Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03177202 2022-09-27
WO 2021/223012
PCT/CA2021/050543
TITLE OF THE INVENTION
ULTRA-VIOLET A (UVA) AND ULTRA-VIOLET C (UVC) SYSTEM AND METHODS FOR
INACTIVATION, REDUCTION AND INHIBITION OF GROWTH OF CORONAVIRUS
FIELD OF THE DISCLOSURE
[0001] This
disclosure relates to a system and method of inactivating, reducing and
inhibiting growth of coronavirus, in public areas such as areas frequented by
humans
in public transit vehicles and the like, by the use of UVA and UVC light
sources at
levels detrimental to coronavirus but safe for animals, including mammals and
humans.
BACKGROUND
[0002] Seven
coronaviruses can infect humans such as human coronavirus (HCoV)
called HCoV-229E, HCoV-0C43, HCoV-HKU1, HCoV-NL63, Middle East respiratory
syndrome coronavirus (MERS-CoV), severe acute respiratory syndrome coronavirus
(SARS-CoV and SARS-CoV-2). The first human coronavirus (HCoV) strain called
B814 was isolated from the nasal discharge of a patient with a common cold in
1965.
More than 30 additional strains were subsequently identified including HCoV-
229E
that was named so after a student specimen coded 229E. HCoV-229E was isolated
by using the standard tissue culture method. HCoVs including HCoV-299E strain
can
be responsible for 15%-30% of common cold cases in human adults. However
severe respiratory tract infections may also occur in elderly people, infants
or
immunocompromised patient. Exposure
to ultraviolet (UV) light can lead to
antimicrobial activity. Far-UV light (for instance, from 207 to 222 nm) may be
used as
an efficient germicidal approach for killing microorganisms. UVC was found to
provide
the strongest antimicrobial activity among other types of UV radiation. For
instance, it
has been reported that far-UVC light (222 nm) inactivated airborne influenza
virus.
However, the exposure to UVC lamp might be associated with a health risk such
as
eye and skin damage. Furthermore, UVC and UVB could be absorbed by RNA or
DNA molecules and induce photo-chemical fusion of the adjacent pyrimidines
into
covalent-linked dimers such as thymine/cytosine dimers in DNA or
uracil/cytosine
dimers in RNA. UV light may also damage RNA protein cross-linking, energy
transfer
between two proteins and result in site-specific damage to RNA. UVA can
provide
oxidative damage to DNA, lead to production of reactive oxygen species and
induce
membrane damage. However, external UVA (315-400 nm) and UVB (280-315 nm)
are approved by FDA to use for the indication of eczema, psoriasis, skin
lymphoma.
UV light sources are known to be very effective in reducing coronavirus levels
on
1
CA 03177202 2022-09-27
WO 2021/223012
PCT/CA2021/050543
surfaces. However, the typical radiated power and exposure time needed to
reduce
the levels of coronavirus may be deleterious to human eyes and epidermis and
dermis layers.
[0003] There is
a need for a system which will reduce the level of coronavirus on a
surface and inhibit further growth while being safe to human exposure.
SUMMARY
[0004] According
to one aspect, there is provided an alternating UVA/UVC system for
inactivating, reducing and inhibiting further growth, on a surface, of
coronavirus, in
one alternative, human coronavirus (HCoV-229E), wherein said system has no
deleterious effects on an animal, including a human, in particular on a human
eye or
epidermis and dermis, wherein said system comprises:
i) at least one UVA light source;
ii) at least one UVC light source; and
iii) at least one controller connected to each of said at least one UVA light
source
and said at least one UVC light source, for controlling at least one parameter
of
each of said UVA light source and UVC light source selected from light source,
light intensity, radiated power level, wavelength, exposure time and
combinations thereof; wherein said at least one UVC light source emits UVC
light to a surface for a period of time reducing the level of said coronavirus
on
said surface to a level that is safe to animals including humans, and said at
least one UVA light source emits UVA light to a surface for a period of time
inhibiting growth of said coronavirus on said surface, such that during the
time
said at least one UVC light source and said at least one UVA light source is
emitting on said surface, radiation levels from said at least one UVC light
source
and said at least one UVA light source is safe to animals, including humans;
wherein when said at least one UVC light source is emitting UVA light to said
surface, said at least one UVC light is off, and when said at least one UVA
light
source is emitting light to said surface, said at least one UVC light source
is off;
and wherein there is a period of blanking time wherein both said at least one
UVC light source and said at least one UVA light source are off, wherein
cycling
between said at least one UVC light source and said at least one UVA light
source and said blanking time is controlled by said at least one controller.
[0005] According
to one alternative, said at least one UVC light source has an
operating wavelength of from about 275 nanometers (nm) to about 295 nm. In one
alternative, said at least one UVC light source has an operating wavelength of
about
275 nm.
2
CA 03177202 2022-09-27
WO 2021/223012
PCT/CA2021/050543
[0006] According
to one alternative, said at least one UVA light source has an
operating wavelength of from about 385 nm to about 405 nm. In one alternative,
said
at least one UVA light source has an operating wavelength of about 405 nm.
[0007] According
to yet another alternative, said at least one UVC light source is a
light emitting diode (LED).
[0008] According
to yet another alternative, said at least one UVA light source is a
LED.
[0009] In one
alternative, the at least one controller automatically cycles between
emitting light from said at least one UVA light source and from said at least
one UVC
light source and said blanking time.
[00010] In one
alternative, said at least one UVC light source has an emission at a
power level and time duration to reduce coronavirus levels on a surface
exposed to
said at least one UVC light source.
[00011] In one
alternative, the power level is selected to ensure the radiated emission
from said at least one UVC light source is at a safe level for human eyes and
epidermis and dermis.
