Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03200411 2023-04-28
Specification
WATER-BASED AEROGEL EFFICIENT FIRE EXTINGUISHING
AGENT AND PREPARATION METHOD THEREOF
Technical Field
The present invention relates to the field of emergency and security new
materials, particularly relates to a water-based aerogel efficient fire
extinguishing
agent, and further discloses a preparation method thereof.
Background Art
There are always reports that people's lives and property safety are being
severely threatened by shocking fires, so the perfmmances and functions of
fire
extinguishing agents have been attracting more and more attentions. Fire
extinguishing agents refer to substances that can effectively destroy
combustion
conditions and thus stop combustion. The fire extinguishing agents used in
traditional fire extinguishing products in market mainly include water-based
fire
extinguishing agents, dry powder fire extinguishing agents and clean gas fire
extinguishing agents.
Regardless of their formulations, traditional fire extinguishing agents
usually
play a role in extinguishing fire from two aspects: one is temperature
reduction, for
instance, water can be used to bring the temperature of a combustion material
down
below the ignition point, and the liquid precipitated from the foam can cool
the
surface of a comburent; the other is oxygen insulation, for instance, foam or
powder
can be used to cover the surface of a comburent so to isolate it from air
(oxygen),
and the so-called "suffocation" caused by carbon dioxide also belongs to this
principle.
However, there are usually many defects during the process of using
traditional
1
Date recue/Date received 2023-04-28
CA 03200411 2023-04-28
Specification
fire extinguishing agents: 1. incompatible functions: it is often that
traditional fire
extinguishing agents cannot have both "cooling" and "isolation" functions at
the
same time, so different fire extinguishing agents are needed for different
fire
sources, which makes the operation more complicated; 2. limited application
scenarios: the application scenarios of traditional fire extinguishing agents
are
greatly limited, for instance, foam extinguishing agents are not suitable for
extinguishing live equipment fires, metal fires and gas fires, carbon dioxide
extinguishing agents are not suitable for extinguishing chemical fires, metal
fires
and fiber material fires, and direct application of water on oil fires and
combustible
dust may easily cause deflagration; 3. unsatisfactory effects: insufficient
effectiveness of traditional fire extinguishing agents are often shown at
critical
moments of fires, firefighters were powerless when facing the raging flames in
both
the accident of "Shenghua Company Deflagration in Zhangjiakou on 11.28" and
the
accident of "Tianjiayi Company Explosion in Xiangshui on 3.21", and they had
to
wait to carry out fire fighting and rescue only after the burning momentum was
less
fierce; 4. inadequate environmental protection: traditional fire extinguishing
agents
can easily cause injuries during using and have environmental pressures
because
they may cause pollutions when they are due to be recycled, so traditional
fire
extinguishing agents must meet the requirements of green environmental
protection
when the concept of green waters and mountains has been deeply rooted in
people's
hearts.
As a result, although traditional fire extinguishing agents are still
occupying
the market and playing an important role, they need to be upgraded. A new
generation of multi-functional and efficient fire extinguishing agents is
urgently
needed, because traditional water-based fire extinguishing agents cannot take
into
account both general fires and oil fires, edible oil fires and live equipment
fires;
moreover, traditional fire extinguishing agents have such use defects as low
fire
fighting efficiency.
2
Date recue/Date received 2023-04-28
CA 03200411 2023-04-28
Specification
Summary of the Invention
Therefore, the technical problem to be solved by the present invention is to
provide a water-based aerogel efficient fire extinguishing agent, so as to
overcome
the following defects of prior art, such as narrow range of fire extinguishing
objects,
weak flame retardancy and low fire extinguishing efficiency.
The second technical problem to be solved by the present invention is to
provide a preparation method of the above-mentioned water-based aerogel
efficient
fire extinguishing agent.
In order to solve the above-mentioned technical problem, the water-based
aerogel efficient fire extinguishing agent according to the present invention,
based
on the total amount of preparation raw materials for the fire extinguishing
agent,
comprises raw material components with the following mass contents:
Inorganic aerogel powder 2-10 wt%;
Template agent 4-9 wt%;
Phosphorous flame retardant 15-18 wt%;
Nitrogenous flame retardant 13-16 wt%;
Hydrophilic colloid stabilizer 3-5 wt%;
Hydrogen ion concentration buffer 3-6 wt%;
Water the rest.
