Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~8~
This invention relates to the preparation of
l-(alkylamino)alkyl-2-keto-1,4-diazacycloalkanes.
The increasing use of polymers in p~ace of
the more traditional types of structural materials
(e.g., wood, metals, etc.) has necessitated the
compounding of such polymers with a variety of
stabilizers in order to enhance the ability of such
polymers to withstand prolonged exposure to a variety
of degradative forces. Degradation of such
environmental sensitive polymers can be caused by
exposure to light, heat and/or air. Such degradation
is usually manifested by either a partial or total
loss of structural integrity, changes in light
transmission properties, changes in color, loss or
reduction in flexibility and/or resiliency, or any
combination of the above phenomenon. As will be
appreciated, the stabilizers which are used in
conjunction with the above polymeric materials, in
addition to providing protection against such
degradative changes, must also be compatible with the
aesthetic properties of the polymeric article and be
effective at low concentrations. The economics of
the marketplace dictate that these stabilizers be
relatively inexpensive and capable of preparation
from readily available starting materials by simple
and straightforward synthetic techniques.
The 2-keto-1,4-diazacycloalkanes have been
found to be highly effective in the stabilization of
polymeric materials against the photodegradative
forces of ultraviolet light. The efficiency of such
materials in the W stabilization of polymers is
described in U.S. Patents 4,167,512 and 4,190,571.
One particular class of 2-keto-1,4-diazacycloalkanes
~,
1~8955~
is the class of l-(alkylamino)alkyl-2-keto-1,4-
diazacycloalkanes.
It is known that such diazacycloalkanes can
be prepared by non-catalytic base-induced ketoform
5 synthesis as described in U.S. Patent 4,466,915.
~he non-catalytic base-induced ketoform
synthesis includes cyclization,
work-up and purification. The cyclization involves a
combination of an amine, a ketone or a benzaldehyde
and a haloform such as chlor~form or bromoform and a
base such as alkali metal hydroxide such as sodium
hydroxide.
Work-up of the reaction product is done by
working up the reactant by adding a solvent such as
CHC13 and water as disclosed in Example 2 of U.S. Patent
4,466,915. The water layer is extracted with the
solvent and combined with the original solvent layer
wherein the combined solvent is washed with water.
The solvent layer is then dried over anhydrous
magnesium sulfate to leave a yellow liquid which is
purified by fractional distillation.
Using work-up as above involves an expensive
step of using a water immiscible solvent such as
CHC13 from which the crude reaction product has to
be extracted. A solvent such as CHC13 is known to
be toxic and it is desirable to avoid use of such a
solvent. The solvent is used to avoid decomposition
of the product during purification. It also has been
found in the preparation of diazacycloalkanes, as the
molecular weight of the diazacycloalkanes increase so
does the boiling point of the diazacycloalkanes and
there is a tendency of the reactant product of the
cyclization to decompose during distillation unless
certain precautions were followed.
~.'
~:89ro58
-- 3 --
It is the object of the present invention to
provide an improved process for the preparation of
l-(alkylamino)alkyl-2-keto-1,4-diazacycloalkanes
without decomposition during the purification.
SUMMARY OF THE INVENTION
A method of preparing
l-(alkylamino)alkyl-2-keto-1,4-diazacycloalkane
compound has been discovered wherein a cyclized
product is prepared from a carbonyl-containing
compound which is combined with a haloform, an amine
and at least S.O moles of base to each mole of amine
is added. Thereafter, the cyclized product is worked
up without introducing water and without introducing
a water immiscible solvent whereafter the crude
product is purified to the desired
l-(alkylamino)alkyl-2-keto-1,4-diazacycloalkane
compound without decomposition of the compound
occurring during the distillation.
