Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 2021/252626
PCT/US2021/036614
METHODS AND COMPOSITIONS FOR PREVENTING AND TREATING MYOPIA
WITH FENOTEROL HYDROBROMIDE, A 132-ADRENERGIC RECEPTOR
AGONIST, AND DERIVATIVES THEREOF
CROSS REFERENCE TO RELATED APPLICATION
The present application claims priority to U.S. Patent Application Serial No.
63/037,891, filed June 11, 2020, which is hereby incorporated by reference in
its entirety.
FIELD
The disclosure relates to methods and compositions for preventing and/or
treating an
ocular disease. In particular, the disclosure relates to preventing and/or
treating myopia with
systemic or topical administration of fenoterol hydrohromi de, which is af32-
adrenergic receptor
agonist, and derivatives thereof.
BACKGROUND
Myopia (nearsightedness) is the most common ocular disorder in the world. The
prevalence of myopia in the U.S. has increased from 25% to 48% in the last 40
years* 2 In
parts of Asia, more than 80% of the population are affected by myopia.' The
worldwide
prevalence of myopia is predicted to increase from 25% in 2020 to 50% by
2050.4 Myopia
results in 250-billion-dollar worldwide productivity loss a year.
Myopia often leads to serious pathological complications such as chorioretinal
atrophy,
retinoschisis, retinal tears, retinal detachment, and myopic macular
degeneration, which often
lead to blindness.5- 6 It also represents a major risk factor for a number of
other serious ocular
diseases such as cataracts and glaucoma, which also often lead to vision
impairment and vision
loss.7 s Because of the increasing prevalence, myopia is rapidly becoming one
of the leading
causes of vision loss, and the World Health Organization designated myopia as
one of five
priority health conditions.5' 9
Development of myopia is controlled by both environmental and genetic
factors.1
Human population studies suggest that the leading environmental factors
causing human
myopia are nearwork and reading,'" which are associated with hyperopic defocus
produced
by the lag of accommodation, i.e., insufficiently strong accommodative
response to near
objects when the subject performs nearwork tasks.14' 15 The optical blur
produced by the
hyperopic defocus is believed to be the signal that drives excessive eye
growth and causes
myopia.16, 17 For example, analysis of the incidence of myopia in orthodox
Jewish students
1
CA 03182397 2022- 12- 12
WO 2021/252626
PCT/US2021/036614
(who spent the 'majority of the day reading) and secular Jewish students (who
spent less time
reading) found that the orthodox students had a much higher incidence and
degree of myopia
as compared to the secular students,18 which suggests that reading is the
factor that causes
myopia. In addition, there are a number of epidemiological studies that show
that myopia is
more common in urban areas, amongst professionals, educated patients, computer
users,
university students, and associated with increased intelligence.19-23 Myopia
is also increased in
individuals who perform tasks requiring increased use of eyes such as
microscopists.24 The
association between optical defocus and myopia was supported by the numerous
animal
studies, which found that degradation of visual input using either diffusers
or negative lenses
causes excessive eye growth and myopia in species as diverse as fish,
chickens, tree shrews,
monkeys, guinea pigs and mice.25
Although the increase in the prevalence of myopia in recent decades is
primarily
attributed to rapidly increasing exposure of young children to nearwork,26 the
contribution of
genetic factors to myopia development has been estimated to be between 60% and
80%.27 The
incidence of myopia increases when both parents have myopia.2 Numerous
studies have
shown that the refractive error of the parents is the most important predictor
of the development
of myopia.28. 29 Strong support for the role of genetic factors in myopia
development also comes
from studies comparing monozygotic 30 and dizygotic twins.31' 32 Myopia is a
complex genetic
disease, which is controlled by hundreds of genes; similar to height and
weight.27. 33 Genetic
studies have implicated over 900 genes to the development of human myopia.27'
33
Thus, both environmental and genetics factors have been shown to contribute to
myopia
development.10 Moreover, a recent study demonstrated the existence of genes,
which modulate
the impact of myopiagenic environmental factors on refractive eye
development.34 Further
support for gene-environment interaction in the development of myopia comes
from gene-
expression-profiling studies which uncovered that development of myopia is
accompanied by
large-scale changes in gene expression in the eye, suggesting that nearwork
activates molecular
signaling pathways in the eye which stimulate excessive eye growth leading to
the development
of myopia.33' 35-37 Several studies revealed that the eye responds to local
changes in optical
defocus with local changes in growth rate, thus suggesting that information
about optical
defocus is summed up across the entire surface of the retina and the
integrated signal regulates
the growth of the eye.38' 39 Importantly, the eye is able to respond to
myopiagenic optical
defocus even if the optic nerve was severed,39 demonstrating that the
signaling cascade
regulating refractive eye development is located within the eye itself and
does not require a
feedback from the brain.
2
CA 03182397 2022- 12- 12
WO 2021/252626
PCT/US2021/036614
Myopia seems to progress the most during a susceptible period between ages 6-
16 and
then begins to slow down."' 41 In previous generations, myopic progression was
assumed to
end at around age 20. However, that has changed since more students have
entered graduate
school followed by jobs requiring 8 hours of sustained computer work.42 This
conjecture was
recently studied in a cohort of post university graduates with a mean age of
35.43 Myopia was
found to progress in approximately 10% of the cohort who spent a lot of time
in front of
computers. Those subjects who did not spend time in front of computers did not
progress as
much.
Current approved treatment options for myopia are limited to optical
correction using
spectacles or contact lenses. Optical correction using single-vision
corrective lenses, which is
the most widely used treatment option for myopia, does not stop the
progression of myopia and
does not prevent the blinding pathological complications associated with the
disease.44' 45
Several experimental optics-based clinical interventions to slow myopia
progression, such as
spectacles with bifocal lenses, multifocal and Ortho-K contact lenses, have
shown some
promise; however, these treatment options have low efficacy.46
Spectacles with bifocal lenses were the first to be used to control myopia
progression.
The multi-center COMET study, which was designed to determine if bifocals
could slow the
progression of myopia as compared to a single vision spectacle lenses
demonstrated that
bifocals slowed the progression of myopia by 20% in the first year; however,
the effect was
significantly reduced in years 2-4.47
Two separate meta-analyses analyzed the ability of Ortho-K lenses to slow
myopic
progression,48' 49 and found that myopic progression can be reduced by
approximately 45%;
however one study found that there was a considerable rebound effect when
Ortho-K lenses
were di sconti nued.
25 Recently, there has been increasing interest in the use of soft
multifocal contact lenses
to replicate the optics of Ortho-K.51-53 A meta-analysis, which included 587
subjects from 8
studies found that concentric ring and distance centered multifocal contact
lenses slowed
myopia progression by 30-38% over 24 months.54
Currently available pharmacological options for myopia control are essentially
limited
30 to two drugs, atropine and 7-methylxanthine, which have significant side
effects and/or
relatively low efficacy.
