Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
3 4 5 ~ 3
Thi~ is a divisional application of copendîng application
serial no. 470,058, filed Decernber 13, 1984.
_UEL COMPOSITIONS
Compression ignitioll fllel compositions alld
ad~ ive mixtures oE or-3allic nitrat:e i~nitioll
accelerator alld hydrocarbyl-substituted succillimi.de, in
5 amoullts sufficient to resist the coking tendellcies of
coml)ression iynition fuel cornpositions when used in the
operation oE indirect injectioll diesel engines.
l`hrottlillg diesel no%%les have recerltly come into
widespread use in indirect injection automotive and
10 ligllt-duty diesel truck engines, i.e., compression
ignitioll engines in whicll the ~uel is injected into and
ignited in a prechamber or swirl chamber. In this way,
the Elame Eront proceeds Erom the prechamber into the
larger cornpression chamber where the cornbustion is com-
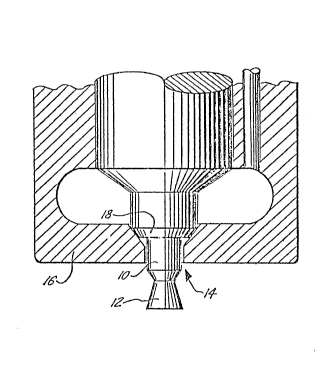
15 pleted. Engines designed in this manner allow ~orquieter and smoother operation. The Figure o the
Drawing illustrates the geometry oE the typical
throttling diesel noz%le (oEten reEerred to as the
"pintle nozzle" ) .
UnEorturlately, the advent oE such en~ines has
giJell rise to a new problem, that o~ excessive cokinc3 on
~ ~458~3
the critical sur~aces o~ the injec~ors that inject Euel
itltO l:he prechalnber or swirl charnber of the engine. In
varticular and with referellce to tlle Figure, the carbon
tends to ~ill in all o~ the available corners and
5 ~surEaces o~ ~he obturator 10 and tlle form 12 until a
smoo~ )rofile is achieved. The carnon also tends to
block the drille(3 oriEice 1~ in the injector ~ody 16 an(3
fill UL~ to the seat 18. In severe cases, carbon buil-3s
up on the ~orm :L2 and tlle obturator lO to such an extent
10 that it interferes with the spray pattern of the fuel
iSSUill9 from around the perimeter oE orifice 14. Such
carbon build up or coking oEten results in such
undesirable consequences as delayed fuel injection,
increased rate of uel injection, increased rate of
lS combustion chamber pressure rise, and increased engine
noise, and can also result in an excessive increase in
emission from the engine of unburned hydrocarbons.
~ ll)ile low fuel cetane number is believed to be a
major contributing factor to the coking prohlem, it is
20 not the only relevant factor. Thermal and oxidative
stability (lacquering tendencies), fuel aromaticity, and
such Euel characteristics as viscosity, surface tension
and relative density have also heen indicated to play a
role in the coking problem.
~n important contributioll to the art would be a
fuel composition which has enhanced resistance to coking
1~3458 3
tendencies when employed in the operation of indirect
injection diesel engines.
In accordance with one of its embodiments, this
invention provides distillate fuel for indirect injection
compression ignition engines containing at least the
combination of (a) organic nitrate ignition accelerator, and
(b) hydrocarbyl-substituted succinimide or succinamide, or
the combination of (a) organic nitrate ignition accelerator,
(c) hydrocarbyl amine having from 3 to 60 carbons and from 1
to 10 nitrogens and (d) N,N'-disalicylidene-1,2-
diaminopropane, or the combination of (b) hydrocarbyl-
substituted succinimide or succinamide, (c) hydrocarbyl amine
having from 3 to 60 carbons and from 1 to 10 nitrogens and
(d) N,N'-disalicylidene-1,2-diaminopropane, said combinations
being separately present in an amount sufficient to minimize
coking, especially throttling nozzle coking, in the
prechambers or swirl chambers of indirect injection
compression ignition engines operated on such fuel.
Another embodiment of the present invention is a
distillate fuel additive fluid composition comprising (a)
oryanic nitrate ignition accelerator, and (b) hydrocarbyl-
substituted succinimide or succinamide, or (a) organic
nitrate ignition accelerator, (c) hydrocarbyl amine having
from 3 to 60 carbons and from 1 to lO nitrogens and (d) N,N'-
rn/
458~3
disalicylidene-1,2-diaminopropane or (b) hydrocarbyl-
substituted succinimide or succinamide, (c) hydrocarbyl amine
having from 3 to 60 carbons and from 1 to 10 nitrogens and
(d) N,N'-disalicylidene-1,2-diaminopropane in an amount
sufficient to minimize the coking characteristics of such
fuel, especially throttling nozzle coking, in the prechambers
or swirl chambers of indirect compression ignition engines
operated on such fuel.
