Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
Description
ELECTROLYTIC CELL, METHOD FOR OPERATING A CELL OF THIS TYPE
AND ELECTROLYSER
The invention relates to an electrolytic cell comprising a cathode half-cell
having a
cathode, an anode half-cell having an anode, and a separator which separates
the
two half-cells from one another and which is permeable to an electrolyte
present in
the half-cells during operation. The invention further relates to a method for
operating such an electrolytic cell and to an electrolyzer comprising a
multiplicity of
such electrolytic cells.
Classically, electrolyzers have an anolyte circuit and catholyte circuit, with
each
half-cell having an electrolyte inlet and an electrolyte outlet. Such
configurations ¨
which are already known from US 4,285,795 for example ¨ are associated with
considerable complexity in terms of providing pipelines, reservoirs, pumps and
instruments.
Moreover, undesired stray currents flow via the electrolyte between
electrolytic cells
which are electrically connected in series and which are connected to one
another
via mutual electrolyte inlets and outlets. Not only are undesired secondary
reactions
caused by such stray currents, but the stray currents are also associated
especially
with a reduction in the efficiency of the electrolysis. Furthermore, it is
known that
stray currents contribute to undesired corrosion and thus to a reduction in
the
service life of the electrolytic cells.
A reduction in stray currents can be brought about by reducing the cross-
sections
of the inlets and outlets for the electrolytes, though this approach can only
be
pursued to a limited extent because of the need to achieve a minimum
electrolyte
volumetric flow rate. Alternatively, it is known to reduce the stray currents
by
increasing the length of the inlets and outlets for the electrolytes. Although
this is
associated with the advantage of increasing the electrical resistance and thus
1
CA 03178248 2022- 11- 8
reducing the stray currents, it is also associated with increasing the space
requirements and the costs.
US 2018/0274110 Al describes a water electrolysis cell having a polymer
electrolyte membrane (PE M). The membrane is composed of a hydrocarbon-based
electrolyte membrane laminated with a composite membrane comprising a
perfluorosulfonic acid-based electrolyte and a superstrong acid metal oxide.
The
membrane forms a solid electrolyte having improved proton conductivity with,
at the
same time, low gas permeability and high mechanical strength.
US 2012/0234676 Al discloses a chlor-alkali electrolysis cell having a
permeable
diaphragm as a separator between the chambers. Brine is supplied to the anode
chamber, and it flows through the pores of the diaphragm and fills the cathode
chamber. In the steady state, the brine level in the anode chamber is higher
than
that in the cathode chamber. Since more brine is supplied than required for
chlorine
production, a portion thereof flows through the diaphragm into the cathode
chamber
and is discharged therefrom mixed with caustic soda.
Proceeding from the prior art described above, it is therefore an object of
the
invention to propose an electrolytic cell which makes it possible to reduce
the space
requirements and the production costs and, at the same time, to reduce the
stray
currents and thus the operating costs, and allows serial connection of cells.
This object is achieved according to the invention by an electrolytic cell of
the type
in question mentioned at the start, wherein the electrolytic cell is suitable
for carrying
out water electrolysis and at least one inlet for electrolyte is provided in a
first ha If-
cell of the two half-cells and at least one outlet for electrolyte and no
inlet for
electrolyte are provided in the second half-cell, so that electrolyte supplied
via the
at least one inlet is dischargeable via the at least one outlet after passing
through
the separator.
2
CA 03178248 2022- 11- 8
Furthermore, the object is operationally achieved according to the invention
by a
method of the type in question mentioned at the start, wherein the
electrolytic cell
is an electrolytic cell according to the invention, which method comprises the
following steps:
- connecting the at least one electrolyte inlet and the at least one
electrolyte outlet to an electrolyte circuit which is closed via the
permeable separator, and filling the two half-cells with electrolyte,
- starting an electrolysis process by closing an electrical circuit via the
cathode and anode of the electrolytic cell and an external power source,
- discharging, during the electrolysis process, product gas formed in the
half-cells,
- applying to the first half-cells, during the electrolysis process, a
positive
pressure compared to the second half-cell in order to promote the
passage of the electrolyte through the separator.
Such an electrolytic cell comprises a cathode half-cell having a cathode and
an
anode half-cell having an anode. Both half-cells are separated from one
another by
a separator which is permeable to an electrolyte present in the half-cells
during
operation and is intended for separation of the gases formed during
electrolysis,
which gases could lead to an undesired oxyhydrogen explosion during water
electrolysis. Said electrolytic cell is distinguished by the fact that at
least one inlet
for electrolyte ¨ an inlet in the context of the invention is to be understood
to mean
a supply of unconsumed, i.e., processed, electrolyte ¨ is provided in a first
half-cell
of the two half-cells and at least one outlet for (consumed) electrolyte and
no inlet
for electrolyte are provided in the second half-cell. Electrolyte supplied via
the at
least one inlet is thus dischargeable via the at least one outlet after
passing through
the separator. Advantageously, not only does this avoid considerable
complexity
with respect to the peripherals, such as pipelines, reservoirs, pumps and
instruments, but it has also been found that, surprisingly, this measure can
significantly reduce stray currents. This not only results in higher
efficiency and thus
economic viability of the particular electrolytic cell, but also increases the
service
3
CA 03178248 2022- 11- 8
life thereof, since undesired corrosion processes and secondary reactions can
be
significantly reduced.
