Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03238264 2024-05-13
WO 2023/102572 PCT/US2022/080951
LIQUID FERTILIZER COMPRISING NITROGEN, MAGNESIUM, AND
CHLORIDE, AND METHODS FOR MAKING AND USING THE SAME
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority from U. S . Provisional Patent
Application No.
63/286,076, filed on December 5, 2021, which is incorporated by reference
herein in its
entirety.
BACKGROUND
[0002] Plant life depends on sunlight to give the plant the energy it
needs to grow and
produce seed or fruit. Through the process of photosynthesis, plants convert
sunlight into
energy, and this energy is used to promote vegetative growth and plant
reproduction. Any
plant nutrient or combination of nutrients that encourages the efficiency of
the photosynthetic
process may give an advantage in plant health and yield.
[0003] Chlorophyll is the green pigment present in all green plants and
is responsible
for the absorption of sunlight that contributes to photosynthesis. In the corn
plant, for example,
several key nutrients are involved in the formation and production of
chlorophyll. Nitrogen
(particularly in the form of urea and ammonium nitrate) and other nutrients
such as magnesium
optimize the production of chlorophyll.
[0004] The availability of these nutrients to plants is a persistent
problem.
[0005] While urea is water soluble, it precipitates or "salts out" at
relatively high
temperatures. For example, urea 46-0-0 can be used to make urea solutions up
to 47.82% by
weight, a grade of 22-0-0 nitrogen solution, which has a salt out temperature
of 11 C. Liquid
fertilizer that sits in aboveground storage tanks through a cold winter can
undergo stratification,
salting out, or both. Stratification results in pockets of varying product
concentrations within
an aboveground storage tank. With cold temperatures, some liquid fertilizers
will salt out,
leaving a combination of salted product and liquid product. The salted product
can clog
sprayers, planters, and applicators.
CA 03238264 2024-05-13
WO 2023/102572 PCT/US2022/080951
[0006] Moreover, contacting magnesium chloride with nitrogen-containing
fertilizers,
e.g., 10-34-0 (ammonium polyphosphate) and 12-0-0-26 (ammonium thiosulfate),
typically
causes precipitation. Thus far, nitrogen and magnesium have been successfully
combined in
very low nitrogen concentrations (e.g., NutriMagTm 5 N-0 P-0 K-5.5 Mg
manufactured by
Innovative Surface Solutions) and/or including less advantageous forms of
nitrogen (e.g.,
ammonium and nitrate, such as in 32-0-0 liquid).
[0007] What is needed is a single fertilizer solution, comprising both
high urea-based
nitrogen concentration and chloride (Cl-) and magnesium (Mg2+) ions, that can
be stored and
applied using conventional storage and application apparatuses as a liquid at
relatively cold (0
C and below) ambient temperatures. It would be particularly beneficial if such
a fertilizer
solution could serve as a base matrix for other nutrients (e.g., potassium or
phosphorus) as well.
SUMMARY
[0008] In one aspect, a liquid composition for use as a fertilizer is
provided, the liquid
composition produced by the steps in the order: (1) providing an about 30%
aqueous solution
of magnesium chloride and heating the solution to a temperature of at least
about 60 C; (2)
providing dry urea 46-0-0 and adding the dry urea 46-0-0 to the heated aqueous
solution of
magnesium chloride to form a solid-liquid mixture; (3) agitating the solid-
liquid mixture to
dissolve the dry urea 46-0-0 and form the liquid composition, wherein the
liquid composition
is characterized in that: (i) it remains in liquid form for more than 24 hours
at a temperature of
at less than 0 C; (ii) it has a pH between 7 and 8; and (iii) it has a
fertilizer ratio of about 23.5
N ¨ 0 P ¨ 0 K ¨ 3.5 Mg. In one aspect, the fertilizer ratio is about 24N ¨ OP
¨ OK ¨ OS ¨ 4Mg
¨ 10C1 ¨ 0.35B. In one aspect, the 30% aqueous solution of magnesium chloride
is provided
in about 49% w/w, and the dry urea 46-0-0 is provided in about 51% w/w.
