Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
1
INTRANASAL DRUG DELIVERY DEVICE, SYSTEM, AND PROCESS
CROSS-REFERENCE
[0001] This application claims priority to U.S. Provisional Application No.
62/914,070, filed
October 11, 2019, U.S. Provisional Application No. 62/914,161, filed October
11, 2019, U.S.
Provisional Application No. 62/914,180, filed October 11, 2019, U.S.
Provisional Application No.
62/914,361, filed October 11, 2019, and U.S. Provisional Application No.
62/914,202, filed October
11, 2019, all of which are incorporated by reference herein in its entirety.
FIELD
[0002] The present disclosure generally relates to the field of drug delivery
and intranasal devices.
BACKGROUND
[0003] Intranasal drug delivery provides an alternative means for drug
administration by a subject
as compared to other forms such as intravenous or oral. The nasal cavity of a
subject comprises
anatomical features that intranasal drug delivery must overcome for effective
drug administration.
[0004] The inventors have determined a need for improved intranasal delivery
devices.
SUMMARY
[0005] In accordance with an aspect, there is provided an intranasal drug
delivery device having
compliant or flexible, soft nib to precisely locate the dosage and provide
comfort for user. The term
drug can also be used herein to refer to other agents such as vitamins,
fragrance, saline or non-
pharmaceutical agents.
[0006] Disclosed herein, in some embodiments, is an intranasal fluid delivery
device comprising:
a) a dispensing tip comprising a flexible nib configured to comply with or
conform to a surface of a
subject's nasal cavity, thereby enabling a fluid delivery orifice of the
dispensing tip to be directed
towards an olfactory region of the subject; b) a shot chamber for carrying a
fluid, the shot chamber
fluidly coupled to the dispensing tip; and c) a plunger configured to drive
the fluid from the shot
chamber and through the dispensing tip, thereby delivering the fluid from the
fluid delivery orifice
of the dispensing tip. Disclosed herein, in some embodiments, is an intranasal
fluid delivery device
comprising: a dispensing tip comprising a flexible nib configured to comply
with or conform to a
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
2
surface of a subject's nasal cavity, thereby enabling a fluid delivery orifice
of the dispensing tip to
be directed towards an olfactory region of the subject for delivering a fluid
therein.
[0007] In some embodiments, for any intranasal delivery device described
herein, the dispensing
tip is configured to deliver the fluid as a liquid jet or a liquid stream. In
some embodiments, the
liquid jet is a laminar flow or the liquid stream is a laminar flow. In some
embodiments, the
dispensing tip is configured to deliver the fluid as a spray, mist, or
aerosol. In some embodiments,
the fluid comprises a powder. In some embodiments, the dispensing tip
comprises an atomizer. In
some embodiments, the dispensing tip comprises a cannula. In some embodiments,
the dispensing
tip is tubular. In some embodiments, the dispensing tip has an inner diameter
from about 0.3 mm to
about 1.5 mm. In some embodiments, the flexible nib comprises a polymer. In
some embodiments,
the flexible nib comprises thermoplastic polyurethane (TPU), high-density
polyethylene (HDPE),
polyvinyl chloride (PVC), a thermoplastic elastomer (TPE), styrene-ethylene-
butylene-styrene
(SEB S), low density polyethylene (LDPE),
silicone polypropylene. comprises
polytetrafluoroethylene (PTFE), or any combinations thereof. In some
embodiments, the dispensing
tip comprises a distal portion having a first rigidity and a proximal portion
having a second rigidity,
and wherein the first rigidity is less than the second rigidity. In some
embodiments, the distal portion
comprising a first rigidity comprises a portion of the dispensing tip that is
about lmm to about 15mm
from the fluid delivery orifice. In some embodiments, the dispensing tip
comprises a distal portion
that is softer than a proximal portion. In some embodiments, the distal
portion that is softer than a
proximal portion comprises a portion of the dispensing tip that is about lmm
to about 15mm from
the fluid delivery orifice. In some embodiments, the dispensing tip comprises
a distal portion having
a first outer diameter and a proximal portion having a second outer diameter,
and wherein the first
outer diameter is less than the second outer diameter. In some embodiments,
the dispensing tip further
comprises a nose cushion to limit over-insertion of the dispensing tip within
the nasal cavity, wherein
the nose cushion configured to provide subject comfort. In some embodiments,
the dispensing tip
comprises a distal portion having a first outer diameter and a proximal
portion having a second outer
diameter, and wherein the first outer diameter is greater than the second
outer diameter. In some
embodiments, the dispensing tip is configured to be inserted to an insertion
depth of about lOmm to
about 90mm within the subject's nasal cavity. In some embodiments, the
dispensing tip is configured
to be inserted to an insertion depth of about 40mm to about 70mm within the
subject's nasal cavity.
In some embodiments, the fluid delivery orifice of the dispensing tip is
configured to be positioned
at or near the anterior entry to the olfactory region. In some embodiments,
the fluid delivery orifice
of the dispensing tip is configured to be positioned in the upper nares of a
subject. In some
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
3
embodiments, the fluid delivery orifice of the dispensing tip is configured to
be positioned within
about lmm to about 25mm from the anterior entry to the olfactory region.
[0008] In some embodiments, for any intranasal delivery device described
herein, the dispensing
tip comprises a hydrophilic coating applied to an outer surface of the
dispensing tip. In some
embodiments, the hydrophilic coating is activated by contact with a hydrating
medium. In some
embodiments, the hydrating medium is water, a gel, a lubricating gel, a
viscous liquid, a vapor, or
any combination thereof In some embodiments, the water is water vapor. In some
embodiments,
activating the hydrophilic coating reduces a surface friction of the
hydrophilic coating. In some
embodiments, the fluid comprises a pharmaceutical agent or a medicament. In
some embodiments,
the fluid comprises ketamine or insulin. In some embodiments, the
pharmaceutical agent or the
medicament is configured to be transported to the central nervous system (CNS)
from the olfactory
region and at least partly through olfactory neuronal pathways. In some
embodiments, the fluid
comprises vitamins, fragrance, saline or non-pharmaceutical agents.
[0009] In some embodiments, for any intranasal delivery device described
herein, the surface of a
subject's nasal cavity comprises anatomical features of a nasal cavity of the
subject. In some
embodiments, the anatomical features comprise nasal turbinates, a nasal valve,
or combinations
thereof In some embodiments, the anatomical features comprise a septum of the
subject. In some
embodiments, the anatomical features comprise an anterior aspect of the nasal
passage.
[0010] In some embodiments, for any intranasal delivery device described
herein, the dispensing
tip has an elliptical cross section. In some embodiments, the dispensing tip
has a distal portion and a
proximal portion, and wherein a first center axis of the distal portion and a
second center axis of the
proximal portion are non-colinear. In some embodiments, the dispensing tip
comprises an off-center
drug dispensing channel. In some embodiments, the dispensing tip comprises a
protruding element.
In some embodiments, the dispensing tip comprises an inflatable balloon
surrounding at least a part
of a distal portion of the dispensing tip. In some embodiments, the inflatable
balloon further
surrounds at least a part of a proximal portion of the dispensing tip. In some
embodiments, a distal
portion of the dispensing tip is curved. In some embodiments, the dispensing
tip has a perforation. In
some embodiments, the dispensing tip has a perforation on a distal portion of
the dispensing tip. In
some embodiments, the perforation is on a single side of the dispensing tip.
In some embodiments, a
distal portion of the dispensing tip has a spiral shape. In some embodiments,
exerting the pressure on
fluid located within the dispensing tip enables an unraveling of the spiral
shape. In some
embodiments, the shot chamber is removable.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
4
[0011] In some embodiments, for any intranasal fluid delivery device described
herein, further
comprising a damping mechanism configured to generate a controlled velocity
profile of the fluid
delivered from the dispensing tip. In some embodiments, the fluid is delivered
from the dispensing
tip with a velocity of about 0.5 m/s to about 15 m/s. In some embodiments, the
fluid is delivered from
the dispensing tip with a velocity of about 1.5 m/s to about 9 m/s. In some
embodiments, the damping
mechanism comprises at least one of a magnet, a spring, a viscous dampener, a
sealed chamber with
an airflow restriction, a container of compressed gas, a valve, a motor, an
elastomeric chamber, a
flow restriction device, and a configuration of the plunger and shot chamber.
In some embodiments,
the damping mechanism comprises a flow restriction fluidically coupling the
shot chamber and the
dispensing tip. In some embodiments, the flow restriction is a constriction
between the shot chamber
and the dispensing tip. In some embodiments, the flow restriction is a
constriction within the
dispensing tip. In some embodiments, the flow restriction is a porous body. In
some embodiments,
the porous body comprises an open cell pore, a closed cell pore, or any
combination thereof In some
embodiments, the porous body is formed of metal, ceramic, plastic, wood, or
any combination
thereof In some embodiments, the flow restriction is an orifice plate within
the dispensing tip,
between the shot chamber and the dispensing tip, or both. In some embodiments,
the flow restriction
is an orifice within the dispensing tip, between the shot chamber and the
dispensing tip, or both. In
some embodiments, the flow restriction comprises flexible washers within the
dispensing tip,
between the shot chamber and the dispensing tip, or both.
[0012] In some embodiments, for any intranasal fluid delivery device described
herein further
comprising a secondary chamber, wherein the secondary chamber comprises a
second plunger at one
end configured to drive the fluid out the dispensing tip. In some embodiments,
a fluidic pathway
between the shot chamber and dispensing tip comprises a one-way valve
configured to prevent
backflow of the fluid into the shot chamber. The intranasal fluid delivery
device of claim 64 or 65,
wherein the second plunger is further configured to drive a secondary fluid
out of the dispensing tip.
In some embodiments, the secondary fluid is a gas.
[0013] In some embodiments, for any intranasal fluid delivery device described
herein, further
comprising an actuator operatively coupled to the plunger and configured to
move the plunger,
thereby enabling the plunger to drive the fluid from the shot chamber. In some
embodiments, the
actuator is operatively coupled to a push rod, such that when a user or the
subject engages the
actuator, the actuator enables the push rod to push against the plunger,
thereby causing the plunger
to move.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
[0014] In some embodiments, for any intranasal fluid delivery device described
herein, further
comprising: a hollow needle coupled to the dispensing tip; and a diaphragm
disposed between the
shot chamber and the hollow needle, the diaphragm providing a fluidic barrier
between the shot
chamber and dispensing tip, wherein the hollow needle is configured to
puncture the diaphragm so
as to provide fluidic communication between the shot chamber and dispensing
tip. In some
embodiments, the hollow needle is configured to be manually pushed towards the
shot chamber by
a user or subject so as to puncture the diaphragm. In some embodiments, for
any intranasal fluid
delivery device described herein, further comprising an actuator operatively
coupled to a push rod
moveable toward the plunger, such that the actuator is configured to move the
plunger, thereby
enabling the plunger to drive the fluid from the shot chamber to the
dispensing tip via the hollow
needle. In some embodiments, any intranasal fluid delivery device described
herein, configured such
that when a user or the subject engages the actuator, the actuator enables the
push rod to push against
the plunger, thereby causing the plunger to move. In some embodiments, for any
intranasal fluid
delivery device described herein, further comprising an actuator configured
such that when a user or
the subject engages the actuator, the actuator enables a push rod to push
against the plunger, causing
the shot chamber to move toward the hollow needle, such that the hollow needle
punctures the
diaphragm. In some embodiments, the actuator is configured to move the plunger
via the push rod,
thereby enabling the plunger to drive the fluid from the shot chamber. In some
embodiments, any
intranasal fluid delivery device described herein, wherein a single actuation
by the user of the actuator
enables the shot chamber to move forward so as to puncture the diaphragm, and
subsequently eject
the fluid within the shot chamber via the plunger being pushed by the push
rod. In some embodiments,
the actuator comprises a locking mechanism, and wherein user or subject
engagement of the actuator
releases the locking mechanism, allowing the push rod to push against the
plunger. In some
embodiments, the locking mechanism comprises one or both of: one or more tabs
comprising a lock
material, configured such that the locking mechanism is released by the user
breaking the lock
material; and one or more pivotable tabs, configured such that the locking
mechanism is released by
the user pivoting the pivotable tabs. In some embodiments, any intranasal
delivery device described
herein, further comprising a spring in alignment with the push rod, wherein
the locking mechanism
is configured to maintain the spring under a pressure condition, wherein
releasing the locking
mechanism releases the spring from the pressure condition, causing the push
rod to push against the
plunger. In some embodiments, the spring is a variable pitch spring. In some
embodiments, the
locking mechanism comprises one or more tabs that break off to release the
push rod, such that the
device is useable only once. In some embodiments, the device is configured to
maintain the fluid
within the shot chamber pressurized prior to being ejected through the
dispensing tip.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
6
[0015] In some embodiments, any intranasal fluid delivery device described
herein, comprising a
cartridge configured for containing, or containing, a pharmaceutical fluid,
wherein the cartridge
comprises the shot chamber, the diaphragm, and the plunger. In some
embodiments, any intranasal
fluid delivery device described herein further comprising a stopping mechanism
configured to limit
a travel distance of the push rod. In some embodiments, any intranasal fluid
delivery device described
herein, further comprising a cocking mechanism configured to be activated by
the user, wherein the
intranasal fluid delivery device is configured such that when the user
activates the cocking
mechanism, pressure is applied to the spring and the spring is thereby placed
under the pressure
condition.
[0016] In some embodiments, any intranasal fluid delivery device described
herein, further
comprising a damping mechanism configured to generate a controlled velocity
profile of the fluid
delivered from the dispensing tip, wherein the damping mechanism comprises an
actuator restriction
coupled to the actuator. In some embodiments, the actuator restriction
comprises a porous cavity. In
some embodiments, the porous cavity comprises an open cell pore, a closed cell
pore, or any
combination thereof. In some embodiments, the porous cavity is formed of
metal, ceramic, plastic,
wood, or any combination thereof
[0017] In some embodiments, any intranasal fluid delivery device described
herein, further
comprising a secondary chamber, wherein the secondary chamber comprises a
second plunger at one
end and a second actuator connected to a second push rod moveable toward the
dispensing tip. In
some embodiments, the hollow needle comprises a one-way valve configured to
prevent backflow.
In some embodiments, the second plunger and the second actuator are configured
to drive the fluid
out of the dispensing tip. In some embodiments, the second plunger and the
second actuator are
further configured to drive a secondary fluid out of the dispensing tip. In
some embodiments, the
secondary fluid is a gas. In some embodiments, any intranasal fluid delivery
device described herein,
further comprising a second needle for providing fluid communication between
the shot chamber and
the secondary chamber. In some embodiments, the second actuator is configured
to control the flow
rate of the fluid out of the dispensing tip.
[0018] In some embodiments, any intranasal fluid delivery device described
herein, wherein the
spring type, dampener type, shot chamber dimensions, dispensing tip length, or
any combinations
thereof is selected based on the fluid characteristics, therapeutic
requirements, surface of a subject's
nasal cavity, or combinations thereof.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
7
[0019] Disclosed herein, in some embodiments, is a method for delivering a
fluid to an olfactory
region of a subject, the method comprising: inserting a compliant dispensing
tip into the subject's
nasal cavity, wherein the dispensing tip comprises a flexible nib configured
to comply with or
conform to a surface of the subject's nasal cavity, thereby enabling a fluid
delivery orifice of the
dispensing tip to be directed towards the olfactory region of the subject; and
ejecting the fluid from
the fluid delivery orifice of the compliant dispensing tip to deliver the
fluid to the olfactory region of
the subject.
[0020] In some embodiments, the dispensing tip is configured to deliver the
fluid as a liquid jet or
a liquid stream. In some embodiments, the liquid jet is a laminar flow or the
liquid stream is a laminar
flow. In some embodiments, the dispensing tip is configured to deliver the
fluid as a spray, mist, or
aerosol. In some embodiments, the fluid comprises a powder. In some
embodiments, the dispensing
tip comprises an atomizer. In some embodiments, the dispensing tip comprises a
cannula. In some
embodiments, n the dispensing tip is tubular. In some embodiments, the
dispensing tip has an inner
diameter from about 0.3 mm to about 1.5 mm. In some embodiments, the flexible
nib comprises a
polymer. In some embodiments, the flexible nib comprises thermoplastic
polyurethane (TPU), high-
density polyethylene (HDPE), polyvinyl chloride (PVC), a thermoplastic
elastomer (TPE), styrene-
ethylene-butylene-styrene (SEBS), low density polyethylene (LDPE), silicone
polypropylene.
comprises polytetrafluoroethylene (PTFE), or any combinations thereof. In some
embodiments, the
dispensing tip comprises a distal portion having a first rigidity and a
proximal portion having a second
rigidity, and wherein the first rigidity is less than the second rigidity. In
some embodiments, the distal
portion comprising a first rigidity comprises a portion of the dispensing tip
that is about lmm to about
15mm from the fluid delivery orifice. In some embodiments, the dispensing tip
comprises a distal
portion that is softer than a proximal portion. In some embodiments, the
distal portion that is softer
than a proximal portion comprises a portion of the dispensing tip that is
about lmm to about 15mm
from the fluid delivery orifice. In some embodiments, the dispensing tip has a
distal portion having
a first outer diameter and a proximal portion having a second outer diameter,
and wherein the first
outer diameter is less than the second outer diameter. In some embodiments,
the dispensing tip has a
distal portion having a first outer diameter, a proximal portion having a
second outer diameter, and
wherein the first outer diameter is greater than the second outer diameter. In
some embodiments, the
dispensing tip further comprises a nose cushion to limit over-insertion of the
dispensing tip within
the nasal cavity, wherein the nose cushion configured to provide subject
comfort. In some
embodiments, the dispensing tip is configured to be inserted to an insertion
depth of about lOmm to
about 90mm within the subject's nasal cavity. In some embodiments, the fluid
delivery orifice of the
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
8
dispensing tip is configured to be positioned at or near the anterior entry to
the olfactory region. In
some embodiments, the fluid delivery orifice of the dispensing tip is
configured to be positioned
within about lmm to about 25mm from the anterior entry to the olfactory
region.
[0021] In some embodiments, for any method described herein, the dispensing
tip comprises a
hydrophilic coating applied to an outer surface of the dispensing tip. In some
embodiments, the fluid
comprises a pharmaceutical agent or a medicament. The method of claim 120,
wherein the fluid
comprises ketamine or insulin. In some embodiments, the pharmaceutical agent
or the medicament
is configured to be absorbed by the central nervous system (CNS) through the
olfactory region. In
some embodiments, the fluid comprises vitamins, fragrance, saline or non-
pharmaceutical agents. In
some embodiments, the surface of a subject's nasal cavity comprises anatomical
features of a nasal
cavity of the subject. In some embodiments, the anatomical features comprise
nasal turbinates, a
nasal valve, or combinations thereof. In some embodiments, the anatomical
features comprise a
septum of the subject. In some embodiments, the anatomical features comprise
an anterior aspect of
the nasal passage.
[0022] In some embodiments, for any method described herein, the dispensing
tip has an elliptical
cross section. In some embodiments, the dispensing tip has a distal portion
and a proximal portion,
and wherein a first center axis of the distal portion and a second center axis
of the proximal portion
are non-colinear. In some embodiments, the dispensing tip comprises an off-
center drug dispensing
channel. In some embodiments, the dispensing tip comprises a protruding
element. In some
embodiments, the dispensing tip comprises an inflatable balloon surrounding at
least a part of a distal
portion of the dispensing tip. In some embodiments, the inflatable balloon
further surrounds at least
a part of a proximal portion of the dispensing tip. In some embodiments, a
distal portion of the
dispensing tip is curved. In some embodiments, the dispensing tip has a
perforation. In some
embodiments, the dispensing tip has a perforation on a distal portion of the
dispensing tip. In some
embodiments, the perforation is on a single side of the dispensing tip. In
some embodiments, a distal
portion of the dispensing tip has a spiral shape. In some embodiments,
exerting the pressure on fluid
located within the dispensing tip enables an unraveling of the spiral shape
[0023] In this respect, before explaining at least one embodiment in detail,
it is to be understood
that the embodiments are not limited in application to the details of
construction and to the
arrangements of the components set forth in the following description or
illustrated in the drawings.
Also, it is to be understood that the phraseology and terminology employed
herein are for the purpose
of description and should not be regarded as limiting.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
9
[0024] Many further features and combinations thereof concerning embodiments
described herein
will appear to those skilled in the art following a reading of the instant
disclosure.
DESCRIPTION OF THE FIGURES
[0025] Specific embodiments of the disclosed devices, delivery systems, or
methods will now be
described with reference to the drawings. Nothing in this detailed description
is intended to imply
that any particular component, feature, or step is essential to the invention.
[0026] Figure 1 shows an example intranasal drug delivery device according to
some
embodiments.
[0027] Figure 2 shows an example intranasal drug delivery device with a lid or
cap according to
some embodiments.
[0028] Figure 3 shows an illustration of the olfactory region.
