Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~3~
O.Z. 0050/38184
Substituted pyrrol-1-ylphenyldihydropyridazinones,
their preparation and their use
The invention relates to novel substituted pyrrol-
1-ylphenyldihyclropyridazinones, processes for their prepa-
ration and their use for the treatment of disorders.
6-Aryldihydropyridazinones having pharmacological
effects are known (cf. for example J. Med. Chem. 17 (1974),
Z73, J. Het. Chem. 11 (1974), 755 and J. Med. Chem. 26
(1983), 800). A number of 6-phenyldihydropyridazinones
which increase myocardial contractivity and have an anti-
hypertensive action are described in Europe~n Laid-Open
Application 75,436 and J. Med. Chem. 28 (1985), 1405-
1413.
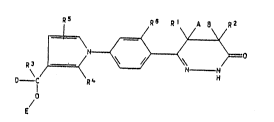
We have found that substituted pyrrol-1-ylphenyl-
dihyclropyridazinones of the formula I
7S R6 Rt A ~ R2
R3
o - I R4 H
E/
where R1 is hydrogen, methyl or hydroxymethyl, R2 is hyd-
rogen or methyl, or R1 and R2 together form a radical
-(CH2)m-, in ~hich m is an integer from 1 to 4~ R3, R4
and R5 independently of one another are each hydrogen or
C1-C6-alkyl, R6 is hydrogen or, together with R1, forms a
radical -(CH2)~-, in which n is 1, 2 or 3, or the rad;cal
-CH=CH-~ A and ~ are each hydrogen or together form a
bond, D is hydrogen, C1-C6-alkyl or phenyl, and E is
hydrogen, or D and E together form a bond, with the
proviso that, if R3 is hydrogen, one or both of the
radicals R4 and R5 are C1-C6-alkyl or D is C1-C6-alkyl
or phenyl~ have useful pharmacological properties.
The specific radica!s, independently of one
LZ93ZS~
- 2 - O.Z. 0050/38184
another have the following preferred meanings.
If A and 8 are each hydrogen, R1 is preferably hydrogen
or methyl, in particular methyl, and R2 is preferably
hydrc~gen, and R1 and RZ together form a group of the
S formula -(CH2)m-, in ~hich m is 1 or 2, in particular 1.
If A and 8 together form a bond, R1 is prefer-
ably hydrogen and R2 is preferably hydrogen or methyl.
R3 is preferably hydrogen or C1-C3-alkyl, in
particular C1-C3-alkyl, R4 is preferably hydrogen or
C1-C3-alkyl, in part`icular hydrogen, R5 is preferably
hydrogen or C1-C3-alkyl, in particular C1-C3-alkyl,
and R6 is preferably hydrogen. If R6 together with R1
forms a radical -tCH2)n- or -CH=CH-, n is preferably
1 or 2. In particular, R6 and R1 together form the
radical -(CH2)z- or -CH=CH-. A and B are each ore-
ferably hydrogen.
D and E together preferably form a bond. Where D
is hydrogen, C1-C6-alkyl or phenyl, it is preferably
C1-C3-alkyl or phenyl, in particular C1-C3-alkyl.
Z0 The novel compounds of the formula I, where D
and E together form a bond, are prepared by a process in
which a p-aminophenyldihydropyr;daz;nane of the formula
II R6 R ~ A 3 R2
H ~= I I
N ~ N
where R1, R2, R6, A and 3 have the mean;ngs described
for formula I, is reacted with a tetrahydrofuran of the
~formula III RS R7
,~
l~ 5 \
:1 1 0
13 2 /
R3 ~ III
i R~ R7
where R3, R4 and RS have the meanings stated in formula
~ ~33Z5~
- 3 - O.Z. 0050/38184
I, RS is bonded to ring atom 4 or 5 of the tetrahydro-
furan ring, and R7 ;s C1-C4-alkoxy, C1-C4-alkanoyloxy,
chlorine or bromine, and, if required, the compound thus
obtained ;s hydrogenated at the C=0 group or reacted with
an organometallic compound F-Met, where F is C1-C6-alkyl
or phenyl and Met is an alkali metal atom or a radical
MgX, where X is chlorine, bromine or iodine.
