Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~B33S6
PHARMACEUTICAL COMPOSITIONS OF MICROBIALLY
PRODUCED INTERLEUKIN-2
This invention is in the field of pharmaceuticals. More
particularly, it relates to pharmaceutical formulations of microbially
produced interleukin-2.
Interleukin-2, a lymphokine which is produced by normal
peripheral blood lymphocytes and induces proliferation of antigen or
mitogen stimulated T cells after exposure to plant lectins, antigens,
or other stimuli, was first described by Morgan, D. A., et al.,
Science (1976) 193:1007-1008. Then called T cell growth factor
because of its ability to induce proliferation of stimulated T
lymphocytes, it is now recognized that in addition to its growth
factor properties it modulates a variety of functions of immune system
cells in vitro and in vivo and has been renamed interleukin-2 (IL-
2)~ IL-2 is one of several lymphocyte-produced messenger-regulatory
molecules that mediate immunocyte interactions and functions.
IL-2 was initially made by cultivating human peripheral
blood lymphocytes (PBL) or other IL-2-producing cell lines. See, for
instance, U.S. Patent No. 4,401,756. Recombinant DNA technology has
;~ provided an alternative to PBLs and cell lines for producing IL-2.
Taniguchi, T., et al., Nature (1983) 302:305-310 and Devos, R.,
~Nucleic Acids Research (1983) 11:4307-4323 have reported cloning the
human IL-2 gene and expressing it in microorganisms.
Belgian Patent No. 898,016, granted 14 November 1983
; describes muteins of IL-2 in which the cysteine normally occurring at
position 125 of the wild-type or native molecule has been deleted or
replaced with a neutral amino acid, such as serine. These muteins
possess IL-2 biological activity. The Belgian patent states that the
recombinant muteins may be formulated and administered as with native
IL-2 by combining them with aqueous vehicles and injecting them
intravenously, subcutaneously, or the like.
~- 30 One aspect of the present invention is an IL-2 composition
suitable for reconstituting in a pharmaceutically acceptable aqueous
.; ~
~,.
` ~1.2~33356
vehicle for parenteral administration to a patient to provide IL-2
therapy comprising a sterile lyophilized mixture of:
(a) a therapeutically effective amount of oxidized
microbially produced recombinant IL-2 that is substantially free of
non-IL-2 protein;
- (b) a pharmaceutically acceptable water soluble carrier
that does not affect the stability of the microbially produced IL-2
adversely; and
(c) a sufficient amount of surface active agent such as
alkali metal sulfates, e.g., sodium dodecyl sulfate (SDS), alkali
metal sarcosinates or sodium deoxycholate to ensure the water
solubility of the microbially produced recombinant IL-2.
Preferably, the recombinant IL-2 has been selectively
~ ; oxidized such that the cysteines at positions 69 and 105 form a
! ~ 15 disulfide bond to render the molecule biologically active.
Another aspect of this invention is a pharmaceutical
composition for providing therapy to a patient comprising a sterile
solution of:
(a) the above described m;xture dissolved in
(b) a pharmaceutically acceptable aqueous parenteral
vehicle, said solution containing in the range of about 0.01 mg to
about 2 mg of the microbially produced recombinant IL-2 per ml.
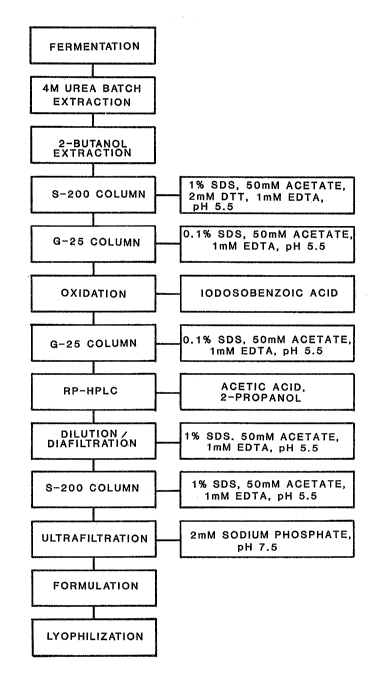
Figure 1 is a flow diagram of a preferred procedure for
processing and purifying microbially produced recombinant IL-2.
