of the expanded or deployed retention portion 123, giving the retention
portion 123 a pyramid
or reversed conical shape. In some examples, a maximum cross-sectional area of
the three-
dimensional shape 32 defined by the deployed or expanded retention portion 123
in a plane
transverse to the central axis A of the deployed or expanded retention portion
132 can range
from about 100 mm2 to 1500 mm2, or about 750 mm2.
[00379] Other examples of a catheter device 10 are shown in FIGS. 1F-1J. The
retention
portion 123 of the catheter device 10 comprises a basket shaped structure or
support cap 212
of a bladder superior wall support 210 or outer periphery 1002, configured to
be disposed
within a distal portion of the tube 12 in a retracted position and to extend
from the distal end
of the tube 12 in a deployed position. The bladder superior wall support 210
comprises a
support cap 212 configured to support a superior wall or bladder tissue 1004
and a plurality
of support members, such as legs 214, connected to a proximal surface of the
support cap
212. The legs 214 can be positioned so that the cap 212 is spaced apart from
an open distal
end of the drainage tube 12. For example, the legs 214 can be configured to
maintain a gap,
cavity, or space of distance D1 between an open distal end 30 of the tube 12
and the support
cap 212. The distance D1 can range from about 1 mm to about 40 mm, or about 5
mm to
about 40 mm. The height D2 of the bladder superior wall support 210 or
retention portion
can range from about 25 mm to about 75 mm, or about 40 mm. The maximum
diameter of
the support cap 212 can range from about 25 mm to about 60 mm in the deployed
state, and
preferably range from about 35 mm and 45 mm.
[00380] In some examples, the legs 214 comprise flexible tines, which can be
formed from
a flexible or shape memory material, such as a nickel titanium. The number of
legs can range
from about 3 to about 8. The length of each leg can range from about 25 mm to
about 100
mm, or longer if the deployment mechanism is external to the patient's body.
The width
and/or thickness, e.g., diameter, of each leg can range from about 0.003
inches to about 0.035
inches.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
84
[00381] In some examples, the support cap 212 can be a flexible cover 216
mounted to and
supported by the legs 214. The flexible cover 216 can be formed from a
flexible, soft and/or
resilient material, such as silicone or Teflon , for preventing fluid from
passing through the
cover 216, a porous material, or combinations thereof. In some examples, the
flexible
material is formed from a material which does not appreciably abrade,
irritate, or damage the
mucosal lining of the bladder wall or the urethra when positioned adjacent to
the mucosal
lining, such as silicone or Teflon materials or porous materials. The
thickness of the cover
216 can range from about 0.05 mm to about 0.5 mm. In some examples, the
flexible cover
216 and legs 214 are sufficiently structurally rigid so that the cover 216 and
legs 214
maintain their form when contacted by the superior wall or bladder tissue
1004. Accordingly,
the legs 214 and flexible cover 216 prevent the bladder from collapsing and
occluding
perforations on the retention portion 6 and/or an open distal end 30 of the
tube 12. Also, the
legs 214 and flexible cover 216 effectively keep the trigone region and
ureteral orifices open
so that negative pressure can draw urine into the bladder and drainage tube
12. As discussed
herein, if the bladder were permitted to collapse too far, flaps of tissue
would extend over the
ureter openings, thereby preventing negative pressure from being transmitted
to the ureteral
catheter(s), ureteral stent(s) and /or ureters and thereby inhibit drawing of
urine into the
bladder.
[00382] In some examples, the catheter device 10 further comprises a drainage
tube 218.
As shown in FIGS. 1G-1J, the drainage tube 218 can comprise an open distal end
220
positioned adjacent to or extending from the open distal end 30 of the tube
12. In some
examples, the open distal end 220 of the drainage tube 218 is the only opening
for drawing
urine from the bladder into the interior of the drainage tube 218. In other
examples, a distal
portion of the drainage tube 218 may comprise perforations (not shown in FIGS.
1G-1I) or
holes, ports or perforations 174 on a sidewall 222 thereon, as shown in FIG.
1J. The holes,
ports or perforations 174 can provide additional spaces for drawing urine into
the interior of
the drainage tube 218, thereby ensuring that fluid collection can continue
even if the open
distal end 220 of the drainage tube 218 is occluded. Also, holes, ports or
perforations 174
can increase surface area available for drawing fluid into the drainage tube
218, thereby
increasing efficiency and/or fluid collection yield.
[00383] In some examples, a distal most portion of the support cap 212 can
comprise a
sponge or pad 224, such as a gel pad. The pad 224 can be positioned to contact
and press
against the superior bladder wall or bladder tissue 1004 for the purpose of
preventing
drainage, aspiration, or other trauma to the bladder 10 during negative
pressure treatment.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
[00384] With reference to FIG. 1J, the bladder superior wall support 210
comprises a
support cap 212 and a plurality of legs 214. As in previously described
examples, the bladder
superior wall support 210 is capable of being moved between a retracted
position, in which
the support 210 is at least partially retracted in a conduit or tube 12, and a
deployed position
to support the superior wall of the bladder. In some examples, the catheter
device 10 also
includes a drainage tube 218 extending from the open distal end 30 of the
conduit or tube 12.
Unlike in the previously-described examples, the support cap 212 shown in FIG.
4 comprises
an inflatable balloon 226. The inflatable balloon 226 can be a substantially
semi-spherical
and can comprise a curved distal surface 228 configured to contact and support
at least a
portion of the superior bladder wall or bladder tissue 1004 when deployed.
[00385] In some examples, the drainage tube 218 comprises a perforated portion
230
extending between the open distal end 30 of the tube 12 and the support
structure 212. The
perforated portion 230 is positioned to draw fluid into an interior of the
drainage tube 218 so
that it can be removed from the bladder 100. Desirably, the perforated portion
230 is
positioned so as not to be occluded either by the deployed support cap 212 or
the bladder wall
when negative pressure is applied thereto. The drainage tube 218 can comprise
or be
positioned adjacent to an inflation lumen 232 for providing fluid or gas to an
interior 234 of
the balloon 226 for inflating the balloon 226 from its contracted position to
the deployed
position. For example, as shown in FIG. 1J, the inflation lumen 232 can be
disposed within
the drainage tube 218.
[00386] With reference to FIG. 1K, an exemplary retention portion 6, 123 of a
urine
collection catheter device 10 including multiple coiled drainage lumens,
generally denoted as
lumens 218, is illustrated. The retention portion 6 comprises the tube 12
having a distal open
end 30. The drainage lumens 218 are positioned partially within the tube 12.
In a deployed
position, the draining lumens 218 are configured to extend from the open
distal end 30 of the
tube 12 and to conform to a coiled orientation. The drainage lumens 218 can be
separate for
the entire length of the catheter device 10, or may empty into a single
drainage lumen defined
by the tube 12. In some examples, as shown in FIG. 6, the drainage lumens 218
can be
pigtail coils having one or more coils 244. Unlike in the previously described
example, the
pigtail coils 244 are coiled about an axis that is not coextensive with an
axis C of an uncoiled
portion of the tube. Instead, as shown in FIG. 6, the pigtail coils can be
coiled about an axis
D that is approximately perpendicular to the axis C of the tube 12. In some
examples, the
drainage lumens 218 can comprise holes, ports or perforations (not shown in
FIG. 1K),
similar to perforations 132, 133 in FIGS. 9A or 9B, for drawing fluid from the
bladder into an
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
86
interior of the drainage lumens 218. In some examples, the perforations can be
positioned on
a radially inwardly facing side 240 and/or outwardly facing side of the coiled
portions of the
drainage lumens. As previously described, perforations positioned on radially
inwardly
facing sides of the drainage lumens 218 or tube 12 are less likely to be
occluded by the
bladder walls during application of negative pressure to the bladder. Urine
can also be drawn
directly into one or more drainage lumens defined by the tube 12. For example,
rather than
being drawing into the drainage lumen(s) 218 through the perforations 230,
urine can be
drawn directly through the open distal end 30 and into a drainage lumen
defined by the tube
12.
[00387] With reference to FIGS. 1L and 1M, another example of a retention
portion 123 is
shown. A fluid receiving portion or distal end portion 30a of the catheter
device 10a is
shown in a contracted position in FIG. 1L, and in a deployed position in FIG.
1M. The distal
end 30a includes opposing bladder wall supports 19a, 19b for supporting the
superior and
inferior bladder walls 1004. For example, the distal end portion 30a can
comprise a proximal
sheath 20a and a distal sheath 22a. Each sheath 20a, 22a extends between a
slidable ring or
collar 24a and stationary or mounted ring or collar 28a. The sheaths 20a, 22a
are formed
from a flexible, non-porous material, such as silicon or any of the materials
discussed herein.
The sheaths 20a, 22a are held together by one or more flexible wires or cables
26a. The
sheaths 20a, 22a can also be connected by one or more rigid members, such as
supports 32a.
In some examples, the supports 32a can be tines formed from a flexible, shape-
memory
material, such as nickel titanium. The supports 32a are positioned to provide
support for the
proximal sheath 20a and to prevent the distal end 30a from collapsing when it
is in the
deployed position. In the contracted position, the collars 24a, 28a are
positioned apart from
one another, such that the sheaths 20a, 22a are stretched or folded against
the cable 26a and
supports 32a. In the deployed position, the slidable collars 24a are moved
toward the
stationary collars 28a, allowing the sheaths 20a, 22a to unfold from the
central cables 26a and
to form a substantially flat disk-shaped structure.
[00388] In use, the distal end 30a of the catheter device 10a is inserted into
the bladder of a
patient in the contracted position. Once inserted in the bladder, the distal
sheath 22a is
released by sliding the slidable collar 24a in a distal direction toward the
stationary collar
28a. Once the distal sheath 22a is deployed, the proximal sheath 20a is
released or deployed
in a similar manner by sliding the slidable collar 24a in the proximal
direction toward the
respective stationary collar 28a. At this point, the proximal sheath 20a is
floating within the
bladder, and is not positioned or sealed against the inferior wall of the
bladder. Pressure
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
87
against the distal sheath 22a caused by collapsing of the bladder is
transferred to the proximal
sheath 20a through the supports 32a and causes the proximal sheath 20a to move
toward the
desired position adjacent to the opening of the urethra. Once the proximal
sheath 20a is in
place, a seal over the urethra opening may be created. The proximal sheath 20a
assists in
maintaining a negative pressure within the bladder and prevents air and/or
urine from exiting
the bladder through the urethra.
[00389] With reference to FIGS. 1N-1T, retention portions 123 comprising an
inflatable
support cap, such as an annular balloon 310, positioned to contact the
superior wall of the
bladder 10 to prevent the bladder 10 from contracting and occluding either
fluid port(s) 312
of the catheter device 10 or the ureteral openings of the bladder. In some
examples, a distal
end portion 30 of the tube 12 extends through a central opening 314 of the
balloon 310. The
distal end portion 30 of the tube 12 can also contact the superior bladder
wall.
[00390] Referring now to FIGS. 1N and 10, in some examples, the tube 12
comprises a
fluid access portion 316 positioned proximal to the balloon 310 and extending
through a
sidewall of the tube 12. The fluid access portion 316 can comprise a filter
318 (shown in
FIG. 10) disposed about a central lumen of the tube 12. In some examples, a
sponge material
320 can be positioned over the filter 318 for increased absorbance of fluid
within the bladder.
For example, the sponge material 320 can be injection molded over the filter
318. In use,
urine is absorbed by the sponge material 320 and, upon application of negative
pressure
through the tube 12, passes through the filter 318 and into the central lumen
of the tube 12.
[00391] Referring now to FIGS. 1P-1R, in another example, the support cap,
such as the
annular balloon 310, comprises a substantially bulbous distal portion 322
configured to
contact and support the superior bladder wall. The balloon 310 further
comprises a plurality
of proximally extending lobes 324. For example, the balloon 310 can comprise
three lobes
324 spaced equidistantly around a portion of the tube 12 proximal to the
balloon 310. As
shown in FIG. 1R, the fluid ports 312 can be positioned between adjacent lobes
324. In this
configuration, the lobes 324 and bulbous distal portion 322 contact the
bladder wall, which
prevents the bladder wall from blocking or occluding the fluid ports 312.
[00392] Referring now to FIGS. 15 and 1T, in another example, the annular
balloon 310 is
provided with a flattened and elongated shape. For example, the annular
balloon 310 can
have a substantially teardrop shaped radial cross section as shown in FIG. 1T,
with a
narrower portion 326 thereof positioned adjacent to the tube 12 and the
enlarged or bulbous
portion 328 positioned on the radially outwardly facing side thereof. The
flatted annular
balloon 310 is configured to span and optionally seal the periphery of the
trigone region of
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
88
the bladder such that when deployed in the bladder, the outer circumference of
the balloon
310 extends radially beyond the ureteral openings. For example, when
positioned in the
patient's bladder, the central opening 314 of the balloon 310 can be
configured to be
positioned above the trigone region. Fluid port(s) 312 can be positioned
proximal to the
central portion balloon 310, as shown in FIG. 1T. Desirably, the fluid port(s)
312 are
positioned between the central opening 314 of the balloon and the trigone
region. When the
bladder contracts from application of negative pressure, the bladder wall is
supported by the
outer circumference of the balloon 310 to avoid blocking the ureter openings.
