Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
PROCESSES FOR IMPROVED PERFORMANCE OF
DOWNSTREAM OIL CONVERSION
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] The present application claims priority to U.S. Provisional
Application Number
filed on 63/027052 filed on May, 19, 2020, the contents of which are
incorporated herein in
their entirety.
FIELD OF THE TECHNOLOGY
[0002] The present technology relates to processes for reducing sulfur and
asphaltene
content in hydrocarbon feedstocks as well as other impurities to improve
performance of
downstream oil conversion processes. Both catalytic and thermal conversion
performance
may be improved.
BACKGROUND OF THE TECHNOLOGY
[0003] Hydrocarbon oils, including many oil feedstocks, often contain
difficult-to-
remove impurities such as sulfur in the form of organosulfur compounds as well
as metals
and other heteroatom-containing compounds that hinder usage of the
hydrocarbons. The
undesired impurities present in hydrocarbon oils can be concentrated in the
resins and
asphaltenes found in the vacuum residue distillation fraction, generally
defined by a boiling
point of 510 C to 565 C (950 F to 1050 F) or greater. Traditional refining
configurations
further concentrate the undesired impurities by separating the high value, low
boiling point
distillation fractions (gasoline, diesel, jet, and gasoils) from the low
value, high boiling point
bottoms fractions (atmospheric and vacuum residues). The low boiling point
distillation
fractions can be easily treated and converted into finished products using
established
processes such as hydrotreating, alkylation, catalytic reforming, catalytic
cracking and the
like. High boiling point residuum streams cannot be easily treated because the
disproportionately high metals content fouls catalysts and the polyaromatic
structure of the
asphaltenes hinders access to impurities.
[0004] The sulfur species present in hydrocarbons can be characterized as
asphaltenic
sulfur (i.e., sulfur-containing asphaltene species) and non-asphaltenic sulfur
(i.e., sulfur-
containing non-asphaltene species). Non-asphaltenic sulfur typically includes
thiols, sulfides,
benzothiophene and dibenzothiophene (DBT) derivatives among others, is
primarily located
1
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
in the vacuum residuum fraction, but may also be present in the saturates,
aromatics and resin
components located in any distillation fraction. These sulfur species,
especially those located
within the gasoline, naphtha, kerosene, diesel, and gasoil fractions, can
generally be removed
using conventional catalytic treatment or conversion processes such as
hydrotreating,
hydrodesulfurization or hydrocracking. Asphaltenic sulfur, located in the
asphaltenes within
the heaviest residuum distillation fraction, is primarily characterized by
layers of condensed,
sulfur-containing polynuclear aromatic compounds linked by saturated species
and sulfur.
DBT and DBT derivatives and sulfur bridges may account for a large proportion
of the
asphaltenic sulfur species. Additionally, metals, including nickel, vanadium
and iron, are
often concentrated within porphyrin metal complexes located in the asphaltene
fraction.
Sulfur cannot be easily removed from asphaltenes without subjecting the
asphaltenic sulfur to
severe operating conditions.
[0005] Residuum thermal or catalytic conversion units operate under severe
operating
conditions, typically high temperatures (>350 C/662 F), high hydrogen partial
pressures
(500-3000 psig) and with specialized catalysts that are deactivated by metals
and coke
deposition. Even after subjecting the residuum streams to the most severe
operating
conditions, a fraction of sulfur and metals is not removed and remains in the
oil. As a result,
low value residuum bottoms streams are either 1) converted into asphalt, 2)
processed in a
thermal conversion unit (like a coker) to extract as many high value
intermediates as possible
or 3) blended into high sulfur bunker fuel.
BRIEF SUMMARY OF THE TECHNOLOGY
[0006] Surprisingly, processes for preferentially removing the sulfur and
metals from
sulfur-containing asphaltenes and/or converting a portion of the asphaltene
fraction in
hydrocarbon feedstocks into non-asphaltene liquid products have been
discovered. The
processes provide a converted feedstock with a reduced sulfur (and other
heteroatom) content
and a reduced metals content, especially within the asphaltene fraction. Using
the present
technology, impurities concentrated in an asphaltene fraction of a hydrocarbon
feedstock may
be best removed by contacting such feeds with sodium metal while impurities
concentrated
elsewhere may be best removed by traditional refining processes. Furthermore,
the operation
of downstream process units may be optimized by reducing the high
concentration of
2
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
impurities within the asphaltene fraction, resulting in improved refinery
operability and
profitability.
[0007] Thus, in a first aspect, the present technology provides a process
for improving the
yield of liquid hydrocarbons from a thermal conversion process comprising:
contacting a
hydrocarbon feedstock with an effective amount of sodium metal and an
effective amount of
exogenous capping agent at a temperature of 250-500 C, to produce a mixture of
sodium
salts and a converted feedstock, wherein the hydrocarbon feedstock comprises
hydrocarbons
with a sulfur content of at least 0.5 wt%, an asphaltene content of at least 1
wt% and micro
carbon residue content of at least 5 wt%; the converted feedstock comprises
hydrocarbons
with a sulfur content less than that in the hydrocarbon feedstock, a micro
carbon residue
content less than that in the hydrocarbon feedstock and/or an asphaltene
content less than that
in the hydrocarbon feedstock; and subjecting the converted feedstock to a
thermal conversion
process to produce a gaseous product, a purified product and a residual
product, wherein the
proportion of purified product to residual product is greater than that
produced by subjecting
the hydrocarbon feedstock to the same thermal conversion process. In
embodiments where
the thermal conversion process is, e.g., a coking process, the present process
provides
improved yield of vacuum gasoil, reduced coke losses, and lowers sulfur
content of all
subsequent streams. The lower sulfur content translates into less H2S to be
handled by, e.g., a
Claus plant, lower loads for gasoil hydrotreaters and sweeter coke. Moreover,
the amount of
sodium metal used in the process can be varied to optimize downstream yields
and
economics.
[0008] In a second aspect, the present technology provides a process for
preparing anode
grade coke from dirtier feeds than previously possible. The process comprises:
contacting a
hydrocarbon feedstock with an effective amount of sodium metal and an
effective amount of
exogenous capping agent at a temperature of 250-500 C, to produce a mixture of
sodium
salts and a converted feedstock, wherein the hydrocarbon feedstock comprises
hydrocarbons
with a sulfur content of at least 0.5 wt%, an asphaltene content of at least 1
wt%, a vanadium
content of at least 15 ppm and a micro carbon residue content of at least 5
wt%; the converted
feedstock comprises hydrocarbons with a sulfur content less than that in the
hydrocarbon
feedstock, micro carbon residue less than that in the hydrocarbon feedstock
and/or an
asphaltene content less than that in the hydrocarbon feedstock; and subjecting
the converted
3
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
feedstock to a thermal conversion process to produce a premium anode grade
coke product
with less than 0.5% wt% sulfur and less than 150 ppm vanadium.
[0009] In a third aspect, the present technology provides a process for
preparing needle
grade coke from dirtier feeds than previously possible. The process comprises:
contacting a
hydrocarbon feedstock with an effective amount of sodium metal and an
effective amount of
exogenous capping agent at a temperature of 250 C - 500 C, to produce a
mixture of sodium
salts and a converted feedstock, wherein the hydrocarbon feedstock comprises
hydrocarbons
with a sulfur content of at least 0.5 wt%, an asphaltene content of at least 1
wt%, a nickel
content of at least 10 ppm and a micro carbon residue content of at least 5
wt%; the converted
feedstock comprises hydrocarbons with a sulfur content less than that in the
hydrocarbon
feedstock, micro carbon residue less than that in the hydrocarbon feedstock
and/or an
asphaltene content less than that in the hydrocarbon feedstock; and subjecting
the converted
feedstock to a thermal conversion process to produce a high purity needle coke
product with
less than 0.5 wt% sulfur, less than 0.7 wt% nitrogen, less than 10 ppm nickel,
a coefficient of
thermal expansion greater than 2.5x107/ C and an electrical resistivity of 320
x 106 Ohm-In.
[0010] In a fourth aspect, the present technology provides a process for
improving the
conversion of a hydrocarbon feedstock in catalytic conversion processes. The
process
comprises: combining a hydrocarbon feedstock with an effective amount of
sodium metal and
an effective amount of exogenous capping agent at a temperature of 250 C - 500
C, to
produce a mixture of sodium salts and a converted feedstock, wherein the
hydrocarbon
feedstock comprises hydrocarbons with a sulfur content of at least 0.5 wt%, an
asphaltene
content of at least 1 wt% and a total metal content of at least 100 ppm; the
converted
feedstock comprises a hydrocarbon having a sulfur content less than 0.5 wt%, a
vanadium
content less than 50 ppm, a nickel content less than 50 ppm, a lower
concentration of
asphaltenes than that in the hydrocarbon feedstock, and/or a greater
proportion of lower
boiling point hydrocarbons (<538 C) to residual hydrocarbons (>538 C) than
that in the
hydrocarbon feedstock; optionally subjecting the converted feedstock to a
thermal conversion
process to provide a double-converted product; and subjecting the converted
feedstock or
double-converted feedstock to a catalytic conversion process (e.g., catalytic
hydroprocessing)to produce a fuel grade product without blending or further
conversion
processing. This process improves conversion and performance of hydrocarbon
feeds,
4
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
including residual streams, in downstream catalytic processing by a) improving
catalyst life,
b) providing higher gasoline yield, and c) allowing the coker to be bypassed
completely.
[0011] In a fifth aspect, the present technology provides a process for
producing a low
sulfur fuel-grade product from an out-of-specification hydrocarbon feedstock
with little or no
blending. The process comprises: combining a hydrocarbon feedstock with an
effective
amount of sodium metal and an effective amount of exogenous capping agent at a
temperature of 250 C - 500 C, to produce a mixture of sodium salts and a
converted
feedstock, wherein the hydrocarbon feedstock comprises hydrocarbons with a
sulfur content
of at least 0.5 wt%, an asphaltene content of at least 1 wt% and fails to meet
one or more
fuel-grade specifications selected from the group consisting of viscosity,
density, micro
carbon residue, metals content and cleanliness/compatibility; the converted
product
comprises a hydrocarbon having a sulfur content less than 0.5 wt%, and meets
one or more
fuel grade specifications selected from the group consisting of viscosity,
density, micro
carbon residue, metals content and compatibility; and the fuel-grade
specifications are
viscosity of less than 380 cSt @ 50 C, a density of less than 991 kg/m', a
micro carbon
residue content less than 18 wt%, a vanadium content less than 350 mg/kg and a
cleanliness
spot test result of 1 or 2 as measured by ASTM D4740. For example, low sulfur
bunker fuel
may be prepared via the disclosed method. In some embodiments, the product is
a near fuel
grade product that may be brought up to specification by blending a nominal
amount of
blendstock, e.g., blending 0.5 wt% -10 wt%.
