Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 2022/087732
PCT/CA2021/051512
1
DETECTING THERMITE REACTIONS IN AN ELECTROLYTIC CELL
Cross-Reference to Related Applications
[0001] The present patent application claims the benefits of
priority of U.S.
Provisional Patent Application No. 63/106,517 entitled "SYSTEM AND METHOD
FOR DETECTING THERMITE REACTIONS IN AN ELECTROLYTIC CELL-, and
filed at the United States Patent and Trademark Office on October 28th, 2020,
the
content of which is incorporated herein by reference.
Technical Field
[0002] The present application generally relates to the detection and/or
prevention
of thermite reactions in electrolytic cells.
Background
[0003] Aluminum is the third most common element in the
earth's crust. The
aluminum is extracted from aluminum oxide, al so known as alumina, by an
electrolysis
process. The electrolysis process takes place inside an electrolytic cell
comprising a
plurality of cathodes, one or more anode assemblies, and an electrolytic bath
containing
molten cryolite in which the alumina is dissolved.
[0004] During the electrolysis process, aluminum ions flow
towards the cathodes
where they gain electrons and become aluminum metal. The oxide ions move
towards
the anodes where they lose electrons and pair up producing dioxygen molecules
02.
Hall-Heroult process implies the use of anodes made of carbon or graphite
materials.
The dioxygen molecules react with the carbon atoms of the carbon anodes
producing
carbon dioxide CO2. This results in the anode being corroded and consumed
during the
electrolysis process. Besides, the use of carbon anodes have an environmental
cost
because of the CO2 molecules that are released in the air.
[0005] "Inert anodes- have been used to replace the carbon
graphite anodes during
el ectrochemi cal reduction o f metal oxides as they are insoluble in the
electrolyte under
the conditions of the electrolysis. The inert anodes are, thus, non-consumable
during
the electrolysis process. Moreover, the reaction taking place on the inert
anodes does
not produce CO2 but rather 02, making the use of inert anodes a greener
technology.
However, in the case of use of oxide-based inert anodes for electrochemical
reduction
of metals, such as aluminum, there is a possibility of a thermite reaction.
- 1 -
CA 03193088 2023- 3- 17
WO 2022/087732
PCT/CA2021/051512
2
[0006] A thermite reaction is a reaction that involves a
metal reacting with a
metallic or a non-metallic oxide to form a more stable oxide and the con-
esponding
metal or non-metal of the reactant oxide.
[0007] The thermite reaction that occurs during aluminum
electrolysis is described
by the equation:
Fe2O3 + 2 Al ¨> 2Fe + A1203+ heat
[0008] Thermite reactions are thus highly exothermic, self
sustaining at high
temperatures, and pose risks to personnel and equipment.
[0009] Detecting and mitigating and/or suppressing thermite
reactions during the
electrolysis process implies monitoring the electrolytic cell. As disclosed in
U.S. patent
no. 9,982,355 B2 (D'Astolfo et al.), the content of which is incorporated
herein by
reference, monitoring the cell can be done by installing several probes on the
conductive
elements of the cell (for example, anodes) to measure the voltage drop of one
or more
anodes and to detect the thermite reaction. In this technique, the voltage
probes may be
placed at a maximum distance apart to maximize the voltage drop relative to
the noise
and therefore to obtain a more sensitive response. This technique has the
disadvantage
of the high cost of installation and continuous monitoring of a large number
of voltage
drops necessary for the detection system.
[0010] There is thus a need for a more simple system and
method for monitoring
electrolytic cell, detecting and mitigating and/or suppressing thermites
reactions.
Summary
[0011] It is first disclosed herein a method for detecting a
thermite reaction in an
electrolytic cell comprising at least one anode assembly of one or more metal-
oxide
anodes, at least one cathode, an electrolytic bath, and a current supply buss
providing a
cun-ent to the at least one anode assembly through a distinct anode rod for
each anode
assembly. The method comprises: measuring a voltage drop using a pair of
voltage
probes located on the anode rod of each anode assembly, the voltage drop
corresponding to a current flow in each anode assembly; computing from said
measured
voltage drop at least one of: a voltage drop derivative, a voltage drop
variance across
the one or more anode assemblies; and a derivative of the voltage drop
variance across
the one or more anode assemblies, wherein said voltage drop variance and
derivative
of the voltage drop variance may be computed when the electrolytic cell
comprises a
- 2 -
CA 03193088 2023- 3- 17
WO 2022/087732
PCT/CA2021/051512
3
plurality of anode assemblies. The method also comprises: detecting the
thermite
reaction upon occurrence of one or more of: a voltage drop exceeding at least
one
voltage threshold level, wherein each voltage threshold level is a
predetermined voltage
drop previously associated with a thermite reaction; a variation in the
voltage drop
derivative; a variation in the variance of the voltage drop across the anode
assemblies;
and a variation in the derivative of the voltage drop variance across the
anode
assemblies. The method may further comprise, upon detection of the thermite
reaction,
optionally adjusting at least one operational parameter of the electrolytic
cell to mitigate
and/or suppress the thermite reaction.
