Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 2022/194801 PCT/EP2022/056586
New Thiazolopyrimidinone Derivatives
The present invention relates to new organic compounds useful for therapy
and/or
prophylaxis in a mammal, and in particular to compounds that reduce the
protein level of
huntingtin (HTT) and which are useful in the treatment of Huntington's
disease.
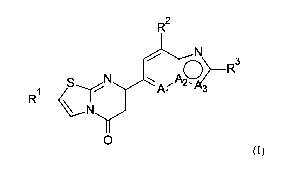
In particular, the present invention relates to a compound of formula (I)
R2
R3
2QA>
R1 I ArA 3
Nr
0
(I)
wherein
RI is hydrogen, alkyl, cycloalkyl, aryl, heteroaryl or heterocycloalkyl,
wherein each
instance of cycloalkyl, aryl, heteroaryl and heterocycloalkyl is optionally
substituted with one, two, three or four substituents independently selected
from R4;
R2 is hydrogen, cycloalkyl, alkenyl, cyano, amino, hydroxy, halogen, alkyl,
haloalkyl, haloalkoxy or alkoxy;
R3 is alkyl, hydrogen, halogen or haloalkyl;
R4 is halogen, alkyl, heterocycloalkyl, heterocycloalkylalkyl,
alkylheterocycloalkyl,
haloheterocycloalkyl, cycloalkyl, cycloalkylalkyl, alkylcycloalkyl,
halocycloalkyl, cycloalkylamino, aryl, arylalkyl, alkylaryl, haloaryl, cyano,
hydroxy, oxo, haloalkyl, alkylcarbonyl, alkoxy, haloalkoxy, alkoxyalkyl,
alkoxycarbonyl, amino, alkylamino, dialkylamino, aminoalkyl,
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 2 -
alkylaminoalkyl, dialkylaminoalkyl, aminoalkylamino, alkoxyalkylamino,
alkylcarbonylamino, alkoxycarbonylamino, hydroxyalkyl, hydroxyalkoxyalkyl
or hydroxyalkylamino; and
(i) Al is -N-, A2 is -N- and A3 is -CH-;
(ii) Ai is -N-, A2 is -C- and A3 is -0-; or
(iii) Ai is -CH-, A2 is -C- and A3 is -0-;
or a pharmaceutically acceptable salt thereof;
provided that
2-(4,7-diazaspiro[2.5]octan-7-y1)-7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-
yl)thiazolo[3,2-a]pyrimidin-5-one;
is excluded.
Huntington's Disease (HD) is an inherited autosomal dominant neurodegenerative
disease caused due to a CAG bases repeat expansion in the huntingtin (HTT)
gene. Several
lines of evidence indicate that the mutant HTT gene together with its gene
product mHTT
protein contributes to HD pathogenesis via a toxic gain of function mechanism.
The triplet repeat expansion in the exon 1 of the HTT gene translates into a
polyglutamine repeat in the HTT protein which is prone to misfolding and
aggregating in
the cells. While the exact mechanisms of how mutant HTT disrupts cellular
function is
unclear, several processes ranging from interruption of RNA translation, toxic
RNA
species, protein aggregates, RNA translation, and stress granules have been
implicated.
At a neural circuit level, HD has been shown to affects deep brains structures
like the
striatum as well as cortical regions to different extents. Seminal mouse
genetic
experiments coupled with human imaging experiments point to a key role of
cortico-
striatal connections in the pathogenicity of HD (Wang et al., "Neuronal
targets of mutant
huntingtin genetic reduction to ameliorate Huntington's disease pathogenesis
in mice"
Nature medicine 20.5 (2014): 536; Tabrizi et al.; "Potential endpoints for
clinical trials in
premanifest and early Huntington's disease in the TRACK-HD study: analysis of
24 month
observational data." The Lancet Neurology 11.1 (2012): 42-53).
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 3 -
HD typically manifests around 30-50 years of age characterized by a multitude
of
symptoms spanning the motor, cognitive and affective domains eventually
leading to death
in 10-20 years after the onset of motor symptoms. CAG repeat length negatively
correlates
with age of onset of motor symptoms, however this only accounts for 50-70% of
the
variance in age of onset. In an effort to identify genetic modifiers of age of
onset in HD,
Lee et al. (2019, Huntington's disease onset is determined by length of
uninterrupted CAG,
not encoded polyglutamine, and is modified by DNA maintenance mechanisms.
Bioarxiv
doi: https://doi.org/10.1101/529768) conducted a large GWAS (genome-wide
association
study) that has uncovered additional genetic modifiers of age of onset.
Various mouse models have been characterized to model aspects of HD. The
YAC128 mice expressing the full length mutant HTT transgene with 128 CAG
repeats,
BACHD mice expressing the full length mutant HTT genomic sequence with 97
CAG/CAA repeats, the R6/2 mice expressing exon 1 of the mutant human HTT gene
with
110-135 CAG repeats). In addition to these mice that express the human
transgene, there
are also a series of mouse models, like the frequently used Q111, the Q175
knock in mice
where the expanded repeats are knocked-in in the context of the mouse HTT
locus.
There are currently no disease modifying therapies for Huntington's Disease
while
several are in development. The core disease process behind the symptomatology
characterized by motor, cognitive and behavioral symptoms remains unmet by the
various
symptomatic treatments currently approved. Tetrabenazine and tiapride are
currently
approved for the treatment of motor symptoms namely HD-associated chorea. In
addition,
anticonvulsants, benzodiazepines, antidepressants, and antipsychotics are also
used off-
label to treat the motor, cognitive and psychiatric symptoms associated with
HD.
Several therapeutic strategies targeting DNA and RNA are being investigated
for
HTT lowering (E. J. Wild, S. Tabrizi, Lancet Neurol. 2017 16(10): 837-847).
HTT
lowering is a promising therapeutic approach that aims to slow disease
progression by
getting at the core cause of Huntington's Disease. HTT lowering is thought to
be
transformative when treated in the pre-manifest or manifest stages of disease
onset, thus
preventing major neurodegenerative processes in the brain. However, the
challenge lies in
identifying the patients at the right disease stage, as age of onset is quite
variable across the
population (S. J. Tabrizi, R. Ghosh, B. R. Leavitt, Neuron, 2019, 102(4),
899).
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 4 -
The current clinical approaches are mainly based on anti sense
oligonucleotides
(AS0s). In addition, a few allele specific lowering strategies such as SNP
(single-
nucleotide polymorphism) based ASO and zinc finger based gene editing
approaches are
investigated. While the use of small molecules to lower HTT expression has
been
postulated, this strategy has not yet been validated and none has proved
successful so far.
Small molecules provide an opportunity to allow for HTT lowering in the brain
as
well as the periphery. In addition, a small molecule modality allows access to
patient
populations that could be difficult to reach with modalities like ASOs or gene
therapy.
There is thus the need for new compounds capable of lowering mHTT and with
favourable brain penetration properties.
The applicant has surprisingly found that the compounds of the invention are
active
in lowering mHTT and are therefore useful in the treatment of HD.
All publications, patent applications, patents, and other references mentioned
herein
are incorporated by reference in their entirety.
In the present description the term "alkyl", alone or in combination,
signifies a
straight-chain or branched-chain alkyl group with Ito 8 carbon atoms,
particularly a
straight or branched-chain alkyl group with 1 to 6 carbon atoms and more
particularly a
straight or branched-chain alkyl group with 1 to 4 carbon atoms. Examples of
straight-
chain and branched-chain Cl-C8 alkyl groups are methyl, ethyl, propyl,
isopropyl, butyl,
isobutyl, tert.-butyl, the isomeric pentyls, the isomeric hexyls, the isomeric
heptyls and the
isomeric octyls. Particular examples of "alkyl" are methyl, ethyl and
isopropyl. Methyl and
ethyl are particular examples of "alkyl" in the compound of formula (1).
In the present description the term "alkenyl", alone or in combination,
signifies a
straight-chain or branched-chain alkenyl group with 2 to 8 carbon atoms and
further
comprising at least one double bond, particularly a straight or branched-chain
alkenyl
group with 2 to 4 carbon atoms and further comprising at least one double
bond. Particular
examples of "alkenyl" are ethenyl, propenyl, isopropenyl, butenyl, isobutenyl
and
tertbutenyl. Isopropenyl is a particular example of "alkenyl" in the compound
of formula
(D.
Date regue/date received 2023-08-25
WO 2022/194801
PCT/EP2022/056586
- 5 -
The term "cycloalkyl", alone or in combination, signifies a cycloalkyl ring
with 3 to
carbon atoms and particularly a cycloalkyl ring with 3 to 6 carbon atoms.
Examples of
"cycloalkyl" are cyclopropyl, cyclobutyl, cyclopentyl and cyclohexyl,
cycloheptyl,
cyclooctyl, cyclononyl and cyclodecyl. A particular example of "cycloalkyl" is
5 cyclopropyl.
The term "aryl", alone or in combination, signifies an aromatic mono- or
bicyclic
ring system comprising 6 to 10 carbon ring atoms. Examples of "aryl" include,
but are not
limited to, phenyl and naphthyl.
The term "heteroaryl", alone or in combination, signifies an aromatic mono- or
10 bicyclic ring system with 5 to 12 ring atoms, comprising 1, 2, 3 or
4 heteroatoms each
independently selected from N, 0 and S, the remaining ring atoms being carbon.
Examples
of heteroaryl include, but are not limited to, furanyl, thiophenyl, 1H-
pyrazolyl, 1H-
imidazolyl, 1H-1,2,3-triazolyl, 4H-1,2,4-triazolyl, 1,2,4-oxadiazolyl, 1,3,4-
oxadiazolyl,
pyridinyl, pyridazinyl, pyrimidinyl, pyrazinyl, 1H-indolyl, 2H-indolyl, 1H-
indazolyl, 2H-
indazolyl, indolizinyl, benzofuranyl, 1H-benzimidazolyl, 1,3-benzoxazolyl,
furo[2,3-
b]pyridinyl, furo[2,3-c]pyridinyl, furo[3,2-b]pyridinyl, furo[3,2-c]pyridinyl,
1H-
pyrrolo[2,3-b]pyridinyl, 1H-pyrrolo[2,3-c]pyridinyl, pyrrolo[1,2-
a]pyrimidinyl,
pyrrolo[1,2-a]pyrazinyl, pyn-olo[1,2-b]pyridazinyl, pyrazolo[1,5-a]pyridinyl,
1H-
pyrazolo[4,3-b]pyridinyl, 2H-pyrazolo[4,3-b]pyridinyl, 2H-pyrazolo[4,3-
c]pyridinyl,
pyrazolo[1,5-a]pyrazinyl, pyrazolo[1,5-a]pyrimidinyl, imidazo[1,2-a]pyridinyl,
imidazo[1,2-a]pyrimidinyl, imidazo[1,2-a]pyrazinyl, imidazo[1,2-b]pyridazinyl,
imidazo[1,2-c]pyrimidinyl, imidazo[1,5-a]pyridinyl, imidazo[2,1-
b][1,3]thiazolyl,
imidazo[2,1-b][1,3,4]thiadiazolyl, [1,3]oxazolo[4,5-b]pyridinyl,
[1,2,4]triazolo[1,5-
a]pyridnyl, [1,2,4]triazolo[1,5-b]pyridazinyl, benzo[d]oxazoly1 and
quinolinyl.
The term "alkoxy" or "alkyloxy", alone or in combination, signifies a group of
the
formula alkyl-0- in which the term "alkyl" has the previously given
significance, such as
methoxy, ethoxy, n-propoxy, isopropoxy, n-butoxy, isobutoxy, sec-butoxy and
tert.-
butoxy. A particular example of "alkoxy" is methoxy.
The term "oxy", alone or in combination, signifies the -0- group.
The terms "halogen" or "halo", alone or in combination, signifies fluorine,
chlorine,
bromine or iodine and particularly fluorine, chlorine or bromine. One
preferred example of
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 6 -
halogen is fluorine. The term "halo", in combination with another group, if
not otherwise
specified, denotes the substitution of said group with at least one halogen,
particularly
substituted with one to five halogens, particularly one to four halogens, i.e.
one, two, three
or four halogens.
The term "haloalkyl", alone or in combination, denotes an alkyl group
substituted
with at least one halogen, particularly substituted with one to five halogens,
particularly
one to three halogens. Particular "haloalkyl" are fluoromethyl,
trifluoromethyl,
difluoromethyl, fluoroethyl, fluoropropyl and fluorobutyl. Further particular
"haloalkyl"
are difluoromethyl and trifluoromethyl.
The term "haloalkoxy", alone or in combination, denotes an alkoxy group
substituted
with at least one halogen, particularly substituted with one to five halogens,
particularly
one to three halogens. Particular "haloalkoxy" are fluoromethoxy,
fluoroethoxy,
difluoromethoxy, difluoroethoxy, trifluoromethoxy and trifluoroethoxy. A
further
particular "haloalkoxy" is difluoromethoxy.
The terms "hydroxyl" and "hydroxy", alone or in combination, signify the -OH
group.
The term "carbonyl", alone or in combination, signifies the -C(0)- group.
The term "amino", alone or in combination, signifies the primary amino group (-
NI-12), the secondary amino group (-NH-), or the tertiary amino group (-N-).
The term "cyano", alone or in combination, signifies the -CN group.
The term "heterocycloalkyl", alone or in combination, signifies a monocyclic
or
bicyclic saturated or monounsaturated ring system with 3 to 12 ring atoms,
comprising 1,
2, 3 or 4 heteroatoms each independently selected from N, 0 and S, and the
remaining ring
atoms being carbon. In some particular embodiments the term
"heterocycloalkyl", alone or
in combination, can signify a monocyclic or bicyclic saturated or
monounsaturated ring
system with 5 to 10 ring atoms, comprising 1 or 2 nitrogen atoms and the
remaining ring
atoms being carbon. Examples of "heterocycloalkyl" are piperazinyl,
azetidinyl,
morpholinyl, tetrahydropyranyl, tetrahydrofuranyl, 1,2,3,6-tetrahydropyridin-4-
yl, 4,7-
diazaspiro[2.5]octan-7-yl, (8aS)-3,4,6,7,8,8a-hexahydro-1H-pyrrolo[1,2-
a]pyrazin-2-yl,
(8a.R)-3,4,6,7,8,8a-hexahydro-1H-pyrrolo[1,2-a]pyrazin-2-yl, 3,8-
diazabicyclo[3.2.1]octan-
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 7 -
8-yl, (1S,4S)-2,5-diazabicyclo[2.2.1]heptan-2-yl, 2,3,3a,4,6,6a-hexahydro-1H-
pyrrolo[3,4-
c]pyrrol-5-yl, pyrrolidinyl, 2,8-diazaspiro[4.5]decan-2-yl, piperidyl, (7R)-4-
azaspiro[2.5]octan-7-yl, (7S)-4-azaspiro[2.5]octan-7-yl, 4-azaspiro[2.5]octan-
7-yl, [rac-
(1R,5S)-9-oxa-3-azabicyclo[3.3,1]nonan-7-y1], [rac-(1 S,5R)-9-oxa-3-
azabicyclo[3.3.1]nonan-7-yl, and [rac-(1S,5R)-3-azabicyclo[3.1.0]hexan-6-y1].
Particular
examples of "heterocycloalkyl" are piperazin-l-yl, 4-piperidyl, pyrrolidin-l-
yl, 1,2,3,6-
tetrahydropyridin-4-yl, (7S)-4-azaspiro[2.5]octan-7-y1 and (8aR)-3,4,6,7,8,8a-
hexahydro-
1H-pyrrolo[1,2-a]pyrazin-2-yl.
The term "pharmaceutically acceptable salt" refers to those salts which retain
the
biological effectiveness and properties of the free bases or free acids, which
are not
biologically or otherwise undesirable. The salts are formed with inorganic
acids such as
hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid, phosphoric
acid, particularly
hydrochloric acid, and organic acids such as formic acid, acetic acid,
propionic acid,
glycolic acid, pyruvic acid, oxalic acid, maleic acid, malonic acid, succinic
acid, fumaric
acid, tartaric acid, citric acid, benzoic acid, cinnamic acid, mandelic acid,
methanesulfonic
acid, ethanesulfonic acid, p-toluenesulfonic acid, salicylic acid, N-
acetylcystein and
trifluoroacetic acid. In addition these salts may be prepared form addition of
an inorganic
base or an organic base to the free acid. Salts derived from an inorganic base
include, but
are not limited to, the sodium, potassium, lithium, ammonium, calcium,
magnesium salts.
Salts derived from organic bases include, but are not limited to salts of
primary, secondary,
and tertiary amines, substituted amines including naturally occurring
substituted amines,
cyclic amines and basic ion exchange resins, such as isopropylamine,
trimethylamine,
diethylamine, triethylamine, tripropylamine, ethanolamine, lysine, arginine, N-
ethylpiperidine, piperidine, polyamine resins. The compound of formula (I) can
also be
present in the form of zwitterions. Particularly preferred pharmaceutically
acceptable salts
of compounds of formula (1) are the salts formed with trifluoroacetic acid or
hydrochloric
acid.
If one of the starting materials or compounds of formula (1) contain one or
more
functional groups which are not stable or are reactive under the reaction
conditions of one
or more reaction steps, appropriate protecting groups (as described e.g. in
"Protective
Groups in Organic Chemistry" by T. W. Greene and P. G. M. Wuts, 3rd Ed., 1999,
Wiley,
New York) can be introduced before the critical step applying methods well
known in the
art. Such protecting groups can be removed at a later stage of the synthesis
using standard
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 8 -
methods described in the literature. Examples of protecting groups are tert-
butoxycarbonyl
(Boc), trityl (Trt), 2,4.dimethoxybenzyl (Dmb), 9-fluorenylmethyl carbamate
(Fmoc), 2-
trimethylsilylethyl carbamate (Teoc), carbobenzyloxy (Cbz) and p-
methoxybenzyloxycarbonyl (Moz). A particular example of a protecting group is
tert-
butoxycarbonyl (Boc).
A certain embodiment of the invention relates to the compound of formula (I)
asdescribed herein, or a pharmaceutically acceptable salt thereof, wherein at
least one
substituent comprises at least one radioisotope. Particular examples of
radioisotopes are
2H, 3H, 13C, 14C and 18F.
Furthermore, the invention includes all optical isomers, i.e.
diastereoisomers,
diastereomeric mixtures, racemic mixtures, all their corresponding enantiomers
and/or
tautomers as well as their solvates, wherever applicable, of the compound of
formula (I).
The compound of formula (I) may contain one or more asymmetric centers and can
therefore occur as racemates, racemic mixtures, single enantiomers,
diastereomeric
mixtures and individual diastereomers. Additional asymmetric centers may be
present
depending upon the nature of the various substituents on the molecule. Each
such
asymmetric center will independently produce two optical isomers and it is
intended that
all of the possible optical isomers and diastereomers in mixtures and as pure
or partially
purified compounds are included within this invention. The present invention
is meant to
encompass all such isomeric forms of these compounds. The independent
syntheses of
these diastereomers or their chromatographic separations may be achieved as
known in the
art by appropriate modification of the methodology disclosed herein. Their
absolute
stereochemistry may be determined by the x-ray crystallography of crystalline
products or
crystalline intermediates which are derivatized, if necessary, with a reagent
containing an
asymmetric center of known absolute configuration. If desired, racemic
mixtures of the
compounds may be separated so that the individual enantiomers are isolated.
The
separation can be carried out by methods well known in the art, such as the
coupling of a
racemic mixture of compounds to an enantiomerically pure compound to form a
diastereomeric mixture, followed by separation of the individual diastereomers
by standard
methods, such as fractional crystallization or chromatography.
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 9 -
The term "asymmetric carbon atom" means a carbon atom with four different
substituents. According to the Cahn-Ingold-Prelog Convention an asymmetric
carbon atom
can be of the "R" or "S" configuration.
The invention thus also relates in particular to:
A compound according to the invention wherein It' is heterocylcoalkyl
optionally
substituted with one, two, three or four substituents independently selected
from R4;
A compound according to the invention wherein RI is heterocylcoalkyl
optionally
substituted with one or two substituents independently selected from R4;
A compound according to the invention wherein IV is piperazin-l-yl, 1,2,3,6-
tetrahydropyridin-4-yl, 4,7-diazaspiro[2.5]octan-7-yl, (8aS)-3,4,6,7,8,8a-
hexahydro-1H-
pyrrolo[1,2-a]pyrazin-2-yl, 3,8-diazabicyclo[3.2.1]octan-8-yl, (8aR)-
3,4,6,7,8,8a-
hexahydro-1H-pyrrolo[1,2-a]pyrazin-2-yl, (1S,4S)-2,5-diazabicyclo[2.2.1]heptan-
2-y1õ
2,3,3a,4,6,6a-hexahydro-1H-pyrrolo[3,4-c]pyrrol-5-yl, pyrrolidin-l-yl, 2,8-
diazaspiro[4.5]decan-2-yl, (7R)-4-azaspiro[2.5]octan-7-yl, (7S)-4-
azaspiro[2.5]octan-7-yl,
4-piperidyl, [rac-( 1R, 5 S)-9-oxa-3 -azabicyclo[3 .3. 1 ]nonan-7-y1], [rac-(1
S,5R)-9-oxa-3-
azabicyclo[3.3.1]nonan-7-y1 or [rac-(1S,5R)-3-azabicyclo[3.1.0]hexan-6-y1],
and wherein
IV is optionally substituted with one or two substituents independently
selected from R4;
A compound according to the invention wherein RI is piperazin-l-yl, 1,2,3,6-
tetrahydropyridin-4-yl, 4,7-diazaspiro[2.5]octan-7-yl, (8aS)-3,4,6,7,8,8a-
hexahydro-1H-
pyrrolo[1,2-a]pyrazin-2-yl, 3,8-diazabicyclo[3.2.1]octan-8-yl, (8aR)-
3,4,6,7,8,8a-
hexahydro-1H-pyrrolo[1,2-a]pyrazin-2-yl, (1S,4S)-2,5-diazabicyclo[2.2.1]heptan-
2-y1õ
2,3,3a,4,6,6a-hexahydro-1H-pyrrolo[3,4-c]pyrrol-5-yl, pyrrolidin-l-yl, 2,8-
diazaspiro[4.5]decan-2-yl, (7R)-4-azaspiro[2.5]octan-7-yl, (7S)-4-
azaspiro[2.5]octan-7-y1
or 4-piperidyl, and wherein RI is optionally substituted with one or two
substituents
independently selected from R4;
A compound according to the invention wherein RI is piperazin-l-yl, 4-
piperidyl,
pyrrolidin-l-yl, 1,2,3,6-tetrahydropyridin-4-yl, (7S)-4-azaspiro[2.5]octan-7-
yl, (7R)-4-
azaspiro[2.5]octan-7-y1 or (8aR)-3,4,6,7,8,8a-hexahydro-1H-pyrrolo[1,2-
a]pyrazin-2-yl,
and wherein RI. is optionally substituted with one or two substituents
independently
selected from R4;
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 1 0 -
A compound according to the invention wherein RI is piperazin-l-yl optionally
substituted with one or two substituents independently selected from R4;
A compound according to the invention wherein RI is 4-piperidyl optionally
substituted with one or two substituents independently selected from R4;
A compound according to the invention wherein RI is (7S)-4-azaspiro[2.5]octan-
7-y1
optionally substituted with one or two substituents independently selected
from R4;
A compound according to the invention wherein RI is (7R)-4-azaspiro[2.5]octan-
7-y1
optionally substituted with one or two substituents independently selected
from R4;
A compound according to the invention wherein RI- is [rac-(1R,5S)-9-oxa-3-
azabicyclo[3.3.1]nonan-7-y1], [rac-(1S,5R)-9-oxa-3-azabicyclo[3.3.1]nonan-7-y1
or [rac-
(1S,5R)-3-azabicyclo[3.1.0]hexan-6-y1], and wherein 10- is optionally
substituted with one
or two substituents independently selected from R4;
A compound according to the invention wherein R1 is cycloalkyl, aryl,
heteroaryl or
heterocycloalkyl, wherein each instance of cycloalkyl, aryl, heteroaryl and
heterocycloalkyl
is optionally substituted with one, two, three or four substituents
independently selected
from R4;
A compound according to the invention wherein RI is hydrogen or alkyl;
A compound according to the invention wherein R2 is hydrogen, cycloalkyl,
alkenyl,
halogen, alkyl, haloalkyl, haloalkoxy or alkoxy;
A compound according to the invention wherein R2 is hydrogen, cyclopropyl,
isopropenyl, fluor , methyl, ethyl, isopropyl, trifluoromethyl,
difluoromethoxy or methoxy;
A compound according to the invention wherein R2 is alkyl, haloalkyl or
alkoxy;
A compound according to the invention wherein R2 is methyl, ethyl,
trifluoromethyl
or methoxy;
A compound according to the invention wherein R3 is alkyl or halogen;
A compound according to the invention wherein R3 is methyl or chloro;
A compound according to the invention wherein R4 is halogen, alkyl or
heterocycloalkyl;
A compound according to the invention wherein R4 is alkyl;
A compound according to the invention wherein R4 is methyl;
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 1 1 -
A compound according to the invention wherein R4 is halogen;
A compound according to the invention wherein R4 is fluor , methyl or
pyrrolidinyl;
A compound according to the invention wherein R4 is fluoro;
A compound according to the invention wherein Ai is -N-, A2 is -N- and A3 is -
CH-;
A compound according to the invention wherein Ai is -N-, A2 is -C- and A3 is -
0-;
and
A compound according to the invention wherein Ai is -CH-, A2 is -C- and A3 is -
0-.
The invention further also relates in particular to:
A compound of formula (Ia) according to the invention
R2
pe3
N N
R1
µ,N
0
(Ia)
wherein the compound of formula (Ia) is a compound of formula (I) wherein A1
is -
N-, A2 is -N- and A3 is -CH-; and
A compound of formula (lb) according to the invention
R2
R3
R
0
(Ib)
wherein the compound of formula (lb) is a compound of formula (I) wherein A2
is -
C- and A3 is -0-.