[0029] Figure 4 shows examples of intranasal drug delivery devices according
to some
embodiments.
[0030] Figure 5 shows an example ejection stroke and reload strokes of an
intranasal drug delivery
device according to some embodiments.
[0031] Figure 6 shows an example internal view of a tip and tip mechanism of
an intranasal drug
delivery device according to some embodiments.
[0032] Figure 7 shows an example intranasal drug delivery device with a
removable reservoir
according to some embodiments.
[0033] Figure 8 shows an example intranasal drug delivery device according to
some embodiments
with the tip inserted in the nasal cavity.
[0034] Figure 9 shows an illustration of an integrated intranasal drug-
delivery platform.
[0035] Figure 10 shows an example single use intranasal drug delivery device
according to some
embodiments.
[0036] Figure 11 shows an example intranasal drug delivery device according to
some
embodiments.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
[0037] Figure 12 shows an example intranasal drug delivery device according to
some
embodiments.
[0038] Figure 13 shows an example intranasal drug delivery device according to
some
embodiments.
[0039] Figure 14 shows an example intranasal drug delivery device according to
some
embodiments.
[0040] Figure 15 shows an example intranasal drug delivery device according to
some
embodiments.
[0041] Figure 16 shows an external view of an example intranasal drug delivery
device according
to some embodiments.
[0042] Figure 17 shows an external view of an example intranasal drug delivery
device according
to some embodiments.
[0043] Figure 18 shows an example intranasal drug delivery device according to
some
embodiments.
[0044] Figures 19 a-c show an example intranasal drug delivery device
according to some
embodiments.
[0045] Figures 20 a-c show an example intranasal drug delivery device
according to some
embodiments.
[0046] Figure 21 shows an example intranasal drug delivery device according to
some
embodiments.
[0047] Figure 22 shows an example intranasal drug delivery device according to
some
embodiments.
[0048] Figure 23 shows an example intranasal drug delivery device according to
some
embodiments.
[0049] Figure 24 shows an example intranasal drug delivery device according to
some
embodiments.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
11
[0050] Figure 25 shows an example intranasal drug delivery device according to
some
embodiments.
[0051] Figures 26 a-b show an example intranasal drug delivery device
according to some
embodiments.
[0052] Figures 27 a-b show an example intranasal drug delivery device
according to some
embodiments.
[0053] Figure 28 shows an example intranasal drug delivery device according to
some
embodiments.
[0054] Figures 29 a-c show an example intranasal drug delivery device
according to some
embodiments.
[0055] Figures 30 a-c show an example intranasal drug delivery device
according to some
embodiments.
[0056] Figure 31 shows an example intranasal drug delivery device according to
some
embodiments.
[0057] Figure 32 shows an example intranasal drug delivery device according to
some
embodiments.
[0058] Figures 33 a-c show an example intranasal drug delivery device
according to some
embodiments.
[0059] Figure 34 shows an example intranasal drug delivery device with a
dispensing tip having a
bulbous end portion according to some embodiments.
[0060] Figure 35 shows an example intranasal drug delivery device with a
dispensing tip having
an alpha loop according to some embodiments.
[0061] Figure 36 shows images from a scan of a test subject during testing of
a prototype device
with a tracer fluid.
[0062] Figures 37A-C show an exemplary embodiment of a dispensing tip
according to some
embodiments used with an intranasal drug delivery device described herein.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
12
[0063] Figures 38A-B show an exemplary embodiment of a dispensing tip
according to some
embodiments used with an intranasal drug delivery device described herein.
[0064] Figures 39A-B show an exemplary embodiment of a dispensing tip
according to some
embodiments used with an intranasal drug delivery device described herein.
[0065] Figure 40 shows an exemplary accessory that could be used with
dispensing tip described
herein.
[0066] Figures 41A-B show an exemplary embodiment of a dispensing tip
according to some
embodiments used with an intranasal drug delivery device described herein.
[0067] Figures 42A-B show an exemplary embodiment of a dispensing tip
according to some
embodiments used with an intranasal drug delivery device described herein.
[0068] Figures 43A-D show an exemplary embodiment of a dispensing tip
according to some
embodiments used with an intranasal drug delivery device described herein.
[0069] Figures 44A-C show an exemplary embodiment of a dispensing tip
according to some
embodiments used with an intranasal drug delivery device described herein.
[0070] Figures 45A-C show an exemplary embodiment of a dispensing tip
according to some
embodiments used with an intranasal drug delivery device described herein.
[0071] Figures 46A-B show an exemplary embodiment of a dispensing tip
according to some
embodiments used with an intranasal drug delivery device described herein.
[0072] Figures 47A-C show an exemplary embodiment of a dispensing tip
according to some
embodiments used with an intranasal drug delivery device described herein.
[0073] Figures 48A-D show an exemplary method for improving patient comfort
while inserting
a dispensing tip used with an intranasal drug delivery device described herein
into the nose.
[0074] Figures 49A-C show an exemplary method for improving patient comfort
while inserting
a dispensing tip used with an intranasal drug delivery device described herein
into the nose.
[0075] Figures 50A-D show an exemplary method for improving patient comfort
while inserting
a dispensing tip used with an intranasal drug delivery device described herein
into the nose.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
13
[0076] Figures 51A-C show an exemplary method for improving patient comfort
while inserting
a dispensing tip used with an intranasal drug delivery device described herein
into the nose.
[0077] Figures 52A-D show an exemplary method for improving patient comfort
while inserting
a dispensing tip used with an intranasal drug delivery device described herein
into the nose.
[0078] Figures 53A-D show an exemplary method for improving patient comfort
while inserting
a dispensing tip used with an intranasal drug delivery device described herein
into the nose.
[0079] Figures 54A-C show an exemplary method for improving patient comfort
while inserting
a dispensing tip used with an intranasal drug delivery device described herein
into the nose
[0080] Figures 55A-C show an exemplary method for improving patient comfort
while inserting
a dispensing tip used with an intranasal drug delivery device described herein
into the nose.
[0081] Figures 56A-D show an exemplary method for improving patient comfort
while inserting
a dispensing tip used with an intranasal drug delivery device described herein
into the nose.
[0082] Figure 57 shows a damper useful to control ejection velocity from an
intranasal drug
delivery device described herein.
[0083] Figures 58A-B shows a damper useful to control ejection velocity from
an intranasal drug
delivery device described herein.
[0084] Figure 59 shows a damper useful to control ejection velocity from an
intranasal drug
delivery device described herein.
[0085] Figure 60 shows a damper useful to control ejection velocity from an
intranasal drug
delivery device described herein.
[0086] Figure 61 shows a damper useful to control ejection velocity from an
intranasal drug
delivery device described herein.
[0087] Figure 62A shows a damper useful to control ejection velocity from an
intranasal drug
delivery device described herein.
[0088] Figures 62B-D shows alternate variations to the damper of Figure 62A.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
14
[0089] Figure 62E shows a relationship between velocity, force, and
progressive and digressive
damping profiles.
[0090] Figures 63A-C show an exemplary intranasal drug delivery device
according to some
embodiments.
[0091] Figures 64A-D show an exemplary intranasal drug delivery device
according to some
embodiments.
[0092] Figures 65A-C show an exemplary intranasal drug delivery device
according to some
embodiments.
[0093] Figures 66A-D show an exemplary intranasal drug delivery device
according to some
embodiments.
[0094] Figures 67A-C show an exemplary intranasal drug formulation delivery
device according
to some embodiments
[0095] Figures 68A-C show an exemplary intranasal drug formulation delivery
device according
to some embodiments
[0096] Figures 69A-D show an exemplary intranasal drug formulation delivery
device dispensing
tip according to some embodiments
[0097] Figure 70 shows an exemplary intranasal drug formulation delivery
device accessory
according to some embodiments
[0098] Figure 71 shows an exemplary intranasal drug formulation delivery
device according to
some embodiments
[0099] Figure 72 shows an exemplary intranasal drug delivery device according
to some
embodiments.
[00100] Figures 73 shows an exemplary intranasal drug delivery device
according to some
embodiments.
[00101] Figure 74 shows an exemplary intranasal drug delivery device according
to some
embodiments.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
[00102] Figures 75A-B show an exemplary intranasal drug delivery device
according to some
embodiments.
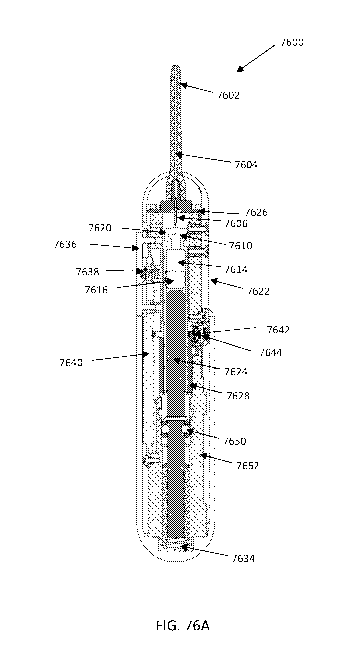
[00103] Figures 76A-D show an exemplary intranasal drug delivery device
according to some
embodiments.
[00104] Figures 77A-C show the intranasal drug delivery device of Figure 76A
with a cap disposed
thereon.
[00105] Figures 78A-C show intranasal drug delivery device of Figure 76A with
a cap removed
therefrom, and a trigger in an undepressed and depressed positions.
[00106] Figures 79A-B show intranasal drug delivery device of Figure 76A with
a ring oriented and
positioned perpendicular to a longitudinal axis of the device.
DETAILED DESCRIPTION
[00107] Embodiments of methods, systems, and apparatus are described through
reference to the
drawings.
[00108] Currently disposable intranasal drug delivery devices are
characterized by low
accuracy/uniformity of drug dosing, no design for anatomic variability and
poor design for human
factors ¨ efficacy and safety. The applications where these shortcomings are
most detrimental are:
direct-to-brain delivery path (uptake through olfactory epithelium into CSF,
action in brain),
systemically acting drugs (uptake through mucosa into vasculature, systemic
action), vaccines
(uptake and action in mucosa), and topically acting drugs (uptake and action
in mucosa).
[00109] The following provides for intranasal delivery of new and existing
drugs, with the
following benefits: increased dosing precision, greater efficacy, less cost,
increased effectiveness,
increased safety (both to patient and society), and increased convenience (in
terms of health care).
For any embodiment described herein, a drug comprises a fluid. In some
embodiments, the fluid
comprises a liquid, gel, solid, powder, or any combination thereof. In some
embodiments, for any
device disclosed herein, the drug delivered comprises a powder.
[00110] The following provides for opportunities in terms of design for
markets where access to
health care is challenged (humanitarian impact) and in terms of design for
prevention of drug misuse.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
16
[00111] Figure 1 shows an example intranasal drug delivery device 100
according to some
embodiments.
[00112] The device 100 has a compliant or flexible, soft nib 102 (as opposed
to a hard nib) to
precisely locate the dosage. The soft nib 102 also provides comfort for user
and may minimize
blocking by the nasal wall or congestion. In some embodiments, the nib
comprises a polymer. In
some embodiments, the nib comprises thermoplastic polyurethane (TPU). In some
embodiments, the
nib comprises TPU at grade 65D, 57D, 95A, 90A, 80A, or any combination thereof
In some
embodiments, the nib comprises high-density polyethylene (HDPE). In some
embodiments, the nib
comprises polyvinyl chloride (PVC). In some embodiments, the nib comprises a
thermoplastic
elastomer (TPE). In some embodiments, the nib comprises styrene-ethylene-
butylene-styrene
(SEBS). In some embodiments, the nib comprises low density polyethylene
(LDPE). In some
embodiments, the nib comprises silicone (e.g., liquid silicone rubber (LSR)).
In some embodiments,
the nib comprises polypropylene. In some embodiments, the nib comprises
polytetrafluoroethylene
(PTFE), such as for example, Teflon. In some embodiments, the nib comprises
thermoplastic
polyurethane (TPU), high-density polyethylene (HDPE), polyvinyl chloride
(PVC), a thermoplastic
elastomer (TPE), styrene-ethylene-butylene-styrene (SEBS), low density
polyethylene (LDPE),
silicone polypropylene. comprises polytetrafluoroethylene (PTFE), or any
combinations thereof.
[00113] Septal deviation can cause different health related problems. In some
embodiments
compliant, soft nib 102 conforms to the anterior aspect of the intranasal
passage. In some
embodiments the soft nib 102 is biased to follow the patient's septum. This
allows the tip 110 to be
placed in a location in the nasal cavity to discharge medicine targeting the
olfactory region and
accommodates differences in nasal cavity anatomy such as the nasal valve,
nasal turbinates and the
septum.
[00114] In some embodiments compliant, soft nib 102 has a kiss-cut valve near
the tip 110. The
valve reduces the partial discharge at the front and backend of the actuation.
The tip 110 also reduces
or eliminates air or contaminates from contacting the line-fill remaining in
the dispensing tip between
dosing. In some embodiments the orientation of the kiss cut is off set from
the end of the tip 110 for
directing the medicine in the direction of the olfactory region of the nasal
anatomy. The nib 102 can
be a multiple material over-moulded nib in some embodiments. As shown in
Figure 34, in some
embodiments the nib may have a bulbous or ball shape tip 3400 to ease the
insertion, improve
localization, open tight passageways, and facilitate better liquid jet or
stream flow to a target region
in the nasal cavity. As shown in Figure 35, in some embodiments, the compliant
nib utilizes an 'alpha
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
17
loop' 3500 to facilitate delivery past an obstruction. One of the tricks in
interventional cardiology to
pass a guidewire past a stricture or calcified obstruction is to force the
flexible tip guide wire into the
obstruction. The tip will naturally bend back on itself and the wire finds its
way through the
obstruction with the alpha loop leading. The larger bearing surface helps to
steer the wire to the point
of least resistance and it will slip through the stricture/obstruction. This
embodiment may be utilized
in trauma where a nose may be less than perfect, this could be the shape that
would help the compliant
nib find its mark.
[00115] The device 100 has an actuator 106 (e.g. button, trigger) and cocking
mechanism 108 to
release dosage that is reproducible to reduce human error/variation. Use of a
cock-and-release
mechanism in some embodiments promotes steady positioning during delivery and
reduces the need
for priming of the device 100, thereby reducing the possibility of operator
error. In some
embodiments a finger press button actuation discharges the shot chamber. This
method of actuating
the device 100 requires very little dexterity or fine motor skills which may
be of particular importance
to patients whose motor skills may be impaired e.g. patients with Parkinson's.
Priming can refer to
ensuring full liquid filling dosing/metering mechanism suitable for pumping of
the liquid including
but not limited to positive displacement pumping.
[00116] The device 100 has an internal reservoir that can be under pressure
constantly in some
embodiments to enable dosing independent of orientation (e.g. the user can be
standing up or laying
down and it will work). The reservoir may be a bag and may be collapsible by
external pressure,
including ambient air pressure. The pressure within the reservoir may change
depending on the spring
used, but it can always be under some amount of pressure.
[00117] In some embodiments the device 100 has no air-port for filling,
storing or actuating the
device 100. This allows for travel or transport by air, particularly
unpressurized aircraft or higher
elevations and may be useful for oxygen sensitive medicine and extending shelf
life of certain
medicines, particularly where there is no cold-chain infrastructure. In
addition, this makes the device
difficult to tamper with. In some embodiments, there can be an air bleed port.
[00118] In some embodiments the shape of the device 100 allows for correct
dispensing tip
positioning and ergonomic grip that does not engage the shoulder, wrist, or
any part of the other arm
not activating the device 100. The design of device 100 promotes minimal use
of shoulder and arm
movement.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
18
[00119] In some embodiments the design of device 100 is made highly ergonomic
in form, taking
inspiration both from a wider remote controller design and a more dexterous
pen design.
[00120] The ergonomics and considered human factors create a step change in
the state of the art
for nasal delivery devices. The design minimizes human error, allowing for a
targeted, repeatable,
and metered dose delivery. The design accommodates a consumable drug reservoir
for short to long
term use, while allowing for a low cost single patient consumable. This gives
the ability for a wide
variety of drugs to be filled at the point of care or by pharmaceutical
filling lines. The design allows
for, as an example, a compliant, soft nib 102 with an ultra-soft, matte
finish, elastomeric shroud.
[00121] The compliant, soft nib 102 of the device is entered into the
intranasal cavity and uses the
common internal nasal geometry to guide the tip proximate to the olfactory
region. The compliant
soft nib 102 stops at a distance from the olfactory region and the ejected
liquid containing a drug is
guided to the olfactory by the native geometry of the nasal anatomy. The
device mechanism supports
a pocketable form being based on compact and low-cost injection-mouldable
parts.
[00122] Figure 2 shows an example intranasal drug delivery device 100 with a
lid 202 or cap
according to some embodiments.
[00123] In some embodiments the lid 202 may be used with the cocking mechanism
108, or instead
of cocking mechanism 108, as part of reloading the intranasal drug delivery
device 100. The addition
of the lid 202 increases the grip size of the drug delivery device 100 and
prevents misfiring of the
drug delivery device 100. In some embodiments lid 202 may provide extra space
for full hand grip
when attached to bottom of device 100. In some embodiments lid 202 is shaped
to increase the surface
area without obstruction by hand when in use so that machine readable indicia
(i.e. URL code) can
be added to the increased surface area.
[00124] In some embodiments, the device 100 may include rechargeable energy
storage to provide
motive energy with separate actuation. Rechargeable energy may include
electrical, chemical or
pressurized fluid storage.
[00125] Figure 3 shows an illustration of the nasal cavity 300 including the
olfactory region 306,
upper nares 308 and lower nares 310.
[00126] In topical drug delivery, drug is delivered to the entire mucosa, i.e.
both the upper nares
308 and lower nares 310. In systemic drug delivery, drug is delivered through
the mucosa of the
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
19
upper nares 308 into the vasculature. In direct-to-brain drug delivery, drug
is delivered mainly
through the olfactory region 306 by diffusion into the olfactory mucosa and
transport through the
cribriform plate along the olfactory neuron pathways to the Central Nervous
System. Drug transport
from the olfactory region to the Central Nervous System may also involve the
participation of
trigeminal nerves.
[00127] Current drug formulations for nasal delivery use standard sprays with
no specificity to the
olfactory region 306, relatively small molecules are used, and formulations
are mainly water-based
with some alcohols. For non-active ingredients in drug formulations for nasal
delivery a wide variety
of functionality is used: solvents, mucoadhesive, agents, absorption
enhancers, viscosity modifiers,
pH buffers, antioxidants, preservatives, surfactants and more.
[00128] The majority of airflow passes through the lower nares 310. Therefore,
sneezing would
likely not expel liquids deposited in the olfactory region 306. Nasal
congestion may affect mainly
the lower nares 310 while the olfactory region 306 stays clear.
[00129] Targeted direct-to-brain drug delivery may be achieved through
saturation of the olfactory
region 306 with an excipient/drug combination. The drug may travel via
intracellular or extracellular
neuronal transport to the Central Nervous System, via the cribriform plate.
This targeted delivery is
intended to reduce both topical and systemic delivery, allowing for safer and
more effective drug
delivery.
[00130] In some embodiments the device 100 may be adapted by the addition of a
lateral atomizer
tip to achieve the current state of the art of topical drug delivery by
saturating the entire mucosa, or
systemic drug delivery by targeting the Upper Nares 308.
[00131] The olfactory plateau is generally located to the posterior aspect of
the Radix line. This
correlates to the Nasal Bridge length, which is measured from the soft tissue
of the Nasion (SeIlion)
to the Subnasale.
[00132] Figure 4 shows examples of intranasal drug delivery devices according
to some
embodiments with reservoir 402 and compliant tip 404.
[00133] Figure 5 shows an example release and reload mechanism 500 according
to some
embodiments. Release and reload mechanism 500 may be incorporated into an
intranasal drug
delivery devices such as, for example, device 100.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
[00134] The release and reload mechanism 500 has a reservoir 502 containing a
drug for delivery
into the nasal cavity.
[00135] The release and reload mechanism 500 has an insertion needle 504 for
insertion into the
reservoir 502.
[00136] In some embodiments reservoir 502 is a bag and may be collapsible by
external pressure,
including ambient air pressure.
[00137] In some embodiments, reservoir 502 is removable and insertion needle
504 is inserted
through a silicon stopper in the top of reservoir 502 for drawing the
substance into the device 100.
The silicon stopper has re-sealing properties for air sensitive medicine. The
insertion needle 504 can
be left in the bottle from which the medicine for the device was obtained. The
filling process can
eliminate the need for a separate syringe. In some embodiments, this may be
referred to as a lure
lock.
[00138] The release and reload mechanism 500 has actuator 506 connected to
release spring 508.
[00139] The release and reload mechanism 500 has plunger 510, load valves 512
and load chambers
514.
