Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
METHOD OF RAPIDLY ACHIEVING THERAPEUTIC CONCENTRATIONS OF
ZOLMITRIPTAN FOR TREATMENT OF MIGRAINES AND CLUSTER HEADACHES
TECHNICAL FIELD
[0001] The present invention relates to the field of transdermal
or intracutaneous
delivery of pharmaceutical agents, and more particularly to the delivery of
triptans, including
zolmitriptan.
BACKGROUND
[0002] According to the Migraine Research Foundation, migraine
affects 30
million men, women and children in the United States Most migraines last
between four and 24
hours, but some last as long as three days. According to published studies,
63% of migraine
patients experience between one and four migraines per month. The prevalence
in women
(about 18%) is on par with asthma and diabetes combined. Approximately one-
third of those
afflicted with migraines have three or more migraines per month and over half
report severe
impairment or the need for bed rest. Migraines are most prevalent in the third
decade of life,
affecting both productivity and quality of life. In surveys of desirable
attributes for therapies for
migraine, fast relief consistently scores very high as one of the most
important factors for a
migraine therapy.
[0003] Acute migraine is an incapacitating headache disorder
that is
characterized by episodic attacks of moderate to severe headache together with
various
combinations of neurological, gastrointestinal and autonomic symptoms.
Migraine without aura
is usually associated with nausea, vomiting, sensitivity to light, sound or
movement, and can last
for 4-72 hours if untreated. Previously termed "common migraine," migraine
without aura is
experienced by approximately 65% of patients. Migraine with aura is
experienced by about 15-
20% of patients; individuals suffer from transient focal neurological
symptoms, usually visual.
The visual symptoms are known as an aura. The remainder of migraine patients
experience both
types of migraine.
[0004] About a quarter of migraine patients experience one or
more episodes per
week. The most unpleasant symptoms associated with acute migraine are nausea
and vomiting.
The majority (about 90%) of patients experience nausea; about 70% experience
vomiting; and a
third typically experience both symptoms with every attack.
1
Date Recue/Date Received 2023-12-18
[0005] Cluster headaches are excruciating headaches that recur
in a cyclical
pattern (a "cluster"), usually daily, for a period of 1 week or more, usually
for six to twelve
weeks. In chronic cluster headache, clusters last for a year or more without
remission. Cluster
headaches are considered to be among the worst headaches known to medical
science, and are
known to result in pain more severe than migraines. In approximately 47% of
cases, cluster
headache attacks recur at the same time of day or night, and, in particular,
during sleep. Clusters
can start at the same time and have a similar duration year after year. These
recurrence patterns
suggest a role of the hypothamlamus (the body's biological clock), although
the exact cause is
unknown.
[0006] The pain of a cluster headache is typically on one side
(unilateral) or
localized and is around or above the eye. During an attack, patients may
become agitated and
restless, and they may have trouble resting in one place. As patients may
prefer to move around,
they may rock, sit, pace, crawl, scream in pain, or even bang their head
against a hard surface. In
some patients, due to the severity and recurrence of cluster headaches, there
is a heightened risk
of alcohol and drug abuse, self-injury, and even suicide. Notably, cluster
headaches are
sometimes referred to as "suicide headaches."
[0007] Treatment options for cluster headaches are extremely
limited, and there
is a strong need for treatments which provide rapid and effective responses.
See, e.g., Law et al,
Triptans for acute cluster headache, Cochrane Database of Systematic Reviews
(2013), Issue 7.
While migraines are different from cluster headaches, it is believed that
certain treatments for
migraines including triptans could also be effective for cluster headaches.
However, oral and
nasal routes of administration are not particularly effective due to slow
absorption rates and the
short duration of the headache. Injected triptans have shown some efficacy,
but they are limited
by needle phobia, lack of portability, complex preparation, the requirement
for sharps disposal,
and safety issues related to needle prick, cutting, and cross contamination
infection.
[0008] The acute treatment of migraine was revolutionized in
1991 by the
introduction of the triptan class, such as sumatriptan and zolmitriptan. The
triptans are serotonin
derivatives displaying highly selective and potent agonist activity at the
vascular 5-HT1B receptor
and the neuronal .5-HT1D receptor. The mode of action of the triptans is
hypothesized to be
three-fold: (1) binding of postsynaptic vascular 5-HT1B receptors, to
stimulate vasoconstriction
2
Date Recue/Date Received 2023-12-18
of meningeal vessels; (2) binding of presynaptic neuronal 5-HTID receptors, to
inhibit release of
pro-inflammatory neuropeptides; and (3) binding of presynaptic neuronal 5-HTID
receptors, to
diminish the firing rate in trigeminal neurons and the trigeminal nucleus
caudalis (central action).
Triptans have similarly shown improved efficacy in treating cluster headaches
over placebos.
[0009] Each of the currently available methods of administering
triptans,
including oral, nasal spray, subcutaneous injection and iontophoretic
intracutaneous patch (which
is a device that delivers medicine through the skin by a low electrical
current), has significant
disadvantages. Some migraine patients fail to respond consistently to oral
triptans, and oral
treatments may be ineffectual and/or unpleasant for patients who are suffering
from the nausea,
vomiting, or gastric stasis that can be associated with migraine. Oral, nasal
and iontophoretic
patch triptan products are also characterized by delayed absorption and
relatively slow onset of
action causing insufficient relief, especially early in the episode. This
delay is a critical failure in
cluster headache, as the fact that cluster headache episodes often last for 30
minutes or less
implies a complete lack of treatment for oral triptans, and significantly
reduced efficacy for nasal
when compared to subcutaneously delivered triptans. Cluster headaches are
often accompanied
by stuffy or runny nose, which may impact absorption following nasal delivery.
Nasal sprays
may be unpleasant in taste, and use of injectables can cause discomfort.
[0010] Sumatriptan (IMITREXO) has been commercially available in
a number
of dosage forms, such as a tablet, subcutaneous (SC) injection, nasal spray
and by transdermal
electrophoresis. Oral administration (as a succinate) suffers from poor
bioavailability (about
15%) due to poor absorption and pre-systemic metabolism. The time to reach
maximum
concentration in the bloodstream (Tmax) after oral tablet administration is
about 2 hours. A rapid-
release tablet formulation has roughly the same bioavailability, although the
T. is achieved on
average 10--1 5 minutes earlier than the conventional tablet. When injected,
sumatriptan is faster-
acting (usually within 10 minutes), but the duration of action is lower.
Although SC is faster, the
tablet formulations of sumatriptan have been much more widely prescribed than
the injection
because many patients do not like injecting themselves.
[0011] The triptans have an excellent safety profile when used
appropriately and
their adverse effect profile is similar to that observed with placebo in
clinical trials. Like other
compounds in the triptan class, zolmitriptan has been shown to be effective
and well-tolerated in
3
Date Recue/Date Received 2023-12-18
placebo-controlled clinical trials. It is available in a number of commercial
formulations
(ZOMIG ): (a) a conventional release tablet (2.5 mg and 5.0 mg); (b) a "fast
melt" orally
disintegrating tablet (2.5 mg and 5.0 mg); and (c) a nasal spray (5.0 mg).
[0012] The bioavailability of zolmitriptan conventional release
tablets has been
found to be between 41 and 48%, and administration with food reduced C.,, and
AUC by 13-
16% (Seaber et al., "The absolute bioavailability and metabolic disposition of
the novel
antimigraine compound zolmitriptan (311C90)," Br. J. Clin. Pharmacol. 1997;
43(6): 579-87;
Seaber et al., "The absolute bioavailability and effect of food on the
pharmacokinetics of
zolmitriptan in healthy volunteers," Br. J. Clin. Pharmacol. 1998; 46: 433-
439). The Tmax of the
conventional tablet is about 1.5 hours. Absorption is reported to be lower
during an actual
migraine attack so the Tmax may be higher during a migraine. Zolmitriptan is
converted to an
active N-desmethyl metabolite such that the metabolite concentrations are
about two-thirds that
of zolmitriptan. Because the 5HTiBilD potency of the metabolite is 2 to 6
times that of the parent,
the metabolite may contribute a substantial portion of the overall effect
after zolmitriptan
administration.
[0013] The bioavailability of the orally disintegrating tablets
is similar to that of
the conventional tablets but the Tõ.õ is (somewhat surprisingly) higher, at
about 3 hours for the
disintegrating tablets compared with 1.5 hours for the conventional tablet.
The disintegrating
tablets may also exacerbate nausea often concomitant with a migraine attack.
[0014] Zolmitriptan has significant advantages over other
triptans when
contemplated for alternate delivery routes and methods. Only three triptans,
zolmitriptan,
naratriptan, and frovatriptan have a lowest oral dose less than 5 mg. However,
at this lowest
dose, zolmitriptan significantly outperforms naratriptan in terms of pain
relief at 2 hours (62%
vs. 49%), and pain freedom at 2 hours (29% vs. 18%). (C. Asseburg, P. Peura,
T. Oksanen, J.
Turunen, T. Purmonen and J. Martikainen (2012); Cost-effectiveness of oral
triptans for acute
migraine: Mixed treatment comparison. International Journal of Technology
Assessment in
Health Care, 28, pp 382-389), and frovatriptan "has the lowest efficacy in 2-
hour response, 2-
hour pain free compared to the other triptans" (Neuropsychiatr Dis Treat. 2008
Feb; 4(1): 49-
54).
4
Date Recue/Date Received 2023-12-18
[0015] Nasal administration of zolmitriptan was used in an
attempt to overcome
the disadvantages of oral delivery described above, and doses of 2.5 mg and
5.0 mg have been
commercialized. However, the T.. of zolmitriptan is only improved slightly (to
about 1.5
hours), and a large portion of the dose is swallowed and still subject to
first pass metabolism.
[0016] Other non-oral routes of administration such as
transdermal
iontophoresis, patches and liquid injectors have the disadvantages of skin
irritation and scarring,
pain and inability to deliver a therapeutically effective dose. Subcutaneously
injected
sumatriptan has been available for years but has never been widely used
because it is difficult to
prepare and due to issues related to needle phobia, sharps disposal, and
accidental pricking,
cutting, and cross contamination that are related to delivery with a needle.
[0017] Effectiveness of treatment of migraine with triptans has
been shown to
be more dependent on rate of absorption than on dose or plasma concentration.
More rapid
absorption and earlier Trnax leads to better pain relief, not just early in a
migraine episode but
surprisingly even later when the plasma concentration from the slower absorbed
therapy is
higher. In cluster headache, in addition to the above effect, there is a need
for rapid absorption
simply because of the short duration of the headache event. Rapid absorption
can further reduce
required application time of a transdermal patch, and lead to more efficient
delivery of the
packaged dose, which facilitates reproducibility, for example by reducing
variability caused by
variation in patch wear time.
[0018] Therefore, advantages could be achieved by a therapeutic
alternative to
currently available migraine and cluster headache treatments that: (a) has an
onset of action
faster than oral and comparable to SC formulations but without issues related
to needles; (b)
avoids the oral route that may limit absorption caused by the gastric effects
of migraine (gastric
stasis, nausea and vomiting); (c) mitigates the potential for food
interactions, avoids first-pass
metabolism and reduces the potential for drug interactions; (d) is preferred
by patients (rapid
onset but not injected or with unpleasant taste/smell e.g., nasal sprays); (e)
has lower absorption
that reduces triptan side effects, e.g., chest constriction while still
effective at mitigating
migraine related headache and nausea; (f) can be conveniently carried by the
patient for use at
the first sign of a migraine or cluster headache; and (g) can be quickly,
conveniently, and safely
self-administered. Additionally, because nausea is present in 60-70% of
migraine attacks, it
Date Recue/Date Received 2023-12-18
would be advantageous for physicians and patients to have a product that can
be administered
without using the gastrointestinal system and not susceptible to lack of
absorption due to emesis.
Similarly, stuffy and runny nose in cluster headache may impact the efficacy
of nasally delivered
drugs.
[0019] Thus, there is a need in the art for a route of
administration that can
accommodate the relatively large doses of triptans, such as zolmitriptan,
typical of oral doses but
lacks the side effects of orally delivered doses. The present disclosure meets
these challenges
and needs, among others For instance, Applicant has surprisingly discovered
that transdermal
delivery of a triptan, such as zolmitriptan as described herein, can rapidly
deliver the relatively
large doses of zolmitriptan typical of oral doses with plasma concentrations
in the range of or
higher than those seen following oral administration, despite the difficulty
of the skin's highly
impermeable nature.
[0020] There is furthermore a need for a route of triptan
administration that has
improved efficacy due to an absorption rate comparable to injection and is
portable and easy to
prepare while avoiding the issues of needle phobia, sharps disposal, and
accidental pricking,
cutting, and cross contamination that are related to delivery with a needle.
[0021] Further, there is a need in the art for an effective
method of triptan
administration through transdermal delivery in which the patch can be
accurately and evenly
coated, without causing issues of residual drug on the array or issues of
manufacturing
inconsistencies, such as uneven formulation coating on a patch or difficulty
with formulation
sticking to the patch. Many attempts have been made to use transdermal
microneedle patches for
effective drug delivery; however, achieving rapid release of drug from and
rapid treatment with
microneedle systems, optimizing and developing effective microneedle shapes
and sizes, while
also containing a sufficient dosage of drug has proved elusive. Applicants,
through significant
development efforts, developed a system of effective triptan delivery by
addressing issues of
viscosity, drug loading, surface tension, shape and size of microneedles, and
common
manufacturing defects.
SUMMARY
6
Date Recue/Date Received 2023-12-18
[0022] The present disclosure relates to compositions, devices,
methods of
treatment, kits and methods of manufacture of pharmaceutical products useful
in the treatment of
migraines and other conditions, including cluster headaches. More
specifically, the disclosure is
directed to administration of a triptan, such as zolmitriptan, as the active
pharmaceutical
ingredient to a subject in need thereof In particular, the present disclosure
is directed to
transdermally or intracutaneously, or otherwise through the skin,
administering a therapeutically
effective dose of the active ingredient that is more rapidly available in the
subject's bloodstream
as compared to a therapeutically effective oral dose of the active ingredient,
in a format that is
easy to use and portable for rapid administration. In one embodiment, the
transdermal delivery
of a triptan, such as zolmitriptan, generally comprises a patch assembly
having a microprojection
member that includes a plurality of microprojections (or "needles" or
"microneedles" or "array-)
that are coated with, in fluid contact with a reservoir of, or otherwise
comprise the drug. The
patch assembly further comprises an adhesive component, and in a preferred
embodiment the
microprojection member and adhesive component are mounted in a retainer ring.
The
microprojections are applied to the skin to deliver the drug to the
bloodstream or, more
particularly, are adapted to penetrate or pierce the stratum corneum at a
depth sufficient to
provide a therapeutically effective amount to the bloodstream. In one
embodiment, the insertion
of the drug-coated microneedles into the skin is controlled by a hand-held
applicator that imparts
sufficient impact energy density in less than about 10 milliseconds.
[0023] Preferably, the microprojection member includes a
biocompatible coating
formulation comprising the drug, such as zolmitriptan, in a dose sufficient to
provide therapeutic
effect. The coating may further comprise one or more excipients or carriers to
facilitate the
administration of the drug across the skin. For instance, the biocompatible
coating formulation
comprises zolmitriptan and a water-soluble carrier that is first applied to
the microprojections in
liquid form and then dried to form a solid biocompatible coating.
[0024] In a preferred embodiment, zolmitriptan, excipients, the
coating and
drying process lead to a drug coating that is non-crystalline (amorphous) with
a surprisingly
rapid dissolution rate. In this embodiment, the coating, upon its application
to the skin via the
microneedles, dissolves at a rate sufficient for rapid uptake of the drug into
the epidermis and
bloodstream. In one embodiment, such rate is less than 20 minutes, or less
than 15 minutes, or
7
Date Recue/Date Received 2023-12-18
less than 10 minutes, or less than 5 minutes, or less than 2.5 minutes, or
less than 1 minute. This
rate leads to rapid migraine and cluster headache relief. Preferably, this
rapid uptake leads to
greater than about 10% of patients being pain free in 1 hour after
administration, more preferably
greater than about 20% of patients, most preferably about 25% of patients or
more are pain free.
In another embodiment, this rapid uptake leads to greater than 40% of patients
achieving pain
relief in 1 hour after administration, or greater than 50 percent of patients,
or about 65% of
patients or more achieve pain relief 1 hour after administration. Preferably,
the drug coating
remains amorphous for 1 year, more preferably 2 years, following gamma or e-
beam irradiation.
[0025] Such intracutaneous delivery system may be in the form of
a device that
is adapted for easy use directly by the patient. For example, the system may
be a drug-device
combination product comprising: (a) a disposable microprotrusion member with
titanium
microneedles that are coated with a drug product formulation and dried, the
microprotrusion
member being centered on an adhesive backing thus forming a patch, and (b) a
reusable
handheld applicator that ensures the patch is applied to the skin with a
defined application energy
sufficient to press the microneedles into the stratum corneum thereby
resulting in drug
absorption. In one embodiment, the delivery system comprises a patch
comprising about 0.2 mg
to about 10 mg zolmitriptan, or about 1 mg to about 4 mg, or about 1 mg, or
about 1.9 mg, or
about 2 mg, or about 3 mg, or about 3.8 mg, or about 4 mg, or about 5 mg, or
about 6 mg, or
about 7 mg, or about 8 mg, or about 9 mg zolmitriptan. In one embodiment, the
delivery system
is designed to deliver about 0.2 mg to about 10 mg zolmitriptan
intracutaneously, or about 1 mg
to about 4 mg, or about 1 mg, or about 1.9 mg, or about 2 mg, or about 3 mg,
or about 3.8 mg, or
about 4 mg, or about 5 mg, or about 6 mg, or about 7 mg, or about 8 mg, or
about 9 mg, or more
than about 1 mg, or more than about 1.9 mg, or more than about 2 mg, or more
than about 3 mg,
or more than about 3.8 mg, or more than about 4 mg, or more than about 5 mg,
or more than
about 6 mg, or more than about 7 mg, or more than about 8 mg or more than
about 9 mg
zolmitriptan.
[0026] In another embodiment, the present disclosure relates to
a method for
transderrnally or intracutaneously administering a triptan to a patient in
need thereof, comprising
the steps of: (a) providing a transdermal patch adapted to intracutaneously
deliver a triptan,
comprising a microprojection member having a plurality of microprojections
that are adapted to
8
Date Recue/Date Received 2023-12-18
penetrate or pierce the stratum comeum of the patient, wherein the
microprojections comprise a
biocompatible coating partially or fully disposed on the microprojections, the
coating comprising
a therapeutically effective amount of the triptan; and (b) applying the
microprojection member of
the device to the skin of the patient, whereby the plurality of
microprojections penetrate or pierce
the stratum comeum and deliver the triptan to the patient's bloodstream. In
one embodiment, the
triptan is zolmitriptan and is coated on the microprojections in a total
amount of approximately
0.2 to 10 mg of which approximately 50%, or 60%, or 65%, or 75%, or 80%, or
85%, or 90%, or
95%, or 100% of such dose reaches the bloodstream of the patient after
administration,
preferably wherein more than approximately 50%, or 60%, or 65%, or 75%, or
80%, or 85%, or
90%, or 95% of such dose reaches the bloodstream of the patient after
administration.
[0027] The present disclosure encompasses a method for treatment
or alleviation
of migraine or cluster headache in a human patient in need thereof, comprising
the transdermal
or intracutaneous administration of a therapeutically effective amount of
zolmitriptan that
produces a therapeutic concentration of zolmitriptan in the bloodstream faster
than
therapeutically effective doses administered orally, intranasally,
sublingually, or
iontophoretically. In one aspect, the method for treatment or alleviation of
migraine or cluster
headache in a patient results in a plasma Tmax as quick as about 2 minutes and
not later than
about 30-40 minutes in most subjects. In another aspect, the method results in
a maximum
plasma concentration (Cmõ) of zolmitriptan of less than 50 ng/ml.
[0028] In one embodiment, the zolmitriptan-coated microneedle patch
as disclosed
herein achieves rapid blood plasma concentrations after application during a
migraine or cluster
headache attack. Such patch provides pain freedom and freedom from bothersome
migraine or
cluster headache symptoms for at least 45 minutes post administration. A
patient's most
bothersome migraine symptom in addition to pain is usually selected from
sensitivity to light,
particularly bright lights (photophobia), sensitivity to sound, particularly
loud sounds
(phonophobia), and nausea, although migraines can also be accompanied by
vomiting,
sensitivity to smell, aura, vision changes, numbness, tingling, weakness,
vertigo, feeling
lightheaded or dizzy, puffy eyelid, difficulty concentrating, fatigue,
diarrhea, constipation, mood
changes, food cravings, hives, and/or fever. Common symptoms of cluster
headache include
excruciating pain, often on one side of the head and generally situated in or
around one eye, but
9
Date Recue/Date Received 2023-12-18
which may radiate to other areas of face, head, neck and shoulders,
restlessness, excessive tear
production and redness in the eye on the affected side, stuffy or runny nose,
forehead or facial
sweating, pale skin (pallor), facial flushing, swelling around the eye on the
affected side, and/or
drooping eyelid.
[0029] Additional embodiments of the present devices,
compositions, methods
and the like will be apparent from the following description, drawings,
examples, and claims. As
can be appreciated from the foregoing and following description, each and
every feature
described herein, and each and every combination of two or more of such
features, is included
within the scope of the present disclosure provided that the features included
in such a
combination are not mutually inconsistent. In addition, any feature or
combination of features
may be specifically excluded from any embodiment or aspect. Additional aspects
and
embodiments are set forth in the following description and claims,
particularly when considered
in conjunction with the accompanying examples and drawings.
REFERENCE TO COLOR DRAWINGS
[0030] This application file contains at least one drawing
executed in color.
Copies of this patent application publication with color drawings will be
provided by the Office
upon request and payment of the necessary fee.
BRIEF DESCRIPTION OF THE DRAWINGS
[0031] The foregoing features of embodiments will be more
readily understood
by reference to the following detailed description, taken with reference to
the accompanying
drawings, in which:
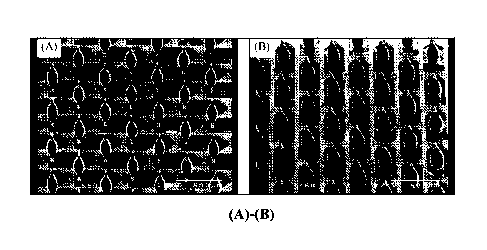
[0032] Figure 1(A) and (B) are scanning electron micrographs
(SEM) of
MF1663 array design coated with 1.9 mg zolmitriptan.
[0033] Figure 2(A)-(B) show views of the patch and the retainer
ring structure.
(A) provides a top view of the patch and retainer ring. (B) provides a bottom
perspective view of
the patch attached to a retainer ring.
[0034] Figure 3(A)-(B) illustrates the patch assembly, comprised
of a patch in a
retainer ring. (A) provides a side view of the patch assembly. (B) illustrates
an exploded view of
a patch assembly.
Date Recue/Date Received 2023-12-18
[0035] Figure 4(A)-(B) illustrates how the used plastic retainer
ring is removed
from the applicator and discarded. The fingers are used to pull the used
retainer ring off the
applicator. (A) provides a side view of the retainer ring attached to the
applicator. (B) provides a
side view of the retainer ring separated from the applicator.
[0036] Figure 5(A)-(E) are photographs of the steps for
application of the patch
of the present invention. (A) illustrates step 1: snap patch assembly onto
applicator. (B) further
illustrates step 1 and provides a bottom front perspective of the patch
assembly with the
applicator. (C) illustrates step 2: twist applicator cap clockwise from
position 1 to position 2 to
unlock for patch application. (D) illustrates step 3: press applicator
downward to apply patch to
skin. (E) illustrates step 4: patch is applied to the patient's skin and the
retainer ring remains
attached to the applicator.
[0037] Figure 6(A)-(C) provides in vitro release profiles of ZP-
Zolmitriptan
M207 1.9 mg patches. (A), top left, provides in vitro release profiles of ZP-
Zolmitriptan M207
1.9 mg patches that have been E-beam irradiated and stored at RT for 10
months, L/N0164004.
(B), top right, provides in vitro release profiles of ZP-Zolmitriptan M207 1.9
mg patches that
have been non-irradiated and stored at 40 C/75% RH for 10 months, L/N0203149-
NI. (C),
bottom left, provides in vitro release profiles of ZP-Zolmitriptan M207 1.9 mg
patches that have
been E-beam irradiated and stored at 40 C/75% RH for 10 months, L/N0203149-IR.
10038] Figure 7 is a line graph of mean zolmitriptan and
sumatriptan plasma
concentrations over time (zero to 24 hours) in normal human volunteers,
wherein Treatment A is
the M207 system (0.48 mg); Treatment B is the M207 system (0.48 mg x 2);
Treatment C is the
M207 system (1.9 mg); Treatment D is the zolmitriptan (2.5 mg oral tablet);
Treatment E is the
Sumatriptan (6.0 mg SC using auto-injector pen); Treatment F is the
Zolmitriptan system (1.9
mg x 2); and Treatment G is the Zolmitriptan system (3.8 mg). Sumatriptan was
scaled 6/90 to
show the sumatriptan concentration-time profile relative to other treatments.
[0039] Figure 8 is a line graph of mean zolmitriptan and
sumatriptan plasma
concentrations over time (zero to two hours), wherein Treatment A is the M207
system (0.48
mg); Treatment B is the M207 system (0.48 mg x 2); Treatment C is the M207
system (1.9 mg);
Treatment D is Zolmitriptan (2.5 mg oral tablet); Treatment E is the
Sumatriptan (6.0 mg SC
using auto-injector pen); Treatment F is the Zolmitriptan system (1.9 mg x 2);
and Treatment G
11
Date Recue/Date Received 2023-12-18
is the Zolmitriptan system (3.8 mg). In the graph, sumatriptan was scaled 6/90
to show the
sumatriptan concentration-time profile relative to other treatments.
[0040] Figure 9 is a line graph of dose linearity of M207 Cmax,
for single patch
and multiple patches, excluding 3.8 mg.
[0041] Figure 10 is a line graph of dose linearity of M207 AUCt,
for single patch
and multiple patches, excluding 3.8 mg.