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 12 -
The invention further relates to a compound selected from
7-(2,8-dimethylimidazo[1,2-1Apyridazin-6-y1)-2-piperazin-1-yl-thiazolo[3,2-
a]pyrimidin-5-
one;
7-(2,8-dimethylimidazo[1,2-Npyridazin-6-y1)-2-(1,2,3,6-tetrahydropyridin-4-
yOthiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-13]pyridazin-6-y1)-2-(3,3-dimethylpiperazin-1-
yl)thiazolo[3,2-
a]pyrimidin-5-one;
7-(4-fluoro-2-methy1-1,3-benzoxazol-6-y1)-2-piperazin-1-yl-thiazolo[3,2-
a]pyrimidin-5-
one;
7-(2,8-dimethylimidazo[1,2-1Apyridazin-6-y1)-2-[(3S)-3-methylpiperazin-1-
yl]thiazolo[3,2-
a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-1Apyridazin-6-y1)-2-[(3R)-3-methylpiperazin-l-
yl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-13]pyridazin-6-y1)-2-(3,5-dimethylpiperazin-1-
yl)thiazolo[3,2-
a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-13]pyridazin-6-y1)-2-[(8aS)-3,4,6,7,8,8a-hexahydro-
1H-
pyrrolo[1,2-a]pyrazin-2-yl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2-methyli mi dazo[1,2 -Npyridazin-6-y1)-2-piperazin-l-yl-thi azol o[3,2-
a]pyrimidin-5-
one;
742-methy1-8-(trifluoromethyl)imidazo[1,2-13]pyridazin-6-y11-2-piperazin-l-yl-
thiazolo[3,2-a]pyrimidin-5-one;
7-(8-ethy1-2-methyl-imidazo[1,2-1Apyridazin-6-y1)-2-piperazin-1-yl-
thiazolo[3,2-
a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-1Apyridazin-6-y1)-2-piperazin-1-yl-
thiazolo[3,2-
a]pyrimidin-5-one;
2-(3,8-diazabicyclo[3.2.1]octan-8-y1)-7-(2,8-dimethylimidazo[1,2-1Apyridazin-6-
yl)thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(8aR)-3,4,6,7,8,8a-hexahydro-
1H-
pyrrolo[1,2-a]pyrazin-2-ylithiazolo[3,2-a]pyrimidin-5-one;
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 13 -7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(1S,4S)-2,5-
diazabicyclo[2.2.1]heptan-2-
yl]thiazolo[3,2-a]pyrimidin-5-one;
2-(2,3,3a,4,6,6a-hexahydro-1H-pyrrolo[3,4-c]pyrrol-5-y1)-7-(2,8-
dimethylimidazo[1,2-
b]pyridazin-6-yl)thiazolo[3,2-a]pyrimidin-5-one;
7-(8-cyclopropy1-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-piperazin-1-yl-
thiazolo[3,2-
a]pyrimidin-5-one;
7-(8-isopropeny1-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-piperazin-1-yl-
thiazolo[3,2-
a]pyrimidin-5-one;
7-(2,7-dim ethyl oxazol o[5,4-b]pyridin-5-y1)-2-piperazin-1-yl-thiazol o[3,2-
a]pyrimi din-5-
one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3S)-3-pyrrolidin-1-
ylpyrrolidin-1-
yl]thiazolo[3,2-a]pyrimidin-5-one;
2-(2,8-diazaspiro[4.5]decan-2-y1)-7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-
yl)thiazolo[3,2-a]pyrimi din-5-one;
7-(8-isopropy1-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-piperazin-1-yl-
thiazolo[3,2-
a]pyrimidin-5-one;
7-(2,8-dim ethylimidazo[1,2-b]pyridazin-6-y1)-2-(4-piperi dyl)thiazol o[3,2-
a]pyrimi din-5-
one;
742-methy1-8-(trifluoromethypimidazo[1,2-b]pyridazin-6-y1]-2-[(8aR)-
3,4,6,7,8,8a-
hexahydro-1H-pyrrolo[1,2-a]pyrazin-2-yl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-(4-fluoro-4-
piperidyl)thiazolo[3,2-
a]pyrimidin-5-one;
748-(difluoromethoxy)-2-methyl-imidazo[1,2-b]pyridazin-6-y1]-2-piperazin-l-yl-
thiazolo[3,2-a]pyrimidin-5-one;
742-methy1-7-(trifluoromethyl)oxazolo[5,4-b]pyridin-5-y1]-2-piperazin-1-yl-
thiazolo[3,2-
a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-(4-
piperidyl)thiazolo[3,2-
a]pyrimidin-5-one;
7-(2,7-dimethyloxazolo[5,4-b]pyridin-5-y1)-2-(4-piperidyl)thiazolo[3,2-
a]pyrimidin-5-one;
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 1 4 -
7-(2-chloro-8-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-(4-piperidyl)thiazolo[3,2-
a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3R,4R)-3-fluoro-4-
piperidyl]thiazo1o[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3S,4S)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(3R,4R)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
-fluoro-4-
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(7S)-4-azaspiro[2.5]octan-7-
yl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(7R)-4-azaspiro[2.5]octan-7-
yllthiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3S,4R)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3R,4S)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
octan-7-
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(7R)-4-
azaspiro[2.5]octan-7-
yl]thiazolo[3,2-a]pyrimidin-5-one;
2-(4-fluoro-4-piperidy1)-7-(8-methoxy-2-methyl-imidazo[1,2-13]pyridazin-6-
yOthiazolo[3,2-a]pyrimidin-5-one;
7-(2-chloro-8-methyl-imidazo[1,2-1Apytidazin-6-y1)-2-[(3R,4R)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,7-dimethyloxazolo[5,4-b]pyridin-5-y1)-2-[(3R,4R)-3-fluoro-4-
piperidyl]thiazolo[3,2-
a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(3S,4R)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
Date recue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 1 5 -7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(3R,4S)-3-fluoro-
4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[rac-(1R,5S)-9-oxa-3-
azabicyclo[3.3.1]nonan-7-yl]thiazolo[3,2-a]pyrimidin-5-one
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[rac-(1S,5R)-9-oxa-3-
azabicyclo[3.3.1]nonan-7-yl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[rac-(1R,5S)-9-oxa-3-
azabicyclo[3.3.1]nonan-7-yl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[rac-(1S,5R)-9-oxa-3-
azabicyclo[3.3.1]nonan-7-yl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[rac-(3R,4S)-3,4-
difluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[rac-(3S,4S)-3,4-
difluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[rac-(3R,4S)-3,4-difluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[rac-(3S,4S)-3,4-difluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[rac-(1S,5R)-3-
azabicyclo[3.1.0]hexan-6-
yl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(3R,4S)-3,4-difluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(3S,4S)-3,4-difluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(3R,4R)-3,4-difluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one; and
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(3S,4R)-3,4-difluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
or a pharmaceutically acceptable salt thereof.
The invention further relates to a compound selected from
Date recue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 16 -7-(2,8-dim ethylimidazo[1,2-b]pyridazin-6-y1)-2-piperazin-1-yl-
thiazolo[3,2-a]pyrimi din-5-
one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-(1,2,3,6-tetrahydropyridin-4-
yl)thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-(3,3-dimethylpiperazin-1-
yl)thiazolo[3,2-
a]pyrimidin-5-one;
7-(4-fluoro-2-methy1-1,3 -benzoxazol-6-y1)-2-piperazin-l-yl-thiazolo[3,2-
a]pyrimidin-5-
one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3 S)-3-methyl piperazin-1-
yl]thiazolo[3,2-
a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3R)-3-methylpiperazin-1-
yl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-(3,5-dimethylpiperazin-1-
yl)thiazolo[3,2-
a]pyrimidin-5-one;
7-(2,8-dim ethylimidazo[1,2-b]pyri dazin-6-y1)-2-[(8aS)-3,4,6,7,8,8a-hexahydro-
1H-
pyrrolo[1,2-a]pyrazin-2-yl]thiazolo[3,2-a]pyrimi din-5-one;
7-(2-methylimidazo[1,2-b]pyri dazin-6-y1)-2-piperazin-1-yl-thiazol o[3,2-
a]pyrimidin-5-
one;
742-methy1-8-(trifluoromethypimidazo[1,2-b]pyridazin-6-y1]-2-piperazin-l-yl-
thiazolo[3,2-a]pyrimidin-5-one;
7-(8-ethy1-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-piperazin-1-yl-
thiazolo[3,2-
a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-piperazin-l-yl-
thiazolo[3,2-
a]pyrimidin-5-one;
2-(3,8-diazabicyclo[3.2.1]octan-8-y1)-7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-
yl)thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(8aR)-3,4,6,7,8,8a-hexahydro-
1H-
pyrrolo[1,2-a]pyrazin-2-ylithiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(1S,4 S)-2,5-
diazabicyclo[2.2.1]heptan-2-
yl]thiazolo[3,2-a]pyrimi din-5-one;
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 1 7 -
2-(2,3,3 a,4,6,6a-hexahydro- 1H-pyrrol o[3 ,4-c]pyrrol-5-y1)-7-(2,8-
dimethylimi dazo[ 1,2-
b]pyridazin-6-yl)thiazolo[3 ,2-a]pyrimidin-5 -one;
7-(8-cyclopropy1-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-piperazin-1-yl-
thiazolo[3,2-
a]pyrimidin-5-one;
7-(8-isopropeny1-2-methyl-imidazo[1,2-13]pyridazin-6-y1)-2-piperazin-1-yl-
thiazolo[3,2-
a]pyrimidin-5-one;
7-(2,7-dim ethyloxazolo[5,4-b]pyri din-5-y1)-2-piperazin- 1 -yl-thiazolo[3,2-
a]pyrimidin-5-
one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3 S)-3 -pyrroli din- 1 -
ylpyrroli din- 1-
yl]thiazolo[3,2-a]pyrimidin-5-one;
2-(2,8-diazaspiro[4.5]decan-2-y1)-7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-
yl)thiazolo[3,2-a]pyrimidin-5-one;
7-(8-isopropy1-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-piperazin-1-yl-
thiazolo[3,2-
a]pyrimidin-5-one;
7-(2,8-dim ethylimidazo[ 1,2-b]pyri dazin-6-y1)-2-(4-piperidyl)thiazolo[3,2-
a]pyrimidin-5-
one;
7[2-methy1-8-(trifluoromethyl)imi dazo[ 1,2-b]pyri dazin-6-y1]-2-[(8aR)-3
,4,6,7, 8,8a-
hexahydro- 1H-pyrrolo[ 1,2-a]pyrazin-2-yl]thiazolo[3 ,2-a]pyrimidin-5 -one;
7-(2,8-dimethylimidazo[ 1,2-b]pyridazin-6-y1)-2-(4-fluoro-4-
piperidyl)thiazolo[3
a]pyrimidin-5-one;
7-[8-(difluoromethoxy)-2-methyl-imidazo[ 1,2-b]pyridazin-6-y1]-2-piperazin- 1 -
yl-
thiazolo[3 ,2-a]pyrimidin-5-one;
742-methy1-7-(trifluoromethyl)oxazolo[5,4-Npyridin-5-y1]-2-piperazin-l-yl-
thiazolo[3,2-
a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-(4-
piperidyl)thiazolo[3,2-
a]pyrimidin-5-one;
7-(2,7-dimethyloxazolo[5,4-b]pyridin-5-y1)-2-(4-piperidyl)thiazolo[3,2-
a]pyrimidin-5-one;
7-(2-chloro-8-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-(4-piperidyl)thiazolo[3,2-
a]pyrimidin-5-one;
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 1 8 -7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3R,4R)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3S,4S)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(3R,4R)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-24(3S,4S)-3-fluoro-4-
piperidyllthiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(7S)-4-azaspiro[2.5]octan-7-
yl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(7R)-4-azaspiro[2.5]octan-7-
yl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3S,4R)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-24(3R,4S)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(7S)-4-
azaspiro[2.5]octan-7-
yl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(7R)-4-
azaspiro[2.5]octan-7-
yl]thiazolo[3,2-a]pyrimidin-5-one;
2-(4-fluoro-4-piperidy1)-7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-
yOthiazolo[3,2-a]pyrimidin-5-one;
7-(2-chloro-8-methyl-imidazo[1,2-b]pyridazin-6-y1)-24(3R,4R)-3-fluoro-4-
piperidyllthiazolo[3,2-a]pyrimidin-5-one or 7-(2,7-dimethyloxazolo[5,4-
b]pyridin-5-y1)-2-
[(3S,4S)-3-fluoro-4-piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,7-dimethyloxazolo[5,4-b]pyridin-5-y1)-2-[(3R,4R)-3-fluoro-4-
piperidyl]thiazolo[3,2-
a]pyrimidin-5-one or 7-(2,7-dimethyloxazolo[5,4-b]pyridin-5-y1)-24(3S,4S)-3-
fluoro-4-
piperidyllthiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(3S,4R)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one; and
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 1 9 -
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(3R,4S)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
or a pharmaceutically acceptable salt thereof.
The invention further relates to a compound selected from
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[rac-(1R,5S)-9-oxa-3-
azabicyclo[3.3.1]nonan-7-yl]thiazolo[3,2-a]pyrimidin-5-one ;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[rac-(1S,5R)-9-oxa-3-
azabicyclo[3.3.1]rionan-7-yl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[rac-(1R,5S)-9-oxa-3-
1 0 azabicyclo[3 .3 . 1 ]nonan-7-yl]thiazolo[3 ,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[rac-(1S,5R)-9-oxa-3-
azabicyclo[3.3.1]nonan-7-yl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[rac-(3R,4S)-3,4-
difluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[rac-(3S,4S)-3,4-
difluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[rac-(3R,4S)-3,4-difluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[rac-(3S,4S)-3,4-difluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[rac-(1S,5R)-3-
azabicyclo[3.1.0]hexan-6-
yl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(3R,4S)-3,4-difluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(3S,4S)-3,4-difluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(3R,4R)-3,4-difluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one; and
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-R3S,4R)-3,4-difluoro-4-
piperidylithiazolo[3,2-a]pyrimidin-5-one;
Date recue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 20 -
or a pharmaceutically acceptable salt thereof.
The invention further relates to a compound selected from
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-piperazin-1-yl-thiazolo[3,2-
a]pyrimidin-5-
one;
7-(2,8-dimethylimidazo[1,2-13]pyridazin-6-y1)-2-(1,2,3,6-tetrahydropyridin-4-
yl)thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-1Apyridazin-6-y1)-2-R3R)-3-methylpiperazin-1-
yllthiazolo[3,2-a]pyrimidin-5-one;
7-[2-methyl-8-(trifluoromethyl)imidazo [1,2-13]pyridazin-6-y1]-2-piperazin-l-
yl-
thiazolo[3,2-a]pyrimidin-5-one;
7-(8-ethy1-2-methyl-imidazo[1,2-1Apyridazin-6-y1)-2-piperazin-l-yl-
thiazolo[3,2-
a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-1Apyridazin-6-y1)-2-piperazin-l-yl-
thiazolo[3,2-
a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-1Apyridazin-6-y1)-2-R8aR)-3,4,6,7,8,8a-hexahydro-1
H-
p y rro 1 o [ 1,2-a]pyrazin-2-yl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,7-dimethyloxazolo[5,4-b]pyridin-5-y1)-2-piperazin-1-yl-thiazolo[3,2-
a]pyrimidin-5-
one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-(4-piperidyl)thiazolo[3,2-
a]pyrimidin-5-
one;
7-(2,8-dimethylimidazo[1,2-13]pyridazin-6-y1)-2-(4-fluoro-4-
piperidypthiazolo[3,2-
a]pyrimidin-5-one;
7[8-(difluoromethoxy)-2-methyl-imidazo[1,2-1Apyridazin-6-y1]-2-piperazin- I -
yl-
thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-(4-
piperidyl)thiazolo[3,2-
a]pyrimidin-5-one;
7-(2,7-dimethyloxazolo[5,4-b]pyridin-5-y1)-2-(4-piperidyl)thiazolo[3,2-
a]pyrimidin-5-one;
7-(2-chloro-8-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-(4-piperidyl)thiazolo[3,2-
a]pyrimidin-5-one;
Date regue/date received 2023-08-25
WO 2022/194801
PCT/EP2022/056586
-21 -
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3R,4R)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3S,4S)-3-fluoro-4-
piperidyl]thiazo1o[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(3R,4R)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(3S,4S)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
octan-7-
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(7R)-4-azaspiro[2.5]octan-7-
yl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3S,4R)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3R,4S)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(7S)-4-
azaspiro[2.5]octan-7-
yl]thiazolo[3,2-a]pyrimidin-5-one;
]octan-7-
2-(4-fluoro-4-piperidy1)-7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-
yl)thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(3S,4R)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(3R,4S)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[rac-(1S,5R)-9-oxa-3-
azabicyclo[3.3.1]nonan-7-yl]thiazo1o[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[rac-(3R,4S)-3,4-
difluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
Date recue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 22 -7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[rac-(1S,5R)-3-
azabicyclo[3.1.0]hexan-6-
yl]thiazolo[3,2-a]pyrimidin-5-one; and
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(3R,4S)-3,4-difluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
or a pharmaceutically acceptable salt thereof.
The invention further relates to a compound selected from
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-piperazin-1-yl-thiazolo[3,2-
a]pyrimidin-5-
one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-(1,2,3,6-tetrahydropyridin-4-
yl)thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3R)-3-methylpiperazin-1-
yl]thiazolo[3,2-a]pyrimidin-5-one;
742-methy1-8-(trifluoromethyl)imidazo[1,2-b]pyridazin-6-y1]-2-piperazin-l-yl-
thiazolo[3,2-a]pyrimidin-5-one;
7-(8-ethy1-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-piperazin-1-yl-
thiazolo[3,2-
a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-piperazin-1-yl-
thiazolo[3,2-
a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(8aR)-3,4,6,7,8,8a-hexahydro-1
H-
pyrrolo[1,2-a]pyrazin-2-ylithiazolo[3,2-a]pyrimidin-5-one;
7-(2,7-dimethyloxazolo[5,4-b]pyridin-5-y1)-2-piperazin-1-yl-thiazolo[3,2-
a]pyrimidin-5-
one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-(4-piperidyl)thiazolo[3,2-
a]pyrimidin-5-
one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-(4-fluoro-4-
piperidyl)thiazolo[3,2-
a]pyrimidin-5-one;
7-[8-(difluoromethoxy)-2-methyl-imidazo[1,2-b]pyridazin-6-y1]-2-piperazin- I -
yl-
thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-(4-
piperidyl)thiazolo[3,2-
a]pyrimidin-5-one;
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 23 -
7-(2,7-dimethyloxazolo[5,4-b]pyridin-5-y1)-2-(4-piperidyl)thiazolo[3,2-
a]pyrimidin-5-one;
7-(2-chloro-8-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-(4-pipendyl)thiazolo[3,2-
a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3R,4R)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3S,4S)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(3R,4R)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(3S,4S)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(7S)-4-azaspiro[2.5]octan-7-
yl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(7R)-4-azaspiro[2.5]octan-7-
ylithiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3 S,4R)-3-fluoro-4-
pipetidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3R,4S)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(7S)-4-
azaspiro[2.5]octan-7-
yl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(7R)-4-
azaspiro[2.5]octan-7-
yl]thiazolo[3,2-a]pyrimidin-5-one;
2-(4-fluoro-4-piperidy1)-7-(8-methoxy-2-methyl-imidazo[1,2-13]pyridazin-6-
yl)thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-243S,4R)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one; and
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-243R,4S)-3-fluoro-4-
piperidylithiazolo[3,2-a]pyrimidin-5-one;
or a pharmaceutically acceptable salt thereof
Date recue/date received 2023-08-25
WO 2022/194801
PCT/EP2022/056586
-24 -
The invention further relates to a compound selected from
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-(1,2,3,6-tetrahydropyridin-4-
yl)thiazolo[3,2-a]pyrimidin-5-one; and
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3R)-3-methylpiperazin-1-
yl]thiazolo[3,2-a]pyrimidin-5-one;
or a pharmaceutically acceptable salt thereof.
The invention further relates to a compound selected from
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(7R)-4-
azaspiro[2.5]octan-7-
yl]thiazolo[3,2-a]pyrimidin-5-one; and
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(7S)-4-
azaspiro[2.5]octan-7-
yl]thiazolo[3,2-a]pyrimidin-5-one;
or a pharmaceutically acceptable salt thereof
The invention further relates to a compound selected from
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-(4-fluoro-4-
piperidyl)thiazolo[3,2-
a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3R,4R)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3S,4S)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(3R,4R)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one;
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(3 S,4S)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one; and
2-(4-fluoro-4-piperidy1)-7-(8-methoxy-2-methyl-imidazo[1,2-13]pyridazin-6-
yl)thiazolo[3,2-a]pyrimidin-5-one;
or a pharmaceutically acceptable salt thereof
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 25 -
The synthesis of the compound of formula (I) can, for example, be accomplished
according to the following schemes. 10-R4 and A1-A3 are as defined above,
unless
otherwise specified.
Scheme 1
Br sN H2 0 H
H2
,
-Cfr
\ N -Fr
R __
11
0
2 3 4
R2 1
xLr-N
R2
0 (OR)2B¨A3 6
R3
sN 0
R1 c- I R1 Trsq
0 0
5 (I)
The compound of formula (I) wherein RI is a N-linked heterocycloalkyl or N-
linked
heteroaryl, can be made according to scheme L The preparation of such
compounds is
commenced by an aromatic nucleophilic substitution reaction of the
commercially
available 5-bromothiazol-2-amine (or a suitable derivate thereof) 2 with a It'
heterocycle
or heteroaryl (and wherein RI may further contain a protecting group) in
presence of a base
such as K2CO3 and a polar solvant as DMF, leading to 3. Following a
condensation with
commercially available bis(2,4,6-trichlorophenyl) propanedioate, the resulting
intermediate
4 was obtained, which readily reacted with tosyl chlorid in presence of an
organic base
such as triethyl amine to yield 5. A Suzuki-cross coupling reaction between 5
and a
suitable boronic acid 6, wherein in -B(OR)2 each R is independently selected
from
hydrogen and alkyl or -B(OR)2 is optionally substituted dioxaborolanyl. The
Suzuki-cross
coupling between 5 and 6 in 1,4-dioxane, acetonitrile, water or a mixture
thereof, in
presence of a base such as K2CO3 and a suitable palladium catalyst yields the
compound of
formula (1) (after an eventual removal of a protecting group on the It'
moiety).
Scheme 2
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
-26 -
R2
0 R H 2 -sr
3
0)¨R
R1
7 3 0 (I)
The compound of formula (I) wherein It" is a C-linked heterocycloalkyl, C-
linked
heteroaryl, cycloalkyl, aryl or alkyl can be made according to scheme 2. The
synthesis of
such compounds starts with a commercially available aldehyde (or as descrived
herein
below and wherein IV may further contain a protecting group) 7, which is
converted
readily into the aminothiazole derivatives of formula 3 following a sequential
bromination
and cyclisation with thiourea. The same synthetic sequence is then perfomed as
in scheme
1 from intermediate 3 to form the compound of formula (I) (after an eventual
removal of a
protecting group on the moiety).
Scheme 3
R2
H2 Br¨ a A AP
R1-6 (OH)2 R3 =
7
Ri I
2 3 0
(I)
Alternatively, the compound of formula (I) wherein It' is a C-linked
heterocycloalkyl, C-
linked heteroaryl, cycloalkyl aryl or alkyl can be made according to scheme 3.
This
alternative synthesis starts from the 5-bromothiazol-2-amine compound (or a
derivative
thereof) 2 which underoes a Suzuki-cross coupling with a boronic acid of
formula
RIB(OH)2 to yield the intermediate of formula 3 (wherein IV may further
contain a
protecting group). The same synthetic sequence is then perfomed as in scheme 1
from
intermediate 3 to form the compound of formula (I) (after an eventual removal
of a
protecting group on the RI moiety).
The invention thus also relates to a process for the preparation of a compound
according to the invention, comprising at least one of the following steps:
(a) the reaction of a compound of formula (B1)
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 27 -
¨
PG ______________________________ Ri_c-r
n N-Y
0
(B1)
with a compound of formula (B2)
R2
R
_________________________________________________ 3
A2... A>
(OR)2B Ac 3
(B2)
in a suitable solvent in the presence of a base and a suitable palladium
catalyst,
wherein n is 0 or 1, X is 0-tosylate, 0-triflate, 0-mesylate or halogen, and
wherein
in -B(OR)2 each R is independently selected from hydrogen and alkyl, or -
B(OR)2 is
optionally substituted dioxaborolanyl, to arrive at a compound of fomula (B3)
R2
3
R
" 1 r1.3
PG ______________________ R
\ 1 A
0
(B3)
(b) the reaction of the compound of formula (B3), wherein n is 1, in a
suitable
solvent and in presence of an acid to yield the compound of formula (I)
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 28 -
R2
A20,A3 >¨ 3
R1
0
(I)
wherein in the process R2, R3, R4, Ai, Az and A3 are as defined above,
and PG is
a protecting group.
The reaction of step (a) can be conveniently carried out in a solvent. The
solvent can
be for example 1,4-dioxane, acetonitrile, water or a mixture thereof;
In the reaction of step (a), the base can be for example K2CO3, Li2CO3,
Na2CO3,
KOtBu, Cs2CO3, NaOtBu or LiOtBu, in particular K2CO3;
In the reaction of step (a), the palladium catalyst can be for example
Pd(dppf)C12 -
CH2C12 (CAS#95464-05-4) or XPhos PdG4 (CAS#1599466-81-5);
In the reaction of step (a), X is conveniently 0-tosylate or chloro, in
particular 0-
tosylate;
In the reaction of step (a), B(OR)2 can be for example dioxaborolanyl
optionally
substituted with one, two, three or four alkyl, in particular 4,4,5,5-
tetramethy1-1,3,2-
dioxaborolan-2-y1;
Convenient conditions for the reaction of step (a) are around 20 C ¨ 150 C,
particularly around 40 C ¨ 130 C, more particularly around 60 C ¨ 110 C,
in particular
around 90 C;
Particular conditions for the reaction of step (a) are the use of K2CO3 in 1,4-
dioxane,
acetonitrile, water or a mixture thereof at around 90 C for around 2 hrs - 8
hrs;
The reaction of step (b) can be conveniently carried out in a solvent. The
solvent can
be for example CH2C12 or 1,4-dioxane;
In the reaction of step (b) the acid can be for example 11-,A or HC1;
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 29 -
Convenient conditions for the reaction of step (b) are around 0 C ¨ 100 C,
particularly around 5 C ¨ 80 C, more particularly around 10 C ¨ 60 C, in
particular
around 15 C ¨ 40 C;
Particular conditions for the reaction of step (b) are the use of TFA in
CH2C12 at
around 15-40 C for around 1 hrs - 24 hrs, in particular for around 1 h - 3
hrs;
In the process, the protecting group can be for example Boc, Trt or Dmb, in
particular Boc.