[00140] The release and reload mechanism 500 has shot chamber 516, fluid
chamber 518, release
valves 520 and dispensing tip 522. The dispensing tip 522 may be in fluid
communication with the
nib 102 such that fluid is ejected from dispensing tip 522 and through nib 102
or as described below.
[00141] In some embodiments, release valves 520 may comprise a check valve in
the dispensing tip
to reduce and valve the line/dead volume. In some embodiments release valves
520 may comprise an
elongated duckbill valve in tip to reduce and valve the line/dead volume.
[00142] In some embodiments, reservoir 502 is held under tension by
compression spring 524. A
constant and predetermined fluid pressure may be maintained by compression
spring 524 pushing up
from the bottom of the reservoir towards the shot chamber 516 and dispensing
tip 522 and plunger
510. This constant liquid pressure charges the load chamber 514 without
exposing the medicine to
air or metal springs typical in most nasal pumps. In some embodiments, this
may avoid the use of
tubing between the reservoir 502 and shot chamber 516. This can reduce dead
volume of medication
or medication left in line after use. This can ensure dosing accuracy is not
compromised by air
entering the shot chamber 516 and no content remains in the shot chamber 516
or reservoir 502 after
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
21
the last usable medicine was administered. The constant pressure enables
dosing independent of user
orientation.
[00143] In some embodiments the compliant, soft nib 102 is designed to
discharge a liquid jet or
liquid stream. In some embodiments the liquid jet or liquid stream is a
laminar flow and this may
include a turbulent boundary, discreet liquid slug ideally suited for
maximizing dose delivery to the
flat narrow section of nasal cavity leading up to the olfactory region.
Delivery of laminar liquid slug
assists in capillary action required for maximum medicine reaching the
olfactory region. In some
embodiments, the laminar stream is created by tube array or hydrodynamic
focusing. In some
embodiments, the liquid jet or liquid stream is delivered through the
dispensing tip with a controlled
velocity profile to limit shear forces on the fluid. In some embodiments, the
liquid jet or liquid stream
is delivered at a velocity from about 0.5 m/s to about 15 m/s. In some
embodiments, the fluid is
delivered at a velocity from about 1.5 m/s to about 9 m/s. In some
embodiments, the fluid is delivered
at a velocity from about 0.5 m/s to about 15 m/s. In some embodiments, the
fluid is delivered at a
velocity from about 0.5 m/s to about 1.5 m/s, about 0.5 m/s to about 3 m/s,
about 0.5 m/s to about 5
m/s, about 0.5 m/s to about 9 m/s, about 0.5 m/s to about 12 m/s, about 0.5
m/s to about 15 m/s, about
1.5 m/s to about 3 m/s, about 1.5 m/s to about 5 m/s, about 1.5 m/s to about 9
m/s, about 1.5 m/s to
about 12 m/s, about 1.5 m/s to about 15 m/s, about 3 m/s to about 5 m/s, about
3 m/s to about 9 m/s,
about 3 m/s to about 12 m/s, about 3 m/s to about 15 m/s, about 5 m/s to about
9 m/s, about 5 m/s to
about 12 m/s, about 5 m/s to about 15 m/s, about 9 m/s to about 12 m/s, about
9 m/s to about 15 m/s,
or about 12 m/s to about 15 m/s, including increments therein. In some
embodiments, the fluid is
delivered at a velocity from about 0.5 m/s, about 1.5 m/s, about 3 m/s, about
5 m/s, about 9 m/s, about
12 m/s, or about 15 m/s. In some embodiments, the fluid is delivered at a
velocity from at least about
0.5 m/s, about 1.5 m/s, about 3 m/s, about 5 m/s, about 9 m/s, or about 12
m/s. In some embodiments,
the fluid is delivered at a velocity from at most about 1.5 m/s, about 3 m/s,
about 5 m/s, about 9 m/s,
about 12 m/s, or about 15 m/s.
[00144] In some embodiments the design of the chamber and fluid path can
promote high accuracy
in ejected volume.
[00145] In some embodiments device 100 is cocked by pushing down, or
compressing, the bottle.
This method of preparing the device for actuating requires very little
dexterity or fine motor skills.
This method of preparing the device for administrating medicine may be of
particular importance to
patients whose motor skills may be impaired e.g. patients with Parkinson's.
The device can be
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
22
oriented in any direction and the reloading of the shot chamber and the shot
performance will not be
affected i.e. the device is not gravity sensitive.
[00146] In some embodiments the compliant, soft nib 102 is extended by cocking
the device. This
reduces over length profile of the device for shipping, shelf space and
pocketing. In the resting
position the device has a less 'menacing' look.
[00147] In some embodiments cocking the device 100 may activate a dose
counter. In some
embodiments cocking may activate a separate shot counter for each dosing
session.
[00148] In some embodiments cocking may activate a dose delay. In some
embodiments cocking
may activate a timer to remind patient when to activate between shots needed
for dosing session. The
delay between shots accommodates drug dosing indications including the timing
of maximum drug
absorption via the olfactory tight junction and the natural clearing of the
mucosa cilia.
[00149] In some embodiments cocking may change the exposed color 112 between
the upper bottle
sleeve 104 and base 108. This, along with an extended dispensing tip (which in
some embodiments
does not fit in the lid 202 while cocked) gives the patient or care giver a
clear visual and/or feel the
device is ready for dosing or storage. In some embodiments exposed color 112
is made with glow
plastic for darkness which promotes ease and convenience of nighttime use and
for patients sensitive
to light e.g. for administering medicine that dilates pupils.
[00150] In some embodiments the dispensing tip has an adjustable nostril stop
114. This stop gives
patient feedback the dispensing tip has arrived at the optimum nostril depth.
The stop also reduces
sniffing/snorting during activation.
[00151] In some embodiments, the drug may be delivered by the intranasal drug
delivery device
100 by delivery of a liquid jet, stream, burst or plug, rather than a spray.
In some embodiments the
design of the compliant, soft nib 102, the dispensing tip 522, and the valves
in the reload mechanism
500 may be designed to optimize laminar ejection of drug.
[00152] Technology for liquid delivery works for a wide variety of liquid
properties. This
technology may be adapted to olfactory, systemic and topical delivery of drugs
through an intranasal
drug delivery device 100.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
23
[00153] In some embodiments intranasal drug delivery device 100 may use
particular liquid
properties (such as viscosity and surface tension) to ensure prolonged
residence of the delivered
liquid in the target area (i.e. the olfactory region) partially enabled by
capillary bridging.
[00154] In some embodiments intranasal drug delivery device 100 may include
excipients in the
liquid drug for delivery with particular characteristics. For example,
excipients may have
thixotropicity (higher viscosity at rest which improves residence time in the
olfactory region 306,
and lower viscosity at under shear which improves ease of metering and
delivery) through additives
such as cellulose. As a further example, excipients used may impact surface
tension of a drug to
promote wetting and capillary bridging in olfactory region. As a further
example, excipients used
may be pre-approved by the Federal Drug Administration for shorter development
time.
[00155] In some embodiments intranasal drug delivery device 100 may include a
measurement
method or accessory to determine the ideal compliant, soft nib 102 size, or
dispensing tip 522 type.
[00156] In some embodiments intranasal drug delivery device 100 may include a
mechanical or
electronic timer and/or lock mechanism to prevent overdosing. Intranasal drug
delivery device 100
may incorporate use of mobile technology for identifying users and tracking
use to prevent
overdosing. Intranasal drug delivery device 100 may incorporate use of a cock-
and-release
mechanism to promote steady positioning during drug delivery. These additions
assist with patient
compliance.
[00157] In some embodiments intranasal drug delivery device 100 may be used in
one or more of
the following applications: 1) drugs directly targeting the brain via the
olfactory region, 2)
systemically acting drugs (e.g. better systemic bioavailability or less
degradation than via the GI
tract), 3) vaccines eliciting a mucosal immune response, and 4) topically-
acting drugs. In some
embodiments, the drug delivered is a drug formulation. In some embodiments,
for any embodiment
herein, the drug comprises a fluid. In some embodiments, for any embodiment
described herein, the
fluid comprises a liquid, gel, powder, or combinations thereof In some
embodiments, the drug
comprises a powder suspended within a liquid or gaseous fluid. In some
embodiments, the drug
comprises a powder that is delivered by the device.
[00158] In some embodiments the intranasal drug delivery device 100 may have
one or more of the
following features: 1) hand held, 2) useable with a single hand, 3) designed
for ambidextrous use, 4)
the priming mechanism is simple and intuitive to the user, 5) there is a clear
indication when the dose
is primed, 6) the form promotes proper positioning in the nasal cavity, 7)
designed to require a single
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
24
user action to deliver a primed dose, 8) designed to prevent the user from
dispensing partial doses,
and 9) useable for multiple doses.
[00159] In some embodiments the intranasal drug delivery device 100 is
intended to be filled by a
pharmacist or other medical professional. In some embodiments the intranasal
drug delivery device
100 shall contain means for preventing unintended refills of the reservoir
502.
[00160] In some embodiments the intranasal drug delivery device 100 is
designed for multiple uses.
In some embodiments the intranasal drug delivery device 100 uses a disposable
or a refillable
reservoir 502. In some embodiments the compliant, soft nib 102 is disposable.
[00161] In some embodiments intranasal drug delivery device 100 is designed
with a floating gasket
in a disposable or reusable reservoir 502.
[00162] In some embodiments, the drug delivery device 100 may integrate with a
system involving
mobile technology such as, for example, face recognition and position
tracking, Gyroscopic position
tracking of device and correlation with facial position, use of NFC to track
number of shots.
[00163] In some embodiments, the drug delivery device 100 may enable
electrically activated drug
delivery such as Iontophoresis. In some embodiments, the drug delivery device
100 may involve
applying an ionic charge to the drug molecule to enhance transport. In some
embodiments, the drug
delivery device 100 may involve an extending tip that telescopes.
[00164] In some embodiments, intranasal drug delivery device 100 is designed
to use a foam as an
excipient to assure residence time in target area yet allow air to pass.
[00165] In some embodiments intranasal drug delivery device 100 has barbs to
lock a gasket at the
end of travel to prevent misuse by refilling.
[00166] In some embodiments intranasal drug delivery device 100 has a piston
that scores the
chamber walls as it travels to the top of the reservoir with each actuation.
This renders the device
useless after a single use.
[00167] In some embodiments intranasal drug delivery device 100 is a multi-
dose device with a
sterile barrier to avoid contamination.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
[00168] Figure 6 shows an example intranasal drug delivery device 100
according to some
embodiments including fluid chamber 602, dispensing tip 604, compliant, soft
nib 606, actuator 608,
exposed colour 610 and base 612.
[00169] Figure 7 shows an example intranasal drug delivery device 700 708, 710
according to some
embodiments: with the base 702 connected to the intranasal drug device 700,
with the base 702
removed and the removable reservoir 704 inserted into the intranasal drug
delivery device 708, and
with the removable reservoir 704 partially removed from intranasal drug
delivery device 710. In some
embodiments, a latch mechanism 706 retains the removable reservoir 704 in the
device.
[00170] Figure 8 shows an intranasal drug delivery device 100 inserted into
the nasal cavity of a
patient with the tip positioned proximate or about the olfactory region 306 or
an anterior entry to the
olfactory region. In some embodiments, the tip is positioned from about 0.1 mm
to about 30 mm
from the olfactory region or an anterior entry to the olfactory region. In
some embodiments, the tip
is positioned from about 0.1 mm to about 25 mm from the olfactory region or an
anterior entry to the
olfactory region. In some embodiments, the tip is positioned from about 0.1 mm
to about 3 mm, about
0.1 mm to about 5 mm, about 0.1 mm to about 9 mm, about 0.1 mm to about 12 mm,
about 0.1 mm
to about 18 mm, about 0.1 mm to about 20 mm, about 0.1 mm to about 25 mm,
about 3 mm to about
5 mm, about 3 mm to about 9 mm, about 3 mm to about 12 mm, about 3 mm to about
18 mm, about
3 mm to about 20 mm, about 3 mm to about 25 mm, about 5 mm to about 9 mm,
about 5 mm to about
12 mm, about 5 mm to about 18 mm, about 5 mm to about 20 mm, about 5 mm to
about 25 mm,
about 9 mm to about 12 mm, about 9 mm to about 18 mm, about 9 mm to about 20
mm, about 9 mm
to about 25 mm, about 12 mm to about 18 mm, about 12 mm to about 20 mm, about
12 mm to about
25 mm, about 18 mm to about 20 mm, about 18 mm to about 25 mm, or about 20 mm
to about 25
mm, including increments therein. In some embodiments, the tip is positioned
from about 0.1 mm,
about 3 mm, about 5 mm, about 9 mm, about 12 mm, about 18 mm, about 20 mm, or
about 25 mm.
In some embodiments, the tip is positioned from at least about 0.1 mm, about 3
mm, about 5 mm,
about 9 mm, about 12 mm, about 18 mm, or about 20 mm. In some embodiments, the
tip is positioned
from at most about 3 mm, about 5 mm, about 9 mm, about 12 mm, about 18 mm,
about 20 mm, or
about 25 mm from the olfactory region or an anterior entry to the olfactory
region. In some
embodiments, the tip is touching the olfactory region. In some embodiments a
speculum may be used
as an accessory to open the nostril. In some embodiments the device 100 may be
include an accessory
part to guide the tip.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
26
[00171] The compliant, soft nib 102 of the device is entered into the
intranasal cavity and uses the
common internal nasal geometry to self-guide the compliant, soft nib 102
towards the olfactory
region. The compliant, soft nib 102 is held from lateral deviation via the
flanking medial septum, and
the lateral nasal wall.
[00172] In some embodiments when the device 100 is activated, an internal
metering chamber ejects
a repeatable and metered dose into the superior/posterior aspect of the
olfactory region. In some
embodiments, a liquid jet or liquid stream is produced, as opposed to
conventional spray, mist or
aerosol, to ensure that the ejected dose gets delivered to the target area,
rather than spreading in the
entire intranasal space. In some embodiments, a laminar flow is produced. In
some embodiments,
due to the Coanda effect, the ejected excipient adheres to the medial, lateral
and superior aspect of
the olfactory corridor while still motive.
[00173] In some embodiments, when the motive energy of the ejected liquid has
dissipated, the
excipient coats part of or all of the olfactory region surface. In some
embodiments, when the motive
energy of the ejected liquid has dissipated, opposing wall capillary motion
allows the excipient to fill
a part of the entire olfactory region. This is due to the combination of
excipient surface tension (which
is caused by cohesion within the excipient) and mucoadhesive properties
between the excipient and
olfactory mucosa wall.
[00174] To achieve residence time, and as a result of capillary action, the
excipient will be held in
the olfactory corridor due to a capillary bridge effect caused by the opposing
walls of the medial,
lateral and superior aspect of the olfactory corridor, thus preventing the
excipient from draining to
the inferior aspect of the nasal vault. An adequately high viscosity or
thixotropic property of the
excipient helps prolonging residence time.
[00175] In one embodiment the proposed method for targeted drug delivery using
the device 100 is
as follows: 1) The compliant tip is placed to the anterior aspect of the
olfactory corridor; 2) The
excipient is ejected out of the tip in a liquid jet or liquid stream and
towards the posterior aspect of
the olfactory region; 3) When the motive energy of the ejected liquid has
dissipated, the excipient
coats all or a portion of the olfactory region surface. This is due to the
combination of excipient
surface tension (which is caused by cohesion within the excipient) and
mucoadhesive properties
between the excipient and olfactory mucosa wall. In one embodiment the
proposed method for
targeted drug delivery using the device 100 is as follows: 1) The compliant
tip is placed to the anterior
aspect of the olfactory corridor; 2) The excipient is ejected out of the tip
in a liquid jet or liquid stream
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
27
and towards the posterior aspect of the olfactory corridor; 3) When the motive
energy of the ejected
liquid has dissipated, opposing wall capillary motion allows the excipient to
fill all or a portion of
the entire olfactory region. This is due to the combination of excipient
surface tension (which is
caused by cohesion within the excipient) and mucoadhesive properties between
the excipient and
olfactory mucosa wall; 4) To achieve residence time, and as a result of
capillary action, the excipient
will be held in the olfactory region due to a capillary bridge effect caused
by the opposing walls of
the medial, lateral and superior aspect of the olfactory region, thus
preventing the excipient from
draining to the inferior aspect of the nasal vault. An adequately high
viscosity or thixotropic property
of the excipient helps prolonging residence time. In some embodiments, the
liquid jet is a
"reasonably" laminar jet.
[00176] Figures 75 A-B illustrate another exemplary embodiment of an
intranasal drug delivery
device 7500 according to some embodiments. The device 7500 comprise a
dispensing tip 7502 as
described herein. In some embodiments, the device 7500 further comprises a
trigger 7504 for delivery
of a fluid to within a nasal cavity of a subject.
[00177] Figure 9 illustrates an integrated intranasal drug-delivery platform
900 including an
intranasal drug delivery device 902, a mobile device 904, an intranasal device
software application
906, a core application program interface 908, and device generated data 910
that may be shared with
shareholders 912.
[00178] The device 902 can connect to a software application 906 installed on
a mobile device 904
for data logging to flag or track misuse and compliance. For example, the
intranasal device software
application 906 can capture images up the nasal cavity to flag misuse,
implement user biometric
authentication for compliance, capture timing data of dosage for compliance,
provide alerts or
reminders to user and so on.
[00179] In some embodiments a software application will be available in
association with the device
100 to create an integrated hardware and software intranasal drug-delivery
platform 900. This
includes a database for the storage of data generated from device 100 that
serves as a basis for
extension to a permission-based personal data ecosystem platform.
[00180] In some embodiments the software application may be extended to become
a platform for
more broad data aggregation and permission-based sharing. A patient's personal
data could be
collected and exchanged with permission to/from all parties who have a role
and accountability for
administering (dispensed and applied) intranasal treatments. The data exchange
portal would provide
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
28
patient insight aimed at aligning and continuously influencing positive
behavior for optimum health
care delivery. The extension will facilitate sharing of different types of
smartphone-based personal
data to different stakeholders such as other patients, guardians, doctors,
clinics, clinical trial
researches, health care providers, patient medical insurers, doctor insurers,
health care insurers, drug
developers, pharmacies, patient peer support groups, disease/disorder
researchers, disease/disorder
NGO's, government regulators, law enforcement/first responders. Privacy and
control of personal
data are important. A user may wish to share data in certain circumstances,
based on incentives or
goodwill.
[00181] In some embodiments components of an integrated intranasal drug-
delivery platform 900
may comprise an intranasal drug delivery device 902 that is inextricably
linked with a specified
medicine and an individual patient through device and patient verification;
intranasal drug delivery
device 902 that provides machine readable signals (fiducial markers) at time
of scrip writing, scrip
filling, patient dosing, patient possession, and device redemption (i.e.
patient life cycle events); on-
going data harvesting, transit, storage and retrieval capability; aggregation
and anonymization of
personal data into mineable and usable data sets e.g. reporting, analytics,
gamification, incentivizing,
etc.; personal data for optimizing patient's immediate and ongoing healthcare
and a permission-based
sharing system.
[00182] Categories of data that an integrated intranasal drug-delivery
platform 900 may utilize
include a patient profile; stakeholder profiles to manage data that has been
shared with them; non-
medical passive personal data (recovery of which may be ongoing); medical /
biometric personal data
(recovery of which may be ongoing); event driven personal data at time of
scrip writing, scrip filling,
patient dosing, patient possession, and device redemption (i.e. patient
lifecycle); and event driven
prompting to influence immediate behavior.
[00183] For an example of an integrated intranasal drug-delivery platform 900
for a user that has
been prescribed a drug that is dispensed with intranasal drug delivery device
902, 1) the user receives
an alert on his/her mobile device 904 signaling that it's time to take a
scheduled dose of drug, 2) the
user unlocks the mobile device 904 using native identity authentication
(passcode, fingerprint or
facial recognition) and the intranasal device software application 906 opens
on the mobile device, 3)
the user touches the mobile device 904 to the intranasal drug delivery device
902 or initiates another
form of recognition, 4) the user uses the mobile device 902 for facial
recognition validation, 5) the
intranasal device software application 906 prompts the user for measuring pre-
actuation
metrics/biometrics (relevant metrics may be determined by clinician, for
example, cognition survey,
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
29
HR measurement, short video capture to determine emotional state/impairment
etc.), 6) the user
completes any inputs needed to complete pre-actuation tests, 7) the intranasal
device software
application 906 determines that the intranasal drug delivery device 902 has
been actuated (the action
may be timestamped and recorded, methods for confirming actuation include
Bluetooth connectivity,
visual image, sound, colour change, artificial intelligence that recognizes
actuation), 8) the intranasal
device software application 906 prompts the user for measurements of post-
actuation biometrics
(relevant metrics may be determined by clinicians); 9) the user is taken back
to dashboard as part of
an interface controlled by software application 906 where he/she can track
different metrics and
manage permissions (who can see what data).
