Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2~37~33
The use of guanidine derivatives for the preparation
of a pharmaceutical product having NPY
antagonistic activity
Descri~tion
Neuropeptide Y (NPY) is a peptide of 36 amino acids
which was originally isolated from pigs' brains
(K. Tatemoto, Proc. Natl. Sci., USA 79, 5485 (1982)) but
; has also been found in the human central and peripheral
nervous system.
NPY controls the vascular sympathetic tone together
with noradrenalin. The systematic use of NPY leads to
prolonged rise in the vascular resistance. Boublik et al
(J.H. Boublik, N.A. Scott, M.R. Brown and J.E. Rivier,
J. Med Chem. 32, 597 (1989)) also proved the participation
of NPY in the production of high blood pressure.
NPY antagonists therefore constitute a potential
new method in the treatment of high blood pressure, but no
NPY antagonists have hitherto been known.
Guanidine derivatives having the following basic
~0 structure
l/
( `
2~37~33
which have histamine-H2 agonistic and histamine-H
antagonistic activities are known from DE-OS 35 12 084,
35 28 214, 35 28 215 and 36 31 334 and from EP-OS
0 199 845. According to the information given in the said
documents, these compounds are suitable, by virtue of
their pharmacological properties, for use as cardiotonic
agents, i.e. compounds which increase the force of
contraction of the heart. They are therefore proposed for
the therapy of acute and chronic caxdiac insufficiency.
10 It has now been found that the compounds described
above surprisingly also have neuropeptide-Y antagonistic
activities independently of the above mentioned cardio-
tonic and positive inotropic activity.
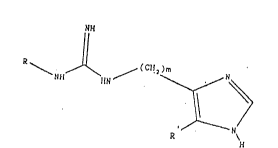
- The present invention therefore relates to the use
of guanidine derivatives corresponding to the general
formula II
~111
R~ ~ ~(CI
¢~
N~
R il
in which R denotes the group
Rl \
N-(cH2)n
R2
wherein Rl denotes a phenyl group which may be unsub-
stituted or mono- or disubstituted with halogen atoms,
2~3 ~4~3
C1-C3-alkyl groups or Cl-C3-alkoxy groups, or a pyridine
ring which may be unsubstituted or mono- or disubstituted
with halogen atoms, C1-C3-alkyl groups or C1-C3-alkoxy
groups, R2 denotes a hydrogen atom, a C1-C3-alkyl group, a
phenyl optionally mono- or disubstituted with halogen
atoms, C1-C3-alkyl groups or C1-C3-alkoxy groups, or a
benzyl or hetero aryl methyl group which may be unsub-
stituted or mono- or disubstituted with halogen atoms,
Cl-C3-alkyl groups or Cl-C3-alkoxy groups, and n has the
value 2, 3 or 4,
or in which R denotes the group
R4-c-z-(cH2)
~5
wherein R3 stands for a pyridine ring or phenyl ring which
may be unsubstituted or mono- or disubstituted with
halogen atoms, C1-C3-alkyl groups or C1-C3-alkoxy groups,
R4 denotes a hydrogen atom or a phenyl group optionally
s mono- or disubstituted with halogen atoms, C1-C3-alkyl
groups or C1-C3-alkoxy groups, R5 stands for a h ydrogen
atom or a methyl or hydroxyl group and Z stands for a
single bond, an oxygen atom or a sulphur atom, and p has
the value 2 or 3,
m has the value 2 or 3 and R' denotes a hydrogen atom or a
methyl group,
and the physiologically acceptable salts thereof,
for the preparatlon of a pharmaceutical product having NPY
antagonistic activity.
The substances considered according to the
invention are suitable in particular for the treatment of
high blood pressure due to their neuropeptide-Y-
antagonistic activity.
2037~33
Another object of this invention is therefore the
use of the above-defined guanidine derivatives for the
preparation of a pharmaceutical product for the treatment
of high blood pressure.
In the general formula II indicated above, R may
denote the group
Rl
\ N-(CH2)n~
R2
In this group, Rl stands for an unsubstituted or mono- or
disubstituted phenyl group. In the case of substitution,
the substituents may in particular be 1 or 2 halogen
atoms such as fluorine, chlorine or bromine atoms,
preferably fluorine or chlorine atoms, 1 or 2 C1-C3-alkyl
groups, preferably methyl or ethyl groups, and 1 or 2
C1-C3-alkoxy groups such as methoxy or ethoxy groups.
