Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
PHOTOPOLYMERIZA~LE MIXTURE ~ND
PHOTOPOLYMERIZABLE COPYING MATERIAL CONTAINING SAME
Backqround of the Invention
The invention relates to a photopolymerizable
mixture. More particularly, the invention relates
to a mixture which comprises a polymeric binder, a
polymerizable compound having at least one terminal
olefinic double bond and having a boiling point
above 100~C at normal pressure, and a 9-arylacridine
compound as photoinitiator.
DE-C-2,027,467 discloses photopolymerizable
mixtures of the composition specified above which
contain derivatives of acridine and phenazine as
photoinitiators. Some representatives of this class
of compound, for example 9-phenylacridine, are
notable for a high photosensitivity. The preferred
representatives have the disadvantage that they tend
to migrate out of photopolymerizable coatings which
are in contact with polyethylene films into said
films and through them. As a result, the coating
becomes depleted of initiator and loses sensitivity.
2~
The initiator may also migrate out of photocured
photoresist coatings into certain treatment baths,
for example acidic ele~troplating baths and produce
a troublesome yellow coloration therein.
Summary of the Invention
Accordingly, it is an object o~ the present
invention to provide a photopolymerizable mixture
having a photosensitivity and image reproduction as
good as the preferred known mixtures, but with
photoinitiators having a lesser tendency to migrate
out of the photopolymerizable or photopolymerized
coating.
In accomplishing the foregoing objectives,
there has been provided, in accordance with one
aspect of the present invention, a
photopolymerizable mixture which comprises a
polymeric binder, a polymerizable compound having at
least one terminal olefinic double bond and having
a boiling point above 100~C at normal pressure, and
a 9-phenylacridine compound as photoinitiator,
wherein the acridine compound conforms to the
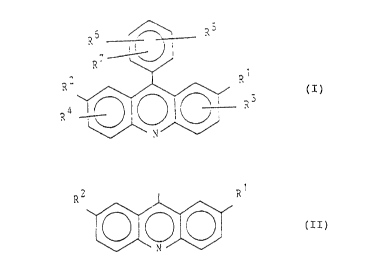
general formula I
R6 = - 2i
~ - ~ ) (I)
,,~..~1
-2-
7~L
in which
R1 denotes an optionally substituted alkyl
. or acyl group,
R , R are identical or different and denote
and R4 hydrogen or halogen atoms or optionally
substituted alkyl or acyl groups,
~5, R6 are identical or different and denote
and R7 hydrogen or halogen atoms or optionally
substituted alkyl, aryl or acyl groups,
or groups of the formula II
R~ (II)
In accordance with another aspect of the
present invention, there is provided a
photopolymerizable copying material which comprises
a coating base and a photopolymerizable layer
comprising the photopolymerizable mixture defined
above.
Other objects, features and advantages of the
present invention will become apparent to those
skilled in the art from the following detailed
description. It should be understood, however, that
the detailed description and specific examples,
while indicating preferred embodiments of the
p-esent inven~ion, are given by way of illustration
and not limitation. Many changes and modifications
within ~he scope of the present invention may be
made without departing from the spirit thereof, and
~he inventlon includes all such modifications.
Detailed Description of Preferred ~mbodiments
In the compounds of the general formula I, Rl
is an alkyl group containing preferably 1 to 10
carbon atoms, an aliphatic acyl group containing 2
to 10 carbon atoms or an aromatic acyl group
containing 7 to 15 carbon atoms.
These groups may be substituted by halogen
atoms, in particular fluorine, chlorine or bromine,
hydroxyl groups, alkoxy groups, alkoxycarbonyl
groups, alkyl groups, aryl groups, aryloxy groups,
acyl groups, acyloxy groups, primary, secondary or
tertiary amino groups, alkoxycarbonylamino groups or
alkylaminocarbonyloxy groups. In g~neral R1 has a
molecular weight of about 15 to 200, preferably of
60 to 150. R2 is a hydrogen or halogen atom or a
group having the meaning of Rl.
Preferably R2 is a group having the meaning
of R1, it being possible for R1 and R~ to be
identical or different.
; R3 and R4 have the same general meaning as
R2, but preferably at least one of these radicals is
a hydrogen atom.
Rs, R6 and R7 may have the same meaning as R3
and R4 and may additionally be aryl groups or groups
7~
of the formula II, in particular halogen atoms
(especially fluorine, chlorine or bromine) or alkyl
groups. Preferably, at least one of these radicals
is a hydrogen atom.