[0009] In another aspect, a method for preparing a liquid composition for
use as a
fertilizer is provided, the method comprising the steps in the order: (1)
providing an about 30%
CA 03238264 2024-05-13
WO 2023/102572 PCT/US2022/080951
aqueous solution of magnesium chloride and heating the solution to a
temperature of at least
about 60 C; (2) providing dry urea 46-0-0 and adding the dry urea 46-0-0 to
the heated aqueous
solution of magnesium chloride to form a solid-liquid mixture; (3) agitating
the solid-liquid
mixture to dissolve the dry urea 46-0-0 and form the liquid composition,
wherein the liquid
composition is characterized in that: (i) it remains in liquid form for more
than 24 hours at a
temperature of at less than 0 C; (ii) it has a pH between 7 and 8; and (iii)
it has a fertilizer ratio
of about 23.5 N¨OP¨OK¨ 3.5 Mg. In one aspect, the fertilizer ratio is about
24N ¨ OP ¨ OK
¨ OS ¨ 4Mg ¨ 10C1 ¨ 0.35B. In one aspect, the 30% aqueous solution of
magnesium chloride
is provided in about 49% w/w, and the dry urea 46-0-0 is provided in about 51%
w/w.
BRIEF DESCRIPTION OF THE FIGURES
[0010] FIG. 1 is a flow chart showing the steps of the method that lead
to the claimed
liquid fertilizer compositions comprising a complex of magnesium chloride and
urea with mild
hydration.
[0011] FIG. 2 is an example plant set-up for carrying out the steps of
the method that
lead to the claimed liquid fertilizer compositions.
[0012] Figure 3 shows the FTIR spectrum of urea.
[0013] Figure 4 shows the TGA profile for urea.
[0014] Figure 5 shows the FTIR spectra of MgC120)<H20 and MgC1202H20.
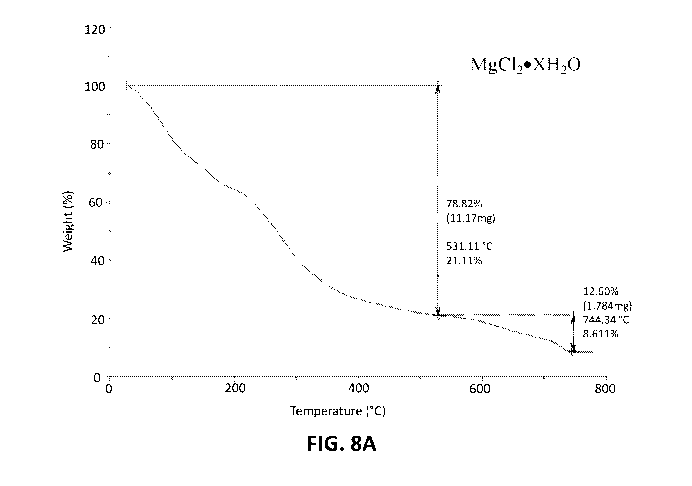
[0015] Figures 6A and 6B show the TGA spectra of MgC120)<H20 and
MgC1202H20.
[0016] Figure 7 shows the FTIR spectra of: (i) an example claimed complex
of
magnesium chloride and urea with mild hydration ("N-Mag"); (ii) N-Mag
crystallized at 40
C; and (iii) N-Mag crystallized at 60 C.
[0017] Figures 8A and 8B show the TGA spectra of N-Mag and N-Mag
crystallized at
60 C.
[0018] Figure 9 shows mass data for several molecular formulae.
CA 03238264 2024-05-13
WO 2023/102572 PCT/US2022/080951
[0019] Figure 10 shows the FTIR spectra of N-Mag compared to two simple
solutions
of MgCl2 and urea.
[0020] Figure 11 shows TGA spectra of N-Mag compared to two simple
solutions of
MgCl2 and urea, in solution.
