Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2036~3~
SU3STITUT~D PHENY~SU~PHOi~RIAZIl~UREAS,
SALTS THEREO~ AND AGEN~ FOR P ~JT GROWTH
STI~i~ULATIO~ AI~ IiEIBITION BAS~D THEREON
The ?resent invention relates to organic chemistry and, mo-
re specifically, to novel compounds - substituted phenylsul-
phonyltriazinylureas, their salts and an agent for plant growth
stimulation and inhibition based thereon.
An important problem in agriculture is increasing yield of
crops. ~or this purpose plant-gro~-rth stimulants and herbicides
are employed.
A simultaneous use of herbicides and growth stimulants
brings about certain difficulties, since specificity of their
effect depends on the variety of the treated farm crop.
Known in the art are different compounds featuring a her-
bicidal activity. Thus, known in the art is a compound, viz.
1-(2-chlorobenzenesulphonyl)-3-(4-methyl-6-metho~y-1,3,5-tria-
zin-2-yl)-urea. A preparation based on this compound is useful
in agriculture for protection of sawings of wheat, barley, rye,
oats and lin from weeds (U~, A, 4127405).
Also known are compounds which possess, apart from their
herbicidal activity, properties of plant growth regulators as
well. As a rule, the effect of these compounds is accompanied
by retardation, growth-inhibition and formative effects. Such
compounds may be e~emplified by arylsulphonylurea derivatives
of the general formula:
~'
- 20~63~
N(CH3)2
R N ~ ~
S02NHCO~H ~ OCH3
~herei~: R = Cl, CO2CH3, CH3
In a dose of from 60 to 400 g/ha such compounds display
herbicidal properties &nd possess retardation, growth-inhibi-
tion or growth-formation effects (US, A, 4231784).
However, these prior art compounds inhibit gro~th of farm
crops.
The compounds according to the present invention are no~el
and hitherto u~nown from the literature.
It is an obj~ct of the present invention to provide novel
compounds possessing growth-stimulation activity with a simul-
taneous herbicidal effect.
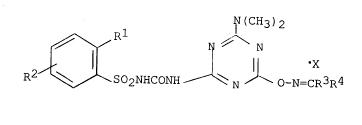
This object is accomplished by ~lovel compounds, viz. sub-
stituted phenylsulpho~yltriazinylureas and salts thereof cor-
responding to the general formula:
~J(CH3)2
R~ ~ SO2NHCO~H ~ ~ ~ o-N=CR3R4
R' = Cl, NO2, COOOH3,
R _ H, Cl,
R3 = H, CH3
R CH3, C2~5, C6H5~ C6H4Cl~2; X is absent, or
- 2~3e334
/C2H5
X = K, Na, HN - C2H5
\ C2H40H
The compounds according to the present inven-
tion show growth-stimulant and herbicidal activity.
An agent for stimulation and inhibition of
plant growth incorporating an active principle and a
diluent, according to the present invention contains
as the active principle the compounds according to
the present invention - substituted phenylsulphonyl-
triazinylurea or salts thereof. Preferably, the
agent according to the present invention incorpo-
rates, as the active principle, 1-(2-chlorobenzene-
sulphonyl)-3-(4-dimethylamino-6-isopropylidene-
iminooxy-1,3,5-triazin-2-yl)-urea or its potassium,
sodium or diethylethanolammonium salt of the follow-
ing formula:
S02NHCONH ~ ~-N=C~ 3
CH3
/C2H5
wherein X is absent or X = K, Na, HN C2H5
C2H40H
The compound according to the present invention
may also incorporate, preferably, as the active
principle 1-(2-chlorobenzenesulphonyl)-3-[4-di-
methylamino-(a-methyl)-propylideneiminooxy-1,3,4-
triazin-2-yl]-urea or its potassium, sodium or
203~33~
-- 4 --
diethy~mmOnium salt of the following ~ormula:
~(C~I3)2
Cl ~ ~ ~
S02~HCONH ~ ~ ~ O-N=C 3
C ~ 5
2H5
herein X is absent, or X = K, Na, HN \ C2~5
C2X40H
The compounds according to the present invention have a
high activity and are used for stimulation and inhivition of
plant growth.
The novel compounds accordi~g to the present invent_on -
substituted phenyl~ulphonyltriazinylureas are stable ~ihite
crystalline substances sparingly solub~e in ~ater and i~ or-
~anic sol-~ents.
/~he c~mpounds according to the present invention - substi-
tuted phenylsulphonyltriazinylureas are prepared oy reacting
substituted benzenesulpho~ylisocyanates with substituted 2-
ami~o-1,3,5-triazines:
~(CH3~2
~ - 5~ ~ICO + ~H ~ ~`~ ~ ,R34
R~ N(CH3)2
R S02~HCOw~ CB.3F~4
herein R' = Cl, NO2, COGH3,
R2 = X,Cl,
-- 5 --
2~36334
R3 = H, CH3
R4 = CH3, C2~s~ C6X5~ C6H4
The starting components are employed in the ~ei~ht ratio
of 1~ he process is conducted in an organic solvent (ben-
e r
zene, xylene, toluene, actonit~ile~ tetrahydrofuran) in tne
presence of catalytical amounts of tetramethylethylenediamine
at a temperature of from 50 to 60C for 2 hours. T~len the re-
action mass temperature is lowered to 20C and the mass is
stirred for aaditional 2 hours till completion of the reaction.
The precipi-tate is filteredoff, washed with the solvent in
which the reaction has been carried out and dried.
The compounas accorcing to -the present inven~ion can be al-
so prepared '3~ reacti-ng a substi~uted arylsulphonamide with
chlorosulphonylisocJanate and substituted 2-amino-1,3,5-tria-
zine in an organic solvent (toluene, ~ylene) at a temperature
of 60-70C for 3 hours.
N(CH3)2
R~ R1 + ClS02NC0 + N~
S2NH2 H2N ~ ~l o-M=CR3R4
R 1 (CR3)
wherein: R1 = Cl1~02, C~OCH3
R2 = H Cl 2~3~334
- H, ~H3
i~.4 = ~I3, C2II5~ ~6~ 6~4
The structures of the synthesized substituteQ phenylsul-
p'nonyltriazinylureas have been proven by methods of elemental
analysis, IR- and PMR-spectroscopy. IR-spectra were taken by
means of a "PY~ UlJIC~I" instrument using ~3r tablets. Pi~iIR-
spectra were taken by means of a "~I-250'1 instrument in deute-
roacetone using tetramethylsilane as an internal s~andard re-
~erence. Given as an example is a ~F~.-spectrum of 1-(2-chloro-
benzenesulhonyl)-3-(4-dimethylamino-6-isopropylideneiminoo~y
-1,3,5-triazi-n-2-yl)-urea, wherein there are signals from pro-
tons of the isopropylidene group at 2.04 ppm (~), 6H of dime-
thylamino group at 3.11 ppm (d), 6I1 signals from protons of
the phen~l ring at 7.~-7.7 ppm (m), 3.I and ~.22 ppm (d, d) H6.
The signals from protons of two ~ groups are observed at
9.33 ppm (s) 1H and 13.59 ppm (~) 1H. ~he location of these
signals was unambiguously proven by means of sy~thesized sul-
phonylurea tagged b~ 15N isotope at the nitrogen atom of the
sulphamide group. The Spi~-3pi~ cleavage 15~-H signal at
13.59 pp~ equal to 90 Hz has made it possible to u~ambiguously
assign this resonance signal to hydrogen of the ~ulph~mide group.
The salts of substituted phenylsulphonyltriazinylureas are
crystalline substances soluble in ~iater and in some organic
solvents such as alcohols, glycols, sulphoxides.
~ he salls according to the present invention are prepared by
reacting substituted phenylsulphonyltriazinylureas with an al-
~ 7 ~ 2Q 3G334
kali metal (potassium or sodium) hydroxide or diethylethano-
lamine at the ~ss ratio of 1:1.1 in an organic solvent (al-
cohols) or in ~rater at a temperature of from 35 to 40C for 3
hours. ~he structure of the c~-aimed salts was verified by me-
thods of P,ilR and IR-spectroscopy. Thus, in a P"~-spectrum of
soaium salt of 1-(2-shlorobenzenesulphonyl)-3-(4-dimethylami-
no-6-isop~opylideneiminooxy-1,3,5-tria~in-2-yl) urea taken in
deu~eroacetone the sig~al from proton at I~-1 a~om disappears.
In the reaction of the substituted ureas according to the pre-
sent invention with fatty amines oil~ compound~ are formed.
~lemental analysis of, for example, a product of the reac-tion
~hY~,J
of d~'e~nolamine and the above mentioned substituted u~ea po-
ints -to tihe formation of an adduct of the reactants i~ the ra-
tio of 1:1. In a Pr~;~R-spectrum o~ this adduct taken in deutero-
acetone there are no signals of prolons of both amide groups
which points to the formation of a compound having a salt-liXe
character.
Given hereinbelow are the data of l~J~-spectra of the above-
-mentioned substitut ~ ea according to the present invention
and i~s sodium and a olamine salts:
2.03d, C---'~3
~ CH3
~(CH )
Cl / 3 2 3.11d, N(CH3)2
1 2 ~; ~ 7.69-3.13m, Ar
~' ~o~HCO~H~
O-N=C(cH3)2 10.69c 2
13.61c,
2Q3633~
-- 8 --
Cl ~ ( 3)2 ~- 2.05 c, C C C~3
SO l~T - CO~X ~ / CH
2~a+ N/_ O`l`~=C~ 3 3.08 d, N(CH3)2
7.5~-~.03 m, Ar
9.13 c, I~L
1.1~ c, CH3(C2H5)
Cl N(CH3)2
S02NHCO~H- ~/
O-~=C(CH3)2 2.02 d, C(CH3~(Het)
' 2.98 q, CH2(C2X5)
(C2H5)2 N CH2cH20H 3.15 d, ~(CH3)2
3.3 m, CII2CH2
- 6.35 c, OH
7.5-~.1 m, Ar
','Ile ~ I spec'ra ~-Jere take-n by me`ans of a "WM-250" ins-
trument at the freGuency of 250.13MHz for H+.