Atropine, a nonselective rnuscarinic antagonist, is an alkaloid produced by
Atropa
belladonna, which has been traditionally used in ophthalmic practice as a
mydriatic and
cycloplegic drug. Several clinical trials have evaluated the effects of
different concentrations
3
CA 03182397 2022- 12- 12
WO 2021/252626
PCT/US2021/036614
of atropine on myopia progression and its long-term effects on visual function
in children. The
first trial, Atropine for the treatment of Myopia 1 (ATOM1), revealed that the
1% atropine eye
drops retard the progression of myopia by approximately 76% over the 2-year
treatment
period.55 However, the follow up study found that the discontinuation of
treatment led to a
strong rebound effect resulting in the 300% increase in the myopia progression
rate compared
to placebo during the first 12 months after the cessation of atropine, which
eliminated
approximately 60% of the 2-year treatment effect.56 Moreover, 1% atropine
caused
uncomfortable side effects such as photophobia, reduced accommodation
amplitude, and
blurred vision. The follow up trial, ATOM2, evaluated the effects of 0.5%,
0.1%, and 0.01%
atropine on the progression of myopia in children and found that 0.5% atropine
suppressed the
progression of myopia by 75%, while 0.1% and 0.01% atropine retarded
progression by 68%
and 59% respectively.57 The cessation of treatment caused a 218% rebound
increase in the
progression rate compared to placebo in the group treated with 0.5% atropine
and 170%
increase in the group treated with 0.1 % atropine during the first 12 months
after stopping the
administration of the drug.58 However, the progression rate dropped by
approximately 30% in
the group treated with 0.01% atropine.58 These findings were reinforced by the
recent 5-year
follow up study, which revealed that a higher initial atropine dose
predisposed children to
greater myopia progression after the cessation of treatment and suggested that
0.01% atropine
provides the best long-term outcome with approximately 30% suppression
effect.59 These
findings were refined by a recent trial, Low-Concentration Atropine for Myopia
Control
(LAMP) study, which suggested that low-dose atropine has a dose-dependent
suppressive
effect on myopia progression 6 . This study found that 0.01% atropine retarded
progression of
myopia by 27% over 1-year period, compared to 43% and 67% achieved with 0.025%
and
0.05% atropine respectively. However, a recent study found that the use of
atropine in juvenile
primates has long-term adverse effects on the development of ocular components
and
emmetropization, which puts in doubt the utility of atropine as anti-myopia
drug.61
7-methylxanthine (7-MX), a nonselective adenosine receptor antagonist, is a
natural
metabolite of caffeine and theobromine, two alkaloids produced by several
plant species and
major constituents of cacao, coffee, and tea. The first indication that 7-MX
might be a potential
medication for myopia control came from an observation that 7-MX causes
thickening of the
sclera and an increase in the diameter of the scleral collagen fibrils,62
i.e., it causes changes in
the sclera opposite to those observed in myopic eyes. A small follow-up
clinical trial analyzed
the effect of a daily oral consumption of 400 mg (-15 mg/kg) of 7-MX on the
progression of
myopia in children and revealed that 7-MX can potentially suppress myopia by
approximately
4
CA 03182397 2022- 12- 12
WO 2021/252626
PCT/US2021/036614
22% in subjects with slow progressing myopia, while had no effect on myopia
progression in
the subjects with high rates of progression.63 In guinea pigs, a 300 mg/kg
dose of 7-MX was
shown to suppress myopia by 49%.64 Similarly, a 30 mg/kg dose of 7-MX reduced
the extent
of induced myopia in rabbits by approximately 67%.65 Recent data from a study
in monkeys
also suggested that 7-MX can suppress myopia in primates, but the effect
strongly depended
on the genetic background of a specific subject.66 Thus, preliminary data
suggest that 7-MX
has therapeutic potential for myopia control in subjects with slow progressing
myopia, but the
questions of the effective dose and efficacy in humans remain to be clarified.
The safety profile
and long-term effects of daily oral consumption of 7-MX in children is
currently unknown.
Several other compounds have been suggested to suppress myopia to various
degrees.
The muscarinic receptor antagonists pirenzepine and himbacine were shown to
inhibit the
development of experimental myopia in tree shrews, rhesus monkeys, and
chickens.67' 6B While
piren zepine was found to suppress the progression of myopia in children by
40%, clinical trials
were eventually discontinued due to serious side effects.69 Several GABAB and
GABAc
receptor antagonists, such as (1,2,5,6-tetrahydropyridin-4y1) methylphosphinic
acid (TPMPA),
CGP46381, and (3 -aminocyclopentanyl) butylphosphinic acid (3 -ACPBPA) were
shown to
suppress myopia development in chickens and guinea pigs.70-72 Further, a-
adrenergic agonists,
such as clonidine and guanfacine, were shown to inhibit experimentally induced
myopia in
chickens," while brimonidinc suppressed myopia in chickens." and guinea
pigs.74 Moreover,
apomorphine, a dopamine receptor agonist, was found to inhibit myopia
development in
several animal models, such as chicken, mouse and non-human primates,75' 76
and an
intraocular-pressure-lowering drug latanoprost was found to reduce progression
of myopia in
guinea pigs.77 Finally, a recent drug screen in a mouse model of myopia
identified crocetin, a
natural carotenoid found in the crocus flowers and Gardenia jasminoides
fruits, as a potential
anti-myopia agent.78
The prevalence of myopia has been increasing exponentially throughout the
world in
recent years and already reached epidemic proportions in many countries. With
the prevalence
of myopia projected to increase to 50% of the world's population by 2050, the
world will soon
face a public health crisis in vision loss because 8% of low to moderate
myopes and 29% of
high myopcs will develop myopic macular degeneration and will lose sight.79
Currently
available optics-based treatment options for myopia have low efficacy and can
only slow the
progression of myopia, but not stop it. Currently available pharmacological
options have either
low efficacy and/or serious adverse effects. Clearly, there is an urgent
medical need to develop
5
CA 03182397 2022- 12- 12
WO 2021/252626
PCT/US2021/036614
a product for myopia control that, compared to the currently available
products, can achieve
much greater efficacy and can be safely used in children.
SUMMARY
The disclosure provides a method for preventing and/or treating myopia in a
subject in
need thereof by suppressing ocular signaling pathways underlying the
development of myopia
using an oral composition, extended drug release formulations or compositions,
extended drug
delivery by contact lenses, or eye drops comprising a drug compound or agent
identified using
pharmacogenomic pipeline for anti-myopia drug development.
Thus, one embodiment is a method of preventing and/or treating myopia in a
subject in
need thereof comprising administering to the subject a therapeutically
effective amount of a
composition comprising an active drug compound identified using
pharmacogenomic pipeline
for anti-myopia drug development.
In one embodiment, the active drug compound is a 02-adrenergic receptor
agonist,
fenoterol hydrobromide, having the structure:
OH
014
or a derivative thereof.
In some embodiments, the disclosure provides methods for preventing and/or
treating
myopia by administering to a subject a therapeutically effective amount of
fenoterol
hydrobromide or a derivative thereof, in a form of oral composition, extended
drug release
formulation or composition, extended drug delivery by contact lenses, or eye
drops during a
susceptible period for myopia development.
In some embodiments, the disclosure provides methods for preventing and/or
treating
myopia by administering to a subject a repeating dose of a therapeutically
effective amount of
a fenoterol hydrobromide or a derivative thereof, in a form of oral
composition, extended drug
release formulation or composition, extended drug delivery by contact lenses,
or eye drops
during a susceptible period for myopia development.
In further embodiments, the active drug compound is a 132-adrenergic receptor
agonist.