[0021] Figure 12 shows TGA spectra of crystals formed from N-Mag compared
to
crystals formed from two simple solutions of MgCl2 and urea.
DETAILED DESCRIPTION
[0022] In one aspect, a liquid composition for use as a fertilizer is
provided, the liquid
composition produced by the steps in the order: (1) providing an about 30%
aqueous solution
of magnesium chloride and heating the solution to a temperature of at least
about 60 C; (2)
providing dry urea 46-0-0 and adding the dry urea 46-0-0 to the heated aqueous
solution of
magnesium chloride to form a solid-liquid mixture; (3) agitating the solid-
liquid mixture to
dissolve the dry urea 46-0-0 and form the liquid composition, wherein the
liquid composition
is characterized in that: (i) it remains in liquid form for more than 24 hours
at a temperature of
at less than 0 C; (ii) it has a pH between 7 and 8; and (iii) it has a
fertilizer ratio of about 23.5
N ¨ 0 P ¨ 0 K ¨ 3.5 Mg. In one aspect, the fertilizer ratio is about 24N ¨ OP
¨ OK ¨ OS ¨ 4Mg
¨ 10C1 ¨ 0.35B. In one aspect, the 30% aqueous solution of magnesium chloride
is provided
in about 49% w/w, and the dry urea 46-0-0 is provided in about 51% w/w.
[0023] In another aspect, a method for preparing a liquid composition for
use as a
fertilizer is provided, the method comprising the steps in the order: (1)
providing an about 30%
aqueous solution of magnesium chloride and heating the solution to a
temperature of at least
about 60 C; (2) providing dry urea 46-0-0 and adding the dry urea 46-0-0 to
the heated aqueous
solution of magnesium chloride to form a solid-liquid mixture; (3) agitating
the solid-liquid
mixture to dissolve the dry urea 46-0-0 and form the liquid composition,
wherein the liquid
composition is characterized in that: (i) it remains in liquid form for more
than 24 hours at a
CA 03238264 2024-05-13
WO 2023/102572 PCT/US2022/080951
temperature of at less than 0 C; (ii) it has a pH between 7 and 8; and (iii)
it has a fertilizer ratio
of about 23.5 N¨OP¨OK¨ 3.5 Mg. In one aspect, the fertilizer ratio is about
24N ¨ OP ¨ OK
¨ OS ¨ 4Mg ¨ 10C1 ¨ 0.35B. In one aspect, the 30% aqueous solution of
magnesium chloride
is provided in about 49% w/w, and the dry urea 46-0-0 is provided in about 51%
w/w.
[0024] In one aspect, the product is not simply a mixture or super-
saturated mixture of
the two starting products. Rather, the liquid composition comprises a complex
of MgCl2 and
urea (U) with mild hydration. In one aspect, the structure comprises
MgC12U4=XH20, wherein
X = 1 to 6.
[0025] Urea, also known as carbamide, is an organic compound having the
chemical
formula CO(NH2)2. Urea 46-0-0, or urea 46% nitrogen, is a white crystalline
solid containing
46% nitrogen. Urea 46-0-0 is widely used in the agriculture industry as a
fertilizer. The
designation "46-0-0" refers to a fertilizer ratio, in this case, an NPK
(nitrogen-phosphorus-
potassium) fertilizer ratio of 46 N:0 P:0 K. "Fertilizer ratio" means the
ratio of two or more
nutrients to another in 100 pounds of either liquid or dry material.
[0026] Urea is readily commercially available or may be manufactured by
feeding
ammonia and carbon dioxide into a reactor at 180 ¨ 210 C and 150 bar
pressure. After
stripping the reaction mixture of ammonia, the urea solution is concentrated
by evaporation or
crystallization.
[0027] MgCl2 is also commercially available, typically in the form of
MgC12=6H20
crystals. Water is added to the crystals to achieve a desired concentration.
For example, for a
30% MgCl2 solution, 14.88 lbs of MgC12=6H20 are mixed per gallon of water.