'rhe above-given data e-nable assumption of the following
struc-ture for the salt accordin~ to the pre~ent invention:
2~3633~
O ~(CH ~2
S02~ 01~1=C(CH3)~
-M'
C2H5 ¦ CH2 C~20H
C2H5
All tlle compounds according to the present invention eY~hi-
bi-t a plant ~lowth-s-timulant and growt~ ~ibiting activity.
~ he compi~unds accoraing to tne pre3ent inven-tion are active
principles of compositions inte~ded for stimulation a~d inni-
bi-tion of pLant ~rowth, ~he activity of the compounds accor-
~ing to the present invention and of agents based thereon tVas
studied on ~lan-t~ under laboratory and ~ield conditions.
The compounds according to the present invention are capa-
~le of substantially accelerating the development of some farm
plants durin~ earlier stages of or~ano~ene3is, thus resulting
ill the formation of larger-size plants, i.e. the compounds ac-
cording to the present invention selectively stimulate o~ly
farm crops simultaneously inhibitinsthe gro~rth and cevelopment
of weeds
The compounds accordin~ to the present lnvention ~rere sub-
jected to biological tests on grain crops (for e~ample, wheat,
barley), corn and cotton, It was revealed that the compounds
according to the presen-t invention were superior to the known
preparations such as GLIN*, GIBBERELIN* and ATRAZIN* in their
* Trade Mark
- 10 -
- 2Q3i~334
herbicidal and growth-stimulant activity.
Gibberelin, ~rhile causing elongation of the stem, produced
no positive ef~:ect on the biomass of the above-earth part of
the pl2n' ~ wherefore they Y~ere formed thinned and lodging.
Atrazin produced an effect similar to that of the compounds
according to the present invention, but in 10 and 25 times
greater doses.
All -the compounds accordin~ to the present invention are
lo~ toxic, t'ne ~50 on white mice intramuscularl~ is over
5,000 mg/kg. An agent ~asea on the compounds according to the
present invention can be used as wettable po~ders, granules
and solutions. The compound according to the present invention
is intended for the treatment of both green plants and tile so-
il by methods of spraJing, powdering, wetting or pelletizing
of seeds.
~ or a better understanding of the present invention, ~o~e
specific e~amples illustrating preparation of the compounds,
testing of their activity and use are given hereinbelow.
~ ample 1
1-(2-Chlorobenzenesulphonyl)-3-(4-dimethylamino-6-isopropyli-
deneiminoo~v-1,3,5--triazin-2-yl)-urea /Compound I/.
~ o a suspension of 9.57 g (G.05 mol) of 2-chlorobenzenesul-
phonamide and 0.3 ml of tetramethylethylenediamine in 100 ml
of o-xylene there were added 7.1 g (0.05 mol) of chlorosulpho-
nylisocyanate at the temperature of 20C. ~he reaction mixture
~as heated for 3 hours at a temperature of 95-100C, cooled
and added with 11.2 g (0.05 mol~ of 2-amino-4-dimethyl-amino-
2Q~33~
- 11 -
6-isopropylideneiminooxy-1,3,5-triazine; the mixture was hea-
ted for 3 hours at a temperature of 50 to 60C, cooled; the
precipitate was filtered-off to give 17.0 g (~0~) of compound
I, m.p. 184-186C (f ~m ac~tonitrile).
~i'ound, ,~0: C 41.94, ~ 4.34, Il 22.o1~ Cl 6.36~ ~ 8.14.
C1 5'118CLi.704S.
Calculated, ): C 42.11, H 4.24, l~ 22.92, Cl 3.2~, ~ 7.50.
~ ample 2
1-(2-Chlorobenzenesulphonyl)-3-(4-dimetllylamirlo-6-isopropyli-
deneiminooxy-1,3,5--triazin-2-yl)-urea (Compound I).
To a suspension of 19.0 ~ (0.09 mol) of 2-amino-4-dimethy-
lamino-6-iso?ropylideneiminooxy-1,3,5-triazine in 5G ml of
oenzene neated ~o the rempelature of 60C a sulution of 21.7 g
(0.1 mol) of 2-chlorobenzenesulphonylisocyanate in 100 ml of
benzene ~as gradually added. The reaction mass ~as stirred for
3 hours at a temperature of 60-70C and then for 2 hours at
room temperature. The precipitate ~as filtered-off, washed
with benzene and dried to give 36.5 g (95jO) of 1-(2-chloroben-
zenesulphonyl~-3-(4-dimethylamino-6-isopropylideneiminooxy-
1,3,5-triazin-2-yl)-~rea, m.p. 1~4-186C. ~he data of elemen-
tal analysis were similar to those give in 3xample 1 hereina-
bove.
3xample 3
1-(2-Chlorobenzenesulphonyl)-3-[4-Qimethylamino-6-(C~-methul)-
propylideneiminooxy-1,3,5-triazin-2-yl] -urea (Compound 2).
~ 'o a suspension of 2.06 ~ (0.0092 ~ol) of 2-amino-4-dime-
thylamilo-6-(cC -methyl)propylideneiminooxy-1,3,5-triazine in
203633~
40 ml of dry benzene 1 drop of tetramethylethylenediamine ~ a~
added, the mixture ~ras heated to a temperature of 50-60C and
added with 2,15 g (0.0099 mol) of 2-chlorobenzenesulphonyli-
socyan2te. The reaction mi~ture was stirred for 2 hours at
the temperature of 60C and for 2 hours at tile te:lperature of
20~, the precipitate ~i~ras filtered-off to give 4.0 ~; (95~) of
compound 2, m.p. 172-173~ (decomp.).
Found, %: C 43.22, H 4.68, N 22.31, Cl 7.81, ~ 7.46.
C1 6I~20Cl~i704S.
Calculated, ~: C ~3.49, ~1 4.56, ~T 22.19, Cl ,~.02, ~ 7.25.
E~ample ~.
1-(2-Cl1lorooenzenesulhonyl)-3-(4-dimethylamino-6-benzylide-
neirninooxy-1,3,~-triazin-2-yl)-urea ~Compound 3).
In a manner similar to that dcscribed in Example 2 hereinbe-
fore from 2-chlorobenzenesulphonJlisocyanate and 2-amino-4-
di.me'-hylamino-6-benzylideneiminoo~vr-1,3,5-triazine the above-
icien~ified urea /as obtained, m.p. 173C, the yield quantita-
tive.
~ ound, -,0: " 47.67, H 4.05, N 20.65, Cl 7.61, ~ G.75
C l Cl -~ O '~
Calculated, i: ~ 47.95, ~-~ 3.~ 20.65, Cl 7.45, ~1 6.73.
~:~ample
1-(2-chlorobenzenesulphonvrl~-3- [4-dimethylamino-6-(2-c~îloro)
-benzylideneiminoo~y-1,3,5-triazin-2-~ -urea (~ompound 43.
In a manner similar to ~ha-t discribed in ~xample 2 herein-
before, from 2-chlorobe-nzenesulphonglisocyanate and 2-amino-
; - 13 -
2~)3~334
4-dimethylamino-6-(2-chloro)-benzylideneiminooxy-
1,3,5-triazine the above-identified urea was
obtained, m.p. 167-168C, the yield 96.5%.
Found, %: C 44.80, H 3.64, N 18.94, Cl 13.76,
S 6.34.
ClgH17C12N704S -
Calculated, %: C 44.71, N 19.22, H 3.36, Cl
13.89,
S 6.28.
Example 6
1-(2-Nitrobenzenesulphonyl)-3-(4-dimethylamino-6-
isopropylideneiminooxy-1,3,5-triazin-2-yl)urea
(Compound 5).
In a manner similar to that described in
Example 2 hereinbefore, from 2-nitrobenzene-
sulphonylisocyanate and 2-amino-4-dimethylamino-6-
isopropylideniminooxy-1,3,5-triazine the above-
identified urea was obtained, m.p. 190-192C, yield
88%.
Found, %: C 40.87, H 4.21, N 25.35, S 7.46.
Cl 5H1 8N86S -
Calculated, %: C 41.09, H 4.14, N 25.87,
S 7.31.
Example 7
1-(2-Nitrobenzenesulphonyl)-3-[4-dimethylamino-6-(a-
methyl)-propylideneiminooxy-1,3,5-triazin-2-yl]urea
(compound 6).
In a manner similar to that of Example 2, from
2-nitrobenzenesulphonylisocyanate and 2-amino-4-di-
methylamino-6-(a-methyl)-propylideneiminooxy-1,3,5-
triazine the above-identified urea was obtained,
m.p. 187-189C, yield 85%.
Found, %: C 41.92, H 4.39, N 24.16, S 7.11.
Cl 6H2 oN86S -
Calculated, %: C 42.48, H 4.46, N 24.77,
S 7.08.
- 14 -
2Q3G33~
Example 8
1-(2-Nitrobenzenesulphonyl)-3-(4-dimethylamino-6-
benzylideneiminooxy-1,3,5-triazin-2-yl)-urea
(Compound 7).
In a manner similar to that of Example 2, from
2-nitrobenzenesulphonylisocyanate and 2-amino-4-di-
methylamino-6-benzylideneiminooxy-1,3,5-triazine the
above-identified urea was obtained, m.p. 194-196C,
yield 82%.
Found, %: C 46.73, H 3.69, N 23.02, S 6.61.
Cl gHl 8N806S -
Calculated, %: C 46.91, H 3.73, N 23.03,
S 6.59.
Example 9
1-(2-Methopxycarbonylbenzenesulphonyl)-3-(4-di-
methylamino-6-isopropylideneiminooxy-1,3,5-triazin-
2-yl)-urea (Compound 8).
In a manner similar to that of the foregoing
Example 2, from 2-methoxycarbonylbenzenesul-
phonylisocyanate and 2-amino-4-dimethylamino-6-iso-
propylideneiminooxy-1,3,5-triazine the above-
identified urea was obtained, m.p. 187-189C, yield
90% .
Found, %: C 45.5, H 5.0, N 21.57, S 7.24.
Cl 7H2 lN706S -
Calculated, %: C 45.23, H 4.69, N 21.72,
S 7.10~
Example 10
1-(2-Methoxycarbonylbenzenesulphonyl)-3-[4-di-
methylamino-6-(a-methyl)-propylideneiminooxy-1,3,5-
triazin-2-yl]-urea (Compound 9).