[00012] In one
alternative, the time duration is selected to ensure the radiated
emission from said at least one UVC light source is at a safe exposure time
for
human eyes and epidermis and dermis.
[00013] In one alternative,
said at least one UVA light source has an emission at a
power level to inhibit growth of coronavirus on a surface exposed to said at
least one
UVC light source, while safe for human eyes and epidermis and dermis,
regardless of
the exposure time.
[00014] In one
alternative, said at least one UVC light source has a power rating of
from about 10 mW to about 100 W. In one alternative, said at least one UVC
light
source has a power rating of 236 mW.
[00015] In one
alternative, said at least one UVA light source has a power rating of
from about 10 mW to about 100 W. In one alternative, said at least one UVA
light
source has a power rating of 74 mW.
[00016] In one alternative,
said system reduces the level of active coronavirus on a
surface exposed to said system by 1 to about 100%. In one alternative, by 10
to
about 20%.
[00017] In yet
another alternative, there is provided a method of inactivating, reducing
levels, on a surface, and inhibiting further growth of coronavirus, on said
surface,
wherein said method has no deleterious effects on an animal, including a
human, in
particular on a human eye or epidermis and dermis, wherein said method
comprises:
3
CA 03177202 2022-09-27
WO 2021/223012
PCT/CA2021/050543
i) Exposing said surface to at least one UVC light source for a period of
time to
reduce the level of coronavirus on said surface;
ii) Terminating the exposure of the at least one UVC light source on said
surface;
iii) Exposing said UVC exposed surface to at least one UVA light source for
a
period of time to inhibit growth of said coronavirus on said surface;
iv) Terminate the exposure of the at least one UVA light source on said
surface;
v) Providing a period of blanking time wherein said at least one UVA light
source
and said at least one UVC light source are off;
vi) Optionally repeating steps i) to v) in order to maintain a desired
level of the
coronavirus, on said surface.
[00018] In one
alternative, said at least one UVC light source has an operating
wavelength of from about 275 nanometers (nm) to about 295 nm. In one
alternative,
said at least one UVC light source has an operating wavelength of about 275
nm.
[00019] According
to one alternative, said at least one UVA light source has an
operating wavelength of from about 385 nm to about 405 nm. In one alternative,
said
at least one UVA light source has an operating wavelength of about 405 nm.
[00020] According
to yet another alternative, said at least one UVC light source is a
light emitting diode (LED).
[00021] According
to yet another alternative, said at least one UVA light source is a
LED.
[00022] In one
alternative, steps i) to v) are controlled by at least one controller
automatically cycling between emitting light from said at least one UVA light
source
and from said at least one UVC light source and providing said blanking time.
[00023] In one
alternative, said at least one UVC light source has an emission at a
power level and time duration to reduce coronavirus on a surface exposed to
said at
least one UVC light source.
[00024] In one
alternative, the power level is selected to ensure the radiated emission
from said at least one UVC light source is at a safe level for human eyes and
epidermis and dermis.
[00025] In one alternative,
the time duration is selected to ensure the radiated
emission from said at least one UVC light source is at a safe exposure time
for
human eyes and epidermis and dermis.
[00026] In one
alternative, said at least one UVA light source has an emission at a
power level to inhibit growth of coronavirus on a surface exposed to said at
least one
UVC light source, while safe for human eyes and epidermis and dermis,
regardless of
the exposure time.
4
CA 03177202 2022-09-27
WO 2021/223012
PCT/CA2021/050543
[00027] In one alternative, said at least one UVC light source has a
power rating of
from about 10 mW to about 100 W. In one alternative, said at least one UVC
light
source has a power rating of 236 mW.
[00028] In one alternative, said at least one UVA light source has a
power rating of
from about 10 mW to about 100 W. In one alternative, said at least one UVA
light
source has a power rating of 74 mW.
[00029] In one alternative, said method reduces the level, and in
another alternative
inhibits growth, of active coronavirus on a surface by 1 to 100%. In one
alternative,
by at least one of the following ranges: 10 to 20%, 20 to 30%, 30 to 40%, 40
to 50%,
50 to 60%, 60 to 70%, 70 to 80%, 80 to 90% and 90 to 100%.
[00030] In one alternative, said method includes said at least one UVC
light source is
on for about 6 seconds, then off and followed immediately by at least one UVA
light
source on for about 6.5 hours, then both said at least one UVC light source
and said
at least one UVA light source off for about 1.5 hours for a blanking period
before
recommencing cycling of UVC and UVA light exposure, as required.
[00031] In one alternative, the UVA light source may remain on at levels
safe to
animals including humans to inhibit coronavirus growth and UVC is turned on at
intervals to reduce coronavirus levels should coronavirus growth inhibition
meet its
limit, if any.
[00032] In yet another alternative, said system and method with blanking
intervals are
considered Risk exempt when tested to the IEC 62471 standard.
[00033] Herein the term coronavirus may include HCoV-229E.
[00034] Herein the term surface includes surfaces typically found in
public places such
as bathrooms and kitchens, including but not limited to countertops, hard
counters,
wood counters, concrete, plastic, rubber, leather, material and the like.
BRIEF DESCRIPTION OF THE FIGURES
[00035] Figure 1 is a block diagram of the system, according to one
alternative.
[00036] Figure 2 is a block diagram of the system, according to another
alternative.
