Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
i81~1
ALPHA~ALXYL POLYOLEFINIC CARBOXYLIC ACIDS AND
DERIVATIVES THEREOF USEFUL IN THE TREATMENT
OF PSORIASIS AND ALLERGIC RESPONSES
The present invention relates to novel alpha-alkyl
polyolefinic carboxylic acids derived from such polyolefinic
intermediates as retinal(3,7-dimethyl-9-(2,6,6-trimethyl-1-
cyclohexen-l-yl)-2,4,6,8-nonatetraenal; vitamin A aldehyde)
which possesses the structure
~ ~ ~ ~ ~CHO
~ J ~
A synthesis of retinal from beta-ionone and
propargyl halide is described in U.S. Patent No. 3,060,229.
A number of alpha-substituted polyolefinic
carboxylic aldehydes, acids and esters are described in the
scientific literature. Japanese Patent 10,124 (1964); C.A.,
62, 2798 g (1965) describes 2,7-dimethyl-9-(2,6,6-trimethyl-1-
- cyclohexen-l-yl)-2,4,6,8-nonatetraenonic acid and 2,7,11-tri-
methyl-13-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8,10,12-
tridecahexanenoic acid; Machleidt, et al., Justus Liebigs Ann.
Chem., 679, 20 (1964) describe a-fluoropolyolefinic acids and
esters; Chan, et al., J.A.C.S., 96, 3642 (1974) describe
polyolefinic carboxaldehydes; ~aeck, et al. Recuil, 85 (1966)
pp. 334-338 describe 5,9-dimethyl-11-(2,6,6-trimethyl-1-cyclo-
hexen-l-yl)-2,4,6,8,10-undecapentaenoic acid and corresponding
2,4,6,8,10,12-tridecahexanenoic aclds as well as the
corresponding~-cyano and ~-carboxy substituted compounds.
Buchta, et al., Naturw1ssenschaften, 46, 74 (1959) describe
~methyl-2-methyl-7-phenyl)-2,4,6,-heptatrienoate.
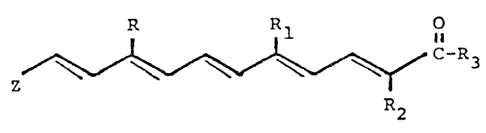
The present invention is directed to novel alpha-
alkyl, polyolefinic carboxylic acids and derivatives thereof
of the general formula
R
C-~3
I R2
in which R and Rl are each hydrogen or an alkyl group of from
1 to 5 carbon atoms; R2 is an alkyl group of from 1 to 5
carbon atoms; R3 is hydroxyl, alkoxy of from 1 to 5 carbon
atoms, NH2, NHR2 or NR2R2 and Z is a cycloalkyl, cycloalkenyl
or cycloalkdienyl group substituted with from 0 to 5 alkyl
groups, a keto group or a hydroxyl group, or a phenyl g~oup
substituted with from 0 to 4 hydroxy, alkoxy, alkyl or
trifluoromethyl groups or halogen atoms or combinations
thereof; and the pharmaceutically-acceptable salts thereof.
The invention includes compoun~s wherein the double bonds are
in the cis or trans configuration.
The foregoing compounds have been found to be useful
in therapeutic compositions for the treatment of psoriasis,
acne, and cellular and humoral immunodeficiency.
Therapeutic compositions containing compounds of the
foregoing invention have also been found active in regulating
the formation of lipoxygenase and as such possess therapeutic
value in the treatment of inflammatory conditions and allergic
responses.
3~
. ..~
21
--3--
A preferred group of compounds within the aforesaid
general formula are those in which Rl is methyl, R3 is
hydroxyl or alkoxy of from 1 to 5 carbon atoms and z is a
cycloalkenyl group substituted with from 0 to 3 alkyl groups,
or a phenyl group substituted with from 1 to 4 alkoxy or alkyl
groups containing up to 5 carbon atoms or combinations of the
foregoing, including those compounds in which one or more of
the double bonds are in the cis configuration. Within this
preferred group of compounds, still more preferred are
compounds in which Z is the group 2,6,6-trimethyl-1-
cyclohexen-l-yl.
The compounds of this invention can be prepared from
known polyolefinic materials, e.~., retinal, employing known
synthetic procedures of from analogous polyolefinic compounds
which can be prepared in accordance with methods known by
those skilled in the art.
