Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03204880 2023-06-09
2021-12-20 PG22105PC00 / P00196PCT
WO 2022/136353 PCT/EP2021/086955
1
A POUCHED PRODUCT FOR ORAL USE COMPRISING A LIQUID PERMEABLE
COVER MATERIAL AND A FILLING MATERIAL
TECHNICAL FIELD
The present disclosure relates to a pouched product for oral use comprising a
liquid
permeable cover material and a portion sized amount of a filling material
comprising a
liquid insoluble particulate material, and one or more water-soluble
components in
addition to the particulate material, the filling material being enclosed by
the liquid
permeable cover material, the particles of the particulate material having a
particle density
and a bulk density.
BACKGROUND
An oral pouched product as disclosed herein, is intended for use in the oral
cavity, such
as by buccal placement e.g., by placing the pouched product between the upper
or lower
gum and the lip or cheek. A pouched smokeless tobacco product may also be
referred to
as a portion-packed smokeless tobacco product for oral use. The pouched
product is
normally sized and configured to fit comfortably and discreetly in a user's
mouth between
the upper or lower gum and the lip or cheek.
Traditionally, oral pouched products are used in the oral cavity of a consumer
to provide a
user with the benefits of an active substance such as nicotine, caffeine,
and/or different
flavors. A common type of nicotine containing oral pouched products is oral
smokeless
tobacco products. Such products generally comprise water, salt, pH adjuster(s)
and
additional components such as flavors and humectants. Commonly, these products
are
called snuff.
Oral pouched nicotine containing products comprising no tobacco, or only a
small amount
of tobacco, are now becoming increasingly popular among consumers due to inter
alia
their appealing appearance, freshness, and taste. Moreover, this kind of
product allows a
user to enjoy nicotine without being exposed to tobacco. The tobacco free or
almost
tobacco free oral pouched products are usually flavored compositions
comprising a filling
material which may e.g., comprise microcrystalline cellulose or fiber material
derived from
plants other than tobacco.
CA 03204880 2023-06-09
2021-12-20 PG22105PC00 / P00196PCT
WO 2022/136353 PCT/EP2021/086955
2
The tobacco free oral pouched products are generally relatively dry products,
with a pre-
use moisture content below 35% by weight of the filling material and often
below 20% by
weight of the filling material. Oral pouched products having even lower
moisture content,
in the order of 4-10 % by weight of the filling material are also known in the
art.
Further types of oral pouched products are those which only deliver a flavor
into the oral
cavity and those which are designed for delivering active substances other
than nicotine.
Oral pouched products are typically used by a consumer by placing the pouch
between
the upper or lower gum and the lip and retaining it there for a limited period
of time. The
product is configured to fit comfortably and discreetly in the user's mouth.
The pouch
material holds the filling material in place allowing saliva to pass into the
filling material
and allowing flavors and active substances such as nicotine to diffuse from
the filling
material into the consumer's mouth.
An objective with the disclosure herein is to offer an oral pouched product
containing a
filling material having improved properties, such as mouthfeel, user
satisfaction and rapid
release of components, such as active agents and flavors.
SUMMARY
The above object may be achieved with an oral pouched product according to
claim 1.
Variations of the disclosure are set out in the dependent claims and in the
following
description.
The pouched product for oral use as disclosed herein comprises a liquid
permeable cover
material and a filling material comprising a particulate material consisting
of water
insoluble particles, and one or more water-soluble components in addition to
the
particulate material. The filling material is enclosed by the liquid permeable
cover material.
The particles of the particulate material have a particle density and a bulk
density. The
particles of the particulate material have a particle density in the range of
from 0.9 g/cm3
to 2.0 g/cm3. The particle density is at least 1.4 times greater than the bulk
density of the
particulate material. The filling material has a pre-use moisture content of
from 1% by
weight of the filling material to 35% by weight of the filling material. The
filling material is
CA 03204880 2023-06-09
2021-12-20 PG22105PC00 / P00196PCT
WO 2022/136353 PCT/EP2021/086955
3
free from tobacco material, or the filling material comprises tobacco material
in an amount
within the range of from 0.05 wt% to 10 wt% based on the total weight of the
filling
material.