The inlet for electrolyte is provided in the cathode half-cell and the outlet
for
electrolyte is provided in the anode half-cell. In water electrolysis, such a
configuration has the advantage that the product purity of the hydrogen formed
in
the cathode half-cell is improved. Owing to the flow of the electrolyte
through the
separator, product gases dissolved in the electrolyte are entrained to a
certain
extent. If the electrolyte flow is directed from the cathode half-cell to the
anode half-
cell, oxygen dissolved in the electrolyte reaches the cathode half-cell
through the
separator only to a limited extent. Before re-entry into the cathode half-
cell, the
product gases dissolved in the electrolyte can be removed as part of
electrolyte
processing.
In preferred embodiments, the separator is hydrophilic. The hydrophilicity of
the
separator increases the capillary forces, which lead to complete wetting of
the
separator with electrolyte. Owing to the wetting of the separator with
electrolyte, the
pores of the separator are also closed in the gas space present above the
electrolyte level in the cell, and so no product gases can pass through the
separator.
At the same time, the hydrophilicity of the separator ensures an increase in
the
opening pressure of the pores (i.e., the gas pressure at which the pores
reopen)
and an improved permeability of the separator to electrolyte.
Preferably, the separator has a permeability in the range from 17 to 175
liters of
electrolyte per hour per square meter of active separator surface at a
pressure
difference between the two half-cells of up to 500 mbar. What can be achieved
with
a flow rate in this range is that an adequate supply of electrolyte to the
cell is
ensured and, at the same time, the heat of reaction produced can also be
dissipated
from the cell via the electrolyte.
In a preferred development of the electrolytic cell according to the
invention, a gas
separator for separating a product gas from the electrolyte is arranged in the
first
4
CA 03178248 2022- 11- 8
and/or in the second half-cell. Said gas separator is connected to an
electrolyte
return, by means of which electrolyte which has entered the gas separator is
returnable to the respective half-cell. Electrolyte enters the gas separator
primarily
through the product gases which are formed during electrolysis and which
entrain
electrolyte as a flow of droplets, which electrolyte can be recovered through
a return
from the gas separator in which the electrolyte is separated from the product
gas
generated.
According to a particularly preferred embodiment of the invention, said return
runs
inside the half-cell or outside the half-cell. Whereas, in the case of a
return inside
the half-cell, the electrolyte recovered in the gas separator is conducted
back to the
electrolyte reservoir through a line inside the half-cell, a return outside
the half-cell
is distinguished by the fact that the electrolyte first exits from the half-
cell or the gas
separator through a pipeline and is then either coupled to the already
existing
electrolyte inlet or forms a second electrolyte inlet for the first half-cell.
A return
inside the half-cell is particularly preferred with regard to reducing space
requirements, whereas the return outside the half-cell has the advantage of
less
influence on the processes within the half-cell, for example the rise of
product gas.
The electrolytic cell according to the invention is suitable for carrying out
alkaline
water electrolysis. In such a configuration, a particular reduction in stray
currents
was observed, which is particularly advantageous in view of the increasing
importance of water electrolysis.
In practical terms, the electrolytic cells according to the invention are
combined in
an electrolyzer to form a multiplicity of electrolytic cells which are
electrically
connected in series and hydraulically connected in parallel in an electrolyte
circuit,
so that an economically relevant quantity of product gases can be generated.
The electrolyzer preferably comprises only one electrolyte circuit for
supplying
electrolyte to the cathode half-cells and the anode half-cells. Electrolyte is
supplied
5
CA 03178248 2022- 11- 8
to the anode half-cells through the separator. The electrolyte circuit is thus
closed
through the separator.
The electrolyzer preferably further comprises means for generating a positive
pressure, by means of which a positive pressure is appliable to the cathode
half-
cells in relation to the anode half-cells. A positive pressure in the cathode
half-cell
can additionally reduce passage of product gases formed on the anode side
through the separator and promote the passage of electrolyte in the opposite
direction.