Since the invention also embodies the operation of
an indirect injection compression ignition engine in a manner
which results in reduced coking, a still further embodiment
of the present invention is a method of inhibiting coking,
especially throttling nozzle coking, in the prechambers or
swirl chambers of an indirect injection compression ignition
engine, which comprises supplying said engine with a
distillate fuel containing at least the combination of (a)
organic nitrate ignition accelerator, and (b) hydrocarbyl-
substituted succinimide or succinamide, or the combination of
(a) organic nitrate ignition accelerator, ~c) hydrocarbyl
amine having from 3 to 60 carbons and from 1 to 10 nitrogens
and (d) N,N'-disalicylidene-1,2-diaminopropane or the
combination of (b) hydrocarbyl-substituted succinimide or
succinamide, (c) hydrocarbyl amine having from 3 to 60
carbons and from 1 to 10 nitrogens and (d)
rn/
58 3
~,N'-disalicyclidene-1,2-diaminopropane, said comb.ina-
t;ons being separately present in an ainount su~ficient
to millimize such coking in an engine operate~ on such
~uel.
A feature o~ this invention is that the
combillation oE additives utilized in its practice ls
capable o~ suppressing co~ing l:endencies o~ Euels used
to operate indirect injection compression ignitioll
ellgines. SUCI1 behavior was exhibited in a series oE
stan(lard engine dynamometer tests conducted as des-
cribed in Examples I, II and III hereinafter.
~ wide variety of organic nitrate ignition
accelerators, compollent (a) "nay be employed in the
fuels of this invention. Preferred nitrate esters are
the aliphatic or cycloaliphatic nitrates in which the
aliphatic or cycloaliphatic group is saturated, con-
tains up to about 12 carbons and, optionally "nay be
substituted with one or more oxygen atoms.
Typical organic nitrates that rnay be used are
20 methyl nitrate, ethyl nitrate, propyl nitrate, isopropyl
nitrate, allyl nitrate, butyl nitrate, isohutyl nitrate,
sec-butyl nitrate, tert-butyl nitrate, amyl nitrate,
isoamyl nitrate, 2-amyl nitrate, 3-amyl nitrate, hexyl
nitrate, heptyl nitrate, 2-heptyl nitrate, octyl
25 nitrate, isooctyl nitrate, 2-ethylhexyl nitrate, nonyl
nitrate, decyl nitrate, undecyl nitrate, dodecyl
.
~.
~458~
ni~rate, cyclopentyl nitrate, cyclollexyl nitrate, methyl-
cyclohexyl nitrate, cyclododecyl nitrate, 2-ethoxyethyl
nitrate, 2-(2-ethoxy-ethoxy)ethyl nitrate, tetra-
hydro~uranyl nitrate, and the like. Mixtures of such
Inaterials Inay also be used. The preferred i~nitioll
accelerator Eor use in t~le ~uels oE this invention is a
Inixture oE octyl nitrates available as an article oE
comlnerce ~roln Ethyl Corporation under the trade mark
DII-3 ignition improver.
~rhe hydrocarbyl-substituted succinimides,
colnponellt ~b) of the fuels of this invention, are well
knowll. They are readily made ~y ~irst reacting an
oleEillically unsaturated llydrocarbon of the desired
molecular weight with maleic anllydride to form a
hydrocarbyl-substituted succinic anhydride. Reaction
telnpcratures oE 100-250C are used. ~ith higher boilinc~
oleEinically-unsaturated hydrocarbons, ~ood results are
obtailled at 200-250C This reaction can be promoted by
the addition oE chlorine. Typical oleEins include
cracked wax oleEins, linear alpha olefins, branched
chaill alplla oleEins, polymers and copolymers of lower
oleCills. These include polymers oE ethylene, pro-
pylelle, isobutylene, l-hexelle, l-decene and the like.
UseEul copolylners are ethylene-propylene copolymers,
ethyleile-isobutylene copolymers, propylene-isobutylene
copolylners, ethylelle-l-decene copolylnees and the like.
`
~4~8 3
llydrocarbyl substituel1ts have also been made ~ror
olel~in terpol;~mers. Very use~ul products have been made
f etl1ylene-C3 12 alpl1a olel~in - C5~12
col1ju(Jclted diene terpolylners; SllCil as ethylel1e--
5 propyl ne-l,4~11e~adiene terpolymer; etl1ylel1e-propylel1e-
],5--cyclooctadiene terpol-ymer; ethylene-propylene--
norl~orm1ene terpolymers and the like.
Of the foregoin-3, by Lar the most useEul hydro-
carbyl substituents are derived Erom butene polymers,
lO especially polymers of isobutylene.
The molecular weight of the hydrocarbyl sub-
stituent can vary over a wide range. It is desirable
that the liydrocarbyl group have a moleclllar weig11t of at
least 500. Although there is no critical upper limit, a
15 preerred range is 500-sno,ooo number average molecular
weig11t. The more preferred average molecular weight is
700-5,000 and most preEerably 900-3,000.
