Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 2023/107356
PCT/US2022/051770
TITLE OF THE INVENTION
ANTIMALARIAL AGENTS
FIELD OF THE INVENTION
The present invention relates to compounds of Formula (I), or pharmaceutically
acceptable salts thereof, useful for the treatment of Plasmodium infections.
More specifically, the
present invention relates to compounds of Formula (I), or pharmaceutically
acceptable salts
thereof, useful for the treatment of Plasmodium infections, more particularly
for the treatment of
malaria.
B A CK GR OUND OF THE INVENTION
Malaria is a major disease in humans with several hundred million infections
and
over 450,000 deaths each year. The most lethal form of malaria is caused by
Plasmodium
falciparum. This protozoan parasite is responsible for almost all malarial
deaths with most
occurring in Africa. P. falciparum has a complex life cycle starting in the
Anopheles mosquito
vector when sporozoite forms are injected into the human host during a blood
feed. These
sporozoites migrate to the liver and invade hepatocytes in which they develop
to form thousands
of liver merozoites that egress into the blood where they invade erythrocytes
to commence the
asexual cycle of the parasite responsible for the symptoms of malaria. The
parasite develops
within the protected niche of the red cell to form 16-32 merozoites that, once
mature, egress
from the host cell to invade new red blood cells. Some of these parasites
differentiate to form
gametocytes, the sexual form of the parasite. These can be taken up by the
mosquito where male
and female gametes form, fuse and differentiate into oocysts on the mosquito
midgut
extracellular matrix. Sporozoites form within the oocyst and upon egress
migrate to the salivary
gland for delivery to the next host during blood feeding for perpetuation and
survival of the
parasite.
Other forms of malaria include a relapsing form of malaria caused by P. vivax
which is responsible for significant morbidity, can cause virulent forms of
this disease with some
deaths and is mainly a problem outside Africa. P. knowlesi is found in South
East Asia and is a
zoonotic parasite that normally infects long-tailed macaques but has been
shown to infect
humans in Malaysian Borneo.
Artemisinin combined with partner drugs have become a mainstay in the
treatment and control of malaria. However, due to the increasing threat of
artemisinin-based
combination therapy (ACT) drug resistance, the development of new
antimalarials with novel
1
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
targets that inhibit multiple steps in the parasite life cycle is an urgent
priority for the malaria
control field. Such novel antimalarials, as monotherapies or ACT partner
drugs, could make
strides towards malaria elimination as there is a reduced likelihood of
parasites with preexisting
resistance mutations being present in the parasite population.
Currently, aspartic acid proteases are prime targets for drug development: the
111V aspartic acid protease has been successfully targeted with a drug in
clinical use; inhibitors
that target human renin, BACE1 and gamma-secretase have been or are in
clinical development.
In the antimalarial drug space, P. falciparum aspartic acid proteases
plasmepsin X and IX (PMX
and PMIX) have been identified as potential targets since inhibitors block
parasite egress and
invasion of the host cell and prevent maturation of some rhoptry and
micronemal proteins
required for this process (Pino P, Caldelari R, Mukherjee B, Vahokoski J,
Klages N, Maco B, et
al. A multistage antimalarial targets the plasmepsins IX and X essential for
invasion and egress.
Science. 2017;358(6362):522-8.)
SUMMARY OF THE INVENTION
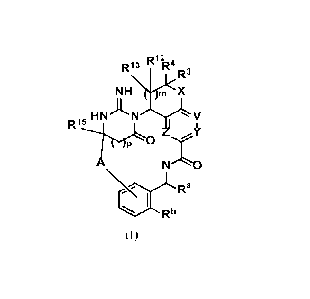
The present invention is directed to compounds of Formula (I):
R13 FQ12 R4R3
N HW-k< X
HNAN V
.5,1I
N'"1/4O
I Ra
(I)
wherein A, X, V, Y, Z, Ra, Rb, R3, R4, It', RI', 105, m and p are described
below.
Also described herein are methods of treatment of Plasmodium infections
comprising administering to a subject in need thereof a compound of Formula
(I), or a
pharmaceutically acceptable salt thereof. Also described herein are methods of
treatment of
Plasmodium infections comprising administering to a subject in need thereof a
compound of
Formula (I), or a pharmaceutically acceptable salt thereof, and a
pharmaceutically acceptable
carrier.
2
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
Also described herein are methods of treatment of malaria comprising
administering to a subject in need thereof a compound of Formula (I), or a
pharmaceutically
acceptable salt thereof.
The present invention further provides the use of compositions, including
pharmaceutical compositions, comprising one or more compounds of the invention
(e.g., one
compound of the invention), or a tautomer thereof, or a pharmaceutically
acceptable salt or
solvate of said compound(s) and/or said tautomer(s), optionally together with
one or more
additional therapeutic agents, optionally in an acceptable (e.g.,
pharmaceutically acceptable)
carrier or diluent, for the treatment of malaria.
Moreover, the present invention provides methods for the use of pharmaceutical
compositions comprising one or more of said compounds in the free form or in
pharmaceutically
acceptable salt form, together with one or more customary pharmaceutical
excipient(s), for the
treatment of Plasmodium infections, the treatment of malaria, the inhibition
of plasmepsin X, or
the dual inhibition of plasmepsin X and plasmepsin IX. Methods for the use of
combinations of
the compounds or salts of the invention together with one or more additional
pharmaceutically
active agents are also provided.
The present invention further provides methods for the inhibition of
plasmepsin
X, or the dual inhibition of plasmepsin X and plasmepsin IX activity and of
treatment,
prevention, amelioration and/or delaying onset of diseases or disorders in
which the inhibition of
plasmepsin X and/or plasmepsin IX has or may have a therapeutic effect, e g ,
malaria
The present invention further provides methods for the inhibition of P.
falciparum
aspartic acid proteases. The present invention further provides methods for
blocking P.
fakiparum growth by inhibiting plasmepsin X. The present invention further
provides methods
for blocking P. .fakiparum growth by inhibiting both PMX and Plasmepsin IX.
The present invention further provides methods for the treatment of malaria by
inhibiting plasmepsin X. The present invention further provides methods for
the treatment of
malaria by inhibiting both PMX and Plasmepsin IX.
These and other embodiments of the invention, which are described in detail
below or will become clear to those of ordinary skill in the art, are included
within the scope of
the invention.
DETAILED DESCRIPTION OF THE INVENTION
Described herein are compounds having the structural Formula (I):
3
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
R13 R14 RR3
N H*\/=-=< X
R1J NCO
b Ra
Rb
(I)
wherein A is a straight or branched, saturated or unsaturated (C3-
Cio)alkylene, phenyl(C3-
Cio)alkylene or cycloalkyl(C3-Cio)alkylene comprising at least one -CH2-
group, wherein one or
more additional -CH2- groups in A are optionally and independently replaced
with a moiety
selected from the group consisting of 0, S, NR, CONR, NRCO, S02, and SO2NR and
wherein
one or more of the hydrogens along A can be replaced with a group
independently selected from
hydroxyl, halogen and C1-3 haloalkyl,
X is a bond, C(R14)2, 0, S. SO, SO2 or NH;
Y is CR9 or N, wherein when Y is N, Z is CR11 and V is CR1 ;
V is CR1 or N, wherein when V is N, Z is CR11 and Y is CR9;
Z is CR" or N, wherein when Z is N, V is CR1 and Y is CR9,
R is hydrogen, C1-C6alkylCOOH, COOH, C3-C6cycloalkyl, C,-C6alkyl, haloCi-
C6alkyl, Ci-
C6alkylOH, COCi-C6alkyl or COOCi-C6alkyl;
Ra is hydrogen, halogen, CN, OH, C,-C6alkoxy, Ci-C6alkylOC1-C6alkyl, C,-
C6alkylCOOH,
COOH, oxo, COOC1-C6alkyl, C1-C6alkylCOOCi-C6alkyl, C3-C6cycloalkyl, C1-
C6alky1C3-
C6cycloalkyl, Ci-C6alkyl, -Ci-C6alkylOhaloCi-C6alkyl, haloCi-C6alkyl, Ci-
C6alkylOH,
CON(R7)(R8), N(R7)(R8) or C,-C6alkylN(R7)(1V) or when taken with Rb forms a C3-
C6cycloalkyl
or heterocycloalkyl, wherein the C3-C6cycl alkyl or heterocycloalkyl is
unsubstituted or
substituted with one or two substituents selected from the group consisting of
halogen, CN, OH,
Ci-C6alkoxy, C1-C6alkylOCi-Coalkyl, C,-C6alkylCOOH, COOH, oxo, COOCi-Coalkyl,
Ci-
C6alkylCOOCi-C6alkyl, C3-C6cycloalkyl, Ci-C6alky1C3-C6cycloalkyl, Ci-C6alkyl, -
Ci-
C6alkylOhaloCi-C6alkyl, haloCi-C6alkyl, C,-Coalky101-1, CON(R7)(R8), N(R7)(R8)
and Ci-
C6alkylN(R7)(R8),
Rb is hydrogen, halogen, CN, OH, CI-C6alkoxy, Ci-C6alkylOCI-C6alkyl, CI-
C6alkylCOOH,
COOH, oxo, COOCi-Coalkyl, C1-C6alkylCOOCi-C6alkyl, C3-C6cycloalkyl, Ci-
C6alky1C3-
4
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
C6cycloalkyl, Ci-C6alkyl, -Ci-C6a1ky1Oha1oCi-C6a1ky1, haloCi-C6alkyl, C1-
C6a1ky1OH,
CON(R7)(R8), N(R7)(R8) or CI-C6alkylN(R7)(R8) or when taken with Ra forms a C3-
C6cycloalkyl
or heterocycloalkyl, wherein the C3-C6cycloalkyl or heterocycloalkyl is
unsubstituted or
substituted with one or three sub stituents selected from the group consisting
of halogen, CN, OH,
Ci-C6a1koxy, C1-C6alky1OC1-C6alkyl, C1-C6alkylCOOH, COOH, oxo, COOCi-C6alkyl,
Ci-
C6alky1COOC1-C6alkyl, C3-C6cycloalkyl, Ci-C6alky1C3-C6cycloalkyl, Ci-C6alkyl, -
Ci-
C6a1ky1OhaloC1-C6alkyl, haloCi-C6alkyl, Ci-C6alkylOH, CON(R7)(R8), N(R7)(R8)
and Ci-
C6alkylN(R7)(R8);
R3 is hydrogen, halogen, CN, OH, Ci-Coalkoxy, Ci-CoalkylOCi-Coalkyl, Ci-
CoalkylCOOH,
COOH, C3-Cocycloalkyl, Ci-C6alkyl, haloCi-C6alkyl, Ci-C6alkylOH, CON(R7)(R8),
N(R7)(R8),
Ci-C6alkylN(R7)(R8), Ci-Coalkyl(OCH2CH2)nN(R7)(R8) or Ci-CoalkylOhaloCi-
Coalkyl or when
taken with R4 forms a C3-C6cycloalkyl or C3-C6heterocycloalkyl;
R4 is hydrogen, halogen, CN, OH, C1-C6alkoxy, CI-C6alkylOCi-C6alkyl, Ci-
C6alkylCOOH,
COOH, C3-C6cycloalkyl, CI-C6alkyl, haloCI-Coalkyl, CI-C6alkylOH, CON(R7)(R8),
N(R7)(R8),
Ci-C6alkylN(R7)(R8), Ci-C6alkyl(OCH2CH2)nN(R7)(R8) or Ci-C6alkylOhaloCi-
C6alkyl or when
taken with R3 forms a C3-C6cycloalkyl or C3-C6heterocycloalkyl;
R7 is hydrogen, Ci-C6alkylCOOH, COOH, C3-C6cycloalkyl, Ci-C6alkyl, haloCi-
C6alkyl, Ci-
C6a1ky1OH, COCi-C6alkyl or CO0C1-C6alkyl;
R8 is hydrogen, C1-C6alkylCOOH, COOH, C3-C6cycloalkyl, Ci-C6alkyl, haloCi-
C6alkyl, Ci-
C6alkylOH, COCi-C6alkyl or COOCI-C6alkyl;
R9 is hydrogen, halogen, CN, OH, Cl-C6alkoxy, CI-C6alkylOCi-C6alkyl, Ci-
C6alkylCOOH,
COOH, C3-C6cycloalkyl, CI-C6alkyl, haloCI-C6alkyl, CI-C6alkylOH, CON(R7)(R8),
N(R7)(R8)
or Ci-C6alkylN(R7)(R8);
R1 is hydrogen, halogen, CN, OH, C1-C6a1koxy, C1-C6alky1OC1-C6alkyl, Ci-
C6alkylCOOH,
COOH, C3-C6cycloalkyl, C1-C6alky1, haloCi-C6alkyl, Ci-C6a1kylOH, CON(R7)(R8),
N(R7)(R8)
or CI-C6alkylN(R7)(R8);
R11 is hydrogen, halogen, CN, OH, Ci-C6alkoxy, Ci-C6alkylOCi-C6alkyl, Ci-
C6alkylCOOH,
COOH, C3-Cocycloalkyl, C1-C6alky1, haloCi-C6a1kyl, Ci-C6alkylOH, CON(R7)(R8),
N(R7)(R8)
or Ci-C6alkylN(R7)(R8);
R12 is hydrogen, halogen, CN, OH, C1-C6a1koxy, C1-C6alky1OC1-C6alkyl, C1-
C6alkylCOOH,
COOH, C3-Cocycloalkyl, Ci-Coalkyl, haloCi-Coalkyl, Ci-CoalkylOH, CON(R7)(R8),
N(R7)(R8)
or Ci-CoalkylN(R7)(R8);
R13 is hydrogen, halogen, CN, OH, Ci-C6alkoxy, Ci-C6alkylOCi-C6alkyl, Ci-
C6alkylCOOH,
COOH, C3-C6cycloalkyl, Ci-C6alkyl, haloCi-C6alkyl, Ci-C6alkylOH, CON(R7)(R8),
N(R7)(R8)
or Ci-C6alkylN(R7)(R8);
5
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
each occurrence of R" is independently selected from the group consisting of
hydrogen,
halogen, CN, 01-1, CI-C6alkoxy, Ci-C6alky1OCI-C6alkyl, CI-C6alkylCOOH, COOH,
C3-
C6cycloalkyl, Ci-Coalkyl, haloCi-C6alkyl, Ci-C6alkylOH, CON(R7)(R8), N(R7)(R8)
and Ci-
C6alkylN(R7)(R8);
R15 is hydrogen, halogen, CN, OH, C1-C6alkoxy, Ci-C6alkylOCi-C6alkyl, C1-
C6alkylCOOH,
COOH, C3-C6cycloalkyl, Ci-C6alkyl, haloCi-C6alkyl, Ci-C6alkylOH, CON(R7)(R8),
N(R7)(R8)
or Ci-C6alkylN(R7)(R8);
each occurrence of R'6 is independently selected from the group consisting of
hydrogen,
halogen, CN, OH, Ci-C6alkoxy, C1-C6alkylOC1-C6alky1, CI-C6alkylCOOH, COOH,
C6cycloalkyl, Ci-C6alkyl, hal oCi-C6alkyl, Ci-CoalkylOH, CON(R7)(R8),
N(R7)(R8) and Ci-
C6alkylN(R7)(R8);
m is 0 or 1,
n is 1, 2, 3 or 4; and
p is 0 or I.
In the embodiments described herein, X is a bond, C(R14)2, 0, S, SO, SO2 or
NH.
In certain embodiments described herein X is a bond. In certain embodiments, X
is C(R14)2,
wherein R" is discussed in further detail below. In certain embodiments, X is
a bond, CH2,
CH(CH3), C(CH3)2, 0, CH(OCH3), SO2 or CF2. In other embodiments X is CH2, 0,
S, SO, SO2
or NH Tn certain embodiments, Xis CH2 In the embodiments described herein, Xis
0 Tn
certain embodiments described herein, X is S. In certain embodiments described
herein, X is SO.
In other embodiments described herein, X is SO2. In certain embodiments
described herein, X is
NH.
In the embodiments described herein, Y is CR9 or N. In certain embodiments, Y
is
CR9, wherein R9 is discussed in detail below. In certain embodiments, Y is N.
In certain
embodiments, Y is CH. In certain embodiments, wherein when Y is N, Z is CR"
and V is CR').
In the embodiments described herein, V is CRN or N. In certain embodiments, V
is CR19, wherein 10-9 is discussed in detail below. In certain embodiments, V
is N. In certain
embodiments, V is CH. In certain embodiments, wherein when V is N, Z is CR"
and Y is CR9.
In the embodiments described herein, Z is CR" or N. In certain embodiments, Z
is CR", wherein R" is discussed in detail below. In certain embodiments, Z is
CH. In certain
embodiments, Z is N. In certain embodiments, wherein when Z is N, V is CR1
and Y is CR9.
In certain embodiments, Xis 0, Y and V are each CH and Z is N. In certain
embodiments, X is 0, Y and Z are each CH and V is N. In certain embodiments, X
is 0 and V, Y
and Z are all simultaneously CH.
6
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
In the compounds described herein, Ra is hydrogen, halogen, CN, OH, Ci-
C6alkoxy, CI-C6alkylOCI-C6alkyl, CI-C6alkylCOOH, COOH, oxo, COOC1-C6alky1, CI-
C6alkylCOOCi-C6alkyl, C3-C6cycloalkyl, Ci-C6alky1C3-C6cycloalkyl, Ci-C6alkyl, -
Ci-
C6alkylOhaloCt-Coalkyl, haloCt-C6alkyl, Ci-C6alkylOH, CON(R7)(R8), N(R7)R8) or
Ci-
C6alkylN(R7)(R9) or when taken with Rb forms a C3-C6cycloalkyl or
heterocycloalkyl, wherein
the C3-C6cycloalkyl or heterocycloalkyl is unsubstituted or substituted with
one or two
sub stituents selected from the group consisting of halogen, CN, OH, Ci-
C6alkoxy, Ci-
C6alkylOCI-C6alkyl, CI-C6alkylCOOH, COOH, oxo, COOCt-C6alkyl, Ct-C6alkylCOOCI-
C6alkyl, C3-C6cycloalkyl, CI-CoalkylC3-Cocycloalkyl, Ci-Coalkyl, -C1-
CoalkylOhaloCt-Coalkyl,
haloCt-C6alkyl, C1-C6alkylOH, CON(R7)(R8), N(R7)(R8) and Ci-C6alkylN(R7)(R8).
In certain embodiments described herein, Ra is hydrogen.
In certain embodiments, Ra is halogen. Examples of suitable halogens include
chlorine, bromine, fluorine and iodine.
In certain embodiments, It is CN
In certain embodiments, Ra is OH.
In certain embodiments, Ra is Ci-C6alkoxy. Suitable alkoxys include, but are
not
limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy.
In certain embodiments, Ra is Ci-C6alkylOC1-C6alkyl.
In certain embodiments, Ra is Ci-C6alkylCOOH.
In certain embodiments, Ra is COOH
In certain embodiments, Ra is an oxo group.
In certain embodiments, Ra is COOCt-C6alkyl.
In certain embodiments, Ra is Ci-C6alkylCOOC1-Ccalkyl.
In certain embodiments, Ra is C3-C6cycloalkyl. Suitable examples of
cycloalkyls
include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl.
In certain embodiments, Ra is CI-C6alkylC3-C6cycloalkyl. Suitable examples of
cycloalkyls include, but are not limited to
In certain embodiments, Ra is Ci-C6alkyl. Examples of Ci-C6alkyl groups can
include but are not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl,
isobutyl, sec-butyl,
tert-butyl, n-pentyl, isopentyl, neopentyl, tert-pentyl, 1-methylbutyl, 2-
methylbutyl, 1,2-
dimethylpropyl, 1-ethylpropyl, n-hexyl, isohexyl, 1-methylpentyl, 2-
methylpentyl, 3-
methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1-
ethylbutyl, 1,1,2-
tri methyl propyl, 1,2,2-trim ethylpropyl, 1 -ethyl-2-m ethyl propyl and 1 -
ethyl -1 -methyl propyl. In
certain embodiments, IV is methyl.
7
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
In certain embodiments, Ra is Ci-C6alkylOhaloCi-C6alkyl. Suitable examples of
)(F
F
C1-C6alkylOhaloCi-C6alkyls include, but are not limited to,
In certain embodiments, R is hal oCt-C6alkyl. Suitable examples of haloalkyls
include, but are not limited to, fluoromethyl, difluoromethyl,
trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and 2,2-difluoroethyl.
In certain embodiments, Ra is Ci-CoalkylOH. Suitable alcohols include, but are
not limited to, methanol, ethanol, propanol, butanol and iso-butanol.
In certain embodiments, Ra is CON(R7)(R8). In certain embodiments, Ra is
N(R7)(R8). In certain embodiments, Ra is Ci-C6a1kylN(R7)(Rg), wherein R7 and
R8 will be
described in detail below.
In certain embodiments, the compounds described herein have the Formula (II):
R13 Ri2 R4 R3
NH m X
HNAN
N =
14111 (II)
IS In certain embodiments, Ra is taken with Rb and forms a C3-
C6cycloalkyl or
heterocycloalkyl, wherein the C 3 - C6cycloalkyl or heterocycloalkyl is
unsubstituted or substituted
with one or two substituents selected from the group consisting of halogen,
CN, OH, Ci-
C6alkoxy, Ci-C6alkylOC1-C6alkyl, C1-C6alkylCOOH, COOH, oxo, COOC1-C6alkyl, Ci-
C6alkylCOOCI-C6alkyl, C3-C6cycloalkyl, Ct-C6alky1C3-C6cycloalkyl, C1-C6alkyl, -
CI-
C6alkylOhaloCt-C6alkyl, haloCi-C6alkyl, CI-C6alkylOH, CON(R7)(R8), N(R7)(1e)
and Ci-
CoalkylN(R7)(R8).
In certain embodiments, Ra is taken with Rb and forms a C3-C6cycloalkyl,
wherein
the cycloalkyl is unsubstituted or substituted with one or two sub stituents
selected from the
group consisting of halogen, CN, OH, C1-C6alkoxy, C1-C6alkylOC1-C6alkyl, C1-
C6alkylCOOH,
8
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
COOH, oxo, COOC1-C6a1kyl, C1-C6a1ky1COOC1-C6alkyl, C3-C6cycloalkyl, C1-
C6alky1C3-
C6cycloalky1, C1-C6alkyl, -CI-C6a1kylOhaloC1-C6a1kyl, haloCi-Coalkyl, CI-
C6alkylOH,
CON(R7)(R8), N(R7)(R8) and Ci-C6alkylN(R7)(R8).
Suitable examples of cycloalkyls include, but are not limited to, cyclopropyl,
cyclobutyl, cyclopentyl and cyclohexyl.
In certain embodiments, the cycloalkyl is unsubstituted. In certain
embodiments,
the heterocycloalkyl is substituted with one or two substituents selected from
the group
consisting of halogen, CN, OH, CI-C6alkoxy, C1-
C6alkylCOOH,
COOH, oxo, COOCI-Coalkyl, Ci-CoalkylCOOCI-Coalkyl, C3-Cocycloalkyl, C1-
C6alky1C3-
C6cycloalky1, Ci-C6alkyl, -C1-CoalkylOhaloCl-Coalkyl, haloCi-C6alkyl, C1-
C6alkylOH,
CON(R7)(R8), N(R7)(R8) and CI-CoalkylN(R7)(R8).
In certain embodiments, the cycloalkyl is substituted with one substituent
selected
from the group consisting of halogen, CN, OH, C1-C6alkoxy, C1-C6alkylOC1-
C6alkyl, Ci-
C6alky1COOH, COOH, oxo, COOCI-C6alkyl, CI-C6alkylCOOCI-C6alkyl, C3-
C6cycloalkyl, CI-
C6alky1C3-C6cycloalkyl, Ci-C6alkyl, -Ci-C6alkylOhaloCi-C6alkyl, haloCi-
C6alkyl, Ci-
C6alkylOH, CON(R7)(R8), N(R7)(R8) and C1-C6a1kylN(R7)(R8).
In certain embodiments, the cycloalkyl is substituted with two substituents
selected from the group consisting of halogen, CN, OH, Ci-C6alkoxy, Ci-
C6alkylOCi-C6alkyl,
Cl-C6alkylCOOH, COOH, oxo, COOCi-C6alkyl, Ci-C6alkylCOOC1-C6alkyl, C3-
C6cycloalkyl,
CI-C6alkylC3-C6cycloalkyl, CI-C6alkyl, haloCt-C6alkyl, CI-
C6alky1OH, CON(R7)(R8), N(R7)(R8) and Ci-C6a1kylN(R7)(R8).
In certain embodiments, the cycloalkyl is substituted with OH.
In certain embodiments, It is taken with Rb and forms a heterocycloalkyl,
wherein the heterocycloalkyl is unsubstituted or substituted with one or two
substituents selected
from the group consisting of halogen, CN, OH, Ci-C6alkoxy, Ci-C6alkylOCi-
C6alkyl, Ci-
C6alky1COOH, COOH, oxo, COOCI-C6alkyl, CI-C6alkylCOOCI-C6alkyl, C3-
C6cycloalky1, CI-
C6alky1C3-C6cycloalkyl, Ci-C6alkyl, -Ci-C6alkylOhaloCi-C6alkyl, haloCi-
C6alkyl, Ci-
C6alkylOH, CON(R7)(R8), N(R7)(R8) and Ci-C6alkylN(R7)(10.
Non-limiting examples of monocyclic heterocycloalkyl groups include piperidyl,
oxetanyl, pyrrolyl, piperazinyl, morpholinyl, thiomorpholinyl, thiazolidinyl,
1,4-dioxanyl,
tetrahydrofuranyl, tetrahydrothiophenyl, beta lactam, gamma lactam, delta
lactam, beta lactone,
gamma lactone, delta lactone, and pyrrolidinone, and oxides thereof. Non-
limiting examples of
heterocycloalkyl groups include, but are not limited to,
9
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
c0) 0) 0
and
Non-limiting examples of bicyclic heterocycloalkyl groups include, but are not
limited to,
c0) 0
and
In certain embodiments, Ra is taken with Rb and forms:
0.
In certain embodiments, the heterocycloalkyl is unsubstituted. In certain
embodiments, the heterocycloalkyl is substituted with one or two substituents
selected from the
group consisting of halogen, CN, OH, Ci-C6alkoxy, C1-C6alkyl0C1-C6alkyl, Ci-
C6alkylCOOH,
COOH, oxo, COOCi-Coalkyl, Ci-C6alkylCOOCi-C6alkyl, C3-C6cycloalkyl, Ci-
C6a1ky1C3-
C6cycloalkyl, CI-Coalkyl, -Ct-CoalkylOhaloCt-Coalkyl, haloCt-Coalkyl, CI-
C6alkylOH,
CON(R7)(R8), N(R7)(R8) and Ct-C6alkylN(R7)(1e).
In certain embodiments, the heterocycloalkyl is substituted with one
substituent
selected from the group consisting of halogen, CN, OH, Cl-C6alkoxy, Cl-
C6alkylOCt-C6alkyl,
Ci-C6alkylCOOH, COOH, oxo, COOCi-Coalkyl, Ci-C6alkylCOOCI-C6alkyl, C3-
C6cycloalky1,
Ct-C6alky1C3-C6cycloalkyl, Ci-C6alkyl, -C1-C6alkylOhaloCt-Coalkyl, haloCt-
C6alkyl, Ct-
C6alkylOH, CON(R7)(R8), N(R7)(R8) and Ci-C6alkylN(R7)(R8).
In certain embodiments, the heterocycloalkyl is substituted with two
substituents
selected from the group consisting of halogen, CN, OH, Ci-C6alkoxy, Ci-
C6alkylOCi-C6alkyl,
Ci-C6alkylCOOH, COOH, oxo, COOCi-C6alkyl, Ci-C6alkylCOOCI-C6alkyl, C3-
C6cycloalkyl,
Ct-C6alky1C3-C6cycloalkyl, Ci-C6alkyl, -C1-C6alkylOhaloCt-Coalkyl, haloCt-
C6alkyl, Ct-
C6alkylOH, CON(R7)(R8), N(R7)(R8) and Ci-C6alkylN(R7)(R8).
In certain embodiments, the heterocycloalkyl is substituted with two
substituents
selected from the group consisting of CI-C6alky1.
In the compounds described herein, Rb is hydrogen, halogen, CN, OH, C1-
C6alkoxy, Ci-C6alkyl0C1-C6alkyl, Ci-Coalky1C001-1, COOH, oxo, COOC1-C6alkyl,
Ci-
C6alkylCOOCi-C6alkyl, C3-C6cycloalkyl, Ci-C6alky1C3-C6cycloalkyl, Ci-C6alkyl, -
Ci-
C6alkylOhaloCi-C6alkyl, haloCi-C6alkyl, Ci-C6alkylOH, CON(R7)(R8), N(R7)(R8)
or Ci-
C6alkylN(R7)(R9) or when taken with Ra forms a C3-C6cycloalky1 or
heterocycloalkyl, wherein
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
the C3-C6cycloalky1 or heterocycloalkyl is unsubstituted or substituted with
one or two
sub stituents selected from the group consisting of halogen, CN, OH, CI-
C6alkoxy, CI-
C6alkylOCt-C6alkyl, Ci-C6alkylCOOH, COOH, oxo, COOCI-C6alkyl, Cl-C6alkylCOOC1-
C6alkyl, C3-C6cycloalkyl, Ci-Coalky1C3-C6cycloalkyl, Ci-Coalkyl, -Ct-
CoalkylOhaloCt-C6alkyl,
haloCt-C6alkyl, C1-C6alkylOH, CON(R7)(R8), N(R7)(1V) and Ci-C6alkylN(R7)(1e).
In certain embodiments described herein, Rb is hydrogen.
In certain embodiments, Rb is halogen. Examples of suitable halogens include
chlorine, bromine, fluorine and iodine.
In certain embodiments, Rb is CN.
In certain embodiments, Rb is OH.
In certain embodiments, Rb is Ct-C6alkoxy. Suitable alkoxys include, but are
not
limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy.
In certain embodiments, RI' is Ct-C6alkyl0C1-C6alkyl.
In certain embodiments, Rb is Ct-C6alkylCOOH.
In certain embodiments, le is COOH.
In certain embodiments, Rb is an oxo group.
In certain embodiments, Rb is COOCt-C6alkyl.
In certain embodiments, Rb is CI-C6alkylCOOC1-C6alkyl.
In certain embodiments, Rb is C3-C6cycloalkyl. Suitable examples of
cycloalkyls
include, but are not limited to, cyclopropyl, cycl butyl, cyclopentyl and
cyclohexyl
In certain embodiments, Rb is CI-C6a1ky1C3-C6cycloalkyl. Suitable examples of
cycloalkyls include, but are not limited to
In certain embodiments, Rb is Ct-C6alkyl. Examples of Ci-C6alkyl groups can
include but are not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl,
isobutyl, sec-butyl,
tert-butyl, n-pentyl, isopentyl, neopentyl, tert-pentyl, 1-methylbutyl, 2-
methylbutyl, 1,2-
dimethylpropyl, 1-ethylpropyl, n-hexyl, isohexyl, 1-methylpentyl, 2-
methylpentyl, 3-
methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1-
ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethyl-2-methylpropyl and 1-ethyl-1-
methylpropyl. In
certain embodiments, Rb is methyl.
In certain embodiments, Rb is Ct-C6alkylOhaloCi-C6alkyl. Suitable examples of
Ci-C6alkylOhaloCt-C6alkyls include, but are not limited to, F
11
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
In certain embodiments, Rb is haloCt-C6alkyl. Suitable examples of haloalkyls
include, but are not limited to, fluoromethyl, difluoromethyl,
trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and 2,2-difluoroethyl.
In certain embodiments, Rb is CI-C6alkylOH. Suitable alcohols include, but are
not limited to, methanol, ethanol, propanol, butanol and iso-butanol.
In certain embodiments, Rb is CON(R7)(R8). In certain embodiments, kb is
N(R7)(R8). In certain embodiments, Rb is C1-C6alkylN(R7)(R8), wherein R7 and
R8 will be
described in detail below.
In certain embodiments, Rb is taken with Ra and forms a cycloalkyl or
heterocycloalkyl, wherein the cycloalkyl or heterocycloalkyl is unsubstituted
or substituted with
one or two substituents selected from the group consisting of halogen, CN, OH,
C t-C6alkoxy,
C1-C6alkyl0C1-C6alkyl, C1-C6alkylCOOH, COOH, oxo, COOC1-C6alkyl, CI-
C6alkylCOOC1-
C6alkyl, C3-C6cycloalkyl, C1-C6alky1C3-C6cycloalkyl, C1-C6alkyl, -C1-
C6alkylOhaloC1-C6alkyl,
haloCt-C6alkyl, C1-C6alkylOH, CON(R7)(R8), N(R7)(R8) and CI-C6alkylN(R7)(R8).
In certain embodiments, le is taken with Ra and forms a cycloalkyl, wherein
the
cycloalkyl is unsubstituted or substituted with one or two substituents
selected from the group
consisting of halogen, CN, OH, C1-C6alkoxy, C1-C6alkylOC1-C6alkyl, Ci-
C6alkylCOOH,
COOH, oxo, COOCt-C6alkyl, C1-C6alkylCOOC1-C6alkyl, C3-C6cycloalkyl, C1-
C6alkylC 3-
C6cycloalkyl, C1-C6alkyl, -Ct-C6alkylOhaloCt-C6alkyl, haloCi-C6alkyl, C1-
C6alkylOH,
CON(R7)(R8), N(R7)(R8) and CI-C6alkylN(R7)(R8)
Suitable examples of cycloalkyls include, but are not limited to, cyclopropyl,
cyclobutyl, cyclopentyl and cyclohexyl.
In certain embodiments, the cycloalkyl is unsubstituted. In certain
embodiments,
the heterocycloalkyl is substituted with one or two substituents selected from
the group
consisting of halogen, CN, OH, C1-C6alkoxy, C1-C6alkylOC1-C6alkyl, C1-
C6alkylCOOH,
COOH, oxo, COOCI-Coalkyl, CI-C6alkylCOOCI-C6alkyl, C3-Cocycloalkyl, CI-
C6alky1C3-
C6cycloalkyl, -Ct-C6alkylOhaloCt-C6alkyl, haloCt-C6alkyl, Ci-
C6alkylOH,
CON(R7)(R8), N(R7)(R8) and Ct-C6alkylN(R7)(R8).
In certain embodiments, the cycloalkyl is substituted with one substituent
selected
from the group consisting of halogen, CN, OH, C1-C6alkoxy, C1-C6alkylOC1-
C6alkyl,
C6alkylCOOH, COOH, oxo, COOCI-C6alkyl, CI-C6alkylCOOCI-C6alkyl, C3-
C6cycloalkyl,
Ci-
CoalkylC3-Cocycloalkyl, -CI-C6alkylOhaloCt-C6alkyl, haloCt-
C6alkyl, Ci-
C6alkylOH, CON(R7)(R8), N(R7)(R8) and C1-C6alkylN(R7)(R8)
In certain embodiments, the cycloalkyl is substituted with two substituents
12
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
selected from the group consisting of halogen, CN, OH, C1-C6alkoxy, C1-
C6alkylOCi-C6alkyl,
Ci-C6alkylCOOH, COOH, oxo, COOCi-C6alkyl, Ci-C6alkylCOOCi-C6alkyl, C3-
C6cycloalkyl,
Ci-C6alky1C3-C6cycloalkyl, Ci-C6alkyl, -Ci-C6alkylOhaloCi-C6alkyl, haloCi-
C6alkyl, CI-
C6alkylOH, CON(R7)(R8), N(R7)(R8) and C1-C6alkylN(R7)(R8).
In certain embodiments, the cycloalkyl is substituted with OH.
In certain embodiments, Rb is taken with Ra and forms a heterocycloalkyl,
wherein the heterocycloalkyl is unsubstituted or substituted with one or two
substituents selected
from the group consisting of halogen, CN, OH, C t-C6alkoxy, CI-C6alkylOCI-
C6alkyl, Ci-
C6alky1COOH, COOH, oxo, COOCI-Coalkyl, Ci-CoalkylCOOCI-Coalkyl, C3-
Cocycloalkyl, Ci-
C6a1ky1C3-C6cycloalkyl, C1-C6alkyl, -C1-C6alkylOhaloCi-C6alkyl, haloCi-
C6alkyl, Ci-
CoalkylOH, CON(R7)(R8), N(R7)(R8) and Ci-CoalkylN(R7)(R8).
Non-limiting examples of monocyclic heterocycloalkyl groups include piperidyl,
oxetanyl, pyrrolyl, piperazinyl, morpholinyl, thiomorpholinyl, thiazolidinyl,
1,4-dioxanyl,
tetrahydrofuranyl, tetrahydrothiophenyl, beta lactam, gamma lactam, delta
lactam, beta lactone,
gamma lactone, delta 'octane, and pyrrolidinone, and oxides thereof. Non-
limiting examples of
heterocycloalkyl groups include, but are not limited to,
OD a 0
and
Non-limiting examples of bicyclic heterocycloalkyl groups include, but are not
limited to,
and 0.
In certain embodiments, Rb is taken with Ra and forms:
a.
In certain embodiments, the cycloalkyl or heterocycloalkyl is unsubstituted.
In
certain embodiments, the cycloalkyl or heterocycloalkyl is substituted with
one or two
sub stituents selected from the group consisting of halogen, CN, OH, Ci-
C6alkoxy, Ci-
C6alkylOCi-C6alkyl, Ci-C6alkylCOOH, COOH, oxo, COOCi-C6a1kyl, C1-C6alkylCOOCi-
C6alky1, C3-C6cycloalkyl, Ci-C6alky1C3-C6cycloalkyl,
-Ci-C6alkylOhaloCi-C6alkyl,
haloCi-C6alkyl, Ci-C6alkylOH, CON(R7)(R8), N(R7)(R8) and Ci-C6alkylN(R7)(R8).
In certain
embodiments, the cycloalkyl or heterocycloalkyl is substituted with one
substituent selected
13
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
from the group consisting of halogen, CN, OH, C1-C6alkoxy, C1-C6alkyl0C1-
C6alkyl, Ci-
C6alky1COOH, COOH, oxo, COOC1-C6alkyl, CI-C6alkylCOOCI-C6alkyl, C3-
C6cycloalkyl, C1-
C6alky1C3-C6cycloalkyl, Ci-C6alkyl, -Ct-C6alkylOhaloCt-C6alkyl, haloCt-
C6alkyl, Ci-
C6alkylOH, CON(R7)(R8), N(R7)(R8) and C1-C6alkylN(R7)(R8). In certain
embodiments, the
cycloalkyl or heterocycloalkyl is substituted with two substituents selected
from the group
consisting of halogen, CN, OH, Ct-C6alkoxy, C1-C6alkyl0C1-C6alkyl, C1-
C6alkylCOOH,
COOH, oxo, COOC1-C6alkyl, C1-C6alkylCOOCi-C6alkyl, C3-C6cycloalkyl, C1-
C6alky1C3-
C6cycloalkyl, CI-C6alkyl, -Ct-C6alkylOhaloCt-C6alkyl, haloCi-C6alkyl, C1-
C6alkylOH,
CON(R7)(R8), N(R7)(R8) and Ct-CoalkylN(R7)(R8).
In certain embodiments, the heterocycloalkyl is substituted with two
substituents
selected from the group consisting of Ci-C6alkyl. In certain embodiments, the
cycloalkyl is
substituted with two substituents selected from the group consisting of Ct-
C6alkyl. In certain
embodiments, the cycloalkyl is unsubstituted.
In the embodiments described herein, 11_3 is hydrogen, halogen, CN, OH, Ci-
C6a1koxy, Ci-C6alkylOCi-C6alkyl, Ci-C6alkylCOOH, COOH, C3-C6cycloalkyl, Ci-
C6alkyl,
haloCi-C6alkyl, C1-C6alkylOH, CON(R7)(R8), N(117)(R8), Ci-C6alkylN(R7)(R8), Ci-
C6alkyl(OCH2CH2),,N(R7)(R8) or Ci-C6alkylOhaloCi-C6alkyl or when taken with R4
forms a C3-
C6cycloalkyl or C3-C6heterocycloalkyl.
In certain embodiments of the compounds described herein, IV is hydrogen,
halogen, CN, 01-1, CI-C6alkoxy, CI-C6alky1OCt-C6alkyl, Ct-C6alkylC0OH, COON,
C3-
C6cycloalkyl, Cl-C6alkyl, haloCt-C6alky1, Ci-C6alkylOH, CON(R7)(R8), N(R7)(R8)
or CI-
C6alkylN(R7)(11_8) or when taken with R4 forms a C3-C6cycloalkyl or C3-
C6heterocycloalkyl. In
certain embodiments, R3 is hydrogen. In certain embodiments, R3 is halogen.
Suitable halogens
include fluorine, chlorine, bromine, and iodine. In certain embodiments, R3 is
CN. In certain
embodiments, R3 is OH.
In certain embodiments, R3 is Ct-C6alkoxy. Suitable alkoxys include, but are
not
limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy. In certain
embodiments, R3 is
Ct-C6alkyl0C1-C6alkyl. In certain embodiments, IV is COOH. In certain
embodiments, R3 is Ci-
C6alkylCOOH. In certain embodiments, R3 is C3-C6cycloalkyl. Suitable examples
of cycloalkyls
include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl. In certain
embodiments, R3 is Ci-C6alkyl. Examples of Ci-C6alkyl groups can include but
are not limited
to, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-
butyl, n-pentyl, isopentyl,
neopentyl, tert-pentyl, 1-methylbutyl, 2-methylbutyl, 1,2-dimethylpropyl, 1 -
ethylpropyl, n-hexyl,
isohexyl, 1-methylpentyl, 2-methylpentyl, 3-methylpentyl, 1,1-dimethylbutyl,
1,2-dimethylbutyl,
14
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
2,2-dimethylbutyl, 1-ethylbutyl, 1,1,2-trimethylpropyl, 1,2,2-
trimethylpropyl, 1-ethyl-2-methylpropyl and 1-ethyl-1-methylpropyl. In certain
embodiments, R3
is haloCi-C6a1kyl. Suitable examples of haloalkyls include, but are not
limited to, fluoromethyl,
difluoromethyl, trifluoromethyl, 2-fluoroethyl, 1,2-difluoroethyl and 2,2-
difluoroethyl. In certain
embodiments, R3 is C1-C6alkylOH. Examples of suitable alcohols include, but
are not limited to,
methanol, ethanol, propanol, butanol and iso-butanol. In certain embodiments,
R3 is
CON(R7)(R8). Suitable examples of N(R7)(R8) include, but are not limited to,
CONH2 and
CON(CH3)2. In certain embodiments, R3 is N(R7)(R8). Suitable examples of
N(R7)(R8) include,
but are not limited to, NH2 and N(CH3)2. In certain embodiments, R3 is C t-
CoalkylN(R7)(R8).
Suitable examples of Ci-C6alkylN(R7)(R8) include, but are not limited to, 57"
0 0
0
and
. R7 and R8 are discussed in further detail
below.
In certain embodiments, R3 is CI-C6alkylOhaloCi-C6alkyl. Suitable examples of
)<F
`222.-"0 F
haloalkyls include, but are not limited to,
In certain embodiments, R3 is CI-C6alkyl(OCH2CH2),6N(R7)(R8). R7, R8 and n are
discussed in detail below. Suitable examples of C1-C6alkyl(OCH2CH2)11N(R7)(1e)
include, but
0
NH2
ro,.0
are not limited to, , and
0
o
With regard to the compounds described herein, n is 1, 2, 3 or 4. In certain
embodiments, n is 1. In certain embodiments, n is 2. In certain embodiments, n
is 3. In certain
embodiments, n is 4.
In certain embodiments, R3 is taken with R4 and forms a C3-C6cycloalkyl or C3-
C6heterocycloa1kyl. In certain embodiments, R3 is taken with R4 and forms a C3-
C6cycloalkyl.
Suitable examples of cycloalkyls include, but are not limited to, cyclopropyl,
cyclobutyl,
cyclopentyl and cyclohexyl. In certain embodiments, R3 is taken with R4 and
forms a C3-
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
C6heterocycloalkyl. Suitable examples of heterocycloalkyls include, but are
not limited to,
piperidyl, oxetanyl, pyrrolyl, piperazinyl, morpholinyl, thiomorpholinyl,
thiazolidinyl, 1,4-
dioxanyl, tetrahydrofuranyl, tetrahydrothiophenyl, beta lactam, gamma lactam,
delta lactam,
beta lactone, gamma lactone, delta lactone, and pyrrolidinone, and oxides
thereof.
In certain embodiments, It3 is hydrogen, fluorine, methyl, ethyl, OH, methoxy,
0
OH NH2 \.
CON(CH3)2,
0 k
F 0 N H2
µ2,/-=.0F
,
0 0
)*L
0
(3µ(o
or
In certain embodiments, R3 is hydrogen, methyl, ethyl or
In certain embodiments, R3 is taken with R4 to form oxetanyl.
In certain embodiments described herein, R4 is hydrogen, halogen, CN, OH, CI-
C6alkoxy, Ci-C6alkylOCi-C6alkyl, Ci-C6alkylCOOH, COOH, C3-C6cycloalkyl, Ci-
C6alkyl,
haloCi-C6alkyl, C1-C6alkylOH, CON(R7)(R8), N(R7)(R8), C1-C6alkylN(R7)(R8), Ci-
C6alkyl(OCH2CH2),,N(R7)(R8) or C1-C6alkylOhaloC1-C6alkyl or when taken with R3
forms a C3-
C6cycloalkyl or C3-C6heterocycloalkyl. In certain embodiments of the compounds
described
herein, R4 is hydrogen, halogen, CN, OH, CI-C6alkoxy, CI-
C6alkylCOOH, COOH, C3-C6cycloalkyl, Cl-C6alkyl, haloCi-C6alkyl, Ci-C6alkylOH,
CON(R7)(R8), N(R7)(R8) or Ci-C6alkylN(R7)(R8) or when taken with R3 forms a C3-
C6cycloalkyl
or C3-C6heterocycloalkyl. In certain embodiments, R4 is hydrogen. In certain
embodiments, R4 is
halogen. Suitable halogens include fluorine, chlorine, bromine, or iodine. In
certain
embodiments, R4 is CN. In certain embodiments, R4 is OH.
In certain embodiments, R4 is CI-C6alkoxy. Suitable alkoxys include, but are
not
limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy. In certain
embodiments, R4 is
Ci-C6alkylOCi-C6alkyl. In certain embodiments, R4 is COOH. In certain
embodiments, 10 is Ci-
C6alkylCOOH. In certain embodiments, R4 is C3-C6cycloalkyl. Suitable examples
of cycloalkyls
include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl. In certain
16
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
embodiments, R4 is C1-C6alkyl. Examples of C1-C6alkyl groups can include but
are not limited
to, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-
butyl, n-pentyl,
isopentyl, neopentyl, tert-pentyl, 1-methylbutyl, 2-methylbutyl, 1,2-
dimethylpropyl, 1-
ethylpropyl, n-hexyl, isohexyl, 1-methylpentyl, 2-methylpentyl, 3-
methylpentyl, 1,1-
dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1-ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-
trimethylpropyl, 1-ethyl-2-methylpropyl and 1-ethyl-1-methylpropyl. In certain
embodiments, R4
is haloCi-C6a1kyl. Suitable examples of haloalkyls include, but are not
limited to, fluoromethyl,
difluoromethyl, trifluoromethyl, 2-fluoroerhyl, 1,2-difluoroethyl and 2,2-
difluoroethyl. In certain
embodiments, R4 is Ci-C6alkylOH. Examples of suitable alcohols include, but
are not limited to,
methanol, ethanol, propanol, butanol and iso-butanol. In certain embodiments,
R4 is
CON(R7)(R8). Suitable examples of N(R7)(R8) include, but are not limited to,
CONH2 and
CON(CH3)2. In certain embodiments, R4 is N(R7)(R8). Suitable examples of
N(R7)(R8) include,
but are not limited to, N112 and N(CH3)2. In certain embodiments, R4 is Ci-
C6alkylN(R7)(R8).
NH2
Suitable examples of C1-C6alkylN(R7)(R8) include, but are not limited to,
0 0
N
and . R7 and R8 are
discussed in further detail
below.
In certain embodiments, R4 is CI-C6alkylOhaloCi-C6alkyl. Suitable examples of
)..F
F
haloalkyls include, but are not limited to,
In certain embodiments, R4 is CI-C6alkyl(OCH2CH2),1N(R7)(R8). R7, R8 are
discussed in detail below and n is discussed above. Suitable examples of Ci-
r. o NH 2
C6alky1(OCH2CH2)11N(R7)(R8) include, but are not limited to,
0 0
000
=-=..õ--""'"N 0
, and
In certain embodiments, R4 is taken with R3 and forms a C3-C6cycloalkyl or C 3
-
C6heterocycloalkyl. In certain embodiments, R4 is taken with R3 and forms a C3-
C6cycloalkyl.
Suitable examples of cycloalkyls include, but are not limited to, cyclopropyl,
cyclobutyl,
cyclopentyl and cyclohexyl. In certain embodiments, R4 is taken with R3 and
forms a C 3 -
17
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
C6heterocycloalkyl. Suitable examples of heterocycloalkyls include, but are
not limited to,
piperidyl, oxetanyl, pyrrolyl, piperazinyl, morpholinyl, thiomorpholinyl,
thiazolidinyl, 1,4-
dioxanyl, tetrahydrofuranyl, tetrahydrothiophenyl, beta lactam, gamma lactam,
delta lactam,
beta lactone, gamma lactone, delta lactone, and pyrrolidinone, and oxides
thereof.
In certain embodiments, R4 is hydrogen or methyl. In certain embodiments, R4
is
hydrogen, methyl, ethyl or . In certain embodiments, R4 is taken
with R3 to form
oxetanyl. In certain embodiments, R3 and R4 are both hydrogen, methyl or
ethyl.
In certain embodiments, R3 is hydrogen and R4 is hydrogen.
In certain embodiments, R3 and R4 are both halogen, where the halogen is
selected from fluorine, chlorine, bromine and iodine. In certain embodiments,
R3 and R4 are both
fluorine.
In the embodiments described herein, R5 is hydrogen, halogen, CN, OH, Ci-
C6alkoxy, C1-C6alkylOC1-C6alkyl, C1-C6alkylCOOH, COOH, oxo, COOC1-C6alkyl, Ci-
C6alkylCOOC1-C6alkyl, C3-C6cycloalkyl, Ct-C6alky1C3-C6cycloalkyl, C1-C6alkyl, -
Ci-
CoalkylOhaloCi-Coalkyl, haloCi-Coalkyl, Ci-CoalkylOH, CON(R7)(R8), N(R7)(R8)
or CI-
C6alkylN(R7)(R8).
In certain embodiments described herein, R5 is hydrogen.
In certain embodiments, R5 is halogen. Examples of suitable halogens include
chlorine, bromine, fluorine and iodine.
In certain embodiments, R5 is CN.
In certain embodiments, R5 is OH.
In certain embodiments, R5 is Ct-C6alkoxy. Suitable alkoxys include, but are
not
limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy.
In certain embodiments, R5 is Ci-CoalkylOCi-C6alkyl.
5 In certain embodiments, R5 is Ct-C6alkylCOOH.
In certain embodiments, R5 is COOH.
In certain embodiments, R5 is an oxo group.
In certain embodiments, R5 is COOCt-C6alkyl.
In certain embodiments, R5 is Ct-C6alkylCOOCi-C6alkyl.
In certain embodiments, R5 is C3-C6cycloalkyl. Suitable examples of
cycloalkyls
include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl.
In certain embodiments, R5 is Ct-C6alky1C3-C6cycloalkyl. Suitable examples of
cycloalkyls include, but are not limited to .
18
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
In certain embodiments, R5 is CI-C6alkyl. Examples of C1-C6alkyl groups can
include but are not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl,
isobutyl, sec-butyl,
tert-butyl, n-pentyl, isopentyl, neopentyl, tert-pentyl, 1-methylbutyl, 2-
methylbutyl, 1,2-
dimethylpropyl, 1-ethylpropyl, n-hexyl, isohexyl, 1-methylpentyl, 2-
methylpentyl, 3-
methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1-
ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethyl-2-methylpropyl and 1-ethyl-1-
methylpropyl. In
certain embodiments, R5 is methyl.
In certain embodiments, R5 is Ct-C6alkylOhaloCt-C6alkyl. Suitable examples of
FF
"ear--0 F
Ct-C6alkylOhaloCt-C6alkyls include, but are not limited to,
In certain embodiments, R5 is haloCi-C6alkyl. Suitable examples of haloalkyls
include, but are not limited to, fluoromethyl, difluoromethyl,
trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and 2,2-difluoroethyl.
In certain embodiments, R5 is CI-C6alkylOH. Suitable alcohols include, but are
not limited to, methanol, ethanol, propanol, butanol and iso-butanol.
In certain embodiments, R5 is CON(R7)(R8). In certain embodiments, RI- is
N(R7)(R8). In certain embodiments, R5 is Ci-C6alkylN(R7)(R8), wherein R7 and
Rg will be
described in detail below.
In certain embodiments, R5 is hydrogen, methyl, ethyl or t-butyl.
In the embodiments described herein, R6 is hydrogen, halogen, CN, OH, Ci-
C6alkoxy, C1-C6alkylCOOH, COOH, oxo, COOCt-C6alkyl, Ci-
C6alkylCOOCI-C6alkyl, C3-C6cycloalkyl, Ct-C6alky1C3-C6cycloalkyl, C1-Coalkyl, -
CI-
C6alkylOhaloCt-C6alkyl, haloCt-C6alkyl, C1-C6alkylOH, CON(R7)(R8), N(R7)(R8)
or Cl-
C6alkylN(R7)(10)
In certain embodiments described herein, R6 is hydrogen.