Monosubstitution is preferably in the 4-position and
disubstitution is preferably in the 3- and 4-position or
the 3- and 5-position of the phenyl ring.
The substituent R1 may also be an unsubstituted or
a mono- or disubstituted pyridine ring. Examples of
suitable substituents on the pyridine ring include halogen
atoms such as fluorine, chlorine or bromine atoms,
preferably bromine or chlorine atoms, most preferably
bromine atoms, C1-C3-alkyl groups such as methyl or ethyl
groups and C1-C3-alkoxy groups such as methoxy, ethoxy or
propoxy groups, preferably methoxy groups.
Linkage of the pyridine ring denoted by Rl to the
nitrogen atom in the group R may take place in the 2-, 3-
or 4-position of the pyridine ring, the 2- or 3-position
being preferred. Linkage in the 2-position of the pyridine
ring is particularly preferred.
R2 stands for a hydrogen atom, a C1-C3-alkyl group,
in particular a methyl, ethyl or propyl group, a phenyl
2(~3r~ ~33
group, which may be unsubstituted or mono- or disubstitut-
ed, a benzyl group, which may be unsubstituted or mono- or
disubstituted, or a hetero aryl methyl gxoup, which may be
unsubstituted or mono- or disubstituted. In the case of
substitution, the phenyl group denoted by R2 may be
substituted in the same manner and with the same sub-
stituents as described above in connection with the
substitution of the phenyl group denoted by R1.
In the case of substitution, the benzyl group may
be substituted with 1 or 2 halogen atoms such as fluorine,
chlorine or bromine atoms, preferably chlorine or fluorine
atoms, or C1-C3-alkoxy groups, such as methoxy or ethoxy
groups, preferably methoxy groups. In the case of mono-
substitution of the benzyl group denoted by R2, the
substituent is preferably attached in the para position to
the methylene group whereas in the case of disubstitution
the 3- and 4-positions of the benzyl group are preferred.
When R2 stands for a hetero aryl methyl group, this
group is preferably a thiophenylmethyl, a furanomethyl or
a pyridinomethyl group. The heteroarylmethyl group may also
be u~substituted or, preferably, mono- or disubstitut-
ed. The substituents may be halogen atoms such as
fluorine, chlorine or bromine atoms, C1-C3-alkyl groups
such as methyl or ethyl groups and linear C1-C3-alkoxy
groups such as methoxy groups.
The index n has the value 2, 3 or 4, the value 3
being preferred.
R may also stand for the group
R4 f z (CH2,p
R5
2~37~33
In this group, R3 may denote an unsubstituted or a mono-
or disubstituted phenyl group or an unsubstituted or mono-
or disubstituted pyridine ring. In the case of substitu-
- tion, suitable substituents are in particular one or two
halogen atoms such as fluorine, chlorine or bromine atoms,
preferably fluorine or chlorine atoms, 1 or 2 C1-C3-alkyl
groups, preferably methyl or ethyl groups, and 1 or 2 Cl-
C3-alkoxy groups, such as methoxy or ethoxy groups.
Monosubstitution and disubstitution are preferred.
Substitution in the 4-position of the phenyl ring is
preferred in the case of monosubstitution and substitution
in the 3- and 4-position of the phenyl ring is preferred
in the case of disubstitution.
The substituent R3 may also be an unsubstituted or a mono-
or disubstituted pyridine ring, preferably an unsub-
stituted pyridine ring or a monosubstituted pyridine ring.
The substituents of the pyridine ring may be, for example,
halogen atoms such as fluorine, chlorine or bromine atoms,
preferably bromine or chlorine atoms, most preferably
bromine atoms, Cl-C3-alkyl groups such as methyl or ethyl
groups and C1-C3-alkoxy groups such as methoxy, ethoxy or
propoxy groups, preferably methoxy groups.
Linkage of the pyridine ring denoted by R3 to the
carbon atom in the group R may take place in the 2-, 3- or
4-position of the pyridine ring, the 2- or 3-position
being preferred. Linkage in the 2-position of the pyridine
ring is particularly preferred.
R4 denotes a hydrogen atom or an unsubstituted or
mono- or disubstituted phenyl group. In the case of
substitution, the phenyl group denoted by R4 is sub-
stituted in the same manner as the phenyl group denoted
by R3. R5 denotes a hydrogen atom or a methyl or hydroxyl
group. Z stands for a single bond, an oxygen atom or a
sulphur atom and p has the value 2 or 3.