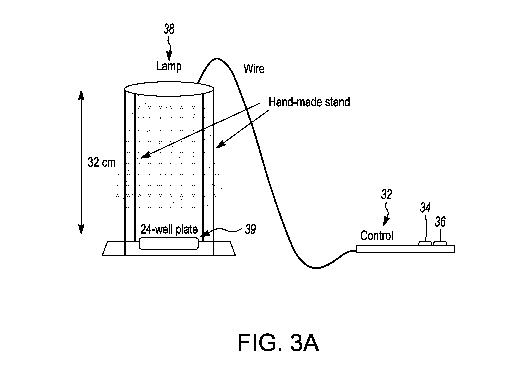
[00037] Figure 3a is a schematic of the setup for Example 1
[00038] Figure 3b is a photograph of the interior of the setup for
Example 1.
[00039] Figure 4 is a schematic representation of the serial dilution
carried out for
Example 1.
[00040] Figure 5 depicts Non-infected (left) and infected (right) with
HCoV-229E MRC-
5 cells of Example 1.
[00041] Figure 6 depicts the effect of UVA and UVC light on infectivity
of HCoV-229E
in 96-well plate of Example 1 following protocol 1.
5
CA 03177202 2022-09-27
WO 2021/223012
PCT/CA2021/050543
[00042] Figure 7 Percentage of infected MRC-5 cells after 0, 1 and 3
cycles using
protocol 1 in 96-well plate of Example 1 following protocol 1.
[00043] Figure 8 Effect of UVA and UVC light on infectivity of HCoV-229E
in 96-well
plate following protocol 2.
[00044] Figure 9 Percentage of infected MRC-5 cells after 0, 1 and 3 cycles
in 96-well
plate following protocol 2.
[00045] Figure 10 Effect of UVA and UVC light on infectivity of HCoV-
229E in 96-well
plate following protocol 2a.
[00046] Figure 11 Percentage of infected MRC-5 cells after 0, 1 and 3
cycles in 96-
well plate following protocol 2a.
[00047] Figure 12 Effect of protocol 1, 2 and 2a on infectivity of HCoV-
229E in 96-well
plate.
[00048] Figure 13 depicts images showing MRC-5 cells at different
experimental
stages: non-infected control (a), infected cells with HCoV-229E before UV
treatment
(b), infected cells after 1 cycle of UV treatment (c) and infected cells after
3 cycles of
UV treatment (d) using protocol 2a.
[00049] Figure 14 Effect of protocol 1, 2 and 2a on infectivity of HCoV-
229E in 24-well
plate. Control is considered as "0 cycles". Data shown represent mean of two
independent experiments with error bars of standard deviation. Control was
compared to treatments with P-values being<0.5, >0.1 and <0.01 for protocol 1,
2 and
2a, respectively.
[00050] Figure 15 Effect of protocol 1, 2 and2a on infectivity of HCoV-
229E in 24-well
plate. Control is considered as "0 cycles".
DETAILED DESCRIPTION
[00051] Referring now to FIG. 1, there is depicted a block diagram of a
two continuous
Pulse Width Modulation (PWM) example of one alternative for the system
described
herein. A PWM generator 10 generates a continuous PWM which feeds into two
circuits 20 and 30. The first circuit is an optional logic buffer circuit 20
for controlling
the pulsing of the UVC emitter 40. The logic buffer circuit 20 ensures that
the UVC
emitter 40 is emitting when the PWM generator 10 is outputting a high logic
level, and
off when the PWM generator 10 is outputting a low logic level. See the output
curve
22. The second circuit is a logic inverter 30 which feeds into an OR circuit
40', along
with the output of the second PWM generator 10', wherein the output of the OR
circuit 40' controls the UVA emitter 50, ensuring that the UVA emitter is off
when the
PWM generator 10' is outputting a high logic level, and UVA emitter is on when
the
PWM generator 10' is outputting a low logic level. See the inverted output
curve 32.
6
CA 03177202 2022-09-27
WO 2021/223012
PCT/CA2021/050543
[00052] Referring
now to FIG. 2, there is depicted a block diagram of a three timer
controlled system, according to one alternative. In this example, there is a
UVC timer
circuit 100, a UVA timer circuit 200 each controlling the UVC emitter 40 and
UVA
emitter 50 respectively, and a blanking timer circuit 300 for controlling the
blanking
period. The UVC timer circuit 100 is set for 6 seconds on and the UVA timer
circuit
200 is set for 6.5 hours and the blanking timer circuit 300 is set for 1.5
hours. During
start up, the UVC timer circuit 100 is enabled and outputs a logic high which
is fed
into a first logic buffer 110 and first logic inverter 120. The first logic
buffer 110
controls the UVC emitter 40 to be on with a high logic output and the UVC
emitter 40
to be off with a low logic output, while the first logic inverter 120 is used
to ensure the
UVA timer circuit 200 is off. Once the UVC timer circuit 100 completes the 6
seconds, the output changes state to turn off the UVC emitter 40 and turn on
the UVA
timer circuit 200 for 6.5 hours. Once enabled, UVA timer circuit 200 outputs a
logic
high which is fed into a second logic buffer 210 and second logic inverter
220. The
second logic buffer 210 controls the UVA emitter 50 to be on, while the second
logic
inverter 220 is used to ensure the UVC timer circuit 100 is off. Once the 6.5
hours is
completed, the output changes state to turn off the UVA emitter 50 and turn on
the
blanking timer circuit 300 which ensures both UVC emitter 40 and UVC emitter
50
remain off for 1.5 hours, and the cycle repeats as required. Once enabled, the
1.5
hour timer circuit 300 outputs a logic high and this output is fed into logic
inverter 310
wherein the output of logic inverter 310 is combined with the output of logic
inverter
220 and fed into logic AND circuit 60 producing a rising edge of the output of
the logic
AND circuit 60 which feeds in to the 6 second timer 100 to restart the 6
second timer
100 once the 1.5 hour timer circuit 300 completes the time. The time value of
each
time may be determined by a variety of factors including, power level of UV
light
source, size of room, etc.
