Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03205490 2023-06-16
USE OF PHARMACEUTICAL COMPOSITION FOR TREATING LUNG CANCER
Technical Field
The present disclosure relates to a use of a pharmaceutical composition. More
particularly, the present disclosure relates to a use of a pharmaceutical
composition
including a diarylheptanoid compound or a pharmaceutically acceptable salt
thereof
for treating lung cancer.
Description of Related Art
Cancer, also known as malignant tumor, is an abnormal proliferation of cells,
and
these proliferating cells may invade other parts of the body, which is a
disease caused
by abnormal control of cell division and proliferation mechanisms. There is an
increasing trend in the number of people suffering from cancer worldwide, and
about
20% of the cancer population in the world is lung cancer patients. The 5-year
survival rate of lung cancer patients after treatment is still as low as about
15%, which
has been the cancer with the highest death rate in the world for many years.
According to different biological characteristics, treatment and prognosis,
lung
cancer can be divided into small cell lung cancer and non-small cell lung
cancer
(NSCLC).
About 85-90% of lung cancers are NSCLC, of which lung
adenocarcinoma is the most common type of lung cancer in women and non-smoking
patients. Treatment for lung cancer often depends on the age of patient, past
medical history, current health status, type of cancer cells, and stage of the
disease.
Generally speaking, small cell lung cancer has the characteristics of rapid
division
1
Date Regue/Date Received 2023-06-16
CA 03205490 2023-06-16
and proliferation, and metastases may occur in a short period of time, so that
systemic
chemotherapy or radiation therapy is the main treatment. The growth of NSCLC
is
slower, and the occurrence of metastasis is also slower, so that the principle
of
treatment depends on the clinical stage of the disease. The radical treatment
of
early stage (stage I, II) NSCLC is still based on complete resection of the
tumor by
surgery. The treatment principle is mainly chemical drug therapy or chemical
drug
combined with radiation therapy for the locally extended stage (stage III)
including
patients with malignant pericardium or hydropleural effusion and distant
metastases
(stage IV) or patients whose physical condition cannot be surgically removed.
However, it is a thorny problem in the treatment of metastatic or advanced
NSCLC that has undergone chemotherapy and relapsed. Current clinical studies
have confirmed that epidermal growth factor-tyrosine kinase inhibitors (EGFR-
TKIs)
can be used as the second-line treatment after the first-line chemotherapy
fails. But
about 40-80% of NSCLC patients have the EGFR gene mutation, which
overexpresses the epidermal growth factor receptor, leading to rapid growth,
metastasis and drug resistance of cancer. Almost all patients with the EGFR
gene
mutation relapse within two years after clinical treatment with EGFR-TKIs, and
no
effective drugs are available after relapse so far.
SUMMARY
In view of this, one of the objectives of the present disclosure is to provide
a use
of a pharmaceutical composition, which can be used to manufacture a drug for
treating a lung cancer. The pharmaceutical composition includes a
diarylheptanoid
2
Date Regue/Date Received 2023-06-16
CA 03205490 2023-06-16
compound or a pharmaceutically acceptable salt thereof, which can inhibit the
growth
of the epidermal growth factor receptor-tyrosine kinase inhibitors resistant
non-small
cell lung cancer cells. Therefore, the pharmaceutical composition can be used
alone or in combination with clinically used epidermal growth factor receptor-
tyrosine
kinase inhibitors to treat a EGFR gene mutation with epidermal growth factor
receptor-tyrosine kinase inhibition drug-resistant lung cancer.
According to one aspect of the present disclosure is to provide a use of a
pharmaceutical composition, the pharmaceutical composition is used to
manufacture
a drug for treating a lung cancer, wherein the pharmaceutical composition
includes a
diarylheptanoid compound or a pharmaceutically acceptable salt thereof, the
diarylheptanoid compound has a structure represented by Formula (I):
0 0
Ra R '
a
RC Rc
Rb Rbi
Re Re
Formula (I),
wherein Ra, Rb, Ra' and Rh' are independently H, C1-C2 alkyl, C1-C3 alkoxy,
OH,
or -0C(=0)Rd, wherein Rd is C1-C3 alkyl or C1-C3 alkanol; Rc is H, C1-C2
alkyl,
C3-C6 unsaturated alkyl or C7-C12 arylalkyl with double or triple bonds; and
Re and
Re' are independently H, C1-C6 alkyl or C1-C6 alkoxy.
