Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~90~3~;
C-I-L 714
--1--
Calcium hypochlorite is used primarily in the solid
form as a bleaching and sanitizing agent, particularly for
swimming pool chlorination. The typical commerci~al product
contains 65-75~ calcium hypochlorite with the balance being
: 5 mainly sodium chloride, small quantities of cal:cium
hydroxide and moisture.
Calcium hypochlorite is produced industrially by
reacting an aqueous mixture of calcium hydroxide and ~odium
hydroxlde with chlorine accordlng to the overall equatl~on
Ca(OH32 + 2NaOH + 2C12 ~ t Ca(OCl)2 + 2NaCl + 2H20
~: :
: The crystalline calcium hypochlorite produced is
separated from the aqueous phase as a wet cake and contains : ~ .; : : a~slgnificant quantity of the aqueous phase, or mother
liquor, which ~onsists mainly of calcium hypochlorite and
sodium chloride in solution. When the crystalline calcium
hypochlorite cake is dried thi.s qodium chloride remains in
the product and, typically, constitutes between 15 and 20%
of the final product by weight.
Thé sodium chloride content in the solid final product
does not constitute all the sodium chloride produced in the
primary reaction and the sodium chloride remaining in
: solution in the mother~liquor must be removed from the
~: ~ process either by disposition of the mother liquor or by
:
:: ::: `
.. ~, ~, , ~ , "
~29~135 C-I-L 714
--2--
.
; some other ~eans. However, disposal o~ the mother liguor
is not economically attractive as the mother liquor
contains significant quantities of calcium hypochlorite in
solution.
Two of the raw materials for the calcium hypochlorite
process, sodium hydroxide and chlorine, are produced
industrially by electrolyzing an aqueous solution of sodium
chloride to produce sodium hydroxide, chlorine and
hydrogen, viz:-
2NaCl -~ 2H2O J 2NaOH ~ C12 + H2
Since sodium chloride is inevitably a by-product of
- the calcium hypochlorite process it would be advantageous
if this sodium chloride by-product were suitable for sodium
hydroxide and chlorine production. However, in this
respect, it is important that the sodium chloride contain
minimal quantities of calcium ion as calcium ion is
deleterious to the operation of the electrolytic cells used
for sodium hydroxide and chlorine production and, thus,
must be reduced to low concentrations in the feed brin~ to
these cells.
Sodium hydroxide is produced industrially in
electrolytic cells of the mercury, diaphragm or m~mbrane
type~. Mercury cells typically produce sodium hydroxide at
approximately 50% strength directly. Diaphragm cells
produce a low strength sodium hydroxide mixed with sodium
chloride and this mixture is evaporated to ~ive a 50~
caustic product and to remove most of the sodium chloride.
~embrane cells produce sodium hydroxide at up to 35%
concentration. As sodium hydroxide is a raw material for
the production of calcium hypochlorite it follows that,
using commercial strength caustic, water will also be
introduced along with the sodium hydroxide. This quantity
of water, plus that produced during the chemical reaction
, ~ , ' ,, , ;~ ' , ' :
. . ,, : , : ,
~;~9~35
C-I-L 714
--3--
to manufacture calcium hypochlorite exceeds the water that
leaves the process with the product calcium hypochlorite
(typically 3-10% by weight o~ the final product). This
water imbalance could be managed by the use of highly
concentrated sodium hydroxide (greater than 50~ strength~
to reduce the water entering the process or by evaporation
of the excess water from within the process. However, high
concentration sodium hydroxide is produced by the
evaporation of commercial strength solution under vacuum
and, thus, hi~h concentration sodium hydroxide is a more
expensive feed material than concentrations commercially
available.
It is known that calcium hypochlorite may be partially
recovered from mother liquor containing calcium
hypochlorite, sodium chloride and water by the addition of
calcium hydroxide. The addition of calcium hydroxide
- causes the precipitation of dibasic calcium hypochlorite,
according to the equation.