~` 25 As used herein the term "IL-2" denotes an unglycosylated
protein that is (a) produced by a microorganism that has been
transformed with a human int rleukin-2 DNA sequence or a modification
of the human interleukin-2 DNA sequence that encodes a protein
having: (a) an amino acid sequence that is at least substantially
identical to the amino acid sequence of native human interleukin-2
including the disulfide bond of the cystines at positions 58 and 105~
~ and (b) has biological activity that is common to native human
; interleukin-2. Substantial identity of amino acid sequences means the
.. ,~. . ~,~. . " .. ..
128~3~;~
sequences are identical or differ by one or more amino acid
alterations (deletions, additions, substitutions) that do not cause an
adverse functional dissimilarity between the synthetic protein and
native human interleukin-2. Examples of such proteins are the
recombinant IL-2s described in European patent application 8310103500
filed February 3, 1983 (published October 19, 1983 under publication
no. 91539) and European patent application 82307036.2 filed December
22, 1982 (published September 14, 1983 under no. 88195), the
recombinant IL-2 muteins described in European patent application
83306221.9 filed October 13, 1983 (published May 30, 1984 under no.
109748) which is the equivalent to Belgian Patent No. 893,016, and the
recombinant IL-2s described in this application.
As used herein the term "transformed microorganism" denotes
a microorganism that has been genetically engineered to produce a
pro~ein that possesses native human interleukin-2 activity. Examples
of transformed microorganisms are described in said European patent
;~ publications 88,198; 91,539 and 109,748. Bacteria are preferred
microorganisms for producing IL-2. A typical transformed
microorganism useful in the present invention is E. coli K-12 strain
ZO MM294 transformed with plasmid pLW1 (deposited at the American Type
Culture Collection on August 4, 1983 by Cetus Corporation under the
provisions of the Budapest Treaty and having accession number
39,405). Synthetic recombinant IL-2 may also be made by suitably
transformed yeast and mammalian cells~ E. coli is particularly
preferred host organism.
The transformed microorganisms are grown in a suitable
growth medium, typically to an optical density (OD) of at least about
` 30 to 680 nm, and preferably between about 20 and 40 at 680 nm. The
composition of the growth medium will depend upon the particular
microorganism involved. The medium is an aqueous medium containing
compounds that fulfill the nutritional requirements of the
microorganism. Growth media will typically contain assimilable
sources of carbon and nitrogen, energy sources, magnesium, potassium
and sodium ions, and optionally amino acids and purine and pyrimidine
bases. (See Review of Medical Biology, Lange Medical Publications,
;
~LZ~3~3~i6
14th Ed pp 80-95 (1980.)) In expression vectors involving the trp
promoter, the tryptophane concentration in the medium is carefully
controlled to become limiting at the time IL-2 expression is
desired. Growth media for E. coli are well known in the art. A
preferred growth method is described in U.S. Patent No. 4,499,188,
granted 12 February 1985.
- After the cells are harvested from the culture, they may be
concentrated, if nesessary, to about ZO to 150 mg/ml, preferably 80 to
100 mg/ml (OD 40 to 300, preferably 160 to 200 at 680 nm) by
filtration, centrifugation, or other conventional methods.
Following concentration the cell membranes of the
microorganisms are disrupted. The main purpose of disruption is to
facilitate the following extraction and solubilization steps.
Conventional cell disruption techniques such as homogenization,
sonication, or pressure cycling may be used in this step of the
process. Preferred methods are sonication or homogenization with a
Manton-Gaulin homogenizer. The end point of the disruption step may
be monitored by optical density, with the optical density of the
suspensiion typically decreasing about 65% to 85%. In any event, the
disruption should break substantially all of the cells so that
substantially no intact cells are carried through to the
solubillzation step. Before the disruption, the pH of the liquid
phase of the concentrate is adjusted, if necessary, to a level that
~; ~ facilitates removal of E. coli proteins in subsequent steps, while
retaining recombinant IL-2 protein as an insoluble complex ir, the
; ~ cellular debris. The pH may be so adjusted by adding suitable
buffers. In most instances pHs in the range of about 8 to about 8.5
will be used.
