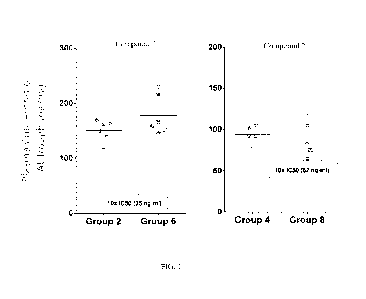Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 2021/202731
PCT/US2021/025171
COMPOSITIONS AND METHODS FOR TREATING INFLAMMATORY BOWEL
DISEASE USING CCR9 INHIBITOR AND ANTI-IL-23 BLOCKING ANTIBODIES
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority to U.S. Provisional
Application No. 63/002,747,
filed March 31, 2020, the disclosure of which is hereby incorporated by
reference in its entirety
for all purposes.
BACKGROUND
[0002] Inflammatory bowel disease (IBD) is a group of chronic
inflammatory conditions
that affects part or all of the gastrointestinal (GI) tract such as the mouth,
esophagus, stomach,
small intestines, large intestines (colon), rectum, and anus. TED includes
Crohn's disease (CD),
ulcerative colitis (UC), and indeterminate colitis. CD and UC can be
distinguished by clinical,
endoscopic and pathological features.
[0003] CD is a disease of chronic inflammation that can involve
any part of the GI tract.
Characteristic symptoms of the disease include severe abdominal pain, frequent
diarrhea, rectal
bleeding, rectal urgency, and swelling of the lower right abdomen.
[0004] UC is a chronic remitting and relapsing inflammatory
disease of the colon. The
disease is characterized by recurring episodes of inflammation primarily
involving superficial
mucosal lesions that extend through the rectum and upwards through the colon.
Acute episodes
are characterized by chronic diarrhea or constipation, rectal bleeding,
cramping and abdominal
pain.
[0005] IBD is characterized by inflammation and the infiltration
of leukocytes such as
lymphocytes, granulocytes, monocytes and macrophages from the blood to the
mucosal or
epithelial lining of the intestines. Multiple inflammatory cell types
including lymphocytes,
neutrophils, macrophages and dendritic cells contribute to IBD. T lymphocytes,
for instance,
infiltrate the mucosa of the gastrointestinal tract through coordinated
interactions between
adhesion molecules on the surface of the T lymphocyte and their cognate
ligands on the
endothelium. Chemokine receptors and ligands, e.g-., the receptor CCR9 and its
ligand CCL25,
1
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
also play a role in the migration of inflammatory cells, e.g., effector memory
T helper cells, into
the intestinal epithelium in IBD.
[0006] In view of the above, it is apparent that effective
treatment regimens for IBD that
are able to block multiple pathways and/or multiple cell types associated with
infiltration of
lymphocytes into intestinal tissue can be useful for treating the disease. The
present invention
provides such therapies along with pharmaceutical compositions and related
methods of
treatment.
BRIEF SUMMARY
[0007] In one aspect, the present disclosure provides a method
of treating or reducing the
development of inflammatory bowel disease in a mammal, said method comprising
administering a suitable amount of the CCR9 inhibitor with an anti-IL-23 or an
anti-IL-23
receptor (anti-IL-23R) blocking antibody. In some embodiments, the
inflammatory bowel
disease is Crohn's disease (CD) or ulcerative colitis (UC).
[0008] In some embodiments, the CCR9 chemokine receptor
inhibitor is a small
molecule receptor inhibitor having a molecular weight of less than 1500. The
CCR9 small
molecule receptor inhibitor can have a molecular weight of about 1495, 1450,
1400, 1300, 1200,
1100, 1000, 900, 800, 700, 600, 500, or less.
[0009] In some embodiments, the CCR9 small molecule inhibitors
provided herein may
be represented by formula (I) or salts thereof:
xl
x2 1101 )(4
x3 x5 (I)
0
--s
y3
Z1
y2 y4
yl
2
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
where X', X2, X3, X4, X5, Y', Y2, Y3, )(4, L', and Z' are as defined below.
[0010] In some embodiments, the CCR9 small molecule inhibitors
provided herein may
be represented by formula (II) or salts thereof:
Oz.-s
'NH
4
R5 -cLN,
¨N Afl
R6 A5
1-1
\A7 -A
where R1, R2, R3, R4, R5, R6, L, Al, A2, A3, A4, A5, A6, A7, and A8 are as
defined below.
[0011] In some embodiments, the anti-IL-23 or the anti-IL-23R
blocking antibody is
risankizumab, guselkumab (TREMFYAg), ustekinumab, briakinumab, brazikumab,
mirikizumab, tildrakizumab, or a biosimilar, biobetter, or bioequivalent
thereof.
[0012] In some embodiments, the CCR9 inhibitor and the anti-IL-
23 blocking antibody
are administered in a combination formulation. In other embodiments, the CCR9
inhibitor and
the anti-IL-23 blocking antibody are administered sequentially. In yet other
embodiments, the
CCR9 inhibitor is administered prior to the anti-IL-23 blocking antibody. In
another
embodiment, the CCR9 inhibitor is administered after administration of the
anti-IL-23 blocking
antibody.
[0013] In some embodiments, the CCR9 inhibitor is a compound
having the formula:
11101
0 ces---"NH
0=S¨NH
0
CI or ci
3
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
[0014] In some embodiments, the CCR9 inhibitor is a compound
selected from:
F
1111
0' NH
0
N =N
-N
N
and
NH2.
[0015] In another aspect, the present disclosure provides a
composition for treating or
reducing the development of inflammatory bowel disease in a mammal, said
composition
comprising a therapeutically effective amount of the CCR9 inhibitor, a
therapeutically effective
amount of an anti-IL-23 or the anti-IL-23R blocking antibody, and a
pharmaceutically acceptable
carrier or excipicnt.
[0016] In some embodiments, the inflammatory bowel disease is
Crohn's disease (CD) or
ulcerative colitis (UC).
[0017] In yet another aspect, the present disclosure provides a
kit for treating or reducing
the development of inflammatory bowel disease in a mammal, said kit comprising
a
therapeutically effective amount of the CCR9 inhibitor, a therapeutically
effective amount of an
anti-IL-23 or the anti-IL-23R blocking antibody, and instructions for
effective administration.
[0018] In some embodiments, the CCR9 inhibitor and the anti-IL-
23 or the anti-IL-23R
blocking antibody are formulated for sequential administration. In other
embodiments, the
CCR9 inhibitor and the anti-IL-23 or the anti-IL-23R blocking antibody are
formulated for
concomitant administration.
[0019] In some embodiments, the anti-IL-23 or the anti-IL-23R
blocking antibody is
risankizumab, guselkumab (TREMFYAg), ustekinumab, briakinumab, brazikumab,
mirikizumab, tildrakizumab, or a biosimilar, biobetter, or bioequivalent
thereof.
[0020] Other objects, features, and advantages of the present
invention will be apparent
to one of skill in the art from the following detailed description and
figures.
4
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
BRIEF DESCRIPTION OF THE DRAWINGS
[0021] FIG. 1 shows Compounds 1 and 2 plasma concentrations at
trough on day 3.
[0022] FIG. 2 shows colon weight-to-length ratio for wild-type
compared to MDR-/-
mice.
[0023] FIG. 3 shows colon weight-to-length ratio for Compound 1-
treated groups and
controls.
[0024] FIG. 4 shows colon weight-to-length ratio for Compound 2-
treated groups and
controls.
DETAILED DESCRIPTION
I. Introduction
[0025] The present disclosure is based, in part, on the
unexpected discovery that a
combination therapy of a CCR9 inhibitor, e.g., a small molecule inhibitor of
CCR9, and an
antibody against IL-23 or against the IL-23 receptor can act synergistically
in the treatment of
inflammatory bowel disease such as Crohn's disease, ulcerative colitis, and
indeterminate colitis.
Provided herein are methods, compositions and kits for treating IBD in a
subject, e.g., human or
animal subject, in need thereof. In some embodiments, the method includes
administering
therapeutically effective amounts of CCR9 inhibitor and an anti-IL-23 or the
anti-IL-23R
antibody to a subject with [BD to elicit a clinical response or maintain
clinical remission in the
subject.
Definitions
[0026] When describing the compounds, compositions, methods and
processes of this
invention, the following terms have the following meanings, unless otherwise
indicated.
[0027] The terms "a," "an," or "the" as used herein not only
include aspects with one
member, but also include aspects with more than one member. For instance, the
singular forms
"a," "an," and "the" include plural referents unless the context clearly
dictates otherwise. Thus,
for example, reference to "a cell" includes a plurality of such cells and
reference to "the agent"
includes reference to one or more agents known to those skilled in the art,
and so forth.
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
[0028] The terms "about" and "approximately" shall generally
mean an acceptable
degree of error for the quantity measured given the nature or precision of the
measurements.
Typical, exemplary degrees of error are within 20 percent (%), preferably
within 10%, and more
preferably within 5% of a given value or range of values. Alternatively, and
particularly in
biological systems, the terms "about" and -approximately" may mean values that
are within an
order of magnitude, preferably within 5-fold and more preferably within 2-fold
of a given value.
Numerical quantities given herein are approximate unless stated otherwise,
meaning that the
term "about" or "approximately" can be inferred when not expressly stated.
[0029] The term "inflammatory bowel disease" or "IBD" includes
gastrointestinal
disorders such as, e.g., Crohn's disease (CD), ulcerative colitis (UC),
indeterminate colitis (IC),
and IBD that is inconclusive for CD vs. UC ("Inconclusive"). Inflammatory
bowel diseases
(e.g., CD, UC, IC, and Inconclusive) are distinguished from all other
disorders, syndromes, and
abnormalities of the gastroenterological tract, including irritable bowel
syndrome (IBS).
Examples of IBD-related diseases include collagenous colitis and lymphocytic
colitis.
[0030] The term "ulcerative colitis" or "UC" refers to a chronic
remitting and relapsing
inflammatory bowel disease (IBD) of the colon or large bowel characterized by
superficial
mucosal lesions that extend through the rectum and progress upstream. The
different types of
ulcerative colitis are classified according to the location and extent of
inflammation. Examples
of UC include, but are not limited to, ulcerative proctitis,
proctosigmoiditis, left-sided colitis, and
pan-ulcerative (total) colitis.
[0031] The term "Crohn's Disease" or "CD" refers to a disease of
chronic inflammation
that can involve any part of the gastrointestinal tract. Commonly, the distal
portion of the small
intestine, i.e., the ileum, and the cecum are affected. In other cases, the
disease is confined to the
small intestine, colon, or anorectal region. CD occasionally involves the
duodenum and
stomach, and more rarely the esophagus and mouth. Examples of UC include, but
are not
limited to, ileocolitis, ileitis, gastroduodenal Crohn's disease,
jejunoileitis, and Crohn's
(granulomatous) colitis.
[0032] The term -subject," -individual" or -patient" refers to
an animal such as a
mammal, including, but not limited to, primates (e.g., humans), cows, sheep,
goats, horses, dogs,
cats, rabbits, rats, mice and the like.
6
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
[0033] The term "C-C chemokine receptor type 9," "CCR9" or "CCR9
chemokine
receptor" refers to a receptor for the chemokine CCL25 which is also known as
TECK and
SCYA25. The human CCR9 polypeptide sequence is set forth in, e.g., GenBank
Accession Nos.
NP 001243298, NP 006632, NP 112477, and XP 011531614. The human CCR9 mRNA
(coding) sequence is set forth in, e.g., GenBank Accession Nos. NM 001256369,
NM 006641,
NM 031200, and XM 011533312.
[0034] The term "C-C chemokine receptor 9 inhibitor," "CCR9
inhibitor" or "CCR9
chemokine receptor inhibitor" refers to an inhibitor or antagonist of a CCR9
receptor
polypeptide, variants thereof, or fragments thereof.
[0035] The term "small molecule inhibitor" refers to a small
molecule or low molecular
weight organic compound that inactivates, inhibits, or antagonizes a target
molecule,
biomolecule, protein or other biological product.
[0036] The term "anti-IL-23 blocking antibody" or "anti-IL-23
neutralizing antibody"
refers to an antibody or a fragment thereof that specifically binds to IL-23
polypeptide or a
fragment thereof. Fragments of IL-23 include IL12b and IL23a. In some cases,
an IL-23
blocking antibody blocks the interaction of IL-23 with any one of its ligands.
The term "anti-IL-
23R blocking antibody" refers to an antibody or a fragment thereof that
specifically binds the IL-
23 receptor or a fragment thereof IL-23, as used herein, is intended to refer
to a human cytokine
that exists as a heterodimeric composed of subunits IL12b and IL23a. The
structure of IL-23 is
described further in, for example, Oppmann B, et al., Immunity, November 2000,
13 (5): 715-
725.
[0037] The term "biosimilar" refers to a biological product that
is highly similar to an
FDA-approved biological product (reference product) and has no clinically
meaningful
differences in terms of pharmacokinetics, safety and efficacy from the
reference product.
[0038] The term -bioequivalent" refers to a biological product
that is pharmaceutically
equivalent and has a similar bioavailability to an FDA-approved biological
product (reference
product). For example, according to the FDA the term bioequivalence is defined
as "the absence
of a significant difference in the rate and extent to which the active
ingredient or active moiety in
pharmaceutical equivalents or pharmaceutical alternatives becomes available at
the site of drug
action when administered at the same molar dose under similar conditions in an
appropriately
7
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
designed study" (United States Food and Drug Administration, "Guidance for
Industry:Bioavailability and Bioequicalence Studies for Orally Administered
Drug Products ¨
General Considerations," 2003, Center for Drug Evaluation and Research).
[0039] The term "biobetter" refers a biological product that is
in the same class as an
FDA-approved biological product (reference product) but is not identical and
is improved in
terms of safety, efficacy, stability, etc. over the reference product.
[0040] The term "therapeutically effective amount" refers to
that amount of the
therapeutic agent sufficient to ameliorate the targeted condition or symptoms.
For example, for
the given parameter, a therapeutically effective amount will show an increase
or decrease of at
least 5%, 10%, 15%, 20%, 25%, 40%, 50%, 60%, 75%, 80%, 90%, or at least 100%.
Therapeutic efficacy can also be expressed as "-fold" increase or decrease.
For example, a
therapeutically effective amount can have at least a 1.2-fold, 1.5-fold, 2-
fold, 5-fold, or more
effect over a control.
[0041] The term "administering" or "administration" and
derivatives thereof refers to the
methods that may be used to enable delivery of agents or compositions to the
desired site of
biological action. These methods include, but are not limited to parenteral
administration (e.g.,
intravenous, subcutaneous, intraperitoneal, intramuscular, intravascular,
intrathecal, intranasal,
intravitreal, infusion and local injection), transmucosal injection, oral
administration,
administration as a suppository, and topical administration. One skilled in
the art will know of
additional methods for administering a therapeutically effective amount of a
compound of the
present invention for preventing or relieving one or more symptoms associated
with a disease.