Ca(OCl)~ + 2Ca(OH)2 ~ Ca(OCl)2O2Ca~OH)2
The dibasic calcium hypochlorite so produced may be
separated by conventional separation techniques and
recycled to a slurry chlorinator. In the slurry
chlorinator the dibasic compound is chlorinated together
with sodium hydroxide to form calcium hypochlorite, sodium
chloride and water. All or part o~ the calcium hydroxide
required for the overall reaction may be added to the
slurry chlorinator. The total quantity of calcium
hydroxide required for the process is in excess of that
required for the production of dibasic calcium
hypochlorite, and therefore, if all the calcium hydroxide
required for the overall reaction is added to the dibasic
calcium hypochlorite reactor, the dibasic calcium
hypochlorite after separation from the mother liquor will
~ ' , .
.
' -
.
.
129~35
C-I-L 714
--4--
contain excess calcium hydroxide.
The mother liquor remaining after separation of the
dibasic calcium hypochlorite contains residual calcium
hypochlorite and sodium chloride in solution and thus a
S complete recovery of the total quantity of calcium
hypochlorite produced is not obtained.
The patent literature describes many processes for the
production of calcium hypochlorite of which the following
are relevant to the present invention.
U.S. Patent No. 1,718,284 describes a process in which
calcium hydroxide and sodium hydroxide are mixed with a
eutectic solution of calcium hypochlorite and sodium
chloride and then chlorinated. Sodium chloride cystallizes
from the metastable solution of calcium hypochlorite and is
carefully separated. Crystallization of calcium
hypochlorite from the remaining liquor is then initiated.
U.S. Patent No. 3,572,989 describes a process in which
recycled mother liquor containing calcium hypochlorite and
sodium chloride is mixed with sodium hydroxide of high
concentration and chlorinated. Sodium chloride crystals
are precipitated and removed. The remaining liquor is
mixed with calcium hydroxide and chlorinated to produce
crystalline calcium hypochlorite which is separated. The
mother liquor is recycled
U.S. Patent No. 3,767,775 decr;bes a process in which
sodium hydroxide, calcium hydroxide, water and a mother
liquor saturated with calcium hypochlorite and sodium
chloride from a later stage in the process are chlorinated.
Calcium hypochlorite and sodium chloride are co-crystaI-
lized, separated from each other and the mother liquor
recovered and recycled to the chlorinator.
U.S. Patent No. 3,~95,099 describes a process for the
manufacture of calcium h~pochlorite in which the mother
liquor containing dissolved calcium h~pochlorite and sodium
chloride is reacted with sodium hydroxide to produce a
~ . .
~29~l35
C-I-L 714
--5--
slurry of calcium hydroxide in sodium hypochlorite and
sodium chloride. The calcium hydroxide is separated and
recycled to the primary reactor.
V.S. Patent No. 3,950,499 describes a process for the
manufacture of calcium hypochlorite in which mother liquor
containing calcium hypochlorite and sodium chloride is
mixed with sodium hydroxide and chlorinated. Sodium
chloride crystals are produced and are separated from the
aqueous liquor. Calcium hydroxide and sodium hydroxide are
added to this aqueous liquor and the mixture is
chlorinated. A slurry containing sodium chloride and
calcium hypochlorite crystals is produced. The crystals of
calcium hypochlorite and sodium chloride are separated from
each other and the mother liquor recycled.
U.S. Patent No. 3,954,948 describes a process for the
manufacture of calcium hypochlorite in which calcium
hydroxide and sodium hypochlorite are chlorinated to form a
solution containing crystals of calcium hypochlorite. The
calcium hypochlorite crystals are separated and the
solution reacted with sodium hydroxide to produce a calcium
hydroxide slurry. The calcium hydro~ide is separated and
recycled. The remaining solution may be used as bleach
liquor or processed to produce solid sodium chloride and a
sodium hypochlorite solution which may be recycled.
U.S. Patent No. 4,196,184 describes an improved
version of the above process in which the calcium hydroxide
slurry is produced under closely controlled conditions
which result in the formation of some hemi-basic calcium
hypochlorite which improves the separation characteristics
of the calcium hydroxide.