DETAILED DESCRIPTION
miS invention relates to the purification
of l-(alkylamino)alkyl-2-keto-1,4-diazacycloalkanes
and derivatives thereof which find utility as W
stabilizers and as intermediates in the preparation
of relatively high molecular weight stabilizers. The
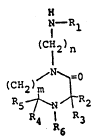
25 1- (alkylamino)alkyl-2-keto-1,4-diazacycloalkanes may
be represented by the general formula
Il
~--R
(CH2 )
I n
(CH2)m ~ (I)
R5 ~ ~ R2
4 R 3
1~89558
wherein m repreSents an integer of 1 or 2, being the
number of methylene groups forming a bridge of
variable length; when m is 1, (I) represents a
substituted l-(alkylamino)alkyl-2-piperazinone; n
represents an integer in the range from 2 to about
10; Rl representS alkyl having from 1 to about 24
carbon atoms, cycloalkyl having from 5 to 12 carbon
atoms, aralkyl having from 7 to about 20 carbon
atoms, azaalkyl having from 1 to about 24 carbon
; R2, R3, R4 and R5 independently
represent alkyl, haloalkyl, cyanoalkyl, cycloalkyl,
hydroxy-cycloalkyl, aminoalkyl and alkenyl; R6
represents hydrogen, alkyl, hydroxyalkyl, oxy,
hydroxy, haloalkyl, cyanoalkyl, aminoalkyl, alkenyl,
aralkyl and carboalkoxy.
Preferably, R2, R3, R4 and Rs
independently represent alkyl or haloalkyl having
from 1 to about 12 carbon atoms, cyanoalkyl or
aminoalkyl having from 2 to about 12 carbon atoms,
20 cycloalkyl or hydroxycycloalkyl having from 5 to
about 14 carbon atoms, and alkenyl having from 7 to
about 14 carbon atoms; R6 represents hydrogen,
hydroxy, oxy, alkyl having from 1 to about 24 carbon
atoms, hydroxyalkyl, haloalkyl, or aminoalkyl having
from 1 to about 12 carbon atoms, cyanoalkyl having
from 2 to about 12 carbon atoms and alkenyl or
aralkyl having from 7 to about 14 carbon atoms.
The more preferred substituted
l-(alkylamino)alkyl-2-keto-1,4-diazacycloalkanes are
30 those wherein R2, R3, R4, and R5 are selected
from the group consisting of alkyl having from 1 to
about 12 carbon atoms, and polymethylene having from
5 to 6 carbon atoms which are cyclizable; examples
which include methyl, ethyl, propyl, n-butyl,
35 isobutyl, n-hexyl, 2-ethylheptyl, n-decyl, and where
9~8
-- 5
the substituents are cyclizable, cyclopentyl,
rnethylcyclopentyl, cyclohexyl, methylcyclonexyl,
dimethyl cycloheptyl, piperidyl, 2,2,6,6-tetramethyl
piperidyl, and the like.
Examples of most preferred substituted
l-(alkylamino)alkyl-2-keto-l~4-diazacycloalkanes
are:
1-[2-(2-butylamino)ethyl]-3,3,5,5-tetramethyl-2-piperaz
inone;
1-[2-(isopropylamino)ethyl]-3,3,5,5-tetramethvl-2-piper
azinone;
1-~2-(1,3-dimethylbutylamino)ethyl]-3,3,5,5-tetramethyl
-2-piperazinone;
1-[2-(cyclohexylamino)ethyl]-3,3,5,5-tetramethyl-2-pipe
razinone; and
1-[2-(cyclohexylamino)propyl]-3,3,5,5-tetramethyl-2-pip
erazinone. The
l-(alkylamino)alkyl-2-keto-1,4-diazacycloalkanes are
generally prepared in a process which involves
cyclization and purification. A reactant compound
consisting of an amine nucleophilic agent, a
haloform, a ketone and a base are mixed in the
cyclization process. The amine which can be the
primary or secondary amine is chosen to provide the
desired substituents at preselected locations in the
reactant compound, and to provide upon cyclization,
the desired number of C atoms in the bridge (between
N atoms) on one side of the ring, and also to provide
the desired substituents on preselected C atoms of
this bridge.
