Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
SYSTEMS AND METHODS FOR ANALYZING RNA TRANSCRIPTS
CROSS-REFERENCE TO RELATED APPLICATIONS
100011 This application claims the benefit of and priority to U.S. Patent
Application
Serial No. 16/408,970, filed May 10, 2019, which claims priority to U.S.
Provisional
Patent Application Serial No. 62/669,575, filed May 10, 2018, and entitled
"ALZHEIMER'S SCREENING TECHNIQUE AND DEVICE," and U.S. Patent
Application Serial No. 16/345,175, filed April 25, 2019, which is a national
stage entry
under Section 371 of International Patent Application Serial No.
PCT/U52017/058559,
filed October 26, 2017, which claims priority to U.S. Provisional Patent
Application
Serial No. 62/413,144, filed October 26, 2016 and entitled "AUTOMATED NUCLEIC
ACID DETECTION AND QUANTITATION WITH OPTICAL SENSING," the entirety
of each which is incorporated herein by reference.
BACKGROUND
10002] The subject matter disclosed herein relates to detecting target
molecules, such as
nucleic acid molecules and, more particularly, to systems for optical sensing
of the target
molecules.
[0003] Various methods have developed for analyzing biological samples and
detecting
the presence of target molecules, such as nucleic acid molecules. These
methods can be
used, for example, in detecting pathogens in samples.
[0004] Typically, detection methods use disruption techniques, such as
Polymerase
Chain Reaction (PCR) to extract and replicate nucleic acid molecules from a
sample.
PCR is a technique that allows for replicating and amplifying trace amounts of
DNA
fragments into quantities that are sufficient for analysis. As such, PCR can
be used in a
variety of applications, such as DNA sequencing and detecting DNA fragments in
samples.
[0005] An electronic sensor for detection of specific target nucleic acid
molecules can
include capture probes immobilized on a sensor surface between a set of paired
electrodes. An example of a system and method for detecting target nucleic
acid
molecules is described in U.S. Patent No. 7,645,574, the entirety of which is
herein
incorporated by reference. Following PCR, amplified products or amplicons
derived
1
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
from targeted pathogen sequences are captured by the probes. Nano-gold
clusters,
functionalized with a complementary sequence, are used for localized
hybridization to the
amplicons. Subsequently, using a short treatment with a gold developer
reagent, the
nano-gold clusters serve as catalytic nucleation sites for metallization,
which cascades
into the development of a fully conductive film. The presence of the gold film
shorts the
gap between the electrodes and is measured by a drop in resistance, allowing
the presence
of the captured amplification products to be measured. However, such sensors
can be
insensitive to small quantities of target molecules, resulting in false
negative results or a
failure to detect the target molecules.
SUMMARY
[0006] In one embodiment, a system for analyzing molecules in a sample is
presented.
The system includes an imager, a flow cell, a magnet, and a processor. The
flow cell
includes a functionalized surface comprising a capture probe configured to
bind
molecules comprising a first RNA transcript. The magnet is positioned opposite
the
functionalized surface. The magnet is configured to direct the molecules
comprising the
first RNA transcript to the functionalized surface to bind to the capture
probe. The light
source configured to direct a light beam at the bound molecules comprising the
first RNA
transcript. The imager is configured to capture light from the bound molecules
comprising the first RNA transcript. A processor configured to determine a
quantity of
the molecules in the sample comprising the first RNA transcript. The process
is further
configured to determine an expression level of the first RNA transcript in the
sample
based on the quantity of the molecules.
[0007] In another embodiment, a method for analyzing molecules in a sample is
presented. The method uses an imager, a flow cell including a functionalized
surface
having a capture probe coupled to the functionalized surface, a magnet, and a
light
source. The method includes the following steps. Binding molecules comprising
a first
RNA transcript to magnetic particles. Directing the molecules comprising the
first RNA
transcript to the functionalized surface via the magnet. Binding the molecules
comprising
the first RNA transcript to the capture probe. Directing a light beam from the
light
2
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
source at the bound molecules comprising the first RNA transcript. Capturing
light from
the bound molecules comprising the first RNA transcript. Determining a
quantity of the
molecules in the sample comprising the first RNA transcript. Determining an
expression
level of the first RNA transcript in the sample based on the quantity of the
molecules.
[0008] In another embodiment, a method for analyzing molecules in a sample is
presented. The method uses an imager, a magnet, a light source, and a flow
cell that
includes a functionalized surface having a plurality of capture probes. Each
of the
plurality of capture probes is configured to bind molecules in the sample
comprising one
of the plurality of RNA transcripts. The method includes the following steps.
Binding
molecules in the sample to a magnetic particle. Directing the molecules to the
functionalized surface using the magnet. Binding each specific molecule of the
molecules
to one of the plurality of capture probes configured to bind the RNA
transcript of the
specific molecule. Directing a light beam from the light source at bound
molecules
bound on each of the plurality of capture probes. Capturing light from the
bound
molecules. Determining a quantity of the bound molecules bound on each of the
plurality
of capture probes based on the captured light. Determining a plurality of
expression
levels corresponding to the plurality of RNA transcripts based on the quantity
of the
bound molecules bound on each of the plurality of capture probes configured to
bind each
of the plurality of RNA transcript.
[0009] The above embodiments are exemplary only. Other embodiments are within
the
scope of the disclosed subject matter.
BRIEF DESCRIPTION OF THE DRAWINGS
[0010] So that the manner in which the features of the invention can be
understood, a
detailed description of the invention may be had by reference to certain
embodiment,
some of which are illustrated in the accompanying drawings. It is to be noted,
however,
that the drawings illustrate only certain embodiments of this invention and
are therefore
not to be considered limiting of its scope, for the scope of the disclosed
subject matter
encompasses other embodiments as well. The drawings are not necessarily to
scale,
emphasis generally being placed upon illustrating the features of certain
embodiments of
3
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
the invention. In the drawings, like numerals are used to indicate like parts
throughout
the various views.
[0011] FIG. 1 is a perspective view of a portable diagnostic assay system
operative to
accept one of a plurality of disposable cartridges configured to test fluid
samples of
collected blood/food/biological samples;
[0012] FIG. 2 is an exploded perspective view of one of the disposable
cartridges
configured to test a blood/food/biological sample;
[0013] FIG. 3 is a top view of the one of the disposable cartridges
illustrating a variety of
assay chambers including a central assay chamber, one of which contains an
assay
chemical suitable to breakdown the fluid sample to detect a particular
attribute of the
tested fluid sample;
[0014] FIG. 4 is a bottom view of the disposable cartridge shown in FIG. 3
illustrating a
variety of channels operative to move at least a portion of the fluid sample
from one
chamber to another for the purpose of performing multiple operations on the
fluid
sample.