In a manner similar to that described in Exam-
ple 2 hereinbefore from 2-methoxycarbonylbenzene-
sulphonylisocyanate and 2-amino-4-dimethylamino-6-
(a-methyl)-propylideneiminooxy-1,3,5-triazine the
above-mentioned urea was obtained, m.p. 170C
(decomp.), yield 92%.
- 15 - 2036331
Found, %: C 46.51, H 5.08, N 21.08, S 6.62.
Cl 8H2 3N76S -
Calculated, %: C 46.45, H 4.98, N 21.06,
S 6.89.
Example 11
1-(2-Methoxycarbonylbenzenesulphonyl)-3-t4-di-
methylamino-6-benzylideneiminooxy-1,3,5-triazin-2-
yl)-urea (Compound 10).
In a manner similar to that described in Exam-
ple 2 hereinbefore, from 2-methoxycarbonylbenzene-
sulphonylisocyanate and 2-amino-4-dimethylamino-6-
benzylideneiminooxy-1,3,5-triazine the above-
mentioned urea was obtained, m.p. 163-164C
(decomp.), yield 91%.
Found, %: C 50.27, H 4.36, N 19.84, S 6.13.
C2 lH2 lN7o6s -
Calculated, %: C 50.50, H 4.24, N 19.68,
S 6.42.
Example 12
1-(2-Methoxycarbonylbenzenesulphonyl~-3-[4-di-
methylamino-6-(2-chloro)-benzylideneiminooxy-1,3,5-
triazin-2-yl]-urea (Compound 11).
In a manner similar to that of Example 2
hereinbefore, from 2-methoxycarbonylbenzene-
sulphonylisocyanate and 2-amino-4-dimethylamino-6-
(2-chloro)-benzylideneiminooxy-1,3,5-triazine the
above-identified urea was obtained, m.p. 162-163C
(decomp.), yield 96%.
Found, %: C 47.64, H 3.93, N 18.64, Cl 6.26,
S 5.75.
C2 1H2 oClN706S -
Calculated, %: C 47.24, H 3.78, N 18.36,
Cl 6.64, S 6Ø
Example 13
1-(2,5-Dichlorobenzenesulphonyl)-3-(4-dimethylamino-
6-isopro-
- 16 - 203~334
pylideneiminooxy-1,3,5-triazin-2-yl)-urea (~ompound 12)
In a manner similar to that of Example 2 from 2,5-dichlor-
benzene9ulphonylisocyanate and 2-animo-4-dimethylamino-6-iso-
propylideneiminooxy-1,3,5-triazine the above-mentioned urea
was obtained, m.p. 170-172~, yield 90~.
~ ound, ~: C 38.81, H 3.48, Cl 15.01, N 20.96.
C 1 5H1 7C12N704S .
Calculated, ~: C 38.97, H 3.77, Cl 15.96, N 21.21.
3gample 14
1-(2,5-Dichlorobenzenesulphonyl)-3- [4-dimethylamino-6-(~ -
methyl)-propylideneiminooxy-1,3,5-triazin-2-yl¦ -urea (Compo-
und 13).
In a manner similar to that described in ~xample 2, from
2,5-dichlorobenzene~ulphonylisocyanate and 2-amino-4-dimethy-
lamino-6-(cL-methyl)-propylideneiminoogy-1~3,5-triazine the
above-iden-tified urea was obtained, m.p. 154-156C, yield 92~o.
~ ound, ~: C 40.68, H 4.05, N 20.55, Cl 14.54, S 6.15.
C1 6H1 gC12N704S
Calculated, ~ 40.34, H 4.02, N 20.59, Cl 14.88, S 6.73.
~ ample 15
1-(2,5-Dichlorobenzenesulphonyl)-3-(4-dimethylamino-6-benzyl-
ide~eiminooxy-1,3,5-triazin-2-yl)-urea (Compound 14).
In a manner similar to that described in Example 2 herein-
before, from 2~5-dichlorobenzenesulphonylisocyanate and 2-ami-
no-4-dimethylamino-6-benzylideneiminoogy-1,3,5-triazine the
above-mentioned urea was obtained, m.p. 180-182~, yield 8~%.
- 17 - 203e33~
Found, %: C 44.51, H 3.08, Cl 13.82, N 18.96.
C1 gH1 7C12I~704S .
Calculated, ~: C 44.72, H 3.36, Cl 13.89, N 19.22.
Example 16
1-(2,6-Dichlorobenzenesulphonyl)-3-(4-dimethylamino-6-i~opro-
pylideneiminooxy-1,3,5-triazin-2-yl) urea (Compound 15).
In a ma~ner similar to that described in ~xample 2, from
2,6-dichlorobenzenesulphonyli~ocyanate and 2-amino-4-dimethyl
-amino-6-isopropylideneiminooxy-1,3,5-triazine the above-men-
tioned urea ~as obtained, m.p. 168-170C, yield 85,o.
Found, %: C 40.12, H 3.9, Cl 15.54, N 21.54.
C15Ht7Cl2N704S.
Calculated, ~: C 38.97, H 3.71, Cl 15.34, N 21.21.
Example 17
1-(2,6-Dichlorobenzene~ulphonyl)-3- L4-dimeihylamino-6-(~-me-
thyl)-propylideneiminooxy-1,3,5-triazin-2-yl~ -urea (Compound
16).
In a manner similar to that de~cribed in Example 2 herein-
before, ~rom 2,6-dichlorobenzenesulphonylisocyanate and 2-ami-
no-4-dimethylamino-6-(~-methyl)-propylideneiminooxy-1,3,5-
triazine the above-mentioned urea Y~a~ obtained, m.p. 164-166C
yield 86~o.
~ ound, ~0: C 40.62, H 4.08, Cl 15.20, IJ 20.81.
C16H19Cl2N704S.
Calculated, %: C 40.34, H 4.02, Cl 14.88, N 20.59.
20~6334
- 18 -
Egample 18
1-(2,6-Dichlorobenzenesulphonyl)-3-(4-dimethylamino-6-benzyl-
ideneiminoogy-1,3,5-triazin-2-yl)-urea (Compound 17)
In a manner similar to that of ~xample 2, from 2,6-dichlo-
robenzenesulphonylisocyanate and 2-amino-4-dimèthylamino-6-
benzylideneiminooxy-1,3,5-triazine the above-mentioned urea
was obtained, m.p. 171-173C, yield 8g~.
~ ound, ~0: C 44.98, H 3.70, Cl 14.12, 1~ 19.49.
Cl gH1 7C12N704~ .
Calculated, ~: C 44.72, H 3.36, Cl 13.83, I~ 19.22.
The Il~- and Pr~R-spectra of substituted phenylsulphonyltria-
zinylureas are shown in Table 1 hereinbelow.
2Q36334
a) ~
.
~q
0
a) c
D~ ~Lf~ ~D G~
~ IC~
.
~ ~ O
o ~ ~ O h'~ L
~2~/ ^~;
\ ~11
~ ~ O ~O COO Lr~ O Lr~
~ ~ h 1~u~ Lr~ ~ ~D O ~ O
O ` HC~
O O ~ ) L~ O Lf~ G~
h ~t J
C) ~ C~
&) I
~ ~ L~ V C~l ~)
P~ V V ~
V
V V
~;
H C~l
V V V V
.
O ~
203G33 1
L~ L~ L~ O O C~l
O~ ~ 0 ~ 0 C3 ~ C~.l (~J
~. . . . .
O O ~ 0 0 0 0
O ~ ~C3 ~ 0 0 N
o o 5~ 0 ~ ~O
. . . . . . . .
0 ~t~ C~ N'r~ L~ 0 ~ O
O C~l O ~ OC~l O O ~
U~ ~O OO O C~J ~ L~ ~DL~ '~ Ir~ O O 1~ L~ O
L~ O~D 00 ~ D (~U~ O
o
c~l
~l t~ ~ L" O '~ ~\ Il~ O C\~l O O
0 ~D ~ ~O ~ ~ ~ ~D ~ ~ 0 0 ~D
L~ Lr~ L~ r--l ~ L'~
L~ ,' ~ r,~r ',~, V '~
V C~ V C~J
V V V V V $ V
V
~r ~ ~--r ~r
V V C~ V V V
H H
V V
~r
r~ ~ .
C~ O O O~, ~' ~, ',I;
~r ~ ~ V VV
CU r--l ~1
a) oo oo v v
r-l VV VV
.~
o
203G334
-
o~ ~ ~ C--
~ -
L'~ 0
L" ~ ~D
-
0 L'~ C;) L"
O O O O
~ ~ _ _
O L~ OO Od~ O
~ OL'~ O~D O~D
C\l
O L" C~ OO ~J O O
L" L" Il~
~D X
V V V V
V
H H H H
V V V V
I I I I
L~ ~D ~S)
~r
o
V V V V
a~
,(~
E~ . . -
- 22 - 2 0 3 ~33~
Egample 19
~odium salt of 1-(2-chlorobenzenesulphonyl)-3-(4-dimethylami_
no-6-isopropylideneiminooxy-1,3,5-triazin-2-yl)-urea (Compo-
und 18).
To a solution of 0.6 g (0.015 mol) of sodium hydroxide in
20 ml of water 4.28 g (0.01 mol) of 1-(2-chlorobenzenesulpho-
nyl)-3-(4-dimethylamino-6-isopropylide~eiminoogy-1,3,5-tria-
zin-2-yl)-urea were portion-wise added under stirring. ~he
reactio~ mi~ture ~as stirred at a temperature of 40-45C for
3 hours till dissolution of sulphonylurea. The resulting re-
action mass was filtered, water ~ras evaporated under a reduced
pressure and at a,emperature of not over 50C. The precipi-
tate was washed wi~h ether, dried in vacuum to give 4.3 g
(90,~0) of the above-specified salt, m.p. 178C (decomp~.
~ ound, ',j'0: C 40.21, rI 3.96, Il 22.0, S. 7.36. C15H17ClN704SNa.
Calculated, C,v: C 40.04, ~ 3.78, Il 21.8, S 7.12.
~ xample 20
Sodiun salt of 1-(2-chlorobenzylsulphonyl)-3- L4-dimethylami-
no-6-(-~ -meth-yl)propylideneimi~ooxy-1,3,5-triazin-2-~l ¦ -urea
(Compound 19).
