Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
Polyethylene composition and use thereof, and polyolefin microporous
breathable film prepared therefrom
Technical field
The present invention relates to the field of polyolefin compositions, in
particular to a
polyethylene composition and use thereof and a polyolefin microporous
breathable film
prepared therefrom, and further to a breathable composite article comprising
the
polyolefin microporous breathable film.
Background art
A breathable film is also called "water-blocking breathable microporous film"
and has the
characteristic of being permeable to gas (such as air, water vapor, etc.) and
impermeable
to water (water droplets, sweat and other liquids). The breathable film in the
prior art is
generally a composite material with a thermoplastic plastic as the matrix and
filled with a
certain proportion of solid particles (such as calcium carbonate, barium
sulfate, titanium
dioxide, talc powder and other mineral fillers), which composite material is
prepared into
a thin film via methods such as blowing moulding, calendering or casting,
which thin film
is then uniaxially or biaxially stretched under appropriate conditions to
obtain a
microporous thin film material with smaller pores. A microporous breathable
film allows
gases such as water vapor and air to pass through while blocking liquids, can
be widely
used in medical supplies such as medical protective clothing, wound care
bandages and
dressings, in hygiene products such as baby diapers and adult care products,
in food
packaging and daily products, and in other fields, and is a new material that
has
developed rapidly in the past two decades.
Theoretically, any thermoplastics can be used as the matrix resin of
breathable film
materials, but from the overall market value of microporous breathable films,
polyethylene
breathable films account for the largest proportion. Polyethylene has the
advantages of
rich sources of starting materials, easy processing and molding, good film-
forming effect,
comfortable and soft feel, etc., and is widely used as the matrix resin of
breathable film
materials.
The sanitary product industry is the largest end market for polyethylene
breathable films,
CA 03236535 2024- 4- 26
1
so the safety and comfort of the breathable films must be taken into
consideration.
Therefore, high quality is required for a porogen used in the preparation
process of
polyethylene breathable films. Inorganic fillers, particularly calcium
carbonate, have
become the preferred material as the porogen due to their abundant resources,
low price,
and good overall properties. However, inorganic porogens have hydrophilic and
oleophobic and extremely polar surface, and have poor compatibility with the
polymer
matrix, which makes it difficult to disperse uniformly in the matrix. At the
same time, the
dispersion uniformity of the porogen in the matrix will have an impact on the
mechanical
properties, surface evenness, pore size uniformity of micropores, porosity and
other
parameters of the breathable film. In the traditional production process,
surfactants are
often used to pretreat inorganic fillers to increase their compatibility with
the matrix, and
at the same time coupling agents and dispersants are added to solve the
dispersion
problem of inorganic fillers.
CN102336940A discloses a composition for a breathable film with a low
permeation
volume and a preparation method thereof. The composition comprises a
polyolefin resin
mixture, a permeation volume regulator, surface-modified micro-scale inorganic
particles,
an antioxidant, a lubricant and a coupling agent. The advantage of this
composition lies
in that the micron-scale inorganic filler can improve the mechanical
properties of the
breathable film, and at the same time the coupling modification of the surface
of the
inorganic particles can increase their compatibility with polyolefin resin,
while the
disadvantage lies in that the micron-scale inorganic particles cannot be
dispersed
uniformly in the matrix resin, thus affecting the breathability uniformity of
the film.
CN1176986C discloses a special resin for a polyolefin highly breathable cast
film and a
preparation method of the film. The special resin is obtained by using a
polyolefin resin
(compounded by the three polymers of LDPE, LLDPE and propylene copolymer) and
an
ultra-fine mineral filler (calcium carbonate, talc powder, etc., having a
particle size of 3-
10 pm) as the main starting materials, adding a Ti- Al composite coupling
agent, POE
modifier and OPE dispersant, and subjecting to the processing by coupling
treatment,
compatibilization and dispersion, internal mixing and extrusion granulation.
Via the
casting and stretching processes, this special resin produces uniform and fine
pores
between the two phases of mineral filler particles and polyolefin in the thin
film, and then
after cooling and crystallization, a polyolefin highly breathable cast film is
formed. This
method cannot effectively form pores and control the pore size, and moreover
uses an
CA 03236535 2024- 4- 26
2
internal mixer, which makes the process cumbersome and complicated.
CN101747548A discloses a compound for the preparation of a high-strength
polyolefin
breathable film and a preparation method thereof. The compound comprises a
polyolefin
resin, micron-scale and nano-scale inorganic fillers, an antioxidant, a
processing aid, and
a coupling agent. The nano-scale inorganic filler can improve the mechanical
properties
of the polyolefin breathable film and reduce the thickness of the breathable
film. In
addition, breathable film with high strength and high air permeability can be
prepared by
adjusting process conditions. However, the poor compatibility between the nano
inorganic
filler and the polyolefin resin would cause uneven breathability of the
material, thus
affecting the use of the breathable film.
At present, the inorganic porogens used in the production of breathable films
on the
market have the following problems: (1) they are easy to agglomerate; (2) the
breathable
film has a poor pore size uniformity due to the large particle size and uneven
particle size
distribution, or even a large number of pinhole defects occur; (3) the filler
particles have
a poor surface modification effect and a poor compatibility with the matrix,
thus affecting
the strength of the film; and (4) the filler particles have a poor dispersity
and will migrate
to the surface of the breathable film, causing color differences or spots on
the breathable
film.
In recent years, there have also been a few publications reporting on the use
of organic
porogens in breathable films. For example, Bo Shi et al. ("New Breathable
Films Using a
Themoplastic Cross-Linked Starch as a Porogen", J. Appl. Polym. SCI, 2014,
41016,
pages 1-8) discloses a biodegradable breathable film prepared by using natural
corn and
chemically cross-linked starch as the organic porogen, and poly(butylene
adipate
terephthalate) as the matrix resin. The film does not use polyolefin as the
matrix resin. In
addition, although no inorganic porogen is used, mono and diglycerides must be
used as
the surfactant to convert the two starch porogens into thermoplastic starch or
thermoplastic cross-linked starch before blending with the copolyester in film
preparation.
Summary of the invention
In order to overcome the defects existing in the prior art, one object of the
present
invention is to provide a polyethylene composition for preparing a polyolefin
nnicroporous
CA 03236535 2024- 4- 26
3
breathable film, which composition can achieve a good dispersion of a porogen
in the
polyethylene matrix resin without agglomeration in the case of not using
coupling agents,
dispersants and/or surfactants, and thus can avoid the disadvantages of uneven
pore
size distribution, poor dispersion, poor compatibility, etc. caused by
inorganic porogens.
When the composition is used to prepare a breathable film, uniform
breathability and
improved water vapor transmission rate can be achieved.
Another object of the present invention is to provide a polyolefin
rnicroporous breathable
film and a breathable composite article comprising the film. The breathable
film is
characterized by widely adjustable physical properties and air permeability,
wide
adaptability, simple operation, and stable product performance.
In the present invention, it was unexpectedly found out that the above objects
were
achieved by using maleic anhydride copolymer microspheres as an organic
porogen in a
polyethylene matrix resin, which copolymer microspheres have an appropriate
compatibility and binding force with the polyethylene matrix resin,
particularly, which
copolymer microspheres even have a uniform particle size distribution
(rnonodisperse
particle size distribution) and preferably have a spherical shape, so that the
porogen is
well and uniformly dispersed in the polyethylene matrix resin, does not
agglomerate
therein and binds properly therewith without the use of a coupling agent, a
dispersant
and/or a surfactant, and thus rnicropores permeable to air and water vapor but
impermeable to liquid water can be generated in a film during the process of
preparing
the film by casting and stretching and such micropores even have uniform pore
sizes.
Thus according to a first aspect, the present invention provides a
polyethylene
composition, comprising a polyethylene matrix resin and a porogen, wherein the
porogen
is used in an amount of 30-110 parts by weight based on 100 parts by weight of
the
polyethylene matrix resin, and the porogen comprises maleic anhydride
copolymer
microspheres, wherein the copolymer in the microspheres comprises a structural
unit A
from maleic anhydride, a structural unit B from a vinyl-containing comonomer
M, and
optionally a cross-linking structural unit from a cross-linking agent, and the
average
particle size of the maleic anhydride copolymer microspheres is 500-2000 nm.
According to a second aspect, the present invention provides use of the
polyethylene
composition of the present invention for preparing a polyolefin microporous
breathable
CA 03236535 2024- 4- 26
4
film.
According to a third aspect, the present invention provides a polyolefin
microporous
breathable film prepared from the polyethylene composition according to the
present
invention.
According to a fourth aspect, the present invention provides a breathable
composite
article comprising the polyolefin rnicroporous breathable film according to
the present
invention.
Description of the drawings
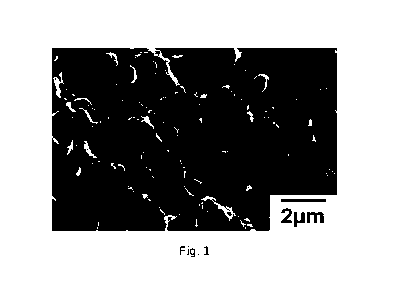
Fig. 1 is an SEM photograph of the cross-section of the polyethylene
composition sample
sheet prepared in Example 1-1;
Fig. 2 is an SEM photograph of the cross-section of the polyethylene
composition sample
sheet prepared in Example 1-3;
Fig. 3 is an SEM photograph of the cross-section of the polyethylene
omposition sample
sheet prepared in Example 1-5;
Fig. 4 is an SEM photograph of the cross-section of the polyethylene
composition sample
sheet prepared in Example 1-6;
Fig. 5 is an SEM photograph of the cross-section of the polyethylene
composition sample
sheet prepared in Comparative Example 1-1;
Fig. 6 is an SEM photograph of the cross-section of the polyethylene
composition sample
sheet prepared in Comparative Example 1-2;
Fig. 7 is an SEM photograph of the cross-section of the polyethylene
composition sample
sheet prepared in Comparative Example 1-3;
Fig. 8 is an SEM photograph of the cross-section of the polyethylene
composition sample
sheet prepared in Example 11-1;
Fig. 9 is an SEM photograph of the cross-section of the polyethylene
composition sample
sheet prepared in Example 11-3;
Fig. 10 is an SEM photograph of the cross-section of the polyethylene
composition
sample sheet prepared in Example 11-4;
Fig. 11 is an SEM photograph of the cross-section of the polyethylene
composition
sample sheet prepared in Example 11-6;
Fig. 12 is an SEM photograph of the cross-section of the polyethylene
composition
CA 03236535 2024- 4- 26
5
sample sheet prepared in Comparative Example 11-1;
Fig. 13 is an SEM photograph of the cross-section of the polyethylene
composition
sample sheet prepared in Comparative Example 11-2;
Fig. 14 is an SEM photograph of the cross-section of the polyethylene
composition
sample sheet prepared in Comparative Example 11-3.
Detailed description of the invention
According to a first aspect, the present invention provides a polyethylene
composition,
comprising a polyethylene matrix resin and a porogen, wherein the content of
the porogen
is 30-110 parts by weight based on 100 parts by weight of the polyethylene
matrix resin,
and the porogen comprises maleic anhydride copolymer microspheres, wherein the
copolymer in the microspheres comprises a structural unit A from maleic
anhydride and
a structural unit B from a vinyl-containing comonomer M, and the average
particle size of
the maleic anhydride copolymer microspheres is 500-2000 nnn.
Herein, the porogen refers to an additive added to a composition for preparing
a
breathable film, which is capable of generating micropores in a film during
the process of
preparing the film through orientation. The micropores have the size which
allows gases,
including air and water vapor, to penetrate, but does not allow liquids, such
as water
droplets and sweat, to penetrate.
Herein, the microspheres refer to particles having micron-scale size, which
may be in the
form of spherical, near-spherical or non-spherical particles.
Herein, the average particle size is characterized by the number average
particle size,
and measured by means of a scanning electron microscope. Specifically, 500
microspheres are selected from the scanning electron microscope photograph,
measured
for their diameters and calculated for their average particle size using the
arithmetic
average method. For spherical particles, the diameter is that of the sphere.
For near-
spherical particles, the diameter is the diameter of the sphere equivalent to
the near-
spherical body. For non-spherical particles, the diameter refers to the
longitudinal axis of
the particle (the distance between the two furthest points on the particle
profile).
Herein, the terms "spherical" and "near-spherical" mean that the aspect ratio
of the
CA 03236535 2024- 4- 26
6
particle is substantially in the range of from 1 to 2.
Porogen
In the polyethylene composition of the present invention, based on 100 parts
by weight
of the polyethylene matrix resin, the porogen may be used in an amount of 30-
110 parts
by weight, preferably 35-110 parts by weight, more preferably 50-100 parts by
weight, still
more preferably 50-90 parts by weight.
The polyethylene composition of the present invention comprises maleic
anhydride
copolymer microspheres as an organic porogen, and preferably comprises only
maleic
anhydride copolymer microspheres as the porogen.
The average particle size of the maleic anhydride copolymer microspheres used
in the
present invention is smaller than the average particle size of the inorganic
porogen (for
example, calcium carbonate) used in the prior art, and can be 500-2000nm,
preferably
500-1700nm, preferably 500-1600nm, preferably 500-1500nm, more preferably 600-
1300nm, more preferably 800-1300nm, and more preferably 900-1200nm.
The maleic anhydride copolymer microspheres are preferably spherical or near
spherical.