[0042] The term "treating" or "treatment" refers to the treating
or treatment of a disease
or medical condition (such as inflammation) in a patient, such as a mammal
(particularly a
human or an animal) which includes: ameliorating the disease or medical
condition, i.e.,
eliminating or causing regression of the disease or medical condition in a
patient; suppressing the
disease or medical condition, i.e., slowing or arresting the development of
the disease or medical
condition in a patient; or alleviating the symptoms of the disease or medical
condition in a
patient. The term encompasses the prophylactic treatment of a disease as to
prevent or reduce
the risk of acquiring or developing a specific disease, or to prevent or
reduce the risk of disease
recurrence.
8
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
100431 "Alkyl" by itself or as part of another sub stituent
refers to a hydrocarbon group
which may be linear, cyclic, or branched or a combination thereof having the
number of carbon
atoms designated (i.e., CI-8 means one to eight carbon atoms). The term
"cycloalkyl" by itself or
as a part of another substituent refers to a cyclic alkyl group having the
number of carbons
designated and is a subset of the term "alkyl." Other subsets of the term
"alkyl" include "linear"
and "branched" alkyl groups which refer to two different types of acyclic
alkyl groups.
Examples of alkyl groups include methyl, ethyl, n-propyl, isopropyl, n-butyl,
t-butyl, isobutyl,
sec-butyl, cyclohexyl, cyclopentyl, (cyclohexyl)methyl, cyclopropylmethyl,
bicyclo[2.2.1]heptane, bicyclo[2.2.2]octane, etc. In this list of examples,
the methyl, ethyl, n-
propyl, and n-butyl alkyl examples are also examples of "linear alkyl- groups.
Similarly,
isopropyl and t-butyl are also examples of "branched alkyl" groups.
Cyclopentyl, cyclohexyl,
(cyclohexyl)methyl, cyclopropylmethyl, bicyclo[2.2.1]heptane,
bicyclo[2.2.2]octane are
examples of -cycloalkyl" groups. In some embodiments, cyclopropyl may be used
as a bridging
group between two other moieties and represeneted as ¨CH(CH2)CH¨. "Alkyl
groups can be
substituted or unsubstituted, unless otherwise indicated. Examples of
substituted alkyl include
haloalkyl, thioalkyl, aminoalkyl, and the like. Additional examples of
suitable substituted alkyl
include, but are not limited to, hydroxy-isopropyl, ¨C(CH3)2-0H, aminomethyl,
2-nitroethyl, 4-
cyanobutyl, 2,3-dichloropentyl, and 3 -hydroxy-5-carboxyhexyl, 2-aminoethyl,
pentachloroethyl,
trifluoromethyl, 2-diethylaminoethyl, 2-dimethylaminopropyl,
ethoxycarbonylmethyl,
methanylsulfanylmethyl, methoxymethyl, 3-hydroxypentyl, 2-carboxybutyl, 4-
ehlorobutyl, and
pentafluoroethyl. Suitable substituents for substituted alkyl, include
halogen, ¨CN, ¨CO2R',
¨C(0)R', ¨C(0)NR'R", oxo (=0 or ¨0), ¨OR', ¨0C(0)R', ¨0C(0)NR'R" ¨NO2,
¨NR' C(0)R" , ¨NR' " C(0)NR'R", ¨NR'R" , ¨NR' CO2R" , ¨NR' S(0)R", ¨NR' S
(0)2R" ' ,
¨NR"'S(0)NR'R", 1¨NR" ' S(0)2NR'R", ¨SR' ,¨S(0)R', ¨S(0)2R', ¨S(0)2NR'R",
¨NR'¨
C(NHR")=NR"', ¨0SiR'R"R", ¨N3, substituted or
unsubstituted C6-10 aryl,
substituted or unsubstituted 5- to 10-membered heteroaryl, and substituted or
unsubstituted 3- to
10-membered heterocyclyl. The number of possible substituents range from zero
to (2m'+1),
where m' is the total number of carbon atoms in such radical. With respect to
substituted alkyl,
R', R" and R" each independently refer to a variety of groups including
hydrogen, substituted or
unsubstituted C1_8 alkyl, substituted or unsubstituted C2-8 alkenyl,
substituted or unsubstituted C2-
8 alkynyl, substituted or unsubstituted aryl, substituted or unsubstituted
heteroaryl, substituted or
9
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
unsubstituted heterocyclyl, substituted or unsubstituted arylalkyl,
substituted or unsubstituted
aryloxyalkyl. When R' and R" are attached to the same nitrogen atom, they can
be combined
with the nitrogen atom to form a 3-, 4-, 5-, 6-, or 7-membered ring (for
example, ¨NR'R"
includes 1 -pyrrolidinyl and 4-morpholiny1). Furthermore, R' and R", R" and
R", or R' and
R" may together with the atom(s) to which they are attached, form a
substituted or
unsubstituted 5-, 6-, or 7-membered ring.
[0044] "Alkoxy" refers to ¨0¨alkyl. Examples of an alkoxy group
include methoxy,
ethoxy, n-propoxy etc.
[0045] "Alkenyl" refers to an unsaturated hydrocarbon group
which may be linear, cyclic
or branched or a combination thereof. Alkenyl groups with 2-8 carbon atoms are
preferred. The
alkenyl group may contain 1, 2 or 3 carbon-carbon double bonds. Examples of
alkenyl groups
include ethenyl, n-propenyl, isopropenyl, n-but-2-enyl, n-hex-3-enyl,
cyclohexenyl,
cyclopentenyl and the like. Alkenyl groups can be substituted or
unsubstituted, unless otherwise
indicated.
[0046] "Alkynyl" refers to an unsaturated hydrocarbon group
which may be linear, cyclic
or branched or a combination thereof. Alkynyl groups with 2-8 carbon atoms are
preferred. The
alkynyl group may contain 1, 2 or 3 carbon-carbon triple bonds. Examples of
alkynyl groups
include ethynyl, n-propynyl, n-but-2-ynyl, n-hex-3-ynyl and the like. Alkynyl
groups can be
substituted or unsubstituted, unless otherwise indicated.
[0047] "Alkylamino" refers to ¨N(alkyl)2 or ¨NH(alkyl). When the
alkylamino group
contains two alkyl groups, the alkyl groups may be combined together to form a
carbocyclic or
heterocylic ring. It is to be understood that the alkyl groups of the
alkylamino group may be
substituted or unsubstituted. Examples of an alkylamino group include
methylamino, tert-
butylamino, dimethylamino, di-isopropylamino, morpholino, and the like.
[0048] -Aminoalkyl", as a substituted alkyl group, refers to a
monoaminoalkyl or
polyaminoalkyl group, most typically substituted with from 1-2 amino groups.
Examples
include aminomethyl, 2-aminoethyl, 2-diethylaminoethyl, and the like.
[0049] "Aryl" refers to a polyunsaturated, aromatic hydrocarbon
group haying a single
ring (bicyclic) or multiple rings (preferably bicyclic) which can be fused
together or linked
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
covalently. Aryl groups with 6-10 carbon atoms are preferred, where this
number of carbon
atoms can be designated by C6-10, for example. Examples of aryl groups include
phenyl and
naphthalene-l-yl, naphthalene-2-yl, biphenyl and the like. Aryl groups can be
substituted or
unsubstituted, unless otherwise indicated. Substituted aryl may be substituted
with one or more
substituents. Suitable substituents for awl include substituted or
unsubstituted C1-8 alkyl and
those substituents as discussed above for substituted alkyl
[0050] "Halo" or "halogen", by itself or as part of a
substituent refers to a chlorine,
bromine, iodine, or fluorine atom.
[0051] "Haloalkyl", as a substituted alkyl group, refers to a
monohaloalkyl or
polyhaloalkyl group, most typically substituted with from 1-3 halogen atoms.
Examples include
1-chloroethyl, 3-bromopropyl, trifluoromethyl and the like.
[0052] "Heterocycly1" refers to a saturated or unsaturated non-
aromatic group containing
at least one heteroatom (typically 1 to 5 heteroatoms) selected from nitrogen,
oxygen or sulfur.
Preferably, these groups contain 0-5 nitrogen atoms, 0-2 sulfur atoms and 0-2
oxygen atoms.
More preferably, these groups contain 0-3 nitrogen atoms, 0-1 sulfur atoms and
0-1 oxygen
atoms. Examples of heterocycle groups include pyrrolidine, piperidine,
imidazolidine,
pyrazolidine, butyrolactam, valerolactam, imidazolidinone, hydantoin,
dioxolane, phthalimi de,
piperidine, 1,4-dioxane, morpholine, thiomorpholine, thiomorpholine-S-oxide,
thiomorpholine-
S,S-dioxide, piperazine, pyran, pyridone, 3-pyrroline, thiopyran, pyrone,
tetrahydrofuran,
tetrahydrothiophene, quinucli dine and the like . Preferred heterocyclic
groups are monocyclic,
though they may be fused or linked covalently to an awl or heteroaryl ring
system.
[0053] Exemplary heterocyclic groups may be represented by
formula (AA) below:
(CRaRb)j
im2
(CRGRd)k
(AA)
where formula (AA) is attached via a free valence on either M2 or N42;
M' represents 0, Nit', or
S(0)';
M2 represents CRfkg, 0, S(0)1, or Nit', where it may be necessary to omit one
Rf, Rg, or
Re to create a free valence on or M2 such as, for example CRY, CRg, or N;
1 is 0, 1 or 2; j is 1,
2 or 3 and k is 1, 2 or 3, with the proviso that j + k is 3, 4, or 5; and Ra,
Rb, Re, Rd, Re,
and Rg
11
CA 03174350 2022- 9- 30
WO 2021/202731 PCT/US2021/025171
are independently selected from the group consisting of hydrogen, halogen,
unsubstituted or
substituted C1-8 alkyl, unsubstituted or substituted C2-8 alkenyl,
unsubstituted or substituted C2-
8 alkynyl, ____________ CORh, _____ CO2R1', __________ CONRhiti, __ NRhCOR',
.. S02R1' .. SO2NRhitl,
-NRhS02W-
New, OR", siRhRiRi, osiRhRiRj, Q _co-1-c A,1 Q1CO2R11, -Q1CON-RhRI,
-Q1NRI1COW -Q1s02R11, Qt so2NRh-
QINRhS02R, tik wherein Q1 is a
member selected from the group consisting of C1-4 alkylene, C2-4 alkenylene
and C2-4 alkynylene,
and Rh, R' and R1 are independently selected from the group consisting of
hydrogen and C1-8
alkyl, and wherein the aliphatic portions of each of the Ra, Rb, Re, Rd, Re,
le, Rg,
_I( Wand R
substituents are optionally substituted with from one to three members
selected from the group
consisting of halogen, -OH, -0C(0)NHRn, -0C(0)NWW, -SH,
-S(0)W,
-S(0)2Rn-S(0)2NHRn, -S(0)2NWR", -NHS(0)2Rn, -NWS(0)2R , -C(0)NH2,
-C(0)NHIV, -C(0)NWW, -C(0)R11, -NHC(0)W, -NRnC(0)R , -NHC(0)NH2,
-NRnC(0)NH2, -Nli11C(0)NHR , -NHC(0)NHR", -NRnC(0)NR RP, -NHC(0)NwiRo,
-CO2H, -0O2R11, -NHCO2Rn, - NRnCO2R , -CN, -NO2, -NH2, -Nfliftn, -NRnit ,
-NWS(0)NH2 and -NR"S(0)2N1-TR , where R", R and RP are independently an
unsubstituted
C1-8 alkyl. Additionally, any two of It', Rb, Re, Rd, -e,
Rf, and Rg may be combined to form a
bridged or spirocyclic ring system.
[0054]
Preferably, the number of R" Rb + RC + Rd,groups that are other than
hydrogen is
0, 1 or 2. More preferably, Re, Rb, Re, Rd., -C,
Rf, and Rg are independently selected from the
group consisting of hydrogen, halogen, unsubstituted or substituted C1-8
alkyl, C(0)Rh,
-CO2Rh, -C(0)NR(Rh, NRtic oRi, SO2Rh, -S02 "R', -NSO2R1'Ri, -NR111V, and
-0R11, where Rh and Ware independently selected from the group consisting of
hydrogen and
unsubstituted Ci-8 alkyl; and where the aliphatic portions of each of the It',
Rb, Re, Rd, -e,
Rf, and
W substituents are optionally substituted with from one to three members
selected from the
group consisting of halogen, -OH, -OW, -0C(0)NHW, -0C(0)NWR , -SH,
-S(0)R , -SO2R", -SO2NH2, -SO2NHR", -SO2NR"OW, -NHSO2R", -NWSO2R ,
-C(0)NH2, -C(0)NHR", -C(0)NR"R , -C(0)R", -NHC(0)R", -NWC(0)R ,
-NHC(0)NH2, -NRnC(0)NH2, -NWC(0)NHR , -NHC(0 )1\111Rn, -NWC(0)NWR ,
-NHC(0)NWR , -CO2H, -CO2Rn, -NHCO2W, -NWCO2W, -CN, -NO2, -NH2,
-NRnR , -NWS(0)NH2 and -NWS(0)2NHR , where R11, R and RP are
independently an unsubstituted Ci-8 alkyl.
12
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
[0055] More preferably, Ra, Rb, Re, Rd, Re, Rf, and Rg are
independently hydrogen or
C1-4 alkyl. In another preferred embodiment, at least three of Ra, Rb, Rc, Rd,
Re, Rf, and
Rg are hydrogen.
[0056] "Heteroaryl" refers to an aromatic group containing at
least one heteroatom.
Examples include pyridyl, pyridazinyl, pyrazinyl, pyrimidinyl, triazinyl,
quinolinyl,
quinoxalinyl, quinazolinyl, cinnolinyl, phthalazinyl, benzotriazinyl, purinyl,
benzimidazolyl,
benzopyrazolyl, benzotriazolyl, benzisoxazolyl, isobenzofuryl, isoindolyl,
indolizinyl,
benzotriazinyl, thienopyridinyl, thienopyrimidinyl, pyrazolopyrimidinyl,
imidazopyridines,
benzothiazolyl, benzofuranyl, benzothienyl, indolyl, quinolyl, isoquinolyl,
isothiazolyl,
pyrazolyl, indazolyl, pteridinyl, imidazolyl, triazolyl, tetrazolyl, oxazolyl,
isoxazolyl,
oxadiazolyl, thiadiazolyl, pyrrolyl, thiazolyl, furyl or thienyl. Preferred
heteroaryl groups are
those having at least one aryl ring nitrogen atom, such as quinolinyl,
quinoxalinyl, purinyl,
benzimidazolyl, benzopyrazolyl, benzotriazolyl, benzothiazolyl, indolyl,
quinolyl, isoquinolyl
and the like. Preferred 6-ring heteroaryl systems include pyridyl,
pyridazinyl, pyrazinyl,
pyrimidinyl, triazinyl and the like. Preferred 5-ring heteroaryl systems
include isothiazolyl,
pyrazolyl, imidazolyl, thienyl, furyl, triazolyl, tetrazolyl, oxazolyl,
isoxazolyl, oxadiazolyl,
thiadiazolyl, pyrrolyl, thiazolyl and the like.