In a manner similar to that de~cribed in the foregoing
~ample 19, Irom sodium hydroxide and 1-(2-chlorobenzene-sul-
phonyl)-3-(4-dimethylamino-6-(~-methyl)-propylideneiminoogy-
-1,3,5-triazin-2-yl)-urea the above-identified salt was obtai-
ned in the yield of 92~, m.p. 161C (decomp.).
~ ound, %: C 41.42, H 4.09, Cl 7.66, I~ 21.14.
C16~I19ClN704 a.
203~334
-
-- 23 --
Calculated, ~9: C 41,48, II 4.13, Cl 7.59, N 21.28.
ample 21
Sodium salt of 1-(2-chlorobenzene~ulphonyl)-3-(4-dimethylami-
no-6-benzylideneiminooxy-1,3,5-triazin-2-yl)-urea (Compound 20)
In a manner similar to that of 13xample 19, from sodium hy-
droxide and 1-(2-chlorobenzenesulphonyl)-3-(4-dimethylamino-
6-benzylideneiminooxy-1,3,5-triazin-2yl)-urea the above-iden-
tified salt was obtained in the yield of 9~, m.p. 156C (de-
comp.).
Found, ~: C 45.~3, H 3.42, Cl 7.13, M 19.69.
C 1 gH1 7C11~704SNa .
Calculated, %: C 45.91, H 3.48, Cl 7.31, N 19.66.
Example 22
Sodium salt of 1-(2-chlorobenzenesulphonyl)-3- ¦4-dimethylami-
no-6-(2-chloro)benzylideneiminooxy-1,3,5-triazin-2-ylJ -urea
(Compound 21).
In a manner similar to that described in 2xample 19, from
sodium hydroxide and 1-(2-chlorobenzenesulphonyl)-3-(4-dimet-
hylamino-6-(2-chloro)benzylideneiminooxy-1,3,5-triazin-2-yl)
urea the above-mentioned salt lNas obtained in the yield of 95~,
m.p. 159C (decomp.).
~ ound, ~0: C 42.73, H 3.G6, Cl 13.41, N 13.56.
C1 gH16C12IJ704SlJa.
Calculated, 9S: C 42.~6, H 3.00, Cl 13.35, M 18.42.
3xample 23
Sodiuun salt of 1-(2-nitrobenzenesulphonyl)-3-(4-dimethylamino-
6-isopropylideneiminooxy-1,3,5-triazin-2-yl)-urea (Compound 22).
-
- 24 _ 2 Q 3 633~
In a manner similar to that of Example 19 hereinbefore,
f~om sodium hydroxide a~d 1-(2-nitrobenzenesulphonyl)_3-(4-
dimethylamino-6-isopropylideneiminooxy-1,3,5-triazin-2-yl)-
urea the above-mentioned salt ~as obtained in the yield of
95%, m.p. 184C (decomp.).
~ ound, ~: C 39.23, H 3.73, Il 24.63, S 6.73. C15H17N806SI~a.
Calculated, ~: C 39.1, H 3.59, Tl 24.35, S 6.96.
Egample 24
Sodium salt of 1-(2-nitrobenzenesulphonyl)-3-(4-dimethylamino
-6-(~ -methyl)-propylideneiminooxy-1,3,5-triazin-2-yl)-urea
(Compound 23).
In a manner similar to that of ~xample 19, from sodium hy-
droxide and 1-(2-nitrobenzenesulphonyl)-3- ¦4-dimethylamino-
6-(~-methyl)-propylideneiminooxy-1, 3,5-triazin-2-yl~ urea
the above-mentioned salt ~as obtained in the yield of 92~,
m.p. 184C (decomp.).
l1'ound, ~: C 40.5, H 4.01, N 23.63, S 6.75~ C16~19~06~IJa.
Calculated,%: C 40.61, H 4.19, N 23.72, S 6.69.
~ xample 25
Sodium salt of 1-(2-nitrobenze~esulp~onyl)-3-(4-dimethylamino-
-6-benzylideneiminooxy-1,3,5-triazin-2-yl)-urea (Compound 24).
In a manner similar to that of 3xample 19, from sodium hy-
droxide and 1-(2-nitrobenzenesulphonyl)-3-(4-dimethylamino-6-
-benzylideneiminooxy-1,3,5-triazin-2-yl)-urea the above-menti-
oned salt was obtained in the yield of 9 ~, m.p. 185C (decomp.)
~'ound~ %: C 44-91, H 3.88, N 22.31. C19H17N806SNa.
Calculated, ~: C 44.88, H 3.74, N 22.04.
2~3633~
-
- 25 -
Example 26
Sodium qalt of 1-(2-methoxycarbonylbenzenesulphonyl)-3-(4-di-
methylamino-6-isopropylideneiminooxy-1,3,5-triazin-2-yl)-urea
(Compound 25).
In a manner ~imilar to that o~ Example 19, from ~odium hy-
droxide and 1-(2-methoæycarbonylbenzenesulphonyl-3-(4-dimethy-
lamino-6-isopropylideneiminooxy-1,3,5-triazin-2-yl)-urea the
above-mentioned 3alt was obtained in the yield of 94~, m.p.
162C (decomp.).
~ ound, ~ C 43.12, H 4.23, N 21.72, S 6.76. C17E20IJ706SNa.
Calculated, ~: C 43.06, ~I 4.39, IJ 21.83, S 6.82.
3xample 27
Sodium salt of 1-(2-methoxycarbonylbenzenesulphonyl)-3- ~ 4-di-
methylamino-6-~C~-methyl3-propylideneiminooxy-1,3,5-triazin-
2-yl~ -urea (Compond 26).
In a manner similar to that described`in ~xample 19, from
sodium hydroxide and 1-(2-methoxycarbonylbenzenesulphonyl-3-
dimethylamino-6-(~ -methyl)-propylideneiminooxy-1,3,5-
triazin-2-yl~ -urea tne abo~e-mentioned salt was obtained in
the yiled o~ 92~, m.p. 151C (decomp.)
~ ound, ~: C 44.35, ~ 4.52, N 20.12, S 6.57. C18H22N706SNa.
Calculated, %: C 44.49, H 4.41, ;l 20.18, S 6.83.
Example 28
Sodium ~alt of 1-(2-methoxycarbonylbenzene3ulphony1)-3-(4-di-
methylamino-6-benzylideneiminooxy-1,3,5-triazin-2-yl)-urea
(Compound 27).
- 26 - 2~3633~
In a manner similar to that of Example 19, from
sodium hydroxide and 1-(2-methoxycarbonylbenzene-
sulphonyl)-3-(4-dimethylamino-6-benzylideneiminooxy-
1,3,5-triazin-2-yl)-urea the above-mentioned salt
was obtained in the yield of 93%, m.p. 154C
(decomp.).
Found, ~: C 48.37, H 3.83, N 18.81, S 6.14.
C2 lH2 0N706SNa .
Calculated, %: C 48.43, H 3.96, N 18.91,
S 6.29.
Example 29
Sodium salt of 1-(2-methoxycarbonylbenzene-
sulphonyl)-3-[4-dimethylamino-6-(2-chloro)-benzyl-
ideneiminooxy-1,3,5-triazin-2-yl]-urea (Compound
28).
In a manner similar to that described in Exam-
ple 19, from sodium hydroxide and l-(2-methoxy-
carbonylbenzenesulphonyl)-3-[4-dimethylamino-6-(2-
chloro)-benzylideneiminooxy-1,3,5-triazin-2-yl]-urea
the above-mentioned salt was obtained in the yield
of 95%, m.p. 157C (decomp.).
Found, ~: C 45.36, H 3.42, Cl 6.39, N 17.64.
C2 lHl gClN706SNa .
Calculated, %: C 45.53, H 3.48, Cl 6.44,
N 17.59.
Example 30
Sodium salt of 1-(2,5-dichlorobenzenesulphonyl)-3-
(4-dimethylamino-6-isopropylideneiminooxy-1,3,5-
triazin-2-yl)-urea (Compound 29).
In a manner similar to that of Example 19, from
sodium hydroxyide and 1-(2,5-dichlorobenzene-
sulphonyl)-3-(4-dimethylamino-6-isopropylidene-
iminooxy-1,3,5-triazin-2-yl)-urea the above-
mentioned salt was obtained in the yield of 91%,
m.p. 165C (decomp.).
Found, %: C 37.19, H 3.3, Cl 14.67, N 20.25.
C15H16C12N74SNa .
- 27 - 2Q36334
Calculated, %: C 37.41, H 3.29, Cl 14.36,
N 20.43.
Example 31
Sodium salt of 1-(2,5-dichlorobenzenesulphonyl)-3-
[4-dimethylamino-6-(a-methyl)-propylideneiminooxy-
1,3,5-triazin-2-yl]-urea (Compound 30).
In a manner similar to that of Example 19, from
sodium hydroxide and 1-(2,5-dichlorobenzene-
sulphonyl)-3-[4-dimethylamino-6-(a-methyl)-
propylideneiminooxy-1,3,5-triazin-2-yl]-urea the
above-identified salt was obtained in the yield of
92%, m.p. 151C (decomp.).
Found, %: C 38.55, H 3.61, Cl 14.26, N 19.68.
C16H18Cl2N74SNa-
Calculated, %: C 38.41, H 3.79, Cl 14.31,
N 19.74.
Example 32
Sodium salt of 1-(2,5-dichlorobenzenesulphonyl)-3-
(4-dimethylamino-6-benzylideneiminooxy-1,3,5-
triazin-2-yl)-urea (Compound 31).
In a manner similar to that of Example 19, from
sodium hydroxide and 1-(2,5-dichlorobenzene-
sulphonyl)-3-(4-dimethylamino-6-benzylideneiminooxy-
1,3,5-triazin-2-yl)-urea the above-mentioned salt
was obtained in the yield of 93%, m.p. 174C
(decomp.).
Found, %: C 42.86, H 3.01, Cl 13.35, N 18.42.
Cl9H16Cl2N74SNa-
Calculated, %: C 42.71, H 3.31, Cl 13.21,
N 18.31.
Example 33
Sodium salt of 1-(2,6-dichlorobenzenesulphonyl)-3-
(4-dimethylamino-6-isopropylideneiminooxy-1,3,5-
triazin-2-yl)-urea (Compound 32).