R7 is preferably C1-C4-alkoxyu
Where R3 and R4 are not two identical radicals
(Ra and Rb), the isomeric products I a and I b may be
formed
R5 R~ Rt A a R2
~ ~ ~ I a
;C ~ b ~ - N
Il
o
R5 R5 Rl A ~ R2 I b
b ~ ~ o
a \~ N
li R
where R1, R2, R5, R6, A and ~ have the meanings sta15 ted for formula I.
The novel compounds of the formula I where D and
E together form a bond are prepared under ac;dic condi-
tions, either in a lower alkanecarboxylic acid, such as
acetic acid, or in an organic solvent with the addition
of an acid, such an inorganic or organic carboxylic acid
or a sulfonic acid. The preparation may also be carried
out in the presence of an acidic ion exchanger. The acid
is used in an amoun~ of from 0.01 to 300, preferably from
0.1 to 20, mol X. The reaction can be carried out under
atmospheric or superatmospheric pressure at from room
temperature to the reflux temperature of the solvent used.
Temperatures of from 20 to 160C, preferably from 6û
Z~3;~:5~
- 4 - O.Z. OO50t38184
to to 120C, are usually employed. Suitable organic
solvents are aromatic hydrocarbons, such as benzene, tolu-
ene, ethylbenzene, dichlorobenzene, o-, m- and p-xylene
and methylnaphthalene, aliphatic or cycloaliphatic hydro-
carbons, such as naphtha, heptane or cyclohexane, ethers,such as diethyl ether or tetrahydrofuran, amides, such as
N,N-dimethylformamide, N,N-dimethylacetamide or N-methyl-
pyrrolidone, and mixtures of these solvents.
The preparation is preferably carried out in a
lower alkanecarboxylic acid, in particular in acetic acid,
at from 60 to 120C, in particular from 70 to 100C.
Compounds of the formula I where D and E are each
hydrogen are obtained by reducing the compounds of the
formula I where D and E together form a bond. This re-
duction can be carried out, for example, using hydrogenand a metal catalyst, such as a platinum~ palladium,
nickel or cobalt catalyst, by using an organometallic
hydride, such as sod;um borohydr;de, or lithim aluminum
hydride, ;n a solvent which is ;nert under the reaction
conditions.
The reaction of the keto compounds ~I, where D
; and E together form a ~ond) with an organometallic com-
pound can be carr;ed out by a convent;onal method for
Grignard reactions~ Particularly suitable alkal; metals
for the rad;cal F are l;th;um and sod;um.
The ;nvent;on embraces all enantiomeric, diastere-
omeric and tautomeric forms of compounds of the formula I.
The start;ng mater;als of the formulae ~I and III
are known. rhose wh;ch are unknown can be synthesized
by methods similar to those known from the Literature
(for the preparation of comp~unds of the formula III, cf.
Z. Chemie 13 (1973), 290-291 and Collect. Czech. Chem.
Commun. 45 (1980~, 888-891).
The compounds accord;ng to the invention has use-
ful pharmacologicaL effects. In particular, they seLect-
ively inhibit the phosphodiesterase III, which is cAMP-
specific and has a high affinity (Low Km) for cAMP (cf.
~Z~32S~
.
- 5 - O.Z. 0050t38184
review on phosphodiesterase inhibitors: J. Med. Chem. Z8
(1985), $37-545)-
The compounds according to the invention inhibitplatelet aggregation and have positive inotropic, vaso-
dilatory and coronary dilatory actions. They also reduceblood pressure, inhibit the secre~ion of gastric acid and
have cytoprotective effects on the gastric mucous mem-
brane. They are therefore useful as antihypertensives
and for the treatment of thrombotic disorders, cardiac
insufficiency and disorders accompanied by pathological
increase in the secretion of gastric acid, such as gastric
and duodenal ulcers~
The novel compounds can be administerea orally or
parenterally (subcutaneously, intravenously, intramuscu-
larly or intraperitoneally) in a conventional manner.
