Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03210001 2023-07-27
WO 2022/187822 PCT/US2022/070910
MICROSPHERE FORMULATIONS COMPRISING BTK INHIBITORS AND
METHODS FOR MAKING AND USING THE SAME
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority from U.S. Provisional Patent
Application No.
63/156,020, filed on March 3, 2021, which is incorporated by reference herein
in its entirety.
BACKGROUND
[0002] B-cells account for up to 25% of all cells in some cancers. By
inhibiting the Bruton's
Tyrosine Kinase ("BTK") enzyme involved in B-cell receptor signaling, BTK
inhibitors cause
detachment of malignant B-cells from cancer sites into blood, which results in
cell death. BTK
inhibition reduces the proliferation of malignant B-cells and decreases the
survival of malignant
B-cells.
[0003] Ibrutinib (chemical formula C25H24N602; CAS Number 936563-96-1),
characterized
by the general structure:
0Q
NH2
N
ii N N N
0
is a BTK inhibitor. Ibrutinib, alone and in combination with other drugs, has
been approved by
the U.S. Food and Drug Administration (the "FDA") for the treatment of mantle
cell lymphoma
("MCL"), chronic lymphocytic leukemia ("CLL"), Waldenstrom' s
macroglobulinemia, small
1
CA 03210001 2023-07-27
WO 2022/187822 PCT/US2022/070910
lymphocytic lymphoma ("SLL"), relapsed/refractory marginal zone lymphoma in
patients who
require systemic therapy and have received at least one prior anti-CD20-based
therapy, and graft-
versus-host disease, among other diseases.
[0004] Two other BTK inhibitors have been approved by the FDA:
acalabrutinib (approved
for treatment of relapsed MCL) and zanubrutinib (approved for treatment of
MCL). Several other
drugs that inhibit BTK are in clinical trials, including evobrutinib for
multiple sclerosis; ABBV-
105 for systemic lupus erythematosus; fenebrutinib for rheumatoid arthritis,
systemic lupus
erythematosus, and chronic spontaneous urticaria; GS-4059 for non-Hodgkin's
lymphoma and/or
CLL; Spebrutinib (AVL-292, CC-292); and HM71224 for autoimmune diseases.
[0005] All of the currently approved BTK inhibitors are oral formulations.
Oral formulations
may have several disadvantages. For example, oral formulations may require
closely timed,
successive dosages under the supervision of a physician. Further, some BTK
inhibitors may have
low and variable oral bioavailability. For example, ibrutinib may have an oral
bioavailability of
only 2.9% in the fasted state, but this can vary from patient to patient.
[0006] A need exists for a high-bioavailability formulation comprising a
BTK inhibitor that
may be administered by a long-acting, sustained release injection, without the
need for patients to
administer closely timed, successive dosages under supervision from their
physician.
SUMMARY
[0007] Microsphere formulations comprising a BTK inhibitor are provided.
The microsphere
formulations comprise polymer microspheres, each polymer microsphere
comprising: (i) a BTK
inhibitor; and (ii) a biodegradable polymer, wherein each polymer microsphere
comprises a drug
load of the BTK inhibitor of greater than 40% by weight of the polymer
microsphere, and wherein
the polymer microspheres have an average particle size of less than 110 i_tm
(D5o). In another
2
CA 03210001 2023-07-27
WO 2022/187822 PCT/US2022/070910
aspect, the microsphere formulations are characterized in that they have a low
initial burst release,
that is, not more than 50% of the BTK inhibitor is released within about 4
hours of injection into
a subject.
[0008] In one aspect, the microsphere formulations may be made by a method,
the method
comprising: (A) mixing: (i) the biodegradable polymer; (ii) a primary solvent;
and (iii) a BTK
inhibitor, to form a dispersed phase; (B) mixing: (i) water; and (ii) a
surfactant, to form a
continuous phase; and (C) combining the dispersed phase with the continuous
phase in a
homogenizer.
[0009] In one aspect, a method for treating cancer, including a B-cell
malignancy, is provided.
The method may comprise administering by intramuscular or subcutaneous
injection to a patient
in need thereof a microsphere formulation made according to the methods
described herein.
[0010] In another aspect, use is disclosed of a microsphere formulation
comprising polymer
microspheres, each polymer microsphere comprising: (i) a BTK inhibitor; and
(ii) a biodegradable
polymer, wherein each polymer microsphere comprises a drug load of the BTK
inhibitor of greater
than 40% by weight of the polymer microsphere, and wherein the polymer
microspheres have an
average particle size of less than 110 i_tm (D5o), in the manufacture of a
medicament for the
treatment of cancer, including a B-cell malignancy.
[0011] In another aspect, a microsphere formulation comprising polymer
microspheres, each
polymer microsphere comprising: (i) a BTK inhibitor; and (ii) a biodegradable
polymer, wherein
each polymer microsphere comprises a drug load of the BTK inhibitor of greater
than 40% by
weight of the polymer microsphere, and wherein the polymer microspheres have
an average
particle size of less than 110 i_tm (D5o), is provided for use as a medicament
for the treatment of
cancer, including a B-cell malignancy.
3
CA 03210001 2023-07-27
WO 2022/187822 PCT/US2022/070910
[0012] In another aspect, a kit is provided, the kit comprising polymer
microspheres, each
polymer microsphere comprising: (i) a BTK inhibitor; and (ii) a biodegradable
polymer, wherein
each polymer microsphere comprises a drug load of the BTK inhibitor of greater
than 40% by
weight of the polymer microsphere, and wherein the polymer microspheres have
an average
particle size of less than 110 i_tm (D5o).
