Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
Nano-scale Energy Conversion Device
BACKGROUND
[0001] The present embodiments relate to electric power generation,
conversion, and
transfer. More specifically, the embodiments disclosed herein are related to
nano-scale
energy conversion devices that generate electric power through thermionic
energy conversion
and thermoelectric energy conversion, and methods of making and using the
same.
[0002] Portable electric power generating devices are often used to power
devices where
access to electric power from the electric power grid is not practical (e.g.,
mobile phones and
tablets in a shopping complex and satellites in orbit about the Earth). Such
devices are also
used when access to the grid is not possible (e.g., remote installations with
intermittent or no
grid availability). In addition, such devices are used as a backup power
supply to support
continued operation of critical equipment during a grid event (e.g., a
blackout or a brownout).
[0003] Standard, portable electric power generation devices include
gasoline engines and
diesel engines. However, when in operation, these devices require frequent
monitoring to
ensure that necessary refueling is performed to maintain the devices in
operation.
Photoelectric devices, e.g., solar cells are effective only when sufficient
light is available.
Commercially available electric power storage devices include, for example,
electrochemical
batteries and solid state batteries. However, due to limited charges on
electrochemical and
solid state batteries, frequent replacement and/or recharging are required. In
addition,
electrochemical batteries and solid state batteries have a relatively large
footprint when used
for emergency and backup power supplies in industrial facilities. Similarly,
microelectronic
devices are not always compatible with the employment of electrochemical
batteries and
solid state batteries. One example of a microelectronic device possibly
requiring a compact,
long-life, low-current, electric power device is a low power electronic sensor
which is
installed for long-term unattended operation in an inaccessible location.
Another example of
a microelectronic device possibly requiring a compact, long-life, minimal
power draw, is a
nonvolatile memory circuit of a compact computing device.
[0004] Other portable power generation devices include fuel cells and
nuclear batteries.
Fuels cells require hydrogen replacement after a period of time and, similar
to
1
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
electrochemical and solid state batteries, fuel cells have a relatively large
footprint when used
as emergency and backup power supplies in industrial facilities. Nuclear
batteries rely on
processes that include fission, fusion, or radioactive decay of the nuclei of
atoms. These
processes are relatively inefficient and require shielding to reduce the
emissions of ionizing
radiation that are a natural by-product of the nuclear processes.
SUMMARY
[0005] An apparatus and a method are provided to generate electric power.
[0006] In one aspect, the apparatus is provided with first and second
electrodes, the first
electrode having a first work function value and the second electrode having a
second work
function value, with the first and second work function values being
different. The second
electrode is positioned a distance from the first electrode. A cavity is
formed by at least part
of the distance and a dielectric medium is disposed in the cavity. A plurality
of nanoparticles
are suspended in the dielectric medium.
[0007] In another aspect, a method for generating electric power involves
providing a
first electrode with a first work function value, providing a second electrode
with a second
work function value, positioning the first electrode a distance from the
second electrode,
establishing a contact potential difference between the first electrode and
the second
electrode, and providing a plurality of nanoparticles within a cavity defined
by the distance.
A plurality of electrons is emitted from the first electrode, and the
electrons are transmitted to
the second electrode through Brownian motion of the nanoparticles.
[0008] These and other features and advantages will become apparent from
the following
detailed description of the presently exemplary embodiment(s), taken in
conjunction with the
accompanying drawings.
BRIEF DESCRIPTION OF THE SEVERAL VIEWS OF THE DRAWINGS
[0009] The drawings referenced herein form a part of the specification and
are
incorporated herein. Features shown in the drawings are meant as illustrative
of only some
embodiments, and not of all embodiments, unless otherwise explicitly
indicated.
2
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
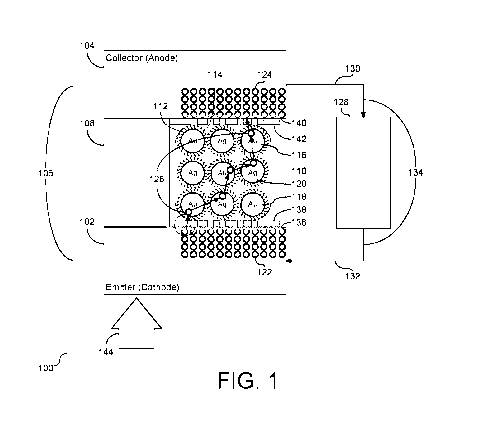
[0010] FIG. 1 depicts a cutaway view of an embodiment of a nano-scale
energy
conversion device.
[0011] FIGS. 2A and 2B depict a flow chart illustrating a process for
manufacturing a
nano-scale energy conversion device.
[0012] FIG. 3 depicts a cutaway view of an embodiment of a partially
constructed nano-
scale energy conversion device.
[0013] FIG. 4 depicts a table of work function values of elemental bulk
materials.
[0014] FIG. 5 depicts a perspective view of a process for depositing a
thermionic electron
emissive material on an electrode substrate.
[0015] FIG. 6 depicts a graphical representation of work function values as
a function of
particle size.
[0016] FIG. 7 depicts an overhead view of a covalently-bonded dipole
deposited on the
surface of an electrode through an electrospray deposition of a thermionic
electron emissive
material on the electrode substrate.
[0017] FIG. 8 depicts a cutaway view of an embodiment of a nanofluid
including a
plurality of nanoparticle clusters suspended in a dielectric medium.
[0018] FIG. 9 depicts a flow chart illustrating a process for generating
electric power with
the nano-scale energy conversion device.
[0019] FIG. 10 depicts a cutaway view of an embodiment of the nano-scale
energy
conversion device showing a relationship between the work functions of the
electrodes and
nanofluid therein.
3
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
[0020] FIG. 11 depicts a graphical representation of the effect of the
emitter work
function value being larger than the collector work function value.
[0021] FIG. 12 depicts a schematic view of electron transfer through
collisions of the
nanoparticle clusters.
[0022] FIG. 13 depicts a schematic view of an embodiment of employment of
the nano-
scale energy conversion device.
[0023] FIG. 14 depicts a schematic view of a system of stacked or grouped
nano-scale
energy conversion devices to that generates electric power from waste heat.
[0024] FIG. 15 depicts a cutaway view of waste heat harvesting system that
includes a
nano-scale energy conversion device coupled to an electronic chip that
harvests electrical
energy from waste heat from the electronic chip.
[0025] FIG. 16 depicts an exploded view of an electric power generation
system that
includes an array of nano-scale energy conversion devices coupled to an array
of solar cells
that harvests electrical energy from waste heat from the solar cell array.
DETAILED DESCRIPTION
[0026] It will be readily understood that the components of the present
embodiments, as
generally described and illustrated in the Figures herein, may be arranged and
designed in a
wide variety of different configurations. Thus, the following detailed
description of the
embodiments of the apparatus, system, and method of the present embodiments,
as presented
in the Figures, is not intended to limit the scope of the embodiments, as
claimed, but is
merely representative of selected embodiments.
[0027] Reference throughout this specification to "a select embodiment,"
"one
embodiment," or "an embodiment" means that a particular feature, structure, or
characteristic
described in connection with the embodiment is included in at least one
embodiment. Thus,
appearances of the phrases "a select embodiment," "in one embodiment," or "in
an
4
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
embodiment" in various places throughout this specification are not
necessarily referring to
the same embodiment. The various embodiments may be combined with one another.
[0028] The illustrated embodiments will be best understood by reference to
the drawings,
wherein like parts are designated by like numerals throughout. The following
description is
intended only by way of example, and simply illustrates certain selected
embodiments of
devices, systems, and processes that are consistent with the embodiments as
claimed herein.
[0029] Thermionic power conversion provides a method to convert heat into
electrical
energy. Thermionic power conversion generators convert heat energy to
electrical energy by
an emission of electrons from a heated emitter electrode (i.e., a cathode).
Electrons flow from
an emitter electrode, across an inter-electrode gap, to a collector electrode
(i.e., an anode),
through an external load, and return back to the emitter electrode, thereby
converting heat to
electrical energy. Recent improvements in thermionic power converters include
selecting
materials with lower work functions for the electrodes and using a fluid to
fill the inter-
electrode gap. The electron transfer density is limited by the materials of
the electrodes and
the materials of the fluid in the inter-electrode gap (i.e., the associated
work functions).
[0030] Construction of the Nano-scale Energy Conversion Device
[0031] To provide additional details for an improved understanding of
selected
embodiments of the present disclosure, reference is now made FIG. 1
illustrating a cutaway
view of an embodiment of a nano-scale energy conversion device (100) that is
configured to
generate electrical power. The nano-scale energy conversion device (100) is
sometimes
referred to as a cell or a layer. A plurality of devices (100) may be
organized as a plurality of
cells or a plurality of layers in series or parallel, or a combination of both
to generate
electrical power at the desired voltage, current, and power output. The nano-
scale energy
conversion device (100) includes an emitter electrode (cathode) (102) and a
collector
electrode (anode) (104). The emitter electrode (102) and collector electrode
(104) are
collectively referred to as the electrodes (106) of the nano-scale energy
conversion device
(100). A plurality of insulator posts, also referred to herein as columns,
standoffs, or micro-
pillars, (108) (only one shown) maintain separation between the electrodes
(106) such that the
electrodes (106) and the insulator posts (108) define a cavity (110). In an
embodiment, the
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
insulator posts (108) are fabricated with a dielectric material, such as, and
without limitation,
alkanethiol, sol-gel with aerogel-like properties, corona dope, super corona
dope, silicon,
silicon-oxide, polymer, any dielectric material, or a combination including
one or more of the
foregoing. In an embodiment, one or more insulator walls (not shown) are
formed that divide
the cavity (110). In an embodiment, the cavity (110) extends between the
electrodes (106) for
a distance in the range from 1 nanometer (nm) to less than about 10 nm. An
interstitial
nanofluid (112) is maintained within the cavity (110). Accordingly, nano-scale
energy
conversion device (100) includes two opposing electrodes (106) separated by
insulator posts
(108) with a cavity (110) filled with a nanofluid (112) between the electrodes
(106).