[0042] Figure 11 is a line graph of mean plasma zolmitriptan
concentrations in
females over zero to two hours.
[0043] Figure 12 is a line graph of mean plasma zolmitriptan
concentrations in
males over zero to two hours.
[0044] Figure 13 is a line graph of mean plasma N-desmethyl
zolmitriptan
concentration over zero to two hours.
[0045] Figure 14 is a line graph of mean N-desmethyl zolmitriptan
plasma
concentrations over zero to twenty-four hours.
[0046] Figure 15 is a line graph of dose linearity of M207 Cm.
for single patch
and multiple patches.
[0047] Figure 16 is a line graph of dose linearity of M207 AUCt
for single patch
and multiple patches.
[0048] Figure 17 is a line graph of dose linearity of M207 AUCmf
for single
patch and multiple patches.
[0049] Figure 18 is a line graph of dose linearity of M207 AUCmf
for single
patch and multiple patches, excluding 3.8 mg patch.
[0050] Figure 19 is a line graph of N-desmethyl zolmitriptan dose
linearity Cma.
as a function of M207 dose for single patch and multiple patches.
[0051] Figure 20 is a line graph of N-desmethyl zolmitriptan dose
linearity AUCt
as a function of M207 dose for single patch and multiple patches.
[0052] Figure 21 is a line graph of N-desmethyl zolmitriptan dose
linearity
AUC,,,f as a function of M207 dose for single patch and multiple patches.
[0053] Figure 22 is a line graph of N-desmethyl zolmitriptan dose
linearity Cmax
as a function of M207 dose for single patch and multiple patches, excluding
3.8 mg.
12
Date Recue/Date Received 2023-12-18
[0054] Figure 23 is a line graph of N-desmethyl zolmitriptan dose
linearity AUCt
as a function of M207 dose, for single patch and multiple patches, excluding
3.8 mg.
[0055] Figure 24 is a line graph of N-desmethyl zolmitriptan dose
linearity
AUCilif as a function of M207 dose, for single patch and multiple patches,
excluding 3.8 mg.
[0056] Figure 25 is a graphical comparison of "% pain free" at 1, 2,
and 4 hours
after treatment.
[0057] Figure 26 is a graphical comparison of "% pain relief' at
1,2, and 4
hours after treatment.
[0058] Figure 27 is a graphical comparison of "% pain free" at 1, 2,
and 4 hours
after treatment.
[0059] Figure 28 is a graphical comparison of "% pain relief' at 1,
2, and 4
hours after treatment.
[0060] Figure 29 is a graphical comparison of "% subjects with pain
freedom"
for up to 4 hours after treatment.
[0061] Figure 30 is a graphical representation of the mean flux
results from ex
vivo human skin samples.
[0062] Figure 31 depicts the interaction of microprojections with the
skin, and,
specifically, how the microprojections penetrate the stratum comeum for
effective drug
delivery.
[0063] Figure 32 demonstrates an embodiment of a microprojection with
a shape
and dimensions before the microprojection is bent outward from the substrate
and coated with
drug
DETAILED DESCRIPTION OF SPECIFIC EMBODIMENTS
[0064] The various aspects and embodiments will now be fully
described herein.
These aspects and embodiments may, however, be embodied in many different
forms and should
not be construed as limiting; rather, these embodiments are provided so the
disclosure will be
thorough and complete, and will fully convey the scope of the present subject
matter to those
skilled in the art.
13
I. Introduction
[0065] Applicant surprisingly found, inter alia, that a dose of
zolmitriptan
typical of that of an oral delivery was well tolerated in delivery routes
other than oral delivery,
e.g., such as the intracutaneous or transdermal delivery of zolmitriptan as
described herein. In
accordance with this disclosure, the delivery of zolmitriptan generally
comprises a delivery
system comprising a microprojecti on member (or system) that includes a
plurality of
microprojections (or array thereof) that are adapted to penetrate or pierce
the stratum corneum
into the underlying epidermis layer, or epidermis and dermis layers.
Applicant, through
significant trial and error and development efforts, customized the
transdermal delivery of
zolmitriptan. In one embodiment, the microprojection member includes a
biocompatible coating
comprising zolmitriptan. This system provides superior pharmacokinetics and
pharmacodynamics over existing therapies and can be extended to other triptans
useful for
treating migraines, cluster headaches, and other diseases or conditions.
Definitions
[0066] Unless defined otherwise, all terms and phrases used
herein include the
meanings that the terms and phrases have attained in the art, unless the
contrary is clearly
indicated or clearly apparent from the context in which the term or phrase is
used. Although any
methods and materials similar or equivalent to those described herein can be
used in the practice
or testing of the present invention, particular methods and materials are now
described.
[0067] Unless otherwise stated, the use of individual numerical
values are stated
as approximations as though the values were preceded by the word "about" or
"approximately."
Similarly, the numerical values in the various ranges specified in this
application, unless
expressly indicated otherwise, are stated as approximations as though the
minimum and
maximum values within the stated ranges were both preceded by the word "about"
or
"approximately." In this manner, variations above and below the stated ranges
can be used to
achieve substantially the same results as values within the ranges. As used
herein, the terms
"about" and "approximately" when referring to a numerical value shall have
their plain and
ordinary meanings to a person of ordinary skill in the art to which the
disclosed subject matter is
most closely related or the art relevant to the range or element at issue. The
amount of
broadening from the strict numerical boundary depends upon many factors. For
example, some
14
Date Recue/Date Received 2023-12-18
of the factors which may be considered include the criticality of the element
and/or the effect a
given amount of variation will have on the performance of the claimed subject
matter, as well as
other considerations known to those of skill in the art. As used herein, the
use of differing
amounts of significant digits for different numerical values is not meant to
limit how the use of
the words "about" or "approximately" will serve to broaden a particular
numerical value or
range. Thus, as a general matter, "about" or "approximately" broaden the
numerical value. Also,
the disclosure of ranges is intended as a continuous range including every
value between the
minimum and maximum values plus the broadening of the range afforded by the
use of the term
"about" or "approximately." Consequently, recitation of ranges of values
herein are merely
intended to serve as a shorthand method of referring individually to each
separate value falling
within the range.
[0068] The term "amorphous" means a non-crystalline solid, i.e.,
a solid that
lacks the long-range order that is characteristic of a crystal.
[0069] The term "area under the curve" or "AUC" means the area
under the
curve (mathematically known as definite integral) in a plot of concentration
of drug in blood
plasma against time. Typically, the area is computed starting at the time the
drug is administered
and ending when the concentration in plasma is negligible. In practice, the
drug concentration is
measured at certain discrete points in time and the trapezoidal rule is used
to estimate AUC.
[0070] The teini "biocompatible coating," as used herein, means
and includes a
coating formed from a "coating formulation" that has sufficient adhesion
characteristics and no
(or minimal) adverse interactions with the biologically active agent (a/k/a
active pharmaceutical
ingredient, or therapeutic agent, or drug).
[0071] The term "bioequivalent," as used herein, denotes a
scientific basis on
which two or more pharmaceutical products, compositions or methods containing
same active
ingredient are compared with one another. "Bioequivalence" means the absence
of a significant
difference in the rate and extent to which the active agent in pharmaceutical
equivalents or
pharmaceutical alternatives becomes available at the site of action when
administered in an
appropriately designed study. Bioequivalence can be determined by an in vivo
study comparing
a pharmacokinetic parameter for the two compositions. Parameters often used in
bioequivalence
Dat
studies are T,, C, AUC AUCO.t. In the present context, substantial
bioequivalence of
two compositions or products is established by 90% confidence intervals (CI)
of between 0.80
and 1.25 for AUC and Cmax=
[0072] The term "cluster" as used herein refers to a series of
recurring cluster
headaches. Cluster duration is usually from one week to one year, although in
chronic cluster
headache the duration exceeds one year. The end of a cluster is identified by
at least 1 month of
remission.
[0073] The term "cluster headache" as used herein refers to a
condition
characterized by excruciating headache pain that recurs, usually daily
(although bouts may recur
up to 8 times per day), for a period of a week or longer. Cluster headaches
pain is usually
localized on one side of the head ("unilateral") usually the same side
although in some patients
the side can vary. The pain usually reaches full intensity in under 10 minutes
and lasts for
between 15 minutes and 3 hours (usually between 30 and 60 minutes). Because of
the rapid
onset of symptoms and short duration, treatment via a route by which the drug
is rapidly
absorbed is required.
[0074] The term "coating formulation," as used herein, means and
includes a
freely flowing composition or mixture, which is employed to coat a delivery
surface, including
one or more microprojections and/or arrays thereof.
[0075] The term "degradation," as used herein, means the purity
of the
biological agent decreases from an initial time point.
[0076] The term "desiccant," as used herein, means an agent that
absorbs water,
usually a chemical agent.
[0077] The term "deteriorate," as used herein, means that the
biologically active
agent is diminished or impaired in quality, character, or value.
[0078] The term "electrotransport" refers, in general, to the
passage of a
beneficial agent, e.g., a drug or drug precursor, through a body surface such
as skin, mucous
membranes, nails, and the like. The transport of the agent is induced or
enhanced by the
application of an electrical potential, which results in the application of
electric current, which
delivers or enhances delivery of the agent, or, for "reverse"
electrotransport, samples or enhances
16
Date Recue/Date Received 2023-12-18
sampling of the agent. The electrotransport of the agents into or out of the
human body may be
attained in various manners.
[0079] The term "half life" as used herein refers to the time
required for a drug's
blood or plasma concentration to decrease by one half. This decrease in drug
concentration is a
reflection of its excretion or elimination after absorption is complete and
distribution has reached
an equilibrium or quasi-equilibrium state. The half life of a drug in the
blood may be determined
graphically from a pharmacokinetic plot of a drug's blood-concentration time
plot, typically after
intravenous administration to a sample population. The half life can also be
determined using
mathematical calculations that are well known in the art. Further, as used
herein the term "half
life" also includes the "apparent half-life" of a drug. The apparent half life
may be a composite
number that accounts for contributions from other processes besides
elimination, such as
absorption, reuptake, or enterohepatic recycling.
[0080] The term "headache pain scale" as used herein means a
scale used to
allow patients to quantify their level of pain. Preferably a scale of 0 ¨ 3 is
used, wherein severe
pain has a pain score of 3, moderate pain has a score of 2, mild pain has a
score of I, and no pain
(also referred to as "pain freedom") has a score of 0.
[0081] The word "intracutaneous" as used herein, is a generic
term that refers to
delivery of an active agent (e.g., a therapeutic agent, such as a drug,
pharmaceutical, peptide,
polypeptide or protein) through the skin to the local tissue or systemic
circulatory system without
substantial cutting or penetration of the skin, such as cutting with a
surgical knife or piercing the
skin with a hypodermic needle. Intracutaneous agent delivery includes delivery
via passive
diffusion as well as delivery based upon external energy sources, such as
electricity (e.g.,
iontophoresis) and ultrasound (e.g., phonophoresis).
[0082] The term "intracutaneous flux," as used herein, means the
rate of
intracutaneous delivery of a drug.
[0083] The term "microprojection member" or "mieroneedle array,"
and the like
as used herein, generally connotes a microprojection grouping comprising a
plurality of
microprojections, preferably arranged in an array, for penetrating or piercing
the stratum
corneum. The microprojection member can be formed by etching or punching a
plurality of
microprojections from a thin sheet of metal or other rigid material, and
folding or bending the
17
Date Recue/Date Received 2023-12-18
microprojections out of the plane of the sheet to form a configuration. The
microprojection
member could alternatively be fabricated with other materials, including
plastics or polymers,
such as polyetheretherketone (PEEK). The microprojection member can be formed
in other
known techniques, such as injecting molding or micro-molding,
microelectromechanical systems
(MEMS), or by forming one or more strips having microprojections along an edge
of each of the
strip(s), as disclosed in U.S. Pat. Nos. 6,083,196; 6,091,975; 6,050,988;
6,855,131; 8,753,318;
9,387,315; 9,192,749; 7,963,935; 7,556,821; 9,295,714; 8,361,022; 8,633,159;
7,419,481;
7,131,960; 7,798,987; 7,097,631; 9,421,351; 6,953,589; 6,322,808; 6,083,196;
6,855,372;
7,435,299; 7,087,035; 7,184,826; 7,537,795; 8,663,155, and U.S. Pub. Nos.
US20080039775;
US20150038897; US20160074644; and US20020016562. As will be appreciated by one
having
ordinary skill in the art, when a microprojection array is employed, the dose
of the therapeutic
agent that is delivered can also be varied or manipulated by altering the
microprojection array
size, density, etc.
[0084] The term "microprojections" and" microneedles," as used
interchangeably herein, refers to piercing elements that are adapted to
penetrate, pierce or cut
into and/or through the stratum corneum into the underlying epidermis layer,
or epidermis and
dermis layers, of the skin of a living animal, particularly a mammal and, more
particularly, a
human. In one embodiment of the invention, the piercing elements have a
projection length less
than 1000 microns. In a further embodiment, the piercing elements have a
projection length of
less than 500 microns, more preferably less than 400 microns. The
microprojections further have
a width in the range of approximately 25 to 500 microns and a thickness in the
range of
approximately 10 to 100 microns. The microprojections may be formed in
different shapes, such
as needles, blades, pins, punches, and combinations thereof.
[0085] The terms "minimize" or "alleviate" as used herein means
reduce.
[0086] "Most bothersome other symptom" means a symptom, usually
a
migraine symptom, that is most bothersome to a patient, in addition to pain.
Preferably, a most
bothersome other symptom is identified by a patient at the start of a clinical
trial. Usually, most
bothersome other symptom is selected from nausea, photophonia, and
phonophobia.
[0087] "Most bothersome other symptom freedom" means the patient
reports an
absence of the most bothersome other symptom at one or more pre-specified
times after drug
18
Date Recue/Date Received 2023-12-18
administration. Preferred times for migraine patients are 1 hour, 2 hours, and
4 hours. Preferred
times for cluster headache patients are 15 minutes and 30 minutes.
[0088] "Nausea freedom" means the patient reports the absence of
nausea at a
pre-specified time period after drug administration.
[0089] "Optional" or "optionally" means that the subsequently
described
element, component or circumstance may or may not occur, so that the
description includes
instances where the element, component, or circumstance occurs and instances
where it does not.
[0090] "Pain freedom" means the patient reports an absence of
headache pain
(headache pain score = 0) at one or more pre-specified time after drug
administration. Preferred
times for migraine patients are 1 hour, 2 hours, and 4 hours. Preferred times
for cluster headache
patients are 15 minutes and 30 minutes.
[0091] "Pain relief' means the patient reports a reduction in
headache pain, a
reduction from moderate or severe pain (headache pain score = 3 or 2) to mild
or no pain
(headache pain score = 1 or 0), at one or more pre-specified time period after
drug
administration. Preferred times for migraine patients are 1 hour, 2 hours, and
4 hours. Preferred
times for cluster headache patients are 15 minutes and 30 minutes.
[0092] "Phonophobi a" refers to a fear of or aversion to sounds,
especially loud
sounds.
[0093] "Phonophobia freedom" means the patient reports the
absence of
phonophobia at a pre-specified time period after drug administration.
[0094] "Photophobia" refers to increased, often painful
sensitivity to light,
especially bright light.
[0095] "Photophobia freedom" means the patient reports the
absence of
photophobia at a pre-specified time period after drug administration.
[0096] "Partial AUC" means an area under the drug concentration-
time curve
(AUC) calculated using linear trapezoidal summation for a specified interval
of time, for
example, AUC(0-1hr), AUC(0-2hr), AUC(0-4hr), AUC(0-6hr), AUC(0-8hr) etc.
[0097] A drug "release rate," as used herein, refers to the
quantity of drug
released from a dosage form or pharmaceutical composition per unit time, e.g.,
milligrams of
drug released per hour (mg/hr) Drug release rates for drug dosage foi ____ ins
are typically measured
19
Date Recue/Date Received 2023-12-18
as an in vitro rate of dissolution, i.e., a quantity of drug released from the
dosage form or
pharmaceutical composition per unit time measured under appropriate conditions
and in a
suitable fluid.
[0098] The term "stable," as used herein, refers to an agent
formulation, means
the agent formulation is not subject to undue chemical or physical change,
including
decomposition, breakdown, or inactivation. "Stable" as used herein, refers to
a coating also
means mechanically stable, i.e., not subject to undue displacement or loss
from the surface upon
which the coating is deposited.
[0099] The terms "subject" or "patient" are used interchangeably
herein and
refer to a vertebrate, preferably a mammal. Mammals include, but are not
limited to, humans.
[00100] The terms "therapeutic-effective" or "therapeutically-
effective amount,"
as used herein, refer to the amount of the biologically active agent needed to
stimulate or initiate
the desired beneficial result. The amount of the biologically active agent
employed in the
coatings of the invention will be that amount necessary to deliver an amount
of the biologically
active agent needed to achieve the desired result. In practice, this will vary
widely depending
upon the particular biologically active agent being delivered, the site of
delivery, and the
dissolution and release kinetics for delivery of the biologically active agent
into skin tissues.
[00101] The term "transdermal," as used herein, means the
delivery of an agent
into and/or through the skin for local or systemic therapy.
[00102] The term "transdermal flux," as used herein, means the
rate of
transdermal delivery.
[00103] The term "Tmax" refers to the time from the start of
delivery to C..õ the
maximum plasma concentration of the biologically active agent.
[00104] The term "package" or "packaging" will be understood to
also include
reference to "storage" or "storing."
[00105] The term "zolmitriptan" includes, without limitation,
zolmitriptan salts,
simple derivatives of zolmitriptan and closely related molecules.
111. Intractitaneotis Delivery System
[00106] In one embodiment, the intracutaneous delivery system is
a transdermal
or intracutaneous drug delivery technology which comprises a disposable patch
comprised of a
Date Recue/Date Received 2023-12-18
microprojection member centered on an adhesive backing. The microprojection
member
comprises titanium (or other rigid material, including a plastic or polymeric
material like
polyetheretherketone (PEEK)) microneedles that are coated with a dry drug
product formulation.
The patch is mounted in a retainer ring to form the patch assembly. The patch
assembly is
removably mounted in a handheld applicator to form the intracutaneous delivery
system. The
applicator ensures that the patch is applied with a defined application speed
and energy to the site
of intracutaneous administration. The applicator may be designed for single
use or be reusable.
[00107] More particularly, the patch can comprise an array of
about 3 to 6 cm2
of titanium microneedles approximately 200-350 microns long, coated with a
hydrophilic
formulation of the relevant drug, and attached to an adhesive backing. The
maximum amount of
active drug that can be coated on a patch's microneedle array depends on the
active moiety of the
drug formulation, the weight of the excipients in the drug formulation, and
the coatable surface
area of the microneedle array. For example, patches with about 1 cm2, 2 cm2, 3
cm2, 4 cm2, 5
cm2, and 6 cm2 microneedle arrays may be employed. The patch is applied with a
hand-held
applicator that presses the microneedles into the skin to a substantially
uniform depth in each
application, close to the capillary bed, allowing for dissolution and
absorption of the drug
coating, yet short of the nerve endings in the skin The typical patch wear
time is about 15 to 45
minutes or less, decreasing the potential for skin irritation. Nominal
applicator energies of about
0.20 to 0.60 joules are generally able to achieve a good balance between
sensation on impact and
array penetration. The actual kinetic energy at the moment of impact may be
less than these
nominal values due to incomplete extension of the applicator's spring, energy
loss from breaking
away the patch from its retainer ring, and other losses, which may comprise
approximately total
25% of the nominal.
A. Array Design
[00108] A number of variables play a role in the type of array
utilized for a
particular active agent. For example, different shapes (e.g., shapes similar
to an arrowhead as
shown in Figure 31, hook, conical, or the Washington monument, Figure 1(A)-
(B)) may enable
higher drug loading capacity, while the length of the microprojections may be
increased to
provide more driving force for penetration. The stratum corneum has a
thickness of about 10-40
um, and microprojections must have an adequate size, thickness, and shape to
penetrate and
21
Date Recue/Date Received 2023-12-18
effect drug delivery through the stratum comeum. Figure 31, not drawn to
scale, demonstrates
how an array interacts with the skin, such that the microprojections penetrate
the stratum
corneum and the substrate interfaces with the surface of the skin. It is
advantageous to achieve
a thicker coating on the microprojections, which will penetrate the stratum
corneum, while
avoiding applying coating to the substrate or the base ("streets") of the
array, which will not
penetrate the stratum corneum. A larger surface area allows for a thicker
coating without
extending to the base or streets of the array. The coating is applied only to
the microprojections.
Further, the higher penetration force required for a more bulky projection
with coating may be
compensated by a longer length and lower density of projections per cm2.
[00109] Exemplary intracutaneous delivery systems that may be used
in the
present disclosure include the drug delivery technologies described in U.S.
Patent Nos.
6,083,196; 6,091,975; 6,050,988; 6,855,131; 8,753,318; 9,387,315; 9,192,749;
7,963,935;
7,556,821; 9,295,714; 8,361,022; 8,633,159; 7,419,481; 7,131,960; 7,798,987;
7,097,631;
9,421,351; 6,953,589; 6,322,808; 6,083,196; 6,855,372; 7,435,299; 7,087,035;
7,184,826;
7,537,795; 8,663,155, and U.S. Pub. Nos. US20080039775; US20150038897;
US20160074644;
and US20020016562. The disclosed systems and apparatus employ piercing
elements of various
shapes and sizes to pierce the outermost layer (i.e., the stratum corneum) of
the skin, and thus
enhance the agent flux. The piercing elements generally extend perpendicularly
from a thin, flat
substrate member, such as a pad or sheet. The piercing elements are typically
small, some having
a microprojection length of only about 25 to 400 microns and a microprojection
thickness of
about 5 to 50 microns. These tiny piercing/cutting elements make
correspondingly small
microslits/microcuts in the stratum corneum for enhanced
transdermal/intracutaneous agent
delivery. The active agent to be delivered is associated with one or more of
the microprojections,
preferably by coating the microprojections with a triptan- or zolmitriptan-
based formulation to
form a solid, dry coating, or optionally, by the use of a reservoir that
communicates with the
stratum corneum after the microslits are formed, or by forming the
microprojections from solid
triptan-based formulations that dissolve after application. The
microprojections can be solid or
can be hollow, and can further include device features adapted to receive
and/or enhance the
volume of the coating, such as apertures, grooves, surface irregularities or
similar modifications,
wherein the features provide increased surface area upon which a greater
amount of coating can
22
Date Recue/Date Received 2023-12-18
be deposited. The microneedles may be constructed out of stainless steel,
titanium, nickel
titanium alloys, or similar biocompatible materials, such as polymeric
materials.
1001101 The
present disclosure therefore encompasses patches and microneedle
arrays haying the following features:
= Patch size: About 1 to 20 cm2, or about 2 to 15 cm2 , or about 4 to 11
cm2, or
about 5 cm2, or about 10 cm2.
= Substrate size: About 0.5 to 10 cm2, or about 2 to 8 cm2, or about 3 to 6
cm2, or
about 3 cm2, or about 3.13 cm2, or about 6 cm2.
= Array size: About 0.5 to 10 cm2, or about 2 to 8 cm2 , or about 2.5 to 6
cm2, or
about 2.7 cm2, or about 5.5 cm2. or about 2.74 cm2, or about 5.48 cm2.
= Density (microprojections/cm2): At least about 10 microprojections/cm2,
or in the
range of about 200 to 2000 microprojections/cm2, or about 500 to 1000
microprojections/cm2, or about 650 to 800 microprojections/cm2, or
approximately 725 microprojections/cm2
= Number of microprojections/array: About 100 to 4000, or about 1000 to
3000, or
about or about 1500 to 2500, or about 1900 to 2100, or about 2000, or about
1987,
or about 200 to 8000, or about 3000 to 5000, or about or about 3500 to 4500,
or
about 4900 to 4100, or about 4000, or about 3974.
= Microprojection length: About 25 to 600 microns, or about 100 to 500
microns,
or about 300 to 450 microns, or about 320 to 410 microns, or about 340
microns,
or about 390 microns, or about 387 microns. In other embodiments, the length
is
less than 1000 microns, or less than 700 microns, or less than 500 microns.
Accordingly, the microneedles penetrate the skin to about 25 to 1000 microns.
= Tip length: About 100 to 250 microns, or about 130 to about 200 microns,
or
about 150 to 180 microns, or about 160 to 170 microns, or about 165 microns.
= Microprojection width: About 10 to 500 microns, or about 50 to 300
microns, or
about 75 to 200 microns, or about 90 to 160 microns, or about 250 to 400
microns, or about 300 microns, or about 100 microns, or about 110 microns, or
about 120 microns, or about 130 microns, or about 140 microns, or about 150
microns
23
Date Recue/Date Received 2023-12-18
= Microprojection thickness: about 1 micron to about 500 microns, or about
5
microns to 300 microns, or about 10 microns to 100 microns, or about 10
microns
to 50 microns, or about 20 microns to 30 microns, or about 25 microns.
= Tip angle: about 10-70 degrees, or about 20-60 degrees or about 30 to 50
degrees, or about 35 to 45 degrees, or about 40 degrees.
= Total active agent per array: About 0.1 mg to 10 mg, or about 0.5 mg to 5
mg, or
about 1 mg to 4 mg, or about 1 mg, or about 1.9 mg, or about 3.8 mg.
= Amount of inactive ingredient per array: About 0.1 to 10 mg, or about 0.2
to 4
mg, or about 0.3 mg to 2 mg, or about 0.6 mg, or about 0.63 mg, or about 1.3
mg,
or about 1.26 mg. Alternatively, the amount of inactive ingredient is from one
to
three times less than the active agent, or from about 0.033 mg to about 3.33
mg.
= Coating Thickness: about 100 gm to about 500 gm, or about 200 gm to about
350
gm, or about 250 gm to about 290 gm, or about 270 gm.
= Active agent per microprojection: About 0.01 to about 100 jig, or about
0.1 to 10
g, or about .5 to 2 jig, or about 1 jig, or about 0.96 pg.
[001111 A particularly preferred embodiment has a patch area of
about 5 cm2
adhered to a titanium substrate with an area of about 3.1 cm2 and a thickness
of about 25 gm.
The substrate is comprised of a microprojection array with an area of about
2.74 cm2 containing
about 1987 microprojections at a density of about 725 microprojections/cm2.