The particle density of the particulate material may be from 1.4 to 5 times
greater than the
bulk density of the particulate material, such as from 1.5 to 4 times greater
than the bulk
density of the particulate material.
With a particle density of 0.9 g/cm3 the bulk density of the particulate
material is 0.6 g/cm3
or less, and with a particle density of 2 g/cm3, the bulk density of the
particulate material is
1.4 g/cm3 or less.
The difference in particle density and bulk density reflects the amount of
void volume in
the filling material which is created by the interstices between the water
insoluble particles
of the particulate material. When the difference in particle density and bulk
density is low,
the amount of void volume in the filling material is low. For instance, when
there is no or
substantially no difference in particle density and bulk density, there is no
or substantially
no void volume. In contrast, when the difference in particle density and bulk
density is
high, the amount of void volume will be high. A large void volume allows a
correspondingly large amount of saliva to be contained within the bulk of the
filling
material. The interstices between the particles form a macro-scale network of
channels
within the filling material, in which channels saliva can flow into and out of
the filling
material. The water-soluble components in the filling material can readily
dissolve in the
saliva and can be rapidly transported out of the filling material in the macro-
scale
channels formed by the interstices between the water insoluble particles,
resulting in a
rapid release of water-soluble components into the oral cavity of a user.
Mechanical
working of the pouch during use may further aid in promoting saliva transport
in the filling
material and rapid release of water-soluble components by changing the
configuration of
the channel network, thereby creating a "pumping action" within the mass of
the filling
material.
The filling material in the oral pouched products as disclosed herein have a
pre-use
moisture content as determined by the method disclosed herein of from 1% by
weight of
the filling material to 35% by weight of the filling material.The filling
material in the oral
pouched products as disclosed herein may have a pre-use moisture content as
CA 03204880 2023-06-09
2021-12-20 PG22105PC00 / P00196PCT
WO 2022/136353 PCT/EP2021/086955
4
determined by the method disclosed herein of from 1% by weight of the filling
material to
30% by weight of the filling material, such as from 1% by weight of the
filling material to
25% by weight of the filling material, such as from 1% by weight of the
filling material to
20% by weight of the filling material, such as from 1% by weight of the
filling material to
15% by weight of the filling material, such as from 1% by weight of the
filling material to
7% by weight of the filling material, such as from 5% by weight of the filling
material to
30% by weight of the filling material, such as from 5% by weight of the
filling material to
25% by weight of the filling material, such as from 5% by weight of the
filling material to
20% by weight of the filling material, such as from 5% by weight of the
filling material to
15% by weight of the filling material, such as from 10% by weight of the
filling material to
20% by weight of the filling material, such as from 10% by weight of the
filling material to
15% by weight of the filling material.
It may be preferred that the moisture content of the filling material in the
oral pouched
products as disclosed herein is less than 20% by weight.
A filling material in an oral pouched product as disclosed herein and having a
relatively
low pre-use moisture content as set out above, is perceived by users to be
fresh and
agreeable to handle when taking it out of a user container and tucking it in,
e.g., between
the upper or lower lip and the gum of the user. A relatively low pre-use
moisture content
also allows the particles of the particulate material constituting the bulk
volume of the
filling material to move more freely in relation to each other, which makes
the oral
pouched product easier to shape and to be made to conform to the shape of the
space
where the oral pouched product is placed in the oral cavity of the user.
The particulate material in the filling material are water insoluble
particles. The water
insoluble particles may be particles of microcrystalline cellulose, water
insoluble starch,
silica, or a mixture thereof.