Accordingly,
in this configuration, the balloon 310 contacts and prevents the bladder wall
from blocking or
occluding the fluid ports 312. In a similar manner, as discussed herein, the
balloon 310 keeps
the trigone region open so that urine can be drawn from the ureters into the
bladder through
the ureteral openings.
[00393] With reference to FIG. 41, in another example of a bladder catheter,
an
expandable cage 530 can anchor the bladder catheter in the bladder. The
expandable cage
530 comprises a plurality of flexible members or tines extending
longitudinally and radially
outward from a catheter body of a bladder catheter which, in some examples,
can be similar
to those discussed above with respect to the retention portion of the ureteral
catheter of FIG.
41. The members can be formed from a suitable elastic and shape memory
material such as
nitinol. In a deployed position, the members or tines are imparted with a
sufficient curvature
to define a spherical or ellipsoid central cavity. The cage is attached to an
open distal open
end of the catheter tube or body, to allow access to a drainage lumen defined
by the tube or
body. The cage is sized for positioning within the lower portion of the
bladder and can define
a diameter and length ranging from 1.0 cm to 2.3 cm, and preferably about 1.9
cm (0.75 in).
[00394] In some examples, the cage further comprises a shield or cover over
distal portions
of the cage to prevent or reduce the likelihood that tissue, namely, the
distal wall of the
bladder, will be caught or pinched as a result of contact with the cage or
member. More
specifically, as the bladder contracts, the inner distal wall of the bladder
comes into contact
with the distal side of the cage. The cover prevents the tissue from being
pinched or caught,
may reduce patient discomfort, and protect the device during use. The cover
can be formed
at least in part from a porous and/or permeable biocompatible material, such
as a woven
polymer mesh. In some examples, the cover encloses all or substantially all of
the cavity. In
some examples, the cover covers only about the distal 2/3, about the distal
half, or about the
distal third portion or any amount, of the cage 210.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
89
[00395] The cage and cover are transitionable from a contracted position, in
which the
members are contracted tightly together around a central portion and/or around
the bladder
catheter 116 to permit insertion through a catheter or sheath to the deployed
position. For
example, in the case of a cage constructed from a shape memory material, the
cage can be
configured to transition to the deployed position when it is warmed to a
sufficient
temperature, such as body temperature (e.g., 37 C). In the deployed position,
the cage has a
diameter D that is preferably wider than the urethral opening, and prevents
patient motion
from translating through the ureteral catheters 112, 114 to the ureters. The
open arrangement
of the members 212 or tines does not obstruct or occlude the distal opening
248 and/or
drainage ports of the bladder catheter 216, making manipulation of the
catheters 112, 114
easier to perform.
[00396] It is understood that any of the above-described bladder catheters may
also be
useful as ureteral catheters.
[00397] The bladder catheter is connected to the vacuum source, such as pump
assembly
710 by, for example, flexible tubing 166 defining a fluid flow path.
Exemplary fluid sensors:
[00398] With reference again to FIGS. 1A, 1B, 1C, 1F, 1P, 1U, 2A, 2B, in some
examples,
the system or assembly 100, 700, 1100 further comprises one or more sensors
174 for
monitoring physical parameters or fluid characteristics of fluid or urine
being collected from
the ureters 6, 8 and/or bladder 10. The one or more physiological sensors 174
associated with
the patient can be configured to provide information representative of at
least one physical
parameter to a controller. As discussed herein in connection with FIG. 44,
information
obtained from the sensors 174 can be transmitted to a central data collection
module or
processor and used, for example, to control operation of an external device,
such as the pump
710 (shown in FIG. 44). The sensors 174 can be integrally formed with one or
more of the
catheters 112, 114, 116 such as, for example, embedded in a wall of the
catheter body or tube
and in fluid communication with drainage lumens 124, 140. In other examples,
one or more
of the sensors 174 can be positioned in a fluid collection container 712
(shown in FIG. 44) or
in internal circuitry of an external device, such as the pump 710.
[00399] Exemplary sensors 174 that can be used with the urine collection
assembly 100
can comprise one or more of the following sensor types. For example, the
catheter assembly
100 can comprise a conductance sensor or electrode that samples conductivity
of urine. The
normal conductance of human urine is about 5-10 mS/m. Urine having a
conductance
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
outside of the expected range can indicate that the patient is experiencing a
physiological
problem, which requires further treatment or analysis. The catheter assembly
100 can also
comprise a flow meter for measuring a flow rate of urine through the
catheter(s) 112, 114,
116. Flow rate can be used to determine a total volume of fluid excreted from
the body. The
catheter(s) 112, 114, 116 can also comprise a thermometer for measuring urine
temperature.
Urine temperature can be used to collaborate the conductance sensor. Urine
temperature can
also be used for monitoring purposes, as urine temperature outside of a
physiologically
normal range can be indicative of certain physiological conditions. In some
examples, the
sensors 174 can be urine analyte sensors configured to measure a concentration
of creatinine
and/or proteins in urine. For example, various conductivity sensors and
optical spectrometry
sensors may be used for determining analyte concentration in urine. Sensors
based on color
change reagent test strips may also be used for this purpose.
Method of insertion of a system:
[00400] Having described the system 100 comprising the ureteral catheter(s)
and/or
ureteral stent(s) and bladder catheter, some examples of methods for insertion
and
deployment of the ureteral stent(s) or ureteral catheter(s) and bladder
catheter will now be
discussed in detail.
[00401] In some examples, a method for inducing negative pressure in a portion
of a
urinary tract of a patient is provided, the method comprising: deploying a
ureteral catheter
into a ureter of a patient to maintain patency of fluid flow between a kidney
and a bladder of
the patient, the ureteral catheter comprising a distal portion for insertion
within the patient's
kidney and a proximal portion; deploying a bladder catheter into the bladder
of the patient,
wherein the bladder catheter comprises a distal portion for insertion within
the patient's
bladder and a proximal portion for application of negative pressure, the
proximal portion
extending outside of the patient's body; and applying negative pressure to the
proximal end
of the bladder catheter to induce negative pressure in a portion of the
urinary tract of the
patient to remove fluid from the patient. In some examples, at least one of
the ureteral
catheter or the bladder catheter comprises (a) a proximal portion; and (b) a
distal portion, the
distal portion comprising a retention portion that comprises one or more
protected drainage
holes, ports or perforations and is configured to establish an outer periphery
or protective
surface area that inhibits mucosal tissue from occluding the one or more
protected drainage
holes, ports or perforations upon application of negative pressure through the
catheter.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
91
[00402] With reference to FIG. 42A, an example of steps for positioning a
system in a
patient's body and, optionally, for inducing negative pressure in a patient's
urinary tract, such
as bladder, ureter and/or kidneys are illustrated. As shown at box 610, a
medical professional
or caregiver inserts a flexible or rigid cystoscope through the patient's
urethra and into the
bladder to obtain visualization of the ureteral orifices or openings. Once
suitable
visualization is obtained, as shown at box 612, a guidewire is advanced
through the urethra,
bladder, ureteral opening, ureter, and to a desired fluid collection position,
such as the renal
pelvis of the kidney. Once the guidewire is advanced to the desired fluid
collection position,
a ureteral stent or ureteral catheter of the present invention (examples of
which are discussed
in detail above) is inserted over the guidewire to the fluid collection
position, as shown at box
614. In some examples, the location of the ureteral stent or ureteral catheter
can be
confirmed by fluoroscopy, as shown at box 616. Once the position of the distal
end of the
ureteral stent or ureteral catheter is confirmed, as shown at box 618, the
retention portion of
the ureteral catheter can be deployed. For example, the guidewire can be
removed from the
catheter, thereby allowing the distal end and/or retention portion to
transition to a deployed
position. In some examples, the deployed distal end portion of the catheter
does not entirely
occlude the ureter and/or renal pelvis, such that urine is permitted to pass
outside the catheter
and through the ureters into the bladder. Since moving the catheter can exert
forces against
urinary tract tissues, avoiding complete blockage of the ureters avoids
application of force to
the ureter sidewalls, which may cause injury.
[00403] After the ureteral stent or ureteral catheter is in place and
deployed, the same
guidewire can be used to position a second ureteral stent or second ureteral
catheter in the
other ureter and/or kidney using the same insertion and positioning methods
described herein.
For example, the cystoscope can be used to obtain visualization of the other
ureteral opening
in the bladder, and the guidewire can be advanced through the visualized
ureteral opening to
a fluid collection position in the other ureter. A second ureteral stent or
second ureteral
catheter can be drawn alongside the guidewire and deployed in the manner
described herein.
Alternatively, the cystoscope and guidewire can be removed from the body. The
cystoscope
can be reinserted into the bladder over the first ureteral catheter. The
cystoscope is used, in
the manner described above, to obtain visualization of the ureteral opening
and to assist in
advancing a second guidewire to the second ureter and/or kidney for
positioning of the
second ureteral stent or second ureteral catheter. Once the ureteral stents or
catheters are in
place, in some examples, the guidewire and cystoscope are removed. In other
examples, the
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
92
cystoscope and/or guidewire can remain in the bladder to assist with placement
of the bladder
catheter.
[00404] In some examples, once the ureteral catheters are in place, as shown
at box 620,
the medical professional, caregiver or patient can insert a distal end of a
bladder catheter in a
collapsed or contracted state through the urethra of the patient and into the
bladder. The
bladder catheter can be a bladder catheter of the present invention as
discussed in detail
above. Once inserted in the bladder, as shown at box 622, an anchor connected
to and/or
associated with the bladder catheter is expanded to a deployed position. In
some examples,
the bladder catheter is inserted through the urethra and into the bladder
without using a
guidewire and/or cystoscope. In other examples, the bladder catheter is
inserted over the
same guidewire used to position the ureteral stents or catheters.
[00405] In some examples, the ureteral stent or ureteral catheter is deployed
and remains in
the patient's body for at least 24 hours or longer. In some examples, the
ureteral stent or
ureteral catheter is deployed and remains in the patient's body for at least
30 days or longer.
In some examples, the ureteral stent(s) or ureteral catheter(s) can be
replaced periodically, for
example every week or every month, to extend the length of therapy.
[00406] In some examples, the bladder catheter is replaced more often that the
ureteral
stent or ureteral catheter. In some examples, multiple bladder catheters are
placed and
removed sequentially during the indwell time for a single ureteral stent or
ureteral catheter.
For example, a physician, nurse, caregiver or patient can place the bladder
catheter(s) in the
patient at home or in any healthcare setting. Multiple bladder catheters can
be provided to
the healthcare professional, patient or caregiver in a kit, optionally with
instructions for
placement, replacement and optional connection of the bladder catheter(s) to
the negative
pressure source or drainage to a container, as needed. In some examples,
negative pressure is
applied each evening for a predetermined number of evenings (such as for 1 to
30 evenings or
more). Optionally, the bladder catheter can be replaced each evening before
application of
negative pressure.
[00407] In some examples, the urine is permitted to drain by gravity or
peristalsis from the
urethra. In other examples, a negative pressure is induced in the bladder
catheter to facilitate
drainage of the urine. While not intending to be bound by any theory, it is
believed that a
portion of the negative pressure applied to the proximal end of the bladder
catheter is
transmitted to the ureter(s), renal pelvis or other portions of the kidney(s)
to facilitate
drainage of the fluid or urine from the kidney.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
93
[00408] With reference to FIG. 42B, steps for using the system for inducement
of negative
pressure in the ureter(s) and/or kidney(s) are illustrated. As shown at box
624, after the
indwelling portions of the ureteral stents or ureteral catheters and bladder
catheters are
correctly positioned and any anchoring/retention structures, if present, are
deployed, the
external proximal end of the bladder catheter is connected to a fluid
collection or pump
assembly. For example, the bladder catheter can be connected to a pump for
inducing
negative pressure at the patient's bladder, renal pelvis and/or kidney.
[00409] Once the bladder catheter and pump assembly are connected, negative
pressure is
applied to the renal pelvis and/or kidney and/or bladder through the drainage
lumen of the
bladder catheter, as shown at box 626. The negative pressure is intended to
counter
congestion mediated interstitial hydrostatic pressures due to elevated intra-
abdominal
pressure and consequential or elevated renal venous pressure or renal
lymphatic pressure.
The applied negative pressure is therefore capable of increasing flow of
filtrate through the
medullary tubules and of decreasing water and sodium re-absorption.