Specifically, the inorganic aerogel powder comprises at least one of the
aerogel
powders such as silicon oxide, alumina, zirconia, silica-aluminum binary
hybrid and
silica-zirconia binary hybrid; preferably, the inorganic aerogel needs to be
preprocessed into a powder with a particle size of 10-100 microns.
Specifically, the template agent comprises at least one of
cetyltrimethylammonium bromide, sodium do decylbenzene sulfonate or
3
Date recue/Date received 2023-04-28
CA 03200411 2023-04-28
Specification
cetyltrimethylammonium chloride.
Specifically, the phosphorus flame retardant comprises at least one of
ammonium dihydrogen phosphate, tri-(2,3-dibromopropyl) phosphate ester or
triisocyanate thiophosphate.
Specifically, the nitrogenous flame retardant comprises a mixture of at least
two of urea, ammonium pentaborate (NH4B508-4H20), ammonium bicarbonate
(NH4HCO3) or melamine cyanurate.
Specifically, the hydrophilic colloid stabilizer comprises at least one of
carbomer, alginate or carboxymethyl cellulose.
Specifically, the hydrogen ion concentration buffer comprises a mixture of at
least two of sodium dihydrogen phosphate, dipotassium hydrogen phosphate,
acetic
acid, sodium acetate, ammonia water and ammonium chloride.
The present invention further discloses a preparation method of the water-
based
aerogel efficient fire extinguishing agent, including the following steps of:
(1) preparing aerogel sol: take a selected amount of aerogel powder and inlet
nitrogen at 180-250 C for heat treatment of surface modification, add a
selected
amount of the template agent for mixing and full grinding, and then add water
and
stir well, thereby obtaining needed aerogel sol, which is ready for use;
(2) preparing water-based flame retardant agent: take a selected amount of the
phosphorus flame retardant and the nitrogenous flame retardant and mix them,
add
water for full mixture, and then add a selected amount of the hydrophilic
colloid
stabilizer and stir well, thereby obtaining a needed mixed liquor of water-
based
flame retardant agent, which is ready for use;
(3) adjusting pH value: stir the obtained aerogel sol and mixed liquor of
water-based flame retardant agent well, and add the hydrogen ion concentration
buffer so as to adjust the pH value of the mixed liquor to be neutral (6.5-
7.5); and
4
Date recue/Date received 2023-04-28
CA 03200411 2023-04-28
Specification
(4) curing: cure the obtained mixed material liquor at room temperature,
thereby obtaining the needed fire extinguishing agent.
Specifically, in the step (1):
processing time for the heat treatment of surface modification for the aerogel
powder is at least 3h; and
processing time for the grinding is at least 3h.
Specifically, in step (4), processing time for the curing step is 8-9h.
With inorganic aerogel powder, template agent, phosphorous flame retardant,
nitrogenous flame retardant and hydrophilic colloid stabilizer as raw
materials, the
water-based aerogel efficient fire extinguishing agent of the present
invention can
significantly enhance the efficiency of fire extinguishing agent by using the
ultra-low thermal conductivity and ultra-high adsorption capacity of inorganic
nano-porous aerogels; meanwhile, a combination of phosphorous flame retardant
and nitrogenous flame retardant serve as a relevant functional component of
the fire
extinguishing agent, and the colloid stabilizer and the icon buffer are added
into the
water-based aerogel so as to enhance the uniformity and shelf life of the
flame
retardants, so that the fire extinguishing agent not only has efficient fire
extinguishing function, excellent fire resistance function and flame retardant
function, but also has a comparatively long quality guaranty and storage life,
thereby
having an effectively expanded fire extinguishing types, efficiency and
application
range.
