Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03199289 2023-04-20
Reactor Assembly, Sulfur-Containing Waste Treatment System, Method for Burning
Sulfur-Containing Waste, and Method for Making Sulfuric Acid by Regenerating
Sulfur-Containing Waste
CROSS REFERENCE TO RELATED APPLICATIONS
The present application claims the benefits of the Chinese Patent Application
Nos.
202011148953.4 and 202011150297.1 filed on Oct. 23, 2020 and the Chinese
Patent
Application Nos. 202110739636.8, 202110736744.X, 202110736751.X,
202110736752.4,
202121481507.5 and 202110736743.5 filed on Jun. 30, 2021, all of which are
incorporated
herein by reference.
FIELD
The present disclosure relates to sulfur-containing waste treatment, in
particular to a reactor
assembly, a sulfur-containing waste treatment system, a method for burning
sulfur-containing
waste and a method for making sulfuric acid by regenerating sulfur-containing
waste.
BACKGROUND
Concentrated sulfuric acid is widely used as a catalyst in the processes in
the petrochemical and
organic synthesis industries, in which a large amount of waste sulfuric acid
is produced. In some
organic synthesis processes, such as methyl methacrylate (MMA) synthesis
process and
acrylonitrile (AN) synthesis process, about 30 wt% - 45 wt% waste ammonium
sulfate is
produced, in addition to waste sulfuric acid. These sulfur-containing wastes
may cause serious
environmental pollution. Therefore, it is necessary to purify industrial waste
acids and sulfur-
containing waste liquors and recycle them as much as possible.
Existing sulfur-containing waste treatment methods mainly include high-
temperature
concentration, solvent extraction, alkali neutralization, chemical oxidation,
and high-
temperature combustion cracking, etc. At present, although the high-
temperature combustion
method is thorough and clean, it has some drawbacks, such as low combustion
efficiency, high
operating cost and complicated operation.
1
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
SUMMARY
To overcome the drawbacks in the prior art, the present disclosure provides a
reactor assembly
and a sulfur-containing waste treatment system. The reactor assembly can burn
and treat sulfur-
containing waste and inhibit the generation of nitrogen oxides.
To attain the above object, the present disclosure provides a reactor
assembly, which comprises
a reactor body having a hearth for performing a combustion reaction of sulfur-
containing waste
and a fuel gas inlet and a process gas outlet that are in communication with
the hearth, wherein
the hearth is of a cylindrical structure, the fuel gas inlet and the process
gas outlet are arranged
spaced apart from each other at two ends of the hearth in an axial direction
of the hearth, and
.. the fuel gas inlet is configured to be able to supply the hearth with fuel
flowing in the axial
direction of the hearth; the reactor assembly comprises a combustion air
supply mechanism,
which is configured to be able to supply the hearth with combustion air
flowing in the
circumferential direction of the inner wall of the hearth.
Optionally, the combustion air supply mechanism comprises a plurality of
groups of
combustion air inlets that are arranged at intervals in the axial direction of
the hearth.
Optionally, the plurality of groups of combustion air inlets comprise a first
group of combustion
air inlets and a second group of combustion air inlets, wherein the first
group of combustion air
inlets are arranged near the fuel gas inlet, and the second group of
combustion air inlets are
arranged near the process gas outlet; the reactor assembly comprises a control
device for
controlling the combustion air supply mechanism, and the control device is
configured to:
control the air to enter via the first group of combustion air inlets, so that
the oxygen content at
the first group of combustion air inlets is a first oxygen content; and
control the air to enter via
the second group of combustion air inlets, so that the oxygen content at the
second group of
combustion air inlets is a second oxygen content, wherein the second oxygen
content is equal
to a theoretical oxygen demand of a normal combustion process of the sulfur-
containing waste,
the first oxygen content is smaller than the second oxygen content, and the
first oxygen content
and the second oxygen content are controlled so that the fuel and the sulfur-
containing waste to
be burned have combustion for at least two times, including a first combustion
corresponding
to the first oxygen content and a second combustion corresponding to the
second oxygen
2
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
content, thereby a gas containing sulfur dioxide is obtained finally.
Optionally, the first combustion has an oxygen coefficient X1 and a
temperature of 1,100-
1,250 C; the last combustion has an oxygen coefficient X3 and a temperature of
1,000-1,100 C;
the optional remaining combustions have an oxygen coefficient X2 and a
temperature of
.. 1,100-1,200 C respectively and independently, and 0.5<X1<0.85, 0.7<X1+X2<1,
and
l<X1+X2+X3<1.15; the oxygen coefficient refers to a ratio of the molar volume
of the oxygen-
containing combustion gas measured in the molar content of oxygen to the molar
content of
oxygen required for complete combustion of the fuel.
According to the above technical scheme, the fuel gas can enter the hearth
through the fuel gas
.. inlet and flow in the axial direction of the hearth; at the same time, the
combustion air supplied
by the combustion air supply mechanism to the hearth flows in the
circumferential direction of
the inner wall of the hearth; thus, the mixture of fuel gas and combustion air
flows in a spiral
form toward the process gas outlet. Therefore, in the spiral flow process, the
residence time of
the gas mixture in the hearth is longer, and the gas mixture has a combustion
reaction with the
mixed liquor of sulfur-containing waste extensively, thereby the combustion
efficiency of the
reactor assembly is improved. Moreover, since the detention time of the gas
mixture in the
hearth is longer, the distance between the fuel gas inlet and the process gas
outlet can be
shortened relatively, so that the reactor assembly in the present disclosure
can be miniaturized.
The present disclosure further provides a sulfur-containing waste treatment
system, which
comprises:
the reactor assembly described above, which is configured to enable the sulfur-
containing waste
to perform a combustion reaction, so as to obtain a first gas containing
sulfur dioxide; a heat
recovery unit configured to recover heat from the first gas to obtain a second
gas; a purifying
and cooling unit configured to purify and cool down the second gas to obtain a
third gas; and a
drying unit configured to dry the third gas to obtain a fourth gas; an
oxidation and absorption
unit configured to oxidize and absorb the fourth gas to obtain sulfuric acid
and exhaust gas.
According to the above technical scheme, with the sulfur-containing waste
treatment system
and the reactor assembly provided by the present disclosure, combustion in a
lean-oxygen
3
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
environment is carried out first in the reactor to inhibit the generation of
nitrogen oxides; then
combustion in a rich-oxygen environment is carried out at the terminal to
complete the expected
combustion process; however, since the temperature at the terminal is
relatively low, the
generation of nitrogen oxides is also inhibited. Since nitrogen oxides are
reduced, the content
of sulfur dioxide in the process gas can be increased; in addition, since the
expected combustion
process is completed, there is no adverse effect on the normal process.
The present disclosure further provides a method based on the above reactor
assembly for
burning sulfur-containing waste and a method based on the above sulfur-
containing waste
treatment system for making sulfuric acid by regenerating sulfur-containing
waste.
The present disclosure attains the following beneficial effects: the present
disclosure provides
optimized and improved process and device for mixed treatment of waste
sulfuric acid, sulfur-
containing solid waste, sulfur-containing liquid waste and sulfur-containing
gas waste, with
which the process flow is shortened as far as possible, less equipment is
required, the operation
is simpler, and the utilization ratio of heat energy is higher. The sulfur
element in the raw
material is regenerated into 93%-100% sulfuric acid and fuming sulfuric acid.
BRIEF DESCRITION OF THE DRAWINGS
Fig. 1 is a schematic diagram of the reactor assembly in an embodiment of the
present disclosure;
Fig. 2 is a side view of the hearth of the reactor assembly in the present
disclosure;
Fig. 3 is a front view of the hearth of the reactor assembly in the present
disclosure;
Fig. 4 is a schematic diagram of the heating device of the reactor assembly in
the present
disclosure;
Fig. 5 is a flow chart of the reactor combustion control method of the sulfur-
containing waste
treatment system provided in an embodiment of the present disclosure;
Fig. 6 is a flow chart of the reactor combustion control method of the sulfur-
containing waste
treatment system provided in an embodiment of the present disclosure;
Fig. 7 is a process flow diagram of the sulfur-containing waste treatment
system in an
embodiment of the present disclosure;
4
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
Fig. 8 is a flow chart of the method for making sulfuric acid by regenerating
sulfur-containing
waste in the present disclosure;
Fig. 9 is a schematic diagram of the dust removal unit and the heat recovery
unit;
Fig. 10 is a schematic diagram of the quenching and humidifying column;
Fig. 11 is a schematic diagram of the cooling and absorption column;
Fig. 12 is a schematic diagram of the oxidation and absorption unit; and
Fig. 13 is a schematic diagram of a converter.
