Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03179861 2022-10-07
WO 2021/207698
PCT/US2021/026714
TITLE
DEVICES FOR BLEEDING REDUCTION AND METHODS OF MAKING AND USING
THE SAME
CROSS-REFERENCE TO RELATED APPLICATION
100011 This application claims the benefit of priority to U.S. Provisional
Application No.
63/007,543, filed on April 9, 2020, which is herein incorporated by reference
in its entirety
for all purposes.
FIELD OF INVENTION
100021 The present invention relates generally to devices having topical
applicators, and
more specifically to devices having topical applicators for reducing bleeding
of a wound
and/or achieving hemostasis at a wound.
BACKGROUND OF THE INVENTION
100031 Health professionals continue to encourage patients with minor injuries
to seek
medical attention at primary care clinics, urgent care centers, and hospital
emergency rooms.
Many urgent care centers are not open twenty-four hours per day and seven days
per week
(24/7). Additionally, rural areas may have limited availability of these
urgent care centers. A
common type of visit to a hospital emergency room or urgent care center is a
wound
requiring cessation of bleeding, especially patients on a variety of
anticoagulant or
antiplatelet medications. As the population ages, more patients require these
medications for
a variety of conditions,
100041 Better methods and devices are needed to allow patients, family
members, and/or
caretakers to safely treat at home, or at the place of the injury, minor
wounds requiring the
cessation of bleeding.
SUMMARY OF INVENTION
100051 Some of the main aspects of the present invention are summarized below.
Additional
aspects are described in the Detailed Description of the invention, Drawings,
and Claims
CA 03179861 2022-10-07
WO 2021/207698
PCT/US2021/026714
sections of this disclosure. The description in each section of this
disclosure is intended to be
read in conjunction with the other sections. Furthermore, the various
embodiments described
in each section of this disclosure can be combined in various different ways,
and all such
combinations are intended to fall within the scope of the present invention.
100061 The invention provides a device and methods for treating wounds and
reducing
bleeding of a wound.
100071 In one aspect, the invention provides devices for delivering a
medication to a wound.
In embodiments of the invention, the devices comprise: (a) a reservoir
comprising a closed
end, an open end, walls between the closed end and open end that have an outer
surface and
an inner surface, and a longitudinal axis extending from the closed end to the
open end; (b) an
ampule contained within the reservoir, wherein the ampule comprises the
medication and is
comprised of a material that can be punctured or broken; (c) a release
mechanism comprised
in the reservoir, the release mechanism being configured to apply a mechanical
force to the
ampule upon activation of the release mechanism, wherein application of the
mechanical
force to the ampule directly or indirectly causes the ampule to break or
puncture, and the
medication to be released; and (d) a topical applicator attached to the open
end of the
reservoir, wherein the topical applicator comprises a material capable of
retaining the
medication released from the ampule.
[0008] In some embodiments, the ampule comprises a body portion and a neck
portion, in
which the neck portion is thinner than the body portion. In certain
embodiments, the ampule
is oriented in the reservoir such that the neck portion is positioned closer
to the open end of
the reservoir and the body portion is positioned closer to the closed end of
the reservoir. In
preferred embodiments, the ampule comprises a glass ampule.
[0009j In some embodiments, the inner surface of the wall of the reservoir
comprises an
abutment located adjacent to the ampule. In some embodiments, the abutment is
located
adjacent to the neck portion of the ampule. In certain embodiments, the
abutment comprises
an incline plane. In further embodiments, the release mechanism is positioned
at the closed
end of the reservoir, and the application of the mechanical force to the
ampule upon
activation of the release mechanism causes the ampule, preferably the neck
portion of the
ampule, to contact the abutment and results in the ampule breaking, preferably
breaking at the
neck portion.
2
CA 03179861 2022-10-07
WO 2021/207698
PCT/US2021/026714
10010j In some embodiments, the release mechanism is positioned at the wall of
the
reservoir, and the application of the mechanical force to the ampule upon
activation of the
release mechanism causes the ampule to break, preferably break at the neck
portion.
100111 In embodiments of the invention, the devices comprise: (a) a reservoir
comprising a
closed end, an open end, walls between the closed end and open end that have
an outer
surface and an inner surface, and a longitudinal axis extending from the
closed end to the
open end; in which the reservoir is configured to contain an ampule comprising
a medication;
(b) a release mechanism comprised in the reservoir, the release mechanism
being configured
to apply a mechanical force to the ampule upon activation of the release
mechanism, wherein
application of the mechanical force to the ampule directly or indirectly
causes the ampule to
break or puncture, and the medication to be released; and (c) a topical
applicator attached to
the open end of the reservoir, wherein the topical applicator comprises a
material capable of
retaining the medication released from the ampule.
100121 In some embodiments, the reservoir is configured to contain an ampule
that comprises
a body portion and a neck portion, in which the neck portion is thinner than
the body portion.
In certain embodiments, the reservoir is configured to contain an ampule that
is oriented in
the reservoir such that the neck portion is positioned closer to the open end
of the reservoir
and the body portion is positioned closer to the closed end of the reservoir.
In preferred
embodiments, the reservoir is configured to contain an ampule comprising a
glass ampule.