6
CA 03182397 2022- 12- 12
WO 2021/252626 PCT/US2021/036614
In some embodiments, the 132-adrenergic receptor agonist includes but is not
limited to,
bitolterol, isoprenaline, levosalbutamol, levalbuterol, orciprenaline,
metaproterenol, pirbuterol,
procaterol, ritodrine, salbutamol, albuterol, terbutaline, arformoterol,
bambuterol, clenbuterol,
formoterol, salmeterol, and derivatives thereof
Thus, in further embodiments, the disclosure provides methods for preventing
and/or
treating myopia by administering to a subject a therapeutically effective
amount of a 132-
adrenergic receptor agonist, in a form of oral composition, extended drug
release formulation
or composition, extended drug delivery by contact lenses, or eye drops during
a susceptible
period for myopia development.
In some embodiments, the disclosure provides methods for preventing and/or
treating
myopia by administering to a subject a repeating dose of a therapeutically
effective amount of
a 132-adrenergic receptor agonist, in a form of oral composition, extended
drug release
formulation or composition, extended drug delivery by contact lenses, or eye
drops during a
susceptible period for myopia development.
In some embodiments, the composition is administered to the subject once a
day. In
some embodiments, the composition is administered once a week. In some
embodiments, the
composition is administered twice a week. In some embodiments, the composition
is
administered three times a week. In some embodiments, the composition is
administered to the
subject continuously or intermittently for about 5 years to about 10 years.
In some embodiments, the subject is a young adult, i.e., under 30 years of
age. In some
embodiments, the subject is a child, i.e., under the age of 18. In some
embodiments, the subject
is about 4 years of age to about 30 years of age. In some embodiments, the
subject is about 6
years of age to about 20 years of age. In some embodiments, the subject is
about 8 years of age
to about 15 years of age. In some embodiments, the subject is about 10 years
of age to about
12 years of age.
In some embodiments, the subject has myopia. In some embodiments, the subject
is at
risk for myopia. In some embodiments, the subject is susceptible to myopia.
In some embodiments, the subject is monitored for suppression of myopia and
the
therapeutically effective amount and/or frequency of administration of the
drug compound is
adjusted depending on the degree of suppression. Suppression of myopia may be
monitored
using methods known in the art.
A further embodiment of the present disclosure are kits comprising
compositions and
agents for practicing the disclosed methods.
7
CA 03182397 2022- 12- 12
WO 2021/252626
PCT/US2021/036614
BRIEF DESCRIPTION OF THE FIGURES
For the purpose of illustrating the invention, there are depicted in drawings
certain
embodiments of the invention. However, the invention is not limited to the
precise
arrangements and instrumentalities of the embodiments depicted in the
drawings.
Fig. 1. Experimentally induced myopia in mice has features of human myopia.
Fig. lA
shows a mouse induced to have myopia with -25 D lenses. Fig. 1B is a graph
show the
statistically significant myopic shift in refraction observed in the eyes of
the mice treated with
-25 D lenses for 21 days. Fig. 1C shows that the lens-induced myopia in mice
is due to a
statistically significant increase in the vitreous chamber depth, as in human
myopia. Fig. 1D
shows the power simulations demonstrating the relationship between statistical
power and a
number of animals for induced myopia experiments. ACD, anterior chamber depth;
CRC,
corneal radius of curvature; LT, lens thickness; VCD, vitreous chamber depth;
OD, right
(myopic) eye; OS, left (control) eye. En-or bars, SD. P, significance value.
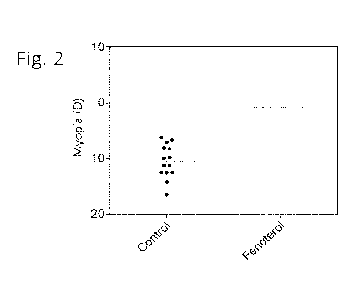
Fig. 2 shows that systemic administration of 1 mg/kg fenoterol hydrobromide
suppresses development of myopia in mice with experimentally induced myopia by
approximately 90%.
DETAILED DESCRIPTION
Definitions
The following definitions and explanations are meant and intended to be
controlling in
any construction unless clearly and unambiguously modified in the following
examples or
when application of the meaning renders any construction meaningless or
essentially
meaningless. In cases where the construction of the term would render it
meaningless or
essentially meaningless, the definition should he taken from Wehster's
Dictionary or a
dictionary known to those of skill in the art, such as the Oxford Dictionary
of Biochemistry
and Molecular Biology or similar.
The contents of any patents, patent applications, and references cited
throughout this
specification are hereby incorporated by reference in their entireties.
As used herein and unless otherwise indicated, the temis "a" and "an" are
taken to mean
"one", "at least one' or "one or more". Unless otherwise required by context,
singular terms
used herein shall include pluralities and plural terms shall include the
singular.
The term "myopia" or "myopic" shall mean eye disease condition in which the
posterior
segment of the eye is too large for the optical power of the eye and the focal
point is located in
front of the retina; thus, producing blurred distant vision.
8
CA 03182397 2022- 12- 12
WO 2021/252626
PCT/US2021/036614
The term "hyperopia" or "hyperopic" shall mean eye condition in which the
posterior
segment of the eye is too small for the optical power of the eye and the focal
point is located
behind the retina; thus, producing blurred near vision.
The term "negative lens" shall mean a lens which shifts focal point of the eye
towards
the back of the eye; thus, rendering the eye hyperopic.
The term "genetic network" shall mean a network of interconnected genes which
regulate a physiological or biological process.
The term "differentially expressed" shall mean changes in gene expression
level
induced by environmental factors, changes in genetic background, or other
internal or external
insult or influence.
The term "experimentally induced myopia" is used here to describe myopia
induced in
animal models by experimental manipulations, such as the application of
negative lenses over
the eye.
The term "whole-genome gene expression profiling" refers to a method of
analyzing
differential gene expression at the level of the entire genome; thus,
providing information about
expression of all genes encoded by the genome.
Thc term "gene-bascd genome-wide association study" refers to a genetic study
which
analyzes statistical associations between genetic variations in the genome and
a disease at the
level of specific genes, found previously to be involved in a disease process
by other
experimental approaches such as whole-genome gene expression profiling.
The term "positive optical defocus' shall mean the condition when focal point
of the
eye is located in front of the retina.
The term "negative optical defocus" shall mean the condition when focal point
of the
eye is located behind the retina.
The term "derivative" refers to structural analog of a compound that is
derived from a
compound by a chemical reaction. A structural analog is a compound having a
structure similar
to that of another compound but differing from it in respect to a certain
component. It can differ
in one or more atoms, functional groups, or substructures, which are replaced
with other atoms,
groups, or substructures. A structural analog can also differ from another
compound in one or
more atoms, functional groups, or substructures, which arc added to or
subtracted from another
compound. A structural analog can be imagined to be formed by those skilled in
art, at least
theoretically, from the other compound.
9
CA 03182397 2022- 12- 12
WO 2021/252626
PCT/US2021/036614
The term "subject" as used in this application means a human subject. In some
embodiments of the present invention, the "subject" has myopia, is at risk for
myopia or is
susceptible to myopia.
The terms "treat", "treatment", and the like refer to a means to slow down,
relieve,
ameliorate or alleviate at least one of the symptoms of the disease, or
reverse the disease after
its onset.