According to the use of the pharmaceutical composition, wherein at least one
of
Ra, Rh, Ra', and Rh' can be -0C(=0)Rd, wherein Rd is C1-C3 alkyl or C1-C3
alkanol.
3
Date Recue/Date Received 2023-06-16
CA 03205490 2023-06-16
According to the use of the pharmaceutical composition, wherein the
diarylheptanoid compound can be interconvertible between keto and enol forms,
when Rc is H.
According to the use of the pharmaceutical composition, wherein the
diarylheptanoid compound can be selected from Compound 1, Compound 21a,
Compound 21b, Compound 22a, Compound 22b, Compound 23a, Compound 23b,
Compound 24a, Compound 24b, Compound 25, Compound 26, Compound 27,
Compound 31 and Compound 33 having a structure represented by Formula (II):
0 OH
1
R2
nn
R3 rs.3
Formula (II);
wherein Ri, Ri' are OCH3 respectively, R2, R2' are H respectively, R3, R3' are
H
respectively in Compound 1; Ri, Ri' are OCH3 respectively, R2, R2' are 0R4
respectively, R3, R3' are H respectively in Compound 21a; Ri, Ri' are OCH3
respectively, R2 is OH, R2' is 0R4, R3, R3' are H respectively in Compound
21b; Ri,
Ri' are OCH3 respectively, R2, R2' are 0R5 respectively, R3, R3' are H
respectively in
Compound 22a; Ri, Ri' are OCH3 respectively, R2 is OH, R2' is 0R5, R3, R3' are
H
respectively in Compound 22b; Ri, Ri' are H respectively, R2, R2' are 0R5
respectively, R3, R3' are H respectively in Compound 23a; Ri, Ri' are H
respectively,
R2 is 0R5, R2' is OH, R3, R3' are H respectively in Compound 23b; Ri, Ri' are
0R4
4
Date Recue/Date Received 2023-06-16
CA 03205490 2023-06-16
respectively, R2, R2' are OCH3 respectively, R3, R3' are H respectively in
Compound
24a; Ri is 0R4, Ri' is OH, R2, R2' are OCH3 respectively, R3, R3' are H
respectively in
Compound 24b; Ri is 0R5, Ri' is OH, R2, R2' are OCH3 respectively, R3, R3' are
H
respectively in Compound 25; Ri, Ri' are 0C2H5 respectively, R2, R2' are 0R4
.. respectively, R3, R3' are H respectively in Compound 26; Ri, Ri' are 0C2H5
respectively, R2, R2' are 0R5 respectively, R3, R3' are H respectively in
Compound 27;
Ri, Ri' are C2H5 respectively, R2, R2' are 0R5 respectively, R3, R3' are C2H5
respectively in Compound 31; and Ri, Ri' are OCH3 respectively, R2 is OCH3,
R2' is
0R4, R3, R3' are H respectively in Compound 33; wherein R4 is a structure
represented by Formula (i), and R5 is a structure represented by Formula (ii):
0 0
OH OH
Formula (i); Formula (ii).
According to the use of the pharmaceutical composition, wherein the
diarylheptanoid compound is selected from Compound 35a, Compound 35c,
Compound 35d, Compound 35e, Compound 36 and Compound 37 having a structure
represented by Formula (III):
0 0
R6 R6'
'=,,
R8 R8
N7
Formula (III);
5
Date Recue/Date Received 2023-06-16
CA 03205490 2023-06-16
wherein R6, Re are OCH3 respectively, R7, R7' are 0R4 respectively, R8 is CH3
in
Compound 35a; R6, R6' are OCH3 respectively, R7, R7' are 0R4 respectively, R8
is
benzyl in Compound 35c; R6, Re are OCH3 respectively, R7, R7' are 0R4
respectively,
R8 is propargyl in Compound 35d; R6, Re are OCH3 respectively, R7, R7' are 0R4
respectively, R8 is allyl in Compound 35e; R6, Re are H respectively, R7, R7'
are 0R4
respectively, R8 is CH3 in Compound 36; and R6, Re are 0R4 respectively, R7,
R7' are
OCH3 respectively, R8 is CH3 in Compound 37; wherein R4 is a structure
represented
by Formula (i):
0
'''jts's4:0H
OH
Formula (i).
According to the use of the pharmaceutical composition, wherein the
diarylheptanoid compound can be selected from:
. 0
Ai
mectsoõ J ( .H Ofis
0 '-
1 Mg __
He>510 IP 7\ ' W-
1-le'"irk0 ---
HO ii HO
"'OH ,
7
0 0
''.