[0012] The foregoing is a summary of the disclosure and thus by necessity
contains
simplifications, generalizations, and omissions of detail. Consequently, those
skilled in the
art will appreciate that the summary is illustrative only and is not intended
to be in any way
limiting. Other aspects, features, and advantages of the processes described
herein, as
defined by the claims, will become apparent in the detailed description set
forth herein and
taken in conjunction with the accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
[0013] In order that the manner in which the above-recited and other features
and advantages
of the technology are obtained will be readily understood, a more particular
description of the
technology briefly described above will be rendered by reference to specific
embodiments
thereof that are illustrated in the appended drawings. Understanding that
these drawings
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
depict only typical embodiments of the technology and are not therefore to be
considered to
be limiting of its scope, the technology will be described and explained with
additional
specificity and detail through the use of the accompanying drawings in which:
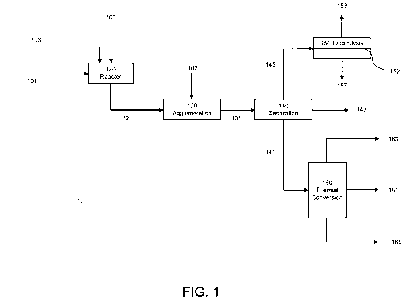
[0014] FIG. 1 is a flow diagram of an illustrative embodiment of a first,
second, or third
aspect of a process of the present technology, including optional separation
and electrolysis
steps.
[0015] FIG. 2 is a flow diagram of an illustrative embodiment of a fourth
aspect of a
process of the present technology, including optional separation and
electrolysis steps.
[0016] FIG. 3 is a flow diagram of another illustrative embodiment of a
fourth aspect of a
process of the present technology, including optional separation and
electrolysis steps.
[0017] FIG. 4 is a flow diagram of an illustrative embodiment of a process
of the present
technology including optional pretreatment steps, and optional separation and
electrolysis
steps, and a refinery processing step.
DETAILED DESCRIPTION OF THE TECHNOLOGY
[0018] The following terms are used throughout as defined below.
[0019] As used herein, singular articles such as "a" and "an" and "the" and
similar
referents in the context of describing the elements (especially in the context
of the following
claims) are to be construed to cover both the singular and the plural, unless
otherwise
indicated herein or clearly contradicted by context. Recitation of ranges of
values herein are
merely intended to serve as a shorthand method of referring individually to
each separate
value falling within the range, unless otherwise indicated herein, and each
separate value is
incorporated into the specification as if it were individually recited herein.
All methods
described herein can be performed in any suitable order unless otherwise
indicated herein or
otherwise clearly contradicted by context. The use of any and all examples, or
exemplary
language (e.g., "such as") provided herein, is intended merely to better
illuminate the
embodiments and does not pose a limitation on the scope of the claims unless
otherwise
stated. No language in the specification should be construed as indicating any
non-claimed
element as essential.
6
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
[0020] As
used herein, "about" will be understood by persons of ordinary skill in the
art
and will vary to some extent depending upon the context in which it is used.
If there are uses
of the term which are not clear to persons of ordinary skill in the art, given
the context in
which it is used, "about" will mean up to plus or minus 10% of the particular
term.
[0021] As
used herein, "asphaltenes" refers to the constituents of oil that are
insoluble in
any of the C3-8 alkanes. Asphaltenes include polyaromatic molecules that
comprise one or
more heteroatoms selected from S, N, and 0. Sulfur species found in
asphaltenes are
collectively referred to herein as "asphaltenic sulfur." All other sulfur
species found in the
non-asphaltenic fractions of hydrocarbon oils and fractions thereof, are
collectively referred
to herein as "non-asphaltenic sulfur." The
latter may include, e.g., thiols, sulfates,
thiophenes, including benzothiophenes and dibenzothiophenes, hydrogen sulfide
and other
sulfides.
[0022] As
used herein, "hydrocarbon feedstocks" refers to any material that may be an
input for refining, conversion or other industrial process in which
hydrocarbons are the
principal constituents. Hydrocarbon feedstocks may be solid or liquid at room
temperature
and may include non-hydrocarbon constituents such as heteroatom-containing
(e.g., S, N, 0,
P, metals) organic and inorganic materials. Crude oils, refinery streams,
chemical plant
streams (e.g. steam cracked tar) and recycling plant streams (e.g., lube oils
and pyrolysis oil
from tires or municipal solid waste) are non-limiting examples of hydrocarbon
feedstocks.
[0023] The
present technology provides processes for improving the yield of downstream
oil conversion processes. Thus, in a first aspect is provided a process for
improving the yield
of liquid hydrocarbons from a thermal conversion process comprising:
contacting a
hydrocarbon feedstock with an effective amount of sodium metal and an
effective amount of
exogenous capping agent at a temperature of 250-500 C, to produce a mixture of
sodium
salts and a converted feedstock. The hydrocarbon feedstock comprises
hydrocarbons with a
sulfur content of at least 0.5 wt%, an asphaltene content of at least 1 wt%
and micro carbon
residue content of at least 5 wt%. The converted feedstock comprises
hydrocarbons with a
sulfur content less than that in the hydrocarbon feedstock, a micro carbon
residue content less
than that in the hydrocarbon feedstock and/or an asphaltene content less than
that in the
hydrocarbon feedstock. Micro carbon residue (MCR), measured according to ASTM
D4530,
indicates the tendency of a hydrocarbon to form carbonaceous deposits after
exposure to high
temperatures. MCR is numerically equivalent to the Conradson carbon residue
(CCR),
7
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
measured according to ASTM D189, and may be used interchangeably. In any
embodiments,
the converted feedstock comprises hydrocarbons with a sulfur content less than
that in the
hydrocarbon feedstock, a micro carbon residue content less than that in the
hydrocarbon
feedstock and an asphaltene content less than that in the hydrocarbon
feedstock. The
converted feedstock is subjected to a thermal conversion process (e.g., a
coking or
visbreaking process) to produce a gaseous product (e.g., steam, H2S, Ci-C4
saturated gases,
C2-C4 olefins and isobutane), a purified product (e.g., naphtha, diesel,
gasoils and light and
heavy cycle oils) and a residual product (e.g., coke or visbreaker tar),
wherein the proportion
of purified product to residual product is greater than that produced by
subjecting the
hydrocarbon feedstock to the same thermal conversion process.
[0024] In
a second aspect, a process for producing premium anode grade coke or needle
coke is provided. The process includes contacting a hydrocarbon feedstock with
an effective
amount of sodium metal and an effective amount of exogenous capping agent at a
temperature of 250-500 C, to produce a mixture of sodium salts and a converted
feedstock.
The hydrocarbon feedstock comprises hydrocarbons with a sulfur content of at
least 0.5 wt%,
an asphaltene content of at least 1 wt%, a vanadium content of at least 15 ppm
and a micro
carbon residue content of at least 5 wt%. The converted feedstock comprises
hydrocarbons
with a sulfur content less than that in the hydrocarbon feedstock, micro
carbon residue less
than that in the hydrocarbon feedstock and/or an asphaltene content less than
that in the
hydrocarbon feedstock. In
any embodiments, the converted feedstock comprises
hydrocarbons with a sulfur content less than that in the hydrocarbon
feedstock, micro carbon
residue less than that in the hydrocarbon feedstock and an asphaltene content
less than that in
the hydrocarbon feedstock. The converted feedstock is subjected to a thermal
conversion
process to produce a premium anode grade coke product with less than 0.5% wt%
sulfur and
less than 150 ppm vanadium.
[0025] In
a third aspect, there is provided a process for producing needle coke,
comprising contacting a hydrocarbon feedstock with an effective amount of
sodium metal
and an effective amount of exogenous capping agent at a temperature of 250 C -
500 C, to
produce a mixture of sodium salts and a converted feedstock. The hydrocarbon
feedstock
comprises hydrocarbons with a sulfur content of at least 0.5 wt%, an
asphaltene content of at
least 1 wt%, a nickel content of at least 10 ppm and a micro carbon residue
content of at least
wt%; the converted feedstock comprises hydrocarbons with a sulfur content less
than 0.5
8
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
wt%, micro carbon residue less than that in the hydrocarbon feedstock, an
asphaltene content
less than 0.25 wt% and/or ash content <0.1 wt%. In any embodiments, the
converted
feedstock comprises hydrocarbons with a sulfur content less than 0.5 wt%,
micro carbon
residue less than that in the hydrocarbon feedstock, an asphaltene content
less than 0.25 wt%
and ash content <0.1 wt%. The converted feedstock is treated in a thermal
conversion process
to produce a high purity needle coke product with less than 0.5 wt% sulfur,
less than 0.7 wt%
nitrogen, less than 10 ppm nickel, a coefficient of thermal expansion greater
than 2.5x107/ C
and an electrical resistivity of 320 x 106 Ohm-In.
[0026] In a fourth aspect, the present technology provides processes for
improving the
conversion of feedstocks in a catalytic conversion or a treatment process. The
process may
include: combining a hydrocarbon feedstock with an effective amount of sodium
metal and
an effective amount of exogenous capping agent at a temperature of 250 C ¨ 500
C, to
produce a mixture of sodium salts and a converted feedstock; optionally
further subjecting
the converted feedstock to a thermal conversion process to provide a double-
converted
product; and subjecting the converted feedstock or double-converted feedstock
to a catalytic
conversion process (e.g., catalytic hydroprocessing, fluid catalytic cracking,
etc.) to produce a
fuel grade product without blending or further conversion processing. In this
process, the
hydrocarbon feedstock comprises hydrocarbons with a sulfur content of at least
0.5 wt%, an
asphaltene content of at least 1 wt% and a total metal content of at least 100
ppm. The
converted feedstock comprises a hydrocarbon having a sulfur content less than
0.5 wt%, a
vanadium content less than 50 ppm, a nickel content less than 50 ppm, a lower
concentration
of asphaltenes than that in the hydrocarbon feedstock, and/or a greater
proportion of lower
boiling point hydrocarbons (<538 C) to residual hydrocarbons (>538 C) than
that in the
hydrocarbon feedstock. In any embodiments, the converted feedstock comprises a
hydrocarbon having a sulfur content less than 0.5 wt%, a vanadium content less
than 50 ppm,
a nickel content less than 50 ppm, a lower concentration of asphaltenes than
that in the
hydrocarbon feedstock, and a greater proportion of lower boiling point
hydrocarbons
(<538 C) to residual hydrocarbons (>538 C) than that in the hydrocarbon
feedstock.
[0027] In the fourth aspect, when the converted feedstock has a micro
carbon residue
content of at least 5 wt%, the process may further include subjecting the
converted feedstock
to a thermal conversion process (e.g., in a coker) to provide the double-
converted feedstock
and a solid coke product. In such embodiments, the double-converted product
comprises a
9
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
liquid hydrocarbon having a lower concentration of impurities than that in the
hydrocarbon
feedstock, and a proportion of lower boiling point hydrocarbons (<538 C) to
higher boiling
point residuum hydrocarbons (>538 C) greater than that of the converted
feedstock. In any
embodiments of the fourth aspect, the fuel grade product may be gasoline,
diesel, kerosene,
jet fuel, petroleum naphtha, or LPG.