The invention also relates to a compound according to the invention when
manufactured according to a process of the invention.
The invention thus also relates in particular to:
A compound according to the invention for use as therapeutically active
substance;
A pharmaceutical composition comprising a compound according to the invention
and a therapeutically inert carrier;
A compound according to the invention for use in the treatment or prophylaxis
of a
neurodegenerative disease;
A compound according to the invention for use in the treatment or prophylaxis
of
Huntington's disease;
The use of a compound according to the invention for the treatment or
prophylaxis of
a neurodegenerative disease, in particular Huntington's disease;
The use of a compound according to the invention for the preparation of a
medicament for the treatment or prophylaxis of a neurodegenerative disease, in
particular
Huntington's disease; and
A method for the treatment or prophylaxis of a neurodegenerative disease, in
particular Huntington's disease, which method comprises administering an
effective
amount of a compound according to the invention to a patient in need thereof
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 30 -
A certain embodiment of the invention relates to a pharmaceutical composition
comprising the compound of formula (I) as described herein, or a
pharmaceutically
acceptable salt thereof, and a phainiaceutically acceptable auxiliary
substance.
Furthermore, the invention includes all substituents in its corresponding
deuterated
form, wherever applicable, of the compound of formula (I).
Furthermore, the invention includes all optical isomers, i.e.
diastereoisomers,
diastereomeric mixtures, racemic mixtures, all their corresponding enantiomers
and/or
tautomers as well as their solvates, wherever applicable, of the compound of
formula (I).
The compound of formula (I) may contain one or more asymmetric centers and can
therefore occur as racemates, racemic mixtures, single enantiomers,
diastereomeric
mixtures and individual diastereomers. Additional asymmetric centers may be
present
depending upon the nature of the various substituents on the molecule. Each
such
asymmetric center will independently produce two optical isomers and it is
intended that
all of the possible optical isomers and diastereomers in mixtures and as pure
or partially
purified compounds are included within this invention. The present invention
is meant to
encompass all such isomeric forms of these compounds. The independent
syntheses of
these diastereomers or their chromatographic separations may be achieved as
known in the
art by appropriate modification of the methodology disclosed herein. Their
absolute
stereochemistry may be determined by the x-ray crystallography of crystalline
products or
crystalline intermediates which are derivatized, if necessary, with a reagent
containing an
asymmetric center of known absolute configuration. If desired, racemic
mixtures of the
compounds may be separated so that the individual enantiomers are isolated.
The
separation can be carried out by methods well known in the art, such as the
coupling of a
racemic mixture of compounds to an enantiomerically pure compound to form a
diastereomeric mixture, followed by separation of the individual diastereomers
by standard
methods, such as fractional crystallization or chromatography.
In the embodiments, where optically pure enantiomers are provided, optically
pure
enantiomer means that the compound contains > 90 % of the desired isomer by
weight,
particularly > 95 % of the desired isomer by weight, or more particularly > 99
% of the
desired isomer by weight, said weight percent based upon the total weight of
the isomer(s)
of the compound. Chirally pure or chirally enriched compounds may be prepared
by
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 31 -
chirally selective synthesis or by separation of enantiomers. The separation
of enantiomers
may be carried out on the final product or alternatively on a suitable
intermediate.
In a particular embodiment, the compounds of the present invention have
favourable
brain penetration properties.
Also an embodiment of the present invention is a compound of formula (I) as
described herein, when manufactured according to any one of the described
processes.
The compound of formula (I) or a pharmaceutically acceptable salt thereof can
be
used as a medicament (e.g. in the form of a pharmaceutical preparation). The
pharmaceutical preparation can be administered internally, such as orally
(e.g. in the form
of tablets, coated tablets, dragees, hard and soft gelatin capsules,
solutions, emulsions or
suspensions), nasally (e.g. in the form of nasal sprays), rectally (e.g. in
the form of
suppositories) or topical ocularly (e.g. in the form of solutions, ointments,
gels or water
soluble polymeric inserts). However, the administration can also be effected
parenterally,
such as intramuscularly, intravenously, or intraocularly (e.g. in the form of
sterile injection
solutions).
The compound of formula (I) or a pharmaceutically acceptable salt thereof can
be
processed with pharmaceutically inert, inorganic or organic adjuvants for the
production of
tablets, coated tablets, dragees, hard gelatin capsules, injection solutions
or topical
formulations Lactose, corn starch or derivatives thereof, talc, stearic acid
or its salts etc.
can be used, for example, as such adjuvants for tablets, dragees and hard
gelatin capsules.
Suitable adjuvants for soft gelatin capsules, are, for example, vegetable
oils, waxes,
fats, semi-solid substances and liquid polyols, etc.
Suitable adjuvants for the production of solutions and syrups are, for
example, water,
polyols, saccharose, invert sugar, glucose, etc.
Suitable adjuvants for injection solutions are, for example, water, alcohols,
polyols,
glycerol, vegetable oils, etc.
Suitable adjuvants for suppositories are, for example, natural or hardened
oils,
waxes, fats, semi-solid or liquid polyols, etc.
Suitable adjuvants for topical ocular formulations are, for example,
cyclodextrins,
mannitol or many other carriers and excipients known in the art.
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 32 -
Moreover, the pharmaceutical preparation can contain preservatives,
solubilizers,
viscosity-increasing substances, stabilizers, wetting agents, emulsifiers,
sweeteners,
colorants, flavorants, salts for varying the osmotic pressure, buffers,
masking agents or
antioxidants. The pharmaceutical preparation can also contain still other
therapeutically
valuable substances.
The dosage can vary in wide limits and will be fitted to the individual
requirements
in each particular case. In general, in the case of oral administration a
daily dosage of about
0.1 mg to 20 mg per kg body weight, preferably about 0.5 mg to 4 mg per kg
body weight
(e.g. about 300 mg per person), divided into preferably 1-3 individual doses,
which can
consist, for example, of the same amounts, should it be appropriate. In the
case of topical
administration, the formulation can contain 0.001% to 15% by weight of
medicament and
the required dose, which can be between 0.1 and 25 mg in can be administered
either by
single dose per day or per week, or by multiple doses (2 to 4) per day, or by
multiple doses
per week It will, however, be clear that the upper or lower limit given herein
can be
exceeded when this is shown to be indicated.
Pharmaceutical Compositions
The compound of formula (I) or a pharmaceutically acceptable salt thereof can
be
used as a therapeutically active substance, e.g. in the form of a
pharmaceutical preparation.
The pharmaceutical preparation can be administered orally, e.g. in the form of
tablets,
coated tablets, dragees, hard and soft gelatin capsules, solutions, emulsions
or suspensions.
The administration can, however, also be effected rectally, e.g. in the form
of
suppositories, or parenterally, e.g. in the fol m of injection solutions.
The compound of formula (I) or a pharmaceutically acceptable salt thereof can
be
processed with pharmaceutically inert, inorganic or organic carriers for the
production of a
pharmaceutical preparation. Lactose, corn starch or derivatives thereof, talc,
stearic acids
or its salts and the like can be used, for example, as such carriers for
tablets, coated tablets,
dragees and hard gelatin capsules. Suitable carriers for soft gelatin capsules
are, for
example, vegetable oils, waxes, fats, semi-solid and liquid polyols and the
like. Depending
on the nature of the active substance no carriers are however usually required
in the case of
soft gelatin capsules. Suitable carriers for the production of solutions and
syrups are, for
example, water, polyols, glycerol, vegetable oil and the like. Suitable
carriers for
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 33 -
suppositories are, for example, natural or hardened oils, waxes, fats, semi-
liquid or liquid
polyols and the like.
The pharmaceutical preparation can, moreover, contain pharmaceutically
acceptable
auxiliary substances such as preservatives, solubilizers, stabilizers, wetting
agents,
emulsifiers, sweeteners, colorants, flavorants, salts for varying the osmotic
pressure,
buffers, masking agents or antioxidants. They can also contain still other
therapeutically
valuable substances.
Medicaments containing a compound of formula (I) or a pharmaceutically
acceptable
salt thereof and a therapeutically inert carrier are also provided by the
present invention, as
is a process for their production, which comprises bringing a compound of
formula (I)
and/or pharmaceutically acceptable salts thereof and, if desired, one or more
other
therapeutically valuable substances into a galenical administration form
together with one
or more therapeutically inert carriers.
The dosage can vary within wide limits and will, have to be adjusted to the
individual
requirements in each particular case. In the case of oral administration the
dosage for
adults can vary from about 0.01 mg to about 1000 mg per day of a compound of
general
formula (I) or of the corresponding amount of a pharmaceutically acceptable
salt thereof.
The daily dosage may be administered as single dose or in divided doses and,
in addition,
the upper limit can also be exceeded when this is found to be indicated.
The following examples illustrate the present invention without limiting it,
but serve
merely as representative thereof. The phamiaceutical preparation conveniently
contains
about 1-500 mg, particularly 1-100 mg, of a compound of formula (I). Examples
of
compositions according to the invention are:
Example A
Tablets of the following composition are manufactured in the usual manner:
ingredient mg/tablet
5 25 100 500
Compound of formula (I) 5 25 100 500
Date regue/date received 2023-08-25
WO 2022/194801
PCT/EP2022/056586
- 34 -
Lactose Anhydrous DTG 125 105 30 150
Sta-Rx 1500 6 6 6 60
Microcrystalline Cellulose 30 30 30 450
Magnesium Stearate 1 1 1 1
Total 167 167 167 831
Table 1: possible tablet composition
Manufacturing Procedure
1. Mix ingredients 1, 2, 3 and 4 and granulate with purified water.
2. Dry the granules at 50 C.
3. Pass the granules through suitable milling equipment.
4. Add ingredient 5 and mix for three minutes; compress on a suitable
press.
Example B-1
Capsules of the following composition are manufactured:
ingredient mg/capsule
5 25 100 500
,
Compound of formula (I) 5 25 100 500
,
Hydrous Lactose 159 123 148 -
Corn Starch 25 35 40 70
Talk 10 15 10 25
Magnesium Stearate 1 2 2 5
Total 200 200 300 600
Table 2: possible capsule ingredient composition
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 35 -
Manufacturing Procedure
1. Mix ingredients 1, 2 and 3 in a suitable mixer for 30 minutes.
2. Add ingredients 4 and 5 and mix for 3 minutes.
3. Fill into a suitable capsule.
The compound of formula (1), lactose and corn starch are firstly mixed in a
mixer and
then in a comminuting machine. The mixture is returned to the mixer; the talc
is added
thereto and mixed thoroughly. The mixture is filled by machine into suitable
capsules, e.g.
hard gelatin capsules.
Example B-2
Soft Gelatin Capsules of the following composition are manufactured:
ingredient mg/capsule
Compound of formula (I) 5
Yellow wax 8
Hydrogenated Soya bean oil 8
Partially hydrogenated plant oils 34
Soya bean oil 110
Total 165
Table 3: possible soft gelatin capsule ingredient composition
ingredient mg/capsule
Gelatin 75
Glycerol 85 % 32
Karion 83 8 (dry matter)
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 36 -
Titan dioxide 0.4
Iron oxide yellow 1.1
Total 116.5
Table 4: possible soft gelatin capsule composition
Manufacturing Procedure
The compound of formula (I) is dissolved in a warm melting of the other
ingredients
and the mixture is filled into soft gelatin capsules of appropriate size. The
filled soft
gelatin capsules are treated according to the usual procedures.
Example C
Suppositories of the following composition are manufactured:
ingredient mg/supp.
Compound of formula (I) 15
Suppository mass 1285
Total 1300
Table 5: possible suppository composition
Manufacturing Procedure
The suppository mass is melted in a glass or steel vessel, mixed thoroughly
and
cooled to 45 C. Thereupon, the finely powdered compound of formula (I) is
added thereto
and stirred until it has dispersed completely. The mixture is poured into
suppository
moulds of suitable size, left to cool; the suppositories are then removed from
the moulds
and packed individually in wax paper or metal foil.
Example D
Injection solutions of the following composition are manufactured:
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 37 -
ingredient mg/injection solution.
Compound of formula (I) 3
Polyethylene Glycol 400 150
acetic acid q.s. ad pH 5.0
water for injection solutions ad 1.0 ml
Table 6: possible injection solution composition
Manufacturing Procedure
The compound of formula (I) is dissolved in a mixture of Polyethylene Glycol
400
and water for injection (part). The pH is adjusted to 5.0 by acetic acid. The
volume is
adjusted to 1.0 ml by addition of the residual amount of water. The solution
is filtered,
filled into vials using an appropriate overage and sterilized.
Example E
Sachets of the following composition are manufactured:
ingredient mg/sachet
Compound of formula (I) 50
Lactose, fine powder 1015
Microcrystalline cellulose (AVICEL PH 102) 1400
Sodium carboxymethyl cellulose 14
Polyvinylpyrrolidon K 30 10
Magnesium stearate 10
Flavoring additives 1
Total 2500
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 38 -
Table 7: possible sachet composition
Manufacturing Procedure
The compound of founula (I) is mixed with lactose, microcrystalline cellulose
and
sodium carboxymethyl cellulose and granulated with a mixture of
polyvinylpyrrolidone in
water. The granulate is mixed with magnesium stearate and the flavoring
additives and
filled into sachets.
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 39 -
Examples
Abbreviations:
AcCl: acetyl chloride; ACN: acetonitrile; AcOH: acetic acid; Boc: tert-
butyloxycarbonyl;
DAST: diethylaminosulfur trifluoride; DCM: dichloromethane; DIBAL-H:
diisobutylaluminium hydride; DMAP: 4-dimethylaminopyridine; DMF:
dimethylformamide; DMSO: dimethyl sulfoxide; dppf:
bis(diphenylphosphino)ferrocene;
dppp: 1,3-bis-(diphenylphosphino)-propan; ES+: positive electronspray
ionization; Et0Ac:
ethyl acetate; Et0H: ethanol; HPLC: high performance liquid chromatography;
HTRF:
homogeneous time resolved fluorescence; iPrOH: isopropanol; LAH: lithium
aluminium
hydride; LCMS: liquid chromatography mass spectrometry; MeOH: methanol; MS:
mass
spectrometry; PPTS: pyridinium p-toluenesulfonate; RP-HPLC: reversed phase-
high
performance liquid chromatography; RT: room temperature; SFC: supercritical
fluid
chromatography; TBME: tert-butyl methyl ether; TEA: triethylamine; TFA:
trifluoroacetic
acid; THE: tetrahydrofuran.
The following examples are provided for illustration of the invention. They
should
not be considered as limiting the scope of the invention, but merely as being
representative
thereof.
Synthesis of intermediates of formula 3
Intermediate 3-1
tert-butyl 4-(2-aminothiazol-5-yl)piperazine-1-carboxylate
mu2
s¨(t
o
NJ
In a stirred solution of DMSO (28.0 mL) was added (5-bromothiazol-2-yl)amine
(5.0 g,
27.9 mmol), Et3N (11.7 mL, 83.8 mmol) and commercially available 1-boc-
piperazine (7.8
g, 41.9 mmol). The reaction mixture was heated at 100 C for 6 hours before
cooling down
to RT. The crude reaction mixture was partitioned between Et0Ac (100 mL) and
H20 (100
mL) and the separated. The aqueous phase was extracted with Et0Ac. The
combined
organic phases were dried over Na2SO4, filtered and concentrated under vacuo.
The solid
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
-40 -
residue was triturated in EtOAC/n-heptane and the solid collected by
filtration and dried
under vacuo to yield tert-butyl 4-(2-aminothiazol-5-yl)piperazine-1-
carboxylate as a light
brown solid (130 g, 42 % yield) . MS (ES+) m/z: 285.2 [(M+H)+].
Intermediate 3-2
tert-butyl 4-(2-aminothiazol-5-y1)-3,6-dihydro-211-pyridine-1-carboxylate
NH2
NH2 1- %BILL T71SCI OH 8 NH2 IT FA
g 2. N-Bac P Boc20
step >r 2
3-2
Step 1: Carbinols such as 4-(2-aminothiazol-5-y1)-4-hydroxy-piperidine-1 -
carboxylic acid
tert-butyl ester are obtained following the experimental procedures described
in details by
Katritzky et.al. CAN J. CHEM. volume 66, 1988.
Step 2: A solution of 4-(2-aminothiazol-5-y1)-4-hydroxy-piperidine-1-
carboxylic acid tert-
butyl ester (300 mg, 0.962 mmol) in TFA (5 mL) was stirred at 65 C until Boc
deprotection and dehydration had reached completion, usually between 4 and 16
hours.
Volatiles were evaporated and then azeotroped with toluene (15 mL x 3), the
residue is
then resuspended in 9 mL of a 1:1 mixture of 1M NaHCO3 and 1,4-dioxane, cooled
to 0 C
and treated with Boc anhydride (231 mg, 1.06 mmol). Upon completion, the
reaction is
diluted with ethyl acetate and extracted, dried over Na2SO4, filtered and
purified on silica
gel, eluting with Me0H in DCM from 0 to 20% in 25 minutes.
4-(2-aminothiazol -5-y1)-3 , 6-di hydro-2H-pyridine- 1-carboxylic
acid tert-butyl ester
derivative 3-2 (160 mg, 59 % yield) was obtained as a white solid. MS (ES+)
m/z: 282.2
[M+H]t
Intermediate 3-3
tert-butyl 4-(2-aminothiazol-5-y1)-2,2-dimethyl-piperazine-1-carboxylate
NH2
0
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
-41 -
In a stirred solution of DMF (10.0 mL) was added (5-bromothiazol-2-yl)amine
(1.0 g, 5.59
mmol), K2CO3 (3.09 g, 22.3 mmol) and commercially available 2,2-
dimethylpiperazine-1-
carboxylic acid tert-butyl ester (1.44 g, 6.7 mmol). The reaction mixture was
heated at 70
C for 1 hours before cooling down to RT and DMF mostly evaporated. The crude
reaction
mixture was partitioned between Et0Ac (50 mL) and a 1M aqueous solution of
NaHCO3
(50 mL) and the separated. The aqueous phase was extracted with Et0Ac. The
combined
organic phases were dried over Na2SO4, filtered and concentrated under vacuo
and a
purification by column chromatography (CH2C12 / Me0H) under yielded tert-butyl
4-(2-
aminothiazol-5-y1)-2,2-dimethyl-piperazine-1-carboxylate as an orange solid
(1.06 g, 60 %
yield). MS (ES+) m/z: 313.3 [(M+H)+].
Intermediate 3-4
tert-butyl 7-(2-aminothiazol-5-y1)-4,7-diazaspiro12.51octane-4-carboxylate
N H2
0 IC:r\ N4S
In analogy to the preparation of the intermediate 3-3, from commercially
available 4,7-
diazaspiro[2.5]octane-4-carboxylic acid tert-butyl ester, the title compound
was prepared
as a red oil (360 mg, 55% yield). MS (ES+) m/z: 311.5 [(M+H)+].
Intermediate 3-5
tert-butyl (2S)-4-(2-aminothiazol-5-y1)-2-methyl-piperazine-1-carboxylate
N H2
S
0 \f+jk,'
In analogy to the preparation of the intermediate 3-3, from commercially
available (2S)-2-
methylpiperazine-1 -carboxylic acid tert-butyl ester, the title compound was
prepared as a
red oil (408 mg, 64% yield). MS (ES+) m/z: 299.2 [(M+H)+].
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
-42 -
Intermediate 3-6
tert-butyl (2R)-4-(2-aminothiazol-5-y1)-2-methyl-piperazine-1-carboxylate
N H2
9
0
In analogy to the preparation of the intermediate 3-3, from commercially
available (2R)-2-
methylpiperazine- 1-carboxylic acid tert-butyl ester, the title compound was
prepared as a
red solid (339 mg, 53% yield). MS (ES+) m/z: 299.2 [(M+H)+].
Intermediate 3-7
tert-butyl 4-(2-aminothiazol-5-y1)-2,6-dimethyl-piperazine-1-carboxylate
4011-12
s
0 \r"\N----44N
In analogy to the preparation of the intermediate 3-3, from commercially
available 2,6-
dimethylpiperazine-1-carboxylic acid tert-butyl ester, the title compound was
prepared as a
red oil (328 mg, 53% yield). MS (ES+) m/z: 313.3 [(Md-H)+].
Intermediate 3-8
5-[(8aS)-3,4,6,7,8,8a-hexahydro-1H-pyrrolo[1,2-alpyrazin-2-ylithiazol-2-amine
N H2
In analogy to the preparation of the intermediate 3-3, from commercially
available (8a5)-
1,2,3,4,6,7,8,8a-octahydropyrrolo[1,2-a]pyrazine, the title compound was
prepared as a red
oil (240 mg, 50% yield). MS (ES+) m/z: 225.1 [(M+H)+].
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
-43 -
Intermediate 3-9
tert-butyl 8-(2-aminothiazol-5-y1)-3,8-diazabicyclop.2.11octane-3-carboxylate
NH2
)70
¨141D ¨7(/if
0
In analogy to the preparation of the intermediate 3-3, from commercailly
available 3,8-
diazabicyclo[3.2.1]octane-3-carboxylic acid tert-butyl ester, the title
compound was
prepared as a light red solid (357 mg, 59% yield). MS (ES+) m/z: 311.3
[(M+H)+].
Intermediate 3-10
5-1(8aR)-3,4,6,7,8,8a-hexahydro-1H-pyrrolo11,2-alpyrazin-2-yllthiazol-2-amine
N H2
--(I.
N.\,1
In analogy to the preparation of the intermediate 3-3, from (8aR)-
1,2,3,4,6,7,8,8a-
octahydropyrrolo[1,2-a]pyrazine, the title compound was prepared as a red oil
(200 mg,
55% yield). MS (ES+) m/z: 225.1 [(M+H)+].
Intermediate 3-11
tert-butyl (1S,4S)-5-(2-aminothiazol-5-y1)-2,5-diazabicyclo12.2.11heptane-2-
carboxylate
pH:
S
s2atig,-4:4z)N
0
In analogy to the preparation of the intermediate 3-3, from commercial
available (1S,4S)-
2,5-diazabicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester, the title
compound was
prepared as a red oil (295 mg, 51% yield). MS (ES+) m/z: 297.2 [(M+H)+].
Intermediate 3-12
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
-44 -
tert-butyl 2-(2-aminothiazol-5-y1)-1,3,3a,4,6,6a-hexahydropyrrolo[3,4-
c]pyrrole-5-
carboxylate
NHst
41.1
N
0
In analogy to the preparation of the intermediate 3-3, from 2,3,3a,4,6,6a-
hexahydro-1H-
pyrrolo[3,4-c]pyrrole-5-carboxylic acid tert-butyl ester, the title compound
was prepared as
a red solid (219 mg, 33% yield). MS (ES+) m/z: 311.2 [(M+H)].
Intermediate 3-13
5-1(3S)-3-pyrrolidin-1-ylpyrrolidin-1-ylithiazol-2-amine
NH2
In analogy to the preparation of the intermediate 3-3, from 1-[(35)-pyrrolidin-
3-
yl]pyrrolidine, the title compound was prepared as a red solid (517 mg, 90%
yield). MS
(ES+) m/z: 239.2 [(M+H)+].
Intermediate 3-14
tert-butyl 2-(2-aminothiazol-5-y1)-2,8-diazaspiro[4.51decane-8-carboxylate
N H2
S
In analogy to the preparation of the intermediate 3-3, from 2,8-
diazaspiro[4.5]decane-8-
carboxylic acid tert-butyl ester, the title compound was prepared as a red
solid (161 mg,
15% yield). MS (ES+) m/z: 339.2 [(M+H)].
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
-45 -
Intermediate 3-15
tert-butyl 4-(2-aminothiazol-5-yl)piperidine-1-carboxylate
44112
S
--c ;N
0
N
\is0
Step 1: In a two-necked round bottom flask oxalyl chloride (1.4 mL, 16.05
mmol), diluted
in anhydrous CH2C12 (20 mL), was cooled to ¨ 78 C and treated over the course
of 20
minutes with a solution of DMS0 (2.28 mL, 32.09 mmol) diluted in anhydrous
CH2C12 (20
mL). The resulting solution was stirred at the same temperature for 20 minutes
before
adding 4-(2-hydroxyethyl)piperidine-1-carboxylic acid tert-butyl ester (3.2 g,
13.95 mmol)
dissolved in anhydrous CH2C12 (20 mL), over the course of 15 minutes. The
resulting
milky solution was left stirring for 45 minutes before dropwise addition of
TEA (9.72 mL,
69.77 mmol). The resulting milky suspension was warmed to room temperature to
obtain a
foamy suspension.
The mixture was diluted with DCM and water, layers were separated and the
organic layer
was treated with Na2SO4, filtered and reduced to small volume. The residue was
loaded
onto a Silica cartridge and purified with a gradient of Et0Ac in heptane from
0 to 100 % in
minutes, flow 30 mL/min, ISCO system to give 4-(2-ketoethyl)piperidine-1-
carboxylic
acid tert-butyl ester (2. 8 g, 88.3% yield) as white crystalline powder. MS
(ES+) m/z: 172.1
[M+H-Buten].
20 Step 2: To a solution of 4-(2-ketoethyl)piperidine-1-carboxylic acid
tert-butyl ester (2.8. g,
12.32 mmol) in TI-IF (72 mL) and cooled to 0 C. Trimethylphenylammonium
tribromide
(4.72 g, 12.56 mmol) was added portionwise over the course of 20 minutes to
give a white
suspension. Upon completion, the reaction was quenched with 1M solution of
sodium
thiosulfate, diluted with diethylether and 1 M NaHCO3. Extract and dry with
Na2SO4, filter
25 and purified via silica gel to provide 4-(1-bromo-2-keto-
ethyl)piperidine-1-carboxylic acid
tert-butyl ester (2.6 g, 69 % yield) as a colorless solid.