U.S. Patent No. 4,258,024 describes a process in which
calcium hydroxide is added to a recycled mother liquor
containing calcium hypochlorite. Crystalline dibasic
calcium hypochlorite is produced and separated from the
mother liquor. The dibasic hypochlorite is mixed with high
.
. .
: . .
. . . .. .
129~35 C-I-L 714
--6--
strength sodium hydroxide and chlorinated to produce a
slurry containing calcium hypochlorite and sodium chloride
crystals. The crystals are separated from each other and
the mother liquor recycled.
U.S. Patent Nos. 4,328,200 and 49390,512 describe a
process in which sodium chloride and calcium hypochlorite
are co-crystallized and the crystals then separated. A
recycled aqueous solution of sodium chloride and calcium
hypochlorite is mixed with sodium hydroxide and calcium
hydroxide chlorinated in the presence of a seed bed of
sodium chloride and calcium hypochlorite crystals. The two
types of crystal are then separated in a classification
zone. The crystals are separated romm the mother liquor
which is then recycled.
~rom a review of the above prior art and from a review
of patents not described hereinabove which relates only to
improvements in specific process steps, it is clear that
processes in which sodium chloride and calcium hypochlorite
crystals are co-crystallized from a~solution are inherently
difficult to operate. Many techniques are described to
enhance the separation efficiency, particularly with regard
to the crystallizing conditions necessary to grow crystals
o~ different sizes which may be physically separated. It
is also clear that there will be some contamination of the
calcium hypochlorite crystals with sodium chloride crystals
and vice versa. Use of wash water to improve the quality
of the products is not practical as these processes operate
under a tight water balance, maintained by using high
strength caustic soda, to obtain the required product
yield.
It is/ thus, an object of this invention to provide an
improved industrial process ~or the production of calcium
hypochlorite in which the calcium hypochlorite produced is
effectively all recovered as product and the excess
co-produced sodium chloride lS recovered in crystalline
,
,:' .
:
.~
:
,
:,: ,- , ` ' ` . : :
129~5 C-I-L 714
-7-
form containing calcium impurities in concentrations
similar to those typically present in sodium chloride used
for chlorine and sodium hydroxide production.
It is a further object of the present invention to
s provide a commercial process of improved flexibility in the
production of calcium hypochlorite.
It is a further object to provide a process using a
single step chlorination.
It is a further object to provide a process which
avoids the separation of co-crystallized calcium
hypochlorite and sodium chloride and avoids the
precipitation and separation of calcium hydroxide as part
of the recovery process.
It is a further object to provide a process utilizing
the common commodity 50~ sodium hydroxide as a raw material
without further evaporation, or to use sodium hydroxide at
lower than 50% concentration.
It is a further object to provide a process in which
effectively all of the calcium and hypochlorite values
produced in the overall reaction are accounted for in the
desired solid calcium hypochlorite product.
It is a further object to provide a process which does
not produce a solution containing calcium and hypochlorite
values either as a by-product stream of lesser value, or as
a waste stream of zero or negative value due to disposal
costs.
It is a further object to provide a process which is
free of objectionable effluents, wherein the only outputs
from the process are the desired solid calcium hypochlorite
product, solid sodium chloride, and condensed water vapour.
~ccordingly, the invention provides
(a) reacting aqueous sodium hydroxide, calcium hydroxide
and dibasic calcium hypochlorite in a slurry
chlorinator with chlorine to produce crystalline
calcium hypochlorite and a primary mother liquor
.
.. .
, ', . '' i' . :,
.
,,
: ~ :
~90135
C-I-L 714
--8--
containing calcium hypochlorite;
(b) separating said crystalline calcium hypochlorite from
said primary mother liquor;
(c) adding calcium hydroxide and an evaporator mother
liquor, as hereinafter defined, to said primary
mother liquor to effect production of crystalline
dibasic calcium hypochlorite and a dibasic mother
liquor;
(d) separating said crystalline dibasic calcium
hypochlorite from said dibasic mother liquor;
(e) feeding said crystalline dibasic calcium hypochlorite
to said slurry chlorinator;
(f) evaporating said dibasic mother liquor to produce
crystallized sodium chloride and said evaporator
mother liquor;
(g) separating said crystaIlized sodium chloride from said
evaporator mother liquor; and
(h) feeding said evaporator mother liquor to said primary :~ :
mother liquor as hereinbefore de~fined in step (c).
