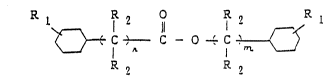Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
3~
--1--
S~NT~ETIC ~UBRICATING FL~JID
IE~ OF TH~ INVENTION
Thi~ invention relates to a syDthetic l~bricating
fluid comprising a monoester compou~d of cyclohexanol with
cyclohexanecarboxylic acid, or said ester and a branc~ed
poly-~ -olefin incorporated therein.
BACKGROUND OF THE INVENTl;ON
Traction drive power transmissions which transmit
power to a driven part through a traction drive mechanism
have attracted attention in the field of automobiles and
industrial machinery, and in recent years extensive
research and development has been conducted in this area.
The traction drive mechanism is a power transmitting
mechanism. Unlike conventional drive mechanisms it does
not use any gears. This results in a reduction in
vibration and noise as well as a smooth speed change in
high-speed rotation. An important goal in the automobile
industry is improvement in the fuel consumption of
automobiles. It has been suggested that if the traction
drive is applied to the transmission of automobiles in
order to convert the transmission to a continuous vari-
able-speed transmission the fuel consumption can be reduced
by at least 20~ compared with conventional transmission
syste~s. This is due to the fact that the drive can always
be in the optimum fuel consumption region of an engine.
Recent studies have been conductèd in the areas of
development of materials having high fatigue resistance and
theoretical analysis of traction mechanisms. Regarding the
traction fluid, the correlation of traction coefficients is
gradually being understood on a level of the molecular
structure of the components. The term "traction coef-
'
-~ 2-
~icien~l' as used hexein is defined as the ratio of the
tracti~ al ~orce which is caused by slipping at the contact
point~ between rotators which are in contact with each
oth~r in a power transmission o~ the rolling friction type
to the normal load.
The traction fluid is required to be comprised of
a lubricating oil having ~ high traction coefficient. It
has been confirmed that a traction Xluid possessing a
molecular structure having a naphthene ring exhibits a high
performance. 'ISantotrack~" manufactured by the Monsanto
Chemical Company is widely known as a commercially avail-
able traction fluid. Japanese Patent Publication ~o.
35763/1972 discloses di(cyclohexyl)alkane and dicyclohexane
as traction fluids having a naphthene ring. This patent
publication discloses that a fluid obtained by incor-
porating the above-mentioned alkane compound in perhy-
drogenated (~~methyl)styrene polymer, hydrindane compound
or the like has a high traction coefficient. Japanese
Patent Laid-Open No. 191797/1984 discloses a traction fluid
containing an ester compound having a naphthene ring. It
- discloses that an ester obtained by the hydrogenation of
the aromatic nucleus of dicyclohexyl cyclohexane-
dicarboxylate or dicyclohexyl phthalate is a preferred
traction fluid.
As mentioned above the development of continuous
variable-speed transmissions has advanced in recent years.
The higher the traction coefficient of the traction fluid
the larger the transmission force. This allows a reduction
in the size of the device which in turn results in a
reduction in emission of polluting exhaust gases.
Therefore, there is a strong demand for a fluid having a
traction coefficient which is as high as possible.
HowQv~r, the use of a conventional traction fluid which
exhibit~ th~ highest performance of all the currently
commercially available fluids in such a traction drive
device provides un~ati6factory pexformance with respect to
the traction coef~icient. Such conventional fluids are
also expensive. The traction fluid which has been proposed
in Japanese Patent Pub~ication No. 35763/1971 contains
San~otrack~ or its analogue as a compon~nt and, therefore,
is also unsatisfactory with respect to performance and
cos~.
The present inventors have made extensive and
intensive studies with a view to developing a traction
fluid which not only exhibits a high traction coefficient
but is also inexpensive~ As a result, the present inven-
tors have discovered that the incorporation of an ester
having two cyclohexyl rings or its derivative, or said
ester in combination with a branched poly-~-olefin, can
provide an economical, high-performance base oil fluid.
The present invention is based on this discovery.
SUMMARY OF THE INVENTION
Synthetic lubricating fluids comprising (i) an
ester or its derivative containing two cyclohexyl radicals
obtained from a monohydric alcohol containing a cyclohexyl
or C1 to C8 alkyl substituted cyclohexyl moiety and
cyclohexane carboxylic acid; or (ii) the ester of (i) and
from 1 t~ 70% by weight of at least one branched poly-
~-olefin or its hydrogenation product.
