Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
The invention concerns 4-/4'-halophenyl/-2-methyl-
1,2,3,4,-tetrahydroisoquinolines racemates or optically
active antipodes and a method for their preparation.
It is known from R. Kunstmen, H. Gerhards, H.
Kruse, M. Leven, E. Paulus, U. Schacht, K. Schmitt, J. Med.
Chem. 1987, 30, 7988-804 that racemic or optically active
4-phenyl-8-amino-2-methyl-1,2,3,4,-tetrahydroisoquinolines,
possess inhibitory effect on the uptake of dopamine/DA/,
noradrenaline/NA/ and serotonin/5-HT/ from synaptozomes of
rats' cerebral cortex.
It is also known from M. Bickel, Arzneim-Torsch;
Drug Res., 30(1), No 1, 69, 1980, that the racemic 4-phenyl-
8-amino-2-methyl-1,2,3,4-tetrahydroisoquinoline possesses
moderate inhibitory effect towards stress inducing stomach
ulcers in rats.
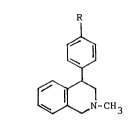
The object of the invention is to create new
4-/4'-halophenyl/-2-methyl-1,2,3,4,-tetrahydroisoquinolines
having general formula (1):
R
1~1 ,, ~ ,,
-CH3 la R = Br
lb R = F
where R means brome or fluor, and their physiologically
acceptable salts, which have high inhibitory effect towards
dopamine, noradrenaline or serotonin uptake, as well as a
high antiulcer activity.
The essence of the invention comprises in the fact
that the racemic compounds with general formula 1 are
; obtained according to the following three-stage reaction
scheme:
- 2
X - C ,i~,"- o ~ /~>_ ~ . .s
R
_;
~3 c~ J C~Z - C~ R ` - " J ~
, _,
Oh~ .
ai ALkyldti..~n of bt=n~-ylt=thy].~;l~.ine ~'. free ~a5~ aik~1l ir~
twi._e m~llar e~GSS wit~ C-h~lo~en~ e~- ar~;l aliF~hat:i.c.
ket:~n~s _~ in a boil:ir~ ~n_ol c~ a~ ~t~nt~ m2~iium til.l tha
F.~ arati.orl c~f c.--"'N-m2thyl-N-~en~ylan~ir{~f--4-hal~a~ ~to-
ph~ rlc~ t, wher~ 1~ has th~ showr, ~bov~ ~anin~. Kr~lones ~
~}~: i9~ t.;~ ~Crrll ot h~td~ .h~ori~ 3~,t~, pr~areiJ 3-'y
~i-cOtmenr ~i- ~`aJ ~ SF'5 with ~;tly di~thYl ~th2r ~satura~e,~ wit
h~
t,) .-~edu~_tii;,r. cf ket~n~s ~ tc~ N--~n, y].--~-fm~thylamir~ ~.f-l-
~-ha:l~_r.~h~n~,lf-l.-ethar-,o~s ~ u~ing ;~ thr~e-l:inl~ molar ~,~c--sg
c~f si~dium ~c~rhYdr~t~ Ths: r~a~-.ti~ ,arrl,=d c~u t in l.-~wer
ali ~ ~h.~ls medium at a tt---m~!erature ir-ter~Ja 1 f rc,m 10 t-o
,
.~i.: : . :: : : : . .: :
2 ~
Thc pre~;1r-d .:-J,,iinols 5 ~re s~ rate-l in th~ ic,Jm --,f well.
cr~st~ i~irl~ tree t~ses. wr.ictl ar-.- clear enou~h /~ccording
t~ ~7-2 Ol- TL~ for the l-ext ~t-~e o~~ c~/cli~.ati(~n
c) cyclizaci-~nl ot- Gart~in~ s 5 tc .4-aryl-
t~tr~hy~rois~-Jqu~ ines 1 i~ ~arrie~ out in a dichlormethane
or dichl~re~lane medi~lm in the ~reser.ce ~f ~oncentrated
sulph~ri-_ ~ciA at a tempeI~ture interval from O to 30~~
pr~erar,l-~ from ~ o ;5~ f-~. 1 t~ ~', hours, p~e~er3bly 1 t~
hours
R~ ba~es 1 ~re is~lat~d l-r-om ~he ..eacliorl mixtures by
c~3tir,~ -h~ l~rter in ice cold water f~llowÆd t~y
neu-~.raliza tiOIl ~al -th~ prepare~ so~u~.ons i~ ~5 per cent
aquec,L~s sc~iu._~r:.-.. amm~ni.ull ~nd extr3ction with ~ .c.uitable
~rgar,i,- soivent.