Ilydrocarbyl-substituted succinimides and
succinalnides are made by reaction of the desired
20 hydrocarbyl-substituted succinic an11ydride with an amine
havin~ at least one reactive hydrogen atom bonded to an
amine nitrogen atom. E:~amples oE these are methyl
anine, dimethyl arnine, n-butyl arnine, di-(n-dodecyl)
alnine, N-(aminoethyl) piperidine, piperazine, ~1-(3-amino-
25 L)ro~yl) piperazine, and the lilce.
~L~f3458~3
Preferably, the amine has at least one reactivepriilary aTnine group capa~le o~ reacting to ~orm ~he
preerred succinimides. ~xalnples oE such primary amines
are rl-octyl amine, N,N-dimetll-yl-1,3-propane diamine,
I~-(3-~aminopropyl) piperazine, 1,6-hexane diamine, alld
the ~ike.
Ilydroxyalkyl amines can also be use-3 to make the
suc_iniinide-succinaJnide compol-ents of the invention
~hicll contain some ester grouus. These amines include
10 ethallo]. amine, diethanol amine, 2-hydroxypropyl amine,
~l-hydroxyethyl ethylenediamine and the like. Such
hydroxyalkyl a-nines can be rnade by reacting a lower
alkylene oxide, such as ethylene oxide, prol~ylene oxi-3e
or butylene oxide with a:nmonia or a primary or secondary
alnine such as ethylene diamine, dethylene triamine,
tri(~ llylene tetramine, tetraetllylellepentamine an~ the
]ike.
A more preferred class oE primary amines used to
make tlle succinimide, succinamide or mixtures thereoE
are the polyalkylene amines. These are polyamines and
mixl:ures of polyamines whicll llave the general formula
H2N-~ R - N~ nH
458~3
wl~ereill ~ is a divalent aliphatic hydrocarbon group
having 2-4 carbon atoms an(~ n is an integer from 1-10
inclu~ g mixtures of sucll polyalkylene amines.
In a highly preferred embodimellt, the poly-
alkylene a,-nine is a polyethyleneamine containillg 2-6
etllylelleamine Ullits. T},ese are eepresented by tlle above
~oLmula in whicll R is tlle group -CH2Cl~2- and n has a
value of 2-6.
The alnine used to make the succinimide,
~;uccinaJnide or mixture thereof need not be all amine.
mono or poly-hydroxyalcohol may be included in the
reaction. Such alcohols can be reacted concurrently
with the amine or tlle two alcohol and amine may be
reacted sequentially. UseEul alcohols are methallol,
ethallol, n-dodecanol~ 2-ethyl hexanol, ethylene glycol,
propylene glycol, diethylene glycol, 2-ethoxy ethanol,
trilnethylol propane, pentaerythritol, dipentaerytllritol
arld the like.
Use~ul amine-alcohol products are described in
U.S. 3,184,474; U.S. 3,576,7~3; U.S. 3,632,511; U.S.
3,804,763; U.S. 3,836,471; U.S. 3,936,480; U.S.
3,9~ 00; U.S. 3,950,341; U.S. 3,957,854; U.S.
3,957,855; U.S. 3,991,098; U.S. 4,071,548 and U.S.
4,173,5~0.
The reaction between the hydrocarbyl-substituted
SUCCilliC anllydride and the amine can be carried out by
~ 458~3
-- 10 --
mixil~g the components and heating the mixture to a
temperatllre l~igh enougl~ to cause a reaction to occur but
not so higll as to cause 3ecolnposition o~ the reactarlts
or products or the anhydride may be heated to reaction
ter;ll?irature and tlle arnine added over an extende(1
peL iod. A useful ternperature is 100-250C. Best
results are obtained by conductill~ tlle reaction at a
temperature high enough to distill out water ~ormed in
tl-e reaction.
~ preferred succininide-succinamide cornponellt is
available as an article oE commerce froln the Edwin
Cooper Company under the tr~de mark IIITEC E-6~4. This
product comprises a mixture of active ingrediellts and
solvent. Tllus, whell I~ITI~C~ E-644 is used as component
(b) in formulating the ~uels of this invelltion, the
product as received should be used at a concentration o~
at least about 40 YTB (pounds per thousarld barrels)
O.11~36 grams per liter - to insure that the fillished
blend contains an adequate quantity of the foregoing
20 succinimide-succina1nide ingredient although smaller
alnounts may be successfully emoloyed.
The nitrate ignition accelerator--cornponent
(a)--should be present in an amount of at least 100 to
1000 PTB (pounds per thousan~ barrels) - 0.2859 to 2 859
grans per liter - of the base fuel. Preferably, the
~4~8~
concelltration of the ignition accelerator is 400 to 600
PTl3 (1.1~36 to 1.715~ gralns per l.iter).
It is not believed that there is anytlling
critical as regards the Inaximum arnoullt oE cornponellts (a)
and (b) used in the Euel. Tl1US, the maxilnum amount oE
tl~ese components will probably ~e governed in ally qiven
sitllatiotl by matters oE clloice and economics.