The dry
formulation contained on each microprojection may have the approximate shape
of an American
football with a thickness that tapers down from a maximum of about 270 gm and
consists of
about 0.96 jig of zolmitriptan and about 0.32 of tartaric acid, or about 1.9
mg of zolmitriptan and
about 0.63 mg of tartaric acid per patch.
[001121 Figure 32 demonstrates the shape of the microprojection, in
a preferred
embodiment, prior to bending (forming). Array forming is a process that bends
the individual
microprojections at right angles to the plane of the substrate. An array is
placed over the forming
tool, which contains cavities that are registered with the microprojections.
An elastomeric
forming disk is placed on top of the array and forced under pressure into the
cavities in the
forming tool. The elastomer flows into the cavities, causing the
microprojections to be bent to
24
Date Recue/Date Received 2023-12-18
the desired angle. The use of the elastomer has the advantage that no careful
registration of the
forming disk to the microprojections and the cavities is required in order to
have effective array
forming. As shown in Figure 32, the microprojections may be substantially
rectangular, with a
width of about 120 13 gm and a thickness of about 25.4 2.5 gm. The
microprojections end
with a triangular tip to facilitate penetration. The tip has an angle of 40
5 degrees, and is about
165 25 microns long. Prior to bending (forming) out from the substrate, the
microprojections
have a length of about 387 13 gm, and after bending, they protrude
perpendicular to the
substrate about 340 pm.
[00113] Another preferred embodiment has a patch area of about 5
cm2 adhered to
a titanium substrate of about 6 cm2 to and a thickness of about 25 p.m. The
substrate is
comprised of an array with an area of about 5.5 cm' containing about 4000
microprojections at a
density of about 725 microprojections/cm2. The dry formulation contained on
each
microprojection is in the approximate shape of an American football with a
thickness that tapers
down from a maximum of about 270 and consists of about 0.96 jig of
zolmitriptan and about
0.32 of tartaric acid, or about 3.8 mg of zolmitriptan and about 1.3 mg of
tartaric acid per patch.
The microprojections have a length of about 387 13 gm, a width of about 120
13 gm, and a
thickness of about 25.4 2.5 gm. The microprojections are rectangular, with a
triangular tip to
facilitate penetration. The tip has an angle of 40 5 degrees, and is about
165 25 microns
long.
[00114] The exact combination of bulk, length, and density that
produces the
desired penetration will vary, and may depend on the drug, its dose, the
disease or condition to
be treated and the frequency of administration. Thus, the drug delivery
efficiency of a particular
array (i.e., the amount of drug delivered to the bloodstream) will vary
between about 40% to
100%, or about 40%, or about 50%, or about 60%, or about 70%, or about 80%, or
about 90%, or
about 100%.
B. Impact Applicator
1001151 As illustrated in Figures 4(A)-(B), 5(A)-(E), the
intracutaneous drug
delivery system of the present disclosure may further comprise an impact
applicator having a
body and a piston movable within the body, wherein the surface of the piston
impacts the patch
Date Recue/Date Received 2023-12-18
against the skin causing the microprojections to pierce the stratum corneum.
The applicator is
adapted to apply the microneedle array to the stratum corneum with an impact
energy density of
at least 0.05 joules per cm2 in 10 milliseconds or less, or about 0.26 joules
per cm2 in 10
milliseconds or less, or about 0.52 joules per cm2 in 10 milliseconds or less.
[00116] As illustrated in Figures 2(A) and 2(B), the intracutaneous
delivery system
comprises a patch having an adhesive backing on one surface and a shiny metal
surface on the
other side comprised of the array of drug-coated microneedles. The patch may
be applied to the
skin by pressing the shiny metal surface against the skin either manually, or
preferably by an
applicator. Preferably, the applicator applies the patch to the skin with an
impact energy density
of 0.26 joules per cm2 in 10 milliseconds or less. As shown on Figures 2A, 2B,
3A and 3B, the
patch may be connected to and supported by a retainer ring structure forming a
patch assembly.
The retainer ring is adapted to fit onto the impact adaptor and removably
attach the patch to the
applicator. The retainer ring structure may comprise an inner ring and outer
ring, which are
designed to receive the adhesive patch and microneedle array. Figures 5(A)-(E)
demonstrate one
embodiment of the claimed invention, in which the user facilitates the
connection of the impact
applicator to the retainer ring, which is already loaded with the patch and
the microneedle array.
As shown, once the retainer ring and impact applicator are connected, a user
can unlock the
impact applicator by twisting the applicator cap Figure 5(C) shows that the
user may then press
the applicator downward on the skin to dispense the patch and apply it to the
skin. The patch
will removably attach to the patient's skin, and the retainer ring remains
attached to the
applicator. As shown in Figures 4(A) and 4(B), the retainer ring reversibly
attaches to the impact
applicator such that the impact applicator can be reused during subsequent
dosing events with
additional patch assemblies and potentially for other active ingredients and
disease states.
[00117] In another embodiment, the patch and applicator are
supplied as a single,
integrated unit, with packaging that ensures the stability and sterility of
the formulation. The
user removes the system from the packaging and applies the patch much as
described above.
The used applicator is then disposed of This embodiment, while somewhat higher
cost per dose,
provides a system that is less complex, smaller, lighter, and easier to use.
26
Date Recue/Date Received 2023-12-18
[00118] The present disclosure can also be employed in conjunction
with a wide
variety of active transdermal systems (as opposed to passive, manual
intracutaneous delivery
devices described herein), as the disclosure is not limited in any way in this
regard.
[00119] Some active transdermal systems utilize electrotransport.
Illustrative
electrotransport drug delivery systems are disclosed in U.S. Pat. Nos.
5,147,296; 5,080,646;
5,169,382 and 5,169,383.
One widely used electrotransport process, iontophoresis, involves the
electrically
induced transport of charged ions. Electroosmosis, another type of
electrotransport process
involved in the transdermal transport of uncharged or neutrally charged
molecules (e.g.,
transdermal sampling of glucose), involves the movement of a solvent with the
agent through a
membrane under the influence of an electric field. Electroporation, still
another type of
electrotransport, involves the passage of an agent through pores formed by
applying an electrical
pulse, a high voltage pulse, to a membrane. In many instances, more than one
of the noted
processes may be occurring simultaneously to different extents. Accordingly,
the term
"electrotransport" is given herein its broadest reasonable interpretation, to
include the electrically
induced or enhanced transport of at least one charged or uncharged agent, or
mixtures thereof,
regardless of the specific mechanism(s) by which the agent is actually being
transported with.
[00120] In addition, any other transport enhancing method, including
but not
limited to chemical penetration enhancement, laser ablation, heat, ultrasound,
or piezoelectric
devices, can be used in conjunction with the disclosure herein.
IV. Active Agents and Biocompatible Coating
[00121] The coating formulations applied to the microprojection member
described above to form solid coatings are comprised of a liquid, preferably
an aqueous
formulation having at least one biologically active agent, which can be
dissolved within a
biocompatible carrier or suspended within the carrier. The biologically active
agent may be a
triptan, including zolmitriptan, sumatriptan, rizatriptan, naratriptan,
eletriptan, almotriptan,
frovatriptan, avitriptan, and donitriptan, and pharmaceutically acceptable
salts, fragments,
analogs, or prodrugs thereof. Preferably, the biologically active agent is
zolmitriptan.
[00122] Examples of pharmaceutically acceptable salts include, without
limitation,
acetate, propionate, butyrate, pentanoate, hexanoate, heptanoate, levulinate,
chloride, bromide,
27
citrate, succinate, maleate, glycolate, gluconate, glucuronate, 3-
hydroxyisobutyrate,
tricarballylate, malonate, adipate, citraconate, glutarate, itaconate,
mesaconate, citramalate,
dimethylolpropionate, tiglate, glycerate, methacrylate, isocrotonate,13-
hydroxibutyrate,
crotonate, angelate, hydracrylate, ascorbate, aspartate, glutamate, 2-
hydroxyisobutyrate, lactate,
malate, pyruvate, fumarate, tartrate, nitrate, phosphate, benzene sulfonate,
methane sulfonate,
sulfate and sulfonate.
1001231 The concentration of biologically active ingredient and
excipients must be
carefully controlled to achieve the desired amount of the active ingredient
with an acceptable
coating thickness, avoid wicking of the coating formulation onto the base of
the microneedle
array, maintain the uniformity of the coating, and ensure stability. In one
embodiment, the
active agent is present in the coating formulation at a concentration of
between about 1% w/w to
about 60% w/w, preferably between about 15% and 60%, or more preferably
between 35% and
45%. The foi __ mulation may further comprise an acid at a concentration of
between about 0.1%
w/w to about 20% w/w. Such acid may be selected from tartaric acid, citric
acid, succinic acid,
malic acid, maleic acid, ascorbic acid, lactic acid, hydrochloric acid, either
individually or in
combination. In another embodiment, in the coating formulation, the active
agent to acid ratio
is about 1:1, about 2:1, about 3:1, about 4:1, or about 5:1. The present
disclosure further
encompasses a coating formulation comprising about 33% w/w zolmitriptan base
and about
11% w/w tartaric acid. In some embodiments, the acid is one of tartaric acid,
citric acid,
succinic acid, malic acid or maleic acid, and is present in an amount of about
0.33% to 10%
w/w, or about 8.33% to about 16.67% w/w, or about 13.33% w/w, or about 15%
w/w, or about
6.67% w/w. In some embodiments, the coating formulation comprises 45% w/w of
the active
agent, 15% w/w of the acid, and 40% w/w of water.
[00124] Surfactants may be included in the coating formulation.
Surfactants
suitable for inclusion in the coating formulations include, but are not
limited to, polysorbate 20
and polysorbate 80. Surfactants are commonly used to improve drug delivery as
penetration
enhancers. However, Applicant found that surfactants resulted in undulations
in the coating
formulation, which is indicative of an uneven film and is highly
disadvantageous. Applicant
found that the need for surfactants and other penetration enhancers can be
avoided through the
use of the claimed invention¨specifically, through the claimed zolmitriptan
transdermal
28
Date Recue/Date Received 2023-12-18
delivery patches. Furthermore, Applicant surprisingly found that microneedle
coating avoided
wicking, and the coating sufficiently adhered to the microprojections during
the manufacturing
process of the microneedle arrays, despite the lack of a surfactant.
[00125]
Antioxidants may be included in the coating formulation. Antioxidants
suitable for inclusion in the coating formulations include, but are not
limited to, methionine,
ascorbic acid, and EDTA.
[00126] The coating formulation further comprises a liquid,
preferably water, in an
amount sufficient (qs ad) to bring the formulation to 1009/0 prior to being
dried onto the
microneedles. The pH of the liquid coating folinulation may be below about pH
8. In other
cases, the pH is between about pH 3 and 7.4, or between about pH 3.5 to 4.5.
1001271
Representative examples of liquid coating formulations according to the
present disclosure are set forth in Table 1 below. The coatings generally
contain at least one
acid.
Table 1: Coating Formulations
Ingredient* 1 2 3 4 5 6 7 8 9 10
Zolmitriptan 25-50 1-30 45 40 45 40 45 40 45 40
Tartaric acid 0- 0- 0- 0- 0-
0-10 0-15 0-15 0-15 0-15
16.67 13.33 13.33 13.33
13.33
Citric acid 0- 0- 0- 0- 0-
0-10 0-15 0-15 0-15 0-15
16.67 13.33 13.33 13.33
13.33
Succinic acid 0- 0- ' 0- 0- ' 0-
0-10 0-15 0-15 0-15 0-15
16.67 13.33 13.33 13.33
13.33
Malic acid 0- 0- 0- 0- 0-
0-10 0-15 0-15 0-15 0-15
16.67 13.33 13.33 13.33
13.33
Maleic acid 0- 0- 0- 0- 0-
0-10 0-15 0-15 0-15 0-15
16.67 13.33 13.33 13.33
13.33
Ascorbic acid 0-5 0-5 0-5 0-5 0-5 0-5 0-5 0-5 0-5
0-5
Lactic acid 0-10 0-10 0-10 0-10 0-
10 0-10 , 0-10 , 0-10 , 0-10 , 0-10
Surfactants (e.g.
polysorbate 20, 0-0.2 0 0 0 0.2 0.2 0 0 0 0
polysorbate 80) -
EDTA
(Antioxidant 0-0.01 0 0 0 0 0 0.01 0.01 0 0
Chelator)
Methionine
0-1 0 0 0 0 0 0 0 1 1
(Antioxidant)
Deionized water qs qs qs qs qs
qs qs 100 100 100 100 100 qs qs
qs
100% 100% % % %
100% 100% 100%
% %
29
Date Recue/Date Received 2023-12-18
* Ingredients are expressed in% w/w
[00128] The present disclosure contemplates that sumatriptan or
other triptan may
be substituted for zolmitriptan in similar amounts or proportions as described
above.
[00129] The liquid coating formulations according to the present
disclosure
generally exhibit the ability to consistently coat the microneedles with
adequate content and
morphology, and result in a stable solid-state (dried) formulation, containing
less than 5% water,
preferably less than 3%. The liquid formulations are applied to the
microneedle arrays and the
microprojection tips thereof using an engineered coater which allows accurate
control of the
depth of the microprojection tips dipping into the liquid film. Examples of
suitable coating
techniques are described in U.S. Patent No. 6,855,372.
Accordingly, the viscosity of the liquid plays a role in microprojection
member
coating process as has been described. See Amen, M.; Fan, Sc.; Maa, YF (2010);
"Parathyroid
hormone PTH(1-34) formulation that enables uniform coating on a novel
transdermal
microprojection delivery system;" Pharmaceutical Research, 27, pp. 303-313;
see also Ameni
M, Wang X, Maa YF (2010); "Effect of irradiation on parathyroid hormone PTH(I -
34) coated
on a novel transdermal microprojection delivery system to produce a sterile
product--adhesive
compatibility;" Journal of Pharmaceutical Sciences, 99, 2123-34.
[00130] The coating formulations comprising zolmitriptan have a
viscosity less
than approximately 500 centipoise (cP) and greater than 3 cP, or less than
approximately 400 cP
and greater than 10 cP, or less than approximately 300 cP and greater than 50
cP, or less than
250 cP and greater than approximately 100 cP. In some embodiments, the
viscosity of the
liquid formulation prior to coating is at least 20 cP. In other embodiments,
the viscosity is about
25 cP, or about 30 cP, or about 35 cP, or about 40 cP, or about 45 cP, or
about 50 cP, or about
55 cP, or about 60 cP, or about 65 cP, or about 70 cP, or about 75 cP, or
about 80 cP, or about
85 cP, or about 90 cP, or about 95 cP, or about 100 cP, or about 150 cP, or
about 200 cP, or
about 300 cP, or about 400 cP, or about 500 cP. In other embodiments, the
viscosity is more
than about 25 cP, or a more than about 30 cP, or more than about 35 cP, or
more than about 40
cP, or more than about 45 cP, or more than about 50 cP, or more than about 55
cP, or more than
about 60 cP, or more than about 65 cP, or more than about 70 cP, or more than
about 75 cP, or
more than about 80 cP, or more than about 85 cP, or more than about 90 cP, or
more than about
Dat,
95 cP, or more than about 100 cP, or more than about 150 cP, or more than
about 200 cP, or
more than about 300 cP, or more than about 400 cP, or less than about 500 cP.
In a preferred
embodiment, the viscosity of the coating formulation is more than about 80 cP
and less than
about 350 cP; in another preferred embodiment, the viscosity is more than
about 100 cP and less
than about 350 cP; and, in another preferred embodiment, the viscosity is more
than about 100
cP and less than about 250 cP.
[00131] Once applied to the microprojections, the coating
formulation may have
an average thickness of about 10 to about 400 microns, or from about 30 to
about 300 microns,
or from about 100 microns to about 175 microns, or from about 115 to about 150
microns, or
about 135 microns, as measured from the microprojection surface. Although it
is preferable that
the coating formulation have a uniform thickness covering the microprojection,
the formulation
may vary slightly as a result of the manufacturing process. As shown in Figure
31, the
microprojections are generally coated uniformly because they penetrate the
stratum comeum. In
some embodiments, the microprojections are not coated the entire distance from
the tip to the
base; instead, the coating covers a portion of the length of the
microprojection, measured from
tip to the base, of at least about 10% to about 80%, or 20% to about 70%, or
about 30% to about
60%, or about 40% to about 50% of the length of the microprojection.
[00132] The liquid coating formulation is applied to an array of
microprojections
so as to deliver a dose of the active agent in the amount of about 0.1 mg to
10 mg per array. In
the case of zolmitriptan, the dose is about 0.25 mg to about 10 mg, or about 1
mg or more, or
about 1.9 mg or more, or about 2 mg or more, or about 3 mg or more, or about
3.8 mg or more,
or about 4 mg or more, or about 5 mg or more delivered to the stratum comeum
per array (via a
patch or other form). In one embodiment, the amount of the zolmitriptan
contained in coating
formulation is 1-10001.1g or 10-100 us. In one embodiment, the array size is
about 5.5 cm2
comprising a dose of about 3.8 mg zolmitriptan, or the array size is about 3
cm2 comprising a
dose of about 3.8 mg, or the array size is about 3 cm2, comprising a dose of
about 1.9 mg. The
amount of zolmitriptan or similar active agent per microprojection could range
from about 0.001
to about 1000 g, or about 0.01 to about 100 jig, or about 0.1 to about 10
jig, or about 0.5 to
about 2 pg. In one embodiment, the amount of zolmitriptan or similar active
agent per
31
Date Recue/Date Received 2023-12-18
microprojection is about 1 jig. The microprojection shape and size has a
significant bearing on
the drug loading capacity and on the effectiveness of drug delivery.
[00133] Importantly, the formulations of the present disclosure do
not primarily
rely on penetration enhancers to facilitate absorption of the active agent
into the bloodstream.
Penetration enhancers, such as Azone and fatty acids, often cause skin
irritation and have
other disadvantages. Thus, the systems of the present disclosure are either
completely free of a
penetration enhancer, or are substantially free thereof. In other embodiments,
there is less than
15% w/w of penetration enhancer present, or less than 10% w/w, or less than 5%
w/w, or less
than 2.5% w/w, or less than 1% w/w present in the dried formulation.
[00134] The biologically active agent formulations are generally
prepared as a
solid coating by drying a coating formulation on the microprojection, as
described in U.S.
Application Pub. No. 2002/0128599. The coating formulation is usually an
aqueous
formulation. During a drying process, all volatiles, including water are
mostly removed;
however, the final solid coating may still contain about 1% w/w water, or
about 2% w/w water,
or about 3% w/w water, or about 4% w/w water, or about 5% w/w water. The
oxygen and/or
water content present in the formulations are reduced by the use of a dry
inert atmosphere
and/or a partial vacuum. In a solid coating on a microprojection array, the
drug may be present
in an amount of less than about 10 mg per unit dose or less than about 4 mg or
less than about 3
mg or less than about 2 mg or less than about 1 mg. With the addition of
excipients, the total
mass of solid coating may be less than about 15 mg per unit dose.
[00135] The microprotrusion member is usually present on an
adhesive backing,
which is attached to a disposable polymeric retainer ring. This assembly is
packaged
individually in a pouch or a polymeric housing. In addition to the assembly,
this package
contains a dead volume that represents a volume of at least 3 mL. This large
volume (as
compared to that of the coating) acts as a partial sink for water. For
example, at 20 C, the
amount of water present in a 3 mL atmosphere as a result of its vapor pressure
would be about
0.05 mg at saturation, which is typically the amount of residual water that is
present in the solid
coating after drying. Therefore, storage in a dry inert atmosphere and/or a
partial vacuum will
further reduce the water content of the coating resulting in improved
stability.
32
Date Recue/Date Received 2023-12-18
[00136] According to the disclosure, the coating can be applied to
the
microprojections by a variety of known methods. For example, the coating may
be only applied
to those portions of the microprojection member or microprojections that
pierce the skin (e.g.,
tips). The coating is then dried to form a solid coating. One such coating
method comprises dip-
coating. Dip-coating can be described as a method to coat the microprojections
by partially or
totally immersing the microprojections into a coating solution By use of a
partial immersion
technique, it is possible to limit the coating to only the tips of the
microprojections.
[00137] A further coating method comprises roller coating, which
employs a roller
coating mechanism that similarly limits the coating to the tips of the
microprojections. The
roller coating method is disclosed in U.S. Application Pub. No. 2002/0132054.
As discussed in
detail therein, the disclosed roller coating method provides a smooth coating
that is not easily
dislodged from the microprojections during skin piercing.
[00138] A further coating method that can be employed within the
scope of the
present invention comprises spray coating. Spray coating can encompass
formation of an
aerosol suspension of the coating composition. In one embodiment, an aerosol
suspension
having a droplet size of about 10 to 200 picoliters is sprayed onto the
microprojections and then
dried.
[00139] Pattern coating can also be employed to coat the
microprojections. The
pattern coating can be applied using a dispensing system for positioning the
deposited liquid
onto the microprojection surface. The quantity of the deposited liquid is
preferably in the range
of 0.1 to 20 nanolitersimicroprojection. Examples of suitable precision-
metered liquid
dispensers are disclosed in U.S. Patent Nos. 5,916,524; 5,743,960; 5,741,554;
and 5,738,728.
[00140] Microprojection coating formulations or solutions can also
be applied
using ink jet technology using known solenoid valve dispensers, optional fluid
motive means
and positioning means which is generally controlled by use of an electric
field. Other liquid
dispensing technology from the printing industry or similar liquid dispensing
technology known
in the art can be used for applying the pattern coating of this invention.
[00141] In one embodiment of the disclosure, the thickness of the
dried coating
formulations comprising zolmitriptan range from about 10 to 100 microns as
measured from the
33
Dat
microprojection surface, or from about 20 to 80 microns, or from about 30 to
60 microns, or
from about 40 to 50 microns. The desired coating thickness is dependent upon
several factors,
including the required dose and, hence, coating thickness necessary to deliver
the dose, the
density of the microprojections per unit area of the sheet, the viscosity, the
solubility and
concentration of the coating composition and the coating method chosen. The
thickness of
coating applied to microprojections can also be adapted to optimize stability
of the zolmitriptan.
Known formulation adjuvants can also be added to the coating formulations
provided they do
not adversely affect the necessary solubility and viscosity characteristics of
the coating
formulation nor the physical integrity of the dried coating.
[00142] The coating is applied to the microneedles, which protrude
from the base,
or streets, of the microneedle array. The coating is applied to the tips of
the microneedles, and
is not intended to cover the microneedles and the surface of the microneedle
array. This reduces
the amount of drug per transdermal patch, which is advantageous in light of
FDA Guidance on
the danger of residual drug on transdermal delivery systems, which suggests
that the amount of
residual drug in a system should be minimized. See FDA Guidance for Industry,
Residual Drug
in Transdermal and Related Drug Delivery Systems (August 2011). Applicant's
strategy was to
maximize drug release into skin per unit area, without using an excess of drug
for coating.
[00143] After a coating has been applied, the coating formulation is
dried onto the
microprojections by various means. The coated microprojection member may be
dried in
ambient room conditions. However, various temperatures and humidity levels can
be used to
dry the coating formulation onto the microprojections. Additionally, the
coated member can be
heated, stored under vacuum or over desiccant, lyophilized, freeze dried or
similar techniques
used to remove the residual water from the coating.
[00144] Coating was conducted at ambient temperature utilizing a
roller drum,
rotating at 50 rpm, in a drug formulation reservoir (2 mL in volume) to
produce a film of
controlled thickness of around 270 um in thickness. Further information about
the coating
process can be found in U.S. Pat, No. 6,855,372.
Microprojection arrays are dipped into the drug film, and the amount of
coating is controlled by
the number of dips (passes) through the drug film.
34
[00145] During the drying process, there may be issues related to
forming a
uniform coating the microprojection with a controlled and consistent
thickness. One common
issue in transdermal patch coating, called "dripping" or "teardrop"
formations, occurs when the
coating is drying and the coating accumulates at the end of the
microprojections in a "teardrop"
shape. This teardrop shape can blunt the sharp end of the microneedle,
potentially impacting the
effectiveness and uniformity of penetration. Uneven layers of formulation on
the
microprojections results in uneven, and sometimes inadequate drug delivery.
Additionally, the
issues in the drying process cause issues of quality control in formulation
coating. Preferred
liquid coating formulations comprise zolmitriptan in an amount of 30% w/w to
about 60% w/w,
preferably about 40% w/w to about 50% w/w, more preferably about 45% w/w, and
tartaric acid
in an amount of about 5% w/w to about 25% w/w, preferably about 10% w/w to
about 20%
w/w, more preferably about 15% w/w, in a liquid carrier, preferably water,
more preferably
deionized water. With these liquid coating formulations, Applicant
surprisingly found that
maintaining a viscosity of about 150 cP to about 350 cP, preferably about 200
cP to about 300
cP, more preferably about 250 centipoise, and a surface tension of about 50
mNnfl to about 72
mNm-1, preferably about 55 mNm-1 to about 65 mNm-1 , more preferably about
62.5 mNrn1 was
required to avoid dripping. Teardrop foi 'nation could be avoided while
simultaneously allowing
each dip of microprojections into the liquid coating formulation to pick up
sufficient volume of
liquid coating formulation, thereby achieving the desired drug dose with a
minimum number of
dips. When the viscosity and surface tension of the coating solution are high
enough, the
coated liquid does not quickly drip back or foim a teardrop shape after
dipping and before
drying.
[00146] The products and methods described herein with respect to
delivery of
zolmitriptan in a method of rapidly achieving therapeutic concentrations of
zolmitriptan for
treatment of migraine or cluster headache also can be applied to other
triptans, including
sumatriptan, rizatriptan, naratriptan, eletriptan, almotriptan, frovatriptan,
avitriptan, and
donitriptan.
