Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~~~~~)~~
~»1' ~~llJ US
POLYMERIZATION CATALYST SYSTEM
BACKGROUND OF THE INVENTION
Field of the Invention
The present invention relates to the polymerization of
olefins using a catalyst system comprising a compound of a
Group IVB metal and an aluminoxane.
Description of the Prior Art
Various prior art references exist which describe olefin
polymerization catalysts, comprising a compound of a transition
metal belonging to Group IVB of the Periodic Table and an
aluminoxane. Included are U.S. Patent Nos. 4,404,344,
4,42,199, 4,544,762, 4,665,208 arid 4,752,597. Also included
is PCT Publication No. WO 88/03932. It would be of commercial
interest to achieve an increase in the activity of such
catalysts.
U.S. Patent No. 3,740,384 mentions use of 1,1,3,3-tetra-
phenylsiloxane-1,3-diol to improve the olefin polymerization
activity of an organometallic zirconium complex. The catalyst
described in this patent is devoid of an aluminoxane
component.
SUMMARY OF THE INVENTION
The present invention relates to an improved (more
active) catalyst of the aforementioned type which also
contains a hydroxysiloxane compound to achieve such activity
increase.
DETAILED DESCRIPTION OF THE INVENTION
The catalyst component of the present invention
comprises, as one essential component, a transition metal
compound of a Group IVB metal, such as titanium, zirconium,
- 1 -
~~~Jf9~i~
and hafnium. Zirconium is an especially preferred metal.
These compounds can have a ligand which is a group having a
conjugated pi_-electron such as cycloalkadienyl. The other
ligands can be cycloalkadienyl aryl, alkyl, aralkyl, halogen
or hydrogen. These compounds can also have a ligand which is
a multidentate compound having at least two groups connected
through a lower alkylene group or a substituted silicon atom.
These two groups can be the indenyl group, a substituted
indenyl group and the partial hydride thereof. The
concentration of the metal atoms in the reaction system can
range from about 10-~ to about 10-3 moles/L. A
representative transition metal compound is
bis(cyclopentadienyl)dichlorozirconium.
The second essential component of the catalyst is an
aluminoxane which is formed by reaction of water with a
trialkylaluminum, such as trimethylaluminum. The oligomeric
linear and/or cyclic alkyl aluminoxanes known in the art are
intended to be included. Generally speaking, this component
can be present at from about 0.01 to about 5 moles/L in the
reaction system.
Both of the foregoing components are generally known to
persons of ordinary skill in the pertinent art and it is
intended that the instant disclosure in regard to them is to
encompass such generally known components and their
equivalents.
In order to achieve the increased activity contemplated
by the present invention, the novel catalyst system of this
invention also contains an effective amount of a hydroxy-
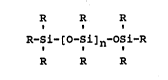
siloxane component. This component is of the general formulae
R R R
R-Si-[O-Si]n-OSi-R
R R R
- 2 -
l ~~~la>s:~'~
where at least one R is OH and the others are independently
selected from -OH, alkyl, and aryl and where n can preferably
range from 0 to about 10. It is generally present at from
about 1% to about 10% of the weight of the aluminoxane.
Representative compounds of this type include such siloxane
diols as 1,3-dihydroxytetramethylsiloxane, 1,3-dihydroxy-
1,3-dimethyl-1,3-diphenyldisiloxane, and 1,3-dihydroxy-1,3-
tetraphenyldisiloxane.
The hydroxysiloxane additive can be advantageously
incorporated into the catalyst system by first being admixed
with the aluminoxane. This can be done as shown in Example 1
by mixing the aluminoxane and hydroxysiloxane compound in a
suitable organic solvent.
The catalyst system described before is useful in the
polymerization of olefins such as ethylene and ethylene-alpha-
olefin copolymers. Such polymerizations may be performed in
either the gas or liquid phase (e.g., in a solvent, such as
toluene, or in a diluent, such as heptanej. The
polymerization can be conducted at conventional temperatures
(e.g., 0° to 120°C) and pressures (e.g., ambient to 50
kg/cm2) using conventional procedures as to molecular weight
regulation and the like.
The Examples which follow illustrate certain embodiments
of the invention.
- 3 -
~~GJ~C)t1
EXAMPLE 1
Methylaluminoxane, MAO, (330 mg) prepared from trimethyl-
aluminum (TMAL) and hydrated magnesium sulfate was dissolved
in several milliliters of toluene and was treated with 9 mg of
1,3 dihydroxytetramethyldisiloxane (DHMS) to form a slightly
gelatinous mixture. The modified aluminoxane mixture was
injected into a 250 ml toluene charge in a pressure bottle
followed by 1.5 x 10-8 mole bis(cyclopentadienyl)dichloro
zirconium (Cp2ZrC12). Ethylene was poly~cnerized for nine
minutes at 85.5oC. The yield of polyethylene was
8.7 grams corresponding to an activity of
14 x 106gmPE~gmZr 1'hr-1'atm.-1.