The coking-inhibiting components (a) and (b) oE
the invention can be added to the Euels by any means
k~lown in the art Eor incorpoeatillg small quantities of
additi.ves into distillate Euels. Components ta) and ~b)
can be added separately or they can be combined and
added together. It is convelliellt to utilize additive
fluid Inixtures whicll consist of organic nitrate ignition
accelerator and hydrocarbyl-substituted succinimide-
succinamide agents. These additive fluid mixtures are
added to distillate Euels. In other words, part oE the
present invention are coking inllibiting fluids whicl
comprise organic nitrate ignition accelerator an~
llyc3rocarbyl-substituted succinirnide-succinaJnide.
U.se oE such fluids in addition to resulting in
great convenience in storage, handlin~, teansportation,
blellding with fuels, and so Eorth, also are potent
concelltrates which serve the Eunction oE inhibiting or
min.irni~ing the coking characteris~ics o.E compres.sion
4S~'3
i911i~iOIl distillate fuels used to operate inclirect
compressioll ignition engines.
In these Eluid compositions, the amount of com-
pOllell~S (a) and (b) can vary widel~. In general, the
fluid compositions contain 5 to 95~ by weigllt of the
organic nitrate igllitioll accelerator componellt and 5 to
95~ by weight oE the hydrocarbyl-substitute(i
succini,nide-succinamide compollellt. Typically, Erom .01
by weight up to 1.0% by weight oE the combillation will
be suEficiellt to provide good coking-inhibiting proper-
ties to the distillate fuel, A preEerred distillate
fuel composition contains rom 0.1 to 0.5~ '~y weigllt of
the combination containing from 25~ to 95% by weight of
the organic nitrate ignition accelerator and from 75~ to
5% by weigllt of the hydrocarbyl-substituted succinimide-
succinamide component
The additive Eluids, as well as the distillate
fuel compositions of tlle presellt inventioll may also
contain other additives such as, corrosion inhibitors,
antioxidants, metal deactivators, detergents, cold ~low
improvers, inert solvents or diluents, an~l the like.
Accordingly, a more preferred distillate fuel
composition includes a hydrocarbyl amine in combinatio
witll l;he present additives.
458~
~ hile a variety of hydrocarbyl arnines rnay be used
in tlle ~uel compositiolls oE this invention, a primary
aliL)Ilatic amine, the aliphatic grouu of whicil is
tertiary, e.g., an amine of t}le Eorlnula:
i~-NI~ 2
whereill R is one or a mixtllre of tertiary aliphatic
grollps containing 8 to 18 or more (preferably 12-16)
carboll atorns is preferred. Most preferably, these
tertiary aliphatic groups are tertiary alkyl groups. It
10 is also preEerred that hydrocarbyl amine component (c)
include in addition to the above-depicted amine one or
more llydrocarbyl amines difering therefrom.
U.S. Pat. ~o. 3,9n~,2l5 gives a description oE
the various hydrocarbyl amines having from 3 to 60
carbolls and ~rorn 1 to 10 nitroyells whicll may be employed
in t!le fuels of this invention. A few additional
exam~les of desirable amines include 2,6-di-tert-
butyl-~-dilnethylamino-p-cresol, ~-cyclohexyl-N,~l-
dimethylamine, and N-alkyl,N,N-dimethylamilles in which
20 the alkyl group is one or a combillation of alkyl groups
preEerably ~laving 8 to 18 or more carbon atoms.
~ particularly preEerred llydrocarbyl arnine is
available commercially ~rom the Rohrn and llaas ~ompany
d(r the trade mark Primene 81R. The Primene 81R*is
* tra~le mark
s~
- 14 -
believed to be a mixture oE primary aliphatic amines inWjliCIl the aliphatic groups are predomillantly C12 and
Cl~ tertiary alkyl groups.
~rhe ~uels oE this invention should contain at
least 1.5 to ~0 PTs (0.00429 to 0.1143 gralns/litec oE
col.l?ollent (c), the hydrocarbyl amine.
~ ccordillyly, another embodiment oE t:he present
invention is distillate Euel for indirect injection
com,~ression igllition engines containillg at least the
combination oE (a) organic nitrate ignition accelerator,
(b) hydrocarbyl-substituted succinimide, and (c)
hyd[ocarbyl amine, said combinatioll being present in an
amount suEEiciellt to rninimize coking, especially
thro~tling nozzle coking in the prechambe~s or swirl
chamb~rs in indirect injectioll compression ignition
engilles operated on such Euel.
Also included as a urther embodirnent o~ the
illvention is a distillate uel additive composition
comprising (a) or~anic nitrate i~nition accelerator, (b)
llydrocarbyl-substituted succinimide an-3 (c) hydrocarbyl
amine in an arnollnt suEficient to mini.mize the coking
characteristics of such Euel, especially throttling
nozzle coking in the prechambers or swirl chambers in
in(lirect injection cornpression ignition en.3ines operated
~5 on .such Euel.