[00147] In one aspect, the route of administration of zolmitriptan
is
intramuscularly, intracutaneously, subcutaneously, intranasally, oral
inhalation, transdermally,
buccally, pulmonary, or sublingually. For example, a formulation designed for
intramuscular or
Date Recue/Date Received 2023-12-18
subcutaneous delivery would contain 1 mg of zolmitriptan (base) and 0.3 mg of
tartaric acid in 1
mL of 0.9 % w/v saline. Further, a formulation designed for pulmonary delivery
would be in
the form of zolmitriptan salt dissolved or suspended in water or a
zolmitriptan powder
generated using milling, supercritical fluid process, spray drying or spray
freeze drying for
inhalation delivery and would produce respirable particles with a controlled
particle size of
about 0.5- 5.8 prn mass median aerodynamic diameter (MTV1AD) to ensure that a
significant
fraction of zolmitriptan would be deposited in the lung. The processes to
produce zolmitriptan
powder can be used directly by metering in from a powder reservoir or
premetering into a dry
powder inhaler (DPI) format, or the particulates may be suspended/dispersed
directly into a
suspending media, such as a pharmaceutically acceptable propellant e.g.,
hydrofluoralkanes
(selected from the group consisting of: 1,1,1,2-tetrafluoroethane,
1,1,1,2,3,3,3-heptafluoro-n-
propane and a mixture of 1,1,1,2-tetrafluoroethane and 1,1,1,2,3,3,3-
heptafluoro-n-propane or a
mixture of thereof), in a metered dose inhaler (MDI) format. The particles
produced may be
crystalline or may be amorphous depending on the process to generate the
zolmitriptan powder.
In one aspect, the zolmitriptan dose ranges from 0.5 to 4 mg, administered at
the onset of
migraine or cluster headache.
V. Packaging, Sterilization
[00148] Improved physical stability of the dry coated formulations
provides not
only the benefit of an increased storage or shelf life for the therapeutic
agent itself, but enhances
efficacy in that once stabilized in accordance with the compositions of and
methods for
formulating and delivering of the present invention, the therapeutic agents
become useful in a
greater range of possible formulations, and with a greater variety of
therapeutic agent delivery
means.
[00149] The present disclosure comprises an active agent
formulation wherein the
deterioration by oxygen and/or water is minimized and/or controlled by the
manufacture and/or
packaging of the active agent formulation in a dry inert atmosphere. The
formulation may be
contained in a dry inert atmosphere in the presence of a desiccant, optionally
in a chamber or
package comprising a foil layer.
[00150] The desiccant can be any known to those skilled in the art.
Some common
desiccants include, but are not limited to molecular sieve, calcium oxide,
clay desiccant, calcium
36
Date Recue/Date Received 2023-12-18
sulfate, and silica gel. The desiccant may be one that can be placed with the
biologically active
agent-containing formulation in the presence of an inert atmosphere in a
package comprising a
foil layer.
[00151] In another aspect, the active agent formulation is packaged
in a chamber
comprising a foil layer after the formulation is coated onto the
microprojection array delivery
device. In this embodiment, a desiccant is contained in the chamber,
preferably attached to a
chamber lid which comprises a foil layer, and the chamber is purged with dry
nitrogen or other
inert gas such as a noble gas prior to the delivery device-containing foil
chamber being sealed
by the foil lid. Any suitable inert gas can be used herein to create the dry
inert atmosphere.
[00152] In one embodiment, the compositions of and methods for
formulating and
delivering zolmitriptan suitable for intracutaneous delivery utilize a patch
assembly. This patch
assembly is manufactured and/or packaged in a dry inert atmosphere, and in the
presence of a
desiccant. In one embodiment, the patch assembly is manufactured in a dry
inert atmosphere
and/or packaged in a chamber comprising a foil layer and having a dry inert
atmosphere and a
desiccant. In one embodiment, the patch assembly is manufactured and/or
packaged in a partial
vacuum. In one embodiment, the patch assembly is manufactured and/or packaged
in a dry inert
atmosphere, and a partial vacuum. In one embodiment, patch assembly is
manufactured in a dry
inert atmosphere under a partial vacuum and/or packaged in a chamber
comprising a foil layer
and having a dry inert atmosphere, a partial vacuum, and a desiccant,
[00153] Generally, in the noted embodiments of the present
invention, the inert
atmosphere should have essentially zero water content. For example, nitrogen
gas of essentially
zero water content (dry nitrogen gas) can be prepared by electrically
controlled boiling of liquid
nitrogen. Purge systems can be also used to reduce moisture or oxygen content.
A range for a
partial vacuum is from about 0.01 to about 0.3 atmospheres.
[00154] In an aspect of this embodiment, the zolmitriptan further
comprises a
biocompatible carrier. In another embodiment, there is an intracutaneous
delivery system,
adapted to deliver zolmitriptan, comprising: (a) a microprojection member
including a plurality
of microprojections that are adapted to pierce the stratum corneum of a
patient; (b) a hydrogel
formulation comprised of zolmitriptan, wherein the hydrogel formulation is in
communication
with the microprojection member; and (c) packaging purged with an inert gas
and adapted to
37
Date Recue/Date Received 2023-12-18
control environmental conditions sealed around the microprojection member,
wherein the sealed
package has been exposed to radiation to sterilize the microprojection member.
[00155] In another embodiment, there is an intracutaneous delivery
system,
adapted to deliver zolmitriptan, comprising: (a) a microprojection member
including a plurality
of microprojections that are adapted to pierce the stratum corneum of a
patient; (b) a solid film
disposed proximate the microprojection member, wherein the solid film is made
by casting a
liquid formulation comprising zolmitriptan, a polymeric material, a
plasticizing agent, a
surfactant and a volatile solvent; and (c) packaging purged with an inert gas
and adapted to
control environmental conditions sealed around the microprojection member,
wherein the sealed
package has been exposed to radiation to sterilize the microprojection member.
1001561 The present disclosure is also to a method for terminally
sterilizing a patch
assembly adapted to deliver zolmitriptan, comprising the steps of: (a)
providing a
microprojection member having a plurality of microprojections that are adapted
to pierce the
stratum corneum of a patient having a biocompatible coating comprising
zolmitriptan disposed
on the microprojection member; and (b) exposing the microprojection member to
radiation
selected from the group consisting of gamma radiation and e-beam, wherein the
radiation is
sufficient to reach a desired sterility assurance level. Such sterility
assurance level may be 10'6
or 10'5. The method may further comprise sealing the microprojection member
with a desiccant
inside packaging purged with an inert gas and exposing the packaged
microprojection member
to radiation selected from the group consisting of gamma radiation and e-beam
radiation,
wherein the radiation is sufficient to reach a desired sterility assurance
level.
[00157] In an aspect of this embodiment, the method further
comprises the step of
mounting a patch comprised of a microprojection member attached to an adhesive
backing on a
pre-dried retainer ring to form a patch assembly, and subsequently sealing the
microprojection
member inside the packaging. In an aspect of this embodiment, the system
further comprises a
desiccant sealed inside the packaging with the patch assembly, and/or the
packaging is purged
with nitrogen, and/or the packaging comprises a pouch comprised of a foil
layer. Preferably, the
foil layer comprises aluminum.
[00158] The step of exposing the microprojection member to
radiation may occur
at approximately -78.5 to 25 C, or the member may be exposed to radiation at
ambient
38
Date Recue/Date Received 2023-12-18
temperature. The radiation may be in the range of approximately 5 to 50 kGy,
or approximately
to 30 kGy, or approximately 15 to 25 kGy, or approximately 21 kGy, or
approximately 7
kGy. In one aspect of this embodiment, the radiation is delivered to the
microprojection
member at a rate of at least approximately 3.0 kGy/hr.
[00159] As described herein, Applicant developed a zolmitriptan
formulation
which, when coated on the microneedle members of the present disclosure, is
stable and
maintains its amorphous character for at least 6 months, or at least 9 months,
or at least 12
months, or at least 18 months, or at least 24 months after being exposed to
radiation as described
above.
[00160] In one embodiment, the dried zolmitriptan formulation on
the
microneedles retains for at least 6 months approximately 100% of initial
purity, or
approximately 99% of initial purity, or approximately 98% of initial purity,
or approximately
97% of initial purity, or approximately 96% of initial purity, or
approximately 95% of initial
purity, or approximately 90% of initial purity. In other aspects, such purity
is retained for at
least 9 months, or at least 12 months, or at least 18 months, or at least 24
months after
packaging. In a further embodiment, the zolmitriptan coating on the
microneedles retains its
purity as described in this paragraph, and also substantially maintains its
amorphous character
for at least 6 months, or at least 9 months or at least 12 months, or at least
18 months, or at least
24 months after packaging.
[00161] In one embodiment, a method for manufacturing a patch
assembly for an
intracutaneous delivery system adapted to deliver a zolmitriptan, comprises
the steps of:
providing a microneedle member having a plurality of microneedles that are
adapted to
penetrate or pierce the stratum corneum of a patient having a biocompatible
coating disposed on
the microneedle member, the coating being formed from a coating formulation
having
zolmitriptan and disposed thereon; sealing the microneedle member with a
desiccant inside
packaging purged with nitrogen and adapted to control environmental conditions
surrounding
the microneedle and exposing the microneedle member to radiation selected from
the group
consisting of gamma radiation, e-beam and x-ray wherein the radiation is
sufficient to reach a
desired sterility assurance level.
39
Date Recue/Date Received 2023-12-18
[00162] In accordance with another embodiment of the invention, a
method for
delivering stable biologically active agent formulations comprises the
following steps: (i)
providing a microprojection member having a plurality of microprojections,
(ii) providing a
stabilized formulation of biologically active agent; (iii) forming a
biocompatible coating
formulation that includes the formulation of stabilized biologically active
agent, (iv) coating the
microprojection member with the biocompatible coating formulation to form a
biocompatible
coating; (v) stabilizing the biocompatible coating by drying; and (vi)
applying the coated
microprojection member to the skin of a subject.
[00163] Additionally, optimal stability and shelf life of the agent
is attained by a
biocompatible coating that is solid and substantially dry. However, the
kinetics of the coating
dissolution and agent release can vary appreciably depending upon a number of
factors. It will
be appreciated that in addition to being storage stable, the biocompatible
coating should permit
desired release of the therapeutic agent.
[00164] Encompassed herein is a method for terminally sterilizing a
transdermal
device adapted to deliver a zolmitriptan, comprising the steps of: providing a
microprojection
member having a plurality of microprojections that are adapted to penetrate or
pierce the
stratum corneum of a patient having a biocompatible coating disposed on the
microprojection
member, the coating being formed from a coating formulation having at least
one triptan,
preferably zolmitriptan, disposed thereon; and exposing the microprojection
member to
radiation selected from the group consisting of gamma radiation and e-beam,
wherein the
radiation is sufficient to reach a desired sterility assurance level. A
further aspect of this method
comprises the further step of sealing the microprojection member inside
packaging adapted to
control environmental conditions surrounding the microprojection member. In
one aspect the
packaging comprises a foil pouch. A further aspect of this method, comprises
the further step of
sealing a desiccant inside the packaging. Further, the method comprises the
step of mounting
the microprojection member on a pre-dried retainer ring prior to sealing the
microprojection
member inside the packaging. A further aspect of this method comprises the
step of purging the
packaging with an inert gas prior to sealing the packaging. In one embodiment,
the inert gas
comprises nitrogen.
VI. In Vivo Pharmacokinetics (PK)
Date Recue/Date Received 2023-12-18
[00165] The intracutaneous/transdermal systems of the present
invention provide
serum concentrations to the bloodstream faster and with less overall drug
exposure as compared
to oral doses of the same drug. For example, the absorption of
intracutaneously administered
zolmitriptan delivered via the systems of the present disclosure results in a
Cmax of less than 50
mg/mL and the Traax is between about 2 minutes and 30 minutes. In another
embodiment, the
plasma zolmitriptan AUC for the first 2 hours is greater than that seen
following oral
administration, but the plasma zolmitriptan AUC(0-24hr) is less than that seen
after oral
administration.
[00166] In another aspect, the absorption of the zolmitriptan
results in an increase
in the maximum plasma zolmitriptan, but the N-desmethyl zolmitriptan
production (AUCO-2411.)
is reduced and thus has a lower likelihood for metabolite accumulation. The
intracutaneous
administration of triptans, including zolmitriptan, avoids the first pass
metabolism in the liver
found with oral administration, resulting in higher bioavailability. In
particular, metabolism is
significantly reduced resulting in at least about 20% less serum concentration
of N-desmethyl
zolmitriptan at time points (e.g., 1.5 hours, 2 hours, 5 hours, 10 hours) post-
application than
seen in oral products. Further, zolmitriptan plasma levels may be increased,
but the N-
desm ethyl zolmitriptan production is reduced relative to that produced upon
oral administration
of a comparable dose of zolmitriptan. Therefore, there is a lower likelihood
for metabolite
accumulation. However, because N-desmethyl zolmitriptan is more active at the
target sites
than zolmitriptan, the present invention is surprisingly effective at treating
migraine or cluster
headache as detailed below. In addition, the apparent half-life of
zolmitriptan is reduced
compared to oral administration, such that the duration of side effects may be
reduced.
[00167] In another aspect, the plasma concentration of N-desmethyl
zolmitriptan is
about 0.05 to 0.9 ng/ml after about 15 minutes after application, or about 0.1
to 1.4 ng/ml after
about 30 minutes, or about 0.1 to 1.6 ng/ml after about 1 hour, or about 0.1
to 1.4 ng/ml after
about 1.5 hours, or about 0.1 to 1.3 ng/ml after about 2 hours, or less than
about 0.7 ng/ml after
hours, or less than about 02 ng/ml after 10 hours,
[00168] Further, the intracutaneously delivered biocompatible
coating comprises a
dose of the zolmitriptan in the range of approximately 0.2 to 10 mg,
preferably1 to 5 mg, more
preferably approximately 1.9 or 3.8 mg, wherein intracutaneous delivery of the
zolmitriptan
41
Date Recue/Date Received 2023-12-18
results in a plasma C.,õ of at least 2 ng/mL zolmitriptan, at least 3.6 ng/mL
zolmitriptan, at least
4 ng/mL zolmitriptan, at least 6 ng/mL zolmitriptan, at least 9 ng/mL
zolmitriptan, at least 10
ng/mL zolmitriptan, at least 12 ng/mL zolmitriptan, at least 14 ng/mL
zolmitriptan, at least 16
ng/mL zolmitriptan, at least 18 ng/mL zolmitriptan, at least 20 ng/mL
zolmitriptan, at least 25
ng/mL zolmitriptan, at least 30 ng/mL zolmitriptan, at least 40 ng/mL
zolmitriptan, at least 45
ng/mL zolmitriptan, at least 50 ng/mL zolmitriptan, less than 50 ng/mL
zolmitriptan, at least 55
ng/mL zolmitriptan, at least 60 ng/mL zolmitriptan or at least 65 ng/mL
zolmitriptan after one
application or two applications.
[00169] Also, the intracutaneous delivery of the zolmitriptan
results in a plasma
Tina), of no more than 1 minute, no more than 2 minutes, no more than 3
minutes, no more than 4
minutes, no more than 5 minutes, no more than 8 minutes, no more than 10
minutes, no more
than 12 minutes, no more than 15 minutes, no more than 20 minutes, no more
than 30 minutes,
no more than 35 minutes, no more than 40 minutes, no more than 45 minutes, no
more than 50
minutes, no more than 55 minutes, is between 2 minutes and 30 minutes, or is
no more than 60
minutes after one application.
[00170] In one embodiment, the Tmax of intracutaneously
administered
zolmitriptan via the inventive systems occurs about 2 hours or more before
conventional release
oral zolmitriptan tablets, or about 1.8 hours or more before such tablets, or
about 1.6 hours or
more before such tablets, or about 1.4 hours or more before such tablets, or
about 1.2 hours or
more before such tablets, or about 1.0 hours or more before such tablets, or
about 0.8 hours or
more before such tablets, or about 0.6 hours or more before such tablets, or
about 0.4 hours or
more before such tablets, or about 0.2 hours or more before such tablets.
[00171] In another embodiment, the Tm of intracutaneously
administered
zolmitriptan via the inventive systems occurs about 3 hours or more before
ZOMIG
(zolmitriptan) orally disintegrating tablets, or about 2.5 hours or more
before such tablets, or
about 2.0 hours or more before such tablets, or about 1.5 hours or more before
such tablets, or
about 1.0 hours or more before such tablets, or about 0.5 hour before such
tablets.
[00172] In further embodiments, the Tnõ, of intracutaneously
administered
zolmitriptan via the inventive systems occurs about 3 hours or more before
zolmitriptan nasal
spray, or about 2.5 hours or more before such spray, or about 2.0 hours or
more before such
42
Date Recue/Date Received 2023-12-18
spray, or about 1.5 hours or more before such spray, or about 1.0 hour or more
before such
spray, or about 0.5 hour or more before such spray.
[00173] In another embodiment, the elimination rate (ty) for
intracutaneously
administered zolmitriptan via the inventive systems is about 0.75 hour, or 1.0
hour, or 1.1 hour,
or 1.2 hour, or 1.3 hour, or 1.4 hour, or 1.5 hour, or 1.6 hour, or 1.7 hour,
or 1.8 hour, or 1.9
hour, or 2.0 hours. Such elimination rate (N) is approximately three times the
rate of
zolmitriptan conventional tablets, or approximately twice the rate of
zolmitriptan conventional
tablets.
[00174] In further embodiments, the Cmax for intracutaneously
administered
zolmitriptan via the inventive systems is about 1 to about 8 times higher than
the Crnax of
conventional oral 2.5 mg zolmitriptan tablets, or about 1.5 to about 7 times
higher, or about 2 to
about 6 times higher, or about 3 to about 5 times higher, or about 4 times
higher.
[00175] Further, the mean peak exposure (C.) is about 2 to about 5
times higher
for intracutaneous zolmitriptan relative to the oral tablets. In a further
aspect, the mean peak
exposure (C.) for the intracutaneous zolmitriptan of the present invention is
about 1.0 to about
40.0 mg/mL, or about 5.0 to about 35.0 mg/mL, or about 10.0 to about 30.0
mg/mL, or about
15.0 to about 25.0 mg/mL, or about 20.0 to about 30.0 mg/mL, or about 25
mg/mL.
[00176] Additionally, compared to conventional oral zolmitriptan
2.5 mg,
intracutaneous zolmitriptan of the invention at doses ranging from about 0.5
mg to about 4.0 mg
have a bioavailability of about 50% to about 100% of the oral bioavailability.
In other
embodiments, the bioavailability of intracutaneous is about 55% to about 95%,
or about 60% to
about 90%, or about 65% to about 85%, or about 70% to about 80%, or about 75%
of the oral
bioavailability.
[00177] Finally, the present invention encompasses formulations and
devices that
are bioequivalent to the M207 Intracutaneous Delivery System described herein.
Thus, the
disclosure covers products where bioequivalence is established by (i) a 90%
Confidence Interval
(Cl) for AUC which is between 0.80 and 1.25; and (ii) a 90% Cl for C. which is
between 0.80
and 1.25.
43
Date Recue/Date Received 2023-12-18
VII. Methods of Treatment
[00178] The drug-device combinations of the present invention can
be used to treat
a variety of diseases and conditions, including migraine and cluster headache.
In one
embodiment of the present invention, there is a method for treatment or
alleviation of migraine
or cluster headache to an individual in need thereof, comprising
administration of a
therapeutically effective amount of a zolmitriptan-based agent, wherein the
absorption of the
zolmitriptan-based agent results in a plasma C.õ of less than 50 ng/mL. Doses
include about
0.2 mg to about 10 mg zolmitriptan. The dose may also be 0.48 mg, 0.96 mg, 1.9
mg, and 3.8
mg zolmitriptan. Doses also include a single patch administration of either
1.0 mg, 1.9 mg, or
3.8 mg, or two patches of 1.9 mg. These doses can be delivered utilizing the
patch(es) described
herein and can be applied to the skin of any part of the body. In a preferred
embodiment, the
zolmitriptan dose(s) is delivered via the patch to the upper arm to treat a
single migraine or
cluster headache attack.
[00179] In certain embodiments, the methods of treatment of
migraine or cluster
headache as described herein result in improvement with respect to the
following therapeutic
endpoints: Migraine Pain freedom at 1 hour, 2 hours, or 4 hours after dosing;
Cluster headache
pain freedom at 15 or 30 minutes after dosing, most bothersome other migraine
symptom
freedom at 1 hour or 2 hours after dosing; freedom from a patient's previously
identified most
bothersome other cluster headache symptom at 15 or 30 minutes after dosing,
migraine pain
relief at 1 hour, 2 hours or 4 hours, Cluster headache pain relief at 15 or 30
minutes after dosing,
pain relief at 30 minutes; photophobia freedom at 2 hours; phonophobia freedom
at 2 hours;
pain relief at 15 minutes; pain relief at 3 hours; pain relief at 4 hours;
nausea freedom at 2 hours;
pain freedom at 30 minutes; pain freedom at 24 hours; and pain freedom at 48
hours. Further,
there is an improvement in terms of treated patients requiring rescue
medication. Improvement
as to pain, most bothersome other symptom, photophobia, phonophobia, nausea,
and other
bothersome symptoms, is assessed sequentially, in a fixed-sequential testing
method.
[00180] Tables 45-48 demonstrate effectiveness of the claimed
invention for
reducing or eliminating pain from migraine or cluster headaches, as compared
to triptans and
alternative forms of zolmitriptan. These results are based on one embodiment
of the claimed
invention, but are not so limited.
44
Date Recue/Date Received 2023-12-18
[00181] Shown in Table 45, methods described herein demonstrate
that the one
embodiment of the claimed invention shows significant improvement in patients
being pain free
at 1 hour after dosing, as compared to a tablet of zolmitriptan. The results
shown in Table 45
are merely one example of the significant efficacy that the claimed invention
provides over the
known methods for treating migraine or cluster headache with zolmitriptan. In
one embodiment
of the claimed invention, with a zolmitriptan dose of 1 mg, more than 15% of
patients were pain
free at 1 hour after treatment. In another embodiment (1.9 mg), more than 20%
of patients were
pain free at 1 hour. In a third embodiment (3.8 mg), more than 25% of patients
were pain free at
1 hour. This shows improved efficacy over nasal treatment of zolmitriptan,
with which it has
been shown that only about 10% of patients are pain free at 1 hour. The
current invention is
also significantly more efficacious than 2.5 mg, 5 mg, and 10 mg tablets and
2.5 mg orally
dissolving tablets, all of which only achieve pain freedom after 1 hour of 10%
or less, The
claimed invention also shows significant improvements in pain free results at
2 hours and 4
hours after treatment. In one embodiment, at 2 hours after treatment, more
than thirty percent of
patients were pain free. In another embodiment, at 2 hours, more than forty
percent of patients
were pain free. In a third embodiment, at 4 hours after treatment, more than
fifty percent of
patients were pain free. These are significant improvements over nasal
treatments using
zolmitriptan, in which less than twenty five and forty percent of patients are
pain free after two
and four hours from treatment, respectively. These results are also comparable
to other
zolmitriptan dosage forms and delivery routes, and at forty percent pain free
at two hours better
than all other zolmitriptan dosage forms and delivery routes. These results
are also shown
graphically in Figure 25.
[00182] Table 46 provides a comparison of resulting pain relief
between the
claimed invention and the current methods for treating migraine or cluster
headaches with
zolmitriptan. In the 1 mg, 1.9 mg, and 3.8 mg embodiments, pain relief of over
45%, 55%, and
65% respectively was achieved at just one hour after dosing. More than 65%,
68%, and 80%
respectively experienced pain relief at two hours after dosing. With the 3.8
mg embodiment,
over 80% of patients experienced pain relief at four hours after dosing. All
three embodiments
demonstrated significant improvements in pain relief at 1 hour when compared
to the other
zolmitriptan dosage forms and delivery routes, and the 3.8 mg embodiment was
also superior to
Date Recue/Date Received 2023-12-18
the other zolmitriptan dosage forms at 2 and 4 hours. These results are also
shown graphically in
Figure 26.
[00183] Tables 47 and 48 demonstrate the significant improvements
of the
claimed inventions over other triptans used for treating migraine or cluster
headaches, for
eliminating or reducing migraine or cluster headache pain. As shown in Table
47, the claimed
invention shows significant improvements in pain free results over other
triptans which are
currently used in the art. At 17.7%, 20.5%, and 26.8% pain freedom at 1 hour
for the 1 mg, 1.9
mg, and 3.8 mg embodiments respectively, all three strengths achieved higher
levels of pain
freedom than any of the other triptans. At 41.5% and 54.9% pain freedom at 2
and 4 hours, the
3.8 mg embodiment was still superior to all of the other triptans. These
results are also shown
graphically in Figure 27. As shown in Table 48, the claimed invention shows
significant
improvements in pain relief results, over other triptans which are currently
used in the art. At
46.8%, 55.4%, and 68.3% pain relief at 1 hour for the 1 mg, 1.9 mg, and 3.8 mg
embodiments
respectively, all three strengths achieved higher levels of pain relief than
any of the other
triptans. At 80.5% and 82.9% pain relief at 2 and 4 hours, the 3.8 mg
embodiment was still
superior to all of the other triptans. These results are also shown
graphically in Figure 28. In
another aspect, the plasma Tmax of the administered zolmitriptan based agent
is between about 2
minutes and 30 minutes. In one embodiment, administration of the zolmitriptan
based agent is
by transdermal or intracutaneous administration. Alternatively, the route of
administration of a
zolmitriptan based agent is intravenously, subcutaneously, orally,
intranasally, oral inhalation,
intracutaneously, transdermally, buccally, or sublingually.
[00184] In another embodiment, there is a method for treatment or
alleviation of
migraine or cluster headache in an individual in need thereof, comprising
administering a
therapeutically effective amount of a zolmitriptan based agent, wherein the
plasma zolmitriptan
AUC for the first 2 hours is greater than the plasma zolmitriptan AUC
following oral
administration of an equivalent dose of zolmitriptan, but the plasma
zolmitriptan AUC0-in1
following intracutaneous administration of a therapeutically effective amount
of a zolmitriptan
based agent is less than the plasma zolmitriptan AUCof seen after the oral
administration of an
equivalent dose of zolmitriptan. In one aspect of this embodiment,
administration of the
zolmitriptan based agent is transdermal or intracutaneous administration. In
one aspect of this
46
Date Recue/Date Received 2023-12-18
embodiment, the route of administration of a zolmitriptan based agent is
intravenously,
subcutaneously, orally, intranasally, oral inhalation, intracutaneously,
transdermally, buccally,
or sublingually.