[00184] Figure 10 shows an example single use intranasal drug delivery device
1000, pump 1002
incorporating a reservoir, a pump locking mechanism 1004, and compliant, soft
nib 1008, with a nib
locking mechanism 1006, shot chamber 1010 and spray tip 1012. In some
embodiments the pump
1002 would be a spring actuated piston and the pump locking mechanism 1004
would lock with the
nib locking mechanism 1006.
[00185] In some embodiments the device can include an olfactory marker that
will be included with
the excipient/drug that will provide biofeedback to the user. This may take
the form of olfactory
active marker that can signal to the user that the drug/excipient has been
delivered to the olfactory
region. This may include, but not be limited to markers which provide feedback
of missed, un-
deployed, deployed or over deployed drug/excipient. The marker can be included
in the
drug/excipient formulation or in some embodiments be added during the ejection
process. In some
embodiments, the marker may be included without the active drug agent to
provide feedback to the
user that an application and dosage (without the drug agent) was successful
soliciting a psychological
response.
[00186] Figure 11 shows an example intranasal drug delivery device 1100
according to some
embodiments. The device 1100 comprises an outer chassis 1108 with a dispensing
opening at a first
end and an actuating opening at a second end. A dispensing tip is coupled to
the dispensing opening,
and an actuator 1130 is coupled to the actuating opening. As described below,
fluid can be delivered
to a nasal volume through the dispensing tip by pressing on the actuator 1130.
In some embodiments,
for any intranasal drug delivery device described herein, the fluid comprises
a liquid, a gel, a powder,
or any combinations thereof In some embodiments, the fluid is delivered from
the dispensing tip
through a fluid delivery orifice disposed at a distal end of the dispensing
tip. In some embodiments,
the drug comprises a powder that is delivered by the device.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
[00187] In some embodiments, the device 1100 is configured to receive a
cartridge, which may be
pre-filled with a fluid for delivery into an intranasal cavity of a subject.
In some embodiments, for
any intranasal delivery device described herein, a cartridge comprises a
carpule. In some
embodiments, the cartridge 1120 (which comprises a diaphragm 1110, tube 1112,
shot chamber 1114,
and plunger 1116 as described below) pre-filled with a fluid, such as for
example a pharmaceutical
fluid. In the Figure 11 example, the device 1100 comprises an enclosure 1122
slidably received
within the outer chassis 1108 and shaped to accept a cartridge 1120.
[00188] The cartridge 1120 comprises a tube 1112 with an interior shot chamber
1114 that contains
a fluid. In some embodiments, shot chamber 1114 may carry medication, such as
ketamine of other
pharmaceuticals, for delivery to a patient's nasal cavity or olfactory region.
In some embodiments,
for any device disclosed herein, the shot chamber is removable from the
cartridge. In some
embodiments, the shot chamber may be removed and replaced with another shot
chamber. In some
embodiments, the shot chamber is refillable. In some embodiments, the shot
chamber comprises a
refillable cartridge. The shot chamber 1114 has a plunger 1116 on one end, and
a diaphragm 1110
on the opposite end from the plunger 1116. The device 1100 is configured such
that when a user
engages the actuator 1130, the fluid in the shot chamber 1114 is delivered
through the dispensing tip
with predetermined flow characteristics. In some embodiments, the dispensing
tip comprises a
flexible cannula or nib 102 configured to deliver a liquid jet, stream, burst
or plug. In some
embodiments, the liquid jet comprises laminar flow. In some embodiments, the
dispensing tip
comprises a flexible cannula or nib 102 configured to deliver a laminar liquid
slug, as described
above. In some embodiments, the fluid is delivered through the dispensing tip
with a controlled
velocity profile to limit shear forces on the fluid. In some embodiments, the
fluid is delivered at a
velocity from about 0.5 m/s to about 15 m/s. In some embodiments, the fluid is
delivered at a velocity
from about 1.5 m/s to about 9 m/s. In some embodiments, the fluid is delivered
at a velocity from
about 0.5 m/s to about 15 m/s. In some embodiments, the fluid is delivered at
a velocity from about
0.5 m/s to about 1.5 m/s, about 0.5 m/s to about 3 m/s, about 0.5 m/s to about
5 m/s, about 0.5 m/s to
about 9 m/s, about 0.5 m/s to about 12 m/s, about 0.5 m/s to about 15 m/s,
about 1.5 m/s to about 3
m/s, about 1.5 m/s to about 5 m/s, about 1.5 m/s to about 9 m/s, about 1.5 m/s
to about 12 m/s, about
1.5 m/s to about 15 m/s, about 3 m/s to about 5 m/s, about 3 m/s to about 9
m/s, about 3 m/s to about
12 m/s, about 3 m/s to about 15 m/s, about 5 m/s to about 9 m/s, about 5 m/s
to about 12 m/s, about
5 m/s to about 15 m/s, about 9 m/s to about 12 m/s, about 9 m/s to about 15
m/s, or about 12 m/s to
about 15 m/s, including increments therein. In some embodiments, the fluid is
delivered at a velocity
from about 0.5 m/s, about 1.5 m/s, about 3 m/s, about 5 m/s, about 9 m/s,
about 12 m/s, or about 15
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
31
m/s. In some embodiments, the fluid is delivered at a velocity from at least
about 0.5 m/s, about 1.5
m/s, about 3 m/s, about 5 m/s, about 9 m/s, or about 12 m/s. In some
embodiments, the fluid is
delivered at a velocity from at most about 1.5 m/s, about 3 m/s, about 5 m/s,
about 9 m/s, about 12
m/s, or about 15 m/s.
[00189] As described herein, in some embodiments and for any intranasal
delivery device described
herein, the dispensing tip is configured to be inserted into a subject's nasal
cavity. In some
embodiments, the dispensing tip comprises a fluid discharge orifice configured
to discharge fluid
from the dispensing tip to the olfactory region. As described herein, in some
embodiments, the
dispensing tip comprises a flexible nib or cannula configured to comply with
or conform to a surface
of a subject's intranasal passage, thereby enabling the fluid delivery orifice
of the dispensing tip to
be positioned within the intranasal cavity of a subject. In some embodiments,
the dispensing tip is
configured to be positioned in or near an olfactory region of the subject. In
some embodiments, the
dispensing tip is configured to be inserted within a subject's nasal cavity to
an insertion depth of at
least about lOmm to about 85mm. In some embodiments, the dispensing tip is
configured to be
inserted within a subject's intranasal cavity to an insertion depth of about 5
mm to about 100 mm. In
some embodiments, the dispensing tip is configured to be inserted within a
subject's intranasal cavity
to an insertion depth of about 5 mm to about 25 mm, about 5 mm to about 50 mm,
about 5 mm to
about 70 mm, about 5 mm to about 85 mm, about 5 mm to about 100 mm, about 25
mm to about 50
mm, about 25 mm to about 70 mm, about 25 mm to about 85 mm, about 25 mm to
about 100 mm,
about 50 mm to about 70 mm, about 50 mm to about 85 mm, about 50 mm to about
100 mm, about
70 mm to about 85 mm, about 70 mm to about 100 mm, or about 85 mm to about 100
mm, including
increments therein. In some embodiments, the dispensing tip is configured to
be inserted within a
subject's intranasal cavity to an insertion depth of about 5 mm, about 25 mm,
about 50 mm, about 70
mm, about 85 mm, or about 100 mm. In some embodiments, the dispensing tip is
configured to be
inserted within a subject's intranasal cavity to an insertion depth of at
least about 5 mm, about 25
mm, about 50 mm, about 70 mm, or about 85 mm. In some embodiments, the
dispensing tip is
configured to be inserted within a subject's intranasal cavity to an insertion
depth of at most about 25
mm, about 50 mm, about 70 mm, about 85 mm, or about 100 mm.
[00190] In some embodiments, for any embodiment herein, the dispensing tip
comprises a distal
portion that is softer than a proximal portion. In some embodiments, the
distal portion that is softer
than a proximal portion comprises a portion of the distal tip between about
lmm to about 15mm from
a distal end of the dispensing tip. In some embodiments, for any embodiment
herein, the dispensing
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
32
tip comprises a distal portion having a first rigidity and a proximal portion
having a second rigidity,
and wherein the first rigidity is less than the second rigidity. In some
embodiments, the distal portion
comprising a first rigidity comprises a portion of the dispensing tip that is
about lmm to about 15mm
from a distal end of the dispensing tip. In some embodiments, the dispensing
tip further comprises a
nose cushion to limit over-insertion of the dispensing tip within the
intranasal passage. In some
embodiments, the nose cushion is configured to provide a user comfort when the
dispensing is
inserted into a user's intranasal cavity. In some embodiments, the nose
cushion is removably attached
to the dispensing tip. In some embodiments, the dispensing tip has a proximal
portion having an outer
diameter that tapers towards the distal portion to provide comfort for the
user or to limit insertion
distance.
[00191] In some embodiments, the dispensing tip comprises a polymer. In some
embodiments, the
dispensing tip comprises thermoplastic polyurethane (TPU). In some
embodiments, the dispensing
tip comprises TPU at grade 65D, 57D, 95A, 90A, 80A, or any combination
thereof. In some
embodiments, the dispensing tip comprises high-density polyethylene (HDPE). In
some
embodiments, the dispensing tip comprises polyvinyl chloride (PVC). In some
embodiments, the
dispensing tip comprises a thermoplastic elastomer (TPE). In some embodiments,
the dispensing tip
comprises styrene-ethylene-butylene-styrene (SEBS). In some embodiments, the
dispensing tip
comprises low density polyethylene (LDPE). In some embodiments, the dispensing
tip comprises
silicone (e.g., liquid silicone rubber (LSR)). In some embodiments, the
dispensing tip comprises
polypropylene. In some embodiments, the dispensing tip comprises
polytetrafluoroethylene (PTFE),
such as for example, Teflon. In some embodiments, the dispensing tip comprises
thermoplastic
polyurethane (TPU), high-density polyethylene (HDPE), polyvinyl chloride
(PVC), a thermoplastic
elastomer (TPE), styrene-ethylene-butylene-styrene (SEBS), low density
polyethylene (LDPE),
silicone polypropylene. comprises polytetrafluoroethylene (PTFE), or any
combinations thereof.
[00192] In some embodiments and for any intranasal delivery device described
herein, the
dispensing tip comprises an inner diameter of at most about 1.0 mm. In some
embodiments, the
dispensing tip comprises an inner diameter of at most about 0.7 mm. In some
embodiments, the
dispensing tip comprises an inner diameter from about 0.5mm to about 1.0mm. In
some
embodiments, the dispensing tip comprises an inner diameter from about 0.3mm
to about 1.5mm. In
some embodiments, the dispensing tip comprises an inner diameter of at least
about 0.3 mm.
[00193] In some embodiments, plunger 1116 may be engaged by a push rod 1124.
In the Figure 11
example, the push rod 1124 is located at the bottom of the enclosure 1112, and
a spring 1134 is
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
33
compressed between the push rod 1124 and a push button 1132. In some
embodiments, the spring is
a variable pitch spring. A locking mechanism 1128 holds the push rod 1124 and
prevents it from
engaging with plunger 1116 until the push button 1132 is pressed. In the
illustrated example, the
locking mechanism 1128 comprise a pair of pivotable tabs with inner ends
engaging the push rod and
outer ends extending past the outer edges of the enclosure 1122 such that when
the enclosure 1122
is pushed into the chassis 1108 by pressing on the push button 1132 the tabs
pivot to release the push
rod 1124. In other embodiments, the locking mechanism may comprise one or more
tabs of a lock
material which is breakable by pressing on the push button 1132.
[00194] The diaphragm 1110 is puncturable by the needle 1106. Needle 1106
connects to channel
1104 in flexible nib 102, which may be inserted into the nasal cavity for
fluid delivery as described
above. When engaged, the fluid in shot chamber 1114 is forced through needle
1106 and channel
1104 into the nasal cavity. Arms 1126 may assist the user in gripping device
1100 and engaging push
button 1132.
[00195] In some embodiments, to assemble device 1100, cartridge 1120 may be
inserted into the
cartridge enclosure 1122. The cartridge enclosure 1122 may then be inserted
into outer chassis 1108.
In the illustrated example, the chassis 1108 comprises a resilient lip 1109
and the actuator opening
deforms slightly to receive the cartridge enclosure 1122 and cartridge 1120,
then holds them within
the chassis 1108. In other embodiments, seals may be added to assist in
detection of tampering.
[00196] Use of a cartridge may be advantageous in certain situations because
it is a commonly
manufactured vessel for medication and may be made of a material that is non-
reactive with
medication, such as glass.
[00197] Figure 12 shows an example intranasal drug delivery device 1100
according to some
embodiments, wherein cartridge 1120 is inserted in cartridge enclosure 1122
and the cartridge
enclosure 1122 is inserted in outer chassis 1108, but the actuator 1130 has
not been engaged by the
user and locking mechanism 1128 holds push rod 1124 such that plunger 1116 is
not engaged and
fluid in shot chamber 1114 is not under pressure. Arms 1126 may be folded
outward or inward against
outer chassis 1108. The device 1100 may be stored without the fluid in shot
chamber 1114 being
under pressure. Flexible nib 102 may be placed in the nasal cavity of the
patient prior to the actuator
1130 being engaged by the user.
[00198] Figure 13 shows an example intranasal drug delivery device 1100
according to some
embodiments, wherein the user has engaged the push button 1132, for example,
by pushing it with
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
34
their thumb. The user may hold the device 1100 in their hand using arms 1126
in a folded out
orientation. When user pushes the push button 1132, the locking mechanism 1128
releases push rod
1124. In some embodiments, the locking mechanism may comprise one or more tabs
that break off
to release push rod 1124, making the device 1100 useable only once. In other
embodiments, the
locking mechanism may comprise one or more tabs that fold or cantilever out of
the way to release
push rod 1124. When the locking mechanism 1128 is engaged it prevents the push
rod 1124 from
exerting pressure on the plunger 1116.
[00199] When the push rod 1124 presses against the plunger 1116 it puts the
fluid in shot chamber
1114 under pressure, and will move the cartridge 1120 toward the needle. In
some embodiments, a
spring 1134 may be included to such that the push rod 1124 exerts even
pressure on plunger 1116,
and once the locking mechanism 1128 is released the spring 1134 will cause
cartridge 1120 to move
further into outer chassis 1108 toward needle 1106 until needle 1106 punctures
diaphragm 1110. In
some embodiments a user continues to push on the push button 1132 to move the
cartridge 1120 into
outer chassis 1108 until the needle 1106 punctures diaphragm 1110.
[00200] In some embodiments, actuator 1130 may be a push button located at the
bottom of device
1100, in other embodiments, actuator 1132 may be located on the side of outer
chassis 1108.
[00201] In some embodiments, device 1100 may be designed for one-time use,
with a locking
mechanism 1128 comprising tabs that break off, or other sacrificial clips or
structures such that
cartridge enclosure 1122 may not be removed from outer chassis 1118 to replace
the spent cartridge
1120 with a new cartridge 1120 without the device 1100 being damaged.
[00202] Figure 14 shows an example intranasal drug delivery device 1100
according to some
embodiments, wherein the user has pushed the actuator 1130 such that it causes
the needle 1106 to
puncture diaphragm 1110 so that the tip of needle 1106 is in contact with the
fluid in shot chamber
1114. The fluid in shot chamber 1114 is under pressure from the plunger 1106
and may enter needle
1106 and flow through channel 1104 in nib 102. Fluid may flow through channel
1104 to be deposited
in the nasal cavity or olfactory region of a patient.
[00203] Figure 15 shows an example intranasal drug delivery device 1100
according to some
embodiments, wherein the user has pushed the actuator 1130 such that push rod
1124 has pushed
plunger 1116 to reach diaphragm 1110, ending the ejection of fluid. In some
embodiments, upon
pushing the actuator 1130, the push rod will first push the cartridge such
that the needle penetrates
the diaphragm, followed by the plunger being pushed by the push rod to
discharge the contents of
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
the shot chamber. In some embodiments, upon pushing the actuator 1130, the
push rod will push the
cartridge such that the needle penetrates the diaphragm, and the plunger being
pushed by the push
rod will discharge the contents of the shot chamber, wherein the device is
actuate through a single
actuation (or engagement) by the user.
[00204] In some embodiments, an intranasal drug delivery device as depicted in
FIG. 11 is provided,
wherein the cartridge does not comprise a diaphragm, such that actuation of
the device will engage
the push rod with the plunger, thereby pushing the fluid in the shot chamber
through the dispensing
tip and into the nasal cavity or olfactory region of a patient.
[00205] Figure 16 shows an external view of an example intranasal drug
delivery device 1100
according to some embodiments, wherein arms 1126 are hinged with hinge 1602
and may be folded
against outer chassis 1108 for storage, packing and transport. Hinge 1602 may
be a living hinge
comprised of thin material, for example.
[00206] Figure 17 shows an external view of an example intranasal drug
delivery device 1100
according to some embodiments, wherein arms 1126 are folded outward from the
outer chassis 1108,
providing a grip for the user when using the device 1100. In the folded out
position arms 1126 may
provide a grip for a user wearing gloves or a user with dexterity challenges.
[00207] Figure 18 shows an example intranasal drug delivery device 1100
according to some
embodiments, wherein the dispensing tip comprises an atomizer 1103 designed to
deliver a spray of
fluid into the nasal cavity rather than a laminar liquid slug.
[00208] In some embodiments, components and the configuration for any
intranasal drug delivery
device disclosed herein can be specified based on delivery fluid
characteristics, therapeutic
requirements, physiology of a patient's nasal anatomy, or combinations
thereof, so as to improve
drug delivery accuracy. In some embodiments, fluid characteristics include
volume, viscosity,
density, weight, or combinations thereof In some embodiments, components and
configuration that
can be specified include spring type and characteristics, dimensions of the
shot chamber, dispensing
tip length, dispensing tip flexibility, damper type, or combinations thereof.
[00209] FIGS. 76A to 79B illustrate an exemplary embodiment of an intranasal
drug delivery device
7600 according to some embodiments. FIG. 76A shows the device 7600 comprising
a dispensing tip
7602. In some embodiments, the dispensing tip is flexible, and configured to
comply with an
intranasal cavity of a subject, as described herein. In some embodiments, the
dispensing tip comprises
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
36
a nib. The dispensing tip may comprise a channel 7604. In some embodiments,
the device 7600 is
configured to receive a cartridge, which may be pre-filled with a fluid for
delivery into an intranasal
cavity of a subject. The device may further comprise a cartridge holder 7628.
As described herein, in
some embodiments, a cartridge comprises a cartridge 7620. In some embodiments,
the cartridge 7620
comprises a diaphragm 7610, shot chamber 7614, and plunger 7616. In some
embodiments, the shot
chamber 7614 is pre-filled with a fluid, such as for example a pharmaceutical
fluid. In some
embodiments, the device comprises a needle 7606 configured to penetrate the
diaphragm 7610.
[00210] The device 7600 may further be configured with a trigger 7636 to
depress a trigger paddle
7640. In some embodiments, the trigger 7636 is configured with a trigger
spring 7638 that is
configured to return the trigger 7636 to its original position. In some
embodiments, the trigger spring
7638 is a compliant member built directly into the trigger 7636. In some
embodiments, the device
further comprises a latch 7642 and latch spring 7644. The device may further
comprise a push rod
7624 that can be moved forward by a spring 7634. The device may comprise a
cartridge stop 7626 at
the top of the device.
[00211] FIG. 76B illustrates the device 7600 with the shot chamber moved
forward such that the
needle 7606 has penetrated the diaphragm 7610. In some embodiments the
cartridge holder 7628
bottoms out on the internal frame 7652. In other embodiments, the cartridge
holder 7628 bottoms
out through the cartridge 7620 and the cartridge stop 7626. In some
embodiments, the trigger paddle
7640 released the trigger latch 7642. In other embodiments, the trigger 7636
release the trigger latch
7642 directly. In some embodiments the device features an internal release
7650 that separates the
motions of the push rod 7624 from the cartridge holder 7628 only when the
cartridge holder has fully
bottomed out and the needle 7606 penetrates the diaphragm 7610. In some
embodiments, the internal
release comprises of a compliant clip as part of the cartridge holder 7628. In
some embodiments, the
compliant clip may be part of the push rod 7624. FIG. 76C illustrates the
device 7600 wherein the
push rod 7624 has moved the plunger 7614 forward so as to deliver a fluid
through the dispensing
tip 7602. FIG. 76D illustrates the device 7600 wherein a ring 7646 is shown to
be oriented and
positioned perpendicular to a longitudinal axis of the device 7600. In some
embodiments, the ring
7646 features a pin that prevents the cartridge 7620 from unintentionally
coming in contact with the
needle 7606. In some embodiments, the device 7600 comprises a cap 7648 which
included
protrusions that prevent the trigger 7636 from being unintentionally
depressed.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
37
[00212] FIG. 77A illustrates a side view of the device 7600. FIG. 77B
illustrates a side perspective
view of the device 7600. FIG. 77C illustrates a front view of the device 7600,
with a trigger 7636
visible.