Example 1 UVA and UVC effect on coronavirus
[00053] In order
to reduce risk associated with the experiments, the UV lamp was
located in a sealed light box (See FIGS. 3a and 3b). Eyes were covered with a
UV
protective shield and a lab coat was worn at all times during the experiments.
Experiments involving Human coronavirus (HCoV-229E) were carried out in a
safety
level 2 hood. Appropriate risk assessments and Control of Substances Hazardous
to
Health Regulations (COSHH) forms were completed prior to the experiment.
7
CA 03177202 2022-09-27
WO 2021/223012
PCT/CA2021/050543
Materials
[00054] MRC-5 (ATCC CCL-171TM) Human fibroblast cells were obtained from
American Type Culture Collection and used to multiply HCoV-229E and for
subsequent assays. HCoV-229E (ATCC VR-740) was also purchased from American
type culture collection. Eagle's Minimum Essential Medium (EMEM) (ATCC 30-
2003TM) containing both glucose and L-glutamine was purchased from American
Type Culture Collection (ATCC) as well as Dimethylsulfoxide (DMSO) (ATCC 4-
XTm). Trypsin-EDTA sterile-filtered solution (0.25%) and surface cell culture,
rectangular flasks (CelIBIND, 25cm2) were obtained from Merck Life Science UK
Limited. Dimethyl sulfoxide (DMSO) was obtained from ChemCruz. Dulbecco's
phosphate-buffered saline (DPBS) was purchased from Sigma Aldrich.
[00055] UVA (405 nm and 74 mW and 147 mW) and UVC (275 nm and 236 mW) light
equipment was provided by Helios Shield LTD.
Maintenance of MRC-5 cells (human lung fibroblast cells)
[00056] Short-
term frozen storage of the cells at -80 C was carried out by re-
suspending cell pellets produced by centrifugation for 5 minutes at 1200 rpm,
in 1 ml
of a solution of 900 pl of fetal calf serum (FCS) and 100 pl of dimethyl
sulfoxide
(DMSO).
[00057] Eagle's minimum
essential medium (EMEM) (Eagle's minimum essential
medium contains Earle's Balanced Salt Solution, nonessential amino acids, 2 mM
Glutamine, 1 mM sodium pyruvate, and 1500 mg/L sodium bicarbonate) was used to
maintain MRC-5 cells. The medium was supplemented with a mixture of penicillin-
streptomycin antibiotic (1% of penicillin streptomycin antibiotic) and FCS
(10% of FCS
(fetal calf serum) to give a final concentration of 1 vol. % and 10 vol. %,
respectively.
8
CA 03177202 2022-09-27
WO 2021/223012
PCT/CA2021/050543
Reviving MRC-5 cells from frozen
[00058] The MRC-5 Cells were thawed (thawed from freezer to lab room
temperature.
Limited control. Thawing of frozen cells was performed as follows: a tube
containing
1 ml of frozen cells was taken out of the freezer (-80 C) and left inside a
tissue culture
hood at room temperature (around 19 C). Once there was a small bit of ice left
in the
vial (usually after about a minute), transferred the cell suspension into a
centrifuge
tube and diluted to 1:10 with EMEM medium, and centrifuged at 1200 rpm for 5
minutes. Pellets were then re-suspended in 1 ml of fresh EMEM medium, diluted
to
1:6 with the fresh EMEM medium, and incubated in 25 cm2 tissue culture flasks
at
37 C for up to 72 hours.
Passaging of MRC-5 cells
[0001] The spent
EMEM medium from the flasks was discarded. The cells were
washed once with sterile DPBS (1 mL of DPBS was added to the flasks shaken and
discarded). Then, 1 ml of trypsin was transferred into each flask, and cells
were
incubated for 5 minutes at 37 C. Once all cells had disassociated from the
flask
(rounded), 9 ml of fresh EMEM medium was added to the same flask. The cells
were
centrifuged at 1200 rpm for 5 minutes. Cell pellets were re-suspended into 1
ml of
fresh EMEM medium. Further passaging was carried out by diluting cells to 1:6
in
fresh medium and incubated at 37 C.
[0002] Cells at the
passage 4 were used for the subsequent experiments (in other
words, from the beginning, we have a first cell line, then multiply 4x to
derive
generation 4. This generation was then frozen. This procedure was repeated
throughout all the tests). If cells were required for experiments, cells were
counted
using a haemocytometer after centrifugation and re-suspension of cell pellets
into 1
ml of fresh EMEM medium, but before the passaging. The cells were seeded into
sterile plastic ware at appropriate concentrations, where 1 x 104 cell/ml was
used for
experiments in both 96-well plates and 24-well plates, respectively.
Infecting MRC-5 cells with HCoV-229E
[0003] MRC-5 lung
fibroblast cells were seeded at a concentration of 1 x 104 cell/ml
into two 24-well plates 48 hours prior to the experiment. The initial
purchased stock of
HCoV-229E in a volume of 100 pl was serially diluted to 10-9 in EMEM media
(serial
dilution is shown in FIG. 4). After 48 hours, once the cells were about 50%
confluent,
the old medium was replaced with each dilution of HCoV-229E in a fresh medium.
9
CA 03177202 2022-09-27
WO 2021/223012
PCT/CA2021/050543
Treatment of infected cells using UV light
[0004] Referring
to FIG. 3a, the schematic representation of the experimental set up
is shown. The control 32 had two green buttons: the UVA 34 and UVC 36
switches.