[00185] In another embodiment, there is a method for treatment or
alleviation of
migraine or cluster headache in an individual in need thereof, of a
therapeutically effective
amount of a zolmitriptan based agent, wherein, in comparison to oral
administration of an
equivalent dose of zolmitriptan, the zolmitriptan plasma levels are increased,
but the N-
desmethyl zolmitriptan production is reduced, thereby reducing the likelihood
for metabolite
accumulation. In one aspect of this embodiment, administration of the
zolmitriptan based agent
is transdermal or intracutaneous administration. In one aspect of this
embodiment, the route of
administration of a zolmitriptan based agent is intravenously,
intramuscularly, intracutaneously,
subcutaneously, orally, intranasally, oral inhalation, transdermally,
buccally, or sublingually.
[00186] In another embodiment, there is a method for treatment or
alleviation of
migraine or cluster headache in an individual in need thereof, comprising the
administration of a
therapeutically effective amount of a zolmitriptan based agent, wherein, in
comparison to oral
administration of an equivalent dose of zolmitriptan, the apparent half-life
of zolmitriptan is
reduced, thereby indicating a likelihood of a reduced duration of side
effects. In one aspect of
this embodiment, administration of the zolmitriptan based agent is transdermal
or intracutaneous
administration. In one aspect of this embodiment, the route of administration
of a zolmitriptan
based agent is intravenously, intramuscularly, intracutaneously,
subcutaneously, orally,
intranasally, oral inhalation, transdermally, buccally, or sublingually.
[00187] In any of the embodiments disclosed herein, the route of
administration of
a zolmitriptan based agent is selected from the group consisting of
intravenously,
intramuscularly, intracutaneously, subcutaneously, orally, intranasally, oral
inhalation,
transdermally, buccally, and sublingually.
[00188] In an aspect of this embodiment, the intracutaneously
administered
zolmitriptan based agent provides a pharmacokinetic profile similar to the
pharmacokinetic
profile provided by subcutaneous administration of an equivalent dose to the
intracutaneously
administered sumatriptan based agent.
47
Date Recue/Date Received 2023-12-18
[00189] In one aspect of the method where the zolmitriptan is
administered, the
administration of the zolmitriptan is not associated with effects on blood
pressure greater than
those seen with oral zolmitriptan, despite faster absorption.
[00190] In one embodiment, there is a method for treatment or
alleviation of
migraine or cluster headache in an individual in need thereof, comprising
administration of a
therapeutically effective amount of a zolmitriptan based agent, wherein the
time to achieve
maximum plasma concentration (Tmax) was comparable to or less than the Tmax of
an equivalent
oral dose of zolmitriptan. In one aspect of this embodiment, administration of
the zolmitriptan
based agent is transdermal or intracutaneous administration. In one aspect of
this embodiment,
the route of administration of a zolmitriptan based agent is intravenously,
subcutaneously,
orally, intranasally, oral inhalation, intracutaneously, transdermally,
buccally, or sublingually. In
one aspect of these embodiments, the generation of N-desmethyl zolmitriptan is
reduced relative
to the generation of N-desmethyl zolmitriptan resulting from an oral dose of
an equivalent
amount of the zolmitriptan based agent. In another aspect of these
embodiments, the absorption
of the intracutaneously administered zolmitriptan based agent results in a
Cinax of less than 50
ng/mL.
vm. Examples
[00191] The following examples are included to demonstrate certain
embodiments
of the invention. Those of skill in the art should, however, in light of the
present disclosure,
appreciate that modifications can be made in the specific embodiments that are
disclosed and
still obtain a like or similar result without departing from the spirit and
scope of the invention.
Therefore all matter set forth is to be interpreted as illustrative and not in
a limiting sense.
[00192] In the examples below, unless stated otherwise, the
microprojection arrays
were fabricated by a photo/chemical etching and formed using a controlled
manufacturing
process. The method is substantially similar to that described in M. Cormier
et al., "Device for
enhancing transdermal agent delivery or sampling," EP0914178B1 .
Drug formulation coating on the microprojection array was conducted
at ambient temperature utilizing a roller drum, rotating at 50 rpm, in a drug
formulation
reservoir (2 mL in volume) to produce a drug coating formulation film of
controlled thickness.
The method is substantially similar to that described in J.C. Trautman et al.,
"Method and
48
Da
apparatus for coating skin piercing microprojections," U.S. Patent No.
6,855,372; J.C. Trautman
et al., "Method and apparatus for coating skin piercing microprojections,"
U.S. Patent No.
7,435,299.
Microprojections are dipped into
the film. The amount of coating is controlled by the number of dips (passes)
through the drug
film as well as the drug coating formulation properties. The time between each
dip was a few
seconds which was sufficient to dry the coated liquid formulation under
ambient conditions.
The reservoir was circulated with coolant to maintain a film temperature of 1
C. Since the
reservoir is open to the ambient air, the coating apparatus was positioned
inside a dew-point
control system. Dew point control minimizes moisture condensation into or
evaporation from
the liquid formulation during coating. The zolmitriptan-coated microneedle
arrays were
assembled with adhesive backing to form a patch, and mounted on a retainer
ring to form a
patch assembly. The patch assembly was packaged in an aluminum pouch (Mangar,
New
Britain, PA, USA), purged with dry nitrogen and heat-sealed with a Multivac
heat sealer
(model C400) (Multivac, Kansas City, MO, USA).
Example 1¨Zolmitriptan Coating Formulations, Characterization, Physical
Properties
100193] Zolmitriptan is a weak base with a pKa of 9.6. Solubility
measurements
were conducted by adding excess zolmitriptan base to 0.5 ml of 0.1 M acid and
rotating the
suspension overnight at 2-8 C. The suspension was then centrifuged. The
supernatant was then
collected and subsequently the concentration of zolmitriptan dissolved was
determined. Table 2
presents the solubility results of zolmitriptan in the various acids.
Table 2: Solubility of Zolmitriptan in Various Acids at 2-8 C
Aqueous Solvent Solubility (mg/mL)
Citric acid 88.6
Tartaric acid 63.3
Maleic acid 50.5
Succinic acid 59.1
HCl 33.3
De-ionized water 1.3
100194] Zolmitriptan exhibits good solubility in the various acids.
It was noted
that the rheological behavior of the zolmitriptan solution was affected by the
counterion in the
formulation for pH control. Several weak acid buffers, including one triacid
(citric acid), two
49
Dat
diacids (maleic acid and tartaric acid) were tested. The zolmitriptan
formulations that were
prepared with citric, maleic and tartaric acids were at pH 5.2, 4.3 and 6.2
respectively, at the
pKa of the acids. The viscosity profiles of formulations including these acids
were measured as
a function of time. Citric and maleic acid buffered formulations exhibited
rheopectic behavior,
i.e., an increase in viscosity as a function of time, while formulations
buffered by tartaric acid
maintained relatively uniform viscosity with time. Given the overall
rheological effect, tartaric
acid was selected as the counterion for pH adjustment.
[00195] A liquid coating formulation of 33 %w/w zolmitriptan, 11
%w/w tartaric
acid and 56 %w/w de-ionized water formulation was prepared at pH 4.5 and
contact angle on
titanium substrate was determined to be 65.8 degrees indicative of poorly
wettable formulation.
To improve wettability of formulation on titanium, polysorbate 20 at
concentration of 0.2 %
w/w was added to the zolmitriptan formulation. Contact angle decreased to 51.6
degrees.
[00196] Static contact angle of drug solution formulations on
titanium surface was
determined using a FDS contact angle meter (Model OCA15) employing an optical
contact
angle method called "Sessile drop". For static contact angle measurement, a
photo snapshot is
taken once a drop of the solution (5 L) is dispensed from the syringe and
laid on a clean
titanium foil surface. The angle between the baseline of the drop and the
tangent at the drop
boundary is measured on both sides Complete measurement was obtained by
averaging the two
numbers. At least five readings were recorded for each sample.
[00197] Coating trials with 33 % w/w zolmitriptan, 11 %w/w tartaric
acid, 0.2 %
w/w polysorbate 20, qs ad deionized water were conducted. Drug formulation
coating on the
microprojection array was conducted at ambient temperature utilizing a roller
drum, rotating at
50 rpm, in a drug formulation reservoir (2 mL in volume) to produce a drug
formulation film of
controlled thickness. Microprojections were dipped into the drug film. The
amount of coating
was controlled by the number of dips (passes) through the drug coating
formulation film. The
time between each dip was only a few seconds which was sufficient to dry the
coated liquid
formulation under the ambient conditions. Since the reservoir was open to the
ambient air, the
coating apparatus was positioned inside a dew-point control system. The
process is designed to
match the drug film temperature to the air dew point, which prevents
evaporation of the coating
formulation over the duration of the manufacturing run. However, undulations
in the
Date Recue/Date Received 2023-12-18
zolmitriptan liquid formulation were noted visually, which is symptomatic of
an uneven film.
Concentration of zolmitriptan in the liquid formulation was increased up to 51
% w/w (tartaric
acid in the formulation was 17 % w/w and 0.2 % w/w polysorbate 20).
Undulations in film
were still noted with the higher solids content formulations. Subsequently,
the polysorbate 20
was removed, and it was noted that the undulations were no longer present.
This is a surprising
and non-obvious result, because conventional teachings in pharmaceutics
supported the use of
surfactants to facilitate the production of a smooth, uniform coating.
[00198] In another coating embodiment, a 33% w/w zolmitriptan, 11%
w/w
tartaric acid and 56% w/w deionized water foimulation caused high incidence of
wicking on the
particular microprojection array utilized, whereby the drug did not adhere to
the
microprojections. Although the viscosity of the formulation was 22 cP, the
design of the
microprojection (width of 120 um, length 340 1.1m) and the thick drug film
(calculated film
thickness 270 m) are such that, in each dip into the drug film the
microprojections would pick
a volume of liquid that cannot be dried fast enough, which leads to the drug
film spreading onto
the base of the microprojections. A higher solids content formulation 40 % w/w
zolmitriptan,
13.3 % w/w tartaric acid and 46.7% w/w de-ionized water (M207), with a
viscosity of 85 cP
coated the microprojections uniformly and no wicking was noted. This
formulation was utilized
for further evaluation. Representative batch formulas are provided in Tables 3
and 4 for two
strengths of microneedle array patches (internal product name M207), based on
the nominal
batch size of 45 g (zolmitriptan base).
Table 3: Batch Formula for M207 1 mg
Component Quantity (mg/patch) Quantity (g/batch)
Zolmitriptan 1 45
Tartaric Acid 0.3 15
Table 4: Batch Formula for M207 1.9 mg
Component Quantity (mg/patch) Quantity (g/batch)
Zolmitriptan 1.9 45
Tartaric Acid 0.6 15
Viscoelastic Properties
51
Date Recue/Date Received 2023-12-18
[00199] The 40 %w/w zolmitriptan liquid formulation was evaluated for
viscoelastic properties. Viscoelastic characterization of a fluid can be a
useful tool for predicting
the fluid's gelation tendency. H. A. Barnes, J. F. Hutton, and K. Walters, An
Introduction to
Rheology (Elsevier, New York, 1989). Measurement of viscoelasticity (i.e.,
elastic and viscous
components) in a viscometer is based on a complex, theoretical model. Briefly
subjecting the
material to an oscillatory stress or strain, whose value is small enough not
to destroy the
material's structure, produces the output of phase angle. The phase angle is
the ratio between
the viscous modulus and the elastic modulus. A phase angle of 0 degrees
corresponds to a fully
elastic material, following Hooke's law of elasticity, hence suggesting a more
rigid, and ordered
structure. A phase angle of 90 degrees corresponds to a material with fully
viscous behavior,
indicating a less ordered structure which is less prone to gelation. The 40 %
w/w zolmitriptan
liquid formulation exhibited high phase angle around 83 degrees indicating
that the formulation
is not susceptible to gelation.
Characterization of Mechanical Properties of ZP-Zolmitriptan Patches by
Nanoindentation
[00200] Mechanical properties of zolmitriptan coating such as hardness and
toughness were evaluated by nanoindentation for M207 1.9 mg patches.
Nanoindentation
testing was performed on individual microprojections coated with zolmitriptan
after the
microprojections were broken off at the base of the titanium array. For
hardness measurements,
the coated microprojections were sampled from the center and two edge
locations of the array
and 10 indentation measurements were made for each of the three locations.
Hardness and
reduced elastic modulus were determined using a Berkovich indenter by a
Nanomechanical Test
System, TriboIndenter. Toughness was determined by fracture toughness using
TriboIndenter
with a cube corner indenter. Five indents were made for each patch sample.
Table 5: Nanoindentation results for gamma-irradiated M207
1.9 mg (L/N0203154-gamma)
Stability avg H avg H S.D. avg Er avg Er S.D. K, K,
S.D.
Condition (MPa) (MPa) (GPa) (GPa) (kPa*m1/2) (kPa*m1/2)
TO 380.89 29.27 8.20 0.33 157.02 17.87
T3M-
25 C/60% RH 357.85 6.27 8.19 0.33 135.76 2.94
52
Date Recue/Date Received 2023-12-18
Stability avg H avg H S.D. avg Er avg Er S.D. K, K,
S.D.
Condition (MPa) (MPa) (GPa) (GPa) (kPa*m 1/2) (kPa*m1/2)
T3M-
329.34 42.84 7.80 0.47 126.64 3.08
40 C/75% RH
T6M-
279.89 8.70 9.09 0.89 117.67 12.03
25 C/60% RH
25 C/60% RH T12M-
283.61 4.01 7.15 0.05 164.96 9.20
Dynamic Vapor Sorption
[00201] Water sorption and desorption isotherms of M207 1.9 mg patches were
determined at 25 C by DVS Intrinsic (Surface Measurement Systems, Ltd.). Each
patch was
exposed to a cycle of controlled relative humidity (RH) at incremental steps
ascending from 0%
to 65% and subsequently descending from 65% to 0%. The change in weight was
continually
measured by a microbalance with a 0.1 jig resolution. At each RH step the
sample was allowed
to reach equilibrium with dm/dt criterion of 0.0004 before moving to the next
RH step. An
uncoated patch was analyzed under the same conditions to determine background
water uptake
by the patch components other than the coated drug formulation.
Crystallinity
[00202] X-Ray diffraction (VW) analysis was performed to characterize the
solid
state phases of dried zolmitriptan coating on patch for the M207 1.9 mg
system. Non-irradiated
and gamma- irradiated M207 patches were analyzed and compared to an uncoated
patch of the
same array design. For each patch sample to be analyzed, approximately 45-50
microprojections with zolmitriptan coating were broken off at the base of the
titanium array and
analyzed as bulk by XRD. XRD data was collected by a coupled Theta: 2-Theta
scan on a
Bruker D8 Vantec diffractometer equipped with a micro-focus copper x-ray tube
with Montel
optics monochromator, 0.5mm collimator, a Vantec 500 2-D area detector and
laser alignment
system. XRD pattern of zolmitriptan coated microprojections were compared to
that of
uncoated microprojections. All the sharp peaks present in zolmitriptan coated
patch samples
were matched with those in the uncoated patch sample. Those sharp peaks were
identified as
titanium (Ti) metal based on the reference XRD data from ICDD/IC SD database,
indicating the
crystalline phase result from the Ti microprojection substrate. Zolmitriptan
coated patches
showed a broad, wide peak centered at about 18 2-Theta, which is absent in
the uncoated patch
53
Date Recue/Date Received 2023-12-18
sample, indicating the drug coating is amorphous material for both non-
irradiated and gamma-
irradiated patches. Percent crystallinity was calculated with peak profile
fitting, the results are
summarized in Table 6, and was monitored on stability as described below.
Table 6: Phase Identification and Percent Crystallinity for M207 Patch Samples
Sample Phases Present % Crystallinity
Non-irradiated M207 Ti = Titanium Hexagonal, S.G: 100% (Ti)
(LN 0203154-NI) P63/mmc (194) Phase Info 0% drug coating
[01-089-3725] Amorphous
material
Gamma-irradiated Ti = Titanium Hexagonal, S.G: 100% (Ti)
M207 P63/mmc (194) Phase Info 0% drug coating
(LN 0203154-Gamma) [01-089-3725] Amorphous
material
Uncoated Patch Ti = Titanium Hexagonal, S.G: 100% (Ti)
(Array Design: P63/mmc (194) Phase Info
MF1663) [01-089-3725]
[00203] Mechanical properties of zolmitriptan coating were
evaluated as function
of time and storage condition. Mechanical properties such as hardness, which
is a measure of a
material's resistance to localized plastic deformation, elastic modulus a
measure of material's
resistance to being deformed elastically when a force is applied to it
(measure of material's
stiffness) and fracture toughness, which describes the ability of a material
containing a crack to
resist fracture, were evaluated. Multiple coated microprojections from
different areas of
individual ZP-Zolmitriptan patches were sampled for testing. Table 5
summarizes the results of
nanohardness (H), reduced modulus elastic modulus (Er), and fracture toughness
(1(,) for
gamma irradiated M207 1.9 mg patches stored at 25 C/60% RR for up to 12 months
and at
40 C/75% RH for up to 3 months. The stability results suggest a decreasing
trend in hardness
and fracture toughness, and an increasing trend in the elastic modulus.
Zolmitriptan Purity and Content Quantitation
[00204] Purity of
zolmitriptan was determined by the reverse phase high
performance liquid chromatography (RPHPLC) method (TM-601) at wavelength of
225 nm.
Chromatography for the assay was performed using a Phenomenex Kinetex EVO C18,
(4.6 mm
1D x150mm, 5 um) maintained at 30 C. The mobile phase involved a gradient
elution, with
solvent A: Ammonium Dihydrogen Phosphate buffer: MeOH:Acetonitrile, 70: 20: 10
(v/v), and
54
Date Recue/Date Received 2023-12-18
solvent B: Ammonium Dihydrogen Phosphate buffer: Acetonitrile, 30: 70 (v/v),
and was
pumped at the flow rate of 0.6 mL/min on an HPLC system (Water Alliance 2695)
equipped
with a binary pump, a thermostatted autosampler, column compartment, and a PDA
detector.
Data were collected and analyzed using Empower Pro (Empower 2 software, Waters
Corporation).
In Vitro Dissolution
[00205] In vitro dissolution of M207 1.9 mg patches were evaluated
using standard
USP Paddle over Disk apparatus (USP apparatus 5) with Distek 2100C 6-position
dissolution
tester. The paddle height was set at 25 mm above patch and rotated at 50 RPM.
An USP vessel
was filled with degassed 500 mL PBS dissolution medium and the temperature was
controlled at
32 C. A full patch assembly containing the coated patch adhered to the center
of an inner ring
and then attached to an outer ring was inserted along the vessel wall into the
vessel with the
coated microprojections facing upright. The release of zolmitriptan from the
coated patch was
continually monitored via quantitation of zolmitriptan concentration of the
dissolution medium
by UV absorbance using Pion Rainbow 6-Ch Fiber Optic System with 14 cm dip
probe and 10
mm pathlength.
[00206] M207 patches stored at room temperature and at 40 C/75%
relative
humidity for 10 months were evaluated. The results illustrated in Figures 6(A)-
(C) and Table 7
show instantaneous release of zolmitriptan for all the patches tested with a
steep slope reaching
concentration plateau of complete dissolution in less than one minute.
[00207]
Table 7: Concentration-Time of Dissolution of Zolmitriptan
Time (s) Percent Dissolution (%D)
0 0
20 60-80
E-beam irradiated and stored
40 90-100
at RT for 10 months
60 100
80 100
100 100
Non-irradiated and stored at Time (s) Percent Dissolution (%D)
Date Recue/Date Received 2023-12-18
40 C/75% RH for 10 months 0 0
20 0
40 10-40
60 90-100
80 100
100 100
Time(s) Percent Dissolution (%D)
0 0
20 5-10
E-beam irradiated and stored 40 10-50
at 40 C/75% RH for 10 months
60 50-100
80 100
100 100
Example 2--Ex vivo Human Skin
[00208] The in vitro, Franz, human skin finite dose model is a tool
for the study of
percutaneous absorption of topically applied drugs. The model uses ex vivo,
human torso skin
mounted in specially designed diffusion cells allowing the skin to be
maintained at a
temperature and humidity that match typical in vivo conditions. A finite dose
(for example, 2
mg/cm2 ¨ 10 mg/cm2 of a semisolid, or a transdermal delivery system) of
formulation is applied
to the outer surface of the skin and drug absorption is measured by monitoring
its rate of
appearance in the receptor solution bathing the inner surface of the skin.
Data defining total
absorption, rate of absorption, as well as skin content can be determined in
this model.
[00209] Dosing. The zolmitriptan patches were applied to the ex-
vivo skin with
use of an applicator. Following dosing, the patch and the skin were
immediately mounted onto a
Franz Diffusion Cell.
[00210] Dermal Receptor Medium. Normal phosphate buffered saline
(pH 7.4
0,1) with 0,008% gentamicin sulfate (PBSg) solution was utilized when the
diffusion cells were
first mounted.
56
Date Recue/Date Received 2023-12-18
[00211] Diffusion Cell and Skin Preparation. Percutaneous
absorption was
measured using the in vitro, human skin, Franz finite dose technique. Ex vivo,
dermatomed,
human torso skin, without obvious signs of skin disease or damage was used in
this study. The
skin was provided to the testing facility as dermatomed, cryopreserved, and
sealed in a water-
impermeable bag with continuous storage at ¨ -70 C. Prior to use, it was
thawed in ¨37 C water
and then rinsed in distilled, de-ionized water (ddH20) to remove any adherent
blood or other
material from the surface.
[00212] Skin from each donor was cut into multiple smaller sections
large enough
to fit on nominal 7 cm2 static Franz diffusion cells. The actual thickness of
each skin section
was measured in triplicate using a Digital Pocket Thickness Gauge (results in
Table 2). Each
skin section was then mounted onto a diffusion cell.
[00213] The dermal receptor compartment was filled to capacity with
PBSg. The
epidermal chamber (also known as the chimney or donor compartment) was left un-
occluded
with exposure to the ambient laboratory environment. The cells were then
placed within a rack
system and attached to a water circulation system from which the receptor
solution was stirred
magnetically at approximately 600 RPM, and its temperature was maintained to
achieve a skin
surface temperature of 32 1 C (data on file). Skin was left to equilibrate
for a minimum of 1
hour prior to the barrier integrity test.
[00214] One additional skin section per donor was prepared and
underwent all
study activities, but dosed with a placebo patch, to serve as a negative
sample control.
[00215] Barrier Integrity Test. To ensure the barrier integrity of
each skin
section, its desorption of water was measured for trans-epidermal water loss
(TEWL). A Delfin
Vapometer probe was activated, placed onto the skin surface, and the TEWL
value recorded.
Skin mounted in diffusion cells in which TEWL was less than 25 g/m2/h were
considered
acceptable. Skin sections that were determined to be unacceptable for dosing
may have been
used as non-dosed negative sample control cells, if needed. After the barrier
integrity test was
complete, the receptor solution was replaced with the designated stock
receptor solution of 0.1x
PBSg.
[00216] Donor Demographics. The demographics of the skin donors are
summarized in table 8.
57
Date Recue/Date Received 2023-12-18
Table 8: Donor Demographics
Skin Integrity Test
Donor ID Age Race Sex Thickness
(mm) (g/m2/h)
NS021715 51 Black Male 0.40 0.08 8.78 0.66
SM081016 50 Caucasian Male 0.49 0.16 8.18 2.63
SG100316 50 Black _ Female 0.50 0.21 8.80 0.29
[00217] .. Dose Administration and Sample Collection. Prior to administration
of
the patches to the skin sections, a pre-dose (0 hour) sample was collected as
the entirety of the
receptor solution volume was withdrawn with an approximate 5 mL aliquot of the
collected
sample saved for subsequent analysis. The receptor solution was replaced with
the designated
stock receptor solution of 0.1x PBSg. The chimney was then temporarily removed
from the
Franz diffusion cell to allow full access to the epidermal surface of the
skin.
[00218] Immediately following patch application, the skin-patch combination
and
the donor compartment (chimney) were replaced onto the receptor compartment of
the Franz
diffusion cell.
[00219] At the scheduled sampling time points (3,5, 10, 15, 30, 45, 60, 90,
120,
150, 180, 210, 240, 270, and 300 minutes), the receptor solution was removed
in its entirety,
refilled with stock receptor solution, and an approximate 5 mL aliquot of the
collected sample
was saved for subsequent analysis. For sample analysis, a 5 mL aliquot was
lyophilized using
vacuum centrifugation and reconstituted in 0.25 mL of ddH70.
[00220] After the last receptor sample was collected, the patch was removed
for
subsequent extraction and analysis. The skin surface wash was performed using
two successive
refluxing washes of ddH20. Each wash cycle consisted of at least 10 refluxes.
The two wash
volumes from each donor cell were pooled to generate a single surface wash
sample for the
diffusion cell.
[00221] .. Following the surface wash, the skin was allowed to dry for no less
than
minutes. Subsequently, the skin was tape stripped with up to ten (10)
sequential tapes (e.g.
3M Transporee tape) to remove and collect the stratum corneum. Tape strips
were extracted
overnight in ddH20. The skin was then dismounted from the cell and separated
by manual
58
Date Recue/Date Received 2023-12-18
dissection into epidermis and dermis for subsequent extraction and analysis.
Skin sections were
extracted overnight in ddH20.
[00222] Sample Analysis. Quantification of zolmitriptan in the
collected samples
was accomplished using a validated HPLC method.
[00223] Samples were analyzed on a Shimadzu Series LC System. The
HPLC
UV/Vis method used a solvent system consisting of a mobile phase gradient
using (Solvent A)
0.1% ammonium acetate with 0.1% acetic acid in H20 and (Solvent B) methanol
and was run
through a Phenomenex Luna C18(2) column, (100 x 4.6 mm, 3 ) at a flow rate of
0.5 mL/min
for the analysis of zolmitriptan. The column was maintained at 40 C.
[00224] Mean Flux Results. Percutaneous absorption of zolmitriptan
through ex
vivo human torso skin over 300 minutes from a single application (Mean SE).
The mean flux
( g/cm2/hr) is summarized in table 9 and in coordinating figure 30. This is an
across donor
summary, showing results of percutaneous absorption of zolmitriptan through ex
vivo human
torso skin over 300 minutes from a single application (Mean, 1\1= 3 donors).