He>ft0L
11 'EOH Ho=-=>)., 1-cii0H
HO OH HO
,and
o o 0
110">fk0 rigki 'N, ..--- OH
IHO Rip 40 '
If'OH
i-t3c0 OCH3 .
6
Date Recue/Date Received 2023-06-16
CA 03205490 2023-06-16
According to the use of the pharmaceutical composition, wherein the
pharmaceutical composition can further include epidermal growth factor
receptor-tyrosine kinase inhibitors (EGFR-TKIs). Preferably, the EGFR-TKIs can
be
osimertinib, gefitinib, erlotinib or afatinib.
According to the use of the pharmaceutical composition, wherein the lung
cancer
can be a non-small cell lung cancer (NSCLC).
According to the use of the pharmaceutical composition, wherein the lung
cancer
can be resistant to epidermal growth factor receptor-tyrosine kinase
inhibitors.
The above summary is intended to provide a simplified summary of the
disclosure to provide the reader with a basic understanding of the disclosure.
The
summary is not an exhaustive overview of the disclosure, and it is not
intended to
identify key/critical elements of embodiments of the present disclosure or to
delineate
the scope of the present disclosure.
BRIEF DESCRIPTION OF THE DRAWINGS
The present disclosure can be more fully understood by reading the following
detailed description of the embodiment, with reference made to the
accompanying
drawings as follows:
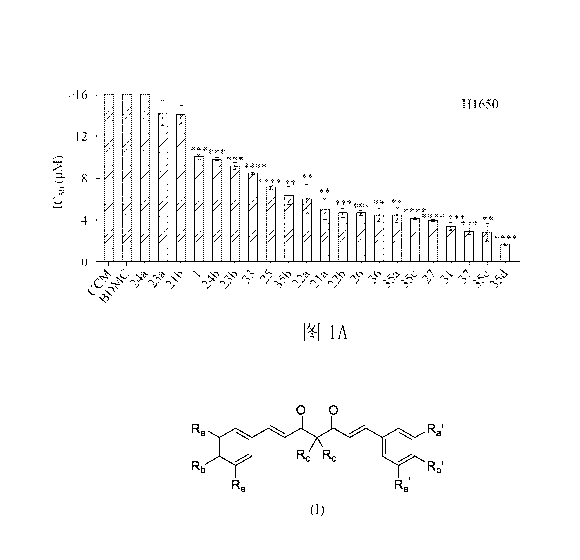
Fig. 1A shows the analysis result of the inhibition of H1650 cell growth by
the
diarylheptanoid compounds of the present disclosure;
Fig. 1B, Fig. 1C and Fig. 1D show the analysis results of the inhibition of
the
growth of the epidermal growth factor receptor-tyrosine kinase inhibitors
resistant
7
Date Regue/Date Received 2023-06-16
CA 03205490 2023-06-16
non-small cell lung cancer (NSCLC) cells by the diarylheptanoid compounds of
the
present disclosure;
Fig. 2A shows the analysis result of the inhibition of tumor growth in the GR6
tumor mice by treating Compound 35d alone;
Fig. 2B shows the analysis result of the inhibition of tumor growth in the GR8
tumor mice by treating Compound 35d alone;
Fig. 2C shows the analysis result of the inhibition of tumor growth in the
HCC827
tumor mice by treating Compound 35d alone;
Fig. 2D shows the statistical chart of body weight changes in the tumor mice
by
io treating Compound 35d alone;
Fig. 3A shows the analysis result of the inhibition of tumor re-progression in
the
GR6 tumor mice by treating a combined treatment of Compound 35d and
osimertinib;
and
Fig. 3B shows the statistical chart of body weight changes in the GR6 tumor
mice
by treating the combined treatment of Compound 35d and osimertinib.
DETAILED DESCRIPTION
The present disclosure provides a novel use of a pharmaceutical composition,
and the pharmaceutical composition includes a diarylheptanoid compound or a
pharmaceutically acceptable salt thereof, which can be used to manufacture a
drug
for treating a lung cancer. The diarylheptanoid compound of the present
disclosure
has a structure represented by Formula (I):
8
Date Regue/Date Received 2023-06-16
CA 03205490 2023-06-16
0 0
Re R '
a
I
Rc Rc
I ,
Re Re
Formula (I),
wherein Ra, Rb, Rai and RID' are independently H, C1-C2 alkyl, C1-C3 alkoxy,
OH,
or -0C(=0)Rd, wherein Rd is C1-C3 alkyl or C1-C3 alkanol; Rc is H, C1-C2
alkyl,
.. C3-C6 unsaturated alkyl or C7-C12 arylalkyl with double or triple bonds;
and Re and
Re' are independently H, C1-C6 alkyl or C1-C6 alkoxy.