The hydroct.ic,~ide ~alt~ of 1 are prepared .~rom raw bases
~y dlssoluti~n ~f the lart_r in absolute ethan~l and
treatment of ttle prepared solutiQns with dry diethyl ether.
satl-ra-~d ~ith HCl ~as.
The structure or the compounds with general. ~rmula 1 is
proved by data Erom IR, lH-NMi~ and ~ass spectroscopy. as
weil as from data of the elemental analysis.
The optic~lly~ aGtive forms of la, /~=Br/ are obtained by
treatment of ~ la free base with /+~-O.O-dibenzoyl-D-
tartariG acid or ,~-f-O.~-di~2n oyl-L-tar~aric acid in 98 per
c,ent alcch2. med,u~.
The compouncls with a veneral formula 1 are studied Eor
influenc~ on dopamine ~DA/~ r,oraclrenalin~ ~NA~ and serotonin
j5HT/ uptake from synaptozomes. isolated from cerebral
cortex o~ rats. Thes~ bioc~lemical tes~s, a~ it is known, are
reg~rded as basic criterià for presence of antidepressant
activity As it is seen fro~l the pres~nted result.s on ~ge10
itabLe 1~, the racemiG ~a and 1~ p2ssess not 2nly,high
inhi~it.~ry efl'ec,t in reg,3ri to ~A ancl NA u~tai~e. but the~e
are also str~n~ inhihitors oE 5HT uptak2 for ~iffeI-ence t~
the ~.nown antidepre~ant Noinifenzine, which is a wea~.
serotonin inhibitor
r~ - arlti~ ,ti~ ~ t ~l ' r~ d dl~ or~ dn
.:L?~l-im~l~t,~1 m~-"3~i -). w~r-inlm~T~ . ~tr~ uc~-:~ C,t~m~
~llcers in ra~s is d,L~o .,~ ie-i ~t~ , As a pr-pdrarion
f~r c.o~palis.ori 13 used H~-~ntd~ni.~ Rar.it~
The re~ults ~rc,m th-se stu.lies show that the compound
1~ a~plie~-1 oraliy ~s~esses hi~h antiulcer activity ever. at
se :J 05(-l m~k~, with a suppressi~r of the u~cer index
perGentage o-f 2~. l'he av~rage effective do~.e i~ O.~CIO m~,~k~
a, which the inh~bi.ion per-~l.t~Ee i~ ~,' With ,he incre~
.-.f the d._,se als~ ncrea~es re~;.l.arlY the ,~nhit~itorY ~ffect
~n~ t.he maximum is aohieved at ~o~e ~ m~k~.
The results ot the c~-~mparalive stud~es ~it.h Raniti~ir-
sh~ J that la h~c several time~ higher ~ntiulcer ef.eGt T~u~-.
~c.r,example at c~05e 0 1~0 mg,f'~ the p~rc~entage of ~hi~ition
of ~h~ ul~e-~- inclex i~ 'or la. while at five times
hi~her ~aniti~iir ~ose ~I ',00 m~.~k~) the percenta~e of
inhibiti~n is onl~; ~7 At dc~s~ Cl 500 m~k~ of la the
per~er.ta~e o~ inhibit}~n is ~ while L~e same p~rcentage is
achieved at Ranitidin d~se o~ lu mgJkg This rasult shows
that la possesses ~ times stronger antiul._er effect than
the taken for co~parison Ranitidin Similar result~ are
~bt~i~ed for comp~un~ lb. too.
I'he following examples illustrate the inventic~n without
lim~.ting i~
Example 1. Pr~paration o~ N-methyl-N-benzylaminQ/-4-
brom-ace-topherlone ~a ~R-Br~
To a sol.utlon of 6 ~5 g ~50 mmol~ ben~ylmethyla~nin free
base in lS ml acetone at temperature 35-4~C i3 added a
solution of 6 ~5 ~ J~5 mmolf w-brom-4-~rc~macetoph2none in
~,5 ml acetone The reaction ~i~ture is boilin~ f~r 8 hours,
and aft~r that it is left tc, stay for c,ne ni~ht in a
refrigerator The crystalli~ed hydrobromide of the initial
ben~ylmethylam~ne is filtered The fil-tr~te is evaporated
under vacuo to give an oil re.siclue The latter i~ d:iss~lved
in 5 ml absolut2 ethanc.l and tc. the prepared ~olution is
added ,iry diethyl ether ~aturated with H~l gas For the
`:~ :.. . : : : :
~ ~ ~, r '~
complete ~edimentation o~ the produced hydrochloride t~ 4,a
are further added SO ml diethyl et~er. The raw hydrochloride
is recrystallized from ethanol, and after that are isolated
6 09 g /70 per cent/ from 4a hydrochloride with M.P. 133-
138C ~:.