The maleic anhydride copolymer microspheres preferably have a uniform particle
size
distribution. Preferably, the copolymer microspheres are monodispersed
copolymer
microspheres, particularly those having a particle size dispersion coefficient
of 1.05-
1.0001, preferably 1.035-1.002, and more preferably 1.028-1.002.
The particle size dispersion coefficient (U) is measured by the following
method: taking a
sample from the polymer dispersion system and observing the morphology of the
polymer
microspheres using a scanning electron microscope. The size of the
microspheres is
expressed by the average particle diameter (Dn), and the particle size
distribution is
expressed by the dispersion coefficient. The formulae are as follows:
( 1 )
CA 03236535 2024- 4- 26
7
k 4 /k D3 Dv, =10=1Di /
(2)
=Dw I Dn (3)
wherein Di is the diameter (nm) of the ith particle, N is the sample size, Dn
is the defined
mathematical average diameter, and Dw is the defined weight average diameter.
Such a uniform particle size distribution enables a more uniform distribution
of micropores
in the prepared breathable film.
The molar ratio of the structural unit A from maleic anhydride to the
structural unit B from
the vinyl-containing comonomer M in the maleic anhydride copolymer microsphere
can
be in the range of (0.5:1) to (1:0.5), preferably (0.75:1) to (1:0.75).
Based on the total molar amount of structural units A and B in the copolymer,
the molar
content of structural unit A may be 48-55%, preferably 48-53%, and the molar
content of
structural unit B may be 45-52%, preferably 47-52%. More preferably, the molar
content
of the structural unit A is 49-51%, and the molar content of the structural
unit B is 49-51%,
that is, it is preferred that the copolymer has an alternating
copolymerization structure.
The vinyl-containing comonomer M is preferably at least one selected from the
group
consisting of styrene, a-methyl styrene, vinyl acetate, mixed C4 and mixed C5.
In the present invention, the mixed C4 refers to the general name of
hydrocarbon
compounds having four carbon atoms (mainly including butene). Generally,
besides
butenes of various different structures (such as trans-2-butene, cis-2-butene,
n-butene,
and isobutene), C4 also includes a certain amount of alkanes (e.g., n-butane)
and other
possible impurities. In the present invention, the content of olefins in the
mixed C4 is in
the range of 60-75% by weight.
In the present invention, the mixed C5 refers to the general name of
hydrocarbon
compounds having five carbon atoms (mainly including pentene). Generally,
besides
pentenes of various different structures (such as dienes (isopentadiene,
cyclopentadiene,
1,4-pentadiene and piperylene) and monoolefins (1-pentene, 2-pentene,
cyclopentene,
2-methyl-1-butene and 2-methyl-2-butene)), C5 also includes a certain amount
of alkanes
CA 03236535 2024- 4- 26
8
(such as n-pentane, isopentane, cyclopentane and 2-methylbutane), alkynes
(such as
butyne-2 and 3-penten-1-yne) and other possible impurities. In the present
invention, the
content of olefins in the mixed C5 is 55-65% by weight.
The mixed C4 and mixed C5 can come from petroleum refining or petrochemical
production process, such as the C4 and C5 fractions obtained from the ethylene
cracking
process, liquefied fuels produced in the petroleum refining process, cracked
gases
produced from naphtha cracking, gases produced by the preparation of olefins
from
methanol, etc.
In one embodiment, the maleic anhydride copolymer microspheres may be cross-
linked
maleic anhydride copolymer microspheres cross-linked by a cross-linking agent.
The
copolymer in the microspheres additionally comprises a cross-linking
structural unit from
the cross-linking agent. The cross-linking degree of the copolymer
microspheres may be
65')/0, more preferably 70%. The cross-linking degree is determined by an
extraction
method using tetrahydrofuran.
The structural unit A and the structural unit B in the cross-linked maleic
anhydride
copolymer microspheres are also as described above.
The cross-linking agent described herein can be a variety of common vinyl-
containing
monomers with difunctionality or higher functionality which are capable of
free radical
polymerization. Preferably, the cross-linking agent is at least one selected
from the group
consisting of divinylbenzene and an acrylate cross-linking agent containing at
least two
acrylate groups. The structural formula of the acrylate group is preferably: -
0-C(0)-
C(R')=CH2, R' being H or a C1-C4 alkyl (for example, methyl). More preferably,
the cross-
linking agent is at least one selected from the group consisting of
divinylbenzene,
propylene glycol bis(meth)acrylates (such as 1,3-propanediol dimethacrylate,
1,2-
propanediol dimethacrylate, 1,3-propanediol diacrylate and 1,2-propanediol
diacrylate),
ethylene glycol bis(meth)acrylates (ethylene glycol dimethacrylate, ethylene
glycol
diacrylate, diethylene glycol dimethacrylate, diethylene glycol diacrylate,
triethylene glycol
dimethacrylate, triethylene glycol diacrylate, tetraethylene glycol
dimethacrylate and
tetraethylene glycol diacrylate), trimethylolpropane
tri(meth)acrylate,
bistrimethylolpropane tetra(meth)acrylate, polyethylene glycol
bis(meth)acrylate, phthalic
acid diethylene glycol diacrylate, pentaerythritol tetra(meth)acrylate,
dipentaerythritol
CA 03236535 2024- 4- 26
9
penta(meth)acrylate, dipentaerythritol hexa(meth)acrylate
and ethoxylated
multifunctional acrylate. More preferably, the cross-linking agent is
divinylbenzene.
In the cross-linked maleic anhydride copolymer microspheres, the molar ratio
of the
structural unit A, the structural unit B and the cross-linking structural unit
may be
100:(100-120):(1-40) , preferably 100:(100-105):(10-30).
The maleic anhydride copolymer microspheres can be prepared by methods known
in
the prior art, especially by the two methods described below.
In the first preparation method, maleic anhydride copolymer microspheres can
be
prepared by a method comprising the following steps:
(1) in an inert atmosphere, dissolving maleic anhydride, a comonomer and an
initiator
in a reaction medium to form a homogeneous solution, wherein the comonomer can
be
at least one selected from the group consisting of styrene, a-methyl styrene
and vinyl
acetate;
(2) subjecting the homogeneous solution to polymerization reaction to obtain a
copolymer emulsion suspension, followed by solid-liquid separation to obtain
the maleic
anhydride copolymer microspheres.
Based on the total weight of the maleic anhydride and comonomer, the maleic
anhydride
is used in an amount of 50-90wt%, and the comonomer is used in an amount of 10-
50wt%;
preferably, the maleic anhydride is used in an amount of 50-60wt%, and the
comonomer
is used in an amount of 40-50wt%.
In step (1) of this method, the total mass concentration of the maleic
anhydride and the
comonomer may be 5-25 wt%, preferably 5-20 wt%, based on the total weight of
the
homogeneous solution. The mass concentration of the initiator may be 0.01-
5wt%,
preferably 1-4wt%, based on the total weight of the homogeneous solution.
The reaction medium may be selected from ketones and mixtures thereof with
alkanes,
preferably a mixture of a compound represented by formula (1) and an alkane:
CA 03236535 2024- 4- 26
0
II
R1-C- R2
formula (1)
wherein Ri and R2 are each independently an alkyl having 1 to 6 carbon atoms,
preferably
1 to 4 carbon atoms, and more preferably methyl or ethyl.
The alkane is preferably an alkane having 6 to 12 carbon atoms, preferably at
least one
selected from the group consisting of hexane, heptane and octane, and more
preferably
hexane. Based on the total volume of the reaction medium, the amount of the
alkane may
be 50-90 vol%, preferably 60-80 vol%.
In this method, by using a mixture of a compound represented by formula (1)
and an
alkane as the reaction medium, which can interact with a specific amount of
maleic
anhydride and comonomer, self-stabilizing precipitation polymerization
reaction of the
maleic anhydride and comonomer can be achieved, wherein the polymerization
reaction
system requires no stabilizer and co-stabilizer to be added and has a self-
stabilizing
dispersion effect. The obtained polymer microspheres have uniform particle
sizes and
clean surfaces without pollution (as can be seen by observing the surface in
the SEM
photograph).
The polymerization reaction in step (2) is carried out in an inert atmosphere,
which can
be provided by a conventional inert gas in the prior art, for example,
nitrogen. The
conditions for the polymerization reaction may include: a polymerization
temperature of
61-100 C, preferably 70-90 C; and a polymerization time period of 1-24h,
preferably 2-
12h.
The solid-liquid separation may be in the manner of a conventional solid-
liquid separation
in the art, for example, centrifugal separation or flash separation. When
centrifugal
separation is adopted, the centrifugal rotational speed can be 1500-
5000rad/min, and the
centrifugal time can be 5-60 minutes. When flash separation is adopted, it can
be carried
out in a flash separator, the flash temperature can be 10-40 C, and the flash
pressure
can be about OMPa.
In this preparation method, the maleic anhydride monomer and the comonomer are
CA 03236535 2024- 4- 26
11
copolymerized in a specific ratio, so that the produced maleic anhydride
copolymer takes
on microspheres with excellent uniformity, and the copolymer is characterized
by a clean
surface, has a good dispersion in the medium and does not agglomerate.
Particularly
when the amount of the maleic anhydride is 50-60wt% and the amount of the
comonomer
is 40-50wt%, based on the total weight of polymerized monomers, the prepared
maleic
anhydride copolymer microspheres have uniform particles, excellent particle
morphology,
and clean particle surface, and the copolymer microspheres have better overall
performance.
In the second preparation method, the maleic anhydride copolymer microspheres
can be
prepared by a method comprising the following step:
under an inert atmosphere, in an organic solvent and in the presence of an
initiator,
subjecting maleic anhydride, a comonomer M and optionally a cross-linking
agent to
copolymerization reaction to obtain optionally cross-linked maleic anhydride
copolymer
microspheres, wherein the comonomer M can be at least one selected from the
group
consisting of styrene, a-methyl styrene, vinyl acetate, mixed C4 and mixed C5.
Herein, in
the case of preparing non-crosslinked maleic anhydride copolymer microspheres,
the
comonomer M is preferably selected from mixed C4 and/or mixed C5.
In this preparation method, the copolymerization reaction may be a one-step
reaction
using a precipitation polymerization method. The copolymerization reaction can
be
carried out at a temperature of 50-100 C, preferably 60-90 C, more preferably
70-90 C,
under a pressure of 0.1-2MPa, preferably 0.2-2MPa, preferably 0.5-1MPa, and
for a
reaction time period of 3-15h, preferably 5-12h, more preferably 5-10h,
preferably 6-9h.
Unless otherwise stated, the pressure described herein refers to gauge
pressure.
The weight ratio of the maleic anhydride to the comonomer may be 1:(0.2-3),
preferably,
1:(0.8-3). Alternatively, relative to 100 mol of maleic anhydride, the amount
of the
comonomer M may be 50-150 mol, more preferably 75-100 mol.
The organic solvent may include an organic acid alkyl ester, for example, it
may be
selected from an organic acid alkyl ester, or a mixture of an organic acid
alkyl ester and
an alkane, or a mixture of an organic acid alkyl ester and an aromatic
hydrocarbon. The
organic acid alkyl esters include, but are not limited to: at least one of
methyl formate,
CA 03236535 2024- 4- 26
12
ethyl formate, propyl formate, butyl formate, isobutyl formate, amyl formate,
methyl
acetate, ethyl acetate, propyl acetate, butyl acetate, isobutyl acetate, sec-
butyl acetate,
amyl acetate, isoamyl acetate, benzyl acetate, methyl propionate, ethyl
propionate, butyl
propionate, methyl butyrate, ethyl butyrate, butyl butyrate, isobutyl
butyrate, isoamyl
butyrate, isoamyl isovalerate, methyl benzoate, ethyl benzoate, propyl
benzoate, butyl
benzoate, isoamyl benzoate, methyl phenylacetate and ethyl phenylacetate,
preferably
at least one selected from isoamyl acetate, butyl acetate, isopropyl acetate
and ethyl
acetate. The alkane includes, but is not limited to: n-hexane and/or n-
heptane. The
aromatic hydrocarbon includes, but is not limited to: at least one of benzene,
toluene and
xylene.
The amount of the organic solvent is not particularly limited, as long as it
can provide a
medium for the reaction. Preferably, relative to 100 mol of maleic anhydride,
the amount
of the organic solvent can be 50-150L, more preferably 75-100L; or based on
the total
weight of the organic solvent, the concentration of the maleic anhydride can
be 5-25 wt%;
preferably 10-20 wt%.
In these two preparation methods, the amount of the initiator is not
particularly required.
Preferably, based on the total amount of maleic anhydride, the amount of the
initiator can
be 0.05-20 mol%, preferably 0.05-10 mol%, and more preferably 1-1.5 mol%.
The initiator can be a common reagent in the art used for initiating the
polymerization
reaction of maleic anhydride and a vinyl-containing monomer such as a-methyl
styrene
(or styrene), and can be a thermal decomposition initiator, for example,
organic peroxides
and/or azo compounds. The organic peroxide can be at least one selected from
the group
consisting of dibenzoyl peroxide, dicumyl peroxide, di-tert-butyl peroxide,
dodecanoyl
peroxide, tert-butyl peroxybenzoate, diisopropyl peroxydicarbonate and
dicyclohexyl
peroxydicarbonate. The azo compound can be selected from
azobisisobutyronitrile
and/or azobisisoheptanitrile.