[0032] The emitter electrode (102) and the collector electrode (104) are
each fabricated
with different materials, with the different materials having separate and
different work
function values. As used herein, the work function of a material (or a
combination of
materials) is the minimum thermodynamic work (i.e., minimum energy) needed to
remove an
electron from a solid to a point in a vacuum immediately outside a solid
surface of the
material. The work function is a material-dependent characteristic. Work
function values are
typically expressed in units of electron volts (eV). Accordingly, the work
function of a
material determines the minimum energy required for electrons to escape the
surface, with
lower work functions generally facilitating electron emission.
[0033] The emitter electrode (102) has a higher work function value than
the collector
electrode (104). The difference in work function values between the electrodes
(106) due to
the different electrode materials induces a contact potential difference
between the electrodes
(106) that has to be overcome to transmit electrons through the nanofluid
(112) within the
cavity (110) from the emitter electrode (102) to the collector electrode
(104). Both electrodes
(106) emit electrons; however, as explained in more detail elsewhere herein,
once the contact
potential difference is overcome, the emitter electrode (102) will emit
significantly more
electrons than the collector electrode (104). A net flow of electrons will be
transferred from
the emitter electrode (102) to the collector electrode (104), and a net
electric current will flow
from the emitter electrode (102) to the collector electrode (104). This net
current causes the
emitter electrode (102) to become positively charged and the collector
electrode (104) to
become negatively charged. Accordingly, the nano-scale energy conversion
device (100)
6
CA 03131367 2021-08-24
WO 2020/176345
PCT/US2020/019232
generates an electron current that is transmitted from the emitter electrode
(102) to the
collector electrode (104).
[0034] The nanofluid (112) includes a dielectric medium (114) and a
plurality of
nanoparticle clusters (116) suspended in the dielectric medium (114). The
nanofluid (112)
minimizes ohmic heating and eliminates formation of space charges in the
cavity (110) such
that arcing in the medium (114) is prevented. In some embodiments, and without
limitation,
the dielectric medium (114) is one of water, silicone oil, or alcohol. Also,
in at least one
embodiment, the dielectric medium (114) is a sol-gel with aerogel-like
properties and low
thermal conductivity values that reduce heat transfer therethrough, e.g.,
thermal conductivity
values less than 1.0 watts per meter-degrees Kelvin (W/(m= K)). In at least
one embodiment,
the thermal conductivity of the dielectric medium (114) is as low as 0.013
watts per meter-
degrees Kelvin (W/(m-K)), as compared to the thermal conductivity of water at
20 degrees
Celsius ( C) of 0.6 W/(m-K). Accordingly, the nanofluid (112) minimizes heat
transfer
through the cavity (110) with low thermal conductivity values. The heat
transport in the
thermal conductivity nanofluid (112) is proportional to the temperature
difference between
the electrodes (102) and (104). For example, if the heat transport in a low
thermal
conductivity nanofluid is small, a high temperature difference between the two
electrodes
(102 and 104) can be maintained during operation. As discussed elsewhere
herein, the
electrical conductivity of the nanofluid (112) changes with operation of the
corresponding
device.
[0035] The nanoparticle clusters (116) may be fabricated from metal and
metal alloys,
ceramics, cermet, composites, and other materials. Some of the nanoparticle
clusters (116)
may include materials dissimilar from other nanoparticle clusters (116). In an
embodiment,
and without limitation, the nanofluid (112) includes nanoparticle clusters
(116) of gold (Au)
(118) and silver (Ag) (120). As used herein, "nanoparticle clusters" refers to
a grouping of 6
to 8 atoms of the associated materials, e.g., Au and Ag, where the number of
atoms is non-
limiting, The nanoparticle clusters (116) have work function values that are
greater than the
work function values for the electrodes (106). Specifically, the work function
values of the
Au nanoparticle clusters (118) and the Ag nanoparticle clusters (120) are 4.1
eV and 3.8 eV,
respectively. As explained in more detail elsewhere herein, charge transport
through electron
hopping and Brownian motion is facilitated by the greater work function values
of the
7
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
nanoparticle clusters (116) and use of at least two types of nanoparticle
clusters (116), each
type with a different work function value. The Brownian motion of the
nanoparticle clusters
(116) includes collisions between the nanoparticle clusters (116) among
themselves and
collisions between the nanoparticle clusters (116) and the two electrodes
(102) and (104).
[0036] The nanoparticle clusters (116) are coated with alkanethiol to form
a dielectric
barrier thereon, where the selection of alkanethiol is non-limiting. In at
least one
embodiment, dodecanethiols are used. In at least one other embodiment, at
least one other
alkane shorter than dodecanethiol and decanethiol is used. The length of the
alkane chain is
limited by the need for the nanoparticle conductive cores to be within 1 nm to
transfer
electrons from one conductive surface to another. The alkanethiol coating
reduces
coalescence of the nanoparticle clusters (116). In an embodiment, the
nanoparticle clusters
(116) have a diameter in the range of 0.5 nm to 5 nm. In at least one
embodiment, the
nanoparticle clusters (116) have a diameter in the range of 1-3 nm. In an
embodiment, the
nanoparticle clusters (116) have a diameter of 2 nm. The nanoparticle clusters
(116) of Au
(118) and Ag (120) are tailored to be electrically conductive with charge
storage features.
Accordingly, the nanofluid (112), including the suspended nanoparticle
clusters (116),
provides a conductive pathway for electrons to travel across the cavity (110)
from the emitter
electrode (102) to the collector electrode (104) through charge transfer.
[0037] A plurality of emitter electrons (122) and a plurality of collector
electrons (124)
are shown proximate to the cavity (110) within the respective emitter
electrode (102) and
collector electrode (104). An electron (126) is shown as leaving the emitter
electrode (102),
hopping across the nanoparticle clusters (116), and entering the collector
electrode (104).
FIG. 1 illustrates an external circuit (128) connected to the two electrodes
(106). Specifically,
a first electrical conductor (130) is connected to the collector electrode
(104) and the external
circuit (128) and a second electrical conductor (132) is connected to the
external circuit (128)
and the emitter electrode (102). When nano-scale energy conversion device
(100) is in
service generating electricity, external circuit current (134) is transmitted
through external
circuit (128), and the same amount of electron current as flowing through the
external circuit
(134) will flow from the emitter electrode (102) to the collector electrode
(104). For example,
a single cell or layer, such as the configuration shown and described in FIG.
1, can generate a
voltage within a range extending between about 0.25 volts and 6.0 volts,
depending on the
8
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
contact potential difference (discussed further herein) induced between the
emitter electrode
(102) and the collector electrode (104) as a function of the materials used
for each. In some
embodiments, the device (100) nominally generates between about 0.75 volts and
5.0 volts.
In an embodiment, the device generates about 0.75 volts. Also, for example, a
single cell or
layer, such as the configuration shown and described in FIG. 1, the device
(100) can generate
an electrical current within a range of approximately 1 milliampere (ma) to
approximately 10
ma. Further, in some embodiments, the device (100) generates approximately
0.75 milliwatts
per square centimeter (mW/cm2). Accordingly, nano-scale energy conversion
device (100)
generates sufficient electrical current to power small loads, e.g., a micro-
circuit.
[0038] In an embodiment, the emitter electrode (102) is manufactured with a
tungsten
(W) nanoparticle surface (136) and a cesium oxide (Cs20) coating (138) that at
least partially
covers the W nanoparticle surface (136). The collector electrode (104) is
manufactured with a
gold (Au) nanoparticle surface (140) and a Cs20 coating (142) that at least
partially covers
the Au nanoparticle surface (140). As discussed further herein, during
manufacturing of the
electrodes (106), W nanoparticles are electrosprayed onto one side of polymer
base that
includes a nanometal film, i.e., an atomic-scale lattice of nanometal atoms,
on one side of the
polymer base. The W nanoparticles form a surface layer on the nanometal film.
The Cs20
coating (138) on the W nanoparticle surface (136) is layered on the surface
(136) through a
template. The polymer base is a sacrificial component for assembly that is
removed with
acetone (described further herein). Similarly, in an embodiment the Au
nanoparticles are
electrosprayed onto the nanometal film on a polymer base to form the Au
nanoparticle
surface (140) and the Cs20 coating (142) is layered on the Au nanoparticle
surface (140)
through a template, and the polymer base is removed. The use of the templates
with particular
electrospray and nanofabrication techniques form deposited layers of Cs20
(138) and (142) in
one or more predetermined patterns on the W nanoparticle surface (136) and the
Au
nanoparticle surface (140). A percentage of coverage of each of the two
surfaces (136) and
(140) with the respective Cs20 coating layers (138) and (142) is within a
range of at least
50% up to 70%, and in at least one embodiment, is about 60%. The Cs20 coatings
(138) and
(142) reduce the work function values of the electrodes (102) and (104) from
the work
function values of W (typically 4.55 eV) and Au (typically 5.1 eV),
respectively. Specifically,
the emitter electrode (102) has a work function value of 0.88 electron volts
(eV) and the
collector electrode (104) has a work function value of 0.65 eV. Accordingly,
the lower work
9
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
function values of the electrodes (102) and (104) are essential to the
operation of the nano-
scale energy conversion device (100) as described herein.
[0039] In an exemplary embodiment, W and Au are selected for the electrodes
(106) due
to at least some of their metallic properties (e.g., strength and resistance
to corrosion) and the
measured change in work function when the thermionic emissive material of Cs20
is layered
thereon. Alternative materials may be used, such as noble metals including,
and without
limitation, rhenium (Re), osmium (Os), ruthenium (Ru), tantalum (Ta), iridium
(Jr), rhodium
(Rh), palladium (Pd), and platinum (Pt), or any combination of these metals.
In addition, and
without limitation, non-noble metals such as aluminum (Al) and molybdenum (Mo)
may also
be used. For example, and without limitation, Al nanoparticles may be used
rather than W
nanoparticles to form surface (136), and Pt nanoparticles may be used rather
than Au
nanoparticles to form surface (140). Accordingly, the selection of the
materials to use to form
the nanoparticle surfaces (136) and (140) is principally based on the work
functions of the
electrodes (106), and more specifically, the difference in the work functions
once the
electrodes (106) are fully fabricated.