As for the water-based aerogel efficient fire extinguishing agent of the
present
invention, inorganic nano-porous aerogel is first applied to the fire
extinguishing
agent, wherein the inorganic nano-porous aerogel includes at least one of the
aerogels such as silica, alumina, zirconia, silicon aluminum binary hybrid,
silicon
zirconium binary hybrid aerogel, and the aerogel is used after being
preprocessed
into particles with a powder with a particle size of 10-100 microns. After
full
Date recue/Date received 2023-04-28
CA 03200411 2023-04-28
Specification
absorption of fire extinguishing agent, a surface of the aerogel particle is
uneven due
to the abundant of holes, so the surface area is much greater than that of
liquid
particle (droplet) of the same volume, as a result, the volatilization of the
fire
extinguishing agent is also much faster than that of droplets under the same
conditions, heat can be taken away much faster by the volatilization of the
fire
extinguishing agent than by that of pure liquid fire extinguishing agents,
temperature is lowered more rapidly, and thus the fire center can be reached
directly.
Moreover, aerogel particles with full absorption of fire extinguishing agent
are
formed with a layer of aerogel thermal insulation layer after volatilization
of the fire
extinguishing agent on the surface, which avoids over fast volatilization of
the fire
extinguishing agent in the center part and makes it possible to directly reach
the fire
center through the flame. Aerogel particles are adhered onto the surface of
the
comburent, while the fire extinguishing agent in the center continues to
directly
lower the temperature of the fire center, thereby overcoming defects of
traditional
pure liquid fire extinguishing agent, namely, in case of a fierce fire,
traditional pure
liquid fire extinguishing agents will be fully volatilized before reaching the
fire
center, so traditional pure liquid fire extinguishing agents can hardly be
sprayed into
the fire center. In addition, with a small volume, a light weight and a large
surface
area, the aerogel particles have an extremely high adsorption (adhesion), and
can be
rapidly adhered onto the surface of a comburent to cut off the air (oxygen),
and can
rapidly float and cover the surface of burning oil so as to cut off the air
(oxygen),
thereby rapidly extinguishing fire. The huge specific surface area of aerogel
can also
efficiently adsorb a variety of toxic and harmful gases. This agent is capable
of
rapidly adsorbing all kinds of smoke right after being sprayed for fire
extinguishing,
so that thick black fire smoke disappears rapidly and lives are effectively
protected.
In addition, aerogel is currently a material with the lowest thermal
conductivity in
the world and has an efficient heat insulation effect; meanwhile, inorganic
aerogel is
also an excellent fireproof and flame retardant material. When it is adhered
to cover
6
Date recue/Date received 2023-04-28
CA 03200411 2023-04-28
Specification
the surface of a comburent, it can effectively extinguish fires, as well as
can retard
flames and prevent re-burning. For forest, grassland and other mass fires, the
present
fire extinguishing agent can be sprayed to rapidly and efficiently form a fire
barrier
zone. To sum up, the water-based aerogel efficient fire extinguishing agent of
the
present invention effectively solves the problems of narrow application range
of fire
extinguishing objects, insufficient flame retardant performance and low fire
extinguishing efficiency in the prior art, and has an extremely high
application
value.
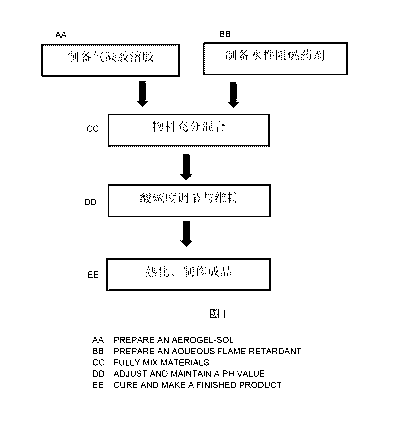
Description of Drawings
For easy and clear understanding, details of the present invention will be
further described according to the embodiments of the present invention and
with
reference to the drawing.
Fig. 1 is a flowchart for preparing the water-based aerogel efficient fire
extinguishing agent of the present invention.
Embodiments
Embodiment 1
The water-based aerogel efficient fire extinguishing agent in this embodiment,
based on the total amount of preparation raw materials, comprises raw material
components with the following mass contents:
Silica inorganic aerogel powder (a particle size being 20-30 micron) 2.5
wt%;
Template agent (cetyltrimethylammonium bromide) 6 wt%;
Phosphorous flame retardant (ammonium dihydrogen phosphate) 16 wt%;
Nitrogenous flame retardant (urea: ammonium pentaborate=1:1) 15 wt%;
Hydrophilic colloid stabilizer (Carbomer) 3.5 wt%;
7
Date recue/Date received 2023-04-28
CA 03200411 2023-04-28
Specification
Hydrogen ion concentration buffer (sodium dihydrogen phosphate, acetic acid)
4 wt%;
Deionized water the rest.