1~345~3'3
In gelleral, these additive fuel compositions will
contain as mllch as sn~ by weight of the combination oE
orgallic nitrate ignitioll accelerator and llydrocarbyl-
sul~stituted succinimide and up to 50~ o the hydrocarbyl
alnine or other additives wllen they are present.
In a still ~urtiler elnbo(3i.ment of the invention
there is provided a method oE inhibiting cokiny,
especially throttling nozzle coking in the prechambers
or swirl chalnbers oE an indirect injection compression
10 igllition engine which comprises supplyin~ said engine
Wit]l a distillate fuel containing at least the com-
bination of (a) organic nitrate ignition acc~lerator,
(b) hydrocarbyl-substituted succinimide alld (c)
hydrocarbyl amine, said co!nbination being present in an
amoullt suEicient to Ininirnize such coking in an engine
operated on such fuel.
~ nother additive whicll can be used to advantage
in the present inVention i5 a Inetal deactivator.
E~amples oE these are salicylidene-o-aminophenol,
20 disalicylidene ethylenediamine and disalicylidene
propylenediamine. ~ particularly preferred rnetal
deactivator is ~,N'-disalicylidene-1,2-diaminopropalle
(80 weigllt percent active in 20 ~eight percent toluene
solvellt) which is available as an article oE colnrnerce
~rom Ethyl Corporation under the trade mark `'Etllyl" MDA.
5~3~
- 16 -
The Euels of this invel-tion should eontain at
lea.st 0.2 to 5 pTs (0.00572 to 0.012 grams per liter) o~
compollent td), the metal deactivator, pre~erably N,N'-
disc~licylidene-1~2-dialninopropalle.
~ccordingly, another embodiment oE the present
invelltion i.s distillate fuel for ;.ndirect injection
compressioll ignitioll enc3ines containing at least the
combination oE (a) organie nitrate ignition aeeelerator,
(b) hydroeaLbyl-substituted sueeinimide, (e) hydroearbyl
10 a-nill~, and (d) N,N'-disalicylidene-1,2-diamillopropane,
said combination being present in an amount suf~ieient
to minimize coking, expeeially throttling nozzle eoking
in the preehambers or swirl ehambers in indireet
injection eompression ignition engilles operated on sueh
15 ~Uel.
~ lso ineluded as a Eurther embodiment oE the
invention is a distillate Euel additive eo~nposition
comprising (a) organie nitrate ignition aeeelerator, (b)
hydroearbyl-substituted sueeinimide, (e) hydroearbyl
20 amill~, and (d) N,N'-disalieylidene-1,2-diaminopropane in
an al~oullt su~ieient to minimiæe the eoking eharae-
teristies oE suell Euel, espeeially throttlinc~ nozzle
eoking in the preehalnbers or swirl ehambers of indireet
injection compression ignition engines operated on sueh
25 fu~ls.
In general, these additive fuel compositions
eontain as mueh as 50~ by weight of the combina - n
. ~
~4S~
or~anLc nitrate ignition accelerator and hydrocarbyl-
sub.stituted succinimide-succinamide and up to sn~ of the
combination oE hydrocarbyl amine an-l N,N'-disalicylidene-
1,2--diaminopropane or otiher additive.s when they are
5 ~reienl:.
In a still Eurther elnbodi!nent of the invention
there is pLovide~ a met)lod o~ inllibitillg coking,
especially throttling nozzle coking in the prechambers
or swirl chambers in an indirect injectioll compression
L0 i'.JIlitiOIl enyi.ne which cornprises supp.lying said engille
with a distillate uel containin~J at least the com-
bincltioll oE ~a) organic nitrate ignition accelerator,
(b) ilydrocarbyl-substituted succinimide, (c) hydrocarbyl
amine and (d) N,~'-disalicylidene-1,2-diaminopropane,
15 said combination being presellt in an amount to minimize
such coking in an en-~ine operated on such Euel.
In another embodiment of t]liS invention, the
coking-i~ ibiting componellts (a), (c~ and (d) of the
invelltion can be added to the .Euels by any means known
in the art Eor incorporating srnall quantities oE
additives into distillate Euels. Compollents (a), (c)
and (d) can be added separately or they can be combined
and added togetller. It is convenient to utilize
addil:ive Eluid mixtures ~/llich consist oE organic nitrate
ignition accelerator, hydrocarbyl amine and metal
deactivator agents. These additive Eluid mixt:ures are
458'.~
- 18 -
adde~1 to distillate Euels. In other words, part of thc
presel1t invention are coking inl1ibitil1g Eluids which
coml)rise organic nitrate i911itiOIl accelerator,
hydrocarbyl amine having Eroln 3 to 60 carbons and Erom l
to 10 nitrogens and metal deactivator, preEerahly N,~'-
di:-;a,licylidene-l,2-~:~iaminopropalle.