The water insoluble particles of the particulate material are relatively
dense, non-porous
particles having a particle density in the range of from 0.9 g/cm3 to 2.0
g/cm3, such as
from 1.0 g/cm3 to 1.7 g/cm3, such as from 1.0 g/cm3 to 1.5 g/cm3, such as from
1.1 g/cm3
to 1.4 g/cm3.
CA 03204880 2023-06-09
2021-12-20 PG22105PC00 / P00196PCT
WO 2022/136353 PCT/EP2021/086955
Using dense non-porous water insoluble particles may enhance rapid release of
water-
soluble components from the filling material as essentially no, or only a
minor amount of
water-soluble components will be present in pores within the water insoluble
particles of
the particulate material in the filling material.
5
The water insoluble particles of the particulate material may constitute 75%
by dry weight
to 99% by dry weight of the filling material, such as 85% by dry weight to 98%
by dry
weight of the filling material or 95% by dry weight to 98% by dry weight of
the filling
material. The particles of the particulate material may constitute 85% by dry
weight to
99% by dry weight of the filling material.
The volume of the water insoluble particulate material in the filling material
preferably
defines the volume of the filling material, with any additional component of
the filling
material of the oral pouched product contributing only to a negligible or very
small extent
to the volume of the filling material. By "a very small extent" as used
herein, is implied a
contribution to the volume of the filling material by components other than
the particles of
the particulate material of less than 5%, such as of less than 3%, of less
than 2%,
preferably of less than 1%.
The oral pouched products of the present disclosure may have a volume after
use which
is in the same order as the volume before use of the oral pouched product.
This means
that the volume of the oral pouched product may remain largely unchanged
during use of
the oral pouched product, which is a property of the oral pouched product that
has been
found to be appreciated by many users. An oral pouched product which does not
lose
volume during use may be perceived as retaining the mouthfeel of a new fresh
product
and to be more malleable and satisfactory to keep in the mouth for a longer
time of use.
The particles of the particulate material in the filling material are
preferably relatively large
particles and may have an average particle size within the range of from 0.3
mm to 3.0
mm, such as from 0.4 mm to 3.0 mm, such as from 0.3 mm to 2.5 mm, such as from
0.4
mm to 2.5 mm such as from 0.5 mm to 2.5 mm, such as from 0.6 mm to 2.5 mm,
such as
from 0.7 mm to 2 mm, such as from 0.8 mm to 1.5 mm, such as from 0.85 mm to
1.2 mm.
The particles of the particulate material in the filling material may be of
generally the same
size, with a narrow particle size distribution profile.
CA 03204880 2023-06-09
2021-12-20 PG22105PC00 / P00196PCT
WO 2022/136353 PCT/EP2021/086955
6
The particles of the particulate material in the filling material are
preferably spherical or
generally spherical particles having a sphericity within the range of from 0.7
to 1.0, such
as from 0.8 to 1.0 and a diameter of from 0.3 mm to 3 mm, such as from 0.4 mm
to 3 mm
such as from 0.7 mm to 3 mm.
Sphericity and particle size may be determined with the aid of a QicPic image
analysis
instrument from 2012, Sympatec GmbH, ID No. 290-D, with Rodos/L dispersion
line ID
NO 214D and Vibri/L sample feeding ID NO 273, or equivalent equipment. A well
dispersed particle flow is led through the image plane of the instrument. If
the particles are
small, a high number of particles per image frame may be captured, such as in
the order
of 50,000-100,000 particles per image frame. For larger particles, such as
particles having
a particle size from 300 pm to 3000pm, the number of particles per image frame
may be
substantially less and may be in the order of from 300 to 2000 particles per
image frame.
The particulate material in the filling material as disclosed herein may
contain less than
0.5% of particles which are small enough to pass through a sieve having a mesh
size of
250 pm.
A mesh size of 250 pm corresponds to a particle size in the order of a small
to medium-
sized grain of sand. Such particles are extremely unpleasant if they escape
out through
the cover material into the oral cavity of a user as they give rise to a
gritty and dry
mouthfeel which may linger for a long time after the product has been placed
in the oral
cavity, especially if the particles are non-soluble particles.