[0040] The nano-scale energy conversion device (100) generates electric
power through
harvesting heat energy (144). As described in further detail herein, the
emitter electrode (102)
receives the heat energy (144) from sources that include, without limitation,
heat generating
sources and ambient environments, and generates electrons (126) that traverse
the cavity
(110) via the nanoparticle clusters (116). The electrons (126) reach the
collector electrode
(104) and external circuit current (134) is transmitted to the external
circuit (128). In some
embodiments, nano-scale energy conversion device (100) generates electrical
power through
placement in ambient, room temperature environments. Accordingly, the nano-
scale energy
conversion device (100) harvests heat energy (144), including waste heat, to
generate useful
electrical power.
[0041] Assembly of the Nano-scale Energy Conversion Device
[0042] Referring to FIGS. 2A and 2B, a flow chart (200) is provided
illustrating a process
for manufacturing a nano-scale energy conversion device. Referring to FIG. 3,
a diagram
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
(300) is provided illustrating a cutaway view of an embodiment of a partially
constructed
nano-scale energy conversion device (300) (not shown to scale).
[0043] Fabrication of the Emitter Electrode (Cathode)
[0044] As shown and described in FIGS. 2A, 2B, and 3, an emitter electrode
(302) is
fabricated (202) by positioning (204) a nanometal film (328) on one side of a
base, such as a
polymer base (330) (shown in phantom). The polymer base (330) is approximately
2 nm
thick (where this value should be considered non-limiting) and the nanometal
film (328) is
approximately 3.7 angstroms thick. The polymer base (330) is used as a
sacrificial assembly
component. The nanometal film (328) is positioned proximate to an electrospray
nozzle of an
electrospray device (see FIG. 5, electrospray nozzle (512)). Emitter electrode
nanoparticles
(not shown) are selected (206) for deposition (208) on the nanometal film
(328). In an
exemplary embodiment for selection (206) of the nanoparticle material(s) for
the emitter
electrode (302), the combination of the nanoparticle material and the
deposited thermionic
emissive material has a combined work function value greater than the work
function value
of a collector electrode (306) (fabricated as described further elsewhere
herein). In an
exemplary embodiment, the difference of the work function values of the two
electrodes
(302) and (306) (as measured in eV) is above a predetermined value to maximize
electron
transfer between the two electrodes (302) and (306). A partial list of the
appropriate
nanoparticle materials for the emitter electrode (302) is provided elsewhere
herein. In the
exemplary embodiment, at least one layer of W nanoparticles (338) is deposited
(208)
through electrospray onto the nanometal film (328) to form a nanoparticle
surface (304) on
the nanometal film (328). In at least one embodiment, the thickness of the
layer of
nanoparticles to form nanoparticle surface (304) is approximately 2 nm (i.e.,
the approximate
thickness of a nanoparticle), where the 2 nm value should be considered non-
limiting.
Accordingly, one or more metal materials in the form of nanoparticles are
selected as the
nanoparticle surface (304) for the emitter electrodes (302) at least partially
as a function of
decreasing the work function value of the electrodes (302) and (306) and
maintaining a work
function value differential between the electrodes (302) and (306) above a
predetermined
value.
11
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
[0045] Once the W nanoparticle surface (304) is formed (208), the polymer
base (330) is
removed (210) through an acetone solution, thereby rendering the polymer base
(330) as a
sacrificial material. Accordingly, the W nanoparticle surface (304) on the
nanometal film
(328) is ready to receive a thermionic emissive material thereon.
[0046] At least one layer of a thermionic electron emissive material (308)
is deposited
(212) on at least a portion of the W nanoparticle surface (304). In an
embodiment, a
monolayer of the thermionic electron emissive material (308) is deposited
(212) on about at
least 50% to about 70% of the surface of the W nanoparticle surface (304). In
another
embodiment, about 60% of the surface of the W nanoparticle surface (304)
receives a
monolayer of the material (308). In yet another embodiment, a plurality of
layers of the
thermionic electron emissive material (308) is deposited on about 60% of the W
nanoparticle
surface (304). The deposited thermionic electron emissive material (308) is
selected to
decrease the work function value of the emitter electrode (302) to a value
below that of the
work function value of the material selected for the W nanoparticle surface
(304).
[0047] Referring to FIG. 4, a table (400) is provided illustrating
elemental work function
values of bulk materials. For example, the work function value of W is 4.55
eV. As described
further herein, the deposition of Cs20 (308) on the W nanoparticle surface
(304) decreases
the work function value of emitter electrode (302) to about 0.88 eV.
Accordingly, the
combination of materials is positioned to create the desired work function
values, and
modifying the combination of materials can change the work function value of
the
combination.
[0048] The selection of the thermionic electron emissive material (308) to
deposit on the
nanoparticle surface (304) is partially based on the desired work function
value of the
electrode (302) (and electrode (306)) and chemical compatibility between the
nanoparticle
surface (304) and the deposited material (308). Deposition materials include,
but are not
limited to, thorium, aluminum, cerium, and scandium, as well as oxides of
alkali or alkaline
earth metals, such as cesium, barium, calcium, and strontium.
[0049] Referring to FIG. 5, a diagram is provided illustrating a
perspective view of a
process (500) for depositing a thermionic electron emissive material on an
electrode
12
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
substrate, i.e., a nanoparticle surface such as W nanoparticle surface
(136/304) and Au
nanoparticle surface (140/310). In a first step (502), a template (504) is
positioned proximate
to a substrate (506). The template (504) includes a pattern (508) of openings
corresponding to
a desired deposition pattern on the substrate (506). The template (504) is
positioned over one
side of the substrate (506) as shown by the arrow (524). The substrate (506)
is grounded to
facilitate direct deposition of droplets on the substrate (506) to form a
pattern of the deposited
deposition material(s).
[0050] In a second step (510), the substrate (506) with the overlaid
template (504) is
positioned proximate to an electrospray nozzle (512) of an electrospray device
(not shown).
An emission of the thermionic electron emissive material issues from the
electrospray nozzle
(512) as monodispersed droplets (514) is characterized by nanoparticles of
uniform size in a
dispersed phase. An electrospray of the droplets (514), hereinafter referred
to as electrospray,
produces monodisperse particles to support deposition of the thermionic
electron emissive
material in the nanometric scale range. In an embodiment, the electrospray
(514) includes a
solution of 0.1 molar (M) Cs20 nanoparticles in ethanol, where the droplet
diameter is 10
microns. Also, in an embodiment, the pattern (508) includes a range of 30-50
micron
diameter holes with a center-to-center distance ranging from about 60-200
microns, staggered
at about 30-60 degree angles, and preferably 45 degree angles. The
electrospray (514) with
Cs20 nanoparticles is directed toward the template (504) to form a monolayer
(516) as the
template (504) and the substrate (506) traverse the electrospray (514) as
shown by the arrow
(526). In an embodiment, 1014 Cs20 atoms per square centimeter (cm2) is the
target
concentration for the substrate (506).
[0051] In a third step (518), the template (504) is separated from the
substrate (506) as
shown by the arrow (528) to leave a patterned thermionic electron emissive
material (520) on
electrode substrate (506) to form an emitter electrode (522). As shown, four
distinct lines of
deposited material, collectively referred to as deposited material (520), are
overlaid on the W
substrate (506). This pattern is merely an example pattern and should not be
considered
limiting. In an embodiment, the pattern of thermionic electrons may vary as
long as the
pattern enables operation of the electrode (522). Accordingly, a monolayer of
patterned
thermionic electron emissive material (520), i.e., Cs20, is formed on a W
substrate (506) to
achieve coverage of the substrate (506) in excess of at least 50% to about
70%, with 60%
13
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
being an optimal coverage. In at least one embodiment, the thickness of the
layer of patterned
thermionic electron emissive material (520) is approximately 2 nm, where the 2
nm value
should be considered non-limiting.
[0052] Exemplary electrospray and nano-fabrication technique(s) and
associated
equipment, including three-dimensional printing and four-dimensional printing
(in which the
fourth dimension is varying the nanoscale composition during printing to
tailor properties) for
forming the layers, films, and coatings discussed herein, are set forth in
U.S. Application
Publication No. 2015/0251213.
[0053] Referring to FIG. 6, a diagram is provided illustrating a graphical
representation
(600) of work function values as a function of particle size. The graph (600)
includes a first
axis (602), represented herein as a vertical axis (602) that indicates a
change in work function
value (A), where the vertical axis has a range from 0 to 1.2 in increments of
0.2 and in units
of eV. The graph (600) also includes a second axis (604), represented herein
as a horizontal
axis that indicates particle size, where the second axis (604) extends from 0
to 60 in
increments of 10 and in units of nanometers (nm). The graph (600) also
includes a curve
(606) representing the relationship between the particle size and the change
in work function
value associated with the size. As indicated in the graph (600), the selection
of nanoparticle
size impacts its work function value. For example, the work function value
changes more
than 1 eV as the nanoparticle cluster approaches that of a single atom.
Conversely, as the
nanoparticle size enlarges, the work function value approaches measurements
associated with
bulk materials. This property of a work function value of a material
increasing with
decreasing particle size enables the tailoring of each individual application
of the nano-scale
energy conversion device (100). Accordingly, selecting the particular
nanoparticle sizes for
the spray depositions (520) on the emitter and collector electrodes
(102)/(302) and
(104)/(306), respectively, is performed to increase the work function
difference between the
electrodes to increase the number of electrons transferred during operation of
the nano-scale
energy conversion device (100).
[0054] Referring to FIG. 7, a diagram is provided illustrating an overhead
view (700) of a
covalently-bonded dipole (702) deposited on the nanoparticle surface
(136/304/506/704) of
an electrode (302/306) through an electrospray (514) deposition of the
thermionic electron
14
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
emissive material on the electrode nanoparticle surface/substrate
(136/304)/(506/704). The
nanoparticles in the Cs20 deposited on the electrode nanoparticle surface
(704) form covalent
bonds that in turn form the surface dipoles (702) (only one shown in FIG. 7).
