Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 2021/095030 PCT/11,2020/051161
1
Monovalent brines for use as wellbore fluids
Aqueous metal bromide brines are used by the oil and gas well
drilling industries for many decades. These industries primarily
seek clear, high density brines to serve as completion, packer and
workover fluids. High density is achieved by dissolving soluble
salt(s) in water, to create single salt or multiple salts
solutions. A list of water-soluble salts, including halide salts,
that are suitable for the preparation of high-density brines can
be found in US 2,898,294, Table 1.
Another important consideration in formulating high density
wellbore fluids is their stability against crystallization down to
low temperatures, to guarantee that the brines remain operative
over a broad temperature range. The property of interest is the
true crystallization temperature (TCT) of the brine. The TCT is
slightly higher than the point at which crystals first appear in
the solution, because of release of heat during crystallization
which leads to a slight increase of temperature; this increased
temperature is the TOT, which is the property normally reported to
describe a brine under consideration.
Leading halide brines found in the marketplace are calcium
bromide/calcium chloride, zinc bromide/calcium bromide, and zinc
bromide/calcium bromide/calcium chloride brines, which can be
formulated to achieve high densities and low TCT, as described,
for example, in US 4,292,183. It should be noted that in response
to environmental concerns which may drive the industry away from
zinc-containing brines, a few zinc-free metal bromide wellbore
fluids have been recently described in EP 3,180,408, US
2018/0298266 and WO 2019/168562, e.g., chiefly based on the
combination of calcium bromide and an auxiliary water soluble salt.
CA 03157732 2022-5-9
WO 2021/095030 PCT/11,2020/051161
2
Clear aqueous alkali bromides such as sodium bromide are also
being used in the oil and gas well drilling industries.
Nevertheless, the applicability of monovalent bromides is
restricted by difficulties in formulating them to meet
simultaneously the high-density and low-TCT requirements. Figure
1 is a TCT versus concentration plot of sodium bromide clear
fluid, exhibiting a characteristic minimum at 39% by weight sodium
bromide in solution: the TCT of such brine is as low as -3000.
However, the density of such a brine is usually not sufficiently
high (1.40 g/cc). It is seen from the graph that increasing the
concentration of the salt, i.e., increasing the density of the
fluid, is accompanied by a sharp increase of the TCT. The product
currently on the market is sodium bromide clear brine of 1.485
gr/cc density, which contains 45% by weight sodium bromide in
solution. The TCT of the brine is -2 C.
Although sodium bromide cannot match the density achieved by
divalent bromide salts, e.g., calcium bromide, its use is of
importance in sites where calcium bromide is precluded due to the
scaling tendency of the formation water, e.g., when formation
waters contain high levels of carbonate and sulfate, because the
presence of these anions may lead to precipitation of the
corresponding, poorly water soluble, calcium salts.
Therefore, there exists a need to formulate monovalent brines,
for example, sodium bromide-based clear fluids, to meet
simultaneously the high-density and low-TOT requirements.
We have found that the addition of alkali nitrates (e.g., sodium
nitrate, potassium nitrate) to alkali bromide brines affords
monovalent brines with reduced TCT. That is, sodium bromide and
blends thereof with other alkali bromides, such as sodium
bromide/potassium bromide blend, can benefit from the addition of
alkali nitrate to achieve - over a useful density range, say, up
CA 03157732 2022-5-9
WO 2021/095030 PCT/11,2020/051161
3
to 1.56 g/cc - clear monovalent brines that are stable against
crystallization down to low ambient temperatures, e.g., down to
-15 C. The experimental results reported below indicate that the
TOT of the alkali bromide/alkali nitrate systems is reduced by as
much as 20 C, relative to the equivalent (i.e., of equal density)
alkali bromide (nitrate free) clear fluids.
The alkali nitrates employed as TOT-reducing agents in monovalent
bromide brines according to this invention, e.g., sodium nitrate
and potassium nitrate, are non-toxic materials (in fact, they are
used as fertilizers, food additives and pharmaceutical
excipients). Sodium nitrate has the advantage of higher water
solubility whereas potassium nitrate is much less hygroscopic and
therefore remains in the form of a free-flowing powder even under
high humidity. Thus, both sodium and potassium nitrate are easy
to formulate in monovalent brines, as shown below.