According to the preparation flowchart shown in Fig. 1, the preparation method
for the water-based aerogel efficient fire extinguishing agent in this
embodiment
includes the following steps of:
(1) preparing aerogel sol: first, perform surface modification for the silica
aerogel powder and inlet clean nitrogen at 220 C for heat treatment of 4h;
then, add
a selected amount of the template agent for mixing and full grinding for 4h;
add a
proper amount of deionized water and stir for 2.5h after grinding and mixing,
wherein the amount of deionized water is no less than 25 wt% of the total
amount
and the rotation speed of the mixer is controlled to be 120-150 r/min, thereby
obtaining needed aerogel sol, which is ready for use;
(2) preparing water-based flame retardant agent: take another amount, which is
no less than 15 wt% of the total amount, of deionized water and add it to the
mixer,
then add a selected amount of the phosphorus flame retardant and the
nitrogenous
flame retardant, adjust the rotation speed of the mixer to be 100-120 r/min,
and then
add the hydrophilic colloid stabilizer and keep on stirring for 1.0-1.2h,
thereby
obtaining a stable mixed liquor of water-based flame retardant agent, which is
ready
for use;
(3) adjusting pH value: mix the aerogel sol with the mixed liquor of
water-based flame retardant agent, and add the mixture to a mixer, adjust the
rotation speed of the mixer to be 100-120 r/min and stir for at least 2h at
room
temperature; deionized water can be supplemented and added to a specified
amount
during stirring, and is stirred well so as to obtain the uniform mixed liquor;
then add
the hydrogen ion concentration buffer to adjust the pH value of the mixed
liquor to
be neutral (6.5-7.5) and maintain the pH value; cure the mixed liquor with
adjusted
8
Date recue/Date received 2023-04-28
CA 03200411 2023-04-28
Specification
and maintained pH value at room temperature for 8-9h, thereby obtaining the
needed fire extinguishing agent product.
Embodiment 2
The water-based aerogel efficient fire extinguishing agent in this embodiment,
based on the total amount of preparation raw materials, comprises raw material
components with the following mass contents:
Silica inorganic aerogel powder (a particle size being 50-100 micron) 4 wt%;
Template agent (cetyltrimethylammonium bromide) 5 wt%;
Phosphorous flame retardant (ammonium dihydrogen phosphate: triisocyanate
thiopho sphate=1: 1) 15
wt%;
Nitrogenous flame retardant (urea: ammonium bicarbonate =1:1) 15
wt%;
Hydrophilic colloid stabilizer (alginate) 4
wt%;
Hydrogen ion concentration buffer (dipotassium hydrogen phosphate, acetic
acid)
wt%;
Deionized water the rest.
According to the preparation flowchart shown in Fig. 1, the preparation method
for the water-based aerogel efficient fire extinguishing agent in this
embodiment
includes the following steps of:
(1) preparing aerogel sol: first, perform surface modification for the silica
aerogel powder and inlet clean nitrogen at 250 C for heat treatment of 3h;
then, add
a selected amount of the template agent for mixing and full grinding for 4h;
add a
proper amount of deionized water and stir for 2.5h after grinding and mixing,
wherein the amount of deionized water is no less than 30 wt% of the total
amount
and the rotation speed of the mixer is controlled to be 150-180 r/min, thereby
obtaining needed aerogel sol, which is ready for use;
9
Date recue/Date received 2023-04-28
CA 03200411 2023-04-28
Specification
(2) preparing water-based flame retardant agent: take another amount, which is
no less than 15 wt% of the total amount, of deionized water and add it to the
mixer,
then add a selected amount of the phosphorus flame retardant and the
nitrogenous
flame retardant, adjust the rotation speed of the mixer to be 100-120 r/min,
and then
add the hydrophilic colloid stabilizer and keep on stirring for 1.0-1.2h,
thereby
obtaining a stable mixed liquor of water-based flame retardant agent, which is
ready
for use;
(3) adjusting pH value: mix the aerogel sol with the mixed liquor of
water-based flame retardant agent, and add the mixture to a mixer, adjust the
rotation speed of the mixer to be 120-150 r/min and stir for at least 2h at
room
temperature; deionized water can be supplemented and added to a specified
amount
during stirring, and is stirred well so as to obtain the uniform mixed liquor;
then add
the hydrogen ion concentration buffer to adjust the pH value of the mixed
liquor to
be neutral (6.5-7.5) and maintain the pH value; cure the mixed liquor with
adjusted
and maintained pH value at room temperature for 8-9h, thereby obtaining the
needed fire extinguishing agent product.