[0057] Heterocycly1 and heteroaryl can be attached at any
available ring carbon or
heteroatom_ Each heteroeycly1 and heteroaryl may have one or more rings When
multiple rings
are present, they can be fused together or linked covalently. Each
heterocyclyl and heteroaryl
must contain at least one heteroatom (typically 1 to 5 heteroatoms) selected
from nitrogen,
oxygen or sulfur. Preferably, these groups contain 0-5 nitrogen atoms, 0-2
sulfur atoms and 0-2
oxygen atoms. More preferably, these groups contain 0-3 nitrogen atoms, 0-1
sulfur atoms and
0-1 oxygen atoms. Heterocyclyl and heteroaryl groups can be substituted or
unsubstituted,
unless otherwise indicated. For substituted groups, the substitution may be on
a carbon or
heteroatom. For example, when the substitution is oxo (=0 or 0¨), the
resulting group may
have either a carbonyl (¨C(0)-) or a N-oxide (¨N+-0¨) or ¨S(0)¨ or ¨S(0)2¨.
[0058] Suitable sub stituents for substituted alkyl, substituted
alkenyl, and substituted
alkynyl include halogen, ¨CN, ¨CO2R', ¨C(0)R', ¨C(0)NR'R", oxo (=0 or ¨0¨),
¨OR', ¨0C(0)R', ¨0C(0)NR'R"¨NO2, ¨NIVC(0)R", ¨NR'"C(0)NR'R", ¨NR'R",
13
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
-NR'CO2R", -NR'S(0)2R", -SR', -S(0)R', -S(0)2R', -S(0)2NR'R", -SiR'R"R'",
N3, substituted or unsubstituted C6-10 awl, substituted or unsubstituted 5- to
10-membered
heteroaryl, and substituted or unsubstituted 3- to 10-membered heterocyclyl,
in a number ranging
from zero to (2m'+1), where m' is the total number of carbon atoms in such
radical.
[0059] Suitable sub stituents for substituted aryl, substituted
heteroaryl and substituted
heterocyclyl include halogen, _____ CN, __ CO2R', __ C(0)R', ________________
C(0)NR'R", oxo (=0 or 0-),
-OR', -0C(0)R', -0C(0)NR'R", -NO2, -NR'C(0)R", -NR'C(0)NR"R'", -NR'R",
NR'CO2R", ____________ NR'S(0)2R", __ SR', __ S(0)R', ______ S(0)2R',
S(0)2NR'R",
NR' ___________ C(NHR")=NR'", __ SiR'R"R'", _________________________________
N3, substituted or unsubstituted C1-8 alkyl,
substituted or unsubstituted C2-8 alkenyl, substituted or unsubstituted C2-8
alkynyl, substituted
or unsubstituted C6-10 awl, substituted or unsubstituted 5-to 10-membered
heteroaryl, and
substituted or unsubstituted 3- to 10-membered heterocyclyl. The number of
possible
substituents range from zero to the total number of open valences on the
aromatic ring system.
[0060] As used above, R', R" and R'" each independently refer to
a variety of groups
including hydrogen, substituted or unsubstituted C1-8 alkyl, substituted or
unsubstituted C2-
8 alkenyl, substituted or unsubstituted C2-8 alkynyl, substituted or
unsubstituted awl, substituted
or unsubstituted heteroaryl, substituted or unsubstituted heterocyclyl,
substituted or unsubstituted
arylalkyl, and substituted or unsubstituted aryloxyalkyl. When R' and R" are
attached to the
same nitrogen atom, they can be combined with the nitrogen atom to form a 3-,
4-, 5-, 6-, or 7-
membered ring (for example, _______ NR'R" includes 1-pyrrolidinyl and 4-
morpholiny1).
Furthermore, R' and R", R" and R'", or R' and R" may together with the atom(s)
to which they
are attached, form a substituted or unsubstituted 5-, 6-, or 7-membered ring.
[0061] Two of the substituents on adjacent atoms of an awl or
heteroaryl ring may
optionally be replaced with a substituent of the formula -T-C(0)-(CH2)q-U-,
where T and U
are independently -NR"-, -0-, -CH2- or a single bond, and q is an integer of
from 0 to
2. Alternatively, two of the substituents on adjacent atoms of the aryl or
heteroaryl ring may
optionally be replaced with a substituent of the formula -A'-(CH2)r-B'-, where
A' and B' are
independently -CH2-, -0-, -NR""-, -S-, -S(0)-, -S(0)2-, -S(0)2NR"-- or
a single bond, and r is an integer from 1 to 3. One of the single bonds of the
new ring so formed
may optionally be replaced with a double bond. Alternatively, two of the
substituents on
14
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
adjacent atoms of the aryl or heteroaryl ring may optionally be replaced with
a substituent of the
formula _________ (CH2)s __ X __ (CH2)t , where s and t are independently
integers of from 0 to 3, and
X is _________ 0 __ , __ NR" __ , __ S ___ , __ S(0) __ , __ S(0)2 __ , or
S(0)2NR' . The substituent R"
in ¨NR"¨ and ¨S(0)2NR""¨ is hydrogen or unsubstituted C1-8 alkyl.
[0062] "Heteroatom- is meant to include oxygen (0), nitrogen
(N), sulfur (S) and silicon
(Si).
[0063] "Pharmaceutically acceptable" carrier, diluent, or
excipient is a carrier, diluent, or
excipient compatible with the other ingredients of the formulation and not
deleterious to the
recipient thereof.
[0064] "Pharmaceutically-acceptable salt" refers to a salt which
is acceptable for
administration to a patient, such as a mammal (e.g., salts having acceptable
mammalian safety
for a given dosage regime). Such salts can be derived from pharmaceutically-
acceptable
inorganic or organic bases and from pharmaceutically-acceptable inorganic or
organic acids,
depending on the particular substituents found on the compounds described
herein. When
compounds of the present invention contain relatively acidic functionalities,
base addition salts
can be obtained by contacting the neutral form of such compounds with a
sufficient amount of
the desired base, either neat or in a suitable inert solvent. Salts derived
from pharmaceutically-
acceptable inorganic bases include aluminum, ammonium, calcium, copper,
ferric, ferrous,
lithium, magnesium, manganic, manganous, potassium, sodium, zinc and the like.
Salts derived
from pharmaceutically-acceptable organic bases include salts of primary,
secondary, tertiary and
quaternary amines, including substituted amines, cyclic amines, naturally-
occurring amines and
the like, such as arginine, betaine, caffeine, choline, N,N1-
dibenzylethylenediamine,
diethylamine, 2-diethylaminoethanol, 2-dimethylaminoethanol, ethanolamine,
ethylenediamine,
N-ethylmorpholine, N-ethylpiperidine, glucamine, glucosamine, histidine,
hydrabamine,
isopropylamine, lysine, methylglucamine, morpholine, piperazine, piperidine,
polyamine resins,
procaine, purines, theobromine, triethylamine, trimethylamine, tripropylamine,
tromethamine
and the like. When compounds of the present invention contain relatively basic
functionalities,
acid addition salts can be obtained by contacting the neutral form of such
compounds with a
sufficient amount of the desired acid, either neat or in a suitable inert
solvent. Salts derived from
pharmaceutically-acceptable acids include acetic, ascorbic, benzenesulfonic,
benzoic,
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
camphosulfonic, citric, ethanesulfonic, fumaric, gluconic, glucoronic,
glutamic, hippuric,
hydrobromic, hydrochloric, isethionic, lactic, lactobionic, maleic, malic,
mandelic,
methanesulfonic, mucic, naphthalenesulfonic, nicotinic, nitric, pamoic,
pantothenic, phosphoric,
succinic, sulfuric, tartaric, p-toluenesulfonic and the like.
[0065] Also included are salts of amino acids such as arginate
and the like, and salts of
organic acids like glucuronic or galactunoric acids and the like (see, for
example, Berge, S. M.,
et at, "Pharmaceutical Salts", J. Pharmaceutical Science, 1977, 66:1-19).
Certain specific
compounds of the present invention contain both basic and acidic
functionalities that allow the
compounds to be converted into either base or acid addition salts.
[0066] The neutral forms of the compounds may be regenerated by
contacting the salt
with a base or acid and isolating the parent compound in the conventional
manner. The parent
form of the compound differs from the various salt forms in certain physical
properties, such as
solubility in polar solvents, but otherwise the salts are equivalent to the
parent form of the
compound for the purposes of the present invention.
[0067] "Salt thereof' refers to a compound formed when the
hydrogen of an acid is
replaced by a cation, such as a metal cation or an organic cation and the
like. Preferably, the salt
is a pharmaceutically-acceptable salt, although this is not required for salts
of intermediate
compounds which are not intended for administration to a patient.
[0068] In addition to salt forms, the present invention provides
compounds which are in a
prodrug form. Prodrugs are often useful because, in some situations, they may
be easier to
administer than the parent drug. They may, for instance, be bioavailable by
oral administration
whereas the parent drug is not. The prodrug may also have improved solubility
in
pharmaceutical compositions over the parent drug. A wide variety of prodrug
derivatives are
known in the art, such as those that rely on hydrolytic cleavage or oxidative
activation of the
prodrug. An example, without limitation, of a prodrug would be a compound of
the present
invention which is administered as an ester (the "prodrug"), but then is
metabolically hydrolyzed
to the carboxylic acid, the active entity. Additional examples include
peptidyl derivatives of a
compound of the invention.
[0069] Prodrugs of the compounds described herein are those
compounds that readily
undergo chemical changes under physiological conditions to provide the
compounds of the
16
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
present invention. Additionally, prodrugs can be converted to the compounds of
the present
invention by chemical or biochemical methods in an ex vivo environment. For
example,
prodrugs can be slowly converted to the compounds of the present invention
when placed in a
transdermal patch reservoir with a suitable enzyme or chemical reagent.
[0070] Prodrugs may be prepared by modifying functional groups
present in the
compounds in such a way that the modifications are cleaved, either in routine
manipulation or in
vivo, to the parent compounds. Prodrugs include compounds wherein hydroxyl,
amino,
sulfhydryl, or carboxyl groups are bonded to any group that, when administered
to a mammalian
subject, cleaves to form a free hydroxyl, amino, sulfhydryl, or carboxyl group
respectively.
Examples of prodrugs include, but are not limited to, acetate, formate and
benzoate derivatives
of alcohol and amine functional groups in the compounds of the invention.
Preparation,
selection, and use of prodrugs is discussed in T. Higuchi and V. Stella, "Pro-
drugs as Novel
Delivery Systems," Vol. 14 of the A.C.S. Symposium Series; "Design of
Prodrugs", ed. H.
Bundgaard, Elsevier, 1985; and in Bioreversible Carriers in Drug Design, ed.
Edward B. Roche,
American Pharmaceutical Association and Pergamon Press, 1987, each of which
are hereby
incorporated by reference in their entirety.
[0071] The compounds of the invention may be present in the form
of pharmaceutically
acceptable metabolites thereof. The term "metabolite" refers to a
pharmaceutically acceptable
form of a metabolic derivative of a compound of the invention (or a salt
thereof). In some
aspects, the metabolite may be a functional derivative of a compound that is
readily convertible
in vivo into an active compound. In other aspects, the metabolite may be an
active compound.
[0072] The term "acid isosteres" refers to, unless otherwise
stated, a group which can
replace a carboxylic acid, having an acidic functionality and steric and
electronic characteristics
that provide a level of activity (or other compound characteristic such as
solubility) similar to a
carboxylic acid. Representative acid isosteres include: hydroxamic acids,
sulfonic acids, sulfinic
acids, sulfonamides, acyl-sulfonamides, phosphonic acids, phosphinic acids,
phosphoric acids,
tetrazole, and oxo-oxadiazoles.
[0073] Certain compounds of the present invention can exist in
unsolvated forms as well
as solvated forms, including hydrated forms. In general, both solvated forms
and unsolvated
forms are intended to be encompassed within the scope of the present
invention. Certain
17
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
compounds of the present invention may exist in multiple crystalline or
amorphous forms (i.e., as
polymorphs). In general, all physical forms are equivalent for the uses
contemplated by the
present invention and are intended to be within the scope of the present
invention.
[0074] Certain compounds of the present invention possess
asymmetric carbon atoms
(optical centers) or double bonds; the racemates, diastereomers, geometric
isomers and
individual isomers (e.g., separate enantiomers) are all intended to be
encompassed within the
scope of the present invention. The compounds of the present invention may
also contain
unnatural proportions of atomic isotopes at one or more of the atoms that
constitute such
compounds. For example, the compounds may be radiolabeled with radioactive
isotopes, such as
for example tritium (3H), iodine-125 (1251) or carbon-14 (14C). All isotopic
variations of the
compounds of the present invention, whether radioactive or not, are intended
to be encompassed
within the scope of the present invention.
[0075] The compounds of the present invention may include a
detectable label. A
detectable label is a group that is detectable at low concentrations, usually
less than micromolar,
probably less than nanomolar and possibly less than picomolar, and that can be
readily
distinguished from other molecules, due to differences in a molecular property
(e.g. molecular
weight, mass to charge ratio, radioactivity, redox potential, luminescence,
fluorescence,
electromagnetic properties, binding properties, and the like). Detectable
labels may be detected
by spectroscopic, photochemical, biochemical, immunochemical, electrical,
magnetic,
electromagnetic, optical or chemical means and the like.
[0076] A wide variety of detectable labels are within the scope
of the present invention,
including hapten labels (e.g., biotin, or labels used in conjunction with
detectable antibodies such
as horse radish peroxidase antibodies); mass tag labels (e.g., stable isotope
labels); radioisotopic
labels (including 3H, 1251, 35S, 14C, or 32P); metal chelate labels;
luminescent labels including
fluorescent labels (such as fluorescein, isothiocyanate, Texas red, rhodamine,
green fluorescent
protein, and the like), phosphorescent labels, and chemiluminescent labels,
typically having
quantum yield greater than 0.1; electroactive and electron transfer labels;
enzyme modulator
labels including coenzymes, organometallic catalysts horse radish peroxidase,
alkaline
phosphatase and others commonly used in an ELISA; photosensitizer labels;
magnetic bead
labels including Dynabeads; colorimetric labels such as colloidal gold,
silver, selenium, or other
18
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
metals and metal sol labels (see U.S. Pat. No. 5,120,643, which is herein
incorporated by
reference in its entirety for all purposes), or colored glass or plastic
(e.g., polystyrene,
polypropylene, latex, etc.) bead labels; and carbon black labels. Patents
teaching the use of such
detectable labels include U.S. Pat. Nos. 3,817,837; 3,850,752; 3,939,350;
3,996,345; 4,277,437;
4,275,149; 4,366,241; 6,312,914; 5,990,479; 6,207,392; 6,423,551; 6,251,303;
6,306,610;
6,322,901; 6,319,426; 6,326,144; and 6,444,143, which are herein incorporated
by reference in
their entirety for all purposes.
[0077] Detectable labels are commercially available or may be
prepared as known to one
skilled in the art. Detectable labels may be covalently attached to the
compounds using a
reactive functional group, which can be located at any appropriate position.