DETAILED DESCRIPTION OF THE INVENTION
In accordance with a first embodiment of the
present invention there is provided a synthetic lubricating
fluid comprising an ester compound or its derivative of
cyclohexanol with cyclohexanecarboxylic acid, represented
.~
-4~ 3
by the ~ollowing general formula
R 1 1 2
~} C,--C - O ' C ~
R ~ R 2
wherei~ n is an integer of O to 5 and m is an integer of O
t~ 5, provided that n + m is 1 to 10, each Rl ~ay be the
same or different and are each a hydroge,n atom or an alkyl
group having 1 to 8 carbon atoms, and ~ach R2 may be the
same or different and is a hydrogen atom or an alkyl group
having 1 to 3 carbon atoms. In a second embodiment of the
present invention there is provided a synthetic lubricating
fluid comprising the above mentioned ester compound or its
derivative of cyclohexanol with cyclohexanecarboxylic acid
and 1 to 70~ by weight of a branched poly-~-olefin.
A first object of the present invention is to
provide a synthetic lubricating fluid having excellent
properties. A second object of the present invention is to
provide a synthetic lubricating fluid which i9 not only
economical but also readily available and easily applicable
to transmissions.
The traction fluid of the present invention
comprises an ester or its derivative having two cyclohexyl
rings at both ends (hereinafter often referred to as
"component A"), or said ester and a specific amount of a
branched poly-~-olefin (hereinafter often referred to as
"component B").
The component A is an ester represented by the
above structural formula. In the ~ormula n is an integer
of O to 5 and m is an integer of O to 5, provided that n +
m is 1 to 10. n is preferably an integer of 1 to 3, while
m is preferably an integer of 1 to 3. When n + m is ~ero,
the traction coefficient of the fluid is low
_5- .~ ?~
while when the sum of n ~ m is 11 or more the viscosity of
the fluid is unfavorably high. Rl is a hydrogen atom or
an alkyl group having 1 to 8 carbon atoms and is pre~erably
a hydrogen atom or a methyl group. R2 is a hydrogen atom
or an alkyl group having 1 to 3 carbon ~toms and is
preferably a hydrogen atom. When Rl is an alkyl group
having 9 or more carbon atoms or whlQn R2 is an alkyl
group having ~ or more carbon atom~ the fluid is not only
susceptible to decomposition but also has a viscosity which
is too high.
The esters or their derivatives have a viscosity
of 5 to 50 cst, particularly preferably 10 to 30 cst at
40C and 1 to 10 cst, particularly preferably 2 to 5 cst at
100C. Examples of the derivatives of the esters include
their amination products and ether compounds~
The ester can be produced by the following
method. Specifically, the ester is produced by the
esterification reaction of a monohydric alcohol with a
cyclohexanecarboxylic acid compound. The monohydric
alcohol compound is a compound having a cyclohexyl ring and
is represented by the following structural formula:
Rl R2
~}t C j m H
R 2
wherein Rl is independently selected from hydrogen and
alkyl groups having 1 to 8 carbon atoms, R2 is indepen-
dently selected from hydrogen or an alkyl group having 1 to
3 carbon atoms, and m is an integer of 0 to 5. A parti-
cularly preferred monohydric alcohol is a compound in which
R2 is hydrogen or a methyl group, R1 is hydrogen or an
alkyl group having 1 to 4 carbon atoms, and m is an integer
of 0 to 2. Examples of such a compound include cyclo-
hexanol, methylcyclohexanol, and cyclohexylcarbinol. The
cyclohexanecarboxylic acid is a compound represented by the
-6~ 34t~
following ~tructural ~ormula~
R 1 R2
F ~c_o ~
R2
wherein R1 is independently selected fr~m hydrogen and
alkyl groups having 1 to 8 carbon atoms, R2 is
independently selected from hydrogen or an alkyl group
having 1 to 3 carbon atoms, and n is an integer of o to 5.
