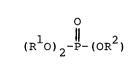Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
1336185
T 5617 FF
PHOSPHATE ESTER LUBRICANTS
The present process relates to the use of
certain phosphate ester compounds as lubricants, in
p~rticular their use in traction drives.
These lubricants can be used in a variety of
engineering applications, being of particular value
in traction drives. Traction is broadly defined as
the adhesive friction of a body on a surface on which
it moves. A traction drive is a device in which
torque is transmitted from an input element to an
output element through nominal point or line contact
typically with a rolling action by virtue of the
traction between the contacting elements. While
traction elements are commonly spoken of as being in
contact, it is generally accepted that a fluid film
is present therebetween. Almost all traction drives
r~quire fluids to remove heat, to prevent wear at the
contact surfaces and to lubricate bearings and other
moving parts associated with the drive. Thus,
ihstead of metal to metal rolling contact there is a
film of fluid introduced into the contact zone and
interposed between the metal elements. The nature of
this fluid determines to a large extent the limits in
pçrformance and the capacity of the drive. Most
traction drives are designed to operate with a
traction fluid which preferably has a coefficient of
PS13005
- 2 - 1336185
63293-3123
traction above about 0.06, a viscosity in the range of about
4-20,000 mPa.s over a temperature range of 40C to -20C and
good thermal and oxidative stability. The fluid should also be
noncorrosive to common materials of construction and have good
load-bearing and low wear-rate properties.
Mineral base oils are rather unsatisfactory lubricants
for traction drives since in general their traction (friction)
coefficient is low, which means that for any given load applied
to the gears the maximal tangential force that may be trans-
mitted by the friction wheels is low.
Accordingly, the present invention provides the use aslubricants, and especially as traction fluids, or organophosphate
esters of the general formula I
1 ll 2
IR O)2-P-(OR )
wherein Rl and R2 are independently selected from 2-methylcyclo-
hexyl and 3-methylcyclohexyl groups.
Organophosphate esters are known compounds, and there-
fore may be prepared by known procedures, such as the reaction
of phosphoryl chloride with the appropriate alcohol in the
presence of a base, such as pyridine or triethylamine, and
suitably also a solvent.
It has been found that the viscosity characteristics
of the above ester compounds are very suitable for use in e.g.
friction wheel gears (traction drives) in which application they
may be admixed with conventional grease thickeners. Such
thickeners can be of any number of materials commonly used to
` _ 3 _ 1336185
~ 63293-3123
thicken mineral oils to lubricating viscosity, including both
organic and inorganic compositions such as metallic soaps,
synthetic polymers, organosiloxanes, clays, bentonite, and
colloidal silica. Suitably, the viscosity properties of
compounds to be used in traction drives are such that the
compounds are operable between -30 and 150C.
The compounds can be used as lubricants in various
engineering applications. Since the above ester compounds show
excellent lubricating performance in traction drives, the
invent~ion in particular provides the use of these ester compounds
as traction fluids, and also the operation of a traction drive
wherein such esters form the traction fluid.
The ester compounds of the present invention can be used
se as lubricants. They can be mixed with other lubricants
such as mineral or synthetic oils, and various additives can be
added to the ester compounds, such as VI-improvers, pour point
depressants, dispersants, detergents, anti-oxidants and the
like. A mixture that can be of particular interest for traction
fluid applications is a blend with a polyolefin, in particular a
poly-alpha-olefin, especially polyisobutylene, since the
presence of the polymer can usefully enh~e the traction
coefficient of the fluid blend. The molecular weight of such
polyolefin blend components is conveniently in the range 500-
10,000, a specific example of a suitable polyisobutylene being
"Hyvis", and the proportion of polyolefin may vary from zero to
70~ by weight.
The following Examples illustrate the preparation of
compounds used in the present invention (III and IV), and of
-- 4
- 1 33 6 18~3293-3l23
comparative compounds (I, II and V), together with their
frictional and other physical properties.
Example 1 - TricyclohèXyl phosphate
A solution of cyclohexanol (751.2g, 7.5m) and pyridine
(595.0g, 7.5m) in dichloromethane (3.5L) was stirred under an
atmosphere of dry nitrogen and phosphoryl chloride (382.5g,
2.5m) was added dropwise over 1-2 hours, whilst maintaining the
temperature of the reaction mixture at between 15 and 25C
throughout the addition period. On completion of the addition,
stirring was continued at ambient temperature fora further 18
hours, and then the resultant mixture was filtered. The organic
solution was washed with water (3 x SL) and dried (over MgSO4),
and the solvent was evaporated off ln-vacuo at 30C to give a
pale-yellow viscous oil (730.3g) which slowly solidifed on
standing. The crude oil was dissolved with stirring in diethyl
ether ~750ml) and cooled with stirring to -40 to -50C in a dry
ice/acetone cooling bath. The precipitate was filtered and
dried in vacuo at 25C to give tri(cyclohexyl) phosphate (520.0g)
as an off-white solid, m.p. 48-50C.