At least one of Ra, Rb, Rai, and RID' of the diarylheptanoid compound of the
present
disclosure can be -0C(=0)Rd, and Rd is C1-C3 alkyl or C1-C3 alkanol. In
addition,
the diarylheptanoid compound can be interconvertible between keto and enol
forms,
when Rc is H.
The pharmaceutical composition of the present disclosure can further include
epidermal growth factor-tyrosine kinase inhibitors (EGFR-TKIs), which can be
combined with the diarylheptanoid compound or a pharmaceutically acceptable
salt
thereof. The EGFR-TKIs can be osimertinib, gefitinib, erlotinib or afatinib.
The
lung cancer treated by the pharmaceutical composition of the present
disclosure can
be non-small cell lung cancer (NSCLC). In addition, the lung cancer can be
resistant
to the EGFR-TKIs.
Unless otherwise noted, all terms, symbols or other scientific terms or terms
used
in the present disclosure have the meanings that are commonly understood by
person
having ordinary skill in the art. In some cases, terms with conventional
meanings
9
Date Regue/Date Received 2023-06-16
CA 03205490 2023-06-16
are defined herein for clarity and/or immediate reference, and the definitions
incorporated herein should be construed as not necessarily substantial
different from
the conventional meanings in the art. Many of the techniques and procedures
described or referenced herein are well known and routinely used by those
skilled in
the art. Where appropriate, unless otherwise stated, procedures for the use of
commercially available kits and reagents are generally performed according to
instructions and/or parameters defined by the manufacturer.
Unless contraindicated or noted otherwise, in these descriptions and
throughout
this specification, the terms "a" and "an" mean one or more (that is at least
one).
Furthermore, genera are recited as shorthand for a recitation of all members
of the
genus; for example, the recitation of C1-C3 alkyl is shorthand for a
recitation of all
C1-C3 alkyls including methyl, ethyl, propyl, and isomers thereof.
The diarylheptanoid compounds disclosed in the present disclosure and the
pharmaceutically acceptable salt thereof can be verified by in vitro
experiments,
which can inhibit the growth of the EGFR-TKIs resistant NSCLC cells. It can
further
be verified by in vivo experiments that the compound disclosed in the
specification
and/or at least one pharmaceutically acceptable salt thereof can be
administered to
animals suffering from EGFR-TKIs resistant lung cancer (such as mouse model),
and
can obtain therapeutic effects. A positive result in one or more tests is
sufficient to
demonstrate the actual utility of the tested compound and/or salt, and an
appropriate
dosage range and administration route for animals (such as humans) can be
determined based on test results.
Date Recue/Date Received 2023-06-16
CA 03205490 2023-06-16
Useful pharmaceutical dosage forms for administering the diarylheptanoid
compound of the present disclosure and the pharmaceutically acceptable salt
thereof
include, but are not limited to, hard and soft gelatin capsules, tablets,
parenteral
injections and oral suspensions. The dosage administered can depend on factors
including the age of the subject, the health and weight of the subject, the
extent of
the disease, the type of concomitant treatment (if any), the frequency of
treatment
and the nature of the desired effect. Usually the daily dose of active
ingredient may
vary, for example from 0.1 to 2000 mg per day. For example, 10-500 mg one or
more times per day can be effective to achieve the desired results.
The same dosage form can generally be used when the diarylheptanoid
compounds of the present disclosure and pharmaceutically acceptable salt
thereof
are administered stepwise or in combination with at least one other
therapeutic agent.
When drugs are administered in a physical combination, the dosage form and
route
of administration should be selected based on the compatibility of the
combined drugs.
Therefore, "co-administration" in the specification should be understood to
include
the concomitant or sequential administration of at least two agents, or as a
fixed-dose
combination of at least two active ingredients.
The diarylheptanoid compound and the pharmaceutically acceptable salt thereof
in the specification can be used as the active ingredient alone, or
administered in
combination with at least one second active ingredient, the second active
ingredient
can be selected from, for example, other active ingredients known to be useful
in the
treatment of patients with NSCLC, in particular the EGFR-TKIs.
11
Date Regue/Date Received 2023-06-16
CA 03205490 2023-06-16
The following specific examples are used to further illustrate the present
disclosure, in order to benefit the person having ordinary skill in the art,
and can fully
utilize and practice the present disclosure without excessive interpretation.