It should be noted that nitrates (see US 8,003,578) were suggested
for use in wellbore fluids. US 8,003,578 explains that efforts to
use nitrate-based brines as completion and packer fluids were
abandoned in the 1950s. Example 1 of US 8,003,578 illustrates the
dissolution of sodium nitrate in sodium bromine brine, to achieve
13.12 ppg (1.57 g/cc) density. The 13.12 ppg (1.57 g/cc) sodium
bromide/sodium nitrate brine was then thickened, e.g., with guar
gum in an alkaline pH and its viscosity and thermal stability were
evaluated. It is reported in US 8,003,578 that the sodium
bromide/sodium nitrate clear brine of 1.57 gr/cc (13.12 ppg)
density has crystallization point of 60 F (+15 C)
Accordingly, the present invention is primarily directed to a
wellbore fluid, which is a monovalent brine comprising one or more
alkali bromide salt(s) and one or more TCT-reducing additive(s)
selected from the group consisting of alkali nitrates.
CA 03157732 2022-5-9
WO 2021/095030
PCT/11,2020/051161
4
The invention further provides a method of preparing wellbore fluid
with improved stability against crystallization, comprising
formulating one or more alkali bromide(s) and at least one alkali
nitrate in water, where the nitrate is present in an amount
sufficient to achieve a TOT-lowering effect of not less than 3 C,
e.g., TOT is reduced by more than 5 C, or more than 8 C and
sometimes more than 10 C, compared to the corresponding nitrate-
free brine of equal density. Owing to the presence of the alkali
nitrate, the crystallization of the monovalent brine on cooling
(and hence its transformation into unpumpable slurry) is avoided.
The invention also provides the use of alkali nitrate as a TCT-
lowering agent in monovalent brines of alkali bromides.
One variant of the invention relates to a wellbore fluid, which is
a monovalent brine comprising water and a binary salt mixture
consisting of sodium bromide and alkali nitrate [namely, sodium
nitrate, lithium nitrate or potassium nitrate], especially a
sodium bromide/sodium nitrate binary mixture formulated in water
to give a clear monovalent brine of density in the range from 1.47
to 1.55 g/ml, e.g., from 1.47 to 1.53 g/ml, displaying lower TOT
compared to the corresponding nitrate free brine, e.g., TOT below
-5.0 C, below -7.0 C, and even down to below -10 C.
Monovalent brines, in which sodium bromide and sodium nitrate
constitute the major and minor components, respectively, are
formulated to meet the density and TCT requirements set out above,
e.g., a monovalent brine which contains from 30 to 45% by weight
sodium bromide and from 3 to 18% by weight sodium nitrate (e.g.,
from 5 to 15%) in solution. Experimental results reported below
indicate a linear relationship between the reduction achieved in
TOT and the amount of alkali nitrate in solution (TOT reduction is
measured relative to the corresponding nitrate-free sodium bromide
brine of equal density). For example, with the aid of - 4-5%,
-8-10% and -13-15% by weight sodium nitrate in solution, TCT-
CA 03157732 2022-5-9
WO 2021/095030 PCT/11,2020/051161
lowering effects of - 8-9 C, 13-14 C and 20-21 C were respectively
measured.
Specific monovalent brines comprising binary mixtures of sodium
bromide and sodium nitrate for use as wellbore fluids according to
the invention are:
Sodium bromide/sodium nitrate brine of density from 1.47 gr/ml to
1.49 gr/ml and TOT below -5.0 C, especially below -7.0 C (e.g.,
from 38 to 45% by weight sodium bromide and from 4 to 12% by weight
sodium nitrate in aqueous solution);
Sodium bromide/sodium nitrate brine of density from 1.49 gr/ml to
1.52 gr/ml and TOT below -5.0 C, especially below -7.0 C (e.g.,
from 35 to 38% by weight sodium bromide and from 13 to 18% by
weight sodium nitrate in aqueous solution).
Improving (lowering) TCT was also achieved with the aid of other
alkali nitrates, e.g., lithium nitrate and potassium nitrate. The
invention therefore also provides monovalent brines comprising
binary mixtures of sodium bromide and lithium or potassium nitrate
for use as wellbore fluids:
Sodium bromide/lithium nitrate brine of density from 1.47 gr/ml to
1.49 gr/ml and TOT below -5.0 C, especially below -7.0 C (e.g.,
from 35 to 42% by weight sodium bromide and from 7 to 10% by weight
lithium nitrate in aqueous solution);
Sodium bromide/potassium nitrate brine of density from 1.48 gr/ml
to 1.51 gr/ml and TOT below -5.0 C, especially below -7.0 C (e.g.,
from 35 to 42% by weight sodium bromide and from 7 to 10% by weight
potassium nitrate in aqueous solution).
