Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
,
Descri~tion
Fines Recirculating Fluid Bed Co~bustor
Method and Apparatus
Background of the Invention
Fluid bed boilers burning high sulfur coal are
well known in the art. These boilers use classical
bubbling bed technology whereby the fluid bed operates
with superficial velocities in the range of 4 to 12
ft/sec and the bed is composed of particles with an
average diameter of approximately 1000 microns. Coal
is burned in the bubbling bed and limestone or dolomite
sorbent is added to suppress the sulfur oxide
emissions. The sorbent is added in particle sizes of
1000 to 3000 microns and the bed is composed largely of
coal ash, spent sorbent, partially spent sorbent and
partially burned fuel particles. The bubbling bed
contains tubes within it to transfer heat to the steam.
Tubes are also mounted above the bed in the freeboard
to transfer heat from the hot combustion gases, thus
cooling them. In operation, the bed elutriates fine
particulates comprised of char, ash and partially spent
sorbent. Many of these particles are captured by a
recycle cyclone located downstream of the convective
heat exchanger and these particles are returned to the
bed in order to burn the fuel particles and allow
unused sorbent to absorb more sulfur oxides. Very fine
particles escape the recycle cyclone and are trapped in
a filter system. The flow rate in the recycle loop is
approximately equal to the total solids flow rate of
the fuel and the sorbent fed into the combustor.
Conventional fluid bed boilers have several
disadvantages. One disadvantage is that the combustion
efficiency is low, approximately 97~, because small
particles of unburned fuel escape to the combustion
system. This problem would be vastly exacerbated if
j~_
~; , . ' .
~7~345
--2--
the boiler were to attempt to burn a low volatile
content fuel such as petroleum coke which has 90~ fixed
carbon compared to 42~ fixed carbon for coal. The
second disadvantage is low sorbent utilization. A
calcium to sulfur molar ratio of at least 3:1 must be
maintained to produce sulfur oxide suppression of 90%
to meet typical air pollution requirements. The reason
for this is that the relatively large particles of
sorbent only absorb sulfur oxides on their surface,
leaving their interior material largely unused. A
third disadvantage is that these boilers emit nitrogen
oxides as a pollutant; the nitrogen oxides are
generated from fuel-bound nitrogen. In many parts of
the country the nitrogen oxide emissions do not exceed
local limits but in some areas, such as Southern
California, they do.
To improve combustion efficiency of conventional
fluid bed boilers, Stewart et. al. in U~S. Patent
Number 4,177,741 teaches the agglomeration of the
recycled fines before reintroducing them into the
bubbling bed. The agglomerated fines are thus
prevented Erom being blo~n out of the bed and are thus
encouraged to burn in the bed. Jones, U.S. Patent
Number 4,259,911 teaches agglomeration of coal fines
plus recycled material before injection into the bed.
To improve the utilization of sorbent, Jones U.S.
Patent Number 4,329,234 teaches the removal of a
portion of the fluid bed and grinding the sorbent
particles to 50 microns in diameter to fracture them,
exposing new surface for additional sorption of sulfur
oxides. The fractured particles are reintroduced into
bed by being agglomerated with the coal ~fuel). All of
these approaches are simple modifications of the
classic bubbling bed boiler described earlier.
Reh et. al. in German Patent Number DE 3,023,480
describes a different approach to obtain good sorbent
utilization in suppressing sulfur oxides from
:
,
--3--
combustion gases. Reh et. al. passes combustion gas
through a fluidized bed of sorbent with particle size
of 30 to 200 microns and a superficial velocity of 3 to
30 t/second, producing an entrained bed with a
particle density 0.1 to 1Okg/cu m. The particulate
entrained by the high gas velocity is removed by a
recycle cyclone and returned to the bed, which is
between 1300F and 2000F in temperature. The hourly
recycle rate is approximately five times the bed
weight. This approach achieves good sulfur oxide
suppression by the use of fine particulate with large
surface area and vigorous mixing. Reh however, does
not teach combustion in the entrained bed of heat
recovery with tubes from the entrained bed.