For example, employing retinal as starting compound,
condensation through the aldehyde group with the active
methylene group of suitable acids or acid derivatives of the
formula:
2C 21l 3
will result in the corresponding undecapentaenoic acid
derivative. Activating substituents on the alpha carbon atom
of the said compounds, e.g., trialkylphosphono derivatives,
facilitate the condensation reaction.
The condensation reaction is usually carried out by
reacting the selected starting materials in a suitable solvent
preferably in the presence of a strong base such as sodium
hydride, sodamide, sodium ethoxide and similar alkali metal
compounds. The reaction is usually exothermic and is
.~,;.7
/~`,
~2681~
--4--
consequently cooled to control the rate of reaction. After
the initial reaction has subsided, the reaction mixture is
heated at reflux to assure completeness of reaction.
A variety of reaction solvents can be employed
including dioxane, tetrahydrofuran (THF), dimethylformamide,
dimethylacetamide and similar water-miscible organic solvents.
The solvents employed are preferably anhydrous, particularly
when the alkali metal bases are used, to avoid secondary
reactions.
The present new compounds can also be prepared from
corresponding compounds containing only alpha hydrogen by
alkylation using alkylating agents such as dialkyl sulfates,
e.g., dimethyl and diethyl sulfate and alkyl halides, e.g.,
propyl bromide and ethyl bromide, in the presence of alkali
metals cr alkali metal compounds which react with alpha
halogen, e.g., sodium hydride, lithium, potassiu~, sodamide
and alkali metal alkoxides such as sodium or potassium
ethoxide.
The compounds of this invention are also prepared by
partial reduction of corresponding compounds containing
acetylenic in lieu of ethylenic bonds. In addition, the
dehydrohalogenation of corresponding alpha-halo acid with no
ethylenic bond between alpha and beta carbon atoms also leads
to the present compounds.
A further preparative method involves condensation
of appropriate side chains with the appropriate side chains
with the appropriately substituted cyclohexanone with, for
example, an omegahaloundecapentaenoate, preferably in the form
of the corresponding Grignard reagent, followed by hydrolysis
of the product to form the alpha-substituted cyclohexanol and
then dehydration to the cyclohexenyl compound. The side
chain, i.e., the eleven carbon side chain can be formed
~1~
i812i
--5--
piecemeal by suitable condensation employing the half aldehyde
of a dicarboxylic acid of suitable carbon content to condense
with a side chain of suitable carbon content with groups
suitable to react with the aldehyde functional group.
A still further process can be used involving
oxidation of derivatives of the desired undecapentaenoic acid
with mild oxidants such as hypochlorite, e.g., sodium
hypochlorite. The oxidants selected should preferably avoid
secondary reactions with the remainder of the substrate
molecule, or the oxidation should be carried out under
controlled conditions to avoid appreciable secondary
reactions, as by conducting oxidation with hypochlorite
solution at or below about 10C and preferably between 0 and
5C. For example, a compound of the formula
[~ _ ~C-CH3
25 on oxidation with hypochlorite yields the corresponding acid
of formula I herein. These new compounds can also be prepared
by dehydration of corresponding ~ or ~ hydroxy acids or
esters to form an alpha-beta ethylenic bond. The beta hydroxy
acids or esters can be formed by condensation of an alpha-
3Q halo-carboxylic acid (or ester) with an aldehyde of two
carbons less than the desired side chain in the presence of
zinc (the Reformatsky Reaction).
The present compounds can also be prepared by
oxidation of the corresponding aldehyde and alcohol of the
same carbon content using oxidizing agents known for such
reaction, e.g., hypochlorite, as previously described.
1~:68~21
--6--
EXAMPLE 1
Ethyl 2,5,9-Trimethyl-11-(2,6,6-trimethyl-1-cyclohexen-1-yl)-
2,4,6,8,10-Undecapentaenoate
~ C02C2~5
Sodium hydride (4.03 g, 503 dispersion in mineral oil) was
washed with dry pentane three times and suspended in 50 ml of
anhydrous THF under nitrogen. The stirred mixture was cooled
in an ice-water bath and 20.6 g of triethyl 2-phosphono-
propionate was added dropwise. The resulting mixture was
stirred for additional two hours while allowing the reaction
mixture to warm up slowly to room temperature. The mixture
was then cooled in an ice-water bath and a solution of retinal
(16 g) in 50 ml of anhydrous THF was added dropwise. The
resulting dark red mixture was stirred for four hours at room
temperature; 700 ml of cold water was added and the mixture
was extracted with three 200 ml portions of ether. The
combined ethereal solution was washed with 100 ml of water and
dried over sodium sulfate. Removal of solvent gave the crude
ester (20 g, 97~) as a dark red oily substance. This material
was used for the preparation of the free acid of Example 2
! without further purification.
