Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 2021/071980
PCT/US2020/054625
METHODS AND COMPOSITIONS FOR TREATMENT OF CONCRETE
RECLAIMED WATER
CROSS-REFERENCE
[0001] This application is related to_PCT Application
No. PCT/CA2018/050750, filed
June 20, 2018, PCT Application No. PCT/CA2017/050445, filed April 11, 2017,
U.S.
Provisional Patent Application No. 62/321,013, filed April 11, 2016, U.S.
Provisional Patent
Application No. 62/522,510 filed June 20, 2017, U.S. Provisional Patent
Application No.
62/554,830 filed September 6, 2017, U.S. Provisional Patent Application No.
62/558,173
filed September 13, 2017, U.S. Provisional Patent Application No. 62/559,771
filed
September 18, 2017, U.S. Provisional Patent Application No. 62/560,311 filed
September 19,
2017, U.S. Provisional Patent Application No. 62/570,452 filed October 10,
2017, U.S.
Provisional Patent Application No. 62/675,615 filed May 23, 2018, U.S.
Provisional Patent
Application No. 62/652,385 filed April 4, 2018, and to U.S. Provisional Patent
Application
No. 62/573,109 filed October 16, 2017 all of which are incorporated herein by
reference in
their entirety. This application also claims priority to U.S. Provisional
Patent Application No.
62/911,871 filed October 7, 2019, which is incorporated herein by reference in
its entirety.
BACKGROUND OF THE INVENTION
[0002] Wash water, produced in the making of concrete,
poses a significant problem in
terms of use and/or disposal. Methods and compositions to better manage
concrete wash
water are needed.
SUMMARY OF THE INVENTION
[0003] In one aspect, provided herein are compositions.
[0004] In certain embodiments, provided herein is an apparatus for
introducing a gas into
concrete reclaimed water comprising (i) a first conduit operably connected to
a source of
concrete reclaimed water at a proximal end of the first conduit, wherein the
first conduit
allows the reclaimed water to flow through it from the proximal end and out of
it at a distal
end; and (ii) a second conduit situated inside the first conduit, wherein the
second conduit is
operably connected to a source of a gas and is configured to allow the gas to
flow into it and
to flow out of it into the reclaimed water in the first conduit. In certain
embodiments, the gas
comprises carbon dioxide. In certain embodiments, the diameter of the first
conduit is 0.5-5
inches and the diameter of the second conduit is 0.3-3 inches. In certain
embodiments, the
-1-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
first conduit is operably connected to the source of concrete reclaimed water
at its proximal
end by a third conduit, wherein the diameter of the first conduit is greater
than the diameter of
the third conduit. In certain embodiments, the first conduit is operably
connected at its distal
end to a reclaimer by a fourth conduit, wherein the diameter of the first
conduit is greater than
the diameter of the fourth conduit. In certain embodiments, the apparatus
further comprises a
control system comprising (iii) a sensor to sense the specific gravity of the
reclaimed water
and transmit information regarding the specific gravity to (iv) a controller
that processes the
information from the sensor. The control system can further comprise (v) an
actuator that
receives a signal from the controller based, at least in part, on the
processed information from
the sensor. In certain embodiments, the actuator comprises a valve that can
modulate the
flow of the gas into the second conduit. In certain embodiments, the second
conduit
comprises perforations that are configured to allow the gas to pass from the
second conduit to
the reclaimed water in the first conduit when the gas exceeds a threshold
pressure in the
second conduit, but that do not allow reclaimed water from the first conduit
into the second
conduit. In certain embodiments, the apparatus further comprises at least one
of a sensor to
sense a level of reclaimed water in a reclaimed water holding tank, a sensor
to sense a
temperature of the reclaimed water, a sensor to sense rate of flow of the gas
into the second
conduit, a sensor to sense whether and/or how much admixture is added to the
reclaimed
water, a device that indicates whether a pump to pump reclaimed water through
the first
conduit is activated, or a timer, wherein the sensor, device or timer is
configured to send
information to the controller, which processes the information. In certain
embodiments, the
apparatus further comprises at least two of a sensor to sense a level of
reclaimed water in a
reclaimed water holding tank, a sensor to sense a temperature of the reclaimed
water, a sensor
to sense rate of flow of the gas into the second conduit, a sensor to sense
whether and/or how
much admixture is added to the reclaimed water, a device that indicates
whether a pump to
pump reclaimed water through the first conduit is activated, or a timer,
wherein the sensor,
device or timer is configured to send information to the controller, which
processes the
information. In certain embodiments, the apparatus further comprises at least
three of a
sensor to sense a level of reclaimed water in a reclaimed water holding tank,
a sensor to sense
a temperature of the reclaimed water, a sensor to sense rate of flow of the
gas into the second
conduit, a sensor to sense whether and/or how much admixture is added to the
reclaimed
water, a device that indicates whether a pump to pump reclaimed water through
the first
conduit is activated, or a timer, wherein the sensor, device or timer is
configured to send
information to the controller, which processes the information. In certain
embodiments, the
-2-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
apparatus further comprises at least four of a sensor to sense a level of
reclaimed water in a
reclaimed water holding tank, a sensor to sense a temperature of the reclaimed
water, a sensor
to sense rate of flow of the gas into the second conduit, a sensor to sense
whether and/or how
much admixture is added to the reclaimed water, a device that indicates
whether a pump to
pump reclaimed water through the first conduit is activated, or a timer,
wherein the sensor,
device or timer is configured to send information to the controller, which
processes the
information. In certain embodiments, the controller further receives
information about the
composition of the reclaimed water, wherein the information includes a
proportion of the
reclaimed water that is cementitious material.
10005] In another aspect, provided herein are methods
10006] In certain embodiments, provided herein is a
method of treating concrete
reclaimed water with a gas comprising (i) flowing the reclaimed water from a
source of the
reclaimed water into a first conduit at a proximal end of the first conduit
and out of the first
conduit at a distal end of the first conduit; (ii) flowing a gas from a source
of the gas into a
second conduit situated inside the first conduit; and (iii) flowing the gas
out of the second
conduit into the reclaimed water in the first conduit. In certain embodiments,
the gas
comprises carbon dioxide_ In certain embodiments, the diameter of the first
conduit is 0.5-5
inches and the diameter of the second conduit is 0.3-3 inches. In certain
embodiments, the
reclaimed water is flowed into the first conduit from the source of concrete
reclaimed water
via a third conduit operably connected to the source of concrete reclaimed
water and
connected to the first conduit at the proximal end of the first conduit,
wherein the diameter of
the first conduit is greater than the diameter of the third conduit. In
certain embodiments, the
reclaimed water is flowed out of the first conduit into a fourth conduit
operably connected to
the distal end of the first conduit, wherein the diameter of the first conduit
is greater than the
diameter of the fourth conduit. In certain embodiments, the method further
comprises
determining the specific gravity of the reclaimed water and transmitting
information
regarding the specific gravity to a controller that processes the information.
In certain
embodiments, the method further comprises sending a signal to from the
controller to an
actuator wherein the signal is based, at least in part on the processed
information. In certain
embodiments, the actuator comprises a valve that modulates the flow of the gas
into the
second conduit based, at least in part, on the signal received from the
controller. In certain
embodiments, the gas moves from the second conduit into the reclaimed water in
the first
conduit via perforations that are configured to allow the gas to pass from the
second conduit
to the reclaimed water in the first conduit when the gas exceeds a threshold
pressure in the
-3-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
second conduit, but that do not allow reclaimed water from the first conduit
into the second
conduit. In certain embodiments, the method further comprises sending
information to the
controller from at least one of a sensor to sense a level of reclaimed water
in a reclaimed
water holding tank, a sensor to sense a temperature of the reclaimed water, a
sensor to sense
rate of flow of the gas into the second conduit, a sensor to sense whether
and/or how much
admixture is added to the reclaimed water, a device that indicates whether a
pump to pump
reclaimed water through the first conduit is activated, or a timer, and
processing the
information at the controller. In certain embodiments, the method further
comprises sending
information to the controller from at least two of a sensor to sense a level
of reclaimed water
in a reclaimed water holding tank, a sensor to sense a temperature of the
reclaimed water, a
sensor to sense rate of flow of the gas into the second conduit, a sensor to
sense whether
and/or how much admixture is added to the reclaimed water, a device that
indicates whether a
pump to pump reclaimed water through the first conduit is activated, or a
timer, and
processing the information at the controller. In certain embodiments, the
method further
comprises sending information to the controller from at least three of a
sensor to sense a level
of reclaimed water in a reclaimed water holding tank, a sensor to sense a
temperature of the
reclaimed water, a sensor to sense rate of flow of the gas into the second
conduit, a sensor to
sense whether and/or how much admixture is added to the reclaimed water, a
device that
indicates whether a pump to pump reclaimed water through the first conduit is
activated, or a
timer, and processing the information at the controller. In certain
embodiments, the method
further comprises sending information to the controller from at least four of
a sensor to sense
a level of reclaimed water in a reclaimed water holding tank, a sensor to
sense a temperature
of the reclaimed water, a sensor to sense rate of flow of the gas into the
second conduit, a
sensor to sense whether and/or how much admixture is added to the reclaimed
water, a device
that indicates whether a pump to pump reclaimed water through the first
conduit is activated,
or a timer, and processing the information at the controller. In certain
embodiments, the
controller further receives information about the composition of the reclaimed
water, wherein
the information includes a proportion of the reclaimed water that is
cementitious material.
INCORPORATION BY REFERENCE
100071 All publications, patents, and patent
applications mentioned in this specification
are herein incorporated by reference to the same extent as if each individual
publication,
-4-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
patent, or patent application was specifically and individually indicated to
be incorporated by
reference.
BRIEF DESCRIPTION OF THE DRAWINGS
[0008] The novel features of the invention are set
forth with particularity in the appended
claims. A better understanding of the features and advantages of the present
invention will be
obtained by reference to the following detailed description that sets forth
illustrative
embodiments, in which the principles of the invention are utilized, and the
accompanying
drawings of which:
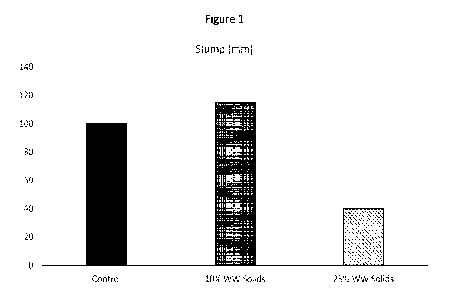
[0009] Figure 1 shows slump for concrete mixes produced
with 10% w/w dried
carbonated washwater solids, 20% w/w dried carbonated washwater solids, or
control (no
dried carbonated washwater solids).
[0010] Figure 2 shows compressive strength for concrete
mixes produced with 10% w/w
dried carbonated washwater solids, 20% w/w dried carbonated washwater solids,
or control
(no dried carbonated washwater solids), at 1, 7, and 28 days.
[0011] Figure 3 shows calorimetry, as power vs. time, for concrete mixes
produced with
10% w/w dried carbonated washwater solids, 20% w/w dried carbonated washwater
solids, or
control (no dried carbonated washwater solids).
[0012] Figure 4 shows compositions of various concrete
mixes produced with 10% w/w
dried carbonated washwater solids, 20% w/w dried carbonated washwater solids,
or control
(no dried carbonated washwater solids).
[0013] Figure 5 shows slump for concrete mixes produced
with washwater exposed to
carbon dioxide by exposure to simulated flue gas and various levels of cement,
or cement and
water reduction, compared to control.
[0014] Figure 6 shows compressive strength for concrete
mixes produced with
washwater exposed to carbon dioxide by exposure to simulated flue gas and
various levels of
cement, or cement and water reduction, compared to control, at 1, 7, and 28
days.
[0015] Figure 7 shows calorimetry, as power vs. time,
for concrete mixes produced with
washwater exposed to carbon dioxide by exposure to simulated flue gas and
various levels of
cement, or cement and water reduction, compared to control.
[0016] Figure 8 shows compositions of various concrete mixes produced with
washwater
exposed to carbon dioxide by exposure to simulated flue gas and various levels
of cement, or
cement and water reduction, compared to control.
-5-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
100171 Figure 9 shows slump for a control concrete made
with no washwater; concrete
batch made with 1.10 specific gravity treated washwater with full washwater
replacement and
1.5% sodium gluconate; a concrete batch made with 1.075 specific gravity
treated washwater
with full washwater replacement and 1.5% sodium gluconate; and a concrete
batch made with
1.05 specific gravity treated washwater batch with full washwater replacement
and 1.5%
sodium gluconate.
[0018] Figure 10 shows compressive strength for a
control concrete made with no
washwater; concrete batch made with 1.10 specific gravity treated washwater
with full
washwater replacement and 1.5% sodium gluconate; a concrete batch made with
1.075
specific gravity treated washwater with full washwater replacement and 1.5%
sodium
gluconate; and a concrete batch made with 1.05 specific gravity treated
washwater batch with
full washwater replacement and 1.5% sodium gluconate, at 1, 7, and 28 days.
[0019] Figure 11 shows calorimetry, as power vs. time,
for a control concrete made with
no washwater; concrete batch made with 1.10 specific gravity heated washwater
with full
washwater replacement and 1.5% sodium gluconate; a concrete batch made with
1.075
specific gravity treated washwater with full washwater replacement and 1.5%
sodium
gluconate; and a concrete batch made with 1.05 specific gravity treated
washwater batch with
full washwater replacement and 1.5% sodium gluconate.
[0020] Figure 12 shows compositions of various concrete
mixes produced as a control
concrete made with no washwater; concrete batch made with 1.10 specific
gravity treated
washwater with full washwater replacement and 1.5% sodium gluconate; a
concrete batch
made with 1.075 specific gravity treated washwater with full washwater
replacement and
1.5% sodium gluconate; and a concrete batch made with 1.05 specific gravity
treated
washwater batch with full washwater replacement and 1.5% sodium gluconate.
[0021] Figure 13 shows slump for various concrete mixes, including a
control mix (no
washwater), and mixes made with untreated washwater comprising no gluconate;
untreated
washwater comprising gluconate which was added after 3 hours of hydration;
untreated
washwater comprising gluconate which was added after 24 hours of hydration and
immediately before concrete batching; treated washwater comprising no
gluconate; treated
washwater comprising gluconate which was added before treatment and after 3
hours of
hydration; and treated washwater comprising gluconate which was added after 24
hours and
immediately before concrete batching.
[0022] Figure 14 shows compressive strength for various
concrete mixes, including a
control mix (no washwater), and mixes made with untreated washwater comprising
no
-6-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
gluconate; untreated washwater comprising gluconate which was added after 3
hours of
hydration; untreated washwater comprising gluconate which was added after 24
hours of
hydration and immediately before concrete batching; treated washwater
comprising no
gluconate; treated washwater comprising gluconate which was added before
treatment and
after 3 hours of hydration; and treated washwater comprising gluconate which
was added
after 24 hours and immediately before concrete batching, at 7 days and 28
days.
[0023] Figure 15 shows calorimetry, as power vs. time,
for various concrete mixes,
including a control mix (no washwater), and mixes made with untreated
washwater
comprising no gluconate; untreated washwater comprising gluconate which was
added after 3
hours of hydration, untreated washwater comprising gluconate which was added
after 24
hours of hydration and immediately before concrete botching; treated washwater
comprising
no gluconate; treated washwater comprising gluconate which was added before
treatment and
after 3 hours of hydration; and treated washwater comprising gluconate which
was added
after 24 hours and immediately before concrete batching,
[0024] Figure 16 shows compositions of various concrete mixes, including a
control mix
(no washwater), and mixes made with untreated washwater comprising no
gluconate;
untreated washwater comprising gluconate which was added after 3 hours of
hydration;
untreated washwater comprising gluconate which was added after 24 hours of
hydration and
immediately before concrete batching; treated washwater comprising no
gluconate; treated
washwater comprising gluconate which was added before treatment and after 3
hours of
hydration; and treated washwater comprising gluconate which was added after 24
hours and
immediately before concrete batching.
[0025] Figure 17 shows slump for a control concrete
batch made with no washwater; a
concrete batch made with untreated washwater with 0.6% sodium gluconate, with
full
washwater replacement; a concrete batch made with untreated washwater with
1.2% sodium
gluconate with full washwater replacement; a concrete batch made with treated
washwater
batch with 3% sodium gluconate and 5% cement reduction, and full washwater
replacement;
and a concrete batch made with treated washwater with 3% sodium gluconate and
10%
cement reduction, with full washwater replacement.
[0026] Figure 18 shows compressive strength for a control concrete batch
made with no
washwater; a concrete batch made with untreated washwater with 0.6% sodium
gluconate,
with full washwater replacement; a concrete batch made with untreated
washwater with 1.2%
sodium gluconate with full washwater replacement; a concrete batch made with
treated
washwater batch with 3% sodium gluconate and 5% cement reduction, and full
washwater
-7-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
replacement; and a concrete batch made with treated washwater with 3% sodium
gluconate
and 10% cement reduction, with full washwater replacement.
[0027] Figure 19 shows calorimetry, as power vs. time,
for a control concrete batch made
with no washwater; a concrete batch made with untreated washwater with 0.6%
sodium
gluconate, with full washwater replacement; a concrete batch made with
untreated washwater
with 1.2% sodium gluconate with full washwater replacement; a concrete batch
made with
treated washwater batch with 3% sodium gluconate and 5% cement reduction, and
full
washwater replacement; and a concrete batch made with treated washwater with
3% sodium
gluconate and 10% cement reduction, with full washwater replacement.
[0028] Figure 20 shows the composition of concrete batches made a control
concrete
batch made with no washwater; a concrete batch made with untreated washwater
with 0_6%
sodium gluconate, with full washwater replacement; a concrete batch made with
untreated
washwater with 1.2% sodium gluconate with full washwater replacement; a
concrete batch
made with treated washwater batch with 3% sodium gluconate and 5% cement
reduction, and
full washwater replacement; and a concrete batch made with treated washwater
with 3%
sodium gluconate and 10% cement reduction, with full washwater replacement.
[0029] Figure 21 shows slump for a control concrete
batch made with no washwater; a
concrete batch made with aged treated washwater with no sodium gluconate and
full
washwater replacement; a concrete batch made with aged treated washwater
comprising 2.4%
sodium gluconate with full washwater replacement; and a concrete batch made
with aged
treated washwater comprising 4.8% sodium gluconate with full washwater
replacement.
[0030] Figure 22 shows compressive strength for a
control concrete batch made with no
washwater; a concrete batch made with aged treated washwater with no sodium
gluconate
and full washwater replacement; a concrete batch made with aged treated
washwater
comprising 2.4% sodium gluconate with full washwater replacement; and a
concrete batch
made with aged treated washwater comprising 4.8% sodium gluconate with full
washwater
replacement, at 1, 7, and 28 days.
[0031] Figure 23 shows compositions for a control
concrete batch made with no
washwater; a concrete batch made with aged treated washwater with no sodium
gluconate
and full washwater replacement; a concrete batch made with aged treated
washwater
comprising 2.4% sodium gluconate with full washwater replacement; and a
concrete batch
made with aged treated washwater comprising 4.8% sodium gluconate with full
washwater
replacement.
-8-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
100321 Figure 24 shows slump for concrete batches made
as follows: Control (no
washwater); Untreated washwater control (2.7% gluconate immediately before
hatching);
Treated washwater (no gluconate); Treated washwater (2.7% gluconate added
immediately
before hatching); Treated washwater control (8.1% lignosulfonate added
immediately before
hatching).
100331 Figure 25 shows compressive strength for
concrete batches made as follows:
Control (no washwater); Untreated washwater control (2.7% gluconate
immediately before
hatching); Treated washwater (no gluconate); Treated washwater (2.7% gluconate
added
immediately before batching); Treated washwater control (8.1% lignosulfonate
added
immediately before hatching), at 3, 7, and 28 days.
100341 Figure 26 shows calorirnetry, as power v. time,
for concrete batches made as
follows: Control (no washwater); Untreated washwater control (2.7% gluconate
immediately
before batching); Treated washwater (no gluconate); Treated washwater (2.7%
gluconate
added immediately before batching); Treated washwater control (8.1%
lignosulfonate added
immediately before batching)
100351 Figure 27 shows compositions for concrete
batches made as follows: Control (no
washwater); Untreated washwater control (2.7% gluconate immediately before
hatching);
Treated washwater (no gluconate); Treated washwater (2.7% gluconate added
immediately
before hatching); Treated washwater control (8.1% lignosulfonate added
immediately before
batching).
100361 Figure 28 shows slump for concrete batches made
as follows: Control (no
washwater); Treated washwater batch, full washwater replacement, 1.4% sodium
gluconate;
Treated washwater batch, full washwater replacement, 1.4% sodium gluconate
before
carbonation and 0.7% sodium gluconate after carbonation; Treated washwater
batch with 5%
cementitious reduction, full washwater replacement, 1.4% sodium gluconate.
100371 Figure 29 shows compressive strength for
concrete batches made as follows:
Control (no washwater); Treated washwater batch, full washwater replacement,
1.4% sodium
gluconate; Treated washwater batch, full washwater replacement, 1.4% sodium
gluconate
before carbonation and 0.7% sodium gluconate after carbonation; Treated
washwater batch
with 5% cementitious reduction, full washwater replacement, 1.4% sodium
gluconate, at 1, 7,
and 28 days.
100381 Figure 30 shows calorimetry, as power vs. time,
for concrete batches made as
follows: Control (no washwater); Treated washwater batch, full washwater
replacement,
1.4% sodium gluconate; Treated washwater batch, full washwater replacement,
1.4% sodium
-9-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
gluconate before carbonation and 0.7% sodium gluconate after carbonation;
Treated
washwater batch with 5% cementitious reduction, full washwater replacement,
1.4% sodium
gluconate.
[0039] Figure 31 shows compositions of concrete batches
made as follows: Control (no
washwater); Treated washwater batch, full washwater replacement, 1.4% sodium
gluconate;
Treated washwater batch, full washwater replacement, 1.4% sodium gluconate
before
carbonation and 0.7% sodium gluconate after carbonation; Treated washwater
batch with 5%
cementitious reduction, full washwater replacement, 1.4% sodium gluconate.
[0040] Figure 32 shows slump over time (minutes) for
concrete batches made as follows:
Control (no washwater); Treated washwater, full replacement, 1.4% gluconate by
weight of
washwater solids; Treated washwater, full replacement, 2% gluconate by weight
of
washwater solids.
[0041] Figure 33 shows compressive strength for
concrete batches made as follows:
Control (no washwater); Treated washwater, full replacement, 1.4% gluconate by
weight of
washwater solids; Treated washwater, full replacement, 2% gluconate by weight
of
washwater solids.
[0042] Figure 34 shows calorimetry, as power vs. time,
for concrete batches made as
follows: Control (no washwater); Treated washwater, full replacement, 1.4%
gluconate by
weight of washwater solids; Treated washwater, full replacement, 2% gluconate
by weight of
washwater solids.
[0043] Figure 35 shows compositions for concrete
batches made as follows: Control (no
washwater); Treated washwater, full replacement, 1.4% gluconate by weight of
washwater
solids; Treated washwater, full replacement, 2% gluconate by weight of
washwater solids.
[0044] Figure 36 shows slump for concrete batches made
as follows: Control (no
washwater); Treated washwater, full replacement, no gluconate; Treated
washwater, full
replacement, 1.6% gluconate by weight of washwater solids.
[0045] Figure 37 shows compressive strength for
concrete batches made as follows:
Control (no washwater); Treated washwater, full replacement, no gluconate;
Treated
washwater, full replacement, 1.6% gluconate by weight of washwater solids, at
1, 7, and 28
days.
[0046] Figure 38 shows calorimetry, as power vs. time,
for concrete batches made as
follows: Control (no washwater); Treated washwater, full replacement, no
gluconate; Treated
washwater, full replacement, 1.6% gluconate by weight of washwater solids.
-10-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
[0047] Figure 39 shows the composition for concrete
batches made as follows: Control
(no washwater); Treated washwater, full replacement, no gluconate; Treated
washwater, full
replacement, 1.6% gluconate by weight of washwater solids.
[0048] Figure 40 shows slump for concrete batches made
as follows: Control (no
washwater); Treated washwater, full replacement, 1.7% gluconate by weight of
washwater
solids; Treated washwater, full replacement, 1.7% gluconate by weight of
washwater solids
with a 5% cementitious reduction.
[0049] Figure 41 shows compressive strength for
concrete batches made as follows:
Control (no washwater); Treated washwater, full replacement, 1.7% gluconate by
weight of
washwater solids; Treated washwater, full replacement, 1.7% gluconate by
weight of
washwater solids with a 5% cementitious reduction, at 1 day and 28 days.
[0050] Figure 42 shows calorimetry, as power vs. time,
for concrete batches made as
follows: Control (no washwater); Treated washwater, full replacement, 1.7%
gluconate by
weight of washwater solids; Treated washwater, full replacement, 1.7%
gluconate by weight
of washwater solids with a 5% cementitious reduction.
[0051] Figure 43 shows compositions for concrete
batches made as follows: Control (no
washwater); Treated washwater, full replacement, 1.7% gluconate by weight of
washwater
solids; Treated washwater, full replacement, 1.7% gluconate by weight of
washwater solids
with a 5% cementitious reduction.
[0052] Figure 44 shows slump for concrete batches made as follows: Control
(no
washwater); Treated washwater, half replacement, 2% gluconate by weight of
washwater
solids, washwater mixed with potable water and added upfront; Treated
washwater, half
replacement, 2% gluconate by weight of washwater solids, washwater added
upfront with the
potable water added later; Treated washwater, half replacement, 2% gluconate
by weight of
washwater solids, potable added upfront with the washwater added later.
[0053] Figure 45 shows compressive strength for
concrete batches made as follows:
Control (no washwater); Treated washwater, half replacement, 2% gluconate by
weight of
washwater solids, washwater mixed with potable water and added upfront;
Treated
washwater, half replacement, 2% gluconate by weight of washwater solids,
washwater added
upfront with the potable water added later, Treated washwater, half
replacement, 2%
gluconate by weight of washwater solids, potable added upfront with the
washwater added
later.
[0054] Figure 46 shows calorimetry as power vs time for
concrete batches made as
follows: Control (no washwater); Treated washwater, half replacement, 2%
gluconate by
-11-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
weight of washwager solids, washwater mixed with potable water and added
upfront; Treated
washwater, half replacement, 2% gluconate by weight of washwater solids,
washwater added
upfront with the potable water added later, Treated washwater, half
replacement, 2%
gluconate by weight of washwater solids, potable added upfront with the
washwater added
later.
[0055] Figure 47 shows compositions for concrete
batches made as follows: Control (no
washwater); Treated washwater, half replacement, 2% gluconate by weight of
washwater
solids, washwater mixed with potable water and added upfront; Treated
washwater, half
replacement, 2% gluconate by weight of washwater solids, washwater added
upfront with the
potable water added later; Treated washwater, half replacement, 2% gluconate
by weight of
washwater solids, potable added upfront with the washwater added later.
[0056] Figure 48 shows slump for concrete batches made
as follows: Control (no
washwater). Treated washwater, full replacement, 2.5% gluconate by weight of
washwater
solids; Treated washwater, 75% replacement, 2.5% gluconate by weight of
washwater solids;
Treated washwater, 50% replacement, 2.5% gluconate by weight of washwater
solids.
[0057] Figure 49 shows compressive strength for
concrete batches made as follows:
Control (no washwater). Treated washwater, full replacement, 2.5% gluconate by
weight of
washwater solids; Treated washwater, 75% replacement, 2.5% gluconate by weight
of
washwater solids; Treated washwater, 50% replacement, 2.5% gluconate by weight
of
washwater solids, at 7 and 28 days.
10058] Figure 50 shows calorimetry, as power vs. time,
for concrete batches made as
follows: Control (no washwater). Treated washwater, full replacement, 2.5%
gluconate by
weight of washwater solids; Treated washwater, 75% replacement, 2.5% gluconate
by
weight of washwater solids; Treated washwater, 50% replacement, 2.5% gluconate
by weight
of washwater solids.
[0059] Figure 51 shows compositions for concrete
batches made as follows: Control (no
washwater). Treated washwater, full replacement, 2.5% gluconate by weight of
washwater
solids; Treated washwater, 75% replacement, 2.5% gluconate by weight of
washwater solids;
Treated washwater, 50% replacement, 2.5% gluconate by weight of washwater
solids.
[0060] Figure 52 shows slump for concrete batches made as follows: Control
(no
washwater; Untreated washwater, full replacement, 2% gluconate by weight of
washwater
solids; Treated washwater, full replacement, 2% gluconate by weight of
washwater solids;
Untreated washwater, 50% replacement, 2% gluconate by weight of washwater
solids;
Treated washwater, 50% replacement, 2% gluconate by weight of washwater
solids.
-12-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
100611 Figure 53 shows compressive strength for
concrete batches made as follows:
Control (no washwater; Untreated washwater, full replacement, 2% gluconate by
weight of
washwater solids; Treated washwater, full replacement, 2% gluconate by weight
of
washwater solids; Untreated washwater, 50% replacement, 2% gluconate by weight
of
washwater solids; Treated washwater, 50% replacement, 2% gluconate by weight
of
washwater solids.
100621 Figure 54 shows calorimetry as power vs. time
for concrete batches made as
follows: Control (no washwater; Untreated washwater, full replacement, 2%
gluconate by
weight of washwater solids; Treated washwater, full replacement, 2% gluconate
by weight of
washwater solids; Untreated washwater, 50% replacement, 2% gluconate by weight
of
washwater solids; Treated washwater, 50% replacement, 2% gluconate by weight
of
washwater solids.
100631 Figure 55 shows compositions for concrete
batches made as follows: Control (no
washwater; Untreated washwater, full replacement, 2% gluconate by weight of
washwater
solids; Treated washwater, full replacement, 2% gluconate by weight of
washwater solids;
Untreated washwater, 50% replacement, 2% gluconate by weight of washwater
solids;
Treated washwater, 50% replacement, 2% gluconate by weight of washwater
solids.
100641 Figure 56 shows carbon dioxide uptake for
washwater treated with carbon
dioxide at low, medium, and high flow rates.
100651 Figure 57 shows 7-day compressive strength, compared to control,
for mortar
batches made as follows (test was repeated for each flow rate): Control ;
Untreated
washwater, full replacement, no CO2; Treated washwater, full replacement, 7-8%
CO2;
Treated washwater, full replacement, 10-13% CO2; Treated washwater, full
replacement, 14-
15% CO2; Treated washwater, full replacement, 16-17% CO2;Treated washwater,
full
replacement, 19-21% CO2.
100661 Figure 58 shows slump, compared to control, for
mortar batches made as follows
(test was repeated for each flow rate): Control ; Untreated washwater, full
replacement, no
CO2; Treated washwater, full replacement, 7-8% CO2; Treated washwater, full
replacement,
10-13% CO2; Treated washwater, full replacement, 14-15% CO2; Treated
washwater, full
replacement, 16-17% CO2;Treated washwater, full replacement, 19-21% CO2.
100671 Figure 59 shows calorimeter setting time
relative to control for mortar batches
made as follows (test was repeated for each flow rate): Control ; Untreated
washwater, full
replacement, no CO2; Treated washwater, full replacement, 7-8% CO2; Treated
washwater,
-13-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
full replacement, 10-13% CO2; Treated washwater, full replacement, 14-15% CO2;
Treated
washwater, full replacement, 16-17% CO2;Treated washwater, full replacement,
19-21% CO2.
10068] Figure 60 shows calorimeter peak energy output
relative to control for mortar
batches made as follows (test was repeated for each flow rate): Control ;
Untreated
washwater, full replacement, no CO2; Treated washwater, full replacement, 7-8%
CO2;
Treated washwater, full replacement, 10-13% CO2; Treated washwater, full
replacement, 14-
15% CO2; Treated washwater, full replacement, 16-17% CO2;Treated washwater,
full
replacement, 19-21% CO2
100691 Figure 61 shows washwater temperature for mortar
batches made as follows (test
was repeated for each flow rate): Control ; Untreated washwater, full
replacement, no CO2
Treated washwater, full replacement, 7-8% CO2; Treated washwater, full
replacement, 10-
13% CO2; Treated washwater, full replacement, 14-15% CO2; Treated washwater,
full
replacement, 16-17% CO2;Treated washwater, full replacement, 19-21% CO2
10070] Figure 62 shows washwater pH for mortar batches
made as follows (test was
repeated for each flow rate): Control ; Untreated washwater, full replacement,
no CO2;
Treated washwater, full replacement, 7-8% CO2; Treated washwater, full
replacement, 10-
13% CO2; Treated washwater, full replacement, 14-15% CO2; Treated washwater,
full
replacement, 16-17% CO2;Treated washwater, full replacement, 19-21% CO2.
10071] Figure 63 shows carbon dioxide uptake vs. time
for washwatets of various
specific gravities treated with carbon dioxide.
100721 Figure 64 shows temperature vs. time for
washwaters of various specific gravities
treated with carbon dioxide.
10073] Figure 65 shows compressive strengths of mortar
that contained a blend of 70%
cement and 30% class C fly ash. The class C fly ash was all in prepared wash
waters, treated
with 1.2, 2.2, 2.4, 3.2, or 3_5% carbon dioxide.
10074] Figure 66 shows calorimetry, as power vs. time,
of mortar that contained a blend
of 70% cement and 30% class C fly ash. The class C fly ash was all in prepared
wash waters,
treated with 1.2, 2.2,2.4, 3.2, or 3.5% carbon dioxide.
10075] Figure 67 shows the compositions of mortars made
with mortar that contained a
blend of 70% cement and 30% class C fly ash. The class C fly ash was all in
prepared wash
waters, treated with 1.2, 2.2, 2_4, 3.2, or 3.5% carbon dioxide.
10076] Figure 68 shows slump in concrete mixes made as
follows: Control, no
washwater; Untreated washwater, full replacement; Treated washwater, 20
minutes of CO2
injection; Treated washwater, 40 minutes of CO2 injection.
-14-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
100771 Figure 69 shows compressive strength in concrete
mixes made as follows:
Control, no washwater; Untreated washwater, full replacement; Treated
washwater, 20
minutes of CO2 injection; Treated washwater, 40 minutes of CO2 injection.
100781 Figure 70 shows calorimetry as power vs. time in
concrete mixes made as
follows: Control, no washwater; Untreated washwater, full replacement; Treated
washwater,
20 minutes of CO2 injection; Treated washwater, 40 minutes of CO2 injection.
100791 Figure 71 shows the compositions of concrete
mixes made as follows: Control, no
washwater; Untreated washwater, full replacement; Treated washwater, 20
minutes of CO2
injection; Treated washwater, 40 minutes of CO2 injection.
100801 Figure 72 shows slump for concrete mixes made as follows: Control,
no
washwater, 15g air entraining admixture; Treated washwater, full replacement,
15g air
entraining admixture; Treated washwater, full replacement, 15g air entraining
admixture,
sodium gluconate added 2% by weight of washwater solids.
100811 Figure 73 shows air entrained for concrete mixes
made as follows: Control, no
washwater, 15g air entraining admixture; Treated washwater, full replacement,
15g air
entraining admixture; Treated washwater, full replacement, 15g air entraining
admixture,
sodium gluconate added 2% by weight of washwater solids.
100821 Figure 74 shows compressive strength atl, 7, and
28 days for concrete mixes
made as follows: Control, no washwater, 15g air entraining admixture; Treated
washwater,
full replacement, 15g air entraining admixture; Treated washwater, full
replacement, 15g air
entraining admixture, sodium gluconate added 2% by weight of washwater solids.
100831 Figure 75 shows calorimetry as power vs. time
for concrete mixes made as
follows: Control, no washwater, 15g air entraining admixture; Treated
washwater, full
replacement, 15g air entraining admixture; Treated washwater, full
replacement, 15g air
entraining admixture, sodium gluconate added 2% by weight of washwater solids.
100841 Figure 76 shows compositions for concrete mixes
made as follows: Control, no
washwater, 15g air entraining admixture; Treated washwater, full replacement,
15g air
entraining admixture; Treated washwater, full replacement, 15g air entraining
admixture,
sodium gluconate added 2% by weight of washwater solids.
100851 Figure 77 shows slump for concrete mixes made as follows: Control,
no
washwater; Treated washwater, full replacement, added assuming 12% of the
washwater was
unavailable for concrete hydration; Treated washwater, full replacement, added
assuming
17% of the washwater was unavailable for concrete hydration.
-15-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
[0086] Figure 78 shows compressive strength at 1, 7,
and 28 days for concrete mixes
made as follows: Control, no washwater; Treated washwater, full replacement,
added
assuming 12% of the washwater was unavailable for concrete hydration; Treated
washwater,
full replacement, added assuming 17% of the washwater was unavailable for
concrete
hydration.
[0087] Figure 79 shows calorimetry as power vs. time
for concrete mixes made as
follows: Control, no washwater; Treated washwater, full replacement, added
assuming 12%
of the washwater was unavailable for concrete hydration; Treated washwater,
full
replacement, added assuming 17% of the washwater was unavailable for concrete
hydration.
[0088] Figure 80 shows compositions for concrete mixes made as follows:
Control, no
washwater; Treated washwater, full replacement, added assuming 12% of the
washwater was
unavailable for concrete hydration; Treated washwater, full replacement, added
assuming
17% of the washwater was unavailable for concrete hydration.
[0089] Figure 81 shows slump for concrete mixes
produced as follows: Control, no
washwater; Treated washwater, full replacement, sodium gluconate added 1% by
weight of
washwater solids; Treated washwater, full replacement, Daratard 17 added 5% by
weight of
washwater solids; Treated washwater, full replacement, Recover added 5% by
weight of
washwater solids.
[0090] Figure 82 shows compressive strength at 1, 7,
and 28 days for concrete mixes
produced as follows: Control, no washwater; Treated washwater, full
replacement, sodium
gluconate added 1% by weight of washwater solids; Treated washwater, full
replacement,
Daratard 17 added 5% by weight of washwater solids; Treated washwater, full
replacement,
Recover added 5% by weight of washwater solids.
[0091] Figure 83 shows ca1orimetry as power vs. time
for concrete mixes produced as
follows: Control, no washwater; Treated washwater, full replacement, sodium
gluconate
added 1% by weight of washwater solids; Treated washwater, hill replacement,
Daratard 17
added 5% by weight of washwater solids; Treated washwater, full replacement,
Recover
added 5% by weight of washwater solids.
[0092] Figure 84 shows compositions for concrete mixes
produced as follows: Control,
no washwater; Treated washwater, full replacement, sodium gluconate added 1%
by weight
of washwater solids; Treated washwater, full replacement, Daratard 17 added 5%
by weight
of washwater solids; Treated washwater, full replacement, Recover added 5% by
weight of
washwater solids.
-16-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
[0093] Figure 85 shows slump for concrete mixes made as
follows: Control, no
washwater; Treated washwater, full replacement, washwater from pump system;
Treated
washwater, full replacement, washwater from drill system.
[0094] Figure 86 shows compressive strength for
concrete mixes made as follows:
Control, no washwater; Treated washwater, full replacement, washwater from
pump system;
Treated washwater, full replacement, washwater from drill system.
[0095] Figure 87 shows calorimetry as power vs. time
for concrete mixes made as
follows: Control, no washwater; Treated washwater, full replacement, washwater
from pump
system; Treated washwater, full replacement, washwater from drill system.
[0096] Figure 88 shows compositions for concrete mixes made as follows:
Control, no
washwater; Treated washwater, full replacement, washwater from pump system;
Treated
washwater, full replacement, washwater from drill system.
[0097] Figure 89 shows carbon dioxide uptake and
efficiency of uptake for different
flowrates and total carbon dioxide added to a slurry.
[0098] Figure 90 shows carbon dioxide uptake and efficiency of uptake for
Milne vs. no
inhne mixing as carbon dioxide is added.
[0099] Figure 91 shows carbon dioxide uptake and
efficiency of uptake for 1 vs. 2
carbon dioxide injection points.
[0100] Figure 92 shows an apparatus for adding carbon
dioxide to a wash water slurry.
[0101] Figure 93 shows workability (slump) for concrete prepared with
washwater at
high specific gravity (1.15) and low replacement levels (10,20, and 30%).
[0102] Figure 94 shows calorimetry (power v time) for
concrete prepared with
washwater at high specific gravity (1.15) and low replacement levels (10, 20,
and 30%).
[0103] Figure 95 shows compressive strength at 1, 7,
and 28 days for concrete prepared
with washwater at high specific gravity (1.15) and low replacement levels (10,
20, and 30%).
[0104] Figure 96 shows mix designs for concrete
prepared with washwater at high
specific gravity (1.15) and low replacement levels (10,20, and 30%).
[0105] Figure 97 shows workability (slump) for concrete
prepared with two different
batches of washwater, at specific gravities of 1.10 and 1.05, respectively.
[0106] Figure 98 shows calorimetry (power v time) for concrete prepared
with two
different batches of washwater, at specific gravities of 1.10 and 1.05,
respectively.
[0107] Figure 99 shows compressive strength at 1, 7,
and 28 days for concrete prepared
with two different batches of washwater, at specific gravities of 1.10 and
1.05, respectively.
-17-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
[0108] Figure 100 shows mix designs for concrete
prepared with two different batches of
washwater, at specific gravities of 1.10 and 1.05, respectively.
[0109] Figure 101 shows x-ray diffraction analysis for
washwater at a specific gravity of
1.10 and treated with CO2 to 0,5, 10, 15, 20 and 25% by weight of cement, at 0
hours.
[0110] Figure 102 shows x-ray diffraction analysis for washwater at a
specific gravity of
1.10 and treated with CO2 to 0, 5, 10, 15, 20 and 25% by weight of cement, at
3 hours.
[0111] Figure 103 shows x-ray diffraction analysis for
washwater at a specific gravity of
1.10 and treated with CO2 to 0, 5, 10, 15, 20 and 25% by weight of cement, at
6 hours.
[0112] Figure 104 shows x-ray diffraction analysis for
washwater at a specific gravity of
1.10 and treated with CO2 to 0, 5, 10, 15, 20 and 25% by weight of cement, at
24 hours.
[0113] Figure 105 shows x-ray diffraction analysis for
washwater at a specific gravity of
1.10 and treated with CO2 to 0, 5, 10, 15, 20 and 25% by weight of cement, at
72 hours.
[0114] Figure 106 shows slump for concrete made with
treated washwater at two
different treatment levels (5, 25%) and compared to potable water reference
and an untreated
washwater reference. All conditions were made with and without a 3% reduction
in cement.
[0115] Figure 107 shows calorimetry (power v time) for
concrete prepared with treated
washwater at two different treatment levels (5, 25%) and compared to potable
water reference
and an untreated washwater reference. All conditions were made with and
without a 3%
reduction in cement.
[0116] Figure 108 shows compressive strength at 1, 7, and 28 days for
concrete prepared
with treated washwater at two different treatment levels (5, 25%) and compared
to potable
water reference and an untreated washwater reference. MI conditions were made
with and
without a 3% reduction in cement.