' The dose depends on the age, condition and weightof the patient and on the route of administration. As a
rule, the daily dose of active compound per patient is
about 0.1-1a, preferably û.S-5, mg in the case of paren-
teral administration and 1-1ûO, preferably 5-50, mg in
the case of oral admin;stration.
The novel compounds may be employed in the con-
vent;onal solid or liquid pharmaceutical forms, such as
tablets, film tablets, capsules, granules, coated tablets,
solutions or suppositories. These are prepared in a con-
ventional manner, and to do so the active compounds can
be mixed with the conventional pharmaceutical auxiliaries,
such as tablet binders, fillers, preservatives, tablet
disintegrators, flow regulators, plasticizers, wetting
agents, dispersants, emulsifiers, solvents, retarding
agents and/or antioxidants (cf. H. Sucker et al: Pharma-
zeutische Technologie, Thieme Verlag,'Stuttgart 1978).
The formulations thus obtained normally contain from 0.1
to 99~ by weight of ~he active compoundO
The Examples ~hich follow illustrate the inven-
tion.
zg325~
- 6 - O.Z. 0050/38184
EXAMPLE 1A
6-~4-(4-Acetyl-2-methylpyrrol-1-yl)-phenyl]-4,5-dihydro-
pyridazin-3-one
Compound I, where R1, R2r R4, R6, A and B are each H,
R3 is CH3, R5 is 5-CH3 and D and E together form a
bond~
A solution of 2.8 9 ~15 millimoles) of 6-(4-amino-
phenyl)-4,5-dihydropyridazinone and 3.1 9 (16.5 milli-
moles) of 2,5-dimethyl-2,5-dimethoxytetrahydrofuran-3-
carbaldehyde in 30 ml of acetic acid ~as stirred forZ hours at 80C. Thereafter, the reaction solution was
poured into saturated potassium carbonate solution and
extracted se~eral times with methylene chloride. The
organic phase was dried over Na2S04, the solvent was
stripped off in a rotary evaporator and the crude product
was then recryitallized from dimethylformamide/water.
3.4 9 of 6-~4-(4-acetyl-2-methylpyrrol-1-yl)-phenyl]-
4,5-dihydropyridazinone of melting point 188-189C were
abtained.
EXAMPLES 1B to 1H
The compounds of Examples 1B to 1H were prepared
sim;larly to Example lA (compounds of the formula I, where
R3 is CH3, R4 is H, R5 ;s 5-CH3 and D and E together form
a bond):
25 Example R6 R1 R2 A B m.p. C
13 H H CH3 H H 159-160
1C H CH3 H H H 202-203
1D H -CH2- H H 230-231
1E H -CH2-CH2 H H 208-209
1F H H H common 231-232
bond
1G -CH2-CH2 H H H 219-221
1H H H CH3 H H 159-160
EXAMPLES 1K to 1S
The compounds of Examples 1K to 1S were prepared
similarly to Example 1A (compounds of the formula I, where
3;~5~
- 7 - O.Z. 0050/38184
R3 is CH3, R4 is H, RS is 5-CH3 and D and E together form
a bond):
Example R6 R1 R2 A 9
1K H CH3 H common bond
1L H H CH3 common bond
1M H CH20H H H H
lN H -(CH2)4- H H-
-CH2- H H H
1P -CH2- H common bond
1Q -CHzCH2- H common bond 228 (decom-
position)
1R -CH=CH- H H H
1S -CH=CH- H common bond
EXAMPLE 2A
15 6-t4-(3-n-Butanoylpyrrol-1-yl)-phenyl]-S-methyl-4,5-di-
; hydropyridazin-3-one (2 A1) and 6-t4-(3-formyl-2-n-pro-
pylpyrrol-1-yl)-phenyl]-5-methyl-4,5-dihydropyr;dazin-3-
one (2 A2)
A mixture of 5.0 g (24.6 m;llimoles) of 6-tp-
aminophenyl)-S-methyl-4,5-dihydropyridazinone and 6.5 9
(3Z millimoles) of 3-n-butanoyl-2,5-dimethoxytetrahydro-
furan in 100 ml of acetic acid was stirred for Z hours at
80C. After the addition of a further 2.0 9 t10 milli-
moles) of 3-n-butanoyl-2,5-dimethoxytetrahydrofuran,
st;rring was continued for a further 2 hours at 80C.