Reference Numbers
100 - reactor assembly; 110 - reactor body; 111 - hearth; 112 - fuel gas
inlet; 113 - process gas
outlet; 114 - first combustion air inlet; 115 - second combustion air inlet;
116 - fluid sprayer
120 - heating device; 121 - heating shell; 122 - electric heating mechanism;
123 - heating
chamber; 124 - heating gas inlet; 125 - heating gas outlet; 130 - blower fan;
200 - dust removal unit; 210 - cyclone dust collector; 220 - ceramic membrane
filter;
300 - heat recovery unit; 310 - waste heat boiler; 320 - steam superheater;
400 - quenching and humidifying column; 410 - first column body; 411 - cooling
chamber; 412
- chamber inlet; 413 - chamber outlet; 420 - spraying assembly; 421 - first
spraying port; 422 -
second spraying port; 430 - unidirectional spray head;
500 - cooling and absorption column; 510 - second column body; 511 - gas
inlet; 512 - gas
outlet; 513 - partition; 520 - first spraying mechanism; 530 - second spraying
mechanism; 540
.. - first filler; 550 - second filler; 560 - water pump; 570 - cooler;
600 - electrostatic mist precipitator;
700 - drying unit;
800 - oxidation and absorption unit; 810 - blower fan; 820 - heating furnace;
830 - first external
heat exchanger; 840 - converter; 841 - converter shell; 842a - first
conversion gas inlet; 842b -
second conversion gas inlet; 843a - first conversion gas outlet; 843b - second
conversion gas
5
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
outlet; 844a - first catalyst layer; 844b - second catalyst layer; 844c -
third catalyst layer; 844d
- fourth catalyst layer; 845a - first heat exchange pipeline; 845b - second
heat exchange pipeline;
846 - grating; 847 - heat-resistant ceramic ball; 850 - first heat exchanger;
860 - second heat
exchanger; 870 - second external heat exchanger; 880 - multi-stage absorption
column; 890 -
sulfuric acid cooler.
DETAILED DESCRIPTION
Hereunder some embodiments of the present disclosure will be detailed with
reference to the
accompanying drawings. It should be understood that the embodiments described
herein are
only provided to describe and explain the present disclosure, but are not
intended to constitute
any limitation to the present disclosure.
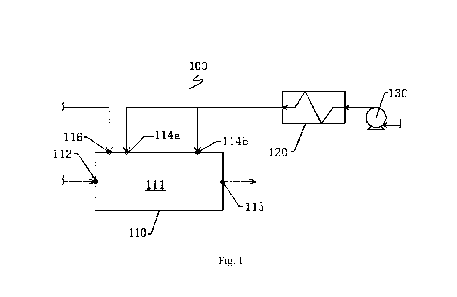
As shown in Figs. 1-3, the reactor assembly in the present disclosure
comprises a reactor body
100, which has a hearth 111 for the sulfur-containing waste to perform a
combustion reaction,
and the hearth 111 is of a cylindrical structure. The reactor body 100 is
further provided with a
fuel gas inlet 112 and a process gas outlet 113 that are in communication with
the hearth 111.
The fuel gas inlet 112 and the process gas outlet 113 are arranged space apart
from each other
at the two ends of the hearth 111 in the axial direction of the hearth 111,
and the fuel gas inlet
112 is configured to supply the hearth 111 with a fuel gas flowing in the
axial direction of the
hearth 111. The reactor assembly 100 further comprises a combustion air supply
mechanism,
which is configured to be able to supply the hearth 111 with combustion air
flowing in the
circumferential direction of the inner wall of the hearth 111.
In the present disclosure, the fuel gas can enter the hearth 111 through the
fuel gas inlet 112 and
flow in the axial direction of the hearth 111; at the same time, the
combustion air supplied by
the combustion air supply mechanism to the hearth 111 flows in the
circumferential direction
of the inner wall of the hearth 111; thus, the mixture of fuel gas and
combustion air flows in a
spiral form (as shown in Fig. 2) toward the process gas outlet 113. Therefore,
in the spiral flow
process, the residence time of the gas mixture in the hearth 111 is longer,
and the gas mixture
has a combustion reaction with the mixed liquor of sulfur-containing waste
extensively, thereby
the combustion efficiency of the reactor assembly is improved. Moreover, since
the detention
time of the gas mixture in the hearth 111 is longer, the distance between the
fuel gas inlet 112
6
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
and the process gas outlet 113 can be shortened relatively, so that the
reactor assembly in the
present disclosure can be miniaturized.
It should be understood that the combustion air supply mechanism can be
designed in a variety
of forms to drive the combustion air to flow in the circumferential direction
of the inner wall of
the hearth 111. For example, the combustion air supply mechanism may comprise
a combustion
air nozzle for supplying combustion air into the hearth 111, and a draft fan
arranged in the hearth
111 to change the flow direction of the combustion air, so that the combustion
air can flow in
the circumferential direction of the inner wall of the hearth 111 under a
drafting effect of the
draft fan after it flows out of the combustion air nozzle. In order to further
reduce the cost and
simplify the structure of the reactor assembly, in an embodiment of the
present disclosure, the
combustion air supply mechanism comprises a group of combustion air inlets,
including a first
combustion air inlet 114 and a second combustion air inlet 115 in
communication with the
hearth 111 respectively. The first combustion air inlet 114 and the second
combustion air inlet
115 are configured to supply the hearth 111 with combustion air flowing in a
tangential direction
of the hearth 111 respectively, and the flow direction of the combustion air
supplied via the first
combustion air inlet 114 is the same as the flow direction of the combustion
air supplied via the
second combustion air inlet 115. Since both the first combustion air inlet 114
and the second
combustion air inlet 115 are arranged in a tangential direction of the inner
wall of the hearth
111, both the combustion air jetted from the first combustion air inlet 114
and the combustion
air jetted from the second combustion air inlet 115 can flow in the
circumferential direction of
the inner wall of the hearth 111, thereby other parts can be omitted, and the
structure is
simplified and can be serviced more conveniently.
Of course, in addition to the first combustion air inlet 114 and the second
combustion air inlet
115, the group of combustion air inlets may further include a third combustion
air inlet, and a
fourth combustion air inlet, etc. All these combustion air inlets are
positioned in the same radial
plane of the hearth 111 and arranged in the same orientation (i.e., clockwise
or
counterclockwise), so as to ensure that the gas mixture flows in a spiral form
in the hearth 111.
It should be noted that in an embodiment of the present disclosure, as shown
in Figs. 2 and 3,
both the opening direction of the first combustion air inlet 114 and the
opening direction of the
7
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
second combustion air inlet 115 are perpendicular to the axial direction of
the hearth 111, which
is to way, the flow direction of the fuel gas is perpendicular to a plane
formed by the flow
direction of the combustion air. In another embodiment of the present
disclosure, both the
opening direction of the first combustion air inlet 114 and the opening
direction of the second
combustion air inlet 115 are inclined toward the fuel gas inlet 112. An
advantage of such an
arrangement is that the flow direction of the combustion air into the hearth
111 is opposite to
the flow direction of the fuel gas, thereby the combustion air and the fuel
gas can be mixed
more extensively, and the flow speed of the gas mixture is decreased, thus the
duration of flow
of the gas mixture in the hearth 111 is further increased, thereby the reactor
assembly can be
further miniaturized. Of course, the opening direction of the first combustion
air inlet 114 and
the opening direction of the second combustion air inlet 115 only has to be
slightly inclined
toward the fuel gas inlet 112; for example, they can be inclined by 20-40
degrees toward the
fuel gas inlet 112.
In order to supply the combustion air more plentifully, in an embodiment of
the present
disclosure, as shown in Fig. 2, the combustion air supply mechanism includes a
plurality of
groups of combustion air inlets, which are arranged at intervals in the axial
direction of the
hearth 111. In the illustrated preferred embodiment, the reactor body 100 is
provided with two
above-mentioned first combustion air inlets 114 and two above-mentioned second
combustion
air inlets 115 respectively, wherein the combustion air inlets 114a and 115a
near the fuel gas
inlet 112 constitute a first group of combustion air inlets, and combustion
air inlets 114b and
115b near the process gas outlet 113 constitute a second group of combustion
air inlets. Thus,
the combustion of the sulfur-containing waste can be controlled by controlling
the amount of
combustion air introduced into the hearth 111 via the first group of
combustion air inlets and
the second group of combustion air inlets.