COMPARATIVE EXAMPLE 2
Example 1 was repeated except that the siloxane treatment
was omitted and the polymerization lasted fifteen minutes.
The polyethylene product (7.3 grams) was collected,
corresponding to an activity of only
6.9 x 106gmPE~gmZr-1~hr 1~atm. 1.
EXAMPLE 3
Example 1 was repeated using 340 mg MAO prepared
from hydrated magnesium chloride treated with 8.4 mg DHMS,
2.4 x 108 moles of Cp2ZrC12, and with a polymerization
time of eight minutes. The polymer yield was 9.9 grams
giving an activity of 1Ox106gmPE'gmZr 1'hr 1'at~i 1.
COMPARATIVE EXAMPLE 4
Example 3 was repeated omitting the siloxane
treatment, using 1.9 x 10-8 moles of Cp2ZrCl2 and
polymerizing for fifteen minutes. The polymer yield was
7.6 grams giving an activity of
5.4 x 106gmPE'gmZr-1'hr-1°atm-1.
EXAMPLE 5
MAO (330 mgj prepared from hydrated magnesium
chloride was dissolved in several milliliters of toluene and
was treated with 11 mg of 1,3- dihydroxy-1,3-dimethyl
-1,3-diphenyldisiloxane (DHDMDPDS) to form a clear nonviscous
solution. This solution was injected into a 250 ml toluene
charge in a pressure bottle followed by 1.35 x l0-8 mole of
bis(cyclopentadienyl)dichloro zirconium Ethylene was
polymerized for six minutes. The yield of polyethylene
was 9.55 grams which corresponds to an activity of
24 x 106gmPE'gmZr~l.hr 1'atm 1~
s
3 a ~1 irl
EXAMPLE 6
Example 5 was repeated using 22 mg of
1,3-dihydroxy-1,3- tetraphenyldisiloxane in place of
DHDMDPDS. The amount of Cp2ZrC12 catalyst charge was
1.38 x 18-8 moles and the polymerization time was nine
minutes. The polymer yield was 9.2 grams corresponding to
an activity of 17 x 106gmPE°gmZr-1'hr 1°atm 1.
COMPARATIVE EXAMPLE 7
Example 5 was repeated omitting the siloxane
treatment, using 1.26 x 10-8 moles of Cp2ZrCl2 and
polymerizing for fifteen minutes. The polymer yield was
5.93 grams corresponding to an activity of only
6.4 x 106gmPE'gmZr°l.h~ 1'atm 1.
-6-
EXAMPLE 8
DIiDMDPDS (12 mg) was added to 3.4 grams of a
toluene solution of MAO containing 4.51% A1, Then 0.0049
gram of a toluene solution containing 1.8 x 10-3 mole
Cp2ZrC12 was added to the MAO solution. The mixed
solution was injected into a pressure bottle containing 250
ml heptane at 82oC. The vessel was maintained at 88oC
for twenty minutes at 47 psig ethylene. After three
minutes ethylene uptake became noticeable. Polyethylene
(5.7 grams) was collected corresponding to an actieity of
3.2 x 105PE/gmZr'1'hr 1'atm'1. The polymer had an
MFI (melt flow index) of 3.3 grams/ten minutes.
fOMPARATIVE EXAMPLE 9
By syringe, 0.004 gram of a toluene solution
containing 1.8 x 10'7 mole Cp2ZrC12 was added to 3.4
grams of a toluene solution of MAO containing 4.51% A1.
The mixed solution was injected into a pressure bottle
containing 250 ml heptane at 80oC. The vessel was
maintained at 88oC for twenty minutes at 46 prig
ethylene. There was no ethylene uptake for the first six
minutes after which the polymerization began. Polyethylene
(3.6 grams) was collected corresponding to an activity of
2.1 x 105gmPE/gmZr 1'hr'1°atm 1. The polymer had
an MFI of 3.4 grams/ten minutes.
~~~a~~
~~.~ 1 .r ~e ~ :J U:~
COMPARATIVE EXAMPLE 10
By syringe, 0.0062 mg of a toluene solution
containing 1.6 x 10-8 moles of Cp2ZrC12 was added to
3.4 grams of a toluene solution of MAO containing 4.51% A1
and 0.0881 gram of polydimethylsiloxane silanol terminated,
approximately equal to 1700 molecular weight, containing
2.5% OH. The mixed solution was infected into a pressure
bottle containing 250 ml of toluene and was pressurized to
45 psig with ethylene at 85°C. No polymerization occurred.
The foregoing Examples have been presented for
illustrative reasons and should not, therefore, be
construed in a limiting sense. The scope of protection
sought is set forth in the claims which follow.
_ g _