Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
1 337285
ELECTROCHEMICAL RE-ALKALIZATION OF CONCRETE
The present invention concerns a method of re-
alkalizing carbonated zones in concrete, and the like, in
order to rehabilitate the condition of such concrete.
Reinforcement and other steel objects which are cast
in concrete, cements or calcareous materials, such as
mortar, plaster or gunite etc., are normally protected
against corrosion because of the alkaline environment in
the mass. Gradually however, the alkaline concentration
is reduced by the absorption dioxide and sulphur
trioxide. This absorption leads to a gradual
neutralization of the alkaline environment. When the pH-
value of the mass reaches 9,5 (approx.) the steel is no
longer protected and corrosion is initiated.
The most well-known neutralization reaction is
caused by the absorption of carbon dioxide, and is known
as "carbonation".
Carbonation is already a serious problem today as is
evidence by the structural damage to buildings caused by
the corrosion of reinforced steel, which is turn reduces
the cross-section of the steel, and leads to the
penetration of the concrete overlay as well as reductions
in strength.
Damage caused by carbonation easily becomes a
serious matter and is both difficult and expensive to
repair. Traditionally this type of damage is repaired by
chopping away the carbonated zone, sandblasting the
exposed steel, and then concreting or using gunite.
Sealing combined with elutriation or a filler is another
approach. The result is seldom satisfactory as far as
durability and load-carrying capability are concerned.
The purpose of the present invention is to find a
means of repairing carbonated zones in concrete etc.
which produce better results than the methods already in
existence.
. ;~, ~
1 337285
One of the characteristics of carbonation is that
the damage is usually restricted to the surface zones of
the structure, i.e., in the zone down to the first layer
of reinforcement. This is normally a thin zone in
relation to the remainder of the concrete cross-section,
which is non-carbonated and has a surplus of alkaline
matter.
Aspects of the invention are as follows:
The method of re-alkalizing a carbonated zone of a
concrete structure from an adjacent zone of alkaline
medium, wherein the carbonated zone has a pH
substantially below 12, which comprises,
(a) applying between said zones a direct current
voltage of at least six volts to effect a migration of
alkaline ions from said alkaline medium to said
carbonated zone,
(b) generally maintaining said voltage until said
carbonated zone reaches a pH of about 12, and
(c) thereafter discontinuing the application of
said voltage.
A method of re-alkalizing carbonated zones in
concrete etc., is characterized by an electric charge
being passed between one reinforcement element in an area
which has been carbonated and an electrode in an area
with an alkaline environment.
The carbonated zone is supplied with alkaline either
from the fresh interior of the concrete etc., or from an
external electrolytic medium by employing electricity.
The re-alkalization from this alkaline matter re-
establishes the corrosive protection of the steel.
1 337285
2a
The procedure is based on the following principle:
When a base electrolytic solution which contains
calcium, sodium and potassium hydroxide, for example, is
exposed to an electrical field between two electrodes, ions
will be transported between the electrodes, which will make
the area in the immediate vicinity of one of the electrodes
highly alkaline. This alkaline content remains after the
removal of the electrical field.
This procedure is executed in practice in the following
way:
1. If the concrete etc., contains a double layer of
reinforcement, where one layer is in carbonated concrete
and the other is in fresh concrete, the reinforcement in
the carbonated zone is connected to one pole on a rectifier
or battery, whilst supplied, the alkaline hydroxyl-ions
migrate to the reinforcement
1 337285
existing methods, such as pH-sensitive tracers or pH-
electrodes. When the desired pH value is reached
(usually over 12), the current can be disconnected.
2. If the concrete contains one layer of
reinforcement, or possibly a second layer which is
unaccessible, or if the distance between the two layers
is too great for the techn;que described in point 1 to
be feasible, an external electrode is located in a
suitable electrolytic medium on the surface of the
structure. This electrode can consist of wires, cords,
plates, foil or sheet metal, conducting plastics or
other conductive materials. The electrolyte can be an
aqueous solution of calcium, sodium, potassium and their salts
either in a liquid or absorbed in a porous medium such
as rock wool, cellulose, sawdust, sand, clay and the
like, or it can be strongly retarded concrete, mortar,
cement grout, lime paste etc. When a cement-based
concrete, mortar or paste is used, a strongly retarding
substance such as sucrose is added to prevent the mass
from setting, thus once the treatment is completed, this
mass can be removed by scraping or flushing.
The external electrode is connected to one pole of
the rectifier or battery, whilst the reinforcement in
the carbonated zone is connected to the other pole in
the manner described in point 1, above.
The speed of the re-alkalization process depends on
the direct voltage which is applied, which is in turn
dependent on the conductivity of the concrete and
electrolyte, the density of the electrodes, and the
distance between them. For normal building structures
this voltage should be between 6-20 V, which will ensure
that the re-alkalization is completed within a matter of
days or weeks, depending on the overall conditions and
the degree and depth of carbonation.
1 337285
The method is illustrated in the enclosed drawings,
where:
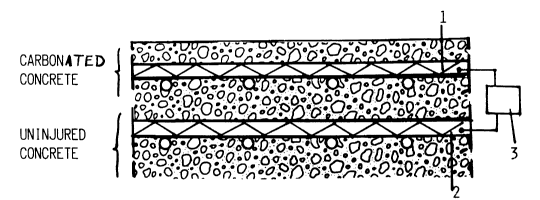
Fig. 1 shows a section through part of a concrete
structure, where electricity is applied to the
reinforcement in fresh and carbonated concrete,
respectively, whilst
Fig. 2 shows an equivalent section through a
concrete structure where electricity is supplied by
means of an external electrode in an electrolytic medium
and part of the reinforcement in carbonated concrete.
The procedure will be described in more detail with
reference the enclosed illustrations. In Fig. 1, there
is a layer of reinforcement 1 in an area with carbonated
concrete and a layer of reinforcement 2 is in fresh
concrete. The reinforcement l is connected to one pole
of a rectifier or battery 3, and the reinforcement 2
connected to the other pole. When the current is
supplied the alkaline hydroxyl-ions migrate to the
reinforcement 1 in the carbonated zone.
In Fig. 2, the reinforcement 4 is in a carbonated
zone in the concrete. A suitable electrolytic medium 5
is applied to the surface of the structure. An
electrode 6 has been located in this medium 5. The
electrode 6 is connected to one pole of the battery or
rectifier 7, whilst the reinforcement 4 in the
carbonated zone is connected to the other pole. When
the current is supplied, the alkaline hydroxyl-ions will
migrate from an external medium 5 to the reinforcement 4
in the carbonated zone.
As a rule the polarity is selected so that the
reinforcement in the carbonated concrete is connected to
the negative pole of the battery. In special cases it
could be an advantage to reverse the polarity (this is
dependent on factors such as the type of salt in the
electrolytic medium).