[0028] In a fifth aspect, the present technology provides processes for
producing low
sulfur fuel-grade products. The processes include combining a hydrocarbon
feedstock with
an effective amount of sodium metal and an effective amount of exogenous
capping agent at
a temperature of 250 C - 500 C, to produce a mixture of sodium salts and a
converted
feedstock. The hydrocarbon feedstock comprises hydrocarbons with a sulfur
content of at
least 0.5 wt%, an asphaltene content of at least 1 wt% and fails to meet one
or more fuel-
grade specifications selected from the group consisting of viscosity, density,
micro carbon
residue, metals content and cleanliness/compatibility. The converted product
comprises a
hydrocarbon having a sulfur content less than 0.5 wt%, and meets one or more
fuel grade
specifications selected from the group consisting of viscosity, density, micro
carbon residue,
metals content and compatibility, wherein the fuel-grade specifications are
viscosity of less
than 380 cSt @ 50 C, a density of less than 991 kg/m3, a micro carbon residue
content less
than 18 wt%, a vanadium content less than 350 mg/kg and a cleanliness spot
test result of 1
or 2 as measured by ASTM D4740. In some embodiments, the converted product
meets two
or more, three or more, four or more or all five fuel grade specifications. In
some
embodiments, the product is a near fuel grade product that may be brought up
to specification
by blending a nominal amount of blendstock, e.g., blending 0.5 wt%, 1 wt%, 2
wt%, 3 wt%,
4 wt%, 5 wt%, 6 wt%, 7 wt%, 8 wt%, 9 wt%, or 10 wt%, or a range between and
including
any two of the foregoing values such as 0.5-10 wt%, 1-10 wt% or 2-7 wt%.
[0029] In any embodiments of the processes described herein, the processes
may further
include pretreating the hydrocarbon feedstock before the contacting step to
provide a purified
feedstock and a pretreated hydrocarbon feedstock. The purified feedstock
comprises a lower
concentration of impurities than the hydrocarbon feedstock before
pretreatment, the
pretreated hydrocarbon feedstock comprises a higher concentration of
impurities than the
purified feedstock; and the pretreated hydrocarbon feedstock is the feedstock
subjected to the
contacting step to produce the converted feedstock. In any embodiments of the
present
processes including a pretreating step, the pretreatment step may include
phase separation by
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
an externally applied field, separation by addition of heat, hydroconversion,
thermal
conversion, catalytic conversion or treatment, solvent extraction, solvent
deasphalting or a
combination of any two or more thereof In any embodiments, the pretreatment
step may
include contacting the hydrocarbon feedstock with exogenous hydrogen and/or a
catalyst to
remove one or more of sulfur, nitrogen, oxygen, metals and asphaltenes.
Examples of
pretreatment steps to produce a purified feedstock and a pretreated
hydrocarbon feedstock
include atmospheric distillation, vacuum distillation, steam cracking,
catalytic cracking,
thermal cracking, fluid catalytic cracking (FCC), solvent deasphalting,
hydrodesulfurization,
visbreaking, pyrolysis, catalytic reforming, alkylation, and combinations of
any two or more
of the foregoing. It will be understood that certain of the foregoing
processes, such as
atmospheric distillation and vacuum distillation directly yield a purified
feedstock and a
pretreated hydrocarbon feedstock, while others require a subsequent separation
step. For
example, steam cracking, catalytic cracking, thermal cracking, FCC and
pyrolysis yield a
mixture of products that can be subsequently separated into a purified
feedstock and a
pretreated hydrocarbon feedstock by distillation or other separation process.
[0030] In any aspects or embodiments of the processes described herein
including a
thermal conversion process, the thermal conversion process may be or include
visbreaking,
delayed coking, fluid coking, FlexicokingTM, pyrolysis, a variant thereof or a
combination of
any two or more thereof In any embodiments, the thermal conversion process may
be
operated at a temperature about 400 C to about 570 C. In any embodiments, the
thermal
conversion process may be operated at a pressure of about 10 to about 200
psig. In any
embodiments, the thermal conversion process may be operated at about 450 C to
about
500 C and at about 20-100 psig, e.g., as in a coker.
[0031] In any aspects or embodiments of the processes described herein
including a
catalytic conversion process, the catalytic conversion process comprises fluid
catalytic
cracking (FCC), residual FCC, hydrotreating, residual hydrotreating,
hydrocracking, catalytic
reforming, hydrodesulfurization, hydrodenitrogenation, hydrodemetallation, or
residue
upgrading/ hydroconversion (e.g., ARDS , LC-Fining , H-Oil ), their variants
or a
combination of any two or more thereof. The catalyst may comprise cobalt,
molybdenum,
nickel, tungsten, platinum, palladium, alumina, silica, zeolites, their
isomers, oxides, sulfides
or combinations of any two or more thereof. In any embodiments, the catalytic
conversion
process may be operated at a temperature from about 250 C to about 575 C. In
any
11
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
embodiments, the catalytic conversion process may be operated at a pressure of
about 10 to
about 3000 psig. In any embodiments, the catalytic conversion process may be
operated at
about 400 C to about 575 C and at about 1000 to about 3000 psig. In any
embodiments, the
catalytic conversion process may be operated at about 450 C to about 575 C and
at about 15
to about 100 psig.
[0032] In any aspects or embodiments of the processes described herein, it
will be
understood that other refinery processes such as distillation (both
atmospheric or vacuum
distillation) may be employed as part of the process. Alternatively, in
certain aspects or
embodiments, other refinery processes may be used in place of thermal or
catalytic
conversion processes (see, e.g., FIG. 4).
[0033] Hydrocarbon feedstocks for the present processes are or may be
derived from
virgin crude oils (for example petroleum, heavy oil, bitumen, shale oil and
oil shale).
Hydrocarbon feedstocks may also be a residual feedstock, e.g., the product of
a thermal
cracking process. Residual feedstocks may be produced by various pretreatment
processes of
the present technology and will be referred to as "pretreated hydrocarbon
feedstocks," which
may also be contacted with sodium metal and exogenous capping agent. Thus,
pretreated
feedstocks may include distillation products of hydrocarbon feedstocks,
(atmospheric or
vacuum residuums, gasoline, diesel, kerosene, and gas oils), and refinery
intermediate
streams. In any embodiments, the pretreated hydrocarbon feedstock may include
hydrotreated
products, hydrocracker residue, hydroconversion residue (e.g., LCFiner
(Chevron Global
Lummus) residue, or HOil (Axens) residue), FCC slurry, residual FCC slurry,
atmospheric
or vacuum residuums, solvent deasphalting tar, deasphalted oil, steam cracked
tar, visbreaker
tar, high sulfur fuel oil, low sulfur fuel oil, asphaltenes, asphalt and coke.
The foregoing
hydrocarbon feedstocks (including pretreated hydrocarbon feedstocks) may be
derived from
any geological formation (oil sand, conventional or tight reservoirs, shale
oil, oil shale) or
geographical location (North America, South America, Middle East, etc.). In
certain aspects
and embodiments of the present processes, especially the second and third
aspects, the
hydrocarbon feedstock includes a significant amount of aromatic compounds,
e.g., at least 10
wt%, at least 20 wt%, at least 25 wt%, at least 30 wt%,
[0034] In processes of the present technology, the hydrocarbon feedstock
includes
hydrocarbons (e.g., a hydrocarbon oil) and impurities. Similarly, the residual
feedstock
includes hydrocarbons and impurities. In some embodiments, the residual
feedstock has a
12
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
higher concentration of impurities than the hydrocarbon feedstock.
"Impurities" as used
herein refer to heteroatoms (i.e., atoms other than carbon and hydrogen), such
as sulfur,
oxygen, nitrogen, phosphorous, and metals. Impurities may be found in or
include
substances such as naphthenic acids, water, ammonia, hydrogen sulfide, thiols,
thiophenes,
benzothiophenes, porphyrins, Fe, V, Ni, and the like. In any embodiments of
the present
processes, the hydrocarbon feedstock or residual feedstock includes
hydrocarbons with a
sulfur content of at least 0.5 wt% and an asphaltene content of at least 1
wt%. The sulfur
content comprises asphaltenic sulfur and non-asphaltenic sulfur, but is
measured as the wt%
of sulfur atoms in the feedstock. In any embodiments, the sulfur content may
range from 0.5
wt% to 15 wt%, including for example, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5,
6, 7, 8, 9, 10, 11,
12, 13, 14, or 15 wt% or a range between and including any two of the
foregoing values.
Thus, the sulfur content may range may be, in any embodiments, 1 wt% to 15
wt%, 0.5 wt%
to 8 wt%, or 1.5 wt% to 10 wt%.
[0035] In processes of the present technology, the asphaltene content
refers to the total
amount of asphaltenes in a feedstock measured as the n-pentane insoluble
fraction of the
feedstock. However, in some aspects or embodiments of the present processes,
the
asphaltene content may be measured as the insoluble fraction precipitated or
otherwise
separated from the feedstock, after mixing with a sufficient quantity of one
or more C3-8
alkanes. The C3-8 alkanes may be propane, butane, pentane, hexane, heptane,
octane, isomers
thereof, or mixtures of any two or more thereof. In any embodiments, the
asphaltene content
of a feed may be defined as the constituents insoluble in heptane. A detailed
discussion of
the physical properties and structure of asphaltenes and the process
conditions (temperatures,
pressures, solvent/oil ratios) required to produce a specific asphaltene is
described in J.G.
Speight, "Petroleum Asphaltenes Part 1: Asphaltenes, Resins and the Structure
of
Petroleum", Oil & Gas Science and Technology - Rev IFP, Vol 59 (2004) pp. 467-
477
(incorporated herein by reference in its entirety and for all purposes). The
standard test
method for determining heptane (C7) insoluble asphaltene content is described
by ASTM
standard D6560-17 and can be extended to any alkane, including pentane.
[0036] In any embodiments of the present processes, the asphaltene content
of
hydrocarbon feedstock or residual feedstock may be at least 1 wt%, at least 2
wt%, at least 3
wt%, at least 4 wt% or at least 5 wt%. For example, the asphaltene content may
range from 1
wt% to 100 wt%, such as 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 25, 30, 35, 40,
45, 50, 55, 60, 70,
13
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
80, 90, 95 or 100 wt% or between and including any two of the foregoing
values. Thus, in
any embodiments, the asphaltene content may range from 2 wt% to 100 wt %, 1
wt% to 30
wt%, 2 wt% to 30 wt%, 5 wt% to 100 wt%, 10 wt% to 100 wt%, or 20 wt% to 100
wt%.
[0037] In any embodiments of the present process, it may be necessary to
dilute the
hydrocarbon feedstock with a diluent if an elevated asphaltene content in the
hydrocarbon
feedstock leads to a viscosity that is too high for the sodium treatment
process. Because of
the aromatic nature of asphaltenes, a diluent will typically include aromatics
(i.e., compounds
having aromatic rings). The diluent may be a single compound (e.g., benzene,
toluene,
xylene, ethylbenzene, cumene, naphthalene, 1-methylnaphthalene), mixtures of
any two or
more thereof, or a refinery intermediate that is aromatic (e.g., light cycle
oil, reformate). The
amount of diluent needed will vary with the asphaltene content of the
feedstock and the
viscosity required for processing. Higher asphaltene content in a feedstock
may require more
diluent than a feedstock with lower asphaltene content. It is within the skill
in the art to
select an appropriate amount of diluent to permit processing of asphaltenes in
the present
processes.