Reh in U.S. Patent Number 4,111,158 describes a
fluid bed combustor based upon the principle of an
entrained fluid bed which offers improvements in
combustion efficiency, sulfur oxide suppression,
nitrogen oxide control and turn-down. Whereas bubbling
bed combustors operate with superficial velocities in
the range of 4-12 ft/second and have a clearly defined
upper surface, entrained bed combustors operate at
superficial velocities of 15 to 45 ft/second and have
no clearly defined upper surface but rather a gradation
of particulate density from the bottom to the top of
the combustor. The particulate is entrained with the
gas flow in the reactor and separated from it by a
recycle cyclone downstream of the reactor whereupon the
particulate is reintroduced into the base of the
reactor. Particle size ranges from 30 to 250 microns
and the particle density is 10 to 40 kg/cu m in the
upper portion of the reactor. Heat is not recovered
from the particulate or gases in the reactor or recycle
loop. Tubes in the reactor would be subject to high
erosion and would not be effective in transferring heat
because of the low particle density compared to that of
a bubbling bed (500 kg/cu m). Heat is recovered by
,~
. ,
~'~7~45
--4--
draining a portion of the bed from the base of the
reactor and cooling it in a separate fluid bed heat
exchanger optimized for that process. High combustion
efficiency is obtained by completely burning small
diameter fuel particles in the highly turbulent reactor
and the hot recycle loop. Good sorbent usage is also
obtained by using fine particulate and maintaining it
at an effective temperature throughout the reactor and
recycle loop. Limited nitrogen oxide control is
obtained by progressively introducing co~bustion air
along the length of the reactor. The disadvantage of
the system is the need for the separate fluidized bed
heat exchanger and large recycle cyclones.
Ammonia injection to suppress nitrogen oxides
without a catalyst is taught by Lyon in ~.S. Patent
~umber 3,900,554. Lyon describes the basic gas phase
reaction whereby ammonia selectively reduces nitrogen
oxide in the presence of oxygen at 1742~F to 1832F and
predicts a suppression of 20% at an ammonia/nitrogen
oxide molar ratio of 2, Lyon does not teach the
benefits of good mixing, as in the recycle cyclone,
which produced nitrogen oxide ions of 95% at the same
molar ratio of 2.
Disclosure of the Invention
. . ~_
The object of the present invention is to achieve
the benefits of high combustion efficiency and good
~ sorbent utilization without using a separate fluidi~ed
- bed heat exchanger with a large recycle cyclone.
The present invention utilizes a bubbling fluid
bed combustor with tubes in the bed for heat transfer
but with bed particles whose average diameter is in the
range of 100 to 800 microns wherein 20% to 40% of the
particles, respectively, are less than 200 microns in
diameter. The superficial velocity of the bed is 3 to
7 ft/second, well below the 15 to 45 ft/second of the
entrained bed. The result of the relatively low
~7~
--5--
superficial velocity combined with a bed of small
diameter particulate is to produce a bubbling bed but
with a high rate of elutriation of the fines component
of the bed. A particle density loading of 0.5 kg/cu m
5 is achieved at the top of the bed. This compares to ~
the 10-40 kg/cu meter typical of an entrained bed.
Hence, the present invention uses particulate sizes
typical of entrained beds but a much lower superficial
velocity and hence produces a bubbling bed with
substantially reduced transport of bed material.
Compared to a conventional bubbling bed combustor,
it uses a much smaller average particle size (500
microns versus 1000 microns) and has considerably
higher bed transport. The recycle rate of a
conventional bubbling fluid bed boiler is approximately
equal to the combined solids feed rates whereas the
recycle rate of the present invention is 20 times that
value, equivalent to changing the bed every ~0 minutes.
Unlike the bub~ling bed combustors but similar to the
entrained bed combustors, the present invention has no
heat transfer surfaces between the bed and the recycle
cyclone to cool the gas and particulate, hence contains
an isothermal recycle loop operating at the ideal
temperature for combustion or sulfur sorption. Unlike
either the bubbling bed combustor or the entrained
combustor the subject invention uses ammonia injection
at the inlet of the recycle cyclone for control of
nitrogen oxide emissions. Other benefits are a 100~ to
300% increase in heat transfer coefficient on the tubes
in the bed because of the small particle size in the
bed and a re~uction in tube erosion (compared to
conventional bubbling beds) because of the low
superficial velocities. (Tube erosion increases
exponentially with superficial velocity). Another
attractive feature is a 10:1 range of fluidization
velocities which allows for a full fluidized start up
at low system throughputs.