CA 03157732 2022-5-9
WO 2021/095030 PCT/11,2020/051161
6
The TOT-lowering effect of alkali nitrate is observed also in
mixed alkali bromide monovalent brine. Accordingly, another
variant of the invention relates to a monovalent brine comprising
water and a ternary salt mixture consisting of a first alkali
bromide, a second alkali bromide and alkali nitrate, such as
sodium nitrate. For example, the first alkali bromide is sodium
bromide and the second alkali bromide is either lithium bromide,
potassium bromide or cesium bromide.
Specific monovalent brines comprising a first alkali bromide, a
second alkali bromide and sodium nitrate for use as wellbore
fluids, on account of their high density and stability down to
low ambient temperatures (TCT below -7 C) include:
Sodium bromide/ lithium bromide/sodium nitrate brine of density
higher than 1.47 gr/ml, e.g., from 1.47 grim' to 1.49 grim' and
TOT below -5.0 C, especially below -7.0 C [formulated to contain
from 30 to 35% by weight sodium bromide (e.g., 33-35%); from 5 to
10% by weight lithium bromide (e.g., 5-7%);. and from 6 to 10% by
weight sodium nitrate (e.g., 7-9%) in aqueous solution];
Sodium bromide/ potassium bromide/sodium nitrate brine of density
higher than 1.51 gr/ml, e.g., from 1.51 gr/ml to 1.54 gr/ml and
TOT below -5.00C, especially below -7.00C [formulated to contain
from 30 to 37% by weight sodium bromide (e.g., 33 to 35%); from 5
to 10% by weight potassium bromide (e.g., 7-9%); and from 6 to 10%
by weight sodium nitrate (e.g., 7-9.%) in aqueous solution];
Sodium bromide/ cesium bromide/sodium nitrate brine of density
higher than 1.53 gr/ml, e.g., from 1.53 grim' to 1.57 grim' and
TOT below -5.0C, especially below -7+0 C [formulated to contain
from 33 to 37% by weight sodium bromide, from 5 to 10% by weight
cesium bromide (e.g., 7-9%) and from 5 to 10% by weight sodium
nitrate (e.g., 7-9%) in aqueous solution].
CA 03157732 2022-5-9
WO 2021/095030
PCT/11,2020/051161
7
The monovalent brines described herein are readily prepared by
combining in water one or more alkali bromide(s) and one or more
alkali nitrates(s). One convenient method
to formulate the
monovalent brine consists of adding alkali nitrate to a clear,
nearly saturated or saturated aqueous solution of alkali
bromide(s) (solubility of sodium bromide in water is 48% by
weight), with the addition of water to dissolve the added nitrate,
followed by filtration to remove undissolved solids if needed and
further dilution with water and/or the addition of dry salts to
adjust the density within the desired range.
The alkali bromide solution required for the preparation of the
monovalent brine of this invention is obtained either by dissolving
solid sodium bromide in water to reach saturation, by reacting
elemental bromine with a suitable sodium compound in aqueous
solution under conditions preventing bromate formation or after
removal of the bromate by-product, or by reacting hydrogen bromide
with sodium hydroxide by the methods known in the art.
It should be noted that alkali nitrates, such as sodium nitrate,
exhibit high solubility in sodium bromide brines and the
dissolution of solid sodium nitrate in saturated sodium bromide
solution is achieved rather easily, at ambient temperature under
stirring, whereby significant amount of sodium nitrate can be
loaded into the monovalent brine, e.g., up to 15% by weight.
Heating of the solution to facilitate dissolution of the nitrate
is normally not required, unless preparation of the brine takes
place on-site under conditions of cold temperature. Higher nitrate
concentration may require addition of water and heating to achieve
dissolution.
Another procedure to produce the monovalent brine of the invention
with desired density and TCT is by mixing concentrated, nearly
saturated aqueous solutions of alkali bromide and alkali nitrate
CA 03157732 2022-5-9
WO 2021/095030 PCT/11,2020/051161
8
in varying amounts and adjusting the density by further addition
of the salts in solid form.
The mixed monovalent bromide/nitrate brine displays good
stability, i.e., sodium bromide/sodium nitrate brines with varying
loading of the nitrate, which were examined for their stability
under severe conditions - held in an oven at 120 C for six days -
showed no signs of incompatibility or instability (neither color
change nor evolution of gas). In contrast, as indicated by
experimental results reported below, dissolution of sodium nitrate
in calcium bromide or manganese bromide saturated solutions, to
create clear brines, is difficult or impossible to achieve.