[0038] The present processes may also reduce/remove the naphthenic acid
content and/or
the metals content in converted feedstocks compared to the hydrocarbon and
pretreated
hydrocarbon feedstocks. In any embodiments the hydrocarbon feedstock or
pretreated
hydrocarbon feedstock includes (on an aggregate or individual basis) about 1
to about 10,000
ppm metals. The metals may be naturally occurring metals bound to the
hydrocarbon
structure or residual metal fragments entrained in the pretreated hydrocarbon
feedstock
during upstream processing (e.g., corrosion products or catalyst fragments).
In any
embodiments, the metal is selected from the group consisting alkali metals,
alkali earth
metals, transition metals, post transition metals, and metalloids having an
atomic weight
equal to or less than 82. In any embodiments, the metal is selected from the
group of
vanadium, nickel, iron, arsenic, lead, cadmium, copper, zinc, chromium,
molybdenum,
silicon, calcium, potassium, aluminum, magnesium, manganese, titanium, mercury
and
combinations of any two or more thereof In any embodiments, the metal is
selected from the
group consisting of vanadium, nickel, iron, and combinations of any two or
more thereof. In
any embodiments, the metals concentration of the hydrocarbon feedstock or the
pretreated
hydrocarbon feedstock may be (in the aggregate or on an individual basis)
about 2 to about
14
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
10,000 ppm, about 10 to about 10,000 ppm, about 100 to about 10,000 ppm, about
100 to
about 5,000 ppm, about 10 to about 1,000 ppm, about 100 to about 1,000 ppm,
and the like.
[0039] The processes of the present technology not only upgrade the
hydrocarbon and
pretreated hydrocarbon feedstocks employed by removal/reduction of impurities,
but may
also improve physical properties such as viscosity and density. The
hydrocarbon feedstock or
pretreated hydrocarbon feedstock may have a viscosity between 1 to 10,000,000
cSt at 50 C.
For example, the viscosity may 1, 10, 25, 50, 100, 200, 300, 400, 500, 1,000,
2,000, 5,000,
10,000, 25,000, 50,000, 100,000, 500,000, 1,000,000, 2,000,000, 3,000,000,
4,000,000,
5,000,000, 6,000,000, 7,000,000, 8,000,000, or 9,000,000 cSt or a range
between and
including any two of the foregoing values. Thus, in any embodiments, the
viscosity of the
hydrocarbon feedstock or the pretreated hydrocarbon feedstock may be, e.g.,
100 to
10,000,000 cSt, 380 to 9,000,000 cSt, 500 to 9,000,000 cSt, or 500 to
5,000,000 cSt, among
others.
[0040] The hydrocarbon feedstock or pretreated hydrocarbon feedstock may a
density
from 800 to 1200 kg/m3 at 15.6 C or 60 F. For example, the density may be
800, 825, 850,
875, 900, 925, 975, 1000, 1050, 1100, 1150, or 1200 kg/m3 or a range between
and including
any two of the foregoing values. Thus, in any embodiments, the density may be,
e.g., from
850 to 1200 kg/m3, 900 to 1200 kg/m3, 950 to 1200 kg/m3, or 925 to 1100 kg/m3.
[0041] In processes of the present technology, the hydrocarbon feedstock or
pretreated
hydrocarbon feedstock is contacted with an effective amount of sodium metal
and an
effective amount of exogenous capping agent. Any suitable source of sodium
metal may be
used, including, but not limited to electrochemically generated sodium metal,
e.g., as
described in US 8,088,270, incorporated by reference in its entirety herein.
By "effective
amount" is meant the amount of a material or agent to bring about a desired
consequence.
For example, an effective amount of sodium metal in the present processes may
include a
stoichiometric, suprastoichiometric or substochiometric amount of sodium metal
sufficient to
reduce the amount of asphaltene and/or sulfur in the hydrocarbon feedstock.
[0042] The exogenous capping agent used in the present processes is
typically used to
cap the radicals formed when sulfur and other heteroatoms have been abstracted
by the
sodium metal during the contacting step. Although some feedstocks may
inherently contain
small amounts of naturally occurring capping agents ("endogenous capping
agents"), such
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
amounts are insufficient to cap substantially all of the free radicals
generated by the present
processes. Effective amounts of exogenous (i.e., added) capping agents are
used in the
present processes, such as 1-1.5 moles of capping agent (e.g., hydrogen) may
be used per
mole of sulfur, nitrogen, or oxygen present. It is within the skill of the art
to determine an
effective amount of exogenous capping agent needed to carry out the present
processes for
the particular hydrocarbon feedstock or pretreated hydrocarbon feedstock being
used based
on the disclosure herein. The exogenous capping agent may include hydrogen,
hydrogen
sulfide, natural gas, methane, ethane, propane, butane, pentane, ethene,
propene, butene,
pentene, dienes, isomers of the forgoing, or a mixture of any two or more
thereof In any
embodiments, the exogenous capping agent may be hydrogen and/or a C1-6 acyclic
alkanes
and/or C2-6 acyclic alkene or a mixture of any two or more thereof.
[0043] The effective amount of sodium in its metallic state and used in the
contacting
step will vary with the level of heteroatom, metal, and asphaltene impurities
of the
hydrocarbon and pretreated hydrocarbon feedstocks, the desired extent of
conversion or
removal of an impurity, the temperature used and other conditions. In any
embodiments,
stoichiometric or greater than stoichiometric amounts of sodium metal may be
used to
remove all or nearly all sulfur content, e.g., 1-3 mole equivalents of sodium
metal versus
sulfur content. In any embodiments, the hydrocarbon feedstock or pretreated
hydrocarbon
feedstock is contacted with more than 1 mole equivalent of sodium metal versus
the sulfur
content therein, e.g., 1.1, 1.15, 1.2, 1.25, 1.3, 1.4, 1.5, 2, 2.5 or 3 mole
equivalents of sodium
metal.
[0044] Surprisingly, a sub-stoichiometric ratio of metallic sodium to
sulfur content (in the
hydrocarbon/pretreated hydrocarbon feedstocks) may be used to preferentially
lower the
amount of asphaltenic sulfur versus the non-asphaltenic sulfur. Thus, in any
embodiments,
the pretreated hydrocarbon feedstock (or alternatively the hydrocarbon
feedstock) may be
contacted with a less than stoichiometric amount of sodium metal to the sulfur
content
therein. In the present technology, it will be understood that the
stoichiometric amount of
sodium metal to sulfur content is the theoretical amount of sodium metal
required to convert
all sulfur content in the pretreated hydrocarbon (or hydrocarbon) feedstock to
sodium sulfide.
For example, it will be appreciated by those of skill in the art that the
stoichiometric amount
of sodium metal necessary to convert all of the sulfur to sodium sulfide in a
feedstock
containing about 1 mole of sulfur atoms is 2 moles of sodium metal. A less
than
16
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
stoichiometric amount of sodium metal to sulfur content in such an example
would be less
than 2 moles of sodium metal, such as 1.6 moles, or 0.8 mole equivalents of
sodium metal.
In any embodiments, the less than stoichiometric amount of sodium metal to
sulfur content
may be 0.1 equivalents to less than 1 equivalent. Examples of such sub-
stoichiometric
amounts include 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9 or less than 1
equivalents of sodium
metal to sulfur content or a range between and including any two of the
foregoing values.
Thus, in any embodiments the sub-stoichiometric amounts may range from 0.1 to
0.9
equivalents, 0.2 to 0.8 equivalents, 0.4 to 0.8 equivalents, or the like.
[0045] As the contacting step takes place at a temperature of about 250 C
to about
500 C, the sodium metal will be in a molten (i.e., liquid) state. For example
the contacting
step may be carried out at about 250 C, about 275 C, about 300 C, about 325 C,
about
350 C, about 375 C, about 400 C, about 425 C, about 450 C, about 500 C, or a
range
between and including any two of the foregoing temperatures. Thus, in any
embodiments the
contacting may take place at about 275 C to about 425 C, or about 300 C to
about 400 C
(e.g., at about 350 C).
[0046] In any embodiments, the contacting step may take place at a pressure
of about 400
to about 3000 psi, e.g., at about 400 psi, about 500 psi, about 600 psi, about
750 psi, about
1000 psi, about 1250 psi, about 1500 psi, about 2000 psi, about 2500 psi,
about 3000 psi or a
range between and including any two of the foregoing values.
[0047] The reaction of sodium metal with heteroatom contaminants in the
hydrocarbon/pretreated hydrocarbon feedstocks is relatively fast, being
complete within a
few minutes, if not seconds. Mixing the combination of feedstock and metallic
sodium
further speeds the reaction and is commonly used for this reaction on the
industrial scale.
However, certain embodiments may require an extended residence time to improve
the extent
of conversion or adjust the operating conditions to target removal of a
specific heteroatom
impurity. Hence, in any embodiments the contacting step is carried out for
about 1 minute to
about 120 minutes, e.g., about 1, about 5, about 7, about 9, about 10, about
15 minutes, about
30, about 45 about 60, about 75, about 90, about 105, or about 120 minutes, or
is conducted
for a time ranging between and including any two of the foregoing values.
Thus, in any
embodiments the time may range from about 1 to about 60 minutes, about 5
minutes to about
60 minutes, about 1 to about 15 minutes, about 60 minutes to 120 minutes, or
the like.
17
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
[0048] The
present processes produce a converted feedstock that include a hydrocarbon
oil with a sulfur content less than that in the hydrocarbon feedstock (or
pretreated
hydrocarbon feedstock). In any embodiments, the sulfur content of the
converted feedstock
may be less than 0.5 wt%, e.g., less than or about 0.4 wt%, less than or about
0.3 wt%, less
than or about 0.2 wt%, less than or about 0.1 wt%, and even less than or about
0.05 wt%, or a
range between and including any two of the foregoing values. Removal
efficiency of the
sulfur content (a.k.a., conversion efficiency) from the hydrocarbon or
pretreated hydrocarbon
feedstock compared to the converted feedstock may be at least 40%, at least
50%, at least
60%, at least 70%, at least 80%, at least 90%, at least 95%, at least 96%, at
least 97%, at least
98%, at least 99% or 100% by weight, or a range between and including any two
of the
foregoing values. Where the effective amount of sodium metal is greater than a
stoichiometric amount, the sulfur content conversion efficiency can be very
high, e.g., at least
90%.
[0049]
When sub-stoichiometric amounts of sodium metal are used in the present
processes (including but not limited to processes of the first, second, third
and fourth
aspects), lower conversion efficiencies are observed, but the sulfur content
from asphaltenic
sulfur is preferentially reduced compared to that from non-asphaltenic sulfur.