100121 According to a preferred embodiment, the method further comprises
sending a signal to an operator of the electrolytic cell upon detection of the
thermite
reaction.
[0013] According to a preferred embodiment, the threshold
voltage levels are
based on past operational data of the electrolytic cell.
[0014] According to a preferred embodiment, the threshold voltage levels
are
computer derived threshold levels derived from at least one of past
operational data of
the electrolytic cell, operation parameters, and composition of the
electrolytic cell.
[0015] According to a preferred embodiment, the thermite
reaction is detected
when the variation in voltage drop derivative exceeds a threshold variation.
[0016] According to a preferred embodiment, the thermite reaction is
detected
when the variation in variance of the voltage drop across the anode assemblies
exceeds
a threshold variation.
100171 According to a preferred embodiment, the thermite
reaction is detected
when the variation in derivative of the voltage drop variance across the anode
assemblies exceeds a threshold variation.
[0018] According to a preferred embodiment, adjusting at
least one operational
parameter of the electrolytic cell to mitigate and/or suppress the thermite
reaction
comprises one or more of:
changing an anode to cathode overlap (ACO) of one or more anode assemblies;
removing one or more anode assemblies from the electrolytic bath;
changing the current supplied to at least one of the one or more anode
assemblies
- 3 -
CA 03193088 2023- 3- 17
WO 2022/087732
PCT/CA2021/051512
4
or the electrolytic cell;
changing a temperature of the electrolytic bath; and
changing a chemistry of the electrolytic bath.
[0019] According to a preferred embodiment, when the voltage
drop of one of the
anode assemblies exceeds the at least one voltage threshold level, adjusting
at least one
operational parameter of the electrolytic cell takes into account one or more
of the
exceeded voltage threshold levels.
[0020] According to a preferred embodiment, upon detection of
the thermite
reaction, adjusting at least one operational parameter of the electrolytic
cell takes into
account a magnitude of the voltage drop.
[0021] According to a preferred embodiment, upon detection of
the thermite
reaction, adjusting at least one operational parameter of the electrolytic
cell takes into
account a magnitude of the voltage drop derivative.
[0022] According to a preferred embodiment, upon detection of
the thermite
reaction, adjusting at least one operational parameter of the electrolytic
cell takes into
account a magnitude of the variance of the voltage drop.
100231 According to a preferred embodiment, upon detection of
the thermite
reaction, adjusting at least one operational parameter of the electrolytic
cell takes into
account a magnitude of the derivative of the voltage drop variance.
[0024] According to a preferred embodiment, the method further comprises
filtering the voltage drop, the voltage drop derivative, the variance of the
voltage drop,
and/or the derivative of the voltage drop variance.
[0025] It is also disclosed herein a system for detecting a
thermite reaction in an
electrolytic cell comprising at least one anode assembly of one or more metal-
oxide-
containing anodes, at least one cathode, an electrolytic bath, and a current
supply buss
providing a current to the at least one anode assembly through a distinct
anode rod for
each anode assembly. The system comprises:
a pair of voltage probes located on the anode rod of each anode assembly for
measuring a voltage drop, the voltage drop corresponding to a current flow in
the anode
assembly;
- 4 -
CA 03193088 2023- 3- 17
WO 2022/087732
PCT/CA2021/051512
a processor module for:
computing from said measured voltage drop at least one of:
a voltage drop derivative;
a voltage drop variance across the one or more anode assemblies;
5 and
a derivative of the voltage drop variance across the one or more
anode assemblies;
wherein said voltage drop variance and derivative of the voltage
drop variance are computed when the electrolytic cell comprises
a plurality of anode assemblies; and
detecting the thermite reaction upon occurrence of one or more of:
a voltage drop exceeding at least one voltage threshold
level, wherein each voltage threshold level is a predetermined
voltage drop previously associated with a thermite reaction;
a variation in voltage drop derivative;
a variation in variance of the voltage drop across the
anode assemblies; and
a variation in derivative of the voltage drop variance
across the anode assemblies.
[0026] According to a preferred embodiment, the system further comprises a
communication module for sending a signal to an operator of the electrolytic
cell upon
detection of the thermite reaction.
[0027] According to a preferred embodiment, the threshold
voltage levels are
based on past operational data of the electrolytic cell.
[0028] According to a preferred embodiment, the threshold voltage levels
are
computer derived threshold levels derived from at least one of past
operational data of
the electrolytic cell, operation parameters, and composition of the
electrolytic cell.
[0029] According to a preferred embodiment, the processor
module is configured
to detect the thermite reaction when the variation in voltage drop derivative
exceeds a
- 5 -
CA 03193088 2023- 3- 17
WO 2022/087732
PCT/CA2021/051512
6
threshold variation.
100301 According to a preferred embodiment, the processor
module is configured
to detect the thermite reaction when the variation in variance of the voltage
drop across
the anode assemblies exceeds a threshold variation.
[0031] According to a preferred embodiment, the processor module is
configured
to detect the thermite reaction when the variation in derivative of the
voltage drop across
the anode assemblies exceeds a threshold variation.
