Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03177643 2022-09-28
WO 2021/211636 PCT/US2021/027161
System and Method of Measuring Venous Oxygen Saturation Using
Intelligent Pulse Averaging With Integrated EKG and PPG Sensors
Related Applications:
The present application claims priority to U.S. Provisional Patent
Applications Serial
Number 63/009,470, entitled PULSE WAVE TRANSIT TIME (PWTT) MEASUREMENT
SYSTEM USING INTEGRATED EKG AND PPG SENSORS, filed April 14, 2020, and to U.S.
Provisional Application Serial Number 63/067,147, entitled, SYSTEM FOR
IMPROVED
MEASUREMENT OF OXYGEN SATURATION, NON-INVASIVE DETECTION OF
VENOUS AND ARTERIAL PULSE WAVEFORMS, AS WELL AS DETECTION OF
CARBOXYHEMOGLOBIN, HYPERTROPHIC CARDIOMYOPATHY AND OTHER
CARDIAC CONDITIONS, filed August 18, 2020, and to U.S. Patent Application
Serial number
17/135,936, entitled SYSTEMS FOR SYNCHRONIZING DIFFERENT DEVICES TO A
CARDIAC CYCLE AND FOR GENERATING PULSE WAVEFORMS FROM
SYNCHRONIZED ECG AND PPG SYSTEMS, filed December 28, 2020, the entire
disclosures
of which are incorporated herein by reference in their entireties for all
purposes.
Technical Field of the Invention:
The present system relates to cardiac sensing systems using combined
electrocardiographic (EKG) and photoplethysmographic (PPG) sensing systems.
Brief Description of the Clinical Problem:
Venous hemoglobin oxygenation in health is often greater than 80%. While this
may
seem surprising, this high level of oxygenation represents a metabolic reserve
that the body can
dip into even though a deep breath has not been taken in the last few seconds.
In states of stress
that reserve will be whittled away; as such venous saturation is clinically
useful as it provides a
measure of the body's oxygen reserve. Currently this can only be obtained via
an invasive
venous blood gas measurement. Venous oxygen saturation and serum lactate are
both used to
1
CA 03177643 2022-09-28
WO 2021/211636 PCT/US2021/027161
measure a patient's degree of metabolic reserve and stress, as venous
saturation is depressed in
times of metabolic stress. Serum lactate will rise when tissues are not
receiving sufficient oxygen
to meet the metabolic needs, and the tissues turn to anaerobic use of glucose.
Currently, the most
common measure for evaluation of metabolic stress is a serum lactate, though
recent studies such
as Serum Lactate Poorly Predicts Central Venous Oxygen Saturation In
Critically Ill Patients: A
Retrospective Cohort Study by Bisara et. al., PMID: 21516712, DOT:
10.1186/s40560-019-0401-
5, suggest venous oxygen saturation may be a better early measure of stress
before onset of
critical decompensation. Serum measurement of lactate, or a venous blood gas,
requires aseptic
blood drawing capacity and a qualified laboratory nearby capable of
expeditiously running a
venous sample that has been put on ice after blood draw. The capability of non-
invasively
measuring venous oxygenation saturation therefore has tremendous implications
for assessing
metabolic stress in both resource-rich and resource-limited situations.
Summary of the Invention and Cardiac Physiology Germane to the Invention:
In preferred aspects, the present system determines venous oxygen saturation
in a system that
comprises: (a) a device positionable against a person's skin; (b) at least one
PPG sensor mounted
on the device for measuring the person's PPG signal at multiple wavelengths of
light; (c) a
plurality of electrodes for measuring the person's EKG signal; (d) a computer
logic system for
receiving and analyzing the PPG signal and the EKG signal, wherein the
computer logic system
further comprises: (i) a system for identifying cardiac cycles in the EKG
signal; (ii) a system for
segmenting the PPG signal into a series of PPG signal segments based upon
features in the
identified cardiac cycles, (iii) a system for sorting the PPG signal segments
into a plurality of
bins, each bin based upon durations of prior R-to-R cardiac cycles and current
R-to-R cardiac
cycles, (iv) a system for generating a composite signal for each of the
plurality of bins, and (v) a
system for measuring a person's venous oxygen saturation by: (a) calculating
arterial oxygen
saturation by comparing composite signals measured at different wavelengths of
light, (b) sub-
sampling composite signals at two consecutive signal maxima measured at
different wavelengths
of light, and (c) comparing the sub-sampled composite signals measured at
different wavelengths
of light to the calculated arterial oxygen saturation to determine venous
oxygen saturation.
Preferably, the arterial oxygen saturation is calculated by comparing
composite signals measured
2
CA 03177643 2022-09-28
WO 2021/211636 PCT/US2021/027161
at different wavelengths of light which comprises comparing composite Signal
Prime Over
Signal (SPOS) signals, each composite SPOS signal being the derivative of a
composite signal
normalized by the composite signal itself.
In preferred aspects, the present system for measuring a person's venous
oxygen saturation
selects preferred bins from which the composite signals are used when
calculating the person's
venous oxygen saturation, and the preferred bins correspond to the bins having
the largest
number of PPG signal segments therein and/or the largest difference between
current and prior
R-to-R values. A composite signal may be generated for each bin by summing or
averaging the
PPG signal segments in the bin. In addition, the composite signal may be used
to generate a
composite Signal Prime Over Signal (SPOS) which is the derivative of the
composite signal
normalized by the composite signal itself. In such aspects, a system for
calculating arterial
oxygen saturation by comparing composite SPOS signals measured at different
wavelengths of
light may be included.
In preferred aspects, the system for generating a composite signal for each of
the plurality of bins
comprises a system for removing aberrant PPG signal segments from the
calculation of the
composite signal, for example, by iteratively re-calculating the composite
signal, by: comparing
a SPOS of each of the PPG signal segments used to calculate a composite signal
against the
SPOS of the calculated composite signal, removing outlier PPG signal segments,
re-calculating
the composite signal with the outlier PPG signal segments removed, and
repeating the iteration
until there are no more outlier PPG signal segments.