For example,
the (total) sulfur content conversion efficiency may range from about 10% to
about 80%,
including, about 10%, about 20%, about 30%, about 40%, about 50%, about 60%,
about
70%, about 80%, or a range between and including any two of the foregoing
values. At the
same time, the corresponding sulfur content conversion efficiency of
asphaltenic sulfur is
higher at each point than the total sulfur-conversion efficiency. For example
sulfur content
conversion efficiency of asphaltenic sulfur for any given feed may range from
1% to 40%
higher (e.g., 1%, 2%, 3%, 4%, 5%, 6%, 7%, 8%, 9%, 10%, 11%, 12%, 13%, 14%,
15%,
16%, 17%, 18%, 19%, 20%, 22%, 24%, 25%, 27%, 30%, 32%, 35%, 37%, or 40% higher
or
a range between and including any two of the foregoing values) than the
corresponding
overall sulfur content conversion efficiency.
[0050] The
converted feedstocks of the present technology have a reduced concentration
of metals compared to the hydrocarbon or pretreated hydrocarbon feedstocks.
The metals
content of the converted feedstock may be reduced by at least 20% compared to
the
hydrocarbon feedstock or pretreated hydrocarbon feedstock, for example, by 20%
to 100%.
Examples of the percent reduction in metals (collectively or individually) in
the converted
18
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
feedstock compared to the hydrocarbon feedstock or the pretreated hydrocarbon
feedstock
include 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 95%, 97%, 98%, 99%, 100%, or a
range between and including any two or more of the foregoing values. Thus, in
any
embodiments the percent reduction may be from 20% to 99%, from 20% to 95%,
from 70%
to 99% or to 100%. The metals may be any of those disclosed herein. In some
embodiments,
the metals are selected from iron, vanadium, nickel or combinations of any two
or more
thereof. For example, the iron and vanadium content of the converted feedstock
has been
reduced by at least 20% compared to the hydrocarbon feedstock or pretreated
hydrocarbon
feedstock. Similarly, in any embodiments, the nickel content of the converted
feedstock has
been reduced by at least 20% compared to the hydrocarbon feedstock or
pretreated
hydrocarbon feedstock.
[0051] The present processes also provide converted feedstocks with
improved physical
properties compared to the hydrocarbon feedstock or pretreated hydrocarbon
feedstock.
However, it has been discovered that the physical properties of converted
feedstocks of the
present processes do not necessarily change proportionately to the sodium to
sulfur ratio. For
example, the extent of metals demetallization, especially metals detrimental
to catalyst life
including iron, vanadium and nickel will generally be greater than the extent
of
desulfurization for a given sodium to sulfur ratio. Example 6 demonstrates the
insensitivity of
sodium treatment to initial metals content, unlike catalytic conversion
processes. Sodium
demetallization at low sodium/total sulfur addition ratio could be highly
advantageous for
pre-treating a hydrocarbon feed with an undesirably high metals content prior
to catalytic
conversion or treatment.
[0052] Additional physical properties that greatly reduce the value of
heavy residual
feedstocks are improved after treatment with sodium. Desulfurization of the
asphaltene
fraction occurs without the hydrogen saturation observed in hydroconversion or
the carbon
rejection exhibited by thermal cracking processes. As a result, at least a
portion of the
asphaltene content is converted by the present processes into a soluble,
stable and
desulfurized converted liquid product, increasing the yield of higher value
liquid products
(e.g., hydrocarbon oils derived from asphaltenes. Thus, the converted
feedstock produced by
the present processes may have an asphaltene content less than that in the
hydrocarbon
feedstock (or pretreated hydrocarbon feedstock). In any embodiments, the
present processes
convert at least some asphaltenes to a hydrocarbon oil, such as paraffins. In
any
19
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
embodiments, at least 5%, at least 10%, at least 15%, at least 20% or more of
the asphaltene
content in the pretreated hydrocarbon feedstock is converted to a liquid
hydrocarbon oil in the
converted feedstock. Conversion efficiency for the asphaltene content removed
from the
hydrocarbon or pretreated hydrocarbon feedstocks varies with the amount of
sodium used,
but is generally high, e.g., at least 70%, at least 75%, at least 80%, at
least 85%, at least 90%,
at least 95%, up to 98%, up to 99% or even up to 99.9% or 100%, or a range
between and
including any two of the foregoing values (e.g., 70% to 100%, or 75% to 99.9%,
etc.).
[0053] The conversion of asphaltenes to smaller, lower molecular weight
components
with fewer attached functional groups typically results in a reduction in
viscosity of at least
40% to as much as 5 orders of magnitude (10000x) and an increase in the API
gravity by
about 1 to about 3 units for each wt% sulfur removed. In any embodiments, the
viscosity of
the converted feedstock may be reduced by at least 50 cSt at 50 C or by at
least 40%. In any
such embodiments, the viscosity is reduced at 50 C by at least 100 cSt, at
least 200 cSt, at
least 300 cSt, or more. For any of the hydrocarbon feedstocks or pretreated
hydrocarbon
feedstocks disclosed herein with viscosities above 1,000 cSt (see above), the
reductions are
particularly great and may be at least 50%, at least 60%, at least 70%, at
least 80%, at least
90%, at least 95%, at least 99%, or even 100% (e.g., at least a 40 to 99%
reduction in
viscosity. In any embodiments, the density of the converted feedstock is
decreased by about
to about 25 kg/m' per wt% reduction in sulfur content of the converted
feedstock compared
to the hydrocarbon feedstock or pretreated hydrocarbon feedstock. For example,
the decrease
in density may be about 5, about 10, about 15, about 20, about 25 kg/m3 or a
range between
and including any two of the foregoing values (such as about 5 to about 20
kg/m' or about 10
to about 25 kg/m3, etc.).
[0054] As noted above, in any embodiments, the present processes may
include
pretreating a hydrocarbon feedstock containing impurities prior to contacting
with sodium
metal. In some cases, a hydrocarbon feedstock may be pretreated to concentrate
the
impurities in the pretreated hydrocarbon feedstock and therefore reduce the
volume of
feedstock to process. For example, a virgin crude oil may be distilled to
produce one or more
light distillate cuts as the purified feedstock and an atmospheric residuum
(the pretreated
hydrocarbon feedstock) with a higher sulfur content and higher asphaltene
content than that
in both the purified feedstock and the virgin crude (hydrocarbon feedstock).
Alternatively, a
hydrocarbon feedstock may be pre-treated to remove a portion of the undesired
impurities to
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
provide for a purified feedstock with a lower concentration of impurities and
a pretreated
hydrocarbon feedstock with impurities that remain after pre-treatment. The
pretreated
hydrocarbon feedstock may comprise impurities because of the chosen level of
conversion or
because the pre-treatment process cannot remove the impurity. For example, a
vacuum
residuum may be treated in a hydroprocessing reactor (such as an LC-Fining
unit or HOil
unit) to remove sulfur and convert the residuum fraction to higher value
products. However,
after hydroprocessing at operating conditions exceeding 350 C and 1500-3000
psig in the
presence of catalysts, a recalcitrant sulfur and asphaltene fraction remains
in the
hydroprocessed bottoms stream. The pre-treatment process may comprise a
separation
process, a thermal or catalytic conversion process or a treatment process, or
combinations of
any two or more thereof.
[0055] In any embodiments, the pretreatment process may include a
separation process
that comprises one or more of a physical separation using energy (heat), phase
addition
(solvent or absorbent), a change in pressure, or application of an external
field or gradient to
concentrate the impurity in the pretreated hydrocarbon feedstock. The
separation process may
include gravity separation, flash vaporization, distillation, condensation,
drying, liquid-liquid
extraction, stripping, absorption, centrifugation, electrostatic separation
and their variants.
The separation process may further comprise solvent extraction processes,
including solvent
deasphalting processes, such as Residuum Oil Supercritical Extraction (ROSE ).
For
example, a hydrocarbon feedstock may be desalted to remove salt and water, an
API
separator may be used to separate water and solids from oil or a distillation
column may be
used to separate low sulfur, low boiling point products from high sulfur, high
boiling point
products in crude oil. The separation process may also require a solid agent
or barrier, such as
adsorption, filtration, osmosis or their variants. Each of the disclosed
separation processes
results in a purified feedstock with a lower concentration of impurities than
the hydrocarbon
feedstock and a pretreated hydrocarbon feedstock with a higher concentration
of impurities
than the purified feedstock. In any embodiments, the pretreated hydrocarbon
feedstock
comprises impurities at a higher concentration than in the hydrocarbon
feedstock.
[0056] In any embodiments, the pretreatment process may include thermal or
catalytic
processes that modifies the molecular structure or results in rejection of at
least a portion of
the carbon content of the hydrocarbon feedstock. The thermal conversion
process may
include a coker, a visbreaker or other process to increase the yield of
cracked distillates by
21
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
rejecting carbon as coke. The catalytic processes may include fixed bed and
fluidized bed
processes such as, but not limited to catalytic crackers (FCC or Residuum
FCC),
hydrocrackers, residuum hydrocrackers and hydroconversion (e.g., LC Fining , H-
0i1 ).
The conversion process may be a hydroprocessing process that requires both
hydrogen and
catalysts.
[0057] The
pretreatment step of the present processes may include a treatment process
that results in hydrocarbon saturation or removal of a specific impurity on a
whole feed basis.
Thus, in any embodiments, the pretreating process may include solvent
deasphalting,
hydrotreating, residuum hydrotreating
(RHT), hy drode sul furi z ati on (RD S),
hydrodemetallization (HDM) or hydrodenitrification (HDN) or a combination of
two or more
thereof. While the overall concentration of an impurity (or impurities) may be
reduced,
treatment processes generally produce a purified feedstock with a lower
concentration of
impurities than the hydrocarbon feedstock and a pretreated hydrocarbon
feedstock with a
higher concentration of impurities than the purified feedstock. Nevertheless,
the
concentration of impurities in the pretreated hydrocarbon feedstock may be
lower than the
hydrocarbon feedstock. Additionally, catalytic treatment processes cannot
typically process
feedstocks with high concentrations of impurities in asphaltenes because of
accelerated
catalyst deactivation from the metals and micro-carbon residue.
[0058]
Processes of the present technology produces a mixture that includes the
converted feedstock and sodium salts. The present processes may further
include separating
the sodium salts from the converted feedstock. The sodium salts are comprised
of particles,
which can be quite fine (e.g., < 10 [tm) and cannot be completely removed by
standard
separation techniques (e.g., filtration or centrifugation). In any
embodiments, the separating
may include a. heating the mixture of sodium salts and converted feedstock
with elemental
sulfur to a temperature from about 150 C to 500 C to provide a sulfur-treated
mixture
comprising agglomerated sodium salts; and separating the agglomerated sodium
salts from
the sulfur treated mixture, to provide a desulfurized liquid hydrocarbon and
separated sodium
salts. This separation may be carried out as described in US Patent No.
10,435,631, the entire
contents of which are incorporated by reference herein for all purposes.