The steps in the recovery process subsequent to the
disruption step as shown in Fig. 1 are primarily designed to separate
the IL-2 from E. coli proteins to a high level of purity (preferably
at 1east about 95% and more preferably at least about 98%) in good
yields while maintaining the IL-2 in a reduced state. Simultaneously,
these purificat~on processes, in combination, also reduce pyrogenic
substances in the final product to a level believed to be acceptable
for parenteral administrtion to patients.
~2833~
After the cells have been disrupted the particulate matter
may be separated from the liquid phase of the disruptate and
resuspended in an aqueous medium buffered to the optimal pH for the
extraction. The particulate matter may optionally be washed with
buffer at this stage to remove any water soluble E. coli proteins
therein. In any event, the protein concentration of the cell
suspension subjected to the extraction will usually be in the range of
about 5 to about 60 mg/ml, preferably 20 to ~0 mg/ml.
The extraction of E. coli proteins from the particulate
cellular material may be carried out concurrently with the disruption
or sequentially following the disruption. It is preferably carried
out as a step following the disruption. The extractant is an aqueous
solution of a chaotropic agent (i.e., a mild protein denaturant that
dissociates hydrogen bonds and affects the tertiary structure of
proteins). The extractant selectivley removes the bulk of the E. coli
proteins from the cellular debris leaving at least a substantial
portion of the recombinant IL-2 associated (contained in or bound to)
with the cellular debris. The selectivity is facilitated by the
hydrophobicity of the recombinant IL-2 and the fact that it is in a
reduced, insoluble state at a pH near the isoelectric point of the
protein. In addition, a substantial portion of the recombinant IL-2
may be present ln in vivo as inclusion bodies of significant mass, as
has been the case with other cloned proteins expressed at high levels
in E. coli. Examples of extractants are urea and guanidinium
hydrochloride (guanidinium hydrochloride should not be used when SDS
is used as a solubilizing agent). Urea is preferred. The
concentration of the chaotropic agent in the extraction mixture will
depend upon the particular agent that is used and the amount of
cellular material in the extraction mixture. In the case of urea,
concentrations (final) between about 3.5 M and 4.5 M, preferably about
4 M, will be used in batch processes at 25C. If the extraction is
run on a continuous basis over longer time periods it may be desirable
to use lower concentrations. Temperatures in the range of 20C to
25C will normally be used in extraction, with room temperature being
used for convenience. Mixing will typically be used to enhance
.
". ~L2~333S~
-
contact between the solution and particulate matter and thus decrease
the time required to extract non-IL-2 proteins from the cellular
debris. Kinetic analysis of the extraction process was performed on
the supernatants using SDS-PAGE, and the extraction was found to be
essentially complete by 15-30 minutes.
Following the extraction, the mixture is separated into
solid and liquid phases. The recombinant IL-2 in the solid phase is
then selectively solubilized by contacting the solid phase with a
neutral, aqueous buffer containing a reducing agent and a solubilizing
agent. Physiologically acceptable surface active agents (detergents)
that have a suitable hydrophobic-hydrophilic balance to solubilize the
hydrophobic recombinant IL-2 may be used. Alkali metal sulfates
containing 10 to 14 carbon atoms and alkali metal alkyl sarcosinates
are preferred solubilizing agents, with SDS and sarcosyl being
particularly preferred.
The amount of solubilizing agent used in the solubilization
will depend upon the particular agent. When SDS or sarcosyl are used,
the preferred ratio (w/w) of SDS/sarcosyl to solid phase protein is
about 0.5:1 to 1.4:1. The solubilizing medium also contains a
sufficient amount of reducing agent to prevent the solubilized IL-2
from undergoing oxidation to any significant degree. Protein reducing
agents such as dithiothreitol (DTT) and 2-mercaptoethanol may be
used. The concentration of reducing agent such as DTT in the medium
will usually range between about 5 to 20 mM. The solubilization will
typically be carried out at temperatures in the range of 20C to 25C
with mixing to facilitate contact ~between the solid phase and the
solubilizing medium. Higher temperatures may solubilize unwanted E.
coli proteins. The solubilization is considered complete when the
sample has sat 15 minutes or the solution turns translucent.