EXAMPLES
Example 1: Preparation and characterization of MgC12U4=XH20
[0028] With reference to FIGs. 1 and 2, a 30% MgCl2 solution (Compass
Minerals)
sourced from the Great Salt Lake in Utah is placed into a commercial
fertilizer blender and
CA 03238264 2024-05-13
WO 2023/102572 PCT/US2022/080951
heated to about 60 C. Granular urea 46-0-0 (CF Industries) (51% urea to 49%
MgCl2 w/w) is
added slowly to the heated MgCl2 solution by hopper with forceful agitation.
After the mixture
becomes a homogeneous, slightly viscous, light brown liquid free of foreign
matter, agitation
is ceased, and the liquid is cooled and prepared for transport. The liquid
product has a fertilizer
ratio of about 23.5 N¨OP¨OK¨ 3.5 Mg, or about 24N¨ OP ¨ OK ¨ OS ¨ 4Mg ¨ 10C1 ¨
0.35B, a pH of about 7.5-7.8, and remains in liquid form below 0 C for
sustained periods.
[0029] The product ("N-Mag" or "NitroMag") was evaluated by:
[0030] Fourier Transform Infrared (FTIR) Spectroscopy
Bruker Tensor II
MIRacleATR sampling accessory
4 cm' resolution, 16 scans per spectrum, 4000-600 cm'
[0031] Thermogravimetric Analysis (TGA)
TA Instruments, Hi-Res TGA 2950 Thermogravimetric Analyzer
30 C/minute ramp to 800 C
Oxygen atmosphere
[0032] Figure 3 shows the FTIR spectrum of urea. Figure 4 shows the TGA
profile
for urea. Figure 5 shows the FTIR spectra of MgC120)<H20 and MgC1202H20.
Figures 6A
and 6B show the TGA spectra of MgC120)<H20 and MgC1202H20.
[0033] Figure 7 shows the FTIR spectra of: (i) N-Mag; (ii) N-Mag
crystallized at 40
C; and (iii) N-Mag crystallized at 60 C. N-Mag is a combination of MgCl2 and
urea with
features of both represented in the FTIR spectrum. As the product is dried and
crystallized,
there is a slight decrease in absorbance in the 3000-3600 cm' region,
indicating that the final
product is mildly hydrated (in contrast to the MgC12.)<H20 starting product
that shows more
dramatic changes in this region as the product is dried).
CA 03238264 2024-05-13
WO 2023/102572 PCT/US2022/080951
[0034] Figures 8A and 8B show the TGA spectra of N-Mag and N-Mag
crystallized at
60 C. TGA of N-Mag does not show clear transitions that can be correlated to
fragmentation
of the molecule. TGA of the dried sample, however, shows two transitions.
Because the weight
change does not begin until the temperature reaches almost 200 C, the initial
weight loss is
not attributed to water. If, instead, the mass loss of 72% is attributed to
urea, a complex of
MgC12U4 would correlate to these data.
[0035] Figure 9 shows mass data for several molecular formulae. In TGA,
molecules
rarely break down to the bare metal atom, Mg in this case. The residue is
generally an oxide,
like MgO, or salt, like MgCl2. Therefore, the mass percent of Mg or Cl alone
is unlikely to
correlate to the final stages of TGA.
[0036] N-Mag was next compared to simple mixtures of starting materials.
First,
MgCl2 (49% w/w) and urea (51% w/w) were combined at room temperature and mixed
thoroughly until homogeneous. Second, MgCl2 heated to 60 C prior to the
addition of urea.
[0037] Figure 10 shows the FTIR spectra of N-Mag compared to the two
simple
solutions. FTIR spectra of the simple MgCl2-urea solutions are very similar
(the small baseline
shift is not significant). When overlaid with the spectrum of the N-Mag
product, there is a
distinctive absorbance around 1100 cm' that is not present in the simple
mixtures. This unique
spectral feature supports that the N-Mag is a new complex, not simply a
mixture of the starting
materials.