In various preferred physical embodiments illustrated herein, the present
system is a hand-
held device with the at least one PPG sensor mounted thereon and a plurality
of electrode wires
extending therefrom or mounted thereon. Alternatively, the present system may
be positioned
within a strap or band disposed around the person's chest or limb with at
least one PPG sensor
and the plurality of electrodes are disposed within the strap or band.
Alternatively, the present
system may be disposed in a patch with the at least one PPG sensor and at
least one of the
plurality of electrodes positioned therein. Systems are also provided for data
transmission.
3
CA 03177643 2022-09-28
WO 2021/211636 PCT/US2021/027161
The present system provides information regarding the metabolic reserve/stress
of a given
patient, inexpensively and non-invasively. Such knowledge can provide
clinicians with critical
point-of-care information about the clinical trajectory of a patient's
recovery or decline quickly
and safely, without having to wait on laboratory results. At heart is the
analysis of FIG. 1
showing the system top-level flow diagram for estimating venous saturation,
wherein the
hemoglobin saturation of arterial blood is obtained from the main-pulse region
of a composite
PPG signal shape (101), i.e. the region of greatest slope change, and a
separate estimation of
venous blood hemoglobin oxygenation is obtained using only end-pulse signal
maxima/arterial
pulse minima (102). The two results are then compared to determine how much
oxygen reserve
is apparent.
The key to this analysis is understanding that the dynamics of the "tissue
sandwich", through
which the PPG signal is filtered, changes slightly at the end of the pulse.
This seen in FIG. 2. The
curve 201 is an experimentally obtained arterial waveform, derived from a
composite IR PPG
signal. As one can see, the waveform is incredibly clean, the result of
intelligent similar pulse
averaging over a number of minutes. The salient point is that, were the
arterial pulse descent
from the peak (or "roll-off') a simple exponential, or even a steady decline,
the curve would not
result in the "hump" seen prior to the pulse minimum just prior to the onset
of the next pulse
(202).
Rather than passive draining, in this time frame there is active filling of
arterioles, in essence
priming an "hour glass" structure consisting of the arterioles, capillaries
(through which blood
cells pass one-by-one), and venules. This priming effect causes a change in
the composition of
the blood measured by PPG oximetry. Capitalizing on this observation, that the
blood
composition is changing just prior to the end of the pulse, signal maxima
(corresponding to
arterial pulse minima) are gathered together and analyzed independently from
the PPG signal
obtained through the pulse. A pictorial depiction of this approach is provided
in FIG. 3, showing
LED sampling in FIG. 3A, standard PPG arterial hemoglobin oximetry, and FIG.
3B end-
pulse/signal maxima sampling. Line 302 shows the arterial pulse sampled via
standard oximetry,
through which LED signal (301) sampling occurs. Line 303 is the resultant PPG
signal. Point
304 shows a signal maxima/pulse minima. FIG. 3B shows the different sampling
done in end-
4
CA 03177643 2022-09-28
WO 2021/211636 PCT/US2021/027161
pulse/signal maxima oximetry. Line 306 shows the arterial pulse, through which
LED signal
(305) sampling occurs, though only at end-pulse, signal maxima. Line 307 is
the resultant PPG
signal with such sampling.
Further depiction of the structure being measured at arterial end-pulse can be
seen in FIG. 4.
In FIG. 4A the arteriole/capillary/venule structure is shown as an hourglass.
Through the main
portion of the arterial pulse, an hourglass representing the
arteriole/capillary/venule structure
measured in reflective oximetry is dominated by the maximally oxygenated, pre-
capillary
arteriole blood (401). However, at end-pulse there is piling up of the venule,
post-capillary
blood, depicted in 402. FIG. 4B gives another representation of the structure,
with the structure
measured in reflective oximetry is shown as a shaded area (403). Also seen is
a graph with a
curve of the velocity of blood movement (404). This shows the blood cells
slowing to their
lowest velocity as they pass through the capillaries, with an asymmetry in
velocity prior and post
capillaries, explaining the change in arteriole to venule blood at end-pulse.
The approach is further explained in FIGS. 5A and 5B, showing changes between
the
sampling points (the R-to-R duration) as linear, and how the PPG signal may
change. Area 501
shows the end-pulse arterial (pre-capillary) blood, and area 502 the end-pulse
venous (post-
capillary) blood; area 503 is the fixed elements of the structure (largely
connective tissue). In
FIG. 5A LED sampling through the structure at end-pulse/signal maxima (504)
before and after
long pulses yields PPG signals 505 and 506, separated by time 507 (R-to-R
duration). In FIG.
5B LED sampling through the structure at end-pulse/signal maxima (504) before
and after short
pulses yields PPG signals 508 and 509, separated by time 510 (R-to-R
duration).
The present system uses combined electrocardiography (EKG) and
photoplethysmography
(PPG) signals (PPG is also commonly referred to as oximetry and the two terms
will be used
interchangeably throughout this specification). The former senses voltage
produced by heart
muscle contraction, and the latter measures light absorbed by tissues. Changes
in PPG signal
reflect changes in blood volume and measurement at different wavelengths
allows determination
of oxygen saturation.
CA 03177643 2022-09-28
WO 2021/211636 PCT/US2021/027161
The present system allows different insight than is currently available using
hand-held,
portable PPG systems/devices. The combination of EKG and PPG signals in this
system utilize
Pulse Wave Transit Time (or PWTT), and PPG Signal Prime Over Signal (SPOS)
curves. PWTT
is the period of time taken between a heartbeat as measured by the onset of
the QRS complex
and the time at which the blood from the aorta reaches an extremity or other
body part, as
determined by the negative spike generated in the SPOS curve, also described
as the derivative
of the LED signal divided by the signal. Use of the signal derivative to
determine the change in a
LED signal heralding the arrival of an arterial pulse has been described in
U.S. Patent
10,213,123, assigned to MocaCare Corporation of Palo Alto, California, however
use of the
signal prime over signal (SPOS) allows for greater insight, as it normalizes
each wavelength
signal and thus allows for comparisons between different wavelength SPOS
curves.