[0015] FIG. 5 is a diagram of an embodiment of a sensor system having a
functionalized
surface;
[0016] FIG. 6 is a flowchart illustrating an embodiment of a method of
detecting a target
molecule;
[0017] FIG. 7 is a diagram of the sensor system of FIG. 5 with target
molecules bound to
magnetic particles;
[0018] FIG. 8A is cross-sectional illustration of an embodiment of a magnetic
particle;
[0019] FIG. 8B is a cross-sectional illustration of another embodiment of a
magnetic
particle;
[0020] FIG. 8C is an illustration of an embodiment of a magnetic particle
bound with
nanoparticles;
[0021] FIG. 8D is an illustration of another embodiment of a magnetic particle
bound
with nanoparticles;
4
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
[0022] FIG. 8E is an illustration of an embodiment of a target molecule bound
with a
magnetic particle and a nanoparticle;
[0023] FIG. 8F is an illustration of another embodiment of a target molecule
bound with
a magnetic particle and nanoparticles;
[0024] FIG. 8G is an illustration of yet another embodiment of a target
molecule bound
with a nanoparticle and magnetic particles;
[0025] FIG. 9 is a diagram of the sensor system of FIGS. 5 and 7 with the
target
molecules bound to the functionalized surface;
[0026] FIG. 10 is a diagram of the sensor system of FIGS. 5 and 7-8 with
functionalized
nanoparticles bound to the target molecules;
[0027] FIG. 11 is a diagram of the sensor system of FIGS. 5, 7-8, and 10 with
a light
source directed at the functionalized nanoparticles;
[0028] FIG. 12A is an embodiment of scattering signatures of 50 nm
monodispersed
nanoparticles under dark field microscopy;
[0029] FIG. 12B is an embodiment of scattering signatures of 100 nm
monodispersed
nanoparticles under dark field microscopy;
[0030] FIG. 13 is a comparison of scattering signatures of developed
nanoparticles versus
undeveloped nanoparticles under dark field microscopy;
[0031] FIG. 14 is an illustration of an embodiment of an optical sensor
system;
[0032] FIG. 15A is an enlarged partial illustration of the optical sensor
system of FIG. 14
with the magnet retracted;
[0033] FIG. 15B is an enlarged partial illustration of the optical sensor
system of FIG. 14
with the magnet extended;
[0034] FIG. 16 is a side view illustration of an embodiment of an optical
instrument
incorporating the optical sensor system of FIG. 14;
[0035] FIG. 17A is a diagram of another embodiment of a sensor system having a
functionalized surface;
[0036] FIG. 17B is a diagram of the sensor system of FIG. 17A with target
molecules
bound to the functionalized surface;
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
[0037] FIG. 17C is a diagram of the sensor system of FIGS. 17A-17B having
nanoparticles bound to the target molecules;
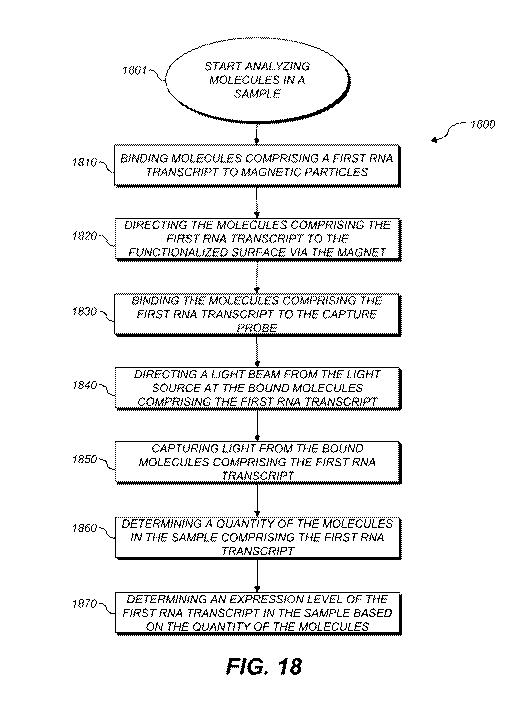
[0038] FIG. 18 depicts a method for analyzing a plurality of RNA transcripts
in a sample;
[0039] FIG. 19 depicts a method for determining a risk level of a
neurodegenerative
disease by analyzing a plurality of RNA transcripts of a sample; and
[0040] FIG. 20 depicts another working example.
[0041] Corresponding reference characters indicate corresponding parts
throughout
several views. The examples set out herein illustrate several embodiments, but
should
not be construed as limiting in scope in any manner.
DETAILED DESCRIPTION
[0042] A disposable cartridge is described for use in a portable/automated
assay system
such as that described in commonly-owned, U.S. Patent Application Ser. No.
15/157,584
filed May 18, 2016 entitled "Method and System for Sample Preparation" which
is
hereby included by reference in its entirety. While the principal utility for
the disposable
cartridge includes DNA testing, the disposable cartridge may be used to detect
any of a
variety of diseases which may be found in either a blood, food or biological
detecting
hepatitis, autoimmune deficiency syndrome (AIDS/HIV), diabetes, leukemia,
graves,
lupus, multiple myeloma, etc., just naming a small fraction of the various
blood borne
diseases that the portable/automated assay system may be configured to detect.
Food
diagnostic cartridges may be used to detect salmonella, e-coli, staphylococcus
aureus or
dysentery. Diagnostic cartridges may also be used to test samples from insects
and
specimen. For example, blood diagnostic cartridges may be dedicated cartridges
useful
for animals to detect diseases such as malaria, encephalitis and the west Nile
virus, to
name but a few.
[0043] More specifically, and referring to FIGS. 1 and 2, a portable assay
system 10
receives any one of a variety of disposable assay cartridges 20, each
selectively
configured for detecting a particular attribute of a fluid sample, each
attribute potentially
providing a marker for a blood, food or biological (animal borne) disease. The
portable
assay system 10 includes one or more linear and rotary actuators operative to
move fluids
6
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
into, and out of, various compartments or chambers of the disposable assay
cartridge 20
for the purpose of identifying or detecting a fluid attribute. More
specifically, the
cartridge 20 includes a flow cell 21 extending horizontally therefrom. A
rotary actuator
(not shown) of the portable assay system 10 aligns one of a variety of ports
18P, disposed
about a cylindrical rotor 18, with a syringe barrel 22B of a stationary
cartridge body 22.
The linear actuator 24 displaces a plunger shaft 26 so as to develop pressure
i.e., positive
or negative (vacuum) in the syringe barrel 22. That is, the plunger shaft 26
displaces an
elastomer plunger 28 within the syringe 22 to move and or admix fluids
contained in one
or more of the chambers 30, 32. In addition, system 10 includes one or more
processors
11 housed within a control board 12 of the body of the system 10 for receiving
signals
from the various components of the system 10.
[0044] The disposable cartridge 20 provides an automated process for preparing
the fluid
sample for analysis and/or performing the fluid sample analysis. The sample
preparation
process allows for disruption of cells, sizing of DNA and RNA, and
concentration/clean-
up of the material for analysis. More specifically, the sample preparation
process of the
instant disclosure prepares fragments of DNA and RNA in a size range of
between about
100 and 10,000 base pairs. The chambers can be used to deliver the reagents
necessary
for end-repair and kinase treatment. Enzymes may be stored dry and rehydrated
in the
disposable cartridge 20, or added to the disposable cartridge 20, just prior
to use. The
implementation of a rotary actuator allows for a single plunger 26, 28 to draw
and
dispense fluid samples without the need for a complex system of valves to open
and close
at various times. This greatly reduces potential for leaks and failure of the
device
compared to conventional systems. Finally, it will also be appreciated that
the system
greatly diminishes the potential for human error.
[0045] In FIGS. 3 and 4, the cylindrical rotor 18 includes a central chamber
30 and a
plurality of assay chambers 32, 34 surrounded, and separated by, one or more
radial or
circumferential walls. In the described embodiment, the central chamber 30
receives the
fluid sample while the surrounding chambers 32, 34 contain a premeasured assay
chemical or reagent for the purpose of detecting an attribute of the fluid
sample. The
7
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
chemical or reagents may be initially dry and rehydrated immediately prior to
conducting
a test. Some of the chambers 32, 34 may be open to allow the introduction of
an assay
chemical while an assay procedure is underway or in-process. The chambers 30,
32, 34
are disposed in fluid communication, i.e., from one of the ports 18P to one of
the
chambers 30, 32, 34, by channels 40, 42 molded along a bottom panel 44, i.e.,
along
underside surface of the rotor 18. For example, a first port 18P,
corresponding to
aperture 42, may be in fluid communication with the central chamber 30, via
aperture 50.
[0046] FIG. 5 illustrates an embodiment of a sensor system 70. The sensor
system 70
includes an imager 72 configured to capture still images, video, or a
combination thereof
For example, the imager 72 can be configured to capture high resolution still
images. In
the illustrated embodiment, the imager 72 includes a pixel array 74 and array
circuitry 76.
The pixel array 74 can include any suitable number of pixels. For example, the
pixel
array 74 can be a high density array including at least six (6) megapixels. In
a further
example, the camera can have a large field of view. The pixel array 74 is a
light sensitive
pixel array, such as an active array, passive array, planar Fourier capture
array, angle
sensitive array, photodiode array, a charge coupled device, a complementary
metal-oxide
semiconductor (CMOS), or a charge injection device.
[0047] The sensor system 70 also includes a flow cell 78. The flow cell 78 can
be
formed of any suitable material, such as a polypropylene or polystyrene
polymer or glass,
among others. In an embodiment, the flow cell is formed by injection molding.
The flow
cell 78 includes a transparent or optically clear surface 80 and a transparent
functionalized surface 82. The functionalized surface 82 includes a plurality
of capture
probes 84 in the form of a functionalized oxide surface allowing attachment
and
immobilization of capture probe molecules 84 on the surface 82. The capture
probes 84
are designed to capture or bind target molecules 86 (FIG. 7) by interaction
between
complementary sequences. The target molecules 86 can be collected from a
biological
sample. The biological sample could be any suitable type of materials, such as
blood,
mucous, and skin, among others. For example, the target molecules 86 can be
protein
ligands or DNA segments.
8
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
[0048] An objective or lens 75 can optionally be positioned between the imager
72 and
the flow cell 78. A magnet 88 can be positioned opposite the functionalized
surface 82.
The magnet 88 can be a single magnet or an array of magnets.