i~i8~
--7--
EXAMPLE 2
52,5,9-Trimethyl-11-(2,6,~-trimethyl-1-cyclohexen-1-yl)-
2,4,6,8,10-Undecapentaenoic Acid
~ COOH
lS The crude ethyl ester (20 g) from Example 1 was dissolved in
50 ml of ethanol and a solution of potassium hydroxide (5.12
g) in 45 ml of ethanol and 5 ml of water was added dropwise
with stirring under nitrogen. The resulting mixture was
stirred for 12 hours at room temperature. The reaction
mixture was partially concentrated under reduced pressure and
then mixed with 500 ml of water. The resulting mixture was
extracted with three 150 ml portions of ether. The ethereal
layer was discarded, the aqueous layer was acidified to p~ 3
with 10 N aqueous hydrochloric acid. The resulting product
was extracted into ether. The ethereal solution was washed
with water and dried over sodium sulfate. Concentration and
filtration of this solution afforded the desired product as
orange-red powders. Recrystallization in acetone/ethanol gave
9.3 g (50.6%) of pure product, m.p. 197-199C, UV spectrum
(methanol) max 380 nm.
1;~6~31Z~
In like manner to the procedure described in
Examples 1 and 2, the following compounds were prepared:
Ethyl 2-ethyl-5,9-dimethyl-11-(2,6,6-trimethyl-1-cyclohexen-1-
yl)-2,4,6,8,10-undecapentaenoate (an oil);
Ethyl 2-propyl-5,9 dimethyl-11-(2,6,6-trimethyl-1-cyclohexen-
l-yl)-2,4,6,8,10-undecapentaenoate (an oil);
2-Ethyl-5,9-dimethyl-11-(2,6,6-trimethyl-1-cyclohexen-1-yl)-
2,4,6,8,10-undecapentaenoic acid (m.p. 162-165C);
2-Propyl-5,9-dimethyl-11-(2,6,6-trimethyl-1-cyclohexen-1-yl)-
2,4,6,8,10-undecapentaenoic acid (m.p. 172-175C).
EXAMPLE 3A
Triethyl 2-Phosphonobutyrate
((Eto)3p + Br-CH-CO2Et >(Eto)~p - CHCO2Et)
Et Et
A mixture of ethyl 2-bromobutyrate (100 g, 0.5 mole)
and triethyl phosphite (85.2 g, 0.5 mole) was heated in an oil
bath at 145C for 2 hrs. After cooling to room temperature,
the reaction mixture was distilled at atmospheric pressure to
remove the bulk of the ethyl bromide. The desired product was
then distilled at 80-95C (0.15 mm of Hg). Obtained: 60 g of
triethyl 2-phosphonobutyrate as colorless clear liquid.
~2~
_9_
EXAMPLE 3B
5,9-Dimethyl-2-Ethyl-11-(2,6,6-trimethyl-1-cyclohexen-1-yl)-
2,4,6,8,10-Undecapentaenoic Acid
i~--~ + (E'o) 2P CIHCo2Et NaH
retinal
[~ C 2 E t ~ ~ CO 2 E~
In analogy to the procedure given in Example 1:
Triethyl 2-phosphonobutyrate was reacted with retinal to give
ethyl 5,9-dimethyl-2-ethyl-11-(2,6,6-trimethyl-1-cyclohexen-1-
yl)-2,4,6,8,10-undecapentaenoate, which was converted by the
procedure of Example 2 to 5,9-dimethyl-2-ethyl-11-(2,6,6-
trimethyl-l-cyclohexen-l-yl~-2,4,6,8,10-undecapentaenoic acid,
m.p. = 164-165C.
--10--
EXAMPLE 4A
Triethyl 2-Phosphonovalerate
(Eto)3p + Br-CH-CO2Et > (Eto)2p-CHCO2Et
C3H7 C3H7
In analogy to the procedure given in Example 3:
Ethyl 2-bromovalerate was treated with triethylphosphite to
give triethyl 2-phosphonovalerate as a colorless clear liquid,
b.p. = 95-110C (0.175 mm of Hg).
lZ~.
