Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~3~
A method of controlling alkaline pulping by means
of a rapid analyzer measuring organic and inorganic
cooking liyuor components
The invention relates to a method of controlling
alkaline pulping processes, especially sulphate pulping,
in which method the components dissolved from wood and
the recidual inorganic cooking chemical are measured
from the process liquors by means of a rapid analyzer
apparatus.
The object of the invention was to provide a
method of obtaining measuring data on the concentrations
of the cooking chemical and the reaction product of
alkaline pulping processes at short intervals for
controlling the cooking on the basis of the factual
proceeding thereof.
: The object of the invention is achieved by
feeding a small sample taken from the process into a
separate analyzer liquid flow having accurately
uniform conditions, in which flow the sample, after
having been diluted therein under the influence
of diffusion, flows as a peak-shaped zone having a
nearly normally distributed concentration through
different kind of measuring dP~ices operated on the
flow-through principle. Lignin dissolved from wood
is measured on the basis of the absorption of the
ultraviolet radiation at the wave length of an
extreme point (e.g. 205 or 280 nm) of the UV-spectrum
of lignin; the total amount of the dry matter
dissolved from wood is measured on the basis of the
difference in the re~raction index in relation to a
pure flowing medium by means of a differential
xefractometer; the residual active alkali being measured
by a conductivity measurement. In addition, the liquid
flow can be passed through any measurement carried out
.~ .
' ~ ~
'' ~ .
~.
~26~
on the flow-through principle in order to provide
further measurings. Obtaining data from the
above-mentioned measurements involves the principle
that the alkali concentration measured at the beginning
of the cooking and during -the proceeding thereof is
used as grounds for set-ting an objective for -the end
point of the cooking, the attainment of said objective
being then observed and, if required, corrected by means
of measurements of the reaction products. In other
words, the cooking is here controlled both by means
of a "feed forward" control directed forwards and
a "feed back" control connected backwards. Both ways
of controlling have been suggested to be effected by
means of different kinds of direct measurements carried
out from the process pipelines or by means of automatic
titrators. The novelty of the present invention lies
in that the arrangement according to the present
method combines all ways of control by providing the
necessary data by means of a single apparatus and from
one and the same sample. The present measuring tech-
nique is particularly advantageous in that the
measurements are independent of any changes of the
zero point, because there is always returned to the
zero between the measurements and there are measured
only the differences caused by the sample in the
quantity to be measured in relation to time. Further,
the measuring devices are not at all liable to
become contaminated, as the sample is diluted and a
pure measuring medium flows between the samples.
At present, controlling of alkaline pulping
processes, sulphate pulping in particular, is based on
the fact that it has been possible to develop a
ma-thematical model, a so called H-factor, on lignin
dissolution occuring during the cooking, said model
taking into consideration the cooking chemical charge,
., .
, : ,
,
- :. ,, : :;
,.
; ~,',
6~5
the digestlng temperature and the digesting time, as
appears from the publication Rekunen, S., Jutila, E.,
LXhteenmaki, E., Lonnberg, B., Virkola, N-E.,
Examination of reaetion kinetics in kraft cooking.
Paperi ja Puu 62 (1980):2, 80-90). Thls kind oE
model operates fairly satisfac-torily, provided that
the cooking conditions as well as the quali-ty and
humidity of the wood ehips are known. However, this
is not usually the ease at any single plant, but the
conditions vary at random, and, although important
for the process control, such quantities as humidity,
quality and particle si~e distribution characteri~ing
the raw material, i.e. chips, are left beyond the
possibilities of the present measuring techniques.
Attempts have been made to improve the situation
by carrying out different kind of measurements from the
digester liquors. The amount of the eooking ehemieal
or active alkali has been measured either by conduct-
ivity measurement directly from a process pipeline
system in a manner disclosed in the publication
Lundqvist, G., Alkali analysis by conductivity
measurements. Svensk Papperstid 76 (1972): 9, 524-527
or by an automatic microprocessor-controlled titration
from the cooking liquid sample, which, in turn, is
known from ~.S. Patent Specifications 4,104,028 and
4,012,197 as well as from the publication Wallin, G.,
Noreus, S., Computer control of bateh digesters.
Svensk Papperstid 76 (1973): 9, 329-334. A
disadvantage of a direet eonduetivity measurement,
however, is that the proeess pipelines are extremenly
liable to ge-t eontaminated, whieh results in slipping
of the zero point and diffieulties in the eompensation
thereof. Fairly reliable information is obtained on
the aetive alkali by means of titrators, whieh, however,
are slow and, besides, a single point per process in
~1~2t~ 05
the initial stayes thereof does not yet disclose
the profile of the alkali consumption nor the recidual
alkali providing perhaps the most important grounds for
:Eorward control in view of the determination of the
end point.