[0032] According to a preferred embodiment, the processor
module is configured
to adjust at least one operational parameter of the electrolytic cell to
mitigate and/or
suppress the thermite reaction by:
changing an anode to cathode overlap (ACO) of one or more anode assemblies;
removing one or more anode assemblies from the electrolytic bath;
changing the current supplied to at least one of the one or more anode
assemblies
or the electrolytic cell;
changing a temperature of the electrolytic bath; and/or
changing a chemistry of the electrolytic bath.
[0033] According to a preferred embodiment, when the voltage
drop of one of the
anode assemblies exceeds the at least one voltage threshold level, the
processor module
is configured to adjust at least one operational parameter of the electrolytic
cell by
taking into account one or more of the exceeded voltage threshold levels.
100341 According to a preferred embodiment, upon detection of
the thermite
reaction, the processor module is configured to adjust at least one
operational parameter
of the electrolytic cell taking into account a magnitude of the voltage drop.
[0035] According to a preferred embodiment, upon detection of
the thermite
reaction, the processor module is configured to adjust at least one
operational parameter
of the electrolytic cell taking into account a magnitude of the voltage drop
derivative.
[0036] According to a preferred embodiment, upon detection of
the thermite
reaction, the processor module is configured to adjust at least one
operational parameter
of the electrolytic cell taking into account a magnitude of the variance of
the voltage
drop.
- 6 -
CA 03193088 2023- 3- 17
WO 2022/087732
PCT/CA2021/051512
7
[0037] According to a preferred embodiment, upon detection of
the thermite
reaction, the processor module is configured to adjust at least one
operational parameter
of the electrolytic cell taking into account a magnitude of the derivative of
the voltage
drop variance.
[0038] According to a preferred embodiment, the processor module is further
configured to filter the voltage drop, the voltage drop derivative, the
variance of the
voltage drop, and/or the derivative of the voltage drop variance.
[0039] Another aspect is directed to an electrolytic cell
comprising at least one
anode assembly of one or more metal-oxide-containing anodes, at least one
cathode, an
electrolytic bath, and a current supply buss providing a current to the at
least one anode
assembly through a distinct anode rod for each anode assembly, and the system
for
detecting a thermite reaction as defined herein. Preferably, the electrolytic
cell is used
for the making of a metal, such as, but not limited to, aluminum (Al).
100401 The method, system and electrolytic cell as disclosed
herein are particularly
advantageous as they allow reducing the number of voltage signals/ drops
necessary for
detecting a thermite reaction by a factor of 10. Other advantages are detailed
herein.
Brief Description of the Drawings
[0041] The above and other aspects, features and advantages
will become more
readily apparent from the following description, reference being made to the
accompanying drawings in which:
[0042] Figure 1 is a schematic illustration of an anode
assembly for an electrolytic
cell with a horizontal configuration of the anode rod, according to a
preferred
embodiment;
[0043] Figure 2 is a schematic illustration of an anode
assembly for an electrolytic
cell with a vertical configuration of the anode rod, according to a preferred
embodiment;
[0044] Figure 3 shows a graphic of voltage drops for a cell
according to a preferred
embodiment, in which the cell comprises four anode assemblies in which one of
the
assemblies is experiencing a simulated thermite reaction;
[0045] Figure 4 shows a graphic of voltage drop derivatives
for a cell according to
a preferred embodiment, in which the cell comprises four anode assemblies in
which
one of the assemblies was experiencing a simulated thermite reaction;
- 7 -
CA 03193088 2023- 3- 17
WO 2022/087732
PCT/CA2021/051512
8
[0046] Figure 5 shows a graphic of variance of the voltage
drop for a cell according
to a preferred embodiment in which the cell comprises four anode assemblies in
which
one of the assemblies was experiencing a simulated thermite reaction;
[0047] Figure 6 shows a graphic of the derivative of the
voltage drop variance for
a cell according to a preferred embodiment in which the cell comprises four
anode
assemblies in which one of the assemblies was experiencing a simulated
thermite
reaction;
[0048] Figure 7 is a flow chart for illustrating a method for
detecting a thermite
reaction in an electrolytic cell, according to a preferred embodiment; and
[0049] Figure 8 shows a logical modular representation of a system for
detecting a
thermite reaction in an electrolytic cell in accordance with the teachings of
the present
invention.
Detailed Description of Preferred Embodiments
[0050] A novel system, method and related electrolytic cell
will be described
hereinafter. Although they are described in terms of specific illustrative
embodiments,
it is to be understood that the embodiments described herein are by way of
example
only and that the scope of the invention is not intended to be limited
thereby.
[0051] The terminology used herein is in accordance with
definitions set out
below.
[0052] The description which follows, and the embodiments described therein
are
provided by way of illustration of an example of particular embodiments of
principles
and aspects of the present invention. These examples are provided for the
purposes of
explanation and not of limitation of the principles of the invention. In the
description
that follows, like parts and/or steps are marked throughout the specification
and the
drawing with the same respective reference numerals.