[00410] As a result of the applied negative pressure, as shown at box 628,
urine is drawn
into the bladder catheter at the drainage port(s) at the distal end thereof,
through the drainage
lumen of the bladder catheter, and to a fluid collection container for
disposal. As the urine is
being drawn to the collection container, at box 630, optional sensors disposed
in the fluid
collection system can provide a number of measurements about the urine that
can be used to
assess physical parameters, such as the volume of urine collected, as well as
information
about the physical condition of the patient and composition of the urine
produced. In some
examples, the information obtained by the sensors is processed, as shown at
box 632, by a
processor associated with the pump and/or with another patient monitoring
device and, at box
634, is displayed to the user via a visual display of an associated feedback
device.
Exemplary fluid collection system:
[00411] Having described an exemplary system and method of positioning such a
system
in the patient's body, with reference to FIG. 44, a system 700 for inducing
negative pressure
to a patient's bladder, ureter(s), renal pelvis and/or kidney(s) will now be
described. The
system 700 can comprise the ureteral stent(s) and/or ureteral catheter(s),
bladder catheter or
the system 100 described hereinabove. As shown in FIG. 44, the bladder
catheter 116 of the
system 100 is connected to one or more fluid collection containers 712 for
collecting urine
drawn from the bladder. The fluid collection container 712 connected to the
bladder catheter
116 can be in fluid communication with an external fluid pump 710 for
generating negative
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
94
pressure in the bladder, ureter(s) and/or kidney(s) through the bladder
catheter 116 and/or
ureteral catheter(s) 112, 114. As discussed herein, such negative pressure can
be provided for
overcoming interstitial pressure and forming urine in the kidney or nephron.
In some
examples, a connection between the fluid collection container 712 and pump 710
can
comprise a fluid lock or fluid barrier to prevent air from entering the
bladder, renal pelvis or
kidney in case of incidental therapeutic or non-therapeutic pressure changes.
For example,
inflow and outflow ports of the fluid container can be positioned below a
fluid level in the
container. Accordingly, air is prevented from entering medical tubing or the
catheter through
either the inflow or outflow ports of the fluid container 712. As discussed
previously,
external portions of the tubing extending between the fluid collection
container 712 and the
pump 710 can include one or more filters to prevent urine and/or particulates
from entering
the pump 710.
[00412] As shown in FIG. 44, the system 700 further comprises a controller
714, such as a
microprocessor, electronically coupled to the pump 710 and having or
associated with
computer readable memory 716. In some examples, the memory 716 comprises
instructions
that, when executed, cause the controller 714 to receive information from
sensors 174 located
on or associated with portions of the assembly 100. Information about a
condition of the
patient can be determined based on information from the sensors 174.
Information from the
sensors 174 can also be used to determine and implement operating parameters
for the pump
710.
[00413] In some examples, the controller 714 is incorporated in a separate and
remote
electronic device in communication with the pump 710, such as a dedicated
electronic device,
computer, tablet PC, or smart phone. Alternatively, the controller 714 can be
included in the
pump 710 and, for example, can control both a user interface for manually
operating the
pump 710, as well as system functions such as receiving and processing
information from the
sensors 174.
[00414] The controller 714 is configured to receive information from the one
or more
sensors 174 and to store the information in the associated computer-readable
memory 716.
For example, the controller 714 can be configured to receive information from
the sensor 174
at a predetermined rate, such as once every second, and to determine a
conductance based on
the received information. In some examples, the algorithm for calculating
conductance can
also include other sensor measurements, such as urine temperature, to obtain a
more robust
determination of conductance.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
[00415] The controller 714 can also be configured to calculate patient
physical statistics or
diagnostic indicators that illustrate changes in the patient's condition over
time. For example,
the system 700 can be configured to identify an amount of total sodium
excreted. The total
sodium excreted may be based, for example, on a combination of flow rate and
conductance
over a period of time.
[00416] With continued reference to FIG. 44, the system 700 can further
comprise a
feedback device 720, such as a visual display or audio system, for providing
information to
the user. In some examples, the feedback device 720 can be integrally formed
with the pump
710. Alternatively, the feedback device 720 can be a separate dedicated or a
multipurpose
electronic device, such as a computer, laptop computer, tablet PC, smart
phone, or other
handheld electronic devices. The feedback device 720 is configured to receive
the calculated
or determined measurements from the controller 714 and to present the received
information
to a user via the feedback device 720. For example, the feedback device 720
may be
configured to display current negative pressure (in mmHg) being applied to the
urinary tract.
In other examples, the feedback device 720 is configured to display current
flow rate of urine,
temperature, current conductance in mS/m of urine, total urine produced during
the session,
total sodium excreted during the session, other physical parameters, or any
combination
thereof.
[00417] In some examples, the feedback device 720 further comprises a user
interface
module or component that allows the user to control operation of the pump 710.
For
example, the user can engage or turn off the pump 710 via the user interface.
The user can
also adjust pressure applied by the pump 710 to achieve a greater magnitude or
rate of
sodium excretion and fluid removal.
[00418] Optionally, the feedback device 720 and/or pump 710 further comprise a
data
transmitter 722 for sending information from the device 720 and/or pump 710 to
other
electronic devices or computer networks. The data transmitter 722 can utilize
a short-range
or long-range data communications protocol. An example of a short-range data
transmission
protocol is Bluetooth . Long-range data transmission networks include, for
example, Wi-Fi
or cellular networks. The data transmitter 722 can send information to a
patient's physician
or caregiver to inform the physician or caregiver about the patient's current
condition.
Alternatively, or in addition, information can be sent from the data
transmitter 722 to existing
databases or information storage locations, such as, for example, to include
the recorded
information in a patient's electronic health record (EHR).
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
96
[00419] With continued reference to FIG. 44, in addition to the urine sensors
174, in some
examples, the system 700 can further comprise one or more patient monitoring
sensors 724.
Patient monitoring sensors 724 can include invasive and non-invasive sensors
for measuring
information about the patient's physical parameters, such as urine
composition, as discussed
in detail above, blood composition (e.g., hematocrit ratio, analyte
concentration, protein
concentration, creatinine concentration) and/or blood flow (e.g., blood
pressure, blood flow
velocity). Hematocrit is a ratio of the volume of red blood cells to the total
volume of blood.
Normal hematocrit is about 25% to 40%, and preferably about 35% and 40% (e.g.,
35% to
40% red blood cells by volume and 60% to 65% plasma).
[00420] Non-invasive patient monitoring sensors 724 can include pulse oximetry
sensors,
blood pressure sensors, heart rate sensors, and respiration sensors (e.g., a
capnography
sensor). Invasive patient monitoring sensors 724 can include invasive blood
pressure sensors,
glucose sensors, blood velocity sensors, hemoglobin sensors, hematocrit
sensors, protein
sensors, creatinine sensors, and others. In still other examples, sensors may
be associated
with an extracorporeal blood system or circuit and configured to measure
parameters of blood
passing through tubing of the extracorporeal system. For example, analyte
sensors, such as
capacitance sensors or optical spectroscopy sensors, may be associated with
tubing of the
extracorporeal blood system to measure parameter values of the patient's blood
as it passes
through the tubing. The patient monitoring sensors 724 can be in wired or
wireless
communication with the pump 710 and/or controller 714.
[00421] In some examples, the controller 714 is configured to cause the pump
710 to
provide treatment for a patient based information obtained from the urine
analyte sensor 174
and/or patient monitoring sensors 724, such as blood monitoring sensors. For
example, pump
710 operating parameters can be adjusted based on changes in the patient's
blood hematocrit
ratio, blood protein concertation, creatinine concentration, urine output
volume, urine protein
concentration (e.g., albumin), and other parameters. For example, the
controller 714 can be
configured to receive information about a blood hematocrit ratio or creatinine
concentration
of the patient from the patient monitoring sensors 724 and/or analyte sensors
174. The
controller 714 can be configured to adjust operating parameters of the pump
710 based on the
blood and/or urine measurements. In other examples, hematocrit ratio may be
measured from
blood samples periodically obtained from the patient. Results of the tests can
be manually or
automatically provided to the controller 714 for processing and analysis.
[00422] As discussed herein, measured hematocrit values for the patient can be
compared
to predetermined threshold or clinically acceptable values for the general
population.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
97
Generally, hematocrit levels for females are lower than for males. In other
examples,
measured hematocrit values can be compared to patient baseline values obtained
prior to a
surgical procedure. When the measured hematocrit value is increased to within
the
acceptable range, the pump 710 may be turned off ceasing application of
negative pressure to
the ureter or kidneys. In a similar manner, the intensity of negative pressure
can be adjusted
based on measured parameter values. For example, as the patient's measured
parameters
begin to approach the acceptable range, intensity of negative pressure being
applied to the
ureter and kidneys can be reduced. In contrast, if an undesirable trend (e.g.,
a decrease in
hematocrit value, urine output rate, and/or creatinine clearance) is
identified, the intensity of
negative pressure can be increased in order to produce a positive
physiological result. For
example, the pump 710 may be configured to begin by providing a low level of
negative
pressure (e.g., between about 0.1 mmHg and 10 mmHg). The negative pressure may
be
incrementally increased until a positive trend in patient creatinine level is
observed.
However, generally, negative pressure provided by the pump 710 will not exceed
about 50
mmHg.
[00423] With reference to FIGS. 45A and 45B, an exemplary pump 710 for use
with the
system is illustrated. In some examples, the pump 710 is a micro-pump
configured to draw
fluid from the catheter(s) 112, 114 (shown, for example, in FIGS. 1A, 1B, 1C,
1F, 1P, 1U,
2A, 2B) and having a sensitivity or accuracy of about 10 mm Hg or less.
Desirably, the
pump 710 is capable of providing a range of flow of urine between 0.05 ml/min
and 3 ml/min
for extended periods of time, for example, for about 8 hours to about 24 hours
per day, for
one (1) to about 30 days or longer. At 0.2 ml/min, it is anticipated that
about 300 mL of urine
per day is collected by the system 700. The pump 710 can be configured to
provide a
negative pressure to the bladder of the patient, the negative pressure ranging
from about 0.1
mmHg and about 150 mmHg, or about 0.1 mmHg to about 50 mmHg, or about 5 mmHg
to
about 20 mmHg (gauge pressure at the pump 710). For example, a micro-pump
manufactured by Langer Inc. (Model BT100-2J) can be used with the presently
disclosed
system 700. Diaphragm aspirator pumps, as well as other types of commercially
available
pumps, can also be used for this purpose. Peristaltic pumps can also be used
with the system
700. In other examples, a piston pump, vacuum bottle, or manual vacuum source
can be used
for providing negative pressure. In other examples, the system can be
connected to a wall
suction source, as is available in a hospital, through a vacuum regulator for
reducing negative
pressure to therapeutically appropriate levels.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
98
[00424] In some examples, at least a portion of the pump assembly can be
positioned
within the patient's urinary tract, for example within the bladder. For
example, the pump
assembly can comprise a pump module and a control module coupled to the pump
module,
the control module being configured to direct motion of the pump module. At
least one (one
or more) of the pump module, the control module, or the power supply may be
positioned
within the patient's urinary tract. The pump module can comprise at least one
pump element
positioned within the fluid flow channel to draw fluid through the channel.
Some examples
of suitable pump assemblies, systems and methods of use are disclosed in U.S.
Patent
Application No. 62/550,259, entitled "Indwelling Pump for Facilitating Removal
of Urine
from the Urinary Tract", filed on August 25, 2017, which is incorporated by
reference herein
in its entirety.
[00425] In some examples, the pump 710 is configured for extended use and,
thus, is
capable of maintaining precise suction for extended periods of time, for
example, for about 8
hours to about 24 hours per day, or for 1 to about 30 days or longer, except
for replacement
time of bladder catheters. Further, in some examples, the pump 710 is
configured to be
manually operated and, in that case, includes a control panel 718 that allows
a user to set a
desired suction value. The pump 710 can also include a controller or
processor, which can be
the same controller that operates the system 700 or can be a separate
processor dedicated for
operation of the pump 710. In either case, the processor is configured for
both receiving
instructions for manual operation of the pump and for automatically operating
the pump 710
according to predetermined operating parameters. Alternatively, or in
addition, operation of
the pump 710 can be controlled by the processor based on feedback received
from the
plurality of sensors associated with the catheter.
[00426] In some examples, the processor is configured to cause the pump 710 to
operate
intermittently. For example, the pump 710 may be configured to emit pulses of
negative
pressure followed by periods in which no negative pressure is provided. In
other examples,
the pump 710 can be configured to alternate between providing negative
pressure and
positive pressure to produce an alternating flush and pump effect. For
example, a positive
pressure of about 0.1 mmHg to 20 mmHg, and preferably about 5 mmHg to 20 mmHg
can be
provided followed by a negative pressure ranging from about 0.1 mmHg to 50
mmHg.