[00213] FIG. 78A illustrates a side view of the device 7600. FIG without a
cap, thereby exposing
the dispensing tip 7602. FIG. 78B illustrates a side perspective view of the
device 7600 without a
cap, while FIG. 78C illustrates a side perspective view of the device 7600
with the trigger 7636
pushed in. In some embodiments, the top portion of the device is to act as a
nose pillow to prevent
inserting the cannula too far. In some embodiments, the top portion of the
device 7600 is adjustable
to either increase or decrease the depth of insertion of the cannula 7602.
[00214] FIGS. 79A-B illustrates a side view and side perspective view of the
device 7600
respectively, wherein the ring 7646 is shown to be oriented and positioned
perpendicular to a
longitudinal axis of the device.
[00215] In some embodiments, an intranasal drug delivery device as depicted in
FIG. 11 is provided
with a two-stage triggering mechanism wherein the needle and dispensing tip
are manually pushed
towards the cartridge, such that the needle pierces the diaphragm. The
actuator is then actuated, such
that push rod engages with the plunger to push the fluid within the shot
chamber through the
dispensing tip and into the nasal cavity or olfactory region of the patient.
[00216] Figures 19 a-c show an example intranasal drug delivery device 1900
according to some
embodiments, wherein a two-stage triggering mechanism is executed with a
single button push.
[00217] When actuator 1902 is first pushed by a user, the cartridge 1904 is
pressed into a needle
1906. The needle 1906 pierces the diaphragm 1908 (i.e. the cartridge septum)
and opens a fluid path
through the channel 1910 (cannula) as shown in Figure 19b. Actuator 1902 is
connected directly to
plunger 1914. When the actuator 1902 is pressed a second time by a user,
spring 1912 releases and
depresses the plunger 1914, ejecting fluid through the channel 1910 as shown
in Figure 19c.
[00218] Spring 1912 may be released by breaking a shear pin 1916 into pieces
1918 and 1920, as
shown in Figures 19b and 19c. In other embodiments the spring 1912 may be
released when injection
molded breakoff points or wings snap off of the plunger 1914. In other
embodiments the spring 1912
may be released by a ball detent mechanism, molded snap fit component or other
mechanism that is
activated by reaching a pre-set force. In still other embodiments the spring
1912 may be released by
the press force separating a magnet in the plunger from a magnet in the system
body.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
38
[00219] The travel of plunger 1914 is limited by a stop mechanism 1904 to set
a total dose. Stop
mechanism may comprise actuator projections 1922 that engage the base of the
cartridge 1924.
[00220] Figures 20 a-c show an example intranasal drug delivery device 1900A
according to some
embodiments, wherein a two-stage triggering mechanism is executed with a
single pushing motion.
In this embodiment, the actuator 1902A is connected to spring 1912A, which is
connected to plunger
1914A. After actuator 1902A is pushed by a user, the cartridge 1904A is
pressed into a needle 1906A
and the needle 1906A pierces the diaphragm 1908A and opens a fluid path
through the channel
1910A (cannula) as shown in Figure 20b, wherein as the user continues to push
on the actuator
1902A, this builds up spring force in the user's hand (or other method used to
press the button). When
sufficient spring force is achieved, the actuator 1902A is released. The
actuator 1902A may be
released by several different methods, as described above. The spring force
built up behind the
actuator 1902A then rapidly compresses the spring 1912A between the actuator
1902A and the
plunger 1914A. The spring 1912A then dispenses the fluid from the channel
1910A.
[00221] In some embodiments, the device comprises a damping mechanism,
examples of which are
described further below with reference to Figures 21-33. Elements such as the
dispensing tip, the
needle that pierces the diaphragm, and an outer body are not shown in all
views, but may be included
in some embodiments. In each of these example embodiments, the device
2100/2200/2300/2400/2500/2600/2700/2800/2900/3000/3100/3200/33 00 is
configured to eject a jet
of fluid through a channel with a controlled velocity profile. This assists in
preventing excessive
shear on the delivered drug, some of which may be damaged by shear. For
example, in some
embodiments the device is configured to eject a jet of fluid starting at a
high initial velocity but
dropping linearly to a near zero jet velocity at the end of j et dispensing.
[00222] Figure 21 shows an example device 2100 according to some embodiments,
wherein a
plunger 2102 is pushed by a spring 2104. In the Figure 21 embodiment, the
velocity of the plunger
2102 is controlled by an eddy current brake connected to the traveling end of
the spring 2104. In the
Figure 21 embodiment, the damping mechanism comprises a magnet 2106 connected
to the plunger
2102 moves through a conductive jacket 2108, generating eddy currents and
limiting the maximum
plunger speed. In another embodiment the velocity of the plunger 2102 may be
controlled by having
magnet 2106 spun by a helix on a shaft connected to the traveling end of the
spring (not shown). By
changing the geometry of the conductive jacket to generate more or less eddy
current at different
locations along the plunger travel, the velocity profile for the ejected
payload can be controlled.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
39
[00223] Figure 22 shows an example device 2200 according to some embodiments,
wherein the
velocity of the plunger 2202 travel is controlled by a damping mechanism
inherently formed by the
construction of the device 2200 and the materials chosen. For example, in some
embodiments part
tolerances and material variations are controlled to provide a plunger 2202
friction and spring 2204
K value configured to ensure desired jet velocity profile.
[00224] Figure 23 shows an example device 2300 according to some embodiments,
wherein the
velocity of the plunger 2302 is controlled by a damping mechanism comprising a
viscous damper
2304 connected to the traveling end of the spring 2306. The damper 2304 is
filled with air or with
viscous liquid (e.g. oil). The damper 2304 controls the velocity of the
traveling end of the spring
2306. Maximum velocity is limited by the damper 2304, and as the spring 2306
extends, it's driving
force decreases. This provides an initially high velocity followed by a
decrease in velocity over the
total dispensed volume. It may also provide a constant velocity over the total
dispensing.
[00225] Figure 24 shows an example device 2400 according to some embodiments,
wherein the
velocity of the plunger 2402 is controlled by a damping mechanism comprising a
sealed chamber
2404 attached to the back of the device 2400 connected to a spring 2408, which
is connected to the
plunger 2402. Air must be drawn into the chamber 2404 to allow the plunger
2402 to advance, but
air flow into the chamber 2404 is limited by ether 1) a flow control valve
(not shown) or 2) a simple
flow restriction 2406 (e.g. narrow channel, orifice plate).
[00226] Figure 25 shows an example device 2500 according to some embodiments,
wherein the
damping mechanism comprises a spring 2502 used to compress a body of air (e.g.
pushing on a
bellows, pushing on a diaphragm, pushing a piston) into a sealed chamber 2504.
The compressed air
flow through a flow restriction 2506 that controls air flow rate to the device
2500. The outside of the
device 2500 body seals to the sealed chamber 2504 (e.g. 0-ring seal). The air
then pushes on the back
side 2508 of the piston 2510, pushing the drug out of the channel 2512.
Because the flow rate of air
is controlled by the flow restriction 2506, the rate of travel for the piston
2510 is controlled. The flow
restriction 2506 may be simple, like an orifice plate, narrow tube, or narrow
drilled hole, but it may
also be a pneumatic device like a pressure relief valve, or flow control
valve.
[00227] Figures 26 a-b show an example device 2600 according to some
embodiments, wherein
control over the velocity of the plunger 2602 is be achieved by a damping
mechanism comprising a
container 2604 of compressed gas (e.g. CO2 canister, sealed canister of air,
N2, etc.). The container
2604 of compressed gas is connected to the flow restriction 2606 by piercing a
membrane 2608 or
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
septum or by connecting with a valve. A leak point may be added to the chamber
to cause pressure
applied to the device 2600 to dissipate over time. This provides a decreasing
velocity profile for the
fluid jet. The compressed gas container may be connected to the device 2600
chamber by piercing a
membrane on the canister, by a valve, or by a similar mechanism.
[00228] Figures 27 a-b show an example device 2700 according to some
embodiments, wherein the
damping mechanism comprises a piston 2702, sealed chamber 2704, pin and ball
valve 2706. In this
embodiment, the piston 2702 is moved and compressed gas in sealed chamber 2704
is provided
instantaneously using a mechanically operated valve such as pin and ball valve
2706. When the piston
2702 reaches the top of the chamber 2704, a pin 2708 is pushed by the piston
2702, opening a ball
valve 2710 to release pressure into the shot chamber 2712.
[00229] Figure 28 shows an example device 2800 according to some embodiments,
wherein a
plunger 2802 is pushed by an electric motor 2804 (e.g. stepper motor, DC
motor, brushless motor,
etc.) which provides the function of both actuating force and a damping
mechanism. Circuitry
onboard the electric motor 2804 controls the plunger 2802 velocity to set the
desired ejected fluid
velocity profile. Control of the electric motor 2804 may be open loop or
closed loop. Motor 2804
may be a liner motor, or a rotary motor combined with gearing, a linkage, cam,
lead screw, or other
mechanical element to drive the plunger 2802.
[00230] Figures 29 a-c show an example device 2900 according to some
embodiments, wherein
controlled jet velocity is provided by a damping mechanism comprising an
elastomeric chamber
2902. This occurs in two steps. First, the plunger 2904 is depressed to fill
the elastomeric chamber
2902, as shown in Figure 29b. Second the fluid path to the channel 2906 is
opened, now spring force
stored in the stretched elastomeric chamber 2902 forces the fluid out of the
channel 2906 as shown
in Figure 29c.
[00231] The flow resistance of the fluid path out of the elastomeric chamber
2902 is matched to the
stiffness of the elastomeric chamber 2902 to provide a controlled jet velocity
profile. As the
elastomeric chamber 2902 relaxes, the pressure on the fluid decreases, so this
provides an initial high
velocity followed by a decrease in jet velocity.
[00232] Figures 30 a-c show an example device 3000 according to some
embodiments, wherein a
cartridge 3002 is depressed to fill the elastomeric chamber 3004 and the fluid
path to channel 3006
is opened with a single motion. In this embodiment, a needle 3008 is partially
embedded in a septum
3010 to seal the end of the needle 3008, as shown in Figure 30a. First, as the
plunger 3016 moves,
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
41
the diaphragm 3012 is pierced. As the plunger 3016 continues to move as shown
in Figure 30b, the
elastomeric chamber 3004 is loaded with fluid. The spring 3014 prevents travel
of the cartridge 3003
until the plunger 3016 is sufficiently depressed. Third, the plunger 3016
travel ends, the spring 3014
is compressed, and the septum 3010 is pierced by needle 3008 as shown in
Figure 30c. Fourth, the
elastomeric chamber 3004 forces fluid out through the channel 3006. As the
elastic elastomeric
chamber 3004, pressure drops, providing a decreasing velocity profile. Chamber
geometry can be
varied to make a linear or non-linear decreasing velocity profile.
[00233] Figure 31 shows an example device 3100 according to some embodiments,
wherein a large
spring 3102 with a limited initial travel is used to break static friction in
the piston 3106 and a second
spring 3104 provides the force to fully dispense the drug. Large spring 3102
is a higher force spring
than second spring 3104. The flow path out of the channel 3108 is long enough
that the high velocity
travel from the large spring 3102 does not cause fluid to leave the channel
3108.
[00234] Figure 32 shows an example device 3200 according to some embodiments,
wherein the
flow rate of the jet is controlled by a flow restriction device 3202 between a
cartridge 3204 and a
channel 3206. The flow restriction device 3202 can be long and gradual to keep
a laminar flow
profile. This will prevent excessive shear on the delivered drug (e.g.
protecting the viability of
vaccines). The flow restriction device 3202 could also be more compact but
producing a turbulent
flow. The flow restriction could also be an orifice plate. This would make a
more compact device
suitable for delivering robust therapeutic agents. The flow restriction device
3202 could also be
replaced by an active element like a constant velocity flow control valve, a
pressure relief valve, or
a pressure control valve.
[00235] Figure 33 a-c show an example device 3300 according to some
embodiments, wherein the
plunger 3302 is driven by a spring 3304, but piston velocity is controlled by
bellows 3306 filled with
air. As the piston 3302 travels up, the bellows 3306 are compressed, and air
is forced through a flow
restriction 3308 (e.g. simple orifice plate, small drilled hole, pressure
control valve, flow rate control
valve). The rate that the bellows 3306 can deform is controlled by the rate of
air flow through the
flow restriction 3308. This could be accomplished by an arrangement where air
is contained in a
diaphragm 3310, rolling diaphragm or a piston as shown in Figures 33b and 33c.
It may also be
accomplished in the same configuration shown in Figure 33a but with a
diaphragm, rolling
diaphragm, or piston.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
42
[00236] Air may vent externally to the device, or it may vent into a secondary
chamber to avoid the
need for an external vent.
[00237] A prototype device including a cannula and damping mechanism has been
tested to
demonstrate targeted delivery of the fluid. The testing comprised inserting
the cannula into the upper
nares of a patient and ejecting a liquid jet or stream of fluid through the
cannula. In the testing,
technicium 99 was used as a tracer fluid. A scan of the patient performed
following the delivery of
the fluid show that the fluid is deposited at the olfactory region of the
patient 3600, as shown in
Figure 36. The presence of the technicium 99 appears as a light region on the
scan shown in Figure
36.
[00238] Figure 37A shows an exemplary embodiment of the dispensing tip 3700
used with an
intranasal drug delivery device described herein. In some embodiments, the tip
is flexible and has a
circular cross-section. In some embodiments, the tip is flexible and has a non-
circular cross-section.
In this embodiment (FIG. 37A), the tip has an elliptical cross section to
provide stiffness in the plane
of the major axis (of the elliptical shape) while maintaining flexibility in
the plane of the minor axis.
In some embodiments, the tip is inserted with additional stiffness in the
sagittal plane (e.g., along the
major axis of an elliptical cross-section), so it can maintain its shape and
stay aligned with the target
site (Figure 37B). However, the tip's flexible plane (e.g., along the minor
axis of an elliptical cross-
section) allows the tip to conform to patient anatomy perpendicular to the
sagittal plane (Figure 37C)
[00239] Figure 38A shows an exemplary embodiment of the dispensing tip 3800
used with an
intranasal drug delivery device described herein. In some embodiments, the tip
3800 includes a bent
section 3801 to direct an ejected drug away from a longitudinal axis of a
straight section 3802 of the
dispensing tip. This allows a more vertical insertion of the tip while still
directing a drug ejection
toward a target site (Figure 38B) within a patient's nasal cavity. As
described herein, for any
embodiment, delivery of a drug may comprise delivery of a fluid. In some
embodiments, the fluid
comprises a liquid, a gel, a powder, or any combinations thereof In some
embodiments, the drug
comprises a powder suspended in a liquid or gaseous fluid. In some
embodiments, the drug comprises
a powder that is delivered by the device.
[00240] Figure 39A shows an exemplary embodiment of the dispensing tip 3900
used with an
intranasal drug delivery device described herein. In some embodiments, the tip
3900 is shaped with
several curves to conform to the shape of the patient's nasal valve 3901 while
still directing drug
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
43
ejection to a target site. In some embodiments, the tip 3900 may conform to
the nasal valve with
flexible sections.
[00241] Figure 40 shows a possible accessory for a dispensing tip used with an
intranasal drug
delivery device described herein. In some embodiments, the dispensing tip 4001
further comprises a
base of compliant material 4000 (e.g. foam, silicone, memory foam). In some
embodiments, the base
of compliant material 4000 protrudes from a dispensing tip body 4001. In some
embodiments, when
inserted, the compliant base contacts the nasal valve, which locates the
dispensing tip and prevents
over insertion of the dispensing tip within the nasal cavity.
[00242] Figure 41A shows an exemplary embodiment of the dispensing tip 4100
used with an
intranasal drug delivery device described herein. In some embodiments, the tip
4101 is curled over,
and the curled section of the tip includes one (or more) perforations 4102. In
some embodiments, the
curved shape aids in insertion and orientation within the nasal cavity. In
some embodiments, the tip
4100 is made from a soft material, such that the tip could be placed in
contact with a target site within
a patient's nasal cavity as shown in Figure 41B and used to gently dispense
the drug directly to the
target site.
[00243] Figures 42A-B show additional exemplary embodiments of the dispensing
tip used with an
intranasal drug delivery device described herein. In some embodiments, the
dispensing tip comprises
a soft end 4200 or a ball end 4201 to prevent accidental damage to the patient
and to improve patient
comfort on insertion.
[00244] Figures 43A-B shows an exemplary embodiment of the dispensing tip 4300
used with an
intranasal drug delivery device described herein. In some embodiments, the end
of the dispensing tip
includes a rounded end 4301, an off-center drug dispensing channel 4302, and a
flexible section 4303.
When inserted, the rounded end 4301 contacts a surface within a patient's
nasal cavity (Figure 43B)
and the tip 4300 flexes (via flexible section 4303) to align the drug
dispensing channel 4302 with a
target site within the patient's nasal cavity (Figure 43C).
[00245] Figure 44A shows an exemplary embodiment of the dispensing tip 4400
used with an
intranasal drug delivery device described herein. In some embodiments, the tip
includes a flexible
section 4401 and a soft protruding element 4402. When inserted (Figure 44B),
the protruding element
contacts the nasal valve of a patient. As the tip 4400 is further inserted,
the flexible section 4401
bends to point the tip at the target site (Figure 44c).
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
44
[00246] Figure 45A shows an exemplary embodiment of the dispensing tip 4500
used with an
intranasal drug delivery device described herein. In some embodiments, the
dispensing tip includes
a balloon 4501. After insertion (Figure 45B), the balloon is inflated (Figure
45C). When inflated, the
balloon conforms to the patient's unique anatomy and points the tip towards a
target site within a
patient's nasal cavity. The balloon may also be used to open up the nasal
cavity to aid in drug
dispensing.
[00247] Figure 46A shows an exemplary embodiment of the dispensing tip 4600
used with an
intranasal drug delivery device described herein. The tip has a curled over
tip 4601. In some
embodiments, when a drug is forced into the tip 4600, fluid pressure causes
the curled over portion
4601 of the tip to unroll gently within a patients' nasal cavity (Figure 46B).
In some embodiments,
when completely unrolled (Figure 46B), the tip opens under pressure and the
drug is dispensed to a
target site with the patient's nasal cavity.
[00248] Figure 47A shows an example embodiment of the dispensing tip 4700 used
with an
intranasal drug delivery device described herein. In some embodiments, the
dispensing tip 4700
comprises an inflatable balloon 4701 at a terminal end of the dispensing tip
4700 with an exit port
4702 integrated into the balloon 4701. After insertion (Figure 47B), the
balloon 4701 is inflated.
When inflated, the balloon 4701 fills at least a portion of the nasal cavity
near a target site and creates
a sealed chamber around the target site (Figure 47C). Drug is then dispensed
through the integrated
exit port 4703 directly onto the target site. This technique provides the
opportunity to cause a flow
of drug into the target site with positive pressure which can increase the
rate of uptake for the drug.
[00249] Figures 48A-D show an exemplary method for improving patient comfort
while inserting
a dispensing tip into a patient's nasal cavity. In some embodiments, a
hydrophilic coating 4801 is
applied to the outer surface of a dispensing tip 4802 used with an intranasal
drug delivery device
described herein, wherein the dispensing tip is contained in a packaging 4803
(Figure 48A). When
this coating 4801 is activated by contact with a hydrating medium (e.g.,
water, a gel), the coating
4801 activates to become a low friction surface that aids in insertion into a
patient's nasal cavity. The
most common form of this product is where a sterile, individually packaged
single or multi use
dispensing tip is packaged in a dry state or condition (Figure 48A). The user
opens 4805 the package
and fills the package with a hydrating medium 4804 (Figure 48bB). After
waiting an appropriate time
(e.g. 30 seconds), the coating 4801 is activated (Figure 48C). The user then
removes the dispensing
tip 4802 from the package, and the tip is ready for insertion (Figure 48D).
Rather than filling the
package with hydrating medium, the user may provide a container of hydrating
medium, remove the
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
dispensing tip from the package, and place the dispensing tip into the
container of hydrating medium.
The user submerges the tip in hydrating medium, waits for the hydrophilic
coating 4801 to activate,
and then removes the tip for use (Figure 48D)
[00250] Figures 49A-C show an exemplary method for improving patient comfort
while inserting
a dispensing tip into a patient's nasal cavity 4900. In some embodiments, a
hydrophilic coating 4901
is applied to the outer surface of a dispensing tip 4902 used with an
intranasal drug delivery device
described herein, wherein the dispensing tip is contained in a package 4903
(Figure 49A). The
dispensing tip is submerged in a hydrating medium 4904 (e.g., water, a gel)
within the package 4903.
In some embodiments, the hydrophilic coating 4901 is allowed to activate
during
manufacture/transport so that the dispensing tip 4902 is ready for use before
the package is given to
the user. The user opens 4905 the package (Figure 49B) and removes the
dispensing tip 4902 ready
for use (Figure 49C).