[0117] Figure 109 shows mix designs for concrete
prepared with treated washwater at
two different treatment levels (5, 25%) and compared to potable water
reference and an
untreated washwater reference. All conditions were made with and without a 3%
reduction
in cement.
[0118] Figure 110 shows slump for concrete made with
washwater treated with 0, 3, 6,
and 9% CO2 by weight solids; washwater was produced at a large scale (1000L)
and treated
in a way to simulate the treatment that would be used in a reclaimer--
washwater was
transferred from one tank to another with CO2 being injected in the transfer
line. The
washwater was transferred/treated every 30 minutes.
[0119] Figure 111 shows calorimetry (power v time) for
concrete prepared with
washwater treated with 0, 3, 6, and 9% CO2 by weight solids; washwater was
produced at a
-18-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
large scale (1000L) and treated in a way to simulate the treatment that would
be used in a
reclaimer--washwater was transferred from one tank to another with CO2 being
injected in
the transfer line. The washwater was transferred/treated every 30 minutes.
101201 Figure 112 shows compressive strength at 1, 7,
and 28 days for concrete prepared
with washwater treated with 0, 3,6, and 9% CO2 by weight solids; washwater was
produced
at a large scale (WOOL) and treated in a way to simulate the treatment that
would be used in a
reclaimer--washwater was transferred from one tank to another with CO2 being
injected in
the transfer line. The washwater was transferred/treated every 30 minutes.
101211 Figure 113 shows mix designs for concrete
prepared with washwater treated with
0, 3, 6, and 9% CO2 by weight solids; washwater was produced at a large scale
(1000L) and
treated in a way to simulate the treatment that would be used in a reclaimer--
washwater was
transferred from one tank to another with CO2 being injected in the transfer
line. The
washwater was transferred/treated every 30 minutes.
101221 Figure 114 shows slump for concrete made with
washwater treated with 0, 3, 6,
and 9% CO2 by weight solids; washwater was produced at a large scale (1000L)
and treated
in a way to simulate the treatment that would be used in a reclaimer--
washwater was
transferred from one tank to another with CO2 being injected in the transfer
line. The
washwater was transferred/treated every 30 minutes, then allowed to age for 24
hours after
treatment.
101231 Figure 115 shows calorimetry (power v time) for concrete prepared
with
washwater treated with 0, 3, 6, and 9% CO2 by weight solids; washwater was
produced at a
large scale (1000L) and treated in a way to simulate the treatment that would
be used in a
reclaimer--washwater was transferred from one tank to another with CO2 being
injected in
the transfer line. The washwater was transferred/treated every 30 minutes,
then allowed to
age for 24 hours after treatment.
101241 Figure 116 shows compressive strength at 1, 7,
and 28 days for concrete prepared
with washwater treated with 0, 3, 6, and 9% CO2 by weight solids; washwater
was produced
at a large scale (1000L) and treated in a way to simulate the treatment that
would be used in a
reclaimer--washwater was transferred from one tank to another with CO2 being
injected in
the transfer line. The washwater was transferred/treated every 30 minutes,
then allowed to
age for 24 hours after treatment.
101251 Figure 117 shows mix designs for concrete
prepared with washwater treated with
0, 3, 6, and 9% CO2 by weight solids; washwater was produced at a large scale
(1000L) and
treated in a way to simulate the treatment that would be used in a reclaimer--
washwater was
-19-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
transferred from one tank to another with CO2 being injected in the transfer
line. The
washwater was transferred/treated every 30 minutes, then allowed to age for 24
hours after
treatment.
[0126] Figure 118 shows slump of mortar made with 100%
water replacement with
washwater treated with CO2 to an uptake of 8% CO2 by weight washwater solids,
either as is
or treated with two different concentrations of a commercial set retarding
admixture.
[0127] Figure 119 shows calorimetry (power v time) of
mortar made with 100% water
replacement with washwater treated with CO2 to an uptake of 8% CO2 by weight
washwater
solids, either as is or treated with two different concentrations of a
commercial set retarding
admixture.
[0128] Figure 120 shows compressive strength at 1, 7,
and 28 days of mortar made with
1000/n water replacement with washwater treated with CO2 to an uptake of 8%
CO2 by
weight washwater solids, either as is or treated with two different
concentrations of a
commercial set retarding admixture.
[0129] Figure 121 shows mix designs of mortar made with 100% water
replacement with
washwater treated with CO2 to an uptake of 8% CO2 by weight washwater solids,
either as is
or treated with two different concentrations of a commercial set retarding
admixture.
[0130] Figure 122 shows X-ray diffraction for washwater
prepared with 100% cement
treated with 0, 5, 10, 15, 20, and 25% CO2 by weight of cement at 0 hours.
[0131] Figure 123 shows X-ray diffraction for washwater prepared with 100%
cement
treated with 0, 5, 10, 15, 20, and 25% CO2 by weight of cement at 24 hours.
[0132] Figure 124 shows X-ray diffraction for washwater
prepared with 75% cement and
25% slag treated with 0, 5, 10, 15, 20, and 25% CO2 by weight of cement at 0
hours.
[0133] Figure 125 shows X-ray diffraction for washwater
prepared with 75% cement and
25% slag treated with 0, 5, 10, 15, 20, and 25% CO2 by weight of cement at 24
hours.
[0134] Figure 126 shows X-ray diffraction for washwater
prepared with 100% cement
and treated with CO2 at a flow rate of 5 LPM for 0, 5, 10, 15, 20, and 25% CO2
by weight
cement solids at 0 hours.
[0135] Figure 127 shows X-ray diffraction for washwater
prepared with 100% cement
and treated with CO2 at a flow rate of 5 LPM for 0, 5, 10, 15, 20, and 25% CO2
by weight
cement solids at 24 hours.
[0136] Figure 128 shows X-ray diffraction for washwater
prepared with 100% cement
and treated with CO2 at a flow rate of 10 LPM for 0, 5, 10, 15, 20, and 25%
CO2 by weight
cement solids at 0 hours.
-20-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
101371 Figure 129 shows X-ray diffraction for washwater
prepared with 100% cement
and treated with CO2 at a Dow rate of 10 LPM for 0, 5, 10, 15, 20, and 25% CO2
by weight
cement solids at 24 hours.
101381 Figure 130 shows X-ray diffraction for washwater
prepared with 100% cement to
a specific gravity of 1.05, treated with CO2 for 0, 5, 10, 15, 20, 25 and 30%
CO2 by weight
of cement solids at 0 hours.
[0139] Figure 131 shows X-ray diffraction for washwater
prepared with 100% cement to
a specific gravity of 1.05, treated with CO2 for 0, 5, 10, 15, 20, 25 and 30%
CO2 by weight
of cement solids at 24 hours.
[0140] Figure 132 shows X-ray diffraction for washwater prepared with 100%
cement to
a specific gravity of L05, treated with CO2 for 0, 5, 10, 15, 20, 25 and 30%
CO2 by weight
of cement solids at 48 hours.
[0141] Figure 133 shows X-ray diffraction for washwater
prepared with 100% cement to
a specific gravity of 1.15, treated with CO2 for 0, 5, 10, 15, 20, 25 and 30%
CO2 by weight
of cement solids at 0 hours.
[0142] Figure 134 shows X-ray diffraction for washwater
prepared with 100% cement to
a specific gravity of 1.15, treated with CO2 for 0, 5, 10, 15, 20, 25 and 30%
CO2 by weight
of cement solids at 24 hours.
[0143] Figure 135 shows X-ray diffraction for washwater
prepared with 100% cement to
a specific gravity of 1.15, treated with CO2 for 0, 5, 10, 15, 20, 25 and 30%
CO2 by weight
of cement solids at 48 hours.
DETAILED DESCRIPTION OF THE INVENTION
[0144] Wash water, also called grey water or reclaimed
water herein, is produced as a
byproduct of the concrete industry. This water, which may contain suspended
solids in the
form of sand, aggregate and/or cementitious materials, is generated through
various steps in
the cycle of producing concrete structures. Generally a large volume of
concrete wash water
(reclaimed water) is produced by the washing-out of concrete mixer trucks
following the
delivery of concrete. This water is alkaline in nature and requires
specialized treatment,
handling and disposal. As used herein, "wash water" includes waters that are
primarily
composed of concrete drum wash water; such water may contain water from other
parts of
the concrete production process, rain runoff water, etc., as is known in the
art. As will be
clear from context, "wash water" includes water used to clean the drum of a
ready-mix truck
-21-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
and/or other mixers, which contains cement and aggregate, as well as such
water after
aggregate has been removed, e.g., in a reclaimer, but still containing solids,
such as
cementitious solids. Typically at least a portion of such solids are retained
in the wash water
for re-use in subsequent concrete batches.
[0145] While this water can be suitable for reuse in the production of
concrete, it has
been documented that the wash water can result in negative impacts on the
properties of
concrete, for example, set acceleration and loss of workability. Wash water is
mainly a
mixture of cement and, in many cases, supplementary cementitious materials
(SCMs) in
water. It becomes problematic as a mix water because as the cement hydrates it
changes the
chemistry of the water. These changes in chemistry, along with the hydration
products, cause
a host of issues when the water is used as mix water, such as acceleration,
increased water
demand, reduced 7-day strength, and the like. These issues generally worsen as
the amount
of cement in the water increases, and/or the water ages.
[0146] The methods and compositions of the invention
utilize the application of CO2 to
concrete wash water to improve its properties for reuse in the production of
concrete. Thus,
wash water that has a cement content (e.g., specific gravity) and/or that has
aged to a degree
that would normally not allow its use as mix water can, after application of
carbon dioxide,
be so used.
[0147] Without being bound by theory, it is thought
that by carbonating wash water,
several results may be achieved that are beneficial in terms of using the
water as part or all of
mix water for subsequent batches of concrete:
[0148] 1) Maintain a pH of-7: This effectively
dissolves the cement due to the acidity
of CO2. This helps deliver a grey water of consistent chemistry and removes
the "ageing
effects". In certain embodiments, a pH of less than or greater than 7 may be
maintained,
as described elsewhere herein.
[0149] 2) Precipitate any insoluble carbonates: CO2
actively forms carbonate reaction
products with many ions. This removes certain species from solution, such as
calcium,
aluminum, magnesium and others. This is another step that helps provide a grey
water of
consistent chemistry.
[0150] 3) Change solubility of cement ions: The solubilities of many ions
depend on
pH, By maintaining the pH at -7 with CO2 the nature of the water chemistry is
changed,
potentially in a favorable direction. In certain embodiments, a pH of less
than or greater
than 7 may be maintained, as described elsewhere herein.
-22-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
[0151] 4) Shut down pozzolanic reactions: By
maintaining the pH around 7 no
Ca(OH)2 is available to react with slag and/or fly ash in the grey water. This
can mean
that these SCMs are unaltered through the treatment and reuse of the grey
water, thus
reducing the impact of the grey water substantially. In certain embodiments, a
pH of less
than or greater than 7 may be maintained, as described elsewhere herein.
[0152] 5) Reduce amount of anions left behind: The
formation of carbonate
precipitates using CO2 is advantageous over other common acids, like HO or
H2SO4
whose anions, if left soluble in the treated water_ can adversely impact the
chemistry of
the grey water for concrete hatching.
[0153] 6) Cause retardation: By saturating the grey water with CO)/HCO3-
retardation
can be achieved when used as batch water.
[0154] 7) Nature of precipitates: The process may
potentially be altered to form
precipitates that have less effects on the water demand of concrete prepared
with grey
water. In particular, conditions of carbonation may be used that produce
nanocrystalline
carbonates, such as nanocrystalline calcium carbonate, that are known to be
beneficial
when used in concrete products.
[0155] In certain embodiments, the invention provides a
method of providing a mix water
for a batch of concrete, where the mix water comprises wash water from one or
more
previous batches of concrete that has be exposed to carbon dioxide in an
amount above
atmospheric concentrations of carbon dioxide, to carbonate the wash water
("carbonated
wash water"). The mix water may contain at least 10, 20, 30, 40, 50, 60, 70,
80, 90, 95, 99,
or 99.5% carbonated wash water. Alternatively or additionally, the mix water
may contain
no more than 20, 30, 40, 50, 60, 70, 80, 90, 95, 99, 99.5, or 100% carbonated
wash water. In
certain embodiments, the mix water is 100% carbonated wash water. In certain
embodiments, the mix water is 1-100% carbonated wash water. In certain
embodiments, the
mix water is 1-80% carbonated wash water. In certain embodiments, the mix
water is 1-50%
carbonated wash water. In certain embodiments, the mix water is 1-30%
carbonated wash
water. In certain embodiments, the mix water is 10-100% carbonated wash water.
In certain
embodiments, the mix water is 20-100% carbonated wash water. In certain
embodiments, the
mix water is 50-100% carbonated wash water. In certain embodiments, the mix
water is 70-
100% carbonated wash water. In certain embodiments, the mix water is 90-100%
carbonated
wash water.
-23-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
101561 In certain embodiments, a first portion of mix
water that is plain water, e.g., not
wash Of other water that has been carbonated, such as plain water as normally
used in
concrete mixes, is mixed with concrete materials, such as cement, aggregate,
and the like, and
then a second portion of mix water that comprises carbonated water, which can
be carbonated
plain water or, e.g., carbonated wash water is added. The first portion of
water may be such
that an acceptable level of mixing is achieved, e.g., mixing without clumps or
without
substantial amounts of clumps. For example, the first portion of mix water
that is plain water
may be more than 1, 2, 5, 10,20, 30, 40, 50, 60, 70, 80, or 90%, and/or less
than 2, 5, 10, 20,
30, 40, 50, 60, 70, 80, 90 or 95%, such as % 1-90%, or 1-80%, or 1-75%, or 1-
70%, or 1-
65%, or 1-60%, or 1-55%, or 1-50%, or 1-45%, or 1-40%, or 1-30%, or 1-20%, or
1-10% of
the total mix water used in the concrete mix, while the remainder of the mix
water used in the
concrete mix is the second portion, i.e., carbonated mix water. The first
portion of water may
be added at one location and the second portion at a second location. For
example, in a ready
mix operation, the first portion may be added to concrete materials which are
mixed, then the
mixed materials are transferred to a drum of a ready-mix truck, where the
second portion of
water is added to the concrete in the drum of the ready-mix truck. However, it
is also
possible that both the first and the second locations are the same location,
e.g., a mixer prior
to deposit into a ready-mix truck, or the drum of the ready-mix truck. The
second portion of
water may be added at any suitable time after the addition of the first
portion_ In general, the
second portion of water is added at least after the first portion and the
concrete materials have
mixed sufficiently to achieve mixing without clumps or without substantial
amounts of
clumps. In certain embodiments, the second portion of water is added at least
I, 2, 3, 4, 5, 6,
7, 8, 9, 10, 12, 15, 20, 25, 30,40, 50, or 60 minutes after the first portion
of water, and/or not
more than , 2, 3, 4, 5, 6,7, 8,9, 10, 12, 15, 20, 25, 30, 40, 50, or 60
minutes, or 1,2, 3,4, 5,
or 6 hours after the first portion of water.
101571 The wash water may be carbonated at any suitable time, for example,
right after its
production, at some time after production, or just before use in the concrete,
or any
combination thereof Without being bound by theory, it is probable that at time
0
(immediately after formation of the wash water), added carbon dioxide will
react with
unhydrated cement phases (C3S, C2S, C3A, etc) while at later ages added carbon
dioxide
will react with hydrated cement phases (CSI-1, ettringite, etc.). Providing
dosage later can
result in different properties than when the dosage is applied earlier,
potentially leading to
different properties when the wash water is reused in concrete production. In
addition, the
phases reacting in wash water at later ages can be generally more
thermodynamically stable
-24-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
and thus have lower heats of reaction when reacting with carbon dioxide; the
inventors have
observed that the exothermic heat rise (e.g., as measured by temperature) can
be greater when
treating fresh wash water with carbon dioxide than when treating aged wash
water. It can be
advantageous to have a lower heat rise because a treated water that becomes
heated may have
to be cooled before it can be used as a mix water. Hence, certain embodiments
provide
methods and apparatus that cause a cooling of the wash water due to production
of gaseous
carbon dioxide for treatment of the wash water from liquid carbon dioxide,
e.g., piping or
conduits that contact the wash water and absorb heat necessary to convert
liquid to gaseous
carbon dioxide and thus cooling the wash water. These are described in more
detail
elsewhere herein. In addition, when treating an aged wash water with carbon
dioxide, it can
be possible that less carbon dioxide is required to achieve a stable wash
water than with wash
water that is fresh. The amount of carbon dioxide to create a stable wash
water (e.g.,
properties are relatively unchanged after further aging) can depend on the
relative
contributions of Ca(OH)2, ettringite, CSH, and/or unreacted cement (e.g.,
unreacted Ordinary
Portland Cement, OPC) to the undesirable properties of wash water. In
addition, different
phases can have different carbon dioxide reaction kinetics, which in turn can
influence
choices of carbon dioxide delivery settings, approaches (e.g., type of
delivery system or
adjustments to delivery system), and the like.
[0158] Thus, for example, in certain embodiments, carbonation of wash water
can
commence no later than 1, 2,5, 10, 20, 30, 40, 60, 80, 100, 120, 150, 180,
240, 300, 360, 420,
or 480 minutes, or 7, 8,9, 10, 11, 12, 14, 16, 18, or 24 hours, or 1.5, 2, 3,
4, or 5 days after
formation of the wash water, and/or no sooner than 0, 0.5, 1, 2, 5, 10, 20,
30, 40, 60, 80, 100,
120, 150, 180, 240, 300, 360, 420, 480, or 540 minutes or 8,9, 10, 11, 12, 14,
16, 18, or 24
hours, or 1.5, 2, 3, 4, 5, or 6 days after formation of the wash water. The
carbonation can
continue for any suitable period of time, for example, in certain embodiments
wash water is
continuously exposed to carbon dioxide for a period of time after carbonation
commences.
Alternatively or additionally, wash water can be carbonated just before its
use as mix water,
for example, no more than 1, 2, 5, 10, 20, 30, 40, 60, 80, 100, 120, 150, 180,
240, 300, 360,
420, or 480 minutes before its use as mix water (e.g., before contacting the
concrete mixture),
and/or no sooner than 0,0,5, 1, 2,5, 10, 20, 30, 40, 60, 80, 100, 120, 150,
180, 240, 300,
360, 420, 480, or 540 minutes before its use as mix water. Additionally or
alternatively, the
wash water may be aged for some amount of time after addition of carbon
dioxide before it is
used as wash water, for example, carbonated wash water can be used as mix
water no later
than 1, 2, 5, 10, 20, 30, 40, 60, 80, 100, 120, 150, 180, 240, 300, 360, 420,
or 480 minutes, or,
-25-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
7, 8, 9, 10, 12, 18, or 24 hours, or 1,5, 2, 3, 4, 5, or six days after
carbonation of the wash
water, and/or no sooner than 0,0.5, 1, 2,5, 10, 20, 30, 40, 60, 80, 100, 120,
150, 180, 240,
300, 360, 420, 480, or 540 minutes or 8, 10, 12, 18, 24 hours, or 1.5, 2, 3,
4, 5, 6, 7, 8, 10, 12,
or 14 days after carbonation of the wash water; for example, at least 3 hours,
at least 6 hours,
at least 12 hours, at least one day, at least 3 days, or at least 5 days after
carbonation of the
wash water.
101591 The water used for washing may be clean water or recycled wash water.
In certain
embodiments, the water that is used to wash out trucks may be carbonated
before and/or
during the wash process, i.e., before the wash water enters a reclamation
tank. Concrete
trucks typically have 10-15 min of mixing when washing out. Carbon dioxide can
be, e.g.,
injected into the water pump line on its way to the truck (fresh water input),
or from the
settlement pond/reclamation system pump (recycled water input).
101601 Additionally or alternatively, after a truck is emptied and water is
added to the truck
for washing, carbon dioxide can be added to the truck. The carbon dioxide
reacts with the
slurry, and the carbon dioxide can "put the cement to sleep" (e.g., halt or
retard most or all
deleterious reactions, and react with most or all deleterious materials, as
outlined herein). In
certain embodiments, the slurry can be reused in a new batch. In certain
embodiments, the
slurry need not even leave the truck. Carbon dioxide can be added as a solid,
liquid, or gas,
or combination thereof For example, carbon dioxide may be added as a solid. In
certain
embodiments, carbon dioxide is added as a mixture of solid and gas, produced
when liquid
carbon dioxide is released to atmospheric pressure. A conduit carries liquid
carbon dioxide
from a container to an injector, which is configured so as to cause a desired
conversion to gas
and solid. The mixture of gaseous and solid carbon dioxide is directed into
the drum of a
ready mix truck. The amount of carbon dioxide added may be a predetermined
amount,
based, e.g., on typical residual amounts of concrete left in the truck. The
amount of carbon
dioxide added may also be regulated according to the condition of the wash
water, e.g.,
according to pH as the carbon dioxide mixes and reacts with components of the
wash water.
Using this method, it is possible to eliminate the need to discharge wash
water from the
mixer. This allows the wash water to be used as mix water in the next batch of
concrete
produced and prevents the residual plastic concrete from hardening. In certain
embodiments,
the treatment allows stabilization of the wash water, so that it can be used
as mix water for
the next batch, after at least 0.5, 1, 2, 3, 4, 5, 6, 12, 18, 24, 30, 36, 42,
48, 54, 60, 66, 72, 78,
86, or 92 hours and/or not more than 12, 18, 24, 30, 36, 42, 48, 54, 60, 66,
72, 78, 86, 92, or
104 hours. The carbon dioxide treatment may be used alone or used with other
treatments
-26-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
that are designed to stabilize wash water and allow reuse, such as Recover,
GCP Applied
Technologies, Inc., Cambridge, Mass., or similar admixture.
101611 In certain embodiments, the wash water is circulated before its use as
a mix water.
For example, part or all of the wash water that is carbonated may be
circulated (e.g., run
through one or more loops to, e.g., aid in mixing and/or reactions, or
agitated, or stirred, or
the like). This circulation may occur continuously or intermittently as the
water is held prior
to use. In certain embodiments the wash water is circulated for at least 5,
10, 20, 50, 70, 80,
90, 95, or 99% and/or not more than 10, 20, 50, 70, 80, 90, 95, 99 or 100% of
the time it is
held prior to use as mix water.
[0162] It will be appreciated that many different wash waters are typically
combined and
held, for example, in a holding tank, until use or disposal. Carbonation of
wash water may
occur before, during, or after its placement in a holding tank, or any
combination thereof.
Some or all of the wash water from a given operation may be carbonated. It is
also possible
that wash water from one batch of concrete may be carbonated then used
directly in a
subsequent batch, without storage. In general, the tank will be outfitted or
retrofitted to allow
circulation of the water in such a way that sedimentation does not occur, to
allow reuse of
materials in the wash water as it is carbonated.
101631 Any suitable method or combination of methods may be used to carbonate
the wash
water. For example, the wash water may be held in a container and exposed to a
carbon
dioxide atmosphere while mixing. Carbon dioxide may be bubbled through mix
water by any
suitable method; for example, by use of bubbling mats, or alternatively or
additionally, by
introduction of carbon dioxide via one or more conduits with one or a
plurality of openings
beneath the surface of the wash water. The conduit may be positioned to be
above the sludge
that settles in the tank and, in certain embodiments, regulated so as to not
significantly
impede settling. Catalysts may also be used to accelerate one or more
reactions in the
carbonating wash water. In certain embodiments, liquid carbon dioxide
injection is used. A
vaporizer can be set inside the tank and converts liquid carbon dioxide to
gas, drawing heat
from the water to do so, and thereby cooling the water. For example, a series
of metal tubes
may be submerged in the water that are configured to ensure gas rises to the
top and is pushed
out of a nozzle. Pipes run vertically, but with the heat capacity and transfer
rate in water
being so much higher than air, fins that are normally be present in a
cryogenic carbon dioxide
heat exchanger that operates in air may not be needed.
Impeller blades
-27-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
[0164] In certain embodiments, carbon dioxide is added to a slurry tank by
injecting it
through a specially designed agitator blade. As known in the water treatment
industry, a flash
mixing style blade can be used that is designed to create turbulence,
vortices, vacuum pockets
and high shear behind the mixer blades to promote rapid mixing action. See,
e.g., blades
supplied by Dynamix Inc., 14480 River Road, Unit 150, Richmond, British
Columbia,
Canada V6V 1L4, such as the P4 Pitch Impeller Blade. This is merely exemplary
and those
of skill in the art will recognize that various types, such as pitch-blade
impellers or airfoil
impellers may be used.
[0165] Injection of carbon dioxide at a particular location along the blade
edge can increase
mixing action and contact time. The blade action forces the carbon dioxide
bubbles to
undergo more mixing rather than being buoyantly forced towards the surface.
Fine dispersed
bubbles can be assured through selecting the proper hole size. It is important
to ensure that
the holes remain unplugged. Whereas a perforated hose in the bottom of a tank
with have
solids settle upon it when the slurry is unagitated, the agitator blade holes
will not be at the
bottom of a tank and get covered by the settling solids. Further the holes can
be placed on the
sides or bottom of the agitator element to avoid vertical settlement buildup.
Augur
[0166] In a pond where an auger is used for mixing, injection can be through
the central axis
of the auger shaft. In certain embodiments, to ensure serviceability and
possibly to reduce
the occurrence of buildup, a retractable injection pipe with a gas
distribution nozzle at the end
can be routed through the central axis of the mixing auger shaft The carbon
dioxide can be
injected, e.g., when a control system calls for it and then the injector can
retract out of the
water when the system has determined that the amount of carbon dioxide is
sufficient.
Alternately, a retractable injector is not routed through the shaft, but the
shaft is simply
hollow. Carbon dioxide can be injected down the center of the mixing auger
shaft. An
orifice at the injection point can promote the formation of finely dispersed
bubbles. Either
way, the injector nozzle positioning, direction, and injection speed are such
that they do not
interfere with normal mixing, so that sedimentation does not occur.
Submersible pump
[0167] A suitably efficient or powerful pump can both circulate the slurry and
also, in some
cases, send the slurry to the concrete batching process. Carbon dioxide can be
integrated
with the pump via, for example, injection into the impeller housing at a
location chosen to
maximize mixing, or, for example, just under the intake to allow the suction
to bring the gas
into the housing. The impeller blades mix up and pressurize the carbon
dioxide/wastewater
-28-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
mix, providing better uptake of carbon dioxide, and pump the slurry through a
long hose. The
transport in the hose provides additional time to promote uptake. The shiny
can be directed
back into the tank or pumped directly into the batch process.
[0168] The CO2 injection rate can be tied to the flow rate/density of the
slurry. If one cycle
through the loop is insufficient to provide the desired degree of carbon
dioxide uptake, then it
can be recirculated through the same loop or through another loop, e.g.., via
a secondary,
smaller pump, until the desired amount of CO2 has been absorbed.
[0169] Carbon dioxide injection can take place near an impeller. Carbon
dioxide injection
can also take place in a discharge pipe line, near the pump itself or at any
point in the pipe
line. Carbon dioxide injection can be achieved with single or multiple
injection points and
carbon dioxide can be injected at 90 degrees or any suitable angle relative to
the direction of
flow. Directing the carbon dioxide exit parallel to the rising liquid flow
will increase liquid
flow as the buoyancy of the carbon dioxide displaces the wash water upwards.
Eductor nozzles
[0170] In certain embodiments, one or more eductor nozzles are used. Eductor
nozzles are
well-known in the art. An eductor nozzle mixes and agitates, and increases
overall water
flow, thus allowing a smaller pump to move sufficient water to ensure adequate
mixing to
prevent sedimentation. The nozzle allows high pressure into a first stage
nozzle to increase
velocity, then the eductor provides a venturi effect of high velocity flow
which creates low
pressure, pulling added liquid into the stream of flow, and allowing higher
volume lower
velocity output Such nozzles are supplied by, e.g., Bete Ltd., P.O. Box 2748,
Lewes, Fast
Sussex, United Kingdom. Such a nozzle can incorporate carbon dioxide injection
into its
operation. If carbon dioxide is injected as nanobubbles in solution
(supersaturated carbon
dioxide water, see elsewhere in this application, e.g., systems supplied by
Gaia USA Inc.,
Scottsdale, Arizona) then the buoyancy that acts upon coarse bubbles may be
avoided.
Pumps can be used for mixing, provided they are placed strategically and
provide sufficient
flow.
[0171] In certain embodiments, a combination of mixing blades and sump pump
with
eductor may be used, so long as the pump or pumps is in a non-intrusive
location and does
not impede the mixing action required. The discharge of water and carbon
dioxide (eductor)
is in a location that does not disturb the blade mixing action. Most reclaimer
blades push
material downward so it is preferred to discharge the pump water/carbon
dioxide near the
axis of the blades to help promote mixing. In certain embodiments, an
integrated mixing and
injection process is used: Strategically placed eductor nozzles can be used to
carbonate water
-29-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
and maintain sufficient fluid flow. The eductors are fed by a pump or pumps
which can
incorporate carbon dioxide in several ways, as described herein. For
retrofitting of existing
wash water settlement ponds, a series of eductors can be configured to mix the
pond. It is
important to ensure the eductor configuration keeps the water flow throughout
the tank above
the settlement velocity of suspended solids.
Head space integration
[0172] If the treatment vessel is a closed container then increased efficiency
can be had by
recycling gas from the headspace into the injection hardware. As bubbles rise
through the
liquid to join the headspace such an approach allows the carbon dioxide
molecules another
chance to dissolve and react. The process can monitor the headspace gas for
carbon dioxide
and pressure. For a given fixed mass of carbon dioxide injected the carbon
dioxide content
and pressure will initially increase. As reaction proceeds the carbon dioxide
concentration
and pressure will decrease. This can be a signal that causes another dose of
carbon dioxide.
The dosing efficiency of the dose is in direct response to the absorption.
Super-saturated carbon dioxide
[0173] In certain cases, mix water, e.g., wash water may be treated with
carbon dioxide in
such a manner that the carbon dioxide content of the water increases beyond
normal
saturation, for example, at least 10, 20, 30,40, 50, 70, 100, 150, 200, or
300%, or not more
than 10, 20, 30, 40, 50, 70, 100, 150, 200, 300, 400, or 500% beyond normal
saturation,
compared to the same water under the same conditions that is normally
saturated with carbon
dioxide. Normal saturation is, e.g., the saturation achieved by, e.g.,
bubbling carbon dioxide
through the water, e.g., wash water, until saturation is achieved, without
using manipulation
of the water beyond the contact with the carbon dioxide gas. For methods of
treating water to
increase carbon dioxide concentration beyond normal saturation levels, see,
e.g., U.S. Patent
Application Publication No. 2015/0202579.
[0174] In certain embodiments, washwater is exposed to carbon dioxide in a
conduit, where
wash water is pulled from a source of washwater, such as a slurry pond,
through an input into
the conduit, and moved through the conduit to an output. In certain
embodiments, the treated
washwater is conducted from the output to a concrete mixing operation; that
is, exposure to
carbon dioxide occurs outside the source of washwater, and the system can
operate as an on-
demand washwater carbonation system. The water thus carbonated can be used in
a concrete
mix, disposed of, or used in any other appropriate manner. This type of system
can be easily
retrofitted into virtually any existing washwater system, since most or all of
the injection
system stands alone from the source of washwater, e.g., slurry pond. The
conduit is operably
-30-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
connected to a source of carbon dioxide at one or more injection points for
carbon dioxide,
such as at least 1, 2, 3, 4, 5, 6,7, 8, 9, 10, 12, 15, or 20 injection points,
and/or not more than
2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 15, 20, or 30 injection points. At each
injection point, carbon
dioxide is injected into a flowing stream of washwater slurry. If more than
one injection
point is used, the injection points are sufficiently spaced from one another
that, with the
appropriate flow rate for the slurry and injection rate for the carbon
dioxide, together with the
diameter of the conduit, and cement content of the washwater, carbon dioxide
is injected as
bubbles at the injection point, with each bubble separated from each other (or
at least 50, 60,
70, 80, or 90% of the bubbles separated from each other), and by the end of
the conduit
section the carbon dioxide from the injection is at least 20, 30, 40, 50, 60,
70, 80, 90, 95, or
99% absorbed and/or reacted by the slurry, and/or not more than 30, 40, 50,
60, 70, 80, 90,
95, 99, or 100% absorbed and/or reacted by the slurry. See Figure 92 for an
illustration of
one section of an injector system. The conduit can comprise any suitable
number of injection
points, as described, thus allowing for carbon dioxide to be added in each
section and a
desired carbon dioxide uptake to be achieved. Thus, merely as an example, a
single section
may allow for, e.g., 2% carbon dioxide uptake and a desired carbon dioxide
uptake may be
10%, so the conduit would have 5 sections/injection points. In certain
embodiments, the
sections are contiguous; however, it is also possible to have one or more
sections separated
from the others, with non-contiguous sections operably connected by a conduit;
this may help
to utilize available space, e.g., allow for multiple sections to be used with
minimal height
requirement, compared to a contiguous system. Additionally or alternatively,
to increase
carbon dioxide uptake, washwater may be recirculated through the system, so
that with each
pass the washwater more carbon dioxide is taken up; thus in certain
embodiments, the
washwater is recirculated at least 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10 times
and/or not more than 2, 3,
4, 5, 6, 7, 8, 9, 10, 15, or 20 times. Any suitable orientation of the system
may be used. In
certain embodiment, the conduit (or conduit sections, if sections are non-
contiguous) is
positioned to be vertical, such as within 1, 2, 5, 10, 15, 20, 30, 40, or 50%
of vertical. In
certain embodiments, one or more of the sections is configured to mix the
washwater as it
moves through. Sample calculations for system parameters and additional
description are
given in Example 27.
101751 In certain embodiments, the invention allows the use of wash water
substantially "as
is," that is, without settling to remove solids. Carbonation of the wash water
permits its use
as mix water, even at high specific gravities.
-31-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
101761 This technology can allow the use of grey (wash) water as mix water,
where the grey
(wash) water is at specific gravities of at least 1.01, 1.02, 1.03, 1.04,
1.05, 1.06, 1.07, 1.08,
1.09, 1.10,1.11, 1.12, 1.13, 1.14, 1.15, 1.16, 1.17, 1.18, 1.19, 1.20, 1.22,
1.25, 1.30, 1.35,
1.40, or 1.50, and/or not more than 1.02, 1.03, 1.04, 1.05, 1.06, 1.07, 1.08,
1.09, 1.10, 1.11,
1.12, 1.13, 1.14, 1.15, 1.16, 1.17, 1.18, 1.19, 1.20, 1.22, 1.25, 1.30, 1,35,
1.40, 1.50 or 1.60;
e.g., 1.0-1.2, or 1.0 to 1.3, or 1.0 to 1.18, or 1.0 to 1.16, or 1.0 to 1.15,
or 1.0 to 1.14, or 1.010
1.13, or 1.0 to 1.12, or 1.0 to 1.10, or 1.0 to 1.09, or 1.0 to 1.08, or 1.0
to 1.07, or 1.0 to 1.06,
or 1.0 to 1.05, or 1.010 1.04, or 1.0 to 1.03, or 1.0 to 1.02, 1.01-1.2, or
1.01 to 1.3, or LO1 to
1.18, or 1.01 to 1.16,01 1.01 to 1.15, or 1.01 to 1.14, or 1.01 to 1.13, or
1.01 to 1.12, or 1.01
to 1.10, or LO1 to 1.09, or 1.01 to 1.08, or 1.01 to L07, or 1.01 to 1.06, or
LO1 to L05, or
1.01 to 1.04, or 1.01 to 1.03, or 1.01 to 1.02, or 1.02-1.2, or 1.02 to 1.3,
or 1.02 to 1.18, or
1.02 to 1.16, or 1.0210 1.15, or 1.02 to 1.14, or 1.02 to 1.13, or 1.02 to
1.12, or 1.02 to 1.10,
or 1.02 to 1.09, or 1.02 to 1.08, or 1.02 to 1.07, or 1.02 to 1.06, or 1.02 to
1.05, or 1.02 to
1.04, or 1.02 to 1.03,01 1.03-1,2, or 1.03 to 1.3, or 1.03 to 1.18, or 1.03 to
1.16,01 1.03 to
1.15, or 1.03 to 1.14, or 1.03 to 1.13, or 1.03 to 1.12, or 1.03 to 1.10, or
1.03 to 1.09, or 1.03
to 1.08, or 1.03 to 1.07, or 1.03 to 1.06, or 1.03 to 1.05, or 1.03 to 1.04,
or 1.05-1.2, or 1.05 to
1.3, or 1.0510 1.18, or 1.05 to 1.16, or 1.05 to 1.15, or 1.05 to 1.14, or
1.05 to 1.13, or 1.05 to
1,12, or 1.05 to1.10, or 1.05 to 1,09, or 1.05 to 1,08, or 1.05 to 1.07, or
1.05 to 1.06. In
certain embodiments the methods and compositions of the invention allow the
use of grey
(wash) water as mix water, where the grey water has a specific gravity of at
least 1.01, 1.02,
1.03, 1.04, 1.05, 1.06, 1.07, 1.08, 1.09, 1.10, 1.11, 1.12, 1.13, 1.14, 1.15,
1.16, 1.17, 1.18,
1.19, or 1.20. The methods and compositions of the invention can reduce or
even eliminate
the need to further treat wash water, beyond carbonation, for the wash water
to be suitable for
use as mix water in a subsequent batch. In certain embodiments, after grey
(wash) water is
carbonated, it is used in subsequent batches of concrete with no more than 5,
10, 15, 20, 30,
40, 50, 60, 70, 80, 90, 01 95% of remaining solids removed. In certain
embodiments, none of
the remain solids are removed. The carbonated wash water may be combined with
non-wash
water, e.g., normal mix water, before or during use in a subsequent concrete
batch, to provide
a total amount of water used in the batch; in certain embodiments, the
carbonated wash water
comprises at least 5, 10, 15, 20, 30, 40, 50, 60, 70, 80, 90, 95, or 99% of
the total amount of
water used in the batch; in certain embodiments, 100% of the total amount of
water used in
the batch is carbonated wash water, excluding water used to wash down
equipment and, in
some cases, excluding water added at the job before or during pouring of the
concrete mix.
-32-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
10177] The use of wash water in a concrete mix, especially carbonated wash
water, often
results in enhanced strength of the resulting concrete composition at one or
more times after
pouring, for example, an increase in compressive strength, when compared to
the same
concrete mix without carbonated wash water, of at least 1, 2, 3, 4, 5, 6, 7,
8, 9, 10, 12, 14, 16,
18, 20, 22, or 25% at 1-day, 7-days, and/or 28-days. This increase in early
strength, as well
as additionally or alternatively the presence of cementitious materials in the
carbonated wash
water that can replace some of the cementitious materials in a subsequent mix,
often allows
the use of less cement in a mix that incorporates carbonated wash water than
would be used
in the same mix that did not incorporate carbonated wash water; for example,
the use of at
least 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 20, 22,
25, 30, 35, or 40% less
cement in the mix where the mix retains a compressive strength at a time after
pouring, e.g.,
at 1, 7, and/or 28-days, that is within 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 15,
20, 30, 40, or 50% of
the compressive strength of the mix that did not incorporate carbonated wash
water, e.g.,
within 5%, or within 7%, or within 10%.
10178] In addition, the carbonation of wash water can allow the use of wash
water at certain
ages that would otherwise not be feasible, e.g., wash water that has aged at
least 1, 2, 3,4, 5,
6, 7, 8, 9, 10, 12, or 15 days. Wash water that has been carbonated may be
used in concrete
at an age where it would otherwise produce a concrete mix without sufficient
workability to
be used.
10179] The CO2 treatment produces carbonate reaction products that likely
contain some
amount of nano-structured material. Of the carbonated products in the wash
water, e.g.,
calcium carbonate, at least 1, 2, 5, 7, 10, 12, 15, 20, 25, 30, 25, 40, 45,
50, 60, 70, 80, or 90%
may be present as nano-structured materials, and/or not more than 5, 7, 10,
12, 15, 20, 25, 30,
25, 40, 45, 50, 60, 70, 80, 90, 95, or 100% may be present as nano-structured
material. A
"nano-structured material," as that term used herein, includes a solid product
of reaction of a
wash water component with carbon dioxide whose longest dimension is no more
than 500
nm, in certain embodiments no more than 400 rim, in certain embodiment no more
than 300
rim, and in certain embodiments no more than 100 nm.
10180] Carbon dioxide treatment of wash water can result in a solid material
that is distinct
from untreated wash water in terms of the coordination environment of aluminum
and silicon
crosslinking, e.g., as measured by NMR. Without being bound by theory, it is
thought that
carbon dioxide treatment of the wash water can create a carbonate shell around
the particle,
and that this shell can have an inhibiting effect on the phases contained
therein, perhaps
physically inhibiting dissolution.
-33-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
[0181] The CO2 treatment has the further benefit of sequestering carbon
dioxide, as the
carbon dioxide reacts with components of the wash water (typically cement Of
supplementary
cementitious material), as well as being present as dissolved carbon
dioxide/carbonic
acid/bicarbonate which, when the wash water is added to a fresh concrete mix,
further reacts
with the cement in the mix to produce further carbon dioxide-sequestering
products. In
certain embodiments, the carbon dioxide added to the wash water results in
products in the
wash water that account for at least 1, 2, 5, 7, 10, 12, 15, 20, 25, 30, 25,
40, 45, 50, 60, 70, 80,
Of 90% carbon dioxide by weight cement (bwc) in the wash water, and/or not
more than 2, 5,
7, 10, 12, 15, 20, 25, 30, 25, 40, 45, 50, 60, 70, 80, 90, 95, or 100% carbon
dioxide by weigh
cement (bwc) in the wash water.
[0182] Embodiments include applying CO2 immediately after the wash water is
generated,
in a tank, and/or as the grey water is being loaded for batching.
[0183] Alternatively or additionally, carbonation of grey (wash) water can
allow use of aged
wash water as mix water, for example, wash water that has aged at least 1, 2,
3, 4, 5, 6, 7, 8,
9, or 10 days.
[0184] The source of the carbon dioxide can be any suitable source. In certain
embodiments, some or all of the carbon dioxide is recovered from a cement kiln
operation,
for example, one or more cement kiln operations in proximity to the concrete
production
facility, e.g., one or more cement kiln operations that produce cement used in
the concrete
production facility. In certain embodiments, wash water is transported from a
concrete wash
station or similar facility where concrete wash water is produced, to a cement
kiln, or a power
plant and flue gas from the cement kiln or power plant is used to carbonate
the wash water.