[0038] Figure 11 shows TGA spectra of N-Mag compared to the two simple
solutions,
in solution. TGA profiles of the simple solutions and N-Mag are similar until
temperatures
exceed 550 C. Above 550 C, there is a distinctive shift in the profile for N-
Mag. This shift
supports that the N-Mag is a new complex, not simply a mixture of the starting
materials.
[0039] Figure 12 shows TGA spectra of crystals formed from N-Mag compared
to the
two simple solutions. Crystals formed from the simple mixtures produced very
similar TGA
CA 03238264 2024-05-13
WO 2023/102572 PCT/US2022/080951
profiles. There is a distinctive shift in the profile for the N-Mag throughout
the temperature
ramp. This shift further supports that the N-Mag crystals represent a new
complex, not co-
crystallization of the starting materials.
Example 2: Fertility Test (Plot Scale)
[0040] The liquid composition from Example 1 was compared to other
formulations
and tested on corn as follows:
Table 1: Formulations
Formulation Formulation
No.
1 Dry urea
2 Urea (first application) and 32-0-0 (liquid)
3 Urea (first application) and 28-0-0-5S (combination of 32-0-0 (10
ga.) and
ammonium thiosulfate (12-0-0-26) (2 ga.) (both liquids))
4 Urea (first application) and 32-0-0 (liquid) combined with 30%
MgCl2
solution (51:49 w/w)
Urea (first application) and 46-0-0 (solid) combined with 30% MgCl2
solution (51:49 w/w) as described in Example 1
6 Urea (first application) and 32-0-0 (liquid) combined with 30%
MgCl2
solution (51:49 w/w), plus nitrogen stabilizer
7 Urea (first application) and 46-0-0 (solid) combined with 30%
MgCl2
solution (51:49 w/w) as described in Example 1, plus nitrogen stabilizer
Table 2: Application Method
Application Application Method
Method No.
1 250 lb N (dry urea) spread between rows ("up front")
2 150 lb N (dry urea) up front; with corn at approximately 1 ft., 50
lb N
[according to Formulation No.] along the sides of the corn in the rows ("by
side-dress"); with corn at approximately 4-5 ft., 50 lb N [according to
Formulation No.] along the sides of the corn in the rows ("by y-drop")
CA 03238264 2024-05-13
WO 2023/102572 PCT/US2022/080951
Table 3: Plot* Results
Row Formulation Application Bu./Acre Avg
No. Method No. Bu./Acre
1 1 1 233
2 1 1 229
3 1 1 263 242
4 1 1 244
1 2 2 242
2 2 2 241
3 2 2 261 252
4 2 2 264
1 3 2 271
2 3 2 271
3 3 2 263 269
4 3 2 273
1 4 2 271
2 4 2 263
3 4 2 272 270
4 4 2 273
1 5 2 281
2 5 2 301
3 5 2 284 291
4 5 2 296
1 6 2 280
CA 03238264 2024-05-13
WO 2023/102572 PCT/US2022/080951
2 6 2 275
3 6 2 268 272
4 6 2 264
1 7 2 280
2 7 2 301
3 7 2 292 291
4 7 2 291
*Plot size: 4 rows, 47.5 ft/per row, 30" between rows
[0041] As shown in Table 3, the liquid fertilizer according to Example 1
(Formulation
Nos. 5 and 7) resulted in consistent, significant increases over urea alone
(20%) and the other
nitrogen-containing blends (7-15%).
Example 3: Fertility Test (Field Scale)
[0042] Formulation No. 5 (NitroMag) was tested on #2 yellow corn. Each
test was
evaluated for yield results, plant health, stock quality, and nutrient uptake.
Four test sites were
evaluated during different corn plant growth stages.