In general, the radicals R1 to R7 contain in
total at least S, preferably 12 to 40 carbon atoms.
The molecular weight of the compound-of the formula
I is increased by these substituents in general by
60 to 800, preferably by 100 to 700, in particular
by 200 to 600, compared with 9-phenylacridine.
Compounds in which at least one of the
radicals Rl to R7 is or contains an aromatic group
are preferred. In general, at least one of the
radicals R2 to R7 is different from hydrogen, and
preferably it is the radical R2. Advantageously,
compounds are also used which contain a halogen
atom, in particular a fluorine, chlorine or bromine
atom, in at least one of the substituents R1 to R4.
Preferably, those substituents are used which
contain (optionally in addition to the aromatic
group) at least one oxygen atom which may be an
ethereal, carbonyl or ester oxygen atom.
The compounds of the formula I may be present
and be used in pure form or as mixtures with one
another, for example as substance mixtures ~hich are
produced during the synthesis. Mixtures of this
type usually have the advantage of a better
solubility than the pure compounds in the coating
solvents.
Some of the compounds of the formula I are
already known, for example 2,7-dibenzoyl-9-
7~
phenylacridine from J. Org. Chem. 29, 2857 (1964) or
from J. Chem. Soc. (London) C 1967, 2071 and 1968,
2900. No usability as photoinitiators is mentioned
therein.
The compounds of the formula I are
synthesized by reacting diphenylamine or its simple
substitution products, for e~ample 4,4'-dimethyl-
diphenylamine, 3-methyldiphenylamine or ~-chloro-
diphenylamine, with benzoic acid or simple benzoic
acid derivatives, for example tert-butylbenzoic
acid, benzophenone-4-carboxylic acid, diphenyl-4-
carboxylic acid, 4-aminomethylbenzoic acid or
terephthalic acid in a suitable reaction medium such
as polyphosphoric acid at about 150 - 200~C.
With diphenylamine, acridine compounds of the
formula I are obtained in which R1 is an optionally
substituted benzoyl group whose carbonyl group can
be further reacted, for example, reduced to the
-CHOH group with sodium boranate.A multiplicity of
further derivatives is possible in the sequence:
reaction of the OH group with acid anhydrides,
isocyanates, condensation reactions with phenols or
esterifications.
Examples of suitable compounds of the formula
I are 2,7-dibenzoyl-9-phenylacridine, 2,7-bis(~-
hydrcxybenzyl)-g-phenylacridine, 2,7-bis (Q-
acetoxybenzyl)-g-phenylacridine, 2,7-dimethyl-9-(4-
methylphenyl)acridine, 2,7-dimethyl-9-phenyl-
acridine, 2,7-bis(3,4-dimethylbenzoyl)-9-(3,4-
dimethylphenyl)acridine~ 2,7-bis(~-acetoxy-4-tert-
butylbenzyl)-9-(4-tert-butylphenyl)acridine, 2,7-
6C37~L
dimethyl-9-(3,4-dichlorophenyl)acridine, 2,7-
dimethyl-9-(~-benzoylphenyl)acridine, 2,7-bis~2-
chlorobenzoyl)-9-(2-chlorophenyl~acridine, 2~
hydroxy-3-bromoben~yl)-6-methyl-9-(3-bromophenyl)-
acridine, 2,5-bis(4-tert-butylbenzoyl)-9-(4-tert-
butylphenyl)acridine, 1,4-bis(2,7-dimethyl-9-
acridinyl)benzene, 2,7-bis(~-phenylaminocarbonyloxy-
3,4-dimethylbenzyl)-9-(3,4- dimethylphenyl~acridine
and 2,7-bis(3,5-dimethyl-4-hydroxy-4'-fluorodi-
phenylmethyl)-9-(4-fluorophenyl)acridine.
The quantitative proportion of the compounds
of the formula I in the mixture according to the
invention is in general about 0.01 to 10, preferably
0.1 to 5% by weight, based on the nonvolatil~
constituents.
For the purposes of the invention, suitable
polymerizable compounds are known and are described,
for example, in US-A 2,760,863 and 3,060,023.