Specifically, as illustrated in the flow chart of the method for reactor
combustion control of the
sulfur-containing waste treatment system shown in Fig. 5, the method
comprises:
Step Sll: detecting the oxygen content at the first group of combustion air
inlets and the second
group of combustion air inlets;
For example, oxygen sensors may be provided at the first group of combustion
air inlets and
8
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
the second group of combustion air inlets to detect the oxygen content.
Step S12: controlling the air to enter into the first group of combustion air
inlets, so that the
oxygen content at the first group of combustion air inlets is a first oxygen
content;
For example, air can be supplemented/reduced at the first group of combustion
air inlets with
reference to the oxygen content at the first group of combustion air inlets,
so that the oxygen
content at the first group of combustion air inlets reaches a first oxygen
content, which is, for
example, 60% of the theoretical oxygen demand in the normal combustion process
of the sulfur-
containing waste, thereby the sulfur-containing waste is burned in an lean-
oxygen environment,
so that the generation of nitrogen oxides from the substances containing
nitrogen element in the
sulfur-containing waste, such as (N114)2504 and/or N114}1SO4, is inhibited
during the reaction.
Step S13: controlling the air to enter into the second group of combustion air
inlets, so that the
oxygen content at the second group of combustion air inlets is a second oxygen
content, which
is the theoretical oxygen demand in the normal combustion process of the
sulfur-containing
waste, wherein the first oxygen content is smaller than the second oxygen
content.
For example, in order to complete the normal combustion process, air is
supplemented at the
second group of combustion air inlets at the terminal of the reactor, so that
the oxygen content
at the second group of combustion air inlets reaches a second oxygen content,
which, for
example, is the theoretical oxygen demand in the normal combustion process of
the sulfur-
containing waste; thus, the normal combustion process of the sulfur-containing
waste can be
completed. Owing to the fact that the amount of generated nitrogen oxides is
related with the
temperature, i.e., increases as the temperature rises, the amount of nitrogen
oxides generated at
the terminal of the reactor away from the flame cone will also be reduced even
though the
oxygen content is adequate if the temperature is relatively low there.
With the method described above, since nitrogen oxides are reduced, the
content of sulfur
dioxide in the process gas in the reactor can be increased; in addition, since
the expected
combustion process is completed, there is no adverse effect on the normal
process.
Fig. 6 is a flow chart of the method for reactor combustion control of the
sulfur-containing
waste treatment system in another embodiment of the present disclosure. In
this embodiment,
9
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
the reactor body further comprises a third combustion air inlet arranged in
the middle of the
reactor body, and the method comprises:
Step S21: detecting the oxygen content at the first group of combustion air
inlets and the second
group of combustion air inlets;
Step S22: controlling the air to enter into the first group of combustion air
inlets, so that the
oxygen content at the first group of combustion air inlets is a first oxygen
content;
For example, the embodiment of steps S21-22 is similar to the above embodiment
of steps Sll-
12, and will not be described further here.
Step S23: controlling the air to enter into the third group of combustion air
inlets, so that the
oxygen content at the third group of combustion air inlets is a third oxygen
content. The third
oxygen content may be, for example, 95% of the second oxygen content. The
sulfur-containing
waste is still burned in a lean-oxygen environment, so that the generation of
nitrogen oxides
from the substances containing nitrogen element in the sulfur-containing
waste, such as
(N114)2504 and/or N}14}1SO4, is inhibited.
Step S24: controlling the air to enter into the second group of combustion air
inlets, so that the
oxygen content at the second group of combustion air inlets is a second oxygen
content, which
is the theoretical oxygen demand in the normal combustion process of the
sulfur-containing
waste, wherein the first oxygen content is smaller than the second oxygen
content, and the third
oxygen content is smaller than the second oxygen content.
For example, similarly, in order to complete the normal combustion process,
air is supplemented
at the second group of combustion air inlets at the terminal of the reactor,
so that the oxygen
content at the second group of combustion air inlets reaches a second oxygen
content, thereby
the normal combustion process of the sulfur-containing waste can be completed.
It can be understood that although an embodiment having two group of
combustion air inlets
and an embodiment having three group of combustion air inlets are described
above, it is also
possible that the reactor have more air inlets; for example, the reactor may
have a plurality of
third groups of combustion air inlets, and these air inlets are used to supply
air at the same time,
as long as the oxygen content at the air inlets at the terminal of the reactor
meets the theoretical
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
oxygen demand in the normal combustion process of the sulfur-containing waste,
while the
oxygen contents at the other air inlets are lower than the theoretical oxygen
demand in the
normal combustion process of the sulfur-containing waste. The specific
arrangement of the air
inlets will not be further detailed here.
In the above process, the fuel and the sulfur-containing waste to be burned
are controlled to
have combustion for two times, including a first combustion corresponding to
the first oxygen
content and a second combustion corresponding to the second oxygen content,
and finally a gas
containing sulfur dioxide is obtained.
The first combustion has an oxygen coefficient X1 and a temperature of 1,100-
1,250 C; the last
combustion has an oxygen coefficient X3 and a temperature of 1,000-1,100 C;
the optional
remaining combustions has an oxygen coefficient X2 and a temperature of 1,100-
1,200 C
respectively and independently, and 0.5<X1<0.85, 0.7<X1+X2<1, 1<X1+X2+X3<1.15.
The
oxygen coefficient refers to a ratio of the molar volume of the oxygen-
containing combustion
gas measured in the oxygen contained in the oxygen-containing combustion gas
to the molar
volume of oxygen required for complete combustion of the fuel.
In the present disclosure, the oxygen coefficient refers to a ratio of the
molar volume of the
oxygen-containing combustion gas measured in the oxygen contained in the
oxygen-containing
combustion gas to the molar volume of oxygen required for complete combustion
of the fuel in
each combustion process. Here, the fuel refers to the initial total fuel
rather than the remaining
fuel after the previous combustion.
According to the present disclosure, by controlling the volume of the oxygen-
containing
combustion gas used in each combustion process, the oxygen coefficient in each
combustion
process can be adjusted. By controlling the oxygen coefficient in combination
with other
process conditions, e.g., temperature, in each combustion process, normal
combustion of the
sulfur-containing waste can be realized, and a process gas with high sulfur
dioxide content can
be obtained.
In addition, especially for reaction materials containing nitrogen element,
with the method
provided by the present disclosure, the content of nitrogen oxides (NO) in the
process gas can
11
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
be decreased significantly, without additional denitration treatment.
Therefore, the method is
environment-friendly and cost-efficient.
Preferably, in the first combustion, the oxygen coefficient is Xl, and the
temperature is 1,150-
1,250 C; in the last combustion, the oxygen coefficient is X3, and the
temperature is 1,050-
1,100 C; in the optional remaining combustions, the oxygen coefficient is X2
respectively and
independently, the temperature is 1,100-1,200 C respectively and
independently, and
0.7<X1<0.85, 0.8<X1+X2<1, and 1<X1+X2+X3<1.15. Thus, the inventor of the
present
disclosure has found: by particularly controlling the oxygen coefficient and
the temperature in
each combustion reaction process to be within the above-mentioned ranges in
combination, the
sulfur-containing waste will be burned more fully, the content of sulfur
dioxide in the resulting
process gas containing sulfur dioxide will be higher; especially, for reaction
materials
containing nitrogen element, the content of NO in the resulting process gas
will be lower.
According to the present disclosure, the at least two times of combustion
means that the
combustion can be carried out for more than two times (e.g., three times, four
times, or five
times, etc.). That is to say, the optional existence means that the remaining
combustion
processes may exist or don't exist.
Preferably, the combustion is carried out for 2-3 times.
According to the present disclosure, it should be noted: when there are two
times of combustion,
only the first combustion and the last combustion exist, without any remaining
combustion
process.
According to a preferred embodiment of the present disclosure, the combustion
is carried out
for two times, and the method comprises:
In the present of the oxygen-containing combustion gas, controlling the fuel
and the sulfur-
containing waste to be burned to have a first combustion and a second
combustion, to obtain a
gas containing sulfur dioxide.
Preferably, the conditions of the first combustion include: the oxygen
coefficient is X1 , and the
temperature is 1,150-1,250 C; the conditions of the second combustion include:
the oxygen
coefficient is X3, and the temperature is 1,050-1,100 C; and 0.7<X1<0.85, and
l<X1+X3<1.15.
12
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
According to another preferred embodiment of the present disclosure, the
combustion is carried
out for three times, and the method comprises:
In the present of the oxygen-containing combustion gas, controlling the fuel
and the sulfur-
containing waste to be burned to have a first combustion, a second combustion,
and a third
combustion, to obtain a gas containing sulfur dioxide.