These
examples should not be regarded as limiting the scope of the present
disclosure, but
is used to illustrate how to implement the materials and methods of the
present
disclosure.
1. Structure of the diarylheptanoid compound of the present disclosure
The diarylheptanoid compound of the present disclosure uses curcumin (CCM)
as a guiding compound to design a curcuminoid diarylheptanoid compound, which
has a structure represented by Formula (I):
0 0
Re R '
a
R R
c
Rbi
Re Re
Formula (I),
Please refer to the following Table 1, which shows Ra, Rb, Ra',
Rc, Re and Re'
of examples of the diarylheptanoid compound of the present disclosure -
Compound
1, Compound 21a, Compound 21b, Compound 22a, Compound 22b, Compound 23a,
Compound 23b, Compound 24a, Compound 24b, Compound 25, Compound 26,
Compound 27, Compound 31, Compound 33, Compound 35a, Compound 35c,
Compound 35d, Compound 35e, Compound 36 and Compound 37.
12
Date Recue/Date Received 2023-06-16
CA 03205490 2023-06-16
Table 1
Compound Ra Ra' Rb RID' Rc Re,
Re'
1 OH OH OCH3 OCH3 H H
O E0H 0 E0H
21a OCH3 OCH3 ¨0-8 cH3 ¨0-8 cH3 H H
OH OH
O -OH
21b OCH3 OCH3 ¨0-8 cH3
OH H H
-OH
OH 0 -OH
22a OCH3 OCH3 ¨0-g Ic2H5 ¨0-6 c2H5 H H
OH -OH
22b OCH3 OCH3 0 () c2H5OH H -- H
LOH
O [OH 0 [OH
23a H H ¨0-8 cH3 ¨0-8 cH3 H -- H
OH OH
O [OH
23b H H ¨0-8 8H3
OH H H
OH
0 -OH 0 -OH
24a ¨0-8
OCH3 OCH3 H H
-OH -OH
0 -OH
24b ¨0-8 cH,
OH OCH3 OCH3 H H
-OH
0 1-002HH5
25 OH OCH3 OCH3 H H
LOH
O [OH 0 [OH
26 0C2H5 0C2H5 0-8 cH3 ¨0-8 cH3 H H
OH OH
O LOH 0 LOH
27 0C2H5 0C2H5 0-8 c2H5 ¨0-8 c2H5 H H
OH OH
O -OH 0 -OH
31 C2 H5 C2 H5 ¨0- CH3 ¨0- CH3 H C2H5
-OH -OH
O [OH
33 OCH3 OCH3 ¨0-8 cH3
OCH3 H H
OH
13
Date Regue/Date Received 2023-06-16
CA 03205490 2023-06-16
9 -CH 9 -CH
35a OCH3 OCH3 -(3-C CH3 H
-OH -OH
9 -CH 9 -CH
35c OCH3 OCH3 -(3-c cH3 -0-c cH3
benzyl H
-OH -OH
2 E0H 2 E0H
35d OCH3 OCH3 -o-c cH3 -o-c cH3 p-ropargyl H
OH OH
9 -CH 9 -CH
35e OCH3 OCH3 -(3-c cH3 -0-c cH3
allyl H
-OH -OH
2 E0H 2 E0H
36 H H -o-c cH3 -o-c cH3
CH3 H
OH OH
2 -0H 2 E0H
37 -0-c cH, -o-c cH3 OCH3 OCH3 CH3
H
-OH OH
Structures of Compound 21a-((1E,3Z,6E)-3-hydroxy-5-oxohepta- 1,3,6-triene-
1,7-diy1)bis(2-methoxy-4,1-phenylene)bis(3-hydroxy-2-hydroxymethyl)-2-
methylpropanoate, Compound 35a-((1E,6E)-4,4-dimethyl-3,5-dioxohepta-1, 6-diene-
1,7-diy1)bis(2-methoxy-4,1-phenylene)bis(3-hydroxy-2-hydroxymethyl)-2-
methylpropanoate, Compound 35d-((1E,6E)-3,5-dioxo-4,4-di(prop-2-yn-1-y1) hepta-
1,6-diene-1,7-diy1)bis(2-methoxy-4,1-phenylene)bis(3-hydroxy-2-(hydroxymethyl)-
2-
methylpropanoate), Compound 36-((1E,6E)-4,4-dimethy1-3,5- dioxohepta-1,6-diene-
1,7-diy1)bis(4,1-phenylene)bis(3-hydroxy-2-(hydroxymethyl)-2-
methylpropanoate),
and Corn pound 37-((1E,6E)-4,4-dimethy1-3,5- dioxohepta-1,6-dien-1,7-
diy1)bis(2-
io methoxy-5,1-phenylene)bis(3-hydroxy-2-(hydroxymethyl)-2-methylpropanoate)
are
shown in Table 2.