`''
~7~45
--6--
The subject invention has demonstrated an 86
reduction in nitrogen oxides twithout the use of
ammonia) by operating at a low combustion temperature
of 1450F which reduces the evolution of nitrogen oxides
from fuel bound nitrogen. In addition, it is also well
known that char at 1450F will reduce nitrogen oxides
in the presence of oxygen. At 1450F large quan,tities
of char are elutriated from the bed and circulate in
the recycle loop. A portion of the ion of the 86~
nitrogen oxides is contributed by its reaction with hot
char but the degree of contribution is unknown.
Thus, we have discovered a unique fluid bed
combustion method and apparatus producing superior
performance and efficiency.
Brief Description of Drawings
. .
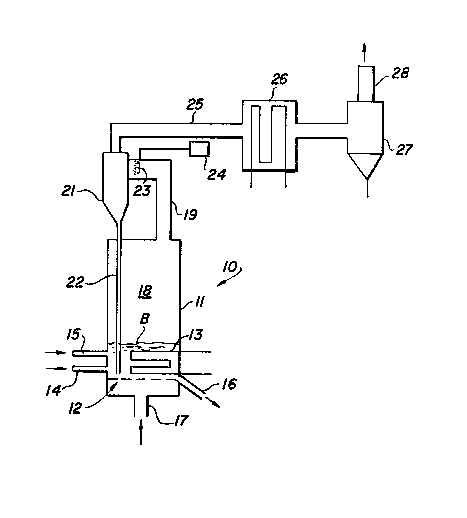
Figure 1 is a schematic elevational sectional view
of a complete system in which the present invention is
embodied and utilized.
Best Mode for Carryinq Out the Invention
The present invention is embodied and employed in
a system comprised of a fluid bed combustor 10 having a
combustion chamber 11 for containing a fluid particle
bed B supported on a distribution plate 12. The
combustor 10 includes cooling tubes 13 in the fluid bed
B as well as a fuel feed 14, sorbent feed 15 and bed
drain 16. Fluidizing air is introduced in the bottom
of the combustor 10 at 17. The hot gas and elutriated
particulate leaving the surface of the bed B pass
through the freeboard 18 and are directed via a conduit
19 to a recycle cyclone 21 mounted above the bed to
provide a straight dip leg 22 with adequate head in the
dip-leg 22 to provide a free flowing return of fine
particulate to the bed. The cut point of the recycle
cyclone 21 is approximately 12 microns~
,
~,
.
--7--
Ammonia is injected into the hot gas stream in the
conduit 19 immediately upstream of the recycle cyclone
by an ammonia injector 23 to suppress nitrogen oxides
when burning fuel with fuel-bound nitrogen. Ammonia is
supplied from a supply tank 24.
Hot gas leaving the recycle cyclone 21 via a
conduit 25 passes through a convection heater 26 where
the remaining heat is removed from the hot gas.
Downstream of the convection heater 26 the ~as passes
through a filter system 27, such as a baghouse filter,
to remove dust before being exhausted to the atmosphere
through the stack 28~
According to this invention we provide fluidized
bed combustion system method and apparatus which
comprises a bubbling fluid bed combustor with a
superficial velocity in the range of 0.5 ft/second to 7
ft/second but with bed material in the size range of 45
microns to 2000 microns in diameter whereby ~0~ to 20
of the bed material, respectively, is less than 200
microns in diameter. The large fraction is associated
with dolomite feedstock size when dolomite is used.
The bubbling bed contains tubes to transfer heat from
the hot fluid bed; the heat transfer coefficient on the
outside of those tubes is in the range of 100 to 200
BTU/FT2-HR-F because of the fine particulate in the
bed. Solid fuel or liquid fuel is fed directly into
the bed with the solid fuel. If sulfur sorbent such as
limestone or dolomite is used it is fed directly into
the b~d as well.
The fluid bed combustor has a hot freeboard with
no heat transfer surfaces. A large number of fines are
elutriated from the fluid bed into the hot freeboard
wherein excellent mixing conditions exist between the
particles and the gas, and adequate residence time is
available for chemical reactions7 Most of the
particulate elutriated from the bed into the freeboard
falls back into the bed but a significant amount is
. ~ .