EXAMPLE 4B
5,9-Dimethyl~ (2,6,6-cyclohexen-1-yl)-2-PrOpyl-2,4,6,8,10-
Undecapentaenoic Acid
co2H
In analogy to the procedure described in Example 1:
Triethyl 2-phosphonovalerate was reacted with retinal to give
ethyl 5,9-dimethyl-11-(2,6,8-cyclohexen-1-yl)-2-propyl-
2,4,6,8,10-undecapentaenoate, which was converted by the
procedure of Example 2 to 5,9-dimethyl-11-(2,6,6-cyclohexen-
l-yl)-2-propyl-2,4,6,8,10-undecapentaenoic acid, m.p. =
172-175C.
The compounds of this invention are active against
various skin disorders, such as acne and psoriasis, when
tested according to models considered to be predictive of the
clinical condition in humans. The models used were the rhino
mouse procedure (Rligman, et al., J Investigative
Dermatology, 73, 354 [1979]), the rabbit comedolytic procedure
(Mills OH, Kligman AM: Assay of Comedolytic Agents in the
Rabbit Ear, Animal Models in Dermatology; Relevance to Human
Dermatopharmacology and Dermatoxicology, edited by H.I.
Maibach, New York Churchill-Livingston, 1975, pp. 176-183) and
the mouse epidermal cell culture procedure (Marcelo, et al.,
J Cell Biol., 79, 356 [1978]). Testing was done
-
comparatively against standard retinoids known to be effective
in these disorders and against a known ~-methyl retinoid
(2,7-dimethyl-9-2,6,6-trimethyl-1-cyclohexene-1-yl-2,4,6,8-
nonatetraenoic acid, referred to as DTCNA).
/~
12~i8~2~
- 12 -
Activity equal to or greater than the standards and
the known compound was shown by 2,5,9~trimethyl~ (2,6,6,-
trimethyl-l-cyclohexene-l-yl)-2,4,6,8,10-undecapentaenoic acid
(TTCUA). Thus, in the rabbit ear model at a concentration of
0.05%, it was equal to trans retinoic acid (TR~) in ability to
reduce comedone size. In the rhino mouse model at the same
concentration, it was equal to TRA in ability to reduce
significantly the size of utriculi (pseudocomedones) and the
amount of horny impaction in the utriculi. The skin of these
mice showed moderate epidermal hyperplasia and significantly
less wrinkling than the untreated control animals.
In the mouse epidermal cell culture at a
concentration of 12 ~g/ml, it reduced cell proliferation, as
measured by inhibition of the uptake of tritiated thymidine
into DNA. Table I shows percentage takeup relative to vehicle
control (100%).
TABLE I
20Day Culture
Exposure to DrugsTRACRA TTCUA DTCNA
3 77 47 31 53
53 75 15 61
36 64 21 60
Percentage uptake with TTCUA is seen to be up to five fold
less at all times points in comparison to both standards.
Known compound DTCNA in contrast is seen to give about the
same percentage uptake as the standard drugs at all three time
points. Likewise TTCVA showed high anti-differentiation
acitivity at 12~g/ml in the mouse epidermal cell culture, as
shown in Table II.
TABLE II
Day of Culture Vehicle
Exposure to Drug Control TRA CRA TTCUA DTCNA
53 3/6 3/5 2/7 2/6 3/6
5 3/5 3/5 2/70.5/8.5 2/6
10 7.5/2 2/6 2/6.51/7.5 2/5
The ratios in the table represent scoring of two measured
parameters, culture staining by the Kreyberg technique
Imaximum differentiation 10) and nuclei enumeration (maximum
differentiation 0). Thus, the highest possible anti-
differentiative activity would be given by the ratio 0/10.
TTCUA is seen to be more active in both parameters than the
two standards whereas the known compound is about the same as
the standards.
Compounds of the present invention were found to
have potent activity in regulating the formation of
lipoxygenase and as such possess therapeutic value in the
treatment of inflammatory conditions and allergic responses
such as anaphylaxis and asthma.
Lipoxygenases in mammals have been found in the
lung, platelets and white cells. They are enzymes capable of
oxidizing arachidonic acid into hydroperoxyeicosatetraenoic
acids (HPETEs) and their stable products hydroxyeico-
satetraenoic acids (HETEs). Lipoxygenases are classified
according to the position in the arachidonic acid which is
oxygenated. Platelets metablize arachidonic acid to 12-HETE,
while polymorphonuclear leukocytes contain 5 and 15
lipoxygenases. It is known that 12-HETE and 5,12-diHETE are
chemotactic for human neutrophils and eosinophils, and may
augment and inflammation process. 5-HPETE is known to be a
precursor of slow-reacting substance of anaphylaxis (SRS-A).