In a manner similar to that described in exam-
ple 19 hereinbefore, from sodium hydroxide and 1-
(2,6-dichlorobenzenesulphonyl)-3-(4-dimethylamino-6-
- 28 -
2Q3633~
isopropylideneiminooxy-1,3,5-triazin-2-yl)-urea the
above-mentioned salt was obtained in the yield of
92%, m.p. 157C (decomp.).
Found, %: C 37.19, H 3.3, Cl 14.67, N 20.24.
Cl gHl 8Cl2N704sNa -
Calculated, %: C 37.48, H 3.49, Cl 14.53,
N 20.44.
Example 34
Sodium salt of 1-(2,6-dichlorobenzenesulphonyl)-3-
[4-dimethylamino-6-(a-methyl)-propylideneiminooxy-
1,3,5-triazin-2-yl]-urea (Compound 33).
In a manner similar to that of Example 19, from
sodium hydroxide and 1-(2,6-dichlorobenzene-
sulphonyl)-3-[4-dimethylamino-6-(a-methyl)-
propylideneiminooxy-1,3,5-triazin-2-yl]-urea the
above-mentioned salt was obtained in the yield of
92%, m.p. 160C (decomp.).
Found, %: C 38.55, H 3.61, Cl 14.25, N 19.68.
Cl 6H1 8C12N704SNa -
Calculated, %: C 38.63, H 3.76, Cl 14.59,
N 19.67.
Example 35
Sodium salt of 1-(2,6-dichlorobenzenesulphonyl)-3-
(4-dimethylamino-6-benzylideneiminooxy-1,3,5-
triazin-2-yl)-urea (Compound 34).
In a manner similar to that of Example 19, from
sodium hydroxide and 1-(2,6-dichlorobenzene-
sulphonyl)-3-(4-dimethylamino-6-benzylideneiminooxy-
1,3,5-triazin-2-yl)-urea the above-mentioned salt
was obtained in the yield of 92%, m.p. 167C
(decomp.).
Found, %: C 42.86, H 3.01, Cl 13.35, N 18.42.
Cl gHl 6cl2N7o4sNa -
Calculated, %: C 42.93, H 3.13, Cl 13.49,
N 18.91.
,~
2~3633~
- 29 -
Example 36
Potassium salt of 1-(2-chlorobenzenesulphonyl)-3-(4-
dimethylamino-6-isopropylideneiminooxy-1,3,5-
triazin-2-yl)-urea (Compound 35).
To a solution of 0.84 g (0.015 mol) of
potassium hydroxide in 25 ml of water 4.28 g
(0.01 mol) of 1-(2-chlorobenzenesulphonyl)-3-(4-di-
methylamino-6-isopropylideneiminooxy-1,3,5-triazin-
2-yl)-urea were portion-wise added under stirring.
The reaction mixture was stirred at a temperature of
40-45C for 3 hours till dissolution of sul-
phonylurea. The resulting reaction mass was
filtered, water was evaporated under a reduced pres-
sure and at a temperature of not more than 50C. The
precipitate was washed with ether, dried in vacuum
to give 4.5 g (90%) of the above-mentioned salt,
m.p. 181C (decomp.).
Found, %: C 38.81, H 3.63, N 20.97, S 6.98.
C15H17ClN704SK.
Calculated, %: C 38.67, H 3.65, N 21.05,
S 6.87.
Example 37
Potassium salt of 1-(2-chlorobenzenesulphonyl)-3-[4-
dimethylamino-6-(a-methyl)-propylideneiminooxy-
1,3,5-triazin-2-yl]-urea (Compound 36).
In a manner similar to that of the foregoing
Example 36, from potassium hydroxide and 1-(2-
chlorobenzenesulphonyl)-3-[4-dimethylamino-6-(a-
methyl)-propylideneiminooxy-1,3,5-triazin-2-yl]-urea
the above-mentioned salt was obtained in the yield
of 92%, m.p. 163C (decomp.).
Found, %: C 40.15, H 3.90, Cl 7.19, N 20.51.
C16H1gClN704SK.
Calculated, %: C 40.04, H 3.96, Cl 7.4,
N 20.43.
- 30 -
20~33~
Example 38
Potassium salt of 1-(2-chlorobenzenesulphonyl)-3-(4-
dimethylamino-6-benzylideneiminooxy-1,3,5,-triazin-
2-yl)-urea (Compound 37).
In a manner similar to that described in the
foregoing Example 36, from potassium hydroxide and
1-(2-chlorobenzenesulphonyl)-3-(4-dimethylamino-6-
benzylideneiminooxy-1,3,5-triazin-2-yl)-urea the
above-mentioned salt was obtained in the yield of
94%, m.p. 169C (decomp.).
Found, %: C 44.59, H 3.43, Cl 6.72, N 18.95.
ClgH17ClN704SK.
Calculated, %: C 44.4, H 3.31, Cl 6.91,
N 19.08.
Example 39
Potassium salt of 1-(2-chlorobenzenesulphonyl)-3-[4-
dimethylamino-6-(2-chloro)-benzylideneiminooxy-
1,3,5-triazin-2-yl]-urea (Compound 38).
In a manner similar to that of Rxample 36, from
potassium hydroxide and 1-(2-chlorGbenzenesulphonyl-
3-[4-dimethylamino-6-(2-chloro)-benzylideneiminooxy-
1,3,5-triazin-2-yl]-urea the above-mentioned salt
was obtained in the yield of 91%, m.p. 164C
(decomp.).
Found, ~: C 41.74, H 2.85, Cl 12.82, N 17.70.
ClgH17ClN704SK.
Calculated, %: C 41.6, H 2.92, Cl 12.96,
N 17.88.
Example 40
Potassium salt of 1-(2-nitrobenzenesulphonyl)-3-(4-
dimethylamino-6-isopropylideneiminooxy-1,3,5-
triazin-2-yl)-urea (Compound 39).
In a manner similar to that described in Exam-
ple 36, from potassium hydroxide and 1-(2-nitro-
benzenesulphonyl)-3-(4-dimethylamino-6-iso-
propylideneiminooxy-1,3,5-triazin-2-yl)-urea the
j - 31 - 2Q3~4
above-mentioned salt was obtained in the yield of
93%, m.p. 186C (decomp.).
Found, %: C 37.69, H 3.49, N 23.68, S 6.59.
C15H17N806SK .
Calculated, %: C 37.82, H 3.57, N 23.53,
S 6.72.
Example 41
Potasslum salt of 1-(2-nitrobenzenesulphonyl)-3-[4-
dimethylamino-6-ta-methyl)-propylideneiminooxy-
1,3,5-triazin-2-yl]-urea (Compound 40).
In a manner similar- to that of Example 36, from
potassium hydroxide and 1-(2-nitrobenzenesulphonyl)-
3-[4-dimethylamino-6-(a-methyl)-propylidene-
iminooxy-1,3,5-triazin-2-yl]-urea the above-
mentioned salt was obtained in the yield of 95%,
m.p. 189C (decomp.).
Found, %: C 39.35, H 3.76, N 22.72, S 6.70.
Cl 6Hl gN806SK .
Calculated, %: C 39.18, H 3.88, N 22.86,
S 6.53.
Example 42
Potassium salt of 1-(2-nitrobenzenesulphonyl)-3-(4-
dimethylamino-6-benzylideneiminooxy 1,3,5-triazin-2-
yl)-urea (Compound 41).
In a manner similar to that described of
Example 36, from potassium hydroxide and 1-(2-
nitrobenzenesulphonyl)-3-(4-dimethylamino-6-
benzylideneiminooxy-1,3,5-triazin-2-yl)-urea the
above-mentioned salt was obtained in the yield of
91%, m.p. 187C (decomp.).
Found, %: C 43.65, H 3.71, N 21.25, S 6.06.
ClgH17N806SK.
Calculated, %: C 43.51, H 3.62, N 21.73,
S 6.11.
- 32 -
2036334
-
Example 43
Potassium salt of 1-(2-methoxycarbonylbenzene-
sulphonyl~-3-(4-dimethylamino-6-isopropylidene-
iminooxy-1,3,5-triazin-2-yl)-urea (Compound 42).
In a manner similar to that of Example 36, from
potassium hydroxide and 1-(2-methoxycarbonylbenzene-
sulphonyl)-3-(4-dimethylamino-6-isopropylidene-
iminooxy-1,3,5-triazin-2-yl)-urea the above-
mentioned salt was obtained in the yield of 92%,
m.p. 168C (decomp.).
Found, %: C 41.58, H 4.02, N 20.34, S 6.58.
Cl 7H2 oN706SK .
Calculated, %: C 41.71, H 4.08, N 20.16,
S 6.54.
Example 44
Potassium salt of 1-(2-methoxycarbonylbenzene-
sulphonyl)-3-[4-dimethylamino-6-(a-methyl)-
propylideneiminooxy-1,3,5-triazin-2-yl]-urea
(Compound 43).
In a manner similar to that of Example 36, from
potassium hydroxide and 1-(2-methoxycarbonylbenzene-
sulphonyl)-3-[4-dimethylamino-6-(a-methyl)-
propylideneiminooxy-1,3,5-triazin-2-yl]-urea the
above-mentioned salt is obtained in the yield of
93%, m.p. 153C (decomp.).
Found, %: C 42.71, H 4.42, N 19.61, S 6.22.
Cl 8H22N706SK .
Calculated, %: C 42.9, H 4.37, N 19.48, S 6.36.
Example 45
Potassium salt of 1-(2-methoxycarbonylbenzene-
sulphonyl)-3-(4-dimethylamino-6-benzylideneiminooxy-
1,3,5-triazin-2-yl)-urea (Compound 44).
In a manner similar to that of Example 36, from
potassium hydroxide and 1-(2-methoxycarbonylbenzene-
sulphonyl)-3-(4-dimethylamino-6-benzylideneiminooxy-
1,3,5-triazin-2-yl)-urea the above-mentioned salt
,~
,~
20~34
- 33 -
was obtained in the yield of 95%, m.p. 159C
(decomp.).
Found, %: C 46.84, H 3.65, N 18.09, S 5.81.
C2 lH2 oN706SK .
Calculated, %: C 46.93, H 3.72, N 18.25,
S 5.96.
Example 46
Potassium salt of 1-(2-methoxycarbonylbenzene-
sulphonyl)-3-[4-dimethylamino-6-~2-chloro)-
benzylideneiminooxy-1,3,5-triazin-2-yl]-urea
(Compound 45).