[0059] The
present processes may further include recovering metallic sodium from the
separated sodium salts. In any embodiments, the present processes may further
include
22
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
electrolyzing the separated sodium salts to provide sodium metal. The
separated sodium salts
may comprise one or more of sodium sulfide, sodium hydrosulfide, or sodium
polysulfide.
The electrolyzing may be carried out in an electrochemical cell in accordance
with, e.g., US
Patent No. 8,088,270, or US Provisional Patent Application No. 62/985,287, the
entire
contents of each of which are incorporated by reference herein for all
purposes. The
electrochemical cell may include an anolyte compartment, a catholyte
compartment, and a
NaSICON membrane that separates the anolyte compartment from the catholyte
compartment. A cathode comprising sodium metal is disposed in a catholyte in
the catholyte
compartment. An anode comprising the sodium salts are disposed in anolyte in
the anolyte
compartment. An electrical power supply is electrically connected to the anode
and cathode.
In any embodiments, the separated sodium salts are dissolved in an organic
solvent prior to
electrolyzing the salts to provide sodium metal.
[0060] Current thermal and catalytic desulfurization processes produce a
hydrogen
sulfide byproduct that must be treated in a sulfur recovery unit such as a
Claus plant. Sulfur
recovery units (SRU) are very efficient, but release sulfur emissions during
operation;
therefore, refining complexes are subject to stringent sulfur emission limits
that are regulated
by local and national authorities. In many cases, refining complexes operate
near or at their
sulfur emission limits. Desulfurization using sodium produces an elemental
sulfur product
that can be stored as a solid or liquid and sold to the market. Each
organically bound sulfur
removed using sodium displaces an equivalent amount of sulfur as H25 that must
be
processed in the SRU. As a result, a refining complex gains operational
flexibility to either
reduce the throughput and operating cost of (and resulting sulfur emissions
from) the existing
SRU or to increase the sulfur processing capacity of the facility by
desulfurizing at least a
part of the hydrocarbon feedstock with sodium. In any embodiments, the present
processes
include a sulfur recovery step employing a sulfur recovery unit (e.g., Claus
Plants, SCOT
units, or the like). In any such embodiments, the capacity of the sulfur
recovery unit is
increased proportionately to the sulfur recovered during treatment of the
hydrocarbon
feedstock with sodium.
[0061] Illustrative embodiments of processes of the present technology will
now be
described with reference to the flow diagrams of FIGS. 1-4. With respect to
the purification
and conversion system 10 of FIG. 1, the hydrocarbon feedstock 101, containing
sulfur and
asphaltene impurities as described herein (e.g., a sulfur content of at least
0.5 wt% (herein,
23
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
"wt%" means "weight percent") and an asphaltene content of at least 1 wt%), is
charged to
reactor 120 (continuous or batch) along with effective amounts of sodium metal
103 and an
exogenous capping agent 105 as described herein. The reaction may be carried
out at
elevated temperature and pressure as described herein and is typically
complete within
minutes to give a mixture 121 of sodium salts and converted feedstock,
although higher
asphaltene-containing feeds may require longer times as disclosed herein. The
converted
feedstock includes a hydrocarbon oil with a sulfur content less than that in
the hydrocarbon
feedstock and may include an asphaltene content less than that in the
hydrocarbon feedstock
as described herein. Additionally, the proportion of asphaltenic sulfur to non-
asphaltenic
sulfur in the converted feedstock is lower than in the hydrocarbon feedstock.
Optionally, the
mixture 121 is transported from the reactor 120 to another vessel 130 where
the sodium salts
are agglomerated to particles large enough to be easily separated from the
converted
feedstock. Although any suitable agglomeration method may be used,
agglomeration with
elemental sulfur 107 at elevated temperature as described herein may be used.
The resulting
mixture 131 of agglomerated sodium salts, metals and converted feedstock may
then be
separated by any suitable process and device 140, such as by a centrifuge, to
give the
converted feedstock 141, free of metals 143 and sodium salts 145. Optionally,
as described
herein, the sodium salts 145 may be subjected to electrolysis in an
electrolytic cell 150 with a
sodium ion-selective ceramic membrane 152 such as a NaSiCON membrane to
provide
sodium metal 153 and elemental sulfur 157. The sodium metal 153 and elemental
sulfur 157
may be reused as 103 and 107, respectively, in the present process. The
converted feedstock
141 may be subjected to a thermal conversion process, 160, e.g., a coking
process, a
visbreaking process, or other such processes as described herein, to provide a
purified
product 161 (e.g., naphtha, diesel, gasoils and light and heavy cycle oils), a
gaseous product
163 (e.g., steam, H2S, Ci-C4 saturated gases, C2-C4 olefins and isobutane),
and a residual
product 165 (e.g., coke or visbreaker tar). The proportion of purified product
161 to residual
product 165 is greater than that produced by subjecting the hydrocarbon
feedstock 101 to the
same thermal conversion process without first desulfurizing the feed using
sodium as
described herein.
[0062] In some embodiments of the present processes utilizing the
purification and
conversion system 10 of FIG. 1, the hydrocarbon feedstock 101 comprises
hydrocarbons with
a sulfur content of at least 0.5 wt%, an asphaltene content of at least 1 wt%,
a vanadium
content of at least 15 ppm and a micro carbon residue content of at least 5
wt%. The
24
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
converted feedstock 121/141 comprises hydrocarbons with a sulfur content less
than that in
the hydrocarbon feedstock 101, micro carbon residue less than that in
feedstock 101, and may
contain an asphaltene content less than that in the hydrocarbon feedstock 101.
After
subjecting the converted feedstock 141 to a suitable thermal conversion
process, a premium
anode grade coke may be produced with less than 0.5% wt% sulfur and less than
150 ppm
vanadium.
[0063] FIG. 2 illustrates another process embodiment of the present
technology using the
purification and conversion system 20. The impure hydrocarbon feedstock 201
may include
hydrocarbons with a sulfur content of at least 0.5 wt%, an asphaltene content
of at least 1
wt% and a total metal content of at least 100 ppm. This feedstock is charged
to a reactor 220,
along with sodium metal 203 and an exogenous capping agent 205, analogous to
the process
illustrated in FIG. 1 and as described herein. The resulting mixture 221 of
sodium salts and
converted feedstock may be processed to agglomerate 230 (with elemental sulfur
207) and
separate 240 the sodium salts 245 from the converted feedstock 241 as
described herein. The
converted feedstock 241 includes hydrocarbons having a sulfur content less
than 0.5 wt%, a
vanadium content less than 50 ppm, a nickel content less than 50 ppm, a lower
concentration
of asphaltenes than that in the hydrocarbon feedstock, and/or a greater
proportion of lower
boiling point hydrocarbons (<538 C) to residual hydrocarbons (>538 C) than
that in the
hydrocarbon feedstock 201. Again, the sodium salts 245 may be electrolyzed 250
to provide
sodium metal 253 and elemental sulfur 257 as described herein. The converted
feedstock
241, is then subjected to a catalytic conversion process (such as
hydrotreatment 270 using
hydrogen 265), or other such processes as described herein. Fuel grade
products 272 are
produced in this fashion.
[0064] Alternatively, as shown in FIG. 3, the same process embodiment may
be carried
out using purification and conversion system 30, but employing an optional
thermal
conversion step 360 to produce a double converted product 361, which is then
subjected to a
catalytic conversion process 370 using hydrogen to again produce fuel grade
products 372.
Analogously to FIG. 2, the thermal conversion process 360 also produces,
gaseous product
363 and residual product 365.
[0065] FIG. 4 illustrates another process embodiment of the present
technology using the
purification and conversion system 40, wherein the impure hydrocarbon
feedstock 401 is
pretreated in a process/device 400 to provide a first residual feedstock 402
and a purified
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
feedstock 404. Any suitable pretreatment process resulting in a first residual
feedstock 402
and a first purified feedstock 404 may be used as described herein. The
residual feedstock
402 may optionally be further pretreated (410) to provide a second residual
feedstock 411 and
a purified feedstock 413. Optionally, one or more impurities (e.g., gaseous
impurities such as
H2S, NH3, water, light hydrocarbons, etc.) may be removed in a separate stream
415 during
the first and/or second (as shown) pretreatment step. The second residual
feedstock 411 may
then be treated with sodium metal 403 and exogenous capping agent 405 in
reactor 420 as
described herein to provide a mixture 421 of sodium salts and converted
feedstock. The
sodium salts of mixture 421 may then be agglomerated (430) and separated (440)
as
described before to provide the converted feedstock 441, metals 443, and
sodium salts 445.
The sodium salts 445 may be electrolyzed in an electrolytic cell 450 with a
sodium ion-
selective ceramic membrane 452 (e.g., NaSiCON) as described herein to provide
recovered
sodium metal 453 and elemental sulfur 457. The converted feedstock 441 may be
subjected
to any refinery process(es) 480 (e.g., distillation, thermal conversion,
catalytic conversion, or
the like) to give fuel grade products 482 and a residual product 481.
EXAMPLES
Example 1 ¨ De-sulfurization of hydrocarbon feedstocks with sodium
[0066] A variety of hydrocarbon feedstocks were treated with sodium metal to
demonstrate
the wide applicability of sodium metal treatment for removing impurities and
improving
physical properties. The hydrocarbon feedstocks included virgin crude oils
from different
geographical locations and geological formations, and a variety of converted
and treated
feedstocks located within typical refining and upgrading facilities. 700g of
the hydrocarbon
feedstock was treated with an appropriate mass of sodium in a 1.8L Parr
continuously stirred
tank reactor using a batch or semi-batch system under the following conditions
to yield a
mixture of converted hydrocarbon and sodium salts. The reaction conditions,
feed and
product properties are shown in Table 1.
[0067] The results from Table 1 clearly indicate that molten sodium metal
effectively
removes impurities and improves the physical properties of the converted
feedstock, therefore
improving the fungibility of the converted feedstock. The converted feedstock
may now be
sold directly as a fuel grade product or converted to higher value products in
downstream
refinery units rather than be sold as asphalt or high sulfur bunker fuel oil.
26
Table 1
0
Feedstock Alberta Bitumen American VR
Colombian VR Middle Eastern VR SDA Tar Blend Asp haltenes
Visbreaker Residue Hydrocracker Hydroconversion N
Residue
Bottoms 0
N
Reaction Conditions
Temperature ( C) 350 350 357 358 350
358 350 350 350 i=-=.-)
W
Pressure (psig) 1500 750 1500 1500 750
1500 750 750 750 cA
oe
Mole equivalents of sodium 1.15 1.35 1.34 1.13 1.12
1.07 1.34 1.35 1.35 h.)