100131 In some embodiments, the inner surface of the wall of the reservoir
comprises an
abutment that is configured to be located adjacent to the ampule when the
ampule is
contained in the reservoir. In some embodiments, the abutment is configured to
be located
adjacent to the neck portion of the ampule. In certain embodiments, the
abutment comprises
an incline plane. In further embodiments, the release mechanism is positioned
at the closed
end of the reservoir and, when an ampule is installed and contained in the
reservoir, the
application of the mechanical force to the ampule upon activation of the
release mechanism
causes the ampule, preferably the neck portion of the ampule, to contact the
abutment and
results in the ampule breaking, preferably breaking at the neck portion.
100141 In some embodiments, the release mechanism is positioned at the wall of
the reservoir
and, when an ampule is installed and contained in the reservoir, the
application of the
mechanical force to the ampule upon activation of the release mechanism causes
the ampule
to break, preferably break at the neck portion.
3
CA 03179861 2022-10-07
WO 2021/207698
PCT/US2021/026714
100151 In embodiments of the invention, the release mechanism is activated by
applying a
force to the release mechanism, such as the force that can be generated by a
thumb or finger
of one hand.
100161 In some embodiments, one or more portions of the outer surface of the
reservoir of the
devices comprise a surface feature that aids in gripping or holding the
device. In certain
embodiments, the surface feature comprises one or more ridges. In certain
embodiments, the
surface feature comprises a textured surface.
100171 In some embodiments, the reservoir comprises one or more tabs on the
inner surface
of the walls of the reservoir that maintain, or are configured to maintain,
the position of the
ampule within the reservoir.
100181 In some embodiments, the medication comprises an antifibrinolytic. In
certain
embodiments, the antifibrinolytic is selected from tranexamic acid,
aminocaproic acid, and
the medication protamine sulfate. In preferred embodiments, the
antifibrinolytic is
tranexamic acid.
100191 In some embodiments, the topical applicator is attached to the
reservoir via a fixation
plate. In certain embodiments, the fixation plate is attached to the reservoir
by an adhesive or
welding.
100201 In some embodiments, the topical applicator comprises a foam or sponge.
In
preferred embodiments, the topical applicator comprises a foam.
100211 In some embodiments, the topical applicator is removably attached to
the reservoir.
100221 In some embodiments, the topical applicator comprises a medication pad
and a blood
absorption pad, and a divider therebetween. In certain embodiments, the
topical applicator is
configured such that only the medication pad is positioned to receive the
medication from the
ampule.
100231 in some embodiments, a filter is positioned above the topical
applicator.
100241 In some embodiments, the devices further comprise an applicator cap
that is
configured to fit over the topical applicator.
100251 In another aspect, the invention provides methods involving the devices
of the
invention. In embodiments of the invention, the method is of treating a wound
in a subject in
need thereof, reducing bleeding of a wound in a subject in need thereof,
controlling bleeding
4
CA 03179861 2022-10-07
WO 2021/207698
PCT/US2021/026714
of a wound in a subject in need thereof, treating a wound having excess
bleeding of a wound
in a subject in need thereof, delivering a medication to a wound in a subject
in need thereof,
treating post-surgical or post-procedural external bleeding in a subject in
need thereof, or
treating a post-surgical or post-procedural wound in a subject in need
thereof.
100261 In some embodiments, the method comprises obtaining the device of the
invention
comprising a reservoir, ampule, release mechanism, and topical applicator;
activating the
release mechanism of the device to release the medication in the ampule of the
device and
expose the topical applicator to the medication; and applying the topical
applicator of the
device to the wound.
100271 In some embodiments, the method comprises obtaining the device of the
invention
comprising a reservoir, ampule, release mechanism, and topical applicator;
applying the
topical applicator of the device to the wound; and activating the release
mechanism of the
device to release the medication in the ampule of the device and expose the
topical applicator
to the medication.
100281 In some embodiments, the method comprises obtaining the device of the
invention
comprising a reservoir, release mechanism, and topical applicator, and
obtaining an ampule;
installing the ampule into the device; activating the release mechanism of the
device to
release the medication in the ampule of the device and expose the topical
applicator to the
medication; and applying the topical applicator of the device to the wound.
100291 In some embodiments, the method comprises obtaining the device of the
invention
comprising a reservoir, release mechanism, and topical applicator, and
obtaining an ampule;
installing the ampule into the device; applying the topical applicator of the
device to the
wound; and activating the release mechanism of the device to release the
medication in the
ampule of the device and expose the topical applicator to the medication.
10030j In some embodiments, the method further comprises removing the topical
applicator
from the device and fixating the topical applicator to the wound. In certain
embodiments, the
topical applicator is fixated to the wound using a bandage, patch, film, or
surgical dressing.
100311 In some embodiments, the method is performed by the subject in need of
the
treatment, the reduction in bleeding, the controlling of bleeding, or the
delivery of the
medication. In certain embodiments, the subject was taking, was prescribed, is
taking, or is
CA 03179861 2022-10-07
WO 2021/207698
PCT/US2021/026714
prescribed, an anticoagulant therapy. In certain embodiments, the subject was
administered,
is administered, or is to be administered, one or more antiplatelet therapies.
[0032] In yet another aspect, the invention provides a kit. In embodiments of
the invention,
the kit comprises (a) a device of the invention comprising a reservoir,
ampule, release
mechanism, and topical applicator; and (b) instructions on how to use the
device according to
the methods of the invention.