In addition, the invention relates to a method for operating an electrolytic
cell
according to the invention, comprising the following steps:
- connecting the at least one electrolyte inlet and the at least one
electrolyte outlet to an electrolyte circuit which is closed via the
permeable separator, and filling the two half-cells with electrolyte,
- starting an electrolysis process by closing an electrical circuit via the
cathode and anode of the electrolytic cell and an external power source,
- discharging, during the electrolysis process, product gas formed in the
half-cells,
- applying to the first half-cells, during the electrolysis process, a
positive
pressure compared to the second half-cell in order to promote the
passage of the electrolyte through the separator.
It has been found to be particularly advantageous if, in one of the half-
cells,
preferably the cathode half-cell in the case of water electrolysis, a positive
pressure
prevails relative to the other half-cell. This promotes the passage of the
electrolyte
through the separator, and so the electrolysis can be carried out at a higher
throughput.
In a particularly preferred development of the method according to the
invention,
the electrolyte which has entered the gas separator(s) is returned to the
respective
6
CA 03178248 2022- 11- 8
half-cell, thereby making it possible to reduce electrolyte consumption in a
not
inconsiderable manner.
Advantageous developments will become apparent from the dependent claims, the
following description and the figures.
The invention is described below on the basis of exemplary embodiments with
reference to the accompanying drawings. In the figures:
Fig. 1: shows a schematic representation of an already known electrolytic cell
having an electrolyte inlet and outlet for each half-cell and a permeable
separator,
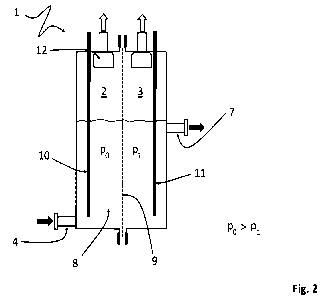
Fig 2: shows a schematic representation of an electrolytic cell according to
the
invention having an electrolyte inlet and outlet for each electrolytic cell
and a
separator permeable to the electrolyte,
Fig. 3: shows a schematic representation of an electrolytic cell according to
the
invention with an internal return of the electrolyte recovered in the gas
separator,
and
Fig. 4: shows a schematic representation of an electrolytic cell according to
the
invention with an external return of the electrolyte recovered in the gas
separator,
Fig. 5: shows a schematic representation of an electrolyzer according to the
invention having a multiplicity of electrolytic cells according to the
invention that are
hydraulically connected in parallel in an electrolyte circuit.
In the various figures, identical parts are always provided with the same
reference
signs and are therefore also generally each named or mentioned only once.
Fig. 1 shows a classic electrolytic cell which has an anolyte circuit and a
catholyte
circuit, i.e., comprises an inlet and outlet for each half-cell. The permeable
separator
7
CA 03178248 2022- 11- 8
separates the two half-cells from one another, said separator being permeable
to
ions in order to close the circuit. During electrolysis, product gases rise
and entrain
droplets of the electrolyte, the respective electrolytes being separated from
the
respective product gases in the gas separators.
An electrolytic cell 1 shown in Fig. 1 comprises two electrolyte circuits ¨
one for
each half-cell 2, 3 ¨ with a total of four pipelines for inlets 4, 5 and
outlets 6, 7 for
processed or consumed electrolyte 8. This results in a high degree of
technical
complexity, especially the installation of pipelines. Not only does this drive
up the
production costs and the complexity of the system, but the multiplicity of
pipelines
also causes a high degree of stray currents, which lower the efficiency of the
electrolytic cell and which moreover lead to undesired corrosion.
A preferred embodiment of the electrolytic cell 1 according to the invention
that is
depicted in Fig. 2 comprises a separator 9 permeable to electrolyte 8, and
also, in
the first of the two half-cells 2 besides an electrode 10, an inlet for
electrolyte 4 and,
in the second half-cell 3 besides an electrode 11, at least one outlet 7 for
electrolyte
8 and no inlet for electrolyte 8. This means that electrolyte 8 supplied via
the one
inlet 4 is dischargeable via the at least one outlet 7 after passing through
the
separator 9. With regard to the already known electrolytic cell 1, this means
that the
function of the outlet 6 of one of the half-cells 2 and the function of the
inlet 5 of the
other half-cell 3 have been taken over by the separator 9 permeable to
electrolyte
8. This not only significantly lowers the number of electrolyte circuits or
necessary
pipelines, but also achieves a reduction in stray currents. This in turn
advantageously leads to a reduction in secondary reactions and corrosion.
In the case of the electrolytic cell 1 according to the invention that is
depicted in Fig.
2, a positive pressure compared to the second half-cell is applied to the
first half-
cell 2 during the electrolysis process in order to promote the passage of the
electrolyte 8 through the separator 9. This significantly raises the
throughput of the
electrolytic cell 1 relative to a flow of electrolyte 8 through the separator
9 that is not
driven by the pressure conditions in the half-cells 2, 3. Moreover, the
separator 9
8
CA 03178248 2022- 11- 8
permeable to electrolyte 8 guarantees separation of the different product
gases,
which, for example, is necessary in water electrolysis to avoid an oxyhydrogen
reaction.