A water bath and/or an oil bath may be used to provide the heat required for
the
polymerization.
In the method of preparing cross-linked maleic anhydride copolymer
nnicrospheres,
relative to 100 mol of maleic anhydride, the amount of the comonomer M can be
50-150
CA 03236535 2024- 4- 26
13
mol, preferably 75-100 mol; the amount of the cross-linking agent can be 1-40
mol,
preferably 10-20 mol, further preferably 15-20 mol; and the amount of the
initiator can be
0.05-10 mol, preferably 1-1.5 mol.
In the method of preparing cross-linked maleic anhydride copolymer
microspheres, the
reaction conditions may include: performing the reaction in the presence of an
inert
atmosphere, at a reaction temperature of 50-90 C, preferably 60-70 C, for a
reaction time
period of 3-15h, preferably 5-12h, and under a reaction pressure of 0.1-1MPa,
preferably
0.1-0.5MPa. The product (the suspension) obtained by the polymerization
reaction is
subjected to post-treatment steps such as separation, washing, drying, etc. to
obtain
cross-linked maleic anhydride copolymer microspheres.
The copolymer emulsion suspension obtained by the polymerization reaction can
be
separated by a solid-liquid separation method to obtain composite
microspheres.
Conventional solid-liquid separation methods in the prior art can be used,
preferably,
centrifugal separation is used. When adopting centrifugal separation, the
centrifugal
rotational speed is 1500-5000rad/min, and the centrifugation time is 5-60min.
By adding maleic anhydride copolymer microspheres as a porogen to the
composition of
the present invention, the porogen can be uniformly dispersed in the
polyethylene matrix
without using coupling agents, dispersants and/or surfactants, and the
micropores have
appropriate and uniform pore sizes; and by adjusting the maleic anhydride
copolymer
microspheres, breathable film characterized by widely adjustable physical
properties and
air permeability, wide adaptability, simple operation, and stable product
performance can
be achieved.
In addition, by adding maleic anhydride copolymer microspheres as organic
porogen, the
melting enthalpy AHmpE of the polyethylene matrix resin and the melting
enthalpy AHm
composition Of the polyethylene composition satisfying the following
relationship can be
particularly achieved: the difference between AHm PE and AHm composition is in
the range of
8-48J /g, preferably 10-45J /g, more preferably 20-451/g.
When the difference between the melting enthalpy of the polyethylene and the
melting
enthalpy of the polyethylene composition is well within the above range, the
maleic
anhydride copolymer microspheres are uniformly distributed in the polyethylene
CA 03236535 2024- 4- 26
14
composition, and the phenomenon of significant decrease in the melting
enthalpy of the
composition due to the agglomeration of the maleic anhydride copolymer
microspheres
does not occur, so that the breathable film prepared from the composition has
both
excellent physical properties and breathable performance.
In the present invention, the melting enthalpy of the polyethylene and of the
polyethylene
composition is measured by differential scanning calorimetry.
In addition, the density of the polyethylene composition of the present
invention Pcomposition
and the density of polyethylene pPE can satisfy the relationship formula: the
difference
between ()composition and ppE is in the range of 0.045-0.155g/cm3. When the
maleic
anhydride copolymer microspheres are non-crosslinked maleic anhydride
copolymer
microspheres, the difference is preferably 0.055-0.155g/cm3, and more
preferably 0.068-
0.145g/cm3; when the maleic anhydride copolymer microspheres are cross-linked
maleic
anhydride copolymer microspheres, it is preferably 0.045-0.120g/cm3, and more
preferably 0.050-0.110g/cm3.
The density of polyethylene is measured according to the method specified in
GBP'
1033.2-2010 and using the density gradient column method.
The density of the polyethylene composition is determined by using a melt flow
rate meter
to press out a bubble-free molten specimen at 190 C and under a load of 2.16kg
according to the method specified in GBiT 3682-2000, followed by the mass-
volume
method using a balance, a 25m L volumetric flask and anhydrous ethanol.
Polyethylene matrix resin
The composition of the present invention uses polyethylene as the matrix
resin. The
polyethylene matrix resin can be selected from ethylene homopolymers (such as
low
density polyethylene (LDPE), high density polyethylene (HDPE) and ultra-high
molecular
weight polyethylene (UHMWPE)), ethylene copolymers (such as linear low density
polyethylene (LLDPE), very low density polyethylene (VLDPE), high density
polyethylene
(HDPE), medium density polyethylene (MDPE), ethylene-vinyl acetate copolymer
and
ethylene-methyl acrylate copolymer) and mixtures thereof or blends with other
polyolefins
(such as propylene homopolymers, propylene-based copolymers, polyolefin
plastomers,
CA 03236535 2024- 4- 26
polyolefin elastomers and polybutylene).
The polyethylene matrix resin is preferably selected from a mixture of linear
low density
polyethylene and low density polyethylene, wherein the amount of linear low
density
polyethylene can be 60-99 parts by weight, preferably 70-95 parts by weight,
and the
amount of low density polyethylene can be 1-40 parts by weight, preferably 5-
30 parts by
weight, based on 100 parts by weight of polyethylene.
The linear low density polyethylene can be a copolymer of ethylene and a-
olefin.
Preferably, the a-olefin can be at least one selected from the group
consisting of butene,
hexene and octene.
The linear low density polyethylene may have a density of 0.905g/cm3-
0.935g/cm3.
Preferably, the linear low density polyethylene has a melt flow rate at 190 C
and under a
load of 2.16kg of 0.5g/10min-10g/10min, preferably 2g/lOmin-6g/lOmin.
Preferably, the
linear low density polyethylene has a molecular weight distribution of 2-12,
preferably 2-
10.
The density of the low density polyethylene may be 0.913g/m3-0.934g/cm3. The
melt
flow rate of the low density polyethylene at 190 C and under a load of 2.16kg
is preferably
0.1g/10min-12g/10min, preferably 2g/lOmin-9g/lOmin. The molecular weight
distribution
of the low density polyethylene is preferably 5-11, preferably 6-10.
Herein, the melt flow rate MFR is determined at 190 C and under a load of
2.16kg
according to the method specified in GBTT 3682-2000; the density is determined
according to the method specified in GB/T 1033.2-2010 and using the density
gradient
column method; and the molecular weight distribution is determined by GPC.
The ethylene homopolymers and copolymers are commercially available or can be
prepared by methods known in the art. The linear low density polyethylene is
preferably
prepared by catalytic polymerization using a Ziegler-Natta catalyst and/or a
metallocene
catalyst.
Antioxidant
CA 03236535 2024- 4- 26
16
The polyethylene composition may additionally comprise an antioxidant. The
amount of
the antioxidant is preferably 0.1-2.5 parts by weight, preferably 0.1-2 parts
by weight,
more preferably 0.1-1.5 parts by weight, still more preferably 0.2-1.5 parts
by weight,
based on 100 parts by weight of the polyethylene matrix resin.
The antioxidant may be an antioxidant known in the art that is suitable for
polyolefin
compositions, for example, at least one, preferably at least two, of the
antioxidants of
hindered phenols, phosphites and thioesters, and preferably at least one
selected from
the group consisting of pentaerythritol
tetrakis[13-( 3, 5-d i-tert-b utyl-4-
hydroxyphenyl)propionate], tris(2,4-di-tert-butylphenyl)phosphite, octadecyl
propionate
(for example, n-octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) and
alkylated
polyphenols.
In a preferred embodiment, the antioxidant is an antioxidant composite
additive
comprising an antioxidant and an acid acceptor. The weight ratio of the
antioxidant to the
acid acceptor may be 1-6:1, preferably 2-5:1. The acid acceptor may be a
stearate salt,
preferably at least one selected from the group consisting of calcium
stearate, zinc
stearate and sodium stearate.
Preferably, the antioxidant composite additive is a mixture of pentaerythritol
tetrakis[f3-
(3,5-d i-tert-butyl-4-hydroxyphenyl)propionate], tris(2,4-di-tert-
butylphenyl)phosphite and
calcium stearate, wherein the weight ratio of pentaerythritol tetrakis[13-(3,5-
di-tert-butyl-4-
hydroxyphenyl)propionate], tris(2,4-di-tert-butylphenyl)phosphite and calcium
stearate
can be (1-3):(1-3):1, preferably (1-2):(1-2):1.
Other additives
By using maleic anhydride copolymer microspheres as organic porogen, it is
possible for
the polyethylene composition of the present invention not to comprise a
coupling agent,
a dispersant or a surfactant or two or three of them, for example, not to
comprise a
coupling agent and a dispersant.
A coupling agent refers to an additive commonly used in the art to improve the
interface
property between a matrix resin and an inorganic filler or a reinforcing
material in plastic
compounding, for example, stearic acid, zinc stearate, calcium stearate,
magnesium
CA 03236535 2024- 4- 26
17
stearate, titanate ester coupling agent, aluminate ester coupling agent,
silane coupling
agent, lanthanum stearate and other rare earth carboxylate coupling agents, as
well as
Ti-Al and Al-Zr composite coupling agents, used in the polyethylene breathable
films
using an inorganic porogen in the prior art.
A dispersant refers to an additive that can enhance and improve the dispersion
performance of a solid or liquid material in a medium, thereby reducing the
aggregation
of the solid or liquid particles in the dispersion system, for example,
paraffin oil, PE wax,
oxidized polyethylene (OPE) dispersant, higher alcohols (C16-C18), etc. used
in the
polyethylene breathable films using an inorganic porogen in the prior art.
A surfactant refers to a compound that can reduce the surface tension (or
interfacial
tension) between two liquids, between a gas and a liquid, or between a liquid
and a solid,
for example, the mono- and di-glycerides used in the polyester breathable
films using a
starch as the porogen in the prior art.
However, the composition of the present invention may additionally comprise an
inorganic
porogen selected from calcium carbonate, barium sulfate, titanium dioxide,
talc, clay, etc.
as a secondary porogen, and may also comprise other conventional additives
suitable for
breathable films.
Preparation and product forms of polyethylene compositions
The polyethylene composition of the present invention may be in the form of
powders or
pellets.
The powder can be prepared by mixing the various components. Any suitable
mixing
equipment can be used here.
The pellets can be obtained by melt blending, extruding, pelletizing and
drying the
polyethylene composition. Any suitable extruder, preferably a twin-screw
extruder, can
be used here. The pellets can be prepared using conventional process
conditions.
Specifically, the pellets can be prepared by a method comprising the following
steps:
(1) mixing a polyethylene matrix resin, a porogen and optionally other
additives to
CA 03236535 2024- 4- 26
18
obtain a mixture; and
(2) melt-blending, extruding, pelletizing and drying the mixture in a twin-
screw
extruder to obtain the polyethylene composition pellets.
The rotational speed of the twin-screw extruder can be 150-400 r/min,
preferably 180-350
r/min. The temperatures of the feed section, melting section, homogenizing
section and
die of the twin-screw extruder can be 150-180 C, 165-200 C, 180-215 C and 175-
210 C,
respectively. Preferably, the temperatures of the feed section, melting
section,
homogenizing section and die of the twin-screw extruder are 160-175 C, 175-190
C, 190-
210 C and 180-205 C, respectively.
Breathable film and its applications
According to a second aspect, the present invention provides the use of the
above
polyethylene composition in a polyolefin microporous breathable film.
The polyethylene composition of the present invention can be used to prepare a
polyolefin
microporous breathable film. The obtained breathable film is significantly
improved in
physical properties and air permeability, and thus can be used as a water-
blocking and
moisture-permeable material for various breathable composite articles,
particularly
sanitary articles, medical articles, food packaging or building articles, such
as disposable
medical and sanitary products, baby diapers, adult care products, medical
protective
clothing, wound care bandages, dressings, food packaging films, industrial
protective
clothing, etc.
According to a third aspect, the present invention provides a polyolefin
microporous
breathable film, prepared from the polyethylene composition described above.
The method for the preparation of the polyolefin microporous breathable film
is not
particularly limited. It can be prepared by selecting a suitable method
according to need,
preferably by extrusion casting and stretching. For example, a polyethylene
composition
is added to an extrusion casting machine to melt and cast a cast sheet, with
the extrusion
temperature being 210-240 C and the temperature of the cooling roller being 20-
60 C,
thereby to produce a polyethylene cast film. Then, the cast film is uniaxially
stretched 2-
5 times to obtain a polyethylene microporous breathable film having an average
thickness
CA 03236535 2024- 4- 26
19
of 25-35 pm, wherein the heat setting temperature is 70-120 C.
The polyolefin microporous breathable film of the present invention has at
least one of
the following characteristics:
1) a thickness of 25-35pm;
2) a water vapor transmission rate of greater than or equal to 3060g/(m2=24h);
3) a porosity of greater than or equal to 38%;
4) a longitudinal tensile strength 25MPa, a transverse tensile strength 5MPa,
a
longitudinal elongation at break 125%, and a transverse elongation at break
355%;
and
5) a surface density of the breathable film of less than or equal to 24.5g/m2
(measured
for a sample having a thickness of 30 microns).
The polyolefin microporous breathable film prepared from the above
polyethylene
composition does not only keep the excellent physical properties of the
polyethylene
composition, and more importantly, the polyolefin microporous breathable film
has a high
air permeability and can be used as a water-blocking and moisture-permeable
material
for use in various breathable composite articles.