[0053] As aforesaid, disclosed herein is a detection system
and method for
detecting a thermite reaction in an electrolytic cell. The electrolytic cell
typically
comprises at least one cathode assembly having at least one cathode, such as,
but not
limited to vertical cathodes, and configured for receiving or interacting with
at least one
corresponding anode assembly having at least one anode, such as, but not
limited to
vertical anodes. The electrolysis cell is also configured to receive an
electrolytic bath
- 8 -
CA 03193088 2023- 3- 17
WO 2022/087732
PCT/CA2021/051512
9
of a molten electrolyte (such as cry olite) for the electrolytic production of
metals, such
as aluminum.
[0054] The "cathode" is an electrode negatively charged and
the "anode" is a
positively charged electrode. Anodes used in electrolytic processes can be
consumable
(e.g. carbon and graphite anodes used with the Hall-Heroult process) or
alternatively
non-consumable anodes, such as inert or oxygen evolving anodes. "Inert anodes"
are
not oxidized during the electrolysis process and are thus insoluble in the
electrolytic
bath during the electrolysis process. Inert anodes can be made of single
compounds,
composite, or alloy-type materials. Examples of inert anodes include: ceramic,
cermet,
metal anodes, and any combination thereof The inert anodes may be constructed
of
electrically conductive materials such as metal oxides. By "metal-oxide-
containing
anodes" used herein, it is meant an anode comprising at least a portion of
metal oxides.
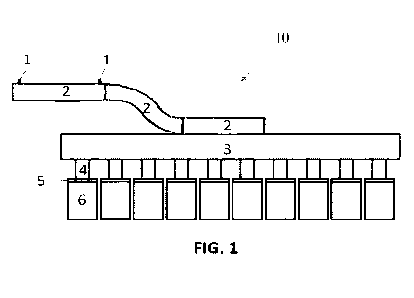
[0055] Figures 1 and 2 are schematic illustrations of an
anode assembly in
accordance with preferred embodiments. Figure 1 shows a horizontal
configuration of
the anode rod of the anode assembly whereas Figure 2 shows a vertical
configuration
of the anode rod.
[0056] More specifically, the anode assembly 10 of Figures 1
and 2 comprises an
anode rod 2 for feeding the electrical current to a supporting yoke or beam 3.
The
electrical current flowing through the anode rod 2 is provided via a current
supply buss
(not shown) that feeds the electrical current to all the anode assemblies of
an electrolytic
cell. The electrical current is then distributed through stubs 4 and
distribution plates 5
to a plurality of anodes 6. The anode assembly further comprises a pair of
voltage
probes 1 used to measure a voltage drop in the anode assembly.
100571 As explained above, thermite reactions may occur when
oxide-based inert
anodes are used for electrochemical reduction of metals, such as aluminum.
Thermite
reactions need to be rapidly detected and stopped as they pose risks to
personnel and
equipment.
[0058] Thermite reactions can be caused by direct contact
between the anodes and
a molten metal source, such as a metal pad. Thermite reactions may be
initiated by an
electrical short where more current flows through the path of the electrical
short. An
electrical short may be detected as a voltage drop increase between one or
more voltage
probes. With reference to the present disclosure, the voltage drop can be used
to detect
- 9 -
CA 03193088 2023- 3- 17
WO 2022/087732
PCT/CA2021/051512
a potential thermite reaction and initiate appropriate responses, such as
moving the
anodes away from the metal pad, and/or reducing current supply.
[0059] By "voltage drop", it is meant a voltage difference
between two objects or
two points of the same object.
5 [0060] A "voltage probe" measures the voltage drop and outputs an
electrical
signal representative of the measured voltage drop.
[0061] A system is described herein comprising one voltage
probe, for each anode
assembly, in an electrochemical reduction cell that can detect and help
prevent thermite
reactions from occurring between a metal-oxide-containing anode and a liquid
metal,
10 such as aluminum. Reduction in total number of signals required to
detect a potential
thermite condition compared to prior solution is targeted herein. Such a
reduction may
be achieved by probe placement to maximize signal-to-noise ratio, and the
consideration of time-dependent derivative signals and signal variance to
enhance the
interpretation of the signals.
[0062] In a preferred embodiment, a pair of voltage probes is used to
measure the
voltage drop in each anode assembly.
100631 An ideal voltage probe preferably provides: connection
ease and
convenience, absolute signal fidelity, zero signal source loading, and
complete noise
immunity. Real voltage probes may raise several problems such as: physical
attachment
of the probe on the circuit, impact of the probe on the circuit operation, and
signal
fidelity. Real voltage probes, inherently, induce background noise in the
measurement
data. Background noise may be caused by different factors such as: measurement
errors,
movement of the circuit, imperfection of the measurement devices, etc.
[0064] Measuring the -signal-to-noise ratio" (SNR) allows
comparing the level of
a desired signal (e.g., a voltage drop) to the level of background noise.
[0065] In a preferred embodiment, the pair of voltage probes
is located on the
anode rod. The location of the voltage probes is optimized to maximize the
signal-to-
noise ratio (SNR). The two voltage probes are placed as far apart as possible
so that
they detect larger voltage drop signals. As the noise is assumed to be
constant through
the anode assembly, the signal-to-noise ratio (SNR) is thus maximized.