Within the
dipoles (702), a positive charge (706) and an opposing negative charge (708)
are formed by
charged nano-droplets in the electrospray (514) as they collide with the
surface (704). The
charges (706) and (708) induce an electric field (710) between the charges
(706) and (708)
such that the dipole (702) acts as a nano-antenna that modifies the proximate
dipole moment
in the vicinity of the dipole (702) through inducement of electromagnetic
waves proximate
thereto as a function of the induced electric field (710). Accordingly,
covalently-bonded
dipoles (702) deposited on an electrode nanoparticle surface (704) create a
low work function
electrode when the coverage area is optimized.
[0055] In at least one embodiment, the electrodes (e.g., the W emitter
electrode (302) and
the Au collector electrode (306)) have dimensions of 20-50 millimeters (mm)
long by 20-50
mm wide and 4-100 nm thick. The 100nm thickness is based on charge penetration
from the
emitter electrode (102/302) into the nanofluid (112) and from the collector
electrode
(104/306) into the second electrical conductor (132). In general, to maximize
the charge
penetration through the associated electrodes (102/302) and (104/306), a
smaller value of the
associated thickness is preferred. Therefore, in at least one embodiment,
electrodes (102/302)
and (104/306) are approximately 4 nm thick.
[0056] Fabrication of the Collector Electrode (Anode)
[0057] Referring again to FIGS. 2A, 2B, and 3, the collector electrode
(306) is fabricated
(214) in a manner substantially similar to that for the emitter electrode
(302). A nanometal
film (332) is positioned (216) on one side of a polymer base (334) (shown in
phantom). The
polymer base (334) is approximately 2 nm thick (where this value should be
considered non-
limiting) and the nanometal film (332) is approximately 3.7 angstroms thick.
The nanometal
film (332) is positioned proximate to an electrospray nozzle of an
electrospray device (see
FIG. 5, electrospray nozzle (512)). Collector electrode nanoparticles (not
shown) are selected
(218) for deposition (220) on the nanometal film (332). In an exemplary
embodiment for
selection (218) of the nanoparticle material(s) for the collector electrode
(306), the
combination of the nanoparticle material and the deposited thermionic emissive
material has
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
a combined work function value less than the work function value of the
emitter electrode
(302) (fabricated as described elsewhere herein). In an exemplary embodiment,
the difference
of the work function values of the two electrodes (302) and (306) (as measured
in eV) is
above a predetermined value to maximize electron transfer between the two
electrodes (302)
and (306). A partial list of the appropriate nanoparticle materials for the
collector electrode
(306) is provided elsewhere herein. In the exemplary embodiment, at least one
layer of Au
nanoparticles (336) is deposited (220) through electrospray onto the nanometal
film (332) to
form a nanoparticle surface (310) on the nanometal film (332). In at least one
embodiment,
the thickness of the layer of nanoparticles to form nanoparticle surface (332)
is approximately
2 nm (i.e., the approximate thickness of a nanoparticle), where the 2 nm value
should be
considered non-limiting.
[0058] Once the Au nanoparticle surface (310) is formed (220), the polymer
base (334) is
removed (222) through an acetone solution, thereby rendering the polymer base
(334) as a
sacrificial material. Accordingly, the Au nanoparticle surface (310) on the
nanometal film
(332) is ready to receive a thermionic emissive material thereon.
[0059] At least one layer of a thermionic electron emissive material (312)
is deposited
(224) on at least a portion of the Au nanoparticle surface (310). In an
embodiment, a
monolayer of the thermionic electron emissive material (312) is deposited on
about at least
50% to about 70% of the surface of the Au nanoparticle surface (310). In
another
embodiment, about 60% of the surface of the Au nanoparticle surface (310)
receives a
monolayer of the material (312). In yet another embodiment, a plurality of
layers of the
thermionic electron emissive material (312) is deposited on about 60% of the
Au nanoparticle
surface (310). The deposited thermionic electron emissive material (312) is
selected to
decrease the work function value of the collector electrode (306) to a value
below that of the
work function value of the material selected for the Au nanoparticle surface
(310). Referring
to FIG. 4, the table (400) indicates that the work function value of Au is 5.1
eV. Deposition
of Cs20 (312) on the Au nanoparticle surface (310) decreases the work function
value of the
collector emitter electrode (306) to 0.65 eV. Accordingly, similar to the
emitter electrode
(302), one or more metal materials in the form of nanoparticles are selected
as the
nanoparticle surface (310) for the collector electrodes (306) at least
partially as a function of
decreasing the work function value of the electrodes (302) and (306) and
maintaining a work
16
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
function value differential between the electrodes (302) and (306) above a
predetermined
value.
[0060] Fabrication of the Insulator Posts
[0061] Once the electrodes (302) and (306) are fabricated, assembly (200)
of the nano-
scale energy conversion device (100)/(300) continues. Specifically, one of the
collector
electrode (306) (as shown in FIG. 3) or the emitter electrode (302) is
positioned on a surface
(not shown) for support. A template (not shown in FIG. 2) is employed to
fabricate (226) a
plurality of insulator posts (or columns, standoffs, or micro-pillars) (314)
through
electrospray deposition to maintain separation between the electrodes (302)
and (306) such
that the electrodes (302) and (306) and the insulator posts (314) define a
cavity (316). In at
least one embodiment, the template is a graphite template. In an embodiment,
the insulator
posts (314) are fabricated with a dielectric material, such as, and without
limitation,
alkanethiol, sol-gel with aerogel-like properties, corona dope, super corona
dope, silicon,
silicon-oxide, polymer, any dielectric material, or a combination including at
least one of the
foregoing. The templates are constructed to allow for electro-spraying of the
alkanethiol such
that overspray onto the thermionic electron emissive materials (308) and (312)
is minimized.
In an embodiment, one or more insulator walls are formed that divide the
cavity (316) into
multiple cavities (316).
[0062] The height (318) of the insulator posts (314) may be in a range of,
for example, 1
nanometer (nm) to less than 10 nm. Therefore, the cavity (316) extends between
the
electrodes (302) and (306) for a distance in the range from 1 nanometer (nm)
to less than 10
nm. The width (320) of the insulator posts (314) may be in a range of, for
example, 1
nanometer (nm) to 10 nm. The width (322) of the cavity (322) may be in a range
of, for
example, 1 nanometer (nm) to 10 nm. The insulator posts (314) are shown as
cubical or
cubical like structures and substantially similar in shape and size to that of
the cavity (322),
although this configuration is not limiting. The distance between insulator
posts (314) is
within a range defined by about 5-6 nm to about 1 cm. The dimensions and
configurations of
the insulator posts (314) and cavities (316) are determined based on the
planned employment
of the nano-scale energy conversion devices (100)/(300). Accordingly,
insulator posts (314)
(or walls) are fabricated on one of the two electrodes (302) and (306) at a
predetermined
17
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
height to maintain a predetermined distance between the two electrodes (302)
and (306)
within the nano-scale energy conversion device (100)/(300).
[0063] Coupling the Electrodes and Insulator Posts Together
[0064] The electrodes (302) and (306) and the insulator posts (314) are
coupled (228)
together. As described above, and shown in FIG. 3, the insulator posts (314)
are deposited on
the collector electrode (306). The electrode on which the insulator posts
(314) were not
formed, as shown in FIG. 3, the emitter electrode (302), is lowered to rest on
top of the
insulator posts (314) as shown by the arrows (326). An adhesive material (324)
is spot-
deposited at the outside ends of the nano-scale energy conversion device
(100)/(300) to
adhere the electrodes (302) and (306) and insulator posts (314) as a unit. In
an embodiment,
this aspect of the assembly is completed when the device (100)/(300) is sealed
or encased
with one of silicon or silicon dioxide casing segments, a sealant such as a
hot melt adhesive,
or by electro-spraying an alkanethiol film gasket around the edge of the
device on all sides,
with the exception of one side remaining unsealed to facilitate addition of a
nanofluid (as
discussed further elsewhere herein). The positioning of the electrodes (302)
and (306) and the
insulator posts (314) define (230) a cavity therebetween (316).
[0065] Manufacturing the Nanofluid and Adding the Nanofluid to the Cavities
[0066] Referring to FIGs. 2A and 2B, the nanofluid (112) is manufactured
(232).
Referring to FIG. 8, a diagram is provided illustrating a cutaway view of an
embodiment of a
nanofluid (800) including a plurality of Au and Ag nanoparticle clusters (802)
and (804),
respectively, suspended in a dielectric medium (806). In some embodiments, and
without
limitation, the dielectric medium (806) is one of water, silicone oil, or
alcohol. Also, in at
least one embodiment, the dielectric medium (806) is a sol-gel with aerogel-
like properties
and low thermal conductivity values that reduce heat transfer therethrough,
e.g., thermal
conductivity values as low as 0.013 watts per meter-degrees Kelvin (W/m-K) as
compared to
the thermal conductivity of water at 20 degrees Celsius ( C) of 0.6 W/m-K.
Appropriate
materials are selected prior to fabricating the nanoparticle clusters (802)
and (804). The
nanoparticle clusters (802) and (804) have work function values that are
greater than the work
function values for the electrodes (234). Specifically, the work function
values of the Au
18
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
nanoparticle clusters (802) and the Ag nanoparticle clusters (804) are 4.1 eV
and 3.8 eV,
respectively.
[0067] At least one layer of a dielectric coating, such as a monolayer of
alkanethiol
material (808), is deposited, e.g., electrosprayed, on the nanoparticles (236)
to form a
dielectric barrier thereon. The alkanethiol material at step (236) includes,
but is not limited to
dodecanethiol and decanethiol. The deposit of the dielectric coating, such as
alkanethiol,
reduces coalescence of the nanoparticle clusters (802) and (804). In at least
one embodiment,
the nanoparticle clusters (802) and (804) have a diameter in the range of 1 nm
to 3 nm. In an
embodiment, the nanoparticle clusters (802) and (804) have a diameter of 2 nm.
The
nanoparticle clusters of Au (802) and Ag (804) are tailored to be electrically
conductive with
charge storage features (i.e., capacitive features), minimize heat transfer
through the cavities
(110) and (316) with low thermal conductivity values, minimize ohmic heating,
eliminate
space charges in the cavities (110) and (316), and prevent arcing. The
plurality of Au and Ag
nanoparticle clusters (802) and (804), respectively, are suspended (238) in
the dielectric
medium (806). Accordingly, the nanofluid (800), including the suspended
nanoparticle
clusters (802) and (804), provides a conductive pathway for electrons to
travel across the
cavities (110) and (316) from the emitter electrode (102) and (302) to the
collector electrode
(104) and (306) through charge transfer.