Thereby, according to a fourth aspect, the present invention provides a
breathable
composite article comprising the polyolefin microporous breathable film,
particularly
sanitary articles, medical articles, food packaging or building articles, such
as disposable
medical and sanitary products, baby diapers, adult care products, medical
protective
clothing, wound care bandages, dressings, food packaging films, industrial
protective
clothing, etc.
The endpoints of ranges and any values disclosed herein are not limited to the
precise
ranges or values, but these ranges or values are to be understood to include
values close
to these ranges or values. For numerical ranges, the endpoint values of each
range, the
endpoint values of each range and individual point values, and the individual
point values
can be combined with each other to obtain one or more new numerical ranges,
which
numerical ranges shall be deemed to be specifically disclosed herein.
Examples
CA 03236535 2024- 4- 26
The present invention will be described in detail below by means of examples.
It should
be understood that the specific examples described here are only used to
illustrate the
present invention and are not intended to limit the present invention.
The relevant data in the present invention and examples are obtained according
to the
following test methods:
1. The content of each structural unit in the maleic anhydride copolymer
microspheres
was tested by 'H NMR using the Avance 300 nuclear magnetic resonance
spectrometer
from the company Bruker in Switzerland. The test method involves calculating
the content
of each structural unit via the proportion of the peak area corresponding to
the
characteristic hydrogen in the corresponding structural unit in 1H NMR.
2. Polymerization yield of the maleic anhydride copolymer (Cp): Cp=Mpx100%/Mm
where Mp was the mass of the polymer obtained; and Mm was the total mass of
monomers added.
3. Method for testing the particle size of the copolymer microsphere: After
gold spraying
under vacuum, the copolymer microsphere powder was observed for its morphology
on
a Hitachi S4800 field emission scanning electron microscope from the company
Hitachi.
500 microspheres were selected from the electron microscope photograph,
measured for
their diameters and calculated for their average particle size using the
arithmetic average
method. For spherical particles, the diameter was the diameter of the sphere.
For near-
spherical particles, the diameter was the diameter of the sphere equivalent to
the near-
spherical body. For particles having other shapes, the diameter referred to
the length of
the longitudinal axis of the particle (the distance between the two furthest
points on the
particle profile).
4. Particle size distribution of the maleic anhydride copolymer microspheres:
It was
obtained by measuring and calculating at least 500 microspheres in the
scanning electron
microscope photograph using the Nano Measurer 1.2 software. Particle size and
particle
size distribution of the polymer microspheres were calculated according to
Formulae (1)
to (3):
D, = p IN
Formula ( )
CA 03236535 2024- 4- 26
21
=E k . Et I E!'
Formula (2)
=(), I D, Formula (3)
wherein Di was the diameter (nm) of the it" particle, N was the sample size,
Dn was the
defined mathematical average diameter, and Dw was the defined weight average
diameter.
5. Specific surface area of the copolymer microspheres: determined by the
nitrogen
physical adsorption BET method in accordance with the international test
standard ISO-
9277 using the ASAP2020C+M fully automatic specific surface and porosity
analyzer
from the company Micromeritics, USA.
6. Conversion efficiency of the mixed C4: weighing the polymer after the
reaction;
conversion efficiency (%) = [(4 xmass of the polymer actually obtained)/(11
xmass of C4
actually used)] x 100%.
7. Molecular weight of the copolymer microspheres: The molecular weight of the
sample
was determined using the Waters 2410 gel permeation chromatograph manufactured
by
the company WATERS, USA, wherein the chromatographic column was three WATERS
Styragel columns in series, the standards were polystyrene (PS), a DMF solvent
system
was used and the flow rate was 1.0m L/min.
8. Morphology of the cross-section of the polyethylene composition sample
sheet and
dispersion of the porogen: A sample was soaked in liquid nitrogen for 15
minutes,
followed by brittle fracture, the fractured section was subjected to gold
spraying treatment,
and then the fractured section of the material was characterized by the
Hitachi S4800
field emission scanning electron microscope from the company Hitachi, to
obtain a
microscopic morphology photograph.
9. Melt flow rate MFR: determined at 190 C and under a load of 2.16kg
according to the
method specified in GB/T 3682-2000 using the UPXRZ-400C melt flow rate meter
from
the company Ceast, Italy.
10. Density of polyethylene: determined according to the method specified in
GB/T
1033.2-2010 and using the density gradient column method.
CA 03236535 2024- 4- 26
22
11. Tensile properties: The tensile properties of the polyethylene composition
were
determined in accordance with the method specified in GB/T 1040.2-2006 and
using the
5966 universal material testing machine from the instrument company INSTRON,
USA;
and the tensile properties of the breathable film were determined in
accordance with the
method specified in GIEWT 1040.3-2006 and using the XLW intelligent electronic
tensile
testing machine from Labthink Instruments Co. Ltd., J man, China.
12. Tc, Tm and AHm: A DSC25 differential scanning calorimeter from the company
TA
Instruments, USA was used to analyze the melting process and crystallization
process of
the material. The specific operation involves measuring 5-10nng of a sample
(diameter
of 5.4mm, thickness of 1-1.5mm) in a three-stage raising/lowering temperature
measurement procedure from 20 C to 200 C under nitrogen protection, wherein
the
changes in heat flow reflects the melting and crystallization processes of the
material;
and calculating the crystallization temperature Tc, melting temperature Tm and
melting
enthalpy AHm according to GB/T 19466.3-2004.
13. Density of the polyethylene composition: The polyethylene composition was
pressed
by the UPXRZ-400C melt flow rate meter from the company Ceast, Italy at 190 C
and
under a load of 2.16kg according to the method specified in GB/T 3682-2000
into a
bubble-free molten specimen, which was cooled, cut into pieces, and then baked
in a
vacuum oven at 100 C for 8 hours to serve as a composition sample for density
testing.
A 25 mL volumetric flask was placed on a balance, which was removed of tare
and
cleared; the polyethylene composition sample was put on and recorded as weight
Ml;
anhydrous ethanol was added slowly with a dropper into the volumetric flask,
so that the
polyethylene composition sample was completely soaked into the anhydrous
ethanol;
after the dropwise addition was completed, the resultant was allowed to stand
still for two
hours, the liquid level was kept flush with the scale line of the volumetric
flask, and weight
M2 was recorded; the volume of anhydrous ethanol in the volumetric flask
should be
Vethanol = (M2-M1)/Pethanol; the volume of the polyethylene composition was
V=25-Vethand;
the density of the sample was ()composition= M 1/V.
14. Water vapor transmission rate (WVTR): determined according to the method
specified
in GBfT 12704-1991.
CA 03236535 2024- 4- 26
23
15. Porosity of the breathable film: determined with ASTM-D2873-1994. A
breathable film
was immersed in n-hexadecane for 1 hour and then taken out, the n-hexadecane
remaining on the surface was wiped dry with a filter paper, the change in mass
of the
breathable film before and after immersion was weighed, and the porosity was
calculated
according to formula (4)
ma ______________________ /Pa
0 = formula (4)
ma /Pa 4- mb 'Pb
wherein ma was the mass of n-hexadecane in the breathable film after liquid
absorption;
mb was the mass of the breathable film before liquid absorption; pa was the
density of n-
hexadecane; pb was the density of the specific material.
16. Surface density of the breathable film: determined according to GBP' 31729-
2015,
with the sample thickness being 30pm.
17. Cross-linking degree of the cross-linked maleic anhydride copolymer: The
cross-
linking degree was characterized by gel content. 2-3 grams of polymer
microspheres was
weighed out (the weight was recorded as wl), wrapped with a medium-speed
qualitative
filter paper, placed into a Soxhlet extractor, and extracted with
tetrahydrofuran for 24
hours, the extracted polymer was dried and weighed (the weight was recorded as
w2),
and the cross-linking degree was calculated by w2/wl.
18. The content of each structural unit in the cross-linked copolymer: For the
cross-linked
maleic anhydride-a-methyl styrene copolymer microspheres, the amount of the
monomer
or cross-linking agent that did not participate in the reaction was analyzed
using LC-MC
(2695 liquid chromatograph and Q-Tof Micro Mass spectrometer from the company
Waters, USA), and the molar ratio of structural unit A, structural unit B and
cross-linking
structural unit was calculated based on the feed amount; for the cross-linked
mixed C4-
maleic anhydride copolymer microspheres, the contents of carbon and oxygen in
the
polymer were determined by X-ray fluorescence spectroscopic analysis; the
clear liquid
after centrifugation was analyzed by the Agilent 7890A gas chromatograph from
the
company Agilent to determine the contents of the remaining maleic anhydride
and the
cross-linking agent; the results were comprehensively analyzed to calculate
the molar
ratio of structural unit A, structural unit B and cross-linking structural
unit in the polymer.
CA 03236535 2024- 4- 26
24
The starting materials used in the examples and comparative examples were
commercially available products, unless otherwise clearly stated.
The solvents and liquid monomers were treated to be oxygen- and water-free.
The pressure was gauge pressure.
Preparation Example 1
Preparation of porogen Al
302.5g of maleic anhydride, 51.3g of azobisisobutyronitrile, 299.9g of
styrene, 1.462L of
acetone and 4.614L of hexane (the amount of hexane: 75.9vo1%) were added into
a 20L
reaction kettle (wherein with respect to the polymerized monomers, the amount
of maleic
anhydride was 50.2wt%, and the amount of styrene was 49.8wt%). After the
materials
were mixed homogeneously (wherein in the homogeneous solution, the mass
concentrations of maleic anhydride and styrene were both 6.2wt%, and the mass
concentration of the initiator was 1.1wt%), nitrogen was introduced for 20
minutes, and
the temperature of the reaction kettle was raised to 70 C; the reaction was
carried out for
6 hours. After the reaction was completed, the obtained polymer emulsion
suspension
was centrifuged via a centrifuge at a rotational speed of 2000rad/min for 20
minutes to
obtain 542g of the maleic anhydride-styrene copolymer microspheres Al as the
porogen,
wherein the corresponding polymerization yield was 90%.
The copolymer microspheres Al were analyzed by 1H NMR to show that based on
the
total molar amount of the various structural units in the polymer, the molar
content of the
structural unit A from maleic anhydride was 49.5%, and the molar content of
the structural
unit B from styrene was 50.5%.
The maleic anhydride copolymer microspheres Al as the porogen had an average
particle size of about 1000nm, a particle size distribution U of 1.0026, a
molecular weight
Mw of 352x104, and a BET specific surface area of 6.39m2/g.
Preparation Example 2
CA 03236535 2024- 4- 26
Preparation of porogen A2
The mass percentages of the various components in the mixed C4 (from SINOPEC
Beijing Yanshan Company, China) were as follows: 1,3-butadiene: 0.06%; trans-2-
butene:
12.67%; isobutane: 37.09%; isobutene: 19.48 %; cis-2-butene: 27.79%; 1-butene:
1.02%;
others: 1.89%. 13.5kg of the mixed C4 having the above composition was
introduced into
a 200L reaction kettle containing an organic reaction liquid consisting of
10kg of maleic
anhydride, 4kg of dibenzoyl peroxide and 100L of isoamyl acetate and subjected
to free
radical copolymerization reaction (the concentration of maleic anhydride in
the organic
solvent: 10.2wt%), wherein the copolymerization reaction pressure was 1MPa,
the
copolymerization reaction temperature was 80 C, and the copolymerization
reaction time
was 6h; the weight ratio of the maleic anhydride to the mixed C4 was 1:1.35.
The copolymerization reaction product was passed into a flash separator for
gas-liquid
separation under the conditions of 30 C and OMPa. The obtained liquid-solid
mixture was
continued to be centrifuged in a centrifugal separator at 4000rad/min for 20
minutes for
liquid-solid separation to obtain a solid product, and the liquid returned to
the reaction
kettle. The solid product was washed with hexane, and the filter cake obtained
by suction
filtration through a fritted glass funnel was vacuum dried at 90 C for 8 hours
to obtain
20.5 kg of maleic anhydride-mixed C4 copolymer microspheres A2.
The maleic anhydride-mixed C4 copolymer microspheres A2 as the porogen was
analyzed by 1H NMR to show that based on the total molar amount of the various
structural units in the polymer, the molar content of the structural unit A
from maleic
anhydride was 49 mol%, the molar content of the structural unit B from mixed
C4 was 51
mol%, wherein the conversion efficiency of mixed C4 calculated by gravimetric
method
was 55wt%.
The maleic anhydride-mixed C4 copolymer microspheres A2 had an average
particle size
of about 1200nm, a particle size distribution U of 1.0048, a molecular weight
Mw of
3.06 x104, and a BET specific surface area of 3.57m2/g.
Preparation Example 3
CA 03236535 2024- 4- 26
26
Preparation of porogen A3
376.4g of maleic anhydride, 48.8g of azobisisobutyronitrile, 256.6g of
styrene, 1.165L of
acetone and 4.614L of hexane (the amount of hexane: 79.8v01%) were added into
a 20L
reaction kettle (wherein with respect to the polymerized monomers, the amount
of maleic
anhydride was 59.5wt%, and the amount of styrene was 40.5wt%). After the
materials
were mixed homogeneously (wherein in the homogeneous solution, the mass
concentrations of maleic anhydride and styrene were 8.1wt% and 5.5wt%,
respectively,
and the mass concentration of the initiator was 1.1wt%), nitrogen was
introduced for 20
minutes, and the temperature of the reaction kettle was raised to 70 C; the
reaction was
carried out for 8 hours. After the reaction was completed, the obtained
polymer emulsion
suspension was centrifuged via a centrifuge at a rotational speed of
2000rad/min for 20
minutes to obtain 493g of the maleic anhydride-styrene copolymer microspheres
A3 as
the porogen, wherein the corresponding polymerization yield was 78%.