[00251] Figures 50A-D shows an exemplary method for improving patient comfort
while inserting
a dispensing tip into the nose 5000. In some embodiments, a hydrophilic
coating 5001 is applied to
the outer surface of the dispensing tip 5002 used with an intranasal drug
delivery device described
herein, wherein the dispensing tip is contained in a package 5003(Figure 50A).
In some
embodiments, the package further comprises a separated compartment 5006 that
contains a sufficient
amount of hydrating medium 5004 (e.g., water, a gel) to activate the
hydrophilic coating 5001. In
some embodiments, the separated compartment 5006 is a different compartment
from a compartment
containing the dispensing tip 5002. In some embodiments, the separated
compartment 5006 is
configured to be opened, so as to allow the hydrating medium 5004 to cover the
hydrophilic coated
surface 5001 (Figure 50B). In this embodiment, a membrane separating the
separated compartment
5006 from the compartment containing the dispensing tip 5002 is burst 5005
(e.g., via pressure build-
up within the compartment 5006 by a user pressing the compartment 5006). In
some embodiments,
the user continues to press on the compartment 5006 until the dispensing tip
5002 is at least partially
submerged in the hydrating medium 5004. Depending on the product, and on the
amount of hydrating
medium in the separate compartment 5006, the package may be manipulated to
fully cover the
dispensing tip 5002 in the hydrating medium 5004. After waiting an appropriate
time (e.g. 30
seconds) after submerging the dispensing tip 5002 at least partially in the
hydrating medium 5002,
the hydrophilic coating 5001 is activated. In some embodiments, the package
5003 (Figure 50C) is
opened 5007. In some embodiments, the dispensing tip 5002 is removed from the
package, and the
tip 5002 is ready for use (Figure 50D). In some embodiments, the package may
include features to
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
46
prevent the hydrating medium 5004 from flowing back into the compartment 5006
(e.g. the
compartment 5006 may be configured to be rolled up and held in place, the
compartment 5006 may
have a one way valve, the upper and lower half of the compartment 5006 may
snap together, or the
c0mpartment55006 may be separated from the compartment containing the
dispensing tip by a
clamp)
[00252] In some embodiments, for any of the methods disclosed in Figures 48A
to 50D, the
hydrating medium may be a gel or a viscous liquid that is less likely to spill
when handling the
package (e.g., handling the dispensing tip and removing the dispensing tip
from the package) than a
liquid hydrating medium like water.
[00253] Figures 51A-D show an exemplary method for improving patient comfort
while inserting
a dispensing tip into a patient's nasal cavity, wherein the method reduces the
risk of spilling a
hydrating medium used to activate a hydrophilic coating. Here, the hydrophilic
coating 5101 is
activated by a hydrating vapor 5105 (e.g. water vapor) rather than by liquid
or gel. A hydrophilic
coating 5101 is applied to the outer surface of the dispensing tip 5102 used
with an intranasal drug
delivery device described herein, and the dispensing tip is contained in a
package 5103. The package
contains a body of a hydrating medium 5104 (e.g., water, gel) that is
contained within a piece of foam
5106. In some embodiments, the foam 5106 prevents loose liquid from presenting
a spill hazard. The
hydrating medium 5104 may also be contained in a piece of fabric, a porous
plastic body, a porous
ceramic body, or a similar wicking body. The hydrating medium 5104 may also be
contained in a
solid material that is saturated with the hydrating medium 5104 (e.g. nylon
plastic soaked in water).
The hydrating medium 5104 may also be a gel. The hydrating medium 5104 may
also be placed in a
separate pocket within the package that is separated from the dispensing tip
by a vapour permeable
but hydrating medium impermeable membrane. The hydrating medium 5104 may also
be contained
within a separate tortuous chamber that is directly connected to a chamber
containing dispensing tip
5102. The tortuous path minimizes the chance of spilling the hydrating medium
5104. During
manufacture or transport, the package 5103 is left closed long enough such
that the package 5103
fills with hydrating vapor 5105, the vapor activates the hydrophilic coating
5101, and the dispensing
tip 5102 becomes ready for use before the package reaches a user (Figure 51A).
The package (Figure
51B) is then opened 5107, wherein the dispensing tip 5102 is removed and ready
for use (Figure
51C)
[00254] Figures 52A-D show an exemplary method for improving patient comfort
while inserting
a dispensing tip into a patient's nasal cavity. In some embodiments, a user is
provided with a package
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
47
5201 that contains lubrication gel 5202 and may include a narrow dispensing
channel 5203 (Figure
52A). In some embodiments, the user may also be provided with any other
standard package of gel
(e.g. blow mold fill ampule, square tear open pouch, etc.). In some
embodiments, the user opens the
package of gel (Figure 52B) and applies the lubrication gel 5202 as a coating
to the surface of the
dispensing tip 5204 used with an intranasal drug delivery device described
herein (Figure 52C). The
dispensing tip 5204 is now ready for use (Figure 52D).
[00255] Figures 53A-D show an exemplary method for improving patient comfort
while inserting
a dispensing tip into a patient's nasal cavity. In some embodiments, a user is
provided with a package
5301 that contains lubrication gel 5302 (Figure 53A). In some embodiments, the
user opens the
package5301 of the lubrication gel 5302 (Figure 53B) and dips a dispensing tip
5303 used with an
intranasal drug delivery device described herein into the lubrication gel 5302
(Figure 53C). In some
embodiments, the user then removes the dispensing tip 5303 (Figure 53D) ready
for use.
[00256] Figures 54A-C show an exemplary method for improving patient comfort
while inserting
a dispensing tip into a patient's nasal cavity. In some embodiments, a
dispensing tip 5401 used with
an intranasal drug delivery device described herein is contained in a package
5402 that is filled with
lubricating gel 5403. In some embodiments, a user opens the package 5402
(Figure 54B) and removes
the dispensing tip 5401 (Figure 54C) containing a coating of the lubricating
gel 5403, ready for use.
[00257] Figures 55A-C show an exemplary method for improving patient comfort
while inserting
a dispensing tip into a patient's nasal cavity. In some embodiments, a
dispensing tip 5501 used with
an intranasal drug delivery device described herein is contained in a package
5502. In some
embodiments, a user opens the package 5502 and fills the package with
lubricating gel 5503 (Figure
55B). The user then removes the dispensing tip 5501 from the package 5502, and
the dispensing tip
5501 containing a coating of the lubricating gel 5503 is ready for use (Figure
55C). In some
embodiments, the user may be instructed to manipulate the package 5502 to
ensure the dispensing
tip 5501 is fully covered in the lubricating gel 5503. The user may be
supplied with a separate package
(pouch, bottle, etc.) of the lubricating gel, or they may provide their own
gel.
[00258] Figures 56A-D show an exemplary method for improving patient comfort
while inserting
a dispensing tip into a patient's nasal cavity. In some embodiments, the
dispensing tip 5601 used with
an intranasal drug delivery device described herein is contained in a package
5602 (Figure 56A). In
some embodiments, the package further comprises a separate compartment 5605
containing a
sufficient amount of lubricating gel 5603 to coat the dispensing tip 5601. In
some embodiments, the
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
48
separated compartment 5605 is a different compartment from a compartment
containing the
dispensing tip 5601. In some embodiments, a user enables for a pathway between
the separate
compartment 5605 and the compartment containing the dispensing tip 5601 to be
opened, allowing
a hydrating medium (e.g., lubricating gel 5603) to cover the at least a
portion of the dispensing tip
(Figure 56B). In this embodiment, the user presses on the compartment 5605
causing a membrane
5604 to burst. The user continues to press on the compartment 5605 until the
dispensing tip is at least
partially submerged in the lubricating gel 5603. Depending on the product and
on the amount of gel
5603 in the separate chamber 5605, to the package 5602 may be manipulated to
fully cover the
dispensing tip 5601 in the hydrating medium (e.g., lubricating gel 5603). The
user may open the
package (Figure 56C), and then remove the dispensing tip 5601 from the package
5602, wherein the
tip 5601 is ready for use (Figure 56D). The package may include features to
prevent the lubricating
gel from flowing back into the separate compartment 5605 (e.g. the compartment
5605 may be rolled
up and held it in place, the compartment 5605 may have a one way valve, the
upper and lower half
of the compartment 5605 may snap together, the compartment 5605 may be
separated from the
compartment containing the dispensing tip 5601 by a clamp).
[00259] Figures 57 to 61 and Figures 42A to 42c illustrate exemplary flow
restriction mechanisms
that could be used with an intranasal drug delivery device described herein
(e.g., a device shown in
Figure 4, 5, 11, 12, 13, 14 15, 18, 19a to 19c, 20a to 20c, 22, 29a to 29c,
30a to 30c, 31, 32, 34, and
35). In some embodiments, the flow restriction mechanism(s) described herein
could also be applied
to the flow path of the drug out of devices shown in Figures 26a, 26b, 27a,
and 27b.
[00260] In Figure 57, in one embodiment of the device 5700, the drug 5701 is
forced through the
flow restriction 5702 as part of its path out of the dispensing tip 5703. In
this device embodiment
3700, the flow restriction 5702 is formed as part of the general architecture
of the device 5700. In
this device 3700, the flow restriction is a needle piercing a septum to
connect the dispensing tip 5703
to the drug container, wherein the flow restriction comprises a narrow inner
diameter. The flow
restriction 5702 could also be a general flow path molded into the dispensing
tip or the device 5700,
or it could be an element with a narrow internal pathway added as an insert.
[00261] In Figures 58A-B, an exemplary flow restriction mechanism is provided,
wherein a drug is
forced through the flow restriction 5802 as part of its path out of the
dispensing tip. In this
embodiment, the flow restriction 5802 is a constant flow rate valve. As fluid
pressure applied to the
flow restriction 5802 increases, it will constrict (FIG. 58A) so as to limit
the flow therethrough and
maintain a constant or nearly constant flow rate of fluid over a range of
pressures. As the fluid
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
49
pressure reduces, the flow restriction 5802 will allow more the flow
therethrough (FIG. 58B). This
effect can be used to maintain a constant drug ejection velocity as pressure
applied to the flow
restriction shifts due to component tolerances and environmental conditions.
[00262] In Figure 59, a drug 5901 is forced through the flow restriction 5902
as part of its path out
of the dispensing tip 5903. In this embodiment of the device 5900, the flow
restriction 5902 comprises
a body of porous media (e.g. ceramic, open cell foam, etc). The porous media
provides an advantage
over other damping elements by allowing large pressure drops within a small
volume, and by
providing large pressure drops within a liquid jet or laminar flow regime.
[00263] In Figure 60, a drug 6001 is forced through the flow restriction 6002
as part of its path out
of the dispensing tip 6003 of the device 6000. In some embodiments, the flow
restriction 6002 is a
layer of porous membrane. In some embodiments, the porous membrane allows for
a large pressure
drop, and producing a diffuse flow over the whole area of the flow channel,
which may allow for
faster development of liquid jet or laminar flow within the dispensing tip
compared to other damping
methods (e.g., an orifice plate). Thus, in some embodiments, the porous
membrane may result in a
more compact device 6000 since a shorter distance of a dispensing tip is
needed to achieve laminar
flow of a drug exiting the device (if desired).
[00264] In Figure 61, the drug 6101 is forced through the flow restriction
6102 as part of its path
out of the dispensing tip 6104 of the device 6100. In this device 6100, the
flow restriction 6102 is
configured with an orifice plate 6103. The orifice plate flow restriction 6102
allows for large pressure
drops over a small distance, wherein the dynamics of an orifice plate are well
understood.
[00265] In Figure 62A, a drug 6201 is forced through the flow restriction 6202
as part of its path
out of the dispensing tip of the device 6200. In some embodiments, the flow
restriction 6200 is
replaced by a first alternate variable flow restriction 6202a (Figure. 62B), a
second alternate variable
flow restriction 6202b (Figure. 62C), or a third alternate variable flow
restriction 6202c (Figure.
62D). In some embodiments, the first alternate variable flow restriction 6202a
is constructed from a
stack of flexible washers, and it provides a progressive damping response
where additional force
reduces the flow restriction (top depiction provides a closed position of flow
restriction 6202a, and
bottom depiction provides open position). In some embodiments, the second
alternate variable flow
restriction 6202b is constructed from a stack of flexible washers, and it
provides a digressive damping
response where additional force results in additional flow restriction (top
depiction provides a closed
position of flow restriction 6202b, and bottom depiction provides open
position). In some
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
embodiments, the third alternate variable flow restriction 6202c is
constructed as a single molded
part and provides a progressive damping response (top depiction provides a
closed position of flow
restriction 6202c, and bottom depiction provides open position). An exemplary
depiction of the
relationship between the force applied by progressive and digressive damping
and the resulting fluid
velocities is shown in Figure 62C.
[00266] Flow restrictions 5700/5800/5900/6000/6100/6200/6202a/6202b/6202c,
could also be
placed in the air pathway of concepts shown in Figure 24, 25, and 33a to 33b
to control travel rate of
the plunger.
[00267] Figure 63A shows an exemplary intranasal drug delivery device 6300
according to some
embodiments. In some embodiments, a mass 6301 is accelerated by spring 6302.
In some
embodiments, the mass stops accelerating once the spring travel is stopped at
a hard stop 6303 (e.g.,
a protrusion of a casing about a spring that contacts another portion of the
device 6300, thereby
preventing the spring to continue to move towards the dispensing tip 6306). In
some embodiments,
the spring 6302 and location of the hard stop 6303 are configured to stop the
mass 6301 accelerating
at a predetermined velocity. In some embodiments, the mass 6301 strikes a
plunger 6304 of a drug
container 6304 containing a drug 6307, forcing the drug through a flow
restriction 6305 and into the
dispensing tip 6307. The flow rate of the drug 6307 out of the device 6300 is
determined by the travel
rate of the mass 6301 as it decelerates. With low mass and high pressure drop,
the mass will
experience significant deceleration and the drug will be ejected with a
decreasing velocity profile.
With a large mass and a low pressure drop, an approximately constant velocity
profile of the drug
being ejected can be achieved.
[00268] Figure 74 shows an exemplary intranasal drug delivery device 7400
according to some
embodiments. In some embodiments, a drug is driven out of the drug container
7401 by a spring 7402
connected to a block of open cell foam 7403. In some embodiments, as the
spring dispenses the drug,
air trapped within the open cell foam 7403 is forced out of the pores of the
foam. In some
embodiments, the flow of air through the pores is restricted because of the
small size of the pores. In
some embodiments, the restricted flow of air provides viscous damping that
controls the speed of
travel of the drug container plunger and thus controls the flow rate of drug
out of the device.
[00269] Figures 64A-D shows an exemplary intranasal drug delivery device 6400
according to
some embodiments. In some embodiments, activation of a first spring 6404 of
the device 6400
enables a drug container containing a drug 6405 to be in fluidic communication
with a delivery
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
51
chamber 6401 (e.g. the body of the dispensing tip), wherein the drug is pushed
out of the drug
container by the first spring 6404 into the delivery chamber 6401 (Figures 64A-
B). In some
embodiments, a second spring 6406 pushes a secondary fluid 6402 (e.g. air)
into the delivery chamber
6401 (Figure 64C) via a second plunger or piston 6407, wherein the secondary
fluid is stored in
another chamber. In some embodiments, a pump is used to push the secondary
fluid 6402 into the
delivery chamber 6401 (e.g., a piston pump with piston 6407). In some
embodiments, the path for
the drug back into the drug container is blocked by a one-way valve, closed
valve, or similar feature.
In some embodiments, the drug container is a cartridge and the cartridge
plunger 6408 closes the path
back into the drug container by contacting the needle at the end of its
travel. In some embodiments,
the drug 6405 is forced out of the dispensing tip by the secondary fluid 6402
by the secondary spring
6406 and/or when a pump is activated (Figure 64D). In some embodiments, the
secondary fluid 6402
(e.g., air) is moved by a spring driven piston pump and the flow rate of the
piston pump is controlled
by a flow restriction 6403 in the air path to the delivery chamber 6401.
[00270] Figures 65A-C shows an exemplary intranasal drug delivery device 6500
according to some
embodiments. In some embodiments, a drug container 6508 containing a drug 6505
is a ridged
container toped with a septum (e.g. an ampule). In some embodiments, upon
activation of the device
6500, two needles are inserted into the drug container 1) a first needle 6501
in fluidic communication
with a dispensing tip 6506 and 2) a second needle 6502 in fluidic
communication with a secondary
chamber 6507 containing a secondary fluid 6503 (e.g., air) (Fig 65B). In some
embodiments, a pump
pushes the secondary fluid 6503 into the drug container 6508, and this forces
the drug 6505 out of
the dispensing tip 6506 (Fig 65C). The flow rate of the drug 6505 exiting the
device 6500 is controlled
by controlling the flow rate of secondary fluid 6503 into the drug container.
In some embodiments,
the secondary fluid (e.g., air) is moved by a spring driven piston pump 6509
and the flow rate of the
secondary fluid 6503 is controlled by a flow restriction 6504 in the air path
to the drug container.
[00271] Figures 66A-D shows an exemplary intranasal drug delivery device 6600
according to
some embodiments. In some embodiments, a drug 6603 is dispensed from a drug
container using a
pump to propel the drug 6603 out of the device 6600. ((Figures 66C-D). In this
embodiment, the
pump is a peristaltic pump integrated into the dispensing tip body 6601
(Figures 66A-B), but the
pump may be a piston pump, peristaltic pump, gear pump, bellows, elastic
chamber, or similar device.
The flow rate for ejecting the drug 6603 is controlled by or determined by the
operation of the pump.
Here flow rate of the drug 6603 exiting the device 6600 is determined by the
rate of travel of roller
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
52
6602. In some embodiments, the roller 6602 is driven by a spring combined with
a dashpot damper
to provide a controlled flow rate. The pump may also be driven by electronic
drive system.
[00272] Figure 67A shows an exemplary intranasal drug delivery device 6700
consisting of a
disposable element 6701 and a reusable dispensing tool 6702. In some
embodiments, the disposable
element 6701 contains a drug 6705 for storage and provides clean and sterile
surfaces to contact the
patient. In some embodiments, only the disposable element 6701 comes in
contact with the drug
6705. In some embodiments, the disposable element 6701 may come in a sterile
package ready for
use. The disposable element 6701 may provide a surface that covers the end of
the dispensing tool
6702 for contacting the exterior of the patients nose and the patients face,
or portions of the dispensing
tool 6702 may be wiped down or otherwise cleaned/sterilized (e.g. UV
sterilization) to ensure safe
contact with the patient and safe use between patients. In some embodiments,
the disposable element
6701 contains a dispensing tip 6703, drug formulation container 6704, a volume
of drug 6705, and a
burst membrane 6706. The reusable dispensing tool 6702 contains an interface
to connect to the
disposable element 6701 (e.g. a taper fit, a socket, a snap fit, a threaded
stud), a moving element 6707
to press on the disposable element 6701, a drive system 6708 for the moving
element, and a trigger
6709. In this embodiment, the disposable element 6701 is contained in a
socket, the moving element
6707 is a roller, the drug container 6704 is a flexible bag, and the drive
system 6708 is an electric
motor. The moving element may also be a plunger, a cam, or a bag/bellows
inflated by the drive
system. The drug container may be any standard drug container with at least
one movable surface
(e.g. cartridge, blister pack, blow mold fill container), or it may be a
custom molded chamber with at
least one movable or flexible surface (e.g. a ridged molded cartridge capped
with a flexible
membrane). The burst membrane may be a plastic membrane, a one-way valve, a
ridged cap that
disconnects with a snap fit, a pressure relief valve, or any other mechanism
that allows fluid to flow
only after a prescribed minimum pressure is applied. The drive system may
consist of a spring,
pressurized gas, or vacuum chamber. The drive system may be energized by force
applied by the user
in an activation step. In some embodiments, to use the device, a user attaches
the disposable element
6701 to the dispensing tool 6702 and inserts the dispensing tip 6703 into
their nose. In some
embodiments, when the user presses trigger 6709, the moving element 6707
presses on the drug
container 6704 (Figure 67B). This pressurizes the drug container body and
causes the burst
membrane 6706 to break (Figure 67B). In some embodiments, the moving element
6707 then
continues to move at a controlled velocity (Figure 67C), which dispenses the
drug 6705 out of the
device 6700 with a controlled flow rate into the nose. Velocity control for
the moving element may
be provided by feedback or control, open loop control if the drive element is
electric. Velocity control
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
53
may also be provided by a viscous damping element if the system is driven
mechanically. The moving
element may pause momentarily after the burst membrane breaks. The reusable
tool may include an
eject button 6710 to easily remove the disposable element 6701. The eject
button may both eject the
disposable element 6701 and energize the drive system with one press. Both the
trigger and the eject
button may be a button, a slider, or a switch.