The calcium hydroxide of use in:the slurry:chlorinator
may be added directly as such or, preferably, added with
the dibasic calcium hypochlorite as a solid mixture of
dibasic calcium hypochlorite - calcium hydroxide produced
: by .the addition of calcium hydroxide in excess to produce
said solid mixture of the formula: ~
. .
: Ca(OC12~2.2 Ca(OH)2 / Ca(OH)2 ~ :
The resultant dibasic mother liquor is subjected to
30 evaporation as hereinbefore defined. .
Accordingly, in a preferred feature the invention
; provides a process as hereinbefore defined wherein said
¢alcium hydroxide is added in step (c) in stoichiometric
excess of said calcium hypochIorite in said primary mother
: 35 liquor to effect production of a solid mixture of
:
,
.. . ..
: .. ,: . :
.. ~, ,, ~. .. ..
~90135 C-I L 714
crystalline dibasic calcium hypochlorite-calcium hydroxide
and dibasic mother liquor; and further comprising
separating said solid mixture from said dibasic mother
liquor and feeding said solid mixture to said slurry
S chlorinator and evaporating said dibasic mother liquor as
hereinbefore defined.
In an alternative preferred feature, the invention
provides a process as hereinbefore defined wherein said
calcium hydroxide is added in step ~c) in stoichiometric
excess of calcium hypochlorite present in the primary
mother liquor and the evaporator mother liquor to effect
production of said solid mixture of crystalline dibasic
calcium hypochlorite - calcium hydroxide.
It is well known that hypochlorite solutions are
subject to loss of active chlorine values, the rate of loss
being dependent on hypochlorite concentration, alkalinity,
temperature and the presence of catalysts such as cobalt
and nickel. The loss of active chlorine values can be by
decomposition to chloride and oxygen or by chemical
conversion ~o chlorate. The rate of loss of active
chlorine values can be minimized ~y selecting conditions of
high pH and low temperature in the absence of catalysts.
It is a preferred feature of the invention that the
evaporation of the dibasic mother liquor step takes place
at low hypochlorite concentration and at a pH of 10.5
A low temperature is maintained by carrying out the
evaporation under vacuum. The combination of low
temperature and short evaporator residence time leads to an
insignificant loss o~ active chlorine in the evaporation
step. It is clear that the selection of said appropriate
evaporation conditions resides in the ~kill of the art.
By the ~traight forward design adjustment of
increasin~ the water evaporation in the evaporator the use
of any required amount of water for washing crystal streams
within the process is permitted. This water is then
', :
--
~ . ., ~ . . , . - . . . :
- . , , , ., .. ,: .
. ' , ', ": ' ",
.. , , .. ,
~29~35 C-I-L 714
--10--
evaporated, condensed and recycled to the washing stages.
In this way sodium chloride crystals of high purity can be
produced. In contrast, processes using high strength
caustic and not employing evaporation cannot accommodate
the addition of wash water to the system and thus can
co-produce only relatively impure product sodium chIoride.
The separation steps may be carried out using
conventional separation equipment such as settlers,
filters, centrifuges and screens. The preparation of
crystals of a specific crystal size to permit the
separations is not necessary. The typical complex process
steps of the art re~uired to simultaneously co-crystallize
sodium chloride and calcium hypochlorite and subsequently
separate the two crystal types are thus avoided in the
present process.
The process can use sodium hydroxide at all commercial
streng~hs including the low strength (30 to 35%) sodium
hydroxide typically produced by membrane cells. The
additional water introduced with the lower strength caustic
may be evaporated in the evaporator and pure condensate
recycled to a chloralkali plant.