Methods for
attaching a detectable label are known to one skilled in the art. When the
reactive group is
attached to an alkyl, or substituted alkyl chain tethered to an aryl nucleus,
the reactive group may
be located at a terminal position of an alkyl chain.
III. Detailed Descriptions of Embodiments
A. Treating Inflammatory Bowel Disease with a Combination
Therapy
[0078] The present disclosure provides methods, compositions,
and kits based on a
combination therapy that includes a CCR9 inhibitor and an anti-IL-23 or an
anti-IL-23R
antibody. This therapy is useful for treating IBD such as Crohn's disease (CD)
and ulcerative
colitis (UC) in a subject. The present invention is based, in part, on the
unexpected discovery
that the synergistic combination of CCR9 inhibitor and an anti-IL-23 or an
anti-IL-23R antibody
is effective at treating IBD.
1. Crohn's disease
[0079] The compositions, methods and kits of the present
invention can be used to a
subject with CD, including all types of CD. The combination therapy of a CCR9
inhibitor and
an anti-IL-23 or an anti-IL-23R antibody can be administered at an effective
amount to induce a
clinical response or maintain clinical remission in a subject with CD. In some
embodiments, the
combi-nation therapy mitigates, reduces or minimizes the severity of one or
more symptoms of
CD.
19
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
[0080] Symptoms of CD include diarrhea, fever, fatigue,
abdominal pain or cramping,
blood in stool, mouth sores, reduced appetite, weight loss, and perianal
disease. Additional
symptoms or characteristics of CD can be evaluated by endoscopy, e.g.,
esophagogastroduodenoscopy, col onoscopy, sigmoidoscopy, endoscopic retrograde
cholangiopancreatography, endoscopic ultrasound, and balloon endoscopy, and
histology of
biopsies form the GI tract. The severity of the disease can be categorized as
mild to moderate,
moderate to severe, and severe/fulminant disease. Additional descriptions
about CD found in,
for example, Lichtenstein et al., Am J Gastroenterol, 2009, 104(2):2465-83.
[0081] Severity of CD as well as clinical response to
combination therapy can be
determined using a clinical index such as the Crohn's Disease Activity Index
or CDAI (Best et
al, Gastroenterology, 1976, 70:439-44). The index is used to quantify the
symptoms of patients
with CD. The CDAI can be used to define clinical response or remission of CD.
The CDAI
consists of eight factors, each added together (summed) after adjustment with
a weighting factor
or multiplier. The eight factors include number of liquid stools, abdominal
pain, general well-
being, extraintestinal complications, antidiarrheal drugs, abdominal mass,
hemacrit, and body
weight. Remission of Crohn's disease is generally defined as a fall or
decrease in the CDAI of
less than 150 points. Severe disease is typically defined as a value of
greater than 450 points. In
certain aspects, response to a particular medication in a Crohn's disease
patient is defined as a
fall of the CDAI of greater than 70 points from baseline (week 0 of
treatment).
[0082] Clinical index such as the CDAI can be used to determine
whether the
combination therapy described herein induces a clinical response or clinical
remission in a
patient with Crohn's disease. In some embodiments, if the patient's CDAI score
decreases by 70
point or more from baseline upon receiving the combination therapy, the
patient is having a
clinical response. If the patient's CDAI score decreases to less than 150
points at the end of the
induction phase of therapy, the patient is in clinical remission of CD.
2. Ulcerative Colitis
[0083] The compositions, methods and kits of the present
invention can be used to a
subject with UC, including all types of UC. The combination therapy of a CCR9
inhibitor and
an anti-IL-23 or an anti-IL-23R antibody can be administered at an effective
amount to induce a
clinical response or maintain clinical remission in a subject with UC. In some
embodiments, the
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
combi-nation therapy mitigates, reduces or minimizes the severity of one or
more symptoms of
UC.
[0084] Symptoms of UC include, but are not limited to, diarrhea,
abdominal pain and
cramping, rectal pain, rectal bleeding, urgency to have a bowel movement,
inability to have a
bowel movement, weight loss, fatigue, fever, or anemia. The severity of the
disease can be
categorized as mild to moderate, moderate to severe, and severe/fulminant
disease. See, e.g.,
Kornbluth et at., Am J Gastroenterol, 2004, 99(7):1371-85.
[0085] Disease activity of UC and response to treatment can be
assessed by quantitative
analysis using a composite index scoring system. Generally, clinicians
consider at least four
factors or variables when assessing UC disease activity: clinical symptoms,
quality of life,
endoscopy evaluation, and histology assessment. For example, the colitis
activity index (CAI) is
a quantitative measurement of incorporates the following disease symptoms:
inflammation in the
colon based on colonoscopy, diarrhea, abdominal pain and cramping, and blood
stool.
Standardized endoscopic score systems such as the UC Endoscopic Index of
Severity (UCEIS)
are useful for establishing a patient's disease index score. Other useful
disease activity indices
include the Mayo Clinic Score (see, e.g., Rutgeert et al., N Eng J Med, 2005,
353(23):2462-76)
and the modified Mayo Disease Activity Index (MMDAI; see, e.g., Schroeder et
al., N Eng J
Med, 1987, 317(26):1625-9). The four factors used in the Mayo Clinic scoring
system include
stool (bowel) frequency, rectal bleeding, endoscopic findings, and the
physician's global
assessment of disease severity (e.g., daily abdominal discomfort and general
sense of well-
being).
[0086] Compared to the Mayo Clinic Score, MMDAI includes the
removal of "friability"
from the endoscopy score of 1. Therefore, the presence of friability reflects
an endoscopy score
of 2 or 3. The 1VIMDAI evaluates 4 subscores (bowel frequency, rectal
bleeding, endoscopic
appearance, and physician's global assessment), each on a scale of 0 to 3 with
a maximum total
score of 12.
[0087] In some embodiments, clinical response by a subject with
UC to a combination
therapy provided herein corresponds to a decrease of 2 points or greater from
baseline in the
M_MDAI score and a 25% or greater decrease from baseline, and/or a decrease of
a 1 point or
21
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
greater from baseline in the rectal bleeding subscore. In other embodiments,
clinical response
corresponds to a decrease of 3 points or greater in Mayo Clinic Score and 30%
from baseline
[0088] Clinical remission by a UC subject administered the
combination therapy can
correspond to a score of 0 for rectal bleeding and a combined score of 2 point
or lower for bowel
frequency and physician's assessment using the MMDAI subscale. In other
embodiments,
clinical remission in a subject with UC refers to having a Mayo Clinic Score
of 2 point or less
and no individual subscore (bowel frequency, rectal bleeding, endoscopic
appearance, and
physician's global assessment) of more than 1 point.
B. Combination Therapy of a CCR9 Inhibitor and Anti-IL-23
Antibodies
[0089] Provided herein are methods, compositions and kits that
take advantage of the
synergistic effect of CCR9 inhibitors and anti-IL-23 or anti-IL-23R antibodies
in reducing
inflammation in subjects with IBD. A combination treatment that includes both
a CCR9
inhibitor and an anti-IL-23 or an anti-IL-23R antibody is more effective at
treating one or more
symptoms of 113D compared to either compound/antibody alone.
1. Chemokine Receptor Type (CCR9) Inhibitors
[0090] The present invention provides compounds that modulate
CCR9 activity.
Specifically, the invention provides compounds having anti-inflammatory or
immunoregulatory
activity. The compounds of the invention are thought to interfere with
inappropriate T-cell
trafficking by specifically modulating or inhibiting a chemokine receptor
function. Chemokine
receptors are integral membrane proteins which interact with an extracellular
ligand, such as a
chemokine, and mediate a cellular response to the ligand, e.g., chemotaxis,
increased
intracellular calcium ion concentration, etc. Therefore, modulation of a
chemokine receptor
function, e.g., interference with a chemokine receptor-ligand interaction, can
inhibit or reduce a
chemokine receptor mediated response, as wells as treat or prevent a chemokine
receptor
mediated condition or disease
[0091] Without being bound by any particular theory, it is
believed that the compounds
provided herein interfere with the interaction between CCR9 and its ligand
CCL25. For
example, compounds of this invention act as potent CCR9 antagonists, and this
antagonistic
activity has been further confirmed in animal testing for inflammation, one of
the hallmark
22
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
disease states for CCR9. Compounds contemplated by the invention include, but
are not limited
to, the exemplary compounds provided herein and salts thereof.
[0092] For example, useful compounds act as potent CCR9
antagonists, and this
antagonistic activity has been further confirmed in animal testing for
inflammation, one of the
hallmark disease states for CCR9. Accordingly, the compounds provided herein
are useful in
pharmaceutical compositions and methods for the treatment of inflammatory
bowel disease, e.g.,
ulcerative colitis and Crohn's disease.
[0093] In some embodiments, the CCR9 small molecule inhibitors
provided herein may
be represented by formula (I) or salts thereof:
X1
X2 X4
X3 X5 (I)
o
¨,S
0
Y3 NH
Ll
y2 y4
y 1
where X', X2, X', X4, X5 are each independently selected from the group
consisting of hydrogen,
halogen, -CN, -NO2, -OR -C(0)Ria, -CO2Ria, -0(CO)Rla, -0C(0)NR1aR2a,
-SO2Ria, -NR1aC(0)R2a, -NR1aC(0)2R2a, -NR11(CO)NRIaR2a,
unsubstituted or
substituted C1-8 alkyl, unsubstituted or substituted C2-8 alkenyl,
unsubstituted or substituted C2-8
alkynyl, unsubstituted or substituted C6-10 aryl, unsubstituted or substituted
5- or 6-membered
heteroaryl, and unsubstituted or substituted 4- to 7-membered heterocyclyl,
with the proviso that
one of Xl, X2, X', X4, X5 is other than hydrogen.
[0094] Rth and R2a are each independently selected from the
group consisting of
hydrogen, C1-8 alkyl, C2-8 alkenyl, C2-8 alkynyl, aryl-C1-4 alkyl, aryloxy-C1-
4 alkyl, C6-10 aryl, 5-
to 10-membered heteroaryl, and 3- to 10-membered heterocycle, or where Ria and
R2a, may
23
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
together with the atom(s) to which they are attached, form an substituted or
unsubstituted 5-, 6-,
or 7-membered ring;
[0095] When X1, X2, )0, X4, or X5 is substituted C1-8 alkyl,
substituted C2-8 alkenyl and
substituted C2-8 alkynyl, it may have from 1 to 3 substituents independently
selected from the
group consisting of halogen, -CN, =0, -0C(0)R1a, Qla, -C(0)R1a, -CONRlaR2a,
_NR2acor la, _
IC CO2R1a, -
NR1aR2a,_SR1',_s(0)R1a, _S(0)2R', -NRiaSO2R2a, unsubstituted or
substituted aryl, unsubstituted or substituted heterocyclyl, and unsubstituted
or substituted
heteroaryl. Preferred substituted C1-8 alkyl have from 1 to 3 substituents
independently selected
from the group consisting of halogen, -CN, =0, -0R1a, -C(0)R1a, -CONRlaR2a,
_NR2acor la, _
IC CO2R1a, -
aNR1 R2a, -S02R1a, unsubstituted or substituted 5- or 6-membered
heteroaryl and 5- or 6-membered unsubstituted or substituted heterocyclyl.
[0096] When Xl, X2, X3, X4, or X5 is substituted C6-10 aryl,
substituted 5- or 6-
membered heteroaryl and substituted 4- to 7-membered heterocycle, it may have
from 1 to 3
substituents independently selected from the group consisting of halogen, -CN,
-0R1a, =0, -
0C(0)R1 a,
-CO2R1 a, -C(0)R1 a, -CONRI aR2a, -NR2aC(0)R1 a, -NR1 aR2a, - SR1 a, -S(0)R1
a, -S(0)2R1 a,
-NR1aS02R2a, unsubstituted C1-8 alkyl, and unsubstituted C1-8 haloalkyl.
[0097] When Xl, X2, X3, X4, or X5 is substituted substituted 5-
or 6-membered
heteroaryl or substituted 5- or 6-membered heteroaryl, it preferably has from
1 to 3 substituents
independently selected from the group consisting of halogen, -0R1 a, -C(0)R1a,
-CONR1 aR2a, -
NR2aC(0)R1 a, -NR1aR2a, -S02R1 a, unsubstituted or substituted C1-8 alkyl, and
unsubstituted
or substituted C1-8 haloalkyl.
[0098] In one embodiment of formula (I), at least one of Xl, X2,
X3, X4, and X5 is other
than hydrogen.
100991 In one embodiment of formula (I), X1 is other than
hydrogen and at least 2 of X2,
X3, X4, and X5 are hydrogen. Preferably, at least 3 of X2, X3, X4, and X5 are
hydrogen; more
preferably, X2, X3, X4, and X5 are hydrogen.
24
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
[00100] In one embodiment of formula(I), Yl, Y2, Y3 and Y4 are
each independently
selected from the group consisting of hydrogen, halogen, -CN, -NO2, -0R3a, -
C(0)R3a, -SR3a,
- CF3, -SOR3a, -SO2R3a, and substituted or unsubstituted C1-4 alkyl.
[00101] In one embodiment of formula(I), R3a is independently
selected from the group
consisting of hydrogen, unsubstituted C1-8 alkyl, unsubstituted C2-8 alkenyl,
unsubstituted C2-8
alkynyl, unsubstituted C6-10 aryl, and unsubstituted 5- to 10-membered
heteroaryl.
[00102] In one embodiment of formula (I), Li -C(0)-, ¨S-, -S(0)-,
or -S(0)2-. In one
embodiment, Li is preferably -C(0)-. In another embodiment of formulae (I), Li
is preferably
¨S-, -S(0)-, or -S(0)2-.
[00103] In another embodiment of formulae (I), when Z1 is
substituted phenyl or
substituted or unsubstituted 5-membered heteroaryl, Li is preferably ¨S-, -
S(0)-, or -S(0)2-.
[00104] In one embodiment of formulae (I), Z1 represents a
substituted phenyl,
unsubstituted or substituted 5- or 6-membered heteroaryl, or substituted 4- to
7-membered
heterocyclyl. Suitably substituted 5- or 6-membered heteroaryls may have from
1 to 3
substituents independently selected from the group consisting of halogen,
unsubstituted or
substituted C1-8 alkyl, unsubstituted or substituted C2-8 alkenyl,
unsubstituted or substituted
C2-8 alkynyl, =0, -CN, -NO2, -0R4a, -0C(0)R4a, -CO2R4a, -C(0)R4a, -
C0NR4aR5a, -NR4aC(0)R5a, -NR4aR5a, -SR4a, -SOR4a, -S02R4a, -S02NR4aR5a, -
NR4aS02R5a, unsubstituted or substituted phenyl, unsubstituted or substituted
5- or 6-
membered heteroaryl, and unsubstituted or substituted 4- to 7-membered
heterocyclyl. If
present, one substituent is preferably located alio to one of the heteroatoms
in the ring or is an
oxygen atom directly connected to a ring heteroatom (i.e. N-oxide).