100331 In other embodiments, the kit comprises (a) a device of the invention
comprising a
reservoir, release mechanism, and topical applicator; (b) an ampule of the
invention; and
(c) instructions on how to use the device and ampule according to the methods
of the
invention.
[0034] In some embodiments, the kit further comprises a container for disposal
of the device.
BRIEF DESCRIPTION OF THE DRAWING FIGURES
100351 The present disclosure will be further explained with reference to the
attached
drawing figures, wherein like structures are referred to by like numerals
throughout the
several views. The drawing figures shown are not necessarily to scale, with
emphasis instead
generally being placed upon illustrating the principles of the present
disclosure, and some
features may be exaggerated to show details of particular components. In
addition, any
measurements, specifications, and the like shown in the drawing figures, or
described below,
are intended to be illustrative, and not restrictive. Therefore, specific
structural and
functional details disclosed herein are not to be interpreted as limiting, but
merely as a
representative basis for teaching one skilled in the art to variously employ
the devices of the
present invention and methods of their use.
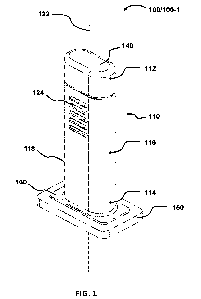
100361 FIG. I is a perspective view of a device according to embodiments of
the present
invention, in which the release mechanism is at the closed end of the
reservoir and is
activated by applying a mechanical force to the release mechanism.
[0037] FIG. 2 is a cut-away view of a device according to embodiments of the
present
invention, in which the device comprises a reservoir, ampule, release
mechanism, and topical
applicator; and in which the release mechanism is at the closed end of the
reservoir.
6
CA 03179861 2022-10-07
WO 2021/207698
PCT/US2021/026714
100381 FIG. 3 is a cut-away view of a device according to embodiments of the
present
invention, in which the device comprises a reservoir, ampule, release
mechanism, and topical
applicator; and in which the release mechanism is at the closed end of the
reservoir and
comprises at outer portion and an inner portion.
100391 FIG. 4 is a perspective view of a device according to embodiments of
the present
invention, in which the release mechanism is at the wall of the reservoir.
100401 FIG. 5 is a cut-away view of a device according to embodiments of the
present
invention, in which the device comprises a reservoir, ampule, release
mechanism, and topical
applicator; and in which the release mechanism is at the wall of the
reservoir.
100411 FIG. 6 is a perspective view of a device according to embodiments of
the present
invention, in which the release mechanism is at the closed end of the
reservoir and is
activated by rotating the release mechanism.
100421 FIG. 7 is a cut-away view of a device according to embodiments of the
present
invention, in which the device comprises a reservoir, ampule, release
mechanism, and topical
applicator; and in which the release mechanism is at the closed end of the
reservoir and is
activated by rotating the release mechanism.
100431 FIG. 8 is a cut-away view of a device according to embodiments of the
present
invention, in which the device comprises a reservoir, ampule, release
mechanism, and topical
applicator; and in which the release mechanism is at the closed end of the
reservoir and is
activated by rotating the release mechanism, which activates a spring.
100441 FIG. 9 is a perspective view of a topical applicator according to
embodiments of the
present invention, in which the topical applicator comprises a medication pad
and a blood
absorption pad.
100451 FIG. 10 is a cut-away view of a device according to embodiments of the
present
invention, in which the device comprises a reservoir, release mechanism, and
topical
applicator; and in w hich the release mechanism is at the closed end of the
reservoir.
100461 FIG. 11 is a cut-away view of a device according to embodiments of the
present
invention, in which the device comprises a reservoir, release mechanism, and
topical
applicator; and in which the release mechanism is at the wall of the
reservoir.
7
CA 03179861 2022-10-07
WO 2021/207698
PCT/US2021/026714
100471 FIG. 12 is a cut-away view of a device according to embodiments of the
present
invention, in which the device comprises a reservoir, release mechanism, and
topical
applicator; and in which the release mechanism is at the closed end of the
reservoir and is
activated by rotating the release mechanism.
100481 FIG. 13 is a cut-away view of a device according to embodiments of the
present
invention, in which the device comprises a reservoir, release mechanism, and
topical
applicator; and in which the release mechanism is at the closed end of the
reservoir and is
activated by rotating the release mechanism, which activates a spring.
DETAILED DESCRIPTION OF THE INVENTION
100491 The present invention relates to devices and their use to treat and/or
reduce bleeding
of a wound.
KAM The devices are used to deliver medication to the wound in order to treat
or reduce
bleeding of the wound. The devices are advantageously easy to use, and can be
operated by
persons with or without medical training, including at home or any other non-
hospital/medical facility' setting.
Definitions
10051j The phraseology or terminology in this disclosure is for the purpose of
description
and not of limitation, such that the terminology or phraseology of the present
specification is
to be interpreted by the skilled artisan in light of the teachings and
guidance.
100521 As used in this specification and the appended claims, the singular
forms "a," "an,"
and "the" include plural referents, unless the context clearly dictates
otherwise. The terms
"a" (or "an") as well as the terms "one or more" and "at least one" can be
used
interchangeably.
100531 Furthermore, "and/or" is to be taken as specific disclosure of each of
the two specified
features or components with or without the other. Thus, the term "and/or" as
used in a phrase
such as "A and/or B" is intended to include A and B, A or B, A (alone), and B
(alone).