Embodiment 3
The water-based aerogel efficient fire extinguishing agent in this embodiment,
based on the total amount of preparation raw materials, comprises raw material
components with the following mass contents:
Alumina inorganic aerogel powder (a particle size being 30-50 micron) 6 wt%;
Template agent (cetyltrimethylammonium bromide) 7 wt%;
Phosphorous flame retardant (ammonium dihydrogen phosphate:
tri-(2,3-dibromopropyl) phosphate ester =1:1) 16 wt%;
Nitrogenous flame retardant (ammonium bicarbonate: melamine cyanurate =1:1)
15 wt%;
Date recue/Date received 2023-04-28
CA 03200411 2023-04-28
Specification
Hydrophilic colloid stabilizer (carboxymethyl cellulose)
4.5 wt%;
Hydrogen ion concentration buffer (ammonia water, acetic acid)
3.5 wt%;
Deionized water
the rest.
According to the preparation flowchart shown in Fig. 1, the preparation method
for the water-based aerogel efficient fire extinguishing agent in this
embodiment
includes the following steps of:
(1) preparing aerogel sol: first, perform surface modification for the silica
aerogel powder and inlet clean nitrogen at 200 C for heat treatment of 5h;
then, add
a selected amount of the template agent for mixing and full grinding for 3h;
add a
proper amount of deionized water and stir for 2.5h after grinding and mixing,
wherein the amount of deionized water is no less than 25 wt% of the total
amount
and the rotation speed of the mixer is controlled to be 120-150 r/min, thereby
obtaining needed aerogel sol, which is ready for use;
(2) preparing water-based flame retardant agent: take another amount, which is
no less than 15 wt% of the total amount, of deionized water and add it to the
mixer,
then add a selected amount of the phosphorus flame retardant and the
nitrogenous
flame retardant, adjust the rotation speed of the mixer to be 100-120 r/min,
and then
add the hydrophilic colloid stabilizer and keep on stirring for 1.0-1.2h,
thereby
obtaining a stable mixed liquor of water-based flame retardant agent, which is
ready
for use;
(3) adjusting pH value: mix the aerogel sol with the mixed liquor of
water-based flame retardant agent, and add the mixture to a mixer, adjust the
rotation speed of the mixer to be 120-150 r/min and stir for at least 2h at
room
temperature; deionized water can be supplemented and added to a specified
amount
during stirring, and is stirred well so as to obtain the uniform mixed liquor;
then add
the hydrogen ion concentration buffer to adjust the pH value of the mixed
liquor to
be neutral (6.5-7.5) and maintain the pH value; cure the mixed liquor with
adjusted
11
Date recue/Date received 2023-04-28
CA 03200411 2023-04-28
Specification
and maintained pH value at room temperature for 8-9h, thereby obtaining the
needed fire extinguishing agent product.
Embodiment 4
The water-based aerogel efficient fire extinguishing agent in this embodiment,
based on the total amount of preparation raw materials, comprises raw material
components with the following mass contents:
Zirconia inorganic aerogel powder (a particle size being 15-20 micron) 9 wt%;
Template agent (sodium dodecylbenzene sulfonate) 6 wt%;
Phosphorous flame retardant (triisocyanate thiophosphate) 15
wt%;
Nitrogenous flame retardant (ammonium bicarbonate: melamine cyanurate =1:1)
13 wt%;
Hydrophilic colloid stabilizer (alginate)
4.5 wt%;
Hydrogen ion concentration buffer (sodium acetate, acetic acid) 5
wt%;
Deionized water
the rest.
