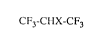Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2036Z;~0
The hexafluoropropanes mentioned in the title have
previously only been obtainable by expensive processes,
using starting materials which are difficult to obtain
and/or in poor yields. Thus, J. Org. Chem. 28, 112 (1963)
describes that 1,1,1,3,3,3-hexafluoropropane can be
obtained only in about 20 ~ yield from hexachloropropane
and pentachloropropene by reaction with potassium fluor-
ide in the presence of a polar solvent.
J. Org. Chem. 54, 1432 (1989) describes the preparation
of 2-chloro-1,1,1,3,3,3-hexafluoropropane from
1,1,1,3,3,3-hexafluoro-2-propanol, which is first conver-
ted to the corresponding nonaflate compound (i.e. a
nonafluorobutanesulphonate) into which a chlorine atom is
then introduced in the 2-position using lithium chloride
in the presence of a crown ether.
1,1l1,3l3,3-Hexafluoropropane and 2-chloro-1,1,1,3,3,3-
hexafluoropropane increasingly are gaining interest in
industry - the former compound as a propellant which does
not endanger the ozone layer of the atmosphere (see
Bild der Wissenschaften 2, 49 (1988)) and the latter
compound as a heat exchanger liquid (see EP-A 72,308).
There is therefore a need for a technically advantageous
preparation process for these substances.
A process has now been found for the prep~ration of
hexafluoropropanes of the fo 1~ (I)
Le A 27 473 - 1 -
20~6;;~20
CF3-CHX-CF3 (I)
in which
X represents hydrogen or chlorine,
which process is characterised in that hexachloropropene
is reacted with hydrogen fluoride in the gas phase in the
presence of a catalyst.
The fitarting material hexachloropropene is available at
low cost, since it is obtainable from simple base chemi-
cals (for example from chloroform and tetrachloroethane).
A suitable fonm of hydrogen fluoride i8 commercially
available hydrogen fluoride. It can be used as such but
also in dilute form, for example mixed with nitrogen.
According to the invention, both reactants are reacted in
the gas phase. Suitable reaction temperatures are, for
example, those in the range from 250 to 600~C. Prefer-
ably, the reaction is carried out in the range from 300
to 550~C, in particular in the range from 350 to 500~C.
The process according to the invention can be carried out
at any desired pressures in the reaction zone, as long as
the reactants r i n in the gas phase at the pressure
chosen in each case. Preferably, the pressure used is
1 to 3 bar, in particular atmospheric pressure or super-
atmospheric pressure corresponding to the flow resistance
of the apparatus used.
Le A 27 473 - 2 -
X03~i~220
The relative amounts of hexachloropropene and hydrogen
fluoride used can be varied wilthin wide limits. It is
advantageous to use the hydrogen fluoride in excess, for
example 5 to 100 mol of hydrogen fluoride per 1 mol of
hexachloropropene. It is particularly preferred to use
10 to 50 mol of hydrogen fluoride per 1 mol of hexa-
chloropropene.
Examples of suitable catalysts for the process according
to the invention are halides and oxides of metals and
transition metals. In particular, chlorides, fluorides
and/or oxides of copper, chromium, iron, bismuth, zinc,
lanthanum, cerium, zirconium, vanadium, molybdenum,
tungsten and/or platinum which may have been mixed are
suitable. Preference is given to chromium(III~ salts
alone or in a mixture with the metal chlorides, fluorides
and/or oxides mentioned. The catalysts can be used as
such, for example in pallet form, but can also be depo-
sited on a support, for example on alumina, magnesium
oxide, magnesium fluoride, calcium fluoride, zinc chlor-
ide and/or activated carbon.
Particular preference is given to chromium(III) salts, inparticular chromium(III) fluorides, chromium(III) chlor-
ides and chromium(III) oxides on one of the support
materials mentioned.
The flow rate of the reaction mixture and the catalyst
amount can be chosen such, for example, that catalyst
charges of 50 to 1000 g/l x h, preferably 150 to
Le A 27 473 - 3 -
~3~i2;~0
500 g/l x h, are obtained.
Suitable materials for the reaction and secondary appara-
tuses are material~ which are resistant to the attack of
hydrogen fluoride and hydrogen chloride even at high
temperature~, for example nickel, chromium and/or molyb-
denum steels, and pure nickel.
The reaction according to the invention can be carried
out, for example, by heating the starting materials
combined or separately to the reaction temperature, then
passing them through a reaction zone (for example a
heatable tube containing the catalyst), if desired
washing the g~fi mixture leaving the reaction zone and
cooling it, so that at least the organic components are
condensed and, if desired, separating them further by
distillation and purifying them.
After carrying out the process according to the inven-
tion, mixtures of fluorine- and/or chlorine-containing
propanes and propenes are in ~eneral obtained. The
propenes can be recycled into the reaction according to
the invention.
The composition of the mixture leaving the reaction zone
can be influenced by the reaction temperature. Particu-
larly high proportions of 1,1,1,3,3,3-hexafluoropropane
(formula (I), X = hydrogen) are obtAine~ by carrying out
the reaction at relatively high temperatures, for example
at 435 to 525~C, in particular at 450 to 500~C.
Le A 27 473 - 4 -
X03~0
Particularly high proportions of 2-chloro-1,1,1,3,3,3~
hexafluoropropane are obtained by carryinq out the
reaction at relatively low temperatures, for example at
325 to 415~C, in particular at 350 to 400~C.