In certain embodiments, R6 is halogen. Examples of suitable halogens include
chlorine, bromine, fluorine and iodine.
In certain embodiments, R6 is CN.
In certain embodiments, R6 is OH.
In certain embodiments, R6 is CI-C6alkoxy. Suitable alkoxys include, but are
not
limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy.
In certain embodiments, R6 is Cl-C6alkylOCi-C6alkyl.
In certain embodiments, R6 is Cl-C6alkylCOOH.
19
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
In certain embodiments, R6 is COOH.
In certain embodiments, R6 is an oxo group.
In certain embodiments, R6 is COOCi-C6alkyl.
In certain embodiments, R6 is CI-C6alkylCOOC1-Coalkyl.
In certain embodiments, R6 is C3-C6cycloalkyl. Suitable examples of
cycloalkyls
include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl.
In certain embodiments, R6 is CI-C6alky1C3-C6cycloalkyl. Suitable examples of
cycloalkyls include, but are not limited to .
In certain embodiments, R6 is CI-C6alkyl. Examples of C1-C6alkyl groups can
include but are not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl,
isobutyl, sec-butyl,
tert-butyl, n-pentyl, isopentyl, neopentyl, tert-pentyl, 1-methylbutyl, 2-
methylbutyl, 1,2-
dimethylpropyl, 1-ethylpropyl, n-hexyl, isohexyl, 1-methylpentyl, 2-
methylpentyl, 3-
methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1-
ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethyl-2-methylpropyl and 1-ethyl-l-
methylpropyl In
certain embodiments, R6 is methyl.
In certain embodiments, R6 is CI-C6alkylOhaloCi-C6alkyl. Suitable examples of
rk.F
F
C1-C6alkylOhaloC1-C6alkyls include, but are not limited to,
In certain embodiments, R6 is haloCi-C6alkyl. Suitable examples of haloalkyls
include, but are not limited to, fluoromethyl, difluoromethyl,
trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and 2,2-difluoroethyl.
In certain embodiments, R6 is CI-C6alkylOH. Suitable alcohols include, but are
not limited to, methanol, ethanol, propanol, butanol and iso-butanol.
In certain embodiments, R6 is CON(R7)(R8). In certain embodiments, RI- is
N(R7)(10). In certain embodiments, R6 is Ci-C6alkylN(R7)(10), wherein It7 and
It8 will be
described in detail below.
In certain embodiments, R6 is hydrogen, methyl, ethyl or t-butyl.
In the embodiments described herein, R7 is hydrogen, Ci-CoalkylCOOH, COOH,
C3-C6cycloalkyl, C1-C6alkyl, haloCA-C6alkyl, Ci-C6alkylOH, COCI-C6alkyl or
COOC1-C6alkyl.
In certain embodiments, IC is hydrogen, Ci-C6alkylCOOH, COOH, C3-C6cycloalkyl,
C1-C6alkyl,
haloCi -C6alkyl or C -C6alkyl OH.
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
In certain embodiments, R7 is hydrogen. In certain embodiments, R7 is Ci-
C6alkylCOOH. In certain embodiments, 117 is COOH. In certain embodiments, R7
is C3-
C6cycloalky1. Suitable examples of cycloalkyls include, but are not limited
to, cyclopropyl,
cyclobutyl, cyclopentyl and cyclohexyl. In certain embodiments, R7 is C1-
C6alkyl. Examples of
Ct-C6alkyl groups can include but are not limited to, methyl, ethyl, n-propyl,
isopropyl, n-butyl,
isobutyl, sec-butyl, tert-butyl, n-pentyl, isopentyl, neopentyl, tert-pentyl,
1-methylbutyl, 2-
methylbutyl, 1,2-dimethylpropyl, 1-ethylpropyl, n-hexyl, isohexyl, 1-
methylpentyl, 2-
methylpentyl, 3-methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-
dimethylbutyl, 1-
ethylbutyl, 1,1,2-trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethyl-2-
methylpropyl and 1-ethy1-1-
methylpropyl. In certain embodiments, R7 is haloCi-C6alkyl. Suitable examples
of haloalkyls
include, but are not limited to, fluoromethyl, difluoromethyl, trifluorom
ethyl, 2-fluoroethyl, 1,2-
difluoroethyl and 2,2-difluoroethyl. In certain embodiments, R7 is C1-
C6a1kylOH. Examples of
suitable alcohols include, but are not limited to, methanol, ethanol,
propanol, butanol and iso-
butanol.
In certain embodiments, R7 is COCi-C6alkyl. Suitable examples include, but are
not limited to, COCH3. In certain embodiments, R7 is COOC1-C6alkyl. Suitable
examples
include, but are not limited to, COOCH3.
In the embodiments described herein, R8 is hydrogen, C1-C6alkylCOOH, COOH,
C3-C6cycloalkyl, Ci-C6alkyl, haloCi-C6alkyl, C1-C6alkylOH, COC1-C6alkyl or
COOCi-C6alkyl.
In certain embodiments, R8 is hydrogen, CI-C6a1kylCOOH, COOH, C3-C6cycloalkyl,
CI-C6alkyl,
haloCi -C6alkyl or C -C6alkyl OH.
In certain embodiments, R8 is hydrogen. In certain embodiments, R8 is CI-
C6alkylCOOH. In certain embodiments, R8 is COOH. In certain embodiments, R8 is
C3-
C6cycloalky1. Suitable examples of cycloalkyls include, but are not limited
to, cyclopropyl,
cyclobutyl, cyclopentyl and cyclohexyl. In certain embodiments, R8 is C1-
C6alkyl. Examples of
CI-C6alkyl groups can include but are not limited to, methyl, ethyl, n-propyl,
isopropyl, n-butyl,
isobutyl, sec-butyl, tert-butyl, n-pentyl, isopentyl, neopentyl, tert-pentyl,
1-methylbutyl, 2-
methylbutyl, 1,2-dimethylpropyl, 1-ethylpropyl, n-hexyl, isohexyl, 1-
methylpentyl, 2-
methylpentyl, 3-methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-
dimethylbutyl, 1-
ethylbutyl, 1,1,2-trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethy1-2-
methylpropyl and 1-ethyl-l-
methylpropyl. In certain embodiments, R8 is haloCi-C6alkyl. Suitable examples
of haloalkyls
include, but are not limited to, fluoromethyl, difluoromethyl,
trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and 2,2-difluoroethyl In certain embodiments, R8 is Ci-
C6alkylOH. Examples of
suitable alcohols include, but are not limited to, methanol, ethanol,
propanol, butanol and iso-
butanol.
21
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
In certain embodiments, R8 is COCt-C6alkyl. Suitable examples include, but are
not limited to, COCH3. In certain embodiments, R8 is COOCt-C6alkyl. Suitable
examples
include, but are not limited to, COOCH3
In the embodiments described herein, R9 is hydrogen, halogen, CN, OH, Ci-
C6alkoxy, C1-C6alkylOC1-C6alkyl, C1-C6alkylCOOH, COOH, C3-C6cycloalkyl, C1-
C6alkyl,
haloCi-C6alkyl, C1-C6alkylOH, CON(R7)(R8) and N(R7)(R8). In certain
embodiments, R9 is
hydrogen. In certain embodiments, R9 is halogen. Suitable halogens include
fluorine, chlorine,
bromine, or iodine. In certain embodiments, R9 is CN. In certain embodiments,
R9 is OH.
In certain embodiments, R9 is Ci-C6alkoxy. Suitable alkoxys include, but are
not
limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy. In certain
embodiments, R9 is
Ci-CGalkyl OCi-Coalkyl. In certain embodiments, R9 is COOH. In certain
embodiments, R9 is Ci-
C6alkylCOOH. In certain embodiments, R9 is C3-C6cycloalkyl. Suitable examples
of cycloalkyls
include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl. In certain
embodiments, R9 is CI-C6alkyl. Examples of CA-C6alkyl groups can include but
are not limited
to, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-
butyl, n-pentyl, isopentyl,
neopentyl, tert-pentyl, 1-methylbutyl, 2-methylbutyl, 1,2-dimethylpropyl, 1-
ethylpropyl, n-hexyl,
isohexyl, 1-methylpentyl, 2-methylpentyl, 3-methylpentyl, 1,1-dimethylbutyl,
1,2-dimethylbutyl,
2,2-dimethylbutyl, 1-ethylbutyl, 1,1,2-trimethylpropyl, 1,2,2-trimethylpropyl,
1-ethy1-2-
methylpropyl and 1-ethyl-l-methylpropyl. In certain embodiments, R9 is haloCi-
C6alkyl.
Suitable examples of haloalkyls include, but are not limited to, fluoromethyl,
difluoromethyl,
trifluoromethyl, 2-fluoroethyl, 1,2-difluoroethyl and 2,2-difluoroethyl. In
certain embodiments,
R9 is CI-C6alkylOH. Examples of suitable alcohols include, but are not limited
to, methanol,
ethanol, propanol, butanol and iso-butanol. In certain embodiments, R9 is
CON(R7)(R8). In
certain embodiments, R9 is N(R7)(R8). In certain embodiments, R9 is C t-
C6alkylN(R7)(R8).
With regard to the compounds described herein, Ri is hydrogen, halogen, CN,
OH, CI-C6alkoxy, Ci-C6alkylOCI-C6alkyl, Ci-C6alkylCOOH, COOH, C3-C6cycloalkyl,
CI-
C6alkyl, haloCi-C6alkyl, CI-C6alkylOH, CON(R7)(R8) and N(R7)(R8). In certain
embodiments,
Ri is hydrogen. In certain embodiments, Ri is halogen. Suitable halogens
include fluorine,
chlorine, bromine, or iodine. In certain embodiments, Ri is CN. In certain
embodiments, Ri is
OH.
In certain embodiments, Ri is Ci-C6alkoxy. Suitable alkoxys include, but are
not
limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy. In certain
embodiments, Ri
is CI-C6alkylOCA-C6alkyl. In certain embodiments, RI is COOH. In certain
embodiments, RI is
Ci-C6alkylCOOH. In certain embodiments, Rio is C3-C6cycloalkyl. Suitable
examples of
22
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
cycloalkyls include, but are not limited to, cyclopropyl, cyclobutyl,
cyclopentyl and cyclohexyl.
In certain embodiments, R1 is CI-Coalkyl. Examples of CI-C6alky1 groups can
include but are
not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-
butyl, tert-butyl, n-pentyl,
isopentyl, neopentyl, tert-pentyl, 1-methylbutyl, 2-methylbutyl, 1,2-
dimethylpropyl, 1-
ethylpropyl, n-hexyl, isohexyl, 1-methylpentyl, 2-methylpentyl, 3-
methylpentyl, 1,1-
dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1-ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-
trimethylpropyl, 1-ethyl-2-methylpropyl and 1-ethyl-l-methylpropyl. In certain
embodiments,
R1 is haloCi-C6alkyl. Suitable examples of haloalkyls include, but are not
limited to,
fluoromethyl, difluoromethyl, trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and 2,2-
difluoroethyl. In certain embodiments, R1 is C1-C6alkylOH. Examples of
suitable alcohols
include, but are not limited to, methanol, ethanol, propanol, butanol and iso-
butanol. In certain
embodiments, R1 is CON(R7)(R8). In certain embodiments, R1 is N(R7)(R8). In
certain
embodiments, R1 is C1-C6alkylN(R7)(R8).
In the embodiments, described herein, R11 is hydrogen, halogen, CN, OH, C1-
C6alkoxy, Ci-C6alkylOC1-C6alkyl, CI-C6alkylCOOH, COOH, C3-C6cycloalkyl, CI-
C6alkyl,
haloCi-C6alkyl, C1-C6alkylOH, CON(R7)(R8) and N(R7)(R8). In certain
embodiments, R11 is
hydrogen. In certain embodiments, R11 is halogen. Suitable halogens include
fluorine, chlorine,
bromine, or iodine. In certain embodiments, R11 is CN. In certain embodiments,
R11 is OH.
In certain embodiments, R11 is C1-C6alkoxy. Suitable alkoxys include, but are
not
limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy. Tn certain
embodiments, R"
is CI-C6alkylOC1-C6alkyl. In certain embodiments, R11 is COOH. In certain
embodiments, RIA is
CI-C6alkylCOOH. In certain embodiments, Rll is C3-C6cycloalkyl. Suitable
examples of
cycloalkyls include, but are not limited to, cyclopropyl, cyclobutyl,
cyclopentyl and cyclohexyl.
In certain embodiments, R11 is C1-C6alkyl. Examples of C1-C6alky1 groups can
include but are
not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-
butyl, tert-butyl, n-pentyl,
isopentyl, neopentyl, tert-pentyl, 1-methylbutyl, 2-methylbutyl, 1,2-
dimethylpropyl, 1-
ethylpropyl, n-hexyl, isohexyl, 1-methylpentyl, 2-methylpentyl, 3-
methylpentyl, 1,1-
dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1-ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-
trimethylpropyl, 1-ethyl-2-methylpropyl and 1-ethyl-l-methylpropyl. In certain
embodiments,
R11 is haloCi-C6alky1. Suitable examples of haloalkyls include, but are not
limited to,
fluoromethyl, difluoromethyl, trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and 2,2-
difluoroethyl. In certain embodiments, R11 is CI-CoalkylOH. Examples of
suitable alcohols
include, but are not limited to, methanol, ethanol, propanol, butanol and iso-
butanol. In certain
embodiments, R11 is CON(R7)(R8). In certain embodiments, R11 is N(R7)(R8). In
certain
23
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
embodiments, R" is C1-C6alkylN(R7)(R8).
In the embodiments described herein, R12 is hydrogen, halogen, CN, OH, CI-
C6alkoxy, Ci-C6alkylOCi-C6alkyl, Ci-C6alkylCOOH, COOH, C3-C6cycloalkyl, Ci-
C6alkyl,
haloCi-C6alkyl, C1-C6alkylOH, CON(R7)(R8), N(R7)(R8) or C1-C6alkylN(R7)(R8).
In certain
embodiments, R12 is hydrogen. In certain embodiments, R12 is halogen. Suitable
halogens
include fluorine, chlorine, bromine, or iodine. In certain embodiments, R12 is
CN. In certain
embodiments, R12 is OH.
In certain embodiments, R12 is CI-C6alkoxy. Suitable alkoxy groups include,
but
are not limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy. In
certain
embodiments, R12 is Cl-C6alkyl0C1-C6alkyl. In certain embodiments, R12 is
COOH. In certain
embodiments, R12 is C1-C6alkylCOOH. In certain embodiments, R12 is C3-
C6cycloalkyl. Suitable
examples of cycloalkyls include, but are not limited to, cyclopropyl,
cyclobutyl, cyclopentyl and
cyclohexyl. In certain embodiments, R12 is C1-C6alkyl. Examples of C1-C6alkyl
groups can
include but are not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl,
isobutyl, sec-butyl,
tert-butyl, n-pentyl, isopentyl, neopentyl, tert-pentyl, 1-methylbutyl, 2-
methylbutyl, 1,2-
dimethylpropyl, 1-ethylpropyl, n-hexyl, isohexyl, 1-methylpentyl, 2-
methylpentyl, 3-
methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1-
ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethyl-2-methylpropyl and 1-ethyl-1-
methylpropyl. In
certain embodiments, R12 is haloCi-C6alkyl. Suitable examples of haloalkyls
include, but are not
limited to, fluoromefhyl, difluoromethyl, trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and
2,2-difluoroethyl. In certain embodiments, R12 is Ci-C6a1kylOH. Examples of
suitable alcohols
include, but are not limited to, methanol, ethanol, propanol, butanol and iso-
butanol. In certain
embodiments, R12 is CON(R7)(R8). In certain embodiments, R12 is N(R7)(R8). In
certain
embodiments, R12 is Cl-C6alkylN(R7)(R8).
In certain embodiments, R12 is hydrogen, methyl, ethyl, methoxy, OH or
In certain embodiments, R12 is hydrogen or
In the embodiments described herein, R" is hydrogen, halogen, CN, OH, Ct-
C6alkoxy, Ci-C6alkylOC1-C6alkyl, Cl-C6alkylCOOH, COON, C3-C6cycloalkyl, Cl-
C6alkyl,
haloCi-C6alkyl, Cl-C6alkylOH, CON(R7)(R8), N(R7)(R8) or Cl-C6alkylN(R7)(R8).
In certain
embodiments, R13 is hydrogen. In certain embodiments, R13 is halogen. Suitable
halogens
include fluorine, chlorine, bromine, or iodine. In certain embodiments, R13 is
CN. In certain
embodiments, R13 is OH
24
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
In certain embodiments, R13 is C1-C6alkoxy. Suitable alkoxy groups include,
but
are not limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy. In
certain
embodiments, R13 is Ci-C6alkylOC1-C6alkyl. In certain embodiments, R13 is
COOH. In certain
embodiments, R13 is Ci-C6alkylCOOH. In certain embodiments, R13 is C3-
C6cycloa1kyl. Suitable
examples of cycloalkyls include, but are not limited to, cyclopropyl,
cyclobutyl, cyclopentyl and
cyclohexyl. In certain embodiments, R13 is C1-C6alkyl. Examples of C1-C6alkyl
groups can
include but are not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl,
isobutyl, sec-butyl,
tert-butyl, n-pentyl, isopentyl, neopentyl, tert-pentyl, 1-methylbutyl, 2-
methylbutyl, 1,2-
dimethylpropyl, 1-ethylpropyl, n-hexyl, isohexyl, 1-methylpentyl, 2-
methylpentyl, 3-
methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1-
ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethyl-2-methylpropyl and 1-ethyl-l-
methylpropyl. In
certain embodiments, R13 is haloCi-C6alky1. Suitable examples of haloalkyls
include, but are not
limited to, fluoromethyl, difluoromethyl, trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and
2,2-difluoroethyl. In certain embodiments, R13 is CI-C6a1kylOH. Examples of
suitable alcohols
include, but are not limited to, methanol, ethanol, propanol, butanol and iso-
butanol. In certain
embodiments, R13 is CON(R7)(R8). In certain embodiments, R13 is N(R7)(R8). In
certain
embodiments, R13 is C1-C6alkylN(R7)(R8).
In certain embodiments, R13 is hydrogen, methyl, ethyl, methoxy, OH or
In certain embodiments, R13 is hydrogen or
In certain embodiments, R12 and R13 are independently selected from the group
consisting of hydrogen and Ci-C6alkylOCI-C6alkyl, Ci-C6alkyl.
In certain embodiments described herein, each occurrence of R'4 is
independently
selected from the group consisting of hydrogen, halogen, CN, OH, C1-C6alkoxy,
C1-C6alkylOC1-
C6alkyl, C1-C6a1kylCOOH, COOH, C3-C6cycloalkyl,
haloCi-C6alkyl, C1-C6alkylOH,
CON(R7)(R8), N(R7)(R8) or C1-C6alkylN(R7)(R8). In certain embodiments, R14 is
hydrogen. In
certain embodiments, R14 is halogen. Suitable halogens include fluorine,
chlorine, bromine, or
iodine. In certain embodiments, R14 is CN. In certain embodiments, R14 is OH.
In certain embodiments, R14 is C1-C6alkoxy. Suitable alkoxy groups include,
but
are not limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy. In
certain
embodiments, R14 is C1-C6alkylOC1-C6alkyl. In certain embodiments, R" is COOH.
In certain
embodiments, R14 is C,-C6alkylCOOH. In certain embodiments, R14 is C3-
C6cycloa1kyl. Suitable
examples of cycloalkyls include, but are not limited to, cyclopropyl,
cyclobutyl, cyclopentyl and
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
cyclohexyl. In certain embodiments, R" is C1-C6alkyl. Examples of C1-C6alkyl
groups can
include but are not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl,
isobutyl,
sec-butyl, tert-butyl, n-pentyl, isopentyl, neopentyl, tert-pentyl, 1-
methylbutyl, 2-methylbutyl,
1,2-dimethylpropyl, 1-ethylpropyl, n-hexyl, isohexyl, 1-methylpentyl, 2-
methylpentyl, 3-
methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1-
ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethyl-2-methylpropyl and 1-ethyl-1-
methylpropyl. In
certain embodiments, R14 is haloCi-C6alkyl. Suitable examples of haloalkyls
include, but are not
limited to, fluoromethyl, difluoromethyl, trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and
2,2-difluoroethyl. In certain embodiments, R14 is CI-C6alkylOH. Examples of
suitable alcohols
include, but are not limited to, methanol, ethanol, propanol, butanol and iso-
butanol. In certain
embodiments, R14 is CON(R7)(R8). In certain embodiments, R14 is N(R7)(R8). In
certain
embodiments, R14 is Cl-C6alkylN(R7)(R8).
In certain embodiments, wherein X is C(R14)2, R14 is independently selected
from
the group consisting of hydrogen, halogen, OH, CI-CoalkylOH, CI-C6alkylalkoxy,
Ci-
C6alkyl0C1 -C6alkyl and Ci-C6alkyl.
In certain embodiments, R14 is hydrogen, methyl, ethyl, methoxy, OH or
In the embodiments described herein, R15 is hydrogen, halogen, CN, OH, Ci-
C6alkoxy, C1-C6alkylOCi-C6alkyl, C1-C6alkylCOOH, COOH, C3-C6cycloalkyl, C1-
C6alkyl,
haloCi-C6alkyl, C1-C6alkylOH, CON(R7)(R8) and N(R7)(R8). In certain
embodiments, R15 is
hydrogen. In certain embodiments, R15 is halogen. Suitable halogens include
fluorine, chlorine,
bromine, or iodine. In certain embodiments, R15 is CN. In certain embodiments,
R15 is OH.
In certain embodiments, R15 is C1-C6alkoxy. Suitable alkoxys include, but are
not
limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy. In certain
embodiments, R15
is CI-C6alkylOCi-C6alkyl. In certain embodiments, R15 is COOH. In certain
embodiments, R15 is
C1-C6alkylCOOH. In certain embodiments, R15 is C3-C6cycloalkyl. Suitable
examples of
cycloalkyls include, but are not limited to, cyclopropyl, cyclobutyl,
cyclopentyl and cyclohexyl.
In certain embodiments, R" is C1-C6alkyl. Examples of Ci-C6alkyl groups can
include but are
not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-
butyl, tert-butyl, n-pentyl,
isopentyl, neopentyl, tert-pentyl, 1-methylbutyl, 2-methylbutyl, 1,2-
dimethylpropyl, 1-
ethylpropyl, n-hexyl, isohexyl, 1-methylpentyl, 2-methylpentyl, 3-
methylpentyl, 1,1-
dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1-ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-
trimethylpropyl, 1-ethyl-2-methylpropyl and 1-ethyl-l-methylpropyl. In
certain
embodiments, R'5 is ethyl.
26
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
In certain embodiments, R15 is haloCt-C6alkyl. Suitable examples of haloalkyls
include, but are not limited to, fluoromethyl, difluoromethyl,
trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and 2,2-difluoroethyl. In certain embodiments, R1-5 is CI-
C6alkylOH. Examples of
suitable alcohols include, but are not limited to, methanol, ethanol,
propanol, butanol and iso-
butanol. In certain embodiments, R15 is CON(R7)(R8). In certain embodiments,
R15 is N(R7)(R8).
In certain embodiments, R15 is C1-C6alkylN(R7)(R8).
In certain embodiments, R15 is methyl or ethyl.
In the embodiments described herein, R16 is hydrogen, halogen, CN, OH, Ci-
C6alkoxy, Ci-C6alkylOCI-C6alkyl, Ci-C6alkylCOOH, COOH, C3-C6cycloalkyl, Ci-
Coalkyl,
haloCi-C6alkyl, C1-C6alkylOH, CON(R7)(R8) and N(R7)(R8). In certain
embodiments, R16 is
hydrogen. In certain embodiments, R16 is halogen. Suitable halogens include
fluorine, chlorine,
bromine, or iodine. In certain embodiments, R16 is CN. In certain embodiments,
R16 is OH.
In certain embodiments, R16 is C1-C6alkoxy. Suitable alkoxys include, but are
not
limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy. In certain
embodiments, R16
is CI-C6alkylOC1-C6alkyl. In certain embodiments, R16 is COOH. In certain
embodiments, R16 is
C1-C6alkylCOOH. In certain embodiments, R16 is C3-C6cycloalkyl. Suitable
examples of
cycloalkyls include, but are not limited to, cyclopropyl, cyclobutyl,
cyclopentyl and cyclohexyl.
In certain embodiments, R16 is C1-C6alkyl. Examples of C1-C6alkyl groups can
include but are
not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-
butyl, tert-butyl, n-pentyl,
isopentyl, neopentyl, tert-pentyl, 1-methylbutyl, 2-methylbutyl, 1,2-dim ethyl
propyl, 1-
ethylpropyl, n-hexyl, isohexyl, 1-methylpentyl, 2-methylpentyl, 3-
methylpentyl, 1,1-
dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1-ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-
trimethylpropyl, 1-ethyl-2-methylpropyl and 1-ethyl-l-methylpropyl. In certain
embodiments,
R16 is haloCi-C6alkyl. Suitable examples of haloalkyls include, but are not
limited to,
fluoromethyl, difluoromethyl, trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and 2,2-
difluoroethyl. In certain embodiments, RI-6 is CI-C6alkylOH. Examples of
suitable alcohols
include, but are not limited to, methanol, ethanol, propanol, butanol and iso-
butanol. In certain
embodiments, R16 is CON(R7)(R8). In certain embodiments, R16 is N(R7)(10. In
certain
embodiments, R16 is Ci-C6alkylN(R7)(R8).
In the embodiments of the compounds described herein, m is 0 or 1. In certain
embodiments, m is 0. In certain embodiments, m is 1.
In the embodiments of the compounds described herein, p is 0 or 1. In certain
embodiments, p is 0 In certain embodiments, p is 1
In the embodiments described herein, A is a straight or branched, saturated or
27
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
unsaturated (C3-C1o)alkylene, phenyl(C3-C1o)alkylene or cycloalkyl(C3-
C1o)alkylene comprising
at least one ¨CH2- group, wherein one or more additional ¨CH2- groups in A are
optionally and
independently replaced with a moiety selected from the group consisting of 0,
S, NR, CONR,
NRCO, SO2, and SO2NR and wherein one or more of the hydrogens along A can be
replaced
with a group independently selected from hydroxyl, halogen and C1-3 haloalkyl.
In certain
embodiments, A is a straight or branched, saturated or unsaturated (C3-
C1o)alkylene or
cycloalkyl(C3-C1o)alkylene, wherein one or more ¨CH2- groups in A are
optionally and
independently replaced with a moiety selected from the group consisting of 0,
S and NH. In
certain embodiment, A will always have at least one ¨CH2- group.
In certain embodiments, A is a straight (C3-C1o)alkylene. Examples of straight
(C3-C1o)alkylenes include,
õ,..\ ;22,
.,..-\
)72, )721
X ..-
(---- ...- ..--
f
-,-, ....-
,v
\. ...-
I ..-
...--
,3,.,,,,
I---- ,----
In certain embodiments, A is a branched (C3-C1o)alkylene. Suitable branched
(C3-
Cio)alkylenes include but are not limited to:
f\ '221
221 X x\ scrry
X?'
''''''
..\...-^,.....õ ,..., ..v
"222- ...\..."....... N.
In certain embodiments, A is a saturated (C3-C1o)alkylene. Examples include,
28
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
,,..N
,i;
X ..-
r- ,.. ....,
....- ....-
f
--r- ..---
:\...-
r ,- ....- ....--
õL..... ..---
,----
-..., r
,
(.....c *".............)221 )221
\ .7, r .....-
'''.---
,
...õ..., ....... ,,,,..,..
"22.. ...y
In certain embodiments, A is an unsaturated (C3-C1o)alkylene. Suitable
unsaturated (C3-Cio)alkylenes include any of the saturated (C3-C1o)alkylene,
wherein hydrogens
have been removed and one or more double or triple covalent bonds exist
between adjacent
carbon atoms. Examples of unsaturated (C3-Cio)alkylenes include, but are not
limited to,
Jr'
I
.....4=- ...-- ___...1
r
In other embodiments, A is a straight cycloalkyl(C3-C1o)alkylene. Suitable
straight cycloalkyl(C3-Cio)alkylenes include a cycloalkyl(C3-Cio)alkylene
wherein two carbons
in a chain are included in a (C3-C1o)cycloalkyl. Examples of straight
cycloalkyl(C3-C1o)alkylenes
include, but are not limited to,
29
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
LN,
In certain embodiments, A is a branched cycloalkyl(C3-C1o)alkylene. Suitable
branched cycloalkyl(Ci-Cio)alkylenes include a branched (C3-Cio)alkylene
wherein two carbons
in a chain are included in a (C3-C1o)cycloalkyl. Examples of cycloalkyl(C3-
C1o)alkylenes
include, but are not limited to,
In certain embodiments, A is a saturated cycloalkyl(C3-C1o)alkylene. Examples
of
saturated cycloalkyl(C3-C1o)alkylenes include, but are not limited to,
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
r<r2"1, tzel,
.:3111 "luv
In certain embodiments, A is an unsaturated cycloalkyl(C3-C1o)alkylene.
Examples of unsaturated cyclo(C3-C1o)alkylenes include, but are not limited
to,
In certain embodiments, A is an unsaturated or saturated phenyl(C3-
C1o)alkylene.
Examples of unsaturated and saturated phenyl(C3-C1o)alkylenes include, but are
not limited to,
c??,
101
4111:1 11111
`371,
In other embodiments, one or more ¨CH2- groups in A are optionally and
independently replaced with a moiety selected from the group consisting of 0,
S. NR, CONR,
NRCO, S02, and SO2NR. In other embodiments, one or more ¨CH2- groups in A are
optionally
and independently replaced with a moiety selected from the group consisting of
0, S and NH. In
other embodiments, one or more ¨CH2- groups in A are optionally and
independently replaced
with 0. In other embodiments, one or more ¨CH2- groups in A are optionally and
independently
replaced with S. In other embodiments, one or more ¨CH2- groups in A are
optionally and
independently replaced with NR. In other embodiments, one or more ¨CH2- groups
in A are
31
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
optionally and independently replaced with CONR. In other embodiments, one or
more ¨CH2-
groups in A are optionally and independently replaced with NRCO. In other
embodiments, one
or more ¨CH2- groups in A are optionally and independently replaced with S02.
In other
embodiments, one or more ¨CH2- groups in A are optionally and independently
replaced with
and SO2NR. R will be described in further detail below.
In the embodiments described herein, R is hydrogen, C1-C6alkylCOOH, COOH,
C3-C6cycloalkyl, C1-C6alkyl, haloCi-C6alkyl, C1-C6alkylOH, COC1-C6alkyl or
COOC1-C6alkyl.
In certain embodiments, R is hydrogen, CI-C6alkylCOOH, COOH, C3-C6cycloalkyl,
CI-C6alkyl,
haloCi-Coalkyl or Ci-CoalkylOH.
In certain embodiments, R is hydrogen. In certain embodiments, R is Ci-
C6alkylCOOH. In certain embodiments, R is COOH. In certain embodiments, R is
C3-
C6cycloalkyl. Suitable examples of cycloalkyls include, but are not limited
to, cyclopropyl,
cyclobutyl, cyclopentyl and cyclohexyl. In certain embodiments, R is C1-
C6alkyl. Examples of
Ci-C6alkyl groups can include but are not limited to, methyl, ethyl, n-propyl,
isopropyl, n-butyl,
isobutyl, sec-butyl, tert-butyl, n-pentyl, isopentyl, neopentyl, tert-pentyl,
1-methylbutyl, 2-
methylbutyl, 1,2-dimethylpropyl, 1-ethylpropyl, n-hexyl, isohexyl, 1-
methylpentyl, 2-
methylpentyl, 3-methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-
dimethylbutyl, 1-
ethylbutyl, 1,1,2-trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethyl-2-
methylpropyl and 1-ethyl-l-
methylpropyl. In certain embodiments, R is haloCi-C6alkyl. Suitable examples
of haloalkyls
include, but are not limited to, fluoromethyl, difluoromethyl, trifluorom
ethyl, 2-fluoroethyl, 1,2-
difluoroethyl and 2,2-difluoroethyl. In certain embodiments, R is Ci-
C6alkylOH. Examples of
suitable alcohols include, but are not limited to, methanol, ethanol,
propanol, butanol and iso-
butanol.
In certain embodiments, R is COC1-C6alkyl. Suitable examples include, but are
not limited to, COCH3. In certain embodiments, R is COOCi-C6alkyl. Suitable
examples include,
but are not limited to, COOCH3.
Examples of such embodiments include, but are not limited to,
rw
r)1,
gf- 6 (!)
:72;_ o)
In certain embodiments, A is
32
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
.....,.. ,
...-- ..--- õ,.... ....-
=%,..
.,õ,,õ. .õ....... r,..... r
.A.r. "I.'''.
In certain embodiments, one or more of the hydrogens along A can be replaced
with a group independently selected from hydroxyl, halogen and C1-3 haloalkyl.
Examples of
suitable halogens include chlorine, bromine, fluorine and iodine. In certain
embodiments, A is
,
WV-V V-VIIV ,,,,,,,
..,-' Fsf F.,..-
-... F
..--'
-"'C' F I õ ,, ;2( I =-
:%''''
'12_
"I V V '
Also described herein are compounds haying the structural Formula (III):
12 .
R13 n p ' R4R3
NHX
HNANVILV
R1.51NrLo µ,1
P
Nl0
1R1 R2
R5
-..,
Q41/1--
R6
(III)
wherein, A is a straight or branched, saturated or unsaturated (C3-
C1o)alkylene, phenyl(C3-
Cio)alkylene or cycloalkyl(C3-C1o)alkylene comprising at least one ¨CH2-
group, wherein one or
more additional ¨CH2- groups in A are optionally and independently replaced
with a moiety
selected from the group consisting of 0, S, NR, CONK, NRCO, S02, and SO2NR and
wherein
one or more of the hydrogens along A can be replaced with a group
independently selected from
hydroxyl, halogen and C1-3 haloalkyl;
Q is C(R16)2, 0, S, SO, SO2 or NH;
33
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
X is a bond, C(R")2, 0, S, SO, SO2 or NH;
Y is CR9 or N, wherein when Y is N, Z is CR" and V is CR";
V is CR1 or N, wherein when V is N, Z is CR11 and Y is CR9;
Z is CR11 or N, wherein when Z is N, V is CR1 and Y is CR9;
R is hydrogen, Ci-C6a1ky1COOH, COOH, C3-C6cycloalkyl, C,-C6alkyl, haloCi-
C6alky1, Ci-
C6alky1OH, COCi-C6alkyl or COOCi-C6alkyl;
R1 is hydrogen, halogen, CN, OH, C,-C6alkoxy, CI-C6alky1OC1-C6alkyl, C,-
C6alkylCOOH,
COOH, oxo, COOCI-C6alkyl, CI-C6alkylCOOCI-C6alkyl, C3-C6cycloalkyl, CI-
C6alky1C3-
Cocycloalkyl, Ci-Coalkyl, -Ci-CoalkylOhaloCi-Coalkyl, haloCi-Coalkyl, Ci-
C6alkylOH,
CON(R7)(R8), N(R7)(R8) or C,-C6alkylN(R7)(R8);
R2 is hydrogen, halogen, CN, OH, C,-Coalkoxy, Ci-CoalkylOCi-C6alkyl, C,-
CoalkylCOOH,
COOH, oxo, COOCi-C6alkyl, Ci-C6alkylCOOCi-C6alkyl, C3-C6cycloalkyl, Ci-
C6alkylC 3-
C6cycloalkyl, Ci-C6alkyl, -Ci-C6alkylOhaloCi-C6alkyl, haloCi-C6alkyl, Ci-
C6a1kylOH,
CON(R7)(R8), N(R7)(R8) or CI-C6alkylN(R7)(R8);
R3 is hydrogen, halogen, CN, OH, Cl-C6alkoxy, CI-C6a1kylOCi-C6alkyl, Cl-
C6alkylCOOH,
COOH, C3-C6cycloalkyl, Ci-C6alkyl, haloCi-C6alkyl, Ci-C6alkylOH, CON(R7)(R8),
N(R7)(R8),
Ci-C6alkylN(R7)(R8), Ci-C6alkyl(OCH2CH2)fiN(R7)(R8) or Ci-C6alkylOhaloCi-
C6alkyl or when
taken with R4 forms a C3-C6cycloa1kyl or C3-C6heterocycloa1kyl;
R4 is hydrogen, halogen, CN, OH, C,-C6alkoxy, Ci-C6alkylOCi-C6alkyl, C,-
C6alkylCOOH,
COOH, C3-C6cycloalkyl, haloCI-Coalkyl, CI-C6alkyl OH, CON(R7)(R8),
N(R7)(R8),
Ci-C6alkylN(R7)(R8), Ci-C6alkyl(OCH2CH2),,N(R7)(R8) or Ci-C6alkylOhaloCi-
C6alkyl or when
taken with R3 forms a C3-C6cycloalkyl or C3-C6heterocycloalkyl;
R5 is hydrogen, halogen, CN, OH, C,-Coalkoxy, Ci-CoalkylOCi-C6alkyl, C,-
CoalkylCOOH,
COOH, C3-C6cycloalkyl, Ci-C6alky1, haloCi-C6alkyl, Ci-C6a1kylOH, CON(R7)(R8),
N(R7)(R8)
or C,-C6alkylN(R7)(R8);
R6 is hydrogen, halogen, CN, OH, CI-C6alkoxy, Ci-C6alkylOCI-C6alkyl, CI-
C6alkylCOOH,
COOH, C3-C6cycloalkyl, Ci-C6alky1, haloCi-C6a1kyl, Cl-C6alkylOH, CON(R7)(R8),
N(R7)(R8)
or C,-C6alkylN(R7)(R8);
R7 is hydrogen, C,-CoalkylCOOH, COOH, C3-Cocycloalkyl, C1-C6alkyl, haloCi-
C6alkyl, Ci-
C6a1ky1OH, COCi-C6alkyl or COOCi-C6alkyl;
R8 is hydrogen, C,-CoalkylCOOH, COOH, C3-Cocycloalkyl, Ci-C6alkyl, haloCi-
Coalkyl, Ci-
CoalkylOH, COCi-C6alkyl or COOCi-C6alkyl;
R9 is hydrogen, halogen, CN, OH, C,-C6alkoxy, Ci-C6alkylOCi-C6alkyl, C,-
C6alkylCOOH,
COOH, C3-C6cycloalkyl, Ci-C6alkyl, haloCi-C6alkyl, Ci-C6alkylOH, CON(R7)(R8),
N(R7)(R8)
34
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
or C1-C6alkylN(R7)(1e);
RI' is hydrogen, halogen, CN, OH, CI-C6alkoxy, CI-C6alky1OCI-C6alkyl, CI-
C6alkylCOOH,
COOH, C3-C6cycloa1kyl, Ci-C6alky1, haloCi-C6a1kyl, Cl-C6alkylOH, CON(R7)(R8),
N(R7)(It8)
or C1-C6alkylN(R7)(R8);
Rll is hydrogen, halogen, CN, OH, C1-C6alkoxy, C1-C6alkylOC1-C6alkyl, C1-
C6alkylCOOH,
COOH, C3-C6cycloa1kyl, Ci-C6alkyl, haloCi-C6alkyl, Cl-C6alkylOH, CON(R7)(R8),
N(R7)(R8)
or C1-C6alkylN(R7)(R8);
R12 is hydrogen, halogen, CN, OH, CI-C6alkoxy, CI-C6alky1OCI-C6alkyl, CI-
C6alkylCOOH,
COOH, C3-Cocycloalkyl, Ci-Coalkyl, haloCi-Coalkyl, CI-CoalkylOH, CON(R7)(R8),
N(R7)(R8)
or C1-C6alkylN(R7)(R8);
R13 is hydrogen, halogen, CN, OH, Ci-Coalkoxy, C1-Coalky1OC1-C6alkyl, C1-
C6alkylCOOH,
COOH, C3-C6cycloalkyl, C1-C6alkyl, haloCi-C6alkyl, C1-C6alkylOH, CON(R7)(R8),
N(R7)(R8)
or Ci-C6alkylN(R7)(R8);
each occurrence of It34 is independently selected from the group consisting of
hydrogen,
halogen, CN, OH, CI-C6alkoxy, Ci-C6alky1OCI-C6alkyl, CI-C6alkylCOOH, COOH, C3-
C6cycloalkyl, Ci-C6alkyl, haloC1-C6alkyl, C1-C6alkylOH, CON(R7)(R8), N(R7)(R8)
and Ci-
C6alkylN(R7)(R8);
R15 is hydrogen, halogen, CN, OH, C1-C6alkoxy, C1-C6alkylOC1-C6alkyl, C1-
C6alkylCOOH,
COOH, C3-C6cycloalkyl, C1-C6alkyl, haloCi-C6alkyl, Cl-C6alkylOH, CON(R7)(R8),
N(R7)(R8)
or CI-C6alkylN(R7)(R8);
each occurrence of It' is independently selected from the group consisting of
hydrogen,
halogen, CN, OH, CI-C6alkoxy, CI-C6alkylOCt-C6alkyl, CI-C6alkylCOOH, COOH, C3-
C6cycloalkyl, Ci-C6alkyl, haloCl-C6alkyl, Ci-CcalkylOH, CON(R7)(R8), N(R7)(R8)
and Ci-
C6alky1N(R7)(R8);
1 is 0 or 1;
m is 0 or 1;
n is 1, 2, 3 or 4; and
p is 0 or 1.
In the embodiments described herein, Q is C(R16)2, 0, S, SO, SO2 or NH. In
certain embodiments, Q is C(R16)2, wherein 106 is discussed in further detail
below. In certain
embodiments, Q is CH2, CH(CH3), C(CH3)2, 0, CH(OCH3), SO2 or CF2. In other
embodiments
Q is CH2, 0, S, SO, SO2 or NH. In certain embodiments, Q is CH2. In certain
embodiments
described herein, Q is 0. In certain embodiments described herein, Q is S. In
other embodiments
described herein, Q is SO. In other embodiments described herein, Q is SO2. In
certain
35
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
embodiments described herein, Q is NH. In other embodiments described herein,
Q is 0
or S02. In still other embodiments described herein, Q is 0 or CH2.
In the embodiments described herein, X is a bond, C(R14)2, 0, S, SO, SO2 or
NH.
In certain embodiments described herein X is a bond. In certain embodiments, X
is C(R14)2,
wherein R14 is discussed in further detail below. In certain embodiments, X is
a bond, CH2,
CH(CH3), C(CH3)2, 0, CH(OCH3), SO2 or CF2. In other embodiments X is CH2, 0,
S, SO, SO2
or NH. In certain embodiments, X is CH2. In the embodiments described herein,
X is 0. In
certain embodiments described herein, X is S. In certain embodiments described
herein, X is SO.
In other embodiments described herein, X is SO2. In certain embodiments
described herein, X is
NH.
In the embodiments described herein, Y is CR9 or N. In certain embodiments, Y
is
CR9, wherein R9 is discussed in detail below. In certain embodiments, Y is N.
In certain
embodiments, Y is CH. In certain embodiments, wherein when Y is N, Z is CRll
and V is CR".
In the embodiments described herein, V is CR' or N. In certain embodiments, V
is CR", wherein R1 is discussed in detail below. In certain embodiments, V is
N. In certain
embodiments, V is CH. In certain embodiments, wherein when V is N, Z is CR11
and Y is CR9.
In the embodiments described herein, Z is CR11 or N. In certain embodiments, Z
is CR11, wherein R11 is discussed in detail below. In certain embodiments, Z
is CH. In certain
embodiments, Z is N. In certain embodiments, wherein when Z is N, V is CR' and
Y is CR9.
In certain embodiments, Xis 0, Y and V are each CH and Z isN In certain
embodiments, X is 0, Y and Z are each CH and V is N. In certain embodiments, X
is 0 and V, Y
and Z are all simultaneously CH.
In certain embodiments, X is a bond, Y and V are each CH and Z is N. In
certain
embodiments, X is a bond, Y and Z are each CH and V is N. In certain
embodiments, X is a
bond and V, Y and Z are all simultaneously CH.
Also described herein are compounds of Formula III represented by structural
Formula
(IIIA):
36
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
-4
NH R3
H N "jj'N (PO
=
R2
R5
R6
(IIIA)
wherein R1, R2, R3, R4, R5, R6, Rls, A, and Q, are as described herein. An
embodiment of this invention is realized when RI, R2, R3, R4, R5, R6, R15, A,
and Q, are as
described in Formula III. Another embodiment of Formula IRA is realized when
R3 and R4 are
both hydrogen, methyl, ethyl, or halogen. Another embodiment of Formula IIIA
is realized when
R3 and R4 are both halogen selected from chlorine and fluorine. Another
embodiment of Formula
IIIA is realized when Q is CH2, CH(CH3), C(CH3)2, 0, CH(OCH3), SO2 or CF2. In
other
embodiments described herein, Q in Formula IIIA is 0 or S02. In still other
embodiments
described herein, Q in Formula IIIIA is 0 or CH2. Another embodiment of
Formula IIIA is
realized when A is a straight or branched, saturated or unsaturated (C3-
C1o)alkylene comprising
at least one ¨CH2- group, wherein one or more additional ¨CH2- groups in A are
optionally and
independently replaced with a moiety selected from the group consisting of 0,
S, NR, CONR,
NRCO, S02, and SO2NR and wherein one or more of the hydrogens along A can be
replaced
with a group independently selected from hydroxyl, halogen and C1-3 haloalkyl.
Also described herein are compounds of Formula III represented by structural
Formula
(IIIB):
R4
HNANH allo R3
N
Rl.Lso
=
R1
R2
R5
R6
(II1B)
37
CA 03240145 2024- 6-5
WO 2023/107356 PCT/US2022/051770
wherein R', R2, R3, R4, R5, R6, RI', A, and Q, are as described herein. An
embodiment of this invention is realized when R', R2, R3, R4, IV, R6, R", A,
and Q, are as
described in Formula III. Another embodiment of Formula IIIB is realized when
R3 and R4 are
both hydrogen, methyl, ethyl, or halogen. Another embodiment of Formula IIIB
is realized when
R3 and R4 are both halogen selected from chlorine and fluorine. Another
embodiment of Formula
IIIB is realized when Q is CH2, CH(CH3), C(CH3)2, 0, CH(OCH3), SO2 or CF2. In
other
embodiments described herein, Q in Formula IIIB is 0 or S02. In still other
embodiments
described herein, Q in Formula IIIIB is 0 or CH2. Another embodiment of
Formula IIIB is
realized when A is a straight or branched, saturated or unsaturated (C3-
C1o)alkylene comprising
at least one ¨CH2- group, wherein one or more additional ¨CH2- groups in A are
optionally and
independently replaced with a moiety selected from the group consisting of 0,
S. NR, CONR,
NRCO, SO2, and SO2NR and wherein one or more of the hydrogens along A can be
replaced
with a group independently selected from hydroxyl, halogen and C1-3 haloalkyl.
Certain embodiments are represented as Formulas IV - VI:
R94
, R1 2
R1 3 i 2 R4R3 R3 R4 R'3 R4R3
NH\)10 NH 0 NH 0
R19J R12 HNAN
1110
0
=
RI RI R2 RI R2
\
I R2 crtirRs
R5
R5
Q I Q I
R6 (w) R6 (v)
R6 (VI)
In the embodiments described herein, RI is hydrogen, halogen, CN, OH, Ci-
C6alkoxy, C1-C6alkylOCI-C6alkyl, CI-C6alkylCOOH, COOH, oxo, COOCi-C6alkyl, CI-
C6alkylCOOCi-C6alkyl, C3-C6cycloalkyl, Ci-C6alky1C3-C6cycloalkyl, Ci-C6alkyl, -
Ci-
C6alkylOhaloCi-Coalkyl, haloCi-C6alkyl, Ci-C6alkylOH, CON(R7)(1e), N(R7)R8) or
Ci-
C6alkylN(R7)(R8).
In certain embodiments described herein, R1 is hydrogen.
In certain embodiments, RI- is halogen. Examples of suitable halogens
include chlorine, bromine, fluorine and iodine.
38
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
In certain embodiments, It' is CN.
In certain embodiments, It' is OH.
In certain embodiments, TO is CI-C6alkoxy. Suitable alkoxys include, but are
not
limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy.
In certain embodiments, It' is CI-C6alkylOC1-C6a1kyl.
In certain embodiments, is CI-C6alkylCOOH.
In certain embodiments, is COOH.
In certain embodiments, 1:11 is an oxo group.
In certain embodiments, It' is COOCI-Coalkyl.
In certain embodiments, R1 is Ct-C6alkylCOOC1-C6alkyl.
In certain embodiments, RI is C3-Cocycloalkyl. Suitable examples of
cycloalkyls
include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl.
In certain embodiments, RI is CI-C6alky1C3-C6cycloalkyl. Suitable examples of
cycloalkyls include, but are not limited to
In certain embodiments, It' is Ct-C6alkyl. Examples of Ci-C6alkyl groups can
include but are not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl,
isobutyl, sec-butyl,
tert-butyl, n-pentyl, isopentyl, neopentyl, tert-pentyl, 1-methylbutyl, 2-
methylbutyl, 1,2-
dimethylpropyl, 1-ethylpropyl, n-hexyl, isohexyl, 1-methylpentyl, 2-
methylpentyl, 3-
methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1-
ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethyl-2-methylpropyl and 1-ethyl-1-
methylpropyl.
In certain embodiments, It' is Ct-C6alkylOhaloCi-C6alkyl. Suitable examples of
)(F
F
Ci-C6alkylOhaloCi-C6alkyls include, but are not limited to, In certain
embodiments, is haloCi-C6alkyl. Suitable examples of haloalkyls
include, but are not limited to, fluoromethyl, difluoromethyl,
trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and 2,2-difluoroethyl.
In certain embodiments, RI is CI-C6alkylOH. Suitable alcohols include, but are
not limited to, methanol, ethanol, propanol, butanol and iso-butanol.
In certain embodiments, R1 is CON(R7)(R'). In certain embodiments, R' is
N(10(10). In certain embodiments, It" is Ci-C6alkylN(10(10), wherein It' and
It8 will be
described in detail below.
In certain embodiments, is hydrogen, bromine, fluorine,
chlorine, methyl, OH,
39
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
halogen, CN oxo, methoxymethyl, COOCH2CH3 or trifluoromethyl.
In the embodiments described herein, R2 is hydrogen, halogen, CN, OH, Ci-
C6alkoxy, Ci-C6alkylOCi-C6alkyl, Ci-C6alkylCOOH, COOH, oxo, COOCi-C6alkyl, Ci-
CoalkylCOOCi-C6alkyl, C3-C6cycloalkyl, C1-CoalkylC3-C6cycloalkyl, Ci-C6alkyl, -
Ci-
C6alkylOhaloC1-C6alkyl, haloCi-C6alkyl, Ci-C6alkylOH, CON(R7)(R8), N(R7)(R8)
or Ci-
C6alkylN(R7)(R8).
In certain embodiments described herein, R2 is hydrogen.
In certain embodiments, R2 is halogen. Examples of suitable halogens include
chlorine, bromine, fluorine and iodine.
In certain embodiments, R2 is CN.
In certain embodiments, R2 is OH.
In certain embodiments, R2 is CI-C6alkoxy. Suitable alkoxys include, but are
not
limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy.
In certain embodiments, R2 is CI-C6alkylOCI-C6alkyl.
In certain embodiments, R2 is CI-C6alkylCOOH.
In certain embodiments, R2 is COOH.
In certain embodiments, R2 is an oxo group.
In certain embodiments, R2 is COOC1-C6alkyl.
In certain embodiments, R2 is CI-C6alkylCOOC1-C6alkyl.
In certain embodiments, R2 is C3-C6cycloalky1 Suitable examples of cycloalkyls
include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl.
In certain embodiments, R2 is CI-C6alkylC3-C6cycloalkyl. Suitable examples of
µCDcycloalkyls include, but are not limited to
In certain embodiments, R2 is Ci-Coalkyl. Examples of Ci-C6alkyl groups can
include but are not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl,
isobutyl, sec-butyl,
tert-butyl, n-pentyl, isopentyl, neopentyl, tert-pentyl, 1-methylbutyl, 2-
methylbutyl, 1,2-
dimethylpropyl, 1-ethylpropyl, n-hexyl, isohexyl, 1-methylpentyl, 2-
methylpentyl, 3-
methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1-
ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethyl-2-methylpropyl and 1-ethyl-1-
methylpropyl.
In certain embodiments, R2 is Ct-C6alkylOhaloCi-C6alkyl. Suitable examples of
Ci-C6alkylOhaloCi-C6alkyls include, but are not limited to, F
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
In certain embodiments, R2 is haloCi-C6alkyl. Suitable examples of haloalkyls
include, but are not limited to, fluoromethyl, difluoromethyl,
trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and 2,2-difluoroethyl.
In certain embodiments, R2 is CI-C6alkylOH. Suitable alcohols include, but are
not limited to, methanol, ethanol, propanol, butanol and iso-butanol.
In certain embodiments, R2 is CON(R7)(R8). In certain embodiments, RI- is
N(R7)(R8). In certain embodiments, R2 is C1-C6alkylN(R7)(R8), wherein R7 and
R8 will be
described in detail below.
In certain embodiments, R2 is hydrogen, bromine, fluorine, chlorine, methyl,
OH,
halogen, CN oxo, methoxymethyl, COOCH2CH3 or trifluoromethyl.