Table 9: Mean Flux (pg/cm2/hr) Results: Across Donor Summary
Time (hr)* ZP-Zolmitriptan 1.9 mg
0.025 385.3 95.9
0.067 849.4 42.1
0.125 730.5 41.7
0.208 716.5 46.7
0.375 408.9 26.1
0.625 284.8 15.0
0.875 187.7 11.8
1.250 107.3 8.3
1.750 63.77 3.70
2.250 41.34 1.00
2.750 26.22 0.34
3.250 17.14 0.89
3.750 11.70 1.22
4.250 8.333 0.751
4.750 6.436 1 0.819
[00225] Total Absorption and Mass Balance Results. Results of
percutaneous
absorption of zolmitriptan into and through ex vivo human, torso skin over 300
minutes, from a
59
Date Recue/Date Received 2023-12-18
single application is summarized in table 10. Mean SE as percent of applied
dose (%) and
total mass (lig).
Table 10: Total Absorption and Mass Balance Results across Donor Summary
Parameter ZP-Zolmitriptan
1.9 mg
Receptor (lug) 1585 25
Dermis (lug) 6.081 0.460
Epi (jig) 6.054 2.258
St. Corn. (fig) 0.929 0,303
S. Wash (pig) 48.74 20.74
Patch (lug) 62.11 6.90
Receptor (%) 83.41 1.33
Dermis (%) 0.320 0.024
Epi (%) 0.319 0.119
St. Corn. (%) 0.049 0.016
S. Wash (%) 2.565 1.092
Patch (%) 3.269 + 0.363
Total Recovery (%) 89.94 2.41
1002261 Conclusion and Discussion. Total absorbed zolmitriptan
through the
skin to the receptor solution was found to be 83.41 1.33 % for the 1.9 mg
patch. Peak flux
occurred at approximately 4 minutes after application with a peak flux of
849.4 42.1
lig/cm2/hr. Less than 1% of the zolmitriptan was recovered from the three skin
layers at the end
of the dose duration period. Mass balance was found to be approximately 90% of
the applied
dose.
Example 3 ¨ M207 Patch Stability
[00227] M207 patch assemblies were irradiated by e-beam and gamma
irradiation
up to 25 kGy dose. Subsequent irradiated patch assemblies were placed on
stability at storage
conditions of 25 C /60% RH and 40 C/75% RH. Results of the e-beam and gamma
irradiated
M207 patches are shown in Tables 11-17.
Table 11: Purity of non-irradiated and e-beam irradiated Zolmitriptan Patches
stored at 25 C/ 60 %RH and 40 C/75% RH (L/N 203149)
Purity by Time (Month)
Temperature
Treatment RP-
( C) 0 1 3 6 9 12 18
HPLC
Date Recue/Date Received 2023-12-18
(%)
Control 99.93 100.00 99.90 99.91
99.88 99.92 99.90
25 avg std 0.03 0.00 0.06 0.03 0.03 0.03 0.01
(Non-
99.93 100.00 99.91 99.91
Irradiated) 40 avg std
0.03 0.00 0.01 0.02
99.89 100.00 99.93 99.88 99.84 99.77 99.82
25 avg std
0.05 0.00 0.01 0.02 0.03 0.02 0.01
Irradiated
99.89 100.00 99.93 99.88
40 avg std
0.05 0.00 0.01 0.01
Table 12: ZP-Zolmitriptan content of non-irradiated and e-beam irradiated
Zolmitriptan Patches stored at 25 C/ 60 (VoRH and 40 C/75% RH (L/N 203149)
Treatment Temperature Content Time (Month)
( C) (mg/patch) 0 1 3 6 9 12
18
avg 1.709 1.698 1.740 1.771 1.701 1.758 1.870
%RSD 6.0 3,8 8.0 2.2 5.9 12.6
2.7
Non-1R
avg 1.709 1.657 1.729 1.778
%RSD 6.0 9.2 12.1 7.2
avg , 1.671 1.813 1.714 1.687 1.670 1.927 1.818
E-beam 25
%RSD 7.9 8.6 5.8 10.1 5,3 5.6
5.4
IR
(19-24 avg 1.671 1.686 1.801 1.781
40 .
kGy)
%RSD 7.9 7,7 ' 4.3 5.3
Table 13: Purity of gamma irradiated Zolmitriptan Patches stored at 25 C/ 60
/012H
and 40 C/75% RH (L/N 203154)
Temperature Purity Time (Month)
Treatment
(CC) (%) 0 1 3 6 9 12
avg 100.00 99.95 99.90 99.87 99.77 99.79
std 0.00 0,00 0.01 0.00 0,02 0,01
Irradiated
avg 100,00 99.93 99.90 99.86
std 0.00 0.01 0.01 0.00
1
Table 14: ZP-Zolmitriptan content of non-irradiated and 7- irradiated
Zolmitriptan
Patches stored at 25 C/60% RH and 40 C/75% RH (L/N 203154)
Temperature Content Time (Month)
Treatment
- ( C) (mg/patch) 0 1 3 6 9 12 .
Gamma IR 25 avg 1.855 1.690 1.771
2.006 1.719 1.876
%RSD 4.2 23.5 2.4 1.8
13.5 4.5
(25 kGy)
40 avg 1.855 1.895 1.818
1.817
%RSD 4.2 _ 7.6 11.6 1.1
25 avg 1.918 1.874
2.001 1.840 1.950
%RSD 10.0 ND 7.4 4.3 3.4
6.7
Non-1R
40 avg 1.918 1.955 1.895
%RSD 10.6 5.1 7.5
61
Date Recue/Date Received 2023-12-18
ND= Not Determined.
Table 15: Total Impurity of non-irradiated and e-beam irradiated Zolmitriptan
Patches
stored at 25 C/60% RH and 40 C/75% RH (L/N 203122)
Temperature Total Time (Month)
Treatment
( C) Impurities (%) 0 1 3 6 9 12
0.06 + 0.06 0.07 0.01 0.00
0.00
25 avg + std 002 + + + + +
0.-02 0.-02 0.-03 0.-00 0.-00
Non-1R
0.06 + 0.06 0.05 0.02
40 avg + std
0.02- + + +
0.-03 0.-01 0.-04
0.06 + 0.06 0.06 0.01 0.00
0.00
25 avg + std 001- . + + +
E-beam IR 0.- + 00 0.-00 0.-03
0.-00 0.-00
(21 kGy) 0.06 + 0.09 0.10 0.02
40 avg + std 0.01 + +
0.03 0.04 0.04
Table 16: ZP-Zolmitriptan content of non-irradiated and e-beam irradiated
Zolmitriptan Patches stored at 25 C/60% RH and 40 C/75% RH (L/N 203122)
Temperature Content Time (Month)
Treatment
( C) (mg/patch) 0 I 3 6 9 12
avg 1.194 1.235 1.198 1.343 1.286 1.326
%RSD 10.4 10.7 7.5 6.4 8.5 5.7
Non-IR
40 avg 1.194 1.236 , 1.279 1.315
%RSD 10.4 7.5 8.7 5.7
avg 1.212 1.130 1.169 1.255 1.251 1.283
%RSD 6.0 3.3 6.3 7.7 7.7 4.9
E-beam IR
(21 kG = avg 1.212 1.181 1.191 1.144
y)
40 60 6.2 11.7 3.1
%RSD .
[00228] Solid-state physical stability was evaluated by XRD. Phase
changes in
amorphous vs. crystalline for zolmitriptan coating were examined by XRD
analysis. M207 1.9
mg patches at initial time point (TO), 6 month and 12 month storage were
analyzed. The drug
coating was amorphous for both non-irradiated and gamma irradiated patches at
TO. Gamma
irradiated zolmitriptan patches stored at 25 C/60% RH for 12 months and 40
C/75% RH
showed similar XRD pattern to that of TO patches. Percent crystallinity was
calculated with
peak profile fitting and the results are summarized in Table 16, below. No
crystalline phase was
detected for zolmitriptan formulation solids coated on gamma-irradiated
patches stored under
62
Date Recue/Date Received 2023-12-18
both intended (25 C /60% RH) and accelerated storage conditions (40 C/75% RH)
for 12 and 6
months respectively.
Table 17: Phase identification and percent crystallinity for gamma-irradiated
M207 1.9 mg (L/N0203154-gamma)
Stability
Phase Present % Crystallinity
Condition
TO Ti = Titanium 100% (Ti)
Hexagonal, S.G: P63/mmc (194)
Phase Info [01-089-3725] 0% (drug)
Amorphous material (drug
coating)
6 months Ti = Titanium 100% (Ti)
storage at Hexagonal, S.G: P63/mmc (194)
25 C/60 /O RH Phase Info [01-089-3725] 0% (drug)
Amorphous material (drug
coating)
6 months Ti = Titanium 100% (Ti)
storage at Hexagonal, S.G: P63/mmc (194)
40 C/75% RH Phase Info [01-089-3725] 0% (drug)
Amorphous material (drug
coating)
12 months Ti = Titanium 100% (Ti)
storage at Hexagonal, S.G: P63/mmc (194)
25 C/60% RH Phase Info [01-089-3725] 0% (drug)
Amorphous material (drug
coating)
[00229] As described herein, zolmitriptan coated microneedles were
exposed to a
dose of radiation in the range of approximately 7-30 kGy. More preferably in
the range of 15-
30 kGy to a sterility assurance level of 10-5 to 10-6. Table 17 shows 12 month
stability results of
irradiated and non-irradiated zolmitriptan patches that were stored at 25 C
and 40 C.
Example 4¨Drug-Device Combination Product
[00230] A novel drug-device combination product (M207) was made
according to
the present disclosure. M207 is an intracutaneous delivery system comprising a
disposable
titanium microprojection member centered on an adhesive backing to form a
patch. This patch
was mounted in a plastic retainer ring to form a patch assembly. The patch is
comprised of
microneedles that are coated with the drug product formulation and dried. The
retainer ring
63
Date Recue/Date Received 2023-12-18
facilitates mounting of the patch to the bottom of a handheld applicator. This
applicator ensures
the patch is applied with a defined application energy to the site of
administration. The
combination of the patch assembly and the applicator comprises the
intracutaneous delivery
system. The applicator is held in one's hand to apply the patch. The
applicator cap is twisted
to unlock the applicator. When the applicator is pressed against the skin, a
plunger pushes the
patch out of the retainer ring and applies it to the skin.
[00231] When one applies the patch to the skin, the patch stays on
the skin and the
plastic ring stays on the applicator and is later detached and thrown away.
The delivery system
was designed to rapidly deliver a 1 mg, 1.9 mg, or 3.8 mg dose of zolmitriptan
intracutaneously.
The unit formulas for the M207 drug products are provided in Table 18.
Table 18: Unit Formula for M207 Drug Product
Amount per
Amount per 1 mg 1.9 mg Unit
Component Unit (mg/patch) (mg/patch) Function
Zolmitriptan 1 1.9 Active
Tartaric acid 0.3 0.6 pH modifier
Nitrogen N/A N/A Inert atmosphere for storage
[00232] The zolmitriptan-coated titanium microneedle array is a 3
cm2 array
consisting of about 1987 or about 997 titanium microneedles for the 1.9 mg or
1 mg drug
product, respectively. It is affixed to an approximately 5 cm2 adhesive patch.
The patch may
be mounted inside a polycarbonate plastic retainer ring with a co-molded
desiccant. The
desiccant may alternatively attached to the lid of foil pouch. The completed
patch assembly is
packaged in a dry nitrogen-purged foil pouch. The user prepares the patch for
application by
pressing the handheld applicator onto the patch assembly. The applicator
comprises a spring-
loaded piston for applying the patch to the user's skin (Figures 4(A), 4(B),
and 5(A)-(E)). The
applicator is unlocked by twisting the outer grip relative to the base from
the #1 position to #2
position (Figure 5(C)). The user applies the patch by pressing the applicator
mounted patch
assembly onto the skin site. The applicator releases its piston at a
sufficient impact energy, for
example, about 0.26 Joules. The piston breaks the patch from the retainer ring
and applies the
patch to the skin with the prescribed impact energy density to ensure
reproducible patch
64
Date Recue/Date Received 2023-12-18
application. The applicator is designed to ensure that the same force is
applied for each delivery
and across different users,
[00233] The drug-coated microneedles penetrate or pierce the
stratum corneum of
the skin, enabling drug delivery, Upon administration, the solid zolmitriptan
coating rapidly
dissolves off of the microneedles in the interstitial fluid in the skin to
form a solution and is
available for absorption. The patch is removed after about 30 minutes,
[00234] The M207 system components are listed in Table 19:
Table 19: M207 System Components
Component Formulation Contact Material Function
Patch Assembly and Pouch Primary Container Closure
Microneedles hold drug formulation and
Microneedle pierce the stratum corneum to enable
Titanium
Array delivery. Base of array adheres to
adhesive
patch.
Affixes the microneedle array to the inner
Acrylate adhesive with
Adhesive Patch ring prior to delivery. Holds array
in position
polyethylene backing
during wear period.
Holds adhesive patch. Assembled to Outer
Inner Ring None
Ring for attachment to Applicator.
Desiccant co-molded with the outer ring
removes residual moisture from patch and
maintains low-moisture environment during
Outer Ring None storage. Ring engages with
applicator to
enable application to patient's skin. Creates
desired domed skin profile when pressed
against skin just prior to patch application.
Low oxygen and low vapor
Pouch None
permeability, protects against light.
Inert, low-moisture atmosphere for drug
Nitrogen Nitrogen
stability
Desiccant Maintains low moisture atmosphere
Provides consistent energy for repeatable
Applicator patch application to a defined
depth of
penetration
Top None Covers internal workings.
Limits travel of piston. Engages with cap to
Upper Post None create ratchet mechanism for
unidirectional
rotation and indexing.
Date Recue/Date Received 2023-12-18
Component Formulation Contact Material Function
Contains ledge for lockout function, and
Twist Cup, Inner None indexing cams which force rotation
and align
window with indicators on inner cup.
Grip surface for user interface. Contains
Twist Cup, Outer None
window for visualizing indicators.
Provides consistent force to dome skin and
trigger the device. After patch application
Doming Spring None
and when removed from skin, resets the
device for next use.
Provides structural support and bearing
Inner Cup None
surface for doming spring.
)rovides guidance for piston and holds the
Lower Post None
trigger mechanism.
Latches on piston to retain during
compression of piston spring. During user
actuation, at point of full compression, the
Trigger None
head of the Trigger contacts the Upper Post
causing the Trigger to pivot on the Lower
Post, releasing the Piston.
Engages with Outer Ring of Patch. Holds
Clear Bottom None
applicator assembly together.
Provides energy for application of Adhesive
Piston Spring None
Patch and Microneedle Array.
Motive member which transfers energy from
Piston None Piston Spring to Adhesive Patch,
enabling
consistent penetration of the Microneedles.
Example 5¨Human PK Clinical Trial
[00235] An evaluation in humans of the M207 product was performed.
In this
Phase 1 study, commercially available oral zolmitriptan tablet 2.5 mg and
subcutaneous
sumatriptan 6.0 mg were included as comparators. As described in the examples
above, M207
consists of a titanium array of microneedles coated with zolmitriptan,
administered
intracutaneously via a patch applied by a specialized applicator. The aim of
this trial was to
provide information on the pharmacokinetics and tolerability of the M207
system. Assessment
of the tolerability of various doses of intracutaneous zolmitriptan to a
standard oral dose (2.5
mg) of zolmitriptan was also completed together with an assessment of
reactions at the
application site.
66
Date Recue/Date Received 2023-12-18
[00236] Specifically, the study compared single administrations of
five regimens
of M207, as well as 2.5 mg of oral zolmitriptan tablet and 6,0 mg of
subcutaneous sumatriptan
in a 7-way crossover design in 20 healthy volunteers. Analysis of the plasma
samples for
concentrations of zolmitriptan, N-desmethyl zolmitriptan and sumatriptan were
performed at
Quest Pharmaceutical Services in Groningen, Holland, by assays known in the
art. In this study,
the administration of M207 systems resulted in a rapid time to maximum
concentration (Tmax),
comparable exposure to orally administered zolmitriptan, but displayed reduced
exposure to the
major metabolite, N-desmethyl zolmitriptan. The doses assessed in this study
using the M207
system were 0.48 mg, 0.96 mg, 1.9 mg, and 3.8 mg.
[00237] The first 4 administrations of zolmitriptan utilized 5cm2
patches and a 0.26
Joule applicator in the intracutaneous microneedle system described herein.
The final treatment
administered was 3.8 mg on a 10 cm' patch using an applicator with 0.52 Joule
of application
energy in the intracutaneous microneedle system described herein. The products
tested were:
[00238] M207 0.48 mg patch assembly: The zolmitriptan 0.48 mg patch
consisted
of a 3 cm' titanium array of microprojections that were nominally 340 p.m in
length coated with
0.48 mg of zolmitriptan. The array was applied to the center of a 5 cm' tan
adhesive backing to
form the patch. The patch was attached to the interior of a white to off-white
polycarbonate ring
co-molded with a desiccant, and this patch assembly was packaged in a foil
pouch.
[00239] M207 1.9 mg patch assembly: The zolmitriptan 1.9 mg patch
consisted of
a 3 cm' titanium array of microprojections that were nominally 340 1.im in
length coated with
1.9 mg of zolmitriptan. The array was applied to the center of a 5 cm' tan
adhesive backing to
form the patch. The patch was attached to the interior of a white to off-white
polycarbonate ring
co-molded with a desiccant, and this patch assembly was packaged in a foil
pouch.
[00240] M207 3.8 mg patch assembly: The zolmitriptan 3.8 mg patch
consisted of
a 5.5 cm' titanium array of microprojections that were nominally 340 [im in
length coated with
3.8 mg of zolmitriptan. The array was applied to the center of a 10 cm2 tan
adhesive backing to
form the patch. The patch was attached to the interior of a white to off-white
polycarbonate ring
co-molded with a desiccant, and this patch assembly was packaged in a foil
pouch.
Study Design
67
Date Recue/Date Received 2023-12-18
[00241] This was a single-center, open-label, randomized five-way
crossover study
(Part 1) followed by a sequential study of two additional treatments (Parts 2
and 3). After
obtaining informed consent and establishing eligibility, each subject received
each of the seven
study treatments once, followed by in-clinic monitoring and extensive blood
sample collection
for pharmacolcinetic analysis. Dosing days in Part 1 occurred between 48-120
hours apart, until
completion of dosing for Treatments A-E (see Table 20) in randomized order per
the treatment
sequence tables. Plasma samples from the initial dosing days were sent to the
analytical
laboratory for analysis, and tolerability for each of the dose levels was
summarized.
Tolerability was judged to be acceptable, and subjects returned for Part 2.
During Part 2,
subjects received intracutaneous administered zolmitriptan in 1.9 mg x 2
patches (applied with
the same 0.26 J applicator used in Part 1), and completed identical procedures
to Part 1. During
Part 3, subjects received a single 3.8 mg patch (applied with a 0.52 J
applicator) and also
completed identical procedures to the previous dosing days. After completion
of the seven
dosing days, subjects were assessed one final time and dismissed from the
study.
[00242] The treatments used in the trial were as listed in Table 20
below:
Table 20: Treatments Used In Trial
TREATMENTS
Part 1 (Cross-Over Design)
Treatment A M207 intracutaneous system 0.48 mg
Treatment B M207 intracutaneous system 0.48 mg x 2
Treatment C M207 intracutaneous system 1.9 mg
Treatment D Zolmitriptan 2.5 mg oral
Treatment E Sumatriptan 6.0 mg SC
Part 2
Treatment F Zolmitriptan intracutaneous system 1.9 mg x2
Part 3
Treatment G Zolmitriptan intracutaneous system 3.8 mg
1002431 Twenty subjects were enrolled in the study, 10 males and 10
females. The
subjects mean age was 29 years 3.5 years with a mean BMI of 24.4 3.5. With
the exception
of one subject who missed one treatment visit, all subjects completed all 7
treatment visits of the
study, and received all 7 study treatments. In two subjects (#1010 in
Treatment A and #2010 in
Treatment D), very few post-dose blood samples were collected at one visit due
to difficulties
68
Date Recue/Date Received 2023-12-18
with venous access, but for all the rest of the subjects, virtually all of the
scheduled
pharmacokinetic blood samples (14 per visit) were collected for analyses.
[00244]
Tolerability in Part 1 was considered acceptable, and following a review
of the safety data and pharmacokinetic data from the first five dosing
periods, and a discussion
between the sponsor and the Principal Investigator, subjects proceeded to
Parts 2 and 3 and
completed those visits. The collected serum was analyzed for zolmitriptan and
N-desmethyl
zolmitriptan using methods well known in the art, such as using a liquid
chromatography-mass
spectrometry (LC-MS-MS) method.
Pharmacokinetics
[00245] The
M207 patch was well-tolerated and rapid absorption was observed
which were believed to potentially translate to fast pain relief for migraine
or cluster headache
patients. The Phase 1 results demonstrating the fast absorption of M207 that
is characteristic of
Zosano's microneedle patch and applicator system are illustrated below:
Table 21: M207 Characteristic of Zosano's Microneedle Patch
Cmai, (SD) Luiz( (range) A UCO-21n- (SD)
AUCoriast(SD)
ng/ml min ng/ml hour
ng/ml hour
A M207 0.48 mg 1.8 (0.53) 20 (2-30) 2.1 (0.73) 2.8
(1.36)
M207 2 x 0.48 mg_ 3.7 (1.05) 20 (2-30) 4.2 (0.95) 6.5
(1.97)
M207 1.9 mg 6.8 (2.75) 20 (2-30) 7.4 (2.53) 12.3
(4.31)
M207 2 x 1.9 mg 14.6 (4.46) 17.5 (2-30) 16.4 (5.34)
27.8 (9.93)
M207 3.8 mg 22.6 (14.00) 15 (2-30) 19.3 (5.37)
31.7 (8.35)
D Zolmitriptan 2.5 mg Oral Tablet 3.8 (1.51) 60(30-240) 4.7
(2.24) 22.2 (10.79)
[00246] The
mean plasma concentration versus time data, for each of the six (6)
zolmitriptan regimens administered are shown in Figures 7 and 8. Figure 7
shows the results for
the entire 24 hours sampling period and Figure 8 shows the results for the
first two hours post
study drug administration only. Both figures include the subcutaneous
sumatriptan
concentration vs. time data (scaled for display purposes to illustrate time
course). The results
following SC sumatriptan were similar to several published studies of this
dose and route of
administration.
[00247] Based on the results presented in Figures 7 and 8, plasma
levels of
zolmitriptan following zolmitriptan intracutaneous application were dose-
dependent, and the
absorption following patch application was much faster than that seen
following administration
69
Date Recue/Date Received 2023-12-18
of the 2.5 mg tablet. The plasma levels seen after the single larger 3.8 mg
patch were higher
than those seen following 2 x 1.9 mg patches. Plots of dose linearity for
zolmitriptan C,
AUCt, and AUCinf are shown in Figures 9, 10, and 18 (excluding the larger 3.8
mg patch). Plots
of dose linearity for N-desmethyl zolmitriptan Cõ,µõ,, AUCt, and AUCiff are
shown in Figures 22-
24 (excluding the larger 3.8 mg patch). Excellent dose linearity was observed
over the range of
doses evaluated. The calculated key mean (median for Trnax) pharmacokinetic
parameters for
the zolmitriptan regimens and subcutaneous sumatriptan are shown in the
following Table 22.
Table 22: Zolmitriptan Group PK values
GROUP PARAMETER Tula% t1/2 Cma, AUCt AUCinr AUC/his
A N 19 19 19 19 19 19
,
(0.48 Mean 17.32 69.06 1.84 2.81 3.81 2 11
mg) (SD) (12.10) (16.26) (0.53) (1.36) (1.46)
(0.73)
Median 20 64.62 1.8 2.78 3.78 2.11
Range 2, 30 46.2, 0.64, 2.93 0.39, 6.23 1.37,
7.5 0.39, 3.53
101.46
CV% 69.90% 23.50% 29.00% 48.30% 38.30%
34.40%
B N _ 20 20 20 . 20 20 20
(0.48 Mean 18.35 77.22 3.70 6.45 7.71 4.15
mg x 2) (SD) (11.23) (17.46) (1.05) (1.97) (2.03)
(0.95)
Median 20 81.96 3.63 6.55 7.85 4.19
Range 2, 30 41.76, 1.96, 6.32 3.16, 9.52
4.22, 10.98 2.46, 5.81
96.84
. ,
CV% 61,20% 22.60% 28.40% 30.50% 26.30%
22.90%
C N _ 20 20 20 20 20 20
(1.9 Mean 17.85 87.84 6.76 (2.75) 12.29 (4.31)
14.14 (4.54) 7.36 (2.53)
mg) (SD) (12.58) (16.74)
Median 20.00 84.72 6.40 12.69 14.50 7.75
Range 2, 30 61.20, 3.14, 13.20 5.01 19.59
6.55, 20.95 3.44, 11.46
124.98
CV% 70.50% 19.10% 40.60% 35.10% 32.10% 34.40%
D N _ 19 18 19 19 18 19
(2.5 mg Mean 107.37 196.44 3.77 22.20 27.19 4.72
(2.24)
oral) (SD) _ (76.37) (48.18) (1.51) , (10.79)
(11.34)
Median 60 196.8 3.7 22.65 27.1 4.92
Range 30, 240 117.84, 1.66, 6.77 7.47, 46.85
14.33, 55.14 1.73, 9.97
288.72
CV% 71.10% 24.50% 40.00% 48.60% 41.70%
47.50%
F N _ 20 20 20 20 20 20
(1.9 mg Mean 17.10 91.70 14.61 27.77 (9.93) 30.12
16.44 (5.34)
x 2) (SD) _ (11.82) (18.7) (4.46) (10.13)
Median 17.5 96.2 14.15 28.05 30.61 16.68
Range 2, 30 60.90, 7.09, 25.50 13.24, 51.33
14.50, 53.38 7.38, 29.32
123.10
CV% 69.10% 20.40% 30.50% 35.70% 33.60%
32.50%
G N 20 20 20 20 20 20
Date Recue/Date Received 2023-12-18
_
GROUP PARAMETER Tn. ti, Cmax AUC, AUCH,f
AUC2hrs.