BRIEF DESCRIPTION OF THE FIGURES
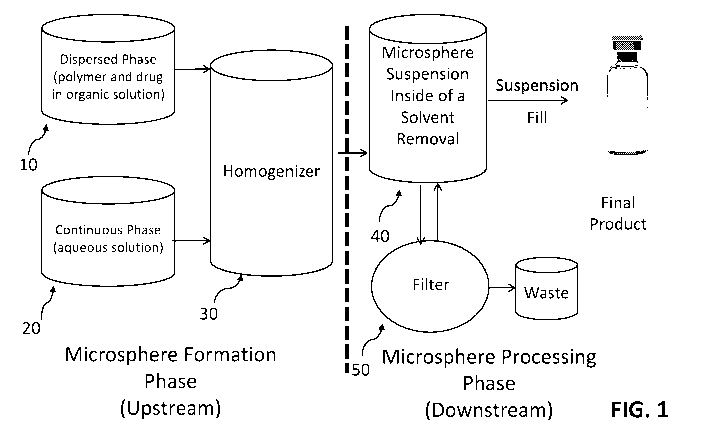
[0013] Figure 1 is a schematic depicting a method for making BTK inhibitor-
encapsulated
polymer microspheres.
[0014] Figure 2 is a graph showing in vitro cumulative ibrutinib release
over time from
ibrutinib-encapsulating polymer microspheres comprising a 50:50 poly (D,L-
lactide-co-glycolide)
("PLGA") as the biodegradable polymer.
[0015] Figure 3 is a graph showing in vitro cumulative ibrutinib release
over time from
ibrutinib-encapsulating polymer microspheres comprising a 75:25 PLGA with an
inherent
viscosity ("IV") of 0.26 dL/g as the biodegradable polymer.
[0016] Figure 4 is a graph showing in vitro cumulative ibrutinib release
over time from
ibrutinib-encapsulating polymer microspheres comprising a 75:25 PLGA with IVs
between 0.41
dL/g and 0.70 dL/g as the biodegradable polymer.
[0017] Figure 5 is a graph showing in vitro cumulative ibrutinib release
over time from
ibrutinib-encapsulating polymer microspheres comprising an 85:15 PLGA as the
biodegradable
polymer.
[0018] Figure 6 is a graph showing in vitro cumulative ibrutinib release
over time from
ibrutinib-encapsulating polymer microspheres comprising a poly(D,L-lactide)
("PLA") as the
biodegradable polymer.
4
CA 03210001 2023-07-27
WO 2022/187822 PCT/US2022/070910
DETAILED DESCRIPTION
[0019] Microsphere formulations comprising a BTK inhibitor are provided.
The microsphere
formulations comprise polymer microspheres, each polymer microsphere
comprising: (i) a BTK
inhibitor; and (ii) a biodegradable polymer, wherein each polymer microsphere
comprises a drug
load of the BTK inhibitor of greater than 40% by weight of the polymer
microsphere, and wherein
the polymer microspheres have an average particle size of less than 110 jim
(D5o). In another
aspect, the microsphere formulations are characterized in that they have a low
initial burst release,
that is, not more than 20% of the BTK inhibitor is released within about 24
hours of injection into
a subject.
[0020] In one aspect, the microsphere formulations may be made by a method,
the method
comprising: (A) mixing: (i) the biodegradable polymer; (ii) a primary solvent;
and (iii) a BTK
inhibitor, to form a dispersed phase; (B) mixing: (i) water; and (ii) a
surfactant, to form a
continuous phase; and (C) combining the dispersed phase with the continuous
phase in a
homogenizer.
BTK inhibitors
[0021] In one aspect, the BTK inhibitor is selected from the group
comprising, consisting
essentially of, or consisting of ibrutinib, acalabrutinib, zanubrutinib,
evobrutinib, ABBV-105,
fenebrutinib, GS-4059, or spebrutinib, or combinations thereof
[0022] In one aspect, the composition consists essentially of ibrutinib. In
one aspect, the
ibrutinib is supplied by ScinoPharm or MSN. In one aspect, the ibrutinib is
hydrophobic. In one
aspect, the ibrutinib is supplied as a free base. In another aspect, the
ibrutinib is supplied as a
pharmaceutically acceptable salt. In one aspect, the ibrutinib is
characterized by an aqueous
solubility of <2.5 mg/g. In one aspect, the ibrutinib is characterized by a
solubility in
CA 03210001 2023-07-27
WO 2022/187822 PCT/US2022/070910
dichloromethane ("DCM") of >300 mg/g. In one aspect, the ibrutinib is
characterized by a pKa
of about 3.74.
[0023] The BTK inhibitor may be in various polymorphic forms. Polymorphic
forms may
include hemihydrates, monohydrates, dihydrates, and other polymorphic forms as
known in the
art. Salts may include hydrochloride, sulfate, acetate, phosphate,
diphosphate, chloride, maleate,
citrate, mesylate, nitrate, tartrate, gluconate, or other salts as known in
the art. In one aspect, the
BTK inhibitor is in an amorphous form.
[0024] In an aspect wherein the BTK inhibitor comprises ibrutinib or
another BTK inhibitor
with similar solubility characteristics, a complex salt may be used to
decrease solubility, such as,
for example, palmitate, benzoic acid, tosylic acid, camphor-sulfonic acid, or
other salt complexes
as one of skill in the art can readily envision.
Biodegradable Polymers
[0025] In one aspect, the dispersed phase may include a biodegradable
polymer, such as a
PLGA or a PLA, although it is contemplated that other suitable biodegradable
polymers may be
used. The biodegradable polymer may be hydrophobic or hydrophilic.
[0026] In some aspects, the biodegradable polymer comprises a PLGA. In one
aspect, the
PLGA comprises a lactide:glycolide ratio of 50:50, 75:25, or 85:15. In one
aspect, the PLGA is
acid-terminated. In one aspect, the PLGA is ester-terminated. In one aspect,
the PLGA has an IV
of from about 0.1 dL/g to about 0.8 dL/g, including from about 0.1 dL/g to
about 0.3 dL/g, from
about 0.16 dL/g to about 0.24 dL/g, from about 0.2 dL/g to about 0.4 dL/g,
from about 0.4 dL/g to
about 0.6 dL/g, from about 0.6 dL/g to about 0.8 dL/g, about 0.20 dL/g, 0.26
dL/g, 0.41 dL/g, 0.56
dL/g, 0.66 dL/g, 0.7 dL/g, and any value or range between any two of those IV
values.