9~
--8--
transported by the gas flow into the recycle cyclone
where it is separated from the gas and returned to the
bed, all at substantially the same temperature as
exists in the fluid bed. Particle loading of the gas
entering the cyclone is approximately 0.5 kg/cu m. The
extended residence time and the excellent mixing
between the fine particulate char and the oxygen rich
combustion gas in both the freeboard and recycle
cyclone cause the char particles to burn to completion
before they can exit the recycle cyclone. A benefit of
the recycle char is believed to be the enhancement for
highly efficient nitrogen oxide suppression at
temperatures down to 1400F. Similarly, the extended
residence time and the excellent mixing between the
fine sorbent particles and the sulfur oxides in the
combustion gas in the hot freeboard and the recycle
cyclone promote good sulfur oxide capture by the
sorbent. The fine particulate spend approximately 1
second in the bed and 3 seconds in the freeboard and
recycle cyclone. The recycle cyclone is designed with
a cut point of 5 microns and with a highly efficient
dip leg to easily recycle most of the particles
substantially larger than 5 microns, preventing their
escape to the filter system. The flow rate of the
captured particulate around the recycle loop is
approximately twenty times the combined low of fuel
and sorbent into the fluid bed. Its hourly flow rate
is twlce the weight of the fluid bed itself.
If the fuel contains fuel-bound nitrogen and
nitrogen oxide suppression is required, ammonia is
sprayed into the hot combustion gas stream immediately
upstream of the inlet to the recycle cyclone.
Although, ammonia selectively and most efficiently
reduces nitrogen oxides without a catalyst in the range
of 17~3 F to 1832F, efficient reduction is normally
achieved in the present invention by operating the
fluid bed at 1450F-1650F and achieving 0 to 150F of
;
~.~7~345
_g
after burning above the bed. Injection of ammonia in
ammonia/nitrogen oxide molar ratios of 1.5 to 2
provides nitrogen oxide suppression of 80~ to 95~
because of the excellent mixing occuring in the recycle
cyclone. Under certain conditions nitrogen oxides are
suppressed without the use of ammonia injection. When
operating at low combustion temperatures of 1450F with
fuel with a high percentage of fixed carbon, a
substantial part of the recycled particulate is char.
This hot fine particulate char reduces nitrogen oxides
such that a nitrogen suppression of 86% has been
achieved by the present invention.
The hot combustion gas and dust escaping the
recycle cyclone pass through a convective heat
exchanger where the gases are cooled to their exit
temperature~ Finally, a filter system removes the dust
before discharging the combustion gas to the
atmosphere.
The present invention thus provides the capability
to burn cleanly a wide variety of solid and liquid
fuels, some of which may be very difficult to burn
(such as petroleum coke with 90~ fixed carbon, i.e.,
low volatiles) or fuels which may contain sulfur or
nitrogen or the combination of sulfur and nitrogen, all
of which cause air pollution. The present invention
burns these fuels by using a conventional bubbling bed
with a fine particulate composition and recycling a
large portion of those fines through a hot recycle loop
above the bubbling bed. Combustion efficiency of 99.4%
is obtained with petroleum coke with 90% fixed carbon,
and 98~ suppression of sulfur oxides is obtained with a
calcium sulfur molar ratio of 1.8. ~ 95~ suppression
of nitrogen oxides is obtained with an ammoniafnitrogen
oxide molar ratio of 2. All this occurs within the
framework of the fluid bed recycle system and occurs
simultaneous.
,
- 1 o -
A further benefit of the present invention is a
large fluidization range of up to 15:1. Because the
bubbling fluid bed is composed of fine particulate, its
minimum fluidization velocity is as low as 0.5
ft/second.
- EXAMPLE - PETROLEUM COKE
Petroleum coke was burned with air in a fluidized
bed combustor whose configuration is described in
Figure 1. The fluid bed combustor was three feet in
diameter and twelve feet tall with the recycle c~clone
mounted above it. The combustor was refractory lined.
The bubbling bed was operated 3-1/2 to 4 feet deep and
contained air tubes to transfer heat out of the bed.
The petroleum coke used in the test had the
following composition and heating value:
Fixed Carbon 89.7~ by weight
Nitrogen 1.9%
Sulfur 2.1~
Other Volatiles 4.4%
Ash 0.3~
Moisture 1.6%
HHV 14,270 BT~/LB
.