(3.8 Mean 16.10 91.00 22.56 31.65 (8.35)
33.81 (7.95) 19.33 (5.37)
mg) (SD) (11.63) _ (18.80) (14.00)
Median 15 87.20 19.9 29.93 32.2 18.42
Range 2, 30 60.90, 9.03, 70.4 16.90, 44.33
19.01, 46.41 9.96, 29.55
130.60
CV% 72.20% 20.60% 62.10% 26.40% 23.50%
27.80%
[00248] Likely most relevant to the potential utility of this
product for the
treatment of migraine or cluster headache is the Tim, for the intracutaneous
administered
zolmitriptan regimens, showing much more rapid absorption of the zolmitriptan
from
intracutaneous administration, than from oral administration.
[00249] The
pharmacokinetic parameters following intracutaneous administered
zolmitriptan were on average very similar when comparing the results in male
subjects with the
results seen in female subjects, as shown in Figures 11 and 12.
[00250] The active metabolite, N-desmethyl zolmitriptan was
detectable in all
subjects dosed at the five higher dose regimens. The N-desmethyl zolmitriptan
phainiacokinetic
parameters for each of the zolmitriptan regimens are shown in the following
Table 23.
Table 23: N-desmethyl Zolmitriptan Metabolite Group PK value
GROUP PARAMETER TIMM tin Cõ,õA AUC,
AUC,õf AUC2hrs
A N 18 16 18 18 16 18
(0.48 mg) Mean (SD) 65.00 (18.55) 198.41 0.22 (0.05)
0.70 (0.31) 1.38 0.31 (0.10)
(98.20) (0.476)
Median 60.00 173.70 0.22 , 0.78 1.42
0.32
_
Range 30.00, 120.00 86.23, 0.14, 0.37
0.19,1.26 0.68,2.50 0.10,0.51
_ 511.18 ,
,
CV% 28.5% 49.5% 24.6% 44.5% 34.5% 31.8%
B N 20 20 20 , 20 20 , 20
(0.48 mg Mean (SD) 57.75 (14.00) 196.71 0.42 (0.11)
1.56 (0.57) 2.43 (0.78) 0.62 (0.17)
x 2) (104.70) ,
Median 60.00 168.2 0.43 1.42 2.49 0.61
Range 15.00, 90.00 122.4, 0.23, 0.60
0.94, 3.39 1.44, 4.42 0.32, 0.94
592.5
CV% 24.2% 53.2% 27.0% 36.4% 31.9% 26.9%
C N 20 20 20 , 20 20 20
(1.9 mg) Mean (SD) 61.50 (18.14) 182.9 0.74 (0.31)
3.01 (1.29) 3.65 (1.22) 1.07 (0.47)
(59.2)
Median 60.00 165.7 0.81 3.03 3.62 1.12
Range 30.00, 90.00 87.3, 0.29, 1.12
1.10,5.37 1.58, 5.91 0.41, 1.64
282.8
CV% 29.5% 32.3% 42.8% 42.8% 33.4% 44.3%
D N 19 19 19 19 19 19
71
Date Recue/Date Received 2023-12-18
(2.5 mg Mean (SD) 162.63 192.9 2.08 (0.50) 13.71
(2.91) 14.55 2.31 (0.93)
oral) (77.02) _ (68.1) (3.06) ,
Median 120.00 164.6 2.04 13.76 14.31
2.24
Range 60,00, 240.00 127.9, 1.40, 3.40
9.39, 19.90 9.90, 0.73, 4.64
348.9 20.58
CV% 47.4% 35.3% 24.1% 21.3% 21.0%
40.3%
20 20 20 20 20 20
(1.9 mg x Mean (SD) 63.00 (13.42) 169.0 1.41 (0.46) 6.50
(2.30) 7.22(2.34) 2.15(0.72)
2) (27.3)
Median 60.00 162.2 1.62 6.75 7.52 2.24
Range 30.00, 90.00 117.1, 0.65, 2.05
2.68, 10.74 3.43, 0.92, 3.45
215.3 11.45
CV% 21.3% 16.1% 32.4% , 35.4%
32.5% , 33.3%
20 , 20 20 20 20 20
(3.8 mg) Mean (SD) 54.74 (16.11) 162.0 1.77 (0.63) 7.55
(1.98) 8.17 (1.96) 2.66 (0.83)
(31.30)
Median 60.00 155.3 1.78 7.48 8.16 2.69
Range 20.00,90.00 111.1, 0.77, 3.54
3.39, 10.56 3.84, 1.19, 4.64
239.1 11.18
CV% 29.4% 19.3% 35.5% 26.3% 24.0%
31.0%
[00251] The levels of N-desmethyl zolmitriptan were significantly
lower after
M207 zolmitriptan intracutaneous administration than those seen following oral
administration
(Treatment D).
[00252] PK parameters were summarized by treatment group using
descriptive
statistics (arithmetic means, standard deviations, coefficients of variation,
sample size,
minimum, maximum, and median). In addition, geometric means and 95% confidence
intervals
(CIs) were calculated for AUCR, AUCt, AUCnif and C. For each of the
zolmitriptan
treatments, the ratio of AUCir,f N-desmethyl zolmitriptan/AUCinf zolmitriptan
was calculated for
each subject; a group mean was determined.
[00253] Dose proportionality was evaluated for the three doses of
M207; dose
proportionality was not based solely on a strict statistical rule. The
relationship between dose
and PK parameters of zolmitriptan were examined using a graphical approach and
by
descriptive statistics. Graphs of apparent dose linearity and proportionality
of PK parameters
(AUCt, AUCinf and C.) were compiled.
[00254] Rapid absorption of zolmitriptan was seen after
intracutaneous patch
application; mean peak plasma concentrations (T.) occurred between 16.1 and
18.4 minutes.
This was similar to sumatriptan SC injection (12.5 4.4 minutes) and
considerably quicker than
zolmitriptan tablets (107.4 76.4 minutes [1.8 1.27 hours]).
72
Date Recue/Date Received 2023-12-18
[00255] The mean ( SD) elimination half life (t1/2) for M207
systems was 1.15
0.27 hours up to 1.53 0.31 hours across the dose range of 0.48 mg to 3.8 mg,
respectively.
Elimination of zolmitriptan following zolmitriptan tablets (3.27 0.8 hours)
was almost twice
as slow as M207.
[00256] The mean ( SD) maximum plasma concentration (C.) of
zolmitriptan
tablets was 3.77 1.51 ng/mL. The administration of 2 x 0.48 mg patches
provided an almost
equivalent maximum concentration of 3.70 1.05 ng/mL; Cm ax for Group C (1.9
mg)
administered as a single patch was almost double (6.76 2.75 ng/mL). Groups F
and G
produced maximum plasma concentrations 3.9 times (14.61 4.46 ng/mL) and 6
times (22.56
14.0 ng/mL) that of zolmitriptan tablets, respectively.
[00257] Mean ( SD) total exposure (AUCinf) was 3.81 1.46 ng.H/mL
for M207
0.48 mg and 33.81 7.95 ng.H/mL for M207 3.8mg applied as single patches.
Treatment with
M207 patches in Groups F and G produced a similar exposure (AUCinf) to
zolmitriptan tablets
(30.12, 33.81 ng.H/mL, respectively). The mean exposure (AUCinf) for M207 was
proportional
(y=8.31) to the dose for single patch administration. The concentration-time
curve over zero to
two hours for all treatments is displayed in Figure 8 and zero to twenty-four
hours in Figure 7.
[00258] Plasma concentrations were slightly higher in males than
females for the
higher doses, Group F (2 x 1.9 mg) and Group G (3.8 mg). There did not appear
to be a
difference between genders at lower doses (Groups A [0,48 mg] to C [1.9 mg]).
[00259] The relative bioavailability of M207 systems was compared
to
zolmitriptan tablets using the following formula:
Fre = AUCf (M207) x Dose (Group D)
AUCinf (Group D) x Dose (M207)
[00260] The mean total exposure for M207 intracutaneous microneedle
systems
was less, relative to zolmitriptan tablets (range: 0.70-0.86). However, the
mean peak exposure
was 2.35 to 3.73 fold higher for intracutaneous zolmitriptan compared to
zolmitriptan tablets.
[00261] A summary of the key calculated pharmacokinetic parameters
from the
study are shown in Table 24.
73
Date Recue/Date Received 2023-12-18
Table 24: Mean (SD) PK parameters (0-24 hours) for All Treatments
Group
11,.. t1,2 C... AUCia AUC, AUCTh. Fw Frd
Formulation (dose) Parameter
(min) (11) (ng,/mL) (ng.H/mL) (ng.H/mL)
(ng.H/mL) AUChff C...
A
ZP-Zolmitriptan Mean 17.3 1.15 1.84 3.81 2.81 2.11
0.73 2.60
(0.48 mg) (SD) (12.1) (0.27) (0.53) (1.46) (1.36)
(0.73)
N-19
B
ZP-Zolmitriptan Mean 18.4 1.29 3.70 7.71 6.45 4.15
0.77 2.65
(0.48 mg x2) (SD) (11.2) (0.29) (1.05) (2.03) (1.97)
(0.95)
N=20
_
C
ZP-Zolmitriptan Mean 17.9 1.46 6.76 14.14 12.29 7.36
0.70 2.35
(1.9 mg) (SD) 12.6 (0.28) (2.75) (4.54) (4.31) (2.53)
N=20
li
Zolmitriptan oral Mean 107.4 3.27 3.77 27.19 22.20 4.72
- -
tablet (2.5 mg) (SD) 76.4 (0.80) (1.51) (11.34) (10.79)
(2.24)
1µ1=19
E
Sumatriptan SC Mean 12.5 1.14 88.80 105.23 100.88 70.88
- -
(6.0 mg/0.5 m1,) (SD) 4.4 (0.31) (27.56) (23.14) (23.29)
(14.15)
1s1=20 . .
F
ZP-Zolmitriptan Mean 17.1 1.53 14.61 30.12 27.77 16.44
0.74 2.63
(1.9 mg x 2) (SD) (11.8) (0.31) (4.46) (10.13) (9.93)
(5.34)
N=20
G
ZP-Zolmitriptan Meats 16.1 1.52 22.56 33.81 31.65 19.33
0.86 3.73
(3.8 mg) (SD) (11.6) (0.31) (14.00) (7.95) (8.35)
(5.37)
N=20
[00262] Approximately twice the amount of the active metabolite, N-
desmethyl
zolmitriptan was formed following zolmitriptan oral administration (mean 59.8
16%)
compared to those seen following M207 (Table 25).
Table 25: N-desmethyl Zolmitriptan/Zolmitriptan Metabolite Ratio
PARAMETER GROUP
A B C D F G
Metabolite ratio (0.48 mg) (0.48 mg x 2) (1.9 mg)
(2.5 mg oral) (1,9 mg x (3.8 mg)
N 16 20 20 18 20 20
Mean (%) 35.1 31.7 25.9 59.8 24.3 24.2
(SD) (10.1) (5.8) (3.6) (16.0) (4.2) (3.8)
Median (%) , 33.6 31.1 25.2 56.9 23.4 24.0 ,
Range (min, max %) 23.1, 61.9 22.4,46.2 19.5, 35.7 29.9, 89.4
17.6,34.1 19.0, 32.1
[00263] The relative bioavailability of the active metabolite, N-
desmethyl
zolmitriptan produced for M207 patches was compared to zolmitriptan tablets
using the
following formula:
74
Date Recue/Date Received 2023-12-18
Frei = N-desmethvl zolmitriptan AUC (M207) x Dose (Group D)
N-desmethyl zolmitriptan AUCinf (Group D) x Dose (M207)
[00264] There was less conversion to the N-desmethyl zolmitriptan
metabolite for
M207 patches compared to zolmitriptan tabs (Frei AUC range: 0.32-0.46) and
approximately
50% less rate of exposure for M207 patches compared to zolmitriptan tablets
based the relative
bioavailability of Cmax.
[00265] Plasma concentrations of the N-desmethyl metabolite reached
maximum
at around 1 hour (range: 54.7-65.0 minutes) for M207 administered via the
intracutaneous route
compared to (162.6 minutes [2.71 H] for zolmitriptan tablets, Figure 13. The
elimination half
life (t112) for the metabolite was comparable for all treatments including
oral administration
(range 2.7 H to 3.31 H]). The concentration-time curve from 0-24 hours for N-
desmethyl
zolmitriptan is displayed in Figure 14.
[00266] Mean maximum plasma concentration (Cõõõ) for the M207 0.48
mg dose
was 0.22 ng/mL and 1.77 ng/mL for the 3.8 mg strength compared to 2.08 ng/mL
for
zolmitriptan tablets. Mean AUCinf was 1.38 ng.H/mL for the 0.48 mg strength up
to 8.17
ng.H/mL for the 3.8 mg strength versus 14.55 ng.H/mL for zolmitriptan tablets.
The extent
(C.õ and AUC) of the N-desmethyl metabolite for M207 patches were directly
proportional
(y=0.4127 and 2.022, respectively) to the dose and considerably lower than
that for zolmitriptan
tablets. See Figures 19 and 21.
1002671 A summary of the mean pharmacokinetic parameters for N-desm
ethyl
zolmitriptan is detailed in Table 26.
Table 26: Mean (SD) PK parameters for N-desmethyl zolmitriptan metabolite
Group
TmaK tin AUC ia AUC, F,d Fõi
Formulation Parameter
(min) (H) (ng/mL) (ng.H/mL) (ng.H/mL) AUCinf
Cmai
(dose)
A
ZP-Zolmitriptan Mean 65.0 3.31 0.22 1.38 0.70 0.46 0.52
(0.48 mg) SD (18.6) (1.64) (0.05) (0.48) (0.31)
ZP-Zolmitriptan Mean 57.8 3.28 0.42 2.43 1.56 0.43 0.52
(0.48 mg x 2) SD (14.0) (1.75) (0.11) (0.78) (0.57)
ZP-Zolmitriptan Mean 61.5 3.05 0.74 3.65 3.01 0.32 0.43
(1.9 mg) SD (18.1) (0.99) (0.32) (1.22) (1.29)
Zolmitriptan oral Mean 162.6 3.22 2.08 14.55 13.71
Date Recue/Date Received 2023-12-18
tablet (2.5 mg) SD (77.0) (1.14) (0.50) (3.06) (2.91)
ZP-Zolmitriptan Mean 63.0 2,82 1.41 7.21 6.50 0.32 0.43
(1.9 mg x 2) SD (13.4) (0.45) (0.48) (2.34) (2.30)
ZP-Zolmitriptan Mean 54.7 2.70 1.77 8.17 7.55 0.36 0.52
(3.8 mg) SD (16.1) (0.52) (0.63) (1.96) (1.98)
[00268] M207 also tended to have less intragroup variability (as
indicated by the
CV/0s) for the AUCtrit- parameter compared to the zolmitriptan tablets.
Dose Linearity for M207 system administration
[00269] A positive linear association and was seen for Ctnax
(y=4.81), AUCt
(y-7.61) and AUCmf (y=8.31) for both single (0.48 mg, 1.9 mg and 3.8 mg) and
multiple (0.48
mg x 2 and 1.9 mg x 2) system administration. See Figures 15-17. At the lower
end of the
dosing range (0.48 mg x 2), concentrations of both the zolmitriptan and the N-
desmethyl
zolmitriptan metabolite were very comparable. However, at the highest dose
(1,9 mg x 2),
plasma concentrations achieved with multiple patch administration were
slightly less than that
with a single patch (and the 0.52J administration force) for both zolmitriptan
and the N-
desmethyl zolmitriptan. For N-desmethyl zolmitriptan, see Figures 19-21.
[00270] M207 patch administration resulted in rapid peak plasma
concentrations
(Tmax) that occurred within 20 minutes of patch application. This compared
favorably with 12.5
minutes for SC sumatriptan and offers a considerable improvement over
conventional release
oral zolmitriptan tablets (1.8 hours). Elimination rate (tw) for M207 was
shorter, approximately
twice the rate of zolmitriptan tablets (1.2-1.5 hours versus 3.3 hours).
[00271] Cmax for zolmitriptan tablets was 3.77 ng/mL. Treatment
with M207
patches in Groups C (1.9 mg), F (1.9 mg x 2) and G (3.8 mg) produced 1.8, 3.9
and 6 times
higher mean peak plasma concentration than zolmitriptan 2.5 mg tablets.
Multiple patch
administration with 2 x 0.48 mg M207 produced a comparable Cmõõ (3.70 ng/mL)
to oral
zolmitriptan tablets.
[00272] Treatment with M207 patches in Groups F and G produced a
similar
exposure (AUCtilf) to oral zolmitriptan tablets (30.12, 33.81 and 27.19
ng.H/mL, respectively).
However, the mean total exposure (AUCA) for M207 patches was less (0.700-0.86)
and the
mean peak exposure (Cmax) was 2.35 to 3.73 fold higher, relative to oral
zolmitriptan tablets.
76
Date Recue/Date Received 2023-12-18
[00273] The time to peak plasma concentration of the N-desmethyl
metabolite
from M207 was considerably faster at around 1 hour versus 2.7 hours for oral
zolmitriptan.
However, the extent of metabolite produced from M207 was about 50% less than
oral
zolmitriptan tablets. The key PK findings are summarized in Table 27 below.
Table 27: Key Pharmacokinetic Parameters
Dose Crnaõ (SD)
T.õ Med AUC9-2h, (SD) AUC0-last (SD) AUC0-1.1 RA v
(mg) ng/mL (Range) ng,/mL
hour ng/mL hour _ Dose Oral
A (ZP Zolmi) 0.48 1.8 (0.53) 20 (2-30) 2.1 (0.73)
2.8 (1.36) 5.8 67%
B (ZP Zolmi) 0.48 x 2 3.7 (1.05) 20(2-30) 4.2(0,95) 6.5
(1.97) 7.5 87%
C (ZP Zolmi) 1.9 6.8 (2.75) 20 (2-30) 7.4 (2.53)
12.3 (4.31) 6.5 76%
F (ZP Zolmi) 1.9 x 2 14.6 (4,46) 17.5 (2-30) 16.4 (5,34)
27,8 (9,93) 7,3 85%
G (ZP Zolmi) 3.8 22.6 (14.00) 15 (2-30) 19.3 (5.37)
31.7 (8.35) 8.3 97%
D (Oral Zolmi) 2.5 3.8 (1.51) 60 (30-240) 4.7 (2.24)
22.2 (10.79) 8.6 100%
E (SC Suma) 6.0 88.8 (27.56) 10 (5-20) 70.9
(14,15) .. 100.9 (23.29) .. 16.8
[00274] Perhaps most relevant to the potential utility of this
product for the
treatment of migraine or cluster headache is the T. for the M207 regimens,
showing much
more rapid absorption of the zolmitriptan from intracutaneous administration,
than from oral
administration.
[00275] A comparison of exposure is provided in Table 28 below.
Table 28: Comparison of exposure - M207 vs. Oral Zolmitriptan
(Phase 1 Study and Literature Comparisons)
Treatment (Study) Cmay, (ng/ml) AUC0-1ast (ng/ml*hr)
M207 0.96 mg (Study) 3.73 6.5
M207 1.9 mg (Study) 6.40 12.3
M207 2 x 1.9 mg (Study) 14.6 27.8
Zolmitriptan 2.5 mg oral (Study) 3.8 22.2
Zolmitriptan 10 mg oral (Seaber1997) 16.6(M) -20.9(F) 84.4(M)-108.6(F)
[00276]
There was excellent dose linearity observed for high and low dose for C.,
and AUCill. M207 was well-tolerated. Adverse events (AE) were predominantly
mild (87%), of
a short (<24 hour) duration and the majority were consistent with events
previously reported
with zolmitriptan (88%). There were no severe or serious AEs. Transient
changes in both
systolic and diastolic blood pressure occurred, and for both systolic and
diastolic blood pressure,
the pressure values returned to pre treatment levels 1-2 hours after drug
administration. No
77
Date Recue/Date Received 2023-12-18
significant ECG changes occurred. Application of the patch was tolerated well
with mostly mild
to moderate reactions that resolved after 24 hours. Local tolerability of the
3.8 mg patch applied
with greater force (0.52J) was not as favorable as the other regimens.
[00277] The M207 intracutaneous delivery system offers
pharmacokinetic
advantages over zolmitriptan tablets that should result in a faster onset of
action, comparable
exposure and reduced first-pass metabolism with the lowered potential for drug
interactions and
adverse events. Importantly, delivery is via a method that does not involve
the gastrointestinal
route or the injection method. Further comparison to the zolmitriptan
conventional oral tablet is
set forth below in Tables 29 and 30.
Table 29: Ratios of M207 vs. Group D
(oral zolmitriptan 2.5 mg tablets)
PARAMETER GROUP (dose)
Ratios vs. Group D A
(2.5 mg) (0.48 mg) (0.48 mg x 2) (1.9 mg) (1.9
mg x 2) (3.8 mg)
Ratio vs. D 0.50 1.02 1.79 4.00 5.56
Crnax
90% CI 0.42, 0.60 0.85, 1.2 1.52, 2.13 3.23,
5.00 4.55, 7.14
Ratio vs. D 0.12 0.31 0.58 1.32 1.55
AUCI
90% CI 0.10, 0.15 0.25, 0.38 0.47, 0.71 1.09,
1.59 1.28, 1.87
Ratio vs. D 0.14 0.3 0.53 1.15 1.32
AUCia
90% CI 0.12, 0.16 0.25, 0.35 0.45, 0.62 0.97,
1.35 1.12, 1.56
Ratio vs. D 0.46 0.96 1.63 3.69 4.41
AUC2hrs 90% CI 0.38, 0.55 0.80, 1.15
1.36, 1.96 3.04, 4.48 3.63, 5.36
Table 30: Ratios of N-desmethyl Zolmitriptan vs. Group D
(oral zolmitriptan 2.5 mg tablets)
PARAMETER GROUP (dose)
Ratio vs. Group D A
(2.5 mg oral) (0.48 mg) (0.48 mg x 2) (1.9 mg) (1.9
mg x 2) (3.8 mg)
Cmax Ratio vs. D 0.10 0.02 0.32 0.65
0.79
90% CI 0.09, 0.11 0.18, 0.23 0.26, 0.37
0.56, 076 0.68, 0.93
AUCi Ratio vs. D 0.04 0.11 0.20 0.45
0.54
90% CI 0.04, 0.05 0.09, 0.13 0.17, 0.24
0.39, 0.53 0.46, 0.63
AUCinf Ratio vs. D 0.09 0.16 0.24 0.48 0.55
90% CI 0.08, 0.10 0.14, 0.19 0.21, 0.28
0.42, 0.55 0.48, 0.63
78
Date Recue/Date Received 2023-12-18
PARAMETER GROUP (dose)
AUC2h., Ratio vs. D 0.13 0.28 0.45 0.93 1.14
90% CI 0.11, 0.15 0.24, 0.32 0.38, 0.52 0.78, 1.13
0.95, 1.38
Example 5¨in vivo Pig Study
[00278] .. The M207 patch was tested in swine.
Determination of Zolmitriptan by LC/MS/MS Method
[00279] The analysis for zolmitriptan from used patches, skin swabs and
plasma
PK samples was performed using established in-house LC/MS/MS methods. The low
limit of
quantitation (LLOQ) and high-limit of quantitation (HLOQ) were 0.1 and 1000
ng/mL,
respectively. A four minute method utilizing high performance liquid
chromatography-
electrospray ionization-tandem mass spectrometry (HPLC-ESI-MS/MS) was
developed for
quantitation of zolmitriptan and its two major metabolites in HGP plasma. The
instrumentation
consist of Agilent 1200 pumps, CTC PAL Autosampler with cooling stack, AB
Sciex
TurboVe ESI source, and API 40004i mass spectrometer.
[00280] All plasma samples and standards were first protein precipitated
using
100% methanol and then diluted with 30% solution of acetonitrile at 1:2 ratio
(Plasma: 30%
ACN), 204 of this solution was injected into autosample. The autosample was
equipped with
a 100 iL syringe and a 100 [IL loop. The syring and the loop were washed twice
with solvent 1
(0.2% formic acid) and solvent 2 (0.2% formic acid in acetonitrile) between
each injections.
[00281] A 10-port 2 position Valco valve was placed in-line with the mass
spectrometer equipped with a guard cartridge (Zorbax 300SB-C8, 12.5x4.6mm)
used for on-line
solid phase extraction (SPE). This valve was setup such that in the "load"
position, the sample
was injected onto the cartridge and was washed with 10% mobile phase B for 30
seconds, next
the valve was switched into the "inject" position, where the cleaned sample
would eluted from
the SPE cartridge in reversed direction onto the analytical column (Luna
PFP(2), 100 A, 5 1.tm,
50x2 mm) and into the mass spectrometer.
[00282] The HPLC method used was a reversed phase gradient method (0.0 min,
10%B; 0.5 min, 10%B; 1.0 min, 40%B; 1.7 min, 40%B; 2.1 min, 10%B; 4.0 min,
10%B).
I-IPLC mobile phases consist of 20 mM Ammonium Acetate as mobile phase A, and
80%
acetonitrile in 20% 20 mM Ammonium Acetate as mobile phase B. The source
setting for all
79
Date Recue/Date Received 2023-12-18
analysis were the following: Collision gas (CAD) = 12; Curtain gas (CUR) = 25;
Ion source gas
1 (GS1) = 60; ion source gas 2 (GS2) = 60; Ion spray Voltage (IS) = 5500;
Temperature (TEM)
= 600; interface Heater (ihe) ¨ on. During the mass spectrometer acquisition,
eleven MRM
transitions were monitored. All eleven MRM transitions were used for data
processing. The
peak areas under each corresponding MRM transitions were summed and compared
to that of
spiked standard plasma samples to calculated concentration for each sample. A
quadratic (1/x)
fitting was used for all calculations.
Anesthesia
[00283] Animals were induced with Telazol intramuscular and
maintained with
isoflurane on a ventilator.