6
CA 03210001 2023-07-27
WO 2022/187822 PCT/US2022/070910
[0027] In one aspect, the PLGA comprises Resomerg 502 H, poly(D,L-lactide-
co-glycolide),
acid terminated, lactide:glycolide 50:50, manufactured by Evonik, having IV =
0.20 ("502 H"). In
one aspect, the PLGA comprises Resomerg 502, poly(D,L-lactide-co-glycolide),
ester terminated,
lactide:glycolide 50:50, manufactured by Evonik, having IV = 0.20 ("502"). In
one aspect, the
PLGA comprises ViatelTM DLG 7503 A, poly(D,L-lactide-co-glycolide), acid
terminated,
lactide:glycolide 75:25, manufactured by Ashland, having IV = 0.26 ("7503 A").
In one aspect,
the PLGA comprises ViatelTM DLG 7503 E, poly(D,L-lactide-co-glycolide), ester
terminated,
lactide:glycolide 75:25, manufactured by Ashland, having IV = 0.26 ("7503 E").
In one aspect,
the PLGA comprises ViatelTM DLG 7505 A, poly(D,L-lactide-co-glycolide), acid
terminated,
lactide:glycolide 75:25, manufactured by Ashland, having IV = 0.56 ("7505 A").
In one aspect,
the PLGA comprises ViatelTM DLG 7505 E, poly(D,L-lactide-co-glycolide), ester
terminated,
lactide:glycolide 75:25, manufactured by Ashland, having IV = 0.41 ("7505 E").
In one aspect,
the PLGA comprises ViatelTM DLG 7507 A, poly(D,L-lactide-co-glycolide), acid
terminated,
lactide:glycolide 75:25, manufactured by Ashland, having IV = 0.70 ("7507 A").
In one aspect,
the PLGA comprises ViatelTM DLG 7507 E, poly(D,L-lactide-co-glycolide), ester
terminated,
lactide:glycolide 75:25, manufactured by Ashland, having IV = 0.66 ("7507 E").
In one aspect,
the PLGA comprises ViatelTM DL 8503 A, poly(D,L-lactide-co-glycolide), acid
terminated,
lactide:glycolide 85:15, manufactured by Ashland, having IV = 0.24 ("8503 A").
In one aspect,
the PLGA comprises ViatelTM DL 8503 E, poly(D,L-lactide-co-glycolide), ester
terminated,
lactide:glycolide 85:15, manufactured by Ashland, having IV = 0.25 ("8503 E").
[0028] In some aspects, the biodegradable polymer is a PLA. In one aspect,
the PLA is acid-
terminated. In one aspect, the PLA is ester-terminated. In one aspect, the PLA
has an IV of
7
CA 03210001 2023-07-27
WO 2022/187822 PCT/US2022/070910
between about 0.1 dL/g and about 0.4 dL/g, including about 0.16 dL/g, about
0.18 dL/g, and about
0.32 dL/g, and any value or range between any two of those IV values.
[0029] In one aspect, the PLA comprises a ViatelTM DL 02 A, poly(D,L-
lactide), acid
terminated, manufactured by Ashland, having IV = 0.16 ("DL 02 A"). In one
aspect, the PLA
comprises a ViatelTM DL 02 E, poly(L-lactide), ester terminated, manufactured
by Ashland, having
IV = 0.18 ("DL 02 E"). In one aspect, the PLA comprises a ViatelTM DL 03 A,
poly(L-lactide),
acid terminated, manufactured by Ashland, having IV = 0.32 ("DL 03 A").
[0030] In one aspect, the biodegradable polymer is mixed with the BTK
inhibitor to form
microspheres, which are injectable and formulated to release the BTK inhibitor
to the patient over
the intended duration of release. In another aspect, the biodegradable polymer
is used to
encapsulate the BTK inhibitor into microspheres, which are injectable and
formulated to release
the BTK inhibitor to the patient over the intended duration of release, via a
controlled rate of
release from the spheres, or release from different spheres at different times
based upon particle
size, thickness of the biodegradable polymer encapsulating the BTK inhibitor,
molecular weight
of the biodegradable polymer, polymer composition such as co-monomer ratio,
end-cap, and drug
load, or combinations of such release-affecting factors.
Dispersed Phase
[0031] In one aspect, the dispersed phase comprises a primary solvent. In
one aspect, the
primary solvent comprises DCM. The dispersed phase may also include up to
about 50% by
weight of a co-solvent capable of optimizing the solubility of the BTK
inhibitor in the primary
solvent. In one aspect, the co-solvent may be benzyl alcohol, dimethyl
sulfoxide, dimethyl
formamide, dimethyl acetamide, acetonitrile, ethanol, N-methyl pyrrolidone,
ethyl acetate, or any
other solvent that increases the solubility of the BTK inhibitor in the
dispersed phase containing
8
CA 03210001 2023-07-27
WO 2022/187822 PCT/US2022/070910
DCM. A microsphere is "essentially free" of organic solvent if the microsphere
meets the
standards set forth in the "ICH Harmonised Guideline, Impurities: Guideline
for Residual Solvents
Q3C(R8), Current Step 4 version dated 22 April 2021," which is incorporated
herein by reference
in its entirety.
Continuous Phase
[0032] The dispersed phase may be combined with an aqueous continuous phase
that
comprises water and, optionally, a buffer, a surfactant, or both.
[0033] In one aspect, the buffer may be added to the continuous phase to
maintain a pH of the
solution of about 7.0 to about 8Ø In one aspect, the buffer may be a
phosphate buffer or a
carbonate buffer. In one aspect, the buffer may be a 10 mM phosphate or
carbonate buffer solution
and may be used to create and maintain a system pH level of about 7.6.