[0049] In one embodiment, the functionalized surface 82 includes an array of
many
different capture probes 84, and each capture probe 84 is configured to
capture molecules
having different RNA transcripts. Thus, the array of probes 84 can be deployed
to
capture, say, between 10-20 different RNA transcripts. In another example,
multiple
probes 84 may be configured to capture molecules having the same RNA
transcripts, so
that there is redundancy to allow for error checking of the results. Thus, an
array of
capture probes 84 can be designed to capture many different RNA transcripts to
analyze
the relative proportion of molecules in the sample that are expressing those
RNA
transcripts. In another example, one or more of the array of probes 84 can be
configured
to capture so-called housekeeping genes, which can serve as a baseline by
which to
normalize the number of other molecules found on other genes. For instance, if
400
molecules of the housekeeping gene are captured, then the number of a
different captured
molecule can be compared to 400 to give an absolute sense of how many of those
molecules are captured as a percentage compared to the housekeeping genes,
thus
establishing an absolute scale.
[0050] FIG. 6 illustrates an embodiment of a method 90 for detection of a
target
molecule. The method 90 can be employed by a sensor system, such as the sensor
system
70. At block 92, target molecules 86 are bound to magnetic particles 110, as
illustrated in
FIG. 7. In an embodiment, the target molecules 86 are bound to the magnetic
particles
110 before being introduced to the flow cell 78. In another embodiment, the
target
molecules 86 and magnetic particles 110 are introduced to the flow cell 78 in
an unbound
state and the target molecules 86 bind to the magnetic particles 110 within
the flow cell
78.
[0051] FIGS. 8A-8B illustrate two embodiments of magnetic particles 110. As
illustrated in FIG. 8A, in one embodiment the magnetic particle 110A is a
composite
particle that has a magnetic core 112, formed of a magnetic material such as
iron, and a
9
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
nanoparticle coating 114. For example, the coating 114 can be a gold coating.
The
coating 114 can be configured to act as a nucleation site for further
nanoparticle
development. The magnetic particle 110A includes at least one binding site for
a ligand
A for binding to the target molecules 86. A chemical reactive group such as a
thiol,
amine, or aldehyde, can mediate or facilitate ligand binding.
[0052] As illustrated in FIG. 8B, in another embodiment, the magnetic particle
110B can
have a magnetic body 116 formed of a magnetic material, such as iron. The
magnetic
particle 110B includes at least one binding site or ligand A for binding to a
target
molecule. In the illustrated embodiment, the magnetic particle 110B further
includes at
least one binding site or ligand B for binding a magnetic nanoparticle. It is
to be
understood that the magnetic particle can include any suitable combination of
binding
sites A, B. For example, the magnetic particle can include both types of
binding sites A,
B or the magnetic particle can include only target molecule binding sites A.
[0053] As illustrated in FIG. 8C, rather than a nanoparticle coating over a
magnetic core,
the magnetic particle 110 can include a magnetic body 112 and a plurality of
nanoparticles 122 bound to the magnetic body 112. Alternatively, as
illustrated by FIG.
8D, the magnetic particle 110 can be an alloy, such as a heterogeneous alloy,
including a
plurality of magnetic bodies 112 bound with a plurality of nanoparticles 122.
[0054] As illustrated in FIGS. 8E-8G, the target molecule 86, magnetic
particle 110, and
nanoparticle 122 can be bound in a variety of arrangements. As illustrated in
FIG. 8E,
the magnetic particle 110 and nanoparticle 122 can each be bound directly to
the target
molecule 86. Alternatively and as illustrated in FIG. 8F, the magnetic
particle 110 can be
bound directly to the target molecule 86 and one or more nanoparticles 122 can
be bound
to the magnetic particle 110. Alternatively and as illustrated in FIG. 8G, a
nanoparticle
122 can be bound directly to the target molecule 86 and one or more magnetic
particles
110 can be bound to the nanoparticle 122.
[0055] Returning to FIG. 6, at block 94, the bound magnetic particles 110 and
target
molecules 86 are directed or moved to the functionalized surface 82. Referring
to FIG. 7,
the magnet 88 is coupled to an actuator (not shown) configured to move the
magnet 88
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
toward (retracted) and away from (extended) the functionalized surface 82. As
the
magnet 88 is moved away 118 from the functionalized surface 82, the magnetic
particles
110, attracted to the magnet 88, move 120 toward the functionalized surface
82. As the
target molecules 86 are bound to the magnetic particles 110, the target
molecules 86 are
directed or drawn by the magnetic particles 110 toward the functionalized
surface 82.
[0056] Returning to FIG. 6, at block 96, the target molecules 86 are bound to
the capture
probes 84 of the functionalized surface 82 as illustrated in FIG. 9. In the
illustrated
embodiment, the magnetic particles 110 remain bound to the bound target
molecules 86.
Alternatively, the target molecules 86 can be denatured to unbind the magnetic
particles
110 from the target molecules 86 when the target molecules 86 reach the
functionalized
surface 82, following which the target molecules 86 can bind to the
functionalized
surface 82.
[0057] Returning to FIG. 6, at block 98, functionalized nanoparticles 122 are
introduced
to the flow cell 78 and are bound to the target molecules 86, as illustrated
in FIG. 10. In
an embodiment, the functionalized nanoparticles 122 are bound directly to the
target
molecules 86. Alternatively, the functionalized nanoparticles 122 are bound to
the
magnetic particles 110 bound to each target molecules 86. In an embodiment, a
plurality
of functionalized nanoparticles 122 are bound to each target molecule 86. Any
suitable
method of hybridizing or binding the nanoparticles 122 to the target molecules
86 can be
used. In an embodiment, the functionalized nanoparticle 122 is a gold
particle. In
another embodiment, the functionalized nanoparticle 122 is a catalytic
nanoparticle, such
as a gold catalyst reagent. In an embodiment, the nanoparticles 122 are in the
form of
catalyst clusters.
[0058] In the illustrated embodiment, the nanoparticles 122 are bound to the
target
molecules 86 after the target molecules 86 are bound to the functionalized
surface 82. In
an alternative embodiment, the nanoparticles 122 can be bound to the target
molecules 86
or magnetic particles 110 prior to binding of the target molecules 86 to the
functionalized
surface 82.
11
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
[0059] Following binding of the nanoparticles 122 to the target molecules 86,
an optional
metallization step can be performed to metallize the nanoparticles 122 and
develop or
form enlarged nanoparticles or even a film. The developed nanoparticles can
improve
detection of the target molecules. In this metallization step, the
nanoparticles 122 serve
as nucleation sites for development of enlarged nanoparticles 124.
[0060] Returning to FIG. 6, at block 100, a light source 126 directs a light
beam 128 at
the target molecules 86 and functionalized nanoparticles 122, 124 in the flow
cell 78.
The light beam 128 is aimed so that light is directed solely at the target
molecules 86 and
nanoparticles 122, 124 and no light 128 from the light source 126 is captured
by the
imager 72. In an embodiment, the flow cell 78 is configured to prevent
diffusion of the
light beam 128 toward the imager 72.
[0061] Referring to FIG. 6, at block 102, light 130 (FIG. 11) from the
nanoparticles 122,
124, target molecules 86, magnetic particles 110, or a combination thereof, is
captured by
the imager 72. In an embodiment, the light 130 can be reflected or emitted
from the
particles 86, 110, 122, 124, or a combination thereof. At block 104, the light
130
captured by the imager 72 is analyzed to detect the number of target molecules
86
present. For example, the captured light 130 can be analyzed using dark field
microscopy. In this embodiment, the spots of detected light are counted and
quantified to
determine the number of target molecules 86 present. Counting and quantifying
is
accomplished using the one or more processors 11 (see FIG. 1).
[0062] In one embodiment, a single imager 72 and light beam 128 may be used as
described above to sequentially examine each of the capture probes 84. Such
sequential
analysis may be achieved by providing, for example, for translation of the
imager 72 and
light beam 128 across the flow cell to allow each capture probe 84 to be
examined one at
a time. In another embodiment, adjustable mirrors can be used to direct the
light from the
light beam 128 to one of the capture probes 84 and the imager 72 one at a time
by
adjusting the mirrors. In a further embodiment, an imager with a larger
imaging
capability can be fixed with the light beam 128 moving to each capture probe
84 one at a
12
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
time. In another embodiment, multiple imagers 72 can be used, with each one
dedicated
to one or several capture probes 84.
[0063] FIGS. 12A-13 illustrate embodiments of light or scattering signatures
or captured
images of gold nanoparticles 122 captured under dark field microscopy. FIG.
12A is a
scattering signature of 50 nm gold nanoparticles and FIG. 12B is a scattering
signature of
100 nm gold nanoparticles. FIG. 13 is an image comparing the scattering
signature 132
of undeveloped (20nm) nanoparticles 122 with the scattering signature 134 of
developed
(100nm) nanoparticles 124. In an embodiment, a series of dark field images can
be
captured. In this embodiment, a first image can be captured prior to
development of the
nanoparticles 122 and at least one additional image can be captured as the
nanoparticles
are developed. Alternatively, a first image can be captured prior to binding
of the target
molecules 86 and at least one additional image can be captured following
binding of the
nanoparticles 122. The captured images can be compared to removed background
artifacts and improve analysis of the dark field images.