Step 3: To a solution of 4-(1-bromo-2-keto-ethyl)piperidine-1 -carboxylic acid
tert-butyl
ester (2.6 g, 8.49 mmol) in Et0H (57 mL) was added thiourea (711 mg, 9.34
mmol)
and the mixture was then brought to reflux. Upon completion, the mixture was
evapotared
to dryness and the residue purified via silica gel yielding 4-(2-aminothiazol-
5-
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
-46 -
yl)piperidine- 1-carboxylic acid tert-butyl ester (1.3 g, 54.2 % yield) as a
white solid. MS
(ES+) in/z: 284.4 [M+H].
Intermediates 3-16
tert-butyl (3R,4R)-4-(2-aminothiazol-5-y1)-3-fluoro-piperidine-1-carboxylate
and tert-
butyl (3S,4S)-4-(2-aminothiazo1-5-y1)-3-fluoro-piperidine-1-carboxylate
H2
N S--*õ
N
0 \ H 2 0 \
and
Step 1: Preparation of 4-(2-ethoxy-2-keto-ethyli dene)-3-fluoro-piperi dine-l-
carboxyli c
acid tert-butyl ester
0 __________________________________________ )/-0/
0
In a flame dried 500 mL 4-necked flask, equipped with a magnetic stirrer bar,
dropping
funnel and a theiniometer, sodium hydride 60 % dispersion in mineral oil (2.0
g, 46.0
mmol, 1 eq.) were added portion wise to a solution of triethyl
phosphonoacetate (9.8 g, 8.8
mL, 43.7 mmol, 0.950 eq.) in tetrahydrofuran, extra dry (200 mL) over 25
minutes at 0-5
C. The ice-bath was removed and the mixture was stirred for 60 min. 3-fluoro-4-
keto-
piperidine-1 -carboxylic acid tert-butyl ester (10 g, 46.0 mmol, 1 eq.)
dissolved in
tetrahydrofuran, extra dry (100 mL) was added dropwise over 30 minutes. The
reaction
was quenched with saturated NH4C1 solution (160 mL) and then partitioned
between water
(250 mL) and ethyl acetate (200 mL). The organic layer was separated. The
aqueous layer
was extracted with one 100-ml portion of ethyl acetate. The combined organic
layers were
washed with one 200-ml portion of brine, dried over anhydrous sodium sulfate,
filtered and
concentrated in vacuo. Purification by flash chromatography with heptane /
ethyl acetate as
eluent gave 4-(2-ethoxy-2-keto-ethylidene)-3-fluoro-piperidine-1-carboxylic
acid tert-butyl
ester (8.51 g, 64%) as colourless oil. MS (ES+) nilz: 188.0 ([M+H-05f1802]+).
Step 2: Preparation of tert-butyl (3 SR,4RS)-4-(2-eth oxy-2-oxo-ethyl)-3 -fl
uoro-pi p eri di ne-
1-carb oxylate and tert-butyl (3 SR,4 SR)-4-(2-ethoxy-2-oxo-ethyl)-3 -flu oro-
p i p eri dine-1-
carboxyl ate
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
F
0
0 0
In a 2 L round-bottomed flask, a solution of 4-(2-ethoxy-2-keto-ethylidene)-3-
fluoro-
piperidine-1-carboxylic acid tert-butyl ester (8.51 g, 29.6 mmol, 1 eq.) in
ethyl acetate (567
mL) was degassed by three vacuum / argon cycles. Addition of palladium on
charcoal 10%
(3.15 g, 2.96 mmol, 0.100 eq.). The reaction mixture was back-filled with
hydrogen stirred
under a hydrogen atmosphere (1 bar, RT) for 1 h. The catalyst was removed by
filtration
over a pad of Decalite. The filtrate was evaporated in vacuo. Purification by
flash
chromatography with methyl tert-butylether / heptane gave tert-butyl (3SR,4SR)-
4-(2-
ethoxy-2-oxo-ethyl)-3-fluoro-piperidine-1-carboxylate (2.18 g, 25 %) as a
colourless
viscous oil and tert-butyl (3SR,4RS)-4-(2-ethoxy-2-oxo-ethyl)-3-fluoro-
piperidine-1-
carboxylate (5.87 g, 69 %) as a colourless viscous oil.
Step 3: Preparation of (3SR,4SR)-3-Fluoro-4-(2-hydroxyethyl)piperidine-1-
carboxylic acid
tert-butyl ester
0 0 H
In a flame-dried 500 mL four necked flask, equipped with a thermometer and a
dropping
funnel, (3 SR,4RS)-4-(2-ethoxy-2-oxo-ethyl)-3 -fluoro-piperidine-l-carboxyl
ate (5.87 g,
20.3 mmol, 1 eq) was dissolved in tetrahydrofuran, extra dry (298 mL). 1 M
lithium
aluminum hydride in THF (20.3 mL, 20.3 mmol, 1 eq.) was added over 30 minutes
at 0-5
C. Stirring was continued for 2 h. The reaction mixture was quenched by
subsequent
addition of water (0.770 mL), 2M aqueous NaOH (0.770 mL) and water (2.31 mL).
The
ice-bath was removed and the resulting slurry was stirred overnight. A white
precipitate
was formed, which was removed by filtration. The filter cake was washed with
THF. The
filtrate was evaporated to give the crude title compound (4.72 g, 94%) as
colorless oil.
Step 4: Preparation of (3SR,4RS)-3-Fluoro-4-(2-ketoethyl)piperidine-1-
carboxylic acid
tert-butyl ester
0 ¨0
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
-48 -
To a solution of (3 SR,4RS)-3 -Fl uoro-4-(2-hydroxyethyl)pipefi di ne-l-carb
oxyli c acid tert-
butyl ester (1.83 g, 7.4 mmol, 1 eq.) in dichloromethane (140 mL) was added
1,1,1-
tfis(acetyloxy)-1,1-dihydro-1,2-benzodioxo1-3-(1H)-one (3.14 g, 7.4 mmol, 1
eq.). The
reaction mixture was stirred for 5 h at RT. TBME was added. The solids were
removed by
filtration. The filtrate was concentrated in vacuo. The residue was
partitioned between
ethyl acetate and water. The layers were separated. The aqueous layer was
extracted with
two portions of ethyl acetate. The combined organic layers were washed with
one portion
of brine, dried over anhydrous sodium sulfate and concentrated in vacuo to
give the crude
tilte compound (2 g) as white semisolid which was used in the next step
without further
purification.
Step 5: Preparation of tert-butyl (3SR,4SR)-4-(2-aminothiazol-5-y1)-3-fluoro-
piperidine-1-
carboxyl ate
H2
S-1=1/
0 \ N
In analogy to intermediate 3-15, steps 2 and 3, from tert-butyl (3SR,4RS)-3-
fluoro-4-(2-
oxoethyl)piperidine-1-carboxylate, was produced (3 SR,4SR)-4-(2-aminothiazol-5-
y1)-3-
fluoro-piperidine-1-carboxylate (1.22 g, 28% yield) as a white solid. MS (ES+)
m/z: 302.1
[M+H]+.
Step 6: Preparation of tert-butyl (3R,4R)-4-(2-aminothiazol-5-y1)-3-fluoro-
pipefidine-1-
carboxylate and tert-butyl (3 S,4S)-4-(2-aminothiazol-5 -y1)-3 -fluoro-pipefi
dine-1 -
carboxylate
H2 ,N H2
N 0 I\ N
0
and
From 1.2 g of (3SR,4SR)-4-(2-aminothiazol-5-y1)-3-fluoro-piperidine-1-
carboxylate, using
a chiral SFC separation (column: 5 m, 250*20 mm, chiral amylose-2, and
20%Me0H +
0.2% Et2NH) were obtained tert-butyl (3R,4R)-4-(2-aminothiazol-5-y1)-3-fluoro-
piperidine-1-carboxylate (MS (ES+) m/z: 302.1 [M+H]) and tert-butyl (35,45)-4-
(2-
ami nothi az ol -5 -y1)-3 -fluoro-pi pefi di ne-l-carb oxyl ate (MS (ES+) m/z:
302.1 [M+H]+)
separately as off-white solids.
Intermediate 3-17
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
-49 -
tert-butyl (3SR,4RS)-4-(2-aminothiazol-5-y1)-3-fluoro-piperidine-1-carboxylate
H2
0 \
In analogy to the preparation of the intermediates 3-16, starting from tert-
butyl (3SR,4RS)-
4-(2-ethoxy-2-oxo-ethyl)-3-fluoro-piperidine-1-carboxylate (obtained in step 2
as
intermediate 3-16), the title compound tert-butyl (3 SR,4RS)-4-(2-aminothiazol-
5-y1)-3-
fluoro-piperidine-1-carboxylate (800 mg) was obtained as an off-white solid.
MS (ES+)
m/z: 302.2 [M+H].
Intermediate 3-18
tert-butyl (7RS)-7-(2-aminothiazol-5-y1)-4-azaspiro[2.5joetane-4-earboxylate
)0 F12
In analogy to the preparation of the intermediate 3-16, starting from tert-
butyl 7-oxo-4-
azaspiro[2.5]octane-4-carboxylate, the title compound was prepared tert-butyl
(7RS)-7-(2-
aminothiazol-5-y1)-4-azaspiro[2.5]octane-4-carboxylate as a white solid. MS
(ES+) m/z:
310.2 [M+H].
Intermediates 3-19
tert-butyl rae-(1R,5S)-7-(2-aminothiazol-5-y1)-9-oxa-3-azabicyclo[3.3.11nonane-
3-
carboxylate and tert-butyl rac-(1S,5R)-7-(2-aminothiazol-5-y1)-9-oxa-3-
azabicyclo[3.3.11nonane-3-carboxylate
H2
N 0
0 H
0 \ ________________________________________________________
and
In analogy to the preparation of the intermediate 3-16, starting from tert-
butyl 7-oxo-9-oxa-
3-azabicyclo[3.3.1]nonane-3-carboxylate, the title compounds were prepared,
tert-butyl
rac-(1R,5S)-7-(2-aminothiazol-5-y1)-9-oxa-3-azabicyclo[3.3.1]nonane-3-
carboxylate and
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 50 -
tert-butyl rac-(1S,5R)-7-(2-aminothiazol-5-y1)-9-oxa-3-azabicyclo[3.3.1]nonane-
3-
carboxylate as a white solid. MS (ES+) m/z: 326.1 [M+H].
Intermediate 3-20:
benzyl(1S,5R)-6-(2-aminothiazol-5-y1)-3-azabicyclo[3.1.0]hexane-3-carboxylate
N H 2
S¨(
Cbz¨N
Step 1 (cvclopropanation): In a two-necked round bottom flask 3-pyrroline-l-
carboxylic
acid benzyl ester (2 g, 9.84 mmol), dissolved in anhydrous Et20 (20 mL) was
treated with
Rhodium acetate dimer (22 mg, 0.049 mmol) while stirring at RT. The mixture
was then
treated over the course of 3 hours with a solution of ethyl diazoacetate (1.53
mL, 14.76
mmol) diluted in anhydrous Et20 (10 mL), upon addition the mixture was left
stirring
overnight at RT.
The mixture was diluted with 30 mL Et20, filtered over Celite and the flow
through
evaporated first under house vacuum and then under high vacuum. The brown-
green
residue was taken up in 5 mL CH2C12 loaded onto a 80 g silica cartridge and
purified with
a gradient of EA in heptane: 5 minutes 100% heptane then linear gradient to
60% EA in 35
minutes, flow 40 mL/min, ISCO system to give (1R,5S)-3-azabicyclo[3.1.0]hexane-
3,6-
dicarboxylic acid 03-benzyl ester 06-ethyl ester (890 mg, 32% yield) as a
yellow oil. The
compound is described in: Synlett, 1996 Katherine E. Brighty and Michael J.
Castaldi. The
second diastereomere (520 mg, 19%) formed was separated during the column
chromatography
Step 2 (Ester reduction): To a solution of (1R,5S)-3-azabicyclo[3.1.0]hexane-
3,6-
dicarboxylic acid 03-benzyl ester 06-ethyl ester (890 mg, 3.08 mmol) in
anhydrous TI-IF
(12 mL) cooled to 0 C, lithium Borohydride 2M in THE (6.18 mL, 12.30 mmol)
was
added in three portions upon stirring briefly to RT and then to 65 C for 16
hours. Upon
completion, the reaction was cooled to RT and treated with 7 mL of methanol in
a
dropwise manner. Gas evolution started upon stirring for 15 minutes. The
mixture was
cooled to 0 C and treated carefully with 25 mL of a 1M solution of HC1,
diluted with
diethylether and exctracted. Organic layers were pooled and treated with
Na2SO4, filtered
and purified via silica gel to provide 4(1S,5R)-6-methylo1-3-
azabicyclo[3.1.0]hexane-3-
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 51 -
carboxylic acid benzyl ester (580 mg, 77 % yield) as a yellow oil. MS (ES+)
m/z: non
detectable.
Step 3 (alcohol oxidation): To a solution of 4(1S,5R)-6-methylo1-3-
azabicyclo[3.1.0]hexane-3-carboxylic acid benzyl ester (580 mg, 2.35 mmol) in
anhydrous
DCM (13 mL) cooled to 0 C, Dess- Martin periodinane was added in three
portions (1.19
g, 2.81 mmol) and the mixture was left stirring at the same temperature for 1
hour. Upon
completion, the reaction was cooled to RT, diluted with 30 mL of DCM and 25 mL
of 1 M
NaHCO3, the biphasic mixture was stirred at RT for 10 minutes before adding
another 25
mL of 1 M NaHCO3 and extracted. The water layer was further extracted with 50
mL
DCM, the combined organic layer was washed with 30 mL water and then brine 2 x
25
mL, treated with Na2SO4, filtered and dried under vacuum. The residue was
taken up in
1% Me0H in CH2C12, loaded onto a 40 g silica cartridge, and purified with a
gradient of
EA in heptane (3 minutes 100% heptane, then linear gradient to 100% EA in 7
minutes,
continued 100% EA for another 10 minutes; flow 40 mL/min, ISCO system), to
give
(1R,5S)-6-formy1-3-azabicyclo[3.1.0]hexane-3-carboxylic acid benzyl ester (550
mg, 91%
yield) as a colorless oil. MS (ES+) m/z: non detectable.
Step 4 (Wittig homologation): (Methoxymethyl)triphenylphosphonium chloride
(3.4 g,
9.86 mmol) was suspended in 5 mL of anhydrous THF under Argon atmosphere and
the
suspension cooled to -78 C. Upon stirring for 10 minutes, a 2 M solution of
NaHIVIDS in
TI-IF (5.30 mL, 10.65 mmol) was added to the Wittig salt in a dropwise manner.
The
resulting orange paste was stirred at -78 C for 1.5 hours and then treated
with a solution of
(1R,5S)-6-formy1-3-azabicyclo[3.1.0]hexane-3-carboxylic acid benzyl ester (550
mg, 2.13
mmol) in 5 mL anhydrous THF. The resulting thick suspension was brought to RT
and
stirred for 1.5 hours. Upon completion, the reaction was diluted with 30 mL of
EA, cooled
to 0 'V treated with 25 mL water and stirred for 15 minutes. Upon warimg to
RT, the
mixture is separated and the aqueous layer is extracted with 2 x 30 mL EA, the
combined
organic was washed with 20 mL water and then 20 mL brine , treated with
Na2SO4, filtered
and dried under vacuum. The residue is taken up in CH2C12, loaded onto a 40 g
silica
cartridge and purified with a gradient of EA in heptane: 3 minutes 100%
heptane then
linear gradient to 80% EA in 17 minutes, flow 30 mL/min, ISCO system to give
(1S,5R)-
6-[(E)-2-methoxyviny1]-3-azabicyclo[3.1.0]hexane-3-carboxylic acid benzyl
ester (370 mg,
64% yield) as a colorless oil. 1H NMR shows a 3:1 mixture of geometirc
isomers.
Step 5 (Enol ether hydrolysis): To a solution of (1S,5R)-6-[(E)-2-
methoxyviny1]-3-
azabicyclo[3.1.0]hexane-3-carboxylic acid benzyl ester (650 mg, 2.38 mmol) in
reagent
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 52 -
grade Acetone (15 mL) cooled to 0 C, 4 mL of 25% w/vol aqueous HC1 were added
dropwise and the mixture left stirring at the same temperature for 30 minutes.
Upon
completion, the mixture was poured into 50 mL of NaFIC03(sai), diluted with 75
mL EA,
stirred for 15 minutes and extracted. The organic layer was washed with 30 mL
of
NaHCO3(sat) and then 30 mL brine, treated with Na2SO4, filtered and dried
under vacuum.
The residue was taken up in 8 mL CH2C12, loaded onto a 40 g silica cartridge
and purified
with a gradient of EA in heptane (3 minutes 100% heptane, then linear gradient
to 80% EA
in 17 minutes, continued with 80% EA for another 5 minutes; flow 35 mL/min,
ISCO
system) to give (1R, 5 S)-6-(2-ketoethyl)-3 -azabi cycl o [3 .1. 0] hexane-3 -
carboxyli c acid
.. benzyl ester (570 mg, 93% yield) as a colorless oil.
Step 6 (a-chlorination/thiazole formation): To a solution of (1R,5S)-6-(2-
ketoethyl)-3-
azabicyclo[3.1.0]hexane-3-carboxylic acid benzyl ester (570 mg, 2.20 mmol) in
anhydrous
DCM (6 mL) cooled to 0 C, N-Chlorosuccinimide was added in one portion (308
mg,
2.31 mmol). The mixture is stirred at the same temperature for 10 minutes
before being
treated with L-Proline (50.6 mg, 0.44 mmol), the resulting suspension is
stirred at 0 C for
15 minutes then brought to RT and stirred for 3 hours. Upon completion, the
reaction was
quenched with 25 mL of 0.1 M NaHCO3, the mixture separated and the DCM layer
treated
with Na2SO4, filtered and dried under vacuum.
The crude chloro-aldehyde residue was taken up in anhydrous Me-TI-IF (10 mL),
treated
with water (800 [IL, 44 mmol), N-methylmorpholine (360 tiL, 3.3 mmol),
thiourea (670
mg, 8.8 mmol), and the mixture heated to 84 'V with vigorous stirring. Upon
overnight
stirring, the mixture was cooled to RT, diluted with 50 mL of Me-TI-IF and 30
mL water,
extracted and the organic layer back washed with a second portion of water (30
mL). The
organic layer was dried in vacuo and the yellow resiude taken up in 6 mL DCM
(precipitation occours), treated with 1 mL 7N ammonia in Me0H and loaded onto
a 40 g
silica cartridge and purified with a gradient of Me0H in DCM: 5 minutes 100%
DCM then
linear gradient to 20% Me0H in 25 minutes, flow 40 mL/min, ISCO system to give
(1R,5S)-6-(2-aminothiazol-5-y1)-3-azabicyclo[3.1.0]hexane-3-carboxylic acid
benzyl ester
(320 mg, 46% yield) as a yellow powder. MS (ES+) m/z: 316.2 [(M+H)].
Synthesis of intermediates 5
Intermediate 5-1
tert-butyl 4- [5-oxo-7-(p-tolyls ulfonyloxy)thiazolo [3,2-a] pyrimidin-2-yll
piperazine-1-
carboxylate
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 53 -
CZ+ 1.11
0
0)z\
Step 1: Preparation of tert-butyl 4-(7-hydroxy-5-oxo-thiazolo[3,2-a]pyrimidin-
2-
yl)piperazine-1-carb oxyl ate
A suspension of 4-(2-aminothiazol-5-yl)piperazine-1-carboxylic acid tert-butyl
ester
(intermediate 3-1, 3.30 g, 11.6 mmol) and commercially available malonic acid
bis(2,4,6-
trichlorophenyl) ester (5.37 g, 11.6 mmol) in toluene was heated at reflux for
2 h. The
reaction mixture was allowed to return to RT and stirred for 1 h. The
resulting suspension
was collected by filtration, washed with three 10-mL portions of toluene and
dried in
vacuo to give the title compound (3.38 g, 83%) as light brown solid. MS (ES+)
m/z: 353.2
[(M+H)+].
Step 2: Preparation of tert-butyl 445-oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-
a]pyrimidin-
2-yl]piperazine-l-carboxylate
To a solution of tert-butyl 4-(7-hydroxy-5-oxo-thiazolo[3,2-a]pyrimidin-2-
yppiperazine-1-
carboxylate (3.38 g, 9.59 mmol) and Et3N (1.94 g, 2.67 mL, 19.18 mmol) in
CH2C12 (96
mL) was added p-toluenesulfonyl chloride (2.19 g, 11.51 mmol) at RT and
stirred for 16 h.
The mixture was washed successively with a 200-mL solution of 2% aqueous HC1,
a 200-
ml solution of 2% aqueous sodium hydroxide followed by 200 mL of water. The
organic
layer was directly concentrated in vacuo. The residue was triturated in wai __
iii ethyl acetate /
n-heptane (2:1,75 ml). The precipitate was collected by filtration, washed
with three
25 ml-portions of ethyl acetate / n-heptane (2:1) and dried in vacuo to give
the title
compound (3.87 g, 80%) as light brown solid. MS (ES+) m/z: 507.3 [(M+H) ].
Intermediate 5-2
tert-butyl 4-[5-oxo-7-(p-tolyls ulfonyloxy)thiazolo[3,2-a]pyrimidin-2-y11-3,6-
dihydro-
2H-pyridine-1-carboxylate
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 54 -
11110
RS
0 0
\>--NO¨cci
Ov_
In analogy to the preparation of the intermediate 5-1, from tert-butyl 4-(2-
aminothiazol-5-
y1)-3,6-dihydro-2H-pyridine-1-carboxylate (intermediate 3-2) was prepared the
title
compound (120 mg) as a white solid. MS (ES+) m/z: 504.2 [(M+H)+].
Intermediate 5-3
tert-butyl 2,2-dimethy1-445-oxo-7-(p-tolylsulfonyloxy)thiazolo13,2-alpyrimidin-
2-yll
piperazine-l-carboxylate
RI% 1101
0 s 0
0)¨ 1,45
In analogy to the preparation of the intermediate 5-1, from tert-butyl 4-(2-
aminothiazol-5-
y1)-2,2-dimethyl-piperazine-1-carboxylate (intermediate 3-3) was prepared the
title
compound (600 mg) as an orange solid. MS (ES+) m/z: 535.3 [(M+H)+].
Intermediate 5-4
tert-butyl 745-oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-alpyrimidin-2-y11-4,7-
diazaspiro12.51 octane-4-carboxylate
0 01
0 77--\ 0-1
0
)-\
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 55 -
In analogy to the preparation of the intermediate 5-1, from tert-butyl 7-(2-
aminothiazol-5-
y1)-4,7-diazaspiro[2.5]octane-4-carboxylate (intermediate 3-4) was prepared
the title
compound (300 mg) as an orange solid. MS (ES+) m/z: 533.3 [(M+H)+].
Intermediate 5-5
tert-butyl (2S)-2-methy1-445-oxo-7-(p-tolylsullonyloxy)thiazolo13,2-
alpyrimidin-2-
yllpiperazine-1-carboxylate
11110111
0 6
0
In analogy to the preparation of the intermediate 5-1, from tert-butyl (2S)-4-
(2-
aminothiazol-5-y1)-2-methyl-piperazine-1-carboxylate (intermediate 3-5) was
prepared the
title compound (469 mg) as a brown solid. MS (ES+) m/z: 521.3 [(M+H)-].
Intermediate 5-6
tert-butyl (2R)-2-methy1-4-15-oxo-7-(p-tolylsulfonyloxy)thiazolo13,2-
alpyrimidin-2-
yllpiperazine-l-carboxylate
0
IN
0
In analogy to the preparation of the intermediate 5-1, from tert-butyl (2R)-4-
(2-
aminothiazol-5-y1)-2-methyl-piperazine-1-carboxylate (intermediate 3-6) was
prepared the
title compound (410 mg) as an orange solid. MS (ES+) m/z: 521.3 [(M+H)+].
Intermediate 5-7
tert-butyl 2,6-dimethy1-4-15-oxo-7-(p-tolylsulfonyloxy)thiazolo13,2-
alpyrimidin-2-
yllpiperazine-1-carboxylate
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 56 -
I:1k fasi
0 N> cc
N 0
)-N -i
0,()__/
0
In analogy to the preparation of the intermediate 5-1, from tert-butyl 4-(2-
aminothiazol-5-
y1)-2,6-dimethyl-piperazine-1-carboxylate (intermediate 3-7) was prepared the
title
compound (160 mg) as an orange solid. MS (ES+) m/z: 535.4 [(M+H)].
Intermediate 5-8
[5-oxo-2-[(8aS)-3,4,6,7,8,8a-hexahydro-1H-pyrrolo[1,2-a[pyrazin-2-
yllthiazolo[3,2-
alpyrimidin-7-yl] 4-methylbenzenesulfonate
9.% (11101
H Orli
0
In analogy to the preparation of the intemediate 5-1, from 5-[(8aS)-
3,4,6,7,8,8a-
hexahydro-1H-pyrrolo[1,2-alpyrazin-2-yl]thiazol-2-amine (intermediate 3-8) was
prepared
the title compound (200 mg) as an orange solid. MS (ES+) nez: 447.6 [(M+H)].
Intermediate 5-9
tert-butyl 8-15-oxo-7-(p-tolylsulfonyloxy)thiazolo13,2-alpyrimidin-2-y11-3,8-
diazabicyclo13.2.1]octane-3-carboxylate
R% 1111
0
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 57 -
In analogy to the preparation of the intermediate 5-1, from tert-butyl 8-(2-
aminothiazol-5-
y1)-3,8-diazabicyclo[3.2.1]octane-3-carboxylate (intermediate 3-9) was
prepared the title
compound (150 mg) as an orange solid. MS (ES+) m/z: 533.4 [(M+H)+].
Intermediate 5-10
[5-oxo-2-1(8aR)-3,4,6,7,8,8a-hexahydro-113-pyrrolo[1,2-alpyrazin-2-
yllthiazolo[3,2-
alpyrimidin-7-yll 4-methylbenzenesulfonate
cy-L\
01 1
,e.N*14
0
In analogy to the preparation of the intermediate 5-1, from 5-[(8aR)-
3,4,6,7,8,8a-
hexahydro-1H-pyrrolo[1,2-a]pyrazin-2-yl]thiazol-2-amine (intermediate 3-10)
was
prepared the title compound (150 mg) as an orange solid. MS (ES+) m/z: 447.6
[(M+H)+].