The presence of a haloform, such as
chloroform or bromoform, takes part in the reaction
as a necessary reagent, but may also have some
function as a catalyst, though the precise mechanism
or the manner in which the haloform affects the
5~8
-- 6
reaction, is not understood. Since the haloform is
reactant, it is essential that at least an equal
molar amount (as the amine) be used if good yields of
the reaction product are to be obtained. Lesser
amounts of the haloform will yield product, but not
in an amount desired.
For a l-(alkylamino)alkyl-2-
keto-1,4-diazacycloalkane to be formed, useful
carbonyl containing groups are those which cyclize
forming a fixed 2-carbon bridge between the Nl and
N4 atoms of the diaza ring.
Preferably, ketones are cycloalkanenones,
dialkylketones and arylalkylketones are useful
carbonyl containing groups. As described in
U.S. Patent 4,466,915, aromatic monoaldehydes
appear to be effective in the synthesis.
The amines, haloforms and carbonyl-
containing compound and use thereof are described
in U.S. Patent 4,466,915.
The preferred base for inducing the reaction
according to the present invention is an alkali metal
hydroxide such as sodium hydroxide or potassium
hydroxide. The alkali metal hydroxide is used in
solid form, either in finely divided powder form, or
commercial bead form. Preferably, at least 5.0 moles
of base to mole of amine nucleophilic agent is used
and more preferably between 5.0 and 6.0 moles of base
is used. In theory, it has been determined that 4.0
moles of base would be sufficient in the cyclization,
but it has been found that 25% to about 50% excess is
required to practice the present invention.
Typically, in the cyclization portion of the
synthesis, the reactants are mixed and the base is
added last. In a more preferred embodiment, the
carbonyl containin9 group and the halogen are
combined into a mixture wherein the mixture is
generally cooled to less than 0C by suitable means
such as a circulatOry bath. Thereafter, cooled amine
is added to the mixture while the temperature is
maintained at between -5C and 10C and more
preferably between 0C and 10C. After the amine was
added, the base is added in an incremental basis. A
small amount of water can be added with the initial
addition of the base to assist the reaction. The
base is added to the system with stirring, and the
heat is removed by cooling since the reaction is
generally exothermic. Momentary increases of
temperature up to 20C may occur at the early stage
of the NaOH addition.
The reaction can proceed at the
subatmospheric or superatmospheric pressures and
pressure considerations are not critical to the
practice of the invention except as to requirements
of the system may dictate. Operation at atmospheric
is most preferred because there appears to be no
substantial advantage to be gained from operating at
higher pressures.
The cyclized product is in the form of a
slurry which requires a work-up which includes
suction filtration to separate the solid and the
liquid which contains the product. The cake is
rinsed with acetone to insure all the organic
materials are in the filtrate. No water or water
immiscible solvent is introduced in the work up. A
trace of water layer (if any present) is removed from
the filtrate whereafter the organic layer was
stripped by an evaporator. The crude product is then
purified by distillation.
1~89~S8
-- 8 --
The decomposition of the product can be
detected by regular means of a gas chromatograph (GC)
and by visual observation wherein a vapor (smoke
like) is noted in the distillation column during
purification of the crude product.
It has been found that even if at least 5.2
molar ratio of base to amine is used in the
cyclization portion of the synthesis, a decomposition
of product in the purification step occurs if water
is introduced to dissolve inorganic compounds during
the work-up. The occurrence of this is shown in
Example 1 below. Example 3 illustrates that
decomposition during purification will occur if 4.9
molar ratio of base to amine is used even when the
product is worked up and purified according to the
present invention.
As a precaution against decomposition during
distillation, it has further been found that an
alkali metal hydroxide such as sodium hydroxide can
be placed in the distillation pot.
The following examples are presented to
illustrate this invention, and are intended in the
illustrative and not limitative sense.