Likewise, the term "and/or" as used in a phrase such as "A, B, and/or C" is
intended to
8
CA 03179861 2022-10-07
WO 2021/207698
PCT/US2021/026714
include A, B, and C; A, B, or C; A or B; A or C; B or C; A and B; A and C; B
and C; A
(alone); B (alone); and C (alone).
100541 Wherever embodiments are described with the language "comprising,"
otherwise
analogous embodiments described in terms of "consisting of' and/or "consisting
essentially
of' are included.
100551 Units, prefixes, and symbols are denoted in their Systeme International
de Unites (SI)
accepted form. Numeric ranges are inclusive of the numbers defining the range,
and any
individual value provided herein can serve as an endpoint for a range that
includes other
individual values provided herein. For example, a set of values such as 1, 2,
3, 8, 9, and 10 is
also a disclosure of a range of numbers from 1-10, from 1-8, from 3-9, and so
forth.
Likewise, a disclosed range is a disclosure of each individual value
encompassed by the
range. For example, a stated range of 5-10 is also a disclosure of 5, 6, 7, 8,
9, and 10.
100561 A "subject" or "individual" or "patient" is any subject, particularly a
mammalian
subject, for whom diagnosis, prognosis, or therapy is desired. Mammalian
subjects include
humans, domestic animals, farm animals, sports animals, and laboratory animals
including,
e.g., humans, non-human primates, canines, felines, porcines, bovines,
equines, rodents,
including rats and mice; rabbits, etc.
[00571 As used herein, the term "bleeding" refers to conditions where blood
flows through a
break in the skin or mucosa of a subject. Non-limiting examples of bleeding
include cuts and
abrasions from trauma including from surgery.
100581 As used herein, the term "minor cuts and abrasions" is intended to
include any and all
cuts and abrasions that do not require hospitalization. Cuts and abrasions
that do not require
hospitalization my include those that do not require stitches, liquid
stitches, or butterfly
stitches to close and heal.
[00591 As used herein, the terms "treating" and "treatment" refer to reduction
in severity
and/or frequency of symptoms, elimination of symptoms and/or underlying cause,
prevention
of the occurrence of symptoms and/or their underlying cause, and improvement
or
remediation of damage.
100601 As used herein; the term "reduce" is used to refer to any decrease in
occurrence or
activity, including full blocking of the occurrence or activity.
9
CA 03179861 2022-10-07
WO 2021/207698
PCT/US2021/026714
Devices
[00611 An aspect of the invention relates to devices that can deliver to a
wound a medication
that treats wounds or reduces wound bleeding. Embodiments of the devices of
the present
invention are shown in FIGS. 1-13.
100621 Devices 100 may comprise a reservoir 110, an ampule 130, a release
mechanism 140,
and a topical applicator 150.
100631 The reservoir 110 comprises a closed end 112, an open end 114, walls
116 between
the closed end 112 and open end 114 wherein the walls 116 have an outer
surface 118 and an
inner surface 120, and a longitudinal axis 122 extending from the closed end
112 to the open
end 114. One or more portions of the outer surface 118 of the wall 116 of the
reservoir 110
may comprise surface features 124 that aid in holding or gripping the device
100. Surface
features 124 may include, but are not limited to, one or more ridges (as shown
in, for
example, FIGS. 1, 2, 4, and 5), concave and/or convex curvatures (not shown),
and a textured
or course surface (not shown).
100641 The reservoir 110 may comprise a shape that is ergonomic and easy to
hold within
one hand. Examples of such shape include, but are not limited to, cylinder,
including a
circular cylinder, oval cylinder, or elliptical cylinder; and a prism,
including a cube, rounded
square prism, rectangular prism, rounded rectangular prism, triangular prism,
pentagonal
prism, hexagonal prism, etc. In some embodiments, the reservoir comprises one
or more
rounded or beveled edges. In certain embodiments, the reservoir comprises a
rounded
rectangular prism shape with one or more rounded edges, as shown in FIGS. 1
and 4. In
certain embodiments, the reservoir comprises a cylindrical shape, as shown in
FIG. 6.
[0065] The reservoir 110 may be comprised of a rigid polymer material.
Examples of rigid
polymer materials include, but are not limited to, polyethylene,
polypropylene, polyamide,
polycarbonate, and the like.
[0066] The reservoir 110 is configured to contain the ampule 130 (see FIGS. 2,
3, 5, 7,8,
and 10-13). In some embodiments, the inner surface 120 of the reservoir 110
may comprise
one or more tabs or other features that maintain the general position of the
ampule 130 within
the reservoir 110.
100671 The ampule 130 contains the medication 132. The ampule 130 is comprised
of a
material that can be broken or punctured in order to release the medication
132. In some
CA 03179861 2022-10-07
WO 2021/207698
PCT/US2021/026714
embodiments, the ampule 130 is comprised of a plastic or glass. In preferred
embodiments,
the ampule is a glass ampule.
[0068] The ampule 130 may comprise a neck portion 134 and a body portion 136,
and a
longitudinal axis 138 extending between the neck portion 134 and the body
portion 136. The
neck portion 134 may be narrower than the body portion 136. In some
embodiments, the
ampule 130 is oriented in the reservoir 110 such that the neck portion 134 is
positioned closer
to the open end 114 of the reservoir 110 and the body portion 136 is
positioned closer to the
closed end 112 of the reservoir 110. (see, e.g, FIGS. 2, 5, 7, and 8).