In the general formula II, m has the value 2 or 3,
~37~33
preferably 3, and R' denotes a hydrogen atom or a methylgroup, preferably a hydrogen atom.
According to the invention, it is preferred to use
guanidine derivatives corresponding to the above general
formula II in which R stands for one of the following
groups:
2-(Diphenylmethoxy)ethyl, 2-[bis-(4-fluorophenyl)-
methoxy]ethyl,
2-[bis-(4-chlorophenyl)methoxy]ethyl,
3-(4-fluorophenyl)-3-(pyridin-2-yl)propyl,
3-(3,4-difluorophenyl)-3-(pyridin-2-yl)propyl,
3-(3,5-difluorophenyl)-3-(pyridin-2-yl)propyl,
3-(4-chlorophenyl)-3-(pyridin-2-yl)propyl,
3-(3,4-dichlorophenyl)-3-(pyridin-2-yl)propyl,
3-(4-fluorophenyl)-3-(pyridin-3-yl)propyl,
2-[N-(5-bromo-3-methyl-pyridin-2-yl)-benzylamino]ethyl,
2-[N-(5-bromo-3-methyl-pyridin-2-yl)-(4-chlorobenzyl)-
amino]-ethyl,
4-(5-bromo-3-methyl-pyridin-2-yl)butyl,
3-(5-bromo-3-methyl-pyridin-2-yl)propyl,
4-(5-bromo-pyridin-2-yl)butyl,
3-(5-bromo-pyridin-2-yl)propyl,
3-(4-chlorophenyl)-3~phenylpropyl,
3-(4-fluorophenyl)-3-phenylpropyl,
3,3-bis-(4-fluorophenyl)propyl or
3,3-bis-(4-chlorophenyl)propyl.
The use of the individual compounds indicated below
is particularly preferred:
N1-[3-(lH-Imidazol-4-yl)propyl~-N2-[2-[(pyridin-3-
yl)methylthio]ethyl]-guanidine
Nl-[3-(lH-imidazol-4-yl)propyl]-N2-(3,3-diphenylpropyl)-
guanidine
Nl-[3-(lH-imidazol-4-yl)]propyl]-N2-[2-[(pyridin-2-yl)-
amino]ethyl]-guanidine
Nl-[3-[(5-bromo-3-methyl-pyridin-2-yl)amino]propyl]-N2-[3-
~3~3~
~lH-imidazol-4-yl)propyl]-guanidine
Nl-[3-(lH-imidazol-4-yl)propyl]-N2-[2-(diphenylmethoxy)-
ethyl]-guanidine (Compound A)
- N1-[3-(3,5-difluorophenyl)-3-(pyridin-2-yl)propyl]-N2-[3-
(lH-imidazol-4-yl)propyl]-guanidine (Compound B)
N1-[2-[N-(5-bromo-3-methyl-pyridin-2-yl)-benzylamino]-
ethyl]-N2-[3-(lH-imidazol-4-yl)propyl]-guanidine
(Compound C)
N1-[4-(5-bromo-3-methyl-pyridin-2-yl)butyl]-N2-[3(lH-
imidazol-4-yl)propyl]-guanidine (Compound D).
The compounds used according to the invention are
known compounds which may be prepared by the processes
described in the above-mentioned documents.
The neuropeptide-Y antagonistic action of the
compounds used according to the invention was demonstrated
by the method of Motulsky and Michel (H.J. Motulsky,
M.C. Michel, Am. J. Physiol. 255, 880 (1988)).
In this method, the rise in intracellular Ca++
concentration in HEl cells (human erythroleukemia cells)
induced by NPY was measured fluorimetrically, using fura-2
as indicator. Under the given conditions, NPY produces a
concentration-dependent rise in the intracellular Ca++
concentration by stimulation of the specific NPY receptor.
To measure the inhibitory action of the antagonists
to be tested, the latter are added to the incubation
medium at concentrations of from 10-4 to 10-6 and the NPY
activity curve is then again determined.
The guanidine compounds used according to the
invention shift the NPY concentration activity curve to
the right. According to ~child-Plot analysls, the shift to
the right is competitive so that the substances antagonise
the NPY action by competition on the specific NPY
receptor.