Blood Sample Collection for Pharmacokinetic Studies
[00284] Blood samples were collected from one or more of the
following blood
vessels in the animals: marginal ear veins/artery (left/right), saphenous
veins (left/right),
mammary veins (left/right), and femoral artery (left/right). Indwelling
catheters/sheaths were
placed to access the preferred blood vessel and were secured in place for the
duration of the
blood collection period. All procedures were performed by trained staff per
approved protocols
and SOPs at the Testing Facilities. A 5-mL blank blood collection was obtained
from each
animal prior to the first dosing. One-mL blood samples were collected up to 5
hours after patch
application dosing. Heparinized microtainer tubes were used to collect all
blood samples. Blood
volume drawn was replaced with equal volume of heparinized saline with
catheter's dead
volume accounted. In general, blood collection on a daily basis did not exceed
3-5% of the
animal's total blood volume.
Intravenous Dosing of Zolmitriptan
[00285] Zolmitriptan solution for IV dosing was prepared in-house
with maximum
zolmitriptan concentration of 3 mg/mL. Less than 3 mL of dosing volume was
injected using a
28-30G needle into a marginal ear vein of the animal. Pressure was applied
momentarily with
gauze immediately after injection to prevent bleeding at the site of
injection.
Determination of Patch Delivery Performance
ZP-Zolmitriptan Delivery Determination Using Residual Drug Analysis
Date Recue/Date Received 2023-12-18
[00286] Zolmitriptan residual was determined from the used patch
and swabbing
from the treated skin site. Once peeled off the skin, the used patches were
trimmed of the
adhesive band outside the microprojection array area and saved for
zolmitriptan residual
analysis. To recover residual zolmitriptan from the treated skin site, three
synthetic fiber swabs
were used. The first swab was pre-wetted by insertion into a vial containing 1
mL of swab
buffer. The first swab was applied over the patch treatment skin site with
slight pressure (using
rolling motion) in several directions and up to the periphery of the treatment
site. The second
and third swabs were dry and were used to capture all residual buffer from the
skin site that was
wetted by the first swab. All three swabs were placed in the original vial
containing the swab
buffer. The amounts of zolmitriptan left on the microprojection array and skin
surface after each
application were compared against the original coated amounts on the array,
allowing for the
determinations of total zolmitriptan Delivery and Delivery Efficiency.
Equations to determine
the total drug delivered and drug delivery efficiency are shown below as (/)
and (2)
respectively.
Total Residual = Patch Residual + Skin Residual
Total Drug Delivered = Nominal Coated amount ¨ Total Residual (I)
Delivery Efficiency (%) = (1)
Nominal Coated amount) * 100 (2)
Study Design
[00287] Three prepubescent female Yorkshire swine were used in the
studies.
Naïve animals were purchased from approved vendors per Test Facilities'
approved protocol
and SOPs and quarantined for up to 7 days. Each group of animals was dosed up
to 5 treatments
(crossover) with a recovery/washout period of up to 5 days between treatments.
At first dosing,
animals had weight ranging from 18-25 kg and at last dosing, animals had
weights ranging from
25-41 kg. In general, treatments per group included one to two controls and
one to three ZP-
Zolmitriptan dosing. Control included IV zolmitriptan. ZP-Zolmitriptan patch
placement was
rotated from the left and right ventral thighs of the hind limbs in each group
of animals where
more than one patch application treatments were conducted.
Results
Table 31: In vivo Zolmitriptan patch delivery results
Coated arnduntoti)'.(s0). 1.89 (0.14) 2.02 0Ø6
81
Date Recue/Date Received 2023-12-18
''Mean Delivered amount (mg) (SDI 1.72 (0.05) 1.79 (0.12)
Mean Delivery Efficiency (%) 90 4 88 7
3 7
Min amount delivered (mg) 1.68 1.68
Max amount delivered (mg) 1 78 1 92
,.N 3 3
1002881 Mean delivery was > 1.7 mg for all coated both in vivo
studies. Delivery
efficiency were >85 % and were consistent for both studies. Pharmacokinetic
study was
subsequently conducted with MF1663 array design coated with 1.9 mg. Table 32
summarizes
the patch and IV results.
Table 32: PK parameters of zolmitriptan patch in animal studies
PK parameters IV ZP-Zolmitriptan
Patch Coated Dose (mg) N/A 1.95
Dose (mg/Kg) 0.074 0.061
Cmax (ng/mL)
251.7 11.4 8.4 1.7
(Mean SE)
Median Tniax (min) 1
(10-30)
AUG (ng x h/mL)
27.4 1.9 16.8 1 5
(Mean SE)
Absolute Bioavailability
N/A 76.9 8 2
(%) (Mean SE)
1002891 In vivo evaluation showed rapid systemic absorption of
zolmitriptan with
patch administration Plasma levels were maintained over 5 h with median 7' max
of 15 minutes
Zolmitriptan patches had an absolute bioavailability of 77 % ZP-Zolmitriptan
patch showed
that the requisite amount of drug could be coated, high delivery efficiency
consistently attained
and coated zolmitriptan was well absorbed with fast time to Guy,.
Example 6¨Human Efficacy Clinical Trial
82
Date Re cue/Date Received 2023-12-18
[00290] The ZOTRIP pivotal efficacy study was a multicenter, double-
blind,
randomized, placebo-controlled trial comparing three doses of M207 (1.0mg,
1.9mg, and
3.8mg) to placebo for the treatment of a single migraine attack. Subjects were
enrolled in the
ZOTRIP trial at 36 centers across the United States. Those recruited into the
trial had a history
of at least one year of migraine episodes with or without aura. Upon
recruitment, the subjects
entered a run-in period that ensured they met the key eligibility criteria of
2-8 migraine attacks
per month, which was documented using an electronic diary or an app on their
cell phone.
Subjects also identified their most bothersome other symptom selected from
nausea,
photophobia, and phonophobia, and indicated the presence or absence of nausea,
phonophobia
or photophobia, during the episodes in the run-in period. Successfully
screened subjects were
then randomized into the treatment/dosing period in which they had 8 weeks to
confirm and
receive blinded treatment for a single migraine attack, termed "qualifying
migraine," in which
their previously identified most bothersome other symptom had to be present.
[00291] During a qualifying migraine, subjects scored the severity
of pain on the
4-point headache pain scale, and the presence or absence of migraine
associated symptoms
(photophobia, phonophobia or nausea), starting pre-dose and then at several
intervals over 48
hours post-dose. The co-primary endpoints for the study were those defined in
the October 2014
FDA Draft Guidance ¨ "Migraine: Developing Drugs fbr Acute Treatment" on pain
and most
bothersome other symptom freedom. Subjects recorded their migraine symptoms in
a patient
diary, prior to treatment, and at varying intervals following treatment, out
to 48 hours. Safety
was assessed by adverse events reported and other standard safety measures.
[00292] Five hundred and eighty nine (589) subjects were enrolled
in this study, of
which 365 were randomized. Of those randomized, 333 subjects were treated and
were included
in the safety analysis, and 321 qualified for the modified intent-to-treat
(mITT) population.
Fifty-one percent (51%) of the subjects randomized were found to have severe
migraine pain
pre-treatment. Also at the time of treatment, 70% reported nausea, 37% aura,
and 51% waking
up with their migraine (morning migraine). With the multiple doses and
multiple endpoints in
the trial, a sequential testing procedure was used beginning with the highest
dose and the co-
primary endpoints. Since statistical significance was not achieved for most
bothersome other
83
Date Recue/Date Received 2023-12-18
symptom in the 1.9 mg group, p-values for secondary endpoints should be
considered nominal
p-values.
1002931 All three doses of M207 (1 mg, 1.9 mg, and 3.8 mg) achieved
statistically
significant pain freedom at 2 hours. The 3.8 mg dose achieved both co-primary
endpoints of
pain freedom and most bothersome other symptom freedom at 2 hours. The 3.8 mg
dose also
achieved significance in the secondary endpoints of pain freedom at 45 minutes
and 1 hour and
showed durability of effect on pain freedom at 24 and 48 hours. Additionally,
M207 was not
associated with any Serious Adverse Events (SAEs).
[00294] The 3.8 mg dose of M207 achieved statistical significance
for both co-
primary endpoints at two hours, as shown in Table 33:
Table 33: Primary Endpoint
Primary endpoint Placebo
3.8mg M207 p-value
Pain freedom 14.3% 41.5% 0.0001
Most bothersome other symptom 42.9% 68.3% 0.0009
free
[00295] Furthermore, secondary endpoints measuring pain freedom at
additional
time points for the 3.8 mg dose of M207 showed M207 superior to placebo with a
nominal p-
value less than 0.05, as shown in Table 34:
Table 34: Pain Freedom
Pain Freedom Placebo 3.8mg M207 p-value*
Pain freedom at 45 minutes 5.2% 17.1% 0.0175
Pain freedom at 60 minutes 10.4% 26.8% 0.0084
Pain freedom at 24 hours 39.0% 69.5% 0.0001
Pain freedom at 48 hours 39.0% 64.6% 0.0013
[002961 Overall, only 13 subjects (3.9%) reported pain at the
application site;
application site pain was reported as mild in all but three subjects. The most
frequently reported
adverse event was redness at the application site (18.3% of subjects). All
cases of redness
resolved. Further, five (1.5%) patients across M207-treated groups reported
dizziness vs. 0%
on placebo.
84
Date Recue/Date Received 2023-12-18
[00297] Additional data from the results of the clinical trial are
set forth in the
Tables below.
Table 35: Co-Primary Endpoints: Primary Endpoint Analysis
mITT Population (LOCF) Treatment Group
Pain Freedom at 2 hours Placebo 1 mg 1.9 mg 3.8
mg
(N=77) (N=79) (N=83)
(N=82)
14.3% 30.4% 27.7% 41.5%
% (n/N)
(11/77) (24/79) (23/83)
(34/82)
Difference from Placebo 16.1% 13.4% 27.2%
P-value 0.0149 0.0351
0.0001
Freedom from most bothersome other symptom at 2 hours
42.9% 57.0% 53.0% 68.3%
0/0 (n/N)
(33/77) (45/79) (44/83)
(56/82)
Difference from Placebo 14.1% 10.2% 25.4%
P-value 0.0706 0.1694
0.0009
1002981 In Table 35, above, the 3.8 mg dose group met both co-
primary endpoints
with a p-value < 0.05. The 1.9 mg dose group met the pain freedom endpoint
with a p-value <
0.05. For the freedom from most bothersome other symptom at 2 hours endpoint,
the 1.9 mg
dose group had a p-value of? 0.05. The 1 mg dose group met the pain freedom
endpoint with a
p-value <0.05. For the 1 mg dose group, the freedom from most bothersome other
symptom
endpoint at 2 hours had a p-value > 0.05.
Table 36: Co-Primary Endpoints: Multiple Imputation
mITT Population (LOCF) Treatment Group
Pain Freedom at 2 hours Placebo 1 mg 1.9 mg 3.8 mg
(N=77) (N=79) (N=83) (N=82)
Average % 14.7% 29.8% 29.4% 39.8%
Difference from Placebo 15.1% 14.7% 25.1%
P-value 0.0297 0.0344 0.0012
Freedom from most bothersome other symptom at 2 hours
% (n/N) 42.6% 59.9% 56.5% 68.2%
Difference from Placebo 17.3% 13.9% 25.6%
P-value 0.0294 0.0755 0.0013
Date Recue/Date Received 2023-12-18
[00299] Table 36, above, is consistent with co-primary endpoint
analyses (mITT
LOCF), Table 35, below, provides the fixed-sequence for testing each of the
multiple endpoints
that are described for migraines to assess whether the study was successful.
For doses of 3.8 mg,
1.9 mg, and 1.0 mg, the efficacy of treatment was tested for the co-primary
and secondary
endpoints in Table 35. As shown, all endpoints at or after testing order 4 are
not significant
under the MCP methodology.
Table 37: CP2016-001: MCP ¨ Fixed Sequential Testing
Statistically
Testing Co-Primary/
Efficacy Endpoint Dose P-value significant
Order Secondary
Under MCP
1 co-primary Pain free at 2 hours 3.8 mg 0.0001 Yes
Most bothersome other symptom free
2 co-primary 3.8 mg 0.0009 Yes
at 2 hours
3 co-primary Pain free at 2 hours 1.9 mg 0.0351 Yes
Most bothersome other symptom free
4 co-primary 1.9 mg 0.1694 No
at 2 hours
secondary Pain relief at 30 minutes 3.8 mg 0.1024 No
6 secondary Pain relief at 30 minutes 1.9 mg, 0.8642 No
7 secondary Pain relief at 2 hours 3.8 mg 0.0013 No
8 secondary Pain relief at 2 hours 1.9 mg 0.1109 No
9 co-primary Pain free at 2 hours 1.0 mg, 0.0149 No
Most bothersome other symptom free
co-primary 1.0 mg 0.0706 No
at 2 hours
11 secondary Pain relief at 30 minutes 1.0 mg 0.7839 No
[00300] Tables 38-46 provide results of a clinical study of
treating with one
embodiment of the claimed invention. In this embodiment, as shown in Tables 36-
40, endpoints
were evaluated sequentially, as described in Table 35, including pain freedom,
pain relief,
photophobia freedom, phonophobia freedom, and nausea freedom for treatment of
1 mg, 1.9
mg, and 3.8 mg at time points of 15 minutes, 30 minutes, 45 minutes, 1 hour, 2
hours, 3 hours, 4
hours, 12 hours, 24 hours, and 48 hours after treatment. As shown in Tables 41-
44,
investigators also made visual assessments of the skin after patch removal for
adverse events
like bruising, edema, and erythema.
Table 38: Pain Freedom
86
Date Recue/Date Received 2023-12-18
Pain Freedom (mITT/LOCF) Treatment Group
Time-point Placebo 1 mg 1.9 mg 3.8 mg
15 Minutes 0% 0% 0% 0%
30 minutes 2.6% 2.5% 3,6% 7,3%
45 minutes 5.2% 3.8% 13.3% 17.1%*
1 hour 10.4% 17.7% 20.5% 26.8%*
2 hour 14.3% 30.4%* 27.7%*
41.5%*
3 hour 26.0% 40.5%* 37.3%
51.2%*
4 hour 28.6% 45.6%* 47.0%*
54.9%*
12 hours 32.5% 54.4%* 53.0%*
62.2%*
24 hours 39.0% 59.5%* 61.4%*
69.5%*
48 hours 39.0% 63.3%* 66.3%*
64.6%*
* Indicates p-value < 0.05; however not significant under MCP
Table 39: Pain Relief
Pain Relief (mITT/LOCF) Treatment Group
Time-point Placebo 1 mg 1.9 mg 3.8 mg
15 Minutes 14.3% 12.7% 16.9% 23.2%
30 minutes 33.8% 31.6% 32.5% 46.3%
45 minutes 45.5% 44.3% 45.8% 56.1%
1 hour 53.2% 46.8% 55.4% 68.3%*
2 hour 57.1% 65.8% 68.7% 80.5%*
3 hour 51.9% 75.9%* 66.3% 81.7%*
4 hour 51.9% 73.4%* 72.3%* 82.9%*
12 hours 48.1% 75,9%* 72.3%* 80.5%*
24 hours 46.89/o 72.2%* 72.3%* 78,0%*
48 hours 41.6% 72.2%* 71.1%* 70.7%*
* Indicates p-value < 0.05; however not significant under MCP
Table 40: Photophobia Freedom
Pain Freedom (mITT/LOCF) Treatment Group
Time-point Placebo 1 mg 1.9 mg 3.8 mg
15 Minutes 9.1% 11.4% 9.6% 11.0%
30 minutes 22.1% 27.8% 24.1% 26.8%
45 minutes 24.7% 43.0%* 33.7% 41.5%*
1 hour 33.8% 48.1%* 44.6% 53.7%*
2 hour 41.6% 60.8%* 56.6%* 69.5%*
3 hour 44,2% 65.8%* 61.4%* 72.0%*
4 hour 45.5% 65.8%* 63.9%* 74.4%*
87
Date Recue/Date Received 2023-12-18
12 hours 42.9% - 7= 4.7%* 66.3%* 75.6%*
24 hours 42.9% 72.2%* 68.7%* 73.2%*
48 hours 40.3% 7= 0.9%* 67.5%* 68.3%*
* Indicates p-value < 0.05; however not significant under MCP
Table 41: Phonophobia Freedom
Pain Freedom (mITT/LOCF) Treatment Group
Time-point Placebo 1 mg 1.9 mg 3.8 mg
15 Minutes 14.3% 20.3% 15,7% 25.6%
30 minutes 35.1% 34.2% 28.9% 45.1%
45 minutes 37.7% 44.3% 43.4% 57.3%*
1 hour 46.8% 48.1% 57.8% 61.0%
2 hour 55.8% 58.2% 61.4% 69.5%
3 hour 54.5% 63.3% 71.1%* 73.2%*
4 hour 57.1% 69.6% 69.9% 74.4%*
12 hours 44.2% 73.4%* 68.7%* 78.0%*
24 hours 42.9% 69.6%* 68.7%* 76.8%*
48 hours 42.9% 72.2%* 68.7%* 70.7%*
* Indicates p-value < 0.05; however not significant under MCP
Table 42: Nausea Freedom
Nausea Freedom (mITT/LOCF) Treatment Group
Time-point Placebo 1 mg 1.9 mg 3.8
mg
15 Minutes 36.4% 39.2% 41.0% 32.9%
30 minutes 59.7% 55.7% 49.4%
45 minutes 63.6% 58.2% 66.3% 62.2%
1 hour 63.6% 68.4% 71.1% 76.8%
2 hour 63.6% 75.9% 74.7% 81.7%*
3 hour 58.4% 78.5%* 74.7%* 79.3%*
4 hour 54.5% 78.5%* 73.5%* 79.3%*
12 hours 45.5% 75.9%* 71.1%* 80.5%*
24 hours 44.2% 72.2%* 74,7%* 79.3%*
48 hours 41.6% 72.2%* 72.3%* 70.7%*
* Indicates p-value <0.05; however not significant under MCP
Table 43: Investigator: Visual Dermal Assessment: PRSPB
Treatment Group
Patch-Related Superficial Punctate
B (PRSPB) Placebo 1 mg 1.9 mg 3.8 mg
ruising
(N=83) (N=78) (N=84) (N=83)
None 82 (98.8%) 73 (93.6%) 72 (85.7%) 74
(89.2%)
88
Date Recue/Date Received 2023-12-18
<=25% ZP patch application site has
1(1.2%) 5 (6.4%) 9 (10.7 A)
4 (4.8%)
punctate bruising spots
>=26% to <50% ZP patch application
0 (0.0%) 0 (0.0%) 2 (2.4%) 2
(2.4%)
site has punctate bruising spots
>50% ZP patch application site has
0 (0.0%) 0 (0.0%) 1 (1.2%) 3
(3.6%)
punctatc bruising spots
Note: Investigator assessment occurs at End-of Study; Day 2-8 (treatment on
Day 1)
Table 44: Investigator: Visual Dermal Assessment: Edema
Treatment Group
Edema Placebo 1 mg 1.9 mg 3.8 mg
, (N=83) (N=78) (N=84) (N=83)
None 83 (100.0%) 77 (98.7%)
81(96.4%) 82 (98.8%)
Slight Edema 0 (0.0%) 1(1.3%) 3 (3.6%) 1(1.2%)
Moderate Edema 0 (0.0%) 0 (0.0%) 0 (0.0%)
0 (0.0%)
Severe Edema 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%)
Note: Investigator assessment occurs at Visit 4; End-of Study
Table 45: Investigator: Visual Dermal Assessment: Erythema
Treatment Group
Erythema Placebo 1 mg 1.9 mg 3.8 mg
(N=83) (N=78) (N=84) (N=83)
None 78 (94.0%) 71(91.0%) 71(84.5%)
64 (77.1%)
Mild Redness 5(6.0%) 7(9.0%) 10(11.9%)
16(19.3%)
Well-defined Redness 0 (0.0%) 0 (0.0%) 3 (3.6%)
3 (3.6%)
Beet Redness 0(0.0%) 0(0.0%) 0(0.0%)
1 0(0.0%)
Note: Investigator assessment occurs at Visit 4; End-of Study
Table 46: General Disorders/Administration Disorders TEAEs
System Organ Class/Preferred Treatment Group
Term N (%) of Subjects Placebo 1 mg 1.9 mg 3.8
mg
General disorders and 12 23 31 38
administration site conditions (14.5%) (28.8%) (35.6%)
(45.8%)
Application site erythema 9 (10.8%) 13 (16.3%) 17
(19.5%) 22 (26.5%)
Application site bruise 3 (3.6%) 5 (6.3%) 12 (13.8%)
12 (14.5%)
Application site pain 1(1.2%) 2 (2.5%) 2 (2.3%) 8
(9.6%)
Application site hemorrhage 0 (0.0%) 3 (3.8%) 5 (5.7%) 4
(4.8%)
Application site swelling 3 (3.6%) 1(1.3%) 3 (3.4%) 2
(2.4%)
89
Date Recue/Date Received 2023-12-18
Application site edema 0 (0.0%) 1(1.3%) 3 (3.4%) 2
(2.4%)
Application site discoloration 1(1.2%) 1(1.3%) 1(1.1%)
1(1.2%)
Note: TEAEs occurring in > 1 active treated subject
[00301] Tables 47-
50 and Figures 25-28 demonstrate the efficacy of one
embodiment of the claimed invention against published results of treatments
that are currently
used in the art. Until the claimed invention, the state of the art included
nasal treatments and
standard and orally dissolving tablets.
Table 47: Pain Free Zolmitriptan
ZOLMITRIPTAN COMPARISON, % PAIN FREE
Dosage Form Dose 1 hour 2 hour 4 hour Reference
M207 Patch 3.8 mg 26.8% 41.5% 54.9%
M207 Patch 1.9 mg 20.5% 27.7% 47.0%
M207 Patch 1.0 mg 17.7% 30.4% 45.6%
NASAL 2.5 mg 10.6% 21.0% 38.4% Charlesworth et al,
2003
TABLET 2.5 mg 10.4% 35.6% Pascual et al , 2000
TABLET 5 mg 10.0% 39.0% Dahlof et
al 1998
TABLET 10 mg 9.0% 39.0% Dahlof et
al 1998
ODT 2.5 mg 7.8% 27.0% 37.0% Dowson et al, 2002
TABLET 5 mg 7.8% 29.3% 54.6% Geraud et al, 2000
TABLET 2.5 mg 5.7% 26.3% Steiner et
al, 2003
Table 48: Pain Relief Zolmitriptan
ZOLMITRIPTAN COMPARISON, % PAIN RELIEF
Dosage Form Dose 1 hour 2 hour 4 hour Reference
M207 Patch 3.8 mg 68.3% 80.5% 82.9?/o
M207 Patch 1.9 mg 55,4% 68.7% 72,3%
M207 Patch 1.0 mg 46.8% 65.8% 73.4%
ODT 2.5 mg 45.0% 63.0% Dowson et
al, 2002
TABLET 5 mg 44.0% 66.0% Dahlof et
al 1998
NASAL 2.5 mg 40.2% 55.4% 63.4% Charlesworth et al, 2003
TABLET 10 mg 40.0% 71.0% Dahlofct al
1998
TABLET 2.5 mg 35.3% 66.8% Pascual et
al , 2000
TABLET 5 mg 34.2% 58.7% 80.5% Geraud
eta!, 2000
TABLET 2.5 mg 25.1% 59.6% 25.1%
Steiner et al, 2003
Date Recue/Date Received 2023-12-18
Table 49: Pain Free Triptans
COMPARISON TO OTHER TRIPTANS, %PAIN FREE
Drug Dosage Form Dose , 1 hour 2 hour 4 hour Reference
Zolmitriptan ZSAN Patch 3.8 mg 26.8% 41.5% 54.9%
Zolmitriptan ZSAN Patch L9 mg 20.5% 27.7% 47.0%
Zolmitriptan ZSAN Patch 1.0 mg 17.7% 30.4% 45.6%
Rizatriptan WAFER 10 mg 13.0% 42.2% Ahrens et al, 1999
Zolmitriptan NASAL 2,5 mg
10.6% 21.0% 38.4% Charlesworth et al, 2003
Rizatriptan TABLET 10 mg 10.4% 40.3% Tfelt-
Hansen et al, 1998
Eletriptan TABLET 80 mg 10.0% 27.0% 49.0% Sheftell et al 2003
Zolmitriptan ODT 2.5 mg 7.8% 27.0% 37.0% Dowson et al, 2002
Zolmitriptan TABLET 5 mg 7.8% 29.3% 54.6% Geraud et al, 2000
100
Sumatriptan TABLET 7.8% 32.8% Tfelt-
Hansen et al, 1998
mg
Rizatriptan WAFER 5 mg 7.7% 34.8% Ahrens et al, 1999
Naratriptan TABLET 2.5 mg 3.3% 20.7% Bomhof et al, 1999
Table 50: Pain Relief Triptans
COMPARISON TO OTHER TRIPTANS, % PAIN RELIEF
Drug Dosage Form Dose 1 hour 2 hour
4 hour Reference
Zolmitriptan ZSAN Patch 3.8 mg 68.3% 80.5% 82.9%
Zolmitriptan ZSAN Patch 1.9 mg 55.4% 68.7% 72.3%
Zolmitriptan ZSAN Patch 1.0 mg 46.8% 65.8% 73.4%
Zolmitriptan ODT 2.5 mg 45.0% 63.0% Dowson
et al, 2002
Rizatriptan WAFER 10 mg 44.9% 74.1% Ahrens
eta!, 1999
Zolmitriptan , NASAL 2.5 mg 40.2% 55.4% 63.4%
Charlesworth eta!, 2003
Rizatriptan WAFER 5 mg 39.8% 58.6% Ahrens
et al, 1999
Rizatriptan TABLET 10 mg 36.6% 67.0%
Tfelt-Hansen et al, 1998
Zolmitriptan TABLET 5 mg 34.2% 58.7% 80.5%
Geraud et al, 2000
Eletriptan TABLET 80 mg 32.0% 59.0% 79.0%
Sheftell et a1 2003
Sumatriptan TABLET 100 mg 27.9% 61.8%
Tfelt-Hansen et al, 1998
Naratriptan TABLET 2.5 mg 27.7% 48.4% Bomhof
et al, 1999
1003021 The
embodiments of the invention described above are intended to be
merely exemplary; numerous variations and modifications will be apparent to
those skilled in
the art. All such variations and modifications are intended to be within the
scope of the present
invention as defined in any appended claim.
91
Date Recue/Date Received 2023-12-18