Insoluble material is separated after completing the solubilization.
After the IL-2 is solubilized the IL-2 may optionally be
extracted from the aqueous solution under reducing conditions with 2-
butanol or 2-methyl-2-butanol to remove additional E. coli proteins,
notably including certain contaminants that have molecular weights
very close to the IL-2. Conditions (e.g., ionic strengths in the
Z833~6
range of 0.05 and 0.15) at which the aqueous solution and butanol are
substantially immiscible are used. In carrying out the organic
extraction the protein concentration of the aqueous solution is
preferably adjusted, if neces~ary, ko less than about 6 mg/mlg
preferably about 0.5 to 4 mg/ml. Reducing conditions are maintained
by carrying out the extraction in the presence of a reducing agent
(e.g~, DTT). The butanol will normally be added to the aqueous
solution of solubilized IL-2 in volume ratios in the range of about
1:1 to about 3:1 (estractant:aqueous solution), preferably about
1:1. The extraction may be carried out in a batch or continuous
operation. The temperature will normally be in the range of 20C to
100C and the pH will normally be about 4 to 9, preferably about 5 to
6. The time of contact between the solution and the butanol is not
critical and relatively short times on the order of a few minutes may
be used. After the extraction is complete, the aqueous phase and
butanol phase are separated and the IL-2 is separated from the butanol
phase. A preferred procedure for separating the IL-2 from the butanol
phase is acid precipitation. This is done by adding the butanol phase
; to aqueous buffer, pH 7.5 until the organic phase is dissolved
(approx. 2-3 vol buffer per vol of organic), and then lowering the pH
to about 5.5 to 7~0, preferably 6.0 to 6.2, to cause the IL-2 to
~ ~ precipitate.
- ~ The next step in the process is to separate the recombinant
,~ ~ IL-2 and any E. coli contaminants remaining after the extraction(s)and optimally from the solubilizing agent. Gel filtration
; chromatography, RP-HPLC, or a combination of gel filtration
chromatography and RP-HPLC are used. The gel -filtration
chromatographic is preferably carried out in two stages that remove
pyrogenic components and protein contaminants having molecular weights
higher or lower than recombinant IL-2. (Recombinant IL-2 has a
molecular weight of about 15.5K daltons.) Gels that are capable of
fractionating the solution to permit separation of the IL-2 from these
contaminants are commercially available. Sephacryl~ S-200 is a
preferred gel for removing the higher molecular weight components and
Sephadex G-25, G-75 or G-100 gels are preferred for removing the low
~ T~lde m~K
;
- , . .
.
833S~
molecular weight contaminants. The gel filtrations will typically be
run in buffered solutions (pH 5.5 to 7.0) containing about 0.170 to
1.0% solubilizing agent and about 1 to 10 mM reducing agent. The
column will be sized to permit suitable resolution of the desired
components.
RP-HPLC is an alternative to gel filtration. Also, RP-HPLC
is capable of removing molecules from the solution that have molecular
; weights close to recombinant IL-2 and cannot, therefore, be removed
completely by gel filtration. In addition, contaminants such as
bacterial endotoxin are also removed effectively by RP-HPLC.
Therefore, RP-HPLC may also be used as a final purification step after
gel filtration. Supports (stationary phases) that provide good
resolution of proteins may also be used as a final purfication step
after gel filtration. Supports (stationary phases) that provide good
resolution of proteins may be used in the RP-HPLC. C-4, C-8, or C-18
; on 300 angstrom pore-size supports are examples of preferred
supports. The separation is carried out at an acidic pH of less than
about 2.3, usually 2.1 to 2.3 in order to keep the IL-2 in solution.