[0034] The surfactant component may be present in the continuous phase in
an amount of
about 0.35% to about 1.0% by weight in water. In one aspect, the surfactant
component comprises
polyvinyl alcohol ("PVA") in a concentration of 0.35% by weight in water.
[0035] In some aspects, the dispersed phase flow rate to the homogenizer
may be from about
mL/min to about 30 mL/min, including about 20 mL/min and about 25 mL/min. In
some
aspects, the continuous phase flow rate to the homogenizer may be about 2
L/min. Thus, in one
aspect, the continuous phase:dispersed phase ratio may be from about 66:1 to
about 200:1,
including about 100:1 and about 80:1. Larger scale batches may require higher
flow rates.
[0036] The continuous phase may be provided at room temperature or above or
below room
temperature. In some aspects, the continuous phase may be provided at about 40
C, about 37 C,
about 35 C, about 30 C, about 25 C, about 20 C, about 15 C, about 10 C,
about 5 C, about
0 C, and any value or range between any two of those temperature values.
9
CA 03210001 2023-07-27
WO 2022/187822 PCT/US2022/070910
Homogenizer
[0037] For brevity, and because the methods are equally applicable to
either, the phrase
"homogenizer" contemplates a system or apparatus that can homogenize the
dispersed phase and
the continuous phase, emulsify the dispersed phase and the continuous phase,
or both, which
systems and apparatuses are known in the art. For example, in one aspect, the
homogenizer is an
in-line SiIverson Homogenizer (commercially available from SiIverson Machines,
Waterside UK)
or a Levitronix BPS-i100 integrated pump system used, e.g., as described in
U.S. Patent No.
11,167,256, which is incorporated by reference herein in its entirety. In one
aspect, the
homogenizer is a membrane emulsifier or a static mixer. In one aspect, the
homogenizer runs at
an impeller speed of about 1,000 to about 4,000 revolutions per minute
("RPM"), including about
2,000 RPM, about 3,000 RPM, and any value or range between any two of those
RPM values.
Drug Load
[0038] The drug load of each polymer microsphere in a drug to polymer
ratio, expressed as a
percentage, may be greater than 40 wt/wt%, including from about 40 wt/wt% to
about 70 wt/wt%,
from about 45 wt/wt% to about 70 wt/wt%, from about 45 wt/wt% to about 65
wt/wt%, from about
50 wt/wt% to about 65 wt/wt%, greater than 50 wt/wt%, and any value or range
between any two
of those drug loads.
[0039] In some particular aspects, it is contemplated that the drug load
may be as low as 20
wt/wt%.
Particle Size
[0040] In one aspect, the polymer microspheres may have an average particle
size of less than
110 i_tm (D5o), including between about 30 i_tm (D5o) and about 60 i_tm (D5o),
between about 30 i_tm
(D5o) and about 50 i_tm (D5o), between about 35 i_tm (D5o) and about 60 i_tm
(D5o), between about
CA 03210001 2023-07-27
WO 2022/187822 PCT/US2022/070910
45 tm (D5o) and about 60 i_tm (D5o), about 30 im (D5o), about 35 i_tm (D5o),
about 40 i_tm (D5o),
about 45 i_tm (D5o), about 50 i_tm (D5o), about 55 i_tm (D5o), about 60 i_tm
(D5o), less than about 60
jim (D5o), and any value or range between any two of those average particle
sizes.
[0041] In some particular aspects, it is contemplated that average particle
sizes may be as large
as 150-200 m.
Extended Release
[0042] In one aspect, the microsphere formulations are characterized in
that they have an in
vivo duration of release of less than about 7 days in humans. In one aspect,
the microsphere
formulations are characterized in that they have an in vivo duration of
release of between about 7
days to about 14 days in humans. In one aspect, the microsphere formulations
are characterized
in that they have an in vivo duration of release of between about 14 days to
about 28 days in
humans. In one aspect, the microsphere formulations are characterized in that
they have an in vivo
duration of release of about 28 days in humans. In one aspect, the microsphere
formulations are
characterized in that they have an in vivo duration of release of greater than
about 28 days in
humans.
[0043] In one aspect, the microsphere formulations are characterized in
that at least about 50%,
at least about 60%, at least about 70%, at least about 80%, at least about
90%, or 100%, and any
range between any of those values, of the BTK inhibitor is released within <7,
7-14, 14-28, or >28
days (as described in the preceding paragraph) of injection into a subject.
For example, in one
aspect, the microsphere formulations are characterized in that about 75% to
100% of the BTK
inhibitor is released over the designated period after injection into a
subject. In another aspect, the
microsphere formulations are characterized in that they have a low initial
burst release, that is, not
11
CA 03210001 2023-07-27
WO 2022/187822 PCT/US2022/070910
more than about 20% of the BTK inhibitor is released within about 24 hours of
injection into a
subj ect.
[0044] A further aspect includes a sustained release injectable formulation
of ibrutinib that is
pharmacologically comparable to oral doses of: 70 mg, 140 mg, 280 mg, 420 mg,
and 560 mg, in
sustained release injectable formulations that release over approximately 7,
14, 21, or 28 days.
[0045] Another aspect includes a method of treating a human patient for
MCL, CLL/SLL, and
other diseases or conditions that may be treated by the BTK inhibitors. The
method may comprise
providing an injectable form of ibrutinib in a dosage strength that is
pharmacologically comparable
to 70 mg, 140 mg, 280 mg, 420 mg, and 560 mg per day orally, the injectable
form with a duration
of continuous release such that patient compliance is assured, the medical
consequences of missing
a dose or doses are avoided, and the pharmacokinetic profile is improved as
compared with the
oral dosage form.
Therapeutic Benefits
[0046] Possible conditions that may be treated using the microsphere
formulations comprising
a BTK inhibitor include cancer, including B-cell malignancies, including MCL,
CCL, and SLL.
In one aspect, a B-cell malignancy may be treated using the microsphere
formulations comprising
a BTK inhibitor, wherein the microsphere formulations are administered about
every <7, 7-14, 14-
28, or >28 days.