IR spectre ~K8r/ cm~ 90 /COf.
Elemental analysis: calculated per cent C 54 ~8,
N 3 93~ Cl 9 96, Br 22 46, C16H18NOClBr, found per cent
C 54 20. H 4 98, N 4 01, Cl 9.80, Br 22 30
In a similar way i~ prepared 2-/N-methyl-N-benzylamino/-
4-fluoracetophenone 4b fR=F~ with a yield of 79 per Gent
Elemental analysis: calculated per cent C 65 19,
H 6 15, N 4.75-, Cl 12.03. C16H18NOClF, found per cent
C 65 ~2, H 6 10, N 4 80, Cl 12 10 :,
E,x,am,~le_2,, Preparation of N-benzyl-2-/methylamino/-1-/4-
bromphen~ 1-ethanol 5 ~R=Br~
12 30 g /37 mmol/ 2-/N-methyl-N-benzylamino/-4-
b~omacetophenone hydroch~oride are dissolved in 1?0 ml
methanol and to the prepared solution are added 5 32 g
/139 mmol~ sodium bo~hydrate at temperature about ~0C and
stirring for 30 minutes The stirring continues at room
temperature for 30 minutes more, and after that the reaction
mixture is boiling ror 6 hours The solvent is removed under
vacuo and 150 ml water are added to the residue and the
separated oil is extracted four time~ with dichlormethane
The extract is dried with Na2S04 and evaporated in vacuo to
dive ~.5 g /82 per cent~ TLC pure 5,a free base with M P . 60-
61.5C.
IR spectre /KBr/ cm~l: 3200 /OH/
In a-similar way is prepared N-benzyl-2-~methylamino/-1-
/fluorphenyl/-1-ethanol 5b f~=F~ with a yield of 94 per .
cent
~ lemental ~nalysis for Sa: calculated per cent C ~9.02, ~
H 5 66. N 4 37, Br 24 qS, C~6H18NOBr~ found per cent ~-
C ~0 ~0, H 5 40~ N 4 20, Br 24 85 :;
.. ; .
..; ~
2 ~
Elemental analysi~ for 5~: calculated per cent C 74 19
H ~ 9g N 5 40 C16H18NBr fo~nd per cent C 74 30 H 7 10
N 5 20
Exampl~ 3 Preparation of 4-/4 -bromphenyl/-2-methyl-
1 2 3 4-tetrahydroisoquinoline hydrochloride la /~=Br/
To 11 7 ml concentrated sulphuric acid cooled to 5C i~
added drop by drop at intense stirrin~ for 45 minutes a
sQlution of 2 ~8 ~ /9 3 mmol/ N-~e~zyl-~-Jmethylamino~ 4
bromphenyl~-1-ethanol in 25 ml dichlorm~thane The stirring
continues for 1 hour more at the ~ame temperature and after
that the r~eaction-mixture i-~ poured on 50 ~ ground ice and
50 ml water The organic layer i~ ~eparated and the aqueous
phase is basified w~th 5 per cent aqueous solution o~
ammonium and the prepared oil is extr~cted with
dichlormethane After drying of the extr~ct with sodi~l~
~ulphat~ and re~oval of the Qolv~nt under vacuo is ot~ined
la fr~e base in the form of oil. The latter i3 di3301ved in
a minimum quantity methanol and is changed to hydrochloc~ide
bly treatment with dry diethyl ether saturated with HCl ~as
~he raw hydrochloride is recrystallized from methanol-
diethyl ether and 2.5 g ~82 per cent/ la hydrochlorid~ are
obtained with M.P ~20-224C
1H-NMR ~ppm ~DMS0-d6~:2 65 (s 3H)-N-C~3 ~ 32-3 76
lm 2H) and 4.53 (m lH)-~H2f3/-~ ~ 4.24 and ~ 36 ~m 2H
J=8.~ Hz)-1-CH2 6.65-7.40 ~two m 9H Ar)- o-C6H4 ~nd
p--C6H4-
MS /70 eV/: m~z = 303/~301 ~1:1/ for 81Brf79Br ~base)
Elemental analysis: ~alculated per cent C 56 74
H 5 06 N ~ 13 C16H17NClBr found per .-ent C 56 64 H 5 20 -~
N 4 22
In a similar way is obtained 4-/4 -fluorphenyl/-~- -
methyl-1 2 3 4-tetrahydroi~oquinoline hydrochloride lb ~R=F~
with a yield of 86 per cent
M P 136-140C ;;~
`' ~
.