A particularly preferred carboxylic acid is a compound in
which R2 is hydrogen or a methyl group, Rl is hydrogen
or an alkyl group having 1 to 4 carbon atoms, and n is an
integer of 0 to 2. Examples o~ the carboxylic acid
compounds include cyclohexanecarboxylic acid,
cyclohexylacetic acid and cyclohexylpropionic acid. The
esterification reaction is conducted in the presence of an
excess amount of the alcohol using a catalyst, such as
phosphoric acid, or in the presence of an excess amount of
the acid. It is preferred that the esterification reaction
be conducted in the presence of an excess amount of the
acid. Specifically, 1 mol of the alcohol compound i5
reacted with 1.2 to 2 mol (particularly preferably 1.5 to
1.8 mol) of the acid. The reaction temperature is about
150 to 250C, preferably 170 to 230C, and the reaction
time is 10 to 40 hr, preferably 15 to 25 hr. Although the
esterification reaction may be conducted under either
elevated or reduced pressures it is preferred that the
reaction be conducted at atmospheric pressure from the
standpoint of ease of reaction operation. Under this
condition the excess acid serves as a catalyst. An
alkylbenzene such as xylene or toluene can be added in a
suitable amoun~ as a solvent. The addition of the solvent
enables the reaction temperature to be easily controlled.
As the reaction proceeds, water which has been formed
during this reaction evaporates. The reaction is
terminated when an equimolar amount, with respect to the
~7~ ~3~
alcohol, o~ the water has evaporated. Th~ excess acid is
neutralized with an aqueous alkaline solution and removed
by washinq ~ith water. Phosphoric acld, p-toluenesulfonic
acid, sul~uric acid or the like is used as the catalyst.
The most preferred catalyst is phosphoric acid because it
enhances the reaction rate and increase.~ the yield of the
ester. The reaction product is finally distilled under
reduced pre~ure to remove water and thl~ solvent, thereby
obtaining the ~ster compound of the present invention.
The ester of the present invent:ion per se exhibits
a high traction coefficient. However, it may be blended
with a second component, e.g., a poly- ~ -olefin such as
polybutene or other ester, which provides a further
improved traction fluid.
The poly-~-olefin as the second component has
either a quaternary carbon atom or a tertiary carbon atom
in its main chain and is a polymer of anOf-olefin having 3
to 5 carbon atoms or the hydrogenation product thereof.
Examples of the poly-~-olefins include polypropylene,
polybutene, polyisobutylene and polypentene and the
hydrogenation products thereof. Particularly preferred are
polybutene and polyisobutylene and the hydrogenation
products thereof. T~e polyisobutylene is represented by
thP following ~tructural formula:
C ~ 3 C ~ 3 C ~ 3
C E 3 -- C ~ C ~ ~ - C ' -~- C E '' -- C ~ C E 2
C E 3 C E~ 3
The hydrogenation product of the polyisobutylene is
represented by the following structural formula:
C E 3 C E 3 C E 3
-~ C EE 3 -- C ~ C E '' -- C -- C E " -- C E -- C ~ 3
f I ~
C E 3 C ~ 3
-8~
In the above formulae the degree of polymerization, n, is 6
to ~oo.
Although the polybutene and polyisobutylene are
commercially available, they may also be produced by
conventional polymerization methods. The hydrogenation
product thereof is produced by reacting polyisobutylene or
the like in the presence of hydrogen. The molecular weight
of the poly-~-olefin is preferably in the range of 300 to
10,000, more preferably in the range of 500 to 5,000. The
molecular weight can be adjusted by suitable methods such
as decomposition of a poly-~-olefin having a high molecular
weight and mixing of poly-~-olefins having different
molecular weights. Although an~ -olefin copolymer (OCP) is
a kind of a poly-~-olefin, it is unsuited for use as
component B in the present invention. This is because OCP
is obtained by polymerization of two or more ~-olefins and
has a structure in which these ~-olefins are irregularly
linked, as opposed to polybutene etc., which have a regular
gem-dialkyl structure.
In the present invention an ester having at least
two cyclohexyl rings and 1 to 3 ester linkages (hereinafter
referred to as "ester B") may also be used as the second
component. Examples of the ester B include a monoester,
diester or triester obtained by the esteriPication of a
cyclohexanol compound with a carboxylic acid. A
particularly preferred ester B is a monoester or diester
having 1 to 10 carbon atoms in its center and having one
cyclohexyl ring at each end.
The detailed structure and process for preparation
of the ester B are described in publishea
Japanese Patent Application.Nos. 27832/1985, 294424/1985,
and 19226/1986, having the same inventors as in the instant
application.
The ester of the present invention, e.g., a
monoester of cyclohexylacetic acid with cyclohexyl
carbinol, exhibits a traction coefficient oP 0.104 to
0.106; the component B, e.g., polybutene, exhibits a
traction coe~ficient o~ 0.075 to 0.085; and the ester B (a
monoe~ter of cyclohexanecarboxylic acid with cyclohexanol)
e~hibits a traction coefficient of 0.090 to 0.092.