[0043] Trial #1: The first trial with NitroMag was side dressed
(fertilizer put in the
ground by the root) on corn at the V5 leaf stage. The comparison was done
against regular
32% nitrogen. The test fields were visited weekly throughout the summer to
observe the
different stages of growth and how they compared to one another. The roots
were healthy and
well established. The ears on the NitroMag and 32% nitrogen-treated corn both
filled all the
way to the end and had similar girth and length. Toward the end of summer,
NitroMag-treated
plants were a darker color of green compared to the 32% nitrogen-treated
plants. The darker
green indicates that the NitroMag plant was healthier. For plant health,
healthy chlorophyll
molecules mean healthier corn leaves and more productive photosynthesis, which
results in
better stock health, nutrient uptake, and higher yields. The yield results
validated the visual
CA 03238264 2024-05-13
WO 2023/102572 PCT/US2022/080951
inspections: on an equal nitrogen basis, NitroMag had a five bushel/acre
advantage over the
32% nitrogen.
[0044] Trial #2: The NitroMag was applied through Y-Drop (product sprayed
on the
ground, right beside where the corn plant emerges from the ground) on corn at
the V8 stage.
The plants had a very strong green color. On an equal nitrogen basis, NitroMag
had a 4.5
bushel/acre advantage over the 32% nitrogen.
[0045] Trial #3: The NitroMag was applied by fertigation (fertilizer was
applied
through irrigation pivots during watering) over two applications, pre-tassel
and post-
pollination. The plant and stock health were consistent throughout the season.
This trial was
a comparison between 28-0-0-5 and NitroMag. The products were applied at the
exact same
time with the same amount of nitrogen. Both sides of the field were equally
healthy and the
ears on both sides had very comparable length and girth. The NitroMag yielded
nine
bushels/acre more than the 28-0-0-5. Stock quality was monitored throughout
the trial, and the
NitroMag had a very health pith (inside of the corn stalk), while the 28-0-05
showed signs of
stock rot.
[0046] Trial #4: This trial between NitroMag and ammonia was delayed due
to rain and
should have been applied sooner, which could have provided higher yields. The
NitroMag was
applied once by fertigation at brown silk, and the plant and stock health were
very good.
NitroMag showed a four bushel/acre yield increase.
Example 4: Stability Tests
[0047] The liquid composition from Example 1 was compared to other
formulations
and tested for temperature resistance:
Formulation Salt-out at 0 C? (S/O temp, if
measured)
46-0-0 (solid) combined with 30% MgCl2 No salt-out, even after 24 hours
solution (51:49 w/w)
CA 03238264 2024-05-13
WO 2023/102572 PCT/US2022/080951
46-0-0 solution in water (50/50 wt/wt) Salted-out at 15 C
Example 5: N-Mag as Base Matrix #1, N-Mag Plus Potassium (NK-Mag)
[0048] The addition of potassium to N-Mag improves N-Mag's efficiency for
late
season pivot applications and for use on other crops that require more
potassium. The base
matrix is N-Mag, which is blended back with dry soluble potash (0-0-60)
((i.e., put back in the
blender and remixed with water and soluble potash in the correct amounts) as a
re-blended mix
to formulate a 19N-OP-3K-0S-3Mg-7C1-0.25 B liquid product.
[0049] The same blend can be manufactured at the time the N-Mag is being
made, by
adding the correct amount of soluble potash to the other products used to
formulate N-Mag
liquid.
[0050] For back blending of N-Mag with soluble potash, the formula is:
80% Nitro-
Mag + 15-20% H20 + 5.3% Soluble Potash (0-0-60)
[0051] The amount of water may have to be increased if temperatures are
colder. For
example, at 20 lbs of water, potassium settles out of the mix. In warmer
temperatures, 15 lbs
will float the potassium.
[0052] A ton of NK-Mag product includes:
Nitro-Mag 80# x 20 = 1600 lbs
Water @ 15# x 20 = 300 lbs
Potash @ 5.3# x 20 = 106 lbs
Total amount = 2006 lbs
[0053] Making the NK-Mag product with this method permits existing N-Mag
to be
taken out of storage and blended in a timely fashion for delivery to, e.g., a
farm, avoiding long
term storage of the product. If long term storage is required, the water may
be increased to 400
lbs in a ton batch. The NK-Mag can be cold blended with the proper agitation
and adding the
water first.