Attempts have also been made to measure lignin
dissolved from wood and to determine the end poin-t of
the cooking on said grounds. In particular, the
absoxption of the ultraviolet radiation has been used
in Kleinert, T.N., Joyce, C. S., Short wawelength
ultraviolet absorption of various lignins and related
substances. IV lignin determination in sulphate
pulping liquor. Pulp and pap.mag.of Can. 58 (1957):
11, 147-152i Williams, D. J., The application of the
ultraviolet absorption characteristic of lignin to the
control of pulp uniformity. Appita 22 (1968):2,
45-52~ Capart, R., Obese -Dec-ty, K-, Le Cardinal, G.,
Gelus, M., Contribution to on-like kraft pulping control.
IFAC PRP 4 automation, Ghent, Belgium 1980, p. 121-128.
These methods are greatly disadvantageous in that they
are continuously operated, whereby they are difficult
to control with respect to contamination and, even the
more, with respect to the extensive continuous dilution.
On account of technical difficulties, lignin
measurements have not been put to constant use in
process controlO Besides, it can be estimated that
a mere lignin concentration without any information
on the alkali concentration and the properties of the
chip filling can not provide a reliable basis for the
determination of the end point.
No mention of a combined use of the above-
mentioned measurements nor of carrying out the same
by a single apparatus and from one and the same sample
has been found in literature.
The present invention combines the separate
;
:
: .:. .: . .; , .. .
.' ' '`' ' ' ' `, ' ' ' ' ' ",1 ' . '. ' ', , ,
,.-: : .
` '' .,:', . ~
.:.. . ::: .~.
. .: :
determinatiorls mentioned in literature above by means
of a novel technique and by elimination of certain
previously encountered factors which reduce the
reliability of the measuring results, and simultaneously
enables in principle an unlimited number of different
flow-through measurements to be carried out from one
and the same sample.
The following figures are illustrative of the
invention: _
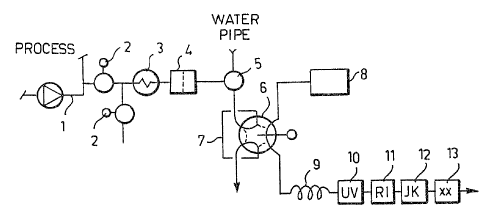
Figure 1 illustrates an analyzer apparatus or
observing and controlling alkaline cooks,
Figure 2 illustrates proceeding of a plant-
scale sulphate batch pulping as a function of the
digesting time, curve A representing the lignin content,
curve B the dry matter content and curve F the residual
alkali content
Figures 3A and 3B show lignin dissolution curves
calculated on the basis of the lignin measuring results
of the analyzer, said curves concerning a plant-scale
sulphate pulping process as a function of the pulping
time,
Figures ~A and 4B show a relative alkali
concentratlon measured by the analyzer from a
plant-scale sulphate batch pulping as a function of
an alkali titration carried out in a laboratory,
Figure 5 shows a relative dry matter content
measured by the analyzer from a plant-scale sulphate
batch pulping as a function of a dry matter content
determination carried out in a laboratory,
Figure 6 illustrates observation of a
continuously operated sulphate pulping process,
curve D representing the alkali concentration
equalizing cycle, curve E the resi~ual alkali
concentration in the outgoing black liquor, curve F -
the lignin concentration in the outgoing black liquor,
,,j~ ~ .
~ . .
.. .
,
,,, - ' ' "' '"'"'` -
~ ~ .
:~ .
.. .
~2~ 5
curve G the lignin concentration in the terminating cycle,
curve H the lignin concentration in the washing cycle,
curve J the lignin concentration equalizing cycle and
curve k kappa number of pulp from digester.
According to Figure 1, a small sample is taken from
a process pipeline, e.g. from a delivery-side 1 of a pump
(a circulation pump of a batch digester or a circulating
pump o~ one cycle of a continuously operated digester) by
means of a valve 2, which sample is cooled in a condenser
3 and filtered in a sinter filter 4 and then passed
through a normal position of a wash valve 5 to a loop 7 of
a loop-injection valve 6. When a necessary amount of the
sample, from the point of view of representativeness (with
a tube size of 1/8" about 50 ml) 7 has passed through the
loop, the injection valve 6 turns over 6~ and a
separate liquid flow 8 of the analyzer (a pump or a
pressurized liquid container) is conducted through the
loop, thus taking therewith a uniform volume (the loop
being 25 microliters) of the sample. In the analyzer
liquid flow ~usually pure water but also a diluted alkali
or an alkalic buffer solution is possible), the sample is
diluted in a long capillary tube 9 (5-6 m 0 0.8 rnm teflon
capillary) under the influence of diffusion without any
artificial dilution steps and the diluted sample zone
flows through different kinds of measuring devices: an
ultraviolet or UV-detector 10 measuring the lignin
concentration of the sample on the basis ~f the absorption
of the ultraviolet radiation (205 or 280 nm); a refraction
index or RI-detector measuring the dry matter content of
the sample on the basis of a difference in the refraction
indices; and a conductivity detector measuring the
concentration of the residual active alkali on the basis of
the conductivity o~ hydroxyl ions. In addition, the flow
can be passed throuyh other measurements xx 13 operated
: , ,. ~ .