100691 in preferred embodiments, the ampule comprises a glass ampule.
[0070] The ampule 130 may comprise a metered dose of the medication 132
ranging from
about 1 mL to about 20 mL, or about 1 mL to about 1.5 mL, or about 2.5 mL to
about 5 mL,
or about 5 mL to about 10 ml.õ or about 10 mL to about 15 mL, or about 15 mL
to about
20 nil,. For example, the ampule 130 may comprise a metered dose of the
medication 132 of
about 1 mL, or about 2 mL, or about 3 mL, or about 4 mL, or about 5 mL, or
about 6 mL, or
about 7 mL, or about 8 ml.õ or about 9 mL, or about 10 mL, or about 11 ml.õ or
about 12 mL,
or about 13 mL, or about 14 mL, or about 15 mL, or about 16 mL, or about 17
mL, or about
18 mL, or about 19 mL, or about 20 inL.
[0071] The medication 1.32 may comprise an antifibrinolytic. Examples of
antifibrinolytics
include, but are not limited to, tranexamic acid, aminocaproic, and a
medication equal or
similar to protamine sulfate. In preferred embodiments, the medication 132
comprises
tranexamic acid.
100721 In some embodiments, the medication 132 also comprises a carrier,
wherein the
carrier comprises sterile water and/or a saline solution. In certain
embodiments, the
medication 132 may comprise between about 50% and about 100% tranexamic acid,
and
between about 50% and about 0% of the carrier. In certain embodiments, the
medication 132
may comprise between about 10% and about 50% tranexamic acid, and between
about 90%
and about 50% of the carrier.
100731 In some embodiments, the medication 132 may comprise an
antifibrinolytic in an
amount of about 50 mg/mL to about 300 mg/mL, including about 50 mg/mL, about
100 mg/mLõ about 150 mg/mL, about 200 mg/mL, about 250 mg/mL, or about 300
m2/mL.
11
CA 03179861 2022-10-07
WO 2021/207698
PCT/US2021/026714
10074j In some embodiments, the medication 132 may be comprised of a liquid or
gel. For
example, the medication 132 may have a iscosity of between about 0.75 mPas and
about
0.98 mPas at approximately 25 C.
100751 In some embodiments, the medication 132 may also include a colored dye
to indicate
that the medication 132 has been dispensed to the wound and is of a
composition to be human
viewable based on skin color and blood color (before and after clotting). In
other
embodiments, the medication 132 may not contain a colored dye.
100761 The release mechanism 140 is comprised in the reservoir 110 and is
configured to,
upon activation, apply a mechanical force that directly or indirectly causes
the ampule 130 to
break and the medication 132 to be released. In some embodiments, as shown in
FIGS. 1
and 2, the release mechanism 140 is at the closed end 112 of the reservoir 110
and is
mechanically coupled to the ampule 130. In such embodiments, the reservoir 110
may
further comprise an abutment 126, such as an inclined plane, on the inner
surface 120 of the
reservoir 110, in which the abutment 126 is adjacent to ampule 130, preferably
to the neck
portion 134 of the ampule 130, in the reservoir 110. Activation of the release
mechanism 140
by application of a force on the release mechanism 140 in the direction along
the longitudinal
axis 122 towards the open end 114 of the reservoir 110 in turn applies a force
on the ampule
130 in the direction along its longitudinal axis 138. Such a force can move
the ampule 130
towards the open end of the reservoir 110, causing the ampule 130, preferably
the neck
portion 134 of the ampule 130, to contact against the abutment 126, and the
ampule 130 to
therefore break, preferably to break at the neck portion 134, and release the
medication 132.
100771 In some embodiments, as shown in FIG. 3, the release mechanism 140 is
at the closed
end 112 of the reservoir 110 and comprises an outer portion 140a and an inner
portion 140b.
The release mechanism 140 may comprise a plunger, a lever, or a twisting, or
the like, and is
configured such that activation of outer portion 140a causes the inner portion
140b to apply a
force that directly or indirectly breaks the ampule 130 and release the
medication 132.
100781 In certain embodiments, the release mechanism 140 may be completely
internal
within the reservoir 110, such that squeezing the reservoir 110 causes the
ampule 130 to
compress and break, thereby releasing the medication 132 (not shown).
100791 In some embodiments, as shown in FIGS. 4 and 5, the release mechanism
140 is on
the wall 116 of the reservoir 110. Activation of the release mechanism 140 by
application of
12
CA 03179861 2022-10-07
WO 2021/207698
PCT/US2021/026714
a force on the release mechanism 140 in the direction perpendicular, or
generally
perpendicular, to the longitudinal axis 122 of the reservoir 110 in turn
applies a force on the
ampule 130, preferably on the neck portion 134 of the ampule 130, in the
direction
perpendicular, or generally perpendicular, of its longitudinal axis 138. Such
a force can
break the ampule 130, preferably the neck portion 134 of the ampule 130,
causing the ampule
130 to break, preferably at the neck portion 134, and release the medication
132.