The terms "prevent", "prevention", and the like refer to acting prior to overt
disease
onset, to prevent the disease from developing or minimize the extent of the
disease or slow its
course of development.
The term "in need thereof' would be a subject known to be, or suspected of,
suffering
from myopia.
A subject in need of treatment would be one that has already developed the
disease or
condition. A subject in need of prevention would be one with risk factors of
the disease or
condition.
The term "agent" as used herein means a substance that produces or is capable
of
producing an effect and would include, but is not limited to, chemicals,
pharmaceuticals,
biologics, small organic molecules, antibodies, nucleic acids, peptides, and
proteins.
The phrase "therapeutically effective amount" is used herein to mean an amount
sufficient to cause an improvement in a clinically significant condition in
the subject, or delays
or minimizes or mitigates one or more symptoms associated with the disease, or
results in a
desired beneficial change of physiology in the subject.
Identifying anti-myopia drugs using a pharmacogenomic pipeline
Shown herein is the results of the use of a pharmacogenomic pipeline developed
by the
inventors for the identification of drug compounds capable of suppressing
myopia
developments A systems genetics approach was used to identify genes, genetic
networks, and
signaling pathways underlying refractive eye development and the development
of myopia.
The systems genetics approach comprised identification of genes differentially
expressed in
the eyes of animals with experimentally induced myopia using whole-genome gene
expression
profiling and identification of genes linked to myopia in humans using gene-
based genome-
wide association studies. One of the inventors' studies found that signaling
pathways
underlying eye's responses to positive optical defocus (which suppresses
myopia) and negative
optical defocus (which promotes myopia development) propagate via two largely
distinct
signaling cascades, described in U.S. Provisional Application No. 62/730,301.
CA 03182397 2022- 12- 12
WO 2021/252626
PCT/US2021/036614
The inventors extended this observation to several vertebrate species and
demonstrated
that signaling cascades underlying myopia development are highly
evolutionarily conserved
across vertebrate species, including humans. The inventors then used their
vast myopia-
associated gene dataset (which included over 3,500 genes) described in
Tkatchenko et al.
2019,81 and computational tools to reconstruct the genetic networks that
control myopia
development and to identify drug compounds, which can suppress signaling
pathways that
promote myopia development and stimulate the pathways that inhibit the
development of
myopia.
A total of 138 drug compounds with anti-myopic potential were identified.
Using the
gene pathways and z-scores, these drug compounds were assigned to top 10, top
20, top 40,
top 80, and low priority categories based on their predicted potential to
suppress myopia and
known or predicted side effects. These drug compounds were then tested on a
mouse model of
myopia (Example 1).
Methods and Compositions for the Prevention and/or Treatment of Myopia using
Fenoterol Hydrobromide, a I32-adrenergic Receptor Agonist, and Derivatives
Thereof
The disclosure provides in some aspects methods of preventing and/or treating
myopia
comprising administering to a subject in need thereof a therapeutically
effective amount of
tbnotcrol hydrobromide or a derivative thereof.
In certain embodiments, the fenoterol hydrobromide or derivative is
administered
systemically. In certain embodiments, the fenoterol hydrobromide or derivative
is administered
orally. In certain embodiments, the fenoterol hydrobromide or derivative is
administered
locally. In some embodiments, the fenoterol hydrobromide or derivative is
administered
directly to or into the eye. In some embodiments, the fenoterol hydrobromide
or derivative is
administered via injection. In other embodiments, the fenoterol hydrobromide
or derivative is
administered as extended drug release formulations or compositions, extended
drug delivery
by contact lenses, Or eye drops.
In certain embodiments, the fenoterol hydrobromide is used directly as the
active
ingredient in the drug. In other embodiments, the fenoterol hydrobromide can
be chemically
modified to improve its efficacy, reduce side effects, improve penetration
through ocular
tissues, increase stability, or improve bioavailability.
In certain embodiments, the fenoterol hydrobromide (or its derivative) is a
sole
component of the drug. In other embodiments, the methods and compositions
described herein
11
CA 03182397 2022- 12- 12
WO 2021/252626
PCT/US2021/036614
comprise the use of pharmaceutical formulations comprising the fenoterol
hydrobromide (or
its derivative).
The term "pharmaceutical formulation" refers to preparations. which include
the
fenoterol hydrobromide (or its derivative) and additional ingredients, such as
other drugs
capable of suppressing myopia or excipients (vehicles, additives,
preservatives, buffers), which
can reasonably be administered to a subject to improve the efficacy of the
active ingredient(s)
or increase stability of the active ingredient(s). A formulation is stable if
the active ingredient(s)
essentially retain their physical properties, and/or chemical properties,
and/or biological
activity at room temperature (15-30 C) for at least a week, or at 2-8 C for
3 months to 1 year.
Fenoterol hydrobromide (or its derivative) is considered to retain its
physical properties
in a pharmaceutical formulation if it meets defined specifications for
degradation, and/or
aggregation, and/or precipitation upon visual examination of color and/or
clarity, or as
measured by light scattering or other suitable art recognized methods.
Fenoterol hydrobromide (or its derivative) is considered to retain its
chemical stability
in a pharmaceutical formulation if the active ingredient content within about
90% of the amount
at the time the pharmaceutical formulation was prepared. Some types of
chemical degradation
include oxidation and hydrolysis, which can be evaluated, for example, by LC-
MS/MS-based
methods.
Fenoterol hydrobromide (or its derivative) is considered to retain its
biological stability
in a pharmaceutical formulation if the active ingredient at a given time is
within about 90% of
the biological activity exhibited at the time the pharmaceutical formulation
was prepared as
determined by in vivo testing, for example.
In the context of the present disclosure, the therapeutically effective dose
of the
fenoterol hydrobromide (or its derivative) is the amount sufficient to at
least partially prevent
and/or treat myopia. A therapeutically effective dose is sufficient if it can
produce even an
incremental change in the symptoms or conditions associated with the disease.
The
therapeutically effective dose does not have to completely cure the disease or
completely
eliminate symptoms. Preferably, the therapeutically effective dose can
significantly slow the
progression of myopia in a subject suffering from the disease. The dose and
frequency of drug
administration effective for this use will depend on the severity of the
disease (i.e., low
progressing versus high progressing myopia), type of myopia (i.e., syndromic
myopia versus
common myopia), subject age, body mass of the subject, and route of
administration among
other factors. The dose and frequency of the drug administration can be
adjusted using well
understood and commonly used state of art in optometric and ophthalmologic
practices.
12
CA 03182397 2022- 12- 12
WO 2021/252626
PCT/US2021/036614
The fenoterol hydrobromide described herein can be co-administered with other
agents
including additional agents for the prevention and/or treatment of myopia. The
co-
administration of agents can be by any administration described herein.
Moreover, the
additional agent can be in the same composition as the fenoterol hydrobromide.
The additional
agent can be in a separate composition from the fenoterol hydrobromide. The
administration
of more than one composition can be simultaneous, concurrently or
sequentially.
The disclosure further provides in some aspects methods of preventing and/or
treating
myopia comprising administering to a subject in need thereof a therapeutically
effective
amount of a f32-adrenergic receptor agonist.
In some embodiments, the 132-adrenergic receptor agonist includes but is not
limited to,
bitolterol, isoprenaline, levosalbutamol, levalbuterol, orciprenaline,
metaproterenol, pirbuterol,
procaterol, ritodrine, salbutamol, albuterol, terbutaline, arformoterol,
bambuterol, clenbuterol,
formoterol, salmeterol, or derivatives thereof.