The polymer microspheres were analyzed by 1H NMR to show that based on the
total
molar amount of the various structural units in the polymer, the molar content
of the
structural unit A from maleic anhydride was 48%, and the molar content of the
structural
unit B from styrene was 52%.
The maleic anhydride copolymer microspheres A3 as the porogen had an average
particle size of about 1200nm, a particle size distribution U of 1.0034, a
molecular weight
Mw of 3.89x104, and a BET specific surface area of 5.67m2/g.
Preparation Example 4
Preparation of porogen A4
262.6g of maleic anhydride, 51.3g of azobisisobutyronitrile, 235.1g of
styrene, 1.253L of
acetone and 4.614L of hexane (the amount of hexane: 78.6v01%) were added into
a 20L
reaction kettle (wherein with respect to the polymerized monomers, the amount
of maleic
anhydride was 52.8wt%, and the amount of styrene was 47.2wt%). After the
materials
were mixed homogeneously (wherein in the homogeneous solution, the mass
concentrations of maleic anhydride and styrene were 5.7wt% and 5.1wt%,
respectively,
and the mass concentration of the initiator was 1.1wt%), nitrogen was
introduced for 20
CA 03236535 2024- 4- 26
27
minutes, and the temperature of the reaction kettle was raised to 70 C and the
reaction
was carried out for 5 hours. After the reaction was completed, the obtained
polymer
emulsion suspension was centrifuged via a centrifuge at a rotational speed of
2000rad/min for 20 minutes to obtain 438g of the maleic anhydride copolymer
microspheres A4 as the porogen, wherein the corresponding polymerization yield
was
88%.
The copolymer microspheres were analyzed by 1H NMR to show that based on the
total
molar amount of the various structural units in the polymer, the molar content
of the
structural unit A from maleic anhydride was 49%, and the molar content of the
structural
unit B from styrene was 51%.
The maleic anhydride copolymer microspheres A4 as the porogen had an average
particle size of about 700nm, a particle size distribution U of 1.0038, a
molecular weight
Mw of 3.18x104, and a BET specific surface area of 7.98m2/g.
Preparation Example 5
Preparation of porogen A5
124.6g of maleic anhydride, 51.3g of azobisisobutyronitrile, 129.9g of
styrene, 2.307L of
acetone and 2.645L of hexane (the amount of hexane: 53.4v01%) were added into
a 20L
reaction kettle (wherein with respect to the polymerized monomers, the amount
of maleic
anhydride was 49.0wt%, and the amount of styrene was 51.0wt%). After the
materials
were mixed homogeneously (wherein in the homogeneous solution, the mass
concentrations of maleic anhydride and styrene were 3.2wt% and 3.4wt%,
respectively,
and the mass concentration of the initiator was 1.3wt%), nitrogen was
introduced for 20
minutes, and the temperature of the reaction kettle was raised to 70 C and the
reaction
was carried out for 4 hours. After the reaction was completed, the obtained
polymer
emulsion suspension was centrifuged via a centrifuge at a rotational speed of
2000rad/min for 20 minutes to obtain 191g of the maleic anhydride copolymer
microspheres AS as the porogen, wherein the corresponding polymerization yield
was
75%.
The copolymer microspheres were analyzed by 1H NMR to show that based on the
total
CA 03236535 2024- 4- 26
28
molar amount of the various structural units in the polymer, the molar content
of the
structural unit A from maleic anhydride was 47%, and the molar content of the
structural
unit B from styrene was 53%.
The maleic anhydride copolymer microspheres A5 as the porogen had an average
particle size of about 460nm, a particle size distribution U of 1.0098, a
molecular weight
Mw of 2.03x104, and a BET specific surface area of 9.67m2/g.
Preparation Example 6
Preparation of porogen B1
In a 20L reaction kettle, 400g of maleic anhydride, 472g of a-methyl styrene,
95g of
divinylbenzene and 8g of azobisisobutyronitrile were dissolved in 4L of
isoamyl acetate,
and reacted for 5 hours at 0.1MPa and 70 C under a nitrogen atmosphere,
wherein the
amount of a-methyl styrene was 97.9 mol, the amount of divinylbenzene was 17.9
mol,
and the amount of the initiator was 1.2 mol relative to 100 mol of maleic
anhydride. The
system after reaction was centrifuged via a centrifuge at 5000 rad/min for 30
minutes to
obtain cross-linked maleic anhydride-a-methyl styrene copolymer microspheres
B1,
which were washed and purified with n-hexane and dried under vacuum.
The cross-linking degree of the cross-linked maleic anhydride-a-methyl styrene
copolymer microspheres B1 was 82%, the molar ratio of the structural unit A,
the
structural unit B and the cross-linking structural unit was 100:101:25, and
the copolymer
microspheres B1 had an average particle size of 1200nm, a particle size
distribution U of
1.0134, and a BET specific surface area of 5.97m2/g.
Preparation Example 7
Preparation of porogen B2
In a 20L reaction kettle, 400g of maleic anhydride, 472g of a-methyl styrene,
104g of
divinylbenzene and 8g of azobisisobutyronitrile were dissolved in 4L of
isoamyl acetate,
and reacted for 5 hours at 0.1MPa and 70 C under a nitrogen atmosphere,
wherein the
amount of a-methyl styrene was 97.9 mol, the amount of divinylbenzene was 19.6
mol,
CA 03236535 2024- 4- 26
29
and the amount of the initiator was 1.2 mol relative to 100 mol of maleic
anhydride. The
system after reaction was centrifuged via a centrifuge at 5000 rad/min for 30
minutes to
obtain cross-linked maleic anhydride-a-methyl styrene copolymer microspheres
B2,
which were washed and purified with n-hexane and dried under vacuum.
The cross-linking degree of the cross-linked maleic anhydride-a-methyl styrene
copolymer microspheres B2 was 84%, the molar ratio of the structural unit A,
the
structural unit B and the cross-linking structural unit was 100:101:28, and
the copolymer
microspheres B2 had an average particle size of 900nm, a particle size
distribution U of
1.0228, and a BET specific surface area of 6.58m2/g.
Preparation Example 8
Preparation of porogen B3
In a 20L reaction kettle, 1000g of maleic anhydride, 1200g of a-methyl
styrene, 200g of
divinylbenzene and 20g of azobisisobutyronitrile were dissolved in 10L of
isoamyl acetate,
and reacted for 5 hours at 0.1MPa and 70 C under a nitrogen atmosphere,
wherein the
amount of a-methyl styrene was 99.6 mol, the amount of divinylbenzene was 15.1
mol,
and the amount of the initiator was 1.2 mol relative to 100 mol of maleic
anhydride. The
system after reaction was centrifuged via a centrifuge at 5000 rad/nnin for 30
minutes to
obtain cross-linked maleic anhydride-a-methyl styrene copolymer microspheres
B3,
which were washed and purified with n-hexane and dried under vacuum.
The cross-linking degree of the cross-linked maleic anhydride-a-methyl styrene
copolymer microspheres B3 was 70%, the molar ratio of the structural unit A,
the
structural unit B and the cross-linking structural unit was 100:101:23, and
the copolymer
microspheres B3 had an average particle size of 1500nm, a particle size
distribution U of
1.0304, and a BET specific surface area of 4.94m2/g.
Preparation Example 9
Preparation of porogen B4
The gas composition of mixed butenes (from SINOPEC Beijing Yanshan Company,
CA 03236535 2024- 4- 26
China) was as follows: trans-2-butene: 40.83 wt%; cis-2-butene: 18.18 wt%; n-
butane:
24.29 wt%; n-butene: 9.52 wt%; isobutene: 2.78 wt%; and others: 4.4 wt%. In a
20L
reaction kettle, the metered mixed butenes (the molar ratio of maleic
anhydride to the
effective components (terminal olefins) in the mixed olefins: 1:1) were
introduced into 1L
of an isoamyl acetate solution containing maleic anhydride in a concentration
of 1mol/L,
azobisisobutyronitrile of 0.05 mol/L and divinylbenzene of 0.2 mol/L under a
nitrogen
atmosphere (wherein relative to 100 mol of maleic anhydride, the amount of the
mixed
butenes (effective components) was 100 mol, the amount of divinylbenzene was
20 mol,
and the amount of the initiator was 5 mol), and charged with nitrogen and
pressurized to
a relative pressure of 0.5 MPa; and the system was reacted at 70 C for 6
hours. The
system after reaction was centrifuged via a centrifuge at 5000 rad/min for 30
minutes to
obtain cross-linked mixed butenes-maleic anhydride copolymer microspheres B4,
which
were washed and purified with n-hexane and dried under vacuum.
The cross-linking degree of the cross-linked mixed C4-maleic anhydride
copolymer
microspheres B4 was 76%, and the molar ratio of the structural unit A, the
structural unit
B and the cross-linking structural unit was 100:100:25. The copolymer
microspheres B4
had an average particle size of 1100nm, a particle size distribution U of
1.0415, and a
BET specific surface area of 3.46m2/g.
Preparation Example 10
Preparation of porogen B5
Cross-linked copolymer microspheres were prepared according to the method of
Preparation Example 1, except that the amount of divinylbenzene was 155 g,
thereby
cross-linked maleic anhydride-a-methyl styrene copolymer microspheres B5 were
obtained. Therein, relative to 100 mol of maleic anhydride, the amount of a-
methyl styrene
was 97.9 mol, the amount of divinylbenzene was 29.2 mol, and the amount of the
initiator
was 1.2 mol.
The cross-linking degree of the cross-linked maleic anhydride-a-methyl styrene
copolymer microspheres B5 was 89%, the molar ratio of the structural unit A,
the
structural unit B and the cross-linking structural unit was 100:102:31, and
the copolymer
microspheres B5 had an average particle size of 400nm, a particle size
distribution U of
CA 03236535 2024- 4- 26
31
1.0676, and a BET specific surface area of 9.14m2/g.
Preparation Example 11
Preparation of porogen B6
In a 20L reaction kettle, 600g of maleic anhydride, 722g of a-methyl styrene,
110g of
divinylbenzene and 12g of azobisisobutyronitrile were dissolved in 6L of
isoannyl acetate,
and reacted for 5 hours at 0.1MPa and 70 C under a nitrogen atmosphere,
wherein the
amount of a-methyl styrene was 99.8 mol, the amount of divinylbenzene was 13.8
mol,
and the amount of the initiator was 1.2 mol relative to 100 mol of maleic
anhydride. The
system after reaction was centrifuged via a centrifuge at 5000 rad/min for 30
minutes to
obtain cross-linked maleic anhydride-a-methyl styrene copolymer microspheres
B6,
which were washed and purified with n-hexane and dried under vacuum.
The cross-linking degree of the cross-linked maleic anhydride-a-methyl styrene
copolymer microspheres B6 was 67%, the molar ratio of the structural unit A,
the
structural unit B and the cross-linking structural unit was 100:101:19, and
the copolymer
microspheres B6 had an average particle size of 2150nnn, a particle size
distribution U of
1.0688, and a BET specific surface area of 4.02m2/g.
Preparation of polyethylene compositions
Example 1-1
90 parts by weight of a linear low-density polyethylene (MFR of 3.5g/lOmin,
density of
0.917g/cm3, and molecular weight distribution of 4; M2330 from ZhongKe
(Guangdong)
Refinery & Petrochemical Co. Ltd., China), 10 parts by weight of a low-density
polyethylene (MFR of 7.5g/10min, density of 0.919g/cm3, and molecular weight
distribution of 7.2; 1C7A from SINOPEC Beijing Yanshan Company, China), 70
parts by
weight of the porogen Al and 1 part by weight of an antioxidant composite
additive were
blended in a high-speed mixer, wherein the antioxidant composite additive was
a mixture
of pentaerythritol tetrakis(13-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate),
tris(2,4-di-tert-
butylphenyl)phosphite and calcium stearate in a weight ratio of 2:2:1.
CA 03236535 2024- 4- 26
32
After being mixed homogeneously, the resultant was added to a twin-screw
extruder for
melt blending, extrusion and pelletization, with the rotational speed of the
screw being
200r/min, and the temperatures of the feed section, melting section,
homogenizing
section and die being: 170 C, 180 C, 200 C and 190 C respectively, thereby a
polyethylene composition was obtained. The prepared polyethylene composition
was
dried. The properties of the polyethylene composition are shown in Table 1.
The
polyethylene composition was molten at 200 C and pressed into a sheet, wherein
the
sample sheet had a thickness of 120-160 pm. Fig. 1 is an SEM photograph of the
cross-
section of the sample sheet of the polyethylene composition. It can be seen
from Fig. 1
that the porogen had uniform particle size (wherein the size of the porogen in
the
polyethylene composition was close to the size of the porogen itself) and was
uniformly
distributed in the polyethylene matrix.