Carbon dioxide concentrations in cement kiln flue gas or power plant flue gas
may be
sufficient that no additional carbon dioxide is needed to carbonate the wash
water; it is also
possible that the flue gas need not be completely treated before exposure to
wash water; i.e.,
it will be appreciated that cement kiln and power plant flue gas, in addition
to containing
carbon dioxide, may also contain S0x, NOx, mercury, volatile organics, and
other substances
required to be removed, or brought to an acceptable level, before the flue gas
is released to
the atmosphere. In certain embodiments, the flue gas is treated to remove one
or more of
these substances, or bring them to acceptable levels, before it is exposed to
the wash water.
In certain embodiments, one or more of these substances is left in the flue
gas as it contacts
the wash water, and after contacting the wash water the amount of the
substance in the flue
gas is reduced, so that further treatment for that substance is decreased or
eliminated. For
example, in certain embodiments, the flue gas comprises S0x, and treatment of
the wash
-34-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
water with the flue gas decreases the amount of SOx in the flue gas (e.g., by
formation of
insoluble sulfates) so that the flue gas after wash water treatment requires
decreased
treatment to remove SO-x, or no treatment. Additionally or alternatively, one
or more of
NOx, volatile organics, acids, and/or mercury may be decreased in the flue gas
by contact
with wash water so that the need for treatment of the flue gas for the
substance is reduced or
eliminated. After treatment with the flue gas, the carbonated wash water may
be transported
to a concrete production facility, either the same one where it was produced
and/or a different
one, and used in producing concrete at the facility, e.g., used as an
admixture, e.g., to reduce
cement requirements in the concrete due to the cement in the wash water.
[0185] The wash water may be monitored, e.g., as it is being carbonated. Any
suitable
characteristic, as described herein, may be used to determine whether to
modify carbon
dioxide delivery to the wash water. One convenient measurement is pit For
example, in
certain embodiments, a carbonated wash water of pH less than 8.0, 7.9, 7.8,
7.7, 7.6, 7.5, 7.4,
7.3, 7.2, 7.1, or 7.0 is desired, e.g., to be used as a mix water. The pH may
be monitored and
brought to a suitable pH or within a suitable range of pHs before, e.g., its
use as a mix water.
For example, the pH can be at least 6.0, 6.1, 6.2, 6.3, 6.4,6.5, 6.6, 6.7,
6.8, 6.9, 7.0, 7.1, 7.2,
7.3, 7.4, 7.5, 7.6, 7.7, 7.8, 7.9, 8.0, 8.1, 8.2, 8.3, 8.4, or 8.5, and/or not
more than 6.1, 6.2, 6.3,
6,4, 6.5, 6.6, 6.7, 6.8, 6.9, 7.0, 7.1, 7,2, 7.3, 7.4, 7.5, 7.6, 7.7, 7.8,
7.9, 8.0, 8.1, 8.2, 8,3, 8.4,
8.5, 8.7, 9.0, 9.3, 9.5,9.7, 10, 10.3, 10.5, 10.7, 11.0, 12.0, or 13Ø
[0186] In addition, it is desirable that gas flow in a wash water, e.g., in a
holding tank, not
be increased to a level high enough that the rate of supply exceeds the rate
of
absorption/reaction; if this occurs, typically, bubbles will be observed at
the surface of the
wash water. If the rate of supply is equal to or less than the rate of
absorption/reaction, then
no bubbles are observed at the surface of the wash water. The rate of
absorption and reaction
may change with time, for example, decreasing as more of particles react or
become coated
with reaction products. Thus, appearance of bubbles may be used as an
indicator to adjust
carbon dioxide flow rate, and an appropriate sensor or sensors may be used to
determine
whether or not bubbles are appearing. Alternatively, or additionally, carbon
dioxide content
of the air above the surface of the wash water may be monitored using
appropriate sensor or
sensors and be used as a signal to modulate delivery of carbon dioxide to the
wash water,
e.g., slow or stop delivery when a certain threshold concentration of carbon
dioxide in the air
above the surface is reached. Rate of change of concentration can also be used
as an
indicator to modulate flow rate of carbon dioxide.
-35-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
101871 Bubble formation, in particular, is to be minimized or avoided, because
in a tank
where water is agitated to prevent settling of solids, it is desired to use
the minimum amount
of energy to cause the water to move in a pattern with sufficient motion that
solids remain
suspended; bubbles, which automatically rise to the surface no matter where
they are in the
overall flow pattern of the tank, can disrupt the flow, and cause more energy
to be required
for sufficient agitation. In a holding tank in which, e.g., an augur is used
for agitation,
systems of the invention may pull water from the tank into a recirculation
loop where carbon
dioxide is introduced. The rate of introduction, length of the loop, and other
relevant factors
are manipulated so that carbon dioxide is absorbed into the water and/or
reacts with
constituents of the water before it's released back into the tank. The carbon
dioxide can be
input into the loop near or at the start of the loop, so that there is maximum
distance for the
carbon dioxide to be absorbed and/or react. It is also advantageous to inject
the carbonated
water at a downward location in the tank.
101881 Additional characteristics that can be useful to monitor include
temperature of the
wash water (reaction of carbon dioxide with cement products is typically
exothermic), ionic
concentration of the wash water, electrical conductivity of the wash water,
and/or optical
properties of the wash water (e.g., it has been observed that carbon dioxide
can change the
color of the wash water). Appropriate sensors for one or more of these
characteristics may be
included in an apparatus of the invention. Other characteristics and sensors
are also
appropriate as described herein.
101891 Compositions include an apparatus for carbonating concrete wash water
in a wash
water operation that includes a source of carbon dioxide operably connected to
a conduit that
runs to a wash water container containing wash water from a concrete
production site, where
one or more openings of the conduit are positioned to deliver carbon dioxide
at or under the
surface of wash water in the container, or both, and a system to transport the
carbonated wash
water to a concrete mix operation where the carbonated wash water is used as
mix water in a
concrete mix, e.g. a second conduit that can be positioned to remove
carbonated wash water
from the wash water container and transport it to a concrete mix operation,
where the
carbonated wash water is used as part or all of mix water for concrete
batches. Generally, the
carbon dioxide will be delivered directly to the wash water tank as described
elsewhere
herein, though in some embodiments carbonation may occur outside the tank and
the
carbonated water returned to the tank. The apparatus may further include a
controller that
determines whether or not to modify the delivery of carbon dioxide based at
least in part on
one or more characteristics of the wash water or wash water operation. The
characteristics
-36-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
may include one or more of pH of the wash water, rate of delivery of carbon
dioxide to the
wash water, total amount of wash water in the wash water container,
temperature of the wash
water, specific gravity of the wash water, concentration of one or more ions
in the wash
water, age of the wash water, circulation rate of the wash water, timing of
circulation of the
wash water, bubbles on surface, carbon dioxide concentration of air above
surface, optical
properties, electrical properties, e.g., conductivity, or any combination
thereof One or more
sensors may be used for monitoring one or more characteristics of the wash
water,
additionally, or alternatively, manual measurements may be made periodically,
e.g., manual
measurements of specific gravity, pH, or the like. The apparatus may further
comprise one or
more actuators operably connected to the controller to modify delivery of
carbon dioxide to
the wash water, or another characteristic of the wash water, or both. The
apparatus may
include a system for moving the wash water, such as by circulating or
agitating the wash
water, either continuously or intermittently. The composition may further
include a delivery
system for delivering carbon dioxide to the source of carbon dioxide, where
some or all of the
carbon dioxide is derived from a cement kiln operation in proximity to the
concrete
production site, for example, a cement kiln operation that produces some or
all of cement
used in the concrete production site.
101901 In certain embodiments, solids are removed from the carbonated wash
water, for
example, by filtration. These solids, which mostly comprise carbonated cement
particles, can
be further treated, e.g., dried. The dried solids can then be, e.g., re-used
in new concrete
batches.
Carbonation of wash water in ready-mix truck, reclaimer, and/or lines.
101911 In certain embodiments, concrete wash water is carbonated directly in
the drum of a
ready-mix truck and/or before it reaches a holding tank, e.g., during cycling
in a reclaimer, or
in the line between a reclaimer and a holding tank.
101921 In a typical operation, a ready-mix truck is loaded at a hatching
facility; the load may
be a partial load or a full load. A hill load may be several cubic meters,
e.g., 8 m3, depending
on the size of the truck. However, regardless of the size of the load, a large
portion, in some
cases virtually all, of the drum and interior components of the drum (e.g.,
fins, etc.), come in
contact with the wet cement. The load is then released at the job site and the
truck returns to
a wash facility, usually at the hatching facility, where it is cleaned prior
to further hatching.
After the load is released at the job site, a certain amount of water that is
carried in containers
on the truck (typically called saddlebags) can be released into the truck and
mixed in the
truck at the site and during the trip back to the wash station, to prevent the
wet concrete from
-37-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
hardening during the time before the truck is cleaned at the wash station.
Additional water is
then introduced into the drum at the wash station, with spraying and mixing to
thoroughly
clean the interior of the drum, and the resultant wash water is then either
dumped, or, more
commonly, sent to one or more tanks to be treated prior to disposal and/or
reuse.
101931 Typically, around 100-160 (e.g., 120) L wash water/m3 of concrete is
used to wash
the truck; however, as stated, since partial loads result in a coating of the
empty truck that is a
greater part of the truck than the proportion of the load to a full load, and
in some cases result
in a completely coated empty truck drum, in some cases in which there has been
a partial load
a more realistic estimate of the amount of water needed is larger than the
120L/m3 of
concrete. For example, if the total capacity of the truck is 8 m3 and a 4 m3
load is delivered,
it is possible that the amount of wash water will be greater than 4 x 120 L,
perhaps as much
as that used for a full load, e.g., 8 x 120 L or 960 L. For any particular
operation, the amount
of water needed for a particular size load and mix type is generally known and
can be used in
any calculations required.
101941 In some facilities, a reclaimer is used to separate out aggregate
(e.g., sand and gravel)
from the wash water, generally for reuse in further concrete batches. The
remainder of the
wash water is generally sent to a settlement pond to settle out further
solids, or, alternatively,
it is pumped into a slurry tank where it is kept suspended with paddles and
diluted to a
specific gravity and otherwise treated so that at least some of the water may
be used again in
concrete production. In a conventional reclaimer process, not all of the
treated wash water
produced can be reused, e.g. in concrete, and the overflow is sent to a
holding pond, where it
is disposed of in the conventional manner
101951 In certain embodiments, provided herein are methods and compositions
for
carbonating wash water in a system that includes a reclaimer, i.e., a system
that includes a
mechanism for removing aggregate from wash water. The methods and compositions
can be
used to create a de novo system, but are advantageously also used in retrofits
of existing
systems. Generally, in a reclaimer system, wash water is passed through an
apparatus to
remove a portion of the aggregate and other solids in the wash water; an
exemplary system is
a rotating perforated drum from which treated wash water, with a lower
proportion of solids,
is sent to a holding tank. As a new truck comes in to be cleaned, water can be
provided to
wash the truck drum and/or other components, for example, with a portion or
all of the water
coming from the holding tank. The drum and/or other components are washed, the
washwater moves through a system to remove a portion of solids, then is sent
back to the
tank. In many systems, a recirculation loop is provided back from the tank to
the system for
-38-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
removing solids, so that water can have a plurality of passes through the
solid-removing
system In certain embodiments, a portion of this recirculation line is used to
carbonate the
wash water. For example, a section of the line can be replaced with a system
that comprises
two conduits. The first is a conduit for the wash water and the second is a
conduit, within the
first conduit, to supply carbon dioxide to the wash water. In order to keep
flow unimpeded,
the first conduit can be of a larger diameter than the conduit leading up to
and away from the
carbonation section, i.e., the conduit used in the system before carbonation.
For example, if
the system uses a 2 inch-diameter conduit, the first conduit of the
carbonation section may be
more than 2 inch-diameter, for example, 3 inches. This is merely exemplary,
and the
diameter of the first conduit relative to the portion of conduit that is not
in the carbonation
section can be any suitable multiple of non-carbonation conduit, so long as
flow through the
first conduit is not impeded or not substantially impeded, e.g., so long as
flow through the
first conduit is sufficient for the purposes of the system. In general, unless
otherwise
indicated, diameters are outside diamters. For example, the diameter of the
first conduit of
the carbonation section can be at least 1.01, 1.02, 1.05, 1.07, 1.1, 1.12,
1.15, 1.17, 1.2,1.25,
1.3, 1.35, 1.4, 1.45, 1.5, 1.6, 1.7, 1.8, 1.9, 2.0, 2.2, 2.5, 3.0, 3.5, 4, 5,
6, 7, 8, or 9 times the
diameter of the non-carbonation portion of conduit, e.g, the diameter of the
recirculation
conduit leading up to the carbonation section, and/or not more than 1.02,
1.05, 1.07, 1.1, 1.12,
1.15, 1.17, 1.2, 1.25, 1.3, 1.35, 1.4, 1.45, 1.5, 1.6, 13, 1.8, 1.9, 2.0, 2.2,
2.5, 3.0, 3.5, 4, 5, 6,
7, 8 9, or 10 times the diameter of the non-carbonation portion of conduit,
e.g, the diameter of
the recirculation conduit leading up to the carbonation section, for
example,1.1-3x, or 1.2-
2.x, or 1.3-2x the diameter. In certain embodiments, the first conduit is 0.5-
10 inches, or 1-8
inches, or 1.5-7 inches, or 1.5-5 inches, or 2-5 inches, or 2-4 inches in
diameter. The first
conduit may join the non-carbonation sections of the recirculation loop by any
suitable
fixture; in some embodiments, the first conduit is fitted to the non-
carbonation conduit at its
proximal and/or distal ends so that the low point (bottom) of the first
conduit is even with, or
not substantially offset from, the low point (bottom) of the non-carbonation
conduit. Without
being bound by theory it is thought that this arrangement wherein the centers
of the conduits
are offset but the low points are even or substantially even prevents settling
or trapping of
solids as the non-carbonation conduit expands into the first conduit. However,
any suitable
configuration that prevents or limits solid accumulation in the first conduit
may be used.
10196] The second conduit in the carbonation section is situated inside the
first conduit, and
supplies carbon dioxide gas to carbonate the wash water flowing through the
first conduit.
The second conduit is configured to allow carbon dioxide gas supplied in the
second conduit
-39-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
to flow into the first conduit but not to allow wash water from the first
conduit to flow into
the second conduit. For example, the second conduit may be made of pliable
material that
comprises perforations that essentially act as one-way valves, closing off and
not allowing
water into the second conduit but allowing gas from the second conduit through
into the first
conduit when gas is supplied to the second conduit. A suitable number,
diameter, and density
of perforations may be used to allow carbonation of the wash water. The second
conduit has
a smaller diameter than the first conduit; any suitable diameter relative to
the first conduit
may be used so long as it is sufficient to allow transfer of carbon dioxide to
the wash water
flowing through the first conduit. Thus, in certain embodiments, the diameter
of the second
conduit is less than 0.99, 0.95, 0.9, 0.85, 0.8, 0.75, 0.7, 0.65, 0.6, 0.55,
0.5, 0.45, 0.4, 0.35,
0.3, 0.25, 0.2, 0.15, or 0.1 times the diameter of the first conduit, and/or
more than 0.95, 0.9,
0.85, 0.8, 0.75, 0.7, 0.65, 0.6,0+55, 0.5, 0.45, 0.4,0.35, 0.3, 0.25, 0.2,
0.15, 0.1, or 0.05 times
the diameter of the first conduit, for example, 0.1-0.9x, or 0.2-0.8x, or 0.3-
0.7x the diameter
of the first conduit. In certain embodiments the second conduit has a diameter
of 0.2-5
inches, or 0.5-2 inches, or 0.5-1.5 inches. Appropriate fittings can be used
to connect the
second conduit to the first conduit, and to connect to further conduits that
lead to a source of
carbon dioxide gas; in some cases, a conduit leading from the second conduit
leads to waste
and in some cases a conduit can lead back to the source of carbon dioxide gas
in order to
recycle gas that is not taken up in a first pass.
[0197] In embodiments in which a reclaimer system is retrofitted, a section of
the
recirculation line in the current system is removed and replaced with the
first and second
conduits, as described, appropriate fittings, a source of carbon dioxide,
generally, appropriate
sensors as described below, and, generally, a control system that receives
information from
the sensors and/or from sensors already present in the system, and that
modulate delivery of
carbon dioxide according to the information received. In retrofitted or de
novo systems, the
length of carbonation conduit section is any suitable length, such as at least
0.5, 1, 2, 3, 4, 5,
6, 7, 8, 9, 10, 11, 12, 15, 20, 25, 30, 40, or 50 feet, and/or not more than
1, 2, 3, 4, 5, 6, 7, 8,
9, 10, 11, 12, 15, 20, 25, 30, 40, 50, or 100 feet, such as 0.5-50 feet, or 1-
20 feet, or 2-15 feet,
or 5-15 feet.
[0198] Thus the carbonation section of the recirculation loop between
reclaimer and holding
tank allows for carbonation of wash water without a large amount of additional
apparatus, but
merely by replacing a section of conduit with a carbonation section and
installing appropriate
sensors, control system, and source of carbon dioxide.
-40-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
101991 Generally, the system is configured to provide information regarding
relevant
parameters. Such information may be determined by sensors, human input, or any
other
suitable method. In certain embodiments, at least one, two, three, four, five,
six, or all of the
1) operation of the pump for the recirculation loop (e.g., on/off and/or
circulation rate); 2) the
level of washwater in the holding tank; 3) temperature; 4) specific gravity,
e.g., of wash
water in the holding tank or other relevant area; 5) composition of solids in
holding tank (e.g.,
cement vs. aggregate); 6) amount of new wash water received from incoming
trucks or other
sources; 7) carbon dioxide content of washwater in the carbonation section or
in other areas
(which can be monitored directly and/or indirectly); 8) flow rate of carbon
dioxide sent to
second conduit; 9) time of carbon dioxide flow; 10) type, amount, and/or
timing of admixture
addition, and any other suitable characteristics. Some or all of this
information can be sent to
a controller, which can process the information, compare it to pre-determined
parameters, and
send output to appropriate actuators to modulate the process. Actuators can
include one, two,
three or more of 1) one or more valves to regulate carbon dioxide flow, 2) one
or more pumps
to regulate washwater flow through recirculation stection; 3) one or more
systems to add
admixture to the system; and any other suitable actuators. The control system
can be tied into
the overall control system for the reclaimet
102001 In operation, the system monitors appropriate characteristics of the
wash water and
adjusts carbon dioxide delivery accordingly, in order to carbonate the wash
water to a desired
level; generally, the desired level will be such that allows a higher specific
gravity of treated
wash water to be used in concrete production operations than could otherwise
be used; in
certain embodiment, it also allows less new cement to be used in subsequent
batches of
concrete because batches made with the carbonated washwater can have higher
compressive
strength than those made without carbonated wash water and, thus, less cement
is needed to
provide the same compressive strength. Thus, in a typical reclaimer system,
wash water is
produced that, with dilution with city water, is at a specific gravity of,
e.g., 1.03 or less and
can then be used in concrete. The present methods and compositions can produce
wash water
that requires less dilution before use in concrete production, for example,
wash water that can
be used in concrete production at a specific gravity of at least 1.02, 1.03,
L04, 1.05, 1.06,
1.07, 1.08, 1.09, 1.10, 1.11, 1.12, 1.13, 1.14, 1.15, 1.16, 1.17, 1.18, 1.19,
or 1.2 and/or not
more than 1.03, 1.04, 1.05, 1.06, 1.07, 1.08, 1.09, 1.10, 1.11, 1.12, 1.13,
1.14, 1.15, 1.16,
1.17, 1.18, 1.19, 1.2, 1.25, 1.3, 1.4, or 1.5, for example, 1.03-1.25, or 1.04-
1.2, or 1.05-1.15.
In addition or alternatively, the present methods and compositions can produce
wash water
that, when used in subsequent batches of cement, allows the use of at least
0.5, 1, 1.5, 2, 2.5,
-41-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
3, 15, 4, 4.5, 5, 5,5, 6,6.5, 7, 8, 9, 10, 11, 12, 13, 14, 15, 17, 20, 25, 30,
35, or 40%, and/or
not more than 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 5.5, 6, 6.5, 7, 8, 9, 10, 11,
12, 13, 14, 15, 17, 20,
25, 30, 35, 40, or 50% less cement than would be used without the use of
carbonated wash
water to produce concrete with the same or substantially the same compressive
strength at,
e.g., 1, 2, 7, 14, or 28 days, or at any other suitable time point. In
addition, the use of
carbonated wash water produced by the system can also allow less and/or
different admixture
to be used than would be required if non-carbonated wash water were used in a
concrete
batch.
10201] Carbon dioxide can be introduced into the carbonation section at
suitable time
intervals, and at suitable flow rates and times, depending on conditions in
the system. It is
desirable that the rate of flow of carbon dioxide is such that lithe or no
carbon dioxide is
wasted, but any suitable flow rate may be used and/or time interval may be
used to achieve
the desired carbonation. In systems in which the carbonation section is new,
or has not been
used for a significant time, carbon dioxide can be added to bring the wash
water currently
present to the desired level of carbonation. Typically, so long as new wash
water is not
added, the wash water in the system will retain carbonation and require little
or no "touch-up"
carbon dioxide. In certain cases, admixture, such as one or more set
retarders, for example a
carbohydrate set retarder such as sodium gluconate, is added to the wash
water. In these
cases, not only is the amount of set retarder monitored, but the time interval
from last
addition may also be monitored; unlike carbonation, set retarders may require
additional
amounts to be added over time. For both carbon dioxide and admixture (if
used), when
additional, new, wash water is added, an appropriate amount of carbon dioxide
and admixture
(if used) is added. The amount of carbon dioxide to be added can depend on,
e.g., specific
gravity of the new wash water (directly measured and/or calculated from change
in SG of
wash water in system, e.g., in holding tank, or determined by any other
suitable method),
volume of wash water added (directly measured and/or calculated from change in
level of
wash water in system, e.g., in holding tank, or determined by any other
suitable method), in
some cases also determined by composition of wash water (e.g., percent solids
as
cementitious material vs. inert material such as aggregates; and by any other
parameters.
Addition of carbon dioxide is commenced mid is halted at a suitable time,
e.g., when the
amount of carbon dioxide added reaches a predetermined amount, when one or
more
characteristics of the wash water indicate desired level of carbonation has
been achieved,
and/or by any other suitable method. The amount of admixture to be added can
be
determined by similar characteristics and can also be modified based on time
from last
-42-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
addition of admixture; in some cases additional admixture is added even if new
wash water
has not been added, based on time from previous addition.
102021 A further advantage of the carbonation system is that it can allow the
use of smaller
holding tanks; in some cases, holding tanks can be eliminated altogether. In
the latter case, in
current setups trucks come in at the end of the day and the water must be held
until the next
day, to be treated and released and/or to be reused in additional batches.
With carbonation,
the wash water can be treated with carbon dioxide and/or admixture so that is
ready to be
batched, and stored, e.g., in the drums of the trucks themselves. In addition
or alternatively, a
smaller holding tank can be used and, in some retrofit embodiments,
replacement of the
current holding tank with a smaller one can be included in the retrofit.
102031 An exemplary wash water control system is as follows:
Quantity of Cementitious Solids
CO2 treatment targets are dependent on the quantity of cementitious solids
contained within a
reclaimer tank. This is a function of: Tank volume; Tank specific gravity
(SG), or solids
content; Solids characterization (fraction cement, fraction fly ash, fraction
non-cementitious
e.g. sand). Exemplary control protocols for determining quantity of
cementitious solids
contained in reclaimer tank are as follows:
1, Continuous measurement of reclaimer tank volume;
2. Semi-continuous measurement of reclaimer tank SG; and/or
3. Monitor all tank inflows and outflows (current sensors on all pumps
providing
infeed / drawing from reclaimer tank)
[0204] OPTION 1 assumes all material inflows can be monitored and measured.
OPTION 2
assumes that this is not possible due to equipment limitations. Both options
assume
continuous monitoring of tank level.
[0205] OPTION 1: total volume and SG of all inflows are measured
4. For tank outflows: TANK SG(n) = TANK SG(n-1)
[0206] Where n is Tank SG following tank outflow, and TANK_SG(n-1) is Tank SG
prior
to tank outflow. Previous SG setpoint is maintained in control logic.
5. For tank inflows: TANK_SG(n) = VOL_TANK(n-1)*TANK_SG(n-1)4VOL-
TANK(n-1)*VOL [FLOW] + VOL INFLOW*INFLOW SG/WOL-TANK(n-
1)*VOL_INFLOW]
[0207] SG of inflows can be setpoints or measured. For example: If inflow is
city water, SG
setpoint would be 1; If inflow is a washout inflow, SG can be measured OR can
be
established as a setpoint.
-43-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
[0208] New SG setpoint is established based on total volume and SG of inflow.
6. For both inflows and outflows:
QUANTITY_SOLIDS (n) = TANK_SG(n)*VOL TANK(n)
QUANTITY_CEMENTITIOUS_SOLIDS (n) =
QUANTITY_SOLIDS(n)*%_CEMENTITIOUS_SOLIDS
[0209] Where %_CEMENTITIOUS SOLIDS is a setpoint or continuously revised
setpoint
based on historical batch records or quantitative washout solids data This can
be further
characterized as %_CEMENT, %_FLYASH, and %_SLAG depending on characterization
requirements. % CAC03 will be discussed below.
[0210] OPTION 2: volume and SG of inflows not measured
[0211] Some systems do not allow for measurement of all material inflows (use
gravity
drainage or overflow from preceding unit operations to manage material flows).
7. For tank outflows: Same as (4) above.
8. For tank inflows: Tank SG is measured semi-continuously.
9. For both inflows and outflows: Same as (6) above.
[0212] CO2 Treatment Knowing quantity of cementitious solids within reclaimer
tank,
quantity of CO2 injected is determined based on the established setpoint. The
setpoint will be
described as MASS_CAC03_CAO/MASS_CEMENT_CAO ratio in the reclaimer system,
which is described below.
[0213] STARTUP SCENARIO: Consider that, for a given reclaimer tank system,
there is a
known characterization of tank solids. For example:
= TANK SG = 1.1
= VOL_TANK = 100,000 L
10. With Solids characterized (as pre-defined setpoint) as follows:
= MASS%_CEM ENT (of solids fraction) = 80% 4 SG_CEMENT = 3.15
= MASS% FLYASH (of solids fraction) = 10% 4 SG_FLYASH = 2.2
= MASS% SLAG (of solids fraction) = 5% 4 SG_SLAG = 2.9
= MASS% SAND (of solids fraction) = 5% 4 SG_SAND = 1.6
= MASS% CAC03 (of solids fraction) = 0% 4 SG_CAC03 = 2.6
11. Conversion to vol% is required for TANK_SG conversion to solids content:
= VOL% CEMENT (of solids fraction) = [0.8/3.15] / [0.8/3.15 + 0.1/2.2 +
0.05/2.9 +
0.05/1.6 +0/2.6] = 72.6%
= VOL%_FLYASH (of solids fraction) = [0.1/2.2] / [0.8/3.15 + 0.1/2.2 +
0.05/2.9 +
0.05/1.6 + 0/2.6] = 13%
-44-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
= VOL%_SLAG (of solids fraction) = 5%
= VOL%_SAND (of solids fraction) = 9%
= VOL%_CAC03 (of solids fraction) = 0%
12. Determine solids fraction of slurry in reclaimer tank using TANK_SG and
%VOL_consituents:
= SG_SOLI DS = [VOL%_CEMENT*SG_CEMENT + VOL%_FLYASH*SG_FLYASH +
VOL% SLAG*SG SLAG + VOL% SAND*SG SAND + + VOL% CAC03*SG CAC03]
= SG SOLIDS = [0.73*3.15 + 2.2*0.13 + 2.9*0.05 + 1.6*0.05 + 0*0] = 2.87
13. Therefore,
= MASS%SOLIDS_TANK = [SG_SOLIDS*RANK_SG -1]/(SG_SOLIDS -111/TANK_S6
= MASS%SOLIDS_TANK = [2.8711.1-1]/[2.87-1]1/1.1 = 0.1395 = 13.95%
14. Therefore,
= MASS% CEMENT = 0.8*0.1395 = 11.2%
= KG_SLURRY = TANK_SG*VOL_TANK = 1.1*100,000 = 110,000 KG
= KG CEMENT = 0.112*110,000 = 12,320 KG
Other constituents:
= KG FLYASH = 1,534.5 kg
= KG SLAG = 767.25 kg
= KG SAND = 767.25 kg
= KG WATER = 94,611
102141 With a known amount of cement in the system, the quantity of CO2
injection
required can be determined based on the stoichiometric reaction of CaO with
CaCO3.
[0215] Consider, using the above example, that the known quantity of cement
has a known
fraction of CaO.
10216] The treatment system would then establish a target treatment level
based on the
target MASS_CAC03_CAO/MASS_CEMENT_CAO setpoint for the system.
[0217] Consider, using the above example, that the
MASS_CAC03_CAOMASS_CEMENT_CAO setpoint is 0.4.
16. Therefore, using above example:
= KG CAO = KG CEMENT_CAO + KG CAC03 CAO
= 8,008 = KG_CEMENT_CAO + 0.4*KG_CEMENT_CAO
= KG CEMENT_CAO = 8,008/1.4 = 5,720 KG
= KG CAC03 CAO = 0.4* 5,720 = 2,288 KG
= KMOL CAC03 CAO = 2,288/56.08 = 40.8 KMOL
-45-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
17. Therefore, using stoichiometry of reaction CO2 + CaO = CaCO3 and molar
mass of
CO2 (44.01):
= Where KMOL CO2 = KMOL CAO
= KG CO2 = KMOL_CO2"44.01= 40.8'44.01= 1,795.6 KG
[0218] In this example, 1,795.6 CO2 is required to treat to desired level.
18. Resulting solids characterization:
Resulting solids characterization is as follows for this example, where (n) is
post-
treatment, n-1 is pre-treatment with CO2.
= KG_CEMENT(n) = KG_CEMENT(n-1) ¨ KG_CAC03_CAO(n) = 12,320 ¨ 2,288 = 10,032
KG
= KG FLYASH (n) = KG_FLYASH(n-1) = 1,534.5 KG
= KG_SLAG (n) = KG_SLAG(n-1) = 767.25 KG
= KG_SAND (n) = KG_SAND(n-1) = 767.25 KG
= KG CAC03 (n) = KG_CAC03(n-1) + KG_CO2 + KG_CAC03_CAO(n) = 0 + 1795.6 +
2,288 = 4,083 KG
19. Resulting MASS%_XX (of solids fraction) Is determined:
= MASS%_CEMENT(n) = 58.4%
= MASS%_FLYASH (n) = 9%
= MASS%_SLAG (n) = 4.4%
= MASS%_SAND (n) = 4.4%
= MASS% CAC03 (n) = 23.8%
AND solids% =
= %SOLIDS_TANK = (10,032 + 1534.5 + 767.25 + 767.25 + 4,083)/ [(10,032 +
1534.5 +
767.25 + 767.25 + 4,083) + 94,6111 = 15.4%
STEADY-STATE SCENARIO:
[0219] Consider the above example, now comprising a fraction of solids as
CaCO3, with
known inflow material characterization from truck washouts as follows:
= MASS% CEMENT (of solids fraction) = 80% 4 SG_CEMENT= 3.15
= MASS% FLYASH (of solids fraction) = 10% 4 SG_FLYASH = 2.2
= MASS% SLAG (of solids fraction) = 5% 4 SG_SLAG = 2.9
= MASS% SAND (of solids fraction) = 5% 4 SG_SAND = 1.6
= MASS% CAC03 (of solids fraction) = 0% 3 SG_CAC03 = 2.6
-46-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
And consider a scenario for which the following daily operating parameters are
known
/measured:
= TANK_SG (end of day) = 1.12
= VOL_TANK = 98,000 L
= VOL SLURRY OUTFLOW = 20,000 L (measured and monitored through batching of
concrete)
= VOL_WATER_IN FLOW = 10,000 L (monitoring of city / fresh water inflows to
reclaimer tank)
Assuming steady-state material balance in reclaimer tank at beginning of
operating day as
established in previous example:
= MASS%_CEMENT(n) (of solids fraction) = 58.4%
= MASS%_FLYASH (n) (of solids fraction) = 9%
= MASS%_SLAG (n) (of solids fraction) = 4.4%
= MASS%_SAND (n) (of solids fraction) = 4.4%
= MASS%_CAC03 (n) (of solids fraction) = 23.8%
20. Therefore, using SG_SOLIDS calculations as above:
= SG SOLIDS TANK (n-1) = 2.85
Where n-1 is SG SOLIDS TANK and beginning of operating day
21. Therefore, using Volume balance and SG_SOLIDS calculations as above:
= VOL_SLURRY_INFLOW = VOL_TANK(n) - [VOL_TANK(n-1) + VOL_WATER_INFLOW -
VOL_SLURRY_OUTFLOW]
= 98,000 ¨ 11100,000 + 10,000 -20,0001 = 8,000 L
22. From Steps (10) to (12) above:
= SG SOLIDS SLURRY_INFLOW = 2.87
= SG SOLIDS_TANK (n-1) = 2.85
= SG WATER INFLOW = 1
Therefore, Volume of original slurry from beginning of day with SG 1.1 is:
= VOL_SLURRY(n-1) = 100,000 ¨ 20,000 = 80,000 L 3 SG 1.1
Including inflows:
= VOL_WATER_IN FLOW = 10,000 L 4 SG 1
= VOL_SLURRY INFLOW = 8,000 L 3 SG UNKNOWN
23. With SG measurement of 1.12, it follows:
-47-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
= SG_TANK(n) = [VOL_SLURRY(n-1)*SG_SLURRY(n-1) +
VOL_WATER_INFLOW*SG_WATER + VOL_SLURRY_INFLOW* [SG SLURRY
INFLOW]/(VOL TANK(n)]
= 1.12 = [80,000*1.1 + 10,000*1 + 8,000*SG_SLURRY_INFLOW]/98,000
= SG_SLURRY_INFLOW = 1.47
24. With SG of slurry inflows calculated at 1.47, it follows from (13) above:
= MASS%_SOLIDS_SLURRY_INFLOW = = [2.8711.47-11/[2.87-1]1/1.47 = 0.49 = 49%
25. Therefore, from (14) above:
= MASS% CEMENT = 0.8*0.49 = 39.2%
= KG_SLURRY = SG_SLURRY_INFLOW*VOL_SLURRY_INFLOW = 1.47*8,000 = 11,760 KG
= KG_CEMENT = 0.392*11,760 = 4,609.9 KG
Other constituents:
= KG FLYASH = 576.24 kg
= KG_SLAG = 288.1 kg
= KG_SAND = 288.1 kg
26. From 15 above, CEMENT_CAO_IN is determined as follows:
= KG_CEME NT CAO = KG_CEMENT*0.65 = 4,609.9*0.65 = 2,996.4 KG
27. New material balance is then determined at end of operating day, prior to
treatment of reclaimer tank with CO2 using balance of inflows and outflows.
For this
example, resulting balance is as follows:
-OUT
= KG_CEMENT = 10,032 ¨ (20,000 L)*(1.1 KG/L)*0.154*0.584 = 8,053 KG
= KG_FLYASH = 1,534.5 - (20,000 L)*(1.1 KG/L)*0.154*0.09 = 1,229.6 KG
= KG_SLAG = 614.8 KG
= KG_SAND = 614.8 KG
= KG_CAC03 = 4,083 KG ¨ 806.34 = 3,276.7 KG
= KG_WATER = 94,611 - (20,000 L)*(1.1 KG/L)*(1-0.154) = 75,999 KG
-FIN
= KG_CEMENT = 8,053 + 4,609.9 = 12,692.9 KG
= KG FLYASH = 1,229.6 + 576.24 = 1,805.84 KG
-48-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
= KG SLAG = 614.8 KG + 288.1 KG = 902.9 KG
= KG SAND = 614.8 KG + 288.1 KG = 902.9 KG
= KG CAC03 = 3,276.7 + 0 KG = 3,276.7 KG
= KG WATER = 75,999 + 10,000 + (8,000 L)*(1.47 KG/L)*(1-0.49) = 91,996 KG
28. MASS_CAC03_CAO/MASS_CEMENT_CAO:
Using molar mass of CaO (56.08) and CaCO3 (100.9), it follows:
= MASS_CAC03_CAO = 3,276.7 KG * (56.08/100.9) = 1,821.2 KG
Using CAO_fraction (Cement) for cement as a constant = 65% (as (15) above):
= MASS_CEMENT_CAO = 12,692.9 KG * 0.65 = 8,250.4 KG
Therefore ratio is:
MASS_CAC03_CAO/MASS_CEMENT_CAO = 1821.2/8250.4 = 0.22
TOTAL CAO = 1821.2+ 8250.4= 10,071.6
29. If desired ratio is 0.4 it follows from (16) above that the amount of
KG_CAC03_CAO
is 2,877.4. With 1,821.2 KG CAO already present as CAC03_CAO, the net amount
of CAO
required for reaction is 2,877.4 ¨ 1821.2 = 1,056.2 KG = 18.83 KMOL.
30. Using stoichiometry as in (17) and (18) above, the required amount of CO2
for
reaction to the desired setpoint is:
= Where KMOL CO2 = KMOL CAO
= KG CO2 = KMOL_CO2*44.01 = 18.83*44.01 = 828.7 KG
In this example, 8283 KG of CO2 is required to treat to desired level.
31. Resulting solids characterization:
Resulting solids characterization is as follows for this example, where (n) is
post-treatment,
n-1 is pre-treatment with CO2.
= KG_CEMENT(n) = KG_CEMENT(n-1) ¨ KG_CAC03_CAO_NET(n) = 12,6919 ¨ 1,056.2 =
11,636.7 KG
= KG FLYASH (n) = KG_FLYASH(n-1) = 1,805.84
= KG_SLAG (n) = KG_SLAG(n-1) = 902.9 KG
= KG SAND (n) = KG_SAND(n-1) = 902.9 KG
= KG_CAC03 (n) = KG_CAC03(n-1) + KG_CO2 + KG_CAC03_CAO(n) = 3,276.7 + 828.7
+
1056.2 = 5,161.6 KG
-49-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
32. Resulting MASS%_XX (of solids fraction) Is determined:
= MASS%_CEMENT(n) = 57.0%
= MASS%_FLYASH (n) = 8.8%
= MASS%_SLAG (n) = 4.4%
= MASS% SAND (n) = 4.4%
= MASS% CAC03 (n) = 25.3%
AND solids% =
= %SOLIDS_TANK = (11,636.7 + 1805.84 + 902.9 + 902.9 + 5161.6)! [(11,636.7 +
1805.84 + 902.9 + 902.9 + 5161.6) + 91,966] = 18.2%
[0220] This Tank Solids characterization now becomes the new beginning of day
material
balance for the following day of operation at the concrete plant.
[0221] Additional or alternative scenarios are as follows:
An exemplary method for monitoring and controlling the reaction mechanism of
CO2 with
concrete washwater slurry is as follows. Concrete wash water slurry with a
known specific
gravity / solids content and cementitious fraction of solids content can be
treated with carbon
dioxide to produce nano-calcium carbonate. Without being bound by theory, the
reaction
mechanism is dependent on a number of factors, including: 1) Ionic calcium
concentration, or
the amount of free calcium in solution; 2) Rate of carbon dioxide injection;
3) Residence time
of reaction.
10222] Relative impact and mechanistic control strategy for each element is
described
below.
1) Ionic Calcium: Hydrating and hydrated portland cement is known to contain
Calcium
hydroxide (Ca(OH)2), which dissociates in water to release Can ions in
solution (as well as
01-11, resulting in a caustic solution. Solubility of Ca(OH)2 decreases with
increasing
temperature. Data presented as saturated solubility in grams per 100 grams of
water.
T (deg C) CaO
Ca(OH)2
0 0.140
0.185
10 0.133
0.176
20 0.125
0.165
25 0.120
0.159
0.116 0.153
-50-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
40 0.106
0.140
50 0.097
0.128
60 0.088
0.116
70 0.079
0.104
80 0.070
0.092
90 0.061
0.081
100 0.054
0.071
[0223] Carbon dioxide reacts with the calcium oxide portion in cement /
calcium hydroxide
to create calcium carbonate Without being bound by theory, in a slurry, the
two CO2
mineralization mechanisms are believed to occur are as follows: a) Pathway 1:
Reaction of
carbon dioxide with free calcium ions in solution and OH- to form discrete
nano-calcium
carbonate in solution (solution mechanism); b) Pathway 2: Reaction of carbon
dioxide with
cement solids to form calcite crystals on the surface of cement particles
(surface reaction
mechanism) The reaction pathway may be controlled by predicting the ionic
calcium
concentration in the wash water shiny and subsequently controlling the rate of
carbon
dioxide injection for a given residence time of reaction. 2) Rate of Carbon
Dioxide
Injection: The rate of carbon dioxide injection can be managed and controlled
to ensure (1)
maximum reaction efficiency and (2) targeted control of the reaction between
carbon dioxide
and calcium.
[0224] The rate of reaction of Pathway 1 as described above is hypothesized to
be faster
than Pathway 2. Further, the Pathway 1 reaction is hypothesized to be more
predictable than
Pathway 2, and to create a more predictable product in the form of "free" nano-
calcium
carbonate. This can lead to higher reaction efficiencies, enhanced control and
consistency in
the application of produced nano-calcium carbonate, and greater predictability
in the
hydration characteristics and kinetics of the remaining cementitious fines.
Consequently, a
method for controlling the rate of carbon dioxide injection and method of
reaction based on
predicted ionic calcium concentration, carbon dioxide bubble size, and
reaction length can be
created.
[0225] As described previously, the injection apparatus used for this
application is
comprised of a section of pipe length (the "injection length", first conduit
as described
previously) installed as a sub-section of a longer conduit that has an inner
diameter suitable to
allow for the insertion of a length of perforated expandable fine bubble
expanding hose
(second conduit as described previously), examples of which are used to create
nano-bubbles
-51-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
such as for water oxygenation in fish farming/aquaculture applications. This
method ensures
an even distribution of nano-bubbles across the entire injection length. An
increase in inner
diameter of the pipe section accounts for lost volume due to the insertion of
the expanding
bubble hose, as well as the addition of CO2 via injection. Alternately, the
diameter can be
sized to slow the fluid velocity and hence increase the residence time that
the slurry has in
direct contact with the bubbled CO2. The pressure drop (high-to-low) at the
inlet of the
injection length can encourage interruption in laminar flow in the preceding
pipe section and
results in turbulence, which encourages effective mixing of slurry with the
injected CO2.
Scenario Example
[0226] Consider a slurry with the following properties flowing through an 11-
metre length
of 2" I.D. pipe at 160 Gallons per Minute or GPM (equivalent to 607 litres per
minute or
LPM). CO2 injection begins at injection length 0-metres and ends at injection
length 1-metre
in a 3" section of pipe. Outer diameter of the expanding bubble hose is 1
inch. CO2 is injected
evenly across said injection length using the injection mechanism described
above. This is
followed by 10-metres of contained reaction length flow prior to discharge to
the atmosphere
and into a recirculation through.