In certain embodiments, RI and R2 are both hydrogen. In certain embodiments,
R'
is OH and R2 is hydrogen.
In the embodiments described herein, R3 is hydrogen, halogen, CN, OH, Ci-
C6alkoxy, C1-C6alkylOCI-C6alkyl, CI-C6alkylCOOH, COOH, C3-C6cycloalkyl, C1-
Coalkyl,
haloCi-C6alkyl, Ci-C6alkylOH, CON(R7)(R8), N(R7)(IV), Ci-C6alkylN(R7)(R8), Ci-
C6alkyl(OCH2CH2)nN(R7)(R8) or C1-C6alkylOhaloC1-C6alkyl or when taken with R4
forms a C3-
C6cycloalkyl or C3-C6heterocycloalkyl.
In certain embodiments of the compounds described herein, IV is hydrogen,
halogen, CN, OH, C1-C6alkoxy, C1-C6alkylOC1-C6alkyl, CI-C6alkylCOOH, COOH, C3-
C6cycl alkyl, CI-C6a1kyl, ha] oC1-C6alkyl, CI-C6a1kyl OH, CON(R7)(R8),
N(R7)(128) or CI-
C6alkylN(R7)(R8) or when taken with R4 forms a C3-C6cycloalkyl or C3-
C6heterocycloalkyl. In
certain embodiments, R3 is hydrogen. In certain embodiments, R3 is halogen.
Suitable halogens
include fluorine, chlorine, bromine, and iodine. In certain embodiments, R3 is
CN. In certain
embodiments, R3 is OH.
In certain embodiments, R3 is CI-C6alkoxy. Suitable alkoxys include, but are
not
limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy. In certain
embodiments, R3 is
Ci-C6alkylOCi-C6alkyl. In certain embodiments, R3 is COOH. In certain
embodiments, R3 is Ci-
C6alkylCOOH. In certain embodiments, R3 is C3-C6cycloalkyl. Suitable examples
of cycloalkyls
include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl. In certain
embodiments, R3 is Ci-C6alkyl. Examples of C1-C6alkyl groups can include but
are not limited
to, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-
butyl, n-pentyl, isopentyl,
neopentyl, tert-pentyl, 1-methylbutyl, 2-methylbutyl, 1,2-dimethylpropyl, 1-
ethylpropyl, n-hexyl,
isohexyl, 1-methylpentyl, 2-methylpentyl, 3-methylpentyl, 1,1-dimethylbutyl,
1,2-dimethylbutyl,
2,2-dimethylbutyl, 1-ethylbutyl, 1,1,2-trimethylpropyl, 1,2,2-
41
CA 03240145 2024- 6-5
WO 2023/107356 PC
T/US2022/051770
trimethylpropyl, 1-ethyl-2-methylpropyl and 1-ethyl-1-methylpropyl. In certain
embodiments, R3
is haloCt-C6a1kyl. Suitable examples of haloalkyls include, but are not
limited to, fluoromethyl,
difluoromethyl, trifluoromethyl, 2-fluoroethyl, 1,2-difluoroethyl and 2,2-
difluoroethyl. In certain
embodiments, R3 is Ci-C6alkylOH. Examples of suitable alcohols include, but
are not limited to,
methanol, ethanol, propanol, butanol and iso-butanol. In certain embodiments,
R3 is
CON(R7)(R8). Suitable examples of N(R7)(R8) include, but are not limited to,
CONH2 and
CON(CH3)2. In certain embodiments, R3 is N(R7)(R8). Suitable examples of
N(R7)(R8) include,
but are not limited to, NI-I2 and N(CH3)2. In certain embodiments, R3 is CI-
C6alkylN(R7)(R8).
N H2
Suitable examples of Ci-C6alkylN(R7)(R8) include, but are not limited to, `2-
0 0
N'iL" N
and . R7 and R8 are
discussed in further detail
below.
In certain embodiments, It3 is CI-C6alkylOhaloC1-C6alkyl. Suitable examples of
)<F
`V"---- 0 F
haloalkyls include, but are not limited to,
In certain embodiments, R3 is CI-C6alkyl(OCH2CH2)11N(R7)(R8). R7, Rg and n are
discussed in detail below. Suitable examples of C1-C6alkyl(OCH2CH2)11N(R7)(R8)
include, but
0
N H2 10O0N
are not limited to, ,
and
0 ly
With regard to the compounds described herein, n is 1, 2, 3 or 4. In certain
embodiments, n is 1. In certain embodiments, n is 2. In certain embodiments, n
is 3. In certain
embodiments, n is 4.
In certain embodiments, R3 is taken with R4 and forms a C3-C6cycloalkyl or C3-
C6heterocycloalkyl. In certain embodiments, R3 is taken with R4 and forms a C3-
C6cycloalkyl.
Suitable examples of cycloalkyls include, but are not limited to, cyclopropyl,
cyclobutyl,
cyclopentyl and cyclohexyl. In certain embodiments, R3 is taken with R4 and
forms a C3-
C6heterocycloa1kyl. Suitable examples of heterocycloalkyls include, but are
not limited to,
42
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
piperidyl, oxetanyl, pyrrolyl, piperazinyl, morpholinyl, thiomorpholinyl,
thiazolidinyl, 1,4-
dioxanyl, tetrahydrofuranyl, tetrahydrothiophenyl, beta lactam, gamma lactam,
delta lactam,
beta lactone, gamma lactone, delta lactone, and pyrrolidinone, and oxides
thereof.
In certain embodiments, R3 is hydrogen, fluorine, methyl, ethyl, OH, methoxy,
0
OH NH2 '722.
, CON(CH3)2,
0 k
0 )<.F
\-^0 F I
0 0
0 )*L
N
co
JVVVV, or
In certain embodiments, R3 is hydrogen, methyl, ethyl or
In certain embodiments, R3 is taken with R4 to form oxetanyl.
In certain embodiments described herein, R4 is hydrogen, halogen, CN, OH, Ci-
C6alkoxy, Ci-C6alkylOCi-C6alkyl, Ci-C6alkylCOOH, COOH, C3-C6cycloalkyl, Ci-
C6alkyl,
haloCi-C6alkyl, Ci-C6alkylOH, CON(R7)(R8), N(R7)(R8), Ci-C6alkylN(R7)(R8), Ci-
C6alkyl(OCH2CH2),,N(R7)(R8) or Ci-C6alkylOhaloCi-C6alkyl or when taken with R3
forms a C3-
C6cycloalkyl or C3-C6heterocycloalkyl. In certain embodiments of the compounds
described
herein, le is hydrogen, halogen, CN, OH, Ci-C6alkoxy, C1-C6alkylOC1-C6alkyl,
Ci-
C6alkylCOOH, COOH, C3-C6cycloalkyl, Ci-C6alkyl, haloCI-C6alkyl, CI-CoalkylOH,
CON(R7)(R8), N(R7)(R8) or Ci-C6alkylN(R7)(R8) or when taken with R3 forms a C3-
C6cycloalkyl
or C3-C6heterocycloalkyl. In certain embodiments, R4 is hydrogen. In certain
embodiments, R4 is
halogen. Suitable halogens include fluorine, chlorine, bromine, or iodine. In
certain
embodiments, It4 is CN. In certain embodiments, 11:4 is OH.
In certain embodiments, R4 is CI-C6alkoxy. Suitable alkoxys include, but are
not
limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy. In certain
embodiments, R4 is
Ci-C6alkylOCi-C6alkyl. In certain embodiments, R4 is COOH. In certain
embodiments, R4 is Ci-
C6alkylCOOH. In certain embodiments, R4 is C3-C6cycloalkyl. Suitable examples
of cycloalkyls
include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl. In certain
embodiments, R4 is C1-C6alkyl. Examples of C1-C6alkyl groups can include but
are not limited
to, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-
butyl, n-pentyl, isopentyl,
43
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
neopentyl, tert-pentyl, 1-methylbutyl, 2-methylbutyl, 1,2-dimethylpropyl, 1-
ethylpropyl, n-hexyl,
isohexyl, 1-methylpentyl, 2-methylpentyl, 3-methylpentyl, 1,1-dimethylbutyl,
1,2-dimethylbutyl,
2,2-dimethylbutyl, 1-ethylbutyl, 1,1,2-trimethylpropyl, 1,2,2-trimethylpropyl,
1-ethy1-2-
methylpropyl and 1-ethyl-1-methylpropyl. In certain embodiments, R4 is haloCi-
C6alkyl.
Suitable examples of haloalkyls include, but are not limited to, fluoromethyl,
difluoromethyl,
trifluoromethyl, 2-fluoroethyl, 1,2-difluoroethyl and 2,2-difluoroethyl. In
certain embodiments,
R4 is C1-C6alkylOH. Examples of suitable alcohols include, but are not limited
to, methanol,
ethanol, propanol, butanol and iso-butanol. In certain embodiments, R4 is
CON(R7)(R8). Suitable
examples of N(R7)(R8) include, but are not limited to, CONH2 and CON(CH3)2. In
certain
embodiments, R4 is N(R7)(R8). Suitable examples of N(R7)(R8) include, but are
not limited to,
NI-12 and N(CH3)2. In certain embodiments, R4 is Ci-C6alkylN(R7)(R8). Suitable
examples of Ci-
0
rk
L.0
C6alkylN(R7)(R8) include, but are not limited to, µ2-
and
. R7 and R8 are discussed in further detail below.
In certain embodiments, R4 is CI-C6alkylOhaloCi-C6alkyl. Suitable examples of
õk=F
haloalkyls include, but are not limited to, F
In certain embodiments, R4 is CI-C6alkyl(OCH2C1-17),N(R7)(R8). R7, R8 are
discussed in detail below and n is discussed above. Suitable examples of Ci-
ro
N H 2
C6alky1(OCH2CH2)11N(R7)(1e) include, but are not limited to,
0 0
J'L
0
, and
In certain embodiments, R4 is taken with R3 and forms a C3-C6cycloalkyl or C3-
C6heterocycloalkyl. In certain embodiments, R4 is taken with R3 and forms a C3-
C6cycloalkyl.
Suitable examples of cycloalkyls include, but are not limited to, cyclopropyl,
cyclobutyl,
cyclopentyl and cyclohexyl. In certain embodiments, R4 is taken with R3 and
forms a C3-
C6heterocycloa1kyl. Suitable examples of heterocycloalkyls include, but are
not limited to,
44
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
piperidyl, oxetanyl, pyrrolyl, piperazinyl, morpholinyl, thiomorpholinyl,
thiazolidinyl, 1,4-
dioxanyl, tetrahydrofuranyl, tetrahydrothiophenyl, beta lactam, gamma lactam,
delta lactam,
beta lactone, gamma lactone, delta lactone, and pyrrolidinone, and oxides
thereof.
In certain embodiments, R4 is hydrogen or methyl. In certain embodiments, R4
is
hydrogen, methyl, ethyl or c) . In certain embodiments, R4 is taken with R3
to form
oxetanyl. In certain embodiments, R3 and R4 are both hydrogen, methyl or
ethyl.
In certain embodiments, R3 is hydrogen and R4 is hydrogen.
In the embodiments described herein, R5 is hydrogen, halogen, CN, OH, Ci-
C6alkoxy, CI-C6alkylCOOH, COOH, oxo, COOCi-C6alkyl,
Ci-
C6alkylCOOCi-C6alkyl, C3-C6cycloalkyl, Ci-C6alky1C3-C6cycloalkyl, Ci-C6alkyl, -
Ci-
C6alkylOhaloCi-C6alkyl, haloCi-C6alkyl, Ci-C6alkylOH, CON(R7)(R8), N(R7)(R8)
or Ci-
C6alkylN(R7)(R8).
In certain embodiments described herein, R5 is hydrogen.
In certain embodiments, R5 is halogen. Examples of suitable halogens include
chlorine, bromine, fluorine and iodine.
In certain embodiments, R5 is CN.
In certain embodiments, R5 is OH.
In certain embodiments, R5 is Ct-Coalkoxy. Suitable alkoxys include, but are
not
limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy.
In certain embodiments, R5 is Ci-C6alkylOCi-C6alkyl.
In certain embodiments, R5 is Ci-C6alkylCOOH.
In certain embodiments, R5 is COOH.
In certain embodiments, R5 is an oxo group.
In certain embodiments, R5 is COOCi-C6alkyl.
In certain embodiments, R5 is CI-C6alkylCOOCi-C6alkyl.
In certain embodiments, R5 is C3-C6cycloalkyl. Suitable examples of
cycloalkyls
include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl.
In certain embodiments, R5 is Ci-C6alky1C3-C6cycloalkyl. Suitable examples of
cycloalkyls include, but are not limited to .
In certain embodiments, R5 is Ct-C6alkyl. Examples of Ci-C6alkyl groups can
include but are not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl,
isobutyl, sec-butyl,
tert-butyl, n-pentyl, isopentyl, neopentyl, tert-pentyl, 1-methylbutyl, 2-
methylbutyl, 1,2-
CA 03240145 2024- 6-5
WO 2023/107356 PCT/US2022/051770
dimethylpropyl, 1-ethylpropyl, n-hexyl, isohexyl, 1-methylpentyl, 2-
methylpentyl, 3-
methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1-
ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethyl-2-methylpropyl and 1-ethyl-1-
methylpropyl. In
certain embodiments, R5 is methyl.
In certain embodiments, R5 is CI-C6alkylOhaloCi-C6alkyl. Suitable examples of
rk.F
F
C1-C6alkylOhaloC1-C6alkyls include, but are not limited to, 't
In certain embodiments, R5 is haloCi-C6alkyl. Suitable examples of haloalkyls
include, but are not limited to, fluoromethyl, difluoromethyl,
trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and 2,2-difluoroethyl
In certain embodiments, R5 is CI-C6alkylOH. Suitable alcohols include, but are
not limited to, methanol, ethanol, propanol, butanol and iso-butanol.
In certain embodiments, R5 is CON(117)(R8). In certain embodiments, RI- is
N(R7)(R8). In certain embodiments, R5 is Ci-C6alkylN(R7)(R8), wherein R7 and
R8 will be
described in detail below.
In certain embodiments, R5 is hydrogen, methyl, ethyl or t-butyl.
In the embodiments described herein, R6 is hydrogen, halogen, CN, OH, Ci-
C6alkoxy, Ci-C6alkylOC1-C6alkyl, C1-C6alkylCOOH, COOH, oxo, COOCi-C6alkyl, Ci-
C6alky1COOC1-C6alkyl, C3-C6cycloalkyl, Ci-C6alky1C3-C6cycloalkyl, Ci-C6alkyl, -
Ci-
C6alkylOhaloCi-C6alkyl, haloCi-C6alkyl, C1-C6alkylOH, CON(R7)(R8), N(R7)(R8)
or Ci-
C6alkylN(R7)(R8).
In certain embodiments described herein, R6 is hydrogen.
In certain embodiments, R6 is halogen. Examples of suitable halogens include
chlorine, bromine, fluorine and iodine.
In certain embodiments, R6 is CN.
In certain embodiments, R6 is OH.
In certain embodiments, R6 is CI-C6alkoxy. Suitable alkoxys include, but are
not
limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy.
In certain embodiments, R6 is CI-C6alkylOCi-C6alkyl.
In certain embodiments, R6 is CI-C6alkylCOOH.
In certain embodiments, R6 is COOH.
In certain embodiments, R6 is an oxo group.
In certain embodiments, R6 is COOC1-C6alkyl.
46
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
In certain embodiments, R6 is CI-C6alkylCOOC1-C6alkyl.
In certain embodiments, R6 is C3-C6cycloalkyl. Suitable examples of
cycloalkyls
include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl.
In certain embodiments, R6 is CI-C6alkylC3-C6cycloalkyl. Suitable examples of
cycloalkyls include, but are not limited to
In certain embodiments, R6 is CI-C6alkyl. Examples of C1-C6alkyl groups can
include but are not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl,
isobutyl, sec-butyl,
tert-butyl, n-pentyl, isopentyl, neopentyl, tert-pentyl, 1-methylbutyl, 2-
methylbutyl, 1,2-
dimethylpropyl, 1-ethylpropyl, n-hexyl, isohexyl, 1-methylpentyl, 2-
methylpentyl, 3-
methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1-
ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethyl-2-methylpropyl and 1-ethyl-1-
methylpropyl. In
certain embodiments, R6 is methyl.
In certain embodiments, R6 is CI-C6alkylOhaloCi-C6alkyl. Suitable examples of
F
C1-C6alkylOhaloC1-C6alkyls include, but are not limited to,
In certain embodiments, R6 is haloCi-Coalkyl. Suitable examples of haloalkyls
include, but are not limited to, fluoromethyl, difluoromethyl,
trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and 2,2-difluoroethyl.
In certain embodiments, R6 is CI-C6alkylOH. Suitable alcohols include, but are
not limited to, methanol, ethanol, propanol, butanol and iso-butanol.
In certain embodiments, R6 is CON(R7)(R8). In certain embodiments, 10- is
N(R7)(R8). In certain embodiments, R6 is Ci-C6alkylN(R7)(R8), wherein R7 and
R8 will be
described in detail below.
In certain embodiments, R6 is hydrogen, methyl, ethyl or t-butyl.
In the embodiments described herein, R7 is hydrogen, Ci-C6alkylCOOH, COOH,
C3-C6cycloalkyl, Ci-C6alkyl, haloCt-C6alkyl, Ci-C6alkylOH, COC1-C6alkyl or
COOCi-C6alkyl.
In certain embodiments, R7 is hydrogen, Ci-C6alkylCOOH, COOH, C3-C6cycloalkyl,
Ci-C6alkyl,
haloCi-C6alkyl or Ci-C6alkylOH.
In certain embodiments, R7 is hydrogen. In certain embodiments, R7 is Ci-
C6alkylCOOH. In certain embodiments, IC is COOH. In certain embodiments, R7 is
C3-
C6cycloalkyl. Suitable examples of cycloalkyls include, but are not limited
to, cyclopropyl,
cyclobutyl, cyclopentyl and cyclohexyl. In certain embodiments, R7 is C1-
C6alkyl. Examples of
47
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
Cl-C6a1kyl groups can include but are not limited to, methyl, ethyl, n-propyl,
isopropyl, n-butyl,
isobutyl, sec-butyl, tert-butyl, n-pentyl, isopentyl, neopentyl, tert-pentyl,
1-methylbutyl, 2-
methylbutyl, 1,2-dimethylpropyl, 1-ethylpropyl, n-hexyl, isohexyl, 1-
methylpentyl, 2-
methylpentyl, 3-methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-
dimethylbutyl, 1-
ethylbutyl, 1,1,2-trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethyl-2-
methylpropyl and 1-ethyl-l-
methylpropyl. In certain embodiments, R7 is haloCi-C6alkyl. Suitable examples
of haloalkyls
include, but are not limited to, fluoromethyl, difluoromethyl,
trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and 2,2-difluoroethyl. In certain embodiments, R7 is CI-
C6alkylOH. Examples of
suitable alcohols include, but are not limited to, methanol, ethanol,
propanol, butanol and iso-
butanol.
In certain embodiments, R7 is COCI-C6alkyl. Suitable examples include, but are
not limited to, COCH3. In certain embodiments, R7 is COOCi-C6alkyl. Suitable
examples
include, but are not limited to, COOCH3.
In the embodiments described herein, It8 is hydrogen, CI-C6alkylCOOH, COOH,
C3-C6cycloalkyl, Ci-C6alkyl, haloCi-C6alkyl, Ci-C6alkylOH, COCi-C6alkyl or
COOCi-C6alkyl.
In certain embodiments, R8 is hydrogen, C1-C6alky1COOH, COOH, C3-C6cycloalkyl,
C1-C6alkyl,
haloCi-C6alkyl or Ci-C6alkylOH.
In certain embodiments, R8 is hydrogen. In certain embodiments, R8 is Ci-
C6a1kylCOOH. In certain embodiments, R8 is COOH. In certain embodiments, R8 is
C.3-
C6cycl alkyl Suitable examples of cycloalkyl s include, but are not limited
to, cyclopropyl,
cyclobutyl, cyclopentyl and cyclohexyl. In certain embodiments, R8 is Ci-
C6alkyl. Examples of
CI-C6a1kyl groups can include but are not limited to, methyl, ethyl, n-propyl,
isopropyl, n-butyl,
isobutyl, sec-butyl, tert-butyl, n-pentyl, isopentyl, neopentyl, tert-pentyl,
1-methylbutyl, 2-
methylbutyl, 1,2-dimethylpropyl, 1-ethylpropyl, n-hexyl, isohexyl, 1-
methylpentyl, 2-
methylpentyl, 3-methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-
dimethylbutyl, 1-
ethylbutyl, 1,1,2-trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethy1-2-
methylpropyl and 1-ethyl-l-
methylpropyl. In certain embodiments, R8 is haloCi-C6a1kyl. Suitable examples
of haloalkyls
include, but are not limited to, fluoromethyl, difluoromethyl,
trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and 2,2-difluoroethyl. In certain embodiments, R8 is Ci-
CoalkylOH. Examples of
suitable alcohols include, but are not limited to, methanol, ethanol,
propanol, butanol and iso-
butanol.
In certain embodiments, R8 is COCI-C6alkyl. Suitable examples include, but are
not limited to, COCH3. In certain embodiments, R8 is COOCi-C6alkyl. Suitable
examples
include, but are not limited to, COOCH3.
48
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
In the embodiments described herein, R9 is hydrogen, halogen, CN, OH, Ci-
C6alkoxy, C1-C6alkylOCI-C6alkyl, C1-C6alky1COOH, COOH, C3-C6cycloalkyl, C1-
Coalkyl,
haloCi-C6alkyl, Cl-C6alkylOH, CON(R7)(R8) and N(R7)(R8). In certain
embodiments, R9 is
hydrogen. In certain embodiments, R9 is halogen. Suitable halogens include
fluorine, chlorine,
bromine, or iodine.
In certain embodiments, R9 is CN. In certain embodiments, R9 is OH.
In certain embodiments, R9 is CI-C6alkoxy. Suitable alkoxys include, but are
not
limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy. In certain
embodiments, R9 is
Ci-CoalkylOCI-Coalkyl. In certain embodiments, R9 is COOH. In certain
embodiments, R9 is Ci-
C6alkylCOOH. In certain embodiments, R9 is C3-C6cycloalkyl. Suitable examples
of cycloalkyls
include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl. In certain
embodiments, R9 is C1-C6alkyl. Examples of C1-C6alkyl groups can include, but
are not limited
to, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-
butyl, n-pentyl, isopentyl,
neopentyl, tert-pentyl, 1-methylbutyl, 2-methylbutyl, 1,2-dimethylpropyl, 1-
ethylpropyl, n-hexyl,
isohexyl, 1-methylpentyl, 2-methylpentyl, 3-methylpentyl, 1,1-
dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1-ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-
trimethylpropyl, 1-ethyl-2-methylpropyl and 1-ethyl-1-methylpropyl. In certain
embodiments, R9
is haloCi-C6alkyl. Suitable examples of haloalkyls include, but are not
limited to, fluoromethyl,
difluoromethyl, trifluoromethyl, 2-fluoroethyl, 1,2-difluoroethyl and 2,2-
difluoroethyl. In certain
embodiments, R9 is CI-C6alkyl OH Examples of suitable alcohols include, but
are not limited to,
methanol, ethanol, propanol, butanol and iso-butanol. In certain embodiments,
R9 is
CON(R7)(R8). In certain embodiments, R9 is N(R7)(R8). In certain embodiments,
R9 is C1-
C6alkylN(10(R8).
With regard to the compounds described herein, Rl is hydrogen, halogen, CN,
OH, C1-C6alkoxy, C1-C6alkylOCi-C6alkyl, CI-C6alkylCOOH, COOH, C3-C6cycloalkyl,
Ci-
C6alkyl, haloCI-C6alkyl, CI-C6alkylOH, CON(R7)(R8) and N(R7)(R8). In certain
embodiments,
R1 is hydrogen. In certain embodiments, Rm is halogen. Suitable halogens
include fluorine,
chlorine, bromine, or iodine. In certain embodiments, Rl is CN. In certain
embodiments, R1 is
OH.
In certain embodiments, 10 is C1-C6alkoxy. Suitable alkoxys include, but are
not
limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy. In certain
embodiments, Rth
is Ci-CoalkylOCI-Coalkyl. In certain embodiments, Rio is COOH. In certain
embodiments, Rl is
Ci-C6alkylCOOH. In certain embodiments, RI is C3-C6cycloalkyl. Suitable
examples of
cycloalkyls include, but are not limited to, cyclopropyl, cyclobutyl,
cyclopentyl and cyclohexyl.
49
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
In certain embodiments, R1 is C1-C6alkyl. Examples of C1-C6alkyl groups can
include but are
not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-
butyl, tert-butyl, n-pentyl,
isopentyl, neopentyl, tert-pentyl, 1-methylbutyl, 2-methylbutyl, 1,2-
dimethylpropyl, 1-
ethylpropyl, n-hexyl, isohexyl, 1-methylpentyl, 2-methylpentyl, 3-
methylpentyl, 1,1-
dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1-ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-
trimethylpropyl, 1-ethyl-2-methylpropyl and 1-ethyl-l-methylpropyl. In certain
embodiments,
R1 is haloCi-C6alkyl. Suitable examples of haloalkyls include, but are not
limited to,
fluorom ethyl, difluorom ethyl, trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and 2,2-
difluoroethyl. In certain embodiments, R1 is CI-CoalkylOH. Examples of
suitable alcohols
include, but are not limited to, methanol, ethanol, propanol, butanol and iso-
butanol. In certain
embodiments, R1 is CON(R7)(R8). In certain embodiments, Rm is N(R7)(R8). In
certain
embodiments, R1 is C1-C6alkylN(R7)(R8).
In the embodiments, described herein, R" is hydrogen, halogen, CN, OH, Ci-
C6alkoxy, C1-C6alkylOCI-C6alkyl, CI-C6alkylCOOH, COOH, C3-C6cycloalkyl, CI-
Coalkyl,
haloCi-C6alkyl, CI-C6alkylOH, CON(R7)(R8) and N(R7)(1e). In certain
embodiments, R11 is
hydrogen. In certain embodiments, R11 is halogen. Suitable halogens include
fluorine, chlorine,
bromine, or iodine. In certain embodiments, R11 is CN. In certain embodiments,
R11 is OH.
In certain embodiments, R11 is C1-C6alkoxy. Suitable alkoxys include, but are
not
limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy. In certain
embodiments, R"
is CI-C6alkylOCI-C6alkyl In certain embodiments, R11 is COOH In certain
embodiments, R" is
Ci-C6a1kylCOOH. In certain embodiments, R11 is Cl-C6cycloalkyl. Suitable
examples of
cycloalkyls include, but are not limited to, cyclopropyl, cyclobutyl,
cyclopentyl and cyclohexyl.
In certain embodiments, R" is C1-C6alkyl. Examples of C1-C6alkyl groups can
include but are
not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-
butyl, tert-butyl, n-pentyl,
isopentyl, neopentyl, tert-pentyl, 1-methylbutyl, 2-methylbutyl, 1,2-
dimethylpropyl, 1-
ethylpropyl, n-hexyl, isohexyl, 1-methylpentyl, 2-methylpentyl, 3-
methylpentyl, 1,1-
dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1-ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-
trimethylpropyl, 1-ethyl-2-methylpropyl and 1-ethyl-l-methylpropyl. In certain
embodiments,
R11 is haloCi-C6alkyl. Suitable examples of haloalkyls include, but are not
limited to,
fluoromethyl, difluoromethyl, trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and 2,2-
difluoroethyl. In certain embodiments, R11 is CI-C6alkylOH. Examples of
suitable alcohols
include, but are not limited to, methanol, ethanol, propanol, butanol and iso-
butanol. In certain
embodiments, R" is CON(R7)(R8). In certain embodiments, R11 is N(R7)(R8). In
certain
embodiments, R" is C1-C6alkylN(R7)(R8).
50
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
In the embodiments described herein, 111-2 is hydrogen, halogen, CN, OH, Ci-
C6alkoxy, Ci-C6alkylOCI-C6alkyl, Ci-C6alkylCOOH, COOH, C3-C6cycloalkyl, Ci-
Coalkyl,
haloCi-C6alkyl, Ci-C6alkylOH, CON(R7)(R8), N(R7)(R8) or Ci-C6alkylN(R7)(R8).
In certain
embodiments, R12 is hydrogen. In certain embodiments, R12 is halogen. Suitable
halogens
include fluorine, chlorine, bromine, or iodine. In certain embodiments, R12 is
CN. In certain
embodiments, R12 is OH.
In certain embodiments, R12 is C1-C6alkoxy. Suitable alkoxy groups include,
but
are not limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy. In
certain
embodiments, R12 is Ci-CoalkylOCI-Coalkyl. In certain embodiments, R12 is
COOH. In certain
embodiments, R12 is Ci-C6alkylCOOH. In certain embodiments, R12 is C3-
C6cycloalkyl. Suitable
examples of cycloalkyls include, but are not limited to, cyclopropyl,
cyclobutyl, cyclopentyl and
cyclohexyl. In certain embodiments, R12 is C1-C6alkyl. Examples of Ci-C6alkyl
groups can
include but are not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl,
isobutyl, sec-butyl,
tert-butyl, n-pentyl, isopentyl, neopentyl, tert-pentyl, 1-methylbutyl, 2-
methylbutyl, 1,2-
dimethylpropyl, 1-ethylpropyl, n-hexyl, isohexyl, 1-methylpentyl, 2-
methylpentyl, 3-
methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1-
ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethyl-2-methylpropyl and 1-ethyl-1-
methylpropyl. In
certain embodiments, R12 is haloCi-C6alkyl. Suitable examples of haloalkyls
include, but are not
limited to, fluoromethyl, difluoromethyl, trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and
2,2-difluoroethyl. Tn certain embodiments, R12 is CI-C6alkylOH. Examples of
suitable alcohols
include, but are not limited to, methanol, ethanol, propanol, butanol and iso-
butanol. In certain
embodiments, R12 is CON(R7)(R8). In certain embodiments, R12 is N(R7)(R8). In
certain
embodiments, R12 is Ci-C6alkylN(R7)(R8).
In certain embodiments, R12 is hydrogen, methyl, ethyl, methoxy, OH or
?5
In certain embodiments, R12 is hydrogen or
In the embodiments described herein, R13 is hydrogen, halogen, CN, OH, Ci-
C6alkoxy, C1-C6alkyl0C1-C6alkyl, C1-C6alkylCOOH, COOH, C3-C6cycloalkyl, C1-
C6alkyl,
haloCi-C6alkyl, Ci-CoalkylOH, CON(R7)(R8), N(R7)(R8) or Ci-C6alkylN(R7)(R8).
In certain
embodiments, R13 is hydrogen. In certain embodiments, R13 is halogen. Suitable
halogens
include fluorine, chlorine, bromine, or iodine. In certain embodiments, R13 is
CN. In certain
embodiments, R13 is OH.
In certain embodiments, R13 is C1-C6alkoxy. Suitable alkoxy groups include,
but
51
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
are not limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy. In
certain
embodiments, R13 is CI-C6alkylOC1-C6alkyl. In certain embodiments, R13 is
COOH. In certain
embodiments, R13 is CI-C6alkylCOOH. In certain embodiments, R13 is C3-
C6cycloalkyl. Suitable
examples of cycloalkyls include, but are not limited to, cyclopropyl,
cyclobutyl, cyclopentyl and
cyclohexyl. In certain embodiments, R13 is C1-C6alkyl. Examples of C1-C6alkyl
groups can
include but are not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl,
isobutyl,
sec-butyl, tert-butyl, n-pentyl, isopentyl, neopentyl, tert-pentyl, 1-
methylbutyl, 2-methylbutyl,
1,2-dimethylpropyl, 1-ethylpropyl, n-hexyl, isohexyl, 1-m ethylpentyl, 2-
methylpentyl, 3-
methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1-
ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethy1-2-methylpropyl and 1-ethyl-1-
methylpropyl. In
certain embodiments, R13 is haloCi-C6alkyl Suitable examples of haloalkyls
include, but are not
limited to, fluoromethyl, difluoromethyl, trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and
2,2-difluoroethyl. In certain embodiments, R13 is C1-C6alkylOH. Examples of
suitable alcohols
include, but are not limited to, methanol, ethanol, propanol, butanol and iso-
butanol. In certain
embodiments, R13 is CON(R7)(R8). In certain embodiments, R13 is N(R7)(118). In
certain
embodiments, R13 is Ci-C6alkylN(R7)(R8).
In certain embodiments, R13 is hydrogen, methyl, ethyl, methoxy, OH or
In certain embodiments, R13 is hydrogen or
In certain embodiments, R12 and R13 are independently selected from the group
consisting of hydrogen and Ci-C6alkylOCi-C6alkyl, Ci-C6alkyl.
In certain embodiments described herein, each occurrence of R14 is
independently
selected from the group consisting of hydrogen, halogen, CN, OH, Ci-C6alkoxy,
C1-C6alkylOCi-
C6alky1, Ci-C6alkylCOOH, COOH, C3-C6cycloalkyl, Ci-C6alkyl, haloCi-C6alkyl, Ci-
C6alkylOH,
CON(R7)(R8), N(R7)(R8) or Ci-C6alkylN(R7)(R8). In certain embodiments, R14 is
hydrogen. In
certain embodiments, R14 is halogen. Suitable halogens include fluorine,
chlorine, bromine, or
iodine. In certain embodiments, R14 is CN. In certain embodiments, R14 is OH.
In certain embodiments, R14 is C1-C6alkoxy. Suitable alkoxy groups include,
but
are not limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy. In
certain
embodiments, RN is Ci-CoalkylOCi-C6alkyl. In certain embodiments, R14 is COOH.
In certain
embodiments, RN is Ci-C6alkylCOOH. In certain embodiments, R14 is C3-
C6cycloalkyl. Suitable
examples of cycloalkyls include, but are not limited to, cyclopropyl,
cyclobutyl, cyclopentyl and
cyclohexyl. In certain embodiments, R14 is Cl-C6alkyl. Examples of Ci-C6alkyl
groups can
52
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
include but are not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl,
isobutyl,
sec-butyl, tert-butyl, n-pentyl, isopentyl, neopentyl, tert-pentyl, 1-
methylbutyl, 2-methylbutyl,
1,2-dimethylpropyl, 1-ethylpropyl, n-hexyl, isohexyl, 1-methylpentyl, 2-
methylpentyl, 3-
methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1-
ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethyl-2-methylpropyl and 1-ethyl-1-
methylpropyl. In
certain embodiments, R14 is haloCi-C6alkyl. Suitable examples of haloalkyls
include, but are not
limited to, fluoromethyl, difluoromethyl, trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and
2,2-difluoroethyl. In certain embodiments, R14 is CI-C6alkylOH. Examples of
suitable alcohols
include, but are not limited to, methanol, ethanol, propanol, butanol and iso-
butanol. In certain
embodiments, R14 is CON(R7)(R8). In certain embodiments, R14 is N(R7)(R8). In
certain
embodiments, R14 is C1-C6alkylN(R7)(R8).
In certain embodiments, wherein X is C(R14)2, R14 is independently selected
from
the group consisting of hydrogen, halogen, OH, C1-C6alkylOH, C1-C6alkylalkoxy,
Ci-
C6alkylOCI-C6alkyl and CI-Coalkyl.
In certain embodiments, R14 is hydrogen, methyl, ethyl, methoxy, OH or
In the embodiments described herein, R15 is hydrogen, halogen, CN, OH, Ci-
C6alkoxy, Ci-C6alkylOCi-C6alkyl, C1-C6alkylCOOH, COOH, C3-C6cycloalkyl, Ci-
C6alkyl,
haloCi-C6alkyl, C1-C6alkylOH, CON(R7)(R8) and N(R7)(R8). In certain
embodiments, R15 is
hydrogen. In certain embodiments, R15 is halogen. Suitable halogens include
fluorine, chlorine,
bromine, or iodine. In certain embodiments, R15 is CN. In certain embodiments,
R15 is OH.
In certain embodiments, R15 is Ci-C6alkoxy. Suitable alkoxys include, but are
not
limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy. In certain
embodiments, R15
is Ci-C6alkylOCi-C6alkyl. In certain embodiments, R15 is COOH. In certain
embodiments, R15 is
C1-C6alkylCOOH. In certain embodiments, R15 is C3-C6cycloalkyl. Suitable
examples of
cycloalkyls include, but are not limited to, cyclopropyl, cyclobutyl,
cyclopentyl and cyclohexyl.
In certain embodiments, R15 is Ci-Coalkyl. Examples of Ci-Coalkyl groups can
include but are
not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-
butyl, tert-butyl, n-pentyl,
isopentyl, neopentyl, tert-pentyl, 1-methylbutyl, 2-methylbutyl, 1,2-
dimethylpropyl, 1-
ethylpropyl, n-hexyl, isohexyl, 1-methylpentyl, 2-methylpentyl, 3-
methylpentyl, 1,1-
dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1-ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-
trimethylpropyl, 1-ethyl-2-methylpropyl and 1-ethyl-l-methylpropyl. In
certain
embodiments, R15 is ethyl.
53
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
In certain embodiments, R15 is haloCt-C6alkyl. Suitable examples of haloalkyls
include, but are not limited to, fluoromethyl, difluoromethyl,
trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and 2,2-difluoroethyl. In certain embodiments, R1-5 is CI-
C6alkylOH. Examples of
suitable alcohols include, but are not limited to, methanol, ethanol,
propanol, butanol and iso-
butanol. In certain embodiments, R15 is CON(R7)(R8). In certain embodiments,
R15 is N(R7)(1e).
In certain embodiments, R15 is C1-C6alkylN(R7)(R8).
In certain embodiments, R15 is methyl or ethyl.
In the embodiments described herein, R16 is hydrogen, halogen, CN, OH, CI-
C6alkoxy, Ci-C6alkylOCI-C6alkyl, Ci-C6alkylCOOH, COOH, C3-C6cycloalkyl, Ci-
Coalkyl,
haloCi-C6alkyl, C1-C6alkylOH, CON(R7)(R8) and N(R7)(R8). In certain
embodiments, R16 is
hydrogen. In certain embodiments, R16 is halogen. Suitable halogens include
fluorine, chlorine,
bromine, or iodine. In certain embodiments, R16 is CN. In certain embodiments,
R16 is OH.
In certain embodiments, R16 is C1-C6alkoxy. Suitable alkoxys include, but are
not
limited to, methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy. In certain
embodiments, R16
is CI-C6alkylOC1-C6alkyl. In certain embodiments, R16 is COOH. In certain
embodiments, R16 is
C1-C6alkylCOOH. In certain embodiments, R16 is C3-C6cycloalkyl. Suitable
examples of
cycloalkyls include, but are not limited to, cyclopropyl, cyclobutyl,
cyclopentyl and cyclohexyl.
In certain embodiments, R16 is C1-C6alkyl. Examples of C1-C6alkyl groups can
include but are not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl,
isobutyl, sec-butyl,
tert-butyl, n-pentyl, isopentyl, neopentyl, tert-pentyl, 1-methylbutyl, 2-m
ethyl butyl , 1,2-
dimethylpropyl, 1-ethylpropyl, n-hexyl, isohexyl, 1-methylpentyl, 2-
methylpentyl, 3-
methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1-
ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethyl-2-methylpropyl and 1-ethyl-1-
methylpropyl. In
certain embodiments, R16 is haloCi-C6alkyl. Suitable examples of haloalkyls
include, but are not
limited to, fluoromethyl, difluoromethyl, trifluoromethyl, 2-fluoroethyl, 1,2-
difluoroethyl and
2,2-difluoroethyl. In certain embodiments, R16 is CI-C6alkylOH. Examples of
suitable alcohols
include, but are not limited to, methanol, ethanol, propanol, butanol and iso-
butanol. In certain
embodiments, R16 is CON(R7)(R8). In certain embodiments, R16 is N(R7)(10. In
certain
embodiments, R16 is Ci-C6alkylN(R7)(R8).
In the embodiments of the compounds described herein, 1 is 0 or 1. In certain
embodiments, 1 is 0. In certain embodiments, 1 is 1.
In the embodiments of the compounds described herein, m is 0 or 1. In certain
embodiments, m is 0 In certain embodiments, m is 1
In the embodiments of the compounds described herein, p is 0 or 1. In certain
54
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
embodiments, p is 0. In certain embodiments, p is 1.
In certain embodiments, m and p are 1 and X is 0.
In certain embodiments, m and p are 1 and X is CH2.
In certain embodiments, m is 0, p is 1 and X is 0.
In certain embodiments, m and p are 1 and X is S02.
In certain embodiments, m is 0, p is 1 and X is C(R14)2, wherein each
occurrence
of R14 is independently selected from the group consisting of hydrogen,
halogen, OH, Ci-
C6alkoxy and Ci-C6alkyl.
In certain embodiments, m is 1 and X is 2
C(R14,),
wherein each occurrence of R14
is independently selected from the group consisting of hydrogen, halogen, OH,
Ci-C6alkoxy and
C1-C6alkyl.
For example, in certain embodiments of Formula (I), 1 is 0; m is 1; p is 1;
Xis 0;
V, Y and Z are CH; and Q is CH2 as shown in formula (VII).
p12
R13 114R3
NH 0
HN A N
101
R11.5,,Aso =
R1 R2
I
(VII).
For example, in certain embodiments of Formula (I),1, m and p are 1; X is 0;
V,
Y and Z are CH; and Q is 0 as shown in Formula (VIII).
R12
R13 WI R3
NH 0
HN N =
.10
=
2
R5
0
R6 (VIII).
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
In the embodiments described herein, A is a straight or branched, saturated or
unsaturated (C3-C1o)alkylene, phenyl(C3-Cto)alkylene or cycloalkyl(C3-
COalkylene comprising
at least one ¨CH2- group, wherein one or more additional ¨CH2- groups in A are
optionally and
independently replaced with a moiety selected from the group consisting of 0,
S, NR, CONR,
NRCO, S02, and SO2NR and wherein one or more of the hydrogens along A can be
replaced
with a group independently selected from hydroxyl, halogen and C1-3haloalkyl.
In certain
embodiments, A is a straight or branched, saturated or unsaturated (C3-
C10)a1ky1ene or
cycloalkyl(C3-Cto)alkylene, wherein one or more ¨CH2- groups in A are
optionally and
independently replaced with a moiety selected from the group consisting of 0,
S and NH. In
certain embodiment, A will always have at least one ¨CH2- group.
In certain embodiments, A is a straight (C3-C1o)alkylene. Examples of straight
(C3-C1o)alkylenes include,
;221
;221
X ,
.--- .--
f
-,-- ..,-
..õ
1----
L.
r.- -I- V
.
In certain embodiments, A is a branched (C3-C1o)alkylene. Suitable branched
(C3-
Cto)alkylenes include but are not limited to:
,
r.õ( --,,,,,,z, ;221
221
;221 ( 2
24 art 1 ,"'...
.."'-' ../.
'..../...
'111.
..µzza ....--- ',......... ...õ -V X.
.2.2z.
\ .
In certain embodiments, A is a saturated (C3-C1O)alkylene. Examples include,
56
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
,,..N
,i;
X ..-
r- ,.. ....,
....- ....-
f
--r- ..---
:\...-
r ,- ....- ....--
õL..... ..---
,----
-..., r
,
(.....c *".............)221 )221
\
'''.---
,
...õ..., ....... ,,,,..,..
"22.. ...y
In certain embodiments, A is an unsaturated (C3-C1o)alkylene. Suitable
unsaturated (C3-Cio)alkylenes include any of the saturated (C3-C1o)alkylene,
wherein hydrogens
have been removed and one or more double or triple covalent bonds exist
between adjacent
carbon atoms. Examples of unsaturated (C3-Cio)alkylenes include, but are not
limited to,
Jr'
I
.....4=- ...-- ___...1
r
In other embodiments, A is a straight cycloalkyl(C3-C1o)alkylene. Suitable
straight cycloalkyl(C3-Cio)alkylenes include a cycloalkyl(C3-Cio)alkylene
wherein two carbons
in a chain are included in a (C3-C1o)cycloalkyl. Examples of straight
cycloalkyl(C3-C1o)alkylenes
include, but are not limited to,
57
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
LN,
In certain embodiments, A is a branched cycloalkyl(C3-C1o)alkylene. Suitable
branched cycloalkyl(Ci-Cio)alkylenes include a branched (C3-Cio)alkylene
wherein two carbons
in a chain are included in a (C3-C1o)cycloalkyl. Examples of cycloalkyl(C3-
C1o)alkylenes
include, but are not limited to,
In certain embodiments, A is a saturated cycloalkyl(C3-C1o)alkylene. Examples
of
saturated cycloalkyl(C3-C1o)alkylenes include, but are not limited to,
58
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
r<r2"1, tzel,
.:3111 "luv
In certain embodiments, A is an unsaturated cycloalkyl(C3-C1o)alkylene.
Examples of unsaturated cyclo(C3-C1o)alkylenes include, but are not limited
to,
In certain embodiments, A is an unsaturated or saturated phenyl(C3-
C1o)alkylene.
Examples of unsaturated and saturated phenyl(C3-C1o)alkylenes include, but are
not limited to,
c??,
101
4111:1 11111
`371,
In other embodiments, one or more ¨CH2- groups in A are optionally and
independently replaced with a moiety selected from the group consisting of 0,
S. NR, CONR,
NRCO, S02, and SO2NR. In other embodiments, one or more ¨CH2- groups in A are
optionally
and independently replaced with a moiety selected from the group consisting of
0, S and NH. In
other embodiments, one or more ¨CH2- groups in A are optionally and
independently replaced
with 0. In other embodiments, one or more ¨CH2- groups in A are optionally and
independently
replaced with S. In other embodiments, one or more ¨CH2- groups in A are
optionally and
independently replaced with NR. In other embodiments, one or more ¨CH2- groups
in A are
59
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
optionally and independently replaced with CONR. In other embodiments, one or
more ¨CH2-
groups in A are optionally and independently replaced with NRCO. In other
embodiments, one
or more ¨CH2- groups in A are optionally and independently replaced with S02.
In other
embodiments, one or more ¨CH2- groups in A are optionally and independently
replaced with
and SO2NR. R will be described in further detail below.
In the embodiments described herein, R is hydrogen, C1-C6alkylCOOH, COOH,
C3-C6cycloalkyl, C1-C6alkyl, haloCi-C6alkyl, C1-C6alkylOH, COC1-C6alkyl or
COOC1-C6alkyl.
In certain embodiments, R is hydrogen, CI-C6alkylCOOH, COOH, C3-C6cycloalkyl,
CI-C6alkyl,
haloCi-Coalkyl or Ci-CoalkylOH.
In certain embodiments, R is hydrogen. In certain embodiments, R is Ci-
C6alkylCOOH. In certain embodiments, R is COOH. In certain embodiments, R is
C3-
C6cycloalkyl. Suitable examples of cycloalkyls include, but are not limited
to, cyclopropyl,
cyclobutyl, cyclopentyl and cyclohexyl. In certain embodiments, R is C1-
C6alkyl. Examples of
Ci-C6alkyl groups can include but are not limited to, methyl, ethyl, n-propyl,
isopropyl, n-butyl,
isobutyl, sec-butyl, tert-butyl, n-pentyl, isopentyl, neopentyl, tert-pentyl,
1-methylbutyl, 2-
methylbutyl, 1,2-dimethylpropyl, 1-ethylpropyl, n-hexyl, isohexyl, 1-
methylpentyl, 2-
methylpentyl, 3-methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-
dimethylbutyl, 1-
ethylbutyl, 1,1,2-trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethyl-2-
methylpropyl and 1-ethyl-l-
methylpropyl. In certain embodiments, R is haloCi-C6alkyl. Suitable examples
of haloalkyls
include, but are not limited to, fluoromethyl, difluoromethyl, trifluorom
ethyl, 2-fluoroethyl, 1,2-
difluoroethyl and 2,2-difluoroethyl. In certain embodiments, R is Ci-
C6alkylOH. Examples of
suitable alcohols include, but are not limited to, methanol, ethanol,
propanol, butanol and iso-
butanol.
In certain embodiments, R is COC1-C6alkyl. Suitable examples include, but are
not limited to, COCH3. In certain embodiments, R is COOCi-C6alkyl. Suitable
examples include,
but are not limited to, COOCH3.
Examples of such embodiments include, but are not limited to,
rw
r)1,
gf- 6 (!)
:72;_ o)
In certain embodiments, A is
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
"1.4 z .., "-,... I ....... ,V. I /".
!=-=
-42,../-
,,,,,, r r
.A.r. "I.'''.
In certain embodiments, one or more of the hydrogens along A can be replaced
with a group independently selected from hydroxyl, halogen and C1_3 haloalkyl.
Examples of
suitable halogens include chlorine, bromine, fluorine and iodine. In certain
embodiments, A is
"'WV
4,1\11/ VVIIV I
)
,J1I ' F
41.11.1V
..,-' Fsf
..--'
-\.."
-1z_ 0 ..\..., =,-----F,.../
" IV V '
In each of the various embodiments of the invention, in the compounds used in
the methods herein, each variable (including those in each of Formulae (I) -
(VIII), and the
various embodiments thereof) it shall be understood that each variable is to
be selected
independently of the others unless otherwise indicated.
In each of the various embodiments of the invention, the compounds described
herein, including those in each of Formulae (I) - (VIII)and the various
embodiments thereof, may
exit in different forms of the compounds such as, for example, any solvates,
hydrates,
stereoisomers, and tautomers of said compounds and of any pharmaceutically
acceptable salts
thereof
In certain embodiments, compounds described herein include:
NH 0
HNJ.L.N
0
HN 0
OH
61
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH 0
HNAN
0
HN 0
OH
NH 0
HNAN
0
HN 0
0
NH 0
HNAN
HN 0
0
0
NH 0
HNAN
JL
HN 0
r". OH
NH 0
HNAN
0
HN 0
OH
62
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH 0
HN'ic
0
HN 0
7-
0
NH 0
HNAN
Jó
HN 0
0
NH 0
HNAN
0
HN 0
0
NH 0
HNAN
JU
0 NH
--....
0
NH 0
HNAN
0
HN 0
/
0
63
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH 0
HWII.'N
0
NH
0
NH 0
HNAN
0
HN 0
0
0
NH 0
HNAN
0
HN 0
OH
0'
NH 0
HNAN 4110
0
HN 0
0
64
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH 0
HNAN
0
HN 0
0
NH
HNAN
HN 0
0
NH 0
HNAN
0
HN 0
0
NH 0
HNAN
0
HN 0
0
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
HNAN
Já
HN
0
NH
HNAN
0
HN 0
0
NH
HNAN
Ió
HN
NH
HNAN
Iá
ID
HN
66
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH 0
HN)-(N /1101
0
HN 0
0
NH 0
NW-11'N 1101
HN 0
0
NH
/"'. 0
NH
0
In certain embodiments, compounds described herein include:
NH
HNJ-N
0
HN 0
,,,OH
67
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH /--'''0
HNAN ¨
/t,õ
0
HN 0
NH 0
HNAN'
/H..
0
HN 0
0
NH --'0
HNAN -
/1,..
0
HN 0
0
NH
HNAN
17L
HN 0
0
NH 0
HNAN's.
/ " 0
0
"t-'-
HN 0
0
68
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
HNAN -
/oõ
0
HN 0
NH
HN N
//,,.
0
HN 0
NH
HNAN
/1,..
0
HN 0
0
NH
HNAN
0
NO
0
NH
HNAN
HN 0
0
69
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH 0
HNAN -
/I ..=
0
0 NH
--,_
0
NH
HN'll'N
1110
HN 0
/
0
NH --'-'0
HN A N
0 NH
0
NH "...'(:)
'
HNA N 0
,---L.
HN 0
0
NH /.---'10
HN AN
/1,. =
0
HN 0
0
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH 0
HKN's.
0
HN 0
NH
HN,;k1"N"'
0
HN 0
0
NH"= 0
HN
* HN 0
0
NH
HN ANN'.
/11Lo
.=
HN 0
0
71
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH 0
HN 'IL Nµ
LT
/II..
0
HN 0
0
NH------0
A =
HN* N
0
HN 0
-...._
0
NH 0
HNAN'.
/1,..
0
* HN 0
*
0
NH
7
HNA N lb
zoõ
0
HN 0
0
NH -'0
)- 7
HN* N
Ii
HN
- 0
72
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
A 7
HN* N
0
HN 0
0
NH 0
0
HN 0
0
NH 0
0
HN 0
0
NH
HNANo.
/1.,.
0
NH
or a pharmaceutically acceptable salt thereof.
73
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
Definitions and Abbreviations:
The terms used herein have their ordinary meaning and the meaning of such
terms
is independent at each occurrence thereof. That notwithstanding and except
where stated
otherwise, the following definitions apply throughout the specification and
claims. Chemical
names, common names and chemical structures may be used interchangeably to
describe that
same structure. These definitions apply regardless of whether a term is used
by itself or in
combination with other terms, unless otherwise indicated. Hence the definition
of "alkyl" applies
to "alkyl" as well as the "alkyl" portion of "hydroxyalkyl", "haloalkyl",
arylalkyl-, alkylaryl-,
-alkoxy" etc.
It shall be understood that, in the various embodiments of the invention
described
herein, any variable not explicitly defined in the context of the embodiment
is as defined in
Formula (I).
In the various embodiments described herein, each variable is selected
independently of the others unless otherwise indicated.
"Drug resistant" means, in connection with a Plasmodium parasite strain, a
Plasmodium species which is no longer susceptible to at least one previously
effective drug;
which has developed the ability to withstand attack by at least one previously
effective drug. A
drug resistant strain may relay that ability to withstand to its progeny. Said
resistance may be due
to random genetic mutations in the bacterial cell that alters its sensitivity
to a single drug or to
different drugs
"Patient" includes both human and non-human animals. Non-human animals
include those research animals and companion animals such as mice, rats,
primates, monkeys,
chimpanzees, great apes, dogs, and house cats.