Preferably, the conditions of the first combustion include: the oxygen
coefficient is X1 , and the
temperature is 1,150-1,250 C; the conditions of the second combustion include:
the oxygen
coefficient is X2, and the temperature is 1,100-1,200 C; the conditions of the
third combustion
include: the oxygen coefficient is X3, and the temperature is 1,050-1,100 C;
and 0.7<X1<0.85,
0 .8<X1+X2<1, and 1<X1+X2+X3<1.15.
Preferably, the oxygen-containing combustion gas is selected from at least one
of air (containing
21 mol% oxygen), oxygen-enriched air (containing 21-40 mol% oxygen), pure
oxygen and
liquid oxygen. The inventor of the present disclosure has found: by using
oxygen-enriched air
or pure oxygen gas as the combustion gas, the fuel consumption can be reduced
greatly, and
more sulfur-containing waste can be treated in a device at an equivalent
scale, thus the
equipment investment can be reduced, and the energy consumption and the
operating cost can
be reduced.
Preferably, the sulfur-containing waste is selected from at least one of waste
sulfuric acid,
sulfur-containing waste liquor, and sulfur-containing waste gas.
There is no particular restriction on the source and kind of the sulfur-
containing waste in the
present disclosure. For example, the sulfur-containing waste liquor may be
liquid sulfur, sulfur-
containing waste liquor containing ammonium sulfate, sulfur-containing waste
liquor
containing ammonium hydrogen sulfate, sulfur-containing waste liquor
containing ferric sulfate,
sulfur-containing waste liquor containing methyl sulfate, or sulfur-containing
waste liquor
containing gypsum, etc.; the sulfur-containing waste gas may be hydrogen
sulfide, sulfur
dioxide, or other sulfur-containing waste gas, etc., for example; preferably,
the mass
concentration of sulfuric acid in the waste sulfuric acid is 50-99%.
Preferably, the water content
of the sulfur-containing waste is less than 15 wt%.
13
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
According to a preferred embodiment of the present disclosure, the reactor
assembly further
comprises a fluid sprayer 116, which is configured to fully atomize the sulfur-
containing waste
(e.g., sulfur-containing waste liquor). Thus, the sulfur-containing waste is
atomized under the
high-pressure atomizing air flow from the fluid sprayer and then sprayed into
the hearth for
combustion with the oxygen-containing combustion gas and the fuel, thereby the
combustion
of the sulfur-containing waste is more intensive and the content of sulfur
dioxide in the process
gas is improved. Preferably, the pressure of the fluid sprayer is 0.4-0.8 MPa,
more preferably is
0.5-0.7 MPa.
According to a preferred embodiment of the present disclosure, the sulfur-
containing waste
contains sulfur-containing solid waste (e.g., sulfur-containing ore), and the
method in the
present disclosure comprises: controlling the sulfur-containing solid waste to
have combustion
(the combustion conditions include: 800-1,050 C temperature) for one time, to
obtain a gas
containing sulfur dioxide II; controlling the sulfur-containing waste liquor
and/or the sulfur-
containing waste gas to have combustion for at least two times, to obtain a
gas containing sulfur
dioxide I; and merging the gas containing sulfur dioxide II with the gas
containing sulfur
dioxide Ito obtain the gas containing sulfur dioxide in the present
disclosure.
According to the present disclosure, the fuel is a high-heat combustible
material that can
combust to provide heat for the combustion of the sulfur-containing waste.
Preferably, the heat
value of the fuel is higher than or equal to 500 kcal/Nm3; for example, the
heat value of natural
gas is 9,700 kcal/Nm3.
Preferably, the fuel is selected from at least one of natural gas, sulfur,
liquefied hydrocarbons,
hydrogen sulfide sour gas, and heavy oil organic substances.
Preferably, the liquefied hydrocarbons are selected from at least one of
liquefied ethylene,
liquefied ethane, liquefied propylene, liquefied propane, liquefied butene and
liquefied butane.
Preferably, the heavy oil organic substances are selected from at least one of
gasoline, kerosene
and diesel oil.
According to the present disclosure, the consumption of the fuel may be
selected and adjusted
according to the amount of the sulfur-containing waste.
14
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
According to the present disclosure, it should be noted specially that the
sulfur-containing waste
and the fuel may be the same substance (e.g., both of them may be hydrogen
sulfide). Those
skilled in the art should appreciate: in the case that the sulfur-containing
waste is hydrogen
sulfide sour gas, the hydrogen sulfide sour gas itself may be used as the
fuel, without the need
for any other additional fuel. Such a case still belongs to the inventive
concept of the present
disclosure and should not be understood by those skilled in the art as
constituting any limitation
to the present disclosure.
Preferably, with the method for burning sulfur-containing waste in the present
disclosure, in the
resulting gas containing sulfur dioxide, the content of sulfur dioxide is 3-12
mol%; the content
of NO is lower than or equal to 100 mg/m3, and the content of oxygen is 0.5-5
mol%.
In the present disclosure, by controlling the conditions of each combustion
reaction process,
especially by controlling the temperature and the oxygen coefficient, the
first combustion
reaction process of the fuel and the sulfur-containing waste to be burned is
carried out under
the conditions of high temperature and lean oxygen, thereby the sulfur-
containing waste can be
burned to generate sulfur dioxide, while the nitrogen-containing material
generates nitrogen
under the lean oxygen condition; the subsequent combustion is carried out
under the conditions
of lower temperature and rich oxygen, thereby the normal combustion of the
fuel and the sulfur-
containing waste is ensured, while the nitrogen generated in the previous
combustion can't react
further to generate nitrogen oxides. Thus, through coordinated combustion
reaction processes,
a process gas with higher sulfur dioxide content and lower NO content is
obtained, and the
obtained gas containing sulfur dioxide doesn't contain any combustible
component.
According to the present disclosure, preferably, at least one of the oxygen-
containing
combustion gas, the fuel and the sulfur-containing waste contains nitrogen
element; wherein,
the sulfur-containing waste is a nitrogen-containing and sulfur-containing
substance, such as
ammonium hydrogen sulfate and ammonium sulfate, etc., for example; the fuel is
a combustible
material containing nitrogen, for example; the oxygen-containing combustion
gas is air, for
example.
According to a preferred embodiment of the present disclosure, the method in
the present
disclosure further comprises: treating the sulfur-containing waste by water
removal first, and
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
then controlling the sulfur-containing waste and the fuel to have combustion
for at least two
times in the presence of the oxygen-containing combustion gas to obtain a gas
containing sulfur
dioxide.
There is no particular restriction on the specific operation of the water
removal treatment, as
long as the water in the sulfur-containing waste is removed at least partially
in the water removal
treatment. For example, the water removal treatment may be carried out by
evaporation and
concentration.
In the method provided by the present disclosure, the combustion conditions of
the sulfur-
containing waste are controlled especially, and the combustion reaction
processes are
coordinated, thereby the combustion of the sulfur-containing waste is more
complete, a process
gas with higher sulfur dioxide content is obtained, and the obtained gas
containing sulfur
dioxide doesn't contain any combustible component.
Particularly, for nitrogen-containing reaction materials, by particularly
controlling the
conditions of each combustion process with the method provided by the present
disclosure, a
process gas with higher sulfur dioxide content and lower NO content is
obtained, and almost
all nitrogen content in the nitrogen-containing reaction materials is emitted
in the form of
nitrogen gas, except for trace NOR. With the method provided by the present
disclosure, the
content of nitrogen oxides (NOR) in the resulting process gas can be reduced
significantly,
without any additional denitration treatment.
On that basis, the present disclosure further provides a method for making
sulfuric acid from
sulfur-containing waste, which comprises the following steps:
(1) burning the sulfur-containing waste to obtain a gas containing sulfur
dioxide;
(2) oxidizing the gas containing sulfur dioxide to obtain a gas containing
sulfur trioxide;
(3) absorbing the gas containing sulfur trioxide to obtain sulfuric acid.
Hereunder the present disclosure will be detailed in examples.
Unless otherwise specified, all the raw materials used in the following
examples are
commercially available.
16
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
Unless otherwise specified, the following examples were carried out in the
aforementioned
reactor assembly. In the reactor assembly, two groups of combustion gas inlets
are distributed
in the axial direction of the reactor (in the axial direction of the reactor,
the axial length of a
straight cylindrical section of the hearth is L, and the length-diameter ratio
is 4-10 (e.g., the
length-diameter ratio is 5), and two combustion gas inlets are arranged at
0.25L from the fuel
inlet end of the hearth and 0.5L from the gas outlet end of the hearth
respectively), so as to
supply oxygen-containing combustion gas required for the combustion process,
and the
oxygen-containing combustion gas inlets are configured to supply oxygen-
containing
combustion gas flowing in the tangential direction of the inner wall of the
hearth into the hearth.