;81~
-14-
The SRS family of molecules, such leukotrienes B, C and D,
have been shown to be potent bronchoconstrictors (see, NATURE
288, 484-486 [1980]).
The following protocol describes an assay to detect
inhibitors of the lipoxygenase pathway. Such inhibitors are
believed to be capable of modulating the biosynthesis of the
leukotrienes, a property believed to be useful in treating
asthma and inflammatory disease states.
PROTOCOL
A homogenate of human neutrophils containing
lipoxygenase activity is incubated for 5 minutes at 37 with
4C-arachidonic acid (AA). Citric Acid (2M) is used to quench
the reaction. Following the addition of a trace amount of 3H-
lS AA together with an excess of unlabeled AA to each tube, the
mixture is extracted with chloroform/methanol. The organic
layer is washed with dilute acid, and an aliquot is
transferred to glass tubes and dried. The residue is
dissolved in a small volume of chloroform, and an aliquot is
spotted on silica gel TLC sheets. The sheets are developed
with an ethyl acetate/isooctane/water/acetic acid solvent
system. The AA-spots are identified with iodine vapors, cut
out and placed in scintillation vials for counting. After
adjusting for the extraction efficiency, the amount (pmole) of
14`C-AA in each of the tubes is quantitated. The pmoles of
oxidized AA are obtained by subtracting the pmoles of AA
remaining in the tubes containing active enzyme (control) from
the pmoles of AA in the tubes acidified prior to the addition
of enzyme (blank). The ability of the test compounds to
modulate the activity of this enzyme is determined by an
increase or decrease in the net amount of AA oxidized.
~ I2~L
A representative compound of the invention
~ ~ ~ ~ ~ ~ ~ ~C2H
1~ ~
~/
was found to require a concentration of 13 ~M (I50 = 13 ~M) to
inhibit 50% of the enzyme.
The therapeutic agents of this invention may be
administered alone or in combination with pharmaceutically
acceptable carriers, the proportion of which is determined by
the solubility and chemical nature of the compound, chosen
route of administration and standard pharmaceutical practice.
For example, they may be administered orally in the form cf
tablets or capsules containing such excipients as starch,
milk, sugar, certain types of clay and so forth. They may be
administered orally in the form of solutions which may contain
coloring and flavoring agents, or they may be injected
parenterally, that is, intramuscularly, intravenously or
subcutaneously. For parenteral administration, they may be
used in the form of a sterile solution containing other
solutes, for example, enough saline or glucose to make the
solution isotonic. ~hen applied topically for treating skin
disorders, the present new products can be provided in the
form of dusting powders, aerosol sprays, ointments, aqueous
compositions including solutions and suspensions, cream
lotions and the like. In this regard, any of the commonly
employed extending agents can be used depending on the nature
of the product as is well known in the art. Generally
speaking, the preferred dosage form is a soft gelatin capsule
~ . . ~
, ~
`"` 12~i~12~
- 16 -
for oral administration containing the active ingredient and a
surfactant, such as Polysorbate 80, encapsulated therein.
Capsules containing 1 mg, 10 mg or 40 mq of active ingredient/
capsule are most useful.
The physician will determine the dosage of the
present therapeutic agents which will be most suitable, and it
will vary with the form of administration and the particular
compound chosen, and furthermore, it will vary with the
particular patient under treatment. The dosage range will
usually be about 0.1-10 mg of active ingredient/kilogram of
body weight. He will generally wish to initiate treatment
with small dosages substantially less than the optimum dose of
the compound and increase the dosage by small increments until
the optimum effect under the circumstances is reached. It
will generally be found that when the composition is
administered orally, larger quantities of the active agent
will be required to produce the same effect as a smaller
quantity given parenterally.
A convenient form for administration of the present
new compounds are salts of those compounds in which R3 is OH,
particularly salts with alkali metals such as sodium and
potassium, the ammonium salt and salts with organic amines,
! particularly those commonly employed in pharmaceutical
formulations. The salts, of course, should be
pharmaceutically acceptable, that is, the salt formation does
not appreciably increase the toxicity of the therapeutic agent
nor cause a toxic reaction in the host.