SG Shiny: 1.1
Temp_Slurry: 20 degrees Celsius
SG Cement: 115
SG FlyAsh: 2.2
Cement_fraction of solids (vol%): 85%
FlyAsh_fraction of solids: (vol%): 15%
Determination of Solids Content of Slurry:
%Solids = [(SG_Cement*Cement_fraction +
SG FlyAsh*FlyAsh fraction)]*[(SG Slurry - 1)/((SG_Cement*Cement fraction +
SG FlyAsh*FlyAsh fraction)-1)]/(SG Sluny)
%Solids = 13.6%
%Water = 86.4%
Volume of slurry and CO2 in injection length:
-52-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
slurry flowrate = [(160 gal/min) * (3.7854 L/gal) * (linin/60 sec)] = 6.3 L/s
CO2 flowrate = (100 L/min) * (1min/ 60 sec) = 1.6 L/s
volumetric flow ratiosiuny/co2 = vol slurry / vol CO2
volumetric flow ratiosinny/co2 = 6.3 Lis /1.6 L/s
volumetric flow ratiosinny/co2 = 3.9375
volumetric flow ratioconstany = 0.2540
2-inch pipe _cross_sectional_area = a *(d12)2
2-inch pipe _cross_sectional_area = a * [(2 in * 0.0254 m/in)/212
2-inch pipe cross sectional area = 0.002027m2
3-inch pipe _cross_sectional_area = a*(cU2)2
3-inch pipe _cross_sectional_area = it * [(3 in * 0.0254 m/in)/212
3-inch pipe _cross_sectional_area = 0.00456 m2
3-inch pipe volume = pipe cross sectional area * pipe length
3-inch pipe volume = a [(3 in * 0.0254 m/in)/212* (1 m)
3-inch pipe volume = 0.00456 m3= 4.56 L
expanding bubble hose volume = hose cross section * hose length
expanding bubble hose volume = it * [(1 in * 0.0254 ailin)/2]2 * (1 m)
expanding bubble hose volume = 0.000507 m3= 0.507 L
injection_length_volume_available = pipe volume - hose volume
injection_length_volume_available = 4.56 L - 0.507 = 4.05 L
injection length volume available = vol slurry + vol CO2
where: vol CO2 = 0.2540 * vol slurry
-53-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
injection_length_volume_available = vol_slurry + 0.254 * vol_slurry
injection_length_volume_available = vol_slurry * (1 + 0.2540)
vol_slurry = injection_length_volume_available / (1 + 0.2540)
vol_slurry = (4.05 L) / (1 + 0.2540)
vol_slurry = 3.23 L
CO2 volume = vol_slurry * Volumetric flow ratiocovskny
CO2 volume = (3.23 L) * 0.2540
CO2 volume = 0.820 L
slurry mass = slurry volume * slurry specific gravity
slurry mass = 3.23 L * 1.1 kg/L
slurry mass = 3.55 kg
water fraction = slurry mass * water fraction of slurry
water fraction = 3.55 kg * 0.864
water_fraction = 3,07 kg = 3.07 L
Slurry velocity change in pipe:
For 2-inch diameter section prior to injection:
slurry_flowrate = 6.3 Lis = 0.0063 m3/s
pipe cross sectional area= 0.002027m2
slurry velocity in 2-in pipe = sluny_flowrate / pipe _cross_sectional_area
slurry velocity in 2-in pipe = 0.0063 m3/s / 0.002027 m2
slurry velocity in 2-in pipe = 3.11 m/s
For 3-inch diameter section where injection occurs:
slurry flowrate = [(160 gal/min) * (3.7854 L/gal) * (ltnin / 60 sec)]
-54-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
slurry flowrate = 6.3 Us = 0.0063 m3/s
vol_slurry = 3.23 L = 0.00323 m3
effective_cross_sectional_area_slurry = slurry_volume / injection length
effective_cross_sectional_area slurry = 0.00323 m3/ lm = 0.00323 m2
slurry velocity in 3-in pipe = sluny_flowrate / effective_cross_sectional_area
slurry
slurry velocity in 3-in pipe = 0.0063 m3/s / 0.00323 M2
slurry velocity in 3-in pipe = 1.95 m/s
10227] The slurry slows down in injection length which results in a pressure
drop and
encourages turbulence/disturbs laminar or plug flow. In another embodiment of
the invention,
a pitot tube sight glass assembly can be used to measure / monitor pressure
drop across each
pipe ID change.
10228] Slurry/CO2 mixture velocity in 2-in pipe following injection length:
Assuming a
negligible conversion of CO2 within injection length (i.e. majority of
reaction occurs in the
10-metres of 2-in pipe section to follow injection length)
vol flowrate_mixture = vol_flowrate_slurry + vol flowrate_CO2
vol flowrate mixture = 6.3 L/s + 1.6 L/s
vol flowrate mixture = 7.9 Lis = 0.0079 m3/s
2-inch pipe cross sectional area = 0.002027 m2
slurry velocity in 2-in pipe = vol_flowrate_rnixture / 2-inch pipe
cross sectional area
slurry velocity in 2-in pipe = 0.0079 m3/s / 0.002027 m2
slurry velocity in 2-in pipe = 3.9 m/s
[0229] Increase in velocity results in pressure drop (high-to-low) in
direction of flow when
leaving 3-inch diameter injection length and entering the 2-inch diameter
reaction length. In
another embodiment, additional venturis are installed along the reaction
length to disrupt
laminar / plug flow and encourage turbulence, thus increasing mixing and
potentially the
reaction efficiency.
[0230] Stoichiometric balance of Cao and CO2:
10231] CaO:
-55-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
At 20 degrees celsius, the saturated solubility of Ca(OH)2 in 100 grams water
is 0.165 grams.
[0232] Therefore, it is predicted that there would be
mass Ca(OH)2 = saturated solubility of Ca(OH)2* volume of solution
mass Ca(OH)2 = (0.165 grams Ca(OH)2 / 100 grams ) * 3070 grams of solution
mass Ca(OH)2 = 5.065 grams of Ca(OH)2 available in solution
moles Ca(OH)2 = 5.065 g Ca(OH)2 / molar mass of Ca(OH)2
moles Ca(OH)2 = 5.064 grams / 74.093 g/mol
moles Ca(OH)2 = 0.0684 moles
mass Ca = 0.0343 moles * 40.08 g/mol Ca = 2.74 grams Ca
CO2:
mass CO2= mass of CO2 in injection length
mass CO2= volume of CO2 in injection length * gas density
mass CO2= 0.820 L * (1.98 g/L) = 1.624g
moles CO2= mass CO2 / molar mass CO2
moles CO2= 1.624 g /(44.01 g/mol) = 0.0369 moles
[0233] Simplified stoichiometric reaction of CO2 with CaO is as follows:
Ca0 + CO2= CaCO3
[0234] The molar stoichiometric ratio (CaO:CO2) is 1:1
For this example, this would indicate that the stoichiometric excess of CaO in
solution
is: stoichiometric excess = (moles Ca(OH)2/ moles COO - 1
stoichiometric excess = (0.0684 moles / 0.0369 moles) -1
stoichiometric excess = +85.4%
[0235] In this example, the reaction is expected to be controlled via Pathway
1, as the CO2 is
expected to react more readily / have a higher affinity for free calcium ions
in solution, versus
surface reaction with cement solids to form calcite crystals. For the purposes
of this
discussion, the reaction length that follows the injection length (10-metres),
and based on the
slurry flowrate, is assumed to provide a sufficient residence time to allow
for 100% reaction
efficiency - i.e., there would be no unreacted C0z discharged to the
atmosphere at the end of
the reaction length.
[0236] It is assumed that the replenishment rate of free calcium ions is low
enough such that
it can be deemed negligible in the reaction length (residence time = ¨3-3.5
seconds in this
-56-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
example). Free calcium replenishment will occur as a result of free calcium
consumption /
reaction with CO2 during injection and subsequent reaction in the injection
and reaction pipe,
lowering CaO content below its saturated solubility point.
[0237] Free calcium can be predicted using solubility and flow
characteristics, or
alternatively it can be measured using a calcium ion sensor.
[0238] To provide further clarification of the proposed method of monitoring
and
controlling the incidence of Pathway 1 reaction vs Pathway 2 reaction, Let's
consider the
CO2 flowrate that would equate to the stoichiometric equivalent of CaO in the
reaction
producing CaCO3:
stoichiometric CO2= 0.0684 moles
mass CO2 = 0_0684 moles *(44.01 g/mol CO2) = 3.01 grams CO2
vol CO2= 3.01 grams / (1.98 g/L) = 1.52 L
vol Slurry = 4.05 L - 1.52 L = 2.53 L
Volumetric flow ratio (Vol slurryNol_CO2) = 2.53 L slurry /1.52 L CO2 = 1.664
Stoichiometric_equivalent_CO2_flowrate = slurry flow rate / volumetric flow
ratioshanyteo2
Stoichiometric_equivalent_CO2 flowrate = 6.3 L slurry/s / 1.664 L slurry/L CO2
Stoichiometric_equivalent_CO2 flowrate = 3.79 L CO2/s = 227 SLPM
[0239] This would indicate, based upon the specific inputs, that the maximum
CO2 flowrate
allowed for stoichiometric reaction of free calcium ions in solution would be
227 SLPM. This
does not address the required residence time following injection to achieve
100% reaction
efficiency, which, as mentioned, would be dependent on rate of reaction and
overall reaction
length residence time.
[0240] In practice, a stoichiometric excess that maximizes the incidence of
Pathway 1
reaction vs Pathway 2 would be targeted. A method for determining extent of
reaction (with
respect to quantities of CO2 consumed / CaCO3 generated per pass) is
described.
[0241] es CaCO3 generated per reaction pass:
Assuming 100% reaction efficiency in the reaction length, and Pathway 1
reaction only, the
amount of CaCO3 generated during each pass using a CO2 flowrate of 100 SLPM
can be
calculated. The Pathway 1 reaction is ensured by maintaining a target
stoichiometric excess
of CaO in solution (in this example 85.4% excess).
CO2 flowrate = 100 SLPM
CO2 flowrate = (100 L/min)*(1min/ 60 sec)
CO2 flowrate = 1.6 L/s
-57-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
CO2_flowrate = 1.6 L/s * (1.98 g/L) = 3.168 g CO2/s
rate moles_CaCO3_generated = CO2_flowrate * molar_mass_CO2
rate moles_CaCO3_generated =3.1168 g/s / (44/.01 g/mol)
rate moles_CaCO3_generated = 0.0720 moles CO2/s
moles_CaCO3_generated = moles CO2 consumed
rate _CaCO3 _generated = moles_CaCO3_generated * molar mass_CaCO3
rate_CaCO3 _generated = 0.0720 moles CaCO3* (100.0869 g/mol CaCO3)
rate_CaCO3 _generated = 7.21 grams CaCO3/s
[0242] For a given period of time, it can then be predicted the amount of nano-
CaCO3
generated "in-situ" in a washwater tank.
[0243] For a 10 hr overnight treatment period of an 80,000 L tank containing a
slurry with a
specific gravity of 1.1, for example, the mass CaCO3 generated / CaCO3
concentration would
be as follows:
CaCO3 _generated = 7.211 grams CaCO3/s *3600 sec/hr *10 hr
CaCO3 _generated = 259,560 grams
CaCO3 _generated = 260 kg
sluriy_mass_(before treatment) = shirry_volume_(before treatment) *
slurry specific_gravity
slurry mass (before treatment) = 80,000 L * 1.1 kg/L
slurry mass_(before treatment) = 88,000 kg
slurry_mass_(after treatment) = slurry_mass_(before treatment) + CaCO3
_generated
slurry mass (after treatment) = 88,000 kg untreated slurry + 260 kg CaCO3
slurry mass_(after treatment) = 88,260 kg treated slurry
Assuming the specific gravity of the slurry does not change,
slurry volume (after treatment) = slurry mass (after treatment) /
slurry specific_gravity
slurry volturie (after treatment) = 88,259 kg / 1.1 kg/L
slurry_volume_(after treatment) = 80,236 L
-58-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
nano-CaCO3 concentration = CaCO3 generated / slurry_volume_(after treatment)
nano-CaCO3 concentration = 260 kg CaCO3 / 80,236 L treated slurry
nano-CaCO3 concentration = 3.23 g/L shiny
nano-CaCO3 concentration = ¨323 ppm
102441 The incidence of reaction Pathway 1 vs Pathway 2 reaction can be
identified by:
Monitoring free calcium concentration in solution via a calcium probe;
evaluating particle
size of an experimental slurry following controlled treatment.
[0245] Consider a test that circulates slurry through an injection apparatus
at a flowrate of
50 gallons per minute (GPM). In a mixing vessel of capacity 55 gallons the
tank turnover is
55 gallons /50 GPM = 1.1 min. The likelihood of short-circuiting is high in
this system, so
the control of Pathway 1 vs Pathway 2 reaction is difficult. Further, the
replenishment rate of
free calcium ions in solution is likely more than 1.1 minutes, especially as
higher levels of
treatment occur (CO2 injection creates an exothermic reaction, and Ca(OH)2
solubility drops
with increased temperature).
102461 At a commercial scale, following discharge to the atmosphere and into
the
recirculation conduit, the slurry will return to the washwater slurry tank.
With proper
management of tank infeed point for recirculation and tank out-feed point for
CO2 injection
and reaction, shod-circuiting of slurry is assumed to be negligible.
3) Reaction length of pipe I residence time of reaction
[0247] Using the scenario provided in the example, consider a system that
monitors the
energy inputs generated by the exothermic formation CaCO3 (either Pathway 1 or
Pathway
2). It is assumed that Pathway 1 reaction occurs at a much higher rate, and
thus for a given
length of pipe, at a greater efficiency than Pathway 2. Consequently, if CO2
injection is
controlled such that Pathway 1 is maintained, the incidence of over-injection
with CO2 (or
alternatively, a reduction in the required stoichiometric excess of free
calcium ions in
solution) can be observed by a loss in efficiency. This loss in efficiency can
be observed by a
reduction in exothermic reaction in a given pipe section, and from an
operational / control
level, this would be observed using temperature probes before / after
injection and reaction.
For this to be observed, it would be assumed that the residence time of
reaction (in the
provided example, 3-3.5 seconds) would be low enough such that any ambient /
environmental effects on slurry temperature would be negligible, and the only
identifiable/measurable temperature change would result from the exothermic
reaction.
-59-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
102481 A predictive model is provided, whereby laafiReall*.T.af can be changed
to
show the impact of variations of btf4t on AttaittWiceittgat based on a
theoretical
calculation of 100% reaction efficiency. See Example 38.
102491 Maintaining / controlling for Pathway 1 reaction results in a more
predictable slurry
in a concrete production environment. Predictability of cementitious solids in
reclaimed
washwater management systems is a significant challenge that impacts the rate
of reuse in
concrete production. This typically results in significant dilution of
washwater to mitigate the
impacts of hydration variability of cementitious solids on fresh properties of
produced
concrete. This in tum leads to a requirement to hold larger volumes in
reclaimed washwater
tanks, which then leads to larger sustained loads of solids and greater
variability in age of
cementitious solids contained in the reclaimer tank. With greater
predictability in the
hydration effects of cementitious solids, complemented by the "in-situ"
generation of nano-
calcium carbonate, the CO2 treated washwater slurry can be used with little or
no dilution, at
higher replacement levels, and in a greater portion of concrete production,
which leads to
smaller sustained loads in reclaimer tanks and thus reduced variability in age
of cementitious
solids contained in the reclaimer tank.
102501 Any reaction in the form of Pathway 2 is challenging to measure, and
the
corresponding impact on the reactivity / dynamics of Pathway 2 created solids
creates even
more challenges with respect to predictability. Conversely, if reaction is
maintained as
Pathway 1, the residual (unreacted) cementitious solids perform as expected as
typical
cementitious solids in water, and hydration kinetics can be managed using
industry proven
hydration stabilization / set retardation techniques. Further, the generation
of nano-CaCO3
results in a distinct "in-situ" product that carries an intrinsic performance
enhancing value-
add with it is currently accepted in the industry as such.
102511 Introduction of carbon dioxide to the drum of the truck. In certain
embodiments of
the invention, carbon dioxide is introduced into the water in the drum of the
ready mix truck,
before the water leaves the drum. The carbon dioxide can be in any form, and
introduced in
any suitable manner.
102521 1) Introduction of carbon dioxide after concrete load has been poured
and before
truck reaches wash station. For example, carbonated water may be used as
saddlebag water
and/or as wash water at a wash station. Supersaturated carbonated water may be
used, as
described elsewhere (see, e.g., U.S. Patent Application Publication No.
2015/0202579). In
addition, or alternatively, solid carbon dioxide may be introduced into the
water. For
example, a certain amount of dry ice may be added at the job site, before,
during, or after the
-60-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
addition of saddlebag water, and mix with the saddlebag water and residual
concrete in the
drum of the ready-mix truck during the drive back to the wash station; the dry
ice will
sublimate in the water and provide a steady source of carbon dioxide as the
cement in the
residual concrete reacts to produce reaction products, e.g., carbonates. The
dry ice may be
added as one dose or as more than one dose, e.g., as 2, 3, 4, 5, 6, 7, 8, 9,
10, or more than 10
doses, or continuously or semi-continuously. In addition or alternatively,
gaseous carbon
dioxide may be introduced into the drum, either as a single addition, or
multiple additions, or
as a stream of carbon dioxide that is injected into the drum, e.g., for some
or all of the
transport time from the job site. For example, carbon dioxide gas may be added
as one dose
or as more than one dose, e.g., as 2, 3,4, 5, 6, 7, 8,9, 10, or more than 10
doses, or
continuously or semi-continuously. Carbon dioxide can also be introduced as
mix of gaseous
and solid carbon dioxide, e.g., by use of a snow horn; this can also be as one
or more
additions or continuous addition. For example, carbon dioxide as a mix of gas
and solid may
be added as one dose or as more than one dose, e.g., as 2, 3, 4, 5, 6, 7, 8,
9, 10, or more than
10 doses, or continuously or semi-continuously. In embodiments in which dry
ice is used,
there can be a further effect of cooling the wash water as cementitious
materials react. It will
be appreciated that one or more of the above options may be used for any given
load.
102531 For example, it is possible to add carbon dioxide to the drum after
saddlebag water
has been added, and while the truck is moving from the job site to a wash
station: In one
option, a certain amount of dry ice may be carried with the truck and
introduced into the
drum at the time that the saddlebag water is introduced; this is an easy and
convenient
method to get a relatively large amount of carbon dioxide into the drum. The
dry ice may be
used as pieces of a certain size, or within a certain range of sizes, that may
be determined by,
e.g., one or more of the volume of saddlebag water, the amount of cement in
the mix, the
expected amount of concrete coating the interior of the truck, the expected
transport time
back to the wash station, the desired level of carbon dioxide uptake, the
efficiency of uptake,
the temperature that the truck is likely to encounter, and the like, so that
the dry ice
sublimates at a rate that will match the expected rate of reaction with
concrete residue and, in
particular, with cement. This will tend to keep more of the carbon dioxide in
the drum of the
truck, since it will be reacting at approximately the rate that it is
sublimated into gaseous
form. In a second option, the saddlebag water is carbonated, or super-
saturated, with carbon
dioxide, generally at the hatching facility before being loaded into its
containers. The
containers may be modified as necessary to preserve the carbonation of the
water for the
necessary time before use. Supersaturated solutions have been found to retain
a large
-61-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
percentage of introduced carbon dioxide over relatively long time periods;
thus, little or no
modification of the saddlebags may he necessary if a supersaturated solution
is used. See,
e.g., U.S. Patent Application Publication No. 2015/0202579. In a third option,
gaseous carbon
dioxide is added to the drtun of the ready-mix truck, before, after, or during
the addition of
the saddlebag water. As described above, the addition may be in one dose, more
than one
dose, continuous, or a combination. The total amount of carbon dioxide added
may be
metered and regulated based on the same criteria as for dry ice. In a fourth
option, a mixture
of solid and gaseous carbon dioxide is added to the drum, for example by use
of liquid carbon
dioxide passed through a snow horn. Dosing and regulation would be as for
gaseous carbon
dioxide. Any combination of these options may be used, as desired and suitable
for a
particular load, truck, or operation.
102541 Because the truck is empty, the drum provides a very large headspace
for any
gaseous carbon dioxide to be retained. In certain embodiments, the opening of
the drum may
be partially or completely closed in order to retain carbon dioxide within the
drum, either
during transport back to the wash station, or at the wash station, or both.
102551 2) Addition of carbon dioxide at a wash facility. Additionally or
alternatively,
carbon dioxide may be added to the drum of the ready-mix truck during the
washing process
at the wash station. Any or all of the options described above for addition of
carbon dioxide
after the load has been poured and before the truck returns to the wash
facility may also be
used during washing at the wash station: carbonated or super-carbonated wash
water, dry ice,
gaseous carbon dioxide, a mix of gaseous and solid carbon dioxide. If carbon
dioxide has
already been added to the drum prior to the truck reaching the wash station,
one or more
characteristics of the water can be useful to determine the extent of reaction
of the carbon
dioxide. Measurements such as pH, temperature, and the like, as described
elsewhere herein,
can be useful. The amount of additional carbon dioxide that would then be
added can be
calculated from the measurement).
102561 The washing can be done as a single wash, or it can be split into two
or more washes,
one or more of which can include carbonation. Thus, the washing may be done as
1, 2, 3, or
more than 3 washes. Of these, one or more may include carbonation. It is
possible that by
splitting the washes, in combination with carbonation, less water may be
needed than if a
single wash is used. If saddlebag water addition is counted as a wash, then,
typically, a
minimum of two washes would be used (first is saddlebag water, second is at
wash station).
If more than one wash is used at the wash station, then it is 3, 4, etc.
washes. Of these total
washes, one or more may include a carbonation step, e.g., there can be 2 total
washes
-62-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
(saddlebag and wash station) where one wash includes a carbonation step (e.g.,
addition of
saddlebag water at job site, or the wash step at the wash station), or both
washes include a
carbonation step. As another example, there can be 3 washes (saddlebag and two
separate
washes at wash station) in which one wash includes a carbonation step (e.g.,
saddlebag at job
site or one of the 2 washes at the wash station), or 2 washes include a
carbonation step (e.g.,
saddlebag at job site and one of the 2 washes at the wash station, or both
washes at the wash
station), or all three washes include a carbonation step.
[0257] The carbon dioxide may be added manually, or automatically, or a
combination of
the two. If the carbon dioxide is added as carbonated wash water, typically,
the usual wash
routine can be used, and some or all of the wash water is carbonated or
supercarbonated. If
the concrete in the truck is already partially carbonated, e.g., if it has
been carbonated during
the trip to the wash facility, a desired additional amount of carbon dioxide
may be calculated,
possibly based on one or more characteristics as described above, e.g., pH,
and the amount of
carbonated wash water and normal (uncarbonated) wash water adjusted
accordingly. If the
concrete in the truck has not been carbonated, an amount of carbon dioxide may
be calculated
as described below, and the amount of carbonated wash water and normal
(uncarbonated)
wash water adjusted accordingly. Alternatively, the wash water may be used as
normal,
without any particular calculations or adjustments.
[0258] In some cases, additionally or alternatively, carbon dioxide may be
added as solid
carbon dioxide. Thus, dry ice, which may be adjusted to a particular size or
range of sizes,
may be added to the drum in a desired amount. The addition can be a simple as
a manual
addition by the truck driver or other personnel.
[0259] Additionally or alternatively, carbon dioxide may be added as gaseous
carbon
dioxide, or as a mixture of gaseous and solid carbon dioxide. In this case, an
injection system
is used. In these cases, in general, a delivery system for the carbon dioxide
includes a source
of carbon dioxide (e.g., a tank of liquid carbon dioxide), a conduit from the
source to an
injector for placing the carbon dioxide in the truck drum, and a system for
positioning the
injector so that the injection of carbon dioxide directs carbon dioxide into
the drum of the
truck, generally at a desired location in the drum, though in some cases very
little is required
beyond aiming the injector into the drum. A system may include a plurality of
injectors to
handle a plurality of trucks, e.g., simultaneously, such as at least 2, 3, 4,
5, 6, 7, 8, 9, 10, or
more than 10 injectors. The injectors may all utilize the same source of
carbon dioxide, with
appropriate piping and valving. Typically, the system will also include a
controller.
-63-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
102601 The injector is positioned so that delivery of carbon dioxide into the
drum will occur
into the opening of the drum and at a desired location of the drum. This can
be as simple as
the truck driver backing the truck to a designated spot, where the delivery
system is situated
so that it is properly aligned to inject carbon dioxide into the drum with
little or no additional
adjustment (e.g., injector is situated to be in proximity to opening of drum
when truck backed
in, then the truck driver may need to move the injector manually to the final
position). In
certain embodiments, an automated system may be used to assist in positioning
the injector,
or even to completely position it with no human intervention. The system
further includes an
actuator to start and stop delivery of carbon dioxide to the drum, e.g., a
valve, and a
connection between the valve and a controller that controls the start and stop
of delivery.
Generally, the system will also include a system to measure flow rate of the
carbon dioxide.
In a system that uses liquid-)gas and solid, this can be, e.g., a system as
described in US.
Patent No. 9,376,345.
102611 The controller can be as simple as a button or switch that the truck
driver toggles
after backing the truck to the bay. It will be appreciated that such a
"switch" can be any
suitable switch, such as the touchscreen of a wireless device, e.g., a
smartphone. Flow can
continue for a designated time, then halted. Again, the simplest method for
this is for the
truck driver to hit the switch again. However, it can be preferable to have an
automatic
controller, to avoid human error and to more finely modulate delivery, so that
the flow of
carbon dioxide is halted automatically on signal from the controller. This may
be after a
certain time, or a certain amount of carbon dioxide is delivered (from flow
rate and time),
and/or based on one or more characteristics of the wash water which can be
measured, e.g.,
by sensors, such as pH, specific gravity, temperature, etc., and communicated
to the
controller, which then halts or adjusts flow based on a pre-determined
algorithm. The
automatic controller can also automatically start flow when the truck and
injector are
properly positioned, using appropriate positioning sensors to determine this
point The
controller can also alert the truck driver as to when the truck is properly
positioned in relation
to the injector, or when the truck or injector is out of position.
102621 An exemplary control system, which may be used for any suitable system
in which
wash water is treated with carbon dioxide, and, in particular in systems in
which the
carbonated wash water is re-used as mix water, utilizes input regarding one or
more
conditions of a wash water holder and/or its environment, such as at least 2,
3, 4, 5, or 6
conditions, processes the input, then signals one or more actuators, such as
at least 2, 3, 4, 5,
or 6 actuators, e.g., a valve that regulates carbon dioxide flow, based on the
processing.
-64-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
Inputs can include, but are not limited to, one or more of wash water pH, wash
water
temperature, carbon dioxide content of air in contact with wash water (e.g.,
air in a headspace
above a tank), andJor a calculated amount of carbon dioxide to be added. In
the latter case,
the calculation can be based on, e.g., volume of wash water, known or
estimated amount of
concrete in wash water, known or estimated percentage of cement in the
concrete, known or
estimated carbon dioxide uptake required to reach an acceptable endpoint,
e.g., acceptable
pH, and/or acceptable carbon dioxide uptake. Thus, one exemplary control
system utilizes
inputs that include wash water pH, temperature, and/or carbon dioxide
concentration directly
above the water, e.g., in a holding tank or reclaimer. In certain embodiments
all three of pH,
temperature, and carbon dioxide concentration are used; in certain embodiments
two of pH,
temperature, and carbon dioxide concentration are used; in certain embodiments
only one of
pH, temperature, and carbon dioxide concentration are used, for example,
carbon dioxide
concentration above the wash water. Additional sensors and/or information that
may input to
a controller, can include a flow meter to determine carbon dioxide flow rate,
a sensor to
determine the level of water in the holding tank (which level may vary
depending on a variety
of conditions), and/or information from a pump or pumps, such as pumps that
pump new
wash water into a holding tank, e.g., from a reclaimer, and/or such as pumps
that pump water
into a recirculation loop. In the case of a pump from a reclaimer, the pump or
pumps
typically have a fixed flow rate, so information regarding time that the pump
is on can be
sufficient for the controller to determine an amount of new wash water that
has been added to
the system; given the typical amount of cement in a load, the controller can,
e.g., adjust
carbon dioxide flow to wash water to account for the anticipated amount of
material to be
carbonated, and keep ahead of the carbonation demand. Alternatively, or
additionally, the
controller may send signals to other sensors, e.g., pH, temperature, and/or
carbon dioxide, to
read values more frequently so that the system can adjust more quickly to the
added load.
102631 Additional sensors can also include a sensor to monitor pressure behind
a carbon
dioxide control valve (typically used to send an alarm signal if the pressure
is outside
acceptable limits), and a sensor for the temperature of incoming gas, which
indicates whether
the carbon dioxide source, e.g., tank, can keep up with demand; such a sensor
can indicate
whether the source is being overwhelmed by demand, because in such case liquid
carbon
dioxide droplets may form.
102641 For convenience, the system will be described in terms of using all
three sensors; it
will be understood that fewer or more sensors may be used. Thus, in an
exemplary
embodiment, a pH sensor/meter, a temperature sensor such as a thermocouple,
and a CO2
-65-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
sensor/meter are used as sensors. The sensors are operably connected to a
control system,
e.g., wired connection, wireless connection, or a combination. The control
system is also
connected to the carbon dioxide addition equipment for the wash water, and,
optionally, a
pump or pumps. Any suitable control system can be used, such as a programmable
logic
controller (PLC). The control system may be stand-alone, or integrated with an
overall
control system for the wash water facility, or a combination thereof.
Additional equipment
can include a first pneumatic cylinder and a second pneumatic cylinder, one or
both of which
can extend and contract, a mass flow meter for CO2 gas flow metering and
control, and a
water line solenoid in a clean water line, to regulate flow of clean water to
rinse the pH probe.
The system can include a pump; an exemplary pump is one that serves to agitate
the water in
a holding tank, so that solids don't settle. Pumps alternatively or in
addition can include
reclaimer pumps.
102651 The wash water temperature sensor, e.g., thermocouple, can be placed
anywhere in
contact with the wash water in the system, but typically is submerged to
ensure the mass of
the sensor does not impact the reading. A single wash water temperature sensor
may be used,
or more than one temperature sensor may be used, such as at least 2, 3,4, 5,
or 6 wash water
temperature sensors.
102661 The CO2 sensor is placed above the surface of the wash water, e.g., in
a location of
upward-flowing wash water. The distance of the CO2 sensor from the surface of
the water
may be any suitable distance so long as the sensor can detect carbon dioxide
emitted from the
wash water, i.e., carbon dioxide that has been contacted with the wash water
but that has not
been absorbed inlreacted with the wash water, so that it is escaping to the
atmosphere above
the wash water (headspace). For example, the sensor may be 0.1-100, or 1-100,
or 1-50, or 5-
100, or 5-50 cm above the surface of the wash water, or any other suitable
distance. If the
CO2 sensor is in a fixed position, the distance from the surface of the water
can vary as water
level varies, e.g., from additional loads, use of water, etc. Thus, the system
may also include
a sensor to sense the level of the wash water in the tank. The controller may
adjust the
weight given to the carbon dioxide value depending on distance from the
surface, e.g., if the
sensor is further from the surface more carbon dioxide has to build up before
the sensor will
read it, and the controller may adjust flow to a different degree, for
example, reduce flow
more, or at a different rate, for example, more quickly, than if the sensor is
closer to the
surface of the water. Additionally or alternatively, a CO2 sensor may be
configured to stay a
constant distance, or within a constant range of distances, from the surface
of the wash water.
For example, a CO2 sensor may be on a float, with the gas-sensing portion a
certain distance
-66-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
above the waterline of the float, or be provided with a mechanism to move the
sensor based
on, e.g., readings of the level of the wash water. Any other suitable method
and apparatus for
maintaining a constant distance from the surface of the wash water may be
used. The system
may use a single CO2 sensor or more than one, such as at least 2, 3, 4, 5, or
6 CO2 sensors.
102671 Input from a sensor to signal the height of water in the tank may
alternatively or
additionally be used to regulate one or more aspects of the system. For
example, when the
water level is low, changes will tend to be more rapid, and the interval
between samples may
be decreased, and/or carbon dioxide flow rate decreased.
102681 The pH sensor or sensors can be used in any suitable location that
allows accurate
readings of wash water p1-1. Any suitable sensor which can withstand the
conditions typical
of concrete wash water may be used. To obtain an accurate reading and prevent
fouling of
the sensor, the sensor is typically contacted with wash water in which the
solids have been
allowed to settle to a sufficient degree to obtain an accurate reading and to
not foul the
sensor. This may be done in any suitable manner. For example, a portion of
wash water may
be removed from the tank for a pH measurement and, e.g., allowed to settle
before a
measurement is taken. In another example, a pneumatic cylinder can be extended
into the
wash water at a location of downward-flowing wash water, for example, about 12
inches into
the wash water, or any other suitable distance. The water inside the cylinder
will not be
exposed to the motion of the overall wash water, and solids can settle out.
After an
appropriate interval to allow sufficient solids to settle, for example, at
least 5, 10, 15, 20, 30,
40, 50, or 60 seconds, a second pneumatic cylinder, which includes the pH
sensor, is
extended into the first cylinder to take a pH reading of the water inside the
first cylinder.
After a reading is complete, the probe is retracted from the first cylinder,
and is subjected to
appropriate treatment to prepare for the next reading, which can be, e.g.,
rinsing of the probe
with clean water released from a clean water line by action of a solenoid in
the line. The first
cylinder is also retracted from the wash water at some time between samples so
that a fresh
sample can be obtained for the next reading. A single pH sensor may be used,
or more than
one may be used, such as at least 2, 3, 4, 5, or 6 pH sensors.
102691 The sensor or sensors send signals to the control system. The readings
from the
various sensors can be reviewed to ensure that proper sampling has occurred,
for example
confirmation logic checks that the reading is in the expected range based on
reading time, that
change in value between readings is reasonable, i.e., not too high or too low.
If an anomaly is
detected, an error signal can be sent and standby logic to ensure continued
safe operation
(e.g., for temperature, pH); in the case of CO2 sensor malfunctioning, an
alarm may sound
-67-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
and/or the system may be shut down to ensure safety. If readings are
determined to be
proper, then the control system may determine, based on one or more readings,
if any
adjustment to CO2 flow rate should be made.
102701 Generally, the variable or variables will be determined to be within a
suitable range,
and if within the range, at what point in the range it is; this may be any
suitable form of
interpolation. The values for each variable may be combined, either as is or
as weighted
variables. The suitable ranges for each value can be determined by routine
testing at the site.
The range for pH may be any suitable range, such as from 6.0, 6.1, 6.2, 6.3,
6.4, 6.5, 6.6, 6.7,
6.8, 6.9, 7.0, 7.1, 7.2, 7.3, 7.4, 7.5, 7.6, 7.7, 7.8, 7.9, 8.0, 8.1, 8.2,
8.3, 8_4, 8.5, 8.6, 8.7, 8.8,
8.9, 9.0, 9.1, 9.2, 93, 9.4, 9.5, 9.6,9,7, 9.8, 9.9, 10, 10.1, 10.2, 10.3,
10.4, 10.5, 10,6, 10.7,
10.8, 10.9, 11.0, 11.2, 11.4, 11.6, 11.8, 12.0, 12.2, 12.4, 12.6, 12.8, 13.0,
13.2, 114,13.6,
13.8, 14.0, or 14.5 to 6.1, 6.2,6.3, 6.4, 6.5, 6.6, 6.7, 6.8, 6.9, 7.0, 7.1,
7.2, 7.3, 7.4, 7.5, 7.6,
7.7, 7.8, 7.9, 8.0, 8.1, 8.2, 8.3, 8.4, 8.5, 8.6, 8.7, 8.8, 8.9, 9.0, 9.1,
9.2, 9.3, 9.4,9.5, 9.6, 9.7,
9.8, 9.9, 10, 10.1, 10.2, 10.3,10.4, 10.5, 10.6, 10.7, 10.8, 10.9, 11.0, 11.2,
11.4, 11,6, 11.8,
12.0, 12.2, 12.4, 12.6, 12.8, 13.0, 13.2, 13.4, 13.6, 13.8, 14.0, 14.5, or
15Ø The range for
temperature may be any suitable range, such as from 5, 7, 8, 9, 10, 11, 12,
13, 14, 15, 16, 17,
18, 19, 20,21, 22, 23, 24, 25,26, 27, 28, 29, or 30 C to 7, 8,9, 10, 11, 12,
13, 14, 15, 16, 17,
18, 19, 20,21, 22, 23, 24, 25,26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37,
38, 39,40, 41,42,
43, 44, 45,46, 47, 48, 50, 52, or 55 C; generally tanks are run in the open
and the lower limit
may be adjusted according to air temperature, while the upper limit may be
determined by the
concrete production facility, which may not use mix water above a certain
temperature. The
range for carbon dioxide may be any suitable range, such as from 340, 350,
360, 370, 380,
390, 400, 410, 420, 430, 440, 450, 460, 470, 480, 490, 500, 520, 540, 560,
580, 600, 620,
640, 660, 680, 700, 720, 740, 760, 780, 800, 825, 850, 875, 900, 925, 950,
975, 1000, 1050,
1100, 1150, 1200, 1250, 1300, 1350, 1400, 1450, 1500, 1550, 1600, 1700, 1800,
1900, 2000,
2100, 2200, 2400, 2600, 2800, 3000, 3200, 3400, 3600, 3800, 4000, 4200, 4400,
4600, or
4800 ppm to 350, 360, 370, 380, 390, 400, 410, 420, 430, 440, 450, 460, 470,
480, 490, 500,
520, 540, 560, 580, 600, 620, 640, 660, 680, 700, 720, 740, 760, 780, 800,
825, 850, 875,
900, 925, 950, 975, 1000, 1050, 1100, 1150, 1200, 1250, 1300, 1350, 1400,
1450, 1500,
1550, 1600, 1700, 1800, 1900, 2000, 2100, 2200, 2400, 2600, 2800, 3000, 3200,
3400, 3600,
3800, 4000, 4200, 4400, 4600, 4800, or 5000 ppm. Since tanks are generally
open to the
atmosphere, the lower limit typically will not be below the atmospheric level
of carbon
dioxide, which is rising, thus determined at the site or as of date. The
maximum upper limit
may be constrained by regulations regarding worker safety, which vary, and can
be as low as
-68-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
1000 ppm, or may be, e.g., 5000 ppm. However, in general the upper limit will
be lower than
worker safety limits in order to more efficiently control carbon dioxide use
in the system, and
to limit waste. A separate carbon dioxide sensor may be installed at the site
in worker areas
and be set to give an alarm at a certain level, or even to shut down carbon
dioxide feed into
the system. This sensor is not necessarily communicating with the overall
system, e.g., it
may be a standalone alarm.
102711 Samples may be taken at any suitable interval, which may be constant or
may vary
depending on conditions, e.g., as described elsewhere, sampling rate may
increase when a
load from, e.g., a reclaimer is sensed. Exemplary sampling intervals are from
1, 2, 3, 4, 5, 7,
10, 20, 30,40, or 50 seconds, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14,
15, 17, or 20 minutes,
to 2, 3,4, 5, 7, 10, 20, 30, 40, or 50 seconds, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10,
11, 12, 13, 14, 15, 17,
20, 22, or 25 minutes. In order to obtain accurate readings at each sample
time, several
readings may be taken from one or more of the sensors, such as at least 2, 3,
4, 5, 6, 7, 8, 9,
10, 12, 15, 17, or 20 readings. Such readings may be averaged, or the control
system may
contain logic that allows choice of the most likely accurate reading or
readings from the
group.
102721 Exemplary control logic to control CO2 flow rates, based on all three
of pH,
temperature, and CO2 above the surface (e.g., in headspace), is as follows,
using upper and
lower limits that are merely exemplary (any suitable ranges may be used), and
using linear
interpolation (an suitable interpolation may be used):
102731 Adjustable variables
Sensor interval (min) = 5
pH (Lower Limit, LL) =7
pH (Upper Limit) = 13
CO2 PPM (LL) = 400
CO2 PPM (UL) =1000
Temp C (LL) = 20 C
Temp C (UL) = 40 C
MAX FLOW = max flow determined onsite for the configuration
used to ensure 100% uptake in new washwater. May be adjusted
-69-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
according to factors that affect uptake, such as volume of water in the
tank (e.g., water level in the tank).
102741 Below is some of the logic that can be incorporated into the logic to
control flow
rates based on the condition of the wash water. This logic uses a linear
interpolation between
100% and 0% of max uptake flow between expected min/max sensor readings for
simplicity
but changing the CO2 factor, pH factor and temperature factor equations would
be relatively
simple when, e.g., data that supports the change. All variables are given
equal weighting but
that can be adjusted, as well, as appropriate.
102751 Conditions:
- if pH < pH(LL) then pH factor =0
- if pH > pH(UL) then pH factor = 1
- if pH (LL)< pH <pH(UL)then pH factor = (pH - pH(LL) / (pH(UL)-pH(LL)))
- if CO2 <CO2 (LL), then CO2 factor = 1
-if CO2 > CO2 (UL) then CO2 factor = 0
- if CO2 (LL) < CO2 < CO2 (UL) then CO2 factor = (CO2 (UL) - CO2 ) / (Co2
(UL) - Co2 (LL)
- if Temp < Temp C (LL) then Temp factor = 1
- if Temp > Temp C (UL) then Temp factor =0
- if Temp C (LL) < Temp <Temp C (UL) then Temp factor = (Temp (UL)
- Temp) /(Temp (UL) - Temp (LL))
Flow = MAX FLOW x ((pH Factor x CO2 factor x Temp factor)/3).
This flow equation is merely exemplary; it will be appreciated that any
suitable
weighting of factors may be used; in the case of the example equation, a value
of
0 for any factor would shut down carbon dioxide flow, as values are
multiplied,
but any suitable numerical manipulation may be used to produce a desired
result.
In general, the combination of factors should not be above 1.0, i.e., max
flow.
Also, it may be desired, as in the example, that any one of the factors
exceeding
an upper or lower limit, depending on the factor, can shut down carbon dioxide
flow.
-70-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
102761 Thus, in certain embodiments the invention provides a method of
treating waste
concrete in concrete mixer comprising adding water to the mixer to wash out
the mixer and
adding carbon dioxide to the mixer, to produce carbonated wash water in the
mixer. At least
a portion of the carbon dioxide added to the mixer is added as carbon dioxide
dissolved in
wash water for the mixer. The concentration of carbon dioxide in the wash
water can be any
concentration as described herein, such as at least 1, 2, 3, 4, 5, 6, 7, 8, 9,
or 10 g/L water. In
certain embodiments, such as when a supersaturated wash water is used,
concentrations of
carbon dioxide in the wash water can exceed 10 g/L, such as at least 12, 13,
14, 15, 16, 17,
18, 19, or 20 g/L. Additionally or alternatively, at least a portion of the
carbon dioxide added
to the mixer can be added as solid and/or gaseous carbon dioxide. The mixer
can be any
suitable mixer. In certain embodiments, the mixer is a transportable mixer,
such as a drum of
a ready-mix truck. The method can include transporting at least a portion of
the carbonated
wash water to a wash water treatment system. The wash water treatment system
can, e.g.,
treat wash water comprising the carbonated wash water to remove aggregates.