Example II - Tri(methylcyclohexyl) phosphate
A solution of methylcyclohexanol (technical grade,
containing a mixture of isomers; 2.08 kg, 18.2m) and pyridine
(1.44 kg, 18.2m) in dichloromethane (lOL) was stirred under an
atmosphere of dry nitrogen and phosphoryl chloride (930.2g,
6.07m) added dropwise over 1.5 hours, whilst maintaining the
temperature of the stirred reaction mixture at between 15 and
25C throughout the addition period. On completion of the
addition, stirring was continued at ambient temperature for a
~ 5 ~ 1336185
63293-3123
further 18 hours, and then the resuItant mixture was filtered,
washed with water (4 x 6L) and dried (over MgSO4), and the solvent
was evaporated off in vacuo at 30C to give the crude product as
a viscous oil. The crude oil was allowed to stand at room
temperature overnight, was then filtered and unreacted methyl-
cyclohexanol and other volatile impurities were removed by
evaporation on a KDL-4 thin-film evaporator (at 85C and 0.8mmHg
(107 Pa)) to give the product as a clear viscous oil (1.75 kg)
containing a mixture of different isomers.
Examples III to V
By using the process of Example II, but substituting
respectively 2-methylcyclohexanol, 3-methylcyclohexanol and
4-meth~lcyclohexanol (in each case mixtures of CiS- and trans-
isomers) in place of the technical grade methylcyclohexanol,
there were prepared:
III - Tri-(2-methylcyclohexyl)phosphate (oil)
IV - Tri-(3-methylcyclohexyl)phosphate (oil)
V - Tri-(4-methylcyclohexyl)phosphate (oil)
Example VI - Friction coefficient measurement
All friction measurements were performed on a two-disc
machine. Hardened steel discs are fixed on the ends of two
shafts so as to make tangential contact with each other. Radial
forces may be applied to press the discs together with loads of
0-200 kgf. Each disc is driven by an electric motor. The speeds
of rotation of the two discs are different, such that there is a
slip .
Between the electric motor and the shaft carrying the
lower test specimen, a measuring device is fitted which indicates
A
-- 6 --
_ 1 336185 63293-3123
the transmitted friction torque. The measuring device is a gear
dynamo~eter with a pendulum which is swung out of its vertical
balanced position when power is transmitted, the sine of the
angle of inclination being a measure of the torque. The torque
measurement is pre-calibrated through the design and dimensions
of the instrument. The friction coefficient is defined by the
torque measured divided by the product of the radial force times
the radius of the lower disc.
Both discs used had a diameter of 50.Omm, the upper
disc having a width of 3mm, the lower one having a width of lOmm.
The top shaft speed was 606rpm, and the mean tangential (or
surface) velocity was 1.48ms . The slip employed was 9.1%.
All experiments were run at ambient temperature
(21C+2C). The friction readings are provided at loadings
equivalent to Hertzian stresses of 0.69, 0.97, 1.19 and 1.38 GPa.
The friction coefficients of the compounds are
indicated in the following Table. For the compound of Example I,
whose m.pt. is 48-50C, these coefficients were determined on a
supercooled fluid at 21(+2)C.
Example VIII - Friction coefficient measurement
The kinematic viscosity properties of the compounds are
also included in this Table.
It will be noted that the friction coefficients of the
compounds are all good, but that those of Examples III and IV are
very surprisingly superior.
r
~' ~
1 3 3 6 1 8 5 63293-3l23
~ a~ o
o r~ r o o o ,~
~1_1 I . . . .
,1 a~ O o o o
~D ~ O O ~
~1~ u7 o o o o
t~ ~ o ~
~ N _I ~1 ~ _I
O . ~
D 0 0 0 0 0
O O
O I O O~
--I 1~ O O O O
_I O
1~ '~5 N 1-1 ~ U-) 11~ a~ CD ~ CO
~--1~0 O N--~ ~ G t` tl~ 0~ O
tS~ ~ U') ~ ~ O O O _~
~3 o u~ , O O O 'r O O O O
N N N
O O U
o ~ a~
O O O O O
_ _ _ I I I
6 ~
U O
8 X ~ ~ ~, _
O U
o .
~r ~ ~ ~ ~ t~ r- t,
_ c
, O O
U
r~
a a ~ ~ ~ 6~ ~