[0068] The Au nanoparticle clusters (802) are dodecanethiol functionalized
gold
nanoparticles, with a particle size of 1-3 nm, at about 2% (weight/volume
percent) and
suspended in toluene. The Ag nanoparticle clusters (804) are dodecanethiol
functionalized
silver nanoparticles, with a particle size of 1-3 nm, at about 0.25%
(weight/volume percent)
and suspended in hexane. In an embodiment, the particle size of both the Au
and Ag
nanoparticle clusters (802) and (804) is at or about 2 nm. The Au and Ag cores
of the
nanoparticle clusters (802) and (804) are selected for their abilities to
store and transfer
electrons. In an embodiment, a 50%-50% mixture of Au and Ag nanoparticle
clusters (802)
and (804) is used. However, a mixture in the range of 1-99% Au-to-Ag could be
used as well.
Electron transfers are more likely between nanoparticle clusters (802) and
(804) with
different work functions. In an embodiment, a mixture of nearly equal numbers
of two
dissimilar nanoparticle clusters (802) and (804) provides good electron
transfer. Accordingly,
19
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
nanoparticle clusters are selected based on particle size, particle material
(with the associated
work function values), mixture ratio, and electron affinity.
[0069] In some cases, conductivity of the nanofluid (800) can be increased
by increasing
concentration of the nanoparticle clusters (802) and (804). However, an
optimum
concentration for the conductance of the nanofluid can be found at lower
concentrations of
nanoparticles, such as less than 0.5 mole/liter, 0.4 to 0.5 mole/liter, or
even lower
concentrations. The nanoparticle clusters (802) and (804) may have a
concentration within
the nanofluid (800) of about 0.1 mole/liter to about 2 moles/liter. In at
least one embodiment,
the Au and Ag nanoparticle clusters (802) and (804) each have a concentration
of at least 1
mole/liter.
[0070] The stability and reactivity of colloidal particles, such as Au and
Ag nanoparticle
clusters (802) and (804), are determined largely by a ligand shell formed by
the alkanethiol
coating (808) adsorbed or covalently bound to the surface of the nanoparticle
clusters (802)
and (804). The nanoparticle clusters (802) and (804) tend to aggregate and
precipitate, which
can be prevented by the presence of a ligand shell of the non-aggregating
polymer alkanethiol
coating (808) enabling these nanoparticle clusters (802) and (804) to remain
suspended.
Adsorbed or covalently attached ligands can act as stabilizers against
agglomeration and can
be used to impart chemical functionality to the nanoparticle clusters (802)
and (804). Over
time, the surfactant nature of the ligand coatings is overcome and the lower
energy state of
agglomerated nanoparticle clusters is formed. Therefore, over time,
agglomeration may occur
due to the lower energy condition of nanoparticle cluster accumulation and
occasional
addition of a surfactant may be used.
[0071] The nanofluid (802) is loaded (240) into the cavities (110) and
(316) by, for
example, capillary and vacuum processes through the remaining unsealed side of
nano-scale
energy conversion device (100)/(300). Assembly is completed when the remaining
unsealed
side of the nano-scale energy conversion device (100)/(300) is sealed (242) or
encased with
one of silicon or silicon dioxide casing segments, a sealant such as a hot
melt adhesive, or by
electrospraying an alkanethiol film gasket around the remaining unsealed side
of the device.
Accordingly, in at least one embodiment, a plurality of Au and Ag nanoparticle
clusters (802)
and (804) are mixed together in a dielectric medium (806) to form a nanofluid
(112)/(800),
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
the nanofluid (112)/(800) is inserted into the cavities (110)/(316), and the
nano-scale energy
conversion device (100)/(300) is fully sealed.
[0072] In an embodiment, the dimensions of the nano-scale energy conversion
device
(100)/(300) is approximately 20-50 mm long by approximately 20-50 mm wide by
approximately 9-19 nm thick (about 4 nm for each electrode (102/302 and
104/306) and
about 1 nm to less than about 10 nm for the cavity (110/316) therebetween).
The thickness of
the device (100/300) is determined based on the desired electron flow therein
and the other
two dimensions are scalable based on the desired overall power output of the
device
(100/300).
[0073] Principles of Operation of the Nano-scale Energy Conversion Device
[0074] Referring again to FIG. 9, a flow chart is provided illustrating a
process (900) for
generating electric power with the nano-scale energy conversion device (100).
As described
herein, an emitter electrode (102) is provided (902) and a collector electrode
(104) is
provided (904), where the work function value of the collector electrode (104)
is less than the
work function value of the emitter electrode (102). The emitter electrode
(102) and the
collector electrode (104) are positioned (906) a predetermined distance from
each other, i.e.,
about 1 nm to less than about 10 nm.
[0075] Referring to FIG. 10, a diagram is provided illustrating a cutaway
view of an
embodiment of the nano-scale energy conversion device (1000) showing a
relationship
between the work functions of an emitter electrode (1002) (WF,), collector
electrode (1004)
(WF,), and nanofluid (1006) (WFõf) therein. FIG. 10 also shows a pair of
insulator posts
(1008) separating the electrodes (1002) and (1004). The difference between the
WF, and WF,
establishes (908) a contact potential difference (CPD) between the two
electrodes (1002) and
(1004). Specifically, a voltage differential (Vcpp) is induced across the
nanofluid (1006) due
to the dissimilar metals of electrodes (1002) and (1004), e.g., W and Au,
respectively, both
including at least a monolayer of Cs20 over about 60% of the surfaces thereof.
In this
embodiment, the value for WF, is 0.88 eV and the value for WFc is 0.65 eV, to
induce a VCPD
of 0.23 eV. The Vcpp induces an electric field (Ecpp) that has to be overcome
to transmit
electrons through the nanofluid (1006) from the emitter electrode (1002) to
the collector
21
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
electrode (1004). Accordingly, as described further herein, this induced CPD
enables
thermionic emission of electrons from the emitter electrode (1002) toward the
collector
electrode (1004).
[0076] The nanofluid (1006) including a plurality of Au nanoparticle
clusters (1010) and
Ag nanoparticle clusters (1012) is provided (910). In this embodiment, the
work functions of
the Au nanoparticle clusters (1010) and Ag nanoparticle clusters (1012) are
4.1 eV and 3.8
eV, respectively. Therefore, a collective work function WFõf is greater than
WF, which is
greater than WF,. This relationship of the work function values of the Au and
Ag
nanoparticle clusters (1010) and (1012) optimizes the transfer of electrons to
the nanoparticle
clusters (1010) and (1012) through Brownian motion and electron hopping
(discussed further
herein). Accordingly, the selection of materials within the nano-scale energy
conversion
device (1000) optimizes electric current generation and transfer therein
through enhancing
the release of electrons from the emitter electrode (1002) and the conduction
of the released
electrons across the nanofluid (1006) to the collector electrode (1004).
[0077] Referring to FIG. 11, a diagram is provided illustrating a graphical
representation
(1100) of the effect of the emitter work function value being larger than the
collector work
function value. The emitter electrode (1102) and the collector electrode
(1104) are separated
by the cavity (1106) that is filled with nanofluid (1108). A thermal
distribution function
(1110) of the electrons in the emitter electrode (1102) above the
electrochemical potential
(the Fermi level (pe)) as a function of the distance from the surface (1112)
of the electrode
(1102) is shown. The work function (WF,) of the emitter electrode (1102)
extends from the
Fermi level (pe) of the emitter electrode (1102) to an electrical potential of
the emitter
electrode (Es). Similarly, a thermal distribution function (1114) of the
electrons in the
collector electrode (1104) above the electrochemical potential (the Fermi
level (pc)) as a
function of the distance from the surface (1116) of the electrode (1104) is
shown. The work
function (WF,) of the collector electrode (1104) extends from the Fermi level
(pc) of the
collector electrode (1104) to the electrical potential of the collector
electrode (Es). The Fermi
level (pc) of the collector electrode (1104) is shifted upward due to the
electrical potential of
a load (eVioad) connected to the electrodes (1102) and (1104) inducing a
contact potential
difference voltage (Wm) across the cavity (1108), where eVioad and Wm are
equal to each
other. An electrical potential function (E) is shown declining from the
surface (1112) of the
22
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
emitter electrode (1102) to the surface (1116) of the collector electrode
(1104), i.e., from E,
to E, linearly as the electrons traverse the nanofluid (1106) and the cavity
(1108) as indicated
by the arrow (1118). The emitter work function (WF,) is greater than the
collector work
function (WF,) so that electrons are accelerated toward the collector
electrode (1104) and not
accelerated back towards the emitter electrode (1102). Accordingly, the
selection of the
materials for the emitter electrode (1102) and the collector electrode (1104)
with the
associated work function and Fermi level values determining operational
functionality of the
nano-scale energy conversion device (100). In an exemplary embodiment, the
work function
(WF,) of the emitter electrode (1102) equals the combination of an external
load and the
work function (WF,) of the collector electrode (1104).
[0078] Referring to FIG. 12 (and FIGs. 1 and 9), a diagram (1200) is
provided illustrating
electron transfer through collisions of a plurality of nanoparticle clusters.
As shown in FIG.
12, the nanofluid (1202) includes a plurality of nanoparticle clusters (1204)
suspended in a
dielectric medium (1206). In the embodiment shown, the plurality of
nanoparticle clusters
(1204) includes Au nanoparticle clusters (1208) and Ag nanoparticle clusters
(1210). The Au
nanoparticle clusters (1208) have a work function value of about 4.1 eV and
the Ag
nanoparticle clusters (1210) have a work function value of about 3.8 eV. These
work function
values of the nanoparticle clusters (1204) are much greater than the work
function values of
the emitter electrode (102) (0.88 eV) and the collector electrode (104) (0.65
eV). The
nanoparticle clusters (1204) are coated with alkanethiol to form a dielectric
barrier (1212)
thereon to reduce coalescence of the nanoparticle clusters (1204). In an
embodiment, the
nanoparticle clusters (1204) have a diameter of about 2 nm. The nanoparticle
clusters (1204)
are tailored to be electrically conductive with capacitive (i.e., charge
storage) features while
minimizing heat transfer therethrough. Accordingly, suspended nanoparticle
clusters (1204)
provide a conductive pathway for electrons to travel across the cavity (110)
from the emitter
electrode (102) to the collector electrode (104) through charge transfer.