It will be appreciated by those knowledgeable in the -
art that the process of the invention is adaptable and
suited to batch, semi-continuous or continuous operations
and is, thus, suitable for a wide ~ange of plant
capacities.
It can further bé seen that the instant process
required only a single chlorination step. This si~plifies
the process control instrumentation and reduces capital
cost.
In order that the invention may be better understood a
preferred embodiment will now be described, by way of
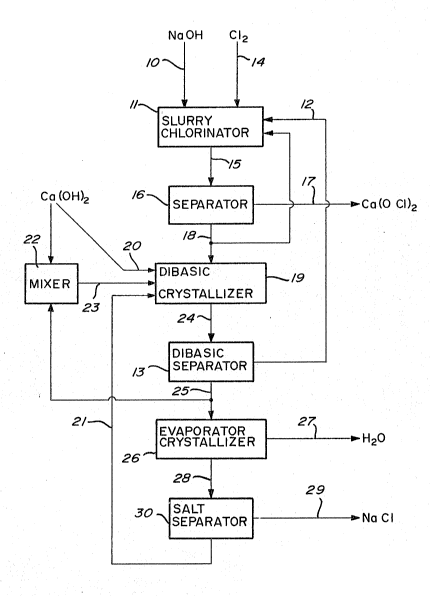
example only, with reference to the accompanying figure
which shows a simplified block diagram of a preferred
35 process according to the in~ention.
~:
:
' " ' ' : ' ,~ ' ': `
.
.
~290~5 C-I-L 714
Commercial strength sodium hydroxide, typically,
30-50% by weight NaOH in water is fed as stream 10 to a
slurry chlorinator 11 wherein it is combined with a mixture
of dibasic calcium hypochlorite-calcium hydroxide fed as
stream 12 from a dibasic separator 13 as hereinafter
described. Chlorine is passed as stream 14 into slurry
chlorinator 11 until essentially all of the calcium
hydroxide present is converted to calcium hypochlorite.
The chlorination process is exothermic and takes place at a
controlled temperature in the range of 15 - 30C with
cooling.
The slurry after chlorination has the general
composition:
Typical Range
Ca(OCl)2 22 % 20-24
NaCl 16 % 15-17 %
Ca(OH)2 0-3% 0.1-0.6%
Miscellaneous0.2% 0.0-0.5%
H2O 61.5~ 60-65
After chlorination, the slurry is fed as stream 15 to
a separator 16 wherein calcium hypochlorite as product is
` removed as stream 17 and the resultant neutral primary
mother liqu~r is transferred as skream 18 to a dibasic
crystallizer 19. Separator 16 may be any suitable -
equipment for separating the crystalline calcium
hypochlorite from the mother liquor, such as a filter or
centrifuge. The type of equipment and the method of its
operation determines the composition of the calclum
hypochlorite stream 17 but this stream typically contains:
Ca(OCl)2 40-60%
NaCl 4-15%
H2O 30-50%
- .
.
,~ , . ..
~9~35
C-I-L 714
--1~--
In a further stage of the industrial process, not
shown in the Figure, the calcium hypochlorite of stream 17
is dried, under carefully controlled conditions to avoid or
minimize product decomposition, to give a final "dry"
product containing less than 10% by weight of water,
typically 5 to 8~.
In a variation on the basic process part of the
neutral primary mother liquor stream 18 may be recycled to
slurry chlorinator 11 to control the desired slurry
strength in the slurry chlorinator 11. The slurry strength
in slurry chlorinator 11 will vary depending upon the
concéntration of the sodium hydroxide used as feed
material. The recycle of neutral primary mother liquor i~
advantageous if the chlorination is performed batchwise.