14
Date Recue/Date Received 2023-06-16
CA 03205490 2023-06-16
Table 2
Compound Structure
= OH
IVI ee0 " r, OM
0
21a HO 0== OH
OH
= 0
Me0 416 OlVie
I 0
35a HO"->ji0 411111)1P 0 OH
HO OH
0 0
0
I
35d HO"--)L0
I I 00H
0 0
0 0
36 He>fk0 0/11-eli
HO OH
0 0 0
gir
37 40
HO"->f0 OH
*INCOH IHO
H3C0 OCH3
The structure of Compound 21a includes a heptadiene-3,5-dione moiety, which
can be readily interconvertible between the keto form and enol form. The 3- or
5-
OH group of the enol form combines with the adjacent 5- or 3-C=0 through
hydrogen
bonds to stabilize the structure thereof. In the present disclosure, two
methyl
functionalities are incorporated onto the 4-position of Compound 21a and
afforded
((1E,6E)-4,4-dimethy1-3,5-dioxohepta-1,6-diene-
1,7-diy1)bis(2-methoxy-4,1-
phenylene)bis(3-hydroxy-2-hydroxymethyl)-2-methylpropanoate (35a), which is
found to possess stable keto form, and is not able to tautomerization. In
addition, in
Date Recue/Date Received 2023-06-16
CA 03205490 2023-06-16
the present disclosure, Compound 35a is used as a new lead compound and
derived
into a series of 4,4-dialkyl derivatives thereof (Compound 35a, Compound 35d,
Compound 36 and Compound 37) with stable keto form.
2. Inhibitory growth effect of the diarylheptanoid compound of the present
disclosure on the EGFR gene mutant NSCLC cells and the EGFR-TKIs resistant
NSCLC cells
The EGFR gene mutant NSCLC cells were treated with the diarylheptanoid
compounds of the present disclosure, and then the cell survival was stained
with
crystal violet to measure the IC50 values of the diarylheptanoid compounds of
the
present disclosure in the EGFR gene mutant NSCLC cells, so as to determine the
growth inhibitory effect of the diarylheptanoid compounds of the present
disclosure
on the EGFR gene mutant NSCLC cells.
EGFR gene mutations in lung cancer are mostly found in exons 18-21, which are
the intracellular tyrosine kinase coding region. The most common mutations
include
E746-A750del in exon 19 and L858R point mutation in exon 21, which account for
about 85%-90% of EGFR gene mutations. Tumor cells with these two mutations are
sensitive to the EGFR-TKIs, known as activating mutations. Secondary mutations
may occur in some tumor cells, and the most common secondary mutation is the
T790M mutation in exon 20, which is a drug resistance mutation. Please refer
to
Fig. 1A, which shows the analysis result of the inhibition of the H1650 cells
growth by
the diarylheptanoid compounds of the present disclosure. The H1650 cells have
the
E746-A750del mutation in exon 19 of the EGFR gene, wherein the CCM represents
curcumin and the BDMC represents bisdemethoxycurcumin. The IC50 value of the
16
Date Recue/Date Received 2023-06-16
CA 03205490 2023-06-16
compound > 16 pM shows that the H1650 cells have not yet reached 50% cell
growth
inhibition after treatment with compounds at concentrations as high as 16 pM,
and
data in Fig. 1A are represented by mean SD (n = 3).
Fig. 1A shows the IC50 values in the H1650 cells treated with 22
diarylheptanoid
compounds (including BDMC) and curcumin for 3 days. 18
diarylheptanoid
compounds including Compound 1, Compound 24b, Compound 23b, Compound 33,
Compound 25, Compound 35b, Compound 22a, Compound 21a, Compound 22b,
Compound 26, Compound 36, Compound 35a, Compound 35c, Compound 27,
Compound 31, Compound 37, Compound 35e and Compound 35d have significantly
io better inhibitory activity than the parent compound - curcumin on
the growth of the
H1650 cells, wherein ** represents p <0.05, *** represents p <0.01, ****
represents
p <0.001.