Intermediate 5-11
tert-butyl (1S,4S)-5-[5-oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-
y11-2,5-
diazabicyclo[2.2.1]heptane-2-carboxylate
C3i.N
H OS
js.--rifirTµ0
0
In analogy to the preparation of the inteunediate 5-1, from tert-butyl (1S,4S)-
5-(2-
aminothiazol-5-y1)-2,5-diazabicyclo[2.2.1]heptane-2-carboxylate (intermediate
3-11) was
prepared the title compound (316 mg) as a brown solid. MS (ES+) m/z: 519.3
[(M+H)+].
Intermediate 5-12
tert-butyl 2-[5-oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-y11-
1,3,3a,4,6,6a-
hexahydropyrrolo[3,4-c]pyrrole-5-carboxylate
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 58 -
s N Cr:01 1 N
flikWi*TC,,Y.
0
In analogy to the preparation of the intermediate 5-1, from tert-butyl 2-(2-
aminothiazol-5-
y1)-1,3,3a,4,6,6a-hexahydropyrrolo[3,4-c]pyrrole-5-carboxylate (intermediate 3-
12) was
prepared the title compound (200 mg) as a brown solid.
Intermediate 5-13
[5-oxo-2-1(3S)-3-pyrrolidin-1-ylpyrrolidin-1-yl]thiazolo[3,2-alpyrimidin-7-yl]
4-
methylbenzenesulfonate
0
In analogy to the preparation of the intermediate 5-1, from 5-[(3S)-3-
pyrrolidin-1-
ylpyrrolidin-l-yl]thiazol-2-amine (intermediate 3-13) was prepared the title
compound
(394 mg) as a brown solid. MS (ES+) nilz: 461.2 [(M+H)+].
Intermediate 5-14
tert-butyl 2-15-oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-y11-2,8-
diazaspiro
-14.51decane-8-carboxylate
*
dpi
In analogy to the preparation of the intermediate 5-1, from tert-butyl 2-(2-
aminothiazol-5-
y1)-2,8-diazaspiro[4.5]decane-8-carboxylate (intermediate 3-14) was prepared
the title
compound (118 mg) as a brown solid. MS (ES+) in/z: 561.3 [(M+H)+].
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 59 -
Intermediate 5-15
tert-butyl 445-oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-alpyrimidin-2-
yllpiperidine-1-
carboxylate
11011
Or-1
0
In analogy to the preparation of the intermediate 5-1, from tert-butyl 4-(2-
aminothiazol-5-
yl)piperidine-1-carboxylate (intermediate 3-15) was prepared the title
compound (100 mg)
as a brown solid. MS (ES+) m/z: 506.4 [(M+H)+]
Intermediate 5-16
tert-butyl 4-hydroxy-4-15-oxo-7-(p-tolylsulfonyloxy)thiazolo13,2-alpyrimidin-2-
yllpiperidine-1-carboxylate
11
0 H 0
0
In analogy to the preparation of the intermediate 5-1, from tert-butyl 4-(2-
aminothiazol-5-
1 5 y1)-4-hydroxy-piperidine-1-carboxylate (which can be prepared in
analogy to intermediate
3-2) was prepared the title compound (310 mg) as a white solid. MS (ES+) m/z:
522.2
[(M+H)+].
Intermediates 5-17
tert-butyl (3R,4R)-3-fluoro-4-15-oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-
a]pyrimidin-2-
yllpiperidine-1-carboxylate and tert-butyl (35,4S)-3-fluoro-4-15-oxo-7-(p-
tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-yllpiperidine-1-carboxylate
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
-60
= s N
0,i? /a
_________________________________________________________ ,s,rõN.õ? 11*
)..nua,) <
0
I I 0
TI
0 0
and
In analogy to the preparation of the intermediate 5-1, the title compounds
were separately
prepared as an off-white solid from tert-butyl (3R,4R)-4-(2-aminothiazol-5-y1)-
3-fluoro-
piperidine-1-carboxylate and tert-butyl (3S,4S)-4-(2-aminothiazol-5-y1)-3-
fluoro-
piperidine-l-carboxylate respectively (intermediates 3-16). MS (ES+) m/z:
524,2 [(M+H)+]
for each enantiomer.
Intermediates 5-18
tert-butyl (3S,4R)-3-11uoro-445-oxo-7-(p-tolylsulfonyloxy)thiazolo13,2-al
pyrimidin-2-
yllpiperidine-1-carboxylate and tert-butyl (3R,4S)-3-fluoro-4-15-oxo-7-(p-
tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-yllpiperidine-1-carboxylate
0,9 =
' S 0
11 0
0
and
Step 1: In analogy to the preparation of the intermediate 5-1, from tert-butyl
(3SR,4RS)-4-
(2-aminothiazol-5-y1)-3-fluoro-piperidine-1-carboxylate (intermediates 3-17)
was
produced tert-butyl (3 SR,4RS)-3-fluoro-445-oxo-7-(p-
tolylsulfonyloxy)thiazolo[3,2-
a]pyrimidin-2-yl]piperidine-1-carboxylate (155 mg) as an off-white solid.
Step 2: This racemic mixture was subjected to a chiral SFC separation (column:
5 p.m,
250*20 mm, chiral IB column, 25%Me0H) yielding tert-butyl (35,4R)-3-fluoro-4-
[5-oxo-
7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-yl]piperidine-1-carboxylate
and tert-butyl
(3R,4S)-3-fluoro-445-oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-
yl]piperidine-
1-carboxylate (68 and 66 mg). MS (ES+) m/z: 524.2 [(M+H)+] for each
enantiomer.
Intermediates 5-19
tert-butyl (7S)-7-15-oxo-7-(p-tolylsulfonyloxy)thiazolo13,2-alpyrimidin-2-y11-
4-
azaspiro[2.5loctane-4-carboxylatecarboxylate and tert-butyl (7R)-7-15-oxo-7-(p-
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
-61 -
tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-y11-4-azaspiro[2.5]octane-4-
carboxylatecarboxylate
c:0 0,
s?
)70,
04 ________ p....*\_ry ),0 s
0 ________ Ny-
0 and 0
Step 1: hi analogy to the preparation of the intermediate 5-1, from racemic
tert-butyl 7-(2-
aminothiazol-5-y1)-4-azaspiro[2.5]octane-4-carboxylate (inteitnediate 3-18)
was prepared
the racemic tert-butyl (7)-745-oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-
a]pyrimidin-2-y1]-4-
azaspiro[2.5]octane-4-carboxylatecarboxylate (133 mg) as a white solid. MS
(ES+) m/z:
532.2 [(M+H) ]
Step 2: The two enantiomeric title compounds have further been separated by
chiral SFC
(chiral AD-H column, 5 gm, 250*20 mm, 45%Me0H + 0.2% Et2NH) yielding tert-
butyl
(7S)-745-oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-y1]-4-
azaspiro[2.5]octane-
4-carboxylatecarboxylate and tert-butyl (7R)-745-oxo-7-(p-
tolylsulfonyloxy)thiazolo[3,2-
a]pyrimidin-2-y1]-4-azaspiro[2.5]octane-4 carboxylatecarboxylate as white
solids. MS
(ES+) m/z: 532.2 [(M+H)+] for each enantiomer.
Intermediates 5-20
tert-butyl rac-(1R,5S)-7-[5-oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-
2-y1]-9-
oxa-3-azabicyclo[3.3.1]nonane-3-carboxylate and tert-butyl rac-(1S,5R)-7-[5-
oxo-7-
(p-tolylsulfonyloxy)thiazolo[3,2-alpyrimidin-2-y1]-9-oxa-3-
azabicyclo[3.3.11nonane-3-
carboxylate
0,p 0,fik
0
NµrY)11
0 \ _____________________________ 0 N\ 0) \
0
and
In analogy to the preparation of intermediates 5-1, from intermediates 3-19
the title
compounds were prepared (2.14 g) as a white foam. MS (ES+) m/z: 548.2
[(M+H)+].
Intermediates 5-21
(1R,5S)-6-(5-keto-7-tosyloxy-thiazolo[3,2-alpyrimidin-2-y1)-3-
azabicyclo[3.1.01hexane-3-carboxylic acid benzyl ester
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
-62 -
q
.õ0
Cbz¨N.,\,XH 0
In analogy to the preparation of the intermediate 5-1, from benzyl(1S,5R)-6-(2-
aminothiazol-5-y1)-3-azabicyclo[3.1.0]hexane-3-carboxylate (intermediate 3-20)
was
prepared the title compound (280 mg) as a white solid. MS (ES+) m/z: 538.2
[(M+H)+].
Preparation of boronic esters 6
Boronic ester 6-1
2,8-dimethy1-6-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-yl)imidazo[1,2-
b]pyridazine
\50c7
0
Boronic ester 6-1 can be prepared according to W02019/057740
Boronic ester 6-2
4-fluoro-2-methyl-6-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-y1)-1,3-
benzoxazole
0 4111/ N
0
Boronic ester 6-2 can be prepared according to W02013/119916
Boronic ester 6-3
2-methyl-6-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-yl)imidazo11,2-
bipyridazine
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
-63 -
...rrr. ......r.spi%1/4
0......B..... 'L...i.re.--14 \c.
Ct
Boronic ester 6-3 can be prepared according to W02015/173181
Boronic ester 6-4
2-methy1-6-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-y1)-8-
(trifluoromethyl)imidazo[1,2-1131pyridazine
F
FZFr
........ .....N
A
Step 1: Preparation of 6-chl oro-2-m ethyl -8-(trifluorom ethyl )imi dazo 1-
1,2-blpyri dazi ne :
In a 10 mL round bottom flask, equipped with a magnetic strrer bar, reflux
condenser and
N2-inlet bubbler, [6-chloro-4-(trifluoromethyppyridazin-3-yl]amine (94 mg,
0.476 mmol)
and pyridinium p-toluene sulfonate (11.9 mg, 0.048 mmol) were combined with
isopropanol (2 mL). 1-bromo-2,2-dimethoxy-propane (104.51 mg, 77.13 uL, 0.571
mmol,
1.2 eq.) was added and the colorless solution was stirred 24 hours at 75 C.
The resulting
dark-brown reaction mixture was cooled to RT, diluted with Et0Ac (10 mL) and
washed
with a saturated aqueous NaHCO3-solution (10 mL). Organic layer was separated
and dried
over sodium sulfate, filtered off and concentrated in vacuo. The crude (120 mg
brownish
viscous oil) was purified by column chromatography yielding 6-chloro-2-methy1-
8-
(trifluoromethyl)imidazo[1,2-13]pyridazine (46 mg, 34% yield) as light yellow
solid. MS
(ES+) m/z: 236.1 [(M+H)+].
Step 2: Preparation of 2-m ethy1-6-(4,4,5,5-tetram ethyl-1,3 ,2-di oxab orol
an-2-y1)-8-
(trifluoromethypimi dazo[1,2-blpyridazine:
In a dry/Argon flushed 20 mL microwave tube with a magnetic stirrer bar and a
cap-
septum, 6-chloro-2-methy1-8-(trifluoromethyl)imidazo[1,2-b]pyridazine (300 mg,
1.2
mmol), bis(pinacolato)diboron (364.7 mg, 1.44 mmol) and potassium acetate
(352.42 mg,
3.59 mmol) were combined with 1,4-dioxane (12 mL). The yellowish fine
suspension was
stirred and degassed with Argon for 10-15 min.
before
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
-64 -
tetrakis(triphenylphosphine)palladium (69.1 mg, 0.060 mmol) was added. The
vial was
sealed and stirred in a heating block (Temp: 100 C) for 22 hours. Further
addition of
tetrakis(triphenylphosphine)palladium (69 mg, 0.060 mmol), after 90 minutes,
3.5 hrs and
6 hrs. The reaction was cooled to room temperature, filtered off and
concentrated in vacuo.
The amber viscous oil was purified by column chromatography to give 2-methy1-6-
(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-y1)-8-(trifluoromethypimidazo[1,2-
b]pyridazine
(428 mg, 48%) as yellow viscous oil.
Boronic ester 6-5
8-ethy1-2-methy1-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-y1)imidazo[1,2-
bipyridazine
)0c77
0
Step 1: Preparation of 8-bromo-6-chl oro-2-m ethyl -imi dazo[1,2-b]pyridazine
To a solution of (4-bromo-6-chloro-pyridazin-3-yl)amine (2000 mg, 9.6 mmol)
and PPTS
(241 mg, 0.96 mmol) in isopropanol (19 mL) was added 1-bromo-2,2-dimethoxy-
propane
(2.11 g, 1.56 mL, 11.5 mmol) at RT. The reaction mixture was heated at reflux
for 30 h.
After cooling down to RT, the mixture was partitioned between ethyl acetate
(50 ml) and
1M Na2CO3 sol (30 m1). The layers were separated, and the organic layer was
washed with
one 30-ml portion of brine and dried over sodium sulfate, filtered and
concentrated in
vacuo to give the crude title compound (2.37 g, 92% yield) as light brown
solid with a
purity of 92%, which was used without further purification. MS (ES+) m/z:
246.0-248.0
[(M+H)+].
Step 2: Preparation of 6-chloro-8-ethy1-2-methyl-imidazoL1,2-blpyridazine:
To a solution of 8-bromo-6-chloro-2-methyl-imidazo[1,2-b]pyridazine (300 mg,
1.22
mmol) and 1.5 M diethylzinc (811 uL, 1.2 mmol) in tetrahydrofuran (4.9 mL) was
added
palladiumtetrakis (70 mg, 0.061 mmol) at RT and stirred for 20 min. The
mixture was
partitioned between ethyl acetate (30 mL) and 1M Na2CO3 sol (30 mL). The
combined
organic layer was washed with one 30-ml portion of brine dried over sodium
sulfate,
filtered concentrated in vacuo. Purification by flash chromatography gave 6-
chloro-8-ethyl-
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
-65 -2-methyl-imidazo[1,2-b]pyridazine (97 mg; 34% yield) as a white solid. MS
(ES+) m/z:
196.0 [(M+H)l.
Step 3: Preparation of 8-ethy1-2-methy1-6-(4,4,5,5-tetramethyl-1,3,2-
dioxaborolan-2-
yl)imi dazo[1,2-b]pyridazine :
To a mixture of 6-chloro-2-methyl-imidazo[1,2-b]pyridazine (100 mg, 0.597
mmol),
bis(pinacolato)diboron (151.5 mg, 0.597 mmol, 1 eq.) and potassium acetate
(150.48 mg,
1.53 mmol) in 1,4-dioxane (1.2 mL) was added of XPhos PdG4 (22 mg, 0.026
mmol). The
reaction mixture was heated at 100 C for 1 h. The reaction mixture was cooled
to RT and
diluted with ethyl acetate (5-10 mL). The solids were removed by filtration.
The filtrate
was concentrated in vacuo to give the crude title compound which was used
directly in the
next step without further purification.
Boronic ester 6-6
8-methoxy-2-methyl-6-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-yl)imidazo11,2-
blpyridazine
0
0
Step 1: Preparation of 6-chloro-8-methoxv-2-methyl-imidazo[1,2-blpvridazine:
To a solution of 8-bromo-6-chloro-2-methyl-imidazo[1,2-b]pyridazine (described
herein
above, 500 mg, 2.03 mmol) and cesium carbonate (1.4 g, 4.3 mmol) in
acetonitrile (10 mL)
was added Me0H (400 uL, 9.89 mmol) at RT and stirring was continued for 4 h.
The
mixture was partitioned between ethyl acetate (30 mL) and water (30 mL). The
combined
organic layer was washed with one 30-mL portion of brine, dried over sodium
sulfate,
filtered and concentrated in vacuo. A purification by flash chromatography
gave 6-chloro-
8-methoxy-2-methyl-imidazo[1,2-b]pyridazine (337 mg; 84% yield) as a white
solid. MS
(ES+) m/z: 198.0 [(M+H) ].
Step 2: Preparation of 8-methoxy-2-methy1-6-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-
yl)imi dazo[1,2-b]pyridazine:
The title compound was prepared from 6-chloro-8-methoxy-2-methyl-imidazo[1,2-
b]pyridazine in analogy to the synthesis of the boronic ester 6-5, step 2.
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
-66 -
Boronic ester 6-7
8-cyclopropy1-2-methy1-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-
yl)imidazo[1,2-
blpyridazine
Step 1: Preparation of 6-chloro-8-cyclopropy1-2-methyl-imidazor1,2-
blpyridazine:
A mixture of 8-bromo-6-chloro-2-methyl-imidazo[1,2-b]pyridazine (prepared
herein
above, 300 mg, 1.2 mmol), cyclopropylboronic acid (209 mg, 2.43 mmol) and
Na2CO3
(387 mg, 3.65 mmol) in 1,4-dioxane (4.9 mL) and water (1.2 mL) was degassed by
three
vacuum / argon cycles followed by the addition of 1,11-
bis(diphenylphosphino)ferrocenedichloro palladium(II) dichloromethane complex
(90.2
mg, 0.122 mmol). The reaction mixture was heated at 90 C and stirred over the
weekend
under an atmosphere of argon. Another portion of cyclopropylboronic acid (209
mg, 2.43
mmol) was added. The mixture was partitioned between ethyl acetate (50 mL) and
an
aqueous 1M solution of NaHCO3 sol (50 m1). The organic layer was washed with
one 50-
mL portion of brine, dried over sodium sulfate, filtered and concentrated in
vacua A
purification by flash chromatography gave 6-chloro-8-cyclopropy1-2-methyl-
imidazo[1,2-
b]pyridazine (64 mg, 15% yield) as light brown solid with a purity of 61%. MS
(ES+) ,n/z:
208.0 [(M+H) ].
Step 2: Preparation of 8-cycl opropyl -2-m ethy1-6-(4,4,5,5-tetram ethyl -1,3
,2-di oxab orol an-
2-yl)imidazo[1,2-blpyridazine:
The title compound was prepared from 6-chloro-8-cyclopropy1-2-methyl-
imidazo[1,2-
b]pyridazine in analogy to the synthesis of the boronic ester 6-5, step 2.
Boronic ester 6-8
8-isopropeny1-2-methy1-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-
yl)imidazo11,2-
blpyridazine
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
-67
Step 1: Preparation of 6-chloro-8-isopropeny1-2-methyl-imidazo[1,2-
b]pyridazine:
A mixture of 8-bromo-6-chloro-2-methyl-imidazo[1,2-b]pyridazine (prepared
herein
above, 300 mg, 1.2 mmol), 2-isopropeny1-4,4,5,5-tetramethy1-1,3,2-
dioxaborolane (245
mg, 274 uL, 1.46 mmol) and K2CO3 (504 mg, 3.65 mmol) in 1,4-dioxane (5.0 mL)
and
water (1.0 mL) was degassed by three vacuum / argon cycles followed by the
addition of
1,11-bis(diphenylphosphino)ferrocenedichloro palladium(11) dichloromethane
complex (90
mg, 0.122 mmol). The reaction mixture was heated at 90 C and stirred over
night under
an atmosphere of argon. The mixture was partitioned between ethyl acetate (50
mL) and
water (50 mL). The organic layer was washed with one 50-mL portion of brine,
dried over
sodium sulfate, filtered and concentrated in vacuo. A purification by flash
chromatography
gave 6-chloro-8-isopropeny1-2-methyl-imidazo[1,2-b]pyridazine (0.180 g, 60%
yield) as
light brown solid. MS (ES+) m/z: 208.1 [(M+H)+].
Step 2: 8-i soprop enyl -2-m ethy1-6-(4,4,5,5-tetram ethy1-1,3,2-di ox ab orol
an-2-
vl)imidazof 1,2-blipyridazine :
The title compound was prepared from 6-chloro-8-isopropeny1-2-methyl-
imidazo[1,2-
b]pyridazine in analogy to the synthesis of the boronic ester 6-5, step 2.
Boronic ester 6-9
2,7-dimethy1-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-yl)oxazolo15,4-
blpyridine
'N Di
¨\(\iciCt
Step 1: Preparation of N-(2,6-dichloro-4-methyl-3-pyridynacetamide:
To a solution of (2,6-dichloro-4-methyl-3-pyridyl)amine (2000 mg, 11.3 mmol)
and Ac20
(11.53 g, 10.66 mL, 112.98 mmol) was added a catalytical amount of DMAP. The
reaction
mixture was stirred for 1 h at RT then over night at 60 C. The reaction
mixture was
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
-68 -
cooled to RT, and ethanol (10 mL, 169.46 mmol) was slowly added and stirring
continued
for 30 min. The solvent were evaporated and residual AcOH was removed by
azeotropic
distillation with one 30-mL portion of toluene. The residue was triturated in
warm ethyl
acetate (15 mL). Heptane (15 mL) was added and the suspension was stirred for
1 h. The
.. precipitate was collected by filtration, washed with two 10 mL-portions of
ethyl acetate / n-
heptane (1:1) and dried in vacuo to give N-(2,6-dichloro-4-methyl-3-
pyridyl)acetamide
(1750 mg, 71% yield) as brown solid. MS (ES+) m/z: 219.1 [(M+H) ],
Step 2: Preparation of 5-chloro-2,7-dimethyl-oxazolo[5,4-blpyridine:
To a solution of N-(2,6-dichloro-4-methyl-3-pyridypacetamide (1750 mg, 7.99
mmol) in
N-methyl-2-pyrrolidinone (16 mL) was added NaH, 55% in oil (348.58 mg, 7.99
mmol) at
RT. The reaction mixture was heated at 120 C and stirred for 20 h. After
cooled down to
RT, acetic acid (959 mg, 914 uL, 15.98 mmol) was slowly added and stirred for
an
additional 30 min. The mixture was partitioned between ethyl acetate (50 mL)
and 1M
NaHCO3 sol (20 mL). The organic layer was washed with one 50-mL portion of
water and
one 30-mL portion of brine, dried over sodium sulfate, filtered and
concentrated in vacuo.
A purification by flash chromatography gave 5-chloro-2,7-dimethyl-oxazolo[5,4-
b]pyridine (85 mg, 6% yield) as a white solid. MS (ES+) m/z: 183.1 [(M+H) ].
Step 3: 2,7-dim ethy1-5 -(4,4,5,5 -tetram ethyl -1,3 ,2-di oxaborolan-2-
yl)oxazol o[5,4-
bbyridine:
The title compound was prepared from 5-chloro-2,7-dimethyl-oxazolo[5,4-
b]pyridine in
analogy to the synthesis of the boronic ester 6-5, step 2.
Boronic ester 6-10
8-isopropy1-2-methy1-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-
yl)imidazo[1,2-
blpyridazine
_\esioszi
0
Step 1: Preparation of 6-chloro-8-isopropy1-2-methyl-imidazo[1,2-blpyridazine:
A solution of 6-chloro-8-isopropeny1-2-methyl-imidazo[1,2-b]pyridazine
(described as
intermediate in the synthesis of the boronic ester 6-8, 150 mg, 0.722 mmol) in
ethyl acetate
(6 mL) was degassed by three vacuum / argon cycles. Addition of platinum (IV)
oxide (16
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
-69 -
mg, 0.072 mmol). The reaction mixture was degassed and purged with hydrogen.
Stirring
was continued for 3 h at RT under an atmosphere of a hydrogen balloon. The
reaction
mixture was diluted with dichloromethane and filtered over a bed of decalite,
then
concentrated in vacuo and give 6-chloro-8-isopropy1-2-methyl-imidazo[1,2-
b]pyridazine
(110 mg, 73% yield) as light yellow oil. MS (ES+) rrt/z: 210.0 [(M+H)+].
Step 2: 8-i sopropy1-2-methy1-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-
yl)imidazo[1,2-
b].pyridazine:
The title compound was prepared from 6-chloro-8-isopropy1-2-methyl-imidazo[1,2-
b]pyridazine in analogy to the synthesis of the boronic ester 6-5, step 2.
Boronic ester 6-11
8-(difluoromethoxy)-2-methy1-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-
yl)imidazo[1,2-b]pyridazine
13)"-F
Step 1: Preparation of 6-chloro-2-methyl-imidazo[1,2-b]pyridazin-8-ol
A suspension of (6-chloro-4-methoxy-pyridazin-3-yl)amine (1765 mg, 11.06
mmol), 1-
bromo-2,2-dimethoxy-propane (2.23 g, 1.64 mL, 12.17 mmol) and PPTS (278 mg,
1.11
mmol) in isopropanol (22 mL) was heated at reflux for 3 days. After cooling
down to RT,
the resulting brown suspension was collected by filtration, washed with three
10-mL
portions of i-PrOH and dried in vacuo to give 6-chloro-2-methyl-imidazo[1,2-
b]pyridazin-
8-ol (1.25 g, 54% yield) as brown solid. MS (ES+) m/z: 184.1 [(M+1-1) ].
Step 2: 6-chloro-8-(difluoromethoxy)-2-methyl-imidazo11,2-blpyridazine
To a solution of 6-chloro-2-methyl-imidazo[1,2-b]pyridazin-8-ol (50 mg, 0.272
mmol) and
sodium chlorodifluoroacetate (83 mg, 0.545 mmol) in N,N-dimethylformamide
(0.91 mL)
was added K2CO3 (113 mg, 0.817 mmol) at RT. The reaction mixture was then
heated at
80 C for lh. The reaction mixture was cooled to RT and water added. The
organic layer
was separated and washed with one 25-ml portion of water and one 25-ml portion
of brine,
then dried over sodium sulfate, filtered and concentrated in vacuo to give 6-
chloro-8-
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 70 -
(difluoromethoxy)-2-methyl-imidazo[1,2-b]pyridazine (34 mg, 45% yield) a a
light brown
solid. MS (ES+) m/z: 234.1 [(M+H)+].
Step 3: 8-(d i fl uorom ethoxy)-2-m ethy1-6-(4,4,5, 5 -tetram ethyl-1,3 , 2-di
ox ab orol an-2-
yl)imi dazo [1,2-1) ]3yri dazine:
The title compound was prepared from 6-chloro-8-(difluoromethoxy)-2-methyl-
imidazo[1,2-b]pyridazine in analogy to the synthesis of the boronic ester 6-5,
step 2.
Boronic ester 6-12
N42-chloro-6-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-y1)-4-(trifluoromethyl)-
3-
pyridylilacetamide
010
I `ir
Step 1: Preparation of N-[2,6-di chl oro-4-(tri fl uorom ethyl)-3 -pyri dyl
acetam i de:
To a solution of [2,6-dichloro-4-(trifluoromethyl)-3-pyridyl]amine (2.0 g,
8.66 mmol) and
AcC1 (5.06 g, 4.59 mL, 64.5 mmol) was added H2SO4 (461 uL, 8.66 mmol) at 0 C.