According to the preparation flowchart shown in Fig. 1, the preparation method
for the water-based aerogel efficient fire extinguishing agent in this
embodiment
includes the following steps of:
(1) preparing aerogel sol: first, perform surface modification for the silica
aerogel powder and inlet clean nitrogen at 220 C for heat treatment of 3h;
then, add
a selected amount of the template agent for mixing and full grinding for 3h;
add a
proper amount of deionized water and stir for 2h after grinding and mixing,
wherein
the amount of deionized water is no less than 30 wt% of the total amount and
the
rotation speed of the mixer is controlled to be 150-180 r/min, thereby
obtaining
needed aerogel sol, which is ready for use;
(2) preparing water-based flame retardant agent: take another amount, which is
12
Date recue/Date received 2023-04-28
CA 03200411 2023-04-28
Specification
no less than 15 wt% of the total amount, of deionized water and add it to the
mixer,
then add a selected amount of the phosphorus flame retardant and the
nitrogenous
flame retardant, adjust the rotation speed of the mixer to be 100-120 r/min,
and then
add the hydrophilic colloid stabilizer and keep on stirring for 1.0-1.2h,
thereby
obtaining a stable mixed liquor of water-based flame retardant agent, which is
ready
for use;
(3) adjusting pH value: mix the aerogel sol with the mixed liquor of
water-based flame retardant agent, and add the mixture to a mixer, adjust the
rotation speed of the mixer to be 150-180 r/min and stir for at least 4h at
room
temperature; deionized water can be supplemented and added to a specified
amount
during stirring, and is stirred well so as to obtain the uniform mixed liquor;
then add
the hydrogen ion concentration buffer to adjust the pH value of the mixed
liquor to
be neutral (6.5-7.5) and maintain the pH value; cure the mixed liquor with
adjusted
and maintained pH value at room temperature for 8-9h, thereby obtaining the
needed fire extinguishing agent product.
Embodiment 5
The water-based aerogel efficient fire extinguishing agent in this embodiment,
based on the total amount of preparation raw materials, comprises raw material
components with the following mass contents:
Silica-aluminum binary hybrid inorganic aerogel powder (a particle size being
20-30
micron) 5 wt%;
Template agent (sodium dodecylbenzene sulfonate) 8 wt%;
Phosphorous flame retardant (ammonium dihydrogen phosphate: triisocyanate
thiopho sphate=1: 1) 15
wt%;
Nitrogenous flame retardant (ammonium pentaborate: melamine cyanurate =1:1)
15 wt%;
13
Date recue/Date received 2023-04-28
CA 03200411 2023-04-28
Specification
Hydrophilic colloid stabilizer (carbomer: alginate=1:1) 5 wt%;
Hydrogen ion concentration buffer (sodium acetate, acetic acid) 5 wt%;
Deionized water the rest.
According to the preparation flowchart shown in Fig. 1, the preparation method
for the water-based aerogel efficient fire extinguishing agent in this
embodiment
includes the following steps of:
(1) preparing aerogel sol: first, perform surface modification for the silica
aerogel powder and inlet clean nitrogen at 250 C for heat treatment of 3h;
then, add
a selected amount of the template agent for mixing and full grinding for 3h;
add a
proper amount of deionized water and stir for 2h after grinding and mixing,
wherein
the amount of deionized water is no less than 25 wt% of the total amount and
the
rotation speed of the mixer is controlled to be 150-180 r/min, thereby
obtaining
needed aerogel sol, which is ready for use;
(2) preparing water-based flame retardant agent: take another amount, which is
no less than 15 wt% of the total amount, of deionized water and add it to the
mixer,
then add a selected amount of the phosphorus flame retardant and the
nitrogenous
flame retardant, adjust the rotation speed of the mixer to be 100-120 r/min,
and then
add the hydrophilic colloid stabilizer and keep on stirring for 1.0-1.2h,
thereby
obtaining a stable mixed liquor of water-based flame retardant agent, which is
ready
for use;
(3) adjusting pH value: mix the aerogel sol with the mixed liquor of
water-based flame retardant agent, and add the mixture to a mixer, adjust the
rotation speed of the mixer to be 150-180 r/min and stir for at least 4h at
room
temperature; deionized water can be supplemented and added to a specified
amount
during stirring, and is stirred well so as to obtain the uniform mixed liquor;
then add
the hydrogen ion concentration buffer to adjust the pH value of the mixed
liquor to
be neutral (6.5-7.5) and maintain the pH value; cure the mixed liquor with
adjusted
14
Date recue/Date received 2023-04-28
CA 03200411 2023-04-28
Specification
and maintained pH value at room temperature for 8-9h, thereby obtaining the
needed fire extinguishing agent product.