[0064] In an alternative embodiment, a dye particle (not shown) is coupled to
the target
molecules 86 for detection of the target molecules 86. In this embodiment, the
light
source 128 is tuned to the wavelength of the dye and regions covered by the
dye will
fluoresce. The fluoresce is detected by the imager 72.
[0065] In another alternative embodiment, to detect the presence of the target
molecules
86, following binding of the target molecules 86 and nanoparticles 122, the
functionalized surface is exposed to a radiation source (not shown). Upon
exposure to
the radiation source, the regions of nanoparticles preferentially absorb the
radiation,
causing localized heating. The localized heating is captured and registered by
the imager
72 to detect the presence of the target molecules 86. Based on the amount of
heating
registered, a count of the number of target molecules present is established.
The system
may be calibrated by allowing a known number of target molecules to be heated,
and
measuring the temperature, for example.
[0066] An example of an optical sensor system 140 is illustrated in FIG. 14.
Similar to
the optical sensor system 70 described above, the optical sensor system 140
includes an
13
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
imager 142 and an objective or lens 144 coupled to the imager 142. In an
embodiment,
the imager 142 is a high resolution imager having a wide angle or large field
of view.
The objective 144 is directed toward the flow cell 146. As discussed above,
the interior
of the flow cell 146 includes a functionalized surface for binding target
molecules. One
or more feeder lines 147 can be coupled to the flow cell 146 to facilitate the
introduction
of various particles to the flow cell 146.
[0067] A light source 148 is directed at the flow cell 146. The light source
148 can be
any suitable light source. For example, the light source 148 can provide light
at a
predetermined frequency. For example, the light source 148 can be a white
light. The
light source 148 is directed or aimed solely at the flow cell 146. In the
illustrated
embodiment, the light source 148 is directed orthogonally to the axis X on
which the
objective is positioned. The flow cell 146 is configured to channel the light
from the
light source 148 toward the particles within the flow cell 146, rather than
toward the
imager 142 and to prevent light diffusion from the light source 148 to the
imager 142.
[0068] A magnet 150 is positioned opposite the flow cell 146 from the
objective 144. An
actuator 152, such as a solenoid, is coupled to the magnet 150 and is
configured to move
the magnet. As illustrated in FIGS. 15A-15B, in an embodiment, the actuator
152 is
configured to retract or move the magnet 150 toward (FIG. 15A) the flow cell
146 and to
extend or move the magnet 150 away (FIG. 15B) from the flow cell 146.
[0069] FIG. 16 illustrates an embodiment of an analysis system 160 including
an optical
system, such as the optical sensor systems 70, 140. In this embodiment, the
analysis
system 160 includes a base 162 and a head 164. The imager 142 and objective
144 are
positioned in the base 162. The flow cell 146 is positioned on the top surface
of the base
162, aligned with the objective 144. The magnet 150 is positioned in the head
164 and is
configured to extend to and engage with the flow cell 146.
[0070] In the illustrated embodiment, in order to minimize the footprint of
the analysis
system 160, the imager 142 and objective 144 are not aligned along an axis, as
illustrated
in FIG. 14. Rather, the imager 142 is aligned along an axis Y extending
longitudinally
through the base 162 between the side surfaces 166, 167 of the base 162. The
objective
14
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
144 is positioned orthogonal to the axis Y and extends upward through the base
162. A
mirror 168 is positioned below the objective 144 and angled toward the imager
142 to
create an optical path 170 between the objective 144 and the imager 142.
[0071] FIGS. 17A-17C illustrate an alternative embodiment of a sensor system
180. In
this embodiment, The sensor system 180 includes a prism type substrate 182
having a
Kretshmann configuration. The substrate 182 has a surface 184 coated with a
metal film
186 suitable for surface plasmon resonance or Raman scattering. For example,
the metal
film can be gold, silver, copper, titanium, or chromium. The film 186 is
functionalized
with a bio-specific coating to include capture probes 84. A light source 188
directs a
light beam 190 through the prism substrate 182 toward the film 186 and a
detector 192
captures light 194 from the film 186, such as light reflected or emitted by
the film 186.
[0072] In operation, a baseline measurement of the captured light is taken. In
an
embodiment, the baseline measurement of the captured light is used to
calibrate the
absorbance angle (FIG. 17A). In addition, the baseline measurement can be sued
to
identify contaminants or debris on the sensor prior to binding of the target
molecules 86
or prior to development of increased nanoparticle size, as discussed below.
Following
the baseline measurement, a sample containing target molecules 86 is
introduced to the
sensor system 180 and the target molecules 86 bind to the capture probes 84
(FIG. 17B).
In an embodiment, the target molecules 86 can be directed to the surface film
186 via
magnetic particles as described above. Functionalized nanoparticles 122 are
introduced
to the system 180 and allowed to bind to the target molecules 86 (FIG. 17C). A
plating
bath can optionally be used to increase the size of the bound nanoparticles
122. To detect
the presence of the target molecules 86, the beam 190 is directed toward the
film 186 and
the light 194 from the film 186, such as reflected or emitted, is captured.
Any difference
in reflectivity or intensity between the baseline measurement and the final
measurement
is observed in order to detect the presence of the target molecules 86. A
quantitative
count of target molecules 86 may be established by comparison with calibrated
tests of
the reflectivity or intensity for known number of target molecules. In an
embodiment, the
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
baseline measurement can be used to subtract particles identified as debris
from the final
measurement.
[0073] FIG. 18 depicts a method 1800 for analyzing a plurality of RNA
transcripts in a
sample.
[0074] By way of background, several technologies have made it possible to
monitor
the expression level of a large number of transcripts within a cell at any one
time (see,
e.g., Schena et al., 1995, Quantitative monitoring of gene expression patterns
with a
complementary DNA micro-array, Science 270:467-470; Lockhart et al.,
1996, Expression monitoring by hybridization to high-density oligonucleotide
arrays,
Nature Biotechnology 14:1675-1680; Blanchard et al., 1996, Sequence to array:
Probing
the genome's secrets, Nature Biotechnology 14, 1649; 1996, U.S. Patent
5,569,588,
issued October 29, 1996 to Ashby et al. entitled "Methods for Drug
Screening").
[0075] Applications of transcript array technology have involved
identification of genes
which are up regulated or down regulated in various diseased states.
Additional uses for
transcript arrays have included the analyses of members of signaling pathways,
and the
identification of targets for various drugs. Transcript arrays can be
beneficial in
monitoring the level of either disease states or effect of therapies.
[0076] RNA profiling is a process useful to monitor disease state or treatment
efficacy by
monitoring the expression levels of key RNA transcripts in a sample. Key RNA
transcripts can be identified using techniques such as an Affymetrix array to
screen all
transcripts in a cell. By profiling multiple patients at different stages of
disease, a set of
key indicator transcripts can be identified and used on an array, such as that
of the current
invention which targets the key indicators. In addition, some housekeeping
genes which
do not vary due to the disease are also monitored to establish a baseline with
which to
compare expression of the key indicators. Statistical methods are used to
determine
which indicators are needed to reliably monitor disease progression.
[0077] Many of these techniques involve large arrays of RNA probes which can
monitor
expression of thousands of genes at a time, such as the Affymetrix array.
Affymetrix
makes quartz chips for analysis of DNA Microarrays called GeneChip arrays.
16
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
Affymetrix's GeneChip arrays assist researchers in quickly scanning for the
presence of
particular genes in a biological sample. Within this area, Affymetrix is
focused on
oligonucleotide microarrays. These microarrays are used to determine which
genes exist
in a sample by detecting specific pieces of mRNA. A single chip can be used to
analyze
thousands of genes in one assay. However, these systems are expensive to run
and their
use can be limited in monitoring disease states and treatment efficacy.
[0078] RNA for analysis in the present techniques can be derived from a
variety of
samples, including but not limited to blood, plasma, leukocytes, other blood
fractions,
sputum, saliva, urine, stool, vaginal swabs, and tissue samples. In various
embodiments,
cartridges have the capability for automated sample disruption and isolation
of RNA.
Advantageously, automation of all sample processing improves reliability of
RNA
isolation without degradation. Furthermore, automation of sample processing
enables
testing to be run outside of traditional laboratories.
[0079] By way of explanation, the method and system described with respect to
FIG. 18
provides a system and methods for determining or monitoring the progression of
disease
states or the efficacy of therapeutic regimens in a subject, preferably a
human patient. In
particular, the technique relates to methods for monitoring disease states or
therapies by
monitoring changes in mRNA expression levels. The current technique utilizes a
simple,
easy to use system which can monitor expression of a number of gene
transcripts for
rapid diagnosis, to enable better treatment. With respect to the present
disclosure,
provided is a system and method for RNA profiling to monitor disease state or
effectiveness of treatment. The identification of changes in gene expression
caused either
by the actions of disease states or by therapeutic regimens, such as drug
regimens, for
disease states is a problem of great commercial and human importance. Most of
the
decisions that need to be made to run efficient clinical trials and to
properly manage the
health of patients rely on assays that monitor changes in cells in the body.