Small particles and fines in a filling material may also cause problems with
dusting during
manufacturing of oral pouched products, as they may impair seal formation and
may
cause clogging of machine parts. It is also desirable to minimize the amount
of dust in the
manufacturing process from a health and hygiene perspective.
The filling material of the oral pouched product as disclosed herein may
comprise
nicotine.
CA 03204880 2023-06-09
2021-12-20 PG22105PC00 / P00196PCT
WO 2022/136353 PCT/EP2021/086955
7
The large void volume between the particles of the particulate material
contributes to
provide the oral pouched product with a high release rate for nicotine and
other active
ingredients, flavours, sweeteners etc., which are present in the oral pouched
product.
The nicotine may be added to the particulate material of the filling material
in the form of a
nicotine compound. The nicotine compound may be a nicotine base and/or may be
selected from the group consisting of nicotine hydrochloride, nicotine
dihydrochloride,
nicotine monotartrate, nicotine bitartrate, nicotine bitartrate dihydrate,
nicotine sulphate,
nicotine zinc chloride monohydrate and nicotine salicylate, nicotine benzoate,
nicotine
polacrilex and any combination thereof.
The filling material of the oral pouched product as disclosed herein is a
tobacco free filling
material or a filling material having a low tobacco content, the filling
material comprising
tobacco material in an amount within the range of from 0.05 wt% to 10 wt%,
such as from
0.05 wt% to 1 wt% or from 0.2 wt% to 1 wt%, based on the total weight of the
filling
material. In such case the tobacco material may be a nicotine source. The
tobacco
material may be the only nicotine source or may be a nicotine source in
addition to one or
more of the nicotine compounds disclosed herein. The particulate material in
the filling
material as disclosed herein is preferably constituted by tobacco free
particles, such as
tobacco free particles of microcrystalline cellulose, water insoluble starch,
silica, or a
mixture of two or more different types of tobacco free particles. A tobacco
free filling
material or tobacco free particles may contain trace amounts of tobacco, below
0.05 wt%.
The filling material of the oral pouched product as disclosed herein may
comprise an
additive selected from the group consisting of a flavouring agent, a
sweetener, a
humectant, and any mixture thereof.
The additive may comprise or consists of a flavouring agent, such as a flavour
oil, such as
a hydrophobic flavour oil, such as a synthetic flavour, such as a nature-
identical flavour.
The filling material of the oral pouched product as disclosed herein may be
free from
tobacco material. A tobacco free filling material may contain material derived
from other
plant sources such as coffee, tea, herbs, etc., and/or any suitable flavouring
agent,
sweetener, etc., as known in the art.
CA 03204880 2023-06-09
2021-12-20 PG22105PC00 / P00196PCT
WO 2022/136353 PCT/EP2021/086955
8
The one or more water-soluble components and other additives are preferably
applied to
the water insoluble particles of the particulate material in the filling
material after the water
insoluble particles have been formed. The one or more water-soluble components
and
other additives may be added as a liquid/aqueous mixture to the water
insoluble particles.
All added components may be comprised in the same mixture. Alternatively,
different
components may be added in different application steps, e.g., a flavorant may
be added in
a separate step from an active agent such as nicotine. By applying the one or
more water-
soluble components and other additives to pre-formed water insoluble
particles, a major
part of the additives will remain on or at the surface of the water insoluble
particles and
will not penetrate into the interior of the water insoluble particles such
that all or
substantially all additives are present on or at the surface of the water
insoluble particles
in the filling material. The water insoluble particles are preferably
homogeneous
hydrophilic particles, such as particles of one or more of microcrystalline
cellulose, water
insoluble starch and silica. It may further be preferred that the water
insoluble particles are
constituted by mono-component particles of one or more of microcrystalline
cellulose,
water insoluble starch and silica.