Preferred examples are acrylic acid esters
and methacrylic acid esters of monohydric or
polyhydric, preferably at least dihydric alcohols
such as ethylene glycol diacrylate, polyethylene
glycol dimethacrylate, acrylates and methacrylates
of trimethylolethane, trimethylolpropane, pentaery-
thritol and dipentaerythritol and of polyhydricalicyclic alcohols or N-substituted acrylic acid
amides and methacrylic acid amides. Advantageously,
reaction products of mono- or diisocyanates with
partial esters of polyhydric alcohols are also used.
Monomers of this type are described in DE-A
2,064,079, 2,361,0~1 and 2,822,190. The
2~ 37~
quantitative proportion of monomers in the coating
is in general about 10 to 80, preferably 20 to 60%
by weight.
The mixture also contains, in addition, a
polymeric binder. A multiplicity of so]uble organic
polymers can be used as binder.
As examples, mention may be made of
polyamides, polyvinyl esters, polyvinyl acetals,
polyvinyl ethers, epoxy resins, polyacrylic acid
esters, polymethacrylic acid esters, polyesters,
alkyd resins, polyacrylamide, polyvinyl alcohol,
polyethylene oxide, polydimethylacrylamide,
polyvinyl pyrrolidone, polyvinylmethylformamide,
polyvinylmethyl acetamide and also copolymers of the
monomers which form the homopolymers listed.
Furthermore, natural substances or modified
natural substances, for example gelatin and
cellulose ethers,are possible as binders.
The use of binders which are water-insoluble
but are soluble, or at least swellable, in aqueous
alkaline solutions is particularly advantageous
since coatings containing such binders can be
developed with the preferred aqueous alkaline
developers. Such binders may contain, for example,
the following groups: -COOH, PO3H2, -SO3H, -SO2NH-,
-SO2-NH-SO2- and -SO2-NH-CO-.
As examples thereof, mention may be made of
maleate resins, polymers of~-(methacryloyloxy)ethyl
N-(p-tolylsulfonyl)carbamate and copolymers of the
latter and similar monomers with other monomers, and
also vinyl acetate/crotonic anhydride and
styrene/maleic anhydride copolymers. Alkyl
methacrylate/methacrylic acid copolymers and
copolymers of methacrylic acid, higher al,;yl
methacrylates and methyl methacrylate and/or
styrene, acrylonitrile etc., such as are described
in DE-A 2,064,080 and 2,363,806, are preferred.
The quantity of binder is in general about ~0
to 90, preferably 40 to 80% by weight of the
constituents of the coating.
Depending on the planned application and
depending on the desired properties, the
photopolymerizable mixtures may contain diverse
substances as additives.
Examples are: inhibitors for preventing
thermal polymerization of the monomers, hydrogen
donors, substances which modify the spectral
photosensitivity of coatings of thls type,
dyestuffs, colored and colorless pigments, color
formers, indicators, and plasticizers, for example
polyglycols or esters of p-hydroxybenzoic acid.
These constituents are advantageously chosen
in a manner such that they have as little absorption
as possible in the actinic radiation range which is
important for the initiation process.
For the purpose of this description, actinic
- radiation shall be understood to mean any radiation
whose energy is equivalent at least to that of short
wave visible light. Longwave UV radiation and also
electron radiation, X-ray radiation and laser
radiation are suitable.
'2~
The photopolymerizable mixture may be used
for a wide variety of applications, for e~ample to
produce safety glass, lacquers which are cured by
light or corpuscular beams, for example electron
beams, in the field of dentistry and, in particular,
as a photosensitive copying material in the field of
reproduction.
The detailed description of the invention is
restricted to the latter field of application, but
the invention is not restricted thereto. As
possible applications in this field, mention may be
made of copying rnaterials for the photomechanical
production of print forms for letterpress printing,
lithographic printir,g, gra~ure printing,screen printing,
of relief images, for example production of texts
in Braille, of single copies,tanned images, pigment
images, etc. Furthermore, the mixtures may be used
for the photomechanical production of etch resists,
for example for manufacturing nameplates, printed
circuits and for chemical milling. The mixtures
according to the invention are particularly
important as copying materials for the photo-
mechanical production of lithographic print forms
and for the photoresist techniques.
For the said application purposes, the
mixture can be utilized commercially in the form of
a liquid solution or dispersion, for example as a
photoresist solution, which is applied by the user
himself to an individual base, for example for
chemical milling, for the production of
prin~ed circuits, of screen printing stencils
--10--
6~
and the llke. The mixture may also take the form of
a solid photosensitive coating on a suitable base in
the form of a storable precoated photosensitive
copying material, for example for production of
print forms. It is also suitable for the production
of dry resist.