In
certain embodiments, the 132 -adren ergi c receptor agonist is administered
systemically. In certain embodiments, the 132-adrenergic receptor agonist is
administered
orally. In certain embodiments, the f12-adrenergic receptor agonist is
administered locally. In
some embodiments, the 132-adrenergic receptor agonist is administered directly
to or into the
eye. In some embodiments, the f32-adrenergic receptor agonist is administered
via injection. In
other embodiments, the 132-adrenergic receptor agonist is administered as
extended drug release
formulations or compositions, extended drug delivery by contact lenses, or eye
drops.
In further embodiments, the 132-acIrenergic receptor agonist is used directly
as the active
ingredient in the drug. In other embodiments, the 132-adrenergic receptor
agonist can be
chemically modified to improve its efficacy, reduce side effects, improve
penetration through
ocular tissues, increase stability, or improve bioavailability.
In certain embodiments, the I32-adrenergic receptor agonist is a sole
component of the
drug. In other embodiments, the methods and compositions described herein
comprise the use
of pharmaceutical formulations comprising the f32-adrenergic receptor agonist.
The term "pharmaceutical formulation" refers to preparations, which include
the 02-
adrenergic receptor agonist and additional ingredients, such as other drugs
capable of
suppressing myopia or excipicnts (vehicles, additives, preservatives,
buffers), which can
reasonably be administered to a subject to improve the efficacy of the active
ingredient(s) or
increase stability of the active ingredient(s). A formulation is stable if the
active ingredient(s)
essentially retain their physical properties, and/or chemical properties,
and/or biological
activity at room temperature (15-30 C) for at least a week, or at 2-8 C for
3 months to 1 year.
13
CA 03182397 2022- 12- 12
WO 2021/252626
PCT/US2021/036614
The 132-adrenergic receptor agonist is considered to retain its physical
properties in a
pharmaceutical formulation if it meets defined specifications for degradation,
and/or
aggregation, and/or precipitation upon visual examination of color and/or
clarity, or as
measured by light scattering or other suitable art recognized methods.
The f32-adrenergic receptor agonist is considered to retain its chemical
stability in a
pharmaceutical formulation if the active ingredient content within about 90%
of the amount at
the time the pharmaceutical formulation was prepared. Some types of chemical
degradation
include oxidation and hydrolysis, which can be evaluated, for example, by LC-
MS/MS-based
methods.
The 132-adrenergic receptor agonist is considered to retain its biological
stability in a
pharmaceutical formulation if the active ingredient at a given time is within
about 90% of the
biological activity exhibited at the time the pharmaceutical formulation was
prepared as
determined by in vivo testing, for example.
In the context of the present disclosure, the therapeutically effective dose
of the 132-
adrenergic receptor agonist is the amount sufficient to at least partially
prevent and/or treat
myopia. A therapeutically effective dose is sufficient if it can produce even
an incremental
change in the symptoms or conditions associated with the disease. The
therapeutically effective
dose does not have to completely cure the disease or completely eliminate
symptoms.
Preferably, the therapeutically effective dose can significantly slow the
progression of myopia
in a subject suffering from the disease. The dose and frequency of drug
administration effective
for this use will depend on the severity of the disease (i.e., low progressing
versus high
progressing myopia), type of myopia (i.e., syndromic myopia versus common
myopia), subject
age, body mass of the subject, and route of administration among other
factors. The dose and
frequency of the drug administration can be adjusted using well understood and
commonly
used state of art in optometric and ophthalmologic practices.
The 132-adrenergic receptor agonist described herein can be co-administered
with other
agents including additional agents for the suppression, prevention and/or
treatment of myopia.
The co-administration of agents can be by any administration described herein.
Moreover, the
additional agent can be in the same composition as the f32- adrenergic
receptor agonist. The
additional agent can be in a separate composition from the f32-adrenergic
receptor agonist. The
administration of more than one composition can be simultaneous, concurrently
or
sequentially.
Oral compositions of the drug can be in a form of capsules, tablets, powders,
granules,
solutions, syrups, suspensions (in non-aqueous or aqueous liquids), or
emulsions. Tablets or
14
CA 03182397 2022- 12- 12
WO 2021/252626
PCT/US2021/036614
hard gelatin capsules may comprise lactose, starch or derivatives thereof,
magnesium stearate,
sodium saccharine, cellulose, magnesium carbonate, stearic acid or salts
thereof. Soft gelatin
capsules may comprise vegetable oils, waxes, fats, semi-solid, or liquid
polyols. Solutions and
syrups may comprise water, polyols, and sugars. An active agent intended for
oral
administration may be coated with or admixed with a material that delays
disintegration and/or
absorption of the active agent in the gastrointestinal tract. Thus, the
sustained release may be
achieved over many hours and if necessary, the active agent can be protected
from degradation
within the stomach. Pharmaceutical compositions for oral administration may be
formulated
to facilitate release of an active agent at a particular gastrointestinal
location due to specific pH
or enzymatic conditions.
It should be understood that, in addition to the ingredients particularly
mentioned
above, the compositions may include other agents conventional in the art
having regard to the
type of formulation in question, for ex ample those suitable for oral
administration may include
flavoring agents.
Extended drug release formulations or compositions can be in a form of a
nanosponge,
patch, gel or other device capable of gradual release of the drug over
extended period of time,
which is injected in the anterior or posterior segment of the eye or
administered or applied to
the anterior or posterior surfaces of the eye.
Extended drug delivery by contact lenses can be in a form of piano contact
lens, single-
vision corrective contact lens, or multi-focal contact lens, in which either
internal surface of
the lens is coated with the chug, or the entire volume of the lens is loaded
with the drug.
Eye drops can be in a form of traditional eye drops well-known and commonly
used by
those skilled in the art, or in a form of a micro-dosing device which delivers
a strictly controlled
amount of the drug to the eye.
In some embodiments, the composition is administered to the subject once a
day. In
some embodiments, the composition is administered once a week. In some
embodiments, the
composition is administered twice a week. In some embodiments, the composition
is
administered three times a week. In some embodiments, the composition is
administered to the
subject continuously or intermittently for about 5 years to about 10 years.
In some embodiments, the composition is administered more than once.
Treatment using the present methods and compositions can continue as long as
needed.
In one embodiment, the efficacy of the treatment in a subject with myopia is
evaluated
every 3-6 months and the dose and/or frequency of drug administration is
adjusted depending
on the degree of myopia suppression. The treatment is discontinued once the
subject does not
CA 03182397 2022- 12- 12
WO 2021/252626
PCT/US2021/036614
exhibit any further progression of myopia, which can be evaluated by
temporarily
discontinuing the treatment and measuring changes in refractive error over 1-6
months using
well understood state of art in optometric and ophthalmologic practices.
In some embodiments, the subject is a child, i.e., under 18 years of age. In
some
embodiments, the subject is a young adult, i.e., under 30 years of age. In
some embodiments,
the subject is about 4 years of age to about 30 years of age. In some
embodiments, the subject
is about 6 years of age to about 20 years of age. In some embodiments, the
subject is about 8
years of age to about 15 years of age. In some embodiments, the subject is
about 10 years of
age to about 12 years of age.