[0047] In one aspect, a method for treating cancer, including a B-cell
malignancy, is provided.
The method may comprise administering by intramuscular or subcutaneous
injection to a patient
in need thereof a microsphere formulation made according to the methods
described herein.
[0048] In another aspect, use is disclosed of a microsphere formulation
comprising polymer
microspheres, each polymer microsphere comprising: (i) a BTK inhibitor; and
(ii) a biodegradable
12
CA 03210001 2023-07-27
WO 2022/187822 PCT/US2022/070910
polymer, wherein each polymer microsphere comprises a drug load of the BTK
inhibitor of greater
than 40% by weight of the polymer microsphere, and wherein the polymer
microspheres have an
average particle size of less than 110 i_tm (D50), in the manufacture of a
medicament for the
treatment of cancer, including a B-cell malignancy.
[0049] In another aspect, a microsphere formulation comprising polymer
microspheres, each
polymer microsphere comprising: (i) a BTK inhibitor; and (ii) a biodegradable
polymer, wherein
each polymer microsphere comprises a drug load of the BTK inhibitor of greater
than 40% by
weight of the polymer microsphere, and wherein the polymer microspheres have
an average
particle size of less than 110 i_tm (D50), is provided for use as a medicament
for the treatment of
cancer, including a B-cell malignancy.
[0050] In another aspect, a kit is provided, the kit comprising polymer
microspheres, each
polymer microsphere comprising: (i) a BTK inhibitor; and (ii) a biodegradable
polymer, wherein
each polymer microsphere comprises a drug load of the BTK inhibitor of greater
than 40% by
weight of the polymer microsphere, and wherein the polymer microspheres have
an average
particle size of less than 110 i_tm (D50).
EXAMPLES
Example 1 ¨ General preparation of polymer microspheres comprising a BTK
inhibitor
[0051] Microsphere Formation Phase. With reference to Figure 1, a dispersed
phase ("DP")
is formed by dissolving a polymer matrix (such as a PLGA or PLA polymer) in an
organic
solvent system (such as DCM), followed by the addition of the BTK inhibitor
with mixing until
completely dissolved. The DP 10 is filtered using a 0.2 i_tm sterilizing PTFE
or PVDF membrane
filter (such as EMFLON, commercially available from Pall or SartoriousAG) and
pumped into a
homogenizer 30 at a defined flow rate. A continuous phase ("CP") 20 comprising
water,
13
CA 03210001 2023-07-27
WO 2022/187822 PCT/US2022/070910
surfactant, and, optionally, a buffer is also pumped into the homogenizer 30
at a defined flow rate.
The speed of the homogenizer 30 is generally fixed to achieve a desired
polymer microsphere size
distribution. A representative continuous "upstream" microsphere formation
phase is described in
U.S. Pat. No. 5,945,126, which is incorporated by reference herein in its
entirety.
[0052] Microsphere Processing Phase. The formed or forming microspheres
exit the
homogenizer 30 and enter a solvent removal vessel ("SRV") 40. Water may be
added to the SRV
40 during microsphere formation to minimize the solvent level in the aqueous
medium. See, e.g.,
U.S. Patent No. 9,017,715, which is incorporated by reference herein in its
entirety. After the DP
has been exhausted, the CP 20 and water flow rates are stopped, and the
washing steps are
initiated. Solvent removal is achieved using water washing and a hollow fiber
filter (commercially
available as HFF from Cytiva) 50. A representative "downstream" microsphere
processing phase
is described in U.S. Pat. No. 6,270,802, which is incorporated by reference
herein in its entirety.
[0053] The washed microspheres are collected and freeze-dried in a
lyophilizer (Virtis) to
remove any moisture. The resulting microspheres are a free-flowing off-white
bulk powder.
Example 2 ¨ Preparation of Ibrutinib-Encapsulated Polymer Microspheres
Comprising a 50:50
PLGA ¨ Batch Nos. 1 and 2 ("Group A")
[0054] Following the general procedure described in Example 1 and
illustrated in Figure 1,
the DP was formed by dissolving 2.5 g of either 502 H polymer (Batch No. 1) or
502 polymer
(Batch No. 2) (IV = 0.20 dL/g) in 11.67 g of DCM, followed by addition of
ibrutinib (2.5 g) with
mixing until completely dissolved. The DP was filtered and pumped at a flow
rate of 25 mL/min
into a Levitronix BPS-i100 integrated pump system operating at 3,000 RPM. The
CP comprising
0.35% PVA was also pumped into the homogenizer at a flow rate of 2 L/min
(CP:DP = 80:1).
14
CA 03210001 2023-07-27
WO 2022/187822 PCT/US2022/070910
[0055] The formed or forming microspheres exited the homogenizer and
entered the SRV.
Deionized water was added to the SRV. Solvent removal was achieved using water
washing and
a hollow fiber filter. The bulk suspension was collected via filtration and
lyophilized to obtain a
free-flowing powder.
[0056] Batch No. 1 had an average particle size of 36 tm (D50), a drug load
of 47.6 wt%, and
a molecular weight of 17.6 kDa. The microspheres contained residual DCM of
3.0%. Batch No.
2 had an average particle size of 44 jim (D50), a drug load of 47.8 wt%, and a
molecular weight of
17.7 kDa. The microspheres contained residual DCM of 3.0%. The parameters and
results are
shown tabularly in Table 1:
t Batch Number 1 2
= Symbol 0 A
Polymer
Evonik
Supplier/Name
gCo-Monomer Ratio 50:50
rt' Polymer IV (dL/g) 0.20
-5
E Polymer Endcap Acid Ester
O Batch Size (g) 5
Mixing Speed (RPM) 3000
Target Drug Load (%) 50.0
Drug Load (%) 47.6 47.8
Encapsulation
95.2 95.6
Efficiency (%)
Tzi Residual Solvent
3.0 3.0
= C- = D M (%)
13,10 17 18
= Particle Size
13,50 36 44
(11m)
13,90 59 79
Sample MW (kDa) 17.6 17.7
Polymer MW (kDa) 17.4 17.6
Table 1
[0057] Figure 2 is a graph showing in vitro cumulative ibrutinib release
over time from the
Group A ibrutinib-encapsulating polymer microspheres.