The solution vas evaporated down in a rotary evaporator~
! after which the residue ~as neutralized with KzC03 solu-
tion and extracted ~ith CHzCl2. The organic phase was
dried over Na2S04, the solvent was stripped off and
the crude product was then chromatographed over 800 9 of
silica gel using a 4:1 me~hylene chloride/methanol mix-
ture. Recrystallization of the appropriate product-con-
taining fractions from ethyl acetate gave 0.8 9 of 6-C4-
3-formyl-2--n-propylpyrrol-1-yl)-phenyl~-5-methyl-4,5-di-
hydropyridazine-3-one of melting point 148-149C (2 A~)
and 1.~5 9 of 6-t4-(3-n-butanoylpyrrol-1-yl)-phenyl-5-
~ z~ 4
- 8 - O.Z. OOS0/38184
methyl-4,5-dihydropyridazin-3-one of melting point 162-
163C (2 A1).
EXAMPLE 2B
6-t4-t2-Ethyl-3-formylpyrrol-1-yl)-phenyl]-S-
S methyl-4,5-dihydropyridazin-3-one of melting point 192-
193C (2 ~2) and 6-~4-t3-prop;onylpyrrol-1-yl)-phenyl]-
S-methyl-4,5-dihydropyridazinone of melting point ...
: t2 B1) were obtained similarly to Example 2A by re-
acting p-aminophenyl-5-methyl-4,5-dihydropyridazin-3-one
with 3-propionyl-Z,5-dimethoxytetrahydrofuran and sub-
jecting the product to column chromatography over alumina,
: using a 4:1 methylene chloride/ethyl acetate mixture as
the mobile phase.
EXAMPLES 2C1-2F2
The compounds of Examples 2C1-2F2 can be ob-
tained similarly to Examples 2A and 28, by reacting amino-
: phenyldihydropyridazinones of the formula II with 3-
alkanoyl-2,5-dimethoxytetrahydrofurans of the formula III
tcompounds I, where D and E together form a bond, and RS
and R6 are each H):
Example Rl R2 R3 R4 A 8
.
2C1 CH3 H CH3 H H H
2C2 CH3 H H CH3 H H
2D1 H H i-C3H7 H H H
25 2D2 H H H i-C3H7 H H
ZE1 -CH2- C2H5 H H H
2E2 -CH2- H C2H5 H H
; 2F1 CH3 Hn-C6-H13 H H H
2F2 CH3 HH n-C6H13 H -H
~30 EXAMPLE 3
6-C4-t?-Methyl-4-formylpyrrol-1-yl)-phenyl]-5-
methyl-4,5-dihydropyridazinone can be obtained similarly
to Example 1A, by reacting 2,5-dimethoxy-4-formyl-2-
methyltetr~hydrofuran with 6-tp-aminophenyl]-S-methyl-
4,5-dihydropyridaz;n-3-one.
L293Z~
~ 9 - O.Z. 0050/38184
EXAMPLES 4 A - 4 D
The compounds of Examples 4 A - 4 D can be ob-
tained similarly to Example 1 A, by reacting 2,5-diethyl-
Z,5-dimethoxy-3-formyltetrahydrofuran with aminophenyldi-
S hydropyridazinones of the formula II (compounds of theformula I, where D and E together form a bond, R3 is C2Hs,
R4 is H, R5 is 5-C2Hs and R6 is H):
Example R1 R2 A a
.