It is in general beneficial to largely
exclude the mixtures from the influence of
atmospheric oxygen during the photopolymerization.
If the mixture is used in the form of thin copying
coatings, it is advisable to apply a suitable top
coat which has low permeability to oxygen. The
latter may be .$elf-supporting and may be peeled off
before the copying coating is developed. Polyester
films, for example, are suitable for this purpose.
The top coat may also be composed of a material
which dissolves in the developer liquid or may be
removed at least at the noncured points during
development. Suitable materials for this purpose
are, for example, waxes, polyvinyl alcohol,
polyphosphates, sugar etc.
Suitable coating bases for copying materials
produced with the mixture according to the invention
are, for example, aluminum, steel, zinc, copper and
plastic films, for example made of polyethylene
terephthalate or cellulose acetate, and also screen
printing bases such as perlon gauze.
The photosensitive materials using the
mixture according to the invention are produced in
a known manner.
Thus, the mixture can ~ taken up in a solvent and the
solution or dispersion may be applied bv pouring,
spraying, immersion, application with rollers, etc.
as a film to the base provided and then dried.
Thick coatings (for example of 250 ~Lm and over) are
advantageously produced by extrusion or pressing as
a self-supporting film which is then possibly
laminated onto the base. In the case of dry resist,
solutions of the mixture are applied to transparent
temporary bases and dried. The photosensitive coatin~s
(thickness approY.imately between 10 and 100 ~m) are
then laminated onto the desired final substrate,
together with the temporary base.
The processing of the materials is carried
out in a known manner. For the purpose of
development, they are treated with a suitable
developer solution, preferably a wea~ly al~aline
aqueous solution, in which process the unexposed
portions of the coating are removed and the exposed
regions of the copying coating remain behind on the
base.
The copying materials according to the
invention are notable for a lower loss in
photosensitivity during storage. This advantage is
effected apparently by a higher resistance to
diffusion of the initiators in the photopoly-
merizable coating compared with unsubstituted 9-
phenylacridine. The diffusion resistance increases
with increasing molecular weight. In this
connection, it is essential that the substituents
-12-
7~L
are in the 2-position or pre~erably in the 2,7-
position of the acridine nucleus. The initiators
also do not migrate, or migrate to a substanti,ally
lesser extent than known initiators, out of the
photocured coating.
Examples oE the mixture according to the
invention are given below. Here the preparation of
compounds of the formula I is first described.
Then Table I specifies photoinitiators which are
used in the photopolymerizable mixtures of the
application examples.
In the examples, parts by weight (pbw) and
parts by volume ~pbv) are in the ratio of g to ccm.
~nless otherwise specified, percentages and quantity
ratios are understood in units of weight.
Preparation examples
1. 2,7-Dibenzoyl-9-phenylacridine~ (compound 1)
1 pbw of diphenylamine and 15 pbw of
polyphosphoric acid are heated to 100~C while
stirring. After adding 2.5 pbw of benzoic
acid, heating of the mixture is continued at
200~C for 45 minutes. After cooling to
lOODC, the mixture is poured into 70 pbw of
water, the product is filtered off and
purified (m.p. 210~C).
)6~)7~
2. 2,7-Bis(~-hYdroxybenzyl)-9-phenylacridine
(compound 2)
1 pbw of 2,7-dibenzoyl-9-phenylacridine is
suspended in 4 pbw of ethanol and re~uced by
adding 0.1 pbw of sodium boranate in portions
at 20-50~C. After 24 hours, the reaction
product is precipitated with water, purified
and dried (m.p. above 280~C).
3. 2~7-Bis(~-acetoxybenzyl)-s-phenylacridine
(compound 3)
1 pbw of 2,7-bis(~-hydroxybenzyl)-9-
phenylacridine is suspended in 4 pbw of
acetone and heated for 1 hour under reflux
with 0.8 pbw of acetic anhydride and 0.001
pbw of 4-dimethylaminopyridine. Water is
then added to the mixture and the product is
filtered off.
4. 2,7-Dimethyl-9-(p-tolyl)acridine(compound4)
l mol of 4,4'-dimethyldiphenylamine and 1 mol
of 4-methylbenzoic acid are heated for 1 hour
at 200~C in 5000 g of polyphosphoric acid.