Example lA
The specific compositions of the fuel used in this example and the sulfur-
containing waste from
the chemical fiber industry are shown in table 1, and the oxygen-containing
combustion gas is
air with 21 mol% oxygen content.
Table 1
Component Waste sulfuric acid Sulfur-containing Natural
gas /wt%
/wt% waste liquor /wt%
H2SO4 16.8
H20 30.2 48.4
(NH4) 2SO4 39.5
N11411SO4 52.2
Polymer (organic 10.2
substance)
Acrylic acid 1.9
Others (methanol, 0.8
MMA)
CH4 96.3
C2H6 2.58
Cl-05 0.72
N2 0.4
The reactor was heated up to 1,200 C, the natural air is pressurized by an air
blower then heated
up to 630 C in an electric heating furnace, and entered the reactor through
the combustion gas
inlets; the sulfur-containing waste was sprayed into the hearth of the reactor
from the sulfur-
containing waste inlet by a fluid sprayer in 0.6 MPa high-pressure atomizing
air; and the fuel
17
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
was introduced into the hearth from the fuel inlet.
The process was as follows:
38.11t sulfur-containing waste (68.22 wt% waste sulfuric acid + 31.78 wt%
sulfur-containing
waste liquor) and 3990 kg fuel were introduced into the reactor through the
sulfur-containing
waste inlet and the fuel inlet respectively, and were moved in the axial
direction of the hearth
to have a first combustion with the oxygen-containing combustion gas
introduced from the first
oxygen-containing combustion gas inlet, and then have a second combustion with
the oxygen-
containing combustion gas introduced from the second oxygen-containing
combustion gas inlet;
wherein the conditions of the first combustion included: the oxygen
coefficient was X1=0.7,
and the temperature was 1,200 C; the conditions of the second combustion
included: the
oxygen coefficient was X3, X1+X3=1.05, and the temperature was 1,100 C; thus a
gas
containing sulfur dioxide was obtained;
In the gas containing sulfur dioxide discharged from the reactor, the content
of sulfur dioxide
was 3.8 mol%, the content of oxygen was 2 mol%, the content of NO was lower
than or equal
to 100 mg/Nm3, and the conversion ratio of sulfur dioxide was 99 mol%.
Example 1B
The method was essentially the same as that in the Example 1A, except that the
oxygen-
containing combustion gas was oxygen-enriched air with 40 mol% oxygen content,
and other
methods were the same as in Example 1.
In the gas containing sulfur dioxide discharged from the reactor, the content
of oxygen was 2
mol%, the content of sulfur dioxide was 6 mol%, the content of NO was lower
than or equal
to 80 mg/Nm3, and the conversion ratio of sulfur dioxide was 99 mol%.
Example 2
The specific compositions of the chlorine-containing waste sulfuric acid,
hydrogen sulfide sour
gas and fuel used in this example are shown in table 2, and the oxygen-
containing combustion
18
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
gas is air with 21 mol% oxygen content.
Table 2
Chlorine-
Waste gas containing
Mass fraction containing waste Natural gas
/wt%
hydrogen sulfide /wt%
sulfuric acid /wt%
H2SO4 50
H20 48
Polymer (organic
2
substance)
H2S 94.98
H20 4.45
CO2 0.47 0.3
Cl-C3 0.1 3
CH4 93.5
N2 3.2
The reactor was heated up to 1,100 C, and natural air was blasted by an air
blaster into the
reactor through the combustion gas inlet. The sulfur-containing waste was
sprayed into the
hearth of the reactor from the sulfur-containing waste inlet by a fluid
sprayer in 0.7 MPa high-
pressure atomizing air; and the fuel was introduced into the hearth from the
fuel inlet.
In this example, 7t sulfur-containing waste (71.43 wt% chlorine-containing
waste sulfuric acid
+ 28.57 wt% hydrogen sulfide sour gas) was treated, wherein the chlorine-
containing waste
sulfuric acid produced by a polytetrahydrofuran production unit had 48 wt%
water content and
less impurities, and was concentrated to 85 wt% by evaporation before it was
introduced into
the reactor 9; although the hydrogen sulfide sour gas was sulfur-containing
waste gas, it only
provided 70 mol% heat for the reactor system, while the lacking 30 mol% heat
is replenished
by natural gas auxiliary fuel.
The specific reaction process was as follows:
The waste sulfuric acid was introduced into the hearth of the reactor through
the sulfur-
containing waste inlet, the hydrogen sulfide sour gas and natural gas were
introduced into the
hearth of the reactor through the fuel inlet; the mixture was moved in the
axial direction of the
hearth to have a first combustion with the oxygen-containing combustion gas
introduced
through the first oxygen-containing combustion gas inlet, and then have a
second combustion
19
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
with the oxygen-containing combustion gas introduced through the second oxygen-
containing
combustion gas inlet; wherein the conditions of the first combustion included:
the oxygen
coefficient was X1=0.75, and the temperature was 1,150 C; the conditions of
the second
combustion included: the oxygen coefficient was X3, Xl+X3=1.05, and the
temperature was
1,050 C; thus a gas containing sulfur dioxide was obtained;
In the gas containing sulfur dioxide discharged from the reactor, the content
of oxygen was 3
mol%, the content of sulfur dioxide was 8.5 mol%, the content of NO was lower
than or equal
to 100 mg/Nm3, and the conversion ratio of sulfur dioxide was 99 mol%.
Example 3
The specific compositions of the sulfur-containing waste and fuel used in this
example are
shown in table 3, and the oxygen-containing combustion gas is air with 21 mol%
oxygen
content.
Table 3
Sulfur-containing ore Waste sulfuric acid
Mass fraction /wt% Liquefied gas
/wt%
/wt%
H2SO4 88
H20 22.723 10
Polymer (organic
2
substance)
S 29.5
Fe 47.75
As 0.002
F 0.025
C3118 21
C3H6 10
C4Hio 50
C4118 6
C4H6 3
CH4 10
The reactor was heated up to 1,250 C, the natural air is pressurized by an air
blower then heated
up to 450 C in an electric heating furnace, and entered the reactor; the
sulfur-containing waste
was sprayed into the hearth of the reactor from the sulfur-containing waste
inlet by a fluid
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
sprayer in 0.5 MPa high-pressure atomizing air; and the fuel was introduced
into the hearth
from the fuel inlet.
The specific reaction process was as follows:
The waste sulfuric acid in 7.5t sulfur-containing waste (80 wt% waste sulfuric
acid + 20 wt%
.. sulfur-containing ore) and 120 kg fuel were introduced into the hearth of
the reactor through
the sulfur-containing waste inlet and the fuel inlet respectively, and were
moved in the axial
direction of the hearth to have a first combustion with the oxygen-containing
combustion gas
introduced from the first oxygen-containing combustion gas inlet, and then
have a second
combustion with the oxygen-containing combustion gas introduced from the
second oxygen-
containing combustion gas inlet; wherein the conditions of the first
combustion included: the
oxygen coefficient was X1=0.85, and the temperature was 1,200 C; the
conditions of the second
combustion included: the oxygen coefficient was X3, Xl+X3=1.05, and the
temperature was
1,050 C; thus a gas containing sulfur dioxide I was obtained; the sulfur-
containing ore was
burned in another reactor (at 800 C temperature) to generate a process gas
containing sulfur
dioxide II;
In the sulfur dioxide gas mixture discharged from the two reactors, the
content of oxygen was
5 mol%, the content of sulfur dioxide was 6 mol%, the content of NO was lower
than or equal
to 100 mg/Nm3, and the conversion ratio of sulfur dioxide was 99 mol%.
Comparative Example 1
The method was similar to that in the Example 1, except that the combustion
was carried out
only for one time.
The specific process was as follows: 38.11t sulfur-containing waste (68.22 wt%
waste sulfuric
acid + 31.78 wt% sulfur-containing waste liquor) and 4,588 kg fuel were
introduced into the
hearth of the reactor through the material inlet, and were moved in the axial
direction of the
hearth to have combustion with the oxygen-containing combustion gas introduced
through the
first oxygen-containing combustion gas inlet, wherein the conditions of the
combustion
included: the oxygen coefficient was 1.04, the temperature was 1,150 C; thus,
a gas containing
21
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
sulfur dioxide was obtained;
The temperature of the gas containing sulfur dioxide discharged from the
reactor was 950-
1,000 C, the content of oxygen in the gas containing sulfur dioxide was 1.8
mol%, the content
of sulfur dioxide was 3.2 mol%, the content of NO was about 500 mg/Nm3, and
the conversion
ratio of sulfur dioxide was 98.8 mol%. The fuel consumption in the comparative
example was
higher than that in the Example 1 by 15 wt%.