The first half-cell 2 forms the cathode half-cell and the second half-cell 3
forms the
anode half-cell, and so the inlet 4 for electrolyte 8 is provided in the
cathode ha If-
cell 2 and the outlet 7 for electrolyte 8 is provided in the anode half-cell
3.
The separator 9 is preferably hydrophilic. The separator can, for example, be
made
of zirconium oxide. The separator 9 preferably has a permeability in the range
from
17 to 175 liters of electrolyte 8 per hour per square meter of active
separator surface
at a pressure difference between the two half-cells 2, 3 of up to 500 mbar.
According to one development of the electrolytic cell 1 according to the
invention
that is depicted in Fig. 3, product gas and electrolyte 7 are separated in a
gas
separator 12 of the first half-cell 2, the recovered electrolyte 8 being
returned to the
electrolyte reservoir through an internal return line 13. In the context of
the
electrolytic cell according to the invention, the gas separator(s) can
alternatively
also be realized in a functional unit with the electrolyte outlet. Owing to
the return
of the electrolyte 8, electrolyte consumption can be significantly reduced,
thereby
also making it possible to reduce the operating costs. The advantage of the
internal
return lies in a compact, space-saving design.
Fig. 4 shows one development of the electrolytic cell 1 according to the
invention
that is an alternative to Fig. 3, in which the return of the electrolyte 8
obtained in the
gas separator 12 is realized as an external return with an external return
line 14 and
a second inlet 15. Such a return configuration does not impair the rise of
product
gas. Likewise, the return line 14 can be directly connected to the inlet 4,
thereby
limiting the number of necessary inlets to one.
Fig. 5 shows an electrolyzer having a multiplicity of electrolytic cells 1
according to
the exemplary embodiment in Fig. 2 that are hydraulically connected in
parallel in
9
CA 03178248 2022- 11- 8
an electrolyte circuit. The electrolytic cells 1 are electrically connected in
series (not
depicted).
The electrolyzer 100 depicted in Fig. 5 comprises only one electrolyte circuit
for
supplying electrolyte 8 to the cathode half-cells 2 and the anode half-cells
3.
Electrolyte 8 is supplied to the anode half-cells 3 as a result of the
electrolyte 8
passing through the separator 9. Particularly preferably, the electrolyte
circuit is
designed in such a way that the entire circulating electrolyte 8 is conducted
through
the separators 9 of the electrolyzer 100.
Starting from an electrolyte processing device 110, the electrolyte 8 is
hydraulically
supplied in parallel to the respective cathode half-cells 2 via an inflow
distributor
120. Inside the electrolytic cells 1, the electrolyte 8 passes through the
separator 9
to supply the anode half-cells 3 with electrolyte 8. The electrolyte 8 leaves
the anode
half-cells 3 via the outlet 7 and is returned to the electrolyte processing
device 110
via a return collector 130.
The electrolyzer 100 further comprises means for generating a positive
pressure
150, by means of which a positive pressure is appliable to the cathode half-
cells 2
in relation to the anode half-cells 3. In the example shown in Fig. 5, the
means for
generating a positive pressure 150 are formed by adjustable pressure control
valves in the gas discharge lines 140 for the product gases. As a result, it
is possible
for the pressures p0 and pl in the cathode half-cells 2 and the anode half-
cells 3,
respectively, to be adjusted separately from one another in order to achieve a
desired pressure drop. In principle, it is also possible to provide a pressure
control
valve only in the gas discharge line of the cathode half-cells 2 and to
operate the
anode half-cells 3 at ambient pressure. A pressure difference of less than 500
mbar
is preferably set between the cathode half-cells 2 and the anode half-cells 3,
particularly preferably less than 100 mbar.
10
CA 03178248 2022- 11- 8
The electrolytic cells 1 shown in Fig. 3 or 4 can also be connected together
to form
an electrolyzer in an analogous manner to Fig. 5. Therefore, all discussions
relating
to Figs. 2 to 4 apply accordingly to the electrolytic cells 1 of the
electrolyzer 100.
List of reference signs
1 Electrolytic cell
2 First half-cell
3 Second half-cell
4 Inlet for electrolyte
5 Inlet for electrolyte
6 Outlet for electrolyte
7 Outlet for electrolyte
8 Electrolyte
9 Separator
10 Electrode
11 Electrode
12 Gas separator
13 Internal return line for electrolyte
14 External return line for electrolyte
15 Inlet for electrolyte
100 Electrolyzer
110 Electrolyte processing device
120 Inflow distributor
130 Return collector
140 Gas discharge
150 Means for generating a positive pressure
11
CA 03178248 2022- 11- 8