CA 03210001 2023-07-27
WO 2022/187822 PCT/US2022/070910
Example 3 ¨ Preparation of Ibrutinib-Encapsulated Polymer Microspheres
Comprising a 75:25
PLGA with a low polymer IV ¨ Batch Nos. 3, 4, 6, 7, and 11 ("Group B")
[0058] Following the general procedure described in Example 1 and
illustrated in Figure 1,
the DP was formed by dissolving 2.5 g (Batch Nos. 3, 4, and 11), 2.0 g (Batch
No. 6), or 1.5 g
(Batch No. 7) of either 7503 A polymer (Batch Nos. 3, 6, 7, and 11) or 7502 E
polymer (Batch
No. 6) (IV = 0.26 dL/g) in 11.67 g of DCM, followed by addition of ibrutinib
(sufficient to provide
a DP weight of 16.67 g, i.e., 2.5 g for Batch Nos. 3, 4, and 11; 3.0 g for
Batch No. 6; and 3.5 g for
Batch No. 7) with mixing until completely dissolved. The DP was filtered and
pumped at a flow
rate of 25 mL/min into a Levitronix BPS-i100 integrated pump system operating
at 3,000 RPM
(Batch Nos. 3, 4, 6, and 7) or 2,000 RPM (Batch No. 11). The CP comprising
0.35% PVA was
also pumped into the homogenizer at a flow rate of 2 L/min (CP:DP = 80:1).
[0059] The formed or forming microspheres exited the homogenizer and
entered the SRV.
Deionized water was added to the SRV. Solvent removal was achieved using water
washing and
a hollow fiber filter. The bulk suspension was collected via filtration and
lyophilized to obtain a
free-flowing powder.
[0060] Batch No. 3 had an average particle size of 39 jim (D50), a drug
load of 48.2 wt%, and
a molecular weight of 29.4 kDa. The microspheres contained residual DCM of
3.1%. Batch No.
4 had an average particle size of 35 jim (D50), a drug load of 48.9 wt%, and a
molecular weight of
25.5 kDa. The microspheres contained residual DCM of 2.1%. Batch No. 6 had an
average
particle size of 34 jim (D50), a drug load of 60.6 wt%, and a molecular weight
of 31.0 kDa. The
microspheres contained residual DCM of 2.7%. Batch No. 7 had an average
particle size of 30
jim (D50), a drug load of 65.6 wt%, and a molecular weight of 30.1 kDa. The
microspheres
contained residual DCM of 1.5%. Batch No. 11 had an average particle size of
61 jim (D5o), a
16
CA 03210001 2023-07-27
WO 2022/187822 PCT/US2022/070910
drug load of 51 wt%, and a molecular weight of 28.9 kDa. The microspheres
contained residual
DCM of 1.4%. The parameters and results are shown tabularly in Table 2:
t Batch Number 3 4 6 7 11
Symbol o A o 0 X
Polymer
Ashland
Supplier/Name
Co-Monomer
75:25
Ratio
() Polymer IV
0.26
2, (dL/g)
ct
Polymer
Acid Ester Acid
! Endcap
o
4-1 Batch Size (g) 5
Mixing Speed
3000 2000
(RPM)
Target Drug
50.0 50.0 60.0 70.0 50.0
Load (%)
Drug Load
48.2 48.9 60.6 65.6 51.0
(A)
Encapsulation
96.4 97.8 101.0 93.7 102.0
Efficiency (%)
Residual
Tzi Solvent DCM 3.1 2.1 2.7 1.5 1.4
0
.-7-,' (%)
-c-zd Particle Dv10 17 15 12 10 29
Size Dv50 39 35 34 30 61
(111n) Dv90 65 59 58 53 103
Sample MW
29.4 25.5 31.0 30.1 28.9
(kDa)
Polymer MW
30.7 25.8 30.7 30.7 28.6
_(kDa)
Table 2
[0061] Figure 3 is a graph showing in vitro cumulative ibrutinib release
over time from the
Group B ibrutinib-encapsulating polymer microspheres.
17
CA 03210001 2023-07-27
WO 2022/187822 PCT/US2022/070910
Example 4 ¨ Preparation of Ibrutinib-Encapsulated Polymer Microspheres
Comprising a 75:25
PLGA with a high polymer IV ¨ Batch Nos. 5, 12, 13, and 14 ("Group C")
[0062] Following the general procedure described in Example 1 and
illustrated in Figure 1,
the DP was formed by dissolving 2.5 g (Batch Nos. 5 and 12) or 2.0 g (Batch
Nos. 13 and 14) of
either 7505 A polymer (Batch No. 5) (IV = 0.56 dL/g), 7505 E polymer (Batch
No. 12) (IV = 0.41
dL/g), 7507 A polymer (Batch No. 13) (IV = 0.7 dL/g), or 7507 E polymer (Batch
No. 14) (IV =
0.66 dL/g) in 11.67 g of DCM, followed by addition of ibrutinib (sufficient to
provide a DP weight
of 16.67 g, i.e., 2.5 g for Batch Nos. 5 and 12; and 3.0 g for Batch Nos. 13
and 14) with mixing
until completely dissolved. The DP was filtered and pumped at a flow rate of
25 mL/min into a
Levitronix BPS-i100 integrated pump system operating at 3,000 RPM. The CP
comprising
0.35% PVA was also pumped into the homogenizer at a flow rate of 2 L/min
(CP:DP = 80:1).
[0063] The formed or forming microspheres exited the homogenizer and
entered the SRV.
Deionized water was added to the SRV. Solvent removal was achieved using water
washing and
a hollow fiber filter. The bulk suspension was collected via filtration and
lyophilized to obtain a
free-flowing powder.