Comparative Example 2
The method was similar to that in the Example 1, except that the oxygen
coefficients in the two
combustion cracking processes were different from the oxygen coefficient in
the Example 1.
Specifically, the oxygen coefficient X1 in the first combustion process was
0.9, and the oxygen
coefficient in the second combustion process was X3, and X1+X3=1.05;
All other conditions were the same as those in the Example 1. In that way, a
gas containing
sulfur dioxide was obtained; in the gas containing sulfur dioxide discharged
from the reactor,
.. the content of oxygen was 2 mol%, and the content of NO was about 200
mg/Nm3.
Furthermore, as shown in Figs. 1 and 4, the combustion air supply mechanism
may further
comprise a heating device 120 and a blower fan 130. The heating device has a
heating shell 121,
which has a heating chamber 123 therein and an electric heating mechanism 122
arranged in
the heating chamber 123. The heating shell 121 is provided with a heating gas
inlet 124 and a
heating gas outlet 125 that are in communication with the heating chamber 123
respectively,
the heating gas inlet 124 is in communication with the blower fan 130, and the
heating gas
outlet 125 is in communication with the hearth 111 of the reactor assembly;
the electric heating
mechanism 122 is configured to be able to raise the temperature inside the
heating chamber 123.
Since the heating gas inlet 124 is in communication with the blower fan 130
and the heating
gas outlet 125 is in communication with the hearth 111 of the reactor
assembly, the electric
heating mechanism 122 can raise the temperature inside the heating chamber
123. Therefore,
the temperature of the combustion air blasted by the blower fan 130 can be
raised when the
22
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
combustion air passes through the heating device 120, thus the temperature of
the combustion
air entering the hearth 111 can be as high as 600-650 C, thereby the
combustion efficiency of
the gas mixture in the reactor assembly is improved.
As shown in Figs. 7 and 8, the present disclosure further provides a sulfur-
containing waste
treatment system, which comprises:
the aforesaid reactor assembly 100, which is configured to enable the sulfur-
containing waste
to perform a combustion reaction to obtain a first gas containing sulfur
dioxide;
a heat recovery unit 300 configured to recover heat from the first gas to
obtain a second gas;
a purifying and cooling unit configured to purify and cool down the second gas
to obtain a third
gas;
a drying unit 700 configured to dry the third gas to obtain a fourth gas; and
an oxidation and absorption unit 800 configured to oxidize and absorb the
fourth gas to obtain
sulfuric acid and exhaust gas.
The specific operating process of the treatment system will be described in
the following
examples:
Although the water content of the sulfur-containing waste is 36 wt% in waste
sulfuric acid and
sulfur-containing waste liquor (68.22 wt% waste sulfuric acid + 31.78 wt%
sulfur-containing
waste liquor), it is difficult to thicken the waste sulfuric acid and the
sulfur-containing waste
liquor in the chemical fiber industry by dehydration owing to the fact that
the waste sulfuric
acid and the sulfur-containing waste liquor contain a lot of particles and
impurities. Therefore,
the waste sulfuric acid and the sulfur-containing waste liquor are directly
fed into the reactor
body 110 without dehydration.
(1) the sulfur-containing waste is atomized and sprayed into the reactor body
110 by a fluid
sprayer under 0.6 MPa high pressure, the reaction temperature in the hearth is
1,200 C,
and the fuel (natural gas) provides heat; air within 21 mol% oxygen content is
used for
supporting combustion, combustion air at normal temperature is pressurized by
a blower
fan 130 and then heated up to 630 C in a heating device 120, and the high-
temperature
23
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
combustion air is introduced into the hearth through different combustion gas
inlets 114
and 115. The sulfur-containing waste has a first combustion with the
combustion gas
introduced through the first group of combustion air inlets 114a and 115a,
then has a
second combustion with the combustion gas introduced through the second group
of
combustion air inlets 114b and 115b; wherein the conditions of the first
combustion
include: the oxygen coefficient is X1=0.7, and the temperature is 1,200 C; the
conditions
of the second combustion include: the oxygen coefficient is X3, Xl+X3=1.05,
and the
temperature is 1,100 C, the remaining oxygen content in the first gas
discharged from the
hearth is 2 mol%, and the residence time of the process gas in the hearth is
greater than or
equal to 5 seconds;
(2) The metal dust in the high-temperature process gas discharged from the
hearth is removed
in the dust removal unit 200 to obtain dedusted high-temperature process gas
(the
operating conditions of the cyclone dust collector 210 include: the inlet
temperature is
1,100 C, the inlet pressure is -1 kPa, and the air speed at the inlet of the
cyclone dust
collector is 30 m/s; the conditions of the dust removal operation of the
ceramic membrane
filter 220 include: the inlet temperature is 1,050 C, the inlet pressure is -1
kPa, and the air
speed at the inlet of the ceramic membrane filter is 15m/s); then the hot
process gas enters
the waste heat boiler 310 for heat recovery (the first heat exchange, the
conditions include:
the pressure at the tube flue gas side is -1.5 kPa, the temperature at the
flue gas inlet of the
boiler is 1,000 C, and the temperature at the flue gas outlet of the boiler is
380 C; the
pressure at the shell steam side is 3.8 MPa, and the temperature is 249 C), 3
it saturated
steam at 3.8 MPa is produced per hour. The saturated steam exchanges heat with
a small
fraction of the solid-free hot process gas in the steam superheater 320 (the
second heat
exchange, the conditions include: the pressure at the flue gas side is -1.5
kPa, the
temperature at the flue gas inlet of the superheater is 1,000 C, and the
temperature at the
flue gas outlet of the superheater is 450 C; the pressure at the steam side is
3.8 MPa, and
the temperature is 350 C), hot steam 16 is obtained, and the super-heated
steam enters the
steam turbine to reduce the power consumption of the device;
(3) After the heat recovery, the process gas enters the quenching and
humidifying column 400
for quenching by adiabatic humidification, and the gas temperature drops
steeply from
24
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
400 C to 77 C. Then the process gas enters the multi-stage filled cooling and
absorption
column 500; first, the process gas enters the first absorption layer and
washed and cooled
by a circulating water cooler to 35 C, then the process gas enters a second
absorption layer
and washed and cooled by a chilled water cooler to 29 C; after the twice
cooling, the
process gas enters the electrostatic mist precipitator 600 to remove the acid
mist of sulfur
trioxide;
(4) After the acid mist of sulfur trioxide is removed, the process gas
enters the drying unit 700
and dried by 93 wt% concentrated sulfuric acid;
(5) The sulfur dioxide content in the dried process gas is 5 mol%, the process
gas is boosted
to 20 kPa by a main blower 810 and then enters a first external heat exchanger
830 and a
second heat exchange pipeline 845b (see Fig. 13) for heat exchange, then the
process gas
37 has an oxidation reaction in the first catalyst layer 844a in a converter
840 at 415 5 C
temperature. After the reaction, the process gas reaches 555 5 C temperature,
then
exchanges heat with the tube side gas in the first heat exchange pipeline 845a
of the
converter 840, thereby the process gas in the second catalyst layer 844b
reaches 455 5 C
temperature and has an oxidation reaction; the first oxidation reaction is
completed in the
first conversion chamber, and the conversion ratio of the first oxidation
reaction is 96
mol%; after the first oxidation reaction, the process gas 38 exchanges heat
with the tube
side gas in the first external heat exchanger 830 and the temperature of the
process gas is
controlled to be higher than or equal to 150 C; then the process gas enters
the first
absorption layer of the multi-stage absorption column 880 for first absorption
with 100 wt%
sulfuric acid, and the absorption ratio is 99.99 wt%;
After the first absorption, the process gas enters the second external heat
exchanger 870 and the
first heat exchange pipeline 845a sequentially for heat exchange, and then
enters the second
conversion chamber. The process gas in the third catalyst layer 844c reaches
415 5 C
temperature and starts an oxidation reaction; after the oxidation reaction,
the process gas
containing sulfur trioxide exchanges heat in the second heat exchange pipeline
845b, then has
an oxidation reaction in the fourth catalyst layer 844d at 415 5 C
temperature, the second
oxidation reaction is completed in the second conversion chamber, the total
conversion ratio of
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
the process gas in the four catalyst layers after the reaction is 99.92 mol%;
then the temperature
of the process gas exiting the second conversion gas outlet after the second
oxidation reaction
is controlled to be higher than or equal to 130 C, and the process gas enters
the second
absorption layer of the multi-stage absorption column 880 for second
absorption with 98 wt%
sulfuric acid, and the absorption ratio is 99.99 wt%.