Improved arterial oxygen saturation estimation is then generated by this
system from an
SPOS curve using a composite sum/average of similar pulses, with the added
ability to generate
oxygen saturation for selected segments of the cardiac cycle, specifically end-
pulse oximetry.
Prior (n-1) EKG R-to-R duration using R-wave peaks are calculated, as are
Current (n) R-to-R
duration, PWTT, and SPOS. These are all used by the present system to
determine similarity of
oximetry pulses, with similar pulses summed/averaged to form composite pulses,
then
comparing differing composite pulses to gain cardiovascular insight.
Reduced PWTT corresponds to greater pulse wave velocity, though the greater
velocity does
not indicate better pump function. This is because the aortic bulb acts as a
"mechanical
capacitor", allowing metered delivery of arterial pulse volume. However,
having obtained the
PWTT for any given monitoring point on the body, this metric remains
relatively stable and
changes only gradually barring a sudden change in cardiovascular state (e.g.
sudden change in
heart rhythm such as onset of atrial fibrillation with rapid ventricular
response). PWTT therefore
provides a means by which to ensure accurate further data collection and
analysis. This allows
more reliable extraction of additional information from the combination of
signals, and
removal/minimization of introduced noise.
6
CA 03177643 2022-09-28
WO 2021/211636 PCT/US2021/027161
Measurement of absorption of light (per Beer-Lambert law) has the form
Measurement(t) = Ke [-MO], and the signal prime over signal (SPOS) of the
measurement
will be: SPOS(t) = -C (cif(t))
dt
The LED signals in plethysmography have the form:
(1) Signal = K * e[-tial(t)*(a*Hb)arteriall * e[-Venous(t)*E(a*Hb)venousl
E(a * Hb)arterial and E (a
* - - - Hb)
venous describe the composition of the blood and
generally change slowly. Therefore, these two terms are constants across time
for the duration of
our sampling. (These terms will be explained in greater detail herein).
Further, in healthy individuals, the venous flow is considered a constant.
Current oximetry
measures assume this, and so will we for this initial exploration. Given this
assumption, the
equation reduces to:
(2) Signal = K1 * e[-Arterial(t)*E(d*Hb)arteriall
Using properties of the exponential function, and of its derivative, we derive
the SPOS for
the PPG Signal at several wavelengths (e.g., IR and Red).
(3) SPOS(t) = (dArterial(t))
dt * (a * Hb)arterial
Using the fact that the conceptual function Arterial(t) is the same for both
Red and IR PPG
signals, we show that the SPOS of the signal from the IR LED (SPOS) is
directly proportional
to the SPOS of the signal from the Red LED (SPOSRed):
(4) SPOSRed = R * SPOS IR or SPOSRed / SPOSIR = R
7
CA 03177643 2022-09-28
WO 2021/211636 PCT/US2021/027161
Returning to the expression: E (a4Hbx* Hbx) This describes how different
wavelengths of
light are absorbed by the blood depending on the relative quantity of the
types of hemoglobin
present within.
Where:
aHbx
õ = absorption coefficient for type of hemoglobin (deoxyhemoglobin,
-
oxyhemoglobin, carboxyhemoglobin, methemoglobin), x, for the wavelength, p.
Hbx= fractional composition of blood of various types of hemoglobin. The Sum
of
fractional components of different types of hemoglobin = 1.0
In the conditions of low levels of carboxyhemoglobin and methemoglobin (e.g.
excepting
situations such as carbon monoxide or cyanide poisoning), and using accepted
standard
absorption coefficients for amHb' aRedyb, Hb = 1 - Hb02.
We end up with the equation:
(5) (aRedHbo2 * lib02) + (aRedim * (1 - Hboj) = R *[(aIRHbo, * Hb02)+ (aIRHb*
(1¨ Hb02))1
The only unknown is HAD,. Solving for Hb02 gives us the fraction of the blood
that is
oxygenated (Arterial oxygenated hemoglobin Fraction, or Arterial Frac 02):
(6) Arterial Frac 02 = (¨a1RHb*R-FaRedHb)
R*(a1RHb02¨alRlib)+(aRedHb¨aRedHb02)
This direct proportionality between SPOS for any wavelength and the summation
of optical
absorption coefficients times the fraction of hemoglobin is used extensively
by the present
system.
Any recording of EKG, or oximetry signals, or their interaction, will have
physiologic
variability, as well as noise. Management of EKG noise have established
protocols that have
been built up over 100 years. Conditioning of oximetry signals do not have as
long a history.
8
CA 03177643 2022-09-28
WO 2021/211636 PCT/US2021/027161
Physiologic oximetry variability can occur from changes in venous flow (due to
volitional
movement, or passive movement from repositioning, or inflation/deflation of a
blood pressure
cuff/sphygmomanometer, etc.), respiration causing changes in intra-thoracic
pressure with
resultant change in blood volume return to the heart, or beat-to-beat duration
variability. Noise,
or non-physiologic variability, can also occur from a range of possibilities,
from variation in the
surface pressure and angle of application of the detector, to ambient light
infection of signal
collection, to DC drift of the detection circuit. Whatever the specific source
of variation, without
an intelligent approach to the signals, one cannot tell physiologic
variability apart from non-
physiologic variability (introduced noise).
Traditional means for dealing with noise introduced into oximetry signals is
to filter. For
example, a commonly used algorithm for detecting signal to noise ratio
utilizes power within the
frequencies below 20 Hz compared with power above this frequency (as described
in
MaximIntegrated AppNote AN6410.pdf provided by Maxim Integrated Corporation of
San Jose,
California). This frequency filtering highlights the underlying primary rhythm
(heart rate) and
smooths the appearance of the displayed waveform. However, pulses are not all
the same, and
treating them as if they are deletes valuable information that can be mined
for deeper insight.