[00105] R4a and R5a are each independently selected from the
group consisting of
hydrogen, unsubstituted C1-8 alkyl, unsubstituted C2-8 alkenyl, unsubstituted
C2-8 alkynyl,
unsubstituted C6-10 aryl, unsubstituted 5 to 10 membered heteroaryl and
unsubstituted 3- to 10-
membered heterocycle.
[00106] In one embodiment of formula (I), Z1 represents a
substituted phenyl. In one
embodiment in any of the formulae (I), at least one sub stituent on the group
Z1 is unsubstituted
C1-6 alkyl (in particular methyl) or C1-6 haloalkyl (in particular ¨CF3).
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
[00107] In one embodiment of any of formula (I), Z1 is the
following residue:
I
0-
[00108] In one embodiment of any of formula (I), Z1 is selected
from one of the following
residues:
N N
N r4,4
1
N
'I NN N C F3 N
CF3
[00109] In another embodiment of any of formula (I), Z1 is
selected from one of the
following residues:
I I N
[00110] In some embodiments, CCR9 inhibitors, e.g., CCR9 small
molecule inhibitors of
the present disclosure are represented by formula (II), or salts thereof:
R1
R2
R3
NH
3
R5 N- 4
R6 A5
µA7"
where R1 is selected from the group consisting of substituted or unsubstituted
C2-8 alkyl,
substituted or unsubstituted C1-8 alkoxy, substituted or unsubstituted C1-8
alkylamino, and
substituted or unsubstituted C3-10 heterocyclyl, and;
R2 is H, F, Cl, or substituted or unsubstituted C1-8 alkoxy; or
R1 and R2 together with the carbon atoms to which they are attached form a non-
aromatic
carbocyclic ring or a heterocyclic ring;
26
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
R3 is H, substituted or unsubstituted C1-8 alkyl, substituted or unsubstituted
alkoxy, or halo;
R4 is H or F;
R5 is H, F, Cl, or _____ CH3;
R6 i s H, halo, -CN, -CO2Ra, -CONH2, substituted or unsubstituted
Ci_g alkyl,
substituted or unsubstituted alkoxy, or substituted or unsubstituted C1-
8 aminoalkyl;
Ra S H or substituted or unsubstituted C1-8 alkyl;
where R5 and le may together form a carbocyclic ring;
L is a bond, or -CI(CH3)-;
each of A', A2, A3, A4, A5, A6, A7, and Ag are independently selected from the
group consisting
of N,N _________ 0, and __ CR8 __ ; where at least one and not more than two
of Al, A2, A3, A4, A5, A6,
A7, and A8 are N or N-0;
R8 is each independently selected from the group consisting of H, halo, -CN, -
OH, oxo,
substituted or unsubstituted C1-8 alkyl, substituted or unsubstituted C1-8
alkoxy, and -NR R
21,
substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl,
and substituted or
unsubstituted heterocyclyl; and
R2 and R21 are each independently H, or substituted or unsubstituted Cl-s
alkyl.
1001111 In some embodiments of formula (II), one of Al or A2 is N
or N 0, and the
remaining of Al, A2, A3, A4, A5, A6, A7, and A8 are -CR8-, where each R8 is
selected
independently.
[00112] In some embodiments, two of, A2, A3, A4, A5 is N or N-0,
and the remaining
of Al, A2, A3, A4, A5, A6, A7, and A8 are -CR8-, where each R8 is selected
independently.
[00113] In some embodiments, the compounds or compositions of
formula (II) are
represented by formula (III) or salts thereof.
27
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
R1
R2
,
R3
// NH
R5 -S"Lr
¨N
R6 (III),
where R1- is selected from the group consisting of substituted or
unsubstituted C2-8 alkyl,
substituted or unsubstituted C1-8 alkoxy, substituted or unsubstituted C1-8
alkylamino, and
substituted or unsubstituted C3-10 heterocyclyl;
R2 is H, F, Cl, or substituted or unsubstituted alkoxy; or
R' and R2 together with the carbon atoms to which they are attached form a non-
aromatic
carbocyclic ring or a heterocyclic ring;
R3 is H, substituted or unsubstituted C1-8 alkyl, substituted or unsubstituted
C,-s alkoxy, or halo;
R4 is H or F;
R5 is H, F, Cl, or ¨CH3;
K6 is H, halo, ¨CN, ¨CONH2, ¨NH2, substituted or unsubstituted
C1-8 aminoalkyl,
substituted or unsubstituted CI-8 alkyl, or substituted or unsubstituted Ci-g
alkoxy;
R' is H or substituted or unsubstituted Ci-s alkyl;
where R5 and R6 may together form a carbocyclic ring,
L is a bond, ¨CH2¨, or ¨CH(CI3)¨; and
Z is selected from the group consisting of:
28
CA 03174350 2022- 9- 30
WO 2021/202731
PC T/US2021/025171
N===== -. -' N
1 I N I
\ . µ
I 1 1
N
1 N
N$
I
\ N
N NN _
N,N
.-'= 1 " ----..
1 I
'22z. 40 \ . µ I.
\ 0
..k......3 I
---N,N
and N-oxides thereof; where the Z group may be unsubstituted or substituted
with 1 to 3
independently selected R8 substituents;
each R8 is independently selected from the group consisting of H, halo, __ CN,
__ OH, oxo,
substituted or unsubstituted C1-8a1ky1, substituted or unsubstituted C1-8
alkoxy, and
¨NR20R21, substituted or unsubstituted awl, substituted or unsubstituted
heteroaryl, and
substituted or unsubstituted heterocyclyl; and
R20 and R21 are each independently H, substituted or unsubstituted C1-8 alkyl.
[00114] In some embodiments of formula (III), Z is selected from
the group consisting of:
substituted or unsubstituted quinolinyl, substituted or unsubstituted
isoquinolinyl, substituted or
unsubstituted 1,6-naphthyridinyl, substituted or unsubstituted cinnolinyl,
substituted or
unsubstituted phthalazinyl, substituted or unsubstituted quinazolinyl.
[00115]
In one embodiment, the compounds or compositions of formula (II) provided
herein are represented by formula (IIIa) or (Mb), or salts thereof:
29
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
R1 R1
R2 R2
R4----1-
R3 R3
NH
,R8) 19z--/PµNH z(R8)n
'
n
R5 N \ R5 ¨ \
R6 (Ma) R6 \
(Mb)
where RI is selected from the group consisting of substituted or unsubstituted
C2-8 alkyl,
substituted or unsubstituted CI-8 alkoxy, substituted or unsubstituted C1-8
alkylamino, and
substituted or unsubstituted C3-10 heterocyclyl;
R2 is H, F, Cl, or substituted or unsubstituted C1-8 alkoxy; or
R1 and R2 together with the carbon atoms to which they are attached form a non-
aromatic
carbocyclic ring or a heterocyclic ring;
R3 is H, substituted or unsubstituted C1-8 alkyl, substituted or unsubstituted
C1-8 alkoxy, or
halo;
R4 is H or F;
R5 is H, F, Cl, or ¨CH3;
R6 is H, halo, ¨CN, ¨CO2Ra, ¨CONH2, ¨NH2, substituted or unsubstituted C1-8
aminoalkyl, substituted or unsubstituted C1-8 alkyl, or substituted or
unsubstituted C1-8 alkoxy,
Ra is H or substituted or unsubstituted C1-8 alkyl;
or where R5 and R6 together with the carbon atoms to which they are attached
form a carbocyclic
ring,
each R8 is independently selected from the group consisting of H, halo, ¨CN,
¨OH, oxo,
substituted or unsubstituted CI-8 alkyl, substituted or unsubstituted C1-8
alkoxy, and
NR2OrsK 21,
substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl,
and
substituted or unsubstituted heterocyclyl;
R2 and R21 are each independently H, or substituted or unsubstituted C1-8
alkyl; and
n is 0, 1, 2 or 3.
CA 03174350 2022- 9- 30
WO 2021/202731 PCT/US2021/025171
[00116] In one embodiment of formula (Ma) or (nth) or salts
thereof, R1 is selected from
the group consisting of: _____ CH2CH3, __ CH(CH3)2, __ C(CH3)3, __
C(CH3)2CH2CH3,
C(CH2CH2)CN, ____ C(OH)(CH3)2, ______ OCH3, ______ OCH2CH3, OCH(CH3)2,
OC(CH3)3,
-OCH2CH(CH3)2, -0CF3, and morpholino; R2 is H, F, or CI; or R1 and R2 may
together
form __________ OC(CH3)2CH2 __ or __ C(CH3)2CH2CH2 __ ; R3 is H, _____ CH3, or
OCH3; R4 is H or
F; R5 is H; R6 is H, -CH3, -CH2CH3, -CH(CH3)2, -C3H7, -CH2F, -CHF2, -
CF2CH3, -CF3,
-CH2OCH3, -CH2OH, -CH2CN, -CN, or -CONH2; and each R8 is independently
selected from the group consisting of H, F, Cl, Br, -CH3, -OH, -OCH3, -
OCH2CH3, -
NH2, -N(CH3)2, and -CN. In some instances, R1 is -C(CH3)3.
[00117] In other embodiments of formula (Ma) or formula (Mb), R2
is H or F; R3 is H;
R4 is H; and R6 is -CH3, -Cl2F, -CI-1F2, or -CF3.
[00118] In one embodiment, the compounds and compositions of
formula (Ma) or (IIIb)
or salts thereof are selected from the group consisting of:
0 F 401 F F
NH2 NH2
01-NH N 0-1-NH ,0
N 01-NH
N
F
F F
,0 00 F
0-1-NH , N 0=--S-NH N
+,0
0 ...,N \ \ :=S-NH
N
/ % g
\
8
_ NH2
F
31
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
F 0 F
111110
õ6
0=---NH N 0=S-NH NI 0=S-NH
N
\
0 0 ..,N1 8
\
--- NH2
,, ----
-- N ---- \
-- N
(1101 40 0
õ0-
õo-
0=-S-NH N
0=S-NH N 0=S-NH
N
8 N \ 0 .,1q \ II
\
NH2
0
-- N -- N
F
F
ION ION lb
õO
0=S-NH N Oz--S -NH N 0=S-NH
N
8 . /,N1 -- \ 8 ..,NI
F \ 8 N -- \
/ %
F F
F and
0
+ 0-
0=-S -NH NI"
8 N -_ \
1001191
In some embodiments, CCR9 inhibitors, e.g., CCR9 small molecule inhibitor
compounds and compositions provided herein are selected from the group
consisting of:
32
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
Me me Me
Me me Me
Me
Me
NH
110 IP
H //¨ N
N
H \ cr,s¨N N ¨114 0'
0-----rN N N¨ b
6 r'N1
/ 1\1%
z
F F FF
F Me
\ iN
F
Me
M MeMe
e Me
r
0 0
,0
01
S
, s I, ---- N
0' NH N \
(1
0=S¨NH --11 \1 6 N 0=S¨NH
N
\
¨N .. il 8 ii
..1\1 --..
Me \ iN ..-
N
Me
Me
Me
Me 40 CN
OMe
0 ION
0
0=S¨NH NH 0=S¨NH N
II ii \ 0=S¨NH
N
0 .,1\1 , 0 0 ..,.N ,
\
N .. N 8 N
Me Me Me
Me
Me
Me
OH
Sc' ION
ION
0=S¨NH N
- \ 0=S¨NH N
8 N \ 0=S¨NH
8
N
\
-- N / % , 6 N
,
,-- N
-- N
Me
Me
Me
33
CA 03174350 2022- 9- 30
WO 2021/202731 PCT/US2021/025171
Me Me
Me Me
Me Me
Me Me
Me
IP I
N,
101 0 Me
01-NH 0=S-NH N
0=S-NH N
.._1\1 6
...- ki 8, ki
Me
Me Me Me Me Me
Me Me
\e%
CY -1\1
0 1101 Me
0 0 ye
0
0=S-NH N 0=S-NH N
8 . 1\1 ---- \ 8 N -- \ O= NH
N
\
-_
M
Me e Me
Me
0
Me Me
Me MeMe
c N Me
)
411101 0 1110
0=S¨NH 4111P \N
0=S¨NH N
0=II
S¨NH N 8
) N --
--- N
Me Me
Me
0
Me Me Me CD MeMe Me
N
0
Oil F
Oil
0=S-NH N N
01-NH
\
8 N .,..N -- \ 0=S-NH N
\
O& -__
-- N 6 _.N
.- N -_
--- N
HO Me
34
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
Me
Me
Me Me
Me Me Me
Me
0
I. Me 1161 OM
01¨NH N
01¨NH N 0=S¨NH N
8 __INI --- \ 0 N 1 II \
---N
0
N ,
Me Me
Me
Me Me Me
Me Me
Me
Me
0 Me
1110
0 a
N/ \
01¨NH --... 01¨NH
8 01¨NH N
\
..-1\1 ,IV N¨ 8
_
, \IMe
Me Me
Me
Me Me
Me Me Me Me Me
Me Me
(110 0 N
11101
III7
--
0=S¨NH N 0=S¨NH .' 0=S¨NH jc;i-,..
1\r.
Me ...N
Me Me
Me
Me Me
Me
MeMe Me
Me Me
1110 0 Me
/N\
110
0=S¨NH N
01¨NH --. \ H
N,
F
Me
Me
Me
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
Me Me
Me
Me me
Me Me
Me
Me
IP
H N
0":1-Nit,Nri 0:=S-NH \ / 0=S-NH
N
\
0 / INI 6 111 8,
N,1 _
N
Me
Me NC
Me
Me Me Me )¨Me
0 Me Me
0
0 CI
4101
0=S-NH N 0=-NH N
0 II \ \ 0=-NH N
N ,
\
.iNi .iN1 (-3
__1=1 ,
Me .N
Me F me
F Me
Me Me
Me Mew
Me Meme
lel
0
INI
0=S-NH N
0 N ---- \ 0=-NH N
II
\ 0=S-NH
N
,-N 0 ./INI
-- N ,
F --kJ
Me
F 0 NH2 F
Me
F
Me
Me MeMe <
Me MeMe /
0 Me
0 neil
Oil
0=S-NH N
ii \ 0=S-
NH N
0=S-NH 0 _.N\ , \
8 1` i i 8 q
,
.' .- ,-
i'\ 1
F
F Me
Me F
36
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
Me
...--Me
0 Me me Me me
Me Me
1110
110 H *
0= 1111
N S-NH H \N N\ me
;1 _
8 N --- \ 0-=S-N N ¨ 0---S-N
0 ,
b ti`N
Me Me Me
Me F\ 7F
Me Me
07.--F Et Me
IIP H = N\ 1104H \ N
111
N
\I
0-1-Nt:r H \
0=-Sõ-N
0 N / ;NI 0:---S-NtI,N
o ,N ¨
0 ,r,`N
Me
Me
Me
Me Me me
Me Me me
Me Me
. N 1104 N lb
H \ H \ H
N
0-=rN N ¨
\
cy.--S-N N _ 0 -N =S
0 i /1\1
,
O 6 =.\,,,,,
F F CN
F
Me
Me me
Me )¨Me Me
m
0 Me me
IP N * H 01
N
H \ \ NI / \
0"---N N ¨ 0-N N<\,>
=-S-N ¨ 0 NH Ni
----=S- .. ¨
N 6 N
8 ,i\i =
F"--- Me
37
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
Me
Me me
Me )¨Me
F 0 Me
OMe Me
IP N 110
N AO
H =
H = \ N
\ H \
0N N
N ¨
_
6 trsN 0 'N 0---sr" N
b ill\I
Me /
Me
F
F
F
Me me
Me Me Me
1110 N )¨Me
0
)¨Me
0
H \
0"----Sõ--N N 1110 H = .