The UV lamp 38 was placed 32 cm away from the 24-well plate 39 containing
infected MRC-5 cells. FIG. 3b is a photograph of the set up during the
experiment
with the UV lamp 38 and well plate 39.
[0005] There
were two protocols for treating the infected cells with UVA and UVC
light at every 8-hour interval (cycle).
Protocol 1
[0006] UVC was
activated for 6 seconds at the rotary position "F" (a light power level
of 236 mW) and then deactivated. UVA was then immediately activated for 6.5
hours
at the rotary position "7" (light power level of 74 mW) and then deactivated.
The last
1.5 hours of the 8 hour interval was a blanking time, where both UVA and UVC
light is
off or deactivated. Such UVC/UVA/blanking interval cycles were repeated up to
11
times. Viral inactivation was analysed after 1, 3, 5, 7, 9, and 11 intervals
as
described below.
Protocol 2
[0007] UVC was activated
for 6 seconds at the rotary position "F" (a light power level
of 236 mW) and then deactivated. UVA was immediately pulsed (or activated) for
8
hours at the rotary position "F" (a light power level of 147 mW) and then
deactivated.
There was no blanking time and the UVC/UVA interval cycle was repeated. Such
intervals were repeated up to 11 times. Viral inactivation was analysed after
1, 3, 5,
7, 9, and 11 intervals as described in below.
Protocol 2a
[0008] UVC was
pulsed for 20 seconds at the rotary position "F" (a light power level
of 236 mW) and then deactivated. UVA was immediately pulsed for 8 hours at the
rotary position "F" (a light power level of 147 mW) and then deactivated).
There was
no blanking time and the UVC/UVA interval cycle was repeated. Such intervals
were
repeated up to 11 times. Viral inactivation was analysed after 1, 3, 5, 7, 9,
and 11
intervals as described in below.
Detection of viral infectivity
[0009] After 1,
3, 5, 7, 9, and 11 intervals or cycles, the suspension containing
infected cells, released virus and medium was transferred into a cryotube and
CA 03177202 2022-09-27
WO 2021/223012
PCT/CA2021/050543
underwent one rapid cycle of freeze and thaw, where the tube was placed for an
hour
at -80 C and subsequently thawed at room temperature for 30 minutes [B.-W.
Kong,
L. K. Foster and D. N. Foster, "A method for the rapid isolation of virus from
cultured
cells," BoTechniques, vol. 44, pp. 1-5, 2018]. Then, the suspension was
centrifuged
at 2000 rpm for 10 minutes to remove cell debris and the culture supernatant.
The
supernatant was filter-sterilised using a 0.45 pm pore size filter and stored
at -80 C
until used for tissue culture infectious dose (TCID50) assay.
Analysis of viral infectivity using TCID50assa
[00010] For TCID50 assay,
HCoV-229E untreated and treated for 1, 3, 5, 7, 9, 11
cycles using either protocol 1 or protocol 2 or 2a was serially diluted in
fresh EMEM
medium. For serial dilution, 100 pl of virus suspension was placed into 900 pl
of the
fresh medium that was corresponded to 1:10 dilution or 10-1 as shown in FIG. 4
as
per the protocol in S. E. Grimes, A Basic Laboratory Manual for the Small-
Scale
Production and Testing of 1-2 Newcastle Disease Vaccine, RAP publications,
2002.
Then, 100 pl of virus/medium suspension from 10-1 was transferred to another
tube
containing 900 pl of fresh medium and classified as 10-2 dilution or 1:100.
This
process was repeated to 10-8 dilution factor.
[00011] MRC-5
cells at a concentration of 1 x 104 were seeded into either 96-well
plate or 24-well plate. Once, the cells reached approximately 50% of
confluence, they
were infected with serially diluted treated coronavirus in 5 repeated wells
for up to 4
days until cytopathic effect (CPE) was observed. Another plate was incubated
with
the serially diluted virus without any treatment in order to obtain control
for tissue
culture infectious dose (TCID50) and will be further called 0 cycles.
[00012] Once CPE was observed, the number of infected wells were counted.
TCID50
was calculated using the Reed and Muench method [L. J. Reed and H. Muench, "A
simple method of estimating fifty per cent endpoints," American Journal of
Epidemiology, vol. 27, no. 3, pp. 493-497, 1938]. The formula for the
calculations is
the following (as per Reed and Muench):
log10 50% Olt dp o int da-utzart ¨
log10 cf cittutLart showing a mortality next aboye 50% ¨
(dif ference of logarithms' X logarithm of dthution factor)
Statistical analysis
[00013] The
statistical analysis was calculated using Minitab software and one-way
ANOVA test (including Tukey test). P-value less than 0.05 was considered
statistically significant.
11
CA 03177202 2022-09-27
WO 2021/223012
PCT/CA2021/050543
Modelling for Cross-Contamination Risks
[00014] Cross-
contamination risk will be calculated using the Exponential model,
which represents a "dose-response" relationship between the dose applied to
hosts
(cells) and the probability of such a host to respond [T. Watanabe, T. A.
Bartrand, M.
H. Weir, T. Omura and C. N. Haas, "Development of a dose-response model for
SARS coronavirus," Risk Anal, vol. 30, pp. 1129-1138, 2010].
[00015] The following equation was used to calculate cross-
contamination:
p(i)=1-exp(-d/k) [C. N. Haas, "Microbial Dose Response Modeling: Past,
Present, and
Future," Environ. Sci. Technol., vol. 49, p. 1245-1259, 2015], [T. Watanabe,
T. A.
Bartrand, M. H. Weir, T. Omura and C. N. Haas, "Development of a dose-response
model for SARS coronavirus," Risk Anal, vol. 30, pp. 1129-1138, 2010]
[00016] Where p
is the risk of contamination, k represents the probability of a single
cell surviving, whereas d is the dose of such cells administered.