[0066] By reducing the number of voltage probes to be
installed in order to detect
- 10 -
CA 03193088 2023- 3- 17
WO 2022/087732
PCT/CA2021/051512
11
a thermite reaction, reducing the number of signals to be monitored is made
possible.
The current method allows reducing the number of voltage signals or drops by a
factor
of ten compared to other solutions, such as, for instance in U.S. patent
9,982,355 B2
(D'Astolfo et al.), the content of which is incorporated herein by reference.
Therefore,
reducing costs related to thermite reaction detection becomes achievable.
[0067] The electrical current passing through an anode /aõode
can be determined
knowing the voltage drop V, the material resistivity p, the length L, and the
area A, by
the equation:
V*A
'anode =
1 0 [0068] The electrical current required to initiate a thermite
reaction on the anode
can be known by experimentation. When the exact location of a potential
electrical
short is not known, a conservative assumption can be made that any rise in
electrical
current for an anode assembly is concentrated on only one anode. Then, one or
more
voltage threshold levels can be calculated based on a current threshold level
for a single
anode in the anode assembly.
[0069] By "voltage threshold level", it is meant a
predetermined voltage drop
previously associated with a thermite reaction.
[0070] In the case where a pair of voltage probes is used to
measure the voltage
drop in an anode assembly, the voltage threshold levels VT i for thermite
reactions may
be calculated using the equation:
I" I
VT . = -R (ni - 1) Til
K E N
wherein: VTi is the voltage drop for level i threshold, Ti is the threshold
current per
anode for level i threshold, N represents the number of anodes in the cell, ni
is the
number of anodes per voltage probe, I is the total cell current, and K, = 17is
a
proportionality constant of the voltage probe.
[0071] The different levels i may be determined
experimentally based on threshold
current levels per anode that are known to cause a thermite reaction or damage
to the
anode.
- 11 -
CA 03193088 2023- 3- 17
WO 2022/087732
PCT/CA2021/051512
12
[0072] The proportionality constant of the voltage probe Ki
may be calculated as a
function of the material resistivity, temperature and the geometry of the
voltage probe.
[0073] When no thermite reaction is taking place in the
electrolytic cell, the
"baseline voltage" measures the voltage drop per voltage probe.
[0074] In the example where a pair of voltage probes is used to measure the
voltage
drop in an anode assembly, the baseline voltage Vb may be given by:
vb Rri )nil
Ki E N
[0075] Therefore, the ratio of the voltage threshold level to
baseline voltage is
given by:
VT . 1 Ti
=
Vb ni (I /N)ni
[0076] For a commercial electrolysis cell, the preceding
equation can be
approximated by the following equation:
VT = 1.5
= 1 ¨
Vb ni
[0077] For a first level threshold voltage VT!, the number of
anodes per voltage
vT,
probe ni can be as many as 100. Therefore, the threshold to base voltage ratio
¨, can
Vb
be as small as 1.015. Thus, the detection system should be capable of
detecting a 1.5%
increase in the voltage drop.
[0078] One approach to increase the sensitivity of the
detection system is by
choosing a location of the voltage probes that optimizes the signal-to-noise
ratio (SNR).
As explained above, the voltage probes are placed as far apart as possible so
that they
detect larger voltage drop signals. As the noise is assumed to be constant
through the
anode assembly, the signal-to-noise ratio (SNR) is thus maximized.
[0079] Figure 3 shows a graphic of voltage drops for a cell
comprising four anode
assemblies in which one of the anode assemblies is experiencing a simulated
thermite
reaction. In this instance, anode assemblies 1 and 2 were sequentially shorted
(first
assembly 1 then 2).
- 12 -
CA 03193088 2023- 3- 17
WO 2022/087732
PCT/CA2021/051512
13
[0080] From Figure 3, it can be seen that when a thermite
reaction is simulated, the
voltage drop increases on the shorted assembly (first assembly 1 then assembly
2). A
clear voltage drop is observed on the shorted assembly, while the other
assemblies
showed a reduction in the voltage drop, as less electrical current flowed to
them.
[0081] As shown in Figure 3, the voltage drop of each anode assembly is
independent of the voltage drops of the other anode assemblies allowing to
identify the
location of the anode assembly experiencing the thermite reaction. Thus, a
targeted
response can be considered in order to mitigate and, ideally, suppress the
thermite
reaction.
[0082] It can be noticed from Figure 3 that each anode assembly may
experience a
different baseline voltage.
[0083] The voltage drop has the characteristics of: having a
variable baseline
voltage, depending on position of anodes and their number, and depending on
the
overlapping dimensions of the anode and cathode (ACO). Indeed, the ACO affects
the
intensity of the electrical current flowing through the anode assembly.
Therefore, a
variation of the ACO affects the voltage drop experienced by the anode
assembly. The
voltage drop may also vary depending on the anode to cathode distance (ACD).
[0084] A thermite condition typically arises suddenly, which
allows for the
consideration of a number of potential signal processing techniques to enhance
detectability of thermite reactions besides the direct voltage reading from
the voltage
probe. One technique is to use the time derivative of the voltage drop to
indicate
occurrence of a potential thermite reaction. A sudden voltage drop variation
would
produce a large spike in the derivative signal (i.e., voltage drop derivative)
thereby
allowing to detect a potential thermite reaction.