Example 1-2
99 parts by weight of a linear low-density polyethylene (MFR of 3.5g/10min,
density of
0.927g/cm3, and molecular weight distribution of 2.7; M2735 from ZhongKe
(Guangdong)
Refinery & Petrochemical Co. Ltd., China), 70 parts by weight of the porogen
Al and 1
part by weight of an antioxidant composite additive were blended in a high-
speed mixer,
wherein the antioxidant composite additive was a mixture of pentaerythritol
tetrakis(f3-
(3,5-di-tert-butyl-4-hydroxyphenyl)propionate), tris(2,4-di-tert-
butylphenyl)phosphite and
calcium stearate in a weight ratio of 2:2:1.
After being mixed homogeneously, the resultant was added to a twin-screw
extruder for
melt blending, extrusion and pelletization, with the rotational speed of the
screw being
200r/min, and the temperatures of the feed section, melting section,
homogenizing
section and die being: 170 C, 180 C, 200 C and 190 C respectively, thereby a
polyethylene composition was obtained. The prepared polyethylene composition
was
dried. The properties of the polyethylene composition are shown in Table 1.
The
polyethylene composition was molten at 200 C and pressed into a sheet, wherein
the
sample sheet had a thickness of 120-160 pm.
Example 1-3
90 parts by weight of a linear low-density polyethylene (MFR of 3.5g/lOmin,
density of
CA 03236535 2024- 4- 26
33
0.917g/cm3, and molecular weight distribution of 4; M2330 from ZhongKe
(Guangdong)
Refinery & Petrochemical Co. Ltd., China), 10 parts by weight of a low-density
polyethylene (MFR of 7.5g/10min, density of 0.919g/cm3, and molecular weight
distribution of 7.2; 1C7A from SINOPEC Beijing Yanshan Company, China), 100
parts by
weight of the porogen Al and 1 part by weight of an antioxidant composite
additive were
blended in a high-speed mixer, wherein the antioxidant composite additive was
a mixture
of pentaerythritol tetrakis(13-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate),
tris(2,4-di-tert-
butylphenyl)phosphite and calcium stearate in a weight ratio of 2:2:1.
After being mixed homogeneously, the resultant was added to a twin-screw
extruder for
melt blending, extrusion and pelletization, with the rotational speed of the
screw being
200r/min, and the temperatures of the feed section, melting section,
homogenizing
section and die being: 170 C, 180 C, 200 C and 190 C respectively, thereby a
polyethylene composition was obtained. The prepared polyethylene composition
was
dried. The properties of the polyethylene composition are shown in Table 1.
The
polyethylene composition was molten at 200 C and pressed into a sheet, wherein
the
sample sheet had a thickness of 120-160 pm. Fig. 2 is an SEM photograph of the
cross-
section of the sample sheet of the polyethylene composition. It can be seen
from Fig. 2
that the porogen had uniform particle size and was uniformly distributed in
the
polyethylene matrix.
Example 1-4
75 parts by weight of a linear low-density polyethylene (MFR of 3.5g/lOmin,
density of
0.917g/cm3, and molecular weight distribution of 4; M2330 from ZhongKe
(Guangdong)
Refinery & Petrochemical Co. Ltd., China), 25 parts by weight of a low-density
polyethylene (MFR of 7.5g/10min, density of 0.919g/cm3, and molecular weight
distribution of 7.2; 1C7A from SINOPEC Beijing Yanshan Company, China), 85
parts by
weight of the porogen A2 and 1 part by weight of an antioxidant composite
additive were
blended in a high-speed mixer, wherein the antioxidant composite additive was
a mixture
of pentaerythritol tetrakis(13-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate),
tris(2,4-di-tert-
butylphenyl)phosphite and calcium stea rate in a weight ratio of 2:2:1.
After being mixed homogeneously, the resultant was added to a twin-screw
extruder for
melt blending, extrusion and pelletization, with the rotational speed of the
screw being
CA 03236535 2024- 4- 26
34
200r/min, and the temperatures of the feed section, melting section,
homogenizing
section and die being: 170 C, 180 C, 200 C and 190 C respectively, thereby a
polyethylene composition was obtained. The prepared polyethylene composition
was
dried. The properties of the polyethylene composition are shown in Table 1.
Example 1-5
75 parts by weight of a linear low-density polyethylene (MFR of 3.5g/lOmin,
density of
0.917g/cm3, and molecular weight distribution of 4; M2330 from ZhongKe
(Guangdong)
Refinery & Petrochemical Co. Ltd., China), 25 parts by weight of a low-density
polyethylene (MFR of 7.5g/lOmin, density of 0.919g/cm3, and molecular weight
distribution of 7.2; 1C7A from SINOPEC Beijing Yanshan Company, China), 100
parts by
weight of the porogen A3 and 1 part by weight of an antioxidant composite
additive were
blended in a high-speed mixer, wherein the antioxidant composite additive was
a mixture
of pentaerythritol tetrakis(6-(3,5-di-tert-buty1-4-hydroxyphenyl)propionate),
tris(2,4-di-tert-
butylphenyl)phosphite and calcium stearate in a weight ratio of 2:2:1.
After being mixed homogeneously, the resultant was added to a twin-screw
extruder for
melt blending, extrusion and pelletization, with the rotational speed of the
screw being
200r/min, and the temperatures of the feed section, melting section,
homogenizing
section and die being: 170 C, 180 C, 200 C and 190 C respectively, thereby a
polyethylene composition was obtained. The prepared polyethylene composition
was
dried. The properties of the polyethylene composition are shown in Table 1.
The
polyethylene composition was molten at 200 C and pressed into a sheet, wherein
the
sample sheet had a thickness of 120-160 pm. Fig. 3 is an SEM photograph of the
cross-
section of the sample sheet of the polyethylene composition. It can be seen
from Fig. 3
that the porogen had uniform particle size and was uniformly distributed in
the
polyethylene matrix.
Example 1-6
90 parts by weight of a linear low-density polyethylene (MFR of 3.5g/lOmin,
density of
0.917g/cm3, and molecular weight distribution of 4; M2330 from ZhongKe
(Guangdong)
Refinery & Petrochemical Co. Ltd., China), 10 parts by weight of a low-density
polyethylene (MFR of 7.5g/lOmin, density of 0.919g/cm3, and molecular weight
CA 03236535 2024- 4- 26
distribution of 7.2; 1C7A from SINOPEC Beijing Yanshan Company, China), 45
parts by
weight of the porogen A4 and 1 part by weight of an antioxidant composite
additive were
blended in a high-speed mixer, wherein the antioxidant composite additive was
a mixture
of pentaerythritol tetrakis(13-(3,5-di-tert-buty1-4-hydroxyphenyl)propionate),
tris(2,4-di-tert-
butylphenyl)phosphite and calcium stearate in a weight ratio of 2:2:1.
After being mixed homogeneously, the resultant was added to a twin-screw
extruder for
melt blending, extrusion and pelletization, with the rotational speed of the
screw being
200r/min, and the temperatures of the feed section, melting section,
homogenizing
section and die being: 170 C, 180 C, 200 C and 190 C respectively, thereby a
polyethylene composition was obtained. The prepared polyethylene composition
was
dried. The properties of the polyethylene composition are shown in Table 1.
The
polyethylene composition was molten at 200 C and pressed into a sheet, wherein
the
sample sheet had a thickness of 120-160 pm. Fig. 4 is an SEM photograph of the
cross-
section of the sample sheet of the polyethylene composition. It can be seen
from Fig. 4
that the porogen had uniform particle size and was uniformly distributed in
the
polyethylene matrix.
Example 1-7
90 parts by weight of a linear low-density polyethylene (MFR of 3.5g/lOmin,
density of
0.917g/cm3, and molecular weight distribution of 4; M2330 from ZhongKe
(Guangdong)
Refinery & Petrochemical Co. Ltd., China), 10 parts by weight of a low-density
polyethylene (MFR of 7.5g/lOmin, density of 0.919g/cm3, and molecular weight
distribution of 7.2; 1C7A from SINOPEC Beijing Yanshan Company, China), 70
parts by
weight of the porogen Al and 1 part by weight of an antioxidant composite
additive were
blended in a high-speed mixer, wherein the antioxidant composite additive was
a mixture
of pentaerythritol tetrakis(6-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate)
and calcium
stearate in a weight ratio of 4:1.
After being mixed homogeneously, the resultant was added to a twin-screw
extruder for
melt blending, extrusion and pelletization, with the rotational speed of the
screw being
200r/min, and the temperatures of the feed section, melting section,
homogenizing
section and die being: 170 C, 180 C, 200 C and 190 C respectively, thereby a
polyethylene composition was obtained. The prepared polyethylene composition
was
CA 03236535 2024- 4- 26
36
dried. The properties of the polyethylene composition are shown in Table 1,
Comparative Example 1-1
90 parts by weight of a linear low-density polyethylene (MFR of 3.5g/lOmin,
density of
0.917g/cm3, and molecular weight distribution of 4; M2330 from ZhongKe
(Guangdong)
Refinery & Petrochemical Co. Ltd.), 10 parts by weight of a low-density
polyethylene
(MFR of 7.5g/lOmin, density of 0.919g/cm3, and molecular weight distribution
of 7.2;
1C7A from Sinopec Yanshan Petrochemical Company, China), 25 parts by weight of
the
porogen Al and 1 part by weight of an antioxidant composite additive were
blended in a
high-speed mixer, wherein the antioxidant composite additive was a mixture of
pentaerythritol tetrakis(0-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate),
tris(2,4-di-tert-
butylphenyl)phosphite and calcium stearate in a weight ratio of 2:2:1.
After being mixed homogeneously, the resultant was added to a twin-screw
extruder for
melt blending, extrusion and pelletization, with the rotational speed of the
screw being
200r/min, and the temperatures of the feed section, melting section,
homogenizing
section and die being: 170 C, 180 C, 200 C and 190 C respectively, thereby a
polyethylene composition was obtained. The prepared polyethylene composition
was
dried. The properties of the polyethylene composition are shown in Table 1.
The
polyethylene composition was molten at 200 C and pressed into a sheet, wherein
the
sample sheet had a thickness of 120-160 pm. Fig. 5 is an SEM photograph of the
cross-
section of the sample sheet of the polyethylene composition. It can be seen
from Fig. 5
that the porogen had uniform particle size, but was less in quantity, and was
non-uniformly
distributed.
Comparative Example 1-2
90 parts by weight of a linear low-density polyethylene (MFR of 3.5g/10min,
density of
0.917g/cm3, and molecular weight distribution of 4; M2330 from ZhongKe
(Guangdong)
Refinery & Petrochemical Co. Ltd., China), 10 parts by weight of a low-density
polyethylene (MFR of 7.5g/lOmin, density of 0.919g/cm3, and molecular weight
distribution of 7.2; 1C7A from SINOPEC Beijing Yanshan Company, China), 100
parts by
weight of a nano CaCO3 powder (Shanghai Yuanjiang Chemical Co., Ltd., China,
10000
meshes, BET specific surface area of 4.41m2/g) and 1 part by weight of an
antioxidant
CA 03236535 2024- 4- 26
37
composite additive were blended in a high-speed mixer, wherein the antioxidant
composite additive was a mixture of pentaerythritol tetrakis(I3-(3,5-di-tert-
buty1-4-
hydroxyphenyl)propionate), tris(2,4-di-tert-butylphenyl)phosphite and calcium
stearate in
a weight ratio of 2:2:1.
After being mixed homogeneously, the resultant was added to a twin-screw
extruder for
melt blending, extrusion and pelletization, with the rotational speed of the
screw being
200r/min, and the temperatures of the feed section, melting section,
homogenizing
section and die being: 170 C, 180 C, 200 C and 190 C respectively, thereby a
polyethylene composition was obtained. The prepared polyethylene composition
was
dried. The properties of the polyethylene composition are shown in Table 1.
The
polyethylene composition was molten at 200 C and pressed into a sheet, wherein
the
sample sheet had a thickness of 120-160 pm. Fig. 6 is an SEM photograph of the
cross-
section of the sample sheet of the polyethylene composition. It can be seen
from Fig. 6
that the porogen appeared to be significantly agglomerated.
Comparative Example 1-3
90 parts by weight of a linear low-density polyethylene (MFR of 3.5g/lOmin,
density of
0.917g/cm3, and molecular weight distribution of 4; M2330 from ZhongKe
(Guangdong)
Refinery & Petrochemical Co. Ltd., China), 10 parts by weight of a low-density
polyethylene (MFR of 7.5g/10min, density of 0.919g/cm3, and molecular weight
distribution of 7.2; 1C7A from SINOPEC Beijing Yanshan Company, China), 100
parts by
weight of a nano BaSO4 powder (Sachtleben, Blanc Fixe Micro, organic coated,
d50=0.7
pm, BET specific surface area of 3.55m2/g) and 1 part by weight of an
antioxidant
composite additive were blended in a high-speed mixer, wherein the antioxidant
composite additive was a mixture of pentaerythritol tetrakis(3-(3,5-di-tert-
buty1-4-
hydroxyphenyl)propionate), tris(2,4-di-tert-butylphenyl)phosphite and calcium
stearate in
a weight ratio of 2:2:1.
After being mixed homogeneously, the resultant was added to a twin-screw
extruder for
melt blending, extrusion and pelletization, with the rotational speed of the
screw being
200r/min, and the temperatures of the feed section, melting section,
homogenizing
section and die being: 170 C, 180 C, 200 C and 190 C respectively, thereby a
polyethylene composition was obtained. The prepared polyethylene composition
was
CA 03236535 2024- 4- 26
38
dried. The properties of the polyethylene composition are shown in Table 1.