The wash
water treatment system can additionally or alternatively add additional carbon
dioxide to the
wash water comprising carbonated wash water. Any suitable method for adding
carbon
dioxide, such as methods described herein, may be used to add the carbon
dioxide.
102771 Dosing of carbon dioxide Regardless of the form of the carbon dioxide,
the total
amount of carbon dioxide to be used in the truck on the drive back to the wash
station and/or
at the station may be determined by the cement content of the concrete mix in
the truck, the
expected amount of concrete that will be coating the inside of the truck, the
expected or
desired level of carbon dioxide uptake by the cement, and the expected
efficiency of uptake
(e.g., carbon dioxide loss due to leakage from the drum of the truck). For
example, a truck
with a capacity of 8 m3 may be carrying concrete with a cement content of 15%,
and it is
known Of estimated that approximately 500 pounds of concrete remains in the
truck after
dumping its load, regardless of load size. A maximum uptake of 50% carbon
dioxide bwc is
expected for this cement type, and an efficiency of uptake of 80% is expected.
The
calculated dose of carbon dioxide for maximum carbonation would be 500 x
0.15/0.50 x
0.80 = ¨188 lb of carbon dioxide. In general, the amount of concrete in the
empty truck will
not be precisely known; a surrogate is the specific gravity of the wash water
as soon as
enough water is added to create a slurry; from the specific gravity and
volume, a mass of
solids may be calculated and, from that and the proportion of cement in the
concrete mix that
was carried in the truck, the amount of cement in the wash water can be
calculated. Thus, in
certain embodiments, the dose of carbon dioxide to be used for wash water
(either in a single
-71-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
truck or in a combination of more than one truck) may be expressed as an
amount by weight
solids, where a percentage of cement and other carbon-dioxide-reacting or -
absorbing
materials is known or estimated, and/or efficiency of carbonation is known or
estimated, e.g.,
at least 1, 2, 5, 7, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75,
80, 85, 90, or 95%
carbon dioxide by weight solids, and/or not more than 2, 5, 7, 10, 15, 20, 25,
30, 35, 40, 45,
50, 55, 60, 65, 70, 75, 80, 85, 90, 95 or 100% carbon dioxide by weight
solids. Higher doses
may be used, e.g., beyond 100% by weight solids, depending on the cement
content of the
wash water, the expected efficiency of carbonation, etc.
10278] Less than a complete (full) dose may be used in any embodiment of the
invention.
This can be for any reason; e.g., the desired or available systems for carbon
dioxide delivery
will not allow sufficient carbon dioxide to be delivered, or it is desired to
keep the carbon
dioxide reactions to a certain level in the time period between dumping the
load of concrete
and final washing at the batching facility, or between washing and further
treatment, etc. As
described elsewhere herein, an aged wash water may require less than a
complete dose (e.g.,
a dose calculated based on fresh concrete in the truck) to provide the
desirable level of
reaction. Although a full or complete dose may be calculated for a given
truck, load, and mix
design, as described elsewhere herein, less than a full or complete dose of
carbon dioxide
may be given, e.g., less than 95, 90, 80, 70, 60, 50,40, 30, 20, or 10% of a
complete dose,
and/or more than 5, 10, 15, 20, 30, 40, 50, 60, 70, 80, or 90% of a full dose.
In certain
embodiments of the invention, the dose of carbon dioxide used to treat wash
water is such
that the total amount of carbon dioxide delivered to a subsequent concrete mix
using the
carbonated mix water (and calculated only from carbon dioxide in the mix
water, ignoring
any other carbon dioxide added to the subsequent concrete mix), is less than
2.0, 1.5, 1.3, 1.0,
0.9, 0.8, 0.7, 0.6, 0.5, 0.4, 0.3, 0.2, or 0.1% by weight cement in the
subsequent mix, for
example, less than 1.0%, or less than 0.8%, or less than 0.5%, or less than
0.3%, or less than
0.1%, such as less than 0.5%. By weight of solids in the washwater, the carbon
dioxide dose
may be at least 0.1, 0.2, 0.4, 0.6,0.8, 1.0, 2.0, 5.0, 10.0, 15.0, 20.0,25,
30, 35, or 40% by
weight of solids in the wash water, and/or not more than 0.2, 0.4, 0.6, 0.8,
1.0, 2.0, 5.0, 10.0,
15.0, 20.0, 25, 30, 35, 40, or 50% by weight of solids in the wash water. The
amount of
carbon dioxide in the wash water may be determined, e.g., by multiplying the
total amount of
carbon dioxide delivered to the wash water by the efficiency (measured or
calculated) of
absorption of carbon dioxide by the wash water and dividing by volume of the
wash water.
Suitable adjustments may be made for the typical case where a holding tank
contains wash
water from multiple trucks, and may be used on an ongoing basis to provide mix
water, based
-72-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
on truck contents and water use, and other appropriate measurements. In
certain
embodiments, the carbon dioxide content the wash water (e.g., carbonates,
bicarbonate,
carbonic acid, and/or dissolved carbon dioxide) may be determined by chemical
or other
suitable measurements. It can be assumed that virtually all of the carbon
dioxide content of a
carbonated wash water, either dissolved or as reaction products with
cementitious materials,
is due to carbonation of the wash water.
102791 It certain embodiments, a full dose, or dose that is calculated to be a
full dose, may
be delivered at the job site and/or during transport to the wash station; in
some cases, less
than a full dose is desired. In some cases, testing at the batching facility
can show whether
carbon dioxide uptake is complete; if not, additional carbon dioxide may be
added at the
batching facility, e.g., during washing of the drum or at a later step, to
achieve a full dose or
the desired less than full dose. In certain embodiments, no carbon dioxide
until the truck is
back at the hatching facility. In certain embodiments, a partial dose is used
at the job site
and/or during the drive back to the hatching facility, and one or more further
partial doses are
delivered at the hatching facility, e.g., during washing or later, as
described above.
102801 In certain embodiments of the invention, the dose of carbon dioxide is
determined
mainly or exclusively by the methods above; e.g., no further pre-testing
beyond, in some
cases, specific gravity, is required. In some cases, dose is calculated simply
from known or
assumed amounts of concrete left in the truck and the mix design of the truck,
including the
amount of cement in the concrete and, in some cases, the type of cement in the
concrete, as
well as known or assumed efficiencies of carbonation, without the need to test
wash water at
all, and in particular, no need for testing for an initial dose of carbon
dioxide.
102811 The carbon dioxide added to the wash water will initially dissolve in
the water and
then form various products from reaction, such as bicarbonates, and carbonates
(e.g., calcium
carbonate). Carbon dioxide in the wash water, in the form of dissolved carbon
dioxide,
carbonic acid, bicarbonates, and carbonates, will be carried over into cement
in which the
which the wash water is used as mix water. Thus, the cement mix will contain a
certain
amount of carbon dioxide (including dissolved carbon dioxide, carbonic acid,
bicarbonate,
and carbonate) contributed by the carbonated wash water, which may be
expressed as percent
by weight cement in the mix. For example, a wash water may have a solids
content 150,000
ppm, or 15%, which would give a specific gravity of approximately 1.10. If
carbon dioxide
is added to the wash water and the uptake by the wash water is 30%, then 4.5%
of the water
is carbon dioxide, mainly as carbonation products. If a concrete mix is then
made using the
carbonated wash water at a water/cement ratio of 0.5, then the amount of
carbon dioxide (as
-73-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
dissolved carbon dioxide, carbonic acid, bicarbonate, and carbonate) in the
concrete mix is
2.25% bwc. These numbers are merely exemplary. Wash water solids content,
efficiency of
uptake, w/c ratio, amount of mix water that is wash water, and the like, can
vary. Thus, the
amount of carbon dioxide provided by carbonated wash water in a concrete mix
that
comprises carbonated wash water can be at least 0.01, 0.05, 0.1, 0.2, 0.5,0.7,
1.0, 1.1, 1.2,
1.3, 1.4, 1.5, 1.6, 1.7, 1.8, 1.9, 2.0, 2.1, 2.2, 2.3, 2.4, 2.5, 2.6, 2.7,
2.8, 2.9, 3.0, 3.1, 3.2, 3.3,
3.4, 3.5, 3.6, 3.7, 3.8, 3.9, 4.0, 4.2, 4.4, 4.6, 4.8, 5.0, 5.5, 6.0, 6.5,
7.0, 7.5, 8.0, 8.5, 9.0, 9.5,
10.0, 10.5, 11.0, 11.5, 12, or 12.5% bwc, and/or not more than 0.05,0.1, 0.2,
0.5, 0.7, 1.0,
1.1, 1.2, 1.3, 1.4, 1.5, 1.6, 1.7, 1.8, 1.9, 2.0, 2.1,2.2, 2.3, 2.4, 2.5, 2.6,
2.7, 2.8, 2.9, 3.0, 3.1,
3.2, 3.3, 3.4, 3.5, 3.6, 3.7, 3.8, 3.9, 4,0, 4.2, 4.4, 4.6, 4.8, 5.0, 5.5,
6.0, 6.5, 7.0, 8.0, 9,0, 10.0,
10.5, 11.0, 11.5, 12.0, 12.5, 13.0, 14, 15, 16, 17, 18, 19, 20, 22, 25, or 30%
bwc. For
example, the invention provides a method of preparing a concrete mix
comprising (i) adding
concrete materials to a mixer, wherein the concrete materials comprise cement;
adding mix
water to the mixer, wherein the mix water comprises carbonated concrete wash
water in an
amount such that the total carbon dioxide or carbon dioxide reaction products
(expressed as
carbon dioxide) supplied by the carbonated mix water to the concrete mix is at
least 0.01,
0.05,0.1, 0.2, 0.5, 0.7, 1.0, 1.1, 1.2, 1.3, 1.4, 1.5, 1.6, 1.7, 1.8, 1.9,
2.0, 2.1, 2.2, 2.3, 2.4, 2.5,
16, 2.7, 2.8, 2.9, 3.0, 3.1, 3.2, 33, 3,4, 3.5, 3.6,3+7, 3.8, 19, 4.0, 4.2,
4.4, 4.6, 4.8, 5,0, 5.5,
6.0, 6.5, 7.0, 7.5, 8.0, 8.5, 9.0, 9.5, 10_0, 10_5, 11.0, 11.5, 12, or 12.5%
bwc, and/or not more
than 0.05, 0.1, 0.2, 0.5, 0.7, 1.0, 1.1, 1.2, 1.3, 1.4, 1.5, 1.6, 1.7, 1.8,
1.9, 2.0, 2.1, 2.2, 2.3, 2_4,
2.5, 2.6, 2.7, 2.8, 19, 3.0, 3.1, 3.2, 33, 3.4, 3.5, 3.6, 3.7, 3.8, 3.9, 4.0,
4.2, 44, 4.6, 4.8, 5.0,
5.5, 6.0, 6.5, 7.0, 8.0,9.0, 10.0,10.5, 11.0, 11.5, 12.0, 12.5, or 13.0% bwc,
for example, at
least 0.5, 1.0, 1.5, or 2.0%, and/or not more than 2.5, 2.0, 1.5, or 1.0%, or
for example, not
more than 2%, or not more than 2.5%, or not more than 3.0%, or not more than
3.5%, or not
more than 4.0%; or, for example, at least 0.01% bwc, or at least 0.05% bwc, or
at least 0.1%
bwc, or at least 0.5% bwc, or at least 1.0% bwc, or at least 2.0% bwc, or at
least 3.0% bwc, or
at least 4.0% bwc, or at least 5.0% bwc; or, for example, in a range of
between 0.01 and
13.0%, bwc, or a range of between 0.01 and 12.0% bwc, or a range of between
0.01 and
11.0%, bwc or a range of between 0.01 and 10.0%, bwc, or a range of between
0.01 and
8.0%, bwc, or a range of between 0.01 and 6.0%, bwc or a range of between 0.01
and 4.0%,
bwc, or in a range of between 0.1 and 13.0%, bwc, or a range of between 0.1
and 12.0% bwc,
or a range of between 0.1 and 11.0%, bwc or a range of between 0.1 and 10.0%,
bwc, or a
range of between 0_1 and 8.0%, bwc, or a range of between 0.1 and 6.0%, bwc or
a range of
between 0.1 and 4.0%, bwc, or in a range of between 1.0 and 13.0%, bwc, or a
range of
-74-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
between 1.0 and 12.0% bwc, or a range of between 1.0 and 11.0%, bwc or a range
of between
1.0 and 10.0%, bwc, or a range of between 1.0 and 8.0%, bwc, or a range of
between 1.0 and
bwc or a range of between 1.0 and 4.0%, bwc and (iii) mixing the water and the
concrete materials to produce a concrete mix. It will be appreciated that the
amount of
carbonated wash water in the total mix water may be any suitable amount, such
as amounts
described herein.
102821 Carbon dioxide delivery in reclaimers and piping from reclaimer to pond
or slurry
tank. Some facilities utilize reclaimers to reclaim aggregate, e.g., sand and
gravel, from the
wash water. The water may then further be used, generally with more
processing, either as
part of mix water or as wash water, any remaining water is disposed of in the
usual manner.
In a typical reclaimer, water with grit and solid components is pumped through
the process,
and sand and gravel are separated out, e.g., by sieving. The water is then
sent to a settlement
pond, and/or to a tank for reuse. In the case of water sent to a settlement
pond, water may be
transported to a tank, where carbon dioxide is added to the water; e.g. a
recirculation line
allows carbon dioxide to be added to the water in the line, then sent back to
the tank; if a tank
is already present, then a carbonation apparatus may be added, for example, a
recirculation
line. This water can be carbonated or super-carbonated, additionally or
alternatively with
carbon dioxide added to the water during the pumping process, so that as
carbon dioxide is
consumed in carbonation reactions, more carbon dioxide is supplied to the
water. Carbon
dioxide can additionally or alternatively be supplied into piping as the water
is pumped to a
settlement pond or a slurry tank. In an optimum situation, sand and gravel are
separated out
as usual, but the water in, e.g., a slurry tank is available for use again
without further dilution,
or with less dilution than would otherwise be required. For example, the
process may
produce water, e.g., water in a slurry tank, from a reclaimer that has a
specific gravity that is
g,reater than, e.g., 1.03, 1.04 1.05, 1.06, 1.07, 1.08, 1.10, 1.11, 1.12,
1.13, 1.14, 1.15, 1.16,
1.17, 1.18, 1.19, or 1.20, but that is suitable for use as mix water. This is
different from
existing reclaimers, where the water in, e.g., a slurry tank, typically
requires dilution to lower
the specific gravity to acceptable levels. In the present process, little or
no additional
processing may be needed (though additionally or alternatively carbonation at
the slurry tank
may be used, if necessary or desired) because the carbonation process halts or
greatly retards
deleterious reactions of the cementitious material while leaving it available
for reaction in a
second concrete batch, and also adjusts the pH of the water to more acceptable
levels. For
example, in the process, filtering and/or settling of solids is generally not
necessary; indeed,
an advantage of the methods and compositions of the invention is that
materials from one
-75-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
batch may be recycled into another batch or batches, potentially allowing less
material, e.g.,
cement to be used, and decreasing or even eliminating costs associated with
disposing of
wash water materials.
[0283] Retrofit of existing facility to provide reclamation: Most concrete
facilities do not
include a reclaimer, but could benefit from being able to reuse wash water
and, potentially,
aggregates from wash water. At present, most solid material is simply allowed
to settle out in
one or more settlement ponds, and is periodically disposed of, with little or
no reuse, while
the water in the settlement pond must be further treated to meet environmental
standards
before disposal. If, instead, wash water is carbonated, either before
placement in the pond, or
during its time in the pond, or both, then some or all of the water may be
used as mix water,
reducing or eliminating the costs and equipment required to treat the water
for disposal. In
addition, some or all of the aggregates may be available for reuse, instead of
hardening and
becoming useless.
[0284] As an example, in one type of operation, wash waters from trucks are
dumped into a
first bay, where solids settle out, harden, and are generally dumped. The top
water from the
first bay goes over a weir into a second bay where, generally, solids are
further allowed to
settle, top water is taken off, often sent to a third bay, and the water, now
essentially free of
solids but still with a high pH, silicates, calcium etc., is treated for
disposal or, in some cases,
for at least partial reuse. In presently available systems, the treatment in
the third bay, where
there are no solids present, may be with carbon dioxide. The present invention
allows for a
retrofit of the first or second bay, where solids are still present, so that
instead of being a
settlement pond, it is a slurry pond where carbonation occurs; the carbonated
wash water is
then suitable for use as mix water, rather than merely being disposed of This
can be done by
the use of agitators, recirculating pumps, or a combination of these, where
carbon dioxide is
added either directly into the pond (e.g., through bubble mats, as described
elsewhere herein)
or in the lines in the recirculation pumps, or both. Other methods of adding
carbon dioxide,
e.g., at impellors or eductors, etc., are as described herein. Other means of
carbon dioxide
addition, such as solid carbon dioxide, or a mixture of gaseous and solid, may
also be used, as
described herein.
[0285] In certain embodiments, a wall is added to the first bay, e.g., a wall
with a notch to
allow water to flow through the notch (e.g., a weir) to an area of the first
tank beyond the
wall. The wall can be placed to provide a division in the first tank to allow
solids, such as
aggregate, to settle, but allow the remaining water, with suspended solids, to
flow over the
notch into a second part of the first bay. Optionally, a second wall can be
added on the other
-76-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
side of the first wall, in order to reduce the volume of the area into which
water flows over
the notch. The water can be pumped out of the area, e.g., with a sump pump or
similar pump,
into a holding tank, where it can be carbonated, e.g., by use of a
recirculation loop, where
water is pumped out of the tank into a pipe and carbon dioxide added to the
water in the pipe,
then the carbonated water is led back into the tank. The carbonated water in
the holding tank
can then be led back to the batching plant, for use in subsequent batches of
concrete.
Addition of carbon dioxide to the water can be controlled as described
elsewhere herein. In
these embodiments, it may not be necessary to have a second or third bay, or
their volumes
may be reduced.
[0286] With this retrofit, some or all of the water from the first or second
pond becomes
useable as mix water, often at a higher specific gravity than would otherwise
be possible, for
example, at a specific gravity greater than, e.g., 1.03, 1.04 1.05, 1.06,
1.07, 1.08, 1.10, 1.11,
1.12,1.13, 1.14, 1.15, 1.16, 1.17, 1.18, 1.19, or 1.20, whereas before the
retrofit, little or none
of the water from the pond was reused as mix water, but instead was disposed
of With the
retrofit, cementitious materials from previous batches also become available
in subsequent
batches (see calculations, below). Appropriate sensors and control systems may
be used to
monitor carbon dioxide addition, as well as monitor appropriate
characteristics of the water,
also as described herein, and to modify carbon dioxide delivery, as well as to
control
redirection of water back into the hatching system for use as mix water. In
this way, as
much as 100% of the wash water may be recycled into mix water, e.g., at least
10, 20, 30, 40,
50, 60, 70, 80, 90, or 95% of the wash water may be recycled into mix water.
For a typical
truck, which uses ¨120L wash water/m3 of concrete carried in the truck to
clean the truck,
and a typical mix, which uses ¨130L water/ in' concrete, it is, indeed,
possible to recycle
100% of the wash water into subsequent batches of concrete.
[0287] A retrofit may additionally or alternatively include a retrofit at the
wash station, or at
the truck, or both, to carbonate wash water before it reaches the ponds. At
the truck level this
includes addition of a source of carbon dioxide, which may be solid, gaseous
(in solution or
free), or a system to deliver both solid and gaseous carbon dioxide, as
described elsewhere
herein. For example, a truck may be retrofitted so that its saddlebags can
hold carbonated
water, if necessary. The backing site may be retrofitted to include a system
for carbonating
water and for supplying it to truck saddlebags (this would include a source of
carbon dioxide,
appropriate piping and injection systems, optionally a system for
supersaturating water with
carbon dioxide, and delivery system to deliver carbonated water to saddlebags,
and
appropriate control systems). Alternatively or additionally, the truck may be
retrofitted to
-77-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
provide a system to carry dry ice for delivery to the drum after the load is
delivered, which
can be as simple as an insulated container. The batching facility may include
a storage
system for the dry ice and, optionally, a system for producing dry ice. If it
is desired to
produce dry ice of appropriate size range for a particular mix or load, as
described elsewhere
herein, the batch facility or the truck itself may further be outfitted with a
system for
producing dry ice of the desired size. Additionally or alternatively, the
truck may be
retrofitted with a system to deliver gaseous carbon dioxide to the drum of the
truck, which
includes a source of carbon dioxide, a conduit to deliver the carbon dioxide
from the source
to the drum, and, typically, a metering and control system to regulate
addition of carbon
dioxide to the drum. All of these retrofits may further include appropriate
control systems,
such as sensors (e.g., pH and other sensors, as described elsewhere herein, or
in the simplest
case, a timer, as well as sensors to determine the flow of carbon dioxide), a
processor, and
one or more actuators (e.g. valves) to control the flow of carbon dioxide
according to the
desired dose/rate, or other parameters. If it is desired to provide a mixture
of solid and
gaseous carbon dioxide to the drum of the truck, then the same basic setup as
for gaseous is
used, except that piping must be such that it can withstand the temperature of
liquid carbon
dioxide, and the injector should be a snow horn of appropriate design to
produce the desired
mix of solid and gaseous carbon dioxide.
[0288] At the wash station level, this includes equipment as described
elsewhere herein for
supplying carbon dioxide at the wash station, including the appropriate source
or sources of
carbon dioxide, appropriate conduits, injectors, positioning, metering, and
control systems if
carbon dioxide is injected into the drum, systems for carbonating or super-
carbonating water
if that method is used, and for delivering the carbonated water to the wash
line.
[0289] It will be appreciated that, if a plant is retrofitted to carbonate the
wash water, either
at the job site/during transport, or at the wash station, or both, sufficient
carbonation of wash
water may occur so that no further carbonation at the ponds need by pursued;
in some cases,
however, additional carbonation at the ponds is necessary. In addition,
through carbonation
in the truck after pouring and during transport, and/or during wash, aggregate
in the concrete
in the truck can become available for reuse. Using the example of a settlement
system with
two ponds, if the wash station and/or truck is equipped to carbonate the
leftover concrete, the
aggregate material in the first pond can remain as discrete particles and be
recovered and
sieved, as appropriate, for use as aggregate in subsequent batches. The water
may be ready
at this point to be used as mix water, or it may require further treatment,
e.g., further
carbonation, to be so used.
-78-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
102901 Further possibilities, e.g., for retrofitting, are as follows:
102911 Agitation of the wash water can be considered in three or more general
approaches
102921 Customer has an existing wash water tank and an agitation system:
retrofit CO2
treatment system can include a pump to move the water to/through the treatment
step (either
inline or in a separate tank). The pump is not the primary source of agitation
and thus only
needs to start when CO2 treatment starts and is controlled based on one or all
of the sensors
(Temp, pH, CO2 level in headspace)
102931 Storage tank with no agitation: Pumps are used to keep material
suspended in the
tank. Pump moves the water to/through the treatment step (either inline, the
same tank or in a
separate tank). The pump is on at any time the CO2 is injected with start/stop
based upon the
sensor logic.
102941 Customer has a pond with no agitation: Retrofit CO2 treatment adapted
to pond.
A pump is used to move the water to/through the treatment step (either inline
or in a separate
tank). The pump would need to be on all the time while CO2 is injected. Pump
and CO2
start/stop are determined by the sensor logic examining the wash water supply.
102951 In addition, there are various possibilities for the location of
addition of carbon
dioxide and/or admixture (described elsewhere herein) to wash water. In an
exemplary
ready-mix operation, wash water is added initially in the truck, after its
load is dumped, to
keep the remaining concrete from hardening. At this point, admixture, e.g., a
set-retarding
admixture, may be added to wash water in the drum of the truck. Alternatively
or
additionally, carbon dioxide may be added to wash water in the drum of the
truck. The truck
then proceeds to a wash station, where further water may be added to the drum.
At this point,
admixture, e.g., a set-retarding admixture, may be added to wash water in the
drum of the
truck. Alternatively or additionally, carbon dioxide may be added to wash
water in the drum
of the truck. The wash water is typically then pumped to a holding tank, and
admixture
and/or carbon dioxide can be added to the wash water in the line from the
truck to the tank.
In an operation in which a reclaimer is used, admixture and/or carbon dioxide
may be added
as described elsewhere herein. In some operations, additional holding tanks
may be used,
and at any one or more of these, admixture and/or carbon dioxide may be added.
As
described herein, the addition may occur in the tank itself or may occur in a
recirculation line
in which wash water is removed from the tank and circulated through a loop;
see, e.g.,
Example 14. At some point, wash water is moved from, e.g., a holding tank,
back to the
drum of a ready-mix truck (or into a central mixer) to be used as part or all
of the mix water
-79-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
for a new batch of concrete. Carbon dioxide and/or admixture may be added in
the line from
the tank to the mixer (truck drum or central mixer).
[0296] The invention also provides kits as appropriate for the various types
and
combinations of retrofits, as described herein. These can be packaged at a
central facility
where appropriate components and sizes are selected, according to the
operation to be
retrofitted, and shipped to the operation, generally with all necessary parts
and fittings so that
installation at the facility is easy and efficient.
[0297] It will be appreciated that the above discussion regarding retrofits
applies equally to
the building of new facilities, though some modifications may not be necessary
when a
facility is built from scratch, whereas other modifications may become
necessary, as will be
apparent to one of skill in the art.
[0298] Benefits of carbonation of wash water The benefits of carbonation of
wash water
include a reduction in the carbon footprint of the concrete operation, reduced
water usage,
reduced waste output, and increased recycled content usage.
[0299] By use of the methods and compositions of the invention, it is possible
to get back
some percentage of cementitious quality of cement, say at least 10, 20, 30,
40, 50, 60, 70, 80,
90, or 95 of cementitious quality. The producer can then reduce amount of
cement in next
batch by corresponding amount. E.g., a truck with 500 lb residual concrete,
15% cement, is
treated by process and compositions of invention and the resultant slurry
contains the cement
with 80% of its cementitious properties retained. If all the wash water can be
transferred over
to the next mix as mix water, then 500 x 0.15 x 0.80 lb, or 60 lb less cement
need be used in
the next batch. If 90% of remainder of the concrete is aggregate that can be
recovered
because of the carbonation process, then an additional 450 lb of aggregate may
be reduced in
the subsequent load. These improvements contribute to a lower carbon
footprint, reduced
waste output, and increased recycled content usage.
10300] In addition, as shown in the Examples and described herein, concrete
made with
wash water treated as described herein exhibits greater strength, especially
greater early
strength, that concrete made with untreated water. The greater strength may
be, in some
cases, over 40% of the strength of concrete made with the same mix design and
procedure,
except with normal mix water rather than carbonated mix water. Thus, less new
cement may
be used in a mix that uses carbonated wash water than in the same mix that
uses normal mix
water, which further reduces carbon footprint; for example, at least 1, 2, 3,
4, 5, 6, 7, 8, 9, 10,
12, 15, 20, 25, 30, 35, or 40% less cement and/or not more than 2, 3, 4, 5, 6,
7, 8, 9, 10, 12,
15, 20, 25, 30, 35, 40 or 50% less cement to achieve the same compressive
strength.
-80-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
[0301] Further, carbonation of a cement mix, even one using normal water,
results in
strength increases in the resultant poured material, and correspondingly less
need for cement
in the batch. See, e.g., U.S. Patent No. 9,388,072. When used in conjunction
with carbonated
wash water, the results can be additive, or even synergistic, thus, with use
of both methods
the operator can reduce carbon footprint while at the same time saving money
on the most
expensive main component of concrete: cement; e.g., combining the two methods
(carbonation of wash water and further carbonation of the concrete mix) can
result in using
for example, at least 1, 2, 3,4, 5, 6, 7, 8, 9, 10, 12, 15, 20, 25, 30, 35,
40, 50, Of 60% less
cement and/or not more than 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 15, 20, 25, 30,
35, 40, 50, 60, or
70% less cement to achieve the same compressive strength
103021 Also as described herein, water reuse at a facility using the methods
and
compositions of the invention can be increased dramatically, in some cases to
100% (e.g.,
reuse of wash water in subsequent mixes of at least 10, 20, 30, 40, 50, 60,
70, 80, 90, or 95%
of the wash water), with a corresponding reduction in waste output, again, in
some cases, at
or near 100% (e.g., decrease of waste water from wash water of at least 10,20,
30,40, 50, 60,
70, 80, 90, or 95% compared to using uncarbonated wash water). This imparts
significant
cost savings, as well as reducing carbon footprint further because of the
reduction in energy
use that would go toward treating and disposing of the wash water.
[0303] Disposal and regulatory costs, as well as cement costs, can be reduced
by using the
methods and compositions described herein. Admixtures, which normally may be
needed,
e.g. when wash water is used as mix water, related to workability, can often
be reduced or
eliminated when carbonated wash water is used.
[0304] In many cases, carbonated wash water may not only be used as mix water,
but can be
recycled as wash water.
103051 Mechanism of carbonation of wash water. Without being bound by theory,
it is
thought that when carbon dioxide is introduced into wash water, it quickly is
converted to
carbonate anion due to the high alkalinity of the wash water; the carbonate
anion reacts with
calcium and forms a coating on suspended cement particles, reducing their
reactivity in the
wash water. They are thus "put to sleep" by the carbon dioxide, thus
reducing/eliminating
acceleration, but contributing to later strength. Variability is also reduced
when using wash
water that has been carbonated.
103061 Sulfates The inventors have found that the methods and compositions of
the
invention also can help to favorably alter sulfate content in a concrete batch
made with mix
water that includes carbonated wash water. Carbon dioxide-treated wash water
can be a tool
-81-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
to deal with undersulfated binder. In general, a concrete mix that contains a
high ratio of
aluminates to sulfates may not be a viable mix when used as is. For example,
the use of
supplementary cementitious materials (SCMs) that contribute aluminates can
mean that a
cement that has a proper aluminate-sulfate balance is now in a cement blend
that is under-
sulfated. Carbonated wash water can contain significant concentrations of
sulfates in
solution. If the sulfate content of the carbonated wash water is known, then
an appropriate
amount of carbonated wash water mixes can be added to compensate for this. In
this case the
wash water could have a low solids content because the sulfates are in
solution.
Compositions.
[0307] Further provided herein are compositions, such as carbonated wash water
compositions. In certain embodiments, the invention provides a carbonated
concrete wash
water composition comprising (i) wash water from concrete; (ii) carbon dioxide
and carbon
dioxide reaction products with the wash water. The wash water can be primarily
composed
of water used to rinse out a concrete mixer, e.g., a drum of a ready mix
truck, or a
combination of wash waters from a plurality of mixers, e.g., a plurality of
ready-mix trucks.
The amount of carbon dioxide and carbon dioxide reaction products in the
carbonated
concrete wash water can be at least 0.1, 0.2, 0.5, 0.7, 1.0,1.2, 1.5, 1.7,
2.0, 2.5, 3.0, 3.5, 4.0,
4.5, 5.0, 5.5, 6.0, 7.0, 8+0,9,0, 10.0, 11.0, 12.0, 13.0, 14.0, 15.0, 17.0,
20.0, or 25% by weight
solids in the wash water composition; for example at least 0.5% by weight
solids in the wash
water composition, in some cases at least 2% by weight solids in the wash
water composition,
such at least 5% by weight solids in the wash water composition, or at least
10% by weight
solids in the wash water composition. The specific gravity of the carbonated
wash water can
be at least 1.01, 1.02, 1.03, 1.04,1.05, 1.06, 1.07, 1.08, 1.09, 1.10, 1.11,
1.12, 1.13, 1.14,
1.15, 1.17, 1.20, or any other specific gravity as described herein; for
example, at least 1.03,
such as at least 1.05, or at least 1.10. The pH of the carbonated wash water
composition can
be any pH or range of pHs as described herein, such as at least 6.0, 6.1, 6.2,
6.3, 6.4, 6.5, 6.6,
6.7, 6.8, 6.9, 7.0, 7.1, 7.2, 7.3, 7.4,7.5, 7.6, 7.7,7+8, 7.9, 8.0, 8.1, 8.2,
8.3, 8.4, or 8.5, and/or
not more than 6.1, 6.2, 6.3, 6.4, 6.5, 6.6, 6.7, 6.8, 6.9, 7.0, 7.1, 7.2, 7.3,
7.4, 7.5, 7.6, 7.7,7.8,
7.9, 8.0, 8.1, 8.2, 8.3, 8.4, 8.5, 8.7, 9.0, 9.3, 9.5,9.7, 10, 10.3, 10.5,
10.7, 11.0, 12.0, or 13.0;
for example, the pH of the carbonated wash water can be less than 9.0, such as
less than 8.5,
or less than 8Ø Compositions can further include (iii) additional cement,
that is not cement
in the wash water, e.g., a cement mix produced from dry cement and carbonated
wash water.
Such mixes can further include aggregates, admixtures, etc.
-82-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
Carbon dioxide sequestration and economic advantages
[0308] A concrete production facility utilizing the methods and compositions
described
herein can incur considerable yearly savings, due to reuse of solids in wash
water (thus
avoiding use of a certain amount of new cement), avoided landfill costs, and
other economic
benefits, such as reduced or no additional water treatment costs because some
or all of wash
water is recycled. In addition, there will be considerable
sequestration/offset of carbon
dioxide. Thus, in certain embodiments, the invention provides a method of
sequestering
and/or offsetting carbon dioxide by treating wash water, concrete byproducts
(such as
returned concrete), or a combination thereof, with carbon dioxide, and
optionally re-using
some or all of the solids in the wash water as cementitious material in
subsequent concrete
batches. See Example 9. In certain embodiments, at least 0.1, 0.5, 1, 1.5, 2,
2.5, 3, 3.5, 4,
4.5, 5, 5.5, 6, 6.5, 7, 7.5, 8, 8.5, 9, 9.5,10, 11, 12, 13, 14, or 15% of the
carbon dioxide
produced in manufacturing cement to be used at a concrete facility,
transportation emissions,
other emissions associated with concrete manufacture and use, or a combination
thereof, is
offset by the process. "Offset," as that term is used herein, includes the
amount of carbon
dioxide emissions avoided (e.g., through reduced cement use), as well as the
amount of
carbon dioxide actually sequestered, e.g., as part of carbonated wash solids
and the like. In
certain embodiments, the process provides a savings of at least 0.1, 0.5, 1,
1.5, 2, 2.5, 3, 3.5,
4, 4.5, 5, 5.5, 6, 6.5, 7, 7.5, 8, 8_5, 9, 9.5, or 10% of the annual
production costs of the
concrete facility (e.g., compared to a period of time before carbonation,
adjusted as
appropriate for fluctuations in loads, costs, etc.). Further cost benefits may
be realized in
areas where there is a price on carbon, e.g., cap and trade or carbon tax,
where the offset
carbon dioxide may be a source of further revenue. Additional or alternative
carbon dioxide
offsets can be achieved by treating concrete produced in the facility with
carbon dioxide
while the concrete is being mixed, e.g., by applying gaseous carbon dioxide,
or solid carbon
dioxide, or a mixture of gaseous and solid carbon dioxide, for example in a
dose of less than
3, 2, 15, 1.2, 1.0, 0.8, 0.6, 0.5, 0.4, 0.3, 0.2, or 0.1 bwc, to the mixing
concrete mix. See,
e.g., U.S. Patent Nos. 9,108,883 and 9,738,562. This treatment can result in a
concrete
product that requires less cement than the uncarbonated product, because, in
addition to the
carbon dioxide directly sequestered in the concrete, the carbonated concrete
product has
greater strength after setting and hardening than uncarbonated concrete
product of the same
mix design, and, consequently, a concrete product that requires at least 1, 2,
3, 4, 5, 6, 7, 8, 9,
10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 22, 25, or 30% less cement than
the uncarbonated
product. In such a case, carbon dioxide offset merely from carbonating the
concrete mix may
-83-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
be at least 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19,
20, 22, 25, or 30%.
When concrete wash water treatment with carbon dioxide and, e.g., re-use of
some or all of
the solids in the wash water in subsequent concrete batches is combined with
carbonation of
concrete batches at a concrete facility, the total carbon dioxide offset can
be at least 1, 2, 3, 4,
5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25,
26, 27, 28, 29, 30,
32, 35, 37, 40, 42 or 45%.
Admixtures
[0309] One or more admixtures may be added to the concrete wash water and/or
to concrete
made with the wash water. The addition may occur at one or more points in the
process, as
described elsewhere herein. Whether or not an admixture is used, the type of
admixture, the
point in the process at which admixture is added, and/or the amount of
admixture added, can
depend, e.g., on the type and amount of cement in the wash water. In some
cases, addition of
carbon dioxide to a wash water from a concrete batch can alter the properties
of a subsequent
batch which is made using the carbonated wash water as part or all of the mix
water.
[0310] A decrease in the particle size of a powder in a binder system can
lead to reduced
workability (silica fume additions are an illustrative example). A workability
impact can be
observed for both CO2-treated and untreated wash water, so the particle size
distribution may
not be pivotal. An admixture that flocculates fine particles to effectively
serve to increase the
median particle size and reduce the effective specific surface area, etc., can
mitigate negative
effects associated with the CO2 induced reduction in particle size.
103111 The use of chemicals in the flocculation of precipitated calcium
carbonate (PCC)
may act favorably on the CO2 treated solids given their outward surface may
effectively
behave as calcium carbonate. With PCC, highly charged polyelectrolytes are
known to
produce strong large flocculants and higher flocculation rates. Both bridging
and charge
neutralization occur in polyelectrolyte induced PCC flocculation. See, e.g.,
R. Gaudreault.,
N. D. Cesare., D. Weitz., T. G. M. van de Ven; "Flocculation kinetics of
precipitated calcium
carbonate"; Colloids and Surfaces A: Physicochem. Eng. Aspects 340, p56-65,
2009
https://do Lorg/10.1016/j. colsu rfa .2000.03.008
[0312] Without being bound by theory, PCC flocculation with positively charged
polyelectrolytes indicates two mechanisms. A polymer with a high charge
density and low
molar mass such as polyethylenimine could induce PCC flocculation by
neutralizing the
charge, thus eliminating the electrostatic repulsive force. Whereas a high
molar weight
polymer with low charge density, such as polyacrylamide, interacts with PCC by
a
combination of electrostatic and bridging forces. See, e.g., A. Vanerek, B.
Mince, T. G. M.
-84-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
van de Ven, "Interaction of calcium carbonate fillers with pulp fibres:
effects of surface
charge and cationic polyelectrolytes", J. Pulp Pap. Sci., 26(9), p317-322,
2000. Natural
carbohydrates can also be used, e.g.,: starch (such as potato, corn, and/or
tapioca starches),
dextran, lignin. A starch derivative Glycidyl tetradecyl dimethylammonium
chloride
(GTDAC) can also be used. See, e.g., Y. Wei, F. Cheng, H. Zheng, "Synthesis
and
flocculating properties of cationic starch derivatives", Carbohydr. Polym.,
74(3), p673-679,
2008, Y. Wei, F. Cheng, H. Zheng, "Synthesis and flocculating properties of
cationic starch
derivatives", Carbohydr. Polym., 74(3), p673-679, 2008. Another possible
admixture is
pectin (a biopolymer of D-galacturonic acid), whereon the addition of Al' and
Fe3+ could
greatly increase pectin's flocculating efficiency. Cationic ions neutralized
and stabilized
negatively charged pectin and bound particles by electrostatic attraction.
See, e.g., H. Yokoi,
T. Obita, J. Hirose, S. Hayashi, Y. Takasalci, "Flocculation properties of
pectin in various
suspensions", Bioresour. Technol., 84(3), p287-290, 2002.
https://doi.org/10.1016/50960-
8524(02)00023-8.
10313] Another potential admixture is cellulose or cellulose derivatives, e.g.
electrosterically
stabilized nanocrystalline cellulose (ENCC);dissolved carboxylated cellulose
(DCC); rod-like
dialdehyde cellulose (DAC) nanofibers, also referred to as sterically
stabilized
nanocrystalline cellulose (SNCC); dissolved DAC as dialdehyde modified
cellulose
(DAMC). ENCC/DCC showed a high flocculation efficiency with PCC particles and
induced
PCC flocculation by a combination of electrostatic and bridging forces.
ENCC/DCC induces
the maximum PCC flocculation when PCC particles reach to isoelectric point.
The
flocculation of PCC induced by SNCC: SNCC particles can bridge PCC to induce
flocculation at low dosage (above 1 mWg). SNCC induced the maximum
flocculation when
its fractional coverage was more than half coverage because SNCC particles
become unstable
after deposition on PCC. Adsorption isotherms of three SNCCs and dialdehyde
modified
cellulose (DAMC) on PCC particles were measured. It was found that DAMC had a
higher
affinity than three SNCCs with different aldehyde contents, and the affinity
of SNCC
increased with reaction time. This indicates DAMC chains adsorb stronger than
nanocrystalline parts of SNCC on PCC. See, e.g., Dezhi Chen, Theo G.M. van de
yen,
Flocculation kinetics of precipitated calcium carbonate induced by
electrosterically stabilized
nanocrystalline cellulose, Colloids and Surfaces A: Physicochemical and
Engineering
Aspects, Volume 504, 2016, Pages 11-17, ISSN 0927-7757,
https://doi.org/10.1016/j.colsurfa.2016.05.023; Chen, Dezhi. "Flocculation
Kinetics Of
-85-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
Precipitated Calcium Carbonate Induced By Functionalized Nanocellulose."
(2015). PhD
Thesis.
[0314] Another useful admixture is cationic polysaccharides, with N-alkyl-N,N-
dimethyl-N-
(2-hydroxypropyl)ammonium chloride pendent groups attached to a dextran
backbone. The
flocculation performance of the hydrophobically modified cationic dextran
highly depended
on its hydrophobicity and charge density, and was less dependent on molar
mass. See, e.g.,
L. Ghimici, M. Nichifor, "Novel biodegradable flocculant agents based on
cationic
amphiphilic polysaccharides", Bioresour. Technol., 101(22), p8549-8554, 2000.
Doi:
10.1016/j.biortech.2010.06.049.
[0315] Another useful admixture is cationic derivatives of dialdehyde
cellulose (CDAC).