Since the ester (first component) of the present
invention exhibits a high traction coe~fficient, its use
alone in a traction drive result~ in a high performance~
However, a further improved traction ~luid can be obtained
by blending with said first component 0.1 to 95% by weight,
particularly 10 ~o 70% by weight, of the second component
comprised of a poly-~-olefin or ester B. Specifically,
although the traction coef~icien~ of the second component
is equal to or lower than that of component A the gem-
dialkyl group or cyclohexyl ring of the second component
cooperates with the cyclohexyl ring o f the first component
to produce a synergistic effect with respect to improvement
in traction coefficient. Further, since the second
component is relatively inexpensive and has excellent
viscosity characteristics a traction fluid can be
economically obtained by blending the first component with
0.1 to 95~ by weight of the second component without
lowering the traction coefficient.
Various additives may also be added to the
traction fluid of the present invention depending on its
applications. Specifically, when the traction device
operates at high temperatures and large loads at least one
additive selected from among an antioxidant, a wear
inhibitor and a corrosion inhibitor may be added in an
amount of 0.01 to 5% by weight. Similarly, when a high
viscosity index is required a known ~iscosity index
improver is added in an amount of 1 to 10% by weight.
However, since the use of polyacrylate and olefin copolymer
unfavorably lowers the traction coefficient, if they are
present it is preferred that they be used in an amount of
4% by weight or less.
The term "synthetic lubricating fluid or traction
fluid" as employed in the present invention is intended to
10--
~293~9~
mean a fluid used in devices which trans~it a rotational
torque through spot contact or line contact, or used in
trans~issions having a similar struc~ure. The synthetic
lubricating fluid of the prese~t invention exhibit~ a
traction coefficient higher than those of conventionally
known fluids, i.e., exhibits a traction coefficient by 5 to
15% higher than those of the conventional fluids, although
the value varîes depending on the viscosity. Therefore,
the synthetic lubricating fluid of the present invention
can be advantageously used for relatively low power drive
transmissions including internal combustion engines of
small passenger cars, spinning machines and food producing
machines, as well as large power drive transmissions such
as industrial machines, etc.
The synthetic lubricating fluid of the present
invention is remarkably superior in traction coefficient to
conventional ~luids. The reason why the traction fluid of
the present invention exhibits a high traction coefficient
is not yet fully understood. However, basically, the
reason is believed to reside in the unique molecular
structure of the synthetic lubricating fluid of the present
invention.
The synthetic lubricating fluid ~first component)
of the present invention is an ester having two cyclohexyl
rings in its molecule. The ester linkages bring about an
interdipolar force between the molecules. It is believed
that the interdipolar force serves to bring the fluid into
a stable glassy state under high load conditions, thereby
increasing the shearing force. Further, when the ester of
the present invention is blended with the second component
this second component possesses a gem-dialkyl quaternary
carbon atom or a cyclohexyl ring. Therefore, when the
traction device is under high load conditions the
cyclohexyl rings of the first component are firmly engaged,
like gears, with the gem-dialkyl portion or cyclohexyl ring
of the second component, while when the device is released
3~9~
from the load the engagement is quickly broken, thereby
causing fluidization.
The following Examples a~e provided ~or
illustrative purposeR only and are not to be construed as
limiting the invention herein described.
E~AMPLES ~ to 19
Ester A1 according to the present invention was
synthesized by the following method. Cyclohexylacetic acid
and cyclohexylcarbinol ( in 3 :1 mole ratio) were charged
into a reactor, followed by addition of 6 g phosphoric acid
as a solvent. The reactor was then heated at 200C, and
the contents of the reactor were allowed to react under
at~ospheric pressure. The heating was stopped at a point
when the amount of water generated during the reaction was
twice by mol the amount of the cyclohexylacetic acid.
The reaction mixture was washed with an allcaline
solution to remove unreacted compounds, i.e.,
cyclohexylacetic acid and cyclohexylcarbinol, from a
mixture of the reaction product i.e., an ester of
cyclohexylacetic acid with cyclohexylcarbinol, and the
unreacted compounds, followed by vacuum distillation,
thereby isolating a pure ester Al.