Percutaneous Urinary Catheters and Systems
[00427] Urine can also be removed from the urinary tract through percutaneous
nephrostomy tubes or urinary bypass catheters deployed via percutaneous
insertion in the
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
99
renal pelvis and/or kidney of a patient. In some examples of the invention,
such urinary
bypass catheters can be adapted to provide negative and/or positive pressure
therapy to a
renal pelvis and/or kidney of a patient to facilitate urine production and to
drain and/or
conduct urine from the renal pelvis and/or kidney to an external fluid
collection container.
Urinary bypass catheters may be used, for example, to remove fluid (e.g.,
urine) from the
body when portions of the urinary tract (e.g., the ureters or urethra) are
blocked. Generally,
such urinary bypass catheters are inserted into an abdomen of a patient at a
percutaneous
access site. Such catheters extend through the kidney and, optionally, into
the renal pelvis. A
common percutaneous access site for accessing the abdominal cavity and kidneys
lies
between about 0.5 cm and about 1.5 cm or approximately 1 cm below a tip of the
twelfth rib
and between about 0.5 cm and about 1.5 cm or approximately 1 cm medially
therefrom. It is
believed that such an access site provides easy access to a lower pole of the
kidney and
avoids injuring other abdominal organs (e.g., the colon, liver, and/or
spleen). A physician
inserting a urinary bypass catheter may also select other access sites on a
patient's torso
based on a patient's size and/or on other therapeutic concerns.
Exemplary urinary catheters
[00428] Deployable retention structures or portions of the bypass catheter are
configured to
maintain a distal portion and/or distal end of the catheter within the kidney,
renal pelvis,
and/or bladder. For example, any of the coils, funnels, expandable cages,
balloons, and/or
sponges described herein can be used us retention portions for maintaining an
end of a
catheter at a desired position within the urinary tract (e.g., within the
renal pelvis, ureters,
and/or kidneys).
[00429] Referring now to FIGS. 52A-54, an exemplary percutaneous nephrostomy
tube or
urinary bypass catheter 7010 will be discussed. It is understood, however,
that any of the
catheters discussed herein can be used in a similar manner as described below.
The
exemplary urinary bypass catheter 7010 is configured to be deployed in a
urinary tract 7100
(shown in FIGS. 54, 55, and 57A-57E) of a patient. The catheter 7010 comprises
an
elongated tube 7018 extending from a proximal end 7020 to a distal end 7022.
The elongated
tube 7018 comprises a proximal portion 7012 configured to pass into an abdomen
of the
patient through a percutaneous opening or access site 7110 (shown in FIG. 54)
and a distal
portion 7014 comprising a retention portion 7016 configured to be deployed in
a renal pelvis
7112, kidney 7102 (shown in FIG. 54), and/or bladder, of the patient. The
percutaneous
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
100
access site 7110 can be formed in a conventional manner, such as by inserting
a tip of a
needle through the skin into the abdomen.
[00430] The tube 7018 can be formed from and/or comprise one or more
biocompatible
polymer(s), such as polyurethane, polyvinyl chloride, polytetrafluoroethylene
(PTFE), latex,
silicone coated latex, silicone, polyglycolide or poly(glycolic acid) (PGA),
Polylactide
(PLA), Poly(lactide-co-glycolide), Polyhydroxyalkanoates, Polycaprolactone
and/or
Poly(propylene fumarate). Portions of the elongated tube 18 can also comprise
and/or be
impregnated with metal materials, such as copper, silver, gold, nickel-
titanium alloy, stainless
steel, and/or titanium,. The elongated tube 7018 should be of sufficient
length to extend from
the renal pelvis 7112, through the kidney and percutaneous access site, and to
the external
fluid collection container. The tube 7018 size can range from about 1 Fr to
about 9 Fr
(French catheter scale), or about 2 Fr to 8 Fr, or can be about 4 Fr. In some
examples, the
tube 18 can have an external diameter ranging from about 0.33 mm to about 3.0
mm, or about
0.66 mm to 2.33 mm, or about 1.0 mm to 2.0 mm, and an internal diameter
ranging from
about 0.165 mm to about 2.40 mm, or about 0.33 mm to 2.0 mm, or about .66 mm
to about
1.66 mm. In one example, the tube 7018 is 6 Fr and has an outer diameter of
2.0 0.1 mm.
A length of the tube 7018 can range from about 30 cm to about 120 cm depending
on the age
(e.g., pediatric or adult) and size of the patient.
[00431] The retention portion 7016 of the bypass catheter 7010 can be
integrally formed
with the distal portion 7014 of the catheter 7010 or can be a separate
structure mounted to the
distal end 7022 of the elongated tube 7018 by a conventional fastener or
adhesive. Many
exemplary retention portions 7016 suitable for retaining the distal end 7022
of the elongated
tube 7018 within the renal pelvis 7112 are provided in previous exemplary
embodiments of
ureteral catheters 7010. For example, retention portions 7016 comprising one
or more of
coils, funnels, cages, balloons, and/or sponges can be adapted for use with
the bypass catheter
7010. In some cases, such retention portions 7016 can be adapted for use with
urinary bypass
catheters 7010 by, for example, inverting the retention portion(s) 7016 to
account for the fact
that a urinary bypass catheter 7010 enters the renal pelvis 7112 through the
kidney 7102,
rather than through the ureters.
[00432] Regardless of the embodiment selected, the retention portion 7016
creates an outer
periphery or protected surface area to prevent urinary tract tissues from
constricting or
occluding a fluid column extending between nephrons of the kidney 7102 and a
lumen of the
elongated tube 7018. In some examples, such a retention portion 7016 could
comprise an
inwardly facing side or protected surface area 7024 comprising one or more
drainage
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
101
openings, perforations, and/or ports 7026 for receiving fluid, such as urine,
produced by the
kidneys 7102 and an outwardly facing side or protective surface area 7028,
which can be free
from or substantially free from the drainage ports 7026. Desirably, the
inwardly facing side
or protected surface area 7024 and the outwardly facing side or protective
surface area 7028
are configured such that, when negative pressure is applied through the
elongated tube 7018,
the urine is drawn into a lumen of the tube 7018 through the one or more
drainage ports 7026,
while mucosal tissues, such as tissue of the ureters and/or renal pelvis 7112,
are prevented
from appreciably occluding the one or more drainage ports 7026. As in
previously described
ureteral catheters, sizes and spacing between the drainage ports 7026 may vary
to achieve
different distributions of negative pressure within the renal pelvis 7112
and/or kidney 7102,
as are disclosed herein. In some examples, each of the one or more drainage
ports 7026 has a
diameter of about 0.0005 mm to about 2.0 mm, or about 0.05 mm to 1.5 mm, or
about 0.5
mm to about 1.0 mm. In some examples, the drainage ports 7026 can be non-
circular, and
can have a surface area of about 0.0002 mm2 to about 100 mm2, or about 0.002
mm2 to about
mm2, or about 0.2 mm2 to about 1.0 mm2. The drainage ports 7026 can be spaced
equidistantly along an axial length of the retention portion 7016. In other
examples, drainage
ports 7026 nearer to the distal end 7022 of the retention portion 7016 may be
spaced more
closely together to increase fluid flow through more distal drainage ports
7026, compared to
examples where the ports 7026 are evenly spaced.
[00433] The proximal portion 7012 of the catheter 7010 generally extends from
the
kidney 7102 of the patient through the percutaneous access site 7110. The
proximal
portion 7012 of the catheter 7010 is free from or substantially free from
perforations,
openings, or drainage ports 7026 to avoid drawing fluids from the abdominal
cavity into the
elongated
tube 7018. Also, the proximal end 7020 of the proximal portion 7012 can be
configured to be
connected to a fluid collection container and/or pump, as shown in FIG. 55.
Exemplary retention portion
[00434] As discussed above, the retention portion 7016 can be any structure
suitable for
maintaining the distal end 7022 of the elongated tube 7018 in the desired
location within the
urinary tract 7100. For example, a sufficiently sized retention portion 7016
can have an axial
length Li ranging from about 5 mm to about 100 mm, or from 20 mm to 80 mm, or
about
50 mm.
[00435] In some examples, the retention portion 7016 comprises an expandable
structure
that transitions from a retracted state, when inserting or removing the
catheter 7010 from the
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
102
patient, to an expanded or deployed state configured to anchor and retain the
retention portion
7016 in the renal pelvis 7112 and/or kidney 7102. In order to sufficiently
retain the catheter
7010 in the desired location within the urinary tract 7100, in some examples,
the retention
portion 7016, when deployed, defines a three-dimensional shape 7032 (shown in
FIG. 53)
sized and positioned to maintain patency of the fluid column flowing between
the kidney
7102 and the proximal end 7020 of the catheter 7010. Further, desirably, at
least a portion of
the fluid produced by the kidneys 7102 flows through the retention portion
7016 and tube
7018, rather than through the ureters. An area of two-dimensional slices 7034
(shown in FIG.
53) of the three-dimensional shape 7032 defined by the deployed expandable
retention
portion 7016 in a plane transverse to a central axis A of the expandable
retention portion
7016 can decrease towards the distal end 7022 of the expandable retention
portion 7016,
giving the retention portion 7016 a pyramid or reversed conical shape. In some
examples, a
maximum cross-sectional area of the three-dimensional shape 7032 defined by
the deployed
expandable retention portion 7016 in a plane transverse to the central axis A
of the
expandable retention portion 7016 is less than or equal to about 500 mm2, or
less than or
equal to about 350 mm2, or from 100 mm2 to 500 mm2, or from 200 mm2 to 350
mm2.
[00436] In some examples, the retention portion 7016 comprises a coiled
retention portion
comprising an inverted helical coil. The coiled retention portion 7016 is
similar to the
retention portions of the ureteral catheters described in connection with
FIGS. 8A-9E, except
that the orientation of the coils is inverted since the retention portion 7016
is inserted into the
renal pelvis through the kidney. The coiled retention portion 7016 can
comprise a plurality of
helical coils 7036, 7038, 7040 arranged such that an outer periphery or outer
region of the
helical
coils 7036, 7038, 7040 contacts and supports tissues of the kidney 7102 and/or
renal pelvis
7112 to inhibit occlusion or blockage of protected drainage holes, ports 7026
or perforations
positioned in inwardly facing sides or protected surface areas of the helical
coils 7036, 7038,
7040.
[00437] The coiled retention portion 7016 can comprise at least the first coil
7036 having a
first diameter D1 (see FIG. 52B), at least a second coil 7038 having a second
diameter D2,
and at least a third coil 7040 having a third diameter D3. In order for the
retention portion
7016 to fit within the renal pelvis 7112, the diameter D3 of the distal-most
or third coil 7040
can be smaller than a diameter of either the first coil 7036 or the second
coil 7038.
Accordingly, a diameter of the coils 7036, 7038, 7040, and/or a step distance
or height
between adjacent coils 7036, 7038, 7040 can vary in a regular or irregular
manner. In some
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
103
examples, the plurality of coils 7036, 7038, 7040 can form a tapered or
reverse pyramid
shape in which D1>D2>D3. In some examples, the coiled retention portion 7016
can
comprise a plurality of similarly sized coils or, for example, can include a
plurality of
proximal similarly sized coils and a distal-most coil having a smaller
diameter than other
coils of the plurality of coils.
[00438] The diameter of the coils 7036, 7038, 7040 and step distance or height
between
adjacent coils is selected so that the retention portion 7016 remains in the
renal pelvis and/or
kidney for a desired period of time. In particular, the coiled retention
portion 7016 is
desirably large enough so that it remains in the renal pelvis 7112 and does
not pass either into
the ureters or back into the kidney 7102 until the catheter 7010 is ready to
be removed. For
example, the outer diameter D1 of the proximal most or first coil 7036 can
range from about
mm to about 30 mm, or about 15 mm to 25 mm, or be about 20 mm. The second coil
38
can have a diameter of about 5 mm to 25 mm, or about 10 mm to 20 mm, or can be
about 15
mm. The distal-most or third coil 40 can have a diameter D3 ranging from about
1 mm to 20
mm, or about 5 mm to 15 mm, or can be about 10 mm.
Additional exemplary retention portions
[00439] Another example of a ureteral catheter 7410 configured for
percutaneous insertion
into the renal pelvis of a patient is shown in FIGS. 58A and 58B. As in
previous examples,
the ureteral catheter 7410 is formed from an elongated tube 7418, and
comprises a proximal
portion 7412 and a distal portion 7414 comprising a retention portion 7416.
The retention
portion 7416 is a coiled retention portion comprising a plurality of coils
wrapped around a
substantially linear or straight segment or portion 7430 of the elongated tube
7418.
[00440] The coiled retention portion 416 further comprises a distal-most coil
7432 formed
from a bend 7434 of from about 90 degrees to 180 degrees at a distal end of
the straight
segment or portion 7430 of the retention portion 7416. The retention portion
7416 further
comprises one or more additional coils, such as a second or middle coil 7436
and a third or
proximal most coil 7438, which are wrapped around the straight portion 7430 of
the tube
7418. The elongated tube 7418 further comprises a distal end 7440 following
the proximal
most coil 7438. The distal end 7440 can be closed or can be open to receive
urine from the
patient's urinary tract.