The following Table shows the values measured in
terms of PA2 values:
2~3~33
¦ Compound I PA2
¦ Inhibition of the rise in Ca++ ¦
5 ¦ A ¦ 4.0
I l I
. I B ¦ 4.72
I C 1 5.88
10 1
¦ D 1 5.04
I _ I !
' The following compounds were used for the above
described test, the results of which are shown in the
Table:
Compound A: N1-[3-(lH-imidazol-4-yl)propyl]-N2-[2-
(diphenylmethoxy)ethyl]-guanidine
N l !
Nll IIN ~
(3 N
2 ~3 3 r~ ~; 3 3
Compound B: Nl-[3-(3,5-difluorophenyl)-3-(pyridin-2-yl~-
propyl]-N2-[3-(lH-imidazol-4-yl)propyl]-
guanidine
F ~ F
Nll
N N ~N,~
~,N H 11 ~1
Compound C: Nl-[2-[N-(5-bromo-3-methyl-pyridin-2-yl)-
5benzylamino]-ethyl]-N2-[3-(1H-imidazol-4-yl)-
propyl]-guanidine
[~
B r~ --I IN N l!--N~--\J
Compound D: N1-[4-(5-brcmo-3-methyl-pyridin-2-yl)butyl]-
N2-[3-(lH-imidazol-4-yl)propyl]-guanidine
ar~CIl3
N~l `Nll NN
The invention is described in the Examples.
2~37~33
Example 1
N1-[3-(lH-Imidazol-4-yl)propyl]-N2-[2-[(pyridin-3-yl)-
methylthio]-ethyl]guanidine-trihydrochloride
-S-CH2-C~2-~l~-C-NH-C~2-CH2-C~2~ l x 3 HCl
0.85 g (2 mmol) of N-Benzoyl-N'-[3-(imidazol-4-yl)-
propyl]-N " -~2-[(pyrid-3-yl)methylthio]ethyl)guanidine are
heated under reflux in 45 ml of 18% hydrochloric acid for
6 hours. When the reaction mixture is cold, the benzoic
acid formed is removed by extraction with ether, the
aqueous phase is evaporated to dryness in a vacuum and
the residue is dried in a high vacuum. 0.78 g (91%) of a
dry, highly hygroscopic foam is obtained.
C15H22N6S 3HCl (427-8)
Molar mass (MS): Calc.: 318.16267; found: 318.16299
MS: m/z (rel. Int. [%]) = 318 (M+, 3), 168(17), 125(29),
95(51), 93(100), 92(57), 44(89).
H-NMR data ~ = 1.87 (m) 2 H,
(d6-DMSO, TMS as 2.62 (t) 2 H,
internal standard) 2.73 (t) 2 H,
3.0-3.7 (m) 4 H,
4.10 (s) 2 H,
7.3 - 8.3 (m) 6 H, 4 H
replaceable by D20
8.5 - 9.1 (m) 4 H, ppm.
'~3~3
Example 2
Nl-[3-(lH-Imidazol-4-yl)propyl]-N2-(3,3-diphenylpropyl)-
guanidine-dihydrochloride
H-cH2-cH2-NH-c-NH-cH2-cH2-c~2 ~ ~ x 2 HCl
0.84 g (1.8 mmol) of N-Benzoyl-N'- r 3-(imidazol-4-
yl)propyl]-N''-(3,3-diphenylpropyl)guanidine are heated
under reflux in 45 ml of 20% hydrochloric acid for 7
hours. The product is worked up as in Example 1~
Yield: 0.67 g (86~) of a hygroscopic, non-crystalline
solid.
C22H27Ns-2HCl (434-4)
MS: m/z (rel. Int. [%]) = 362 ([M+H]+, 84), 167(54),
109(100), 91(60) (FAB method).
H~NMR data: ~ = 1.81 (m) 2 H,
(d6-DMS0, TMS 2.27 (dt~ 2 H,
15 as internal standard) 2.68 (t) 2 H.
3.02 (m) 2 H,
3.16 (m) 2 H,
4.10 (t) 1 H,
7.15 - 7.6 (m) 13 H,
2 H, replaceable by
D20,
7.80 (m) 2 H,
replaceable by D2O,
8.99 (d) 1 H, ppm.