RESULTS
[00017] MRC-5 cells were infected with HCoV-229E and treated with UVA and UVC
light as described. Fig. 5 illustrates non-infected (left) and infected
(right) MRC-5
cells, where CPE could be observed.
Effect of Protocol 1 on HCoV-229E
[00018] TCID50
assay was performed in order to investigate any infectivity of HCoV-
229E after each cycle of the treatment following protocol 1. FIGS. 6 and 7
show the
effect of protocol 1 on viral activity. As seen in FIG. 6, no CPE was observed
after 1
cycle, whereas TCID50 was reduced from 5.1 log TCID50 to 2.5 log TCID50 after
the
first cycle. Control is considered as "0 cycles". Data showed represent the
mean of
two independent experiments with error bars of standard deviation. Control (0
cycles)
was compared to cycle one with P-value >0.05.
[00019] CPE in
MRC-5 cells was still detected in 10-1(log dilution factor -1) diluted viral
suspension after cycle 1, but percentages of infected cells reduced to 71% and
28%
in 10-4 and 10-5 dilutions, respectively (FIG. 7 - Log dilution factor
represents number
of serial dilution as described herein). This means that UVA and UVC exposure
resulted in the inactivation of HCoV-229E after one cycle in concentrations
lower than
1:10 dilutions. In addition, no CPE was observed after 5, 6, 7 and 9 cycles.
Effect of Protocol 2 on HCoV-229E
[00020] Protocol
2 was used for inactivation of HCoV-229E for up to 11 cycles. Data
showed represent the mean of two independent experiments with error bars of
12
CA 03177202 2022-09-27
WO 2021/223012
PCT/CA2021/050543
standard deviation. Control (0 cycles) was significantly different to cycle 1
and cycle 3
(P-value < 0.01), respectively. FIG. 8 shows the effect of each cycle on the
ability of
HCoV-229E to infect at least 50% of MRC-5 cells. TCID50 at 0 cycles was
considered
as the control and corresponded to 7.57 log TCID50. As shown in FIG. 8,
logTCID50
was reduced to 2.34 and 1.16 after the first and third cycle, respectively. No
CPE was
observed after 5, 6, 7 and 9 cycles.
[00021] The
percentage of infected MRC-5 cells was calculated with results presented
in FIG. 9. Log dilution factor represents number of serial dilution as
described herein.
[00022] As shown in FIG. 9, the percentage of MRC-5 cells infected with HCoV-
229E
after a single cycle reduced from 100% to 0% at 10-4 serial dilution. After 3
cycles, the
concentration that infected around 60% of MRC-5 cells was dramatically
decreased
from 10-8 to 10-1 viral stock dilution.
[00023] The data
obtained so far is in agreement with other research studies, where
HCoV-229E was subject to UVC and UVA radiation. It has been found that UVA
decreased HCoV-229E spike protein, which helps virus to bind to a cellular
membrane [R. A, G. G. S. Leite, G. Y. Melmed, R. Mathur, M. J. Villanueva-
Millan
and e. al., "Ultraviolet A light effectively reduces bacteria and viruses
including
coronavirus," PLOS ONE, vol. 15, pp. 1-5, 2020].
Effect of Protocol 2a on HCoV-229E
[00024] TCID50
assay was also used in order to investigate any infectivity of HCoV-
229E after each cycle of the treatment following protocol 2a. FIGS. 10 and 11
show
the effect of UV lamp on CPE caused by HCoV-229E after 11 cycles. As
illustrated in
FIG. 10, TCID50 significantly reduced from 6.1 log TCID50 to 1.6 and 1.4 log
TCID50
after 1 and 3 cycles, respectively. No CPE was observed after 3 cycles.
[00025] As best seen in FIG. 10, there is shown the effect of UVA and UVC
light on
infectivity of HCoV-229E in 96-well plate. The virus was treated with UV
following the
protocol 2. Control is considered as "0 cycles". Data showed represent the
mean of
two independent experiments with error bars of standard deviation. Control (0
cycles)
was compared to cycle 1 and 3 with P-values being <0.05 respectively.
[00026] FIG. 11
represents percentage of infected cells, which dropped from 100% to
60% and 40% at dilution 10-1 after 1 and 3 cycles, respectively.
[00027] As best seen in FIG. 11, there is shown the percentage of infected MRC-
5
cells after 0, 1 and 3 cycles in 96-well plate. Log dilution factor represents
number of
serial dilution as described herein.
Comparison of different protocols used
13
CA 03177202 2022-09-27
WO 2021/223012
PCT/CA2021/050543
[00028] The
effect of different protocols on infectivity of the virus could be compared.
FIG. 12 shows the comparison of protocol 1, 2 and 2a after 11 cycles,
respectively,
on infectivity of HCoV-229E in 96-well plate. Control is considered as "0
cycles". As
seen in FIG. 12, TCID50 reduced from the control to 1.6 log after 1 cycle
using
protocol B2. TCID50 was 2.34 and 2.5 log after 1 cycle using protocols 1 and
2,
respectively. However, there was no CPE detected after 3 cycles using protocol
1,
whereas TCID50 was reduced to 1.6 and 1.4 log by protocols 2 and 2a,
respectively.
This means that protocol 1 might be the most successful setting in order to
inactivate
HCoV-229E after 3 cycles. As protocols 2 and 2a differed by the duration of
UVC, the
longer UVC treatment (20 seconds for protocol 2a) showed a slight change in
TCID50
from 2.34 to 1.6 log.