[00441] As in previous examples, the size and orientation of the coils 7432,
7436, 7438 is
selected so that the retention portion 7416 remains in the renal pelvis and
does not pass into
the ureter or retract back into the kidney. For example, the largest or
proximal most coil
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
104
7438 can be about 10 mm to 30 mm in diameter, or about 15 mm to 25 mm in
diameter, or
about 20 mm in diameter. Coils 7436 and 7438 can have a smaller diameter of,
for example,
mm to 25 mm, or about 10 mm to 20 mm, or about 15 mm. As in previous examples,
the
coiled retention portion 7416 can have a tapered appearance in which the coils
7432, 7436,
7438 become progressively narrower, giving the retention portion 7416 a
reverse pyramid or
reverse conical appearance.
[00442] Also as in previous examples, the retention portion 7416 further
comprises
openings or drainage ports 7442 positioned on a radially inward side or
protected surface area
of the coiled retention portion 7416. Since the coils 7432, 7436, 7438 extend
around the
straight portion 7430 and prevent tissue of the renal pelvis and/or kidneys
from contacting the
straight portion 7430, openings or drainage ports 7442 (shown in FIG. 54B) can
also be
positioned on the straight portion 7430 of the retention portion 7416. As in
previous
examples, the retention portion 7416 is inserted through the kidney and renal
pelvis in a
linear orientation over a guidewire. When the guidewire is removed, the
retention portion
7416 adopts the coiled or deployed configuration.
Urine collection system with percutaneous catheters
[00443] The urinary bypass catheter(s) 7010, 7410 can be used with systems for
inducing
negative pressure in a portion of a urinary tract 7100 of a patient. As shown
in FIG. 55, an
exemplary system 7200 comprises urinary bypass catheters 7010 deployed in the
respective
renal pelvis 7112 of each respective kidney 7102 of the patient. Proximal ends
7020 of the
catheters 7010 are connected directly or indirectly to a pump 7210. For
example, proximal
ends 7020 of the catheters 7010 can be connected to fluid inflow ports of a
rigid fluid
collection container 7212. The pump 7210 can be connected to another port of
the fluid
collection container 7212 to induce negative pressure in the fluid collection
container 7212
and catheters 7010 connected thereto. The pump 7210 can be similar to pumps in
previously
described examples and, particularly, can be configured to deliver gentle
negative pressure to
the urinary tract 7100 of the patient. The pump 7210 can be an external pump.
In other
examples, the pump 7210 can be an indwelling pump as described, for example,
in PCT
Appl. No. PCT/IB2018/056444 to On et al., entitled "Indwelling Pump for
Facilitating
Removal of Urine from the Urinary Tract." Generally, the applied negative
pressure is a
gentle negative pressure, such as a negative pressure of less than 50 mmHg. In
other
examples, negative pressure can be from 2 mmHg to 100 mmHg, or more, depending
on
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
105
therapeutic requirements of particular patients. The pump 7210 desirably has a
sensitivity of
mmHg or less.
[00444] In some examples, the system 7200 further comprises a bladder catheter
7216
deployed within the patient's bladder 7104. The bladder catheter 7216 can be
any suitable
bladder catheter, as described in previous examples. The bladder catheter 7216
comprises an
elongated tube 7218 comprising a proximal portion 7220, which extends through
the
urethra 7106 and from the patient. A proximal end 7222 of the proximal portion
7220 of the
bladder catheter 7216 can be connected to the fluid collection container 7212.
In other
examples, the bladder catheter 7216 can be connected to a separate fluid
collection
container 7224, which is not connected to a pump 7210 for inducing negative
pressure. In
that case, fluid may pass from the patient's bladder 7104 through the bladder
catheter 7216
by gravity.
[00445] In some examples, the system 7200 further comprises a controller 7214
electrically connected to the pump 7210 configured to actuate the pump 7210
and to control
pump operating parameters. As in previous examples, the controller 7214 can be
a
microprocessor of the pump 7210 or a separate electronic device configured to
provide
operating instructions and/or operating parameters for the pump 7210. For
example, the
controller 7214 can be associated with a computer, laptop, tablet, smartphone,
or similar
electronic device.
[00446] The system can further comprise one or more physiological sensors 7226
associated with the patient, fluid collection container 7212, or catheter(s)
710, 7216. The
physiological sensors 7226 can be configured to provide information
representative of at least
one physical parameter of the patient to the controller 7214. In that case,
the controller 7214
can be configured to actuate or stop operation of the pump based on the at
least one physical
parameter.
Deployment methods
[00447] Having described aspects of a urinary bypass catheter 7010 and system
7200 for
applying negative pressure to a patient, a method for insertion and/or
deployment of the
urinary bypass catheter will now be described in connection with the flow
chart of FIG. 56.
Schematic drawings showing different aspects of the catheter deployment method
are shown
in FIGS. 57A-57E. Initially, at box 7510, a needle 7312 (shown in FIGS. 57A-
57C) of a
conical tip catheter 7310 (shown in FIGS. 57A-57E) is inserted into an
abdominal region of
the patient, thereby creating a percutaneous access site. The catheter 7310
and needle 7312
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
106
should be of sufficient size to permit the urinary bypass catheter to pass
through the catheter
7310. For example, the catheter 7310 can be about 3 Fr to about 10 Fr (French
catheter
scale), or from about 5 Fr to about 8 Fr, or about 6 Fr. In some examples, the
catheter 7310
can have an external diameter ranging from about 0.5 mm to about 4 mm, and an
internal
diameter ranging from about 0.2 mm to about 3.5 mm. The needle 7312 can be
from about
gauge to 30 gauge, or about 20 gauge to 25 gauge, and can have an outer
diameter of from
0.3 mm to 3.5 mm, or about 0. 5 mm to 1.0 mm. The needle 7312 can be any
suitable length,
such as from 10 mm to 50 mm, or about 30 mm.
[00448] Once the needle 7312 is inserted through the patient's skin, at 7512,
the needle
7312 is advanced through the abdominal cavity and inserted into the kidney
7102. At 7514,
the needle 7312 is advanced through the kidney 7102 and into the renal pelvis
7112, as
shown in FIG. 57B. Once the needle 7312 is advanced to the renal pelvis 7112,
at box 7516,
a guidewire 7314 can be advanced to the renal pelvis 7112 through the needle
7312, as shown
in FIG. 57C. Once the guidewire 7314 is in position, the needle 7312 can be
retracted back
through the catheter 7310. Next, at 7518, an elongated tube 7318 of the
catheter 7310 can be
inserted into the patient's abdominal cavity through the percutaneous access
site and
advanced to the renal pelvis over a guidewire 7314 and/or needle 7312, as
shown in FIG.
57D. At 7520, once a distal end 7320 and retention portion 7322 of the
elongated tube 7318
reach the renal pelvis, the retention portion 7322 can transition from its
retracted state to the
expanded or deployed state, as shown in FIG. 57E. As described herein,
desirably, when
deployed in the renal pelvis 7112, the retention portion 7322 maintains
patency of fluid flow
from the kidney 7102 into a lumen extending through at least a portion of the
elongated tube
7318.
[00449] In some examples, deploying the retention portion 7322 can comprise
retracting
an outer tube or sheath in a proximal direction away from the retention
portion 7322. Once
the outer tube or sheath is removed, the retention portion 7322 automatically
expands and
returns to an unconstrained shape. In other examples, such as when the
retention portion
7322 comprises a coiled retention portion, retracting the guidewire 7314
causes the retention
portion 7322 to adopt the coiled or deployed configuration. Deploying the
retention portion
can also comprise, for example, blowing up a balloon or releasing a cage-like
structure to
protect the distal end of the elongated tube 7318.
[00450] In some examples, as shown in box 7522, negative pressure can be
applied to the
renal pelvis by attaching a proximal end of the elongated tube 7318 directly
or indirectly to a
fluid pump and actuating the pump to generate the negative pressure. For
example, negative
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
107
pressure can be applied continuously for a predetermined period of time. In
other examples,
negative pressure can be applied as pressure pulses provided for short
duration at
predetermined intervals. In some examples, the pump can alternate between
providing
negative pressure and positive pressure. It is believed that such alternating
pressure therapy
may further stimulate the kidneys resulting in increased urine production. In
other examples,
as described in greater detail above, negative pressure may be delivered to
the renal pelvis
through the elongated tube 7318 due to a pressure distribution or pressure
gradient induced in
the tube 7318, but without use of a pump or negative pressure source. For
example, negative
pressure sufficient to draw fluid, such as urine, into the tube 7318 may be
created in distal
portions of the tube 7318 in response to fluid flow through the tube 7318 by
gravity. While
not intending to be bound by theory, it is believed that the generated suction
force is
dependent on a vertical distance between the retention portion of the catheter
and the
proximal end of the catheter. Accordingly, the created negative pressure can
be increased by
increasing the vertical distance between the retention portion of the deployed
catheter and the
fluid collection container and/or proximal end of the catheter.
Exemplary treatment methods for removing excess fluid
[00451] Steps for removing excess fluid from a patient using the devices and
systems
described herein are illustrated in FIG. 49. As shown in FIG. 49, the
treatment method
comprises deploying a ureteral stent or a urinary tract catheter, such as a
ureteral catheter, in
the ureter and/or kidney of a patient such that flow of urine from the ureter
and/or kidney, as
shown at box 910. The catheter may be placed to avoid occluding the ureter
and/or kidney.
In some examples, a fluid collecting portion of the stent or catheter may be
positioned in the
renal pelvis of the patient's kidney. In some examples, a ureteral stent or
ureteral catheter
may be positioned in each of the patient's kidneys. In other examples, a urine
collection
catheter may be deployed in the bladder or ureter, as shown in box 911. In
some examples,
the ureteral catheter comprises one or more of any of the retention portions
described herein.
For example, the ureteral catheter can comprise a tube defining a drainage
lumen comprising
a helical retention portion and a plurality of drainage ports. In other
examples, the catheter
can include a funnel-shaped fluid collection and retention portion or a
pigtail coil.
Alternatively, a ureteral stent, having, for example, a pigtail coil, can be
deployed.
[00452] As shown at box 912, the method further comprises applying negative
pressure to
at least one of the bladder, the ureter and/or kidney through the bladder
catheter to induce or
facilitate production of fluid or urine in the kidney(s) and to extract the
fluid or urine from the
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
108
patient. Desirably, negative pressure is applied for a period of time
sufficient to reduce the
patient's blood creatinine levels by a clinically significant amount.
[00453] Negative pressure may continue to be applied for a predetermined
period of time.
For example, a user may be instructed to operate the pump for the duration of
a surgical
procedure or for a time period selected based on physiological characteristics
of the patient.
In other examples, patient condition may be monitored to determine when
sufficient
treatment has been provided. For example, as shown at box 914, the method may
further
comprise monitoring the patient to determine when to cease applying negative
pressure to the
patient's bladder, ureter and/or kidneys. In some examples, a patient's
hematocrit level is
measured. For example, patient monitoring devices may be used to periodically
obtain
hematocrit values. In other examples, blood samples may be drawn periodically
to directly
measure hematocrit. In some examples, concentration and/or volume of urine
expelled from
the body through the bladder catheter may be monitored to determine a rate at
which urine is
being produced by the kidneys. In a similar manner, expelled urine output may
be monitored
to determine protein concentration and/or creatinine clearance rate for the
patient. Reduced
creatinine and protein concentration in urine may be indicative of over-
dilution and/or
depressed renal function. Measured values can be compared to the predetermined
threshold
values to assess whether negative pressure therapy is improving patient
condition, and should
be modified or discontinued. For example, as discussed herein, a desirable
range for patient
hematocrit may be between 25% and 40%. In other examples, as described herein,
patient
body weight may be measured and compared to a dry body weight. Changes in
measured
patient body weight demonstrate that fluid is being removed from the body. As
such, a return
to dry body weight represents that hemodilution has been appropriately managed
and the
patient is not over-diluted.
[00454] As shown at box 916, a user may cause the pump to cease providing
negative
pressure therapy when a positive result is identified. In a similar manner,
patient blood
parameters may be monitored to assess effectiveness of the negative pressure
being applied to
the patient's kidneys. For example, a capacitance or analyte sensor may be
placed in fluid
communication with tubing of an extracorporeal blood management system. The
sensor may
be used to measure information representative of blood protein, oxygen,
creatinine, and/or
hematocrit levels. Measured blood parameter values may be measured
continuously or
periodically and compared to various threshold or clinically acceptable
values. Negative
pressure may continue to be applied to the patient's bladder, kidney or ureter
until a measured
parameter value falls within a clinically acceptable range. Once a measured
values fails
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
109
within the threshold or clinically acceptable range, as shown at box 916,
application of
negative pressure may cease.