In addition to alkali bromide(s)/alkali nitrate(s), the wellbore
fluid of the invention may contain conventional constituents, such
as viscosifiers, to increase viscosity, pH modifiers, oxygen
scavengers and corrosion inhibitors. We have found that that the
corrosiveness of the monovalent brine of this invention is
tolerable and in fact comparable or even slightly lower corrosion
rates are expected, compared to a corresponding alkali bromide
(nitrate-free) brine, as shown by a corrosion test reported below
(using an electrochemical corrosion measurement system).
The pH of the monovalent brine of this invention is nearly
neutral, e.g., lying in the range from 6 to 8, e.g., from 7 to 8,
such as - 7.5.
The foregoing monovalent brines of alkali bromide and alkali
nitrate can be used as high density low TCT wellbore fluids, e.g.,
as drilling fluids, completion fluids, workover fluids, packer
fluids, as described, for example, in GB 2089397. Such uses of
the monovalent brines form additional aspects of the invention.
CA 03157732 2022-5-9
WO 2021/095030
PCT/11,2020/051161
9
A drilling fluid is introduced into the wellbore to serve several
functions, such as lubricating and cooling the drilling bit,
carrying away material removed from the formation, and preventing
high pressure subterranean fluids from intruding into the
borehole.
Completion fluid is placed in the well to facilitate final
operations prior to production.
Workover fluid is used when the productivity of the well decreases
or when safety problems are of concern. The workover fluid is
pumped into the wellbore to control the well.
Packer fluid is placed in the annular space between the production
tubing and the well casing to lower the differential pressure on
the wellbore casing and prevent collapse.
The invention further provides a method for treating subterranean
formation comprising delivering the monovalent brine described
herein to a well in the formation, e.g., a method of drilling,
working over, completing or packing a weilbore, by placing in the
wellbore said monovalent brine. As noted above, the monovalent
brine of the invention is suitable for treating subterranean
formations, wherein formation waters contain carbonate and
sulfate.
An apparatus for using the monovalent brine as a subterranean fluid
is described, for example, in US 2018/0298266, e.g., Figures 1 and
2 of US 2018/0298266. The monovalent brine is prepared in a tank
by combining water and salt components as described above. The
clear monovalent brine is supplied via a suitable feed line to a
production tubing penetrating into the subterranean formation,
with the aid of a suitable pump, e.g., a high pressure pump. The
aqueous wellbore fluid can be recirculated back to the surface via
CA 03157732 2022-5-9
WO 2021/095030
PCT/11,2020/051161
an annular space that exists between the wellbore walls and the
tubing, to be regenerated (e.g., by filtration and addition of
fresh salts to restore the required density and TCT, etc.).
In the drawings
Figure 1 is a TCT versus concentration plot of sodium bromide
brine.
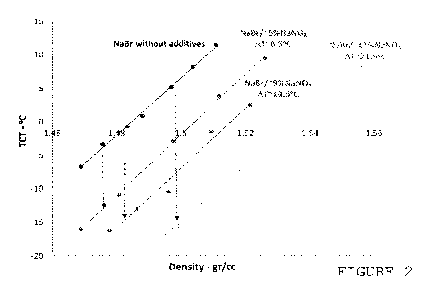
Figure 2 shows TCT versus density plots of sodium bromide/sodium
nitrate brines.
Figure 3 shows TCT versus density plots of sodium bromide/potassium
bromide/sodium nitrate brines.
Figure 4 shows TOT versus density plots of sodium bromide/lithium
bromide/sodium nitrate brines.
Figure 5 shows TCT versus density plots of sodium bromide/cesium
bromide/sodium nitrate brines.
Figure 6 shows TCT versus density plots of sodium bromide/sodium
nitrate, sodium bromide/lithium nitrate and sodium
bromide/potassium nitrate brines.
Figure 7 shows cyclic polarization curves to illustrate the
noncorrosive behavior of sodium bromide/sodium nitrate solution.
CA 03157732 2022-5-9
WO 20211095030
PCT/11,2020/051161
11
Examples
Methods
Density measurements: an empty, clean and dry volumetric flask of
25.00 ml (+0.04 ml) was weighed on an analytical balance (+0.0001
gr). The flask was filled up to 25.00 ml with the desired solution.