[0080] The system provides an automated and closed system for isolation of RNA
from a
patient sample. RNA can be difficult to handle and is susceptible to
degradation by
RNases. The closed system automates disruption of the sample, cleaning and
17
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
concentrating of the RNA. During isolation the sample is treated with a
guanidine
hydrochloride solution, which lyses the cells and disrupts enzymes, thereby
stabilizing
the RNA. By automating the process in a closed cartridge, the risk of
introducing
RNases into the sample during handling is eliminated.
[0081] The system further provides a multiplexed array to monitor the
expression levels
of multiple RNA transcripts. As described below, the sensor array has multiple
sites
specific for individual transcripts. Capture probes specific to a sequence of
nucleotides in
a transcript are printed at a site. The multiplexed array allows for
quantitation of
expression of key indicator transcripts and monitoring of housekeeping genes
in order to
establish baseline expression. The system will then compare expression of the
key
indicator genes against the housekeeping genes to determine expression
relative to the
baseline. Variance of key gene expression will be analyzed using an algorithm
to
determine disease state.
[0082] Rather than monitoring all transcripts in a cell, which requires
thousands of
sensors, the current invention provides an array to monitor between one and
hundreds of
key transcripts. Additional sensors for one to tens of housekeeping genes
would also be
included. Optimally the number of key transcripts to be monitored would be
between
three and twenty. Keeping the number of sites low will minimize the cost of
the array but
allows for targeted testing of key indicators of a disease.
[0083] The current invention has critical functions needed to capture and
quantitate RNA
expression levels rapidly. In particular, the current invention provides a
magnetic
approach to concentrate and move RNAs to the sensors to allow for rapid
testing without
loss of sensitivity. Furthermore, the current system provides a method to
quantitate the
RNA transcripts at each sensor.
[0084] Returning now to FIG. 18, provided is an explanation of how these
features may
be implemented. For instance, the method 1800 may be performed using a sensor
(e.g.,
sensor system 70 of FIG. 5 or any other example set forth herein) comprising
an imager
(e.g., imager 72 of FIG. 5 or any other example set forth herein), a flow cell
(e.g., flow
cell 78 of FIG. 5 or any other example set forth herein) comprising a
functionalized
18
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
surface (e.g., functionalized surface 78 of FIG. 5 or any other example set
forth herein)
having a plurality of capture probes (e.g., probe molecules 84 of FIG. 5 or
any other
example set forth herein) coupled to the functionalized surface, a magnet
(e.g., magnet 88
of FIG. 5 or any other example set forth herein), and a light source (e.g.,
light source 126
of FIG. 6 or any other example set forth herein). In the embodiment of FIG.
18, the
method starts at block 1801 and includes the following steps:
[0085] Step 1810 ¨ Binding molecules comprising a first RNA transcript to
magnetic
particles.
[0086] Step 1820 ¨ Directing the molecules comprising the first RNA transcript
to the
functionalized surface via the magnet.
[0087] Step 1830 ¨ Binding the molecules comprising the first RNA transcript
to the
capture probe.
[0088] Step 1840 ¨ Directing a light beam from the light source at the bound
molecules
comprising the first RNA transcript.
[0089] Step 1850 ¨ Capturing light from the bound molecules comprising the
first RNA
transcript.
[0090] Step 1860 ¨ Determining a quantity of the molecules in the sample
comprising the
first RNA transcript.
[0091] Step 1870 ¨ Determining an expression level of the first RNA transcript
in the
sample based on the quantity of the molecules.
[0092] In one embodiment, the method 1800 further includes calculating a
disease state
or a treatment efficacy based on the plurality of expression levels of the
plurality of RNA
transcripts. In another embodiment, the plurality of capture probes
corresponds to
between 10 and 20 RNA transcripts. In a further embodiment, the method further
includes monitoring a housekeeping gene for determining a baseline for
comparison of
the plurality of expression levels of the plurality of RNA transcripts.
[0093] In one example, the method further includes receiving and processing
the sample
without external exposure. In another example, the method further includes
binding a
plurality of magnetic particles to the molecules and interacting the magnet
with the
19
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
magnetic particles to direct the molecules to the functionalized surface. In
another
example, the method further includes comprising preventing diffusion of the
light beam
toward the imager. In a further example, the method further includes binding
the
molecule to a nanoparticle when the molecule is bound to the functionalized
surface,
wherein the nanoparticle reflects the light beam toward the imager.
[0094] FIG. 19 depicts a method for determining a risk level of a
neurodegenerative
disease by monitoring expression levels of a plurality of RNA transcripts of a
sample.
[0095] By way of background, there are two problems in the diagnosis of
Alzheimer's
disease that would be addressed by the art proposed in this patent
application: 1)
Accuracy of diagnosis of symptomatic persons and 2) Early detection of disease
in
persons without symptoms.
[0096] Problem 1 is the accuracy of diagnosis. The number of conditions that
can cause
cognitive deficits that may look like Alzheimer's disease is pages long. Some
of these
conditions, such as vitamin B deficiency, can be cured, while others may not
be curable
but might be managed with appropriate intervention. The possibility of cure or
potential
management require an accurate diagnosis. Unfortunately, research has
established that
the accuracy of a diagnosis of Alzheimer's disease is poor. If the diagnosis
is made by a
general practitioner, the probability that the diagnosis is correct is about
50% (Connolly,
A., Gaehl, E., Martin, H., Morris, J., Purandare, N., 2011. Under diagnosis of
dementia in
primary care: variations in the observed prevalence and comparisons to the
expected
prevalence. Aging Ment. Health 15, 978e984.). If the diagnosis is made in one
of the ¨30
federally recognized and funded Alzheimer Centers in the United States, the
accuracy is
about 75% (Beach, T.G., Monsell, S.E., Phillips, L.E., Kukull, W., 2012.
Accuracy of the
clinical diagnosis of Alzheimer disease at National Institute on aging
Alzheimer disease
Centers, 2005-2010. J. Neuropathol. Exp. Neurol. 71, 266e273). The system
proposed
here would increase the accuracy of diagnosis to better than 90%.
[0097] Problem 2 is the early detection of disease. We now know that
Alzheimer's
disease, Parkinson's disease and many other age-related neurodegenerative
diseases start
decades before brain damage reaches the point where it is clinically
detectable. In the
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
case of Parkinson's disease, 80% of the neurons in the substantia nigra, the
region most
affected in this disease, are lost before the disease exhibits symptoms that
lead to a
diagnosis of the disease. Examination for the pathology of Alzheimer's disease
in over
3,000 brains of people who died at ages from 10 to 100 showed early Alzheimer
pathology in 20% of the brains of people who died in their late 20s to early
thirties
(Braak, H., Braak, E., 1997. Frequency of stages of Alzheimer-related lesions
in different
age categories. Neurobiol. Aging 18, 351e357). It is not until 50 years later
that 20% of
people are clinically diagnosed with Alzheimer's disease.
[0098] This "silent period" for many age-related neurodegenerative diseases
creates a
window of opportunity for a two-step process. In Step 1 it would be possible
to detect
disease before significant brain damage has occurred. Step 2 would call for
early
effective treatment to halt or slow disease progression so that affected
persons could live
the rest of their lives free of disease symptoms. Free of the shakes and motor
losses of
Parkinson's disease or free of the memory and cognitive defects of Alzheimer's
disease. This application addresses Step 1, accurate diagnosis and early
diagnosis.
[0099] A variety of methods have been used to show that early diagnosis of
Alzheimer's
disease is real. Positron Emission Tomography (PET) scanning of the brain in
living
people has shown Alzheimer pathology in some people as young as the teens or
20s. Microscopic pathological examination of more than 3,000 brains of people
who
died between the ages of 10 and 100 has shown the start of Alzheimer's in
people in their
20's. And sophisticated cognitive testing has been able to establish
indications of
Alzheimer's 15 years before the disease became clinically detectable (REF).
(Kawas,
C.H., Corrada, M.M., Brookmeyer, R., Morrison, A., Resnick, S.M., Zonderman,
A.B.,
Arenberg, D., 2003. Visual memory predicts Alzheimer's dis- ease more than a
decade
before diagnosis. Neurology 60, 1089e1093.) Studies such as these have been
fundamental in establishing that Alzheimer's disease is present in the brain
for decades
before the brain is damaged to the point of exhibiting frank memory and
cognitive
problems. However, their cost, or the fact that they rely on postmortem
samples, make
21
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
them prohibitive for detection of disease, or probability of future disease,
in the general
population.