[00455] In some examples, there is provided a method of removing excess fluid
from a
patient for systemic fluid volume management associated with chronic
edematous,
hypertension, chronic kidney disease and/or acute heart failure. According to
another aspect
of the disclosure, a method for removing excess fluid for a patient undergoing
a fluid
resuscitation procedure, such as coronary graft bypass surgery, by removing
excess fluid
from the patient is provided. During fluid resuscitation, solutions such as
saline solutions
and/or starch solutions, are introduced to the patient's bloodstream by a
suitable fluid
delivery process, such as an intravenous drip. For example, in some surgical
procedures, a
patient may be supplied with between 5 and 10 times a normal daily intake of
fluid. Fluid
replacement or fluid resuscitation can be provided to replace bodily fluids
lost through
sweating, bleeding, dehydration, and similar processes. In the case of a
surgical procedure
such as coronary graft bypass, fluid resuscitation is provided to help
maintain a patient's fluid
balance and blood pressure within an appropriate rate. Acute kidney injury
(AKI) is a known
complication of coronary artery graft bypass surgery. AKI is associated with a
prolonged
hospital stay and increased morbidity and mortality, even for patients who do
not progress to
renal failure. See Kim, et al., Relationship between a perioperative
intravenous fluid
administration strategy and acute kidney injury following off-pump coronary
artery bypass
surgery: an observational study, Critical Care 19:350 (1995). Introducing
fluid to blood also
reduces hematocrit levels which has been shown to further increase mortality
and morbidity.
Research has also demonstrated that introducing saline solution to a patient
may depress renal
functional and/or inhibit natural fluid management processes. As such,
appropriate
monitoring and control of renal function may produce improved outcomes and, in
particular,
may reduce post-operative instances of AM.
[00456] A method of treating a patient for removing excess fluid is
illustrated in FIG. 50.
As shown at box 1010, the method comprises deploying a ureteral stent or
ureteral catheter in
the ureter and/or kidney of a patient such that flow of urine from the ureter
and/or kidney is
not prevented by occlusion of the ureter and/or kidney. For example, a distal
end of the
ureteral stent or fluid collecting portion of the catheter may be positioned
in the renal pelvis.
In other examples, the catheter may be deployed in the kidney or ureter. The
catheter can
comprise one or more of the ureter catheters described herein. For example,
the catheter can
comprise a tube defining a drainage lumen and comprising a helical retention
portion and a
plurality of drainage ports. In other examples, the catheter can include a
pigtail coil.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
110
[00457] As shown at box 1012, a bladder catheter can be deployed in the
patient's bladder.
For example, the bladder catheter may be positioned to at least partially seal
the urethra
opening to prevent passage of urine from the body through the urethra. The
bladder catheter
can, for example, include an anchor for maintaining the distal end of the
catheter in the
bladder. As described herein, other arrangements of coils and helices, funnel,
etc. may be
used to obtain proper positioning of the bladder catheter. The bladder
catheter can be
configured to collect fluid which entered the patient's bladder prior to
placement of the
ureteral catheter(s), as well as fluid collected from the ureters, ureteral
stents, and/or ureteral
catheters during treatment. The bladder catheter may also collect urine which
flows past the
fluid collection portion(s) of the ureteral catheter and enters the bladder.
In some examples, a
proximal portion of the ureteral catheter may be positioned in a drainage
lumen of the bladder
catheter. In a similar manner, the bladder catheter may be advanced into the
bladder using
the same guidewire used for positioning of the ureteral catheter(s). In some
examples,
negative pressure may be provided to the bladder through the drainage lumen of
the bladder
catheter. In other examples, negative pressure may only be applied to the
bladder catheter(s).
In that case, the ureteral catheter drains into the bladder by gravity.
[00458] As shown at box 1014, following deployment of the ureteral stents
and/or ureteral
catheter(s) and the bladder catheter, negative pressure is applied to the
bladder, ureter and/or
kidney through the bladder catheter. For example, negative pressure can be
applied for a
period of time sufficient to extract urine comprising a portion of the fluid
provided to the
patient during the fluid resuscitation procedure. As described herein,
negative pressure can
be provided by an external pump connected to a proximal end or port of the
bladder catheter.
The pump can be operated continually or periodically dependent on therapeutic
requirements
of the patient. In some cases, the pump may alternate between applying
negative pressure
and positive pressure.
[00459] Negative pressure may continue to be applied for a predetermined
period of time.
For example, a user may be instructed to operate the pump for the duration of
a surgical
procedure or for a time period selected based on physiological characteristics
of the patient.
In other examples, patient condition may be monitored to determine when a
sufficient amount
of fluid has been drawn from the patient. For example, as shown at box 1016,
fluid expelled
from the body may be collected and a total volume of obtained fluid may be
monitored. In
that case, the pump can continue to operate until a predetermined fluid volume
has been
collected from the ureteral and/or bladder catheters. The predetermined fluid
volume may be
based, for example, on a volume of fluid provided to the patient prior to and
during the
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
111
surgical procedure. As shown at box 1018, application of negative pressure to
the bladder,
ureter and/or kidneys is stopped when the collected total volume of fluid
exceeds the
predetermined fluid volume.
[00460] In other examples, operation of the pump can be determined based on
measured
physiological parameters of the patient, such as measured creatinine
clearance, blood
creatinine level, or hematocrit ratio. For example, as shown at box 1020,
urine collected
form the patient may be analyzed by one or more sensors associated with the
catheter and/or
pump. The sensor can be a capacitance sensor, analyte sensor, optical sensor,
or similar
device configured to measure urine analyte concentration. In a similar manner,
as shown at
box 1022, a patient's blood creatinine or hematocrit level could be analyzed
based on
information obtain from the patient monitoring sensors discussed hereinabove.
For example,
a capacitance sensor may be placed in an existing extracorporeal blood system.
Information
obtained by the capacitance sensor may be analyzed to determine a patient's
hematocrit ratio.
The measured hematocrit ratio may be compared to certain expected or
therapeutically
acceptable values. The pump may continue to apply negative pressure to the
patient's ureter
and/or kidney until measured values within the therapeutically acceptable
range are obtained.
Once a therapeutically acceptable value is obtained, application of negative
pressure may be
stopped as shown at box 1018.
[00461] In other examples, as shown at box 2024, patient body weight may be
measured to
assess whether fluid is being removed from the patient by the applied negative
pressure
therapy. For example, a patient's measured bodyweight (including fluid
introduced during a
fluid resuscitation procedure) can be compared to a patient's dry body weight.
As used
herein, dry weights is defined as normal body weight measured when a patient
is not over-
diluted. For example, a patient who is not experiencing one or more of:
elevated blood
pressure, lightheadedness or cramping, swelling of legs, feet, arms, hands, or
around the eyes,
and who is breathing comfortably, likely does not have excess fluid. A weight
measured
when the patient is not experiencing such symptoms can be a dry body weight.
Patient
weight can be measured periodically until the measured weight approaches the
dry body
weight. When the measured weight approaches (e.g., is within between 5% and
10% of dry
body weight), as shown at box 1018, application of negative pressure can be
stopped.
[00462] The aforementioned details of treatment using the systems of the
present invention
can be used to treat a variety of conditions that can benefit from increased
urine or fluid
output or removal. For example, a method for preserving renal function by
application of
negative pressure to decrease interstitial pressure within tubules of the
medullar region to
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
112
facilitate urine output and to prevent venous congestion-induced nephron
hypoxia in the
medulla of the kidney is provided. The method comprises: deploying a ureteral
stent or
ureteral catheter into a ureter or kidney of a patient to maintain patency of
fluid flow
between a kidney and a bladder of the patient; deploying a bladder catheter
into the bladder
of the patient, wherein the bladder catheter comprises a distal end configured
to be positioned
in a patient's bladder, a drainage lumen portion having a proximal end, and a
sidewall
extending therebetween; and applying negative pressure to the proximal end of
the catheter to
induce negative pressure in a portion of the urinary tract of the patient for
a predetermined
period of time to remove fluid from the urinary tract of the patient.
[00463] In another example, a method for treatment of acute kidney injury due
to venous
congestion is provided. The method comprises: deploying a ureteral stent or
ureteral catheter
into a ureter or kidney of a patient to maintain patency of fluid flow between
a kidney and a
bladder of the patient; deploying a bladder catheter into the bladder of the
patient, wherein
the bladder catheter comprises a distal end configured to be positioned in a
patient's bladder,
a drainage lumen portion having a proximal end, and a sidewall extending
therebetween; and
applying negative pressure to the proximal end of the catheter to induce
negative pressure in
a portion of the urinary tract of the patient for a predetermined period of
time to remove fluid
from the urinary tract of the patient, thereby reducing venous congestion in
the kidney to treat
acute kidney injury.
[00464] In another example, a method for treatment of New York Heart
Association
(NYHA) Class III and/or Class IV heart failure through reduction of venous
congestion in the
kidney(s) is provided. The method comprises: deploying a ureteral stent or
ureteral catheter
into a ureter or kidney of a patient to maintain patency of fluid flow between
a kidney and a
bladder of the patient; deploying a bladder catheter into the bladder of the
patient, wherein
the bladder catheter comprises a distal end configured to be positioned in a
patient's bladder,
a drainage lumen portion having a proximal end, and a sidewall extending
therebetween; and
applying negative pressure to the proximal end of the catheter to induce
negative pressure in
a portion of the urinary tract of the patient for a predetermined period of
time to remove fluid
from the urinary tract of the patient to treat volume overload in NYHA Class
III and/or Class
IV heart failure.
[00465] In another example, a method for treatment of Stage 4 and/or Stage 5
chronic
kidney disease through reduction of venous congestion in the kidney(s) is
provided. The
method comprises: deploying a ureteral stent or ureteral catheter into a
ureter or kidney of a
patient to maintain patency of fluid flow between a kidney and a bladder of
the patient;
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
113
deploying a bladder catheter into the bladder of the patient, wherein the
bladder catheter
comprises a distal end configured to be positioned in a patient's bladder, a
drainage lumen
portion having a proximal end, and a sidewall extending therebetween; and
applying negative
pressure to the proximal end of the catheter to induce negative pressure in a
portion of the
urinary tract of the patient to remove fluid from the urinary tract of the
patient to reduce
venous congestion in the kidney(s).
[00466] In some examples, a kit is provided for removing fluid from the
urinary tract of a
patient and/or inducing negative pressure in a portion of a urinary tract of a
patient. The kit
comprises: a ureteral stent or ureteral catheter comprising a drainage channel
for facilitating
flow of fluid from the ureter and/or kidney through the drainage channel of
the ureteral stent
or ureteral catheter towards the bladder of the patient; and a pump comprising
a controller
configured to induce a negative pressure in at least one of the ureter, kidney
or bladder of the
patient to draw urine through a drainage lumen of a catheter deployed in the
patient's bladder.
In some examples, the kit further comprises at least one bladder catheter. In
some examples,
the kit further comprises instructions for one or more of the following:
inserting/deploying
the ureteral stent(s) and/or ureteral catheter(s), inserting/deploying the
bladder catheter, and
operating the pump to draw urine through a drainage lumen of the bladder
catheter deployed
the patient's bladder.
[00467] In some examples, another kit comprises: a plurality of disposable
bladder
catheters, each bladder catheter comprising a drainage lumen portion having a
proximal end,
a distal end configured to be positioned in a patient's bladder, and a
sidewall extending
therebetween; and a retention portion extending radially outward from a
portion of the distal
end of the drainage lumen portion, and being configured to be extended into a
deployed
position in which a diameter of the retention portion is greater than a
diameter of the drainage
lumen portion; instructions for inserting/deploying the bladder catheter; and
instructions for
connecting the proximal end of the bladder catheter to a pump and for
operating the pump to
draw urine through the drainage lumen of the bladder catheter, for example by
applying
negative pressure to the proximal end of the bladder catheter.
[00468] In some examples, a kit is provided, the kit comprising: a plurality
of disposable
bladder catheters, each bladder catheter comprising (a) a proximal portion;
and (b) a distal
portion, the distal portion comprising a retention portion that comprises one
or more
protected drainage holes, ports or perforations and is configured to establish
an outer
periphery or protective surface area that inhibits mucosal tissue from
occluding the one or
more protected drainage holes, ports or perforations upon application of
negative pressure
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
114
through the catheter; instructions for deploying the bladder catheter; and
instructions for
connecting the proximal end of the bladder catheter to a pump and for
operating the pump to
draw urine through the drainage lumen of the bladder catheter.
Experimental examples of inducing negative pressure using ureteral catheters:
[00469] Inducement of negative pressure within the renal pelvis of farm swine
was
performed for the purpose of evaluating effects of negative pressure therapy
on renal
congestion in the kidney. An objective of these studies was to demonstrate
whether a
negative pressure delivered into the renal pelvis significantly increases
urine output in a
swine model of renal congestion. In Example 1, a pediatric Fogarty catheter,
normally used
in embolectomy or bronchoscopy applications, was used in the swine model
solely for proof
of principle for inducement of negative pressure in the renal pelvis. It is
not suggested that a
Fogarty catheter be used in humans in clinical settings to avoid injury of
urinary tract tissues.