CDACs showed very good flocculation performance in neutral and acidic
suspensions, while
a low flocculation activity was observed in alkaline suspensions because CDACs
were
broken down into small fragments at alkaline pH. See, e.g., Liimatainen, H,
Sirvit5, J,
Sundman, 0, Visanko, M, Horrni, 0 & Niinimaki, J 2011, 'Flocculation
performance of a
cationic biopolymer derived from a cellulosic source in mild aqueous solution'
BIORESOURCE TECHNOLOGY, vol 102, no. 20, pp. 9626-9632. DO!:
10.1016/j .biortech.2011.07. 099.
103161 Another useful admixture is graft copolymers of carboxymethylcellulose
(CMC) and
polyacrylamide. Copolymers with fewer and longer PAM chains exhibited better
flocculation
performance. See, e.g., D. R. Biswal, R. P. Singh, "Flocculation studies based
on water-
soluble polymers of grafted carboxymethyl cellulose and polyacrylamide", J.
Appl. Polym.
Sci., 102(2), p1000-1007, 2006. doi.:10.1002/app.24016.
103171 The flocculation kinetics of PCC has been studied in relation to
cationic potato starch
(C-starch), anionic potato carboxymethyl starch (A-starch), cationic
polyacrylamide (C-
PAM), Anionic polyacrylamide (A-PAM), Poly(ethylene oxide) (PEO), PEO
cofactor,
PVFA/NaAA, glyoxalated-PAM (PAM-glyoxal), cationic polyacrylamide (C-PAM), and
polyamine (Pam) polyethlylenimine (PEI). See, e.g., Gaudreatdt, R., Di Cesare,
N., Weitz,
D., & van de yen, T. G. (2009). Flocculation kinetics of precipitated calcium
carbonate.
Colloids and Surfaces A: Physicochemical and Engineering Aspects, 340(1-3), 56-
65. doi:
10.1016/j.co1surfa.2009.03.008. During polymer induced flocculation, the
particle size
increases from its initial value to a plateau value. PEO/cofactor, A-PAM and C-
PAM
retention aid systems, are very cost effective in inducing PCC aggregation,
and create very
large aggregates at high polymer dosage. C-PAM, glyoxalated-PAM and the
polyamine
coagulant (Pam) do not significantly induce filler aggregation. Both
PEO/cofactor and C-
-86-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
PAM, gave higher flocculation rates and larger flocculant sizes making them
useful, for
process water clarification. Neither PEO nor cofactor alone, without salt,
induce PCC
aggregation. PCC aggregates induced by PVFA/NaAA and C-starch have floc sizes
less
sensitive to dosage in region I. PEO/cofactor, which is known to cluster, gave
faster
flocculation rate and larger flocs; because the polymer cluster enlarge the
effective polymer
size leading to larger flocs. The A-PAM is highly charged and gives strong
flocs due to
strong binding to PCC. PAM-glyoxal, C-PAM (dry strength), and poly amine cause
little or
no flocculation, because they act as dispersants, similar to PEI.
10318] The effect of cationic polyacrylamide on precipitated calcium carbonate
flocculation:
Kinetics, charge density and ionic strength has also been studied. See, e.g.,
Peng, P. and
Gamier, G., 2012. Effect of cationic polyacrylamide on precipitated calcium
carbonate
flocculation: Kinetics, charge density and ionic strength. Colloids and
Surfaces A:
Physicochemical and Engineering Aspects, 408, pp.32-39. doi:
10.1016/j.colsurfa.2012.05.002. Cationic polyacrylamide (CPAM). The adsorption
kinetics of
CPAM onto PCC can be explained by the balance of the electrostatic and van der
Waals
interactions, hydrogen bonding and steric hindrance between the adsorbed and
dissolved
CPAM molecules and CC. Increasing the ionic strength of the PCC suspension
consistently
screened the charge of CPAM molecules so that the initially dominant
electrostatic attractions
between CPAM and PCC in the absence of salt shifted to hydrogen bonding
dominated
attraction at high ionic strength (I = 0.1). Al low ionic strengths (I =
0.01), both electrostatic
atUactions and hydrogen bonding were important in controlling the interaction
between
CPAM and PCC.
Admixture to retain solids in suspension.
[0319] In certain embodiments, carbonated wash water is treated with one or
more
admixtures to create a mixture where the solids remain suspended with little
or no agitation.
These can include viscosity-modifying admixtures (VMAs). VMAs can be comprised
of a
wide range of different chemistries. Some VMAs are based on fine inorganic
materials like
colloidal silica, while others are comprised of more complex synthetic
polymers such as
styrene-maleic anhydride terpolymers and hydrophobically modified ethoxylated
urethanes
(HEUR). The more common VMAs are based on cellulose-ethers and biopolymers
(xanthan,
welan, and diutan gums), Further VMAs include biopolymer polysaccharides such
as S-657,
welan gum, xanthan, rhamsan, gellan, dextran, pullulan, curdlan, and
derivatives thereof; (b)
marine gums such as algin, agar, carrageenan, and derivatives thereof; (c)
plant exudates such
as locust bean, gum arabic, gum ICaraya, tragacanth, Ghatti, and derivatives
thereof; (d) seed
-87-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
gums such as guar, locust bean, okra, psyllium, mesquite, or derivatives
thereof; and (e)
starch-based gums such as ethers, esters, and derivatives thereof (t)
associative thickeners
such as hydrophobically modified alkali swellable acrylic copolymer,
hydrophobically
modified urethane copolymer, associative thickeners based on polyurethanes,
cellulose,
polyacrylates, or polyethers. In another classification scheme (Khayat, KR.,
1998.
Viscosity-enhancing admixtures for cement-based materials
_______________________________ An overview. Cement and
Concrete Composites 20,171-188. https://doi.org/1O.1016/S0958-9465(98)80006-
1)VMAs
are classified in various clases: Class A are water-soluble synthetic and
natural organic
polymers that increase the viscosity of the mixing water. Class A type
materials include
cellulose ethers, polyethylene oxides, polyacryl- amide, polyvinyl alcohol,
etc. Class B are
organic water-soluble flocculants that become adsorbed onto cement grains and
increase
viscosity due to enhanced inter-particle attraction between cement grains.
Class B materials
include styrene copolymers with car- boxyl groups, synthetic polyelectrolytes,
and natural
gums. Class C are e muls ions of various organic materials which enhance
interparticle
attraction and supply additional superfine particles in the cement paste.
Among the materials
belonging to Class C are acrylic emulsions and aqueous clay dispersions. Class
D are water-
swellable inorganic materials of high surface area which increase the water
retaining capacity
of the paste, such as bentonites, silica fume and milled asbestos. Class E are
inorganic
materials of high surface area that increase the content of the fine particles
in paste and,
thereby, the thixotropy. These materials include fly ash, hydrated lime,
kaolin, various rock
dusts, and diatomaceous earth, etc. In another classification scheme, ICawai
classified water-
soluble polymers as natural, semi-synthetic, and synthetic polymers. Natural
polymers
include starches, guar gum,locust bean gum, alginates, agar, gum arabic, welan
gum, xanthan
gum, rhamsan gum, and gellan gum, as well as plant protein. Semi-synthetic
polymers
include: decomposed starch and its derivatives; cellulose-ether derivatives,
such as
hydroxypropyl methyl cellulose (HPMC), hydroxyethyl cellulose (HEC), and
carboxy methyl
cellulose (CMC); as well as electrolytes, such as sodium alginate and
propyleneglycol
alginate. Finally, synthetic polymers include polymers based on ethylene, such
as
polyethylene oxide, polyacrylamide, polyacrylate, and those based on vinyl,
such as
polyvinyl alcohol. In some cases, a viscosity-modifying agent can be used with
a
superplasticizer, such as a a hydrocolloid such as welan gum or
hydroxypropylmethyl
cellulose and a superplasticizer such as sulfonated naphthalene, sulfonated
melamine,
modified lignosulfate, their derivatives and mixtures thereof Thus, the wash
water can
include a stable hydrocolloid composition in which the hydrocolloid is
uniformly dispersed in
-88-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
a superplasticizer such as sulfonated naphthalene, sulfonated melamine,
modified
lignosulfate, their derivatives and mixtures thereof Suitable hydrocolloids
include welan
gum, methylcellulose, hydroxypropylmethyl cellulose (HPMC), hydroxyethyl
cellulose
(HEC), polyvinyl alcohol (PVA), starch, and the like. The mixture is then
stabilized by a
theological control agent consisting of reticulated cellulose fibers. The
composition is rapidly
hydratable and useful as a stabilizing additive in many cement and drilling
fluid applications.
Further useful admixtures are described in Naik, H.K., Mishra, M.K., Rao
Karanam, U.M.,
2009, The Effect of Drag-Reducing Additives on the Rheological Properties of
Fly Ash-
Water Suspensions at Varying Temperature Environment. Coal Combustion and
Gasification
Products 1, 25-31, doi: 10.4177/CCGP-D-09-00005.1
https://www.researchgate.net/publication/209640967 The_Effect_of Drag-
Reducing_Additives_on the_Rheological_Properties_of Fly_Ash-
Water Suspensions at Varying Temperature Environment.
103201 In this case, the cationic surfactant cetyl
trimethyl ammonium bromide (CTAB)
was selected for its eco-friendly nature. It is less susceptible to mechanical
degradation) and
also known potential to positively influence turbulent flow with very small
amount. It is also
least affected by the presence of calcium and sodium ions in tap water. The
chemical formula
of CTAB is C19H42BrN. The surfactant can be procured from, e.g., LOBA Chemie
Pvt, Ltd.,
Mumbai, India. The molecular weight of the surfactant is 364.46.
For the surfactant drag-reducing additives, the rod-like micelle structures
are thought to be
the key to give complicated theological fluid properties including
viscoelasticity. The
counter-ion acts as a reagent to reduce ion radius of the surfactant to deform
micellar shape
from globular to rod-like micelles. These rod-like micelles entangle together
to make a
certain network structure. Counter-ions will play a role as catalysts for the
breakdown and
reformation of the entanglement points. The counter-ion selected for this
investigation can be,
e.g. sodium salicylate (NaSal) (HOC6H4COONa) having molecular weight 160.10
obtained
from, e.g., LOBA Chemie Pvt. Ltd., Mumbai, India.
Set retarders
103211 In certain embodiments, a set retarder is added
to the wash water before it is
carbonated, e.g., while the wash water is still in the truck, or in any
suitable manner to
introduce the set retarder before carbonation of the wash water. Set retarders
A set retarder
is generally a substance that can delay the time before cement hydrates, for
example, in a
concrete mix. Set retarders are well-known in the concrete industry, and any
suitable set
retarder may be used. Set retarders include carbohydrates, i.e., saccharides,
such as sugars,
-89-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
e.g., fructose, glucose, and sucrose, and sugar acids/bases and their salts,
such as sodium
gluconate and sodium glucoheptonate; phosphonates, such as
nitrilotri(methylphosphonic
acid), 2-phosphonobutane-1,2,4-tricarboxylic acid; and cheating agents, such
as EDTA,
Citric Acid, and nitrilotriacetic acid. Other saccharides and saccharide-
containing admixes
include molasses and corn syrup. An exemplary set retarder is sodium
gluconate. Other
exemplary admixtures that can be of use as set retarders include sodium
sulfate, citric acid,
BASF Pozzolith XR, finned silica, colloidal silica, hydroxyethyl cellulose,
hydroxypropyl
cellulose, fly ash (as defined in ASTM C618), mineral oils (such as light
naphthenic),
hectorite clay, polyoxyalkylenes, natural gums, or mixtures thereof,
polycarboxylate
superplasticizers, naphthalene HRWR (high range water reducer). Additional set
retarders
that can be used include, but are not limited to an oxy-boron compound,
lignin, a
polyphosphonic acid, a carboxylic acid, a hydroxycarboxylic acid,
polycarboxylic acid,
hydroxylated carboxylic acid, such as fumaric, itaconic, malonic, borax,
&conic, and tartaric
acid, lignosulfonates, ascorbic acid, isoascorbic acid, sulphonic acid-acrylic
acid copolymer,
and their corresponding salts, polyhydroxysilane, polyacrylamide. Illustrative
examples of
retarders are set forth in U.S. Pat. Nos. 5,427,617 and 5,203,919,
incorporated herein by
reference.
[0322] The set retarder is added to the concrete or
concrete wash water in any suitable
amount; generally, dosing is well-established for a particular set retarder
and desired effect.
Exemplary percentages for sodium gluconate can be, e.g., at least 0.1, 0.2,
0.5, 1.0, 2.0, 3.0,
4.0, or 5% by weight solids in the washwater, and/or not more than 0.2, 0.5,
1.0, 2.0, 3.0, 4.0,
5, or 10% by weight solids in the washwater It will be appreciated that dosing
may have to
be approximate for some uses, e.g., when used with concrete coated on the
inside of a ready-
mix drum, and often operators will add excess set retarder to ensure that
setting and
hardening do not occur. This excess may be taken into account when carbonating
the
concrete or concrete wash water, and additional carbonation of the new
concrete added to the
old may be used in order to offset the excess set retarder, as necessary.
[0323] Thus, in certain embodiments, the invention
provides methods and compositions
for treating concrete wash water, that has been treated with set retarders,
with carbon dioxide.
This may be used when a truck is returned to the batch site and washed but the
wash water is
not removed from the truck; typically such a truck will sit overnight at the
batching facility,
then a new load of concrete will be introduced into the truck the next day.
The wash water
with set retarder contains components of the load that was in the truck,
including cement
The wash water with set retarder may be treated with carbon dioxide after the
addition of set
-90-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
retarder and before and/or during the addition of a new load of concrete to
the truck. For
example, the concrete wash water may have been exposed to set retarder, and
then have sat,
e.g., in the truck drum, for at least 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11,
12, 13, 14, 15, 16, 17,
18, 19, 20, 22, 24, 28, 32 hours, and/or for not more than 1, 2, 3, 4, 5, 6,7,
8, 9, 10, 11, 12,
13, 14, 15, 16, 17, 18, 19, 20, 22, 24, 28, 32, or 36 hours, then carbon
dioxide is added to the
wash water. This may occur before a new load is added to the truck, e.g., at
least 1, 2, 3, 4, 5,
6, 7, 8, 9, 10, 15, 20, 30, 40, 50, 01 60 minutes before the new load, or at
least 1, 1.5, 2, 2.5, 3,
4, 5, 6, or 8 hours before the new load, and/or not more than 2, 3, 4, 5, 6,
7, 8, 9, 10, 15, 20,
30,40, 50, or 60 minutes before the new load, or not more than 1, 1.5, 2, 2.5,
3, 4, 5, 6, 8, or
10 hours before the new load. Additionally or alternatively, carbon dioxide
may be added as
the new load is added, or carbon dioxide addition may occur both before and
during addition
of the new load. Carbon dioxide may be added in an amount sufficient to
reverse some or all
of the effect of the set retarder on the cement in the wash water with set
retarder; the carbon
dioxide dose may be any suitable dose, calculated as by weight cement in the
wash water; it
will be appreciated that such a calculation often must be based on estimates
of the amount of
concrete sticking to the drum of the truck, and typically in addition the mix
design of the load
or loads that were in the truck prior to washing is also used to estimate
cement content.
Alternatively, a fixed amount of carbon dioxide may be used, such as an amount
known to
provide an excess of carbon dioxide so that all cement will react. The carbon
dioxide dose
may also be adjusted according to the amount of set retarder in the wash
water, which may
be, e.g., recorded by the operator, or may be as specified by protocol, or may
be estimated.
It will be appreciated that if excess set retarder is used in the wash water,
then additional
carbon dioxide may be necessary in order to prevent effects on the next load
added to the
wash water. In such cases, it may be useful to add carbon dioxide as the next
load is added,
or immediately before, so that carbon dioxide will not exit the treated wash
water into the
atmosphere. Exemplary doses are described elsewhere herein, for example, a
dose of 0.001-
5.0% bwc. Additionally or alternatively, carbon dioxide may be added to the
new batch of
concrete; typically, such a dose will be below 2%, such as less than 1.5%, or
less than 1%, or
in some cases less than 0.5% by weight cement (bwc).
[0324] In certain embodiments, concrete wash water is moved to a holding
tank; this
water can be treated with one or more set retarders at some point, either in
the truck, or in the
tank, or a combination thereof, then carbon dioxide can be introduced at a
later point, e.g.,
when it is desired to re-use the wash water in a new batch of concrete_ For
example, wash
water treated with set retarder can be exposed to carbon dioxide before its
use as mix water
-91-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
and/or during its use as mix water. In this way, without being bound by
theory, it is thought
that the cement is kept in a "dormant" state by use of the set retarder, then
that state is
reversed by carbonation reactions from addition of the carbon dioxide.
[0325] In certain embodiments, wash water is treated
with a first dose of a first set
retarder and then, at a later time, with a second dose of a second set
retarder, where the first
and second set retarders may be the same or different. Further doses may be
used as
appropriate. The time of the first dose may be within a few hours of formation
of the wash
water, and the time of the second dose may be, e.g., just before or after
and/or during
exposure of the wash water to carbon dioxide.
[0326] In certain embodiments the invention provides methods and
compositions for
treating concrete, that has been treated with one or more set retarders, with
carbon dioxide.
This can occur, e.g., when a truck returns to a batching facility after only
part of its load is
used at a job site. In this case the concrete may be treated with set retarder
at the job site or
later; thus, the concrete may be batched then set retarder may be added a
certain amount of
time after hatching, for example, at least 0.1, 0.2, 0.5, 0.7, 1, 1.5, 2, 2.5,
3, 3.5, 4, 5, 6, or 8
hours after batching, and/or not more than 0.2, 0.5, 0.7, 1, 1.5, 2, 2.5, 3,
3.5, 4, 5, 6, 8, or 10
hours after hatching. The truck generally returns to the batching facility,
and it may be
desired to load additional concrete into the truck in addition to the returned
concrete. Carbon
dioxide can be added to the returned concrete, that has been treated with one
or more set
retarders, in any suitable dose, as described elsewhere herein; for example,
at a dose of 0.001-
5.0% bwc; the carbon dioxide may be added at any suitable time after set
retarders are added,
though this may be dependent on a number of factors, such as return time to
the batching
facility, storage time at the hatching facility, and the like; thus in certain
embodiments,
carbon dioxide may be added at least 0.1, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5,
6, 7, 8,9, 10, 11, 12,
13, 14, 15, 17, 20, 25, 30, 35, or 40 hours after set retarder is added to the
concrete, and/or
not more than 0.5, 1, 1.5,2, 2.5, 3, 3.5,4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14,
15, 17, 20, 25, 30,
35, or 40 hours after set retarder is added to the concrete. The concrete may
then be used
with additional concrete in a new batch of concrete; such use may occur
simultaneously or
nearly simultaneously with carbon dioxide addition, or may occur at any
suitable time after
carbon dioxide addition, such as at least 1, 2, 5, 7, 10, 15, 20, 25, 30, 40,
or 50 min after
carbon dioxide addition, or at least 1, 1.5, 2, 2.5, 3, 3.5,4, 5, or 6 hours
after carbon dioxide
addition, and/or not more than 2, 5, 7, 10, 15, 20, 25, 30, 40, or 50 min
after carbon dioxide
addition, or not more than 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6 or y hours after
carbon dioxide
addition. The new concrete may additionally be treated with carbon dioxide, so
that in some
-92-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
embodiments both the old concrete and the new concrete are treated with carbon
dioxide; as
discussed, this may happen simultaneously or the old concrete may be treated
with carbon
dioxide, then new concrete is treated, for example, as it is mixed with the
old concrete. The
dose of carbon dioxide for the new concrete may be any suitable dose as
described herein.
103271 In some cases, set retarder is added to a concrete batch at the
batching facility, or
in the truck on the way to the job site, because factors such as expected
traffic on the way to
the job site, temperature, and the like, necessitate that the batch not begin
to set too soon. In
this case, it can be desirable to reverse the effect of the set retarder
before pouring at the job
site, i.e., in this case and other cases described herein, the set retarder
acts as an "off switch,"
and the carbon dioxide acts as an "on switch" for the cement in the concrete.
Carbon dioxide
will be added to the concrete at some other location than the batching
facility in these
embodiments, for example, in the truck on the way to, or at, the job site. A
truck may be
equipped with a portable carbon dioxide delivery system, such as a source of
carbon dioxide
and a conduit for transporting carbon dioxide to the drum of the truck.
Additionally or
alternatively, a carbon dioxide delivery system may be sited at or near the
job site, and trucks
may arrive at the carbon dioxide delivery site, then the concrete contained
therein may be
treated with carbon dioxide at an appropriate time before its use at the job
site; in this way,
trucks may have a larger time window for transporting the concrete and its
use, and factors
such as traffic, delays at the job site, and the like, become less of an
issue; the concrete is
"dormant" due to the set retarder, then activated by use of the carbon
dioxide. The dose of
carbon dioxide may be suitable any dose as described herein, such as a dose of
0.001-5.0%
bwc; also as described elsewhere, the dose may be dependent on the type of
cement in the
concrete, the type and amount of set retarder, the expected time of use of the
concrete after
the addition of carbon dioxide, temperature, and the like. The carbon dioxide
may be added
at any suitable time before the expected time of use of the concrete, for
example, at least 1, 2,
3, 4, 5,7, 10, 15, 20, 30,40, or 50 minutes before the expected time of use,
or at least 1, 1.5,
2, 2.5, or 3 hours before the expected time of use, and/or no more than 2, 3,
4, 5, 7, 10, 15,
20, 30, 40, or 50 minutes before the expected time of use, or no more than 1,
1.5, 2, 2.5, 3, or
3.5 hours before the expected time of use. Thus in certain embodiments the
invention
provides a method of treating concrete comprising treating concrete with a set
retarder, then
treating the concrete with carbon dioxide. The set retarder is generally added
at a batching
facility, though it may be added in the drum of the truck after it has left
the hatching facility,
for example, if traffic delays and/or delays at the job site become known. The
carbon dioxide
is added en route to the job site and/or at the job site; typically it is
added into the drum of the
-93-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
ready-mix truck, though it may also be added during the transport of the
concrete from the
drum to, e.g., the forms at the job site.
103281 In certain embodiments, set retarder and carbon
dioxide are added to a concrete
mix in order to provide a desired combination of improved workability and
acceptable set
time. One or more set retarders may be added to a concrete mix in order to
improve
workability; however, this often comes at the cost of a delayed set time. In
order to shorten
set time but retain workability, a set accelerant admixture may be used.
However, although
set retarders are generally relatively inexpensive, set accelerants are often
expensive and also
often contain undesirable chemical species, such as chloride. Thus, it is
desirable to use a
substance that can accelerate set to within a desired time frame that is not
highly expensive;
carbon dioxide is one such substance. In these cases, carbon dioxide and set
retarder may be
added in any suitable sequence, such as sequentially with set retarder first,
then carbon
dioxide; or as carbon dioxide first, then set retarder; or simultaneously or
nearly
simultaneously, e.g., the timing of addition of set retarder and carbon
dioxide is such that
they are both being added to a concrete mix during at least a portion of their
respective
addition times. Thus, in certain embodiments, carbon dioxide is added to a
concrete mix,
then a set retarder is added after carbon dioxide addition (i.e., after carbon
dioxide addition
begins; depending on the length of time for carbon dioxide addition, set
retarder addition may
start before carbon dioxide addition ends, though this would not typically be
the case); the set
retarder may be added, for example, at least 0.1, 0.5, 1, 2, 3,4, 5, 7, 10,
15, 20, 30, 40, or 50
minutes after carbon dioxide addition, or at least 1, 1.5, 2, 2.5, 3, 3.5, or
4 hours after carbon
dioxide addition; and/or not more than 0_5, 1, 2, 3, 4, 5, 7, 10, 15, 20, 30,
40, or 50 minutes
after carbon dioxide addition, or not more than 1, 1.5, 2, 2.5, 3, 3.5, 4, 5,
or 6 hours after
carbon dioxide addition. In other certain embodiments, set retarder is added
to a concrete
mix, then carbon dioxide is added after set retarder addition (i.e., after set
retarder addition
begins; depending on the length of time for set retarder addition, carbon
dioxide addition may
start before set retarder addition ends, though this would not typically be
the case); the carbon
dioxide may be added, for example, at least 0.1, 0.5, 1, 2, 3, 4, 5,7, 10, 15,
20, 30, 40, or 50
minutes after set retarder addition, oral least 1, 1.5, 2, 2.5, 3, 3.5, or 4
hours after set retarder
addition; and/or not more than 0.5, 1, 2, 3,4, 5, 7, 10, 15, 20, 30, 40, or 50
minutes after set
retarder addition, or not more than 1, 1.5, 2, 2.5, 3, 3.5, 4, or 4.5 hours
after set retarder
addition. It is also possible to add set retarder, carbon dioxide, or both, in
divided doses,
where the timing of each dose of one may be relative to the dose of the other
in any suitable
manner. For example, a certain amount of set retarder may be added, then
carbon dioxide,
-94-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
then a final dose of set retarder; this is merely exemplary, and any suitable
number of doses
for set retarder and/or carbon dioxide, as well as any suitable timing of
addition, may be used.
103291 It will be appreciated that set accelerants are
available as admixtures; such set
accelerants may be used in addition to carbon dioxide. However, these
admixtures tend to be
expensive, and also often contain undesirable chemical species such as
chloride, and it is
desirable to use carbon dioxide as a less expensive alternative as much as
possible.
Dose of carbon dioxide
[0330] The concrete or concrete wash water, with set
retarder, may be exposed to any
suitable dose of carbon dioxide. For example, the dose may be not more than
5%, 4, 3%,
2.5%, 2%, 1.5%, 1.2%, 1%, 0.8%, 0.7%, 0.6%, 0.5%, 0.4%, 0.3%, 0.2%, 0.1%,
0.05%,
0.01%, or 0.05% bwc and/or at least .001, .005, 0.01, 0.05, 0.1, 0.2, 0.3,
0.4, 0.5, 0.6, 0.7, 0.8,
0.9, 1.0, 1.2, 1.5, 2.0, 2.5, 3.0, 4.0, or 4.5% bwc, such as a dose of 0.001-
5%, or 0.001-4%, or
0.001-3%, or 0.001-2%, or 0.001-1.5%, 0.001-1.2%, 0.001-1%, 0.001-0.8%, 0.001-
0_6%,
0.001-0.5%, 0.001-0.4%, 0.001-0,3%, 0.001-0.2%, or 0.001-0.1% bwc, or a dose
of 0.01-5%,
or 0.01-4%, or 0.01-3%, or 0.01-2%, 0.01-1.5%, 0.01-1.2%, 0.01-1%, 0.01-0.8%,
0.01-0.6%,
0.01-0.5%, 0.01-0.4%, 0.01-0.3%, 0.01-0.2%, or 0.01-0.1% bwc, or a dose of
0.02-1.5%,
0.02-1.2%, 0.02-1%, 0.02-0.8%, 0.02-0.6%, 0.02-0.5%, 0.02-0.4%, 0.02-0.3%,
0.02-0.2%, or
0.02-0.1% bwc, or a dose of 0.04-1,5%, 0.04-12%, 0.04-1%, 0.04-0.8%, 0.04-
0.6%, 0.04-
0.5%, 0.04-0.4%, 0.04-0.3%, 0.04-0.2%, or 0.04-0.1% bwc, or a dose of 0.06-
1.5%, 0.06-
1.2%, 0.06-1%, 0.06-0.8%, 0.06-0.6%, 0.06-0.5%, 0.06-0.4%, 0.06-0.3%, 0.06-
0.2%, or
0.06-0.1% bwc, or a dose of 0.1-1.5%, 01-1.2%, 0.1-1%, 0.1-0.8%, 0.1-0.6%, 0.1-
0.5%, 0.1-
0.4%, 0.1-0.3%, or 0.1-0.2% bwc. The dose of carbon dioxide may be dependent
on various
factors, such as the type of cement in the concrete or concrete wash water,
type and amount
of set retarder used, timing of the addition of carbon dioxide after set
retarder, temperature,
expected time between addition of carbon dioxide and use of the concrete, and
the like.
Form of carbon dioxide
[0331] The carbon dioxide may be added to the concrete
or concrete wash water, with set
retarder, in any suitable form, such as a gas, liquid, solid, or supercritical
form; in certain
embodiments, carbon dioxide comprising solid carbon dioxide can be used. This
may be in
the form of a mixture of solid and gaseous carbon dioxide, which can be formed
from liquid
carbon dioxide as it exits a conduit under pressure and is exposed to lower
pressure, such as
atmospheric pressure. See, e.g., U.S. Patent No. 9,738,562. Additionally or
alternatively,
solid carbon dioxide alone may be added, such as as pellets or shavings, or
other suitable
form, which may be determined at least in part by the desired speed of
sublimation of the
-95-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
carbon dioxide and its subsequent entry into solution. See, e.g., U.S. Patent
No. 9,738,562. In
certain embodiments, only gaseous carbon dioxide is used.
Further Admixtures
[0332] This section summarizes some further useful
admixtures for use in the methods
and compositions herein. For additional listings see Report on Chemical
Admixtures for
Concrete, Reported by AC! Committee 212, American Concrete Institute, AC1
212.3R-16,
ISBN 978-1-942727-80-4, incorporated herein by reference in its entirety.
Admixtures useful in the methods and compositions herein include:
[0333] Accelerators: cause increase in the rate of
hydration and thus accelerate setting
and/or early strength development. In general, accelerating admixtures for
concrete use
should meet the requirements of ASTM C494/C494M for Type C (accelerating
admixtures) or Type E (water-reducing and accelerating admixtures). Examples
include
inorganic salts, such as chlorides, bromides, fluorides, carbonates,
thiocyantes, nitrites,
nitrates, thiosulfates, silicates, aluminates, and alkali hydroxides. Of
particular interest
are calcium-containing compounds, such as CaO, Ca(NO2)2, Ca(OH)2, calcium
stearate, or
CaCl2, and magnesium-containing compounds, such as magnesium hydroxide,
magnesium
oxide, magnesium chloride, or magnesium nitrate. Without being bound by
theory, it is
thought that, in the case of carbonated cement, the added calcium or magnesium
compound
may provide free calcium or magnesium to react with the carbon dioxide,
providing a sink for
the carbon dioxide that spares the calcium in the cement mix, or providing a
different site of
carbonation than that of the cement calcium, or both, thus preserving early
strength
development. In addition, the anion, e.g., nitrate from a calcium-containing
admixture may
influence C-S-H particle structure. Other set accelerators include, but are
not limited to, a
nitrate salt of an alkali metal, alkaline earth metal, or aluminum; a nitrite
salt of an alkali
metal, alkaline earth metal, or aluminum; a thiocyanate of an alkali metal,
alkaline earth
metal or aluminum; an alkanolamine; a thiosulfate of an alkali metal, alkaline
earth metal, or
aluminum; a hydroxide of an alkali metal, alkaline earth metal, or aluminum; a
carboxylic
acid salt of an alkali metal, alkaline earth metal, or aluminum (preferably
calcium formate); a
polyhydroxylallcylamine; a halide salt of an alkali metal or alkaline earth
metal (e.g.,
chloride). Stable C-S-H seeds may also be used as accelerators.
[0334] In certain embodiments an accelerator can
include one or more soluble organic
compounds such as one or more alkanolamines, such as triethylamine (TEA),
and/or higher
trialkanolamines or calcium formate. The term "higher trialkanolamine" as used
herein
-96-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
includes tertiary amine compounds which are tri(hydroxyallcyl) amines having
at least one C3
-05 hydroxyalkyl (preferably a C3 -C4 hydroxyalkyl) group therein. The
remaining, if any,
hydroxyalkyl groups of the subject tertiary amine can be selected from CI -C2
hydroxyalkyl
groups (preferably C2 hydroxyalkyl). Examples of such compounds include
hydroxyethyl
di(hydroxypropyl)amine, di(hydroxy ethyl) hydroxypropylamine,
tri(hydroxypropyl)amine,
hydroxyethyl di(hydroxy-n-butyl)amine, tri(2-hydroxybutyl)amine, hydroxy butyl
di(hydroxypropyl)amine, and the like. Accelerators can also include calcium
salts of
carboxylic acids, including acetate, propionate, or butyrate. Other organic
compounds that
can act as accelerators include urea, oxalic acid, lactic acid, various cyclic
compounds, and
condensation compounds of amines and formaldehyde.
[0335] Quick-setting admixtures may be used in some
embodiments, e.g., to produce
quick-setting mortar or concrete suitable for shotcreting or for 3D printing.
These include,
e.g., ferric salts, sodium fluoride, aluminum chloride, sodium aluminate, and
potassium
carbonate.
[0336] Miscellaneous additional accelerating materials include silicates,
finely divided
silica gels, soluble quaternary ammonium silicates, silica fume, finely
divided magnesium or
calcium carbonate. Very fine materials of various composition can exhibit
accelerating
properties. In certain embodiments, admixture can include nucleation seeds
based on
calcium-silicate hydrate (C-S-H) phases; see e.g. Thomas, J.J., et al. 2009 J.
Phys Chem
113:4327-4334 and Diner et al. 2013 BFT International, Jan, pp. 44-51, which
are
incorporated by reference herein in their entireties.
[0337] In certain embodiments, a set accelerator
including one, two, or three of
triisopropanolarnine (TIPA), N,N-bis(2-hydroxyethyl)-N-(2-hydroxypropyl)amine
(BHEHPA) and tri(2-hydroxybutyl) amine (T2BA) is used, for example, a set
accelerator
comprising 11PA. Any suitable dose may be used, such as 0.0001-0.5% bwc, such
as 0.001-
0.1%, or 0.005-0.03% bwc. See U.S. Patent No. 5,084,103.
[0338] In certain embodiments, carbonation of a cement
mix is combined with use of an
admixture comprising an alkanolamine set accelerator, e.g., TIPA, where the
alkanolamine
set accelerator, e.g., TIPA, is incorporated in an amount of 0.0001-0.5% bwc,
such as 0.001-
0.1%, or 0.005-0.03% bwc. In some of these embodiments, the alkanolamine,
e.g., TIPA,-
containing admixture is added before and/or during carbonation, e.g., as part
of the initial mix
water. In some of these embodiments, the alkanolamine, e.g., TIPA,-containing
admixture is
added after and/or during carbonation. In some embodiments, the alkanolamine,
e.g., TIPA,-
containing admixture is added as two or more doses, which may be added at
different times
-97-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
relative to carbonation (e.g., two doses, one before and one after
carbonation, etc.).
Additionally or alternatively, carbonation may proceed in two or more doses
with, e.g., one
or more doses of an alkanolamine, e.g., TIPA,-containing admixture added
before, after, or
during one or more of the carbon dioxide doses. Other components may be
present in the
alkanolamine, e.g., TIPA,-containing admixture, including one or more of
set/strength
controller, set balancer, hydration seed, dispersant, air controller, theology
modifier,
colorant, or a combination thereof Suitable commercially available products
include
BASF Master X-Seed 55 (BASF Corporation, Admixture Systems, Cleveland, OH).
The
total dose of carbon dioxide delivered to the cement mix in these embodiments
may be
any suitable dose, such as those described herein, for example, 0.001-2% bwe,
such as
0.001-1.0% bwc, or 0.001-0.5% bwc.
103391 Air detrainers: also called defoamers or
deaerators, decrease air content.
Examples include nonionic surfactants such as phosphates, including
tributylphosphate,
dibutyl phosphate, phthalates, including diisodecylphthalate and dibutyl
phthalate, block
copolymers, including polyoxypropylene-polyoxyethylene-block copolymers, and
the
like, or mixture thereof Air detrainers also include octyl alcohol, water-
insoluble esters
of carbonic and boric acid, and silicones. Further examples of air detrainers
include
mineral oils, vegetable oils, fatty acids, fatty acid esters, hydroxyl
functional compounds,
amides, phosphoric esters, metal soaps, polymers containing propylene oxide
moieties,
hydrocarbons, alkoxylated hydrocarbons, alkoxylated polyalkyIene oxides,
acelylenic
diols, polydimethylsiloxane, dodecyl alcohol, octyl alcohol, polypropylene
glycols,.
water-soluble esters of carbonic and boric acids, and lower sulfonate oils.
103401 Air-entraining admixtures: The term air
entrainer includes any substance that
will entrain air in cementitious compositions. Some air entrainers can also
reduce the
surface tension of a composition at low concentration. Air-entraining
admixtures are used
to purposely entrain microscopic air bubbles into concrete. Air-entrainment
dramatically
improves the durability of concrete exposed to moisture during cycles of
freezing and
thawing. In addition, entrained air greatly improves concrete's resistance to
surface
scaling caused by chemical deicers. Air entrainment also increases the
workability of
fresh concrete while eliminating or reducing segregation and bleeding.
Materials used to
achieve these desired effects can be selected from wood resin and their salts,
natural resin
and their salts, synthetic resin and their salts, sulfonated lignin and their
salts, petroleum
-98-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
acids and their salts, proteinaceous material and their salts, fatty acids and
their salts,
resinous acids and their salts, alkylbenzene sulfonates, sulfortated
hydrocarbons, vinsol
resin, anionic surfactants, cationic surfactants, nonionic surfactants,
natural rosin,
synthetic rosin, an inorganic air entrainer, synthetic. detergents, and their
corresponding
salts, and mixtures thereof. Solid materials can also be used, such as hollow
plastic
spheres, crushed brick, expanded clay or shale, or spheres of suitable
diatomaceous earth.
Air entrainers are added in an amount to yield a desired level of air in a
cementitious
composition. Examples of air entrainers that can be utilized in the admixture
system
include, but are not limited to MB AF 90, MB VR and MICRO AIR.RT1µ.4., all
available
from BASE Admixtures Inc. of Cleveland, Ohio.
[0341] Alkali-aggregate reactivity inhibitors: Reduce
alkali-aggregate reactivity
expansion. Examples include barium salts, lithium nitrate, lithium carbonate,
and lithium
hydroxide.
[0342] Antiwashout admixtures: Cohesive concrete for
underwater placements.
Examples include cellulose and acrylic polymer.
[0343] Bonding admixtures: Increase bond strength.
Examples include polyvinyl
chloride, polyvinyl acetate, acrylics, and butadiene-styrene copolymers.
[0344] Coloring admixtures: Colored concrete. Examples
include modified carbon
black, iron oxide, phthalocyanine, umber, chromium oxide, titanium oxide,
cobalt blue,
and organic coloring agents.
[0345] Corrosion inhibitors: reduce steel corrosion
activity in a chloride-laden
environment. Examples include calcium nitrite, sodium nitrite, sodium
benzoate, certain
phosphates or fluosilicates, fluoalurninates, and ester amines.
[0346] Dampproofing admixtures: retard moisture
penetration into dry concrete.
Examples include soaps of calcium or ammonium stearate or oleate, butyl
stearate, and
petroleum products.
[0347] Foaming agents: produce lightweight, foamed
concrete with low density
Examples include cationic and anionic surfactants, and hydrolyzed protein.
[0348] Fungicides, germicides, and insecticide& Inhibit
or control bacterial and fungal
growth. Examples include polyhalogenMed phenols, dieldrin emulsions, and
copper
compounds.
-99-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
[0349] Gas formers: Gas formers, or gas-forming agents,
are sometimes added to
concrete and grout in very small quantities to cause a slight expansion prior
to hardening.
The amount of expansion is dependent upon the amount of gas-forming material
used and
the temperature of the fresh mixture. Aluminum powder, resin soap and
vegetable or
animal glue, saponin or hydrolyzed protein can be used as gas formers.
[0350] Hydration control admixtures: Suspend and
reactivate cement hydration with
stabilizer and activator Examples include carboxylic acids and phosphorus-
containing
organic acid salts.
[0351] Permeability reducers: Decrease permeability.
Examples include latex and
calcium stearate.
[0352] Pumping aids: Improve pumpability. Examples
include organic and synthetic
polymers, organic flocculents, organic emulsions of paraffin, coal tar,
asphalt, acrylics,
bentorite and pyrogenic silicas, and hydrated lime_
[0353] Retarders: Retard setting time, and can include
water-reducing set-retarding
admixtures, which reduce the water requirements of a concrete mixture for a
given slump
and increase time of setting (see water reducers), or those that increase set
time of
concrete without affecting the water requirements. In general, set retarders
can be
classified in four categories, any of which may be used in embodiments herein:
I)
lignosulfonic acids and their salts and modifications and derivatives of
these; 2)
lwdroxylated carboxylic acids and their salts and modifications and
derivatives of these;
3) carbohydrate-based compounds such as sugars, sugar acids, and
polysaccharides, and
4) inorganic salts such as borates and phosphates. Thus, set retarders include
carbohydrates,
i.e., saccharides, such as sugars, e.g., fructose, glucose, and sucrose, and
sugar acids/bases
and their salts, such as sodium gluconate and sodium glueoheptonate;
phosphonates, such as
nitrilotri(nethylphosphonic acid), 2-phosphonobutane-1,2,4-tricarboxylic acid;
and chelating
agents, such as EDTA, Citric Acid, and nitrilotriacetic acid. Other
saccharides and
saccharide-containing admixes include molasses and corn syrup. In certain
embodiments, the
admixture is sodium gluconate Other exemplary admixtures that can be of use as
set
retarders include sodium sulfate, citric acid, BASF Pozzolith XR, firmed
silica, colloidal
silica, hydroxyethyl cellulose, hydroxypropyl cellulose, fly ash (as defined
in ASTM C618),
mineral oils (such as light naphthenic), hectorite clay, polyoxyalkylenes,
natural gums, or
mixtures thereof, polycarboxy late superplasticizers, naphthalene HRWR (high
range water
reducer). Additional set retarders that can be used include, but are not
limited to an oxy-
-100-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
boron compound, lignin, a polyphosphonic acid, a carboxylic acid, a
hydroxycarboxylic acid,
polycarboxylic acid, hydroxylated carboxylic acid, such as ftumaric, itaconic,
malonic, borax,
gluconic, and tartaric acid, lignosulfonates, ascorbic acid, isoascorbic acid,
sulphonic acid-
acrylic acid copolymer, and their corresponding salts, polyhydroxysilane,
polyacrylaimide.
Further retarders include nitrilotri(methylphosphonic acid), and 2-
phosphonobutane-1,2,4-
tricarboxylic acid.Illustrative examples of retarders are set forth in US. Pat
Nos. 5,427,617
and 5,203,919, incorporated herein by reference.
10354] Shrinkage reducers: Reduce drying shrinkage.
Examples include
polyoxyalkylenes alkyl ether and propylene glycol.
10355] Water reducers: Water-reducing admixtures (also called
dispersants, especially
HRWR) are used to reduce the quantity of mixing water required to produce
concrete of a
certain slump, reduce water-cement ratio, reduce cement content, or increase
slump_ Typical
water reducers reduce the water content by approximately 5-10%; high range
water reducers
(HRWR) reduce water content even further. Adding a water-reducing admixture to
concrete
without reducing the water content can produce a mixture with a higher slump;
for example,
in certain cases in which high doses of carbon dioxide are used to carbonate a
cement mix.
slump may be reduced, and use of a water reducer may restore adequate
slump/workability.