The
cooling bath was removed and the reaction stirred for 1 hour. The mixture was
poured in
crushed ice and stirred. The resulting precipitate was collected by
filtration, washed with
water and dried in vacuo to give N-[2,6-dichloro-4-(trifluoromethyl)-3-
pyridyl]acetamide
(2.03 g, 86% yield) as white solid. MS (ES+) m/z: 273.1 [(M+H)+].
Step 2: N-[2-chloro-6-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-y1)-4-
(trifluoromethyl)-3-
pyridyljacetami de:
The title compound was prepared from N-[2, 6-di chloro-4-(tri fl u orom
ethyl)-3 -
pyridyl] acetami de in analogy to the synthesis of the boronic ester 6-5, step
2.
Boronic ester 6-13
2-chloro-8-methy1-6-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-yl)imidazo[1,2-
blpyridazine
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
-71
CI
0
Step 1: Preparation of 6-chloro-8-methyl-imidazo11,2-blpyridazin-2-ol
A mixture of 6-chloro-4-methyl-pyridazin-3-amine (1.15 g, 8.0 mmol), methyl
bromoacetate (1.35 g, 8.8 mmol) and PPTS (0.200 g, 0.80 mmol) in 1-butanol (26
mL)
was heated at reflux for 6 hours. After cooling down to RT, the resulting
suspension was
collected by filtration, washed with iPrOH and dried under vacuo, yielding the
title product
(730 mg, 47% yield) as a light green solid. MS (ES+) in/z: 184.1 [(M+H)-].
Step 2: Preparation of 2,6-dichloro-8-methyl-imidazo[1,2-blpyridazine
6-chloro-8-methyl-imidazo[1,2-b]pyridazin-2-ol (663 mg, 3.6 mmol) in POC13
(6.7 mL,
72.2 mmol) was heated at reflux for 24 hours. After cooling down to RT, the
volatiles were
removed under vacuo, and the residue partionned between Et0Ac and an aqueous
saturated solution of NaHCO3. The organic phase was collected, washed with
brine, dried
over Na2SO4 and concentrated under vacuo. A column chromatography afford the
title
compound (129 mg, 18% yield) as a light brown solid. MS (ES+) m/z: 201.9
[(M+H) ].
Step 3: Preparation of 2-chl oro-8-m ethy1-6-(4,4,5,5 -tetram ethyl -1,3 ,2-di
ox ab orol an-2-
yl)imi dazo[1,2-b]pyri dazine
The title compound was prepared from 2,6-dichloro-8-methyl-imidazo[1,2-
b]pyridazine in
analogy to the synthesis of the boronic ester 6-5, step 2.
General procedures
1) Suzuki cross coupling reaction between a tosylate intermediate of general
formula 5 and
a boronic ester/acid 6 (as prepared herein above):
To a solution of an intermediate 5 in 1,4-dioxane, was added 1.5 equivalent of
a boronic
ester intermediate 6 as a 0.33 M solution in CH3CN and 4.0 equivalent of K2CO3
delivered
as a 2 M aqueous solution. As a solvent either 1,4-dioxane, CH3CN, water or a
mixture
thereof were used. The reaction mixture was degased with Argon for 5 minutes
and a
palladium catalyst [either Pd(dppf)C12-CH2C12 (0.2 eq. CAS#95464-05-4) or
XPhos PdG4
(CAS#1599466-81-5)] was added. The reaction mixture was heated at 90 C until
completion (usually between 2 and 8 hours), cooled to room temperature,
filtered over a
pad of Celite preconditioned with CH3CN, washed with CH3CN and concentrated in
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 72 -
vacua A purification was performed either by column chromatography or reverse
phase
preparative HPLC to afford the desired product.
2) General procedure for the Boc protecting group removal:
To a solution of a boc-protected derivative of the compound of formula (I) in
CH2C12 (2
mL) was added TFA (2 mL) and the reaction mixture was stirred at room
temperature until
completion, usually between 1 and 3 hours. The mixture was then evaporated in
vacuo and
purified via reverse phase preparative HPLC, using a ACN / Water + 0.1% Et3N
gradient
on a Gemini NX, 12 nm, 5 rim, 100 x 30 mm column to afford the compound of
formula
Example 1
7-(2,8-dimethylimidazo11,2-b]pyridazin-6-y1)-2-piperazin-1-yl-thiazolo[3,2-
a]pyrimidin-5-one
s N
HN I N
\__/ = N
0
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 4-[5-
oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-yl]piperazine-1-
carboxylate
(intermediate 5-1) and
2,8-dim ethyl -6-(4,4, 5,5 -tetram ethyl -1,3,2-di ox ab orol an-2-
yl)imidazo[1,2-b]pyridazine (boronic ester 6-1) gave tert-butyl 44742,8-
dimethylimidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-
yl]piperazine-1-
carboxylate. MS (ES+) m/z: 482.4 [(M+H)+].
Step 2: A deprotection using the general procedure 2, gave 7-(2,8-
dimethylimidazo[1,2-
b]pyridazin-6-y1)-2-piperazin-1-yl-thiazolo[3,2-a]pyrimidin-5-one (20 mg) as a
light
yellow solid. MS (ES+) m/z: 382.1 [(M+H)+].
Example 2
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 73 -
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-(1,2,3,6-tetrahydropyridin-4-
yl)thiazolo[3,2-a]pyrimidin-5-one
-doe"
H C.N -- I
\
0
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 4-[5-
oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-y1]-3,6-dihydro-2H-
pyridine-1-
carboxylate (intermediate 5-2) and 2,8-dimethy1-6-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-y1)imidazo[1,2-b]pyridazine (boronic ester 6-1) gave tert-butyl
44742,8-
dimethylimidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a] pyrimidin-2-y1]-
3,6-dihydro-
2H-pyridine-1-carboxylate. MS (ES+) m/z: 479.3 [(M+H)+].
Step 2: A deprotection using the general procedure 2, gave 7-(2,8-
dimethylimidazo[1,2-
b]pyridazin-6-y1)-2-(1,2,3,6-tetrahydropyridin-4-yl)thi azolo[3,2-a]pyrimi din-
5-one (20 mg)
as a light yellow solid. MS (ES+) m/z: 379 [(M+H)+].
Example 3
7-(2,8-dimethylimidazo[1,2-blpyridazin-6-yl)-2-(3,3-dimethylpiperazin-1-
yl)thiazolo[3,2-alpyrimidin-5-one
N
---e N`
0
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 2,2-
di methy1-4-[5-oxo-7-(p-tolylsulfonyl oxy)thiazol o [3 ,2-a] pyri mi di n-2-
yl] piperazine-1-
carboxylate (intermediate 5-3) and 2,8-dimethy1-6-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-y1)imidazo[1,2-13]pyridazine (boronic ester 6-1) gave tert-
butyl 4-[7-(2,8-
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 74 -
dimethylimidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-y1]-2,2-
dimethyl-
piperazine-1-carboxylate (478 mg, 55% yield) as a yellow solid. MS (ES+) m/z:
510.3
[(M+H)+].
Step 2: A deprotection using the general procedure 2, gave 7-(2,8-
dimethylimidazo[1,2-
b]pyridazin-6-y1)-2-(3,3-dimethylpiperazin-1-yl)thiazolo[3,2-a]pyrimidin-5-one
(71 mg.
42% yield) as a light yellow solid. MS (ES+) m/z: 410.3 [(M+H)+].
Example 4
2-(4,7-diazaspiro [2.5] octan-7-y1)-7-(2,8-dimethylim id azo [1,2-b] pyridazin-
6-
1 0 yl)thiazolo [3,2-a] pyrimidin-5-one
8
HN N¨Cr14\ I
0
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 7-[5-
oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-y1]-4,7-diazaspiro[2.51
octane-4-
carboxylate (intermediate 5-4) and 2,8-dimethy1-6-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-yl)imidazo[1,2-b]pyridazine (boronic ester 6-1) gave tert-butyl
74742,8-
dimethylimidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a] pyrimidin-2-y1]-
4,7-
diazaspiro[2.5]octane-4-carboxylate (50 mg) as a yellow solid. MS (ES+) m/z:
508.5
[(M 1-1) ]-
Step 2: A deprotection using the general procedure 2, gave 2-(4,7-
diazaspiro[2.5]octan-7-
y1)-7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-yl)thiazolo[3,2-a]pyrimidin-5-one
(40 mg)
as a light yellow solid. MS (ES+) m/z: 408.2 [(M+H)+].
Example 5
7-(4-fluoro-2-methy1-1,3-benzoxazol-6-y1)-2-piperazin-1-yl-thiazolo[3,2-
alpyrimidin-
5-one
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 75 -
N
0
HN N I
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 4-[5-
oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-yl]piperazine-1-
carboxylate
(intermediate 5-1) and 4-fluoro-2-m ethyl -6-(4,4,5,5-tetram ethyl -1,3 ,2-di
ox ab orol an-2-y1)-
1,3-benzoxazole (boronic ester 6-2) gave tert-butyl 4-[7-(4-fluoro-2-methy1-
1,3-
benzoxazol-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-yl]piperazine-1-carboxylate
(26 mg,
12% yield) as a yellow solid. MS (ES+) m/z: 486.2 [(M+H)+].
Step 2: A deprotection using the general procedure 2, gave 7-(4-fluoro-2-
methy1-1,3-
benzoxazol-6-y1)-2-piperazin-1-yl-thiazolo[3,2-a]pyrimidin-5-one (143 mg, 82%
yield) as
a light yellow solid. MS (ES+) m/z: 386.2 [(M+H)].
Example 6
7-(2,8-dimethylimidazo[1,2-131pyridazin-6-y1)-2-1(3S)-3-methylpiperazin-1-
yl]thiazolo[3,2-alpyrimidin-5-one
N-
HN N _________ S\ N I
0
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl (2S)-2-
methy1-4-[5-oxo-7-(p-tolylsulfonyl oxy)thiazolo [3 ,2 -a] pyrimi din-2-yl]
piperazine-1-
carb ox ylate (intermediate 5-5) and
2,8-dimethy1-6-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-y1)imidazo[1,2-13]pyridazine (boronic ester 6-1) gave tert-
butyl (2S)-4-[7-
(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-
y1]-2-
methyl-piperazine-1-carboxylate (578 mg) as a yellow solid. MS (ES+) ,n/z:
496.3
[(M+H)+].
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 76 -
Step 2: A deprotection using the general procedure 2, gave 7-(2,8-
dimethylimidazo[1,2-
b]pyridazin-6-y1)-2-[(3S)-3-methylpiperazin-1-yl]thiazolo[3,2-a]pyrimidin-5-
one (128 mg,
28% yield) as a light yellow solid. MS (ES+) m/z: 396.3 [(M+H)].
Example 7
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-1(3R)-3-methylpiperazin-1-
yllthiazolo[3,2-a]pyrimidin-5-one
N
FIN\
0
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl (2R)-2-
methy1-4-[5-oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-yl]piperazine-
1-
carboxylate (intermediate 5-6) and 2,8-dimethy1-6-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-y1)imidazo[1,2-13]pyridazine (boronic ester 6-1) gave tert-
butyl (2R)-447-
(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-
y1]-2-
methyl-piperazine-1-carboxylate (378 mg) as a yellow solid. MS (ES+) m/z:
496.3
[(W-HY] .
Step 2: A deprotection using the general procedure 2, gave 7-(2,8-
dimethylimidazo[1,2-
b]pyridazin-6-y1)-243R)-3-methylpiperazin-1-ylithiazolo[3,2-a]pyrimidin-5-one
(129 mg,
41% yield) as a light yellow solid. MS (ES+) m/z: 396.3 [(M+H)+].
Example 8
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-(3,5-dimethylpiperazin-1-
y1)thiazolo [3,2-a] pyrimid in-5-one
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 77 -
s
HN) i
0
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 2,6-
dimethy1-4-[5-oxo-7-(p-tolylsulfonyl oxy)thiazol o pyrimi din-2-
yl]piperazine-1-
carboxylate (intel ___________________________________________________________
mediate 5-7) and 2,8-dimethy1-6-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-yl)imidazo[1,2-b]pyridazine (boronic ester 6-1) gave tert-butyl
4-[7-(2,8-
dimethylimidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-y1]-2,6-
dimethyl-
piperazine-1-carboxylate (270 mg) as a yellow solid. MS (ES+) nilz: 510.4
[(M+H)+].
Step 2: A deprotection using the general procedure 2, gave 7-(2,8-
dimethylimidazo[1,2-
b]pyridazin-6-y1)-2-(3,5-dimethylpiperazin-1-yl)thiazolo[3,2-a]pyrimidin-5-one
(65 mg,
30% yield) as a light yellow solid. MS (ES+) m/z: 410.3 [(M+H)+].
Example 9
7-(2,8-dimethylimidazo[1,2-b[pyridazin-6-y1)-2-1(8aS)-3,4,6,7,8,8a-hexahydro-
1H-
pyrrolo11,2-al pyrazin-2-yl]thiazolo [3,2-a] pyrimidin-5-one
CyLm. s )4,1¨
I N
\\,...N I
0
Using the general procedure 1, a cross coupling reaction between [5-oxo-2-
[(8aS)-
3,4,6,7,8,8a-hexahydro-11-1-pyrrolo[1,2-a]pyrazin-2-yl]thiazolo[3,2-
a]pyrimidin-7-yl] 4-
methylbenzene-sulfonate (intermediate 5-8) and 2,8-dimethy1-6-(4,4,5,5-
tetramethy1-1,3,2-
dioxaborolan-2-y1)imidazo[1,2-13]pyridazine (boronic ester 6-1) gave 742,8-
dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(8aS)-3,4,6,7,8,8a-hexahydro-1H-
pyrrolo[1,2-
a]pyrazin-2-yl]thiazolo[3,2-a]pyrimidin-5-one (10 mg, 7% yield) as a yellow
solid. MS
(ES+) m/z: 422.3 [(M+H)+].
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 78 -
Example 10
7-(2-methylimidazo [1,2-b] pyridazin-6-y1)-2-piperazin-1-yl-thiazolo [3,2-a]
pyrimid in-
5-one
s N
, HN N\
0
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 4-[5-
oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-yl]piperazine-1-
carboxylate
(intermediate 5-1) and 2-methyl-6-(4,4,5,5-tetramethyl -1,3 ,2-di
oxab orol an-2-
yl)imidazo[1,2-b]pyridazine (boronic ester 6-3) gave tert-butyl 447-(2-
methylimidazo[1,2-
b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-yl]piperazine-1-carboxylate
(98 mg,
70% yield) as a yellow solid. MS (ES+) m/z: 468.3 [(M+H)l.
Step 2: A deprotection using the general procedure 2, gave 7-(2-
methylimidazo[1,2-
b]pyridazin-6-y1)-2-piperazin-l-yl-thiazolo[3,2-a]pyrimidin-5-one (82 mg) as a
light
yellow solid. MS (ES+) m/z; 368.3 [(M+H)+].
Example 11
742-methy1-8-(trifluoromethyl)imidazo11,2-blpyridazin-6-y1]-2-piperazin-l-yl-
thiazolo13,2-alpyrimidin-5-one
HN\ N
0
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 4-[5-
oxo-7-(p-tolylsulfonyl oxy)thi azol o[3 ,2-a] pyri mi di n-2-y1 ]pi perazi ne-
l-carboxyl ate
(intermediate 5-1) and 2-methyl-6-(4,4,5,5-tetramethy1-1,3 ,2-di oxab
orol an-2-y1)-8-
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 79 -
(trifluoromethyl)imidazo[1,2-b]pyridazine (boronic ester 6-4) gave tert-butyl
44742-
methy1-8-(trifluoromethyl)imidazo[1,2-b]pyridazin-6-y1]-5-oxo-thiazolo[3,2-
a]pyrimidin-
2-yl]piperazine- 1 -carboxylate (42 mg, 57% yield) as a yellow solid. MS (ES+)
m,/z: 536.3
[(M+H)+].
Step 2: A deprotection using the general procedure 2, gave 742-methy1-8-
(trifluoromethypimi dazo[1,2-b]pyridazin-6-y1]-2-piperazin-l-yl-thiazolo[3,2-
a]pyrimidin-
5-one (13 mg, 53% yield) as a light orange solid. MS (ES+) m/z: 436.3 [(M+H)-
].
Example 12
7-(8-ethyl-2-methyl-imidazo[1,2-b] pyridazin-6-y1)-2-piperazin-1-yl-thiazolo
[3,2-
a[pyrimidin-5-one
N
HN _____________________________ tU I
0
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 4-[5-
oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-ylipiperazine-1-
carboxylate
(intermediate 5-1) and 8-ethyl-2-methyl-6-(4,4, 5,5 -tetram ethyl -1,3 ,2-di
oxab orol an-2-
yl)imidazo[1,2-b]pyridazine (boronic ester 6-5) gave tert-butyl 447-(8-ethy1-2-
methyl-
imidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-yl]piperazine-1-
carboxylate (66 mg, 73% yield) as a yellow solid. MS (ES+) m/z: 496.4
[(M+H)+].
Step 2: A deprotection using the general procedure 2, gave 7-(8-ethyl-2-methyl-
imidazo[1,2-b]pyridazin-6-y1)-2-piperazin-1-yl-thiazolo[3,2-a]pyrimi din-5-one
(58 mg,
98% yield) as a light yellow solid. MS (ES+) m/z: 396.3 [(M+H)+].
Example 13
7-(8-methoxy-2-methyl-imidazo [1,2-b] pyridazin-6-y1)-2-piperazin-1-yl-
thiazolo 13,2-
a[pyrimidin-5-one
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 80 -
HN\s_ JN¨ Nicv I
0
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 4-[5-
oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-yl]piperazine-1-
carboxylate
(intermediate 5-1) and 8-methoxy-2-methy1-6-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-
yl)imidazo[1,2-b]pyridazine (boronic ester 6-6) gave tert-butyl 4-[7-(8-
methoxy-2-methyl-
imidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-yl]piperazine-1-
carboxylate (16 mg, 18% yield) as a yellow solid. MS (ES+) m/z: 498.3 [(M+H)-
].
Step 2: A deprotection using the general procedure 2, gave 7-(8-methoxy-2-
methyl-
imidazo[1,2-b]pyridazin-6-y1)-2-piperazin-1-yl-thiazolo[3,2-a]pyrimidin-5-one
(9 mg, 71%
yield) as a light yellow solid. MS (ES+) rn/z: 398.2 [(M+H) ].
Example 14
2-(3,8-diazabicyclo13.2.11octan-8-y1)-7-(2,8-dimethylimidazo11,2-b]pyridazin-6-
yl)thiazolo13,2-a]pyrimidin-5-one
FINcON
I
=
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 8-[5-
oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-y11-3,8-
diazabicyclo[3.2.1]octane-3-
carboxylate (intermediate 5-9) and 2,8-dimethy1-6-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-yl)imidazo[1,2-b]pyridazine (boronic ester 6-1) gave tert-butyl
8-[7-(2,8-
dimethylimidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-y1]-3,8-
diazabicyclo[3.2.1]octane-3-carboxylate (189 mg, 43% yield) as a yellow solid.
MS (ES+)
in/z: 508.4 [(M+H)+].
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 81 -
Step 2: A deprotection using the general procedure 2, gave 2-(3,8-
diazabicyclo[3.2.1]octan-8-y1)-7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-
yl)thiazolo[3,2-
a]pyrimidin-5-one (33 mg, 21% yield) as a light yellow solid. MS (ES+) m/z:
408.3
[(M+H)+].
Example 15
7-(2,8-dimethylimidazo[1,2-b[pyridazin-6-y1)-2-[(8aR)-3,4,6,7,8,8a-hexahydro-
1H-
pyrrolo[1,2-all pyrazin-2-yl]thiazolo [3,2-a] pyrimidin-5-one
N I
0
Using the general procedure 1, a cross coupling reaction between [5-oxo-2-
[(8aR)-
3,4,6,7,8,8a-hexahydro-1H-pyrrolo[1,2-a]pyrazin-2-yl]thiazolo[3,2-a]pyrimidin-
7-yl] 4-
methylbenzene-sulfonate (intermediate 5-10) and 2,8-dimethy1-6-(4,4,5,5-
tetramethy1-
1,3,2-dioxaborolan-2-y1)imidazo[1,2-b]pyridazine (boronic ester 6-1) gave 7-
(2,8-
dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(8aR)-3,4,6,7,8,8a-hexahydro-1H-
pyrrolo[1,2-
a]pyrazin-2-yl]thiazolo[3,2-a]pyrimidin-5-one (12 mg, 8% yield) as a yellow
solid. MS
(ES+) m/z: 422.3 [(M+H)l.
Example 16
7-(2,8-dimethylimidazo[1,2-b[pyridazin-6-y1)-2-1(1S,4S)-2,5-
diazabicyclo 12.2.11heptan-2-ylIthiazolo [3,2-a] pyrimidin-5-one
H r I
0
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 82 -
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl
(IS,4S)-545-oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-y1]-2,5-
diazabicyclo[2.2.1]heptane-2-carboxylate (intermediate 5-11) and 2,8-dimethy1-
6-(4,4,5,5-
tetramethy1-1,3,2-dioxaborolan-2-ypimidazo[1,2-b]pyridazine (boronic ester 6-
1) gave
tert-butyl (1 S,4 S)-547-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-5-oxo-
thiazolo[3 ,2-
a]pyrimidin-2-y1]-2,5-diazabicyclo[2.2.1]heptane-2-carboxylate (211 mg, 45%
yield) as a
yellow solid. MS (ES+) m/z: 494.3 [(M+H)+].
Step 2: A deprotection using the general procedure 2, gave 7-(2,8-
dimethylimidazo[1,2-
b]pyridazin-6-y1)-2-[(1S,4 5)-2,5-diazabicyclo[2.2.1]heptan-2-yl]thiazolo[3,2-
a]pyrimi din-
5-one (150 mg) as a light yellow solid. MS (ES+) m/z: 394.3 [(M+H)+].
Example 17
2-(2,3,3a,4,6,6a-hexahydro-1H-pyrrolo [3,4-c] pyrrol-5-y1)-7-(2,8-
dimethylimidazo [1,2-
b]pyridazin-6-yl)thiazolo[3,2-alpyrimidin-5-one
I
0
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 2-[5-
oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-y1]-1,3,3a,4,6,6a-
hexahydropyrrolo[3,4-c]pyrrole-5-carboxylate (intermediate 5-12) and 2,8-
dimethy1-6-
(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-y1)imidazo[1,2-1Apyridazine (boronic
ester 6-1)
gave tert-butyl 2-[7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-5-oxo-
thiazolo[3,2-
a]pyrimidin-2-y1]-1,3 ,3 a,4,6,6a-hexahydropyrrol o[3 ,4-c]pyrrol e-5-carboxyl
ate (105 mg,
33% yield) as a yellow solid. MS (ES+) m/z: 508.2 [(M+H)l.
Step 2: A deprotection using the general procedure 2, gave 2-(2,3,3a,4,6,6a-
hexahydro-1H-
pyrrolo[3 ,4-c]pyrrol -5-y1)-7-(2, 8-dimethylimi dazo[1,2-b]pyri dazin-6-
yl)thiazolo[3,2-
a]pyrimidin-5-one (110 mg.98% yield) as a light yellow solid. MS (ES+) m/z:
408.2
[(M+H)+].
Example 18
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 83 -
7-(8-cyclopropy1-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-piperazin-1-y1-
thiazolo[3,2-a]pyrimidin-5-one
N
\N¨..:141r I FIN
0
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 4-[5-
oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-yl]piperazine-1-
carboxylate
(intermediate 5-1) and 8-cycl opropy1-2-m ethy1-6-(4,4,5, 5 -tetram ethyl -1,3
,2-di ox ab orol an-
2-yl)imidazo[1,2-b]pyridazine (boronic ester 6-7) gave tert-butyl 447-(8-
cyclopropy1-2-
methyl-imidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-
yl]piperazine-1-
carboxylate (62 mg, 70% yield) as a yellow solid. MS (ES+) m/z: 508.4
[(M+H)+].
Step 2: A deprotection using the general procedure 2, gave 7-(8-cyclopropy1-2-
methyl-
imidazo[1,2-b]pyridazin-6-y1)-2-piperazin-l-yl-thiazolo[3,2-a]pyrimidin-5-one
(5 mg, 10%
yield) as a light yellow solid. MS (ES+) m/z: 408.4 [(M+H) ].
Example 19
7-(8-isopropeny1-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-piperazin-l-yl-
thiazolo[3,2-a]pyrimidin-5-one
Hfiki--7¨ea I
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 4-[5-
oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-ylipiperazine-1-
carboxylate
(intermediate 5-1) and 8-i sopropenyl -2-m ethyl -6-(4,4,5, 5 -tetram ethy1-
1,3,2-di oxaborolan-
2-yDimidazo[1,2-13]pyridazine (boronic ester 6-8) gave tert-butyl 4-[7-(8-
isopropeny1-2-
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 84 -
methyl-imidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-
yl]piperazine-l-
carboxylate (119 mg, 74% yield) as a yellow solid. MS (ES+) m/z: 508.4
[(M+H)+].
Step 2: A deprotection using the general procedure 2, gave 7-(8-isopropeny1-2-
methyl-
imidazo[1,2-b]pyridazin-6-y1)-2-piperazin-l-yl-thi azol o[3,2-a]pyrimi din-5-
one (66 mg,
69% yield) as a light yellow solid. MS (ES+) m/z: 408.2 [(M+H)+].
Example 20
7-(2,7-dimethyloxazolo [5,4-1311pyridin-5-y1)-2-piperazin-1-yl-thiazolop,2-al
pyrimidin-
5-one
N
I
S N 0
HNI¨\N¨ar I
0
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 4-[5-
oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-yl]piperazine-1-
carboxylate
(intermediate 5-1) and 2,7-dim ethyl -5 -(4,4,5,5 -tetramethyl -
1,3,2-di oxab orol an-2-
yl)oxazolo[5,4-b]pyridine (boronic ester 6-9) gave tert-butyl 4-[7-(2,7-
1 5 dimethyloxazolo[5,4-b]pyridin-5-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-
yl]piperazine-1-
carboxylate (36 mg, 44% yield) as a yellow solid. MS (ES+) m/z: 483.3
[(M+H)+].