[0079] Thermally-induced Brownian motion causes the nanoparticle clusters
(1204) to
move within the dielectric medium (1206), and during this movement they
occasionally
collide with each other and with the electrodes (102) and (104). As the
nanoparticle clusters
(1204) move and collide within the dielectric medium (1206), the nanoparticle
clusters
(1204) chemically and physically transfer charge. The nanoparticle clusters
(1204) transfer
23
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
charge chemically when electrons (1214) hop from the electrodes (102) and
(104) to the
nanoparticle clusters (1204) and from one nanoparticle cluster (1204) to
another nanoparticle.
The hops primarily occur during collisions. Due to differences in work
function values,
electrons (1214) are more likely to move from the emitter electrode (102) to
the collector
electrode (104) via the nanoparticle clusters (1204) rather than in the
reverse direction.
Accordingly, a net electron current from the emitter electrode (102) to the
collector electrode
(104) via the nanoparticle clusters (1204) is the primary and dominant current
of the nano-
scale energy conversion device (100).
[0080] The nanoparticle clusters (1204) transfer charge physically (i.e.,
undergo transient
charging) due to the ionization of the nanoparticle clusters (1204) upon
receipt of an electron
and the electric field generated by the differently charged electrodes (102)
and (104). The
nanoparticle clusters (1204) become ionized in collisions when they gain or
lose an electron
(1214). Positive and negative charged nanoparticle clusters (1204) in the
nanofluid (1202)
migrate to the negatively charged collector electrode (104) and the positively
charged emitter
electrode (102), respectively, providing a current flow. This ion current flow
is in the
opposite direction from the electron current flow, but much less in magnitude
than the
electron flow.
[0081] Some ion recombination in the nanofluid (1202) does occur, which
diminishes
both the electron and ion current flow. Electrode separation may be selected
at an optimum
width to maximize ion formation and minimize ion recombination. In the
exemplary
embodiment, the electrode separation is slightly less than10 nm. The
nanoparticle clusters
(1204) have a maximum dimension of about 2 nm, so the electrode separation is
about 3 to 5
nanoparticle clusters (1204). This separation distance provides sufficient
space within the
cavity for nanoparticle clusters (1204) to move around and collide, while
minimizing ion
recombination. For example, in an embodiment, an electron can hop from the
emitter
electrode (102) to a first nanoparticle cluster (1204) and then to a second,
third, fourth, or
fifth nanoparticle cluster (1204) before hopping to the collector electrode
(104). A reduced
quantity of hops mitigates ion recombination opportunities. Accordingly, ion
recombination
in the nanofluid (1202) is minimized through an electrode separation distance
selected at an
optimum width to maximize ion formation and minimize ion recombination.
24
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
[0082] When the emitter electrode (102) and the collector electrode (104)
are initially
brought into close proximity, the electrons of the collector electrode (104)
have a higher
Fermi level than the electrons of the emitter electrode (102) due to the lower
work function of
the collector electrode (104). The difference in Fermi levels drives a net
electron current that
transfers electrons from the collector electrode (104) to the emitter
electrode (102) until the
Fermi levels are equal, i.e., the electrochemical potentials are balanced and
thermodynamic
equilibrium is achieved. The transfer of electrons between the emitter
electrode (102) and the
collector electrode (104) results in a difference in charge between the
emitter electrode (102)
and the collector electrode (104). This charge difference sets up the voltage
of the contact
potential difference (Vcpp) and an electric field between the emitter
electrode (102) and the
collector electrode (104), where the polarity of the Vcpp is determined by the
material having
the greatest work function. With the Fermi levels equalized, no net current
will flow between
the emitter electrode (102) and the collector electrode (104). Accordingly,
electrically
coupling the emitter electrode (102) and the collector electrode (104) with no
external load
results in attaining the contact potential difference between the electrodes
(102 and 104) and
no net current flow between the electrodes (102) and (104) due to attainment
of
thermodynamic equilibrium between the two electrodes (102) and (104).
[0083] The nano-scale energy conversion device (100) can generate electric
power (e.g.,
at room temperature) with or without additional heat input. Heat added to the
emitter
electrode (102) will raise its temperature and the Fermi level of its
electrons. With the Fermi
level of the emitter electrode (102) higher than the Fermi level of the
collector electrode
(104), a net electron current will flow from the emitter electrode (102) to
the collector
electrode (104) through the nanofluid (1202). If the external circuit (128) is
connected, the
same amount of electron current will flow through the external circuit current
(134) from the
collector electrode (104) to the emitter electrode (102). The heat energy
added to the emitter
electrode (102) is carried by the electrons (1214) to the collector electrode
(104). The bulk of
the added energy is transferred to the external circuit (128) for conversion
to useful work and
some of the added energy is transferred in collisions to the collector
electrode (104) and
eventually lost to ambient as waste energy. As the energy input to the emitter
electrode (102)
increases, the temperature of the electrode (102) decreases as the highest
energy electrons are
emitted, and the electron transmission from the emitter electrode (102)
increases, thereby
generating more current. As the emitter electrode (102) releases (912)
electrons onto the
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
nanoparticle clusters (1204), energy is stored in the nano-scale energy
conversion device
(100). Accordingly, the nano-scale energy conversion device (100) generates,
stores, and
transfers charge and moves heat energy if a temperature difference exists,
where added
thermal energy causes the production of electrons to increase from the emitter
electrode (102)
into the nanofluid (1202).
[0084] The nanofluid (1202) is used to transfer charges from the emitter
electrode (102)
to one of the mobile nanoparticle clusters (1204) (via intermediate contact
potential
differences) from the collisions of the nanoparticle cluster (1204) with the
emitter electrode
(102) induced by Brownian motion (914) of the nanoparticle cluster (1204). The
selection of
dissimilar nanoparticle clusters (1204) that include Au nanoparticle clusters
(1208) and Ag
nanoparticle clusters (1210) that have much greater work functions of about
4.1 eV and about
3.8 eV, respectively, optimizes transfer of electrons to the nanoparticle
clusters (1204) from
the emitter electrode (102) and to the collector electrode (104). Unitary
nanoparticle clusters
aggregate more quickly than mixed nanoparticle clusters.
[0085] As the electrons (1214) hop from nanoparticle cluster (1204) to
nanoparticle
cluster (1204), single electron charging effects that include the additional
work required to
hop another electron (1214) on to a nanoparticle cluster (1204) if an electron
(1214) is
already present on the nanoparticle cluster (1204) determine if hopping
additional electrons
(1214) onto that particular nanoparticle cluster (1204) is possible.
Specifically, the
nanoparticle clusters (1204) include a voltage feedback mechanism that
prevents the hopping
of more than a predetermined number of electrons to the nanoparticle cluster
(1204). This
prevents more than the allowed number of electrons from residing on the
nanoparticle cluster
simultaneously. In an embodiment, only one electron (1214) is permitted on any
nanoparticle
cluster (1204) at any one time. Therefore, during conduction of current
through the nanofluid
(1202), a single electron (1214) hops onto the nanoparticle cluster (1204).
The electron
(1214) does not remain on the nanoparticle cluster (1204) indefinitely, but
hops off to either
the next nanoparticle cluster (1204) or the collector electrode (104) through
collisions
resulting from the Brownian motion of the nanoparticle clusters (1204).
However, the
electron (1214) does remain on the nanoparticle cluster (1204) long enough to
provide the
voltage feedback required to prevent additional electrons (1214) from hopping
simultaneously onto the nanoparticle cluster (1204). The hopping of electrons
(1214) across
26
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
the nanoparticle clusters (1204) avoids resistive heating associated with
current flow in a
media. Notably, the nano-scale energy conversion device (100) does not require
pre-charging
by an external power source in order to introduce electrostatic forces. This
is due to the
device (100) being self-charged with triboelectric charges generated upon
contact between
the nanoparticle clusters (1204) due to Brownian motion. Accordingly, the
electron hopping
across the nanofluid (1202) is limited to one electron (1214) at a time
residing on a
nanoparticle cluster (1204).
[0086] As the electrical current starts to flow through the nanofluid
(1202), a substantial
energy flux away from the emitter electrode (102) is made possible by the net
energy
exchange between the emitted (1214) and replacement electrons. The replacement
electrons
from the second electrical conductor (132) connected to the emitter electrode
(102) do not
arrive with a value of energy equivalent to an average value of the Fermi
energy associated
with the material of emitter electrode (102), but with an energy that is lower
than the average
value of the Fermi energy. Therefore, rather than the replacement energy of
the replacement
electrons being equal to the chemical potential of the emitter electrode
(102), the electron
replacement process takes place in the available energy states below the Fermi
energy in the
emitter electrode (102). The process through which electrons are emitted above
the Fermi
level and are replaced with electrons below the Fermi energy is sometimes
referred to as an
inverse Nottingham effect. Accordingly, the low work function value of about
0.88 eV for the
emitter electrode (102) allows for the replacement of the emitted electrons
with electrons
with a lower energy level to induce a cooling effect on the emitter electrode
(102).
[0087] A plurality of nano-scale energy conversion devices (100) are
distinguished by at
least one embodiment having the thermoelectric energy conversion features
described herein.
In general, the nanofluid (1202) is selected for operation of the nano-scale
energy conversion
devices (100) within more than one temperature range. In an embodiment, the
temperature
range of the associated nano-scale energy conversion device (100) is
controlled to modulate a
power output of the device (100). In general, as the temperature of the
emitter electrode (102)
increases, the rate of thermionic emission therefrom increases. The
operational temperature
ranges for the nanofluid (1202) are based on the desired output of the nano-
scale energy
conversion device (100), the temperature ranges that optimize thermionic
conversion, the
temperature ranges that optimize thermoelectric conversion, and fluid
characteristics.