In the pouched product disclosed herein, at least one of the one or more water-
soluble
components may be present on a surface of at least some of the particles of
the
particulate material in the filling material, such as on 20% to 100 % of the
particles, or
50% to 100% of the particles, or 80% t0100% of the particles.
In the pouched product disclosed herein, at least one of the one or more water-
soluble
components may be present in interstices between the particles of the
particulate material
in the filling material.
If all or at least a major part of the one or more water-soluble components
are present on
the surfaces and/or in the interstices between the particles of the
particulate material in
the filling material as opposed to being trapped in pores within the particles
of the
particulate material, almost complete release of active substances from the
filling material
may ideally be achieved.
At least one of the one or more water-soluble components may be present both
on a
surface of at least some of the particles of the particulate material in the
filling material
and in interstices between the particles in the filling material.
CA 03204880 2023-06-09
2021-12-20 PG22105PC00 / P00196PCT
WO 2022/136353 PCT/EP2021/086955
9
It may be preferred that no or substantially no water-soluble component of the
filling
material such as nicotine, flavouring agents, sweeteners, etc., is present in
an internal
pore structure in the particles of the particulate material of the filling
material.
The filling material of the oral pouched product as disclosed herein may
comprise more
than one type of particles. Thereby, the water insoluble particles of the
particulate material
constitute a first type of particles and a second type of particles may differ
from the first
type of particles in one or more properties such as size, shape, or
composition. The
second type of particles may be water insoluble particles or fully or
partially water-soluble
particles.
The liquid permeable cover material of the oral pouched product may be a
nonwoven
material, such as a nonwoven material comprising staple fibres of regenerated
cellulose.
The staple fibres may be viscose staple fibres and the nonwoven material may
further
comprise a binder.
DEFINITIONS
The terms "oral" and "oral use" refer to a use of a product in contact with
mucous
membranes in the oral cavity of a human being, such as buccal placement of the
product
in the oral cavity. The products for oral use as disclosed herein are intended
to be placed
in their entirety in the oral cavity and are not intended to be swallowed.
As used herein the terms 'pouched product for oral use" or "oral pouched
product" refer to
a portion of a smokeless composition containing saliva extractables and being
packed in a
saliva-permeable pouch material.
A "particle" as used herein is a three-dimensional piece of material having a
maximum
dimension of less than 5 mm and an aspect ratio of from 0.3 to 1. The "aspect
ratio", AR,
as used herein, is calculated as the width, w, of the particle divided by the
length I, of the
particle where the length is determined as the largest dimension of the
particle and the
width is determined as the largest dimension orthogonal to the length: AR = W
/ I. A particle
having an aspect ratio of 1 may e.g., be a perfect sphere or cube. The
particles which are
useful as the particulate material in the filling material of the oral pouched
products
CA 03204880 2023-06-09
2021-12-20 PG22105PC00 / P00196PCT
WO 2022/136353 PCT/EP2021/086955
disclosed herein may have a regular shape such as a spherical shape, a cubic
shape, a
cylindrical shape, etc., or may have an irregular shape with regular or near-
regular shapes
being generally preferred. The particles may have generally smooth outer
surfaces or may
have small aberrations in the outer surfaces.
5
A "water insoluble particle" as referred to herein is a particle which does
not dissolve
when subjected to saliva in the oral cavity of a user and which retains or
substantially
retains its shape when incorporated in a pouched product for oral use. The
water
insolubility also means that the particle size of the water insoluble
particles as referred to
10 herein does not diminish or at least does not diminish by more than 1%
during use of an
oral pouched product incorporating the water insoluble particles. The shape
and the size
of the water insoluble particles may remain substantially unaffected during
use. However,
a certain amount of swelling of the water insoluble particles may be permitted
or even
desired. The swelling should preferably be less than 30 % of the pre-use
volume of the
water insoluble particles and more preferably less than 20 % of the pre-use
volume of the
water insoluble particles.