This Euel is difficult to burn because of the high
fixed carbon with few volatiles. It also contains the
elements of nitrogen and sulfur which produce nitrogen
oxides and sulfur oxides as air pollutants. The fuel
was introduced to the fluid bed through a fuel feed,
the majority of the fuel being between 50 and 400
microns in diameter. Dolomite, a sulfur sorbent, was
introduced into the bed through the sorbent feed. Its
composition was:
. .
. . , -.; .
" - ,.
". ~ .
~7~4~
--1 1--
Calcium Carbonate 56.6% by weight
Magnesium Carbonate 45.5~
Inerts 0.9%
Its size was between 4700 microns and 1200 -
microns. This particular dolomite decrepitated in the
bed into fine particles.
The fluid bed was initially composed of crushed
dolomite with an average size of 800 microns.
After testing for approxi~ately 500 hours the bed
was comprised of ash, spent sorbent and partially spent
sorbent; average particle size had stabilized at
approximately 300 microns. The fluld bed operated at
an average superficial velocity of 4 ft/second. It was
necessary to drain the bed periodically to maintain a
constant level.
The recycle cyclone was designed to hold the
majority of particles greater than 5 microns within the
fluid bed combustor and was designed with a free
; flowing dip leg to provide little resistance in the
~0 particulate return path. As a result, high recycle
flow rates of fines were achieved whereby the
recirculation per hour was approximately twice the
weight of the bed and twenty times the combined solids
feed rate. The fuel particulate and sorbent
particulate, unable to leave the fluid bed with the gas
stream until they had reached a very small size, were
contained in the bed and comminuted by the action of
the bedO Fuel particles, restrained from leaving the
fluid bed combustor, burned to completion providing
high combustion efficiency even with a difficult fuel
containing approximately 90% fixed carbon. Combustion
efficiency was further enhanced by the isothermal
nature of the recycle path. The fuel particle is
heated to full combustion temperature in the bed and is
not cooled either in the freeboard or the recycle
cyclone. Operating at a bed temperature of 1600~F with
~7~ ~ ~ 5
-12-
20~ to 30% excess air, combustion efficiencies of 99.4
were achieved. Afterburning above the bed was in the
range of 50 F to 100 F.
Comminution and retention of the sorbent particles
provided a large surface area of the sorbent to absorb
sulfur from gases in the fluid bed combustor. Ninety-
eight percent sulfur oxide suppression was achieved at
a calcium to sulfur molar ratio of 1.8. A further
benefit of the fine particle size in the combustor was
the increase in heat transfer coefficient on the
surface of the tubes immersed in the bed. Heat
transfer coefficients on the outside of the tubes
ranging from 100 to 200 BTU/HR-FT2-F were observed
compared to 40-60 BTU/HR-FT2-F for a conventional fluid
bed boiler.
To suppress nitrogen oxides to meet local
pollution control codes in Southern California, ammonia
was in]ected upstream of the cyclone to mix with the
combustion gas and selectively reduce nitrogen oxide to
nitrogen and water according to the well-known
reactions. At an NH3-to-NO molar ratio of 2,
approximately 95% of the NO was suppressed.
EXAMPLE-UTAH COAL
Utah coal was burned in the same fluid bed
combustor as previously described in the earlier
example. The composition of the coal and its heating
value were as follows:
Fixed carbon 43%
Nitrogen 1.3%
Sulfur 0.6%
Other Volatiles 37.1%
Ash 8.0%
Moisture 10.0%
HHV 11,500 BTU/LB
5 - 1 3-
" The Utah coal had substantially less fixed carbon
and substantially greater volatiles and hence was
easier to burn than petroleum coke. The size of the
coal was minus 1 5/8 inchesO The sulfur sorbent was
the same dolomite as used in the prior example. Its
composition was as follows:
Calcium carbonate 56.6% by weight
Magnesium carbonate 45.5%
Inerts o.9%
Its size was between 1,200 microns and 4,700
microns but it decrepitated into fire particles in the
bed.
Combustion efficiency with coal was 99~8% with 20%
excess air at a bed temperature of 1600F. For coal,
the combustor could be operated as cool as 1400F with
only 20% excess air and yet maintain good combustion
characteristics. With petroleum coke, acceptable
combustion characteristics could only be maintained at
1450F by increasing the excess air to 60~. For coal
at 1600F, afterburning above the bed was reduced to
10-20F. Suppression of sulfur oxides and s~itrogen
oxides was similar to that on petroleum coke.
. . ~