100801 In some embodiments, as shown in FIGS. 6-8, the release mechanism 140
is at the
closed end 112 of the reservoir 110 and is activated by rotating or otherwise
activating the
release mechanism 140. The release mechanism 140 is mechanically coupled to
the ampule
130. In such embodiments, the reservoir 110 may further comprise an abutment
126, such as
an inclined plane, on the inner surface 120 of the reservoir 110, in which the
abutment 126 is
adjacent to the ampule 130, preferably to the neck portion 134 of the ampule
130, in the
reservoir 110. In certain embodiments, turning the release mechanism 140
applies a force on
the ampule 130 in the direction along its longitudinal axis 138, and such a
force moves the
ampule 130 towards the open end of the reservoir 110, causing the ampule 130,
preferably
the neck portion 134 of the ampule 130, to contact against the abutment 126,
and the ampule
130 to therefore break, preferably break at the neck portion 134, and release
the medication
132 (see FIG. 7). Alternatively, rotating or otherwise activating the release
mechanism 140
activates a spring 142, which applies a force on the abutment 126 in the
direction along the
longitudinal axis 138 of the ampule 130 and towards the body portion 136 of
the ampule 130,
and such a force contacts the abutment 126 against the ampule 130, preferably
against the
neck portion 134 of the ampule 130, causing the ampule 130 to break,
preferably break at the
neck portion 134, and release the medication 132. (see FIG. 8).
100811 In some embodiments, the device 100 may comprise a means of preventing
the
releasing mechanism 140 from being inadvertently activated. Such means
include, but are
not limited to, one or more tabs within the device 100 that prevent the
release mechanism 140
from being able to apply a force to the ampule 130 unless a particular level
of force is applied
to the release mechanism 140; or a locking mechanism that requires the release
mechanism
140 to be unlocked before it is activated (not shown).
100821 in some embodiments, the device 100 may comprise a means of preventing
the neck
portion 134 of the ampule 130 from inadvertently contacting the abutment 126.
Such means
include, but are not limited to, one or more tabs 128 within the device 100,
for example on
13
CA 03179861 2022-10-07
WO 2021/207698
PCT/US2021/026714
the inner surface 120 of the reservoir 110 that prevents ampule 130 from
moving to contact
the abutment 126 unless a sufficient force is applied to the release mechanism
140.
100831 The topical applicator 150 is attached to the open end 114 of the
reservoir 110, and
comprises a porous material. In some embodiments, the topical applicator 150
comprises a
sponge or foam pad, preferably a foam pad, capable of retaining the medication
132, to which
the topical applicator 150 is exposed when the ampule 130 inside the reservoir
110 is broken.
In certain embodiments, the foam pad is capable of releasing the retained
medication 132 by
physical contact.
100841 in some embodiments, the topical applicator 150 is attached to the
reservoir via a
fixation plate 160, as shown in FIGS. 1, 4, and 6. The fixation plate 160 is
attached to the
closed end of the reservoir via an adhesive or welding, and creates a seal to
ensure that the
medication 132 is directed to the topical applicator 150. Thus, the fixation
plate 160
comprises an opening (not shown) through which the medication 132 can contact
the topical
applicator 150.
100851 In some embodiments, the topical applicator 150 may comprise a surface
area of
between about 2 cm2 and about 25 cm2. In certain embodiments, the surface area
may be
between about 3 cm2 and about 10 cm2, or between about 10 cm2 and about 20
cm2. For
example, the surface area may be about 2 cm2, or about 3 cm2, or about 4 cm2,
or about
cm2, or about 6 cm2, or about 7 cm2, or about 8 cm2, or about 9 cm2, or about
10 cm2, or
about 11 cm2, or about 12 cm2, or about 13 cm2, or about 14 cm2, or about 15
cm2, or about
16 cm2, or about 17 cm2, or about 18 cm2, or about 19 cm2, or about 20 cm2, or
about 21 cm2,
or about 22 cm2, or about 23 cm2, or about 24 cm2, or about 25 cm2.
100861 The topical applicator 150 may comprise a shape appropriate for
contacting and
covering a wound. Examples of shapes include, but are not limited to, a
circle, oval, ellipse,
square, rounded square, rectangle, rounded rectangle, triangle, pentagon,
hexagon, etc.
100871 In some embodiments, the topical applicator 150 is removable from the
reservoir 110
or the fixation plate 160. For example, the topical applicator 150 may be
attached to the
reservoir 110 or the fixation plate 160 via an adhesive, and may be removed by
peeling or by
otherwise applying a force.
100881 In some embodiments, the topical applicator 150 comprise a medication
pad 150a and
a blood absorption pad 150b. The medication pad 150a and the blood absorption
pad 150b
14
CA 03179861 2022-10-07
WO 2021/207698
PCT/US2021/026714
may comprise the same material or may be different materials. The medication
pad 150a and
the blood absorption pad 1.50b may be configured such that only the medication
pad 150a can
be accessed by the medication. In such embodiments, the medication pad 150a
may be
adjacent, or may be concentric, with the blood absorption pad, as shown in
FIG. 9. A divider
152 may be positioned between the medication pad 150a and the blood absorption
pad 150b.
100891 In some embodiments, the topical applicator 150 may contain a colored
dye to
indicate that the medication 132 has been dispensed to the wound and is of a
composition to
be human viewable based on skin color and blood color (before and after
clotting).
100901 in some embodiments, the device may further comprise a filter (not
shown). The
filter can be positioned within the reservoir, between the ampul and the
topical applicator.