In some embodiments, the subject has myopia. In some embodiments, the subject
is at
risk for myopia. In some embodiments, the subject is susceptible to myopia.
Risk factors for myopia can include but are not limited to having one or more
parents
with myopia.
Kits
Also within the scope of the present disclosure are kits for practicing the
disclosed
methods.
In some embodiments, the kit can comprise instructions for use in any of the
methods
described herein. The included instructions can comprise a description of
administration of the
agents to a subject to achieve the intended activity in a subject. The kit may
further comprise
a description of selecting a subject suitable for treatment based on
identifying whether the
subject is in need of the treatment.
The instructions relating to the use of the drugs described herein generally
include
information as to dosage, dosing schedule, and route of administration for the
intended
treatment. The containers may be unit doses, bulk packages (e.g., multi-dose
packages) or sub-
unit doses. Instructions supplied in the kits of the disclosure are typically
written instructions
on a label Or package insert. The label or package insert indicates that the
pharmaceutical
compositions are used for treating, delaying the onset, and/or alleviating a
disease or disorder
in a subject.
The kits provided herein are in suitable packaging. Suitable packaging
includes, but is
not limited to, vials, bottles, jars, flexible packaging, and the like.
Kits optionally may provide additional components such as buffers and
interpretive
information. Normally, the kit comprises a container and a label or package
insert(s) on or
16
CA 03182397 2022- 12- 12
WO 2021/252626
PCT/US2021/036614
associated with the container. In some embodiment, the disclosure provides
articles of
manufacture comprising contents of the kits described above.
EXAMPLES
The following examples are included to demonstrate preferred embodiments of
the
invention. In light of the present disclosure, it should be appreciated by
those of skill in the art
that many changes can be made in the specific embodiments which are disclosed
and still obtain
a like or similar result without departing from the spirit and scope of the
invention.
Example 1. Myopia can be induced in mammals using negative spectacle lenses
Myopia was induced in 24-days old C57BL/6,1 (B6) mice by attaching -25 diopter
(D)
lens placed in a plastic 3D-printed frame over right eye. The contralateral
eye served as control.
Mice were raised with lenses for 3 weeks. After 3 weeks, the lenses were
removed and
refractive errors in the lens-treated eyes and contralateral control eyes were
compared. Lens-
treatment produced myopia in the lens-treated eyes (average refractive error =
-14.6 0.3 D)
relative to the control eyes (average refractive error = +0.6 0.6 D) (Fig.
1); the interocular
difference in refractive error (-15.2 0.7 D) was highly significant (P <
0.0001). High-
resolution MRI revealed enlargement of the eye and the vitreous chamber in the
treated eyes.
The diameter of lens-treated eyes was on average 65 8 pm larger (P < 0.0001;
Fig. 1C), and
the vitreous chamber depth in the lens-treated eyes was 61 4 pm longer (P <
0.0001; Fig.
1D), than that of the control fellow eyes. No significant interocular
differences were observed
in the anterior chamber depth, corneal radius of curvature and crystalline
lens thickness (Fig.
1D), suggesting that changes induced in the mouse eyes treated with negative
lenses, are
primarily confined to the posterior segment of the eye, similar to human
myopia. Statistical
power analysis revealed that differences as small as 0.5 diopters in
refractive error between the
eyes can be identified with 90% statistical power with the sample size of 22
mice.
17
CA 03182397 2022- 12- 12
WO 2021/252626
PCT/US2021/036614
Example 2. Fenoterol hydrobromide suppresses myopia in subjects with lens-
induced
myopia
Fenoterol hydrobromide was identified as one of the top 10 drug candidates
using the
pharmacogenomic pipeline for anti-myopic drug development. It was discovered
that systemic
oral administration of fenoterol hydrobromide inhibited myopia by 92% (Fig.
2).
The experimental group of B6 mice was raised with -25 D lenses over right eye
on a
diet supplemented with 1 mg/kg of fenoterol hydrobromide for 3 weeks, while
the control
group of B6 mice with -25 D lenses over right eye was raised on a regular non-
medicated diet.
The interocular difference in refractive error between lens-treated eyes and
control eyes in the
fenoterol-treated animals after 3 weeks of lens treatment was -0.8 1.80 D
versus -10.47
3.02 D in the control group, P = 2.47 x les. See Fig. 2.
REFERENCES
1. Kempen JH, Mitchell P, Lee KE, et al. The prevalence of refractive
errors among
adults in the United States, Western Europe, and Australia. Arch Ophthalmol
2004;122:495-
505.
2. Vitale S, Sperduto RD, Ferris FL, 3rd. Increased prevalence
of myopia in the United
States between 1971-1972 and 1999-2004. Arch Ophthalmol 2009;127:1632-1639.
3. Lin LL, Shih YF, Hsiao CK, Chen CJ. Prevalence of myopia in Taiwanese
schoolchildren: 1983 to 2000. Ann Acad Med Singapore 2004;33:27-33.
4. Holden BA, Fricke TR, Wilson DA, et al. Global Prevalence of
Myopia and High
Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016;123:1036-
1042.
5. Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated
pathological
complications. Ophthalmic Physiol Opt 2005;25 :381-391.
6. Verhoeven VJ, Wong KT, Buitendijk CH, Hofman A, Vingerling
JR, Klaver CC.
Visual consequences of refractive errors in the general population.
Ophthalmology
2015;122:101-109.
7. Qiu M, Wang SY, Singh K, Lin SC. Association between myopia and glaucoma
in the
United States population. Invest Ophthalmol Vis Sci 2013;54:830-835.
8. Praveen MR, Vasavada AR, Jani UD, Trivedi RH, Choudhary PK.
Prevalence of
cataract type in relation to axial length in subjects with high myopia and
emmetropia in an
Indian population. Am T Ophthalmol 2008;145:176-181.
18
CA 03182397 2022- 12- 12
WO 2021/252626
PCT/US2021/036614
9. Pizzarello L, Abiose A, Ffytche T, et al. VISION 2020: The Right to
Sight: a global
initiative to eliminate avoidable blindness. Arch Ophthalmol 2004;122:615-620.
10. Wojciechowski R. Nature and nurture: the complex genetics of myopia and
refractive
error. Clin Genet 2011;79:301-320.
11. Parssinen 0, Lyyra AL. Myopia and myopic progression among
schoolchildren: a
three-year follow-up study. Invest Ophthahnol Vis Sci 1993;34:2794-2802.
12. Goss DA. Nearwork and myopia. Lancet 2000;356:1456-1457.
13. Saw SM, Chua WH, Hong CY, et al. Nearwork in early-onset myopia. Invest
Ophthalmol Vis Sci 2002;43:332-339.
14. Gwiazda J, Thorn F, Bauer J, Held R. Myopic children show insufficient
accommodative response to blur. Invest Ophthalmol Vis Sci 1993;34:690-694.
15. Seidemann A, Schaeffel F. An evaluation of the lag of accommodation
using
photorefraction. Vision Res 2003;43:419-430.
16. Charman WN. Near vision, lags of accommodation and myopia. Ophthalmic
Physiol
Opt 1999;19:126-133.
17. Gwiazda JE, Hyman L, Norton TT, et al. Accommodation and related risk
factors
associated with myopia progression and their interaction with treatment in
COMET children.