.--.1
Residence Time 60 60 60 60 60 60
60 60 60
Physical Properties Feed Product Feed Product Feed Product
Feed Product Feed Product Feed Product Feed Product
Feed Product Feed Product
Sulfur (wt%) 4.50 0.50 3.40 0.03 3.90 0.01 5.10
0.02 5.00 0.04 8.20 0.02 2.00 0.30 3.80 0.30
2.40 0.50
API Gravity 8.3 17.8 1.2 4.3 5.8 13.3 4.71
8.3 6.3 13.1 -13. 13 9.3 12.3 3.9 9.2 8.9 12.5
Density (kg/m3) 1012 948 1066 1042 1031 977 1039
1012 1027 979 1174 979 1005 984 1045 1006
1008 983
Viscosity @ 50 C (cSt) 2,871,000 568 4,714,000
284,000 432,000 706 8,947,478 32,380 2,123 N/A 1,944
656 501,200 27,980 658 375
Viscosity @ 80 C (cSt) 690
Resid Cut (524+C) 50% 35% 900% 83.0% 75.9% 52.3%
92.3% 40.4% 70.0% 63.0% 90.5% 46.8% 71.0% 66.0%
85.0% 75.0% 39.0% 33.0%
C5 Asphaltenes (wt%) 14.1% 3.6% 23.3% 10.9%
C7 Asphaltenes (wt%) 10.7% 1% 25.0% 16.0% 20.1% 13.9%
14.2% 4.8% 16.8% 6.6% 64.9% 11.0% 8.2% 17.0% 11.0%
7.9% 5.6%
MCRT (wt%) 35.06 2&0% 28.3%
19.5% 12% 18.0% 14.8% 26.1% 19.3% 13.3% 10.3%
P
Vanadium (ppm) 146 3.7 515 3 195 3
200 52 675 5 ao 7 192 50 140 49 .
Nickel (ppm) 63 21 115 4 53 4 64
21 201 18 41 11 115 40 67 24 L.
i-i
Iron (ppm) 56 13 51 3
47 2 25 6 644 0 L.
h.) Total Metals (ppm) 289 5 265 51 629 7 252 7
315 76 926 23 128 21 332 96 3136 160
.
"
Conversion Efficiency
n,
6,
Sulfur (wt%) 88.9% 99.1% 99.7% 99.6% 99.2%
99.8% 85.0% 92.1% 79.2% "
n,
i
Total Metals (ppm) 98.3% 80.8% 98.9% 97.2% 76.0%
97.5% 84.0% 71.1% 94.9%
C5 Asphaltenes (wt%) 90.7% 36.0% 30.8% 66.3% 60.7%
100.0% 25.5% 35.3% 29.1%
i
i-i
Product Quality Improvement
...1
Density (kg/m3 per wt% S removed) 16.10 7.22 13.72 5.25
9.74 23.84 12.33 11.25 13.26
Viscosity Reduction @ 50 C (cSt) 1 2,870,432 4,430,000 431,294
30,257 1,288 473,220 283
Viscosity Reduction @ 50 C (%) 100.0% 94.0% 99.8%
93.4% 66.3% 94.4% 43.0%
IV
n
cp
w
w
-a-,
w
.6.
.6.
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
Example 2 ¨ De-sulfurization of hydrocarbon feedstocks with sodium
[0068] A variety of hydrocarbon feedstocks and pretreated hydrocarbon
feedstocks were
treated with sodium metal in a pilot plant using a continuous system
essentially as shown, e.g.,
in FIG. 4 to further demonstrate the wide applicability of sodium metal
treatment for removing
impurities and improving physical properties during continuous operation. The
hydrocarbon and
pretreated hydrocarbon feedstocks included virgin crude oil, vacuum residuums
and partially
converted feedstocks produced within typical refining and upgrading
facilities. Each feedstock
was treated with an appropriate mass of sodium in a 12L continuously stirred
tank reactor under
the following conditions to yield a mixture of converted hydrocarbon and
sodium salts.
Hydrogen was the exogenous capping agent for all test campaigns. The reaction
conditions, feed
and product properties are shown in Table 2.
[0069] Similar to Example 1, the results from Table 2 clearly indicate that
molten sodium metal
effectively removes impurities and improves the physical properties of the
converted feedstock,
therefore improving the fungibility of the converted feedstock. The converted
feedstock may
now be sold directly as a fuel grade product or converted to higher value
products in
downstream refinery units rather than be sold as asphalt or high sulfur bunker
fuel oil.
28
Table 2
0
t.)
o
n.)
Conventional Hydroconversion
Diluted Vacuum Residual Crude Oil
Bitumen Vacuum Residual Bottoms c,.)
c:
Reaction Conditions
oe
n.)
-4
Feed Oil Quantity (kg/hr)
65.7 65 67.1
50.5 45.1
Sodium flow rate (kg/hr) 2.1 3.65 5.69
2.6 1.69
Reaction Temperature ( C) 380 360 370
360 360
Pressure (psig) 732 749 700
749 750
Mole equivalents of sodium 1.24 1.24 1.29
1.24 1.24
Reactor Residence Time (min) 4.6 4.5 4.3
6.5 6.7
Physical Properties Feed Product Feed Product
Feed Product Feed Product Feed Product
API Gravity 11.3 12.9 12.5 15.9 10.3
14.7 7.5 10.8 7.9 10.6 P
Density (kg/m3) 991 980 983 960 998
968 1018 994 1015 996
,
.3
n.) Kinematic Viscosity (cSt @ 50 C) 650 374 603 254
2,308 492 191,500 21,510 1,075 592
r.,
Kinematic Viscosity (cSt @ 80 C) 111 72 105 56 293
91 7,120 1,400 137 91
Sulfur (wt%) 1.76 0.49 3.14 0.48 4.57
0.49 2.88 0.61 2.1 0.51 " r.,
,
Iron (wppm) 90 13 6 3 22
0 105 7 105 0 ,
,
,
Nickel (wppm) 32 10 39 15 88
24 61 18 65 21 ,
..,
Vanadium (wppm) 69 23 100 43 240
59 123 53 85 19
Total Metals (wppm) 323 110 212 70 369
101 359 90 699 42
Product Quality Improvement Feed Product Feed Product
Feed Product Feed Product Feed Product
Density reduction (kg/m3 per wt% S
removed) 8.65 8.52
7.36 10.40 12.13
Viscosity Reduction @ 50 C (cSt) 275 350
1,816 169,990 483
Viscosity Reduction @ 50 C (%) 42.4% 58.0%
78.7% 88.8% 45.0% Iv
n
c 4
k ..,
=
k ..,
k ..,
. 6 .
. 6 .
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
Example 3: Preferential removal of sulfur in asphaltene fraction
[0070] A blended vacuum residuum stream was treated with an increasing molar
equivalent
of sodium in 5 separate experiments. 700 g of vacuum residuum was contacted
with sodium
at 350 C and 750 psig of hydrogen partial pressure. Key results are summarized
in Table 3.
The effect of treatment with sodium on preferential removal of sulfur from the
asphaltene
fraction is summarized by:
1. the fraction of total sulfur located in the asphaltenes was reduced from
28.5% to 7.9%
of the converted product at a sodium to sulfur ratio of 0.94.
2. The proportion of asphaltenic sulfur vs non-asphaltenic sulfur is reduced
as a function
of increasing moles equivalents of sodium. The reduction in proportion
demonstrates
that a greater proportion of sulfur has been removed from asphaltenic sulfur
than from
non-asphaltenic sulfur at all practical sodium to sulfur ratios - a critical
result for
unloading hydroprocessing catalysts in downstream refining processes (e.g., in
the
process shown in FIG. 2).
Table 3: Removal of Sulfur from various oil fractions
EiniggginEanigiNinEnaliNiffigi.
Product Oil
Total Sulfur Content (wt% feed) 2.17% 1.92% 1.63% 1.31%
1.01% 0.70%
Total Asphaltene content (wt% feed) 12.6% 11.10% 10.30% 9.85%
8.54% 7.73%
Asphaltenic sulfur content (wt% of
4.9% 3.85% 2.90% 2.12% 1.28%
0.71%
asphaltene fraction)
Extent of Desulfurization
Overall (wt%) 12% 25% 40% 54% 68%
non-Asphaltenic sulfur content (wt% of
3.9% 14.3% 29.1% 42.0%
58.8%
initial non-asphaltenic sulfur content)
Asphaltenic sulfur content (wt% of initial
30.8% 51.6% 66.2% 82.3%
91.1%
asphaltenic sulfur content)
Ratio of asphaltenic sulfur to non-
7.9 3.6 2.3 2.0 1.5
asphaltenic sulfur removed (wt/wt)
Fraction of sulfur in converted
hydrocarbon as asphaltenic sulfur content 28.5% 22.3% 18.3%
15.9% 10.8% 7.9%
(wt%)
Proportion of asphaltenic sulfur to non-
0.40 0.29 0.22 0.19 0.12 0.09
asphaltenic sulfur (g/g)
Example 4: Improving the conversion of hydrocarbons in catalytic conversion
processes by pre-treating with sodium
CA 03183922 2022-11-17
WO 2021/236827
PCT/US2021/033244
[0071]
Refinery intermediate streams (i.e., pretreated hydrocarbon feedstocks) were
treated with various molar equivalents of sodium to demonstrate how the choice
of molar
equivalent of sodium can be used to improve the conversion of hydrocarbons in
downstream
catalytic conversion processes. 700 g of each refinery intermediate was
treated with sodium
at 350 C and 750 psig or 400 C and 1500 psig of hydrogen partial pressure. Key
results are
summarized in Table 4.
[0072]
Treatment of hydrocarbon feedstock with a sub-stoichiometric molar equivalent
of
sodium may be preferable for pre-treating FCC or Residuum Hydrotreater (RHT)
feedstocks
by preferentially removing the impurities that foul catalysts: asphaltenic
sulfur, metals and
asphaltenes. A greater proportion of sulfur was removed from the asphaltene
fraction in all
cases. Additionally, the fraction of metals removed exceeds the fraction of
total sulfur
removed, indicating that a low sodium/sulfur addition ratio may be favorable
to produce a
partially converted product with low metals and asphaltenic sulfur content for
further
processing in downstream refinery processes.
Table 4
Blended
Feedstock Vacuum
Hydroconversion Hydroconversion Hydroconversion
Residuum Residue Residue Residue
Molar equivalent of Sodium 0.20 0.20 0.65
1.08
Sulfur Removal
Total Sulfur wt% 19% 17% 47%
70%
Non-Asphaltene Sulfur wt% 7% 12% 40%
64%
Asphaltene Sulfur wt% 36% 21% 66%
89%
Metals Removal
Iron wt% 100.0% 99.4% 98.0%
99.0%
Nickel wt% 76.2% 39.0% 61.0%
76.0%
Vanadium wt% 74.7% 85.3% 97.0%
98.0%
Asphaltene Conversion yield
Feed Asphaltene fraction wt% 12.6% 15.5%
15.1% 15.1%
Product Asphaltene fraction wt% 9.8% 12.6%
12.2% 12.8%
Asphaltene Conversion wt% 22.1% 18.7% 19.2%
15.2%
Example 5: Producing on-spec low sulfur fuel oil from a vacuum residuum
feedstock
[0073] A
vacuum residuum feedstock was treated with sodium metal in a pilot plant
using a continuous system, e.g., as shown in FIG. 1, to demonstrate the
production of on-spec
low sulfur fuel oil. The vacuum residuum feedstock was treated with an
appropriate mass of
31
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
sodium in a 12L continuously stirred tank reactor under the following
conditions to yield a
mixture of converted hydrocarbon and sodium salts. Hydrogen was the exogenous
capping
agent for all test campaigns. The reaction conditions, feed and product
properties are shown
in Table 5.