Preferably, the filter is positioned adjacent to the topical applicator. The
filter may be
configured to capture particles or pieces (e.g., glass particles or pieces)
generated from the
breaking of the ampul, and prevent such particles or pieces from entering or
contacting the
topical applicator.
100911 In some embodiments, the device 100 may further comprise an applicator
cap 170
(see, e.g., FIG. 3). The applicator cap 170 comprises the same general shape
as the topical
applicator 150, and is configured to fit over the topical applicator 150. The
applicator cap
170, when positioned on the topical applicator 150, protect the topical
applicator 150 from
the external environment until the device 100 is used as intended.
100921 In some embodiments, the device 100 may further comprise an information
label (not
shown). The information label may be adhered to the outside of the reservoir
110 and/or to
the applicator cap 1.70. The information label may contain user instructions
similar to the
following:
Only apply only to superficial bleeding wounds 3 inches or less in length.
Push the
release mechanism to break the medication. Allow the medication to fully cover
the
topical applicator. Press down with the device over the wound for 10 minutes.
Place
an adhesive bandage over the wound. If bleeding does not stop, you may repeat
up to
1 time as long as bleeding is not pulsatile or vascular in nature. If bleeding
does not
stop after 2 applications or if you feel dizzy, weak, or fatigued, seek
professional
medical treatment. Not to be used in the mouth. Not to be used on
arteriovenous
fistulas or deep wounds. Do not use if you have a history of seizures. Do not
use for
CA 03179861 2022-10-07
WO 2021/207698
PCT/US2021/026714
penetrating wounds or puncture wounds (gunshot; knife, etc.). Do not drink
this
medication. Keep away from children or infants.
In some embodiments, the information label may further comprise one or more
statements
indicating one or more of the following: (i) remove the applicator cap before
pressing down
with the device over the wound; and (ii) peel the topical applicator from the
rest of the device
and use an adhesive bandage to keep the topical applicator on the wound.
100931 In some embodiments, the device 100 may be labeled or stamped with an
expiration
date. For example, the device 100 may have a shelf life from a date of
manufacture of three
years. In other embodiments, the shelf life may be greater or less than three
years. The
device may also be labeled or stamped with a lot number and/or a date of
manufacture.
Additionally, the device 100 may be labeled with a bar code and/or Quick
Response (QR)
code.
100941 In other embodiments, the device 100 may be implemented as a pipette.
The pipette
may be configured in a manner most suitable for the accuracy and precision
needed for the
size and location of the wound. For example, the pipette may be configured to
be positioned
within a patient's nostril.
10095j Alternative embodiments relate to devices 100-1 comprising a reservoir
110 as
described above, a release mechanism 140 as described above, and a topical
applicator 150 as
described above. In such embodiments, the ampule 130 is not in the reservoir
1.1.0, but the
reservoir 110 is configured to receive and contain the ampule 130, which may
be installed
into the reservoir 110 when the device 100-1 is ready for use. In some
embodiments, the
closed end 11.2 of the device 100-1. may be removable and allow the ampule 130
to be
installed within the reservoir 110. Examples of devices 1.00-1 are provided
in. FIGS. 10-13,
which depict the same reservoir, release mechanism, and topical applicator as
shown in
FIGS. 2, 5, 7, and 8, respectively.
Uses of the Device
100961 An aspect of the invention relates to use of the device of the
invention to deliver to a
wound a medication that treats wounds or reduces wound bleeding.
100971 Therefore, the present invention is directed to (i) a method of
treating a wound; (ii) a
method of reducing bleeding of a wound; (iii) a method of controlling bleeding
of a wound;
16
CA 03179861 2022-10-07
WO 2021/207698
PCT/US2021/026714
(iv) a method of treating a wound having excess bleeding of a wound; (v) a
method of
delivering medication to a wound; (vi) a method of treating post-surgical or
post-procedural
external bleeding; or (vii) a method of treating post-surgical or post-
procedural wound. The
invention is also directed to the use of the device of the present invention
to (i) treat a wound;
(ii) reduce bleeding of a wound; (iii) control bleeding of a wound; (iv) treat
a wound having
excess bleeding of a wound; (v) deliver medication to a wound; (vi) treat post-
surgical or
post-procedural external bleeding; or (vii) treat post-surgical or post-
procedural wound.
Further the present invention is directed to a device of the present invention
for use in
(i) treating a wound; (ii) reducing bleeding of a wound; (iii) controlling
bleeding of a wound;
(iv) treating a wound having excess bleeding of a wound; (v) delivering
medication to a
wound; (vi) treating post-surgical or post-procedural external bleeding; or
(vii) treating post-
surgical or post-procedural wound. The methods and uses are performed with a
device
according to the present invention.
100981 In embodiments of the invention, these methods and uses may comprise
activating the
release mechanism of the device, and applying the topical applicator of the
device to the
wound. In other embodiments of the invention, these methods and uses may
comprise
applying the topical applicator of the device to the wound, and activating the
release
mechanism of the device.
100991 In embodiments of the invention, the methods and uses may also comprise
obtaining
the device of the invention.
101001 In embodiments of the invention in which the device comprises a topical
applicator
cap, the methods and uses of the invention may further comprise removing the
applicator cap
before activating the release mechanism of the device, or before applying the
topical
applicator to the wound.