In a manner similar to that of Example 36, from
potassium hydroxide and 1-(2-methoxycarbonylbenzene-
sulphonyl)-3-[4-dimethylamino-6-(2-chloro)-
benzylideneiminooxy-1,3,5-triazin-2-yl]-urea the
above-mentioned salt was obtained in the yield of
92%, m.p. 166C (decomp.).
Found, %: C 44.22, H 3.43, Cl 6.15, N 17.02.
C2 lHl gClN706SK .
Calculated, %: C 44.09, H 3.32, Cl 6.21,
N 17.14.
Example 47
Potassium salt of 1-(2,5-dichlorobenzenesulphonyl)-
3-(4-dimethylamino-6-isopropylideneiminooxy-1,3,5-
triazin-2-yl)-urea (Compound 46).
In a manner similar to that of Example 36, from
potassium hydroxide and 1-(2,5-dichlorobenzene-
sulphonyl)-3-(4-dimethylamino-6-isopropylidene-
iminooxy-1,3,5-triazin-2-yl)-urea the above-
mentioned salt was obtained in the yield of 94% m.p.
168C (decomp.).
Found, %: C 35.87, H 3.27, Cl 14.32, N 19.44,
C155H16C12N704SK.
Calculated, %: C 36.00, H 3.2, Cl 14.2, N 19.6.
X
- 34 -
~ 20;~334
Example 48
Potassium salt of 1-(2,5-dichlorobenzenesulphonyl)-
3-[4-dimethylamino-6-(a-methyl)-propylideneiminooxy-
1,3,5-triazin-2-yl]-urea (Compound 47).
In a manner similar to that described in Exam-
ple 36, from potassium hydroxide and 1-(2,5-di-
chlorobenzenesulphonyl)-3-[4-dimethylamino-6-(a-
methyl)-propylideneiminooxy-1,3,5-triazin-2-yl]-urea
the above-mentioned salt was obtained in the yield
of 93%, m.p. 153C (decomp.~.
Found, %: C 37.29, H 3.43, Cl 13.90, N 19.21.
Cl 6H1 8C12N704SK -
Calculated, %: C 37.75, H 3.5, Cl 13.81, N
19.06.
Example 49
Potassium salt of 1-(2,5-dichlorobenzenesulphonyl-3-
(4-dimethylamino-6-benzylideneiminooxy-1,3,5-
triazin-2-yl)-urea (Compound 48).
In a manner similar to that of Example 36
hereinbefore, from potassium hydroxide and 1-(2,5-
dichlorobenzenesulphonyl-3-(4-dimethylamino-6-
benzylideneiminooxy-1,3,5-triazin-2-yl)-urea the
above-mentioned salt was obtained in the yield of
95%, m.p. 178C (decomp.).
Found, %: C 41.69, H 2.95, Cl 12.81, N 17.76.
Cl9H16C12N74SK-
Calculated, %: C 41.6, H 2.92, Cl 12.95,
N 17.88.
Example 50
Potassium salt of 1-(2,6-dichlorobenzenesulphonyl)-
3-(4-dimethylamino-6-isopropylideneiminooxy-1,3,5-
triazin-2-yl)-urea (Compound 49).
In a manner similar to that of Example 36
hereinbefore, from potassium hydroxide and 1-(2,6-
dichlorobenzenesulphonyl)-3-(4-dimethylamino-6-iso-
propylideneiminooxy-1,3,5-triazin-2-yl)-urea the
~ 35 ~ 203~33~
.
above-mentioned salt was obtained in the yield of
96%, m.p. 163C (decomp.).
Found, %: C 35.91, H 3.12, Cl 14.08, N 19.78.
Cl 5H1 8C12N704SK .
Calculated, %: C 36.00, H 3.2, Cl 14.2, N 19.6.
Example 51
Potassium salt of 1-(2,6-dichlorobenzenesulphonyl)-
3-[4-dimethylamino-6-(a-methyl)-propylideneiminooxy-
1,3,5-triazin-2-yl]-urea (Compound 50).
In a manner similar to that described in Exam-
ple 36, from potassium hydroxide and 1-(2-dichloro-
benzenesulphonyl)-3-[4-dimethylamino-6-(a-methyl)-
propylideneiminooxy-1,3,5-triazin-2-yl]-urea the
above-mentioned salt was obtained in the yield of
95%, m.p. 162C (decomp.).
Found, %: C 37.50, H 3.61, Cl 13.74, N 18.89,
Cl 6H1 8C12N704SK -
Calculated, %: C 37.35, H 3.5, Cl 13.81,
N 19.07.
Example 52
Potassium salt of 1-(2,6-dichlorobenzenesulphonyl)-
3-(4-dimethylamino-6-benzylideneiminooxy-1,3,5-
triazin-2-yl)-urea (Compound 51).
In a manner similar to that of Example 36
hereinbefore, from potassium hydroxide and 1-(2,6-
dichlorobenzenesulphonyl)-3-(4-dimethylamino-6-
benzylideneiminooxy-1,3,5-triazin-2-yl)-urea the
above-identified salt was obtained in the yield of
91%, m.p. 169C (decomp.).
Found, %: C 41.52, H 2.88, Cl 12.76, N 17.71.
Cl gHl 6C12N704SK -
Calculated, %: C 41.6, H 2.92, Cl 12.95,
N 17.88.
Example 53
Diethylethanolammonium salt of 1-(2-chlorobenzene-
sulphonyl)-3-(4-dimethylamino-6-isopropylidene-
iminooxy-1,3,5-triazin-2-yl)-urea (Compound 52).
~ - 36 - 2036334
To a solution of 1.8 g (0.015 mol) of di-
ethylethanolamine in 20 ml of water 4.3 g (0.1 mol)
of 1-(2-chlorobenzenesulphonyl)-3-(4-dimethylamino-
6-isopropylideneiminooxy-1,3,5-triazin-2-yl)-urea
were portion-wise added under stirring. The reaction
mixture was stirred at a temperature of 40-45C for
3 hours till dissolution of sulphonylurea. The
resulting reaction mass was filtered, water evapo-
rated under a reduced pressure at a temperature of
not more than 50C. The oily residue was 2 times
washed with ether to remove the excess of di-
ethylethanolamine, dried in vacuum to a constant
weight at room temperature to give 5.4 g (100~) of
the above-mentioned salt.
Found, %: C 46.48, H 5.97, N 20.87, Cl 6.92.
C21H33ClN805S
Calculated, %: C 46.28, H 5.87, N 20.57,
Cl 6.52.
Example 54
Diethylethanolammonium salt of 1-(2-chlorobenzene-
sulphonyl)-3-[4-dimethylamino-6-(a-methyl)-
propylideneiminooxy-1,3,5-triazin-2-yl]-urea
(Compound 53).
In a manner similar to that described in the
foregoing Example 53, from diethylethanolamine and
1-(2-chlorobenzenesulphonyl)-3-[4-dimethylamino-6-
(a-methyl)-propylideneiminooxy-l~3~5-triazin-2-yl]
urea the above-mentioned salt was obtained in the
form of an oily residue with the yield of 92%.
Found, %: C 47.45, H 6.60, Cl 6.70, N 20.38,
S 6.01.
C22H35ClN805S
Calculated, %: C 47.21, H 6.27, Cl 6.36,
N 20.01, S 5.73.
203~334
- 37 -
Example 55
Diethylethanolammonium salt of 1-(2-chlorobenzene-
sulphonyl)-3-(4-dimethylamino-6-benzylideneiminooxy-
1,3,5-triazin-2-yl)-urea (Compound 54).
In a manner similar to that described in Exam-
ple 53, from diethylethanolamine and 1-(2-chloro-
benzenesulphonyl)-3-(4-dimethylamino-6-benzylidene-
iminooxy-1,3,5-triazin-2-yl)-urea the above-
mentioned salt was obtained in the form of an oily
residue in the yield of 94.5%.
Found, %: C 50.39, H 5.18, Cl 6.31, N 18.54.
C2sH33clN8O5s
Calculated, %: C 50.72, H 5.58, Cl 6.00,
N 18.93.
Example 56
Diethylethanolammonium salt of 1-(2-chlorobenzene-
sulphonyl)-3-[4-dimethylamino-6-(2-chloro)-
benzylideneiminooxy-1,3,5-triazin-2-yl]-urea
(Compound 55).
In a manner similar to that described in Exam-
ple 53 hereinbefore, from diethylethanolamine and 1-
(2-chlorobenzenesulphonyl)-3-[4-dimethylamino-6-(2-
chloro)-benzylideneiminooxy-1,3,5-triazin-2-yl]-urea
the above-mentioned salt was obtained in the form of
an oily residue in the yield of 96%.
Found, %: C 47.50, H 5.48, Cl 11.65, N 18.03.
C25H32C12N805S -
Calculated, %: C 47.85, H 5.10, Cl 11.32,
N 17.86.
Example 57
Diethylethanolammonium salt of 1-(2-nitrobenzene-
sulphonyl)-3-(4-dimethylamino-6-iospropylidene-
iminooxy-1,3,5-triazin-2-yl)-urea (Compound 56).
In a manner similar to that of Example 53, from
diethylethanolamine and 1-~2-nitrobenzenesulphonyl)-
3-(4-dimethylamino-6-isopropylideneiminooxy-1,3,5-
triazin-2-yl)-urea the above-mentioned salt was
~1
- 38 - 2Q3G33~
obtained in the form of an oily residue in the yield
of 94%.
Found, %: C 45.79, H 5.80, N 22.31, S 5.48.
C21H33N97S .
Calculated, %: C 45.40, H 5.94, N 22.70,
S 5.76.
Example 58
Diethylethanolammonium salt of 1-(2-nitrobenzene-
sulphonyl)-3-[4-dimethylamino-6-(a-methyl)-
propylideneiminooxy-1,3,5-triazin-2-yl]-urea
(Compound 57).
In a manner similar to that described in Exam-
ple 53, from diethylethanolamine and 1-(2-nitro-
benzenesulphonyl-3-[4-dimethylamino-6-(a-methyl)-
propylideneiminooxy-1,3,5-triazin-2-yl]-urea the
above-mentioned salt was obtained in the form of an
oily residue in the yield of 98%.
Found, %: C 46.11, H 6.50, N 22.51, S 5.90.
C22H35N9O7s
Calculated, %: C 46.40, H 6.15, N 22.14,
S 5.62.