[00029] FIGS. 13A-
13D illustrates MRC-5 cells at different conditions: non-infected
(FIG. 13A), infected, but not treated (FIG. 13B), infected and treated for 1
cycle (FIG.
13C) and infected and treated for 3 cycles (FIG. 13D). Images showing MRC-5
cells
at different experimental stages: non-infected control (FIG. 13A), infected
cells with
HCoV-229E before UV treatment (FIG. 13B), infected cells after 1 cycle of UV
treatment (FIG. 13C) and infected cells after 3 cycles of UV treatment (FIG.
13D)
using protocol 2a. According to FIGS. 13A-13D, cells appeared rounded upon the
infection, which was referred to CPE. There were a small number of alive cells
remained after cycle 1, whereas cells could likely be dead by the end of cycle
3. It
has been discovered elsewhere that many enveloped viruses including SARS-CoV-2
and HCoV-229E might not remain viable for a long time once they left either
liquid
medium or host that are necessary for their replication [C. S. Heilingloh, U.
W.
Aufderhost, L. Schipper, U. Dittmer, 0. Witzke, D. Yang, X. Zheng, K. Sutter,
M.
Trilling, M. Alt, E. Steinmann and A. Krawczyk, "Susceptibility of SARS-CoV-2
to UV
irradiation," American Journal of Infection Control, vol. 48, pp. 1273-1275,
2020].
[00030] FIGS. 14
and 15 represent effect of different protocols on TCID50 of MRC-5
cells after 1 cycle in 24-well plates. According to FIGS. 14 and 15, the
effect of
protocols on CPE caused by HCoV-229E in 24-well plates was similar to 96-well
plates.
[00031] FIG. 14
depicts the effect of protocol 1, 2 and 2a on infectivity of HCoV-229E
in 24-well plate. Control is considered as "0 cycles". Data shown represent
mean of
two independent experiments with error bars of standard deviation. Control was
compared to treatments with P-values being<0.5, >0.1 and <0.01 for protocol 1,
2 and
2a, respectively.
[00032] FIG. 15
depicts the effect of protocol 1, 2 and 2a on infectivity of HCoV-229E
in 24-well plate. Control is considered as "0 cycles".
14
CA 03177202 2022-09-27
WO 2021/223012
PCT/CA2021/050543
Cross-contamination
[00033] The exponential model was used to calculate the impact of UV on
approximately 3.5 PFU/ml, 3.34 PFU/ml, 2.28 PFU/ml concentration of virus
(PFU=0.7TCID50) using protocol 1, 2 and 2a, respectively. Using a value of k =
2.92
[T. Watanabe, T. A. Bartrand, M. H. Weir, T. Omura and C. N. Haas,
"Development
of a dose-response model for SARS coronavirus," Risk Anal, vol. 30, pp. 1129-
1138,
2010], the pulsing programme with the UVA and UVC results in exponential risk
p =
0.7, 0.68 and 0.42 for protocol 1, 2 and 2a that might represent 30%, 32% and
58%
decrease in cross contamination risk for MRC-5 cells after one cycle.
[00034] Diseases associated with coronaviruses are a major worldwide concern
and
might be fatal. There are different ways of spreading viral particles such as
through
air droplets or via touching contaminated surfaces. It was found that
pathogenic
HCoV-229E could be infectious in a human lung cell culture such as MRC-5 for
at
least 5 days as well as on nonbiocidal surface materials:
polytetrafluoroethylene,
glass, polyvinyl chloride (PVC), silicone rubber, ceramic tiles and stainless
steel [C.
S. Heilingloh, U. W. Aufderhost, L. Schipper, U. Dittmer, 0. Witzke, D. Yang,
X.
Zheng, K. Sutter, M. Trilling, M. Alt, E. Steinmann and A. Krawczyk,
"Susceptibility of
SARS-CoV-2 to UV irradiation," American Journal of Infection Control, vol. 48,
pp.
1273-1275, 2020]. Furthermore, SARS-CoV-2 may be still infectious on surfaces
such as on plastic surface for 3-4 days at a room temperature, SARS-CoV-1 can
survive on the surface of polystyrene petri dish for at least 6 days at room
temperature, but loss it's activity after 9 days [M. E. R. Darnell, K.
Subbarao, S. M.
Feinstone and D. R. Taylor, "Inactivation of the coronavirus that induces
severe acute
respiratory syndrome, SARS-CoV," J Virol Methods, vol. 121, pp. 85-91, 2004].
However, infectivity of coronaviruses on surfaces depends on not only on a
type of
surface, but also on both temperature and humidity. It was observed that MERS-
CoV
and HCoV-229E possessed shorter survivability at room temperature compared to
SARS-CoV-1 and SARS-CoV-2 on plastic, whereas HCoV-229E has found to have a
longer persistence on both polytetrafluoroethylene (Teflon), glass, ceramic
and
polyvinyl chloride (PVC) for up to 5 days [M. E. R. Darnell, K. Subbarao, S.
M.
Feinstone and D. R. Taylor, "Inactivation of the coronavirus that induces
severe acute
respiratory syndrome, SARS-CoV," J Virol Methods, vol. 121, pp. 85-91, 2004].