[0085] Figure 4 shows a graphic of the voltage drop derivatives computed
from the
voltage drops of Figure 3. From Figure 4, it can be seen that a distinct
voltage drop
derivative initially positive, then negative is obtained for each simulated
thermite
reaction. The positive voltage drop derivative corresponds to the initial rise
of the
electrical current flowing through the shorted anode assembly. Adversely, the
negative
voltage drop derivative corresponds to the fall of the electrical current at
the end of the
simulated thermite reaction.
100861 As shown in Figure 4, the voltage drop derivative of
each anode assembly
- 13 -
CA 03193088 2023- 3- 17
WO 2022/087732
PCT/CA2021/051512
14
is independent of the voltage drop derivative of the other anode assemblies
allowing to
identify the location of the thermite reaction (i.e., which anode assembly is
experiencing
a thermite reaction). Thus, a targeted response can be considered in order to
mitigate
and, ideally, suppress the thermite reaction.
[0087] The voltage drop derivative takes into account the variation of the
voltage
drop rather than its magnitude. Therefore, the voltage drop derivative has a
zero
baseline voltage and is more sensitive to sudden changes in the voltage drop.
[0088] One disadvantage of the voltage drop derivative is
that it may not provide
a clear information as to the end of the thermite reaction.
[0089] Another technique would be to use the variance of multiple voltage
drops
at each time. "Variance" of a system measures how far a set of values are
spread out
from their average value.
[0090] Since a commercial electrolysis cell contains multiple
anodes or anode
assemblies, a comparison of the voltage drops from each of these assemblies
can be
performed. Generally, if an anode assembly is experiencing an electrical
short, more
current will flow to this assembly and less to the other anode assemblies.
Therefore, the
magnitude of the variance will change for the whole cell. This condition is a
tell-tale
condition for an isolated electrical short. In cases where the voltage drops
across the
anode assemblies are in a narrow range, a sudden change in the voltage drop of
an anode
assembly will increase the variance of the group. However, in cases where the
voltage
drops have high variability, a sudden change in a voltage drop may not produce
a
predictable change in variance of the whole cell.
[0091] Figure 5 shows a graphic of the voltage drop variance
computed from the
voltage drops of Figure 3. A voltage drop is measured for each anode assembly
at each
moment (or time interval). The variance of the voltage drop is computed for
each
moment (or time interval) based on the voltage drop signal. The signal thus
obtained is
the voltage drop variance.
[0092] In the case of the first shorted anode assembly, the
variance actually
dropped when the anode assembly was shorted. This can be due to the fact that
there
was already a low electrical current flowing through the assembly and the
short caused
it to come closer to the other assemblies, thus reducing the variance. In the
case of the
second shorted anode assembly, the current was similar to the three others, so
the
- 14 -
CA 03193088 2023- 3- 17
WO 2022/087732
PCT/CA2021/051512
electrical shorting caused the variance to increase.
100931 The voltage drop variance technique offers the benefit
of tracking a single
signal for the electrolytic cell instead of tracking multiple signals.
[0094] The voltage drop variance takes into account the
voltage drop of each anode
5 assembly of the electrolytic cell. Therefore the voltage drop variance is
dependent on
all anode assemblies of the electrolytic cell. The voltage drop variance does
not allow
to identify the location of the thermite reaction.
[0095] The voltage drop variance has the characteristics of:
having a variable
baseline voltage, depending on position of anodes and their number, and
depending on
10 the overlapping dimensions of the anode and cathode ACO, etc.
[0096] A further enhancement could be to use the derivative
of the variance as a
signal processing technique. This technique would reliably predict a sudden
change in
a voltage drop even if the initial variance is high.
[0097] Figure 6 shows a graphic of the derivative of the
voltage drop variance
15 computed from the voltage drops of Figure 3.
[0098] Figure 6 illustrates a derivative of the voltage drop
variance initially
positive, then negative for each simulated thermite reaction. The positive
derivative of
the voltage drop variance corresponds to the initial rise of the electrical
current flowing
through the shorted anode assembly. The negative derivative of the voltage
drop
variance corresponds to the fall of the electrical current at the end of the
simulated
thermite reaction. In this case, the sign of the variance can be ignored since
the
derivative depends essentially on the rate of change.
[0099] From Figure 6, it can be appreciated that the peaks in
the derivative of the
voltage drop variance have a high magnitude. Thereby, allowing for a more
sensitive
detection of a thermite reaction.
1001001 The technique based on the derivative of the voltage
drop variance offers
the benefit of tracking a single signal for the electrolytic cell instead of
tracking multiple
signals.
1001011 The derivative of the voltage drop variance takes into
account the voltage
drop of each anode assembly of the electrolytic cell. Therefore the derivative
of the
voltage drop variance is dependent on all anode assemblies of the electrolytic
cell.
- 15 -
CA 03193088 2023- 3- 17
WO 2022/087732
PCT/CA2021/051512
16
Consequently, the derivative of the voltage drop variance may not allow to
identify the
location of the thermite reaction.