In this regard, the pH of the solution from the solubilization (gel
filtration) will preferably be adjusted to this range. The solution
is loaded into the RP-HPLC column and is absorbed onto the stationary
phase. A gradient solvent system comprising an organic acid such as
acetic acid or trifluoracetic acid and organic solvent such as
propanol or acetonitrile is used to elute the recombinant IL-2 from
the column. Acetic acid-propanol, trifluoroacetic acid-propanol, and
trifluoroacetic acid-acetonitrile are preferred solvent systems.
Recombinant IL-2 elutes in the acetic acid-propanol system at about
40% propanol, in the trifluoroacetic acid-propanol system at about 50%
propanol, and in the trifluoroacetic acid-acetonitrile system at about
62% acetonitrile. For convenience, the organic solvent content of the
elutant will usually be increased rapidly to a level somewhat below
the solvent concentration at which the recombinant IL-2 elutes
followed by a slow gradient change in the range of about 0.1% to
1.0%/min.
.. ,
33~i6
g
As soon as the recombinant IL-2 is recovered from the
chromatography step3 it is lyophilized and resuspended in a neutral
aqueous buffer containing the reducing agent (to keep the recombinant
IL-2 in a reduced state) and the solubilizing agent (to keep it in
solution). The recombinant IL-2 is stable in this form and may be
stored for further treatment and formulation before being used.
An alternative and preferred procedure is to selectively
oxidizeJ under controlled conditions, the recombinant IL-2 after it
has been separated by gel filtration and purify the oxidized product
by RP-HPLC or gel filtration followed by RP-HPLC. This results in
efficient removal of contaminants surviving the gel filtration as well
as unwanted oxidation products. A preferred oxidation procedure is to
selectively oxidize a fully reduced microbially produced synthetic
recombinant IL-2 protein having an amino acid sequence substantially
identical to the recombinant IL-2 protein which sequence includes
cysteines which in the useful protein are linked intramolecularly at
positions 58 and 105 to form a cystine in a controlled manner so that
the cysteines are oxidized selectively to form the cystine at
positions 58 and 105. The efficiency of the controlled and selective
oxidation is improved if a recombinant IL-2 mutein is used such as
, described and claimed in Belgian Patent No. 898,016. In such case the
~; cysteine at position 125 is deleted or replaced with a neutral aminoacid thus preventing incorrect intramolecular bonds and/or
intermolecular bonds with the cysteine at position 125 during
oxidation which may also form dimers or polymers of IL-2. In this
process the fully reduced microbially produced synthetic recombinant
IL-2~ protein is preferably reacted~ with o-iodosobenzoateJ which
oxidizes cysteines selectively in an aqueous mediumJ at a pH at least
about one-half pH unit below the PKa of said cysteines, wherein the
concentration of synthetic protein in the reaction mixture is less
; than about 5 mglml and the mol ratio of o-iodosobenzoate to protein is
at least stoichiometricJ with the proviso that the o-iodosobenzoate is
in excess in the kerminal portion of the reaction. This selective
oxidation produces a biologically active molecule. RP-HPLC
purificatlon of the selec~ively oxidized product may be carried out
:,
: ' ~
1~33356
under the conditions described above in the absence of a reducing
agent and presence of a detergent at a concentration equal to or less
than those used in the above described gel filtration.
The purity of the recombinant IL-2 after the chromatography
step(s) is at least about 95% and usually at least about 98%. This
highly pure material contains less than about 5 ng endotoxin, usually
less than about 0.01 ng endotoxin per 100,000 Units Il-2 activity.
The formulation of recombinant IL-2 in accordance with this
invention may be carried out as a separate operation using purified,
selectively oxidized IL-2 or in an operation that is integrated with
the purification of the selectively oxidized IL-2. In the latter
case, the starting material for the formulation is a recombinant IL-2-
containing product from a reverse phase high performance liquid
chromatography (RP-HPLC) treatment of the selectively oxidized
; 15 product, preferably one selectively oxidized by the RP-HPLC product(pool) will comprise a solution of recombinant IL-2 in a water-organic
solvent mixture. The nature of the organic solvent will depend upon
the solvent system used in RP-HPLC. Examples of systems that may be
used are combinations of an organic acid such as acetic acid or
trifluoroacetic acid and organic solvent such as propanol or
acetonitrile.