[0064] Batch No. 5 had an average particle size of 53 jim (D5o), a drug
load of 47.5 wt%, and
a molecular weight of 66.4 kDa. The microspheres contained residual DCM of
4.1%. Batch No.
12 had an average particle size of 47 jim (D5o), a drug load of 51.2 wt%, and
a molecular weight
of 49.8 kDa. The microspheres contained residual DCM of 0.8%. Batch No. 13 had
an average
particle size of 52 jim (D5o), a drug load of 62.2 wt%, and a molecular weight
of 87.7 kDa. The
microspheres contained residual DCM of 1.3%. Batch No. 14 had an average
particle size of 53
jim (D5o), a drug load of 61.0 wt%, and a molecular weight of 90.8 kDa. The
microspheres
contained residual DCM of 1.3%. The parameters and results are shown tabularly
in Table 3:
18
CA 03210001 2023-07-27
WO 2022/187822 PCT/US2022/070910
t Batch Number 5 12 13 14
Symbol o A 0
Polymer
Ashland
Supplier/Name
Co-Monomer
7525
Ratio
() Polymer IV
0.56 0.41 0.70 0.66
ct
Polymer
Acid Ester Acid Ester
Endcap
4-1 Batch Size (g) 5
Mixing Speed
3000
(RPM)
Target Drug
50.0 60.0
Load (%)
Drug Load
47.5 51.2 62.2 61.0
(%)
Encapsulation
95.0 102.4 103.7 101.7
Efficiency (%)
Residual
Tzi Solvent DCM 4.1 0.8 1.3 1.3
c.)
(%)
-c-zd Particle Dv10 15 17 14 15
Size Dv50 53 47 52 53
(un) Dv90 102 84 106 106
Sample MW
66.4 49.8 87.7 90.8
(kDa)
Polymer MW
66.2 50.0 91.0 92.9
(kDa)
Table 3
[0065] Figure 4 is a graph showing in vitro cumulative ibrutinib release
over time from the
Group C ibrutinib-encapsulating polymer microspheres.
Example 5 ¨ Preparation of Ibrutinib-Encapsulated Polymer Microspheres
Comprising an 85:15
PLGA ¨ Batch Nos. 18 and 19 ("Group D")
[0066] Following the general procedure described in Example 1 and
illustrated in Figure 1,
the DP was formed by dissolving 2.5 g of either 8503 A polymer (Batch No. 18)
(IV = 0.24 dL/g)
19
CA 03210001 2023-07-27
WO 2022/187822 PCT/US2022/070910
or 8503 E polymer (Batch No. 19) (IV = 0.25 dL/g) in 11.67 g of DCM, followed
by addition of
ibrutinib (2.5 g) with mixing until completely dissolved. The DP was filtered
and pumped at a
flow rate of 25 mL/min into a Levitronix BPS-i100 integrated pump system
operating at 3,000
RPM. The CP comprising 0.35% PVA was also pumped into the homogenizer at a
flow rate of 2
L/min (CP:DP = 80:1).
[0067] The formed or forming microspheres exited the homogenizer and
entered the SRV.
Deionized water was added to the SRV. Solvent removal was achieved using water
washing and
a hollow fiber filter. The bulk suspension was collected via filtration and
lyophilized to obtain a
free-flowing powder.
[0068] Batch No. 18 had an average particle size of 36 jim (D5o), a drug
load of 49.8 wt%, and
a molecular weight of 22.7 kDa. The microspheres contained residual DCM of
0.5%. Batch No.
19 had an average particle size of 35 jim (D5o), a drug load of 49.2 wt%, and
a molecular weight
of 25.8 kDa. The microspheres contained residual DCM of 0.3%. The parameters
and results are
shown tabularly in Table 4:
t Batch Number 18 19
Symbol 0
Polymer
Ashland
Supplier/Name
Co-Monomer Ratio 85:15
2, Polymer IV (dL/g) 0.24 0.25
ct
= Polymer Endcap Acid Ester
Batch Size (g) 5
4-1 Mixing Speed (RPM) 3000
Target Drug Load
(%)
Drug Load (%) 49.8 49.2
Encapsulation
o 99.6 98.4
Efficiency (%)
-c-d Residual Solvent
= DCM (%) 0.5 0.3
Particle Size Dv10 15 13
CA 03210001 2023-07-27
WO 2022/187822 PCT/US2022/070910
(11m) 130,50 36 35
130,90 62 61
Sample MW (kDa) 22.7 25.8
Polymer MW (kDa) 22.7 26.4
Table 4
[0069] Figure 5 is a graph showing in vitro cumulative ibrutinib release
over time from the
Group D ibrutinib-encapsulating polymer microspheres.
Example 6 ¨ Preparation of Ibrutinib-Encapsulated Polymer Microspheres
Comprising a PLA ¨
Batch Nos. 8, 9, 10, 16, and 17 ("Group E")
[0070] Following the general procedure described in Example 1 and
illustrated in Figure 1,
the DP was formed by dissolving 2.5 g (Batch Nos. 8, 9, 16, and 17) or 2.0 g
(Batch No. 10) of
either DL 02 A polymer (Batch Nos. 8 and 17) (IV = 0.16 dL/g), DL 02 E polymer
(Batch Nos. 9
and 10) (IV = 0.18 dL/g), or DL 03 A polymer (Batch No. 16) (IV = 0.32 dL/g)
in 11.67 g of DCM,
followed by addition of ibrutinib (sufficient to provide a DP weight of 16.67
g, i.e., 2.5 g for Batch
Nos. 8, 9, 16, and 17; and 3.0 g for Batch No. 10) with mixing until
completely dissolved. The
DP was filtered and pumped at a flow rate of 25 mL/min into a Levitronix BPS-
i100 integrated
pump system operating at either 3,000 RPM (Batch Nos. 8, 9, 10, and 16) or
2,000 RPM (Batch
No. 17). The CP comprising 0.35% PVA was also pumped into the homogenizer at a
flow rate of
2 L/min (CP:DP = 80:1).
[0071] The formed or forming microspheres exited the homogenizer and
entered the SRV.