CA 03238264 2024-05-13
WO 2023/102572 PCT/US2022/080951
[0054] For blending NK-Mag from scratch:
Nutri-Boost added at desired level
Magnesium Chloride 39% or 19,500 lbs in a 50,000 lb load.
Urea (46-0-0) 41% or 20,500 lbs in 50,000 lbs
Water at 15% or 7500# in 50,000 lbs
Soluble Potash (0-0-60) 5.3% or 2,650 lbs in 50,000 lb
[0055] Mixing instructions:
Nutri-Boost liquid stabilizer is added to the reactor first at the desired
amount
(e.g., 600 lbs).
Place 19,500 pounds of MgCl2 in the reactor to heat to 60-70 C.
Place 7,500 lbs of H20 to the blend, continuing to heat and agitate.
Once the correct temperatures have been achieved, add 20,500 lbs of dry urea
(46-0-0) to the mix while agitating.
[0056] As the mixture blends and a heat of at least 110 F is maintained,
add in the
2,650 lbs of dry soluble potassium (0-0-60). Continue to agitate until product
is blended.
[0057] These instructions produce a 19 N-0 P-3 K-0 S -3Mg-7C1-0.25B mix
of NK-
Mag. The NK-Mag product weighs 10.9 pounds per gallon.
Example 6: N-Mag as base matrix #2; various blends as corn starters or liquid
phosphate
category of products
[0058] Other than liquid nitrogen 32% and side dressing products such as
28-0-0-5S,
the highest usage of liquid fertility products is in the corn starter or
liquid phosphate category
of products. The most widely used phosphate product is a polyphosphate known
as 10-34-0.
In 100 pounds of product, 10-34-0 contains ten pounds of nitrogen and 34
pounds of phosphate.
10-34-0 is made by reacting a 68% phosphoric acid with anhydrous ammonia in an
exothermic
reaction.
CA 03238264 2024-05-13
WO 2023/102572 PCT/US2022/080951
[0059] Unfortunately, the combination of MgCl2 with 10-34-0 product
precipitates,
resulting in clogged hoses and solids that must be removed from the bottom of
large tanks.
[0060] Surprisingly, however, a stable phosphate source (e.g., 54%
Merchant Grade
Acid) and Nitro-Mag mix suitably with MgCl2. Three such blends were prepared:
[0061] 10-30-0
[0062] For a 10-30-0 blend, pump in the desired amount of 54% Merchant
Grade
Phosphoric Acid into a tank or tanker transport in a 55% ratio of the final
gallon quantity
needed. If blending a 4,500 gallon batch, 55% of those 4,500 gallons would be
the phosphoric
acid, or 2,475 gallons.
[0063] The next step is to back blend in the Nitro-Mag liquid by pumping
it into the
tank or tanker to freely mix with the 54% Phosphoric Acid. In this case, that
would be 2,025
gallons or 45% of the blend with the Nitro-Mag liquid fertilizer.
[0064] The end result is a I ON-30P-OK-OS-3Mg-5C1 +Boron product.
[0065] 20-10-0 blend
[0066] For the 20-10-0 blend, the same procedure is followed, except that
the ratios of
the two reagents will change due to the different amounts of nutrients
desired. To make a 4,500
gallon batch, first introduce the 54% Phosphoric Acid by pumping 18% of the
phosphoric acid
or 810 gallons into the tank.
[0067] The next step is to back blend in the Nitro-Mag liquid at 82% of
the 4,500
gallons, or 3,690 gallons.
[0068] The end result is a 20N- I OP-OK-OS-4Mg-7C1 +Boron product.
[0069] 17-15-0 blend
[0070] For the 17-15-0 blend, the same procedure may be used. Start by
taking 28%
of the 54% Phosphoric Acid or 1,260 gallons of a 4,500 gallon batch and
pumping it into the
tank.
CA 03238264 2024-05-13
WO 2023/102572 PCT/US2022/080951
[0071] The next step is to back blend 72% or 3,240 gallons of Nitro-Mag
into the same
tank, letting it blend freely.