Invest Ophtlzalmol Vis Sci 2004;45:2143-2151.
18. Zylbcrmann R, Landau D, Berson D. The influence of study habits on
myopia in
Jewish teenagers. Journal of pediatric ophthalmology and strabismus
1993;30:319-322.
19. Williams C, Miller LL, Gazzarcl G, Saw SM. A comparison of measures of
reading
and intelligence as risk factors for the development of myopia in a UK cohort
of children. Br
J Ophthalmol 2008;92:1117-1121.
20. Pan CW, Ramarnurthy D, Saw SM. Worldwide prevalence and risk factors
for
myopia. Ophthalmic Physiol Opt 2012;32:3-16.
21. Saw SM, Cheng A, Fong A, Gazzard G, Tan DT, Morgan I. School grades and
myopia. Ophthalmic Physiol Opt 2007;27:126-129.
22. Zadnik K, Mutti DO. Refractive error changes in law students. Am J
Optom Physiol
Opt 1987;64:558-561.
23. Ip JM, Rose KA, Morgan IG, Burlutsky G, Mitchell P. Myopia and the
urban
environment: findings in a sample of 12-year-old Australian school children.
Invest
Ophthalmol Vis Sci 2008;49:3858-3863.
24. Ting PW, Lam CS, Edwards MH, Schmid KL. Prevalence of myopia
in a group of
Hong Kong microscopists. Optom Vis Sci 2004;81:88-93.
19
CA 03182397 2022- 12- 12
WO 2021/252626
PCT/US2021/036614
25. Troilo D, Smith EL, 3rd, Nickla DL, et al. IMI - Report on Experimental
Models of
Emmetropization and Myopia. Invest Ophtholmol Vis Sci 2019;60:M31-M88.
26. Huang HM, Chang DS, Wu PC. The Association between Near Work Activities
and
Myopia in Children-A Systematic Review and Meta-Analysis. PLoS One
2015;10:e0140419.
27. Tedja MS, Haarman AEG, Meester-Smoor MA, et al. IMI - Myopia Genetics
Report.
Invest Ophthalmol Vis Sci 2019;60:M89-M105.
28. Dirani M, Shekar SN, Baird PN. Evidence of shared genes in
refraction and axial
length: the Genes in Myopia (GEM) twin study. Invest Ophthalmol Vis Sci
2008;49:4336-
4339.
29. Jones-Jordan LA, Sinnott LT, Manny RE, et al. Early childhood
refractive error and
parental history of myopia as predictors of myopia. Invest Ophthalmol Vis Sci
2010;51:115-
121.
30. Dirani M, Shekar SN, Baird PN. Adult-onset myopia: the Genes
in Myopia (GEM)
twin study. Invest Ophthalmol Vis Sci 2008;49:3324-3327.
31. Tsai MY, Lin LL, Lee V, Chen CJ, Shih YF. Estimation of heritability in
myopic twin
studies. Japanese journal of ophthalmology 2009;53:615-622.
32. Lyhnc N, Sjolic AK, Kyvik KO, Green A. The importance of
genes and environment
for ocular refraction and its determiners: a population based study among 20-
45 year old
twins. Br T Ophthalmol 2001;85:1470-1476.
33. Tkatchenko TV, Shah RL, Nagasaki T, Tkatchenko AV. Analysis of genetic
networks
regulating refractive eye development in collaborative cross progenitor strain
mice reveals
new genes and pathways underlying human myopia. BMC Med Genomics 2019;12:113.
34. Tkatchenko AV, Tkatchenko TV, Guggenheim JA, et al. APLP2
Regulates Refractive
E11-01" and Myopia Development in Mice and Humans. PLUS Genet
2015;11:e1005432.
35. Shelton L, Troilo D, Lerner MR, Gusev Y, Brackett DJ, Rada JS.
Microarray analysis
of choroid/RPE gene expression in marmoset eyes undergoing changes in ocular
growth and
refraction. Mo/ Vis 2008;14:1465-1479.
36. Tkatchenko AV, Walsh PA, Tkatchenko TV, Gustincich S, Raviola E. Form
deprivation modulates retinal neurogenesis in primate experimental myopia.
Proc Natl Acrid
Sci USA 2006;103:4681-4686.
37. Tkatchenko TV, Troilo D, Benavente-Perez A, Tkatchenko AV. Gene
expression in
response to optical defocus of opposite signs reveals bidirectional mechanism
of visually
guided eye growth. PLoS biology 2018:16:e2006021.
CA 03182397 2022- 12- 12
WO 2021/252626
PCT/US2021/036614
38. Smith EL, 3rd. Prentice Award Lecture 2010: A case for peripheral
optical treatment
strategies for myopia. Optorn Vis Sci 2011;88:1029-1044.
39. Troilo D, Gottlieb MD, Wallman J. Visual deprivation causes myopia in
chicks with
optic nerve section. Curr Eye Res 1987;6:993-999.
40. Fan DS, Lam DS, Lam RF, et al. Prevalence, incidence, and progression
of myopia of
school children in Hong Kong. Invest Ophthalmol Vis Sci 2004;45:1071-1075.
41. Donovan L, Sankaridurg P, Ho A, Naduvilath T, Smith EL, 3rd,
Holden BA. Myopia
progression rates in urban children wearing single-vision spectacles. Op torn
Vis Sci
2012;89:27-32.
42. Cortinez MF, Chiappe JP, Iribarren R. Prevalence of refractive errors
in a population
of office-workers in Buenos Aires, Argentina. Ophthalmic epidemiology
2008;15:10-16.
43. Fernandez-Montero A, Olmo-Jimenez JM, Olmo N, et al. The impact of
computer use
in myopia progression: a cohort study in Spain. Prey Med 2015;71:67-71.
44. Ong E, Grice K, Held R, Thorn F, Gwiazda J. Effects of spectacle
intervention on the
progression of myopia in children. Optom Vis Sci 1999;76:363-369.
45. Walline JJ, Jones LA, Sinnott L, et al. A randomized trial of the
effect of soft contact
lenses on myopia progression in children. Invest Ophthalmol Vis Sci
2008;49:4702-4706.
46. Wildsoet CF, Chia A, Cho P, et al. IMI - International Myopia
Institute: Interventions
for Controlling Myopia Onset and Progression Report. Invest Ophthalmol Vis Sci
2019;60:M106-M131.
47. Gwiazda JE, Hyman L, Everett D, Norton T, Kurtz D, Manny R. Five-year
results
from the correction of myopia evaluation trial (COMET). Investigative
Ophthalmology and
Visual Science 2006;47: E-abstract 1166.
48. Sun Y, Xu F, Zhang T, et al. Orthokeratology to control myopia
progression: a meta-
analysis. PLoS One 2015;10:e0124535.
49. Si JK, Tang K, Bi HS, Guo DD, Guo JG, Wang XR. Orthokeratology for
myopia
control: a meta-analysis. Optom Vis Sci 2015;92:252-257.
50. Cho P, Cheung SW. Discontinuation of orthokeratology on eyeball
elongation
(DOEE). Coln Lens Anterior Eye 2017;40:82-87.
51. Woods J, Guthric SE, Kcir N, et al. Inhibition of defocus-induced
myopia in chickens.
Invest Ophthalmol Vis Sci 2013;54:2662-2668.