In these E1uid cornpositions, the alnollnt oE
compol1ents (a), (c) and (d) can vary widely. In
gel1era1, the E1uid compositions contain 10 to 97.9~ by
10 weigllt of the organic nitrate ignition accelerator
compol1ent, 2 0 to 75~ by weight o~ the hydLocarbyl amine
and O.l to 15% by weight metal deactivator. Typically,
from 0.01~ by weight up to 1.0~ by weight of the
combil1ation of the components (a), (c) and a(d) will be
15 sufEicient to provide good coking-inhibitil1g properties
to tl1e distillate fuel. ~ preEerred distillate ~uel
composition contains Erom O.l to 0.S% by weight o~ the
com'~il1ation containil1g Erom 50 to 97.9% b~ weight oE the
orgal1ic nitrate ignitiol1 accelerator, from 2 0 to ~5~ by
20 ~lei~ht oE the hydrocarbyl amine and Erom 0.1 to 5. n~ by
weigllt of the metal deactivator component.
In another embodiment of tl-is invention, the
c~i;in-J-inhibiting components (b), (c) and (d) of the
invel1tion can be added to the Euels by any means know
25 in the art for incorporating small quantities oE
additives into di~stillate Euels. Components (b), (c)
58'3
-- 19 --
and (d) can be added separately or they can be combined
alld a(~ded tO9etiler. It is co~venient to utili~e
ad(~itive fluid mixtures wllich COllSiSt oE hydrocarbyl-
sub~tituted succinimide-succinamide agents, hydrocarbyl
5 anline and ~,N'-disalicylidene-1,2-diaminopropane. rrhese
a~itive Eluid mixtures are ad-3ed to distillate fuels.
In (~tller word.~, part of tlle present invention aee coking
inhibiting fluids which comprise hydrocarb-~l-substituted
succinimide-succinamide, hydrocarbyl amine having from 3
10 to G0 carbons alld 1 to lO nitrogens, and metal deacti-
vator, preferably N,N'-disalicylidene-1,2-diaminopropane.
In these fluid compositions, the amount of
compollellts (b), (c) and (d) can vary widely. In
general, the fluid compositions contain lO to 97.9% by
15 weight oE the hydrocarbyl-substituted succinimide-
succinamide component., 20 ~o 75% by weight of the
hydrocarbyl amine and n. l to 15% by weight metal
deactivator. Typically, Erom 0.01~ by weight up to l.0
by weight of the combination will be sufficient to
20 provide good coking-inhibiting properties to the dis-
tillate fuel. A preferred distillate fuel composition
contains from 0.1 to 0.5% by weight of the combinatio
containing ~rom 50~ to 97.9~ by weight of the hydro-
carbyl succini.mide-succinarnide componellt and from 2.0
25 to 45~ by weigllt of the hydrocarbyl amine and from n.
to 5.0~ by weight of the metal deactivator, preferably
N,~'-disalicylidene-1,2-diaminopropane.
5~3~3
- 20 -
The practice and advantages o~ t)liS invention
will become still further apparellt Erom the ~ollowillg
illu!,trative example.
EXAMPLE l
-
In order to determine the e~fect oE the fuel
comi?ositiolls of the present invelltion on the col;ing
tendellcy of diesel injectors in indirect injection
compression ignition engines, use was made of a com-
lnercial diesel engine oL~erated on a coking test cycle
develoued by Institute Francais Petrole and as prac-
ticed by Peugeot .S A. The arnount of coking together
with a quantitative indication oE the adverse conse-
quellces oE such coking \~as determined by means of (i)
in-jector air flow per~ormance, (ii) emission o~ unl~urlled
hydrocarbons, (iii) engine noise, and (iv) injector
deposit ratinc3s. The engine employed in the tests was a
19~2 Peugeot 2.3 liter, ~-cylinder, turbo-charged XD2S
diesel engine connected to a Mid~est dynamometer through
an engine clutch This enyine is equipued with Bosch
injectors positioned within prechalnbers, and is deemed
representative of the indirect injection compression
ignitioll engines widel-y used in ~utomobiles and light-
duty trucks.
The base fuel employed in these engine tests ~as
a commercially-available diesel fuel havillg a no.nillal
cetane rating oE 42. FIA analysis indicated the fuel
..
~4S8~3
-- 21 --
was composed by volume oE 31.5g aromatiçs, 3.0~ olefins
and G5.5~ sa~urates. Its distillation range (~ST~l
D-158) ~,~as as ~ollows:
Barometer 29.46 incnes oE 1l9 (0.9987 Bars)
Initial 406F - 207.78C
_aporated at F - at C
~39 226.11
450 232.22
456 235.56
463 239.4~
480 248.~9
499 259.44
521 271.67
545 285.0
572 300.0
- 503 317.22
6~1 327.22
643 339.~4
678 358.89
Final 678F 358.89
Xe c ove ry 97.5%
~e s i ~u e 2.5
I,oss None
~3458`3
- 22 -
Other inspectioll data on the base Euel were as
followc;
Kinelnsltic Viscosity, (ASTM D~945) . . . 3.50 Centi-
stokes, 40C
Pour Point (ASTI~ D - 97) .. . . . . . . .-26C
Cloud Point (~ST~ D-97) ................. 33C
Flash Point (AsrrM D-93) ................. 91~C
Stearn Jet Gum . . . . . . . . . . . . . 2.4 mg/100 )nl
~niline Point (ASTM D-611). . . . . . . 143.4F (61.89C)