The outer wall of the flask was cleaned and dried. The flask was
observed to ascertain that there were no air bubbles in the
solution. The full flask was weighed on the analytical balance
and its weight was recorded. The temperature of the solution was
also measured, and the density was calculated (temperature of
measurements reported herein is 26 1 C)
TOT measurements: 25-30 ml of the test sample, pinch of a
nucleation agent and magnetic stirrer were placed in a 50 ml
beaker. Silicon oil (-50 ml) was introduced into a 250 ml jacketed
glass cup. The 50 ml beaker with the sample was inserted into the
250 ml jacketed glass cup (with the silicon oil inside) connected
to a Huber silicon oil circulation thermostat in such a way that
the sample was cooled by the silicon oil in the cup; the
thermocouple was immersed into the solution and the cup was
covered with the cap. The 250 ml jacked glass cup with the beaker
inside was placed on the stirring plate and stirring of the
solution was started. The initial temperature in the thermostat
was set at 10 C and was decreased gradually during the measurements
down to -20 C if needed. When crystals began to form, the
corresponding temperature was written down (FCTA - first crystal
to appear), afterwards the temperature slightly increased,
indicating the TOT.
Bromide concentration in the solution: measurements were made by
direct potentiometric titration using a silver electrode and 0.1M
AgNO3 titrant solution after adding 2N HNO3.
Nitrate concentration in the solution: analysis by Ion
Chromatography (IC). The analysis was done against an external
standard, Dionex Seven Anion standard P.N. 057590. The instrument
used was a Dionex ICS-2100 with an AS-9HC column.
CA 03157732 2022-5-9
m4)202110954930
PCT/11,2020/051161
12
Example 1
Solubility of NaNO3 in NaBr solution and
properties of the NaBr/NaNCt salt solution
Sodium bromide clear brine of 1.496 gr/cc (12.48 ppg) density
contains 46.4% by weight sodium bromide in solution. The TCT of
the brine is 6.5 C and its pH is 7.3.
To this clear sodium bromide brine (100 g) was added sodium
nitrate (11 gr). The added sodium nitrate dissolved swiftly in
the sodium bromide brine at room temperature under stirring, to
form NaBr /NaNO3 -10% solution of 1.55 gr/cc (12.94 ppg) density.
Water (7.8 gr) was added, to afford a NaBr/NaNO3 clear solution
of 1.496 gr/cc (12.48 ppg) density with TCT of -8.7 C, i.e.,
reduction of about 16 degrees compared to the single salt sodium
bromide solution of 1.496 gr/cc (12.48 ppg) density.
Example 2 (comparative)
Solubility of NaNO3 in CaBr2 52% solution
Calcium bromide clear brine of 1.71 gr/co (14.2 ppg) density
contains 52% by weight calcium bromide in solution. The TCT of
the brine is -17 C and its pH is 6.5.
To this clear calcium bromide brine (100 g) was added sodium
nitrate (11 gr). Only partial dissolution of the added salt was
observed. The experiment was repeated, this time with a lesser
amount of sodium nitrate (-5 gr). Most of the added amount
dissolved at room temperature. However, to achieve full
dissolution of the -5 gr sodium nitrate in calcium bromide 52%
solution, it was necessary to heat the solution.
CA 03157732 2022-5-9
m4)202110954930
PCT/11,2020/051161
13
Example 3 (comparative)
Solubility of NaNO3 in MhEr2 58% solution
Manganese bromide clear brine of 1.90 gr/cc (15.86 ppg) density
contains -58% by weight manganese bromide in solution. The TCT
of the brine is 4.7 C and its pH is 3.
To this clear manganese bromide brine (100 g) was added sodium
nitrate (11 gr). Dissolution of sodium nitrate was observed,
followed by color change, from pink-reddish to brown, and
formation of a brown precipitate a few hours later. The
observations indicated oxidation of Mn2 to Mn3' and generation
of a brown precipitate of Mn203.
Example 4
Preparing and testing NaSr/NaNO3 solutions with
varying NaNO3 concentration
A saturated sodium bromide solution was prepared by charging a
vessel with solid sodium bromide (530 gr) and water (470 gr). The
mixture was stirred to achieve dissolution of the salt. To obtain
full dissolution, an additional amount of water was gradually
added, affording a clear fluid which contained - 48% by weight
sodium bromide in solution. The total weight was >1000g.
Three portions were taken from the saturated sodium bromide
solution, each consisting of 320 gr. Sodium nitrate was then added
to each of these sodium bromide solutions in the following
amounts: 16 gr, 32 gr and 48 gr, to create three stock sodium
bromide solutions that contained -5%, -9% and -13% by weight
sodium nitrate, respectively.
Each of the three stock solutions was divided into 50 gr portions,
which were diluted by the addition of small amounts of water (1-
1.5 gr, 2-2.5 gr, 3-3.5 gr, 4-4.5 gr, 5-5.5 gr).