The first step in formulating the recombinant IL~2 from such
: ~ :
an RP-HPLC pool is to render the mixture aqueous by resuspending
(diluting) the pool in an aqeuous buffer containing a detergent, such
as SDS or sarcosyl, that enhances the solubility of the recombinant
IL-2 in water. Following this dilution the organic phase is removed
from the recombinant IL-2 containing aqeuous phase and the detergent
concentration is reduced by diafiltration using an appropriate
buffer. When SDS is used, the SDS is reduced to a level of about 100
3~ to 250, preferably approximately 200, ~g/mg IL-2. Following
diafiltration, the IL-2 concentration is readjusted to a concentration
in the range of about 0.01 to 2 mgiml and the water soluble carrier is
added to the desired level. The carrier will typically be added such
that it is present in the solution at about 1 to 10% by weight,
preferably about 5% by weight. The exact amount of carrier added is
~'
~ '
~LZ~3~3~;6
11
not critical. Conventional solid bulking agents that are used in
pharmaceutical tablet formulation may be used as the carrier. These
materials are water soluble, do not react with the IL-2, and are
themselves stable. They are also preferably non-sensitive (i.e.,
nonhygroscopic) to water. Examples of carriers that may be added are
lactose, mannitol, and other reduced sugars such as sorbitol, starches
- and starch hydrolysates derived from wheat, corn, rice, and potato,
- microcrystalline celluloses, and albumin such as human serum
albumin. Mannitol is preferredO
The carrier adds bulk to the formulation such that when unit
dosage amounts of the solution are lyophilized in containers, such as
sterile vials, the freeze-dried residue will be clearly discernible to
the naked eye. In this regard the preferred carrier, mannitol, yields
an aesthetically acceptable (white, crystalline) residue that is not
sensitive to water. The nonsensitivity of mannitol to water may
enhance the stability of the formulation.
; After adding the carrier the unit dosage amounts (i.e.,
volumes that will provide 0.01 to 2 mg, preferably 0.2 to 0.3 mg, IL-2
per dose) of the solution are dispensed into containers, the
containers are capped with a slotted stopper, and the contents are
; lyophilized using conventional freeze-drying conditions and apparatus.
The lyophilized, sterile product consists of a mixture of
(1) recombinant IL-2, (2) carrier (mannitol), (3) detergent (SDS), and
(4) a small amount of buffer that will provide a physiological pH when
the; mixture is reconstituted. The recombinant IL-2 will typically
constitute about 0.015% to 3.85% by weight of the mixture, more
preferably about 0.4% to 0.6% of the mixture. Storage tests of this
product indicate that the IL-2 is stable in this form for more than
three months at 2~C to 8C.
0 The lyophilized mixture may be reconstituted by injecting a
conventional parenteral aqueous injection such as water for injection,
Ringer's injection, dextrose injection, dextrose and salt injection,
or the like, into the vial. The injection should be added against the
side of the vial to avoid excess foaming. The amount of injection
''`' .
: .
.
~Z~333S6
12
added to the via1 will typically be in the range of 1 to 5 ml,
preferably 1 to 2 ml.
The reconstituted formulation is suitable for parenteral
administration to humans or other mammals to provide IL-2 therapy
thereto. Such therapy is appropriate for a variety of
immunomodulatory indications such as T cell mutagenesis, induction of
cytotoxic T cells~ augmentation of natural killer cell activity,
induction of IFN-gamma, restoration or enhancement of cellular
immunity (e.g., treatment of immune deficient conditions), and
augmentation of cell mediated anti-tumor activity.
The following example further illustrates the invention.
This example is not intended to limit the invention in any manner.
EXAMPLE
The recombinant IL-2 used in this example is des-ala IL-
15 2Serl25. The amino acid sequence of this IL-2 differs from the amino
acid sequence of native human IL-2 in that it lacks the initial
alanine of the native molecule and the cysteine at position 125 has
been changed to serine. Samples of E. coli that produce this IL-2
have been deposited by Cetus Corporation in the American Type Culture
: 20 Collection, 12301 Parklawn Drive, Rockville, Maryland, USA, on
September 26, 1983 under accession number 39452 and on March 6, 1984
- ~ under accession number 39626 under the provisions of the Budapest; ~ Treaty.