"Pharmaceutical composition" (or "pharmaceutically acceptable composition")
means a composition suitable for administration to a patient. Such
compositions may contain the
neat compound (or compounds) of the invention or mixtures thereof, or salts,
solvates, prodrugs,
isomers, or tautomers thereof, and one or more pharmaceutically acceptable
carriers or diluents.
The term -pharmaceutical composition" is also intended to encompass both the
bulk composition
and individual dosage units comprised of one or more (e.g., two)
pharmaceutically active agents
such as, for example, a compound of the present invention and an additional
agent selected from
the lists of the additional agents described herein, along with any
pharmaceutically inactive
excipients. The bulk composition and each individual dosage unit can contain
fixed amounts of
the afore-said "more than one pharmaceutically active agents" The bulk
composition is material
that has not yet been formed into individual dosage units. An illustrative
dosage unit is an oral
74
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
dosage unit such as tablets, pills and the like. Similarly, the herein-
described method of treating a
patient by administering a pharmaceutical composition of the present invention
is also intended
to encompass the administration of the afore-said bulk composition and
individual dosage units.
"Halogen" and "halo" mean fluorine, chlorine, bromine, or iodine. Preferred
are
fluorine, chlorine and bromine.
"Alkylene," by itself or as part of another substituent means a divalent
hydrocarbon chain radical having the stated number of carbon atoms. For
example, -(Ci-
05)alkylene, would include, e.g., -CH2-, -CH2CH2-, -CH2CH2C+12-, -CH2CH2CH2CH2-
, -
CH2CH(CH3)CH2- Of -CH2CH2CH2CH2CH2-. A straight alkylene means a divalent
straight
hydrocarbon chain radical having the stated number of carbon atoms. A branched
alkylene
means a divalent branched hydrocarbon chain radical having the stated number
of carbon atoms.
A saturated alkylene means a divalent saturated hydrocarbon chain radical
having the stated
number of carbon atoms. An unsaturated alkylene means a divalent hydrocarbon
chain radical
having the stated number of carbon atoms and one or more double or triple
covalent bonds
within the chain. A cycloalkylene means a divalent hydrocarbon chain radical
having the stated
number of carbon atoms and a cycloalkyl moiety within the chain.
"Alkyl" means an aliphatic hydrocarbon group which may be straight or branched
and comprising about 1 to about 20 carbon atoms in the chain. Preferred alkyl
groups contain
about 1 to about 12 carbon atoms in the chain. More preferred alkyl groups
contain about 1 to
about 6 carbon atoms in the chain Branched means that one or more lower alkyl
groups such as
methyl, ethyl or propyl, are attached to a linear alkyl chain. "Lower alkyl"
means a group having
about 1 to about 6 carbon atoms in the chain which may be straight or
branched. Non-limiting
examples of suitable alkyl groups include methyl, ethyl, n-propyl, isopropyl
and t-butyl.
"Haloalkyl" means an alkyl as defined above wherein one or more hydrogen
atoms on the alkyl is replaced by a halo group defined above.
"Aryl" means an aromatic monocy clic or multi cycli c ring system comprising
about 6 to about 14 carbon atoms, preferably about 6 to about 10 carbon atoms.
The aryl group
can be optionally substituted with one or more "ring system substituents"
which may be the same
or different, and are as defined herein. Non-limiting examples of suitable
aryl groups include
phenyl and naphthyl. "Monocyclic aryl" means phenyl.
"Cycloalkyl" means a non-aromatic mono- or multicyclic ring system comprising
about 3 to about 12 carbon atoms, preferably about 3 to about 10 carbon atoms.
Preferred
cycloalkyl rings contain about 5 to about 10 ring atoms. The cycloalkyl can be
optionally
substituted with one or more sub stituents, which may be the same or
different, as described
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
herein. Monocyclic cycloalkyl refers to monocyclic versions of the cycloalkyl
moieties described
herein. Non-limiting examples of suitable monocyclic cycloalkyls include
cyclopropyl,
cyclopentyl, cyclohexyl, cycloheptyl and the like. Multicyclic cycloalkyls
refers to multicyclic,
including bicyclic, rings that include a non-aromatic ring. Non-limiting
examples of suitable
multicyclic cycloalkyls include 1-decalinyl, norbornyl, adamantyl and the
like. In certain
embodiments, a non-aromatic ring is fused to an aromatic ring.
"Heterocycloalkyl" (or "heterocyclyl") means a non-aromatic, saturated or
partially saturated monocyclic or multicyclic ring system comprising about 3
to about 10 ring
atoms, preferably about 5 to about 10 ring atoms, in which one or more of the
atoms in the ring
system is an element other than carbon, for example nitrogen, oxygen or
sulfur, alone or in
combination There are no adjacent oxygen and/or sulfur atoms present in the
ring system.
Preferred heterocyclyls contain about 5 to about 6 ring atoms. The prefix aza,
oxa or thia before
the heterocyclyl root name means that at least a nitrogen, oxygen or sulfur
atom respectively is
present as a ring atom. Any ¨NH in a heterocyclyl ring may exist protected
such as, for example,
as an -N(Boc), -N(CBz), -N(Tos) group and the like; such protections are also
considered part of
this invention. The heterocyclyl can be optionally substituted by one or more
substituents, which
may be the same or different, as described herein. The nitrogen or sulfur atom
of the heterocyclyl
can be optionally oxidized to the corresponding N-oxide, S-oxide or S,S-
dioxide. Thus, the term
"oxide," when it appears in a definition of a variable in a general structure
described herein,
refers to the corresponding N-oxide, S-oxide, or S,S-di oxide "Heterocycly1"
also includes rings
wherein =0 replaces two available hydrogens on the same carbon atom (i.e.,
heterocyclyl
includes rings having a carbonyl group in the ring). Such =0 groups may be
referred to herein as
HNO
0
cc oxo." An example of such a moiety is pyrrolidinone (or pyrrolidone): .
As used
herein, the term "monocyclic heterocycloalkyl" refers monocyclic versions of
the
heterocycloalkyl moieties described herein and include a 4- to 7-membered
monocyclic
heterocycloalkyl groups comprising from 1 to 4 ring heteroatoms, said ring
heteroatoms being
independently selected from the group consisting of N, N-oxide, 0, S, S-oxide,
5(0), and S(0)2.
The point of attachment to the parent moiety is to any available ring carbon
or ring heteroatom.
Non-limiting examples of monocyclic heterocycloalkyl groups include piperidyl,
oxetanyl,
pyrrolyl, piperazinyl, morpholinyl, thiomorpholinyl, thiazolidinyl, 1,4-
dioxanyl,
tetrahydrofuranyl, tetrahydrothiophenyl, beta lactam, gamma lactam, delta
lactam, beta lactone,
76
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
gamma lactone, delta lactone, and pyrrolidinone, and oxides thereof. A non-
limiting example of
a monocyclic heterocycloalkyl group include the moiety: O.
Non-limiting examples of multicyclic heterocycloalkyl groups include, bicyclic
heterocycloalkyl
groups. Specific examples include, but are not limited to,
0
- and
"Alkoxy" means an alkyl-0- group in which the alkyl group is as previously
described. Non-limiting examples of suitable alkoxy groups include methoxy,
ethoxy, n-
propoxy, isopropoxy and n-butoxy. The bond to the parent moiety is through the
ether oxygen.
The term "substituted" means that one or more hydrogens on the designated atom
is replaced with a selection from the indicated group, provided that the
designated atom's normal
valency under the existing circumstances is not exceeded, and that the
substitution results in a
stable compound. Combinations of substituents and/or variables are permissible
only if such
combinations result in stable compounds. By "stable compound' or "stable
structure" is meant a
compound that is sufficiently robust to survive isolation to a useful degree
of purity from a
reaction mixture, and formulation into an efficacious therapeutic agent.
The term "optionally substituted" means optional substitution with the
specified
groups, radicals or moieties.
When a variable appears more than once in a group, e.g., R8 in ¨N(R8)2, or a
variable appears more than once in a structure presented herein, the variables
can be the same or
different.
A solid line ¨, as a bond generally indicates a mixture of, or either of, the
possible isomers, e g , containing (R)- and (S)-stereochem stry For exam pl e-
0 0
0
41
means containing either one of or both N and --hr
The wavy line '-u-v1A), as used herein shown crossing a line representing a
chemical bond, indicates a point of attachment to the rest of the compound.
Lines drawn into the
ring systems, such as, for example 0¨indicates that the indicated line (bond)
may be
attached to any of the substitutable ring atoms.
77
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
"Oxo" is defined as an oxygen atom that is double bonded to a ring carbon in a
cycloalkyl, cycloalkenyl, heterocycloalkyl, heterocycloalkenyl, or another
ring described herein,
___________________ 0
e.g., H
In this specification, where there are multiple oxygen and/or sulfur atoms in
a ring
system, there cannot be any adjacent oxygen and/or sulfur present in said ring
system.
As well known in the art, a bond drawn from a particular atom wherein no
moiety
is depicted at the terminal end of the bond indicates a methyl group bound
through that bond to
the atom, unless stated otherwise For example.
H3c cH,
represents
=
In another embodiment, the compounds useful in the methods of the invention,
and/or compositions comprising them useful in said methods, are present in
isolated and/or
purified form. The term "purified", "in purified form" or "in isolated and
purified form" for a
compound refers to the physical state of said compound after being isolated
from a synthetic
process (e.g. from a reaction mixture), or natural source or combination
thereof Thus, the term
"purified", "in purified form" or -in isolated and purified form" for a
compound refers to the
physical state of said compound (or a tautomer or stereoisomer thereof, or
pharmaceutically
acceptable salt or solvate of said compound, said stereoisomer, or said
tautomer) after being
obtained from a purification process or processes described herein or well
known to the skilled
artisan (e.g., chromatography, recrystallization and the like), in sufficient
purity to be suitable for
in vivo or medicinal use and/or characterizable by standard analytical
techniques described
herein or well known to the skilled artisan.
It shall be understood that any carbon as well as heteroatom with unsatisfied
valences in the text, schemes, examples and tables herein is assumed to have
the sufficient
number of hydrogen atom(s) to satisfy the valences.
When a functional group in a compound is termed "protected", this means that
the
group is in modified form to preclude undesired side reactions at the
protected site when the
compound is subjected to a reaction. Suitable protecting groups will be
recognized by those with
ordinary skill in the art as well as by reference to standard textbooks such
as, for example, T. W.
Greene et al., Protective Groups in Organic Synthesis (1991), Wiley, New York.
Another embodiment provides prodrugs and/or solvates of the compounds of the
78
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
invention. A discussion of prodrugs is provided in T. Higuchi and V. Stella,
Pro-drugs as Novel
Delivery Systems (1987) 14 of the A.C.S. Symposium Series, and in
Bioreversible Carriers in
Drug Design, (1987) Edward B. Roche, ed., American Pharmaceutical Association
and
Pergamon Press. The term "prodrug" means a compound (e.g., a drug precursor)
that is
transformed in vivo to yield a compound of the invention or a pharmaceutically
acceptable salt,
hydrate or solvate of the compound. The transformation may occur by various
mechanisms (e.g.,
by metabolic or chemical processes), such as, for example, through hydrolysis
in blood. A
discussion of the use of prodrugs is provided by T. Higuchi and W. Stella,
"Pro-drugs as Novel
Delivery Systems," Vol. 14 of the A.C.S. Symposium Series, and in
Bioreversible Carriers in
Drug Design, ed. Edward B. Roche, American Pharmaceutical Association and
Pergamon Press,
1987.
For example, if a compound useful in the methods of the invention or a
pharmaceutically acceptable salt thereof, contains a carboxylic acid
functional group, a prodrug
can comprise an ester formed by the replacement of the hydrogen atom of the
acid group with a
group such as, for example, (C1¨C8)alkyl, (C2-C12)alkanoyloxymethyl, 1-
(alkanoyloxy)ethyl
having from 4 to 9 carbon atoms, 1-methyl-1-(alkanoyloxy)-ethyl having from 5
to 10 carbon
atoms, alkoxycarbonyloxymethyl having from 3 to 6 carbon atoms, 1-
(alkoxycarbonyloxy)ethyl
having from 4 to 7 carbon atoms, 1-methyl-1-(alkoxycarbonyloxy)ethyl having
from 5 to 8
carbon atoms, N-(alkoxycarbonyl)aminomethyl having from 3 to 9 carbon atoms, 1-
(N-
(alkoxycarbonyl)amino)ethyl having from 4 to 10 carbon atoms, 3-phthalidyl, 4-
crotonolactonyl,
gamma-butyrolacton-4-yl, di-N,N-(Ci-C2)alkylamino(C2-C3)alkyl (such as 13-
dimethylaminoethyl), carbamoy1-(C1-C2)alkyl, N,N-di (C1-C2)alkylcarbamoy1-(C1-
C2)alkyl and
piperidino-, pyrrolidino- or morpholino(C2-C3)alkyl, and the like.
Similarly, if a compound used in the methods of the invention contains an
alcohol
functional group, a prodrug can be formed by the replacement of the hydrogen
atom of the
alcohol group with a group such as, for example, (Ci-C6)alkanoyloxymethyl, 1-
((Ci-
C6)alkanoyl oxy)ethyl, 1-methyl-1-((C1-C6)alkanoyloxy)ethyl, (C1-
C6)alkoxycarbonyloxymethyl,
N-(C1-C6)alkoxycarbonylaminomethyl, succinoyl, (C1-C6)alkanoyl, a-amino(C1-
C4)alkanyl,
arylacyl and a-aminoacyl, or a-aminoacyl-a-aminoacyl, where each a-aminoacyl
group is
independently selected from the naturally occurring L-amino acids, P(0)(OH)2, -
P(0)(0(Ci-
C6)alky1)2 or glycosyl (the radical resulting from the removal of a hydroxyl
group of the
hemiacetal form of a carbohydrate), and the like.
If a compound used in the methods of the invention incorporates an amine
functional group, a prodrug can be formed by the replacement of a hydrogen
atom in the amine
79
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
group with a group such as, for example, R-carbonyl, RO-carbonyl, NRR'-
carbonyl where R and
R' are each independently (Ci-Cio)alkyl, (C3-C7) cycloalkyl, benzyl, or R-
carbonyl is a natural a-
aminoacyl or natural a-aminoacyl, -C(OH)C(0)0Y1 wherein Y1 is H, (CI -C6)alkyl
or benzyl,
-C(0Y2)Y3 wherein Y2 is (Ci-C4) alkyl and Y3 is (CI-C6)alkyl, carboxy (Ci-
C6)alkyl, amino(Ci-
C4)alkyl or mono-N- or di-N,N-(C1-C6)alkylaminoalkyl, -C(Y4)Y5 wherein Y4 is H
or methyl
and Y5 is mono-N- or di-N,N-(C1-C6)alkylamino morpholino, piperidin-l-yl or
pyrrolidin-l-yl,
and the like.
One or more compounds used in the methods of the invention may exist in
unsolvated as well as solvated forms with pharmaceutically acceptable solvents
such as water,
ethanol, and the like, and it is intended that the invention embrace both
solvated and unsolvated
forms. "Solvate" means a physical association of a compound of the invention
with one or more
solvent molecules. This physical association involves varying degrees of ionic
and covalent
bonding, including hydrogen bonding. In certain instances, the solvate will be
capable of
isolation, for example when one or more solvent molecules are incorporated in
the crystal lattice
of the crystalline solid. "Solvate" encompasses both solution-phase and
isolatable solvates. Non-
limiting examples of suitable solvates include ethanolates, methanolates, and
the like. "Hydrate"
is a solvate wherein the solvent molecule is H20.
One or more compounds used in the methods of the invention may optionally be
converted to a solvate. Preparation of solvates is generally known. Thus, for
example M. Caira et
al., J. Pharmaceutical Sci ., 1993, 3, 601-611, describe the preparation of
the solvates of the
antifungal tluconazole in ethyl acetate as well as from water. Similar
preparations of solvates,
hemisolvate, hydrates and the like are described by E. C. van Tonder et at.,
AAPS
PharmSciTech., 5(1), article 12 (2004); and A. L. Bingham et al., Chem.
Commun., 603-604
(2001). A typical, non-limiting, process involves dissolving the inventive
compound in desired
amounts of the desired solvent (organic or water or mixtures thereof) at a
higher than ambient
temperature, and cooling the solution at a rate sufficient to form crystals
which are then isolated
by standard methods. Analytical techniques such as, for example I. R.
spectroscopy, show the
presence of the solvent (or water) in the crystals as a solvate (or hydrate).
"Effective amount" or "therapeutically effective amount" is meant to describe
an
amount of compound or a composition used in the methods of the present
invention effective in
inhibiting the above-noted diseases or enzyme activity and thus producing the
desired
therapeutic, ameliorative, inhibitory or preventative effect.
Another embodiment provides pharmaceutically acceptable salts of the
compounds to be used in the methods of the invention. Thus, reference to a
compound used in
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
the methods of the invention herein is understood to include reference to
salts thereof, unless
otherwise indicated. The term "salt(s)", as employed herein, denotes acidic
salts formed with
inorganic and/or organic acids, as well as basic salts formed with inorganic
and/or organic bases.
In addition, when a compound of the invention contains both a basic moiety,
such as, but not
limited to a pyridine or imidazole, and an acidic moiety, such as, but not
limited to a carboxylic
acid, zwitterions ("inner salts") may be formed and are included within the
term "salt(s)" as used
herein. Pharmaceutically acceptable (i.e., non-toxic, physiologically
acceptable) salts are
preferred, although other salts are also useful. Salts of the compounds used
in the methods of the
invention may be formed, for example, by reacting a compound of the invention
with an amount
of acid or base, such as an equivalent amount, in a medium such as one in
which the salt
precipitates or in an aqueous medium followed by lyophilization.
Exemplary acid addition salts include acetates, ascorbates, benzoates,
benzenesulfonates, bisulfates, borates, butyrates, citrates, camphorates,
camphorsulfonates,
fumarates, hydrochlorides, hydrobromides, hydroiodides, lactates, maleates,
methanesulfonates,
naphthalenesulfonates, nitrates, oxalates, phosphates, propionates,
salicylates, succinates,
sulfates, tartarates, thiocyanates, toluenesulfonates (also known as
tosylates,) and the like.
Additionally, acids which are generally considered suitable for the formation
of
pharmaceutically useful salts from basic pharmaceutical compounds are
discussed, for example,
by P. Stahl et al., Camille G. (eds.) Handbook of Pharmaceutical Salts.
Properties, Selection and
Use. (2002) Zurich. Wiley-VCH; S Berge et al., Journal of Pharmaceutical
Sciences (1977)
66(1) 1-19; P. Gould, International .1 of Pharmaceutics (1986) 33 201-217;
Anderson et al., 'The
Practice of Medicinal Chemistry (1996), Academic Press, New York; and in The
Orange Book
(Food & Drug Administration, Washington, D.C. on their website). These
disclosures are
incorporated herein by reference thereto.
Exemplary basic salts include ammonium salts, alkali metal salts such as
sodium,
lithium, and potassium salts, alkaline earth metal salts such as calcium and
magnesium salts,
salts with organic bases (for example, organic amines) such as
dicyclohexylamines, t-butyl
amines, and salts with amino acids such as arginine, lysine and the like.
Basic nitrogen-
containing groups may be quarternized with agents such as lower alkyl halides
(e.g. methyl,
ethyl, and butyl chlorides, bromides and iodides), dialkyl sulfates (e.g.
dimethyl, diethyl, and
dibutyl sulfates), long chain halides (e.g. decyl, lauryl, and stearyl
chlorides, bromides and
iodides), aralkyl halides (e.g. benzyl and phenethyl bromides), and others.
All such acid salts and base salts are intended to be pharmaceutically
acceptable
salts within the scope of the invention and all acid and base salts are
considered equivalent to the
81
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
free forms of the corresponding compounds for purposes of the invention.
Another embodiment provides pharmaceutically acceptable esters of the
compounds used in the methods of the invention. Such esters include the
following groups: (1)
carboxylic acid esters obtained by esterification of the hydroxy groups, in
which the non-
carbonyl moiety of the carboxylic acid portion of the ester grouping is
selected from straight or
branched chain alkyl (for example, acetyl, n-propyl, t-butyl, or n-butyl),
alkoxyalkyl (for
example, methoxymethyl), aralkyl (for example, benzyl), aryloxyalkyl (for
example,
phenoxymethyl), aryl (for example, phenyl optionally substituted with, for
example, halogen, CI-
4alkyl, or C1-4alkoxy or amino); (2) sulfonate esters, such as alkyl- or
aralkylsulfonyl (for
example, methanesulfonyl); (3) amino acid esters (for example, L-valyl or L-
isoleucyl); (4)
phosphonate esters and (5) mono-, di- or triphosphate esters. The phosphate
esters may be
further esterified by, for example, a C1-20 alcohol or reactive derivative
thereof, or by a 2,3-di
(C6-24)acyl glycerol.
As mentioned herein, another embodiment provides tautomers of the compounds
of the invention to be used in the methods herein, and salts, solvates, esters
and prodrugs of said
tautomers. It shall be understood that all tautomeric forms of such compounds
are within the
scope of the compounds used in the methods of the invention. For example, all
keto-enol and
imine-enamine forms of the compounds, when present, are included in the
invention.
The compounds used in the methods of the invention may contain asymmetric or
chiral centers, and, therefore, exist in different stereoisomeric forms Tt is
intended that all
stereoisomeric forms of the compounds used in the methods of the invention as
well as mixtures
thereof, including racemic mixtures, form part of the present invention. In
addition, the present
invention embraces use of all geometric and positional isomers. For example,
if a compound
used in the methods of the invention incorporates a double bond or a fused
ring, both the cis- and
trans-forms, (E) and (Z) forms, as well as mixtures, are embraced within the
scope of the
invention.
Another embodiment provides for diastereomeric mixtures and individual
enantiomers of the compounds used in the methods of the invention.
Diastereomeric mixtures
can be separated into their individual diastereomers based on their physical
chemical differences
by methods well known to those skilled in the art, such as, for example, by
chromatography and/or fractional crystallization. Enantiomers can be separated
by converting
the enantiomeric mixture into a diastereomeric mixture by reaction with an
appropriate optically
active compound (e.g., chiral auxiliary such as a chiral alcohol or Mosher's
acid chloride),
separating the diastereomers and converting (e.g., hydrolyzing) the individual
diastereomers to
82
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
the corresponding pure enantiomers. Also, some of the compounds used in the
methods of the
invention may be atropisomers (e.g., substituted biaryls) and are considered
as part of this
invention. Enantiomers can also be separated by use of chiral HPLC column.
All stereoisomers (for example, geometric isomers, optical isomers and the
like)
of the compounds used in the methods of the invention (including those of the
salts, solvates,
esters and prodrugs of the compounds as well as the salts, solvates and esters
of the prodrugs),
such as those which may exist due to asymmetric carbons on various
substituents, including
enantiomeric forms (which may exist even in the absence of asymmetric
carbons), rotameric
forms, atropisomers, and diastereomeric forms, are contemplated as embodiments
within the
scope of this invention, as are positional isomers (such as, for example, 4-
pyridyl and 3-pyridy1).
(For example, if a compound of the invention incorporates a double bond or a
fused ring, both
the cis- and trans-forms, as well as mixtures, are embraced within the scope
of the invention.
Also, for example, all keto-enol and imine-enamine forms of the compounds are
included in the
methods of the invention).
Individual stereoisomers of the compounds of the invention may, for example,
be
substantially free of other isomers, or may be admixed, for example, as
racemates or with all
other, or other selected, stereoisomers. The chiral centers of the present
invention can have the S
or R configuration as defined by the IUPAC 1974 Recommendations. The use of
the terms "salt",
"solvate", "ester", "prodrug" and the like, is intended to equally apply to
the salt, solvate, ester
and prodrug of enantiomers, stereoisomers, rotamers, tautomers, positional
isomers, racemates or
prodrugs of the inventive compounds.
Another embodiment provides isotopically-labelled compounds to be used in the
methods the invention. Such compounds are identical to those recited herein,
but for the fact that
one or more atoms are replaced by an atom having an atomic mass or mass number
different
from the atomic mass or mass number usually found in nature. Examples of
isotopes that can be
incorporated into compounds of the invention include isotopes of hydrogen,
carbon, nitrogen,
oxygen, phosphorus, fluorine and chlorine, such as 2H, 3H, 13C, 14C, 15N, 180,
170, 31p, 32p, 35s,
18F, and 36C1, respectively.
Certain isotopically-labelled compounds of the invention (e.g., those labeled
with
3H and 1-4C) are useful in compound and/or substrate tissue distribution
assays. Tritiated (i.e., 3H)
and carbon-14 (i.e., 4C) isotopes are particularly preferred for their ease of
preparation and
detectability. Further, substitution with heavier isotopes such as deuterium
(i.e., 41) may afford
certain therapeutic advantages resulting from greater metabolic stability (e g
, increased in vivo
half-life or reduced dosage requirements) and hence may be preferred in some
circumstances.
83
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
Isotopically labelled compounds of the invention can generally be prepared by
following
procedures analogous to those disclosed in the Schemes and/or in the Examples
hereinbelow, by
substituting an appropriate isotopically labelled reagent for a non-
isotopically labelled reagent.
In the compounds used in the methods of the invention, the atoms may exhibit
their natural isotopic abundances, or one or more of the atoms may be
artificially enriched in a
particular isotope having the same atomic number, but an atomic mass or mass
number different
from the atomic mass or mass number predominantly found in nature. The present
invention is
meant to include all suitable isotopic variations of the compounds of the
invention. For example,
different isotopic forms of hydrogen (H) include protium ('H) and deuterium
(2H). The presence
of deuterium in the compounds of the invention is indicated by "D". Protium is
the predominant
hydrogen isotope found in nature Enriching for deuterium may afford certain
therapeutic
advantages, such as increasing in vivo half-life or reducing dosage
requirements, or may provide
a compound useful as a standard for characterization of biological samples.
Isotopically-enriched
compounds of the invention can be prepared without undue experimentation by
conventional techniques well known to those skilled in the art or by processes
analogous to those
described in the schemes and examples herein using appropriate isotopically-
enriched reagents
and/or intermediates.
Polymorphic forms of the compounds used in the methods of the invention, and
of the salts, solvates, esters and prodrugs of the compounds of the invention,
are intended to be
included in the present invention
Methods of Treatment
The present invention is directed to methods of treatment of Plasmodium
infections comprising administering to a subject in need thereof a compound
described herein, or
a pharmaceutically acceptable salt thereof. More specifically, the methods of
the invention
comprise administration of a compound of Formula (I), or a pharmaceutically
acceptable salt
thereof. In certain embodiments, the compounds of Formula (I), or a
pharmaceutically acceptable
salt thereof, are administered in the form of a pharmaceutical composition,
further comprising a
pharmaceutically acceptable carrier or excipient.
The present invention provides a method for treating a Plasmodium infection,
or
for treating malaria, or for inhibiting plasmepsin X which comprises
administering to a subject in
need of such treatment a therapeutically effective amount of a compound, or a
pharmaceutically
acceptable salt thereof, said compound having the structural Formula (I)
described in the
Summary of the Invention In some embodiments, the compounds of Formula (I), or
pharmaceutically acceptable salts thereof, are administered with a
pharmaceutically acceptable
84
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
carrier, as a pharmaceutical composition. Also provided herein are various
embodiments of these
methods, as described, infra.
The invention also relates to the use of a compound of Formulae (I) - (VIII)
or a
pharmaceutically acceptable salt thereof for inhibiting plasmepsin X activity,
for treating a
Plasmodium infection, or for treating malaria. The invention further relates
to the use of a
compound of Formulae (I) - (VIII) or a pharmaceutically acceptable salt
thereof in the
manufacture of a medicament for inhibiting plasmepsin X activity, for treating
a Plasmodium
infection, or for treating malaria. The compounds of Formulae (I) - (VIII) or
pharmaceutically
acceptable salts thereof described in any of the embodiments of the invention
herein are useful
for any of the uses above.
The present invention provides a method for treating a Plasmodium infection,
or
for treating malaria, or for inhibiting plasmepsin IX which comprises
administering to a subject
in need of such treatment a therapeutically effective amount of a compound, or
a
pharmaceutically acceptable salt thereof, said compound having the structural
Formula (I)
described in the Summary of the Invention. In some embodiments, the compounds
of Formula
(I), or pharmaceutically acceptable salts thereof, are administered with a
pharmaceutically
acceptable carrier, as a pharmaceutical composition. Also provided herein are
various
embodiments of these methods, as described, infra.
The invention also relates to the use of a compound of Formulae (I) - (VIII)
or a
pharmaceutically acceptable salt thereof for inhibiting plasmepsin TX
activity, for treating a
Plasmodium infection, or for treating malaria. The invention further relates
to the use of a
compound of Formulae (I) - (VIII) or a pharmaceutically acceptable salt
thereof in the
manufacture of a medicament for inhibiting plasmepsin IX activity, for
treating a Plasmodium
infection, or for treating malaria. The compounds of Formulae (I) - (VIII) or
pharmaceutically
acceptable salts thereof described in any of the embodiments of the invention
herein are useful
for any of the uses above.
The present invention provides a method for treating a Plasmodium infection,
or
for treating malaria, or for inhibiting plasmepsin X and plasmepsin IX which
comprises
administering to a subject in need of such treatment a therapeutically
effective amount of a
compound, or a pharmaceutically acceptable salt thereof, said compound having
the structural
Formula (I) described in the Summary of the Invention. In some embodiments,
the compounds
of Formula (I), or pharmaceutically acceptable salts thereof, are administered
with a
pharmaceutically acceptable carrier, as a pharmaceutical composition. Also
provided herein are
various embodiments of these methods, as described, infra.
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
The invention also relates to the use of a compound of Formulae (I) - (VIII)
or a
pharmaceutically acceptable salt thereof for inhibiting plasmepsin X and
plasmepsin IX activity,
for treating a Plasmodium infection, or for treating malaria. The invention
further relates to the
use of a compound of Formulae (I) - (VIII) or a pharmaceutically acceptable
salt thereof in the
manufacture of a medicament for inhibiting plasmepsin X and plasmepsin IX
activity, for
treating a Plasmodium infection, or for treating malaria. The compounds of
Formulae (I) - (VIII)
or pharmaceutically acceptable salts thereof described in any of the
embodiments of the
invention herein are useful for any of the uses above.
The methods of the present invention are useful for treating malaria in that
they
inhibit the onset, growth, or progression of the condition, ameliorate the
symptoms of the
condition, cause regression of the condition, cure the condition, or otherwise
improve the general
well-being of a subject afflicted with, or at risk of, contracting the
condition. Thus, in accordance
with the presently disclosed subject matter, the terms "treat", "treating",
and grammatical
variations thereof, as well as the phrase "method of treating", are meant to
encompass any
desired therapeutic intervention, including but not limited to a method for
treating an existing
infection in a subject of infection, such as in a subject that has been
exposed to a parasite as
disclosed herein.
Embodiments of the invention also include one or more of the compounds of
Formulae (I) - (VIII) or a pharmaceutically acceptable salt thereof (i) for
use in, (ii) for use as a
medicament or composition for, or (iii) for use in the preparation of a
medicament for. (a)
therapy (e.g., of the human body); (b) medicine; (c) inhibition of
parasite/Plasmodium growth,
(d) treatment or prophylaxis of infection by Plasmodium species; (e) reduction
of the
progression, onset or severity of pathological symptoms associated with
Plasmodium infection
and/or reduction of the likelihood of severe Plasmodium infection or, (f)
treatment, prophylaxis
of, or delay in the onset, severity, or progression of Plasmodium -associated
di sease(s),
including, but not limited to: malaria.
Accordingly, another embodiment provides methods for the treatment of malaria
or for the treatment of Plasmodium infection, comprising administration of
combinations
comprising an amount of at least one compound of Formulae (I) - (VIII), or a
pharmaceutically
acceptable salt, solvate, ester or prodrug thereof, and an effective amount of
one or more
additional agents described below. In certain embodiments, described herein
are methods for the
treatment of malaria or for the treatment of Plasmodium infection, comprising
administration of
combinations comprising an amount of at least one compound of Formulae (I) -
(VIII), or a
pharmaceutically acceptable salt, solvate, ester or prodrug thereof, and an
effective amount of
86
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
one or more additional anti-malarial agents. In certain embodiments, described
herein are
methods for the treatment of malaria by inhibition of plasmepsin X, IX and at
least one other
mechanism, comprising administration of combinations comprising an amount of
at least one
compound of Formulae (I) - (VIII), or a pharmaceutically acceptable salt,
solvate, ester or
prodrug thereof, and an effective amount of one or more additional anti-
malarial agents, wherein
the additional anti-malarial agents act through a different mechanism than
inhibiting plasmepsin
IX or plasmepsin X. The pharmacological properties of the compounds of
Formulae (I) - (VIII),
or a pharmaceutically acceptable salt thereof may be confirmed by several
pharmacological
assays.
Dosage and Administration
Another embodiment provides suitable dosages and dosage forms of the
compounds used in the methods of the invention. Suitable doses for
administering compounds
used in the methods of the invention to patients may readily be determined by
those skilled in the
art, e.g., by an attending physician, pharmacist, or other skilled worker, and
may vary according
to patient health, age, weight, frequency of administration, use with other
active ingredients,
and/or indication for which the compounds are administered. Doses may range
from about 0.001
to 500 mg/kg of body weight/day of the compound of the invention. In one
embodiment, the
dosage is from about 0.01 to about 25 mg/kg of body weight/day of a compound
of the
invention, or a pharmaceutically acceptable salt or solvate of said compound.
In another
embodiment, the quantity of active compound in a unit dose of preparation may
be varied or
adjusted from about 1 mg to about 100 mg, in specific embodiments from about 1
mg to about
50 mg, in specific embodiments from about 1 mg to about 25 mg, according to
the particular
application. In another embodiment, a typical recommended daily dosage regimen
for oral
administration can range from about 1 mg/day to about 500 mg/day, in specific
embodiments 1
mg/day to 200 mg/day, in two to four divided doses.
As discussed above, the amount and frequency of administration of the
compounds of the invention and/or the pharmaceutically acceptable salts
thereof will be
regulated according to the judgment of the attending clinician considering
such factors as age,
condition and size of the patient as well as severity of the symptoms being
treated.
Liquid form preparations include solutions, suspensions and emulsions. As an
example, may be mentioned water or water-propylene glycol solutions for
parenteral injection or
addition of sweeteners and opacifiers for oral solutions, suspensions and
emulsions. Liquid form
preparations may also include solutions for intranasal administration.
87
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
Aerosol preparations suitable for inhalation may include solutions and solids
in
powder form, which may be in combination with a pharmaceutically acceptable
carrier, such as
an inert compressed gas, e.g., nitrogen.
Also included are solid form preparations that are intended to be converted,
shortly before use, to liquid form preparations for either oral or parenteral
administration. Such
liquid forms include solutions, suspensions and emulsions.
Another embodiment provides for use of compositions comprising a compound of
Formulae (I) - (VIII), or a pharmaceutically acceptable salt thereof
formulated for transdermal
delivery. The transdermal compositions can take the form of creams, lotions,
aerosols and/or
emulsions and can be included in a transdermal patch of the matrix or
reservoir type as are
conventional in the art for this purpose.
Another embodiment provides for use of compositions comprising a compound of
Formulae (I) - (VIII), or a pharmaceutically acceptable salt thereof
formulated for subcutaneous
delivery. Another embodiment provides for use of compositions suitable for
oral delivery. In
some embodiments, it may be advantageous for the pharmaceutical preparation
comprising one
or more compounds of Formulae (I) - (VIII), or a pharmaceutically acceptable
salt thereof to be
prepared in a unit dosage form. In such forms, the preparation is subdivided
into suitably sized
unit doses containing appropriate quantities of the active component, e.g., an
effective amount to
achieve the desired purpose. Each of the foregoing alternatives is considered
as included in the
various embodiments of the invention
When used in combination with one or more additional therapeutic agents
("combination therapy"), the compounds used in the methods of this invention,
i.e., the
compounds of Formulae (I) - (VIII), may be administered together or
sequentially. When
administered sequentially, compounds of the invention may be administered
before or after the
one or more additional therapeutic agents, as determined by those skilled in
the art or patient
preference.
If formulated as a fixed dose, such combination products employ the compounds
of Formulae (I) - (VIII), or a pharmaceutically acceptable salt thereof within
the dosage range
described herein and the other
pharmaceutically active agent or treatment within its dosage range.
Combination Therapy
Another embodiment provides for methods of treatment using pharmaceutically
acceptable compositions comprising a compound of the invention, either as the
neat chemical or
optionally further comprising additional ingredients. Such compositions are
contemplated for
88
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
preparation and use alone or in combination therapy. For preparing
pharmaceutical compositions
from the compounds of the invention, inert, pharmaceutically acceptable
carriers can be either
solid or liquid. Solid form preparations include powders, tablets, dispersible
granules, capsules,
cachets and suppositories. The powders and tablets may be comprised of from
about 5 to about
95 percent active ingredient. Suitable solid carriers are known in the art,
e.g., magnesium
carbonate, magnesium stearate, talc, sugar or lactose. Tablets, powders,
cachets and capsules can
be used as solid dosage forms suitable for oral administration. Examples of
pharmaceutically
acceptable carriers and methods of manufacture for various compositions may be
found in A.
Gennaro (ed.), Remington's Pharmaceutical Sciences, 18th Edition, (1990), Mack
Publishing
Co., Easton, Pennsylvania.
Non-limiting examples of additional drugs and active agents useful in
combination therapies for the treatment of malaria, include the following:
Coartem (Novartis
International AG, Basel, Switzerland; artemether + lumefantrine), Eurartesim
(Sigma-Tau
Pharmaceuticals, Inc., Rome, Italy; dihydroartemisinin-piperaquine), Pyramax
(Shin Poong
Pharmaceutical Co., Ltd., Seoul, Korea; pyronaridine-artesunate), ASAQ
Winthrop (Sanofi SA
(Gentilly, France)/DNDi (Geneva, Switzerland); artesunate + amodiaquine), ASMQ
(Cipla
Limited (Mumbai, India)/DNDi, artesunate + mefloquine), SPAQ-COTM (Guilin
Pharmaceutical
Co., Ltd. (Shanghai), amodiaquine + sulfadoxine, pyrimethamine), Artesun
(Guilin
Pharmaceutical, artesunate), artemether, artesunate, dihydroartemisinin,
lumefantrine,
am odi aquine, mefloquine, piperaquine, quinine, chloroquine, atovaquone and
proguanil and
sulfadoxine-pyrimethamine, Tafenoquine (Glaxosmithkline), 0Z439/PQP (Sanofi),
0Z439/FQ
(Sanofi), KAE609 (Novartis), KAF156 (Novartis), D5M265 (NITI/Takeda), and 1V1K-
4815
(Merck & Co., Inc., Powles et at., Antimicrobial Agents and Chemotherapy
56(5): 2414-
2419(2012)). Selection of such additional active ingredients will be according
to the diseases or
disorders present for which treatment is desired, as determined by the
attending physician or
other health care provider.
Thus, the invention also provides methods of using the compounds of Formulae
(I) - (VIII), or a pharmaceutically acceptable salt thereof to inhibit
plasmepsin X, plasmepsin IX
or plasmepsin X and IX, to treat Plasmodium infection or treat malaria wherein
the method
further comprises administering to a subject in need thereof, one or more
additional anti-malarial
agents. In some embodiments, the one or more additional anti-malarial agents
are selected from
the group consisting of: artemether, lumefantrine, dihydroartemisinin,
piperaquine, pyronaridine,
artesunate, amodiaquine, metloquine, sulfadoxine, pyrimethamine, lumefantrine,
quinine,
chloroquine, atovaquone, and proguanil.
89
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
EXAMPLES
The meanings of the abbreviations in Examples are shown below.
ACN = MeCN = CH3CN = acetonitrile
AcOH = acetic acid
AIBN = Azobisisobutyronitrile
Ar = argon
BBr3 = Boron tribromide
BF3Et20 = Boron trifluoride etherate
Boc20 = di-tert-butyl dicarbonate
Cbz = carboxybenzyl
Cbz0Su = N-(B enzyl oxycarbonyloxy)succinimi de
CBr4 = Tetrabromomethane
CC14= carbontetrachloride
CELITE = diatomaceous earthConc. = concentrated
Cs2CO3 = Cesium carbonate
DBU = 1,8-Diazabicyclo[5.4.0]undec-7-ene
DCE = dichloroethane
DCM = dichloromethane
DDQ = 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone
DM ALH = di i sobutyl alum i num hydride
DIPEA = DIEA= N, N-Diisopropylethylamine, or Htinig's base
DMA = dimethylacetamide
DMAP = 4-dimethylaminopyridine
DMF = N,N-Dimethylformamide
DMP = Dess¨Martin periodinane
DMSO = dimethyl sulfoxide
DPPE = 1,2-Bis(diphenylphosphino)ethane
DPPF = 1,1 '-Bis(diphenylphosphino)ferrocene
EDCI = EDC = 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide
Et20 = diethyl ether
Et0Ac = ethyl acetate
Et0H = ethanol
Et3SiH = Tri ethyl silane
h = hours
H2= hydrogen
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
HATU = 1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-
oxid
hexafluorophosphate
HC1= hydrochloric acid
HFBA= Heptafluorobutyric acid
HOAc = acetic acid
12= iodine
IPA= isopropyl alcohol
[Ir(cod)C1]2 = cyclooctadiene iridium chloride dimer
K2CO3= potassium carbonate
K3PO4 = Tripotassium phosphate
KF = Potassium fluoride
KIIMDS = Potassium bis(trimethylsilyl)amide
KOTMS = Potassium trimethylsilanolate
LCMS = Liquid chromatography¨mass spectrometry
LDA = Lithium diisopropylamide
LHMDS = LiflIVIDS= lithium bis(trimethylsilyl)amide
LiA1H4= lithium aluminum hydride
LiOH = lithium hydroxide
min = minutes
Me = methyl
MeCN = Acetonitrile
Me0H = CH3OH = methanol
MgSO4 = Magnesium sulfate
MsCl= methanesulfonyl chloride
N2= nitrogen
NaBH4 = sodium borohydrate
NaH = sodium hydride
NaHCO3 = Sodium hydrogencarbonate
NaI04= sodium periodate
NaOH = sodium hydroxide
Na2CO3 = sodium carbonate
Na2S03= sodium sulfite
Na7SO4= sodium sulfate
NH4C1 = Ammonium chloride
NH4OH = Ammonium hydroxide
91
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH40Ac = Ammonium acetate
NaHMDS = sodium bis(trimethylsilyl)amide
OMs = mesylate
OTs = tosylate
OTf = trifluoromethanesulfonyl
Pd(OH)2/C = Pearlman's Catalyst - Palladium hydroxide on carbon-C
Pd-C = Palladium on carbon-C
[Pd(C3H5)C12] = Allylpalladium(II) chloride dimer
PdC12(dppf)-CH2C12 = [1,1'-
Bis(diphenylphosphino)ferrocene]dichloropalladium(II)
PPh3 = Triphenylphosphine
Ru02.H20 = ruthenium(IV)oxide hydrate
RP-HPLC = reverse phase high performance liquid chromatography
SFC = Supercritical Fluid Chromatography
TBDPS-Cl = TB SCI = tert-Butyl (chloro)di phenyl si lane
TEA = Et3N = triethylamine
TBAF = Tetra-n-butylammonium fluoride
TFA = trifluoroacetic acid
THF = tetrahydrofuran
Ti(Et0)4 = titanium ethoxide
TMS = Tri methyl si 1 ylb
TMSOTf = Trimethylsilyl trifluoromethanesulfonate
Ts0H = p-Toluenesulfonic acid
CDC13 = heavy chloroform
CD3OD = heavy methanol
1 Standard atmosphere [atm] = 101325 pascal [Pa] = 14.6959488 psi
The meanings of the abbreviations in the nuclear magnetic resonance spectra
are shown
below:
s = singlet, d = doublet, dd = double doublet, dt = double triplet, ddd =
double doublet, Sept =
septet, t = triplet, m = multiplet, br = broad, brs = broad singlet, q =
quartet
J = coupling constant and Hz = hertz.
Several methods for preparing the compounds of this disclosure are described
in the
following Schemes and Examples. Starting materials and intermediates were
purchased
commercially from common catalog sources or were made using known procedures,
or as
92
CA 03240145 2024- 6-5
WO 2023/107356 PCT/US2022/051770
otherwise illustrated. Some frequently applied routes to the compounds of
Formula I are
described in in the Schemes that follow. In some cases, the order of carrying
out the reaction
steps in the schemes may be varied to facilitate the reaction or to avoid
unwanted reaction
products.
SCHEME 1
H N N NH
HN N
0
macroiactamization .. 0
)n 02H
NH2 ) n HN 0
R' R'
R" R"
S-1 S-2
Compounds of Formula S-2 are prepared from S-1 by macrolactamization using
amide coupling reagents.
SCHEME 2
NH NH
HNAN HN NH
HN
0 0
RCM ( reduction
0
( n
HN 0 HN 0
H,Me
HN 0
R' R'
R'
H,Me
R" R"
R"
S-3 S-4
S-5
Intermediate compounds of Formula S-4 are prepared from S-3 after ring closing
metathesis (RCM) reactions using catalysts such as the 2nd generation Grubbs',
Zhan's and
Hoveyda/Grubbs' catalysts. Double bonds in S-4 can be reduced under for
example
hydrogenation conditions to yield the products of Formula S-5
93
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
SCHEME 3
R" R¨
R"'
NH NH NH
HNAN HNAN
HNAN
I ntramolecular R
0 0
coupling n reduction
n
HN 0
X
R' R'
R'
R" R"
R"
S-6 S-7 S-8
Intermediate compounds of Formula S-7 are prepared from S-6, in which X is a
halogen
such as Cl, Br and I, after transition metal catalyzed intramolecular cross-
coupling reactions such
as Heck reactions. Resulting double bonds in S-7 can be reduced under for
example
hydrogenation conditions to yield the products of Formula S-8.
SCHEME 4
R"' R"'
NH NH
HNAN HNAN
I ntramolecular Rro
R )n 0
coupling
HO HN 0
HN 0
X
411 R' R'
R" R''
S-9 S-10
Products of Formula S-10 are prepared from S-9, in which X is a halogen such
as Cl, Br
and I, after transition metal catalyzed intramolecular cross-coupling
reactions such as palladium
catalyzed C-0 coupling reactions.
94
CA 03240145 2024- 6-5
WO 2023/107356 PCT/US2022/051770
SCHEME 5
NH NH
HNN HN.-11,N
(ir.'=-=,-"LO OH,/ HN 0 etherification (0
HN
OH,X
R R.
R" R"
S-11 S-12
Products of Formula S-12 are prepared from S-11 after intramolecular SN2
reactions
between an alcohol and X, in which X is a leaving group such as Cl, Br, I,
OMs, OTs or OTf.
Products of Formula S-12 are also prepared from S-11 diols after dehydration
conditions using
an acid or other dehydration reagents.
SCHEME 6
NH NH NH
HN.11..N HNAN HN N
reductive
c(
0
Or c(
etherification
(r
HN 0 0 HN 0
HN 0
HO
R' R'
" R'
Rõ R" R
5-13 8-14
5-12
Products of Formula S-12 are prepared from intermediates S-13 or S-14 after
intramolecular reductive etherification using conditions such as TMSOTf and
Et3SiH.
Reactions sensitive to moisture or air were performed inside a glove-box or
under
nitrogen or argon using anhydrous solvents and reagents. The progress of
reactions was
determined by either analytical thin layer chromatography (TLC) usually
performed with E.
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
Merck pre-coated TLC plates, silica gel 60E-254, layer thickness 0.25 mm or
liquid
chromatography-mass spectrometry (LC/MS).
Typically, the analytical LC-MS system used consisted of a Waters ZQTM
platform
with electrospray ionization in positive ion detection mode with an Agilent
1100 series HPLC
with autosampler. The column was commonly a Waters Xterra MS C18, 3.0 x 50 mm,
5 pm or a
Waters Acquity UPLC BEH C18 1.0 x 50 mm, 1.7 pm. The flow rate was 1 mL/min,
and the
injection volume was 10 [IL. UV detection was in the range 210-400 nm. The
mobile phase
consisted of solvent A (water plus 0.05% TFA) and solvent B (MeCN plus 0.05%
TFA) with a
gradient of 100% solvent A for 0.7 min changing to 100% solvent B over 3.75
min, maintained
for 1.1 min, then reverting to 100% solvent A over 0.2 min.
Preparative HPLC purifications were usually performed using either a mass
spectrometry directed system or a non-mass guided system. Usually they were
performed on a
Waters Chromatography Workstation configured with LC-MS System consisting of:
Waters
ZQTM single quad MS system with Electrospray Ionization, Waters 2525 Gradient
Pump, Waters
2767 Inject /Collector, Waters 996 PDA Detector, the MS Conditions of: 150-
750 amu,
Positive Electrospray, Collection Triggered by MS, and a Waters SUNFIRE C-18
5-micron, 30
mm (id) x 100 mm column. The mobile phases consisted of mixtures of
acetonitrile (10-100%)
in water containing 0.1% TFA. Flow rates were maintained at 50 mL/min, the
injection volume
was 1800 pL, and the UV detection range was 210-400 nm. An alternate
preparative HPLC
system used was a Gilson Workstation consisting of Gil son GX-281
Injector/Collector, Gilson
UV/VIS-155 Detector, Gilson 333 and 334 Pumps, and either a Phenomenex Gemini-
NX C-18
5-micron, 50 mm (id) x 250 mm column or a Waters XBridgeTM C-18 5-micron
OBDTM, 30 mm
(id) x 250 mm column. The mobile phases consisted of mixtures of acetonitrile
(0-75%) in water
containing 5mmo1 (NH4)HCO3. Flow rates were maintained at 50 mL/min for the
Waters
XbridgeTM column and 90 mL/min for the Phenomenex Gemini column. The injection
volume
ranged from 1000-8000 pL, and the UV detection range was 210-400 nm. Mobile
phase
gradients were optimized for the individual compounds. Reactions performed
using microwave
irradiation were normally carried out using an Emrys Optimizer manufactured by
Personal
Chemistry, or an Initiator manufactured by Biotage. Concentration of solutions
was carried out
on a rotary evaporator under reduced pressure. Flash chromatography was
usually performed
using either a Biotage' Flash Chromatography apparatus (Dyax Corp.), an ISCO
CombiFlashe
Rf apparatus, or an ISCO CombiFlashC Companion XL on silica gel (32-63 litM,
60 A pore size)
in pre-packed cartridges of the size noted. IHNMR spectra were acquired at 500
MHz
spectrometers in CDC13 solutions unless otherwise noted. Chemical shifts were
reported in parts
96
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
per million (ppm). Tetramethylsilane (TMS) was used as internal reference in
CDC13 solutions,
and residual CH3OH peak or TMS was used as internal reference in CD3OD
solutions. Coupling
constants (J) were reported in hertz (Hz). Chiral analytical chromatography
was most commonly
performed on one of CHIRALPAK AS, CH1RALPAK AD, CHIRALCEL OD,
CHIRALCEL IA, or CHIRALCEL OJ columns (250x4.6 mm) (Daicel Chemical
Industries,
Ltd.) with noted percentage of either ethanol in hexane (%Et/Hex) or
isopropanol in heptane
(%IPA/Elep) as isocratic solvent systems. Chiral preparative chromatography
was conducted on
one of CHIRALPAK AS, of CHIRALPAK AD, CHIRALCELI)0D, CHIRALCELITA,
CHIRALCEL 0.1 columns (20x250 mm) (Daicel Chemical Industries, Ltd.) with
desired
isocratic solvent systems identified on chiral analytical chromatography or by
supercritical fluid
(SFC) conditions.
It is understood that a chiral center in a compound may exist in the "S" or
stereo-configuration, or as a mixture of both. Within a molecule, each bond
drawn as a straight
line from a chiral center includes both the (R) and (S) stereoisomers as well
as mixtures thereof
INTERMEDIATE 1
Preparation of Intermediate 1-2
HO1i0 0
j DPPA, DBU
N3"
0 0 0 0
INT1-1 INT1-2
DBU (21.72 mL, 144 mmol) and diphenylphosphinyl was added to a mixture of
methyl (S)-4-hydroxychromane-6-carboxylate (INT1-1) (10 g, 48.0 mmol) in THF
(80 mL).
Azide (35.0 g, 144 mmol) was then added under N2. The mixture was stirred at
50 C for 12 h.
The mixture was quenched with water (100 mL), and extracted with Et0Ac (3 x
100 mL). The
organic layers were washed with brine (80 mL), dried over Na2SO4, filtered and
concentrated.
The crude product was purified by flash silica gel chromatography (ISCO ; 330
g SepaElash
Silica Flash Column, Eluent of 15% Et0Ac/Pet.ether gradient @ 50 mL/min) to
give methyl (R)-
4-azidochromane-6-carboxylate (INT1-2).
MS (ESI) m/z 234.0(M+H )
NMR (500 MHz, CHLOROFORM-d) 6 7.93-7.96 (m, 2H), 6.91 (d, J=8.5 Hz, 1H), 4.65
(t,
J=3.5 Hz, 1H), 4.29-4.35 (m, 2H), 3.90 (s, 3H), 2.14-2.26 (m, 1H), 2.06-2.12
(m, 1H)
97
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
Preparation of Intermediate 1-3
0 0
Pd/C, H2 H2N1`..