Experimentally, another 7 EGFR-TKIs resistant NSCLC cells were treated with
Compound 21a, Compound 35a, Compound 35d, Compound 36 or Compound 37,
and then the cell survival assay was performed to measure the IC50 values of
the
diarylheptanoid compounds of the present disclosure in the EGFR-TKIs resistant
NSCLC cells, so as to determine the growth inhibitory effect of the
diarylheptanoid
compounds of the present disclosure on the EGFR-TKIs resistant NSCLC cells.
The
EGFR-TKIs resistant NSCLC cells used in the test include the H1975 cells, the
GR2
cells, the GR5 cells, the GR6 cells, the GR8 cells, the GR9 cells and the GR10
cells,
in which the H1975 cells have a point mutation of L858R in exon 21 and a
secondary
mutation of T790M in exon 20; the GR2 cells, the GR5 cells, the GR6 cells, the
GR8
cells, the GR9 cells and the GR10 cells are gefitinib-resistant cell lines
obtained after
17
Date Recue/Date Received 2023-06-16
CA 03205490 2023-06-16
treating gefitinib with the HCC827 cells as parental cells, while the HCC827
cells and
the H1650 cells are the NSCLC cells with E746-A750del mutation of EGFR exon
19.
Please refer to Fig. 1B, Fig. 1C and Fig. 1D. Fig. 1B shows the analysis
result
of the inhibition of the H1975 cells growth by the diarylheptanoid compounds
of the
present disclosure. Fig. 1C shows the analysis result of the inhibition of the
GR2
cells, the GR5 cells, the GR6 cells, the GR8 cells, the GR9 cells and the GR10
cells
growth by the diarylheptanoid compounds of the present disclosure. Fig. 1D
shows
the percentage changes of the IC50 values after the GR2 cells, the GR5 cells,
the
GR6 cells, the GR8 cells, the GR9 cells and the GR10 cells were treated with
Compound 35d and gefitinib respectively. Data in Fig. 1B and Fig. 1C are
presented
as mean SD (n = 3), while the IC50 value > 16 pM show that the test cells
have not
yet reached 50% cell growth inhibition after treatment with compounds at
concentrations as high as 16 pM. Gef in Fig. 1D represents gefitinib.
Fig. 1B shows the measured the IC50 values of the H1975 cells treated with
Compound 21a, Compound 35a, Compound 35d, Compound 36, Compound 37 and
curcumin for 3 days, respectively. The results showed that Compound 21a,
Compound 35a, Compound 35d, Compound 36 and Compound 37 of the present
disclosure had significantly better inhibitory activity than curcumin on the
growth of
the H1975 cells.
Fig. 1C shows the measured the IC50 values of the GR2 cells, the GR5 cells,
the
GR6 cells, the GR8 cells, the GR9 cells and the GR10 cells after treatment
with
Compound 21a, Compound 35a, Compound 35d, Compound 36, Compound 37 and
curcumin for 3 days, respectively.
The results show that Compound 21a,
18
Date Recue/Date Received 2023-06-16
CA 03205490 2023-06-16
Compound 35a, Compound 35d, Compound 36 and Compound 37 of the present
disclosure have significantly better inhibitory activity than curcumin on the
growth of
the GR2 cells, the GR5 cells, the GR6 cells, the GR8 cells, the GR9 cells and
the
GR10 cells.
The results in Fig. 1B and Fig. 1C show that all EGFR-TKIs resistant NSCLC
cells are more sensitive to Compound 21a, Compound 35a, Compound 35d,
Compound 36 and Compound 37 than curcumin, and can achieve 50% inhibition of
cell growth at significantly lower concentrations.
Fig. 1D shows that the analysis results obtained by comparing the IC50 values
io measured by the GR2 cells, the GR5 cells, the GR6 cells, the GR8
cells, the GR9
cells and the GR10 cells with the IC50 value measured by the HCC827 cells, in
which
the HCC827 cells, the GR2 cells, the GR5 cells, the GR6 cells, the GR8 cells,
the
GR9 cells and the GR10 cells were treated with Compound 35d and gefitinib for
3
days and then measured the IC50 values. The results in Fig. 1D show that all
is gefitinib-resistant cell lines are indirectly sensitive to Compound 35d.
All
gefitinib-resistant cell lines were >200-fold more resistant to gefitinib
(Gef) than their
parental cells, the HCC827 cells.