N
0 / ;NI
0=-----N N
0 / 1\1 2N H
07---SC-N N
F_ \
F z
)[N
F F
F
F F
Me me
Me
Me Me Me me
Me
H
Me
N
. H
IP
N
CI
H \
0-1¨N N ¨ \ 110 F
N
0 y 0.___.,__N N _ 0-,,
,,
,
_\
OMe
Me Me
Me me
Me Me me
Me Me me
IP NH 2 Me
H \ N 1110 H N
0 N
0=SA¨Nr(\i ¨ \ NH2
NI' \
0--s,-",(,, H
N
b / 1\I 0=-
So¨N ¨.
/
Me
Me
Me
38
CA 03174350 2022- 9- 30
WO 2021/202731 PCT/US2021/025171
Me Me me Me Me Me me
Me
IP N
/ \
/ \
H
N H
0":-.Sµ, --N:\(1 0=S;N=cr 2
rNi
0---:-S,-Nt;i
0 / 1\1 b / 1\I
, b
Me Me
Me
Me Me me Me
Me Me )¨Me
0
110
H = \ Il __
N H
110
H i
c N > N 1104
0=SrN N 0=-6- c
b i\I
= N\
r'l \J , õ
01--S,-N
N
t
(N
M
F-k-F e
F¨F
F
F
Me me
Me Me me Me me
Me Me
0
IP HN
N'
Me 100 H2N
H /
N
\
H \ N
H \
0=Sk-N,Ni
b(
/z1\I 0":=,-N\I s-N
0=
tr.I.:1,N
/
Me
Me Me
Me m Me me
e
Me Me me
Me Me
OMe
al N-N
/ \
0 N NH2
/ \
. H N / \ H
H
0--=SI-N N 07---Sõ-N N .
0:=S" -Nt.:\rj
rsf\J
10 0 rµl \I 0 / `N
/
Me
Me Me
39
CA 03174350 2022- 9- 30
WO 2021/202731 PCT/US2021/025171
Me me Me me Me me
Me Me Me
F
I
CI
/ N\ F P N 11104
\
H _ H \
H Ni
01-N N 07---Sõ-N ¨ 0=S"-N.:\rj
0 ,:N 0 )1 IN
0 / 'N
Me Me Me
Me Me Me me Me me
Me Me Me
F F
Me NH2
1110 N AP H N
H \ H
\
0--1-Nr\ri
01---Ni.:(\i ¨
/ ;N 0 / / N 0 / `N
F F
Me Me
Me Me Me Me Me me
Me Me Me
F F
. , N
/ \ 10H 11104H
N
H H 0 N\ H \ NH2
F
07-"SC-N N 0"---3," N ¨ N=S-N ¨
b rs1\1 b v 0 tt,,`N
Me Me
F
Me me Me me Me me
Me Me Me
110 F
N IP +6 40 CI
H \ H 410. 14\ H N
\
N - 01--NI.,/\(1 0=-1-N.Nri -
0
Me Me Me
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
Me me
Me Me me Me me
Me Me
104 F
OEt
F
H \ + -
N-0 H \ N 1101
0,s1-N\c:(i4 H
\ N
/ ,I NI ,-s,
0--N
ve cµN _
0=-SCN N
b / 'N
r
Me
Me
Me
Me me Me me
Me Me me Me
Me
1110 H = N\ 1110H OMe
N *
H Me
N
\
--Nr\(1N \ 0=--S"-NN(i
' \
0 / r(j
b / /1\1 ¨ 0 i 'N
Me
Me
Me
Me
Me me )¨Me Me me
Me Me
0
Br F F
Br
104 H N . H N 1104
N
\ \ H
\
0=1-N N
b b 0":3SI-N 0=-3,-N(1 ¨ ¨
/ ;NI
/ 1\1
/
b / 'NI
z
Me Me
Me
Me me Me me Me me
Me Me Me
NH2 F CN CN
IPH \ N 1.4 N 1110 H
N
H \
\
0=SI-N.Nci ¨ 0--1-Ni(NNI ossSI-N\c:(
_
b /NI 0 / ' b
z
Me Me Me
and N-oxides thereof
[00120] In some embodiments, the preferred It' substituents are
as follows. In formula (II,
III, Ma, and Mb), Itl is selected from the group consisting of substituted or
unsubstituted C2-8
alkyl, substituted or unsubstituted C1-8 alkoxy, substituted or unsubstituted
C1-8 alkylamino, and
substituted or unsubstituted C3-10 heterocyclyl. When Itl is substituted
alkyl, the alkyl group is
41
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
preferably substituted with halo or hydroxy. When RI- is substituted alkoxy,
the alkoxy group is
preferably substituted with halo. Preferably, RI- is unsubstituted C2-8 alkyl,
including C3-8
cycloalkyl, C2-8 haloalkyl, C1-8 hydroxyalkyl, unsubstituted C1-8 alkoxy, C1-8
haloalkoxy, and C1-8
alkylamino; more preferably unsubstituted C2-8 alkyl, C2-8 haloalkyl,
unsubstituted Ci-g alkoxy,
and C1-8 alkylamino; even more preferably unsubstituted C2-8 alkyl,
unsubstituted CI-8 alkoxy,
and morpholino; still more preferably unsubstituted C2-8; and most preferably
t-butyl.
[00121] In some embodiments, the preferred R6 substituents are as
follows. In formula
(II, III, Ma, and Mb), R6 is H, halo, ¨CN, ¨CO2Ra, ¨CONH2, ¨NH2, substituted
or
unsubstituted C1-8 alkyl, substituted or unsubstituted C1-8 alkoxy, or
substituted or
unsubstituted C1-8 aminoalkyl. When R6 is substituted alkyl, the alkyl group
is preferably
substituted with halo, hydroxy, alkoxy, or cyano. Preferably R6 is ¨CN,
¨CONH2, ¨NH2,
unsubstituted C1-8 alkyl, unsubstituted C1-8 haloalkyl, and unsubstituted C1-8
alkoxy; more
preferably unsubstituted C1-8 alkyl, or unsubstituted C1-8 haloalkyl, even
more preferably
unsubstituted C1-8 alkyl, most preferably methyl.
[00122] In one embodiment, the CCR9 small molecule inhibitor is
Compound 1.
JLCLcI
CI
Compound 1
[00123] In one embodiment, the CCR9 small molecule inhibitor is
Compound 2.
42
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
Lr
cy NH
N
NH2
Compound 2
[00124] In one embodiment, the CCR9 small molecule inhibitor is
Compound 3.
0=S¨NH
FF
- -
N
Compound 3
[00125] In one embodiment, the CCR9 small molecule inhibitor is
Compound 4.
0
0=S¨NH
0
CI
Compound 4
[00126] Detailed descriptions of the CCR9 inhibitor compounds
provided herein and
methods for preparing such compounds is found in, for example, U.S. Patent No.
6,939,885, U.S.
43
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
Patent No. 8,916,601, and U.S. Patent Application Publication Nos.
2013/0267492,
2013/0059893, 2012/0245138, 2012/0165303, 2011/0021523, 2010/0331302,
2010/0227902,
2010/0190762, 2010/0152186, 2010/0056509, 2009/0163498 and 2009/0005410, the
disclosures
of which are herein incorporated by reference in their entirety for all
purposes.
[00127] The compounds provided herein may be may be synthesized
using a variety of
standard organic chemistry transformations. Certain general reaction types
employed widely to
synthesize target compounds in this invention are summarized in the examples.
Specifically,
generic procedures for sulfonamide formation and aza-aryl N-oxide formation
are described
within and were employed routinely.
[00128] While not intended to be exhaustive, representative
synthetic organic
transformations which can be used to prepare compounds of the invention are
included herein.
These representative transformations include; standard functional group
manipulations;
reductions such as nitro to amino; oxidations of functional groups including
alcohols and aza-
aryls; aryl substitutions via IPSO or other mechanisms for the introduction of
a variety of groups
including nitrile, methyl and halogen; protecting group introductions and
removals; Grignard
formation and reaction with an electrophile; metal-mediated cross couplings
including but not
limited to Buckwald, Suzuki and Sonigashira reactions; halogenations and other
electrophilic
aromatic substitution reactions; diazonium salt formations and reactions of
these species;
etherifications, cyclative condensations, dehydrations, oxidations and
reductions leading to
heteroaryl groups; aryl metallations and transmetallations and reaction of the
ensuing aryl-metal
species with an electrophile such as an acid chloride or Weinreb amide;
amidations;
esterifications; nucleophilic substitution reactions; alkylations; acylations;
sulfonamide
formation; chlorosulfonylations; ester and related hydrolyses, and the like.
[00129] Certain molecules claimed in this patent can exist in
different enantiomeric and
diastereomeric forms and all such variants of these compounds are within the
scope of the
invention.
[00130] In the descriptions of the syntheses that follow, some
precursors were obtained
from commercial sources. These commercial sources include Aldrich Chemical
Co., Acros
Organics, Ryan Scientific Incorporated, Oakwood Products Incorporated,
Lancaster Chemicals,
44
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
Sigma Chemical Co., Lancaster Chemical Co., TCI-America, Alfa Aesar, Davos
Chemicals, and
GFS Chemicals.
2. Pharmaceutical Formulations of CCR9 Inhibitors
[00131] In another aspect, the present disclosure provides
compositions or formulations
that modulate CCR9 activity. Generally, the compositions or formulations for
modulating
chemokine receptor activity in a subject such as a human or animal will
comprise a compound
provided herein and a pharmaceutically acceptable excipient or diluent.
[00132] The term "composition" as used herein is intended to
encompass a product
comprising the specified ingredients in the specified amounts, as well as any
product which
results, directly or indirectly, from combination of the specified ingredients
in the specified
amounts. By "pharmaceutically acceptable" it is meant the carrier, diluent or
excipient must be
compatible with the other ingredients of the formulation and not deleterious
to the recipient
thereof.
[00133] The pharmaceutical compositions for the administration of
the compounds of this
invention may conveniently be presented in unit dosage form and may be
prepared by any of the
methods well known in the art of pharmacy. All methods include the step of
bringing the active
ingredient into association with the carrier which constitutes one or more
accessory ingredients.
In general, the pharmaceutical compositions are prepared by uniformly and
intimately bringing
the active ingredient into association with a liquid carrier or a finely
divided solid carrier or both,
and then, if necessary, shaping the product into the desired formulation. In
the pharmaceutical
composition the active object compound is included in an amount sufficient to
produce the
desired effect upon the process or condition of diseases.
[00134] In some embodiments, the CCR9 inhibitor of the present
disclosure is a
pharmaceutical compound having a crystalline form. A non-limiting example of
such a
crystalline form of a CCR9 inhibitor is described in, e.g., U.S. Patent No.
9,133,124, the
disclosure of which is herein incorporated by reference in its entirety for
all purposes.
[00135] The pharmaceutical compositions containing the active
ingredient may be in a
form suitable for oral use, for example, as tablets, troches, lozenges,
aqueous or oily suspensions,
dispersible powders or granules, emulsions and self-emulsifications as
described in U.S. Patent
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
No. 6,451,399, hard or soft capsules, or syrups or elixirs. Compositions
intended for oral use
may be prepared according to any method known to the art for the manufacture
of
pharmaceutical compositions. Such compositions may contain one or more agents
selected from
sweetening agents, flavoring agents, coloring agents and preserving agents in
order to provide
pharmaceutically elegant and palatable preparations. Tablets contain the
active ingredient in
admixture with other non-toxic pharmaceutically acceptable excipients which
are suitable for the
manufacture of tablets. These excipients may be, for example, inert diluents
such as cellulose,
silicon dioxide, aluminum oxide, calcium carbonate, sodium carbonate, glucose,
mannitol,
sorbitol, lactose, calcium phosphate or sodium phosphate, granulating and
disintegrating agents,
for example, corn starch, or alginic acid; binding agents, for example PVP,
cellulose, PEG,
starch, gelatin or acacia, and lubricating agents, for example magnesium
stearate, stearic acid or
talc. The tablets may be uncoated or they may be coated enterically or
otherwise by known
techniques to delay disintegration and absorption in the gastrointestinal
tract and thereby provide
a sustained action over a longer period. For example, a time delay material
such as glyceryl
monostearate or glyceryl distearate may be employed. They may also be coated
by the
techniques described in the U.S. Patent Nos. 4,256,108; 4,166,452; and
4,265,874 to form
osmotic therapeutic tablets for control release.
[00136] Formulations for oral use may also be presented as hard
gelatin capsules where
the active ingredient is mixed with an inert solid diluent, for example,
calcium carbonate,
calcium phosphate or kaolin, or as soft gelatin capsules where the active
ingredient is mixed with
water or an oil medium, for example peanut oil, liquid paraffin, or olive oil.
Additionally,
emulsions can be prepared with a non-water miscible ingredient such as oils
and stabilized with
surfactants such as mono-diglycerides, PEG esters and the like.
[00137] Aqueous suspensions contain the active materials in
admixture with excipients
suitable for the manufacture of aqueous suspensions. Such excipients are
suspending agents, for
example sodium carboxymethylcellulose, methylcellulose,
hydroxypropylmethylcellulose,
sodium alginate, polyvinyl-pyrrolidone, gum tragacanth and gum acacia;
dispersing or wetting
agents may be a naturally-occurring phosphatide, for example lecithin, or
condensation products
of an alkyl ene oxide with fatty acids, for example polyoxyethylene stearate,
or condensation
products of ethylene oxide with long chain aliphatic alcohols, for example
heptadecaethyleneoxycetanol, or condensation products of ethylene oxide with
partial esters
46
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
derived from fatty acids and a hexitol such as polyoxyethylene sorbitol
monooleate, or
condensation products of ethylene oxide with partial esters derived from fatty
acids and hexitol
anhydrides, for example polyethylene sorbitan monooleate. The aqueous
suspensions may also
contain one or more preservatives, for example ethyl, or n-propyl, p-
hydroxybenzoate, one or
more coloring agents, one or more flavoring agents, and one or more sweetening
agents, such as
sucrose or saccharin.
[00138] Oily suspensions may be formulated by suspending the
active ingredient in a
vegetable oil, for example arachis oil, olive oil, sesame oil or coconut oil,
or in a mineral oil such
as liquid paraffin. The oily suspensions may contain a thickening agent, for
example beeswax,
hard paraffin or cetyl alcohol. Sweetening agents such as those set forth
above, and flavoring
agents may be added to provide a palatable oral preparation. These
compositions may be
preserved by the addition of an antioxidant such as ascorbic acid.