CA 03157732 2022-5-9
WO 20211095030
PCT/11,2020/051161
14
Compositions and properties (density and TCT) of the stock
solutions and diluted solutions are tabulated in Table 1 (because
test solutions were prepared by dilution of stock solutions of
-5%, -9% and -13% NaNO3 concentration as described above, NaNO3
concentrations are actually slightly lower; for simplicity, the
-5%, -9% and -13% values are indicated in Table 1 and the
accompanying graph).
Table 1
NaBr single NaBr/-5% NaNO3
NaBr/-9% NaNO3 NaBr/-13% NaNO3
NaBr d TCT NaBr d TCT Naar d
TCT Naar d TCT
g/cc oc g/cc oc
g/cc oc g/cc C
47.0 1.510 11.5 43.9 1.526 9.5 ND
1.555 14.7 39.7 1.556 8.5
46.6 1.503 8.2 43.1 1.512 3.9 42.1
1.522 2.5 38.5 1.537 0.1
46.4 1.497 5.2 41.8 1.497 -2.9 40.4
1.509 -1.4 38 1.526 -3.4
45.5 1.488 0.9 41.0 1.481 -10.9 40.2
1.496 -10.4 37.2 1.512 -10.2
45.2 1.483 -0.7 40.5 1.469 -16 38.9
1.486 -13 36.6 1.499 -15.6
44.6 1.476 -3.3 38.6
1.478 -16.2 36.5 1.494 -16.7
Results are presented in graphical form in Figure 2. TCT versus
density plots show a linear relationship across the density range
under consideration for all four systems, with roughly comparable
slopes, as indicated by the four parallel straight lines.
However, the TCT of sodium bromide/sodium nitrate brines are lower
than that of the corresponding (i.e., of comparable density)
sodium bromide brine. Furthermore, lower TCTs can be achieved
with an increasing concentration of sodium nitrate in the multi-
salt solution: the presence of -5%, -9% and -13% by weight of
sodium nitrate in sodium bromide solution leads to a TCT reduction
of -.8.5 C, -'13.5 C and -'21.5 C, respectively, compared to the
single salt solution.
Owing to the effect of sodium nitrate on TCT, sodium bromide and
sodium nitrate can be formulated, over a useful density range
(say, 1.47 g/cc up to 1.54 g/cc), to give solutions stable against
crystallization down to approximately -2000.
CA 03157732 2022-5-9
WO 2021/095030
PCT/11,2020/051161
Example 5
Preparing and testing NaBr/KEr/NaNC13 solutions
To a saturated sodium bromide 48% solution (300 gr) prepared as
described above was added potassium bromide (30 gr), followed by
the addition of water to obtain a clear fluid. A few samples (50
gr each) were taken and diluted with water to produce sodium
bromide/potassium bromide solutions spanning a density range from
1.536 to 1.470 g/cc.
Another set of samples was prepared by the addition of sodium
nitrate (35 gr) to a stock sodium bromide/potassium bromide
solution, followed by addition of water and removal of non-
dissolved solids by filtration, to obtain a clear brine of 1.593
gr/cc density, which contained 36.7% by weight sodium bromide, 8%
by weight potassium bromide and 8.6% sodium nitrate in solution.
This clear brine served as a stock solution to create a series of
diluted solutions by the addition of small quantities of water.
Compositions and properties of the sodium bromide/potassium
bromide and sodium bromide/potassium bromide/sodium nitrate
solutions are set out in Table 2.
Table 2
NaBr/KBr
NaBr/KBr/NaNO3
NaBr KBr d TCT NaBr
KBr NaNO d TCT
g/cc cc %
% % g/cc 0C
40.0 8.7 1.536 14.3 36.7
8 8.6 1.593 14.7
ND ND 1.520 8.8 ND
ND ND 1.568 5.4
ND ND 1.503 2.3 35.2
7.8 8.2 1.560 1.6
38.3 8.3 1.489 -2.2 ND
ND ND 1.546 -3
ND ND 1.479 -5.8 ND
ND ND 1.530 -7.7
36.6 8.7 1.470 -8.9 33.5
7.4 7.4 1.517 -13.5
CA 03157732 2022-5-9
WO 2021/095030
PCT/11,2020/051161
16
The graph of Figure 3 indicates that a ternary blend composed of
sodium bromide/potassium bromide/sodium nitrate can be formulated
in water to give a clear brine of >1.5 g/cc density, exhibiting
surprisingly low TCT, i.e., from -15 C to -5 C. By contrast, in
the absence of sodium nitrate, solids are crystallized out of
sodium bromide/potassium bromide binary solutions of >1.5 g/cc
density at a temperature in the range from +5 C to +15 C. That is,
across a useful density range [>1.5 g/cc], TCT can be reduced by
-200C with the aid of sodium nitrate.