After cooling, water and ammonia are added to
the reaction mixture and the product is
filtered off by suction and purified (m.p.
210-211~C).
-14-
2~6~i7~
Table
Compounds of the formula I having R3 = R4 = R7 = H
Compound R1 ~2 R5 R6
1 Benzoyl Benzoyl H H
2 ~ Hydroxybenzyl ~-Hydroxybenzyl H H
3 ~-Acetoxybenzyl ~-Acetoxybenzyl H H
4 Methyl Methyl 4-Methyl H
Methyl Methyl H H
6 3,4-Dimethyl~ 3,4-Dimethyl- 4-Methyl 3-Methyl
benzoyl benzoyl
7 ~-Acetoxy-p- ~-Acetoxy-p- 4-tert- H
tert-butyl tert-butyl- butyl
benzyl benzyl
Application example l
The following three coating solutions were
prepared from
pbw of a terpolymer of methyl methacrylate,
n-hexylmethacrylate and methacrylic acid
(5:60:35) with a mean molecular weight M~ =
70,000,
11 pbw of the diurethane formed from 2 mol of
hydroxyethyl methacrylate and 1 mol of 2,2,4-
trimethylhexamethylene diisocyanate,
39 pbw of the reaction product formed from 1 mol
of hydroxyethyl acrylate, 2 mol of capro-
lactone and l mol of n-butyl isocyanate.
0.1 pbw of the blue dyestuff 1,4-bis-iSO
butylamino-anthraquinone (C.I. 61551),
160 pbw of butanone and
15-
pbw o~ ethanol,
to which
a 0.51 pbw of 9-phenylacridine (molecular
weight 255, comparison)
b 0.926 pbw of compound 1 (molecular weight
464), or
c 1.102 pbw of compound 3 (molecular weight
552)
were added as photoinitiator.
The solutions were applied to biaxially
oriented and heat-set polyethylene terephthalate
films having a thickness of 25 ~m in a manner such
that a coating weight of 45 g/m2 was always obtained
after drying at 100~C.
To protect the dry resist coatings from
contamination with dust and against damage, they
were clad with a 23 ~m thick polyethyl~ne top film
and rolled up. They can then be stored with light
excluded for a prolonged period of time. The rolls
~polyethylene film outwards) were fastened with a
commercial adhesive tape.
After the rolls had been stored for 24 hours
at 40~C, the resists were processed.
On peeling off the adhesive tape it was found
that, although separated from the resist by the
polyethylene top film, the tape was colored light
blue by anthraquinone dyestuff which had diffused.
In order to test whether the adhesive tape also
-16-
)66~7~
contained traces of photolnitiators, a UV spectrum
was recorded at 350-450 nm usin~ a spectrophoto-
meter, tvpe Perkin-Elmer Lambda 3. Compared with a
comparison sample, the adhesive tape of sample a
exhibited a peak (extinction = 0.12) at 358 nm. The
light blue adhesive tapes b and c exhibited no
additional extinction between 350 and 450 nm. They
did not contain any photoinitiator.
The dry resist films were laminated in a
commercial laminating apparatus at 115~C onto
phenolic laminate panels clad with copper foil
having a thickness of 35 ~m and were exposed for 4
seconds by means of a 5 kW metal halide lamp with a
distance of 110 cm between lamp and vacuum copying
frame. The master used was a 13-step exposure wedge
which contained density increments of 0.15. In this
operation, the exposure wedge was sited so that it
was positioned on the parts which had originally
been covered by polyethylene film and adhesive tape.
After exposure, the polyester films were
peeled off and the coatings were developed with a
1%-strength sodium carbonate solution in a spray
development apparatus in the course of 60 s.
The sample a was underexposed, the resist
being cured only up to step 5; lines reproduced were
10~ too narrow. The step wedge of samples b and c,
on the other hand, was fully cured up to step 6. A
test master having 80 ~m wide clear and dark lines
was reproduced true to scale as a raised image.
-17-
2~6~1i7~
Application example 2
a 0.70 pbw of 9-~3,4-dimethylphenyl)acridine
(molecular weight 283, comparison),
b 0.70 pbw of compound 5 (molecular weight
283), or
c 1.35 pbw of compound 6 (molecular weight 547)
were dissolved as photoinitiators in coating
solutions composed of
pbw of a copolymer of methyl methacrylate and
methacrylic acid (acid number 115),
pbw of trimethylolpropane triacrylate and
0.5 pbw of Disperse Blue 134 (C.I. 61551) in
520 pbw of 2-methoxyethanol.