Example 3
Nl-[3-(lH-Imidazol-4~yl)propyl]-N2-!2-(pyridin-2-yl-
amino)-ethyl~-guanidine-trihydrochloride
12
~037~33
~ NHCH2CH2-NH-C-NH-CH2cH2cH2 ~
x 3 HCl
0.93 g (76~) of a colourless, hygroscopic solid are
obtained from 1.21 g (3.1 mmol) of N1-benzoyl-N2-[3-(lH-
imidazol-4-yl)propyl]-N3-[2-(pyridin-2-yl-amino)-ethyl]-
guanidine and 20 ml of conc. hydrochloric acid.
C14H24C13N7 (396-75)
H-NMR data: ~ = 1.80 - 2.21 (m) 2 H,
(CD30D, TMS as 2.69 - 3.00 (m) 2 H,
internal standard) 3.37 (t) 2 H,
3.57 - 3.83 (m~ 4 H,
4.8 (broad) 8 H, replace
able by D20,
6.96 (t) 1 H,
7.22 (d) 1 H,
7.44 (s) 1 H,
7.83 - 8.16 (m) 2 H,
8.87 (s) 1 H, ppm.
Example 4
N1-[3-[(5-Bromo-3-methyl-pyridin-2-yl)amino]-propyl]-N2-
[3-(lH-imidazol-4-yl)propyl]-guanidine-hydroiodide
~37~3
~r ~'~;~3 N~
N I IN /--~111 1 IN ~ ~>
x Il~ N~
1.50 g (3.37 mmol) of 3-[~5-Bromo-3-methyl-pyridin-
2-yl)amino)propyl]-isothiuronium iodide and 0.42 g (3.37
mmol) of 3-(lH-imidazol-4-yl)propylamine are boiled under
reflux in 20 ml of acetonitrile for 3 hours.
After cooling, the reaction mixture is concentrated
by evaporation under vacuum and the residue is purified
chromatographically on silica gel, using ethyl
acetate/methanol (70:30) as solvent. Concentration of the
main fraction by evaporation under vacuum yields 0.41 g
(23%) of a colourless, amorphous solid.
C16H2sBrIN7 (522-24)
H-NMR data: ~ = 1.93 (m) 4 H
(CD30D, TMS as 2.12 (s) 3 H
internal standard 2.69 (t) 2 H
3.2 - 3.6 (m) 6 H
4.9 (broad) 6 H,
replaceable by D20,
6.95 (s) 1 H
7.40 (d) 1 H
7.69 (s) 1 H
7.93 (d) 1 H, ppm.
14
~37~3~
Example 5
Nl-[3-(lH-Imidazol-4-yl)propyl]-N2-[2-(diphenylmethoxy)-
- ethyl]-guanidine-hydroiodide
IH
~; ~IH CJI CH l\IH-~-,YHCH C~l OCH
H x H~
a) N1-Benzoyl-N2-[2-(diphenylmethoxy)ethyl]-thiourea
7.8 g (34 mmol) of 2-(diphenylmethoxy)-ethylamine
and 5.6 g (34 mmol) of benzoyl isothiocyanate are stirred
in 60 ml of ethyl acetate for 2 hours at room temperature.
- The precipitated solid is suction filtered, washed with
ethyl acetate and recrystallised from ethanol. 11.1 g
(83%) of colourless crystals, m.p.126-1270C, are obtained.
C23H22N2o2s (390.5)
'.J
b) S-Methyl-N-[2-(diphenylmethoxy)ethyl]-isothiuronium
iodide
11.1 g -(28 mmol) of Nl-benzoyl-N2-[2-(diphenyl-
methoxy)ethyl]-thiourea are boiled up with 4.15 g
(30 mmol) of potassium carbonate in 200 ml of methanol and
ml of water for 40 minutes. After removal of the
solvent by evaporation under vacuum, the residue is taken
up with 20 ml of water and the aqueous phase is extracted
four times with 30 ml of dichloromethane. The combined
organic phases are dehydrated with sodium sulphate,
filtered and concentrated by e~Japoration under ~ac~um. The residue is
ta~en up with 100 ml of e~anol ana with 2.1 ml(33 ~mol) ~ethyl iodide
2~337~33
stirred up for 20 hours at room temperature. 11.4 g (94%) of a
colourless, hi~hly viscous oil are obtained after removal of the
solvent by evaporation under vacuum.