10 Total Sulfur. . . . . . . . . . . . . . 0.41 wt.
Ramsbottom Carbon, ~ (ASTM D-524~ . . . 0.1460 on 10
Residuum
Gravity (ASTM ~-287). . . . . . . . . . 31. 8 API
Speci~ic Gravity @ 25C . . . . . . . . 0.86
Cetane rating . . . . . . . . . . . . . 41
A test bLend was prepared Erom this base fuel
(Fuel A). Fuel A contained a combinatioll oE (ij 506 PTB
~ 7 grams/liter) of mixed octyl nitrates (a com-
mercial product available Erom Ethyl Corporation under
20 tile tr~de mark DII-3 Igllition Improver), ( ii) 41 PT3
(0.117 gram/liter) of HITXC~ ~-G44, a product oE ~dwin
Cooper, Inc., believed to he a hydrocarbyl
succinimide-succinamide made by reacting two moles oE a
polyisobutenyl succinic anhydride (PIBSA) with one mole
o~ a polyethylene amine mixture llaving an average
composition correspondillg to tetraetllylene pentamine,
4~8 3
23 -
(iii) 14 PTB (0.04 grams/liter) o~ a hydrocarbyl amine
availahle commercially from Rohm alld Haas Company url-ler
the tracle mark Primelle 81R and (iv) 1.7 P'1'13 (O.On486
grams/liter) oE Ethyl* Metal neactivator, a product oE
Ethyl Corporation, the active ingrediellt o~ wllicll is
N,l~ disalicylidelle-1,2-dialninopropane. The ;nanu-
Eacl,urer gives the ~ollowing typical properties ~or its
HIT~C~ E-644 product:
~ppearance ~ark brown Viscous
li~luid
Nitrogen, wt. ~ 2.0
Speci~ic Gravity
at 60/60F 0.928
Viscosity at 210F, cs 3~0
(98.890C)
The Primelle 81R*i~s believed to be a mixture oE
primary aliphatic amines in ~hich the aliDhatic groups
are predominall~ly C12 and C14 tertiary alkyl yrouUs-
The manuEacturer gives the ~ollowing typical
properties for its Ethyl* metal l)eactivator:
Form Li~uid
Color Amber
Density, at 68F
g/ml 1.0672
lb/gal ~.91
~ctive ingredient, wt % 80
* trade mark
~3458'3
.
- 2~ -
Solvent vehicle (toluene), wt % 20
Llash point, open cup, F 84 (28.89C)
Fire point, F 100 (37.7~C)
Solubility
In gasoline (Typical) Saturated solulion
contains 9 '1
In water, wt. ~ 0.04
Shell Rotella T*, an SAE 30, SF/CD oil was used
as the crankcase lubricant.
Before starting each test, new Bosch ~NOSD -
1510*nozzles were installed using new copper gaskets and
flalne rin~s. The Cuel line wa~s Cluslled witll the new
test fuel composition to be tested and the Euel Cilter
bowl and Cuel return reservoir were emptied to avoid
additive carry-over Crom test-to-test.
At the start of each test, the engine was
opecated at 1000 rpm, light load ~or 15 minutes. AEter
this warm-up, the engine was subjected to the followillg
autolnatic cycle:
Event R Beam Load Minutes EGR
1 750 0 4 o~f
2 2750 12.0 6 on
3 1500 6.2 6 on
~ ~000 16.2 4; o~f
* trade Inark
~3458`3
The above 20-minute cycle was repeated 60 times and the
tes~ was completed by running the engine at idle for
allother 30 minutes. The total elapsed time was thu
20.5 llours per test.
Wllell passin~ from one event to the next event in
the above cycle, some tilne, oE course, was re~uired to
enal)le the engine to accelerate or decelerate ~rom one
speed to the next. Thus, Inore speciEically, the al~ove
cycle was proyramlned as Eollows:
lOSegmellt Seconds rpm ~eam Load
1 2 750 0
2 200 750 0
3 3* 2500 12
4 7* 2750 12
15 5 350 2750 12
6 3* 2275 6.2
7 7* 1500 6.2
8 330 1500 6.2
9 3* 3500 16.2
2010 7* 4000 16.2
11 230 ~000 16.2
12 .3* 2000 0
13 7* 750 0
14 30 750 0
25 * l~epresents two mode periods ~or acceleration or deceleration
to the next condition.
5~3 3
2 6
'Iydrocarbon exhaust emissions were measured at
the start oE each test (after the Eirst 20-minute
cycle), at the 6-hour test interval .~nd at tlle end of
tl-e test. These measurements were ma-3e at 750t 1000,
and 1400 rpm idle. Noise level rea-lings were made at a
location tllrte ~eet Eroln the en~iTle exhaust side. The
rneasurelllents were made at the start and at the en~ oE
the test ~hile operatillg at three idle speeds, viæ.,
750, 1000 and 1400 rpm.