27
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
Therefore, different embodiments of the nanofluid (1202) are designed for
different energy
outputs of the device (100). For example, for the nanofluid (1202), when the
dielectric
medium (1206) is silicone oil, the temperature of the nanofluid (1202) should
be maintained
at less than 250 C to avoid deleterious changes in energy conversion due to
the viscosity
changes of the silicone oil above 250 C. In an embodiment, the temperature
range of the
nanofluid (1202) for substantially thermionic emission is approximately room
temperature
(i.e., about 20 C to about 25 C) up to about 70-80 C, and the temperature
range of the
nanofluid for thermionic and thermo-electric conversion is above 70-80 C, with
the principle
limitations being the temperature limitations of the materials. The nanofluid
(1202) for
operation including thermoelectric conversion includes a temperature range
that optimizes the
thermoelectric conversion through optimizing the power density within the nano-
scale energy
conversion device (100), thereby optimizing the power output of the device
(100). In at least
one embodiment, a mechanism for regulating the temperature of the nanofluid
(1202)
includes diverting some of the energy output of the device (100) into the
nanofluid (1202).
Accordingly, the cavities (110) of specific embodiments of the nano-scale
energy conversion
device (100) may be filled with the nanofluid (1202) to employ thermoelectric
energy
conversion with thermionic energy conversion above a particular temperature
range, or
thermionic energy conversion by itself below that temperature range.
[0088] As described elsewhere herein, in at least one embodiment, the
dielectric medium
(1206) has thermal conductivity values less than about 1.0 watts per meter-
degrees Kelvin
(W/(m= K)). In at least one embodiment, the thermal conductivity of the
dielectric medium
(1206) is as low as about 0.013 watts per meter-degrees Kelvin (W/(m-K)), as
compared to
the thermal conductivity of water at about 20 degrees Celsius ( C) of 0.6 W/(m-
K).
Accordingly, the nanofluid (1202) minimizes heat transfer through the cavity
(110) with low
thermal conductivity values. Since the heat transport in a low thermal
conductivity nanofluid
(1202) can be small, a high temperature difference between the two electrodes
(102) and
(104) can be maintained during operation. These embodiments are designed for
those nano-
scale energy conversion devices (100) that employ thermionic emission only,
where minimal
heat transfer through the nanofluid (1202) is desired.
[0089] In some alternative embodiments of nano-scale energy conversion
devices (100),
greater heat transfer through the nanofluid (1202) is desired. The nano-scale
energy
28
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
conversion device (100) has a cavity (110) dimension of less than about 10 nm.
In this
predetermined distance range of about 1 nm to less than about 10 nm, thermal
conductivity
values and electrical conductivity values of the nanofluid (1202) are enhanced
over thermal
and electrical conductivity values of the nanofluid (1202) when the
predetermined distance of
the cavity is greater than about 100 nm. This increase of thermal and
electrical conductivity
values of the nanofluid (1202) is due to a number of factors. The first factor
is that of
enhanced phonon and electron transfer between the plurality of nanoparticle
clusters (1204)
within the nanofluid (1202), enhanced phonon and electron transfer between the
plurality of
nanoparticle clusters (1204) and the first electrode (102), and enhanced
phonon and electron
transfer between the plurality of nanoparticle clusters (1204) and the second
electrode (104).
A second factor is the enhanced influence of Brownian motion of the
nanoparticle clusters
(1204) in the more confining volume seen in the scale of less than about 10
nm. As the
distance between the electrodes (106) decreases below about 10 nm, the fluid
continuum
characteristics of the nanofluid (1202) with the suspended nanoparticle
clusters (1204) is
altered. For example, as the ratio of particle size to volume of the cavity
(110) increases, the
random and convection like effects of Brownian motion in a dilute solution
dominate.
Therefore, collisions of the nanoparticle clusters (1204) with the surfaces of
other
nanoparticle clusters (1204) and the electrodes (102) and (104) increase
thermal and electrical
conductivity values due to the enhanced phonon and electron transfer. A third
factor is the at
least partial formation of nanoparticle cluster (1204) matrices within the
nanofluid (1202). In
an embodiment, the formation of the matrices is based on the factors of time
and/or
concentration of the nanoparticle clusters (1204) in the nanofluid (1202).
Under certain
conditions, the nanoparticle clusters (1204) will form matrices within the
nanofluid (1202) as
a function of close proximity to each other with some of the nanoparticle
clusters (1206)
remaining independent from the matrices. A fourth factor is the predetermined
nanoparticle
cluster (1204) density, which in an embodiment is about one mole per liter.
Accordingly, the
very small dimensions of the cavity (110) of less than about 10 nm causes an
increase in the
thermal and electrical conductivity values of the nanofluid (1202) therein.
[0090] In addition, the nanoparticle clusters (1204) are extremely thin and
they are often
considered to have only one dimension, i.e., their characteristic length. This
extreme thinness
restricts electrons and holes in a process called quantum confinement, which
increases
electrical conductivity. The collision of particles with different quantum
confinement
29
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
facilitates transfer of charge to the electrodes (102) and (104). A
nanoparticle cluster's (1204)
small size also increases the influence of its surfaces, thereby tending to
increase thermal
conductivity. The embodiments of nano-scale energy conversion device (100)
have an
enhanced electrical conductivity value greater than about 1 Siemens per meter
(S/m). Also,
the embodiments of device (100) with the enhanced thermal conductivity have a
thermal
conductivity value greater than about 1 W/m-K.
[0091] The thermionic emission of electrons (1214) from the emitter
electrode (102) and
the transfer of the electrons (1214) across the nanofluid (1202) from
nanoparticle cluster
(1204) to nanoparticle cluster (1204) through hopping are both quantum
mechanical effects.
[0092] The release of electrons from the emitter electrode (102) through
thermionic
emission as described herein is an energy selective mechanism. A Coulombic
barrier in the
cavity (110) between the emitter electrode (102) and the collector electrode
(104) is induced
through the interaction of the nanoparticles (1204) with the electrodes (102)
and (104) inside
the cavity (110). The Coulombic barrier is at least partially induced through
the number and
material composition of the plurality of nanoparticle clusters (1204). The
Coulombic barrier
induced through the nanofluid (1202) provides an energy selective barrier on
the order of
magnitude of about 1 eV. Accordingly, the nanofluid (1202) provides an energy
selective
barrier to electron emission and transmission.
[0093] To overcome the thermionic barrier and allow electrons (1214) to be
emitted from
the emitter electrode (102) above the energy level needed to overcome the
barrier, selection
of the materials for the emitter electrode (102) and the collector electrode
(104) are selected
for their work function values and Fermi level values. The Fermi levels of the
two metal
electrodes (102) and (104) and the nanoparticle cluster (1204) will try to
align by tunneling
electrons (1214) from the electrodes (102) and (104) to the nanoparticle
cluster (1204). The
difference in potential between the two electrodes (102) and (104) (described
elsewhere
herein) overcomes the thermionic barrier and the emission of electrons (1214)
from the
emitter electrode (102) occurs with sufficient energy to overcome the
thermionic block.
Notably, for cooling purposes, removing higher energy electrons from the
emitter electrode
(102) causes the emission of electrons (1214) to carry away more heat energy
from the
emitter electrode (102) than is realized with lower energy electrons.
Accordingly, the energy
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
selective barrier is overcome through the thermionic emission of electrons at
a higher energy
level than would be otherwise occurring without the thermionic barrier.
[0094] Once the electrons (1214) have been emitted from the emitter
electrode (102)
through thermionic emission, the thermionic barrier continues to present an
obstacle to
further transmission of the electrons (1214) through the nanofluid (1202).
Smaller gaps on the
order of about 1-10 nm facilitates electron hopping, i.e., field emission, of
short distances
across the cavity (110). The energy requirements for electron hopping are much
lower than
the energy requirements for thermionic emission, therefore the electron
hopping has a
significant effect on the energy generation characteristics of the device
(100). The design of
the nanofluid (1202) enables energy selective tunneling (hopping) that is a
result of the
special form of the barrier (which has wider gap for low energy electrons)
which results in
electrons above the Fermi level being the principal hopping component. The
direction of the
electron hopping is determined through the selection of the different
materials for the
electrodes (102) and (104) and their associated work function and Fermi level
values. The
electron hopping across the nanofluid (1202) transfers heat energy with
electrons (1214)
across the cavity (110) while maintaining a predetermined temperature gradient
such that the
temperature of the fluid (1214) is relatively unchanged during the electron
transfer.
Accordingly, the emitted electrons transport heat energy from the emitter
electrode (102)
across the cavity (110) to the collector electrode (104) without increasing
the temperature of
the nanofluid (1202).
[0095] Sample Applications of the Nano-scale Energy Conversion Devices
[0096] Referring to FIG. 13, a diagram is provided illustrating an
embodiment of
employment of the nano-scale energy conversion device (1300). Heat energy
(1302) from a
source enters the emitter electrode (1304). Electrons (1306) are
thermionically emitted (1318)
from the emitter electrode (1304). The electrons (1306) traverse the cavity
(1308) that is
filled with nanofluid (1310) as described herein. The electrons (1306) reach
(1320) the
collector electrode (1312) that collects the electrons (1306) to generate an
output electron
flow (1314) that is transmitted through a first electrical conductor (1322) to
a load (1316) to
perform work. The load (1316) is connected by a second electrical conductor
(1324) to the
emitter (1304). Electrical current represented by arrow (1326) flows in the
opposite direction
31
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
to electron flow (1314). Accordingly, the nano-scale energy conversion device
(1300)
harvests heat energy (1302), including waste heat and ambient heat, to
generate electrical
power (1314).
[0097] Referring to FIG. 14, a diagram is provided illustrating a system
(1400) of stacked
or grouped nano-scale energy conversion devices (1402) that generates electric
power from
heat (1404), which in an embodiment is waste heat or waste heat by-product.
The system
(1400) includes a plurality of nano-scale energy conversion devices (1402)
within an
insulated casing (1406). Each nano-scale energy conversion device (1402) is
capable of
producing at least 0.024 watts/cell and the system (1400) can reach a power
density of about
1550 watts/liter, therefore, about 64,583 devices (1402) would be involved.