As used herein, the term "moisture content" refers to the percent by weight,
wt%, of oven
volatile substances, such as water and other oven volatiles (e.g., propylene
glycol) which
is present in a component material, a composition or a product and is
determined
according to the Loss On Drying (LOD) method disclosed herein.
The "thy weight" of a material, a composition, or a product is calculated by
detracting the
amount of moisture from the total weight of the material, composition or
product, the
moisture content being determined by the Loss On Drying (LOD) method as
disclosed
herein.
As used herein, the term "water content" refers to the percent by weight, wt%,
of water in
a component material, a composition, or a product. The water content may be
determined
by using a standardized method for water analysis, such as Karl Fischer
titration or gas
chromatography, GC.
The term "additional component" refers to any component except water, which is
present
in addition to the particles of the particulate material in the filling
material as disclosed
herein, such as salts (e.g. sodium chloride, potassium chloride, magnesium
chloride,
CA 03204880 2023-06-09
2021-12-20 PG22105PC00 / P00196PCT
WO 2022/136353 PCT/EP2021/086955
11
calcium chloride and any combinations thereof), pH adjusters (e.g. sodium
hydroxide,
potassium hydroxide, potassium carbonate, sodium carbonate or sodium
bicarbonate),
flavouring agents, sweeteners, colorants, humectants (e.g. propylene glycol or
glycerol),
antioxidants, preservatives (e.g. potassium sorbate), binders, tobacco and non-
tobacco
plant material. The water-soluble component or water-soluble components which
are part
of the filling material in the oral pouched products as disclosed herein
constitute one or
more additional components.
The terms "flavour" or "flavouring agent" are used herein for substances used
to influence
the aroma and/or taste of the oral pouched product. The flavours may be any
food-grade
natural or synthetic flavour as known in the art and may include without
limitation,
essential oils, single flavour compounds, compounded flavourings, and
extracts.
By "tobacco" or "tobacco material" is meant any part, e.g., leaves, stems,
stalks, and
flowers, of any member of the genus Nicotiana.
By a "cover material" as used herein is implied any suitable saliva permeable
packaging
material as known in the art. The cover material may also be referred to as
'pouch
material" and may be a nonwoven material, a material made by conventional
textile
production methods such as weaving or knitting or may be an apertured plastic
film or
netting. A nonwoven material suitable for use as cover material may be a
nonwoven
material comprising staple fibres, such as staple fibres of regenerated
cellulose e.g.,
viscose rayon staple fibres and a binder, such as a polyacrylate binder. Such
nonwoven
materials are commonly produced by carding the staple fibres to form a fibrous
web,
followed by consolidating the carded fibrous web by means of the binder.
Alternatively,
the nonwoven material may comprise fibres which are formed into a nonwoven web
by
spunbonding, hydroentangling, meltblowing, etc. The fibres used in such
processes are
generally thermoplastic fibres which are thermally bonded to form a coherent
nonwoven
web. The covering material may optionally comprise additional components such
as
flavouring agents and/or colorants.
Pouched products for oral use are normally sized and configured to fit
comfortably and
discreetly in a user's mouth between the upper or lower gum and the lip. In
general,
pouched products for oral use have a generally rectangular shape. Some typical
shapes
(length x width) of commercially available pouched products for oral use are,
for instance,
CA 03204880 2023-06-09
2021-12-20 PG22105PC00 / P00196PCT
WO 2022/136353 PCT/EP2021/086955
12
35 mm x 20 mm, 34/35 mm x 14 mm, 33/34 mm x 18 mm, 27/28 mm x 14 mm, 34 mm x
mm and 38 x 14 mm. Typical pouched products for oral use may have a maximum
length within the range of from 25 mm to 40 mm along the longitudinal
direction of the
product and a maximum width within the range of from 5 mm to 20 mm along the
5 transverse direction of the product. The pre-use thickness of the pouched
product is
normally within the range of from 2 mm to 8 mm. The total weight of
commercially
available pouched products for oral use is typically within the range from
about 0.3 g to
about 3.5 g, such as from about 0.5 g to 1.7 g, per pouched product. The
volume of a
portion of filling material in a pouch may be in the range of from 0.5 cm3 to
1.5 cm3,
10 depending on the size of the pouch.