'
2 ~
H-NMR, ~ ppm (DMS0-d6):2 52 (s,~H)-t~C~3, 3,20-3 Oo
~m~,H~ and 4 40 (m,lH)-C~2/3~-CH/1/, ~ i2 and ~ ~5 '~1,2H
J=10 Hz)-1-CH2, 6 78-7 36 (two m, 8H Ar~- o-C6H4 and p-C~H4
~S /70 e'v',': m/7- - ?J~ M+~
~ lemental analysis: calculated per ?,ent C 5g.1~,
H 6 17, N 5 ~4, C16H17NC~F, ~ound per cent C 6~ ~-0. H 6 3S,
N 5 15
E,xa,m~,1~,, Preparation of optica~ly ~ctive ~o~ms o~
/4'-bromphenyl~2-methyl-1,2,~,4-tetrahydroisoquir.oline ,la
9 g ~0 ~3 mol,' racemic 1a free base are dis~clved at
boil.n~ in 90 ml ethanol 10 75 ~ fO 03 mol~ -O,O-
diben~ovl-n-tartaric acid in ~0 ml ethanol are ad~ed tc the
prepared qolution After ~taying for one day at temperature
8-lO~C 9 5 g /96 4 per cent ~rom the 'theoretical yield/
diben~cyl-D-~+f- tartarate of la crystallize with ~ P 115-
ll9~C and ~C ~ = +68 0~ ~c=0 2~. CH30H~, t~]~ of the
~ree base is t31 3~ ~c=0 ~S, C~Cl3; Af~ar one
recrystalli~,ation of the raw tartarate from ethanol ~ 2 ~
/87 per cFnt from the theoretical yield/ la tartara~e are
j obtained with [~]~ = 3~ 38 and ~ P il~-121~ A'ter
treatment of the tartarate with an aquéous ~olution of
ammonium and following extrac-tion with chlorophor.~ is
obtained a free base /+~ ,~a with [~3~ = ~3~ 7~- fc-~ ~5,
i cHcl3/-
The mother li~ors of the raw tartarate and these of the
first cr~rstallizatiQn are combined and are evaporated ~nder
vacuo to dryness The produced residue i~ dissolvsd in
water, basified ~ith an aque~u~ ~olution of ~mmonium and the
obtained oil i8 extracted from chlorof~rm After drying of --
the ~xtract with sodium ~ulphate and following distillation '
' of the solvent under ~acuo are obtained 7 5 g J-~ la raw
', base The latter is di~olved in 37 ~ ml ~oilir.g ethanol and ~-
8 96 g /0 02~ mol/ /-/-0,9-~ibenzoyl-L-tartaric acid are
added to the prepared solution After ~tayin~ at temperature
B-10C S ~ g raw tartarate o~ /-/ la are obtained with
C d]~ = -~8 08 /c=0 25, C~3~f After one recrystallization
2 ~
-- 8
of the raw tartarate from ethanol are prepared 4.8 g/92 per
cent from the theoretical yield/ /-/ la tartarate with ~D -~
= -74.89/c=0.25, CH30H/ and M.P~ 120-124 C /-/'a free base:
~1D = -30.64/c=0.25, CHCl3/.
Example 5: Determination of the inhibitory effect of 4-/4'-
halophenyl/-2-methyl-1,2,3,4,-tetrahydroisoquinolines la and
lb on DA, NA and 5HT uptake from the synaptozomes isolated
from cerebral cortex of rats.
Synaptozomes are isolated from cerebral cortex of
white male rats "Wister" species by method from E.C. Gray,
V.R. Whittaker, J. Anatomy, 96, 79, 1962. The tissue is
homogenized in 10 volumes 0.32 M saccharose. The homogenite
is centrifuged at 3000 g for 10 minutes at 0C. The
prepared supernatant is centrifuged at 17 000 g for 60 min.
The fraction enriched with synaptozomes, P-2 fraction, is
flushed only once with 0.32 M saccharose and is separated at
11 000 g for 20 minutes. The synaptozome fraction is
resuspended in buffer containing 134 mM sodium chloride;
4.1 mM potassium chloride; 1.1 mM acide calcium phosphate;
1.2 mM magnesium chloride; 5.5 mM /+/ gl~cose; 23.6 mM tris
base 1.3 mM calcium dichloride; 0.3 mM EDTA-Na; 0.1 mM
ascorbic acid; 0.01 mM nialamid. The buffer pH is
maintained to 7.4 with sulphuric acid at 37C. The ~`
synaptozomes are resuspended in concentration about 500 ug
protein per 0.1 ml.