Step 2: A deprotection using the general procedure 2, gave 7-(2,7-
dimethyloxazolo[5,4-
b]pyridin-5-y1)-2-piperazin-1-yl-thiazolo[3,2-a]pyrimidin-5-one (7 mg, 23%
yield) as a
light yellow solid. MS (ES+) m/z: 383.2 [(M+H)+].
Example 21
7-(2,8-dimethylimidazo[1,2-131pyridazin-6-y1)-2-1(3S)-3-pyrrolidin-1-
ylpyrrolidin-1-
yllthiazolo[3,2-alpyrimidin-5-one
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 85 -
N
N
cieCNI,:i I
0
Using the general procedure 1, a cross coupling reaction between [5-oxo-2-
[(3S)-3-
pyrrolidin-1-ylpyrrolidin-1-yl]thiazolo[3,2-a]pyrimidin-7-yl]
4-methylbenzenesulfonate
(intermediate 5-13) and 2,8-dimethy1-6-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-
2-
yl)imidazo[1,2-b]pyridazine (boronic ester 6-1) gave 7-(2,8-
dimethylimidazo[1,2-
b]pyridazin-6-y1)-243S)-3-pyrrolidin-1-ylpyrrolidin-1-yllthiazolo[3,2-
a]pyrimidin-5-one
(7 mg, 3% yield) as a yellow oil. MS (ES+) ,n/z: 436.2 [(M+H)+].
Step 2: A deprotection using the general procedure 2, gave 2-(2,3,3a,4,6,6a-
hexahydro-1H-
pyrrolo[3,4-c]pyrrol-5-y1)-7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-
yl)thiazolo[3,2-
a]pyrimidin-5-one (110 mg.98% yield) as a light yellow solid. MS (ES+) in/z:
408.2
[(M+H) ].
Example 22
2-(2,8-diazaspiro14.5]decan-2-y1)-7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-
yl)thiazolo[3,2-a]pyrimidin-5-one
FII41,114
0
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 2-[5-
oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-y1]-2,8-diazaspiro
-[4.51decane-8-
carboxylate (intermediate 5-14) and 2,8-dimethy1-6-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-yl)imidazo[1,2-b]pyridazine (boronic ester 6-1) gave tert-butyl
24742,8-
dimethylimidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a] pyrimidin-2-y1]-
2,8-
diazaspiro[4.5]decane-8-carboxylate (44 mg, 37% yield) as a yellow solid. MS
(ES+) m/z:
536.4 [(M+H)].
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 86 -
Step 2: A deprotection using the general procedure 2, gave 2-(2,8-
diazaspiro[4.5]decan-2-
y1)-7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-yl)thiazolo[3,2-a]pyrimidin-5-one
(61 mg,
98% yield) as a light red oil. MS (ES+) m/z: 436.2 [(M+H)].
Example 23
7-(8-isopropyl-2-methyl-imidazo [1,2-131pyridazin-6-y1)-2-piperazin- 1-yl-
thiazolo [3,2-
alpyrimidin-5-one
N
HN,\_./N
0
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 4-[5-
oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-ylipiperazine- 1 -
carboxylate
(intermediate 5-1) and 8-i sopropy1-2-m ethyl -644,4,5,5 -tetram ethyl -1,3 ,2-
di oxab orol an-2-
yl)imidazo[1,2-b]pyridazine (boronic ester 6-10) gave tert-butyl 447-(8-
isopropy1-2-
methyl-imidazo[1,2-13]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-
yl]piperazine-l-
carboxylate (86 mg, 64% yield) as a yellow solid. MS (ES+) m/z: 510.3
[(M+H)+].
Step 2: A deprotection using the general procedure 2, gave 7-(8-isopropy1-2-
methyl-
imidazo[1,2-b]pyridazin-6-y1)-2-piperazin-1-yl-thiazolo[3,2-a]pyrimi din-5-one
(65 mg,
94% yield) as a light yellow solid. MS (ES+) m/z: 410.3 [(M+H)+].
Example 24
7-(2,8-dimethylimidazo [1,2-131pyridazin-6-y1)-2-(4-piperidyl)thiazolop,2-al
pyrimidin-
5-one
0
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 87 -
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 4-[5-
oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-yl]piperidine-1-
carboxylate
(intermediate 5-15) and 2,8-dimethy1-6-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-
2-
yl)imidazo[1,2-b]pyridazine (boronic ester 6-1) gave tert-butyl 4-[7-(2,8-
dimethylimidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a] pyrimidin-2-
yl]piperidine-1-
carboxyl ate (35 mg, 53% yield) as a yellow solid. MS (ES+) m/z: 481.2
[(M+H)+].
Step 2: A deprotection using the general procedure 2, gave 7-(2,8-
dimethylimidazo[1,2-
b]pyridazin-6-y1)-2-(4-piperidyl)thiazolo[3,2-a]pyrimidin-5-one (3 mg, 6%
yield) as a light
red oil. MS (ES+) m/z: 381 [(M+H) ].
Example 25
742-methy1-8-(trifluoromethyl)imidazo[1,2-blpyridazin-6-y1]-2-1(8aR)-
3,4,6,7,8,8a-
hexahydro-1H-pyrrolo [1,2-a] pyrazin-2-yllthiazolo [3,2-a] pyrimidin-5-one
N
ICY¨\1/4 I
I I
0
Using the general procedure 1, a cross coupling reaction between [5-oxo-2-
[(8aR)-
3,4,6,7, 8,8a-hexahydro-1H-pyrrolo[1,2-a]pyrazin-2-yl]thiazolo[3,2-a]pyrimidin-
7-y1 ] 4-
methylbenzene-sulfonate (intermediate 5-10) and 2-methy1-6-(4,4,5,5-
tetramethy1-1,3,2-
dioxaborolan-2-y1)-8-(trifluoromethyl)imidazo[1,2-13]pyridazine (boronic ester
6-4) gave 7-
[2-methy1-8-(trifluoromethyl)imi dazo[1,2-b]pyri dazin-6-y1]-2-[(8aR)-
3,4,6,7,8,8a-
hexahydro-1H-pyrrolo[1,2-a]pyrazin-2-ylithiazolo[3,2-a]pyrimidin-5-one (6 mg,
20%
yield) as a yellow solid. MS (ES+) m/z: 476.2 [(M+H)+].
Example 26
7-(2,8-dimethylimidazo11,2-b]pyridazin-6-y1)-2-(4-fluoro-4-
piperidyl)thiazolo13,2-
alpyrimidin-5-one
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 88 -
N
HNOZ.<5.:11
\ N I
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 4-
hydroxy-4- [5-oxo-7-(p-tol yl sul fonyl oxy)thi azol o[3,2-a]pyri mi di n-2-
yl]piperi di ne-1-
carboxylate (intermediate 5-16) and 2,8-dimethy1-6-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-yl)imidazo[1,2-b]pyridazine (boronic ester 6-1) gave tert-butyl
4-[7-(2,8-
dimethylimidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-y1]-4-
hydroxy-
piperidine-1-carboxylate (220 mg, 77% yield) as a light brown oil. MS (ES+)
m/z: 497.3
[(M+H)].
Step 2: To a stirred solution of tert-butyl 4-[7-(2,8-dimethylimidazo[1,2-
b]pyridazin-6-y1)-
5-oxo-thiazolo[3,2-a]pyrimidin-2-y1]-4-hydroxy-piperidine-1-carboxylate (220
mg, 0.44
mmol) in CH2C12 at -78 C was added DAST (176 uL, 1.33 mmol in 3 mL of CH2C12)
and
the reaction was gently warmed up to 0 C. The reaction mixture was
partitioned between
CH2C12 and an aqueous saturated solution of NaHCO3. The organic phase was
dried and
concentrated under vacuo. The residue was purified by column chromatography to
give
tert-butyl 4-[7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-5-oxo-
thiazolo[3,2-
a]pyrimidin-2-y1]-4-fluoro-piperidine-1-carboxylate (80 mg, 33% yield) as a
light yellow
powder. MS (ES+) m/z: 499.6 [(M+H)+].
Step 3: A deprotection using the general procedure 2, gave 7-(2,8-
dimethylimidazo[1,2-
b]pyridazin-6-y1)-2-(4-fluoro-4-piperidyl)thiazolo[3,2-a]pyrimidin-5-one (23
mg, 71%
yield) as a light red oil. MS (ES+) m/z: 399.2 [(M+H)+].
Example 27
7-18-(difluoromethoxy)-2-methyl-imidazo11,2-151pyridazin-6-y11-2-piperazin-1-
yl-
thiazolo13,2-alpyrimidin-5-one
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 89 -
= `1""f
N
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 4-[5-
oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-yl]piperazine-1-
carboxylate
(intermediate 5-1) and 8-(difluoromethoxy)-2-methy1-6-(4,4,5,5-tetramethy1-
1,3,2-
dioxaborolan-2-yl)imidazo[1,2-b]pyridazine (boronic ester 6-11) gave tert-
butyl 4-[7-[8-
(difluoromethoxy)-2-methyl-imidazo[1,2-b]pyridazin-6-y1]-5-oxo-thiazolo[3,2-
a]pyrimidin-2-yl]piperazine-1-carboxylate (143 mg, 71% yield) as a yellow
solid. MS
(ES+) rn/z: 534.2 [(M+H)+].
Step 2: A deprotection using the general procedure 2, gave 7-[8-
(difluoromethoxy)-2-
methyl-imidazo[1,2-b]pyridazin-6-y1]-2-piperazin-1-yl-thiazolo[3,2-a]pyrimidin-
5-one (70
mg, 60% yield) as a light yellow solid. MS (ES+) m/z: 434.2 [(M+H)+].
Example 28
742-methyl-7-(trifluoromethyl)oxazolo15,4-blpyridin-5-y11-2-piperazin-l-yl-
thiazolo[3,2-alpyrimidin-5-one
F F
N
SY1 '141 0
HN
th
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 4-[5-
oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-yl]piperazine-1-
carboxylate
(intermediate 5-1) and N-[2-chl oro-6-(4,4, 5,5-tetram ethyl-1,3 ,2-di ox ab
orol an-2-y1)-4-
(trifluoromethyl)-3-pyridyl]acetamide (boronic ester 6-12) gave tert-butyl 447-
[2-methyl-
7-(trifluorom ethypox azolo[5,4 -b]pyridin-5-y1]-5-oxo-thiazolo[3 ,2-a]pyrimi
din-2-
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 90 -
yl]piperazine-1-carboxylate (102 mg, 35% yield) as a yellow solid. MS (ES+)
m/z: 537.2
[(M+H)l.
Step 2: A deprotection using the general procedure 2, gave 742-methy1-7-
(trifluoromethyl)oxazolo[5,4-b]pyri din-5-y1]-2-piperazin-l-yl-thi azol o[3,2-
a]pyrimi din-5-
one (56 mg, 35% yield) as a light yellow solid. MS (ES+) m/z: 437.2 [(M+H)+].
Example 29
7-(8-methoxy-2-methyl-imidazo[1,2-bipyridazin-6-yl)-2-(4-
piperidyl)thiazolo[3,2-
alpyrimidin-5-one
s N
H N
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 4-[5-
oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-yl]piperidine-1-
carboxylate
(intermediate 5-15) and 8-methoxy-2-methy1-6-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-
y1)imidazo[1,2-b]pyridazine (boronic ester 6-6) gave tert-butyl 4-[7-(8-
methoxy-2-methyl-
imidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-yl]piperidine-1-
carboxylate.
Step 2: A deprotection using the general procedure 2, gave the title compound
7-(8-
methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-(4-piperidyl)thiazolo[3,2-
a]pyrimidin-
5-one. MS (ES+) m/z: 397.1 [(M+H)+].
Example 30
7-(2,7-dim ethyloxazolo[5,4-b]pyridin-5-yl)-2-(4-piperidyl)thiazolo[3,2-
a]pyrimidin-5-
one
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 91 -
I
H )
Nyl
0
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 4-[5-
oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-yl]piperidine-1-
carboxylate
(intermediate 5-15) and 2,7-dimethy1-5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-
2-
yl)oxazolo[5,4-13]pyridine (boronic ester 6-9) gave tert-butyl 44742,7-
dimethyloxazolo[5,4-1D]pyridin-5-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-
yl]piperidine-1-
carb ox yl ate.
Step 2: A deprotection using the general procedure 2, gave the title compound
742,7-
dimethyloxazolo[5,4-13]pyridin-5-y1)-2-(4-piperidyl)thiazolo[3,2-a]pyrimidin-5-
one. MS
(ES+) m/z: MS (ES+) mk: 382.1 [(M+H)+].
Example 31
7-(2-ehloro-8-methyl-imidazo11,2-blpyridazin-6-y1)-2-(4-piperidyl)thiazolo13,2-
alpyrimidin-5-one
CI
s
HN/ I N
0
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 445-
oxo-7-(p-tolylsulfonyl oxy)thi azol o pyri mi di n-2-y1 ]pi pen di ne-l-
carboxyl ate
(intermediate 5-15) and 2-chloro-8-methy1-6-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-
y1)imidazo[1,2-13]pyridazine (boronic ester 6-13) gave tert-butyl 4-[7-(2-
chloro-8-methyl-
imidazo[1,2-1Thyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-yl]piperidine-1-
carboxylate.
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 92 -
Step 2: A deprotection using the general procedure 2, gave the title compound
7-(2-chloro-
8-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-(4-piperidyl)thiazolo[3,2-a]pyrimidin-
5-one.
MS (ES+) m/z: 401.1 [(M+H)+].
Examples 32 & 33
7-(2,8-dim ethylimidazo[1,2-131pyridazin-6-y1)-2-1(3R,4R)-3-fluoro-4-
piperidyllthiazolop,2-al pyrimidin-5-one and 7-(2,8-dimethylimidazo 11 ,2-
b]pyridazin-6-yl)-2-1(3S,4S)-3-fluoro-4-piperidyllthiazolo[3,2-alpyrimidin-5-
one
s
HN HN
Fl
0 0
and
Step 1: Using the general procedure 1, separately, a cross coupling reaction
between tert-
butyl
(3R,4R)-3-fluoro-445-oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-
yl]piperidine-1-carboxylate or tert-butyl
(3 S,4 S)-3 -fluoro-445-oxo-7-(p-
tolylsulfonyloxy)thi azol o[3 ,2-a]pyrimi din-2-yl]piperidine- 1 -carboxyl ate
(intel mediates 5-
17) and
2,8-dimethy1-6-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-ypimi dazo[1,2-
b]pyridazine (boronic ester 6-1) gave separately tert-butyl (3R,4R)-447-(2,8-
dimethylimidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-y1]-3-
fluoro-
piperidine-1-carboxylate and tert-butyl
(3S,4S)-447-(2,8-dimethylimidazo[1,2-
b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-y1]-3-fluoro-piperidine-1-
carboxylate
respectively.
Step 2: A deprotection using the general procedure 2, gave the title compounds
742,8-
dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3R,4R)-3-fluoro-4-piperidyl]thi azol
o[3,2-
a]pyrimidin-5-one and 7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3S,4S)-
3-fluoro-
4-piperidyl]thiazolo[3,2-a]pyrimidin-5-one respectively. MS (ES+) m/z: 399.2
[(M+H)+]
for each enantiomer. Optical rotation for the two enantiomers was measured in
Me0H (20
C, 589 nm): Example 32 is (-)-enantiomer, optical rotation: -47'; Example 33
is (+)-
enantiomer, optical rotation: +37 .
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 93 -
Examples 34 & 35
7-(8-methoxy-2-methyl-imidazo [1,2-b] pyridazin-6-y1)-2-1(3R,4R)-3-fluoro-4-
piperidyllthiazolo [3,2-a] pyrimidin-5-one and 7-(8-methoxy-2-methyl-
imidazo[1,2-
blpyridazin-6-yl)-2-1(3S,4S)-3-fluoro-4-piperidyllthiazolo [3,2-a] pyrimidin-5-
one
=
=
,
I NI'
H
N I HN
N
N I
0 0
and
Step 1: Using the general procedure 1, separately, a cross coupling reaction
between tert-
butyl
(3R,4R)-3 -fluoro-4[5-oxo-7-(p-tolylsul fonyl oxy)thi azol o [3,2-a] pyrimi di
n-2-
yl]piperidine-l-carb oxyl ate or tert-butyl
(3S,4 S)-3 -fluoro-4-[5-oxo-7-(p-
tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-yl]piperidine- 1 -carboxylate
(intermediates 5-
17) and 8-methoxy-2-methyl -6-(4,4,5,5-tetramethyl -1,3,2-di oxaborolan-2-
yl)imi dazo[1,2-
b]pyridazine (boronic ester 6-6) gave separately tert-butyl (3R,4R)-3-fluoro-4-
[7-(8-
methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a] pyrimidin-
2-
yl]piperidine-l-carboxylate and tert-butyl (3S,4S)-3-fluoro-4-[7-(8-methoxy-2-
methyl-
imidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-yl]piperidine-1-
carboxyl ate respectively.
Step 2: A deprotection using the general procedure 2, gave separately the
title compounds
7-(8-methoxy-2-methyl-imidazo[1,2-b] pyridazin-6-y1)-2-[(3R,4R)-3-fluoro-4-
piperi dyl]thi azol o[3,2-a]pyrimi din-5-one
and 7-(8-methoxy-2-methyl -imi dazo[1,2-
b]pyridazin-6-y1)-2-[(3S,45)-3-fluoro-4-piperidyl]thiazolo[3,2-a]pyrimidin-5-
one
respectively. MS (ES+) m/z: 415.2 [(M+H)] for each enantiomer. Optical
rotation for the
two enantiomers was measured in Me0H (20 C, 589 nm): Example 34 is (-)-
enantiomer,
optical rotation: -43'; Example 35 is (+)-enantiomer, optical rotation: +47 .
Examples 36 & 37
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 94 -7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-1(7S)-4-
azaspiro[2.51octan-7-
yllthiazolop,2-alpyrimidin-5-one and 7-(2,8-dimethylimidazo 11,2-blpyridazin-6-
y1)-
2-1(7R)-4-azaspiro [2.5] octan-7-yllthiazolo[3,2-alpyrimidin-5-one
S N
N
S N
Hp N H N
I I I I
0 0
and
Step 1: Using the general procedure 1, separately, a cross coupling reaction
between tert-
butyl (7S)-745-oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-
a]pyrimidin-2-y1]-4-
azaspiro[2.5]octane-4-carboxylatecarboxylate or tert-butyl
(7R)-7-[5-oxo-7-(p-
tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-y1]-4-azaspiro[2 .5] octane-4-
carboxyl atecarb oxyl ate (intermediates 5-19) and 2,8-dimethy1-6-(4,4,5,5-
tetram ethyl -1,3,2-
dioxaborolan-2-yl)imidazo[1,2-b]pyridazine (boronic ester 6-1) gave separately
tert-butyl
(7S)-7-[7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-
a]pyrimidin-2-
y1]-4-azaspiro[2.5]octane-4-carboxylate and tert-butyl (7S)-747-(2,8-
dimethylimidazo[1,2-
b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-y1]-4-azaspiro[2.5] octane-
4-
carboxylate respectively.
Step 2: A deprotection using the general procedure 2, gave separately the
title compounds
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(75)-4-azaspiro[2.5] octan-7-
yl]thiazolo[3,2-a]pyrimi din-5-one and 7-(2,8-dimethylimidazo[1,2-b]pyridazin-
6-y1)-2-
[(7R)-4-azaspiro[2.5]octan-7-yl]thiazolo[3,2-a]pyrimidin-5-one respectively.
MS (ES+)
m/z: 407.1 [(M+H)-] for each enantiomer. Optical rotation for the two
enantiomers was
measured in Me0H (20 C, 589 nm): Example 36 is (-)-enantiomer, optical
rotation: -39';
Example 37 is (+)-enantiomer, optical rotation: +39 .
Examples 38 & 39
7-(2,8-dimethylimidazo [1,2-blpyridazin-6-y1)-2-1(3S,4R)-3-fluoro-4-
piperidyllthiazolo[3,2-a]pyrimidin-5-one and 7-(2,8-dimethylimidazo[1,2-
b]pyridazin-6-y1)-24(3R,4S)-3-fluoro-4-piperidyllthiazolo13,2-al pyrimidin-5-
one
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 95 -
s N N
I
HN HN
0 0
and
Step 1: Using the general procedure 1, separately, a cross coupling reaction
between tert-
butyl
(3 S,4R)-3 -fluoro-445-oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-
yl]piperidine-l-carboxylate or tert-butyl
(31R,4 S)-3 -fluoro-445-oxo-7-(p-
tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-yl]piperidine-1-carboxylate
(intermediates 5-
18) and
2,8-dimethy1-6-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-ypimidazo[1,2-
b]pyridazine (boronic ester 6-1) gave separately tert-butyl (3S,4R)-4-[7-(2,8-
dimethylimidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-y1]-3-
fluoro-
piperidine-1-carboxylate and tert-butyl
(3R,4S)-4-[7-(2,8-dimethylimidazo[1,2-
b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-y1]-3-fluoro-piperidine-l-
carboxylate
respectively.
Step 2: A deprotection using the general procedure 2, gave separately the
title compounds
7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[(3 S,4R)-3-fluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one and 7-(2,8-dimethylimidazo[1,2-
b]pyridazin-6-
y1)-2-[(3R,4S)-3-fluoro-4-piperidyl]thiazolo[3,2-a]pyrimidin-5-one
respectively. MS (ES+)
m/z: 399.2 [(M+H)+] for each enantiomer. Optical rotation for the two
enantiomers was
measured in Me0H (20 C, 589 nm): Example 38 is (-)-enantiomer, optical
rotation: -12';
Example 39 is (+)-enantiomer, optical rotation: +7 .
Examples 40 & 41
7-(8-methoxy-2-methyl-imidazo [1,2-b] pyridazin-6-y1)-2-1(7S)-4-azaspiro [2.5]
octan-7-
yllthiazolo13,2-al pyrimid in-5-one and 7-(8-methoxy-2-methyl-imidazo [1,2-
b] pyridazin-6-y1)-2-1(7R)-4-azaspiro [2.5] octan-7-yll thiazolo 13,2-al
pyrimidin-5-one
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 96
= 0
N".
0 0
and
Step 1: Using the general procedure 1, separately, a cross coupling reaction
between tert-
butyl
(78)-745 -oxo-7-(p-toly1 sulfonyloxy)thiazolo[3,2-a]pyrimidin-2-y1]-4-
azaspiro[2.5]octane-4-carboxylatecarboxylate or tert-butyl
(7R)-7-[5-oxo-7-(p-
.. tolyl sulfonyloxy)thi azol o[3,2-a] pyrimi din-2-yl] -4-azaspiro [2 .5]
octane-4-
carboxylatecarboxylate (intel ________________________________________________
mediates 5-19) and 8-methoxy-2-methy1-6-(4,4,5,5-
tetramethyl-1,3 ,2-dioxab orol an-2-yl)imi dazo[1,2-b]pyri dazine (boronic
ester 6-6) gave
tert-butyl
(75)-747-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-5-oxo-
thiazolo[3,2-a]pyrimidin-2-y1]-4-azaspiro[2.5]octane-4-carboxylate and tert-
butyl (7R)-7-
[7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-
a]pyrimidin-2-
y1]-4-azaspiro[2.5]octane-4-carboxylate respectively.
Step 2: A deprotection using the general procedure 2, gave separately the
title compounds
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(75)-4-
azaspiro[2.5]octan-7-
yl]thiazolo[3,2-a]pyrimidin-5-one and 7-(8-methoxy-2-methyl-imidazo[1,2-
b]pyridazin-6-
y1)-2-[(7R)-4-azaspiro[2 . 5] octan-7-y1 ]thiazolo[3 ,2 -a] pyrimidin-5-one
respectively. MS
(ES+) m/z: 423.2 [(M+H)+] for each enantiomer. Optical rotation for the two
enantiomers
in Me0H (20 C, 589 nm): -650 and +30 respectively. Optical rotation for the
two
enantiomers was measured in Me0H (20 C, 589 nm): Example 40 is (-)-
enantiomer,
optical rotation: -65'; Example 41 is (+)-enantiomer, optical rotation: +30 .
Example 42
2-(4-fluoro-4-piperidy1)-7-(8-methoxy-2-methyl-imidazo11,2-b]pyridazin-6-
yl)thiazolo13,2-alpyrimidin-5-one
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 97 -
=
N
_______________________________ F s N =
H< y
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl 4-
hydroxy-4- [5 -ox o-7-(p-tol yl sul fonyl oxy)thi azol o[3,2-a] pyrim i di n-2-
yl] pi p eri din e-1-
carboxylate (intermediate 5-16) and 8-methoxy-2-methy1-6-(4,4,5,5-tetramethyl-
1,3,2-
dioxaborolan-2-yl)imidazo[1,2-b]pyridazine (boronic ester 6-6) gave tert-butyl
4-hydroxy-
4-[7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-
a]pyrimidin-
2-yl]piperidine-1-carboxylate (775 mg, 79% yield). MS (ES+) m/z: 513.3
[(M+H)+].
Step 2: In analogy to example 26, step 2, from tert-butyl 4-hydroxy-447-(8-
methoxy-2-
methyl-imidazo[1,2 -b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-
yl]piperidine-1-
carboxylate was prepared tert-butyl 4-fluoro-4-[7-(8-methoxy-2-methyl-
imidazo[1,2-
b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-yl]piperidine-1-carboxylate
as a white
solid (23 mg, 30% yield). MS (ES+) m/z: 515.2 [(M+H)+].
Step 3: A deprotection using the general procedure 2, gave the title compound
2-(4-fluoro-
4-piperidy1)-7-(8-methoxy-2-methyl-imidazo[1,2-b]pyri dazin-6-yl)thi azol o[3
,2-
a]pyrimidin-5-one. MS (ES+) m/z: 415.1 [(M+H)+].