After the second absorption, the process gas is exhausted. In the exhaust gas,
the SO2
concentration is lower than or equal to 50 mg/Nm3, the NO concentration is
lower than or equal
to 50 mg/Nm3, the acid mist concentration is lower than or equal to 5 mg/Nm3,
and the particle
concentration is lower than or equal to 30 mg/Nm3.
Fig. 9 shows the connection structure of the dust removal unit 200 and the
heat recovery unit
300 in the above sulfur-containing waste treatment system, wherein the heat
recovery unit 300
is connected downstream of the dust removal unit 200. The dust removal unit
200 includes at
least two filter groups arranged in parallel, and each filter group includes
two filters, namely a
cyclone dust collector 210 and a ceramic membrane filter 220, and the ceramic
membrane filter
220 is connected in series downstream of the cyclone dust collector 210; the
shell of the cyclone
dust collector 210 is made of high-alloy steel; the inner wall of the cyclone
dust collector 210
is provided with a heat insulation layer and a fire resistance layer
sequentially, and the heat
insulation layer is made of a light-weight cast material and/or light-weight
refractory bricks;
the fire resistance layer is made of at least one of corundum bricks, corundum
mullite bricks,
chrome corundum bricks and silicon carbide; the air inlet of the cyclone dust
collector has a
spiral surface structure; the ash outlet of the cyclone dust collector employs
a star-shaped ash
valve structure or overflow spiral structure. The heat recovery unit 300
includes a waste heat
boiler 310 and a steam superheater 320, both of which are in communication
with the dust
removal unit 200, and the waste heat boiler 310 and the steam superheater 320
are in
communication with each other, so that the saturated steam obtained from the
waste heat boiler
310 can enter the steam superheater 320.
By using a combination of specific dust removal unit and heat recovery unit
and arranging
specific heat insulation layer and fire resistance layer on the inner wall of
the cyclone dust
collector, in conjunction with specific device structure and combination, the
requirement of the
26
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
high-temperature ash-laden flue gas (e.g., the flue gas containing sulfur
dioxide at 900-1,200 C
temperature from the high-temperature cracking furnace in the high-temperature
combustion
cracking process of the sulfur-containing waste) for the equipment is met,
high dust removal
efficiency is achieved, and the heat loss of the ash-laden high-temperature
flue gas in the dust
removing device can be reduced significantly.
The purifying and cooling unit in the above sulfur-containing waste treatment
system may
comprise a quenching and humidifying column 400, a multi-stage cooling and
absorption
column 500 and an electrostatic mist precipitator 600 that are in
communication with each other
sequentially, and the quenching and humidifying column 400 is in communication
with the heat
recovery unit 300.
Fig. 10 is a schematic diagram of a quenching and humidifying column 400. The
quenching
and humidifying column 400 comprises a first column body 410 and a spraying
assembly 420;
the first column body 410 comprises a cooling chamber 411, and a chamber inlet
412 and a
chamber outlet 413 that are in communication with the outer wall of the first
column body 410
and the cooling chamber 411, wherein the chamber inlet 412 is positioned in
the lower part of
the column body 410, and the chamber outlet 413 is positioned in the upper
part of the column
body 410; the spraying assembly 420 is arranged inside the cooling chamber 411
and comprises
a first spraying port 420 and a second spraying port 421, wherein the first
spraying port 422 is
configured to be able to spray a cooling fluid downward, the second spraying
port 421 is
configured to be able to spray the cooling fluid upward, and the first
spraying port 422 and the
second spraying port 421 are arranged opposite to each other.
Since the first spraying port 421 of the spraying assembly 420 is configured
to spray the cooling
fluid downward and the second spraying port 422 is configured to spray the
cooling fluid
upward, and the first spraying port 421 and the second spraying port 422 are
arranged opposite
to each other, the cooling fluid sprayed from the first spraying port 421 and
the second spraying
port 422 can respectively cool the high-temperature process gas from above and
below as the
high-temperature process gas enters the cooling chamber 411 from the chamber
inlet 412 and
flows toward the chamber outlet 130, and the humidity of the high-temperature
process gas is
increased at the same time, so that the temperature of the high-temperature
process gas can drop
27
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
sharply from 320 C-350 C to 75 C-80 C, thereby the process gas is cooled
rapidly and
effectively, and the system resistance is lower, usually at 1 Kpa, thus the
treatment period of the
sulfur-containing wastes is shortened.
Furthermore, the quenching and humidifying column 400 may further comprise a
unidirectional
.. spray head 430, which is arranged between the spraying mechanism 420 and
the chamber outlet
413 and configured to spray the cooling fluid downward, thereby the cooling
effect is improved.
Fig. 11 is a schematic diagram of a cooling and absorption column 500. The
cooling and
absorption column comprises a second column body 510, a first absorption
layer, and a second
absorption layer; the second column body 510 is arranged in the vertical
direction and has a
cooling and absorption chamber extending in the vertical direction; the second
column body
510 comprises a gas inlet and a gas outlet that are in communication with the
cooling and
absorption chamber respectively, wherein the gas inlet is arranged in the
lower part of the
second column body 510 and the gas outlet is arranged in the upper part of the
second column
body 510; the first absorption layer and the second absorption layer are
arranged spaced apart
.. from each other in the cooling and absorption chamber in the vertical
direction, wherein the
first absorption layer comprises a first spraying mechanism 520 capable of
spraying an
absorption fluid into the cooling and absorption chamber, and the second
absorption layer
comprises a second spraying mechanism 530 capable of spraying an absorption
fluid into the
cooling and absorption chamber.
.. Since the second column body 510 is arranged in the vertical direction and
has a cooling and
absorption chamber extending in the vertical direction, the footprint of the
cooling and
absorption column can be reduced, thereby the operation cost can be reduced.
In addition, since
a first absorption layer and a second absorption layer are arranged spaced
apart from each other
in the vertical direction in the cooling and absorption chamber, the first
absorption layer
includes a first spraying mechanism 520 capable of spraying an absorption
fluid into the cooling
and absorption chamber, and the second absorption layer includes a second
spraying mechanism
530 capable of spraying the absorption fluid into the cooling and absorption
chamber, the
process gas entering the cooling and absorption chamber from the gas inlet can
have an
absorption reaction with the absorption fluid sprayed by the first spraying
mechanism 520 and
28
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
the absorption fluid sprayed by the second spraying mechanism 530
respectively, and the
absorption fluid can fully absorb the sulfur trioxide in the process gas, thus
an effect of washing
the process gas is attained.
Furthermore, the cooling and absorption chamber of the second column body 510
may be
provided with a partition 513, which divides the cooling and absorption
chamber into a first
chamber and a second chamber from bottom to top, with the first absorption
layer arranged in
the first chamber and the second absorption layer arranged in the second
chamber; the second
column body 510 is provided with a gas inlet 511, a gas outlet 512, and an air
vent in
communication with the first chamber and the second chamber, wherein the gas
inlet 511 is in
communication with the first chamber and is positioned in the lower part of
the first chamber
for receiving external process gas; the gas outlet 512 is in communication
with the second
chamber and is positioned in the upper part of the second chamber for
discharging the process
gas to the downstream process. The first spraying mechanism 520 and the second
spraying
mechanism 530 are configured to be able to spray a refrigerating fluid that is
at a temperature
lower than the temperature of the cooling fluid while they spray the
absorption fluid in the first
chamber and the second chamber. An advantage of such an arrangement is that
the temperature
of the process gas can be further decreased by the refrigerating fluid; for
example, the
temperature of the cooling fluid is 28-32 C, the process gas is washed and
cooled to about 35 C
after passing through the first absorption layer, and then the process gas can
be washed and
cooled to 15 C by the refrigerating fluid at 7-10 C temperature after passing
through the second
absorption layer, thus the cooling effect is greatly improved.
In order to improve the utilization efficiency of the cooling fluid, save
energy and reduce the
production cost, the cooling and absorption column 500 includes a water pump
560 and a cooler
570, thereby the absorption fluid and the refrigerating fluid are cooled and
then circulated back
.. to the cooling chamber.