~he solutions were applied by spinning to
15 electrolytically roughened 0.3 mm thick aluminum
which had been hardened by anodizing. The coating
was dried for 2 minutes at 100~C, a coating weight
of 2.4 g/m~ being obtained.
For the purpose of protection against
contamination, the coated, slightly sticky
photosensitive panels were clad at room temperature
with a 20 ~m thick polyethylene film.
The printing plates thus obtained were
exposed for 15 seconds with a 5 kW metal halide lamp
at a distance of 110 cm under a negative master
.~
18-
together wlth a 13-step exposure wedge which
contained density increments of 0.15.
The parts no~ cured by light were removed by
wiping over with a developer solution of the
following composition:
3 pbw of sodium metasilicate nonahydrate,
0.05 ~pbw of strontium chloride,
0.03 pbw of nonionogenic wetting agent
(coconut butter alcohol/polyoxyethylene
ether containing approx. 8 oxyethylene
units),
0.003 pbw of antifoaming agent,
lO0 pbw of demineralized water.
The number of completely cured steps of the
exposure wedge provides a coefficient of measurement
for the photosensitivity. The values are listed
under (A) in the table below.
In order to elucidate the diffusion of the
initiator through the polyethylene film, a
commercial colorless transparent adhesive tape was
pressed onto the polyethylene film on one sample
panel in each case and the composite was stored for
24 hours at room temperature (23~C). The adhesive
film was then peeled off again and (B) the increase
in the optical density at 358 nm was measured. The
exposure test wedge having 13 gray steps was
furthermore placed at the position where the
adhesive film had been removed (experiment C), and
--19--
the printing plate was exposed for 15 seconds and
then developed just as described above.
The results listed in the following table
show that the initiator diffuses most strongly out
of the sample a, to a markedly lesser extent out of
sample b and does not diffuse out of sample c.
(A) Wedge (B) Density(C) Wedge
steps differencestep
a 6 0~17 2
b 7 0.11 4
c 7 0 7
Example 3
a l pbw of compound 7 or
b 1 pbw of isopropylthioxanthone and
2 pbw of p-dimethylaminobenzoate (initiator
mixture of a commercial photoresist)
were added as initiators to coating solutions
composed of:
pbw of a copolymer of methyl methacrylate,
butyl acrylate, styrene and methacrylic acid
(35:40:5:20),
pbw of trimethylolpropane triacrylate,
l pbw of leuco crystal violet and
0.05 pbw of crystal violet in
pbw of butanone and
-20-
Z0~961~
75 pbw of ethanol.
The solutions were applied tO polyethylene
terephthalate film as in ~xample 1, dried, laminated
onto copper foil, e~posed and developed. Then the
optimum exposure time was determined.
In a further experiment,boards produced and
developed as above were rinsed for 30 s with tap
water, incipiently etched for 30 s in a 15~ strength
ammonium pero~odisulfate solution, rinsed with water
again, immersed for 30 s in 10%-strength sulfuric
acid and then electroplated consecutively in the
following electrolyte baths.
1~ 60 minutes in copper electrolyte bath
supplied by Schloetter, Geislingen/Steige,
"bright copper bath" type
current density: 2.5 A/dm2
metal buildup: approx. 30 ~m
temperature: room temperature
2. 15 minutes in a lead-tin bath LA supplied by
Schloetter, Geislingen/Steige,
current density: 2 A/dm2
metal buildup: 15 ~m
temperature: room temperature
The plates did not exhibit any undercuttingor
damage.
To test whether the resist constituents
diffuse into the electroplating baths, 0.075m 2
-21~
~6~7a~
~lass plates were coated with resist (45~/m2) and immersed for
one hour in one liter of 20%-strength sulfuric acid.
In both cases the sulfuric acid remained colorless:
a U~7 spectrum (200 - 800 nm) of both sulfuric acid
extractC war then recorded with the
spectrophotometer (1 cm cell); b sho~!ed a di.stinct
peak at 225 nm (extinction = 0.9'5), some of the
initiator system having migrated into the sulfuric
acid; a showed no contamination.
-22-