An alternative means by which to minimize variability is to average the
oximetry over many
pulses, as described in U.S. Patent 10,485,433, assigned to Intel Corporation.
This allows for
minimization of introduced noise, but eliminates any information that could be
gleaned from
physiologic variability. This approach produces a single, homogenized, and
representative pulse
at the end of the process. However, pulses are not all the same, and treating
them as if they are
effectively obliterates some of the available information.
With the observation of FIG. 2, a re-evaluation of the assumption of a flat
venous blood
profile (as in equation (1)) can be done. The combination of more or less
arteriole
filling/emptying and more or less right ventricular filling with differing R-
to-R will be seen in
varying PPG signal maxima, with both venule volume and arteriole volume
changing at signal
maxima (FIG. 5). The ratio of arteriole and venule composition in this delta
volume is not yet
entirely clear. However, the approach shows that an enlarging difference
between end-pulse
9
CA 03177643 2022-09-28
WO 2021/211636 PCT/US2021/027161
oximetry and arterial hemoglobin oxygen saturation calculated from the rising
pulse (main-pulse
oximetry) reflects falling venous hemoglobin oxygen saturation.
Brief Description Of The Drawings:
FIG. 1 is a top-level flow diagram of the operation of the present system.
FIG. 2 shows a PPG signal filtered through a "tissue sandwich", showing slight
changes at the
end of the pulse.
FIG. 3A is an illustration of LED sampling for standard PPG arterial
hemoglobin oximetry.
FIG. 3B is an illustration of end-pulse/signal maxima sampling.
FIG. 4A illustrates arteriole/capillary/venule structure is shown as an
hourglass.
FIG. 4B corresponds to FIG. 4A, with the structure measured in reflective
oximetry.
FIG. 5A illustrates LED sampling through the structure of FIGS. 4A and 4B at
end-pulse/signal
maxima before and after long pulses.
FIG. 5B illustrates LED sampling through the structure of FIGS. 4A and 4B at
end-pulse/signal
maxima before and after short pulses.
FIG. 6 shows the process of R-wave peak refinement used to generate tOn.
FIG. 7 shows the nomenclature and data structures used in the description of
the present system.
FIG. 8 also shows the nomenclature and data structures used in the description
of the present
system.
CA 03177643 2022-09-28
WO 2021/211636 PCT/US2021/027161
FIG. 9 is an exemplary illustration of various physical components of the
present system.
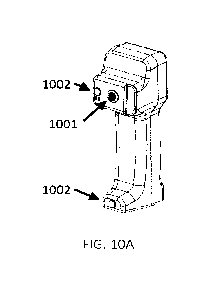
FIGS. 10A to 10D show various views of a hand-held embodiment of the present
system, having
PPG and EKG sensors mounted thereon or attached thereto.
FIG. 11 is a is a cut-away view of a portion of the device of FIGS. 10A to
10D, showing an
optical waveguide adjacent to a PPG sensor.
FIG. 12A is an illustration of the system of FIGS. 10A to 11 collecting PPG
signals from a
person's fingers.
FIG. 12B is an illustration of the system of FIGS. 10A to 11 collecting PPG
signals from the
outside of a person's arm.
FIG. 13 is a is an illustration of EKG and PPG signals measured over time and
generated SPOS
signals corresponding thereto.
FIG. 14 is a is an illustration of one-sided Gaussian fitting.
FIG. 15 illustrates the time relationship of the end-pulse/pre-pulse area of
interest relative to the
SPOS negative spike fitting window.
FIG. 16 illustrates a time-correlated comparison of EKG and PPG signals
showing the
relationships in creation of two-beat dependencies, showing current and prior
"R-to-R".
FIG. 17A illustrates the number of pulses in each bin for a run of a patient
with normal sinus
rhythm.
FIG. 17B illustrates filling of the Current R-to-R versus Prior R-to-R matrix
for purposes of
determining end-pulse oximetry (the greatest difference in R-to-R duration),
and the "fall-back"
11
CA 03177643 2022-09-28
WO 2021/211636
PCT/US2021/027161
or second tier bin choices using intermediate bins providing PPG signal maxima
differences
allowing for venous oxygen saturation estimation.
FIG. 18 is a top-level block diagram for the end-pulse/venous oxygen
saturation calculation.
FIG. 19 is a is an exemplary algorithm for preparing Pulse Data Sets in
accordance with the
present system.
FIG. 20 illustrates the derivation of the Pulse Wave Transit Time (PWTT).
FIG. 21 shows the calculation of end-pulse/venous oxygen saturation.
FIG. 22 illustrates an exemplary embodiment of the present system disposed in
a chest strap.
FIG. 23 is a is a sectional view through the patient corresponding to FIG. 22.
FIG. 24 illustrates an exemplary embodiment of the present system
incorporating a bicep strap
with an electrode extending therefrom.
FIG. 25 is a is a sectional view through the patient corresponding to FIG. 24.
Detailed Description of the Invention:
The central element of the system is the identification and manipulation of
PPG signals on
the basis of Prior R-to-R and CurrentR-to-R duration. The system them
generates composite
pulses from similar pulses.
In accordance with preferred aspects disclosed in U. S. Provisional patent
application
62/955,196, entitled A System For Synchronizing Different Devices To A Cardiac
Cycle, filed
December 30, 2019 and in U.S. Patent Application Serial number 17/135,936,
entitled SYSTEMS FOR SYNCHRONIZING DIFFERENT DEVICES TO A CARDIAC
12
CA 03177643 2022-09-28
WO 2021/211636 PCT/US2021/027161
CYCLE AND FOR GENERATING PULSE WAVEFORMS FROM SYNCHRONIZED ECG
AND PPG SYSTEMS, filed December 28, 2020, incorporated herein by reference in
their
entireties, the present system uses a specific trigger to set time = 0 for
each beat (e.g. EKG R-
wave peak) and then stores each pulse from this start point until completing a
full cycle of sensor
data, such as with LED oximetry signals from maximum to minimum and back to
maximum ¨
which will be a waveform longer than a single pulse length. The next pulse
waveform will have
a t = 0 at the next EKG R-wave peak, thus recording of the next beat will
start before the
recording of the last pulse waveform has completed. In absolute terms, the
time corresponding
to t=0 for the nth pulse will be referred to as time tOn throughout the rest
of the specification.