[00273] Figure 68A shows an exemplary intranasal drug delivery device 6800
comprising a
disposable element 6801, a reusable dispensing tool 6802, and a separate drug
container 6803. In
some embodiments, the disposable element 6801 comprises a dispensing tip 6804
and may contain a
membrane or similar barrier 6805 to a) prevent fluid moving into the reusable
dispensing tool 6802
and/or b) prevent contaminants (e.g. dust, bacteria, oils, etc.) entering the
disposable element 6801.
In some embodiments, the disposable element 6801 may have an open construction
without a barrier.
The disposable element 6801 may be a single molded piece of plastic or
elastomer. Here the
disposable element 6801 can provide clean and sterile surfaces to contact the
patient. In some
embodiments, only the disposable element 6801 comes in contact with the drug.
The disposable
element may come in a sterile package ready for use. The disposable element
6801 may provide a
surface that covers the end of the dispensing tool 6802 for contacting the
exterior of the patients nose
and the patients face, or portions of the dispensing tool 6802 may be wiped
down or otherwise
cleaned/sterilized (e.g. UV sterilization) to ensure safe contact with the
patient and safe use between
patients. In this embodiment, the drug container 6803 is a screw top vial. In
some embodiments, the
drug container 6803 may be a blow mold fill ampule, blister pack, or any other
standard drug
container. In some embodiments, the reusable dispensing tool 6802 comprises a
means to draw a
vacuum and/or to pump air into the disposable element. In this embodiment,
vacuum and air pressure
is generated using a piston 6806, a drive element 6807, and a trigger 6808.
The drive element 6807
may be an electric motor, solenoid, voice, coil, spring/damper system, spring,
or other similar
mechanism. In some embodiments, to use the device 6800, the disposable element
6801 is attached
to the reusable tool 6802 (Figure 68A). In this embodiment, the disposable
element 6801 force fits
onto the reusable tool 6802 with a taper fit. The disposable element 6801 may
also be coupled to the
reusable tool 6802 using a screw, socket, ball detent, or a standard pneumatic
or hydraulic interface.
In some embodiments, the drug container 6803 is opened and the disposable
element is inserted into
the volume of a drug 6810 (Figure 68B). In some embodiments, once the trigger
6808 is pressed, the
piston 6806 draws a vacuum. In some embodiments, the vacuum draws a measured
dose of the drug
6810 into the dispensing tip 6804 of the disposable element (Figure 68B). In
some embodiments, the
device is then removed from the drug container 6803 and inserted into a
patient's nasal cavity. In
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
54
some embodiments, the trigger is pressed a second time (or a secondary trigger
is pressed), and then
the device pumps air into the dispensing tip 6804 of the disposable element
6801 with a controlled
flow rate. In some embodiments, the air pushes the drug 6810 out of the
disposable element and into
the patient's nasal cavity (Figure 68C) at a controlled flow rate. The
reusable tool 6802 may also
contain an eject button 6809 to quickly remove the disposable element 6801.
The eject button 6809
may both eject the disposable element 6801 and energize the drive system 6807
with one press. Both
the trigger and the eject button may be a button, a slider, a switch, or other
standard user interface.
This device may be used without a drug formulation container 6803. In one
embodiment, the device
is used without a drug formulation container in a setting where the drug (e.g.
stem cells) is prepared
on site, transferred to an open container, loaded into the device as shown in
Figure 68B, and given
immediately to the patient.
[00274] Figure 69A shows an exemplary dispensing tip 6900 for an intranasal
drug delivery device
3900 described herein. In some embodiments, the dispensing tip 6900 is
integrated into an endoscope
so the user can control the shape of the dispensing tip as it is inserted
(Figure 69B-D). In some
embodiments, this allows a user to compensate for anatomical variation between
patients and
accurately aim the dispensing tip at a target site within a patient's nasal
cavity. The dispensing tip
may also include a fiber optic cable to visualize the target site.
[00275] Figure 70 shows an accessory that could be integrated into an
intranasal drug formulation
delivery device 4000 described herein. In some embodiments, the accessory
comprises an eye piece
7001, internal optics, an internal light source, and a narrow viewing element
(e.g. a fiber optic cable)
7002. In some embodiments, the accessory is integrated into an intranasal
delivery device described
herein so that the viewing element is aligned with the corresponding device
dispensing tip 7003. In
some embodiments, the internal light source illuminates where the dispensing
tip 7003 is pointing,
and a user is able to visualize where the dispensing tip 7003 is pointing to
help guide device insertion
into a patient's nasal cavity. In some embodiments, this also allows the user
to confirm that drug
delivery was successful. The system may also use a digital camera rather than
an eye piece with
optics, and the operator will visualize the target site of a patient's nasal
cavity on a screen.
[00276] Figure 71 shows an exemplary intranasal drug delivery device 7100. In
some embodiments,
the device 7100 comprises an eye piece 7101, a dispensing tip 7102, a patient
positioning rest 7103,
a disposable dispensing tip, a disposable drug cartridge, and positioning
controls 7104. In some
embodiments, the disposable dispensing tip and disposable drug cartridge are
loaded into the device.
In some embodiments, the drug cartridge contains a drug to be dispensed into a
patient's nasal cavity.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
In some embodiments, a patient then rests their head on the positioning rest
7103, and an operator
guides the dispensing tip 7102 into the patient's nasal cavity while
visualizing the procedure through
an eye piece. In some embodiments, the operator can then dispense a drug out
of the drug cartridge
(located in the device 7100), through the dispensing tip 7102, and into a
target site of the patient's
nasal cavity. In some embodiments, the operator can visualize through the eye
piece that the drug
delivery to the target site was successful. In some embodiments, the system
may also use a digital
camera rather than an eye piece with optics, and the operator will visualize
the target site on a screen.
[00277] Figure 72 shows an exemplary intranasal drug deliver device 7200
according to some
embodiments. In some embodiments, the device comprises a dispensing tip 7201 a
syringe 7202, and
a cap 7303. In some embodiments, the cap 7303 is a single injection molded
part that attaches to the
syringe 7202 with a snap fit. In some embodiments, the cap 7203 includes a
shearing element (e.g.
shear pin, shear plane, stress concentration, snap lock) 7204. In some
embodiments, a user presses
on the shearing element 7204 until it breaks. In some embodiments, breaking
the shearing element
7204 delivers a controlled force to the syringe plunger and a drug is forced
out of the syringe 7202
and out of the dispensing tip 7201. In some embodiments, the dispensing tip
7201 includes a flow
restriction (e.g. orifice plate, narrow channel, constant flow rate valve)
that controls the drug
dispensing rate and provides an appropriate velocity profile to eject the drug
to a target site within a
patient's nasal cavity. The shearing element 7204 may also be an overcenter
mechanism, ball detent,
or other mechanical element that releases under a specific force.
[00278] Figure 73 shows an exemplary intranasal drug delivery device 7300
according to some
embodiments. In some embodiments, the device 7300 comprises a mouth piece
7301, and dispensing
tip 7302. In some embodiments, a user inserts the dispensing tip 7302 into the
nose and the mouth
piece 7301 mouth as shown in Figure 73. In some embodiments, the user then
applies positive
pressure to the device 7300 by blowing into the mouth piece 7301. In some
embodiments, the flow
of air into the mouth piece 7301 dispenses a drug through the dispensing tip
7302 into a target site of
a user's nasal cavity. In some embodiments, the drug may be held in a flexible
container (e.g. bag,
blister pack, bellows) that is directly compressed by air flow from the user.
The drug may be driven
by a mechanism that is driven by the air flow from the user (e.g. air from the
user drives a large
diaphragm that moves a plunger of a drug cartridge). In some embodiments, flow
rate of the drug
may be regulated by a flow restriction (e.g. orifice plate, narrow channel,
constant flow rate valve)
in the path of air from the user or in the path of drug out of the dispensing
tip 7302. The air flow may
fill a compliant chamber where pressure in the chamber builds to a specific
pressure regulated by a
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
56
valve (burst valve, pressure relieve valve, etc) before pushing the drug out
of the device. The device
may also include a priming step to move the drug out of the drug container
(e.g. cartridge, ampule)
and into a secondary chamber. In some embodiments, air flow into the secondary
chamber directly
contacts the drug and forces it through the dispensing tip 7302. This priming
step may be driven by
air pressure from the user, so the sequence of priming then ejection occurs
with a single user breath.
Exemplary Embodiments
[00279] In accordance with an aspect, there is provided an intranasal drug
delivery device having
compliant or flexible, soft nib to precisely locate the dosage and provide
comfort for user. The term
drug can also be used herein to refer to other agents such as vitamins,
fragrance, saline or non-
pharmaceutical agents.
[00280] In accordance with an aspect, there is provided an intranasal drug
delivery device having a
cocking mechanism and actuator to load and release dosage.
[00281] In accordance with an aspect, there is provided an intranasal drug
delivery device having a
non-air interface mechanically pressurized fluid reservoir to enable dosing
independent of orientation
and to load shot chamber. In some example embodiments, reservoir can be
collapsible from external
pressure, including ambient air pressure.
[00282] In accordance with an aspect, there is provided an intranasal drug
delivery device
connectable to a facial or device recognition application to prevent
intentional or unintentional
misuse.
[00283] In accordance with an aspect, there is provided an intranasal fluid
delivery device
comprising a dispensing tip connected to a hollow needle, a shot chamber
carrying a fluid, the shot
chamber having a diaphragm at one end and a plunger at the other end, and an
actuator connected to
a push rod moveable toward the shot chamber and having a locking mechanism,
wherein pushing the
actuator releases the locking mechanism, allowing the push rod to push against
the plunger, exerting
pressure on the fluid and forces the needle through the diaphragm into the
shot chamber such that the
fluid flows out of the needle into the dispensing tip.
[00284] In accordance with an aspect, there is provided apparatus for
delivering fluid to a nasal
volume comprising a housing having a first end with a dispensing opening and a
second end with an
actuating opening, a dispensing tip coupled to the dispensing opening, a
capsule within the housing
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
57
between the actuating opening and the dispensing opening, the capsule
comprising a tube pre-filled
with fluid between a diaphragm and a plunger, and, an actuator coupled to the
actuating opening, the
actuator comprising a push rod moveable into contact with the plunger and held
back by a locking
mechanism, and a spring urging the push rod toward the plunger.
[00285] In accordance with an aspect, there is provided a method for targeted
intranasal fluid
delivery. The method comprises inserting a compliant dispensing tip into a
nasal cavity, and ejecting
a fluid from the compliant dispensing tip to deliver a liquid jet to a
targeted region within the nasal
cavity. The targeted region may be an olfactory region of the nasal cavity.
Inserting the compliant
dispensing tip into the nasal cavity may comprises inserting the compliant
dispensing tip at least into
an upper nares. Inserting the compliant dispensing tip into the nasal cavity
may comprise positioning
an end of the compliant dispensing tip proximate to the olfactory region or
the anterior entry to the
olfactory region. The compliant dispensing tip may comprise a cannula.
Ejecting the fluid may
comprise ejecting the fluid with a controlled velocity profile to limit shear
forces on the fluid.
[00286] In some embodiments, the actuator is connected to a spring and the
spring is connected to
the push rod. In some embodiments, the locking mechanism comprises one or more
tabs made from
a lock material, and the locking mechanism is released by breaking the lock
material. In some
embodiments, the locking mechanism comprises one or more pivotable tabs, and
the locking
mechanism is released by pivoting the tabs. In some embodiments, the device
comprises an outer
chassis having a pair of foldable arms on opposed sides thereof. In some
embodiments, the dispensing
tip comprises a cannula. In some embodiments, the dispensing tip comprises an
atomizer. In some
embodiments, pushing the actuator forces the needle through the diaphragm into
the shot chamber
and then releases the locking mechanism allowing the push rod to push against
the plunger. In some
embodiments, the intranasal fluid delivery device further comprises a damping
mechanism to create
a controlled velocity profile of the fluid when it exits the dispensing tip.
In some embodiments, the
damping mechanism comprises at least one of a magnet, a spring, a viscous
damper, a sealed chamber
with an airflow restriction, a container of compressed gas, a valve, a motor,
an elastomeric chamber,
a flow restriction device, and a configuration of the plunger and shot
chamber. In some embodiments,
intranasal fluid delivery device comprises compliant or flexible, soft nib
designed to conform to
aspects of the nasal cavity anatomy such as the nasal valve, nasal turbinates
and the septum and to
precisely locate the dosage and provide comfort for user. In some embodiments,
the dispensing tip is
flexible In some embodiments, the dispensing tip has an elliptical cross
section. In some
embodiments, the dispensing tip has a distal portion and a proximal portion,
and wherein a first center
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
58
axis of the distal portion and a second center axis of the proximal portion
are non-colinear. In some
embodiments, the dispensing tip has a distal portion having a first rigidity
and a proximal portion
having a second rigidity, and wherein the first rigidity is less than the
second rigidity. In some
embodiments, the dispensing tip has a distal portion having a first softness
and a proximal portion
having a second softness, and wherein the first softness is less than the
second softness. In some
embodiments, the dispensing tip comprises an off-center drug dispensing
channel distal to the distal
portion. In some embodiments, the dispensing tip comprises a protruding
element. In some
embodiments, the dispensing tip comprises an inflatable balloon surrounding at
least a part of the
distal portion of the dispensing tip. In some embodiments, the inflatable
balloon further surrounds at
least a part of the proximal portion of the dispensing tip. In some
embodiments, a distal portion of
the dispensing tip is curved. In some embodiments, the dispensing tip has a
distal portion having a
first outer diameter and a proximal portion having a second outer diameter,
and wherein the first
outer diameter is less than the second outer diameter. In some embodiments,
the dispensing tip has a
distal portion having a first outer diameter, a proximal portion having a
second outer diameter, and
wherein the first outer diameter is greater than the second outer diameter. In
some embodiments, the
dispensing tip has a proximal portion having an outer diameter that tapers
towards the distal portion
to provide comfort for the user or to limit insertion distance. In some
embodiments, the dispensing
tip has a perforation. In some embodiments, the perforation is on a distal
portion of the dispensing
tip. In some embodiments, the perforation is on a single side of the
dispensing tip. In some
embodiments, a distal portion of the dispensing tip has a spiral shape. In
some embodiments, exerting
the pressure on the fluid increases a radius of the spiral shape.
[00287] In accordance with an aspect, there is provided apparatus for
delivering fluid to a nasal
volume comprising a housing having a first end with a dispensing opening and a
second end with an
actuating opening, a dispensing tip coupled to the dispensing opening, a
capsule within the housing
between the actuating opening and the dispensing opening, the capsule
comprising a tube pre-filled
with fluid between a diaphragm and a plunger, and, an actuator coupled to the
actuating opening, the
actuator comprising a push rod moveable into contact with the plunger and held
back by a locking
mechanism, and a spring urging the push rod toward the plunger.
[00288] In some embodiments, the apparatus further comprises means for damping
a flow of fluid
ejected from the dispensing tip. In some embodiments, the locking mechanism
comprises pivotable
tabs. In some embodiments, the locking mechanism comprises breakable tabs. In
some embodiments,
the dispensing tip is flexible. In some embodiments, the dispensing tip has an
elliptical cross section.
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
59
In some embodiments, the dispensing tip has a distal portion and a proximal
portion, and wherein a
first center axis of the distal portion and a second center axis of the
proximal portion are non-colinear.
In some embodiments, the dispensing tip has a distal portion having a first
rigidity and a proximal
portion having a second rigidity, and wherein the first rigidity is less than
the second rigidity. In some
embodiments, the dispensing tip comprises an off-center drug dispensing
channel distal to the distal
portion. In some embodiments, the dispensing tip comprises a protruding
element. In some
embodiments, the dispensing tip comprises an inflatable balloon surrounding at
least a part of the
distal portion of the dispensing tip. In some embodiments, the inflatable
balloon further surrounds at
least a part of the proximal portion of the dispensing tip. In some
embodiments, a distal portion of
the dispensing tip is curved In some embodiments, the dispensing tip has a
distal portion having a
first outer diameter and a proximal portion having a second outer diameter,
and wherein the first
outer diameter is less than the second outer diameter. In some embodiments,
the dispensing tip has a
distal portion having a first outer diameter, a proximal portion having a
second outer diameter, and
wherein the first outer diameter is less than the second outer diameter. In
some embodiments, the
dispensing tip has a perforation. In some embodiments, the perforation is on a
distal portion of the
dispensing tip. In some embodiments, the perforation is on a single side of
the dispensing tip. In some
embodiments, a distal portion of the dispensing tip has a spiral shape. In
some embodiments, exerting
the pressure on the fluid increases a radius of the spiral shape.
[00289] In accordance with an aspect, there is provided a method for targeted
intranasal fluid
delivery. The method comprises inserting a compliant dispensing tip into a
nasal cavity and ejecting
a fluid from the compliant dispensing tip to deliver a liquid jet or stream to
a targeted region within
the nasal cavity. The targeted region may be an olfactory region of the nasal
cavity. Inserting the
compliant dispensing tip into the nasal cavity may comprises inserting the
compliant dispensing tip
at least into an upper nares. Inserting the compliant dispensing tip into the
nasal cavity may comprise
positioning an end of the compliant dispensing tip proximate to the olfactory
region or to the anterior
entry to the olfactory region. The compliant dispensing tip may comprise a
cannula. Ejecting the fluid
may comprise ejecting the fluid with a controlled velocity profile to limit
shear forces on the fluid.
[00290] In various further aspects, the disclosure provides corresponding
systems and devices, and
logic structures such as machine-executable coded instruction sets for
implementing such systems,
devices, and methods.
[00291] In one aspect, provided herein, is a dispensing tip for an apparatus
for delivering fluid to a
nasal volume comprising a hydrophilic coating applied to an outer surface of
the dispensing tip,
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
wherein the hydrophilic coating is activated by contact with a hydrating
medium. In some
embodiments, the hydrating medium is water, a gel, a viscous liquid, a vapor,
or any combination
thereof In some embodiments, the water is water vapor. In some embodiments,
activating the
hydrophilic coating reduces a surface friction of the hydrophilic coating.
[00292] In one aspect, provided herein, is a packaging system for a dispensing
tip comprising: a. a
sealed packaging material; and b. a dispensing tip provided herein contained
in a first compartment
of the packaging material. In some embodiments, the hydrating medium is
contained inside the first
compartment. In some embodiments, the hydrating medium is contained in a
second compartment of
the packaging material. In some embodiments, the packaging system further
comprises a membrane
between the first compartment and the second compartment. In some embodiments,
piercing the
membrane forms a fluid connection between the first compartment and the second
compartment. In
some embodiments, the packaging system further comprises a one-way valve, a
clamp, or both
between the first compartment and the second compartment. In some embodiments,
the hydrating
medium is a vapor released by a hydrating medium body. In some embodiments,
the hydrating
medium body comprises a foam material, a fabric material, a porous plastic
material, a porous
ceramic material, a wicking material, or any combination thereof In some
embodiments, the
hydrating medium body is encapsulated by a vapor permeable membrane. In some
embodiments, the
first compartment and the second compartment in fluidically connect through a
tortuous chamber.
[00293] In one aspect, provided herein, is a kit comprising a. a dispensing
tip provided herein and
b. a hydrating medium provided herein disposed within a packaging material. In
some embodiments,
the packaging material is an ampule, a pouch, a square treat open pouch, or
any combination thereof.
In some embodiments, the hydrating medium is a gel. In some embodiments, the
packaging material
delivers the hydrating medium the outer surface of the dispensing tip. In some
embodiments, the
packaging material is configured to allow insertion of the dispensing tip into
the hydrating medium.
[00294] In this respect, before explaining at least one embodiment in detail,
it is to be understood
that the embodiments are not limited in application to the details of
construction and to the
arrangements of the components set forth in the following description or
illustrated in the drawings.
Also, it is to be understood that the phraseology and terminology employed
herein are for the purpose
of description and should not be regarded as limiting.
Dispensing tip with hydrophilic coating, Packaging System, and Kit Embodiments
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
61
[00295] Disclosed herein, in some embodiments, is a dispensing tip for an
apparatus for delivering
fluid to a nasal volume comprising a hydrophilic coating applied to an outer
surface of the dispensing
tip, wherein the hydrophilic coating is activated by contact with a hydrating
medium. In some
embodiments, the hydrating medium is water, a gel, a lubricating gel, a
viscous liquid, a vapor, or
any combination thereof In some embodiments, the water is water vapor. In some
embodiments,
activating the hydrophilic coating reduces a surface friction of the
hydrophilic coating. In some
embodiments, the dispensing tip comprises a compliant nib, configured to
comply with an intranasal
passage of a subject.