10356] Water reducers for use in the compositions and
methods herein may meet one of
the seven types of water reducers of ASTM C494/C494M, which defines seven
types: 1)
Type A¨water reducing admixtures; 2) Type B¨retarding admixtures (described
above); 3)
Type C¨accelerating admixtures (also described above); 4) Type D¨water-
reducing and
retarding admixtures; 5) Type E¨water reducing and accelerating admixtures; 6)
Type F¨
water-reducing, high range admixtures; or 7) Type G
____________________________ water-reducing, high-range, and
retarding admixtures. Materials generally available for use as water-reducing
admixtures
typically fall into one of seven general categories, and formulations useful
herein may
include. but are not limited to, compounds from more than one category: 1)
lignosulfonic
acids and theirs salts and modifications and derivatives of these; 2)
hydroxylated carboxylic
acids and their salts and modifications and derivatives of these; 3)
carbohydrate-based
compounds such as sugars, sugar acids, and polysaccaharides; 4) salts of
Sulfonated
melamine polycondensation products; 5) salts of sulfonated napthalene
polycondensation
products: 6) polycarboxylates; 7) other materials that can be used to modify
formulations,
including nonionic surface-active agents; amines and their derivatives;
organic phosphonates,
-101-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
incluing zinc salts, borates, phosphates: and certain polymeric compounds,
including
cellulose-ethers, silicones, and Sulfonated hydrocarbon acrylate derivatives.
103571 An increase in strength is generally obtained
with water-reducing admixtures as
the water-cement ratio is reduced. For concretes of ecpEal cement content, air
content, and
slump, the 28-day strength of a water-reduced concrete containing a water
reducer can be
10% to 25% greater than concrete without the admixture. Type A water reducers
can have
little effect on setting, while Type D admixtures provide water reduction with
retardation
(generally a retarder is added), and Type E admixtures provide water reduction
with
accelerated setting (generally an accelerator is added). Type D water-reducing
admixtures
usually retard the setting time of concrete by one to three hours. Some water-
reducing
admixtures may also entrain some air in concrete_
103581 High range water reducer (HRWR, also called
superplasticizer or plasticizer),
Type F (water reducing) and Ci (water reducing and retarding), reduce water
content by at
least 12%.
103591 Examples of water reducers include lignosulfonates, casein,
hydroxylated
carboxylic acids, and carbohydrate& Further examples, including HRWR
(superplasticizers
or plasticizers) include polycarboxylic ethers, polycarboxylates,
polynapthalene sulphonates
(sulfonated napthalene formaldehyde condensates(for example LOMAR DTM+
dispersant
(Cognis Inc., Cincinnati, Ohio)), polymelatnine sulphonates (sulfonated
melamine
formaldehyde condensates), polyoxyethylene phosphonates (phosphonates-
terminated PEG
brushes), vinyl copolymers. Further examples include beta naphthalene
sulfonatesõ
polyaspartates, or oligomeric dispersants.
103601 Polycarboxylate dispersants (water reducers
which are also called polycarboxylate
ethers, polycarboxylate esters) can be used, by which is meant a dispersant
having a carbon
backbone with pendant side chains, wherein at least a portion of the side
chains are attached
to the backbone through a carboxyl group or an ether group. Examples of
polycarboxylate
dispersants can be found in U.S. Pub. No. 2002/0019459 Al, U.S. Pat, No.
6,267,814, U.S.
Pat. No. 6,290,770, U.S. Pat. No. 6,310,143, U.S. Pat. No. 6,187,841, U.S.
Pat. No.
5,158,996, U.S. Pat No. 6,008,275, U.S. Pat. No. 6,136,950, U.S. Pat. No.
6,284,867, U.S.
Pat No. 5,609,681, U.S. Pat. No. 5,494,516; U.S. Pat. No. 5,674,929, U.S. Pat,
No.
5,660,626, U.S. Pat No. 5,668,195, U.S. Pat. No. 5,661,206, U.S. Pat. No.
5,358,566, U.S.
Pat. No. 5,162,402, U.S. Pat. No, 5,798,425, U.S. Pat. No. 5,612,396, U.S.
Pat. No.
6,063,184, U.S. Pat No. 5,912,284, U.S. Pat. No. 5,840,114, U.S. Pat. No.
5,753,744, U.S.
Pat. No. 5,728,207, U.S. Pat No. 5,725,657, U.S. Pat No. 5,703,174, U.S. Pat.
No.
-102-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
5,665,158, U.S. Pat No. 5,643,978, U.S. Pat. No. 5,633,298, U.S. Pat. No,
5,583,183, and
U.S. Pat. No. 5,393,341 The polycarboxylate dispersants of interest include
but are not
limited to dispersants or water reducers sold under the trademarks
GLENIUM®
3030N5, GLENIUM.RTM, 3200 HES, GLENIUM 3000NS.RTNI. (BASF Admixtures Inc.,
Cleveland, Ohio), ADVA® (W. R. Grace Inc., Cambridge, Mass.),
VISCOCRETE® (Sika, Zurich, Switzerland), and SUPERFLUX® (Axim Concrete
Technologies Inc., Middlebranch, Ohio).
[0361] Viscosity and theology modifying admixtures.
Viscosity-modifying admixtures
(VMAs) are typically water-soluble polymers used in concrete to modify its
Theological
properties. VMAs influence the rheology of concrete by increasing its plastic
viscosity; the
effect of yield stress widely varies with the type of VMA, from no increase to
a significant
one. Plastic viscosity is defined ass the property of a material that resists
change in the shape
or arrangement of its elements during flow, and the measure thereof, and yield
stress is
defined as the critical shear stress value below which a viscoplastic material
will not flow
and, once exceed, flows like a viscous liquid. Rheology modifying agents can
be used to
modulate, e.g., increase, the viscosity of cementitious compositions. Suitable
examples of
theology modifier include firmed silica, colloidal silica, cellulose ethers
(e.g., hydroxyethyl
cellulose, hydroxypropyl methylcellulose), fly ash (as defined in ASTM C618),
mineral oils
(such as light naphthenic), hectorite clay, polyoxyalkylenes, polysaccharides,
polyethylene
oxides, polyaciylamides or polyvinyl alcohol, natural and synthetic gums,
alginates (from
seaweed), or mixtures thereof Other materials include finely divided solids
such as starches,
clays, lime, and polymer emulsions. Rheology-modifying admixtures (RMA) are
admixtures
that affect the flow characteristics of concrete by lowering the yield stress
or force required to
initiate flow without necessarily changing the plastic viscosity. The addition
of an RMA to
concrete might not alter its slump but will improve workability and flow
characteristics.
RMAs have been used in low-slump concrete applications, for example, when
concrete is
placed using slipform paving machines to place concrete pavements, curbs, and
barriers, and
potentially in 3D printing. The can also be used in self-consolidating
concrete (SCC) or
highly workable concretes. Rheology-modifying admixtures include those
reported by Bury
and Bury, 2008, Concrete International, 30:42-45, incorporated herein by
reference in its
entirety.
103621 Shrinkage reduction and compensation admixtures.
The shrinkage compensation
agent which can be used in the cementitious composition can include but is not
limited to
RO(A0)1-101-1, wherein R is a Ci-s alkyl or C5-6 cycloalkyl radical and A is a
C2-3 alkylene
-103-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
radical, alkali metal sulfate, alkaline earth metal sulfates, alkaline earth
oxides, preferably
sodium sulfate and calcium oxide. TETRAGUARD® is an example of a shrinkage
reducing agent and is available from BASF Admixtures Inc. of Cleveland, Ohio.
Exemplary
shrinkage reduction admixtures (SFtAs) include polyoxyallcylenes alkyl ethers
or similar
compositions. Exemplary shrinkage compensation admixtures (SCAs) include
calcium
sulfoaluminate and calcium aluminate, calcium hydroxide, magnesium oxide, hard-
burnt and
dead-burnt magnesium oxide.
103631 Extended set-control admixtures. Extended set-
control admixtures (ESCAs) or
hydration-controlling admixtures (HCAs) are sued to stop or severely retard
cement
hydration process in unhardened concrete. They may be used to shut down
ongoing
hydration of cementitious products in returned/waste concrete or in wash water
that has been
treated in the truck or in a concrete reclaimer system, which allows these
products to be
recycled back into concrete production so that they need not be disposed of;
or to stabilize
freshly batched concrete to provide medium- to very long-term set retardation,
which allows
concrete to remain plastic during very long hauls or in long-distance pumping
situations that
require long slump life in a more predictable fashion than normal retarders.
These differ
from conventional set control admixtures because they stop the hydration
process of both the
silicate and aluminate phases in Portland cement. Regular set-control
admixtures act only on
the silicate phases. Examples include carboxylic acids and phosphorus-
containing organic
acids and salts.
103641 Workability-retaining admixtures. Help retain
workability retention of concrete.
Examples include hydration-controlling and retarding admixtures that meet the
requirements
of ASTM C494/C494M Type B or D, or neutral set workability-retaining
admixtures meeting
the requirements of ASTM C494/C494M Type S. See, e.g., Daczlco, 2010,
Proceedings fro
the 6th International Symposium on Self-compacting Concrete and the 4th North
American
Concerence on the Design and Use of Self-Consolidating Concrete, Sept.
103651 Corrosion-inhibiting admixtures. Reduces
corrosion of steel in concrete, e.g.,
rebar. Examples include chromates, phosphates, hydrophosphates, alkalies,
nitrites, and
fluorides; aine carboxylate, amine-ester organic emulsion, and calcium
nitrite.
103661 Permeability-reducing admixtures. Permeability-reducing admixtures
(PRAs)
have been developed to improve concrete durability though controlling water
and moisture
movement, as well as by reducing chloride ion ingress and permeability. These
typically
include, but are not limited, to: 1) hydrophobic water repellants, such as
materials based on
soaps and long-chain fatty acid derivatives, vegetable oils such as tallows,
soya-based
-104-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
materials, and greases, and petroleum such as mineral oil and paraffin waxes.,
e.g, calcium,
ammonium, and butyl stearates; 2) polymer products, such as organic
hydrocarbons supplied
either as emulsions (latex) or in liquid form, such as coal tar pitches,
bitumen or other
resinous polymer, or prepolymer materials; 3) finely divided solids, such as
inert and
chemically active fillers such as talc, bentonite, silicious powders, clay,
lime, silicates, and
colloidal silica Supplementary cementitious materials (SCMs) such as fly ash,
raw or
calcined natural pozzolans, silica fume, or slag cement, although not
technically chemical
admixtures, can contribute to reducing concrete permeability be be a
complementary
component; 4) hydrophobic pore blockers; 5) crystalline products, which can be
proprietary
active chemicals provided in a carrier of cement and sand.
[0367] Bonding admixtures include an organic polymer
dispersed in water (latex).
[0368] Coloring admixtures include natural or synthetic
materials, in liquid or dry forms.
Pigments include black iron oxide, carbon black, phthalocyanine blue, cobalt
blue, red iron
oxide, brown iron oxide, raw burnt umber, chromium oxide, phtalocyanine green,
yellow iron
oxide, and titanium dioxide.
[0369] Flocculating admixtures include synthetic
polyelectrolytes, such as vinyl acetate-
maleic anhydride copolymer.
[0370] Fungicidal. germicidal. and insecticidal
admixtures include polyhalogenated
phenols, dieldrin emulsion, and copper compounds.
[0371] Lithium admixtures to reduce deleterious expansion from alkali-
silica reaction.
Deleterious expansions from alkali-silica reaction (ASR) can occur in concrete
when
susceptible siliceous minerals are present in the aggregate. Exemplary
admixtures that
prevent these deleterious expansion reactions include solid forms (lithium
hydroxide
tnonohydrate and lithium carbonate) and liquid form (30 percent by weight
lithium nitrate
solution in water). Additional examples include lithium nitrite.
[0372] Expansive/gas forming admixtures include
metallic aluminum, zinc or
magnesium, hydrogen peroxide, nitrogen and ammonium compounds, and certain
forms of
activated carbon or fluidized coke.
[0373] Admixtures for cellular concrete/flowable fill
include those based on protein or on
synthetic surfactants.
[0374] Shotcrete admixtures. Shotcrete is define as
"mortar or concrete pneumatically
projected at high velocity onto a surface." Materials useful as shotcrete
admixtures include
accelerators, such as alkali-based accelerators, e.g., aqueous silicate or
aluminate solutions or
alkali-free accelerators such as those based on aluminum sulfates and aluminum
-105-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
hydroxysulfates; high-range water-reducing admixtures such as those known in
the art
specifically formulated for shotcrete mixtures; and extended set-control
admixtures.
103751 Admixtures for manufactured concrete products.
These may be used to add
production efficiency, improve or modify surface texture, enhance and maintain
visual
appeal, or provide value-added performance benefits. These include
plasticizers such as
soaps, surfactants, lubricants, and cement dispersants; accelerators both
calcium chloride and
non-chloride-based; and water-repellant/efflorescence control admixtures such
as
calcium/aluminum stearates, fatty acids, silicone emulsions, and wax
emulsions.
103761 Admixtures for flowing concrete. Flowing
concrete is defined as "concrete that is
characterized as having a slump greater than 7-1/2 in (190 mm) while
maintaining a cohesive
nature." Various admixtures may be used, such as mid-range water reducers and
high-range
water reducers, viscosity-modifying admixtures, set retarders, set
accelerators, and
workability-retaining admixtures, as described herein.
103771 Admixtures for self-consolidating concrete
(SCC). Exemplary admixtures for
inclusion in SCC include high-range water-reducing admixtures, e.g.,
polycarboxylate-based
HRWRAs such as blends of different polycarboxylate polymers that have
different rates of
absorption on the powder substrates; and viscosity-modifying admixtures.
103781 Admixtures for very cold weather concrete, These
allow placement of concrete in
temperatures below freeing, and include water reducers, accelerators,
retarders, corrosion
inhibitors, and shrinkage reducers (for their added freezing point
depression).
103791 Admixture for very-high-early-strength concrete.
VHESC is designed to achieve
extremely high early strengths within the first few hours after placement.
Admixture systems
can include a high-range water reducer, set accelerator, and optionally air-
entraining
admixture. Also include may be workability-retaining admixtures.
103801 Admixtures for pervious concrete. Pervious concrete is a low-slump,
open-graded
material consisting of portland cement, uniform-sized aggregate, little or no
fine aggregate,
chemical admixtures, and water, which, when combined, produces hardened
concrete with
interconnected pores, or voids, that allow water to pass through the concrete
easily.
Exemplary admixtures include air-entraining admixtures, extended set-control
admixtures,
water-reducing admixtures, internal curing admixtures, viscosity-modifying
admixtures, and
latex admixtures.
103811 Admixtures for 3D printing concrete. These
include admixtures that allow the
printed concrete to stand without forms and other admixtures suited to the
requirements of 3D
printing.
-106-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
[0382] Modification or influence on calcium carbonate
In certain embodiments, an
admixture is used that modulates the formation of calcium carbonate, e.g., so
that one or
more polymorphic forms is favored compared to the mixture without the
admixture, e.g.,
modulates the formation of amorphous calcium carbonate, e.g., aragonite, or
calcite.
Exemplary admixtures of this type include organic polymers such as
polyactylate and
polycarboxylate ether, phosphate esters such as hydroxyamino phosphate ester,
phosphonate
and phosphonic acids such as nitrilotri(methylphosphonic acid), 2-
phosphonobutane-1,2,4-
tricarboxylic acid, chelators, such as sodium gluconate,
ethylenediaminetetraacthc acid
(EDTA), and citric acid, or surfactants, such as calcium stearate.
[0383] Further admixtures of interest include those that influence calcium
carbonate
formation, reactions, and other aspects of calcium carbonate. For example,
magnesium can
be a strong inhibitor to calcite growth, and the Mg/Ca ratio may affect the
lifetime of
amorphous calcium carbonate, e.g., high ratios may increase lifetime, and may
influence the
type of crystalline polymorph that forms as the initial and long-term product.
C032-1Ca2
may also affect these, as may physical mixing, either or both of which may be
manipulated.
See, e.g., see Blue, C.R., Giuffre, A., Mergelsberg, S., Han, N., De Yoreo,
J.J., Dove, P.M.,
2017. Chemical and physical controls on the transformation of amorphous
calcium carbonate
into crystalline CaCO3 polymorphs. Geochimica et Cosmochimica Acta 196, 179-
196,
https://doi.org/10.1016/j.gca.2016.09.004, incorporated herein by reference in
its entirety.
[0384] In certain embodiments, admixture can include one or more 2D
substrates
terminated with functional groups, which may also influence crystal phase,
size, shape,
and/or orientation. Exemplary strategies for preparing functional group
substrates include
Langmuir monolayer, surface carbonylation, and alkanethiol self-assembling
monolayer
(SAM). For example, a steafic acid monolayer has been used to direct CaCO3
crystallization.
Various functional groups can be micro-patterned on a substrate to guide CaCO3
crystallization. Thus, in certain embodiments 2D substrates with ¨COOH, -NH2, -
OH, S0314,
-CH3, -SH, and/or or P04112, can be used to control CaCO3 mineralization. The
physical
andlor chemical properties of the substrate may be manipulated as suitable for
desired
outcome. These include chemical character, hydrophilicity, charge (or
coordination number)
and geometry (or spatial structure) of terminated functional groups, substrate
metals and
length of alkanethiol molecule. Additionally or alternatively, environmental
factors such as
temperature and/or initial concentration of can may be manipulated. ACC
formation and
transformation may be preferred on strong hydrophilic surfaces, for example,
on ¨OH or ¨SH
terminated SAMs. Without being bound by theory, it is thought that CaCO3
nucleates via the
-107-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
same mechanism on ¨OH. NI12, and ¨CH3 terminated SAMs. Double-hydrophilic
block
copolymers based on poty(ethylerieglycolXPEG), carboxylated polyanilines (c-
PANIs) can
be used to mediate CaCO3 crystallization, and can provide control over crystal
size, shape,
and modification_ e.g., promote production of purely crystalline calcite
and/or vaterite.
Addition of¨OH and ¨COOH tailored functional polymer can potentially stabilize
ACC
precursor phase_ which may aradually transform to calcites, if desired.
Additionally or
alternatively, charged functional groups can be coupled with Ca2* ions to
facilitate CaCO3
crystallization. See, e.g._ Deng, H., Shen, X.-C., Wang, X.-M., Du, C., 2013.
Calcium
carbonate crystallization controlled by functional groups: A mini-review.
Frontiers of
Materials Science 7, 62-68. htlps://doi.org/10.1007/s11706-013-0191-y,
incorporated herein
by reference in its entirety; in particular, see Table 1 for potential
influences of various
admixtures on morphologies.
103851 In certain embodiments admixture may include one
or more complexing agents,
such as Ethylenediaminetetraaceticacid (EDTA) andlor 1-hydroxyethv- lidene-1,1-
dinhosnhonic acid (HEDP). For example, without being bound by theory, EDTA is
reported to retard the crystal growth of calcite and aragonite. Aquasoft 330,
a commercial
grade HEDP is reported to control the morphology of CaCO3 and calcium oxalate.
See, e.g.,
Gopi, S.P., Subramanian, V.K., Palanisamy, IC, 2015. Synergistic Effect of
EDTA and
HEDP on the Crystal Growth, Polymorphism, and Morphology of CaCO 3. Industrial
&
Engineering Chemistry Research 54, 3618-3625.
https://doi.org/10.1021/ie5034039,
incorporated herein by reference in its entirety.
103861 In certain embodiments, admixture may include
low molecular weight and
polymeric additives, such as block copolymers, poly(ethylene glycol) (PEG),
polyelectrolyte,
polyacrylamide and cellulose, which can exhibit large influence on the
crystallization of
CaCO3. See, e.g., Xie et al., 2006; Xu et al., 2008; Xu et al., 2011, Sadowski
et al., 2010; Su
et al., 2010, all of which are incorporated by reference herein in their
entireties. Among
various templates, PEG is of particular interest because its molecules contain
hydrophilic
groups, which can act as a donor to metal ions to form metal complexes with
diverse
conformation. CaCO3 mineralized without PEG polymer formed rhombohedral
calcite
crystals of an average size of 12.5 and 21.5 pm after 5 min and 24 h of
incubation,
respectively. In contrast, CaCO3 precipitates obtained in the presence of PEG
but collected
after 24 hours of incubation exhibited particles with diameters ranging from
13.4 to 15.9 Rm.
The slight increase in the particle size observed at a high polymer
concentration may be
-108-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
caused by the flocculation effect. Thus, without being bound by theory, it is
thought that the
presence of poly(ethylene glycol) inhibits the growth of CaCO3 particles in
the system. It is
known that low and high molecular weight additives can stabilize
nonequilibrium
morphologies by changing the relative growth rates of different crystal faces
through
molecular, specific interactions with certain surfaces that modify the surface
energy or
growth mechanism, or both. Further without being bound by theory, it is also
thought that in
aqueous solution, Ca2+ and C032- firstly form ACC, which quickly transforms
into vaterite
and calcite within minutes, but at the same time the polymer molecules adsorb
on the surface
of the particles, which can inhibit the growth of crystal during the process
resulting in
formation small particles. See, e.g., Polowczyk, I., Bastrzyk, A., Kozlecki,
T., Sadowski, Z.,
2013. Calcium carbonate mineralization. Part 1: The effect of poly(ethylene
glycol)
concentration on the formation of precipitate. Faculty of Geoengineering,
Mining and
Geology, Wroclaw University of Technology, Wroclaw.
https://doi.org/10.5277/ppmp130222, which is incorporated by reference herein
in its
entirety.
[0387]
In certain embodiments, admixture
may include water-soluble macro-molecules as
soluble additives which may, e.g., affect the crystallization of CaCO3; such
additives may be
present with insoluble matrices. Exemplary soluble additives include
poly(aerylic acid)
(PAA); PAAm: Poly(allylamine); PGA: Poly(glutatnic acid) sodium salt; DNA:
deoxyribonucleic acid, such as sodium salt from salmon sperm (DNA); these
admixtures can
be used with one or more substrates, when suitable, such as glass,
Poly(ethylene- co-acrylic
acid) (PEAA) (20wt% acrylic acid), or chitosan. PEAA and chitosan contain
carboxylic acid
and amino groups, respectively. These polymers can be spin-coated on glass
substrates. In
the absence of soluble additives, rhombohedral calcite crystals can grow on
all three
substrates. Different substrate/macro-molecule combinations can have different
effects. For
example, for glass, there may be no crystallization with PAA or PAAtn, whereas
spherical
crystals may be obtained with PGA additive (vaterite and calcite) or DNA
(calcite). The
same effects can be seen with additives on PEAA. With chitosan, PAA and PGA
may give
thin film states of CaCO3. Without being bound by theory, the carboxylic acid
of PAA and
PGA and the amino group of chitosan may cause interactions, which results in
the formation
of thin film crystals. Spherical particles sporadically grow on the surfaces
in the presence of
DNA. For further discussion of these potential admixtures see, e.g., Kato, T.,
Suzuki, T.,
Arnamiya, T., hie, T., Komiyarna, M., Yui, H., 1998. Effects of macromolecules
on the
-109-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
crystallization of CaCO3 the Formation of Organic/Inorganic Composites.
Supramolecular
Science 5,411-415. https://doi.org/10.1016/S0968-5677(98)00041-8, incorporated
by
reference herein in its entirety.
[0388] The admixture (or each admixture) may be added to any suitable final
percentage
(bwc), such as in the range of 0.01-0.5%, or 0.01-0.3%, or 0.01-0.2%, or 0.01-
0.1%, or 0.01-
1.0%, or 0.01-0.05%, or 0.05% to 5%, or 0.05% to 1%, or 0.05% to 0.5%, or 0.1%
to 1%, or
0.1% to 0.8%, or 0.1% to 0.7% per weight of cement. The admixture (or each
admixture in a
combination of admixtures) may be added to a final percentage of greater than
0.0001,
0.0002, 0.0005, 0.001, 0.002,0005, 0.01, 0.02,0.03, 0.04, 0.05, 0.06, 0.07,
0.08, 0.09, 0.1,
0.15, 0.2, 0.3, 04, 0.5%, 0.6%, 0.7%, 0.8%, 0.9, or 1.0% bwc; in certain cases
also less than
10,5, 4, 3,2, 1,0.9, 0.8, 0.7, 0.6,0.5, 0.4, 0.3, 0.2, 0.1,0.09, 0.08, 0.07,
0.06,0.05, 0.04,
0.03, 0.02, 0.01, 0.005, 0.002, 0.001, 0.0005, or 0.002% bwc. Other ranges and
quantities are
as described herein.
[0389] In certain embodiments, sodium gluconate is used as a set-retarding
admixture, in
combination with carbonation of wash water. The sodium gluconate can be added
at one or
more times in the process as described herein. Any suitable timing and/or
amount of sodium
gluconate can be used, which, as with any admixture, may depend on the mix
design, e.g.,
type and amount of cement, in the concrete that is in the wash water, and/or
the mix design,
e.g., type and amount of cement, in the concrete that is produced in a
subsequent batch from
the carbonated mix water. The exact amount of sodium gluconate can be
important and may
be determined in testing with the mix designs to be treated. In certain
embodiments, the
amount of sodium gluconate, expressed by weight cement in the wash water, may
be 0.1-5%,
or 0.2-4%, or 0.5-3%, or 0.7-2%, or 1.0-2.0%, or 1.2-1.8%, or 1.4-1.6%.
[0390] In certain embodiments, carbonated wash water may itself be used to
accelerate set,
e.g., to produce a concrete that will stick to a desired surface when used as,
e.g., shotcrete. In
a shotcrete operation concrete mix can be sent to the nozzle as a wet mix,
i.e., already mixed
with water, or as a dry mix that is mixed with water just before ejection from
the nozzle. In
the latter case, some or all of the mix water may be carbonated wash water,
and the use of
carbonated wash water may reduce or eliminate flow of the concrete delivered
to the desired
surface by the nozzle.
-110-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
EXAMPLES
EXAMPLE 1
103911 For Examples 1-23 the following general protocols were used, unless
otherwise
noted:
103921 Concrete Mixing Procedure:
1. Add sand and stone, mix for 15 seconds
2. Add 80% of the water, mix for 15 seconds
1 Add cementitious material, mix 15 seconds
4. Add remaining water/concrete admixtures, mix 3 minutes
5. Rest 3 minutes
6. Mix 2 minutes
[0393] Washwater preparation procedure
1. Weigh out cetnentitious material and water in separate buckets at ratios
required to
produce desired specific gravity.
2. Add the cement to the water and mix with drill with a grout mixing paddle
attachment
for approximately 15 seconds.
3. Mix again every 30 minutes to prevent sealing.
4. At appropriate time add to the treatment reactor for CO2 injection (as
shown in
specific Examples) and/or add sodium &neonate (as shown in specific Examples).
103941 Washwater carbon dioxide injection system
103951 The washwater treatment injection system included a steel container
(oil drum) to
hold the water, a standing sump pump for washwater agitation, a CO2 line
tapped into PVC
piping and a copper cooling coil. The washwater sits in the container and is
pumped
continuously through the PVC piping system, which acts as a reaction chamber
for the CO2
and the washwater slurry. The CO2 is controlled with a flowmeter which is
attached to a CO2
gas line. The copper coil has water passed through it to cool the system
during the CO2
reaction.
10396] All admixture concentrations are as % w/w with washwater solids, unless
otherwise
noted.
[0397] Provided herein is an exemplary embodiment of the use of dried treated
washwater
solids as a replacement for cement at levels of 10% and 25%. In this exemplary
embodiment,
washwater was made at a specific gravity of 1.10 and allowed to hydrate for
three hours.
After the initial hydration, the washwater was added to the CO2 injection
system and was
treated to a CO2 uptake of 24%. After 3 days, the washwater solids were dried
out using hot
plates. The solids were then used as a cement replacement.
[0398] Concrete batches were made as follows: a control concrete batch made
with no
washwater solids; a concrete batch made with treated washwater solids that
replaced at 10%
cement; and a concrete batch made with treated washwater solids replaced at
25% cement.
-1 1 1-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
[0399] Figures 1-4 show the impact for treated washwater solids on concrete.
The batches
of concrete with the dried washwater solids saw a loss in workability (Figure
1). There was
also an acceleration in setting time and the compressive strength was reduced
significantly at
24 hours, but the reduction decreased with time (Figure 2; Figure 3 shows
calorimetry, as
power vs. time). After 28 days, the samples were still not as strong as the
control. Figure 4
shows the composition of the concrete batches used in this Example.
EXAMPLE 2
[0400] In another exemplary embodiment, concrete washwater was treated using a
lab
simulated flue gas to see if the washwater and produced concrete properties
would be the
same. General conditions were as in Example 1.
[0401] The washwater was made at a specific gravity of 1.05 and allowed to
hydrate for
three hours. After the initial hydration, the washwater was added to the CO2
injection system
and was treated with the simulated flue gas. The flue gas was a combination of
compressed
air and CO2 where 85% of the flow was air and 15% was CO2. The simulated flue
gas was
injected into the washwater until a CO2 uptake of 27% was achieved.
[0402] Concrete batches made were as follows: a control batch made with no
washwater; a
concrete batch made with flue gas treated washwater with full washwater
replacement; a
concrete batch made with flue gas treated washwater with full washwater
replacement and
with a cement reduction; and a concrete batch made with flue gas treated
washwater with full
washwater replacement and with a cement and water reduction.
[0403] Figures 5-8 show the impact of flue gas treatment. The concrete saw
comparable
workability (Figure 5) and a slight acceleration in the setting time. There
was a large strength
reduction at 24 hours that was lessened at 7 and 28 days (Figure 6; Figure 7
shows
calorimetry, as power vs. time). The Final compressive strength was still
lower than the
control, with the exception of the batch with a cement and water reduction.
The simulated
flue gas appears to have had a negative impact on the concrete strength that
is not seen with
pure CO2. Figure 8 shows the composition of the concrete batches used in this
Example.
EXAMPLE 3
[0404] In another exemplary embodiment, concrete was made with full treated
washwater
replacement, with sodium gluconate being added after treatment. Concrete
batches were
made with varying specific gravity levels in the treated washwater. Desired
specific gravity
-112-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
was achieved by diluting down the washwater with potable water. General
conditions were
as in Example 1.
[0405] The washwater was made at a specific gravity of 1.10 and allowed to
hydrate for
three hours. After the initial hydration, the washwater was added to the CO2
injection system
and was treated to a CO2 uptake of 25%. After 24 hours, the washwater was used
in concrete
production with 1.5% sodium gluconate by weight of washwater solids added to
the
washwater before batching.
[0406] Concrete batches made were as follows: a control concrete made with no
washwater;
concrete batch made with 1.10 specific gravity treated washwater with full
washwater
replacement and 1.5% sodium gluconate; a concrete batch made with 1.075
specific gravity
treated washwater with full washwater replacement and 1.5% sodium gluconate;
and a
concrete batch made with 1.05 specific gravity treated washwater batch with
full washwater
replacement and 1.5% sodium gluconate.
[0407] Figures 9-12 show the impact for specific gravity and sodium gluconate
on concrete.
The concrete produced had a reduction in workability that was lessened with
the decrease of
washwater specific gravity (Figure 9). There was some set acceleration in all
the washwater
batches and a minor strength increase in all cases at 28 days (Figures 10 and
11). The strength
was also impacted by specific gravity, as the specific gravity increased so
did the increase in
compressive strength. Figure 12 shows the compositions of the various concrete
mixes used.
EXAMPLE 4
[0408] In an exemplary embodiment, the addition timing of sodium gluconate to
both
treated and untreated washwater was determined. Addition of gluconate before
carbonation
(at three hours of hydration) was compared with addition of gluconate after
carbonation
(immediately before batching). General conditions were as in Example 1.
[0409] The washwater batches were made as follows: untreated washwater
comprising no
gluconate; untreated washwater comprising gluconate which was added after 3
hours of
hydration; untreated washwater comprising gluconate which was added after 24
hours of
hydration and immediately before concrete batching; treated washwater
comprising no
gluconate; treated washwater comprising gluconate which was added before
treatment and
after 3 hours of hydration; and trcated washwater comprising gluconate which
was added
after 24 hours and immediately before concrete batching MI gluconate dosages
were 3% by
weight of washwater solids. The treated washwater was treated to a CO2 uptake
of 24% by
weight of cement.
-113-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
104101 Concrete batches made were as follows: a control concrete batch made
with no
washwater; a concrete batch made with untreated washwater with no gluconate; a
concrete
batch made with untreated washwater with gluconate added after 3 hours; a
concrete batch
made with untreated washwater with gluconate added after 24 hours; a concrete
batch made
with treated washwater with no gluconate; a concrete batch made with treated
washwater
with gluconate added before treatment; and a concrete batch made with treated
washwater
with gluconate added after treatment
[0411] Figures 13-16 show the impact of the addition timing of sodium
gluconate to treated
and untreated washwater on the properties of concrete. Concrete batches
comprising treated
washwater with gluconate had comparable workability to the control (Figure
13), slight set
retardation and a large compressive strength increase (Figures 14 and 15). The
concrete
batches comprising washwater without gluconate had a reduction in workability
(Figure 13),
set acceleration and a minor strength improvement (Figures 14 and 15). The
concrete batches
comprising untreated washwater with gluconate saw a very large set
retardation.
Compositions of the various concrete mixes are shown in Figure 16.
EXAMPLE 5
[0412] In other exemplary embodiments, the properties of concrete comprising
untreated
washwater with the addition of sodium gluconate were compared with those of
concrete
comprising treated washwater with sodium gluconate and cement reductions.
General
conditions were as in Example 1.
[0413] The washwater was made at a specific gravity of 1.10 and allowed to
hydrate for
three hours. After the initial hydration, sodium gluconate was added to two
samples of
washwater at a dosage of 0.6% and 1.2% by weight of washwater solids. The
remaining
washwater was added to the CO2 injection system and was treated to a CO2
uptake of 29%.
After 24 hours, the washwater was used in concrete production. In the treated
washwater
concrete batches, sodium gluconate was added to the water immediately before
batching at a
dosage of 3% by weight of washwater solids.
[0414] The concrete batches made were as follows: a control concrete batch
made with no
washwater; a concrete batch made with untreated washwater with 0.6% sodium
gluconate,
with full washwater replacement; a concrete batch made with untreated
washwater with L2%
sodium gluconate with full washwater replacement; a concrete batch made with
treated
washwater batch with 3% sodium gluconate and 5% cement reduction, and full
washwater
-114-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
replacement; and a concrete batch made with treated washwater with 3% sodium
gluconate
and 10% cement reduction, with full washwater replacement.
104151 Figures 17-20 show the results. The concrete produced in all batches
had acceptable
workability (Figure 17) with some set retardation in the washwater batch with
1.2% sodium
gluconate and the treated washwater batches. The set retardation could be
adjusted in future
batches with less sodium gluconate. There was a compressive strength increase
in all
washwater batches (Figures 18 and 19). The treated washwater batch with a 5%
cement
reduction was the strongest batch and the one with a 10% cement reduction was
equivalent to
the untreated batches. Compositions of the various concrete batches are shown
in Figure 20.
EXAMPLE 6
104161 Provided herein is exemplary concrete made with full treated washwater
replacement
with sodium gluconate being added before treatment. The washwater was aged 6
days before
being batched in concrete. General conditions were as in Example 1.
10417] The washwater was made at a specific gravity of 1.10 and allowed to
hydrate for
three hours. After the initial hydration, the washwater was added to the CO2
injection system
and was treated to a CO2 uptake of 24%. After 6 days, the washwater was used
in concrete
production. Sodium gluconate was added to the washwater of two concrete
batches before
production at a dosage of 2.4 and 4.8% by weight of washwater solids.
10418] The concrete batches were made as follows: a control concrete batch
made with no
washwater; a concrete batch made with aged treated washwater with no sodium
gluconate
and full washwater replacement; a concrete batch made with aged treated
washwater
comprising 2.4% sodium gluconate with full washwater replacement; and a
concrete batch
made with aged treated washwater comprising 4.8% sodium gluconate with full
washwater
replacement.
104191 Figures 21-23 reveal that the concrete produced saw workability issues
in the batch
without gluconate and the batch with the lower amount of gluconate (Figure
21). Setting time
data was not able to be gathered for this test. There was a large 7- and 28-
day compressive
strength increase in both gluconate samples (Figure 22). This shows the
possible benefit of
treated washwater can exceed 6 days of storage. Compositions of concrete mixes
are shown
in Figure 23.
-115-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
EXAMPLE 7:
[0420] This Example compares the addition of sodium gluconate or a
lignosulfonate added
after washwater treatment immediately before batching. General conditions were
as in
Example 1.
[0421] Washwater: Washwater was made at a specific gravity of 1.10 and
hydrated for three
hours. After the initial hydration it was added to the treatment system and
injected with CO2
until it achieved a CO2 uptake of 23%.
[0422] A small separate batch of untreated washwater was produced. The
washwater was
made at the same time and same specific gravity.
[0423] Concrete: The concrete batches made were as follows: Control (no
washwater);
Untreated washwater control (2.7% gluconate immediately before batching);
Treated
washwater (no gluconate); Treated washwater (2.7% gluconate added immediately
before
batching); Treated washwater control (8.1% lignosulfonate added immediately
before
batching)
[0424] Results are shown in Figures 24-27. All samples had comparable
workability (Figure
24). The untreated sample had a very large set retardation (Figure 26). The
treated sample
without admixture saw some set acceleration and the admixture batches had
comparable set
to the control. All washwater batches saw a large compressive strength
increase (Figure 26).
Compositions of the concrete mixes are shown in Figure 27). This Example
demonstrates
that admixtures comparable to sodium gluconate, such as lignosulfate, may be
added to
carbon dioxide-treated washwater with comparable results.
EXAMPLE 8:
[0425] In this Example, concrete was made with full treated washwater
replacement with
sodium gluconate being added before treatment. One batch had additional
gluconate added
after treatment and another had a cement reduction. General conditions were as
in Example
1.
[0426] Washwater: Washwater was made at a specific gravity of 1.10 and allowed
to
hydrate for three hours. After the initial hydration sodium gluconate was
added to the
washwater (dosage =1.4% by weight of washwater solids). The washwater was
added to the
CO2 injection system and was treated to a CO2 uptake of 26%. After 24 hours
the washwater
was used in concrete production.
[0427] Concrete: The concrete batches made were as follows: Control (no
washwater);
Treated washwater batch, full washwater replacement, 1.4% sodium gluconate;
Treated
-116-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
washwater batch, full washwater replacement, 1.4% sodium gluconate before
carbonation
and 0.7% sodium gluconate after carbonation; Treated washwater batch with 5%
cementitious reduction, full washwater replacement, 1.4% sodium gluconate.
[0428] The results are shown in Figures 28-31. The concrete produced in all
batches had
acceptable workability (Figure 28). The setting time of the washwater batches
were
comparable to the control, with the exception of the batch with gluconate
before and after
carbonation, which saw some set retardation. The set retardation could be
adjusted in future
batches with less sodium gluconate. There was a large compressive strength
increase in the
treated washwater samples, even the sample with the cementitious reduction
(Figures 29 and
30). Compositions of the various concrete mixes are shown in Figure 31.
EXAMPLE 9:
[0429] This Example relates to testing the slump life of treated washwater
concrete with and
without gluconate relative to a control. General conditions were as in Example
1.
[0430] Washwater: Washwater was made at a specific gravity of 1.10 and allowed
to
hydrate for three hours. After the initial hydration the washwater was added
to the CO2
injection system and was treated until it achieved a CO2 uptake of 24% by
weight of cement.
The washwater was used 24 hours later for concrete production.
[0431] Concrete: The concrete batches made were as follows: Control (no
washwater);
Treated washwater, full replacement, 1.4% gluconate by weight of washwater
solids; Treated
washwater, full replacement, 2% gluconate by weight of washwater solids
[0432] The results are shown in Figures 32-35. The treated washwater samples
lost slump
slightly faster than the control (Figure 32). The batch with the higher
gluconate dosage had a
longer slump life (Figure 32). The samples had a slight set retardation
(Figure 34) with an
increase in compressive strength (Figure 33). Compositions of the concrete
mixes are shown
in Figure 35.
EXAMPLE 10:
[0433] This Example demonstrates effects on concrete mixes using treated
washwater to
make concrete with a cement from Lyon, France and a mix design that uses
limestone filler.
General conditions were as in Example 1.
[0434] Washwater: Washwater was made at a specific gravity of 1.10 and allowed
to
hydrate for three hours. After the initial the washwater was added to the CO2
injection system
-117-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
and was treated until it achieved a CO2 uptake of 31% by weight of cement. The
washwater
was used 24 hours later for concrete production.
[0435] Concrete: The concrete batches made were as follows: Control (no
washwater);
Treated washwater, full replacement, no gluconate; Treated washwater, full
replacement,
1.6% gluconate by weight of washwater solids.
The results are shown in Figures 36-39. The treated washwater batches required
more
admixture but were able to achieve the same workability as the control (Figure
36). The
treated washwater sample without gluconate had the same setting time as the
control, whereas
the sample with gluconate had some set retardation (Figure 38). The
compressive strength
was significantly increased in both treated washwater batches (Figure 37). The
compositions
of the various concrete batches are shown in Figure 39.
EXAMPLE 11
10436] This Example demonstrates using treated washwater to make concrete with
a low
w/c cement ratio with and without a cementitious reduction.
[0437] Washwater: Washwater was made at a specific gravity of 1.10 and allowed
to
hydrate for three hours. After the initial the washwater was added to the CO2
injection system
and was treated until it achieved a CO2 uptake of 24% by weight of cement. The
washwater
was used 24 hours later for concrete production.
[0438] Concrete: The concrete batches made were as follows: Control (no
washwater);
Treated washwater, full replacement, 1.7% gluconate by weight of washwater
solids; Treated
washwater, full replacement, 1.7% gluconate by weight of washwater solids with
a 5%
cementitious reduction.
[0439] The results are shown in Figures 40-43. The workability of all the
batches was
comparable (Figure 40). There was a slight retardation in the treated
washwater set times
(Figure 42), but they had an increase in compressive strength, even in the
sample with the
cementitious reduction (Figure 41). Compositions of the various concrete
batches are shown
in Figure 43.
EXAMPLE 12
10440] This Example demonstrates adding treated washwater as mix water in
concrete with
different addition timings. The batches were produced with a 50/50 mix of
potable water and
treated washwater. The goal was to determine if there is a difference when
potable water or
treated washwater is added first. General conditions are as in Example 1.
-118-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
104411 Washwater: Washwater was made at a specific gravity of 1.10 and allowed
to
hydrate for three hours. After the initial hydration the washwater was added
to the CO2
injection system and was treated until it achieved a CO2 uptake of 28% by
weight of cement.
The washwater was used 24 hours later for concrete production.