For a particular
residential dwelling that uses 3 kilowatts (kW), in an embodiment a system
(1400) to power a
typical home would require about a 2 liter system. Stacked nano-scale energy
conversion
devices (1402) (in series or in parallel) define the power flux to obtain an
electric power
system of desired current and voltage characteristics. In addition, heat
removal capabilities
are enhanced through the additional devices (1402). Accordingly, the nano-
scale energy
conversion devices (1402) are scalable and configurable to provide electric
power under a
variety of uses.
[0098] Referring to FIG. 15, a diagram is provided illustrating a waste
heat harvesting
system (1500) that includes a nano-scale energy conversion group (1502)
coupled to an
electronic chip (1504), such as a semi-conductor chip that harvests electrical
energy from
waste heat from the electronic chip (1504). Such a system (1500) is suitable
for use in mobile
phones and other portable electronic devices. The electronic chip (1504) is
affixed to the
nano-scale energy conversion group (1502) with an adhesive (1506), e.g.,
without limitation,
an epoxy adhesive. The group of stacked nano-scale energy conversion devices
(1502)
includes, e.g., and without limitation, about 35 stacked nano-scale energy
conversion devices.
In an embodiment, the quantity of stacked nano-scale energy conversion devices
may range
depending on the size and dimensions of the semi-conductor chip. For example,
in an
embodiment, the quantity may range from a minimum of 1 to in excess of 1,000
stacked
nano-scale energy conversion devices. Similarly, in an embodiment, the
stacking of multiple
nano-scale energy conversion devices forms layers thereof. The nano-scale
energy
conversion group (1502) is cooled by both radiation heat transfer (1508) and
natural
32
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
convection heat transfer (1510). The nano-scale energy conversion group (1502)
is driven by
a temperature difference where the electronic chip (1504) is operating at
about 100 C. The
harvested electrical power does not need to be used to cool the system (1500).
The nano-scale
energy conversion group (1502) includes a predetermined number of nano-scale
energy
conversion devices for multiplying currents and voltages as necessary.
Accordingly, a nano-
scale energy conversion group (1502) can be cooled by combined natural
convection and
radiation, and there may be no need to install any power-consuming fluid
movers.
[0099] Referring to FIG. 16, a diagram is provided illustrating an electric
power
generation system (1600) that includes an array (1602) of nano-scale energy
conversion
devices (1604) coupled to an array of solar cells (1606) that harvests
electrical energy from
heat byproduct (1608) from the solar cell array (1606). An energy storage
device (1610), such
as, and without limitation, an ultra-capacitor, is electrically connected to
the array (1602) of
nano-scale energy conversion devices (1604). Integration of the nano-scale
energy
conversion devices (1604) into electric power generation system (1600) allows
the array
(1602) to both cool the solar cell array (1606) and synergistically generate
power to augment
photovoltaic production. In addition to enhancing photovoltaic power
generation, the
integration of the nano-scale energy conversion devices (1604) augments other
thermal
power sources such as hot water, geothermal sources, and automotive waste heat
sources, to
enhance the generation of electrical power. Accordingly, the nano-scale energy
conversion
devices (1604) can be integrated with multiple energy-harvesting devices to
produce a greater
energy-density device that would otherwise not be accomplished without the
integration.
33
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
[0100] In at least one embodiment described herein, the present disclosure
is directed
generally to an ultra-long life energy source, such as a battery, and more
particularly is directed
to a nano-scale energy conversion device. Ionization is provided therein by
the combination of
electron tunneling and thermionic emission of the nano-scale energy conversion
device. Charge
transfer therein is effected through conductive nanoparticles suspended in a
fluid (e.g., a
nanofluid) undergoing collisions driven by thermally-induced Brownian motion.
The design of
this device enables ambient energy extraction at low and elevated temperatures
(including room
temperature). To this end, the electrodes are very close to each other to
allow electrons to travel
the distance between the electrodes. These electrons emitted at a wide range
of temperatures
proceed across the gap due to the nanofluid providing a conductive pathway for
electrons,
minimizing heat transfer to maintain a nanoscale heat engine, and preventing
arcing. With
respect to thermionic converters, the electrical efficiency of these devices
depends on the very
low work function materials deposited on the emitter electrode (cathode) and
the collector
electrode (anode). The efficiency of two low work function electrodes can be
increased by
developing cathodes with sufficient thermionic emission of electrons operating
even at room
temperature. These low work function cathodes and anodes provide copious
amounts of
electrons. Similarly, a tunneling device comprises two low work function
electrodes separated by
a designed nanofluid. Cooling by electrode emission refers to the transport of
hot electrons
across the nanofluid gap, from the object to be cooled (cathode) to the heat
rejection electrode
(anode). In an embodiment, the coupling of several technologies, including:
the electrospray-
deposition of two low work function electrodes including cesium-oxide on
tungsten and gold,
respectively; an energy selective electron-transfer thermionic emission and
quantum hopping of
electrons; a nanofluid that is tailored as a thermoelectric element to conduct
electricity while
minimizing heat transfer within the device; and thermal communication from the
anode electrical
connection that is in thermal contact with the device and the outside heat
reservoir. This coupling
of technology produces a viable thermionic power generator.
[0101] The nano-scale energy conversion devices of embodiments described
herein facilitate
generating electrical energy via a long-lived, constantly-recharging battery
for any size-scale
electrical application. The devices of embodiments described herein provide a
battery having a
conversion efficiency superior to presently available single and double
conversion batteries. In
34
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
addition, the devices of embodiments described herein may be fabricated as an
integral part of,
and provide electrical energy for, an integrated circuit. The devices of
embodiments described
herein are a light-weight and compact multiple-conversion battery having a
relatively long
operating life with an electrical power output at a useful value. Furthermore,
in addition to the
tailored work functions, the nanoparticle clusters of embodiments described
herein are
multiphase nanocomposites that include thermoelectric materials. The
combination of
thermoelectric and thermionic functions within a single device further
enhances the power
generation capabilities of the nano-scale energy conversion devices of
embodiments described
herein.
[0102] The conversion of ambient heat energy into usable electricity
enables energy
harvesting capable of offsetting, or even replacing, the reliance of
electronics on conventional
power supplies, such as electrochemical batteries, especially when long-term
operation of a large
number of electronic devices in dispersed locations is required. Energy
harvesting distinguishes
itself from conventional batteries and hardwire power owing to inherent
advantages, such as
outstanding longevity measured in years, little maintenance, and minimal
disposal and
contamination issues. The nano-scale energy conversion devices described
herein demonstrate a
novel electric generator with low cost for efficiently harvesting thermal
energy (without the need
for an initial temperature differential or thermal gradient to start the
electron flow).
[0103] The nano-scale energy conversion devices of embodiments described
herein are
scalable across a large number of power generation requirements. The devices
may be designed
for applications requiring electric power in the milliwatts (mW), watts (W),
kilowatts (kW), and
megawatts (MW) ranges. Examples of devices for the mW range include, but are
not limited to,
those devices associated with the Internet of Things (IoT) (home appliances,
vehicles
(communication only)), handheld portable electronic devices (mobile phones,
medical devices,
tablets), and embedded systems (RFIDs and wearables). Examples of devices for
the watts range
include, but are not limited to, handheld sensors, networks, robotic devices,
cordless tools,
drones, appliances, toys, vehicles, utility lighting, and edge computing.
Examples of devices in
the kW range include, but are not limited to, residential off-grid devices
(rather than backup
fossil fuel generators), resilient/sustainable homes, portable generators,
electric and silent
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
transportation (including water-faring), and spacecraft. Examples of devices
in the MW range
include, but are not limited to, industrial/data center/institutional off-grid
devices (e.g.,
uninterruptible power supplies), resilient complexes, urban centers,
commercial and military
aircraft, flying cars, and railway/locomotive/trucking/shipboard
transportation.
[0104] Aspects of the present embodiments are described herein with
reference to one or
more of flowchart illustrations and/or block diagrams of methods and apparatus
(systems)
according to the embodiments. The embodiments are combinable with one another,
such that, for
example, an embodiment may be used to modify another embodiment.
[0105] The terminology used herein is for the purpose of describing
particular embodiments
only and is not intended to be limiting of the embodiments. As used herein,
the singular forms
"a", "an" and "the" are intended to include the plural forms as well, unless
the context clearly
indicates otherwise. It will be further understood that the terms "comprises"
and/or
"comprising," when used in this specification, specify the presence of stated
features, integers,
steps, operations, elements, and/or components, but do not preclude the
presence or addition of
one or more other features, integers, steps, operations, elements, components,
and/or groups
thereof.
[0106] The corresponding structures, materials, acts, and equivalents of
all means or step
plus function elements in the claims below are intended to include any
structure, material, or act
for performing the function in combination with other claimed elements as
specifically claimed.
The description of the present embodiments has been presented for purposes of
illustration and
description, but is not intended to be exhaustive or limited to the
embodiments in the form
disclosed. Many modifications and variations will be apparent to those of
ordinary skill in the art
without departing from the scope and spirit of the embodiments. The
embodiments were chosen
and described in order to best explain the principles of the embodiments and
the practical
application, and to enable others of ordinary skill in the art to understand
the embodiments for
various embodiments with various modifications as are suited to the particular
use contemplated.
The implementation of the nano-scale energy conversion devices as heat
harvesting devices that
efficiently convert waste heat energy to usable electric energy facilitates
flexible uses of the
36
CA 03131367 2021-08-24
WO 2020/176345 PCT/US2020/019232
minute power generators. Accordingly, the nano-scale energy conversion devices
and the
associated embodiments as shown and described in FIGS. 1-16, provide
electrical power through
conversion of heat in most known environments, including ambient, ambient
temperature
environments.
[0107] It will be appreciated that, although specific embodiments have been
described herein
for purposes of illustration, various modifications may be made without
departing from the spirit
and scope of the embodiments. In particular, the nano-scale energy conversion
devices are
shown as configured to harvest waste heat from stationary or relatively
stationary conditions.
Alternatively, the nano-scale energy conversion devices may be configured to
harvest heat or
waste heat while in motion. Accordingly, the scope of protection of the
embodiment(s) is limited
only by the following claims and their equivalents.
37