101011 In embodiments of the invention in which the device comprises a locking
mechanism
that prevents activation of the release mechanism, the methods and uses of the
invention may
further comprise unlocking the locking mechanism before activating the release
mechanism.
101021 In embodiments of the invention in which the topical applicator is
removable from the
device, the methods and uses of the invention may further comprise removing
the topical
applicator from the device and fixating the topical applicator to the wound.
For instance, the
topical applicator may be fixated to the wound using a bandage, patch, film,
or surgical
dressing.
17
CA 03179861 2022-10-07
WO 2021/207698
PCT/US2021/026714
101031 in embodiments of the invention in which the device is not prepared
with an ampule
in the reservoir, the methods and uses of the invention may further comprise
installing an
ampule into the reservoir, for example, before activating the release
mechanism of the device,
and/or before applying the topical applicator of the device to the wound.
101041 In some embodiments, the wound is a superficial wound. Examples of a
superficial
wound includes, but is not limited to, a minor cut, abrasion, laceration, or
break in the skin no
greater than about 3 inches (e.g., about 1 to about 3 inches, about 1 inch,
about 2 inches,
about three inches, less than 1 inch) in any direction. In some embodiments,
the device may
be used for post-vascular access or post-procedural bleeding (e.g., central
venous catheter
insertion, arterial cannulation, chest thoracostomy tube, paracentesis,
tracheostomy site, or
other types of vascular access), hemostasis, or wound care.
101051 In some embodiments, the wound is not one or more of: a penetrating
wound, a
puncture wound, a deep wound, and an arteriovenous fistula. In some
embodiments, the
wound is not on the mouth or on any mucosal surface.
101061 In embodiments of the invention, the methods and uses are performed on
a subject in
need thereof. In some embodiments, the methods and uses are performed by the
subject who
is in need thereof. In some embodiments, the subject is a mammalian subject.
In certain
embodiments, the subject is a domestic animal. In preferred embodiments, the
subject is
human.
101071 In some embodiments, the subject was taking or was prescribed, or is
taking or is
prescribed, an anticoagulant therapy. Examples of an anticoagulant therapies
include, but are
not limited to, heparin, warfarin, factor Xa inhibitor, and thrombin
inhibitors.
101081 In some embodiments, the subject may be administered or is to be
administered one
or more antiplatelet therapies. Examples of antiplatelet therapies include,
but are not limited
to, glycoprotein platelet inhibitors such as abciximab, eptifibatide, and
firofiban; platelet
aggregation inhibitors such as aspirin, cangrelor, cilostazol, clopidogrel,
dipyridamole,
prasugrel, ticlopidine, and ficagrelor: and protease-activated receptor-1
antagonists such as
vorapaxar.
101091 In embodiments of the invention, the methods and uses can be performed
by the
subject with the wound. In some embodiments, the methods and uses can be
performed
outside of a hospital or medical facility, for example, at home or outside.
18
CA 03179861 2022-10-07
WO 2021/207698
PCT/US2021/026714
101101 In some embodiments, the device is intended for a single user and one
wound
application. Therefore, the method and uses may further comprise discarding
the device after
a single use. The device may be discarded in a container such as a re-sealable
bag, or the
like.
Manufacturing the Device
[01111 Another aspect of the invention relates to methods of manufacturing the
device of the
invention.
101121 in some embodiments, the method of manufacturing the device comprises
installing
the release mechanism to the reservoir, introducing the ampule to the
reservoir, and coupling
the topical applicator to the reservoir. In certain embodiments, the method
may further
comprise installing the applicator cap onto the topical applicator. These
steps may be
reordered as needed by the manufacturer.
101131 Additional steps may include, but are not limited to, affixing user
instruction labels to
the reservoir and/or topical applicator cap; applying barcodes and/or QR codes
to the
reservoir and/or topical applicator cap; and labeling the reservoir and/or
applicator cap with
an expiration date.
Kits
101141 An aspect of the invention relates to kits that comprise the device of
the invention,
and instructions on how to use the device according to the invention.
[0115] In embodiments in which the device is not manufactured with the ampule
in the
reservoir, the kit may comprise the device, one or more ampules, and
instructions on how to
use the device.
101161 In some embodiments, the kit may also comprise a container for disposal
of the
device after use. The container may be a resealable bag or the like. In
certain embodiments,
the kit may further comprise a biohazard label and/or instructions for
disposal.
19
CA 03179861 2022-10-07
WO 2021/207698
PCT/US2021/026714
* * * * *
The foregoing description is given for clearness of understanding only, and no
unnecessary limitations should be understood therefrom, as modifications
within the scope of
the invention may be apparent to those having ordinary skill in the art.
Detailed embodiments of the present methods and devices are disclosed herein;
however, it is to be understood that the disclosed embodiments are merely
illustrative and
that the methods and devices may be embodied in various forms. In addition,
each of the
examples given in connection with the various embodiments of the systems and
methods are
intended to be illustrative, and not restrictive.
The practice of a method disclosed herein, and individual steps thereof, can
be
performed manually and/or with the aid of or automation provided by electronic
equipment.
Although processes have been described with reference to particular
embodiments, a person
of ordinary skill in the art will readily appreciate that other ways of
performing the acts
associated with the methods may be used. For example, the order of various
steps may be
changed without departing from the scope or spirit of the method, unless
described otherwise.
In addition, some of the individual steps can be combined, omitted, or further
subdivided into
additional steps.