[00139] Dispersible powders and granules suitable for preparation
of an aqueous
suspension by the addition of water provide the active ingredient in admixture
with a dispersing
or wetting agent, suspending agent and one or more preservatives. Suitable
dispersing or wetting
agents and suspending agents are exemplified by those already mentioned above.
Additional
excipients, for example sweetening, flavoring and coloring agents, may also be
present.
[00140] The pharmaceutical compositions of the invention may also
be in the form of oil
in water emulsions. The oily phase may be a vegetable oil, for example olive
oil or arachis oil, or
a mineral oil, for example liquid paraffin or mixtures of these. Suitable
emulsifying agents may
be naturally-occurring gums, for example gum acacia or gum tragacanth,
naturally-occurring
phosphatides, for example soy bean, lecithin, and esters or partial esters
derived from fatty acids
and hexitol anhydrides, for example sorbitan monooleate, and condensation
products of the said
partial esters with ethylene oxide, for example polyoxyethylene sorbitan
monooleate. The
emulsions may also contain sweetening and flavoring agents.
[00141] Syrups and elixirs may be formulated with sweetening
agents, for example
glycerol, propylene glycol, sorbitol or sucrose. Such formulations may also
contain a demulcent,
a preservative, and flavoring and coloring agents. Oral solutions can be
prepared in combination
with, for example, cyclodextrin, PEG and surfactants.
47
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
[00142] The pharmaceutical compositions may be in the form of a
sterile injectable
aqueous or oleaginous suspension. This suspension may be formulated according
to the known
art using those suitable dispersing or wetting agents and suspending agents
which have been
mentioned above. The sterile injectable preparation may also be a sterile
injectable solution or
suspension in a nontoxic parenterally acceptable diluent or solvent, for
example as a solution in
1,3-butane di ol . Among the acceptable vehicles and solvents that may be
employed are water,
Ringer's solution and isotonic sodium chloride solution. In addition, sterile,
axed oils are
conventionally employed as a solvent or suspending medium. For this purpose
any bland fixed
oil may be employed including synthetic mono- or diglycetides. In addition,
fatty acids such as
oleic acid find use in the preparation of injectables.
[00143] The compounds disclosed herein may also be administered
in the form of
suppositories for rectal administration of the drug. These compositions can be
prepared by
mixing the drug with a suitable non-irritating excipient which is solid at
ordinary temperatures
but liquid at the rectal temperature and will therefore melt in the rectum to
release the drug. Such
materials are cocoa butter and polyethylene glycols. Additionally, the
compounds can be
administered viaocular delivery by means of solutions or ointments. Still
further, transdermal
delivery of the subject compounds can be accomplished by means of
iontophoretic patches and
the like.
[00144] For topical use, creams, ointments, jellies, solutions or
suspensions containing the
compounds of the present invention are employed. As used herein, topical
application is also
meant to include the use of mouth washes and gargles.
[00145] The pharmaceutical compositions and methods of the
present invention may
further comprise other therapeutically active compounds as noted herein, such
as those applied in
the treatment of the above mentioned pathological conditions.
3. Anti-IL-23 Blocking Antibodies
[00146] Anti-IL-23 antibodies suitable for use in the treatment
of inflammatory bowel
disease, e.g., Crohn's disease and ulcerative colitis include antibodies from
any desired source
that inhibits the binding of IL-23 to any one of its ligands. Anti-IL-23R
antibodies bind the IL-23
receptor or a fragment thereof Anti-IL-23 or anti-IL-23R antibodies can be
human antibodies,
mouse antibodies, rabbit antibodies, engineered antibodies such as chimeric
antibodies,
48
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
humanized antibodies, and antigen-binding fragments of antibodies such as Fab,
Fv, scFv, Fab'
and F(ab')2 fragments.
[00147] Non-limiting examples of an anti-IL-23 antibody for use
in the method or
composition described herein include risankizumab, guselkumab (TREMFYMO),
ustekinumab,
briakinumab, brazikumab, mirikizumab, tildrakizumab, or a biosimilar,
biobetter, or
bioequivalent thereof. Additional useful anti-IL-23 antibodies include
bioequivalents,
biosimilars, and biobetters of any of the anti-IL-23 antibodies described
herein. In some
embodiments, the anti-IL-23 blocking antibody is risankizumab. In some
embodiments, the anti-
IL-23 blocking antibody is guselkumab. In some embodiments, the anti-IL-23
blocking antibody
is ustekinumab. In some embodiments, the anti-IL-23 blocking antibody is
briakinumab. In some
embodiments, the anti-IL-23R blocking antibody is brazikumab. In some
embodiments, the anti-
IL-23 blocking antibody is mirikizumab In some embodiments, the anti-IL-23
blocking antibody
is tildrakizumab.
[00148] In some embodiments, the anti-IL-23 antibody of the
present disclosure is an
antibody with an amino acid sequence that has at least 70%, at least 80%, at
least 90%, at least
95% or more sequence identity to an anti-IL-23 reference antibody such as
risankizumab or other
anti-IL-23 antibody that is known to one skilled in the art. In some
embodiments, the anti-IL-
23R antibody of the present disclosure is an antibody with an amino acid
sequence that has at
least 70%, at least 80%, at least 90%, at least 95% or more sequence identity
to an anti-IL-23R
reference antibody such as brazikumab or other anti-IL-23R antibody that is
known to one
skilled in the art. In some instances, the antibody variant has one or more
amino acid
substitutions, deletions and/or additions at certain amino acid positions of
the reference antibody,
but retains antigen binding activity.
[00149] One of skill in the art recognizes that "percent of
sequence identity" can
determined by comparing two optimally aligned sequences over a comparison
window or
designated region of the sequence, wherein the portion of the polypeptide
sequence in the
comparison window may comprise additions or deletions (i.e., gaps) as compared
to the
reference sequence (which does not comprise additions or deletions) for
optimal alignment of the
two sequences. The percentage can be calculated by determining the number of
positions at
which the identical nucleic acid base or amino acid residue occurs in both
sequences to yield the
49
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
number of matched positions, dividing the number of matched positions by the
total number of
positions in the window of comparison and multiplying the result by 100 to
yield the percentage
of sequence identity. Percent sequence identity can be measured using a BLAST
or BLAST 2.0
sequence comparison algorithms, with default parameters, or by manual
alignment and visual
inspection.
[00150] Antibodies, fragments thereof, variants thereof and
derivatives thereof may be
generated using a variety of standard methods recognized by those skilled in
the art. See, e.g.,
Harlow, E. and Lane DP. Antibodies: A Laboratory Manual. Cold Spring Harbor:
Cold Spring
Harbor Laboratory Press, 1988. Antigen-binding fragments such as Fab and
F(ab')2 fragments
may be produced by genetic engineering. Procedures for the production of
chimeric and further
engineered monoclonal antibodies include those described in Riechmann et al.,
Nature,
1988,332:323, Liu et al., Proc. Nat. Acad. Sci. USA, 1987, 84:3439, Larrick et
al.,
Bio/Technology, 1989, 7:934, and Winter et al., TIPS, 1993, 14:139. Examples
of techniques for
production and use of transgenic animals for the production of human or
partially human
antibodies are described in, e.g., Davis et al., 2003, Production of human
antibodies from
transgenic mice in Lo, ed. Antibody Engineering Methods and Protocols, Humana
Press, NJ:191-
200.
4. Pharmaceutical Formulations of Anti-IL-23
Antibodies
[00151] Provided herein are formulations of the anti-IL-23 or the
anti-IL-23R antibody
that can stabilize the antibody, reduce the formation of antibody aggregates,
retard the
degradation of the antibody, and/or minimize the immunogenicity of the
antibody. The
formulation can include an antioxidant or chelator, at least one free amino
acid, a surfactant, a
non-reducing sugar, and/or a buffering agent.
[00152] The antioxidant or chelator can be citrate,
ethylenediaminetetraacetic acid
(EDTA), ethyleneglycoltetraacetic acid (EGTA), dimercaprol,
diethylenetriaminepentaacetic
acid, or N,N-bis(carboxymethyl)glycine; preferably citrate or EDTA. The free
amino acid can
be histidine, alanine, arginine, glycine, glutamic acid and combinations
thereof. The surfactant
can be polysorbates 20; polysorbate 80; TRITON (t-
octylphenoxypolyethoxyethanol, nonionic
detergent; sodium dodecyl sulfate (SDS); sodium laurel sulfate; sodium octyl
glycoside; lauryl-,
myristyl-, linoleyl-, or stearyl-sulfobetaine; lauryl-, myristyl-, linoleyl-
or stearyl-sarcosine;
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
linoleyl-, myristyl-, or cetyl-betaine; lauroamidopropyl-, cocamidopropyl-,
linoleamidopropyl-,
myristamidopropyl-, palmidopropyl-, or isostearamidopropyl-betaine (e.g.
lauroamidopropyl);
myristamidopropyl-, palmidopropyl-, or isostearamidopropyl-dimethylamine;
sodium methyl
cocoyl-, or di sodium methyl oleyl-taurate; sorbitan monopalmitate; polyethyl
glycol (PEG),
polypropylene glycol (PPG), and copolymers of poloxyethylene and
poloxypropylene glycol;
preferably polysorbates 80.
[00153] The buffering agent can be a buffer that can adjust the
pH of the formulation to
about 5.0 to about 7.5, to about pH 5.5 to about 7.5, to about pH 6.0 to about
7.0, or to a pH of
about 6.3 to about 6.5. Non-limiting examples of a buffering agent include
acetate, succinate,
gluconate, histidine, citrate, phosphate, maleate, cacodylate, 2[N-
morpholino]ethanesulfonic
acid (MES), bis(2-hydroxyethyl)iminotris[hydroxymethydmethane (Bi s-Tris), N42-
acetamido]-
2-iminodi acetic acid (ADA), glycylglycine and other organic acid buffers,
preferably hi sti dine or
citrate.
[00154] In some embodiments, the anti-IL-23 or the anti-IL-23R
antibody is in a
lyophilized formulation, e.g., a dry form. In some cases, the lyophilized
formulation includes the
antibody and one or more excipients, such as a non-reducing sugar, a buffering
agent, a free
amino acid, and/or a surfactant.
[00155] In some cases, the lyophilized formulation contains at
least about 50 mg, at least
about 60 mg, at least about 70 mg, at least about 80 mg, at least about 90 mg,
at least about 100
mg, at least about 120 mg, at least about 140 mg, at least about 180 mg, at
least about 200 mg, at
least about 220 mg, at least about 240 mg, at least about 280 mg, at least
about 300 mg, at least
about 400 mg, at least about 500 mg, at least about 600 mg, at least about 700
mg, at least about
800 mg, at least about 900 mg of antibody. In some cases, the lyophilized
formulation is stored
as a single dose in one vial.
[00156] In some embodiments, the anti-IL-23 or anti-IL-23R
antibody is a liquid
formulation. Such a formulation can include the antibody, a buffering agent, a
non-reducing
sugar, and/or a free amino acid.
[00157] The amount of antibody present in a liquid formulation
can be at least about 25
mg/ml to about 200 mg/ml anti-IL-23 antibody, e.g., 25 mg/ml to about 200
mg/ml, 25 mg/ml to
about 150 mg/ml, 25 mg/ml to about 100 mg/ml, 50 mg/ml to about 200 mg/ml, 50
mg/ml to
51
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
about 150 mg/ml, 50 mg/ml to about 100 mg/ml 100 mg/ml to about 200 mg/ml or
150 mg/ml
to about 200 mg/ml of the antibody.
[00158] The non-reducing sugar can be, but not limited to,
mannitol, sorbital, sucrose,
trehalose, raffinose, stachyose, melezitose, dextran, maltitol, lactitol,
isomaltulose, palatinit and
combinations thereof. In some embodiments, the ratio of the non-reducing sugar
to the anti-IL-
23 antibody is at least 400:1 (mole:mole), at least 400:1 (mole:mole), at
least 400:1
(mole:mole), at least 600:1 (mole:mole), at least 625:1 (mole:mole), at least
650:1 (mole:mole),
at least 700:1 (mole:mole), at least 750:1 (mole:mole), at least 800:1
(mole:mole), at least
1000:1 (mole:mole), at least 1100:1 (mole:mole), at least 1200:1 (mole:mole),
at least 1300:1
(mole:mole), at least 1400:1 (mole:mole), at least 1500:1 (mole:mole), at
least 1600:1
(mole:mole), at least 1700:1 (mole:mole), at least 1800:1 (mole:mole), at
least 1900:1
(mole:mole), or at least 2000:1 (mole:mole).
C. Methods of Administration of Combination Therapy
[00159] In another aspect, the present disclosure provides a
combination therapy for the
treatment of IBD, e.g., CD and UC. The combination therapy includes a
therapeutically
effective amount of a CCR9 inhibitor and a therapeutically effective amount of
an anti-IL-23 or
anti-IL-23R blocking antibody. The combination of therapeutic agents can act
synergistically to
effect the treatment or prevention of the various disorders. Using this
approach, therapeutic
efficacy can be achieved using lower dosages of each agent, thus reducing the
potential for
adverse side effects.
[00160] The term "therapeutically effective amount" means the
amount of the subject
compound that will elicit the biological or medical response of a cell,
tissue, system, or animal,
such as a human, that is being sought by the researcher, veterinarian, medical
doctor or other
treatment provider.
[00161] Depending on the disease status and the subjects
condition, the compounds,
antibodies, and formulations of the present disclosure may be administered by
oral, parenteral
(e.g., intramuscular, intraperitoneal, intravenous, ICV, intracistemal
injection or infusion,
subcutaneous injection, or implant), inhalation, nasal, vaginal, rectal,
sublingual, or topical routes
of administration. In addition, the compounds and antibodies may be
formulated, alone or
together, in suitable dosage unit formulations containing conventional
nontoxic pharmaceutically
52
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
acceptable carriers, adjuvants and vehicles appropriate for each rouse of
administration. The
present disclosure also contemplates administration of the compounds and
antibodies of the
present disclosure in a depot formulation.
[00162] In the treatment of IBD such as Crohn's disease and UC,
an appropriate dosage
level of a CCR9 inhibitor will generally be about 0.001 to 100 mg per kg
patient body weight per
day which can be administered in single or multiple doses. Preferably, the
dosage level will be
about 0.01 to about 50 mg/kg per day; more preferably about 0.05 to about 10
mg/kg per day. A
suitable dosage level may be about 0.01 to 50 mg/kg per day, about 0.05 to 10
mg/kg per day, or
about 0.1 to 5 mg/kg per day. Within this range the dosage may be 0.005 to
0.05 mg/kg per day,
0.05 to 0.5 mg/kg per day, 0.5 to 5.0 mg/kg per day, or 5.0 to 50 mg/kg per
day.
[00163] For oral administration, the CCR9 inhibitor is preferably
provided in the form of
tablets containing 1.0 to 1000 milligrams of the active ingredient,
particularly 1.0 mg, 5.0 mg,
10.0 mg, 15.0 mg, 20.0 mg, 25.0 mg, 50.0 mg, 75.0 mg, 100.0 mg, 150.0 mg,
200.0 mg, 250.0
mg, 300.0 mg, 400.0 mg, 500.0 mg, 600.0 mg, 750.0 mg, 800.0 mg, 900.0 mg, and
1000.0 mg of
the active ingredient for the symptomatic adjustment of the dosage to the
patient to be treated.