3. Anticancer activity of Compound 35d against the GR6 tumor, the GR8 tumor
and the HCC827 tumor
20 In order to verify the anticancer effect of the diarylheptanoid
compound of the
present disclosure in vivo, the GR6 tumor mouse model, the GR8 tumor mouse
model
and the HCC827 tumor mouse model of xenotransplantation were first
established,
and the GR6 tumor mice, the GR8 tumor mice, and the HCC827 tumor mice were
19
Date Regue/Date Received 2023-06-16
CA 03205490 2023-06-16
treated with 100 mg/kg of Compound 35d daily for 35 days, and the tumor size
and
body weight of the GR6 tumor mice, the GR8 tumor mice, and the HCC827 tumor
mice were recorded.
Please refer to Fig. 2A to Fig. 2D, Fig. 2A shows the analysis result of the
inhibition of tumor growth in the GR6 tumor mice by treating Compound 35d
alone,
Fig. 2B shows the analysis result of the inhibition of tumor growth in the GR8
tumor
mice by treating Compound 35d alone, Fig. 2C shows the analysis result of the
inhibition of tumor growth in the HCC827 tumor mice by treating Compound 35d
alone,
and Fig. 2D shows the statistical chart of body weight changes in the tumor
mice by
io treating Compound 35d alone, wherein data in Fig. 2A to Fig. 2C are
presented as
mean SEM (n = 10).
The results in Fig. 2A to Fig. 2C show that Compound 35d significantly
inhibits
the tumor growth of the GR6 tumor mice and the GR8 tumor mice, but has less
significant effect on inhibiting the tumor growth of the HCC827 tumor mice.
However,
the results in Fig. 2D show that the body weight of the tumor mice did not
decrease
significantly after being treated with Compound 35d for more than 1 month.
4. Combined treatment of Compound 35d and osimertinib inhibits the
re-progression of the GR6 tumor
EGFR-TKIs are currently the standard treatment for NSCLC patients with the
EGFR gene mutation. To
further test whether the combined use of the
diarylheptanoid compound of the present disclosure and known ones can enhance
the therapeutic effect of NSCLC, the GR6 tumor mice were divided into 4
groups.
One group received 100 mg/kg Compound 35d treatment per day (represented as
Date Recue/Date Received 2023-06-16
CA 03205490 2023-06-16
35d), another group received 1 mg/kg osimertinib treatment per day
(represented as
Osi), still another group received the combined treatment of 100 mg/kg
Compound
35d and 1 mg/kg osimertinib per day (represented as 35d+Osi), and the other
group
was the control group without drug treatment.
Please refer to Fig. 3A and Fig. 3B, Fig. 3A shows the analysis result of the
inhibition of tumor re-progression in the GR6 tumor mice by treating the
combined
treatment of Compound 35d and osimertinib, and Fig. 3B shows the statistical
chart
of body weight changes in the GR6 tumor mice by treating the combined
treatment
of Compound 35d and osimertinib, wherein data in Fig. 3A and Fig. 3B are
.. represented by mean SEM (n = 10).
The results in Fig. 3A show that although the tumor size of the GR6 tumor mice
in the Osi group was initially reduced by osimertinib treatment, the treated
tumors
subsequently re-growth, indicating that the tumors of the GR6 tumor mice have
EGFR-TKIs resistance. However, no matter in the group treated with Compound
35d alone or in the group treated with the combined treatment of Compound 35d
and
osimertinib, tumor re-progression in the GR6 tumor mice was significantly
inhibited.
And the results in Fig. 3B show that neither the single treatment of Compound
35d
nor the combined treatment of Compound 35d and osimertinib can significantly
reduce the body weight of mice.
To sum up, the present disclosure provides a new use of the pharmaceutical
composition, which can be used to manufacture a drug for treating lung cancer.
The
pharmaceutical composition includes the diarylheptanoid compound or the
pharmaceutically acceptable salt thereof, which can inhibit the growth of the
EGFR
21
Date Recue/Date Received 2023-06-16
CA 03205490 2023-06-16
gene mutant NSCLC cells and the EGFR-TKIs resistant NSCLC cells, so that can
be
used to manufacture the drug for treating lung cancer.
Moreover, the
pharmaceutical composition can have a synergistic effect when used in
combination
with EGFR-TKIs, which can increase the effectiveness of treating lung cancer,
especially for the treatment of lung cancer with the EGFR gene mutation and
EGFR-TKIs, and has the potential to be used in the medical and health care
market.
Although the present disclosure has been described as above by way of
embodiments, it is not intended to limit the present disclosure. Person having
skilled
in the art can make various changes and modifications without departing from
the
spirit and scope of the present disclosure. Therefore, the scope of protection
of the
present disclosure should be defined by the scope of the appended patent
application.
22
Date Recue/Date Received 2023-06-16