Example 43
7-(2-chloro-8-methyl-imidazo[1,2-13ipyridazin-6-y1)-2-1(3R,4R)-3-fluoro-4-
piperidylithiazolo[3,2-alpyrimidin-5-one
CI
s
/ N
H
I I
0
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 98 -
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl
(3R,4R)-3-fluoro-4-[5-oxo-7-(p-tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-
yl]piperidine-
1-carboxylate (intermediate 5) and 2-chloro-8-methy1-6-(4,4,5,5-tetramethy1-
1,3,2-
dioxaborolan-2-yl)imidazo[1,2-b]pyridazine (boronic ester 6-13) gave tert-
butyl ((3R,4R)-
.. 4-[7-(2-chloro-8methyl-imidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]
pyrimidin-2-
y1]-]-3 -fluoro-piperidine- 1 -carboxylate.
Step 2: A deprotection using the general procedure 2, gave the title compound
7-(2-chloro-
8-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(3R,4R)-3-fluoro-4-
piperidyl]thiazolo[3,2-
a]pyrimidin-5-one. MS (ES+) m/z: 419.2 [(M+H)+].
Example 44
7-(2,7-dimethyloxazolo[5,4-131pyridin-5-y1)-2-1(3R,4R)-3-fluoro-4-
piperidyllthiazolo[3,2-alpyrimidin-5-one
HN S
N
0
Step 1: Using the general procedure 1, a cross coupling reaction between tert-
butyl
(3R,4R)-3 -fluoro-4-[5 -oxo-7-(p-toly1 sulfonyl oxy)thiazolo[3 ,2-a]pyrimidin-
2-yl] piperi dine-
1-carboxylate (intermediate 5) and 2,7-dimethy1-5-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-yl)oxazolo[5,4-b]pyridine (boronic ester 6-9) gave tert-butyl
((3R,4R)-4-
[74,7-dim ethyl oxazol o[5,4-b]pyridinpyri din-5-y1)-5-oxo-thi azol o[3,2-
a]pyrimidin-2-y1H-
.. 3-fluoro-piperidine-1-carb oxyl ate.
Step 2: A deprotection using the general procedure 2, gave the title compound
7-(2õ7-
dimethyloxazolo[5,4-b]pyridin-5-y1)-2-[(3R,4R)-3-fluoro-4-
piperidyl]]thiazolo[3,2-
a]pyrimidin-5-one. MS (ES+) m/z: 400.2 [(M+H)+].
Examples 45 & 46
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 99 -7-(8-methoxy-2-methyl-imidazo [1,2-b] pyridazin-6-y1)-2-1(35,4R)-3-
fluoro-4-
piperidyl] thiazolo 13,2-al pyrimidin-5-one and 7-(8-methoxy-2-methyl-imidazo
[1,2-
b] pyridazin-6-y1)-2-1(3R,4S)-3-fluoro-4-piperidyllthiazolo[3,2-al pyrimidin-5-
one
=
s /N N
HN/
\ N N HN )--CrrN
N
0 0
and
Step 1: Using the general procedure 1, separately, a cross coupling reaction
between tert-
butyl
(3 S,4R)-3 -fluoro-4-[5-oxo-7-(p-tolylsulfonyloxy)thi azolo [3,2-a]pyrimidin-2-
yl]piperidine-1-carboxylate or tert-butyl
(3R,4 S)-3 -fluoro-4-[5-oxo-7-(p-
tolyl sul fonylox y)thi azol o[3,2-a] pyrim i di n-2-yl] pip eri di ne-l-carb
oxyl ate (intermediates 5-
18) and 8-methoxy-2-methyl-6-(4,4,5, 5-tetramethy1-1,3 ,2-dioxab orolan-2-
yl)imidazo[1,2-
b]pyridazine (boronic ester 6-6) gave (3S,4R)-3-fluoro-447-(8-methoxy-2-methyl-
imidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-yl]piperidine-1-
carboxylate and (3R,4S)-3-fluoro-447-(8-methoxy-2-methyl-imidazo[1,2-
b]pyridazin-6-
y1)-5 -oxo-thi azolo[3 ,2-a] pyrimidin-2-yl]piperidine-1-carboxyl ate
respectively.
Step 2: A deprotection using the general procedure 2, gave separately the
title compounds
7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[(3 S,4R)-3 -fluoro-4-
piperi dyl]thi azol o[3 ,2 -a]pyrimi din-5-one
and 7-(8-m ethoxy-2-methyl -imi dazo[1,2-
b]pyridazin-6-y1)-2-[(3R,4 S)-3 -fluoro-4-piperidyl]thiazolo[3 ,2-a]pyrimidin-
5 -one
respectively. MS (ES+) m/z: 415.2 [(M+H)+] for each enantiomer. Optical
rotation for the
two enantiomers was measured in Me0H (20 C, 589 nm): Example 45 is (-)-
enantiomer,
optical rotation: -15'; Example 46 is (+)-enantiomer, optical rotation: +39 .
Examples 47 & 48
7-(2,8-dim ethylimidazo[1,2-b]pyridazin-6-y1)-2-1rac-(1R,55)-9-oxa-3-
azabicyclo [3.3.1] nonan-7-yl]thiazolo [3,2-al pyrimidin-5-one and 742,8-
dimethylimidazo 11,2-b] pyridazin-6-y1)-2- Irac-(1S,5R)-9-oxa-3-
azabicyclo [3.3.1] nonan-7-yllthiazolo [3,2-a] pyrimidin-5-one
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 100 -
____________________________ ----r NKN = / FS ,..11 S...,..r,
......N -..N,N-....."
HI\ 0)
11
H 0 o
and H
Using the general procedure 1, a cross coupling reaction between the
intermediates 5-20
and 2,8-dim ethyl-6-(4,4, 5,5-tetram ethyl-1,3 ,2-dioxab orolan-2-yl)imi
dazo[1,2-b] pyridazine
(boronic ester 6-1) followed by a deptrotection of the Boc protective group,
gave separately
after column chromatography the title compounds 7-(2,8-dimethylimidazo[1,2-
b]pyridazin-6-y1)-2-[rac-(1R, 5 S)-9-oxa-3 -azabicyclo[3 .3 .1]nonan-7-
yl]thiazolo[3,2-
a]pyrimidin-5-one (71 mg) as light yellow solid MS (ES+) m/z: 423.1 [(M+H)+]
as the
first compound to elute (relative structure assigned by 1H-NMR spectroscopy)
and 7-(2,8-
dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[rac-(1 S,5R)-9-oxa-3 -azabicyclo[3 .3
.1]nonan-7-
yl]thiazolo[3,2-a]pyrimidin-5-one (68 mg) (2nd compound to elute, relative
stereochemistry assigned by 1H-NIVIR spectroscopy) as a light yellow solid. MS
(ES+)
m/z: 423.1 [(M+H)+].
Examples 49 & 50
7-(8-methoxy-2-methyl-imidazo11,2-13] pyridazin-6-yl)-2-Irac-(1R,5S)-9-oxa-3-
azabicyclo [3.3.1] nonan-7-yllthiazolo [3,2-al pyrimid in-5-one and 7-(8-
methoxy-2-
methyl-im id azo 11,2-131pyridazin-6-y1)-2-Irac-(1S,5R)-9-oxa-3-azabicyclo
[3.3.1] nonan-
7-yl] thiazolo [3,2-a] pyrimidin-5-one
____________________ c --- _ \
N ,) / 11)
HN s 0 N -N ' HN 0 YH
\ H) \ H)
0 o
and
In analogy to the preparation of examples 47 and 48, from the intermediates 5-
20 and 8-
m ethoxy-2-methy1-6-(4,4,5,5-tetram ethyl-1,3 ,2-di oxab orol an-2-yl)i m i
dazo[1,2-
b]pyridazine (boronic ester 6-6) the title compounds were obtained separately
as an off
white solid (96 mg and 86 mg respectively). MS (ES+) m/z: 439.1 [(M+H)+] for
each of
the meso compounds. Example 49 was the first one to elute from the column, and
the
relative stereochemistry was established by 1H NMR spectroscopy.
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
-101 -
Examples 51 & 52
7-(8-methoxy-2-methyl-imidazo [1,2-b] pyridazin-6-y1)-2-1rac-(3R,4S)-3,4-
difluoro-4-
piperidyllthiazolo [3,2-a] pyrimidin-5-one and 7-(8-methoxy-2-methyl-
imidazo[1,2-
b]pyridazin-6-yl)-2-Irae-(3S,4S)-3,4-difluoro-4-piperidyl]thiazolo [3,2-a]
pyrimidin-5-
one
N N
s
0
and
Step 1: Preparation of tert-butyl rac-(3R,4S)-4-(2-aminothiazol-5-y1)-3-fluoro-
4-hydroxy-
piperidine-1-carboxylate and tert-butyl rac-(3S,4S)-4-(2-aminothiazol-5-y1)-3-
fluoro-4-
hydroxy-piperidi ne-1-carboxyl ate
ON N 0 H Sy)1 H2 N/-1H/S.._TrN H2
___________________________________ \ N
r r
and
Thiazol-2-ylamine (2.4 g, 24 mmol, 1 eq.) were dissolved in dry THY' (120 mL)
and cooled
to -75 C. A solution of n-BuLi (1.6 M in hexane, 30 mL, 48 mmol, 2 eq.) was
added
dropwise over 30 min and stirring was continued for 1 hour at -75 C. A
solution of TMS-
Cl (5.21 g, 6.13 mL, 48 mmol, 2 eq.) in dry THE (15 mL) was then added
dropwise over
15min. The dry-ice/bath was replaced by an ice-ethanol bath and stirred for 45
minutes
between -30 C and -20 C. The mixture was cooled to -75 C again, and 1.6 M n-
BuLi in
hexane (15 mL, 24 mmol, 1 eq.) was added over 15 minutes and stirring
continued for 15
min. at -75 C. A solution of 3-fluoro-4-keto-piperidine-1-carboxylic acid
tert-butyl ester
(5.21 g, 24 mmol, 1 eq) in THE (20 mL) was added over 20 min. After the
addition, the
dry-ice bath was removed and the reaction was allowed to warm over 45 minutes
to 0 C.
Stirred 30 minutes at 0-5 C. The reaction was quenched with sat.NH4C1-
solution (150
mL) and diluted with water (100 mL) and extracted twice with Ethyl acetate
(2x100 mL).
The organic layers were washed with brine (1x100 mL), dried over Na2SO4,
filtered off
and concentrated in vacuo. The crude product was purified by silica gel
chromatography
with 2 M NH3/Methanol in DCM (gradient from 2-7%) to afford tert-butyl rac-
(3R,4S)-4-
(2-aminothiazol-5-y1)-3-fluoro-4-hydroxy-piperidine-1-carboxylate (3800 mg,
49.4%) as
off-white solid. MS (ES+) nilz: 318.1 [(M+H)+] and tert-butyl rac-(35,45)-4-(2-
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 102 -
aminothiazol-5-y1)-3-fluoro-4-hydroxy-piperidine-1-carboxylate (1200 mg,
15.7%) as light
brown foam. MS (ES+) m/z: 318.1 [(M+H)].
Step 2: Preparation of tert-butyl rac-(3R,4S)-3-fluoro-4-hydroxy-4-(7-hydroxy-
5-oxo-
thi azolo[3 ,2-a]pyrimidin-2-yppiperidine-1-carb oxyl ate
.....)....-or N./
0
tert-butyl rac-(3R,4 S)-4-(2-am inothi az 01-5 -y1)-3 -fluoro-4-
hydroxy-pi p e ri di ne-1-
carboxylate (1.2 g, 3.74 mmol, 1 eq) and malonic acid bis(2,4,6-
trichlorophenyl)ester (1.91
g, 4.12 mmol, 1.1 eq.) were combined in toluene (20 mL). The suspension was
stirred in an
oil bath at 50 C for 22 hours. The thick suspension was cooled in an ice-bath
(0-5 C) and
diluted with tert. butyl methyl ether (20 mL). The resulting solid was
collected by filtration
and washed with cold tert. butyl methyl ether (3x10 mL). The filter cake was
dried in
vacuo at 45 C to yield the title compound (1.23 g, 85.2%) as off-white solid.
MS (ES-)
m/z: 384.3 [(M-H)].
Step 3: Preparation of tert-butyl rac-(3R,45)-3-fluoro-4-hydroxy-4-[5-oxo-7-(p-
tolylsulfonyloxy)thiazolo[3,2-a]pyrimidin-2-yl]piperidine- 1 -carboxylate
o
S-----N...1
o
At room temperature, tert-butyl rac-(3R,4S)-3-fluoro-4-hydroxy-4-(7-hydroxy-5-
oxo-
thiazolo[3,2-a]pyrimidin-2-yppiperidine-1-carboxylate (1.23 g, 3.19 mmol, 1
eq.) and
trimethylamine (0.40 g, 3.99 mmol, 1.25 eq.) were combined with
dichloromethane (15
mL) and Tosyl-Cl (0.67 g, 3.51 mmol, 1.1 eq.) was added and stirring was
continued for 3
hours at RT, resulting in a suspension. Upon addition of DCM (100 mL) the
solid was
dissolved and washed with an aqueous solution of sat. NaHCO3 (30 mL). The
aqueous
layer was back-extracted twice with DCM (50 mL). The combined organic layers
were
dried over Na2SO4, filtered off and concentrated in vacuo. The obtained solid
residue was
suspended in Heptane: tert. butyl methyl ether 3:1(20 mL) for 10 minutes.
Filtered off and
washed with Heptane: tert. butyl methyl ether 3:1 (4x5 mL). The filter cake
was dried in
vacuo at 45 C to yield the title compound (1.55 g, 90.0%) as an off-white
solid. MS (ES+)
m/z: 540.3 [(M+H)+].
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 103 -
Step 4: Preparation of tert-butyl rac-(3R,45)-3-fluoro-4-hydroxy-4-[7-(8-
methoxy-2-
methyl-imidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-
yl]piperidine-1-
carboxylate
õsr _______________________________
N \ 4 I
0 \ ________________________________
Using the general procedure 1, a cross coupling between tert-butyl rac-(3R,4S)-
3-fluoro-4-
hydroxy-4- [5-oxo-7-(p-tol yl sul fonyl oxy)thi azol o[3,2-a]pyrimi di n-2-
yl]piperi dine-1-
carboxylate and 8-methoxy-2-methy1-6-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-
yl)imidazo[1,2-b]pyridazine (boronic ester 6-6) gave the title product (395
mg, 67%) as an
off white solid. MS (ES+) m/z: 531.1 [(M+H)+]
Step 5: Preparation of 7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-
[rac-
(3R,4S)-3,4-difluoro-4-piperidyl]thiazolo[3,2-a]pyrimidin-5-one 51 and 7-(8-
methoxy-2-
methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[rac-(3 S,4S)-3,4-difluoro-4-
piperidyl]thiazolo[3,2-
a]pyrimidin-5-one 52
HN\N
/
0
and
Step 5a) Fluorination: Dichloromethane, extra dry (5 mL) was placed and cooled
in an ice-
ethanol bath to ca. -10 C. Triethylamine trihydrofluoride (91 mg, 92 uL,
0.565 mmol, 1.5
eq.) was added and stirred for 2-3 min. XtalFluor-E (172 mg, 0.754 mmol, 2
eq.; CAS
Number: 63517-29-3) was added and stirred for 2-3 min. Then tert-butyl rac-
(3R,45)-3-
fluoro-4-hydroxy-447-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-5-oxo-
thiazolo[3,2-a]pyrimidin-2-yl]piperidine-11-carboxylate (200 mg, 0.377 mmol, 1
eq.) was
added as solid and stirred for 10 minutes at -10 C. The ice-bath was removed
and stirred
overnight (17 hrs) at room temperature. The light yellowish solution was added
to a stirred
sat. Na.HCO3-solution (30 mL). The bi-layer mixture was stirred for 10
minutes. The
organic layer was separated and the aqueous layer was back-extracted with DCM
(2x20
mL). The combined organic layers were dried over Na2SO4, filtered off and
concentrated in
vacuo. The crude product was purified by NH2-silica gel chromatography (20 g
cartridge,
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 104 -
Ethyl acetate in Heptane gradient from 15-100%). The obtained product (182 mg,
90%) as
a mixture of diastereomers.
Step 5b) Deprotection: The previous mixture (step 5a) (374 mg, 0.702 mmol, 1
eq.) was
dissolved in DCM (5 mL) and TFA (800 mg, 7.02 mmol, 10 eq) was added. After 2
hours
at RT, the reaction mixture was evaporated in vacuo. The residue was dissolved
in
Methanol (5 mL) and purified by preparative HPLC. Column: AGILENT ECLIPSE XDB-
C18, 250 x 30 mm, 7 micron. Eluent: Water (+0.05% HCOOH): Acetonitrile 85:15%
to
50:50% in 8 min. Flow: 40 mL. (Triggered with UV 254 nm and ELSD). Product
containing fractions were combined and evaporated in vacuo. The obtained
products were
dissolved in Methanol. Treated with 7 M NI-13/Methanol (3-4 mL) and aq. 28% NI-
140H (1
mL). Stirred for 10 minutes at room temperature. Addition of 'solute
Evaporated in
vacuo to dryness and finally purified by silica gel chromatography with 2 M
NH3/Methanol
in DCM increased the gradient from 2-7%.
51: 748-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[rac-(3 R,45)-3 ,4-
difluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one (53.7 mg, 17.7%). MS (ES+) m/z: 433.1
RM+H)-1.
52: 7-(8-methoxy-2-methyl-imidazo[1,2-b]pyridazin-6-y1)-2-[rac-(3S,4S)-3,4-
difluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one (59.7 mg, 19.7%). MS (ES+) m/z 433.1
RM+H)1.
Examples 53 & 54
7-(2,8-dim ethyl imidazo [1,2-b]pyridazin-6-yI)-2- [rac-(3R,4S)-3,4-difluoro-4-
piperidyll thiazolo [3,2-al pyrimidin-5-one and 7-(2,8-dimethylimidazo[1,2-
blpyridazin-6-y1)-2-1rac-(3S,45)-3,4-difluoro-4-piperidyllthiazolo[3,2-
a]pyrimidin-5-
one
_________________________________________________ F
I HI\
0 0
and
The compounds 53 and 54 were prepared in a similar manner as the compounds 51
and 52,
with a difference on step 4, using 2,8-dimethy1-6-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-
2-y1)imidazo[1,2-13]pyridazine as boronic ester (intermediate 6-1).
53: 7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[rac-(3R,45)-3,4-difluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one (69 mg) as light yellow solid. MS
(ES+) m/z:
417.2 [(M+H)+].
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 105 -
54: 7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-y1)-2-[rac-(3S,4S)-
3,4-difluoro-4-
piperidyl]thiazolo[3,2-a]pyrimidin-5-one (75 mg) as light yellow solid. MS
(ES+) m/z:
417.2 [(M+H)+].
Example 55
7-(2,8-dimethylimidazo[1,2-blpyridazin-6-y1)-2-1rac-(1S,5R)-3-
azabicyclo13.1.01hexan-6-yllthiazolo[3,2-alpyrimidin-5-one
Hisa>,...,..r( I N
0
Step 1: Using the general procedure 1, a cross coupling reaction between
(1R,5S)-6-(5-
keto-7-tosyloxy-thiazolo[3,2-a]pyrimidin-2-y1)-3-azabicyclo[3.1.0]hexane-3-
carboxylic
acid benzyl ester (intermediate 5-21) and 2,8-dimethy1-6-(4,4,5,5-tetramethy1-
1,3,2-
dioxaborolan-2-y1)imidazo[1,2-b]pyridazine (boronic ester 1) gave tert-butyl 4-
[7-(2,8-
dimethylimidazo[1,2-b]pyridazin-6-y1)-5-oxo-thiazolo[3,2-a]pyrimidin-2-y1]-4-
hydroxy-
piperidine-1-carboxylate (76 mg, 59% yield) as a light yellow solid. MS (ES+)
m/z: 513.4
[(M+H)+].
Step 2: A deprotection using the general procedure 3, gave 2-[(1R,5S)-3-
azabicyclo[3.1.0]hexan-6-y1]-7-(2,8-dimethylimidazo[1,2-b]pyridazin-6-
yl)thiazolo[3,2-
a]pyrimidin-5-one (56 mg, 75% yield) as a white lyophilized powder. MS (ES+)
m/z: 379.3
[(M+H)+].
Examples 56, 57, 58 and 59
7-(8-methoxy-2-methyl-imidazo11,2-blpyridazin-6-y1)-2-1(3R,4S)-3,4-difluoro-4-
piperidyl]thiazolo13,2-alpyrimidin-5-one (56), 7-(8-methoxy-2-methyl-
imidazo[1,2-
b]pyridazin-6-y1)-2-1(3S,4S)-3,4-difluoro-4-piperidyllthiazolo13,2-alpyrimidin-
5-one
(57), 7-(8-methoxy-2-methyl-imidazo[1,2-blpyridazin-6-y1)-2-1(3R,4R)-3,4-
difluoro-4-
piperidyl]thiazolo13,2-alpyrimidin-5-one (58) and 7-(8-methoxy-2-methyl-
imidazo[1,2-b]pyridazin-6-yl)-2-[(3S,4R)-3,4-difluoro-4-piperidyl]thiazolo[3,2-
a]pyrimidin-5-one (59)
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 106
HN
0 56 0 57
and
___________________ F N F _____________________________ N:1)
/ fsr
HN - HN __ õc.....1!,
58 and 0 59
The four optically pure enantiomeres 56-59 were obtained using similar
procedures and
sequences as for the preparation of the corresponding racemic compounds 51 and
52. Here
we started from the optically pure tert-butyl (3R,4S)-4-(2-aminothiazol-5-y1)-
3-fluoro-4-
hydroxy-piperidine-1-carboxylate and tert-butyl (3R,4R)-4-(2-aminothiazol-5-
y1)-3-fluoro-
4-hydroxy-piperidine-1-carboxylate (obtained from a chiral separation: Column
chiral IG,
5 m, 250 x 20 mm. SFC, 30%Me0H+0.2% DEA) Their absolute configuration was
established upon derivatization to derivatives of known absolute
stereochemistry. tert-
Butyl (3R,4 S)-4-(2-aminothi azol-5-y1)-3 -fluoro-4-hydroxy-piperi dine-1 -
carb oxylate was
used to produce the compounds 56 and 58 and tert-butyl (3R,4R)-4-(2-
aminothiazol-5-y1)-
3-fluoro-4-hydroxy-piperidine-1-carboxylate was used to produce compounds 57
and 59
56: 50 mg, off white solid. MS (ES+) m/z: 433.2 [(M+H)+].
57: 40 mg, off white solid. MS (ES+) m/z: 433.2 [(M+H)].
58: 145 mg, off white solid. MS (ES+) m/z: 433.2 [(M+H)+].
59: 125 mg, off white solid. MS (ES+) m/z: 433.2 [(M+H)+].
Example 60
Homogeneous Time Resolved Fluorescence for HTT lowering
The HTRF assay was adapted from Weiss et al. (Analytical Biochemistry Volume
395,
Issue 1, 1 December 2009, Pages 8-15 and Analytical Biochemistry Volume 410,
2011,
Pages 304-306) to cells from GENEAe020-A cell line (https://hpscreg.eu/cell-
line/GENEAe020-A).
Compounds were tested for the effect of mutant HTT levels in Huntington
patient human
cells (GENEAe020-A cell line) using Homogeneous Time Resolved Fluorescence
(HTRF)
Date regue/date received 2023-08-25
WO 2022/194801 PCT/EP2022/056586
- 107 -
directed towards mutant HTT protein (mHTT). The GENEAe020-A cell line was
derived
by Genea Biocells from human blastocysts of HD donors. After assessing
viability, cells
were plated into 384 well collagen coated plates in growth media. Once cells
adhered,
media was removed and test compounds dissolved in DMSO were diluted with
buffer
solution and added to the adherent cells. Controls included experiments with
no cells,
DMSO with no compound, and Hsp90 inhibitor control. Cells were incubated with
compounds and controls for 48 hours. Then, the cells were lysed and
transferred to an
assay plate containing HTRF labeled monoclonal antibodies developed by Paul
Patterson
(Ko et al., Brain Research Bulletin, Volume 56, Numbers 3 and 4, 2001, Pages
319-329)
which recognize specific areas of the HTT protein. The terbium labeled "donor"
antibody
(2B7) binds to the N-terminus of the HTT protein and the Alexa488 labeled
"acceptor"
antibody (MW1) is specific for the polyglutamine region of the protein.
Binding of the
acceptor labeled antibody is more efficient for the extended polyglutamine
repeats of
mutant HTT protein which translates into a signal boost which enables the
specific
measurement of mutant HTT protein level. The HTRF donor and acceptor detection
reagents were incubated with the cell lysate and the ratio between the signals
of the two
fluorophores is indicative of the relative quantities of mHTT.
The results of this assay are provided in Table 1. Table 1 provides the ECso
(half maximal
effective concentration) values for the reduction of mHTT obtained for
particular examples
of the present invention as measured by HTRF assay (data shown below is mean
from
three replicates).
Table 1:
HTRF mHTT HTRF mHTT
Exam. Exam.
EC50 ( M) ECso ( M)
1 0.046 19 3.005
2 20
0.054 0.136
3 0.625 21 4.195
4 22
3.533 1.322
Date regue/date received 2023-08-25
WO 2022/194801
PCT/EP2022/056586
- 108 -
HTRF mHTT HTRF mHTT
Exam. Exam.
ECso (p,M) ECso ( M)
5 23
0.499 0.671
6 24
0.268 0.028
7 25
0.052 0.454
8 26
0.386 0.128
9 27
0.796 0.068
10 28
0.283 0.691
11 29
0.019 0.035
12 36
0.104 0.055
13 37
0.118 0.112
14 38
0.931 0.280
15 39
0.123 0.053
16 40
9.538 0.083
17 41
0.421 0.106
18 42-
0.469 0.053
19 43
3.005 0.152
20 44
0.136 0.059
21
4.195 45 0.097
22
1.322 46 0.069
23
0.671 47 0.330
Date regue/date received 2023-08-25
WO 2022/194801
PCT/EP2022/056586
- 109 -
HTRF mHTT HTRF mHTT
Exam. Exam.
ECso ( M) ECso ( M)
24
0.028 48 0.092
0.454 49 0.200
26
0.128 50 0.057
27
0.068 51 0.102
28
0.691 52 0.237
29
0.035 53 0.132
0.040 54 0.556
31
0.071 55 0.065
32
0.033 56 0.050
33
0.086 57 0.239
34
0.022 58 0.099
0.061 59 0.428
Date regue/date received 2023-08-25