The reaction is carried out in glass tubes. The
incubation mixture volume is 1.0 ml. Each test consists of
a blind smaple/incubation is carried out in an ice bath/, a
control sample/ without inhibitors of the uptake/ and a test
sample/ containing the studied substances/. The studied
substances are dissolved in Tween 80 and in the operating
buffer in three concentrations. The samples are reincubated
for 10 minutes at 37C and the reaction starts with the
addition of 100-300 nM /-/ 3H-noradrenaline, 50-300nM/3H/-
.... .
,'j: . `::
2 ~
dopamine or 200-250 nM/3H/-serotonin to each sample. The
incubation continues 3 min for dopamine and 6 min for
noradrenaline and serotonin and stops with the addition of 4
ml glacial solution of sodium chloride 0.9%. The
synaptozome material is separated from the incubation
mixture by filtering through Millipore filter /0.45 ,u/ and
is flushed only once with 4 ml 0.9 per cent solution of
sodium chloride. Each filter is placed in a scintillation
flask containing standard scintillation solution. The
radioactivity is read on liquid scintillation counter.
Example 6: Determination of the inhibitory effect of 4-aryl
tetrahydroisoguinolines la and lb on water immersion stress
induced stomach ulcers in rats.
The tests are carried out on 280 white male rats
"Wister" species with weight between 150 and 200 g by the
method described in K. Takadi, Y. Ischil, Arzneim-Torsch/
Drug Res., 17, 1544, 1967. Eighteen hours prior the test
the animals starve with free access to water. The studied
substance is applied by mouth and the animals are tied still
on their backs on separated stands immersed in water,
termostated to 17-18C. Five hours later the rats are
killed, their stomachs are taken out and the destructive
changes are read by microscope.
; :;
.
2 ~ L. ,~"
- 10 -
.T. n h i ~ . n g r~ F F~ L-. c, ~ . a l _, E t. . =~ ?e ~ , 3, ', -
t~tr~-lh~rA~.,7.~cf31 ir~c,~-.T.. ~ n ~ L~r.~', rloradrf n.~~ *
3,-~ r--.-ot.~ ir~ T~
r~C~iVi ty /~1,'
PC~lr.f~ _ F~ T
la E3r 1~-7 1.~-8 lC-7
lb ':~ 10-7 ~ -8 10-7
n-?ir~if en,7ine ----- -,, -- - -- -- _",1 J-7 _ _ l0 .8 _ 1v
~ f l ur-nr,~ ~ f 4 - ~ l opl~~e ~ h~ ~ f~
t ~l: T -- ~ yr~l- c~ ? r~ ? i T~s ~ ?~P~n~r ~ ~ 1 ~C ~ 'f.--
s~r 3 i ci ~ g ~ ],-,~:,3 S ~ nd~ t: ?!n a,f, ~ i ç ,* ~ y m~
a~:,p ~ ~ ~ 3 ~ 1 ~?
~;
=Br
Per~f-nt~;~e 3f ~ -
- N~ - of suppressl~ f
Df.~G, mg~k~ ~ni~.?3~s Ulcer index _ _ the ul~r indGx :~: ;
1,,,,,, , ........ __ , ,:2, ,,_,, _ , , ,,, " ", , "", " " , , ,, ,.~, , _ "., ".. .. .. . .. . ... .
~, con'~rol 15 74 . f~6 '~ B~g . ~4 -
5C~ q + 1c~ . ~ f~ . 6
o 1~2 ., ', . 4 * ~ S~ 4D, 3
O.50~ 10 ~0.Z + 1.-. 7 5~
'' ~ . 000' ~ 5 . 7 + 1 ~ . f~ ~a . ~
3 . 0 00 1 ~ _ l CI = 5 + ~ g . 3 ~ ~ ~
.' -. ; ~.
.
2~3.~
- 11
~ 4
_ont~-ol 1 ~ ~5 . a_ + 3~2, 19 --
. 0~0 8 ~i . 5 ~ 7 . 75 ~1 .
,, , ,, 3 , . . .
c-~ntrol lC~ ~4 . ~ -
Q . S O O1 ~ 3 ~ '7
1 . OO~.J 1~
3 ~ 1 ~2 ~ 5 ,~ ;
U ~ ~ 4
1 0 1 7