The graph of Figure 3 further depicts a pair of straight lines
representing TCT versus density plots for sodium bromide alone and
sodium bromide/sodium nitrate, demonstrating a decrease of 13.5 C
in TCT owing to the addition of sodium nitrate, as previously
discussed for Example 4.
The results shown in Figure 3 underscore the strong effect of
sodium nitrate addition on the TCT of a sodium bromide/potassium
bromide brine.
Example 6
Preparing and testing NaBr/LiBr/NaNO3 solutions
To a saturated sodium bromide 48% solution (320 gr) prepared as
described above was added lithium bromide (32 gr), followed by
addition of water to obtain a clear fluid. A few samples (50 gr
each) were taken and diluted with water to produce sodium
bromide/lithium bromide solutions spanning a density range from
1.513 to 1.456 g/cc.
Another set of samples was prepared by the addition of sodium
nitrate (32 gr) to a sodium bromide/lithium bromide stock
solution, followed by addition of water, following which non-
dissolved solids were removed by filtration and a clear brine of
CA 03157732 2022-5-9
WO 2021/095030
PCT/11,2020/051161
17
1.528 gr/cc density was collected, which contained 34.9% by weight
sodium bromide, 6.5% by weight lithium bromide and 8.14% sodium
nitrate in solution. This clear brine served as a stock solution
to create a series of diluted solutions by the addition of small
quantities of water.
Compositions and properties of the sodium bromide/lithium bromide
and sodium bromide/lithium bromide/sodium nitrate solutions are
set out in Table 3.
Table 3
NaBr/LiBr
NaBr/LiBr/NaNO3
NaBr LiBr d TCT NaBr LiBr NaNO3 d
TCT
g/cc 0C
g/cc oc
37.9 9.9 1.513 21.1 34.9 6.5 8.14
1.528 9.5
ND ND 1.495 15.8 ND
ND ND 1.512 2.7
36.6 9.7 1.483 10.8 34 6.5 8.14
1.497 -2.9
ND ND 1.470 5.3 ND
ND ND 1.483 -7.2
35.4 9.1 1.456 -1.7 33.2 6.1 7.77
1.476 -11.8
1.468 -14
Addition of lithium bromide to sodium bromide solution increases
significantly the TCT of the mixture compared to the single salt
sodium bromide brine. The effect of the addition of sodium nitrate
on the TCT of sodium bromide/lithium bromide brine is shown
graphically in Figure 4. Also in this type of alkali bromide blend,
sodium nitrate produces a useful effect: sodium bromide/lithium
bromide/sodium nitrate solution is stable against crystallization
down to temperatures of from -15 C to -5 C, i.e., about 18 C lower
than TCTs of equivalent (i.e., of equal density) sodium
bromide/lithium bromide binary solutions. However, it is seen that
the straight line corresponding to the sodium bromide/sodium
nitrate clear fluid is positioned below that of the sodium
bromide/lithium bromide/sodium nitrate system, indicating that
over the density range under consideration, lower TCTs were
measured for the sodium bromide/sodium nitrate binary blends than
for lithium-containing ternary blends.
CA 03157732 2022-5-9
WO 2021/095030
PCT/11,2020/051161
18
Example 7
Preparing and testing NaBr/CsEr/NaNO3 solutions
To a saturated sodium bromide 48% solution (300 gr) prepared as
described above was added cesium bromide (30 gr), followed by
addition of water to obtain a clear fluid. A. few samples (50 gr
each) were taken and diluted with water to produce sodium
bromide/cesium bromide solutions spanning a density range from
1.611 to 1.531 g/cc.
Another set of samples was prepared by the addition of sodium
nitrate (33.5 gr) to a sodium bromide/cesium bromide stock
solution, following which non-dissolved solids were removed by
filtration and a clear brine of 1.615 gr/cc density was collected,
which contained 36.7% by weight sodium bromide, 8.7% by weight
cesium bromide and 8.9% sodium nitrate in solution. This clear
brine served as a stock solution to create a series of diluted
solutions by the addition of small quantities of water.
Compositions and properties of the sodium bromide/cesium bromide
and sodium bromide/cesium bromide/sodium nitrate solutions are
set out in Table 4.