C17H21IN2S (428.3)
c) N1-[3-(lH-Imidazol-4-yl)propyl]-N2-[2-(diphenyl
methoxy)ethyl]-guanidine hydroiodide
1.73 g (4 mmol) of S-methyl-N-[2-(diphenylmethoxy)-
ethyl]-isothiuronium iodide and 0.50 g (4 mmol) of 3-(lH-
imidazol-4-yl)-propylamine are boiled under reflux in 20
ml of acetonitrile for 3 hours. After removal of the
solvent by evaporation under vacuum and chromatographic
purification on silica gel, using dichloromethane/methanol
(80:20) of solvent, 1.41 g (70~) of a colourless,
amorphous solid are obtained.
C22H2gINsO (505-4)
H-NMR data: ~ = 1.7 - 2.1 (m) 2 H,
(CD30D, TMS as 2.7 (t) 2 H,
internal standard) 3.1 - 3.8 (m) 6 H
4.9 (broad) 5 H, replace-
able by D20,
5.6 (s) 1 H,
7.0 (s) 1 H,
7.2 - 7.6 (m) 10 H
8.0 (s) 1 H, ppm.
Example 6
N1-[3-(3,5-Difluorophenyl)-3-(pyrldin-2-yl)propyl]-N2-
[3-(lH-imidazol-4-yl)propyl]guanidine-trihydrochloride
3 ~
F ~ F
~ CH-CH2-CH2-NH-C-~H-CH2-CH2-CH2 ~ NH ~ 3 HCl
a) Nl-Benzoyl-N2-[3-(3,5-difluorophenyl)-3-(pyridin-2-yl)-
propyl]-N3-[3-(lH-imidazol-4-yl)propyl]guanidine
1.24 g of 3-(3,5-Difluorophenyl)-3-(pyridin-2-yl)-
propylamine and 1.59 g (5 mmol) of N-benzoyl-diphenyl-
imidocarbonate are stirred together with 20 ml ofmethylene chloride at room temperature for 20 minutes.
The solvent is then distilled off under vacuum and the
oily residue is taken up with 30 ml of pyridine and is
heated to 1009C for 45 minutes after the addition of
0.65 g (5.2 mmol) of 3-(lH-imidazol-4-yl)propylamine.
The reaction mixture is concentrated by evaporation under
vacuum and the residue is taken up with 5% hydrochloric
acid and extracted with ether. It is then made alkaline
with ammonia and shaken with methylene chloride and the
organic phase is washed with water, dehydrated over sodium
sulphate and concentrated by evaporation under vacuum.
The reaction product is isolated and purified by prepara-
tive layer chromatography on silica gel 60 PF254 contain-
ing gypsum (Solv~nt: chloroform/methanol 99.5:0.5,
ammoniacal atmosphere). 1.3 g (52~) of a colourless,
amorphous solid are obtained after concentration of the
eluates by evaporation.
C28H28F2N60 (502.6)
2~3~33
H-NMR data: ~ = 1.96 (m) 2 H,
(CDCl3, TMS as 2.3 (broad) 1 H,
internal standard) 2.6 - 2.8 (m) 3 H,
3.34 (broad) 2 H,
3.5 (broad) 1 H,
3.9 (broad) 1 H,
4.17 (dd) 1 H,
6.6 - 7.8 (m) 11 H,
8.12 (d) 2 H,
8.58 (d) 1 H,
10.3 - 10.9 (broad)
1 H, replaceable by
D2O, ppm.
-'
b) Nl-[3-(3,5-Difluorophenyl)-3-(pyridin-2-yl)propyl]-N2-
[3-(lH-imidazol-4-yl)propyl]guanidine
0.76 g (1.5 mmol) of Nl-benzoyl-N2-[3-(3,5-
difluorophenyl) -3 - (pyridin-2-yl) propyl] -N3 - [3 - (lH-
imidazol-4-yl)propyl]guanidine are heated under reflux in
40 ml of 20% hydrochloric acid for 10 hours. The hydro-
20 chloric acid solution is then extracted three times withether, concentrated to dryness under vacuum and dried in a
high vacuum.
Yield: 0.65 g (85%) of the trihydrochloride in the form of
a hygroscopic, amorphous solid.
25 C21H24F2N6 3HCl (507.8)
MS (FAB method): m/z (rel. Int. [%]) = 399 ([M+H]+,80),
232 (100), 204 (18), 109 (60), 100 (36), 95 (11).