0 0 0 0
INT1-2 INT1-3
Pd-C (2510 g, 4.72 mmol) was added to a solution of methyl (R)-4-
azidochromane-6-carboxylate (INT1-2) (11 g, 47.2 mmol) in THF (200 mL) under
N2
atmosphere. The mixture was degassed and backfilled with H2 (three times). The
resulting
mixture was stirred at 25 C for 12 h under H2 (15 psi) atmosphere. The
catalyst was filtered off
and the filtrate was concentrated under reduced pressure to give methyl (R)-4-
aminochromane-6-
carboxylate (INT1-3).
MS (ESI) nilz: 191.1 (M-17-41+)
Preparation of Intermediate 1
0 0
=
ooHN NHBoc
H2Ns' B
. BocHNAN`s
NaH, TFAA, THF
0 0 0 0
INT1 -3 INT-1
Sodium hydride (3.77 g, 94 mmol) was added to a solution of N,N-bisboc-
thiourea (16.94 g, 61.3 mmol) in THF (250 mL) at 0 C in portions under N2.
After lhr at this
temperature, 2,2,2-trifluoroacetic anhydride (8.82 mL, 61.3 mmol) was added
dropwise. The
mixture was stirred at 0 C for lh. A solution of methyl (R)-4-aminochromane-6-
carboxylate
(INTI-3) (9_77 g, 47.1 mmol) in THE (50 mL) was added dropwise at 0 C. The
mixture was
stirred at 0 C for 2 h. The mixture was quenched with water (80 mL), and
extracted with Et0Ac
(3 x 50 mL). The organic layers were washed with brine (40 mL), dried over
Na2SO4, filtered,
and concentrated in vacuo. The crude was purified by flash silica gel
chromatography (ISCO ;
220 g SepaFlash Silica Flash Column, Eluent of 20% Et0Ac/Pet.ether gradient @
50 mL/min)
to afford methyl (R)-4-(3-(tert-butoxycarbonyl)thioureido)chromane-6-
carboxylate (INT-1).
MS (ESI) nilz 367.1 (M+11 )
98
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NMR (500 MHz, CHLOROFORM-d) 6 8.01 (d, J=1.5 Hz, 1H), 7.96 (s, 1H), 7.89 (dd,
J=2.0,
9.0 Hz, 1H), 6.89 (d, J=9.0 Hz, 1H), 4.34-4.41 (m, 1H), 4.19-4.26 (m, 1H),
3.88 (s, 3H), 2.25-
2.40 (m, 2H), 1.47 (s, 9H)
INTERMEDIATE 2
Preparation of Intermediate 2-2
MeONHMe=HCI, 0
0 EDCI, HOBt, DIEA),
N-
OH DCM, RI
INT2-1 INT2-2
To a solution of pent-4-enoic acid (INT2-1) (40 g, 400 mmol), EDCI (92 g, 479
mmol), 1H-benzo[d][1,2,3]triazo1-1-ol (64.8 g, 479 mmol) and N-ethyl-N-
isopropylpropan-2-
amine (279 mL, 1598 mmol) in DCM (400 mL) was added N,0-dimethylhydroxylamine
hydrochloride (54.6 g, 559 mmol). The reaction was stirred at 25 C for 12 h
under N2
atmosphere. LCMS showed desired mass. The mixture was quenched with water (300
mL), and
extracted with DCM (3 x 100 mL). The organic layers were washed with brine
(100 mL), dried
over Na2SO4, filtered and concentrated in vacuo, and the crude was purified by
flash silica gel
chromatography (ISCOS; 220g Agela Silica Flash Column, eluent of 8% ethyl
acetate/pet. ether
gradient @ 50 mL/min) to afford N-methoxy-N-methylpent-4-enamide (INT2-2).
MS (ESI) nilz 144.1 (M+H)+
1-1-1 NMR (500 MHz, CHLOROFORM-d) 6 5.83-5.92 (m, 1H), 4.94-5.10 (m, 2H), 3.68
(s, 3H),
3.18 (s, 3H), 2.50-2.56 (m, 2H), 2.35-2.42 (m, 2H)
Preparation of Intermediate 2-3
0 0
EtMgBr
THF
INT2-2 INT2-3
A solution of N-methoxy-N-methylpent-4-enamide (INT2-2) (20 g, 140 mmol) in
THF (200 mL) under N2 atmosphere at 0 C, was then added ethylmagnesium
bromide (69.8
mL, 210 mmol) dropwise at 0 'C. The reaction was stirred at 25 "V for 1 h
under N2 atmosphere.
TLC showed a new spot. The mixture was quenched with Sat. NH4C1 a. q.(100 mL)
and water
(100 mL), extracted with Et0Ac (3 x 100 mL). The organic layers were washed
with brine (50
99
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
mL), dried over Na2SO4, filtered and concentrated in vacuo, and the crude was
purified by flash
silica gel chromatography (ISCOg; 120g Agela Silica Flash Column, eluent of 5%
ethyl
acetate/pet. ether gradient @ 40 mL/min) to afford hept-6-en-3-one (INT2-3).
1H NMR (500 MHz, CHLOROFORM-d) 6 5.78-5.83 (m, 1H), 4.92-5.07 (m, 2H), 2.48-
2.54 (m,
2H), 2.43 (q, J=7.0 Hz, 2H), 2.29-2.37 (m, 2H), 1.06 (t, J= 7.0 Hz, 3H)
Preparation of Intermediate 2-4
0
(R)g
o H2N - %
N-i'
_______________________________________________________ \
Ti(OEt)4., THF
INT2-3 INT2-4
To a solution hept-6-en-3-one (INT2-3) (10 g, 89 mmol) in THF (100 mL) was
added (R)-2-methylpropane-2-sulfinami de (12.97 g, 107 mmol) followed by
Ti(Ft0)4 (37.5 mTõ
178 mmol), then the reaction was stirred at 75 C for 12 h under N2
atmosphere. TLC showed
new spots. The final mixture was cooled to room temperature, then diluted with
DCM (200 mL),
stirred 15 min, then ice cold-saturated aqueous sodium bicarbonate solution
(50 mL) and Na2SO4
then filtered and concentrated in vacuo. The crude was purified by flash
silica gel
chromatography (ISCOg; 120g Agela Silica Flash Column, eluent of 8% ethyl
acetate/pet, ether
gradient @ 40 mL/min) to afford (R,E)-N-(hept-6-en-3-ylidene)-2-methylpropane-
2-sulfinamide
(IN T2-4).
MS (ESI) nilz 216.2 (m+il)
1H NMR_ (500 MHz, CHLOROFORM-d) 6 5.73-5.88 (m, 1H), 4.94-5.10 (m, 2H), 2.64-
2.87 (m,
2H), 2.31-2.58 (m, 4H), 1.22 (s, 9H), 1.05-1.20 (m, 3H)
Preparation of Intermediate 2-5
LDA,
0 0
Ti(Oi-Pr)3C1,
N¨e CH3COOMe ThN
\ -2\ ____________
THF
\COOMe
INT2-4 INT2-5
To a solution of diisopropylamine (19.64 mL, 139 mmol) in anhydrous THF (40
mL) at -78 C was added butyllithium (55.7 mL, 139 mmol) dropwise under N2
atmosphere. The
100
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
reaction was stirred at 0 C for 30 min to make LDA. Methyl acetate (7.48 mL,
93 mmol) and
Ti(OiPr)3C1 (116 mL, 116 mmol) were added to anhydrous THF (90 mL). LDA (76
mL, 93
mmol) was then added dropwise to the mixture at -78 C. After 1 h, a solution
of (R,E)-N-(hept-
6-en-3-ylidene)-2-methylpropane-2-sulfinamide (INT2-4) (10 g, 46.4 mmol) in
anhydrous THF
(20 mL) was then added dropwise and the mixture was stirred at -78 C for 3 h.
The color was no
change and yellow. LCMS showed major DP mass. The mixture was quenched with
ice-cold
half-saturated aqueous ammonium chloride solution (60 mL). The slurry was
diluted with Et0Ac
(200 mL) then filtered, rinsing with Et0Ac and water. The organic layer was
washed with brine
(50 mL), dried over Na2SO4, filtered and concentrated in vacuo. The residue
was purified by
flash silica gel chromatography (ISC08; 120g Agela Silica Flash Column, eluent
of 25% ethyl
acetate/pet. ether gradient @ 40 mL/min) to afford methyl 34(R)-tert-
butylsulfinyl)amino)-3-
ethylhept-6-enoate (INT2-5).
MS (ESI) nilz 290.1 (M+H)+
1-E1 NMR (500 MHz, CHLOROFORM-d) 6 5.76-5.82 (m, 1H), 5.01-5.05 (m, 1H), 4.97
(dd, J =
1.0, 10.0 Hz, 1H), 4.63 (br d, J = 17.0 Hz, 1H), 3.68 (s, 3H), 2.72 (dd, J =
5.0, 16.0 Hz, 1H), 2.52
(dd, J= 2.5, 16.0 Hz, 1H), 2.07-2.13 (m, 1H), 1.82-1.91 (m, 1H), i.76-1.81(m,
1H), 1.71-1.75
(m, 1H), 1.26 (t, J= 7.0 Hz, 2H), 1.24 (s, 9H), 0.87-0.95 (m, 3H)
Preparation of Intermediate 2-6
0 0 0
SFC HIV"
'-
00Me 00Me 00Me
INT2-5 INT2-6 P1 INT2-6 P2
Methyl 3-4(R)-tert-butylsulfinyl)amino)-3-ethylhept-6-enoate (INT2-5) (12 g,
41.5 mmol) was separated by SFC Column DAICEL CHIRALPAK AD(250mm x50mm, 10um)
Condition 0.1%NH3H20 IPA Begin B 15 End B 15 Gradient Time(min) 100%B Hold
Time(min) FlowRate(mL/min) 200 Injections 200) and Column DAICEL CHIRALPAK
AD(250mm x 50mm, 10um) Condition 0.1%NH3H20 IPA Begin B 12 End B 12 Gradient
Time(min) 100%B Hold Time(min) FlowRate(mL/min) 200 Injections 240) to give
methyl (R)-
3-(((R)-tert-butylsulfinyl)amino)-3-ethylhept-6-enoate (INT2-6 P1, desired)
(tR=1.978 min, UV
= 220 nm) and methyl (S)-34(R)-tert-butylsulfinyl)amino)-3-ethylhept-6-enoate
(IN T2-6_P2)
(tR=2.132min, UV = 220 nm).
MS (ESI) nilz 290.1 (M+II)
101
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
INT2-6 P1:1EINMIR (500 MHz, CHLOROFORM-d) 6 5.79 (tdd, J = 6.56, 10.32, 16.99
Hz,
1H), 4.93-5.09 (m, 2H), 3.62-3.73 (m, 3H), 2.72 (d, J= 15.87 Hz, 1H), 2.52 (d,
J= 15.87 Hz,
1H), 2.48 (s, 1H), 2.00-2.09 (m, 2H), 1.76-1.88 (m, 2H), 1.68-1.74 (m, 2H),
1.23 (s, 9H), 0.86-
0.95 (m, 3H). INT2-6_P2: 1-H NMR (500 MHz, CHLOROFORM-d) 6 5.71-5.87 (m, 1H),
4.94-
5.07 (m, 2H), 3.65-3.73 (m, 3H), 2.72 (d, J = 16.02 Hz, 1H), 2.52 (d, J =
16.02 Hz, 1H), 2.00-
2.14 (m, 2H), 1.83-1.91 (m, 1H), 1.74-1.80 (m, 1H), 1.67-1.74 (m, 2H), 1.20-
1.25 (m, 8H), 0.83-
0.89 (m, 3H).
Preparation of Intermediate 2-7
0
HCI-dioxane \ NH2
__________________________________________________ AP- I
Me0H
COOMe tOOMe
INT2-6 P1 INT2-7
A solution of methyl (R)-3-(((R)-tert-buty1sulfinyl)amino)-3-ethylhept-6-
enoate
(INT2-6 Pl) (20 g, 69.1 mmol) in HC1-dioxane(4N) (100 mL) and Me0H (200 mL)
was stirred
at 25 C for 2 h. LCMS showed the reaction was complete. Solvent was
evaporated under
reduced pressure to give the product methyl (R)-3-amino-3-ethylhept-6-enoate
hydrochloride
(INT2-7).
MS (ESI) nilz 186.3 (M+H)
-1-14 NMR (500 MHz, METHANOL-d4) 6 5.80-5.87 (m, 1H), 5.07-5.15 (m, 1H), 5.02-
5.04 (m,
1H), 3.73 (s, 3H), 2.71-2.79 (m, 2H), 2.07-2.18 (m, 2H), 1.75-1.85 (m, 4H),
0.99 (t, ./= 7.6 Hz,
3H)
Preparation of Intermediate 2-8
S Bo:`NI
Bocl-INAN
NH2 -NCI 4142-P HN N
tOOMe EDCI, DIEA, ACN /I". 0
INT2-7 INT2-8
To a solution of DMB-BOC-THIOUREA (5 g, 15.32 mmol), methyl (R)-3-
amino-3-ethylhept-6-enoate hydrochloride (INT2-7) (3.74 g, 16.85 mmol) and EDC
(7.34 g,
38.3 mmol) in Acetonitrile (100 mL) was added D1EA (12.04 mL, 68.9 mmol). The
reaction was
102
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
stirred at 15 C for 12 h under N2 atmosphere. LCMS showed desired mass, and
some ring-
opened byproduct ester. Then warmed to 50 C and stirred at 50 C for 2 h.
LCMS showed only
desired product mass. The mixture was concentrated in vauo. The crude was
dissolved Et0Ac
(100 mL) and water (100 mL), and the layers were separated. The aqueous layer
was extracted
with Et0Ac (2 x 50 mL). The combined organic layers were washed with brine
(100 mL), dried
over Na2SO4, filtered, and concentrated in vacuo. The crude was purified by
flash column
(ISCOe; 80 g Agela Silica Flash Column, eluent of 15% ethyl acetate/pet. ether
gradient @ 30
mL/min) to afford tert-butyl (R,E)-(4-(but-3-en-1 -y 1 ) -1-(2,4-
dimethoxybenzy1)-4-ethy1-6-
oxotetrahydropyrimidin-2(1H)-ylidene)carbamate (INT2-8).
MS (ESI) nilz 390.1 (M+H-56)
1H NMR (400 MHz, CHLOROFORM-d) 6 9.92 (br s, 1H), 7.10 (br d, J= 8.8 Hz, 1H),
6.33-6.50
(m, 2H), 5.63-5.83 (m, 1H), 5.09 (s, 2H), 4.96-5.06 (m, 2H), 3.78 (dd, .1=
1.6, 4.4 Hz, 6H), 2.61
(s, 2H), 1.98-2.12 (m, 2H), 1.59-1.70 (m, 4H), 1.49 (s, 9H), 0.92 (t, J = 7.6
Hz, 3H)
Preparation of Intermediate 2-9
NH
Boc'N '-'0
HN)L-NH
HN N AI TFA
/ "" .õ-k- 0 41Or 0-- 60 C, 16 113m'
\
`=,.--
INT2-8 /"rO
\
INT2-9
A solution of tert-butyl (R,E)-(4-(but-3-en-l-y1)-1-(2,4-dimethoxyb enzy1)-4-
ethy1-6-oxotetrahydropyrimidin-2(1H)-ylidene)carbamate (INT2-8) (1.0 g, 2.244
mmol) in TFA
(10 mL) was stirred at 60 C for 16 h. LCMS showed desired mass. The mixture
was
concentrated in vacuo. The residue was partitioned between Petether/Et0Ac
(v/v=4:1, 10 mL)
and water (10 mL). And LCMS showed the product was only in aqueous phase, and
pretty clean.
(R)-6-(but-3-en-l-y1)-6-ethy1-2-iminotetrahydropyrimidin-4(1H)-one (INT2-9) in
water (10 mL)
was used for the next step directly.
MS (ESI) nilz 196.0 (M+H)
Preparation of Intermediate 2
103
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH Boo
HNANH ),L
r "
\ (Boc)20, NaHCO3
THF, H20, 25 C, 16 h).- HN NH
/" . _______________________________
/".. o
\ INT-2
INT2-9
To a solution of (R)-6-(but-3-en-1-y1)-6-ethy1-2-iminotetrahydropyrimidin-
4(1H)-
one (INT2-9) (438 mg, 2.243 mmol) in Water (10 mL) and THF (3 mL) was added
NaHCO3
(942 mg, 11.22 mmol) and (BOC)20 (1.042 mL, 4.49 mmol) at 0 C in portions. The
reaction
was stirred at 25 C for 16 h. LCMS showed desired mass. The mixture was
extracted with
Et0Ac (3 x 10 mL). The organic layers were washed with brine (10 mL), dried
over Na2SO4,
filtered, and concentrated in vacuo. The crude was purified by flash column
(ISCOk; 12 g Agela
Silica Flash Column, eluent of 20% EE(EtOAC/Et0H = 3:1)/Pet.ether gradient @
30 mL/min) to
afford tert-butyl (R,E)-(4-(but-3-en-1-y1)-4-ethy1-6-oxotetrahydropyrimidin-
2(1H)-
ylidene)carbamate Intermediate 2
MS (ESI) nilz 296.2 (M+H)
1E1 NMR (400 MHz, CHLOROFORM-d) 6 9.37 (br s, 1H), 5.74-5.81 (m, 1H), 4.90-
5.17(m,
2H), 2.59 (s, 2H), 2.07-2.10 (m, 2H), 1.62-1.78 (m, 4H), 1.51 (s, 9H), 0.97
(t, J= 7.2 Hz, 3H)
EXAMPLE 1
NH --0
HNJ-LN '
0
HN 0
OH
(1R,5R,15R,16R)-5-ethyl-15-hydroxy-3-imino-9-methy1-23-oxa-2,4,17-
tri azahexacycl o[17.6.2.22,5.210,13.012,16.022,26]hentriaconta-
10,12,19,21,26,28-hexaene-
18,31-dione
Preparation of Compound 1-2
104
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
Tf0H(5 eq)
NH2 NH2
11011.=N Br
,10H BS (1.1 eq)
=.10H
DCM, 0 C to it, 1.5h
1-1 1-2
Trifluoromethanesulfonic acid (503 mg, 3.35 mmol) and N-bromosuccinimide
(596 mg, 3.35 mmol) was added to a solution of (1R, 2R)-1-amino-2,3-dihydro-1H-
inden-2-ol
(1-1) (500 mg, 3.35 mmol) in DCM (10 mL) at 0 C. The reaction was stirred at
18 C for lh
under N2 atmosphere. The mixture was quenched with saturated sodium
bicarbonate solution (10
mL) at 0 C, and extracted with DCM (1 x 10 mL) then extracted with Et0Ac (3 x
10 mL). The
organic layers were washed with brine (10 mL), dried over Na2SO4, filtered and
concentrated in
vacuum, the crude was purified by purified by flash silica gel chromatography
(ISCO*); 12 g
SepaFlash Silica Flash Column, Eluent of 10% Me0H/DCM (added 1% ammonium
hydroxide)
gradient @ 50 mL/min) with to afford (1R,2R)-1-amino-6-bromo-2,3-dihydro-1H-
inden-2-ol (1-
2).
MS (ESI) nilz: 228.1, 230.1(M+1-1 )
1H N1VIR (400 MHz, METHANOL-d4) 6 7.50 (s, 1H), 7.33 (dd, 1=1.6, 8.0 Hz, 114),
7.10 (d,
J=8.0 Hz, 1H), 4.10 (q, J=6.8 Hz, 1H), 4.01-4.05 (m, 1H), 3.15 (dd, J=6.8,
15.6 Hz, 1H), 2.68
(dd, J=7.2, 15.6 Hz, 1H)
Preparation of Compound 1-3
Boc
7 HN N BOG N
HN'N
Br 2
HO 0 ro
N H
=.10H HN 0
EDCI, HOBt, DIEA, THF
Z
1-2 1-3
DIEA (0.276 mL, 1.582 mmol) was added to a solution of (R)-4-((R,E)-2-((tert-
butoxycarbonyl)imino)-4-ethy1-6-oxo-4-(pent-4-en-1-y1)tetrahydropyrimidin-
1(2H)-
y1)chromane-6-carboxylic acid (1-2) (96 mg, 0.198 mmol), EDC (189 mg, 0.989
mmol), 1H-
benzo[d][1,2,3]triazol-1-ol (134 mg, 0.989 mmol) and (1R,2R)-1-amino-6-bromo-
2,3-dihydro-
1H-inden-2-ol (45.1 mg, 0.198 mmol) in THF (5 mL). The reaction was stirred at
18 C for 2 h.
105
CA 03240145 2024- 6- 5
WO 2023/107356
PCT/US2022/051770
The mixture was quenched with water (5 mL), and extracted with Et0Ac (3 x 5
mL). The
organic layers were washed with brine (5 mL), dried over Na2SO4, filtered and
concentrated in
vacuum, and the crude was purified by prep-TLC (Pet. ether/Et0Ae=1:1) to
afford tert-butyl
((R,E)-1-((R)-6-(((lR,2R)-6-bromo-2-hydroxy-2,3-dihydro-1H-inden-l-
y1)carbamoyl)chroman-
4-y1)-4-ethy1-6-oxo-4-(pent-4-en-1-y1)tetrahydropyrimidin-2(1H)-
ylidene)carbamate (1-3).
MS (ESI) m/z: 695.1, 697.1 (M-41 )
Preparation of Compound 1-4A, 1-4B, and 1-4C
BocN Boc,N
_.,,,c) Boc,N ,, Boc--0 'N --'¨'0
' /¨'=0
HN N di H N N 01 7IN N 10/ ,H, N
N /111.
7"¶ o ilir l 'A
P(oTo1)3G 2, Cy2Nivie
HN 0 dioxane, 70 C, 16 h 16'.
V
,,.
OH \
7 ... 0
HN 0
,,OH
HN 0
.00H
/Br S.'
1-3 1-4A 1-4B 1-
4C
Chloro[tri(o-tolyl)phosphine][2-(2'-amino-1,11-biphenyl)]palladium(II) (8.83
mg,
0.014 mmol) and N,N-dicyclohexylmethylamine (140 mg, 0.719 mmol) was added to
a solution
of tert-butyl ((R,E)-1-((R)-6-(((1R,2R)-6-bromo-2-hydroxy-2,3-dihydro-1H-inden-
1-
yl)carbamoyl)chroman-4-y1)-4-ethyl-6-oxo-4-(pent-4-en-l-y1)tetrahydropyrimidin-
2(1H)-
ylidene)carbamate (1-3) (100 mg, 0.144 mmol) in dioxane (1.5 mL) at 25 C in a
glove box. The
reaction was stirred at 70 C for 16h. The mixture was quenched with water (3
mL), and
extracted with Et0Ac (3 x 5 mL). The organic layers were washed with brine (5
mL), dried over
Na2SO4, filtered and concentrated in vacuum, and the crude was purified by
reverse preparative
HPLC (Instrument ED; Method Column Boston Prime C18 150 x 30mm, Sum; Condition
water(0.04%NH3H20+ I OmM NH4HCO3)-ACN Begin B 65; End B 95 Gradient Tim e(min)
10;
100%B Hold Time(min) 2 FlowRate(mL/min) 25; Injections 5) to afford: tert-
butyl
((4aR,8R,12E,18R,18aR,28E)-8-ethyl-18-hydroxy-6,20-dioxo-
4,4a,7,8,9,10,11,17,18,18a,19,20-
dodecahydro-3H,6H-1,21-(epiethane[1,2]diylidene)-8,5-(epiminomethano)-14,16-
ethenocyclopenta[h]pyrano[4,3-b][1,7]diazacyclononadecin-28-ylidene)carbamate
(1-4A); tert-
butyl ((4aR,8R, 12Z, 18R, 18aR,28E)-8-ethyl-18-hydroxy-6,20-di oxo-
4,4a,7,8,9,10,11,17,18,18a,19,20-dodecahydro-3H,6H-1,21-
(epiethane[1,2]diylidene)-8,5-
(epiminomethano)-14,16-ethenocyclopenta[h]pyrano[4,3-
b][1,7]diazacyclononadecin-28-
ylidene)carbamate (1-4B); and tert-butyl ((4aR,8R,17R,17aR,E)-8-ethyl- 17-
hydroxy-12-
methylene-6,19-dioxo-4,4a,6,7,8,9,10,11,12, 16,17,17a,18,19-tetradecahydro-3H-
1,20-
106
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(epiethane[1,2]diylidene)-8,5-(epiminomethano)-13,15-
ethenocyclopenta[h]pyrano[4,3-
b][1,7]diazacyclooctadecin-27-ylidene)carbamate (1-4C).
MS (ESI) nilz: 486.2 (M-41+)
1-4A:1H NMIR (500 MHz, CHLOROFORM-d) 6 10.09 (s, 1H), 7.93 (dd, 1=1.5, 8.50
Hz, 1H),
7.21-7.26 (m, 2H), 7.14 (d, J=8.0 Hz, 1H), 6.91-6.98 (m, 2H), 6.42-6.54 (m,
3H), 5.82-5.92 (m,
1H), 5.25 (t, J=5.5 Hz, 1H), 4.94 (s, 111), 4.40-4.50 (m, 2H), 4.25-4.35 (m,
1H), 3.34 (dd, 1=8.0,
15.5 Hz, 1H), 2.99-3.05 (m, 1H), 2.67-2.86 (m, 2H), 2.57 (d, 1=13.5 Hz, 1H),
2.40 (d,1=15.5
Hz, 1H), 2.07 (s, 2H), 1.82 (d, 1=11.0 Hz, 2H), 1.62-1.67 (m, 4H), 1.49 (s,
9H), 0.96 (t, 1=7.5
Hz, 3H)
1-4B: IFINVIR (500 MHz, CHLOROFORM-d) 6 10.13 (s, 1H), 7.90 (dd, 1=2.0, 8.50
Hz, 1H),
7.37 (s, 1H), 7.25 (d, J=7.5 Hz, 1H), 7.17 (s, 1H), 7.12 (d, J=7.5 Hz, 1H),
6.96 (d,1=8.50 Hz,
1H), 6.41-6.48 (m, 2H), 6.26 (dd, 1=7. 0, 9.0 Hz, 1H), 5.60-5.70 (m, 1H), 5.34
(t, 1=6.5 Hz, 1H),
4.60 (s, 1H), 4.46-4.54 (m, 1H), 4.42 (td, J=4.0, 11.0 Hz, 1H), 4.21 (dt,
1=2.5, 11.0 Hz, 1H), 3.34
(dd, J=7.5, 16.0 Hz, 1H), 2.97 (dd, J=7.5, 16.0 Hz, 1H), 2.60-2.72 (m, 2H),
2.49-2.53 (m, 2H),
2.22-2.30 (m, 1H), 2.13-2.18 (m, 1H), 2.03-2.12 (m, 2H), 1.72-1.82 (m, 4H),
1.49 (s, 9H), 1.00
(t, J=7.5 Hz, 3H)
1-4C: III NAIR (500 MHz, CHLOROFORM-d) 6 10.04 (s, 1H), 7.81 (dd, 12.0, 8.5
Hz, 1H),
7.52 (d, 1=8.0 Hz, 1H), 7.24 (d,1=8.0 Hz, 1H), 6.99-7.16 (m, 2H), 6.91
(d,1=8.50 Hz, 1H),
6.38-6.50 (m, 2H), 5.44 (s, 1H), 5.31 (t,1=6.5 Hz, 1H), 5.13 (s, 1H), 4.41-
4.57 (m, 2H), 4.36 (s,
1H), 4.23 (dt,J=2.0, 11.50 Hz, 1H), 3.34 (dd, 1=8.0, 16.0 Hz, 1H), 2.98 (dd,
1=8.0, 16.0 Hz,
1H), 2.69-2.79 (m, 1H), 2.59-2.63 (m, 1H), 2.45-2.52 (m, 2H), 1.93-2.09 (m,
2H), 1.86 (d,
1=12.0 Hz, 2H), 1.53-1.65 (m, 4H), 1.45 (s, 9H), 0.98 (t, J=7.5 Hz, 3H)
Preparation of Compound 1-5
Boc
`N Boc
'N
HN N
IIJPd/C, H2 0
HN 0
Me0H, r.t. HN 0
.õOH
1-4C 1-5
107
CA 03240145 2024- 6- 5
WO 2023/107356
PCT/US2022/051770
Pd-C (1.731 mg, 3.25 [tmol) was added to a solution of tert-butyl
((4aR, 8R, 17R,17aR,E)-8-ethy1-17-hydroxy-12-methylene-6, 19-di ox o-
4,4a,6,7,8,9,10,11,12,16,17,17a,18,19-tetradecahydro-3H-1,20-
(epiethane[1,2]diylidene)-8,5-
(epiminomethano)-13,15-ethenocyclopenta[h]pyrano[4,3-
b][1,7]diazacyclooctadecin-27-
ylidene)carbamate (1-4C) (10 mg, 0.016 mmol) in Me0H (2 ml) under N2
atmosphere. The
mixture was degassed and backfilled with H2 (three times). The resulting
mixture was stirred
under H2 (15 psi) at 25 C for 12 h. The catalyst was filtered off and the
filtrate was concentrated
under reduced pressure to give tert-butyl ((4aR,8R,17R,17aR,E)-8-ethy1-17-
hydroxy-12-methyl-
6,19-dioxo-4,4a,6,7,8,9,10,11,12,16,17,17a,18,19-tetradecahydro-3H-1,20-
(epiethane[1,2]diylidene)-8,5-(epiminomethano)-13,15-
ethenocyclopenta[h]pyrano[4,3-
b][1,7]diazacyclooctadecin-27-ylidene)carbamate (1-5).
MS (ESI) m/z: 617.3 (M-41+)
Preparation of Example 1
Boc
`N NH
HN N HNAN 7
/"" 0 ZnBr2 0
__________________________________________________ )0.
HN 0 15 DCM, it. HN
.õOH .00H
1-5 1
Zinc(II) bromide (29.2 mg, 0.130 mmol) was added to a solution of tert-butyl
((4aR, 8R, 17R,17aR,E)-8-ethy1-17-hydroxy-12-methy1-6,19-di ox o-
4,4a,6,7, 8,9, 10,11, 12,16,17,17a,18,19-tetradecahydro-3H- 1,20-
(epiethane[1,2] diylidene)-8,5-
(epi mi nometh ano)-13,15-ethen ocy cl openta[h]pyrano [4,3 -b] [1,7] di
azacycl ooctadeci n-27-
ylidene)carbamate (1-5) (8 mg, 0.013 mmol) in DCM (3 ml), at 22 C under N2
atmosphere. The
mixture was stirred at 22 C for 16 h. The mixture was cooled, the solvent was
evaporated under
reduced pressure to give the crude product. The residue was purified by
reverse preparative
HPLC (Instrument EJ Method Column Boston Green ODS 150 x30mm, 5um Condition
water(TFA)-ACN Begin B 22 End B 52 Gradient Time(min) 10 100%B Hold Time(min)
2
FlowRate(ml/min) 25 Injections 1) to yield Example 1.
MS (ESI) in/z: 517.2 (M+H+)
1-E1 NMR (500 MHz, METHANOL-d4) 6 8.49 (d, J=9.0 Hz, 1H), 7.72 (dd, J=2.0, 8.5
Hz, 1H),
108
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
7.39 (d, J=1.0 Hz, 1H), 7.19-7.24 (m, 1H), 7.14-7.19 (m, 1H), 6.91-6.96 (m,
2H), 5.30-5.39 (m,
2H), 4.38-4.53 (m, 2H), 4.13-4.15 (m, 1H), 3.18-3.29 (m, 1H), 2.75-2.94 (m,
3H), 2.61-2.72 (m,
2H), 2.20-2.30 (m, 1H), 1.74-1.85 (m, 3H), 1.58-1.68 (m, 2H), 1.50-1.57 (m,
1H), 1.31 (d, J=7.0
Hz, 3H), 1.24-1.29 (m, 1H), 1.11-1.20 (m, 1H), 0.97 (t, J=7.5 Hz, 3H)
EXAMPLE 2
NH -"--`0
HN 7
/".. 0
HN 0
(1R,5R,16R,17R)-5-ethy1-16-hydroxy-3-imino-24-oxa-2,4,18-
tri azahexacyclo [18.6.2.22,5 .211,14.013,17.023,27]doniaconta-
11,13,20,22,27,29-hexaene-
19,32-dione
Preparation of Compound 2-1
Boc Boc
'N 'N
HN N HN N
0
Pd/C, H2
HN 0 HN 0
Me0H, r.t.
õ,OH OH
2-1 2
Pd-C (2.250 mg, 4.23 umol) was added to a solution of tert-butyl
((4aR,8R,12E,18R,18aR,28E)-8-ethy1-18-hydroxy-6,20-dioxo-
4,4a,7,8,9,10,11,17,18,18a,19,20-
dodecahydro-3H,6H-1,21-(epiethane[1,2]diylidene)-8,5-(epiminomethano)-14,16-
ethenocyclopenta[h]pyrano[4,3-b][1,7]diazacyclononadecin-28-ylidene)carbamate
(2-1) (13 mg,
0.021 mmol) in Me0H (4 mL) under N2 atmosphere. The mixture was degassed and
backfilled
with H2 (three times). The resulting mixture was stirred under H2 (15 psi) at
20 C for 2 h. The
catalyst was filtered off and the filtrate was concentrated under reduced
pressure to give tert-
butyl ((4aR,8R,18R,18aR,E)-8-ethy1-18-hydroxy-6,20-dioxo-
109
CA 03240145 2024- 6- 5
WO 2023/107356
PCT/US2022/051770
4,4a,7,8,9,10,11,12,13,17,18,18a,19,20-tetradecahydro-3H,6H-1,21-
(epiethane[1,2]diylidene)-
8,5-(epiminomethano)-14,16-ethenocyclopenta[h]pyrano[4,3-
b][1,7]diazacyclononadecin-28-
ylidene)carbamate (2-1).
MS (ESI) nilz: 617.2 (M-41+)
Preparation of Example 2
BocN NH
'
=
HN N HNAN
HN 0
HN 0 DCM, r.t.
2-1 2
Zinc(II) bromide (47.5 mg, 0.211 mmol) was added to a solution of tert-butyl
((4aR, 8R,18R,18aR,E)-8-ethy1-18-hydroxy-6,20-di oxo-4,4a,7,8,9,
10,11,12,13,17, 18,18a,19,20-
tetradecahydro-3H,6H-1,21-(epiethane[1,2]diylidene)-8,5-(epiminomethano)-14,16-
ethenocyclopenta[h]pyrano[4,3-b][1,7]diazacyclononadecin-28-ylidene)carbamate
(2-1) (13 mg,
0.021 mmol) in DCM (3 mL), under N2 atmosphere. The mixture was stirred at 22
C for 16 h.
The mixture was concentrated under reduced pressure to give the crude product.
The residue was
purified by reverse preparative HPLC (Instrument EJ; Method Column Boston
Green ODS 150 x
30mm, Sum, Condition water(TFA)-ACN Begin B 22; End B 52 Gradient Time(min)
10;
100%B Hold Time(min) 2 FlowRate(mL/min) 25; Injections 1) to give
(4aR,8R,18R,18aR)-8-
ethy1-18-hydroxy-28-imino-4,4a,8,9,10,11,12,13,17,18,18a,19-dodecahydro-3H,6H-
1,21-
(epiethane[1,2]diylidene)-8,5-(epiminomethano)-14,16-
ethenocyclopenta[h]pyrano[4,3-
b][1,7]diazacyclononadecine-6,20(7H)-dione Example 2.
MS (ESI) nilz: 517.2 (MAI+)
1-E1 NMR (500 MHz, METHANOL-6) 6 7.76 (dd, J=2.0, 8.5 Hz, 1H), 7.46 (d, J=1.5
Hz, 1H),
7.13 (d, J=8.0 Hz, 1H), 6.99-7.06 (m, 2H), 6.93 (d, J=8.5 Hz, 1H), 5.21 (t,
J=7.5 Hz, 1H), 5.07
(d, J=5.5 Hz, 1H), 4.54-4.62 (m, 111), 4.38-4.49 (m, 1H), 4.09-4.19 (m, 1H),
3.23-3.28 (m, 1H),
2.90 (d, J=16.5 Hz, 1H), 2.79 (dd, J=6.0, 16.0 Hz, 1H), 2.62-2.74 (m, 2H),
2.50-2.61 (m, 2H),
2.20-2.34 (m, 1H), 1.81 (s, 1H), 1.62-1.76 (m, 3H), 1.49 (t, J=11.5 Hz, 1H),
1.12-1.44 (m, 6H),
0.95 (t, J=7.5 Hz, 3H)
110
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
EXAMPLE 3A
NH
HN 0
0
(1R,5R,17S)-5-ethy1-3-imino-15,15-dimethy1-14,24-dioxa-2,4,18-
triazahexacyclo[18.6.2.22,5.210,13.012,17.023,27]dotriaconta-10,12,20,22,27,29-
hexaene-
19,32-dione
Preparation of Compound 3-2
MeONHMe=HCI,
0 EDCI, HOBt, DIEA 0 (!)
N-
OH DCM, r.t.
3-1 3-2
N,0-dimethylhydroxylamine hydrochloride (54.6 g, 559 mmol) was added to a
solution of pent-4-enoic acid (40 g, 400 mmol), EDC (92 g, 479 mmol), 1H-
benzo[d][1,2,3]triazol-1-ol (3-1) (64.8 g, 479 mmol) and N-ethyl-N-
isopropylpropan-2-amine
(279 mL, 1598 mmol) in DCM (400 mL). The reaction was stirred at 25 C for 12
h under N2
atmosphere. The mixture was quenched with water (300 mL), and extracted with
DCM (3 x 100
mL). The organic layers were washed with brine (100 mL), dried over Na2SO4,
filtered and
concentrated in vacuo, and the crude was purified by flash silica gel
chromatography (ISCOO;
220g Agela Silica Flash Column, eluent of 8% ethyl acetate/pet. ether gradient
@ 50 mL/min) to
afford N-methoxy-N-methylpent-4-enamide (3-2).
MS (ESI) nilz 144.1 (M+11 )
11-1 NMR (500 MHz, CHLOROFORM-d) 6 5.83-5.92 (m, 1H), 4.94-5.10 (m, 2H), 3.68
(s, 3H),
3.18 (s, 3H), 2.50-2.56 (m, 2H), 2.35-2.42 (m, 2H)
Preparation of Compound 3-3
0
EtMgBr
THF
3-2 3-3
111
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
Ethylmagnesium bromide (69.8 mL, 210 mmol) was added dropwise to a solution
of N-methoxy-N-methylpent-4-enamide (3-2) (20 g, 140 mmol) in THE (200 mL)
under N2
atmosphere at 0 C. The reaction was stirred at 25 C for 1 h under N2
atmosphere. The mixture
was quenched with saturated NH4C1 aqueous (100 mL) and water (100 mL),
extracted with
Et0Ac (3 x 100 mL). The organic layers were washed with brine (50 mL), dried
over Na2SO4,
filtered and concentrated in vacuo, and the crude was purified by flash silica
gel chromatography
(ISCOe; 120g Agela Silica Flash Column, eluent of 5% ethyl acetate/pet. ether
gradient @ 40
mL/min) to afford hept-6-en-3-one (3-3).
1H NMR (500 MHz, CHLOROFORM-d) 6 5.78-5.83 (m, 1H), 4.92-5.07 (m, 2H), 2.48-
2.54 (m,
2H), 2.43 (q, J=7.0 Hz, 2H), 2.29-2.37 (m, 2H), 1.06 (t, J=7.0 Hz, 3H)
Preparation of Compound 3-4
0
(R) g
o H2N--
N-e
Ti(0E04, THF
3-3 3-4
(R)-2-methylpropane-2-sulfinamide (12.97 g, 107 mmol) was added to a solution
hept-6-en-3-one (3-3) (10 g, 89 mmol) in THF (100 mL). Ti(Et0)4 (37.5 mL, 178
mmol) was
also added and the reaction was stirred at 75 C for 12 h under N2 atmosphere.
The mixture was
cooled to room temperature, diluted with DCM (200 mL) and stirred for 15 min.
Ice cold-
saturated aqueous sodium bicarbonate solution (50 mL) and Na2SO4 was added and
the mixture
was filtered and concentrated in vacuo. The crude was purified by flash silica
gel
chromatography (ISC08; 120g Agela Silica Flash Column, eluent of 8% ethyl
acetate/pet. ether
gradient @ 40 mL/min) to afford (R,E)-N-(hept-6-en-3-ylidene)-2-methylpropane-
2-sulfinamide
(3-4).
MS (ESI) nilz 216.2(M+11 )
1H NIVIR (500 MHz, CHLOROFORM-d)6 5 73-5 88 (m, 1H), 4.94-5_10 (m, 2H), 2.64-
2.87 (m,
2H), 2.31-2.58 (m, 4H), 1.22 (s, 9H), 1.05-1.20 (m, 3H)
Preparation of compound 3-5
112
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
LDA, 0
0 Ti(Oi-P03D1,
\\ N---e CH3COOMe \ HNAIK--
\ 2\ __
THF
HflCOOMe
3-4 3-5
Butyllithium (55.7 mL, 139 mmol), at -78 C, was added dropwise to a solution
of diisopropylamine (19.64 mL, 139 mmol) in anhydrous THE (40 mL) under N2
atmosphere.
The reaction was stirred at 0 "V for 30 min to make LDA. The resulting LDA (76
mL, 93 mmol)
was dropped to a mixture at -78 C of ethyl acetate (7.48 mL, 93 mmol) and
Ti(OiPr)3C1 (116
mL, 116 mmol) in anhydrous THF(90 mL). After 1 h, a solution of (R,E)-N-(hept-
6-en-3-
ylidene)-2-methylpropane-2-sulfinamide (3-4) (10 g, 46.4 mmol) in anhydrous
THE (20 mL)
was then added dropwise and the mixture was stirred at -78 C for 3h. The
mixture was
quenched with ice-cold half-saturated aqueous ammonium chloride solution (60
mL). The slurry
was diluted with Et0Ac (200 mL) then filtered, rinsing with Et0Ac and water.
The organic layer
was washed with brine (50 mL), dried over Na2SO4, filtered and concentrated in
vacuo. The
residue was purified by flash silica gel chromatography (ISCOR; 120g Agela
Silica Flash
Column, eluent of 25% ethyl acetate/pet. ether gradient @ 40 mL/min) to afford
methyl 3-4(R)-
tert-butylsulfinyl)amino)-3-ethylhept-6-enoate (3-5).
MS (ESI) nilz 290.1 (M+H+)
'El NMR (500 MHz, CHLOROFORM-d) 6 5.76-5.82 (m, 1H), 5.01-5.05 (m, 1H), 4.97
(dd,
J=1.0, 10.0 Hz, 1H), 4.63 (br d, J=17.0 Hz, 1H), 3.68 (s, 3H), 2.72 (dd,
J=5.0, 16.0 Hz, 1H), 2.52
(dd, J=2.5, 16.0 Hz, 1H), 2.07-2.13 (m, 1H), 1.82-1.91 (m, 114), 1.76-1.81 (m,
11-1), 1.71-1.75
(m, 1H), 1.26 (t, J=7.0 Hz, 2H), 1.24 (s, 9H), 0.87-0.95 (m, 3H)
Preparation of Compound 3-6
\---\HNAK--' _______________________________ di
0 \
\ , HCI-oxane
__________________________________________ I -µ Me0H * NH2 = HCI
,)\ tOOMe 00Me
3-5 3-6
A solution of methyl 3-(((R)-tert-butylsulfinyl)amino)-3-ethylhept-6-enoate (3-
5)
(9 g, 31.1 mmol) in 4N HC1-dioxane (20 mL) and Me0H (20.00 mL) at 25 C under
N2
atmosphere was stirred for 1 h. Solvent was evaporated under reduced pressure
to give the crude
product methyl 3-amino-3-ethylhept-6-enoate hydrochloride (3-6) used crude for
the next step
directly.
113
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
MS (ESI) rn/z 186.2 (M+11 )
'El NMR (500 MHz, CHLOROFORM-d) 6 5.75-5.80 (m, 1H), 5.08 (dd, J=1.0, 18.0 Hz,
1H),
4.98 (d, J=10.22 Hz, 11-1), 3.72-3.75 (m, 3H), 2.78-2.85 (m, 2H), 2.21-2.29
(m, 2H), 1.84-2.00
(m, 4H), 1.06 (t, J=7.5 Hz, 3H)
Preparation of Compound 3-7
S
BocHNA N :40 Boc
H 'N ----'µO
t., +ICI INT-1 *
0 o HN N
NH2
\coome EDCI, DIEA, ACN
1....00Me
3-6 3-7
N-ethyl-N-isopropylpropan-2-amine (13.79 mL, 79 mmol) was added to a
solution of EDC (9.08 g, 47.4 mmol), methyl 3-amino-3-ethylhept-6-enoate
hydrochloride (3-6)
(3.5 g, 15.79 mmol) and methyl (R)-4-(3-(tert-
butoxycarbonyl)thioureido)chromane-6-
carboxylate (INT-1) (5.78 g, 15.79 mmol) in ACN (30 mL). The reaction was
stirred at 25 C for
12 h under N2 atmosphere. The mixture was quenched with water (50 mL), and
extracted with
Et0Ac (3 x 50 mL). The organic layers were washed with brine (30 mL), dried
over Na2SO4,
filtered and concentrated in vacuo to afford methyl (4R)-4-((Z)-2-(tert-
butoxycarbony1)-3-(3-
ethyl-l-methoxy-l-oxohept-6-en-3-yl)guanidino)chromane-6-carboxylate (3-7)
used crude for
the next step directly.
MS (ESI) nilz 518.2 (M+H+)
Preparation of Compound 3-8
Boc Boc
'N "'N'-(3 'N -,"=-0
II 7 ij,_ 7
DBU
/ -I.-
THF 0
00Me
\
3-7 3-8
DBU (11.90 mL, 79 mmol) was added to a solution of methyl (4R)-4-((Z)-2-(tert-
butoxycarbonyl )-3-(3-ethyl-l-methoxy-1-oxohept-6-en-3 -yl )guani di
no)chromane-6-carb oxyl ate
(3-7) (8.17 g, 15.78 mmol) in THF (50 mL). The mixture was stirred at 50 C
for 16 h under N2
atmosphere. The mixture was quenched with water (50 mL), and extracted with
Et0Ac (3 x 50
114
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
mL). The organic layers were washed with brine (30 mL), dried over Na2SO4,
filtered and
concentrated in vacuo. The crude was purified by flash silica gel
chromatography (ISCOO; 120g
Agela Silica Flash Column, eluent of 25% ethyl acetate/pet. ether gradient @
40 mL/min) to
afford methyl (4R)-4-((E)-4-(but-3-en-1-y1)-2-((tert-butoxycarbonyl)imino)-4-
ethy1-6-
oxotetrahydropyrimidin-1(2H)-yl)chromane-6-carboxylate (3-8).
MS (ESI) in/z 486.2(M+1-1 )
1H NMR (500 MHz, CHLOROFOR1VI-d) 6 7.77 (td, 12.0, 8.5 Hz, 1H), 7.62 (s, 1H),
6.84 (dd,
1=2.0, 8.5 Hz, 1H), 6.33-6.42 (m, 1H), 5.76-5.90 (m, 1H), 5.00-5.14 (m, 2H),
4.42-4.49 (m, 1H),
4.22-4.23 (m, 1H), 3.81-3.86 (m, 3H), 2.70-2.81 (m, 1H), 2.51-2.60 (m, 2H),
2.03-2.16 (m, 4H),
1.75-1.84 (m, 2H), 1.65-1.73 (m, 4H), 1.52 (s, 9H), 0.98-1.04 (m, 3H)
Preparation of Compounds 3-9A & 3-9B
Boc Bo
'N c'N
= =
HN N HN SFC
,
N
/
TI
3-8 3-9A & 3-9B
Methyl (4R)-4-((E)-4-(but-3-en-1-y1)-2-((tert-butoxycarbonyl)imino)-4-ethyl-6-
oxotetrahydropyrimidin-1(2H)-yl)chromane-6-carboxylate (3-8) (4 g, 8.24 mmol)
was separated
by SFC (Column Boston Green ODS 150 x30mm, 5um Condition water(TFA)-ACN Begin
B 48
End B 78 Gradient Time(min) 10 100%B Hold Time (min) 2 FlowRate (mL/min) 25
Injections
1) to give P1 methyl (4R)-4-((E)-4-(but-3-en-1-y1)-2-((tert-
butoxycarbonyl)imino)-4-ethy1-6-
oxotetrahydropyrimidin-1(2H)-yl)chromane-6-carboxylate (3-9A) (tR=2. 105 min,
UV = 220
nm). P2 methyl (4R)-4-((E)-4-(but-3-en-1-y1)-2-((tert-butoxycarbonyl)imino)-4-
ethy1-6-
oxotetrahydropyrimidin-1(2H)-yl)chromane-6-carboxylate (3-9B) (tR=2.251 min,
UV = 220 nm).
MS (ESI) rivZ 486.2 (M+11 )
3-9A: NMR (500 MHz, CHLOROFORM-d) 37.77 (dd, J=1.5, 8.5 Hz, 1H), 7.62 (s, 1H),
6.84 (d, 1=9.0 Hz, 1H), 6.37 (br dd, 1=7.0, 10.0 Hz, 1H), 5.81-5.86 (m, 1H),
5.00-5.12 (m, 2H),
4.42-4.45 (m, 2H), 4.21-4.24 (m, 1H), 3.83 (s, 3H), 2.68-2.78 (m, 1H), 2.07-
2.16 (m, 3H), 1.66-
1.83 (m, 5H), 1.51 (s, 9H), 0.96 (t, J=7.5 Hz, 3H)
115
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
3-9B: 11-1NMR (500 MHz, CHLOROFORM-d) 6 7.77 (br d, J=8.70 Hz, 1H), 7.62 (s,
1H), 6.84
(d, J=8.5 Hz, 1H), 6.39 (hr dd, J=7.0, 10.0 Hz, 1H), 5.74-5.86 (m, 1H), 5.00-
5.10 (m, 2H), 4.41-
4.50 (m, 1H), 4.21-4.25 (m, 1H), 3.83 (s, 31-1), 2.73-2.81 (m, 1H), 2.06-2.12
(m, 3H), 1.68-1.83
(m, 5H), 1.52 (s, 9H), 0.98-1.04 (m, 3H)
Preparation of Compound 3-10
Boc'N Boc
HN N TMSOK HN N
THE 0
CY- 0 OH
3-9A 3-10
Potassium trimethylsilanolate (872 mg, 6.80 mmol) was added to a solution of
methyl (R)-4-((R,E)-4-(but-3-en-l-y1)-2-((tert-butoxycarbonyl)imino)-4-ethy1-6-
oxotetrahydropyrimidin-1(2H)-yl)chromane-6-carboxylate (3-9A) (550 mg, 1.133
mmol) in TI-IF
(5 mL). The reaction was stirred at 25 C for 0.5 h. The solution of (R)-4-
((R,E)-4-(but-3-en-1-
y1)-2-((tert-butoxycarbonyl)imino)-4-cthyl-6-oxotetrahydropyrimidin-1(2H)-
y1)chromane-6-
carboxylic acid (3-10) was used for next step directly without any further
manipulation or
purification.
MS (ESI) nilz 472.2 (M+H+).
Preparation of Compound 3-11
Boc
BocN NH2
-
HN N
0 0
EDCI, HOBt, 31' NH
OH DIEA, THF
0
3-10 3-11
DIEA (1.582 mL, 9.06 mmol) was added to a solution of (R)-44R,E)-4-(but-3-
en-l-y1)-2-((tert-butoxycarbonyl)imino)-4-ethyl-6-oxotetrahydropyrimidin-1(2H)-
y1)chromane-
6-carboxylic acid (3-10) (534 mg, 1.132 mmol), EDC (1085 mg, 5.66 mmol), 1H-
benzo[d][1,2,3]triazol-1-01 (765 mg, 5.66 mmol) and (S)-2,2-dimethy1-6-
yinylchroman-4-amine
116
CA 03240145 2024- 6- 5
WO 2023/107356
PCT/US2022/051770
(253 mg, 1.246 mmol) in THF (50 mL). The reaction was stirred at 25 C for 12
h. The mixture
was quenched with water (20 mL), and extracted with Et0Ac (3 x 20 mL). The
organic layers
were washed with brine (15 mL), dried over Na2SO4, filtered and concentrated
in vacuo, and the
crude was purified by prep-TLC (Pet. ether/Et0Ac=2:1) to afford tert-butyl
((R,E)-4-(but-3-en-
1-y1)-1-((R)-6-(((S)-2,2-dimethy1-6-vinylchroman-4-yl)carbamoyl)chroman-4-y1)-
4-ethy1-6-
oxotetrahydropyrimidin-2(1H)-ylidene)carbamate (3-11).
MS (ESI) nilz 657.3 (M+H+)
Preparation of Compound 3-12
Boc Boc
`N `N
HN N HN N
Hoveyda
0 Grubbs 2nd 0
DCE
NH HN 0
0 0
3-11 3-12
(1,3 -Bi s-(2,4,6-tri methyl phenyl)-2-imi dazoli di nyli dene)dichl oro(o-
isopropoxyphenylmethylene)ruthenium (95 mg, 0.152 mmol) was added to a
solution of tert-
butyl ((R,E)-4-(but-3-en-1-y1)-14(R)-64(S)-2,2-dimethy1-6-vinylchroman-4-
yl)carbamoyl)chroman-4-y1)-4-ethy1-6-oxotetrahydropyrimidin-2(1H)-
ylidene)carbamate (3-11)
(500 mg, 0.761 mmol) in DCE (500 mL). The reaction was stirred at 50 C for 5
h under N2
atmosphere. The mixture was concentrated in vacuo, and the crude was purified
by flash column
(Pet. ether/Et0Ac/Et0H=8:3:1) to afford tert-butyl ((4aR,8R,11E,18aS,28E)-8-
ethy1-17,17-
dimethy1-6,20-dioxo-4,4a,7,8,9,10,18,18a,19,20-decahydro-3H,6H,17H-8,5-
(epiminomethano)-
1,21:13,15-diethenodipyrano[4,3-b:4',3'-h][1,7]diazacyclooctadecin-28-
ylidene)carbamate (3-
12).