[00100] The
present state of the art has several problems. Several technologies
have been used to establish the existence of changes in the brain that precede
a clinical
diagnosis of Alzheimer's disease by many years or decades. Some of these
technologies
require elaborate equipment and expensive professional expertise. PET imaging
is a
prime example of this, with the cost of a PET scan of the order of $5,000.
This is far too
much to be applicable to a population screen, which is what is needed to
detect incipient
Alzheimer's disease.
[00101] Another
technology in current use is analysis of protein in spinal fluid,
which requires the invasive procedure of a spinal tap performed by a
physician. Charges
for this procedure can also be of the order of thousands of dollars.
[00102] A
number of studies have reported that extensive cognitive testing may
reveal early, preclinical, signs of Alzheimer's disease. These studies for the
most part
require extensive time on the part of both patient and tester, also at
significant cost.
[00103]
Recognition of these costs and other drawbacks plus recognition of the
need to improve diagnostic accuracy and detection of pre-clinical disease has
led to a
search for, and descriptions of a blood test for Alzheimer's disease. Some of
these are
directed a detection of a disease that is already apparent. A few also include
an ability to
detect preclinical disease. All currently require that samples be sent to a
central
laboratory for relatively expensive, time consuming procedures. The system
proposed
here can conduct a test for Alzheimer's disease or other neurodegenerative
diseases
quickly and on site for minimal cost.
[00104] We
present here a method for accomplishing the detection of disease or
detection of a probable future diagnosis of disease that is low cost and
minimally
invasive, and, therefore, practical for use on large numbers of persons.
Detection of
early, incipient disease in the members of a population from perhaps age 30
and beyond
requires minimally invasive, inexpensive methods that do not require
professional
personnel. We here present a method that accomplishes these goals. A method
that both
22
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
provides a more accurate diagnosis of persons presenting with the symptoms
that might
signify Alzheimer's disease, and that determines a person's risk for a future
diagnosis of
Alzheimer's disease. The application presented describes methods for obtaining
a blood
sample, for inserting a sample into an apparatus capable of determining levels
of
expression of multiple RNA species which have been determined to be useful in
multivariate analyses to diagnose and predict Alzheimer's disease. The method
described
can provide a diagnosis or probability of a future diagnosis in the field
within about 30
minutes for a cost we estimate to be less than $100. The system presented is
capable of
application to screening of a mass population of people without symptoms but
who may
be at risk of a future diagnosis of Alzheimer's disease.
[00105] Returning now to FIG. 19, the method 1900 may be performed using a
sensor (e.g., sensor system 70 of FIG. 5 or any other example set forth
herein) comprising
an imager (e.g., imager 72 of FIG. 5 or any other example set forth herein), a
flow cell
(e.g., flow cell 78 of FIG. 5 or any other example set forth herein)
comprising a
functionalized surface (e.g., functionalized surface 78 of FIG. 5 or any other
example set
forth herein) having a plurality of capture probes (e.g., probe molecules 84
of FIG. 5 or
any other example set forth herein) coupled to the functionalized surface, a
magnet (e.g.,
magnet 88 of FIG. 5 or any other example set forth herein), and a light source
(e.g., light
source 126 of FIG. 6 or any other example set forth herein).
[00106] In the embodiment of FIG. 19, the method starts at block 1901 and
includes the following steps:
[00107] Step 1910 ¨ Binding molecules in the sample to a magnetic
particle.
[00108] Step 1920 ¨ Directing the molecules to the functionalized surface
using
the magnet.
[00109] Step 1930 ¨ Binding each specific molecule of the molecules to one
of the
plurality of capture probes configured to bind the RNA transcript of the
specific
molecule.
[00110] Step 1940 ¨ Directing a light beam from the light source at bound
molecules bound on each of the plurality of capture probes.
23
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
[00111] Step 1950 ¨ Capturing light from the bound molecules.
[00112] Step 1960 ¨ Determining a quantity of the bound molecules bound on
each
of the plurality of capture probes based on the captured light.
[00113] Step 1970 ¨ Determining a plurality of expression levels
corresponding to
the plurality of RNA transcripts based on the quantity of the bound molecules
bound on
each of the plurality of capture probes configured to bind each of the
plurality of RNA
transcript.
[00114] Step 1980 ¨ Calculating a risk of the neurodegenerative disease
based on
the plurality of expression levels of the plurality of RNA transcripts.
[00115] In one embodiment, the method 1900 further includes calculating
the risk
of a future diagnosis of the neurodegenerative disease. In another embodiment,
the
method 1900 further includes calculating the risk of a present diagnosis of
the
neurodegenerative disease.
[00116] In a further embodiment, the method step 1980 of calculating the
risk
includes using a formula of the form: F = ao + al X + a2X2 + + apXp + e, where
F is
proportional to the risk; p is the number of the plurality of RNA transcripts;
Xp, for n=1
to p, are the expression levels of each of the plurality of RNA transcripts;
an, for n=1 to
p, are discriminant coefficients for each of the plurality of RNA transcripts;
and e is an
error term.
[00117] In a specific embodiment, the plurality of RNA transcripts
comprise eight
DNA transcripts, for n=1 to 8, HSP27, HSP90, GAPDH, FTH, FTL, COX1, COX2, and
TFR, and the discriminant coefficients an for the plurality of RNA transcripts
comprise,
for n=1 to 8, -4.25936, 3.671572, 2.685682, -5.295300, 1.973631, 2.506241,
0.495803,
and -1.392785.
[00118] In one example, the method 1900 further includes binding a
plurality of
magnetic particles to the molecules and interacting the magnet with the
magnetic
particles to direct the molecules to the functionalized surface. In another
example, the
method 1900 further includes preventing diffusion of the light beam toward the
imager.
In a further example, the method 1900 further includes binding the molecule to
a
24
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
nanoparticle when the molecule is bound to the functionalized surface, wherein
the
nanoparticle reflects the light beam toward the imager.
[00119] Further
implementation details are now set forth in a detailed working
example of the present technique.
[00120] By way
of overview, our basic, laboratory research has established the
possibility that quantifying the expression of a number of nucleotide RNA
species in a
sample and then combining these data yields a number that provides an estimate
of a
diagnosis of disease or probability of a future diagnosis of disease. In the
latter case the
measure is of disease biomarkers that are present in sufficient quantity to
distinguish
from absence of disease, but not yet at a level indicating clinically
diagnosable disease.
Using the systems and method set forth herein, in one example, we will
determine the
amount of 10-20 RNA species in each sample The RNA species interrogated will
have
been selected on the basis of prior and ongoing research. The expression level
of each of
these genes will then be multiplied by a number. The number will be based on
prior and
ongoing research and will be different for each RNA species. The multiplier
numbers
will be the same for the same RNA species in all samples. The resulting 10-20
multiplication products will then be combined into a single number by an
algorithm that
will indicate the probability of an Alzheimer's disease diagnosis OR the
probability of a
future diagnosis of Alzheimer's disease.
[00121] Details
of methods for obtaining blood sample: Blood is drawn from a
person for the purpose of determining a diagnosis of or risk of a future
diagnosis of a
neurodegenerative disease such as Alzheimer's disease, Parkinson's disease,
Fronto-
temporal dementia, etc. Blood may be drawn from any vein, usually the median
cubital vein, and drawn into a syringe. Alternately, blood may be obtained
from a finger
stick. If drawn from a finger, discard the first drop, then squeeze finger and
collect blood
onto a Whatman P Card. Let blood dry overnight at room temperature, then store
sample
at -20 C. Isolate RNA from the filter paper using QIAGEN kit of your choice -
we have
used QiAmp and exoRNeasy. If sample is shipped, do so on dry ice. For example,
a
2.5m1 or less blood sample could be from a person from any vein with a syringe
and
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
needle or similar device. Or, less blood (2-3 drops) could be obtained from a
finger stick
with a sterile needle or other device to accomplish the finger stick or blood
source other
than a fmger. If from a "finger stick" the blood would be drawn onto filter
paper or
equivalent. The sample is given an identifying alphanumeric designation that
will follow
the sample through all further processing. RNA is then extracted from the
sample using
one of several methods, that may use trizol, PAXgene tubes, phenol/chloroform,
etc. As
an example, the protocol for blood drawn into a PAXgene tube is as follows.
[00122] PAXgene blood RNA Tube Blood Draw protocol:
[00123] Ensure that the PAXgene Blood RNA Tube is at 18o C to 25o C prior
to
use and properly labeled with patient ID or code.
[00124] If the PAXgene Blood RNA Tube is the only tube to be drawn, blood
should be drawn into a "Discard Tube" prior to drawing blood into the PAXgene
tube.
Otherwise, the PAXgene tube should be the last tube drawn in the phlebotomy
procedure.
[00125] Collect blood into the PAXgene tube per your institution's
recommended
procedure for standard venipuncture technique.