A "user container" typically contains in the range of 10-30 pouched products,
such as in
the range of 20-25 pouched products. The pouched products may be placed
randomly in
the user container or in a pattern, for instance as described in WO
2012/069505 Al. The
user container as disclosed herein is a consumer package having a shape and a
size
adapted for conveniently carrying the consumer package in a pocket or in a
handbag and
may be used for packaging any known type of pouched product for oral use. The
user
container may include a disposal compartment for storage of used oral pouched
products.
The disposal compartment is separated from the compartment in the container
where the
fresh oral pouched products are stored up until use.
BRIEF DESCRIPTION OF THE DRAWINGS
The present invention will be further explained hereinafter by means of non-
limiting
examples and with reference to the appended drawings wherein:
Figure 1 shows a pouched product for oral use;
Figure 2 shows a cross-section along the line II-II through the
pouched product of
Fig. 1;
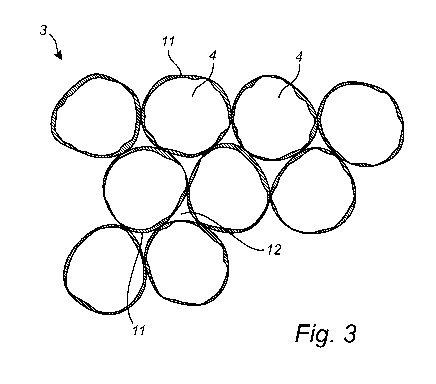
Figure 3 shows generally spherical particles suitable for use in the
oral pouched
products as disclosed herein; and
Figures 4a-4d show some alternative particle shapes.
CA 03204880 2023-06-09
2021-12-20 PG22105PC00 / P00196PCT
WO 2022/136353 PCT/EP2021/086955
13
DETAILED DESCRIPTION
It is to be understood that the drawings are schematic and that individual
components are
not necessarily drawn to scale.
The pouched product 1 for oral use which is shown in Figs. 1 and 2 comprises a
liquid
permeable cover material 2 and a portion sized amount of a filling material 3
comprising a
water insoluble particulate material which is constituted by a plurality of
particles 4
enclosed by the liquid permeable cover material 2. The cover material 2 may be
any
suitable type of cover material as disclosed herein and is formed into a
generally
rectangular pouch into which the filling material 3 has been inserted. The
particulate
material is constituted by water insoluble particles.
A common way of making a pouched product having a generally rectangular pillow-
like
shape, such as the pouched product 1 shown in Figs. 1 and 2, is either to
provide the
cover material as a seamless and endless tube or to form a flat web of cover
material into
an endless tube which is provided with a continuous seal in the longitudinal
direction of
the endless tube. The endless tube is subsequently intermittently sealed in
the transverse
direction of the endless tube while filling the endless tube with filling
material into pockets
which are created between the transverse seals. Individual pouched products
are severed
from the filled and sealed tube of cover material and are usually packed in
user
containers. Sealing of the cover material may be made with any suitable method
or
combination of methods, such as by means of adhesive, heat sealing, ultrasonic
welding,
needling, etc. Heat sealing and ultrasonic welding require the cover material
to contain at
least a functional amount of thermoplastic material, such as thermoplastic
fibres or
thermoplastic binders.
The longitudinal seal created during manufacturing appears as a longitudinal
seal 6
extending along the length I of the pouched product 1 shown in Figs. 1 and 2.
No such
seal will be present if the cover material is provided in the form of an
endless seam-less
tube. The transverse seals form end seals 7 which define the width w of the
pouched
product 1. The pouched product 1 has a first main surface 8 and a second main
surface 9
and a thickness t being defined as the greatest perpendicular distance between
the first
main surface 8 and the second main surface 9.