MS (ESI) nilz 629.3 (M+H+)
IHNMR (500 MHz, CHLOROFORM-d) 6 7.67 (dd, 1.5, 8.5 Hz, 1H), 7.36(s, 1H),
7.19(s,
1H), 7.05-7.10 (m, 1H), 6.91 (d, J=8.5 Hz, 1H), 6.69 (d, J=8.5 Hz, 1H), 6.37
(d, J=15.5 Hz, 1H),
6.09-6.22 (m, 2H), 5.92-5.93 (m, 1H), 5.40-5.42 (m, 1H), 4.49 (td, J=4.0, 11.5
Hz, 1H), 2.93-
3.04 (m, 2H), 2.46-2.53 (m, 2H), 2.35-2.41 (m, 1H), 2.29 (dd, J=6.5, 13.0 Hz,
1H), 1.91-1.99 (m,
1H), 1.71-1.78 (m, 2H), 1.66-1.71 (m, 4H), 1.61-1.66 (m, 1H), 1.50-1.53 (m,
1H), 1.46-1.50 (m,
1H), 1.43 (s, 3H), 1.37 (s, 3H), 1.26 (s, 9H), 0.95 (t, J=7.48 Hz, 3H)
117
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
Preparation of Compound 3-13
Boc Boc
'N 'N
HNNL 11 7
HN--"N
0 Pd/C, H2 0
Jr$IçH
0 Me0H HN 0
0
3-12 3-13
Pd-C (50.8 mg, 0.048 mmol) was added to a solution of tert-butyl
((4aR,8R,11E,18aS,28E)-8-ethy1-17,17-dimethy1-6,20-dioxo-
4,4a,7,8,9,10,18,18a,19,20-
decahydro-3H,6H,17H-8,5-(epiminomethano)-1,21:13,15-diethenodipyrano[4,3-
b:4',3'-
h][1,7]diazacyclooctadecin-28-ylidene)carbamate (3-12) (300 mg, 0.477 mmol) in
Me0H (10
mL), under N2 atmosphere. The mixture was degassed and backfilled with H2
(three times). The
resulting mixture was stirred at 25 C for 0.5 h under H2 (15 psi). The
catalyst was filtered off
and filtrate was concentrated under reduced pressure to give tert-butyl
((4aR,8R,18aS,E)-8-ethyl-
17,17-dimethy1-6,20-dioxo-4,4a,7,8,9,10,11,12,18,18a,19,20-dodecahydro-
3H,6H,17H-8,5-
(epiminomethano)-1,21:13,15-diethenodipyrano[4,3-b:4',3'-
h][1,7]diazacyclooctadecin-28-
ylidene)carbamate (3-13) which was used for the next step directly.
MS (ESI) nilz 631.3(M+11)
Preparation of Example 3A
Boc
'N NH
HN N HNAN
'
0 HCI-dioxane 0
HN 0 HN 0
0 0
3-13 3A
A solution of tert-butyl ((4aR,8R,18aS,E)-8-ethy1-17,17-dimethy1-6,20-dioxo-
4,4a,7,8,9,10,11,12,18,18a,19,20-dodecahydro-3H,6H,17H-8,5-(epiminomethano)-
1,21:13,15-
diethenodipyrano[4,3-b:4',3'-h][1,7]diazacyclooctadecin-28-ylidene)carbamate
(3-13) (280 mg,
0.444 mmol) in HC1-dioxane(4N) (30 mL) was stirred at 25 C for 16 h. The
solvent was
118
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
evaporated under reduced pressure to give the crude product. The residue was
purified by reverse
preparative HITE (Column Boston Green ODS 150 x 30mm, 5um Condition water
(HC1)-ACN
Begin B 30 End B 50 Gradient Time (min) 10 100%B Hold Time (min) 2 FlowRate
(mL/min)
25 Injections 6) to give (4aR,8R,18aS)-8-ethy1-28-imino-17,17-dimethyl-
4,4a,7,8,9,10,11,12,17,18,18a,19-dodecahydro-3H,6H,20H-8,5-(epiminomethano)-
1,21:13,15-
diethenodipyrano[4,3-b:4',3'-h][1,7]diazacyclooctadecine-6,20-dione Example
3A.
MS (ESI) nilz 531.2 (M+H+)
1-fl N1VIR (500 MHz, METHANOL-d4) 6 7.73 (dd, J=2.0, 8.5 Hz, 1H), 7.45 (s,
1f1), 7.02 (s, 1H),
6.92-6.99 (m, 2H), 6.70 (d, J=8.5 Hz, 1H), 5.37-5.44 (m, 2H), 4.44-4.47 (m,
1H), 4.12-4.17 (m,
1H), 2.93 (d, J=16.5 Hz, 1H), 2.55-2.70(m, 4H), 2.23-2.31 (m, 1H), 2.12 (dd,
J=6.5, 13.0 Hz,
1H), 1.81-1.87 (m, 1H), 1.66-1.80 (m, 5H), 1.49-1.59 (m, 1H), 1.42 (s, 3H),
1.34-1.40 (m, 2H),
1.32 (s, 3H), 0.98 (t, J=7.5 Hz, 3H)
EXAMPLE 4
NH 0
HNAN".
0
HN 0
0
(1R,5R,18S)-5-ethy1-3-imino-16,16-dimethyl-10,15,25-trioxa-2,4,19-
tri azahexacyclo [19.6.2.22,5.211,14.013,18.024,28]tritriaconta-
11,13,21,23,28,30-hexaene-20,33-
dione
Preparation of Compound 4-2
MeONHMe=HCI,
0 EDCI, HOBt, DIEA 0 (bi
I
4-1 4-2
DIEA (36.7 mL, 210 mmol) was added to a solution of hex-5-enoic acid (4-1) (8
g, 70.1 mmol), EDC (20.15 g, 105 mmol), 1H-benzo[d][1,2,3]triazol-1-ol (14.21
g, 105 mmol)
and N,0-dimethylhydroxylamine hydrochloride (7.52 g, 77 mmol) in THF (100 mL).
The
119
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
reaction was stirred at 25 C for 5 h. The mixture was quenched with water
(150 mL), and
extracted with Et0Ac (3 x 50 mL). The organic layers were washed with brine
(50 mL), dried
over Na2SO4, filtered and concentrated in yacuo. The crude product was
purified by flash silica
gel chromatography (ISCO(11); 80 g SepaFlashe Silica Flash Column, Fluent of
5%
Et0Ac/Pet.ether gradient @ 80 mL/min) to give N-methoxy-N-methylhex-5-enamide
(4-2).
MS (ESI) in/z 157.7 (MAW)
Preparation of compound 4-3
0
0 cb
EtMgBr
THF
4-2 4-3
Ethylmagnesium bromide (38.2 mL, 114 mmol), at 0 C, was added dropwise to a
solution of N-methoxy-N-methylhex-5-enamide (4-2) (9 g, 57.2 mmol) in THE (120
mL) under
N2 atmosphere. Then the mixture was stirred 20 C for 2 h. The mixture was
quenched with
saturated aqueous NH4C1 (25 mL). The mixture was quenched with water (150 ml)
and extracted
with Et0Ac (3 x 100 mL). The combined organic layers were washed with brine
(80 mL), dried
over Na2SO4, filtered. Solvent was removed under reduced pressure to give
crude product which
was purified by column chromatography (SiO2, Pet.ether: Et0Ac=100:1-10:1) to
afford oct-7-
en-3-one (4-3).
NMR (500 MHz, CHLOROFORM-d) 6 5.76 (m, 1H), 4.93-5.04 (m, 2H), 2.38-2.44 (m,
4H),
2.05 (q, J=7.0 Hz, 2H), 1.68 (m, 2H), 1.04 (t, J=7.5 Hz, 3H).
Preparation of Compound 4-4
0
0 H2N1-1
N--g?
\
Ti(OEt)4, THF,
75 C
4-3 4-4
2-methylpropane-2-sulfinamide (6.34 g, 52.3 mmol) was added to a solution oct-
7-en-3-one (4-3) (5.5 g, 43.6 mmol) in THE (80 mL), followed by Ti(Et0)4
(18.31 ml, 87 mmol).
The reaction was stirred at 75 'V for 16 h under N2 atmosphere. The final
mixture was cooled to
0 C, diluted with DCM (100 mL) and stirred for 15min. Ice cold-saturated
aqueous sodium
bicarbonate solution (15 mL) was added and the solution was filtered and
concentrated in vacuo.
The crude was purified by flash column (SiO2, Pet. ether/Et0Ac = 100:1 to 5:1)
to afford ((E)-2-
120
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
methyl-N-(oct-7-en-3-ylidene)propane-2-sulfinamide (4-4).
MS (ESI) nilz 230.2 (MAW)
1H NMR (500 MHz, CHLOROFORM-d) 6 5.78-5.80 (m, 1H), 4.96-5.07 (m, 2H), 2.62-
2.76 (m,
2H), 2.39-2.50 (m, 2H), 2.04-2.17 (m, 2H), 1.67-1.76 (m, 2H), 1.23 (s, 9H),
1.06-1.21 (m, 3H)
Preparation of Compound 4-5
0
0 LiHMDS,
N_e CH3COOMe HN
\ ?\THF, -78 C).' 00Me
4-4 4-5
Methyl acetate (1.545 ml, 19.18 mmol) at -78 C was added to a solution of
LiHMDS (22.67 ml, 22.67 mmol) in anhydrous THF (35 mL) under N2 atmosphere.
The reaction
was stirred at -78 C for 15min. (E)-2-methyl-N-(oct-7-en-3-ylidene)propane-2-
sulfinamide (4-
4) (4 g, 17.44 mmol) in THY (15 mL) was then added dropwise and the mixture
was stirred at -
78 C for 3h. The mixture was quenched with ice-cold half-saturated aqueous
ammonium
chloride solution (30 mL). The slurry was diluted with Et0Ac (50 mL) then
filtered, rinsing with
Et0Ac and water. The organic layer was washed with brine (50 mL), dried over
Na2SO4, filtered
and concentrated in vacuo. The residue was purified by flash column (SiO2,
Pet.
ether/Et0Ac=100:0 to 10:1) to afford methyl 3-((tert-butylsulfinyl)amino)-3-
ethyloct-7-enoate
(4-5).
MS (ESI) nilz 304.3 (M+11 ).
1H NMIR (500 MHz, CHLOROFORM-d) 6 5 72-5 86 (m, 1H), 4.91-5_06 (m, 2H), 4.59-
4.61 (m,
1H), 3.63-3.76 (m, 3H), 2.68-2.71 (m, 1H), 2.42-2.55 (m, 1H), 1.99-2.13 (m,
2H), 1.58-1.91 (m,
5H), 1.31-1.48 (m, 2H), 1.16-1.24 (m, 9H), 0.80-0.93 (m, 3H)
Preparation of Compound 4-6
0
HN"gl< HCI-dioxane
=HCI
Me0H
COOMe 00Me
4-5 4-6
A solution of methyl 3-((tert-butylsulfinyl)amino)-3-ethyloct-7-enoate (4-5)
(2 g,
6.59 mmol) in HC1-dioxane (4N) (5 mL) and Me0H (5mL) at 15 C under N2
atmosphere was
121
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
stirred at 15 C for 1 h. The solution was concentrated in vacuo to give the
crude product methyl
3-amino-3-ethyloct-7-enoate hydrochloride (4-6) which was used without any
further
purification.
1-E1 NMR (500 MHz, CHLOROFORM-d) 5.77 (m, 1H), 4.93-5.06 (m, 2H), 3.73 (s,
3H), 2.72-
2.88 (m, 2H), 2.02-2.14 (m, 2H), 1.70-2.00 (m, 5H), 1.56 (m, 2H), 1.04 (t, J =
7.50 Hz, 3H)
Preparation of compound 4-7
S -^0
/IL BocHN N 7 BOG 40 -N
=
INT-1 0 0
NH2HCI = Me EDCI, DIEA, ACN pOOMe
00
4-6 4-7
N-ethyl-N-isopropylpropan-2-amine (4.37 ml, 24.56 mmol) was added to a
solution of methyl (R)-4-(3-(tert-butoxycarbonyl)thioureido)chromane-6-
carboxylate (INT-1)
(1.5 g, 4.09 mmol), methyl 3-amino-3-ethyloct-7-enoate hydrochloride (4-6)
(1.448 g, 6.14
mmol) and N-(3-Dimethylaminopropy1)-N'-ethylcarbodiimide hydrochloride (2.354
g, 12.28
mmol) in acetonitrile (30 mL). The mixture was stirred at 15 'V for 10 h. The
mixture was
quenched with water (50 mL), and extracted with Et0Ac (3 x 40 mL). The organic
layers were
washed with brine (20 mL), dried over Na2SO4, filtered, and concentrated in
vacuo to afford
methyl (4R)-4-((Z)-2-(tert-butoxyc arb ony1)-3-(3 -ethyl-l-methoxy-l-oxooct-7-
en-3-
yl)guanidino)chromane-6-carboxylate (4-7) which was used crude for the next
step directly.
MS (ESI) nilz 532.3 (M+11 ).
Preparation of Compound 4-8
Boc
-1\1 Boc
'N
11 7 7
1\1 1-11\r"
DBU
_________________________________________________ )1- 0
00Me THF
0
4-7 4-8
122
CA 03240145 2024- 6-5
WO 2023/107356 PCT/US2022/051770
DBU (2.84 mL, 18.81 mmol) was added to a solution of methyl (4R)-4-((Z)-2-
(tert-butoxycarbony1)-3-(3-ethy1-1-methoxy-1-oxooct-7-en-3-
y1)guanidino)chromane-6-
carboxylate (4-7) (2.0 g, 3.76 mmol) in THF (20 mL). The mixture was stirred
at 50 C for 10 h.
The mixture was quenched with water (40 mL), and extracted with Et0Ac (3 x 40
mL). The
organic layers were washed with brine (20 mL), dried over Na2SO4, filtered,
and concentrated in
vacuo. The crude was purified by flash silica gel chromatography (ISCOg; 20 g
Agela Silica
Flash Column, eluent of 15% ethyl acetate/pet. ether gradient @ 60 mL/min) to
afford methyl
(4R)-4-((E)-2-((tert-butoxycarbanyl)imino)-4-ethyl -6-ox o-4-(pent-4-en-1-
yl)tetrahydropyrimidin-1(2H)-yl)chromane-6-carboxylate (4-8). MS (ES!) m/z:
500.6 (M+11 ).
'El NMR (400 MHz, CHLOROFORM-d) 10.10 (s, 1H), 7.75 (d, J = 8.8 Hz, 1H), 7.61
(s, 1H),
6.83 (ddõI = 0.8, 8.8 Hz, 1H), 6.31-6.46 (m, 11-1), 5.77 (m, 1H), 4.97-5.10
(m, 2H), 4.43 (dõI =
11.2 Hz, 1H), 4.17-4.29 (m, 1H), 3.82 (s, 3H), 2.68-2.86 (m, 1H), 2.47-2.59
(m, 2H), 2.06-2.18
(m, 2H), 1.56-1.81 (m, 5H), 1.51 (s, 9H), 1.45 (s, 3H), 1.40 (s, 1H)
Preparation of Compounds 4-9A & 4-9B
Boc Boc Boc
'N 'N
'N
A 7
HN HN N HN N
SFC
0
0 0,, _____________________________________________ 0 0..
0 0._
4-8 4-9A 4-9B
Methyl (E)-4-(2-((tert-butoxycarbonyl)imino)-4-ethy1-6-oxo-4-(pent-4-en-1-
yl)tetrahydropyrimidin-1(2H)-yl)chromane-6-carboxylate (4-8) (1.8 g, 3.60
mmol) was separated
by SFC (Instrument SFC-22 Method Column DAICEL CHIRALPAK AD (250mm x30mm,
10um) Condition 0.1%NH3H20 IPA Begin B 10% End B 10% Gradient Time (min) 100%B
Hold Time (min) FlowRate(mL/min) 50 Injections 60) to afford product (methyl
(R)-44R,E)-2-
((tert-but oxycarb onyl)imino)-4-ethy1-6-oxo-4-(pent-4-en-l-y1)t etrahydropyri
mi din-1(2H)-
yl)chromane-6-carboxylate (4-9A) (peak 1, Rt=0.816) and (methyl (E)-4-(2-
((tert-
butoxycarb onyl)imino)-4-ethyl -6-oxo-4-(pent-4-en-1-yl)tetrahydropyrimi din-
1(2H)-
yl)chromane-6-carboxylate (4-9B) (peak2, Rt=0.879).
MS (ESI) m/z: 500.2 (M-F11 )
Preparation of Compound 4-10
123
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
Boc Boc"-N
'N 0
0
11 .
11 . 0s04, Na104 HN-- -"N`s
HIV¨'N`'
2,6-lutidine, ________________________________________ /
Z
0
iiz
,0 0 dioxane, H20
)11- ,.... 0
I
4-9A 4-10
2,6-dimethylpyridine (42.9 mg, 0.400 mmol) and osmium (VIII) oxide (5.09 mg,
0.020 mmol) were added to a solution of methyl (R)-44R,E)-2-((tert-
butoxycarbonypimino)-4-
ethy1-6-oxo-4-(pent-4-en-l-y1)tetrahydropyrimidin-1(2H)-y1)chromane-6-
carboxylate (4-9A)
(100 mg, 0.200 mmol) in 1,4-dioxane (4 mL) and water (1 mL). The resulting
solution was
stirred for 0.2 h at 25 'C. Sodium periodate (171 mg, 0.801 mmol) was added
and the resulting
solution was stirred for 2 h at 25 C. The mixture was quenched with Sat.
Na2S03 (10 mL) and
water (5 mL), and extracted with Et0Ac (3 x 10 mL). The organic layers were
washed with brine
(5 mL), dried over Na2SO4, filtered and concentrated in vacuo to afford methyl
(R)-4-((R,E)-2-
((tert-butoxycarbonyl)imino)-4-ethy1-6-oxo-4-(4-oxobutyl)tetrahydropyrimidin-
1(2H)-
yl)chromane-6-carboxylate (4-10) which was used crude for the next step
directly.
MS (E SI) nilz: 502.3 (M-FH+)
Preparation of compound 4-11
Boc`N 0
Boc`N 0 Ii
HN¨N`s.
HN-- ¨N`s NaBH4
0 0
I Me0H, 0 00
C
0
0 0
I
H
4-10 4-11
Nal3H4 (10.75 mg, 0.284 mmol) was added in portions to a solution of methyl
(R)-4-((R, E)-2-((tert-butoxycarbonyl)imino)-4-ethy1-6-oxo-4-(4-
oxobutyl)tetrahydropyrimidin-
1(2H)-yl)chromane-6-carboxylate (4-10) (95 mg, 0.189 mmol) in Me0H (2mL) at 0
C. The
mixture was stirred at 0 C for 1 h. LCMS showed major desired product mass.
The mixture was
quenched with water (5 mL), and extracted with Et0Ac (4 x 5 mL). The combined
organic
layers were washed with brine (5 mL), dried over Na2SO4, filtered, and
concentrated in vacuum,
the crude was purified by prep-TLC (Pet. ether/Et0Ac=3:1) to afford methyl (R)-
4-((R,E)-2-
124
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
((tert-butoxycarbonyl)imino)-4-ethy1-4-(4-hydroxybuty1)-6-
oxotetrahydropyrimidin-1(2H)-
yl)chromane-6-carboxylate (4-11).
MS (ESI) nilz: 504.4 (M-P1-1)
11-1 NMR (400 MHz, CHLOROFORM-d) 10.14 (s, 1H), 7.76 (dd, J= 2.0, 8.4 Hz, 1H),
7.65 (s,
1H), 6.84 (d, J = 8.4 Hz, 1H), 6.36 (dd, J = 7.2, 10.0 Hz, 1H), 4.43-4.46 (m,
1H), 4.22-4.26 (m,
1H), 3.84 (s, 3H), 3.68 (t, J= 6.4 Hz, 2H), 2.69-2.78 (m, 1H), 2.51-2.62 (m,
2H), 1.95-2.24 (m,
2H), 1.64-1.69 (m, 6H), 1.52 (s, 9H), 1.43-1.49 (m, 2H), 0.97 (t, J= 7.6 Hz,
3H)
Preparation of Compound 4-12
Boc`N Boc`N 0 0
HN N"' HN N"
TMSOK
THF, r.t.
0 0 HO 0
HO
1
4-11 4-12
Potassium trimethylsilanolate (81 mg, 0.631 mmol) was added to a solution of
methyl (R)-4-0R,E)-2-((tert-butoxycarbonypimino)-4-ethyl-4-(4-hydroxybuty1)-6-
oxotetrahydropyrimidin-1(2H)-y1)chromane-6-carboxylate (4-11) (53 mg, 0.105
mmol) in THE'
(1 mL). The reaction was stirred at 25 C for 0.5 h. LCMS showed major desired
product mass.
The solution of (R)-4-((R, E)-2-((tert-butoxycarbonyl)imino)-4-ethy1-4-(4-
hydroxybuty1)-6-
oxotetrahydropyrimidin-1(2H)-yl)chromane-6-carboxylic acid (4-12) was used in
the next step
without any further manipulation or purification.
MS (ESI) nilz: 490.2 (M-E11+)
Preparation of Compound 4-13
Boc`N
Boc'N 0
0 NH2
Br ail
HN N"
WI 0/IdJ
HN
" 0"
EDCI, HOBt,
HO 0 DIEA, THF
Br HN 0
HO
0
4-12 4-13
125
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
DIEA (0.143 mL, 0.817 mmol) was added to a solution of (R)-44(R,E)-2-((tert-
butoxycarbonyl)imino)-4-ethy1-4-(4-hydroxybuty1)-6-oxotetrahydropyrimidin-
1(2H)-
yl)chromane-6-carboxylic acid (4-12) (50 mg, 0.102 mmol), EDC (98 mg, 0.511
mmol), 1H-
benzo[d][1,2,3]triazol-1-01 (69.0 mg, 0.511 mmol) and (S)-6-bromo-2,2-
dimethylchroman-4-
amine (28.8 mg, 0.112 mmol) in THE (5 mL). The reaction was stirred at 25 C
for 3 h. The
mixture was quenched with water (5 mL), and extracted with Et0Ac (3 x 5 mL).
The organic
layers were washed with brine (5 mL), dried over Na2SO4, filtered and
concentrated in vacuum,
the crude was purified by prep-TLC (Pet. ether/Et0Ac=3:1) to afford tert-butyl
((R,E)-1-((R)-6-
(((S)-6-bromo-2,2-dimethylchroman-4-yl)carbamoyl)chroman-4-y1)-4-ethyl-4-(4-
hydroxybuty1)-
6-oxotetrahydropyrimidin-2(1H)-ylidene)carbamate (4-13).
MS (ESI) rrilz: 727.2, 729.2 (M-41 )
Preparation of Compound 4-14
BocN Boc'N
JL,
HN N". HN IV*
/II- 0 t-BuXphos G3, Cs2CO3 /I". 0
HN 0 HN
BrJ)ç dioxane, 70 C 0
0 0
4-13 4-14
In a glove box, [(2-Di-tert-butylphosphino-2',4',6'-triisopropy1-1,1'-
bipheny1)-2-
(2'-amino-1,11-bipheny1)] palladium(II) methanesulfonate (7.64 mg, 9.62 umol)
and CsCO3 (37.1
mg, 0.192 mmol) was added to a mixture of tert-butyl ((R,E)-14(R)-6-(((S)-6-
bromo-2,2-
dimethylchroman-4-yl)carbamoyl)chroman-4-y1)-4-ethyl-4-(4-hydroxybuty1)-6-
oxotetrahydropyrimidin-2(1H)-ylidene)carbamate (4-13) (70 mg, 0.096 mmol) in
dioxane (3
mL). The mixture was stirred at 70 C for 3 h. The mixture was diluted with
water (10 mL) and
extracted with Et0Ac (3 x 10 mL). The organic layers were washed with brine (5
mL), dried
over Na2SO4, filtered and concentrated in vacuum. The crude was purified by
prep-TLC (Pet.
ether/Et0Ac=2:1) to afford tert-butyl ((4aR,5R,8R,19aS,E)-8-ethyl -18,18-di m
ethyl -6,21-di ox o-
4,4a,7,8,9,10,11,12,19,19a,20,21-dodecahydro-3H,6H,18H-1,22-
(epiethane[1,2]diylidene)-8,5-
(epiminomethano)-14,16-ethenodipyrano[3,4-d:3',4'-
]][1]oxa[6,12]diazacyclononadecin-29-
ylidene)carbamate (4-14).
MS (ESI) nilz: 647.3 (M-E11)
126
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NMIR (500 MHz, CHLOROFORM-d) 6 7.81 (dd, J= 2.0, 8.5 Hz, 1H), 7.01 (s, 1H),
6.93 (d, J
= 8.5 Hz, 1H), 6.88 (d, J= 1.0 Hz, 1H), 6.78 (s, 2H), 6.40 (dd, J= 7.0,
10.0Hz, 1H), 6.13 (d, J=
8.50 Hz, 1H), 5.37-5.44 (m, 1H), 4.44 (td, J= 3.5, 11.5 Hz, 1H), 4.17-4.23 (m,
2H), 3.82 (dt, J=
5.0, 9.5 Hz, 1H), 2.71-2.79 (m, 2H), 2.53 (d, J = 16.0 Hz, 1H), 2.35 (dd, J =
6.5, 13.0 Hz, 1H),
2.07-2.14 (m, 2H), 1.78 (dd, J= 7.50, 10.50 Hz, 4H), 1.52 (s, 2H), 1.49 (s,
9H), 1.42(s, 3H),
1.37 (s, 3H), 0.95 (t, J= 7.5 Hz, 3H)
Preparation of Example 4
Boo, NH 0
0
HN N". HNAN".
rt-A0
ZoBr2
0
HN 0
HN 0
0 DC M, r.t 0
0
0
4-14 4
Zinc(II) bromide (104 mg, 0.464 mmol) and oxa[6,12]diazacyclononadecin-29-
ylidene)carbamate (4-14) (30 mg, 0.046 mmol) in DCM (3 mL) at 25 C were added
to a
solution of tert-butyl ((4aR,5R,8R,19aS,E)-8-ethy1-18,18-dimethy1-6,21-dioxo-
4,4a,7,8,9,10,11,12,19,19a,20,21-dodecahydro-3H,6H,18H-1,22-
(epiethane[1,2]diylidene)-8,5-
(epiminomethano)-14,16-ethenodipyrano[3,4-d:3',4'-]][1], under N2 atmosphere.
The mixture
was stirred at 25 C for 16 h. The solvent was evaporated under reduced
pressure to give the
crude product and the residue was purified by reverse preparative HPLC
(Instrument e.g.,
Method Column Welch Xtimate C18 150 x 25mm, Sum; Condition water(TFA)-ACN
Begin B
25; End B 55 Gradient Time(min) 11; 100%B Hold Time(min) 2 FlowRate(mL/min)
25;
Injections 1) to give (4aR,5R,8R,19aS)-8-ethy1-29-imino-18,18-dimethyl-
4,4a,7,8,9,10,11,12,18,19,19a,20-dodecahydro-3H,6H,21H-1,22-
(epiethane[1,2]diylidene)-8,5-
(epiminomethano)-14,16-ethenodipyrano[3,4-d:3',4'-
j][1]oxa[6,121diazacyclononadecine-6,21-
dione Example 4.
MS (ESI) nilz: 547.2 (M-Ell+)
1H NMR (500 MHz, METHANOL-d4) 6 8.55 (d, = 8.5 Hz, 1H), 7.73 (dd, .1 = 2.0,
8.5 Hz,
1H), 7.45 (d, J= 1.5 Hz, 1H), 6.94 (d, J= 8.50 Hz, 1H), 6.86 (d, J = 2.5 Hz,
1H), 6.76-6.82 (m,
1H), 6.70 (d, J= 9.0 Hz, 1H), 5.35-5.40 (m, 1H), 5.24-5.32 (m, 1H), 4.47 (td,
J= 4.5, 11.50 Hz,
1H), 4.13-4.21 (m, 2H), 3.90-3.92 (m, 1H), 2.75-2.88 (m, 2H), 2.60-2.72 (m,
1H), 2.22-2.31 (m,
127
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
1H), 2.15 (dd, J= 6.5, 13.0 Hz, 1H), 1.66-1.85 (m, 7H), 1.47-1.60 (m, 2H),
1.40 (s, 3H), 1.33 (s,
3H), 0.97 (t, J= 7.5 Hz, 3H)
EXAMPLE 5
NH rc
HNANµ'µ
NH
(1 1R, 1 4R,14aR,21aS)-11-ethy1-16,16-di fluoro-24-imi n o-2,2-dim ethyl-
1,2,7,8,9,10,11,12,15,16,21,21a-dodecahydro-13H-11,14-(epiminomethano)-
4,6:17,19-
diethenocyclopenta[b]pyrano[4,3-h][1,7]diazacyclooctadecine-13,20(14aH)-dione
Preparation of Compound 5-2
SH
0
Ts0H c\S
Toluene,
Br Br
130 C, 16 h
5-1 5-2
A solution of 5-bromo-2,3-dihydro-1H-inden-1-one (50 g, 237 mmol), ethane-
1,2-dithiol (5-1) (26.6 mL, 317 mmol) and 4-methylbenzenesulfonic acid (8.16
g, 47.4 mmol) in
toluene (500 mL) was heated to 130 C for 16 h using a Dean-Stark apparatus.
TLC showed the
reaction was complete. The cooled solution was washed with 10% NaOH (600 mL),
and the
aqueous layer extracted with DCM (3 x600 mL). The combined organic layer was
washed with
brine (300 mL), dried over Na2SO4, filtered and the solvent was evaporated
under reduced
pressure to give the crude product. The crude product was purified by flash
silica gel
chromatography (ISCO ; 330 g SepaFlash Silica Flash Column, Eluent of 10%
Et0Ac/Pet.ether gradient @ 60 mL/min) to give 5-bromo-2,3-dihydrospiro[indene-
1,2'-
[1,3]dithiolane] (5-2).
MS (ESI) in/z 286.9, 288.9 (M+H)
NMR (500 MHz, CHLOROFORM-d) 6 7.40-7.44 (m, 1H), 7.31-7.38 (m, 2H), 3.50-3.56
(m,
2H), 3.40-3.47 (m, 2H), 2.96 (t, J= 6.5 Hz, 2H), 2.69 (t, J = 6.5 Hz, 2H)
128
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
Preparation of Compound 5-3
C\S HF-Py,
H (4 eq) Br F
DMDB
DCM,
Br -70 C - RT Br
3 h
5-2 5-3
A solution of 1,3-dibromo-5,5-dimethylhydantoin (194 g, 679 mmol) in
anhydrous CH2C12 (700 mL) was cooled to -70 C in a dry ice-acetone bath.
Pyridine
hydrofluoride (57.4 mL, 226 mmol) was added dropwise at a temperature below -
65 C under
N2, and the mixture stirred at -70 C for 30 min. A solution of 5-bromo-2,3-
dihydrospiro[indene-
1,2'41,3]dithiolane] (5-2) (65 g, 226 mmol) in CH2C12 (200 mL) was added
dropwise and the
mixture was stirred at -70 C for 4 h, and then at 25 C overnight. TLC showed
the reaction was
complete. The mixture was poured into NaOH (2 M, 300 mL) containing 39% NaHS03
(600
mL) solution. The aqueous layer was extracted with CH2C12 (2Y600 mL) and the
combined
organic layer washed with brine (300 mL), dried over Na2SO4, filtered and the
solvent was
evaporated under reduced pressure to give the crude product. The crude product
was purified by
flash silica gel chromatography (ISCO(g); 220 g SepaFlash Silica Flash
Column, Eluent of 100%
Pet.ether gradient @ 60 mL/min) to give 2,5-dibromo-1,1-difluoro-2,3-dihydro-
1H-indene (5-3).
No LCMS signal.
NMR (500 MHz, CHLOROFORM-d) 6 7.52-7.58 (m, 1H), 7.47 (d, J= 6.5 Hz, 2H), 4.57
(tt,
= 7.0, 10.5 Hz, 1H), 3.57 (ddd, J = 2.0, 7.5, 16.5 Hz, 1H), 3.27 (dd, J = 7.0,
16.5 Hz, 1H)
Preparation of Compound 5-4
Br DBU
DCM, RT
Br Br
5-3 5-4
To a solution of 2,5-dibromo-1,1-difluoro-2,3-dihydro-1H-indene (5-3) (60 g,
192
mmol) in DCM (600 mL) was added DBU (43.5 mL, 289 mmol). The mixture was
stirred at 25
C for 16 h. LCMS showed desired product mass. Water (600 mL) was added, the
mixture was
acidified with con.HC1 to pH=7. Filtered the mixture with diatomite, and the
aqueous layer was
extracted with CH2C12 (2><600 mL) and the combined organic layers were washed
with brine
(300 mL), dried over Na2SO4, filtered and the solvent was evaporated under
reduced pressure to
129
CA 03240145 2024- 6-5
WO 2023/107356 PCT/US2022/051770
give the crude product. The crude product was purified by flash silica gel
chromatography
(ISCO ; 330 g SepaFlash Silica Flash Column, Eluent of 100% Pet.ether
gradient @ 50
mL/min) to give 5-bromo-1,1-difluoro-1H-indene (5-4).
No LCMS signal.
III NMR (500 MHz, CHLOROFORM-d) 6 7.41 (dd, J= 1.5, 8.0 Hz, 1H), 7.29-7.35 (m,
2H),
6.75 (d, J= 6.0 Hz, 1H), 6.22 (d, J 6.0 Hz, 1H)
Preparation of Compound 5-5
F F
F F
HO
Mn(TMHD)3, PhSiH3'
_______________________________________________ )1.
i-PrOH, 02, Br
Br
Br
5-4 5-5 5-5a
To a solution of 5-bromo-1,1-difluoro-1H-indene (5-4) (15 g, 64.9 mmol) in
iPrOH (250 mL) which was bubble with 02 for 1 h, was added PHENYLSILANE (14.05
g, 130
mmol) and Mn(TM_HD)3 (3.93 g, 6.49 mmol) and stirred at 0 C for 2 h under
02(15 psi). TLC
showed the reaction was complete. The mixture was quenched with water (300
mL), and
extracted with Et0Ac (3 x 300 mL). The organic layers were washed with brine
(300 mL), dried
over Na2SO4, filtered and the solvent was evaporated under reduced pressure to
give the crude
product. The crude product was purified by flash silica gel chromatography
(TSCO ; 40 g
SepaFlash Silica Flash Column, Eluent of 17% Et0Ac/Pet.ether gradient @ 50
mL/min) to give
a mixture of 6-bromo-3,3-difluoro-2,3-dihydro-1H-inden-1-ol (5-5) and 5-bromo-
1,1-difluoro-
2,3-dihydro-1H-inden-2-ol (5-5a); (5-5: 5-5a =7:2).
IH NAIR (500 MHz, CHLOROFORM-d) 6 7.68 (s, 1H), 7.59-7.62 (m, 1H), 7.43-7.46
(m, 1H),
5.31 (q, J= 6.0 Hz, 1H), 3.01-3.12(m, 1H), 2.49 (dq, J= 5.0, 14.5 Hz, 1H)
Preparation of Compound 5-6
F F F F F F F
F
HO Pd(dppOf)C12, HO
TEA, C
_______________________________________________ >
Br Me0H,
Br DMSO, 80 C 00Me
00Me
5-5 5-5a 5-6 5-
6a
To a solution of mix of 6-bromo-3,3-difluoro-2,3-dihydro-1H-inden-1-ol and 5-
bromo-1,1-difluoro-2,3-dihydro-1H-inden-2-ol (11.56 g, 47.3 mmol) (5-5: 5-5a
=7:2) in Me0H
130
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(150 mL) and DMSO (15 mL) was added [1,1'-
Bis(diphenylphospino)ferrocene]dichloropalladium(II) (2.64 g, 3.61 mmol) and
triethylamine
(15.67 mL, 108 mmol) under Ar atmosphere at 20 C and the mixture was stirred
at 80 C for 48
h under CO atmosphere (3.5 mbar). TLC showed the reaction was complete. After
cooled,
filtered the mixture with diatomite, and the mixture was diluted with H20 (200
mL), extracted
with Et0Ac (3x200 mL), dried over Na2SO4, filtered and the solvent was
evaporated under
reduced pressure to give the crude product. The crude product was purified by
flash silica gel
chromatography (ISCO(L); 120 g SepaFlash Silica Flash Column, Eluent of 17%
Et0Ac /
Pet. ether gradient @ 80 mL/min) to give a mixture of methyl 1,1-difluoro-3-
hydroxy-2,3-
dihydro-1H-indene-5-carboxylate (5-6) and methyl 1,1-difluoro-2-hydroxy-2,3-
dihydro-1H-
indene-5-carboxylate (5-6a); (5-6: 5-6a =3:1).
NMR (500 MHz, chloroform-d) 5 8.21 (s, 1H), 8.14 (d, J= 8.0 Hz, 1H), 7.65 (d,
J= 8.0 Hz,
1H), 5.37 (quin, J= 6.0 Hz, 1H), 3.96 (s, 3H), 3.05-3.17 (m, 1H), 2.54 (dq,
.1= 5.0, 14.5 Hz, 1H)
Preparation of Compound 5-7_P1
F F F F F F F F
HO SFC
HO H = 11. HO's'
00Me COOMe 00Me
00Me
5-6 5-6a 5-7 5-
7_P2
The mixture of methyl 1,1-difluoro-3-hydroxy-2,3-dihydro-1H-indene-5-
carboxylate and methyl 1,1-difluoro-2-hydroxy-2,3-dihydro-1H-indene-5-
carboxylate (8.3 g,
36.36 mmol) (5-6: 27-6a = 3:1) was separated by SFC (Column: Chiralpak AD-3
150 x 4.6mm
ID., 3um, Mobile phase: A:CO2 B:iso-propanol (0.05% DEA), Gradient: from 5% to
40% of B
in 5 min and from 40% to 5% of B in 0.5 min, then hold at 5% of B for 1.5 min,
Flow rate:
2.5mL/min, Column temp.: 35 C) to afford product methyl (S)-1,1-difluoro-3-
hydroxy-2,3-
dihydro-1H-indene-5-carboxylate (5-7 Pl, desired) (peakl, Rt=2.577), methyl
(R)-1,1-difluoro-
3-hydroxy-2,3-dihydro-1H-indene-5-carboxylate (5-7_P2) (peak2, Rt=2.944) and a
mixture of
(5-7_132 and 5-6a) (2 g, 8.77 mmol). The mixture of (5-7_P2 and 5-6a) was
separated by SFC
(Column: Cellulose 2 150 x 4.6mm ID., Sum, Mobile phase: A: CO2 B:Me0H (0.05%
DEA)
Gradient: from 5% to 40% of B in 5 min and from 40% to 5% of B in 0.5 min,
then hold 5% of
B for 1.5 min, Column temp.: 35 C) to give methyl 1,1-difluoro-2-hydroxy-2,3-
dihydro-1H-
indene-5-carboxylate (5-6a).
131
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(5-7P1): 1H NMR (500 MHz, chloroform-d) 6 8.21 (s, 1H), 8.15 (d, J= 8.0 Hz,
1H), 7.65 (d, J
= 8.0 Hz, 1H), 5.37 (q, J= 6.0 Hz, 1H), 3.96 (s, 3H), 3.06-3.17 (m, 1H), 2.54
(dq, J= 5.0, 14.5
Hz, 1H)
(5-7_P2): NMR (500 MHz, chloroform-d) .3 8.20 (s, 1H), 8.13 (d, J=
8.0 Hz, 1H), 7.64 (d, J
= 8.0 Hz, 1H), 5.36 (q, J= 5.5 Hz, 1H), 3.95 (s, 3H), 3.05-3.17 (m, 1H), 2.54
(dq, J= 5.0, 14.5
Hz, 1H)
(5-6a): 1H NMR (500 MHz, chloroform-d) 6 8.05 (d, J= 8.0 Hz, 1H), 7.98 (s,
1H), 7.64 (d, J=
8.0 Hz, 1H), 4.53-4.68 (m, 1H), 3.95 (s, 3H), 3.40 (dd, J= 7.0, 16.0 Hz, 1H),
2.95 (dd, J= 5.0,
16.5 Hz, 1H)
Preparation of Compound 5-8
Boc-N
HN NH
Boc'N F F
INT-2
HN N".
ET
DIAD, PPh3 /"" 0
THF, RT 0
00Me
5-7_Pl 5-8
To a solution of methyl (S)-1,1-difluoro-3-hydroxy-2,3-dihydro-1H-indene-5-
carboxylate (5-7 _Pi) (585 mg, 2.56 mmol), tert-butyl (R,E)-(4-(but-3-en-1-y1)-
4-ethy1-6-
oxotetrahydropyrimidin-2(1H)-ylidene)carbamate (INT-2) (757 mg, 2.56 mmol) and
Ph3P (1009
mg, 3.85 mmol) in TIFF (10 mL) was added DIAD (0.997 mL, 5.13 mmol) dropwise
at 0 C
under N2 atmosphere, then the mixture was stirred at 18 C for 2 h. TLC showed
no SM. The
mixture was concentrated in vacuo, and the residue was purified by flash
silica gel
chromatography (ISCO ; 20 g SepaFlash Silica Flash Column, Eluent of 20%
Et0Ac/Pet.ether
gradient @ 60 mL/min) to afford crude product. The crude product was re-
purified by flash silica
gel chromatography (ISC04); 20 g SepaFlash4) Silica Flash Column, Eluent of
DCM gradient @
60 mL/min) to afford product methyl (R)-3-4R,E)-4-(but-3-en-l-y1)-2-((tert-
butoxycarbonyl)imino)-4-ethyl-6-oxotetrahydropyrimidin-1(2H)-y1)-1,1-difluoro-
2,3-dihydro-
IH-indene-5-carboxylate (5-8).
MS (ESI) 111/Z 506.2 (M+H)+
1H NMR (400 MHz, chloroform-d) 6 10.08 (br s, 1H), 8.07 (d, J= 8.0 Hz, 1H),
7.78 (s, 1H),
7.66 (d, J= 8.0 Hz, 1H), 6.74 (q, J= 7.2 Hz, 1H), 5.80-5.87 (m, 1H), 4.95-5.21
(m, 2H), 4.05-
132
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
4.19 (m, 1H), 3.91 (s, 3H), 2.92-3.14 (m, 2H), 2.58 (s, 2H), 2.07-2.19 (m,
2H), 1.63-1.78 (m,
4H), 1.52 (s, 9H), 0.97 (t, J= 7.6 Hz, 3H)
Preparation of Compound 5-9
F F
F F
Boc`N Boc`N
HN N" TMSOK
THF, RTIP / 0
0 OH
\
\ \
5-8 5-9
To a solution of methyl (R)-3-((R,E)-4-(but-3-en-1-y1)-2-((tert-
b utoxy carb onyl)imino)-4-ethy1-6-oxotetrahy dropyrimi din-1(2H)-y1)-1,1-
difluoro-2,3-dihy dro-
1H-indene-5-carboxylate (5-8) (900 mg, 1.780 mmol) in THF (9 mL) was added
potassium
trimethylsilanolate (1370 mg, 10.68 mmol). The reaction was stirred at 18 C
for 0.5 h. LCMS
showed desired mass. Added H3PO4 (0.1 g/mL in H20) to adjust pH to about 6-7,
and the
mixture was quenched with water (10 mL), and extracted with Et0Ac (3 x 10 mL).
The organic
layers were washed with brine (10 mL), dried over Na2SO4, filtered and
concentrated in vacuo to
afford the product (R)-3-((R,E)-4-(but-3-en-1-y1)-2-((tert-
butoxycarbonyl)imino)-4-ethy1-6-
oxotetrahydropyrimidin-1(2H)-y1)-1,1-difluoro-2,3-dihydro-1H-indene-5-
carboxylic acid (5-9).
MS (ESI) in/z 492.1 (M+H)
1H NIVIR (400 MHz, chloroform-d) 6 10.08 (br s, 1H), 8.06 (d, J= 8.0 Hz, 1H),
7.82 (s, 1H),
7.65 (d, J= 8.0 Hz, 1H), 6.72-6.77 (m, 1H), 5.80-5.89 (m, 1H), 4.99-5.16 (m,
2H), 2.98-3.14 (m,
2H), 2.59 (s, 2H), 2.11-2.13 (m, 2H), 1.64-1.83 (m, 4H), 1.53 (s, 9H), 0.98
(t, J= 7.6 Hz, 3H)
Preparation of Compound 5-10
F
F Boc'N F
F
Boc`N NH2
Nr=
HN N"
OH EDCI, HOBt,
DIEA, THF
I
\ /
5-9
5-10
133
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
To a solution of (R)-3-((R,E)-4-(but-3-en-l-y1)-2-((tert-butoxycarbonyl)imino)-
4-
ethy1-6-oxotetrahydropyrimidin-1(2H)-y1)-1,1-difluoro-2,3-dihydro-1H-indene-5-
carboxylic acid
(5-9) (0.87 g, 1.770 mmol), EDC (1.697 g, 8.85 mmol), 1H-
benzo[d][1,2,3]triazol-1-ol (0.718 g,
5.31 mmol) in THF (15 mL) was added DIEA (2.473 mL, 14.16 mmol) and (S)-2,2-
dimethy1-6-
vinylchroman-4-amine (0.396 g, 1.947 mmol). The reaction was stirred at 18 C
for 12 h. LCMS
showed desired mass. The mixture was quenched with water (15 mL), and
extracted with Et0Ac
(3 x 15 mL). The organic layers were washed with brine (10 mL), dried over
Na2SO4, filtered
and concentrated in vacuo, and the crude was purified by flash silica gel
chromatography
(ISCO*; 12 g SepaFlash''' Silica Flash Column, Eluent of 17% Et0Ac/Pet.ether
gradient @ 60
mL/min) to afford product tert-butyl ((R,E)-4-(but-3-en-l-y1)-1-((R)-6-(((S)-
2,2-dimethyl-6-
vinylchroman-4-yl)carbam oy1)-3 ,3 -di fluoro-2,3-di hydro-1H-i nden -1 -y1)-4-
ethyl -6-
oxotetrahydropyrimidin-2(1H)-ylidene)carbamate (5-10).
MS (ESI) nilz 677.4 (M+H)+
1H NMR (400 MHz, CHLOROFORM-d) 6 10.08 (br s, 1H), 7.72-7.78 (m, 1H), 7.61-
7.69 (m,
2H), 7.28-7.34 (m, 2H), 6.81 (d, J = 8.4 Hz, 1H), 6.76-6.77 (m, 1H), 6.58-6.62
(m, 1H), 6.22 (br
d, J= 8.8 Hz, 1H), 5.72-5.77 (m, 1H), 5.46-5.61 (m, 2H), 5.11 (d, J= 11.2 Hz,
1H), 5.06-5.08
(m, 1H), 4.97-5.00 (m, 1H), 2.96-3.14 (m, 2H), 2.58 (s, 2H), 2.29-2.34 (m,
1H), 2.10 (br d, J=
6.0 Hz, 2H), 1.71-1.81 (m, 1H), 1.63-1.74 (m, 4H), 1.52 (s, 9H), 1.46 (s, 3H),
1.38 (s, 3H), 0.96
(t, J = 7.6 Hz, 3H).
Preparation of Compound 5-11
Boc`N Boc`N Boc`N
NH HG-Il 0 0
NH
NH
DOE, 50 C
5-10 5-11 5-11a
To a solution of tert-butyl ((R,E)-4-(but-3-en-1-y1)-1-((R)-6-(((S)-2,2-
dimethy1-6-
vi nyl chrom an-4-yl)carbam oy1)-3 ,3 -di fluoro-2,3 -di hydro-1H-i nden -1 -
y1)-4-ethyl -6-
oxotetrahydropyrimidin-2(1H)-ylidene)carbamate (5-10) (1 g, 1.478 mmol) in DCE
(500 mL)
was added Dichloro[1,3-bis(2,6-isopropylpheny1)-2-imidazolidinylidene](2-
isopropoxyphenylmethylene)ruthenium(II) (0.105 g, 0.148 mmol). The reaction
was stirred at 50
134
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
C for 4 h with N2. LCMS showed desired mass. The mixture was concentrated in
vacuo, and the
crude was purified by flash silica gel chromatography (ISCW; 12 g SepaFlash
Silica Flash
Column, Eluent of 15% Et0Ac/Petether gradient @ 60 mL/min) to afford tert-
butyl
((7Z,11R,14R,14aR,21a S,24E)-11-ethy1-16,16-difluoro-2,2-dimethy1-13,20-di oxo-
1,2,10,11,12,13,14a,15,16,20,21,21a-dodecahydro-9H-11,14-(epiminomethano)-4,6:
17,19-
diethenocyclopenta[b]pyrano[4,3-h][1,7]diazacyclooctadecin-24-
ylidene)carbamate (5-11a, cis
olefin; minor product) and tert-butyl ((7E,11R,14R,14aR,21aS,24E)-11-ethy1-
16,16-difluoro-
2,2-dimethy1-13,20-di oxo-1,2,10,11,12,13,14a,15,16,20,21,21a-dodecahydro-9H-
11,14-
(epiminomethano)-4,6:17,19-diethenocyclopenta[b]pyrano[4,3-
h][1,7]diazacyclooctadecin-24-
ylidene)carbamate (5-11, trans olefin; major product).
(5-11a, cis olefin): MS (ESI)in/z 649.3 (M-41)+
1H NMR (400 MHz, CHLOROFORM-d) 6 10.12 (s, 1H), 8.06 (d, J= 7.6 Hz, 1H), 7.73
(d, 1=
8.0 Hz, 1H), 7.17 (s, 1H), 6.99-7.01 (m, 1H), 6.84 (d, J= 8.4 Hz, 1H), 6.64-
6.76 (m, 1H), 6.39
(d, J= 11.2 Hz, 1H), 6.28 (br d, J= 8.8 Hz, 1H), 5.58-5.61 (m, 1H), 5.41-5.45
(m, 1H), 3.01-
3.13 (m, 2H), 2.66 (d, J= 16.4 Hz, 1H), 2.42-2.45 (m, 2H), 2.20-2.30 (m, 1H),
1.96-2.04(m,
1H), 1.63-1.86 (m, 5H), 1.51 (s, 9H), 1.46 (s, 3H), 1.39 (s, 3H), 1.02 (t, J=
7.2 Hz, 3H)
(5-11, trans olefin): MS (ES!) in/z 649.3 (M+H)
1H NMR (400 MHz, CHLOROFORM-d) 6 10.15 (s, 1H), 7.90 (d, J= 8.0 Hz, 1H), 7.70
(d, 1=
8.0 Hz, 1H), 7.35 (s, 1H), 7.23 (s, 1H), 7.06 (dd, 1= 2.0, 8.4 Hz, 1H), 6.73
(d, J= 8.4 Hz, 1H),
6.61-6.70 (m, 1H), 6.39 (d, 1= 15.6 Hz, 1H), 6.06 (d, 1= 9.2 H7, 1H), 5.68-
5.89 (m, 1H), 5.47-
5.50 (m, 1H), 2.95-3.14 (m, 2H), 2.68 (d, J= 16.4 Hz, 1H), 2.55 (d, J = 16.4
Hz, 1H), 2.32-2.35
(m, 3H), 1.85-1.97 (m, 1H), 1.64-1.76 (m, 4H), 1.47 (s, 3H), 1.41 (s, 9H),
1.40 (br s, 3H), 0.97 (t,
1= 7.6 Hz, 3H)
Preparation of Compound 5-12
Boc'N Boc'N Boc'N
HN
HN N"
Pd/C, H2
NH NH
NH
Me0H
5-11 5-11a 5-12
To a solution of tert-butyl ((7E, 11R,14R,14aR,21aS,24E)-11-ethy1-16,16-
135
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
difluoro-2,2-dimethy1-13,20-dioxo-1,2,10,11,12,13,14a,15,16,20,21,21a-
dodecahydro-9H-11,14-
(epiminomethano)-4,6:17,19-diethenocyclopenta[b]pyrano[4,3-
h][1,7]diazacyclooctadecin-24-
ylidene)carbamate (5-11) (780 mg, 1.202 mmol) in Me0H (10 mL) was added 10%Pd-
C (128
mg, 0.120 mmol) under N2 atmosphere. The mixture was degassed and backfilled
with H2 (three
times). The resulting mixture was stirred at 18 C for 10 min under H2 (15
psi) atmosphere.
LCMS showed the reaction was complete. The mixture was filtered and the
filtrate was
concentrated under reduced pressure to give tert-butyl ((11R,14R,14aR,21aS,E)-
11-ethy1-16,16-
di fluoro-2,2-di m ethyl - 13,20-di oxo-1,2,8,9,10,
11,12,13,14a,15,16,20,21,21a-tetradecahydro-7H-
11,14 -(epiminomethano)-4, 6:17,19-di ethenocyclopenta[b]pyrano[4,3 -
h][1,7]diazacyclooctadecin-24-ylidene)carbamate (5-12), which was used as is
directly in next
step.