In Example 2, the ureteral catheter 112 shown in FIGS. 2A and 2B, and
including a helical
retention portion for mounting or maintaining a distal portion of the catheter
in the renal
pelvis or kidney, was used.
Example 1
Method
[00470] Four farm swine 800 were used for purposes of evaluating effects of
negative
pressure therapy on renal congestion in the kidney. As shown in FIG. 21,
pediatric Fogarty
catheters 812, 814 were inserted to the renal pelvis region 820, 821 of each
kidney 802, 804
of the four swine 800. The catheters 812, 814 were deployed within the renal
pelvis region
by inflating an expandable balloon to a size sufficient to seal the renal
pelvis and to maintain
the position of the balloon within the renal pelvis. The catheters 812, 814
extend from the
renal pelvis 802, 804, through a bladder 810 and urethra 816, and to fluid
collection
containers external to the swine.
[00471] Urine output of two animals was collected for a 15 minute period to
establish a
baseline for urine output volume and rate. Urine output of the right kidney
802 and the left
kidney 804 were measured individually and found to vary considerably.
Creatinine clearance
values were also determined.
[00472] Renal congestion (e.g., congestion or reduced blood flow in the veins
of the
kidney) was induced in the right kidney 802 and the left kidney 804 of the
animal 800 by
partially occluding the inferior vena cava (IVC) with an inflatable balloon
catheter 850 just
above to the renal vein outflow. Pressure sensors were used to measure IVC
pressure.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
115
Normal IVC pressures were 1-4 mmHg. By inflating the balloon of the catheter
850 to
approximately three quarters of the IVC diameter, the IVC pressures were
elevated to
between 15-25 mmHg. Inflation of the balloon to approximately three quarters
of IVC
diameter resulted in a 50-85% reduction in urine output. Full occlusion
generated IVC
pressures above 28 mmHg and was associated with at least a 95% reduction in
urine output.
[00473] One kidney of each animal 800 was not treated and served as a control
("the
control kidney 802"). The ureteral catheter 812 extending from the control
kidney was
connected to a fluid collection container 819 for determining fluid levels.
One kidney ("the
treated kidney 804") of each animal was treated with negative pressure from a
negative
pressure source (e.g., a therapy pump 818 in combination with a regulator
designed to more
accurately control the low magnitude of negative pressures) connected to the
ureteral catheter
814. The pump 818 was an Air Cadet Vacuum Pump from Cole-Parmer Instrument
Company (Model No. EW-07530-85). The pump 818 was connected in series to the
regulator. The regulator was an V-800 Series Miniature Precision Vacuum
Regulator - 1/8
NPT Ports (Model No. V-800-10-W/K), manufactured by Airtrol Components Inc.
[00474] The pump 818 was actuated to induce negative pressure within the renal
pelvis
820, 821 of the treated kidney according to the following protocol. First, the
effect of
negative pressure was investigated in the normal state (e.g., without
inflating the IVC
balloon). Four different pressure levels (-2, -10, -15, and -20 mmHg) were
applied for 15
minutes each and the rate of urine produced and creatinine clearance were
determined.
Pressure levels were controlled and determined at the regulator. Following the
-20 mmHg
therapy, the IVC balloon was inflated to increase the pressure by 15-20 mmHg.
The same
four negative pressure levels were applied. Urine output rate and creatinine
clearance rate for
the congested control kidney 802 and treated kidney 804 were obtained. The
animals 800
were subject to congestion by partial occlusion of the IVC for 90 minutes.
Treatment was
provided for 60 minutes of the 90 minute congestion period.
[00475] Following collection of urine output and creatinine clearance data,
kidneys from
one animal were subjected to gross examination then fixed in a 10% neutral
buffered
formalin. Following gross examination, histological sections were obtained,
examined, and
magnified images of the sections were captured. The sections were examined
using an
upright Olympus BX41 light microscope and images were captured using an
Olympus DP25
digital camera. Specifically, photomicrograph images of the sampled tissues
were obtained at
low magnification (20x original magnification) and high magnification (100x
original
magnification). The obtained images were subjected to histological evaluation.
The purpose
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
116
of the evaluation was to examine the tissue histologically and to
qualitatively characterize
congestion and tubular degeneration for the obtained samples.
[00476] Surface mapping analysis was also performed on obtained slides of the
kidney
tissue. Specifically, the samples were stained and analyzed to evaluate
differences in size of
tubules for treated and untreated kidneys. Image processing techniques
calculated a number
and/or relative percentage of pixels with different coloration in the stained
images.
Calculated measurement data was used to determine volumes of different
anatomical
structures.
Results
Urine output and creatinine clearance
[00477] Urine output rates were highly variable. Three sources of variation in
urine output
rate were observed during the study. The inter-individual and hemodynamic
variability were
anticipated sources of variability known in the art. A third source of
variation in urine output,
upon information and belief believed to be previously unknown, was identified
in the
experiments discussed herein, namely, contralateral intra-individual
variability in urine
output.
[00478] Baseline urine output rates were 0.79 ml/min for one kidney and 1.07
ml/min for
the other kidney (e.g., a 26% difference). The urine output rate is a mean
rate calculated
from urine output rates for each animal.
[00479] When congestion was provided by inflating the IVC balloon, the treated
kidney
urine output dropped from 0.79 ml/min to 0.12 ml/min (15.2% of baseline). In
comparison,
the control kidney urine output rate during congestion dropped from 1.07
ml/min to 0.09
ml/min (8.4% of baseline). Based on urine output rates, a relative increase in
treated kidney
urine output compared to control kidney urine output was calculated, according
to the
following equation:
(Therapy Treated/Baseline Treated) / (Therapy Control / Baseline
Control) = Relative increase
(0.12 ml/min / 0.79 ml/min) / (0.09 ml/min /1.07 ml/min)
= 180.6%
[00480] Thus, the relative increase in treated kidney urine output rate was
180.6%
compared to control. This result shows a greater magnitude of decrease in
urine production
caused by congestion on the control side when compared to the treatment side.
Presenting
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
117
results as a relative percentage difference in urine output adjusts for
differences in urine
output between kidneys.
[00481] Creatinine clearance measurements for baseline, congested, and treated
portions
for one of the animals are shown in FIG. 22.
Gross examination and histological evaluation
[00482] Based on gross examination of the control kidney (right kidney) and
treated
kidney (left kidney), it was determined that the control kidney had a
uniformly dark red-
brown color, which corresponds with more congestion in the control kidney
compared to the
treated kidney. Qualitative evaluation of the magnified section images also
noted increased
congestion in the control kidney compared to the treated kidney. Specifically,
as shown in
Table 1, the treated kidney exhibited lower levels of congestion and tubular
degeneration
compared to the control kidney. The following qualitative scale was used for
evaluation of
the obtained slides.
Con ges ti on
Sem
None: 0
Mild:
Moderate:
_nuked: 3
4
Tubular degeneration
Le$.;.-iort Scow
None.: 0
Mild:
More: 2
Marked: 3
Severe: 4
Table I
TABULATED RESULTS
Hisiolngk IeAnns
Slide
Animal ID/Orpti/Gross lesien Tata& r
nuattwr Congestion Granulomas
kyallue casts
6.34311.,thKRITteyNni=m:,.1 0
6.34.1.eft f.;Lidiley.,Natmai with 0
hinnotriLlgic
634-1Rn-Kikiaey/CongffsUou R1-6-513-3 1
6343/R411i Kidly'Congestion R1.6-51:5-a 2
[00483] As shown in Table 1, the treated kidney (left kidney) exhibited only
mild
congestion and tubular degeneration. In contrast, the control kidney (right
kidney) exhibited
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
118
moderate congestion and tubular degeneration. These results were obtained by
analysis of the
slides discussed below.
[00484] FIGS. 48A and 48B are low and high magnification photomicrographs of
the left
kidney (treated with negative pressure) of the animal. Based on the
histological review, mild
congestion in the blood vessels at the corticomedullary junction was
identified, as indicated
by the arrows. As shown in FIG. 48B, a single tubule with a hyaline cast (as
identified by the
asterisk) was identified.
[00485] FIGS. 48C and 48D are low and high resolution photomicrographs of the
control
kidney (right kidney). Based on the histological review, moderate congestion
in the blood
vessel at the corticomedullary junction was identified, as shown by the arrows
in FIG. 48C.
As shown in FIG. 48D, several tubules with hyaline casts were present in the
tissue sample
(as identified by asterisks in the image). Presence of a substantial number of
hyaline casts is
evidence of hypoxia.
[00486] Surface mapping analysis provided the following results. The treated
kidney was
determined to have 1.5 times greater fluid volume in Bowman's space and 2
times greater
fluid volume in tubule lumen. Increased fluid volume in Bowman's space and the
tubule
lumen corresponds to increased urine output. In addition, the treated kidney
was determined
to have 5 times less blood volume in capillaries compared to the control
kidney. The
increased volume in the treated kidney appears to be a result of (1) a
decrease in individual
capillary size compared to the control and (2) an increase in the number of
capillaries without
visible red blood cells in the treated kidney compared to the control kidney,
an indicator of
less congestion in the treated organ.
Summary
[00487] These results indicate that the control kidney had more congestion and
more
tubules with intraluminal hyaline casts, which represent protein-rich
intraluminal material,
compared to the treated kidney. Accordingly, the treated kidney exhibits a
lower degree of
loss of renal function. While not intending to be bound by theory, it is
believed that as severe
congestion develops in the kidney, hypoxemia of the organ follows. Hypoxemia
interferes
with oxidative phosphorylation within the organ (e.g., ATP production). Loss
of ATP and/or
a decrease in ATP production inhibits the active transport of proteins causing
intraluminal
protein content to increase, which manifests as hyaline casts. The number of
renal tubules
with intraluminal hyaline casts correlates with the degree of loss of renal
function.
Accordingly, the reduced number of tubules in the treated left kidney is
believed to be
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
119
physiologically significant. While not intending to be bound by theory, it is
believed that
these results show that damage to the kidney can be prevented or inhibited by
applying
negative pressure to a ureteral catheter inserted into the renal pelvis to
facilitate urine output.
Example 2
Method
[00488] Four (4) farm swine (A, B, C, D) were sedated and anesthetized. Vitals
for each
of the swine were monitored throughout the experiment and cardiac output was
measured at
the end of each 30-minute phase of the study. Ureteral catheters, such as the
ureteral catheter
112 shown in FIGS. 2A and 2B, were deployed in the renal pelvis region of the
kidneys of
each of the swine. The deployed catheters were a 6 Fr catheter having an outer
diameter of
2.0 0.1 mm. The catheters were 54 2 cm in length, not including the distal
retention
portion. The retention portion was 16 2 mm in length. As shown in the
catheter 112 in
FIGS. 2A and 2B, the retention portion included two full coils and one
proximal half coil.
The outer diameter of the full coils, shown by line D1 in FIGS. 2A and 2B, was
18 2 mm.
The half coil diameter D2 was about 14 mm. The retention portion of the
deployed ureteral
catheters included six drainage openings, plus an additional opening at the
distal end of the
catheter tube. The diameter of each of the drainage openings was 0.83 0.01
mm. The
distance between adjacent drainage openings 132, specifically the linear
distance between
drainage openings when the coils were straightened, was 22.5 2.5 mm.
[00489] The ureteral catheters were positioned to extend from the renal pelvis
of the swine,
through the bladder, and urethra, and to fluid collection containers external
to each swine.
Following placement of the ureteral catheters, pressure sensors for measuring
IVC pressure
were placed in the IVC at a position distal to the renal veins. An inflatable
balloon catheter,
specifically a PTS percutaneous balloon catheter (30 mm diameter by 5 cm
length),
manufactured by NuMED Inc. of Hopkinton, NY, was expanded in the IVC at a
position
proximal to the renal veins. A thermodilution catheter, specifically a Swan-
Ganz
thermodilution pulmonary artery catheter manufactured by Edwards Lifesciences
Corp. of
Irvine, CA, was then placed in the pulmonary artery for the purpose of
measuring cardiac
output.
[00490] Initially, baseline urine output was measured for 30 minutes, and
blood and urine
samples were collected for biochemical analysis. Following the 30-minute
baseline period,
the balloon catheter was inflated to increase IVC pressure from a baseline
pressure of 1-4
mmHg to an elevated congested pressure of about 20 mmHg (+/- 5 mmHg). A
congestion
baseline was then collected for 30 minutes with corresponding blood and urine
analysis.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
120
[00491] At the end of the congestion period, the elevated congested IVC
pressure was
maintained and negative pressure diuresis treatment was provided for swine A
and swine C.