[00102] The derivative of the voltage drop variance has the
advantages of having a
zero baseline voltage and detecting sudden changes in the voltage drop.
[00103] One disadvantage of the derivative of the voltage drop variance is
that it
may not provide a clear information as to the end of the thermite reaction.
[00104] Figure 7 illustrates a detection method 200, based on voltage
measurements, to anticipate and react to a potential thermite condition. The
method 200
also allows to prevent a sustained thermite reaction. The method 200 takes
advantage
of derivative and variance terms to enhance the reliability of the voltage
drop
interpretation.
[00105] The method 200 for detecting a thermite reaction in an
electrolytic cell
comprises measuring 210 a voltage drop for each anode assembly. The voltage
drop
corresponds to the current flow in the anode assembly. The voltage drop is
measured
using a pair of voltage probes located on the anode rod of the anode assembly.
The
method 200 also comprises computing 220 from the measured voltage drop at
least one
of a voltage drop derivative 221, a voltage drop variance 222 across the one
or more
anode assemblies, and a derivative of the voltage drop variance 223 across the
one or
more anode assemblies. The voltage drop variance and derivative of the voltage
drop
variance may be computed when the electrolytic cell comprises a plurality of
anode
assemblies.
[00106] The method 200 may optionally further comprise
filtering 225 the voltage
drop and/or filtering the voltage drop derivative, the variance of the voltage
drop, and
the derivative of the voltage drop variance.
[00107] The method 200 further comprises detecting 230 a thermite reaction
when
the voltage drop exceeds at least one voltage threshold level 231. Each
voltage threshold
level is a predetermined voltage drop previously associated with a thermite
reaction.
The threshold voltage levels may also be based on past operational data of the
electrolytic cell. Alternatively, the threshold voltage levels may be computed
from at
least one of past operational data of the electrolytic cell, operation
parameters, and
composition of the electrolytic cell.
[00108] Alternatively or additionally, detecting 230 a
thermite reaction may be
- 16 -
CA 03193088 2023- 3- 17
WO 2022/087732
PCT/CA2021/051512
17
performed when a variation in the voltage drop derivative occurs 232, such as
for
instance when the variation in voltage drop derivative exceeds a threshold
variation.
[00109] Alternatively or additionally, detecting 230 a
thermite reaction may be
performed when a variation in variance of the voltage drop across the anode
assemblies
occurs 232, such as for instance, when the variation in variance of the
voltage drop
across the anode assemblies exceeds a threshold variation.
[00110] Alternatively or additionally, detecting 230 a
thermite reaction may be
performed when a steep variation in derivative of the voltage drop variance
across the
anode assemblies occurs 234, such, for instance when the variation in
derivative of the
voltage drop across the anode assemblies exceeds a threshold variation.
[00111] As also illustrated on Figure 7, the method 200 may
optionally comprise
adjusting 240 one or more operational parameters of the electrolytic cell to
mitigate
and, ideally, suppress the thermite reaction upon detection of the thermite
reaction.
Adjusting 240 one or more operational parameter of the electrolytic cell may
comprise
changing the anode to cathode overlap (ACO) of one or more anode assemblies.
[00112] According to preferred embodiments, adjusting 240 one
or more
operational parameter of the electrolytic cell to mitigate and, ideally,
suppress the
thermite reaction may also comprise removing one or more anode assemblies from
the
electrolytic bath; changing the current supplied to at least one of the one or
more anode
assemblies or the electrolytic cell; changing a temperature of the
electrolytic bath;
and/or changing a chemistry of the electrolytic bath.
[00113] According to preferred embodimentsõ when the voltage
drop of one of the
anode assemblies exceeds the at least one voltage threshold level, adjusting
240 one or
more operational parameters of the electrolytic cell may take into account one
or more
of the exceeded voltage threshold levels; the magnitude of the voltage drop;
the
magnitude of the voltage drop derivative; the magnitude of the variance of the
voltage
drop; the magnitude of the derivative of the voltage drop variance.
[00114] As aforesaid, the method system and electrolytic cell
as disclosed herein
are particularly advantageous as they allow reducing the number of voltage
signals or
drops necessary for detecting a thermite reaction. From preliminary tests, it
is expected
that the reduction may be achieved by a factor up to 10. Although the method,
system
and electrolytic cell as disclosed herein use fewer voltage signals or drops,
fine tuning
- 17 -
CA 03193088 2023- 3- 17
WO 2022/087732
PCT/CA2021/051512
18
of the location of the voltage probes to gain a better signal to noise ratio
is provided.
Taking advantage of derivative and variance terms contribute to enhancing the
reliability of the signal interpretation. Finally, the system, method and
electrolytic cell
as disclosed herein are expected to permit reduction in maintenance and
operations
costs of the electrolytic cell due to a reduction in the number of signals to
install and
monitor.
[00115] Figure 8 shows a logical modular representation of a
system 1000 for
detecting a thermite reaction in an electrolytic cell in accordance with the
teachings of
the present application. As previously discussed, the electrolytic cell
comprises at least
one anode assembly of one or more metal-oxide oxide-containing anodes, at
least one
cathode, an electrolytic bath, and a current supply buss providing a current
to the at
least one anode assembly through a distinct anode rod for each anode assembly.