[00126] Hold the PAXgene Blood RNA Tube vertically, below the blood
donor's
arm during blood collection.
[00127] Allow at least 10 seconds for a complete blood draw to take place.
Ensure
that the blood has stopped flowing into the tube before removing the tube from
the
holder.
[00128] After blood collection, gently invert the PAXgene tube 8-10 times.
[00129] Store the PAXgene tube in an upright position.
[00130] Tubes can either be left at room temperature for 2 hours then
placed at -
20o C or they can be placed at -20o C upright in a wire rack immediately after
the blood
draw.
[00131] Storage and Shipping Protocol
[00132] To freeze PAXgene tubes, stand them upright in a wire rack. Do not
freeze tubes upright in a Styrofoam tray as this may cause the tubes to crack.
26
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
[00133] For
longer term storage at -80o C, tubes must be stored at -20o for at least
24 hours before putting in the -80o C freezer.
[00134] To ship
tubes on dry ice, they need to be frozen at -20o C for at least 24
hours prior to putting on dry ice.
[00135] NOTE:
Frozen PAXgene Blood RNA Tubes are subject to breakage upon
impact. To reduce the risk of breakage during shipment, frozen tubes should be
treated in
the same manner as glass tubes. It is suggested they be enclosed with bubble
wrap or
some other kind of treatment for protection. When the PaxGene tube is thawed
at room
temperature for study, the tube must be inverted at least 6 times to
thoroughly mix the
contents. Then blood can be withdrawn from the PaxGene tube and inserted
directly into
a TNT supplied cartridge.
[00136] An
example method for isolating RNA from blood collected into EDTA
tubes is as follows: RNA is extracted from leukocytes using the mRNA Isolation
Kit for
Blood/Bone Marrow (Roche) per manufacturer's protocol. In brief, erythrocytes
are
selectively lysed and collected by centrifugation. The leukocytes are then
lysed and the
total nucleic acids is collected by nonspecific adsorption to magnetic beads
and magnetic
separation. Following a series of washes and elution of the nucleic acids,
mRNA is
captured by biotin-labeled oligo(dT) and streptavidin-coated magnetic
particles. After
removal of other nucleic acids (DNA, rRNA, and tRNA) by washing, mRNA samples
are
collected and stored at ¨80 C. RNA quality and abundance are confirmed by
260/280
ratios and by gel electrophoresis. Messenger RNA is amplified and
radioactively labeled
with 32P CTP. The labeled amplified RNA is hybridized to custom cDNA arrays
and
quantified.
[00137] An
example method for isolating RNA from blood collected into
PAXgene tubes is as follows: Approximately, 2.5 mL of fresh whole blood is
collected
into PAXgene Blood RNA tubes (BD Diagnostics/Qiagen) and inverted 4 times.
Total
RNA is extracted from leukocytes with PAXgene Blood RNA Kit (Qiagen) or
PAXgene
Blood miRNA Kit (Qiagen) per manufacturer's directions. Total RNA is stored at
¨80 C
until later use. RNA integrity is determined by analysis with an Agilent 2100
27
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
Bioanalyzer. The suitability of samples for analysis is based on RNA Integrity
Number,
with the rare sample with a RIN a number less than 2 considered not usable.
[00138] An example method for converting RNA to cDNA is as follows: 1.0
lig of
total RNA is reverse transcribed using the High Capacity cDNA Reverse
Transcription
Kit (Applied Biosystems). The resulting cDNA is diluted 1:5 and used as
template in
qRT-PCR reactions using TaqMan Gene Expression Master Mix (Applied
Biosystems)
and TaqMan Gene Expression Assays (Applied Biosystems) and run on an iCycler
iQTM (Bio-Rad) or equivalent equipment. The quantitative RT-PCR amplifications
are
run in triplicate at thermal cycling conditions of 10 minutes at 95 C, 40
cycles of
denaturation at 95 C for 15 seconds, and annealing and extension at 60 C for 1
minute.
Beta glucuronidase (GUSB) is used as an endogenous control since it has been
found to
be the same from sample to sample. Following normalization, data are then
presented as a
ratio using the 2(-Delta Delta C(T)) method (Livak and Schmittgen, 2001).
[00139] in one embodiment, if blood is obtained from a vein, 2.5m1 is
drawn into a
PaxGene tube or equivalent following typical protocols, and then the blood is
inserted in
a cartridge of the present system for internal preparation.
[00140] In one embodiment, the following RNA species may be used for
calculating risk of neurodegenerative disease. RNA species for study are
selected on the
basis of known mechanisms of neurodegenerative diseases and on the basis of
laboratory
findings in preliminary studies. Some examples are shown in the Table 1.
Table 1
Classes of RNA Examples of class
Inflammation IL-17R, TNF-a, Cl Inhibitor
Cell Stress HSP27, COX2, Alphal -ACT
Epigenetics HDAC2, DNMT3a, DNM1,
Cell Cycle PCNA, cdc2/cyclin Bl, cyclin D, cdk4
Nuclear Transport NUPL2, NUP155, RAN
Protein Folding IRE1A, BIP, HSC70
28
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
Mitochondria caspasins, COX5a, ATP5B
Cell Death mTOR, p53,
RAB system RAB1, RAB3a, RAB5, RAB 6a, RAB7
[00141] The
weights assigned to each independent variable are corrected for the
interrelationships among all the variables. The weights are referred to as
discriminant
coefficients. The present apparatus quantifies amount of RNA in a sample
accurately
detect the presence and amount of each of any number of specific RNA or DNA
species.
In the example provided, 8 specific RNA species are specified, but the
identity, specific
species and their number may be different. This information about amount of
each
selected RNA or DNA specie in the sample may be transmitted to an external
device such
as a cell phone or other mobile computing device. Or, the value may be
transmitted to a
computing circuit within the same device. The amount detected by the sensor
elements
for each specie is multiplied in the computing device by a weight that is
unique to that
RNA or DNA specie. The weight may be referred to as the standardized
discriminant
coefficient. (Abbreviations: COX1, prostaglandin-endoperoxide synthase 1;
COX2,
prostaglandin-endoperoxide synthase 2; FTH, ferritin, heavy polypeptide; FTL,
ferritin,
light polypeptide; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HSP27,
heat
shock 27-kDa protein1; HSP90, heat shock protein 90 kDa, class Bl; TFR,
transferrin
receptor).
[00142] In this
example, this multiplication results in 8 values representing the
product of amount of nucleotide specie in the sample multiplied by a weight.
The
weights may be a product of prior investigation or they may be a result of
entering into
the system a method for determining weights on the basis of known identity of
samples
submitted to the system. In the example provided these 8 products of amount of
nucleotide multiplied by the weight for each nucleotide are combined by any
algorithm
that results in a single number that represents a characterization of the
sample entered
into the system. This number then represents the probability for the detection
of a
currently diagnosed or probable future diagnosis of Alzheimer's disease or
some other
29
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
neurodegenerative disease, such as Parkinson's disease or amyotrophic lateral
stenosis.
The resulting number may represent either a diagnosis of a neurodegenerative
disease or
a probability of a clinical diagnosis of that disease at some time in the
future. This
information can be presented to any device such as a display in the equipment
itself, a
local or remote computer or it may wirelessly report data to a secure central
site. In the
latter case these data can be combined with data from multiple sites to
evaluate potential
relationships among disease, geography, socio-economic status, etc.
[00143] The
purpose of discriminant analysis is to obtain a model to predict a
single qualitative variable from one or more independent variable(s).
Discriminant
analysis derives an equation as a combination of the independent variables
that will
discriminate best between the groups in the dependent variable. This
combination is
known as the discriminant function. The weights assigned to each independent
variable
are corrected for the interrelationships among all the variables. The weights
are referred
to as discriminant coefficients.
[00144] The discriminant equation is: F = ao + aiXi + a2X2 + + apXp + e,
[00145] where F
is formed by the linear combination of the dependent variable, Xi,
X2 , ; are the p independent variables, e is the error term and ao, al , a2
ap are the
discriminant coefficients.
[00146]
Standardized Discriminant Coefficients are calculated for each of the 8
transcripts, as set forth in Table 2.
Table 2
RNA specie Weight for that RNA
HSP27 -4.25936
11SP90 3.671572
GAPDH 2.685682
FTH -5.295300
FTL 1.973631
COX1 2.506241
COX2 0.495803
TFR -1.392785
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
[00147] By
comparison, an example of quantifying amount of specific RNA
species in a sample with cDNA or nucleotide arrays is as follows: The cDNA
clones
represented in arrays emphasized those that would test the hypothesis that
transcripts
related to stress, inflammation, and cell cycle would be affected in
leukocytes from AD
cases. 172 cDNAs selected for this purpose were printed on nylon membranes
using a
96-pin replicator (Nalge Nunc) with each cDNA spotted in quadruplicate. Arrays
are
probed with labeled amplified RNA generated from extracted RNA from leukocyte
samples and then exposed to a storage phosphor screen. Hybridization intensity
of each
spot is quantified by laser densitometric scanning (PhosphorImager, Molecular
Dynamics). As a control, the amount of cDNA deposited on each spot in the
array is
quantified by stripping and reprobing the membrane with an oligonucleotide
specific for
the T7 promoter present in all vectors. Any relevant aspects of this example
may be
combined with other examples set forth herein.