Embodiment 6
The water-based aerogel efficient fire extinguishing agent in this embodiment,
based on the total amount of preparation raw materials, comprises raw material
components with the following mass contents:
Silica-zirconia binary hybrid inorganic aerogel powder (a particle size being
15-20
micron) 8
wt%;
Template agent (cetyltrimethylammonium bromide: sodium dodecylbenzene
sulfonate=1 :1) 5
wt%;
Phosphorous flame retardant (ammonium dihydrogen phosphate: triisocyanate
thiopho sphate=1: 1) 18
wt%;
Nitrogenous flame retardant (urea: ammonium pentaborate =1:1) 13
wt%;
Hydrophilic colloid stabilizer (carbomer: carboxymethyl cellulose=1:1) 4
wt%;
Hydrogen ion concentration buffer (ammonium chloride, acetic acid) 4
wt%;
Deionized water
the rest.
According to the preparation flowchart shown in Fig. 1, the preparation method
for the water-based aerogel efficient fire extinguishing agent in this
embodiment
including the following steps of:
(1) preparing aerogel sol: first, perform surface modification for the silica
aerogel powder and inlet clean nitrogen at 250 C for heat treatment of 3h;
then, add
a selected amount of the template agent for mixing and full grinding for 3h;
add a
proper amount of deionized water and stir for 3h after grinding and mixing,
wherein
the amount of deionized water is no less than 25 wt% of the total amount and
the
rotation speed of the mixer is controlled to be 150-180 r/min, thereby
obtaining
needed aerogel sol, which is ready for use;
Date recue/Date received 2023-04-28
CA 03200411 2023-04-28
Specification
(2) preparing water-based flame retardant agent: take another amount, which is
no less than 15 wt% of the total amount, of deionized water and add it to the
mixer,
then add a selected amount of the phosphorus flame retardant and the
nitrogenous
flame retardant, adjust the rotation speed of the mixer to be 100-120 r/min,
and then
add the hydrophilic colloid stabilizer and keep on stirring for 1.0-1.2h,
thereby
obtaining a stable mixed liquor of water-based flame retardant agent, which is
ready
for use;
(3) adjusting pH value: mix the aerogel sol with the mixed liquor of
water-based flame retardant agent, and add the mixture to a mixer, adjust the
rotation speed of the mixer to be 150-180 r/min and stir for at least 4h at
room
temperature; deionized water can be supplemented and added to a specified
amount
during stirring, and is stirred well so as to obtain the uniform mixed liquor;
then add
the hydrogen ion concentration buffer to adjust the pH value of the mixed
liquor to
be neutral (6.5-7.5) and maintain the pH value; cure the mixed liquor with
adjusted
and maintained pH value at room temperature for 8-9h, thereby obtaining the
needed fire extinguishing agent product.
Embodiment 7
The water-based aerogel efficient fire extinguishing agent in this embodiment,
based on the total amount of preparation raw materials, comprises raw material
components with the following mass contents:
Silica inorganic aerogel powder (a particle size being 20-30 micron) 2 wt%;
Template agent (cetyltrimethylammonium bromide) 9 wt%;
Phosphorous flame retardant (ammonium dihydrogen phosphate) 15 wt%;
Nitrogenous flame retardant (urea: ammonium pentaborate=1:1) 16 wt%;
Hydrophilic colloid stabilizer (Carbomer) 3 wt%;
Hydrogen ion concentration buffer (sodium dihydrogen phosphate, acetic acid)
16
Date recue/Date received 2023-04-28
CA 03200411 2023-04-28
Specification
6 wt%;
Deionized water the rest.
According to the preparation flowchart shown in Fig. 1, the preparation method
for the water-based aerogel efficient fire extinguishing agent in this
embodiment is
the same as that in Embodiment 1.