FIG. 6 shows the process of R-wave peak refinement used to generate tOn. The
example
shows how the algorithm has determined the polarity of this collection to be
negative (wires
reversed), and thus the R-wave to be negative. The tOn of the R-wave peak is
found using
polynomial fitting (602) to EKG datapoints (601) and interpolation, then used
to define a Pulse
Data Set.
FIG. 7 and FIG. 8 show the nomenclature and data structures used in the
description (Unless
otherwise specified, PWTT = PWTTIR and PPG signal = PPG signalIR). The tOn
time point is
then used to define a Pulse Data Set with the PPG signals of multiple
wavelengths (here red,
infrared, and green). Stored with the PPG signal are the values for the prior
R-to-R, and current
R-to-R durations, the derived signals for Signal Prime over Signal (SPOS) for
each wavelength,
and the Pulse Wave Transit Time (PWTT) for each wavelength. Note the first PPG
signal
maxima (701) and the second PPG signal maxima (702). FIG. 8 shows the
structure of the
Composite Pulse Data Set, constructed from a group of Pulse Data Sets on the
basis of a defined
criteria (e.g. similar prior R-to-R, or current R-to-R duration). Note how the
PPG waveforms are
of duration longer than a single cardiac cycle, and are long enough to assure
capture of both the
first (801) and second PPG signal maxima (802).
FIGS. 9-11 show a preferred device implementation of the system. The device
block
diagram shows the elements of the device/system, with multiple wavelength LEDs
(901) and a
photodiode detector (902), and EKG input from electrodes (903) applied to the
left and right
13
CA 03177643 2022-09-28
WO 2021/211636 PCT/US2021/027161
chest (or left and right upper extremities. In preferred embodiments, signals
are then fed to a
processing unit (904) carrying out "on-chip" logic that then generates
Composite Pulse Data Sets
from raw signal. The Composite Pulse Data Sets are then communicated via
either wireless or
direct cable connection to an "off-device" display/computing unit (905) that
provides the user
with the final end-pulse oxygenation, arterial main-pulse oxygenation, and
resulting venous
saturation estimation with more graphical options (such as changes over time).
There is also on-
device storage (906) for code as well as buffering and packetized transfer of
data. In an alternate
embodiment, the processing unit simply coordinates communication of raw ECG
and PPG signal
data to the external computing/display device which handles all aspects of the
hydration level
estimation logic. In yet another embodiment, all aspects of hydration level
estimation are carried
out by the processing unit, including rendering of graphics and reporting of
oxygen saturation.
In this case, the external computing/display device provides only the display
function.
FIGS. 10A-D show various view of the PPG collection device. 1001 shows the
optical
waveguide (in front of LEDs and detector); 1002 shows optional incorporated
EKG electrodes;
1003 shows plug-in connector sites for EKG lead wires to adhesive EKG
electrodes (on right and
left chest).
FIG 11 shows detail of the PPG head, with an optical waveguide (1101) that
abuts the LEDs
and detector (1102) on the interior of the device. The optical waveguide
allows for collection of
PPG signals at sites other than the finger.
FIGS. 12A and 12B depict the device in use collecting PPG signals from the
finger (FIG.
12A), and the outside of the upper arm (FIG. 12B). The PPG measurement end of
the device is
applied to the skin in a stable fashion so that PPG measurement can be taken
over the course of
1-2 minutes or more. EKG electrodes are applied to the left and right sides of
the torso (or upper
extremities) and connected to the plug-ins on the smaller end of the device.
Returning to FIG. 1, PPG and EKG signals are collected. PPG waveform selection
is
performed to screen out aberrant beats considerably different than the
majority of pulses, such as
14
CA 03177643 2022-09-28
WO 2021/211636 PCT/US2021/027161
premature ventricular contraction beats, an example where the cardiac
contraction does change
appreciably from the beat prior.
FIG. 13 shows: EKG signal (1301); EKG R-wave peak (1302); PPG signal segments
(contained within Pulse Data Sets) (1303, 1304, 1305); SPOS signals (contained
within Pulse
Data Sets) (1306, 1307, 1308); PWTT using SPOS (also contained within Pulse
Data Sets)
(1309, 1310, 1311). This demonstrates selection of one of the PPG signals (in
the current
implementation red, infrared, and green are used, though the approach is not
limited to using
these alone) with full time length for both PPG signal and SPOS longer than
the R-to-R duration.
The present method and system of intelligent pulse averaging counters the
effect of drift in
"K" (seen in equation 1), related to absorption from fixed elements in the
tissue being analyzed.
With averaging, some pulses will have an upward drift in K, some will have a
downward drift,
leaving the averaged pulse with more options for data point comparisons across
the composite
pulse width.
SPOS generates similar shaped curves for the LED signals for the different
wavelengths,
magnitude differing only by a multiplier that is the E (a * Hb) for the
specific wavelength. The
present system includes the two novel approaches of examining the SPOS signal
in the region of
the "negative spike" to determine:
- the linearity of the rising LED SPOS signal, or
- the fit of the SPOS signal to a combination of Gaussian derivatives
and/or exponential
and/or polynomial equations.