[00148] By
comparison, an example of quantifying amount of specific RNA
species in a sample by quantitative reverse transcriptase polymerase chain
reaction
(qPCR) is as follows: The qRT-PCR may be performed using TaqMan Gene
Expression
Assays (Applied Biosystems, Foster City, CA, USA). In brief, for each sample,
3.0 11,g
total RNA is reverse transcribed using a High Capacity cDNA Reverse
Transcription Kit
(Applied Biosystems). 2 L of a 1:5 dilution of cDNA is combined with TaqMan
Universal PCR Master Mix No AmpErase UNG (Applied Biosystems) and the TaqMan
Gene Expression Assay in a 10-AL reaction set-up by the CAS-1200 liquid
handling
system. The qRT-PCR amplifications may be on an ABI PRISM 7900 HT Sequence
Detection System (Applied Biosystems). Universal thermal cycling conditions
are: 10
minutes at 95 C, 40 cycles of denaturation at 95 C for 15 seconds, and
annealing and
extension at 60 C for 1 minute. Amplification efficiencies is close to 100%
for all assays
according to analyses of different dilutions of the cDNA. Beta glucuronidase
(GUSB) is
used as an endogenous control since its expression is found to be invariant
across all
samples. Following data normalization, data may be then presented as a ratio
using the
31
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
2(-Delta C(T)) method (Livak and Schmittgen, 2001). Any relevant aspects of
this
example may be combined with other examples set forth herein.
[00149] An example result of analysis is set forth in Table 3.
Table 3
Sample Status DCT Std. Dev. Ratio COX1 Fold
COX1/GUSB Change
COX1 - GUSB DCT Relative to
Calibrator
514-1 ND 1.254108400 0.078280830 0.419252590
740-1 Calibrator 1.695213300 0.070636550 0.308808999
HA01-1 0.993282300 0.057280650 0.502333606
HA02-1 0.442682270 0.078432950 0.735765395
578 -0.153747560 0.054353930 1.112455448
703-2 -0.057216644 0.048405107 1.040456496
741-1 0.768585200 0.044472140 0.586992836
1011 0.987758640 0.104300660 0.504260582
0.651290744 1.00 1.00
561-1 AD 1.207212400 0.038268127 0.433104660
0.66 -1.50
712-1 Experi- 1.099462500 0.073005304 0.466690337
0.72 -1.40
mental
765-1 0.940439200 0.059617640 0.521074226
0.80 -1.25
775-1 1.561384200 0.048603410 0.338825838
0.52 -1.92
661-1 0.901712400 0.016683030 0.535251040
0.82 -1.22
698-2 0.969631200 0.078005100 0.510636582
0.78 -1.28
772-3 0.473443980 0.100681660 0.720243190
1.11 1.11
811-2 0.240423200 0.014296981 0.846496965
1.30 1.30
[00150] The table above shows the results of a quantitative PCR analysis
of COX1
(upper left corner) of 8 non-demented (ND) and 8 Alzheimer cases (AD). Each
number
under the column "Sample" is the de-identified number given to each case that
follows
that case through all analyses. The column "Status" indicates whether cases
were ND or
AD. The column labelled COX1-GUSB is the qPCR result of the strength of signal
from
qPCR of COX1 for that case in mathematical relation to the invariant signal
from the
qPCR signal for the enzyme beta glucuronidase (GUSB) which is used as an
endogenous
32
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
control since its expression is invariant across all samples. The next column
labelled "std
Dev" is the standard deviation of each measure in the preceding column, and is
based on
the fact that each measure is made in triplicate. The column labelled "Ratio
COX1/GUSB" is a ratio mathematical expression of the ratio between expression
of
COX1 and GUSB for each case. The column "COX1 relative to calibrator" is
arbitrarily
set at 1 (as is the column labelled "fold change") for purposes of comparisons
with the
values from AD cases.
[00151] The
columns for the AD (Alzheimer disease) cases are similar with the
exceptions of the columns labelled labeled "COX1 relative to calibrator" and
"fold
change" which are now expressions of the relationship between the average
value for the
ND (non-demented) data and the AD (Alzheimer's disease) data. Appendix I
contains all
the corresponding tables for each RNA specie in this demonstration. These data
are then
multiplied by the weights determined for each RNA species.
[00152]
[00153] FIG. 20
depicts another working example, for determining risk of a future
diagnosis of Alzheimer's disease by virtue of being APOE4++ homozygotes. In
this
example we are concerned with the risk of a future diagnosis of AD in persons
who are
presently cognitively intact. Having two copies of the APOE4 gene (one from
each
parent - APOE4++) constitutes significant risk for a future diagnosis of
Alzheimer's
disease. The below bar graph showing our blood test scores for people all of
whom are
cognitively intact. The scores of people who are at increased risk of a future
diagnosis of
Alzheimer's disease by virtue of having two copies of APOE4 (APOE4++ ND) are
clearly separated from the scores of people who do not have the APOE4 gene
variant and
are not at increased risk of a future diagnosis of Alzheimer's disease
[00154] In
summary, the discriminant number resulting from our analysis resulting
may represent either a diagnosis of a neurodegenerative disease in a
symptomatic person
or in the case of a normal control person who is cognitively intact it can
represent the
probability of a clinical diagnosis of that disease at some time in the
future. This
information can be presented to any device such as a display in the equipment
itself, a
33
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
local or remote computer or it may wirelessly report data to a secure central
site. In the
latter case these data can be combined with data from multiple sites to
evaluate potential
relationships among disease, geography, socio-economic status, etc.
[00155]
Possible advantages of the above described method include improved
sensitivity of target molecule detection and improved detection of small
quantities of
target molecules. In addition, the above described method includes increased
speed in
detection of target molecules. For example, the above described method can
permit
detection of target molecules without initial replication of the target
molecules, such as in
a PCR process.
[00156] While
the present invention has been particularly shown and described
with reference to certain exemplary embodiments, it will be understood by one
skilled in
the art that various changes in detail may be effected therein without
departing from the
spirit and scope of the invention that can be supported by the written
description and
drawings. Further, where exemplary embodiments are described with reference to
a
certain number of elements it will be understood that the exemplary
embodiments can be
practiced utilizing either less than or more than the certain number of
elements.
[00157] The
patentable scope of the invention is defined by the claims, and may
include other examples that occur to those skilled in the art. Such other
examples are
intended to be within the scope of the claims if they have structural elements
that do not
differ from the literal language of the claims, or if they include equivalent
structural
elements with insubstantial differences from the literal language of the
claims.
[00158] To the
extent that the claims recite the phrase "at least one of' in reference
to a plurality of elements, this recitation is intended to mean at least one
or more of the
listed elements, and is not limited to at least one of each element. For
example, "at least
one of an element A, element B, and element C," is intended to indicate
element A alone,
or element B alone, or element C alone, or any combination thereof. "At least
one of
element A, element B, and element C" is not intended to be limited to at least
one of an
element A, at least one of an element B, and at least one of an element C.
34
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
PARTS LIST
A target molecule binding site
nanoparticle binding site
X axis
axis
portable assay system
18 rotor
18P port
disposable assay cartridge
21 flow cell
22 cartridge body
22B syringe barrel
24 linear actuator
26 plunger shaft
28 elastomeric plunger
central chamber
32 assay chamber
34 assay chamber
channel
42 channel
44 bottom panel
aperture
70 sensor system
72 imager
74 pixel array
75 lens/objective
76 array circuitry
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
78 flow cell
80 surface
82 functionalized surface
84 capture probes
86 target molecules
88 magnet
90 method
92-104 method steps
110 magnetic particles
110A magnetic particle
110B magnetic particle
112 magnetic core
114 nanoparticle coating
116 magnetic body
118 movement
120 movement
122 nanoparticles
124 enlarged nanoparticles
126 light source
128 light beam
130 light
132 scattering signature (image)
134 scattering signature (image)
140 optical sensor system
36
CA 03139860 2021-11-09
WO 2020/251707
PCT/US2020/032091
142 imager
144 objective/lens
146 flow cell
147 feeder line
148 light source
150 magnet
152 actuator
160 analysis system
162 base
164 head
166 side surface
167 side surface
168 mirror
170 optical path
180 sensor system
182 prism substrate
184 surface
186 film
188 light source
190 light beam
192 detector
194 light
37