The
system 1000 provides an exemplary modular view of the controller 1100 involved
in
the detecting. The system 1000 may also comprise a remote monitoring station
1200.
In some embodiments, the controller 1100 may exchange data with the remote
monitoring station 1200 and the controller 1100 is therefore able to exchange
one or
more message and/or one or more commands with the remote monitoring station
1200.
[00116] In the depicted example of Figure 8, the controller
1100 comprises a
memory module 1120, a processor module 1130 and a network interface module
1140.
The processor module 1130 may represent a single processor with one or more
processor cores or an array of processors, each comprising one or more
processor cores.
The memory module 1120 may comprise various types of memory (different
standardized or kinds of Random Access Memory (RAM) modules, memory cards,
Read-Only Memory (ROM) modules, programmable ROM, etc.). The network
interface module 1140 represents at least one physical interface that can be
used to
communicate with other network nodes. The network interface module 1140 may be
made visible to the other modules of the controller 1100 through one or more
logical
interfaces. The actual stacks of protocols used by the physical network
interface(s)
and/or logical network interface(s) 1142, 1144, 1146, 1148 of the network
interface
module 1140 do not affect the teachings of the present application. The
variants of
processor module 1130, memory module 1120 and network interface module 1140
usable in the context of the present application will be readily apparent to
persons
skilled in the art.
- 18 -
CA 03193088 2023- 3- 17
WO 2022/087732
PCT/CA2021/051512
19
[00117] A bus 1170 is depicted as an example of means for
exchanging data
between the different modules of the controller 1100. The present invention is
not
affected by the way the different modules exchange information between them.
For
instance, the memory module 1120 and the processor module 1130 could be
connected
by a parallel bus, but could also be connected by a serial connection or
involve an
intermediate module (not shown) without affecting the teachings of the present
invention.
[00118] Likewise, even though explicit mentions of the memory
module 1120
and/or the processor module 1130 are not made throughout the description of
the
various embodiments, persons skilled in the art will readily recognize that
such modules
are used in conjunction with other modules of the controller 1100 to perform
routine as
well as innovative steps related to the present invention.
[00119] The controller 1100 may also comprise an optional
Graphical User
Interface (GUI) module 1150 comprising one or more display screen(s) forming a
display system, for the controller 1100. The display screens of the GUI module
1150
could be split into one or more flat panels, but could also be a single flat
or curved
screen visible from an expected user position (not shown). Skilled persons
will readily
understand that the GUI module 1150 may be used in a variety of contexts not
limited
to the previously mentioned examples.
1001201 The system 1000 may comprise a data storage system 1500 that
comprises
data related to brick positioning and may further log data while the
production is
performed. Figure 8 shows examples of the storage system 1500 as a distinct
database
system 1500A, a distinct module 1500B of the controller 1100 or a sub-module
1500C
of the memory module 1120 of the controller 1100. The storage system 1500 may
also
comprise storage modules (not shown) on the remote monitoring station 1200.
The
storage system 1500 may be distributed over different systems A, B, C and/or
the
remote monitoring station 1200 or may be in a single system. The storage
system 1500
may comprise one or more logical or physical as well as local or remote hard
disk drive
(HDD) (or an array thereof). The storage system 1500 may further comprise a
local or
remote database made accessible to the controller 1100 by a standardized or
proprietary
interface or via the network interface module 1140. The variants of the
storage system
1500 usable in the context of the present invention will be readily apparent
to persons
skilled in the art.
- 19 -
CA 03193088 2023- 3- 17
WO 2022/087732
PCT/CA2021/051512
[00121] A measurement input module 1160 and an optional
control module 1161
are provided in the controller 1100. The measurement input module 1160 and the
control module 1161 will be referred to hereinbelow as distinct logical
modules, but
skilled person will readily recognize that a single logical module may have
been shown
5 instead.
[00122] In some embodiment, an optional external input/output
(I/O) module 1162
and/or an optional internal input/output (I/O) module 1164 may be provided
with the
measurement input module 1160 and the control module 1161. The external I/O
module
1162 may be required, for instance, for interfacing with one or more robots,
one or more
10 input device (e.g., measurement probe) and/or one or more output device
(e.g., printer).
The internal input/output (I/O) module 1164 may be required, for instance, for
interfacing the controller 1100 with one or more instruments or controls (not
shown)
typically used in the context of electrolysis cell control (e.g., probes). The
I/O module
1164 may comprise necessary interface(s) to exchange data, set data or get
data from
15 such instruments or controls.
[00123] The measurement input module 1160 and processor module
1130 are
tightly related to the detection of the thermite reaction. In the example of
the system
1000, the measurement input module 1160 and the processor module 1130 may be
involved in various step of a method 200 described hereinabove.
20 1001241 While illustrative and presently preferred embodiments have
been
described in detail hereinabove, it is to be understood that the inventive
concepts may
be otherwise variously embodied and employed and that the appended claims are
intended to be construed to include such variations except insofar as limited
by the prior
art.
- 20 -
CA 03193088 2023- 3- 17