To make the absorption fluid contact with the process gas more extensively,
the first absorption
layer may include a first filler 540, and the first spraying mechanism 520 is
configured to spray
the absorption fluid downward, and the first filler 540 is disposed below the
first spraying
mechanism 520; the second absorption layer may include a second filler 550,
and the second
29
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
spraying mechanism 530 is configured to spray the absorption fluid downward,
and the second
filler 550 is disposed below the second spraying mechanism 530. It should be
understood that
the fillers may be made of a variety of materials, as long as they can make
the absorption fluid
contact with the process gas more extensively. In an embodiment, the first
filler 540 and the
second filler 550 are polypropylene Heilex rings.
The process gas is purified, cooled, and dried by the drying unit 700, and
then introduced into
the oxidation and absorption unit 800 to convert the sulfur dioxide in the
process gas into sulfur
trioxide, which is absorbed to produce sulfuric acid. Fig. 12 is a schematic
diagram of an
oxidation and absorption unit 800, and Fig. 13 is a schematic structural
diagram of a converter
840 in the oxidation and absorption unit 800.
The oxidation and absorption unit 800 comprises a conversion device and an
absorption device,
wherein the conversion device is kept in communication with the drying unit
700, and is
configured to oxidize the sulfur dioxide in the fourth gas obtained in the
drying unit 700 to
obtain a gas containing sulfur trioxide; the absorption device is in
communication with the
conversion device and configured to carry out absorption treatment on the gas
containing sulfur
trioxide to obtain sulfuric acid and exhaust gas. Specifically, in the
illustrated preferred
embodiment, the oxidation and absorption unit 800 includes a first external
heat exchanger 830,
a second external heat exchanger 870, a multi-stage absorption column 880, a
first heat
exchanger 850, a second heat exchanger 860, a sulfuric acid cooler 890, an
absorption
circulating pump, and a converter 840 provided with a first heat exchange
pipeline 845a and a
second heat exchange pipeline 845b. The dotted line represents the heating
pipeline, while the
solid line represents the process pipeline, and the arrow indicates the flow
direction of the
medium during heating. The specific flow process is as follows: the process
gas is blasted into
a heating furnace 820 by the blower fan 810, then enters the first external
heat exchanger 830,
enters the converter 840, and then enters the second external heat exchanger
870, and finally
returns to the blower fan 810 through the second heat exchanger 860, thus a
cycle is completed.
In the heating process, the medium is circulated in the blower fan 810, the
heating furnace 820
and the converter 840 and doesn't enter the multi-stage absorption column 880;
although the
heating pipeline is overlapped with the process pipeline partially, it doesn't
affect the normal
process flow.
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
The converter 840 shown in Fig. 13 comprises a converter shell 841, a catalyst
bed assembly
and heat exchange pipelines 845a and 845b; the converter shell 841 has an
conversion chamber
therein, and is provided with conversion gas inlets 842a and 842b and
conversion gas outlets
843a and 843b that are in communication with the conversion chamber; the
catalyst bed
assembly comprises at least two catalyst layers 844a, 844b, 844c, and 844d,
which are arranged
in the conversion chamber at an interval in the flow direction of the process
gas; the heat
exchange pipelines 845a and 845b match the catalyst layers 844a, 844b, 844c
and 844d in
quantity, and are arranged in the conversion chamber at least partially and
disposed between
adjacent catalyst layers. By arranging the heat exchange pipelines in the
conversion chamber
and between two adjacent catalyst layers, the occupied space can be greatly
reduced and the
operation cost can be effectively reduced.
Specifically, the converter 840 includes a first conversion chamber and a
second conversion
chamber, a first catalyst layer 844a, a second catalyst layer 844b, a third
catalyst layer 844c, a
fourth catalyst layer 844d, a first conversion gas inlet 842a, a second
conversion gas inlet 842b,
a first conversion gas outlet 843a and a second conversion gas outlet 843b
formed in the
converter shell 841; both the first conversion gas inlet 842a and the first
conversion gas outlet
843a are in communication with the first conversion chamber so that the
process gas can flow
from the first conversion gas inlet 842a to the first conversion gas outlet
843a; both the second
conversion gas inlet 842b and the second conversion gas outlet 843b are in
communication with
the second conversion chamber so that the process gas can flow from the second
conversion
gas inlet 842b to the second conversion gas outlet 843b; both the first
catalyst layer 844a and
the second catalyst layer 844b are arranged in the first conversion chamber
and arranged at an
interval in the flow direction of the process gas; both the third catalyst
layer 844c and the fourth
catalyst layer 844d are arranged in the second conversion chamber and arranged
at an interval
in the flow direction of the process gas.
In the present disclosure, the process gas enters the first conversion chamber
through the first
conversion gas inlet 842a, and the process gas reacts with the first catalyst
layer 844a first, and
then reacts with the second catalyst layer 844b. After the reaction, the
process gas enters the
external heat exchanger directly through the first conversion gas outlet 843a;
after the heat
exchange in the external heat exchanger, the process gas containing sulfur
trioxide reaches
31
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
150 C or a higher temperature, and then the process gas enters the first stage
of the multi-stage
absorption column. The conversion ratio of the first conversion is 95%-96%,
the absorption is
carried out with 100 wt% sulfuric acid, and the absorption ratio is 99.99%.
After the absorption,
the process gas enters the external heat exchanger for heat exchange; after
the heat exchange,
.. the process gas reaches 415 C-420 C temperature, and enters the second
conversion chamber
through the second conversion gas inlet 842b, the process gas may react with
the third catalyst
layer 844c first, and the process gas containing sulfur trioxide after the
reaction exchanges heat
in the second heat exchange pipeline 845b and then reacts with the fourth
catalyst layer 844d;
after the reaction, temperature of the process gas is controlled at 130 C or a
higher temperature,
and the process gas enters the second stage of the multi-stage absorption
column through the
second conversion gas outlet 843b. The absorption ratio is 99.99%, and the
process gas is
exhausted after the absorption. In the exhaust gas, the concentration of SO2
is lower than or
equal to 50mg/m3, the concentration of NO is lower than or equal to 100mg/m3,
the
concentration of acid mist is lower than or equal to 5mg/m3, and the
concentration of particle
substances is lower than or equal to 30mg/m3. Therefore, the converter in the
present disclosure
has the advantage of high conversion efficiency.
In order to arrange each catalyst layer more stably in the conversion chamber,
in an embodiment
of the present disclosure, the converter 840 includes a plurality of
supporting assemblies
corresponding to the first catalyst layer 844a, the second catalyst layer
844b, the third catalyst
layer 844c and the fourth catalyst layer 844d in one-to-one correspondence.
Each supporting
assembly includes a grating 846, and the edges of the grating 846 are
connected to the inner
wall of the converter shell 841 to provide support for the corresponding
catalyst layer.
Furthermore, the supporting assembly includes a heat-resistant ceramic balls
847 arranged on
the side of the catalyst layer opposite to the grating 846. In the present
disclosure, the main
function of the heat-resistant ceramic balls 847 is to press the catalyst
layer to keep the catalyst
layer lying on the grating 846, so as to prevent the catalyst layer from being
blown away by the
air flow. Besides, on one hand, the heat-resistant ceramic balls 847 can
exchange heat with the
passing process gas, so that the temperature of the process gas can drop to a
temperature suitable
for reacting with the catalyst layer; and on the other hand, the heat-
resistant ceramic balls 847
can absorb foreign particles in the process gas and play a purifying role.
32
Date recue/Date received 2023-04-20
CA 03199289 2023-04-20
Based on the above-mentioned reactor assembly and sulfur-containing waste
treatment system,
the present disclosure further provides a method for burning sulfur-containing
waste and a
method for making sulfuric acid by regenerating sulfur-containing waste. The
burned sulfur-
containing waste may be waste sulfuric acid, sulfur-containing waste liquid
and sulfur-
containing waste gas, etc., and the fuel may be selected from at least one of
natural gas, sulfur,
liquefied hydrocarbons, hydrogen sulfides and heavy oil organic substances. In
the process gas
generated from combustion, the content of sulfur dioxide is 3-12 mol%, the
content of NO is
lower than or equal to 100 mg/m3, and the content of oxygen is 0.5-5 mol%.
While the present disclosure is described above in detail in some preferred
embodiments with
reference to the accompanying drawings, the present disclosure is not limited
to those
embodiments. Various simple variations may be made to the technical scheme of
the present
disclosure within the technical concept of the present disclosure. To avoid
unnecessary
repetition, various possible combinations are not described specifically in
the present disclosure.
However, such simple variations and combinations shall also be deemed as
having been
disclosed and falling in the scope of protection of the present disclosure.
33
Date recue/Date received 2023-04-20