104421 Concrete: The concrete batches made were as follows: Control (no
washwater);
Treated washwater, half replacement, 2% gluconate by weight of washwater
solids,
washwater mixed with potable water and added upfront; Treated washwater, half
replacement, 2% gluconate by weight of washwater solids, washwater added
upfront with the
potable water added later; Treated washwater, half replacement, 2% gluconate
by weight of
washwater solids, potable added upfront with the washwater added later.
104431 The results are shown in Figures 44-47. The upfront and the delayed
washwater had
an equivalent slump (Figure 44) and setting time (Figure 46). Both samples had
an increase in
compressive strength vs the control, with the strength of the upfront
washwater sample being
slightly higher (Figure 45). Compositions of the concrete mixes are shown in
Figure 47.
EXAMPELE 13:
10444] This Example demonstrates adding treated washwater as mix water in to a
ternary
blend (cement, fly ash, slag) concrete mix with a low water to cement ratio.
The washwater
was added in dosages of 100, 75, and 50% replacement of mix water. General
conditions
were as in Example 1.
104451 Washwater: Washwater was made at a specific gravity of 1.10 and allowed
to
hydrate for three hours. After the initial the washwater was added to the CO2
injection system
and was treated until it achieved a CO2 uptake of 27% by weight of cement. The
washwater
was used 24 hours later for concrete production.
10446] Concrete: The concrete batches made were as follows: Control (no
washwater).
Treated washwater, full replacement, 2.5% gluconate by weight of washwater
solids; Treated
washwater, 75% replacement, 2.5% gluconate by weight of washwater solids;
Treated
washwater, 50% replacement, 2.5% gluconate by weight of washwater solids.
10447] The results are shown in Figures 48-51. The treated washwater batches
all had
comparable slumps to the control (Figure 48) with a bit of set retardation
(Figure 50). All
washwater samples had a compressive strength increase (Figure 49). The
compositions of the
concrete batches are shown in Figure 51.
-119-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
EXAMPLE 14
[0448] This Example demonstrates adding treated washwater as mix water in to a
ternary
blend (cement, fly ash, slag) concrete mix with a low water to cement ratio.
The treated
washwater was compared to untreated washwater at 50 and 100% water
replacement.
General conditions were as in Example 1.
[0449] Washwater: Washwater was made at a specific gravity of 1.10 and allowed
to
hydrate for three hours. After the initial hydration the washwater was added
to the CO2
injection system and was treated until it achieved a CO2 uptake of 28% by
weight of cement.
The washwater was used 24 hours later for concrete production.
[0450] Concrete: The concrete batches made were as follows: Control (no
washwater;
Untreated washwater, full replacement, 2% gluconate by weight of washwater
solids; Treated
washwater, full replacement, 2% gluconate by weight of washwater solids;
Untreated
washwater, 50% replacement, 2% gluconate by weight of washwater solids;
Treated
washwater, 50% replacement, 2% gluconate by weight of washwater solids,
[0451] The results are shown in Figures 52-55. The workability of all mixes
was
comparable (Figure 52) with setting time retardation in all washwater samples
(Figure 54).
All washwater samples also saw a strength increase from the control, with the
treated samples
being stronger than the untreated samples (Figure 53). Compositions of the
concrete mixes
are shown in Figure 55.
EXAMPLE 15
[0452] This Example demonstrates using different flow rates while treating
washwater to
determine if it impacts the washwater properties. General conditions were as
in Example 1.
[0453] Washwater: Washwater was made at a specific gravity of 1.10 and allowed
to
hydrate for three hours. After the initial hydration the washwater was added
to the CO2
injection system and was treated with a flow rate of 2.23 (low), 4.46 (med),
and 6.69 (high)
LPM. The washwater was sampled at intervals calculated to make sure all sample
points have
the same CO2 uptake.
[0454] Mortar: The mortar batches made were as follows (test was repeated for
each flow
rate): Control ; Untreated washwater, full replacement, no CO2; Treated
washwater, full
replacement, 7-8% CO2; Treated washwater, full replacement, 10-13% CO2;
Treated
washwater, full replacement, 14-15% CO2; Treated washwater, full replacement,
16-17%
CO2Treated washwater, full replacement, 19-21% CO2.
-120-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
[0455] The results are shown in Figures 56-62, Figure 56 shows % carbon
dioxide by
weight cement at various time for the different flow rates. The point from 210-
and 360-
minute marks in the low flow sample appear to be outliers in the data. 7-day
compressive
strength is comparable for all conditions except 19-21 min at the low flow
rate, and is about
the same as control (Figure 57). There appears to be a strong correlation
between the
increase in set (Figures 59 and 60), loss in workability (Figure 58) and
temperature increase
in the washwater (Figure 61). The medium and high flow additions showed a
similar effect
on pH, whereas the low flow rate showed a slower decrease in pH (Figure 62).
EXAMPLE 16
[0456] In this Example, washwater was made with increasing specific gravity.
General
conditions for washwater carbon dioxide exposure were as in Example 1, with
modifications
as noted.
10457] Washwater: Washwater was made at a specific gravity of 1.10, 1.20, 1.30
and 1.35
and allowed to hydrate for three hours. After the initial hydration the
washwater was added to
the treatment reactor. The washwater reactor was equipped with a cooling coil
to compensate
for the expected large temperature increase due to the high solids in the
washwater. The
washwater was sampled for carbon analysis every 20-30 minutes depending on the
specific
gravity (1.10 and 1.20 every 20 minutes, 1.30 and 1.35 every 30 minutes).
Temperature was
recorded at all times with a temperature logger.
[0458] The results are shown in Figures 63 and 64. With the addition of proper
cooling and
an on/off switch for the pump and CO2 injection (to allow more cooling with
added heat, see
the dips in the temperature graph, Figure 64) the system was able to treat the
washwater
samples even with the highest specific gravity up to 28% by weight of the
cement (Figure
63). Thus, the system could potential handle a 1.35 specific gravity slurry
provided there was
sufficient cooling.
EXAMPLE 17
[0459] In this Example, a slurry was made with just class C fly ash and water
with the
purpose of being carbonated in a reactor. Class C fly ash is known to be very
variable and the
addition of CO2 may be able to reduce the variability. The slurry was then
used in mortar
mixes.
[0460] Washwater: Washwater (all class C fly ash) was made at a specific
gravity of 1.25
and allowed to hydrate for three hours. The shiny was treated with CO2 at a
flow rate of 10
-121-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
LPM and sampled at 11, 26, 41, 114 and 180 minutes of treatment. The sampled
water was
tested for carbon analysis and used to make mortar.
104611 Mortar: Control, no washwater; Untreated washwater, full replacement;
Treated
washwater, full replacement, 1.2% CO2; Treated washwater, full replacement,
2.2% CO2;
Treated washwater, full replacement, 2.4% CO2; Treated washwater, full
replacement, 3.2%
CO2; Treated washwater, full replacement, 3.5% CO2.
[0462] The mortar made contained a blend of 70% cement and 30% class C fly
ash. The
batches made with the added slurry had no virgin fly ash added. All the fly
ash was added
through the slurry.
[0463] The results are shown in Figures 65-67. There was a reduction in
compressive
strength in all washwater samples vs the control containing the virgin
material (Figure 65).
The addition of CO2 caused set retardation in all samples (Figure 66). This
testing is not able
to determine whether or not this improves variability as all the batches were
made from the
same class C fly ash supply. Figure 67 shows the compositions of various
mortars used in the
Example.
EXAMPLE 18
10464] The Example demonstrates using treated washwater, with lower CO2
dosages, to
make concrete. General conditions were as in Example 1.
[0465] Washwater: Washwater was made at a specific gravity of 1.10 (straight
cement) and
allowed to hydrate for three hours. After the initial hydration 0.8% sodium
gluconate was
added to the washwater by weight of cement. The gluconate was used to prevent
any future
hydration of the cement, "putting it to sleep". After 24 hours, CO2 was
injected in the
washwater at a flow rate of 3LPM. Without being bound by theory, it is thought
that the CO2
reactivates the washwater. The washwater was sampled at 20 minutes of
treatment (estimated
5% uptake) and 40 minutes of treatment (estimated 10% uptake).
10466] Concrete: Control, no washwater; Untreated washwater, full replacement;
Treated
washwater, 20 minutes of CO2 injection; Treated washwater, 40 minutes of CO2
injection.
[0467] The results are shown in Figures 68-71. Slumps were comparable across
all samples
(Figure 68). The washwater sample with 40 minutes of CO2 injection had
acceptable setting
time (Figure 70), workability (Figure 68) and saw a large strength increase
(Figure 69). The
compositions of the various concretes are shown in Figure 71.
-122-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
EXAMPLE 19
[0468] In this Example, treated washwater concrete was produced with the
addition of air
entraining admixture to ensure there were no incompatibilities. General
conditions were as in
Example 1.
[0469] Washwater: Washwater was produced at a specific gravity of 1.10
(straight cement)
and allowed to hydrate for three hours. After the initial hydration the
washwater was added to
the treatment reactor and had CO2 injected at a flow rate of 12.1LPM until it
achieved a CO2
uptake of 27%. The washwater was used after 24 hours to make concrete.
[0470] Concrete: Control, no washwater, 15g air entraining admixture; Treated
washwater,
full replacement, 15g air entraining admixture; Treated washwater, full
replacement, 15g air
entraining admixture, sodium gluconate added 2% by weight of washwater solids
[0471] The results are shown in Figures 72-76. The addition of treated
washwater into a
concrete mix did not reduce the impact of the air entraining admixture on
workability (Figure
72), amount of air entrained (Figure 73), compressive strength (Figure 74), or
set (Figure 75).
The compositions of the various concrete mixes are shown in Figure 76.
EXAMPLE 20
[0472] This Example demonstrates using treated washwater to produce concrete,
in which
increasing amounts of washwater wee added to the concrete to correct the
workability (no
increase in admixtures). This explored the idea of available water in
washwater being less
than the theoretical amount. General conditions were as for Example 1+
[0473] Washwater: Washwater was produced at a specific gravity of 1.05
(straight cement)
and allowed to hydrate for three hours. After the initial hydration it was
added to the reactor
for CO2 injection. The washwater was treated until it achieved a CO2 uptake of
25% by
weight of cement. The washwater was used after 24 hours to make concrete.
[0474] Concrete: Control, no washwater; Treated washwater, full replacement,
added
assuming 12% of the washwater was unavailable for concrete hydration; Treated
washwater,
full replacement, added assuming 17% of the washwater was unavailable for
concrete
hydration.
[0475] Results are shown in Figures 77-80. The theoretical unavailable water
at 1.05
specific gravity is 8%. The concrete produced assuming the washwater had 12%
unavailable
water had comparable setting time (Figure 79) and workability (Figure 77) to
the control with
nearly equal strength after 28 days (Figure 78). Compositions of the concrete
mixes are
shown in Figure 80.
-123-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
EXAMPLE 21
[0476] This Example demonstrates using commercially available admixtures to
stabilize the
washwater and comparing them to the effectiveness of sodium gluconate. The
commercial
admixtures used were hydration stabilizing admixtures. General conditions were
as for
Example 1.
[0477] Washwater: Washwater was produced at a specific gravity of 1.10
(straight cement)
and allowed to hydrate for three hours. After the initial hydration the
washwater was added to
the reactor for CO2 injection. The washwater was treated until it achieved an
uptake of 26%
CO2 by weight of washwater solids. The washwater was used after 24 hours to
produce
concrete. The stabilizing admixtures were added to the washwater 10 minutes
before
hatching.
[0478] Concrete: Control, no washwater; Treated washwater, full replacement,
sodium
gluconate added 1% by weight of washwater solids; Treated washwater, full
replacement,
Daratard 17 added 5% by weight of washwater solids; Treated washwater, full
replacement,
Recover added 5% by weight of washwater solids.
[0479] The results are shown in Figures 81-84. All three admixtures produced
comparable
workability (Figure 81), setting time (Figure 83) and compressive strength
(Figure 82) to
each other. However, the amount of Daratard of Recover required to have the
same impact as
the sodium gluconate is five times more. The compositions of the concrete
mixes are shown
in Figure 84,
EXAMPLE 22
[0480] This Example demonstrates treating washwater with CO2 with two
different injection
methods. The first is the lab reactor that was used in Examples 1-21 (pump
circulating
system), the second is a C0z bubbler system with a fixed drill used to keep
the washwater
solids suspended (carbon dioxide added in same container as wash water, no
circulation).
This testing was completed to see if the high shearing of the pump system
treated the
washwater differently than the low shearing of the drill/bubbler system.
[0481] Washwater: Two sets of washwater were produced at a specific gravity of
1.10
(straight cement) and allowed to hydrate for three hours. After the initial
hydration the
washwater was added to its respective treatment system. Both batches were
treated until
achieving 29% CO2 uptake and 26% uptake for the drill and pump system
respectively. After
24 hours each washwater batch was used to produce concrete.
-124-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
[0482] Concrete: Control, no washwater; Treated washwater, full replacement,
washwater
from pump system; Treated washwater, full replacement, washwater from drill
system.
[0483] The results are shown in Figures 85-88. There was a difference between
the
treatment methods. The drill system saw an increase in washwater sped fie
gravity resulting
in more workability loss in the concrete (Figure 85). Also, the drill system
saw more of an
increase in set acceleration (Figure 87). The compressive strengths of the two
treatment
systems were comparable with the higher strength going to the drill system
(Figure 86). The
compositions of the concrete mixes are shown in Figure 88.
EXAMPLE 23
[0484] The following is the procedure used in Examples 24-26
Washwater inline injection trials (1,000L washwater mixing tank with
continuous
mechanical agitation)
10485] CO2 preparation procedure
Obtain 60 gallon header tank to store CO2 gas required for experimental
trials.
Install inline CO2 gas heaters to ensure full conversion of CO2 supply to gas
prior to infeed to
header tank.
10486] Washwater preparation procedure Fill slurry mixing vessel with water to
80% of
ratio required to produce desired specific gravity and initiate agitator.
Weight out cementitious material and slowly pour into agitated tote.
Add the remaining water required to produce desired specific gravity.
Description of Trial Apparatus and Method
10487] In this trial method, water and cementitious material are received in a
mixing vessel
and continuously agitated using a mechanical agitation method, such as a dual
vane impeller
operating at 1800 rpm_ Concurrently, CO2 in either gas or liquid phase is
heated via an inline
heater and then CO2 gas is collected in a CO2 header tank at a predetermined
pressure, such
as 100 psig, in preparation for experimental trials.
EXAMPLE 24
[0488] Treating a simulated washwater slurry with CO2 gas via inline
injection, without
inline mixing of the CO2 gas and simulated washwater slurry, at varying CO2
gas injection
flowrates.
[0489] Experiment Description: 975 L of simulated washwater was prepared in a
slurry
mixing vessel at a specific gravity of 1.05 (straight cement) and mechanically
agitated for 10
-125-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
minutes. The slurry was then pumped at a flowrate of 115 GPM through a pipe
section with a
CO2 gas injection point, followed by a 20-ft length of hose and collected in a
slurry collection
vessel. The slurry discharged to the atmosphere (i.e. was not submerged in the
slurry
collection vessel) to ensure that any unreacted CO2 gas would be discharged to
the
atmosphere rather than react further in the slurry collection vessel. CO2 gas
was injected into
the slurry at varying flowrates, and the CO2 uptake (by weight of cement) and
uptake
efficiency was measured for each experimental trial.
[0490] Results are shown in Figure 89. Without inline mixing: Uptake increased
slightly
with an increase in CO2 injection flowrate; uptake efficiency dropped 49% with
a doubling of
CO2 injection flowrate.
EXAMPLE 25
[0491] This Example demonstrates treating a simulated washwater slurry with
CO2 gas via
Milne injection, with / without inline mixing of the CO2 gas and simulated
washwater slurry,
at a constant CO2 gas injection flowrate of 400 SLPM.
[0492] Experiment Description: 975 L of simulated washwater was prepared in a
slurry
mixing vessel at a specific gravity of 1.05 (straight cement) and mechanically
agitated for 10
minutes.
[0493] For the first trial, the slurry was then pumped at a flowrate of 115
GPM through a
pipe section with a CO2 gas injection point, followed by a 20-ft length of
hose and collected
in a slurry collection vessel.
[0494] For the second trial, the slurry was then pumped at a flowrate of 115
GPM through a
pipe section with a CO2 gas injection point followed by a series of baffles
inside the pipe
section to facilitate mixing and enhance surface-to-surface interaction of the
simulated
washwater slurry and the CO2 gas immediately after injection.
[0495] For both trials, the simulated washwater slurry / CO2 gas mixture then
passed
through a 20-ft length of hose and collected in a slurry collection vessel.
The slurry
discharged to the atmosphere (i.e. was not submerged in the slurry collection
vessel) to
ensure that any unreacted CO2 gas would be discharged to the atmosphere rather
than react
further in the slurry collection vessel.
[0496] Results are shown in Figure 90. With / without inline mixing: Uptake
increased by
40% with inline mixing compared to without Milne mixing; uptake efficiency
increased by
38% with inline mixing compared to without inline mixing.
-126-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
EXAMPLE 26
[0497] This Example demonstrates treating a simulated washwater slurry with
CO2 gas via
inline injection, with inline mixing of the CO2 gas and simulated washwater
slurry, at a
constant CO2 gas injection flowrate of 400 SLPM per injection point. # of
injection points
was increased from Ito 2 (in series).
[0498] Experiment Description: 975 L of simulated washwater was prepared in a
slurry
mixing vessel at a specific gravity of 1.05 (straight cement) and mechanically
agitated for 10
minutes. The slurry was then pumped at a flowrate of 115 GPM through a pipe
section with a
CO2 gas injection point followed by a series of baffles inside the pipe
section to facilitate
mixing and enhance surface-to-surface interaction of the simulated washwater
slurry and the
CO2 gas immediately after injection.
104991 For each injection point, the simulated washwater slurry / COz gas
mixture then
passed through a 20-ft length of hose and collected in a slurry collection
vessel. The slurry
discharged to the atmosphere (i.e. was not submerged in the slurry collection
vessel) to
ensure that any unreacted CO2 gas would be discharged to the atmosphere rather
than react
further in the slurry collection vessel_
105001 CO2 gas was injected into the slurry at 1 or 2 injection points (in
series) with a CO2
gas flowrate of 400 SLPM per injection point. The CO2 uptake (by weight of
cement) and
uptake efficiency was measured for each experimental trial.
105011 Results are shown in Figure 91. With inline mixing / varying # of
injection points:
Uptake (per injection point / 20-ft hose length) increased slightly with
additional injection
points (in series); uptake efficiency increased by 20% with an increased # of
injection points /
total hose length.
EXAMPLE 27
In-line Injection Predictive Model
1054:121 Design and control of an inline CO2 injection system has been
evaluated via a
predictive model to facilitate the design of experimental programs. Through
this exercise, a
set of theoretical assumptions and critical process variables has been
identified that is
expected to provide the foundation for an inline injection control system
architecture.
Input Variables
105031 An objective of this invention is to develop a CO2 injection system
that can optimize
operation based on a specific set of process inputs. These include, but are
not limited to, the
following:
-127-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
[0504] // ¨> Target CO2 uptake (%by weight of cement) - This will be a process
variable
that is constrained by the physical limitations for a given system, and is to
be manipulated
depending on the desired end-product usage objectives.
[0505] 12 ¨> Washwater slurry flowrate (LPM) - this is anticipated to be
either a
constant/setpoint, or a measurement/input variable depending on the system /
process
availability.
105061 13-) CO2 injection rate (% by weight of cement) - this reflects the
maximum CO2
injection rate per injection point, and will be a variable to be manipulated
on a system-by-
system! day-to-day basis, depending on physical system constraints and daily
process
operating variables.
[0507] Id -. Maximum CO2 bubble diameter (% of Pipe Inner Diameter) - this
will be a
system setpoint to be optimized depending on the physical orientation of
slurry piping and/or
length of pipe available for a given system.
[0508] 15 ¨> Full reaction residence time required (sec) - this will be an
optimized control
system setpoint resulting from experimental and operational data, and will be
dependent on
the pipe size / maximum bubble diameter.
[0509] 16 ¨> Pipe Inner Diameter (in.) - This will be a control system
setpoint to reflect
physical system constraints for a given washwater system/concrete plant.
[0510] 17 ¨> Vertical Pipe length available (11) - This will be a control
system setpoint to
reflect physical system constraints for a given washwater system/concrete
plant.
[0511] IS ¨> Washwater Slurry SG - This will be an online process measurement
that will
impact specific injection system outputs such as max. CO2 injection rate,
slurry flowrate, and
maximum CO2 uptake achieved.
105121 19 ¨> %Cement - This will be a day-to-day process measurement or
setpoint that
will impact specific injection system outputs such as max. CO2 injection rate,
slurry flowrate,
and maximum CO2 uptake achieved.
[0513] 110 ¨> %Fly Ash - This will be a day-to-day process measurement or
setpoint that
will impact specific injection system outputs such as max. CO2 injection rate,
slurry flowrate,
and maximum CO2 uptake achieve&
[0514] Ill ¨> %Slag - This will be a day-to-day process measurement or
setpoint that will
impact specific injection system outputs such as max. CO2 injection rate,
slurry flowrate, and
maximum CO2 uptake achieved.
-128-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
CO2 Uptake Efficiency & Reaction Efficiency
[0515] As documented herein, CO2 uptake efficiency refers to the reaction
efficiency
between a CO2 gas flow and a cenaentitious washwater slurry flow.
Specifically, efficiency
refers to the extent to which the reaction takes place between a CO2 gas flow
and a
cementitious washwater slurry flow for a given pipe section (or hose). As
documented herein,
the reaction pipe section (or length) refers to the length of pipe between two
injection points_
[0516] For the purposes of this predictive model, 100% uptake efficiency is
assumed, and
the length of pipe (or hose) required to achieve 100% uptake efficiency (i.e.
injected CO2 gas
is fully consurnedVconverted to a mineralized byproduct via reaction with
calcium ions
contained in the washwater slurry) is documented herein as the pipe length
required for fill
reaction.
[0517] As documented herein, Reaction efficiency refers to the speed of the
reaction
between a CO2 gas flow and a cernemitious washwater slurry flow to full
conversion (i.e.
injected CO2 gas is fully consumed/converted to a mineralized byproduct). The
primary
output that quantifies the reaction efficiency in such a way that can be used
to control and
optimize the inline CO2 injection system is documented herein as thefull
conversion
residence time.
Plug Flow vs. Encapsulated Flow
[0518] If the volumetric flow of CO2 gas is greater than the volumetric flow
of the slurry,
intermittent/unpredictable plug flow will occur in the vertical pipe sections,
and the reaction
efficiency and effectiveness of inline mixing will be significantly reduced.
Conversely, if the
volumetric flow of CO2 gas is less than the volumetric flow of washwater
slurry, the injected
CO2 gas stream will be broken into a series of bubbles encapsulated entirely
by washwater
slurry inside the pipe section, resulting in a maximized surface area for
reaction.
[0519] The above hypothesis provides the basis for the experimental predictive
modelling
developed herein, and results in the following process assumptions to ensure
an optimized
inline CO2 injection system.
[0520] Maximize reaction efficiency ¨> Minimize full conversion residence time
¨>
Minimize pipe length required for full reaction
[0521] Following these assumptions, a CO2 injection system can be optimized
for a given
washwater system/concrete plant, depending on the process variables and
physical system
constraints. For example, for a given total pipe length at a concrete plant,
minimizing the pipe
length required for full reaction will allow for more injection points and
thus a higher uptake
potential.
-129-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
Injection System Sizing and Control
105221 The system will be sized and controlled with respect to the process
inputs as listed
(but not limited to the) above.
105231 Figure 92 illustrates an inline injection system for a vertical pipe
section. For the
purposes of the theory detailed herein, it is assumed that all CO2 injection
is performed in a
vertical pipe section.
105241 The theory developed is guided by a set of process assumptions to allow
for effective
control and efficient operation of the inline injection system. Based on this
theory, it is
believed that uptake efficiency and reaction efficiency will drop dramatically
if any of the
following performance conditions are not satisfied / validated.
Performance Condition #1 - Encapsulated flow of CO2 and washwater slurry in a
vertical pipe section
105251 The first process assumption involves the ratio of volumetric flow of
washwater
slurry to CO2 gas in a section of vertical pipe.
10526] For a bubble of CO2 gas to exist within a section of vertical pipe, a
maximum bubble
diameter (11) as a function of the vertical pipe inner diameter (1) must be
established to
reduce the risk of plug flow and a corresponding drop in reaction efficiency.
Consequently, a
minimum bubble spacing (Q2) is established to ensure that bubbles remain
separated and that
reaction surface area is not negatively impacted via bubble agglomeration.
This has been
quantified as the bubble section length (03).
EXAMPLE 1
111:15271 Constants:
Cl density of CO2 gas = 1.9 kg/m'3
C2 ¨> 0.0163871 L/cubic-in.
105281 Inputs:
105291 Process calculations:
14 ¨> Maximum bubble diameter = 90% of 16
16 Pipe Inner Diameter =4 inches
Vbubble = 4/3451r3
Wubbie(L) = F4l3*(E*(16.14/2) 3 ]*C2
= 0.28 L
-130-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
Mbubble (g) = Vbubbl e*C 1 *1 000/1000
= 0.28*C 1* 1000/1000
= 0.53 g
Outputs:
02 ¨> Minimum bubble spacing
03 ¨> Bubble section length
(02) Required bubble spacing =16*(1-14)/2
=0.4 in.
(03) Bubble section length = 16*I4+02*2
=4.0 in.
Performance Condition #2- Required slurry velocity
105301 Following the outputs of assumption #1, specifically the bubble section
length (03),
the second process assumption involves the required slurry velocity of a
system to maximize
the reaction of CO2 for each injection point / reaction pipe section.
105311 For a specified bubble mass (as shown in EXAMPLE 1) and a specified CO2
injection rate, the number of bubbles required per second can be determined,
and using the
bubble section length ()3 , a required slurry velocity (Co.),1 can be
determined to ensure
encapsulated flow is maintained. If the actual slurry velocity (05) (which is
a function of
slurry flowrate and pipe diameter) is less than the required slurry velocity
(04), then it cannot
be assumed that encapsulated flow is occurring inside the pipe and thus
reaction efficiency
will begin to be negatively impacted.
EXAMPLE 2
-131-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
Constants:
C2 ¨ 0.0163871 L/cubic-in.
C3¨* SG Cement = 3.15
Inputs:
12 Shirty flowrate = 1,000 LPM
13 ¨> CO2 injection point rate (% by weight cement) = 0.85%
16 Pipe Inner Diameter =4 inches
18 SG Slurry =1.05
19 %Cement in solids = 100%
From EXAMPLE I:
Mbubbie = 0.53 g
03 bubble section length =4 inches
Process calculations:
%Cements luny = [C3*(I8-1)/(C3-1)]/I8
=6.98%
MassFlowc,ement = I2*I8*%Cementskin7
= 73 kg per min.
MassFlowco2 = MassFlowcancm*I3
= 0.92 kg per min
= 0.0153 kg per sec.
-132-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
# of bubbles required (per sec) = MassFlowco2 /(Mbubbie/1000)
= 20 bubbles per second
Outputs:
04 ¨ Required slurry velocity
05 Actual slurry velocity
(04)V elocity shiny -regime(' = 03*I# of bubbles required (per sec)
= 80.3 inches per second
= 6.7 ft per second
(05)V elocity slurry-Actual =12./pC2*60*e(I6/2)21
= 80.9 inches per second
= 6.74 ft per second
Evaluation:
Velocitysiuny-Acium > Velocitysktry-Requared ;
therefore, the system can be assumed to be operating in an encapsulated flow
environment.
-133-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
System Sizing / Maximum Potential
105321 The above performance conditions provide basis for optimizing the
inline CO2
injection system for each injection point. With an optimized system,
projections can be made
as to the maximum CO2 uptake that can be achieved (08) for a given washwater
system/concrete plant.
105331 It should be noted that, if one of the performance conditions cannot be
met, or if the
maximum CO2 uptake that can be achieved (08) is less than required, changes to
the
physical infrastructure of the washwater system / concrete plant may be
required before a
system can be installed (i.e. increased pump flowrate, or increased vertical
pipe length).
EXAMPLE 3
105341 Using the slurry characterization from EXAMPLE 2, and assuming the
system has
been previously optimized as per Performance condition #1 and #2:
Inputs:
12 Sluriy flowrate = 1,000 LPM
13 ¨> CO2 injection rate per injection point (% by weight cement) = 1.25%
15 Full conversion residence time = 0.5 sec
16 Pipe inner diameter =4 inches
17 Vertical pipe length available at concrete plant = 50
ft
From EXAMPLE 2:
05 ¨> Velocityskiny_Acmai = 6.75 ft per second
105351 Outputs:
06 ¨> Pipe length required for full reaction = 0595
= 3.4 ft
07 ¨> Maximum number of injection points / reaction sections =17/06
= 14.8 injection points
-134-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
08¨' Maximum CO2 uptake achieved (% by weight cement) = I3*07
105361
= 18.5%
105371 The following conditions were used for Examples 28-37, except where
otherwise
indicated:
Concrete Mixing Procedure (Pan Mixer):
Add sand, cement and stone, mix for 60 seconds
Add water slowly over 60 seconds
Add remaining water/concrete admixtures, mix 3 minutes
Rest 3 minutes
Mix 2 minutes
Washwater preparation procedure
Weigh out cementitious material and water in separate buckets at ratios
required to produce
desired specific gravity.
Add the cement to the water and mix with drill with a grout mixing paddle
attachment for
approximately 15 seconds.
Mix again every 30 minutes to prevent settling.
After three hours of hydration either add sodium gluconate (if the water is
being treated after
24 hours) or add the treatment reactor for CO2 injection.
EXAMPLE 28
105381 In this Example, washwater was prepared and treated at a high specific
gravity (1.15)
and used to make concrete at low replacement levels (10, 20, 30%).
105391 Washwater:Washwater was prepared at a specific gravity of 1.15 and
allowed to
hydrate for 3 hours. This washwater was then treated with CO2 to an uptake of
15% by
weight of cement. Sodium gluconate was added to the washwater at a dose of
1.5% by weight
of cement immediately before batching.
[0540] Concrete: Control, no washwater; Treated washwater, 10% replacement;
Treated
washwater, 20% replacement; Treated washwater, 30% replacement
[0541] Conclusions: The concrete produced was all comparable in setting time
and
workability. The batches that had the washwater addition saw a large increase
in compressive
strength. See Figures 93-96.
-135-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
EXAMPLE 29
105421 In this Example, two batches of washwater were made at two different
specific
gravities, 1.10 and 1.05. The 1.05 washwater was treated and used to make
concrete and the
1.10 washwater was treated and then diluted down to 1.05 specific gravity. The
diluted
washwater was also used to make concrete and was compared to the batched 1.05
washwater.
105431 Washwater: Two batches of washwater were prepared with straight cement.
Both
washwater batches were allowed to hydrate for 3 hours and were then treated to
full
saturation with CO2. The washwater batches were made at different specific
gravities, one
was batched at 1.05 and the other at 1.10. Both washwater samples were dosed
with 1%
sodium gluconate immediately before batching.
105441 Concrete: Control, no washwater; Treated washwater, 1.05 SG, 100%
replacement;
Treated washwater, 1.10 SG diluted to 1_05 SG, 100% replacement
105451 Conclusions: There was a slump loss in the washwater batches, more
noticeable in
the diluted 1.10 SG sample. The setting time was comparable among all samples
batched and
the compressive strength was increased in both washwater samples, more so in
the diluted
1.10 SG samples. The diluted sample had a larger strength increase and a
larger loss in
workability; this points towards there being less water in the concrete
produced. This
indicates that diluting washwater does not guarantee that it will have the
same available water
as washwater batches at that specific gravity. See Figures 97¨ 100.
EXAMPLE 30
105461 In this Example, washwater was produced at a specific gravity of 1.10
and was
treated with CO2 to 0, 5, 10, 15, 20 and 25% by weight of cement. Each CO2
point was
tested for x-ray diffraction analysis after 0, 3, 6, 24 and 72 hours of
hydration.
105471 Washwater: Washwater was batched at a specific gravity of 1.10 and was
allowed to
hydrate for 3 hours. It was then treated to 0, 5, 10, 15, 20 and 25% CO2 by
weight of cement.
At each CO2 dose it the water was sampled and tested at 0, 3, 6, 24 and 72
hours.
105481 Conclusions: It can be seen that 15% CO2 by weight of cement is enough
to stop the
formation of calcium hydroxide for as much as 3 days. This indicates that once
15% CO2 is
added to washwater, the washwater will stop hydrating for a minimum of 3 day&
See Figures
101-105.
-136-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
EXAMPLE 31
105491 In this Example, concrete samples were produced using treated washwater
for the
purpose of testing durability in treated washwater concrete. Washwater was
made at two
different treatment levels (5, 25%) and compared to a potable water reference
and an
untreated washwater reference. All conditions were made with and without a 3%
cementitious reduction. Samples were tested for compressive strength, flexural
strength,
absorption, carbonation, freeze/thaw, salt scaling, abrasion, corrosion, bulk
diffusion and
chloride penetration.
105501 Washwater: Washwater was made at a specific gravity of 1.10 (75%
cement, 25% fly
ash) and allowed to hydrate for 3 hours. The washwater was treated to 3
different conditions.
The untreated condition was dosed with 0.6% sodium gluconate after initial
hydration and
rested for 24 hours until concrete batching. The low CO2 condition was dosed
with 0.6%
sodium gluconate after initial hydration and rested for 24 hours. Before
batching the
washwater was treated to 5% CO2 by weight of cement solids at a flow rate of 5
LPM. The
high CO2 condition was treated to 25% CO2 by weight of cement solids after its
initial
hydration and rested for 24 hours. Immediately before batching concrete the
high CO2
washwater was dosed with 1.5% sodium gluconate by weight of cement solids.
105511 Concrete: Control, no washwater; Untreated washwater, 100% replacement;
Untreated washwater, 100% replacement, 3% cementitious reduction; 5% treated
washwater,
100% replacement; 5% treated washwater, 100% replacement, 3% cementitious
reduction;
25% treated washwater, 100% replacement; 25% treated washwater, 100%
replacement, 3%
cementitious reduction_
105521 Conclusions: Minor reductions in workability in the samples with
washwater relative
to the potable water control with negligible change in setting time.
Noticeable compressive
strength increases in all washwater samples at 28-days with the most strength
coming from
the highest CO2 dose, low CO2 dose being the next strongest. Cement reduction
samples still
had a strength increase with the exception of the untreated washwater
condition. Still waiting
on results for the flexural strength, absorption, carbonation, freeze/thaw,
salt scaling,
abrasion, corrosion, bulk diffusion and chloride penetration testing. See
Figures 106-109.
EXAMPLE 32
105531 Washwater was produced at a large scale (1000L) and treated in a way to
simulate
the treatment that would be used in a reclaimer. Since a reclaimer is so large
the turnover
time could be multiple hours. Washwater was transferred from one tank to
another with CO2
-137-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
being injected in the transfer line. The washwater was transferred/treated
every 30 minutes.
The washwater was used to make concrete at CO2 levels of 0, 3, 6 and 9% by
weight of
cement solids.
105541 Washwater:Washwater was made at a specific gravity of 1.10 (100%
cement) and
allowed to hydrate for 3 hours. After the initial hydration, Recover
(commercial hydration
stabilizing admixture) was added at a dose of 2% by weight of washwater
solids. After the
admixture addition, the treatment of the washwater was started. The washwater
was sampled
at 0, 3, 6 and 9% CO2 by weight of cement solids.
105551 Concrete: Control, no washwater; Untreated washwater, 100% replacement;
3%
treated washwater, 100% replacement; 6% treated washwater, 100% replacement;
9% treated
washwater, 100% replacement
105561 Conclusions: There was a loss in slump when the CO2 was added to the
washwater
as compared to the control and the untreated case. The untreated and 3%
treated washwater
samples showed retardation in set, whereas the 6% and 9% washwater samples
showed set
acceleration. There was a significant strength increase in all washwater
samples relative to
the control with the largest increase coming from the 6% treated sample. See
Figures 110-
113.
EXAMPLE 33
105571 In this Example, the same experiment and washwater as Example 32 was
conducted
except the washwater samples were allowed to age 24 hours after treatment.
105581 Washwater: Washwater was made at a specific gravity of 1.10 (100%
cement) and
allowed to hydrate for 3 hours. After the initial hydration, Recover
(commercial hydration
stabilizing admixture) was added at a dose of 2% by weight of washwater
solids. After the
admixture addition, the treatment of the washwater was started. The washwater
was sampled
at 0, 3, 6 and 9% CO2 by weight of cement solids. Each sample was allowed to
age for 24
hours and then was used to make concrete.
105591 Concrete: Control, no washwater; Untreated washwater, 100% replacement;
3%
treated washwater, 100% replacement; 6% treated washwater, 100% replacement;
9% treated
washwater, 100% replacement.
105601 Conclusions: There was a loss in slump when the CO2 was added to the
washwater
as compared to the control and the untreated case. The untreated washwater
sample showed
retardation in set, whereas the 3%, 6% and 9% washwater samples showed set
acceleration
(all were within an acceptable range). There was a significant strength
increase in all
-138-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
washwater samples relative to the control at 24 hours. With minor strength
increases at 7- and
28-days. The largest strength increase was in the 9% treated sample. See
Figures 114-117.
EXAMPLE 34
105611 In this Example, washwater was treated to an uptake of 8% CO2 by weight
of
washwater solids. This washwater was used to make mortar at a 100% water
replacement. A
commercial set retarding admixture (Eucon DS) was added to the washwater
before batching
to try to correct for workability and set issues. The admixture was used at
two different
dosages and was compared to -treated washwater without the retarder, untreated
washwater
and a potable water reference.
105621 Washwater:Washwater was prepared at a specific gravity of 1.10
(straight cement)
and allowed to hydrate for 3 hours. It was then treated to an uptake of 8% CO2
by weight of
cement solids over a span of 16 hours at a flow rate of 0.36 LPM. Commercial
set retarding
admixture (Eucon DS) was added to the washwater immediately before batching.
105631 Mortar: Control, no washwater; Untreated washwater, 100% replacement;
8% treated
washwater, 100% replacement; 8% treated washwater, 100% replacement, Eucon DS
2.4%
by weight of washwater solids; 8% treated washwater, 100% replacement, Eucon
DS 7.3%
by weight of washwater solids
105641 Conclusions: There was a reduction in slump with the treated washwater
samples,
the 2.4 and 7.3% Eucon samples had comparable slump to the control but each
received 0.5g
of water reducer to correct workability. The washwater samples with the Eucon
did show set
retardation demonstrating the set could be corrected by the addition of a
commercial
admixture. Compressive strength samples were comparable throughout all
batches. See
Figures 118-121.
EXAMPLE 35
105651 In this Example, two washwater batches were prepared, one with 100%
cement and
the other with a blend of 75% cement and 25% slag. Both washwater samples were
treated to
0, 5, 10, 15, 20 and 25% CO2 by weight of cement. This washwater was tested
for XRD after
3 hours of hydration and again after 24 hours of hydration at each CO2
interval.
105661 Washwater: Two batches of washwater were prepared at a specific gravity
of 1.10
(straight cement and 75/25 slag blend) and allowed to hydrate for 3 hours. The
water was
then treated to 0, 5, 10, 15, 20 and 25% CO2 uptake by weight of cement
solids.
-139-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
105671 Conclusions: The 100% cement sample required 20% CO2 addition to stop
the
hydration (formation of calcium hydroxide) for 24 hours, whereas the blended
sample only
required the addition of 10% CO2. See Figures 122-125.
EXAMPLE 36
105681 In this Example, two batches of washwater were prepared and treated
with two
different flow rates (5LPM and 10LPM). Each washwater batch was treated to 0,
5, 10, 15,
20 and 25% CO2 by weight of cement solids. This washwater was tested for XRD
after 3
hours of hydration and again after 24 hours of hydration.
105691 Washwater: Two batches of washwater were prepared at a specific gravity
of 1.10
(straight cement) and allowed to hydrate for 3 hours. One was treated at a
flowrate of 5LPM
and the other at 10LPM. Each washwater batch was treated to 0, 5, 10, 15, 20
and 25% CO2
by weight of cement solids.
105701 Conclusions: The 5 LPM sample required 20% CO2 addition to stop the
hydration
(formation of calcium hydroxide) for 24 hours, whereas the 10 LPM sample only
required the
addition of 10% CO2. See Figures 126-129.
EXAMPLE 37
105711 In this Example, two batches of washwater were prepared at two
different specific
gravities (L05 and 1.15) and allowed to hydrate for 3 hours. Each washwater
batch was
treated to 0, 5, 10, 15, 20, 25 and 30% CO2 by weight of cement solids. This
washwater was
tested for XRD after 3, 24 and 48 hours of hydration.
105721 Washwater: Two batches of washwater were prepared at different specific
gravities
(straight cement). One was batched at 1.05 and the other at 1.15, they were
both allowed to
hydrate for 3 hours_ After hydration they were treated with CO2 to an uptake
levels of 0, 5,
10, 15, 20,25 and 30% by weight of cement.
105731 Conclusions: The 1.05 sample required 5% CO2 addition to stop the
hydration
(formation of calcium hydroxide) for 48 hours, whereas the 1.15 sample
required the addition
of 10% CO2. See Figures 130-135.
EXAMPLE 38
105741 A trial was performed whereby CO2 was injected at 100 SLPM. Slurry was
circulated at 100 GPM from a 260 gallon vessel, resulting in a tank turnover
of 2.6 minutes.
-140-
CA 03154009 2022-4-7
WO 2021/071980
PCT/US2020/054625
105751 A graph would show the temperature difference between two probes placed
12
meters apart along an injection/reaction length, one measuring temp (To)
before injection of
CO2 (length 0) and one measuring temp (Tn) after injection / reaction of CO2
(length 12 m).
The graph shows that for a period of time, the del-T remained at an elevated
level and
continued to climb - this is as a result of the exothermic reaction
iteratively creating a new T0
for each tank volume iteration (assuming no short circuiting, each injection /
reaction
iteration).
105761 In this trial, there was an observable drop in the delta-T (T11-T0)
after 2 hours of
treatment. This has been attributed to the possibility that at this point in
the reaction, the free
calcium in solution was depleted and the remaining reaction was dominated by
Pathway 2
reaction, such that the rate of free calcium replenishment was less than the
rate of free
calcium consumption via reaction with CO2.. The correlative reaction
efficiency was
measured and a noticeable drop in efficiency was observed at this specific
point in treatment,
and continued to remain at a reduced level thereafter.
105771 While preferred embodiments of the present invention have been shown
and
described herein, it will be obvious to those skilled in the art that such
embodiments are
provided by way of example only. Numerous variations, changes, and
substitutions will now
occur to those skilled in the art without departing from the invention. It
should be understood
that various alternatives to the embodiments of the invention described herein
may be
employed in practicing the invention.
-141-
CA 03154009 2022-4-7