CA 03204880 2023-06-09
2021-12-20 PG22105PC00 / P00196PCT
WO 2022/136353 PCT/EP2021/086955
14
The particles 4 of the particulate material may constitute a very high
proportion of the total
dry weight of the filling material 3, such as 75% by dry weight to 99% by dry
weight of the
filling material, as set out herein.
The filling material 3 further comprises one or more water-soluble components
11, such
as flavours, sweeteners, active ingredients such as nicotine, etc. as
disclosed herein.
A part of a filling material 3 for an oral pouched product as disclosed herein
is shown in
Fig. 3, the filling material 3 comprising a plurality of generally spherical,
water insoluble
particles 4.
The particles 4 of the filling material may have a relatively large average
particle size
within the range of from 0.3 mm to 3.0 mm. By using large water insoluble
particles for the
particles 4 of the particulate material in the filling material 3, a major
part of the water-
soluble components 11, i.e., components which are soluble in water and saliva,
may to a
large extent be present in the filling material 3 on surfaces of the particles
4 which are
facing interstices 12 between the particles 4. In this manner, any water-
soluble
components 12 may be substantially "concealed" within the mass of the filling
material 3
where they do not add, or do not substantially add to the volume of the
filling material 3.
Fig. 3 shows only a very small number of particles 4. In a full portion of
filling material 3 for
an oral pouched product 1, the number of particles 4 in the particulate
material is
considerably higher, such as in the order of 150 particles or more which means
that a
large majority of the particle surfaces will be located in the interior of the
filling material 3.
As disclosed herein, the particles 4 of the particulate material may be dense,
non-porous
particles having a particle density in the range of from 0.9 g/cm3 to 2.0
g/cm3, such as
from 1.0 g/cm3 to 1.5 g/cm3, such as from such as from 1.0 g/cm3 to 1.7 g/cm3,
or from 1.1
g/cm3 to 1.4 g/cm3. In such dense non-porous particles, no, or substantially
no water-
soluble components 11 are present within the particles 4 themselves.
Figs. 4a, 4b, 4c and 4d illustrate some alternative shapes for the particles 4
of the filling
materials 3 as disclosed herein.
CA 03204880 2023-06-09
2021-12-20 PG22105PC00 / P00196PCT
WO 2022/136353 PCT/EP2021/086955
The particles 4 which are shown in Fig. 4a have a substantially cubic shape,
the particles
4 which are shown in Fig. 4b are grain-shaped, the particles 4 which are shown
in Fig. 4c
have a substantially cylindrical shape and the particles 4 which are shown in
Fig. 4d have
an irregular shape. The particles 4 in Fig. 4a, has an aspect ratio AR = w/I
which is
5 approximately 1, while the particles 4 shown in Figs. 4b-4d have a smaller
aspect ratio.
Method for determining moisture content, Loss On Drying, LOD
The moisture content as referred to herein may be determined by using a method
based
10 on literature references Federal Register/ vol.74, no. 4/712-719/Wednesday,
January 7,
2009/Notices "Total moisture determination" and AOAC (Association of Official
Analytical
Chemics), Official Methods of Analysis 966.02: "Moisture in Tobacco" (1990),
Fifth
Edition, K. Helrich (ed). In this method, the moisture content is determined
gravimetrically
by taking 2.5 0.25 g sample and weighing the sample at ambient conditions,
herein
15 defined as being at a temperature of 22 C and a relative humidity of 60%,
before
evaporation of moisture and after completion of dehydration. Mettler Toledo's
Moisture
Analyzer HB43, a balance with halogen heating technology, is used (instead of
an oven
and a balance as in the mentioned literature references) in the experiments
described
herein. The sample is heated to 105 C (instead of 99.5 0.5 C as in the
mentioned
literature references). The measurement is stopped when the weight change is
less than
1 mg during a 90 second time frame. The moisture content as weight percent of
the
sample is then calculated automatically by the Moisture Analyzer HB43.