Deionized water was added to the SRV. Solvent removal was achieved using water
washing and
a hollow fiber filter. The bulk suspension was collected via filtration and
lyophilized to obtain a
free-flowing powder.
[0072] Batch No. 8 had an average particle size of 32 i_tm (D5o), a drug
load of 51.7 wt%, and
a molecular weight of 12.0 kDa. The microspheres contained residual DCM of
0.4%. Batch No.
21
CA 03210001 2023-07-27
WO 2022/187822 PCT/US2022/070910
9 had an average particle size of 29 i_tm (D50), a drug load of 51.8 wt%, and
a molecular weight of
11.7 kDa. The microspheres contained residual DCM of 0.1%. Batch No. 10 had an
average
particle size of 29 i_tm (D50), a drug load of 64.2 wt%, and a molecular
weight of 11.7 kDa. The
microspheres contained residual DCM of 0.2%. Batch No. 16 had an average
particle size of 40
i_tm (D50), a drug load of 48.9 wt%, and a molecular weight of 30.1 kDa. The
microspheres
contained residual DCM of 0.6%. Batch No. 17 had an average particle size of
49 i_tm (D5o), a
drug load of 50.2 wt%, and a molecular weight of 12.4 kDa. The microspheres
contained residual
DCM of 0.8%. The parameters and results are shown tabularly in Table 5:
t Batch Number 8 9 10 16 17
= Symbol o A o 0 X
Polymer
Ashland
Supplier/Name
Co-Monomer
1000
Ratio
() Polymer IV
0.16 0.18 0.32 0.16
=,72, (dL/g)
ct
Polymer
Acid Ester Acid
! Endcap
o
4-1 Batch Size (g) 5
Mixing Speed
3000 2000
(RPM)
Target Drug
50.0 60.0 50.0
Load (%)
Drug Load
51.7 51.8 64.2 48.9 50.2
(%)
Encapsulation
103.4 103.6 107.0 97.8 100.4
Efficiency (%)
Tzi Residual
c.)
= Solvent DCM 0.4 0.1 0.2 0.6
0.8
'' Particle Dv10 15 12 14 18 25
Size Dv50 32 29 29 40 49
(111n) Dv90 53 50 50 66 81
Sample MW
12.0 11.7 11.7 30.1 12.4
_(kDa)
22
CA 03210001 2023-07-27
WO 2022/187822 PCT/US2022/070910
Polymer MW
11.9 11.8 11.8 29.5 11.7
(kD a)
Table 5
[0073] Figure 6 is a graph showing in vitro cumulative ibrutinib release
over time from the
Group E ibrutinib-encapsulating polymer microspheres.
[0074] In use, the microspheres may be suspended in a diluent for
administration (injection).
The diluent may generally contain a thickening agent, a tonicity agent, and a
wetting agent. The
thickening agent may include carboxymethyl cellulose-sodium (CMC-Na) or other
suitable
compounds. An appropriate viscosity grade and suitable concentration of CMC-Na
may be
selected so that the viscosity of the diluent is 3 cps or higher. Generally, a
viscosity of about 10
cps is suitable; however, a higher viscosity diluent may be preferred for
larger microspheres to
minimize the settling of microspheres in the suspension.
[0075] Uniform microsphere suspension without particle settling will result
in a consistent
delivered dose during drug administration by injection. To have a tonicity of
the diluent closer to
the biological system, about 290 milliosmole (mOsm), solutes such as mannitol,
sodium chloride,
or any other acceptable salt may be used. The diluent may also contain a
buffer salt to maintain
the pH of the composition. Typically, the pH is maintained around a
physiologically relevant pH
by adjusting the buffer content as needed (pH about 7 to about 8).
[0076] The aspects disclosed herein are not intended to be exhaustive or to
be limiting. A
skilled artisan would acknowledge that other aspects or modifications to
instant aspects can be
made without departing from the spirit or scope of the invention. The aspects
of the present
disclosure, as generally described herein and illustrated in the figures, can
be arranged, substituted,
combined, separated, and designed in a wide variety of different
configurations, all of which are
contemplated herein.
23
CA 03210001 2023-07-27
WO 2022/187822 PCT/US2022/070910
[0077] Unless otherwise specified, "a," "an," "the," "one or more of," and
"at least one" are
used interchangeably. The singular forms "a", "an," and "the" are inclusive of
their plural forms.
The recitations of numerical ranges by endpoints include all numbers subsumed
within that range
(e.g., 1 to 5 includes 1, 1.5, 2, 2.75, 3, 3.80, 4, 5, etc.). The terms
"comprising" and "including"
are intended to be equivalent and open-ended. The phrase "consisting
essentially of' means that
the composition or method may include additional ingredients and/or steps, but
only if the
additional ingredients and/or steps do not materially alter the basic and
novel characteristics of the
claimed composition or method. The phrase "selected from the group consisting
of' is meant to
include mixtures of the listed group.
[0078] When reference is made to the term "each," it is not meant to mean
"each and every,
without exception." For example, if reference is made to microsphere
formulation comprising
polymer microspheres, and "each polymer microsphere" is said to have a
particular BTK inhibitor
content, if there are 10 polymer microspheres, and two or more of the polymer
microspheres have
the particular BTK inhibitor content, then that subset of two or more polymer
microspheres is
intended to meet the limitation.
[0079] The term "about" in conjunction with a number is simply shorthand
and is intended to
include 10% of the number. This is true whether "about" is modifying a stand-
alone number or
modifying a number at either or both ends of a range of numbers. In other
words, "about 10"
means from 9 to 11. Likewise, "about 10 to about 20" contemplates 9 to 22 and
11 to 18. In the
absence of the term "about," the exact number is intended. In other words,
"10" means 10.
24