.. ' . . :~
; ' ~ ~ :" . .,
,
)5
on the flow through principle, such a e.g. ion
selective eLectrodes, pH-electrodes and an addition
oE a neutralizing chemica~, the active alkali and
sulphidity being thereby determined by means of the
reaction heat and changes in the conductivity.
Measuring signals of the different detectors
from the diluted sample zone are integrated as a
function of time, i.e. the areas of the concentration
peaks are calculated, thus directly obtaining the
relative concentrations of the sample, when -the
analyzer liquor flow is run at a standard rate. If
desired, the relative concentrations can be modified
into the actual ones, e.g. g/l, by running samples
analyzed in a laboratory in order to form a
calibration curve.
When the sample zone has advanced in the
analyzer into the dilution capillary, washing of the
sample line begins immediately. Said valve 5 passes
the water upstream through the filter 4 and the
condenser 3 and the washing water is discharged
-through a valve 14.
The analyzer arrangement according to the
invention has the following advantages:
- the sample amount required is small (50 ml),
which is of advantage as the dry matter content of
such a small sample is low and so the filter can
easily be kept clean by a simple counter-current wash.
In industrial test runs, a sinter filter having a
diameter of 30 mm has remained completely clean
through thousands of analyses.
- complicated dilution devices required by the
UV-devices mentioned in literature are not at all
necessary. The dilution is effected spontaneously in
a long capillary tube under the influence of diffusion.
- the measurements are completely independent
., , ' ' ., ,.:,.:: .: .
`' ` '~
:~` `''`'' ~: '
~: :'' :.
; ` :
,'' ~ : .
~,'2~60~i
of shiftings of the zero points of the measuring
devices, because only a difference caused by the
sample zone is measured and there is always
returned to the zero point between the measurements,
whatever it may be.
- the measuring devices remain almost clean,
because the sample is very diluted and a pure measuring
medium flows between the samples.
- all measuring conditions are made accurately
constant by maintaining the temperature of the measuring
devices and the measuring medium constant.
- by virtue of the integration principle, small
variations if the operation of the apparatus or in the
shape of the dilution zone do not cause any disturbances.
Example 1
Use of the analyzer apparatus for observing a
plant-scale sulphate batch pulping process in accordance
with the invention.
An industrial sulphate batch digester was
observed by means of the analyzer apparatus of
Figure 1 with sampling intervals of 10 minutes by taking
a sample from the delivery-side of a calorisator pump of
the digester. The volume of the digester was 180 cm3,
the wood species was pine, the alkali charge 21~
calculated on the amount of wood, the sulphidity
being 30~ and the digesting temperature 170C. In
the apparatus of Figure 1, the flowing liquid of
the analyzer was pure water; a hose pump was used as
a liquid flow source 8; the flow rate was ~ ml/min,
the volume of the sampling loop 7 was 25 /ul, the
length of the diffusion dilution capillary 9 was 5 m
and the diameter therof 0.8 mm. The UV-detector 10
was a Knauer*UV/VIS Photometer, the wave length being
280 nm; the refxaction index detector was a Knauer
Differential Refractometer; and the conductivity
*Trade Mark
, ,
.. :
.. '~. ... :' ~ ~ , '
. - .: ,
~ ' ~ ' '' : '
- . ` :
~2~6C)S
detector a Vydac*Conductivity Detector. The
electrical signals of the detectors were integrated
by means of an eight-bit SELMA*-microprocessor and
a self-made integrator program. With this way of
measuring, the analyzing time for one sample was
about four minutes.
Example 2
Use of the analyzer apparatus for observing
a plant-scale continuously operated suplhate pulping
in accordance with the invention.
The procedure was similar to Example 1 expect
that different cycles of a conti~uously operated
sulphate digester were observed, said cycles being
equalizing, outgoing black liquor, terminating and
washing. The analyzer took a sample about every
five minutes by moving from one cycle -to another,
whereby the analyzing interval of one cycle was
20 minutes. The results appear from Figure 6.
*Trade Mark
, ~
,.: ; . .: , : ~ `
".