The
infectivity of SARS-CoV-1 and SARS-CoV-2 on glass was limited to 2 and 4 days,
respectively [M. E. R. Darnell, K. Subbarao, S. M. Feinstone and D. R. Taylor,
"Inactivation of the coronavirus that induces severe acute respiratory
syndrome,
SARS-CoV," J Virol Methods, vol. 121, pp. 85-91, 2004]. SARS-CoV-1 and
CA 03177202 2022-09-27
WO 2021/223012
PCT/CA2021/050543
HCoV-229E were detectable in dechlorinated tap water for 3 and 6 days,
respectively
[M. E. R. Darnell, K. Subbarao, S. M. Feinstone and D. R. Taylor,
"Inactivation of the
coronavirus that induces severe acute respiratory syndrome, SARS-CoV," J Virol
Methods, vol. 121, pp. 85-91, 2004]. This means that in order to stop
spreading of
diseases associated with coronaviruses, commonly touched surfaces should be
decontaminated. One of the ways to decontaminate such surfaces could be use of
different types of UV lamps.
[00035] Each
strain of coronavirus might possess different sensitivity to UV. For
instance, it has been reported elsewhere that far-UVC can eliminate beta HCoV-
0C43 virus in 8 minutes (-90% viral inactivation), in -11 minutes (95%), -16
minutes
(99%) or -25 minutes (99.9%) [M. Buonanno, D. Welch, I. Shuryak and J. D.
Brenner,
"Far-UVC light (222 nm) efficiently and safely inactivates airborne human
coronaviruses," Sientific Reports, vol. 10, pp. 1-3, 2020]. Another study
discovered
that 1,048 mJ/cm2 of UVC for 9 minutes is enough to inactivate 5x106 TCID50/m1
of
SARS-CoV-2 [S. L. Warnes, Z. R. Little and W. C. Keevil, "Human Coronavirus
229E
Remains Infectious on Common Touch Surface Materials," American Society of
Microbiology, vol. 6, 2015]. Moreover, exposure of SARS-CoV-2 to 1 and 3
mJ/cm2 of
222-nm UVC could result in 88.5 and 99.7% viral reduction [. A. Aboubakr, T.
A.
Sharafeldin and S. M. Goyal, "Stability of SARS-CoV-2 and other coronaviruses
in the
environment and on common touch surfaces and the influence of climatic
conditions:
A review," Transbound Emerg Dis., pp. 1-17, 2020]. UVA was also used for viral
inactivation. It was observed that UVA (540 pW/cm2 at a distance of 3 cm)
demonstrated weak inactivation of SARS-CoV-2 after 15 minutes, but UVC (1940
pW/cm2) in a 400-fold decrease in infectious virus after 6 minutes [M.
Bueckert, R.
Gupta, A. Gupta, M. Garg and A. Mazumder, "Infectivity of SARS-CoV-2 and Other
Coronaviruses on Dry Surfaces: Potential for Indirect Transmission,"
Materials, vol.
13, pp. 1-16, 2020], [S. L. Warnes, Z. R. Little and W. C. Keevil, "Human
Coronavirus
229E Remains Infectious on Common Touch Surface Materials," American Society
of
Microbiology, vol. 6, 2015]. Another study found a one-log titre reduction of
SARS-
CoV-2 after 9 minutes of UVA exposure (365 nm) [C. S. Heilingloh, U. W.
Aufderhost,
L. Schipper, U. Dittmer, 0. Witzke, D. Yang, X. Zheng, K. Sutter, M. Trilling,
M. Alt, E.
Steinmann and A. Krawczyk, "Susceptibility of SARS-CoV-2 to UV irradiation,"
American Journal of Infection Control, vol. 48, pp. 1273-1275, 2020].
Moreover, it was
reported that UVA significantly affected single-stranded RNA viruses such as
HCoV-
229E spike proteins without major damage to human cells [R. A, G. G. S. Leite,
G. Y.
Melmed, R. Mathur, M. J. Villanueva-Millan and e. al., "Ultraviolet A light
effectively
16
CA 03177202 2022-09-27
WO 2021/223012
PCT/CA2021/050543
reduces bacteria and viruses including coronavirus," PLOS ONE, vol. 15, pp. 1-
5,
2020].
[00036] Different effects of UVC and UVA on coronaviruses could be explained
by
mechanisms of light absorption. UVA light may be weakly absorbed by RNA and
DNA
and subsequently could be less effective in inducing pyrimidine dimers than
either
UVC or UVB. However, UVA was found to cause additional genetic damage via
production of reactive oxygen species that lead to oxidation of bases and
strand
breaks [M. Bueckert, R. Gupta, A. Gupta, M. Garg and A. Mazumder, "Infectivity
of
SARS-CoV-2 and Other Coronaviruses on Dry Surfaces: Potential for Indirect
Transmission," Materials, vol. 13, pp. 1-16, 2020], [R. A, G. G. S. Leite, G.
Y.
Melmed, R. Mathur, M. J. Villanueva-Millan and e. al., "Ultraviolet A light
effectively
reduces bacteria and viruses including coronavirus," PLOS ONE, vol. 15, pp. 1-
5,
2020].
[00037] Effect of UVA and UVC on infectivity of HCoV-229E strain was analysed
using
MRC-5 cell line. UVA and UVC were engaged using three different protocols. The
infectious dose of HCoV-229E was detected using TCID50 assay using protocols
1, 2
and 2a. The results showed that TCI D50 of HCoV-229E reduced from 7.57 log TCI
D50
of the control 2.34, 2.5 and 1.6 log TCID50 using protocols 1, 2 and 2a after
the first
cycle, respectively. No CPE was observed after 5, 6, 7 and 9 cycles.
[00038] In addition, the results from the exponential model calculations
showed that
one cycle of protocol 1, 2 and 2a reduced cross-contamination of MRC-5 cells
to
32%, 30% and 58%, respectively.
[00039] As many changes can be made to the preferred embodiment of the
disclosure
without departing from the scope thereof; it is intended that all matter
contained
herein be considered illustrative and not in a limiting sense.
17