Table 4
NaBr/CsBr
Na3r/CsBr/NaNO3
NaBr CsBr d TCT NaBr CsBr NaNO3
d TCT
g/ce oc
g/cc 0C
43.1 9.8 1.611 16.8 36.7
8.7 8.9 1.615 9.4
ND ND 1.585 8.3 Nd
ND ND 1.589 0.6
ND ND 1.568 1.8 35.3
8.2 8.6 1.571 -7.1
40.0 9.0 1.551 -5.8 ND
ND ND 1.551 -11.8
39.5 8.8 1.531 -11.8 34.5
8.3 8.3 1.546 -13.4
The information gleaned from the graph of Figure 5 is that the
sodium bromide/cesium bromide/sodium nitrate brine displays TOTs
CA 03157732 2022-5-9
WO 20211095030
PCT/11,2020/051161
19
that are about 8 C lower than sodium bromide/cesium bromide brines
of equal density. This magnitude of reduction of TCT is smaller
than previously observed for the other blends of alkali bromides.
Nevertheless, incorporation of cesium bromide shifts the density
towards higher ranges, say, >1.55 g/cc relative to cesium-free
bromide mixtures, such that sodium bromide/cesium bromide /sodium
nitrate blends can be formulated in water to give a clear brine
of >1.55 g/cc density that does not crystallize out solids down
to temperatures in the range from -15 C to -5 C.
Example 8
Preparing and testing NaBr/VB*3 solutions (A-Li, Na and K)
A set of solutions was prepared by the methods described above,
to investigate the effect of the alkali nitrate (LiNO3, NaNO3 and
10103) on the TCT of sodium nitrate/ alkali nitrate systems. The
concentration of the added alkali nitrate was -9%. Compositions
(sodium bromide/alkali nitrate concentrations) and properties of
the solutions (density and TOT) are set out in Table 5.
Table 5
NaBr alone NaBr/-9% LiNO3
NaBr/-9% NaNO3 NaBr /-9% KNO3
NaBr d TCT NaBr LiNO3 d TCT
NaBrNaNO3 d TCT NaBr KNO3 d TCT
g/cc cc % g/cc cc
% g/cc cc % g/cc cc
47.0 1.510 11.5 42.5 10.2 1.544 15.8 ND ND
1.555 14.7 42.2 9.4 1.544 6.2
46.6 1.503 8.2 ND ND 1.530 8.6 42.1
10.4 1.522 2.5 ND ND 1.528 -0.3
46.4 1.497 5.2 ND ND 1.514 3.7 40.4
8.5 1.509 -1.4 40.0 9.1 1.511 -5.3
45.5 1.488 0.9 40.3 8.2 1.500 -2.2 40.2
8.2 1.496 -10.4 ND ND 1.500 -11.5
45.2 1.483 -0.7 ND ND 1.489 -7.0 38.9
8.0 1.486 -13 38.6 8.6 1.484 -16.3
44.6 1.476 -3.3 38.9 9.1 1.476 -12.3 38.6 8.1
1.478 -16.2
1.469 -6.7 ND ND
1.463 <-18
Graphical representation is provided in Figure 6. The results
indicate that a TCT reduction effect is achieved by the addition
of different alkali nitrates to sodium bromide.
CA 03157732 2022-5-9
WO 20211095030
PCT/11,2020/051161
Example 9
Corrosion experiment
The effect of the presence of sodium nitrate in sodium bromide
brine on the corrosivity of the solution was evaluated using
three-electrode cell arrangement, where the working electrode,
(consisting of the tested specimen) was made of carbon steel ST-
37. Platinum and Ag/AgC1 (in 3.5 M KC1) served as counter and
reference electrodes, respectively. The instrument used for the
measurements was VersaSTAT 3 equipped with V3-Studio software
package. Nitrate-free sodium bromide solution of 1.491 gr/cc
density (p11-7.6) and 39.5% sodium bromide/7.7% sodium nitrate
solution of 1.496 gr/cc density were tested.
Cyclic polarization curves for ST-37 in sodium bromide and sodium
bromide/sodium nitrate solution are shown in Figure 7 (red and
blue curves, respectively). Tafel analysis is used to determine
corrosion potential (Ecorr) and corrosion current (Icon), obtained
by the intersection of the linear sections corresponding to anodic
and cathodic currents. Results are tabulated in Table 6.
Table 6
Solution E con (nil)
I corr PA)
NaBr 690.57
146.64
NaBr/NaNO3 738.24
103.2
Generally, the behavior of carbon steel ST-37 in contact with the
two brines is similar, as is demonstrated by the comparable Ecorr
and la= values and the general shape of the curves. A difference
in favor of the NaBr/NaNO3 brine is observed across the anodic
branch, with the mixed NaBr/NaNO3 brine showing increased
passivation range. The formation of negative hysteresis for both
solutions indicates that pitting - significant corrosion
occurring in a small area - has not been developed.
CA 03157732 2022-5-9