MS (ESI) nilz 651.4 (M+H)+
Preparation of Example 5
Boc
NH
H NI`s=
HN N`s=
HCI-dioxane
0 0
NH NH
5-12 5
A solution of tert-butyl ((11R,14R,14aR,21aS,E)-11-ethy1-16,16-difluoro-2,2-
dimethy1-13,20-dioxo-1,2,8,9, 10,11,12,13, 14a,15,16,20,21,21 a-tetradecahydro-
7H-11,14-
(epi mi nometh ano)-4,6 :17,19-di ethenocycl openta[b]pyrano[4,3-h ] [1, 7] di
azacycl ooctadeci n-24-
ylidene)carbamate (5-12) (700 mg, 1.076 mmol) in HC1-dioxane (4N) (10 mL) was
stirred at 18
C for 12 h. LCMS showed the reaction was complete. Solvent was evaporated
under reduced
pressure to give the crude product. The residue was purified by reverse
preparative HPLC
(Instrument 3-101(EK) Method Phase separation Column Boston Uni C18 150 x
40mm, 5um
Condition water (0.04%HC1)-ACN Begin B 33 End B 63 Gradient Time(min) 10 100%B
Hold
Time 2 Flow Rate(mL/min) 60 Injections 2) to afford (11R,14R,14aR,21aS)-11-
ethy1-16,16-
difluoro-24-imino-2,2-dimethyl- 1,2,7,8,9,10,11,12, 15,16,21,21a-dodecahydro-
13H-11,14-
(epiminomethano)-4,6:17,19-diethenocyclopenta[b]pyrano[4,3-
h][1,7]diazacyclooctadecine-
13,20(14aH)-dione Example 5.
MS (ESI) nilz 551.3 (M+H)
136
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
1-H NAIR (400 MHz, METHANOL-d4) 6 7.98 (d, J= 8.0 Hz, 1H), 7.74 (d, J= 8.0 Hz,
1H), 7.62
(s, 1H), 7.03 (s, 1H), 6.97-7.00 (m, 1H), 6.70 (d, J= 8.0 Hz, 1H), 5.72 (hr d,
J= 6.4 Hz, 1H),
5.42 (dd, J= 6.4, 11.6 Hz, 1H), 3.22-3.31 (m, 1H), 2.95-3.15 (m, 2H), 2.46-
2.63 (m, 3H), 2.14-
2.17 (m, 1H), 1.73-1.91 (m, 5H), 1.55-1.72 (m, 2H), 1.39-1.51 (m, 5H), 1.33
(s, 3H), 0.98 (t, J=
7.2 Hz, 3H)
The compounds in Table 1 were prepared in an analogous fashion to that
described for Example 1 and/or Example 5. The isomers were separated by
preparative HPLC
or/and preparative chiral SFC.
An asterisk (*) may be used in a chemical structure drawing that indicates the
location of a chiral center.
TABLE 1
Example Structure MS Observed Chemical Name
(1R,5S,17S)-5-
ethy1-3-imino-
N H
15,15-dimethyl-
14,24-dioxa-
HN N
=2,4,18-
3B 531.2
triazahexacyclo[18
HN 0 .6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
0
10,12,20,22,27,29-
hexaene-19,32-
dione
137
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,5R,9E,16R,17
R)-5-ethy1-16-
NH hydroxy-
3-imino-
HN A N 24-oxa-
2,4,18-
/".' 0
triazahexacyclo[18
5a 515.3 .6.2.22,5.211,14.0
HN 0
13,17.023,27]dotri
aconta-
9,11,13,20,22,27,2
9-heptaene-19,32-
dione
(1R,5R,9Z,16R,17
R)-5-ethy1-16-
NH hydroxy-
3-i mi no-
H N N
24-oxa-2,4,18-
7
/"L ....,___.L0 triazahexacyclo[18
HN
6 515.2 .6.2.22,5.211,14.0
0
13,17.023,27]dotri
õOH
aconta-
9,11,13,20,22,27,2
9-heptaene-19,32-
dione
(1R,5R,9E,18S)-5-
ethy1-3-imino-
NH
16,16-dimethyl-
HNAN
15,25-di oxa-
7
2,4,19-
/" 0
triazahexacyclo[19
7 543.3
HN
.6.2.22,5.211,14.0
13,18 .024,28]tritri
aconta-
0
9,11,13,21,23,28,3
0-heptaene-20,33-
di on e
138
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,5R,18S)-5-
ethy1-3-imino-
NH 16,16-
dimethyl-
HNNL 15,25-dioxa-
2,4,19-
8 545.3
triazahexacyclo[19
HN 0
.6.2.22,5.211,14.0
13,18 .024,28]tritri
LJLJç
aconta-
0
11,13,21,23,28,30-
hexaene-20,33 -
dione
(1R,5S,18S)-5-
ethy1-3-imi no-
NH
16,16-dimethyl-
HN
15,25-dioxa-
/7L
0
2,4,19-
triazahexacyclo[19
9 545.3
HN 0
.6.2.22,5.211,14.0
13,18.024,28]tritri
aconta-
11,13,21,23,28,30-
hexaene-20,33 -
dione
139
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,5R,9Z,19S)-5-
ethy1-3-imino-
17,17-dimethyl-
NH
16,26-dioxa-
HN -
2,4,20-
"" 0
triazahexacyclo[20
557.3 .6.2.22,5.212,15.0
NH
14,19.025,29]tetrat
riaconta-
9,12,14,22(30),23,
25(29),31-
heptaene-21,34-
dione
(1R,5R,9E,20S)-5-
ethy1-3-imino-
18,18-dimethyl-
NH
HN,11-,N 17,27-dioxa-
2,4,21-
triazahexacyclo[21
11 557.3
HN 0
.6.2.22,5.112,16.0
15,20.026,30]tetrat
riaconta-
0
9,12(32),13,15,23,
25,30-heptaene-
22,34-dione
140
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,5R,19S)-5-
ethy1-3-imino-
NH
17,17-dimethyl-
HN.A.N
16,26-dioxa-
2,4,20-
" " 0
triazahexacyclo[20
12 559.3
0 NH
.6.2.22,5.212,15.0
14,19.025,29]tetrat
riaconta-
0
12,14,22(30),23,2
5(29),31-hexaene-
21,34-dione
(1R,5S,20S)-5-
ethy1-3-imi no-
18,18-dimethyl-
N H
HN)1,N -
17,27-dioxa-
17.
2,4,21-
13
triazahexacyclo[21
HN 559.3
.6.2.22,5.112,16.0
15,20.026,30]tetrat
riaconta-
0
12(32),13,15,23,2
5,30-hexaene-
22,34-dione
(1R,5R,20S)-5-
ethy1-3-i mi no-
18,18-dimethyl-
N H
HN
17,27-dioxa-
2,4,21-
" 0
triazahexacyclo[21
14 559.3
HN 0
.6.2.22,5.112,16.0
15,20.026,30]tetrat
riaconta-
0
12(32),13,15,23,2
5,30-hexaene-
22,34-di one
141
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,16R,17R,26R)
-5-ethy1-16-
hydroxy-3-imino-
26-
NH '= 0
(methoxymethy1)-
HN.-2.c`s.
24-oxa-2,4,18-
15A 561.2
triazahexacyclo[18
HN 0
.6.2.22,5.211,14.0
OH
13,17.023,27]dotri
aconta-
11,13,20,22,27,29-
hexaene-19,32-
dione
(1R,16R,17R,26R)
-5-ethyl-16-
hydroxy-3-imino-
1, 26-
NH "O
(methoxymethyl)-
HNN`s.
24-oxa-2,4,18-
15B 0 561.2
triazahexacyclo[18
HN 0
.6.2.22,5.211,14.0
OH
13,17.023,27]dotri
aconta-
11,13,20,22,27,29-
hexaene-19,32-
dione
142
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,18S,27R)-5-
ethy1-3-imino-27-
CY" (methoxymethyl)-
1 16,16-
dimethyl-
NH
15,25-dioxa-
2,4,19-
16A 0 589.3
triazahexacyclo[19
HN 0 .6.2.22,5.211,14.0
13,18.024,28]tritri
aconta-
0
11,13,21,23,28,30-
hexaene-20,33 -
dione
rae-(1R,18S,27R)-
5-ethy1-3-imino-
27-
(methoxymethyl)-
NH 16,16-
dimethyl-
HNN's.
15,25-dioxa-
16B 0 589.3
2,4,19-
triazahexacyclo[19
HN 0
.6.2.22,5.211,14.0
13,18.024,28]tritri
0 aconta-
11,13,21,23,28,30-
hexaene-20,33 -
dione
143
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
rac-
(12R,13R,22S)-8-
ethy1-10-imino-
13-
1,
(methoxymethy1)-
NH 0 24,24-
dimethyl-
15,25-dioxa-
0 601.3
9,11,21-
17
* "HO
triazaheptacyclo[2
0.6.2.28,11.216,19
.02,4.012,17.026,3
0
0]tetratriaconta-
1(29),16(32),17,19
(31),26(30),27-
hexaene-20,33-
dione
rac-
(1R,5R,9E,18S)-5-
NH 0
ethy1-3-imino-
H NN"=
15,25-dioxa-
2,4,19-
triazahexacyclo[19
18 515.2
HN 0
.6.2.22,5.211,14.0
13,18.024,28]tritri
aconta-
9,11,13,21,23,28,3
0-heptaene-20,33-
dione
144
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
rac-(1R,5R,18S)-
5-ethy1-3-imino-
NH
15,25-dioxa-
HNAN".
2,4,19-
/II "
triazahexacyclo[19
19 517.2
.6.2.22,5.211,14.0
HN 0
13,18.024,28]tritri
aconta-
11,13,21,23,28,30-
hexaene-20,33 -
dione
rac-(1R,8E,17S)-
5-ethy1-3-imino-
NH
15,15-dim ethyl -
14,24-dioxa-
LJHN N
2,4,18-
0
triazahexacyclo[18
20 529.2
HN 0
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
0
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
rac-(8R,12R,22S)-
8-ethyl- 1 0-imino-
15,25-di oxa-
NH
HNAN".
9,11,21-
21
triazaheptacyclo[2
/" " 0
0.6.2.28,11.216,19
529.2
* HN 0
.02,4.012,17.026,3
O]tetratriaconta-
1(29),16(32),17,19
(31),26(30),27-
hexaene-20,33-
di on e
145
CA 03240145 2024- 6-5
WO 2023/107356
PC T/US2022/051770
rac-(1R,5R,19S)-
5-ethy1-3-imino-
N H
17,17-dimethyl-
HN N
16,26-dioxa-
2,4,20-
/I'1._..0 triazahexacyclo[20
22 545.2
H N 0 .6.2.22,5.111,15.0
14,19.025,29]tritri
aconta-
0
11(31),12,14,22,2
4,29-hexaene-
21,33-dione
rac-(1R,11Z,21S)-
5-ethy1-3-imino-
N H
19,19-dimethyl-
HN N
18,28-dioxa-
2,4,22-
0 triazahexacyclo[22
23 571.3
H N 0 .6.2.22,5.113,17.0
16,21.027,31Thent
atriaconta-
- 0
11,13(33),14,16,2
4,26,31-heptaene-
23,35-dione
rac-(1R,21S)-5-
ethy1-3-imino-
N H
19,19-dimethyl-
H N* N
18,28-dioxa-
2,4,22-
0 triazahexacyc1o[22
24A 573.3
H N .6.2.22,5.113,17.0
16,21.027,31]pent
atriaconta-
0
13(33),14,16,24,2
6,31-hexaene-
23,35-dione
146
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
rac-(1R,21S)-5-
ethy1-3-imino-
N H
19,19-dimethyl-
A -
H N* N 18,28-dioxa-
2,4,22-
0
triazahexacyclo[22
24B 573.3
HN 0
.6.2.22,5.113,17.0
16,21.027,31Thent
atriaconta-
0
13(33),14,16,24,2
6,31-hexaene-
23,35-dione
rac-(1R,18S,27R)-
3-i mino-27-
(methoxymethyl)-
5,16,16-trim ethyl-
N H
15,25-dioxa-
H N`'.
2,4,19-
25A 0 575.4
triazahexacyclo[19
HN 0
.6.2.22,5.211,14.0
13,18.024,28]tritri
aconta-
0
11,13,21,23,28,30-
hexaene-20,33 -
dione
147
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
rac-(1R,18S,27R)-
3 -imino-27-
(methoxymethyl)-
5,16,16-trim ethyl-
NH 0
15,25-dioxa-
2,4,19-
25B 0 575.3
triazahexacyclo[19
HN 0
.6.2.22,5.211,14.0
13,18.024,28]tritri
aconta-
0
11,13,21,23,28,30-
hexaene-20,33 -
dione
rac-
(1R,9E,185,27R)-
5-ethy1-3 -imino-
27-
(methoxymethy1)-
NH
16,16-dimethyl-
HKjc". 15,25-dioxa-
26A 0 587.5
2,4,19-
HN 0
triazahexacyclo[19
.6.2.22,5.211,14.0
LJJcç 13,18.024,28]tritri
0
aconta-
9,11,13,21,23,28,3
0-heptaene-20,33-
dione
148
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
rac-
(1R,9E,18S,27R)-
5-ethy1-3-imino-
0 27-
(methoxymethy1)-
NH -=
15,25-dioxa-
26B 0 587.3
2,4,19-
HN a
triazahexacyclo[19
.6.2.22,5.211,14.0
13,18.024,28]tritri
0
aconta-
9,11,13,21,23,28,3
0-heptaene-20,33-
dione
(1R,5R,8E,15S)-5-
ethy1-3-imino-15-
NH
HN N`s= methy1-
22-oxa-
2,4,16-
""0
triazapentacyclo[l
28 473.2
HN 0
6.6.2.22,5.110,14.
/
021,25]nonacosa-
8,10,12,14(27),18,
20,25-heptaene-
17,29-dione
(1R,5R,16S)-5-
ethy1-3 -imino-16-
NH methy1-
23-oxa-
HN N= 2,4,17-
triazahexacyc1o[17
29 HN 0 487.2
.6.2.22,5.111,15.0
*
8,10.022,26]triaco
1110 nta-
11,13,15(28),19,2
1,26-hexaene-
18,30-di one
149
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,5R,15R,16R)-
5-ethyl-IS-
H hydroxy-3-imino-
HNANN%µ= 2,4,17-
/1I
" 0 triazahexacyclo[17
30 0 NH 487.2
.5.2.22,5.210,13.0
12,16.022,25]triac
,,,OH
onta-
10,12,19,21,25,27-
hexaene-18,30-
dione
(1R,15R,16R)-15-
hydroxy-3-imino-
NH 5-methy1-23-oxa-
H N*A 2,4,17-
0
triazahexacyclo[17
31 489.2
.6.2.22,5.210,13.0
HN 0
OH
12,16.022,26]hentr
.õ
iaconta-
10,12,19,21,26,28-
hexaene-18,31-
dione
(1R,5R,8Z,14S,16
S)-5-ethy1-3-
NH 0 imino-14-methyl-
HN 23-oxa-
2,4,17-
/"- 0 triazahexacyclo[17
32 HN 499.2
.6.2.22,5.210,13.0
0
12,16.022,26]hentr
8,10,12,19,21,26,2
8-heptaene-18,31-
dione
150
CA 03240145 2024- 6-5
WO 2023/107356
PCT/U52022/051770
(1R,5R,14S,16S)-
5-ethy1-3-imino-
NH 14-
methy1-23-oxa-
HNAN' 2,4,17-
triazahexacyclo[17
33 HN 501.2
.6.2.22,5.210,13.0
0
12,16.022,26]hentr
10,12,19,21,26,28-
hexaene-18,31-
dione
(1R,5R,14R,16S)-
5-ethy1-3-imino-
NH 14-
methy1-23-oxa-
2,4,17-
""
triazahexacyclo[17
34 HN 501.3
.6.2.22,5.210,13.0
0
12,16.022,26]hentr
iaconta-
10,12,19,21,26,28-
hexaene-18,31-
dione
(1R,5R,15R,16R)-
5-ethyl-I5-
NH hydroxy-
3-imino-
HNANN'' 2,4,17-
0
triazahexacyclo[17
HN
35 501.2 .6.2.22,5.210,13.0
0
12,16.022,26]hentr
.,,OH
iaconta-
10,12,19,21,26,28-
hexaene-18,31-
dione
151
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,5R,8Z,17S)-5-
ethy1-3-imino-
NH 14,24-
dioxa-
HN1N
2,4,11,18-
/11
tetrazahexacyclo[l
'.
HN 0
36 502.3
8.6.2.22,5.210,13.
012,17.023,27]dot
riaconta-
0"
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
(1R,5R,15R,16R)-
5-ethyl-IS-
NH hydroxy-
3-imino-
HNAN 23-oxa-
2,4,17-
triazahexacyclo[17
37 503.2 .6.2.22,5.210,13.0
HN 0
12,16.022,26]hentr
iaconta-
10,12,19,21,26,28-
hexaene-18,31-
dione
(1R,5R,8Z,14R,16
S)-5,14-diethy1-3-
NH imino-23-
oxa-
HNWs. 2,4,17-
/1¶. a
triazahexacyclo[17
38 513.2 .6.2.22,5.210,13.0
HN 0
12,16.022,26]hentr
iaconta-
8,10,12,19,21,26,2
8-heptaene-18,31-
dione
152
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,5R,8Z,14S,16
S)-5,14-diethy1-3 -
NH 0 imino-23-
oxa-
HNANS. 2,4,17-
a TLJ
triazahexacyclo[17
39 HN 513.3
.6.2.22,5.210,13.0
0
12,16.022,26]hentr
iaconta-
':
8,10,12,19,21,26,2
8-heptaene-18,31-
dione
(1R,5R,8E,14S,16
S)-5,14-diethy1-3 -
NH imino-23-
oxa-
HNANN'' 2,4,17-
a
triazahexacyclo[17
40 HN 513.2
.6.2.22,5.210,13.0
0
12,16.022,26]hentr
iaconta-
":
8,10,12,19,21,26,2
8-heptaene-18,31-
dione
(1R,5R,8E,14R,16
S)-5,14-diethy1-3 -
NH imino-23-
oxa-
HN 2,4,17-
/11-a
triazahexacyclo[17
41 HN 513.2
.6.2.22,5.210,13.0
0
12,16.022,26]hentr
iaconta-
':
8,10,12,19,21,26,2
8-heptaene-18,31-
dione
153
CA 03240145 2024- 6-5
WO 2023/107356 PCT/US2022/051770
(1R,5R,8Z,15R,17
S)-5-ethy1-3-
NH
imino-15-methyl-
HNAN
14,24-dioxa-
2,4,18-
/1" 0 triazahexacyclo[18
42 515.3
HN 0
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
0
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
(1R,5R,8Z,16S,17
S)-5-ethyl-3-
NH
imino-16-methyl-
HN N`' 0
14,24-dioxa-
A.
2,4,18-
triazahexacyclo[18
43 515.2
HN 0
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
0
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
(1R,5R,8Z,15R,16
R)-5-ethyl -15-
hydroxy-3 -imino-
NH 0
15-methy1-23-oxa-
2,4,17-
triazahexacyclo[17
44 515.2
HN 0
.6.2.22,5.210,13.0
.,,OH
12,16.022,26]hentr
iaconta-
8,10,12,19,21,26,2
8-heptaen e-18,31-
di on e
154
CA 03240145 2024- 6-5
WO 2023/107356
PCT/U52022/051770
(1R,5R,7E,16S)-5-
ethy1-3-imino-
NH
14,14-dimethyl-
0
13,23-dioxa-
HN N".
2,4,17-
/I'= = 0
triazahexacyclo[17
45 515.2
HN 0
.6.2.22,5.29,12.01
1,16.022,26]hentri
aconta-
0
7,9,11,19,21,26,28
-heptaene-18,31-
dione
(1R,5R,8E,15R,17
S)-5-ethyl-3-
NH
imino-15-methyl-
HN N
14,24-dioxa-
2,4,18-
triazahexacyclo[18
46 515.3
HN 0
.6.2.22,5.210,13.0
12,17.023,27]dotri
S
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
(1R,5R,16R,17R)-
5-ethyl-I 6-
NH hydroxy-
3-imino-
HN 24-oxa-
2,4,18-
/ 0
triazaheptacyclo[l
47 515.2
8.6.2.22,5.211,14.
* * HN 0
08,10.013,17.023,
.,,OH
27]dotriaconta-
11,13,20,22,27,29-
hexaene-19,32-
di on e
155
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
1R,5R,9E,18S)-5-
ethy1-3-imino-
NH 15,25-
dioxa-
HNAN". 2,4,19-
/"triazahexacyclo[19
..
48 515.2
.6.2.22,5.211,14.0
HN 0
13,18.024,28]tritri
aconta-
9,11,13,21,23,28,3
0-heptaene-20,33-
dione
(1R,5R,8E,15R,16
R)-5-ethy1-15-
hydroxy-3-imino-
NH 0
HNAN`'. 15-
methy1-23-oxa-
2,4,17-
/II'. 0
triazahexacyclo[17
49 515.2
HN 0
.6.2.22,5.210,13.0
.õOH
12,16.022,26]hentr
iaconta-
8,10,12,19,21,26,2
8-heptaene-18,31-
dione
(1R,5R,8E,16R,17
S)-5-ethy1-3-
NH
imino-16-methyl-
0
HNN`'= 14,24-
dioxa-
2,4,18-
/ 0
triazahexacyclo[18
50 515.2
HN 0
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
0
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
156
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,5R,8E,16S,17
S)-5-ethy1-3-
NH
imino-16-methyl-
HN N`' 0
14,24-dioxa-
2,4,18-
"
triazahexacyclo[18
51 515.2
HN 0
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
0
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
(1R,5R,8E,16R)-
5-ethy1-3-imino-
15,15-dimethyl-
NH
HNAN`'.
14,23-dioxa-
ftJ 2,4,17-
" " 0
triazahexacyclo[17
52 515.3
HN 0
.6.2.22,5.210,13.0
12,16.022,26]hentr
iaconta-
8,10,12,19,21,26,2
8-heptaene-18,31-
dione
(1R,5R,14R,16S)-
5,14-di ethy1-3-
NH imino-23-
oxa-
HNAN' 2,4,17-
/".. 0
triazahexacyclo[17
53 515.3 .6.2.22,5.210,13.0
HN 0
12,16.022,26]hentr
iaconta-
10,12,19,21,26,28-
hexaene-18,31-
di on e
157
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,SR,14S,16S)-
5,14-diethy1-3-
NH imino-23-
oxa-
HNANN'. 2,4,17-
triazahexacyclo[17
54 HN 515.3
.6.2.22,5.210,13.0
0
12,16.022,26]hentr
iaconta-
10,12,19,21,26,28-
hexaene-18,31-
dione
(1R,5R,15R,16R)-
5-ethyl-IS-
NH hydroxy-
3-imino-
HN 15-
methy1-2,4,17-
triazahexacyclo[17
0
55 /....
515.3 .6.2.22,5.210,13.0
HN 0
OH
12,16.022,26]hentr
iaconta-
10,12,19,21,26,28-
hexaene-18,31-
dione
(1R,5R,15R,17S)-
5-ethy1-3-imino-
NH 15-
methy1-14,24-
HNAN dioxa-
2,4,18-
/"" 0
triazahexacyclo[18
56 HN 517.3
.6.2.22,5.210,13.0
0
12,17.023,27]dotri
101 0 aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
158
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,5R,16S)-5-
ethy1-3-imino-
NH
14,14-dimethyl-
0
HNAN", 13,23-
dioxa-
2,4,17-
" "
triazahexacyclo[17
57 517.2
HN 0
.6.2.22,5.29,12.01
1,16.022,26]hentri
aconta-
0
9,11,19,21,26,28-
hexaene-18,31-
dione
(1R,5R,15S,17S)-
5-ethy1-3-imino-
NH I5-
methyl-14,24-
HNAN dioxa-
2,4,18-
0
triazahexacyclo[18
HN 0
58 517.3
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
1111 0
10,12,20,22,27,29-
hexaene-19,32-
dione
(1R,5R,15R,16R)-
5-ethyl-I5-
hydroxy-3-i mi no-
NH
HNAN'. 15-methy1-23-oxa-
µ
2,4,17-
" 0
triazahexacyclo[17
59 517.2
HN 0
.6.2.22,5.210,13.0
12,16.022,26]hentr
iaconta-
10,12,19,21,26,28-
hexaene-18,31-
di on e
159
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,5R)-5-ethyl-
15-hydroxy-3-
NH imino-14-
methyl-
HNANN' 23-oxa-
2,4,17-
triazahexacyclo[17
/"..
60 517.3
.6.2.22,5.210,13.0
JN 0
12,16.022,26]hentr
O
AP.H iaconta-
10,12,19,21,26,28-
hexaene-18,31-
dione
(1R,5R,15R,16R)-
15-hydroxy-3-
NH imino-5-
isopropyl-
HNANN' 23-oxa-
2,4,17-
)11"o
triazahexacyclo[17
61 HN 517.3
.6.2.22,5.210,13.0
0
OH
12,16.022,26]hentr
.õ
iaconta-
10,12,19,21,26,28-
hexaene-18,31-
dione
(1R,5R)-5-ethyl-
15-hydroxy-3-
NH 0 imino-14-
methyl-
HNA IV= 23-oxa-
2,4,17-
/"" 0
triazahexacyclo[17
62 N 517.2
.6.2.22,5.210,13.0
kI 0
OH
12,16.022,26]hentr
iaconta-
10,12,19,21,26,28-
hexaene-18,31-
dione
160
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,5R,16S)-5-
ethy1-3-imino-
15,15-dimethyl-
NH
HNAN 13,23-
dioxa-
2,4,17-
/""
triazahexacyclo[17
63 517.2
HN 0 .6.2.22,5.29,12.01
1,16.022,26]hentri
aconta-
0
9,11,19,21,26,28-
hexaene-18,31-
dione
(1R,5R,16R)-5-
ethy1-3-imino-
15,15-dimethyl-
NH 0
HN N`' 14,23-
dioxa-
A.
2,4,17-
triazahexacyclo[17
64 517.3
HN 0 .6.2.22,5.210,13.0
12,16.022,26]hentr
iaconta-
10,12,19,21,26,28-
hexaene-18,31-
dione
(1R,5R,16R,17S)-
5-ethy1-3-imino-
NH 0 16-
methy1-14,24-
HNANV dioxa-
2,4,18-
/"" 0 1LJ
triazahexacyclo[18
65 517.2
.6.2.22,5.210,13.0
HN 0
12,17.023,27]dotri
aconta-
0 10,12,20,22,27,29-
hexaene-19,32-
dione
161
CA 03240145 2024- 6-5
WO 2023/107356
PCT/U52022/051770
(1R,5R)-5¨ethyl-
15-hydroxy-3 ¨
NH 0 imino-14-
methyl-
H 23-oxa-
2,4,17-
a
triazahexacyclo[17
66 517.2 .6.2.22,5.210,13.0
JN 0
12,16.022,26]hentr
400 OH
iaconta-
10,12,19,21,26,28-
hexaene-18,31-
di one
(1R,5R,16S,17S)-
5-ethy1-3-imino-
NH 0 16-
methyl-I 4,24-
HN 1\l`s. dioxa-
2,4,18-
/1
a
triazahexacyclo[18
67 517.3
.6.2.22,5.210,13.0
HN 0
12,17. 023,27]dotri
Si
.ssµµ
aconta-
10,12,20,22,27,29-
hexaene-19,32-
di one
(1R,5R,18S)-5-
ethy1-3
NH 0 15,25-
dioxa-
HNAN`s. 2,4,19-
" " 0
triazahexacyclo[19
68 517.2 .6.2.22,5.211,14.0
HN 0
13,18. 024,28]tritri
aconta-
0
11,13,21,23,28,30-
hexaene-20,33-
di one
162
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,5R,15R,16R,2
3S)-5-ethy1-15-
0¨
hydroxy-3-imino-
NH 23-
methoxy-
HNN's= 2,4,17-
/".L..__0
triazahexacyclo[17
69 517.3
.5.2.22,5.210,13.0
HN 0
12,16.022,25]triac
onta-
10,12,19,21,25,27-
hexaene-18,30-
dione
(1S,8E,15S,23R)-
5-ethy1-3-imino-
23-
NH o
(methoxymethyl)-
HN N'' 15,23-
dimethyl-
irA
22-oxa-2,4,16-
70 0 517.2
0
triazapentacyclo[l
HN
6.5.2.22,5.110,14.
021,24]octacosa-
8,10,12,14(26),18,
20,24-heptaene-
17,28-dione
(1R,5R,14S,16S)-
5-ethy1-3-imino-
NH 0 14-
methoxy-23-
HN oxa-
2,4,17-
triazahexacyclo[17
71 517.2 .6.2.22,5.210,13.0
HN 0
12,16.022,26]hentr
iaconta-
10,12,19,21,26,28-
hexaene-18,31-
dione
163
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,5R,16S)-5-
ethy1-3-imino-
NH
14,14-dimethyl-
HN N
8,13,23-trioxa-
101
2,4,17-
/"0
triazahexacyclo[17
72 519.2
HN 0
.6.2.22,5.29,12.01
1,16.022,26]hentri
0 aconta-
9,11,19,21,26,28-
hexaene-18,31-
dione
(1R,5R,16S,17R)-
5-ethyl-I 6-
NH
hydroxy-3 -imino-
0
14,24-dioxa-
2,4,18-
" 0
triazahexacyclo[18
73 519.2
HN 0 .6.2.22,5.210,13.0
12,17.023,27]dotri
SO.
10,12,20,22,27,29-
hexaene-19,32-
dione
(1S,15S,23R)-5-
ethy1-3-imi no-23-
(methoxymethyl)-
NH 15,23-
dimethyl-
H N*A N`s. 22-oxa-
2,4,16-
74 0 519.2
triazapentacyclo[l
HN 0
6.5.2.22,5.110,14.
021, 24]octacosa-
10,12,14(26),18,2
0,24-hexaene-
17,28-di one
164
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,15R,16R)-5-
ethy1-23,23-
F difluoro-15-
F
NH hydroxy-
3-imino-
HN*ANs' 2,4,17-
triazahexacyclo[17
0
75A 523.2
0 .5.2.22,5.210,13.0
HN
12,16.022,25]triac
onta-
10,12,19(26),20,2
2(25),27-hexaene-
18,30-dione
(1R,15R,16R)-5-
ethy1-23,23-
difluoro-15-
F
ANH HN* hydroxy-3-
imino-
N's = 2,4,17-
triazahexacyclo[17
0
0 .5.2.22,5.210,13.0
75B
HN 523.2
12,16.022,25]triac
cPonta-
10,12,19(26),20,2
2(25),27-hexaene-
18,30-dione
(1R,5R,8Z)-5-
ethy1-15-hydroxy-
NH
3-imino-spiro[23-
--".0
HN N 7 oxa-
2,4,17-
/""
ftJ
triazahexacyclo[17
0
.6.2.22,5.210,13.0
76 527.2
14.1\1
12,16.022,26]hentr
Ala. OH iaconta-
WIF
8,10,12,19,21,26,2
8-heptaene-14,1'-
cyclopropane]-
18,31-di one
165
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,5R,8E)-5-
ethy1-15-hydroxy-
NH 3-imino-
spiro[23-
oxa-2,4,17-
/""
triazahexacyclo[17
0
.6.2.22,5.210,13.0
77 527.3
0
12,16.022,26]hentr
* OH iaconta-
8,10,12,19,21,26,2
8-heptaene-14,1'-
cyclopropane]-
18,31-dione
(1R,5R)-5-ethyl-
15-hydroxy-3-
NH
imino-spiro[23-
HN N
oxa-2,4,17-
/""
triazahexacyclo[17
0
.6.2.22,5.210,13.0
78 529.2
14.N 0
12,16.022,26]hentr
OH iaconta-
10,12,19,21,26,28-
hexaene-14,1'-
cyclopropane]-
18,31-dione
(1R,5R,8Z,15S,17
S)-5,15-di ethyl-3-
NH imino-
14,24-
HNN = dioxa-2,4,18-
/"" 0
triazahexacyclo[l 8
79 529.2
.6.2.22,5.210,13.0
HN 0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
di on e
166
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(7R,11R,21S,23R)
-7-ethy1-9-imino-
23-methy1-14,24-
NH
H N N
dioxa-8,10,20-
triazaheptacyclo[l
0
9.6.2.27,10.215,18
80 529.3
* * HN 0
.02,4.011,16.025,2
9]tritriaconta-
1(28),15(31),16,18
0
(30),25(29),26-
hexaene-19,32-
dione
(1R,5R,17S)-5-
ethy1-3-imi no-
spiro[14,24-dioxa-
NH 0 2,4,18-
triazahexacyclo[ 1 8
.6.2.22,5.210,13.0
81 529.3 12,17.023,27]dotri
HN 0
aconta-
10(30),11,13(29),2
0 0,22,27-
hexaene-
15,1'-
cyclopropane]-
19,32-dione
167
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(6R,10R,20S)-6-
ethy1-8-imino-
22,22-dimethyl-
NH 13,23-
dioxa-
HN N 7,9,19-
I'== *
triazaheptacyclo[l
82 529.2
8.6.2.26,9.214,17.
HN 0
02,4.010,15.024,2
8]dotriaconta-
0 1(27),14(30),15,17
(29),24(28),25-
hexaene-18,31-
dione
(1R,5R,8Z,17R)-
5-ethy1-3-imino-
14,14-dimethyl-
NH
HN Nv-
15,24-dioxa-
2,4,18-
0
triazahexacyclo[18
83 HN o 529.2
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10(30),11,13(29)
,20,22,27-
heptaene-19,32-
dione
168
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(7R,11R,21S,23S)
-7-ethy1-9-imino-
NH
23-methyl-14,24-
dioxa-8,10,20-
HNAN
triazaheptacyclo[l
9.6.2.27,10.215,18
84 529.3
* * HN 0 .02,4.011,16.025,2
9]tritriaconta-
1(28),15(31),16,18
(30),25(29),26-
hexaene-19,32-
dione
(1R,5R,8E,17R)-
5-ethy1-3-imino-
14,14-dimethyl-
NH
HNN`s 15,24-
dioxa-
=
2,4,18-
/I". 0
triazahexacyclo[18
85 HN 0 529.3
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10(30),11,13(29)
,20,22,27-
heptaene-19,32-
dione
(1R,5R,8E,15R,17
S)-5,15-diethy1-3-
NH imino-
14,24-
dioxa-2,4,18-
0
triazahexacyc1o[18
86 529.2 .6.2.22,5.210,13.0
HN 0
12,17.023,27]dotri
aconta-
0 8,10,12,20,22,27,2
9-heptaene-19,32-
dione
169
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(7R,11R,21S,22S)
-7-ethy1-9-imino-
NH
22-methyl-14,24-
0
dioxa-8,10,20-
HNAW.
triazaheptacyclo[l
o 9.6.2.27,10.215,18
87 529.2
* * HN 0 .02,4.011,16.025,2
9]tritriaconta-
1(28),15(31),16,18
0
(30),25(29),26-
hexaene-19,32-
dione
(1R,8E,17S)-5-
ethy1-3-imi no-
15,15-dimethyl-
NH
14,24-dioxa-
o
HN*AN
2,4,18-
triazahexacyclo[18
88 529.2
HN 0
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
0
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
(8R,12R,22S)-8-
ethy1-10-imino-
15,25-dioxa-
NH
HNAN`s.
9,11,21-
triazaheptacyclo[2
/1". 0
0.6.2.28,11.216,19
89 529.2
* HN 0 .02,4.012,17.026,3
O]tetratriaconta-
1(29),16(32),17,19
0
(31),26(30),27-
hexaene-20,33-
di on e
170
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,5R,8E,15S,17
S)-5,15-diethy1-3-
NH imino-
14,24-
HN dioxa-
2,4,18-
LL/"" 0 triazahexacyclo[18
90 529.2
.6.2.22,5.210,13.0
HN 0
12,17.023,27]dotri
aconta-
0 8,10,12,20,22,27,2
9-heptaene-19,32-
dione
(1R,5R,8Z)-5,14-
diethyl-15-
NH
HNAN".
hydroxy-3-imino-
23-oxa-2,4,17-
/"" 0 triazahexacyclo[17
91 WN 0 529.2
.6.2.22,5.210,13.0
ofp OH
12,16.022,26]hentr
iaconta-
8,10,12,19,21,26,2
8-heptaene-18,31-
dione
(1R,5R,8E)-5,14-
diethyl-15-
NH 0
HNN's= hydroxy-
3-imino-
23-oxa-2,4,17-
/"" 0 triazahexacyclo[17
92 I4N NO 529.2
.6.2.22,5.210,13.0
OH
12,16.022,26]hentr
iaconta-
8,10,12,19,21,26,2
8-heptaene-18,31-
dione
171
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
(1R,5R,8Z,17S)-5-
HNAN ethy1-3-
imino-
/"" 0 S 15,15-
dimethyl-
14,24-dioxa-
HN 0
2,4,18,29-
93 530.3
tetrazahexacyclo[l
8.6.2.22,5.210,13.
012,17.023,27]dot
riaconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
NH
(1R,5R,8E,17S)-5-
HNAN ethy1-3-
imino-
/".= 0 15,15-
dimethyl-
14,24-dioxa-
HN 0
2,4,18,29-
530.3 tetrazahexacyclo[l
94 -N 0
8.6.2.22,5.210,13.
012,17.023,27]dot
riaconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
172
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,8Z,15R,16R,2
NH ", 0 5R)-15-hydroxy-
HN*
,A.N" 3-imino-25-
(methoxymethyl)-
0
HN 0
5-methy1-23-oxa-
2,4,17-
.00H
95 531.3
triazahexacyclo[17
.6.2.22,5.210,13.0
12,16.022,26]hentr
iaconta-
8,10,12,19,21,26,2
8-heptaene-18,31-
dione
NH
(1R,8Z,165,17R)-
HNIõAN\s. 16-hydroxy-3-
0 imino-
5,15,15-
HN 0
trimethy1-14,24-
so0H dioxa-
2,4,18-
96 531.2
triazahexacyclo[18
0
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
173
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
(7R,11R,21R,22S)
HNAN". -7-ethyl-
22-
/ hydroxy-9-imino-
* HN 0
14,24-dioxa-
OH
8,10,20-
triazaheptacyclo[l
0
97 531.2
9.6.2.27,10.215,18
.02,4.011,16.025,2
9]tritriaconta-
1(28),15(31),16,18
(30),25(29),26-
hexaene-19,32-
dione
(13
(1S,8E,15R,16R,2
NH 7 4R)-5-
ethyl-15-
- 0
HN,(1-LN0' hydroxy-
3-imino-
24-
0
HN 0 24-
methy1-23-oxa-
(methoxymethyl)-
2,4,17-
.õ OH
98 531.2
triazahexacyclo[17
.5.2.210,13.12,5.0
12,16.022,25]nona
cosa-
8,10,12,19,21,25,2
7-heptaene-18,29-
dione
174
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH (1R,5R,8E,16S,17
H N N"= R)-5-
ethy1-16-
/".= hydroxy-
3-imino-
HN 0 16-methyl-14,24-
OH dioxa-
2,4,18-
99 1110 0 531.2
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
di one
NH 0
(1R,8E,16S,17R)-
HNõAN" 16-hydroxy-3 -
0 imino-5,15,15-
HN 0
trimethy1-14,24-
OH dioxa-2,4,18-
100 531.2
triazahexacyclo[18
0
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
di one
175
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,5R,7E,14R,15
NH ="- 0 R,24R)-5-ethyl-
HN 14-
hydroxy-3-
/I'= 0 imino-24-
HN 0
(methoxymethyl)-
22-oxa-2,4,16-
101 1410. ,,,OH
531.3 triazahexacyclo[16
.6.2.22,5.29,12.01
1,15 .021,25]triaco
nta-
7,9,11,18,20,25,27
-heptaene-17,30-
dione
NH 0
(1R,5R,8Z)-5-
HNANNs. ethy1-16-hydroxy-
/" 0 3-imino-
15-
methyl-14,24-
E4N 0
* OH dioxa-2,4,18-
102 0 531.2
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
176
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
(1R,5R,8E)-5-
HNAN". ethy1-16-
hydroxy-
/'" 0 3-imino-
15-
methyl-14,24-
E1N 0
OH
dioxa-2,4,18-
103 0 531.2
*
LJL
L.
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
di one
NH
(1S,10R,14R,17Z)
HNANNs'
¨14¨ethyl-30¨
/ 0 fluoro-
12-imino-
NH
0 24,24-
dimethyl-
23-oxa-2,11,13-
triazahexacyclo[17
104 531.2
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4,6,17,19(27),20,2
2(26),30-heptaene-
3,29-dione
NH (1R,5R)-
5,14-
HN A N'- diethyl-
15-
/ I" 0 hydroxy-
3-imino-
N 0
23-oxa-2,4,17-
I4.
triazahexacyclo[17
105 dok
531.2 .6.2.22,5.210,13.0
OH
12,16.022,26]hentr
iaconta-
10,12,19,21,26,28-
hexaene-18,31-
di on e
177
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH 0
(1R,5R,17R)-5-
HN)LN`s. ethy1-3-
imino-
"" 14,14-
dimethyl-
15,24-dioxa-
HN 0
2,4,18-
triazahexacyclo[18
106 531.3
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10(30),11,13(29),2
0,22,27-hexaene-
19,32-dione
NH
(1R,10R,14R,25S)
HN No = -14-
ethyl-25-
hydroxy-12-
0 NH
imino-24,24-
0H dimethy1-
23-oxa-
.0
2,11,13-
triazahexacyclo[17
107 531.2
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4(31),5,7(30),19(2
7),20,22(26)-
hexaene-3,29-
dione
178
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
(1R,5R,15S,17S)-
HN AN 7 5,15-
diethyl-3-
imino-14,24-
dioxa-2,4,18-
HN 0
triazahexacyclo[18
108 531.3
.6.2.22,5.210,13.0
0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
NH
(1R,5R,15R,17S)-
HNN 5,15-
diethy1-3-
/"" 0 imino-
14,24-
dioxa-2,4,18-
HN 0
triazahexacyclo[18
109 531.2
.6.2.22,5.210,13.0
0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
NH
(1R,5R,17S)-5-
HN)-(N"- ethy1-3-
imino-
16,16-dimethyl-
HN 0
14,24-dioxa-
2,4,18-
110 531.3
triazahexacyclo[18
0
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
di on e
179
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH 0 (1R,5R)-5,14-
HN kl. diethyl-
15-
o hydroxy-
3-imino-
N
23-oxa-2,4,17-
14.
triazahexacyclo[17
111 op
531.3 .6.2.22,5.210,13.0
OH
12,16.022,26]hentr
iaconta-
10,12,19,21,26,28-
hexaene-18,31-
dione
NH 0 (1R,5R,17S)-5-
"
H N N'''f--) ethy1-3-
imino-
"
15,15-dimethyl-
HNO 14,24-
dioxa-
2,4,18,28-
112 532.2
tetrazahexacyclo[l
0
8.6.2.22,5.210,13.
012,17.023,27]dot
riaconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
1IINH 0 (1R,5R,17S)-5-
HN'It'N` ethy1-3-
imino-
"
I N
15,15-dimethyl-
"
HN 14,24-
dioxa-
2,4,18,21-
113 532.3
tetrazahexacyclo[l
0
8.6.2.22,5.210,13.
012,17.023,27]dot
riaconta-
10,12,20,22,27,29-
hexaene-19,32-
di on e
180
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
(1R,5R,17S)-5-
HNAN ethy1-3-
imino-
/ 0 S 15,15-
dimethyl-
HN
14,24-dioxa-
0
2,4,18,29-
114 532.3
tetrazahexacyclo[l
8.6.2.22,5.210,13.
012,17.023,27]dot
riaconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
NH
(1R,5R,17S)-5-
HNAN ethy1-3-
imi no-
" 0 15,15-
dimethyl-
HN 0
14,24-dioxa-
2,4,18,30-
115 532.2
tetrazahexacyclo[l
N., I
0
8.6.2.22,5.210,13.
012,17.023,27]dot
riaconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
NH 0
(1R,5R,16S,17R)-
HNAN". 5-ethyl-
I 6-
/ I" 0 hydroxy-
3-imino-
HN 0
16-methyl-14,24-
dioxa-2,4,18-
116 533.2
triazahexacyc1o[18
OH
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
di on e
181
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH (1R,5R,17S)-5-
HN r ethy1-3-
imino-
15,15-dimethyl-
HN
8,14,24-trioxa-
0
0 2,4,18-
117
triazahexacyclo[18
0 533.2
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
NH (1R,5R,17S)-5-
H - ethy1-3-
imi no-
15,15-dimethyl-
9,14,24-trioxa-
HN 0
0 2,4,18-
triazahexacyclo[18
118 0 533.2
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
NH
(1R,16S,17R)-16-
., =
HN1*11 N" hydroxy-3-i mi no-
0 5,15,15-
trimethyl-
HN 0 14,24-
dioxa-
OH2,4,18-
119 533.3
triazahexacyc1o[18
0
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
di on e
182
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
(1R,5R,15S,16R)-
HN1N 7
0
(hydroxymethyl)-
3-imino-15-
HN 0
methyl-14,23-
dioxa-2,4,17-
120 533.2
triazahexacyclo[17
.6.2.22,5.210,13.0
12,16.022,26]hentr
iaconta-
10,12,19,21,26,28-
hexaene-18,31-
dione
NH (1R,5R)-
5-ethyl-
HNAN\'' 16-
hydroxy-3-
/"" 0 imino-15-
methyl-
14,24-dioxa-
FIN 0
* OH 2,4,18-
121A
triazahexacyclo[18
0 533.3
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
183
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH 0 (1R,5R)-
5-ethyl-
HN NV 16-
hydroxy-3 -
"" 0 imino-15-
methyl-
14,24-dioxa-
FiN 0
* OH
2,4,18-
121B
*
triazahexacyclo[18
0 533.3
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
NH (1R,5R)-
5-ethyl-
HNAN" 16-
hydroxy-3 -
"" 0 imino-15-
methyl-
14,24-dioxa-
FiN 0
* OH
2,4,18-
121C
triazahexacyclo[18
0 533.3
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
NH (1R,5R)-
5-ethyl-
HN N`'. 16-
hydroxy-3 -
imino-15-methyl-
14,24-dioxa-
FiN 0
* OH
2,4,18-
121D LjL0..J.533.3
triazahexacyc1o[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
di on e
184
CA 03240145 2024- 6-5
WO 2023/107356 PCT/US2022/051770
NH
(1R,5R,15S,16R)-
H N N"= 5-ethyl-
15-
/I" 0 hydroxy-3-imino-
HN 0
14-dimethyl-
OH
13,23-dioxa-
2,4,17-
0
122 533.2
triazahexacyclo[17
.6.2.22,5.29,12.01
1,16.022,26]hentri
aconta-
9,11,19,21,26,28-
hexaene-18,31-
dione
(1S,14R,15R,23R)
NH 7 - 5 -
ethyl -14-
- o
hydroxy-3-imino-
23-
0
HN 0 23-
methy1-22-oxa-
(methoxymethyl)-
2,4,16-
123 533.2
triazahexacyclo[16
.5.2.22,5.29,12.01
1,15.021,24]nonac
osa-
9,11,18,20,24,26-
hexaene-17,29-
dione
185
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
0
(1 S,15R,16R,24R)
NH 7
- 0 - 5 -
ethyl -15-
H N*A N hydroxy-3-imino-
0 24-
(methoxymethyl)-
HN 0
24-methy1-23-oxa-
, õOH
2,4,17-
124 533.2
triazahexacyclo[17
.5.2.210,13.12,5.0
12,16.022,25]nona
cosa-
10,12,19,21,25,27-
hexaene-18,29-
dione
CY' (1R,15R,16R,25R)
-15-hydroxy-3-
HAN%. = imino-25-
(methoxymethyl)-
HN 0
5-methy1-23-oxa-
OH
2,4,17-
,
125 õ 533.2
triazahexacyclo[17
.6.2.22,5.210,13.0
12,16.022,26]hentr
iaconta-
10,12,19,21,26,28-
hexaene-18,31-
dione
186
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
(1R,5R,17S)-5-
HN Nµs'CrLi. ethy1-3 -
imino-
1\1 N
/ o
N 15,15-
dimethyl-
14,24-dioxa-
2,4,18,21,28-
126
pentazahexacyclo[
0 533.3
18.6.2.22,5.210,13
.012,17.023,27]dot
riaconta-
10,12,20,22,27,29-
hexaene-19,32-
di one
(1S,10R,14R)-14-
ethy1-30-fluoro-
12-imino-24,24-
NH
dimethy1-23-oxa-
H N N". 2,11,13-
".. 0
triazahexacyclo[17
127 NH 533.2
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4,6,19(27),20,22(2
6),30-hexaene-
3,29-dione
(1S,10R,14R,17Z,
NH
HN.A.N". 24R)-14-
ethyl -8,8-
difluoro-12-imino-
/ 0 24-
methy1-23-oxa-
NH
2,11,13-
triazahexacyclo[17
128 535.2
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4,6,17,19(27),20,2
2(26),30-heptaene-
3,29-di one
187
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
(1R,5R,8Z,15R,16
H N N R)-5-
ethyl-23,23-
/"Lo difluoro-15-
..
hydroxy-3 -imino-
H N 0 2,4,17-
129 535.2
.õOH
triazahexacyclo[17
.6.2.22,5.210,13.0
12,16.022,26]hentr
iaconta-
8,10,12,19,21,26,2
8-heptaene-18,31-
dione
NH
(1R,9Z,16R,17R)-
HN*ANµµ= 5-ethyl-
24,24-
ftJ difluoro-
16-
0
hydroxy-3 -imino-
0 NH 2,4,18-
130
triazahexacyclo[18
535.3
.5.2.22,5.211,14.0
13,17.023,26]hentr
iaconta-
9,11,13,20,22,26,2
8-heptaene-19,31-
dione
(1R, 9E,16R,17R)-
NH
HN* N,
5-ethyl-24,24-
-
difluoro-16-
0
hydroxy-3 -imino-
0 NH 2,4,18-
131
triazahexacyclo[18
535.3
.5.2.22,5.211,14.0
13,17.023,26]hentr
iaconta-
9,11,13,20,22,26,2
8-heptaene-19,31-
di on e
188
CA 03240145 2024- 6-5
WO 2023/107356 PCT/US2022/051770
0
(1 S,14R,15R,23R)
NH 7
- 0 J-t -5-ethyl-
14-
,
hydroxy-3-imino-
0
NH 23-
(methoxymethyl)-
23-methyl-8,22-
, õOH
dioxa-2,4,16-
132 535.4
triazahexacyclo[16
.5.2.22,5.29,12.01
1,15 .021,24]nonac
osa-
9,11,18,20,24,26-
hexaene-17,29-
dione
(1 S,10R)-14-ethyl-
NH
8,8-difluoro-12-
H N". imino-23,23-
0 dimethy1-
22-oxa-
NH
2,11,13-
triazahexacyclo[16
133 537.4
.6.2.24,7.211,14.0
6,10.021,25]triaco
nta-
4,6,18(26),19,21(2
5),29-hexaene-
3,28-dione
189
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
( 1 S,10R,14R,24R)
NH
-14-ethy1-8,8-
H N N".
difluoro-12-imino-
24-methy1-23-oxa-
NH
2,11,13-
triazahexacyclo[17
134 537.2
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4,6,19(27),20,22(2
6),30-hexaene-
3,29-dione
(1S,10R,14R,24S)
NH
HNJ-L.N \s=
¨14¨ethyl-8,8¨
difluoro-12-imino-
".. 0 24-
methy1-23-oxa-
NH
2,11,13-
triazahexacyclo[17
135 "", 537.2
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4,6,19(27),20,22(2
6),30-hexaene-
3,29-dione
NH
(1R,16R,17R)-5-
ethyl-24,24-
HN* =
difluoro-16-
0
hydroxy-3 -imino-
0 NH 2,4,18-
õOH
triazahexacyclo[18
136 537.3
.5.2.22,5.211,14.0
13,17.023,26]hentr
iaconta-
11,13,20,22,26,28-
hexaene-19,31-
di on e
190
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
(1R,5R,15R,16R)-
HNAN''. 5-ethyl-
23,23-
/
difluoro-15-
...
0
hydroxy-3-imino-
HN 0 2,4,17-
137 537.2
.õOH triazahexacyclo[17
.6.2.22,5.210,13.0
12,16.022,26]hentr
iaconta-
10,12,19,21,26,28-
hexaene-18,31-
dione
NH F
(1R,5R)-5-ethyl-
HNAN0' "
14,14,23,23-
tetrafluoro-3-
" 0
imino-2,4,17-
0 NH triazahexacyclo[17
138 543.2
.5.2.22,5.210,13.0
12,16.022,25]triac
onta-
10,12,19,21,25,27-
hexaene-18,30-
dione
NH
(7R,11R,21S)-7-
HWIL'N ethy1-9-
imino-
t..