To dibasic crystallizer 19 is also added calcium
hydroxide, as stream 20, and a recycled evaporator mother
liquor, as stream 21, as hereinafter described. In dibasic
crystallizer 19, most of the calcium hypochlorite of the
primary mother liquor is crystallized as dibasic caIcium ;
hypochlorite, Ca(OCl)2.2Ca(OH)2 by the calcium hydroxide
added. The calcium hydroxide may be added directly to
dibasic crystallizer 19 or, to improve the operability by
minimizing the formation of lumps of calcium hydroxide, may
be mixed with a recycled dibasic mother liquor (as
hereinafter described) in a mixer 22 prior to addition to
the dibasic crystallizer as stream 23. The~quantity of
calcium hydroxide~introduced into the dibasic crystallizer
may be any quantity up to the total quantity required ~or
the overall reaction However, it is preferred that the
quantity added iq sufficient to maintain at least a molar
ratio of 2 parts calcium hydroxide to one part calcium
hypochlorite. If the total ~uantity of calcium hydroxide
i9 not added to the dibasic crystallizer l9 the balance of
the lime may be added to slurry chlorinator 11. Although
not advantageous, the source of calcium hydroxide may be
'
.: '
,. :
. .
129~13~ C-I-L 714
-13-
from calcium chloride and a base. Dibasic crystallizer 19
is typically an agitated mixing vessel. The temperature in
the dibasic crystallizer may range from aprroximately 15 -
80C but is preferably in the range of 40 - 50C which
5 provides a crystal slurry of good separation
characteristics.
The crystal slurry from dibasic crystallizer 19 is fed
as stream 24 to dibasic separator 13 and separated into
solids stream 12 consisting of the mixture of dibasic
calcium hypochlorite and calcium hydroxide and dibasic
mother liquor stream 25. The dibasic separator may be any
suitable separation device such as a filter or centrifuge.
The composition of the dibasic calcium hypochlorite/
calcium hydroxide mixture after separation, stream 12, will
vary depending on the equipment selected and depending on
the use of an optional water wash but will typically
analyze as shown below:
Typical ~E~
Ca(OCl)2 21% 16-30%
Ca(OH)2 30% 20-40%
NaCl 11~ 1-15%
H2O 38% 30-50% ~ -
Dibasic mother liquor, stream 25, contains
approximately 3~ dissolved calcium hypochlorite and 20-23%
dissolved sodium chloride. The solution is alkaline,
approximately pH 11. Optionally part of the liquor may be
recycled to prepare a calcium hydroxide slurry as
previously described. The balance of the liquor stream is
evaporated in an evaporator crystallizer 26 to give a
solutlon containing approximately 8 to 10% calcium
hypochlorite in solution. During the evaporation process
sodium chloride is crystallized from the solution. To
minimize decomposition of hypochlorite the evaporation
.
:
.
' ~' ' ' ' ': ' ' ';' ',
'; , ' ~ " ''.', ' :. ' `
~Z90135 C-I-L 714
--14--
process is performed under vacuum so that the operating
temperature of the evaporator is in the range of 30-60C.
The water vapour evaporated is condensed. Part of this
water may be recycled for process use, if required, with
the balance (representing the difference between the water
introduced into the overall process with the sodium
hydroxide and that removed in the dry product) discharged
as condensate, as stream 27.
A salt crystal slurry, stream 28, is withdrawn from
evaporator crystallizer 26 and separated into a salt stream
29 and a concentrated recycled dibasic mother liquor stream
21 as hereinbefore described in a salt separator 30. Salt
separator 30 may be any suitable separation device such as
a filter or centrifuge. Salt is removed from the process
as stream 29. The quality of the salt by-product may be
impoved by washing the salt with water or brine to dispIace
residual debasic mother liquor on the crystals, thereby
reducing the calcium content of the salt stream 29.
Typical analyses are:
Unwashed Washed
NaCl balance balance
Ca 0.3-0.85% 0.1-0.25%
~32 2-5% 2-5%
The concentrated dibasic mother liquor stream 25 is of
similar composition to that of neutral primary mother
liquor stream 18 and may be used in place o~ this primary
mother liquor in, for example, slurry chlorinator 11, if
advantageous.
Thus, it can be readily seen that the process provides
almost complete recovery o~ the calcium and hypochlorite
values in the process streams, thus maximizing the process
yield and minimiæing raw material requirements.
"
. ' ' ' ' :
.. :.,