Example 1
Preparation of
1-[3-(cyclohexylamino)propyl]-3,3,5,5-tetramethyl-2-pip
erazinone having the following structure:
N
(CH2)3
I
H3C ~ ~ CH3
~3C l C~3
1~8~551~
In a 5-liter, 3-necked flask were placed
1833 millileters (24.9 moles) of acetone, and 230
milliliters (2.88 moles) of chloroform wherein the
two ingredients were stirred and cooled to a
temperature of about 6C wherein 562.4 grams (1.92
moles) of
Nl-[3-(cyclohexylamino)propyl~-2-methyl-l~2-propanediam
ine was added. ~hen the temperature of the pot
reached 4C, 2.5 grams of sodium hydroxide (NaOH) and
2.5 grams of H20 were added; 6 minutes later 8
grams of ~aOH and 5.5 grams of H20 were added;
whereafter the rest of the NaOH was added in from 13
gram to 27 gram amounts over the next 4 hours. A
total of 399.4 grams (9.98 moles) of NaOH was added
to the mixture. The molar ratio of base to amine was
5.2. The cyclized product reacted over the next 2
hours (after addition of the ~aOE~ was completed).
The range of the temperature of the reaction during
this time was between about -5C and 10C. Work up
of the cyclized product included addition of 1500 ml
of H20. Two layers formed from which the organic
layer was separated and then stripped using an
evaporator whereafter a GC of the crude product was
taken. The crude product was fractionally
distilled. The first indication of decomposition was
the presence of smoke in the distillation column. A
G.C. of the traction showed decomposition with the
presence of low boils which were not found in the GC
of the original crude product.
Example 2
Preparation of
1-[3-(cyclohexylamino)propyl]-3,3,5,5-tetramethyl-2-pip
erazinone as shown was prepared.
In a 2-liter, 3-necked flask were placed 668
35 milliliters (9.1 moles) of acetone and 133.6 grams
1~8955~
- 10 --
(1.12 moles) of chloroform. Then 165.8 grams (0.7
mole) of
N~-[3-(cyclohexylamino)propyl]-2-methyl-ll2-propanediam
ine was added. To this stirred and cooled mixture
was added the base of sodium hydroxide beads starting
with .5 gram NaOH beads, 1 gram H20, 2.5 grams NaOH
beads followed by 5 grams H20. Then the rest of
the sodium hydroxide was added without any H20
while maintaining the pot temperature of between 1C
to 10C over a period of about 3 hours. In all toll,
146 grams (3.64 moles) of sodium hydroxide beads was
added to the reaction. The molar ratio of base to
amine was 5.2. The cyclized prod~ct slurry was
filtered and the white filter cake was washed with
50n milliliters of acetone and then stripped. The
crude product was fractionally distilled without
decomposition and purity as measured by the G.C.'s
was from 97 to 100~.
Exam~le 3
Preparation of
1-[3-(cyclohexylamino)propyl]-3,3,5,5-tetramethyl-2-pip
erazinone as shown in Example 1 was prepared.
In one 5-liter, 3-necked flask were placed
2100 ml (28.6 moles) of acetone and 264 ml (3.3
25 moles) of chloroform. Then 690.8 grams (2.2 moles)
of
N'-[3-(cyclohexylamino)propyl]-2-methyl-1,2-propanediam
ine was added. To this stirred and cooled mixture
was added 440 grams (11 moles) of NaOH as was done in
Examples 1 and 2. The molar ratio of base to amine
was 5Ø
In a second 5-liter, 3-necked flask were
placed 2100 ml of acetone, 264 ml of chloroform and
690.8 grams of
N'-l3-(cyclohexylamino)propyl]-2-methyl-1,2-propanediam
~8~5sa
ine. To this was added 422.4 grams (10.6 moles) of
~aOH as above. The molar ratio of base to amine was
4.8.
Both processes were worked up as in Example
2 whereafter the cyclized products were combined and
vacuumed distillated. The molar ratio of the base to
amine in the combined cyclized product was 4.9.
When G.C.'s of the tractions was taken,
decomposition was found to occur even though water
was not introduced to dissolve inorganic solids
during the work-up.