Embodiment 8
The water-based aerogel efficient fire extinguishing agent in this embodiment,
based on the total amount of preparation raw materials, comprises raw material
components with the following mass contents:
Silica inorganic aerogel powder (a particle size being 20-30 micron) 10
wt%;
Template agent (cetyltrimethylammonium bromide) 4 wt%;
Phosphorous flame retardant (ammonium dihydrogen phosphate) 18 wt%;
Nitrogenous flame retardant (urea: ammonium pentaborate=1:1) 13 wt%;
Hydrophilic colloid stabilizer (Carbomer) 5 wt%;
Hydrogen ion concentration buffer (sodium dihydrogen phosphate, acetic acid)
3 wt%;
Deionized water the rest.
According to the preparation flowchart shown in Fig. 1, the preparation method
for the water-based aerogel efficient fire extinguishing agent in this
embodiment is
the same as that in Embodiment 1.
Comparative embodiment 1
The technical solution of the fire distinguishing agent in this comparative
embodiment is the same as that in Embodiment 1, and the difference between
them
merely lies in that silica aerogel powder is directly used in this comparative
embodiment without surface modification, while other conditions are the same
as
17
Date recue/Date received 2023-04-28
CA 03200411 2023-04-28
Specification
those in Embodiment 1.
Test Examples
Tests of fire extinguishing performance
According to the test conditions provided in the paper ("Flow characteristics
and effects of water mist fire extinguishing agent modified by nano-SiO2",
pages
119-202 in 28th Supplementary Issue of CHEMICAL INDUSTRY AND
ENGINEERING PROGRESS) written by Xi Yunqin, the fire extinguishing agent in
Embodiment 1 and that in Comparative Embodiment 1 were tested. The ignition
times (an average of five times is taken) for battens, respectively soaked in
the fire
distinguishing agent of Embodiment 1 and in that of Comparative embodiment 1,
in direct contact with the flame, as well as extinguishing times for the wood
with an
ignition time of 60s with a flow rate of the fire distinguishing agent being
0.027kg/s were tested. The results are shown in Table 1. Table 1 further
provides
the test results where water is used for the same test as a contrast.
Table 1 Test results of fire extinguishing performance
Tests Ignition time (s) Extinguishing time
(s)
Embodiment 1 372 15
Comparative embodiment 1 296 22
Water 95 110
According to Table 1, the fire extinguishing agent in Embodiment 1 of the
present invention is more efficient in fire extinguishing and has better
ignition
retardant effects than the fire extinguishing agent in the Comparative
embodiment 1,
namely, the fire extinguishing agent in Embodiment 1 of the present invention
has
better flame retardant effects; relative to pure water, Embodiment 1 and
Comparative embodiment 1 both have excellent flame retardant effects.
Fire distinguishing tests were performed for fire distinguishing agents
prepared
according to Embodiments 1-8, and their fire distinguishing performances were
also
compared with that of water. A board of 1 mx1 m xl cm was selected for the
fire
18
Date recue/Date received 2023-04-28
CA 03200411 2023-04-28
Specification
distinguishing tests, wherein straws with a thickness of about 1 cm was placed
on
the surface of the board, and fire distinguishing was performed 30s after the
straws
were ignited. The control time here is the burning time, and the fire
distinguishing
time is the time needed for each kind of fire distinguishing agent. The
experiment
data are shown in Table 2 below.
Table 2 Performance test results of fire extinguishing agents
Embodiments
Water
1 2 3 4 5 6 7 8
Control time/s 30 30 30 30 30 30 30 30 30
Fire extinguishing time/s 8 8 7 7 9 8 8 9 53
According to Table 2, the fire distinguishing time for Embodiments 1 to 8 are
all less than 10s, which is much faster than that of pure water. It is shown
that the
fire distinguishing agent prepared according to the present invention is
really
efficient.
The above embodiments are examples only for clear illustration rather than
for limiting. Based on the above illustration, other forms of variation or
change can
also be made by ordinary technicians in this field. There is no need to nor
can
exhaustively list all of the embodiments here. Obvious variations or changes
derived
therefrom are still within the scope of protection of the present invention.
19
Date recue/Date received 2023-04-28