Example 59
Diethylethanolammonium salt of 1-(2-nitrobenzene-
sulphonyl)-3-(4-dimethylamino-6-benzylideneiminooxy-
1,3,5-triazin-2-yl)-urea (Compound 58).
In a manner similar to that described in Exam-
ple 53, from diethylethanolamine and 1-(2-nitro-
benzenesulphonyl)-3-(4-dimethylamino-6-benzylidene-
iminooxy-1,3,5-triazin-2-yl)-urea the above-
mentioned salt was obtained in the form of an oily
residue in the yield of 96%.
Found, %: C 50.02, H 5.91, N 21.32, S 5.75.
C23H36N8O7s
Calculated, %: C 49.75, H 5.47, N 20.90,
S 5.31.
~`
~ 39 - 2Q36334
Example 60
Diethylethanolammonium salt of 1-(2-methoxycarbonyl-
benzenesulphonyl)-3-(4-dimethylamino-6-iso-
propylideneiminooxy-1,3,5-triazin-2-yl)-urea
(Compound 59)
In a manner similar to that described in Exam-
ple 53, from diethylethanolamine and 1-(2-methoxy-
carbonylbenzenesulphonyl)-3-(4-dimethylamino-6-iso-
propylideneiminooxy-1,3,5-triazin-2-yl)-urea the
above-identified salt was obtained in the form of an
oily residue in the yield of 92%.
Found, %: C 48.21, H 6.72, N 20.01, S 6.10.
C23H36N8O7s
Calculated, %: C 48.59, H 6.31, N 19.72, S
5.63.
Example 61
Diethylethanolammonium salt of 1-(2-methoxycarbonyl-
benzenesulphonyl)-3-[4-dimethylamino-6-(a-methyl)-
propylideneiminooxy-1,3,5-triazin-2-yl]-urea
(Compound 60).
In a manner similar to that of Example 53
hereinbefore, from diethylethanolamine and 1-(2-
methoxycarbonylsulphonyl)-3-[4-dimethylamino-6-(a-
methyl)-propylideneiminooxy-1,3,5-triazin-2-yl]-urea
the above-mentioned salt was obtained in the form of
an oily residue in the yield of 94%.
Found, ~: C 49.12, H 6.14, N 18.95.
C24H38N8O7s-
Calculated, %: C 52.60, H 5.84, N 18.18.
Example 62Diethylethanolammonium salt of 1-(2-methoxycarbonyl-
benzenesulphonyl)-3-(4-dimethylamino-6-benzylidene-
iminooxy-1,3,5-triazin-2-yl)-urea (Compound 61).
In a manner similar to that of Example 53
hereinbefore, from diethylethanolamine and 1-(2-
methoxycarbonylbenzenesulphonyl)-3-(4-dimethylamino-
6-benzylideneiminooxy-1,3,5-triazin-2-yl)-urea the
~ - 40 -
203633~
above-mentioned salt was obtained in the form of an
oily residue in the yield of 94.5%.
Found, %: C 52.93, H 6.20, N 18.50,
C2 lH2 lN7o6s -
Calculated, %: C 52.60, H 5.84, N 18.18.
Example 63
Diethylethanolammonium salt of 1-(2-methoxycarbonyl-
benzenesulphonyl)-3-[4-dimethylamino-6-(2-chloro)-
benzylideneiminooxy-1,3,5-triazin-2-yl]-urea
(Compound 62).
In a manner similar to that of Example 53, from
diethylethanolamine and 1-(2-methoxycarbonylbenzene-
sulphonyl)-3-[4-dimethylamino-6-(2-chloro)-
benzylideneiminooxy-1,3,5-triazin-2-yl]-urea the
above-mentioned salt was obtained in the form of an
oily residue in the yield of 93%.
Found, %: C 49.59, H 5.01, Cl 5.06, N 16.92,
C2 lH2 oclN7o6s
Calculated, %: C 49.81, H 5.38, Cl 5.46,
N 17.22.
Example 64
Diethylethanolammonium salt of 1-(2,5-dichloro-
benzenesulphonyl)-3-(4-dimethylamino-6-iso-
propylideneiminooxy-1,3,5-triazin-2-yl)-urea
(Compound 63).
In a manner similar to that of Example 53, from
diethylethanolamine and 1-(2,5-dichlorobenzene-
sulphonyl)-3-(4-dimethylamino-6-isopropylidene-
iminooxy-1,3,5-triazin-2-yl)-urea the above-
mentioned salt was obtained in the form of an oily
residue in the yield of 92%.
Found, %: C 43.94, H 5.98, Cl 12.60, N 19.70.
C2 lH32cl2N8o5s -
Calculated, %: C 43.52, H 5.53, Cl 12.26,
N 19.34.
- 41 - ~ 036334
Example 65
Diethylethanolammonium salt of 1-(2,5-dichloro-
benzenesulphonyl)-3-[4-dimethylamino-6-(a-methyl)-
propylideneiminooxy-1,3,5-triazin-2-yl]-urea
(Compound 64).
In a manner similar to that of Example 53
hereinbefore, from diethylethanolamine and 1-(2,5-
dichlorobenzenesulphonyl)-3-[4-dimethylamino-6-(a-
methyl)-propylideneiminooxy-1,3,5-triazin-2-yl]-urea
the above-mentioned salt was obtained in the form of
an oily residue in the yield of 92.5%.
Found, %: C 44.89, H 6.02, Cl 12.34, N 19.21.
C22H34C12N805S
Calculated, %: C 44.52, H 5.73, Cl 11.97,
N 18.89.
Example 66
Diethylethanolammonium salt of 1-(2,5-dichloro-
benzenesulphonyl)-3-(4-dimethylamino-6-benzylidene-
iminooxy-1,3,5-triazin-2-yl)-urea (Compound 65).
In a manner similar to that of Example 53, from
diethylethanolamine and 1-(2,5-dichlorobenzene-
sulphonyl)-3-(4-dimethylamino-6-benzylideneiminooxy-
1,3,5-triazin-2-yl)-urea the above-mentioned salt
was obtained in the form of an oily residue in the
yield of 95%.
Found, %: C 48.08, H 5.48, Cl 11.60, N 18.11.
C25H32C12N805S
Calculated, %: C 47.85, H 5.10, Cl 11.32,
N 17.86.
Example 67
Diethylethanolammonium salt of 1-(2,6-dichloro-
benzenesulphonyl)-3-(4-dimethylamino-6-iso-
propylideneiminooxy-1,3,5-triazin-2-yl)-urea
(Compound 66).
Likewise in Example 53 hereinbefore, from di-
ethylethanolamine and 1-(2,6-dichlorobenzene-
sulphonyl)-3-(4-dimethylamino-6-isopropylidene-
~ - 42 - 203633~
-
iminooxy-1,3,5-triazin-2-yl)-urea the above-
mentioned salt was obtained in the form of an oily
residue in the yield of 97.5%.
Found, %: C 43.14, H 5.19, Cl 11.85, N 19.01.
C2 lH32cl2N8o5s
Calculated, %: C 43.52, H 5.53, Cl 12.26,
N 19.34.
Example 68
diethylethanolamine salt of 1-(2,6-dichlorobenzene-
sulphonyl)-3-[4-dimethylamino-6-(a-methyl)-
propylideneiminooxy-1,3,5-triazin-2-yl]-urea
(Compound 67).
Likewise in Example 53, from diethylethanol-
amine and 1-(2,6-dichlorobenzenesulphonyl)-3-t4-
dimethylamino-6-isopropylideneiminooxy-1,3,5-
triazin-2-yl)-urea the above-mentioned salt was
obtained in the form of an oily residue in the yield
of 96.5%.
Found, %: C 44.19, H 5.31, Cl 11.51, N 18.59.
C22H34C12N805S
Calculated, %: C 44.52, H 5.73, C1 11.97,
N 18.89.
Example 69
Diethylethanolammonium salt of 1-(2,6-dichloro-
benzenesulphonyl)-3-(4-dimethylamino-6-benzylidene-
iminooxy-1,3,5-triazin-2-yl)-urea (Compound 68).
In a manner similar to that described in Exam-
ple 53 hereinbefore, from diethylethanolamine and 1-
(2,6-dichlorobenzenesulphonyl)-3-(4-dimethylamino-6-
benzylideneiminooxy-1,3,5-triazin-2-yl)-urea the
above-mentioned salt was obtained comprising an oily
residue in the yield of 98%.
Found, %: C 47.49, H 4.85, Cl 10.93, N 17.50.
C2 5H32cl2N8o5s
Calculated, %: C 47.85, H 5.lG, Cl 11.32,
N 17.86.
- 43 -
2~3633~
Example 70
The compounds according to the present inven-
tion were subjected to tests of their biological
activity. To this end, the effect of representatives
of these compounds, i.e. 1-(2-chlorobenzene-
sulphonyl)-3-(4-dimethylamino-6-isopropylidene-
iminooxy-1,3,5-triazin-2-yl)-urea (Compound I) and
1-(2-chlorobenzenesulphonyl)-3-[4-dimethylamino-6- (a
-methyl)-propylideneiminooxy-1,3,5-triazin-2-yl]-
urea (Compound 2) was studied relative to the growth
and evelopment of sproutings of corn and cucumber.
Under laboratory experiment conditions the plants
were grown on soils in 0.5 kg (capacity) vessels
filled with a mixture soil-sand-peat (3:1:1). In the
case of pre-sprouting application, the compounds
were ingroduced right after seeding by way of
spraying over the soil surface. In the case of
after-sprouting application the plants in the phase
of 2 leaves were sprayed with the test compounds. An
aqueous solution in the amount of 10 ml containing
0.00062% of the active principle was used for every
vessel which corresponded to the application rate of
20 g/ha.
As standard reference compounds Gibberelin and
Atrazin were used.
The biological activity was assessed by the
stem length increment, amount of green above-ground
mass, rate of appearance and growth of young leaves
relative to the control (without the soil treatment)
3-4 weeks after the treatment. Repetition in
variants was 4 times.
;' - 44 - 2~36334
.
The experiment results are shown in Tables 2 to
4 as percentage of the control.