[0074] The example clearly demonstrates that an on-spec low sulfur fuel oil
can be
produced when a hydrocarbon feedstock is contacted with an effective amount of
sodium and
an effective amount of exogenous capping agent.
Table 5
Diluted Vacuum Residuum
Reaction Conditions
Feed Oil Quantity (kg/hr) 65.8
Sodium flow mte (kg/hr) 2.1
Reaction Temperature ( C) 378
Pressure (psig) 750
Mole equivalents of sodium 1.26
Reactor Residence Time (min) 4.6
ISO 8217 2010 RMG
Physical Properties Feed Product 380 Spec
Sulfur (wt%) 1.76 0.47 0.5 Max
Density (kg/m3) 991 976 991 Max
Kinematic Viscosity (cSt @ 50 C) 650 354 380 Max
Acid Number, mg KOH/g 0 0 2.5 Max
MCRT, wt% 13 10 18 Max
CCAI 838 870 Max
Flash Point, C >700 60 Min
Pour Point, C <24 30 Max
Compatibility, Spot# 1 Not required
Vanadium, wppm 75 20 350 Max
Aluminum + Silicon, wppm 109 6 60 Max
Ash, wt% 0.16 0.01 0.1 Max
Total Sed, Potential, wt% <0.01 0.1 Max
Sodium, wppm 78 11 100 Max
Example 6: Improving the yield and quality of coker products by pre-treating
the coker
feed with sodium
Four feedstocks from Table 1, vacuum residuum, SDA tar, visbreaker residue and
Hydrocracker residue, were treated with sodium metal to demonstrate the
improved yield and
quality of coker products when pre-treating the coker feedstock with sodium
metal prior to
32
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
thermal conversion. 700g of the hydrocarbon feedstock was treated with an
appropriate mass
of sodium in a 1.8L Parr continuously stirred tank reactor using a batch or
semi-batch system
under the following conditions to yield a mixture of converted hydrocarbon and
sodium salts.
The reaction conditions, feed and product properties are shown in Table 1. The
product yield
and quality of the coker products are estimated using accepted industry
correlations (Gary,
J.H., and Handwerk, G.E. (2001). "Petroleum Refining." Marcel Dekker, NewYork)
for
both the 'as received' hydrocarbon feedstock and the converted feedstock after
sodium
treatment. The coker products are summarized in Table 6.
[0075] The results from Table 6 clearly indicate that when compared to the
thermal
conversion of the as-received feedstock, treatment the feed with molten sodium
metal prior to
thermal conversion increases the total liquid yield, reduces the coke yield,
increases the
proportion of purified product to residual product and reduces the sulfur
content of all coker
products.
[0076] Additionally, the results demonstrate how treating a feedstock with
molten sodium
metal can unload sulfur recovery processes (i.e., Claus or SCOT plants). In
the 4 examples,
the sulfur to gas processing (as H25) is reduced by over 90%. This process
configuration is
advantageous to increase the sulfur handling capacity for a refining facility
without
increasing sulfur emissions or exceeding limits.
33
Table 6
0
F:e.:61MfiedMehiixgeMFaitF:&ttMehiiiigegFeed Feed Chtn fietIMF:&tIMCh46geiiii
Total Liquid wrY0 of
oe
Yield feed 31.16
43.37 39% 42.84 58.19 36% 60.81 66.39 9% 46.68 58.54 25%
wt% of
Coke production feed 56.00 44.80 -20% 45.28 31.20 -
31% 28.80 23.68 -18% 41.76 30.88 -26%
Sulphur to gas wt% of
processing feed 1.01 0.08 -92% 1.50 0.12 -92% 0.1
0.01 -84% 1.1 0.09 -92%
Proportion of
Liquid Products/
Residual
Product wt/wt 0.56 0.97 74% 0.95 1.87 97% 2.11
2.80 33% 1.12 1.90 70%
0
.66
.66
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
Example 7: Producing a high purity premium grade anode coke or needle coke by
pretreating with sodium
[0077] The coke product qualities for the same four feedstocks from Example
6, vacuum
residuum, SDA tar, visbreaker residue and Hydrocracker residue, is estimated
using accepted
industry correlations (Gary, J.H., and Handwerk, G.E. (2001). "Petroleum
Refining." Marcel
Dekker, New York) for both the 'as received' hydrocarbon feedstock and the
converted
feedstock after sodium treatment and are summarized in Table 7. None of the
cokes
produced from the as-received feed meet anode grade coke specifications,
whereas all coke
products produced from the converted feedstocks are near or exceed the anode
grade coke
specifications. The vanadium specification can be achieved by slightly
increasing the molar
equivalent of sodium in the sodium contacting step.
Table 7
Sulfur Vanadium
wt% PPm
Anode Coke Spec 0.5 150
Vacuum Residual
Hydrocarbon Feed 1.79 261
Converted Feed 0.17 122
SDA Tar
Hydrocarbon Feed 3.31 442
Converted Feed 0.38 167
Visbreaker Residue
Hydrocarbon Feed 2.06 139
Converted Feed 0.38 31
Hydrocracker Residue
Hydrocarbon Feed 2.70 460
Converted Feed 0.30 162
Example 8: Improving the distillation properties of petroleum products by pre-
treating
the feed with sodium
[0078] A comparison was made of distillation properties for hydrocarbon
feedstocks
before and after treatment with sodium in accordance with the procedure of
Example 1. The
results in Table 8 show improved properties, with a reduction in the resid
fraction of 1-10%
and associated increases in the higher-value distillate and gasoil fractions
of 0.5-3% and 0.5-
8% respectively. This improved product profile provides higher value products
from a given
volume of feed, e.g., when conducting distillations after desulfurization
using sodium.
Table 8
Diluted DSU Conventi DSU DSU Heavy .. DSU ..
Vacuum DSU
Fraction Bitumen
0
VR product onal product
product bottoms product residue product
o
i.)
1-,
Naphtha (C5-177) 0 0 1.60 0.65 1.79 0.64
0 0.36 0.00 0 iZ.1
cr
oe
Distillate (177-343) 22.27 23.14 20.11 21.28 16.45 19.17
12.39 14.95 0.00 2.62 n.)
-4
Gas Oil (343-524) 20.52 21.05 34.09 36.52 29.79 37.42
37.61 39.04 12.66 17.59
Resid (524-0 57.21 55.81 44.20 41.56 51.97 42.77
50.00 45.65 87.34 79.80
Deltas
Naphtha (C5-177) 0 -0.95 -1.15
0.36 0
P
Distillate (177-343) 0.87 1.17 2.72
2.56 2.62 .
,
.3
cr Gas Oil (343-524) 0.53 2.42 7.63
1.43 4.93
r.,
r.,
r.,
,
,
,
,
,
..,
IV
n
c 4
k ..,
=
k ..,
k ..,
. 6 .
. 6 .
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
EQUIVALENT S
[0079] While certain embodiments have been illustrated and described, a
person with
ordinary skill in the art, after reading the foregoing specification, can
affect changes,
substitutions of equivalents and other types of alterations to the processes
of the present
technology and products thereof as set forth herein. Each aspect and
embodiment described
above can also have included or incorporated therewith such variations or
aspects as
disclosed in regard to any or all of the other aspects and embodiments.
[0080] The present technology is also not to be limited in terms of the
particular aspects
described herein, which are intended as single illustrations of individual
aspects of the present
technology. Many modifications and variations of this present technology can
be made
without departing from its spirit and scope, as will be apparent to those
skilled in the art.
Functionally equivalent methods within the scope of the present technology, in
addition to
those enumerated herein, will be apparent to those skilled in the art from the
foregoing
descriptions. Such modifications and variations are intended to fall within
the scope of the
appended claims. It is to be understood that this present technology is not
limited to
particular methods, feedstocks, compositions, or conditions, which can, of
course, vary. It is
also to be understood that the terminology used herein is for the purpose of
describing
particular aspects only, and is not intended to be limiting. Thus, it is
intended that the
specification be considered as exemplary only with the breadth, scope and
spirit of the
present technology indicated only by the appended claims, definitions therein
and any
equivalents thereof.
[0081] The embodiments, illustratively described herein may suitably be
practiced in the
absence of any element or elements, limitation or limitations, not
specifically disclosed
herein. Thus, for example, the terms "comprising," "including," "containing,"
etc. shall be
read expansively and without limitation. Additionally, the terms and
expressions employed
herein have been used as terms of description and not of limitation, and there
is no intention
in the use of such terms and expressions of excluding any equivalents of the
features shown
and described or portions thereof, but it is recognized that various
modifications are possible
within the scope of the claimed technology. Likewise, the use of the terms
"comprising,"
"including," "containing," etc. shall be understood to disclose embodiments
using the terms
"consisting essentially of' and "consisting of." The phrase "consisting
essentially of' will be
understood to include those elements specifically recited and those additional
elements that
37
CA 03183922 2022-11-17
WO 2021/236827 PCT/US2021/033244
do not materially affect the basic and novel characteristics of the claimed
technology. The
phrase "consisting of' excludes any element not specified.
[0082] In addition, where features or aspects of the disclosure are
described in terms of
Markush groups, those skilled in the art will recognize that the disclosure is
also thereby
described in terms of any individual member or subgroup of members of the
Markush group.
Each of the narrower species and subgeneric groupings falling within the
generic disclosure
also form part of the invention. This includes the generic description of the
invention with a
proviso or negative limitation removing any subject matter from the genus,
regardless of
whether or not the excised material is specifically recited herein.
[0083] As will be understood by one skilled in the art, for any and all
purposes,
particularly in terms of providing a written description, all ranges disclosed
herein also
encompass any and all possible subranges and combinations of subranges thereof
Any listed
range can be easily recognized as sufficiently describing and enabling the
same range being
broken down into at least equal halves, thirds, quarters, fifths, tenths, etc.
As a non-limiting
example, each range discussed herein can be readily broken down into a lower
third, middle
third and upper third, etc. As will also be understood by one skilled in the
art all language
such as "up to," "at least," "greater than," "less than," and the like,
include the number
recited and refer to ranges which can be subsequently broken down into
subranges as
discussed above. Finally, as will be understood by one skilled in the art, a
range includes
each individual member.
[0084] All publications, patent applications, issued patents, and other
documents (for
example, journals, articles and/or textbooks) referred to in this
specification are herein
incorporated by reference as if each individual publication, patent
application, issued patent,
or other document was specifically and individually indicated to be
incorporated by reference
in its entirety. Definitions that are contained in text incorporated by
reference are excluded to
the extent that they contradict definitions in this disclosure.
[0085] Other embodiments are set forth in the following claims, along with
the full scope
of equivalents to which such claims are entitled.
38