The
polyethylene composition was molten at 200 C and pressed into a sheet, wherein
the
sample sheet had a thickness of 120-160 pm. Fig. 7 is an SEM photograph of the
cross-
section of the sample sheet of the polyethylene composition. It can be seen
from the
figure that the porogen appeared to be significantly agglomerated.
Comparative Example 1-4
90 parts by weight of a linear low-density polyethylene (MFR of 3.5g/lOmin,
density of
0.917g/cm3, and molecular weight distribution of 4; M2330 from ZhongKe
(Guangdong)
Refinery & Petrochemical Co. Ltd., China), 10 parts by weight of a low-density
polyethylene (MFR of 7.5g/lOmin, density of 0.919g/cm3, and molecular weight
distribution of 7.2; 1C7A from SINOPEC Beijing Yanshan Company, China), 140
parts by
weight of the porogen Al and 1 part by weight of an antioxidant composite
additive were
blended in a high-speed mixer, wherein the antioxidant composite additive was
a mixture
of pentaerythritol tetrakis(13-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate),
tris(2,4-di-tert-
butylphenyl)phosphite and calcium stearate in a weight ratio of 2:2:1.
After being mixed homogeneously, the resultant was added to a twin-screw
extruder for
melt blending, extrusion and pelletization, with the rotational speed of the
screw being
200r/min, and the temperatures of the feed section, melting section,
homogenizing
section and die being: 170 C, 180 C, 200 C and 190 C respectively, thereby a
polyethylene composition was obtained. The prepared polyethylene composition
was
dried. The properties of the polyethylene composition are shown in Table 1.
Comparative Example 1-5
90 parts by weight of a linear low-density polyethylene (MFR of 3.5g/lOmin,
density of
0.917g/cm3, and molecular weight distribution of 4; M2330 from ZhongKe
(Guangdong)
Refinery & Petrochemical Co. Ltd., China), 10 parts by weight of a low-density
polyethylene (MFR of 7.5g/lOmin, density of 0.919g/cm3, and molecular weight
distribution of 7.2; 1C7A from SINOPEC Beijing Yanshan Company, China), 70
parts by
weight of the porogen A5 and 1 part by weight of an antioxidant composite
additive were
blended in a high-speed mixer, wherein the antioxidant composite additive was
a mixture
of pentaerythritol tetrakis(6-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate),
tris(2,4-di-tert-
CA 03236535 2024- 4- 26
39
butylphenyl)phosphite and calcium stearate in a weight ratio of 2:2:1.
After being mixed homogeneously, the resultant was added to a twin-screw
extruder for
melt blending, extrusion and pelletization, with the rotational speed of the
screw being
200r/min, and the temperatures of the feed section, melting section,
homogenizing
section and die being: 170 C, 180 C, 200 C and 190 C respectively, thereby a
polyethylene composition was obtained. The prepared polyethylene composition
was
dried. The properties of the polyethylene composition are shown in Table 1.
Comparative Example 1-6
90 parts by weight of a linear low-density polyethylene (MFR of 3.5g/lOmin,
density of
0.917g/cm3, and molecular weight distribution of 4; M2330 from ZhongKe
(Guangdong)
Refinery & Petrochemical Co. Ltd., China), 10 parts by weight of a low-density
polyethylene (MFR of 7.5g/lOmin, density of 0.919g/cm3, and molecular weight
distribution of 7.2; 1C7A from S1NOPEC Beijing Yanshan Company, China), 70
parts by
weight of a cross-linked starch (National 4302 from Ingredion Food Ingredients
Co., Ltd.)
and 1 part by weight of an antioxidant composite additive were blended in a
high-speed
mixer, wherein the antioxidant composite additive was a mixture of
pentaerythritol
tetrakis(0-(3,5-d i-tert-butyl-4-hydroxyphenyl)propionate),
tris(2,4-di-tert-
butylphenyl)phosphite and calcium stearate in a weight ratio of 2:2:1.
After being mixed homogeneously, the resultant was added to a twin-screw
extruder for
melt blending, extrusion and pelletization, with the rotational speed of the
screw being
200r/min, and the temperatures of the feed section, melting section,
homogenizing
section and die being: 170 C, 180 C, 200 C and 190 C respectively, thereby a
polyethylene composition was obtained. The prepared polyethylene composition
was
dried. The properties of the polyethylene composition are shown in Table 1.
Example 11-1
90 parts by weight of a linear low-density polyethylene (MFR of 3.5g/lOmin,
density of
0.917g/cm3, and molecular weight distribution of 4; M2330 from ZhongKe
(Guangdong)
Refinery & Petrochemical Co. Ltd., China), 10 parts by weight of a low-density
polyethylene (MFR of 7.5g/lOmin, density of 0.919g/cm3, and molecular weight
CA 03236535 2024- 4- 26
distribution of 7.2; 1C7A from S1NOPEC Beijing Yanshan Company, China), 70
parts by
weight of the porogen B1 and 1 part by weight of an antioxidant composite
additive were
blended in a high-speed mixer, wherein the antioxidant composite additive was
a mixture
of pentaerythritol tetrakis(13-(3,5-di-tert-buty1-4-hydroxyphenyl)propionate),
tris(2,4-di-tert-
butylphenyl)phosphite and calcium stea rate in a weight ratio of 2:2:1.
After being mixed homogeneously, the resultant was added to a twin-screw
extruder for
melt blending, extrusion and pelletization, with the rotational speed of the
screw being
200r/min, and the temperatures of the feed section, melting section,
homogenizing
section and die being: 170 C, 180 C, 200 C and 190 C respectively, thereby a
polyethylene composition was obtained. The prepared polyethylene composition
was
dried. The properties of the polyethylene composition are shown in Table 1.
The
polyethylene composition was molten at 200 C and pressed into a sheet, wherein
the
sample sheet had a thickness of 160-200 pm. Fig. 8 is an SEM photograph of the
cross-
section of the sample sheet of the polyethylene composition. It can be seen
from the
figure that the porogen had uniform particle size and was uniformly
distributed in the
polyethylene matrix.
Example 11-2
82 parts by weight of a linear low-density polyethylene (MFR of 3.5g/lOmin,
density of
0.917g/cm3, and molecular weight distribution of 4; M2330 from ZhongKe
(Guangdong)
Refinery & Petrochemical Co. Ltd., China), 10 parts by weight of a low-density
polyethylene (MFR of 7.5g/lOmin, density of 0.919g/cm3, and molecular weight
distribution of 7.2; 1C7A from SINOPEC Beijing Yanshan Company, China), 8
parts by
weight of a random copolymerized polypropylene (MFR of 2.5g/lOmin, and
molecular
weight distribution of 4.9; GM250E from Sinopec Shanghai Petrochemical Co.,
Ltd.,
China), 70 parts by weight of the porogen B1 and 1 part by weight of an
antioxidant
composite additive were blended in a high-speed mixer, wherein the antioxidant
composite additive was a mixture of pentaerythritol tetrakis(13-(3,5-di-tert-
buty1-4-
hydroxyphenyl)propionate), tris(2,4-di-tert-butylphenyl)phosphite and calcium
stearate in
a weight ratio of 2:2:1.
After being mixed homogeneously, the resultant was added to a twin-screw
extruder for
melt blending, extrusion and pelletization, with the rotational speed of the
screw being
CA 03236535 2024- 4- 26
41
200r/min, and the temperatures of the feed section, melting section,
homogenizing
section and die being: 170 C, 180 C, 200 C and 190 C respectively, thereby a
polyethylene composition was obtained. The prepared polyethylene composition
was
dried. The properties of the polyethylene composition are shown in Table 1.
Example 11-3
90 parts by weight of a linear low-density polyethylene (MFR of 3.5g/lOmin,
density of
0.917g/cm3, and molecular weight distribution of 4; M2330 from ZhongKe
(Guangdong)
Refinery & Petrochemical Co. Ltd., China), 10 parts by weight of a low-density
polyethylene (MFR of 7.5g/lOmin, density of 0.919g/cm3, and molecular weight
distribution of 7.2; 1C7A from SINOPEC Beijing Yanshan Company, China), 30
parts by
weight of the porogen B2 and 1 part by weight of an antioxidant composite
additive were
blended in a high-speed mixer, wherein the antioxidant composite additive was
a mixture
of pentaerythritol tetrakis(13-(3,5-di-tert-buty1-4-hydroxyphenyl)propionate),
tris(2,4-di-tert-
butylphenyl)phosphite and calcium stearate in a weight ratio of 2:2:1.
After being mixed homogeneously, the resultant was added to a twin-screw
extruder for
melt blending, extrusion and pelletization, with the rotational speed of the
screw being
200r/min, and the temperatures of the feed section, melting section,
homogenizing
section and die being: 170 C, 180 C, 200 C and 190 C respectively, thereby a
polyethylene composition was obtained. The prepared polyethylene composition
was
dried. The properties of the polyethylene composition are shown in Table 1.
The
polyethylene composition was molten at 200 C and pressed into a sheet, wherein
the
sample sheet had a thickness of 160-200 pm. Fig. 9 is an SEM photograph of the
cross-
section of the sample sheet of the polyethylene composition. It can be seen
from the
figure that the porogen had uniform particle size and was uniformly
distributed in the
polyethylene matrix.
Example 11-4
75 parts by weight of a linear low-density polyethylene (MFR of 3.5g/lOmin,
density of
0.917g/cm3, and molecular weight distribution of 4; M2330 from ZhongKe
(Guangdong)
Refinery & Petrochemical Co. Ltd., China), 25 parts by weight of a low-density
polyethylene (MFR of 7.5g/lOmin, density of 0.919g/cm3, and molecular weight
CA 03236535 2024- 4- 26
42
distribution of 7.2; 1C7A from S1NOPEC Beijing Yanshan Company, China), 50
parts by
weight of the porogen B3 and 1 part by weight of an antioxidant composite
additive were
blended in a high-speed mixer, wherein the antioxidant composite additive was
a mixture
of pentaerythritol tetrakis(13-(3,5-di-tert-buty1-4-hydroxyphenyl)propionate),
tris(2,4-di-tert-
butylphenyl)phosphite and calcium stearate in a weight ratio of 2:2:1.
After being mixed homogeneously, the resultant was added to a twin-screw
extruder for
melt blending, extrusion and pelletization, with the rotational speed of the
screw being
200r/min, and the temperatures of the feed section, melting section,
homogenizing
section and die being: 170 C, 180 C, 200 C and 190 C respectively, thereby a
polyethylene composition was obtained. The prepared polyethylene composition
was
dried. The properties of the polyethylene composition are shown in Table 1.
The
polyethylene composition was molten at 200 C and pressed into a sheet, wherein
the
sample sheet had a thickness of 160-200 pm. Fig. 10 is an SEM photograph of
the cross-
section of the sample sheet of the polyethylene composition. It can be seen
from the
figure that the porogen had uniform particle size and was uniformly
distributed in the
polyethylene matrix.
Example 11-5
90 parts by weight of a linear low-density polyethylene (MFR of 3.5g/lOmin,
density of
0.917g/cm3, and molecular weight distribution of 4; M2330 from ZhongKe
(Guangdong)
Refinery & Petrochemical Co. Ltd., China), 10 parts by weight of a low-density
polyethylene (MFR of 7.5g/lOmin, density of 0.919g/cm3, and molecular weight
distribution of 7.2; 1C7A from S1NOPEC Beijing Yanshan Company, China), 90
parts by
weight of the porogen B4 and 1 part by weight of an antioxidant composite
additive were
blended in a high-speed mixer, wherein the antioxidant composite additive was
a mixture
of pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate),
tris(2,4-di-tert-
butylphenyl)phosphite and calcium stearate in a weight ratio of 2:2:1.
After being mixed homogeneously, the resultant was added to a twin-screw
extruder for
melt blending, extrusion and pelletization, with the rotational speed of the
screw being
200r/min, and the temperatures of the feed section, melting section,
homogenizing
section and die being: 170 C, 180 C, 200 C and 190 C respectively, thereby a
polyethylene composition was obtained. The prepared polyethylene composition
was
CA 03236535 2024- 4- 26
43
dried. The properties of the polyethylene composition are shown in Table 1.
Example 11-6
90 parts by weight of a linear low-density polyethylene (MFR of 3.5g/lOmin,
density of
0.917g/cm3, and molecular weight distribution of 4; M2330 from ZhongKe
(Guangdong)
Refinery & Petrochemical Co. Ltd., China), 10 parts by weight of a low-density
polyethylene (MFR of 7.5g/lOmin, density of 0.919g/cm3, and molecular weight
distribution of 7.2; 1C7A from SINOPEC Beijing Yanshan Company, China), 100
parts by
weight of the porogen B1 and 1 part by weight of an antioxidant composite
additive were
blended in a high-speed mixer, wherein the antioxidant composite additive was
a mixture
of pentaerythritol tetrakis(13-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate),
tris(2,4-di-tert-
butylphenyl)phosphite and calcium stearate in a weight ratio of 2:2:1.
After being mixed homogeneously, the resultant was added to a twin-screw
extruder for
melt blending, extrusion and pelletization, with the rotational speed of the
screw being
200r/min, and the temperatures of the feed section, melting section,
homogenizing
section and die being: 170 C, 180 C, 200 C and 190 C respectively, thereby a
polyethylene composition was obtained. The prepared polyethylene composition
was
dried. The properties of the polyethylene composition are shown in Table 1.