10 AEter the test operation, the injectors were
care{ully removed from the engille so as not to disturb
tlle deposits formed thereon. Measurements were made o~
air Elow through each no~zle at diEferent pintle lifts,
and pintle deposits were rate~ using the CRC deposit
rating system.
The most significallt test results are ~iven in
Table I, in wllich air flow is expres:,ed as CC/lllill and
hydrocarbon emissiolls as ppm.
TABLE I
.~ir Flo~ Pintle Obturator llydrocarbon
@ 0.1 mm Deposits l~oise, ~B Emissions
Fuel Lift (10 = clean) EOT* INC~. EOT* Incr.
Base 36 8.0 83.8 3.0 577 406
A 38 8.6 81.4 1.9 275 143
* Va]ue at end of test; the increase (Incr.) shown is in
comparison to the vallle at start of test.
. .,
4~
- 21 -
The results prQsented in Tabl~ I show that tllere
were less coking deposits (hi~3her air flow rate and
fewer deposits), less engine noise and le.ss hydrocarbo
emissions with Fuel A, tlle Euel of the invention, .~s
comL)ared to the Base Fuel.
E~:~AMPLE :tI
~ test blend was prepared Erom the base fuel o
Example I ~Fuel B). Fuel ~ contained a coml>ination o~
(i) 50G PTB (1.447 grams per liter) of mixed octyl
10. nitrates (a cornmercial product available from Ethyl
Corporation under the trade mark DII-3 Ignition
Improver), (ii) 13.2 PTB (0.0377 grams per liter) of a
hy(3rocarbyl amine available commercially from Rohm and
Haas Company under the trade mark PriJnene 81R and (iii)
1.7 PTB (0 00486 grams per liter) of Ethyl Metal
Deactivator, a product of Ethyl Corporation, the active
ingredient of which is N,N'-disalicylidene-1,2-diamino-
propane.
The test engine was operated under the same con-
ditions as those of Example I.
The most significant test results are given inTable II, in which air flow is expressed as cc/min and
hydrocarbon emissions as ppm.
* trade mark
.
~ 45~
- 2~ ~
T~BLE II
Air Flow Pintle Obturator ~ydrocarbon
Q 0.1 mmDeposits l~oise~ Emiss-~ns
Fuel Lift (10 = clean) EOT* INCR. EOT* Ilicr.
__ . _ _ _ _
sa,e 36 8.0 ~3.~ 3.0 577 406
l3 ~9 ~.~ 81.3 2.~ 282 51
* Value at end oE test; tlle increa.se (Incr.) showll is in
comparisoll to the value at start of test.
'rhe results presented in Table II show tllat there
were less cokin-3 deposits (higller air flow rate and
~ewer 3eposits), less engirle noise and less hydrocarbon
e.nissions witll Fuel B, the fuel oE the inve-ltion, as
colnL)ared to the Base Fuel.
EXAMPLE III
A test blend was prepared ~rom the base ~uel of
Example I (Fuel C). Fuel C contained a combination o~
(i) 41 PTB (0.117 grams per liter) of HI'rEC E-644, a
product of Edwin Cooper, Xnc., helieve~ to be a hydro-
carbyl succinimide-succinarllide made by reacting two
moles of a polyisobutenyl succinic anhydride ~PIBSA)
witll one mole of a polyethylene a,nine mixture having an
average composition corresponding to tetraethylelle
pentamine, (ii) 14 PTB (0.04 grams per liter) of a
hydrocarbyl amine available commercially ~rom Rohlil and
ll~as Company under the trade mark Primene 81~, and
.
1~4~3'3
- 29 -
(iii~ 1.7 Plrl3 (0.00~8fi grams per liter) o~ Ethyl# Metal
Deactivator, a product of r~tllyl Corporation, the active
i.n-3rediellt ol~ which is N~N~-di!,alicylidelle-1~2-dialnirlo-
prvp.llle.
The test engine was operated ullde the same con-
ditions as those oE ~xarnple I. The most signi~icallt
test results are given in Tal~le III, in wllicll air ~low
is exl~ressed as cc/min and hydrocarbon emissions as ppm.
T B 1, E I I I
Air Flow Pintle Obturator Hydrocarbon
@ 0.1 mm Deposits Noise, DB Emissions
Fuel Lift (10 = clean) EOT* INCR EO'r* Incr
Base 36 8.0 83.8 3.0 577 406
C 40 8.5 83.2 3.0 513 278
* Value at end of test the increase (Incr.) showll is in
comparison to the value at start oE test.
The results presented in Table III show that
there were less coking 3eposits (higher air Elow rate
and Eewer deposits), less engine noise and less
I-ydlocarbon emissions ~ith Fuel C, the ~uel oE the
invelltion, as compared to the ~ase Fuel.
# trade mark