4 A H H H H
10 4 8 CH3 H H H
4 C -CHz- H H
4 D H H common bond
EXAMPLE 5 A
6-~4-(4-(hydroxyeth-1-yl)-2-methylpyrrol-1-yl)-phenyl]-
5-methyl-4,5-dihydropyridazin-3-one
A solution ot 3.1 9 t10 millimoles) of the com-
pound from Example 1 C in 100 ml of ethanol was stirred
overnight with 1.5 9 (Z0 millimoles) of NaaH4 at room tem-
perature. The solution was evaporated down in a rotary
evaporator, after which water was added and the mixture
was extracted ~ith ethyl acetate. After drying had been
carried out over Naz~04 and the solvent stripped off
in a rotary evaporator, the crude product was recrystal-
lized from dimethylformamide. 0.8 9 of 6-~4-~4-hydroxy-
2S eth-1-yl)-2-methylpyrrol-1-yl)phenyl~-5-methyl-4,5-di-
hydropyridazin-3-one was obtained as a diastereomeric
mixture of melting point 75-78C~
EXAMPLES 5 9 - 5 H
The compounds of Examples 5 3 - 5 H can be ob-
tained similarly to Example 5 A (starting materials for5 B: 1 a; s c: 1 D; 5 D: 1 F; 5 E: 1 G; 5 F: 2 A2; 5 G:
Z A1; 5 H: 3~. (Compounds of the formula I, where D
and E are each H).
-` ~Z932S4
- 10 - 9~Z. 0050/38184
Example R3 R4 RS R6 R1 R2 A 8
S ~ CH3 H S-CH3 H H H H H
S C CH3 ~ 5-CH3 H -CH2- H H
S D CH3 H 5-CH3 H H H common
bond
5 E CH3 H 5-CH3 -CH2-CH2 H H H
5 F H n-C3H7 H H CH3 H H H
5 G n-C3H7 H H H CH3 H H H
S H H H 5-CH3 H CH3 H H H
EXAMPLE 6 A
Z2 millimoles of phenylmagnesium bromide, as a
solution In diethyl ether, were added to a solution of
2.8 9 (10 millimoles) of 6-C4-(3-formylpyrrol-1-yl)-phe-
nyl]-5-methyl-4,5-dihydropyridazinone (see German Laid-
Open Application DOS 3,425,632) in 100 ml of absolute
tetrahydrofuran at -10C, and the mixture was slowly
warmed up to room temperature. The mixture was stirred
` overnight and then poured into water, and the solution
was brought to pH S with acetic acid and extracted with
ethyl acetate. The crude product was subjected to column
20 chromatography over silica gel, using 98:2-9:1 CH2Cl2/
CH30H, to glve 6-C4-t3-t1-phenyl-1-hydroxy~ethyl)-pyr-
rol-1-yl)-phenyl]-S-methyl-4,S-dihydropyridazinone.
EXAMPLE 6 8
6-C4-t3-t1-Hydroxyhept-1-yl)-pyrrol-1-yl)-phenyl]-
5-methyl-4,5-d;hydrupyridazinone can be obtained similarly
! to Example 6 A, by reacting n-hexylmagnesium bromide with
6-C4-t3-for~ylpyrrol-1-yl)-phenyl]-5-methyl-4,5-dihydro-
pyri~dazinone. ~
EXAMPLE 6 C
6-C4-(4-t2-Hydroxyprop-2-yl)-2-methylpyrrol-1-yl)-phenyl]
S-methyl-4,5-dihydropyridazinone can be obtained simi-
larly to Example 6 A, by reacting methylmagnesium iodide
with the compound fro~ Example 1 C.
~ 35 6-C4-(3-Acetyl-4-methylpyrrol-1-yl)-phenyl]-5-
: '
-~ ~2~32~4
~ O.Z. 0050/38184
EXAMPLE 7
methyl-4,5-dihydropyr;dazinone can be obtained sim;larly
to Example 1 A, by reacting 6-l~4-aminophenyl)-5-methyl-
4,5-dihydropyridazinone with 3-formyl-2,5-dimethoxy-2,4-
dimethyltetrahydrofuran.