Table 2
Effect of Compound I on the Growth of Corn
Stem in the Dose of 20 g/ha
Time of assessment
(day since the 6 8 10 13 15 17 21
sprouting
moment)
Stem height,
% of the 135 144* 125* 123* 123* 117* 109
control
Table 3
Effect of Compound I on the Rate of Development
of Young Corn Plants in the Dose of 20 g/ha
Time of asessment Leaf length in Leaf width in
(day since the the upper tier, the upper tier
Sprouting % of the (leaf middle
moment) control portion), % of
the control
17 125* 128*
4s 203633~
Table 3 (continued)
1 2 3
28 153* 178*
38 173 202*
Table 4
Effect of Compounds 1 and 2 on the ~mount of Above-ground Mass
of Sproutings of ~orn and Cucumber 3 Weeks after Sprouting
Moment ~Oof the non-treated control plants)
~ompou~d dose, g/ha1,000 1,000 50 20
~ulture c o r n
Compound 1 _ 1 14 116 1 42
~ulture ~ u c u m b e r
~ompound 1 1 16 162* 104
Compound 2 123 132* 130~ -
NOTE: * - certain deviation from the control at P=0.95.
As it follows from the data shown in Tables 2 and 3, compo-
und I in the dose of 20 g/ha stimulates the growth of corn
stem at earlier stages of the development of young corn plants,
wherefore the size of leaves of the uppermost (at the moment
of measurement) tier is substantially greater than in the cont-
rol. According to the data of Table 4, acceleration of growth
of sproutings of corn and cucumber results in that the biomass
o~ the plants grown on the soil treated with compounds 1 and 2
is considerably greater than the biomass of the control plants.
Example 71
The biological activity of substituted phenylsulphonyltria-
zinylureas was evaluated by their effect on the growth of
-
~ 4L
2Q3~334
sproutings of corn and cucumber. The procedure o~ testing was
similar to that described in the foregoing E~ample 7C. As an
indicator of acceleration of the development of sproutings
there was used the weight of the abo~e-ground mass of three-
weeks sproutings relative to the control (above-ground mass of
sproutings grown on the untreated soil). The comparison of the
biological acti~ity was effected with compounds having a si-
milar structure possessing herbicidal and growth-regulating
activity and corresponding to the formula:
N(C~3)2
R
~ S02NHCO~H ~ ~ ~ ~ ~ OCH3
wherein R = Cl (compound A), R = CO2CH3(compound B) and with
the herbicide Glin (active principle chlorosulphurone~
CT.~3
~ SO2~JHCONH ~ ~ N ~ OCH3
as well as with the known plant growth stimulant Gibberilin
and corn herbicide and growth stimulan Atrazin (6-isopropyl-
amino-2-chloro-4-ethylamino-1,3,5-triazine). The results are
shown in Table 5 hereinbelow,
47 2036~34
~able 5
~ffect of Substituted Phenylsulphonyltriazin,yl ureas on
Growth of Sprouting~ of corn and cucumbers
Corn Cucumber
Compound I~o. Dose, g/ha
100 20 100 20
1 116 142* 162* 102
2 136* 130~ 132* 125
3 128* 103 96 143*
4 97 104 151* 109
106 114 98 147*
6 92 136* 131* 111
7 131* 123 115 98
8 100 109 95 139*
9 136* 127* 102 146*
105 121 108 155*
11 148* 95 92 140*
12 121 154* 113 88
13 98 97 136* 116
14 90 141* 123 12Q
118 102 120 148*
16 89 135* 98 109
17 127* 125 146* 122
Compound A 47* 83 64* 90
Compound B 31* 60* 20* 53*
Glin 0* 6* 0* 8*
Gibberelin 91 101 86 95
Atrazin dose of 123* 11
1kg/ha ,5 kg~a 4
* Certain deviation from the control at P = 0.95
~rom the data shown in ~able 5 it follows that the compounds
according to the present invention cau~e a certain gro~lth sti-
mulation of the abo~e-earth ma3s of both corn and cucumbers,
~rhereas compound~ A, B and Glin are characterized by growth in-
hibition or retardation effect.
2Q3~334
Gibberelin, while causing elongation of the stem, produced
no positive effect on the biomass of the above-eart part of
the plant, wherefore they were formed thin~ed and lodging.
Atrazin had its effect similar to that produced by the com-
pounds accordin~ to the present invention, but in doses by 10
and 25 times higher.
E~ample 72
~ he effect of compounds 52 and 53 on the growth and deve-
lopment of most widespread weeds was studied. The procedure o~
carrying out the e~periments was similar to that de~cribed he-
reinabove. The effect of the compounds on the growth and deve-
lopment of weeds was assessed by the wèight of the biomass of
the above-earth part of the plants 3 weeks after sot~ing. The
preparation in the form of an aqueo-glycolic solution was in-
troduced into the soil prior to seeding. ~he results are shown
in ~able 6 as percentage of the control (~ithout the soil tre-
atment).
Table 6
Effect of Compounds 52 and 53 on the Growth o~ l`Jeeds
Dose o~ the compound, g/ha
;Jeed variety Compound 52 Compound 53
100 20 100 20
1 2 3 4 5
Pigweed 88 97 81 105
Barnyard millet 75* 89 79 90
Bristle grass 32 107 94 100
Green amaranth 93 102 106 107
~Iatricary 96 1 o3 1 o6 97
* - Certain deviation from the control at P = 0.95.
2~6334
49
~ rom the above-given data it follows that compounds 52 and
53 insignificantly affect the growt~ and development of weeds
in early stages of organogenesis, i.e. they selectively stimu-
late the growth of certain farm crops placing tham into a more
favourable condition in the case of competition with weeds.
Example 73
The effect of compounds 52 and 53 according to the present
invention was assessed in respect of weed-contamination and
yield of corn under field conditio~s. These compounds were em-
ployed as water-glycol solutions in the case of presprouting
introduction i~to the soil in the doses of 20 and 50 g~ha as
calculated for the active principle.
The test plot area was 70 m2, repetition of the treatment-
4 times. The plot soil was dark-grey, podzolized, sandy-loam,
humus content 1.5~, pH = 4Ø The main prevailing varieties of
weeds on the test plot: matricary, shepherd's purse, water pe-
pper, chickweed, field horsetail, couch grass. The following
results were obtained: in 1985 the yield of the above-ground
mass in the case of using compound 52 in the dose of 20 g/ha
was 149~ of the control, in the dose of 50 g/ha - 1 76~o; for
both doses the weed-contamination was reduced by 9 ~. A simi-
lar result was obtained in 1986. In this case the weed-conta-
mination was reduced by 85% for the dose of 20 g/ha and by 7
for the dose of 60 g/ha. The yield of the silage mass was 136
of the control (for the dose o~ 50 g/ha). The effect of these
compounds was revealed in inhibition, to the same extent, of
both one-year dicotyledonous and graminous weeds. The use of
2Q3~334
~o
compound 53 in the dose of 20 g/ha resulted in the yield gain
of green ma~s of corn by 173 c/ha and in the dose of 40 g/ha -
by 86 c/ha. The total yield was 120 c/ha (~ o 5 = 82 c/ha).
*~ED - minimum essential difference.
It is obvious that the use of Glin, highly toxic in respect
of corn and compounds A and 3 inhibiting the growth of corn
under field conditions is inexpedie~t (Table 5 ) .
~ æample 74
The effect of compound 53 was studied in a field experiment
for ~reed-contamination and yield of ~heat and barley.
The compound 53 in the form of an aqueo-glycolic solution
~as used in the pre-sprouting stage of application in the do-
ses of 1C and 20 g/ha as calculated for the active principle.
The test plot area was 36 m2, repetition of the treatment - 4
times. The plot soil ~las common chernozem, clayed loam ~ith
the humus content of 4.5~, pI-I = 7Ø The basic prevailing va-
rieties of weeds on the test plot: bristle grass, pi~reed,
stitchwort species, ~ild buckwhea~, field pennycress, green
amaranth, shepherd's purse, corn bindweed, field horsetail,
sow thistles. The following results were obtained. In ~he dose
of 10 g/ha compound 53 caused death of 45~o of weeds in sowings
of winter wheat, the yield gain was 8.4 c/ha. The ~tandard re-
ference was Glin - 2.1 c/ha (the yield in the control - 25
c/ha, IEDo 5 - 4.0 c/ha). In barley sowings compound 53 caused
death of 4 ~ and 5~o of ~Jeeds for the doses of 10 and 20 g/ha
respectively. The yield gain ~as 0.5 c/ha and 5.2 c/ha respec-
tively. The standard reference Glin (10 g/ha) resulted in the
2~36334
_ 5l
yield gain o~ 2.9 c/ha (the yield in the control was 25.3 c/ha
EDo 5 - 2.0 c/ha)-
Similar results were obtained in the study of the biologi-
cal activity of sodium and potassium salts of substituted phe-
nylsulphonyltriazinyl ureas.
Example 75
The effect of the compounds according to the present inven-
tion was ,studied in respect of the yield of cotton - compounds
2, 9, 10, 11, 14.
~ he preparation of these compounds in the form of an aque-
ous emulsion with a surfactant employed in the amount of 0.1~o
by mass at the rate of the working liquid application of
500 l/ha were applied onto cotton plants in the phase of bud-
formation by way of sprinkling. The size of the test plot was
3 m2, repetition of the treatment - 3 times. The variety of
cotton - Tashkent-1, The obtained results are shov~n in Table
7 hereinbelow.
2036~34
a)
o
h ~ ~ ~ C~l CO
O d ~ u~
O O C~
_ O ~, C~
~
h - G~
O ~
o CO ~ ~ C~ O
O
:;~ O d ~ ~ ~ ~t o
O ~ ~ ~ ~
o ~æ c~
U~
~D ~ C~ ~ ~ O O
_ a) a~
O ~ O Y ~, . . . .
v O ~ ~ ~~o ~ ~
O o C)
rl ~;~(~\ '2 ~ ~ C~J d~
2 ~ r~
~ X ~ Z V '--
6~ C ~ V V V ~ V
~ ~, V
~r ~
~G ~o Es:
O >=~n p~ ~ v v
r-l ~ V V rl
a) o g O
O ~ U
t~
C~J C)
q) O P~ r~
C.)
q~ 11
~Q
¢ ~ ~
:~ ~r ~;
~rl
r I
O
h , ~I ~ O ~ ~ ~ ..
c;5 0 0 ~ ~ ~ ~ ~
o o ~ ~ o