329 mg of an RP-HPLC purified oxidized IL-2 product (protein
concentration 0.94 mg/ml) in 60% 2-propanol, 6% acetic acid was
diluted ten-fold into 50 mM sodium acetate, 1 mM ethylene diamine
tetraacetic acid (EDTA), 0.1% sodium dodecyl sulfate (SDS) at pH 5.5.
The IL-2 solution was then concentrated using a 10 sq. ft.
hollow fiber cartridge (nominal molecular weight cut-off 10,000
daltons) to a volume of 600 ml and then diafiltered for 3 volumes
against 50 mM sodium acetate, 1 mM EDTA, 0.1% SDS at ptl 5.5. The
material was then further diafiltered against 10 mM sodium phosphate
containing 5 ~9 SDS/ml until the residual SDS reached a value of 131
~g SDS/mg protein. Approximately 255 mg IL-2 at a concentration of
0.6 mg/ml were recovered (425 ml).
3356
13
Only 222 mg were used for the formulation which was carried
out as follows: 370 ml of the IL-2 solution (222 mg, 0.6 mg/ml) was
diluted with 10 mM sodium phosphate, pH 7.5 and 20% mannitol such that
the final composition was:
50.25 mg/ml IL-2
) in 10 mM sodium phosphate, pH 7.5
5% mannitol
The solution was then sterile filtered through a 0.2 micron
filter, filled into sterile vials (1.2 ml fill volume) and
lyophilized. lhe product was sealed under vacuum.
The thus produced formulation has been used clinically in
humans and has been well tolerated at dosages up to 2 million units/m2
when administered as a continuous intravenous infusion or up to 1
million units/kg when administered as an intravenous or intramuscular
~ 15 bolus. Suitable indications for use of the recomhinant IL-2 include:
;~ 1) treatment of immunodeficiency states, acquired, inborn, or induced
by chemotherapy, immunotherapy, or irradiation;
2) enhancement of cell-mediated immune responses in the therapy of
viral, parasitic, bacterial, malignant, fungal, protozoal, or
mycobacterial~ or other infectious diseases;
3) ~induction of enhanced immunologic response of cells ex vlvo in the
~; treatment of infectious, malignant, rheumatic, or autoimmune diseases;
4) ;treatment of rheumatoid or other inflammatory arthridites;
5) treatment of diseases of abnormal immune response such as multiple
sclerosis, systemic lupus erythematosis, glomerulonephritis, or
hepatitis;
6) regulation of hematopoietic tumors or pre-malignant or aplastic
abnormalities of hematopoietic tissue;
~.
,. . .. ..
.
.
.: :
. .
". lZ8
14
7) use as an adjuvant in induction of cell-mediated or humoral
response to naturally occurring, administered nautral, chemically
synthesized or modified, or recombinantly engineered vaccines or other
antigens administerd for therapeutic purposes;
~) use as a mediator of neurotransmission or as a psychoactive
therapeutic, as an enkephalin for therapeutic purpose, or as a
modifier of central nervous system function;
9) in a topical applicatior, for the treatment of above-mentioned
disease states;
10) in combination with cytotoxic chemotherapy or irradiation or
surgery in the treatment of malignant or pre-malignant diseases in a
direct therapeutic or adjuvant setting;
11) in combination with agents with direct anti-viral, anti-fungal,
anti-bacterial, or anti-protozoal activity or in combination with drug
therapy for typical and atypical m. tuberculosis;
12) in combination with other immune-modulating drugs, lymphokines,
; (e.g., IL-1,IL-3, CSF-l, alpha-interferons, and gamma-interferons)
naturally occurring or inducible anti-cellular toxins or molecules
which mediate lysis or stasis or malignant cells in the treatment of
0 malignant, infectious, autoimmune, or rheumatic diseases; and
13) for prophylaxis against infectious diseases
: :
:: :
. :
'
.
,,
"