Given the similar shapes for the SPOS curves, any such fitting can be applied
to one
wavelength to yield a fitted curve. Fitting to another wavelength only
requires finding the
magnitude needed to best fit that curve. For example, if f(t) best fits the
infrared LED SPOS,
then "A" needed to best fit A*f(t) to the SPOS for the red LED signal yields
the arterial oxygen
saturation just as with the equation 1. The difference with the standard
formulation is that this
CA 03177643 2022-09-28
WO 2021/211636 PCT/US2021/027161
fitting is based on many more time points (up to 50 at slower heart rates)
than the two (maximum
and minimum) used in the standard formulation.
FIG. 14 shows this concept using a one-sided Gaussian derivative fitting.
Curve 1401 are the
datapoints of a collected Composite IR SPOS signal with a fitting window 1402
selecting out the
negative SPOS "spike". Curve 1403 shows the datapoints for the window in an
expanded plot,
also showing the one-sided Gaussian derivative fitted curve 1404.
The interval of the fitting window selected (the SPOS "negative spike"), or
subset thereof
(e.g. the rising SPOS right half of the "negative spike") represents a unique
period wherein a
single dominant and coherent physiologic event - the contraction of the left
ventricle during the
time of an open aortic valve - is clearly separate from other confounding
physiologic features.
This allows for extraction of parameters, which can then be applied to the
entire PPG sensor
pulse waveform.
The interval just preceding this fitting window for the "negative spike" of
the SPOS
represents yet another unique interval, as described in the summary of the
physiology above.
FIG. 15 shows the time relationship of the end-pulse/pre-pulse area of
interest relative to the
SPOS negative spike fitting window. Time 1501 identifies the end-pulse point;
region 1502
shows the excess SPOS PPG above expected linear or exponential "rolloff' (the
shaded region of
FIG. 15 corresponds to the shaded region of FIG. 2). Region 1503 identifies
the window of the
negative SPOS "spike" used to estimate arterial hemoglobin oxygen saturation
fraction
Two Beat Complex Creation for Venous Saturation Analysis:
2-beat complex selection for a longer train of pulses in atrial fibrillation
(yielding random R-
to-R duration) is shown in FIG. 16. Signal from a two-electrode, single lead
EKG (curve 1601) is
plotted in temporal alignment with an infrared (IR) LED PPG signal (curve
1602). As the
infrared wavelength has relatively equivalent absorption from venous and
arterial blood, this is
the wavelength shown and used to select pulses for further analysis.
16
CA 03177643 2022-09-28
WO 2021/211636 PCT/US2021/027161
With accumulation of similar 2-beat complexes (based on similar n-1 R-to-R and
n R-to-R
duration), composite pulse construction can be taken from one pulse
minima/signal maxima all
the way through to the next pulse minima/signal maxima. With this formulation,
pulse
minima/signal maxima at the start of the pulse and at the end of the pulse can
be compared, with
additional information available regarding the cardiovascular state of the
individual. FIG. 16
shows the top/bottom alignment of EKG (1601) and PPG signals (1602) showing
the steps in the
of generation of a composite PPG wave for purposes of venous oxygen saturation
derivation.
The above EKG (1601) signal shows a series of pulses labeled A through I. Each
of these
pulses has a different duration, though some are closer in duration than
others. 2-beat
dependency ties together two successive beats, with key features being the R-
to-R duration of the
first beat, and the PPG signal of the second beat. This is a dependency (1603)
as depicted in the
bracket tying together the R-to-R duration of beat "B" (1604) and the PPG
signal (1605) of beat
"C". Additionally important in this analysis is the current R-to-R duration,
which for this
complex is the R-to-R duration of pulse "C" (1606). Notable with the bracketed
complex 1603 is
a paring of a long n-1 R-to-R followed by a short n R-to-R.
Pulses B and C are analyzed together, with the R-to-R duration of B and R-to-R
duration of
C putting this 2-beat complex in the long n-1 R-to-R/short n R-to-R "bin".
Next, pulses C and D
are considered together, with the R-to-R duration of C and R-to-R duration of
D putting this 2-
beat complex in the short n-1 R-to-R/long n R-to-R "bin". Next, pulses D and E
are considered
together, with the R-to-R duration of D and R-to-R duration of E putting this
2-beat complex in
the long n-1 R-to-R/intermediate n R-to-R "bin". Next, pulses E and F are
considered together,
with the R-to-R duration of E and R-to-R duration of F putting this 2-beat
complex in the
intermediate n-1 R-to-R/short n R-to-R "bin". Next, pulses F and G are
considered together, with
the R-to-R duration of F and R-to-R duration of G putting this 2-beat complex
in the short n-1 R-
to-R/long n R-to-R "bin". Next, pulses G and H are considered together, with
the R-to-R
duration of G and R-to-R duration of H putting this 2-beat complex in the long
n-1 R-to-
R/intermediate n R-to-R "bin".
17
CA 03177643 2022-09-28
WO 2021/211636 PCT/US2021/027161
As this analysis reveals, atrial fibrillation provides a wide range of
permutations of n-1 R-
to-R and n R-to-R duration. This allows for analysis using short-long and long-
short n-1 and n R-
to-R durations, the combinations that reveals the biggest changes in PPG
signal maxima.
However, with normal sinus rhythm, it is harder to select combinations that
will help reveal
signal maxima differences. FIG. 17A shows the number of pulses in each bin for
a run of a
patient with normal sinus rhythm. Note how the diagonal corresponding to the
same n-1 and n
R-to-R durations are most populated and the short-long and long-short bins are
the least
populated. FIG. 17B shows the preferential filling of the Current R-to-R
versus Prior R-to-R
matrix (1701) for purposes of determining end-pulse oximetry (the greatest
difference in R-to-R
duration), and the "fall-back" or second tier bin choices using intermediate
bins (1702) providing
PPG signal maxima differences allowing for venous oxygen saturation
estimation.
Measuring Venous Oxygen Saturation
Because these 2-beat complexes define both the beginning and ending composite
PPG signal,
both first and second signal maxima (pulse minima) are therefore defined. And
because
accumulation of similar 2-beat complexes reduce the effect of DC drift, the
methods described
here also allow for an estimate of venous saturation. The top-level block
diagram for the end-
pulse/venous oxygen saturation calculation is seen in FIG. 18.