[0072] The end result is a 17N-15P-OK-OS-3.5Mg-6C1 +Boron product.
[0073] The success of these blends is based on the ability of 54%
Merchant Grade
Phosphoric Acid to blend well with MgCl2 in the presence of the Nitro-Mag base
matrix.
Example 7: N-Mag as base matrix #3, various blends as corn starters or liquid
phosphate
category of products
[0074] To make a ton of 20N-12P-OK-OS-3.2Mg-9C1-0.29B configuration:
(1) Add 1,680# of Nitro-Mag in the reactor while agitating.
(2) Add in 320# of a 75% Phosphoric Green Acid and continue to blend
until well mixed.
[0075] To make a ton of 10N-28P-OK-OS-1.7Mg-4.6C1-0.15B configuration:
(1) Add 840# of Nitro-Mag into the reactor with agitation and heat.
(2) Add 740# of 75% Phosphoric Green Acid and continue to agitate and
heat.
(3) Add 420# water to the mix and continue to blend until well mixed.
(4) Heating temperatures on this blend should be at 38 C.
Example 8: N-Mag as base matrix #4, various blends as corn starters or liquid
phosphate
category of products
[0076] To make a ton of 20N-12P-OK-OS-3.2Mg-9C1-0.29B:
(1) Add Nutri-Boost Stabilizer at 25# or more to the reactor.
(2) Add 811# of MgCl2 into the reactor while mixing and heating.
(3) Add 320# of 75% Phosphoric Acid while mixing and heating to 60-70
C.
CA 03238264 2024-05-13
WO 2023/102572 PCT/US2022/080951
(4) Add 844# of Urea to melt while continuing to mix until all solids are
blended.
(5) If final product has unsuitably thick viscosity, up to 300# of water
may
be added per ton.
[0077] To blend a ton of 10-28-0K-OS-1.6Mg-4.6C1-0.15B
(1) Add 25# or more of Nutri-Boost Stabilizer to the reactor.
(2) Add 400# of MgCl2 into the reactor while mixing and heating the
product.
(3) Add 740# of 75% Phosphoric Acid while mixing and heating to 60-70
C.
(4) Add 420# of water while mixing and heating.
(5) Add 415# of urea to melt while continuing to mix until all solids are
blended.
[0078] The aspects disclosed herein are not intended to be exhaustive or
to be limiting.
A skilled artisan would acknowledge that other aspects or modifications to
instant aspects can
be made without departing from the spirit or scope of the invention. The
aspects of the present
disclosure, as generally described herein and illustrated in the figures, can
be arranged,
substituted, combined, separated, and designed in a wide variety of different
configurations, all
of which are contemplated herein.
[0079] Unless otherwise specified, "a," "an," "the," "one or more of,"
and "at least
one" are used interchangeably. The singular forms "a", "an," and "the" are
inclusive of their
plural forms. The recitations of numerical ranges by endpoints include all
numbers subsumed
within that range (e.g., 1 to 5 includes 1, 1.5, 2, 2.75, 3, 3.80,4, 5, etc.).
The terms "comprising"
and "including" are intended to be equivalent and open-ended. The phrase
"consisting
essentially of' means that the composition or method may include additional
ingredients and/or
CA 03238264 2024-05-13
WO 2023/102572 PCT/US2022/080951
steps, but only if the additional ingredients and/or steps do not materially
alter the basic and
novel characteristics of the claimed composition or method. The phrase
"selected from the
group consisting of' is meant to include mixtures of the listed group.
[0080] When reference is made to the term "each," it is not meant to mean
"each and
every, without exception."
[0081] The term "about" in conjunction with a number is simply shorthand
and is
intended to include 10% of the number. This is true whether "about" is
modifying a stand-
alone number or modifying a number at either or both ends of a range of
numbers. In other
words, "about 10" means from 9 to 11. Likewise, "about 10 to about 20"
contemplates 9 to 22
and 11 to 18. In the absence of the term "about," the exact number is
intended. In other words,
"10" means 10.