Specifically, the swine (A, C) were treated by applying a negative pressure of
-25 mmHg
through the ureteral catheters with a pump. As in previously-discussed
examples, the pump
was an Air Cadet Vacuum Pump from Cole-Parmer Instrument Company (Model No. EW-
07530-85). The pump was connected in series to a regulator. The regulator was
a V-800
Series Miniature Precision Vacuum Regulator - 1/8 NPT Ports (Model No. V-800-
10-W/K),
manufactured by Airtrol Components Inc. The swine were observed for 120
minutes, as
treatment was provided. Blood and urine collection were performed every 30
minutes,
during the treatment period. Two of the swine (B, D) were treated as congested
controls
(e.g., negative pressure was not applied to the renal pelvis through the
ureteral catheters),
meaning that the two swine (B, D) did not receive negative pressure diuresis
therapy.
[00492] Following collection of urine output and creatinine clearance data for
the 120-
minute treatment period, the animals were sacrificed and kidneys from each
animal were
subjected to gross examination. Following gross examination, histological
sections were
obtained and examined, and magnified images of the sections were captured.
Results
[00493] Measurements collected during the Baseline, Congestion, and Treatment
periods
are provided in Table 2. Specifically, urine output, serum creatinine, and
urinary creatinine
measurements were obtained for each time period. These values allow for the
calculation of
a measured creatinine clearance as follows:
Creatinine Clearance:CrCl
Urinary Creatinine (mg / dl)
= Urine Output (ml/min) * ___________________________________
Serum Creatinine (mg /dl)
In addition, Neutrophil gelatinase-associated lipocalin (NGAL) values were
measured from
serum samples obtained for each time period and Kidney Injury Molecule 1 (KIM-
1) values
were measured from the urine samples obtained for each time period.
Qualitative histological
findings determined from review of the obtained histological sections are also
included in
Table 2.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
121
Table 2
Animal A
Treatment assignment Treatment Control Treatment
Control
Baseline:
Urine output (ml/min) 3.01 2.63 0.47 0.98
Serum creatinine (mg/di) 0.8 0.9 3.2 1.0
Creatinine clearance (ml/min) 261 172 5.4 46.8
Serum NGAL (ng/ml) 169 963 99
Urinary KIM-1 (ng/ml) 4.11 3.59 1.16
Congestion:
Urine output (ml/min) 0.06 (2%) 0.53 (20%) 0.12
(25%) 0.24 (25%)
Serum creatinine (mg/d1) 1.2 (150%) 1.1 (122%) 3.1 (97%)
1.2 (120%)
Creatinine clearance (ml/min) 1.0 (0.4%) 30.8 (18%) 1.6 (21%)
16.2 (35%)
Serum NGAL (ng/ml) 102 (60%) 809 (84%) 126 (127%)
Urinary KIM-1 (ng/ml) 24.3 (591%) 2.2 (61%) 1.39
(120%)
Treatment:
Urine output (ml/min) 0.54 (17%) 0.47 (101%) 0.35
(36%)
Serum creatinine (mg/d1) 1.3 (163%) 3.1 (97%) 1.7 (170%)
Creatinine clearance (ml/min) 30.6 (12%) ** 18.3 (341%)
13.6 (29%)
Serum NGAL (ng/ml) 197(117%) 1104 (115%) 208
(209%)
Urinary KIM-1 (ng/ml) 260 (6326%) 28.7 (799%) 233
(20000%)
Histological findings:
Blood volume in capillary space 2.4% 0.9% 4.0%
Hyaline casts Mild/Mod ** None Mod
Degranulation Mild/Mod None Mod
Data are raw values (% baseline)
* not measured
** confounded by phenylephrine
[00494] Animal A: The animal weighed 50.6 kg and had a baseline urine output
rate of
3.01 ml/min, a baseline serum creatinine of 0.8 mg/di, and a measured CrC1 of
261 ml/min.
It is noted that these measurements, aside from serum creatinine, were
uncharacteristically
high relative to other animals studied. Congestion was associated with a 98%
reduction in
urine output rate (0.06 ml/min) and a >99% reduction in CrC1 (1.0 ml/min).
Treatment with
negative pressure applied through the ureteral catheters was associated with
urine output and
CrC1 of 17% and 12%, respectively, of baseline values, and 9x and >10x,
respectively, of
congestion values. Levels of NGAL changed throughout the experiment, ranging
from 68%
of baseline during congestion to 258% of baseline after 90 minutes of therapy.
The final
value was 130% of baseline. Levels of KIM-1 were 6 times and 4 times of
baseline for the
first two 30-minute windows after baseline assessment, before increasing to
68x, 52x, and
63x of baseline values, respectively, for the last three collection periods.
The 2-hour serum
creatinine was 1.3 mg/d1. Histological examination revealed an overall
congestion level,
measured by blood volume in capillary space, of 2.4%. Histological examination
also noted
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
122
several tubules with intraluminal hyaline casts and some degree of tubular
epithelial
degeneration, a finding consistent with cellular damage.
[00495] Animal B: The animal weighed 50.2 kg and had a baseline urine output
rate of
2.62 ml/min and a measured CrC1 of 172 ml/min (also higher than anticipated).
Congestion
was associated with an 80% reduction in urine output rate (0.5 ml/min) and an
83% reduction
in CrC1 (30 ml/min). At 50 minutes into the congestion (20 minutes after the
congestion
baseline period), the animal experienced an abrupt drop in mean arterial
pressure and
respiration rate, followed by tachycardia. The anesthesiologist administered a
dose of
phenylephrine (75 mg) to avert cardiogenic shock. Phenylephrine is indicated
for
intravenous administration when blood pressure drops below safe levels during
anesthesia.
However, since the experiment was testing the impact of congestion on renal
physiology,
administration of phenylephrine confounded the remainder of the experiment.
[00496] Animal C: The animal weighed 39.8 kg and had a baseline urine output
rate of
0.47 ml/min, a baseline serum creatinine of 3.2 mg/di, and a measured CrC1 of
5.4 ml/min.
Congestion was associated with a 75% reduction in urine output (0.12 ml/min)
and a 79%
reduction in CrC1 (1.6 ml/min). It was determined that baseline NGAL levels
were >5x the
upper limit of normal (ULN). Treatment with negative pressure applied to the
renal pelvis
through the ureteral catheters was associated with a normalization of urine
output (101% of
baseline) and a 341% improvement in CrC1 (18.2 ml/min). Levels of NGAL changed
throughout the experiment, ranging from 84% of baseline during congestion to
47% to 84%
of baseline between 30 and 90 minutes. The final value was 115% of baseline.
Levels of
KIM-1 decreased 40% from baseline within the first 30 minutes of congestion,
before
increasing to 8.7x, 6.7x, 6.6x, and 8x of baseline values, respectively, for
the remaining 30-
minute windows. Serum creatinine level at 2 hours was 3.1 mg/d1. Histological
examination
revealed an overall congestion level, measured by blood volume in capillary
space, of 0.9%.
The tubules were noted to be histologically normal.
[00497] Animal D: The animal weighed 38.2 kg and had a baseline urine output
of 0.98
ml/min, a baseline serum creatinine of 1.0 mg/di, and a measured CrC1 of 46.8
ml/min.
Congestion was associated with a 75% reduction in urine output rate (0.24
ml/min) and a
65% reduction in Cr Cl (16.2 ml/min). Continued congestion was associated with
a 66% to
91% reduction of urine output and 89% to 71% reduction in CrCl. Levels of NGAL
changed
throughout the experiment, ranging from 127% of baseline during congestion to
a final value
of 209% of baseline. Levels of KIM-1 remained between ix and 2x of baseline
for the first
two 30-minute windows after baseline assessment, before increasing to 190x,
219x, and 201x
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
123
of baseline values for the last three 30-minute periods. The 2-hour serum
creatinine level was
1.7 mg/d1. Histological examination revealed an overall congestion level 2.44x
greater than
that observed in tissue samples for the treated animals (A, C) with an average
capillary size
2.33 times greater than that observed in either of the treated animals. The
histological
evaluation also noted several tubules with intraluminal hyaline casts as well
as tubular
epithelial degeneration, indicating substantial cellular damage.
Summary
[00498] While not intending to be bound by theory, it is believed that the
collected data
supports the hypothesis that venous congestion creates a physiologically
significant impact
on renal function. In particular, it was observed that elevation of the renal
vein pressure
reduced urine output by 75% to 98% within seconds. The association between
elevations in
biomarkers of tubular injury and histological damage is consistent with the
degree of venous
congestion generated, both in terms of magnitude and duration of the injury.
[00499] The data also appears to support the hypothesis that venous congestion
decreases
the filtration gradients in the medullary nephrons by altering the
interstitial pressures. The
change appears to directly contribute to the hypoxia and cellular injury
within medullary
nephrons. While this model does not mimic the clinical condition of AKI, it
does provide
insight into the mechanical sustaining injury.
[00500] The data also appears to support the hypothesis that applying negative
pressure to
the renal pelvis through ureteral catheters can increase urine output in a
venous congestion
model. In particular, negative pressure treatment was associated with
increases in urine
output and creatinine clearance that would be clinically significant.
Physiologically
meaningful decreases in medullary capillary volume and smaller elevations in
biomarkers of
tubular injury were also observed. Thus, it appears that by increasing urine
output rate and
decreasing interstitial pressures in medullary nephrons, negative pressure
therapy may
directly decrease congestion. While not intending to be bound by theory, by
decreasing
congestion, it may be concluded that negative pressure therapy reduces hypoxia
and its
downstream effects within the kidney in a venous congestion mediated AM.
[00501] The experimental results appear to support the hypothesis that the
degree of
congestion, both in terms of the magnitude of pressure and duration, is
associated with the
degree of cellular injury observed. Specifically, an association between the
degree of urine
output reduction and the histological damage was observed. For example,
treated Swine A,
which had a 98% reduction in urine output, experienced more damage than
treated Swine C,
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
124
which had a 75% reduction in urine output. As would be expected, control Swine
D, which
was subjected to a 75% reduction in urine output without benefit of therapy
for two and a half
hours, exhibited the most histological damage. These findings are broadly
consistent with
human data demonstrating an increased risk for AKI onset with greater venous
congestion.
See e.g., Legrand, M. et al., Association between systemic hemodynamics and
septic acute
kidney injury in critically ill patients: a retrospective observational study.
Critical Care
17:R278-86, 2013.
Example 3
Method
[00502] Inducement of negative pressure within the renal pelvis of farm swine
using
ureteral catheters was performed for the purpose of evaluating effects of
negative pressure
therapy on hemodilution of the blood. An objective of these studies was to
demonstrate
whether a negative pressure delivered into the renal pelvis significantly
increases urine output
in a swine model of fluid resuscitation.
[00503] Two pigs were sedated and anesthetized using ketamine, midazolam,
isoflurane
and propofol. One animal (#6543) was treated with a ureteral catheter and
negative pressure
therapy as described herein. The other, which received a Foley type bladder
catheter, served
as a control (#6566). Following placement of the ureretal catheters, the
animals were
transferred to a sling and monitored for 24 hours.
[00504] Fluid overload was induced in both animals with a constant infusion of
saline (125
mL/hour) during the 24 hour follow-up. Urine output volume was measured at 15
minute
increments for 24 hours. Blood and urine samples were collected at 4 hour
increments. As
shown in FIG. 21, a therapy pump 818 was set to induce negative pressure
within the renal
pelvis 820, 821 (shown in FIG. 21) of both kidneys using a pressure of -45mmHg
(+/- 2
mmHg).
Results
[00505] Both animals received 7 L of saline over the 24 hour period. The
treated animal
produced 4.22 L of urine while the control produced 2.11 L. At the end of 24
hours, the
control had retained 4.94 L of the 7 L administered, while the treated animal
retained 2.81 L
of the 7 L administered. FIG. 26 illustrates the change in serum albumin. The
treated animal
had a 6% drop in the serum albumin concentration over 24 hours, while the
control animal
had a 29% drop.
CA 03118062 2021-04-28
WO 2020/110046
PCT/IB2019/060255
125
Summary
[00506] While not intending to be bound by theory, it is believed that the
collected data
supports the hypothesis that fluid overload induces clinically significant
impact on renal
function and, consequently induces hemodilution. In particular, it was
observed that
administration of large quantities of intravenous saline cannot be effectively
removed by even
healthy kidneys. The resulting fluid accumulation leads to hemodilution. The
data also
appears to support the hypothesis that applying negative pressure diuresis
therapy using
ureteral catheters to fluid overloaded animals can increase urine output,
improve net fluid
balance and decrease the impact of fluid resuscitation on development of
hemodilution.
[00507] The preceding examples and embodiments of the invention have been
described
with reference to various examples. Modifications and alterations will occur
to others upon
reading and understanding the foregoing examples. Accordingly, the foregoing
examples are
not to be construed as limiting the disclosure.