~he process according to the invention enables the
preparation of hexafluoropropanes of the formula (I) in
a simple and low-cost manner. If the halogenated propenes
present in the reaction mixture are recycled, hexafluoro-
propanes of the formula (I) can in general be obtained in
yields of more than 80 ~, relative to the hexachloro-
propene used. If desired, CF3-CCl2-CF3 and/or CF3-CHCl-CF3
can be separated off from the reaction mixture and
hydrogenated catalytically either separately or in a
mixture with 1,1,1,3,3,3-hexafluoropropane to give
1,1,1,3,3,3-hexafluoropropane.
This catalytic hydrogenation can be carried out in a
manner known per se, for example by passing a mixture of
hydrogen and CF3-CCl2-CF3 and/or CF3 CHCl-CF3 over a fixed
bed of hydrogenation catalyst. The molar ratio of hydro-
genatable compounds to hydrogen can be, for example, 1:3to 1:50. Preferably it is 1:4 to 1:20.
The hydrogenation can be carried out, for example, at
atmospheric pressure or at superatmospheric pressures,
for example in the range from atmospheric pressure to
20 bar. Preferably, it is carried out at atmospheric
pressure.
Le A 27 473 - 5 -
~(~3~i221~
Suitable hydrogena~ion catalysts are in particular those
containing transition metals on support materials. Of the
transition metals, palladium and platinum are preferred,
in particular palladium. Examples of support materials
are activated carbons, aluminas, silicas, barium sul-
phate, spinels, silicates and titanium dioxide. Activated
carbons and lithium/aluminium spinels are preferred. The
catalysts can contain, for example, 0.5 to 30 g of
transition metal per litre of support material. Prefera-
bly, this content is in the range 2 to 20 g/l.
The flow rate of the hydrogenation mixture and the amountof catalyst can be chosen, for example, such that cata-
lyst charges of 10 to 1000 g/l x h, preferably those of
50 to 500 g/l x h, are obtained. The reaction tempera-
tures are in general above 20~C, preferably in the range100 to 250~C.
The mixture formed in the hydrogenation can be worked up,
for example, by washing it with water or dilute base to
remove the hydrogen chloride formed and condensing the
gaseous products, if desired after drying.
Examples
Examples 1 to 4
40 g of hexachloropropene, 80 g of hydrogen fluoride and
1 1 of nitrogen per hour were passed through a nickel
tube containing 750 ml of a catalyst prepared according
to Example 8 at the temperature given in each case. The
Le A 27 473 - 6 -
;~03~Z~
gas mixture leaving th~ reaction zone was washed with
water, dried, and the condensable portions were condensed
at -78~C.
The content of organic components in the condensate was
determined by gas chromatography and by nuclear magnetic
resonance spectroscopy. The following results were
obtained:
Le A 27 473 - 7 -
~)3~
c
D c~
o ~
o ~ In r~
to
a u
o o ~;
0
U D
o
oo cn ~ a~ ~) ~ .4
O
Z .,~
¢ ~Q
,_ 0
J U a)
O
U~ O ~ ~ U~
~ J
~ O O ~D ~ ~ I ~n
,4-- I ~ ~ 0
~a
.
O
", ~" U ~ 14 14 U ~,
U ~U U U C) U ~ H
Le A 27473 - 8 -
: 03~
Examples 5 to 7
An upright electrically heatable tubular quartz reactor
(length 310 mm, diameter 36 mm) was charged with 200 ml
of a supported catalyst containing 18 g of palladium per
litre of a globular lithium/al~minium spinel ~globule
diameter 3 to 5 mm).
The catalyst was conditioned at 250~C for 6 hours while
passing 20 to 25 ml of hydrogen per hour through it.
After that, the hydrogenations described below were
carried out in each case. The gases leaving the quartz
tube were condensed at -78~C and analysed by means of
l9F-NMR spectroscopy.
Example 5
Amounts used: 0.16 mol/h of CF3-CHCl-CF3 and 2.5 mol/h of
hydrogen
Reaction conditions: 200~C, atmospheric pressure
Catalyst charge: 150 g/l x h
CF3-CH2-CF3 was obtained at a conversion of 95 ~ and a
selectivity of 94 ~.
Example 6
Amounts used: 0.2 mol/h of CF3-CHcl-cF3 and 0.8 mol/h of
hydrogen
Reaction conditions: 200~C, atmospheric pressure
Catalyst charge: 180 g/l x h
Le A 27 473 - 9 -
~0;3~;2~
CF3-CH2-CF3 was obtained at a conversion of 92 ~ and a
selectivity of 85 ~.
Example 7
~ mounts used: 0.21 mol/h of CF3-CC12-CF3 and 1.1 mol/h of
hydrogen
Reaction conditions: 200~C, atmospheric pressure
Catalyst charge: 200 g/l x h
CF3-CH2-CF3 was obtained at a conversion of 89 % and a
selectivity of 87 ~.
Example 8
300 g of CrCl3 x 6 HzO and 30 g of MgF2 were heated to
90~C in lO l of water. After 1 hour, 1300 g of an 11 %
strength aqueous ammonia solution were metered in. The
mixture was then stirred for 1 hour, allowed to cool, and
the precipitated solid was filtered off through a nutsche
filter. The solid was washed twice with water, dried,
powdered and mixed homogeneously with 2 % by weight of
graphite. This mixture was compacted to give tablets of
4 mm in size.
Le A 27 473 - 10 -