[00164] The CCR9 inhibitor may be administered on a regimen of 1
to 4 times per day,
preferably once or twice per day.
[00165] In the treatment of IBD such as Crohn's disease and UC,
an appropriate dosage
level of an anti-IL-23 or anti-IL-23R antibody provides an effective amount of
the antibody or a
formulation thereof to induce remission of IBD in a human patient. In some
embodiments, the
therapeutically effective amount of anti-IL-23 or anti-IL-23R antibody is
sufficient to achieve
about 51.tg/m1 to about 60 [tg/m1 mean trough serum concentration of antibody
at the end of the
induction phase, e.g., about 5 [tg/m1 to about 60 ttg/ml, about 10 mg/m1 to
about 50 mg/ml, about
15 mg/m1 to about 45 jig/ml, about 20 ttg/m1 to about 30 pg/ml, about 25 ig/m1
to about 35
lig/ml, or about 30 lug/m1 to about 60 ps/m1 mean trough serum concentration
of anti-IL-23 or
anti-IL-23R antibody at the end of the induction phase.
[00166] Suitable dosages of antibody can be administered from
about 0.1 mg/kg, about 0.3
mg/kg, about 0.5 mg/kg, about 1 mg/kg, about 2 mg/kg, about 3 mg/kg, about 4
mg/kg, about 5
mg/kg, about 6 mg/kg, about 7 mg/kg, about 8 mg/kg, about 9 mg/kg, or about 10
mg/kg.
53
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
[00167] In some embodiments, the total dose amount is about 6 mg,
about 10 mg, about
20 mg, about 30 mg, about 40 mg, about 50 mg, about 60 mg, about 70 mg, about
80 mg, about
90 mg, about 100 mg, about 125 mg, about 150 mg, about 175 mg, about 200 mg,
about 225 mg,
about 250 mg, about 275 mg, about 300 mg, about 325 mg, about 350 mg, about
375 mg, about
400 mg, about 425 mg, about 450 mg, about 475 mg, about 500 mg, about 525 mg,
about 550
mg, about 575 mg, about 650 mg, or more. In some embodiments, the antibody is
administered
by subcutaneous injection at an initial dose of about 400 mg given as two
subcutaneous
injections of 200 mg. In some embodiments, the antibody is dosed at weeks 2
and 4; if response
occurs then 400 mg of the antibody is administered every four weeks.
[00168] In some embodiment, the induction phase is for at least
about 2 weeks, at least
about 3 weeks, at least about 4 weeks, at least about 5 weeks, at least about
6 weeks, at least
about 7 weeks, at least about 8 weeks, at least about 9 weeks, or at least
about 10 weeks of
treatment.
[00169] The treatment regime during the induction phase can
include administration of a
high dose, frequent administrations, or a combination of a high dose and
frequent administrations
of the anti-IL-23 antibody or a formulation thereof In some cases during the
induction phase, a
dose is administered once per day, every other day, every two days, every
three days, once per
week, every 10 days, once every two weeks, once every three weeks or once a
month.
[00170] In some embodiments, the induction dosing is provided
once at initiation of
treatment (day 0) and once at about two weeks after initiation of treatment.
The induction phase
duration can be six weeks. In other embodiments, the induction phase duration
is six weeks and
a plurality of induction doses are administered during the first two weeks. In
instances, when the
human patient has severe IBD or is not responding to anti-IL-23 therapy, the
induction phase has
longer duration than a patient who has mild to moderate IBD.
[00171] Also, in the treatment of IBD, an appropriate dosage
level of an anti-IL-23
antibody provides an effective amount of the antibody or a formulation thereof
to maintain
remission of IBD in a human patient. As such, during the maintenance phase of
the treatment,
the therapeutically effective amount of anti-IL-23 or anti-IL-23R antibody is
sufficient to achieve
about 1 ug/m1 to about 25 ug/m1 mean steady state trough serum concentration
of anti-IL-23 or
anti-IL-23R antibody during the maintenance phase, e.g., about 1 jig/m1 to
about 25 jig/ml, about
54
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
1 lug/m1 to about 20 lug/ml, about 1 jig/m1 to about 15 lug/ml, about 1 g/m1
to about 10 jig/ml,
about 1 g/m1 to about 5 g/ml, about 5 g/m1 to about 25 g/ml, about 5
jig/m1 to about 20
g/ml, about 5 g/m1 to about 15 g/ml, about 5 g/m1 to about 10 jig/ml, about
15 g/m1 to
about 25 g/ml, about 15 jig/m1 to about 20 jig/ml, about 10 lag/m1 to about
25 g/ml, about 10
g/m1 to about 20 g/ml, about 10 g/m1 to about 15 jig/ml, or about 20 jig/m1
to about 25 g/m1
mean steady state trough serum concentration of anti-IL-23 or anti-IL-23R
antibody at the end of
the induction phase.
[00172] The maintenance dose can be administered once a week,
once every other week,
once every three weeks, once every 4 weeks, once every 5 weeks, once every 6
weeks, once
every 7 weeks, once every 8 weeks, once every 9 weeks, or once every 10 weeks.
In some
embodiments during the maintenance phase, the same dosing amount is
administered. In other
embodiments during the maintenance phase, one or more different dosing amounts
are
administered over the maintenance phase. Additionally, depending on the
disease course, the
dosing frequency can be increased.
[00173] The anti-IL-23 or anti-IL-23R antibody or formulation
thereof can be
administered by injection, e.g., intravenous injection, intramuscular
injection, subcutaneous
injection, intraarterial injection, intraperitoneal injection, intravitreal
injection, and the like. If
the formulation is in a solid or lyophilized form, the process of
administering the antibody can
include reconstituting the dry formulation into a liquid formulation_ In some
embodiments, the
antibody or formulation thereof can be administered topically, e.g., in a
patch, cream, aerosol or
suppository. In other embodiments, the topical routes of administration
include nasal,
inhalational or transdermal administration.
[00174] It will be understood, however, that the specific dose
level and frequency of
dosage for any particular patient may be varied and will depend upon a variety
of factors
including the activity of the specific compound employed, the metabolic
stability and length of
action of that compound, the age, body weight, hereditary characteristics,
general health, sex,
diet, mode and time of administration, rate of excretion, drug combination,
the severity of the
particular condition, and the host undergoing therapy.
[00175] The weight ratio of the CCR9 inhibitor described herein
to the anti-IL-23 or anti-
IL-23R antibody of the present disclosure may be varied and will depend upon
the effective dose
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
of each ingredient. Generally, an effective dose of each will be used. Thus,
for example,
wherein a CCR9 inhibitor is combined with an anti-IL-23 antibody, the weight
ratio of the CCR9
inhibitor to the anti-IL-23 antibody will generally range from about 1000:1 to
about 1:1000,
preferably about 200:1 to about 1:200.
[00176] Combination therapy includes co-administration of the
CCR9 inhibitor and the
anti-IL-23 antibody, sequential administration of the CCR9 inhibitor and the
anti-IL-23 antibody,
administration of a composition containing the CCR9 inhibitor and the anti-IL-
23 antibody, or
simultaneous administration of separate compositions such that one composition
contains the
CCR9 inhibitor and another composition contains the anti-IL-23 antibody.
[00177] Co-administration includes administering the CCR9
inhibitor of the present
invention within 0.5, 1, 2, 4, 6, 8, 10, 12, 16, 20, or 24 hours of the anti-
IL-23 antibody of the
present invention. Co-administration also includes administering
simultaneously, approximately
simultaneously (e.g., within about 1, 5, 10, 15, 20, or 30 minutes of each
other), or sequentially
in any order. Moreover, the CCR9 inhibitor and anti-IL-23 antibody can each be
administered
once a day, or two, three, or more times per day so as to provide the
preferred dosage level per
day.
[00178] The combination therapy can be administered at an
induction phase or
maintenance phase of the treatment regimen. In the induction phase, the
combination therapy
can be administered at an effective amount to induce immune tolerance to the
antibody of the
therapy, induce a clinical response, and/or ameliorate one or more symptoms of
IBD. Also, if
during the maintenance phase, there is a return of one or more symptoms of IBD
or if there is a
relapse from remission of the disease, a patient can be administered an amount
corresponding to
an induction phase treatment. During the maintenance phase, the combination
therapy can be
administered at an effective amount to continue the response achieved during
the induction
therapy and/or prevent the return of symptoms or relapse of IBD.
[00179] In some embodiments, one or more additional active
ingredients such as an anti-
inflammatory compound, e.g., sulfasalazine, azathioprine, 6-mercaptopurine, 5-
aminosalicylic
acid containing anti-inflammatories, a non-steroidal anti-inflammatory
compound, and a
steroidal anti-inflammatory compound; antibiotics commonly administered for
control of IBD,
56
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
e.g., ciprofloxacin and metronidazole; or another biologic agent, e.g., a TNFc
. antagonist can be
administered in conjunction with the combination therapy disclosed herein.
D. Kits
[00180] In some aspects, provided herein are kits containing a
CCR9 inhibitor and an anti-
IL-23 or anti-IL-23R antibody disclosed herein that are useful for treating a
disease or disorder
characterized by inflammation of the gastrointestinal tract such as IBD,
including CD, UC and
indeterminate colitis. A kit can contain a pharmaceutical composition
containing a CCR9
inhibitor compounds, e.g., a small molecule inhibitor of CCR9 and a
pharmaceutical composition
containing an anti-IL-23 or anti-IL-23R antibody. In some embodiments, the
CCR9 inhibitor
compound is a compound of formula (I), (II), (III), (Ma), or (11Th). In some
embodiments, the
CCR9 inhibitor compound is Compound 1. In some embodiments, the CCR9 inhibitor
compound
is Compound 2. In some embodiments, the CCR9 inhibitor compound is Compound 3.
In some
embodiments, the CCR9 inhibitor compound is Compound 4. In some instances, the
kit includes
written materials e.g., instructions for use of the compound, antibody or
pharmaceutical
compositions thereof. Without limitation, the kit may include buffers,
diluents, filters, needles,
syringes, and package inserts with instructions for performing any methods
disclosed herein.
IV. Examples
[00181] The following examples are offered to illustrate, but not
to limit, the claimed
invention.
Example 1: Piroxicam-Accelerated MDRIa-1- Model Of Colitis
[00182] Mice of the FVB strain lacking a functional MDR1 gene
(also known as ABCB1,
P-gp or CD243) develop a spontaneous infammatory bowel disease. This disease
can be
accelerated by adding the drug piroxicam to their food. FVB mice bearing a
functional MDR1
gene are resistant to this disease.
[00183] Piroxi cam is included in the mouse food for ten days,
and the health of the mouse
is monitored for a total of 21 days both during and after the piroxicam
feeding. Symptoms
monitored during this time include severity of diarrhea and changes in body
weight.
57
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
[00184]
The colon inflammation manifests itself as a thickening of the colon wall
and
shortening of the colon itself. The severity of the disease can thus be
assessed via the ratio of the
colon' s weight to its length.
[00185]
The mice used were female 6 week old FVBMDR1a(+/+) and FVBMDR1a(-/-).
Mice were normalized for body weight (BW) upon reaching 7 weeks of age, at
which time their
diet was switched to powdered chow containing piroxicam for a total of 10
days. BW and
diarrhea score was monitored for 21 days (including the 10 days of piroxicam
feeding). Blood
was taken for trough compound levels on day 3 and at takedown. Rat IgG levels
(anti-IL23 or
isotype-matched control) were quantitated by ELISA on terminal blood draw. At
takedown,
colons were photographed and weight/length ratio calculated. Two colons from
each group were
formaldehyde fixed for future pathology assessment and disease scoring.
Remaining colons from
each group were dissociated for flow cytometry analysis of infiltrating immune
cells.
TABLE 1: 21 Day Piroxicam-Accelerated MDR1a-l- Model Of Colitis: anti-IL-23 +
CCR9
inhibitor combination.
Group 1 2 3 4 5 6 7 8 9
10 11 12
Pirox- + + + + + + + + + +
- -
team
Dose Veh. 1 Comp. Vehicle Comp. Veh. 1 Comp.
Veh 2 Comp. 2 - - - -
1 2 2 1
MAb Rat Rat Rat Rat anti- anti- anti-
anti-IL23 - - - -
IgG1 IgG1 IgG1 IgG1 IL23 IL23 IL23
Strain Mdr Mdr Mdr Mdi Mclr Mdr
Mdr Mdr Mdr FVB Mdr FVB
N 8 8 8 8 8 8 8 8 8 8
8 8
[00186]
Compound 1 was dosed at 90 mg/kg in 10% Cremophor-EL, SC, BID, and
Compound 2 was dosed at 30 mg/kg in 1% HPMC, SC, QD. Vehicle 1 was 10%
Cremophor-EL
and was administered SC, BID. Vehicle 2 was 1% HPMC administered SC, QD. Anti-
mouse IL-
23 Clone G23-8 (Rat IgG1) was dosed at 300 mg/mouse, QD/QOD; Isotype-matched
control
Clone EIRPN (Rat IgG1) was dosed at 300 hg/mouse, QD/QOD.
58
CA 03174350 2022- 9- 30
WO 2021/202731
PCT/US2021/025171
[00187] Blood was taken at trough from all Compound 1- and
Compound 2-dosed mice,
and plasma analyzed by Liquid Chromatography-Mass Spectroscopy to determine
the
concentration. Trough blood was taken after the 3rd day of dosing (FIG. 1).
Groups refer to the
designations shown in Table I. Trough Compound 1 (Left panel) and Compound 2
(Right panel)
levels met or exceeded the minimal concentration established for 98% receptor
coverage (dotted
line).
[00188] The difference in colon W:L ratio (i.e. extent of chronic
inflammation) in the
piroxicam response can be clearly observed between wild-type FVB mice (FIG. 2;
open circles)
and FVB mice lacking the MDRla gene (FIG. 2; squares).
[00189] FIG. 3 shows W:L ratio of mice treated with the combined
therapy of Compound
1 and anti-IL-23 were significantly improved from control-treated mice (p =
0.0260, inverted
triangles), but those receiving only anti-IL-23 were not significantly
improved (p = 0.0931,
triangles).
[00190] FIG. 4 shows W:L ratio of mice treated with the combined
therapy of Compound
2 and anti-IL-23 were significantly improved from control-treated mice (p =
0.0190, open
inverted triangles), but those receiving only anti-IL-23 were not
significantly improved (p =
0.2571, open triangles).
[00191] Compounds 1 and 2 synergized with anti-IL-23 to improve
colon inflammation
because only the combined therapy significantly improved the W:L ratio.
[00192] Although the foregoing invention has been described in
some detail by way of
illustration and example for purposes of clarity of understanding, one of
skill in the art will
appreciate that certain changes and modifications may be practiced within the
scope of the
appended claims. In addition, each reference provided herein is incorporated
by reference in its
entirety to the same extent as if each reference was individually incorporated
by reference.
59
CA 03174350 2022- 9- 30