In the same manner as described above esters A2
and A3 of the present invention were synthesized using
` the ~ollowing raw materials:
; A2... cyclohexylcarboxylic acid and cyclohexyl-
carbinol (n=1, m=0 in the aforementioned
structural formula)
A3... cyclohexylacetic acid and cyclohexanol
(n=0, m=1 in the aforementioned structural
formula)
The traction coefficients of the following were
determined: neat esters Al A2 and A3; and blends of
these esters with (i) hydrogenated polybutene (B~) having
an average molecular weight of 1350, (ii~ polybutene (B2)
having an average molecular weight of 900, and (iii)
,~ -12~ L~
polyb~tene ~3) having an average molecular weight of
2350 or polybutene (B4) having an average molecular
weight o~ 420. The measurement conditions ~or determining
traction coefficient are shown below.
measurement equipment: Soda-type four roller
traction testing ~achine.
test conditions: a fluid tempcrature of
20~C; a roller temperature
of 30C; a ~ean Hertzian
pressure of 1.2 GPa; a
rolling velocity of 3.6
m/s; and a percent
slipping ratio of 3.0%.
As illustrated by the data in Table 1 the
synthetic lubricating fluids of the this invention are
superior in traction performance to the conventional fluids
tested.
CO~PARATIVE EXAMPLE5 1 - 6
For comparison, the traction coefficients of a
traction fluid consisting of neat polybutene ~i.e., loO
weight percent) and a commercially available traction fluid
were measured under the same conditions as described in the
above Examples.
The results are shown in Table 1. As can be seen
from Table 1, all the comparative samples exhibited
traction coefficients 5 to 15~ lower than those of the
synthetic lubricating fluids of the present invention.
, .
-13-lZ.~13499
. . .- .
," ... - . .
~ T
~,~ . , .
__ _ __ ~ _ ,
a B V~sco~le~ ~Cst) Vl~coJlty T~ ct~on . .
~o~dln~ 40C 100C lnd~ an~
__ _ __ __ __ __ _
. ~ Al 100 _ 20.3~ 3.59 1~.7 O.lOS
2 a2 100 _ 12.78 2.83 4;2.4 O . L00
3 a3 100 _ 11.~5 2.64 4a~3 O.lOS
: ~ ~1 90 ~1 10 39.10 5.25 40.5 0.110
5 Al 80 2~ 67.93 8.5~ 94.9 0.115
6 Al 70 30 L29.2 13.18 95.4 O . L13
7 A2 90 10 22.93 4.40 100.0 0.103
~ A2 80 20 44.54 7.04 116.7 0.106
9 A2 70 30 90.09 11.27 }12.7 0.106
A2 60 40 L83.9 18.36 110.8 0.104
11 ~ 90 10 20.30 4.1Z 102.7 0.108
12 A3 80 20 39.81 6.62 120~2 0.111
13 A3 70 30 79.81 10.62 118.2 0.109
14 ~3 6C . ,40 161.2 17.15 114.5 0.105
al 80 82 20 61.4 8.41 107 0.108
16 Al 70 1 30 16a .6 1~ . 77 84 0.104
17 Al 90 83 10 50.6 8 ~0 L47 O . L10
18 Al ao l 20 1a7.~ ! ~2.~4 1 1 ~5 0.107
19 Al 35 B4 65 52.3 , 6. ,a ¦ ~6~ 7 0.097
Cau~. . ._ ........... ll .
~x. 1 _ al 100 33,000 j/co 0
.030
2 _ ~4 100 35 , ~ 73.3 0.079
3 hlqh-vLic~ity 8.6 1 2.1 ~ 5 0.086
traction bas~ oil
4 medium-viscosLty 41.0 4.3 -~1 O .096
t~action ~ase oil
S lQw-vi~cosity 69.6 5. ~ -66 0.090
traction base oil
; 6 Santotrack~ L 3.8 2.99 46 O .037
.
-14~ 3 ~
The present i~vention is directed to a synthetic
lubricating fluid containing a base oil comprised of an
ester having two cyclohexyl rings, or said ester and a
specific a~ount of a poly-O(-olefin blended therewith~ The
synthetic lubricating oil not only exhibits an extremely
high traction coefficient but is also inexpensive and has
excellent viscosity characteristics.
Therefore, the use of the traction fluid of the
present invention in a power transmissi.on, particularly a
traction drive device, leads to a remarkable increase in
shearing force under a high load. Thi enables the
reduction in si~e of the device resulting in a reduction in
cost of said device.