18
~6~7~3
H-NMR data: ~ = 1.85 (m) 2 H,
(DMSO-d6, TMS as 2.35 - 2.65 (m) 2 H,
internal standard) 2.72 (t) 2 H,
3.0 - 3.3 (m) 4 H,
4.78 (t) 1 H.
7.16 (dd) 1 H,
7.36 ~d) 2 H,
7.51 (s) 1 H,
7.62 (s) 2 H, replace-
able by D2O,
7.76 (dd) 1 H,
8.02 (m) 3 H, 2 H,
replaceable by D20,
8.32 (dd) 1 H,
8.75 (d) 1 H,
9.05 (s) 1 H,
14.45 (broad) 1 H,
replaceable by D20,
14.8 (broad) 1 H,
replaceable by D2O ppm.
Example 7
Nl-[2-[N-(5-Bromo-3-methyl-pyridin-2-yl)-benzylamino]-
ethyl]-N2-[3-(lH-imidazol-4-yl)propyl]guanidine-tri-
hydrochloride
3r CH3 NH
`N-cH2cH2NH-c-NH-cH2cH2cH2-¢ ~ ,c 3 HCl
CH2 N
19
2~'7433
1.15 g (2.0 mmol) of Nl-benæoyl-N2-[2-[N-(5-bromo-
3-methylpyridin-2-yl)-benzylamino]ethyl]-N3-[3-(lH-
imidazol-4-yl)propyl]-guanidine are boiled in 20 ml of
conc. hydrochloric acid for 20 hours. The aqueous solution
is concentrated by evaporation to about half its volume
and extracted with 3 x 20 ml of diethylether.
The aqueous phase is then filtered, concentrated to
dryness under vacuum and concentrated twice more by
evaporation under vacuum, each time with 20 ml of absolute
ethanol. The residue is recrystallised from isopropanol.
Yield: 0.82 g (71%) of a colourless, highly hygroscopic
solid.
C22H31BrCl3N7 (579.80)
1H-NMR data: ~ = 1.80 - 2.18 (m) 2 H
15 (CD30D, TMS as 2.61 (s) 3 H,
internal standard) 2.89 (t) 2 H,
3.34 (t) 2 H,
3.60 (m) 2 H,
3.83 (m) 2 H,
4.15 (t) 2 H,
4.9 (broad) 7 H,
7.37 - 7.55 (m) 6 H
8.84 (d) 1 H,
8.92 (d) 2 H, ppm.
Example 8
N1-[4-(5-Bromo-3-methyl-pyridin-2-yl)butyl]-N2[3-(lH-
imidazol-4-yl)propyl]guanidine-trihydrochloride
Br~ CH B NH
N J--CH2~CH2CH2CH2-NH-C-I`~H-C~rl2CH2CH2-¢ N x 3 HCl
~1
H
~037433
1.00 g (2 mmol) of Nl-Benzoyl-N2[4-(5-bromo-3-
- methyl-pyridin-2-yl)butyl]-N3-[3-(lH-imidazol-4-yl)-
propyl]-guanidine are boiled in 20 ml of conc. hydro-
chloric acid for 18 hours. The aqueous solution, diluted
to 40 ml after cooling, is extracted with 4 x 20 ml of
diethylether, filtered and concentrated by evaporation
under vacuum. The residue is taken up twice with 20 ml of
absolute ethanol and concentrated by evaporation. The
crude product obtained is then converted into the base
with sodium methylate and chromatographed on aluminium
oxide, using ethyl acetate/methanol (1:1). The main
fraction is taken up with 5 ml of water after concentra-
tion by evaporation, 0.5 ml of conc. hydrochloric acid are
added and the product is concentrated by evaporation under
vacuum. After it has been again concentrated by evapora-
tion with 20 ml of absolute ethanol, 0.62 g (60%) of the
title compound are obtained in the form of a colourless,
hygroscopic solid.
C17H28BrC13N6 (502-71)
,
20 lH-NMR data: ~ = 1.68 - 2.22 (m) 6 H,
(CD30D, TMS as 2.61 (s) 3 H,
internal standard) 2.91 (t) 2 H,
3.05 - 3.52 (m) 6H,
4.95 (broad) 7 H,
7.61 (s) 1 H,
8.89 (d) 1 H,
9.10 (d) 2 H, ppm.