R-wave peak refinement of pulse "n" is done with curve fitting and
interpolation (1801) prior
to determining the prior (n-1) and current (n) R-to-R duration; then prior (n-
1) and current (n) R-
to-R durations for Pulse Data Set "n" are incorporated into Pulse Data Set "n"
(1802). PPG
signals are gathered, and a process of outlier rejection is carried out
(including but not limited to
data determined to be corrupted using accelerometer input, as well as cross-
checking with
multiple LED PPG sensors, 1803). Once the PPG signals of the current Pulse
Data Set have
been selected, the Pulse Data Set is considered together with all available
prior Pulse Data Sets
and their PPG signals (each of which is associated with a prior (n-1) R-to-R
duration and current
(n) R-to-R duration).
18
CA 03177643 2022-09-28
WO 2021/211636 PCT/US2021/027161
Available Pulse Data Sets are then sorted into a 3 by 3 bin matrix of Prior R-
to-R and Current
R-to-R, each of which are considered and deemed to be short, intermediate, or
long duration
(1804). Dynamic boundary adjustment is used to ensure relatively equal numbers
across bins, to
the extent possible: normal sinus rhythm yields few Pulse Data Sets available
for bins off the
diagonal of short-short, intermediate-intermediate, and long-long (see FIG.
17). After all Pulse
Data Sets are allocated into bins, the optimal bins are selected, e.g. those
bins containing the
largest number of Pulse Data Sets and those that reveal the biggest changes in
signal maxima
(1805). With the optimal bins established, and initial Composite Pulse Data
Set is formed by
adding together the corresponding PPG signals of each wavelength for each
Pulse Data Set in the
bin (1808).
With the Pulse Data Sets in a bin and the initial Composite Pulse Data Set in
hand, a pruning
loop is carried out for each bin to weed out Pulse Data Sets with noisy or
otherwise aberrant PPG
signals (1806) that made it through the coarser outlier rejection. For each
Pulse Data Set in the
bin, and for each wavelength in the Pulse Data Set, the PWTT for the
wavelength is compared
against the PWTT for the wavelength for the Composite Pulse Data Set
(aggregate of all the
pulses). If the PWTT of two of the current three wavelengths (red, green, IR)
are within 15% of
the PWTT of the Composite Pulse Data Set, the Pulse Data Set is left in the
composite. If not,
the Pulse Data Set is rejected ("pruned") and the process is run again with
the remaining Pulse
Data Sets. A pruned Pulse Data Set is removed from the bin and subtracted from
the Composite
Pulse Data Set. If the number of Pulse Data Sets falls below a specified
threshold for the number
in the bin (good results have been obtained with numbers down to 4), then an
additional Pulse
Data Set is added prior to reporting any results. The algorithm is seen in
FIG. 19. FIG. 20 shows
the derivation of the Pulse Wave Transit Time (PWTT). This is done using the
Signal Prime
Over Signal (SPOS(t)) curve for each wavelength PPG signal(t), together with
interpolation and
(negative) peak refinement.
The calculation of end-pulse/venous oxygen saturation then proceeds as shown
in FIG. 21.
The venous oxygen saturation calculation follows the derivation shown in
Appendix B using 2-
point composite signals composed of PPG signal maxima corresponding to
incongruent R-to-R
19
CA 03177643 2022-09-28
WO 2021/211636 PCT/US2021/027161
duration from 2-beat complexes (1807). The results are averaged and the venous
fractional
oxygen saturation is reported (1809).
System Operation:
Operational alternative options are presented in the various exemplary
embodiments of the
present system, below. It is to be understood that the present system can be
embodied in any of
the systems described herein, and that the present system is not limited
solely to the various
exemplary embodiments described below:
FIG. 22 illustrates use of a chest strap (2201) across the chest, with
incorporated electrodes
(2202) contacting the left and right chest, and LED device with detector
(2203).
FIG 23 illustrates cross section of a chest strap (2301) across the chest,
with incorporated
electrodes (2302) contacting the left and right chest, and LED device with
detector (2303).
FIG. 24 illustrates use of a bicep strap (2401), with incorporated electrode
(2402) and LED
device with detector (2403). A second electrode piggybacks off existing
telemetry wiring (2404).
FIG 25 illustrates cross section of a bicep strap (2501), with incorporated
electrode (2502)
and LED device with detector (2503). A second electrode piggybacks off
existing telemetry
wiring (2504).
An advantage of a chest or arm strap or band is that the band/strap provides a
normal force
on the LED of the PPG sensor to get a good signal off the chest wall. In
aspects where a chest or
arm strap is used, optional "traction" may also be provided on the inside of
the strap, similar to
the silicone / adhesive bead that is found on the inside of standard bike
shorts to keep the legs
from riding up.
CA 03177643 2022-09-28
WO 2021/211636 PCT/US2021/027161
APPENDIX A: Cardiovascular Physiology Background
In normal health, delivery of oxygen and glucose to tissues is adequate for
tissues to meet
their energy needs by aerobic glycolysis, a process using oxygen to breakdown
glucose that
releases far more energy than anaerobic glycolysis, or fermentation
(metabolism of glucose
without oxygen). Whereas aerobic glycolysis breaks down glucose to water and
carbon dioxide,
anaerobic glycolysis breaks the glucose down to lactic acid. In health lactate
is low, and pH
(affected by the presence of lactic acid) is maintained around 7.4. Anaerobic
metabolism with
production of lactic acid allows muscles to transiently access extra energy
when the tissue needs
are high and there is insufficient delivery of oxygen to "burn" the available
glucose (such a
situation seen when sprinting at maximal effort for short distances). The
lactate thus produced is
then cleared from the blood stream by the liver and converted back to glucose
once the
physiologic stress is resolved. This allows for complete aerobic glycolysis of
the previously
fermented glucose.