[00296] Disclosed herein, in some embodiments, is a packaging system for a
dispensing tip
comprising: a. a sealed packaging material; and b. the dispensing tip of any
embodiment herein
wherein the dispensing tip comprises a hydrophilic coating applied to an outer
surface of the
dispensing tip, wherein the dispensing tip is contained in a first compartment
of the packaging
material. In some embodiments, the hydrating medium is contained inside the
first compartment. In
some embodiments, the hydrating medium is contained in a second compartment of
the packaging
material. In some embodiments, the packaging system further comprising a
membrane between the
first compartment and the second compartment. In some embodiments, wherein
piercing the
membrane forms a fluid connection between the first compartment and the second
compartment. In
some embodiments, the packaging system further comprising a one-way valve, a
clamp, or both
between the first compartment and the second compartment. In some embodiments,
the hydrating
medium is a vapor released by a hydrating medium body. In some embodiments,
the hydrating
medium body comprises a foam material, a fabric material, a porous plastic
material, a porous
ceramic material, a wicking material, or any combination thereof In some
embodiments, the
hydrating medium body is encapsulated by a vapor permeable membrane. In some
embodiments, the
first compartment and the second compartment in fluidically connect through a
tortuous chamber. In
some embodiments, the dispensing tip comprises a compliant nib, configured to
comply with an
intranasal passage of a subject.
[00297] Disclosed herein, in some embodiments, is a kit comprising: a. the
dispensing tip of any
embodiment herein wherein the dispensing tip comprises a hydrophilic coating
applied to an outer
surface of the dispensing tip; and b. any the hydrating medium described
herein disposed within a
packaging material. In some embodiments, the packaging material is an ampule,
a pouch, a square
treat open pouch, or any combination thereof. In some embodiments, the
hydrating medium is a gel.
In some embodiments, the packaging material delivers the hydrating medium to
the outer surface of
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
62
the dispensing tip. In some embodiments, the packaging material is configured
to allow insertion of
the dispensing tip into the hydrating medium. In some embodiments, the
dispensing tip is disposed
within the packaging material such that the hydrophilic coating is in contact
with the hydrating
medium. In some embodiments, the dispensing tip comprises a compliant nib,
configured to comply
with an intranasal passage of a subject.
[00298] Disclosed herein, in some embodiments, is a method of using a kit
disclosed herein, the
method comprising: dispensing the hydrating medium onto the hydrophilic
coating of the dispensing
tip. In some embodiments, the dispensing tip comprises a compliant nib,
configured to comply with
an intranasal passage of a subject.
[00299] Disclosed herein, in some embodiments, is a method of using a kit
disclosed herein, the
method comprising: inserting the dispensing tip into hydrating medium disposed
within the
packaging material. In some embodiments, the dispensing tip comprises a
compliant nib, configured
to comply with an intranasal passage of a subject.
[00300] Disclosed herein, in some embodiments, is a method of using a
packaging system disclosed
herein, the method comprising opening the sealed packaging material; and
adding the hydrating
medium to the first compartment such that the hydrophilic coating is contacted
with the hydrating
medium. In some embodiments, the dispensing tip comprises a compliant nib,
configured to comply
with an intranasal passage of a subject.
[00301] Disclosed herein, in some embodiments, is a method of using a
packaging system disclosed
herein, the method comprising: transferring the hydrating medium from the
second compartment to
the first compartment such that the hydrophilic coating is contacted with the
hydrating medium. In
some embodiments, the dispensing tip comprises a compliant nib, configured to
comply with an
intranasal passage of a subject.
[00302] Disclosed herein, in some embodiments, is a packaging system for a
dispensing tip
comprising: a. a sealed packaging material; and b. the dispensing tip disposed
in a first compartment
of the packaging material. In some embodiments, the packaging system further
comprising a
lubricating gel. In some embodiments, the lubricating gel is disposed within
the first compartment.
In some embodiments, the lubricating gel is contained in a second compartment
of the packaging
material. In some embodiments, the packaging system further comprising a
membrane between the
first compartment and the second compartment. In some embodiments, wherein
piercing the
membrane forms a fluid connection between the first compartment and the second
compartment. In
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
63
some embodiments, the packaging system further comprising a one-way valve, a
clamp, or both
between the first compartment and the second compartment. In some embodiments,
the dispensing
tip comprises a compliant nib, configured to comply with an intranasal passage
of a subject.
[00303] Disclosed herein, in some embodiments, is a method of lubricating a
dispensing tip,
comprising adding a lubricating gel to the surface of the dispensing tip. In
some embodiments, the
lubricating gel is dispensed from a packaging material containing the
lubricating gel. In some
embodiments, the dispensing tip is dipped into a packaging material containing
a lubricating gel. In
some embodiments, the dispensing tip comprises a compliant nib, configured to
comply with an
intranasal passage of a subject.
Alternative Damping Mechanisms Embodiment
[00304] Disclosed herein, in some embodiments, is an apparatus for delivering
fluid to a nasal
volume, the apparatus comprising: a housing having a first end with a
dispensing opening and a
second end with an actuating opening; a dispensing tip coupled to the
dispensing opening; a capsule
within the housing between the actuating opening and the dispensing opening,
the capsule comprising
a tube pre-filled with fluid between a diaphragm and a plunger; and an
actuator coupled to the
actuating opening, the actuator comprising a push rod moveable into contact
with the plunger and
held back by a locking mechanism, and a spring urging the push rod toward the
plunger. In some
embodiments, the apparatus further comprising means for damping a flow of
fluid ejected from the
dispensing tip. In some embodiments, the means for damping a flow of fluid
comprises a flow
restriction fluidically coupling the needle and the dispensing tip. In some
embodiments, the means
for damping a flow of fluid comprises a flow restriction fluidically coupling
the needle and the
dispensing tip. In some embodiments, the flow restriction is a constriction
within the hollow needle.
In some embodiments, the flow restriction is a porous body. In some
embodiments, the porous body
comprises an open cell pore, a closed cell pore, or any combination thereof.
In some embodiments,
the porous body is formed of metal, ceramic, plastic, wood, or any combination
thereof In some
embodiments, the flow restriction is an orifice plate within the needle, the
dispensing tip, or both. In
some embodiments, the flow restriction is an orifice within the needle, the
dispensing tip, or both. In
some embodiments, the flow restriction comprises flexible washers within the
needle, the dispensing
tip, or both. In some embodiments, the damping mechanism comprises an actuator
restriction coupled
to the actuator. In some embodiments, the actuator restriction comprises a
porous cavity. In some
embodiments, the porous cavity comprises an open cell pore, a closed cell
pore, or any combination
thereof In some embodiments, the porous cavity is formed of metal, ceramic,
plastic, wood, or any
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
64
combination thereof In some embodiments, the dispensing tip comprises a
compliant nib, configured
to comply with an intranasal passage of a subject.
Secondary Chamber Embodiment
[00305] Disclosed herein, in some embodiments, is an intranasal fluid delivery
device comprising
a dispensing tip connected to a hollow needle, a shot chamber carrying a
fluid, the shot chamber
having a diaphragm at one end and a plunger at the other end, and an actuator
connected to a push
rod moveable toward the shot chamber and having a locking mechanism, wherein
pushing the
actuator releases the locking mechanism, allowing the push rod to push against
the plunger, exerting
pressure on the fluid and forcing the needle through the diaphragm into the
shot chamber such that
the fluid flows out of the needle into the dispensing tip and a secondary
chamber, wherein the
secondary chamber comprises a second plunger at one end and a second actuator
connected to a
second push rod moveable toward the dispensing tip. In some embodiments, the
needle comprises a
one-way valve configured to prevent backflow. In some embodiments, the second
plunger and the
second actuator are configured to drive the fluid out of the dispensing tip.
In some embodiments, the
second plunger and the second actuator are further configured to drive a
secondary fluid out of the
dispensing tip. In some embodiments, the secondary fluid is a gas. In some
embodiments, the
intranasal fluid delivery device further comprising a second needle for
providing fluid
communication between the shot chamber and the secondary chamber. In some
embodiments, the
second actuator is configured to control the flow rate of the fluid out of the
dispensing tip. In some
embodiments, the dispensing tip comprises a compliant nib, configured to
comply with an intranasal
passage of a subject.
[00306] Disclosed herein, in some embodiments, is an apparatus for delivering
fluid to a nasal
volume, the apparatus comprising: a housing having a first end with a
dispensing opening and a
second end with an actuating opening; a dispensing tip coupled to the
dispensing opening, the
dispensing tip comprising a pump configured to propel fluid out of the
dispensing tip; a capsule
within the housing between the actuating opening and the dispensing opening,
the capsule comprising
a tube pre-filled with fluid between a diaphragm and a plunger; and, an
actuator coupled to the
actuating opening, the actuator comprising a push rod moveable into contact
with the plunger and
held back by a locking mechanism, and a spring urging the push rod toward the
plunger. In some
embodiments, the pump comprises a roller configured to control the flow rate
of fluid out of the
dispensing tip. In some embodiments, the roller is driven by a spring combined
with a dashpot
damper. In some embodiments, the pump is a peristaltic pump, a piston pump, a
gear pump, a bellows,
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
or an elastic chamber, or any combination thereof In some embodiments, the
pump is integrated into
the dispensing tip. In some embodiments, the dispensing tip comprises a
compliant nib, configured
to comply with an intranasal passage of a subject.
Burst Membrane Embodiment
[00307] Disclosed herein, in some embodiments, is an intranasal fluid delivery
system comprising:
a container comprising a dispensing tip and sealed by a burst membrane within
the dispensing tip; a
fluid within the container; and a dispensing mechanism configured to compress
the container and
pressurize the fluid therein to puncture the burst membrane and release the
fluid from the dispensing
tip. In some embodiments, at least a portion of the container is flexible. In
some embodiments, the
container comprises a fastener that removably couples the container to the
dispensing mechanism. In
some embodiments, the dispensing mechanism comprises a container release that
decouples the
container from the dispensing mechanism. In some embodiments, a trigger within
the dispensing
mechanism initiates the compression by the driving mechanism. In some
embodiments, the burst
membrane comprises a thin membrane, a one-way valve, a snap fit, a pressure
relief valve, or any
combination thereof. In some embodiments, the dispensing mechanism comprises a
roller, a motor,
a solenoid, a cam, a plunger, a bellow, a screw drive, a spring, a pressurized
container, a vacuum
chamber or any combination thereof. In some embodiments, the dispensing
mechanism comprises
the roller translated by a motor or a solenoid. In some embodiments, the
dispensing mechanism
continues to compress the chamber after the burst membrane has been punctured.
In some
embodiments, the dispensing mechanism comprises a velocity control controlling
a velocity of the
compression. In some embodiments, the velocity control comprises a damping
element. In some
embodiments, the velocity control pauses the compression of the container
after the burst membrane
is punctured. In some embodiments, the dispensing tip comprises a compliant
nib, configured to
comply with an intranasal passage of a subject.
Fluid Contained in a Container Embodiment
[00308] Disclosed herein, in some embodiments, is an intranasal fluid delivery
system comprising:
a container containing a fluid; a hollow dispensing tip having a distal
opening and a proximal
opening; and a dispensing tool removably coupled to the dispensing; tip and
configured to draw the
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
66
fluid from the container into the proximal opening of the hollow dispensing
tip, and to subsequently
eject the fluid in the hollow dispensing tip intranasally. In some
embodiments, a portion of the interior
of the hollow dispensing tip that is proximal to the dispensing tool comprises
a barrier that is air
permeable and impermeable to the fluid. In some embodiments, the dispensing
tool draws the fluid
from the container by depressurizing a chamber within the dispensing tool and
in fluid
communications with the hollow dispensing tip. In some embodiments, the
dispensing tool
depressurizes the chamber by a motor, a solenoid, a spring, a damper, or any
combination thereof In
some embodiments, the dispensing tool removably coupled to the hollow
dispensing tip via a screw,
a socket, a detent, a hydraulic interface, or any combination thereof In some
embodiments, at least
one of the drawing of the fluid from the container and the ejecting of the
fluid in the hollow dispensing
tip is performed by actuating a trigger of the dispensing tool. In some
embodiments, the hollow
dispensing tip is removable from the dispensing tool by actuating an ejection
trigger. In some
embodiments, the system further comprising a sterility cover encompassing at
least a portion of the
dispensing tool. In some embodiments, the dispensing tip comprises a compliant
nib, configured to
comply with an intranasal passage of a subject.
Dispensing Tip Coupled to an Endoscope Embodiment
[00309] Disclosed herein, in some embodiments, is an intranasal fluid delivery
device comprising:
an endoscope; and a dispensing tip removably coupled to the endoscope. In some
embodiments, the
endoscope and the dispensing tip are concentric when coupled. In some
embodiments, the endoscope
and the dispensing tip are tangent when coupled. In some embodiments, the
endoscope and the
dispensing tip are flexible. In some embodiments, the endoscope comprises an
illuminator. In some
embodiments, the endoscope comprises a fiber optic cable transmitting light
emitted by the
illuminator. In some embodiments, the endoscope further comprises a camera, an
eyepiece, or both.
In some embodiments, the device further comprising a patient positioning rest.
In some embodiments,
the device further comprising an adjustment mechanism translating the
endoscope and the dispensing
tip with respect to the patient positioning rest. In some embodiments, the
device further comprising
a sterility cover encompassing at least a portion of the patient positioning
rest. In some embodiments,
the device further comprising a fluid container containing a fluid and in
fluidic communication with
the dispensing tip. In some embodiments, the dispensing tip is decoupled from
the endoscope by
actuating an ejection trigger. In some embodiments, the dispensing tip
comprises a compliant nib,
configured to comply with an intranasal passage of a subject.
Cap Embodiment
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
67
[00310] Disclosed herein, in some embodiments, is an apparatus for delivering
fluid to a nasal
volume, the apparatus comprising: a housing comprising a first end having a
dispensing opening and
a second end having an actuating opening; a dispensing tip coupled to the
dispensing opening; a
capsule within the housing between the actuating opening and the dispensing
opening, the capsule
comprising a diaphragm, a plunger, and a tube pre-filled with fluid between a
diaphragm and a
plunger; an actuator coupled to the actuating opening, the actuator comprising
a push rod moveable
into contact with the plunger and coupled to a locking mechanism, and a spring
translating the push
rod toward the plunger; and a cap comprising a shearing element covering the
push rod. In some
embodiments, the shearing element comprises a shear pin, a shear plane, a
stress concentration, a
snap lock, an overcenter mechanism, a ball detent, or combinations thereof. In
some embodiments,
the shearing element is configured to break when under force. In some
embodiments, the shearing
element is further configured to deliver a controlled force to the push rod.
In some embodiments, the
cap couples to the housing. In some embodiments, the cap couples to the
housing by a snap fit. In
some embodiments, the dispensing tip comprises a flow restriction. In some
embodiments, the flow
restriction is an orifice plate, a narrow channel, a constant flow rate valve,
or any combination thereof
In some embodiments, the dispensing tip comprises a compliant nib, configured
to comply with an
intranasal passage of a subject.
Mouthpiece Embodiment
[00311] Disclosed herein, in some embodiments, is an apparatus for delivering
fluid to a nasal
volume, the apparatus comprising: a housing having a dispensing opening and an
actuating opening;
a dispensing tip coupled to the dispensing opening; a capsule containing a
first fluid and positioned
within the housing between the actuating opening and the dispensing opening;
and, a mouth piece in
fluid connection with the actuating opening, wherein the actuating opening is
configured to dispense
the first fluid from the dispensing tip when a second fluid is delivered into
the mouthpiece. In some
embodiments, the capsule comprises a flexible capsule. In some embodiments,
the flexible capsule
comprises a bag, a blister pack, a bellows, or combinations thereof In some
embodiments, the
flexible capsule is configured to compress when the second fluid is delivered
into the mouthpiece. In
some embodiments, the apparatus further comprising an actuator coupled to the
actuating opening,
wherein the actuator is configured to drive the first fluid out of the
dispensing tip when the second
fluid is delivered into the mouthpiece. In some embodiments, the actuator is a
plunger. In some
embodiments, the apparatus further comprising a diaphragm between the
mouthpiece and the
actuator. In some embodiments, the diaphragm is configured to drive the
actuator when the second
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
68
fluid is delivered into the mouthpiece. In some embodiments, the mouthpiece,
the dispensing tip, or
both comprise a flow restriction. In some embodiments, the flow restriction is
an orifice plate, a
narrow channel, a constant rate flow valve, or any combination thereof In some
embodiments, the
apparatus further comprising a chamber between the mouthpiece and the capsule,
the chamber
comprising a valve configured to drive the fluid out of the dispensing tip
when a certain pressure of
the second fluid is reached. In some embodiments, the valve is a burst valve
or a pressure release
valve. In some embodiments, the apparatus further comprising a priming chamber
between the
capsule and the dispensing tip. In some embodiments, the priming chamber is in
fluid connection
with the capsule and the dispensing tip. In some embodiments, the mouthpiece
is in fluid connection
with the priming chamber. In some embodiments, the second fluid is air. In
some embodiments, the
dispensing tip comprises a compliant nib, configured to comply with an
intranasal passage of a
subj ect.
[00312] Unless otherwise defined, all technical terms used herein have the
same meaning as
commonly understood by one of ordinary skill in the art to which this
disclosure belongs.
[00313] As used herein, the singular forms "a," "an," and "the" include plural
references unless the
context clearly dictates otherwise. Any reference to "or" herein is intended
to encompass "and/or"
unless otherwise stated.
[00314] As used herein, the term "about" in some cases refers to an amount
that is approximately
the stated amount.
[00315] As used herein, the term "about" refers to an amount that is near the
stated amount by 10%,
5%, or 1%, including increments therein.
[00316] As used herein, the term "about" in reference to a percentage refers
to an amount that is
greater or less the stated percentage by 10%, 5%, or 1%, including increments
therein.
[00317] As used herein, the term "generally" refers to a geometric
relationship between two or more
elements within tolerances of 10%, 5%, or 1%, including increments therein.
[00318] As used herein, the phrases "at least one", "one or more", and
"and/or" are open-ended
expressions that are both conjunctive and disjunctive in operation. For
example, each of the
expressions "at least one of A, B and C", "at least one of A, B, or C", "one
or more of A, B, and C",
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
69
"one or more of A, B, or C" and "A, B, and/or C" means A alone, B alone, C
alone, A and B together,
A and C together, B and C together, or A, B and C together.
[00319] As used herein, the term "subject" can refer to a "patient" or a
"user".
[00320] As used herein, the term "user" can refer to a "patient" or a
"subject".
[00321] As used herein, the term "intranasal passage" and "nasal cavity" may
be used
interchangeably.
[00322] The foregoing discussion provides many example embodiments of the
inventive subject
matter. Although each embodiment represents a single combination of inventive
elements, the
inventive subject matter is considered to include all possible combinations of
the disclosed elements.
In one embodiment comprises elements A, B, and C, and a second embodiment
comprises elements
B and D, then the inventive subject matter is also considered to include other
remaining combinations
of A, B, C, or D, even if not explicitly disclosed.
[00323] The embodiments of the devices, systems and methods described herein
may be
implemented in a combination of both hardware and software. These embodiments
may be
implemented on programmable computers, each computer including at least one
processor, a data
storage system (including volatile memory or non-volatile memory or other data
storage elements or
a combination thereof), and at least one communication interface.
[00324] Program code is applied to input data to perform the functions
described herein and to
generate output information. The output information is applied to one or more
output devices. In
some embodiments, the communication interface may be a network communication
interface. In
embodiments in which elements may be combined, the communication interface may
be a software
communication interface, such as those for inter-process communication. In
still other embodiments,
there may be a combination of communication interfaces implemented as
hardware, software, and
combination thereof
[00325] Throughout the foregoing discussion, numerous references will be made
regarding servers,
services, interfaces, portals, platforms, or other systems formed from
computing devices. It should
be appreciated that the use of such terms is deemed to represent one or more
computing devices
having at least one processor configured to execute software instructions
stored on a computer
readable tangible, non-transitory medium. For example, a server can include
one or more computers
CA 03157487 2022-04-08
WO 2021/069972 PCT/IB2020/000849
operating as a web server, database server, or other type of computer server
in a manner to fulfill
described roles, responsibilities, or functions.
[00326] The technical solution of embodiments may be in the form of a software
product. The
software product may be stored in a non-volatile or non-transitory storage
medium, which can be a
compact disk read-only memory (CD-ROM), a USB flash disk, or a removable hard
disk. The
software product includes a number of instructions that enable a computer
device (personal computer,
server, or network device) to execute the methods provided by the embodiments.
[00327] The embodiments described herein may be implemented by physical
computer hardware,
including computing devices, servers, receivers, transmitters, processors,
memory, displays, and
networks. The embodiments described herein may comprise useful physical
machines and
particularly configured computer hardware arrangements.
[00328] Although the embodiments have been described in detail, it should be
understood that
various changes, substitutions and alterations can be made herein.
[00329] Moreover, the scope of the present application is not intended to be
limited to the particular
embodiments of the process, machine, manufacture, composition of matter,
means, methods and
steps described in the specification.
[00330] As can be understood, the examples described above and illustrated are
intended to be
exemplary only.