52. Walline JJ, Greiner KL, McVey ME, Jones-Jordan LA.
Multifocal contact lens
myopia control. Optom Vis Sci 2013;90:1207-1214.
21
CA 03182397 2022- 12- 12
WO 2021/252626
PCT/US2021/036614
53. Paune J, Morales H, Armengol J, Quevedo L, Faria-Ribeiro M, Gonzalez-
Meijome
JM. Myopia Control with a Novel Peripheral Gradient Soft Lens and
Orthokeratology: A 2-
Year Clinical Trial. BioMed research international 2015;2015:507572.
54. Li SM, Kang MT, Wu SS, et al. Studies using concentric ring bifocal and
peripheral
add multifocal contact lenses to slow myopia progression in school-aged
children: a meta-
analysis. Ophthalmic Physiol Opt 2017;37:51-59.
55. Chua WH, Balakrishnan V, Chan YH, et al. Atropine for the treatment of
childhood
myopia. Ophthalmology 2006;113:2285-2291.
56. Tong L, Huang XL, Koh AL, Zhang X, Tan DT, Chua WH. Atropine for the
treatment of childhood myopia: effect on myopia progression after cessation of
atropine.
Ophthalmology 2009;116:572-579.
57. Chia A, Chua WH, Cheung YB, et al. Atropine for the treatment of
childhood
myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the
Treatment of
Myopia 2). Ophthalmology 2012;119:347-354.
58. Chia A, Chua WH, Wen L, Fong A, Goon YY, Tan D. Atropine for the
treatment of
childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5%. Am J
Ophthalmol
2014;157:451-457 e451.
59. Chia A, Lu QS, Tan D. Five-Year Clinical Trial on Atropine for the
Treatment of
Myopia 2: Myopia Control with Atropinc 0.01% Eycdrops. Ophthalmology
2016;123:391-
399.
60. Yam JC, Jiang Y, Tang SM, et al. Low-Concentration Atropine for Myopia
Progression (LAMP) Study: A Randomized, Double-Blinded, Placebo-Controlled
Trial of
0.05%, 0.025%, and 0.01% Atropine Eye Drops in Myopia Control. Ophthalmology
2019;126:113-124.
61. Whatham AR, Lunn D, Judge SJ. Effects of Monocular Atropinization on
Refractive
Error and Eye Growth in Infant New World Monkeys. Invest Ophthalmol Vis Sci
2019;60:2623-2630.
62. Trier K, Olsen EB, Kobayashi T, Ribel-Madsen SM. Biochemical and
ultrastructural
changes in rabbit sclera after treatment with 7-methylxanthine, theobrornine,
acetazolamide,
or L-ornithinc. Br J Ophthalmol 1999;83:1370-1375.
63. Trier K, Munk Ribel-Madsen S, Cui D, Brogger Christensen S. Systemic 7-
methylxanthine in retarding axial eye growth and myopia progression: a 36-
month pilot
study. J Ocul Biol Dis lnfor 2008:1:85-93.
22
CA 03182397 2022- 12- 12
WO 2021/252626
PCT/US2021/036614
64. Cui D, Trier K, Zeng J, et al. Effects of 7-methylxanthine on the
sclera in form
deprivation myopia in guinea pigs. Acta Ophthalmol 2011;89:328-334.
65. Nie HH, Huo U. Yang X, et al. Effects of 7-methylxanthine on form-
deprivation
myopia in pigmented rabbits. International journal of ophthalmology 2012;5:133-
137.
66. Hung LF, Arumugam B, Ostrin L, et al. The Adenosine Receptor
Antagonist, 7-
Methylxanthine, Alters Emmetropizing Responses in Infant Macaques. Invest
Ophthalmol
Vis Sci 2018;59:472-486.
67. Cottriall CL, McBrien NA. The M1 muscarinic antagonist pirenzepine
reduces
myopia and eye enlargement in the tree shrew. Invest Ophthalmol Vis Sci
1996;37:1368-
1379.
68. Cottriall CL, Truong HT, McBrien NA. Inhibition of myopia development
in chicks
using himbacine: a role for M(4) receptors? Neuro report 2001;12:2453-2456.
69. Siatkowski RM, Cotter SA, Crockett RS, et al. Two-year multicenter,
randomized,
double-masked, placebo-controlled, parallel safety and efficacy study of 2%
pirenzepine
ophthalmic gel in children with myopia. Journal of AAPOS : the official
publication of the
American Association for Pediatric Ophthalmology and Strabismus /American
Association
for Pediatric Ophthalmology and Strabismus 2008;12:332-339.
70. Stone RA, Liu J, Sugimoto R, Capehart C, Zhu X, Pendrak K. GABA,
experimental
myopia, and ocular growth in chick. Invest Ophthalmol Vis Sci 2003;44:3933-
3946.
71. Cheng ZY, Wang XP, Schmid KL, Han XG. Inhibition of form-deprivation
myopia
by a GABAAOr receptor antagonist, (1,2,5,6-tetrahydropyridin-4-y1)
methylphosphinic acid
(TPMPA), in guinea pigs. Graefes Arch Clin Exp Ophthalmol 2014;252:1939-1946.
72. Cheng ZY, Wang XP, Schmid KL, et al. GABAB receptor antagonist CGP46381
inhibits form-deprivation myopia development in guinea pigs. BioMed research
international
2015;2015:207312.
73. Can BJ, Nguyen CT, Stell WK. Alpha2 -adrenoceptor agonists inhibit form-
deprivation myopia in the chick. Clin Exp Optorn 2019;102:418-425.
74. Liu Y, Wang Y, Lv H, Jiang X, Zhang M, Li X. alpha-adrenergic agonist
brimonidine
control of experimentally induced myopia in guinea pigs: A pilot study. Mo/
Vis
2017;23:785-798.
75. Iuvone PM, Tigges M, Stone RA, Lambert S, Laties AM. Effects of
apomorphine, a
dopamine receptor agonist, on ocular refraction and axial elongation in a
primate model of
myopia. Invest Ophthalmol Vis Sci 1991;32:1674-1677.
23
CA 03182397 2022- 12- 12
WO 2021/252626
PCT/US2021/036614
76. Yan T, Xiong W, Huang F, et al. Daily Injection But Not Continuous
Infusion of
Apomorphine Inhibits Form-Deprivation Myopia in Mice. Invest Ophthohnol Vis
Sci
2015;56:2475-2485.
77. El-Nimri NW, Wildsoet CF. Effects of Topical Latanoprost on Intraocular
Pressure
and Myopia Progression in Young Guinea Pigs. Invest Ophthalmol Vis Sci
2018;59:2644-
2651.
78. Mori K, Kurihara T, Miyauchi M, et al. Oral crocetin administration
suppressed
refractive shift and axial elongation in a murine model of lens-induced
myopia. Sci Rep
2019;9:295.
79. Wong YL, Sabanayagam C, Ding Y, et al. Prevalence, Risk Factors, and
Impact of
Myopic Macular Degeneration on Visual Impairment and Functioning Among Adults
in
Singapore. Invest Ophthalmol Vis Sci 2018;59:4603-4613.
80. Tkatchenko TV, Tkatchenko AV. Pharmacogenomic approach to
antimyopia drug
development: pathways lead the way. Trends Pharmacol Sci 2019;40:834-853.
24
CA 03182397 2022- 12- 12