However, when that physiologic stress is sustained rather than transient, many
things begin
to go awry. This can happen when infection causes the metabolism to
drastically increase; or it
can happen when delivery capability is suddenly reduced, as with a heart
attack; it can also
happen in the setting of otherwise moderate stress in the setting of baseline
reduction in heart
pump function. In all cases, the oxygen requirements of the tissues increase
relative to what the
cardiovascular system can deliver. Oxygen obtained in the lungs cannot fully
replace the oxygen
removed from the blood stream in the capillaries. In this situation the
arterial hemoglobin
oxygenation will fall ¨ though the venous saturation will fall even more due
to the body eating
into the oxygen reserve in the venous blood stored up prior to the stress
(oxygen saturation of
venous blood may exceed 80% in unstressed normal health). All of which yields
a growing
difference between arterial and venous oxygen.
As venous oxygenation falls further, lactate levels will eventually begin to
rise, though recent
studies have shown that the rising in lactate is preceded by a measurable fall
in venous
oxygenation, which that fall providing clinically useful information. To
measure the venous
blood oxygen, though, requires a venous blood gas sample. This is obtained by
a blood draw that
21
CA 03177643 2022-09-28
WO 2021/211636 PCT/US2021/027161
is immediately placed on ice and sent to a qualified lab. All of which is a
relatively expensive
and invasive procedure with a minimum turn-around time of around 10-15
minutes, if done
STAT.
Circulatory shock causes inadequate oxygen delivery, resulting in
mitochondrial hypoxia.
With failure of mitochondrial oxidative phosphorylation, energy metabolism
becomes dependent
on anaerobic glycolysis. Anaerobic glycolysis sharply increases the production
of cellular
lactate, and then blood levels. With severe infection, the blood lactate
concentration varies in
proportion to the ongoing deficit in tissue oxygenation. The ability of the
patient to clear blood
lactate indicates restoration of oxygen delivery with resuscitation. Studies
have shown that a
lactate clearance of 10% or more predicts survival from septic shock.
Studies have also shown that falling venous oxygen can provide earlier usable
information
than rising lactate. This unfortunately requiring ongoing invasive monitoring
via a central venous
line (or Swan-Ganz intracardiac catheter) and/or repeated blood draws.
22
CA 03177643 2022-09-28
WO 2021/211636 PCT/US2021/027161
APPENDIX B: Venous Hemoglobin Saturation Calculation
At the pulse minima (LED signal maxima),
(1) Signal max = K * lo * eHa*thickness)connectivetissuel
* eHstaticarterialblood)*E(a*Hb)arterial]
(2) Signal = K * e[-ArterialPulse(t)*E(a*Hb)arteriall * e[-
VenousPulse(t)*E(a*Hb)venous]
where (a * Hb)arterial and (a * Hbl
'venous are the absorption coefficients of the type of
hemoglobin (deoxyhemoglobin, oxyhemoglobin, carboxyhemoglobin, methemoglobin),
and
E (a * Hb)arterial and E (a * Hbl
'venous are summations of absorption coefficient for each type
of hemoglobin times the fraction of each type of hemoglobin making up the
arterial and venous
pulses (as they have different compositions, the arterial blood carrying a
much higher fraction of
oxygenated blood).
Collecting the LED signal maxima (A and B, separated in time by R-to-R
duration), treating
them as the time varying signal, and reordering the equation:
(3) Signalmax(t) = K * lo * eHa*thickness)connectivetissuel
* eHstaticarterialblood)*E(a*Hb)arteriall
* eHdynamicarterialblood)*E(a*Hb)arteriall
* e[-(venous(t))*E(a*Hb)venous]
With arteriole blood priming an "hour glass" structure consisting of the
arterioles, capillaries,
and venules toward the end of the pulse, some change in the composition of the
blood toward the
end of the pulse is present. This suggests that a re-evaluation of the
assumption of flat venous
blood profile can be done by using the following to model the change in blood
composition at
PPG signal maxima:
(4) delta arterial blood = gamma* delta volume
(5) delta venous blood = (1-gamma)* delta volume
23
CA 03177643 2022-09-28
WO 2021/211636
PCT/US2021/027161
Where gamma is somewhere between 1 and 0.5.
(6)
Signalmax(t) =
Kv * eFal-gamma)*volume(t))*E(a*Hb)arteriall * e
[¨(gamma*volume(0)*E(a*Hb)venous]
(7) 2ptSPOS(Signa/max(t)) = ¨
R B¨ t014 R) * (132+A)
(8) 2ptSPOS(Signa1max) = (dVolume(t))
arter tat * [gamma * E(a * Hb) + (1
¨ gamma) *
2*dt
E(a * Hb)venous]
(9) 2ptSPOS(Signa1max)/[gamma * E(a * Hb)arterial + (1¨ gamma) * E(a *
Hb)venousl
(dVolume(t))
2*dt )
Once again assuming that there is only deoxyhemoglobin (Hb) and oxyhemoglobin
(Hb02),
(10)
2ptSPOS(Signalmax(M =
(dVolume(t))
[gamma*Z(Cr*Hb)artertal+(l-gamma)*(aHb+(axb02-aHb)*Hb02)J 2*dt )
Done at two or more different wavelengths (e.g. red, infrared, though not
exclusive to these),
one can solve for Hb02, given that the only unknowns are Hb02 and
dVolume(t)/dt. Note that
E (a * Hb)arterial at each wavelength is known from analyses done elsewhere in
the system
description.
(2ptSPOS(SignalmaxRed(t)))
(11) With R =
(2ptSPOS(SignalmaxIR(M)
(1¨gamma)*[¨amilb*R¨aRedHb]+gamma*[¨Z(cr*Hb)artertalIR*R+Z(IX*Hb)artertaIRedi
(12) ____________________________________________________________________ Hb
02 =
(1¨gamma)[R*(a¨pe
iHb02¨aiRim)+(aRedim¨aRedHb02)1
24