23,23-dimethyl-
14,24-dioxa-
* * HN 0
8,10,20-
triazaheptacyclo[l
0
139A 543.2
9.6.2.27,10.215,18
.02,4.011,16.025,2
9]tritriaconta-
1(28),15(31),16,18
(30),25(29),26-
hexaene-19,32-
di on e
191
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
(7R,11R,21S)-7-
HNAN ethy1-9-
imino-
/I". 23,23-
dimethyl-
14,24-dioxa-
* * HN 0
8,10,20-
triazaheptacyclo[l
0
139B 543.3
9.6.2.27,10.215,18
.02,4.011,16.025,2
9]tritriaconta-
1(28),15(31),16,18
(30),25(29),26-
hexaene-19,32-
dione
NH
(1R,5S,8Z,17S)-5-
J-L '
0
\ HN N 01/ ethy1-3-
imino-
HN
6,15,15-trimethyl-
14,24-dioxa-
0
2,4,18-
543.2 triazahexacyclo[18
140A 0
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
192
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH (1R,5S,8Z,17S)-5-
-
µ HNA N (1110 ethy1-3-
imino-
140B
1,.. 6,15,15-
trimethyl-
0
HN
14,24-dioxa-
0
2,4,18-
543.2 triazahexacyclo[18
0
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
NH
(1R,5S,8E,17S)-5-
HNAN ethy1-3-
imino-
\ it.. 0 6,15,15-
trimethyl-
HN
14,24-dioxa-
0
2,4,18-
141"tZJtIIIIJ 543.2
triazahexacyclo[18
0
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
NH
(1R,5R,8Z,15R,17
HN)I,N 7 S)-5-ethyl-3-
imino-15-
isopropyl-14,24-
HN 0
dioxa-2,4,18-
142
triazahexacyc1o[18
543.3
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
193
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,5R,8E,15R,17
S)-5-ethy1-3-
NH imino-15-
HN,11..N
isopropyl-14,24-
/"L _J.0 LtJ dioxa-
2,4,18-
143 HN 0 543.2
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
NH (7R,11R,21S)-7-
HN N ethy1-9-imino-
N
/".. 23,23-
dimethyl-
14,24-dioxa-
* * H N
8,10,17,20-
tetrazaheptacyclo[
0
144 544.2
19.6.2.27,10.215,1
8.02,4.011,16.025,
29]tritriaconta-
1(28),15(31),16,18
(30),25(29),26-
hexaene-19,32-
dione
194
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1S,8E,15R,16R,2
4R)-5-ethy1-15-
NH 0
hydroxy-3-imino-
HN*
24-
0
(methoxymethyl)-
0
HN 24-
methy1-23-oxa-
2,4,17-
145
545.2
triazahexacyclo[17
.5.2.22,5.210,13.0
12,16.022,25]triac
onta-
8,10,12,19,21,25,2
7-heptaene-18,30-
dione
NH
(1R,5R,8Z,16R,17
HNAN".
imino-16-
HN 0
(methoxymethyl)-
14,24-dioxa-
2,4,18-
0
146 545.2
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
195
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH 0
(1R,5R,8Z,16S,17
HNAN". S)-5-
ethyl-3-
/'oimino-16-
(methoxymethyl)-
HN 0
14,24-dioxa-
0
2,4,18-
147 545.2
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
NH 0
(1R,5R,17S)-5-
HNAN". ethy1-3-
imino-
/"" 0 15,15-
dimethyl-
14,24-dioxa-
HN 0
2,4,18-
triazahexacyclo[18
148 0 545.2
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10(30),11,13(29),2
0,22,27-hexaene-
9,19,32-trione
196
CA 03240145 2024- 6-5
WO 2023/107356 PCT/US2022/051770
NH 0
(7R,11R,21R,22S)
HN)L N`s. -7-ethyl-
22-
/II hydroxy-9-imino-
22-methy1-14
* * HN 0
dioxa-8,10,20-
triazaheptacyclo[l
0
149 545.3
9.6.2.27,10.215,18
.02,4.011,16.025,2
9]tritriaconta-
1(28),15(31),16,18
(30),25(29),26-
hexaene-19,32-
dione
(1R,16R,17R,26R)
NH 0 -16-
hydroxy-3-
--IL = imino-26-
H Nk
(methoxymethyl)-
* * HN
5-methy1-24-oxa-
0
2,4,18-
.õ
150 OH 545.3
triazaheptacyclo[l
8.6.2.22,5.211,14.
08,10.013,17.023,
27]dotriaconta-
11,13,20,22,27,29-
hexaene-19,32-
dione
197
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,5R,8Z,15R,16
R,25S)-5-ethy1-15-
) hydroxy-3 -imino-
25-
NH 0
(methoxymethyl)-
HN 23-oxa-
2,4,17-
151 /"" 0 545.4
triazahexacyclo[17
.6.2.22,5.210,13.0
HN 0
12,16.022,26]hentr
.,,OH
iaconta-
8,10,12,19,21,26,2
8-heptaene-18,31-
di one
Cr"
(1R,5R,8Z,15R,16
NH 0 R,25R)-5-
ethyl-
HN.A.N"= 15-
hydroxy-3-
0
imino-25-
HN 0
(methoxymethyl)-
23-oxa-2,4,17-
, õOH
152 545.3
triazahexacyclo[17
.6.2.22,5.210,13.0
12,16.022,26]hentr
iaconta-
8,10,12,19,21,26,2
8-heptaene-18,31-
di one
198
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
(1R,5R,8E,16S,17
HNAN". R)-5-
ethyl-16-
/'o hydroxy-
3-imino-
HN
15,15-dimethyl-
0
14,24-dioxa-
2,4,18-
153 545.2
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
NH
(1R,5R,8E,16S,17
HNAN`s. S)-5-
ethyl-3-
/ ".. 0 imino-16-
HN 0
(methoxymethyl)-
14,24-dioxa-
2,4,18-
LjL0J
154 545.2
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
199
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH 0
(1R,5R,8E,16R,17
HNAN". S)-5-ethy1-3-
/ = imino-16-
HN
(methoxymethyl)-
0
14,24-dioxa-
=s"0-
2,4,18-
0
155 545.2
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
di one
Cr" (1R,5R,8E,15R,16
NH '''= 0 R,25R)-5-ethyl-
HN)k.. N`s= 15-hydroxy-3-
imino-25-
/ 0
(methoxymethyl)-
HN 0
23-oxa-2,4,17-
õ,OH
156 545.2
triazahexacyclo[17
.6.2.22,5.210,13.0
12,16.022,26]hentr
iaconta-
8,10,12,19,21,26,2
8-heptaene-18,31-
di one
200
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
(7R,11R,20S)-7-
HN AN . ethyl-30-fluoro-9-
/ II0 imino-
22,22-
NH
dimethy1-23-oxa-
8,10,19-
triazaheptacyclo[l
157 545.2
8.6.2.27,10.214,17
.02,4.011,15.024,2
8]dotriaconta-
1(27),14,16,24(28)
,25,29-hexaene-
18,31-dione
C( (1S,9S,10S,14R)-
NH
ià 14-ethyl
-12-
HNN " imino-9-methoxy-
24,24-dimethyl-
23-oxa-2,11,13-
HN
triazahexacyclo[17
158 545.2
.6.2.24,7.211,14.0
0
6,10.022,26]hentri
aconta-
4(31),5,7(30),19(2
7),20,22(26)-
hexaene-3,29-
dione
201
CA 03240145 2024- 6-5
WO 2023/107356 PCT/US2022/051770
NH
(1R,5R,16S,17R)-
HNAN". 5-ethyl-
16-
/1 " 0 hydroxy-3-imino-
HN 0
15,15-dimethyl-
14-oxa-2,4,18-
OH
159
triazahexacyclo[18
0 545.3
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
NH "-""O
(1R,5R,17S,26S)-
HN N= 5-ethy1-
3-imino-
" "
15,15,26-
0
HN 0
trimethy1-14,24-
dioxa-2,4,18-
160 545.3
triazahexacyclo[18
0
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
NH --"0
(1R,5R,15R,17S)-
HN.JI,N 7 5-ethy1-
3-imino-
15-isopropyl-
HN 0
14,24-dioxa-
2,4,18-
161 545.3
triazahexacyc1o[18
0
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
di on e
202
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
(1R,5S,17S)-5-
HN N
\ 1... ethy1-3-
imino-
162A
0 6,15,15-
trimethyl-
HN
14,24-dioxa-
0
2,4,18-
545.2 triazahexacyclo[18
0
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
NH
(1R,5S,17S)-5-
HNAN ethy1-3-
imi no-
\
HN
0 1101 6,15,15-
trimethyl-
14,24-dioxa-
0
2,4,18-
545.2 triazahexacyclo[18
162B 0
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
NH
(1R,5R,15S,17S)-
HN--11,N 5-ethy1-
3-imino-
15-isopropyl-
HN 0
14,24-dioxa-
2,4,18-
163 545.3
triazahexacyc1o[18
0
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
di on e
203
CA 03240145 2024- 6-5
WO 2023/107356 PCT/US2022/051770
C( (1S,9R,10S,14R)-
HN N"' imino-9-methoxy-
NH 14-ethyl-12-
/l'. 0 24,24-
dimethyl-
HN 0
23-oxa-2,11,13-
triazahexacyclo[17
164 545.3 .6.2.24,7.211,14.0
0
6,10.022,26]hentri
aconta-
4(31),5,7(30),19(2
7),20,22(26)-
hexaene-3,29-
dione
NH (5R,17S)-5-ethyl-
HNAN 3 -imino-15,15,26-
/
trimethy1-14,24-
". H y"
dioxa-2,4,18,28-
NO
tetrazahexacyclo[l
165 546.3
8.6.2.22,5.210,13.
0
012,17.023,27]dot
riaconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
204
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1 S,15R,16R,24R)
-5-ethyl-IS -
NH 0
A = hydroxy-3-imino-
HN* N"
24-
0
(methoxymethyl)-
0
HN 24-
methy1-23-oxa-
..,OH 2,4,17-
166 547.2
triazahexacyclo[17
.5.2.22,5.210,13.0
12,16.022,25]triac
onta-
10,12,19,21,25,27-
hexaene-18,30-
dione
NH
(1R,5R,16S,17R)-
HN A N"= 5-ethy1-16-
/...
hydroxy-3-imino-
HN
15,15-dimethyl-
0
14,24-dioxa-
2,4,18-
OH
0
167 547.2
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
205
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH 0
(1R,5R,16S,17S)-
HNAN"' 5-ethy1-
3-imino-
O 16-
(methoxymethyl)-
HN 0
14,24-dioxa-
Cr'
2,4,18-
168 547.3
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
NH 0
(1R,5R,16R,17S)-
HNANNs. 5-ethy1-
3-imino-
0
HN 16-
(methoxymethyl)-
0
14,24-dioxa-
2,4,18-
0
169 547.2
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
206
CA 03240145 2024- 6-5
WO 2023/107356 PCT/US2022/051770
NH 0
(1R,5R,17S)-5-
HN AN". ethy1-8-
hydroxy-
/ " 3-imino-
15,15-
dimethyl-14,24-
HO HN 0
dioxa-2,4,18-
triazahexacyclo[18
170 0 547.3
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10(30),11,13(29),2
0,22,27-hexaene-
19,32-dione
NH
(1R,5R,15S,16R)-
HN A N 5-ethy1-
3-imino-
o 15-
(methoxymethyl)-
HN 0
15-methy1-14,23-
dioxa-2,4,17-
171 547.2
triazahexacyclo[17
.6.2.22,5.210,13.0
12,16.022,26]hentr
iaconta-
10,12,19,21,26,28-
hexaene-18,31-
dione
207
CA 03240145 2024- 6-5
WO 2023/107356 PC
T/US2022/051770
O
(1R,5R,15R,16R,2
NH ''= 0 5R)-5-
ethy1-15-
NW-11'N". hydroxy-
3-imino-
/"== 0 25-
HN
(methoxymethyl)-
0
23-oxa-2,4,17-
OH
172 547.3
triazahexacyclo[17
.6.2.22,5.210,13.0
12,16.022,26]hentr
iaconta-
10,12,19,21,26,28-
hexaene-18,31-
dione
(1R,5R,15R,16R,2
5S)-5-ethyl-15-
NH 0
HN N"µ
hydroxy-3-imino-
25-
(methoxymethyl)-
HN 0 23-oxa-
2,4,17-
173 ,,,OH 547.3
triazahexacyclo[17
.6.2.22,5.210,13.0
12,16.022,26]hentr
iaconta-
10,12,19,21,26,28-
hexaene-18,31-
dione
208
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH 0
(1R,5R,18S)-5-
HNAN`s. ethy1-3-
imino-
/"== o
16,16-dimethyl-
HN 0
9,15,25-trioxa-
2,4,19-
174 547.2
triazahexacyclo[19
0
.6.2.22,5.211,14.0
13,18.024,28]tritri
aconta-
11,13,21,23,28,30-
hexaene-20,33-
dione
NH 0
(1R,5R,17S)-5-
HN.11,,N.
ethy1-9-hydroxy-
3-imino-15,15-
HN 0
dimethy1-14,24-
*
dioxa-2,4,18-
H 175 547.2
triazahexacyclo[18
0
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10(30),11,13(29),2
0,22,27-hexaene-
19,32-dione
(1R,18S)-5-ethy1-
3-imino-16,16-
NH 0 dimethy1-
8,15,25-
HAN`s. trioxa-
2,4,19-
V
triazahexacyclo[19
HN 0
0
176 547.2 .6.2.22,5.211,14.0
13,18.024,28]tritri
aconta-
0
11,13,21,23,28,30-
hexaene-20,33-
dione
209
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH 0 (1R,5R)-
5-ethyl-
HNAN"' 16-
hydroxy-3-
/"" imino-
15,16-
dimethyl-14,24-
1\1 0
* OH dioxa-
2,4,18-
177 547.2
*
triazahexacyclo[18
0
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
(1S,10R,14R)-14-
NH
HNAN"= ethy1-
8,8-di fl uoro-
12-imino-
/"'. 0 spiro[23-
oxa-
NH
2,11,13-
triazahexacyclo[17
.6.2.24,7.211,14.0
178 549.2
6,10.022,26]hentri
aconta-
4,6,19(27),20,22(2
6),30-hexaene-
24,1'-
cyclopropane]-
3,29-dione
210
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
F F
(1S,10R,14R,17Z)
HN
-14-ethy1-8,8-
HWILW
0
0 24,24-
dimethyl-
HN 23-oxa-
2,11,13-
triazahexacyclo[17
179 === 549.4
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4,6,17,19(27),20,2
2(26),30-heptaene-
3,29-dione
(1S,10R,14R,17E)
NH
-14-ethy1-8,8-
HNAN".
0 24,24-
dimethyl-
NH
23-oxa-2,11,13-
180 549.3
triazahexacyclo[17
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4,6,17,19(27),20,2
2(26),30-heptaene-
3,29-dione
211
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
(1R,5R)-5-ethyl-
HNAN"= 23,23-
difluoro-15-
hydroxy-3-hnino-
/"'. spiro[2,4,17-
0
OH
.5.2.22,5.210,13.0 triazahexacyclo[17
181 549.2
12,16.022,25]triac
onta-
10,12,19(26),20,2
2(25),27-hexaene-
14,1'-
cyclopropane]-
18,30-dione
(1S,15R,16R,24R)
NH
-5-ethyl-IS-
0
hydroxy-3-imino-
HN*1 24-
0 (methoxymethyl)-
0
HN 24-
methyl-9,23-
2
dioxa-2,4,17-
182 549.
0
triazahexacyclo[17
.5.2.22,5.210,13.0
12,16.022,25]triac
onta-
10,12,19,21,25,27-
hexaene-18,30-
dione
212
CA 03240145 2024- 6-5
WO 2023/107356 PCT/US2022/051770
(1 S,15R,16R,24R)
NH
-5-ethyl-IS -
HN'"NV
hydroxy-3-imino-
2
24-
O
(methoxymethyl)-
HN 0 24-
methyl-8,23-
dioxa-2,4,17-
183 549.3
triazahexacyclo[17
.5.2.22,5.210,13.0
12,16.022,25]triac
onta-
10,12,19,21,25,27-
hexaene-18,30-
dione
NH
(1S,14R,17Z)-14-
ethy1-8,8-difluoro-
HNAN 12-imino-
24,24-
/1" 0 dimethy1-23-oxa-
0
HN
2,5,11,13-
tetrazahexacyclo[l
184 550.2
7.6.2.24,7.211,14.
06,10.022,26]hentr
iaconta-
4,6,17,19(27),20,2
2(26),30-heptaene-
3,29-dione
213
CA 03240145 2024- 6-5
WO 2023/107356 PCT/US2022/051770
NH
(1S,14R,17E)-14-
ethy1-8,8-difluoro-
HNAN \ 12-imino-
24,24-
/1" 0 dimethy1-23-oxa-
0
HN
2,5,11,13-
tetrazahexacyclo[l
185 550.2
7.6.2.24,7.21 L14.
06,10.022,26]hentr
iaconta-
4,6,17,19(27),20,2
2(26),30-heptaene-
3,29-dione
NH
(1 S,14R)-14-ethyl-
8,8-difluoro-12-
HNAN imino-
24,24-
".. 0 dimethy1-
23-oxa-
0
HN
2,5,11,13-
tetrazahexacyclo[l
186 552.2
7.6.2.24,7.211,14.
06,10.022,26]hentr
iaconta-
4,6,19(27),20,22(2
6),30-hexaene-
3,29-dione
214
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,10R,14R,25S)
NH
-14-ethy1-8,8-
HNANV difluoro-25-
hydroxy-12-
0
HN imino-25-
methyl-
..10H 23-oxa-
2,11,13-
187 553.3
triazahexacyclo[17
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4,6,19(27),20,22(2
6),30-hexaene-
3,29-dione
(1R,10R,24S)-14-
NH
A = ethy1-8,8-difluoro-
HNõ N" 24-hydroxy-12-
0 imino-23,23-
NH
dimethy1-22-oxa-
.
2,11,13-
188 553.2
triazahexacyclo[16
.6.2.24,7.211,14.0
6,10.021,25]triaco
nta-
4,6,18(26),19,21(2
5),29-hexaene-
3,28-dione
215
CA 03240145 2024- 6-5
WO 2023/107356 PCT/US2022/051770
(1 S,14R)-14-ethyl-
NH
8,8,29-trifluoro-
H NAN 12-imino-
23,23-
dimethy1-22-oxa-
NH
2,11,13-
triazahexacyclo[16
189 555.2
.6.2.24,7.211,14.0
6,10.021,25]triaco
nta-
4,6,18(26),19,21(2
5),29-hexaene-
3,28-dione
NH
(1R,5R,14R,16S)-
HNAN 5-ethy1-
3-imino-
/1" 0 14-
H N
(trifluoromethyl)-
0
13,23-dioxa-
2,4,17-
. F
SI 0 'F
190557.3 triazahexacyclo[17
.6.2.22,5.29,12.01
1,16.022,26]hentri
aconta-
9,11,19,21,26,28-
hexaene-18,31-
dione
216
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
0--
(1S,9S,10R,14R,1
NH mik 7Z)-14-
ethyl-12-
HN
.11,N = iipP imino-9-
"
o
(methoxymethyl)-
HN 0
24,24-dimethyl-
23-oxa-2,11,13-
191
triazahexacyclo[17
557.3
0
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4(31),5,7(30),17,1
9(27),20,22(26)-
heptaene-3,29-
dione
0¨
(1S,9R,10R,14R,1
NH 7Z)-14-
ethyl-I2-
HNN"= imino-9-
(methoxymethyl)-
24,24-dimethyl-
HN 0
23-oxa-2,11,13-
192
triazahexacyclo[17
557.3
0
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4(31),5,7(30),17,1
9(27),20,22(26)-
heptaene-3,29-
dione
217
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH (1R,5R)-5-ethyl-
HN1Nµµ '
14,14,23,23-
LLJ
tetrafluoro-15-
/"" 0
hydroxy-3-imino-
0 õNH 2,4,17-
OH triazahexacyclo[17
193 559.2
.5.2.22,5.210,13.0
12,16.022,25]triac
onta-
10,12,19,21,25,27-
hexaene-18,30-
dione
(1S,9Z,16R,17R,2
NH 5R)-5-ethy1-16-
HN* Nµµ
hydroxy-3-imino-
'
25-
0
(methoxymethyl)-
0 NH 25-
methy1-24-oxa-
\ ,õOH
194 559.2
triazahexacyclo[18
2,4,18-
.5.2.22,5.211,14.0
13,17.023,26]hentr
iaconta-
9,11,13,20,22,26,2
8-heptaene-19,31-
dione
218
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
CY-
(1R,5R,7E,16S,25
NH 0 R)-5-
ethy1-3-
HNN`s= imino-25-
(methoxymethyl)-
HN 0
14,14-dimethyl-
13,23-dioxa-
195 559.3 2,4,17-
0
triazahexacyclo[17
.6.2.22,5.29,12.01
1,16.022,26]hentri
aconta-
7,9,11,19,21,26,28
-heptaene-18,31-
dione
0¨
(1R,8R,10R,14R,1
NH
HN)-N 7Z,25S)-
14-ethy1-
25-hydroxy-12-
/"" 0 imino-8-
methoxy-
HN 0 24,24-
dimethyl-
.00H 23-oxa-
2,11,13-
196
triazahexacyclo[17
0 559.4
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4(31),5,7(30),17,1
9(27),20,22(26)-
heptaene-3,29-
dione
219
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
0-
(1R,8R,10R,14R,1
NH _
HNAN 7E,25S)-
14-ethy1-
25-hydroxy-12-
/"" 0 imino-8-
methoxy-
HN 0 24,24-
dimethyl-
OH
23-oxa-2,11,13-
197
triazahexacyclo[17
0 559.4
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4(31),5,7(30),17,1
9(27),20,22(26)-
heptaene-3,29-
dione
0¨
(1S,9S,10R,14R)-
NH ma I4-ethyl-12-
HN
AN = 111, imino-9-
"
0
(methoxymethyl)-
HN 0
24,24-dimethyl-
23-oxa-2,11,13-
198
triazahexacyclo[17
559.3
0 .6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4(31),5,7(30),19(2
7),20,22(26)-
hexaene-3,29-
dione
220
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
0¨
/ (1S,9R,10R,14R)-
--
NH 14-ethyl-
12-
HNN" imino-9-
(methoxymethyl)-
HN 0
24,24-dimethyl-
23-oxa-2,11,13-
199
triazahexacyclo[17
559.3
0
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4(31),5,7(30),19(2
7),20,22(26)-
hexaene-3,29-
dione
NH
(1R,5R,16S,17R)-
HN N's= 16-hydroxy-3-
>1" imino-5-
i sopropyl-
HN 0
0
15,15-dimethyl-
14,24-dioxa-
2,4,18¨
0
200 561.3
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
221
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1S,5R,16R,17R,2
5R)-5-ethy1-16-
hydroxy-3-imino-
(b, 25-
NH 0
(methoxymethyl)-
HNANo= 25-
methy1-24-oxa-
2,4,18-
201 /,561.2
...
triazahexacyclo[18
0 NH
.5.2.22,5.211,14.0
13,17.023,26]hentr
iaconta-
11,13,20,22,26,28-
hexaene-19,31-
dione
(1S,9R,10S)-14-
ethy1-12-imino-9-
(methoxymethyl)-
(I) 9,23,23-
trimethyl-
NH = 8,22-
dioxa-
- 0
HN, 2,11,13-
202
0
triazahexacyclo[16
561.4
.6.2.24,7.211,14.0
HN 0
6,10.021,25]triaco
nta-
0
4(30),5,7(29),18(2
6),19,21(25)-
hexaene-3,28-
dione
222
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,8S,10R,14R,2
NH
HNN`s= 5S)-14-
ethyl-25-
hydroxy-12-
/"" 0 imino-8-
methoxy-
HN 0 24,24-
dimethyl-
23-oxa-2,11,13-
203 561
triazahexacyclo[17
0 .3
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4(31),5,7(30),19(2
7),20,22(26)-
hexaene-3,29-
di one
(1R,5R,16S,25R)-
NH
5-ethy1-3-imino-
HNAN`s.
25-
/".. 0
(methoxymethyl)-
HN 0 14,14-
dimethyl-
.00H 13,23-
dioxa-
204 0 561.3 2,4,17-
triazahexacyclo[17
.6.2.22,5.29,12.01
1,16.022,26]hentri
aconta-
9,11,19,21,26,28-
hexaene-18,31-
dione
223
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(7R,11R,20S)-7-
NH
ethyl-13,13 -
HN NIµs.
difluoro-9-imino-
" = 0 22,22-
dimethyl-
NH
23-oxa-8,10,19-
*
triazaheptacyclo[l
205 563.3
8.6.2.27,10.214,17
.02,4.011,15.024,2
8] dotri ac onta-
1(27),14,16,24(28)
,25,29-hexaene-
18,31-dione
NH
(1R,5R,8Z,17S)-5-
HNANN' ethyl-
24,24-
/'
difluoro-3-imino-
"
15,15-dimethyl-
HN 0 14-oxa-
2,4,18-
triazahexacyclo[18
206 563.3
.6.2.22,5.210,13.0
0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
224
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,10R,14R,17E,
NH
25 S)-14-ethy1-8,8-
HNAN`s. difluoro-
25-
0 hydroxy-12-
NH
0 spH imino-
24,24-
=
dimethy1-23-oxa-
2,11,13-
207 565.3
triazahexacyclo[17
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4,6,17,19(27),20,2
2(26),30-heptaene-
3,29-dione
NH
(1R,5R,17S)-5-
HNAN".
ethyl-24,24-
/11LJ difluoro-3-imino-
-
15,15-dimethyl-
HN 0 14-oxa-2,4,18-
triazahexacyclo[18
208 565.3
.6.2.22,5.210,13.0
0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
225
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
(1R,5R,17S)-5-
HNAN`s. ethy1-
9,9-difluoro-
/"" 0 3-imino-
15,15-
HN 0
dimethy1-14,24-
dioxa-2,4,18-
F
209 0 567.2
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10(30),11,13(29),2
0,22,27-hexaene-
19,32-dione
(1R,10R,14R,25S)
NH
HNAN`s= -14-
ethyl-8,8-
difluoro-25-
/"'= hydroxy-
12-
0 imino-
24,24-
NH
dimethy1-23-oxa-
2,11,13-
210 567.3
triazahexacyclo[17
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4,6,19(27),20,22(2
6),30-hexaene-
3,29-dione
226
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
(1R,5R,17S)-5-
HNAN". ethy1-8,8-difluoro-
/"" 0 3-imino-
15,15-
211 567.3 dimethyl-
14,24-
F HN 0
dioxa-2,4,18-
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10(30),11,13(29),2
0,22,27-hexaene-
19,32-dione
NH (1R,5R,16S)-8-
HN A N (di fluorom ethyl)-
o 5-ethy1-
3-imino-
H N 0 14,14-
dimethyl-
13,23-dioxa-
2,4,17-
0
212 567.5
triazahexacyclo[17
.6.2.22,5.29,12.01
1,16.022,26]hentri
aconta-
9,11,19,21,26,28-
hexaene-18,31-
dione
227
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
(1R,5R,8Z,15S,17
HNAN S)-5-
ethyl-3-
/ 0 imino-15-
HN 0
(trifluoromethyl)-
14,24-dioxa-
2,4,18-
F
0
213 569.3
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
NH
(1R,5R,8Z,15R,17
H N S)-5-
ethyl-3-
/1 ". 0 imino-15-
HN
(trifluoromethyl)-
0
14,24-dioxa-
F
2,4,18-
,
214 0 "irF 569.2
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
228
CA 03240145 2024- 6-5
WO 2023/107356 PCT/US2022/051770
NHFF
(1 S,14R)-14-ethyl-
8,8,30-trifluoro-
HN N 12-imino-
24,24-
/"' 0 dimethy1-23-oxa-
NH
2,11,13-
triazahexacyclo[17
215 569.2
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4,6,19(27),20,22(2
6),30-hexaene-
3,29-dione
NH
(1R,5R,15R,17S)-
HN N 5-ethy1-
3-imino-
" 0 15-
HN 0
(trifluoromethyl)-
14,24-dioxa-
2,4,18-
. F
0 "ir F
216 571.2
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
229
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
(1R,5R,15S,17S)-
HN N (110 5-ethy1-
3-imino-
o 15-
HN
(trifluoromethyl)-
0
14,24-dioxa-
2,4,18-
0
217 571.2
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
(1R,11Z,21S)-5-
ethy1-3-imino-
19,19-dimethyl-
N H /-**-"0
HIA N 18,28-
dioxa-
t
2,4,22-
triazahexacyclo[22
218 571.3
HN 0
.6.2.22,5.113,17.0
16,21.027,31]pent
¨
atriaconta-
0
11,13(33),14,16,2
4,26,31-heptaene-
23,35-dione
230
CA 03240145 2024- 6-5
WO 2023/107356 PCT/US2022/051770
(7R,11R,12R,20S)
NH -7-ethy1-
9-imino-
HNAN
12-
/l. 0
(methoxymethyl)-
* HN
22,22-dimethyl-
*
23-oxa-8,10,19-
219A 571
triazaheptacyclo[l
.3
0 8.6.2.27,10.214,17
.02,4.011,15.024,2
8]dotriaconta-
1(27),14(30),15,17
(29),24(28),25-
hexaene-18,31-
di one
(7R,11R,12R,20S)
NH -7-ethy1-
9-imino-
H N N"= 12-
0
(methoxymethyl)-
22,22-dimethyl-
* * HN 0
23-oxa-8,10,19-
219B 571
triazaheptacyclo[l
.3
0 8.6.2.27,10.214,17
.02,4.011,15.024,2
8]dotriaconta-
1(27),14(30),15,17
(29),24(28),25-
hexaene-18,31-
dione
231
CA 03240145 2024- 6-5
WO 2023/107356 PCT/US2022/051770
NH
(1R,5R,15R,17S)-
H N N 5-ethy1-
3-imino-
/1" 0 y'
HN 15-
(tri fluorom ethyl)-
14,24-dioxa-
2,4,18,28-
, F
220 0 F 572.3
tetrazahexacyclo[l
8.6.2.22,5.210,13.
012,17.023,27]dot
riaconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
NH ,.."O (1R,21
S)-5-ethyl -
HN, N 3-imino-
19,19-
o dimethyl-18,28-
dioxa-2,4,22-
HN 0
triazahexacyclo[22
221A 573.3
.6.2.22,5.113,17.0
16,21.027,31]pent
atriaconta-
13(33),14,16,24,2
6,31-hexaene-
23,35-dione
NH (1R,21
S)-5-ethyl-
A 7
HN* N 3-i mino-
19,19-
o dimethyl-18,28-
dioxa-2,4,22-
HN 0
triazahexacyclo[22
221B 573.3
.6.2.22,5.113,17.0
16,21.027,31]pent
atriaconta-
13(33),14,16,24,2
6,31-hexaene-
23,35-di one
232
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(5R,8Z,17S)-5-
NH : 0 ethy1-3-
imino-26-
(methoxymethyl)-
HNAN \
y-
15,15-dimethyl-
14,24-dioxa-
2,4,18,28-
\
222 574.2
tetrazahexacyclo[l
0
8.6.2.22,5.210,13.
012,17.023,27]dot
riaconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
(1R,8Z,165,17R,2
NH 0 6R)-16-
hydroxy-
,K. = 3-imino-
26-
(methoxymethyl)-
0
HN 0
5,15,15-trimethyl-
0H
14,24-dioxa-
.0
223 575.4 2,4,18-
0
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
233
CA 03240145 2024- 6-5
WO 2023/107356 PCT/US2022/051770
(1R,8E,16S,17R,2
NH"= 0 6R)-16-hydroxy-
.11, = 3-imino-
26-
HN* N`s
(methoxymethyl)-
0
HN 0
5,15,15-trimethyl-
õOH
14,24-dioxa-
224 575.3 2,4,18-
0
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
di one
(1S,9R,10S,14R)-
N40,. 14-ethyl-12-
H N`s= imino-9-
/1... 0
HN
0
(methoxymethyl)-
9,24,24-trimethyl-
8,23 -dioxa-
2,11,13-
0
225 575.4
triazahexacyclo[17
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4(31),5,7(30),19(2
7),20,22(26)-
hexaene-3,29-
di one
234
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,18S,27R)-3-
imino-27-
(methoxymethyl)-
5,16,16-trimethyl-
NH
A = 15,25-dioxa-
HN* NV' 2,4,19-
226 0 575.3
triazahexacyclo[19
HN 0
.6.2.22,5.211,14.0
13,18.024,28]tritri
aconta-
0
11,13,21,23,28,30-
hexaene-20,33-
dione
O (1R,5R,17S,26R)-
NH 0 5-ethy1-
3-imino-
HNN`s= 26-
0
(methoxymethyl)-
15,15-dimethyl-
HN 0 14,24-
dioxa-
2,4,18-
227 575.4
0
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
235
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
O (5R,17S)-5-ethyl-
NH0
3-imino-26-
HW N (methoxymethyl)-
IL''Th-"-L.,
15,15-dimethyl-
..
0
HN-0 14,24-
dioxa-
/1.
2,4,18,28-
228 576.3
tetrazahexacyclo[l
0 8.6.2.22,5.210,13.
012,17.023,27]dot
riaconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
O (5R,16S)-5-ethyl-
NH 3-imino-
25-
(methoxymethyl)-
HN N
==-= 8,14,14-
trimethyl-
/"" 0
HN 13,23-dioxa-
2,4,17,27-
229 576.3
tetrazahexacyclo[l
o 7.6.2.22,5.29,12.0
11,16.022,26]hentr
iaconta-
9,11,19,21,26,28-
hexaene-18,31-
dione
236
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
(7R,11R,21S)-7-
HNAN".
ethyl-14,14-
/I difluoro-
9-imino-
". 0
23,23-dimethyl-
* * HN 0 24-oxa-
8,10,20-
triazaheptacyclo[l
230A 0 577.3
9.6.2.27,10.215,18
.02,4.011,16.025,2
9]tritriaconta-
1(28),15(31),16,18
(30),25(29),26-
hexaene-19,32-
dione
NH
(7R,11R,21S)-7-
HN N"= ethyl-
14,14-
difluoro-9-imino-
0
23,23-dimethyl-
* * HN 0 24-oxa-
8,10,20-
triazaheptacyclo[l
230B 0 577.3
9.6.2.27,10.215,18
.02,4.011,16.025,2
9]tritriaconta-
1(28),15(31),16,18
(30),25(29),26-
hexaene-19,32-
dione
237
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
(7R,11R,21S)-7-
HNANµs. ethyl-14,14-
difluoro-9-imino-
23,23-dimethyl-
HN 0 24-oxa-
8,10,20-
triazaheptacyclo[l
231 0 577.3
9.6.2.27,10.215,18
.02,4.011,16.025,2
9]tritriaconta-
1(28),15(31),16,18
(30),25(29),26-
hexaene-19,32-
dione
\O (1R,5R,16S,17R,2
NH 6R)-5-
ethy1-16-
HN N"= hydroxy-3-imino-
/". 0
LJ 26-
'
HN
(methoxymethyl)-
0
16-methyl-14,24-
ss
dioxa-2,4,18-
OH
232 577.3
0
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
238
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,16S,17R,26R)
NH "= 0 -16-hydroxy-3-
..K. = imino-26-
fti
(methoxymethyl)-
0
HN 0
5,15,15-trimethyl-
OH
14,24-dioxa-
233 577.3
.õ
2,4,18-
0
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
NH
(1R,5R,8Z,16S,17
H N N"= R)-5-
ethy1-24,24-
difluoro-16-
hydroxy-3-imino-
HN OH 0 15,15-
dimethyl-
14-oxa-2,4,18-
234
0<1ZIIIi11IIIJccT579.2 triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
239
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,9R,10S,25S)-
14-ethy1-25-
NH 0
A = hydroxy-
12-
HN* N"
imino-9-
0 (methoxymethyl)-
0
HN9,25 -dimethyl-
8,18,23-trioxa-
0
0
2,11,13-
235 579.2
triazahexacyclo[17
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4(31),5,7(30),19(2
7),20,22(26)-
hexaene-3,29-
dione
F F
(1S,8R,10R,14R,1
NH
7Z)-14-ethyl-12-
mik
= MI
imino-24,24-
HN N"
dimethy1-8-
NH
(trifluoromethyl)-
4,
236 581.2
triazahexacyclo[17
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4,6,17,19(27),20,2
2(26),30-heptaene-
3,29-dione
240
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
(1R,5R,16S,17R)-
HNANµs.
5-ethyl-24,24-
difluoro-16-
/I" = 0
hydroxy-3-imino-
HN 0 15,15-
dimethyl-
14-oxa-2,4,18-
237 0 581.3
triazahexacyclo[18
OH
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
NH
(7R,11R,21S,23S)
HNAN -7-ethy1-
9-imino-
23-
*
(trifluoromethyl)-
* HN 0
14,24-dioxa-
8,10,20-
238
0
triazaheptacyclo[l
583.3
9.6.2.27,10.215,18
.02,4.011,16.025,2
9]tritriaconta-
1(28),15(31),16,18
(30),25(29),26-
hexaene-19,32-
dione
241
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
F F
(1S,8S,10R,14R)-
NH
\L-F
14-ethyl-12-
HNAN imino-
24,24-
dimethy1-8-
/"'. 0
(trifluoromethyl)-
NH
23-oxa-2,11,13-
239 583.3
triazahexacyclo[17
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4,6,19(27),20,22(2
6),30-hexaene-
3,29-dione
F F
F
(1S,8R,10R,14R)-
14-ethyl-12-
NH
HN N"' imino-
24,24-
A 411111
dim ethyl-8-
(trifluoromethyl)-
NH
=
23-oxa-2,11,13-
240 583.2
triazahexacyclo[17
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4,6,19(27),20,22(2
6),30-hexaene-
3,29-dione
242
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
F F
F
(1S,9R,10R,14R)-
NH 14-ethyl-
12-
H NAN"' imino-
24,24-
/".. 0 dimethy1-
9-
NH
(trifluoromethyl)-
23-oxa-2,11,13-
241 583.2
triazahexacyclo[17
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4,6,19(27),20,22(2
6),30-hexaene-
3,29-dione
F F
F
(1S,9S,10R,14R)-
NH 14-ethyl-
12-
HN
AN" = 411. imino-
24,24-
dimethy1-9-
NH
(trifluoromethyl)-
=
23-oxa-2,11,13-
242 583.3
triazahexacyclo[17
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4,6,19(27),20,22(2
6),30-hexaene-
3,29-dione
243
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
(1R,5R,8Z)-5-
HNAN = ethy1-16-
hydroxy-
/"" 3-imino-
15-
(trifluoromethyl)-
14N 0
* OH 14,24-
dioxa-
2,4,18-
F
0
243 585.3
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
di one
NH 0
(5R,15R,17S)-5-
HNAN ethyl-3-
imino-26-
I 0HN 1\1 methyl-
15-
y'
(trifluoromethyl)-
O
14,24-dioxa-
2,4,18,28-
. F
244 0 586.3
tetrazahexacyclo[l
8.6.2.22,5.210,13.
012,17.023,27]dot
riaconta-
10,12,20,22,27,29-
hexaene-19,32-
di one
244
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH (1R,5R)-
5-ethyl-
HNAN 16-
hydroxy-3 -
"" imino-15-
(tri fluorom ethyl)-
EIN 0
* OH 14,24-
dioxa-
2,4,18-
F
0
245 587.2
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
di one
(1R,9E,185,27R)-
NH '=a 5-ethy1-
3-imino-
)1.N`s = 27-
(methoxymethyl)-
16,16-dimethyl-
HN 0 15,25-
dioxa-
2,4,19-
246 587.3
0
triazahexacyclo[19
.6.2.22,5.211,14.0
13,18. 024,28]tritri
aconta-
9,11,13,21,23,28,3
0-heptaene-20,33 -
di one
245
CA 03240145 2024- 6-5
WO 2023/107356 PCT/US2022/051770
O
(1R,5R,8Z,16S,17
NH 0 R,26S)-5-
ethy1-16-
H N". hydroxy-3-imino-
0 26-
(methoxymethyl)-
HN 0
.s.PH 15,15-
dimethyl-
14,24-dioxa-
247 0 589.6 2,4,18¨
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
NO
(7R,11R,12R,21R,
NH 0 22 S)-7-
ethy1-22-
HNN's= hydroxy-
9-imino-
/" LJ 12-
'
(methoxymethy1)-
HN 0
22-methyl-14 24-
OH
dioxa-8,10,20-
248 0 589.3
triazaheptacyclo[l
9.6.2.27,10.215,18
.02,4.011,16.025,2
9]tritriaconta-
1(28),15(31),16,18
(30),25(29),26-
hexaene-19,32-
dione
246
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
O
(1R,5R,8E,16S,17
NH 0 R,26R)-5-
ethyl-
HN.A..N`s= 16-
hydroxy-3-
imino-26-
"
HN
(methoxymethyl)-
0
15,15-dimethyl-
OH
14,24-dioxa-
249 0 589.3 2,4,18-
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
NH 0
[(1R,5R,16S,17R)-
5-ethy1-3-imino-
0 15,15-
dimethyl-
HN 0
19,32-dioxo-
0
o 's% 14,24-
dioxa-
2,4,18-
250 589.2
triazahexacyclo[ 1 8
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaen-16-yl]
acetate
247
CA 03240145 2024- 6-5
WO 2023/107356 PCT/US2022/051770
(1S,9R,10S,14R)-
14-ethyl-12-
imino-9-
0
(methoxymethyl)-
NH
---
9,25,25-trimethyl-
H N N '
:
8,24-dioxa-
r 2,11,13-
251 589.4
triazahexacyclo[18
0 NH
.6.2.24,7.211,14.0
6,10.023,27]clotria
conta-
0
4(32),5,7(31),20(2
8),21,23(27)-
hexaene-3,30-
dione
(1 S,14R,17Z,24R)
NH
HNAN
-14-ethyl-8,8-
difluoro-12-imino-
" " 0 24-
0
HN
(trifluoromethyl)-
\ 23-oxa-
2,5,11,13-
252 590.2
tetrazahexacyclo[l
7.6.2.24,7.211,14.
06,10.022,26]hentr
iaconta-
4,6,17,19(27),20,2
2(26),30-heptaene-
3,29-dione
248
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH (1
S,10R,14R,24R)
-14-ethyl-8,8-
HN
difluoro-12-imino-
/ = 0 24-
NH
0
(trifluoromethyl)-
F 23-oxa-
2,11,13-
253 591.3
triazahexacyclo[17
.6.2.24,7.211,14.0
6,10.022,26]hentri
aconta-
4,6,19(27),20,22(2
6),30-hexaene-
3,29-dione
CY" (1R,5R,16S,17R,2
NH = 0 6R)-5-
ethy1-16-
HN N"= hydroxy-
3 -imino-
26-
HN
/ 0
(methoxymethyl)-
0
15,15-dimethyl-
14,24-dioxa-
254 0 591.3 2,4,18-
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
249
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
O
(1R,5R,16S,17R,2
NH 0 6S)-5-
ethy1-16-
HNAN". 26-
HN
hydroxy-3-imino-
/".. 110/
(methoxymethyl)-
0
15,15-dimethyl-
14,24-dioxa-
255 0 591.3 2,4,18-
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
(1 S,14R,24R)-14-
NH
ethy1-8,8-difluoro-
HN N 12-imino-
24-
(trifluoromethyl)-
0
HN 23-oxa-
2,5,11,13-
tetrazahexacyclo[l
256 592.2
""
7.6.2.24,7.211,14.
06,10.022,26]hentr
iaconta-
4,6,19(27),20,22(2
6),30-hexaene-
3,29-dione
250
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
F F
(7R,11R,13R,20S)
-7-ethy1-9-imino-
NH
HNANµ'. 22,22-
dimethyl-
4111111
13-
/"..
(trifluoromethyl)-
* NH
=
23-oxa-8,10,19-
257 595.3
triazaheptacyclo[l
8.6.2.27,10.214,17
.02,4.011,15.024,2
8]dotriaconta-
1(27),14,16,24(28)
,25,29-hexaene-
18,31-dione
0.
(1S,15R,16R,24R)
NH 0 -15-
hydroxy-3-
.A. = imino-24-
HN* N"
(methoxymethyl)-
0
0 24-
methyl-5-
HN
.õOH pheny1-
23-oxa-
595.2
2,4,17-
258
triazahexacyclo[17
.5.2.22,5.210,13.0
12,16.022,25]triac
onta-
10,12,19,21,25,27-
hexaene-18,30-
dione
251
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
(1R,5R,17S)-8-
HNAN`s. (di 5-
ethyl-8-
HN 0 5-ethy1-
8-
hydroxy-3-imino-
HO
15,15-dimethyl-
14,24-dioxa-
0 2,4,18-
259A 597.3
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10(30),11,13(29),2
0,22,27-hexaene-
19,32-dione
NH
(1R,5R,17S)-8-
HN)L-N`s.
(difluoromethy1)-
0
/...
HN 0
5-ethyl-8-
hydroxy-3-imino-
HO
15,15-dimethyl-
14,24-dioxa-
0 2,4,18-
259B 597.3
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10(30),11,13(29),2
0,22,27-hexaene-
19,32-dione
252
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH (7R,11R)-
7-ethyl-
HIVAN 22-
hydroxy-9-
/' 0 = imino-23-
* *
(trifluoromethyl)-
0
* OH 14,24-
dioxa-
8,10,20-
260
0
triazaheptacyclo[l
599.3
9.6.2.27,10.215,18
.02,4.011,16.025,2
9]tritriaconta-
1(28),15(31),16,18
(30),25(29),26-
hexaene-19,32-
dione
F F
(1R,5R,17S,26S)-
NH 5-ethy1-
3-imino-
HN,Jt.N`s. 15,15-
dimethyl-
/I''. 26-
0
HN
(trifluoromethyl)-
0
14,24-dioxa-
261 599.3
0
triazahexacyclo[18
2,4,18¨
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
253
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
F F
(1R,5R,17S,26R)-
NH 0 5-ethy1-
3-imino-
HN)LN" 15,15-
dimethyl-
,.. = 26-
/1
(trifluoromethyl)-
HN 0 14,24-dioxa-
2,4,18-
262 599.3
0
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
NH
[(1R,5R,16S,17R)-
HN N 's= 5-ethy1-
3-imino-
/"" 15,15-
dimethyl-
H N
19,32-dioxo-
0
0 14,24-dioxa-
2,4,18-
0
263 603.3
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaen-16-yl]
propanoate
254
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
(1R,5R,8Z,15R,17
NH 0 S,26R)-5-
ethy1-3-
HN-jc`s. imino-26-
/1
(methoxymethyl)-
"
15-
HN 0
(trifluoromethyl)-
14,24-dioxa-
F
264 "ir F 613.2 2,4,18-
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
O
(5R,8Z,15R,17S)-
NH 5-ethy1-
3-imino-
26-
H N \
(methoxymethyl)-
/
HN 15-
(trifluoromethyl)-
\
14,24-dioxa-
, F
265 0 F 614.2
2,4,18,28-
tetrazahexacyclo[1
8.6.2.22,5.210,13.
012,17.023,27]dot
riaconta-
8,10,12,20,22,27,2
9-heptaene-19,32-
dione
255
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
NH
(1R,5R,17S)-5-
ethy1-9-hydroxy-
/"" 0 3-imino-
15,15-
F
HN dimethy1-
9-
*
(trifluoromethyl)-
F
14,24-dioxa-
0 2,4,18-
266 615.2
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10(30),11,13(29),2
0,22,27-hexaene-
19,32-dione
O
(1R,5R,15R,17S,2
NH "O 6R)-5-
ethy1-3-
HNAN`s. imino-26-
/
(methoxymethyl)-
15-
F
HN 0
(trifluoromethyl)-
14,24-dioxa-
267 0 F 615.2 2,4,18-
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
256
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
CY- (5R,15R,17S)-5-
NH 0 ethy1-3-
imino-26-
HNN (methoxymethyl)-
...,
`-r 15-
(trifluoromethyl)-
HN 0 14,24-
dioxa-
2,4,18,28-
268 616.2
0 rF
tetrazahexacyclo[l
8.6.2.22,5.210,13.
012,17.023,27]dot
riaconta-
10,12,20,22,27,29-
hexaene-19,32-
dione
NH 0
[(1R,5R,16S,17R)-
HN.1t.N`s. 5-ethy1-
3-imino-
0 15,15-dimethyl-
19,32-dioxo-
HN 0
14,24-dioxa-
2,4,18-
0
269 617.4
triazahexacyclo[18
.6.2.22,5.210,13.0
12,17.023,27]dotri
aconta-
10,12,20,22,27,29-
hexaen-16-yl] 2-
methylpropanoate
Assessing antiparasite potency in a parasite LDH growth assay (Parasite Assay)
The parasite stock was maintained at 4% haematocrit in RPMI-Hepes media 30
buffered with sodium bicarbonate and supplemented with 5% heat inactivated
human serum and
0.5% albumax.
Approximately 42 hours prior to the potency assay being set up, parasites were
synchronized
with 5% sorbitol to select for ring stage parasites. On the day of assay set
up, a blood smear of
257
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
the parasite culture was Giemsa stained and counted. The parasitemia was 35
adjusted to 0.7%
rings and the haematocrit was diluted to 2% in RPMI-Hepes media buffered with
sodium
bicarbonate and supplemented with 5% heat inactivated human serum and 0.5%
albumax. 30u1
of diluted parasites are then added into lOul of media + compound in pre-
prepared Greiner TC
assay plates. Parasite assay plates were placed in gassed humidified boxes in
single layer and
allowed to incubate at 37 C for 72 hours. After 72 hours growth, assay plates
are sealed with
parafilm and frozen flat, in single file at -80 C overnight. On the following
day, assay plates are
allowed to thaw at room temperature for 4 hours to which an LDH assay is
performed to measure
parasite growth.
Assay EC50 results are shown in Table 2.
Table 2
Example EC50 (nM)
1 2.7
2 11.0
3A 0.2
3B 269.8
4 2.0
5 0.2
5a 0.9
6 2.8
7 0.9
8 1.1
9 76.5
10 2.5
11 4.6
12 1.0
13 22.0
14 34.0
15A 9.2
15B 81.0
16A 61.5
16B 3.4
17 18.9
18 6.0
19 4.8
3.6
258
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
21 5.1
22 57.4
23 7.5
24A 5.4
24B 2.0
25A 23.0
25B 0.7
26A 189.7
26B 7.4
28 19.4
29 6.3
30 11.3
31 7.8
32 0.7
33 1.0
34 1.6
35 2.5
36 7.0
37 6.2
38 0.7
39 1.7
40 7.0
41 8.8
42 0.7
43 0.7
44 0.8
45 1.5
46 2.6
47 4.2
48 6.0
49 7.3
50 7.7
51 9.1
52 19.5
53 0.7
54 0.7
55 3.1
56 0.2
57 0.3
259
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
58 0.4
59 0.6
60 0.9
61 1.0
62 1.0
63 1.8
64 1.9
65 2.0
66 2.2
67 2.6
68 4.8
69 12.0
70 18.0
71 0.7
72 0.7
73 2.1
74 5.2
75A 0.8
75B 1.3
76 0.3
77 7.0
78 0.5
79 0.6
80 0.6
81 0.6
82 0.6
83 0.7
84 1.4
85 2.1
86 2.4
87 3.4
88 3.6
89 5.1
90 6.8
91 0.5
92 6.5
93 3.0
94 8.8
95 1.0
260
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
96 1.2
97 1.5
98 2.6
99 3.3
100 7.7
101 8.3
102 0.8
103 6.7
104 0.3
105 0.7
106 0.7
107 0.7
108 0.8
109 1.0
110 1.8
111 0.6
112 0.3
113 0.7
114 1.5
115 3.4
116 0.3
117 0.4
118 0.6
119 1.4
120 1.5
121A 1.6
121B 0.5
121C 0.6
121D 5.7
122 1.9
123 2.3
124 3.8
125 6.0
126 0.8
127 0.6
128 0.3
129 1.1
130 1.9
131 2.1
261
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
132 10.6
133 0.3
134 0.3
135 0.5
136 0.9
137 2.0
138 1.6
139A 0.3
139B 0.8
140A 1.0
140B 1.4
141 7.3
142 1.4
143 7.3
144 0.3
145 0.2
146 0.6
147 0.7
148 0.7
149 0.8
150 0.9
151 1.0
152 1.2
153 5.9
154 6.2
155 6.2
156 9.6
157 1.0
158 0.3
159 0.6
160 0.6
161 0.7
162A 1.4
162B 1.1
163 1.1
164 2.1
165 2.4
166 0.4
167 0.4
262
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
168 1.2
169 1.4
170 2.5
171 2.9
172 3.1
173 3.2
174 3.4
175 5.0
176 5.3
177 0.7
178 0.4
179 0.8
180 6.7
181 0.6
182 0.8
183 2.4
184 0.9
185 19.5
186 0.8
187 0.6
188 0.6
189 19.4
190 0.5
191 1.0
192 0.9
193 1.7
194 0.9
195 1.1
196 3.6
197 6.6
198 0.7
199 1.9
200 0.4
201 0.6
202 1.1
203 1.8
204 3.2
205 0.3
206 0.9
263
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
207 1.9
208 0.7
209 0.4
210 0.7
211 0.7
212 0.9
213 1.2
214 1.9
215 0.6
216 0.2
217 5.6
218 7.5
219A 2.5
219B 6.4
220 1.0
221A 5.4
221B 2.0
222 2.7
223 0.7
224 2.7
225 0.4
226 0.7
227 0.8
228 0.4
229 19.0
230A 0.9
230B 0.8
231 1.1
232 0.2
233 0.8
234 3.1
235 0.4
236 0.8
237 0.7
238 0.8
239 0.2
240 0.6
241 0.7
242 1.2
264
CA 03240145 2024- 6-5
WO 2023/107356
PCT/US2022/051770
243 0.4
244 2.4
245 1.4
246 7.4
247 0.3
248 0.7
249 1.2
250 1.3
251 1.0
252 2.1
253 1.0
254 0.3
255 1.2
256 0.7
257 0.6
258 2.4
259A 9.9
259B 2.3
260 0.8
261 2.3
262 6.7
263 0.9
264 0.8
265 1.0
266 16.0
267 1.0
268 1.9
269 1.1
10
265
CA 03240145 2024- 6-5