The
polyethylene composition was molten at 200 C and pressed into a sheet, wherein
the
sample sheet had a thickness of 160-200 pm. Fig. 11 is an SEM photograph of
the cross-
section of the sample sheet of the polyethylene composition. It can be seen
from the
figure that the porogen had uniform particle size and was uniformly
distributed in the
polyethylene matrix.
Comparative Example 11-1
A polyethylene composition was prepared according to the method of Example 11-
1,
except that the porogen B1 was 15 parts by weight. The properties of the
polyethylene
composition are shown in Table 1. Fig. 12 is an SEM photograph of the cross-
section of
the sample sheet of the polyethylene composition. It can be seen from Fig. 12
that the
porogen had uniform particle size, but was less in quantity, and was non-
uniformly
distributed.
CA 03236535 2024- 4- 26
44
Comparative Example 11-2
90 parts by weight of a linear low-density polyethylene (MFR of 3.5g/10min,
density of
0.917g/cm3, and molecular weight distribution of 4; M2330 from ZhongKe
(Guangdong)
Refinery & Petrochemical Co. Ltd., China), 10 parts by weight of a low-density
polyethylene (MFR of 7.5g/lOmin, density of 0.919g/cm3, and molecular weight
distribution of 7.2; 1C7A from SINOPEC Beijing Yanshan Company, China), 100
parts by
weight of a nano CaCO3 powder (Shanghai Yuanjiang Chemical Co., Ltd., China,
10000
meshes) and 1 part by weight of an antioxidant composite additive were blended
in a
high-speed mixer, wherein the antioxidant composite additive was a mixture of
pentaerythritol tetrakis(13-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate),
tris(2,4-di-tert-
butylphenyl)phosphite and calcium stearate in a weight ratio of 2:2:1.
After being mixed homogeneously, the resultant was added to a twin-screw
extruder for
melt blending, extrusion and pelletization, with the rotational speed of the
screw being
200r/min, and the temperatures of the feed section, melting section,
homogenizing
section and die being: 170 C, 180 C, 200 C and 190 C respectively, thereby a
polyethylene composition was obtained. The prepared polyethylene composition
was
dried. The properties of the polyethylene composition are shown in Table 1.
The
polyethylene composition was molten at 200 C and pressed into a sheet, wherein
the
sample sheet had a thickness of 160-200 pm. Fig. 13 is an SEM photograph of
the cross-
section of the sample sheet of the polyethylene composition. It can be seen
from the
figure that the porogen appeared to be significantly agglomerated.
Comparative Example 11-3
90 parts by weight of a linear low-density polyethylene (MFR of 3.5g/10min,
density of
0.917g/cm3, and molecular weight distribution of 4; M2330 from ZhongKe
(Guangdong)
Refinery & Petrochemical Co. Ltd., China), 10 parts by weight of a low-density
polyethylene (MFR of 7.5g/lOmin, density of 0.919g/cm3, and molecular weight
distribution of 7.2; 1C7A from SINOPEC Beijing Yanshan Company, China), 100
parts by
weight of a nano BaSO4 powder (Sachtleben, Blanc Fixe Micro, organic coated,
d50=0.7
pm) and 1 part by weight of an antioxidant composite additive were blended in
a high-
speed mixer, wherein the antioxidant composite additive was a mixture of
pentaerythritol
tetrakis(I3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate),
tris(2,4-di-tert-
CA 03236535 2024- 4- 26
butylphenyl)phosphite and calcium stearate in a weight ratio of 2:2:1.
After being mixed homogeneously, the resultant was added to a twin-screw
extruder for
melt blending, extrusion and pelletization, with the rotational speed of the
screw being
200r/min, and the temperatures of the feed section, melting section,
homogenizing
section and die being: 170 C, 180 C, 200 C and 190 C respectively, thereby a
polyethylene composition was obtained. The prepared polyethylene composition
was
dried. The properties of the polyethylene composition are shown in Table 1.
The
polyethylene composition was molten at 200 C and pressed into a film, which
had a
thickness of 160-200 pm. Fig. 14 is an SEM photograph of the cross-section of
the sample
sheet of the polyethylene composition. It can be seen from the figure that the
porogen
appeared to be significantly agglomerated.
Comparative Example 11-4
A polyethylene composition was prepared according to the method of Example 11-
1,
except that the porogen B1 was 135 parts by weight. The properties of the
polyethylene
composition are shown in Table 1.
Comparative Example 11-5
A polyethylene composition was prepared according to the method of Example 11-
1,
except that the porogen used was porogen B5. The properties of the
polyethylene
composition are shown in Table 1.
Comparative Example 11-6
A polyethylene composition was prepared according to the method of Example 11-
1,
except that the porogen used was porogen B6. The properties of the
polyethylene
composition are shown in Table 1.
CA 03236535 2024- 4- 26
46
Table 1
Tensile
MFR PcomposItion-PPE TC Tm AHm pE-AN-
composition strength at Elongation at break
Items (g110min) (gIcm3) ( C) ( C) a Ig) break
(%)
(MPa)
Example 1-1 2.26 0.113 105.1 120.6 31.1 18.1
460
Example 1-2 1,95 0.118 106,9 119,3 33.5 19,4
480
Example 1-3 2.05 0,138 104.6 120.1 41.5 17.3
440
Example 1-4 3.04 0.116 103.9 117.1 34.7 16.2
420
Example 1-5 2.83 0.133 102.7 116,9 42.9 15.9
400
Example 1-6 2.43 0.091 106.2 121.1 19.6 18,6
470
Example 1-7 2.35 0.112 105.3 120.7 32.1 17.9
450
Comparative Example 1-1 2.56 0.042 106.6 121.5 4.6
18.9 480
Comparative Example 1-2 1.49 0.329 108.9 122,7 57.4
11.5 280 .
Comparative Example 1-3 1,57 0.624 107,2 121.6 59,8
12,1 300
Comparative Example 1-4 1.59 0.162 104.9 119.4 59.1
12.8 340
Comparative Example 1-5 2.19 0,104 106.7 120.8 49.9
16.5 420
Comparative Example 1-6 0.89 0.144 105.1 119.9 49.7
11.7 300
Example 11-1 2,08 0.079 106,8 121,1 27,7 17.2
430
Example 11-2 2.02 0.082 106.4 126.8 29.2 18.6
460
Example 11-3 2.33 0,057 106.9 120.5 10.8 18.1
440
Example 11-4 3.21 0.066 106.1 119.9 23.4 17.4
430
Example 11-5 1.63 0,094 105.7 119,7 40.5 15.6
410
Example 11-6 1,57 0.103 104,5 119.5 45,1 15,3
400
41
Comparative Example 11-1 2.92 0.029 107.4 1215 4.1
18.8 450
Comparative Example 11-2 1.49 0.329 _ 108.9 122.7 57.4
115 280 _
Comparative Example 11-3 1.57 0.624 107.2 121.6 59.8
12.1 300
Comparative Example 11-4 1.45 0.131 104,8 119,1 58.6
12.2 330
Comparative Example 11-5 1.96 0.087 105.2 119.7 50.6
13.5 350
Comparative Example 11-6 1,98 0.081 105,7 119,8 50,1
13.1 310
48
It can be seen from Table 1 that the melt flow rate (MFR) of the polyethylene
compositions
provided by the present invention met the requirements of the casting process,
and
especially when the difference between AHm PE and AHm composition was in a
specific
range, maleic anhydride copolymer microspheres were uniformly distributed in
the
composition without agglomeration. At the same time, the compositions of the
examples
according to the present invention also had good mechanical properties,
thereby meeting
the requirements for the stretching ratio of the breathable film. The
difference between
the density pcomposition Of the polyethylene composition of the present
invention and the
density PPE of the polyethylene was advantageously within a specific range.
The
compositions of the present invention could achieve good dispersion of the
porogen in
the matrix resin without additional use of a coupling agent, a dispersant and
a surfactant.
Test examples
The polyethylene compositions of the examples and comparative examples were
added
to an extrusion casting machine and molten and cast into cast sheets, with the
extrusion
temperature being 210-240 C and the temperature of the cooling roller being 20-
60 C,
thereby polyethylene cast films were produced. Then, the cast films were
uniaxially
stretched 3 times to obtain polyethylene microporous breathable films having
an average
thickness of 30 pm, wherein the heat setting temperature was 85 C. The
properties of the
polyethylene microporous breathable films are shown in Table 2.
30
CA 03236535 2024- 4- 26
49
Table 2
Tensile Elongation at
Surface Water vapor strength
break
Items density Porositytransmission rate (MPa)
(%)
(%)
(g/m2) (g/(m2.24h))
1-- MD TD MD TD
Example 14 19.06 47.6 3260 27.8 6.8
151.6 406.3
Example 1-2 19.19 47.3 3210 28.5 6.9
146.5 397.5
Example 1-3 19.43 46.8 3240 27.2 6.7
146.2 394.1
Example 1-4 19.12 44.6 3160 26.5 6.3
138.4 385.8
Example 1-5 19.28 45.1 3190 26.0 5.9
132.6 367.3
Example 1-6 18.91 42.4 3110 28.1 7.2
156.1 408.8
Example 1-7 19.01 46.8 3240 27.6 6.6
148.9 397.7
Comparative 18.32 i 31.4 1980 28.2
6.8 147.2 398.4
Example 1-1
r
Comparative
31.14 34.7 2540 19.2 3.7 112.9 311.8
Example 1-2
Comparative 35.68 36.1 2660 21.8 4.1 118.6 320.3
Example 1-3
Comparative
20.17 36.4 2730 23.2 4.4 131.2 329.7
Example 1-4
Comparative 18.97 36.7 2800 26.8 6.5 141.1 389.6
Example 1-5
Comparative
19.74 36.5 2760 19.4 3.4 91.7 262.9
Example 1-6
Example 11-1 18.76 _ 44.4 3170 27.0
6.4 143.1 399.6
Example 11-2 18.88 44.1 3150 27.9 7.1
150.6 391.2
Example 11-3 18.48 42.3 3110 27.5 6.6
147.3 395.6
Example 11-4 18.69 43.9 3150 27.1 6.5
142.8 392.3
1-
Example 11-5 18.96 40.7 3090 25.6 5.8
134.7 378.8
Example 11-6 19.1 40.2 3060 25.3 5.4
129.4 369.1
Comparative 18.25 27.2 1860 28.2 6.8 147.2 398.4
Example 11-1
Comparative 31.14 34.7 2540 19.2 3.7 112.9 311.8
Example 11-2
Comparative
35.28 36.1 2660 21.8 4.1 118.6 320.3
Example 11-3
Comparative 19.28 36.2 2710 22.1 4.2 121.2 322.1
Example 11-4
Comparative 18.92 36.1 2720 22.8 4.6 127.4 332.3
Example 11-5
Comparative 18.85 35.9 2680 22.5 4.4 119.1 320.1
Example 11-6
It can be seen from Table 2 and the SEM photographs in the drawings that in
the
examples according to the present invention, by using maleic anhydride
copolymer
CA 03236535 2024- 4- 26
microspheres as organic porogen in the polyethylene matrix resin, the porogen
could be
well dispersed in the matrix resin without the use of a coupling agent, a
dispersant and a
surfactant, thereby producing quite a lot of uniformly distributed micropores
having a
suitable size in the resulting film, thus making the obtained breathable film
have a higher
breathability.
In the comparative examples using an inorganic porogen or a cross-linked
starch as
organic porogen, without the addition of a dispersant, a coupling agent and a
surfactant,
both the properties of the compositions and the properties of the prepared
breathable
films were worse than those of the examples according to the present
invention, and there
were fewer and non-uniformly distributed micropores in the resulting films,
thus the water
vapor transmission rate was lower.
As can be seen from the comparison between Example 1-1 and Comparative Example
1-
5 and the comparison between Example 11-1 and Comparative Example 11-5, when
nnaleic
anhydride copolymer microspheres having an average particle size of less than
500 nm
were used, the pores were too small, and both the properties of the
polyethylene
compositions and the properties of the resulting films were worse, wherein the
water
vapor transmission rate was lower than 3000g/(m2=24h).
As can be seen from the comparison between Example 11-1 and Comparative
Example
11-6, when the porogen was larger (average particle size >2000nm), the pores
were larger
and their quantity was less, the water vapor transmission rate was worse, and
the
mechanical properties were negatively affected.
As can be seen from the comparison between Example 1-1 and Example 1-2,
compared
to using LLDPE alone as the matrix resin, using a combination of LLDPE and
LDPE as
the matrix resin not only improved the processing performance, but also
promoted
dispersion of the porogen to a certain extent, thereby achieving increased
water vapor
transmission rate.
The results show that the polyethylene breathable films produced by the
examples
according to the present invention had a high air permeability and good
mechanical
properties, thus could be widely used in the field of breathable composite
articles such as
water-blocking and moisture-permeable materials, sanitary articles, medical
articles, food
CA 03236535 2024- 4- 26
51
packaging or building articles, etc.
Although the present invention has been described in detail and illustrated by
examples,
it will be apparent to those skilled in the art that other modifications and
variations can be
made within the spirit and scope of the invention. Furthermore, it should be
understood
that various aspects described in the present invention, various parts of
different
embodiments, and various features as listed may be combined or interchanged in
whole
or in part. Furthermore, those skilled in the art will appreciate that the
foregoing
description is by way of example only and is not intended to limit the present
invention.
CA 03236535 2024- 4- 26
52